Abstract
Chemotherapy, surgery, and radiation are accepted as the preferred treatment modalities against cancer, but in recent years the use of immunotherapeutic approaches has gained prominence as the fourth treatment modality in cancer patients. In this approach, a patient’s innate and adaptive immune systems are activated to achieve clearance of occult cancerous cells. In this review, we discuss the preclinical and clinical immunotherapeutic (e.g., immunoadjuvants (in-situ vaccines, oncolytic viruses, CXC antagonists, device activated agents), organic and inorganic nanoparticles, checkpoint blockade etc.) that are under investigation for cancer therapy and diagnostics. Additionally, the innovations in imaging of immune cells for tracking therapeutic responses and limitations (e.g., toxicity, inefficient immunomodulation, etc.) are described. Existing data suggest that if immune therapy is optimized, it can be a real and potentially paradigm-shifting cancer treatment frontier.
Keywords: Cancer immunology, Immunoadjuvant, Nanoparticles, Checkpoint inhibitor, Immunotherapy
1. Introduction
Advanced stage malignant tumors are typically resistant to chemo- and radiotherapy and are difficult to target, resulting in modest patient survival despite aggressive treatments. To improve clinical outcomes, current cancer research is focused on finding a more efficient and selective cancer therapeutic that overcomes the limitations of tumor evasive factors (Fouad & Aanei, 2017; Vinay, et al., 2015). In this context, immunotherapies are increasingly emerging as an interesting alternative approach due to their superior specificity and efficiency of targeting malignant tumors (Geering & Fussenegger, 2015). Immunotherapies alter the tumor microenvironments by selectively recognizing and binding to immunosuppressive proteins that are expressed on tumor cells, activated T and B cells, macrophages, and regulatory T cells to activate local and systemic anti-tumor immune responses against cancerous cells (Farkona, Diamandis, & Blasutig, 2016; Gong, Chehrazi-Raffle, Reddi, & Salgia, 2018; Kruger, et al., 2019). While both innate and adaptive immunity play key roles in proper immune function, their key differences are important to distinguish. Innate immunity primarily responds to immediate and acute insults such as foreign pathogens, and it is mainly mediated by phagocytic cells such as natural killer cells, monocytes, macrophages, dendritic cells, and neutrophils (Platt & Wetzler, 2013). In contrast, foreign pathogens/agents are processed and presented by the major histocompatibilty complex (MHC) as antigens by innate cells to then activate the adaptive response, which includes cells of humoral and cell-mediated immunity (i.e., helper, cytotoxic, and suppressor-T cells and B cells). Other major differences between innate and adaptive immunity include efficacy, response time, and specificity. Innate response sacrifices specificity and effectiveness for a more immediate response that is always active. In contrast, adaptive response sacrifices speed for high specificity and extreme efficacy to specified antigens built over previous exposure (Boehm & Swann, 2014).
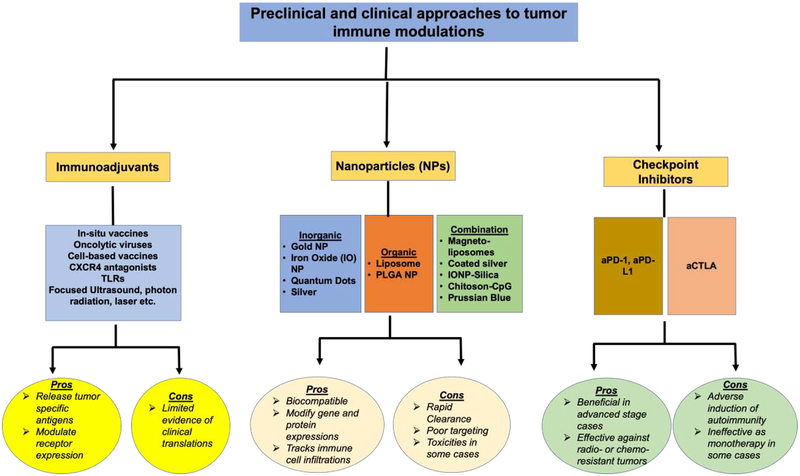
Recent research on immunomodulation as a possible contributor to a targeted design aimed against cancer has resulted in many different methodologies, including (i) immune-targeted nanoparticles (NPs), (ii) in situ vaccinations, (iii) checkpoint inhibitors such as anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) or anti programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1), and (iv) various combinations of these in combination with other known modalities. For example, many recent studies have evaluated the effect of NPs on both innate and adaptive immunity and their mode of action in tumor models. Together, (i) inorganic NPs, (ii) organic NPs, and (iii) combination NPs dominate this newly created niche. Likewise, the mode of action of in-situ vaccinations includes harnessing the ideal immune activation without the need for the labor-intensive biopsy for every individual patient (Hammerich, Bhardwaj, Kohrt, & Brody, 2016). The effectiveness of an in-situ vaccination (ISV) on a per patient basis can be high (Hammerich, et al., 2019). In situ vaccinations utilize the killing of tumor cells, the release of tumor-associated antigens (TAA), and the capturing of those antigens to activate T effector cells to elicit the systemic immune response and recognition of the tumor microenvironment (Pierce, Campbell, Pai, Brody, & Kohrt, 2015). The use of the local tumor as the antigen source in the context of the patients means that all relevant antigens, whether tumor-associated or neo-antigens released by ISV can be included in the vaccine, obviating the need to identify the antigens (Hammerich, Binder, & Brody, 2015). They are used most effectively in combination with other therapies, and extensive research has been focused on combinations with therapies previously listed (Hammerich, et al., 2016; Hammerich, et al., 2015). Beyond this, another goal to push immunomodulation to the forefront of all cancer therapies has been to observe immunotherapy through an imaging system in real time. A common pitfall of immunotherapy is the inability to monitor an improvement in immune responses in real time longitudinally and beyond the treatment window. Noninvasive macrophage or T cell imaging would not only provide peace of mind to the patient by showing that the therapy is effective and active, but it also would lend itself to filling many gaps in knowledge- such as the elusive mechanism of the abscopal effect of some therapies to secondary tumors that were not directly targeted or treated. It would also provide a systematic and mechanistic link to explain how immune cells are directly related to any desired phenomena.
Herein, we review the recent pre-clinical and clinical utilization of immunomodulator therapies using any of the above listed approaches (NPs, ISVs, checkpoint inhibitors and/or immune cell imaging) alone or in combination, and we discuss both their beneficial aspects and their inevitable pitfalls in the broad context of innate versus adaptive immunity within cancer immunotherapeutic modalities (Fig. 1). We also discuss the next-generation combination therapeutic approaches, their associated pathways, and imaging methods to track immune cells for the assessment of therapeutic responses.
Fig. 1.
Pre-clinical or clinical agents in current use for modulation of tumor immune microenvironment.
2. Immuno-Adjuvants
Immune adjuvants are based on modern vaccinations with a slight modification. Modern vaccinations commonly use an antigen and an adjuvant in conjunction to cause and boost an immune response and build antibodies against the chosen antigen. In contrast, immunoadjuvants (e.g., ISVs) use the tumor as the antigen source to elicit a patient-specific immune response (Hammerich, et al., 2015; Lizotte, et al., 2016). In particular, ISVs have the potential to greatly reduce the cost of therapy due to the simplicity of treatment on a per patient basis. A unique feature of the ISV regimen is that it alters an immunosuppressive tumor microenvironment from a low intratumoral T cell infiltration type to a T cell inflamed phenotype (Hammerich, et al., 2015; P. Sharma & Allison, 2015). ISVs also do not preclude other immunotherapies, and with modern image-guidance systems enabling precise visualization and injection into a tumor almost anywhere within the body, this approach can be translatable for treatment of a variety of tumors types.
Among the immunoadjuvant approaches actively under investigation, using oncolytic viruses (OVs) that target cancer themselves (Chiocca & Rabkin, 2014), and using novel antibodies/physical treatments appear to be important. These avenues have been pursued as stand-alone treatments and in a combinatory setting. Examples such as using oncolytic viruses to display TAAs to dendritic cells are described below.
2.1. Oncolytic Viruses
OVs utilize the altered immunogenicity of tumors to evade phagocytic death and replicate exclusively in the cancerous cells (Chiocca & Rabkin, 2014). Both natural non-pathogenic autonomous OVs (e.g., parvoviruses, myxoma virus (MYXV; poxvirus), Newcastle disease virus (NDV; paramyxovirus), reovirus, and Seneca valley virus (SVV; picornavirus); and genetically engineered OVs (e.g., measles virus (MV; paramyxovirus), poliovirus (PV; picornavirus), vaccinia virus (VV; poxvirus), adenovirus (Ad), herpes simplex virus (HSV), VV, and vesicular stomatitis virus (VSV; rhabdovirus)) have been investigated for tumor immune modulation (Chiocca & Rabkin, 2014). The microenvironment of a solid tumor is characterized by the presence of M2 macrophages (Hobson-Gutierrez & Carmona-Fontaine, 2018), release of cytokines such as IL-10 (Mannino, et al., 2015), and downregulation of type-I IFNs that normally promote an inflammatory response against foreign pathogens (Gajewski & Corrales, 2015). OVs can prey on the mutated machinery of solid tumor cells and induce the release of TAAs and pro-inflammatory cytokines to re-activate systemic antitumor immunity. Here we discuss the main contributors in OV class for tumor immune modulations: adenoviruses, poxviruses (Breitbach, Thorne, Bell, & Kirn, 2012), herpes simplex virus (HSV) (Delwar, et al., 2016), coxsackieviruses, poliovirus, and measles virus (Brown, et al., 2014).
Adenoviruses are versatile and useful because they can be modified and infect many different cancer cell types (Uusi-Kerttula, Hulin-Curtis, Davies, & Parker, 2015). In addition, adenoviruses are typically replication incompetent (dies in the infected cells), achieve high gene delivery and expression, and their viral genome do not incorporate with the host genetic modules preventing genotoxicity (Sato-Dahlman & Yamamoto, 2018). Adenoviruses have been widely investigated against a variety of tumor types for oncolytic disruption of immunosuppressive environment (Gallo, Dharmapuri, Cipriani, & Monaci, 2005). For instance, adenovirus overexpressing the immune co-stimulator OX40 ligand (OX40L) was shown to induce immunogenic cell death (ICD) and recruit lymphocytes to inhibit growth of murine glioma (H. Jiang, et al., 2017). Despite significant promises, the challenges of transient expression, clearance, and non-specific targeting remain a key bottleneck in the clinical translation of adenovirus vectors. This has been addressed to some extent by development of targeted adenovirus, paving the way for clinical use of strain H101 in china (Yokoda, Nagalo, & Borad, 2018). Currently, several adenovirus-based gene delivery systems are in clinical trials for treatment of lung cancers (), cutaneous and uveal melanoma (Garcia, et al., 2019), and (pancreatic, biliary, colorectal or ovarian; ). Early Phase I data from uveal melanoma studies in 12 patients indicates effective infection, but demonstrate a lack of tumor regression. Therefore, additional works are needed such that improved viral capsid and tumor cell interaction is attained to promote uptake and replications in the cancerous cells; details of which were covered in a recent review by Baker et al. (Baker, Aguirre-Hernandez, Hallden, & Parker, 2018).
Among the poxviruses, vaccinia viruses can effectively engage the T cells to promote oncolytic activity and bystander effect against secondary metastatic tumor cells without significant toxicities (F. Yu, et al., 2014). For example, a western reserve strain of vaccinia tested via intratumoral injection in a first-in-human phase I clinical trial was found to be non-toxic in the dose-escalation study (Zeh, et al., 2015). Other virus strains in phase I dose-escalation in advanced cancer cases have also shown superior outcomes with reduced toxicities to healthy organs. For instance, a mutant of HSV-1 replicated in tumors without damaging the surrounding normal tissue (Kasuya, et al., 2014). More recently, a promising OV called JX-594, a modified, replication-incompetent vaccinia virus encoding granulocyte-macrophage colony-stimulating factor (GM-CSF) and LacZ, was shown to inhibit tumor vasculature among other multiple pathways (Merrick, Ilett, & Melcher, 2009). Additionally, when combined with sunitinib, a multitargeted kinase inhibitor, JX-594 amplified antitumor activity (M. Kim, et al., 2018). The detailed review of viral properties of vaccinia, herpes simplex virus (HSV), and coxsackievirus was reviewed recently (Z. S. Guo, et al., 2019). Notable findings from the review suggested that the viruses induce immunogenic tumor cell death, enhance STING and Batf3-dependent dendritic cell activation, and synergize with immunotherapies including checkpoint blockages. The main hurdles for these viruses included the sub-optimal propagation in the entire tumor cell population, inadequate systemic antitumor effects against metastatic cells, and interactions with gut microbiota influencing the oncolysis of tumor cells and therapeutic outcomes.
2.2. Focused Ultrasound, and radiation based physical treatments
Adjuvants used as a part of a cancer therapy utilize a similar concept like OVs to create a more efficient systemic response by changing chemokine profiles, TLR modifications, and acting as a receptor agonist/antagonist to promote a certain pathway, thereby enabling immune detection of cancerous cells (Hammerich, et al., 2016). Adjuvancy to tumors can also be provided by physical treatments such as focused ultrasound (FUS) heating, which causes cell stress and tumor antigen release (Maloney & Hwang, 2015), thus promoting cancer recognition by the immune system. Unlike ionizing radiation, which damages collateral tissues and induces oncogenic proteins, FUS generates protein coagulation and non-lethal thermal stress in the tumors less aggressively (Silvestrini, et al., 2017; van den Bijgaart, et al., 2017). We and others have shown that FUS-induced local heating and stress modify the tumor microenvironment to impart several benefits including enhanced solid cancer chemotherapy, tumor antigen release, expression of heat-shock proteins, upregulation of pro-phagocytic signals such as calreticulin (CRT), and overall tumor immunity compared to conventional treatment (T. Chen, Guo, Han, Yang, & Cao, 2009; T. Chen, Guo, Yang, Zhu, & Cao, 2011; de Smet, et al., 2013; Formenti & Demaria, 2013; Z. Hu, et al., 2007; X. Huang, et al., 2012; Kang, Demaria, & Formenti, 2016; F. Liu, et al., 2010; Manzoor, et al., 2012; Ranjan, et al., 2012; Y. Zhang, Deng, Feng, & Wu, 2010). A tumor microenvironment modified with focused ultrasound can also improve combinatorial immunotherapy regimens. In murine models with bilateral B16 flank melanoma, we performed intratumoral injections of anti-CD40 in combination with FUS heating (M. Singh, et al., 2019). CD-40 is a member of the tumor necrosis factor receptor family that is highly expressed in antigen presenting cells (APCs) including macrophages, monocytes, dendritic cells, and B cells (Afreen & Dermime, 2014; Clark, et al., 1988; van Kooten & Banchereau, 2000). The administration of CD-40 agonists can reprogram macrophages and T-cells. We hypothesized that in situ vaccination with a combination of intratumoral CD-40 agonist antibody and local FUS heating (FUS40) will improve T-cell effector function and trigger tumor suppressive M1 macrophage phenotype in murine melanoma to result in superior anti-tumor effects. FUS heating was applied for ~15 min in right flank tumor, and intratumoral injections of CD-40 were performed sequentially within 4h. A total of 3 FUS and 4 anti-CD-40 treatments were administered unilaterally 3 days apart. Mice sacrificed 30 days post-inoculation showed increased population of tumor-specific CD-4+ and CD-8+T cells rich in Granzyme B+, interleukin-2 (IL-2) and IFN-γ production and with poor PD-1 expression upon the FUS40 combinatorial therapy. The resultant immune-enhancing effects of the combinatorial treatment suppressed B16 melanoma growth at the treated site by 2–3-fold compared to control, FUS, and CD-40, and achieved significant regression in untreated tumors relative to CD40 alone. In addition to CD40 agonists, FUS has also been combined with other therapies (e.g., checkpoint blockade (Silvestrini, et al., 2017), TLR agonist (Chavez, et al., 2018). Early data suggests an increased expression of genes associated with T-cell activation and stimulation (Eomes, Prf1 and Icos was 27, 56 and 89-fold higher) with CpG TLRs and curative treatments upon combination of checkpoint inhibitors with FUS ablation in mice with high melanoma burden. This thereby suggests a need to further investigate the FUS-based combinatorial approaches in clinical settings.
Like FUS, radiation exposure of tumors can enhance immune detection by promoting immunogenic cell death, ATP and HMGB1 secretion and the translocation of calreticulin protein; all of which can result in enhanced tumor cell phagocytosis (Gameiro, et al., 2014). Mechanistically, radiation induced calreticulin in tumor cells is processed by dendritic cells facilitating antigen presentation and T-cell cytotoxicity, whereas the HMGB1 trigger the production of the cytokines TNF, IL-1, IL-6, and IL-8 from monocytes to enhance antigen production from the DCs by binding to toll like receptors, and the released ATPs bind to the purine receptors of DCs causing inflammasome release and IL1β (Z. I. Hu, McArthur, & Ho, 2017). An interesting feature of local radiation immune modulations is its ability to induce systemic antitumor effect. Several preclinical and some clinical case studies support the feasibility of this idea (Azami, et al., 2018; Leung, Wang, Jin-Jhih, & Chan, 2018), although such findings are rare and often inconsistent. Most likely, the immunoactivity of radiation is counterbalanced by suppressive cytokines, and cells (e.g. myeloid derived suppressive cells), thereby suggesting the need for combining them with checkpoint blockage, and other immune modulators. The interrelationship of radiation dose and checkpoint blockade, and outcomes against secondary untreated tumors have been covered in detail by other reviews (Buchwald, et al., 2018; Manukian, Bar-Ad, Lu, Argiris, & Johnson, 2019). In general, the reviews propose an overall optimal immune-stimulating radiation dose in the range of 8 and 10 Gy per fraction in 1–3 fractions, however, additional studies may be needed to verify these findings. It also appears that introducing immunotherapy immediately after radiation induced antigen released could be crucial for more durable response rates in patients.
2.3. Toll like, GM-CSF and CXC-chemokine receptors antagonists
The TLRs recognize different kinds of ligands based on their foreign characteristics. TLRs 1, 2, 4, 6, and 10 recognize specific lipid composition, TLRs 5 and 11 recognize foreign proteins, and TLRs 3, 7, 8, and 9 recognize foreign nucleic acids (Seledtsov, Goncharov, & Seledtsova, 2015). Additionally, TLR 2 recognizes Gram positive bacteria, TLR 4 recognizes Gram negative bacteria, and viruses are recognized by TLR 3 (by dsRNA), 7 (by ssRNA), and 9 (by CpG-DNA). TLR agonists of all kinds can be used as potential immunomodulators. For instance, targeted TLR 9/CpG-DNA using a TLR 9 agonist was used to disrupt the tumor microenvironment of a TC-1 cell line with HPV-16 E6 and E7 oncogenes in C57BL/6 mice containing myeloid-derived suppressor cells, tumor-associated macrophages, and T regulatory cells (Chang, et al., 2014). Stimulation of TLR 2 was found to cause colorectal cancer proliferation and growth whereas suppression by blockade or CRISPR modification induced the opposite effect (Y. D. Liu, et al., 2018). Furthermore, recent studies have shown a role for the TLRs in metabolic reprogramming of immune cells, directly impacting immunotherapy outcomes (L. Huang, Xu, & Peng, 2018). Although a detailed mechanistic discussion of TLR for cancer immunotherapy is beyond the scope of this paper, broadly TLRs act by influencing the differentiation and function of different T-cell subsets, including Th1, Th2 and Th17 cell, and through reprogramming of the suppressive function of tumor-derived CD4+, CD8+ and γδ Treg cells. Thus, the combination of TLRs with FUS (and other therapies e.g., hypo-fractionated radiotherapy, checkpoints, etc.) can be a promising approach to cytotoxic therapy of solid tumors.
Other cancer immunotherapy approaches include incorporation of GM-CSF, CXCR antagonism, and heat shock protein modulations in tumors. GM-CSF is a chemokine that derives its antitumor effect through dendritic cell maturation. Colorectal cells that overexpress GM-CSF do not develop tumors in immunocompetent mice. Interestingly, 100% of patients diagnosed with these specific cancers survived at least 5 years after initial diagnosis (Urdinguio, et al., 2013). This finding suggested that immunomodulator therapies that target GM-CSF expression as a biomarker and a therapeutic can be highly promising. However, further investigation in other cancer models, including non-small cell lung cancer, glioma, human bladder cancer cell line 5637, colorectal cancer, melanoma, and bone metastasis, revealed that the prevalence of GMCSF is, in fact, a negative biomarker linked to a poor survival rate (Aliper, Frieden-Korovkina, Buzdin, Roumiantsev, & Zhavoronkov, 2014). Thus, using GM-CSF as a therapeutic or a biomarker may dependent on the type of cancer and the patient status, and needs detailed investigations prior to clinical introduction and use.
CXC-chemokine receptors are transmembrane G protein-coupled receptors that are known to bind cytokines of the CXC-family (e.g., CXCR2 and CXCR4) and are primarily responsible for growth and movement of cells during angiogenesis and neovascularization. Overexpression of CXCR2 and CXCR7 is a predictive indicator of poor outcome and is associated with metastasis and drug-resistance in human colorectal cancers (Desurmont, Skrypek et al. 2015). In one study, colorectal cancer patients (>50) who had CXCR7 expression above 1 had a survival rate of 28.0 ± 4.67 months compared to 64.4 ± 3.0 months for those with expression levels <1. Likewise, esophageal cancer expressing CXCR2 was linked to significantly higher incidences of distant metastasis (p = 0.0052; 57.6%) compared to those with lower CXCR2 expression (36.7%, p = 0.063) (Nishi, Takeuchi et al. 2015). In another study, CXCR4 and CXCR7 expression in breast cancer cells of 100 breast cancer patients showed a positive correlation with progressive worsening condition associated with many roles- primarily metastasis (Wu, Qian et al. 2015). Further, oral cells expression of CXCR1 and CXCR2 was typically higher in cancerous cells. When exposed to IL-8 and GROα, an increase in Matrix Metallopeptidase 7 (MMP-7) was noticed in oral cancer cell lines compared to normal cells. Thus, while still not fully understood, CXCRs seem to play a role in cancer biology (Khurram, Bingle et al. 2014).
Based on the aforementioned study results, researchers have tried to develop antagonists that would counter the effects of CXCRs. The main effect of such therapies is limiting the migration and metastasis of an existing tumor and improving immunotherapy outcomes (S. B. Peng, et al., 2015; Xiang, et al., 2015). For example, Gil et al. blocked CXCR4 using an oncolytic vaccinia virus to inhibit ID8 mouse ovarian epithelial cancer cell growth and metastasis (Gil, Komorowski et al. 2014). Currently, cancers such as osteosarcoma (K. Jiang, et al., 2019), prostate carcinoma (Goltz, et al., 2016) and hepatocellular carcinoma (Y. Chen, et al., 2015) show significant resistance to checkpoint inhibition. While the exact mechanism is not yet fully understood, it is hypothesized that CXCR4/CXCL12 axis and cancer associated fibroblasts may be responsible for checkpoint resistance (Scala, 2015). For example, in murine pancreatic cancer, Fearon et al. showed that the resistance to checkpoints is mediated via carcinoma-associated fibroblasts (CAF) that also influences the CXCR4/CXCL12 axis (Fearon, 2014). In general, an inverse relationship between CXCR4 expression and immune activation have been established in several studies. For instance, in a K7M2 mouse osteosarcoma model overexpressing CXCR4, the myeloid-derived suppressor cells in tumor inhibited T cell proliferation by up to 80% (K. Jiang, et al., 2019). Dietrich et al. found that patients with high PD-L1 expression and aberrant CXCL12 methylation demonstrated significantly shorter survival rates compared to those with low PD-L1 expression or normal CXCL12 methylation, supporting the link between CXCR4/CXCL12 and PD-1/PD-L1 pathways (Goltz, et al., 2016). To overcome these barriers, Zeng et al. combined AMD3100, a known CXCR4 antagonist (Plerixafor), and aPD-1 antibody alone or in combination in an immunocompetent syngeneic mouse model of ovarian cancer to prolong survival rates by enhancing T-cell function, and reprogramming the T regulatory cells into T-helper cells (Zeng, et al., 2019). In sorafenib resistant hepatocellular carcinoma, Chen et al. combined sorafenib, a broad tyrosine kinase inhibitor, PD-L1 antibodies and AMD-3100 to prevent immune cell suppression, enhance immune cell tumor infiltration, increase apoptosis/necrosis of tumor cells and overall delay hepatocellular carcinoma progression/metastasis (Y. Chen, et al., 2015). The K7M2 mouse osteosarcoma earlier mentioned was also treated with a CXCR4 antagonist with aPD-1 to increase survival of their subcutaneous osteosarcoma model in immunocompetent BALB/c mice. Combination of PD-L1 and K7M2 significantly upregulated the Ki67, intracellular granzyme B, IFN-γ, and TNF-α expression in CD8+ TILs relative to AMD3100 and aPD-1 monotherapy respectively. More recently, Wu et al. showed evidence of modulation of the tumor microenvironment and tumor vasculature in murine glioma models and increased survival in vivo in C57 BL/6 mice with anti-CXCR4 and aPD-1 combination compared to monotherapies (A. Wu, et al., 2018). All of these findings suggest suggesting that CXCR4 antagonism can potentially synergize with the immunotherapies (K. Jiang, et al., 2019).
2.3. Cancer cell vaccines
Interest in the use of DCs or other antigen-presenting cells as a possible immunomodulation therapy for solid tumors has grown immensely. For example, targeting dendritic cells using a B16F10-OVA-liposome in combination with a DC maturation signal such as IFN-gamma or GMCSF liposomes was shown to have potent antitumor activity and decreased lung metastasis in vivo (van Broekhoven, Parish, Demangel, Britton, & Altin, 2004). Waeckerleman et al. used PLGA microspheres as delivery tools for antigen loading of human monocyte-derived DC (hMoDC). They found that this process prolonged antigen presentation of the dendritic cells compared to soluble antigens without impacting the migration, cytokine secretion, survival and allostimulation of T cells (Waeckerle-Men & Groettrup, 2005). Similarly, GM-CSF derived-dendritic cells acted as both antigen donor and antigen presenting cells in murine fibrosarcoma (Ebrahimi-Nik, et al., 2018). Other research has shown the benefits of using a combination of photodynamic therapy (to increase antigen-presenting capacity) with dendritic cell vaccination in squamous cell carcinoma to induce an effective immune response (H. Zhang, et al., 2018). Another combinatorial therapy used an adenoviral vector to selectively activate dendritic cells (Chondronasiou, et al., 2018).
2.4. Limitations
In some cases, viruses can replicate in the patient, so development of replication-incompetent virus is crucial (Chiocca & Rabkin, 2014). Some virus or adjuvant treatments require that the therapeutic agent cross the blood-brain barrier, which is a challenge. Potential approaches that may overcome this problem include using smaller viruses (e.g., parvovirus) or injecting the agent directly into the central nervous system. Moreover, the long-term effect of in situ vaccinations as an immunotherapeutic or prophylactic treatment have not been extensively studied to date.
3. Nanoparticles
NPs can be either inorganic (i.e., gold, gadolinium, etc.) or organic based (i.e. carbon, hydrogen, nitrogen, phosphorus, sulfur) on the elements they incorporate. NPs vary widely in size and shape, but a broad spectrum of acceptable sizes is 1 to 100 nm. Typically, spherical NPs (~100–200nm) are translated for biological applications since they are easy to fabricate and load drugs, but non-spherical structures are also starting to emerge due to their ability to achieve superior accumulation in targeted tissues (Jurney, et al., 2017; Truong, Whittaker, Mak, & Davis, 2015). NPs have unique physical characteristic and capabilities, such as a high surface area to volume ratio which gives the ability to conjugate many different moieties like antibodies, antigens, and small molecules for cell labeling/targeting. NPs have been primarily investigated for targeted delivery of a payload (Fischer, Rasley, & Blanchette, 2014). Other unique features of NPs include optical, thermal, and/or magnetic properties. Within this scope, fine-tuning of NP synthesis has led to the development of agents that are endowed with specified, desired properties such as optimal light-scattering for use in sunscreen lotions to limit direct skin exposure to UVA and UVB rays. Depending on the desired use, NPs can be tailored (Hewakuruppu, et al., 2013), and increase the tumoral exposure by the enhanced permeability and retention (EPR) effect (Baetke, Lammers, & Kiessling, 2015). For example, EPR methodology was used to decrease doxorubicin cardiotoxicity as seen in the case of Doxil®, which is FDA-approved while also enhancing tumor delivery and therapeutic effects (Barenholz, 2012).
Whether organic or inorganic, NPs are typically modified with polyethylene glycol (PEG) groups to help evade detection by the immune system and circumvent a systemic response (Amoozgar & Yeo, 2012). In particular, the most significant interaction between NPs and phagocytic cells appears to be through pattern recognition receptors recognizing pathogen-associated molecular patterns (Ward & Rosenthal, 2014). In contrast, B cell activation within current forms of immunomodulation (outside of the dendritic cell cascade) with nanoparticles is not well known or understood to date.
Although initially NPs were primarily designed to evade the immune response, many recent studies have focused on their use as immunotherapeutics due to their tendency to expand the population of immune cells and alter the cytokine profile around the tumor microenvironment (Almeida, Chen, Foster, & Drezek, 2011). These studies utilized NPs primarily in combinatorial regimens to additively or synergistically enhance specific immune effects in comparison to individual modes of therapy. Some NPs can also work as adjuvants to push T-helper cell activation to either the Th1 or Th2 cell phenotype to promote cell-mediated immunity or humoral immunity. For instance, fullerene cages achieved higher Th1/Th2 ratio in comparison to controls in murine models by decreasing the production of Th2 cytokines (IL-4, IL-5, and IL-6) and increasing the production of Th1 cytokines (IL-2, IFN, and TNF) (Y. H. Luo, Chang, & Lin, 2015).
3.1. Inorganic Nanoparticles (INPs)
Metal/metal oxides NPs, referred to here as INPs, have high optical, thermal, and magnetic properties that are amenable to contrast imaging and targeted therapy. As of current cancer therapies/motifs, the most prevalent INPs seem to include gold nanoparticles (GNPs), superparamagnetic iron oxide NPs (SPIONs), gadolinium NPs and quantum dots (QDs). GNPs can be used with photothermal therapies (Hwang, et al., 2014), as a radiosensitizer (Butterworth, McMahon, Currell, & Prise, 2012) (Jain, et al., 2014), or as a therapeutic/therapeutic carrier (Kumar, et al., 2012) (Ekin, Karatas, Culha, & Ozen, 2014). Additionally, a clinical trial in 2015 reported that GNPs are safe to use in combination with laser ablation in prostate cancers (Stern, et al., 2016).
Iron oxide nanoparticles (e.g., SPIONs) are very popular medicinally because of their unique properties (Kandasamy & Maity, 2015), such as a T2-MRI contrast agent (Yoffe, Leshuk, Everett, & Gu, 2013) (Kato, et al., 2015), their accumulation in tumors (Baetke, et al., 2015), and their highly magnetic properties (Tietze, et al., 2015). In the presence of an external alternating magnetic frequency, SPIONs can also generate local heating by molecular vibrations either via Neel mode that rotate its magnetic moment toward the magnetic field or by rotational friction that is provided by the resistance of the suspending medium (Dan, Bae, Pittman, & Yokel, 2015; DeNardo & DeNardo, 2008). If controlled well, mild heating with AMF do not impact the function of both the SPIONs and proteins/antibodies (Solar, et al., 2015; Thirunavukkarasu, et al., 2018). Johnson et al. showed that SPIONS have potential to replace “sentinel lymph node biopsy in the management of early breast cancer with in vivo pre-operative metastasis detection” by mainly accumulating in the lymph node and surrounding areas of metastasis in a clinical trial (Johnson, Pinder, & Douek, 2013). Maier-Hauff et al. showed the safety and efficacy of intratumoral injections of iron oxide NPs in combination with an alternating magnetic field in 66 patients (59 with recurrent glioblastoma); they described significantly longer overall survival after diagnosis of first tumor recurrence, the safety of therapy along with smaller radiation doses, and general safety with minimal adverse side effects (Maier-Hauff, et al., 2011). The exploitation of SPIONS with cancer biomarkers as targets, such as high overexpression of mucin 1 and folate receptors in most malignant adenocarcinomas (Aghanejad, Babamiri, Adibkia, Barar, & Omidi, 2018) or VEGF-A (vascular endothelial growth factor-A) overexpression in tumor angiogenesis also appears promising (Tsoukalas, et al., 2018). Other imageable NPs include those containing gadolinium (Y. Li, Song, Cao, Peng, & Yang, 2018), as it is an ideal f-block metal for maximizing MRI imaging capabilities. Gadolinium oxide NPs can be synthesized readily (T. J. Kim, Chae, Chang, & Lee, 2013). However, to date the research using these NPs has focused mainly on imaging capabilities and less on immunomodulation.
In this review, we focus on designs that have been synthesized and studied in either in vivo or in vitro models and on the data available in the literature. GNPs of all sizes and shapes have been tested for their potential immunomodulatory effects (P. Singh, et al., 2018), but before delving into this topic, we must understand how different cells interact with these NPs. To understand the interaction and uptake of GNPs, Liu et al. examined accumulation of differently charged GNPs (positively or negatively charged) in phagocytic RAW 264.7 macrophage cells and nonphagocytic HepG2 cells. They reported that RAW264.7 cells accumulated similar amounts of positive and negative NPs, but HepG2 cells accumulated more positive than negative GNPs, suggesting that phagocytic immune cells clears the GNP regardless of the type of surface charge (X. Liu, Huang, Li, Jin, & Ji, 2013). In fact, GNPs are known to stay in liver macrophages for up to 6 months post-treatment (Sadauskas, et al., 2009). Additionally, in macrophages GNPs can show size-dependent inhibition of toll-like-receptor (TLR9) function (C. Y. Tsai, et al., 2012), which illustrates their role in inflammatory pathways. As an immunomodulator, GNPs can act as both a pro-inflammatory and an anti-inflammatory agent (Almeida, Figueroa, & Drezek, 2014). Naked GNPs tend to have a pro-inflammatory response and a cytotoxic effect in macrophages in vitro by increasing levels of TNF-α, IL-1, and IL-6 (Yen, Hsu, & Tsai, 2009), whereas modified GNPs, such as those coated in citrate, tend to be immunosuppressive by downregulating IL-1β, a proinflammatory cytokine, both in vitro and in vivo (Sumbayev, et al., 2013). Functionalization of GNPs with peptides molecules typically aids anti-tumor immunity. For example, GNP bound to TGF-B1 decreased IL-10 to alter the tumor microenvironment, significantly reducing the protective immunosuppressive cytokine profile, and increased levels of tumor-infiltrating CD4+ and CD8+ T lymphocytes in murine bladder cancer models (Y. S. Tsai, et al., 2013). Additionally, certain peptide-functionalized GNPs effectively polarizes liver macrophages to either M2 or M1 (Bartneck, et al., 2012). Overall, data indicate that GNPs increase the immune cell numbers and cytokine profiles depending on the synthetic approach (e.g., coating, shape and size to give a specific, desired therapeutic response).
Iron oxide NPs are generally used for their ability to be imaged by MRI or their magnetic properties (Tietze, et al., 2015). Studies focused on altering the immune profile using iron oxide NPs have reported magnetic field-induced T cell activation with 4-fold greater naïve T cell proliferation compared to the no magnet control (Perica, et al., 2014) or magnet-enhancement in T cell activation with 2-fold more T cell receptor signaling with MHC-Ig and anti-CD28 antibodies bound to iron-dextran NPs in murine B16 melanoma models (Yigit, Moore, & Medarova, 2012). Another advantage of magnetic NPs, such as SPIONs, is their ability to initiate hyperthermia in the tumor while minimizing heat transfer to surrounding tissues (Hoopes, et al., 2018). Huang et al. reported a tumor temperature of up to 66 °C while maintaining muscle tissue at no more than 42 °C (H. S. Huang & Hainfeld, 2013). We and others found that combining iron oxide NPs with Alternating Magnetic Field (AMF) heating aids the immune stimulatory potential of radiation therapy (RT), resulting in an extended tumor control and systemic immune effects in canine and rodent models (Hoopes, et al., 2017). In particular, in murine melanoma models, heating B16 primary tumors at 43°C for 30 min with iron oxide/AMF combination activated dendritic cells (DCs) and propagated melanoma specific CD8(+) T cells in the draining lymph node, conferring systemic resistance against re-challenge with melanoma cells (Toraya-Brown, et al., 2014). More recently, the FDA-approved iron oxide nanoparticle compound, ferumoxytol was found to delay mammary cancers, and lung cancer metastases in liver and lungs of murine cancers by inducing a pro-inflammatory M1 phenotype, protecting against cancer recurrence even in the absence of AMF (Zanganeh, et al., 2016). The proposed mechanism was by creating a cascade of reactive oxygen species (ROS) via the induction of Fenton reaction, followed by iron oxide-induced M1 macrophage polarization. This process augmented the production of TNFα and nitric oxide to promote cancer cell apoptosis. In summary, iron oxide NPs undoubtedly enhances anti-tumor immunity, however, more studies are needed to fully understand how to utilize them in combination with other immunomodulators to achieve superior outcomes against a variety of tumor types.
Quantum dots (QDs) are becoming more and more relevant with the increased manufacturing of them for consumer-based applications. Beyond their industrial use, QDs have shown some potential for imaging within live cells (Michalet, et al., 2005). Like the other NPs discussed, QDs can have immunomodulatory effects. Sub-lethal doses of CdSe/ZnS (cadmium selenide/zinc sulfide) QDs in human epidermal keratinocytes and human dermal fibroblasts have inflammatory effects by increasing multiple cytokines and chemokines such as TNF-α, IL-1β, IL-1, IL-10, IL-6, CASP1 and others associated with immune-activated apoptotic responses. Romoser et al. suggested that QDs control immunomodulation primarily through the NF-κβ pathway in human skin cells (Romoser, et al., 2011). Graphene QDs show a similar trend of NF-κβ inducing generation of ROS, autophagy, apoptosis, and inflammation by promoting TNF-α, increasing phosphorylation of p38 and p65, and increasing expression of caspase-3, caspase-9, IL-1β and IL-8 in THP-1 activated macrophages (Qin, et al., 2015). Data about the effects of QDs will increase drastically over the next decade as more cell studies testing newer QD formulations are conducted.
Other metal-based, inorganic NPs can have immunomodulatory effects as well. For example, silver NPs have been shown to alter the IL-1β profile (Yang, Kim, Kim, & Choi, 2012), or titanium dioxide NPs have been shown to increase the Th2 immune response (Larsen, Roursgaard, Jensen, & Nielsen, 2010). However, only the most popular NPs to date are included in this discussion. Other examples can be found in Table 1.
Table 1.
Nanoparticle immunomodulation information, altered immune profile/toxicity profile, and modified cell type
| Type of Nanoparticle | Immune/Toxic Profile | Mode of Action/Cell type | Reference(s) |
|---|---|---|---|
| Copper oxide/Platinum | DNA damage | Dendritic cells | (Karlsson, Cronholm, Gustafsson, & Moller, 2008) (Asharani, Lianwu, Gong, & Valiyaveettil, 2011) |
| Cobalt oxide | ROS production | Unknown (hypothesized Toll-like receptors) | (Chattopadhyay, et al., 2015) |
| Iron oxide (SPIONs) | Little to none found alone, NF-κβ activation with co-exposure with UVB radiation | Co-exposed with UVB rays alters glutathione and oxidative balance internally, creating cytokine release, cell death, and release of LDH | (Murray, et al., 2013) |
| Gold | Immunostimulatory-IL-1↑, IL-6↑ TNF-α↑, IFN-λ↑, MΦ−1 polarization, binds TGF-B1, IL-10↓ Immunosuppressive-IL-1β↓, MΦ−2 polarization, binds TLR9 |
Macrophages, dendritic cells, and neutrophils | (Asharani, et al., 2011) |
| Silver | High levels of DNA damage Immunosuppressive-IL-1β↓ | Toll-like receptors, T lymphocytes, suppression of natural killer cells in spleen | (Asharani, et al., 2011) (Lappas, 2015) |
| Nickel | Less toxic than soluble nickel | Hypothesized Toll-like receptors | (Ispas, et al., 2009) |
| Zinc oxide | DNA damage and decreased cell viability | Toll-like receptors | (Y. H. Luo, et al., 2015) |
| Organic-coated silver | ROS production, DNA damage, inflammation | Damage to cell membrane, mainly observed in epithelial cells | (V. K. Sharma, et al., 2014) |
| Quantum dot | TNF-α↑, IL-1β↑, IL-10↑, IL-6↑, IL-1↑, IL-8↑, caspase-3↑, caspase-9↑ | Skin cells, macrophages | (Almeida, et al., 2011) (Bilan, Fleury, Nabiev, & Sukhanova, 2015) (Michalet, et al., 2005) (Qin, et al., 2015) (Romoser, et al., 2011) |
| Titanium dioxide | ROS production, oxidative stress, mitochondrial damage | Toll-like receptors, T lymphocytes, inflammatory response resulting in T lymphocyte, B cell, and natural killer cell increases | (Czajka, et al., 2015) (Lappas, 2015) (Demir, Burgucu, Turna, Aksakal, & Kaya, 2013) |
| Calcium phosphate | Limited, negligible cytotoxicity | T lymphocytes and T cell maturation stage, negligible liver cell cytotoxicity | (Q. Wang, et al., 2015) (Petrarca, et al., 2015) |
| Zirconium dioxide | Little to none found, including no genotoxic effects | -------- | (Demir, et al., 2013) |
| PLGA | Little to none reported, varies with other moieties involved or attached | Dendritic Cells and other antigen presenting cells through phagocytosis mainly | (D. Singh, Singh, Sahu, Srivastava, & Singh, 2016) |
| Palladium | Increase of stopping cells at checkpoints G0/G1 phase | Tested with human peripheral blood mononuclear cell | (Petrarca, et al., 2014) |
3.2. Organic Nanoparticles
Liposomes, and core-shell polymeric structures are commonly used organic NPs. Liposomes have an internal water-soluble cavity with a phospholipid bilayer membrane (W. D. Wu, et al., 2015). A meta-analysis of 10 randomized controlled trials showed that a water-soluble therapy, such as doxorubicin, could be encapsulated in a liposome to lower cardiotoxicity of normal routes of therapy (Xing, Yan, Yu, & Shen, 2015). Doxorubicin (DOX) can also be attached to the surface of micelles or dendrimers. Functionalization of the surface can be achieved in all cases with specified methodologies. Altering the lipid profile of liposomes lends the ability to burst them in response to hyperthermic conditions and aids in the concept of a targeted, deliverable payload (Kong & Dewhirst, 1999). Additionally, manipulation of the surface with proteins or ligands to target certain processes or cell types further enhances the specificity of targeted delivery for a desired effect.
This review focuses mainly on liposomes and poly(d,l-lactide-co-glycolide) nanoparticles (PLGA) polymeric nanoparticles for immunotherapy. Liposomes can be used as hollow transportation vessels for chemotherapies, but some formulations (e.g., cationic liposomes) can also have immunomodulatory effects via stimulation of the antigen presenting dendritic cells (DC) leading to the expression of co-stimulatory molecules, CD80 and CD86 (Vangasseri, et al., 2006). Collins et al. showed that ovalbumin (OVA)-encapsulated acid-resistant liposomes could generate class I MHC-restricted T cell responses in vivo by lysosomal processing and recycling of immunogenic peptides (D. S. Collins, Findlay, & Harding, 1992; Harding, Collins, Kanagawa, & Unanue, 1991). Suzuki and colleagues used unmethylated cytosine-phosphorothioateguanine oligodeoxynucleotide (CpG-ODNs)-encapsulated cationic liposomes as an adjuvant to actively target antigen presenting cells (e.g., dendritic cells) by binding to TLR9 and increasing serum IL-12 levels, increasing type I innate immunity (Suzuki, et al., 2004). TLR9, the target of CpG typically localizes in the endosomal/vacuolar/vesicular compartments but not to the cell surface. Data suggested that liposome encapsulation of unmodified CpG-ODN enhanced the dendritic cell uptake and IL-12 production. Additionally, this approach induced IFN-γ but not IL-4 production by natural killer cells by ligating TLR9 to CpG-liposome coencapsulated ovalbumin (OVA); features essential for Th1-dependent cytotoxicity against OVA-expressing tumor cells. If used in combination with a stimulatory RNA or DNA, liposomes can also be used for targeted epitope expression and activation of T-cells. RNA encoding tumor associated, or tumor specific epitopes, were encapsulated in liposomes to enhance the targeting of antigen-presenting cells (i.e., splenic macrophages, dendritic cells, Kupffer cells) for epitope encoded activation of the T cell (Sayour, Mendez-Gomez, & Mitchell, 2018). The key benefits of the RNA-liposome approach include immune activation following parenteral injections, simultaneous loading of multiple epitope generating mRNAs and the induction of type I interferon response. Liposomes and/or liposome-type NPs have also shown to deliver plasmid DNAs that normally would be very difficult to achieve (Moon, et al., 2011). For instance, cationic liposomes were shown to enhance the delivery of plasmid DNA to elicit antitumor immunity in murine model of cervical cancer (Sayour, et al., 2018). Unlike viral vectors that risk mutagenic integration with host cells, liposomes are a promising and safer gene drug delivery technology (Seow & Wood, 2009), and thus are starting to enter clinical trials for immunotherapy investigations (Madhusudan, et al., 2004; Wakabayashi, et al., 2008; D. Wang, Sun, Liu, Meng, & Lee, 2018; Yoo, et al., 2001). Studies are currently underway to further improve the transfection and expression efficiencies in a targeted manner in tumor cells while reducing side effects to healthy organs and induction of autoimmunity.
PLGA NPs have been thoroughly investigated and found to be very compatible with cells and tissues, as they have a very low toxicity profile and provide controlled release of encapsulated proteins. Many researchers have investigated different preparation techniques, drug encapsulations, and surface modifications (Sadat Tabatabaei Mirakabad, et al., 2014). PLGAs have been synthesized with a combination of Poloxamer 235/encapsulated docetaxel to treat the multidrug resistant human breast cancer cell line MCF-7/TXT, but they had a significantly higher toxicity profile than the control (Tang, et al., 2015). Several recent studies have assessed potential modalities of PLGA NPs in combination therapy and/or as a targeted delivery transporter (Heit, Schmitz, Haas, Busch, & Wagner, 2007). As an immune therapy, PLGAs can incorporate other moieties, just as liposomes do, to increase potential application in a combination therapy (e.g., as a carrier for a vaccine or drug). Using the B16 cell line, Zhang et al. reported data that support effective induction of an antitumor cytotoxic T cell response when PLGA NPs containing murine melanoma antigenic peptides or TLR4 agonist were used in a prophylactic setting. Moreover, when administered with interferon gamma (IFN-λ), the antitumor activity was significantly augmented (Z. Zhang, et al., 2011). Furthermore, exogenous antigens seem to have a much higher MHC class I presentation in vivo when loaded into PLGA NPs. It was reported that “in primary mouse bone marrow-derived dendritic cells, the MHC class I presentation of PLGA-encapsulated OVA stimulated T cell interleukin-2 secretion at 1000-fold lower concentration than soluble antigen and 10-fold lower than antigen-coated latex beads”(H. Shen, et al., 2006; Waeckerle-Men, et al., 2006). Chen et al. showed that PLGA-NPs coencapsulated with indocyanine green (ICG), a photothermal agent, and imiquimod (R837), a TLR7 agonist was effective in inducing simultaneous photothermal ablation locally, and triggering release of TAAs for superior immunological responses with checkpoint therapies in malignant and orthotopic murine breast and colon cancer models (Q. Chen, et al., 2016). It may be noted that although PLGA-NPs loaded with antigen can enhance anti-tumor immunity, however, they also generate high population of T-regulatory cells, suggesting a need to combine them with checkpoint blockade. In summary, considering that PLGA material is FDA-approved, and achieves multifunctional outcomes as listed above, the improvement in particle design principles hold significant potential for immunotherapy applications.
Interest in the NP approach has even extended into synthesizing combination organic/inorganic NPs and synergizing their therapeutic effects with device applicators. Combinations could include incorporating metals into the film preps of organic NPs, adding polymers during metal NP synthesis, crosslinking metal-containing molecules to previously made organic NPs, or crosslinking proteins to previously synthesized inorganic NPs. Examples of combination NP approaches could include organic-coated silver NPs (V. K. Sharma, Siskova, Zboril, & Gardea-Torresdey, 2014), fluorescent sulfonate-based inorganic-organic NPs (Poss, Zittel, Meschkov, Schepers, & Feldmann, 2018), zinc-doped iron oxide core NPs with a biocompatible mesoporous silica shell with miRNA and DOX (Deng, et al., 2018), hydrophilic surface-functionalized superparamagnetic NPs to be used in magnetic fluid hyperthermia-based thermotherapy (Kandasamy, Sudame, Luthra, Saini, & Maity, 2018), and biocompatible chitosan-functionalized upconverting nanocomposites (Duong, et al., 2018). Many NPs combination have been extended for immunoadjuvant capabilities with increased efficiency. For instance, iron oxide-zinc oxide core-shell nanoparticles with ZnO-binding peptides were used for improved delivery of tumor antigens and the real-time imaging of dendritic cells trafficking in lymph nodes. In vivo studies in murine tumor models by magnetic resonance imaging suggested an effective uptake by the dendritic cells. Additionally, an antigen specific T-cell activation resulting in a superior tumor regressions and survival rates were noted (Cho, et al., 2011). Likewise, core-shell lipidic nanoparticles composed of Fe3O4 magnetic nanoclusters (MNCs) and anti-CD205 decorated shells were shown to facilitate efficient dendritic cell uptake and MHC I cross-presentation in 4T1 murine breast cancer model. The resultant immune activation efficiently propagated CD8+ T cells and tumor control of malignant tumors, while also providing efficient tracking of immunotherapy responses by MRI imaging (F. Li, et al., 2019). Like lipids, iron oxide along with perfluoropentane (PFP) and anti PD-1 (aPD-1)-coated with PLGA shells have been investigated for local checkpoint inhibitor delivery (N. Zhang, et al., 2019). Several other nanoparticles incorporating PLGA have been seen to improve immunotherapy outcomes in murine models not discussed here have also been reported in literature (Cruz, et al., 2014; Jin, Luo, Zhang, & Pang, 2018; Saengruengrit, et al., 2018).
Other combinatorial approach to aid NP immunotherapy includes synergizing its therapeutic effect with photothermal modality. For instance, PLGA NPs effectively delivered aPD-1 locally especially when combined with photothermal therapy and that this combination is effective in tumor control via increased CD8+ T cell infiltration in melanoma (N. Zhang, et al., 2019). Similarly, chitosan-coated-CpG hollow copper sulfide (CuS) NP achieved tumor size reduction in both primary and secondary untreated tumors in a mouse breast cancer model with photothermal therapy (L. Guo, et al., 2014). Therapeutically, following laser irradiation, the intratumorally injected NPs disintegrated and reassemble into chitosan-CpG nanocomplexes, allowing CpG tumor retention and uptake by dendritic cells and enhancing the cross-priming of the tumor antigen-specific T cells. In another study, administration of CpG oligodeoxynucleotide-coated Prussian blue nanoparticles (CpG-PBNPs) induced tumor cell death and antigen release with photothermal therapy in vitro and in murine model of neuroblastoma (Cano-Mejia, Bookstaver, Sweeney, Jewell, & Fernandes, 2019). Importantly, PBNPs alone enhanced the anti-CTLA-4 checkpoint inhibition effect to result in significantly higher survival (55% survival vs. 12.5% aCTLA-4 alone) and increased protection to re-challenge in the neuroblastoma murine model (Cano-Mejia, et al., 2017). Other types of NPs such as PEG-PEI-covered nanosized graphene oxide sheet encapsulating CpG oligodeoxynucleotide has also been found to have immunostimulatory properties with photothermal therapy (evaluated by TNF-α and IL-6) and enhanced survival in vivo in a colon cancer model (Tao, Ju, Ren, & Qu, 2014). In the future, the NP approach with the most usage among the previously listed options could be used in a combination treatment to effectively synergize therapies while limiting negative effects such as toxicity or high excretion rate. INPs can be incorporated in organic materials to increase the immunomodulatory effects and increase stealth delivery and local biological effects.
3.3. Immunotoxicity/Limitations
For the most part, NPs have been thoroughly investigated for their general acute and/or chronic toxicities. Metal or metal oxide NPs exposure by inhalation due to recent industrial and medicinal advancements remains a concern (Bakand, Hayes, & Dechsakulthorn, 2012). Previous studies have shown that oral dosages are significantly less toxic in comparison to the inhalation route of exposure. The general mode of toxicity can be necrotic and/or apoptotic separately and independently (Buerki-Thurnherr, et al., 2013). As a general rule when comparing metal and metal oxide NPs, the toxicity profile increases with a reduction in size; however, there still is a difference depending on the metal used (Sutunkova, et al., 2018). Table 1 shows the immunotoxicity profile of different NPs. Organic NP toxicity is generally less cumbersome because they are naturally occurring biocompatible compounds that tend to be less hazardous than metals. A few cases of NPs with a high level of toxicity have been reported, but many adverse side effects can be decreased and minimized by incorporating some modification of surface chemistry (e.g., lipids or proteins) to make them “stealthier” and not as cytotoxic. Considering that the NP immunomodulation approach is a relatively new field, the continued learning of managing adverse toxic effects should be a key goal. We propose that combination immunotherapies (e.g., modified NP plus checkpoint inhibitor) rather than nanoparticle alone is the way to translate this approach for clinical use. Furthermore, development of targeted approaches such as the use of anti-CD8a F(ab’)2 fragment decorated NPs for CD8+ T cell attachment (Schmid, et al., 2017), and leveraging the inherent physicochemical properties of nanomaterials for modification of innate immunity (Y. Liu, Hardie, Zhang, & Rotello, 2017) can provide innovative ways to improve therapeutic outcomes in patients.
4. Checkpoint Inhibitors
Checkpoint inhibitors are proteins or monoclonal antibodies that function to downregulate the natural immune response mediated by CTLA-4 and PD-1 pathways to foreign cancer cells. The two predominantly discussed checkpoint inhibitors include anti CTLA-4 and anti PD-1/PD-L1. Some less studied and more recent checkpoint inhibitors are briefly discussed as well. Table 2 describes the drugs used for the clinically relevant PD-1 and CTLA-4 pathways, their pathway of inhibition, and which cancer cell lines are most affected to date.
Table 2.
Comparison of current checkpoint inhibitor drugs, mode of inhibition, and cancer treated with anti CTLA-4 and anti PD-1, anti PD-L1 targets
| Drug/Antigen | Inhibition | Cancer Cell Line | Reference(s) |
|---|---|---|---|
| Pembrolizumab | PD-1 | Hodgkin lymphoma, melanoma, non-small cell lung | (Long, et al., 2017), (Deeks, 2016) |
| Nivolumab | PD-1 | Melanoma, non-small cell lung, kidney, colorectal | (Overman, et al., 2017) |
| Atezolizumab | PD-L1 | Non-small cell lung and bladder | (W. Luo, Wang, Tian, & Li, 2018) |
| Durvalumab | PD-L1 | Bladder and urinary tract | (Brower, 2016) |
| Avelumab | PD-1 | Non-small cell lung, Merkel cell carcinoma | (Gulley, et al., 2017), (Kaufman, et al., 2016) |
| Ipilimumab | CTLA-4 | Metastatic melanoma | (Williams, et al., 2017), (Long, et al., 2017) |
| Atezolizumab+vemurafenib, cobimetinib | PD-L1 +BRAF inhibitor and/or MEK inhibitor | BRAFV600-mutated metastatic melanoma | (Sullivan, et al., 2019) |
| Pembrolizumab+Entinostat | PD-1 +class I HDAC inhibitor | Uveal malignant melanoma | (Jespersen, et al., 2019) |
| Pembrolizumab | PD-1 | Resectable NSCLC stage II/IIIA | (Eichhorn, et al., 2019) |
| Nivolumab vs. Everolimus | PD-1 vs. mTOR inhibitor | Advanced renal cell carcinoma | (Tomita, et al., 2019) |
| Nivolumab vs. Ipilimumab | PD-1 vs. CTLA-4 | Advanced esophageal squamous cell cancer | (Meindl-Beinker, et al., 2019) |
| Avelumab+Chemoradiatherapy | PD-L1 | Advanced squamous cell carcinoma of the head and neck | (Y. Yu & Lee, 2019) |
| Nivolumab+Metformin | PD-1 +antihyperglycemic agent | Non-small-cell lung cancer or pancreatic cancer | (Kubo, et al., 2018) |
| Nivolumab+Ipilimumab | PD-1 +CTLA-4 | Stage III or IV Unresecetable advanced melanoma | (Larkin, et al., 2015) |
| Durvalumab+Tremelimumab | PD-L1 +CTLA-4 | Metastatic head and neck carcinoma, Non-small-cell lung cancer | (Bahig, et al., 2019) (Planchard, et al., 2016) (Antonia, et al., 2016) |
4.1. Anti PD-1
PD-1 is a cell surface protein expressed on both activated T cells and professional B cells. This immune checkpoint receptor in the immunoglobulin superfamily and in the extended family of CD28/CTLA4 binds PD-L1, also known as CD274 or B7-H1, which is a transmembrane protein in tumor cells (P. Zheng & Zhou, 2015) (Afreen & Dermime, 2014). The normal function of this receptor interaction is downregulation of apoptosis of T regulatory cells and suppression of antigen-specific immune cells proliferating in the lymph nodes, therefore promoting an immunosuppressive environment. These help reduce autoimmunity complications and maintain homeostasis of immunity. However, certain cancers (e.g., metastatic melanoma, non-small cell lung cancer, and renal cell cancer) can disrupt PD-1/PD-L1 expression to evade immune detection (Hargadon, Johnson, & Williams, 2018). The recent interest in blocking the cascade reaction of immunosuppression from PD-1/PD-L1 by blocking either the protein, PD-L1, or the actual receptor, PD-1, to activate effector T-cell function and tumor cell killing has resulted in a plethora of recently synthesized antagonists (Table 2). The PD-1/PD-L1 axis has also been exploited in HIV-1, which perpetually downregulates the activation of T cells by binding PD-1; this shows the potential of using aPD-1 therapies as an anti-HIV therapy (Gay, et al., 2017). Interestingly, PD-L1 can also be expressed and upregulated in immune cells via IFN-γ–induced adaptive regulation in tumors through pre-existing immunity, and a high PD-L1 expression in tumor immune cells generally correlates with a more durable clinical responses to aPD-L1s (Fehrenbacher, et al., 2016; Kowanetz, et al., 2018; Yamazaki, et al., 2002). A meta-analysis of 18 studies involving 3647 patients in breast cancer, 4,549 cases of non-small lung cancer and 37 stage III malignant melanoma patients showed that the expression of PD-L1 in tumor infiltrating immune cells correlated strongly with enhanced survival rates (Jacquelot, et al., 2017; Kowanetz, et al., 2018; Zhao, Li, Wu, Li, & Zhang, 2017).
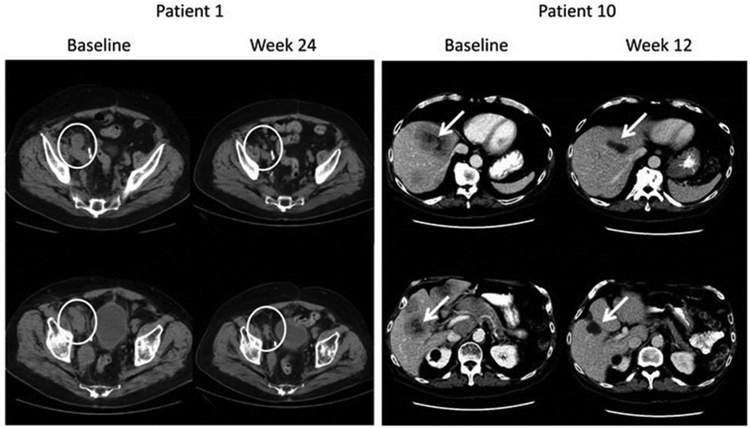
Clinical trials are increasingly being reported for a variety of PD-1 checkpoint inhibitors. For example, aPD1 Nivolumab was tested versus docetaxel in advanced non-small-cell lung cancer during or after platinum-based therapy in over 500 patients to determine if the treatment significantly increased overall survival rates (Borghaei, et al., 2015). Data showed that the median overall survival was significantly higher for nivolumab group (12.2 months; 292 patients) versus the docetaxel group (9.4 months; 290 patients). Lipson et al. reported results from a pilot phase I clinical trial in 3 patients diagnosed with colorectal cancer, renal cell cancer, and melanoma who were treated with aPD 1 antibody BMS-936558; they found a complete response in colon and renal cell cancer, and a partial response in melanoma. Also, the re-induction therapy in melanoma was shown to be effective upon remission (Lipson, et al., 2013). In another study, Lipson et al. characterized the therapeutic efficacy of pembrolizumab (aPD-1) in a patient with metastatic PD-L1 (+) basal cell carcinoma. A partial response was noted up to 14 months after initiation of therapy (Lipson, et al., 2017). Likewise, a phase 3 double-blind clinical trial conducted to evaluate the PD-1 inhibitor, pembrolizumab, as an adjuvant therapy in high-risk stage III melanoma after complete resection showed significantly longer recurrence-free survival in comparison to placebo with a dosage of pembrolizumab every 3 weeks for up to a year (ClinicalTrials.gov number, ) (Eggermont, et al., 2018). Yet another clinical trial showed evidence of aPD-1 activity in enzalutamide-resistant prostate cancer in two out of 10 patients (Graff, et al., 2016) (Fig. 2). Both of these patients partially responded to the treatment and the outcomes correlated with microsatellite instabilities in the genome of prostate cancer cells. Although promising, symptoms of myositis, hypothyroidism, and hypothyroidism were noted in the patients. More recent studies have shown that the toxicities can be addressed in combinatorial regimen, and several inhibitors are starting to enter large scale trials, or receive FDA approval for therapy of malignant therapy (J. M. Collins & Gulley, 2019; X. Shen & Zhao, 2018). Additionally, it has been established that checkpoint therapies are most effective when PD-L1 is expressed in immune cells (T-cell, macrophages) along with tumor cells, personalizing therapies and outcomes.
Fig. 2.
Anti-PD1 (pembrolizumab) therapy induces regression of castration resistant prostate tumors in patients. In patient 10, a significant reduction in the metastatic sites was noted 12-weeks post treatment with anti PD-1 and enzalutamide. Patient 1 also showed significant reduction in tumor size post treatment (Fig. adapted from Graff et al). Reproduced with permissions from Oncotarget, 2016; 7:52810–52817.
4.2. Anti CTLA-4
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a homologue to CD28 on T-cells and binds to CD80 (B7–1) or CD86 (B7–2) on APCs (e.g., dendritic cells) to regulate immune function. Unlike CD28 that stimulates immune response, CTLA-4 has a regulatory/inhibitory function, and demonstrates a higher affinity for APC receptors than CD28. Anti CTLA-4 drugs are in clinical use as an immunomodulatory therapeutic because certain cancer cell lines, such as non-small cell lung cancers (Clinical trial identifier: , , , ), small cell lung cancers (), gastric adenocarcinoma (), hepatocellular carcinoma (), and various others (), inhibit immune activation and cytotoxic killing through this pathway (Walker & Sansom, 2015).
Initial studies with ipilimumab demonstrated the early foundation for this idea (Blank & Enk, 2015). Later in vivo studies in which human CTLA-4 was “knocked-in” in mouse models allowed successful screening of anti-human CTLA-4 therapies (e.g. hu-PBL-SCID mouse models of Epstein-Barr virus-associated lymphoma) (May, et al., 2005). Like anti-PD1, anti-CTL4s enhance survival responses in melanoma, renal cell carcinoma, squamous cell carcinoma, and non-small cell lung cancer patients when compared to conventional chemotherapies (Seidel, Otsuka, & Kabashima, 2018). Importantly, in malignant melanoma, PD-1 blockage is traditionally more effective than CTLA-4 inhibition, and when combined, a significantly longer progression-free survival than monotherapy alone is generally seen. This important finding paved the way for the first checkpoint to receive FDA approval as a combinatorial regimen (Larkin, et al., 2015). Currently, nivolumab (aPD-1) combined with ipilimumab (fully humanized aCTLA-4) is under investigation in over 100 clinical trials in various stages (Rotte, 2019). The proposed mechanisms of enhanced effects of aCTLA-4 and aPD-1 combination are through an enhancement of tumor induced lymphocyte (TIL) activity, and by a decrease in the frequency and function markers of Treg cells (Chae, et al., 2018). A benefit of such an approach is also the reduced immune related adverse events in patients. For example, reduced-dose ipilimumab followed by standard-dose pembrolizumab (aPD1) in 153 melanoma patients was found to provide robust anti-tumor immune effects, and reduced toxicities (Long, et al., 2017). Thus, the current clinical data suggest that checkpoint combinatorial regimen are likely to result in enhanced outcomes against a variety of tumor types.
4.3. Toxicity/Limitations
The efficacy of aPD-L1 therapies have been shown to correlate with PD-L1 expression in patients especially in melanoma patients (PD-L1 positive: 43.6% vs. PD-L1 negative patients: 20.3%) (Daud, et al., 2016). Thus, newer innovations that increase the expression of PD-L1s in tumor are needed to optimize treatment outcomes. Another limitation of using checkpoint inhibitors is the adverse side effects that are commonly experienced. Long-term follow-up of previous clinical data has shown therapy-induced hypophysitis after ipilimumab that involved hormonal imbalances (Albarel, et al., 2015). A phase 2 study using pembrolizumab in advanced soft-tissue and bone sarcoma showed post-treatment adverse events including symptoms of adrenal insufficiency, pneumonitis, and nephritis. The primary endpoint was not completed according to the researchers due to the aforementioned side effects (ClinicalTrials.gov, number ) (Tawbi, et al., 2017). Another phase 2 clinical trial that used nivolumab as a treatment for adult T cell leukemia-lymphoma showed an increase in disease progression in all three of the first three patients within a week rather than it being an effective therapeutic as a PD-1 therapy (ClinicalTrials.gov number, ) (Ratner, Waldmann, Janakiram, & Brammer, 2018). Another pitfall is the lack of an overly expressed PD-1/PD-L1 or CTLA-4 axis for all cancer models. Additionally, despite profound clinical benefits, a large proportion (>50%) of patients still do not respond to the immunotherapy. This is attributed to a lack of a baseline T-cell infiltration, the dependence of immunotherapies on interferon gamma (IFN-γ) producing CD8+ T-cells, and selection of less-immunogenic tumor cells such as those with loss of function mutation of PTEN that blunt tumor-cell antigen cross presentation, and c-Myc signaling that influences expression of checkpoint molecules (Casey, et al., 2016; Horton, Williams, Cabanov, Spranger, & Gajewski, 2018; W. Peng, et al., 2016; Rizvi & Chan, 2016; Spranger, Bao, & Gajewski, 2015; Szczepaniak Sloane, et al., 2017). Furthermore, not all cancer cell lines that express checkpoints respond favorably to inhibitors. In one study, only 45.3% of 72 cases of pulmonary neuroendocrine tumors showed positive expression of PD-L1, and positive expression was not associated with overall survival or progression-free survival (H. Wang, et al., 2018). Overall, the most useful direction for checkpoint inhibitor therapies seem to be in chemoand radio-resistant tumors, but mitigating the adverse side effects evident in the current approaches is an unmet gap; therefore, further research is needed to optimize these approaches.
5. Noninvasive Imaging of Immune Cells
A challenge in current immunotherapy and immunomodulation therapy is the uncertain nature of immune activation depending on the tumor type. In general, it is extremely difficult to assess immune responses longitudinally in solid tumors for multiple reasons. First, the tumor microenvironment is highly adept at generating an immunosuppressive response to avoid immune detection. Second, compared to immune activation, relatively longer time is typically required for tumor regression, so correlating immune modulation with therapeutic outcome is difficult. Thus, it is critical to develop new abilities to determine the immune modulations during and after immunotherapy, so that it can be linked to treatment planning and dosing. Towards this goal, an effective non-invasive imaging approach should image tumors without impacting the immune response, provide feedback to both patient and clinician in a longitudinal manner, and identify immune cell types rank them in order of their tumor infiltration rates to provide efficient tracking of immune therapy success.
5.1. T cell imaging
In terms of imaging a broad, systemic immune response, the most obvious target is the T cell. The tracking of T cells was reported using Cerenkov luminescence imaging (CLI) and radioluminescence imaging in murine tumors in both in vivo and ex vivo models with 32P-ATP labeled T lymphocytes (Boschi, De Sanctis, Ugel, & Spinelli, 2018). CLI is an optical imaging technique that provides detection of beta emitter’s radionuclides with endpoint energy of 261 keV in water and around 219 keV in biological tissues. For the detection of the T-cells in vivo, the research team generated orthotopic Panc02 tumors in C57BL/6 mice and adoptively injected the32P-ATP labeled T lymphocytes by intravenous and intraperitoneal route. Bioluminescent images were acquired at different time points (from 30 minutes to 18 hours) from the pancreatic regions. Results suggested a high increase of light emission in the body compared to pre-injected time and the control group. Similarly, another group used T cells labeled with NIR-797-isothiocyanate as a near-infrared photoacoustic and fluorescent agent to noninvasively determine the location of T cells in diseased tissues at different time points following intravenous injection (S. Zheng, et al., 2018). More recently, a T cell-specific PET agent, [18F]F-AraG was evaluated in a rhabdomyosarcoma model. A high accumulation of the PET agent in CD8+ T cells in the murine tumors was observed. The PET agent could also differentiate the differences in signal between mice that responded to and those that did not respond to aPD-1 therapy (Levi, et al., 2019). In another study, genetically modified CD8+ autologous cytotoxic T lymphocytes (CTLs) co-expressing the chimeric antigen receptor (CAR) interleukin-13 (IL-13) and the herpes simplex virus type 1 thymidine kinase gene (HSV1-tk) were imaged using radiolabeled 9-[4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine ([18F]FHBG) penciclovir (antiherpes drug in humans). Data suggested an increase in PET signal after cytotoxic T lymphocytes (CTL) infusions in patients, suggesting that this approach can provide a means to image immune cells in real-time (Keu, et al., 2017). Alternative approaches that combine contrast-enhanced CT images, RNA-seq genomic data, and histopathological features of tumor biopsies to assess CD8 cell tumor infiltration are also being widely reported for characterization of immune cell phenotypes and likely success to checkpoint therapies (R. Sun, et al., 2018). Furthermore, a variety of approaches has also been reported for imaging of T-regulatory cells with via two-photon (or multiphoton) microscopy; detailed review of which was recently published (Hickey & Chow, 2017).
5.2. Macrophage Imaging
Macrophage phenotypes play an important role in determining effectiveness of immunotherapy. Within the tumor microenvironment, M2 macrophage phenotype can be the predominant macrophage phenotype (Hobson-Gutierrez & Carmona-Fontaine, 2018). Repolarization therapy that modifies macrophages from pro-tumoral M2 macrophage to anti-tumoral M1 phenotype has the capability to sensitize and/or effectively kill tumor cells.
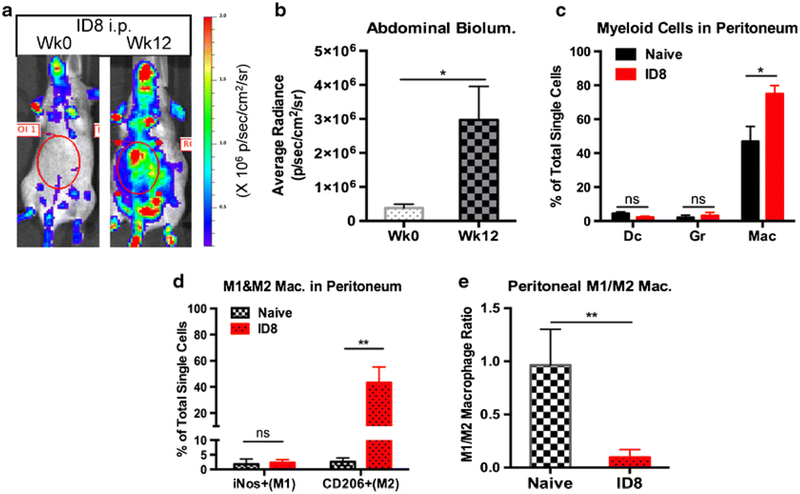
Macrophages’ membrane receptor targeting, or passive uptake can be utilized for their imaging. For example, M2-oriented macrophages were imaged using 18F-labeled camelid single-domain antibody fragments that bind to the macrophage mannose receptor for PET imaging (Blykers, et al., 2015). Such selective phenotype-specific macrophages imaging can allow monitoring of the disappearance or the appearance of M2 macrophages to show the progression of therapy that is otherwise unobservable. In a 4T1 mouse breast cancer cell model, Sun et al. developed NIRF-dyed anti CD-206 antibodies to track the location of M2 macrophages in tumors (X. Sun, et al., 2015). While targeting a specific macrophage subpopulation such as M1 versus M2 has not yet been reported, their carbohydrate linker offers the potential to link affinity ligands to bind to specified or desired locations/cells (Nahrendorf, et al., 2008). Additionally, transgenic mouse model for in vivo imaging of luciferase-expressing macrophages has been reported to understand candidate immunotherapeutics. In one study, B6.129P2-Lyz2tm1(cre)Ifo/J mice expressing Cre recombinase under the control of the lysozyme M promoter (LysM) were crossed with Cre-lox Luc reporter mice (RLG), to produce LysM-LG mice, resulting in generation of mice with macrophages positive for luciferase expressions. Orthotopic induction of B16 melanoma and ID8 ovarian cancer cells in the skin and ovarian tissues of these mice allowed the tracking of macrophages in the tumor milieu. In general, a significant enhancement in the bioluminescence signal in the tumoral regions was noted longitudinally over time, with flow cytometry suggesting the macrophages to be mostly of the M2 origin, mimicking clinical conditions (Fig. 3) (He, et al., 2017).
Fig. 3.
Tracking infiltration of tumor associated macrophages (TAMs) in murine tumors. A genetically engineered mouse model with bioluminescent TAM was developed. a-c) Upon implantation of ovarian cancer (ID8), a significant enhancement in the TAM infiltration in the peritoneal cavity was noted; d-f) The immunosuppressive ID8 tumors skewed the phenotype of macrophages towards pro-tumoral M2 macrophage. Reprinted with permission from Springer: Springer Nature [Molecular Imaging and Biology] [He et al.] (Imaging of Tumor-Associated Macrophages in a Transgenic Mouse Model of Orthotopic Ovarian Cancer, Huanhuan He, Alan C. Chiu, Masamitsu Kanada et al), [COPYRIGHT] (2017)
Another means of noninvasively imaging macrophages can be to track the byproducts of macrophages or other phagocytic cells “at work.” For example, a fluorescent probe to track hypochlorous acid (HClO) as a means of showing “apoptosis” or cell death via phagocyte activation in macrophages was developed (Zuo, et al., 2012). HClO is produced through a respiratory burst in which the cell takes up oxygen and forms superoxide anions. Superoxide reacts with hydrogen ions, or protons, to form hydrogen peroxide. Although HClO can also be formed by neutrophils, this may still be a useful approach for tracking the metabolic profile of macrophages.
5.3. Limitations
An obvious drawback to this approach is the unintended change of function of immune cells while trying to monitor them. Imageable agents binding to T cells or macrophages can alter their function, resulting in flawed results. However, if the imaging process can be discretely controlled, it may dramatically improve our abilities to track the success or failure of immune therapies. For most approaches, intratumoral injection results in a highly localized response, but their application is mostly limited to easily accessible tissues such as skin, breast, and head and neck cancer, limiting their application for deep seated tumors (e.g., pancreas).
6. Conclusion & Perspectives
Many therapeutic approaches that attempt to employ immunomodulation as their main contributor are actively being investigated. NPs can be used as an immune stimulator or adjuvant, whereas checkpoint inhibitors can be used to reverse the immunosuppressive tumor microenvironment. In situ vaccinations can be used in combination with other approaches to further target the solid tumor microenvironment. Such processes can be aided by noninvasive imaging of the immune cells that can help monitor the short and long-term outcome of an immunomodulator therapeutic.
The type of therapy used to treat cancer must be based on an understanding of the tumor microenvironment, such as the presence or absence of T regulatory cells or expression of certain receptors/ligands that may potentially create an interruption in the normal immune response. Such an approach will lead to more personalized therapeutic regimens. The data presented above suggest that combination therapy would be the most efficient and effective approach to treating any solid tumor. It should be accompanied by an individual case-by-case analysis of the best route of therapy available by characterizing the genotypic and phenotypic features of the patient tumor. The topics presented herein may open the door to new possibilities and therapies in the fight against cancer in the near future.
9. Acknowledgements
We thank the seed grant from the Center for Veterinary Health Sciences, National Cancer Institute of the National Institutes of Health under Award Number 1R01CA239150, Focused Ultrasound Foundation, PETCO and the Kerr (Ranjan) Endowed Chair at Oklahoma State University for supporting the immunotherapy research.
Abbreviations
- NPs
nanoparticles
- TAAs
tumor-associated antigens
- MHC
Major Histocompatibility Complex
- CTLA-4
anti cytotoxic T-lymphocyte Antigen 4
- PD-1/PD-L1
programmed death-ligand 1
- ISV
in situ vaccinations
- PLGA
poly lactic-co-glycolic acid
- Th
T-helper
- IL
interleukin
- IFN
interferon
- TNF
tumor necrosis factor
- INPs
inorganic nanoparticles
- GNP
gold nanoparticle
- SPION
superparamagnetic iron oxide nanoparticle
- QD
quantum dot
- MRI
magnetic resonance imaging
- AMF
alternating magnetic field
- DC
dendritic cell
- FDA
Food & Drug Administration
- ROS
reactive oxygen species
- DOX
doxorubicin
- OVA
ovalbumin
- CpG-ODN
cytosine-phosphorothioate-guanine oligodeoxynucleotide
- DNA
deoxyribonucleic acid
- RNA
ribonucleic acid
- TLR
toll-like-receptor
- OV
oncolytic virus
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HSV
herpes simplex virus
- APC
antigen presenting cells
- CXCR
c-xc-chemokine receptor
- Hsp
heat shock proteins
- PET
positron-emission tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that they have no competing interests.
REFERENCES
- Afreen S, & Dermime S (2014). The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther, 7, 1–17. [DOI] [PubMed] [Google Scholar]
- Aghanejad A, Babamiri H, Adibkia K, Barar J, & Omidi Y (2018). Mucin–1 aptamer-armed superparamagnetic iron oxide nanoparticles for targeted delivery of doxorubicin to breast cancer cells. Bioimpacts, 8, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarel F, Gaudy C, Castinetti F, Carre T, Morange I, Conte-Devolx B, Grob JJ, & Brue T (2015). Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol, 172, 195–204. [DOI] [PubMed] [Google Scholar]
- Aliper AM, Frieden-Korovkina VP, Buzdin A, Roumiantsev SA, & Zhavoronkov A (2014). A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med, 3, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JP, Chen AL, Foster A, & Drezek R (2011). In vivo biodistribution of nanoparticles. Nanomedicine (Lond), 6, 815–835. [DOI] [PubMed] [Google Scholar]
- Almeida JP, Figueroa ER, & Drezek RA (2014). Gold nanoparticle mediated cancer immunotherapy. Nanomedicine, 10, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoozgar Z, & Yeo Y (2012). Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 4, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, & Rizvi NA (2016). Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol, 17, 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asharani PV, Lianwu Y, Gong Z, & Valiyaveettil S (2011). Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology, 5, 43–54. [DOI] [PubMed] [Google Scholar]
- Azami A, Suzuki N, Azami Y, Seto I, Sato A, Takano Y, Abe T, Teranishi Y, Tachibana K, & Ohtake T (2018). Abscopal effect following radiation monotherapy in breast cancer: A case report. Mol Clin Oncol, 9, 283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetke SC, Lammers T, & Kiessling F (2015). Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol, 88, 20150207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahig H, Aubin F, Stagg J, Gologan O, Ballivy O, Bissada E, Nguyen-Tan FP, Soulieres D, Guertin L, Filion E, Christopoulos A, Lambert L, Tehfe M, Ayad T, Charpentier D, Jamal R, & Wong P (2019). Phase I/II trial of Durvalumab plus Tremelimumab and stereotactic body radiotherapy for metastatic head and neck carcinoma. BMC Cancer, 19, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakand S, Hayes A, & Dechsakulthorn F (2012). Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal Toxicol, 24, 125–135. [DOI] [PubMed] [Google Scholar]
- Baker AT, Aguirre-Hernandez C, Hallden G, & Parker AL (2018). Designer Oncolytic Adenovirus: Coming of Age. Cancers (Basel), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholz Y (2012). Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release, 160, 117–134. [DOI] [PubMed] [Google Scholar]
- Bartneck M, Ritz T, Keul HA, Wambach M, Bornemann J, Gbureck U, Ehling J, Lammers T, Heymann F, Gassler N, Ludde T, Trautwein C, Groll J, & Tacke F (2012). Peptide - functionalized gold nanorods increase liver injury in hepatitis. ACS Nano, 6, 8767–8777. [DOI] [PubMed] [Google Scholar]
- Bilan R, Fleury F, Nabiev I, & Sukhanova A (2015). Quantum dot surface chemistry and functionalization for cell targeting and imaging. Bioconjug Chem, 26, 609–624. [DOI] [PubMed] [Google Scholar]
- Blank CU, & Enk A (2015). Therapeutic use of anti–CTLA-4 antibodies. Int Immunol, 27, 3–10. [DOI] [PubMed] [Google Scholar]
- Blykers A, Schoonooghe S, Xavier C, D’Hoe K, Laoui D, D’Huyvetter M, Vaneycken I, Cleeren F, Bormans G, Heemskerk J, Raes G, De Baetselier P, Lahoutte T, Devoogdt N, Van Ginderachter JA, & Caveliers V (2015). PET Imaging of Macrophage Mannose Receptor-Expressing Macrophages in Tumor Stroma Using 18F-Radiolabeled Camelid Single-Domain Antibody Fragments. J Nucl Med, 56, 1265–1271. [DOI] [PubMed] [Google Scholar]
- Boehm T, & Swann JB (2014). Origin and evolution of adaptive immunity. Annu Rev Anim Biosci, 2, 259–283. [DOI] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr., Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, & Brahmer JR (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med, 373, 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschi F, De Sanctis F, Ugel S, & Spinelli AE (2018). T-cell tracking using Cerenkov and Radioluminescence imaging. J Biophotonics, e201800093. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Thorne SH, Bell JC, & Kirn DH (2012). Targeted and armed oncolytic poxviruses for cancer: the lead example of JX-594. Curr Pharm Biotechnol, 13, 1768–1772. [DOI] [PubMed] [Google Scholar]
- Brower V (2016). Anti-PD-L1 inhibitor durvalumab in bladder cancer. Lancet Oncol, 17, e275. [DOI] [PubMed] [Google Scholar]
- Brown MC, Dobrikova EY, Dobrikov MI, Walton RW, Gemberling SL, Nair SK, Desjardins A, Sampson JH, Friedman HS, Friedman AH, Tyler DS, Bigner DD, & Gromeier M (2014). Oncolytic polio virotherapy of cancer. Cancer, 120, 3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald ZS, Wynne J, Nasti TH, Zhu S, Mourad WF, Yan W, Gupta S, Khleif SN, & Khan MK (2018). Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front Oncol, 8, 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerki-Thurnherr T, Xiao L, Diener L, Arslan O, Hirsch C, Maeder-Althaus X, Grieder K, Wampfler B, Mathur S, Wick P, & Krug HF (2013). In vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology, 7, 402–416. [DOI] [PubMed] [Google Scholar]
- Butterworth KT, McMahon SJ, Currell FJ, & Prise KM (2012). Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale, 4, 4830–4838. [DOI] [PubMed] [Google Scholar]
- Cano-Mejia J, Bookstaver ML, Sweeney EE, Jewell CM, & Fernandes R (2019). Prussian blue nanoparticle-based antigenicity and adjuvanticity trigger robust antitumor immune responses against neuroblastoma. Biomater Sci, 7, 1875–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Mejia J, Burga RA, Sweeney EE, Fisher JP, Bollard CM, Sandler AD, Cruz CRY, & Fernandes R (2017). Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine, 13, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gutgemann I, Eilers M, & Felsher DW (2016). MYC regulates the antitumor immune response through CD47 and PD-L1. Science, 352, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, & Giles F (2018). Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer, 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LS, Leng CH, Yeh YC, Wu CC, Chen HW, Huang HM, & Liu SJ (2014). Toll -like receptor 9 agonist enhances anti-tumor immunity and inhibits tumor-associated immunosuppressive cells numbers in a mouse cervical cancer model following recombinant lipoprotein therapy. Mol Cancer, 13, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Dash SK, Tripathy S, Das B, Mandal D, Pramanik P, & Roy S (2015). Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem Biol Interact, 226, 58–71. [DOI] [PubMed] [Google Scholar]
- Chavez M, Silvestrini MT, Ingham ES, Fite BZ, Mahakian LM, Tam SM, Ilovitsh A, Monjazeb AM, Murphy WJ, Hubbard NE, Davis RR, Tepper CG, Borowsky AD, & Ferrara KW (2018). Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics, 8, 3611–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xu L, Liang C, Wang C, Peng R, & Liu Z (2016). Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun, 7, 13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Guo J, Han C, Yang M, & Cao X (2009). Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol, 182, 1449–1459. [DOI] [PubMed] [Google Scholar]
- Chen T, Guo J, Yang M, Zhu X, & Cao X (2011). Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol, 186, 2219–2228. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, Fan C, Huang P, Bardeesy N, Zhu AX, Jain RK, & Duda DG (2015). CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology, 61, 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca EA, & Rabkin SD (2014). Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res, 2, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D, Yang JS, Kim S, Kim YK, & Seong SY (2011). A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol, 6, 675–682. [DOI] [PubMed] [Google Scholar]
- Chondronasiou D, Eisden T, Stam AGM, Matthews QL, Icyuz M, Hooijberg E, Dmitriev I, Curiel DT, de Gruijl TD, & van de Ven R (2018). Improved Induction of Anti-Melanoma T Cells by Adenovirus-5/3 Fiber Modification to Target Human DCs. Vaccines (Basel), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Yip TC, Ledbetter JA, Yukawa H, Kikutani H, Kishimoto T, & Ng MH (1988). CDw40 and BLCa-specific monoclonal antibodies detect two distinct molecules which transmit progression signals to human B lymphocytes. Eur J Immunol, 18, 451–457. [DOI] [PubMed] [Google Scholar]
- Collins DS, Findlay K, & Harding CV (1992). Processing of exogenous liposome-encapsulated antigens in vivo generates class I MHC-restricted T cell responses. J Immunol, 148, 3336–3341. [PubMed] [Google Scholar]
- Collins JM, & Gulley JL (2019). Product review: avelumab, an anti-PD-L1 antibody. Hum Vaccin Immunother, 15, 891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz LJ, Tacken PJ, Zeelenberg IS, Srinivas M, Bonetto F, Weigelin B, Eich C, de Vries IJ, & Figdor CG (2014). Tracking targeted bimodal nanovaccines: immune responses and routing in cells, tissue, and whole organism. Mol Pharm, 11, 4299–4313. [DOI] [PubMed] [Google Scholar]
- Czajka M, Sawicki K, Sikorska K, Popek S, Kruszewski M, & Kapka-Skrzypczak L (2015). Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol In Vitro, 29, 1042–1052. [DOI] [PubMed] [Google Scholar]
- Dan M, Bae Y, Pittman TA, & Yokel RA (2015). Alternating magnetic field-induced hyperthermia increases iron oxide nanoparticle cell association/uptake and flux in blood-brain barrier models. Pharm Res, 32, 1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, Joseph R, Gangadhar TC, Dronca R, Patnaik A, Zarour H, Roach C, Toland G, Lunceford JK, Li XN, Emancipator K, Dolled-Filhart M, Kang SP, Ebbinghaus S, & Hamid O (2016). Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J Clin Oncol, 34, 4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet M, Hijnen NM, Langereis S, Elevelt A, Heijman E, Dubois L, Lambin P, & Grull H (2013). Magnetic resonance guided high-intensity focused ultrasound mediated hyperthermia improves the intratumoral distribution of temperature-sensitive liposomal Doxorubicin. Invest Radiol, 48, 395–405. [DOI] [PubMed] [Google Scholar]
- Deeks ED (2016). Pembrolizumab: A Review in Advanced Melanoma. Drugs, 76, 375–386. [DOI] [PubMed] [Google Scholar]
- Delwar ZM, Liu G, Kuo Y, Lee C, Bu L, Rennie PS, & Jia WW (2016). Tumour–specific triple-regulated oncolytic herpes virus to target glioma. Oncotarget, 7, 28658–28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Burgucu D, Turna F, Aksakal S, & Kaya B (2013). Determination of TiO2, ZrO2, and Al2O3 nanoparticles on genotoxic responses in human peripheral blood lymphocytes and cultured embyronic kidney cells. J Toxicol Environ Health A, 76, 990–1002. [DOI] [PubMed] [Google Scholar]
- DeNardo GL, & DeNardo SJ (2008). Update: Turning the heat on cancer. Cancer Biother Radiopharm, 23, 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Zeng S, Qu Y, Luo Q, Guo C, Yin L, Han Y, Li Y, Cai C, Fu Y, & Shen H (2018). BMP4 promotes hepatocellular carcinoma proliferation by autophagy activation through JNK1-mediated Bcl-2 phosphorylation. J Exp Clin Cancer Res, 37, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong HV, Chau TTL, Dang NTT, Vanterpool F, Salmeron-Sanchez M, Lizundia E, Tran HT, Nguyen LV, & Nguyen TD (2018). Biocompatible Chitosan-Functionalized Upconverting Nanocomposites. ACS Omega, 3, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Nik H, Corwin WL, Shcheglova T, Das Mohapatra A, Mandoiu II, & Srivastava PK (2018). CD11c(+) MHCII(lo) GM-CSF-bone marrow-derived dendritic cells act as antigen donor cells and as antigen presenting cells in neoepitope-elicited tumor immunity against a mouse fibrosarcoma. Cancer Immunol Immunother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Maio M, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan P, Ibrahim N, Marreaud S, van Akkooi ACJ, Suciu S, & Robert C (2018). Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med, 378, 1789–1801. [DOI] [PubMed] [Google Scholar]
- Eichhorn F, Klotz LV, Bischoff H, Thomas M, Lasitschka F, Winter H, Hoffmann H, & Eichhorn ME (2019). Neoadjuvant anti-programmed Death-1 immunotherapy by Pembrolizumab in resectable nodal positive stage II/IIIa non-small-cell lung cancer (NSCLC): the NEOMUN trial. BMC Cancer, 19, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekin A, Karatas OF, Culha M, & Ozen M (2014). Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. J Gene Med, 16, 331–335. [DOI] [PubMed] [Google Scholar]
- Farkona S, Diamandis EP, & Blasutig IM (2016). Cancer immunotherapy: the beginning of the end of cancer? BMC Med, 14, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon DT (2014). The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res, 2, 187–193. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, & Rittmeyer A (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet, 387, 1837–1846. [DOI] [PubMed] [Google Scholar]
- Fischer NO, Rasley A, & Blanchette C (2014). Nanoparticles and antigen delivery: understanding the benefits and drawbacks of different delivery platforms. Nanomedicine (Lond), 9, 373–376. [DOI] [PubMed] [Google Scholar]
- Formenti SC, & Demaria S (2013). Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst, 105, 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad YA, & Aanei C (2017). Revisiting the hallmarks of cancer. Am J Cancer Res, 7, 1016–1036. [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, & Corrales L (2015). New perspectives on type I IFNs in cancer. Cytokine Growth Factor Rev, 26, 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo P, Dharmapuri S, Cipriani B, & Monaci P (2005). Adenovirus as vehicle for anticancer genetic immunotherapy. Gene Ther, 12 Suppl 1, S84–91. [DOI] [PubMed] [Google Scholar]
- Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, & Hodge JW (2014). Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget, 5, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Moreno R, Gil-Martin M, Cascallo M, de Olza MO, Cuadra C, Piulats JM, Navarro V, Domenech M, Alemany R, & Salazar R (2019). A Phase 1 Trial of Oncolytic Adenovirus ICOVIR-5 Administered Intravenously to Cutaneous and Uveal Melanoma Patients. Hum Gene Ther, 30, 352–364. [DOI] [PubMed] [Google Scholar]
- Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, Cyktor JC, Coffin JM, Acosta EP, Koup RA, Mellors JW, & Eron JJ (2017). Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis, 215, 1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B, & Fussenegger M (2015). Synthetic immunology: modulating the human immune system. Trends Biotechnol, 33, 65–79. [DOI] [PubMed] [Google Scholar]
- Goltz D, Holmes EE, Gevensleben H, Sailer V, Dietrich J, Jung M, Rohler M, Meller S, Ellinger J, Kristiansen G, & Dietrich D (2016). CXCL12 promoter methylation and PD-L1 expression as prognostic biomarkers in prostate cancer patients. Oncotarget, 7, 53309–53320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Chehrazi-Raffle A, Reddi S, & Salgia R (2018). Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer, 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, Cetnar JP, Ey FS, Bergan RC, Slottke R, & Beer TM (2016). Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget, 7, 52810–52817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, Heydebreck AV, Chin K, Cuillerot JM, & Kelly K (2017). Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol, 18, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, Yan B, & Lu W (2014). Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano, 8, 5670–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, Liu W, Storkus WJ, He Y, Liu Z, & Bartlett DL (2019). Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer, 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerich L, Bhardwaj N, Kohrt HE, & Brody JD (2016). In situ vaccination for the treatment of cancer. Immunotherapy, 8, 315–330. [DOI] [PubMed] [Google Scholar]
- Hammerich L, Binder A, & Brody JD (2015). In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol Oncol, 9, 1966–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, Zhan Y, Ostrowski D, Yellin M, Marsh H, Salazar AM, Rahman AH, Brown BD, Merad M, & Brody JD (2019). Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med, 25, 814–824. [DOI] [PubMed] [Google Scholar]
- Harding CV, Collins DS, Kanagawa O, & Unanue ER (1991). Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol, 147, 2860–2863. [PubMed] [Google Scholar]
- Hargadon KM, Johnson CE, & Williams CJ (2018). Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol, 62, 29–39. [DOI] [PubMed] [Google Scholar]
- He H, Chiu AC, Kanada M, Schaar BT, Krishnan V, Contag CH, & Dorigo O (2017). Imaging of Tumor-Associated Macrophages in a Transgenic Mouse Model of Orthotopic Ovarian Cancer. Mol Imaging Biol, 19, 694–702. [DOI] [PubMed] [Google Scholar]
- Heit A, Schmitz F, Haas T, Busch DH, & Wagner H (2007). Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur J Immunol, 37, 2063–2074. [DOI] [PubMed] [Google Scholar]
- Hewakuruppu YL, Dombrovsky LA, Chen C, Timchenko V, Jiang X, Baek S, & Taylor RA (2013). Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt, 52, 6041–6050. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, & Chow Z (2017). Viewing immune regulation as it happens: in vivo imaging for investigation of regulatory T-cell function. Immunol Cell Biol, 95, 514–519. [DOI] [PubMed] [Google Scholar]
- Hobson-Gutierrez SA, & Carmona-Fontaine C (2018). The metabolic axis of macrophage and immune cell polarization. Dis Model Mech, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes PJ, Moodie KL, Petryk AA, Petryk JD, Sechrist S, Gladstone DJ, Steinmetz NF, Veliz FA, Bursey AA, Wagner RJ, Rajan A, Dugat D, Crary-Burney M, & Fiering SN (2017). Hypo-fractionated Radiation, Magnetic Nanoparticle Hyperthermia and a Viral Immunotherapy Treatment of Spontaneous Canine Cancer. Proc SPIE Int Soc Opt Eng, 10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, Crary-Burney M, Ariaspulido H, Veliz FA, Steinmetz NF, & Fiering SN (2018). Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Mol Pharm, 15, 3717–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BL, Williams JB, Cabanov A, Spranger S, & Gajewski TF (2018). Intratumoral CD8(+) T-cell Apoptosis Is a Major Component of T-cell Dysfunction and Impedes Antitumor Immunity. Cancer Immunol Res, 6, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, Lyerly HK, Clay TM, & Zhong P (2007). Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med, 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZI, McArthur HL, & Ho AY (2017). The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr Breast Cancer Rep, 9, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, & Hainfeld JF (2013). Intravenous magnetic nanoparticle cancer hyperthermia. Int J Nanomedicine, 8, 2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Xu H, & Peng G (2018). TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell Mol Immunol, 15, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yuan F, Liang M, Lo HW, Shinohara ML, Robertson C, & Zhong P (2012). M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS One, 7, e41632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Nam J, Jung S, Song J, Doh H, & Kim S (2014). Gold nanoparticle-mediated photothermal therapy: current status and future perspective. Nanomedicine (Lond), 9, 2003–2022. [DOI] [PubMed] [Google Scholar]
- Ispas C, Andreescu D, Patel A, Goia DV, Andreescu S, & Wallace KN (2009). Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environ Sci Technol, 43, 6349–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelot N, Roberti MP, Enot DP, Rusakiewicz S, Ternes N, Jegou S, Woods DM, Sodre AL, Hansen M, Meirow Y, Sade-Feldman M, Burra A, Kwek SS, Flament C, Messaoudene M, Duong CPM, Chen L, Kwon BS, Anderson AC, Kuchroo VK, Weide B, Aubin F, Borg C, Dalle S, Beatrix O, Ayyoub M, Balme B, Tomasic G, Di Giacomo AM, Maio M, Schadendorf D, Melero I, Dreno B, Khammari A, Dummer R, Levesque M, Koguchi Y, Fong L, Lotem M, Baniyash M, Schmidt H, Svane IM, Kroemer G, Marabelle A, Michiels S, Cavalcanti A, Smyth MJ, Weber JS, Eggermont AM, & Zitvogel L (2017). Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun, 8, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Coulter JA, Butterworth KT, Hounsell AR, McMahon SJ, Hyland WB, Muir MF, Dickson GR, Prise KM, Currell FJ, Hirst DG, & O’Sullivan JM (2014). Gold nanoparticle cellular uptake, toxicity and radiosensitisation in hypoxic conditions. Radiother Oncol, 110, 342–347. [DOI] [PubMed] [Google Scholar]
- Jespersen H, Bagge RO, Ullenhag G, Carneiro A, Helgadottir H, Ljuslinder I, Levin M, All-Eriksson C, Andersson B, Stierner U, Nilsson LM, Nilsson JA, & Ny L (2019). Concomitant use of pembrolizumab and entinostat in adult patients with metastatic uveal melanoma (PEMDAC study): protocol for a multicenter phase II open label study. BMC Cancer, 19, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, Yuan Y, Lang FF, Toniatti C, Hossain MB, & Fueyo J (2017). Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination. Cancer Res, 77, 3894–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Li J, Zhang J, Wang L, Zhang Q, Ge J, Guo Y, Wang B, Huang Y, Yang T, Hao D, & Shan L (2019). SDF-1/CXCR4 axis facilitates myeloid-derived suppressor cells accumulation in osteosarcoma microenvironment and blunts the response to anti-PD-1 therapy. Int Immunopharmacol, 75, 105818. [DOI] [PubMed] [Google Scholar]
- Jin K, Luo Z, Zhang B, & Pang Z (2018). Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B, 8, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Pinder SE, & Douek M (2013). Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology, 62, 481–486. [DOI] [PubMed] [Google Scholar]
- Jurney P, Agarwal R, Singh V, Choi D, Roy K, Sreenivasan SV, & Shi L (2017). Unique size and shape-dependent uptake behaviors of non-spherical nanoparticles by endothelial cells due to a shearing flow. J Control Release, 245, 170–176. [DOI] [PubMed] [Google Scholar]
- Kandasamy G, & Maity D (2015). Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int J Pharm, 496, 191–218. [DOI] [PubMed] [Google Scholar]
- Kandasamy G, Sudame A, Luthra T, Saini K, & Maity D (2018). Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega, 3, 3991–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Demaria S, & Formenti S (2016). Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer, 4, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HL, Cronholm P, Gustafsson J, & Moller L (2008). Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol, 21, 1726–1732. [DOI] [PubMed] [Google Scholar]
- Kasuya H, Kodera Y, Nakao A, Yamamura K, Gewen T, Zhiwen W, Hotta Y, Yamada S, Fujii T, Fukuda S, Tsurumaru N, Kuwahara T, Kikumori T, Koide Y, Fujimoto Y, Nakashima T, Hirooka Y, Shiku H, Tanaka M, Takesako K, Kondo T, Aleksic B, Kawashima H, Goto H, & Nishiyama Y (2014). Phase I Dose-escalation Clinical Trial of HF10 Oncolytic Herpes Virus in 17 Japanese Patients with Advanced Cancer. Hepatogastroenterology, 61, 599–605. [PubMed] [Google Scholar]
- Kato Y, Zhu W, Backer MV, Neoh CC, Hapuarachchige S, Sarkar SK, Backer JM, & Artemov D (2015). Noninvasive Imaging of Liposomal Delivery of Superparamagnetic Iron Oxide Nanoparticles to Orthotopic Human Breast Tumor in Mice. Pharm Res, 32, 3746–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbe C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, & Nghiem P (2016). Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol, 17, 1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R, Williams J, Habte F, Wagner JR, Forman S, Brown C, Allen-Auerbach M, Czernin J, Tang W, Jensen MC, Badie B, & Gambhir SS (2017). Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Nitschke M, Sennino B, Murer P, Schriver BJ, Bell A, Subramanian A, McDonald CE, Wang J, Cha H, Bourgeois-Daigneault MC, Kirn DH, Bell JC, De Silva N, Breitbach CJ, & McDonald DM (2018). Amplification of Oncolytic Vaccinia Virus Widespread Tumor Cell Killing by Sunitinib through Multiple Mechanisms. Cancer Res, 78, 922–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Chae KS, Chang Y, & Lee GH (2013). Gadolinium oxide nanoparticles as potential multimodal imaging and therapeutic agents. Curr Top Med Chem, 13, 422–433. [DOI] [PubMed] [Google Scholar]
- Kong G, & Dewhirst MW (1999). Hyperthermia and liposomes. Int J Hyperthermia, 15, 345–370. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, Kadel EE 3rd, Wistuba I, Chaft J, Rizvi NA, Spigel DR, Spira A, Hirsch FR, Cohen V, Smith D, Boyd Z, Miley N, Flynn S, Leveque V, Shames DS, Ballinger M, Mocci S, Shankar G, Funke R, Hampton G, Sandler A, Amler L, Mellman I, Chen DS, & Hegde PS (2018). Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci U S A, 115, E10119–e10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, Schuebbe G, Renz BW, D’Haese JG, Schloesser H, Heinemann V, Subklewe M, Boeck S, Werner J, & von Bergwelt-Baildon M (2019). Advances in cancer immunotherapy 2019 - latest trends. J Exp Clin Cancer Res, 38, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Ninomiya T, Hotta K, Kozuki T, Toyooka S, Okada H, Fujiwara T, Udono H, & Kiura K (2018). Study Protocol: Phase-Ib Trial of Nivolumab Combined With Metformin for Refractory/Recurrent Solid Tumors. Clin Lung Cancer, 19, e861–e864. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J, Wei T, Cao W, Zou G, & Liang XJ (2012). Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials, 33, 1180–1189. [DOI] [PubMed] [Google Scholar]
- Lappas CM (2015). The immunomodulatory effects of titanium dioxide and silver nanoparticles. Food Chem Toxicol, 85, 78–83. [DOI] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, & Wolchok JD (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med, 373, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen ST, Roursgaard M, Jensen KA, & Nielsen GD (2010). Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin Pharmacol Toxicol, 106, 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HW, Wang SY, Jin-Jhih H, & Chan AL (2018). Abscopal effect of radiation on bone metastases of breast cancer: A case report. Cancer Biol Ther, 19, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi J, Lam T, Goth SR, Yaghoubi S, Bates J, Ren G, Jivan S, Huynh TL, Blecha JE, Khattri R, Schmidt KF, Jennings D, & VanBrocklin H (2019). Imaging of Activated T Cells as an Early Predictor of Immune Response to Anti-PD-1 Therapy. Cancer Res, 79, 3455–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Nie W, Zhang F, Lu G, Lv C, Lv Y, Bao W, Zhang L, Wang S, Gao X, Wei W, & Xie HY (2019). Engineering Magnetosomes for High-Performance Cancer Vaccination. ACS Cent Sci, 5, 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Song K, Cao Y, Peng C, & Yang G (2018). Keratin-templated synthesis of metallic oxide nanoparticles as MRI contrast agents and drug carriers. ACS Appl Mater Interfaces. [DOI] [PubMed] [Google Scholar]
- Lipson EJ, Lilo MT, Ogurtsova A, Esandrio J, Xu H, Brothers P, Schollenberger M, Sharfman WH, & Taube JM (2017). Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer, 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, & Topalian SL (2013). Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res, 19, 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Hu Z, Qiu L, Hui C, Li C, Zhong P, & Zhang J (2010). Boosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturation. J Transl Med, 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang N, Li H, Jin Q, & Ji J (2013). Surface and size effects on cell interaction of gold nanoparticles with both phagocytic and nonphagocytic cells. Langmuir, 29, 9138–9148. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hardie J, Zhang X, & Rotello VM (2017). Effects of engineered nanoparticles on the innate immune system. Semin Immunol, 34, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YD, Ji CB, Li SB, Yan F, Gu QS, Balic JJ, Yu L, & Li JK (2018). Toll -like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-kappaB signaling pathways. Int Immunopharmacol, 59, 375–383. [DOI] [PubMed] [Google Scholar]
- Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, & Fiering S (2016). In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol, 11, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, McNeil CM, Hill AG, Ribas A, Atkins MB, Thompson JA, Hwu WJ, Hodi FS, Menzies AM, Guminski AD, Kefford R, Kong BY, Tamjid B, Srivastava A, Lomax AJ, Islam M, Shu X, Ebbinghaus S, Ibrahim N, & Carlino MS (2017). Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol, 18, 1202–1210. [DOI] [PubMed] [Google Scholar]
- Luo W, Wang Z, Tian P, & Li W (2018). Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YH, Chang LW, & Lin P (2015). Metal-Based Nanoparticles and the Immune System: Activation, Inflammation, and Potential Applications. Biomed Res Int, 2015, 143720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudan S, Tamir A, Bates N, Flanagan E, Gore ME, Barton DP, Harper P, Seckl M, Thomas H, Lemoine NR, Charnock M, Habib NA, Lechler R, Nicholls J, Pignatelli M, & Ganesan TS (2004). A multicenter Phase I gene therapy clinical trial involving intraperitoneal administration of E1A-lipid complex in patients with recurrent epithelial ovarian cancer overexpressing HER-2/neu oncogene. Clin Cancer Res, 10, 2986–2996. [DOI] [PubMed] [Google Scholar]
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, & Jordan A (2011). Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol, 103, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney E, & Hwang JH (2015). Emerging HIFU applications in cancer therapy. Int J Hyperthermia, 31, 302–309. [DOI] [PubMed] [Google Scholar]
- Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, & Fang Y (2015). The paradoxical role of IL-10 in immunity and cancer. Cancer Lett, 367, 103–107. [DOI] [PubMed] [Google Scholar]
- Manukian G, Bar-Ad V, Lu B, Argiris A, & Johnson JM (2019). Combining Radiation and Immune Checkpoint Blockade in the Treatment of Head and Neck Squamous Cell Carcinoma. Front Oncol, 9, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, ten Hagen TL, Needham D, & Dewhirst MW (2012). Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res, 72, 5566–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May KF Jr., Roychowdhury S, Bhatt D, Kocak E, Bai XF, Liu JQ, Ferketich AK, Martin EW Jr., Caligiuri MA, Zheng P, & Liu Y (2005). Anti-human CTLA-4 monoclonal antibody promotes T-cell expansion and immunity in a hu-PBL-SCID model: a new method for preclinical screening of costimulatory monoclonal antibodies. Blood, 105, 1114–1120. [DOI] [PubMed] [Google Scholar]
- Meindl-Beinker NM, Betge J, Gutting T, Burgermeister E, Belle S, Zhan T, Schulte N, Maenz M, Ebert MP, & Haertel N (2019). A multicenter open-label phase II trial to evaluate nivolumab and ipilimumab for 2nd line therapy in elderly patients with advanced esophageal squamous cell cancer (RAMONA). BMC Cancer, 19, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick AE, Ilett EJ, & Melcher AA (2009). JX-594, a targeted oncolytic poxvirus for the treatment of cancer. Curr Opin Investig Drugs, 10, 1372–1382. [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, & Weiss S (2005). Quantum dots for live cells, in vivo imaging, and diagnostics. Science, 307, 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, Goodwin JT, Ramos J, Chiu W, & Irvine DJ (2011). Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater, 10, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AR, Kisin E, Inman A, Young SH, Muhammed M, Burks T, Uheida A, Tkach A, Waltz M, Castranova V, Fadeel B, Kagan VE, Riviere JE, Monteiro-Riviere N, & Shvedova AA (2013). Oxidative stress and dermal toxicity of iron oxide nanoparticles in vitro. Cell Biochem Biophys, 67, 461–476. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, & Weissleder R (2008). Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation, 117, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, & Andre T (2017). Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol, 18, 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SB, Zhang X, Paul D, Kays LM, Gough W, Stewart J, Uhlik MT, Chen Q, Hui YH, Zamek-Gliszczynski MJ, Wijsman JA, Credille KM, & Yan LZ (2015). Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Mol Cancer Ther, 14, 480–490. [DOI] [PubMed] [Google Scholar]
- Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, Williams LJ, Deng W, Chen G, Mbofung R, Lazar AJ, Torres-Cabala CA, Cooper ZA, Chen PL, Tieu TN, Spranger S, Yu X, Bernatchez C, Forget MA, Haymaker C, Amaria R, McQuade JL, Glitza IC, Cascone T, Li HS, Kwong LN, Heffernan TP, Hu J, Bassett RL Jr., Bosenberg MW, Woodman SE, Overwijk WW, Lizee G, Roszik J, Gajewski TF, Wargo JA, Gershenwald JE, Radvanyi L, Davies MA, & Hwu P (2016). Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov, 6, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perica K, Tu A, Richter A, Bieler JG, Edidin M, & Schneck JP (2014). Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano, 8, 2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarca C, Clemente E, Amato V, Pedata P, Sabbioni E, Bernardini G, Iavicoli I, Cortese S, Niu Q, Otsuki T, Paganelli R, & Di Gioacchino M (2015). Engineered metal based nanoparticles and innate immunity. Clin Mol Allergy, 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarca C, Clemente E, Di Giampaolo L, Mariani-Costantini R, Leopold K, Schindl R, Lotti LV, Mangifesta R, Sabbioni E, Niu Q, Bernardini G, & Di Gioacchino M (2014). Palladium nanoparticles induce disturbances in cell cycle entry and progression of peripheral blood mononuclear cells: paramount role of ions. J Immunol Res, 2014, 295092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RH, Campbell JS, Pai SI, Brody JD, & Kohrt HE (2015). In-situ tumor vaccination: Bringing the fight to the tumor. Hum Vaccin Immunother, 11, 1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, Shi K, & Soria JC (2016). A Phase III Study of Durvalumab (MEDI4736) With or Without Tremelimumab for Previously Treated Patients With Advanced NSCLC: Rationale and Protocol Design of the ARCTIC Study. Clin Lung Cancer, 17, 232–236 e231. [DOI] [PubMed] [Google Scholar]
- Platt A, & Wetzler L (2013). Innate immunity and vaccines. Curr Top Med Chem, 13, 2597–2608. [DOI] [PubMed] [Google Scholar]
- Poss M, Zittel E, Meschkov A, Schepers U, & Feldmann C (2018). Fluorescent Sulfonate-Based Inorganic-Organic Hybrid Nanoparticles for Staining and Imaging. Bioconjug Chem. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhou ZW, Pan ST, He ZX, Zhang X, Qiu JX, Duan W, Yang T, & Zhou SF (2015). Graphene quantum dots induce apoptosis, autophagy, and inflammatory response via p38 mitogen-activated protein kinase and nuclear factor-kappaB mediated signaling pathways in activated THP-1 macrophages. Toxicology, 327, 62–76. [DOI] [PubMed] [Google Scholar]
- Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, & Wood BJ (2012). Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. Journal of Controlled Release, 158, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L, Waldmann TA, Janakiram M, & Brammer JE (2018). Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. N Engl J Med, 378, 1947–1948. [DOI] [PubMed] [Google Scholar]
- Rizvi NA, & Chan TA (2016). Immunotherapy and Oncogenic Pathways: The PTEN Connection. Cancer Discov, 6, 128–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romoser AA, Chen PL, Berg JM, Seabury C, Ivanov I, Criscitiello MF, & Sayes CM (2011). Quantum dots trigger immunomodulation of the NFkappaB pathway in human skin cells. Mol Immunol, 48, 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotte A (2019). Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res, 38, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S, Hanifehpour Y, & Joo SW (2014). PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev, 15, 517–535. [DOI] [PubMed] [Google Scholar]
- Sadauskas E, Danscher G, Stoltenberg M, Vogel U, Larsen A, & Wallin H (2009). Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine, 5, 162–169. [DOI] [PubMed] [Google Scholar]
- Saengruengrit C, Ritprajak P, Wanichwecharungruang S, Sharma A, Salvan G, Zahn DRT, & Insin N (2018). The combined magnetic field and iron oxide-PLGA composite particles: Effective protein antigen delivery and immune stimulation in dendritic cells. J Colloid Interface Sci, 520, 101–111. [DOI] [PubMed] [Google Scholar]
- Sato-Dahlman M, & Yamamoto M (2018). The Development of Oncoltyic Adenovirus Therapy in the Past and Future - For the Case of Pancreatic Cancer. Curr Cancer Drug Targets, 18, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayour EJ, Mendez-Gomez HR, & Mitchell DA (2018). Cancer Vaccine Immunotherapy with RNA-Loaded Liposomes. Int J Mol Sci, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala S (2015). Molecular Pathways: Targeting the CXCR4-CXCL12 Axis--Untapped Potential in the Tumor Microenvironment. Clin Cancer Res, 21, 4278–4285. [DOI] [PubMed] [Google Scholar]
- Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, & Goldberg MS (2017). T cell -targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun, 8, 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel JA, Otsuka A, & Kabashima K (2018). Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol, 8, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seledtsov VI, Goncharov AG, & Seledtsova GV (2015). Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum Vaccin Immunother, 11, 851–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow Y, & Wood MJ (2009). Biological gene delivery vehicles: beyond viral vectors. Mol Ther, 17, 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, & Allison JP (2015). The future of immune checkpoint therapy. Science, 348, 56–61. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Siskova KM, Zboril R, & Gardea-Torresdey JL (2014). Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. AdvColloid Interface Sci, 204, 15–34. [DOI] [PubMed] [Google Scholar]
- Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, & Hanlon DJ (2006). Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology, 117, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, & Zhao B (2018). Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. Bmj, 362, k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini MT, Ingham ES, Mahakian LM, Kheirolomoom A, Liu Y, Fite BZ, Tam SM, Tucci ST, Watson KD, Wong AW, Monjazeb AM, Hubbard NE, Murphy WJ, Borowsky AD, & Ferrara KW (2017). Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight, 2, e90521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Singh S, Sahu J, Srivastava S, & Singh MR (2016). Ceramic nanoparticles: Recompense, cellular uptake and toxicity concerns. Artif Cells Nanomed Biotechnol, 44, 401–409. [DOI] [PubMed] [Google Scholar]
- Singh M, Sethuraman NS, Fiering S, Guha C, Malayer J, Ritchey J, & Ranjan A (2019). In-situ vaccination using focused ultrasound heating and anti-CD-40 agonistic antibody enhances T-cell mediated local and abscopal effects in murine melanoma. International Journal of Hyperthermia, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, & Mijakovic I (2018). Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int J Mol Sci, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar P, Gonzalez G, Vilos C, Herrera N, Juica N, Moreno M, Simon F, & Velasquez L (2015). Multifunctional polymeric nanoparticles doubly loaded with SPION and ceftiofur retain their physical and biological properties. J Nanobiotechnology, 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, & Gajewski TF (2015). Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature, 523, 231–235. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kibanov Solomonov VV, Sazykina E, Schwartz JA, Gad SC, & Goodrich GP (2016). Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int J Toxicol, 35, 38–46. [DOI] [PubMed] [Google Scholar]
- Sullivan RJ, Hamid O, Gonzalez R, Infante JR, Patel MR, Hodi FS, Lewis KD, Tawbi HA, Hernandez G, Wongchenko MJ, Chang Y, Roberts L, Ballinger M, Yan Y, Cha E, & Hwu P (2019). Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med, 25, 929–935. [DOI] [PubMed] [Google Scholar]
- Sumbayev VV, Yasinska IM, Garcia CP, Gilliland D, Lall GS, Gibbs BF, Bonsall DR, Varani L, Rossi F, & Calzolai L (2013). Gold nanoparticles downregulate interleukin-1beta-induced pro-inflammatory responses. Small, 9, 472–477. [DOI] [PubMed] [Google Scholar]
- Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S, Hollebecque A, Scoazec JY, Marabelle A, Massard C, Soria JC, Robert C, Paragios N, Deutsch E, & Ferte C (2018). A radiomics approach to assess tumour infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol, 19, 1180–1191. [DOI] [PubMed] [Google Scholar]
- Sun X, Gao D, Gao L, Zhang C, Yu X, Jia B, Wang F, & Liu Z (2015). Molecular imaging of tumor-infiltrating macrophages in a preclinical mouse model of breast cancer. Theranostics, 5, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutunkova MP, Privalova LI, Minigalieva IA, Gurvich VB, Panov VG, & Katsnelson BA (2018). The most important inferences from the Ekaterinburg nanotoxicology team’s animal experiments assessing adverse health effects of metallic and metal oxide nanoparticles. Toxicol Rep, 5, 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, Gyobu H, Kawarada Y, Kondo S, Akira S, Katoh H, Ikeda H, & Nishimura T (2004). Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res, 64, 8754–8760. [DOI] [PubMed] [Google Scholar]
- Szczepaniak Sloane RA, Gopalakrishnan V, Reddy SM, Zhang X, Reuben A, & Wargo JA (2017). Interaction of molecular alterations with immune response in melanoma. Cancer, 123, 2130–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Liang Y, Feng X, Zhang R, Jin X, & Sun L (2015). Co-delivery of docetaxel and Poloxamer 235 by PLGA-TPGS nanoparticles for breast cancer treatment. Mater Sci Eng C Mater Biol Appl, 49, 348–355. [DOI] [PubMed] [Google Scholar]
- Tao Y, Ju E, Ren J, & Qu X (2014). Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials, 35, 9963–9971. [DOI] [PubMed] [Google Scholar]
- Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D’Angelo S, Attia S, Riedel RF, Priebat DA, Movva S, Davis LE, Okuno SH, Reed DR, Crowley J, Butterfield LH, Salazar R, Rodriguez-Canales J, Lazar AJ, Wistuba II, Baker LH, Maki RG, Reinke D, & Patel S (2017). Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol, 18, 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirunavukkarasu GK, Cherukula K, Lee H, Jeong YY, Park IK, & Lee JY (2018). Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials, 180, 240–252. [DOI] [PubMed] [Google Scholar]
- Tietze R, Zaloga J, Unterweger H, Lyer S, Friedrich RP, Janko C, Pottler M, Durr S, & Alexiou C (2015). Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun, 468, 463–470. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Fukasawa S, Shinohara N, Kitamura H, Oya M, Eto M, Tanabe K, Saito M, Kimura G, Yonese J, Yao M, & Uemura H (2019). Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup 3-year follow-up analysis from the Phase III CheckMate 025 study. Jpn J Clin Oncol, 49, 506–514. [DOI] [PubMed] [Google Scholar]
- Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, & Fiering S (2014). Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine, 10, 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong NP, Whittaker MR, Mak CW, & Davis TP (2015). The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv, 12, 129–142. [DOI] [PubMed] [Google Scholar]
- Tsai CY, Lu SL, Hu CW, Yeh CS, Lee GB, & Lei HY (2012). Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages. J Immunol, 188, 68–76. [DOI] [PubMed] [Google Scholar]
- Tsai YS, Chen YH, Cheng PC, Tsai HT, Shiau AL, Tzai TS, & Wu CL (2013). TGF-beta1 conjugated to gold nanoparticles results in protein conformational changes and attenuates the biological function. Small, 9, 2119–2128. [DOI] [PubMed] [Google Scholar]
- Tsoukalas C, Psimadas D, Kastis GA, Koutoulidis V, Harris AL, Paravatou-Petsotas M, Karageorgou M, Furenlid LR, Moulopoulos LA, Stamopoulos D, & Bouziotis P (2018). A Novel Metal-Based Imaging Probe for Targeted Dual-Modality SPECT/MR Imaging of Angiogenesis. Front Chem, 6, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdinguio RG, Fernandez AF, Moncada-Pazos A, Huidobro C, Rodriguez RM, Ferrero C, Martinez-Camblor P, Obaya AJ, Bernal T, Parra-Blanco A, Rodrigo L, Santacana M, Matias-Guiu X, Soldevilla B, Dominguez G, Bonilla F, Cal S, Lopez-Otin C, & Fraga MF (2013). Immune-dependent and independent antitumor activity of GM-CSF aberrantly expressed by mouse and human colorectal tumors. Cancer Res, 73, 395–405. [DOI] [PubMed] [Google Scholar]
- Uusi-Kerttula H, Hulin-Curtis S, Davies J, & Parker AL (2015). Oncolytic Adenovirus: Strategies and Insights for Vector Design and Immuno-Oncolytic Applications. Viruses, 7, 6009–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Broekhoven CL, Parish CR, Demangel C, Britton WJ, & Altin JG (2004). Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res, 64, 4357–4365. [DOI] [PubMed] [Google Scholar]
- van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Futterer JJ, den Brok MH, & Adema GJ (2017). Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother, 66, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten C, & Banchereau J (2000). CD40-CD40 ligand. J Leukoc Biol, 67, 2–17. [DOI] [PubMed] [Google Scholar]
- Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD Jr., & Huang L (2006). Immunostimulation of dendritic cells by cationic liposomes. Mol Membr Biol, 23, 385–395. [DOI] [PubMed] [Google Scholar]
- Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara H, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, & Kwon BS (2015). Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol, 35 Suppl, S185–s198. [DOI] [PubMed] [Google Scholar]
- Waeckerle-Men Y, Allmen EU, Gander B, Scandella E, Schlosser E, Schmidtke G, Merkle HP, & Groettrup M (2006). Encapsulation of proteins and peptides into biodegradable poly(D,L-lactide-co-glycolide) microspheres prolongs and enhances antigen presentation by human dendritic cells. Vaccine, 24, 1847–1857. [DOI] [PubMed] [Google Scholar]
- Waeckerle-Men Y, & Groettrup M (2005). PLGA microspheres for improved antigen delivery to dendritic cells as cellular vaccines. Adv Drug Deliv Rev, 57, 475–482. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, & Yoshida J (2008). A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J Gene Med, 10, 329–339. [DOI] [PubMed] [Google Scholar]
- Walker LS, & Sansom DM (2015). Confusing signals: recent progress in CTLA-4 biology. Trends Immunol, 36, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun Y, Liu Y, Meng F, & Lee RJ (2018). Clinical translation of immunoliposomes for cancer therapy: recent perspectives. Expert Opin Drug Deliv, 15, 893–903. [DOI] [PubMed] [Google Scholar]
- Wang H, Li Z, Dong B, Sun W, Yang X, Liu R, Zhou L, Huang X, Jia L, & Lin D (2018). Prognostic significance of PD-L1 expression and CD8+ T cell infiltration in pulmonary neuroendocrine tumors. Diagn Pathol, 13, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu P, Sun Y, Gong T, Zhu M, Sun X, Zhang Z, & Duan Y (2015). Preparation and properties of biocompatible PS-PEG/calcium phosphate nanospheres. Nanotoxicology, 9, 190–200. [DOI] [PubMed] [Google Scholar]
- Ward AE, & Rosenthal BM (2014). Evolutionary responses of innate immunity to adaptive immunity. Infect Genet Evol, 21, 492–496. [DOI] [PubMed] [Google Scholar]
- Williams NL, Wuthrick EJ, Kim H, Palmer JD, Garg S, Eldredge-Hindy H, Daskalakis C, Feeney KJ, Mastrangelo MJ, Kim LJ, Sato T, Kendra KL, Olencki T, Liebner DA, Farrell CJ, Evans JJ, Judy KD, Andrews DW, Dicker AP, Werner-Wasik M, & Shi W (2017). Phase 1 Study of Ipilimumab Combined With Whole Brain Radiation Therapy or Radiosurgery for Melanoma Patients With Brain Metastases. Int J Radiat Oncol Biol Phys, 99, 22–30. [DOI] [PubMed] [Google Scholar]
- Wu A, Cardarelli P, Oyasu M, Menezes D, Ponath P, Cogswell J, Maxwell R, Luksik A, Hung A, Kim E, Belcaid Z, Brem H, Pardoll D, & Lim M (2018). Abstract 1736: The combination of CXCR4 and checkpoint receptor inhibition improves survival in an orthotopic murine glioma model. Cancer Research, 78, 1736–1736. [Google Scholar]
- Wu WD, Yi XL, Jiang LX, Li YZ, Gao J, Zeng Y, Yi RD, Dai LP, Li W, Ci XY, Si DY, & Liu CX (2015). The Targeted-liposome Delivery System of Antitumor Drugs. Curr Drug Metab, 16, 894–910. [DOI] [PubMed] [Google Scholar]
- Xiang J, Hurchla MA, Fontana F, Su X, Amend SR, Esser AK, Douglas GJ, Mudalagiriyappa C, Luker KE, Pluard T, Ademuyiwa FO, Romagnoli B, Tuffin G, Chevalier E, Luker GD, Bauer M, Zimmermann J, Aft RL, Dembowsky K, & Weilbaecher KN (2015). CXCR4 Protein Epitope Mimetic Antagonist POL5551 Disrupts Metastasis and Enhances Chemotherapy Effect in Triple-Negative Breast Cancer. Mol Cancer Ther, 14, 2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M, Yan F, Yu S, & Shen P (2015). Efficacy and Cardiotoxicity of Liposomal Doxorubicin-Based Chemotherapy in Advanced Breast Cancer: A Meta-Analysis of Ten Randomized Controlled Trials. PLoS One, 10, e0133569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, & Yagita H (2002). Expression of programmed death 1 ligands by murine T cells and APC. J Immunol, 169, 5538–5545. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Kim S, Kim JS, & Choi IH (2012). Inflammasome formation and IL-1beta release by human blood monocytes in response to silver nanoparticles. Biomaterials, 33, 6858–6867. [DOI] [PubMed] [Google Scholar]
- Yen HJ, Hsu SH, & Tsai CL (2009). Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small, 5, 1553–1561. [DOI] [PubMed] [Google Scholar]
- Yigit MV, Moore A, & Medarova Z (2012). Magnetic nanoparticles for cancer diagnosis and therapy. Pharm Res, 29, 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoffe S, Leshuk T, Everett P, & Gu F (2013). Superparamagnetic iron oxide nanoparticles (SPIONs): synthesis and surface modification techniques for use with MRI and other biomedical applications. Curr Pharm Des, 19, 493–509. [PubMed] [Google Scholar]
- Yokoda RT, Nagalo BM, & Borad MJ (2018). Oncolytic Adenoviruses in Gastrointestinal Cancers. Biomedicines, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo GH, Hung MC, Lopez-Berestein G, LaFollette S, Ensley JF, Carey M, Batson E, Reynolds TC, & Murray JL (2001). Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin Cancer Res, 7, 1237–1245. [PubMed] [Google Scholar]
- Yu F, Wang X, Guo ZS, Bartlett DL, Gottschalk SM, & Song XT (2014). T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther, 22, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, & Lee NY (2019). JAVELIN Head and Neck 100: a Phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Future Oncol, 15, 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, & Daldrup-Link HE (2016). Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol, 11, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh HJ, Downs-Canner S, McCart JA, Guo ZS, Rao UN, Ramalingam L, Thorne SH, Jones HL, Kalinski P, Wieckowski E, O’Malley ME, Daneshmand M, Hu K, Bell JC, Hwang TH, Moon A, Breitbach CJ, Kirn DH, & Bartlett DL (2015). First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther, 23, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Li B, Liang Y, Reeves PM, Qu X, Ran C, Liu Q, Callahan MV, Sluder AE, Gelfand JA, Chen H, & Poznansky MC (2019). Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment. Faseb j, 33, 6596–6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang P, Wang X, Shi L, Fan Z, Zhang G, Yang D, Bahavar CF, Zhou F, Chen WR, & Wang X (2018). Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice. Technol Cancer Res Treat, 17, 1533033818785275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Song J, Liu Y, Liu M, Zhang L, Sheng D, Deng L, Yi H, Wu M, Zheng Y, Wang Z, & Yang Z (2019). Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-PD1 antibody and synergizes with antitumor immunotherapy for melanoma. J Control Release, 306, 15–28. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Deng J, Feng J, & Wu F (2010). Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World J Gastroenterol, 16, 3584–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, Reinhold B, Keskin DB, Reinherz EL, & Sasada T (2011). Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials, 32, 3666–3678. [DOI] [PubMed] [Google Scholar]
- Zhao T, Li C, Wu Y, Li B, & Zhang B (2017). Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: A meta-analysis. PLoS One, 12, e0176822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, & Zhou Z (2015). Human Cancer Immunotherapy with PD-1/PD-L1 Blockade. Biomark Cancer, 7, 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Li H, Lai K, Chen M, Fu G, Liu WH, Fu G, & Nie L (2018). Noninvasive photoacoustic and fluorescent tracking of optical dye labeled T cellular activities of diseased sites at new depth. J Biophotonics, e201800073. [DOI] [PubMed] [Google Scholar]
- Zuo QP, Li ZJ, Hu YH, Li B, Huang LH, Wang CJ, Liu SK, & Liao HQ (2012). A highly sensitive fluorescent probe for HClO and its application in live cell imaging. J Fluoresc, 22, 1201–1207. [DOI] [PubMed] [Google Scholar]