Abstract
Background
Osteoarthritis (OA) is the most common joint disease and is characterized by the progressive degeneration of articular cartilage. The molecular basis of OA involves various factors and has not been fully clarified. Autophagy is a conserved catabolic process that involves cellular degradation through the lysosomal machinery.
Material/Methods
We found that miRNA-411 regulates chondrocyte autophagy in OA by targeting hypoxia-inducible factor 1 alpha (HIF-1α) and identified the related molecular mechanism. OA condition in chondrocyte C28/I2 cells was induced by treatment with interleukin 1 beta (IL-1β). The protein expressions of LC3, p62, HIF-1α, ULK-1, and Beclin-1 were assessed by Western blot analysis, and LC3 expression was assessed by immunofluorescence.
Results
TargetScan analysis showed that HIF-1α mRNA is directly targeted by miR-411, which was confirmed by luciferase reporter assay. miR-411 mimic decreased HIF-1α levels in chondrocytes while miR-411 inhibitor increased HIF-1α levels in chondrocytes. Furthermore, expression of LC3, ULK-1, P62, and Beclin-1 in chondrocytes was induced by miR-411 inhibitor and was downregulated by miR-411 mimics. In addition, miR-411 mimics reduced the expression level of LC3, as determined by immunofluorescence analysis.
Conclusions
Our results demonstrate that miR-411 promotes chondrocyte autophagy by targeting HIF-1α, suggesting that regulating HIF-1α by miR-411 might be a therapeutic strategy for OA.
MeSH Keywords: Autophagy, Hypoxia-Inducible Factor-Proline Dioxygenases, MicroRNAs, Osteoarthritis
Background
Osteoarthritis (OA) is the most common joint disease. The etiological factors of OA are multifactorial, and the prevalence of OA is rising, mainly due to increasing obesity and aging of populations [1]. The overall prevalence of symptomatic knee OA is 8.1% in China and is higher in women than in men. OA causes pain and loss of joint function; unfortunately, few treatments are available to cure or delay the disease progression [2]. Extracellular matrix (ECM) catabolism is a key factor and characteristic of the progression of OA [3]. Several lines of evidence have suggested that OA onset and progression are regulated by gene expression and transcription factors [4,5], but the mechanisms involved in the occurrence and progression of OA remain largely unknown.
Autophagy, which is involved in maintaining cellular homeostasis, can protect cells from death that clears and degrades dysfunctional organelles and unwanted proteins[6,7]. Emerging evidence has indicated that autophagy participates in chondrocyte degeneration in OA and that the activation of autophagy protects chondrocytes from OA progression. Expression of autophagy-related proteins such as ULK-1, LC3, and Beclin1 is increased in cartilage, but their expression decreases during OA [8]. However, the exact mechanisms by which autophagy attenuates OA remain unclear.
miRNAs are abundant, single-stranded, small, non-coding RNAs that can act as post-transcriptional regulators of protein-coding gene expression by repressing protein translation, promoting RNA degradation, or acting via other mechanisms after binding to 3′ untranslated regions (UTRs) of RNAs [9]. Various factors affect OA, including aging, obesity, apoptosis, and inflammation, and regulation of miRNA expression and miRNAs regulatory autophagy play important roles in OA through various networks [10,11]. Chen et al. [12] found that miR-146a upregulates ULK-1, HIF-1α, and ATG-5 expression by targeting Traf6/IRAK1 in hypoxia, which slows OA progression. Additionally, miR-204 and miR-211 have been shown to regulate homeostasis and OA progression by affecting nerve growth factor (NGF) expression in a Runx2-dependent manner [13]. Therefore, miRNAs are essential in maintaining homeostasis because they target different pathways.
In our previous study, we showed that miR-411 is aberrantly expressed in articular cartilage and is specifically decreased in OA [14]. In the present study, we explored the roles of miR-411 in autophagy and found that HIF-1α is a target of miR-411. Our findings suggest that miR-411 regulates chondrocyte autophagy by targeting HIF-1α and may be a pathway involved in the pathogenesis of OA.
Material and Methods
Cell cultures
The human chondrocyte C28/I2 line was obtained from Uncoiling (Shandong, China) and maintained in DMEM (Thermo Fisher Scientific, USA). C28/I2 cells were incubated in a humidified atmosphere with 10% fetal bovine serum containing 5% CO2 at 37°C for 7 days before treatment.
IL-1β-induced cellular OA model and miRNA transfection
C28/I2 cells were seeded in 6-well plates and treated with 10 ng/mL IL-1β (Thermo Fisher Scientific, USA) for 24 h to generate an OA chondrocyte model. For miRNA transfection, the C28/I2 cells were transfected with miR-411 inhibitor, mimics or negative control (NC) (GenePharma, China):
miR-411 mimic 5′-TAGTAGACCGTATAGCGTACG-3′
miR-411 inhibitor 5′-CGTACGCTATACGGTCTACTA-3′
miR-411 NC 5′-TTCTCCGAACGTGTCACGT-3′
Dual luciferase report assay
The human HIF-1α 3′-UTR fragments and miR-411 were co-transfected into chondrocytes using Lipofectamine3000 (Invitrogen). Transfection efficiency was normalized using the pRL-TK vector. A Dual Luciferase Assay Kit (Promega, USA) was using to measure luciferase activities at 48 h after transfection.
Protein extraction and Western blot analysis
Cells were collected at 48 h and washed 3 times with phosphate-buffered saline (PBS). Next, the cells were centrifuged at 14 000 rpm for 15 min, and the supernatants were collected. We used a BCA protein assay kit (Uncoiling, China) to measure the total protein concentrations. The extracted protein samples were separated by SDS-PAGE (8–12%) and transferred onto PVDF membranes. The membranes were incubated with primary antibodies (Abcam, UK) overnight at 4°C after being blocked in 5% non-fat milk. The membranes were then washed twice with PBS with 0.05% Tween-20 and incubated for 1.5 h with a secondary antibody at room temperature. GAPDH (Abcam, UK) served as a loading control. Finally, the signals were analyzed using an ECL detection kit (Uncoiling, China). ImageJ software was used for semi-quantitative calculations.
RT-PCR analysis
Isolated total RNA was extracted from C28/I2 cells by Trizol reagent (Thermo Fisher Scientific, USA). A cDNA synthesis kit (Thermo Fisher Scientific, USA) was using to reverse-transcribe total RNA to cDNA. Generated cDNA of miR-411 and HIF-1α was performed using SYBR Green (Roche, Switzerland). The 2−ΔΔCT method was used to calculate the relative expression:
HIF-1α forward: 5′-CACCACAGGACAGTACAGGAT-3′;
reverse: 5′-CGTGCTGAATAATACCACTCACA-3′.
GAPDH forward: 5′-TCAGTGGTGGACCTGACCTG-3′;
reverse: 5′-TGCTGTAGCCAAATTCG TTG-3′.
Immunofluorescence
C28/I2 cells were seeded for 3 days in coverslips. Cells were fixed with 4% paraformaldehyde for 30 min, then 3% bovine serum albumin (BSA) was blocked for 1 h at 25°C. Next, the cells were incubated with primary antibodies (LC3) for 1 h at 37°C and with secondary antibodies for 1.5 h at room temperature. Then, the cells were incubated with DAPI for 15 min. Fluorescence signals were detected by microscopy.
Statistics analysis
All data are presented as the mean±S.D. GraphPad Prism Software 5.0 was used to conducted statistical analyses. We used the t test to compare significant differences. * P<0.05, ** P<0.01, and *** P<0.001 were used to indicate a statistically significant difference.
Results
HIF-1α is a direct target of miR-411 in chondrocytes
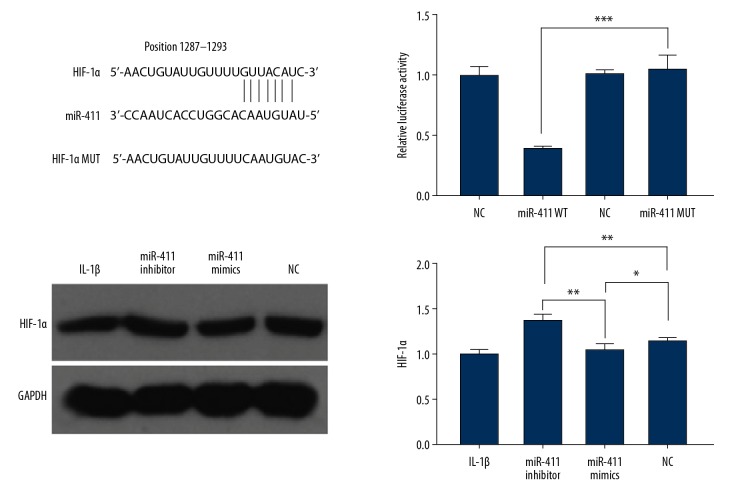
To explore the potential miR-411-targeting genes related to autophagy in human chondrocytes, we first predicted miR-411 targets by online TargetScan analysis. We found that HIF-1α may be a candidate miR-411 target. The interaction between miR-411 and targeting sites within the 3′-UTR of HIF-1α is shown in Figure 1. In support of this prediction, we found that miR-411 suppressed the luciferase activity of HIF-1α with the WT sequence but not MUT 3′-UTR of HIF-1α by the luciferase reporter assays. Further supporting this finding, the protein levels of HIF-1α were significantly induced in chondrocyte C28/I2 cells by miR-411 inhibitor. These results demonstrated that miR-411 directly recognizes the predicted HIF-1α mRNA site and suppresses HIF-1α expression in chondrocytes.
Figure 1.
HIF-1α is a target of miR-411. We screened the candidate target genes using TargetScan and identified HIF-1α by luciferase reporter assays. Sequence alignment between miR-411, WT and MUT of 3′-UTR of HIF-1α. Immunoblotting of HIF-1α protein in human chondrocyte C28/I2 cell OA model. Relative expression of HIF-1α by RT-qPCR in human chondrocyte C28/I2 cell OA model. * P<0.05; ** P<0.01; *** P<0.001.
miR-411 and HIF-1α expression in C28/I2 cells
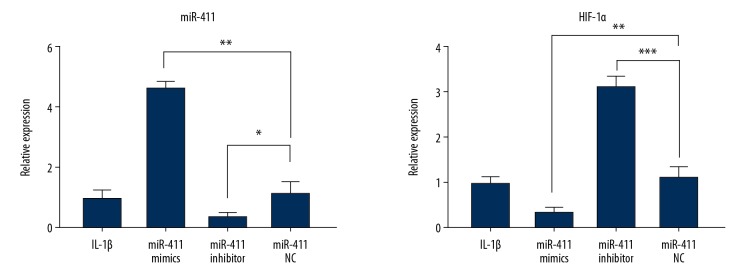
In our previous study, we found that miR-411 levels were lower in OA patients [14]. To test whether miR-411 downregulation leads to increased levels of HIF-1α in OA, we treated C28/I2 cells with IL-1β to induce OA, followed by transfection with miR-411 inhibitor, mimics, or NC. miR-411 in C28/I2 cells was significantly increased by miR-411 mimics but was decreased by miR-411 inhibitor (Figure 2). Compared with the NC group, HIF-1α mRNA expression was higher in the miR-411 inhibitor group and was lower in the miR-411 mimic group, which was consistent with the protein level of HIF-1α in chondrocyte C28/I2 cells (Figure 1). These results demonstrated that HIF-1α is negatively regulated by miR-411 in chondrocytes treated with IL-1β.
Figure 2.
miR-411 promotes HIF-1α expression in C28/I2 cells. The mRNA expression levels of miR-411 and HIF-1α as determined by RT-qPCR in human chondrocyte C28/I2 cell OA model. * P<0.05; ** P<0.01; *** P<0.001.
MiR-411 promotes autophagy in C28/I2 cells
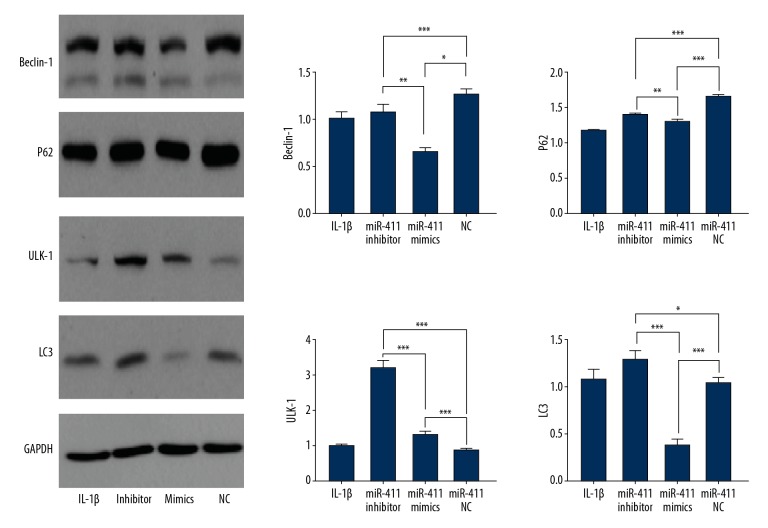
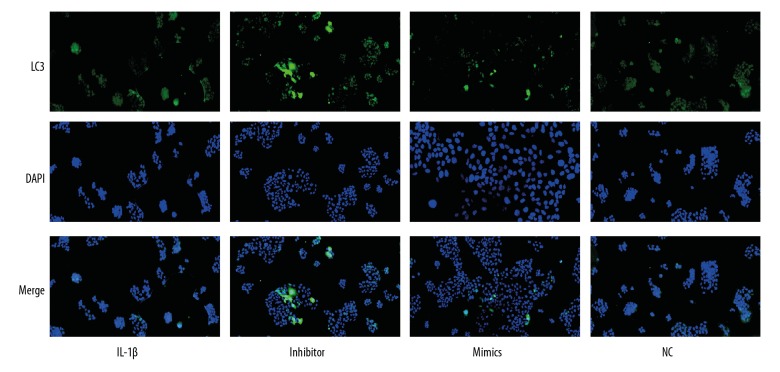
As shown in Figure 3, miR-411 inhibitor increased the expression of Beclin-1, P62, ULK-1, and LC3 and the number of cells with punctate LC3, but upregulation of miR-411 decreased the expression of Beclin-1, P62, ULK-1, and LC3 and reduced the number of cells with punctate LC3. Immunofluorescence staining was used to directly visualize chondrocyte autophagy changes, showing that the expression of miR-411 was associated with the level of autophagy and demonstrating that autophagy was decreased in the miR-411 mimics group; however, autophagy was increased in the miR-411 inhibitor group (Figure 4). Taken together, these results suggest that miR-411 alleviates autophagy.
Figure 3.
miR-411 regulates the autophagy of C28/I2 cells. Immunoblotting of the indicated proteins in human chondrocyte C28/I2 cell OA model. The relative expressions levels of the indicated proteins as quantificated by ImageJ. * P<0.05; ** P<0.01; *** P<0.001.
Figure 4.
Effect of miR-411 on the analysis of LC3 in C28/I2 cells by immunofluorescence staining. C28/I2 cells were transferred with miR-411 mimics, inhibitor or NC for 48 h. LC3 was checked by immunofluorescence staining. LC3 puncta were counted under confocal microscopy. Green indicates LC3. Blue indicates nuclei as stained by DAPI.
Discussion
OA is a painful, chronic joint disease with multiple pathological manifestations, typically including progressive destruction of articular cartilage, synovitis, osteophyte formation, chronic inflammation, and subchondral bone remodeling. Imbalance of homeostasis in the ECM of articular cartilage is essential for the development and progression of OA [15,16]. Although the molecular mechanisms are unclear, signaling pathways, increased expression of inflammation, apoptosis, and defective autophagy are all important factors leading to development of OA [4]. A review of the published literature indicated that miRNAs are important regulators of genes involved in cartilage degeneration, proteolytic enzyme synthesis, inflammation, apoptosis, autophagy, and signaling pathways. It was recently reported that many miRNAs are aberrantly expressed in OA; miR upregulation primarily targets the nucleus, and miR downregulation primarily targets transcription [17]. However, the pathophysiology role for miRNAs alteration during the onset and progression of OA is largely unknown.
Previous studies have reported that miR-411 plays important roles in a variety of diseases, such as bladder cancer, renal cancer, lung cancer, and ovarian can, and it contributes to cell proliferation and migration [18,19]. However, the biological functions of miR-411 expression in OA are unclear. In our previous study, we found there was decreased miR-411 expression accompanied by increased MMP-13 [14]. Many studies have found that IL-1β has important regulatory roles in the ECM proteins of OA, and has been widely used to induce OA cell models [20]. In the present study, we found that downregulation of miR-411 enhanced IL-1β-induced autophagy, whereas upregulation of miR-411 attenuated autophagy. Immunofluorescence and autophagy-related protein analyses also showed consistent results, demonstrating that miR-411 negatively regulates chondrocyte autophagy.
It is well established that articular cartilage physiologically lacks capillary networks, which results in reduced oxygen levels in joints [21]. Thus, chondrocytes need well-adapted mechanisms to maintain homeostasis. Many recent studies have shown that HIF-1α is overexpressed in hypoxia, which results in chondrocyte apoptosis [22]. HIF-1α has been found to have an important role in cartilage homeostasis. Previous studies have found that HIF-1α levels are increased in cartilage and synovial fluid, and HIF-1α levels are significantly correlated with the severity and progression of knee OA [23,24]. We demonstrated that HIF-1α is a direct target of miR-411 and that HIF-1α expression is negatively regulated by miR-411. Many recent studies have shown that HIF-1α can protect articular cartilage by regulating multiple pathological processes, such as chondrogenesis, autophagy, and apoptosis [25–28]. In this study, we found that miR-411 inhibitor increased HIF-1α expression, and miR-411 mimics decreased HIF-1α expression. Consistently, miR-411 inhibitor increased chondrocyte autophagy, whereas miR-411mimics attenuated chondrocyte autophagy. Additionally, the miR-411 inhibitor group exhibited increased HIF-1α expression, and the miR-411 mimics group exhibited decreased HIF-1α expression. Immunofluorescence analysis further supported these results. In a series of experiments, we showed that miR-411 alleviates autophagy by regulating HIF-1α in chondrocytes.
To further confirm the effects on autophagy, we analyzed 4 autophagy-related proteins (Beclin-1, P62, ULK-1, and LC3) in this study. LC3 is a specific marker of autophagosomes that activates autophagosome biogenesis by binding to ATG7 and other autophagic effectors [29]. Beclin-1 is a key player in autophagy and is thought to be involved in regulating autophagosome synthesis and maturation [30]. Recent reports proved that ULK-1 has crucial roles in autophagy events. Activated ULK-1 can trigger the autophagy cascade by phosphorylating several proteins and/or activating AMPK, thereby promoting autophagy [31]. P62, a protein capable of delivering autophagic cargo, can serve as a measure of autophagy flux, and it is considered to be negatively correlated with autophagic degradation [32]. We found that miR-411 inhibitor increased the expression levels of Beclin-1, p62, ULK-1, and LC3. These results revealed an important role of miR-411 in regulating autophagy in chondrocytes.
Conclusions
We previously found that miR-411 is downregulated in OA chondrocytes, suggesting that reduction of miR-411 can contribute to the progression and pathogenesis of OA. We found that the miR-411-mediated HIF-1α axis plays an essential role in the autophagy of chondrocyte C28/I2 cell and suggests that replenishing miR-411 may provide a novel therapeutic strategy for the treatment of OA. Rapid advances in miRNA delivery methods such as nanoparticle- and exosome-mediated delivery of miRNAs show promise for use in assessing the therapeutic effects of miR-411 in OA, both in vitro and in vivo.
Footnotes
Source of support: Departmental sources
References
- 1.Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386:376–87. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Tang X, Wang S, Zhan S, et al. The prevalence of symptomatic knee osteoarthritis in China: Results from the China health and retirement longitudinal study. Arthritis Rheumatol. 2016;68(3):648–53. doi: 10.1002/art.39465. [DOI] [PubMed] [Google Scholar]
- 3.Luo P, Gao F, Niu D, Sun X, et al. The role of autophagy in chondrocyte metabolism and osteoarthritis: A comprehensive research review. Biomed Res Int. 2019;2019 doi: 10.1155/2019/5171602. 5171602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mobasheri A, Rayman MP, Gualillo O, et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–11. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 5.Jeffries MA, Donica M, Baker LW, et al. Genome-wide DNA methylation study identifies significant epigenomic changes in osteoarthritic cartilage. Arthritis Rheumatol. 2014;66(10):2804–15. doi: 10.1002/art.38762. [DOI] [PubMed] [Google Scholar]
- 6.Lopez de Figueroa P, Lotz MK, Blanco FJ, Carames B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015;67:966–76. doi: 10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouderlique T, Vuppalapati KK, Newton PT, et al. Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann Rheum Dis. 2016;75:627–31. doi: 10.1136/annrheumdis-2015-207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carames B, Taniguchi N, Otsuki S, et al. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Adamo S, Cetrullo S, Minguzzi M, et al. MicroRNAs and autophagy: Fine players in the control of chondrocyte homeostatic activities in osteoarthritis. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/3720128. 3720128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. 2012;8:543–52. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musumeci G, Castrogiovanni P, Trovato FM, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16:20560–75. doi: 10.3390/ijms160920560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Gao X, Wang J, et al. Hypoxia-induced microRNA-146a represses Bcl-2 through Traf6/IRAK1 but not Smad4 to promote chondrocyte autophagy. Biol Chem. 2017;398:499–507. doi: 10.1515/hsz-2016-0211. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Zhao L, Fan Y, et al. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat Commun. 2019;10:2876. doi: 10.1038/s41467-019-10753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G, Zhang Y, Zhao X, et al. MicroRNA-411 inhibited matrix metalloproteinase 13 expression in human chondrocytes. Am J Transl Res. 2015;7(10):2000–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Malemud CJ. Biologic basis of osteoarthritis: State of the evidence. Curr Opin Rheumatol. 2015;27:289–94. doi: 10.1097/BOR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12:632–44. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 17.Cong L, Zhu Y, Tu G. A bioinformatic analysis of microRNAs role in osteoarthritis. Osteoarthritis Cartilage. 2017;25:1362–71. doi: 10.1016/j.joca.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Wang H, Liu X, et al. Oncogenic microRNA-411 promotes lung carcinogenesis by directly targeting suppressor genes SPRY4 and TXNIP. Oncogene. 2019;38:1892–904. doi: 10.1038/s41388-018-0534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu N, Yang W, Liu Y, et al. MicroRNA-411 promoted the osteosarcoma progression by suppressing MTSS1 expression. Environ Sci Pollut Res Int. 2018;25:12064–71. doi: 10.1007/s11356-018-1331-9. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Wu Y, Chen J, et al. miR-10a-5p promotes chondrocyte apoptosis in osteoarthritis by targeting HOXA1. Mol Ther Nucleic Acids. 2019;14:398–409. doi: 10.1016/j.omtn.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: Structure, composition, and function. Sports Health. 2009;1:461–68. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Z, Zhou M, Wang Q, et al. Mechanical and hypoxia stress can cause chondrocytes apoptosis through over-activation of endoplasmic reticulum stress. Arch Oral Biol. 2017;84:125–32. doi: 10.1016/j.archoralbio.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Araldi E, Schipani E. Hypoxia, HIFs and bone development. Bone. 2010;47:190–96. doi: 10.1016/j.bone.2010.04.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qing L, Lei P, Liu H, et al. Expression of hypoxia-inducible factor-1alpha in synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis. Exp Ther Med. 2017;13:63–68. doi: 10.3892/etm.2016.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amarilio R, Viukov SV, Sharir A, et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–28. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 26.Bohensky J, Shapiro IM, Leshinsky S, et al. HIF-1 regulation of chondrocyte apoptosis: induction of the autophagic pathway. Autophagy. 2007;3:207–14. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- 27.Zhang FJ, Luo W, Lei GH. Role of HIF-1alpha and HIF-2alpha in osteoarthritis. Joint Bone Spine. 2015;82:144–47. doi: 10.1016/j.jbspin.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Yudoh K, Nakamura H, Masuko-Hongo K, et al. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: Involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res Ther. 2005;7:R904–14. doi: 10.1186/ar1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang R, Liu W. Identifying an essential role of nuclear LC3 for autophagy. Autophagy. 2015;11:852–53. doi: 10.1080/15548627.2015.1038016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill SM, Wrobel L, Rubinsztein DC. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 2019;26:617–29. doi: 10.1038/s41418-018-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CC, Lin YC, Chen YH, et al. Cul3-KLHL20 ubiquitin ligase governs the turnover of ULK1 and VPS34 complexes to control autophagy termination. Mol Cell. 2016;61:84–97. doi: 10.1016/j.molcel.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Long M, Li X, Li L, et al. Multifunctional p62 effects underlie diverse metabolic diseases. Trends Endocrinol Metab. 2017;28:818–30. doi: 10.1016/j.tem.2017.09.001. [DOI] [PubMed] [Google Scholar]