Abstract
OBJECTIVE
In children with type 1 diabetes (T1D), severe hypoglycemia (SH) is associated with poorer cognition, but the association of SH with cognitive function in late life is unknown. Given the increasing life expectancy in people with T1D, understanding the role of SH in brain health is crucial.
RESEARCH DESIGN AND METHODS
We examined the association between SH and cognitive function in 718 older adults with T1D from the Study of Longevity in Diabetes (SOLID). Subjects self-reported recent SH (previous 12 months) and lifetime history of SH resulting in inpatient/emergency department utilization. Global and domain-specific cognition (language, executive function, episodic memory, and simple attention) were assessed. The associations of SH with cognitive function and impaired cognition were evaluated via linear and logistic regression models, respectively.
RESULTS
Thirty-two percent of participants (mean age 67.2 years) reported recent SH and 50% reported lifetime SH. Compared with those with no SH, subjects with a recent SH history had significantly lower global cognition scores. Domain-specific analyses revealed significantly lower scores on language, executive function, and episodic memory with recent SH exposure and significantly lower executive function with lifetime SH exposure. Recent SH was associated with impaired global cognition (odds ratio [OR] 3.22, 95% CI 1.30, 7.94) and cognitive impairment on the language domain (OR 3.15, 95% CI 1.19, 8.29).
CONCLUSIONS
Among older adults with T1D, recent SH and lifetime SH were associated with worse cognition. Recent SH was associated with impaired global cognition. These findings suggest a deleterious role of SH on the brain health of older patients with T1D and highlight the importance of SH prevention.
Introduction
Severe hypoglycemia (SH) is a common, yet life-threatening, complication of type 1 diabetes (T1D). SH, defined as an episode of low blood glucose requiring external help to recover, affects ∼30–50% of people with T1D annually (1–3). Among older adults with T1D (≥65 years of age) and those with long-standing diabetes (≥40 years’ duration), rates of SH are even higher (4).
With recent advances in treatment, individuals with T1D are living longer and are, thus, at increased risk for a number of aging-related diseases, such as cognitive decline and dementia (5). One of the major concerns surrounding SH is its possible long-term effects on cognition. Indeed, studies in individuals with type 2 diabetes (T2D) have reported associations between SH and cognitive decline and SH and dementia (6–9). In T1D, findings are less clear. Most studies have been conducted in children or young adults. In children, findings have generally supported an association between SH and cognitive impairment (10–13), with some exceptions (14,15). Studies in young adults, among them the Diabetes Control and Complications Trial (DCCT) and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, have not (16–19). To our knowledge, only one small study (n = 36 with T1D) has examined the association between exposure to SH and cognitive function in older adults with T1D (20). This study reported a significant association between SH and worse cognitive functioning. A possible explanation for these discrepant findings is that the brains of children and of older adults are more vulnerable than in middle age and thus more susceptible to the harm associated with SH. Understanding the association between SH and cognition in older adults with T1D is increasingly important given the growing population of older adults with T1D and the increased risk of both SH and cognitive decline in this population. It is especially important to delineate whether SH is differentially associated with certain domains of cognition; given the demanding self-care that is required with T1D, it is relevant to determine whether SH impacts executive function or memory—areas of cognition that, if impaired, interfere with an individual’s ability to properly manage T1D. In this study, we 1) characterize the frequency of SH in a cohort of older adults with T1D and 2) examine the association between SH and cognitive function (overall and across four cognitive domains).
Research Design and Methods
Study Population
The Study of Longevity in Diabetes (SOLID) is a prospective cohort study of aging and diabetes that recruited members of Kaiser Permanente Northern California (KPNC) with T1D and aged ≥60 years. The present analysis focuses only on baseline measures (completed August 2015–June 2017). Eligible individuals with T1D were identified using ICD-9 and ICD-10 diagnosis codes extracted from their electronic medical record. Members were classified as having T1D if ≥75% of their diabetes-related diagnostic codes were for T1D (250.x1, 250.x3, or E10.x) and the member was prescribed insulin. Manual medical record review was conducted for participants reporting the onset of T1D at ≥31 years of age to confirm T1D status. Of the 2,113 total KPNC members with T1D aged ≥60 years, 805 individuals were enrolled and completed baseline interviews (see Supplementary Fig. 1 for study flow). All enrolled participants provided informed consent, and this study was approved by the KPNC Internal Review Board.
Self-reported Hypoglycemia
Self-reported SH (“severe low blood glucose reaction such as passing out or needing help to treat the reaction”) was captured at baseline interview. Participants were asked to self-report exposure to the following categories of SH: 1) SH over the past 12 months regardless of whether it resulted in emergency department (ED) utilization or inpatient utilization (“recent SH”) and 2) exposure to SH that resulted in ED or inpatient utilization over their lifetime (“lifetime SH history”).
Cognitive Function
At baseline interview, all participants were administered a comprehensive cognitive battery by trained interviewers. We conducted factor analysis on cognitive assessments from all participants with T1D through which four cognitive domains were identified: language, executive function, episodic memory, and simple attention. The language domain comprised the phonemic fluency test (F and L), the category fluency test (animals and vegetables), list sorting (two alternative lists), and the Multilingual Naming Test (MINT). The executive function domain comprised the Trail Making Test (parts A and B), the Digit Symbol Substitution Test, and the Stroop Color and Word Test. The episodic memory domain encompassed the word list learning test (immediate and delayed) and the Benson Complex Figure Copy (immediate and delayed). The simple attention domain encompassed the Diamond and TMX cancellation tests. Each test score was converted to a z score (mean = 0, SD = 1). For each domain, a summary score was calculated by summing the z scores for individuals who completed at least 50% of the relevant tests for a given domain; a global cognition score was calculated as the average of the four domain-specific summary scores for individuals who competed at least 50% of all cognitive function tests. Impaired global and domain-specific scores were defined as scores <1.5 SD below the population mean (21–24).
Covariates
Sex was obtained from KPNC records. Date of baseline interview and date of birth were used to calculate age at baseline interview. Age of diabetes onset was obtained via participant self-report and was used, in conjunction with age at baseline interview, to estimate diabetes duration. Race/ethnicity was based on self-report and was categorized as white, black, Hispanic, Asian, and other. Educational attainment was based on self-report and was categorized as “college degree or greater” or “less than a college degree.” The following baseline health conditions were based on self-reported history of a physician’s diagnosis: retinopathy, nephropathy, neuropathy, and stroke/cerebrovascular event. Participants also self-reported lifetime history of a head injury resulting in loss of consciousness or requiring medical care and current alcohol use. Depression symptoms were assessed at baseline using the Beck Depression Inventory, which is a 21-item measure of depression (25). Each item is scored based on symptom severity on a scale from 0 to 3 and all items are summed, resulting in a total score ranging from 0 to 63 with higher scores indicated more severe depression. Total Beck Depression Inventory score was used as a continuous covariate. Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality in participants. The PSQI measures seven areas of sleep over the past month to differentiate between “good” and “poor” quality sleep. Global PSQI scores range from 0 to 21 with higher scores indicating worse sleep quality. The PSQI was used as a continuous covariate.
Analytic Sample
The present analysis utilizes baseline measures for the subset of participants with T1D. Of the 805 individuals with T1D who were enrolled in SOLID, we excluded 24 participants who were missing hypoglycemia measures, 36 participants who were missing the global cognition score, and 27 participants who were missing key covariates, resulting in a final analytic sample of 718. The following numbers of people completed at least 50% of domain-specific cognitive tests for the relevant domain and are included in analyses specific to those domains: language, 704 participants; executive function, 703 participants; episodic memory, 683 participants; and simple attention, 703 participants.
Statistical Analyses
First, we examined the distribution of baseline characteristics in the sample, overall and by recent SH status. Next, we examined mean standardized scores on global and domain-specific cognitive measures across categories of SH exposure without covariate adjustment. For our main analysis, we specified linear regression models to examine the association between the two SH exposure variables and performance on global and domain-specific measures of cognition in two separate models with varying degrees of confounder adjustment. First, we fit a base model in which we adjusted for covariates that we felt reasonably certain would temporally precede SH exposure (age at baseline interview, sex, race/ethnicity, education, diabetes duration). Next, we fit a model with additional adjustment for a number of covariates that reflect overall well-being at baseline and, thus, may be impacting cognitive performance at baseline, but that we felt may be on the causal pathway from SH to cognitive function (baseline depression, sleep quality, perceived stress, and alcohol use). In secondary analyses, to better understand the association of SH with clinically meaningful cognitive impairment, we used the well-accepted neuropsychological cutoff of 1.5 SD below the population mean as a threshold for impaired cognition and used multivariable logistic regression models (adjusting for the same confounders indicated above) to examine the odds of cognitive impairment associated with each SH exposure (21).
For our main analysis, we conducted a series of sensitivity analyses to examine the robustness of our findings to varying assumptions and in a number of subgroups. To examine the directionality of the association between SH and cognition, we conducted a sensitivity analysis among the subset of participants without impaired cognition at baseline (n = 664 for global cognitive [varies slightly by each domain]). We also tested for statistical interaction between SH and long-term exposure to hyperglycemia (operationalized as self-reported exposure to retinopathy, nephropathy, or neuropathy) and SH and college education status. Finally, to better understand the impact of recent SH on cognition independent of prior SH exposure, we conducted a sensitivity analysis using multivariable linear regression models to examine the association of recent SH with cognition, adjusting for all covariates above plus additionally adjusting for lifetime SH history. All analyses were performed using SAS 9.4.
Results
Of the 718 adults with T1D, 32% reported recent SH and 50% reported lifetime SH history (Table 1). The mean (SD) age at baseline was 67.2 (6.2) years. Participants were predominantly white (86%) and predominantly college educated (62%). The average duration of diabetes in our sample was 39 years (SD 15), with an average age at onset of 28 years (SD 15).
Table 1.
Characteristics of older adults with T1D from SOLID
| Overall, N = 718 | Past 12 months’ exposure to SH episodes | |||
|---|---|---|---|---|
| No exposure, n = 491 | 1–3 episodes, n = 173 | ≥4 episodes, n = 54 | ||
| Age (years) | ||||
| Mean (SD) | 67.21 (6.33) | 67.11 (6.29) | 67.43 (6.47) | 67.48 (6.34) |
| Range | 59–96 | 59–90 | 60–96 | 60–85 |
| Median (IQR) | 66 (62, 71) | 65 (62, 71) | 66 (62, 70) | 67 (62, 70) |
| Female, n (%) | 364 (50.70) | 249 (50.71) | 85 (49.13) | 30 (55.56) |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic white | 615 (85.65) | 416 (84.73) | 152 (87.86) | 47 (87.04) |
| African American | 20 (2.79) | 15 (3.05) | 4 (2.31) | 1 (1.85) |
| Hispanic | 26 (3.62) | 17 (3.46) | 8 (4.62) | 1 (1.85) |
| Asian | 16 (2.23) | 14 (2.85) | 0 (0.00) | 2 (3.70) |
| Other | 41 (5.71) | 29 (5.91) | 9 (5.20) | 3 (5.56) |
| College degree or greater, n (%) | 446 (62.12) | 324 (65.99) | 94 (54.34) | 28 (51.85) |
| Diabetes duration (years) | ||||
| Mean (SD) | 39.05 (15.04) | 38.03 (15.19) | 41.06 (14.52) | 41.86 (14.45) |
| Range | 1–79 | 1–73 | 2–73 | 11–79 |
| Median (IQR) | 41.0 (28.5, 51.0) | 40 (28, 50) | 43 (31, 52) | 41 (33, 55) |
| Age at diabetes onset (years) | ||||
| Mean (SD) | 28.17 (15.19) | 29.08 (15.55) | 26.38 (14.54) | 25.62 (13.21) |
| Range | 0–70 | 0–70 | 3–68 | 5–68 |
| Median (IQR) | 26 (16, 29) | 27 (16, 40) | 25 (15, 35) | 25 (14, 34) |
| History of retinopathy, n (%) | 305 (44.59) | 210 (44.97) | 73 (44.51) | 22 (41.51) |
| History of neuropathy, n (%) | 293 (42.22) | 200 (42.19) | 68 (40.24) | 25 (49.02) |
| History of nephropathy, n (%) | 56 (8.89) | 37 (8.47) | 14 (9.59) | 5 (10.64) |
| History of stroke/cerebrovascular event, n (%) | 62 (8.83) | 35 (7.28) | 17 (10.18) | 10 (18.52) |
| History of head injury resulting in loss of consciousness/medical care, n (%) | 167 (23.26) | 117 (23.83) | 40 (23.12) | 10 (18.52) |
| Current alcohol use, n (%) | 511 (71.27) | 353 (72.04) | 123 (71.10) | 35 (64.81) |
| Beck Depression Inventory score | ||||
| Mean (SD) | 5.42 (4.77) | 5.21 (4.64) | 5.83 (4.98) | 6.02 (5.12) |
| Range | 0–28 | 0–28 | 0–24 | 0–19 |
| Median (IQR) | 4 (2, 8) | 4 (2, 8) | 5 (2, 8) | 4 (2, 9) |
| Perceived Stress Scale | ||||
| Mean (SD) | 9.84 (6.41) | 9.83 (6.31) | 9.62 (6.63) | 10.61 (6.64) |
| Range | 0–34 | 0–34 | 0–30 | 0–26 |
| Median (IQR) | 9 (5, 14) | 9 (5, 14) | 9 (4, 14) | 10 (6, 14) |
| PSQI | ||||
| Mean (SD) | 8.22 (2.83) | 8.15 (2.83) | 8.35 (2.88) | 8.43 (2.66) |
| Range | 3–18 | 3–18 | 3–17 | 4–14 |
| Median (IQR) | 8 (6, 10) | 8 (6, 10) | 8 (6, 10) | 8 (6, 11) |
The following variables were based on participant self-report: race/ethnicity, education, age at diabetes onset, and exposure to past 12 months’ and lifetime SH. History of retinopathy, nephropathy, neuropathy, and stroke/cerebrovascular events was self-reported based on a physician’s diagnosis. History of head injury resulting in loss of consciousness or requiring medical care and alcohol use was based on participant self-report. The Beck Depression Inventory scores range from 0 to 63 with higher scores indicating more severe depression. Scores on the Perceived Stress Scale range from 0 to 40 with higher scores indicating higher perceived stress. Global PSQI scores range from 0 to 21 with higher scores indicating worse sleep quality. IQR, interquartile range.
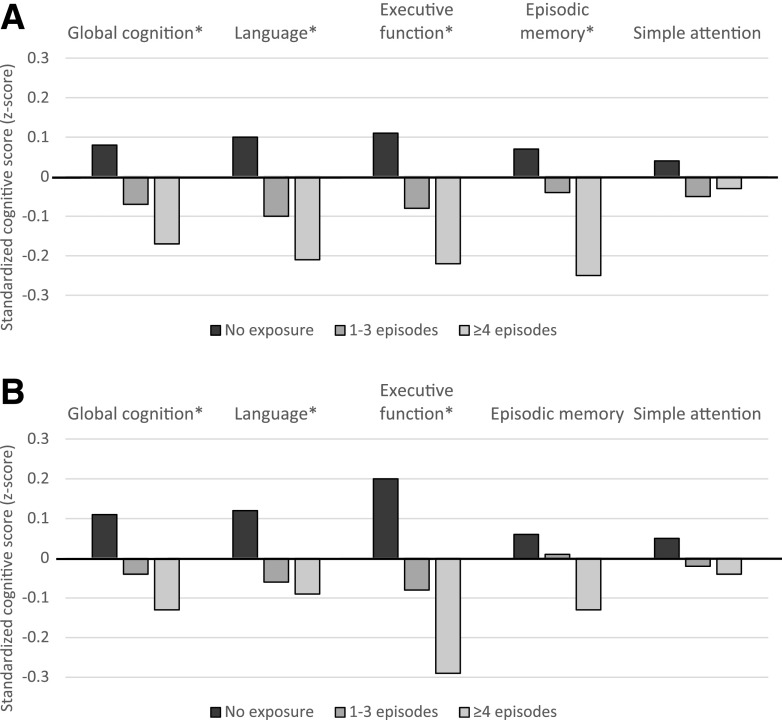
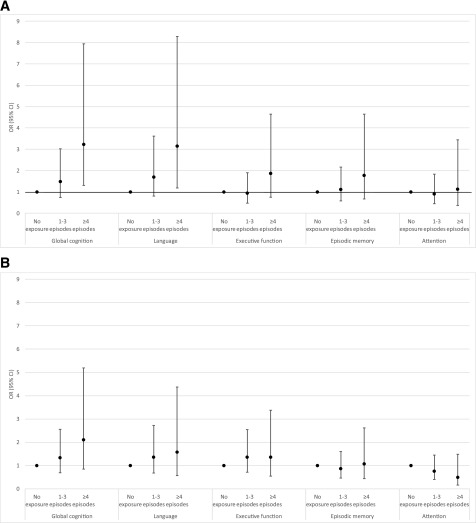
Findings revealed evidence of a gradient association between SH and cognition. Mean scores on global cognition, language, executive function, and episodic memory were highest among those with no recent SH and lowest among those with the highest recent SH (Fig. 1A) (all P values <0.01); there was no association between recent SH and simple attention (P = 0.38). Mean scores on global cognition, language, and executive function were highest among those with no lifetime SH history and lowest among those with the highest lifetime SH history (Fig. 1B) (all P < 0.01); no association was found between mean scores on episodic memory or simple attention and lifetime SH history (P = 0.12 and 0.44, respectively). In adjusted linear regression models, compared with those with no exposure, participants in the highest exposure category of recent SH had significantly lower global cognition scores (Table 2). Domain-specific analyses revealed significantly lower scores on language, executive function, and episodic memory with recent SH exposure and significantly lower scores on executive function with lifetime SH history. In examination of cognitive function as a binary exposure (impaired cognition vs. normal cognition using 1.5 SD as a cutoff [n = 57 individuals with impaired global cognition]), findings revealed a similar pattern of risk (Fig. 2). Compared with those with no recent SH exposure, participants with the highest recent SH exposure were three times more likely to have impaired global cognition and impaired language (odds ratio [OR] for impaired global cognition 3.10, 95% CI 1.26, 7.63; OR for impaired language 3.08, 95% CI 1.17, 8.08). In sensitivity analyses, among the subset of participants without impaired cognition at baseline (n = 664 for global cognition), the association between SH and cognitive function was slightly attenuated but remained significant for the following domains: recent SH was associated with poorer executive function and episodic memory, and lifetime SH was associated with poorer executive function (Supplementary Table 1). We found no difference in SH rates by persistent hyperglycemia status (P = 0.69 for recent SH and P = 0.23 for lifetime SH χ2 test) and no difference in the association between SH and cognition by persistent hyperglycemia status for the global or any domain-specific analyses (all P > 0.10). Additionally, in analyses using educational attainment (college degree, yes or no) as a proxy of intellectual ability, we found no difference in lifetime hypoglycemia rates by college education status (P = 0.64 for χ2 test) and no difference in the association between lifetime SH and cognition by college education status for the global or any domain-specific analyses (all education * hypoglycemia interaction terms P > 0.10). Finally, we found a strong association between recent SH and cognition that was independent of lifetime SH history (Supplementary Table 2). Over and above the risk conferred by lifetime SH history (and with adjustment for all other covariates), recent SH was associated with lower cognitive performance (global and on the language, executive function, and episodic domains) and impaired cognition (global and on the language domain).
Figure 1.
Mean standardized cognitive scores across categories of exposure to recent SH (A) and lifetime exposure to SH resulting in hospitalization or ED visit (B). *P value for trend significant at <0.01.
Table 2.
Association between exposure to recent and lifetime SH and cognitive function
| Model outcome | Exposure to recent SH | Exposure to lifetime SH | ||
|---|---|---|---|---|
| Model 1: adjustment for age, sex, race, age at diagnosis, and education | Model 2: additional adjustment for depression, stress, sleep, and alcohol use at baseline | Model 1: adjustment for age, sex, race, age at diagnosis, and education | Model 2: additional adjustment for depression, stress, sleep, and alcohol use at baseline | |
| Global cognition | ||||
| 0 episodes | REF | REF | REF | REF |
| 1–3 episodes | −0.11 (−0.18, −0.03) | −0.13 (−0.21, −0.04) | −0.09 (−0.16, −0.02) | −0.09 (−0.17, −0.02) |
| ≥4 episodes | −0.20 (−0.33, −0.07) | −0.21 (−0.33, −0.08) | −0.13 (−0.24, −0.01) | −0.10 (−0.22, 0.02) |
| Language | ||||
| 0 episodes | REF | REF | REF | REF |
| 1–3 episodes | −0.14 (−0.25, −0.020 | −0.16 (−0.28, −0.04) | −0.11 (−0.21, −0.002) | −0.11 (−0.22, −0.01) |
| ≥4 episodes | −0.24 (−0.42, −0.05) | −0.27 (−0.46, −0.09) | −0.13 (−0.30, 0.04) | −0.09 (−0.26, 0.09) |
| Executive function | ||||
| 0 episodes | REF | REF | REF | REF |
| 1–3 episodes | −0.14 (−0.25, −0.02) | −0.16 (−0.28, −0.04) | −0.18 (−0.29, −0.08) | −0.17 (−0.28, −0.07) |
| ≥4 episodes | −0.25 (−0.44, −0.06) | −0.24 (−0.43, −0.06) | −0.30 (−0.48, −0.13) | −0.27 (−0.45, −0.10) |
| Episodic memory | ||||
| 0 episodes | REF | REF | REF | REF |
| 1–3 episodes | −0.07 (−0.19, 0.04) | −0.12 (−0.23, −0.0002) | 0.01 (−0.10, 0.11) | −0.01 (−0.12, 0.09) |
| ≥4 episodes | −0.30 (−0.49, −0.12) | −0.29 (−0.48, −0.11) | −0.06 (−0.23, 0.11) | −0.07 (−0.24, 0.10) |
| Attention | ||||
| 0 episodes | REF | REF | REF | REF |
| 1–3 episodes | −0.09 (−0.22, 0.06) | −0.08 (−0.23, 0.07) | −0.06 (−0.19, 0.07) | −0.06 (−0.20, 0.08) |
| ≥4 episodes | −0.08 (−0.30, 0.15) | −0.08 (−0.31, 0.15) | −0.02 (−0.22, 0.19) | 0.01 (−0.20, 0.23) |
Data are β (95% CI). Multivariable linear regression models examining the association between recent SH (past 12 months) and lifetime SH (resulting in ED or inpatient utilization). REF, reference.
Figure 2.
Association between cognitive impairment (1.5 SD below population mean) and exposure to recent SH (A) and lifetime SH resulting in ED or inpatient utilization (B). ORs and 95% CIs presented. All models adjusted for age, sex, race/ethnicity, education, and diabetes duration.
Conclusions
In this study of older adults with T1D, exposure to SH was associated with lower cognitive performance. We found clear evidence of a gradient association between two different measures of SH and cognitive function. Exposure to ≥4 episodes of SH in the past 12 months was associated with significantly lower global cognition scores. The relationship between recent SH exposure and lower cognitive performance was observed on multiple domains: language, executive function, and episodic memory. In contrast, the relationship between lifetime SH history and lower cognitive performance seemed to be primarily driven by poorer performance on the executive function domain. Impaired cognition (defined as 1.5 SD below the population mean) followed the same general pattern of association, however with fewer statistically significant results; recent SH was associated with increased risk of impaired global cognition and impaired cognition (global and on the language domain), while lifetime SH was not significantly associated with impaired cognition globally or on any specific domains.
Of note, we did not observe any association between SH and cognitive performance on the simple attention domain. We examined the distribution of participant scores on each of the cognitive tests for a potential ceiling effect (where a substantial number of participants perform at or near the maximum possible test score). For the two cognitive tests that comprised the simple attention domain (TMX cancellation and Diamond cancellation) there was evidence of a ceiling effect (56% of participants obtained the maximum score on the TMX test and 12% on the Diamond test). This may explain the lack of an association with SH on this domain. Despite this, we chose to use the number of correct answers to score the TMX and Diamond cancellation tests to facilitate comparison with other cohorts.
To our knowledge, this is the first large-scale study to investigate the association between SH and cognition in older adults with T1D. Our results complement and extend previous studies that have reported an association between SH and decreased cognitive function in children and adolescents with T1D (10–12,14). Our findings are also consistent with one previous small-scale study (n = 36 with T1D) that reported an association between SH and cognitive decline among older adults with T1D (mean age at baseline 62 years) and numerous studies in T2D that have reported an association between SH and cognitive decline and dementia in older adults (6–9,20). However, our findings are in contrast to prior studies in middle-aged adults with T1D, notably the DCCT/EDIC, that reported no association between SH and impaired cognition (16). The age differences between DCCT/EDIC and SOLID study participants (mean age at baseline in SOLID = 67 years vs. mean age at EDIC study year 12 = 46 years) may explain the disparate findings. Other possible explanations for our contrasting findings include differences in study design, diabetes management, definitions of SH, cognitive function assessment, or sample population. Indeed, in this study, we found a robust association of past 12-month SH on cognition that was independent of lifetime SH exposure (Supplementary Table 2). These findings suggest that, in older adults with T1D, recent SH exposures contribute to cognitive risk independent of prior adverse effects on the brain from past SH exposures. This also supports the hypothesis that the timing of the SH insult may be particularly important and late adulthood may represent a crucial period during which the aging brain may be more susceptible to insults from SH than the brain in middle age (26).
Strengths of this study include the large sample of older adults with T1D with varying duration of disease and age at onset, the comprehensive cognitive assessment including measures of cognitive function on multiple domains, and the ability to examine SH exposure over differing periods of time. Limitations include reliance on self-reported SH exposures. Despite this concern, the SH exposure measures included in this study (SH over the past 12 months and lifetime SH resulting in hospitalization or ED visit) are likely less prone to recall bias than other, less severe forms of hypoglycemia. Further, we have previously shown that relying on health care utilization data, rather than self-report, to assess SH captures only 5% of SH cases in the prior 12 months, which is also less than optimal (27). Prior research has demonstrated a bidirectional association between SH and cognition such that SH is associated with poorer cognition, and poorer cognition may result in increased SH (8). Because our data are cross-sectional in nature, it is possible that our findings are being driven by reverse causation, whereby decreases in cognitive function are resulting in greater SH exposure as opposed to the other way around; however, we have performed a number of sensitivity analyses (restricting to patients without cognitive impairment at baseline and testing for interaction between lifetime SH and education attainment as a proxy of intellectual ability/lifetime executive function) and our findings of the association between SH and cognition were robust in these additional analyses. An additional limitation was the inability to characterize participants’ long-term glycemic control. In the current study, we did not have access to laboratory data of hemoglobin A1c, a measure that we have previously shown to be associated with dementia risk in older adults with T1D (28). To address this limitation, we conducted a sensitivity analysis examining persistent hyperglycemia as an effect modifier of the association between SH and cognition and found no significant interaction on the global or any domain-specific analyses (all P > 0.10). It is possible, however, that part of the association between cognitive function and SH may actually be explained by variation in hemoglobin A1c that is not fully captured by our measure of persistent hyperglycemia. Finally, the participants with T1D in our study are generally white and well educated, and, thus, our findings may not be generalizable to the broader population of older adults with T1D. Despite this limitation, SOLID is one of the largest cohort studies of older adults with T1D to date and, thus, is well poised to contribute to our understanding of the complex nature of aging, T1D, and cognition.
The mechanisms underlying the association between SH and cognition are clear, and the acute consequences of SH on the brain have been well characterized. Repeated SH episodes cause significant neuronal death, and, in the short term, acute SH interrupts the supply of glucose to the brain, which produces marked cognitive impairment and, if left untreated, can lead to coma and death (29–32). However, the long-term consequences of SH on the brain, and, in particular, the aging brain, remain poorly understood. One reason for this is the lack of large-scale, population-based studies of older adults with T1D. Largely due to advances in treatment of T1D and resulting increases in life span in recent decades, older adults with T1D are a growing yet understudied population. Another reason may be the difficulty that surrounds parsing the effects of SH from the array of confounders that place individuals with T1D at higher risk of both SH and poor cognitive function, among them advanced age, long-standing diabetes duration, higher prevalence of depression, and complications resulting from chronic hyperglycemia (33,34). Despite these challenges, understanding the cognitive impact of exposure to SH is especially important in older adults with T1D given the complex treatment regimens that these individuals usually follow, as studies have shown that even modest deficits in cognitive function may cause difficulties with T1D self-management (35). Our study advances the evidence surrounding SH and cognition in older adults with T1D by providing the first large-scale study of SH exposure over varying time periods and its association with cognition.
In this study of 718 older adults with T1D, we found a strong association between SH and lower scores on global and select domain-specific measures of cognition (language, executive function, and episodic memory). Exposure to ≥4 episodes of SH in the past 12 months was associated with a more than threefold risk of impaired global cognition; this finding remained after adjustment for lifetime exposure to SH. Our findings underscore the importance of continued vigilance and management to prevent SH in this older population, as the aging brain may be particularly susceptible to SH-related cognitive decline.
Supplementary Material
Article Information
Funding. The authors gratefully acknowledge funding from the National Institute on Aging, National Institutes of Health (NIA R01 AG047500 [to R.A.W.]). M.E.L. and C.E. were supported by the University of California, San Francisco, Training for Research on Aging and Chronic Disease program (NIA T32 AG049663). M.E.L. was also supported by the Patient-Centered Outcomes Research Institute through contract PPRN-1306-04709.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.E.L. conducted analyses, wrote the manuscript, and assisted with study design and data interpretation. P.G., C.E., M.S.B., and A.J.K. assisted with study design and data interpretation and reviewed and edited the manuscript. R.A.W. obtained funding, assisted with study design and data interpretation, and reviewed and edited the manuscript. M.E.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Alzheimer’s Association International Conference, Chicago, IL, 22–26 July 2018.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0906/-/DC1.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med 1993;10:238–245 [DOI] [PubMed] [Google Scholar]
- 2.Ratzki-Leewing A, Harris SB, Mequanint S, et al. Real-world crude incidence of hypoglycemia in adults with diabetes: results of the InHypo-DM Study, Canada. BMJ Open Diabetes Res Care 2018;6:e000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev 2004;20:479–486 [DOI] [PubMed] [Google Scholar]
- 4.Weinstock RS, Xing D, Maahs DM, et al.; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 5.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 2012;61:2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinkohl I, Aung PP, Keller M, et al.; Edinburgh Type 2 Diabetes Study (ET2DS) Investigators . Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2014;37:507–515 [DOI] [PubMed] [Google Scholar]
- 7.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaffe K, Falvey CM, Hamilton N, et al.; Health ABC Study . Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013;173:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CH, Sheu WH. Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7-year follow-up study. J Intern Med 2013;273:102–110 [DOI] [PubMed] [Google Scholar]
- 10.Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes--a meta-analysis. J Pediatr Psychol 2009;34:271–282 [DOI] [PubMed] [Google Scholar]
- 11.Hershey T, Craft S, Bhargava N, White NH. Memory and insulin dependent diabetes mellitus (IDDM): effects of childhood onset and severe hypoglycemia. J Int Neuropsychol Soc 1997;3:509–520 [PubMed] [Google Scholar]
- 12.Blasetti A, Chiuri RM, Tocco AM, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol 2011;26:1383–1391 [DOI] [PubMed] [Google Scholar]
- 13.Hershey T, Lillie R, Sadler M, White NH. Severe hypoglycemia and long-term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc 2003;9:740–750 [DOI] [PubMed] [Google Scholar]
- 14.Strudwick SK, Carne C, Gardiner J, Foster JK, Davis EA, Jones TW. Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr 2005;147:680–685 [DOI] [PubMed] [Google Scholar]
- 15.Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156 [DOI] [PubMed] [Google Scholar]
- 16.Jacobson AM, Musen G, Ryan CM, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term effect of diabetes and its treatment on cognitive function [published correction appears in N Engl J Med 2009;361:1914]. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin EJ, Deary IJ. Effects of repeated hypoglycemia on cognitive function: a psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care 1999;22:1273–1277 [DOI] [PubMed] [Google Scholar]
- 18.Kramer L, Fasching P, Madl C, et al. Previous episodes of hypoglycemic coma are not associated with permanent cognitive brain dysfunction in IDDM patients on intensive insulin treatment. Diabetes 1998;47:1909–1914 [DOI] [PubMed] [Google Scholar]
- 19.Reichard P, Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes 1994;43:313–317 [DOI] [PubMed] [Google Scholar]
- 20.Duinkerken Ev, Brands AM, van den Berg E, Henselmans JM, Hoogma RP, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group . Cognition in older patients with type 1 diabetes mellitus: a longitudinal study. J Am Geriatr Soc 2011;59:563–565 [DOI] [PubMed] [Google Scholar]
- 21.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 2005;62:1160–1163; discussion 1167 [DOI] [PubMed] [Google Scholar]
- 23.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol 2005;62:1739–1746 [DOI] [PubMed] [Google Scholar]
- 24.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009;17:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571 [DOI] [PubMed] [Google Scholar]
- 26.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol 2008;7:184–190 [DOI] [PubMed] [Google Scholar]
- 27.Karter AJ, Moffet HH, Liu JY, Lipska KJ. Surveillance of hypoglycemia-limitations of emergency department and hospital utilization data. JAMA Intern Med 2018;178:987–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacy ME, Gilsanz P, Karter AJ, Quesenberry CP, Pletcher MJ, Whitmer RA. Long-term glycemic control and dementia risk in type 1 diabetes. Diabetes Care 2018;41:2339–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNay EC, Cotero VE. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiol Behav 2010;100:234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graveling AJ, Deary IJ, Frier BM. Acute hypoglycemia impairs executive cognitive function in adults with and without type 1 diabetes. Diabetes Care 2013;36:3240–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deary IJ. Symptoms of hypoglycaemia and effects on mental performance and emotions. In Hypoglycaemia in Clinical Diabetes. Frier BM, Fisher BM, Eds. Chichester, U.K., John Wiley & Sons, 1999, pp. 29–54 [Google Scholar]
- 32.Widom B, Simonson DC. Glycemic control and neuropsychologic function during hypoglycemia in patients with insulin-dependent diabetes mellitus. Ann Intern Med 1990;112:904–912 [DOI] [PubMed] [Google Scholar]
- 33.Weinstock RS, DuBose SN, Bergenstal RM, et al.; T1D Exchange Severe Hypoglycemia in Older Adults With Type 1 Diabetes Study Group . Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2016;39:603–610 [DOI] [PubMed] [Google Scholar]
- 34.Trief PM, Xing D, Foster NC, et al.; T1D Exchange Clinic Network . Depression in adults in the T1D Exchange Clinic Registry. Diabetes Care 2014;37:1563–1572 [DOI] [PubMed] [Google Scholar]
- 35.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 2005;28:726–735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.