Abstract
Introduction/Aim
The brain in Alzheimer’s disease shows glucose hypometabolism but may utilize ketones for energy production. Ketone levels can potentially be boosted through oral intake of Medium Chain Triglycerides (MCTs). The aim of this meta-analysis is to investigate the effect of MCTs on peripheral ketone levels and cognitive performance in patients with mild cognitive impairment and Alzheimer’s disease.
Methods
Medline, Scopus and Web of Science were searched for literature up to March 1, 2019. Meta-analyses were performed by implementing continuous random-effects models and outcomes were reported as weighted Mean Differences (MDs) or Standardized Mean Differences (SMDs).
Results
Twelve records (422 participants) were included. Meta-analysis of RCTs showed that, compared with placebo, MCTs elevated beta-hydroxybutyrate (MD = 0.355; 95% CI, 0.286 - 0.424, I2 = 0%), showed a trend towards cognitive improvement on ADAS-Cog (MD = − 0.539; 95% CI, −1.239 - 0.161, I2 = 0%), and significantly improved cognition when combining ADAS-Cog with MMSE (SMD = − 0.289; 95% CI, −0.551- −0.027, I2 = 0%).
Conclusions
In this meta-analysis, we demonstrated that MCTs can induce mild ketosis and may improve cognition in patients with mild cognitive impairment and Alzheimer’s disease. However, risk of bias of existing studies necessitates future trials.
Keywords: Medium Chain Triglycerides, Ketosis, Cognition, Alzheimer’s disease, Systematic review, Meta-analysis
1. Introduction
Alzheimer’s disease (AD) and its prodrome, mild cognitive impairment (MCI), are characterized by brain glucose hypometabolism in brain regions affected by disease pathology (Kapogiannis and Mattson, 2011; Mullins et al., 2018; Mullins et al., 2017; Willette et al., 2015). In contrast, the metabolism of the ketone bodies β-hydroxybutyrate (BHB) and acetoacetate, which are physiological alternative fuels that can be readily utilized by brain cells (Drenick et al., 1972; Owen et al., 1967), does not decline with normal aging (Castellano et al., 2019) and even remains normal in the MCI/AD brain (Castellano et al., 2015; Croteau et al., 2018a). Importantly, brain ketone levels are positively correlated with blood ketone levels (Courchesne-Loyer et al., 2017; Croteau et al., 2018b) providing an easily obtained outcome for interventions aiming to boost brain ketone utilization. Therefore, interventions aimed at increasing blood ketone levels should also increase brain ketone levels and thus increase energy bioavailability, potentially compensating for the glucose under-utilization that occurs in the MCI/AD brain (Croteau et al., 2018b; Cunnane et al., 2011; Cunnane et al., 2016).
The main approaches taken to date to increase ketones in blood and consequently brain, are through fasting (Mattson et al., 2018), exercising (van Praag et al., 2014) and nutritional modification (Cunnane et al., 2016). Nutritional ketosis can be achieved by (i) ketogenic (low carbohydrate-high fat or low calorie) diets (CJ et al., 2018; Meckling et al., 2004), (ii) exogenous administration of ketone esters or salts (Hashim and Vanltallie, 2014; Stubbs et al., 2018) and (iii) supplementation with medium chain triglycerides (MCTs) (CJ et al., 2018; Cunnane et al., 2016). MCTs are fatty acids with the unique property of bypassing the peripheral circulation and entering the liver through the portal vein, where they induce rapid ketone production (Bach and Babayan, 1982). Human breast milk is a natural source of MCTs (Andreas et al., 2015), but, in adult life, the major food-derived sources of MCTs are palm kernel oil and coconut oil (Fernando et al., 2015; Takeuchi et al., 2008). MCT supplements are concentrated palm kernel and coconut oils and are generally considered safe (Bach and Babayan, 1982; Traul et al., 2000). The present systematic review and meta-analysis aims to investigate the effects of MCT oil or coconut oil (which contains a high concentration of MCTs) on ketosis induction and cognitive function in patients with MCI or AD, by examining all the available clinical evidence in the literature.
2. Methods
For our systematic review and meta-analysis, we adopted the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al., 2009).
2.1. Information Sources and Literature Search
Medline, Scopus and Web of Science were searched for literature up to March 1, 2019. The terms “Medium Chain Triglycerides”, “MCTs”, “coconut”, “ketones”, “ketosis”, “beta-hydroxybutyrate”, “Alzheimer’s”, “Mild Cognitive Impairment” and “dementia” were combined for the identification of relevant studies.
2.2. Study Selection
Two reviewers (Drs. Avgerinos and Kapogiannis) independently searched the Electronic Databases for identification of eligible studies published up until 1, March 2019. Disagreements were solved with the help of a third reviewer (Dr. Egan) until consensus was reached. Retrieved records were imported into a reference manager software (Endnote X8, Thomson Reuters). Any irrelevant titles were excluded and after deduplication, the remaining titles and abstracts were screened based on eligibility criteria. After that, we assessed the remaining studies for eligibility by examining their full texts. After exclusion of several studies for specific reasons, we retained the desired studies for our qualitative and quantitative synthesis. An additional literature search was performed just prior the article’s submission (4/25/2019) to identify any recently published articles that may not have been identified in the initial search.
2.3. Eligibility criteria
A study was deemed eligible, if it fulfilled the following inclusion criteria: (1) reported findings in humans (2) was published in any language up to March 1, 2019 (enriched by an additional search on 4/25/2019), (3) included patients with MCI and/or AD, (4) participants were given MCT oil or coconut oil at any dose and for any duration, and they were compared or not to participants taking placebo/nothing, and (5) was designed as randomized control trial (RCT), open label trial, single-arm trial, prospective cohort, case-control, cross-sectional, case series or case report study. A study was excluded if it: (i) reported findings in non-humans, (ii) included patients with diagnoses other than MCI and/or AD, (iii) did not involve supplementation with MCTs/coconut oil in sufficient quantities to induce ketosis [for example studies in which ketosis was produced by modifying the primary nutrient content of the diet (“ketogenic diet”, i.e. restricting carbohydrates/increasing fats) rather than by providing MCTs/coconut oil in sufficient quantities to induce or contribute to ketosis]; also, studies involving exclusively fasting or calorie restriction or exercising or administration of ketone esters/salts to induce ketosis), (iv) was a Review or a Conference Abstract, and, (v) did not report cognitive outcomes.
2.4. Data Collection Process and Data Items
Two reviewers (Drs. Avgerinos and Kapogiannis) collected data independently. Any disagreement was solved with the contribution of a third reviewer (Dr. Egan) until consensus was reached. Extracted data from the eligible studies included the following fields: Title, ID, name of first author and year of study, study design, study duration, number of patients, daily dose and duration of intervention or placebo, levels of beta-hydroxybutyrate (BHB) before and after the treatment/placebo, scores on cognitive scales [Mini Mental State Examination (MMSE), ADAS-Cog (Alzheimer’s Disease Assessment Scale-Cognitive Subscale) or other] before and after intervention.
2.5. Quality and Risk of Bias
To our knowledge there is no official tool for the assessment of Risk of Bias (ROB) in single-arm trials (ROBINS-I tool is designed for ROB assessment of non-randomized studies that include a control group) (Sterne et al., 2016). To provide a methodological assessment of included studies without a control group (3 single arm trials, 1 case series and 2 case reports, subsequently referred to as “non-RCT studies” (Farah, 2014; Maynard and Gelblum, 2013; Newport et al., 2015; Ohnuma et al., 2016; Ota et al., 2019; Taylor et al., 2018)), we used the Newcastle Ottawa Scale (NOS), a quality assessment tool for non-randomized trials (GA Wells, 2019), that was adapted to assess the included non-RCT studies. Notably, the Cochrane Handbook makes a distinction between the assessment of Quality and ROB and favors the latter (see chapter 8.2.2). (Higgins JPT, 2011). It is suggested that a study can be designed according to high standards (good quality) and still be of high ROB in drawing conclusions (Higgins JPT, 2011). To provide a means for assessing results, we wished to provide at least a quality assessment of non-RCT studies to our readers but recommend caution for possible ROB. On the other hand, regarding ROB of RCTs, we used the classical “Cochrane Collaboration’s tool for assessing risk of bias in randomized trials”(Higgins et al., 2011). Quality of non-RCTs and ROB of RCTs was assessed by two independent reviewers (Drs. Avgerinos and Kapogiannis). Any disagreement was solved with the contribution of another reviewer (Dr. Egan) until consensus was reached.
The NOS tool originally examines the domains of selection, comparability and outcome for quality and gives ideally four, two and three points respectively for each domain (with maximum score 9/9) (GA Wells, 2019). For our non-RCT studies which consisted of treatment only group, we didn’t take into consideration the “comparability” domain (accounting for 2 points). Also, we didn’t take into consideration one of the four parts of the “selection” domain (accounting for 1 point), which also refers to comparison of two groups. Consequently, the ideal scoring of our modified tool would be 6 points (3 for “selection”, and 3 for “outcome” domains). A study of good quality would score 2-3 points on each domain. A study of fair quality would score 1 point on one domain but 2 points on the other domain. Finally, a study of poor quality would be a study with 0 point in any of the two domains or 1 point on each of the two domains.
Regarding the ROB tool for RCTs (Higgins et al., 2011), every outcome was evaluated within each study. Possible domains of bias were selection bias, detection bias, attrition bias and reporting bias. If at least one domain of bias was deemed as “high risk”, then the whole study was characterized as of high ROB. If at least one domain was of “unclear risk” while the rest domains were “low risk”, then the whole study was deemed as unclear ROB. A study was characterized as of low ROB, if all domains were “low risk”.
2.6. Synthesis and Statistical Analysis
For the case series and the case report studies, we provide a qualitative report of the results only. Regarding single arm trials, in addition to qualitative assessment, we performed a weighted pooling of the outcomes of interest in the single group (treatment group). Regarding RCTs, in addition to qualitative assessment, we performed various meta-analyses. The tactic of including any human study relevant to the topic regardless of design, was adopted because we wanted to critically examine the entire existing clinical evidence; thus, in addition to “classical” metanalyses of RCTs, we also performed a synthesis of single arm trials, as a complementary piece of information, remaining mindful of and cautioning readers to the fact that the synthesis of single arm trials is lower in the level of evidence than that of RCTs.
We performed statistical synthesis for a specific outcome, only if we had relevant data from at least three studies. Our data was continuous, thus we calculated weighted Mean Differences (MDs) or Standardized Mean Differences (SMDs), and implemented the continuous random-effects model (DerSimonian and Laird, 1986). We performed syntheses using results reported for the latest possible timepoint (last outcome assessment) in each study.
Specifically, to assess ketosis induction by MCTs, we calculated and synthesized MDs of peripheral BHB levels. Similarly, for global cognitive assessment, we calculated and synthesized MDs of ADAS-Cog scores. For ADAS-Cog, lower scores are indicative of better cognition; therefore, a negative change in MD would indicate improvement. Furthermore, for additional syntheses of cognitive outcomes that included studies assessing global cognition on a scale different than ADAS-Cog (such as Delayed Recall or MMSE), we converted all cognitive changes to SMDs and combined them for syntheses.
We adopted the < 0.05 two-tailed statistical significance level for all analyses. To assess statistical heterogeneity, we used the resulting I2 as measurement of the heterogeneity degree (Higgins and Thompson, 2002). Heterogeneity below 75% was considered acceptable (Higgins and Thompson, 2002). For calculations of MDs/SMDs, statistical syntheses and forest plots’ generation we used the OpenMeta[Analyst] statistical software (Wallace et al., 2012).
3. Results
3.1. Literature search results
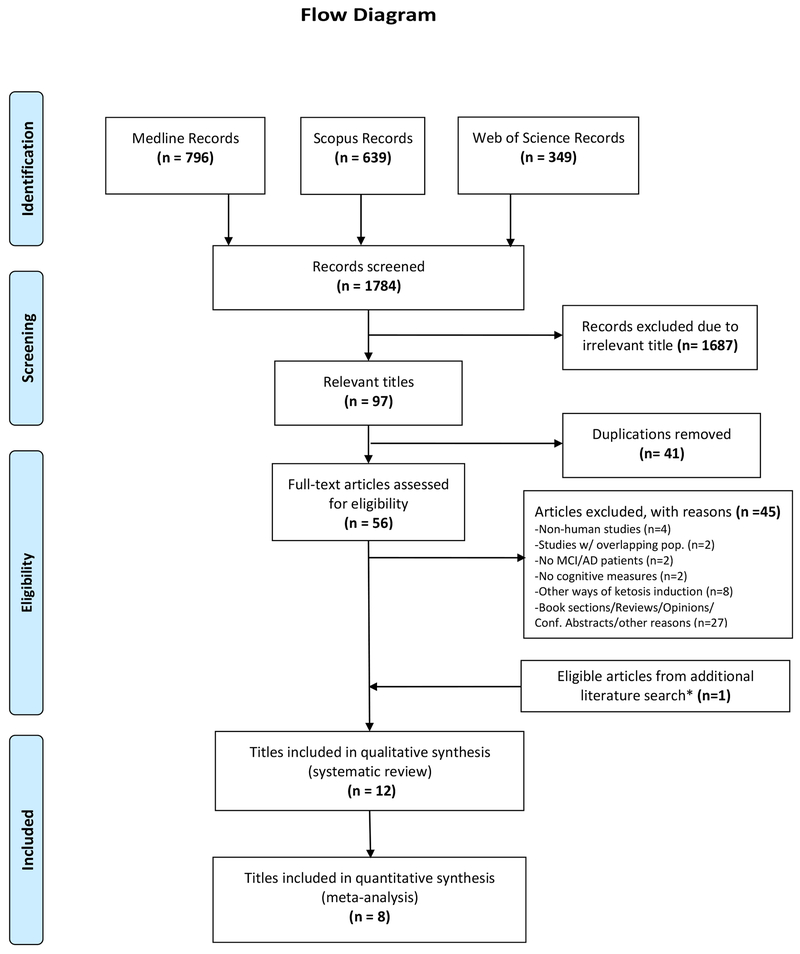
In the initial literature search (up to 1, March 2019), 1784 titles were identified, after searching in the three databases outlined. Of those, 56 titles were retained for full text assessment and, consequently, 11 articles were included in the systematic review and seven articles in the meta-analysis. In an additional literature search (performed just before the article’s submission), we identified one additional eligible article. Collectively, 12 articles were eligible for inclusion into our qualitative synthesis (systematic review). Of those, eight articles were included in the quantitative syntheses (meta-analyses). The full process of study selection and its results are outlined in the flow diagram (figure 1).
Figure 1.
Flow diagram showing the selection process of eligible studies. One of the included titles (Ota et al) consisted of two studies of different design (one RCT and one single-arm trial). The two studies were handled separately in the meta-analysis (Ota part A and Ota part B). Consequently, we included 13 studies (12 titles) for systematic review and 9 studies (8 titles) for meta-analysis. *The additional literature search was performed on 4/25/19 to incorporate any newly published title after the initial literature search (performed on 3/1/19)
3.2. Characteristics of included studies
Twelve publication titles (involving 13 studies, which collectively enrolled a total of 422 patients) were deemed eligible for inclusion (Chan et al., 2017; Farah, 2014; Henderson et al., 2009; Maynard and Gelblum, 2013; Mélanie Fortier, 2019; Newport et al., 2015; Ohnuma et al., 2016; Ota et al., 2019; Rebello et al., 2015; Reger et al., 2004; Taylor et al., 2018; Yang et al., 2015). Of those 13 studies, seven were designed as RCTs, three as single arm trials and three were case series/case reports. The article by Ota el al reported two separate studies, one RCT and one single-arm trial. Thus, this article contributed both an RCT and a single-arm trial. Table 1 depicts the characteristics of included titles in detail.
Table 1.
Characteristics of Included studies
| Study | Country | Study Design | Number and type of patients | Diagnostic criteria for inclusion | Study groups | Intervention dose | Duration of intervention | Plasma beta-hydroxybutyrate measurement method | Cognitive measures |
|---|---|---|---|---|---|---|---|---|---|
| Fortier 2019 | Canada | RCT | 52 with MCI | Subjective memory complaint, MoCA, MMSE | MCTs (C8:C10 ≈ 3:2) vs Placebo | 30gr/d | 6 months | colorimetric assay using an automated clinical chemistry analyzer (Dimension XPand Plus, Dade Behring Inc., Newark, DE) | MMSE, MoCA, 16 item free and cued word learning and recall, Trail Making Test, Stroop Test, Verbal Fluency, Digit Symbol Substitut ion, Boston Naming Test |
| Ota part A (2019) | Japan | RCT | 20 with mild/moderate AD | NINCDS-ADRDA | MCTs (C8:C10 ≈ 3:1) vs Placebo | 20gr/d | 2 days | enzymatic method at SRL Corp. (Tokyo) | WAIS III, WMS-R, Stroop test, Trail Making Test |
| Chan (2017) | Malaysia | RCT | 41 with mild/moderate/ severe AD | MMSE | Coconut oil (C8:C10 NR) vs Placebo | 60 ml/d* | 6 months | NA | MMSE, Clock drawing test |
| Rebello (2015) | USA | RCT | 6 with MCI | National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. | MCTS (C 8:C10 ≈ 5:4) vs Placebo | 56gr/day | 6 months | NR | ADAS-Cog, Trail Making Test Digit Symbol Test |
| Yang (2015) | Spain | RCT | 44 with AD | Institutionalized AD patients (unclear diagnostic criteria) | Coconut oil (C8:C10 ≈ 5:1) vs placebo | 40 ml/d | 3 weeks | NA | MMSE (Spanish version) |
| Henderson (2009) | USA | RCT | 152 with mild/moderate AD | NINCDS-ADRDA and DSM-IV criterial | MCTs (C8) vs Placebo | 20 gr/d | 3 months | method Allied Research International (formerly SFBC) of Miami, FL using the BHB Liquicolor diagnostic kit supplied by Stanbio Laboratories (Boenre, TX)] | MMSE, ADAS-Cog |
| Reger (2006) | USA | RCT | 20 with probable AD or amnestic MCI | NINCDS-ADRDA criteria | MCTs vs (C8) Placebo | 40 ml | 2 days | enzymatically, using procedure 310-UV (Sigma Diagnostics, Inc.) | MMSE, ADAS-cog, Stroop Test, Paragraph recall |
| Ota part B* (2019) | Japan | 1 arm trial | 19 with mild/moderate AD | NINCDS-ADRDA criteria | MCTs (C8:C10 ≈ 3:1) | 20gr/d | 3 months | enzymatic method at SRL Corp. (Tokyo) | WAIS III, WMS-R, Stroop test, Trail Making Test |
| Taylor (2017) | USA | 1 arm trial | 10 with very mild/mild/moderate AD | National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. | MCTs + low carb/high fat diet (C8:C10 ≈ 5:3) | 22.5 - 45 ml/d | 3 months | NR | MMSE ADAS-Cog |
| Ohnuma (2016) | Japan | 1 arm trial | 20 with moderate/ severe AD | NINCDS-ADRDA | MCTs (C8 = 50%; C10 % NR) | 20gr/d | 3 months | ELISA using buffer solution and reaction reagent for total ketone bodies (Kainos Laboratories Inc, Tokyo, Japan). The procedure was performed according to the manufacturer’s protocol using the BioMajesty™ system (JCA-BM8000; JEOL, Tokyo, Japan) at SRL Inc (Tokyo, Japan) | MMSE, ADAS-Cog |
| Maynart (2013) | USA | Case series | 55 with probable mild/moderate AD | MMSE | MCTs (C8) | 20 gr/d | 18.8 ± 9.2 months | NA | MMSE |
| Newport (2015) | USA | Case report | Young-onset sporadic AD | Clinical diagnosis, MMSE scores, MRI, Apoe4 carriage | MCTs + coconut oil (4:3 ratio); C8:C9 NR | 165 ml/d | 2.5 months | Precision Xtra Glucose and Ketone Monitoring System* (Abbott) | MMSE, ADAS-Cog |
| Farah (2014) | USA | Case report | Probable AD | MMSE, MoCA, FDG PET | MCTs (C8) | 20 gr/dl | ~ 3 months | NA | MMSE, MoCA |
MCI, Mild Cognitive Impairment; AD, Alzheimer’s Disease; MCTs, Medium Chain Triglycerides; WAIS, Wechsler Adult Intelligence Scale; WMS-R, Wechsler Memory Scale-Revised; MMSE, Mini Mental State Examination; ADAS-Cog, Alzheimer’s Dis. Assessment Scale-cognitive subscale; MoCA, Montreal Cognitive Assessment Scale. NINCDS-ADRDA, National Institute of Neurological and Communicative Disease and Stroke and the Alzheimer’s Disease and Related Disorder Association; NA, Not Applicable; NR, Not Reported; FDG PET, fluorodeoxyglucose (18F) positron emission tomography; C8, caprylic acid; C10, capric acid
3.3. Quality and Risk of Bias
All non-RCTs, were of good quality (4/6 - 6/6); for most studies there was a loss of a point due to inadequate description of the outcome assessment procedures that had been followed (Table 2). Despite being of good quality, there was a high risk of bias in drawing conclusions from the synthesis of those studies, since they did not include comparison groups, a fact that categorizes them as low in the pyramid of evidence level. Regarding RCTs, three studies were deemed of high ROB because they had at least one domain of high ROB. Three studies were of unknown risk due to multiple domains with unclear ROB, while one study was of low ROB (Table 3).
Table 2.
Quality of non-randomized studies
| SELECTION | COMPARABILITY | OUTCOME | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | Representativeness cohort | Exposure ascertainment | Demonstration that outcome wasn’t present at start of study | Comparability of cohorts | Outcome assessment | Adequate follow up for outcomes to occur | Adequate cohort follow up | Overall score (judgment) |
| Ota part B 2019 | 1 | 1 | 1 | N/A | 0 | 1 | 1 | 5/6 (Good) |
| Ohnuma 2015 | 1 | 1 | 1 | N/A | 0 | 1 | 1 | 5/6 (Good) |
| Taylor 2017 | 1 | 1 | 1 | N/A | 0 | 1 | 1 | 5/6 (Good) |
| Maynart 2013 | 1 | 1 | 1 | N/A | 1 | 1 | 1 | 6/6 (Good) |
| Newport 2015 | 0 | 1 | 1 | N/A | 0 | 1 | 1 | 4/6 (Good) |
| Farah 2014 | 1 | 1 | 1 | N/A | 0 | 1 | 1 | 5/6 (Good) |
N/A, Not applicable; Quality was assessed with Newcastle-Ottawa Quality Assessment Scale
Table 3.
Risk of Bias Assessment of RCTs
| Study | Random sequence generation (Selection Bias) | Allocation concealment (Selection Bias) | Blinding of participants-personnel Bias | Blinding of outcome assessment Bias | Incomplete outcome data (Attrition Bias) | Selective reporting Bias | Other bias | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Fortier 2019 | Low | ? | ? | ? | High | Low | Low | High |
| Ota part A 2019 | ? | ? | ? | ? | ? | Low | Low | ? |
| Chan 2017 | Low | Low | High | ? | High | Low | Low | High |
| Yang 2015 | ? | ? | ? | ? | ? | Low | Low | ? |
| Rebello 2015 | Low | Low | Low | Low | Low | Low | Low | Low |
| Henderson 2009 | High | High | Low | ? | High | Low | ? | High |
| Reger 2006 | ? | ? | ? | ? | ? | Low | Low | ? |
Low, Low Risk of Bias; ?, Unknown Risk of Bias; High, High Risk of Bias; Risk of Bias was assessed with the Cochrane Tool for Risk of Bias
3.4. Ketosis induction and cognitive outcomes
3.4.1. Non-RCTs
Of the non-RCTs, the case series study (Maynard and Gelblum, 2013) and the two case reports (Farah, 2014; Newport et al., 2015) did not assess blood ketone levels after the administration of MCTs. Regarding the case series study, there was an improvement in mean MMSE scores in a 18.8 month-period, but it did not reach statistical significance (Maynard and Gelblum, 2013). However, mean scores were derived only from 23 of all 55 patients due to absence of MMSE score records for 32 participants (Maynard and Gelblum, 2013). Results from caregivers surveys’ showed that for most participants, the ability to recall numbers, names and finding objects remained stable during the MCTs administration period (Maynard and Gelblum, 2013). In the two case reports, there were substantial improvements in neuropsychological testing scores (ADAS-Cog, MMSE, Montreal Cognitive Assessment) (Farah, 2014; Newport et al., 2015). For example, Newport et al reported an 8 points increase in MMSE after a 2.5 months period (Newport et al., 2015), while Farah et al reported a 5 points increase in MMSE after a 3 month period of treatment with MCTs (Farah, 2014).
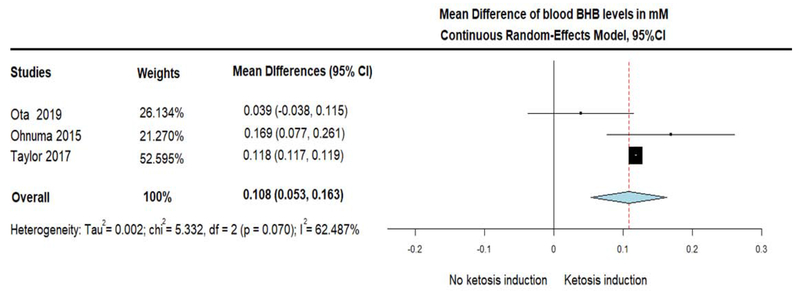
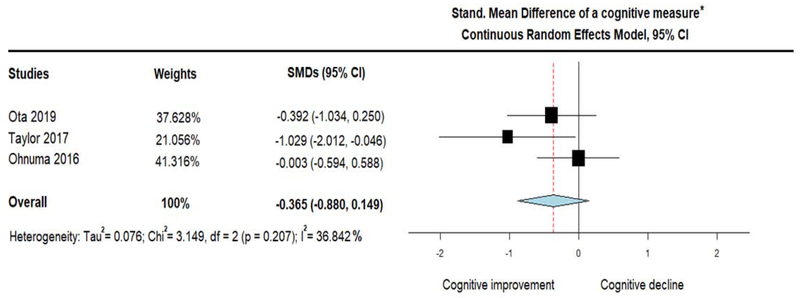
The three single arm trials assessed the change of plasma BHB levels in response to MCTs administration. Of those, two studies reported fasting BHB levels at baseline and at various timepoints during treatment (Ota et al., 2019; Taylor et al., 2018), but one study did no clarify if the measurements were fasting or not (Ohnuma et al., 2016). After pooling the mean differences of plasma BHB, we found a significant increase of BHB (MD = 0.108; 95% CI, 0.053 to 0.163, I2 = 62.49%) in response to MCTs, suggesting that ketosis was induced by the treatment (figure 2). However, a combined cognitive measure (ADAS-Cog and Delayed Logical Memory), showed a trend only for improvement in cognitive performance (note that negative values denote improved performance) (SMD = −0.365; 95% CI, −0.880 to 0.149, I2 = 36.842%) (figure 3).
Figure 2.
Forest plot showing ketosis induction (depicted as positive Mean Difference of beta-hydroxybutyrate (BHB) levels in plasma), in response to the administration of Medium Chain Triglycerides (MCTs). Results from pooling of 3 single arm trials (treatment group only) using Continuous Random-Effects Model.
Figure 3.
Forest plot showing performance on a combined cognitive measure (measured as Standardized Mean Difference on (i) Delayed Logical Memory in Ota et al study and (ii) ADAS-Cog in the rest studies), after the administration of Medium Chain Triglycerides (MCTs). Results from pooling cognitive outcomes of 3 single arm trials (treatment group only) by using Continuous Random-Effects Model. Note that negative results indicate cognitive improvement (similarly to ADAS-Cog scores).
3.4.2. RCTs
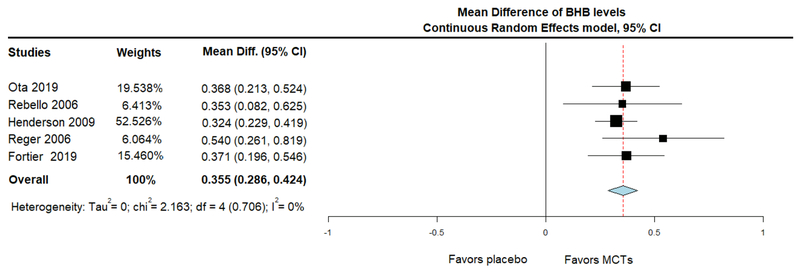
BHB levels of individual RCTs were reported for fasting (before MCTs’ administration) and following MCTs’ administration on the same day, for the baseline and subsequent visits. In two studies, there was no measurement of BHB levels (Chan et al., 2017; Hu Yang et al., 2015). The rest of the RCTs were combined in a meta-analysis of acute change in BHB levels (levels after minus levels before treatment at the same day). This synthesis showed that, compared with placebo, MCTs increased plasma BHB levels acutely (MD = 0.355; 95% CI, 0.286 to 0.424, I2 = 0%), suggesting the induction of ketosis (figure 4).
Figure 4.
Forest plot showing ketosis induction (measured as Mean Difference of beta-hydroxybutyrate (BHB) levels in plasma), after administration of Medium Chain Triglycerides (MCTs) or placebo. Results from meta-analysis of 5 Randomized Controlled Trials, using Continuous Random Effects Model.
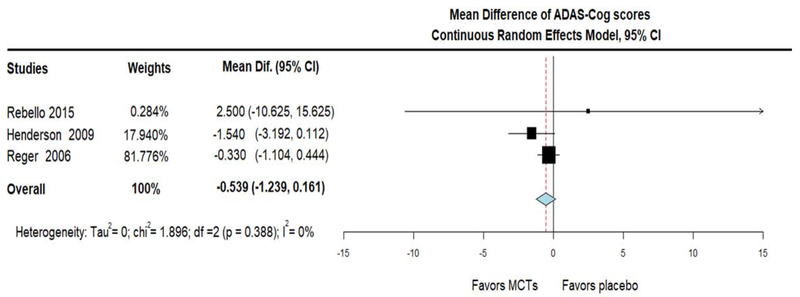
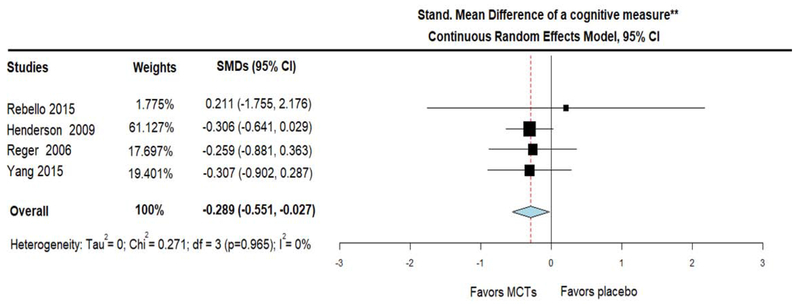
Regarding cognitive function, when compared with placebo, MCTs showed a trend towards decreased ADAS-Cog scores (indicating improvement) (MD = − 0.539; 95% CI, −1.239 to 0.161, I2 = 0%) (figure 5A). To pursue this trend further, we conducted an additional meta-analysis, which included an additional study that assessed cognitive performance with the Spanish version of MMSE (Yang et al., 2015). The cognitive performance on this combined measure was expressed as SMD and negative results indicated improvement in cognitive performance. Compared with placebo, MCTs improved cognitive performance on this combined scale (SMD = − 0.289; 95% CI, −0.551 to −0.027, I2 = 0%) (figure 5B). It is important to mention that two studies reported no difference in general cognitive function (measured with MMSE) between the two groups, but the raw MMSE scores were not available and thus those studies were not included in any of the statistical synthesis of cognitive function (Chan et al., 2017; Mélanie Fortier, 2019). Of note, all performed meta-analyses of RCTs, were highly homogeneous and this was reflected with a statistical measurement of heterogeneity (I2) equal to 0.
Figure 5A.
Forest plot showing cognitive performance change (measured as Mean Difference on ADAS-Cog scale) after administration of Medium Chain Triglycerides (MCTs) or placebo. Results from meta-analysis of 3 Randomized Controlled Trials that had originally used the same assessment scale (ADAS-Cog). We used Continuous Random Effects Model. Note that on ADAS-Cog scale, negative changes indicate cognitive improvement.
Figure 5B.
Forest plot showing cognitive performance (measured with Standardized Mean Difference derived from changes on (i) the Spanish version of MMSE in Yang et al study and (ii) ADAS-Cog for the rest studies). Results from meta-analysis of 4 Randomized Controlled Trials, using Continuous Random-Effects Model. Note that as with ADAS-Cog scale, negative changes here also indicate cognitive improvement
4. Discussion
In the present systematic review and meta-analysis, we investigated the effects of MCTs on peripheral BHB levels and cognitive performance in patients with MCI and AD, by examining all available evidence from human studies. Results from a highly homogeneous meta-analysis of RCTs, indicated that MCTs can induce elevation of plasma BHB acutely and thus resulted in a state of ketosis in MCI/AD. In addition, a homogeneous meta-analysis of RCTs based on a combined cognitive measure indicated that MCTs may be able to improve cognitive performance in MCI/AD. On the other hand, pooling of single-arm trials demonstrated an elevation of BHB but failed to show any cognitive improvement. However, single arm trials offer lower level of evidence compared to RCTs; therefore, we base our conclusions and discussion on the latter.
Ketones are recognized as being neuroprotective (Maalouf et al., 2009) and their positive effects may extend to various neurological conditions characterized by glutamate receptor-mediated excitotoxicity including AD. In animal studies, dietary ketone supplementation has been shown to increase brain BHB (Pawlosky et al., 2017), increase mitochondrial biogenesis (Srivastava et al., 2012), and improve cognitive-behavioral outcomes and decrease beta-amyloid and tau pathologies in a mouse model of Alzheimer’s disease (Kashiwaya et al., 2013). Given the profound abnormalities in glucose metabolism characterizing the AD brain and its preserved ability to utilize ketones, interventions aiming to shift brain metabolism to a state of ketosis have gained attention.
Strict ketogenic diets are effective in inducing ketosis (Hashim and Vanltallie, 2014; Meckling et al., 2004), are widely implemented efficacious treatments for epilepsy (D’Andrea Meira et al., 2019; Lutas and Yellen, 2013; Neal et al., 2008; Sariego-Jamardo et al., 2015) and may result in improved cognitive outcomes (Nordli et al., 2001; Pulsifer et al., 2001) in patients with intractable epilepsy, although it is unclear if this effect is due to seizure frequency reduction or due to ketosis per se. On these grounds, ketogenic diets have also been tried in AD. In a clinical study enrolling MCI patients, dietary ketosis was associated with improved verbal memory performance (Krikorian et al., 2012). However, ketogenic diets can be difficult to follow, and there is a concern of causing malnutrition especially for AD patients (Wlodarek, 2019). Other known ways to induce ketosis, such as fasting and exercise, are practical for MCI patients, but may be problematic for AD patients (Suttanon et al., 2013). A novel concept to induce ketosis with administration of a sodium glucose transporter 2 inhibitor (ClinicalTrials.gov Identifier: ) is currently being studied by us in healthy elderly individuals, but its effectiveness in AD is as yet unknown.
The limitations inherent in the means for endogenous ketone induction has prompted researchers to consider administration of exogenous ketones as a way to exert positive effects in AD. The oral administration of a ketone ester is particularly promising (Kashiwaya et al., 2013; Newport et al., 2015), but its long-term safety and efficacy has not been studied systematically in AD. By far, the most studied exogenous administration approach is supplementation with oral MCTs (CJ et al., 2018; Cunnane et al., 2016; van Praag et al., 2014). MCTs’ administration is considered safe in general populations (Traul et al., 2000). This was confirmed by the few included (in our meta-analysis) studies that reported side effects (Henderson et al., 2009; Maynard and Gelblum, 2013; Ohnuma et al., 2016; Taylor et al., 2018). Most of the reported treatment-related side effects were of gastrointestinal (GI) nature, such as diarrhea, flatulence and abdominal pain; those occurred in a relatively small proportion of participants, in frequencies that varied from study to study, from 13.5% to 50% (Henderson et al., 2009; Maynard and Gelblum, 2013; Ohnuma et al., 2016; Taylor et al., 2018). Factors such as splitting of the total dose into multiple doses and administration of MCTs with food might help in controlling the GI side effects. The degree of ketosis induced by MCTs is not as large as with exogenous ketone ester intake (CJ et al., 2018; Stubbs et al., 2018), but it is comparable to that of ketogenic diets (CJ et al., 2018; Huttenlocher, 1976) and is greater than ketosis occurring after a 12-hour fasting period (Boden et al., 2005; CJ et al., 2018).
Our results (both from the pooling of single arm trials and the meta-analyses of RCTs), confirmed that MCTs can induce acute BHB elevation and thus result in a state of peripheral ketosis (figures 2 and 4). In the synthesis of single-arm trials, one included study (Taylor et al., 2018) implemented low carbohydrate/high fat (ketogenic) diet in addition to the administration of MCTs; the study was included because MCTs administration covered 24-32% of energy intake and likely contributed substantially to ketosis. Nevertheless, the combination of studies implementing different interventions is a limitation for this synthesis. Despite using a slightly different intervention, the level of ketosis achieved in this study was very mild (BHB ~ 0.1 mM) and similar to that of the rest of the included studies (see figure 2)). Interestingly, RCTs’ synthesis (which provide a higher level of evidence) showed a greater level of ketosis induction in comparison to single arm pooling. However, the meta-analysis on BHB, did not include the studies by Chan et al. and Yang et al., because there was no report on this outcome. Specifically, RCTs’ meta-analysis showed an absolute 0.36 mM greater increase in BHB blood levels following MCT consumption, compared with placebo. It has been reported that after fasting for 12-24 hours (corresponding to plasma ketone body concentration of 0.3-0.5 mM), the brain derives 3-5% of its total energy from ketones (Hashim and Vanltallie, 2014). Thus, for the 0.36 mM of BHB elevation that was attributed to the MCTs in our meta-analysis, the brain may receive at least 3-5 % additional energy from ketones (Courchesne-Loyer et al., 2017; Cunnane et al., 2016). This proportion may be even higher in the AD brain given its state of chronic starvation from glucose underutilization (Courchesne-Loyer et al., 2017; Cunnane et al., 2016; Mamelak, 2012) and this additional energy may counterbalance the energy deficit seen in AD due to glucose hypometabolism. Considering that most of the studies included in our meta-analysis implemented a daily MCT dose of 20 grams, there may be physiologic room for administration of higher MCT doses, and thus higher levels of peripheral ketosis induction and brain ketone utilization. For example, several human studies have reported MCT administration of 50 grams or more resulting in higher peripheral BHB levels (Freemantle et al., 2009; Seaton et al., 1986). Theoretically, a daily dose of 40-50 grams MCTs could be divided in two or three doses starting in the morning with the highest dose (for example 20-25 grams of MCTs), so that the resulting BHB increase from MCTs would be additive to the already existing mild ketosis from the overnight fasting. MCTs increase satiety (Bach et al., 1996; Kinsella et al., 2017) and thus individuals could potentially fast for several hours again after their breakfast. Additional doses (for example 10-15 grams each) of MCTs could be given later in the day adding to the already existing mild ketosis from the short-term fasting.
Regarding cognition, our meta-analysis of RCTs based on the combined cognitive score (combined SMDs from ADAS-Cog and MMSE changes) showed that cognitive performance of MCI/AD patients was significantly increased after MCTs administration, compared with placebo. It is important to notice that three RCTs did not provide data on general cognitive measures, so they couldn’t be combined in this meta-analysis. Of those three, Chan et al. and Fortier et al. described no changes in MMSE, despite not providing raw scores or score changes. The negative results of these two studies might be discouraging, but we cannot drive any safe conclusions on what could have been their effects on the combined result. Also, Ota et al. study did not assess general cognition. On the other hand, even though results from pooling single arm trials showed no significant improvement, single arm trials do not include a control group, thus the result from pooling them is of lower quality compared to the meta-analysis of RCTs. Therefore, we consider the cognitive results of the meta-analysis of RCTs as the safest basis to drive conclusions. However, one study (Taylor et al., 2018), which involved a ketogenic diet supplemented with administration of MCTs and which was included in the single-arm trials’ pooling, showed greater cognitive benefit than the rest of the included studies, which exclusively relied on MCTs supplementation (figure 3). Since this study resulted in a similar to other included studies low level of ketosis (figure 2), it could be hypothesized that the combination of MCTs with a ketogenic diet by means of restricting carbohydrates/increasing fats could have some additional cognitive benefits, compared to exclusive MCTs’ administration. However, as described previously, the use of different interventions when performing quantitative syntheses is a limitation that adds to the uncertainty of drawing conclusions from single-arm trials syntheses.
The mechanisms of action responsible for cognitive improvement are likely to be protean. Evidence from animal studies suggests that in addition to providing an extra source of energy to the brain, ketones are associated with a variety of possible beneficial effects to the neurons, including protection from excitotoxicity (Kashiwaya et al., 2000; Maalouf et al., 2009), improved mitochondrial function (Kashiwaya et al., 2000; Maalouf et al., 2009), increased autophagy (Finn and Dice, 2005), stimulation of brain-derived neurotrophic factor production (Marosi et al., 2016) and activation of pathways associated with decreased inflammation and longevity (Elamin et al., 2017).
Meta-analyses of RCTs with low degree of heterogeneity (I2) (as in the present meta-analysis) are considered as providing the highest quality of clinical evidence. To our knowledge this study represents the first attempt to systematically investigate the effects of MCTs on ketosis induction and cognitive performance in MCI/AD based on all available RCTs. For sake of comprehensiveness, we wished to examine and present all existing evidence from human studies. Thus, we performed additional pooling of single-arm trials and qualitative presentation of observational studies and case series/case reports. Another strength was that the included studies were conducted in multiple countries (see table 1), allowing for relative generalizability of our results in terms of race and ethnicity. It is also important that regarding two RCTs included in our syntheses, the duration of MCT treatment was short (1 day for each group) (Ota et al., 2019; Reger et al., 2004) and thus potential chronic benefits of MCTs may not have been shown. Considering the short duration of treatment in some of the included studies we consider the overall results as encouraging. Sensitivity analyses based on the duration of the intervention (acute or chronic MCTs’ administration) would be desirable, but this was not possible due to the small number of available studies that were combined in the meta-analyses. As more studies get published, we hope that it may become possible to assess potential effects of C8:C10 composition through a meta-regression. Despite the encouraging results, there were several limitations in our study. First, the number of included studies in each synthesis was relatively small. Second, there were only seven RCTs among all included studies. Of those, three were deemed as unclear for ROB, and three were high for ROB. Furthermore, several studies provided only descriptive report of the outcomes without report of raw scores of measures of general cognition and/or peripheral levels of BHB; thus, they were not included in the statistical synthesis and forests plots. Finally, available data did not allow us to examine whether there is a change-change correlation between BHB levels and cognitive scores, which would have provided further support to the hypothesis.
5. Conclusions
In this systematic review and meta-analysis, we investigated the effects of oral MCTs administration in patients with MCI/AD. Highly homogeneous meta-analyses of RCTs found that MCTs result in mildly elevated peripheral BHB levels and improved cognitive performance in a combined cognitive measure. This action may have been mediated by the increased availability of ketone bodies to the hypometabolic brain of MCI/AD that is unable to utilize glucose. MCTs can be easily administered without the need for following onerous instructions, such as with ketogenic diets, fasting or exercise. Despite the encouraging results, the relatively small sample size of our synthesis and the potential bias of several included studies necessitate a relatively cautious interpretation of the results. Future high-quality RCTs of increased duration of treatment could add to the existing evidence on MCTs as a promising intervention for MCI/AD.
Highlights.
From meta-analysis of RCTs in Alzheimer’s disease and Mild Cognitive Impairment, oral Medium Chain Triglycerides administration induced mild peripheral ketosis
From meta-analysis of RCTs in Alzheimer’s disease and Mild Cognitive Impairment, oral Medium Chain Triglycerides administration improved cognition on a combined scale of ADAS-Cog and MMSE.
Risk of bias of existing studies of RCTs in Alzheimer’s disease and Mild Cognitive Impairment necessitates future trials.
Acknowledgments
This research was supported entirely by the Intramural research Program of the NIH, National institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None
References
- Andreas NJ, Kampmann B, Mehring Le-Doare K, 2015. Human breast milk: A review on its composition and bioactivity. Early Hum Dev 91, 629–635. [DOI] [PubMed] [Google Scholar]
- Bach AC, Babayan VK, 1982. Medium-chain triglycerides: an update. Am J Clin Nutr 36, 950–962. [DOI] [PubMed] [Google Scholar]
- Bach AC, Ingenbleek Y, Frey A, 1996. The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res 37, 708–726. [PubMed] [Google Scholar]
- Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP, 2005. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 142, 403–411. [DOI] [PubMed] [Google Scholar]
- Castellano CA, Hudon C, Croteau E, Fortier M, St-Pierre V, Vandenberghe C, Nugent S, Tremblay S, Paquet N, Lepage M, Fulop T, Turcotte EE, Dionne IJ, Potvin O, Duchesne S, Cunnane SC, 2019. Links Between Metabolic and Structural Changes in the Brain of Cognitively Normal Older Adults: A 4-Year Longitudinal Follow-Up. Front Aging Neurosci 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, Imbeault H, Turcotte E, Fulop T, Cunnane SC, 2015. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J Alzheimers Dis 43, 1343–1353. [DOI] [PubMed] [Google Scholar]
- Chan SC, Esther GE, Yip HL, Sugathan S, Chin PS, 2017. Effect of cold pressed coconut oil on cognition and behavior among patients with Alzheimer’s disease - A pilot intervention study. National Journal of Physiology, Pharmacy and Pharmacology 7, 1432–1435. [Google Scholar]
- CJ DCH, Schofield GM, Williden M, McQuillan JA, 2018. The Effect of Medium Chain Triglycerides on Time to Nutritional Ketosis and Symptoms of Keto-Induction in Healthy Adults: A Randomised Controlled Clinical Trial. J Nutr Metab 2018, 2630565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne-Loyer A, Croteau E, Castellano CA, St-Pierre V, Hennebelle M, Cunnane SC, 2017. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: A dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab 37, 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau E, Castellano CA, Fortier M, Bocti C, Fulop T, Paquet N, Cunnane SC, 2018a. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Experimental Gerontology 107, 18–26. [DOI] [PubMed] [Google Scholar]
- Croteau E, Castellano CA, Richard MA, Fortier M, Nugent S, Lepage M, Duchesne S, Whittingstall K, Turcotte EE, Bocti C, Fulop T, Cunnane SC, 2018b. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J Alzheimers Dis 64, 551–561. [DOI] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI, 2011. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 27, 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano CA, 2016. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Annals of the New York Academy of Sciences 1367, 12–20. [DOI] [PubMed] [Google Scholar]
- D’Andrea Meira I, Romao TT, Pires do Prado HJ, Kruger LT, Pires MEP, da Conceicao PO, 2019. Ketogenic Diet and Epilepsy: What We Know So Far. Front Neurosci 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N, 1986. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Drenick EJ, Alvarez LC, Tamasi GC, Brickman AS, 1972. Resistance to symptomatic insulin reactions after fasting. J Clin Invest 51, 2757–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin M, Ruskin DN, Masino SA, Sacchetti P, 2017. Ketone-Based Metabolic Therapy: Is Increased NAD(+) a Primary Mechanism? Front Mol Neurosci 10, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah BA, 2014. Effects of caprylic triglyceride on cognitive performance and cerebral glucose metabolism in mild Alzheimer’s disease: A single-case observation. Frontiers in Aging Neuroscience 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando WM, Martins IJ, Goozee KG, Brennan CS, Jayasena V, Martins RN, 2015. The role of dietary coconut for the prevention and treatment of Alzheimer’s disease: potential mechanisms of action. Br J Nutr 114, 1–14. [DOI] [PubMed] [Google Scholar]
- Finn PF, Dice JF, 2005. Ketone bodies stimulate chaperone-mediated autophagy. J Biol Chem 280, 25864–25870. [DOI] [PubMed] [Google Scholar]
- Freemantle E, Vandal M, Tremblay Mercier J, Plourde M, Poirier J, Cunnane SC, 2009. Metabolic response to a ketogenic breakfast in the healthy elderly. J Nutr Health Aging 13, 293–298. [DOI] [PubMed] [Google Scholar]
- Wells GA, S. B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P, 2019. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- Hashim SA, Vanltallie TB, 2014. Ketone body therapy: from the ketogenic diet to the oral administration of ketone ester. J Lipid Res 55, 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC, 2009. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutrition and Metabolism 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods, G., Cochrane Statistical Methods, G., 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, 2002. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, G.S.e., 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, The Cochrane Collaboration, 2011. [Google Scholar]
- Hu Yang I, De la Rubia Orti JE, Selvi Sabater P, Sancho Castillo, S., Rochina MJ, Manresa Ramon N, Montoya-Castilla I, 2015. [COCONUT OIL: NON-ALTERNATIVE DRUG TREATMENT AGAINST ALZHEIMER S DISEASE]. Nutr Hosp 32, 2822–2827. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, 1976. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res 10, 536–540. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP, 2011. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 10, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL, 2013. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiology of aging 34, 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL, 2000. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci U S A 97, 5440–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella R, Maher T, Clegg ME, 2017. Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol Behav 179, 422–426. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ, 2012. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiology of aging 33, 425 e419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutas A, Yellen G, 2013. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci 36, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP, 2009. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev 59, 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamelak M, 2012. Sporadic Alzheimer’s disease: the starving brain. J Alzheimers Dis 31, 459–474. [DOI] [PubMed] [Google Scholar]
- Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP, 2016. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem 139, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A, 2018. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci 19, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SD, Gelblum J, 2013. Retrospective cohort study of the efficacy of caprylic triglyceride in patients with mild-to-moderate Alzheimer’s disease. Neuropsychiatric Disease and Treatment 9, 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckling KA, O’Sullivan C, Saari D, 2004. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metab 89, 2717–2723. [DOI] [PubMed] [Google Scholar]
- Mélanie Fortier C-AC, Croteau Etienne, Langlois Francis, St-Pierre Christian Boctiad Valérie, Vandenberghe Camille, Bernier Michaël, Roy Maggie, Descoteaux Masxime, Whittingstall Kevin, Lepage Martin, Turcotte Éric E., Fulop Tamas, Cunnane Stephen C., 2019. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimer’s & Dementia. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R, Reiter D, Kapogiannis D, 2018. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer’s brain. Ann Clin Transl Neurol 5, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RJ, Diehl TC, Chia CW, Kapogiannis D, 2017. Insulin Resistance as a Link between Amyloid-Beta and Tau Pathologies in Alzheimer’s Disease. Front Aging Neurosci 9, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH, 2008. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 7, 500–506. [DOI] [PubMed] [Google Scholar]
- Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL, 2015. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 11, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordli DR Jr., Kuroda MM, Carroll J, Koenigsberger DY, Hirsch LJ, Bruner HJ, Seidel WT, De Vivo DC, 2001. Experience with the ketogenic diet in infants. Pediatrics 108, 129–133. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Toda A, Kimoto A, Takebayashi Y, Higashiyama R, Tagata Y, Ito M, Ota T, Shibata N, Arai H, 2016. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: a prospective, open-label pilot study. Clinical interventions in aging 11, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H, Yoshida S, Ashida K, Nakamura K, Takahashi T, Kunugi H, 2019. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neuroscience letters 690, 232–236. [DOI] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr., 1967. Brain metabolism during fasting. J Clin Invest 46, 1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlosky RJ, Kemper MF, Kashiwaya Y, King MT, Mattson MP, Veech RL, 2017. Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer’s disease. J Neurochem 141, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM, 2001. Effects of ketogenic diet on development and behavior: preliminary report of a prospective study. Dev Med Child Neurol 43, 301–306. [DOI] [PubMed] [Google Scholar]
- Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL, 2015. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA clinical 3, 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S, 2004. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiology of aging 25, 311–314. [DOI] [PubMed] [Google Scholar]
- Sariego-Jamardo A, Garcia-Cazorla A, Artuch R, Castejon E, Garcia-Arenas D, Molero-Luis M, Ormazabal A, Sanmarti FX, 2015. Efficacy of the Ketogenic Diet for the Treatment of Refractory Childhood Epilepsy: Cerebrospinal Fluid Neurotransmitters and Amino Acid Levels. Pediatr Neurol 53, 422–426. [DOI] [PubMed] [Google Scholar]
- Seaton TB, Welle SL, Warenko MK, Campbell RG, 1986. Thermic effect of medium-chain and long-chain triglycerides in man. Am J Clin Nutr 44, 630–634. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Kashiwaya Y, King MT, Baxa U, Tam J, Niu G, Chen X, Clarke K, Veech RL, 2012. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J 26, 2351–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor d., Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP, 2016. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H, 2018. A Ketone Ester Drink Lowers Human Ghrelin and Appetite. Obesity (Silver Spring, Md.) 26, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttanon P, Hill KD, Said CM, Williams SB, Byrne KN, LoGiudice D, Lautenschlager NT, Dodd KJ, 2013. Feasibility, safety and preli. mar evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer’s disease: a pilot randomized controlled trial. Clin Rehabil 27, 427–438. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekine S, Kojima K, Aoyama T, 2008. The application of medium-chain fatty acids: edible oil with a suppressing effect on body fat accumulation. Asia Pac J Clin Nutr 17 Suppl 1, 320–323. [PubMed] [Google Scholar]
- Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH, 2018. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s & dementia (New York, N. Y.) 4, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traul KA, Driedger A, Ingle DL, Nakhasi D, 2000. Review of the toxicologic properties of medium-chain triglycerides. Food Chem Toxicol 38, 79–98. [DOI] [PubMed] [Google Scholar]
- van Praag H, Fleshner M, Schwartz MW, Mattson MP, 2014. Exercise, energy intake, glucose homeostasis, and the brain. J Neurosci 34, 15139–15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH, 2012. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. 2012 49, 15 %J Journal of Statistical Software. [Google Scholar]
- Willette AA, Modanlo N, Kapogiannis D, Alzheimer’s Disease Neuroimaging I, 2015. Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes 64, 1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarek D, 2019. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IH, Orti JED, Sabater PS, Castillo SS, Rochina MJ, Ramon NM, Montoya-Castilla I, 2015. COCONUT OIL: NON-ALTERNATIVE DRUG TREATMENT AGAINST ALZHEIMER’S DISEASE. Nutricion Hospitalaria 32, 2822–2827. [DOI] [PubMed] [Google Scholar]