Abstract
Endocannabinoids are ancient biomolecules involved in several cellular (e.g., metabolism) and physiological (e.g., eating behaviour) functions. Indeed, eating behaviour alterations in marijuana users have led to investigate the orexigen-ic/anorexigenic effects of cannabinoids in animal/human models. This increasing body of research suggests that the endo-cannabinoid system plays an important role in feeding control. Accordingly, within the endocannabinoid system, canna-binoid receptors, enzymes and genes represent potential therapeutic targets for dealing with multiple metabolic and behav-ioural dysfunctions (e.g., obesity, anorexia, etc.). Paradoxically, our understanding on the endocannabinoid system as a cel-lular mediator is yet limited. For example: (i) only two cannabinoid receptors have been classified, but they are not enough to explain the pharmacological profile of several experimental effects induced by cannabinoids; and (ii) several orphan G pro-tein-coupled receptors (GPCRs) interact with cannabinoids and we do not know how to classify them (e.g., GPR18, GPR55 and GPR119; amongst others).
On this basis, the present review attempts to summarize the lines of evidence supporting the potential role of GPR18, GPR55 and GPR119 in metabolism and feeding control that may explain some of the divergent effects and puzzling data re-lated to cannabinoid research. Moreover, their therapeutic potential in feeding behaviour alterations will be considered.
Keywords: Feeding control, endocannabinoid system, GPR18, GPR55, GPR119
1. PHYSIOLOGY OF FEEDING
1.1. Energy Homeostasis: Hunger and Satiety
Apart from feeding’s biological role in energy supply, growing and cell metabolism, human beings eat for many other reasons including (but not limited to) social interaction, anxiety, hedonic satisfaction, boredom, etc. [1, 2]. Feeding is such an important event that it can be even used as an indicator of brain injury recovery [3]. As any behavioural action, feeding involves levels of several hormones and activity in different areas of the central nervous system (CNS). However, the biological interpretation of peripheral signals is mainly integrated via hypothalamic circuitry which involves nuclei such as dorsomedial hypothalamus (DMH), paraventricular nucleus (PVN), lateral hypothalamic area (LHA), ventromedial hypothalamus (VMH) and other areas out of hypothalamus [e.g., nucleus tractus solitarius (NTS); pituitary gland; ventral tegmental area (VTA); etc.] [4]. In its most simplistic interpretation, feeding behaviour may be understood as the balance of a “hypothalamic centre” in which the main feeding promotion centre is into the lateral hypothalamus [arcuate nucleus (ARC)]; whereas the satiety centre is mainly located in the VMH [5, 6] and also some neuronal populations in the ARC (see below). In the hypothalamus, feeding and satiety are controlled by many factors, including: the gut-brain axis (via neuronal and hormonal signals), leptin-brain interactions, levels of peripheral insulin, endocannabinoids, adiponectin, glucocorticoids, ciliary neurotrophic factor and hedonic mechanisms [7].
In the ARC, the expression of two main neuronal populations plays a major role in energy homeostasis and feeding behaviour, namely: i) neurons co-expressing neuropeptide Y (NPY) and agouti-related protein (AgRP), which promote feeding behaviour [8] (see below); and (ii) neurons that contain proopiomelanocortin hormone (POMC) which, in turn, produce satiety by inhibiting the activity of NPY and AgRP neurons into the ARC [9-12]. Moreover, ARC neurons that contain POMC also co-express the cocaine and amphetamine-related transcript (CART), an endogenous protein that mimics cocaine’s and amphetamines’ anorexigenic effects and, hence, it can induce satiety [13]. In keeping with these findings, Yanik et al. [13] reported an interesting case where all members of a family who carried a genetic mutation (i.e., a Leu34Phe missense mutation) that compromised CART activity were obese; whereas the reported member without the mutation (with a normal weight) and six non-obese controls had normal activity of CART.
Similarly, mutant animals with non-functional hypothalamic POMC neurons could develop obesity [14]. Thus, molecules that induce the release of NPY and AgRP may promote feeding; whereas those that increase CART and POMC activity may be potentially useful to induce satiety. Accordingly, mechanisms related to control of NPY, AgRP, CART and POMC are potential targets for the treatment of feeding pathologies (i.e., orexigenic- and anorexigenic-based alterations, respectively).
1.2. General Mechanisms and Molecules Associated with Feeding Promotion
Execution of feeding behaviour goes beyond the simple food-intake action. In fact, it is the result of several complex interactions that involve genes, hormones, neurotransmitters, enzymes, currently known and even unknown receptors (e.g., the CART receptor) and specialized cells in the CNS and elsewhere [15]. Feeding regulation is integrated through several molecules that exert their effects according to the target cell, cellular receptors involved and interactions with other transduction pathways related to metabolism and energy balance. Interestingly, NPY is essential for inducing feeding in adult animals as, indeed, its experimental ablation results in starvation [16]. NPY receptors are G protein-coupled and are widely expressed in the human body, including the hypothalamus [17]. NPY receptors are classified into Y1R, Y2R, Y4R and Y5R subtypes (Y3R and Y6R have been hypothesized in humans) [18, 19].
In addition, obesity has been related to NPY [20], in view that: (i) studies with Y1R, Y2R and Y5R knockout mice have shown body composition alterations including higher body weight and greater adipose tissue deposition; and (ii) ob/ob mice have augmented levels of mRNA of NPY in the ARC. Moreover, mutant mice lacking Y4R developed lower body weight and fat tissue reduction [20-22]. Interestingly, the absence of Y4R in the ob/ob mice did not prevent obesity [20].
On the other hand, AgRP neuronal activation can rapidly increase feeding behaviours through ghrelin, an orexigenic hormone produced mainly by P/D1 endocrine cells of the stomach fundus [23]. AgRP inhibits the melanocortin system and α-melanocyte stimulating hormone (α-MSH) which are both anorexigenic agents. Thus, AgRP can increase food intake by increasing NPY signaling and by decreasing melanocortin signaling [24, 25]. Moreover, AgRP neurons in the CNS of rodents are activated by peripheral endocrine signals (e.g., ghrelin) after an energetic challenge (usually a long period of food deprivation), and this activation leads to increases in NPY (promoting acute food intake) and, obviously, release of AgRP (promoting food hoarding behaviour) [26].
Other appetite stimulants are endogenous opioids such as β-endorphin, which has a short effect duration on appetite. Moreover, galanin, orexins, glutamate and γ-aminobutyric acid can affect directly or indirectly the feeding behaviour via their interactions with hypothalamic nuclei and/or peripheral tissues [27].
1.3. General Mechanisms and Mediators Associated with Satiety
POMC induces satiety by releasing melanocortin, which in turn is processed by a prohormone convertase (PC-1 & PC-2) to α-MSH by prohormone convertases 1 and 2 (PC1 and PC2). α-MSH is an agonist of the melanocortin receptors MC3/MC4. MC4 promotes feeding; whereas MC3 regulates fat tissue deposition [28]. To support these notions, an MC4 receptor antagonist reduced food intake in animal models [29].
Another hormone that induces severe anorexigenic effects is the corticotropin-releasing hormone (CRH). CRH seems to be involved in the satiety effect induced by leptin [30]. Likewise, glucagon-like peptide-1 (GLP-1) acts as an appetite inhibitor and it is also related to the anorexic signals of leptin [31, 32].
Remarkably, satiety depends on short and long-term signals like cholecystokinin (CCK), GLP-1, leptin, insulin, ghrelin, peptide YY, neurotensin and even cytokines like TNF-α [27]. Low concentrations of any of these mediators may affect the activity of the others (e.g., low leptin and CCK) and this will result in a higher food intake. An attenuated response to meal-related satiety signals is triggered by leptin deficiency and may contribute to an increase in food ingestion [33].
The interpretation of the above findings is further complicated when considering that different food types play also a role in satiety. For example, high energy density foods may increase food consumption due to their palatability properties, but they also may delay the appearance of hunger sensation in the short-term. Moreover, people with high caloric diets may change their gastric capacity over time [34, 35]. This suggests that energy homeostasis is a more complex process that involves multifactorial variables such as food caloric density, macronutrients ratio, gastric capacity, abdominal distension and peripheral and central signaling [34]. At this point, it is important to highlight that societies all over the world have a common alteration in feeding behaviour which results in a high percentage of people living with overweight and obesity [36]. Therefore, it is urgent to identify potential therapeutic targets and to develop novel prophylactic agents to combat this global health problem. In this context, the endocannabinoid system has emerged in the last decades as an interesting alternative to provide promising therapeutic avenues.
2. EFFECTS OF CANNABINOIDS ON METABOLISM AND FEEDING BEHAVIOUR
More than 3000 years ago, a Chinese papyrus documented the consumption of marijuana extracts as a therapeutic option to treat anorexia [37, 38]. However, it was thousands of years later when research in this field emerged upon the isolation and characterization of the first phytocannabinoid, namely, Δ9-tetrahydrocannabinol (Δ9-THC, Fig. (1) by Prof. Mechoulam and his group [39]. In the early 1990s, the cannabinoid receptors CB1 and CB2 were cloned and, subsequently, the endogenous cannabinoid ligands anandamide (AEA) and 2-arachidonoylglycerol (2-AG) were identified [40]. These milestones prompted a plethora of investigations towards understanding the physiopathological role of cannabinoids. However, our current knowledge on the endocannabinoid system (ECS) and its therapeutic targets remains incomplete. Hence, in the present review we have tried to summarize information that supports their potential role in metabolic and behavioural actions which, in turn, control food intake and could have therapeutic implications.
Fig. (1).
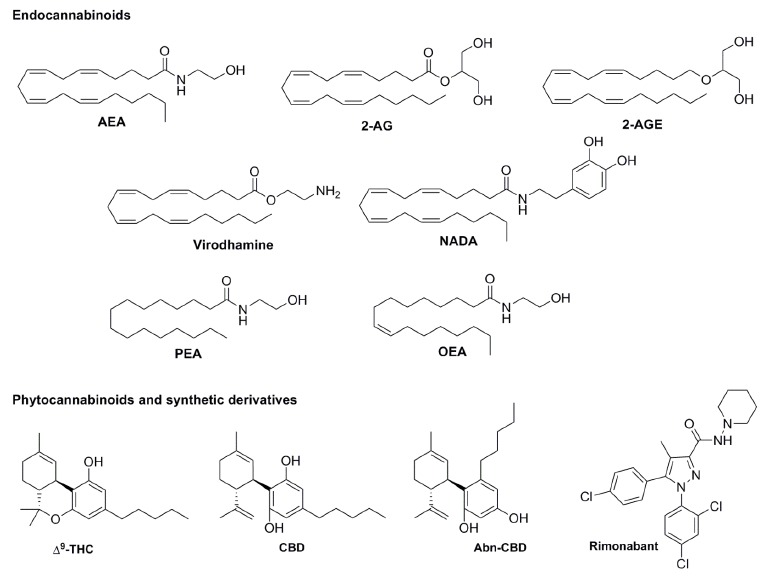
Structure of endocannabinoids: AEA, 2-AG, 2-AGE, virodhamine, NADA, PEA and OEA; phytocannabinoids THC and CBD; and synthetic cannabinoids Abn-CBD and rimonabant.
2.1. Endocannabinoid System: Introduction
Lipids, beyond their structural and energetic functions, are ancient molecules involved in the control of cell processes and belong to a complex family whose members include endocannabinoids [41-43]. Precursors of endocannabinoids are present in living systems since the existence of eukaryotes (i.e., more than 2000 million years ago [43]). Not with standing, endocannabinoids’ physiology remains far from being completely understood.
Endocannabinoids are synthetized on demand from membrane phospholipid precursors, through the activation of specific phospholipases. For instance, AEA synthesis occurs via phospholipase A2, phospholipase C and NAPE-PLD (N-acyl phosphatidylethanolamine phospholipase D) [44]. In addition to the cannabinoid receptors, CB1 and CB2 and their endogenous ligands, the proteins responsible for the biosynthesis, inactivation and transport of endocannabinoids are also part of the ECS. The metabolizing enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) have been extensively studied as reviewed elsewhere [45, 46]. Moreover, transporter proteins for endocannabinoids such as the fatty acid binding proteins (FABPs) have been identified and investigated in the last decade [47-49].
The molecular pharmacology of these endogenous molecules is quite complex and not fully unravelled. In addition to CB1 and CB2 receptors [50], endocannabinoids may interact with a wide variety of receptors, including: (i) TRPV1 [51]; (ii) GPR55 [52]; (iii) GPR18 [53]; (iv) GPR119 [54]; (v) PPARs [55] and (vi) others [56]. As a consequence of this target promiscuity, in general terms, the modulation of the endocannabinoid system has typical major issues that deserve to be carefully considered before the interpretation of any findings. For example: (i) cannabinoid effects in the body usually have opposite effects (i.e., increases or decreases in body functions depending on the dose); (ii) potential side effects are practically as unlimited as the potential therapeutic uses; and (iii) there is a complex pharmacological characterization of the ligands used. Moreover, other lipids mimic classic biological effects of AEA (first endocannabinoid found) and/or phytocannabinoids (e.g., hypothermia, vasodilatation, immunosuppression, etc.). Endocannabinoids and structurally related compounds include: AEA, 2-AG, palmitoylethanolamide (PEA), oleoylethanolamide (OEA), arachidonoylglyceryl ether (noladin ether, 2-AGE), O-arachidonylethanolamine (virodhamine), and N-arachidonoyl-dopamine (NADA), oleamide, amongst others (structures depicted in Fig. (1) [57]. Interestingly, many of them have been shown to mediate direct and/or indirect effects on feeding behaviour and/or metabolism (see below).
2.2. Effect of Cannabinoids on Feeding
Cannabinoids are so important on feeding control that they may be involved in mammal early-life feeding (which is crucial for survival and development). Indeed, immediately after birth, mammals exhibit food-seeking behaviour to get milk [58]. Interestingly, maternal milk contains high levels of endocannabinoids such as 2‑AG, AEA and other fatty acids [59]. On the very day of birth, the rat brain achieves peak concentrations of 2-AG in its whole life, coinciding with peak levels of mRNA of CB1 receptors on the ventromedial and paraventricular nucleus (PVN) of the hypothalamus [60]. It is important to note that the systemic administration of CB1 antagonists dramatically blocks the suckling movement in neonatal pups, decreasing survival. This finding, in addition to confirming the fundamental function of CB1 signaling in early feeding, reveals the complex interactions of cannabinoid ligands with other potential cannabinoid receptors [59, 61, 62].
Furthermore, cannabinoid CB2 receptors are known to be involved in the regulation of the immune system. Paradoxically, this receptor has been reported to promote obesity inflammation [63], whereas JWH-015 (a CB2 receptor agonist) protected against obesity induced by diet [64]. Although this finding could be contradictory, JWH‑015 is also a GPR55 receptor agonist [65]. Thus, effects induced by JWH-015 may involved non-CB2 receptors (e.g., GPR55).
Additionally, it is well documented that, depending on the dose and consumption frequency, marijuana may increase or decrease food intake in humans, particularly of sweet tasting foods [66-69]. This effect, which may be mimicked by synthetic cannabinoids and phyto-cannabinoids [70], has been historically explained in terms of CB1 receptor activation/blockade in central and peripheral tissues (see below). Likewise, hypothalamic circuity is regulated by the ECS via CB1 receptors, the acute activation of which promotes feeding [71, 72]. In fact, CB1 receptor antagonists (such as rimonabant, Fig. (1) and their allosteric modulators are potent anorexigenic drugs in rodents [73, 74]. For this reason, CB1 receptor antagonists have been clinically studied
for obesity treatment [75, 76]. One difficulty for the development of anti-obesity CB1 blocker-based treatments include side effects in the limbic system (particularly, depression). In fact, several preclinical and clinical reports have suggested an important role of the endocannabinoid system in neuropsychiatric disorders, such as anxiety and depression [77, 78]. Thus, these treatments require further basic and clinical evaluation [79]. Indeed, clinical assays that evaluated CB1-antagonist efficacy originally failed due to suicide risk and depression [80].
Admittedly, the pharmacological profile of CB1 and CB2 receptors as well as that of other classical targets (i.e., TRPV1, PPAR and voltage channels; see section 2.1) is insufficient for explaining all actions induced by cannabinoids. For example, cannabidivarin (CBDV) induced anti-seizure effects in different preclinical models in a CB1-independent pathway [81]. In this respect, it is noteworthy that GPR55 is co-expressed along with cannabinoid CB1 receptors in different areas in the CNS (e.g., striatum, hypothalamus, hippocampus, frontal cortex, etc. [52]).
In this scenario, some orphan G-protein coupled receptors have been proposed as putative cannabinoid receptors, namely, GPR18, GPR55 and GPR119 [82]. Accordingly, these receptors may be the key for understanding those controversial actions of cannabinoids in general, and specifically on metabolism and feeding (Table 1).
Table 1. Global deletion of GPR18, GPR55 and GRP119 and metabolic alterations in murine model research.
| Putative Cannabinoid Receptor | Effects after Global Deletion |
|---|---|
| GPR18 | Alterations in intraepithelial lymphocytes physiology of the small intestinea,b |
| GPR55 | Body fat increasec Lower energy expenditurec Insulin resistancec,d Reduced physical activityc |
| GPR119 | Low plasma insulin levelse Lower lean masse GLP-1 release attenuationf |
2.3. Cannabinoid Targets Related to Feeding Control
The complex effects of cannabinoids on eating physiology encompass from cortical, hypothalamic and limbic pathways to adipocytes and islet cells [89-91]. In animal models, exogenous administration of cannabinoids also produces biphasic effects, i.e.: hyperphagia at low doses and hypophagia at high doses [92, 93]. These opposite effects could be explained by, at least, two hypotheses: (i) the pharmacological profile of the receptors involved in these opposite effects include classic cannabinoid and/or other receptors; and (ii) only classic cannabinoid receptors are involved acting in different CNS areas.
A decisive autonomic feedback for hypothalamic circuits in feeding control is the gastric emptying, which can be delayed by oral administration of THC in healthy volunteers [94]. The fact that this effect is not mimicked by intravenous administration of THC [95] suggests a target expressed directly in the epithelial cells on the gastrointestinal tract. The second main component in marijuana, cannabidiol (CBD, Fig. (1), which displays low affinity for CB1/CB2 receptors, has been shown to decrease intestinal motility and, consequently, food intake [96, 97]. A possible explanation for this effect of CBD may include its capability to antagonize GPR55 receptors (Table 2), which seem to participate in promoting upper intestinal transit, particularly during inflammation [98]. In addition, GPR55 may affect feeding via central mechanisms (section 3.3). Lastly, GPR55 could also be involved by acting as a negative modulator of the cannabinoid CB1 receptor in the neuroendocrine cells of the gut [99].
Table 2. Functionality of selected cannabinoids implicated in feeding disorders.
| Compound | Cannabinoid Receptors | Putative Cannabinoid Receptors | |||||
|---|---|---|---|---|---|---|---|
| CB1a | CB2a | GPR18b,c | GPR55d,e | GPR119f,g | |||
| AEA | Agonist | Agonist | Agonist | Agonist* | Weak agonist | ||
| 2-AG | Agonist | Agonist | NR | Agonist* | NE | ||
| PEA | NE | NE | NR | Agonist | Weak agonist | ||
| OEA | NE | NE | NR | Agonist | Agonist | ||
| THC | Partial agonist | Partial agonist | Agonist | Agonist | NE | ||
| CBD | Weak antagonist/ Allosteric modulator** | Weak antagonist/ Allosteric modulator** | Antagonist* | Antagonist | NE | ||
| Abn-CBD | NE | NE | Agonist | Agonist* | NR | ||
| Rimonabant | Inverse agonist | NE | NE | Antagonist* | NR | ||
Abbreviations: NE: no significant effect; NR: Not reported. *Discrepancies in functionality depending on signaling outcome and /or cell system (See Morales and Jagerovic for further detail). **Allosteric effects of CBD have been reported by Laprairie et al., Tham et al. and Martinez-Pinilla et al. Data taken from: aPertwee et al.; bMcHugh et al.; cRajaraman et al.; dRoss [99]; eMorales and Jagerovic; fBrown; gGodlewski et al. *[100-103]. a[82], b[53], c[104], d[105], e[100], f[106], g[107].
Another important molecule, the ghrelin peptide, is produced in the gut to activate an AMP-activated protein kinase (AMPK) on the PVN and ARC in a CB1-dependent manner, resulting in promotion of feeding. In fact, intraperitoneal injection of ghrelin can increase 2-AG and AEA levels in the hypothalamus [108], suggesting a pivotal role of endocannabinoids for interpreting peripheral signals related to feeding behaviour. To support this notion, leptin is under negative control of hypothalamic levels of AEA and 2‑AG; high levels of endocannabinoids reduce leptin secretion and vice versa [109]. Moreover, leptin can disrupt synaptic plasticity on lateral hypothalamic (LH) neurons depending on endocannabinoid tone. Decreased endocannabinoid signaling is associated with decreased feeding and LH excitability which, in turn, control motivated feeding through limbic connections [110, 111].
Acylethanolamides synthesized in the gut such as AEA, OEA, or PEA drive peripheral control of feeding through direct and/or indirect activation of CB1, CB2, TRPV1, PPAR-α, GPR55 and/or GPR119 receptors [54]. Intestinal AEA and OEA levels react to feeding states, namely: after 24 hours of food deprivation, levels of AEA increased; whereas those for OEA decreased [112, 113]. Furthermore, after refeeding, levels of AEA are re-established with a transitory increase in OEA [112, 113]. Additionally, vagal afferents in the stomach and duodenum showed a high expression of CB1 receptors after 12 hours of food deprivation, which decreased after refeeding by a CCK-1 receptor-dependent mechanism [114]. These results strongly support the notion that the simplest change in CB1 receptor expression may affect feeding. In agreement with this view, CB1 receptors seem to have a high level of constitutive activity that, in turn, is sensitive to CB1 receptor inverse agonists [115].
Direct injections of AEA into the ventromedial hypothalamic nucleus may induce hyperphagia in pre-satiated rats [116]. Interestingly, partial hypothalamic deletion of CB1 receptors in adult mice decreased body weight gain without altering food intake by promoting changes in brown adipose activity [117]. In rodent models of obesity, oral administration of CB1 receptor antagonists (e.g., JD5037 or AM6545) with low brain penetration capacity results in hypophagic effects while reducing body weight, hepatic steatosis and insulin resistance [118, 119]. These findings suggest that the CB1 site of action is both peripheral and central. Indeed, Nogueiras et al. [120] reported that peripheral blockade of CB1 receptors induced changes in several metabolic pathways (e.g., sensitivity to insulin, reuptake of glucose in muscle, etc.) in obese rats; whereas central blockade of CB1 receptors only decreased food intake and body weight [120]. Moreover, in obese humans, oral administration of rimonabant induced loss of body weight, reduction in triglycerides, increased high-density lypoprotein (HDL) cholesterol and adiponectin in plasma, but this is not clearly related to food intake inhibition [121, 122]. Evidently, rimonabant (as many other cannabinoids) is not a selective drug for CB1/CB2 receptors Table (2). Thus, all lines of evidence that did not consider other targets should be further analyzed with the advent of selective putative cannabinoid receptor antagonists.
Endocannabinoids are produced by dietary fatty acids [123]. In keeping with this fact, a high linoleic acid diet in rodents raised: (i) 2-AG and AEA levels in the liver; (ii) plasma leptin; (iii) adiposity; and (iv) food intake [124]. Interestingly, oral administration of OEA (a GPR55 and a GPR119 receptor agonist, Table (2) lowered food intake, adipose tissue mass and plasma triglyceride levels in a high fat diet-induced obesity in mice [125]. Such an anti-obesity effect was related to a higher synthesis of the cannabinoid degrader enzyme, FAAH and with the GPR119 receptor in the small intestine [125].
The above findings, taken together, allow us to speculate on several pathways for explaining the effects of cannabinoids on feeding, namely: (i) in view of the promiscuity of endocannabinoids for interacting with multiple targets, the physiology of the endocannabinoid tone requires a dynamic fluctuation among levels of each member of this system at peripheral and central levels; (ii) feeding/satiety includes an active phase of enzymatic transformation of several lipids (including all the endocannabinoids) that results in transitory and local increases and decreases in endocannabinoid levels; (iii) some of the peripheral signals promote changes in the expression of cannabinoid-related receptors and/or in the activity of key tissues (e.g., gut, intestine, vagal nerves, hypothalamic nerves, etc.); (iv) the putative cannabinoid receptors are fundamental for explaining the complex effects of cannabinoids on feeding and energy metabolism. Hence, further research is required (see below).
2.4. Role of Cannabinoids in Energy Expenditure
Apart from their actions on the “hedonic brain”, cannabinoids can also modulate some mechanisms related to energy expenditure. In fact, CB1 mitochondrial receptors (mtCB1) seem to be crucial for the neuronal energy dynamics [126]. In this respect, the fact that even mitochondrion use the cannabinoid system for controlling some of their functions reflects the ancestral and ubiquitous profile of this system. It is possible that mtCB1, in addition to the classic presynaptic axonal CB1 receptors, are responsible for the wide spectrum of cannabinoid actions in the CNS (i.e., learning and memory, anxiety, feeding, attention, etc.) [127]. mtCB1 receptors play also an important role in controlling the oxidative activity of striated muscle respiration [128]. Interestingly, Ruiz de Azua et al. [129] reported a protection against diet-induced obesity and metabolic alterations in mice by using adipocyte-specific inducible deletion of the CB1 gene (Ati-CB1–KO). Hence, CB1 receptors may represent key proteins for controlling all aspects of feeding and energy expenditure. Moreover, both GPR55 and GPR119 have a role in insulin release, feeding and energy homeostasis (see below).
3. POTENTIAL ROLE OF GPR18, GPR55 AND GPR119 IN FEEDING AND ENERGY HOMEOSTASIS
3.1. Evidence for Non-CB1/non-CB2 Receptors Mediating the Metabolic and Feeding Actions of Cannabinoids
Naughton et al. [123] have reported that obesity may be related to: (i) high levels of arachidonic acid (and, consequently, of endocannabinoids); and (ii) an altered pattern of cannabinoid receptors expression. As previously pointed out, drugs originally designed as CB1 receptor antagonists (e.g., rimonabant) have been reported to induce satiety [75, 76] and an increased risk for important psychiatric side effects mainly related to CB1 receptor blockade [130-132]. As the promiscuity of these molecules results in multiple interactions with cannabinoid, orphan and non-cannabinoid receptors (Table (2), their effects may involve other non-CB1 receptors targets (e.g., GPR18, GPR55 and GPR119). Table (2) summarizes the functionality of cannabinoids related to feeding disorders at CB1, CB2 and the putative cannabinoid receptors GPR18, GPR55 and GPR119.
On this basis, it is reasonable to suggest that some therapeutic anti-obesity potential effects induced by rimonabant may be explained via other non-CB1/2 pharmacological receptors. If so, GPR18, GPR55 and GPR119 represent potential targets for exploring the aforementioned effects. Hence, further research is required to identify the potential anti-obesity actions of these putative cannabinoid receptors (see below).
3.2. Potential Role of the G Protein-coupled Receptor 18 (GPR18) in the Control of Adipose Tissue Inflammation
GPR18 is activated by AEA, lipid N-arachidonyl glycine (NAGly) and the plant constitute “abnormal cannabidiol” (Abn-CBD, Table (2), [40, 133]. As activation of this receptor results in actions typically induced by endocannabinoids (e.g., hypotension, immune system inhibition, etc. [134]), it has been suggested as a potential cannabinoid receptor. This requires further evidence before a formal nomenclature is established [134, 135]. The pharmacological profile of GPR18 denotes complex transductional pathways that involve both: (i) negative regulation of cyclic adenosine monophosphate (cAMP) and phosphorylation of extracellular signal-regulated kinase (ERK) [136]; and (ii) a rise in intracellular levels of Ca2+ and MAPK activity and, hence, the potential participation of Gαi/o and Gαq protein-coupling [137]. The last possibility requires confirmation in view that all GPR18 agonists used by Console-Bram et al. [137] are also GPR55 agonists (which involves increases in Ca2+ levels).
In addition, it has recently been reported that GPR18, a receptor for resolvin (Rv)D2 (a potent anti-inflammatory endogenous molecule), is expressed in hypothalamic NPY and POMC neurons [138]. Interestingly, activation of GPR18 protects against hypothalamic inflammation, improves glucose tolerance and provides body mass reduction [138]. Thus, pharmacological manipulation of GPR18 may be useful to prevent metabolic alterations related to metabolic syndrome and obesity. To support this notion, NAGly has been reported as an antinociceptive and anti-inflammatory lipid [139]. Indeed, GPR18 showed high and selective expression on proinflammatory macrophages [140]. Activation of GPR18 by NAGly reduced viability of these proinflammatory macrophages via apoptotic mechanisms related to the MAPK pathway [140] Fig. (2). This kind of macrophages seem to be important in the development of obesity. Thus, activation of GPR18 may play a protective role against obesity. It is worthy of note that GPR18 and GPR55 are activated by O-1602, a synthetic cannabinoid linked with Abn-CBD [53]. Moreover, O-1602 can induce feeding behaviour in mutant GPR55 -/- mice and lipid accumulation in adipocytes (in vitro) [141].
Fig. (2).

Anti-inflammatory actions of GPR18 bafter stimulation by NaGly. NaGly may be produced by macrophages and C6 glyoma cells and/or from dietary fatty acids Bradshaw et al. [162]. NaGly activates MAP/ERK/JNK and mitochondrial signaling pathways via GPR18 for inducing apoptosis of proinflammatory macrophages Takenouchi et al. [140]. In beta cells, NaGly increases intracelullar calcium and induce insulin release through an unknown receptor Ikeda et al. and Takenouchi et al. [163, 164]. NaGly synthesized by two independent ways Bradshaw et al. [162].
GPR18 involves MAPK activity [53], which is an important messenger during inflammatory disorders, including obesity [142]. Hence, the role of peripheral GPR18 for promoting or inhibiting obesogenic mechanisms remains obscure. On the other hand, several free fatty acids, which are increased in obesity [143], are circulating in the whole body inducing their effects via multiple non-identified receptors. Currently, the available findings suggest an anti-obesogenic activity of hypothalamic GPR18; whereas activation of peripheral GPR18 requires further studies.
3.3. GPR55 as a Metabolic Regulator
GPR55 seems to be highly distributed throughout the body and in some brain´s areas involved in motor behaviour, spatial memory, metabolism and other functions [52, 144]. It is expressed on white adipose tissue, hypothalamus, CNS, adrenal glands, gastrointestinal tract, and frontal cortex, amongst other brain structures [52]. This molecule seems to be involved in a variety of processes, such as vasodilatation, energy homeostasis, learning and memory, motor function and pain [52, 65, 144-146]. Mutant GPR55-/- mice studies have shown opposite effects. For example, Meadows et al. [85] reported an increase in body fat and an altered glucose metabolism causing insulin resistance in adult animals, which also showed a reduction in voluntary physical activity; in contrast, Bjursell et al. [147] demonstrated no important metabolic changes as compared with wild-type mice. Indeed, in vitro studies performed in both human and rodent β-cells suggest that GPR55 is importantly involved in the control of insulin release [86, 148-150] (see Fig. (3). Moreover, energy dynamics in adipose tissue seems to be related to GPR55 activity [151]. For example, Moreno-Navarrete et al. [152] have reported: (i) higher levels of lysophosphatidylinositol (LPI; a GPR55 endogenous agonist) and the presence of GPR55 in obese human adipose tissue as compared to lean subjects (a finding also observed in patients with type 2 diabetes); and (ii) that leptin-/- mice and high-fat diet rats showed lower levels of GPR55. These findings, which suggest that GPR55 may play a differential role in humans and rodents, may account for the above opposite effects. Fig. (3). [85, 149, 153].
Fig. (3).
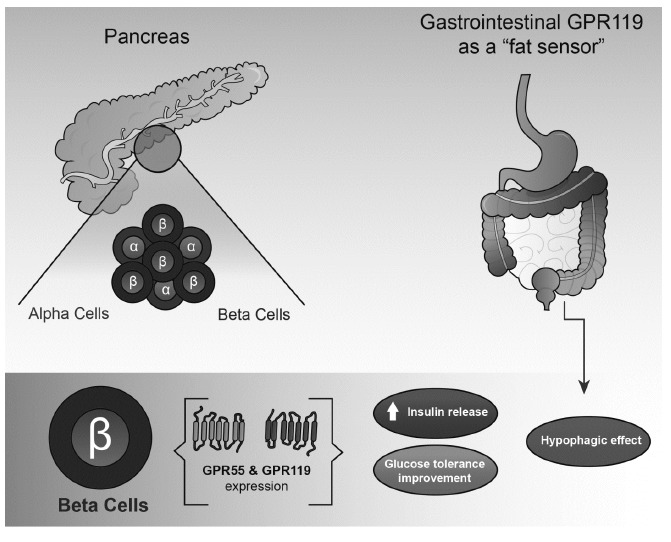
Metabolic effects of gastrointestinal and pancreatic GPR55 and GPR119. Both receptors seem to produce beneficial effects on glucose metabolism and tolerance by controlling positively insulin release McKillop et al. and Soga et al. [149, 153]. On the other hand, GPR119 is also expressed in the gastrointestinal tract (enteroendocrine system) and it plays an important function as a “fat sensor” regulating the expression and secretion of hormones such as GLP-1, GLP-2 and PYY leading to hypophagia Meadows et al. [85].
On the other hand, pharmacological stimulation of GPR55 plus CB1 receptor blockade in rodents (an effect typically induced by classical CB1 receptor antagonists) seems to induce protection against obesity related to anxiety [154]. However, our understanding of GPR55 physiology and its clinical application requires the advent of highly selective GPR55 pharmacological tools and a clear understanding of physiological differences amongst the actions of this protein in humans and in other species.
3.4. Function of GPR119 as a Fat Sensor
Perhaps the most interesting putative cannabinoid receptor in terms of its potential for the control of feeding is GPR119 (see below). At least two major fatty acid derivatives are endogenous ligands for GPR119, namely, lysophosphatidylcholine (LPC) [153] and an endogenous lipid-related to an oleic acid such as oleoylethanolamide (OEA) [155]. This receptor is mainly expressed in pancreatic islets and in the gastrointestinal tract controlling insulin, incretin hormone and GLP-1 secretion, respectively [156, 157]. In keeping with this view, Ha et al. [158] have reported that the GPR119 agonist HD0471042 controlled glucose levels and body weight in a rodent model of obesity induced by diet. Moreover, Panaro et al. [87] have recently shown that the role of GPR119 in controlling glucose levels is beyond the simple pancreatic β-cell activation, suggesting a key role for GPR119 in the intestinal tract [87]. Apart from its role in insulin dynamics, GPR119 seems to play a pancreatic protective role on β-cells [159]. In summary, gastrointestinal GPR119 seems to be a fat sensor that induces satiety via the secretion of GLP-1 from enteroendocrine cells [155] Fig. (3).
Interestingly, oral administration of agonists at GPR55 and GPR119 seems to provide an important metabolic protection against streptozotocin-induced diabetes [160]. Indeed, deletion of GPR55 increased fat mass and insulin resistance and reduced spontaneous physical activity in mice [85]; whereas stimulation of GPR119 suppressed feeding and reduced body weight gain in rats [161].
CONCLUSION
Historically, CB1 receptors were assumed to mediate all the behavioural actions of cannabinoids. Currently, we know that several putative cannabinoid receptors may also be involved. In view of their strategic expression on digestive, metabolic, hypothalamic and other tissues (e.g., vagal afferents), GPR18, GPR55 and GPR119 seem to be directly and/or indirectly involved in the physiology of feeding. In fact, the role of numerous cannabinoid ligands might be due to their interactions with these orphan GPCRs.
While GPR55 and GPR18 have been reported to recognize a variety of cannabinoid ligands, no cannabinoid has been found to modulate GPR119 with significant potency. However, the latter orphan GPCR has a closer phylogenetic relationship with CB1 and CB2.
We are still far from a complete understanding of the physiology of the endocannabinoid system, but these
potential members of the cannabinoid family are clearly related to the pathological relevance of this system. Accordingly, they represent an alternative to further exploring their therapeutic potential in feeding disorders.
ACKNOWLEDGEMENTS
We thank Alejandra Estefanía Santillán Macías for graphics. Finally, we thank Arturo Femat from the Autonomous University of Aguascalientes for his extraordinary institutional support in the management of the PRODEP program.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
B.A.M.C. (UAA-PTC-200) and R.E.O.R. were supported by a grant of the “Programa para el Desarrollo Profesional Docente para el Tipo Superior (PRODEP)”. C.M.V. was supported by a grant of the Consejo Nacional de Ciencia y Tecnología (CONACyT grant No. 219707, Mexico City).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Blundell J.E., Lawton C.L., Cotton J.R., Macdiarmid J.I. Control of human appetite: Implications for the intake of dietary fat. Annu. Rev. Nutr. 1996;16(1):285–319. doi: 10.1146/annurev.nu.16.070196.001441. [http://dx.doi.org/10.1146/ annurev.nu.16.070196.001441]. [PMID: 8839929]. [DOI] [PubMed] [Google Scholar]
- 2.Koball A.M., Meers M.R., Storfer-Isser A., Domoff S.E., Musher-Eizenman D.R. Eating when bored: revision of the emotional eating scale with a focus on boredom. Health Psychol. 2012;31(4):521–524. doi: 10.1037/a0025893. [http://dx.doi.org/10.1037/a0025893]. [PMID: 22004466]. [DOI] [PubMed] [Google Scholar]
- 3.Formisano R., Voogt R.D., Buzzi M.G., Vinicola V., Penta F., Peppe A., Stanzione P. Time interval of oral feeding recovery as a prognostic factor in severe traumatic brain injury. Brain Inj. 2004;18(1):103–109. doi: 10.1080/0269905031000149470. [http://dx.doi.org/10.1080/0269905031000149470]. [PMID: 14660239]. [DOI] [PubMed] [Google Scholar]
- 4.Waterson M.J., Horvath T.L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab. 2015;22(6):962–970. doi: 10.1016/j.cmet.2015.09.026. [http://dx.doi.org/10.1016/j.cmet.2015.09. 026]. [PMID: 26603190]. [DOI] [PubMed] [Google Scholar]
- 5.Elmquist J.K., Elias C.F., Saper C.B. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [http://dx.doi.org/10.1016/S0896-6273(00) 81084-3]. [PMID: 10069329]. [DOI] [PubMed] [Google Scholar]
- 6.Pinto S., Roseberry A.G., Liu H., Diano S., Shanabrough M., Cai X., Friedman J.M., Horvath T.L. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. doi: 10.1126/science.1089459. [http://dx.doi.org/10.1126/science.1089459]. [PMID: 15064421]. [DOI] [PubMed] [Google Scholar]
- 7.Ahima R.S., Antwi D.A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. North Am. 2008;37(4):811–823. doi: 10.1016/j.ecl.2008.08.005. [http://dx.doi.org/10.1016/j.ecl.2008.08.005]. [PMID: 19026933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirth M.M., Giraudo S.Q. Agouti-related protein in the hypothalamic paraventricular nucleus: effect on feeding. Peptides. 2000;21(9):1369–1375. doi: 10.1016/s0196-9781(00)00280-1. [http://dx.doi.org/10.1016/S0196-9781(00)00280-1]. [PMID: 11072124]. [DOI] [PubMed] [Google Scholar]
- 9.Gehlert D.R., Beavers L.S., Johnson D., Gackenheimer S.L., Schober D.A., Gadski R.A. Expression cloning of a human brain neuropeptide Y Y2 receptor. Mol. Pharmacol. 1996;49(2):224–228. [PMID: 8632753]. [PubMed] [Google Scholar]
- 10.Sainsbury A., Cooney G.J., Herzog H. Hypothalamic regulation of energy homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2002;16(4):623–637. doi: 10.1053/beem.2002.0230. [http://dx.doi.org/10.1053/beem.2002.0230]. [PMID: 12468411]. [DOI] [PubMed] [Google Scholar]
- 11.Ollmann M.M., Wilson B.D., Yang Y.K., Kerns J.A., Chen Y., Gantz I., Barsh G.S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. doi: 10.1126/science.278.5335.135. [http://dx.doi.org/10.1126/science.278.5335. 135]. [PMID: 9311920]. [DOI] [PubMed] [Google Scholar]
- 12.Lau J., Herzog H. CART in the regulation of appetite and energy homeostasis. Front. Neurosci. 2014;8:313. doi: 10.3389/fnins.2014.00313. [http://dx.doi.org/ 10.3389/fnins.2014.00313]. [PMID: 25352770]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanik T., Dominguez G., Kuhar M.J., Del Giudice E.M., Loh Y.P. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: A possible cause for obesity in humans. Endocrinology. 2006;147(1):39–43. doi: 10.1210/en.2005-0812. [http://dx.doi.org/10.1210/en.2005-0812]. [PMID: 16210370]. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Lacza Z., Sun Y.E., Han W. Leptin resistance and obesity in mice with deletion of methyl-CpG-binding protein 2 (MeCP2) in hypothalamic pro-opiomelanocortin (POMC) neurons. Diabetologia. 2014;57(1):236–245. doi: 10.1007/s00125-013-3072-0. [http://dx.doi.org/10.1007/ s00125-013-3072-0]. [PMID: 24078059]. [DOI] [PubMed] [Google Scholar]
- 15.Lau J., Farzi A., Qi Y., Heilbronn R., Mietzsch M., Shi Y.C., Herzog H. CART neurons in the arcuate nucleus and lateral hypothalamic area exert differential controls on energy homeostasis. Mol. Metab. 2018;7:102–118. doi: 10.1016/j.molmet.2017.10.015. [http://dx.doi.org/10.1016/j. molmet.2017.10.015]. [PMID: 29146410]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [http://dx.doi.org/10. 1126/science.1115524]. [PMID: 16254186]. [DOI] [PubMed] [Google Scholar]
- 17.Silva A.P., Kaufmann J.E., Vivancos C., Fakan S., Cavadas C., Shaw P., Brunner H.R., Vischer U., Grouzmann E. Neuropeptide Y expression, localization and cellular transducing effects in HUVEC. Biol. Cell. 2005;97(6):457–467. doi: 10.1042/BC20040102. [http://dx.doi.org/10. 1042/BC20040102]. [PMID: 15850450]. [DOI] [PubMed] [Google Scholar]
- 18.Acuna-Goycolea C., Tamamaki N., Yanagawa Y., Obata K., van den Pol A.N. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J. Neurosci. 2005;25(32):7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.1008-05.2005]. [PMID: 16093392]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander S.P., Christopoulos A., Davenport A.P., Kelly E., Marrion N.V., Peters J.A., Faccenda E., Harding S.D., Pawson A.J., Sharman J.L., Southan C., Davies J.A., Collaborators C. The concise guide to pharmacology 2017/18: G protein-coupled receptors. Br J Pharmacol. 2017;174(Suppl 1)(S1):S17–S129. doi: 10.1111/bph.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sainsbury A., Schwarzer C., Couzens M., Jenkins A., Oakes S.R., Ormandy C.J., Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16(9):1077–1088. doi: 10.1101/gad.979102. [http:// dx.doi.org/10.1101/gad.979102]. [PMID: 12000791]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushi A., Sasai H., Koizumi H., Takeda N., Yokoyama M., Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95(26):15659–15664. doi: 10.1073/pnas.95.26.15659. [http://dx.doi.org/10.1073/pnas. 95.26.15659]. [PMID: 9861026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naveilhan P., Hassani H., Canals J.M., Ekstrand A.J., Larefalk A., Chhajlani V., Arenas E., Gedda K., Svensson L., Thoren P., Ernfors P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y-Y2 receptor. Nat. Med. 1999;5(10):1188–1193. doi: 10.1038/13514. [http://dx.doi.org/10.1038/13514]. [PMID: 10502824]. [DOI] [PubMed] [Google Scholar]
- 23.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [http://dx. doi.org/10.1038/45230]. [PMID: 10604470]. [DOI] [PubMed] [Google Scholar]
- 24.Ekblad E., Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23(2):251–261. doi: 10.1016/s0196-9781(01)00601-5. [http://dx.doi.org/10. 1016/S0196-9781(01)00601-5]. [PMID: 11825640]. [DOI] [PubMed] [Google Scholar]
- 25.Hahn T.M., Breininger J.F., Baskin D.G., Schwartz M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1998;1(4):271–272. doi: 10.1038/1082. [http://dx.doi.org/10. 1038/1082]. [PMID: 10195157]. [DOI] [PubMed] [Google Scholar]
- 26.Thomas M.A., Xue B. Mechanisms for AgRP neuron-mediated regulation of appetitive behaviors in rodents. Physiol. Behav. 2018;190:34–42. doi: 10.1016/j.physbeh.2017.10.006. [http://dx.doi.org/10.1016/j.physbeh.2017.10.006]. [PMID: 29031550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malacara J.M. Mecanismos regulatorios de la ingestión de alimentos¿ Al fin un tratamiento a la vista. Rev Endocrin Nutric. 2004;12(4):188–198. [Google Scholar]
- 28.Chen A.S., Marsh D.J., Trumbauer M.E., Frazier E.G., Guan X.M., Yu H., Rosenblum C.I., Vongs A., Feng Y., Cao L., Metzger J.M., Strack A.M., Camacho R.E., Mellin T.N., Nunes C.N., Min W., Fisher J., Gopal-Truter S., MacIntyre D.E., Chen H.Y., Van der Ploeg L.H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000;26(1):97–102. doi: 10.1038/79254. [http://dx.doi.org/10.1038/ 79254]. [PMID: 10973258]. [DOI] [PubMed] [Google Scholar]
- 29.Schiöth H.B., Kask A., Mutulis F., Muceniece R., Mutule I., Mutule I., Mandrika I., Wikberg J.E. Novel selective melanocortin 4 receptor antagonist induces food intake after peripheral administration. Biochem. Biophys. Res. Commun. 2003;301(2):399–405. doi: 10.1016/s0006-291x(02)03065-6. [http://dx.doi.org/10.1016/S0006-291X(02)03065-6]. [PMID: 12565874]. [DOI] [PubMed] [Google Scholar]
- 30.Uehara Y., Shimizu H., Ohtani K., Sato N., Mori M. Hypothalamic corticotropin-releasing hormone is a mediator of the anorexigenic effect of leptin. Diabetes. 1998;47(6):890–893. doi: 10.2337/diabetes.47.6.890. [http:// dx.doi.org/10.2337/diabetes.47.6.890]. [PMID: 9604864]. [DOI] [PubMed] [Google Scholar]
- 31.Brubaker P.L., Anini Y. Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can. J. Physiol. Pharmacol. 2003;81(11):1005–1012. doi: 10.1139/y03-107. [http:// dx.doi.org/10.1139/y03-107]. [PMID: 14719035]. [DOI] [PubMed] [Google Scholar]
- 32.Scott M.M., Williams K.W., Rossi J., Lee C.E., Elmquist J.K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 2011;121(6):2413–2421. doi: 10.1172/JCI43703. [http://dx.doi.org/10.1172/JCI43703]. [PMID: 21606595]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMinn J.E., Sindelar D.K., Havel P.J., Schwartz M.W. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology. 2000;141(12):4442–4448. doi: 10.1210/endo.141.12.7815. [http://dx. doi.org/10.1210/endo.141.12.7815]. [PMID: 11108253]. [DOI] [PubMed] [Google Scholar]
- 34.Stubbs J., Ferres S., Horgan G. Energy density of foods: effects on energy intake. Crit. Rev. Food Sci. Nutr. 2000;40(6):481–515. doi: 10.1080/10408690091189248. [http://dx.doi.org/10.1080/10408690091189248]. [PMID: 11186237]. [DOI] [PubMed] [Google Scholar]
- 35.Geliebter A., Schachter S., Lohmann-Walter C., Feldman H., Hashim S.A. Reduced stomach capacity in obese subjects after dieting. Am. J. Clin. Nutr. 1996;63(2):170–173. doi: 10.1093/ajcn/63.2.170. [http://dx.doi.org/ 10.1093/ajcn/63.2.170]. [PMID: 8561056]. [DOI] [PubMed] [Google Scholar]
- 36.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F., Abraham J.P., Abu-Rmeileh N.M., Achoki T., AlBuhairan F.S., Alemu Z.A., Alfonso R., Ali M.K., Ali R., Guzman N.A., Ammar W., Anwari P., Banerjee A., Barquera S., Basu S., Bennett D.A., Bhutta Z., Blore J., Cabral N., Nonato I.C., Chang J.C., Chowdhury R., Courville K.J., Criqui M.H., Cundiff D.K., Dabhadkar K.C., Dandona L., Davis A., Dayama A., Dharmaratne S.D., Ding E.L., Durrani A.M., Esteghamati A., Farzadfar F., Fay D.F., Feigin V.L., Flaxman A., Forouzanfar M.H., Goto A., Green M.A., Gupta R., Hafezi-Nejad N., Hankey G.J., Harewood H.C., Havmoeller R., Hay S., Hernandez L., Husseini A., Idrisov B.T., Ikeda N., Islami F., Jahangir E., Jassal S.K., Jee S.H., Jeffreys M., Jonas J.B., Kabagambe E.K., Khalifa S.E., Kengne A.P., Khader Y.S., Khang Y.H., Kim D., Kimokoti R.W., Kinge J.M., Kokubo Y., Kosen S., Kwan G., Lai T., Leinsalu M., Li Y., Liang X., Liu S., Logroscino G., Lotufo P.A., Lu Y., Ma J., Mainoo N.K., Mensah G.A., Merriman T.R., Mokdad A.H., Moschandreas J., Naghavi M., Naheed A., Nand D., Narayan K.M., Nelson E.L., Neuhouser M.L., Nisar M.I., Ohkubo T., Oti S.O., Pedroza A., Prabhakaran D., Roy N., Sampson U., Seo H., Sepanlou S.G., Shibuya K., Shiri R., Shiue I., Singh G.M., Singh J.A., Skirbekk V., Stapelberg N.J., Sturua L., Sykes B.L., Tobias M., Tran B.X., Trasande L., Toyoshima H., van de Vijver S., Vasankari T.J., Veerman J.L., Velasquez-Melendez G., Vlassov V.V., Vollset S.E., Vos T., Wang C., Wang X., Weiderpass E., Werdecker A., Wright J.L., Yang Y.C., Yatsuya H., Yoon J., Yoon S.J., Zhao Y., Zhou M., Zhu S., Lopez A.D., Murray C.J., Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [http://dx.doi.org/10.1016/ S0140-6736(14)60460-8]. [PMID: 24880830]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H-L. An archaeological and historical account of cannabis in China. Econ. Bot. 1973;28(4):437–448. [http://dx.doi.org/10. 1007/BF02862859]. [Google Scholar]
- 38.Touw M. The religious and medicinal uses of Cannabis in China, India and Tibet. J. Psychoactive Drugs. 1981;13(1):23–34. doi: 10.1080/02791072.1981.10471447. [http:// dx.doi.org/10.1080/02791072.1981.10471447]. [PMID: 7024492]. [DOI] [PubMed] [Google Scholar]
- 39.Mechoulam R., Gaoni Y. A total synthesis of dl-delta-1-Tetrahydrocannabinol, the active constituent of hashish. J. Am. Chem. Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [http://dx.doi.org/10.1021/ ja01092a065]. [PMID: 14324315]. [DOI] [PubMed] [Google Scholar]
- 40.Pertwee R.G. The pharmacology of cannabinoid receptors and their ligands: an overview. Int. J. Obes. 2006;30(Suppl 1)(S1):S13–S18. doi: 10.1038/sj.ijo.0803272. [http://dx.doi.org/10.1038/sj.ijo.0803272] [DOI] [PubMed] [Google Scholar]
- 41.Elphick M.R., Egertová M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb. Exp. Pharmacol. 2005;(168):283–297. doi: 10.1007/3-540-26573-2_9. [http://dx.doi.org/10.1007/3-540-26573-2_9]. [PMID: 16596778]. [DOI] [PubMed] [Google Scholar]
- 42.Elphick M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1607):3201–3215. doi: 10.1098/rstb.2011.0394. [http://dx.doi.org/10.1098/rstb. 2011.0394]. [PMID: 23108540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McPartland J.M., Matias I., Di Marzo V., Glass M. Evolutionary origins of the endocannabinoid system. Gene. 2006;370:64–74. doi: 10.1016/j.gene.2005.11.004. [http://dx.doi.org/10.1016/j.gene.2005.11.004]. [PMID: 16434153]. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Wang L., Harvey-White J., Huang B.X., Kim H.Y., Luquet S., Palmiter R.D., Krystal G., Rai R., Mahadevan A., Razdan R.K., Kunos G. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54(1):1–7. doi: 10.1016/j.neuropharm.2007.05.020. [http://dx.doi.org/10.1016/j.neuropharm.2007.05.020]. [PMID: 17631919]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R., Pertwee R.G., Griffin G., Bayewitch M., Barg J., Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [http://dx.doi.org/10.1016/0006-2952(95)00109-D]. [PMID: 7605349]. [DOI] [PubMed] [Google Scholar]
- 46.Mechoulam R., Ben Shabat S., Hanus L., Fride E., Vogel Z., Bayewitch M., Sulcova A.E. Endogenous cannabinoid ligands--chemical and biological studies. J. Lipid Mediat. Cell Signal. 1996;14(1-3):45–49. doi: 10.1016/0929-7855(96)01507-6. [http://dx.doi.org/10.1016/0929-7855(96) 01507-6]. [PMID: 8906544]. [DOI] [PubMed] [Google Scholar]
- 47.Fu J., Bottegoni G., Sasso O., Bertorelli R., Rocchia W., Masetti M., Guijarro A., Lodola A., Armirotti A., Garau G., Bandiera T., Reggiani A., Mor M., Cavalli A., Piomelli D. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat. Neurosci. 2011;15(1):64–69. doi: 10.1038/nn.2986. [http://dx.doi.org/10. 1038/nn.2986]. [PMID: 22101642]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marsicano G., Chaouloff F. Moving bliss: a new anandamide transporter. Nat. Neurosci. 2011;15(1):5–6. doi: 10.1038/nn.3011. [http://dx.doi.org/10. 1038/nn.3011]. [PMID: 22193249]. [DOI] [PubMed] [Google Scholar]
- 49.Beltramo M., Stella N., Calignano A., Lin S.Y., Makriyannis A., Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277(5329):1094–1097. doi: 10.1126/science.277.5329.1094. [http://dx.doi.org/10.1126/science.277.5329.1094]. [PMID: 9262477]. [DOI] [PubMed] [Google Scholar]
- 50.Mechoulam R., Ben S.S., Hanus L., Fride E., Vogel Z., Bayewitch M., Sulcova A.E. Endogenous cannabinoid ligands--chemical and biological studies. J. Lipid Mediat. Cell Signal. 1996;14(1-3):45–49. doi: 10.1016/0929-7855(96)01507-6. [http://dx.doi.org/10.1016/0929-7855(96) 01507-6]. [PMID: 8906544]. [DOI] [PubMed] [Google Scholar]
- 51.Tóth A., Blumberg P.M., Boczán J. Anandamide and the vanilloid receptor (TRPV1). Vitam. Horm. 2009;81:389–419. doi: 10.1016/S0083-6729(09)81015-7. [http:// dx.doi.org/10.1016/S0083-6729(09)81015-7]. [PMID: 19647120]. [DOI] [PubMed] [Google Scholar]
- 52.Marichal-Cancino B.A., Fajardo-Valdez A., Ruiz-Contreras A.E., Mendez-Díaz M., Prospero-García O. Advances in the physiology of GPR55 in the central nervous system. Curr. Neuropharmacol. 2017;15(5):771–778. doi: 10.2174/1570159X14666160729155441. [http://dx.doi.org/10.2174/1570159X146661 60729155441]. [PMID: 27488130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McHugh D., Page J., Dunn E., Bradshaw H.B.Δ. (9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012;165(8):2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [http:// dx.doi.org/10.1111/j.1476-5381.2011.01497.x]. [PMID: 21595653]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borrelli F., Izzo A.A. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23(1):33–49. doi: 10.1016/j.beem.2008.10.003. [http://dx.doi.org/10.1016/j.beem.2008.10.003]. [PMID: 19285259]. [DOI] [PubMed] [Google Scholar]
- 55.O’Sullivan S.E., Kendall D.A. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215(8):611–616. doi: 10.1016/j.imbio.2009.09.007. [http://dx.doi.org/10.1016/j.imbio.2009.09.007]. [PMID: 19833407]. [DOI] [PubMed] [Google Scholar]
- 56.Laun A.S., Song Z.H. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem. Biophys. Res. Commun. 2017;490(1):17–21. doi: 10.1016/j.bbrc.2017.05.165. [http://dx.doi.org/10.1016/j.bbrc.2017.05.165]. [PMID: 28571738]. [DOI] [PubMed] [Google Scholar]
- 57.Fonseca B.M., Costa M.A., Almada M., Correia-da-Silva G., Teixeira N.A. Endogenous cannabinoids revisited: a biochemistry perspective. Prostaglandins Other Lipid Mediat. 2013;102-103:13–30. doi: 10.1016/j.prostaglandins.2013.02.002. [http://dx.doi.org/10.1016/j.prostaglandins.2013.02.002]. [PMID: 23474290]. [DOI] [PubMed] [Google Scholar]
- 58.Fride E. Multiple roles for the endocannabinoid system during the earliest stages of life: pre- and postnatal development. J. Neuroendocrinol. 2008;20(Suppl. 1):75–81. doi: 10.1111/j.1365-2826.2008.01670.x. [http://dx.doi.org/10.1111/ j.1365-2826.2008.01670.x]. [PMID: 18426504]. [DOI] [PubMed] [Google Scholar]
- 59.Fride E., Ginzburg Y., Breuer A., Bisogno T., Di Marzo V., Mechoulam R. Critical role of the endogenous cannabinoid system in mouse pup suckling and growth. Eur. J. Pharmacol. 2001;419(2-3):207–214. doi: 10.1016/s0014-2999(01)00953-0. [http://dx.doi.org/10.1016/S0014-2999(01) 00953-0]. [PMID: 11426843]. [DOI] [PubMed] [Google Scholar]
- 60.Berrendero F., Sepe N., Ramos J.A., Di Marzo V., Fernández-Ruiz J.J. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33(3):181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [http://dx.doi.org/10.1002/(SICI)1098-2396 (19990901)33:3<181:AID-SYN3>3.0.CO;2-R]. [PMID: 10420166]. [DOI] [PubMed] [Google Scholar]
- 61.Fride E., Foox A., Rosenberg E., Faigenboim M., Cohen V., Barda L., Blau H., Mechoulam R. Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: evidence for a “CB3” receptor. Eur. J. Pharmacol. 2003;461(1):27–34. doi: 10.1016/s0014-2999(03)01295-0. [http:// dx.doi.org/10.1016/S0014-2999(03)01295-0]. [PMID: 12568912]. [DOI] [PubMed] [Google Scholar]
- 62.Fride E., Braun H., Matan H., Steinberg S., Reggio P.H., Seltzman H.H. Inhibition of milk ingestion and growth after administration of a neutral cannabinoid CB1 receptor antagonist on the first postnatal day in the mouse. Pediatr. Res. 2007;62(5):533–536. doi: 10.1203/PDR.0b013e3181559d42. [http://dx.doi.org/10.1203/PDR.0b013e3181559d42]. [PMID: 17805201]. [DOI] [PubMed] [Google Scholar]
- 63.Deveaux V., Cadoudal T., Ichigotani Y., Teixeira-Clerc F., Louvet A., Manin S., Nhieu J.T., Belot M.P., Zimmer A., Even P., Cani P.D., Knauf C., Burcelin R., Bertola A., Le Marchand-Brustel Y., Gual P., Mallat A., Lotersztajn S. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS One. 2009;4(6):e5844. doi: 10.1371/journal.pone.0005844. [http:// dx.doi.org/10.1371/journal.pone.0005844]. [PMID: 19513120]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verty A.N., Stefanidis A., McAinch A.J., Hryciw D.H., Oldfield B. Anti-obesity effect of the CB2 receptor agonist JWH-015 in diet-induced obese mice. PLoS One. 2015;10(11):e0140592. doi: 10.1371/journal.pone.0140592. [http://dx.doi.org/10.1371/journal.pone.0140592]. [PMID: 26588700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lauckner J.E., Jensen J.B., Chen H-Y., Lu H-C., Hille B., Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA. 2008;105(7):2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abel E.L. Cannabis: effects on hunger and thirst. Behav. Biol. 1975;15(3):255–281. doi: 10.1016/s0091-6773(75)91684-3. [http://dx.doi.org/10.1016/S0091-6773(75) 91684-3]. [PMID: 1106391]. [DOI] [PubMed] [Google Scholar]
- 67.Bouquet J. Cannabis (concluded). Bull. Narc. 1951;3(1):22–45. [Google Scholar]
- 68.Chopra R.N., Brevet C., Chopra G.S. Present position of hemp drug addiction in India. Addiction. 1940;38(2):71–74. [http://dx. doi.org/10.1111/j.1360-0443.1940.tb05405.x]. [Google Scholar]
- 69.Foltin R.W., Fischman M.W., Byrne M.F. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11(1):1–14. doi: 10.1016/s0195-6663(88)80017-5. [http://dx. doi.org/10.1016/S0195-6663(88)80017-5]. [PMID: 3228283]. [DOI] [PubMed] [Google Scholar]
- 70.Bhargava H.N. Potential therapeutic applications of naturally occurring and synthetic cannabinoids. Gen. Pharmacol. 1978;9(4):195–213. doi: 10.1016/0306-3623(78)90037-x. [http://dx.doi.org/10.1016/0306-3623(78)90037-X]. [PMID: 680553]. [DOI] [PubMed] [Google Scholar]
- 71.Koch M., Varela L., Kim J.G., Kim J.D., Hernández-Nuño F., Simonds S.E., Castorena C.M., Vianna C.R., Elmquist J.K., Morozov Y.M., Rakic P., Bechmann I., Cowley M.A., Szigeti-Buck K., Dietrich M.O., Gao X.B., Diano S., Horvath T.L. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519(7541):45–50. doi: 10.1038/nature14260. [http://dx.doi.org/10.1038/ nature14260]. [PMID: 25707796]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lage R., Parisi C., Seoane-Collazo P., Fernø J., Mazza R., Bosch F., Seoane L.M., Nogueiras R., Diéguez C., Quarta C., López M. Lack of hypophagia in CB1 null mice is associated to decreased hypothalamic POMC and CART expression. Int. J. Neuropsychopharmacol. 2015;18(9):1–6. doi: 10.1093/ijnp/pyv011. [http://dx.doi.org/10.1093/ ijnp/pyv011]. [PMID: 25655433]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gamage T.F., Ignatowska-Jankowska B.M., Wiley J.L., Abdelrahman M., Trembleau L., Greig I.R., Thakur G.A., Tichkule R., Poklis J., Ross R.A., Pertwee R.G., Lichtman A.H. In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav. Pharmacol. 2014;25(2):182–185. doi: 10.1097/FBP.0000000000000027. [http:// dx.doi.org/10.1097/FBP.0000000000000027]. [PMID: 24603340]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madsen A.N., Jelsing J., van de Wall E.H., Vrang N., Larsen P.J., Schwartz G.J. Rimonabant induced anorexia in rodents is not mediated by vagal or sympathetic gut afferents. Neurosci. Lett. 2009;449(1):20–23. doi: 10.1016/j.neulet.2008.10.001. [http://dx.doi.org/10.1016/j.neulet.2008.10. 001]. [PMID: 18926875]. [DOI] [PubMed] [Google Scholar]
- 75.Pi-Sunyer F.X., Aronne L.J., Heshmati H.M., Devin J., Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295(7):761–775. doi: 10.1001/jama.295.7.761. [http://dx.doi.org/10.1001/jama.295.7.761]. [PMID: 16478899]. [DOI] [PubMed] [Google Scholar]
- 76.Van Gaal L.F., Rissanen A.M., Scheen A.J., Ziegler O., Rössner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365(9468):1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [http://dx.doi.org/10.1016/S0140-6736(05) 66374-X]. [PMID: 15836887]. [DOI] [PubMed] [Google Scholar]
- 77.Micale V.T.K., Kučerová J., Drago F. Role of the endocannabinoid system in depression: From preclinical to clinical evidence. In: P. Campolongo, Liana Fattore., editors. Cannabinoid Modulation of Emotion, Memory, and Motivation. New York: Springer, New York: 2015. pp. 97–129. [Google Scholar]
- 78.Micale V., Di Marzo V., Sulcova A., Wotjak C.T., Drago F. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol. Ther. 2013;138(1):18–37. doi: 10.1016/j.pharmthera.2012.12.002. [http://dx. doi.org/10.1016/j.pharmthera.2012.12.002]. [PMID: 23261685]. [DOI] [PubMed] [Google Scholar]
- 79.Richey J.M., Woolcott O. Re-visiting the endocannabinoid system and Its therapeutic potential in obesity and associated diseases. Curr. Diab. Rep. 2017;17(10):99. doi: 10.1007/s11892-017-0924-x. [http://dx.doi.org/10.1007/ s11892-017-0924-x]. [PMID: 28913816]. [DOI] [PubMed] [Google Scholar]
- 80.Gadde K.M., Allison D.B. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation. 2006;114(9):974–984. doi: 10.1161/CIRCULATIONAHA.105.596130. [http://dx.doi.org/10.1161/ CIRCULATIONAHA.105.596130]. [PMID: 16940206]. [DOI] [PubMed] [Google Scholar]
- 81.Hill T.D.M., Cascio M.-G., Romano B., Duncan M., Pertwee R.G., Williams C.M., Whalley B.J., Hill A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor- independent mechanism. 2013;170(3):679–692. doi: 10.1111/bph.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G., Mackie K., Mechoulam R., Ross R.A. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [http://dx.doi.org/10.1124/pr.110.003004]. [PMID: 21079038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Sumida H., Cyster J.G. GPR18 is required for a normal CD8αα intestinal intraepithelial lymphocyte compartment. J. Exp. Med. 2014;211(12):2351–2359. doi: 10.1084/jem.20140646. [http://dx.doi.org/10.1084/jem. 20140646]. [PMID: 25348153]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker A.M., Callahan D.J., Richner J.M., Choi J. [Google Scholar]; DiPersio J.F., Diamond M.S., Bhattacharya D. GPR18 controls reconstitution of mouse small intestine intraepithelial lymphocytes following bone marrow transplantation. PLoS One. 2015;10(7):e0133854. doi: 10.1371/journal.pone.0133854. [http://dx.doi.org/10.1371/journal.pone.0133854]. [PMID: 26197390]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meadows A., Lee J.H., Wu C.S., Wei Q., Pradhan G., Yafi M., Lu H.C., Sun Y. Deletion of G-protein-coupled receptor 55 promotes obesity by reducing physical activity. Int. J. Obes. 2016;40(3):417–424. doi: 10.1038/ijo.2015.209. [http://dx.doi.org/10.1038/ijo.2015.209]. [PMID: 26447738]. [DOI] [PubMed] [Google Scholar]
- 86.Romero-Zerbo S.Y., Rafacho A., Díaz-Arteaga A., Suárez J., Quesada I., Imbernon M., Ross R.A., Dieguez C., Rodríguez de Fonseca F., Nogueiras R., Nadal A., Bermúdez-Silva F.J. A role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J. Endocrinol. 2011;211(2):177–185. doi: 10.1530/JOE-11-0166. [http://dx. doi.org/10.1530/JOE-11-0166]. [PMID: 21885477]. [DOI] [PubMed] [Google Scholar]
- 87.Panaro B.L., Flock G.B., Campbell J.E., Beaudry J.L., Cao X., Drucker D.J. β-Cell Inactivation of Gpr119 Unmasks Incretin Dependence of GPR119-Mediated Glucoregulation. Diabetes. 2017;66(6):1626–1635. doi: 10.2337/db17-0017. [http://dx.doi.org/10.2337/db17-0017]. [PMID: 28254842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lan H., Vassileva G., Corona A., Liu L., Baker H., Golovko A., Abbondanzo S.J., Hu W., Yang S., Ning Y., Del Vecchio R.A., Poulet F., Laverty M., Gustafson E.L., Hedrick J.A., Kowalski T.J. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J. Endocrinol. 2009;201(2):219–230. doi: 10.1677/JOE-08-0453. [http://dx.doi.org/10.1677/ JOE-08-0453]. [PMID: 19282326]. [DOI] [PubMed] [Google Scholar]
- 89.Cota D., Marsicano G., Tschöp M., Grübler Y., Flachskamm C., Schubert M., Auer D., Yassouridis A., Thöne-Reineke C., Ortmann S., Tomassoni F., Cervino C., Nisoli E., Linthorst A.C., Pasquali R., Lutz B., Stalla G.K., Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. 2003;112(3):423–431. doi: 10.1172/JCI17725. [http://dx.doi.org/10.1172/JCI17725]. [PMID: 12897210]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keefer S.E., Petrovich G.D. Distinct recruitment of basolateral amygdala-medial prefrontal cortex pathways across Pavlovian appetitive conditioning. Neurobiol. Learn. Mem. 2017;141:27–32. doi: 10.1016/j.nlm.2017.03.006. [http://dx.doi.org/10.1016/j.nlm.2017.03.006]. [PMID: 28288832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viveros M.P., de Fonseca F.R., Bermudez-Silva F.J., McPartland J.M. Critical role of the endocannabinoid system in the regulation of food intake and energy metabolism, with phylogenetic, developmental, and pathophysiological implications. Endocr. Metab. Immune Disord. Drug Targets. 2008;8(3):220–230. doi: 10.2174/187153008785700082. [http:// dx.doi.org/10.2174/187153008785700082]. [PMID: 18782018]. [DOI] [PubMed] [Google Scholar]
- 92.Bellocchio L., Lafenêtre P., Cannich A., Cota D., Puente N., Grandes P., Chaouloff F., Piazza P.V., Marsicano G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010;13(3):281–283. doi: 10.1038/nn.2494. [http://dx.doi.org/10.1038/ nn.2494]. [PMID: 20139974]. [DOI] [PubMed] [Google Scholar]
- 93.Wiley J.L., Burston J.J., Leggett D.C., Alekseeva O.O., Razdan R.K., Mahadevan A., Martin B.R. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br. J. Pharmacol. 2005;145(3):293–300. doi: 10.1038/sj.bjp.0706157. [http://dx.doi.org/10.1038/sj.bjp.0706157]. [PMID: 15778743]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCallum R.W., Soykan I., Sridhar K.R., Ricci D.A., Lange R.C., Plankey M.W. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment. Pharmacol. Ther. 1999;13(1):77–80. doi: 10.1046/j.1365-2036.1999.00441.x. [http:// dx.doi.org/10.1046/j.1365-2036.1999.00441.x]. [PMID: 9892882]. [DOI] [PubMed] [Google Scholar]
- 95.Bateman D.N. Delta-9-tetrahydrocannabinol and gastric emptying. Br. J. Clin. Pharmacol. 1983;15(6):749–751. doi: 10.1111/j.1365-2125.1983.tb01561.x. [http://dx.doi.org/ 10.1111/j.1365-2125.1983.tb01561.x]. [PMID: 6307330]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fride E., Bregman T., Kirkham T.C. Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood. Exp. Biol. Med. (Maywood) 2005;230(4):225–234. doi: 10.1177/153537020523000401. [http://dx.doi.org/ 10.1177/153537020523000401]. [PMID: 15792943]. [DOI] [PubMed] [Google Scholar]
- 97.Morales P., Reggio P.H., Jagerovic N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol. 2017;8:422. doi: 10.3389/fphar.2017.00422. [http://dx.doi.org/10.3389/fphar. 2017.00422]. [PMID: 28701957]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schicho R., Storr M. A potential role for GPR55 in gastrointestinal functions. Curr. Opin. Pharmacol. 2012;12(6):653–658. doi: 10.1016/j.coph.2012.09.009. [http://dx.doi.org/10.1016/j.coph.2012.09.009]. [PMID: 23063456]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laprairie R.B., Bagher A.M., Kelly M.E., Denovan-Wright E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015;172(20):4790–4805. doi: 10.1111/bph.13250. [http://dx.doi.org/10.1111/bph.13250]. [PMID: 26218440]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morales P., Jagerovic N. Advances towards the discovery of GPR55 ligands. Curr. Med. Chem. 2016;23(20):2087–2100. doi: 10.2174/0929867323666160425113836. [http://dx.doi.org/10.2174/0929867323666160425113836]. [PMID: 27109575]. [DOI] [PubMed] [Google Scholar]
- 101.Laprairie R.B., Bagher A.M., Kelly M.E., Dupré D.J., Denovan-Wright E.M. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J. Biol. Chem. 2014;289(36):24845–24862. doi: 10.1074/jbc.M114.557025. [http://dx.doi.org/10.1074/jbc.M114.557025]. [PMID: 25037227]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tham M., Yilmaz O., Alaverdashvili M., Kelly M.E.M., Denovan-Wright E.M., Laprairie R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. doi: 10.1111/bph.14440. In press. [PMID: 29981240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martínez-Pinilla E., Varani K., Reyes-Resina I., Angelats E., Vincenzi F., Ferreiro-Vera C., Oyarzabal J., Canela E.I., Lanciego J.L., Nadal X., Navarro G., Borea P.A., Franco R. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front. Pharmacol. 2017;8:744. doi: 10.3389/fphar.2017.00744. [http://dx.doi.org/10.3389/fphar.2017.00744]. [PMID: 29109685]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajaraman G., Simcocks A., Hryciw D.H., Hutchinson D.S., McAinch A.J. G protein coupled receptor 18: A potential role for endocannabinoid signaling in metabolic dysfunction. Mol. Nutr. Food Res. 2016;60(1):92–102. doi: 10.1002/mnfr.201500449. [http://dx.doi.org/10.1002/mnfr. 201500449]. [PMID: 26337420]. [DOI] [PubMed] [Google Scholar]
- 105.Ross R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009;30(3):156–163. doi: 10.1016/j.tips.2008.12.004. [http://dx.doi.org/10.1016/ j.tips.2008.12.004]. [PMID: 19233486]. [DOI] [PubMed] [Google Scholar]
- 106.Brown A.J. Novel cannabinoid receptors. Br. J. Pharmacol. 2007;152(5):567–575. doi: 10.1038/sj.bjp.0707481. [http://dx.doi.org/10.1038/sj.bjp.0707481]. [PMID: 17906678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Godlewski G., Offertáler L., Wagner J.A., Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009;89(3-4):105–111. doi: 10.1016/j.prostaglandins.2009.07.001. [http://dx.doi.org/10.1016/ j.prostaglandins.2009.07.001]. [PMID: 19615459]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kola B., Farkas I., Christ-Crain M., Wittmann G., Lolli F., Amin F., Harvey-White J., Liposits Z., Kunos G., Grossman A.B., Fekete C., Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3(3):e1797. doi: 10.1371/journal.pone.0001797. [http://dx.doi.org/10.1371/ journal.pone.0001797]. [PMID: 18335063]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z., Fezza F., Miura G.I., Palmiter R.D., Sugiura T., Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–825. doi: 10.1038/35071088. [http://dx.doi.org/ 10.1038/35071088]. [PMID: 11298451]. [DOI] [PubMed] [Google Scholar]
- 110.Jo Y.H., Chen Y.J., Chua S.C., Jr, Talmage D.A., Role L.W. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48(6):1055–1066. doi: 10.1016/j.neuron.2005.10.021. [http:// dx.doi.org/10.1016/j.neuron.2005.10.021]. [PMID: 16364907]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sánchez-Fuentes A., Marichal-Cancino B.A., Méndez-Díaz M., Becerril-Meléndez A.L., Ruiz-Contreras A.E., Prospéro-Garcia O. mGluR1/5 activation in the lateral hypothalamus increases food intake via the endocannabinoid system. Neurosci. Lett. 2016;631:104–108. doi: 10.1016/j.neulet.2016.08.020. [http://dx.doi.org/10.1016/j.neulet.2016.08.020]. [PMID: 27542344]. [DOI] [PubMed] [Google Scholar]
- 112.Gómez R., Navarro M., Ferrer B., Trigo J.M., Bilbao A., Del Arco I., Cippitelli A., Nava F., Piomelli D., Rodríguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J. Neurosci. 2002;22(21):9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [http://dx.doi.org/10.1523/JNEUROSCI.22-21-09612.2002]. [PMID: 12417686]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petersen G., Sørensen C., Schmid P.C., Artmann A., Tang-Christensen M., Hansen S.H., Larsen P.J., Schmid H.H., Hansen H.S. Intestinal levels of anandamide and oleoylethanolamide in food-deprived rats are regulated through their precursors. Biochim. Biophys. Acta. 2006;1761(2):143–150. doi: 10.1016/j.bbalip.2005.12.011. [http://dx.doi.org/10.1016/ j.bbalip.2005.12.011]. [PMID: 16478679]. [DOI] [PubMed] [Google Scholar]
- 114.Burdyga G., Lal S., Varro A., Dimaline R., Thompson D.G., Dockray G.J. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J. Neurosci. 2004;24(11):2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [http://dx.doi.org/10.1523/JNEUROSCI.5404-03.2004]. [PMID: 15028763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bouaboula M., Perrachon S., Milligan L., Canat X., Rinaldi-Carmona M., Portier M., Barth F., Calandra B., Pecceu F., Lupker J., Maffrand J.P., Le Fur G., Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272(35):22330–22339. doi: 10.1074/jbc.272.35.22330. [http://dx.doi. org/10.1074/jbc.272.35.22330]. [PMID: 9268384]. [DOI] [PubMed] [Google Scholar]
- 116.Jamshidi N., Taylor D.A. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br. J. Pharmacol. 2001;134(6):1151–1154. doi: 10.1038/sj.bjp.0704379. [http://dx.doi.org/10.1038/ sj.bjp.0704379]. [PMID: 11704633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cardinal P., Bellocchio L., Clark S., Cannich A., Klugmann M., Lutz B., Marsicano G., Cota D. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology. 2012;153(9):4136–4143. doi: 10.1210/en.2012-1405. [http://dx.doi.org/10.1210/en.2012-1405]. [PMID: 22778221]. [DOI] [PubMed] [Google Scholar]
- 118.Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T., Szanda G., Mukhopadhyay B., Chedester L., Liow J.S., Innis R.B., Cheng K., Rice K.C., Deschamps J.R., Chorvat R.J., McElroy J.F., Kunos G. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–179. doi: 10.1016/j.cmet.2012.07.002. [http://dx.doi.org/10.1016/j.cmet.2012. 07.002]. [PMID: 22841573]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tam J., Vemuri V.K., Liu J., Bátkai S., Mukhopadhyay B., Godlewski G., Osei-Hyiaman D., Ohnuma S., Ambudkar S.V., Pickel J., Makriyannis A., Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Invest. 2010;120(8):2953–2966. doi: 10.1172/JCI42551. [http://dx. doi.org/10.1172/JCI42551]. [PMID: 20664173]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nogueiras R., Veyrat-Durebex C., Suchanek P.M., Klein M., Tschöp J., Caldwell C., Woods S.C., Wittmann G., Watanabe M., Liposits Z., Fekete C., Reizes O., Rohner-Jeanrenaud F., Tschöp M.H. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57(11):2977–2991. doi: 10.2337/db08-0161. [http://dx.doi.org/10. 2337/db08-0161]. [PMID: 18716045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Després J.P., Golay A., Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 2005;353(20):2121–2134. doi: 10.1056/NEJMoa044537. [http://dx.doi.org/10. 1056/NEJMoa044537]. [PMID: 16291982]. [DOI] [PubMed] [Google Scholar]
- 122.Van Gaal L., Pi-Sunyer X., Després J.P., McCarthy C., Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31(Suppl. 2):S229–S240. doi: 10.2337/dc08-s258. [http://dx.doi.org/10. 2337/dc08-s258]. [PMID: 18227491]. [DOI] [PubMed] [Google Scholar]
- 123.Naughton S.S., Mathai M.L., Hryciw D.H., McAinch A.J. Fatty Acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol. 2013;2013:361895. doi: 10.1155/2013/361895. [http://dx.doi.org/10.1155/2013/361895]. [PMID: 23762050]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alvheim A.R., Malde M.K., Osei-Hyiaman D., Lin Y.H., Pawlosky R.J., Madsen L., Kristiansen K., Frøyland L., Hibbeln J.R. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 2012;20(10):1984–1994. doi: 10.1038/oby.2012.38. [http://dx.doi.org/10.1038/oby.2012.38]. [PMID: 22334255]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thabuis C., Destaillats F., Landrier J.F., Tissot-Favre D., Martin J.C. Analysis of gene expression pattern reveals potential targets of dietary oleoylethanolamide in reducing body fat gain in C3H mice. J. Nutr. Biochem. 2010;21(10):922–928. doi: 10.1016/j.jnutbio.2009.07.006. [http://dx.doi.org/ 10.1016/j.jnutbio.2009.07.006]. [PMID: 19954948]. [DOI] [PubMed] [Google Scholar]
- 126.Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L., Soria-Gómez E., Matias I., Delamarre A., Metna-Laurent M., Cannich A., Hebert-Chatelain E., Mulle C., Ortega-Gutiérrez S., Martín-Fontecha M., Klugmann M., Guggenhuber S., Lutz B., Gertsch J., Chaouloff F., López-Rodríguez M.L., Grandes P., Rossignol R., Marsicano G. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012;15(4):558–564. doi: 10.1038/nn.3053. [http://dx.doi.org/10.1038/nn.3053]. [PMID: 22388959]. [DOI] [PubMed] [Google Scholar]
- 127.Djeungoue-Petga M.-A., Hebert-Chatelain E. Linking mitochondria and synaptic transmission: The CB1 receptor. 2017;39(12) doi: 10.1002/bies.201700126. 1700126. [DOI] [PubMed] [Google Scholar]
- 128.Mendizabal-Zubiaga J., Melser S., Bénard G., Ramos A., Reguero L., Arrabal S., Elezgarai I., Gerrikagoitia I., Suarez J., Rodríguez De Fonseca F., Puente N., Marsicano G., Grandes P. Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 2016;7:476. doi: 10.3389/fphys.2016.00476. [http://dx.doi.org/10.3389/fphys.2016. 00476]. [PMID: 27826249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruiz de Azua I., Mancini G., Srivastava R.K., Rey A.A., Cardinal P., Tedesco L., Zingaretti C.M., Sassmann A., Quarta C., Schwitter C., Conrad A., Wettschureck N., Vemuri V.K., Makriyannis A., Hartwig J., Mendez-Lago M., Bindila L., Monory K., Giordano A., Cinti S., Marsicano G., Offermanns S., Nisoli E., Pagotto U., Cota D., Lutz B. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Invest. 2017;127(11):4148–4162. doi: 10.1172/JCI83626. [http://dx.doi.org/10.1172/JCI83626]. [PMID: 29035280]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moreira F.A., Grieb M., Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: Focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23(1):133–144. doi: 10.1016/j.beem.2008.09.003. [http://dx.doi.org/10.1016/j.beem.2008. 09.003]. [PMID: 19285266]. [DOI] [PubMed] [Google Scholar]
- 131.Moreira F.A., Crippa J.A. The psychiatric side-effects of rimonabant. Br. J. Psychiatry. 2009;31(2):145–153. doi: 10.1590/s1516-44462009000200012. [http://dx.doi.org/10. 1590/S1516-44462009000200012]. [PMID: 19578688]. [DOI] [PubMed] [Google Scholar]
- 132.Pan C., Yoo H.J., Ho L.T. Perspectives of CB1 antagonist in treatment of obesity: Experience of RIO-Asia. J. Obes. 2011;2011:957268. doi: 10.1155/2011/957268. [http://dx.doi.org/10.1155/2011/957268]. [PMID: 21253513]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gantz I., Muraoka A., Yang Y.K., Samuelson L.C., Zimmerman E.M., Cook H., Yamada T. Cloning and chromosomal localization of a gene (GPR18) encoding a novel seven transmembrane receptor highly expressed in spleen and testis. Genomics. 1997;42(3):462–466. doi: 10.1006/geno.1997.4752. [http://dx.doi.org/10.1006/geno.1997.4752]. [PMID: 9205118]. [DOI] [PubMed] [Google Scholar]
- 134.Penumarti A., Abdel-Rahman A.A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J. Pharmacol. Exp. Ther. 2014;349(1):29–38. doi: 10.1124/jpet.113.209213. [http://dx.doi.org/10.1124/ jpet.113.209213]. [PMID: 24431468]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alexander S.P. So what do we call GPR18 now? Br. J. Pharmacol. 2012;165(8):2411–2413. doi: 10.1111/j.1476-5381.2011.01731.x. [http://dx.doi.org/10.1111/j.1476-5381.2011.01731.x]. [PMID: 22014123]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Finlay D.B., Joseph W.R., Grimsey N.L., Glass M. GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. PeerJ. 2016;4:e1835. doi: 10.7717/peerj.1835. [http:// dx.doi.org/10.7717/peerj.1835]. [PMID: 27018161]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Console-Bram L., Brailoiu E., Brailoiu G.C., Sharir H., Abood M.E. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br. J. Pharmacol. 2014;171(16):3908–3917. doi: 10.1111/bph.12746. [http://dx.doi.org/10.1111/bph.12746]. [PMID: 24762058]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pascoal L.B., Bombassaro B., Ramalho A.F., Coope A., Moura R.F., Correa-da-Silva F., Ignacio-Souza L., Razolli D., de Oliveira D., Catharino R., Velloso L.A. Resolvin RvD2 reduces hypothalamic inflammation and rescues mice from diet-induced obesity. J. Neuroinflammation. 2017;14(1):5. doi: 10.1186/s12974-016-0777-2. [http://dx.doi.org/ 10.1186/s12974-016-0777-2]. [PMID: 28086928]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Succar R., Mitchell V.A., Vaughan C.W. Actions of N-arachidonyl-glycine in a rat inflammatory pain model. Mol. Pain. 2007;3(24):24. doi: 10.1186/1744-8069-3-24. [PMID: 17727733]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takenouchi R., Inoue K., Kambe Y., Miyata A. N-arachidonoyl glycine induces macrophage apoptosis via GPR18. Biochem. Biophys. Res. Commun. 2012;418(2):366–371. doi: 10.1016/j.bbrc.2012.01.027. [http://dx.doi.org/10. 1016/j.bbrc.2012.01.027]. [PMID: 22266325]. [DOI] [PubMed] [Google Scholar]
- 141.Díaz-Arteaga A., Vázquez M.J., Vazquez-Martínez R., Pulido M.R., Suarez J., Velásquez D.A., López M., Ross R.A., de Fonseca F.R., Bermudez-Silva F.J., Malagón M.M., Diéguez C., Nogueiras R. The atypical cannabinoid O-1602 stimulates food intake and adiposity in rats. Diabetes Obes. Metab. 2012;14(3):234–243. doi: 10.1111/j.1463-1326.2011.01515.x. [http://dx.doi.org/10.1111/j.1463-1326.2011.01515.x]. [PMID: 21981246]. [DOI] [PubMed] [Google Scholar]
- 142.Slattery M.L., Lundgreen A., Wolff R.K. Dietary influence on MAPK-signaling pathways and risk of colon and rectal cancer. Nutr. Cancer. 2013;65(5):729–738. doi: 10.1080/01635581.2013.795599. [http://dx.doi.org/10.1080/ 01635581.2013.795599]. [PMID: 23859041]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Karpe F., Dickmann J.R., Frayn K.N. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441–2449. doi: 10.2337/db11-0425. [http://dx.doi.org/10.2337/db11-0425]. [PMID: 21948998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marichal-Cancino B.A., Fajardo-Valdez A., Ruiz-Contreras A.E., Méndez-Díaz M., Prospéro-García O. Possible role of hippocampal GPR55 in spatial learning and memory in rats. Acta Neurobiol. Exp. (Warsz.) 2018;78(1):41–50. [http://dx.doi.org/10.21307/ane-2018-001]. [PMID: 29694340]. [PubMed] [Google Scholar]
- 145.Marichal-Cancino B.A., Manrique-Maldonado G., Altamirano-Espinoza A.H., Ruiz-Salinas I., González-Hernández A., Maassenvandenbrink A., Villalón C.M. Analysis of anandamide- and lysophosphatidylinositol-induced inhibition of the vasopressor responses produced by sympathetic stimulation or noradrenaline in pithed rats. Eur. J. Pharmacol. 2013;721(1-3):168–177. doi: 10.1016/j.ejphar.2013.09.039. [http:// dx.doi.org/10.1016/j.ejphar.2013.09.039]. [PMID: 24076186]. [DOI] [PubMed] [Google Scholar]
- 146.Marichal-Cancino B.A., Sánchez-Fuentes A., Méndez-Díaz M., Ruiz-Contreras A.E., Prospéro-García O. Blockade of GPR55 in the dorsolateral striatum impairs performance of rats in a T-maze paradigm. Behav. Pharmacol. 2016;27(4):393–396. doi: 10.1097/FBP.0000000000000185. [http://dx.doi. org/10.1097/FBP.0000000000000185]. [PMID: 26292188]. [DOI] [PubMed] [Google Scholar]
- 147.Bjursell M., Ryberg E., Wu T., Greasley P.J., Bohlooly-Y M., Hjorth S. Deletion of Gpr55 results in subtle effects on energy metabolism, motor activity and thermal pain sensation. PLoS One. 2016;11(12):e0167965. doi: 10.1371/journal.pone.0167965. [http://dx.doi.org/10.1371/journal.pone. 0167965]. [PMID: 27941994]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu B., Song S., Ruz-Maldonado I., Pingitore A., Huang G.C., Baker D., Jones P.M., Persaud S.J. GPR55-dependent stimulation of insulin secretion from isolated mouse and human islets of Langerhans. Diabetes Obes. Metab. 2016;18(12):1263–1273. doi: 10.1111/dom.12780. [http://dx.doi.org/10.1111/dom.12780]. [PMID: 27561953]. [DOI] [PubMed] [Google Scholar]
- 149.McKillop A.M., Moran B.M., Abdel-Wahab Y.H., Flatt P.R. Evaluation of the insulin releasing and antihyperglycaemic activities of GPR55 lipid agonists using clonal beta-cells, isolated pancreatic islets and mice. Br. J. Pharmacol. 2013;170(5):978–990. doi: 10.1111/bph.12356. [http://dx.doi.org/10.1111/bph.12356]. [PMID: 23992544]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ruz-Maldonado I., Pingitore A., Liu B., Atanes P., Huang G.C., Baker D., Alonso F.J., Bermúdez-Silva F.J., Persaud S.J. LH-21 and abnormal cannabidiol improve β-cell function in isolated human and mouse islets through GPR55-dependent and -independent signalling. Diabetes Obes. Metab. 2018;20(4):930–942. doi: 10.1111/dom.13180. [http://dx. doi.org/10.1111/dom.13180]. [PMID: 29205751]. [DOI] [PubMed] [Google Scholar]
- 151.Imbernon M., Whyte L., Diaz-Arteaga A., Russell W.R., Moreno N.R., Vazquez M.J., Gonzalez C.R., Díaz-Ruiz A., Lopez M., Malagón M.M., Ross R.A., Dieguez C., Nogueiras R. Regulation of GPR55 in rat white adipose tissue and serum LPI by nutritional status, gestation, gender and pituitary factors. Mol. Cell. Endocrinol. 2014;383(1-2):159–169. doi: 10.1016/j.mce.2013.12.011. [http://dx.doi.org/10.1016/ j.mce.2013.12.011]. [PMID: 24378736]. [DOI] [PubMed] [Google Scholar]
- 152.Moreno-Navarrete J.M., Catalán V., Whyte L., Díaz-Arteaga A., Vázquez-Martínez R., Rotellar F., Guzmán R., Gómez-Ambrosi J., Pulido M.R., Russell W.R., Imbernón M., Ross R.A., Malagón M.M., Dieguez C., Fernández-Real J.M., Frühbeck G., Nogueiras R. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61(2):281–291. doi: 10.2337/db11-0649. [http://dx.doi.org/10.2337/db11-0649]. [PMID: 22179809]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soga T., Ohishi T., Matsui T., Saito T., Matsumoto M., Takasaki J., Matsumoto S., Kamohara M., Hiyama H., Yoshida S., Momose K., Ueda Y., Matsushime H., Kobori M., Furuichi K. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005;326(4):744–751. doi: 10.1016/j.bbrc.2004.11.120. [http://dx.doi.org/10. 1016/j.bbrc.2004.11.120]. [PMID: 15607732]. [DOI] [PubMed] [Google Scholar]
- 154.Romero-Zerbo S.Y., Ruz-Maldonado I., Espinosa-Jiménez V., Rafacho A., Gómez-Conde A.I., Sánchez-Salido L., Cobo-Vuilleumier N., Gauthier B.R., Tinahones F.J., Persaud S.J., Bermúdez-Silva F.J. The cannabinoid ligand LH-21 reduces anxiety and improves glucose handling in diet-induced obese pre-diabetic mice. Sci. Rep. 2017;7(1):3946. doi: 10.1038/s41598-017-03292-w. [http://dx.doi.org/ 10.1038/s41598-017-03292-w]. [PMID: 28638091]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hansen H.S., Rosenkilde M.M., Holst J.J., Schwartz T.W. GPR119 as a fat sensor. Trends Pharmacol. Sci. 2012;33(7):374–381. doi: 10.1016/j.tips.2012.03.014. [http://dx.doi.org/10.1016/j.tips.2012.03.014]. [PMID: 22560300]. [DOI] [PubMed] [Google Scholar]
- 156.Chu Z.L., Jones R.M., He H., Carroll C., Gutierrez V., Lucman A., Moloney M., Gao H., Mondala H., Bagnol D., Unett D., Liang Y., Demarest K., Semple G., Behan D.P., Leonard J. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148(6):2601–2609. doi: 10.1210/en.2006-1608. [http://dx.doi.org/10.1210/ en.2006-1608]. [PMID: 17289847]. [DOI] [PubMed] [Google Scholar]
- 157.Sakamoto Y., Inoue H., Kawakami S., Miyawaki K., Miyamoto T., Mizuta K., Itakura M. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: Predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem. Biophys. Res. Commun. 2006;351(2):474–480. doi: 10.1016/j.bbrc.2006.10.076. [http://dx.doi.org/10.1016/j. bbrc.2006.10.076]. [PMID: 17070774]. [DOI] [PubMed] [Google Scholar]
- 158.Ha T.Y., Kim Y.S., Kim C.H., Choi H.S., Yang J., Park S.H., Kim D.H., Rhee J.K. Novel GPR119 agonist HD0471042 attenuated type 2 diabetes mellitus. Arch. Pharm. Res. 2014;37(5):671–678. doi: 10.1007/s12272-013-0209-0. [http://dx.doi.org/10.1007/s12272-013-0209-0]. [PMID: 23897163]. [DOI] [PubMed] [Google Scholar]
- 159.Yoshida S., Ohishi T., Matsui T., Tanaka H., Oshima H., Yonetoku Y., Shibasaki M. The role of small molecule GPR119 agonist, AS1535907, in glucose-stimulated insulin secretion and pancreatic β-cell function. Diabetes Obes. Metab. 2011;13(1):34–41. doi: 10.1111/j.1463-1326.2010.01315.x. [http://dx.doi.org/10.1111/j.1463-1326.2010.01315.x]. [PMID: 21114601]. [DOI] [PubMed] [Google Scholar]
- 160.McKillop A.M., Moran B.M., Abdel-Wahab Y.H., Gormley N.M., Flatt P.R. Metabolic effects of orally administered small-molecule agonists of GPR55 and GPR119 in multiple low-dose streptozotocin-induced diabetic and incretin-receptor-knockout mice. Diabetologia. 2016;59(12):2674–2685. doi: 10.1007/s00125-016-4108-z. [http://dx.doi.org/ 10.1007/s00125-016-4108-z]. [PMID: 27677765]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Overton H.A., Babbs A.J., Doel S.M., Fyfe M.C., Gardner L.S., Griffin G., Jackson H.C., Procter M.J., Rasamison C.M., Tang-Christensen M., Widdowson P.S., Williams G.M., Reynet C. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3(3):167–175. doi: 10.1016/j.cmet.2006.02.004. [http://dx.doi.org/10. 1016/j.cmet.2006.02.004]. [PMID: 16517404]. [DOI] [PubMed] [Google Scholar]
- 162.Bradshaw H.B., Rimmerman N., Hu S.S., Benton V.M., Stuart J.M., Masuda K., et al. The endocannabinoid anandamide is a precursor for the signaling lipid N-arachidonoyl glycine by two distinct pathways. BMC Biochem. 2009;10:14. doi: 10.1186/1471-2091-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takenouchi R., Inoue K., Kambe Y., Miyata A. Identification of N-arachidonylglycine, U18666A, and 4-androstene-3,17-dione as novel insulin Secretagogues. Biochem. Biophys. Res. Commun. 2005;333(3):778–786. doi: 10.1016/j.bbrc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 164.Ikeda Y., Iguchi H., Nakata M., Ioka R.X., Tanaka T., Iwasaki S., et al. Identification of N-arachidonylglycine, U18666A, and 4-androstene-3,17-dione as novel insulin Secretagogues. Biochem. Biophys. Res. Commun. 2005;333(3):778–786. doi: 10.1016/j.bbrc.2005.06.005. [DOI] [PubMed] [Google Scholar]


