Abstract
Background and Purpose:
Therapeutic strategies that capitalize on the intrinsic capacity for neurological recovery early post-stroke to improve locomotion are uncertain. Emerging data suggest that task-specific stepping practice provided at higher cardiovascular intensities may be critical dosage parameters that could maximize locomotor recovery. The purpose of this investigation was to determine the comparative effectiveness of providing high-intensity training on locomotor capacity early post-stroke as compared to usual care.
Methods:
A quasi-experimental design was used to compare changes in stepping activity (StepWatch®), walking, and balance outcomes during usual care (n=56) versus high-intensity stepping intervention (n=54) in inpatient stroke patients. Primary outcomes assessed weekly included self-selected and fastest gait speed (SSS, FS), 6-minute walk test (6MWT) and the Berg Balance Scale (BBS), with secondary outcomes of Swedish Postural Assessment Scale for Stroke-Norwegian version, Functional Ambulation Category (FAC), 30-s sit-to-stand, strength (average manual muscle testing) and Barthel Index. Regression analyses identified relationships between demographics, baseline function, and training activities (steps/day, duration achieved 70–85% maximum heart rates) and primary outcomes at discharge.
Results:
Following implementation of high-intensity stepping, average steps/day (5777±2784) was significantly greater than during usual care (3917±2656, p<0.001). Statistically different and clinically meaningful changes in SSS (0.39±0.28 vs 0.16±0.26 m/s) and FS (0.47±0.41 vs 0.17±0.38 m/s, both p<0.001) were observed following high-intensity interventions vs usual care, and at every assessment throughout the length of stay. Changes in BBS and 6MWT were also statistically and clinically different between groups, while secondary measures of FAC and strength were also different at discharge. Primary predictors of improved walking capacity were steps/day, baseline impairments, and age.
Conclusion:
Provision of high-intensity stepping training applied during inpatient rehabilitation resulted in significantly greater walking balance outcomes. This training paradigm should be further tested in other contexts to determine the generalizability to real world and community settings.
Keywords: locomotion, rehabilitation, exercise
Introduction
While the majority of people with stroke recover some level of independent walking function, deficits in strength and postural stability often limit mobility in the home or community1–3. Improving community mobility is a priority during stroke rehabilitation, as reduced physical activity is associated with decreased health and increased health-care costs4. Specific thresholds of locomotor capacity (i.e., gait speed) are associated with greater levels of community mobility3, which is critical given the increasing stroke incidence in an aging population.
To improve locomotor capacity, many interventions are employed clinically, although the efficacy of most strategies is uncertain. Recent practice guidelines5, 6 encourage provision of task-specific walking training at higher cardiovascular intensities to improve both cardiovascular health and function. Previous controlled studies also suggest the amount and intensity of stepping training are related to gains in gait speed and distance7, 8. However, these trials were performed in patients >1-month post-stroke, and data from animal models suggest earlier interventions can elicit greater improvements9.
Despite this potential, most observational studies indicate patients early post-stroke receive limited amounts of stepping practice (250–500 steps/session or steps/day10–12) at low aerobic intensities, reaching aerobic thresholds <5% of sessions13–15. While many barriers to clinical translation exist, the safety of high-intensity training during inpatient rehabilitation has recently been addressed. Specifically, patients receiving inpatient stroke rehabilitation performed ~1500 steps/day and achieved higher intensities (~40% of sessions), with substantial gains in locomotor and non-locomotor outcomes, and no increased incidence of adverse events16.
While promising, the limitations of those findings include the lack of a control intervention to evaluate the efficacy of high-intensity training early post-stroke. Strategies to assess intervention efficacy typically involve randomized controlled trial designs, although their utility during inpatient rehabilitation may raise concerns. To begin, evidence for interventions is typically generated in a laboratory setting, and, if results are positive, are often referred to as “evidence-based interventions”. A major goal is to implement evidence-based interventions into the clinical setting once substantial data are generated to support its efficacy. However, when evidence-based interventions are studied during subacute rehabilitation they are often provided in addition to usual care, and the subsequent efficacy of the intervention alone is uncertain due to the clinical therapy provided, which is often not well-described or documented17, 18. To understand the impact of the intervention, concerted efforts of the rehabilitation team are required to ensure the evidence-based interventions are delivered in manner that is consistent to how they were studied (i.e., fidelity). This may necessitate replacement of existing, less effective practice patterns (i.e., de-implementation)19 given the fiscal constraints that limit provision of additional therapy. Most research paradigms do not address implementation strategies needed to integrate evidence-based interventions into clinical care, although such strategies are critical to facilitate adaptation of evidence-based practice in the clinical setting.
To mitigate some of these concerns, the present study details a comparative effectiveness trial using a historical control group to evaluate the benefits of high-intensity training during inpatient stroke rehabilitation. Using a quasi-experimental design, standardized measures and daily stepping activity were systematically collected over a 10-month period in patients with subacute stroke during inpatient rehabilitation, while therapists provided their typical rehabilitation interventions (i.e. usual care). Following specific implementation strategies,20, 21 therapists then attempted to provide high-intensity training during clinical inpatient rehabilitation over the subsequent 11 months, while continuing to monitor outcomes and stepping activity. The specific aims were to: 1) assess the stepping amounts and associated functional outcomes achieved during usual care in inpatient rehabilitation; and 2) assess the impact of high-intensity training on functional outcomes early post-stroke. We hypothesized that the patients who received high-intensity interventions would have greater gains in primary outcomes than those receiving usual care.
Materials and Methods
Because of the sensitive nature of the data collected for this study, as well as consent form restrictions and health privacy laws, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Study Sample and Design
The study represents a quasi-experimental pre-post design22 in which outcomes and stepping activity were monitored prior to and following implementation of high-intensity training interventions during standard clinical care. Two separate inpatient rehabilitation units in Oslo, Norway, that admit patients with many different conditions and diagnoses participated in this study. Oslo University Hospital (OUH) is a 21-bed specialist health service (5.0 physiotherapy positions). Oslo Municipality Services (OMS) is a 23-bed enhanced rehabilitation unit (5.0 physiotherapy positions) within Oslo’s primary health service. To qualify for treatment, patients required services from >1 specialty (physical, occupational, or speech therapy). However, the two sites represent different levels of care in the Norwegian health system, and patients at OUH required services only provided at a specialty hospital. Often, patients undergoing stroke rehabilitation received inpatient care from the specialty hospital as well as the primary health services. Data were collected as part of standard rehabilitation services, which includes one 45–60 minute, physical therapy (PT) session/day, although the time of PT sessions varied daily to accommodate for all rehabilitation services offered. The project was approved by the Southeastern Norway Ethics Committee (approval 2016/873). All participants provided written informed consent. Patients were enrolled in the usual care data collection phase from February-November 2017. Following this phase, implementation activities (including clinician training, strategies to overcome barriers, etc.) occurred in November–December 2017. Patients were enrolled in the high-intensity training phase from January-November 2018. While the assessments and interventions were offered to all patients undergoing stroke rehabilitation, data were included in the analysis if patients provided consent for his/her data to be used in research.
Adults (≥18 years old) who sustained a stroke within the previous 2 months and were receiving inpatient stroke rehabilitation on either unit with goals to improve walking function were included. Exclusion criteria were: FAC scores of 5; BBS score on item #3 (sitting balance) ≤ 2 at admission; the inability to provide consent; use of bracing or instrumentation that limited walking (e.g. ventilator); uncontrolled cardiopulmonary, metabolic, infectious or psychiatric disorders; or previous history of orthopedic or additional neurologic injury that limited walking > 50 m prior to stroke.
Outcomes
Both usual care and high-intensity training phases included collection of outcomes and daily stepping activity for participants throughout inpatient rehabilitation. Clinicians were trained to administer a standardized assessment battery prior to the usual-care phase. Weekly assessments included the 10-meter walk test at self-selected (SSS) and fastest possible speeds (FS), 6-minute walk test (6MWT), and the Berg Balance Scale (BBS). Secondary outcomes tested at admission and discharge included the Swedish Postural Assessment Scale for Stroke-Norwegian Version (SWEPASS-NV), number of sit-to-stand transfers in 30 sec (STS), Barthel Index (Barthel), and Functional Ambulation Capacity (FAC). Additional secondary measures included Patient Reported Outcome Measures Information Systems (PROMIS) scores from anxiety, depression, fatigue and sleep short-forms. Daily stepping activity was recorded during the patients’ waking hours using the StepWatch (Modus Inc, Washington DC) worn on the paretic ankle, with validation studies performed previously.23–25 Steps/session were also estimated, and steps/min calculated as minutes during sessions when stepping practice occurred (≥10 steps/min).
Interventions
Treatments provided during usual care were unaffected by study participation, as therapists were not adequately trained to deliver high-intensity training. A one-day educational session was provided ~8 months prior to starting data collection (June 2016), although managerial staff verified no changes in interventions occurred. Most efforts during usual care focused on processes to ensure documentation of outcomes and collection of stepping data.
During the implementation phase, the research team and clinicians collaborated to ensure delivery of the high-intensity stepping program during clinical practice. Therapists focused on prioritizing stepping practice at higher aerobic intensities during scheduled treatments.7 The amount of personnel or time allotted to physical therapy sessions was consistent with the usual care phase (i.e. no additional personnel or time resources allocated for high-intensity training). The details of this training paradigm have been described previously26–28; stepping was performed on treadmills and over ground, with safety harness systems and body-weight support only as needed to ensure successful stepping (e.g., positive step lengths and lack of limb collapse or postural instability). Specific tasks included walking in different directions, over obstacles or on uneven, compliant surfaces, and on stairs/steps. Tasks were progressed by increasing task difficulty as determined by the therapist. Physical assistance was provided as needed to continue stepping but did not focus on normalizing gait kinematics. Accordingly, practice of non-walking tasks performed during physiotherapy, including bed mobility, transfers, and standing balance/pre-gait activities, was limited. Practice of transfer tasks was reserved for family training or during weekly assessments.
A key feature of the intervention was the focus on achieving higher intensities, defined as 70–85% age-predicted maximum heart rate [HRmax; calculated as 211-(age x 0.64)].29 Heart rates were monitored continuously using the OH1 or H10 (Polar, USA). The Borg Rating of Perceived Exertion30 (RPE; 6–20 scale) was also assessed, with scores ≥14 (“somewhat hard”) targeted, and used in situations when targeted HRs were difficult to achieve due to inter-individual differences, medications, or mobility limitations. Peak HRs and RPEs, and amount of time >70% HRmax and ≥14 RPE, were documented each session. Monitoring HRs and RPEs was purposely not performed during usual care to minimize therapists’ desire to change interventions based on previous education regarding the importance of exercise intensity.
Statistical Analysis
All demographic, training and outcome data were tabulated, with missing weekly outcomes imputed from the previous week, and data were not considered if admission scores were not recorded. Data in tables indicate means ± standard deviations with figures depicting means and confidence intervals. Differences in baseline measures were assessed using unpaired comparisons (unpaired t-tests or Mann-Whitney U, Γ2 analyses). Data were tested for normality using Shapiro-Wilk tests. Changes in primary measures (SSS, FS, 6MWT, and BBS) from admission to week 1 and discharge were assessed using two-way, repeated measures ANOVA with intervention and time (repeated) as main factors. Given differences in length of stay (LOS) between the groups (Table 1), additional repeated-measures ANOVAs were performed comparing 3-week (~22 days) outcomes during high-intensity stepping to usual care discharge outcomes (mean 23 days). Unpaired comparisons of change scores at each time point were performed if ANOVAs were significant. Secondary outcomes were compared only between baseline and discharge. We also calculated the percentage of individuals in each phase who achieved SSS thresholds associated with full community ambulation (0.93 m/s)3. The ratio was determined only for those <0.93 m/s at admission, and was determined at week 1, week 3, and discharge.
Table 1.
Demographics, baseline characteristics, and training characteristics.
| Usual care (n=56) | High-intensity Training (n=54) | p-values | |
|---|---|---|---|
| Demographics/baseline characteristics | |||
| Age (years) | 74±14 | 73±10 | p=0.69 |
| Gender (male/female, n) | 29/27 | 35/20 | p=0.25 |
| Paretic side (right/left, n) | 36/18 | 32/24 | p=0.47 |
| Ischemic/hemorrhagic (n) | 41/13 | 41/15 | p=1.00 |
| Duration post-stroke (days) | 15±11 | 13±10 | p=0.30 |
| CCI (a.u.) | 4.3±2.0 | 4.6±2.0 | p=0.58 |
| Modified Rankin Scale (a.u.) | 3.4±0.78 | 3.3±0.87 | p=0.69 |
| Paretic leg strength (a.u.) | 3.4±1.1 | 3.5±1.0 | p=0.72 |
| Training characteristics | |||
| LOS (days) | 23±9.7 | 35±17 | p<0.001 |
| Stepping activity (steps/day) | 3917±2656 | 5776±2784 | p<0.001 |
| Steps/session | 1167±612 | 1866±653 | p<0.001 |
| Steps/min during sessions | 44±10 | 55±10 | p<0.001 |
| Peak HR (% predicted max) | - | 79±8.3 | - |
| Mean HR (% predicted max) | - | 66±7.4 | - |
| Time in HR range (% session) | - | 34±27 | - |
Associations between selected demographic, training, and admission data with primary and secondary outcomes at discharge and change (Δ) scores from admission were assessed with Pearson and Spearman correlation analyses. Additional regression analyses were performed for 3-week outcomes. Stepwise, multiple linear regression models were calculated to estimate the contributions of independent predictors to discharge and change in outcomes. Specific factors included age, gender, duration post-stroke, LOS, type (ischemic or hemorrhagic) or side (right/left/bilateral) of stroke, steps/day, and admission scores for mean paretic leg strength (averaged for muscles tested), BBS, and the primary outcome. Collinearity was monitored, with variance inflation factors <3.0 considered acceptable.
Conditional logistic regressions were performed to evaluate contributions of independent predictors to achieving specific community mobility thresholds. For this analysis, we also computed the differences between admission SSS and 0.93 m/s to determine their contributions towards surpassing this threshold, consistent with previous work31.
Results
Over the 2-year period of time, a total of 350 patients were admitted with the diagnosis of stroke (usual care n = 150; high-intensity stepping n = 200). Data were collected on 110 patients throughout the 2-year period (usual care, n=56; high-intensity stepping, n=54). Approximately 37% of stroke admissions were enrolled during usual care and 27% during high-intensity stepping. The primary reasons for the increased number of exclusions during the high-intensity phase were an increased number of uncontrolled cardiopulmonary, metabolic, infectious or psychiatric disorders. Reasons for exclusion are listed in Supplementary Table 1. Tables 1–2 detail demographic data and admission measures for both patient cohorts, indicating similar baseline impairments and functional capacity. The results are organized by changes in training activities, and therapy-related changes in primary and secondary outcome measures.
Table 2.
Changes in primary and secondary outcome measures in usual care and high-intensity training phases.
| Usual care | High-intensity training | p-value 3-weeks | p-value DC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ADMIT | Week 1 | DC | ADMIT | Week 1 | Week 3 | DC | |||
| SSS (m/s) | 0.62±0.34 | 0.70±0.35 | 0.79±0.34 | 0.64±0.33 | 0.79±0.37 | 0.90±0.44 | 1.0±0.40 | p=0.03 | p<0.001 |
| FS (m/s) | 0.85±0.49 | 0.93±0.46 | 1.0±0.49 | 0.89±0.49 | 1.11±0.51 | 1.21±0.55 | 1.37±0.52 | p=0.02 | p<0.001 |
| 6MWT (m) | 243±138 | 269±1437 | 303±130 | 243±141 | 301±146 | 339±158 | 378±156 | p=0.07 | p<0.001 |
| BBS | 35±15 | 40±13 | 44±11 | 33±16 | 38±15 | 43±14 | 48±11 | p=0.32 | p<0.001 |
| FAC | 2.7±1.3 | -- | 3.9±1.0 | 2.6±1.3 | -- | -- | 4.3±1.0 | -- | p=0.01 |
| Barthel | 12±3.9 | -- | 17±2.9 | 13±4.8 | -- | -- | 18±3.5 | -- | p=0.07 |
| SWE -PASS NV | 28±4.5 | -- | 31±3.1 | 27±6.5 | -- | -- | 32±3.3 | -- | p=0.17 |
| STS | 5.3±4.3 | -- | 8.8±3.6 | 5.7±5.4 | -- | -- | 9.5±4.4 | -- | p=0.66 |
| Strength | 3.4±1.1 | 3.8±1.1 | 3.5±1.0 | -- | -- | 4.3±0.7 | -- | p=0.01 | |
| PROMIS- Anxiety | 49±10 | 48±10 | 48±9.6 | -- | -- | 45±9.5 | -- | p=0.21 | |
| PROMIS- Depression | 48±9.0 | 47±9.2 | 48±8.4 | -- | -- | 45±8.1 | -- | p=0.34 | |
| PROMIS- Fatigue | 46±9.9 | 45±8.9 | 46±9.3 | -- | -- | 44±8.7 | -- | p=0.73 | |
| PROMIS- Sleep | 51±9.8 | 50±8.5 | 52±10 | -- | -- | 47±8.4 | -- | p=0.05 | |
Training Activities
Training activities are detailed in Table 1. Differences in daily stepping (e.g., steps/day) between cohorts were observed, with nearly 1800 greater steps/day during high-intensity stepping vs usual care (5777±2784 vs 3917±2656 steps/day, p< 0.001). Single-day examples of stepping activity at day 7 post-admission in two individuals with similar admission BBS scores are depicted in Fig 1, with dotted lines indicating stepping during the highest frequency stepping hour which we presume represents a physical therapy session. Differences in the amounts and rates of stepping activity during the highest frequency hour are evident between the usual care (Fig 1A) and high-intensity stepping phases (Fig 1B), consistent with average data across participants (Table 1).
Figure 1.
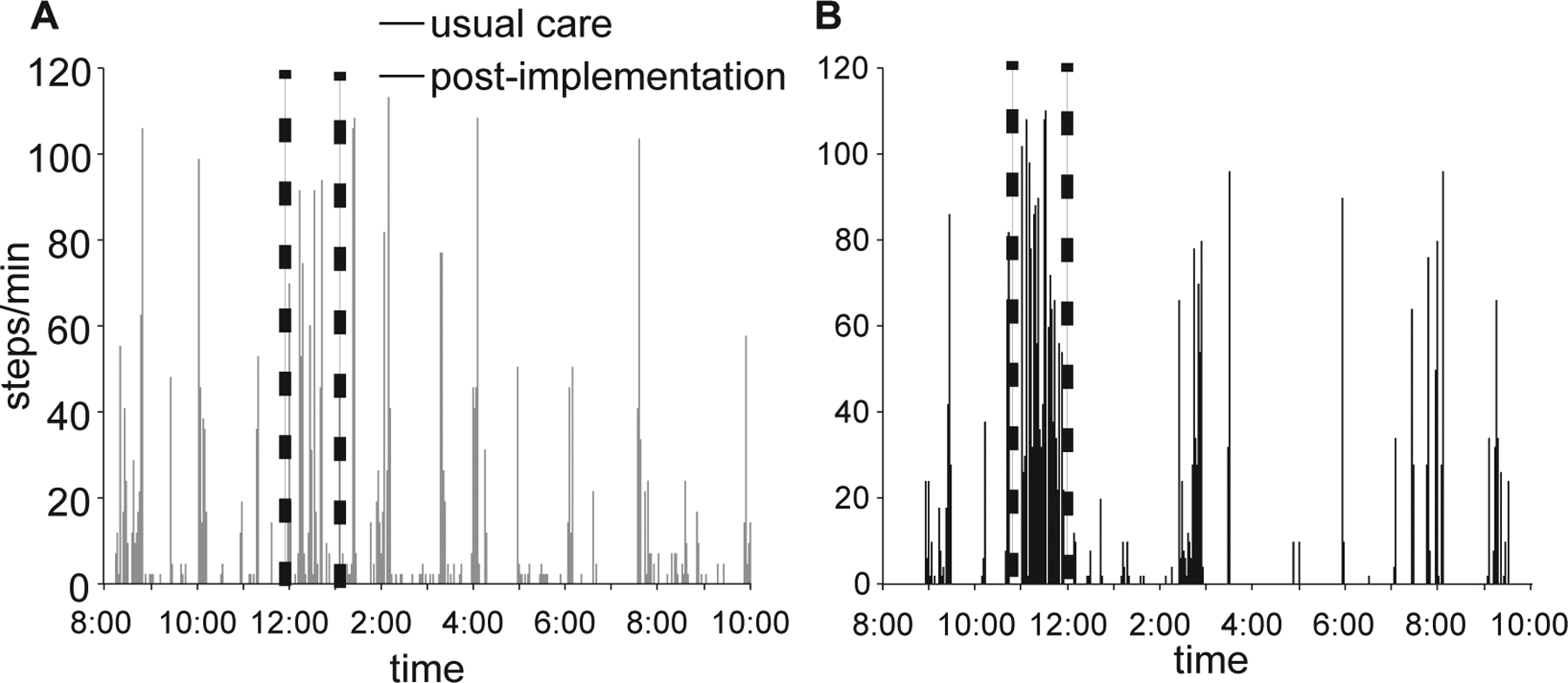
Depiction of single days of daily stepping in two patients with similar admission and walking scores during A) usual care vs B) high-intensity stepping. Dotted lines indicate physical therapy sessions.
Daily stepping provided in either cohort was related to baseline BBS (Fig 2A) and paretic leg strength (Fig 2B). Regressions for these relationships demonstrate large baseline (e.g., y-intercept) shifts between cohorts, with smaller differences in gains (e.g., slopes), indicating consistently greater stepping post-implementation across patients with varying levels of impairment.
Figure 2.
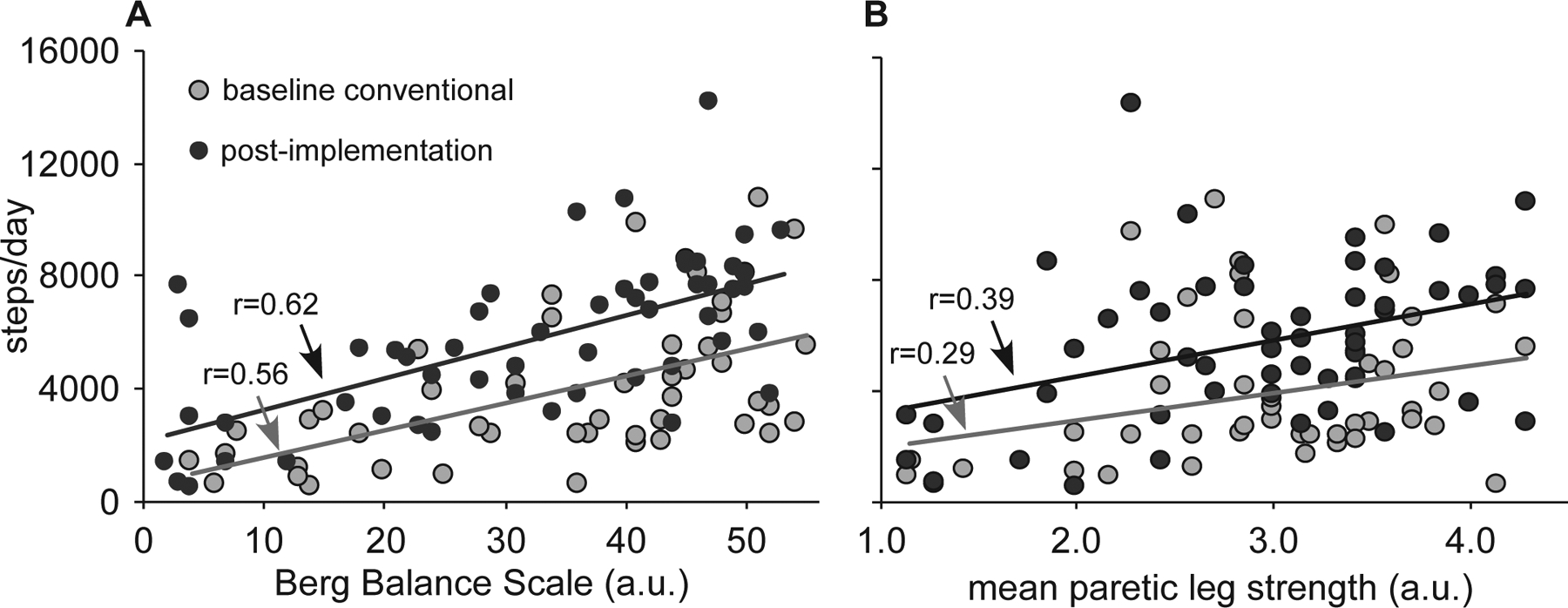
Relations between average steps/day and baseline BBS (1A) and mean paretic leg strength (1B).
Details of cardiovascular intensity measured following implementation (Table 1) suggests patients reached relatively higher HRs throughout training and were able to maintain cardiovascular demands within 70–85% HRmax for over 30% of each session. Monitoring of adverse events was conducted throughout the project, with falls outside of therapy reported most frequently, including 9 non-injury incidents in 8 patients during usual care and 11 falls in 6 patients during high-intensity stepping. Other adverse events occurred only during usual care, including infection (n=1) and transfers to acute care for medical issues (n=4), syncope (n=1), and unknown reasons (n=2).
During the study, many patients were transferred between the two inpatient sites. When this occurred, the combined LOS at the two sites were added together to calculate the patient LOS. There were significant differences in the patient LOS during inpatient rehabilitation favoring high-intensity training vs usual care (35±17 vs 23±9.7 days, p<0.01). This difference was likely due to improved coordination between the two sites, as OUH more frequently discharged to OMS in the high-intensity training (n=25) vs. usual care phase (n=4). Conversely, during usual care, patients were often discharged to other inpatient locations and not tracked further. However, the average stay at each site (i.e. site LOS) was not significantly different in the two phases of the project. The OUH LOS was 21±7.8 days during usual care and 24±10 days during high-intensity training (p=0.15). The OMS LOS was mean (SD) 22±12 days during usual care and 27±13 days during high-intensity stepping (p=0.16).
Primary and secondary outcomes
Changes in primary and secondary outcome measures are delineated in Table 2 and Figure 3. Repeated measures ANOVA and post-hoc unpaired comparisons indicate significant between-group differences in primary outcomes for all walking measures at week 1 and discharge (p<0.05), including gains in SSS (0.39±0.28 vs 0.16±0.26 m/s), FS (0.47±0.41 vs 0.17±0.38 m/s), and 6MWT (130±113 vs 64±93 m) at discharge. For BBS, significant differences were observed at discharge (15±11 vs 8.8±8.8), with no differences at week 1. All differences in changes between high-intensity training and usual care were above thresholds considered clinically meaningful.32 Accounting for differences in LOS by comparing week 3 high-intensity stepping vs usual care at DC, differences in SSS (p=0.03) and FS (p=0.02) were observed, with no differences in 6MWT (p=0.07) and BBS (p=0.42). Additional secondary outcomes measured at admission and discharge indicate that changes in FAC were different between groups (Table 2). While PROMIS sleep disturbance, anxiety, and depression demonstrated a trend toward improvements during high-intensity training, no significant differences occurred (Table 2).
Figure 3.
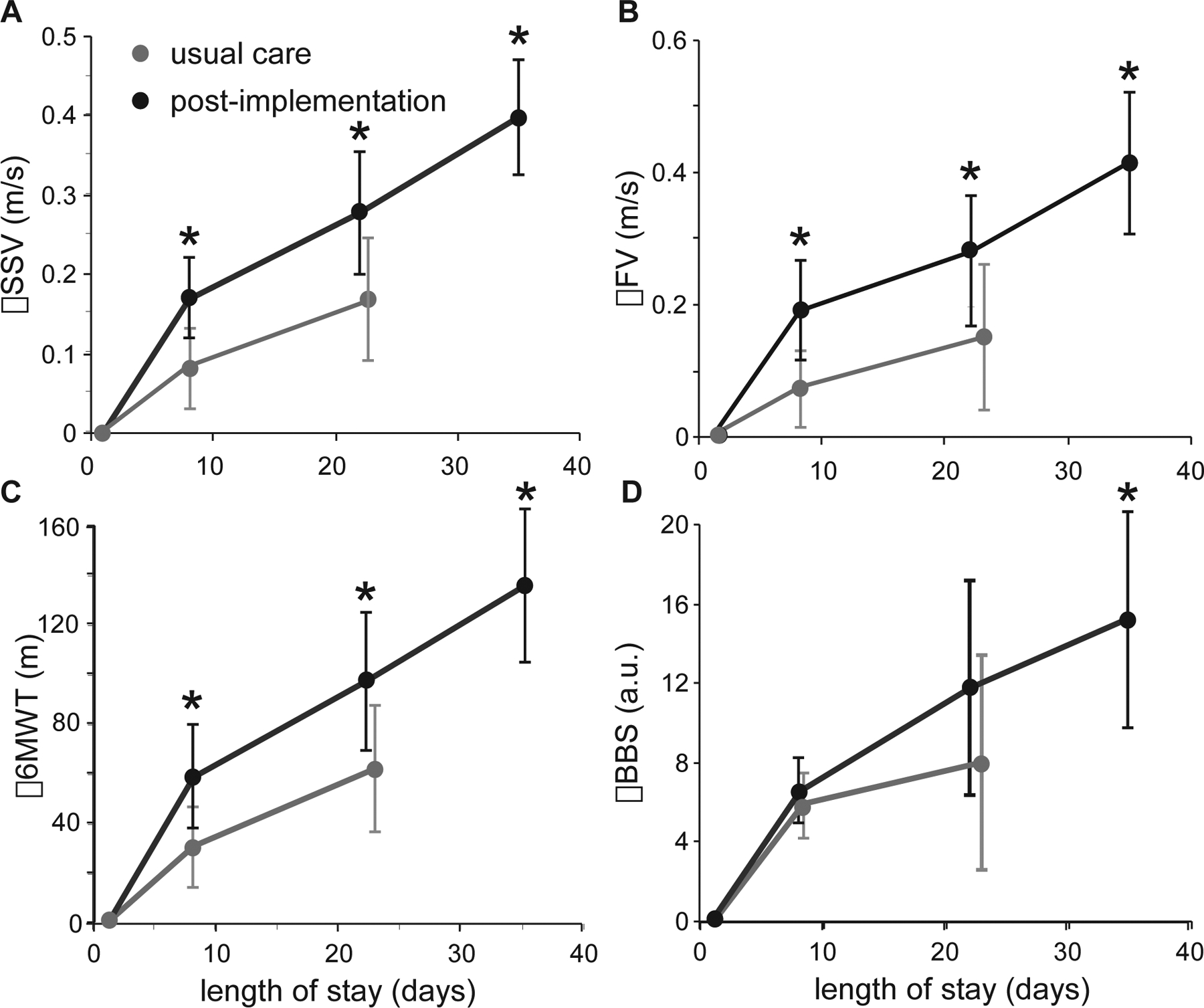
Changes in primary outcomes throughout the LOS, including SSS (2A) and FS (2B), 6MWT (2C), and BBS (2D). (* indicate p<0.05)
To estimate the population of patients who were likely able to ambulate in the community at discharge, we determined the number of participants who surpassed published SSS thresholds associated with full community mobility (SSS > 0.93 m/s).3 Only 6/49 participants (12%) during usual care surpassed this threshold at week 1, and 13 (26%) at discharge. During high-intensity stepping, 9/45 (20%) surpassed the threshold at week 1, 17 (38%) at week 3 and 26 (58%) at discharge, with significant differences only at discharge (p<0.01).
Correlation and regression analyses (Table 3, Equations 1–3) examined associations between primary outcomes at discharge and changes from baseline with demographics, impairments and functional deficits at admission, and training characteristics. Moderate to strong correlations were observed between primary discharge outcomes and impairments or functional deficits at admission. For training variables, only steps/day was consistently related to discharge outcomes. For change scores, low negative correlations were observed with admission impairments and function, with positive low to moderate associations with LOS and steps/day. Of the demographic variables, only age was consistently correlated with walking outcomes.
Table 3.
Results of Pearson and Spearman correlation analyses determining associations between primary discharge outcomes and changes in outcomes with demographics, impairments and functional deficits at admission, and training characteristics.
| SSS | FS | 6MWT | BBS | |||||
|---|---|---|---|---|---|---|---|---|
| DC | Δ | DC | Δ | DC | Δ | DC | Δ | |
| Age | −0.15 | −0.24* | −0.20* | −0.24* | −0.25* | −0.37* | −0.05 | −0.07 |
| Gender | 0.22* | 0.15 | 0.20* | 0.11 | 0.20* | 0.09 | −0.02 | −0.04 |
| Type | −0.05 | 0.03 | −0.07 | 0.05 | −0.05 | 0.05 | 0.06 | 0.16 |
| Side | 0.11 | 0.08 | 0.12 | 0.08 | 0.13 | 0.09 | 0.08 | −0.08 |
| Duration | −0,.10 | −0.06 | −0.05 | 0.15 | 0.03 | 0.16 | −0.17 | 0.01 |
| CCI | −0.06 | −0.18 | −0.03 | −0.12 | −0.14 | −0.20* | −0.09 | −0.19 |
| Strength | 0.54* | 0.02 | 0.53* | −.0.01 | 0.49* | −0.02 | 0.43* | −0.32* |
| BBS | 0.59* | −0.10 | 0.52* | −0.26* | 0.59 | −0.24 | 0.73* | −0.69* |
| FAC | 0.45* | −0.23* | 0.43* | −0.33 | 0.48* | −0.29 | 0.52* | −0.54* |
| Initial scores | 0.70* | −0.22* | 0.67* | −0.32 | 0.74* | −0.27* | -- | -- |
| LOS | −0.22* | 0.24* | −0.14 | 0.33* | −0.20* | 0.31* | −0.19 | 0.56* |
| Steps/day | 0.74* | 0.34* | 0.63* | 0.16 | 0.71* | 0.15 | 0.66* | −0.27* |
DC indicates the association of the variable with discharge score, Δ indicates association with change in outcomes, and
indicates significant finding (p<.05).
Subsequent regression analyses revealed significant associations between steps/day and admission scores, with additional contributions from age, paretic strength, and LOS. Equations 1 and 2 represent regression equations for discharge and changes in SSS, with other regressions for primary variables in the Supplementary Table 2. Regression analyses determining changes in primary outcomes at week 3 for usual care and high-intensity stepping revealed similar predictors for most all variables, with non-significant influences of LOS.
| Equation 1: |
| Equation 2: |
Conditional logistic regressions were performed to identify predictors of the ability to achieve SSS thresholds associated with community mobility. The primary determinant was steps/day with secondary contributions of age (Equation 3). Recalculation of the logistic regressions using 3-week data also indicate similar predictors but also includes the difference from admission SSS to 0.93 m/s (not shown).
| Equation 3: |
Discussion
This study evaluated the comparative effectiveness of high-intensity training during inpatient stroke rehabilitation as compared to a historical usual-care cohort. Consistent with previous efforts16, therapists increased stepping activity and HRs during physical therapy sessions, without increased incidence of adverse events. Outcomes included greater changes in SSS and FS at each assessment, with predictors of improved walking function including steps/day and admission scores, as well as age, baseline deficits, and LOS.
The primary predictors of walking outcomes are consistent with previous implementation studies applied to patients post-stroke16, and the contributions of steps/day reemphasize the potential impact of a modifiable training variable on gains in locomotor capacity. Importantly, prioritizing stepping practice during standard treatment resulted in less attention to other motor skills (balance and transfers), although these outcomes were not compromised (BBS, STS), with no differences between cohorts28. Increasing the intensity of stepping training can influence the stepping amount and rate, which can enhance neuromuscular and cardiopulmonary function.
Of specific interest is the large amounts of stepping activity performed as compared to studies of inpatient stroke rehabilitation. Observational studies at separate institutions revealed that the amount of stepping activity achieved during conventional inpatient therapy sessions was only ~250 steps/session10, 11. In our previous attempts to implement high-intensity training, stepping activity increased to ~1500 steps/day16, which is in stark contrast with the amounts of stepping performed in usual care or high-intensity stepping. Differences may be due to the clinicians attending an educational session regarding high-intensity training provided 8 months prior to the usual care phase, although one-time didactic educational sessions typically do not impact clinical practice33. Greater stepping activity observed during usual care may be due to differences in severity of motor impairments, as admission BBS scores were higher here than those observed previously during inpatient implementation efforts16. Daily stepping achieved is consistent with previous studies assessing the preliminary utility of high-intensity training in variable contexts in patients > 1 month post-stroke26–28, but with similar baseline impairments. Interestingly, during the high-intensity stepping phase, increases in stepping within sessions (~700 steps/sessions) did not completely account for the changes in daily stepping (~1800 steps/day). Reasons underlying this discrepancy are unclear, and daily activity patterns outside of therapy were not documented. Observational data suggest that levels of daily physical activity in Norwegians may be relatively higher as compared to age-matched counterparts in other nations34, which may have contributed to altered exercise tolerance, habits, or patient motivation in this cohort. Regardless, this practice likely accounted for gains in recovery and monitoring of activities outside of therapies should be documented in future studies.
An additional variable of interest was the difference in the patient LOS between the usual care and high-intensity training phases (i.e., 12 days), which appeared to contribute positively to locomotor recovery. These data contrast directly with previous research suggesting greater LOS during inpatient rehabilitation is negatively correlated with walking outcomes16. However, the positive contributions of LOS observed here were likely a result of improved coordination between the two levels of care that participated in the study (i.e. specialty and primary care), as patients were more frequently discharged from OUH to OMS instead of other inpatient facilities. Additionally, the LOS at each site was not significantly different between phases. This improved coordination between the specialty and primary services also demonstrates an important health system level impact. In Norway, a current health system goal is to improve cooperation and coordination between these two levels of care. Therefore, in this project, the two sites successfully achieved better cooperation between specialty and primary care as demonstrated by the increased number of transfers from OUH to OMS during the high-intensity training phase. Further, by using the same assessments and interventions, the two sites collectively demonstrated the patients’ outcomes from the entire inpatient care episode. Clinicians also anecdotally reported that they perceived that transferring from OUH to OMS, as well as extending the patient’s access to high-intensity training, would result in a greater functional capacity to actively contribute to society and participate in life. This uncontrolled factor is a limitation, although these types of confounders are not uncommon during implementation studies performed in clinical practice.35 The contributions of high-intensity training are still evident when attempting to control for LOS differences by comparing week 3 outcomes.
Additional limitations include the sample size and the lack of a contemporaneous control condition, as changes in medical or other rehabilitation services could have influenced recovery. While high-intensity stepping was offered to all patients who met the inclusion criteria, data were only analyzed for patients who consented for the study. In addition, an increased number of admitted patients with stroke were excluded for uncontrolled cardiopulmonary, metabolic, infectious or psychiatric disorders in the high-intensity training phase. While these additional inclusions occurred, the Charlson Comorbidity Index was not significantly different between phases. Regardless, we must be cautious when considering how these results would generalize to all patients undergoing stroke rehabilitation. The context in which high-intensity training program was delivered should also be considered, as the patient population and administrative support may have influenced therapist performance and patient outcomes. Since this project was conducted within clinical practice, a potential bias may have been introduced since the clinicians providing the treatment also collected the outcome data. In order to achieve a high dose of stepping, practice priorities shifted to target mainly gait function in patients with gait-related goals. While secondary outcome measures that assessed ADL tasks were collected at admission and discharge, these data do not reflect potential differences that may have occurred at week 3. Further, data on upper extremity function were not collected. Therefore, the specific impact of shifting practice priorities in physiotherapy away from improving upper extremity function is unknown. Additional implementation trials should consider the influence of duration post-stroke and levels of function. Another limitation is the lack of long-term follow-up, which is critical to understand the durability of gains achieved early post-stroke, particularly when discharged to community settings. The calculation of patients who achieved community mobility thresholds is a positive finding, but does not truly capture actual physical activity.
In summary, these data suggest that delivery of large amounts of stepping practice with focus on achieving higher cardiovascular intensities may result in greater locomotor performance and selected non-locomotor gains as compared to usual care during inpatient stroke rehabilitation. Further work is required to adequately assess the efficacy and effectiveness of these strategies to improve long-term outcomes of patients early post-stroke.
Supplementary Material
Acknowledgements:
We are grateful for support from clinicians, administrative staff, and participants at Oslo University Hospital and City of Oslo, Reinforced Interdisciplinary Rehabilitation Aker.
Sources of Funding: Internal funding was provided by the SouthEastern Norway Knowledge Translation Center, City of Oslo and Oslo University Hospital. Funding also provided by the Norwegian Fund for Post-Graduate Training in Physiotherapy, National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR-H133B031127), and the National Institute of Health (NIH-NINDS-NS079751).
Footnotes
Disclosures: Jennifer Moore receives salary from the Regional Center for Knowledge Translation in Rehabilitation (Sunnaas Rehabilitation Hospital) and also the owner of the Institute for Knowledge Translation. Ingvild Rosseland receives salary from the Reinforced Interdisciplinary Rehabilitation Aker. T. George Hornby is employed by the Indiana University School of Medicine.
Contributor Information
FIRST OSLO TEAM:
Tonje Barkenaes, Hanne Bratlie, Miriam Byhring, Ingvild Grimstad, Magnus Hågå, Joakim Halvorsen, Chris Henderson, Julia-Aneth Mbalilaki, Stein-Arne Rimehaug, Kirsten Saether, Thomas Tomren, and Karen Vergoossen
References
- 1.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: How important and obtainable is it and what measures appear predictive? Archives of physical medicine and rehabilitation. 2004;85:234–239 [DOI] [PubMed] [Google Scholar]
- 2.Preston E, Ada L, Dean CM, Stanton R, Waddington G. What is the probability of patients who are nonambulatory after stroke regaining independent walking? A systematic review. International journal of stroke : official journal of the International Stroke Society. 2011;6:531–540 [DOI] [PubMed] [Google Scholar]
- 3.Fulk GD, He Y, Boyne P, Dunning K. Predicting home and community walking activity poststroke. Stroke; a journal of cerebral circulation. 2017;48:406–411 [DOI] [PubMed] [Google Scholar]
- 4.Rajsic S, Gothe H, Borba HH, Sroczynski G, Vujicic J, Toell T, et al. Economic burden of stroke: A systematic review on post-stroke care. Eur J Health Econ. 2019;20:107–134 [DOI] [PubMed] [Google Scholar]
- 5.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2016;47:e98–e169 [DOI] [PubMed] [Google Scholar]
- 6.Hebert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bagg S, et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. International Journal of Stroke. 2016;11:459–484 [DOI] [PubMed] [Google Scholar]
- 7.Hornby TG, Straube DS, Kinnaird CR, Holleran CL, Echauz AJ, Rodriguez KS, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Topics in stroke rehabilitation. 2011;18:293–307 [DOI] [PubMed] [Google Scholar]
- 8.Hornby TG, Moore JL, Lovell L, Roth EJ. Influence of skill and exercise training parameters on locomotor recovery during stroke rehabilitation. Current opinion in neurology. 2016;29:677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Archives of physical medicine and rehabilitation. 2009;90:1692–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scrivener K, Sherrington C, Schurr K. Exercise dose and mobility outcome in a comprehensive stroke unit: Description and prediction from a prospective cohort study. Journal of rehabilitation medicine. 2012;44:824–829 [DOI] [PubMed] [Google Scholar]
- 12.Scrivener K, Sherrington C, Schurr K. Amount of exercise in the first week after stroke predicts walking speed and unassisted walking. Neurorehabilitation and neural repair. 2012;26:932–938 [DOI] [PubMed] [Google Scholar]
- 13.Kuys S, Brauer S, Ada L. Routine physiotherapy does not induce a cardiorespiratory training effect post-stroke, regardless of walking ability. Physiotherapy research international : the journal for researchers and clinicians in physical therapy. 2006;11:219–227 [DOI] [PubMed] [Google Scholar]
- 14.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: Is the intensity adequate to induce a training effect? Archives of physical medicine and rehabilitation. 2002;83:1378–1383 [DOI] [PubMed] [Google Scholar]
- 15.Prajapati SK, Mansfield A, Gage WH, Brooks D, McIlroy WE. Cardiovascular responses associated with daily walking in subacute stroke. Stroke research and treatment. 2013;2013:612458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornby TG, Holleran CL, Leddy AL, Hennessy P, Leech KA, Connolly M, et al. Feasibility of focused stepping practice during inpatient rehabilitation poststroke and potential contributions to mobility outcomes. Neurorehabilitation and neural repair. 2015;29:923–932 [DOI] [PubMed] [Google Scholar]
- 17.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. The New England journal of medicine. 2011;364:2026–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohl M, Werner C, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: A single-blind, randomized multicentre trial (deutsche gangtrainerstudie, degas). Clinical rehabilitation. 2007;21:17–27 [DOI] [PubMed] [Google Scholar]
- 19.van Bodegom-Vos L, Davidoff F, Marang-van de Mheen PJ. Implementation and de-implementation: Two sides of the same coin? BMJ Qual Saf. 2017;26:495–501 [DOI] [PubMed] [Google Scholar]
- 20.Graham I, Logan J, Harrison M, Straus S, Tetroe J, Caswell W. Lost in knowledge translation: Time for a map? J Contin Educ Heal Prof. 2006;26. [DOI] [PubMed] [Google Scholar]
- 21.Straus S, Tetroe J, Graham I. Knowledge translation in health care: Moving from evidence to practice. West Sussex, UK: BMJ Books; 2013. [Google Scholar]
- 22.Brown CH, Curran G, Palinkas LA, Aarons GA, Wells KB, Jones L. An overview of research and evaluation designs for dissemination and implementation. Annual review of public health. 2017;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudge S, Stott NS, Walt SE. Criterion validity of the stepwatch activity monitor as a measure of walking activity in patients after stroke. Archives of physical medicine and rehabilitation. 2007;88:1710–1715 [DOI] [PubMed] [Google Scholar]
- 24.Mudge S, Stott NS. Test--retest reliability of the stepwatch activity monitor outputs in individuals with chronic stroke. Clinical rehabilitation. 2008;22:871–877 [DOI] [PubMed] [Google Scholar]
- 25.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Physical therapy. 2014;94:222–229 [DOI] [PubMed] [Google Scholar]
- 26.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilitation and neural repair. 2014;28:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Camardo J, et al. Variable intensive early walking poststroke (views): A randomized controlled trial. Neurorehabilitation and neural repair. 2016;30:440–450 [DOI] [PubMed] [Google Scholar]
- 28.Straube DD, Holleran CL, Kinnaird CR, Leddy AL, Hennessy PW, Hornby TG. Effects of dynamic stepping training on nonlocomotor tasks in individuals poststroke. Physical therapy. 2014;94:921–933 [DOI] [PubMed] [Google Scholar]
- 29.Nes BM, Janszky I, Wisloff U, Stoylen A, Karlsen T. Age-predicted maximal heart rate in healthy subjects: The hunt fitness study. Scand J Med Sci Sports. 2013;23:697–704 [DOI] [PubMed] [Google Scholar]
- 30.Borg GA. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise. 1982;14:377–381 [PubMed] [Google Scholar]
- 31.Dobkin BH, Nadeau SE, Behrman AL, Wu SS, Rose DK, Bowden M, et al. Prediction of responders for outcome measures of locomotor experience applied post stroke trial. Journal of rehabilitation research and development. 2014;51:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 33.Jones CA, Roop SC, Pohar SL, Albrecht L, Scott SD. Translating knowledge in rehabilitation: Systematic review. Physical therapy. 2015;95:663–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohne-Seiler H, Hansen BH, Kolle E, Anderssen SA. Accelerometer-determined physical activity and self-reported health in a population of older adults (65–85 years): A cross-sectional study. BMC public health. 2014;14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmqvist R, Philips B, Barkham M. Developing practice-based evidence: Benefits, challenges, and tensions. Psychotherapy research : journal of the Society for Psychotherapy Research. 2015;25:20–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


