Abstract
Background
Vitamin D and related compounds have been used to prevent osteoporotic fractures in older people. This is the third update of a Cochrane review first published in 1996.
Objectives
To determine the effects of vitamin D or related compounds, with or without calcium, for preventing fractures in post‐menopausal women and older men.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (to December 2012), the Cochrane Central Register of Controlled Trials (2012, Issue 12), MEDLINE (1966 to November Week 3 2012), EMBASE (1980 to 2012 Week 50), CINAHL (1982 to December 2012), BIOSIS (1985 to 3 January 2013), Current Controlled Trials (December 2012) and reference lists of articles.
Selection criteria
Randomised or quasi‐randomised trials that compared vitamin D or related compounds, alone or with calcium, against placebo, no intervention or calcium alone, and that reported fracture outcomes in older people. The primary outcome was hip fracture.
Data collection and analysis
Two authors independently assessed trial risk of selection bias and aspects of methodological quality, and extracted data. Data were pooled, where possible, using the fixed‐effect model, or the random‐effects model when heterogeneity between studies appeared substantial.
Main results
We included 53 trials with a total of 91,791 participants. Thirty‐one trials, with sample sizes ranging from 70 to 36,282 participants, examined vitamin D (including 25‐hydroxy vitamin D) with or without calcium in the prevention of fractures in community, nursing home or hospital inpatient populations. Twelve of these 31 trials had participants with a mean or median age of 80 years or over.
Another group of 22 smaller trials examined calcitriol or alfacalcidol (1‐alphahydroxyvitamin D3), mostly with participants who had established osteoporosis. These trials were carried out in the setting of institutional referral clinics or hospitals.
In the assessment of risk of bias for random sequence generation, 21 trials (40%) were deemed to be at low risk, 28 trials (53%) at unclear risk and four trials at high risk (8%). For allocation concealment, 22 trials were at low risk (42%), 29 trials were at unclear risk (55%) and two trials were at high risk (4%).
There is high quality evidence that vitamin D alone, in the formats and doses tested, is unlikely to be effective in preventing hip fracture (11 trials, 27,693 participants; risk ratio (RR) 1.12, 95% confidence intervals (CI) 0.98 to 1.29) or any new fracture (15 trials, 28,271 participants; RR 1.03, 95% CI 0.96 to 1.11).
There is high quality evidence that vitamin D plus calcium results in a small reduction in hip fracture risk (nine trials, 49,853 participants; RR 0.84, 95% confidence interval (CI) 0.74 to 0.96; P value 0.01). In low‐risk populations (residents in the community: with an estimated eight hip fractures per 1000 per year), this equates to one fewer hip fracture per 1000 older adults per year (95% CI 0 to 2). In high risk populations (residents in institutions: with an estimated 54 hip fractures per 1000 per year), this equates to nine fewer hip fractures per 1000 older adults per year (95% CI 2 to 14).
There is high quality evidence that vitamin D plus calcium is associated with a statistically significant reduction in incidence of new non‐vertebral fractures. However, there is only moderate quality evidence of an absence of a statistically significant preventive effect on clinical vertebral fractures. There is high quality evidence that vitamin D plus calcium reduces the risk of any type of fracture (10 trials, 49,976 participants; RR 0.95, 95% CI 0.90 to 0.99).
In terms of the results for adverse effects: mortality was not adversely affected by either vitamin D or vitamin D plus calcium supplementation (29 trials, 71,032 participants, RR 0.97, 95% CI 0.93 to 1.01). Hypercalcaemia, which was usually mild (2.6 to 2.8 mmol/L), was more common in people receiving vitamin D or an analogue, with or without calcium (21 trials, 17,124 participants, RR 2.28, 95% CI 1.57 to 3.31), especially for calcitriol (four trials, 988 participants, RR 4.41, 95% CI 2.14 to 9.09), than in people receiving placebo or control. There was also a small increased risk of gastrointestinal symptoms (15 trials, 47,761 participants, RR 1.04, 95% CI 1.00 to 1.08), especially for calcium plus vitamin D (four trials, 40,524 participants, RR 1.05, 95% CI 1.01 to 1.09), and a significant increase in renal disease (11 trials, 46,548 participants, RR 1.16, 95% CI 1.02 to 1.33). Other systematic reviews have found an increased association of myocardial infarction with supplemental calcium; and evidence of increased myocardial infarction and stroke, but decreased cancer, with supplemental calcium plus vitamin D, without an overall effect on mortality.
Authors' conclusions
Vitamin D alone is unlikely to prevent fractures in the doses and formulations tested so far in older people. Supplements of vitamin D and calcium may prevent hip or any type of fracture. There was a small but significant increase in gastrointestinal symptoms and renal disease associated with vitamin D and calcium. This review found that there was no increased risk of death from taking calcium and vitamin D.
Plain language summary
Vitamin D and related vitamin D compounds for preventing fractures resulting from osteoporosis in older people
Why do older people suffer bone fractures?
Hip fractures and several other types of fractures are very common in post‐menopausal women and older men due to age‐related weakening of their bones (osteoporosis).
What is the impact of bone fractures in older people?
Fractures due to osteoporosis often occur in the hip, wrist or spine and can lead to considerable disability or even death. Those who survive often have reduced mobility and may require greater social and nursing care.
Why might vitamin D help?
Vitamin D is necessary for building strong bone. Older people often have low vitamin D levels because of lack of exposure to sunlight and low consumption of vitamin D in their diet. Therefore, it has been suggested that taking additional vitamin D in the form of supplements may help to reduce the risk of fractures of the hip and other bones.
Purpose of this review
To investigate the effects of vitamin D or vitamin D‐related supplements, taken with or without calcium supplements, for preventing fractures in post‐menopausal women and older men.
Conduct of this review
The review authors searched the medical literature up to December 2012, and identified 53 relevant medical trials, with a total of 91,791 people taking part. The trials reported fracture outcomes in postmenopausal women or men aged over 65 years from community, hospital and nursing‐home settings. These trials compared vitamin D or related supplements with – or without ‐ calcium supplements, against fake supplements (placebo), no supplement or calcium supplements alone.
Findings of this review
The review found reliable evidence that taking vitamin D only, in the forms tested in the trials, is unlikely to prevent fractures. However, reliable evidence showed that vitamin D taken with additional calcium supplements slightly reduces the likelihood of hip fractures and other types of fracture. The review found that there was no increased risk of death from taking vitamin D and calcium.
Although the risk of harmful effects (such as gastrointestinal (stomach) symptoms and kidney disease) from taking vitamin D and calcium is small, some people, particularly with kidney stones, kidney disease, high blood calcium levels, gastrointestinal disease or who are at risk of heart disease should seek medical advice before taking these supplements.
Summary of findings
Summary of findings 1. Vitamin D [D2, D3 or 25(OH)D] plus calcium compared with control or placebo for preventing fractures in older people.
| Vitamin D (D2, D3 or 25(OH)D) plus calcium compared with control or placebo for preventing fractures in older people | ||||||
|
Patient or population: post‐menopausal women and older people at risk of osteoporotic fractures Settings: community or institutional Intervention: vitamin D (D2, D3 or 25(OH)D) plus calcium Comparison: control or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
Comments Notes on assessment of the quality |
|
| Assumed risk | Corresponding risk | |||||
| No vitamin D plus calcium | Vitamin D plus calcium | |||||
|
Persons sustaining new hip fracture (1 year estimate)1 |
Lower risk population2 | RR 0.84 (0.74 to 0.96) | 49,853 participants (9 trials) | ⊕⊕⊕⊕ high | ||
| 8 per 1000 | 7 per 1000 (6 to 8) | |||||
| High risk population3 | ||||||
| 54 per 1000 | 45 per 1000 (40 to 52) | |||||
|
Persons sustaining new non‐vertebral fracture (1 year estimate)1 |
Overall population4 |
RR0.86 (0.78 to 0.96) |
10,380 participants (8 trials) |
⊕⊕⊕⊕ high | ||
| 39 per 1000 | 34 per 1000 (30 to 37) | |||||
|
Persons sustaining new vertebral fracture or deformity (1 year estimate)1 |
Overall population5 (see notes) |
RR0.89 (0.74 to 1.09) |
42,185 participants (4 trials) |
⊕⊕⊕⊝ moderate | Variation and difficulties in the diagnosis and definition of vertebral fractures means that estimates of control risk are likely to be very provisional (and probably underestimates). This also reduces the quality of this evidence, downgraded to moderate evidence | |
| 2 per 1000 | 2 per 1000 (1 to 2) | |||||
|
Persons sustaining any new fracture (1 year estimate)1 |
Lower risk population6 |
RR 0.95 (0.90 to 0.99) |
49,976 participants (10 trials) |
⊕⊕⊕⊕ high | ||
| 26 per 1000 | 25 per 1000 (23 to 26) | |||||
| High risk population7 | ||||||
| 75 per 1000 | 71 per 1000 (68 to 74) | |||||
|
Persons with hypercalcaemia ‐ Vitamin D (D2, D3 or 25(OH)D) plus calcium (1 year estimate)1 |
Overall population8 |
RR 3.29 (0.37 to 29.14) |
3853 participants (2 trials) |
⊕⊕⊝⊝ low | Downgraded for imprecision: confidence interval (0.37 to 29.14) crosses line of no effect | |
| 4 per 10,000 |
13 per 10,000 (1 to 117) |
|||||
|
Persons with renal disease (calculi or insufficiency) ‐ Vitamin D (D2, D3 or 25(OH)D) plus calcium (1 year estimate)1 |
Overall population9 |
RR 1.17 (1.03 to 1.34) |
39,552 participants (2 trials) | ⊕⊕⊕⊕ high | These data are dominated by the WHI 2006 trial, which had a 7 year follow‐up. As noted, the risk of some complications are unlikely to be uniform over time | |
| 8 per 10,000 | 9 per 10,000 (8 to 11) | |||||
|
Deaths ‐ Vitamin D (D2, D3, 25(OH)D) plus calcium (1 year estimate)1 |
Overall population10 | RR 0.94 (0.87 to 1.02) | 46,794 participants (6 trials) | ⊕⊕⊕⊕ high | A similar overall result was found when data were pooled from 29 trials | |
| 57 per 1000 | 54 per 1000 (50 to 58) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1. The included studies had different follow‐ups. All estimates are calculated for 1 year follow‐up. However, the risk of some outcomes may not be uniform over time
2. Control group risk derived from median control group data across community residence studies testing vitamin D [D2, D3 or 25(OH)D] plus calcium; plus data from community studies reported in the Gillespie 2010 Cochrane review: Gillespie WJ, Gillespie LD, Parker MJ. Hip protectors for preventing hip fractures in older people. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No.: CD001255. DOI: 10.1002/14651858.CD001255.pub4
3. Control group risk derived from median control group data across institutional residence studies testing vitamin D [D2, D3 or 25(OH)D] plus calcium; plus data from institutional studies reported in Gillespie 2010
4. Control group risk derived from median control group across studies testing vitamin D [D2, D3 or 25(OH)D] alone and trials testing vitamin D [D2, D3 or 25(OH)D] plus calcium
5. Control group risk derived from median control group across studies testing vitamin D [D2, D3 or 25(OH)D] plus calcium
6. Control group risk derived from median control group data across community residence studies testing vitamin D [D2, D3 or 25(OH)D] plus calcium
7. Control group risk derived from median control group data across institutional residence studies testing vitamin D [D2, D3 or 25(OH)D] plus calcium
8. Control group risk derived from median control group across studies testing vitamin D [D2, D3 or 25(OH)D] alone. Five of 10 trials in this group and the two trials (Chapuy 1992 and Chapuy 2002) reporting on hypercalcaemia, reported no events in the control group
9. Control group risk derived from median control group across studies testing vitamin D [D2, D3 or 25(OH)D] alone and trials testing vitamin D [D2, D3 or 25(OH)D] plus calcium
10. Control group risk derived from median control group across studies testing vitamin D [D2, D3 or 25(OH)D] alone and trials testing vitamin D [D2, D3 or 25(OH)D] plus calcium
Background
Description of the condition
Osteoporosis, a gradual loss of bone mass, is a complex, chronic, multifactorial process, which is a normal part of ageing. It derives its public health importance from its association with the development of characteristic fractures late in life, and because of the increasing number of people living longer, particularly in industrialised societies. Both men and women are affected, but the main burden of disease is in post‐menopausal women. Osteoporotic fractures include those of the hip, wrist and spinal vertebrae, and can lead to considerable disability. Incidence rates for hip fracture vary by a factor of more than ten worldwide, with age‐standardised incidence rates highest in Scandinavia and lowest in Africa and South America (Kanis 2012). Many patients with hip fractures have other medical conditions, but the hip fracture itself may account for 17% to 32% of deaths in patients with hip fracture, and 1.5% of all deaths in people aged 50 years or older (Kanis 2003). Survivors of hip fracture are often disabled by reduced mobility and may require greater social and nursing care (Johnell 2004).
A high proportion of vertebral fractures do not come to clinical attention and may not cause symptoms, but undiagnosed vertebral fractures may be associated with increased back pain and functional limitation (Nevitt 1998). The criteria used to define vertebral fractures in radiographs may differ, but studies suggest that up to half of women over the age of 75 years in Europe and North America have vertebral fractures (Cummings 2002).
Between 1988 and 1998, in England and Wales, the estimated lifetime risk of hip fracture for a woman aged 50 years was 11.4%, and for a man aged 50 years was 3.1% (Van Staa 2001). Similarly, the lifetime risk for a woman aged 50 years for a distal forearm fracture was 16.6% and for a clinically evident vertebral fracture, 3.1%; for a man aged 50 years these risks were 2.9% and 1.2% respectively (Van Staa 2001). In the last 20 years hip fracture rates have tended to stabilise ‐ or even fall ‐ in Europe, North America, Australia and New Zealand, but rates may be rising in Asia (Cooper 2011).
Description of the intervention
The primary goal of the various interventions, such as vitamin D, that have been proposed for osteoporosis is the prevention of fractures. While slowing progressive bone loss plausibly reduces fracture rates, other factors, particularly the fall rate in older people, are clearly involved (Cummings 1995). Effective strategies may require prophylactic measures many years before fractures are likely to occur. The conduct of randomised controlled trials of effectiveness in this context is difficult. Financial, academic and commercial pressures have favoured the selection of short‐term intermediate outcomes, such as changes in bone mineral density (BMD), as evidence of efficacy, but the effectiveness of interventions is best measured using fracture outcomes.
How the intervention might work
Vitamin D is one of a number of agents with known biological effects on mineral homeostasis, acting mainly upon the intestine, kidneys and bone. Intestinal calcium absorption is stimulated and bone mass protected (Norman 1993), although the benefit may be largely lost within two years of discontinuation of the supplement (Dawson‐Hughes 2000). Vitamin D is mostly derived from exposure of the skin to ultraviolet sunlight. Although there are a few dietary sources, such as oily fish, these contribute relatively little vitamin D (known as D3, cholecalciferol), except in people who consume oily fish several times a week. Synthetic vitamin D (known as D2, ergocalciferol) is frequently the form provided in supplements, but this may not be equivalent to vitamin D3 (Houghton 2006).
Administration of vitamin D, and particularly its derivatives (analogues) (see Table 2 for details of nomenclature, synonyms and abbreviations of vitamin D), may carry a risk of hypercalcaemia and hypercalciuria (high levels of calcium in the blood and urine, respectively). There is a winter decline in circulating vitamin D concentrations in older people living at high latitudes that may be correctable by a single injection of cholecalciferol (Khaw 1994). However, the bioavailability of intramuscular vitamin D is variable, and may be very poor; thus intermittent high‐dose oral supplementation may be more reliable (Romagnoli 2008). The rates of hip fracture vary annually with a winter peak in both Northern and Southern hemispheres (Jacobsen 1990; Lau 1995). Inadequate vitamin D levels have been demonstrated in patients with osteoporosis (Lips 2006) ‐ especially those with hip fracture ‐ in many countries, although low levels may be influenced by the fracture itself (Pieper 2007). Adequate calcium intake may also protect bone mass (Cumming 1990), but calcium supplements may provoke gastrointestinal symptoms.
1. Vitamin D nomenclature, synonyms and abbreviations.
| Vitamin D | Synonyms | Graph abbreviations |
| Vitamin D: two forms are vitamin D2 and vitamin D3 | ||
| Vitamin D2 | Ergocalciferol | D2 |
| Vitamin D3 | Cholecalciferol | D3 |
| 25‐hydroxy vitamin D: vitamin D with one hydroxyl group added equivalent to liver activation | Calcidiol | 25(OH)D |
| 1‐alphahydroxyvitamin D3*: vitamin D with one hydroxyl group added equivalent to renal activation | Alfacalcidol | 1‐alpha(OH)D3 |
| 1,25‐dihydroxyvitamin D3*: vitamin D with two hydroxyl groups added equivalent to both liver and renal activation | Calcitriol | 1,25(OH)2D3 |
| 24R,25(OH)2 vitamin D3*: vitamin D with two hydroxyl groups added equivalent to both liver and renal activation |
* denotes analogues/derivatives Ca: abbreviation for calcium in graphs
Why it is important to do this review
Vitamin D is an attractive candidate agent for use in public health interventions, particularly if it can be given intermittently in high dosage. A randomised trial widely quoted as supporting the effectiveness of vitamin D evaluated co‐administration of daily oral vitamin D3 and calcium supplements (Chapuy 1992). Calcium co‐supplementation requires tablets to be taken daily, which may influence compliance; furthermore, calcium may be associated with gastrointestinal side‐effects (RECORD 2005). A systematic review of current evidence to assess for effectiveness of vitamin D analogues, with and without calcium, in fracture prevention in older people should inform practice and research. This is an update of a Cochrane review first published in 1996, and previously updated in 2009 (Avenell 2009a).
Objectives
To determine the effects of vitamin D or related compounds, with or without calcium, for preventing fractures in post‐menopausal women and older men.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised trial or quasi‐randomised (method of allocating participants to a treatment that is not strictly random, e.g. by date of birth, hospital record number, alternation) trial meeting the criteria for participants, interventions or outcomes listed below.
Types of participants
Trials with post‐menopausal women or older men (mean or median population age over 65 years), or both. We included trials whose participants had neurological disease impairing mobility (for example, after stroke or in Parkinson's disease), but excluded studies focused on participants on corticosteroid therapy, which is the subject of another Cochrane review (Homik 1998), and studies where vitamin D was given to patients selected on the basis of renal failure.
Types of interventions
Administration of a vitamin D or a vitamin D‐related compound, either alone or in combination with calcium supplementation, compared with a placebo, no intervention, or the administration of calcium supplements (see Table 2 for details of nomenclature, synonyms and abbreviations of the vitamin D preparations considered here). Interventions incorporating treatments other than vitamin D and calcium were not considered, e.g. vitamin D and hormone replacement therapy (HRT) compared with HRT alone. Interventions examining eldecalcitol (ED‐71, 1alpha,25‐dihydroxy‐2beta‐(3‐hydroxypropoxy) vitamin D3) were also not included.
In defining a comparison, advice only on dietary modification to increase calcium intake was not considered as supplementation.
Types of outcome measures
Primary outcomes
Hip fracture
Secondary outcomes
Any non‐vertebral fracture. Non‐vertebral fractures were defined as all fractures except those of the vertebrae, but including hip fractures.
Vertebral fracture (two outcomes were sought: clinical fracture events, and new vertebral deformity identified by radiological morphometry or semi‐quantitative reading by a radiologist, using routine radiographs, according to a defined experimental protocol. Either of these methods appear to provide a valid approach to defining vertebral deformity (Black 1995))
Any new fracture. In previous versions of the review, the category 'any new fracture' was classified as fractures not covered by hip, vertebral or non‐vertebral categories, or where the site of fracture was unclear. This meant that some of the very large community trials were not analysed together, because they chose to report 'non‐vertebral fractures' or 'hip fractures' or 'vertebral fractures' but not 'all fractures' and these numbers were not available or could not be calculated from the data without risk of double counting. As a new feature in this version of the review for Analyses 1 to 4 (comparisons involving vitamin D), the category 'all fractures' includes data from non‐vertebral fractures (or hip or vertebral fractures if not given), if the data for 'all fractures' are not available (see Differences between protocol and review).
Adverse effects (hypercalcaemia, renal disease, gastrointestinal symptoms, all as defined by the investigators; death)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (to December 2012), the Cochrane Central Register of Controlled Trials (2012 Issue 12), MEDLINE (1966 to November Week 3 2012), EMBASE (1980 to 2012 Week 50), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to December 2012) and BIOSIS (1985 to 3 January 2013).
In MEDLINE (OVID Web), we combined subject specific terms with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011), and modified for use in other databases (see Appendix 1). For this update, the search results were limited to 2007 onwards. Details of the previous search strategies can be found in past versions of the review, most recently Avenell 2009b.
We identified ongoing studies by searching all registers in Current Controlled Trials (December 2012).
Searching other resources
We also checked reference lists of articles and contacted active researchers in the field. We handsearched abstracts published in the Journal of Bone and Mineral Research (1986 to 2012 volume 27), Bone (1998 to December 2012), Calcified Tissue International (1998 to December 2012) and Osteoporosis International (1998 to December 2012).
We placed no restrictions on the language of publication.
Data collection and analysis
Selection of studies
After initial screening by one person, the citations of potentially eligible studies were entered into Review Manager software (RevMan 2012). Full text copies of these references were retrieved and at least two authors independently sorted them into included and excluded studies on the basis of the criteria above.
Data extraction and management
Two review authors extracted data independently using a data extraction form developed for previous versions of this review. Any unresolved disagreement between authors was adjudicated by a third author. One author entered qualitative details and published data describing study population, interventions and outcomes into RevMan, and another author checked data entry for outcomes.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for the two domains of randomisation sequence generation and allocation concealment, according to the Cochrane Collaboration 'Risk of bias' tool (Higgins 2011). For the remaining assessment of risk of bias, two review authors independently assessed methodological quality using a scoring schedule and a coding instruction manual, which was used in previous versions of this review. The assessment protocol scored each item between 0 and 2 (see Table 3 for the quality assessment items, B to J, and possible scores). Disagreement between review authors was adjudicated by a third author.
2. Quality assessment items and possible scores.
| Items | Scores |
| Item B Were the outcomes of participants who withdrew or were excluded after allocation described and included in an "intention‐to‐treat" analysis? | Score 2 if adequate detail of withdrawals and exclusions after randomisation exists, and an intention‐to‐treat analysis has been, or can be carried out Score 1 if number and reasons for withdrawal are mentioned but intention to treat analysis is not possible Score 0 if inadequate detail exists to allow the author to check or carry out an intention to treat analysis, or obvious differences with no adjustment |
| Item C Were the outcome assessors blind to assignment status? | Score 2 if blinding of all possible outcome assessors is clearly established Score 1 if there is a small or moderate chance of unblinding of assessors, or some but not other assessors who could have been blinded were blinded Score 0 if no attempt to blind assessors to the assignment of treatment is reported |
| Item D Were the treatment and control group comparable at entry? | Score 2 if groups are demonstrably comparable in respect of potential confounding factors on inspection of the characteristics on entry (means with some expression of the variation e.g. SD, SE, confidence intervals are required), or differences between groups adjusted for in the analysis (stratification, Mantel‐Haenszel technique, logistic regression, multiple regression, multivariable techniques) Score 1 if confounding appears small: although noted, adjustment has not been made Score 0 if description of the treatment groups at baseline, either in text or table, is inadequate to confirm comparability for all plausibly important confounders, or statistically significant differences between the groups are present but no adjustment has been made in the analysis |
| Item E Were the subjects blind to assignment status following allocation? | Score 2 if effective action has been taken to blind participants to assignment Score 1 if in a drug study, or in a study comparing a physical modality with a control, it is unclear whether participants were made aware, or could have become aware, of their assignment prior to measurement of outcomes, or the nature of the trial intervention is such that it is unlikely that they will have effects which allow identification of assignment (e.g. calcium supplements versus placebo) Score 0 if in a drug study, no treatment rather than a placebo is used, or in a placebo‐controlled drug study or in a study of comparable physical modalities, participants became aware of their allocation before outcome assessment and analysis |
| Item F Were the providers of care blind to assignment status? | Score 2 if the study is clearly double or triple blind Score 1 if it is unclear whether the treatment providers were blinded to the allocation Score 0 if in a placebo controlled drug trial, the providers of care were informed of the treatment allocation before outcome assessment and analysis, or a physical modality was used in one or more arms of the trial |
| Item G Were the care programmes, other than the trial options, identical? |
Score 2 if it is clear that the care programmes other than the trial interventions were identical
Score 1 if differences between the programmes are trivial Score 0 if the nature of the care programmes other than the trial interventions is unclear, or there are important differences between the programmes offered, other than the trial interventions |
| Item H Were the inclusion and exclusion criteria for entry clearly defined? | Score 2 if the inclusion and exclusion criteria are clearly defined and indicate that individuals currently exposed to a trial intervention were excluded e.g. vitamin D analogue, hormone replacement therapy Score 1 if the inclusion and exclusion criteria as described allow the possibility that individuals may have entered the study currently exposed to a trial intervention, or description of the inclusion and exclusion criteria is inadequate to determine how the sample was made up Score 0 if no description, other than age and gender, of inclusion and exclusion criteria was provided |
| Item J Was the ascertainment of fractures and other outcomes active and of clinically appropriate duration? | Score 2 if some form of concurrent collection of data about fracture e.g. subjects given postcards to mail back etc., with confirmation by interview, and by radiograph if positive, or, for vertebral fracture, routine confirmation by radiograph Score 1 if contact was made on a regular basis e.g. 6‐monthly phone call to establish if fracture had occurred or not, with confirmation by radiograph if positive Score 0 if fracture was registered as an outcome without confirmation by radiograph |
Item A, which considered allocation concealment, was removed as this now forms part of the risk of bias assessment
Measures of treatment effect
We calculated risk ratios (RR) and 95% confidence intervals (95% CI) for dichotomous outcomes. For fracture outcomes we used the number, or proportion, of participants with at least one new fracture at the end of the observation period to calculate the RR and 95% CI.
Unit of analysis issues
For meta‐analyses including the cluster‐randomised trial by Law 2006, the review authors made adjustments to the number of participants with outcomes and the denominators in Law 2006 using an intraclass correlation coefficient of 0.026 (derived from Dyer 2004), using methods described in Higgins 2011a. This means that the numbers of participants with outcomes and denominators in the meta‐analyses in which this trial is included do not reflect the total number actually randomised and having events.
Dealing with missing data
Denominators used in calculating the incidence of outcomes for each group in each study were all participants randomised to that group (intention‐to‐treat analysis), unless that information was unavailable from the published reports or after contact with investigators, in which case we used the denominator in the published report.
Assessment of heterogeneity
Heterogeneity was assessed using the I2 test (Higgins 2003), in conjunction with the P value from the Chi2 test and visual inspection.
Assessment of reporting biases
Where there were at least 10 trials in a meta‐analysis, the possibility of publication bias was assessed via visual inspection of funnel plots.
Data synthesis
Where it was possible and appropriate to pool data, the resulting pooled risk ratio was calculated with 95% CIs. The fixed‐effect model was used to pool data unless substantial heterogeneity was present, in which case we used the random‐effects model.
Some trials, such as the RECORD 2005 trial, had a factorial design, e.g. calcium and vitamin D supplementation (group 1) and vitamin D supplementation (group 2) compared with calcium supplementation (group 3) and placebo (group 4). In such cases the data in the meta‐analyses of fractures refer only to the individual groups of the study (group 1 versus group 3, or group 2 versus group 4), and do not make use of the factorial design to explore the full range of combinations of supplements because of the potential interaction of vitamin D and calcium, with the exception of the adverse events meta‐analyses.
Subgroup analysis and investigation of heterogeneity
In previous versions of the review, we set out two secondary hypotheses that anticipated an effect or a greater effect of supplementation with vitamin D or a vitamin D‐related compound, either alone, or in combination with calcium, on the incidence of hip, non‐vertebral, vertebral or any new fracture in a) older people with a history of previous osteoporotic fracture, and b) older people who are more likely to be frail, which we defined as residents in institutions such as nursing homes or residential care homes. Subgroup analyses were undertaken to explore these two secondary hypotheses; i.e. by history of osteoporotic fracture and by residential status.
We investigated whether the results of subgroups were significantly different by inspecting the overlap of CIs and by performing the test for subgroup differences available in RevMan (RevMan 2012).
Sensitivity analysis
We undertook a post hoc sensitivity analysis for vitamin D alone versus placebo or no treatment comparison. We examined the results for meta‐analyses with and without three trials (Law 2006; Smith 2007; Vital D); this was because in Law 2006, the effect of clustering might not have been adequately controlled in our meta‐analyses; and in Smith 2007 and Vital D, there may have been supratherapeutic dosing (Dawson‐Hughes 2010).
'Summary of findings' tables
We have presented the main results of the comparison for vitamin D plus calcium versus placebo or no treatment in a 'Summary of findings' (SoF) table. We graded the evidence as 'very low', 'low', 'moderate' or 'high' in accordance with the GRADE working group criteria.
Results
Description of studies
Results of the search
The search was updated from August 2007 to December 2012. We screened a total of 4028 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (31), the Cochrane Central Register of Controlled Trials (372), MEDLINE (901), EMBASE (1398), CINAHL (362), BIOSIS (729), and Current Controlled Trials (235).
Twenty‐one studies were identified. We included twelve of these: seven new trials (Burleigh 2007; Glendenning 2012; Janssen 2010; Mitri 2011; Ones 2007; Witham 2010; Witham 2013); one study that had been ongoing at the time of the previous update of this review (Vital D); and four studies had previously been awaiting assessment (Hayashi 1992; Nakatsuka 1997; OSTPRE‐FPS 2007; Pfeifer 2009). One study was excluded (Orimo 2011), two were placed in Ongoing studies (ANVITAD; REVITAHIP) and six await classification (Bischoff‐Ferrari 2010a; Papaioannou 2011; Petkakov 1995; TIDE 2012; Wood 2012; Xia 2009).
One previously included trial is now awaiting classification (Nuti 2006), as both trial arms included forms of vitamin D. Three previously included studies have been moved to Studies awaiting classification, whilst clarification is awaited for data queries (Sato 1997; Sato 1999a; Sato 1999b).
Overall, this review now has a total of 53 included trials, 63 excluded studies, two ongoing trials and 13 studies awaiting classification. See Figure 1 for study flow diagram.
1.
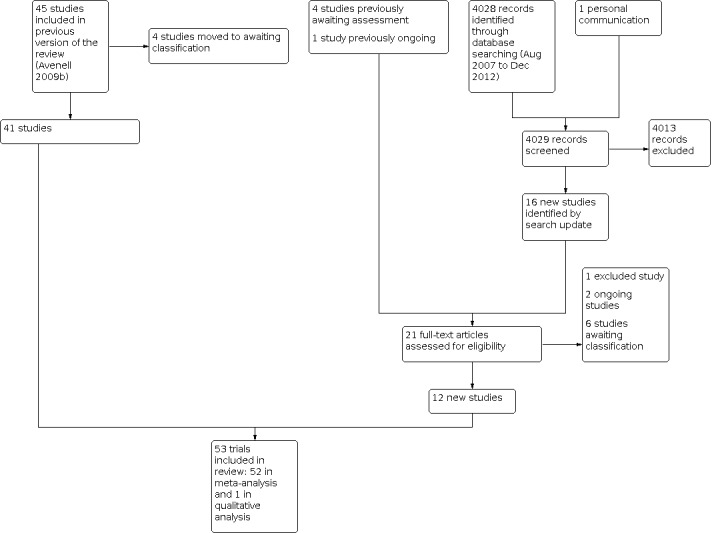
tudy flow diagram for the updated search (December 2012)
Included studies
Fifty‐three trials were included in this review: 49 were individually randomised controlled trials (RCTs), one was a cluster‐randomised trial (Law 2006), and three were quasi‐randomised (Hayashi 1992; Inkovaara 1983; Meyer 2002) (see 'Characteristics of included studies' table for full descriptions). There was a total of 91,791 participants in the 53 trials.
Broadly, the included trials fall into two main groups.
Trials with cholecalciferol, ergocalciferol or 25‐hydroxy vitamin D
In this first group, there were 31 trials; the number of participants ranged from 70 to 36,282. These trials were set in Australasia, Europe, and North America; there were none from the Far East. They examined the use of vitamin D (including 25‐hydroxy vitamin D) with or without calcium in the prevention of fractures (see Table 4 for further details of studies included in comparisons 1 to 4). These trials were set in community, nursing home or hospital inpatient populations, and generally had older participants than in the second group of trials described below. Twelve trials had participants with a mean or median age of 80 years or over (Bischoff 2003; Burleigh 2007; Chapuy 1992; Chapuy 2002; Flicker 2005; Harwood 2004; Janssen 2010; Law 2006; Lips 1996; Lyons 2007; Meyer 2002; Witham 2010). Fifteen trials included women only; none included men only (see Table 4). Only three trials specifically recruited participants who had had a previous fracture (Avenell 2004; Harwood 2004; RECORD 2005). Thirteen trials had more than 1000 participants (Chapuy 1992; Garay Lillo 1997; Law 2006; Lips 1996; Lyons 2007; Meyer 2002; OSTPRE‐FPS 2007; Porthouse 2005; RECORD 2005; Smith 2007; Trivedi 2003; Vital D; WHI 2006).
3. Characteristics of studies in comparisons 1 to 4.
| Comparison 1: Vitamin D [D2, D3 or 25(OH)D] versus control or placebo | ||||||
| Trial name in decreasing order of size | Number recruited | Setting | Previous fracture Y/N | Male or female? | Mean/median age | |
| Smith 2007 | 9440 | Multicentre general practice study in 111 sites, UK | N | Both (4354 women, 5086 men) | 79 | |
|
RECORD 2005 |
5292 | Community‐based study, UK | Y | Both (4481 women, 811 men) | 77 | |
|
Law 2006 |
3717 | Clusters of participants in 30 bedded units in care homes or entire care home if small, UK | N | Both (2825 women, 892 men) | 85 | |
|
Lyons 2007 |
3440 | Residential homes (38%), nursing or dual‐registered home (55%), sheltered accommodation (7%), Wales | N | Both (2624 women, 816 men) | 84 | |
|
Trivedi 2003 |
2686 | Community‐based study, UK | N | Both (2037 men and 649 women) | 75 | |
|
Lips 1996 |
2578 | Community‐based study, The Netherlands | N | Both (1916 women and 662 men) | 80 |
|
|
Vital D |
2258 | Community‐dwelling women in southern Victoria, Australia | Some | Women only | 76 | |
|
Meyer 2002 |
1144 | Nursing homes, Norway | N | Both (868 women, 276 men) | 85 | |
|
Glendenning 2012 |
686 | Community living (from general practice or electoral role), Perth, Australia | N | Women only | 77 | |
|
Peacock 2000 |
438 | Community study, USA |
N | Both (316 women, 122 men) | Mean age women 74 years, men 76 years | |
|
Witham 2013 |
159 | Participants recruited from primary care, secondary care and the press, Scotland, UK | N | Both (82 men, 77 women) | 77 | |
|
Harwood 2004 |
150 | Community‐based study, UK | Y | Women only | 81 | |
|
Avenell 2004 |
134 | Community‐based study, UK | Y | Both (111 women, 23 men) | 78 | |
|
Witham 2010 |
105 | Participants recruited from primary and secondary care, Scotland, UK | N | Both (69 men, 36 women) |
80 | |
|
Mitri 2011 |
92 | Community recruitment, Boston, USA | N | Both (47 women, 45 men) | 57 | |
| Comparison 2: Vitamin D [D2, D3 or 25(OH)D] and calcium versus calcium | ||||||
| Trial name in decreasing order of size | Number recruited | Setting | Previous fracture Y/N | Male or female? | Mean/median age | |
|
Garay Lillo 1997 |
6945 | Community‐based study, Spain | N | Women only | Not available | |
|
RECORD 2005 |
5292 | Community‐based study, UK | Y | Both (4481 women, 811 men) | 77 | |
| Flicker 2005 | 693 | 60 assisted living facilities and 89 nursing homes, Australia | N | Both (594 women, 31 men) | 83 | |
|
Prince 2008 |
302 | Community‐based study, Australia | N | Women only | 77 | |
| Pfeifer 2009 | 242 | Community‐based study, Austria and Germany | N | Both (181 women, 61 men) | 77 | |
|
Burleigh 2007 |
205 | Acute geriatric unit, Glasgow, Scotland, UK | N | Both (121 women, 84 men) | 83 | |
|
Komulainen 1998 |
232 | Community‐based study, Finland | N | Women only | 53 | |
|
Pfeifer 2000 |
148 | Osteology clinic, Germany | N | Women only | 75 | |
|
Avenell 2004 |
134 | Community‐based study, UK | Y | Both (111 women, 23 men) | 78 | |
|
Bischoff 2003 |
122 | Two long‐stay geriatric care units, Switzerland | N | Women only | 85 |
|
|
Janssen 2010 |
70 | Outpatient clinic of the Department of Geriatric Medicine at the University Medical Centre, Utrecht, The Netherlands | N | Women only | 81 | |
| Comparison 3: Vitamin D [D2, D3 or 25(OH)D] versus calcium | ||||||
| Trial name in decreasing order of size | Number recruited | Setting | Previous fracture Y/N | Male or female? | Mean/median age | |
|
RECORD 2005 |
5292 | Community‐based study, UK | Y | Both (4481 women, 811 men) | 77 | |
|
Peacock 2000 |
438 | Community‐based study, USA |
N | Both (316 women, 122 men) | Mean age women 74 years, men 76 years | |
|
Avenell 2004 |
134 | Community‐based study, UK | Y | Both (111 women, 23 men) | 78 | |
| Mitri 2011 | 92 | Community‐based study, USA | N | Both (47 women, 45 men) | 57 | |
| Comparison 4: Vitamin D [D2, D3 or 25(OH)D] and calcium versus control or placebo | ||||||
| Trial name in decreasing order of size | Number recruited | Setting | Previous fracture Y/N | Male or female? | Mean/median age | |
|
WHI 2006 |
36,282 | Community based women, USA |
N | Women only | 62 |
|
| RECORD 2005 | 5292 | Community‐based study, UK | Y | Both (4481 women, 811 men) | 77 | |
|
OSTPRE‐FPS 2007 |
3195 | Population‐based sample, recruited by post, northern Savonia, Finland |
N | Women only | 67 | |
|
Porthouse 2005 |
3314 | Multicentre general practice study, UK |
Some | Women only | 77 | |
|
Chapuy 1992 |
3270 | Residents of nursing homes or apartment houses for elderly people, France |
N | Women only | 84 | |
|
Chapuy 2002 |
583 | Residents of 55 apartment houses for elderly people, France | N | Women only | 85 | |
|
Dawson‐Hughes 1997 |
445 | Community‐based study, USA |
N | Both (199 men, 246 women | 71 | |
|
Harwood 2004 |
150 | Community‐based study, UK | Y | Women only | 81 | |
|
Avenell 2004 |
134 | Community‐based study, UK | Y | Both (111 women, 23 men) | 78 | |
Avenell 2004 was a small open design study, which was conducted in parallel to RECORD 2005. Law 2006 examined three‐monthly vitamin D2 versus no treatment and was the only cluster‐randomised trial; it involved 223 residential units in 118 homes for older people.
The 15 trials comparing vitamin D versus placebo or control, ranged in size from 92 to 9440 participants. The 11 trials comparing vitamin D and calcium versus calcium alone, ranged in size from 70 to 6945 participants. Four trials compared vitamin D versus calcium; these ranged in size from 92 to 5292 participants. The nine trials comparing calcium and vitamin D versus placebo or control, ranged in size from 134 to 36,282 participants.
Trials with calcitriol or alfacalcidol (1‐alphahydroxyvitamin D3)
In this second group, 22 smaller trials contributed data on calcitriol or alfacalcidol (1‐alphahydroxyvitamin D3). Seven of these trials were set in Japan (Gorai 1999; Hayashi 1992; Ishida 2004 (retracted); Nakatsuka 1997; Orimo 1994; Shiraki 1996; Ushiroyama 2001), with the rest being located in Australasia, Europe, Israel and North America. Almost all recruited from referral populations with established osteoporosis or other diseases thought to relate to vitamin D deficiency and were carried out in the setting of outpatient or research clinics. In the majority, osteoporosis had been formally diagnosed, and often the presence of one or more deformed vertebrae on an initial radiograph was required for inclusion in the trial. Most participants underwent bone density measurements, or extensive biochemical analyses of blood and urine, or assessment of musculoskeletal function. Radiological vertebral deformity or changes in bone mineral density were often the principal outcomes, although other fracture data were sometimes available. None of this group of trials had participants with a group mean or median age of 80 years or more. One trial included men only (Ebeling 2001), and three had men and women (Dukas 2004, Geusens 1986, Hayashi 1992); the rest recruited women only. The largest trial had 740 participants (Hayashi 1992). Only four of these trials had more than 200 participants each (Dukas 2004; Gallagher 2001; Hayashi 1992; Tilyard 1992).
Table 5 gives the baseline 25‐hydroxy vitamin D (25(OH)D, vitamin D with one hydroxyl group added equivalent to liver activation) levels in the intervention and control groups of the included studies, where reported. This is a laboratory measure of vitamin D status. These values have to be interpreted with considerable caution, since they depend on the laboratory and method used (Lips 1999).
4. Baseline 25‐hydroxy vitamin D in intervention and control groups.
| Study ID | 25(OH)D nmol/L |
| Aloia 1988 | Intervention 54.8 (SD 17.8); Control 66.5 (SD 29.3) |
| Arthur 1990 | Intervention 30 (SD 7.5); Control 52.5 (SD 22.5)* (Considerable difference between intervention and control) |
| Avenell 2004 | N/A |
| Bischoff 2003 | Intervention 30.8 (interquartile range 23‐55); Control 29 (interquartile range 23‐55) |
| Bolton‐Smith 2007 | Intervention 62.5 (SD 15.5); Control 57 (15.3) |
| Burleigh 2007 | Intervention 21.7 (SD 7.1); Control 24.7 (SD 10.0) |
| Caniggia 1984 | N/A |
| Chapuy 1992 | Intervention 40.0 (SD 27.5); Control 32.5 (SD 22.5) subgroups |
| Chapuy 2002 | Intervention 21.3 (SD 13.3), 22.5 (SD 16.5); Control 22.8 (SD 17.3) |
| Dawson‐Hughes 1997 | Intervention 82.5 (SD 40.8) men, 71.8 (SD 33.3) women; Control 84.0 (SD 31.8) men, 61.3 (SD 25.8) women |
| Dukas 2004 | Intervention 98.8 (SD 30.0); Control 97.8 (SD 27.3)* |
| Ebeling 2001 | Intervention 91 (SD 42); Control 86 (27) |
| Falch 1987 | N/A |
| Flicker 2005 | Intervention 61% in 25‐40 range; Control 54% in 25‐40 range |
| Gallagher 1989 | N/A |
| Gallagher 1990 | N/A |
| Gallagher 2001 | Intervention 78.0 (SD 21.6); Control 80.5 (SD 27.4) |
| Garay Lillo 1997 | Intervention 58.3 (SD 46.3); Control 64.8 (SD 51.3) subgroups |
| Geusens 1986 | N/A |
| Glendenning 2012 | 65.8 (SD 22.7) combined intervention and control subgroup |
| Gorai 1999 | N/A |
| Harwood 2004 | Intervention 28 (range 10‐67), 30 (range 12‐85), 29 (range 6‐75); Control 30 (range 12‐64) |
| Hayashi 1992 | N/A |
| Inkovaara 1983 | N/A |
| Ishida 2004 (retracted) | N/A |
| Janssen 2010 | Vitamin D and calcium 32.6 (SD 11.6); Placebo and calcium 34.3 (SD 11.5) |
| Komulainen 1998 | N/A |
| Law 2006 | Intervention 47 median (35‐102, 90th centile range) subgroup; no data for control group |
| Lips 1996 | Intervention 27 (25th‐75th centile 19‐36); Control 26 (25th‐75th centile 19‐37) subgroups |
| Lyons 2007 | N/A |
| Menczel 1994 | N/A |
| Meyer 2002 | Intervention 47 (SD 26); Control 51 (SD 33) subgroups |
| Mitri 2011 | Vitamin D and calcium 56 (SE 4), Vitamin D and placebo 66 (SE 4), Calcium and placebo 63 (SE 5), Placebos 61 (SE 3) |
| Nakatsuka 1997 | Intervention 58.4 (SD 24.5); Control 69.8 (SD 28.5) |
| Ones 2007 | N/A |
| Orimo 1994 | Intervention 58.0 (SD 22.5); Control 50.3 (SD 16.3) |
| OSTPRE‐FPS 2007 | Intervention 50.0 (SD 18.7); Control 49.1 (SD 17.7) subgroups |
| Ott 1989 | Intervention 66.8 (SD 31.5); Control 65.8 (SD 39.3) |
| Peacock 2000 | Intervention 65.0 (SD 25) men, 57.5 (SD 33) women; Control 65.0 (SD 30) men, 60.0 (SD 30) women |
| Pfeifer 2000 | Interventiion 25.7 (SD 13.6); Control 24.6 (SD 12.1) |
| Pfeifer 2009 | Vitamin D and calcium 55 (SD 18); Calcium 54 (SD 18) |
| Porthouse 2005 | N/A |
| Prince 2008 | Intervention 45.2 (SD 12.5); Control 44.3 (SD 12.7) |
| RECORD 2005 | Intervention 38.0 (SD 16.3); Control 39.5 (SD 14.8) subgroups* |
| Shiraki 1996 | N/A |
| Smith 2007 | Intervention 56.5; Control 62.2 subgroups |
| Tilyard 1992 | N/A |
| Trivedi 2003 | N/A |
| Ushiroyama 2001 | N/A |
| Vital D | Intervention 53 (interquartile range 40‐65); Control 45 (interquartile range 40‐57) subgroups |
| WHI 2006 | 46.0 (SD 22.6) subsequent hip fracture, 48.4 (SD 23.5) Controls, subgroups |
| Witham 2010 | Intervention 20.5 (SD 8.9); Control 23.7 (SD 10.0) |
| Witham 2013 | Intervention 44(SD 16); Control 45(SD 15) |
* reported as D3 N/A: not available
Excluded studies
Sixty‐three studies were excluded (see Characteristics of excluded studies table for details). Most were excluded because the trials did not present fracture data.
Attention is drawn to one particular excluded study, which has been quoted as evidence for effectiveness of single‐dose vitamin D injection in fracture prevention (Heikinheimo 1992). This study was quasi‐randomised (allocation based on month of birth) and there was no attempt at blinding. Only individuals recruited in the autumn and winter were included, and there was no placebo group. Follow‐up varied between two and five years but the cumulative analysis of fracture incidence did not include confidence intervals despite the decreasing numbers with longer follow‐up. Participants who rejected injection were added to the control group. Although considered ineligible for inclusion in this systematic review, this study was important mainly for raising the hypothesis that this relatively inexpensive, practical proposal for fracture prevention should be tested more rigorously.
We also draw attention to an excluded trial that we included in a previous version of this review (Avenell 2005), with a note that its result should be treated with caution. Larsen 2004, a cluster‐randomised study (N = 4 clusters) was not included in our pooled analysis at that time, as the investigators' analysis appeared to be for individually randomised participants. We have now excluded this widely quoted study because it does not meet the inclusion criteria for this review. Participants in each of the three treatment clusters received one or more co‐interventions designed to reduce falls (medication review, environmental hazard and health assessment, and osteoporosis/fall prevention leaflets) but the control group received no intervention. No treatment group received vitamin D and calcium alone. Thus, although the investigators state that this was a factorial study, the reports of the design do not appear to fit that description, and the effect of vitamin D and calcium cannot be separated from the effects of co‐interventions.
Trials of included interventions that do not report fracture data, but do report adverse effects are listed in Table 6 and their details given in the 'Characteristics of excluded studies' table.
5. Selected adverse effects reported in excluded trials.
| Excluded study ID | Adverse effects |
| Aloia 2005 | Death, renal stone, hypercalcaemia |
| Binkley 2007 | Renal insufficiency, hypercalcaemia |
| Brazier 2005 | Death, gastrointestinal event, hypercalcaemia |
| Broe 2007 | Death |
| Chen 1997 | Gastrointestinal event, hypercalcaemia |
| Corless 1985 | Death, hypercalcaemia |
| Daly 2006 | Gastrointestinal event |
| Dawson‐Hughes 1991 | Renal insufficiency and renal stone, hypercalcaemia |
| Doetsch 2004 | Death |
| Grady 1991 | Death, renal insufficiency |
| Jensen 1982 | Hypercalcaemia |
| Johnson 1980 | Hypercalcaemia |
| Keane 1998 | Death |
| Larsen 2004 | Death |
| Latham 2003 | Death |
| Meier 2004 | Death |
| Moschonis 2006 | Gastrointestinal event |
| Ongphiphadhanakul 2000 | Hypercalcaemia |
| Orimo 2011 | Gastrointestinal event, hypercalcaemia |
Ongoing studies
We identified two ongoing studies, details of which are given in the 'Characteristics of ongoing studies' table.
Studies awaiting classification
A further 13 trials have met, or may meet, the inclusion criteria, but require further information or revision to the review protocol before their data can be included (see 'Characteristics of studies awaiting classification' table).
Risk of bias in included studies
The results of the assessment of the methodological quality of each included trial are in Figure 2, Figure 3 and Table 7.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
6. Quality assessment scores.
| Study | Item B | Item C | Item D | Item E | Item F | Item G | Item H | Item J |
| Aloia 1988 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 |
| Arthur 1990 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 2 |
| Avenell 2004 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 1 |
| Bischoff 2003 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 |
| Bolton‐Smith 2007 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Burleigh 2007 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Caniggia 1984 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 2 |
| Chapuy 1992 | 2 | 1 | 2 | 1 | 1 | 0 | 2 | 1 |
| Chapuy 2002 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 |
| Dawson‐Hughes 1997 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 1 |
| Dukas 2004 | 2 | 0 | 2 | 2 | 2 | 0 | 2 | 0 |
| Ebeling 2001 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 |
| Falch 1987 | 1 | 2 | 1 | 1 | 0 | 0 | 2 | 0 |
| Flicker 2005 | 1 | 2 | 1 | 2 | 2 | 0 | 2 | 2 |
| Gallagher 1989 | 1 | 2 | 1 | 2 | 2 | 0 | 2 | 2 |
| Gallagher 1990 | 1 | 2 | 1 | 2 | 2 | 0 | 2 | 2 |
| Gallagher 2001 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 1 |
| Garay Lillo 1997 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Geusens 1986 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Glendenning 2012 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 |
| Gorai 1999 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Harwood 2004 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 1 |
| Hayashi 1992 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 2 |
| Inkovaara 1983 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 0 |
| Ishida 2004 (retracted) | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 1 |
| Janssen 2010 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Komulainen 1998 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| Law 2006 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 2 |
| Lips 1996 | 2 | 1 | 2 | 2 | 2 | 0 | 2 | 1 |
| Lyons 2007 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 |
| Menczel 1994 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Meyer 2002 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 2 |
| Mitri 2011 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Nakatsuka 1997 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 2 |
| Ones 2007 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 2 |
| Orimo 1994 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 |
| OSTPRE‐FPS 2007 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 |
| Ott 1989 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 |
| Peacock 2000 | 0 | 1 | 2 | 2 | 1 | 1 | 2 | 2 |
| Pfeifer 2000 | 1 | 1 | 2 | 2 | 2 | 0 | 2 | 1 |
| Pfeifer 2009 | 2 | 1 | 2 | 2 | 1 | 0 | 2 | 1 |
| Porthouse 2005 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 2 |
| Prince 2008 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 |
| RECORD 2005 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 1 |
| Shiraki 1996 | 1 | 2 | 1 | 2 | 2 | 0 | 2 | 2 |
| Smith 2007 | 2 | 1 | 0 | 2 | 2 | 0 | 2 | 0 |
| Tilyard 1992 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 2 |
| Trivedi 2003 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Ushiroyama 2001 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| Vital D | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| WHI 2006 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 |
| Witham 2010 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 1 |
| Witham 2013 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
Allocation
Figure 2 and Figure 3 show the results for the random sequence generation and allocation concealment for the Cochrane 'Risk of bias' tool. For random sequence generation: 21 (40%) trials were deemed to be at low risk, 28 trials (53%) were at unclear risk and four trials were at high risk (8%). For allocation concealment: 22 trials were at low risk (42%), 29 trials were at unclear risk (55%) and two trials were at high risk (4%).
Blinding
Thirteen trials did not report any attempt to blind assessors to treatment assignment (25%) (item C). In 60% (32 trials) and 57% (30 trials) of trials respectively, the participants (item E) or providers (item F), or both, were blinded to treatment allocation.
Incomplete outcome data
Five trials did not provide information about the number of participants allocated to groups at randomisation (Caniggia 1984; Chapuy 2002; Dawson‐Hughes 1997; Garay Lillo 1997; Geusens 1986); one trial provided this information after we contacted the author (Flicker 2005). One large trial provided results but very sparse information about methods (Garay Lillo 1997). Six trials did not report details of withdrawals and exclusions after treatment assignment adequately (11%) (item B).
Other potential sources of bias
Although item H relates to applicability rather than bias, we note that the inclusion and exclusion criteria were clearly defined in 42 trials (79%). Only 23 trials (43%) collected outcome data on fractures as they occurred and confirmed them by interview and radiograph (item J). The intervention and control groups were demonstrably comparable in 31 trials (58%) (Item D). In the majority of trials (N = 35, 66%) the comparable nature of the care programmes, other than the trial interventions, was not reported (item G).
Effects of interventions
See: Table 1
See Table 2 for a list of vitamin D synonyms and abbreviations. Duration of intervention and follow‐up of individual trials are described in the 'Characteristics of included studies'. Due to the randomisation by cluster in Law 2006, the effective numbers of events and participants have been adjusted by the design effect for inclusion in the relevant meta‐analyses (seeAnalysis 1.1; Analysis 1.4; Analysis 13.2; Analysis 13.1). Therefore the numbers used in these meta‐analyses are lower than those reported in the trial. Throughout the text of the review, the number of participants analysed in each meta‐analysis is reported.
1.1. Analysis.

Comparison 1: Vitamin D [D2, D3 or 25(OH)D] versus control or placebo, Outcome 1: Persons sustaining new hip fracture
1.4. Analysis.

Comparison 1: Vitamin D [D2, D3 or 25(OH)D] versus control or placebo, Outcome 4: Persons sustaining any new fracture
13.2. Analysis.

Comparison 13: Vitamin D [D2, D3 or 25(OH)D] or any analogue with/without calcium: adverse effects, Outcome 2: Persons with hypercalcaemia
13.1. Analysis.

Comparison 13: Vitamin D [D2, D3 or 25(OH)D] or any analogue with/without calcium: adverse effects, Outcome 1: Deaths
Inkovaara 1983, a quasi‐randomised trial with four intervention groups, compared vitamin D, calcium and vitamin D, and calcium versus placebo. Data for fractures in this trial were reported, but as it was unclear whether the data represent fractures or participants with fractures, these data have not been included in the appropriate meta‐analyses. The authors commented that fractures were more common in the placebo group, but the difference was not statistically significant.
Results are presented for fractures for the different comparisons, followed by complications. Results are presented for hip fracture, non‐vertebral fracture, vertebral fracture and any new fracture.
In Porthouse 2005, more than half the participants had had a previous fracture, and so this trial is included in the subgroup analysis of trials where participants were selected on this basis. In Vital D, only a third of participants were selected because there was a previous history of fracture, so this trial is not included in this subgroup.
Vitamin D alone versus placebo or no treatment
Fifteen trials compared vitamin D alone versus placebo or no treatment (Avenell 2004; Glendenning 2012; Harwood 2004; Law 2006; Lips 1996; Lyons 2007; Meyer 2002; Mitri 2011; Peacock 2000: RECORD 2005; Smith 2007; Trivedi 2003; Vital D; Witham 2010; Witham 2013). Pooled data comparing vitamin D alone with placebo or no treatment showed no statistically significant effect on hip fracture (11 trials, 27,693 participants, RR 1.12, 95% CI 0.98 to 1.29, seeAnalysis 1.1), non‐vertebral fractures (12 trials, 22,930 participants, RR 1.05, 95% CI 0.96 to 1.14, seeAnalysis 1.2), vertebral fractures or deformities (six trials, 11,396 participants, RR 1.03, 95% CI 0.76 to 1.39, seeAnalysis 1.3). For any new fracture vitamin D alone produced no statistically significant reduction (15 trials, 28,271 participants, RR 1.03, 95% CI 0.96 to 1.11, seeAnalysis 1.4). There was evidence of very little heterogeneity (I2 less than 30%) for all these outcomes. Funnel plots for hip fracture trials (Figure 4) and any new fracture (Figure 5) do not suggest evidence of publication bias.
1.2. Analysis.

Comparison 1: Vitamin D [D2, D3 or 25(OH)D] versus control or placebo, Outcome 2: Persons sustaining new non‐vertebral fracture
1.3. Analysis.

Comparison 1: Vitamin D [D2, D3 or 25(OH)D] versus control or placebo, Outcome 3: Persons sustaining new vertebral fracture or deformity
4.

Funnel plot of comparison: 1 Vitamin D [D2, D3 or 25(OH)D] versus control or placebo, outcome: 1.1 Persons sustaining new hip fracture
5.

Funnel plot of comparison: 1 Vitamin D [D2, D3 or 25(OH)D] versus control or placebo, outcome: 1.4 Persons sustaining any new fracture
Vitamin D plus calcium versus calcium alone
Eleven trials compared vitamin D plus calcium with calcium alone (Avenell 2004; Bischoff 2003; Burleigh 2007; Flicker 2005; Garay Lillo 1997; Janssen 2010; Komulainen 1998; Pfeifer 2000; Pfeifer 2009; Prince 2008; RECORD 2005).
In the populations studied, vitamin D (including 25‐hydroxy vitamin D) plus calcium was no more effective than calcium alone for hip fracture (seven trials, 7411 participants, RR 0.84, 95% CI 0.63 to 1.13, seeAnalysis 2.1), any non‐vertebral fracture (six trials, 3336 participants, RR 0.96, 95% CI 0.79 to 1.16, seeAnalysis 2.2), and vertebral fracture (two trials, 2681 participants, RR 0.14, 95% CI 0.01 to 2.77, seeAnalysis 2.3). The three vertebral fractures from RECORD 2005 relate only to vertebral fractures presenting to clinical attention, rather than deformities detected by imaging. For any fracture there was no overall statistically significant effect (11 trials, 8812 participants, RR 0.87, 95% CI 0.74 to 1.02, seeAnalysis 2.4). There was some evidence that, for those participants not selected on the basis of a previous fracture, risk for any new fracture was reduced (nine trials, 6131 participants, RR 0.70, 95% CI 0.53 to 0.92) compared with evidence that those participants selected with a previous fracture were less likely to have a reduction in fractures (two trials, 2681 participants, RR 0.98, 95% CI 0.80 to 1.20); test for subgroup differences P value 0.05.
2.1. Analysis.

Comparison 2: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus calcium, Outcome 1: Persons sustaining new hip fracture
2.2. Analysis.

Comparison 2: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus calcium, Outcome 2: Persons sustaining new non‐vertebral fracture
2.3. Analysis.

Comparison 2: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus calcium, Outcome 3: Persons sustaining new vertebral fracture
2.4. Analysis.

Comparison 2: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus calcium, Outcome 4: Persons sustaining any new fracture
Vitamin D versus calcium
Four trials compared vitamin D versus calcium (Avenell 2004; Mitri 2011; Peacock 2000; RECORD 2005).
There was no evidence of a statistically significant difference between vitamin D alone and calcium in the prevention of hip fracture (two trials, 2718 participants, RR 0.90, 95% CI 0.61 to 1.32, seeAnalysis 3.1), non‐vertebral fractures (four trials, 3021 participants, RR 1.10, 95% CI 0.91 to 1.33, seeAnalysis 3.2) or any fracture (four trials, 3021 participants, RR 1.11, 95% CI 0.92 to 1.33, seeAnalysis 3.4). There was evidence that vitamin D alone was less effective than calcium for the prevention of vertebral fracture or deformity (three trials, 2976 participants, RR 2.21, 95% CI 1.08 to 4.53, seeAnalysis 3.3).
3.1. Analysis.

Comparison 3: Vitamin D [D2, D3 or 25(OH)D] versus calcium, Outcome 1: Persons sustaining new hip fracture
3.2. Analysis.

Comparison 3: Vitamin D [D2, D3 or 25(OH)D] versus calcium, Outcome 2: Persons sustaining new non‐vertebral fracture
3.4. Analysis.

Comparison 3: Vitamin D [D2, D3 or 25(OH)D] versus calcium, Outcome 4: Persons sustaining any new fracture
3.3. Analysis.

Comparison 3: Vitamin D [D2, D3 or 25(OH)D] versus calcium, Outcome 3: Persons sustaining new vertebral fracture or deformity
Vitamin D plus calcium versus placebo or no treatment
Hip fracture
Nine trials compared vitamin D plus calcium versus placebo or no treatment for hip fracture (Avenell 2004; Chapuy 1992; Chapuy 2002; Dawson‐Hughes 1997; Harwood 2004; OSTPRE‐FPS 2007; Porthouse 2005; RECORD 2005; WHI 2006).
Pooled data found a statistically significant reduction in the incidence of hip fracture in the population receiving vitamin D plus calcium (nine trials, 49,853 participants, RR 0.84, 95% CI 0.74 to 0.96, seeAnalysis 4.1). Heterogeneity was not evident (I2 = 0%). In studies where a previous osteoporotic fracture was not a selection criterion, the RR was 0.82, 95% CI 0.71 to 0.94 (five trials, 43,719 participants); and in participants with a history of prior fracture, the RR was 1.02, 95% CI 0.71 to 1.47 (four trials, 6134 participants). The test for subgroup differences was not significant (P value 0.26).
4.1. Analysis.

Comparison 4: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus control or placebo, Outcome 1: Persons sustaining new hip fracture
In the subgroup analysis by residential status (institution versus community: seeAnalysis 4.2) there was evidence that hip fracture incidence was reduced in institutional residents with a RR of 0.75, 95% CI 0.62 to 0.92 (two trials, 3853 participants); it was also reduced in the community dwelling group but this was a non‐significant finding (RR of 0.91, 95% CI 0.77 to 1.09, seven trials, 46,000 participants; seeAnalysis 4.2). The test for subgroup differences was not significant (P value 0.15).
4.2. Analysis.

Comparison 4: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus control or placebo, Outcome 2: Persons sustaining new hip fracture: subgroup analysis by residential status (institution vs community)
Non‐vertebral fracture
Eight trials compared vitamin D plus calcium versus placebo or no treatment for non‐vertebral fracture (Avenell 2004; Bolton‐Smith 2007; Chapuy 1992; Chapuy 2002; Dawson‐Hughes 1997; Harwood 2004; OSTPRE‐FPS 2007; RECORD 2005). Overall, administration of vitamin D and calcium was associated with a statistically significant reduction in incidence of new non‐vertebral fracture (eight trials, 10,380 participants, RR 0.86, 95% CI 0.78 to 0.96, seeAnalysis 4.3).
4.3. Analysis.

Comparison 4: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus control or placebo, Outcome 3: Persons sustaining new non‐vertebral fracture
Vertebral fracture
Four trials compared vitamin D plus calcium versus placebo or no treatment for vertebral fracture (Avenell 2004; OSTPRE‐FPS 2007; RECORD 2005; WHI 2006). There was no evidence of a statistically significant preventive effect on clinical vertebral fractures from the administration of vitamin D and calcium (four trials, 42,185 participants, RR 0.89, 95% CI 0.74 to 1.09, seeAnalysis 4.4).
4.4. Analysis.

Comparison 4: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus control or placebo, Outcome 4: Persons sustaining new vertebral fracture
The Women's Health Initiative has recently reported (WHI 2006) ‐ in abstract form ‐ fracture outcomes after seven years of the trial and a further five years of follow‐up (Cauley 2012). For the overall period of follow‐up, no significant differences between treatment and placebo groups were found for hip fractures or total fractures, but clinical vertebral fractures were 13% lower in women randomised to calcium and vitamin D (reported hazard ratio 0.87, 95% CI 0.76 to 0.98).
Any fracture
Ten trials compared vitamin D plus calcium versus placebo or no treatment for any fracture (Avenell 2004; Bolton‐Smith 2007; Chapuy 1992; Chapuy 2002; Dawson‐Hughes 1997; Harwood 2004; OSTPRE‐FPS 2007; Porthouse 2005; RECORD 2005; WHI 2006).
There was evidence of a statistically significant reduction in the incidence of any fracture (10 trials, 49,976 participants, RR 0.95, 95% CI 0.90 to 0.99, seeAnalysis 4.5). In participants selected on the basis of no previous fracture, the RR was 0.95, 95% CI 0.90 to 1.00 (six trials, 43,842 participants); and in those with a previous history of fracture, the RR was 0.93, 95% CI 0.79 to 1.10 (four trials, 6134 participants). The test for subgroup differences was not significant (P value 0.84).
4.5. Analysis.

Comparison 4: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus control or placebo, Outcome 5: Persons sustaining any new fracture
There was evidence for a reduction in any fracture for residents in institutions (RR 0.85, 95% CI 0.74 to 0.98, two trials, 3853 participants; seeAnalysis 4.6), and a non‐significant effect for people living in the community (RR 0.96, 95% CI 0.91 to 1.01, eight trials, 46,123 participants, seeAnalysis 4.6). However, the test for subgroup differences was not significant (P value 0.13).
4.6. Analysis.

Comparison 4: Vitamin D [D2, D3 or 25(OH)D] plus calcium versus control or placebo, Outcome 6: Persons sustaining any new fracture: subgroup analysis by residential status (institution vs community)
Alfacalcidol (1‐alphahydroxyvitamin D3) versus placebo or no treatment
Non‐vertebral fracture
Three trials compared alfacalcidol (1‐alphahydroxyvitamin D3) versus placebo or no treatment for non‐vertebral fracture (Dukas 2004; Gorai 1999; Ushiroyama 2001). There was no statistically significant reduction in non‐vertebral fractures in people with and without pre‐existing osteoporotic fracture (three trials, 526 participants, RR 0.97, 95% CI 0.25 to 3.82, seeAnalysis 5.1).
5.1. Analysis.

Comparison 5: Alfacalcidol [1‐alpha(OH)D3] versus control or placebo, Outcome 1: Persons sustaining new non‐vertebral fracture
Vertebral fracture
One trial compared alfacalcidol (1‐alphahydroxyvitamin D3) versus placebo or no treatment for vertebral fracture (Hayashi 1992). There was a statistically significant reduction in vertebral fractures (one trial, 740 participants, RR 0.56, 95% CI 0.48 to 0.65, seeAnalysis 5.2).
5.2. Analysis.

Comparison 5: Alfacalcidol [1‐alpha(OH)D3] versus control or placebo, Outcome 2: Persons sustaining new vertebral fracture
Alfacalcidol (1‐alphahydroxyvitamin D3) plus calcium versus calcium
Five trials compared alfacalcidol (1‐alphahydroxyvitamin D3) plus calcium versus calcium (Menczel 1994; Nakatsuka 1997; Ones 2007; Orimo 1994; Shiraki 1996).
There was no statistically significant reduction in hip fractures (one trial, 113 participants, RR 0.20, 95% CI 0.01 to 4.00, seeAnalysis 6.1), but there was a statistically significant effect on the development of new vertebral deformity (five trials, 390 participants, RR 0.44, 95% CI 0.21 to 0.96, seeAnalysis 6.2).
6.1. Analysis.

Comparison 6: Alfacalcidol [1‐alpha(OH)D3] plus calcium versus calcium, Outcome 1: Persons sustaining new hip fracture
6.2. Analysis.

Comparison 6: Alfacalcidol [1‐alpha(OH)D3] plus calcium versus calcium, Outcome 2: Persons sustaining new vertebral deformity
Alfacalcidol (1‐alphahydroxyvitamin D3) versus calcium
One trial in participants with osteoporosis found no statistically significant effect of alfacalcidol (1‐alphahydroxyvitamin D3) compared with calcium on people with new vertebral deformities (one trial, 23 participants, RR 0.95, 95% CI 0.52 to 1.74, seeAnalysis 7.1) (Geusens 1986).
7.1. Analysis.

Comparison 7: Alfacalcidol [1‐alpha(OH)D3] versus calcium, Outcome 1: Persons sustaining new vertebral deformity
Calcitriol (1,25‐dihydroxyvitamin D3) versus placebo or no treatment
Three trials compared calcitriol (1,25‐dihydroxyvitamin D3) versus placebo or no treatment (Caniggia 1984; Gallagher 1989; Gallagher 2001).
Calcitriol had no statistically significant effect on hip fracture (one trial, 246 participants RR 0.33, 95% CI 0.01 to 8.10, seeAnalysis 8.1), non‐vertebral fracture (one trial, 246 participants, RR 0.42, 95% CI 0.15 to 1.15, seeAnalysis 8.2), or new vertebral deformity (three trials, 327 participants RR 0.75, 95% CI 0.40 to 1.41, seeAnalysis 8.3).
8.1. Analysis.

Comparison 8: Calcitriol [1,25(OH)2D3] versus control or placebo, Outcome 1: Persons sustaining new hip fracture
8.2. Analysis.

Comparison 8: Calcitriol [1,25(OH)2D3] versus control or placebo, Outcome 2: Persons sustaining new non‐vertebral fracture
8.3. Analysis.

Comparison 8: Calcitriol [1,25(OH)2D3] versus control or placebo, Outcome 3: Persons sustaining new vertebral deformity
Calcitriol (1,25‐dihydroxyvitamin D3) plus calcium versus calcium
One trial compared calcitriol (1,25‐dihydroxyvitamin D3) plus calcium versus calcium (Ott 1989). Additional supplementation with calcitriol in people with osteoporosis already taking calcium showed no statistically significant effect on the incidence of new non‐vertebral fracture (one trial, 86 participants, RR 2.50, 95% CI 0.51 to 12.19, seeAnalysis 9.1), or new vertebral deformity (one trial, 86 participants, RR 1.50, 95% CI 0.58 to 3.85, seeAnalysis 9.2).
9.1. Analysis.

Comparison 9: Calcitriol [1,25(OH)2D3] plus calcium versus calcium, Outcome 1: Persons sustaining new non‐vertebral fracture
9.2. Analysis.

Comparison 9: Calcitriol [1,25(OH)2D3] plus calcium versus calcium, Outcome 2: Persons sustaining new vertebral deformity
Calcitriol (1,25‐dihydroxyvitamin D3) plus vitamin D plus calcium versus vitamin D plus calcium
Two studies that compared calcitriol (1,25‐dihydroxyvitamin D3) plus vitamin D and calcium versus vitamin D plus calcium found no statistically significant effect on the number of people developing new vertebral deformities (two trials, 84 participants, RR 0.79, 95% CI 0.41 to 1.52, seeAnalysis 10.1) (Aloia 1988; Gallagher 1990).
10.1. Analysis.

Comparison 10: Calcitriol [1,25(OH)2D3] plus vitamin D plus calcium versus vitamin D plus calcium, Outcome 1: Persons sustaining new vertebral deformity
Calcitriol (1,25‐dihydroxyvitamin D3) versus calcium
Two trials compared calcitriol (1,25‐dihydroxyvitamin D3) versus calcium (Ebeling 2001; Tilyard 1992).
Overall, there was no statistically significant effect on the incidence of non‐vertebral fractures (two trials, 663 participants, random‐effects RR 1.23, 95% CI 0.10 to 14.78, seeAnalysis 11.1), or vertebral deformities (two trials, 556 participants, random‐effects RR 1.69, 95% CI 0.25 to 11.28, seeAnalysis 11.2). There was, however, considerable heterogeneity for both analyses (I2 values of 67% and 70%, respectively).
11.1. Analysis.

Comparison 11: Calcitriol [1,25(OH)2D3] versus calcium, Outcome 1: Persons sustaining new non‐vertebral fracture
11.2. Analysis.

Comparison 11: Calcitriol [1,25(OH)2D3] versus calcium, Outcome 2: Persons sustaining new vertebral deformity
In Tilyard 1992, the duration of treatment was critical (seeAnalysis 11.3). At the end of one year, no effect could be shown. Fewer vertebral deformities occurred in the calcitriol group during the second year (RR 0.47, 95% CI 0.26 to 0.87), and during the third year (RR 0.28, 95% CI 0.15 to 0.52).
11.3. Analysis.

Comparison 11: Calcitriol [1,25(OH)2D3] versus calcium, Outcome 3: Persons sustaining new vertebral deformity in Tilyard study
Calcitriol (1,25‐dihydroxyvitamin D3) versus vitamin D
Two trials compared calcitriol (1,25‐dihydroxyvitamin D3) versus vitamin D (Arthur 1990; Falch 1987).
When calcitriol was compared with vitamin D in people with pre‐existing osteoporosis no statistically significant effect was seen for non‐vertebral fractures (one trial, 86 participants, RR 1.16, 95% CI 0.40 to 3.37, seeAnalysis 12.1), or vertebral deformities (two trials, 96 participants RR 1.38, 95% CI, 0.55 to 3.47, seeAnalysis 12.2).
12.1. Analysis.

Comparison 12: Calcitriol [1,25(OH)2D3] versus vitamin D (with or without calcium in each group), Outcome 1: Persons sustaining new non‐vertebral fracture
12.2. Analysis.

Comparison 12: Calcitriol [1,25(OH)2D3] versus vitamin D (with or without calcium in each group), Outcome 2: Persons sustaining new vertebral deformity
Reported adverse effects: vitamin D (D2, D3 or 25(OH)D) or any analogue with/without calcium
Death
The risk of death during the studies appeared marginally lower in participants given vitamin D or its analogues with or without calcium than in those given placebo or calcium (29 trials, 71,032 participants, RR 0.97, 95% CI 0.93 to 1.01, Analysis 13.1).
Hypercalcaemia
Only twenty‐one trials reported on the presence or absence of hypercalcaemia, which was usually mild (2.6 to 2.8 mmol/L) (Aloia 1988; Avenell 2004; Bischoff 2003; Burleigh 2007; Chapuy 2002; Dukas 2004; Gallagher 2001; Gorai 1999; Harwood 2004; Law 2006; Menczel 1994; Orimo 1994; Ott 1989; Peacock 2000; Prince 2008; RECORD 2005; Tilyard 1992; Ushiroyama 2001; Vital D; Witham 2010; Witham 2013). Hypercalcaemia was reported more commonly when vitamin D or its analogues were given compared with placebo or calcium (21 trials, 17,124 participants, RR 2.28, 95% CI 1.57 to 3.31, Analysis 13.2). The risk of hypercalcaemia was particularly high for the use of calcitriol (four trials, 988 participants, RR 4.41, 95% CI 2.14 to 9.09, Analysis 13.2).
Gastrointestinal symptoms
Fifteen trials reported on gastrointestinal symptoms (Avenell 2004; Bischoff 2003; Burleigh 2007; Chapuy 1992; Chapuy 2002; Dawson‐Hughes 1997; Ebeling 2001; Gallagher 2001; Janssen 2010; Ones 2007; Prince 2008; RECORD 2005; Tilyard 1992, WHI 2006; Witham 2010). There was evidence of a small increase in gastrointestinal symptoms with interventions containing vitamin D (15 trials, 47,761 participants, RR 1.04, 95% CI 1.00 to 1.08, seeAnalysis 13.3). The risk of gastrointestinal effects was higher for the use of vitamin D and calcium (four trials, 40,524 participants, RR 1.05, 95% CI 1.01 to 1.09).
13.3. Analysis.

Comparison 13: Vitamin D [D2, D3 or 25(OH)D] or any analogue with/without calcium: adverse effects, Outcome 3: Persons with gastrointestinal effects
Occurrence of renal calculi or renal insufficiency
Eleven trials reported on the occurrence of renal calculi or renal insufficiency (Aloia 1988; Avenell 2004; Chapuy 2002; Gallagher 1990; Gallagher 2001; Menczel 1994; Peacock 2000; RECORD 2005; Tilyard 1992; WHI 2006; Witham 2013).
There was evidence of a statistically significant increase in the incidence of renal calculi or renal insufficiency (11 trials, 46,548 participants, RR 1.16, 95% CI 1.02 to 1.33, seeAnalysis 13.4). This was mainly due to the influence of WHI 2006through possibly more intensive follow‐up (WHI 2006 reported 10 times more renal adverse events than the large RECORD 2005 trial). If WHI 2006 is removed from the analysis the difference for this outcome is no longer statistically significant (10 trials, 10,266 participants, RR 0.91, 95% CI 0.44 to 1.86).
13.4. Analysis.

Comparison 13: Vitamin D [D2, D3 or 25(OH)D] or any analogue with/without calcium: adverse effects, Outcome 4: Persons with renal disease (calculi or insufficiency)
A separate Cochrane review has reported adverse effects from trials of vitamin D supplementation (Bjelakovic 2014), where trials were not limited to those for fracture prevention. Adverse effects reported include all cause mortality, cardiovascular disorders and mortality, hypercalcaemia, renal calculi, renal insufficiency and gastrointestinal disorders.
Table 6 lists adverse effects reported in trials of interventions that met the inclusion criteria but were excluded because they did not report fracture data.
Discussion
Summary of main results
Vitamin D alone versus placebo or no treatment
Vitamin D alone, in the formats and doses tested, appears unlikely to be effective in preventing hip fracture (11 trials, 27,693 participants, RR 1.12, 95% CI 0.98 to 1.29). Vitamin D alone, in the formats and doses tested, also appears unlikely to reduce any new fracture (15 trials, 28,271 participants, RR 1.03, 95% CI 0.96 to 1.11). The high quality of this evidence is reinforced by the visual impression of an absence of publication bias shown for both outcomes.
Vitamin D was administered in a variety of formats and doses (oral daily, oral intermittent, intra‐muscular). However, none of the trials in this review tested daily vitamin D3 in doses greater than 800 IU. It has been suggested that higher doses of vitamin D are required, such as 1800 IU/day to 4000 IU/day (Bischoff‐Ferrari 2010), in order to raise 25(OH)D to at least 75 to 110 nmol/L; although the US Institute of Medicine's report on calcium and vitamin D concluded that a 25(OH)D of 50 nmol/L (equivalent to 20 ng/ml) should be adequate for 97.5% of the population (Institute of Medicine 2010). Gallagher and colleagues evaluated doses of 400 IU to 4800 IU cholecalciferol taken daily for one year (Gallagher 2012), and concluded that 800 IU daily increased 25(OH)D to over 50 nmol/L in 97.5% of women (and 1600 IU/d to over 75 nmol/L in 97.5% of women), where baseline 25(OH)D was 37 nmol/L.
Intermittent annual high oral dosing (Vital D) or intra‐muscular injections (Smith 2007) were not effective. In the case of Vital D, one oral dose of 500,000 IU Vitamin D3 appeared to increase the risk of falls, particularly in the three months after dosing, which might have been related to the very high dose leading to lower 1,25‐dihydroxyvitamin D (the active form of the vitamin) (Dawson‐Hughes 2010), possibly accentuated by a lack of regular daily vitamin D replacement (Mak 2010). Neither of these trials appeared to recruit participants with very low baseline 25(OH)D levels (mean or medians of 45 nmol/L to 62 nmol/L) implying that participants may have had transient supratherapeutic levels. However, when these two trials (Smith 2007; Vital D), and the trial by Law 2006 (for which the effects of clustering might not have been adequately controlled in our meta‐analyses) are removed from the results, the conclusions of the meta‐analyses are not changed.
A variety of new vitamin D oral dosing strategies are being tested for their effects on baseline 25(OH)D, namely: loading, daily, weekly, monthly and less frequent dosing strategies, and comparison of ergocalciferol and cholecalciferol (Bacon 2009; Binkley 2011; Gallagher 2012; Ish‐Shalom 2008; Pekkarinen 2010). These newer regimens have not been tested in large trials of fracture prevention, where longer‐term compliance might be improved by intermittent dosing.
Vitamin D plus calcium versus calcium alone
Although there was no firm evidence that vitamin D (including 25‐hydroxy vitamin D) with calcium was any more effective than calcium alone for preventing hip fracture, any non‐vertebral fracture or vertebral fracture, there was some evidence that, for those participants not selected on the basis of a previous fracture, risk for any new fracture was reduced (nine trials, 6131 participants; RR 0.70, 95% CI 0.53 to 0.92) compared with those participants selected with a previous fracture (two trials, 2681 participants, RR 0.98, 95% CI 0.80 to 1.20). Trials generally used a dose of cholecalciferol of 400 IU to 1000 IU given daily. The biological rationale for the subgroup differences is unclear, as is the practical application of this finding, since the decision to add vitamin D to existing calcium supplementation rarely arises. This finding also appears to contradict the results for vitamin D alone, and might suggest that vitamin D and calcium interact in the prevention of fractures.
Vitamin D versus calcium
Few trials examined the comparison of vitamin D versus calcium, and the results are dominated by those from the RECORD 2005 trial. Although calcium appeared to be more effective than vitamin D in the prevention of vertebral fractures or deformity, this result should be interpreted with caution as reflected in the wide confidence intervals (RR 2.21, 95% CI 1.08 to 4.53), where results are dominated by Peacock 2000 with only 258 participants. RECORD 2005 only assessed vertebral fractures that came to clinical attention (seven events in 2654 participants).
Vitamin D plus calcium versus placebo or no treatment
There was high quality evidence that vitamin D and calcium resulted in a small reduction in hip fracture risk (nine trials, 49,853 participants; RR 0.(RR 0.84; 95% CI 0.74 to 0.96) (RR 0.84; 95% CI 0.74 to 0.96) 84, 95% CI 0.74 to 0.96) (seeTable 1). As an illustration, in low‐risk populations (i.e. residents in the community: with an estimated eight hip fractures per 1000 per year), this equates to one fewer hip fracture per 1000 older adults per year (95% CI 0 to 2). In high risk populations (i.e. residents in institutions: with an estimated 54 hip fractures per 1000 per year), this equates to nine fewer hip fractures per 1000 older adults per year (95% CI 2 to 14).
There was high quality evidence that vitamin D and calcium was associated with a statistically significant reduction in incidence of new non‐vertebral fractures. However, there was only moderate quality evidence of an absence of a statistically significant preventive effect on clinical vertebral fractures.
There was high quality evidence that vitamin D and calcium resulted in a small reduction in any new fracture risk (10 trials, 49,976 participants; RR 0.95, 95% CI 0.90 to 0.99) (seeTable 1). As an illustration, in low‐risk populations (i.e. residents in the community: with an estimated 26 fractures per 1000 per year), this equates to one fewer fracture per 1000 older adults per year (95% CI 0 to 3). In high risk populations (i.e. residents in institutions: with an estimated 75 fractures per 1000 per year), this equates to four fewer hip fractures per 1000 older adults per year (95% CI 1 to 7).
Most trials used 400 IU to 800 IU vitamin D3 daily with co‐administration of 1000 mg calcium. Not all trials provided vertebral fracture data, and no statistically significant effect on vertebral fracture was found (four trials, 42,185 participants, RR 0.89, 95% CI 0.74 to 1.09). The very large WHI 2006 trial used a lower dose of 400 IU vitamin D and 1000 mg calcium and showed a trend only for hip fracture reduction. However, 44% of women in WHI 2006 were taking calcium and vitamin D at baseline and 3% were taking vitamin D. Analysis of women with and without the use of personal supplements of calcium and vitamin D at baseline did not suggest that this influenced the outcome for hip, or any fracture (Bolland 2011b). Using data from women who were not taking such supplements in our analyses, instead of the entire WHI 2006 trial population, does not affect the conclusions of the meta‐analyses (data not shown). In a post hoc sensitivity analysis, removal of WHI 2006 data for the entire trial population did not affect the conclusions of any of the fracture outcome analyses, including those of the subgroups, discussed below. We conducted two subgroup analyses. The first separated the data by the residential status of the participants at recruitment. We defined institutional as residence in a nursing home or residential care home. We recognise that differences exist in nomenclature between different countries (for example nursing homes in Australia can mean high‐level residential care homes or residential care homes in general) but found the descriptions provided by trialists sufficient to make a judgement in most cases. Studies with a mixed population were categorised as 'institutional' or 'community' based on the dominant place of residence of participants. For example, we classified RECORD 2005 as a community study, as 94% of participants were resident in their own homes and only 6% were resident in institutions. This subgroup analysis was not pre‐defined in the original review in 1995, but emerged for an earlier update from the accumulation of evidence, from clinical biochemistry and epidemiology, that many frail, institutionalised older people are vitamin D deficient, and likely to have lower 25(OH)vitamin D status. We hypothesised that they, in particular, might benefit from the administration of vitamin D and calcium. This analysis is particularly influenced by the two trials conducted amongst frail people living in nursing homes or apartments for older people in France (Chapuy 1992; Chapuy 2002). Tests for subgroup differences found no statistically significant differences between community or institutional residence populations for hip or any new fracture.
Considerable epidemiologic evidence supports the association between prior osteoporotic fracture and subsequent hip fracture; RECORD 2005 therefore recruited participants with a prior fracture history. We found no evidence from our subgroup analyses (which was heavily dominated by RECORD 2005), that a population with such a history, irrespective of age, benefits in respect of fracture incidence from receiving vitamin D and calcium. Although the dose of vitamin D3 used in RECORD 2005 was the same as was used in Chapuy 1992 and Chapuy 2002, poorer compliance may have reduced the effect in RECORD 2005. Compliance appears to have been very good in both Chapuy 2002 and Chapuy 1992 (see reference in Bischoff‐Ferrari 2012). Ways to improve compliance with bone‐active medication of all forms in this population needs to be researched (Seeman 2007). Pooled data from studies where a previous osteoporotic fracture was not a selection criterion did show a statistically significant reduction in hip fracture or any type of fracture, but tests for subgroup differences between this group and those with a previous fracture did not show statistical significance.
Alfacalcidol
One trial of alfacalcidol suggested that vertebral fractures may be prevented by taking alfacalcidol (one trial, 740 participants, RR 0.56, 95% CI 0.48 to 0.65), although the evidence did not suggest prevention of non‐vertebral fractures. The data for vertebral fractures are from Hayashi 1992, which was quasi‐randomised and thus susceptible to bias. Other small studies, which compared alfacalcidol and calcium with calcium alone, also suggested protection against vertebral fractures.
Calcitriol
The effect of calcitriol in fracture prevention is unclear, with the best evidence for effectiveness coming from the trial of Tilyard 1992 that compared calcitriol with calcium and where vertebral deformities were significantly reduced only in the second and third years (seeAnalysis 11.3). However, the use of calcitriol is associated with a statistically significant increase in risk of hypercalcaemia (four trials, 988 participants, RR 4.41, 95% CI 2.14 to 9.09).
Adverse effects
Not all trials reported adverse effects. Overall, there was no significant effect of vitamin D on mortality, or from groups of supplement types such as vitamin D and calcium (see also Table 1), in the trials examined here, which only encompassed fracture prevention (29 trials, 71,032 participants, RR 0.97, 95% CI 0.93 to 1.01, Analysis 13.1). In the previous version of the review (Avenell 2009a), vitamin D and calcium were associated with a reduction in mortality (RR 0.94, 95% CI 0.89 to 0.99). This partly relates to reclassification of some of the trials in the subgroups in this analysis due to the use of the factorial design, as well as the inclusion of one new trial (OSTPRE‐FPS 2007). A Cochrane review examined all forms of vitamin D supplementation including with calcium (as in our overall meta‐analysis of mortality) in 56 trials and found a similar RR to our study with narrower and statistically significant confidence intervals: RR 0.97, 95% CI 0.94 to 0.99 (Bjelakovic 2014).
Calcium supplements could increase the risk of myocardial infarction (Bolland 2010), particularly in people with a higher dietary calcium intake, suggesting over‐therapeutic usage of supplements. Supplements of calcium and vitamin D could also increase the risk of myocardial infarction and stroke (Bolland 2011a), but might also decrease the risk of total and breast cancer (Bolland 2011b). However, this review found no significant increase in mortality in trials of vitamin D and calcium.
Gastrointestinal effects and renal disease were particularly likely to be not reported. In the past, there has been serious concern that cholecalciferol or ergocalciferol may be associated with hypercalcaemia when given in only moderate doses, which may have led to cautious use of low doses in the trials of vitamin D. There is some evidence that potential toxicity in this respect has been seriously overestimated (Gallagher 2012), and that requirements for vitamin D3 might be more than previously recognised (Vieth 2001). However, hypercalcaemia was found more often in participants taking vitamin D, but this mainly related to the use of calcitriol. Gastrointestinal effects and renal disease (especially calculi) were more common amongst participants receiving vitamin D, and in these cases calcium and vitamin D appeared to be most associated with increased risk (see also Table 1). These analyses were dominated by WHI 2006 in which calcium supplements were also given (with the associated use of off‐study calcium and vitamin D), but there was no significant difference between the subgroups with and without calcium supplementation in our meta‐analysis. Our results for hypercalcaemia, gastrointestinal effects and renal disease were similar to those from the Cochrane review by Bjelakovic 2014, although the latter review also included trials of vitamin D whose aim was not fracture prevention.
Overall completeness and applicability of evidence
This review included a large evidence base from 31 trials that examined vitamin D (including 25‐hydroxy vitamin D) with or without calcium in the prevention of fractures, mostly set in the community, nursing home or hospital inpatient population, and generally with older participants than in the second group of trials described below. Twelve trials had participants with a mean or median age of 80 years or over. Only three trials recruited participants because they had all had a previous fracture (Avenell 2004; Harwood 2004; RECORD 2005).
The second group of 21 smaller trials of calcitriol or alfacalcidol ‐ almost all of which recruited from referral populations with established osteoporosis ‐ were carried out in the setting of institutional referral clinics or hospitals. In the majority of cases, osteoporosis had been formally diagnosed, and often the presence of one or more deformed vertebrae on an initial radiograph was required for inclusion in the trial. None of this group of trials had participants with a mean or median age of 80 years or more.
It is unclear whether the effectiveness of vitamin D and calcium found in the nursing home trials in France by Chapuy 1992 and Chapuy 2002 is transferable to other institutional populations in other countries. Further trials in similar settings in other countries would be valuable, although the adoption of use of vitamin D and calcium in these settings, based on these two studies, might raise ethical issues and make further placebo‐controlled trials difficult to carry out. There is also suggestion that the focus be shifted from high‐dose supplementary calcium to dietary calcium in the calcium/vitamin D supplementation regime, given the evidence provided by Bolland 2010 and Bolland 2011a of increased cardiovascular disease with oral calcium supplementation, with or without co‐supplementation with vitamin D, especially in those who are calcium‐replete in their diet.
Table 5 gives the baseline 25(OH)D levels in the intervention and control groups of the included studies. The values have to be interpreted with caution, since they depend on the laboratory and method used (Lips 1999). It might be expected that those people with the lowest 25(OH)D levels would benefit most from supplementation, and there is some suggestion of this in Chapuy 1992 (25(OH)D of 40 nmol/L) and Chapuy 2002 (22 nmol/L). Lips 1996 (27 nmol/L) also had low values, but had a lower dose of supplementation of 400 IU vitamin D3 daily. The results of the RECORD 2005 trial are somewhat contradictory, given the 25(OH)D3 level of 38 nmol/L. OSTPRE‐FPS 2007 and Vital D had 25(OH)D levels in intervention groups of 50 nmol/L and 53 nmol/L, respectively. The statistically significant treatment effect for all fractures from the trial of Dawson‐Hughes 1997 is also unusual given the high average baseline value of 77 nmol/L.
Despite the ability of injection of vitamin D to reduce the winter decline in serum vitamin D concentrations (Khaw 1994), and the apparently positive findings of Trivedi 2003, there is robust evidence that the administration of vitamin D alone, whether by annual injection, periodic bolus oral dosage, or daily oral dosage, is unlikely to be effective for fracture prevention in the doses used here.
Quality of the evidence
This review included a large body of evidence from 53 trials with 91,791 participants. Our assessments of risk of bias and quality assessment are presented in Figure 2, Figure 3 and Table 7. There were only four trials with high risk of bias for random sequence generation and two trials with high risk for allocation concealment, and these had no influence on the findings for vitamin D or vitamin D and calcium trials. The evidence base from trials examining vitamin D alone versus placebo or no treatment, and vitamin D and calcium together versus placebo or no treatment was of high (“Further research is very unlikely to change our confidence in the estimate of effect”) or moderate quality (“Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.”).
Potential biases in the review process
We believe that selection bias is unlikely in this review. We have searched a wide range of databases and handsearched numerous relevant journals. We note, though, that we have identified 12 reports of studies that may, if further information becomes available, be eligible for inclusion. The contact author for the review is in touch with research groups in this field. Action was taken to minimise bias in the selection of studies for inclusion, and during the process of quality assessment and data extraction, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Authors who had participated in included trials (AA and WJG, a previous author) were not involved in the quality assessment or data extraction relating to those studies.
However the reporting of adverse effects (Analyses 14.1 to 14.4) includes only RCTs in which vitamin D or vitamin D analogues had fracture data. Our search strategy was not designed to identify studies in which vitamin D was administered in studies with no fracture data. Nor would it have identified other study types that might have provided useful data on adverse effects. So, the data used in these analyses are incomplete, although there is no reason to suspect that they are not representative.
Ascertainment bias cannot be completely ruled out. Incomplete information was available to us on the number of drop outs from intervention and control groups in a number of trials. Thus, it is possible that our analyses, based on the principles of intention‐to‐treat, might have under‐estimated the number of outcome events in the intervention or control groups, or both.
Agreements and disagreements with other studies or reviews
There have been a large number of recent trial level and individual patient data meta‐analyses of vitamin D, with or without calcium, for the prevention of fracture. The meta‐analyses (like those here) are limited by the small number of trials and limited combinations of treatments and doses, although this Cochrane review includes more trials than any of the previous systematic reviews. Most reported that calcium was required in addition to vitamin D for effectiveness (Bergman 2010; Chung 2011; DIPART 2010; Lai 2010; Murad 2012). However, Bischoff‐Ferrari and colleagues (Bischoff‐Ferrari 2009b; Bischoff‐Ferrari 2012), in an attempt to correct for compliance and off‐study use of supplements, reported that calcium did not improve the anti‐fracture efficacy of vitamin D. Bischoff‐Ferrari 2012 has been criticised for the methods used, including compromising the benefits of randomisation in the trials analysed (Abrahamsen 2013).
Tang 2007 and colleagues undertook a systematic review of calcium, with and without vitamin D, in the prevention of fractures. The addition of vitamin D to calcium did not significantly change the effect of calcium alone. However, no meta‐analysis of trials with treatment arms of vitamin D and calcium compared with calcium was undertaken, as in our comparison (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4), and we have included several newer trials.
Fractures may result from falls, so we would draw readers' attention to two recent Cochrane reviews on prevention of falls in the community (Gillespie 2012), and in institutions (Cameron 2012), which include many of the trials here. In the community, vitamin D with or without calcium may reduce the rate or risk of falls in people if they have lower 25(OH)D status (25 nmol/L to 55 nmol/L in the trials). In residents in institutions, vitamin D alone appeared to reduce the rate of falls, with two of the trials comparing vitamin D and calcium to calcium alone. Other systematic reviews have examined the effect of vitamin D in preventing falls (Bischoff‐Ferrari 2009a; Kalyani 2010; Murad 2011; O'Donnell 2008), with some finding calcium was required too (Kalyani 2010; Murad 2011), but this was not always clear (Bischoff‐Ferrari 2009a). A systematic review by O'Donnell 2008 and colleagues reported that alfacalcidol or calcitriol could reduce the risk of experiencing one or more falls.
In an individual patient data and trial‐level meta‐analysis of vitamin D with or without calcium, while vitamin D plus calcium was associated with a reduction in mortality, vitamin D alone was not (Rejnmark 2012). An earlier systematic review of randomised trials found that vitamin D was associated with a reduced risk of mortality, which did not appear to relate to additional calcium provision (Autier 2007). In the Cochrane review of vitamin D supplementation and mortality, cholecalciferol and calcium were associated with a reduction in mortality (RR 0.96, 95% CI 0.92 to 0.99), but cholecalciferol alone was not (RR 0.92, 95% CI 0.85 to 1.00) (Bjelakovic 2014).
Authors' conclusions
Implications for practice.
Vitamin D plus calcium can help prevent hip fracture or any type of fracture. The benefits need to be balanced against the risk of kidney stones, kidney disease, gastrointestinal disease or heart disease (Bolland 2010; Bolland 2011a). Vitamin D and calcium together are not associated with an increased risk of dying.
Vitamin D alone, in the doses and formulations that have been used, appears unlikely to be effective in fracture prevention in older people.
Alphacalcidol may protect against vertebral fractures.
Calcitriol appears to be associated with an increased incidence of adverse effects such as hypercalcaemia.
Implications for research.
Newer vitamin D dosing strategies to achieve better long‐term compliance and at least a 25(OH)D level of 75 nmol/L, should be tested in institutional settings for fracture prevention. As the generalisability of the trials from French nursing homes is still unclear, and calcium and vitamin D have more adverse effects and require daily dosing, a third calcium and vitamin D arm could be added to a trial of vitamin D compared to placebo or control intervention.
The design and reporting of any future trials should conform to the CONSORT statement (Schulz 2010), or any future development of it. Trials using cluster randomisation should perform appropriate analyses and include sufficient information in trial reports to aid interpretation by readers and users of such trials (Campbell 2012).
Feedback
Comment sent 19 August 1999,
Summary
I note that the odds ratio is given as 0.68 at three years follow up for the outcome "Persons with new hip fracture in 3 years" for Chapuy 1992. However this differs slightly from the odds ratio presented in the original BMJ publication. Why do these two results differ and why don't the reviewers present odds ratio based on intention‐to‐treat analysis as presented in the analysis in this paper?
Reply
Thank you for pointing out this discrepancy. This occurred because, by mistake, we used the data for the total number of hip fractures sustained rather than those for the number of people sustaining one or more hip fractures. The corrected intention‐to‐treat analysis, presented in the review, yields the same odds ratio as in the BMJ article.
Contributors
Comment sent from: Associate Prof Ivar Sonbo Kristiansen, Odense, Denmark Reply from: Prof William Gillespie, Dunedin, New Zealand Processed by: Dr Helen Handoll, Edinburgh, UK Dr Rajan Madhok, Hull, UK (Criticism Editor)
Comment on vitamin D doses, December 2015
Summary
Your 'Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men' is an excellent example of superb and rigorous activity applied in a way that is completely useless; that is when the process fails to consider whether the original studies are based on flawed assumptions. For example, were you to review the criteria in the studies evaluated, the preponderance of them will show the investigators' lack of understanding of the fundamental nature of cholecalciferol, which they think of it as a "vitamin", and they fail to appreciate that a blood level approximating 50 ng/ml is normal. Instead, they set the bar and the dosage so low as to be very close to proving a negative effect. Any protocol for the effects of cholecalciferol that does not include as a fundamental criterion, dosing in such a manner as DAILY (instead of Stoss‐like frequencies of weekly, monthly, or worst, single‐megadose) in the amounts the human body will generate when given modest UV‐B exposure that more closely mimics the evolutionary stimulii for humans, AND then verifying by frequent testing of the 25(OH)D levels to insure a level of around 50 ng/ml is not only achieved but maintained throughout the study, is useless.
Reply
Thank you for your comments on our Cochrane Review. Cochrane Reviews can only cover the actual trials that have been conducted, and so are limited by the vitamin D formulations, doses and dosing schedules used by those trials.
In the text of our review we do point out that "the administration of vitamin D alone, whether by annual injection, periodic bolus oral dosage, or daily oral dosage, is unlikely to be effective for fracture prevention in the doses used here".
We also state that "Vitamin D was administered in a variety of formats and doses (oral daily, oral intermittent, intra‐muscular). However, none of the trials in this review tested daily vitamin D3 in doses greater than 800 IU. It has been suggested that higher doses of vitamin D are required, such as 1800 IU/day to 4000 IU/day (Bischoff‐Ferrari 2010), in order to raise 25(OH)D to at least 75 to 110 nmol/ L."
This Cochrane Review will be updated when the results of trials with daily higher dose oral vitamin D are available, producing around the 25(OH)D levels you suggest. An example is the US VITAL trial, which aims to provide levels of 90 nmol/L.
Contributors
Feedback submitted by: A Ron Carmichael, USA Reply prepared by: Alison Avenell (review contact author) Editors: Helen Handoll (Co‐ordinating Editor, Cochrane Bone, Joint and Muscle Trauma Group); Cathie Sherrington (Feedback Editor; Cochrane Bone, Joint and Muscle Trauma Group)
What's new
| Date | Event | Description |
|---|---|---|
| 1 June 2021 | Amended | Given the trial report of Ishida 2004, an included trial, has been retracted, changes were made to the review to alert the reader to this, including removing data from the analyses, and relabelling were updated, and the study: Ishida 2004 (Retracted). Concerns with regard to the included studies, Pfeifer 2009 and Pfeifer 2000, were added to Characteristics of studies table entries and references to correspondence added. See Published notes. |
History
Protocol first published: Issue 2, 1995 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 15 February 2016 | Feedback has been incorporated | Feedback incorporated about vitamin D doses tested in the trials. |
| 28 February 2014 | New citation required and conclusions have changed |
|
| 1 December 2012 | New search has been performed |
|
| 13 February 2009 | New citation required but conclusions have not changed | An editorial oversight resulted in the omission of a new citation for the very substantial update of this review, published in Issue 1, 2009. Although there were no changes to the conclusions, the evidence base for this review was substantially augmented by the addition of eight new trials, contributing data from 44,827 participants. |
| 31 October 2008 | New search has been performed | In this update (Issue 1, 2009) we updated the search to October 2007. Eight new trials have been included with 44,827 participants. The conclusions of the review are unchanged for fracture prevention. |
| 30 October 2008 | Amended | Converted to new review format. |
| 26 May 2005 | New search has been performed | This review was substantively updated with 17 new studies in Issue 3, 2005. Eleven studies were awaiting assessment and three ongoing trials were identified. The conclusions were revised. |
| 30 November 2000 | New search has been performed | This review was substantively updated in Issue 1, 2001. Seven new studies were included and six studies awaited further evaluation. Five ongoing trials were identified. Small corrections were made to the results for hip fracture at three years for Chapuy 1992 in response to a reader's comment. The search strategy was updated. The methodological appraisal tool was revised In accordance with review group policy and the included studies re‐scored. Data were analysed and presented as relative risk rather than Peto odds ratio. The reviewers' conclusions remained substantially unchanged. |
Notes
This review has been amended to alert the reader to the changes relating to three included studies. Ishida 2004, has been retracted; reflecting this, the analyses and discussion were updated, and the study was relabelled: Ishida 2004 (retracted). This trial evaluated alfacalcidol and its removal did not change our findings. It also did not appear in the summaries or affect the conclusions. Thus with discussion with Helen Handoll (former Co‐ordinating Editor, Cochrane Bone, Joint and Muscle Trauma Group), we took the pragmatic decision to retain this study as an included study, reflecting its minimal impact on review findings. It will be removed in full for the update, which is presently ongoing.
Concerns with regard to Pfeifer 2000 and Pfeifer 2009 were added to Characteristics of studies table entries and references to published correspondence added. The review update will incorporate the findings related to these two trials.
Acknowledgements
We are very grateful to Prof Bill Gillespie and Mrs Lesley Gillespie for their extensive work on previous versions of this review. We would also like to acknowledge the contribution of Prof David Henry and Dr Jane Robertson as co‐authors of the first version of the review, and Mari Imamura who extracted data for trials published in Japanese.
The authors are grateful to the following who replied to requests for further information about primary studies: Dr HA Bischoff, Dr Liz Burleigh, Dr A Caniggia, Dr MC Chapuy, Prof C Cooper, Dr S Crozier, Dr LC Dukas, Dr P Ebeling, Dr JA Falch, Prof L Flicker, Dr JC Gallagher, Prof AM Grant, Dr Y Hayashi, Dr Y Ishida, Dr R Harwood, Dr H Janssen, Dr A Johansen, Ms C Kennedy, Dr MH Komulainen, Prof MR Law, Dr P Lips, Prof M McMurdo, Prof P Meunier, Prof A Papaioannou, Prof K Sanders, Dr MW Tilyard, Prof DJ Torgerson, Dr MD Witham, and Dr K Zhu.
We would also like to thank Prof Roger Francis, Prof Adrian Grant, Prof Jos Kleijnen, Dr Brendon Rae, Prof David Torgerson for their valuable comments on the protocol, review and review updates. We would also like to thank the following editors of the Cochrane Bone, Joint and Muscle Trauma Group for their comments: A/Prof Peter Herbison, Dr Vicki Livingstone, Prof Rajan Madhok and Dr Janet Wale. We thank Dr Miles Witham for his constructive comments on this review. We particularly thank Dr Helen Handoll from the Cochrane Bone, Joint and Muscle Trauma Group for help with the 'Summary of findings' table.
We acknowledge the internal financial support for earlier versions of the review from: University of Aberdeen, UK; University of Edinburgh, UK; University of Hull, UK; University of Newcastle, Australia; University of Otago and Healthcare Otago Endowment Trust, New Zealand; University of York, UK. We received external financial support for earlier versions from: Australian Institute of Health and Welfare; Health Research Council of New Zealand, Medical Research Council, UK.
Appendices
Appendix 1. Search strategies
Central Register of Controlled Trials (Wiley Online Library)
2012 Issue 12
#1 MeSH descriptor: [Vitamin D] explode all trees (1875) #2 (vitamin D or vitamin D2 or vitamin D3):ti,ab,kw (3242) #3 (al*acalcidol or c*olecalciferol or calcitriol or calcidiol or calcifediol or calciferol or calciol or calderol or dihydrotachysterol or dedrogyl or dihydrotachysterol or dihydroxycolecalciferol or dihydroxycholecalciferol or dihydroxyvitamin D or dihydroxyvitamin D2 or dihydroxyvitamin D3 or doxercalciferol or eldecalcitol or ercalcidiol or ergocalciferol* or hidroferol or hydroxycalciferol or hydroxyl‐calciferol or hydroxycolecalciferol or hydroxycholecalciferol or hydroxyergocalciferol* or hydroxyvitamin D or hydroxyvitamin D2 or hydroxyvitamin D3 or paricalcitol or tachystin):ti,ab,kw (2077) #4 #1 or #2 or #3 (4207) #5 MeSH descriptor: [Fractures, Bone] explode all trees (3421) #6 (fracture*):ti or (fracture*):ab (6539) #7 #5 or #6 (7024) #8 #4 and #7 (592) #9 SR‐muskinj (7174) #10 #8 not #9 [in Trials] (372)
MEDLINE (OVID Web)
Aug 2007 to Dec 2012
1 exp Vitamin D/ (39953) 2 (vitamin D or vitamin D2 or vitamin D3).tw. (35694) 3 (al*acalcidol or c?olecalciferol or calcitriol or calcidiol or calcifediol or calciferol or calciol or calderol or dihydrotachysterol or dedrogyl or dihydrotachysterol or dihydroxycolecalciferol or dihydroxycholecalciferol or dihydroxyvitamin D or dihydroxyvitamin D2 or dihydroxyvitamin D3 or doxercalciferol or eldecalcitol or ercalcidiol or ergocalciferol* or hidroferol or hydroxycalciferol or hydroxyl‐calciferol or hydroxycolecalciferol or hydroxycholecalciferol or hydroxyergocalciferol* or hydroxyvitamin D or hydroxyvitamin D2 or hydroxyvitamin D3 or paricalcitol or tachystin).tw. (20807) 4 or/1‐3 (53551) 5 exp Fractures, Bone/ (133479) 6 fracture*.ti,ab. (150015) 7 or/5‐6 (188023) 8 and/4,7 (4430) 9 Randomized controlled trial.pt. (342334) 10 Controlled clinical trial.pt. (85694) 11 randomized.ab. (244919) 12 placebo.ab. (136550) 13 Drug therapy.fs. (1588363) 14 randomly.ab. (175193) 15 trial.ab. (253825) 16 groups.ab. (1145730) 17 or/9‐16 (2960405) 18 exp Animals/ not Humans/ (3812817) 19 17 not 18 (2515366) 20 and/8,19 (2258) 21 (200708* or 200709* or 200710* or 200711* or 200712* or 2008* or 2009* or 2010* or 2011* or 2012*).ed. (4151978) 22 20 and 21 (901)
EMBASE (OVID Web)
Jan 2007 to Dec 2012
1 exp Vitamin D/ (78052) 2 (vitamin D or vitamin D2 or vitamin D3).tw. (47810) 3 (al*acalcidol or c?olecalciferol or calcitriol or calcidiol or calcifediol or calciferol or calciol or calderol or dihydrotachysterol or dedrogyl or dihydrotachysterol or dihydroxycolecalciferol or dihydroxycholecalciferol or dihydroxyvitamin D or dihydroxyvitamin D2 or dihydroxyvitamin D3 or doxercalciferol or eldecalcitol or ercalcidiol or ergocalciferol* or hidroferol or hydroxycalciferol or hydroxyl‐calciferol or hydroxycolecalciferol or hydroxycholecalciferol or hydroxyergocalciferol* or hydroxyvitamin D or hydroxyvitamin D2 or hydroxyvitamin D3 or paricalcitol or tachystin).tw. (25323) 4 or/1‐3 (88916) 5 exp Fracture/ (178731) 6 fracture*.ti,ab. (181466) 7 or/5‐6 (235005) 8 and/4,7 (10721) 9 exp Randomized Controlled trial/ (334017) 10 exp Double Blind Procedure/ (112280) 11 exp Single Blind Procedure/ (16758) 12 exp Crossover Procedure/ (35737) 13 Controlled Study/ (3923787) 14 or/9‐13 (4004024) 15 ((clinical or controlled or comparative or placebo or prospective$ or randomi#ed) adj3 (trial or study)).tw. (661628) 16 (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$)).tw. (161062) 17 ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw. (149581) 18 (cross?over$ or (cross adj1 over$)).tw. (63934) 19 ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. (202997) 20 or/15‐19 (987275) 21 or/14,20 (4494169) 22 limit 21 to human (2729889) 23 and/8,22 (2601) 24 (2007* or 2008* or 2009* or 2010* or 2011* or 2012*).em. (6433620) 25 23 and 24 (1398)
CINAHL (Ebsco)
Jan 2007 to Dec 2012
S1 (MH "Vitamin D+") (7,530) S2 TX vitamin D or vitamin D2 or vitamin D3 (9,803) S3 TX al*acalcidol or c*olecalciferol or calcitriol or calcidiol or calcifediol or calciferol or calciol or calderol or dihydrotachysterol or dedrogyl or dihydrotachysterol or dihydroxycolecalciferol or dihydroxycholecalciferol or dihydroxyvitamin D or dihydroxyvitamin D2 or dihydroxyvitamin D3 or doxercalciferol or eldecalcitol or ercalcidiol or ergocalciferol* or hidroferol or hydroxycalciferol or hydroxyl‐calciferol or hydroxycolecalciferol or hydroxycholecalciferol or hydroxyergocalciferol* or hydroxyvitamin D or hydroxyvitamin D2 or hydroxyvitamin D3 or paricalcitol or tachystin (1,998) S4 S1 or S2 or S3 (10,187) S5 (MH "Fractures") (10,130) S6 TI fracture* OR AB fracture* (27,212) S7 S5 or S6 (31,188) S8 S4 and S7 (1,319) S9 (MH "Clinical Trials+") (152,382) S10 (MH "Evaluation Research+") (19,032) S11 (MH "Comparative Studies") (69,705) S12 (MH "Crossover Design") (9,958) S13 PT Clinical Trial (74,592) S14 (MH "Random Assignment") (33,960) S15 S9 or S10 or S11 or S12 or S13 or S14 (244,640) S16 TX ((clinical or controlled or comparative or placebo or prospective or randomi?ed) and (trial or study)) (421,108) S17 TX (random* and (allocat* or allot* or assign* or basis* or divid* or order*)) (59,647) S18 TX ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) (649,695) S19 TX ( crossover* or 'cross over' ) or TX cross n1 over (12,502) S20 TX ((allocat* or allot* or assign* or divid*) and (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)) (74,928) S21 S16 or S17 or S18 or S19 or S20 (991,006) S22 S15 or S21 (1,051,168) S23 S8 and S22 (540) S24 EM 2007 OR EM 2008 OR EM 2009 OR EM 2010 OR EM 2011 OR EM 2012 (2,067,963) S25 S23 AND S24 (362)
Data and analyses
Comparison 1. Vitamin D [D2, D3 or 25(OH)D] versus control or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Persons sustaining new hip fracture | 11 | 27693 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.98, 1.29] |
| 1.1.1 Not selected on the basis of previous osteoporotic fracture | 8 | 24873 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.30] |
| 1.1.2 Selected on the basis of previous osteoporotic fracture | 3 | 2820 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.72, 1.62] |
| 1.2 Persons sustaining new non‐vertebral fracture | 12 | 22930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.14] |
| 1.2.1 Not selected on the basis of previous osteoporotic fracture | 10 | 20185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.16] |
| 1.2.2 Selected on the basis of previous osteoporotic fracture | 2 | 2745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.24] |
| 1.3 Persons sustaining new vertebral fracture or deformity | 6 | 11396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] |
| 1.3.1 Not selected on the basis of previous osteoporotic fracture | 4 | 8651 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.74, 1.36] |
| 1.3.2 Selected on the basis of previous osteoporotic fracture | 2 | 2745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.38, 8.37] |
| 1.4 Persons sustaining any new fracture | 15 | 28271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.11] |
| 1.4.1 Not selected on the basis of previous osteoporotic fracture | 12 | 25451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.12] |
| 1.4.2 Selected on the basis of previous osteoporotic fracture | 3 | 2820 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.21] |
Comparison 2. Vitamin D [D2, D3 or 25(OH)D] plus calcium versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Persons sustaining new hip fracture | 7 | 7411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.13] |
| 2.1.1 Not selected on the basis of previous osteoporotic fracture | 5 | 4730 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.45, 1.12] |
| 2.1.2 Selected on the basis of previous osteoporotic fracture | 2 | 2681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.41] |
| 2.2 Persons sustaining new non‐vertebral fracture | 6 | 3336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 2.2.1 Not selected on the basis of previous osteoporotic fracture | 4 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.22] |
| 2.2.2 Selected on the basis of previous osteoporotic fracture | 2 | 2681 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.22] |
| 2.3 Persons sustaining new vertebral fracture | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Selected on the basis of previous osteoporotic fracture | 2 | 2681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.77] |
| 2.4 Persons sustaining any new fracture | 11 | 8812 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 2.4.1 Not selected on the basis of previous osteoporotic fracture | 9 | 6131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.53, 0.92] |
| 2.4.2 Selected on the basis of previous osteoporotic fracture | 2 | 2681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.20] |
Comparison 3. Vitamin D [D2, D3 or 25(OH)D] versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Persons sustaining new hip fracture | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Selected on the basis of previous osteoporotic fracture | 2 | 2718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.32] |
| 3.2 Persons sustaining new non‐vertebral fracture | 4 | 3021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.91, 1.33] |
| 3.2.1 Not selected on the basis of previous osteoporotic fracture | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.62, 2.66] |
| 3.2.2 Selected on the basis of previous osteoporotic fracture | 2 | 2718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.32] |
| 3.3 Persons sustaining new vertebral fracture or deformity | 3 | 2976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.08, 4.53] |
| 3.3.1 Not selected on the basis of previous osteoporotic fracture | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.13, 5.95] |
| 3.3.2 Selected on the basis of previous osteoporotic fracture | 2 | 2718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.29, 5.80] |
| 3.4 Persons sustaining any new fracture | 4 | 3021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.33] |
| 3.4.1 Not selected on the basis of previous osteoporotic fracture | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.62, 2.66] |
| 3.4.2 Selected on the basis of previous osteoporotic fracture | 2 | 2718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.33] |
Comparison 4. Vitamin D [D2, D3 or 25(OH)D] plus calcium versus control or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Persons sustaining new hip fracture | 9 | 49853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.96] |
| 4.1.1 Not selected on the basis of previous osteoporotic fracture | 5 | 43719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.94] |
| 4.1.2 Selected on the basis of previous osteoporotic fracture | 4 | 6134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.47] |
| 4.2 Persons sustaining new hip fracture: subgroup analysis by residential status (institution vs community) | 9 | 49853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.96] |
| 4.2.1 Resident in institution (nursing home, residential care etc) | 2 | 3853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.62, 0.92] |
| 4.2.2 Community dwelling | 7 | 46000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.09] |
| 4.3 Persons sustaining new non‐vertebral fracture | 8 | 10380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.96] |
| 4.3.1 Not selected on the basis of previous osteoporotic fracture | 5 | 7560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 4.3.2 Selected on the basis of previous osteoporotic fracture | 3 | 2820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.13] |
| 4.4 Persons sustaining new vertebral fracture | 4 | 42185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.09] |
| 4.4.1 Not selected on the basis of previous osteoporotic fracture | 2 | 39477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 4.4.2 Selected on the basis of previous osteoporotic fracture | 2 | 2708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.20] |
| 4.5 Persons sustaining any new fracture | 10 | 49976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 0.99] |
| 4.5.1 Not selected on the basis of previous osteoporotic fracture | 6 | 43842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 4.5.2 Selected on the basis of previous osteoporotic fracture | 4 | 6134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.10] |
| 4.6 Persons sustaining any new fracture: subgroup analysis by residential status (institution vs community) | 10 | 49976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 0.99] |
| 4.6.1 Resident in institution (nursing home, residential care etc) | 2 | 3853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 4.6.2 Community dwelling | 8 | 46123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.01] |
Comparison 5. Alfacalcidol [1‐alpha(OH)D3] versus control or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Persons sustaining new non‐vertebral fracture | 3 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.25, 3.82] |
| 5.1.1 Not selected on the basis of previous osteoporotic fracture | 1 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.25, 3.82] |
| 5.1.2 Selected on the basis of previous osteoporotic fracture | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.2 Persons sustaining new vertebral fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Selected on the basis of previous osteoporotic fracture | 1 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.48, 0.65] |
Comparison 6. Alfacalcidol [1‐alpha(OH)D3] plus calcium versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Persons sustaining new hip fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 Selected on the basis of previous osteoporotic fracture | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.00] |
| 6.2 Persons sustaining new vertebral deformity | 5 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.96] |
| 6.2.1 Selected on the basis of previous osteoporotic fracture | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.23, 1.24] |
| 6.2.2 Not selected on the basis of previous osteoporotic fracture | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.44] |
Comparison 7. Alfacalcidol [1‐alpha(OH)D3] versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Persons sustaining new vertebral deformity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 Selected on the basis of previous osteoporotic fracture | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.74] |
Comparison 8. Calcitriol [1,25(OH)2D3] versus control or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Persons sustaining new hip fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1.1 Not selected on the basis of previous osteoporotic fracture | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 8.2 Persons sustaining new non‐vertebral fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.2.1 Not selected on the basis of previous osteoporotic fracture | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.15] |
| 8.3 Persons sustaining new vertebral deformity | 3 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.41] |
| 8.3.1 Not selected on the basis of previous osteoporotic fracture | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.45, 35.28] |
| 8.3.2 Selected on the basis of previous osteoporotic fracture | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.28, 1.10] |
Comparison 9. Calcitriol [1,25(OH)2D3] plus calcium versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Persons sustaining new non‐vertebral fracture | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.51, 12.19] |
| 9.1.1 Selected on the basis of previous osteoporotic fracture | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.51, 12.19] |
| 9.2 Persons sustaining new vertebral deformity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.2.1 Selected on the basis of previous osteoporotic fracture | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.58, 3.85] |
Comparison 10. Calcitriol [1,25(OH)2D3] plus vitamin D plus calcium versus vitamin D plus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Persons sustaining new vertebral deformity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1.1 Selected on the basis of previous osteoporotic fracture | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.41, 1.52] |
Comparison 11. Calcitriol [1,25(OH)2D3] versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Persons sustaining new non‐vertebral fracture | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1.1 Selected on the basis of previous osteoporotic fracture | 2 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.10, 14.78] |
| 11.2 Persons sustaining new vertebral deformity | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.2.1 Selected on the basis of previous osteoporotic fracture | 2 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.25, 11.28] |
| 11.3 Persons sustaining new vertebral deformity in Tilyard study | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.3.1 Year 1 | 1 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.40, 1.58] |
| 11.3.2 Year 2 | 1 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.26, 0.87] |
| 11.3.3 Year 3 | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.52] |
Comparison 12. Calcitriol [1,25(OH)2D3] versus vitamin D (with or without calcium in each group).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Persons sustaining new non‐vertebral fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1.1 Selected on the basis of previous osteoporotic fracture | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.40, 3.37] |
| 12.2 Persons sustaining new vertebral deformity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.2.1 Selected on the basis of previous osteoporotic fracture | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.55, 3.47] |
Comparison 13. Vitamin D [D2, D3 or 25(OH)D] or any analogue with/without calcium: adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Deaths | 29 | 71032 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.01] |
| 13.1.1 Vitamin D [D2, D3, 25(OH)D] | 18 | 22854 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.03] |
| 13.1.2 Vitamin D [D2, D3 or 25(OH)D] plus calcium | 6 | 46794 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 13.1.3 Alfacalcidol [1‐alpha(OH)D3] | 1 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.38] |
| 13.1.4 Calcitriol [1,25(OH)2D3] | 4 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.41, 3.09] |
| 13.2 Persons with hypercalcaemia | 21 | 17124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.57, 3.31] |
| 13.2.1 Vitamin D [D2, D3 or 25(OH)D] | 10 | 11611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.80, 3.05] |
| 13.2.2 Vitamin D [D2, D3 or 25(OH)D] plus calcium | 2 | 3853 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.37, 29.14] |
| 13.2.3 Alfacalcidol [1‐alpha(OH)D3] | 5 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.85, 2.72] |
| 13.2.4 Calcitriol [1,25(OH)2D3] | 4 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.41 [2.14, 9.09] |
| 13.3 Persons with gastrointestinal effects | 15 | 47761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.00, 1.08] |
| 13.3.1 Vitamin D [D2, D3 or 25(OH)D] | 7 | 6230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 13.3.2 Vitamin D [D2, D3 or 25(OH)D] plus calcium | 4 | 40524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.01, 1.09] |
| 13.3.3 Alfacalcidol [1‐alpha(OH)D3] | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.3.4 Calcitriol [1,25(OH)2D3] | 3 | 909 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.58] |
| 13.4 Persons with renal disease (calculi or insufficiency) | 11 | 46548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.02, 1.33] |
| 13.4.1 Vitamin D [D2, D3, 25(OH)D] | 4 | 5978 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.42] |
| 13.4.2 Vitamin D [D2, D3 or 25(OH)D] plus calcium | 2 | 39552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.03, 1.34] |
| 13.4.3 Alfacalcidol [1‐alpha(OH)D3] | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.4.4 Calcitriol [1,25(OH)2D3] | 4 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.61, 10.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aloia 1988.
| Study characteristics | ||
| Methods | Randomisation schedule held off campus by the sponsoring manufacturer Appears adequately blinded 27 of 34 participants completed | |
| Participants | Tertiary hospital, USA
34 women with post menopausal osteoporosis aged 50 to 80 years. Mean age 64.5 years. Sample drawn from media release publicity Inclusion criterion: at least 1 non‐traumatic vertebral compression fracture Disease exclusions: hepatic or renal disease, malignancy, malabsorption, parathyroid or thyroid disorder, inflammatory arthritis, alcoholism, overt vitamin D deficiency, history of renal stones, insulin dependent diabetes, previous long‐term hospitalisation, any other disorder known to affect bone metabolism Drug exclusions: glucocorticoids, anticonvulsants, oestrogens, sodium fluoride, calcium supplements, pharmacologic doses of vitamin D |
|
| Interventions | 1. Calcitriol 0.25 μg, dose titrated, plus vitamin D 400 IU daily
Randomised 17, completed 12 2. Placebo plus vitamin D 400 IU daily Randomised 17, completed 15 Calcium intake adjusted to 1 g/day in each group (diet adjustment). Stepwise increase at 2‐weekly intervals ending at double the initial dose permitted at investigators' control Duration of treatment 24 months |
|
| Outcomes | Measured at 2 years 1. Number of women with new vertebral fractures, measured radiologically 2. Number of new vertebral fractures in each group 3. BMC radius 4. BMD lumbar spine 5. Total body calcium (neutron activation) 6. Radiographic absorptiometry of phalanges 7. Urinary hydroxyproline 8. Vitamin D metabolites 9. PTH radioimmunoassay 10. Serum alkaline phosphatase 11. Serum osteocalcin 12. Bone biopsy 13. Renal dysfunction | |
| Notes | Although authors published separately, trial protocol similar to Gallagher 1990 and Ott 1989 (see personal communication from Gallagher under Gallagher 1990) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized", no other information |
| Allocation concealment (selection bias) | Low risk | States "double‐blind", and "Following completion of 24 months of study, the code was broken" |
Arthur 1990.
| Study characteristics | ||
| Methods | Randomised trial of 2 treatments Normal "controls" described but not randomised Radiologic assessors blinded 10 of 14 participants completed | |
| Participants | Community hospital, USA
10 women over 60 years (mean age 66.5 years) with radiographic and bone biopsy evidence of osteoporosis Disease exclusions: renal or liver disease, malabsorption or surgery that might predispose to malabsorption, hypercalcaemia, malignancy, hyperthyroidism, alcoholism, significant immobilisation Drug exclusions: use of steroids (including oestrogen), heparin or anticonvulsants |
|
| Interventions | 1. Calcitriol 0.25 μg plus 1 g elemental calcium/day orally. Calcitriol dose doubled in all participants by end of study (monitored by serum calcium at 10 mg/dL or less)
Randomised 7, completed 4 2. Ergocalciferol 50,000 units orally twice weekly, plus 1 g elemental calcium daily Randomised 7, completed 6 All in Group 1 and two‐thirds in Group 2 were taking calcium supplements at entry Duration of treatment 12 months |
|
| Outcomes | Measured at 1 year 1. Women sustaining new vertebral fractures during study 2. BMD lumbar spine (CT) 3. Bone biopsy 4. Serum vitamin D 5. Serum Ca, PO4, creatinine 6. Creatinine clearance 7. Daily calcium excretion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no other information |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Avenell 2004.
| Study characteristics | ||
| Methods | Random allocation. Remote site computer generated randomisation Blinding of outcome assessors stated 106 of 134 participants completed | |
| Participants | Community‐based study, UK
134 patients (111 women, 23 men), mean age 78 years Inclusion criteria: osteoporotic fracture within the last 10 years, aged 70 years or over Disease exclusion: bed‐ or chair‐bound prior to fracture, cognitive impairment indicated by an abbreviated mental test score of < 7, suffered from cancer likely to metastasise to bone within the previous 10 years, fracture associated with pre‐existing local bone abnormality, known hypercalcaemia, renal stone in the last 10 years, life expectancy < 6 months, known to be leaving the UK Drug exclusions: taking more than 200 IU (5 μg) vitamin D or more than 500 mg calcium supplements daily; had fluoride, bisphosphonates, calcitonin, tibolone, HRT, selective oestrogen receptor modulators, or any vitamin D metabolite (such as calcitriol) in the last 5 years; vitamin D by injection in the last year |
|
| Interventions | 1. Calcium 1000 mg and vitamin D3 800 IU given as 2 tablets daily
Randomised 35, completed 32 2. Calcium 1000 mg given as 2 tablets daily Randomised 29, completed 25 3. Vitamin D3 800 IU given as 2 tablets daily Randomised 35, completed 20 4. No tablets Randomised 35, completed 29 Duration of treatment: up to 46 months |
|
| Outcomes | Measured over 46 months 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Number of persons with new clinical vertebral fracture 4. Number of persons with hypercalcaemia, renal stone or failure, gastrointestinal adverse events 5. Numbers of persons dying | |
| Notes | Dr Avenell provided longer‐term follow‐up data (1 year data in published trial). Trial is parallel study to the RECORD trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States used "preprogrammed laptop computer to generate random allocation" |
| Allocation concealment (selection bias) | Low risk | States used "preprogrammed laptop computer". Remote site |
Bischoff 2003.
| Study characteristics | ||
| Methods | Statistician generated block randomisation, no further details. Double‐blind trial 89 of 122 participants completed for fracture data | |
| Participants | 2 long‐stay geriatric care units, Switzerland
122 patients (all women), mean age 85.3 years (SD 6.6) Inclusion criteria: 60 years or over, ability to walk 3 meters with or without walking aid Disease exclusions: primary hyperparathyroidism, hypocalcaemia, hypercalciuria, creatinine > 117 μmol/L, fracture or stroke in last 3 months Drug exclusions: HRT, calcitonin, fluoride, bisphosphonates in last 24 months |
|
| Interventions | 1. 1200 mg calcium carbonate and 800 IU vitamin D3 as 2 tablets daily, randomised 62 2. 1200 mg calcium carbonate as 2 tablets daily, randomised 60 Losses to follow‐up 33, numbers for each group unclear Duration of treatment 12 weeks |
|
| Outcomes | Measured over a follow‐up of 12 weeks 1. Number of persons with new hip fracture 2. Number of persons with gastrointestinal adverse events, hypercalcaemia 3. Numbers of persons dying | |
| Notes | Dr Bischoff supplied hip fracture and mortality data according to allocation by e‐mail on 13 July 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomization was performed by an independent statistician", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomization was performed by an independent statistician", no other information provided |
Bolton‐Smith 2007.
| Study characteristics | ||
| Methods | Double‐blind trial. Independent statistician at remote site provided randomisation 106 of 123 participants had fracture data in vitamin D/calcium group and placebo group | |
| Participants | 123 patients (all women), mean age 68.6 years, for vitamin D/calcium group and placebo group only Inclusion criterion: 60 years or over, healthy Disease exclusions: clinical osteoporosis; chronic disease (e.g. diabetes mellitus, cardiovascular disease, cancer, fat malabsorption) Drug exclusions: routine medication interfering with vitamin K, vitamin D or bone metabolism (e.g. warfarin, steroids); supplements over 30 μg/day vitamin K, 10 μg (400 IU)/day vitamin D or 500 mg calcium/day |
|
| Interventions | 1. 1000 mg calcium carbonate and 400 IU vitamin D3 daily and placebo daily
Randomised 62, 50 completed 2 years 2. 1000 mg calcium carbonate and 400 IU vitamin D3 and 200 μg vitamin K1 daily 3. 200 μg vitamin K1 daily and placebo daily 4. Double placebo Randomised 61, 56 completed 2 years Duration of treatment 2 years |
|
| Outcomes | Measured over a follow‐up of 2 years 1. Number of persons with new non‐vertebral fracture | |
| Notes | Prof McMurdo supplied fracture data, collected by self‐report, on 1 October 2007. Groups 2 and 3 not used in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | States "an independent statistician at Hoffman‐La Roche, who had no other connection to the study, provided a computer‐generated randomization list to the researchers" |
Burleigh 2007.
| Study characteristics | ||
| Methods | Double‐blind randomised trial Blinding of outcome assessors stated 199 of 205 participants completed |
|
| Participants | Acute geriatric unit, Glasgow, Scotland 205 patients (121 women, 84 men), mean age 83 years, newly transferred or admitted to wards Inclusion criteria: 65 years or over Exclusion criteria: known hypercalcaemia, urolithiasis, or renal dialysis. Terminally ill or bed‐bound with reduced Glasgow Coma Score, already prescribed calcium and vitamin D, nil by mouth at time of admission |
|
| Interventions | 1. 800 IU vitamin D3 and 1200 mg calcium as calcium carbonate once daily 2. 1200 mg calcium as calcium carbonate once daily Randomised 101/104; 98/101 completed Median duration of treatment 30 days (interquartile range 15 to 71) |
|
| Outcomes | Outcomes measured at a median of 30 days (interquartile range 15 to 71 days) 1. Number of persons with new non‐vertebral fracture 2. Number of persons with new hip fracture 3. Number of persons with hypercalcaemia, gastrointestinal adverse events 4. Number of persons dying |
|
| Notes | Liz Burleigh supplied information on fractures and hypercalcaemia 23 December 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "randomised using a random numbers table" |
| Allocation concealment (selection bias) | Low risk | States "randomisation was known only to the statistician and pharmacist who subsequently issued an appropriate uniquely numbered drug blister pack to each patient's ward" |
Caniggia 1984.
| Study characteristics | ||
| Methods | Allocation concealment technique not clearly described, and not clarified as result of correspondence Blinding appears adequate 22 of 28 participants completed | |
| Participants | Tertiary hospital, Italy
28 women aged 54 to 74 years (mean age not given) with symptomatic post‐menopausal osteoporosis Inclusion criterion: radiolucency of spine with at least 1 crush fracture Disease exclusions: osteomalacia on iliac crest bone biopsy, malabsorption Drug exclusions: adrenocorticosteroids for 3 months or more in last 5 years, anticonvulsants, oestrogens, progestagens, androgens, anabolic drugs (in last 6 months), chlorothiazide and allied diuretics, sodium fluoride, calcium and vitamin D within the last 6 months |
|
| Interventions | 1. 1,25(OH)2vitamin D3 0.5 μg /day with oestrogen placebo
Randomised 7, completed 5 2. Oestradiol valerate 2 mg/day on 21‐on and 7‐off cycle, with vitamin D3 placebo Randomised 7, completed 5 3. Both interventions as in 1 and 2 Randomised 7, completed 7 4. Double placebo Randomised 7, completed 5 Duration of treatment 1 year |
|
| Outcomes | Measured at 1 year 1. Number of new vertebral fractures 2. Variation in standing height 3. BMC of the ulna at 2 measuring points 4. Iliac crest bone histomorphometry 5. Pain relief and improvement of mobility 6. Biochemical parameters: plasma and urinary calcium, phosphate, and creatinine, serum alkaline phosphatase, urinary hydroxyproline, liver enzymes, ESR 7. Blood pressure, vaginal bleeding | |
| Notes | Clarification sought regarding methods. Reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "double‐blind" and "placebo", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "double‐blind" and "placebo", no other information provided |
Chapuy 1992.
| Study characteristics | ||
| Methods | Random allocation. Allocation concealment details following clarification from author Blinding of assessors unclear 2303 of 3270 completed, 3 years | |
| Participants | France
3270 women aged 69 to 106 years (mean 84, SD 6) living in nursing homes or apartment houses for the elderly Inclusion criteria: ambulant, life expectancy of at least 18 months. Previous fracture and thiazide usage not excluded Disease exclusions: "serious medical conditions" Drug exclusions: corticosteroids, anticonvulsants, thyroxine, fluoride, calcium supplementation |
|
| Interventions | 1. Calcium 1.2 g plus vitamin D3 800 IU orally daily
Randomised 1634, 877 completed 18 months 2. Double placebo Randomised 1636, 888 completed 18 months Treatment period 18 months for initial report, continued to complete 3 years |
|
| Outcomes | Outcomes measured up to 3 years 1. Hip fractures at 18 months and 3 years 2. Non‐vertebral fractures at 18 months and 3 years 3. In a subgroup, serum calcium, phosphate, creatinine, total protein, alkaline phosphatase, PTH, 25(OH)D3 (73 treatment, 69 placebo) at baseline and 6‐monthly to 18 months 4. Femoral BMD at baseline and after 18 months in 27 treatment and 29 placebo 5. Adverse effects: gastro intestinal symptoms, renal disease, hypercalcaemia, death |
|
| Notes | Falling status recorded at baseline but no falling data presented in the relevant papers thus far. Allocation concealment details provided following clarification from author 18‐month follow‐up reported in 1992, and 3‐year follow‐up in 1994. There appears to be a discrepancy between the 18‐month and 3‐year report compatible with misclassification of 5 participants at some point | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned. . .in groups of four at each nursing home" |
| Allocation concealment (selection bias) | Low risk | Details provided by author |
Chapuy 2002.
| Study characteristics | ||
| Methods | Random allocation. No further details provided Double‐masked, placebo‐controlled study, blinding of outcome assessors not confirmed Completion rate unclear | |
| Participants | Residents of 55 apartment houses for elderly people, in France
610 or 583 (number unclear) women, mean age 85 years Inclusion criteria: ambulatory (able to walk indoors with cane or walker), life expectancy of at least 24 months Disease exclusions: intestinal malabsorption, hypercalcaemia (serum calcium > 2.63 mmol/L), chronic renal failure (serum creatinine > 150 μmol/L) Drug exclusions: received drugs known to alter bone metabolism, such as corticosteroids, anticonvulsants or a high dose of thyroxine, in the past year. Fluoride salts (> 3 months), bisphosphonates, calcitonin (> 1 month), calcium (> 500 mg daily), vitamin D (> 100 IU daily) in last 12 months |
|
| Interventions | 1. Calcium 1200 mg as tricalcium phosphate and vitamin D3 800 IU daily as 1 sachet 2. Calcium 1200 mg as tricalcium phosphate sachet and 2 pills of vitamin D3 400 IU daily Groups 1 and 2: randomised 389, completed unclear 3. 1 placebo sachet and 2 placebo tablets daily. Randomised 194, completed unclear Duration of treatment 2 years |
|
| Outcomes | Measured over a follow‐up of 2 years 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Numbers of persons developing hypercalcaemia 4. Number of persons dying 5. Number of persons reporting gastrointestinal disorders 6. PTH, 25(OH)D 7. BMD of distal radius, femoral neck BMD, ultrasound of os calcis | |
| Notes | Prof Meunier provided further details on outcomes 28 February 2005, confirming 194 in placebo group and 389 in calcium and vitamin D groups combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Dawson‐Hughes 1997.
| Study characteristics | ||
| Methods | Random allocation. Stratified by gender, race, and decade of age Double‐blind, placebo‐controlled trial 389 of 445 completed |
|
| Participants | Community‐based study, USA
445 enrolled participants (199 men, 246 women, aged 65 years and older (mean age 71 years)). Recruitment was from a mix of volunteers answering advertisement, and presentations on medical care Exclusion criteria: current cancer or hyperparathyroidism, renal stone history within 5 years, bilateral hip surgery, femoral neck BMD more than 2 SD below the mean for age and gender, dietary calcium intake exceeding 1500 mg/day, laboratory evidence of renal or liver disease Drug exclusions: therapy with a bisphosphonate, calcitonin, oestrogen, tamoxifen, or testosterone in the past 6 months, or fluoride within the past 2 years |
|
| Interventions | 1. Calcium 500 mg plus vitamin D3 700 IU orally daily 2. Double placebo Total randomised 445, 389 completed Duration of treatment 3 years |
|
| Outcomes | Final assessment at 3 years
1. Non‐vertebral fractures identified by self report, interview, and validation from case records Also measured at 6‐month intervals, but not considered in this review, were BMD, biochemical assays, and other measures |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
Dukas 2004.
| Study characteristics | ||
| Methods | Random allocation. Randomisation independent of trial Blinding of outcome assessors stated 323 of 380 completed | |
| Participants | Community study, Switzerland
380 (192 women, 188 men), mean age 75 years Inclusion criteria: age 70 years or over, mobile, independent lifestyle Disease exclusions: primary hyperparathyroidism, polyarthritis, inability to walk, active kidney stone disease, history of hypercalciuria, cancer or other incurable disease, dementia, elective surgery within next 3 months, creatinine clearance < 20 mL/min, fracture or stroke in last 3 months Drug exclusions: current calcium supplementation of > 500 mg/day or vitamin D > 200 IU/day |
|
| Interventions | 1. Alfacalcidol D3 1 μg tablet/day
Randomised 193, completed unclear 2. Placebo tablet once daily Randomised 187, completed unclear Duration of treatment 36 weeks |
|
| Outcomes | Measured over follow‐up of 36 weeks 1. Number of persons sustaining new non‐vertebral fracture 2. Number of persons dying 3. Number of persons developing hypercalcaemia 4. PTH, 1,25(OH)2D3 and 25(OH)D3 | |
| Notes | Dr LC Dukas provided fracture data 19 July 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomization was done using numbered containers" |
Ebeling 2001.
| Study characteristics | ||
| Methods | Random allocation Outcome assessors blinded for assessment of vertebral fractures 33 of 41 completed | |
| Participants | Hospital‐based study, Australia
41 patients (41 men), age range 27‐77 years, with primary osteoporosis Inclusion criterion: at least 1 fragility fracture Disease exclusions: disease known to affect bone or mineral metabolism, normal 25(OH)D and BMD T score values Drug exclusions: none given |
|
| Interventions | 1. Calcitriol 0.5 μg twice daily and calcium placebo twice daily
Randomised 21, completed 17 2. Calcium 500 mg twice daily and calcitriol placebo twice daily. Randomised 20, completed 16 Duration of treatment 2 years |
|
| Outcomes | Measured over first and second years and overall 1. Number of persons sustaining new vertebral fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Numbers of persons with adverse events 4. BMD of lumbar spine and femoral neck, total body bone mineral content 5. Biochemical markers of bone formation and breakdown, PTH, 25(OH)D, 1,25(OH)2D | |
| Notes | Dr Ebeling provided details of numbers randomised and details of non‐vertebral fractures 15 February 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized"; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Falch 1987.
| Study characteristics | ||
| Methods | Randomised trial Evaluation at 3 years by blinded observers 76 of 86 completed | |
| Participants | University Hospital, Norway
86 postmenopausal women aged 50 to 65 years (mean age 59.6 years) who had sustained a fracture of the distal left forearm Disease exclusions: if incident fall was from greater than standing height, previous fracture of the right forearm, endocrine disease, malabsorption, gastric surgery, nephrolithiasis, renal failure Drug exclusions: oestrogens, anticonvulsants, glucocorticoids |
|
| Interventions | 1. Calcitriol 0.5 μg daily (reduced to 0.25 μg if serum calcium rose above 2.65 mmol/L)
Randomised 47, completed 39 2. Vitamin D3 400 IU daily (oral) Randomised 39, completed 37 No calcium supplements or manipulation of dietary calcium involved Duration of treatment 3 years |
|
| Outcomes | Measured at 3 years 1. Number of women sustaining new vertebral fracture 2. Number of women sustaining new hip fracture 3. Number of women sustaining other new appendicular fracture 4. BMC distal radius 5. BMC proximal radius | |
| Notes | Additional data provided by Dr Falch by letter on site of appendicular fractures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized"; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Flicker 2005.
| Study characteristics | ||
| Methods | Individual remote from institutions undertook randomisation Double‐blind trial Completed for fracture data: 367 of 625 | |
| Participants | 60 assisted living facilities and 89 nursing homes, Australia
693 randomised, 625 took medication (594 women, 31 men), mean age 83.4 years (SD 6.6) Inclusion criterion: 25(OH)D 25‐90 nmol/L Disease exclusions: 25(OH)D < 25 nmol/L or > 90 nmol/L, thyrotoxicosis in last 3 years, primary hyperparathyroidism treated in last 3 years, multiple myeloma, Paget's disease of bone, history of malabsorption, active malignancy, other disorders affecting bone and mineral metabolism Drug exclusions: warfarin, chronic heparin therapy, vitamin D in previous 3 months, glucocorticoids equivalent to > 5 mg prednisolone for > 1 month in preceding year, current bisphosphonates or HRT |
|
| Interventions | 1. 600 mg daily calcium as calcium carbonate and 10,000 IU vitamin D2weekly initially then 1000 IU vitamin D2 dailyday
Randomised 346, 313 started supplements, of whom 183 completed 2 years 2. 600 mg daily calcium as calcium carbonate and matching vitamin D placebo weekly then daily Randomised 347, 312 started supplements, of whom 184 completed 2 years Duration of treatment 2 years |
|
| Outcomes | Measured over a follow‐up of 2 years 1. Number of persons with any new fracture 2. Number of persons dying | |
| Notes | Additional data provided by Dr Flicker 7 January 2008, Erratum published in 2012, mortality data had been transposed in original paper, data corrected for this update of the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated lists" |
| Allocation concealment (selection bias) | Low risk | States "individual who was not involved in contact with the subjects or the residential care institutions performed randomization" |
Gallagher 1989.
| Study characteristics | ||
| Methods | 2‐centred double‐blind randomised placebo‐controlled trial Placebo patients crossed‐over at 1 year: thus, only 12‐month assessment of controlled administration available Assessors were blinded (2 in each centre), inter‐observer error calculated for each centre 58 of 71 completed | |
| Participants | University Hospital, USA
71 postmenopausal women mean age 63 years. Sampling technique not described
Osteoporosis defined as 1 or more non‐traumatic vertebral fractures Disease exclusions: liver or renal disease, any disease known to be associated with disorder of calcium metabolism Evidence of osteomalacia on biopsy Drug exclusions: drugs associated with disorders of calcium metabolism |
|
| Interventions | 1. Calcitriol 0.25 μg twice daily, increased to up to 1 μg daily under discretion of investigator, monitored by serum calcium
Randomised 33, completed 29 2. Placebo twice daily Randomised 38, completed 29 All participants followed a free calcium intake diet during the study Duration of treatment 1 year |
|
| Outcomes | Measured at 1 year 1. Number of women sustaining new vertebral fracture 2. Total number of new vertebral fractures in each group 3. Number of persons dying | |
| Notes | Further data are reported for a subsequent year in which placebo patients were transferred to the active treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized"; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Gallagher 1990.
| Study characteristics | ||
| Methods | Randomised double‐blind controlled trial Safety monitoring by weekly serum analysis Outcome assessors blinded 40 of 50 completed | |
| Participants | University Hospital, USA
50 post‐menopausal women aged 50 to 78 years (mean 70 years), with 1 or more previous non‐traumatic vertebral fracture. Recruitment from a referral population Disease exclusions: renal failure, malignancy, gastro‐intestinal abnormalities, parathyroid disease, thyroid disease, acromegaly, Cushings syndrome, arthritis, overt vitamin D deficiency (bone biopsy confirmed), history of renal stones, diabetes or alcoholism. Previous prolonged immobilisation Drug exclusions: corticosteroids, anti‐convulsants, oestrogen or calcium supplements within previous 6 months or sodium fluoride within 1 year |
|
| Interventions | 1. Calcitriol 0.25 μg twice daily orally, increased by the investigators at 2‐weekly intervals up to a maximum of 2 μg/day. Mean dose 0.62 μg/day. Plus vitamin D2 400 IU daily orally. Calcium intake adjusted to 1 g daily using calcium supplements if necessary
Randomised 25, completed 18 2. Placebo plus vitamin D2 400 IU daily orally, plus calcium intake adjusted to 1 g daily Randomised 25, completed 22 Duration of treatment 2 years |
|
| Outcomes | Measured at 2 years 1. Number of women sustaining a new vertebral fracture 2. Total number of new vertebral fractures in each group 3. BMD lumbar spine 4. BMD total body 5. Total body calcium 6. Metacarpal index 7. Bone biopsy 8. Renal disease | |
| Notes | See also Aloia 1988, Ott 1989, carried out under similar protocol but published separately Dr Gallagher contacted and provided additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "a list of randomly generated treatment numbers previously prepared by Hoffman La Roche" |
| Allocation concealment (selection bias) | Low risk | States "treatment code was unknown at our center, and numbered medication bottles were dispensed to patients in chronological order" |
Gallagher 2001.
| Study characteristics | ||
| Methods | Random allocation. No further details provided Blinding of outcome assessors reported 213 of 246 completed | |
| Participants | Mailing list, USA
246 women, age range 65 to 77 years Inclusion criterion: femoral neck BMD within 2 standard deviations of the normal range for age Disease exclusions: severe chronic illness, primary hyperparathyroidism, active renal stone disease Drug exclusions: bisphosphonates, anticonvulsants, oestrogen, fluoride, thiazide diuretic in last 6 months |
|
| Interventions | 1. Calcitriol 0.25 μg twice daily
Randomised 123, completed 101 2. Placebo interventions Randomised 123, completed 112 3. Conjugated oestrogen 0.625 mg and medroxyprogesterone 2.5 mg daily if intact uterus (group not used in this review) 4. Calcitriol and HRT (group not used in this review) Duration of treatment 3 years |
|
| Outcomes | Measured over 3 years 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 2. Number of persons sustaining new vertebral fracture 3. Numbers of persons with adverse events (kidney stone, gastrointestinal) 4. Number of persons dying 5. BMD of lumbar spine, proximal femur, total body 6. PTH, 25(OH)D and 1,25(OH)2D; biochemical markers of bone formation and breakdown | |
| Notes | Dr JC Gallagher provided extra fracture data 21 February 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "independent statistical group performed the blinding and randomization", no other information provided |
| Allocation concealment (selection bias) | Low risk | States "independent statistical group performed the blinding and randomization", no other information provided |
Garay Lillo 1997.
| Study characteristics | ||
| Methods | Divided "randomly" Outcome assessment does not appear blinded 3910 of 6945 completed |
|
| Participants | Community‐based study, Spain
6945 ambulant community living women between 65 and 85 years of age Disease exclusions: abnormal renal function (serum creatinine > 144 μmol/L), serious medical problems, thyroid or parathyroid abnormalities, intestinal malabsorption, previous gastrectomy Drug exclusions: administration of calcium or vitamin D in the previous 6 months; administration of corticosteroids, anticonvulsants, or thyroxine in the year prior to enrolment |
|
| Interventions | 1. Tricalcium phosphate 1.2 g daily plus 25(OH)D 16,000 IU per week
Randomised unclear, 2086 completed 1 year 2. Tricalcium phosphate 1.2 g daily Randomised unclear, 2099 completed 1 year Duration of treatment 2 years |
|
| Outcomes | Measured at 1 and at 2 years 1. Number of women sustaining a hip fracture Also measured, but not considered in this review were BMD and biochemical measures | |
| Notes | Unclear in the published report: Details of randomisation Details of losses Details of how fracture outcome was ascertained Letter sent 10 February 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Translation states "divided randomly", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Geusens 1986.
| Study characteristics | ||
| Methods | Randomisation stated but method not defined Double‐blind (triple dummy) design Radiologic outcome assessor blinded 34 of 60 completed | |
| Participants | University Hospital, Belgium
48 women and 12 men (mean age not reported, but median ages for completed participants 65 years for group 1; 75 years for group 3) Inclusion criteria: evidence of vertebral collapse without trauma Disease exclusions: other diseases that might cause osteoporosis All had normal thyroid function, serum cortisol profile, serum creatinine, phosphate, calcium, PTH. Biochemical and radiological signs of osteomalacia were absent |
|
| Interventions | 1. Nandrolone decanoate (deca‐durabolin) 50 mg every 3 weeks
Randomised possibly 20, completed 11 2. 1‐alphahydroxyvitamin D3 1 μg daily orally Randomised possibly 20, completed 11 3. Elemental calcium 15 mg (as calcium gluconate) per kg body weight by IV infusion, daily for 12 days Randomised possibly 20, completed 12 Duration of treatment 2 years Each active agent accompanied by double dummy placebo |
|
| Outcomes | Measured at 2 years 1. Metacarpal cortical thickness and fractional cortical thickness 2. BMC radius 3. Number of patients with new fractures 4. Number of new fractures 5. Biochemical measures: serum calcium, protein, alkaline phosphatase, creatinine, urinary calcium, creatinine, hydroxyproline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly divided"; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Glendenning 2012.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Outcome assessors blinded 638 of 686 completed |
|
| Participants | Community‐living (from general practice or electoral role), Perth, Australia 686 women mean age 76.7 years (SD 4.1) Inclusion criteria: aged over 70 years, registered with a general practitioner, likely to attend 4 study visits in 9 months in investigators' opinion Exclusion criteria: consumption of vitamin D supplements either in isolation or as part of combination treatment, Mini Mental State Score < 24, investigators' opinion unsuitable for study |
|
| Interventions | 1. 150,000 IU cholecalciferol as 3 capsules at baseline, 3 months and 6 months 2. 3 placebo capsules at baseline, 3 months and 6 months Randomised 686, analysed 686 Duration of study 9 months |
|
| Outcomes | Measured over 9 months 1. Number of women sustaining a hip fracture 2. Number of women sustaining a clinical vertebral fracture 3. Number of women sustaining any new fracture 4. Number of deaths |
|
| Notes | Details of fractures obtained from Kathy Zhu 23 January 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated randomization sequence" |
| Allocation concealment (selection bias) | Low risk | States "randomization sequence was generated by a pharmacist. . . allocation to study and placebo was completed by the pharmacist before each subject's baseline visit" |
Gorai 1999.
| Study characteristics | ||
| Methods | Random allocation List of randomly generated treatment codes prepared by 1 of the investigators No blinding of outcome assessors reported Completion rate unclear | |
| Participants | Outpatient study, Japan
44 women, average age 51 years Inclusion criteria: postmenopausal women with at least 1 year but not more than 5 years since last menses Disease exclusions: surgical menopause, chronic disease (renal disease, hyperparathyroidism, diabetes mellitus), compression fracture on thoracic or lumbar spine radiograph Drug exclusions: drug treatment known to affect bone metabolism |
|
| Interventions | 1. 1 μg 1‐alphahydroxyvitamin D3 daily
Randomised 20, completed unclear 2. No intervention Randomised 24, completed unclear 3. 1 μg 1‐alphahydroxyvitamin D3 and 0.625 mg conjugated oestrogen daily (group not used in this review) 4. 0.625 mg conjugated oestrogen daily (group not used in this review) Duration of treatment 2 years |
|
| Outcomes | Measured at 2 years 1. Number of persons sustaining new vertebral fracture 2. Number of persons with hypercalcaemia 3. BMD of lumbar spine and femoral neck 4. Biochemical markers of bone formation and breakdown | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | States "randomly generated treatment codes previously prepared by a controller": Y Misu who was one of the investigators; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Harwood 2004.
| Study characteristics | ||
| Methods | Random allocation Computer‐generated random number lists and opaque, sealed envelopes Blinding of outcome assessors stated 119 of 150 completed | |
| Participants | Community‐based study, UK
150 women, mean age 81.2 years on fast‐tract orthogeriatric rehabilitation ward Inclusion criteria: within 7 days of surgery for hip fracture, community residence and independent in activities of daily living Disease exclusions: institutionalised, diseases known to affect bone metabolism, abbreviated mental test score < 7 at time of recruitment Drug exclusions: medications know to affect bone metabolism |
|
| Interventions | 1. Vitamin D2 300,000 IU by injection once at beginning of trial
Randomised 38, completed 30 2. Vitamin D2 300,000 IU by injection once at beginning of trial and calcium 1000 mg daily as 2 tablets Randomised 36, completed 25 3. Vitamin D3 800 IU and calcium 1000 mg daily as 2 tablets Randomised 39, completed 29 4. No trial treatment Randomised 37, completed 35 Duration of treatment 1 year |
|
| Outcomes | Measured over follow‐up of 1 year 1. Number of persons sustaining new non‐vertebral fracture 2. Number of persons sustaining hew hip fracture 3. Number of persons dying 4. Number of persons developing hypercalcaemia 5. BMD of lumbar spine and proximal femur 6. PTH, 1,25(OH)2D and 25(OH)D3 | |
| Notes | Dr R Harwood provided further details of fractures and deaths 24 January 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated random number lists" |
| Allocation concealment (selection bias) | Low risk | States "Computer‐generated random number lists and opaque, sealed envelopes" |
Hayashi 1992.
| Study characteristics | ||
| Methods | Multicentre open quasi‐randomised trial Blinding of outcome assessors unclear Completion rate unclear |
|
| Participants | 228 medical institutions in Japan 740 men and women with osteoporosis, based on Savile criteria of vertebral trabecular pattern, mean age 74.2 years (SD 8.2) Inclusion criteria: no further details Exclusion criteria: not given |
|
| Interventions | 1. 1 μg alfacalcidol daily 2. No treatment Randomised 740, completed unclear Duration of treatment 1 year |
|
| Outcomes | Number of persons with vertebral fracture on X‐ray | |
| Notes | Author provided fracture data 15 January 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | States randomisation "alternatively based upon the chronological order of consultation" |
| Allocation concealment (selection bias) | High risk | States randomisation "alternatively based upon the chronological order of consultation" |
Inkovaara 1983.
| Study characteristics | ||
| Methods | Quasi‐randomised by date of birth Double‐blind placebo‐controlled trial, blinding of outcome assessors not described 121 of 175 completed in the 4 groups examined in this review | |
| Participants | Community‐based study, Finland
143 women and 32 men living in a municipal home for the aged. Mean age 79.5 years SD 7.1 Exclusions: functional disorders of kidneys (serum creatinine > 150 μmol/L) or liver (AAT > 40 IU/L or APT > 280 IU/L, hypercalcaemia (serum Ca 2.80 mmol/L) or kidney stones |
|
| Interventions | 1. Calcium plus vitamin D3 daily with placebo
Randomised 46 completed 30 2. Vitamin D3 1000 IU daily with double placebo Randomised 45 completed 32 3. Elemental calcium 1.2 g daily, with double placebo Randomised 42 completed 31 4. Placebo Randomised 42 completed 28 4 additional groups had methanedione alone or in combination: these groups are not analysed in this review Duration of treatment 9 months |
|
| Outcomes | Measured at 1 year 1. Fractures of vertebrae or wrist (assessed in a sample N = 10 in each group) 2. Hypercalcaemia 3. Number of persons dying 4. Body weight 5. Serum biochemistry: calcium, phosphate, creatinine, alkaline phosphatase, aspartate aminotransferase | |
| Notes | Unclear whether the data represent fractures or participants with fractures. Data have not been included in the appropriate meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | States "Patients were divided into 8 groups according to year and month of birth" |
| Allocation concealment (selection bias) | Unclear risk | States "A double‐blind trial technique was used. The code was known only to the drug manufacturer" |
Ishida 2004 (retracted).
| Study characteristics | ||
| Methods | Random allocation. No further details Blinding of outcome assessors reported 123 of 132 completed | |
| Participants | Outpatient study, Japan
132 women, age range 50 to 75 years Inclusion criteria: at least 5 years since natural or surgical menopause, 1 or more vertebral fractures (T4‐L4) and BMD of distal third of radius 20% below the mean for young adults (or 30% below the mean for young adults if no fracture) Disease exclusions: recent cancer, another metabolic bone disease, important abnormality in routine blood tests, history of bilateral hip fractures, any physical or mental condition precluding participation Drug exclusions: recent drug treatment known to affect bone |
|
| Interventions | 1. 1‐alphahydroxyvitamin D3 1 μg/day Randomised 66, 63 completed 2. No intervention Randomised 66, 60 completed 3. Conjugated oestrogen 0.625 mg and medroxyprogesterone 2.5 mg daily (group not used in this review) 4. Etidronate 200 mg daily followed by 10‐week medication‐free periods (group not used in this review) 5. Calcitonin derived from eels 20 IU/week (group not used in this review) 6. Vitamin K (menatetrenone) 45 mg daily (group not used in this review) Duration of treatment 2 years |
|
| Outcomes | Measured at 2 years 1. Number of persons sustaining new non‐vertebral fracture 2. Number of persons sustaining new vertebral fracture 3. Number of persons sustaining new hip fracture 4. Numbers of persons with adverse events 5. BMD of distal third of the radius 6. Biochemical markers of bone formation and breakdown | |
| Notes | Dr Y Ishida provided further information on publications 21 February 2005 The main article reporting this trial was retracted in 2021. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "dynamic balancing method (modified minimization method)" |
| Allocation concealment (selection bias) | Unclear risk | States "allocated randomly" no further details |
Janssen 2010.
| Study characteristics | ||
| Methods | Double‐blind 6‐month randomised controlled trial Outcome assessors blinded 59 of 70 completed |
|
| Participants | Outpatient clinic of the Department of Geriatric Medicine at the University Medical Centre, Utrecht, The Netherlands 70 women, mean age 81 years Inclusion criteria: women, > 65 years of age, able to walk and follow simple instructions, serum 25(OH)D concentration between 20 nmol/L and 50 nmol/L Exclusion criteria: treatment with vitamin D or steroids in the previous 6 months, history of hypercalcemia or renal stones, liver cirrhosis, serum creatinine > 200 μmol/L, malabsorptive bowel syndrome, primary hyperparathyroidism, uncontrolled thyroid disease, anticonvulsant drug therapy, and/or presence of any other condition that would probably interfere with the patient’s compliance (i.e. surgery planned) |
|
| Interventions | 1. Vitamin D3 400 IU/d and 500 mg calcium/day 2. Placebo and calcium 500 mg/day Duration of treatment 6 months 28 out of 36, and 31 out of 34 completed |
|
| Outcomes | Measured at 6 months 1. Number of persons sustaining new non‐vertebral fracture 2. Number of persons sustaining new hip fracture 3. Number of persons dying 4. Number of persons with gastrointestinal adverse events |
|
| Notes | Hennie Janssen provided details of allocation of patient with hip fracture 15 January 2013, details of allocation for patients with gastrointestinal symptoms and patient who died 9 February 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned"; no other information provided |
| Allocation concealment (selection bias) | Low risk | States "Trial medication was provided by an independent hospital pharmacist who also performed the randomization. . . No person involved, i.e. subjects, investigators, or physicians who treated the subjects, had access to the randomization procedure." |
Komulainen 1998.
| Study characteristics | ||
| Methods | Randomised open controlled trial, factorial design. Randomisation in blocks of 4, 8 or 12 226 of 232 completed (of groups used here) | |
| Participants | Community‐based study, Finland
464 women whose last menstrual period was 6 to 24 months previously (mean age 52.7 years) Exclusion criteria: contraindications for HRT, history of breast or endometrial cancer, thromboembolic diseases, and medication resistant hypertension |
|
| Interventions | 1. HRT: sequential combination of 2 mg oestradiol valerate days 1 to 21, and 1 mg cyproterone acetate days 12 to 21, treatment free interval days 22 to 28 (group not used in this review) 2. Vitamin D3 (cholecalciferol) 300 IU plus calcium lactate 500 mg/day (equivalent to 93mg elemental calcium), no intake during June to August each year Randomised 116, completed 113 at 5 years 3. Treatments 1 and 2 combined (group not used in this review) 4. "Placebo" (calcium lactate 500 mg daily) Randomised 116, completed 113 at 5 years Duration of treatment 5 years |
|
| Outcomes | Measured at 5 years
1. Number of women with a first non‐vertebral fracture during 5 years
2. Number of fractures
3. Number of persons dying Fractures were secondary outcomes in this study, which was powered for detection of changes in BMD Also measured, but not considered in this review were BMD and biochemical measures |
|
| Notes | Author provided mortality data 11 November 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "random allocation to study groups was carried out blockwise by computer" |
| Allocation concealment (selection bias) | Low risk | States "the personnel involved were unaware of the group allocation" |
Law 2006.
| Study characteristics | ||
| Methods | Cluster randomisation by computer, no further details Blinding of outcome assessors not stated Completed for fracture data 2935 of 3717 | |
| Participants | Clusters of participants in 30 bedded units in care homes or entire care home if small, UK
3717 patients (2825 women, 892 men), mean age 85 years Inclusion criteria: 60 years and over, not temporary residents Drug exclusions: sarcoidosis, malignancy, life‐threatening illness Drug exclusions: already taking calcium/vitamin D or drugs to increase bone density |
|
| Interventions | 1. Ergocalciferol (vitamin D2) 2.5 mg every 3 months (1100 IU/d)
Randomised 1762, completed 1366 2. No treatment Randomised 1955, completed 1569 Mean or median duration of treatment 10 months (interquartile range 7 to 14 months) |
|
| Outcomes | Measured over a follow‐up of mean or median of 10 months 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Number of persons with hypercalcaemia 4. Number of persons dying 5. 25(OH)D and PTH in subgroup of 18 participants | |
| Notes | In the case of meta‐analyses including the cluster randomised trial by Law 2006, adjustments to the number of participants with outcomes and denominators (in Law 2006) were made using an intraclass correlation coefficient of 0.026 Publication reports analysis taking into account cluster randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "cluster randomisation by computer" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Lips 1996.
| Study characteristics | ||
| Methods | Double blind, block randomisation 1626 of 2578 completed | |
| Participants | Community‐based study, The Netherlands
2578 elderly people (1916 women and 662 men) 70 years and older (mean age 80 years, SD 6) recruited from general practitioners, and from apartment houses and homes for the elderly in the vicinity of Amsterdam, The Netherlands Inclusion criterion: reasonably healthy Exclusions: history of hip arthroplasty, known hypercalcaemia, history of hip fracture |
|
| Interventions | 1. Vitamin D3 400 IU daily in a single tablet
Randomised 1291, completed 834 2. Identical placebo daily as a single tablet Randomised 1287, completed 792 All participants received written advice on dairy consumption aimed at assuring a calcium intake of 800 mg to 1000 mg/day Duration of treatment initially 3 years, but to attain numbers some participants continued for 3.5 years |
|
| Outcomes | Measured at 3 years 1. Hip fracture 2. Other appendicular skeleton fracture 3. Serum 25(OH)D concentrations (sample only) 4. Hip BMD (non‐random subsample) 5. Number of persons dying | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computerized random‐number generator" |
| Allocation concealment (selection bias) | Low risk | States "Lists in sealed envelopes were sent to the hospital pharmacy for assignment" |
Lyons 2007.
| Study characteristics | ||
| Methods | Double‐blind randomised trial with secure allocation at remote site Completed for fracture data 1834 of 3440 | |
| Participants | Residential homes (38%), nursing or dual‐registered home (55%), sheltered accommodation (7%), Wales, UK
3440 participants (2624 women, 816 men), mean age 84 years Inclusion criteria: resident in participating residential or nursing homes/sheltered housing, regardless of cognitive, visual, hearing or communication impairment Disease exclusions: taking 400 IU or more vitamin D/day or known contraindication to vitamin D Drug exclusions: none specified |
|
| Interventions | 1. Ergocalciferol (vitamin D2) 2.5 mg (100,000 IU) every 4 months as 2 tablets
Randomised 1725, completed unclear 2. 2 matching placebo tablets every 4 months Randomised 1715, completed unclear Duration of treatment 3 years |
|
| Outcomes | Measured over a follow‐up of 3 years 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Number of persons sustaining new vertebral fracture 4. Number of persons dying 5. 25(OH)D and PTH in subgroup of 102 participants | |
| Notes | Dr Antony Johansen provided further outcome data on 15 March 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "randomisation sequences were computer generated by the central dispensing pharmacy" |
| Allocation concealment (selection bias) | Low risk | States "participants were randomised individually within blocks in homes. . . central dispensing pharmacy" |
Menczel 1994.
| Study characteristics | ||
| Methods | Randomised double‐blind study 46 of 66 completed | |
| Participants | University and tertiary institutions, Israel
66 osteoporotic postmenopausal women, mean age 67 years Inclusion based on interpretation of lateral spine radiographs Exclusion criteria: medical condition or medication known to affect bone metabolism (including HRT), a history of recent kidney stones, creatine clearance < 50 mL/min/1.73 m2, serum calcium > 10.8 mg/dL |
|
| Interventions | 1. 1‐alphahydroxyvitamin D3 0.25 μg plus calcium 500 mg, twice daily
Randomised 24, completed 17 2. Placebo plus calcium 500 mg twice daily Randomised 42, completed 29 Duration of treatment 3 years |
|
| Outcomes | Measured at 3 years 1. New vertebral fractures 2. Radial styloid BMC (SPA) 3. Serum Ca, PO4 4. Creatinine clearance 5. Urinary calcium 6. Clinical side effects (gastro‐intestinal) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomly allocated", no other information provided |
Meyer 2002.
| Study characteristics | ||
| Methods | Quasi‐randomised. Allocation based on date of birth Blinding of patients, nursing staff and study investigators stated 715 of 1144 completed | |
| Participants | Nursing homes, Norway
1144 participants (868 women, 276 men), mean age 84.7 years Inclusion criteria: life expectancy > 6 months, not permanently bedridden, not having difficulties taking medicine Disease exclusion: none given Drug exclusions: vitamin D supplementation of > 10 μg/day |
|
| Interventions | 1. Cod liver oil 5 mL with vitamin D3 2.2 μg/mL
Randomised 569, completed 366 2. Cod liver oil 5 mL with vitamin D3 0.1 to 0.2 μg/mL (control) Randomised 575, completed 349 Duration of treatment 2 years |
|
| Outcomes | Measured over 24 months 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Numbers of persons dying | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | States "days of the month (1‐31 days) were divided randomly into group A and group B", no other information provided |
| Allocation concealment (selection bias) | High risk | States "based on the day of birth, a participant was placed automatically in group A or group B when registered in the study database. The nursing staff was not aware of the details in the allocation procedures", no other information provided |
Mitri 2011.
| Study characteristics | ||
| Methods | 16‐week factorial randomised controlled trial Randomisation by permuted blocks using computer‐generated random‐number sequence, no further details provided States double‐masked design 88 of 92 completed |
|
| Participants | 92 participants (47 women, 45 men), community‐based study, Boston, USA, mean age 57 years Inclusion criteria: ambulatory, ≥ 40 years of age and with a BMI (kg/m2) ≥ 25 (≥ 23 if Asian) with glucose intolerance or early diabetes, defined as a fasting plasma glucose concentration ≥ 100 mg/dL or 2‐h glucose concentration ≥140 mg/dL after 75 g oral dextrose or glycated haemoglobin (Hb A1c) ≥ 5.8% Exclusion criteria: BMI > 40, Hb A1c > 7%, self‐reported diabetes treated with pharmacotherapy, weight change > 4 kg over the previous 6 months, use of supplements that contained vitamin D or calcium in ≤ 8 weeks of screening and an unwillingness to discontinue supplementation for ≥ 2 weeks before the study initiation and during the study; hyperparathyroidism, hypercalcemia, nephrolithiasis, chronic kidney disease, conditions that may have affected vitamin D or calcium metabolism (eg, sarcoidosis), and regular visits to tanning booths |
|
| Interventions | 1. 2000 IU vitamin D3 and 800 mg calcium (as 2 doses calcium carbonate) daily 2. 2000 IU vitamin D3 and 2 placebos daily 3. 800 mg calcium (as calcium carbonate) and 1 placebo daily 4. Matching placebos Randomised 23; 23; 22; 24 Completed 23; 22; 21; 22 Duration of treatment 16 weeks |
|
| Outcomes | Measured over a period of 16 weeks 1. Number of persons sustaining new non‐vertebral fracture 2. Numbers of persons sustaining hypercalcaemia or kidney stone |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated random‐number sequence" |
| Allocation concealment (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
Nakatsuka 1997.
| Study characteristics | ||
| Methods | 2‐year randomised cross‐over trial, cross‐over at end of 1 year Random allocation, no further details Blinding of outcome assessors not reported Completed for fracture data 30 of 33 |
|
| Participants | 33 women, mean age 71 years, in Japan Inclusion criteria: elderly women with lumbar BMD less than ‐2.5 SD of young adults, outpatients or residents in nursing homes Exclusion criteria: had medication that affects bone metabolism in last 2 months, spinal osteophytes, aortic calcification, vertebral deformity, scoliosis |
|
| Interventions | Cross‐over trial of 1 μg alfacalcidol/day and calcium 2 g/day or calcium 2 g/day. 2 year trial with cross‐over at 1 year | |
| Outcomes | Measured over a follow‐up of 12 months
1. Number of persons with new vertebral fracture 2. Urinary calcium 3. BMD lumbar spine |
|
| Notes | Review used data from first year only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised"; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Ones 2007.
| Study characteristics | ||
| Methods | Randomised, open, controlled clinical trial Blinding of outcome assessors not reported 98 of 98 in groups reported here completed |
|
| Participants | Postmenopausal women with osteoporosis, country unclear (possibly Turkey or Switzerland) 98 postmenopausal women for trial arms used in this review, mean age 58 years Inclusion criteria: aged 50 to 70 years, postmenopausal for at least 5 years, lumbar or femoral BMD T‐score < ‐2.5 Exclusion criteria: secondary osteoporosis, other bone diseases, significant concomitant disease, abnormal liver or renal function, hypercalcaemia, hypercalciuria, major gastrointestinal diseases, e.g. peptic ulcer, drugs influencing bone metabolism (oestrogens, progestagens, selective oestrogen receptor modifiers, calcitonin, bisphosphonate, vitamin D and calcium, glucocorticoids) |
|
| Interventions | 1. 10 mg alendronate, 0.5 μg alfacalcidol, 500 mg calcium daily 2. 10 mg alendronate, 500 mg calcium daily 3. 0.5 μg alfacalcidol, 500 mg calcium daily ‐ used in this review 4. 500 mg calcium daily ‐ used in this review Randomisation said to be in ratio 1.5:2:1.5:1 Randomised 68/30, completed 68/30 Duration of treatment 2 years |
|
| Outcomes | Measured over a follow‐up of 2 years
1. Number of persons with new vertebral fracture 2. Number of persons with gastrointestinal effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated random lists)" |
| Allocation concealment (selection bias) | Unclear risk | States "randomized"; no other information provided |
Orimo 1994.
| Study characteristics | ||
| Methods | Multicentre, randomised double‐blind placebo‐controlled trial By implication, and the address of the "controller" means that allocation concealment appears adequate A thorough analysis of withdrawal and exclusion presented 53 of 80 completed | |
| Participants | University and community hospitals, Japan
80 postmenopausal women aged 65 years or older, mean age 71 years Inclusion criteria: established osteoporosis, defined as decreased bone mass, presence of fractures of spine, femoral neck, or radius, with normal levels of serum calcium, phosphate or alkaline phosphatase Disease exclusions: hypercalcaemia, osteomalacia, primary/secondary hyperparathyroidism, rheumatoid arthritis, bone metastases, multiple myeloma, secondary osteoporosis, history of prolonged immobilisation Drug exclusions: any of the following in the previous 2 months: oestrogen, progesterone, androgen, calcitonin, bisphosphonate, vitamin D metabolites or analogues, ipriflavone, vitamin K2, corticosteroids, or anticonvulsants |
|
| Interventions | 1. 1‐alphahydroxyvitamin D3 1 μg, plus elemental calcium 300 mg (as calcium lactate) daily
Randomised 38, completed 25 2. Identical placebo, plus elemental calcium 300 mg daily Randomised 42, completed 28 Duration of treatment 1 year |
|
| Outcomes | Measured at 1 year 1. Number of new vertebral fractures 2. New vertebral fracture rate 3. Lumbar spine BMD (L2‐L4) measured by DEXA 4. Femoral neck BMD 5. Biochemical measures, including hypercalcaemia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "list of randomly generated treatment codes previously prepared by a controller (N. Ogawa, M.D.)", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomized"; no other information provided |
OSTPRE‐FPS 2007.
| Study characteristics | ||
| Methods | 3‐year open‐label, randomised controlled trial Blinding of outcome assessors unclear 2919 of 3195 completed |
|
| Participants | Population‐based sample, recruited by post, northern Savonia, Finland 3432 women randomised (237 excluded after randomisation due to death, withdrawal of consent or failure to contact before treatment began), mean age 67 years Inclusion criteria: 65 years or more, living in northern Savonia Exclusion criteria: taken part in trials or BMD measurements of the OSTPRE study |
|
| Interventions | 1. 800 IU vitamin D3 and 1000 mg calcium daily (as 2 tablets daily of Calcichew‐Forte, Leiras Nycomed Ltd) 2. No treatment Randomised 1586; 1609, completed 1346; 1573 Duration of treatment 3.01 (SD 0.22) years |
|
| Outcomes | Measured at 3.01 (SD 0.22) years
1. Number of persons sustaining new vertebral fractures
2. Number of persons sustaining hip fractures
3. Number of persons sustaining non‐vertebral fractures 4. Number of persons sustaining any new fracture 5. Number of persons dying 6. Number of gastrointestinal adverse events ‐ intervention group only |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "Randomization was performed with SPSS for Windows 11.0. . . statistical software" |
| Allocation concealment (selection bias) | Unclear risk | States "randomized. . . by an independent statistician"; no other information provided |
Ott 1989.
| Study characteristics | ||
| Methods | Said to be a double‐blind randomised controlled trial, but doses adjusted according to results of serum and urinary calcium levels 72 of 86 completed | |
| Participants | Tertiary hospital, USA
86 women with post menopausal osteoporosis aged 50 to 80 years (mean age 67.5 years) Inclusion criterion: at least 1 non‐traumatic vertebral compression fracture Disease exclusions: hepatic or renal disease, malignancy, malabsorption, parathyroid or thyroid disorder, inflammatory arthritis, alcoholism, overt vitamin D deficiency, history of renal stones, insulin dependent diabetes, previous long‐term hospitalisation, any other disorder known to affect bone metabolism Drug exclusions: glucocorticoids, anticonvulsants, oestrogens, sodium fluoride, calcium supplements, pharmacologic doses of vitamin D |
|
| Interventions | 1. Calcitriol [1,25(OH)2 D3] 0.25 μg to 2.00 μg daily (0.25 μg capsules), physician adjusted depending upon serum and urinary calcium levels
Randomised 43, completed 35 2. Placebo. Number of capsules adjusted as in 1 Randomised 43, completed 37 All women had supplement if necessary to bring calcium intake to 1000 mg per day Duration of treatment 2 years |
|
| Outcomes | Measured at 2 years 1. Number of persons sustaining new vertebral fractures 2. Number of persons sustaining hip fractures 3. Number of persons sustaining other appendicular skeleton fractures 4. BMC radius (33 outcomes at 2 years) 5. BMD spine (33 outcomes at 2 years) 6. Total body calcium (neutron activation analysis) (28 outcomes at 2 years) 7. Hypercalcaemia | |
| Notes | See also Aloia 1988 and Gallagher 1990 for separate reports from other centres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized", no other information provided |
| Allocation concealment (selection bias) | Low risk | States "Upon enrollment each woman received a bottle of capsules supplied by Hoffman‐La Roche. . . The bottles had been randomized" |
Peacock 2000.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled trial, blinding of outcome assessors not described Completion rate unclear | |
| Participants | Community study, USA
438 (316 women, 122 men), mean age women 74 years, men 76 years Inclusion criteria: willing to undertake 4‐year study, aged 60 years or over, able to give informed consent as assessed by Short Portable Mental Status Test Disease exclusions: terminal illness, Paget's disease, recurrent urinary stone disease, renal disease requiring specific treatment, excluded by primary physician Drug exclusions: treated with sodium fluoride, bisphosphonates, steroids, dilantin |
|
| Interventions | 1. 25(OH)D3 5 μg 3 times daily
2. 250 mg calcium tablet 3 times daily
3. Placebo 3 times daily
Randomised unclear, completed unclear 2. Calcium 250 mg as calcium citrate malate 3 times daily, vitamin D placebos 3 times daily Randomised unclear, completed unclear 3. Matched placebo tablets daily. Randomised unclear, completed unclear Duration of treatment 4 years |
|
| Outcomes | Measured over a follow‐up of 4 years 1. Number of persons sustaining new vertebral fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Number of persons developing hypercalcaemia 4. Number of persons dying 5. Number of persons reporting gastrointestinal disorders 6. Number of people with renal stones 7. PTH, 25(OH)D and 1,25(OH)2D 8. Markers of bone formation and resorption 9. Femoral neck BMD | |
| Notes | Emailed Dr Peacock for further details on denominators and outcomes 18 February 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Pfeifer 2000.
| Study characteristics | ||
| Methods | Double blind randomised controlled trial 137 of 148 completed | |
| Participants | Osteology Clinic, Germany
148 healthy ambulatory community‐living women aged 70 years or older, recruited through advertisement, mean age 75 years Inclusion criteria: 25(OH)D3 serum level below 50 nmol/litre, not holidaying at a different latitude Disease exclusions: hypercalcaemia, primary hyperparathyroidism, osteoporotic extremity fracture, intolerance to vitamin D or calcium; chronic renal failure; drug, alcohol, caffeine, or nicotine abuse; diabetes mellitus Drug exclusions: treatment with bisphosphonate, calcitonin, vitamin D or metabolites, oestrogen, tamoxifen in past 6 months; fluoride in last 2 years; anticonvulsants or medications possibly interfering with postural stability or balance |
|
| Interventions | 1. Elemental calcium (calcium carbonate) 600 mg plus vitamin D3 400 IU
Randomised 74, completed 70 2. Calcium carbonate 600 mg Randomised 74, completed 67 Supplementation at the end of winter for 8 weeks |
|
| Outcomes | Measured at 1 year 1. The number of persons sustaining non‐vertebral fracture 2. The number of persons sustaining a fall (not part of this review) 3. Number of falls in each group (not part of this review) Also measured, but not considered in this review were body sway parameters, and biochemical measures | |
| Notes | Comment on 1 June 2021. Concerns about the integrity of this trial, particularly with regard to plausibility of the data and some data errors have raised with Journal of Bone and Mineral Research. See also notes for Pfeifer 2009. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
Pfeifer 2009.
| Study characteristics | ||
| Methods | One‐year double‐blind randomised controlled trial Blinding of outcome assessors unclear Number completed of 242 unclear |
|
| Participants | 61 men and 181 women aged over 70 years, community‐based study, Austria and Germany, mean age 77 years Inclusion criteria: healthy ambulatory men and women 70 years or older, 25(OH)D < 78 nmol/L Exclusion criteria: hypercalcaemia, primary hyperparathyroidism, fractures of the extremities due to osteoporosis; thiazides, bisphosphonates, calcitonin, vitamin D and vitamin D metabolites, oestrogen, anti‐oestrogen in last 6 months; fluoride in last 2 years; intolerance to study medication; chronic renal failure (serum creatinine above 20% of the upper limit of the reference range); drug or alcohol abuse; more than 20 cigarettes/day; more than 7 cups of coffee/day; holidays along geographic latitude during the study; diabetes mellitus and cardiovascular disease |
|
| Interventions | 1. 1000 mg calcium as calcium carbonate and 800 IU vitamin D3 as 2 tablets daily (Meda Pharma Inc, Vienna) 2. 1000 mg calcium as calcium carbonate as 2 tablets daily (Meda Pharma Inc, Vienna) Randomised 122;120, numbers enrolled and completed unclear Duration of treatment 1 year with end of follow‐up 8 months later |
|
| Outcomes | Measured at 20 months 1. The number of persons sustaining any new fracture 2. The number of persons sustaining a fall (not part of this review) |
|
| Notes | Email to author November 2007 requesting details of fractures. Tables and text differ on numbers of participants randomised to groups. 122 and 120 presented in Bischoff‐Ferrari 2012, Comment on 1 June 2021. Based on the finding of inconsistencies in treatment group numbers and primary endpoint data for falls, and therefore for fractures, we are not confident that the data from this trial are reliable. Published correspondence with the first author has failed to assuage these concerns (correspondence dated 2015 added to references for this trial). The concerns raised were: 1. The text and Table 1 reports 121 participants in each treatment group, but Table 3 reports 120 for Ca and 122 for CaD. 2. Table 3 reports that 75 participants fell with Ca and 49 with CaD, but the breakdown of fallers in Table 3 sums to 71 for Ca and 53 for CaD. 3. The text reports the total number of falls as 171 with Ca and 76 with CaD, but Table 3 reports 169 with Ca and 106 with CaD. 4. The breakdown of total falls in Table 3 sums to at least 111 with CaD, which is greater than the total falls for CaD reported in the text (76) and Table 3 (106). 5. The text reports the mean number of falls per group as 1.41 with Ca and 0.63 with CaD which are incompatible with the reported total number of falls in the text and Table 3. 6. The text reports 13 participants with fracture with Ca whereas Table 3 reports 12. The first author replied: 1. Page 319, second paragraph, line 5: 63 % of the individuals in the calcium mono group versus 40 % in the calcium plus vitamin D group experienced at least one fall (p<0.001). The mean number of falls per group was 1.41 in the calcium mono group and 0.63 in the calcium plus vitamin D group (p<0.001). This translated into a total number of falls of 169 in the calcium mono group and to 106 falls in the calcium and vitamin D group (p<0.001). The initially published and wrong numbers in the text were 171 and 76, respectively. 2. Page 319, fourth paragraph, line 6: In total, 12 subjects in the calcium mono group and seven participants in the calcium plus vitamin D group experienced at least one fracture. The initially published and wrong number in the text was 13. After factoring in the author’s response, the remaining outstanding issues are: 1. The text and Table 1 reports 121 participants in each treatment group, but Table 3 reports 120 for Ca and 122 for CaD. 2. Table 3 reports that 75 participants fell with Ca and 49 with CaD, but the breakdown of fallers in Table 3 sums to 71 for Ca and 53 for CaD. 3. The corrected text reports the total number of falls as 169 with Ca and 106 with CaD, which is the same as Table 3. However, the breakdown of total falls in Table 3 sums to at least 111 with CaD, which is greater than the reported number of 106. 4. The text reports the mean number of falls per group as 1.41 with Ca and 0.63 with CaD which are incompatible with the reported total number of falls in the corrected text and Table 3. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Porthouse 2005.
| Study characteristics | ||
| Methods | Random allocation, initially 2:1 ratio intervention to control Remote site computer randomisation Blinding of outcome assessors not stated 3199 of 3314 completed | |
| Participants | Multicentre general practice study, UK
3314 patients (all women), mean age 77 years, with at least 1 self‐reported risk factor for hip fracture Inclusion criteria: low body weight (< 58 kg), personal history of fracture, maternal history of hip fracture, current smoker, poor or fair health Disease exclusions: kidney or bladder stones, renal failure, hypercalcaemia, cognitive impairment, life expectancy < 6 months Drug exclusions: current calcium supplementation of > 500 mg/day |
|
| Interventions | 1. Calcium 1000 mg and vitamin D3 800 IU given as 2 tablets daily, nurse gave general lifestyle advice, and information leaflet on calcium and vitamin D and on falls prevention
Randomised 1321, completed 1269 2. Information leaflet on calcium and vitamin D and on falls prevention. Randomised 1993, completed 1930 Duration of treatment 18 to 42 months |
|
| Outcomes | Measured over a median follow‐up of 25 months (range 18 to 42 months) 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Number of persons dying | |
| Notes | Prof DJ Torgerson provided pre‐publication report and further details 9‐16 February 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" |
| Allocation concealment (selection bias) | Low risk | States "randomised (stratified by practice) by computer at the York Trials Unit by an independent person with no knowledge of the participants' characteristics" |
Prince 2008.
| Study characteristics | ||
| Methods | Double blind randomised controlled trial Remote site randomisation 275 of 302 completed | |
| Participants | Community‐based study, Australia
302 participants (all women), mean age 77 years Inclusion criteria: aged 70‐90 years, sustained a fall in last 12 months, ambulant, 25(OH)D < 60 nmol/L Disease exclusions: hip Z score < ‐2.0, medical conditions influencing bone metabolism, creatinine > twice reference range, fracture in past 6 months, Mini Mental State Examination score < 24, marked neurological conditions likely to substantially impair balance or physical activity, e.g. stroke, Parkinson's disease Drug exclusions: current consumption of vitamin D or bone active agents |
|
| Interventions | 1. Calcium 1000 mg (as calcium citrate 2 tablets twice daily) and vitamin D2 1000 IU daily
Randomised 151, completed 136 2. Calcium 1000 mg (as calcium citrate 2 tablets twice daily) and placebo daily Randomised 151, completed 139 Duration of treatment 1 year |
|
| Outcomes | Measured at 1 year 1. Number of persons sustaining any fracture 2. Numbers of persons with hypercalcaemia, gastrointestinal events 3. Number of persons dying | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "the randomization procedure used a random number generator" |
| Allocation concealment (selection bias) | Low risk | States "randomization schedule . . . was generated by an independent research scientist (I.M.D.) and was kept in the pharmacy department. . . where the bottles were labeled and dispensed" |
RECORD 2005.
| Study characteristics | ||
| Methods | Random allocation Remote site computer randomisation Blinding of outcome assessors stated Completed for fracture data 5234 of 5292, questionnaires and tablets 3765 of 5292, at 24 months | |
| Participants | Community‐based study, UK
5292 patients (4481 women, 811 men), mean age 77 years Inclusion criteria: osteoporotic fracture within the last 10 years, aged 70 years or over Disease exclusions: bed‐ or chair‐bound prior to fracture, cognitive impairment indicated by an abbreviated mental test score of < 7, suffered from cancer likely to metastasise to bone within the previous 10 years, fracture associated with pre‐existing local bone abnormality, known hypercalcaemia, renal stone in the last 10 years, life expectancy < 6 months, known to be leaving the UK Drug exclusions: taking more than 200 IU (5 μg) vitamin D or more than 500 mg calcium supplements daily; had fluoride, bisphosphonates, calcitonin, tibolone, HRT, selective oestrogen receptor modulators, or any vitamin D metabolite (such as calcitriol) in the last 5 years; vitamin D by injection in the last year |
|
| Interventions | 1. Calcium 1000 mg and vitamin D3 800 IU given as 2 tablets daily
Randomised 1306, completed 921 at 24 months (questionnaires and tablets) 2. Calcium 1000 mg given as 2 tablets daily Randomised 1343, completed 993 at 24 months (questionnaires and tablets) 3. Vitamin D3 800 IU given as 2 tablets daily Randomised 1311, completed 905 at 24 months (questionnaires and tablets) 4. 2 placebo tablets daily Randomised 1332, completed 946 at 24 months (questionnaires and tablets) Duration of treatment 24 to 62 months |
|
| Outcomes | Measured over a follow‐up of 24 to 62 months 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. Number of persons with new clinical vertebral fracture 4. Number of persons with hypercalcaemia, renal stone or failure, gastrointestinal adverse events 5. Number of persons dying 6. 25(OH)D3 and PTH (subgroup of 60 participants) | |
| Notes | Prof AM Grant provided pre‐publication report, low trauma fracture data used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "Randomisation was centralised; computer‐generated; stratified by centre; and minimised by age" |
| Allocation concealment (selection bias) | Low risk | States "Randomisation was centralised. . . The allocation programme was written by the trial programmer (GCM) and the allocation remained concealed until the final analyses (other than for confidential reports to the data monitoring committee)" |
Shiraki 1996.
| Study characteristics | ||
| Methods | Multi‐centre, randomised, double‐blind placebo‐controlled study 79 of 113 completed | |
| Participants | University and Community Hospitals, Japan
113 community living osteoporotic women (mean age 72.4 years). Analysis based on 79 completing participants mean age 71.4 years) Inclusion criteria: aged 60 years and over, osteoporotic Disease exclusions: presence of disease affecting bone or calcium metabolism, abnormal liver or kidney function Drug exclusions: any treatment for osteoporosis during the previous 6 months |
|
| Interventions | 1. 1‐alphahydroxyvitamin D3 0.75 μg daily 2. Identical placebo Randomised 113, completed 79 (breakdown not given by group) Participants in each group were given calcium lactate 2.3 g daily (300 mg elemental calcium) Duration of treatment 2 years |
|
| Outcomes | Measured at 2 years 1. Number of participants sustaining a radiographic vertebral fracture (diagnosed if anterior or central vertebral height was 20% less than the posterior height) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly‐generated treatment codes previously prepared by a controller" (T Nagai, Ph D) |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Smith 2007.
| Study characteristics | ||
| Methods | Double‐blind random allocation to previously randomised, consecutive ampoules, identical in appearance Blinding of outcome assessors stated 4311 of 4570 completed questionnaires of those assessed at 36 months | |
| Participants | Multicentre general practice study in 111 sites, UK
9440 patients (4354 women, 5086 men), median age 79.1 years Inclusion criteria: aged 75 years and older, consenting and presenting for influenza vaccination at general practice Disease exclusions: history of renal failure, renal stones, hypercalcaemia, sarcoidosis, current cancer, bilateral hip replacement, any history of treated osteoporosis Drug exclusions: taking 10 μg or more vitamin D daily |
|
| Interventions | 1. Intramuscular vitamin D (ergocalciferol) 300,000 IU annually every autumn 2. Identical placebo Duration of treatment 3 years (annual injections), recruited in annual waves Randomised 9440, 3‐year follow‐up data for 4570, 2304 vitamin D, 2266 placebo |
|
| Outcomes | Measured over follow‐up of 3 years 1. Number of persons sustaining new hip fracture 2. Number of persons sustaining new non‐vertebral fracture 3. PTH, 25(OH)D, 1,25(OH)2D | |
| Notes | Prof C Cooper and Dr S Crozier provided further details 23 February 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized", no other information provided |
| Allocation concealment (selection bias) | Low risk | States "previously randomized, numbered ampoules . . . they were allocated consecutive ampoules. . . allocation was concealed from investigators, practice nurses and subjects" |
Tilyard 1992.
| Study characteristics | ||
| Methods | Multi‐centre randomised single‐blind comparison of calcitriol and calcium supplementation. No placebo, and each participating physician (123) had own separate randomisation code. Participant compliance not checked 515 of 622 completed at 1 year, 476 at 2 years, 432 at 3 years | |
| Participants | Community‐based study, New Zealand
622 fully ambulatory post‐menopausal women aged 50 to 79 years (mean 63.7 years) with no evidence of disease or drug known to cause osteoporosis, from a population referred with fracture or other manifestation of effects of osteoporosis Inclusion criteria: presence of 1 or more non‐traumatic vertebral compression fracture seen on a lateral spinal radiograph Exclusion criteria: not specifically described |
|
| Interventions | 1. Calcitriol 0.5 μg daily in 2 doses by mouth
Randomised 314, completed 1 years 262, 2 years 236, 3 years 213 2. Elemental calcium 1 g daily (5.2 g calcium gluconate twice daily). Randomised 308, completed 1 years 253, 2 years 240, 3 years 219 Patients instructed not to take any other calcium supplement, but otherwise diet, and exercise programmes were unsupervised Duration of treatment 3 years |
|
| Outcomes | Measured at 1, 2, and 3 years 1. Number of women with new vertebral fractures 2. Number of fractures of the appendicular skeleton by the end of 3 years of treatment 3. Episodes of hypercalcaemia 4. Renal calculi 5. Number of persons dying 6. Gastro‐intestinal symptoms | |
| Notes | Interim reports published in 1990 and 1991 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomization codes", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
Trivedi 2003.
| Study characteristics | ||
| Methods | Participants and investigators blinded until study ended, when pharmacy revealed coding 2055 of 2686 completed | |
| Participants | Community‐based study, UK
2686 (2037 men and 649 women) mean 75 years, from register of British doctors and register of a general practice Inclusion criteria: age 65 to 85 years, living in the community, from British doctors' study register and general practice register in Ipswich Disease exclusions: contraindications to vitamin D supplementation e.g. renal stones, sarcoidosis, malignancy Drug exclusions: already taking vitamin D supplements |
|
| Interventions | 1. Vitamin D3 (cholecalciferol) 100,000 IU
Randomised 1345, completed 1038 2. Placebo: 1 capsule 4‐monthly Randomised 1341, completed 1017 Duration of treatment 5 years |
|
| Outcomes | 1. Non‐vertebral fractures at 5 years 2. Hip fractures at 5 years 3. Vertebral fractures at 5 years 4. Falls 5. Self‐reported health 6. In a subgroup, 238 had measurement of PTH, 25(OH)D and heel ultrasound at 4 years 7. Compliance with trial medication 8. Adverse effects: death, death from cardiovascular disease, death from cancer | |
| Notes | Discrepancy between text and table 5 in subgroup study (235 and 238 respectively) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no other information provided |
| Allocation concealment (selection bias) | Low risk | Study conducted by post, states "participants and investigators were blinded to the treatment until the study ended, when Ipswich Pharmacy revealed the coding" |
Ushiroyama 2001.
| Study characteristics | ||
| Methods | Random allocation, no further details No mention of blinding of outcome assessors No details of completion rate | |
| Participants | Hospital outpatient‐based study, Japan
102 patients (all women), age range 53 to 58 years, with osteoporosis/osteopenia Inclusion criteria: 6 months or more since last menses, status confirmed by oestradiol and gonadotrophin measurements Disease exclusions: renal failure, metabolic bone disease, urolithiasis Drug exclusions: hormonal contraception or postmenopausal oestrogen |
|
| Interventions | 1. 1‐alphahydroxyvitamin D3 0.5 μg orally twice daily. Randomised 50, number completed unclear 2. No intervention Randomised 52, number completed unclear 3. Calcitonin 10 IU twice a month (group not used in this review) 4. Calcitonin and 1‐alphahydroxyvitamin D3 (group not used in this review) Duration of treatment 2 years |
|
| Outcomes | Measured at 1 year 1. Number of persons sustaining new non‐vertebral fracture 2. Number of persons with hypercalcaemia 3. Vertebral BMD 4. PTH, markers of bone formation and resorption | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
Vital D.
| Study characteristics | ||
| Methods | Placebo‐controlled randomised trial Blinding of outcome assessors stated 2032 of 2258 completed |
|
| Participants | Community‐dwelling women in southern Victoria, Australia 2258 women, mean age 76 years Inclusion criteria: women aged 70+ years on entry, higher risk of hip fracture, e.g. maternal hip fracture, past fracture, self‐reported faller Exclusion criteria: could not provide informed consent or information about falls or fractures; residing permanently at a high‐level care facility; albumin corrected calcium level higher than 2.65 mmol/L; or had a creatinine level higher than 150 μmol/L, or currently took vitamin D doses of 400 IU or more, calcitriol, or antifracture therapy |
|
| Interventions | 1. Annual dose of 500,000 IU cholecalciferol orally as 10 tablets, given annually 3 to 5 times 2. Annual matching placebo orally as 10 tablets, given annually 3 to 5 times Randomised 1131; 1127, completed 1015; 1017 |
|
| Outcomes | Measured up to 1 year after last dose 1. Number of persons with new hip fracture 2. Number of persons with new clinical vertebral fracture 3. Number of persons with all new fractures 4. Number of persons with new non‐vertebral fractures 5. Number of persons dying 6. Number of persons with hypercalcaemia 6. Subgroup 25(OH)D measured |
|
| Notes | Kerrie Sanders provided details on hypercalcaemia (20 December 2010) and further details on fracture outcomes (11 February 2013) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated randomization of numbers" |
| Allocation concealment (selection bias) | Low risk | States "Allocation performed by an independent statistician. . . Treatment allocation status was e‐mailed directly to the hospital clinical trials pharmacist responsible for dispensing study medication" |
WHI 2006.
| Study characteristics | ||
| Methods | Double‐blind trial. No details on method of randomisation Completed for fracture data 33751 of 36282 | |
| Participants | Community‐based women, USA
36,282 participants (all women), mean age 62.4 (SD 7.0) years Inclusion criteria: 50 to 79 years, no medical condition associated with predicted survival of less than 3 years Disease exclusions: hypercalcaemia, renal calculi Drug exclusions: corticosteroid use, calcitriol use, calcium supplements > 1000 mg/day, vitamin D > 600 IU/day (> 1000 IU/day after 1999) |
|
| Interventions | 1. 1000 mg calcium as calcium carbonate and 400 IU vitamin D3 as 2 tablets daily
Randomised 18176, 93% completed 7 (SD 1.4) years 2. 2 placebo tablets daily Randomised 18106, 93% completed 7 (SD 1.4) years Duration of treatment 7 (SD 1.4) years |
|
| Outcomes | Measured over a follow‐up of 7 years 1. Number of persons with new hip fracture 2. Number of persons with new clinical vertebral fracture 3. Number of persons with all new fractures (excluding rib, sternum, skull, face, finger, toe, cervical vertebral fracture) 4. Number of persons with gastrointestinal adverse events, renal calculi 5. Number of persons dying 6. Subgroup of 448 had 25(OH)D measured at 2 years | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomly assigned", no other information provided |
Witham 2010.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled trial Blinding of outcome assessors stated 96 of 105 completed |
|
| Participants | Participants recruited from primary and secondary care, Tayside and Fife, Scotland, UK 105 participants, (69 men, 36 women), mean age 80 years Inclusion criteria: aged ≥ 70 years with recorded clinical diagnosis of chronic heart failure; previously documented left ventricular systolic dysfunction by echocardiography, radionuclide ventriculography, or angiography as part of their usual clinical care; New York Heart Association class II or III symptoms; 25(OH)D level of < 50 nmol/L Exclusion criteria: a clinical diagnosis of osteomalacia, under investigation for recurrent falls, taking vitamin D supplements, moderate to severe cognitive impairment (defined as a Folstein mini‐mental state examination < 15/30), serum creatinine > 200 μmol/L, liver function tests (bilirubin, alanine aminotransferase, and alkaline phosphatase) > 3 times the upper limit of the local reference range, systolic blood pressure < 90 mmHg, albumin‐adjusted calcium (> 2.55 mmol/L or < 2.20 mmol/L), and metastatic malignancy, wheelchair‐bound |
|
| Interventions | 1. 100,000 IU D2 orally at start and 10 weeks 2. Placebo orally at start and 10 weeks Randomised 53; 52, 48; 48 completed 20 weeks Duration of treatment 10 weeks |
|
| Outcomes | Measured over a follow‐up of 20 weeks 1. Number of persons with all new fractures 2. Number of persons with gastrointestinal adverse events, hypercalcaemia 3. Number of persons dying | |
| Notes | Further information on fractures obtained from Miles Witham on 15 January 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated random number tables" |
| Allocation concealment (selection bias) | Low risk | States "Randomization was performed . . . by DHP Pharmaceuticals. . . Code allocation was concealed from the research nurse and investigators until after data analysis was complete" |
Witham 2013.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled trial Blinding of outcome assessors stated 142 of 159 completed |
|
| Participants | Participants recruited from primary care, secondary care and the press, Scotland, UK 159 participants (82 men, 77 women), mean age 77 years Inclusion criteria: age 70 years and over, serum 25(OH)D level < 75 nmol/L, office systolic blood pressure > 140 mmHg Exclusion criteria: diastolic blood pressure > 90 mmHg, systolic blood pressure > 180 mmHg, hypertension known to be due to a correctable underlying medical or surgical cause; estimated glomerular filtration rate < 40 mL/minute, any liver function test (alanine aminotransferase, bilirubin, alkaline phosphatase) > 3 x upper limit of local normal range; albumin‐adjusted serum calcium > 2.60 mmol/L or < 2.15 mmol/L; known metastatic malignancy or sarcoidosis, a history of renal calculi, diagnosis of heart failure with left ventricular systolic dysfunction, atrial fibrillation, already taking vitamin D supplements |
|
| Interventions | 1. 100,000 IU vitamin D3 (Vigantol oil, Merck KgAA) every 3 months for 9 months (4 doses) 2. Matching placebo (Mygliol, Merck KgAA) every 3 months for 9 months (4 doses) Randomised 80; 79, completed 73; 69 Duration of treatment 9 months |
|
| Outcomes | Measured over a follow‐up of 12 months
1. Number of persons with all new fractures 2. Number of persons with hypercalcaemia, rise in serum creatinine > 20% 3. Number of persons dying |
|
| Notes | Data from personal communication from Miles Witham 15 January 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "minimisation algorithm" |
| Allocation concealment (selection bias) | Low risk | States "telephone‐based minimisation system" |
See Table 2 for abbreviations for vitamin D
AAT: aspartate aminotransferase APT: alkaline phosphatase BMC: bone mineral content BMD: bone mineral density BMI: body‐mass index Ca: calcium CT: computerised tomography DEXA: dual energy x‐ray absorptiometry ESR: erythrocyte sedimentation rate h: hour(s) HRT: hormone replacement therapy IV: intravenous(ly) μmol/L: micromoles per litre PO4: phosphate PTH: parathyroid hormone SD: standard deviation SPA: single photon absorptiometry
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aguado 2006 | RCT. Oral 80,000 IU 25(OH)D 3‐monthly plus 1000 mg calcium/day versus oral 800 IU vitamin D3 plus 1000 mg calcium/day. No fracture data |
| ALFA 2006 | RCT. 70 mg alendronate weekly and 500 mg calcium daily, with either 1 μg alfacalcidol or placebo. Protocol currently does not include trials with other drug treatments administered to both arms of the trial |
| Aloia 2005 | RCT. Oral 800 IU vitamin D3 daily plus calcium supplements to give intake of 1200 mg to 1500 mg/day versus placebo plus calcium supplements to give intake of 1200 mg to 1500 mg/day. After 2 years vitamin D increased to 2000 IU D3/day for 1 year. Designed to evaluate effect on bone mineral density, collection of fracture data not described, no fractures reported |
| Baeksgaard 1998 | RCT. Placebo‐controlled. Vitamin D plus calcium, and vitamin D plus calcium plus multivitamins. 2 participants with incident vertebral fracture during the study were excluded from the analysis. No fracture data |
| Binder 1995 | RCT. Bolus of 100,000 IU vitamin D3 orally then 50,000 IU/week plus 1000 mg calcium/day versus 1000 mg calcium/day. No fracture data |
| Binkley 2007 | RCT. 8400 IU vitamin D3 weekly versus placebo. No fracture data |
| Brazier 2005 | RCT. Oral 800 IU vitamin D3/day plus 1000 mg calcium/day as 2 tablets daily versus 2 placebo tablets/day. No fracture data |
| Broe 2007 | RCT. 200 IU vitamin D2/day versus 400 IU vitamin D2/day versus 800 IU vitamin D2/day versus placebo. No fracture data |
| Bunout 2006 | RCT. Oral 400 IU vitamin D3 plus 800 mg calcium/day versus oral 800 mg calcium/day, also randomised to resistance training or control. No fracture data |
| Chen 1997 | RCT. 150 mg calcium plus0.75 μg 1‐alphahydroxyvitamin D3 versus calcium 150 mg. No fracture data |
| Chevalley 1994 | RCT. 800 mg calcium (as calcium carbonate or osseino‐mineral complex) versus placebo in vitamin D‐replete participants. Not a trial of vitamin D supplementation |
| Cooper 2003 | RCT. 10,000 IU vitamin D2/week plus 1000 mg calcium/day versus 1000 mg calcium/day. No fracture data |
| Corless 1985 | RCT. 9000 IU vitamin D2 tablets versus placebo. No fracture data |
| Daly 2006 | RCT. 400 ml/day milk fortified with 1000 mg calcium plus 800 IU vitamin D3 versus control. No fracture data |
| Dawson‐Hughes 1991 | RCT. 400 IU vitamin D versus placebo. No fracture data |
| Dawson‐Hughes 1995 | RCT. 400 IU vitamin D and 377 mg calcium/day versus placebo with 377 mg calcium/day. No fracture data |
| Deroisy 1998 | RCT. Fracture data (not primary outcome). Study of acceptability and effect of formulation of vitamin D with calcium co‐supplementation. (1 g calcium plus vitamin D3 800 IU as 2 tablets/day versus 1.2 g calcium as 2 sachets/day plus 800 IU as 2 chewable tablets/day) |
| Deroisy 2002 | RCT. Vitamin D 200 IU plus calcium 500 mg versus calcium 500 mg. No fracture data |
| Dhesi 2004 | RCT. Injection of 600,000 IU ergocalciferol versus placebo. No fracture data |
| Doetsch 2004 | RCT. Vitamin D3 800 IU plus 1 g calcium versus placebo. No fracture data |
| Francis 1996 | RCT. 0.5 μg alfacalcidol versus up to 160 mg calcium plus 1000 IU vitamin D2. No fracture data |
| Fujita 1989 | RCT. 0.5 μg 1,25(OH)2D plus placebo daily versus 0.25 μg 1,25‐dihydroxyvitamin D daily plus 2 placebos daily versus 1.0 μg alphacalcidol plus 2 placebos daily. No fracture data |
| Gallagher 1982 | RCT. 0.5 μg 1,25‐dihydroxyvitamin D3 daily versus placebo. No fracture data |
| Gloth 1995 | RCT. Calcium v calcium plus vitamin D variable dose. No fracture data |
| Grados 2003 | RCT. 400 IU vitamin D plus 500 mg calcium versus placebo. No fracture data |
| Grady 1991 | RCT. 0.5 μg 1,25‐dihydroxyvitamin D3 versus placebo. No fracture data |
| Hangartner 1985 | Quasi‐randomised trial. No fracture data |
| Harju 1989 | RCT. Calcitonin versus 0.5 μg 1‐alphahydroxyvitamin D versus control. No fracture data |
| Heikinheimo 1992 | This study has been widely quoted as providing evidence for the effectiveness of single dose vitamin D in fracture prevention. It is an open quasi‐randomised trial. As only individuals recruited in the northern hemisphere's autumn and winter were included for practical reasons, allocation was not concealed, being based on month of birth. There was no placebo and enrolment was biased. Follow‐up varied from 2 to 5 years but the cumulative analysis of fracture incidence did not include confidence intervals despite the decreasing numbers with longer follow‐up. This study was, therefore, excluded from the analysis, but is important for raising the hypothesis that this relatively inexpensive, practical method of fracture prevention should be tested more rigorously |
| Honkanen 1990 | RCT. 1558 mg calcium plus 1800 IU vitamin D versus no treatment. No fracture data |
| Hunter 2000 | RCT. 800 IU vitamin D versus placebo. No fracture data |
| Itami 1982 | RCT. 1‐alphahydroxyvitamin D3 0.75 μg daily versus placebo for 30 weeks. No fracture data |
| Iwamoto 1999 | RCT. HRT versus 1(OH)vitamin D3 versus vitamin K2 versus control. No fracture data |
| Iwamoto 2000 | RCT. 1‐alphahydroxyvitamin D3 0.75 μg/day versus vitamin K2 versus 1‐alphahydroxyvitamin D3 0.75 μg/day plus vitamin K versus calcium lactate 2 g/day. No fracture data |
| Jensen 1982 | RCT. 1,25(OH)2D3 0.5 μg plus 500 mg calcium versus calcium versus HRT plus calcium versus calcium, HRT plus 1,25(OH)2D3 0.5 μg. No fracture data |
| Jensen 1985 | RCT that measured overall spinal length, but fracture data were unavailable |
| Johnell 2001 | Unclear if cluster RCT. Email from Olof Johnell 9 September 2004 to say publication being drafted. No reply to email to Ewald Ornstein 27 December 2012 |
| Johnson 1980 | RCT. 2000 IU vitamin D or placebo. No fracture data |
| Keane 1998 | RCT. Milk fortified with vitamin D versus unfortified milk. No fracture data |
| Kenny 2003 | RCT comparing 1000 IU/day plus 500 mg/day calcium versus placebo plus 500 mg/day calcium. No fracture data |
| Krieg 1999 | RCT comparing 440 IU vitamin D3 plus 500 mg calcium/day versus no treatment. No fracture data |
| Larsen 2004 | RCT with 4 clusters. Participants in each of the 3 treatment clusters received a medication review co‐intervention, but the control group received no intervention. The vitamin D plus calcium effect cannot be isolated from the effects of the co‐interventions, so this study does not meet the pre‐defined inclusion criteria |
| Latham 2003 | RCT. 300,000 IU vitamin D or placebo, with and without exercise programme. No fracture data |
| Meier 2004 | RCT. 500 IU vitamin D and 500 mg calcium versus control. No fracture data |
| Moschonis 2006 | RCT. 1200 mg calcium plus 300 IU vitamin D3/day supplemented dairy products versus 600 mg/day calcium supplement versus control. No fracture data |
| Nordin 1985 | RCT. 15,000 IU vitamin D2 weekly versus placebo. No fracture data |
| Ongphiphadhanakul 2000 | RCT. 0.25 μg/day calcitriol versus 0.50 μg calcitriol/day versus low dose oestrogen versus high dose oestrogen (all groups received 750 mg calcium/day. No fracture data |
| Ooms 1995 | RCT. Vitamin D supplementation, placebo controlled, no fracture data (subset of Lips 1996) |
| Orimo 2011 | RCT. Alendronate 5 mg/day plus 1 μg/day alfacalcidol versus alendronate 5 mg/day. Protocol for this review currently does not include trials with other drug treatments administered to both arms of the trial |
| Orwoll 1989 | RCT. 40 μg 25‐OHD plus 1200 mg calcium daily or 1200 mg calcium plus placebo daily for 2 years. Reply from Eric Orwoll on 30 July 2004 (orwoll@ohsu.edu) saying no data available on numbers of participants with fractures |
| Patel 2001 | RCT. 800 IU cholecalciferol versus placebo in first year, crossed‐over for second year. Age range 24 to 70 years. Mean age 47 years, too young for trial of osteoporotic fracture prevention |
| Pedrosa 2006 | RCT. 150,000 IU vitamin D3 monthly for 2 months, then 90,000 IU monthly for 4 months plus 1000 mg/day calcium versus placebo plus 1000 mg/day calcium. No fracture data |
| Riera 2003 | RCT. 1 μg/day alfacalcidol plus 500 mg/day calcium citrate versus placebo plus 500 mg/day calcium citrate. No fracture data |
| Riis 1986 | RCT. 10 μg 24R,25(OH)2 vitamin D3 daily or placebo. No fracture data. 24R,25(OH)2 vitamin D3 is form of vitamin D with two hydroxyl groups |
| Shiraki 1985 | RCT. 1 μg 1,24(R) (OH)2 vitamin D3 versus 1 μg 1, 24(S) (OH)2 vitamin D3, 0.5 μg 1‐alphahydroxyvitamin D3 versus 1 μg 1‐alphahydroxyvitamin D3 daily versus control. No fracture data. 1,24(R) (OH)2 vitamin D3 and 1, 24(S) (OH)2 vitamin D3 are different forms of vitamin D with two hydroxyl groups |
| Shiraki 2004 | RCT. 1 μg alfacalcidol plus 78 mg calcium versus 78 mg calcium. No fracture data |
| Son 2001 | RCT. Calcium 1000 mg/day versus 0.5 μg/day alfacalcidol versus placebo. No fracture data |
| Sorensen 1977 | RCT. 0.5 μg 1‐alphahydroxyvitamin D3 plus 1000 mg calcium versus placebo plus 1000 mg calcium. No fracture data |
| Sosa 2000 | Probably 1‐year randomised controlled trial. 10,640 IU 25hydroxyvitamin D3 per week plus 1000 mg calcium versus 1000 mg calcium. No reply to letter sent 10 February 2005 |
| Thomsen 1986 | RCT. 24R,25‐(OH)2D3 versus placebo. No fracture data |
| Ushiroyama 1995 | RCT. Placebo‐controlled, intervention 1‐alphahydroxyvitamin D. No fracture data |
| Ushiroyama 2002 | RCT. 1‐alphahydroxyvitamin D3 1 μg/day versus vitamin K versus 1‐alphahydroxyvitamin D3 1 μg/day plus vitamin K versus control. No fracture data |
| Zhu 2006 | RCT. 500 mg calcium plus 1000 IU vitamin D2/day versus 500 mg calcium/day plus placebo. No fracture data |
HRT: hormone replacement therapy RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Bischoff‐Ferrari 2010a.
| Methods | Factorial design randomised controlled trial |
| Participants | 173 men and women 65 years or older with acute hip fracture |
| Interventions | 1. 800 IU vitamin D3 plus 1000 mg calcium plus 1200 IU vitamin D3 daily, plus standard physiotherapy 2. 800 IU vitamin D3 plus 1000 mg calcium plus placebo daily, plus standard physiotherapy 3. 800 IU vitamin D3 plus 1000 mg calcium plus 1200 IU vitamin D3 daily, plus extended physiotherapy 4. 800 IU vitamin D3 plus 1000 mg calcium plusplacebo daily, plus extended physiotherapy |
| Outcomes | Hip fracture, non‐vertebral fractures, deaths, hypercalcaemia |
| Notes | Includes comparisons of different vitamin D doses, to be included when protocol revised in next update |
Lappe 2007.
| Methods | 4‐year randomised controlled trial |
| Participants | 1179 community‐dwelling women aged over 55 in USA |
| Interventions | 1. 1100 IU vitamin D3 plus 1400‐1500 mg calcium/day 2. Vitamin D placebo plus 1400‐1500 mg calcium 3. Double placebos |
| Outcomes | Fractures |
| Notes | Cancer incidence data published. Email from Joan Lappe 10 September 2007 saying fracture data publication being drafted |
Nuti 2006.
| Methods | Randomised, double‐blind trial, duration 18 months |
| Participants | 11 clinical centres, Italy 148 participants (all women), mean age 64 years (N = 136) |
| Interventions | 1. Alfacalcidol D3 1 μg tablet/day plus placebo 2. 880 IU vitamin D3 plus 1000 mg calcium/day (as calcium carbonate) plus placebo |
| Outcomes | Persons with new vertebral fracture, gastrointestinal adverse events, renal stones |
| Notes | Comparisons of different vitamin D doses; to be included when protocol revised in next update |
Orimo 1987.
| Methods | Probably randomised controlled trial, duration unclear |
| Participants | 86 women, mean age over 70 years, with osteoporosis, in Japan |
| Interventions | 1 μg alfacalcidol or 1 μg alfacalcidol plus 1 g calcium or 1 g calcium daily or no treatment |
| Outcomes | Fractures as vertebral fractures/1000 patient years |
| Notes | Letter sent to authors 3 January 2013 |
Papaioannou 2011.
| Methods | 90‐day 3‐arm randomised controlled trial comparing different vitamin D dosing strategies |
| Participants | 65 participants with acute fragility hip fracture |
| Interventions | All had 1000 IU oral vitamin D3 daily for 90 days. Additional interventions were 100,000 IU vitamin D2 oral bolus versus 50,000 IU vitamin D2 oral bolus versus placebo oral bolus on day 1 |
| Outcomes | Serum 25(OH)vitamin D, fractures, deaths |
| Notes | Includes comparisons of different vitamin D doses; to be included when protocol revised in next update |
Petkakov 1995.
| Methods | 6‐month randomised controlled trial |
| Participants | 20 postmenopausal women |
| Interventions | 0.25 μg 1‐alphahydroxyvitamin D3 twice daily versus placebo |
| Outcomes | Vertebral fractures |
| Notes | Requires translation |
Sato 1997.
| Methods | Double‐blind randomised study 64 of 84 completed |
| Participants | University Hospital, Japan
84 hospital outpatients who had hemiplegia after stroke. Analysis based on 64 completing participants, (35 men, 29 women) of mean age 68.5 years Disease exclusions: shoulder‐hand syndrome, multiple strokes, history of hip fracture, stroke duration < 1 month Drug exclusions: use of oestrogen, calcium, vitamin D, corticosteroids, thyroxine, or anticonvulsants |
| Interventions | 1. 1‐alpha‐hydroxyvitamin D3 1.0 μg daily
Randomised 45, completed 30 2. Identical placebo Randomised 39, 34 completed Both groups received 300 mg calcium daily Duration of treatment 6 months |
| Outcomes | Measured at 6 months 1. Number of participants sustaining a hip fracture Also measured, but not considered in this review were bone mineral density, and biochemical measures |
| Notes | Emailed Dr Sato 14 October 2005 asking for further details of deaths during the study. Awaiting further clarification with regard to methods |
Sato 1999a.
| Methods | Double‐blind randomised study Losses: none described |
| Participants | University Hospital, Japan
86 (35 men, 51 women) elderly people with Parkinson's disease, mean age 70.6 years Disease exclusions: history of previous non‐vertebral fracture, non‐ambulatory (Hoehn and Yahr Stage 5 disease), hyperparathyroidism, renal osteodystrophy, impaired renal, cardiac or thyroid function Drug exclusions: therapy with corticosteroids, oestrogens, calcitonin, etidronate, calcium, or vitamin D at any time in the previous 2 months, or for > 3 months out of the previous 18 |
| Interventions | 1. 1‐alpha‐hydroxyvitamin D3 1.0 μg daily 2. Identical placebo Duration of treatment 18 months |
| Outcomes | Measured at 18 months
1. Number of participants sustaining a fall associated hip fracture
2. Other fall‐associated non‐vertebral fractures
3. Number of self‐reported falls per subject (not part of this review) Also measured, but not considered in this review were bone mineral density, and biochemical measures |
| Notes | Required: details of randomisation, falls data. Letter sent 14 October 2004 asking for details of reported deaths in trial. Awaiting further clarification with regard to methods |
Sato 1999b.
| Methods | No blinding reported 60 of 69 completed |
| Participants | Outpatient study, Japan
69 patients (39 women, 30 men), average age 71 years Inclusion criteria: post‐stroke hemiplegia, at least 1 year post‐stroke Disease exclusions: congestive heart failure, obstructive pulmonary disease, other known causes of osteoporosis (hyperparathyroidism, renal osteodystrophy), impairment of renal, cardiac, or thyroid function Drug exclusions: corticosteroids, oestrogen, calcitonin, etidronate, calcium or vitamin D3 for 3 months or more in 12 months before study; or any of these in 2 months preceding study |
| Interventions | 1. 1‐alpha‐hydroxyvitamin D3 daily 1 μg
Randomised 34, completed 31 2. No tablets. No intervention Randomised 35, completed 29 3. Ipriflavone 600 mg daily (group not used in this review). Randomised 34, completed 28 Duration of treatment 1 year |
| Outcomes | Measured at 1 year 1. Number of persons sustaining new hip fracture 2. Numbers of persons with adverse events 3. Bone mineral density of second metacarpal 4. Biochemical markers of bone formation and breakdown, PTH, 25(OH) vitamin D, 1,25(OH)2 vitamin D |
| Notes | Awaiting further clarification with regard to methods |
Sato 2005.
| Methods | 2‐year randomised controlled trial |
| Participants | 96 men and women with post‐stroke hemiplegia, in Japan |
| Interventions | 1000 IU vitamin D2 daily or placebo |
| Outcomes | Hip fractures/1000 patient years, deaths |
| Notes | Further details required from authors |
TIDE 2012.
| Methods | 3 x 2 factorial RCT |
| Participants | Type 2 diabetics with HbA1c 6.5% to 9.5%, at risk of cardiovascular disease. 1221 randomised to vitamin D or placebo, 1332 randomised to rosiglitazone, pioglitazone or placebo ‐ 16,000 planned recruitment |
| Interventions | (Placebo versus rosiglitazone 4 mg‐8 mg/day versus pioglitazone 30 mg‐45 mg/day) versus (placebo versus vitamin D3 1000 IU/day) |
| Outcomes | For vitamin D included all cause death; cancers requiring hospitalisation, chemotherapy or surgery, fractures |
| Notes | Trial stopped early due to regulatory concerns over safety of rosiglitazone; data awaited |
Wood 2012.
| Methods | Double‐blind, placebo‐controlled trial |
| Participants | 305 healthy postmenopausal women aged 60 to 70 years |
| Interventions | 400 IU vitamin D3, 1000 IU vitamin D3 or placebo |
| Outcomes | Non‐vertebral fractures, hypercalcaemia, gastrointestinal effects |
| Notes | Includes comparisons of different vitamin D doses; to be included when protocol revised in next update |
Xia 2009.
| Methods | Randomised, open‐label trial |
| Participants | 150 postmenopausal women aged over 65 years with lumbar spine bone mineral density T score of ‐1.0 or less, BMI of 18 kg‐30 kg/m2 |
| Interventions | Caltrate D one tablet daily (500 mg calcium plus 125 IU vitamin D) versus calcitriol 0.25 μg/d plus Caltrate D 1 tablet daily |
| Outcomes | Vertebral and non‐vertebral fractures |
| Notes | Includes comparisons of different vitamin D doses; to be included when protocol revised in next update |
BMI: body‐mass index HbA1c: glycated haemoglobin
Characteristics of ongoing studies [ordered by study ID]
ANVITAD.
| Study name | ANVITAD |
| Methods | RCT |
| Participants | 704 non‐institutionalised people aged 65 years or older |
| Interventions | 1. Chewable tablets of 800 IU vitamin D plus 1000 mg calcium daily 2. Chewable placebo |
| Outcomes | Falls, fractures, need for healthcare, kidney stones, gastrointestinal events |
| Starting date | 2008 |
| Contact information | Jesus Lopez‐Torres Hidalgo (jesusl@sescam.org) Unidad de Investigación de la Gerencia de Atención Primaria de Albacete (Servicio de Salud de Castilla‐La Mancha), Marqués de Villores 6‐8, 02001 Albacete, Spain |
| Notes |
REVITAHIP.
| Study name | REVITAHIP |
| Methods | RCT |
| Participants | 250 adults aged 65 years or over with hip fracture requiring surgery |
| Interventions | Intervention of oral loading dose of 250,000 IU vitamin D3 within 7 days of surgery plus 800 IU vitamin D3 plus 1000 mg calcium daily for 26 weeks, versus 800 IU D3 plus 1000 mg calcium daily for 26 weeks only |
| Outcomes | Gait velocity, falls, fractures, adherence, grip strength, quality of life, health service use |
| Starting date | 01 September 2010 |
| Contact information | Jenson Mak, Department of Geriatric Medicine, Central Coast Area Health Service, Gosford Hospital PO Box 361, Gosford, NSW 2250, Australia, jmak@nsccahs.health.nsw.gov.au |
| Notes |
RCT: randomised controlled trial
Differences between protocol and review
In previous versions of the review, the category 'any new fracture' was classified as fractures not covered by hip, vertebral or non‐vertebral categories, or where the site of fracture was unclear. This meant that some of the very large community trials were not analysed together (Analyses 1 to 4; all vitamin D comparisons), because they chose to report 'non‐vertebral fractures' or 'hip fractures' or 'vertebral fractures' but not 'all fractures' and these numbers were not available or could not be calculated from the data without risk of double counting. In this review for Analyses 1 to 4 the category 'all fractures' includes data from non‐vertebral fractures (or hip or vertebral fractures if not given), if the data for 'all fractures' are not available.
Contributions of authors
In this update, AA and JM contributed to methodological appraisal and data extraction. AA drafted the update. JM commented on the draft review and suggested changes. DO provided statistical support, commented on the draft review and suggested changes. A Avenell is the guarantor of the review.
Sources of support
Internal sources
-
Health Services Research Unit, University of Aberdeen, UK
Computing, administration and library services (AA)
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, UK
Part funding of salary (AA)
Declarations of interest
Alison Avenell (AA) participated in the RECORD 2005 trial. She was the principal investigator for the Avenell 2004 trial. AA did not carry out data extraction or quality assessment on trials with which she was involved. AA was involved in the meta‐analyses of the effects of calcium (Bolland 2010), and calcium and vitamin D (Bolland 2011b), on cardiovascular disease Jenson CS Mak: nothing to declare Dianne O'Connell: nothing to declare
Edited (no change to conclusions)
References
References to studies included in this review
Aloia 1988 {published data only}
- Aloia JF, Vaswani A, Yeh JK, Ellis K, Yasumura S, Cohn SH. Calcitriol in the treatment of postmenopausal osteoporosis. American Journal of Medicine 1988;84(3 Pt 1):401-8. [DOI] [PubMed] [Google Scholar]
- Aloia JF. Role of calcitriol in the treatment of postmenopausal osteoporosis. Metabolism 1990;39(4 Suppl 1):35-8. [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Personal communication 21 February 1995.
Arthur 1990 {published data only}
- Arthur RS, Piraino B, Candib D, Cooperstein L, Chen T, West C, et al. Effect of low-dose calcitriol and calcium therapy on bone histomorphometry and urinary calcium excretion in osteopenic women. Mineral and Electrolyte Metabolism 1990;16(6):385-90. [MEDLINE: ] [PubMed] [Google Scholar]
Avenell 2004 {published and unpublished data}
- Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA for the RECORD Trial Management Group. The effects of an open design on trial participant recruitment, compliance and retention - a randomized controlled trial comparison with a blinded, placebo-controlled design. Clinical Trials Journal 2004;1:490-8. [DOI] [PubMed] [Google Scholar]
- Avenell A. Personal communication 2005.
Bischoff 2003 {published and unpublished data}
- Bischoff-Ferrari HA, Conzelmann M, Stahelin HB, Dick W, Carpenter MG, Adkin AL, et al. Is fall prevention by vitamin D mediated by a change in postural or dynamic balance? Osteoporosis International 2006;17(5):656-63. [DOI] [PubMed] [Google Scholar]
- Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. Journal of Bone and Mineral Research 2003;18(2):343-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bischoff HA. Personal communication 13 July 2003.
- Bischoff HA. Vitamin D deficiency in the elderly. Journal of Bone and Mineral Research 2003;18(7):1343. [MEDLINE: ] [Google Scholar]
- Heaney R. Vitamin D depletion and effective calcium absorption. Journal of Bone and Mineral Research 2003;18(7):1342. [DOI] [PubMed] [Google Scholar]
Bolton‐Smith 2007 {published and unpublished data}
- Bolton-Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on bone health of older women. Journal of Bone and Mineral Research 2007;22(4):509-19. [DOI] [PubMed] [Google Scholar]
- McMurdo ME. Personal communication 1 October 2007.
Burleigh 2007 {published and unpublished data}
- Burleigh E, McColl J, Potter J. Does vitamin D stop inpatients falling? A randomised controlled trial. Age and Ageing 2007;36(5):507-13. [DOI] [PubMed] [Google Scholar]
Caniggia 1984 {published data only}
- Caniggia A, Delling G, Nuti R, Lore F, Vattimo A. Clinical, biochemical and histological results of a double-blind trial with 1,25-dihydroxyvitamin D3, estradiol and placebo in post-menopausal osteoporosis. Acta Vitaminologica et Enzymologica 1984;6(2):117-28. [PubMed] [Google Scholar]
Chapuy 1992 {published data only}
- Chapuy MC, Arlot M, et al. Prevention of non vertebral fractures and cortical bone loss in elderly women: a prospective controlled trial using calcium and vitamin D3 supplements [abstract]. Osteoporosis International 1993;3 Suppl 1:258. [Google Scholar]
- Chapuy MC, Arlot ME, Delmas PD, Meunier PJ. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ 1994;308:1081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. New England Journal of Medicine 1992;327:1637-42. [DOI] [PubMed] [Google Scholar]
- Meunier PJ, Pamphile R, Chapuy MC, Schulten J, Arlot M, Lillu H. A calcium-vitamin D3 combined supplementation is cost-saving for preventing hip fractures in institutionalised elderly women: an economic evaluation from the perspective of seven European countries (Belgium, France, Germany, The Netherlands, Spain, Sweden, United Kingdom). Osteoporosis International 2002;13(Suppl 1):S14. [Google Scholar]
Chapuy 2002 {published and unpublished data}
- Bosworth C, Boer IH, Targher G, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and cholecalciferol supplementation on bone mineral density in elderly women with moderate chronic kidney disease. Clinical Nephrology 2012;77(5):358-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporosis International 2002;13(3):257-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation to prevent hip fracture in elderly women. A confirmatory study: Decalyos II. Osteoporosis International 2002;13(Suppl 1):S25. [DOI] [PubMed] [Google Scholar]
- Meunier PJ. Personal communication 28 February 2005.
Dawson‐Hughes 1997 {published data only}
- Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Additive effect of higher testosterone levels and vitamin D plus calcium supplementation in regard to fall risk reduction among older men and women. Osteoporosis International 2008;19:1307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Archives of Internal Medicine 2006;166(4):424-30. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of vitamin D3 plus calcium on fall risk in older men and women: a 3-year randomized controlled trial. Journal of Bone and Mineral Research 2004;19(Suppl 1):S57. [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. New England Journal of Medicine 1997;337(10):670-6. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of withdrawal of calcium and vitamin D supplements on bone mass in elderly men and women. American Journal of Clinical Nutrition 2000;72(3):745-50. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. American Journal of Clinical Nutrition 2002;75(4):773-9. [DOI] [PubMed] [Google Scholar]
- Krall EA, Randall C, Harris SS, Garcia RI, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Journal of Bone and Mineral Research 2000;15(Suppl 1):S191. [DOI] [PubMed] [Google Scholar]
- Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. American Journal of Medicine 2001;111(6):452-6. [DOI] [PubMed] [Google Scholar]
- Niramitmahapanya S, Harris SS, Dawson-Hughes B. Type of dietary fat is associated with the 25-hydroxyvitamin D3 increment in response to vitamin D supplementation. Journal of Clinical Endocrinology and Metabolism 2011;96(10):3170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dukas 2004 {published and unpublished data}
- Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner-Binder D, Damm TN, et al. Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily. Journal of the American Geriatrics Society 2004;52(2):230-6. [DOI] [PubMed] [Google Scholar]
- Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner-Binder D, Thalman B, et al. Alfacalcidol reduces the number of fallers and falls in community-dwelling elderly provided a minimum total daily intake of 500mg calcium [abstract]. Calcified Tissue International 2003;72:371. [Google Scholar]
- Dukas L, Schacht E, Mazor Z, Stahelin HB. Treatment with alfacalcidol in elderly people significantly decreases the high risk of falls associated with a low creatinine clearance of <65 ml/min. Osteoporosis International 2005;16(2):198-203. [DOI] [PubMed] [Google Scholar]
- Dukas L, Schacht E, Stahelin HB. A significant independent new risk factor for falls in the elderly: a low creatinine clearance of less than 65ml/min. Osteoporosis International 2003;14(Suppl 7):S33. [DOI] [PubMed] [Google Scholar]
- Dukas L, Schacht E, Stahelin HB. High risk of falls related to low D-hormone syndrome and its treatment with alfacalcidol. Osteoporosis International 2004;15(Suppl 1):S8-9. [Google Scholar]
- Dukas L, Schacht E, Stahelin HB. Reduction of falls and fallers in high risk patients by D-hormone analogs. Osteoporosis International 2004;15(Suppl 1):S144-5. [Google Scholar]
- Dukas L, Schacht E, Stahelin HB. Treatment with alfacalcidol significantly decreases the high incidence of fallers and the high risk of falls associated with low creatinine clearance (<65ml/min) in elderly community-dwelling men and women. Osteoporosis International 2003;14(Suppl 7):S33. [DOI] [PubMed] [Google Scholar]
- Dukas L, Schacht E. Reduction of falls and fallers using D-hormone analogs. Osteoporosis International 2006;17(Suppl 1):S122-3. [Google Scholar]
- Dukas LC, Schacht E, Mazor Z, Stahelin HB. The low creatinine clearance associated high risk of falls can significantly be treated with alfacalcidol. Journal of Bone and Mineral Research 2004;19(Suppl 1):S95. [Google Scholar]
- Dukas LC. Personal communication 19 July 2004.
Ebeling 2001 {published and unpublished data}
- Ebeling PR, Wark JD, Yeung S, Poon C, Salehi N, Nicholson GC, et al. Effects of calcitriol or calcium on bone mineral density, bone turnover, and fractures in men with primary osteoporosis: a two-year randomized, double-blind, double placebo study. Journal of Clinical Endocrinology and Metabolism 2001;86(9):4098-103. [DOI] [PubMed] [Google Scholar]
- Ebeling PR. Personal communication 15 February 2005.
Falch 1987 {published and unpublished data}
- Falch JA, Odegaard OR, Finnanger AM, Matheson I. Postmenopausal osteoporosis: no effect of three years treatment with 1,25-dihydroxycholecalciferol. Acta Medica Scandinavica 1987;221(2):199-204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Falch JA, Odegaard OR, Finnanger AM. 3 years treatment with 1.25(OH)2 Vitamin D3 does not reduce bone loss or fracture rate in postmenopausal women with fracture of the distal forearm. In: Norman AW, Schaefer K, Grigoleit H-G, v Herrath D , editors(s). Vitamin D : chemical, biochemical, and clinical update: proceedings of the Sixth Workshop on Vitamin D, Merano, Italy, March 1985. Berlin: de Gruyter, 1985:1004-5.
- Falch JA. Personal communication 1999.
Flicker 2005 {published and unpublished data}
- Flicker L, MacInnis R, Stein M, Scherer S, Mead K, Nowson C, et al. Should all older people in residential care receive vitamin D to prevent falls? Results of a randomised trial [abstract]. Journal of Bone and Mineral Research 2004;19(Suppl 1):S99. [DOI] [PubMed]
- Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, et al. Response letter to Dr. Gau et al. Journal of the American Geriatrics Society 2006;54:1021-2. [Google Scholar]
- Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, et al. Erratum. Journal of the American Geriatrics Society 2012;60:1599. [DOI] [PubMed] [Google Scholar]
- Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. Journal of the American Geriatrics Society 2005;53(11):1881-8. [DOI] [PubMed] [Google Scholar]
- Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA. Should all older people in residential care receive vitamin D to prevent falls? Results of a randomised trial. Journal of Bone and Mineral Research 2004;19(Suppl 1):S99. [DOI] [PubMed] [Google Scholar]
- Flicker L. Personal communication 7 January 2008.
Gallagher 1989 {published data only}
- Gallagher JC, Riggs BL, Recker RR, Goldgar D. The effect of calcitriol on patients with postmenopausal osteoporosis with special reference to fracture frequency. Proceedings of the Society for Experimental Biology and Medicine 1989;191(3):287-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gallagher 1990 {published data only}
- Gallagher JC, Goldgar D. Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Annals of Internal Medicine 1990;113(9):649-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Metabolic effects of synthetic calcitriol (Rocaltrol) in the treatment of postmenopausal osteoporosis. Metabolism: Clinical & Experimental 1990;39(4 Suppl 1):27-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Personal communication 21 February 1995.
Gallagher 2001 {published and unpublished data}
- Gallagher JC, Fowler S. Effect of estrogen, calcitriol and a combination of estrogen and calcitriol on bone mineral density and fractures in elderly women. Journal of Bone and Mineral Research 1999;14(Suppl 1):S209. [Google Scholar]
- Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. Journal of Clinical Endocrinology and Metabolism 2001;86(8):3618-28. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Haynatski G, Fowler S. Calcitriol therapy reduces falls and fractures in elderly women. Calcified Tissue International 2003;72:334. [Google Scholar]
- Gallagher JC, Haynatzki G, Fowler S. Effect of estrogen, calcitriol or the combination of both on falls and non vertebral fractures in elderly women. Journal of Bone and Mineral Research 2001;17(Suppl 1):S210. [Google Scholar]
- Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. Journal of Clinical Endocrinology and Metabolism 2002;87(11):4914-23. [12414850] [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Rapuri PB, Haynatzki G. A comparison of estrogen, calcitriol or both therapies on the relationship between serum parathyroid hormone and 25 hydroxy vitamin. Journal of Bone and MIneral Research 2004;19(Suppl 1):S440. [Google Scholar]
- Gallagher JC. Personal communication 21 February 2005.
- Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. Journal of Steroid Biochemistry and Molecular Biology 2004;89-90(1-5):497-501. [DOI] [PubMed] [Google Scholar]
Garay Lillo 1997 {published data only}
- Garay Lillo J, Parreno J, González Y, González JA. Geminis: a prospective, multicentric, randomised study to evaluate the effect of tricalcium phosphate versus tricalcium phosphate plus 25(OH) Vitamin D on the risk of fractures in older women [Géminis: Estudio prospectivo, multicéntrico y aleatori para valorar el efecto del Fosfato Tricálcico versus Fosfato Tricálcico + 25(OH) Vitamina D sobre el riesgo de fracturas en mujerioes ancianas.]. Geriátrika 1997;13(6):24-8. [Google Scholar]
Geusens 1986 {published data only}
- Geusens P, Dequeker J. Long-term effect of nandrolone decanoate, 1 alpha-hydroxyvitamin D3 or intermittent calcium infusion therapy on bone mineral content, bone remodeling and fracture rate in symptomatic osteoporosis: a double-blind controlled study. Bone and Mineral 1986;1(4):347-57. [PubMed] [Google Scholar]
Glendenning 2012 {published data only}
- Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. Journal of Bone and Mineral Research 2012;27(1):170-6. [DOI] [PubMed] [Google Scholar]
Gorai 1999 {published data only}
- Gorai I, Chaki O, Taguchi Y, Nakayama M, Osada H, Suzuki N, et al. Early postmenopausal bone loss is prevented by estrogen and partially by 1alpha-OH-vitamin D3: therapeutic effects of estrogen and/or 1alpha-OH-vitamin D3. Calcified Tissue International 1999;65:16-22. [DOI] [PubMed] [Google Scholar]
Harwood 2004 {published and unpublished data}
- Harwood RH, Sahota O, Gaynor K, Masud T, Hosking D. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NoNOF) Study. Age and Ageing 2004;33:45-51. [14695863] [DOI] [PubMed] [Google Scholar]
- Harwood RW. Personal communication 24 January 2003.
- Sahota O, Harwood RH, Gaynor K, Masud T, Hosking DJ. A randomised, controlled comparison of different calcium and vitamin D regimes: the NoNOF (Nottingham Neck of Femur) study. Osteoporosis International 2003;14(Suppl 4):S10-11. [Google Scholar]
Hayashi 1992 {published data only}
- Hayashi Y, Fujita T, Inoue T. A multi-center study on efficacy of 1 alpha-hydroxy vitamin D3 in preventing spinal fracture of patients with osteoporosis (abstract). Journal of Bone and Mineral Research 1989;4 Suppl 1:Si84. [Google Scholar]
- Hayashi Y, Fujita T, Inoue T. Decrease of vertebral fracture in osteoporotics by administration of 1alpha-hydroxy-vitamin D3. Journal of Bone and Mineral Metabolism 1992;10(2):50-4. [Google Scholar]
Inkovaara 1983 {published data only}
- Inkovaara J, Gothoni G, Halttula R, Heikinheimo R, Tokola O. Calcium, vitamin D and anabolic steroids in treatment of aged bones: double-blind placebo-controlled long-term clinical trial. Age and Ageing 1983;12:124-30. [DOI] [PubMed] [Google Scholar]
Ishida 2004 (retracted) {published and unpublished data}
- Alpert JS. Concerns about the integrity of Ishida Y, Kawai S. Am J Med. 2004;117:549-555: the reply. American Journal of Medicine 2020;133:e315. [DOI] [PubMed] [Google Scholar]
- Avenell A, Grey A, Gamble GD, Bolland MJ. Concerns about the integrity of the Yamaguchi Osteoporosis Prevention Study (YOPS) report, Am J Med. 2004;117:549-555. American Journal of Medicine 2020;133:e311-4. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kawai S. A two-year randomized controlled trial of hormone replacement therapy, etidronate, calcitonin, vitamin D, or vitamin K, in women with postmenopausal osteoporosis. Journal of Bone and Mineral Research 2002;17(Suppl 1):S478. [Google Scholar]
- Ishida Y, Kawai S. Comparative efficacy of hormone replacement therapy, bisphosphonates, calcitonin, vitamin D and vitamin K in postmenopausal women with osteoporosis. Journal of Bone and Mineral Research 2003;18(Suppl 2):S158. [Google Scholar]
- Ishida Y, Kawai S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: the Yamaguchi osteoporosis prevention study. American Journal of Medicine 2004;117:549-55. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kawai S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, vitamin D and vitamin K in postmenopausal osteoporosis. Bone 2003;33(5 Suppl1):S220. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Soh H, Ogawa S, Kawahara S, Murata H. A one-year randomized controlled trial of hormone replacement therapy, bisphosphonate, calcitonin, vitamin D, and vitamin K, in women with postmenopausal osteoporosis. Journal of Bone and Mineral Research 2000;15(Suppl 1):S310. [Google Scholar]
- Ishida Y, Soh H, Tsuchida S, Kawahara S, Murata H, Kawai S. Effectiveness of hormone replacement therapy, etidronate, calcitonin, vitamin D, and vitamin K in postmenopausal women with osteoporosis [abstract]. Bone 2002;30(3 Suppl 1):50S. [Google Scholar]
- Ishida Y. Personal communication 21 February 2005.
Janssen 2010 {published data only}
- Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: a randomized controlled trial on vitamin D and calcium supplementation. Aging Clinical and Experimental Research 2010;22(1):78-84. [DOI] [PubMed] [Google Scholar]
Komulainen 1998 {published and unpublished data}
- Komulainen M, Kroger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and Vitamin D3 in early postmenopausal women: a population based 5-year randomized trial. Journal of Clinical Endocrinology and Metabolism 1999;84(2):546-52. [DOI] [PubMed] [Google Scholar]
- Komulainen M, Kroger H, Tuppurainen MT, Keikkinen A-M, Honkanen R, Saarikoski S, et al. Identification of early postmenopausal women with no bone response to HRT: results of a five-year clinical trial. Osteoporosis International 2000;11:211-8. [DOI] [PubMed] [Google Scholar]
- Komulainen MH, Kroger H, Tuppurainen MT, Heikkinen A-M, Alhava E, Honkanen R, et al. HRT and Vit D in prevention of non-vertebral fractures in postmenopausal women; a 5 year randomized trial. Maturitas 1998;31(1):45-54. [DOI] [PubMed] [Google Scholar]
- Komulainen MH. Personal communication 11 November 2004.
Law 2006 {published data only}
- Law M, Morris J, Withers H. Cholecalciferol, not ergocalciferol, should be used for vitamin D supplementation [author reply]. Age and Ageing 2006;35(6):645. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Law M, Morris J, Withers H. Vitamin D supplementation and the prevention of fractures and falls. Reply. Age and Ageing 2007;36(2):233. [DOI] [PubMed] [Google Scholar]
- Law M, Morris J, Withers H. Vitamin D supplementation and the prevention of fractures and falls [author reply]. Age and Ageing 2007;36(2):233. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation [comment in: Age and Ageing 2006;35(6):645 and Age and Ageing 2007;36(2):232-3]. Age and Ageing 2006;35(5):482-6. [DOI] [PubMed] [Google Scholar]
Lips 1996 {published data only}
- Graafmans WC, Ooms ME, Hofstee HMA, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. American Journal of Epidemiology 1996;143(11):1129-36. [DOI] [PubMed] [Google Scholar]
- Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. The effect of vitamin D supplementation on the incidence of hip fractures in elderly people. Journal of Bone and Mineral Research 1994;9 Suppl 1:S148. [Google Scholar]
- Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. Annals of Internal Medicine 1996;124:400-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lyons 2007 {published data only}
- Johansen A, Lyons RA, Stone M, Brophy S, Newcombe RG, Phillips CJ, et al. Preventing fractures among older people living in institutional care: a randomised double blind placebo controlled trial of vitamin D supplementation [abstract]. Age and Ageing 2006;35(Suppl 3):i41. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Johansen A, Brophy S, Newcombe RG, Phillips CJ, Lervy B, et al. Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporosis International 2007;18(6):811-8. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Johansen A, Stone MD, Brophy S, Newcombe RG, Philips CJ, et al. Preventing fractures among older people living in institutional care: a randomised double blind placebo controlled trial of vitamin D supplementation [abstract]. Osteoporosis International 2006;17(Suppl 3):372. [DOI] [PubMed] [Google Scholar]
Menczel 1994 {published data only}
- Menczel J, Foldes J, Steinberg R, Leichter I, Shalita B, Bjolah-Abram T, et al. Alfacalcidol (Alpha D3) and calcium in osteoporosis. Clinical Orthopaedics and Related Research 1994;(300):241-7. [PubMed] [Google Scholar]
Meyer 2002 {published data only}
- Meyer HE, Falch JA, Kvaavik E, Smedshaug GB, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomised controlled trial. Osteoporosis International 2000;11(Suppl 2):S114-5. [DOI] [PubMed] [Google Scholar]
- Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. Journal of Bone and Mineral Research 2002;17(4):709-15. [DOI] [PubMed] [Google Scholar]
- Smedshaug GB, Pedersen JI, Meyer HE. Can vitamin D supplementation improve grip strength in elderly nursing home residents? A double-blinded controlled trial. Scandinavian Journal of Food and Nutrition 2007;51(2):74-8. [Google Scholar]
Mitri 2011 {published data only}
- Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. American Journal of Clinical Nutrition 2011;94:486-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakatsuka 1997 {published data only}
- Nakatsuka K, Inaba M, Aratani H, Iba K, Sato T, Koike T, et al. Effects of long-term administration of alfacalcidol on bone mass and bone metabolism in patients with primary osteoporosis - comparison with calcium preparations [Japanese]. Nippon Ronen Igakkai Zasshi - Japanese Journal of Geriatrics 1997;34(7):569-76. [DOI] [PubMed] [Google Scholar]
Ones 2007 {published data only}
- Ones K, Schacht E, Dukas L, Caglar N. Effects of combined treatment with alendronate and alfacalcidol on bone mineral density and bone turnover in postmenopausal osteoporosis: a two-years, randomized, multiarm, controlled trial. Internet Journal of Epidemiology 2007;4(1):DOI: 10.5580/1523. [Google Scholar]
Orimo 1994 {published data only}
- Orimo H, Shiraki M, Hayashi Y, Hoshino T, Onaya T, Miyazaki S, et al. Effects of 1-alpha-hydroxyvitamin D3 on lumbar bone mineral density and vertebral fractures in patients with postmenopausal osteoporosis. Calcified Tissue International 1994;54:370-6. [DOI] [PubMed] [Google Scholar]
OSTPRE‐FPS 2007 {published and unpublished data}
- Karkkainen M, Tuppurainen M, Rikkonen T, Salovaara K, Sirola J, Honkanen R, et al. Vitamin-D 800IU/day + calcium 1000mg supplementation decreases the risk of recurrent falling among postmenopausal ambulatory women - a population-based, randomized, 3-year study (OSTPRE-FPS). Osteoporosis International 2008;19(Suppl 1):S17-18. [Google Scholar]
- Karkkainen M, Tuppurainen M, Rikkonen T, Salovaara K, Sirola J, Honkanen R, et al. Vitamin-D 800IU/day + calcium supplementation decreases the risk of falling among postmenopausal ambulatory women - a population-based, randomized 3-year study (OSTPRE-FPS) [abstract]. Journal of Bone and Mineral Research 2007;22(Suppl 1):S131. [Google Scholar]
- Karkkainen M, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Effect of calcium and vitamin D supplementation on bone mineral density in women aged 65-71 years: a 3-year randomized population-based trial (OSTPRE-FPS). Osteoporosis International 2010;21:2047-55. [DOI] [PubMed] [Google Scholar]
- Karkkainen MK, Tuppurainen M, Salovaara M, Sandini L, Rikkonen T, Sirola J, et al. Does daily vitamin D 800 IU and calcium 1000 mg supplementation decrease the risk of falling in ambulatory women aged 65-71 years? A 3-year randomized population-based trial (OSTPRE-FPS). Maturitas 2010;65:359-65. [DOI] [PubMed] [Google Scholar]
- Kroger HP, Tuppurainen M. OSTPRE-FPS Prevention of Fractures and Falls in Postmenopausal Women With Calcium and Vitamin-D Supplementation - a Randomised Study. http://www.controlled-trials.com/mrct/trial/405493/OSTPRE.
- Salovaara K, Tuppurainen M, Karkkainen M, Rikkonen T, Sandini L, Sirola J, et al. Effect of vitamin D3 and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial - the OSTPRE-FPS. Journal of Bone and Mineral Research 2010;25(7):1487-95. [DOI] [PubMed] [Google Scholar]
- Salovaara KT, Tuppurainen M, Rikkonen T, Karkkainen M, Sirola J, Honkanen R, et al. Effect of vitamin-D3 and calcium on fracture risk in 65-71 year old women in a 3-year randomized clinical trial - preliminary results from the OSTPRE-Fracture Prevention Study. Calcified Tissue International 2008;82:545-6. [Google Scholar]
- Salovaara KT, Tuppurainen M, Rikkonen T, Karkkainen M, Sirola J, Honkanen R, et al. Effect of vitamin-D3 and calcium on fracture risk in 65-71 year old women in a 3-year randomized clinical trial - preliminary results of the OSTPRE-Fracture Prevention Study. Journal of Bone and Mineral Research 2007;22(Suppl 1):S76. [DOI] [PubMed] [Google Scholar]
Ott 1989 {published data only}
- Ott SM, Chesnut CH. Calcitriol treatment is not effective in postmenopausal osteoporosis: see comments. Annals of Internal Medicine 1989;110:267-74. [DOI] [PubMed] [Google Scholar]
Peacock 2000 {published data only}
- Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, et al. Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. Journal of Clinical Endocrinology and Metabolism 2000;85:3011-9. [DOI] [PubMed] [Google Scholar]
Pfeifer 2000 {published data only}
- Minne HW, Pfeifer M, Begerow B, Nachtigall D, Hansen C. Vitamin D and calcium supplementation reduces falls in elderly women via improvement of body sway and normalisation of blood pressure; a prospective, randomized, and double-blind study. Osteoporosis International 2000;11(Suppl 2):S115. [Google Scholar]
- Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. Journal of Bone and Mineral Research 2000;15(6):1113-8. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Nachtigall D, Hansen C. Prevention of falls-related fractures: vitamin D reduces body sway in the elderly - a prospective, randomized, double blind study [abstract]. Bone 1998;23(5 Suppl 1):1110. [Google Scholar]
Pfeifer 2009 {published data only}
- Bolland MJ, Grey A. Different outcomes of meta-analyses and data inconsistency:response to comments by Pfeifer. Archives of Osteoporosis 2015;10:43. [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Grey A. Inconsistent data in text and tables. Osteoporosis International 2015;26:2713. [DOI] [PubMed] [Google Scholar]
- Minne HW, Dobnig H, Pfeifer M, Suppan K. Effects of vitamin D and calcium supplementation on falls and parameters of muscle function: a prospective, randomized, double-blind multicenter study [abstract]. Osteoporosis International 2006;17(Suppl 2):S212. [DOI] [PubMed] [Google Scholar]
- Minne HW, Dobnig H, Pfeifer M, Suppan K. Effects of vitamin D and calcium supplementation on falls and parameters of muscle-function - a prospective, randomized, double-blind multi-center study [abstract]. Osteoporosis International 2006;17(Suppl 1):S21. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporosis International 2009;20:315-22. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Dobnig H, Begerow B, Suppan K. Effects of vitamin D and calcium supplementation on falls and parameters of muscle function: a prospective randomized, double-blind multi-centre study [abstract]. Journal of Bone and Mineral Research 2004;19(Suppl 1):S58. [Google Scholar]
- Pfeifer M, Dobnig H, Minne HW, Suppan K. Effects of vitamin D and calcium supplementation on falls and parameters of muscle function - a prospective, randomized, double-blind multi-center study [abstract]. Osteoporosis International 2005;16(Suppl 3):S45. [DOI] [PubMed] [Google Scholar]
- Pfeifer M. Different outcomes of meta-analyses and data inconsistency. Osteoporosis International 2015;26:2715–2716. [DOI] [PubMed] [Google Scholar]
- Pfeifer M. Randomized controlled clinical trials and meta-analyses. Archives of Osteoporosis 2015;10:42. [DOI] [PubMed] [Google Scholar]
Porthouse 2005 {published and unpublished data}
- Dumville JC, Miles JN, Porthouse J, Cockayne ES, Saxon L, King C, et al. Can vitamin D supplementation prevent seasonal affective disorder? A randomised trial among older women. Osteoporosis International 2004;15:S42-3. [Google Scholar]
- Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ 2005;330(7498):1003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and vitamin D supplementation for fracture prevention in primary care. Osteoporosis International 2004;15(Suppl 2):S13. [Google Scholar]
- Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, et al. Randomised controlled trial of calcium and vitamin D supplementation for fracture prevention in primary care. Osteoporosis International 2005;16:S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffer S. Calcium and vitamin D in primary care: adherence results from a randomised controlled trial [abstract 6.9.3]. In: RCN International Nursing Conference; 2004 Mar 21-31; Cambridge (UK). London: Royal College of Nursing, 2004.
- S Puffer, on behalf of the Calcium and Vitamin D Trial. Calcium and vitamin D in primary care. Compliance results from a randomised controlled trial. Osteoporosis International 2003;14(Suppl 4):S8. [Google Scholar]
- Torgerson D, on behalf of the trial group. Calcium and vitamin D in preventing fractures [author reply]. BMJ 2005;331(7508):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson DJ. Personal communication 9-16 February 2005.
Prince 2008 {published data only}
- Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Archives of Internal Medicine 2008;168(1):103-8. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bruce D, Austin N, Devine A, Ebeling PR, Prince RL. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone-related chemistry in elderly women with vitamin D insufficiency. Journal of Bone and Mineral Research 2008;23(8):1343-8. [DOI] [PubMed] [Google Scholar]
- Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. Journal of Clinical Endocrinology and Metabolism 2008;93(3):743-9. [DOI] [PubMed] [Google Scholar]
RECORD 2005 {published and unpublished data}
- Anderson FH, Grant AM, Avenell A, Campbell MK, Cooper C, Donaldson C, et al. The RECORD Trial: an evaluation of calcium and/or vitamin D in the secondary prevention of osteoporotic fractures. Bone 2005;36(Suppl 2):S122-3. [Google Scholar]
- Francis RM, Grant AM and the RECORD trial group. The RECORD trial: a randomised double-blind study of calcium and/or vitamin D in the secondary prevention of low trauma fractures. Age Ageing 2005;34(Suppl 2):ii15. [Google Scholar]
- Grant A, on behalf of the RECORD Trial Group. Prevention of low-trauma fractures in older people [author reply]. Lancet 2005;366(9485):543-4. [Google Scholar]
- Grant AM, Anderson FH, for the RECORD Trial Group. The Medical Research Council RECORD Trial: an evaluation of calcium and/or vitamin D in the secondary prevention of osteoporotic fractures. Osteoporosis International 2004;15(Suppl 2):S13. [Google Scholar]
- Grant AM, Anderson FH. The RECORD trial: an evaluation of calcium and/or vitamin D in the secondary prevention of osteoporotic fractures. Osteoporosis International 2005;16(Suppl 3):S4-5. [Google Scholar]
- Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial [comment in: Lancet 2005;366(9485):543]. Lancet 2005;365(9471):1621-8. [DOI] [PubMed] [Google Scholar]
- Grant AM. Personal communication 11 March 2004.
Shiraki 1996 {published data only}
- Shiraki M, Kushida K, Yamazaki K, Nagai T, Inoue T, Orimo H. Effects of 2 years' treatment of osteoporosis with 1alpha-hydroxy vitamin D3 on bone mineral density and incidence of fracture: a placebo-controlled, double-blind prospective study. Endocrine Journal 1996;43(2):211-20. [DOI] [PubMed] [Google Scholar]
Smith 2007 {published and unpublished data}
- Anderson FH, Smith HE, Raphael HM, Cooper C. Intramuscular vitamin D increased serum 1,25-dihydroxycholecalciferol but did not affect 25-hydroxy-cholecalciferol levels in health older adults [abstract]. Journal of Bone and Mineral Research 2000;15(Suppl 1):S315. [Google Scholar]
- Anderson FH, Smith HE, Raphael HM, Crozier SR, Cooper C. Effect of annual intramuscular vitamin D3 supplementation on fracture risk in 9440 community-living older people: the Wessex fracture prevention trial [abstract]. Journal of Bone and Mineral Research 2004;19(Suppl 1):S57. [Google Scholar]
- Arden NK, Crozier S, Smith H, Anderson F, Edwards C, Raphael H, et al. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis and Rheumatism 2006;55(4):610-5. [DOI] [PubMed] [Google Scholar]
- Cooper C, Crozier S. Personal communication 23 February 2005.
- Raphael H, Smith H, Anderson F, Cooper C. Tackling the problems of trial management in primary care - experience from the Wessex research network fracture prevention study of annual vitamin D injection in older people [abstract]. Osteoporosis International 2000;11(Suppl 1):S63-4. [Google Scholar]
- Smith H, Anderson F, Raphael H, Cooper C. The Wessex research network fracture prevention study - a large pragmatic trial of annual vitamin D injection in older people [abstract]. Osteoporosis International 2000;11(Suppl 1):S64. [Google Scholar]
- Smith H, Anderson F, Raphael H, Crozier S, Cooper C. Effect of annual intramuscular vitamin D supplementation on fracture risk: population-based, randomised, double-blind, placebo-controlled trial [abstract]. Osteoporosis International 2004;15(Suppl 1):S8. [DOI] [PubMed] [Google Scholar]
- Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology 2007;46(12):1852-7. [DOI] [PubMed] [Google Scholar]
Tilyard 1992 {published data only}
- Morrison NA, George PM, Vaughan T, Tilyard MW, Frampton CM, Gilchrist NL. Vitamin D receptor genotypes influence the success of calcitriol therapy for recurrent vertebral fracture in osteoporosis. Pharmacogenetics and Genomics 2005;15(2):127-35. [DOI] [PubMed] [Google Scholar]
- Tilyard M. Low-dose calcitriol versus calcium in established postmenopausal osteoporosis. Metabolism: Clinical & Experimental 1990;39(4 Suppl 1):50-2. [DOI] [PubMed] [Google Scholar]
- Tilyard MW, Spears GF, Thomson J, Dovey S. Treatment of postmenopausal osteoporosis with calcitriol or calcium. New England Journal of Medicine 1992;326(6):357-62. [DOI] [PubMed] [Google Scholar]
- Tilyard MW, Spears GFS, Thomson J, Dovey S. Calcitriol or calcium for postmenopausal osteoporosis. New England Journal of Medicine 1992;327:284. [DOI] [PubMed] [Google Scholar]
- Tilyard MW. 1,25-dihydroxyvitamin D3 (calcitriol) in the treatment of postmenopausal osteoporosis. Aktuelle Rheumatologie 1994;19(Suppl 1):23-6. [Google Scholar]
Trivedi 2003 {published data only}
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ushiroyama 2001 {published data only}
- Ushiroyama T, Ikeda A, Sakai M, Higashiyama T, Ueki M. Effects of the combined use of calcitonin and 1alpha-hydroxycholecalciferol on vertebral bone loss and bone turnover in women with postmenopausal osteopenia and osteoporosis: a prospective study of long-term and continuous administration with low dose calcitonin. Maturitas 2001;40:229-38. [DOI] [PubMed] [Google Scholar]
Vital D {published and unpublished data}
- Sanders K, Duque G, Ebeling P, McCorquodale T, Herrmann M, Shore-Lorenti C, et al. The efficacy of high-does oral vitamin D3 administered once a year: increased fracture risk is associated with 1,25 vitamin D level at 3-months post dose. In: ASBMR Annual Meeting; 2012 Oct 12-15; Minneapolis, Minnesota. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=6beb2500-4b8e-472b-8b38-d881cadb80e3. 2012.
- Sanders K, Stuart A, Williamson E, Simpson J, Kotowicz M, Nicholson G. The efficacy of high-dose oral vitamin D3 administered once a year: a randomised, double-blind, placebo-controlled trial (Vital D study) for falls and fractures in older women. Osteoporosis International 2011;22(Suppl 1):S104-5. [Google Scholar]
- Sanders KM, Duque G, McCorquodale T, Stuart AL, Williamson EJ, Kotowicz MA, et al. Annual high-dose oral vitamin D3: is the increased risk of falls attributable to changes in muscle strength? Osteoporosis International 2011;22(Suppl 4):S552. [Google Scholar]
- Sanders KM, Ebeling PR, Stuart AL, Williamson EJ, Kotowicz MA, Nicholson GC. Annual high-dose oral vitamin D3: is increased risk of fractures attributable to changes in bone turnover markers? Osteoporosis International 2011;22(Suppl 4):S589. [Google Scholar]
- Sanders KM, Hayles AL, Kotowicz MA, Nicholson GC. Does vitamin D explain the lower fracture rate in rural communities? Bone 2007;40(6 Suppl 2):S195. [Google Scholar]
- Sanders KM, Hayles AL, Kotowicz MA, Nicholson GC. The possible role of vitamin D and falls in relation to lower fracture rates in rural communities. Osteoporosis International 2007;18(Suppl 1):S120-1. [Google Scholar]
- Sanders KM, Stuart AL, Kotowicz MA, Nicholson GC. Annual feedback is an effective tool for a sustained increase in calcium intake among older women. Nutrients 2010;2:1018-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Merriman EN, Read ML, Kotowicz MA, Young D, et al. Trials and tribulations of recruiting 2,000 older women onto a clinical trial investigating falls and fractures: Vital D study. BMC Medical Research Methodology 2009;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Nicholson GC. High-dose oral vitamin D supplementation and risk of falls in older women. JAMA 2010;304(8):856-7. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. British Journal of Psychiatry 2011;198:357-64. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Nicholson GC. Annual high-dose oral vitamin D for falls and fractures in elderly women: a randomised, double-blind, placebo-controlled trial (Vital D study). Osteoporosis International 2010;21(Suppl 1):S30. [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women. A randomized controlled trial. JAMA 2010;303(18):1815-22. [DOI] [PubMed] [Google Scholar]
- Sanders KM et al. Incorrect data. JAMA 2010;303(23):2357. [Google Scholar]
WHI 2006 {published data only}
- Cauley J, Wactawski-Wende J, Robbins J, Rodabough R, Chen Z, Johnson K, et al. The Women's Health Initiative (WHI) calcium and vitamin D supplementation trial: health outcomes 5 years after trial completion. ASBMR Annual Meeting; 2012 Oct 12-15; Minneapolis, Minnesota. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=6450bf3e-c670-4dc0-814c-8842e23cd80e (accessed 27 December 2012).
- Cauley JA, LaCroix A, Wu L, Lee J, Horowitz M, Bauer D, et al. Serum 25 hydroxy vitamin D 25(OH) and the risk of hip fracture: the Women's Health Initiative (WHI). Journal of Bone and Mineral Research 2007;22(Suppl 1):S57. [Google Scholar]
- Chen Z, Beck TJ, Wright NC, LaCroix AZ, Cauley JA, Lewis CE, et al. The effect of calcium plus vitamin D supplement on hip geometric structures: results from the Women's Health Initiative CaD trial. Journal of Bone and Mineral Research 2007;22(Suppl 1):S59. [Google Scholar]
- Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative Calcium-Vitamin D Trial: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13:S98-106. [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures [erratum appears in New England Journal of Medicine 9 March 2006;354(10):1102]. New England Journal of Medicine 2006;354(7):669-83. [DOI] [PubMed] [Google Scholar]
- Jackson RD, Wright NC, Beck TJ, Sherrill D, Cauley JA, Lewis CE, et al. Calcium plus vitamin D supplementation has limited effects on femoral geometric strength in older postmenopausal women: the Women's Health Initiative. Calcified Tissue International 2011;88:198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JA, Jackson RD, Cauley JA, LaCroix AZ. Calcium and vitamin D in the prevention of hip and other fractures: an update on the Women's Health Initiative CaD Trial. Journal of Bone and Mineral Research 2002;17(Suppl 1):S477. [Google Scholar]
- The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19(1):61-109. [DOI] [PubMed] [Google Scholar]
Witham 2010 {published data only}
- Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure. A randomized controlled trial. Circulation: Heart Failure 2010;3:195-201. [DOI] [PubMed] [Google Scholar]
Witham 2013 {unpublished data only}ISRCTN92186858
- Witham M. Can high-dose vitamin D supplementation reduce blood pressure and markers of cardiovascular risk in older people with isolated systolic hypertension? A randomised, double-blind, parallel group placebo controlled trial. http://www.controlled-trials.com/ISRCTN92186858/ (accessed 3 December 2013).
- Witham MD, Price RJ, Struthers AD, Donnan PT, Messow M, Ford I, et al. Vitamin D supplementation to reduce blood pressure in older patients with isolated systolic hypertension (VitDISH) - a randomised controlled trial. Personal communication ahead of publication 15 January 2013.
References to studies excluded from this review
Aguado 2006 {published data only}
- Aguado P, Gonzalez-Casaus ML, Cobo T, Campo T, Martinez ME, Martin-Mola E, et al. The efficacy of two dosing regimens (daily colecalciferol versus cyclical calcidiol) for the prevention of bone mass loss in postmenopausal women with vitamin D insufficiency. Journal of Bone and Mineral Research 2006;21(Suppl 1):S306. [Google Scholar]
ALFA 2006 {published data only}
- Bock O, Boerst H, Runge M, Beller G, Touby F, Tuerk J, et al. Effect of alfacalcidol on volumetric bone mineral density measured by pQCT in alendronate-treated postmenopausal women with osteopenia or osteoporosis: 1 year interim analysis of the ALFA study [abstract]. Journal of Bone and Mineral Research 2007;22(Suppl 1):S215. [Google Scholar]
- Bock O, Boerst H, Runge M, Schacht E, Martus P, Felsenberg D. Effect of alfacalcidol on biochemical bone markers in alendronate-treated postmenopausal women with osteopenia or osteoporosis: 1 year interim analysis of the ALFA study [abstract]. Journal of Bone and Mineral Research 2006;21(Suppl 1):S306. [Google Scholar]
- Boerst H, Bock O, Runge M, Degner C, Stephan-Oelkers M, Umrath F, et al. Effects of alfacalcidol on bone markers and bone mineral density in alendronate-treated postmenopausal women with osteopenia or osteoporosis: one year interim analysis of the ALFA study [abstract]. Osteoporosis International 2007;18(Suppl 1):S90-1. [Google Scholar]
- Felsenberg D, Bock O, Borst H, Armbrecht G, Beller G, Degner C, et al. Additive effect of alfacalcidol on bone mineral density and bone strength in alendronate treated postmenopausal women with reduced bone mass. Journal of Musculoskeletal & Neuronal Interactions 2011;11(1):34-45. [PubMed] [Google Scholar]
Aloia 2005 {published data only}
- Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Archives of Internal Medicine 2005;165(14):1618-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar SA, Aloia JF, Pollack S, Yeh J. Oral vitamin D3 supplementation in postmenopausal African American women. Calcified Tissue International 2004;74(Suppl 1):S102. [Google Scholar]
- Talwar SA, Aloia JF, Pollack S, Yeh J. Oral vitamin D3 supplementation in postmenopausal African American women. Journal of Bone and Mineral Research 2004;19(Suppl 1):S324. [Google Scholar]
Baeksgaard 1998 {published data only}
- Baeksgaard L, Andersen KP, Hylstrup L. Calcium and vitamin supplementation increases spinal BMD in healthy, postmenopausal women. Osteoporosis International 1998;8:255-60. [DOI] [PubMed] [Google Scholar]
Binder 1995 {published data only}
- Binder EF. Implementing a structured exercise program for frail nursing home residents with dementia. Journal of Aging and Physical Activity 1995;3:383-95. [Google Scholar]
Binkley 2007 {published data only}
- Binkley N, Recker R, Holst A, Walliser J, Lips P, Pfeifer M. Treatment effects of vitamin D3 8400IU once weekly on elderly subjects with vitamin D insufficiency. Osteoporosis International 2007;18(Suppl 1):S118. [Google Scholar]
Brazier 2005 {published data only}
- Brazier M, Grados F, Kamel S, Mathieu M, Morel A, Maamer M, et al. Clinical and laboratory safety of one year's use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomized, double-blind, placebo-controlled study. Clinical Therapeutics 2005;27(12):1885-93. [DOI] [PubMed] [Google Scholar]
Broe 2007 {published data only}
- Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. Journal of the American Geriatrics Society 2007;55(2):234-9. [DOI] [PubMed] [Google Scholar]
- Kiel DP, Broe KE, Chen TC, Cupples LA, Bischoff-Ferrari H, Holick MF. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, placebo-controlled, multiple dose study. Journal of Bone and Mineral Research 2004;19(Suppl 1):S462-3. [Google Scholar]
Bunout 2006 {published data only}
- Bunout D, Barrera G, Leiva L, Gattas V, la Maza MP, Avendano M, et al. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Experimental Gerontology 2006;41(8):747-52. [DOI] [PubMed] [Google Scholar]
Chen 1997 {published data only}
- Chen JT, Shiraki M, Hasumi K, Tanaka N, Katase K, Kato T, et al. 1-alpha-hydroxyvitamin D3 treatment decreases bone turnover and modulates calcium-regulating hormones in early postmenopausal women. Bone 1997;20:557-62. [DOI] [PubMed] [Google Scholar]
Chevalley 1994 {published data only}
- Chevalley T, Rizzoli R, Nydegger V, Slosman D, Rapin C-H, Michel J-P, et al. Effects of calcium supplements on femoral bone mineral density and vertebral fracture rate in vitamin-D-replete elderly patients. Osteoporosis International 1994;4:245-52. [DOI] [PubMed] [Google Scholar]
Cooper 2003 {published data only}
- Cooper L, Clifton-Bligh PB, Nery ML, Figtree G, Twigg S, Hibbert E, et al. Vitamin D supplementation and bone mineral density in early postmenopausal women. American Journal of Clinical Nutrition 2003;77:1324-9. [DOI] [PubMed] [Google Scholar]
- Cooper L, Figtree G, Nery L, Twigg S, Hibbert E, Clifton-Bligh P, et al. Vitamin D supplementation and bone mineral density in early postmenopausal women. Journal of Bone and Mineral Research 1999;14(Suppl 1):S538. [DOI] [PubMed] [Google Scholar]
Corless 1985 {published data only}
- Corless D, Dawson E, Fraser F, Ellis M, Evans SJW, Perry JD, et al. Do vitamin D supplements improve the physical capabilities of elderly hospital patients? Age and Ageing 1985;14:76-84. [DOI] [PubMed] [Google Scholar]
Daly 2006 {published data only}
- Daly RM, Bass S, Nowson C. Long-term effects of calcium-vitamin-D3-fortified milk on bone geometry and strength in older men. Bone 2006;39(4):946-53. [DOI] [PubMed] [Google Scholar]
- Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium and vitamin D3 fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomised controlled trial. Journal of Bone and Mineral Research 2005;20(Suppl 1):S97. [DOI] [PubMed] [Google Scholar]
- Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomized controlled trial. Journal of Bone and Mineral Research 2006;21(3):397-405. [DOI] [PubMed] [Google Scholar]
- Kukuljan S, Daly RM, Bass SL, Sanders K, Nicholson GC, Turner CH, et al. Does calcium-vitamin D3 fortified milk enhance the effects of exercise on BMD in older men: an 18 month randomised controlled trial. Journal of Bone and Mineral Research 2006;21(Suppl 1):S184. [Google Scholar]
Dawson‐Hughes 1991 {published data only}
- Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Annals of Internal Medicine 1991;115:505-12. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 1995 {published data only}
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE, Falconer G, Green CL. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. American Journal of Clinical Nutrition 1995;61:1140-5. [DOI] [PubMed] [Google Scholar]
Deroisy 1998 {published data only}
- Deroisy R, Collette J, Chevallier T, Breuil V, Reginster JY. Effects of two 1-year calcium and vitamin D3 treatments on bone remodeling markers and femoral bone density in elderly women. Current Therapeutic Research 1998;59:850-62. [Google Scholar]
Deroisy 2002 {published data only}
- Deroisy R, Collette J, Albert A, Jupsin I, Reginster J-Y. Administration of a supplement containing both calcium and vitamin D is more effective than calcium alone to reduce secondary hyperparathyrodism in postmenopausal women with low 25(OH)vitamin D circulating levels. Aging - Clinical and Experimental Research 2002;14:13-7. [DOI] [PubMed] [Google Scholar]
Dhesi 2004 {published data only}
- Dhesi JK, Jackson SHD, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age and Ageing 2004;33:589-95. [DOI] [PubMed] [Google Scholar]
Doetsch 2004 {published data only}
- Doetsch AM, Faber J, Lynnerup N, Watjen I, Bliddal H, Danneskiold-Samsoe B. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo-controlled study. Calcified Tissue International 2004;75:183-8. [DOI] [PubMed] [Google Scholar]
Francis 1996 {published data only}
- Francis RM, Boyle IT, Moniz C, Sutcliffe AM, Davis BS, Beastall GH, et al. A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporosis International 1996;6:284-90. [DOI] [PubMed] [Google Scholar]
Fujita 1989 {published data only}
- Fujita T, Inoue T, Orimo H, Kumahara Y, Kurokawa K, Takahashi H, et al. Clinical evaluation of the effect of calcitriol on osteoporosis. Multicenter double-blind study using alfacalcitol as the control drug. Igaku No Ayumi - Journal of Clinical and Experimental Medicine 1989;148:833-57. [Google Scholar]
Gallagher 1982 {published data only}
- Gallagher JC, Jerpbak CM, Jee WS, Johnson KA, DeLuca HF, Riggs BL. 1,25-Dihydroxyvitamin D3: short- and long-term effects on bone and calcium metabolism in patients with postmenopausal osteoporosis. Proceedings of the National Academy of Sciences 1982;79:3325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gloth 1995 {published data only}
- Gloth FM III, Smith CE, Hollis BW, Tobin JD. Functional improvement with vitamin D replenishment in a cohort of frail, vitamin D-deficient older people. Journal of the American Geriatrics Society 1995;43:1269-71. [DOI] [PubMed] [Google Scholar]
Grados 2003 {published data only}
- Grados F, Brazier M, Kamel S, Duver S, Heurtebize N, Maamer M, et al. Effects on bone mineral density of calcium and vitamin D supplementation in elderly women with vitamin D deficiency. Joint, Bone, Spine: Revue du Rhumatisme 2003;70(3):203-8. [DOI] [PubMed] [Google Scholar]
Grady 1991 {published data only}
- Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, et al. 1,25 dihydroxy vitamin D3 and muscle strength in the elderly: a randomized clinical trial. Journal of Clinical Endocrinology and Metabolism 1991;73:1111-7. [DOI] [PubMed] [Google Scholar]
Hangartner 1985 {published data only}
- Hangartner TN, Overton TR, Harley CH, den Berg L, Crockford PM. Skeletal challenge: an experimental study of pharmacologically induced changes in bone density in the distal radius, using gamma-ray computed tomography. Calcified Tissue International 1985;37:19-24. [DOI] [PubMed] [Google Scholar]
Harju 1989 {published data only}
- Harju E, Punnonen R, Tuimala R, Salmi J, Paronen I. Vitamin D and calcitonin treatment in patients with femoral neck fracture: a prospective controlled clinical study. The Journal of International Medical Research 1989;17:226-42. [DOI] [PubMed] [Google Scholar]
Heikinheimo 1992 {published data only}
- Harju EJ, Heikinheimo RJ, Haavisto MU, Inkovaara JA, Kaarela RH, Kataja M, et al. Prevention of bone fractures by annual intramuscular injection of ergocalciferol in the aged. British Journal of Surgery 1991;78:1145. [Google Scholar]
- Heikinheimo RJ, Inkovaara JA, Harju EJ, Haavisto MV, Kaarela RH, Kataja JM, et al. Annual injection of vitamin D and fractures of aged bones. Calcified Tissue International 1992;51:105-10. [DOI] [PubMed] [Google Scholar]
Honkanen 1990 {published data only}
- Honkanen R, Alhava E, Parviainen M, Talasniemi S, Monkkonen R. The necessity and safety of calcium and vitamin D in the elderly. Journal of the American Geriatrics Society 1990;38:862-6. [DOI] [PubMed] [Google Scholar]
Hunter 2000 {published data only}
- Hunter D, Major P, Arden N, Swaminathan R, Andrew T, MacGregor AJ, et al. A randomized controlled trial of vitamin D supplementation on preventing postmenopausal bone loss and modifying bone metabolism using identical twin pairs. Journal of Bone and Mineral Research 2000;15(11):2276-83. [DOI] [PubMed] [Google Scholar]
Itami 1982 {published data only}
- Itami Y, Fujita T, Inoue T, Orimo H, Matsuno S, Matsui S, et al. [Effect of alphacalcidol on osteoporosis - a multicentre double-blind study]. [Japanese]. Igaku No Ayumi - Journal of Clinical and Experimental Medicine 1982;123:958-73. [Google Scholar]
Iwamoto 1999 {published data only}
- Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Douchi T, et al. A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy. Maturitas 1999;31:161-4. [DOI] [PubMed] [Google Scholar]
Iwamoto 2000 {published data only}
- Iwamoto J, Takeda T, Ichimura S. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. Journal of Orthopaedic Science 2000;5:546-51. [DOI] [PubMed] [Google Scholar]
Jensen 1982 {published data only}
- Jensen C, Holloway L, Block G, Spiller G, Gildengorin G, Gunderson E, et al. Long-term effects of nutrient intervention on markers of bone remodeling and calciotropic hormones in late-postmenopausal women. American Journal of Clinical Nutrition 2002;75:1114-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jensen GF, Christiansen C, Transbol I. Treatment of post menopausal osteoporosis. A controlled therapeutic trial comparing oestrogen/gestagen, 1,25-dihydroxy-vitamin D3 and calcium. Clinical Endocrinology 1982;16:515-24. [DOI] [PubMed] [Google Scholar]
Jensen 1985 {published data only}
- Jensen GF, Meinecke B, Boesen J, Transbol I. Does 1,25(OH)2D3 accelerate spinal bone loss? A controlled therapeutic trial in 70-year old women. Clinical Orthopaedics and Related Research 1985;(192):215-21. [PubMed] [Google Scholar]
Johnell 2001 {published and unpublished data}
- Johnell O, Billsten M, Sernbo I, Rodine I, Ornstein E. Vitamin D treatment best for the frailest to prevent hip fractures? A prospective controlled clinical trial. Journal of Bone and Mineral Research 2002;16(Suppl 1):S180. [Google Scholar]
- Johnell O. Personal communication 9 September 2004.
Johnson 1980 {published data only}
- Johnson KR, Jobber J, Stonawski BJ. Prophylactic vitamin D in the elderly. Age and Ageing 1980;9:121-7. [DOI] [PubMed] [Google Scholar]
Keane 1998 {published data only}
- Keane EM, Healy M, O'Moore R, Coakley D, Walsh JB. Vitamin D-fortified liquid milk: benefits for the elderly community-based population. Calcified Tissue International 1998;62:300-2. [DOI] [PubMed] [Google Scholar]
Kenny 2003 {published data only}
- Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. Journal of the American Geriatrics Society 2003;51:1762-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krieg 1999 {published data only}
- Krieg MA, Jacquet AF, Bremgartner M, Cuttelod S, Thiebaud D, Burckhardt P. Effect of supplementation with vitamin D3 and calcium on quantitative ultrasound of bone in elderly institutionalized women: a longitudinal study. Osteoporosis International 1999;9(6):483-8. [DOI] [PubMed] [Google Scholar]
Larsen 2004 {published data only}
- Larsen ER, Mosekilde L, Foldspang A. Determinants of acceptance of a community-based program for the prevention of falls and fractures among the elderly. Preventive Medicine 2001;33(2 Pt 1):115-9. [DOI] [PubMed] [Google Scholar]
- Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. Journal of Bone and Mineral Research 2004;19(3):370-8. [DOI] [PubMed] [Google Scholar]
- Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents severe falls in elderly community-dwelling women: a pragmatic population-based 3-year intervention study. Aging Clinical and Experimental Research 2005;17(2):125-32. [DOI] [PubMed] [Google Scholar]
- Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium treatment and environmental adjustment in the prevention of falls and osteoporotic fractures among elderly Danish community residents. Journal of Bone and Mineral Research 2002;17(Suppl 1):S157. [Google Scholar]
Latham 2003 {published data only}
- Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects. Journal of the American Geriatrics Society 2003;51:291-9. [DOI] [PubMed] [Google Scholar]
Meier 2004 {published data only}
- Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation with oral vitamin D3 and calcium during winter season prevents seasonal bone loss: a randomized controlled open-label prospective trial. Journal of Bone and Mineral Research 2004;19:1221-30. [DOI] [PubMed] [Google Scholar]
Moschonis 2006 {published data only}
- Moschonis G, Manios Y. Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. British Journal of Nutrition 2006;96(6):1140-8. [DOI] [PubMed] [Google Scholar]
Nordin 1985 {published data only}
- Nordin BE, Baker MR, Horsman A, Peacock M. A prospective trial of the effect of vitamin D supplementation on metacarpal bone loss in elderly women. American Journal of Clinical Nutrition 1985;42:470-4. [DOI] [PubMed] [Google Scholar]
Ongphiphadhanakul 2000 {published data only}
- Ongphiphadhanakul B, Piaseu N, Sae Tung S, Chailurkit L, Rajatanavin R. Prevention of postmenopausal bone loss by low and conventional doses of calcitriol or conjugated equine estrogen. Maturitas 2000;34:179-84. [DOI] [PubMed] [Google Scholar]
Ooms 1995 {published data only}
- Ooms ME, Poos JC, Bezemer PD, Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double blind trial. Journal of Clinical Endocrinology and Metabolism 1995;80:1052-8. [DOI] [PubMed] [Google Scholar]
Orimo 2011 {published data only}
- Orimo H, Nakamura T, Fukunaga M, Ohta H, Hosoi T, Uemura Y, et al. Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: the Japanese Osteoporosis Intervention Trial (JOINT) - 02. Current Medical Research and Opinion 2011;27(6):1273-84. [DOI] [PubMed] [Google Scholar]
Orwoll 1989 {published data only}
- Orwoll ES, McClung MR, Oviatt SK, Recker RR, Weigel RM. Histomorphometric effects of calcium or calcium plus 25-hydroxyvitamin D3 therapy in senile osteoporosis. Journal of Bone and Mineral Research 1989;4:81-8. [DOI] [PubMed] [Google Scholar]
Patel 2001 {published data only}
- Patel R, Collins D, Bullock S, Swaminathan R, Blake GM, Fogelman I. The effect of season and vitamin D supplementation on bone mineral density: a double-blind crossover study. Osteoporosis International 2000;11(Suppl 1):S28. [DOI] [PubMed] [Google Scholar]
- Patel R, Collins D, Bullock S, Swaminathan R, Blake GM, Fogelman I. The effect of season and vitamin D supplementation on bone mineral density in healthy women: a double-masked crossover study. Osteoporosis International 2001;12:319-25. [DOI] [PubMed] [Google Scholar]
- Patel R, Collins D, Swaminathan R, Blake GM, Fogelman I. The effect of season and vitamin D supplementation on bone mineral density: a double-blind cross-over study. Journal of Bone and Mineral Research 2000;15(Suppl):S315. [Google Scholar]
Pedrosa 2006 {published data only}
- Pedrosa MA, Moreira LD, Barros ER, Kunii I, Lazaretti-Castro M. Cholecalciferol supplementation reverts 25-hydroxyvitamin D (25OHD) insufficiency and increases lower limb muscle strength (LLMS) in elderly people living in long-stay geriatric care (LSC). Osteoporosis International 2006;17(Suppl 2):S229-30. [Google Scholar]
Riera 2003 {published data only}
- Naressi M, Riera G, Ramos J, Marcano L. Bone markers and bone mineral density after two years treatment with alfacalcidol in high remodeling postmenopausal women with low mineral density. Osteoporosis International 2004;15(1 Suppl):S100. [Google Scholar]
- Riera-Espinoza G, Naressi M, Velasquez G, Ramos J, Herrera B. Randomized double blind 12 months study of 1mcg/day of alphacalcidol vs placebo in high bone turnover postmenopausal osteoporosis. Bone 2003;5(Suppl 1):S226. [Google Scholar]
- Riera GS, Naressi M, Velasquez G, Ramos J, Herrera B. Randomized double blind twelve months study of 1mcg/day of alphacalcidol vs placebo in high bone turnover postmenopausal women. Journal of Bone and Mineral Research 2002;17(Suppl 1):S276-7. [Google Scholar]
Riis 1986 {published data only}
- Riis BJ, Thomsen K, Christiansen C. Does 24R,25(OH)2-vitamin D3 prevent postmenopausal bone loss? Calcified Tissue International 1986;39:128-32. [DOI] [PubMed] [Google Scholar]
Shiraki 1985 {published data only}
- Shiraki M, Orimo H, Ito H, Akiguchi I, Nakao J, Takahashi R, et al. Long-term treatment of postmenopausal osteoporosis with active vitamin D3, 1-alpha-hydroxycholecalciferol (1 alpha-OHD3) and 1, 24 Dihydroxycholecalciferol (1,24(OH)2D3). Endocrinologia Japonica 1985;32:305-15. [DOI] [PubMed] [Google Scholar]
Shiraki 2004 {published data only}
- Shiraki M, Fukuchi M, Kiriyama T, Okamoto S, Ueno T, Sakamoto H, et al. Alfacalcidol reduces accelerated bone turnover in elderly women with osteoporosis. Journal of Bone and Mineral Metabolism 2004;22:352-9. [DOI] [PubMed] [Google Scholar]
Son 2001 {published data only}
- Son SM, Chun YN. Effect of oral therapy with alphacalcidol or calcium in Korean elderly women with osteopenia and low dietary calcium. Nutrition Research 2001;21:1347-55. [Google Scholar]
Sorensen 1977 {published data only}
- Sorensen OH, Anderson RB, Christensen MS, Friis T, Hjorth L, Jorgensen FS, et al. Treatment of senile osteoporosis with 1 alpha-hydroxyvitamin D3. Clinical Endocrinology 1977;7 Suppl:169s-75s. [DOI] [PubMed] [Google Scholar]
Sosa 2000 {published data only}
- Sosa M, Lainez P, Arbelo A, Navarro MC. The effect of 25-dihydroxyvitamin D on the bone mineral metabolism of elderly women with hip fracture. Rheumatology 2000;39:1263-8. [DOI] [PubMed] [Google Scholar]
Thomsen 1986 {published data only}
- Thomsen K, Riis B, Christiansen C. Effect of estrogen/gestagen and 24R,25-dihydroxyvitamin D3 therapy on bone formation in postmenopausal women. Journal of Bone and Mineral Research 1986;1:503-7. [DOI] [PubMed] [Google Scholar]
Ushiroyama 1995 {published data only}
- Ushiroyama T, Okamura S, Ikeda A, Ueki M. Efficacy of ipriflavone and 1-alpha vitamin D therapy for cessation of vertebral bone loss. International Journal of Gynaecology and Obstetrics 1995;48:283-8. [DOI] [PubMed] [Google Scholar]
Ushiroyama 2002 {published data only}
- Ushiroyama T, Ikeda A, Ueki M. Effect of continuous combined therapy with vitamin K2 and vitamin D3 on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas 2002;41:211-21. [DOI] [PubMed] [Google Scholar]
Zhu 2006 {published data only}
- Zhu K, Dick I, Devine A, Bruce D, Prince RL. An RCT of vitamin D or placebo on falls in elderly women with low vitamin D status and a falling history. Journal of Bone and Mineral Research 2006;21(Suppl 1):S60. [Google Scholar]
- Zhu K, Dick L, Devine A, Bruce D, Prince RL. An RCT of the effects of ergocalciferol and calcium on PTH and bone structure in elderly women with low vitamin D status. Journal of Bone and Mineral Research 2006;21(Suppl 1):S308. [Google Scholar]
References to studies awaiting assessment
Bischoff‐Ferrari 2010a {published data only}
- Bischoff-Ferrari HA, Dawson-Hughes B, Platz A, Orav EJ, Stahelin HB, Willett WC, et al. Effect of high-dosage cholecalciferol and extended physiotherapy on complications after hip fracture. A randomized controlled trial. Archives of Internal Medicine 2010;170(9):813-20. [DOI] [PubMed] [Google Scholar]
Lappe 2007 {published and unpublished data}
- Lappe J, Davies K, Travers-Gustafson D, Haynatzki G, Heaney R, Recker R. Calcium and vitamin D supplementation improves bone health in a population-based sample of postmenopausal women [abstract]. Journal of Bone and Mineral Research 2007;22(Suppl 1):S320. [Google Scholar]
- Lappe J. Personal communication 10 September 2007.
- Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP. Vitamin D status in a rural postmenopausal female population. Journal of the American College of Nutrition 2006;25(5):395-402. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Barger-Lux MJ, Davies KM, Heaney RP, Recker RR. Population-based evidence that serum 25(OH)D levels below 80nmol/L reflect vitamin D deficiency [abstract]. Journal of Bone and Mineral Research 2005;20(Suppl 1):S379. [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. American Journal of Clinical Nutrition 2007;85(6):1586-91. [DOI] [PubMed] [Google Scholar]
- Zhao L-J, Zhou Y, Bu F, Travers-Gustafson D, Ye A, Xu X, et al. Factors predicting vitamin D response variation in non-Hispanic white postmenopausal women. Journal of Clinical Endocrinology and Metabolism 2012;97(8):2699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhao L-J, Watson P, Zhang Q, Lappe JM. The effect of calcium and vitamin D supplementation on obesity in postmenopausal women: secondary analysis for a large-scale placebo controlled, double-blind, 4-year longitudinal trial. Nutrition and Metabolism 2010;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuti 2006 {published data only}
- Nuti R, Bianchi G, Brandi ML, Caudarella R, D'Erasmo E, Fiore C, et al. Efficacy and safety of alfacalcidol compared to vitamin D plus calcium in post menopausal osteoporosis [abstract]. Osteoporosis International 2005;16(Suppl 3):S75-6. [Google Scholar]
- Nuti R, Bianchi G, Brandi ML, Caudarella R, D'Erasmo E, Fiore C, et al. Superiority of alfacalcidol compared to vitamin D plus calcium in lumbar bone mineral density in postmenopausal osteoporosis. Rheumatology International 2006;26(5):445-53. [DOI] [PubMed] [Google Scholar]
Orimo 1987 {published data only}
- Orimo H, Shiraki M, Hayashi T, Nakamura T. Reduced occurrence of vertebral crush fractures in senile osteoporosis treated with 1 alpha (OH)-vitamin D3. Bone and Mineral 1987;3:47-52. [PubMed] [Google Scholar]
Papaioannou 2011 {published data only}
- Papaioannou A, Kennedy CC, Giangregorio L, Ioannidis G, Pritchard J, Hanley DA, et al. A randomized controlled trial of vitamin D dosing strategies after acute hip fracture: no advantage of loading doses over daily supplementation. BMC Muscloskeletal Disorders 2011;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petkakov 1995 {published data only}
- Petkakov M, Popovic V, Milic D, Damjanovic S, Manojlovic D, Micic J. Treatment of postmenopausal osteoporosis with 1-alpha(OH)D3 [Lecenje postmenopauzne osteoporoze sintetskim analogom vitamina de-tri]. Srpski Arhiv za Celokupno Lekarstvo 1995;123(7-8):171-3. [PubMed] [Google Scholar]
Sato 1997 {published data only}
- Sato Y, Maruoka H, Oizumi K. Amelioration of hemiplegia-associated stroke osteopenia more than four years after stroke by 1-alpha-hydroxyvitamin D3 and calcium supplementation. Stroke 1997;28:736-9. [DOI] [PubMed] [Google Scholar]
Sato 1999a {published data only}
- Sato Y, Manabe S, Kuno H, Oizumi K. Amelioration of osteopenia and hypovitaminosis D by 1-alpha-hydroxyvitamin D3 in elderly patients with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 1999;66:64-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 1999b {published data only}
- Sato S, Kuno H, Kaji M, Saruwatari N, Oizumi K. Effect of ipriflavone on bone in elderly hemiplegic stroke patients with hypovitaminosis D. American Journal of Physical Medicine and Rehabilitation 1999;78:457-63. [DOI] [PubMed] [Google Scholar]
Sato 2005 {published data only}
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovascular Diseases 2005;20(3):187-92. [DOI] [PubMed] [Google Scholar]
TIDE 2012 {published data only}
- The TIDE trial investigators. Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE). Diabetologia 2012;55:36-45. [DOI] [PubMed] [Google Scholar]
Wood 2012 {published data only}
- Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. Journal of Clinical Endocrinology and Metabolism 2012;97:3557-68. [DOI] [PubMed] [Google Scholar]
Xia 2009 {published data only}
- Xia W-B, Zhang Z-L, Wang H-F, Meng X-W, Zhang Y, Zhu G-Y, et al. The efficacy and safety of calcitriol and/or Caltrate D in elderly Chinese women with low bone mass. Acta Pharmacologica Sinica 2009;30(3):372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ANVITAD {published data only}
- Lopez-Torres Hidalgo J, ANVITAD Group. Prevention of falls and fractures in old people by administration of calcium and vitamin D. Randomized clinical trial. BMC Public Health 2011;11:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
REVITAHIP {published data only}
- Mak J. Replenishment of Vitamin D in Hip fractured Patients (REVITAHIP) Trial. http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12610000392066 (accessed 27 March 2012).
- Mak JC, Cameron ID, Mason RS, Klein L, Soong M, Ohn K. Improving mobility and reducing disability in older people through early high-dose vitamin D replacement following hip fracture (the REVITAHIP trial): preliminary results. Osteoporosis International 2011;22(Suppl 4):S586-7. [Google Scholar]
Additional references
Abrahamsen 2013
- Abrahamsen B, Avenell A, Bolland M, Rejnmark L, MacLennan GS, Macdonald HM, et al. Commentary, a pooled analysis of vitamin D dose requirements for fracture prevention. BoneKEy 2013;10:256. [Google Scholar]
Autier 2007
- Autier P, Gandini S. Vitamin D supplementation and total mortality. A meta-analysis of randomized controlled trials. Archives of Internal Medicine 2007;167(16):1730-7. [DOI] [PubMed] [Google Scholar]
Avenell 2009a
- Avenell A, Gillespie WJ, Gillespie LD, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD000227. [DOI: 10.1002/14651858.CD000227.pub3] [DOI] [PubMed] [Google Scholar]
Bacon 2009
- Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporosis International 2009;20:1407-15. [DOI] [PubMed] [Google Scholar]
Bergman 2010
- Bergman GJ, Fan T, McFetridege JT, Sen SS. Efficacy of vitamin D3 supplementation in preventing fractures in elderly women: a meta-analysis. Current Medical Research and Opinion 2010;26:1193-201. [DOI] [PubMed] [Google Scholar]
Binkley 2011
- Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. Journal of Clinical Endocrinology and Metabolism 2011;96:981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2009a
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009;339:b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2009b
- Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav J, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency. Archives of Internal Medicine 2009;169:551-61. [DOI] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2010
- Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporosis International 2010;21:1121-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2012
- Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin dose requirements for fracture prevention. New England Journal of Medicine 2012;367:40-9. [DOI] [PubMed] [Google Scholar]
Bjelakovic 2014
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD007470. [DOI: 10.1002/14651858.CD007470.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 1995
- Black BM, Palermo L, Nevitt MC, Genant HK, Epstein R, San-Valentin R, et al. Comparison of methods for defining prevalent vertebral deformities: the study of osteoporotic fractures. Journal of Bone and Mineral Research 1995;10:890-902. [DOI] [PubMed] [Google Scholar]
Bolland 2010
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplementation on the risk of myocardial infarction and cardiovascular events: a meta-analysis. BMJ 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2011a
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2011b
- Bolland MJ, Grey A, Gamble GG, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. American Journal of Clinical Nutrition 2011;94:1144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 2012
- Cameron ID, Gillespie LD, Robertson MC, Murray GR, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD005465. [DOI: 10.1002/14651858.CD005465.pub3] [DOI] [PubMed] [Google Scholar]
Campbell 2012
- Campbell MK, Piaggio G, Elbourne DR, Altman DG, for the CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
Cauley 2012
- Cauley J, Wactawski-Wende J, Robbins J, Rodabough R, Chen Z, Johnson K, et al. The Women's Health Initiative (WHI) calcium and vitamin D supplementation trial: health outcomes 5 years after trial completion. In: ASBMR Annual Meeting; 2012 Oct 12-15; Minneapolis, Minnesota. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=6450bf3e-c670-4dc0-814c-8842e23cd80e. (accessed 27 December 2012).
Chung 2011
- Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with and without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. preventive services task force. Annals of Internal Medicine 2011;155:827-38. [DOI] [PubMed] [Google Scholar]
Cooper 2011
- Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporosis International 2011;22:1277-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cumming 1990
- Cumming RG. Calcium intake and bone mass. A quantitative review of the evidence. Calcified Tissue International 1990;47:194-201. [DOI] [PubMed] [Google Scholar]
Cummings 1995
- Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. New England Journal of Medicine 1995;332:767-73. [DOI] [PubMed] [Google Scholar]
Cummings 2002
- Cummings SR, Melton LJ III. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761-7. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 2000
- Dawson-Hughes B, Harriss SS, Krall EA, Dallal GE. Effect of withdrawal of calcium and vitamin D supplements on bone-mass in elderly men and women. American Journal of Clinical Nutrition 2000;72(3):745-50. [DOI] [PubMed] [Google Scholar]
Dawson‐Hughes 2010
- Dawson-Hughes B, Harris SS. High-dose vitamin D supplementation. Too much of a good thing? JAMA 2010;303:1861-2. [DOI] [PubMed] [Google Scholar]
DIPART 2010
- The DIPART (vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 2010;340:b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dyer 2004
- Dyer CA, Taylor GJ, Reed M, Dyer CA, Robertson DR, Harrington R. Falls prevention in residential care homes: a randomised controlled trial. Age and Ageing 2004;33:596-602. [DOI] [PubMed] [Google Scholar]
Gallagher 2012
- Gallagher JC, Sai A, Templin T, Smith L. Dose response to vitamin D supplementation in postmenopausal women. A randomized trial. Annals of Internal Medicine 2012;156:425-37. [DOI] [PubMed] [Google Scholar]
Gillespie 2012
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD007146. [DOI: 10.1002/14651858.CD007146.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011a
- Higgins JPT, Green S, editors. Example of incorporating a cluster-randomized trial. Cochrane handbook for Systematic Review of Interventions 5.1.0 [updated March 2011].Section 16.3.5. In: The Cochrane Library, Issue 1, 2014. Chichester, UK: John Wiley & Sons, Ltd.
Homik 1998
- Homik J, Suarez-Almazor ME, Shea B, Cranney A, Wells GA, Tugwell P. Calcium and vitamin D for corticosteroid-induced osteoporosis. Cochrane Database of Systematic Reviews 1998, Issue 2. Art. No: CD000952. [DOI: 10.1002/14651858.CD000952] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houghton 2006
- Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. American Journal of Clinical Nutrition 2006;84(4):694-7. [DOI] [PubMed] [Google Scholar]
Institute of Medicine 2010
- Committee to review dietary reference intakes for vitamin D and calcium. Dietary reference intakes for calcium and Vitamin D. http://www.nap.edu/catalog.php?record_id=13050 (accessed 4th April 2014) 2010.
Ish‐Shalom 2008
- Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg L, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. Journal of Clinical Endocrinology and Metabolism 2008;93:3430-5. [DOI] [PubMed] [Google Scholar]
Jacobsen 1990
- Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Regional variation in the incidence of hip fracture. US white women aged 65 years and older. Journal of the American Medical Association 1990;264:500-2. [PubMed] [Google Scholar]
Johnell 2004
- Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporosis International 2004;15:897-902. [DOI] [PubMed] [Google Scholar]
Kalyani 2010
- Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews D. Vitamin D treatment for the prevention of falls in older adults. Journal of the American Geriatrics Society 2010;58:1299-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanis 2003
- Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone 2003;32:468-73. [DOI] [PubMed] [Google Scholar]
Kanis 2012
- Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporosis International 2012;23:2239-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khaw 1994
- Khaw K-T, Scragg R, Murphy S. Single dose cholecalciferol suppresses the winter increase in parathyroid hormone concentrations in healthy older men and women: a randomized trial. American Journal of Clinical Nutrition 1994;59:1040-4. [DOI] [PubMed] [Google Scholar]
Lai 2010
- Lai JK, Lucas RM, Clements MS, Roddam AW, Banks E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health 2010;10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 1995
- Lau EMC, Gillespie WJ, Valenti L, O'Connell DL. The seasonality of hip fracture and its relationship with weather conditions in New South Wales. Australian Journal of Public Health 1995;19:76-80. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Lips 1999
- Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporosis International 1999;9:394-7. [DOI] [PubMed] [Google Scholar]
Lips 2006
- Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. [erratum appears in J Intern Med. 2007 Apr;261(4):408]. Journal of Internal Medicine 2006;260(3):245-54. [DOI] [PubMed] [Google Scholar]
Mak 2010
- Mak JC. High-dose oral vitamin D supplementation and risk of falls in older women. JAMA 2010;304(8):854. [DOI] [PubMed] [Google Scholar]
Murad 2011
- Murad MH, Elamin KB, Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, et al. The effect of vitamin D on falls: a systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism 2011;96:2997-3006. [DOI] [PubMed] [Google Scholar]
Murad 2012
- Murad MH, Drake MT, Mullan RJ, Mauck KF, Stuart LM, Lane MA, et al. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. Journal of Clinical Endocrinology and Metabolism 2012;97:1871-80. [DOI] [PubMed] [Google Scholar]
Nevitt 1998
- Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Annals of Internal Medicine 1998;128(10):793-800. [DOI] [PubMed] [Google Scholar]
Norman 1993
- Norman AW, Henry HL. Vitamin D: metabolism and mechanism of action. In: Favus MJ, editors(s). Primer on the metabolic bone diseases and disorders of mineral metabolism. 2nd edition. New York: Raven Press, 1993:63-70. [Google Scholar]
O'Donnell 2008
- O'Donnell S, Moher D, Thomas K, Hanley DA, Cranney A. Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. Journal of Bone and Mineral Metabolism 2008;26:531-42. [DOI] [PubMed] [Google Scholar]
Pekkarinen 2010
- Pekkarinen T, Valimaki VV, Aarum S, Turpeinen U, Hamalainen E, Loyttyniemi E, et al. The same annual dose of 292000 IU of vitamin D3 (cholecalciferol) on either daily or four monthly basis for elderly women: 1-year comparative study of the effects on serum 25(OH)D3 concentrations and renal function. Clinical Endocrinology 2010;72:455-61. [DOI] [PubMed] [Google Scholar]
Pieper 2007
- Pieper CF, Colon-Emeric C, Caminis J, Betchyk K, Zhang J, Janning C, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. American Journal of Geriatric Pharmacotherapy 2007;5(4):335-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rejnmark 2012
- Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. Journal of Clinical Endocrinology and Metabolism 2012;97:2670-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Romagnoli 2008
- Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. Journal of Clinical Endocrinology and Metabolism 2008;93(8):3015-20. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seeman 2007
- Seeman E, Compston J, Adachi J, Brandi ML, Cooper C, Dawson-Hughes B, et al. Non-compliance: the Achilles' heel of anti-fracture efficacy. Osteoporosis International 2007;18(6):711-9. [DOI] [PubMed] [Google Scholar]
Tang 2007
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 2007;370:657-66. [DOI] [PubMed] [Google Scholar]
Van Staa 2001
- Van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001;29(6):517-22. [DOI] [PubMed] [Google Scholar]
Vieth 2001
- Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. American Journal of Clinical Nutrition 2001;73:288-94. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Avenell 2005
- Avenell A, Gillespie WJ, Gillespie LD, O'Connell DL. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD000227. [DOI: 10.1002/14651858.CD000227.pub2] [DOI] [PubMed] [Google Scholar]
Avenell 2009b
- Avenell A, Gillespie WJ, Gillespie LD, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD000227. [DOI: 10.1002/14651858.CD000227.pub3] [DOI] [PubMed] [Google Scholar]
Gillespie 1996
- Gillespie WJ, Henry DA, O'Connell DL, Robertson J. Vitamin D and Vitamin D analogues in the prevention of fractures in involutional and post-menopausal osteoporosis. Cochrane Database of Systematic Reviews 1996, Issue 3. [Google Scholar]
Gillespie 2000
- Gillespie WJ, Henry DA, O'Connell DL, Robertson J. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI] [PubMed] [Google Scholar]
Gillespie 2001
- Gillespie WJ, Avenell A, Henry DA, O'Connell DL, Robertson J. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI] [PubMed] [Google Scholar]


