Abstract
The pituitary adenylate cyclase-activating polypeptide (PACAP) system plays a central role in the brain’s emotional response to psychological stress by activating cellular processes and circuits associated with threat exposure. The neuropeptide PACAP and its main receptor PAC1 are expressed in the rodent central amygdala (CeA), a brain region critical in negative emotional processing, and CeA PACAPergic signaling drives anxiogenic and stress coping behaviors. Despite this behavioral evidence, PACAP’s effects on neuronal activity within the medial subdivision of the CeA (CeM, the major output nucleus for the entire amygdala complex) during basal conditions and after psychological stress remain unknown. Therefore, in the present study, male Wistar rats were subjected to either restraint stress or control conditions, and PACAPergic regulation of CeM cellular function was assessed using immunohistochemistry and whole-cell patch-clamp electrophysiology. Our results demonstrate that PACAP-38 potentiates GABA release in the CeM of naïve rats, via its actions at presynaptic PAC1. Basal PAC1 activity also enhances GABA release in an action potential-dependent manner. Notably, PACAP-38’s facilitation of CeM GABA release was attenuated after a single restraint stress session, but after repeated sessions returned to the level observed in naïve animals. A single restraint session also significantly decreased PAC1 levels in the CeM, with repeated restraint sessions producing a slight recovery. Collectively our data reveal that PACAP/PAC1 signaling enhances inhibitory control of the CeM and that psychological stress can modulate this influence to potentially disinhibit downstream effector regions that mediate anxiety and stress-related behaviors.
Keywords: GABA, PACAP-38, PAC1, sIPSC, synaptic transmission, pituitary adenylate cyclase-activating polypeptide, anxiety
1. Introduction
Anxiety disorders are one of the most prevalent forms of mental illness, and manifest as excessive fear, anxiety or avoidance of perceived threats from within the individual or in the surrounding environment (Craske and Stein, 2016). Pituitary adenylate cyclase-activating polypeptide (PACAP) signaling acts as a master regulator of the brain’s emotional response to anxiety and stress, and is thought to be specifically recruited after psychological stress (vs. physical stress) to activate cellular processes and circuits associated with threat exposure (Hammack and May, 2015; Ramikie and Ressler, 2016).
PACAPergic signaling in the central nervous system (CNS) is primarily activated by the neuropeptide PACAP-38, which is expressed 10-fold to 100-fold more abundantly than its truncated form PACAP-27 (Arimura et al., 1991). Chronic stress induces PACAP expression in several rodent brain regions that regulate anxiety, including the bed nucleus of the stria terminalis (BNST) and the paraventricular nucleus of the hypothalamus (PVN) (Hammack et al., 2009), while intracerebroventricular infusion of PACAP-38 activates the hypothalamic pituitary adrenal (HPA) axis, and increases the stress responses and anxiety-like behavior of rats (Agarwal et al., 2005; Dore et al., 2013). PACAP-38 mainly produces these effects by binding selectively and with high affinity to its cognate receptor PAC1, though it can also bind the vasoactive intestinal polypeptide receptors 1 and 2 (VPAC1 and VPAC2) (Shivers et al., 1991). Accordingly, chronic stress and fear-conditioning paradigms induce PAC1 expression in multiple rodent brain regions, including the basolateral amygdala (BLA), BNST, medial prefrontal cortex and PVN (Andero et al., 2014; Hammack et al., 2009; Ressler et al., 2011), while PAC1 deletion (i.e. PAC1 knock-out mice) reduces startle behavior and anxiety-like responses (Otto et al., 2001a; Otto et al., 2001b). Of note, recent studies have linked human allelic variants and site-specific methylation of the gene encoding PAC1 (ADCYAP1R1) with an altered patient susceptibility to post-traumatic stress disorder (PTSD) (Almli et al., 2013; Ressler et al., 2011; Uddin et al., 2013), though some studies are conflicting (Chang et al., 2012).
Amygdala dysfunction is implicated in several anxiety disorders (Shin and Liberzon, 2010), with the central nucleus of the amygdala (CeA), in particular, acting as a hub for negative emotional processing (Gilpin et al., 2015). The CeA is a primarily inhibitory nucleus, with an interconnected network of γ-aminobutyric acid (GABA) interneurons and GABA projection neurons that can inhibit each other via axon collaterals (Haubensak et al., 2010; Lopez de Armentia and Sah, 2004; Marek et al., 2013; Pape and Pare, 2010); the lateral (CeL) and capsular (CeC) subdivisions of the CeA receive glutamatergic input from the BLA and lateral amygdala and send GABAergic afferents to its medial subdivision (CeM) (Jolkkonen and Pitkanen, 1998; Pitkanen et al., 1995; Savander et al., 1995). The CeM, which also receives excitatory input from the BLA, serves as the major output nucleus of the entire amygdala complex and sends GABAergic projections to downstream effector regions that regulate stress and fear responses (e.g BNST, dorsal vagal complex of the brainstem, lateral hypothalamus, locus coeruleus and periaqueductal gray; (Gilpin et al., 2015)). The CeA is innervated by PACAPergic fibers from the lateral parabrachial nucleus and dorsal vagal complex of the brainstem (Cho et al., 2012; Missig et al., 2014), and PAC1 is expressed throughout the CeA (Joo et al., 2004; Piggins et al., 1996). PACAP-38 infusion directly into the rodent CeA activates the animals’ HPA axis (Iemolo et al., 2016), increases their anxiety-like behaviors (Iemolo et al., 2016; Missig et al., 2014), shifts their stress-coping behaviors from active to passive modes (Legradi et al., 2007), and heightens their nociceptive sensitivity (Missig et al., 2014). Moreover, intra-CeA infusion of a PAC1 competitive antagonist (PACAP(6–38)) reduces anxiogenic and nociceptive responses in a model of chronic neuropathic pain (Missig et al., 2017).
Despite the clear role of CeA PACAP/PAC1 signaling in mediating anxiogenic and stress coping behaviors, its mechanisms of action within the CeM (the major output nucleus for the entire amygdala) remain to be investigated. Therefore, in the present study, rats were subjected to either restraint stress (a primarily psychological stressor (Gray et al., 2015)) or control conditions, and PACAPergic regulation of CeM cellular function was assessed using immunohistochemistry and whole-cell patch-clamp electrophysiology. Overall our data demonstrate that PACAP-38 acts via PAC1 to enhance inhibition within the CeM and that psychological stress can modulate this PACAPergic influence over local GABA synaptic activity.
2. Materials and Methods
The Scripps Research Institute (TSRI) Institutional Animal Care and Use Committee (IACUC) and Boston University Medical Campus IACUC approved all protocols involving the use of experimental animals in this study.
2.1. Animals
Male Wistar rats (n=59; 324±5 g) were obtained from Charles River Laboratories (Wilmington, MA) and group-housed in a temperature and humidity-controlled vivarium on a 12 hr/12 hr light/dark cycle with food and water available ad libitum. Rats were exposed to restraint stress for a single session (1 hr) or for 3 repeated homotypic sessions (1 hr per day for 3 consecutive days), as previously described (Varodayan et al., 2018). For each restraint stress session, rats were placed in a vented cylindrical tube made of clear Plexiglas, with sliding plugs to allow adjustment of the tube length for each animal size and a tail slot to prevent unnatural body posture. All rats were returned to their home cages for a 1 hr post-stress recovery period prior to sacrifice for both the electrophysiological and the immunohistochemical studies. Similar protocols (1 hr restraint stress followed by 1–2 hr recovery period) have been shown to increase CeA immediate early gene expression (Crane et al., 2005; Kovacs et al., 2018; Stamp and Herbert, 1999; Sterrenburg et al., 2012).
2.2. Whole-cell recordings
Rats were anesthetized with 3–5% isoflurane and sacrificed, and the isolated brains placed in ice-cold oxygenated (95% O2 and 5% CO2) high-sucrose cutting solution (in mM: sucrose, 206; KCl, 2.5; CaCl2, 0.5; MgCl2, 7; NaH2PO4, 1.2; NaHCO3, 26; glucose, 5; HEPES, 5), as previously described (Varodayan et al., 2017a). Coronal slices (300 μm) containing the CeA were cut using a Leica 1200S vibratome (Leica Microsystems, Buffalo Grove, IL) and incubated in artificial cerebrospinal fluid (in mM: NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4•7H2O, 1.5; CaCl2, 2.0; NaHCO3, 24; glucose, 10) at 37 °C for 30 min and then at room temperature for a minimum of 30 min (all recordings were performed at room temperature). We recorded from 137 neurons in the medial subdivision of the CeA (CeM), that were visualized with infrared differential interference contrast optics, a w60 water immersion objective (Olympus) and a CCD camera (EXi Aqua, QImaging, Surrey, Canada). Neurons were clamped at −60 mV, and recordings were performed in gap-free acquisition mode with a sampling rate per signal of 10 kHz and low-pass filtered at 10k Hz, using a Multiclamp 700B amplifier, Digidata 1440A and pClamp 10 software (Molecular Devices, Sunnyvale, CA). Borosilicate glass patch pipettes (3–6MΏ; King Precision, Claremont, CA) were filled with internal solution (in mM: 145.0 KCl; 5.0 EGTA; 5.0 MgCl2; 10.0 HEPES; 2.0 Na+-ATP; 0.2 Na+-GTP). Spontaneous GABAA-mediated inhibitory postsynaptic currents (sIPSCs) were pharmacologically isolated with 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM), DL-2-amino-5-phosphonovalerate (AP-5, 30 μM) and CGP55845A (1 μM). Miniature IPSCs (mIPSCs) were recorded in the presence of 0.5 μM tetrodotoxin (TTX). Only cells with stable resting membrane potentials >−45 mV, stable holding currents <100 pA, stable series resistances <15 MΏ with <20% change (monitored with a 10 mV pulse), and action potentials with positive overshoots (recorded in current-clamp mode and elicited by incremental depolarizing current injections) were used. PACAP-38 (5 nM) and the PAC1 antagonist PACAP(6–38) (250 nM) were purchased from American Peptide Company (Sunnyvale, CA), and the PAC1 antagonist 2-Amino-5,8-dihydro-5-[3-methoxy-4-(2-propen-1-yloxy)phenyl]pyrido[2,3-d]pyrimidine-4,7(3H,6H)-dione (PA-8; 250 nM) was purchased from Tocris (Bristol, UK). Of note, while PACAP(6–38) is considered a selective PAC1 competitive antagonist and used widely as such (due to few alternatives), it can also inhibit VPAC2 receptors (Dickinson et al., 1997). In contrast, PA-8 selectively inhibits PAC1, but not VPAC1 or VPAC2 (Takasaki et al., 2018). All drugs were applied directly to the bath by adding a known concentration of stock solution, with all s/mIPSC measures taken in the 3 min preceding drug application (baseline) and during the last 3 min of the 15 min drug perfusion. s/mIPSC data were analyzed using Mini Analysis (Synaptosoft Inc., Fort Lee, NJ) and visually confirmed. Only s/mIPSCs >5 pA were accepted for analysis, and average s/mIPSC characteristics were based on 3 min of recording and a minimum 60 events. To control for cell-to-cell variation in baseline electrophysiology properties, drug effects were normalized to their own neuron’s baseline prior to group analyses.
2.3. Immunohistochemistry
Rats were anesthetized with isoflurane and transcardially perfused. Coronal sections (30 μm) were cut on a cryostat and stored in cryoprotectant at −20 °C. Every sixth section (180 μm apart) of the entire CeA (bregma: −2.0 to −3.0 mm) was randomly selected for immunohistochemical processing, with the free-floating sections washed in Tris-buffered saline after every incubation. Briefly, sections were incubated with sodium citrate buffer for 10 min at 95 °C, blocked for 1 hr in blocking solution (5% normal goat serum, 0.2% Trition X-100 in Tris-buffered saline), and then transferred into rabbit anti-PAC1 primary (AVR-003, 1:250, Alomone Labs, Jerusalem, Israel) for 48 hr at 4 °C. Sections were then incubated in anti-rabbit secondary antibody (A21429, 1:500, AF555, Abcam, Cambridge, Massachusetts) for 2 hr at room temperature, and then mounted onto slides and coverslipped with DAPI Hardset mountant (Vector Laboratories, Burlingame, CA). In a second experiment, antibody specificity was confirmed using a peptide competition assay, where primary anti-PAC1 antibody (AVR-003, 1:250, Alomone Labs) was incubated with either blocking peptide (Control Antigen AVR-003, Alomone Labs) added at a 10:1 ratio to antibody or PBS for 1 hr at room temperature. Sections were then processed as described above, using either the blocked primary anti-PAC1R antibody or the control primary antibody (Suppl. Fig. 1). Representative 10X CeA images were taken with an Olympus BX-51 microscope, a Rotiga 2000R camera (QImaging), Stereo Investigator software (MicroBrightField, Williston, VT), and a three-axis MAC6000 XYZ motorized stage (Ludl Electronics, Hawthorne, NY). For PAC1 quantification, Stereo Investigator software was used to outline each CeA subdivision (medial: CeM, lateral: CeL and central: CeC) at 2X magnification by referencing DAPI staining and a rat brain atlas (Paxinos G., 2007), and PAC1+ cells were counted at 20X.
2.4. Statistics
All final values were analyzed with Prism 6.01 (GraphPad, San Diego, CA), with statistical significance accepted at the p<0.05 level. Specifically, pooled electrophysiology data for each experimental condition were analyzed by one-sample t-tests for individual means comparisons to baseline to evaluate single drug effects (e.g. PACAP-38), with significance denoted by *. Unpaired t-tests were used for comparisons of drug effects across two conditions (e.g. PACAP-38 in the absence or presence of PACAP(6–38)) within the same animal treatment group, with significance denoted by #. To compare the electrophysiology or immunohistochemistry data across animal treatment groups, we used one-way ANOVAs with post hoc tests as appropriate, with significance denoted by $. Finally, two-way ANOVAs with post hoc tests, were used to assess multiple drug concentration responses across animal groups, with significance denoted by &. All data are presented as mean ± standard error of the mean (SEM).
3. Results
3.1. Restraint stress does not alter basal GABA transmission in the rat CeM
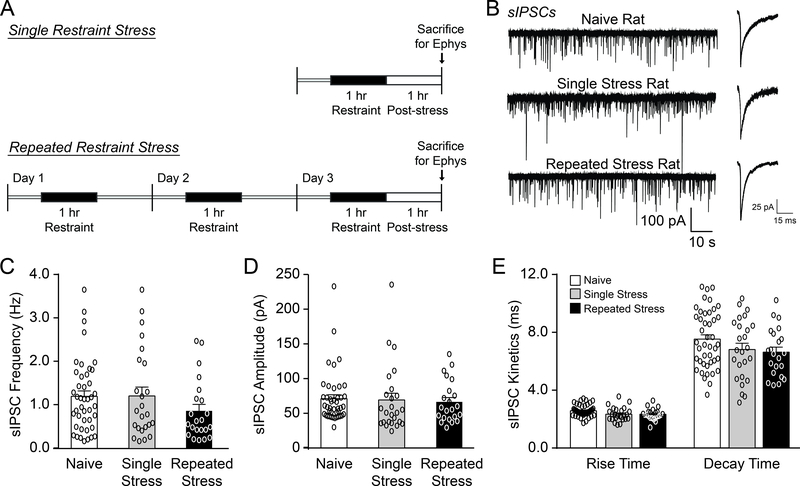
To investigate whether psychological stress alters basal CeM activity we recorded GABAA-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) in naïve rats and rats subjected to either a single (1 hr) or repeated (1 hr per day for 3 consecutive days) restraint stress followed by a 1 hr post-stress recovery period (Fig. 1A). There were no significant differences in baseline sIPSC frequencies, amplitudes, rise times and decay times across all three animal groups (frequency: F(2, 87)=1.30, p=0.28, amplitude: F(2, 87)=0.10, p=0.90, rise time: F(2, 87)=0.54, p=0.58 and decay time: F(2, 87)=1.94, p=0.15, by one-way ANOVA) (Fig. 1B–E). Generally, an increased sIPSC frequency reflects a higher probability of GABA release, while increased sIPSC amplitude/kinetics reflect greater postsynaptic GABAA receptor sensitivity (Otis et al., 1994). Therefore, restraint stress does not impact basal inhibitory transmission across the CeM synaptic network.
Figure 1.
Restraint stress does not alter basal GABAergic transmission in the rat CeM. A. Diagrams illustrating the single and repeated restraint stress protocols. B. Representative sIPSC traces (left) and scaled averages (right) from CeM neurons in naïve rats and rats subjected to single or repeated restraint stress sessions and a 1 hr post-stress recovery period prior to sacrifice. C-E. Baseline sIPSC frequencies (C), amplitudes (D), and kinetics (E) were similar across all three animal groups. For all data presented in this figure, the naïve group comprised 43 cells from 10 rats, the single restraint stress group 25 cells from 8 rats, and the repeated restraint stress group 22 cells from 9 rats. All data are presented as mean±SEM.
3.2. PACAP increases CeM GABA release via PAC1
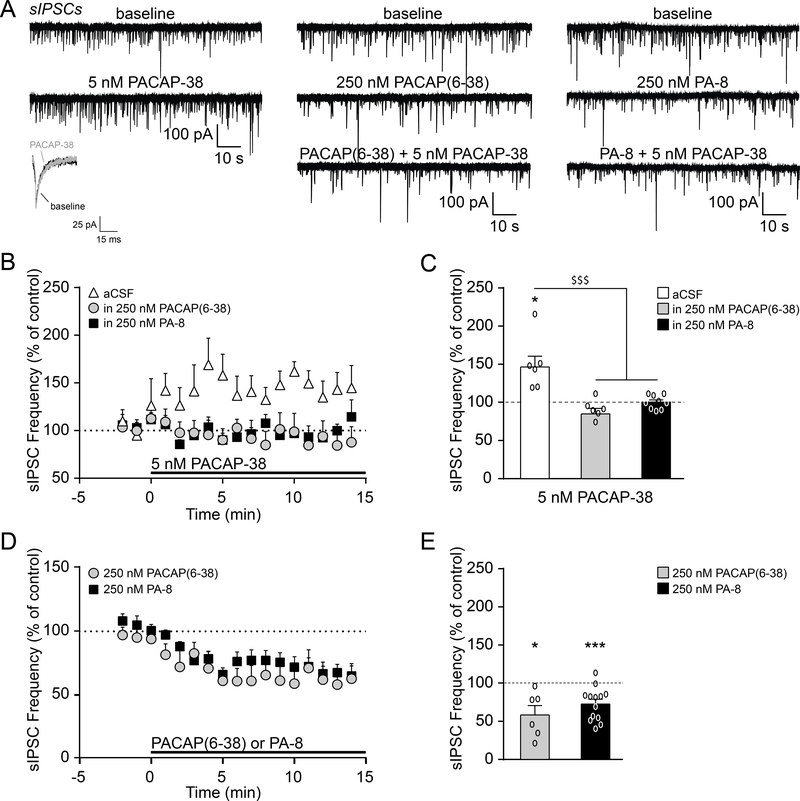
We next assessed whether the PACAP/PAC1 system regulates inhibitory transmission within the CeM. We found that exogenous PACAP-38 application (5 nM for 15 min) significantly increased the mean sIPSC frequency in naïve rats (to 148.7±14.4% of baseline; t(5)=3.37, p<0.05 by one-sample t-test; Fig. 2A–C), but had no effect on sIPSC amplitudes or kinetics. Of note, PACAP-38’s actions were relatively consistent across the CeM, with only 2 out of 27 naïve rat cells not responding to PACAP-38 across the entire study (all cells were included in the final experimental data set). Pretreatment with a PAC1 antagonist (250 nM PACAP(6–38) or 250 nM PA-8 for 15 min) prevented PACAP-38’s enhancement of the sIPSC frequency (F(2, 18)=14.23, p<0.001 by one-way ANOVA and post hoc Tukey’s multiple comparisons test; Fig. 2A–C). In addition, both PAC1 antagonists reduced CeM baseline sIPSC frequencies (PACAP(6–38): 57.8±12.1% of baseline; t(5)=3.48, p<0.05 by one-sample t-test; PA-8: 73.2±6.1%; t(12)=4.383, p<0.001; Fig. 2A and D–E), with no effects on the other sIPSC properties. Thus, PAC1 displays tonic activity in the CeM of naïve rats, and PACAP-38/PAC1 signaling increases local GABA release across the CeM synaptic network.
Figure 2.
PACAP-38 increases CeM GABA release via PAC1. A. Representative sIPSCs and scaled average from naïve rat CeM neurons at baseline and during superfusion of PACAP-38 (5 nM) and PAC1 antagonists (250 nM PACAP(6–38) or 250 nM PA-8). B. Timecourse of PACAP-38 effects on sIPSC frequencies in the absence (white triangle) or presence of either PACAP(6–38) (grey circle) or PA-8 (black square). C. PACAP-38 significantly increased the mean sIPSC frequency, and pretreatment with PACAP(6–38) or PA-8 prevented this facilitation (6–9 cells from 3–4 rats per group). D. Timecourse of the effects of PACAP(6–38) (grey circle) or PA-8 (black square) on the sIPSC frequency. E. PACAP(6–38) and PA-8 reduced the sIPSC frequency in naïve rats (6–13 cells from 4 rats per group). All data are presented as mean±SEM. *p<0.05 and ***p<0.001 by one sample t-test; $ $ $p<0.001 by one-way ANOVA with post hoc Tukey’s multiple comparisons test.
3.3. A single restraint stress reduces PAC1 immunoreactivity in the CeM
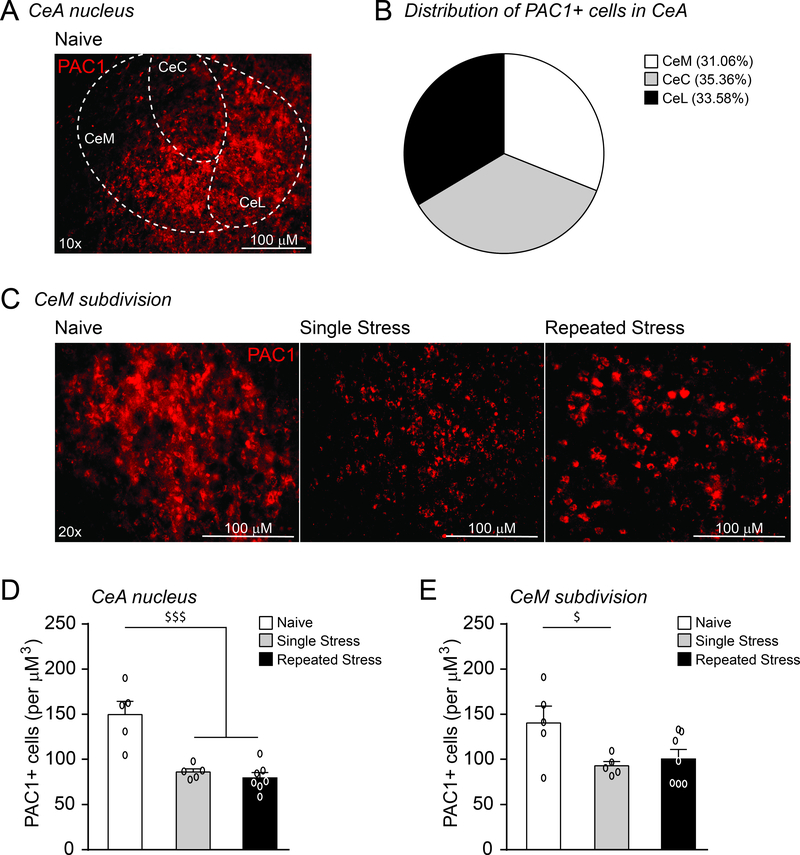
As the CeA PACAP system regulates anxiogenic and stress coping behaviors, we then examined whether psychological stress alters regional PAC1 levels. PAC1 immunoreactivity was found to be dispersed evenly in cell bodies throughout all CeA subdivisions, including the CeM (Fig. 3A–C). Restraint stress significantly altered PAC1 expression globally in the CeA (F(2, 1)= 19.15, p<0.001 by one-way ANOVA and post hoc Newman–Keuls multiple comparisons test; Fig. 3D) and locally within the CeM (F(2, 1)= 3.9779, p<0.05; Fig. 3C and E). Specifically, a single restraint/post-stress period reduced the number of PAC1+ cells in both regions. Of note, this effect persisted in the CeA after repeated restraint stress, but there was a slight recovery in the CeM.
Figure 3.
A single restraint stress reduces PAC1 immunoreactivity in the CeM. A. Image of a coronal section through the CeA showing PAC1 immunoreactivity in the lateral (CeL), capsular (CeC) and medial subdivisions (CeM). B. Each CeA subdivision contained a similar number of PAC1+ cells. C. Image of coronal section showing PAC1 immunoreactivity in the CeM of naive rats and rats subjected to single or repeated restraint stress sessions and a 1 hr post-stress recovery period prior to sacrifice. All scale bars represent 100 μM. D. A single restraint period reduced PAC1+ immunoreactivity in the CeA, and this effect persisted with repeated restraint stress sessions (5–7 rats per group). E. A single restraint period reduced the number of PAC1+ cells in the CeM subdivision and a slight recovery was observed followed repeated restraint (5–7 rats per group). All data are presented as mean±SEM. $p<0.05 and $ $ $p<0.001 by one-way ANOVA with post hoc Newman-Keuls multiple comparisons test.
3.4. A single restraint stress reduces PACAP/PAC1 influence over the CeM GABA network
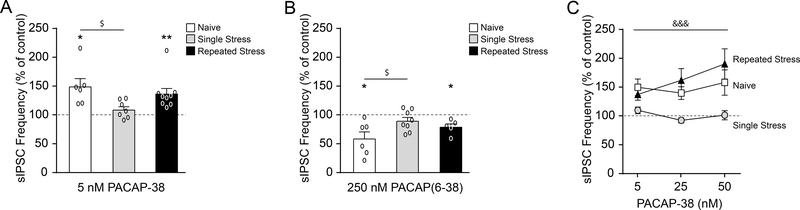
To investigate whether these stress-induced molecular changes in PAC1 signaling are associated with altered CeA network activity, we recorded sIPSCs in the CeM of animals exposed to restraint stress. Restraint stress significantly altered the effects of 5 nM PACAP-38 (F(2, 19)=3.66, p<0.05 by one-way ANOVA and post hoc Tukey’s multiple comparisons test; Fig. 4A) and 250 nM PACAP(6–38) (F(2, 16)=3.97, p<0.05; Fig. 4B) on CeM sIPSC frequencies, with no drug-induced changes in sIPSC amplitudes or kinetics. Specifically, after a single restraint/post-stress period, 5 nM PACAP-38 no longer increased the mean sIPSC frequency (108.5±5.8% of baseline; t(6)=1.48, p=0.19 by one-sample t-test), while PACAP(6–38) no longer decreased it (88.3±6.5%; t(7)=1.82, p=0.11). Since this loss in PACAP-38’s ability to facilitate GABA release may be due to a reduction in PAC1 sensitivity, we also tested higher PACAP-38 concentrations (25 nM or 50 nM; Fig. 4C). Two-way ANOVA showed that there was a significant main effect of stress treatment (F(2, 44)=15.07, p<0.001) on PACAP-38’s modulation of sIPSC frequencies, without any concentration effect (F(2, 44)=1.48, p=0.24) or interaction (F(4, 44)=1.44, p=0.24). Post hoc Tukey’s multiple comparisons testing revealed an attenuation of PACAP-38’s ability to increase the sIPSC frequency after a single restraint stress as compared to either naive or repeated restraint stress rats (p<0.001). Specifically, both 25 nM and 50 nM PACAP-38 did not alter the sIPSC frequency in single restraint stress rats (25 nM: 91.1±4.3%; t(4)=2.07, p=0.11; 50 nM: 100.0±8.1%; t(4)=0.0034, p=0.99 by one-sample t-test), despite the fact that these concentrations continued to increase sIPSC frequencies in naïve rats (25 nM: 138.6±11.1%; t(5)=3.48, p<0.05; 50 nM: 157.0±22.1%; t(5)=2.58, p<0.05). Repeated restraint stress produced adaptation, such that the effects of both PACAP-38 and PACAP(6–38) on sIPSC frequencies were similar to those of naïve animals (5 nM PACAP-38: 136.1±9.8%; t(8)=3.66, p<0.01; 25 nM PACAP-38: 160.8±20.4%; t(4)=2.98, p<0.05; 50 nM PACAP-38: 189.4±26.4%; t(3)=3.38, p<0.05; and PACAP(6–38): 77.9±5.7%; t(4)=3.88, p<0.05; Fig. 4A–C).
Figure 4.
A single restraint stress reduces PACAP/PAC1 regulation of the CeM GABA network. A. 5 nM PACAP-38-induced facilitation of the sIPSC frequency was lost after a single restraint stress, but recovered toward naïve levels with repeated restraint stress sessions (6–9 cells from 3–5 rats per group). The data from naïve rats shown here are the same as in Fig. 2C. B. 250 nM PACAP(6–38) reduced the sIPSC frequency in naïve rats and repeated restraint stress rats, but not in animals subjected to a single restraint stress (5–8 cells from 3–4 rats per group). The data from naïve rats shown here are the same as in Fig. 2E. C. The loss of PACAP-38’s ability to facilitate GABA release after a single restraint stress persisted with higher agonist concentrations (25 nM or 50 nM; 4–9 cells from 3–5 rats per group). The 5 nM PACAP-38 data are the same as in Fig. 2C. All data are presented as mean±SEM. *p<0.05 and **p<0.01 by one sample t-test; $p<0.05 by one-way ANOVA with post hoc Tukey’s multiple comparisons test; &&&p<0.001 by two-way ANOVA with post hoc Tukey’s multiple comparisons test indicating a significant difference between the single restraint stress group as compared with the naive and repeated restraint stress groups.
3.5. PAC1 has a presynaptic site of action at CeM GABA terminals
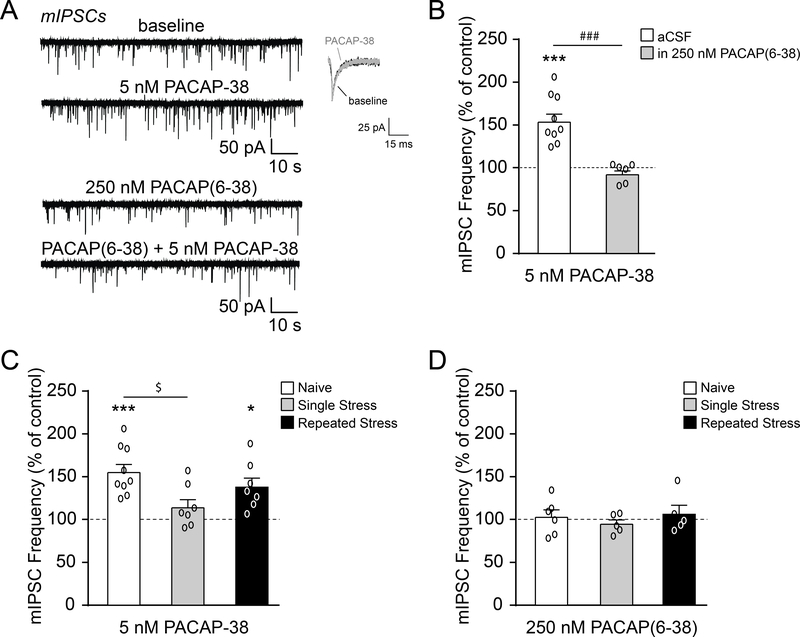
To confirm the presynaptic site of PACAP-38’s actions at GABAergic synapses, we recorded miniature IPSCs (mIPSCs) in the presence of TTX to block action potential generation/propagation. Baseline mIPSC frequencies, amplitudes and rise times were similar across all three animal groups (freq: F(2, 43)=1.84, p=0.17, amp: F(2, 43)=1.53, p=0.23 and rise time: F(2, 43)=0.97, p=0.39 by one-way ANOVA), but there was a significant effect of restraint stress on mIPSC decay times (F(2, 43)=3.23, p<0.05 by one-way ANOVA) with a post hoc Tukey’s multiple comparisons test reporting no significant differences (Table 1). Similar to the sIPSCs, PACAP-38 significantly increased the mIPSC frequency in naïve rats (to 155.8±9.5% of baseline; t(8)=5.85, p<0.001 by one-sample t-test), and this effect was prevented by PACAP(6–38) pretreatment (t(13)=5.04, p<0.001 by unpaired t-test; Fig. 5A–B), supporting our finding that PACAPergic regulation of GABA release is mediated by presynaptic PAC1. Moreover, there was a significant effect of restraint stress on PACAP-38-induced facilitation of vesicular GABA release (F(2, 20)=4.47, p<0.05 by one-way ANOVA), as after a single restraint/post-stress session PACAP-38 no longer increased the mean mIPSC frequency (p<0.05 by post hoc Tukey’s test; Fig. 5C). However, repeated restraint stress produced adaptation, such that PACAP-38’s effect on the mIPSC frequency was similar to that of naïve animals (t(6)=3.62, p<0.05 by one-sample t-test; Fig. 5C). In contrast to the sIPSCs, PACAP(6–38) had no effects on mIPSC frequencies in all three animal groups (F(2, 13)=0.48, p=0.63 by one-way ANOVA), indicating that basal PAC1 regulation of GABA release requires an intact synaptic network (Fig. 5D). There were also no PACAP-38 and PACAP(6–38) effects on mIPSC amplitudes or kinetics in all three animal groups. Overall these data indicate that PACAP-38 acts via PAC1 to potentiate CeM GABAergic synaptic activity, and that restraint stress can alter this neuropeptidergic regulation.
Table 1.
Basal GABAA-mediated mIPSC parameters in the CeA after restraint stress.
| Treatment | Frequency (Hz) | Amplitude (pA) | Rise Time (ms) | Decay Time (ms) | |
|---|---|---|---|---|---|
| mIPSCs |
Naïve (n=21 cells from 9 rats) |
0.37±0.05 | 55.2±3.5 | 2.40±0.07 | 5.10±0.24 |
|
Single Restraint Stress (n=12 cells from 9 rats) |
0.37±0.07 | 47.4±2.7 | 2.31±0.06 | 6.21±0.54 | |
|
Repeated Restraint Stress (n=13 cells from 8 rats) |
0.53±0.08 | 49.1±3.3 | 2.51±0.14 | 6.15±0.41 |
Baseline mIPSC frequencies, amplitudes, rise times and decay times were similar in naïve rats and rats subjected to single or repeated restraint stress sessions, except for a significant effect of restraint stress on mIPSC decay times (p<0.05 by one-way ANOVA). All data are presented as mean±SEM.
Figure 5.
PACAP acts on PAC1 receptors localized to presynaptic terminals of CeM GABA synapses. A. Representative mIPSC traces and scaled averages from naïve rat CeM neurons at baseline and during superfusion of PACAP-38 (5 nM), PAC1 antagonist (250 nM PACAP(6–38)), and PACAP(6–38)+PACAP-38. B. PACAP-38 significantly increased the mean mIPSC frequency in naïve rats, and pretreatment with PACAP(6–38) prevented this facilitation (6–9 cells from 4–6 rats per group). C. PACAP-38-induced facilitation of the mIPSC frequency was lost after a single restraint stress, but recovered toward naïve levels with repeated stress restraint sessions (7–9 cells from 5–6 rats per group). The data from naïve rats shown here are the same as in Fig. 5B. D. PACAP(6–38) had no effect on mIPSC frequencies in naïve rats and rats subjected to single or repeated restraint stress sessions (5–6 cells from 3–4 rats per group). All data are presented as mean±SEM. *p<0.05 and ***p<0.001 by one sample t-test; ###p<0.001 by unpaired t-test; $p<0.05 by one-way ANOVA with post hoc Tukey’s multiple comparisons test.
4. Discussion
PACAPergic signaling directs the brain’s emotional response to psychological stress (Hammack and May, 2015; Ramikie and Ressler, 2016) and, in particular, its actions in the CeA are crucial to anxiogenic and stress coping behaviors (Iemolo et al., 2016; Legradi et al., 2007; Missig et al., 2014). Therefore, we investigated the effects of restraint stress on PACAP’s regulation of neuronal activity in the medial subdivision of the CeA (CeM; the major output nucleus for the entire amygdala complex). We determined that PACAP acts via presynaptic PAC1 to potentiate basal CeM GABA release. A single restraint stress/post-stress period decreased PAC1 levels and dampened the effects of PACAP-38 and PACAP(6–38) on GABA release, strongly suggesting that PACAPergic regulation of CeM neuronal activity may be a critical mediator of acute stress responses. Notably, repeated restraint stress sessions produced adaptation, such that there was a functional recovery of the PACAPergic GABA response. Collectively our data reveal that tonic PACAP/PAC1 signaling enhances inhibitory control of the CeM and that psychological stress can modulate this influence to potentially disinhibit downstream effector regions that mediate anxiety and stress-related behaviors (e.g. BNST, dorsal vagal complex of the brainstem, lateral hypothalamus, locus coeruleus and periaqueductal gray (Gilpin et al., 2015)).
Our immunohistochemical results, although not able to distinguish between pre- vs. post-synaptic location of PAC1, confirm widespread PAC1 presence throughout the CeA, including in the CeM subdivision (see also: Joo et al., 2004; Piggins et al., 1996). This is consistent with previous studies showing PAC1 localized to pre- and postsynaptic sites in the peripheral and central nervous systems, where they regulate neurotransmitter release (Mustafa et al., 2010; Shioda et al., 1997; Stroth et al., 2013; Taupenot et al., 1999). Here we report a novel mechanism for PACAP-38’s direct actions at presynaptic PAC1 on GABAergic synapses in the CeM. Specifically, PACAP-38 (5–50 nM) increased CeM GABA release via PAC1, both in the absence (sIPSC) and presence (mIPSCs) of TTX (which prevents action potential generation/propagation), though future studies are needed to determine the specific site of PACAP/PAC1’s actions (e.g. presynaptic terminals of CeM GABAergic interneurons, presynaptic terminals of CeL GABAergic projections into the CeM, etc). The most parsimonious interpretation of our data is that PACAP-induced GABA release inhibits local interneurons to disinhibit (i.e. activate) CeA inhibitory projections to downstream effector regions. This is consistent with: 1) the work of Cho et al. showing that PACAP-38 acts via postsynaptic VPAC1 receptors to enhance BLA glutamatergic input into the CeL, leading to higher firing rates in CeL GABAergic neurons and potentially increased CeL inhibition of the CeM (Cho et al., 2012), and 2) several recent studies demonstrating that opto/chemogenetic activation of CeA projection neurons enhanced anxiety-like behavior in rodents (McCall et al., 2015; Pomrenze et al., 2018). Moreover, PACAP-38’s actions in the CeM match those of other pro-stress peptides/neurotransmitters, such as CRF, that increase CeM GABA release (Roberto et al., 2010; Varodayan et al., 2017b) and, like PACAP-38 (Iemolo et al., 2016; Missig et al., 2014), are anxiogenic when infused directly into the CeA (Rassnick et al., 1993). We also found that application of just the PAC1 antagonist PACAP(6–38) reduced action potential-dependent GABA release (sIPSC frequency), but not vesicular action potential-independent GABA release (mIPSC frequency), suggesting that endogenous PACAP release requires network activity. While several molecular and cellular physiology studies have observed PACAPergic pre- and postsynaptic regulation of excitatory transmission across the CNS (including: (Cho et al., 2012; Costa et al., 2009; Michel et al., 2006; Pecoraro et al., 2017; Resch et al., 2014; Roberto et al., 2001; Schmidt et al., 2015; Toda and Huganir, 2015)), to our knowledge this is the first study to report a direct effect of the PACAP system on inhibitory transmission.
As CeA PACAP signaling is implicated in anxiogenic and stress coping behaviors, we used restraint to model an acute psychological stress. Similar restraint stress protocols increased immediate early gene expression in the CeA (Crane et al., 2005; Kovacs et al., 2018; Stamp and Herbert, 1999; Sterrenburg et al., 2012), though other studies report only modest or no changes at the same post-stress timepoint (Cullinan et al., 1995; Day et al., 2005; Dayas et al., 2001). Of note, an elegant study by Crane et al. observed that a single 1 hr restraint stress/1 hr recovery period (same protocol as used in our study) produced a simultaneous decrease in the number of Fos+ corticotropin-releasing factor (CRF) cells and increase in the number of Fos+ non-CRF cells in the CeA (Crane et al., 2005). It is unclear how these opposing cell-type specific effects on neuronal activity impact inhibitory information flow through the CeA, but the overall amount of extracellular GABA measured in the CeA by in vivo microdialysis did not change during a 1 hr restraint stress/1 hr recovery period (Reznikov et al., 2009). Likewise, we report no changes in basal GABA synaptic activity in the CeM 1 hr after a single restraint stress session (current study and (Varodayan et al., 2018)), though 6 hr post-stress there was enhanced evoked GABAergic transmission (Ciccocioppo et al., 2014).
While we did not investigate whether restraint stress alters endogenous CeA PACAP release in this study, we did find that a single session reduced PAC1 influence over CeM GABA transmission. Specifically, 1 hr post-stress, there were significantly less PAC1+ cells in the CeM and the region’s GABA synaptic activity was no longer regulated by PAC1 blockade or activation (even with 50 nM PACAP-38, 10-fold the concentration needed to elicit an effect in CeA of naïve animals). PAC1 is a G-protein coupled receptor (GPCR) and its plasma membrane signaling can activate multiple second messenger systems in a temperature-independent manner, including the adenylyl cyclase (AC)/cyclic adenosine monophosphate (cAMP) and the phospholipase C (PLC)/inositol 1,4,5-trisphosphate (IP3) cascades (May et al., 2014). In addition, PACAP-38 can induce CeA PAC1 internalization to promote mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinases (ERK) signaling and produce nociceptive hypersensitivity (May and Parsons, 2017; Missig et al., 2017). This PAC1 endosomal signaling mechanism is suppressed at the lower ambient temperatures (20–24°C) that generally inhibit clathrin/dynamin-mediated GPCR endocytosis (Merriam et al., 2013). While both PAC1 plasma membrane and endosomal signaling have been implicated in PACAP-induced modulation of intrinsic neuronal excitability (Johnson et al., 2019; Parsons and May, 2019), little is known about the intracellular mechanisms underlying PAC1 regulation of synaptic transmission in the CNS (which has generally been studied at non-physiological temperatures similar to the present study (Cho et al., 2012; Michel et al., 2006; Pecoraro et al., 2017; Roberto et al., 2001)). Thus, we speculate that stress-induced PACAP release in the CeA could desensitize plasma membrane PAC1-mediated responses through receptor internalization in vivo, reducing CeM PAC1 immunoreactivity and blunting subsequent PACAPergic influence over GABA synapses (as we observed in vitro with exogenous PACAP-38 application after a single stress restraint session).
Interestingly, this loss of tonic PAC1 activity after a single stress session did not impact baseline GABA release, suggesting that restraint may also induce other adaptive mechanisms in the CeA. One potential source of compensation is the melanocortin 4 receptor (MC4R) system, as Iemolo et al. reported that intra-CeA infusion of a MC4R antagonist prevented PACAP-38-induced anxiety-like behavior (Iemolo et al., 2016). We have observed that the same MC4R antagonist (100 nM SHU 9119) prevents PACAP-38’s facilitation of the CeA sIPSC frequency in naïve rats (F.P. Varodayan and M. Roberto, unpublished data), though the exact cellular mechanism underlying this interaction remains unknown. Alternatively, we have previously shown that several neurotransmitters, peptides and signaling molecules regulate CeA GABA synaptic activity (e.g. CRF (Roberto et al., 2010; Varodayan et al., 2017b), but see (Varodayan et al., 2018); endocannabinoids (Varodayan et al., 2016); orexin/hypocretin (Schmeichel et al., 2017); nociceptin/orphanin FQ (Ciccocioppo et al., 2014); and NPY (Gilpin et al., 2011)), and stress-induced changes in any of these signaling systems could restore levels of basal GABA release either independently or in a compensatory manner.
Finally, we found that repeated restraint stress sessions produced adaptation, such the effects of both PACAP-38 and PACAP(6–38) on GABA release recovered to similar levels as in the CeM of naïve animals. Of note, post-hoc comparisons showed that the single restraint-induced reduction in PAC1 levels persisted in the CeA after repeated restraint stress, but there was a slight recovery in the CeM towards naïve animal levels (i.e. PAC1 expression in the repeated restraint group was not significantly different from the naïve unstressed group). In contrast, PACAP and PAC1 gene expression were higher in rats sacrificed immediately after a short-term (3 day) variable stress protocol (Ergang et al., 2015). Therefore, in our study we speculate that our 3-day protocol of repeated restraint stress either no longer evokes CeA PACAP release and/or PAC1 internalization in vivo, perhaps as a coping mechanism. This would produce a restoration of PACAP’s ability to regulate GABA signaling, as observed in our in vitro electrophysiology studies. Similar protocols of repeated restraint stress have been shown to reduce stress-evoked HPA activity in rats (1 hr restraint stress repeated for 3–5 days (Bhatnagar et al., 2002; Cole et al., 2000; Jaferi and Bhatnagar, 2006); but see (Keim and Sigg, 1976; Rabasa et al., 2015)), though future studies would be needed to confirm whether our specific stress protocol reduces stress-evoked CeA PACAP release, PAC1 internalization, and/or HPA responses.
5. Conclusions
Here we report that the neuropeptide PACAP acts via its receptor PAC1 to tonically regulate GABA release in the CeM of naïve rats, demonstrating a critical role for this peptidergic system in maintaining local inhibitory control within the major output nucleus for the entire amygdala. This influence is stress-sensitive, supporting a critical role for CeA PACAP/PAC1 signaling in mediating an acute psychological stress response. Therefore, the PACAPergic system’s dynamic regulation of CeA inhibitory synapses may represent an adaptive mechanism within a key stress circuit after psychological stress, potentially leading to changes in the activity of downstream brain regions that mediate anxiety and stress-related behaviors.
Supplementary Material
Highlights.
PACAP-38/PAC1 signaling enhances GABA transmission in the central amygdala
A single restraint stress reduced PAC1 immunoreactivity
A single restraint stress dampened PACAP-38’s effects on the GABA response
Repeated restraint stress produced a functional recovery
Acknowledgements
Support for this study was provided by the National Institute on Alcohol Abuse and Alcoholism grants R01 AA015566 (MR), R01 AA006420 (MR), R01 AA025038 (VS), R01 AA024439 (VS), R37 AA017447 (MR), U01 AA013498 (MR), K99 AA025408 (FPV), and T32 AA007456 (MQS) and by the National Institute of Mental Health grant R01 MH093650 (VS). The authors declare no conflict of interest. This is TSRI manuscript number 29710.
Abbreviations
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- CeA
central amygdala
- CeC
capsular subdivision of the CeA
- CeM
medial subdivision of the CeA
- CeL
lateral subdivision of the CeA
- CNS
central nervous system
- CRF
corticotropin-releasing factor
- GABA
γ-aminobutyric acid
- GPCR
G-protein coupled receptor
- mIPSC
miniature GABAA–mediated inhibitory postsynaptic current
- HPA
hypothalamic pituitary adrenal
- IACUC
Institutional Animal Care and Use Committee
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- PAC1
Pituitary adenylate cyclase-activating polypeptide type 1 receptor
- PTSD
posttraumatic stress disorder
- PVN
paraventricular nucleus of the hypothalamus
- sIPSC
spontaneous GABAA–mediated inhibitory postsynaptic current
- SEM
standard error
- TTX
tetrodotoxin
- TSRI
The Scripps Research Institute
- VPAC1
vasoactive intestinal polypeptide receptor 1
- VPAC2
vasoactive intestinal polypeptide receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal A, Halvorson LM, Legradi G, 2005. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res 138, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, Ressler KJ, 2013. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet B Neuropsychiatr Genet 162b, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Dias BG, Ressler KJ, 2014. A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron 83, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C, 1991. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129, 2787–2789. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P, 2002. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 14, 403–410. [DOI] [PubMed] [Google Scholar]
- Chang SC, Xie P, Anton RF, De Vivo I, Farrer LA, Kranzler HR, Oslin D, Purcell SM, Roberts AL, Smoller JW, Uddin M, Gelernter J, Koenen KC, 2012. No association between ADCYAP1R1 and posttraumatic stress disorder in two independent samples. Mol Psychiatry 17, 239–241. [DOI] [PubMed] [Google Scholar]
- Cho JH, Zushida K, Shumyatsky GP, Carlezon WA Jr., Meloni EG, Bolshakov VY, 2012. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci 32, 14165–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, Roberto M, 2014. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci 34, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL, 2000. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol 12, 1034–1042. [DOI] [PubMed] [Google Scholar]
- Costa L, Santangelo F, Li Volsi G, Ciranna L, 2009. Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus. Hippocampus 19, 99–109. [DOI] [PubMed] [Google Scholar]
- Crane JW, French KR, Buller KM, 2005. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress 8, 199–211. [DOI] [PubMed] [Google Scholar]
- Craske MG, Stein MB, 2016. Anxiety. Lancet 388, 3048–3059. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ, 1995. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505. [DOI] [PubMed] [Google Scholar]
- Day HE, Nebel S, Sasse S, Campeau S, 2005. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci 21, 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA, 2001. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM, Mitchell R, Lutz EM, 1997. Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides 31, 175–185. [DOI] [PubMed] [Google Scholar]
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V, 2013. CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology 38, 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergang P, Vodicka M, Sotak M, Klusonova P, Behuliak M, Rehakova L, Zach P, Pacha J, 2015. Differential impact of stress on hypothalamic-pituitary-adrenal axis: gene expression changes in Lewis and Fisher rats. Psychoneuroendocrinology 53, 49–59. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M, 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M, 2011. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry 69, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Chaouloff F, Hill MN, 2015. To stress or not to stress: a question of models. Curr Protoc Neurosci 70, 8.33.31–22. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V, 2009. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, May V, 2015. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol Psychiatry 78, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ, 2010. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Seiglie M, Blasio A, Cottone P, Sabino V, 2016. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the central nucleus of the amygdala induces anxiety via melanocortin receptors. Psychopharmacology (Berl) 233, 3269–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S, 2006. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 147, 4917–4930. [DOI] [PubMed] [Google Scholar]
- Johnson GC, May V, Parsons RL, Hammack SE, 2019. Parallel signaling pathways of pituitary adenylate cyclase activating polypeptide (PACAP) regulate several intrinsic ion channels. Ann N Y Acad Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Pitkanen A, 1998. Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol 395, 53–72. [DOI] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI, 2004. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol 476, 388–413. [DOI] [PubMed] [Google Scholar]
- Keim KL, Sigg EB, 1976. Physiological and biochemical concomitants of restraint stress in rats. Pharmacol Biochem Behav 4, 289–297. [DOI] [PubMed] [Google Scholar]
- Kovacs LA, Schiessl JA, Nafz AE, Csernus V, Gaszner B, 2018. Both Basal and Acute Restraint Stress-Induced c-Fos Expression Is Influenced by Age in the Extended Amygdala and Brainstem Stress Centers in Male Rats. Front Aging Neurosci 10, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM, 2007. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast 2007, 79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P, 2004. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol 92, 1285–1294. [DOI] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW, Sah P, 2013. The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 591, 2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Buttolph TR, Girard BM, Clason TA, Parsons RL, 2014. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol 306, C1068–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Parsons RL, 2017. G Protein-Coupled Receptor Endosomal Signaling and Regulation of Neuronal Excitability and Stress Responses: Signaling Options and Lessons From the PAC1 Receptor. J Cell Physiol 232, 698–706. [DOI] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR, 2015. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 87, 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL, 2013. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci 33, 4614–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS, 2006. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Mei L, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, Hammack SE, May V, 2017. Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal-Regulated Kinase Signaling Regulates the Emotional Component of Pain. Biol Psychiatry 81, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Roman CW, Vizzard MA, Braas KM, Hammack SE, May V, 2014. Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology 86, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Walsh J, Grimaldi M, Eiden LE, 2010. PAC1hop receptor activation facilitates catecholamine secretion selectively through 2-APB-sensitive Ca(2+) channels in PC12 cells. Cell Signal 22, 1420–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I, 1994. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A 91, 7698–7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Grone HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schutz G, 2001a. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci 21, 5520–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G, 2001b. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res 92, 78–84. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D, 2010. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90, 419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RL, May V, 2019. PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability. J Mol Neurosci 68, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, W. C., 2007. The Rat Brain in Stereotaxic Coordinates,. Elsevier Academic Press, San Diego. [Google Scholar]
- Pecoraro V, Sardone LM, Chisari M, Licata F, Li Volsi G, Perciavalle V, Ciranna L, Costa L, 2017. A subnanomolar concentration of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) pre-synaptically modulates glutamatergic transmission in the rat hippocampus acting through acetylcholine. Neuroscience 340, 551–562. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Stamp JA, Burns J, Rusak B, Semba K, 1996. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol 376, 278–294. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG, 1995. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol 356, 288–310. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, Morikawa H, Hopf FW, Messing RO, 2018. A CORTICOTROPIN RELEASING FACTOR NETWORK IN THE EXTENDED AMYGDALA FOR ANXIETY. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabasa C, Gagliano H, Pastor-Ciurana J, Fuentes S, Belda X, Nadal R, Armario A, 2015. Adaptation of the hypothalamus-pituitary-adrenal axis to daily repeated stress does not follow the rules of habituation: A new perspective. Neurosci Biobehav Rev 56, 35–49. [DOI] [PubMed] [Google Scholar]
- Ramikie TS, Ressler KJ, 2016. Stress-related disorders, pituitary adenylate cyclase-activating peptide (PACAP)ergic system, and sex differences. Dialogues Clin Neurosci 18, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF, 1993. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res 605, 25–32. [DOI] [PubMed] [Google Scholar]
- Resch JM, Maunze B, Phillips KA, Choi S, 2014. Inhibition of food intake by PACAP in the hypothalamic ventromedial nuclei is mediated by NMDA receptors. Physiol Behav 133, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V, 2011. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR, 2009. Effects of acute and repeated restraint stress on GABA efflux in the rat basolateral and central amygdala. Brain Res 1256, 61–68. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG., Zorrilla EP., Koob GF., Siggins GR., Parsons LH., 2010. Corticotropin Releasing Factor-Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence. Biol Psychiatry, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Scuri R, Brunelli M, 2001. Differential effects of PACAP-38 on synaptic responses in rat hippocampal CA1 region. Learn Mem 8, 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, Pitkanen A, 1995. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol 361, 345–368. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF, 2017. Hypocretin Neurotransmission Within the Central Amygdala Mediates Escalated Cocaine Self-administration and Stress-Induced Reinstatement in Rats. Biol Psychiatry 81, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SD, Myskiw JC, Furini CR, Schmidt BE, Cavalcante LE, Izquierdo I, 2015. PACAP modulates the consolidation and extinction of the contextual fear conditioning through NMDA receptors. Neurobiol Learn Mem 118, 120–124. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I, 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda S, Shuto Y, Somogyvari-Vigh A, Legradi G, Onda H, Coy DH, Nakajo S, Arimura A, 1997. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci Res 28, 345–354. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Gorcs TJ, Gottschall PE, Arimura A, 1991. Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions. Endocrinology 128, 3055–3065. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Herbert J, 1999. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience 94, 1313–1322. [DOI] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, Peeters BW, Kozicz T, 2012. Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res 90, 179–192. [DOI] [PubMed] [Google Scholar]
- Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE, 2013. PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology 154, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki I, Watanabe A, Yokai M, Watanabe Y, Hayakawa D, Nagashima R, Fukuchi M, Okada T, Toyooka N, Miyata A, Gouda H, Kurihara T, 2018. In Silico Screening Identified Novel Small-molecule Antagonists of PAC1 Receptor. J Pharmacol Exp Ther 365, 1–8. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Mahata M, Mahata SK, O’Connor DT, 1999. Time-dependent effects of the neuropeptide PACAP on catecholamine secretion : stimulation and desensitization. Hypertension 34, 1152–1162. [DOI] [PubMed] [Google Scholar]
- Toda AM, Huganir RL, 2015. Regulation of AMPA receptor phosphorylation by the neuropeptide PACAP38. Proc Natl Acad Sci U S A 112, 6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Keyes KM, McLaughlin KA, Wildman DE, Aiello AE, Koenen KC, 2013. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress Anxiety 30, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, de Guglielmo G, Logrip ML, George O, Roberto M, 2017a. Alcohol Dependence Disrupts Amygdalar L-Type Voltage-Gated Calcium Channel Mechanisms. J Neurosci 37, 4593–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Khom S, Patel RR, Steinman MQ, Hedges DM, Oleata CS, Homanics GE, Roberto M, Bajo M, 2018. Role of TLR4 in the Modulation of Central Amygdala GABA Transmission by CRF Following Restraint Stress. Alcohol Alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Logrip ML, Roberto M, 2017b. P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses. Neuropharmacology 125, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitzer P, Parsons LH, Roberto M, 2016. Chronic ethanol exposure decreases CB1 receptor function at GABAergic synapses in the rat central amygdala. Addict Biol 21, 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.