Abstract
Background
Formate is a one-carbon molecule at the crossroad between cellular and whole body metabolism, between host and microbiome metabolism, and between nutrition and toxicology. This centrality confers formate with a key role in human physiology and disease that is currently unappreciated.
Scope of review
Here we review the scientific literature on formate metabolism, highlighting cellular pathways, whole body metabolism, and interactions with the diet and the gut microbiome. We will discuss the relevance of formate metabolism in the context of embryonic development, cancer, obesity, immunometabolism, and neurodegeneration.
Major conclusions
We will conclude with an outlook of some open questions bringing formate metabolism into the spotlight.
Keywords: Formate metabolism, One-carbon-metabolism, Cancer, Immune system, Neurodegeneration, Obesity
Abbreviations: GSH, glutathione; H-COOH, formate; H-CHO, formaldehyde; CH3-OH, methanol; THF, tetrahydrofolate; CH2-THF, 5,10-methylene-THF; CH3-THF, 5-methyl tetrahydrofolate; 10-CHO-THF, 10-formyl tetrahydrofolate; CH2OH-GSH, S-hydroxymethyl glutathione; CHO-GSH, S-formyl glutathione; ADH2, alcohol dehydrogenase 2; ADH5, alcohol dehydrogenase 5; ALDH1L1, aldehyde dehydrogenase 1 like 1; ALDH1L2, aldehyde dehydrogenase 1 like 2; ALDH2, aldehyde dehydrogenase 2; IDO, indoleamine 2,3-dioxygenase; MTHFD1, methylene-THF dehydrogenase 1; DHFR, dihydrofolate reductase; MTHFD2, methylene-THF dehydrogenase 2; MTHFD2L, methylene-THF dehydrogenase 2L; MTHFD1L, methylene-THF dehydrogenase 1L; MTHFR, methylene-THF reductase; MAT, S-adenosyl-methionine synthetase; MTA, methylthioadenosine; MTAP, methylthioadenosine phosphorylase; MS, methionine synthase; SAM, S-adenosyl-methionine; SSAO, semicarbazide-sensitive amine oxidase; TOD2, tryptophan 2,3-dioxygenase; TYMS, thymidylate synthase; XOR, xanthine oxidoreductase
1. Introduction
Formic acid (HCOOH) was first isolated from distillation of ant bodies, and it was subsequently named using the Latin word for ant, formica [1]. Ants and other insects accumulate formic acid in secretory glands and release their content as a mechanism of defense. In the context of human physiology, formate, the anion of formic acid, is better known as the agent mediating the adverse effects of methanol intoxication [2]. More recently, we are starting to uncover that formate plays a key role in the cellular and whole body metabolism of mammals [3].
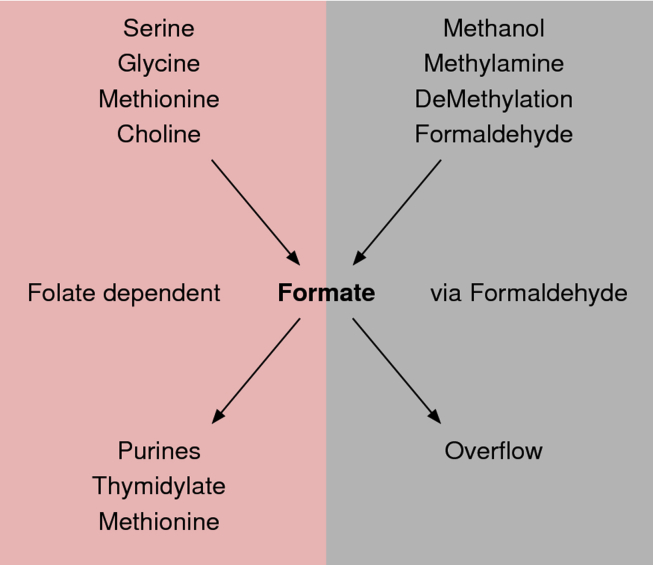
Formate is an intermediate metabolite in one-carbon (1C) metabolism (Figure 1). 1C metabolism brings together the biochemical reactions utilizing, transferring, or producing 1C-units either as free molecules or bound to carrier compounds. This pathway can be divided in different branches depending on co-factor utilization. The branch of 1C metabolism using folates as co-factors is considered the core of 1C metabolism [4], [5], [6]. There is also the folate independent branch associated with formaldehyde metabolism [2].
Figure 1.
Formate metabolism in mammals. Scheme of the central role of formate in mammalian metabolism, showing key sources and sinks.
Formate is a mediator of metabolic interactions between mammalian organisms, the diet and the gut microbiome. Serine, glycine, methionine, choline and methanol can be processed by the endogenous metabolism of mammals to produce formate. Formate is also a by-product of anaerobic fermentation of some bacteria species populating the gut microbiome [7]. The formate generated by the gut bacteria can enter the circulation, adding to the endogenous pool of formate or being used as substrate for the growth of other bacteria with aerobic metabolism [8].
Once in the circulation, formate or its precursors are used in most if not all tissues to fulfill the 1C demand for the synthesis of nucleotides and methyl groups (Figure 1). Given the essential role of nucleotide synthesis during embryonic development, immune cell expansion, and tumor growth, it is not surprising that alterations in formate metabolism have been found in the context of human diseases. There are also human disorders manifesting alterations in the products of formate metabolism (e.g., uric acid), in which the role of formate metabolism remains to be investigated.
Here we review the literature on formate metabolism. In Section 2, we will cover the cellular metabolism of formate, its sources and sinks. This will be followed by the whole body metabolism of formate in Section 3. In Section 4, we will review the relevance of formate metabolism in the context of normal physiology and human disease. In Section 5, we discuss interventions modulating formate metabolism and their potential utilization in the context of prevention and treatment of human diseases. We will conclude with an outlook of open question in the field of formate metabolism.
2. Cellular metabolism of formate
Mammalian cells can produce formate from a variety of nutrients, and formate can be fed into a number of biosynthetic pathways. In this section, we will review the sources and sinks of formate in the context of mammalian cell metabolism.
2.1. Folate dependent formate production
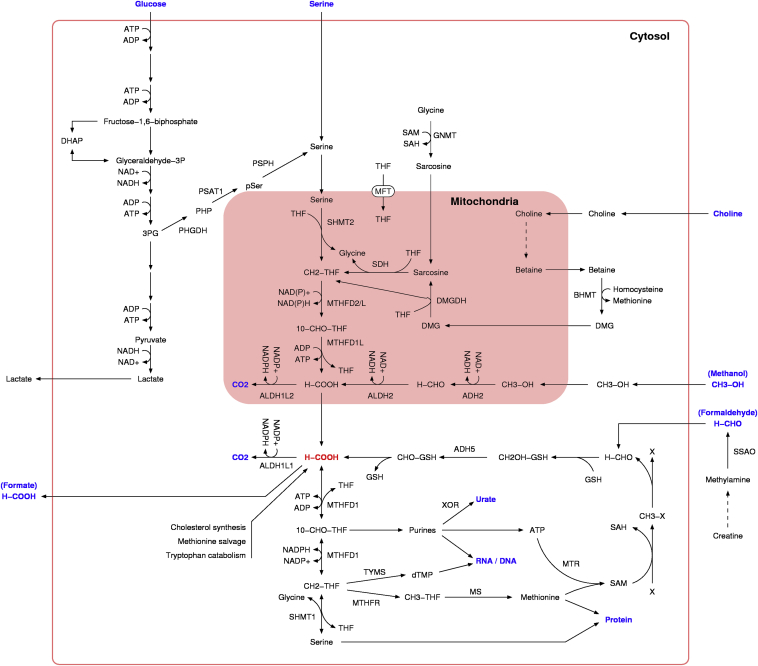
Serine is a major source for formate production in proliferating mammalian cells [4]. Serine deprivation induces cell cycle arrest of proliferating lymphocytes [9], [10] and cancer cells [11]. Serine can be catabolized to formate in a tetrahydrofolate (THF) dependent manner by two complementary pathways located in the cytosol and the mitochondria (Figure 2). Both pathways involve the same biochemical steps: (i) serine hydroxymethyl transferase, (ii) 5,10-CH2-THF dehydrogenase, (iii) 5,10-CH=THF cyclohydrolase and (iv) 10-CHO-THF synthase. In the cytosol, these steps are catalyzed by cytosolic serine hydroxymethyl transferase (SHMT1) and the trifunctional enzyme methylene-THF dehydrogenase 1 (MTHFD1) comprising the remaining enzymatic activities. In the mitochondria, these steps are catalyzed by mitochondrial serine hydroxymethyl transferase (SHMT2), the bifunctional enzyme methylene-THF dehydrogenase 2 (MTHFD2) or 2 like (MTHFD2L) with 5,10-CH2-THF dehydrogenase and 5,10-CH=THF cyclohydrolase activities, and the mitochondrial 10-CHO-THF synthase (methylene-THF dehydrogenase 1 like MTHFD1L).
Figure 2.
Cellular metabolism of formate. The intracellular formate molecule (H-COOH) is highlighted in red. The blue color highlights sources and sinks of formate. Dashed arrows indicate multiple reaction steps.
These pathways are conserved from yeast to mammals [4], indicating that the compartmentalization of 1C metabolism was an early event in the evolution of eukaryote cells. In yeast and mammalian cells, the mitochondrial pathway produces formate at high rates [12], and the cytosolic pathway recaptures formate for its use in serine and nucleotide synthesis [13], [14]. The inactivation of mitochondrial formate production induces a rewiring of 1C metabolism. To compensate for the lack of mitochondrial formate production, the cytosolic pathway switches from serine production to serine catabolism [15]. This switch does not compensate for the lack of mitochondrial glycine production, and cells become dependent on the availability of extracellular glycine [16], [17]. It should be noted, however, that excess glycine supplementation shifts the cytosolic pathway from serine catabolism to serine synthesis, inhibiting the adaptation to inactive mitochondrial 1C metabolism [18].
Glycine is another potential source of mitochondrial formate production. The glycine cleavage system (GCS) catalyzes the catabolism of glycine to CO2 and 5,10-CH2-THF using THF as a co-factor. The GCS is coupled to 1C metabolism via the activity of MTHFD2/MTHFD2L and MTHFD1L, which can contribute to mitochondrial formate production. The GCS is composed of the four proteins T, P, L, and H that are expressed in many tissues including liver, kidney, and brain [19]. Defective glycine cleavage can result in nonketotic hyperglycinemia in human infants. This disorder demonstrates the requirement of the GCS for glycine clearance and, by extrapolation, its contribution to the 1C pool in humans. The requirement of GCS for glycine clearance has been also demonstrated in glioma cell lines cultured in vitro [20]. However, most immortalized cell lines cultured in vitro lack a significant contribution of GCS activity to the 1C pool [18], [21].
Choline catabolism can generate two one-carbon units ([22], Figure 2). Choline is catabolized by a series of enzymatic steps that alternate between cytosolic and mitochondrial localization. The first 1C-unit is generated in the catabolism of choline to sarcosine and the second 1C-unit is generated in the subsequent catabolism of sarcosine. Sarcosine can also be produced from the methylation of glycine, effectively transferring 1C units from the methyl group of SAM to the folate dependent 1C pool (Figure 2).
Demethylation may also contribute to the 1C pool in a THF dependent manner. Histone demethylation by the nuclear amine oxidase homolog LSD1 or the family of JmjC domain-containing proteins generates formaldehyde [23], [24], [25]. LSD1 has a folate binding site near its active site, suggesting that the formaldehyde generated from histone demethylation is transfered to THF generating 5,10-CH2-THF [26]. The latter is confirmed by formation of 5,10-CH2-THF during the course of histone demethylation in the presence of THF [26].
2.2. Formaldehyde dependent formate production
Formaldehyde is an endogenous product of cell metabolism (Figure 2). Liver and other tissues metabolize methanol to formaldehyde [27]. Also in the liver, members of the cytochrome P450 (CYPs) catalyze the demethylation of endogenous metabolites and xenobiotics, releasing the methyl group as formaldehyde or methanol [28], [29]. In neutrophils, the myeloperoxidase (MPO) converts glycine to formaldehyde [30]. The oxidative decomposition of THF, DHF, and 5,10-methenyl-THF releases formaldehyde [31], [32], [33]. The serum semicarbazide sensitive amine oxidase (SSAO) metabolizes methylamine to formaldehyde, H2O2 and ammonia [27], [34]. Methylamine can therefore link the formation of formaldehyde to the catabolism of endogenous amines as adrenaline [35].
Formaldehyde is metabolized to formate by at least two different pathways ([27], Figure 2). The mitochondrial NAD+ dependent aldehyde dehydrogenase 2 (ALDH2) oxidizes formaldehyde to formate, providing a direct route of formaldehyde turnover. The other pathways are associated with the detoxification of products from the spontaneous reaction between formaldehyde and soluble metabolites or chemical groups of proteins and nucleotides. Formaldehyde reacts with the sulfur group of the highly abundant reduced form of glutathione (GSH) forming hydroxymethyl-GSH. Hydroxymethyl-GSH is then converted to formyl-GSH by the cytosolic enzyme alcohol dehydrogenase 5 (ADH5) also known as class III dehydrogenase (ADH3) [36]. Finally, formyl-GSH is hydrolyzed to formate and GSH by the formyl-GSH hydrolase activity of esterase D (ESD) [37]. Formaldehyde may also react with the sulfur groups of cysteine and homocysteine, and unpublished data from our laboratory indicate that reaction with cysteine and homocysteine can become the major route of formaldehyde turnover in cells lacking ALDH2 and ADH5 (Pietzke et al., in preparation).
Recent investigations have raised the question of whether circulating endogenous formaldehyde represents a significant source of formate production [32], [38]. Mammalian cells cultured in medium containing physiological levels of formaldehyde (20–40 μM) generate between 10 and 50% of total formate from formaldehyde [32]. Cells with genetic inactivation of the serine catabolism to formate can grow in media with dialyzed serum lacking extracellular 1C sources, albeit at a slow proliferation rate. In contrast, cells with the additional genetic inactivation of ADH5 do not grow in media with dialyzed serum, indicating that the ADH5 dependent generation of formate can compensate for the absence of serine catabolism to formate.
2.3. Formate production linked to sterol synthesis
A formate molecule is released in the synthesis of cholesterol, downstream of the lanosterol step (Figures 25–59 in Ref. [39]). A formate molecule is also released in the demethylation of androstenedione and testosterone by aromatase to form estrone and estradiol, respectively. Tracing experiments in rats indicate that total sterol synthesis is high in liver, adrenal gland, and ovaries [40]. The total sterol synthesis in these tissues is of the order of 1000 nmol/h/g of wet tissue. Assuming a tissue density of about 1 g/ml this translates to a formate production rate of about 1 mM/h. The latter value is in the range of the 1C demand of proliferating mammalian cells cultured in vitro (0.2–3 mM/h [21]). These numbers suggest that sterol synthesis is a significant source of formate in the liver, adrenal gland, and ovaries.
2.4. Formate production linked to polyamine synthesis
A formate molecule is produced in the methionine salvage pathway linked to polyamine synthesis. S-adenosyl-methionine (SAM) donates a propylamine group to polyamines and it is converted to methylthioadenosine (MTA) [41]. MTA can either be released from cells or salvaged to adenine, methionine, and formate. The first enzyme in the salvage pathway, MTA phosphorylase (MTAP), is often absent in human cancers, and cells lacking MTAP activity release MTA to the extracellular media [41]. Based on estimates for a human fibrosarcoma cell line [41], the methionine salvage pathway can produce about 0.2 mM/h of formate. This value is in the lower range of the 1C demand of proliferating mammalian cells cultured in vitro (0.2–3 mM/h [21]).
2.5. Formate production linked to tryptophan catabolism
A formate molecule is produced in the pathway of tryptophan catabolism (Figures 26–24 in Ref. [39]). Indoleamine 2,3-dioxygenase (IDO1 and 2 in humans) and tryptophan 2,3-dioxygenase (TDO2 in humans) catalyze the conversion of tryptophan to N-formyl-kynurenine. Arylformidase (AFMID in humans) then removes the formyl group producing formate and kynurenine. Kynurenine itself has been implicated in several physiological functions that are beyond the scope of this review [42]. However, the potential contribution of tryptophan to the 1C pool has been less appreciated. In mice injected intraperitoneally with radio-label [ring-2-14C]-l-tryptophan the measured radioactivity in the soluble and nucleic acid pools of the liver, kidney and intestine is similar to that obtained by injection of equimolar amount of [3-14C]-l-serine [43]. These data indicate that tryptophan contributes to the 1C pool of mammalian tissues.
2.6. Formate contribution to purine synthesis
Formate can have different fates depending on the cell type and environmental conditions. In proliferating cells, formate contributes to the 1C demand of purine and thymidylate synthesis [4], [15] (Figure 2). Given that purines are required for the synthesis of RNA, DNA and the free ATP pool, purine synthesis represents the major biosynthetic demand for 1C units in proliferating cells. Purine synthesis may be relevant in non-proliferating cells as well. Non-proliferating differentiated adipocytes release uric acid, a product of purine catabolism, suggesting that these cells have active de novo purine synthesis. Using measurements of uric acid secretion by 3T3-L1 mature adipocytes [44], we have estimated the rate of uric acid secretion by adipocytes to be about 0.17 mM/h. The latter value is of the same order of magnitude than the purine synthesis rate of proliferating mammalian cells cultured in vitro (0.1–1.5 mM/h [21]). To match that rate of uric acid release, adipocytes need to catabolize and therefore synthesize purines at a similar rate.
2.7. Formate contribution to thymidylate synthesis
Thymidylate synthesis represents a smaller demand of 1C units when compared to purine synthesis. Thymilydate represents only one DNA base (T), compared to the two purines A and G, needed in both DNA and RNA. Further the nucleotide demand for RNA synthesis is about two times that for DNA synthesis [45]. This results in a thymidylate/purine demand ratio of about 1/6. There is also a difference between purine and thymidylate synthesis with regard to localization. While purine synthesis takes place in the cytosol, thymidylate synthesis takes place in the nucleus [46] and the mitochondria [47]. This is consistent with the requirement of thymidylate for the synthesis of nuclear and mitochondrial DNA. In summary, thymidylate synthesis represents a lower demand of 1C units when compared to purine synthesis. Nevertheless, thymidylate synthesis is an essential demand that needs to be fulfilled for DNA synthesis to proceed.
2.8. Formate contribution to methylation
Formate can provide 1C units for the generation of methionine via the activity of methionine synthase that can be used to facilitate methylation reactions (Figure 2). During embryonic development, mitochondrial formate production contributes with about 75% of the 1C units used for methylation [48]. In contrast, cancer cells utilize extracellular methionine to satisfy their demand of 1C-units for methylation [49]. The requirement of an ATP molecule for the synthesis of S-adenosyl methionine (SAM), the canonical methyl donor in mammalian cells, provides an additional contribution of formate to methylation metabolism. The relevance of this observation has been demonstrated in the context of serine deprivation. Serine deprivation causes a depletion of purines in cancer cells, and as a consequence a depletion of SAM and hypomethylation [50].
2.9. Formate oxidation to CO2
Mammalian cells have both a cytosolic and a mitochondrial 10-CHO-THF dehydrogenase, catalyzing the oxidation of 10-CHO-THF to CO2 (Figure 2). In humans, these two enzymes are encoded by the genes aldehyde dehydrogenase 1 family member L1 (ALDH1L1) and L2 (ALDH1L2), respectively [51], [52]. 10-CHO-THF dehydrogenases effectively burn 1C units and therefore contribute to the turnover of formate. Consequently, the activity of 10-CHO-dehydrogenase can reduce the availability of 1C units for nucleotide synthesis, leading to inhibited growth and a tumor suppressing function. To avoid this ALDH1L1 and ALDH1L2 are usually underexpressed in cancer [53].
2.10. Formate overflow
Computer simulations of mammalian cell metabolism suggested that mammalian cells could benefit from excess formate production and its release to the extracellular media [54], [55]. Using metabolic flux analysis, it was later shown that the rate of 1C production exceeds the 1C demand of cancer cells [49]. Excess formate production results in formate release from cells and tissues [15], [21], [56], [57]. This evidence suggests that the production and demand of 1C units in proliferating cells may not be balanced but biased towards an excess production that is manifested as formate overflow from cells. Of note, the half-saturation constant for formate efflux by human erythrocytes is about 9 mM [58]. This indicates that cells showing formate overflow should have an intracellular formate concentration in the millimolar range.
2.11. What is the selective advantage of formate overflow?
The evidence reviewed above indicates that formate can have different fates depending on the metabolic demands of cells. From the point of view of anabolism, formate is required for the biosynthesis of nucleotides, the free adenine pool, and SAM. However, the benefit of the formate overflow is still an open question. The catabolism of serine to formate also produces glycine, which is required for the biosynthesis of proteins, purines, and glutathione. Thus, one hypothesis is that the serine catabolism fulfills the biosynthetic demand for glycine, which is larger than that of 1C units, and excess formate is released from cells or burned to CO2 [59]. However, simultaneous secretion of both glycine and formate [21] indicates serine catabolism in true excess.
The serine catabolism to formate can also contribute to the regeneration of NADPH, NADH, and ATP. The cytosolic catabolism of serine to formate produces NADPH [60]. NADPH is in turn required for the biosynthesis of fatty acids and sterols. The mitochondrial catabolism of serine to formate produces NADH. NADH is then oxidized by complex I coupled to the electron transport chain [12], [21], [56], thus contributing to mitochondrial energy production. Since the mitochondrial dehydrogenases MTHFD2 and MTHFD2L can also use NADP+ as a co-factor [61], the mitochondrial catabolism of serine can also produce NADPH. Furthermore, the catabolism of serine to formate contributes to ADP phosphorylation via the reverse activity of 10-CHO-THF synthetase [21], [54].
We are not aware of any physiological context in which any of these glycine and co-factor balance activities plays an essential role. At the present time, we cannot exclude that they are bystanders of an unidentified function. The regulation of mitochondrial protein translation is another possibility. Mitochondrial 5,10-CH2-THF is required for the production of 5-taurinomethyluridine and mitochondrial 10-CHO-THF is required for the formylation of the mitochondrial methyonyl-tRNA. The formylation of methyonyl-tRNA is not an essential requirement for initiation of protein synthesis in bacteria [62] or yeast mitochondria [63]. In agreement with the latter observations, the genetic inactivation of MTHFD2, which is required for the production of mitochondrial 10-CHO-THF (Figure 2), has no effect on mitochondrial activity. In contrast, genetic inactivation of SHMT2, which is required for the mitochondrial production of 5,10-CH2-THF, can result in reduced levels of 5-taurinomethyluridine in mitochondrial tRNA and impaired oxidative phosphorylation [64], [65]. However, in the HAP1 cell line derived from a human leukemia, genetic inactivation of SHMT2 does not result in any significant change in oxidative phosphorylation [57]. These observations indicate that there are other factors modulating the requirement of mitochondrial 1C metabolism for the efficient translation of mitochondrial proteins.
Mitochondrial formate production may be also required to protect the cytosolic pool of folates [66]. The cytosolic folate pool is depleted in cells deficient in mitochondrial formate production, and this phenotype can be rescued by exogenous formate supplementation. However, the generality of these observations remains to be established.
We conclude that, while there are different hypotheses for the selective advantage of excess mitochondrial formate production, we do not have enough evidence to make a definitive conclusion.
3. Whole body metabolism of formate
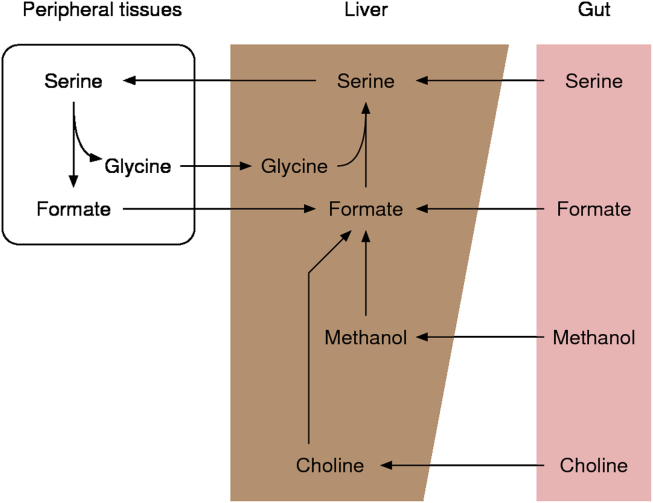
In mammalian organisms, circulating (blood) formate levels are found in the range between 10 and 100 μM, and the blood formate half-life is in the range between 40 and 100 min (Table 1). Circulating formate is subject to a whole body compartmentalization with a striking similarity with the Cori cycle of glucose-lactate metabolism, whereby serine plays the role of glucose and formate/glycine that of lactate (Figure 3). Serine catabolism to formate contributes to the formate production in peripheral tissues and the synthesis of serine in the liver and the kidney closes the loop. The whole body 1C cycle can also gain 1C units from other sources and lose 1C units to various biosynthetic sinks or clearance via the urine. In this section, we review the whole body metabolism of formate, its sources and sinks, and its metabolic functions.
Table 1.
Reported values of circulating formate concentration, formate half-life (t1/2), and whole body formate production for different mammalian species.
| Organism | Circulating formate (μM) | t1/2 (min) | Formate production (mmol/kg/h) | Source |
|---|---|---|---|---|
| Human | 10–100 | 59 | [78], [81], [146] | |
| Swine | 45–113 | [85] | ||
| Micropig | 74 | [156] | ||
| Sheep | 20–50 | [89] | ||
| Rat | 50–100 | 90 | 0.25–0.76 | [88], [157], [158], [159] |
| Mouse | 10–30 | 10a | [21], [57] |
Estimated from a serine bolus injection and consequently may be an overestimate.
Figure 3.
Whole body metabolism of formate. Schematic representation of the tissue compartmentalization of formate metabolism in mammals.
3.1. Formate sources
In mammals, formate can be derived from different dietary sources. Based on a bolus intraperitoneal injection of [3-13C]-l-serine and quantification of 13C fractions of plasma serine and formate, it is estimated that serine catabolism contributes to about 50% of formate production in mice [21]. The microbiome metabolism in the gut could account for the remaining 50% as formate is a fermentation product of anaerobic bacteria in the gut. Formate is found at millimolar concentrations in the intestinal lumen of mice, going down to micromolar levels in germ-free mice [8]. The intestinal formate can enter the circulation and contribute to the circulating formate pool.
Another by-product of the gut microbiome metabolism is methanol, which can be metabolized in the liver to formaldehyde and then to formate [27]. Pectin, a natural component of fruits, is degraded by the gut microbiome to methanol. In humans, the ingestion of 1 kg of apples/day or 10–15 g of pectin generates 0.4–1.4 g of methanol, in the range of the total endogenous production of methanol (0.3–0.6 g/day) [67]. Consumption of a serving of 200 g of Chinese cabbage (Brassica rapa pekinensis) increases circulating plasma methanol from 168 to 225 μM [68]. Alcohol consumption could also represent an important source of methanol. The endogenous production of methanol can be matched by a daily consumption of 0.3 L of 40% brandy [67]. However, we should bear in mind that ethanol inhibits the metabolism of methanol to formate (discussed below). Therefore, the methanol consumed from alcoholic beverages with high ethanol content is most likely cleared via the urine.
Glycine cleavage, choline catabolism, tryptophan catabolism, cholesterol synthesis, and sterol synthesis are further potential sources of formate production in mammals. However, we have a poor understanding about their net contribution to whole body formate production. Some estimates can be obtained by bringing together different literature reports. In rats, the rate of cholesterol synthesis in the liver is about 600 nmol/g of tissue/h [40]. Since one formate is released in the synthesis of cholesterol and rat liver is about 2% of rat body weight, the rate of formate production associated with cholesterol synthesis in the liver is about 0.01 μmol/g of body weight/h. This value is 30 times lower than the overall rate of formate production in rats (0.25–0.36 μmol/g of body weight/h, Table 1), indicating that cholesterol synthesis is only a minor source of whole body formate production in rats. We note that this does not exclude that it can be a relevant source of formate in the liver or that the liver has evolved to take advantage of the formate generated from cholesterol synthesis.
There are other dietary sources of circulating formate that can become relevant when consumed in excess. The sweetener aspartame is metabolized to aspartate, phenylalanine, and methanol. The excess consumption of beverages containing aspartame can therefore act as an extra source of formate via methanol metabolism. Experiments with aspartame supplementation have been conducted in humans [69]. A single aspartame dose of 10 and 34 mg/kg, equivalent to the projected average and 99th percentile if aspartame would replace all sucrose sweeteners in the diet, increases the circulating methanol levels. However, the increase in methanol levels is within the range of the natural variations of circulating methanol. Another study reported no significant changes in circulating formate levels after administration of an abuse aspartame dose of 200 mg/kg [70]. While these data indicate that aspartame use as a sweetener is safe from the point of view of methanol intoxication, it also tells us that aspartame can be a significant source of methanol and consequently of formate. Assuming the production of 0.1 mol methanol per g of aspartame consumed (based on the molar masses of methanol and aspartame), the 34 mg/kg aspartame per day could add up to a formate consumption rate of 0.14 μmol/g/h. The latter value is comparable to the whole body rate of formate production in rats (0.25–0.36 μmol/g of body weight/h, Table 1). These estimates suggest that aspartame can be a significant source of formate in humans consuming large amounts of aspartame-containing beverages. Whether this could contribute to weight gain or other physiological changes remains to be investigated.
Creatine supplementation is another potential exogenous source of formate. Creatine can be metabolized by the gut microbiome to methylamine. Methylamine enters the circulation and it can be metabolized by the serum enzyme semicarbazide sensitive amine oxidase (SSAO) to formaldehyde, H2O2, and ammonia. Creatine monohydrate supplementation has been investigated for its positive effect on physical performance and health [71]. In a creatine monohydrate supplementation experiment where subjects ingested 21 g of creatine/day for 14 days, levels of urine methylamine increased by 90% while formate increased by 13% [72]. Therefore, creatine supplementation can contribute to the whole body intake of formate.
Last but not least, methylated molecules in drinks, foods, and drugs can be demethylated in the liver releasing formaldehyde, which is subsequently converted to formate. A great example is caffeine, also known as 1,3,7-trimethylxanthine. After ingestion of caffeine almost 100% is absorbed through the gut and only about 3% is excreted unchanged [73]. In the liver, caffeine is demethylated at any of the methyl group positions resulting in the formation of paraxanthine (1,7-dimethylxanthine, 80%), theobromine (3,7-dimethylxantine, 12%) and theophylline (1,3-dimethylxanthine, 4%), in each case releasing formaldehyde. For example, an espresso cup contains about 60 mg of caffeine and has the potential to release 0.31 mmol of formaldehyde following absorption and demethylation in the liver. Assuming an intake of 1 espresso cup per day and a typical body weight of 70 kg that would lead to a caffeine dependent formate production rate of 0.18 μmol/h/kg. The latter value is about the rate of total formate production estimate in rats (Table 1). Similar estimates can be derived for soft-drinks containing caffeine (Table 2). In this case, we should consider the use of aspartame as a sweetener, which can also contribute to formaldehyde and formate production. For example, putting together the caffeine and aspartame content, the consumption of 1 can of diet coke per day has the potential for a formate production rate of 0.55 μmol/h/kg. Caffeine is also present in some medications, in some instances exceeding the caffeine content of coffee and soft drinks (Table 2). It is difficult to draw conclusions from these data in the absence of an estimate for the total rate of formate production in humans or the total daily demand of 1C units. Yet, these data suggest that the intake of caffeinated drinks is likely an important dietary source of formate in humans.
Table 2.
The potential for formaldehyde and formate generation from the ingestion of caffeine containing drinks and medications.
| Item | Serving | Aspartamea (mg) | Caffeinea (mg) | Formaldehydeb (mmol) | Formatec (μmol/h/kg) |
|---|---|---|---|---|---|
| Coffee | |||||
| Brewed coffee | 8 oz | 0 | 130 | 0.67 | 0.4 |
| Espresso | 1 oz | 0 | 60 | 0.31 | 0.18 |
| Soft drinks | |||||
| Coke | 12 oz | 0 | 34 | 0.18 | 0.1 |
| Diet coke | 12 oz | 197 | 50 | 0.93 | 0.55 |
| Pepsi | 12 oz | 0 | 31 | 0.16 | 0.1 |
| Diet Pepsi | 12 oz | 161 | 34 | 0.72 | 0.43 |
| Caffeine free diet Pepsi | 12 oz | 170 | 0 | 0.58 | 0.34 |
| Dr. Pepper | 12 oz | 0 | 45 | 0.23 | 0.14 |
| Diet Mountain Dew | 12 oz | 162 | 51 | 0.81 | 0.48 |
| Analgesics | |||||
| Min | 1 Tablet | 0 | 30 | 0.15 | 0.09 |
| Max | 0 | 100 | 0.51 | 0.31 | |
| Decongestant | |||||
| Dristan | 2 Tablets | 0 | 32 | 0.16 | 0.1 |
| Stimulant | |||||
| NoDoz | 1 dose | 0 | 200 | 1.03 | 0.61 |
| Vivarin | 1 dose | 0 | 200 | 1.03 | 0.61 |
| Weight loss | |||||
| Dexatrim | 1 dose | 0 | 200 | 1.03 | 0.61 |
Assuming mono-demethylation of caffeine and metabolism of aspartame to aspartate, phenylalanine, and methanol and of methanol to formaldehyde.
Assuming full conversion of formaldehyde to formate and a body weight of 70 kg. The typical servings of 1 oz (28 ml), 8 oz. (227 ml), and 12 oz (340 ml) are used.
3.2. Formate sinks
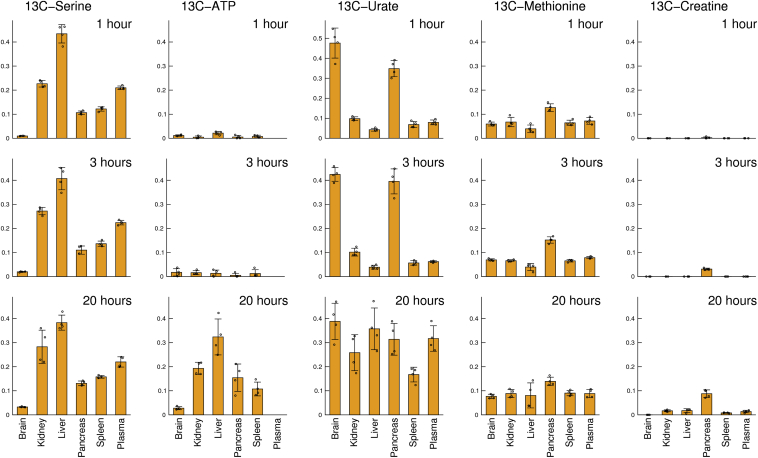
Circulating formate together with the in-tissue formate formation contribute to the tissue specific demands of 1C units. Since methanol is a source of formate in mammals and a large bolus of methanol (3 g/kg) is slowly metabolized to formate in the liver, we can use 13C-methanol to trace the fate of formate in whole organisms [57] (Figure 4). The reverse activity of serine hydroxymethyl transferase and the activity of methionine synthase result in a rapid equilibrium in the 13C-enrichment of serine and methionine, that last from 1 to 20 h in mice. By 20 h, we also observe a significant 13C-enrichment in ATP, reflecting the tissue specific rate of purine synthesis. All tissues exhibit some level of purine synthesis, with high values in the liver and lower in the brain. We also observe 13C incorporation into urate, a product of purine breakdown. Interestingly, the 13C incorporation into urate is faster than that into ATP. This data suggest that, for a given tissue, urate is produced in the tissue compartment where purine synthesis is taking place.
Figure 4.
Fates of methanol derived formate in mice. Incorporation of 13C in tissue metabolites following an intraperitoneal injection of 13C methanol (3 g/kg). The Y-axis shows the 13C-enrichment in the indicated metabolite at the indicated time point after injection.
Based on data reported in Ref. [57].
The incorporation of 13C in methionine is followed by the 13C incorporation into methylated products. Creatine synthesis contains a methylation step; therefore, creatine synthesis acts as a sink of 1C units. By 20 h, there is incorporation of 13C in creatine in most tissues except for the brain. Surprisingly, the 13C-enrichment in creatine is highest in the pancreas, suggesting that the rate of creatine synthesis is higher in the pancreas than the textbook knowledge reporting this reactions to take place in the kidney and the liver [39].
Circulating formate is also excreted in the urine. The anion exchange transporters Pedrin (encoded by the SLC26A4) and CFEX (encoded by the SLC26A6 gene) can exchange Cl− and formate [74], [75], [76]. Based on data from the Human Protein Atlas [77], CFEX has broad tissue expression while Pedrin is mainly expressed in the gastrointestinal track, liver, and male reproductive tissues. These data suggest that CFEX contributes to formate exchange in most tissues and to formate clearance in the kidneys, while Pedrin is responsible for formate absorption from the gut.
4. Formate metabolism in health and disease
Alterations in formate metabolism has been documented in multiple human disorders and pathophysiological conditions [3], [78]. The investigations of environmental and genetic factors causing those alterations have led to major breakthroughs in our understanding of formate metabolism in mammals [4]. Here we review the evidence associated with those conditions, together with indirect evidence for other diseases in which alterations in formate metabolism may play a role as well.
4.1. Methanol intoxication
Faulty fermentation or distillation can lead to the production of alcoholic beverages tainted with methanol [2]. Excessive consumption of methanol-contaminated beverages can lead to different degrees of methanol intoxication. The metabolism of methanol in the liver results in high levels of circulating formate in patients intoxicated with methanol. The concentration of circulating formate is in fact a diagnostic measurement for methanol intoxication [79].
Methanol intoxication can cause visual dysfunction and, in severe cases, death. These clinical symptoms are in part due to the inhibitory effects of formate on cytochrome c oxidase, the complex IV of the mitochondrial electron transport chain. Formate inhibits cytochrome c oxidase with an inhibition constant in the range between 5 and 30 mM [80]. In healthy humans, circulating formate is found below the mM range [78], [81]. Patients that manifest visual dysfunction due to methanol intoxication had a median serum formate concentration of 16 mM at diagnosis [79]. In contrast, patients with serum formate levels below 10 mM at diagnosis survive methanol intoxication without any visual sequelae [79]. Together the clinical and in vitro data support the hypothesis that the toxicity of methanol is in part due to the inhibition of cytochrome c oxidase by formate.
Methanol intoxication is treated with alcohol dehydrogenase inhibitors to inhibit an essential metabolic step in the metabolism of methanol to its toxic by-product formate. Inhibition of alcohol dehydrogenase prevents the accumulation of formate and methanol can be cleared via urine excretion. Since alcohol dehydrogenase catalyzes the conversion of both methanol to formaldehyde and ethanol to acetaldehyde, ethanol acts as a competitive inhibitor of methanol metabolism by alcohol dehydrogenase. Based on this rationale ethanol administration has been used to treat patients with methanol intoxication [82]. The synthetic alcohol dehydrogenase inhibitor fomepizole is also used in the context of methanol poisoning [83]. Yet, alcohol dehydrogenase inhibitors may not be as effective in patients that have already high circulating formate levels at diagnosis. These cases would require treatment strategies to accelerate formate turnover. There are currently investigations of organometallic complexes that catalyze the transfer hydrogenation between formate and NAD+, oxidizing formate to CO2 [84]. These compounds are currently under investigation for their use as cytotoxic agents to treat cancer. The possibility of using these compounds to treat methanol intoxication should be explored.
4.2. Folate deficiency
Folate (in its active form THF) is a co-factor in the catabolism of serine to formate and in the formate incorporation into purines, thymidylate, and methionine. Therefore, folate deficiency should have an impact in both the production and turnover of formate. Pigs subjected to a folate deficiency diet exhibit lower rates of formate turnover than control animals receiving a folate supplemented diet [85]. In contrast, basal plasma formate levels are similar between the two groups [85], indicating that the impairment of formate turnover caused by folate deficiency equally reduces both formate production and incorporation.
4.3. Vitamin B-12 deficiency
Methionine synthase requires vitamin B-12 (cobalamin) as a co-factor, and vitamin B12 deficiency could result in alterations of formate metabolism. The impairment of methionine synthesis may cause an accumulation of its precursors 5-methyl-THF and homocysteine and, as a consequence, a depletion of other cellular folates (methyl-trap hypothesis [86]). Thus, vitamin B-12 deficiency could mimic folate deficiency despite a sufficient total folate pool. In particular, the THF pool depletion causes a decrease in the synthesis of 10-CHO-THF from formate and consequently an increase in the formate overflow from cells. In agreement with this expectation, rats subjected to a vitamin B-12 deficient diet exhibit higher rates of formate production and plasma formate levels than control rats fed a vitamin B-12 replete diet [87], [88].
4.4. Developmental disorders
Both folate and vitamin B-12 deficiency are associated with birth defects, and their supplementation is recommended during pregnancy. Given that formate metabolism is folate- and vitamin B-12 dependent we anticipate a role of formate metabolism in embryonic development. The measurement of formate levels during pregnancy and their association with developmental disorders provides support for this evidence. Formate levels are 3 fold higher in fetal plasma of lambs than in 2–3 months old lambs (191 ± 62 μM vs 62 ± 11 μM [89]). Formate levels are also significantly higher in maternal plasma than in non-pregnant adult sheep (33 ± 13 μM vs 6±7 μM [89]). In humans, reduced maternal urine formate levels at the end of the first trimester are associated with fetal growth restriction [90]. Taken together, these evidences point to a requirement of formate during development.
The formate requirement during development could be obtained from the maternal diet or via the endogenous metabolism of formate sources. Genetic studies in mice have provided evidence for an essential requirement of mitochondrial formate production. As reviewed above, mitochondria can catabolize multiple sources to formate (Figure 2). When serine is used, it requires the activity of the gene products of Shmt2, Mthfd2, and Mthfd1l. Since the serine catabolism to formate is folate dependent there is an additional requirement for the mitochondrial folate transporter MFT, encoded by Slc25a32. The homozygous deletion of Shmt2, Mthfd2, Mthfd1l, or Slc25a32 is embryonic lethal in mice [91], [92], [93]. In fly, knockdown of Shmt2 or Nmdmc, the fly homolog of human MTHFD2, causes mitochondrial dysfunction and developmental defects [94]. Therefore, endogenous formate production from the mitochondrial serine catabolism to formate is an essential requirement during embryonic development.
Interestingly, mitochondria isolated from Mthfd1l−/− mouse embryonic fibroblasts release formate at about 1/3 the values observed in mitochondria isolated from Mthfd1l+/+ mouse embryonic fibroblasts [95]. While the enzyme responsible for mitochondrial formate production in the absence of Mthfd1l remains to be uncovered, these data indicate that a 2/3 reduction in mitochondrial formate production is sufficient to cause neural tube defects in mice.
There is also evidence indicating a role of mitochondrial formate production from glycine via the glycine cleavage system. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans, and it is rescued by formate supplementation [96], [97]. The neural tube defects caused by glycine cleavage deficiency are less severe than of disruption of mitochondrial serine catabolism to formate, resulting in partial penetrance. This evidence suggests that serine is the major source of formate and glycine provides a secondary contribution.
The curly tail mouse provides another model of developmental defects that is not driven by genetic alterations of formate metabolism enzymes. The curly tail (ct) mouse strain carries a hypomorphic allele of Grhl3, encoding the grainyhead-like 3 transcription factor. Homozygous ct/ct or Grhl3−/− embryos develop partially penetrant neural tube defects that can be rescued by sodium formate supplementation in drinking water [98], [99]. At the cellular level, ct/ct or Grhl3−/− embryos are characterized by reduced levels of Mthfd1l, the enzyme responsible for mitochondrial formate production, which is consistent with the rescue by formate supplementation [98], [99].
4.5. Cancer
Cancer cells have metabolic programs supporting tumor growth, invasion, and metastasis [100], [101], [102]. The requirement of purine and thymidylate nucleotides for tumor growth carries as a consequence a demand for 1C unit production [5], [6], [100], [103]. Serine is the major supplier of 1C units for cancer cell proliferation and tumor growth [11], [15], [21], [56]. In contrast, the available experimental data indicate that glycine is not a major source of 1C units in cancer cells [18].
Cancer cells uptake serine from the extracellular media or synthesize serine from the glycolytic intermediate 3-phosphoglycerate (Figure 2). The synthesis of serine takes place in three steps catalyzed by 3-phosphoglycerate dehydrogenase (PHGDH), 3-phospho-hydroxypyruvate transaminase (PSAT1), and phospho-serine phosphatase (PSPH). PHGDH is amplified in a subset of human cancers and cancer cells with PHGDH amplifications are less dependent on media serine for their proliferation [104], [105].
Serine can contribute to 1C metabolism via the cytosolic or mitochondrial folate dependent pathways (Figure 2). The identification of a mitochondrial NAD+ dependent CH2-THF dehydrogenase in cancer cells in the 1980s suggested a role for mitochondrial 1C metabolism [106], [107]. More recent experiments corroborated that cancer cells utilize mainly the mitochondrial pathway [14], [15]. Yet, mitochondrial formate production is not essential for cancer cells, because they can compensate using the cytosolic pathway [15]. That said, there is a distinctive difference between cancer cells with active or inactive mitochondrial formate production. In cancer cells with active mitochondrial 1C metabolism, the mitochondrial formate production exceeds the biosynthetic demand of 1C units [49], and the excess 1C units are released from cells as formate (formate overflow) [21]. Inhibition of mitochondrial formate production by genetic or pharmacological interventions abrogates formate overflow [21], [56] and, to a variable extent, inhibits cancer cell proliferation [15]. These data indicate that cancer cells with active mitochondrial formate production are in a state of excess production of 1C units while cancer cells with inactive mitochondrial 1C metabolism are in a state of 1C insufficiency.
Tumor growth is inhibited when mice are fed a serine and glycine deprived diet compared to tumor bearing mice receiving a regular diet [50]. Genetic disruption of mitochondrial 1C metabolism results in partial tumor growth inhibition [15], [108]. Some tumors in mice exhibit higher rates of serine catabolism to formate than adjacent normal tissues and mice bearing those tumors have significantly higher circulating formate levels than matched healthy controls [57]. There is one noted exception. A mouse model of pancreatic cancer revealed no difference between the rate of serine catabolism to formate in tumors relative to adjacent normal tissue, and no difference in the levels of circulating formate between tumor bearing mice and matched controls [57].
The expression of mitochondrial 1C metabolism genes is altered in human cancers. The mitochondrial genes SHMT2 and MTHFD2 are among the enzyme coding genes manifesting the highest increase in expression in tumor tissues relative to normal tissue controls [109]. The expression of mitochondrial formate production genes is highly correlated with gene signatures of increased cell proliferation [110], a distinctive hallmark of cancer [111].
Circulating formate levels are reduced in breast and lung cancer patients relative to healthy controls [78]. The reduction of circulating formate in human cancer patients could be both a tumor related effect or an effect of the body reaction to the tumor. Human cancers could be starved for 1C units. That hypothesis would explain both the reduction of circulating formate levels and the selective advantage of increasing the expression of mitochondrial serine catabolism enzymes. There are also potential tumor independent explanations. Cancer patients may have altered microbiomes, with a reduced capacity for formate and methanol production. A reduced liver function with regard to the metabolism of methanol to formate can also contribute to reduced formate levels. The tumors could also trigger an immunological response, and the activated immune response could increase the consumption of 1C units by immune cells.
4.6. Immunology
Serine is essential for T cells activation and supplemented formate can partially rescue serine deprivation [10], [112], [113]. Upon activation, T cells exhibit an increase in the expression of genes of the mitochondrial formate metabolism and increased incorporation of the 3rd carbon of serine into purines [10], [113]. Genetic inactivation of SHMT2, the first enzyme in the pathway of mitochondrial serine catabolism to formate, impairs T cell proliferation [113]. During aging in mice, there is a decline in naïve CD4+ T cells, and the remaining cells are impaired upon activation [114]. The naïve CD4+ T cells of aged mice are characterized by a decline in the mitochondrial respiratory capacity and the expression of mitochondrial formate production enzymes, suggesting a defect in mitochondrial formate production. This evidence highlights the importance of formate metabolism during T cell activation. Further work is required to investigate the relationship between circulating formate levels in humans and the strength of the immune system, particularly in the context of aging.
4.7. Obesity
There is hardly any mention of 1C metabolism in the obesity literature, and vice versa. In contrast, there is plenty of literature linking uric acid, or its anion urate, to obesity. Urate is among the circulating metabolites with the highest correlation with obesity and BMI [78], [115], [116], [117]. Higher levels of urate are associated with weight gain [116]. Urate is the end product of purine catabolism via the activity of xanthine oxidoreductase (XOR, Figure 2). XOR is highly expressed in adipose tissue and its activity is increased in the adipose tissue of obese relative to lean mice [44]. The latter report indicates a mechanistic link between increased fat tissue and increased urate levels.
An increase in circulating urate could be due to a number of factors, including increased dietary intake of purines, increased endogenous de novo synthesis of purines, and reduced urate clearance in the urine. Some of these factors have been studied in the context of gout, a type of arthritis caused by the accumulation of urate crystals in the joints [118]. A low purine diet causes about a 25% reduction of serum urate levels in humans [119]. In contrast, drinking beer increases mean urate excretion by 15% in gout patients and by 20% in healthy controls [120]. This evidence indicates that exogenous purines contribute to about ¼ of total purines catabolized to urate. Since diet is quite diverse in the human population, we expect a significant environmental contribution to circulating urate levels.
The endogenous de novo synthesis of purines should account for the remaining ¾ contribution to urate production. Evidence from the field of rheumatoid arthritis links circulating urate levels and purine synthesis. Rheumatoid arthritis is an inflammatory disease that causes pain, swelling, and stiffness in the joints. The antifolate methotrexate is commonly used to treat rheumatoid arthritis patients. Treatment of rheumatoid arthritis with an oral dose of 7.5 mg methotrexate lowers circulating urate levels within 24 h after administration [121]. The methotrexate dependent reduction of circulating urate is associated with a reduction in the number of swollen joints [122]. Additional evidence comes from the study of adipocytes. In vitro cell cultures of 3T3-L1 mature adipocytes release urate at rates comparable to the purine synthesis rates of cancer cells [44]. Since purine is not supplemented in these in vitro cell cultures, we can assume that adipocytes have a high rate of purine synthesis matching the measured rates of urate release.
Where there is an increase of purine synthesis, there is an increase in the demand of 1C units, which could then impinge on circulating formate levels. Formate is a precursor of purine synthesis, and its circulating levels are determined by the whole body formate production and demand. An increase of purine synthesis could shift the balance towards formate consumption, depleting the circulating formate levels. We have recently found that circulating formate levels are significantly decreased in highly obese individuals relative to healthy controls [78]. Circulating formate levels are also significantly decreased in obese colorectal cancer patients relative to leaner patients [123].
Further studies are required to quantify the contribution of purine synthesis in fat tissue to the whole body urate production, in healthy and obese individuals. There is also a need to investigate what is the function of this apparently wasteful pathway of purine synthesis and turnover in adipocytes. Xanthine oxidoreductase has been shown to regulate adipogenesis, providing a context for these investigations [124]. We should also consider that not all fats are equal. Visceral fat is linked to overproduction of urate more than subcutaneous fat is [125]. Environmental and genetic factors can also affect circulating urate and formate levels. For example, rats fed a high fat diet for 81 days have higher fecal formate levels than the day before the intervention [126]. Common polymorphisms in the urate transporters may have functional consequences leading to different capacities of urate clearance [127].
4.8. Neurological disorders
Loss of hypoxanthine-guanine phosphoribosyltransferase (HPRT), a purine recycling enzyme, causes Lesch–Nyhan disease, a neurobehavioral disorder characterized by increased de novo purine synthesis, hyperuricemia, mental retardation, and compulsive aggressive behavior [128], [129]. The neurological symptoms of Lesch–Nyhan disease can be explained by the alterations in purine levels and purinergic signaling associated with loss of HPRT [130]. Some mechanistic insights have been obtained from in vitro biochemical studies using rat PC6-3 cells, a pheochromocytoma cell line that undergoes robust differentiation when treated with nerve growth factor [131]. In contrast to parental PC6-3 cells, HPRT deficient cells are unable to reduce purine synthesis after induction of differentiation, and consequently have reduced levels of several neurotransmitters. This explains the increase in purine synthesis and hyperuricemia in Lesch–Nyhan disease patients. Further work is required to investigate the impact of increased purine on circulating formate levels in the context of Lesch–Nyhan disease.
In contrast to Lesch–Nyhan disease, there is an inverse association between circulating urate levels and Parkinson disease [132], [133], a neurological disease characterized by reduced brain dopamine and motor disorders. From the mechanistic point of view, serum urate is positively correlated with dopamine transporter availability in brain tissue [134]. Biochemical studies in cells with reduced DJ-1 protein levels provide a hypothesis for the reduction of circulating urate in Parkinson disease patients. Loss of DJ-1, encoded by the PARK7 gene, is associated with early onset Parkinson disease. Knockdown of DJ-1 expression induces a reduction in the mitochondrial formate metabolism enzymes SHMT2 and MTHFD2 [135], suggesting that mitochondrial formate production is impaired in DJ-1 dependent Parkinson disease. Since formate is a precursor of purine synthesis and urate is a product of purine catabolism, these data provide a mechanistic hypothesis linking reduced formate production from mitochondrial metabolism and decreased circulating urate in the context of Parkinson's disease associated with DJ-1 deficiency. Further work is required to validate this hypothesis and to investigate its relevance in other forms of Parkinson's disease.
Increased urate levels are also associated with decreased risk of Alzheimer's disease and with good prognosis in Parkinson's disease, amyotrophic lateral sclerosis, Huntington's disease, and multisystem atrophy [136]. These associations are attributed to the antioxidant properties of urate. However, the latter hypothesis contradicts the evidence in Lesch–Nyhan disease, where the association is inverted. This contradicting evidence suggests that both deficiency and excess urate production leads to neurological disorder and, by extrapolation, that purine synthesis in brain tissue should be fine-tuned for optimal neural activity.
Although differentiated neurons do not proliferate, they require de novo purine synthesis for axon regeneration and growth of new axons [137]. In fly, loss of the genes encoding for GMP synthetase or inosine monophosphate dehydrogenase causes severe defects in axon guidance in the retina [138]. Since formate metabolism feeds purine synthesis we hypothesize that local and whole body changes in formate metabolism may affect brain function. 13C-methanol tracing in mice shows 13C incorporation into brain purines, giving a rough estimate of the rate of purine synthesis in the brain relative to other tissues (Figure 4, ATP).
These data indicate that formate supplementation should be explored as a therapeutic option in neurological disorders characterized by low levels of circulating urate. It has been shown that inosine, a purine analog, stimulates axon sprouting and motor recovery after spinal cord injure [139], [140]. Further investigations should address whether formate supplementation could achieve the same effect.
4.9. Cardiovascular diseases
Formate levels are altered in some patients with mitral valve insufficiency. The mitral valve is a heart structure that is required for proper cardiac function. Mitral valve insufficiency is a cardiovascular disorder that can cause heart failure and death [141]. The plasma of patients with mitral valve insufficiency exhibits significantly lower levels of formate and lactate [142]. Formate was indicated as the most discriminatory metabolite between patients with mitral valve insufficiency and healthy controls.
There is currently no mechanistic hypothesis for the association between circulating formate levels and cardiac insufficiency. Yet, there are reports of urate production by human hearts [143]. It has been estimated that human hearts produce 300 nmol of urate/min/100 g of tissue. Assuming a tissue density of 1 kg/l that is equivalent to a urate production rate of 0.18 mM/h, which is again in the range of the purine synthesis rate of immortalized cell lines [21]. Extrapolating from this, one could hypothesize that decreased purine synthesis may be causally related to mitral valve insufficiency. But again, we are confronted with the question of why purine synthesis and catabolism to urate is so active in the first place.
The association between formate levels and cardiac deficiency could be a confounding factor to the reported observation of low formate levels in cancer and obesity patients relative to healthy controls. Mitral regurgitation results in left atrial dilation and left atrial size is positively correlated with BMI [144]. This association suggests that the observation of low circulating formate levels in obese individuals could be mediated by cardiac insufficiency. Whether this is the case should be further investigated.
5. Pharmacological modulators of formate metabolism
5.1. Formate supplementation
The developmental disorders caused by Mthfd1l deficiency are associated with reduced mitochondrial formate production and they can be rescued by formate supplementation [92]. Thus, as it is the case for folic acid and vitamin B12, formate supplementation should be investigated during pregnancy. A potential concern could be the reported formate related toxicity in the context of methanol overdose. Oral administration of a single dose of 3.9 g of calcium formate results in maximum serum formate levels of 30–90 μM in humans [145]. These values are significantly below the 10 mM range associated with sequelae following methanol intoxication [79]. Similar observations have been made in longer term studies of calcium formate supplementation in humans [146]. These studies indicate that calcium-formate supplementation at doses below 3.9 g/day is safe for humans.
The safety of formate supplementation is well documented in animals. Calcium formate is currently authorized by the European Union for its use as a preservative in animal feed up to a maximum dose of 15 g of formate/kg of complete fed (1.5%) [147]. There are, however, organism differences with regard to the nutritional benefits of formate salts supplementation. A diet supplemented with calcium formate (1.2%) significantly improves the growth of piglets [148]. In contrast, a diet supplemented with calcium formate at a dose of 0.5 or 1% has no significant effect on the weight gain of broiler chicks, while a dose of 1.5% even reduces the weight gain [149]. Beyond nutrition, formate salts supplementation may have an effect on the gut microbiome and gut function. The supplementation of calcium formate in piglets indicated a significant increase in the hardness of faeces and a significant reduction in diarrhea events and total Escherichia coli faecal excretion. Another study in rats reported that the supplementation of 250 μM sodium formate in the drinking water alters the gut microbiome [150], resulting in an increase of Bacteroidetes.
Once in the circulation, supplemented formate contributes to the nutritional requirements of 1C units. Interestingly, a study in mice reported a decline of mitochondrial 1C metabolism in the population of CD4+ naïve T cells [114]. This evidence suggests that formate supplementation could rescue the decline of the adaptive immune system during aging.
5.2. Inhibition of 1C metabolism
Inhibition of serine 1C metabolism is currently investigated as a target for cancer therapy. Pharmacological dual inhibitors of SHMT1 and SHMT2 have been developed, showing tumor growth inhibitory activity in mouse models of cancer [151], [152]. Strategies to develop MTHFD2 specific inhibitors have been discussed [153]. LY345899, an antifolate based inhibitor of MTHFD1 with a 96 nM IC50, shows inhibitory activity against MTHFD2 as well, albeit with a 7 times higher IC50 of 663 nM [154]. Carolacton, a natural macrolide keto-carboxylic acid produced by the Sorangium cellulosum bacteria is another dual inhibitor of MTHFD1 and MTHFD2 with in vitro growth inhibitory activity against cancer cells [155]. There are also ongoing investigations of organometallic complexes that catalyze the transfer hydrogenation between formate and NAD+ [84]. These compounds are cytotoxic against cancer cells in part due to their ability to deplete intracellular NAD+, switching cancer cells to a more oxidized state. Whether these compounds could manifest specific activity against tumors with increasing rates of serine catabolism to formate remains to be elucidated.
6. Outlook
The findings reviewed above highlight the central role of formate in mammalian metabolism. There remain a number of open key questions regarding the role of formate metabolism in normal physiology and disease:
-
•
What is the selective advantage of mitochondrial 1C metabolism?
-
•
What is the contribution of diet and the microbiome to the variable intake of formate and methanol in humans?
-
•
Should formate be supplemented during pregnancy to reduce the risks of birth defects?
-
•
What is the role of formate metabolism in diseases characterized by abnormal levels of circulating urate (e.g., gout, obesity, diabetes, neurodegeneration)?
-
•
Does formate supplementation rescue the decline of adaptive immunity during aging?
-
•
Are inhibitors of serine 1C metabolism more effective than established antifolates in the treatment of cancer?
We hope that the answers to these questions will bring more attention to the promising field of formate metabolism.
Conflict of interest
The authors declare they have no conflict of interest.
Acknowledgments
M.P. and A.V. were supported by Cancer Research UK (C596/A21140). J.M. was supported by the FNR-ATTRACT Program (A18/BM/11809970).
References
- 1.Stumper R. L'acide formique. Bulletin – Societe des Naturalistes Luxembourgeois. 1921;31:174–187. [Google Scholar]
- 2.Clary J.J. Wiley; Hoboken, N.J.: 2013. The toxicology of methanol. [Google Scholar]
- 3.Brosnan M.E., Brosnan J.T. Formate: the neglected member of one-carbon metabolism. Annual Review of Nutrition. 2016;36:369–388. doi: 10.1146/annurev-nutr-071715-050738. [DOI] [PubMed] [Google Scholar]
- 4.Tibbetts A.S., Appling D.R. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annual Review of Nutrition. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 5.Meiser J., Vazquez A. Give it or take it: the flux of one-carbon in cancer cells. FEBS Journal. 2016;283:3695–3704. doi: 10.1111/febs.13731. [DOI] [PubMed] [Google Scholar]
- 6.Ducker G.S., Rabinowitz J.D. One-carbon metabolism in health and disease. Cell Metabolism. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham V.T., Lacroix C., Braegger C.P., Chassard C. Lactate-utilizing community is associated with gut microbiota dysbiosis in colicky infants. Scientific Reports. 2017;7:11176. doi: 10.1038/s41598-017-11509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes E.R., Winter M.G., Duerkop B.A., Spiga L., Furtado de Carvalho T., Zhu W. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host & Microbe. 2017;21:208–219. doi: 10.1016/j.chom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen R.W., Moskowitz M. Arrest of cell-growth in G1 phase of cell-cycle by serine deprivation. Experimental Cell Research. 1978;116:127–137. doi: 10.1016/0014-4827(78)90070-8. [DOI] [PubMed] [Google Scholar]
- 10.Ma E.H., Bantug G., Griss T., Condotta S., Johnson R.M., Samborska B. Serine is an essential metabolite for effector T cell expansion. Cell Metabolism. 2017;25:345–357. doi: 10.1016/j.cmet.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Maddocks O.D.K., Berkers C.R., Mason S.M., Zheng L., Blyth K., Gottlieb E. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Martinez L.F., Appling D.R. Characterization of the folate-dependent mitochondrial oxidation of carbon 3 of serine. Biochemistry. 1993;32:4671–4676. doi: 10.1021/bi00068a027. [DOI] [PubMed] [Google Scholar]
- 13.Pasternack L.B., Laude D.A., Jr., Appling D.R. 13C NMR analysis of intercompartmental flow of one-carbon units into choline and purines in Saccharomyces cerevisiae. Biochemistry. 1994;33:74–82. doi: 10.1021/bi00167a010. [DOI] [PubMed] [Google Scholar]
- 14.Lewis C.A., Parker S.J., Fiske B.P., McCloskey D., Gui D.Y., Green C.R. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Molecular Cell. 2014;55:253–263. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ducker G.S., Chen L., Morscher R.J., Ghergurovich J.M., Esposito M., Teng X. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metabolism. 2016;23:1140–1153. doi: 10.1016/j.cmet.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel H., Pietro E.D., MacKenzie R.E. Mammalian fibroblasts lacking mitochondrial NAD+-dependent methylenetetrahydrofolate dehydrogenase–cyclohydrolase are glycine auxotrophs. Journal of Biological Chemistry. 2003;278:19436–19441. doi: 10.1074/jbc.M301718200. [DOI] [PubMed] [Google Scholar]
- 17.McCarty E.A., Titus S.A., Taylor S.M., Jackson-Cook C., Moran R.G. A mutation inactivating the mitochondrial inner membrane folate transporter creates a glycine requirement for survival of Chinese hamster cells. Journal of Biological Chemistry. 2004;279:33829–33836. doi: 10.1074/jbc.M403677200. [DOI] [PubMed] [Google Scholar]
- 18.Labuschagne C.F., van den Broek N.J.F., Mackay G.M., Vousden K.H., Maddocks O.D.K. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Reports. 2014;7:1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi G., Motokawa Y., Yoshida T., Hiraga K. Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 2008;84:246–263. doi: 10.2183/pjab/84.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D., Fiske B.P., Birsoy K., Freinkman E., Kami K., Possemato R.L. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520:363. doi: 10.1038/nature14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meiser J., Tumanov S., Maddocks O., Labuschagne C.F., Athineos D., Van Den Broek N. Serine one-carbon catabolism with formate overflow. Science Advances. 2016;2:e1601273. doi: 10.1126/sciadv.1601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeisel S.H., Blusztajn J.K. Choline and human nutrition. Annual Review of Nutrition. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Jackson M.M. Update on AIDS: the second decade. Today's OR nurse. 1992;14:3. [PubMed] [Google Scholar]
- 25.Walport L.J., Hopkinson R.J., Chowdhury R., Schiller R., Ge W., Kawamura A. Arginine demethylation is catalysed by a subset of JmjC histone lysine demethylases. Nature Communications. 2016;7:11974. doi: 10.1038/ncomms11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luka Z., Pakhomova S., Loukachevitch L.V., Calcutt M.W., Newcomer M.E., Wagner C. Crystal structure of the histone lysine specific demethylase LSD1 complexed with tetrahydrofolate. Protein Science: A Publication of the Protein Society. 2014;23:993–998. doi: 10.1002/pro.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorokhov Y.L., Shindyapina A.V., Sheshukova E.V., Komarova T.V. Metabolic methanol: molecular pathways and physiological roles. Physiological Reviews. 2015;95:603–644. doi: 10.1152/physrev.00034.2014. [DOI] [PubMed] [Google Scholar]
- 28.Axelrod J. The enzymatic N-demethylation of narcotic drugs. Journal of Pharmacology and Experimental Therapeutics. 1956;117:322–330. [PubMed] [Google Scholar]
- 29.Meunier G., Meunier B. Peroxidase-catalyzed O-demethylation reactions. Quinone-imine formation from 9-methoxyellipticine derivatives. Journal of Biological Chemistry. 1985;260:10576–10582. [PubMed] [Google Scholar]
- 30.Hazen S.L., Hsu F.F., d'Avignon A., Heinecke J.W. Human neutrophils employ myeloperoxidase to convert alpha-amino acids to a battery of reactive aldehydes: a pathway for aldehyde generation at sites of inflammation. Biochemistry. 1998;37:6864–6873. doi: 10.1021/bi972449j. [DOI] [PubMed] [Google Scholar]
- 31.Chippel D., Scrimgeour K.G. Oxidative degradation of dihydrofolate and tetrahydrofolate. Canadian Journal of Biochemistry. 1970;48:999–1009. doi: 10.1139/o70-156. [DOI] [PubMed] [Google Scholar]
- 32.Burgos-Barragan G., Wit N., Meiser J., Dingler F.A., Pietzke M., Mulderrig L. Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature. 2017;548:549–554. doi: 10.1038/nature23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Calderon C.B., Bejarano-Garcia J.A., Tinoco-Gago I., Castro M.J., Moreno-Gordillo P., Piruat J.I. Genotoxicity of tetrahydrofolic acid to hematopoietic stem and progenitor cells. Cell Death and Differentiation. 2018;25:1967–1979. doi: 10.1038/s41418-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boor P.J., Trent M.B., Lyles G.A., Tao M., Ansari G.A. Methylamine metabolism to formaldehyde by vascular semicarbazide-sensitive amine oxidase. Toxicology. 1992;73:251–258. doi: 10.1016/0300-483x(92)90067-o. [DOI] [PubMed] [Google Scholar]
- 35.Yu P.H., Lai C.T., Zuo D.M. Formation of formaldehyde from adrenaline in vivo; a potential risk factor for stress-related angiopathy. Neurochemical Research. 1997;22:615–620. doi: 10.1023/a:1022478221421. [DOI] [PubMed] [Google Scholar]
- 36.Sanghani P.C., Stone C.L., Ray B.D., Pindel E.V., Hurley T.D., Bosron W.F. Kinetic mechanism of human glutathione-dependent formaldehyde dehydrogenase. Biochemistry. 2000;39:10720–10729. doi: 10.1021/bi9929711. [DOI] [PubMed] [Google Scholar]
- 37.Uotila L., Koivusalo M. S-Formylglutathione hydrolase. Methods in Enzymology. 1981;77:320–325. doi: 10.1016/s0076-6879(81)77045-9. [DOI] [PubMed] [Google Scholar]
- 38.Bae S., Chon J., Field M.S., Stover P.J. Alcohol dehydrogenase 5 is a source of formate for de novo purine biosynthesis in HepG2 cells. Journal of Nutrition. 2017;147:499–505. doi: 10.3945/jn.116.244467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voet D., Voet J.G. 4th ed. John Wiley & Sons, Inc.; Hoboken, N.J.: 2011. Biochemistry. [Google Scholar]
- 40.Turley S.D., Andersen J.M., Dietschy J.M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. Journal of Lipid Research. 1981;22:551–569. [PubMed] [Google Scholar]
- 41.Shlomi T., Fan J., Tang B., Kruger W.D., Rabinowitz J.D. Quantitation of cellular metabolic fluxes of methionine. Analytical Chemistry. 2014;86:1583–1591. doi: 10.1021/ac4032093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platten M., Nollen E.A.A., Rohrig U.F., Fallarino F., Opitz C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nature Reviews. Drug Discovery. 2019 doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 43.Letter A.A., Zombor G., Henderson J.F. Tryptophan as a source of one-carbon units for purine biosynthesis de novo. Canadian Journal of Biochemistry. 1973;51:486–488. doi: 10.1139/o73-058. [DOI] [PubMed] [Google Scholar]
- 44.Tsushima Y., Nishizawa H., Tochino Y., Nakatsuji H., Sekimoto R., Nagao H. Uric acid secretion from adipose tissue and its increase in obesity. Journal of Biological Chemistry. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez-de-Cossio-Diaz J., Vazquez A. Limits of aerobic metabolism in cancer cells. Scientific Reports. 2017;7:13488. doi: 10.1038/s41598-017-14071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacFarlane A.J., Anderson D.D., Flodby P., Perry C.A., Allen R.H., Stabler S.P. Nuclear localization of de novo thymidylate biosynthesis pathway is required to prevent uracil accumulation in DNA. Journal of Biological Chemistry. 2011;286:44015–44022. doi: 10.1074/jbc.M111.307629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson D.D., Quintero C.M., Stover P.J. Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15163–15168. doi: 10.1073/pnas.1103623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pike S.T., Rajendra R., Artzt K., Appling D.R. Mitochondrial C1-tetrahydrofolate synthase (MTHFD1L) supports the flow of mitochondrial one-carbon units into the methyl cycle in embryos. Journal of Biological Chemistry. 2010;285:4612–4620. doi: 10.1074/jbc.M109.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tedeschi P.M., Johnson-Farley N., Lin H., Shelton L.M., Ooga T., Mackay G. Quantification of folate metabolism using transient metabolic flux analysis. Cancer & Metabolism. 2015;3:6. doi: 10.1186/s40170-015-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddocks O.D., Labuschagne C.F., Adams P.D., Vousden K.H. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Molecular Cell. 2016 doi: 10.1016/j.molcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook R.J., Lloyd R.S., Wagner C. Isolation and characterization of cDNA clones for rat liver 10-formyltetrahydrofolate dehydrogenase. Journal of Biological Chemistry. 1991;266:4965–4973. [PubMed] [Google Scholar]
- 52.Krupenko N.I., Dubard M.E., Strickland K.C., Moxley K.M., Oleinik N.V., Krupenko S.A. ALDH1L2 is the mitochondrial homolog of 10-formyltetrahydrofolate dehydrogenase. Journal of Biological Chemistry. 2010;285:23054–23061. doi: 10.1074/jbc.M110.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krupenko S.A., Oleinik N.V. 10-formyltetrahydrofolate dehydrogenase, one of the major folate enzymes, is down-regulated in tumor tissues and possesses suppressor effects on cancer cells. Cell Growth & Differentiation. 2002;13:227–236. [PubMed] [Google Scholar]
- 54.Vazquez A., Markert E.K., Oltvai Z.N. Serine biosynthesis with one carbon catabolism and the glycine cleavage system represents a novel pathway for ATP generation. PLoS One. 2011;6:e25881. doi: 10.1371/journal.pone.0025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tedeschi P.M., Markert E.K., Gounder M., Lin H., Dvorzhinski D., Dolfi S.C. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death & Disease. 2013;4:e877. doi: 10.1038/cddis.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao X.R., Ong S.E., Goldberger O., Peng J., Sharma R., Thompson D.A. Mitochondrial dysfunction remodels one-carbon metabolism in human cells. eLife. 2016:5. doi: 10.7554/eLife.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meiser J., Schuster A., Pietzke M., Voorde J.V., Athineos D., Oizel K. Increased formate overflow is a hallmark of oxidative cancer. Nature Communications. 2018;9:1368. doi: 10.1038/s41467-018-03777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Himmelreich U., Chapman B.E., Kuchel P.W. Membrane permeability of formate in human erythrocytes: NMR measurements. European Biophysics Journal. 1999;28:158–165. doi: 10.1007/s002490050195. [DOI] [PubMed] [Google Scholar]
- 59.Brosnan M.E., MacMillan L., Stevens J.R., Brosnan J.T. Division of labour: how does folate metabolism partition between one-carbon metabolism and amino acid oxidation? Biochemical Journal. 2015;472:135–146. doi: 10.1042/BJ20150837. [DOI] [PubMed] [Google Scholar]
- 60.Fan J., Ye J.B., Kamphorst J.J., Shlomi T., Thompson C.B., Rabinowitz J.D. Quantitative flux analysis reveals folate-dependent NADPH production (vol 510, pg 298, 2014) Nature. 2014;513:574. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin M., Momb J., Appling D.R. Human mitochondrial MTHFD2 is a dual redox cofactor-specific methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase. Cancer & Metabolism. 2017;5:11. doi: 10.1186/s40170-017-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newton D.T., Creuzenet C., Mangroo D. Formylation is not essential for initiation of protein synthesis in all eubacteria. Journal of Biological Chemistry. 1999;274:22143–22146. doi: 10.1074/jbc.274.32.22143. [DOI] [PubMed] [Google Scholar]
- 63.Li Y., Holmes W.B., Appling D.R., RajBhandary U.L. Initiation of protein synthesis in Saccharomyces cerevisiae mitochondria without formylation of the initiator tRNA. Journal of Bacteriology. 2000;182:2886–2892. doi: 10.1128/jb.182.10.2886-2892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morscher R.J., Ducker G.S., Li S.H., Mayer J.A., Gitai Z., Sperl W. Mitochondrial translation requires folate-dependent tRNA methylation. Nature. 2018;554:128–132. doi: 10.1038/nature25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asano K., Suzuki T., Saito A., Wei F.Y., Ikeuchi Y., Numata T. Metabolic and chemical regulation of tRNA modification associated with taurine deficiency and human disease. Nucleic Acids Research. 2018;46:1565–1583. doi: 10.1093/nar/gky068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng Y., Lin T.Y., Lee G., Paddock M.N., Momb J., Cheng Z. Mitochondrial one-carbon pathway supports cytosolic folate integrity in cancer cells. Cell. 2018;175:1546–1560. doi: 10.1016/j.cell.2018.09.041. e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindinger W., Taucher J., Jordan A., Hansel A., Vogel W. Endogenous production of methanol after the consumption of fruit. Alcoholism: Clinical and Experimental Research. 1997;21:939–943. [PubMed] [Google Scholar]
- 68.Komarova T.V., Petrunia I.V., Shindyapina A.V., Silachev D.N., Sheshukova E.V., Kiryanov G.I. Endogenous methanol regulates mammalian gene activity. PLoS One. 2014;9:e90239. doi: 10.1371/journal.pone.0090239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davoli E., Cappellini L., Airoldi L., Fanelli R. Serum methanol concentrations in rats and in men after a single dose of aspartame. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 1986;24:187–189. doi: 10.1016/0278-6915(86)90227-9. [DOI] [PubMed] [Google Scholar]
- 70.Stegink L.D., Brummel M.C., McMartin K., Martin-Amat G., Filer L.J., Jr., Baker G.L. Blood methanol concentrations in normal adult subjects administered abuse doses of aspartame. Journal of Toxicology and Environmental Health. 1981;7:281–290. doi: 10.1080/15287398109529979. [DOI] [PubMed] [Google Scholar]
- 71.Cooper R., Naclerio F., Allgrove J., Jimenez A. Creatine supplementation with specific view to exercise/sports performance: an update. Journal of the International Society of Sports Nutrition. 2012;9:33. doi: 10.1186/1550-2783-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poortmans J.R., Kumps A., Duez P., Fofonka A., Carpentier A., Francaux M. Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Medicine & Science in Sports & Exercise. 2005;37:1717–1720. doi: 10.1249/01.mss.0000176398.64189.e6. [DOI] [PubMed] [Google Scholar]
- 73.Tang-Liu D.D., Williams R.L., Riegelman S. Disposition of caffeine and its metabolites in man. Journal of Pharmacology and Experimental Therapeutics. 1983;224:180–185. [PubMed] [Google Scholar]
- 74.Karniski L.P., Aronson P.S. Chloride/formate exchange with formic acid recycling: a mechanism of active chloride transport across epithelial membranes. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:6362–6365. doi: 10.1073/pnas.82.18.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scott D.A., Karniski L.P. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. American Journal of Physiology. Cell Physiology. 2000;278:C207–C211. doi: 10.1152/ajpcell.2000.278.1.C207. [DOI] [PubMed] [Google Scholar]
- 76.Knauf F., Yang C.L., Thomson R.B., Mentone S.A., Giebisch G., Aronson P.S. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ponten F., Gry M., Fagerberg L., Lundberg E., Asplund A., Berglund L. A global view of protein expression in human cells, tissues, and organs. Molecular Systems Biology. 2009;5:337. doi: 10.1038/msb.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pietzke M., Fernandez-Arroyo S., Sumpton D., Mackay G.M., Martin-Castillo B., Camps J. Stratification of cancer and diabetes based on circulating levels of formate and glucose. Cancer & Metabolism. 2019;7:3. doi: 10.1186/s40170-019-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zakharov S., Kurcova I., Navratil T., Salek T., Komarc M., Pelclova D. Is the measurement of serum formate concentration useful in the diagnostics of acute methanol poisoning? A prospective study of 38 patients. Basic and Clinical Pharmacology and Toxicology. 2015;116:445–451. doi: 10.1111/bcpt.12338. [DOI] [PubMed] [Google Scholar]
- 80.Nicholls P. Formate as an inhibitor of cytochrome c oxidase. Biochemical and Biophysical Research Communications. 1975;67:610–616. doi: 10.1016/0006-291x(75)90856-6. [DOI] [PubMed] [Google Scholar]
- 81.Brosnan J.T., Mills J.L., Ueland P.M., Shane B., Fan R., Chiu C.Y. Lifestyle, metabolite, and genetic determinants of formate concentrations in a cross-sectional study in young, healthy adults. American Journal of Clinical Nutrition. 2018;107:345–354. doi: 10.1093/ajcn/nqx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bergeron R., Cardinal J., Geadah D. Prevention of methanol toxicity by ethanol therapy. New England Journal of Medicine. 1982;307:1528. doi: 10.1056/nejm198212093072423. [DOI] [PubMed] [Google Scholar]
- 83.Brent J. Fomepizole for ethylene glycol and methanol poisoning. New England Journal of Medicine. 2009;360:2216–2223. doi: 10.1056/NEJMct0806112. [DOI] [PubMed] [Google Scholar]
- 84.Soldevila-Barreda J.J., Romero-Canelon I., Habtemariam A., Sadler P.J. Transfer hydrogenation catalysis in cells as a new approach to anticancer drug design. Nature Communications. 2015;6:6582. doi: 10.1038/ncomms7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sokoro A.A., Zhang Z., Eichhorst J.C., Zello G.A., House J.D., Alcorn J. Formate pharmacokinetics during formate administration in folate-deficient young swine. Metabolism: Clinical and Experimental. 2008;57:920–926. doi: 10.1016/j.metabol.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Shane B., Stokstad E.L. Vitamin B12–folate interrelationships. Annual Review of Nutrition. 1985;5:115–141. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]
- 87.Lamarre S.G., Molloy A.M., Reinke S.N., Sykes B.D., Brosnan M.E., Brosnan J.T. Formate can differentiate between hyperhomocysteinemia due to impaired remethylation and impaired transsulfuration. American Journal of Physiology. Endocrinology and Metabolism. 2012;302:E61–E67. doi: 10.1152/ajpendo.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacMillan L., Tingley G., Young S.K., Clow K.A., Randell E.W., Brosnan M.E. Cobalamin deficiency results in increased production of formate secondary to decreased mitochondrial oxidation of one-carbon units in rats. Journal of Nutrition. 2018;148:358–363. doi: 10.1093/jn/nxx057. [DOI] [PubMed] [Google Scholar]
- 89.Washburn S.E., Caudill M.A., Malysheva O., MacFarlane A.J., Behan N.A., Harnett B. Formate metabolism in fetal and neonatal sheep. American Journal of Physiology. Endocrinology and Metabolism. 2015;308:E921–E927. doi: 10.1152/ajpendo.00046.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maitre L., Fthenou E., Athersuch T., Coen M., Toledano M.B., Holmes E. Urinary metabolic profiles in early pregnancy are associated with preterm birth and fetal growth restriction in the Rhea mother–child cohort study. BMC Medicine. 2014;12:110. doi: 10.1186/1741-7015-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Pietro E., Sirois J., Tremblay M.L., MacKenzie R.E. Mitochondrial NAD-dependent methylenetetrahydrofolate dehydrogenase–methenyltetrahydrofolate cyclohydrolase is essential for embryonic development. Molecular and Cellular Biology. 2002;22:4158–4166. doi: 10.1128/MCB.22.12.4158-4166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Momb J., Lewandowski J.P., Bryant J.D., Fitch R., Surman D.R., Vokes S.A. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:549–554. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim J., Lei Y., Guo J., Kim S.E., Wlodarczyk B.J., Cabrera R.M. Formate rescues neural tube defects caused by mutations in Slc25a32. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:4690–4695. doi: 10.1073/pnas.1800138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Celardo I., Lehmann S., Costa A.C., Loh S.H., Miguel Martins L. dATF4 regulation of mitochondrial folate-mediated one-carbon metabolism is neuroprotective. Cell Death and Differentiation. 2017;24:638–648. doi: 10.1038/cdd.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bryant J.D., Sweeney S.R., Sentandreu E., Shin M., Ipas H., Xhemalce B. Deletion of the neural tube defect-associated gene Mthfd1l disrupts one-carbon and central energy metabolism in mouse embryos. Journal of Biological Chemistry. 2018;293:5821–5833. doi: 10.1074/jbc.RA118.002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narisawa A., Komatsuzaki S., Kikuchi A., Niihori T., Aoki Y., Fujiwara K. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Human Molecular Genetics. 2012;21:1496–1503. doi: 10.1093/hmg/ddr585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pai Y.J., Leung K.Y., Savery D., Hutchin T., Prunty H., Heales S. Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nature Communications. 2015;6:6388. doi: 10.1038/ncomms7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ting S.B., Wilanowski T., Auden A., Hall M., Voss A.K., Thomas T. Inositol- and folate-resistant neural tube defects in mice lacking the epithelial-specific factor Grhl-3. Nature Medicine. 2003;9:1513–1519. doi: 10.1038/nm961. [DOI] [PubMed] [Google Scholar]
- 99.Sudiwala S., De Castro S.C., Leung K.Y., Brosnan J.T., Brosnan M.E., Mills K. Formate supplementation enhances folate-dependent nucleotide biosynthesis and prevents spina bifida in a mouse model of folic acid-resistant neural tube defects. Biochimie. 2016;126:63–70. doi: 10.1016/j.biochi.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Locasale J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nature Reviews. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vazquez A., Kamphorst J.J., Markert E.K., Schug Z.T., Tardito S., Gottlieb E. Cancer metabolism at a glance. Journal of Cell Science. 2016;129:3367–3373. doi: 10.1242/jcs.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metabolism. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nature Reviews. Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 104.Possemato R., Marks K.M., Shaul Y.D., Pacold M.E., Kim D., Birsoy K. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Locasale J.W., Grassian A.R., Melman T., Lyssiotis C.A., Mattaini K.R., Bass A.J. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nature Genetics. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mejia N.R., MacKenzie R.E. NAD-dependent methylenetetrahydrofolate dehydrogenase is expressed by immortal cells. Journal of Biological Chemistry. 1985;260:14616–14620. [PubMed] [Google Scholar]
- 107.Mejia N.R., MacKenzie R.E. NAD-dependent methylenetetrahydrofolate dehydrogenase–methenyltetrahydrofolate cyclohydrolase in transformed cells is a mitochondrial enzyme. Biochemical and Biophysical Research Communications. 1988;155:1–6. doi: 10.1016/s0006-291x(88)81040-4. [DOI] [PubMed] [Google Scholar]
- 108.Pikman Y., Puissant A., Alexe G., Furman A., Chen L.M., Frumm S.M. Targeting MTHFD2 in acute myeloid leukemia. Journal of Experimental Medicine. 2016;213:1285–1306. doi: 10.1084/jem.20151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nilsson R., Jain M., Madhusudhan N., Sheppard N.G., Strittmatter L., Kampf C. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nature Communications. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vazquez A., Tedeschi P.M., Bertino J.R. Overexpression of the mitochondrial folate and glycine-serine pathway: a new determinant of methotrexate selectivity in tumors. Cancer Research. 2013;73:478–482. doi: 10.1158/0008-5472.CAN-12-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 112.Rowe P.B., Sauer D., Fahey D., Craig G., Mccairns E. One-carbon metabolism in lectin-activated human-lymphocytes. Archives of Biochemistry and Biophysics. 1985;236:277–288. doi: 10.1016/0003-9861(85)90627-7. [DOI] [PubMed] [Google Scholar]
- 113.Ron-Harel N., Santos D., Ghergurovich J.M., Sage P.T., Reddy A., Lovitch S.B. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metabolism. 2016;24:104–117. doi: 10.1016/j.cmet.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ron-Harel N., Notarangelo G., Ghergurovich J.M., Paulo J.A., Sage P.T., Santos D. Defective respiration and one-carbon metabolism contribute to impaired naive T cell activation in aged mice. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:13347–13352. doi: 10.1073/pnas.1804149115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hollister L.E., Overall J.E., Snow H.L. Relationship of obesity to serum triglyceride, cholesterol, and uric acid, and to plasma-glucose levels. American Journal of Clinical Nutrition. 1967;20:777–782. doi: 10.1093/ajcn/20.7.777. [DOI] [PubMed] [Google Scholar]
- 116.Menni C., Migaud M., Kastenmuller G., Pallister T., Zierer J., Peters A. Metabolomic profiling of long-term weight change: role of oxidative stress and urate levels in weight gain. Obesity. 2017;25:1618–1624. doi: 10.1002/oby.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cirulli E.T., Guo L., Leon Swisher C., Shah N., Huang L., Napier L.A. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metabolism. 2019;29:488–500. doi: 10.1016/j.cmet.2018.09.022. e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emmerson B.T. The management of gout. New England Journal of Medicine. 1996;334:445–451. doi: 10.1056/NEJM199602153340707. [DOI] [PubMed] [Google Scholar]
- 119.Emmerson B.T. Identification of the causes of persistent hyperuricaemia. Lancet. 1991;337:1461–1463. doi: 10.1016/0140-6736(91)93141-u. [DOI] [PubMed] [Google Scholar]
- 120.Gibson T., Rodgers A.V., Simmonds H.A., Toseland P. Beer drinking and its effect on uric acid. British Journal of Rheumatology. 1984;23:203–209. doi: 10.1093/rheumatology/23.3.203. [DOI] [PubMed] [Google Scholar]
- 121.Smolenska Z., Kaznowska Z., Zarowny D., Simmonds H.A., Smolenski R.T. Effect of methotrexate on blood purine and pyrimidine levels in patients with rheumatoid arthritis. Rheumatology. 1999;38:997–1002. doi: 10.1093/rheumatology/38.10.997. [DOI] [PubMed] [Google Scholar]
- 122.Lee J.J., Bykerk V.P., Dresser G.K., Boire G., Haraoui B., Hitchon C. Reduction in serum uric acid may be related to methotrexate efficacy in early rheumatoid arthritis: data from the Canadian early arthritis cohort (CATCH), clinical medicine insights. Arthritis and Musculoskeletal Disorders. 2016;9:37–43. doi: 10.4137/CMAMD.S38092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bertini I., Cacciatore S., Jensen B.V., Schou J.V., Johansen J.S., Kruhoffer M. Metabolomic NMR fingerprinting to identify and predict survival of patients with metastatic colorectal cancer. Cancer Research. 2012;72:356–364. doi: 10.1158/0008-5472.CAN-11-1543. [DOI] [PubMed] [Google Scholar]
- 124.Cheung K.J., Tzameli I., Pissios P., Rovira I., Gavrilova O., Ohtsubo T. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metabolism. 2007;5:115–128. doi: 10.1016/j.cmet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 125.Matsuura F., Yamashita S., Nakamura T., Nishida M., Nozaki S., Funahashi T. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism: Clinical and Experimental. 1998;47:929–933. doi: 10.1016/s0026-0495(98)90346-8. [DOI] [PubMed] [Google Scholar]
- 126.Lin H., An Y., Hao F., Wang Y., Tang H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Scientific Reports. 2016;6:21618. doi: 10.1038/srep21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kolz M., Johnson T., Sanna S., Teumer A., Vitart V., Perola M. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genetics. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lesch M., Nyhan W.L. A familial disorder of uric acid metabolism and central nervous system function. American Journal of Medicine. 1964;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- 129.Seegmiller J.E., Rosenbloom F.M., Kelley W.N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967;155:1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- 130.Mastrangelo L., Kim J.E., Miyanohara A., Kang T.H., Friedmann T. Purinergic signaling in human pluripotent stem cells is regulated by the housekeeping gene encoding hypoxanthine guanine phosphoribosyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3377–3382. doi: 10.1073/pnas.1118067109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dammer E.B., Gottle M., Duong D.M., Hanfelt J., Seyfried N.T., Jinnah H.A. Consequences of impaired purine recycling on the proteome in a cellular model of Lesch-Nyhan disease. Molecular Genetics and Metabolism. 2015;114:570–579. doi: 10.1016/j.ymgme.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ascherio A., LeWitt P.A., Xu K., Eberly S., Watts A., Matson W.R. Urate as a predictor of the rate of clinical decline in Parkinson disease. Archives of Neurology. 2009;66:1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cipriani S., Chen X., Schwarzschild M.A. Urate: a novel biomarker of Parkinson's disease risk, diagnosis and prognosis. Biomarkers in Medicine. 2010;4:701–712. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moccia M., Pappata S., Erro R., Picillo M., Vitale C., Amboni M. Uric acid relates to dopamine transporter availability in Parkinson's disease. Acta Neurologica Scandinavica. 2015;131:127–131. doi: 10.1111/ane.12295. [DOI] [PubMed] [Google Scholar]
- 135.Meiser J., Delcambre S., Wegner A., Jager C., Ghelfi J., d'Herouel A.F. Loss of DJ-1 impairs antioxidant response by altered glutamine and serine metabolism. Neurobiology of Disease. 2016;89:112–125. doi: 10.1016/j.nbd.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 136.Paganoni S., Schwarzschild M.A. Urate as a marker of risk and progression of neurodegenerative disease. Neurotherapeutics: The Journal of the American Society for Experimental Neurotherapeutics. 2017;14:148–153. doi: 10.1007/s13311-016-0497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Petrausch B., Tabibiazar R., Roser T., Jing Y., Goldman D., Stuermer C.A. A purine-sensitive pathway regulates multiple genes involved in axon regeneration in goldfish retinal ganglion cells. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:8031–8041. doi: 10.1523/JNEUROSCI.20-21-08031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Long H., Cameron S., Yu L., Rao Y. De novo GMP synthesis is required for axon guidance in Drosophila. Genetics. 2006;172:1633–1642. doi: 10.1534/genetics.105.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen P., Goldberg D.E., Kolb B., Lanser M., Benowitz L.I. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim D., Zai L., Liang P., Schaffling C., Ahlborn D., Benowitz L.I. Inosine enhances axon sprouting and motor recovery after spinal cord injury. PLoS One. 2013;8:e81948. doi: 10.1371/journal.pone.0081948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levine R.A., Hagege A.A., Judge D.P., Padala M., Dal-Bianco J.P., Aikawa E. Mitral valve disease – morphology and mechanisms. Nature Reviews. Cardiology. 2015;12:689–710. doi: 10.1038/nrcardio.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang L., Wang J., Li R., Fang Z.M., Zhu X.H., Yi X. Disturbed energy and amino acid metabolism with their diagnostic potential in mitral valve disease revealed by untargeted plasma metabolic profiling. Metabolomics: Official Journal of the Metabolomic Society. 2019;15:57. doi: 10.1007/s11306-019-1518-1. [DOI] [PubMed] [Google Scholar]
- 143.Becker B.F. Towards the physiological function of uric acid. Free Radical Biology & Medicine. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 144.Gottdiener J.S., Reda D.J., Williams D.W., Materson B.J. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Journal of the American College of Cardiology. 1997;29:651–658. doi: 10.1016/s0735-1097(96)00554-2. [DOI] [PubMed] [Google Scholar]
- 145.Altaweel M.M., Hanzlik R.P., Ver Hoeve J.N., Eells J., Zhang B. Ocular and systemic safety evaluation of calcium formate as a dietary supplement. Journal of Ocular Pharmacology and Therapeutics: The Official Journal of the Association for Ocular Pharmacology and Therapeutics. 2009;25:223–230. doi: 10.1089/jop.2008.0128. [DOI] [PubMed] [Google Scholar]
- 146.Hanzlik R.P., Fowler S.C., Eells J.T. Absorption and elimination of formate following oral administration of calcium formate in female human subjects. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2005;33:282–286. doi: 10.1124/dmd.104.001289. [DOI] [PubMed] [Google Scholar]
- 147.EFSA Panel on Additives and Products or Substances used in Animal Feed Scientific opinion on the safety and efficacy of calcium formate when used as a technological additive for all animal species. EFSA Journal. 2014;12:3898. [Google Scholar]
- 148.Bosi P., Sarli G., Casini L., De Filippi S., Trevisi P., Mazzoni M. The influence of fat protection of calcium formate on growth and intestinal defence in Escherichia coli K88-challenged weanling pigs. Animal Feed Science and Technology. 2007;139:170–185. [Google Scholar]
- 149.Patten J.D., Waldroup P.W. Use of organic acids in broiler diets. Poultry Science. 1988;67:1178–1182. doi: 10.3382/ps.0671178. [DOI] [PubMed] [Google Scholar]
- 150.Needell J.C., Ir D., Robertson C.E., Kroehl M.E., Frank D.N., Zipris D. Maternal treatment with short-chain fatty acids modulates the intestinal microbiota and immunity and ameliorates type 1 diabetes in the offspring. PLoS One. 2017;12:e0183786. doi: 10.1371/journal.pone.0183786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ducker G.S., Ghergurovich J.M., Mainolfi N., Suri V., Jeong S.K., Hsin-Jung Li S. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:11404–11409. doi: 10.1073/pnas.1706617114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nonaka H., Nakanishi Y., Kuno S., Ota T., Mochidome K., Saito Y. Design strategy for serine hydroxymethyltransferase probes based on retro-aldol-type reaction. Nature Communications. 2019;10:876. doi: 10.1038/s41467-019-08833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tedeschi P.M., Vazquez A., Kerrigan J.E., Bertino J.R. Mitochondrial methylenetetrahydrofolate dehydrogenase (MTHFD2) overexpression is associated with tumor cell proliferation and is a novel target for drug development. Molecular Cancer Research. 2015;13:1361–1366. doi: 10.1158/1541-7786.MCR-15-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gustafsson R., Jemth A.S., Gustafsson N.M., Farnegardh K., Loseva O., Wiita E. Crystal structure of the emerging cancer target MTHFD2 in complex with a substrate-based inhibitor. Cancer Research. 2017;77:937–948. doi: 10.1158/0008-5472.CAN-16-1476. [DOI] [PubMed] [Google Scholar]
- 155.Fu C., Sikandar A., Donner J., Zaburannyi N., Herrmann J., Reck M. The natural product carolacton inhibits folate-dependent C1 metabolism by targeting FolD/MTHFD. Nature Communications. 2017;8:1529. doi: 10.1038/s41467-017-01671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tephly T.R., Green M.D., Gamble J. Formate metabolism in micropigs. Toxicology and Applied Pharmacology. 1992;116:142–145. doi: 10.1016/0041-008x(92)90155-l. [DOI] [PubMed] [Google Scholar]
- 157.Horton V.L., Higuchi M.A., Rickert D.E. Physiologically based pharmacokinetic model for methanol in rats, monkeys, and humans. Toxicology and Applied Pharmacology. 1992;117:26–36. doi: 10.1016/0041-008x(92)90213-c. [DOI] [PubMed] [Google Scholar]
- 158.MacMillan L., Lamarre S.G., daSilva R.P., Jacobs R.L., Brosnan M.E., Brosnan J.T. Riboflavin deficiency in rats decreases de novo formate production but does not affect plasma formate concentration. Journal of Nutrition. 2017;147:346–352. doi: 10.3945/jn.116.243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morrow G.P., MacMillan L., Lamarre S.G., Young S.K., MacFarlane A.J., Brosnan M.E. In vivo kinetics of formate metabolism in folate-deficient and folate-replete rats. Journal of Biological Chemistry. 2015;290:2244–2250. doi: 10.1074/jbc.M114.600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hathcock J.N. Academic Press; New York: 1982. Nutritional toxicology. [Google Scholar]
- 161.Spiller G.A. CRC Press; Boca Raton, Fla: 1998. Caffeine. [Google Scholar]