Abstract
Background
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria are the gold standard for risk‐stratifying patients with metastatic renal cell cancer (mRCC). We developed a novel risk scoring system for patients with mRCC treated with immune checkpoint inhibitors (ICIs).
Methods
We performed a retrospective analysis of 100 ICI‐treated patients with mRCC at Winship Cancer Institute from 2015 to 2018. Several baseline variables were collected, including markers of inflammation, body mass index (BMI), and sites of metastatic disease, and all were considered for inclusion in our risk scoring system. Upon variable selection in multivariable model, monocyte‐to‐lymphocyte ratio (MLR), BMI, and number and sites of metastases at baseline were used for risk score calculation. Patients were categorized using four‐level risk groups as good (risk score = 0), intermediate (risk score = 1), poor (risk score = 2), or very poor (risk score = 3–4). Cox's proportional hazard model and the Kaplan‐Meier method were implemented for survival outcomes.
Results
Most patients were male (66%) with clear cell renal cell carcinoma (72%). The majority (71%) received anti–programmed cell death protein‐1 monotherapy. Our risk scoring criteria had higher Uno's concordance statistics than IMDC in predicting overall survival (OS; 0.71 vs. 0.57) and progression‐free survival (0.61 vs. 0.58). Setting good risk (MLR <0.93, BMI ≥24, and D_Met = 0) as the reference, the OS hazard ratios were 29.5 (95% confidence interval [CI], 3.64–238.9), 6.58 (95% CI, 0.84–51.68), and 3.75 (95% CI, 0.49–28.57) for very poor, poor, and intermediate risk groups, respectively.
Conclusion
Risk scoring using MLR, BMI, and number and sites of metastases may be an effective way to predict survival in patients with mRCC receiving ICI. These results should be validated in a larger, prospective study.
Implications for Practice
A risk scoring system was created for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. The results of this study have significant implications for practicing oncologists in the community and academic setting. Importantly, these results identify readily available risk factors that can be used clinically to risk‐stratify patients with metastatic renal cell carcinoma who are treated with immune checkpoint inhibitors.
Keywords: Immunotherapy, Prognostic markers, Renal cell carcinoma, Risk scoring system, Inflammation, Body composition
Short abstract
Considering the increasing number of treatment options for patients with metastatic renal cell carcinoma, it is becoming more important to identify factors that increase the likelihood of responses to systemic therapy. This article describes a novel risk scoring system for patients with metastatic renal cell carcinoma treated with ICI, focusing on risk from systemic inflammation, number and sites of metastatic disease, and body composition.
Introduction
Immunotherapy has changed the landscape of treatment of metastatic renal cell carcinoma (mRCC) since nivolumab, a programmed cell death protein‐1 (PD‐1) inhibitor, was approved for vascular endothelial growth factor (VEGF)–refractory mRCC in November 2015 1. The first immune checkpoint inhibitor (ICI) combination regimen, nivolumab and ipilimumab, was recently approved for first‐line intermediate or poor risk mRCC, and results from ongoing trials of ICI and anti‐VEGF combination regimens have been very promising 1, 2, 3, 4. Given the increasing number of treatment options for patients with mRCC, including several ICI‐based regimens, it is becoming increasingly important to identify risk factors that make patients with mRCC more likely to respond to ICI.
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk criteria are currently the gold standard for predicting survival in patients with renal cell cancer (RCC) 5, 6. This method includes six risk factors: time from RCC diagnosis to first‐line systemic therapy <1 year, elevated platelet count, elevated absolute neutrophil count, anemia, hypercalcemia, and Karnofsky performance status <80. The IMDC criteria have been validated in patients treated with VEGF‐targeted therapy 7 and are widely used to risk‐stratify patients in trials of patients with RCC. Although the IMDC criteria capture both laboratory values and clinical findings that accurately predict survival in patients with mRCC, they do not take into account number and sites of metastatic disease, systemic inflammation, or body composition, which all likely affect the host's systemic immune responses to ICI therapy 8, 9, 10, 11.
Patients treated with ICI may require specific risk stratification given the unique mechanism of action of ICI. Updated prognostic models may more accurately predict survival and optimize selection of patients who are most likely to respond to ICI‐based treatment regimens. In this study, we created a novel risk stratification system, the Emory risk scoring system, for patients with mRCC treated with ICI. We categorized patients into four groups using three variables to capture risk from systemic inflammation, number and sites of metastatic disease, and body composition.
Materials and Methods
Patients and Data Collection
We performed a retrospective analysis of 100 patients with mRCC who received ICI at Winship Cancer Institute of Emory University between 2015 and 2018. Overall survival (OS) and progression‐free survival (PFS) were measured from first dose of ICI to date of death or hospice referral and radiographic or clinical progression, respectively. RECIST version 1.1 was used to evaluate response to ICI 12. Neutrophil‐to‐lymphocyte ratio (NLR), monocyte‐to‐lymphocyte ratio (MLR), and platelet‐to‐lymphocyte ratio (PLR) were obtained from the complete blood count prior to treatment with ICI and used as surrogates of systemic inflammation. Body mass index (BMI) at the time of ICI initiation was obtained and used to represent body composition. Number and sites of metastasis at the time of first dose of ICI were obtained from radiology reports and clinic notes. Other variables collected included demographic information, drug allergies, prior lines of systemic therapy, post‐ICI treatments, immune‐related adverse events, eosinophil count, basophil count, and IMDC risk group criteria.
The study was approved by the Emory University Institutional Review Board and was conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. Informed consent for publication has been obtained, and the consent forms are held by the authors. All data generated or analyzed during this study are included in this published article.
Statistical Analysis
The analyses were done using SAS 9.4 and SAS macros 13, and the significance level was set at .05 by two‐sided tests. Given that the presence of liver metastases was associated with shorter OS in univariate analysis (UVA), we created a new variable, D_Met, to more accurately represent risk related to metastatic sites: D_Met = 0 indicates fewer than two metastatic sites, D_Met = 1 indicates at least two metastatic sites without liver metastasis, and D_Met = 2 indicates at least two metastatic sites with liver metastasis. OS was set as the primary outcome. For prognostic factors with continuous values, the optimal cut regarding OS was searched at all unique values, and the value that was chosen was the one that gave the maximum separation 14. The following variables, which were collected prior to treatment with ICI, were under consideration for inclusion in our model: age, gender, race, smoking status, medication allergies, D_Met, Eastern Cooperative Oncology Group performance status, NLR, MLR, PLR, BMI, eosinophils, and basophils. With all possible prognostic variables under consideration, a backward variable selection was implemented in Cox's proportional hazard model regarding OS with p < .05. Based on the parameter estimated in the final model, a score was assigned based on Sullivan's weighting schema 15, 16. The final variables selected were baseline MLR, D_Met, and baseline BMI.
The Emory risk scoring system is shown is Table 1. MLR ≥0.93, BMI <24, and D_Met = 1 each counted as one point in the risk score, whereas D_Met = 2 counted as two points in the risk score. Patients were categorized as good risk (Emory risk score = 0), intermediate risk (Emory risk score = 1), poor risk (Emory risk score = 2), or very poor risk (Emory risk score = 3 or 4). Uno's concordance statistics (C‐statistics) were calculated and compared for the Emory risk scoring system and the IMDC criteria regarding the discrimination for OS or PFS 17. The C‐statistics for each risk scoring system were calculated at the initiation of ICI therapy. Cox's proportional hazard model and the Kaplan‐Meier method were used for association with OS and PFS in UVA and multivariable analysis (MVA) for the Emory risk scoring system.
Table 1.
Emory risk scoring system
| Variable | Score |
|---|---|
| MLR ≥0.93 | 1 |
| MLR <0.93 | 0 |
| Baseline BMI <24 | 1 |
| Baseline BMI ≥24 | 0 |
| D_Met = 2 | 2 |
| D_Met = 1 | 1 |
| D_Met = 0 | 0 |
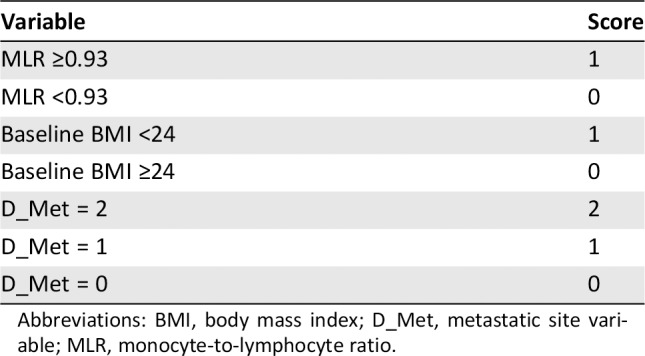
Abbreviations: BMI, body mass index; D_Met, metastatic site variable; MLR, monocyte‐to‐lymphocyte ratio.
Results
Baseline Patient Characteristics
The baseline descriptive statistics are presented in Table 2. Most patients (66%) were male, and the median age was 65 years. The majority (72%) had clear cell renal cell carcinoma histology. The distribution of metastatic sites was as follows: 56% had lymph node metastases, 37% had bone metastases, 25% has liver metastases, 17% had brain metastases, and 71% had lung metastases. Most patients (60%) were overweight or obese at baseline (BMI ≥25), and the median BMI was 26.7. The majority of patients (83%) had at least two sites of metastatic disease at the time of first dose of ICI. The IMDC risk group distribution was as follows: 15% favorable, 55% intermediate, 22% poor risk, and 8% missing. The median baseline NLR, MLR, and PLR were 3.1, 0.40, and 196.02, respectively. Anti–PD‐1 monotherapy (71%) was the most common ICI treatment regimen, and 29% received combination therapy. Most patients (45%) received one prior line of systemic therapy before ICI, and 31% received ICI as first‐line treatment. Less than one‐quarter (24%) of patients received two or more lines of systemic therapy before ICI initiation.
Table 2.
Demographics and baseline patient characteristics
| Variable, category | n (%) |
|---|---|
| Gender | |
| M | 66 (66) |
| F | 34 (34) |
| Race | |
| White/Asian | 83 (83) |
| Black | 17 (17) |
| ECOG PS | |
| 0–1 | 69 (69) |
| 2+ | 15 (15) |
| Missing | 16 (16) |
| Histology | |
| ccRCC | 72 (72) |
| nccRCC | 20 (20) |
| Unknown | 8 (8) |
| Number of metastatic sites | |
| 0–1 | 17 (17) |
| 2+ | 83 (83) |
| IMDC risk group | |
| Favorable | 15 (15) |
| Intermediate | 55 (55) |
| Poor | 22 (22) |
| Missing | 8 (8) |
| Metastatic site distribution | |
| Lymph node | 56 (56) |
| Lung | 71 (71) |
| Brain | 17 (17) |
| Bone | 37 (37) |
| Liver | 25 (25) |
| Baseline BMI (median: 26.7) | |
| ≥25 | 60 (60) |
| <25 | 39 (39) |
| Missing | 1 (1) |
| Type of ICI | |
| PD‐1 monotherapy | 71 (71) |
| ICI combination | 29 (29) |
| Number of prior lines of systemic therapy | |
| 0 | 31 (31) |
| 1 | 45 (45) |
| 2+ | 24 (24) |
| Median NLR | 3.1 |
| Median MLR | 0.40 |
| Median PLR | 196.0 |
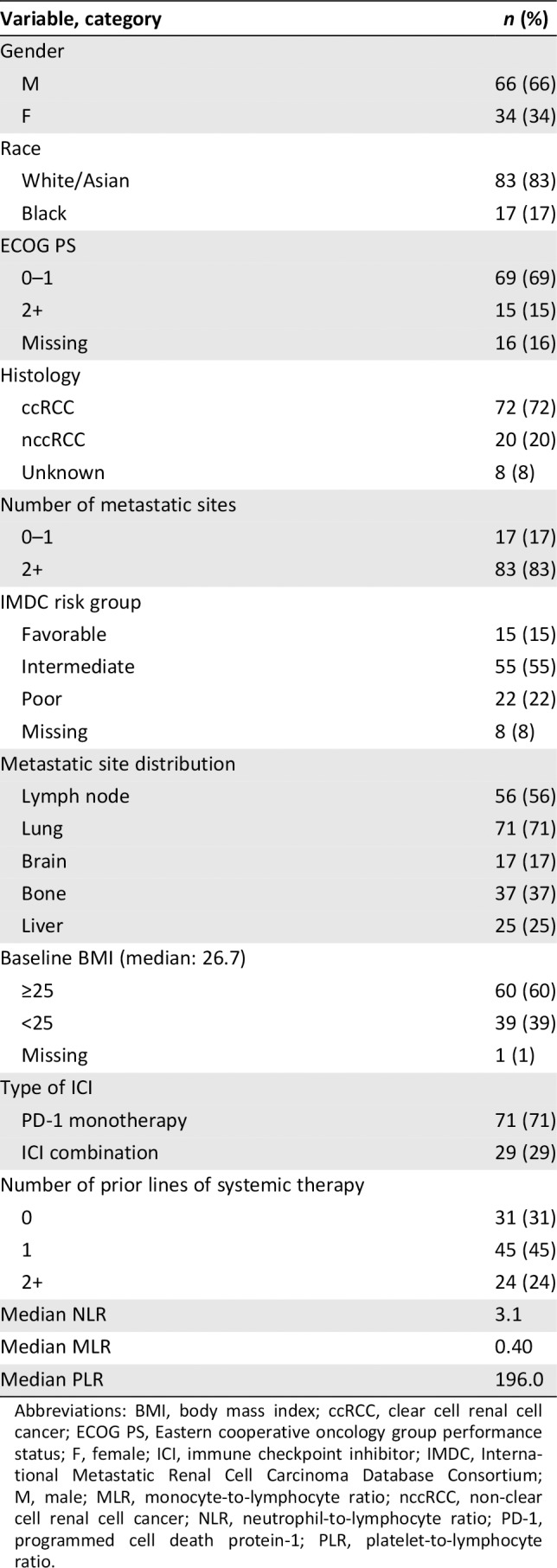
Abbreviations: BMI, body mass index; ccRCC, clear cell renal cell cancer; ECOG PS, Eastern cooperative oncology group performance status; F, female; ICI, immune checkpoint inhibitor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; M, male; MLR, monocyte‐to‐lymphocyte ratio; nccRCC, non‐clear cell renal cell cancer; NLR, neutrophil‐to‐lymphocyte ratio; PD‐1, programmed cell death protein‐1; PLR, platelet‐to‐lymphocyte ratio.
Emory Risk Scoring System Analysis
The UVA and MVA of the association between the Emory risk scoring system and survival are presented in Table 3. Very poor risk patients had significantly shorter OS (hazard ratio [HR], 37.72; 95% confidence interval [CI], 4.76–298.70; p < .001) and PFS (HR, 3.87; CI, 1.50–9.96; p = .005) than good risk patients in UVA. Poor risk patients also had significantly shorter OS than good risk patients (HR, 8.49; CI, 1.11–64.75; p = .039), and they showed a trend toward shorter PFS (HR, 2.13; CI, 0.90–5.02; p = .085) in UVA. In MVA, very poor risk patients had significantly shorter OS (HR, 29.50; CI, 3.64–238.9, p = .002) and PFS (HR, 2.80; CI, 1.10–7.11; p = .030) compared with good risk patients. Poor and intermediate risk patients also trended toward shorter OS (poor risk HR, 6.58; CI, 0.84–51.68; p = .073; intermediate HR, 3.75; CI, 0.49–28.57; p = .203) and PFS (poor risk HR, 1.36; CI, 0.55–3.33; p = .506; intermediate HR, 1.70; CI, 0.73–3.94; p = .218) compared with the good risk group. The median OS and PFS was significantly shorter for very poor risk patients (OS, 4.2 months; PFS, 2.6 months) compared with poor risk (OS, 16.9 months; PFS, 4.5 months), intermediate risk (OS, 29.7 months; PFS, 6.1 months), and good risk patients (OS, not reached; PFS, 12.3 months) per Kaplan‐Meier estimation (Figs. 1 and 2; OS, p < .001; PFS, p = .068).
Table 3.
UVA and MVA of risk groups and survival
| Risk groups | UVA | MVAa | ||||||
|---|---|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | |||||
| HR (CI) | p value | HR (CI) | p value | HR (CI) | p value | HR (CI) | p value | |
| Very poor risk: Risk score = 3–4 (n = 13) | 37.72 (4.76–298.70) | <.001b | 3.87 (1.50–9.96) | .005b | 29.50 (3.64–238.90) | .002b | 2.80 (1.10–7.11) | .030b |
| Median survival: 4.2 months | Median survival: 2.6 months | |||||||
| Poor risk: Risk score = 2 (n = 28) | 8.49 (1.11–64.75) | .039b | 2.13 (0.90–5.02) | .085 | 6.58 (0.84–51.68) | .073 | 1.36 (0.55–3.33) | .506 |
| Median survival: 16.9 months | Median survival: 4.5 months | |||||||
| Intermediate risk: Risk score = 1 (n = 45) | 4.22 (0.55–32.17) | .165 | 1.40 (0.61–3.19) | .422 | 3.75 (0.49–28.57) | .203 | 1.70 (0.73–3.94) | .218 |
| Median survival: 29.7 months | Median survival: 6.1 months | |||||||
| Good risk: Risk score = 0 (n = 13) | 1 | 1 | 1 | 1 | ||||
| Median survival: Not reached | Median survival: 12.3 months | |||||||
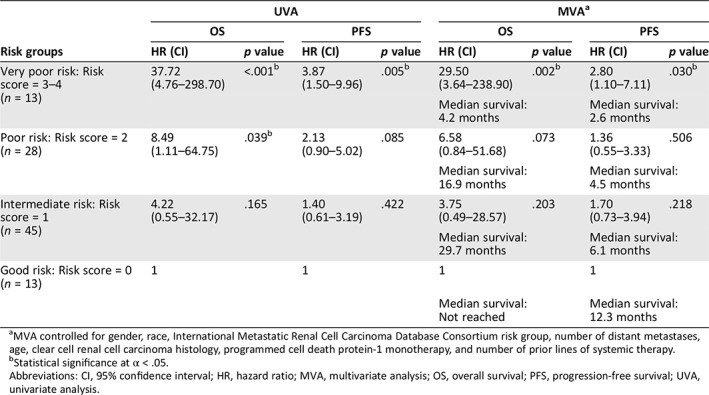
MVA controlled for gender, race, International Metastatic Renal Cell Carcinoma Database Consortium risk group, number of distant metastases, age, clear cell renal cell carcinoma histology, programmed cell death protein‐1 monotherapy, and number of prior lines of systemic therapy.
Statistical significance at α < .05.
Abbreviations: CI, 95% confidence interval; HR, hazard ratio; MVA, multivariate analysis; OS, overall survival; PFS, progression‐free survival; UVA, univariate analysis.
Figure 1.
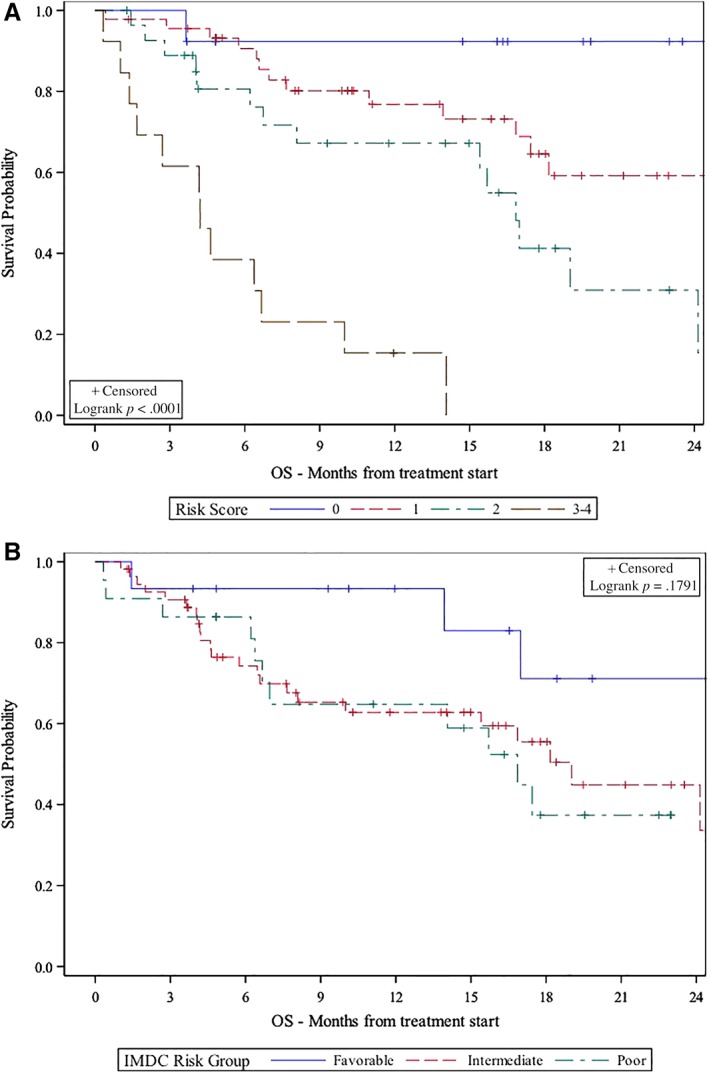
Emory risk group versus IMDC risk group stratification and association with OS. (A): Emory risk group stratification. 0 = good risk, 1 = intermediate risk, 2 = poor risk, 3–4 = very poor risk. (B): IMDC risk group stratification.Abbreviations: IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; OS, overall survival.
Figure 2.
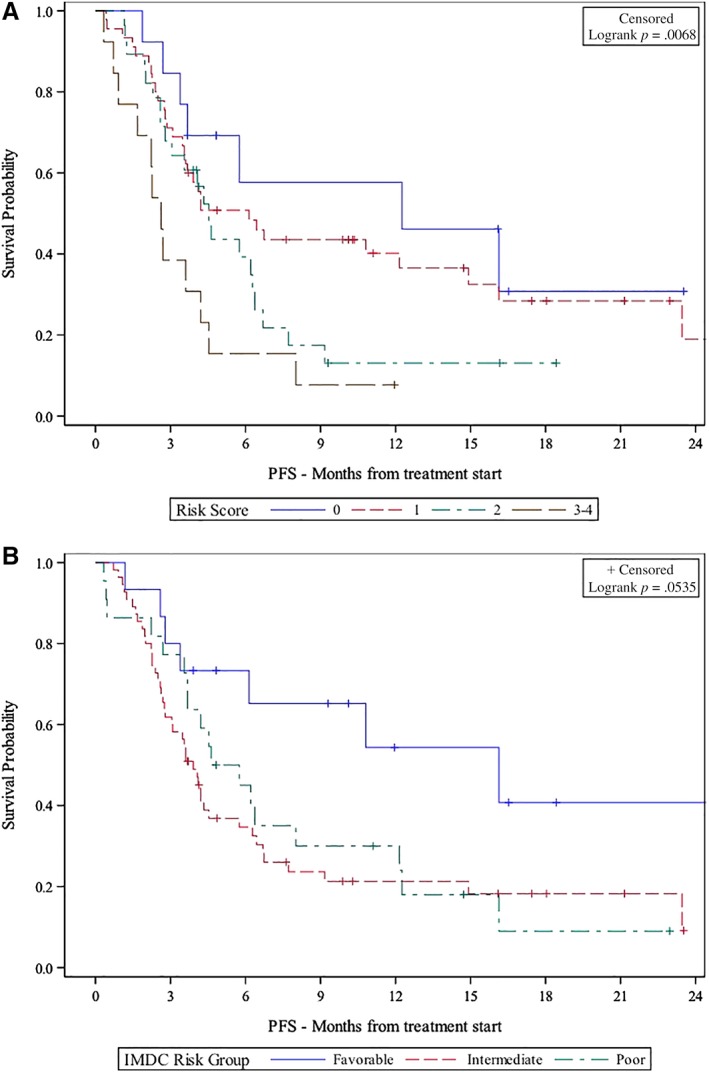
Emory risk group versus IMDC risk group stratification and association with PFS. (A): Emory risk group stratification. 0 = good risk, 1 = intermediate risk, 2 = poor risk, 3–4 = very poor risk. (B): IMDC risk group stratification.Abbreviations: IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; PFS, progression‐free survival.
Given that MLR, PLR, and NLR measure systemic inflammation and were highly correlated (Pearson correlation coefficients >0.678, all p < .0001; supplemental online Fig. 1), risk groups using NLR or PLR instead of MLR were also created. The Kaplan‐Meier plots for the NLR‐based and PLR‐based models are given in supplemental online Figures 2 and 3. The C‐statistics were similar for each of the models in predicting OS (MLR‐based model, 0.711; NLR‐based model, 0.687; PLR‐based model, 0.682) and PFS (MLR‐based model, 0.610; NLR‐based model, 0.611; PLR‐based mode, 0.594).
Comparison with IMDC
A discrimination by the prediction model was measured by Uno's C‐statistics. Regarding predicting OS, the Emory risk scoring system had a C‐statistic of 0.711, compared with 0.566 by IMDC (p = .053). The C‐statistic was also higher for the Emory risk scoring system in predicting PFS (0.610) compared with 0.575 for the IMDC risk grouping (p = .587).
Discussion
Optimal risk stratification is important for practicing oncologists to predict survival and manage treatment for patients. This is becoming increasingly important in mRCC, given that there have been several recently approved treatments with distinct targets such as cabozantanib, nivolumab, and ipilimumab and lenvatinib and everolimus 4, 18, 19, 20. Risk scoring is particularly important for patients treated with immunotherapy because durable clinical benefit is only observed in a subset of patients 21. Furthermore, results from the CA209‐214 study showed that nivolumab and ipilimumab was only superior to sunitinib, a VEGF inhibitor, in intermediate and poor risk patients 19. In this study, we developed a hypothesis‐generating risk scoring system using three variables to represent risk from number and sites of metastatic disease (D_Met), body composition (BMI), and inflammation (MLR). This builds upon previously established data on the effectiveness of the IMDC criteria in predicting survival in patients with mRCC treated with VEGF‐targeted therapy 6, 7, 22. The results from our study indicate that incorporation of risk factors such as sites of metastatic disease, body composition, and a more accurate inflammatory marker may improve prediction of survival in patients with mRCC treated with ICI.
It is becoming increasingly recognized that the tumor microenvironment (TME) influences responses to immunotherapy and that the TME differs significantly between metastatic sites depending on the organ tropism 23. Therefore, sites of metastatic disease likely influence responses to immunotherapy. This is particularly true for the liver, which is a secondary lymphoid organ that synthesizes acute phase reactants, complement proteins, and chemokines and houses a significant population of lymphoid cells such as T lymphocytes 24, 25. Unsurprisingly, liver metastases are a poor prognostic factor in patients treated with ICI 8, 26, 27, 28. A recent study suggests that this may be because liver metastases decrease the ratio of CD8+/Foxp3+ regulatory T cells and the number of activated PD‐1+/CTLA‐4+ T cells, indicating that liver metastases may have a systemic influence on tumor immunity 29. The results from this study as well as several previous studies suggest that liver metastases are a poor prognostic factor in patients treated with ICI. Therefore, updated prognostic models may be improved by including a variable to account for number of metastatic sites and the presence of liver metastases.
Body composition has been an increasingly studied prognostic factor in patients with cancer. The most easily accessible and clinically useful marker of body composition is BMI. Although obesity is a risk factor for the development and progression of some cancers 30, 31, several studies have shown that BMI may be protective in patients with RCC, in both the perioperative and metastatic settings 32, 33. A recent article showed that increased BMI was independently associated with improved survival in patients with melanoma treated with ICI 11. Although the underlying biology explaining these clinical findings has not been elucidated, there are several potential biological explanations for this. Obesity has been shown to alter fatty acid metabolism, which plays a role in both oncogenesis and responses to therapy 34, 35. Adipocyte PD‐L1 expression increases during adipogenesis, suggesting that obesity promotes tumor immune evasion, and this process can be reversed with treatment with ICI via increased activity of effector T cells 36, 37. Taken together, inclusion of a body composition marker such as BMI in updated prognostic models may improve prediction of survival in patients with mRCC treated with ICI.
Inflammation has been described as one of the hallmarks of cancer 38, 39. NLR, MLR, and PLR have been used as markers of systemic inflammation 40. Increased NLR, MLR, and PLR have been shown to be associated with poor outcomes in patients with cancer treated with immunotherapy 40, 41, 42, 43. This may be due to the fact that ICIs rely on the host immune system for their efficacy 44. Therefore, immune dysfunction likely decreases the likelihood that patients will respond favorably to ICI. Although tissue‐based macrophages have differing effects on the inflammatory process depending on whether they are M1 or M2 macrophages 45, MLR was a stronger predictor of survival in our model. It should be noted that NLR, MLR, and PLR were highly correlated (Pearson correlation coefficients >0.678, all p < .0001) and had similar C‐statistics in our model. Therefore, any of these values may provide value as a prognostic biomarker in patients with mRCC treated with ICI. Although the IMDC criteria include thrombocytosis and neutrophilia as risk factors, MLR may be a more effective predictor of survival. The results of this study build upon previous evidence that suggests that the inclusion of a systemic inflammation variable may be helpful in updated prognostic scoring models for patients treated with ICI.
Although this study has significant clinical relevance, the results are hypothesis‐generating, and several limitations should be mentioned. First, this is a retrospective cohort that is subject to selection bias. We attempted to decrease the effect of this on the results of the study by including all patients at our center who received at least one dose of ICI regardless of their RCC histology or other baseline characteristics. It should also be mentioned that BMI is an imperfect marker of body composition, particularly given the increasing prevalence of obesity in the U.S. 46. We chose BMI as a marker of body composition because it is cheap and readily available to practicing oncologists. Finally, we included all patients in our survival analysis regardless of the number of lines of therapy prior to treatment with ICI. Given that survival is likely affected by the number of prior lines of therapy, we attempted to minimize the confounding effect by controlling for this in MVA. Future studies are required to validate the results of this study and to elucidate the underlying biology explaining how metastatic sites, body composition, and systemic inflammation affect host immune responses to ICI.
Conclusion
We developed a novel risk scoring system for patients with mRCC treated with ICI‐based treatment regimens. The results from this study suggest that it may be useful to update prognostic scoring systems for ICI‐treated patients with mRCC to include BMI as a body composition marker, number and sites of metastatic disease to capture the effect of the TME, and MLR as a surrogate of systemic inflammation. Risk scoring may be particularly useful for patients being considered for treatment with immunotherapy, given that durable clinical responses are only seen in a subset of patients. This study is hypothesis generating, and future studies are needed to validate these findings in a larger, prospective study.
Author Contributions
Conception/design: Dylan J. Martini, Yuan Liu, Mehmet Asim Bilen
Provision of study material or patients: Bradley C. Carthon, Emilie Elise Hitron, Greta Anne Russler, Sarah Caulfield, Haydn T. Kissick, Wayne B. Harris, Omer Kucuk, Viraj A. Master, Mehmet Asim Bilen
Collection and/or assembly of data: Dylan J. Martini, Julie M. Shabto
Data analysis and interpretation: Dylan J. Martini, Yuan Liu, Mehmet Asim Bilen
Manuscript writing: Dylan J. Martini, Yuan Liu, Julie M. Shabto, Mehmet Asim Bilen
Final approval of manuscript: Dylan J. Martini, Yuan Liu, Julie M. Shabto, Bradley C. Carthon, Emilie Elise Hitron, Greta Anne Russler, Sarah Caulfield, Haydn T. Kissick, Wayne B. Harris, Omer Kucuk, Viraj A. Master, Mehmet Asim Bilen
Disclosures
Bradley C. Carthon: Astellas Medivation, Pfizer, Blue Earth Diagnostics (C/A), Bristol‐Myers Squibb (other—travel); Mehmet Asim Bilen: Exelixis, Nektar, Genomic health, Sanofi (C/A), Bayer, Bristol‐Myers Squibb, Genentech/Roche, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Peleton, Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Acknowledgements
Data presented in this article were accepted as an abstract at the 2019 American Society of Clinical Oncology Annual Meeting. Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University and NIH/National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Shinji S, Ishiwata T, Tajiri T et al. External whole‐body image of EGFP gene expression. J Nippon Med Sch 2003;70:462–463. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Penkov K, Haanen J et al. Avelumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rini BI, Plimack ER, Stus V et al.; KEYNOTE‐426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 4. Motzer RJ, Tannier NM, McDermott DF et al.; CheckMate 214 Investigators Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heng DY, Xie W, Regan MM et al. External validation and comparison with other models of the International Metastatic Renal‐Cell Carcinoma Database Consortium prognostic model: A population‐based study. Lancet Oncol 2013;14:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka N, Mizuno R, Ito K et al. External validation of the MSKCC and IMDC risk models in patients treated with targeted therapy as a first‐line and subsequent second‐line treatment: A Japanese multi‐institutional study. Eur Urol Focus 2016;2:303–309. [DOI] [PubMed] [Google Scholar]
- 8. Tumeh PC, Hellman MD, Hamid O et al. Liver metastasis and treatment outcome with anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017;5:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berghoff AS, Venur VA, Preusser M et al. Immune checkpoint inhibitors in brain metastases: From biology to treatment. Am Soc Clin Oncol Educ Book 2016;35:e116–e122. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura K, Smyth MJ. Targeting cancer‐related inflammation in the era of immunotherapy. Immunol Cell Biol 2017;95:325–332. [DOI] [PubMed] [Google Scholar]
- 11. McQuade JL, Daniel CR, Hess KR et al. Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol 2018;19:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y, Nickleach DC, Zhang C et al. Carrying out streamlined routine data analyses with reports for observational studies: Introduction to a series of generic SAS macros. F1000Res 2018;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mandrekar JN, Mandrekar SJ, Cha SS. Cutpoint determination methods in survival analysis using SAS. Paper 261‐28 presented at: 28th SAS Users Group International Conference (SUGI); March 30 to April 2, 2003; Seattle, WA.
- 15. Sullivan LM, Massaro JM, D'Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 2004;23:1631–1660. [DOI] [PubMed] [Google Scholar]
- 16. Mehta HB, Mehta V, Girman CT et al. Regression coefficient‐based scoring system should be used to assign weights to the risk index. J Clin Epidemiol 2016;79:22–28. [DOI] [PubMed] [Google Scholar]
- 17. Uno H, Cai T, Pencina MJ et al. On the C‐statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2012;30:1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open‐label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 19. Powles T, Motzer RJ, Escudier B et al. Outcomes based on prior therapy in the phase 3 METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer 2018;119:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motzer RJ, Hutson TE, Glen H et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open‐label, multicentre trial. Lancet Oncol 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 21. Naing A. Being realistic and optimistic in curing cancer. J Immunother Precis Oncol. 2018;1:53–55. [Google Scholar]
- 22. Ko JJ, Xie W, Kroeger N et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first‐line targeted therapy: A population‐based study. Lancet Oncol 2015;16:293–300. [DOI] [PubMed] [Google Scholar]
- 23. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147–163. [DOI] [PubMed] [Google Scholar]
- 25. Nemeth E, Baird AW, O'Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol 2009;31:333–343. [DOI] [PubMed] [Google Scholar]
- 26. Tamiya M, Tamiya A, Inoue T et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non‐small cell lung cancer: A retrospective multicenter trial. PLoS One 2018;13:e0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bilen MA, Shabto JM, Martini DJ et al. Sites of metastasis and association with clinical outcome (CO) in advanced stage cancer patients (pts) treated with immunotherapy (IO). Ann Oncol 2018;29(suppl 8):1221PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shabto JM, Martini DJ, Liu Y et al. Sites of metastases (mets) and their association with clinical outcomes (CO) in urothelial cancer patients (pts) treated with immunotherapy (IO). J Clin Oncol 2019;37(suppl 7):473A. [Google Scholar]
- 29. Lee J. Mehdizadeh S, Tsai K et al. Immunological insights into liver metastasis associated resistance to checkpoint blockade cancer immunotherapy. J Immunol 2018;200(suppl 1):122.26. [Google Scholar]
- 30. De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013;2013:291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bousquenaud M, Fico F, Solinas G et al. Obesity promotes the expansion of metastasis‐initiating cells in breast cancer. Breast Cancer Res 2018;20:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Albiges L, Hakimi AA, Xie W et al. Body mass index and metastatic renal cell carcinoma: Clinical and biological correlations. J Clin Oncol 2016;34:3655–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi Y, Park B, Jeong BC et al. Body mass index and survival in patients with renal cell carcinoma: A clinical‐based cohort and meta‐analysis. Int J Cancer 2013;132:625–634. [DOI] [PubMed] [Google Scholar]
- 34. Balaban S, Lee LS, Schreuder M et al. Obesity and cancer progression: Is there a role of fatty acid metabolism? Biomed Res Int 2015;2015:274585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vriens K, Christen S, Parik S et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 2019;566:403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu B, Sun X, Gupta HB et al. Adipose PD‐L1 modulates PD‐1/PD‐L1 checkpoint blockade immunotherapy efficacy in breast cancer. Oncoimmunology 2018;7:e1500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Z, Aguilar EG, Luna JI et al. Paradoxical effects of obesity on T cell function during tumor progression and PD‐1 checkpoint blockade. Nat Med 2019;25:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 39. Colotta F, Allavena P, Sica A et al. Cancer‐related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009;30:1073–1081. [DOI] [PubMed] [Google Scholar]
- 40. Bilen MA, Martini DJ, Liu Y et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced‐stage cancer treated with immunotherapy. Cancer 2019;125:127–134. [DOI] [PubMed] [Google Scholar]
- 41. Lalani AA, Xie W, Martini DJ et al. Change in neutrophil‐to‐lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer 2018;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diem S, Schmid S, Krapf M et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176–181. [DOI] [PubMed] [Google Scholar]
- 43. Bilen MA, Dutcher GMA, Liu Y et al. Association between pretreatment neutrophil‐to‐lymphocyte ratio and outcome of patients with metastatic renal‐cell carcinoma treated with nivolumab. Clin Genitourin Cancer 2018;16:e563–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–1086. [DOI] [PubMed] [Google Scholar]
- 45. Richards DM, Hettinger J, Feuerer M. Monocytes and macrophages in cancer: Development and functions. Cancer Microenviron 2013;6:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hales CM, Carroll MD, Fryar CD et al. Prevalence of obesity among adults and youth: United States, 2015‐2016. NCHS Data Brief 2017;(288):1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures


