Abstract
Puerto Ricans have high prevalence of obesity, yet little information is available regarding its association with eating patterns in this population. We hypothesized that higher eating frequency and skipping breakfast would be associated with increased odds of abdominal obesity among adults living in Puerto Rico (PR). In a cross-sectional study of adults living in PR aged 30–75y old (n=310), participants reported their frequency of eating meals per day, including snacks and breakfast. Trained interviewers measured waist (WC) and hip circumferences. We calculated the waist-to-hip ratio (WHR) dividing the waist by the hip measurement. Abdominal obesity was defined as either high WC (men≥94cm; women≥80cm) or high WHR (men≥0.90; women≥0.85). We used logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) to assess the association of eating frequency (≤1.5; 1.5–3; ≥3 times/d) and breakfast consumption (vs. non) with abdominal obesity. Models were adjusted for age, sex, income, smoking, physical activity, TV watching, energy intake, diet quality, and eating frequency (only for breakfast consumption). Most participants consumed breakfast (70%), ate 1.5–3 times/d (47%), and had high WC (75%) and WHR (77%). Participants who ate 1.5–3 (OR:2.75, 95%CI:1.23, 6.15) and ≥3 times/d (OR:2.88; 95%CI:1.14, 7.31) were more likely to have high WC compared with participants who ate ≤1.5 times/d (P-trend=0.04). Breakfast consumption was not associated with abdominal obesity. In conclusion, higher eating frequency, but not skipping breakfast, is associated with abdominal obesity among adults in PR. Consuming less frequent meals may help prevent abdominal obesity in this population.
Keywords: eating behavior, breakfast, abdominal obesity, waist circumference, cross-sectional study
1. Introduction
In the United States (U.S.), meal and snack patterns have changed over recent decades. Between 1971–1974 and 2009–2010, the proportion of adults that reported consuming all three main meals (breakfast, lunch, and dinner) decreased from 73% to 59% in men and from 75% to 63% in women [1]. Between 1965 and 1991, the proportion of U.S. adults skipping breakfast increased from 14% to 25%, and the prevalence of snacking increased significantly from 71% to 97% in 2003–2006 [2]. The timeline of this decline in breakfast consumption and the increase in snacking parallels the increase in obesity prevalence. During the same period, the age-adjusted prevalence of obesity (body mass index ≥30 kg/m2) among U.S. adults increased nearly twofold from 12.8% to 22.5% [3]. By 2016, the age-standardized prevalence of obesity among U.S. adults had risen to 40% [4]; the prevalence among Hispanics (47%) was the highest compared to other racial/ethnic groups [5].
Numerous cross-sectional [6–11] and prospective observational studies [12–14] have found an association between skipping breakfast and obesity, which has led to recommendations to consume breakfast as a strategy to prevent weight gain [15]. These recommendations are based on the hypothesis that skipping breakfast leads to higher energy intake later in the day [16]. Snacking or eating frequency can also impact body weight [17]. Greater eating frequency appears to be related to healthier body weight and better health status [18–20]. Proposed mechanisms include better appetite control [21, 22], improved glucose homeostasis [23], and increased diet-induced thermogenesis [24]. However, while some studies have reported benefits of frequent meals, others have not [25, 26]. It has been argued that eating frequent meals is inconsistent with human evolution and circadian rhythms [27]. Less frequent eating and intermittent fasting may have physiological advantages that may improve many health outcomes, including obesity [28].
In the U.S., Hispanics/Latinos are disproportionately affected by obesity. In particular, Puerto Ricans living in Puerto Rico have a high prevalence of obesity and related health disparities. According to the Behavioral Risk Factor and Surveillance System, in 2016, the age-adjusted prevalence of self-reported obesity in Puerto Rico was 30.5%, with an additional 35.2% classified as overweight [29]. Observational studies in Puerto Rico have reported a high prevalence of abdominal obesity among adults [30, 31], which is worrisome as visceral fat deposition can increase risk of multiple chronic diseases [32]. However, the prevalence of skipping breakfast or other eating patterns has not been reported in Puerto Rico, and little information is available regarding behaviors of breakfast consumption and eating frequency in this at-risk ethnic minority group. We hypothesized that higher eating frequency and skipping breakfast would be associated with increased odds of abdominal obesity among adults living in Puerto Rico. To test this hypothesis, we evaluated the associations between breakfast consumption and eating frequency with abdominal obesity, defined as high waist circumference or high waist-to-hip ratio, among adults living in Puerto Rico.
2. Methods and materials
2.1. Study population
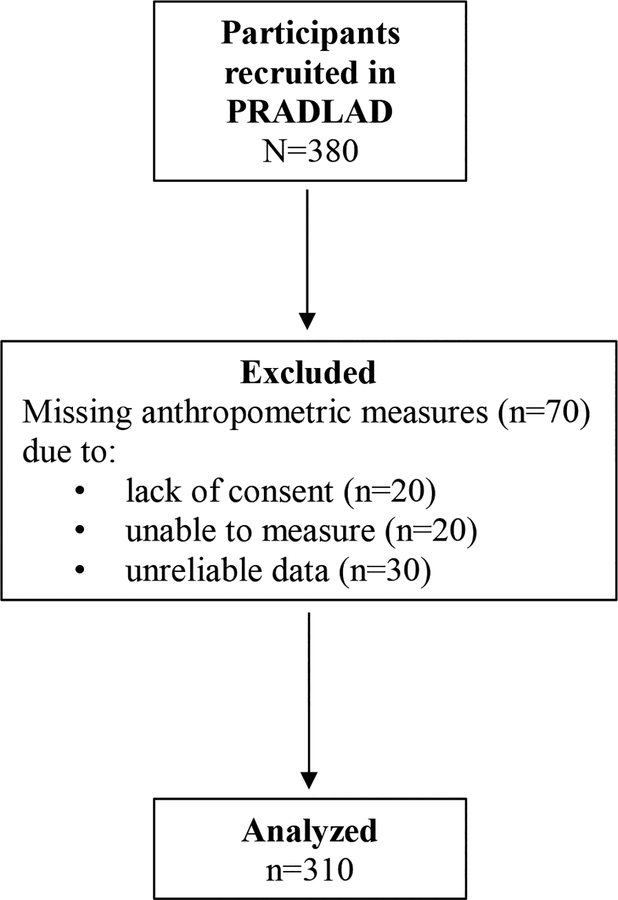
The Puerto Rico Assessment of Diet, Lifestyle and Diseases (PRADLAD) is a cross-sectional pilot study of a convenience sample of 380 men and women, aged between 30–75 years, and residing in Puerto Rico in 2015 for at least 10 months before the study. Detailed recruitment and methodology procedures have been described previously [33]. Briefly, participants were recruited while attending or visiting one of three primary care clinics in the San Juan metro area with a broad representation of patients’ socioeconomic status. We excluded participants with missing anthropometric measures. Missing measures were due to not giving consent (n = 20), not being able to take the measure (n = 20; for example because the person had a physical limitation), or unreliable data (n = 30). The final study population consisted of 310 participants (Figure 1). Participants responded to a set of comprehensive questionnaires administered by trained, bilingual interviewers. Thereafter, the participants underwent anthropometry measurements. The Institutional Review Board at Harvard T.H. Chan School of Public Health, University of Massachusetts-Lowell, Northeastern University, and Ponce Health Sciences University approved the study. Written informed consent was obtained from all participants.
Figure 1. Flowchart of participant selection for the analysis.
PRADLAD, Puerto Rico Assessment of Diet, Lifestyle and Diseases
2.2. Dietary intake and dietary behaviors
Dietary intake was collected using a semi-quantitative food frequency questionnaire (FFQ) adapted and validated for this population [34]. Participants were asked to select their usual intake of a standard portion of each food item; examples of portion sizes were provided. Nine responses were possible, ranging from “never or less than once/month” to “≥6 times/day.” Dietary data were linked to the Minnesota Nutrition Data System for Research (1999, version 25) for energy and nutrient analysis.
A separate questionnaire for dietary behaviors and attitudes included questions about eating frequency: “How often do you eat breakfast (excluding a “bite of something”)?”, “How often do you eat lunch (excluding a “bite of something”)?”, “How often do you eat dinner (excluding a “bite of something”)?”, and “How often do you eat a snack (snack: a bite of something that you do not consider as a complete meal)?”. The response options were ‘never or rarely’, ‘2–4 days/week’, ‘5–6 days/week’, ‘every day’. Because the questions focused on eating, we did not consider beverages a snack in this study.
2.3. Anthropometry measurements
Trained research assistants measured waist and hip circumferences. Waist circumference (WC) was measured at the midway between the lowest rib margin and the iliac crest [35]. Hip circumference was measured around the widest portion of the buttocks, with the tape parallel to the floor [35]. Two measurements rounded to the nearest millimeter were recorded; a third measurement was performed if the differences between the measurements were greater than 1 cm, and the mean of the closest measurements was calculated. We defined abdominal obesity based on the WC cutoff values recommended for use by the International Diabetes Federation (IDF) for populations of European or sub-Saharan Africa heritages: ≥94 cm for males and ≥80 cm for females [36], as well as by current U.S. guidelines (≥102 cm for males and ≥88 cm for females) [37]. We calculated the waist-to-hip ratio (WHR) by dividing the WC by the hip measurement. A WHR of ≥0.90 in men or ≥0.85 in women was considered as high [35].
2.4. Covariates
Age, sex, household income, smoking status, physical activity, and hours spent watching television, were obtained by interview-based questionnaires. A physical activity score was calculated as the sum of hours spent on typical 24-hour activities (sedentary, light, moderate, or heavy activity, and sleeping) multiplied by weighing factors that parallel the rate of oxygen consumption associated with each activity. We used the Alternate Healthy Eating Index 2010 (AHEI-2010), a score created based on nutrients and foods associated with lower risk of chronic disease [38], as an indicator of the participants’ overall diet quality. Briefly, the AHEI-2010 was calculated based on 11 components (vegetables, fruit, nuts and legumes, whole grains, red and processed meats, trans fat, polyunsaturated fatty acids, omega-3 fatty acids, sodium, sugary beverages, and alcohol). Each component contributes 0–10 points to the total score; a score of 10 indicates a perfect adherence to the recommendations, whereas a score of 0 represents the least healthy dietary behavior. Adding all components’ scores results in a total AHEI score of 110, which indicates a perfect adherence to the recommendations, whereas a score of 0 represents the least healthy dietary behavior.
2.5. Statistical analyses
We summed the frequencies that participants reported eating breakfast, lunch, dinner and snacks during the week and divided it by 7 to obtain the total number of eating occasions per day. We categorized participants into groups according to their reported eating frequency: 1.5 or fewer times per day, between 1.5 and 3 times per day, and 3 or more times a day. We used the midpoint in each category to define its median intake of frequency. We categorized participants as breakfast non-consumers (‘Never or rarely’), and breakfast consumers (‘2–4 days/week’, ‘5–6 days/week’, ‘every day’). We imputed the median for missing values for continuous variables, and we created a missing indicator category for categorical variables. Percentages and means with their standard deviation were calculated for descriptive characteristics for all participants and by breakfast consumption. Differences in descriptive characteristics by breakfast consumption were tested using chi-square for categorical variables or Fisher’s exact test for continuous variables.
To examine associations of eating frequency and between breakfast consumption with abdominal obesity, we estimated odds ratios (ORs) and 95% confidence intervals (CIs) using crude, age-adjusted, and multivariable logistic regression models. In multivariable model 1, we adjusted for age (y, continuous), sex (male/female), income ($0-$10,000, $10,001-$20,000 and >$20,000), smoking status (current, past, or never smokers), physical activity score (continuous), and TV watching (h/d, continuous). In multivariable model 2, we further adjusted for dietary variables, including energy intake (kcal/d, continuous), and AHEI-2010 (continuous) because these variables could mediate the association between eating frequency, breakfast consumption, and abdominal obesity. In the models in which breakfast consumption was the main exposure, we also adjusted for the number of eating occasions excluding breakfast (continuous). Tests for trend of eating frequency were calculated by including the median category of eating frequency as an ordinal variable.
In a sensitivity analysis, we used a different definition of breakfast consumption that used three categories: non-consumers (never or rarely), occasional consumers (2–4 days/week) and frequent consumers (5–6 days/week and every day). We also used different WC cutoff values recommended for use by current U.S. guidelines (≥102 cm for males and ≥88 cm for females) [37]. Finally, we separately evaluated the association of the number of complete meals per day (≤1, 2, 3) as well as the number of snacks per week (0.5, 3, 5.5, ≥7) with abdominal obesity. A sample size of 310 participants achieved sufficient power (95%) to detect an odds ratio of 2.5, assuming a two-sided significance level of 5%. All analyses were conducted using SAS version 9.4 (SAS Institute Inc.; Cary, NC), and P<0.05 was considered statistically significant.
3. Results
In this cross-sectional study of 310 adults living in Puerto Rico, the majority of the participants (70%) consumed breakfast at least 2 times per week to every day. In specific, 30% of participants reported “never or rarely” eating breakfast, 31% only “2–4 days/week”, 12% “5–6 days/week”, and 27% were “every day” breakfast consumers. Overall, 32% of participants reported eating three or more times per day; 19% reported “never or rarely” eating lunch, 27% ate lunch “2–4 days/week”, 13% “5–6 days/week”, and 41% had lunch “every day”, while 12% reported “never or rarely” eating dinner, 27% had dinner “2–4 days/week”, 15% “5–6 days/week”, and 46% ate dinner “every day”. Regarding snack consumption, 37% reported “never or rarely” ate snacks, 28% ate snacks “2–4 days/week”, 13% “5–6 days/week”, and 22% had snacks “every day” (not shown). The prevalence of abdominal obesity in the study population defined as high WHR was 76.8%, and 75.5% had high WC using the IDF definition and 61.5% using the U.S. guidelines. The mean (SD) BMI was 25.2 (6.5) kg/m2, and 22.2% were classified with overweight (25.0–29.9 kg/m2) and 20.8% with obesity (≥30 kg/m2). Compared with breakfast consumers, those who skipped breakfast were younger and more sedentary, had lower income, and ate fewer meals per day (Table 1).
Table 1.
Sociodemographic and lifestyle characteristics by breakfast consumption among adults living in Puerto Ricoa
| All (n=310) | Non-breakfast consumers (n=93) | Breakfast consumers (n=217) | P-value | |
|---|---|---|---|---|
| Age (y) | 51.6 (11.3) | 49.6 (11.6) | 52.4 (11.1) | 0.05 |
| Female, % | 65.5 | 73.1 | 62.2 | 0.06 |
| Income, % | 0.01 | |||
| $0-$10,000 | 47.7 | 61.3 | 41.9 | |
| $10,001-$20,000 | 17.8 | 16.1 | 18.4 | |
| ≥$20,000 | 16.1 | 14.0 | 17.1 | |
| Missing | 18.4 | 8.6 | 22.6 | |
| Physical activity, % | 0.05 | |||
| Sedentary | 37.1 | 48.4 | 32.3 | |
| Low | 25.8 | 20.4 | 28.1 | |
| Moderate/Vigorous | 22.3 | 20.4 | 23 | |
| Missing | 14.8 | 10.8 | 16.6 | |
| TV watching (h/d) | 3.6 (2.6) | 3.7 (2.6) | 3.6 (2.6) | 0.51 |
| Smoking status, % | 0.31 | |||
| Never smoker | 64.5 | 65.6 | 64.1 | |
| Former smoker | 15.5 | 12.9 | 16.6 | |
| Current smoker | 16.8 | 20.4 | 15.2 | |
| Missing | 3.2 | 1.1 | 4.1 | |
| Energy intake (kcal/d) | 2179.1 (773.1) | 2128.5 (776.7) | 2200.7 (772.4) | 0.45 |
| Number of meals/d with breakfast | 2.3 (1.0) | 1.4 (0.8) | 2.8 (0.8) | 0.0001 |
| Number of meals/d without breakfast | 1.8 (0.8) | 1.2 (0.8) | 2.0 (0.7) | 0.0001 |
| AHEI-2010b | 59.6 (9.0) | 58.9 (7.7) | 59.9 (9.5) | 0.33 |
| BMI (kg/m2) | 25.2 (6.5) | 25.9 (6.7) | 24.9 (6.3) | 0.23 |
| BMI, % | 0.34 | |||
| <18.5 kg/m2 | 12.4 | 12.5 | 12.4 | |
| 18.5–24.9 kg/m2 | 44.6 | 39.8 | 46.7 | |
| 25.0–29.9 kg/m2 | 22.2 | 20.4 | 22.8 | |
| ≥30 kg/m2 | 20.8 | 27.3 | 18.1 | |
| High WC, %c | 75.5 | 74.2 | 76.0 | 0.73 |
| High WHR, % | 76.8 | 74.2 | 77.9 | 0.48 |
AHEI-2010, alternate healthy eating index 2010; BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio.
All values are shown as percentages (for categorical) or means (SD) for continuous variables.
The AHEI-2010 was calculated based on 11 components (vegetables, fruit, nuts and legumes, whole grains, red and processed meats, trans fat, polyunsaturated fatty acids, omega-3, sodium, sugary beverages, alcohol). Each component contributes 0–10 points to the total score; a score of 10 indicates a perfect adherence to the recommendations, whereas a score of 0 represents the least healthy dietary behavior. The AHEI ranges from 0 (worst) to 110 (best).
High waist circumference using the IDF definition is ≥94 cm in men and ≥80 cm in women.
After adjustment for multiple risk factors, no association was found between breakfast consumption and high WHR or high WC (Table 2). Although not statistically significant, the direction of the association between breakfast consumption and abdominal obesity changed from positive to inverse after adjusting for dietary variables. In a sensitivity analysis, when we used a different definition of breakfast consumption using three categories (non-consumers, occasional consumers, and frequent consumers), no association was found with high WHR or high WC (using the IDF definition and the current U.S. guidelines) (Supplemental Table S1).
Table 2.
Breakfast consumption and odds of high waist-to-hip ratio and high waist circumference among adults living in Puerto Rico
| Non-breakfast consumers (n=93) | Breakfast consumers (n=217) | P-value | |||
|---|---|---|---|---|---|
| High waist-to-hip ratioa | cases=69 | cases=169 | |||
| OR | 95% CI | OR | 95% CI | ||
| Crude | 1.00 | Ref. | 1.23 | 0.70, 2.15 | 0.48 |
| Age-adjusted | 1.00 | Ref. | 1.10 | 0.61, 1.96 | 0.76 |
| Model 1b | 1.00 | Ref. | 1.16 | 0.61, 2.23 | 0.65 |
| Model 2c | 1.00 | Ref. | 0.80 | 0.37, 1.74 | 0.58 |
| High waist circumferenced | cases=69 | cases=165 | |||
| OR | 95% CI | OR | 95% CI | ||
| Crude | 1.00 | Ref. | 1.10 | 0.63, 1.93 | 0.73 |
| Age-adjusted | 1.00 | Ref. | 1.04 | 0.59, 1.83 | 0.90 |
| Model 1b | 1.00 | Ref. | 1.26 | 0.64, 2.48 | 0.50 |
| Model 2c | 1.00 | Ref. | 0.87 | 0.40, 1.89 | 0.72 |
OR, odds ratio; CI, confidence interval; Ref., reference category.
High waist-to-hip ratio was defined as ≥0.90 in men, and ≥0.85 in women.
Model 1 is adjusted for age (y, continuous), sex (male/female), income ($0-$10,000, $10,001-$20,000 and >$20,000), smoking status (current, past, or never smokers), physical activity score (continuous), and TV watching (h/d, continuous).
Model 2 is adjusted for the previous model plus the number of eating occasions excluding breakfast (continuous), energy intake (kcal/d, continuous), and the AHEI-2010 (continuous).
High waist circumference using the IDF definition is ≥94 cm in men and ≥80 cm in women.
Greater eating frequency was associated with higher odds of high WC (Table 3). For instance, compared with participants who ate ≤1.5 times a day, participants who ate between 1.5 and 3 times per day were 2.75 (95% CI: 1.23, 6.15) times more likely to have a high WC and participants who ate 3 or more times a day were 2.88 (95% CI: 1.14, 7.31) times more likely to have a high WC after adjustment for age, sex, income, smoking status, physical activity, TV watching, energy intake, and diet quality. The association between eating frequency and WHR did not reach statistical significance in the fully-adjusted model, although it trended towards higher odds of high WHR. In a sensitivity analysis, when we used a different definition of high WC following current U.S. guidelines that do not account for ethnic heritage, no association was found for either breakfast consumption or eating frequency with abdominal obesity (Supplemental Table S2). We did not find a significant association with either the number of complete meals (Supplemental Table S3) or the number of snacks per week (Supplemental Table S4) with abdominal obesity, when analyzed separately.
Table 3.
Eating frequency (complete meals and snacks) and odds of high waist-to-hip ratio and high waist circumference for among adults living in Puerto Rico
| ≤1.5 times/d (n=66) | 1.5–3 times/d (n=145) | ≥3 times/d (n=99) | P-trend | ||||
|---|---|---|---|---|---|---|---|
| Median (min., max.) | 1 (0.21, 1.36) | 2.14 (1.57, 3.00) | 3.43 (3.42, 4.00) | ||||
| High waist-to-hip ratioa | cases=45 | cases=113 | cases=80 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Crude | 1.00 | Ref. | 1.65 | 0.86, 3.16 | 1.97 | 0.96, 4.04 | 0.08 |
| Age-adjusted | 1.00 | Ref. | 1.49 | 0.77, 2.91 | 1.69 | 0.81, 3.54 | 0.18 |
| Model 1b | 1.00 | Ref. | 1.33 | 0.63, 2.78 | 2.12 | 0.88, 5.09 | 0.09 |
| Model 2c | 1.00 | Ref. | 1.33 | 0.63, 2.81 | 2.32 | 0.94, 5.71 | 0.06 |
| High waist circumferenced | cases=44 | cases=115 | cases=75 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Crude | 1.00 | Ref. | 1.92 | 1.00, 3.68 | 1.56 | 0.79, 3.11 | 0.26 |
| Age-adjusted | 1.00 | Ref. | 1.82 | 0.94, 3.50 | 1.44 | 0.71, 2.89 | 0.42 |
| Model 1b | 1.00 | Ref. | 2.67 | 1.20, 5.93 | 2.59 | 1.06, 6.35 | 0.06 |
| Model 2c | 1.00 | Ref. | 2.75 | 1.23, 6.15 | 2.88 | 1.14, 7.31 | 0.04 |
Min., minimum; Max., maximum; OR, odds ratio; CI, confidence interval; Ref., reference category.
High waist-to-hip ratio was defined as ≥0.90 in men, and ≥0.85 in women.
Model 1 is adjusted for age (y, continuous), sex (male/female), income ($0-$10,000, $10,001-$20,000 and >$20,000), smoking status (current, past, or never smokers), physical activity score (continuous), and TV watching (h/d, continuous).
Model 2 is adjusted for the previous model plus energy intake (kcal/d, continuous), and the AHEI-2010 (continuous).
High waist circumference using the IDF definition is ≥94 cm in men and ≥80 cm in women.
4. Discussion
In this cross-sectional study, higher eating frequency, but not breakfast skipping, was associated with higher odds of high WC in men and women living in Puerto Rico regardless of energy intake or diet quality. Thus, we do not reject our hypothesis that higher eating frequency would be associated with increased odds of abdominal obesity, but we reject the hypothesis that skipping breakfast would also be associated with abdominal obesity among adults living in Puerto Rico. In this study, the self-reported prevalence of obesity (BMI ≥30 kg/m2) was lower (20.8%) than the prevalence reported by the Puerto Rico Behavioral Risk Factor Surveillance System [29] for the same year in which our study was conducted, likely due to misreporting. The prevalence of abdominal obesity, which as objectively measured in our study, was high 61.5% using the IDF definition for high WC. It may be more appropriate to use WC or WHR than BMI because obesity-related health risks are mainly associated with body fat distribution, especially in the midsection region, than with general adiposity [39]. In addition, we found discrepancies among abdominal obesity indicators. The estimated association between eating frequency and abdominal obesity was statistically significant using WC, but it was only marginal for WHR. There are several rationales for this. Measuring WC entails fewer measurements than measuring WHR, which includes the waist and the hip measurements, and thus WC is prone to less measurement error. Furthermore, the biological implications of hip circumference are less clear than for waist circumference, as a large hip may reflect more accumulation of subcutaneous fat, greater gluteal mass, or larger bone structure, and thus an increased WHR can reflect both increased visceral fat mass or a larger bone structure [40].
The findings on breakfast consumption in relation to obesity are inconsistent. In observational studies, skipping breakfast has been associated with increased body mass index [7–11, 41, 42] and weight gain [14]. However, randomized controlled trials have provided mixed results [43–47]. Breakfast is a unique meal because it is the time when fasting ceases. Longer fasting times trigger lower insulin and higher ghrelin concentrations [48, 49], which could induce hunger and eating. Yet, skipping breakfast is one of the first changes in dietary habits people make to lose weight and limit energy intake [44]. Studies have suggested that breakfast consumption is associated with higher energy intake compared to skipping breakfast [8, 50–52]. A recent meta-analysis of randomized controlled trials found a small protective effect of skipping breakfast on weight [53], and showed that breakfast consumption increased total energy intake compared with skipping breakfast. In our study, breakfast consumption was not associated with abdominal obesity. Reverse causality may partly explain the lack of association between breakfast consumption and abdominal obesity. Having obesity may have prompted individuals to skip breakfast as part of energy-restriction strategies, and this may have biased our results towards the null. In this population we cannot reject the possibility that skipping breakfast does not have adverse effects on abdominal obesity. Because numerous international dietary recommendations advocate for breakfast consumption for weight management [53], adults in Puerto Rico may continue including breakfast as part of their usual eating behaviors without increasing or decreasing their likelihood of abdominal obesity, as long as breakfast is comprised of healthy foods and within the energy-balance requirements of the individual.
The evidence of the association between eating frequency and obesity is also inconsistent. While in some cross-sectional studies, higher meal frequency has been associated with lower odds of obesity [18, 41], others have found the opposite [54–56], and a prospective study found a higher risk of weight gain [14]. Our results are consistent with the latter findings after adjustment of multiple confounders, including energy intake and diet quality. Although the exact mechanisms linking eating frequency and obesity are not well established, experimental data suggests that reduced meal frequency (and intermittent fasting) is associated with less oxidative damage and can prevent the development of obesity through the production of protein chaperones and growth factors [57, 58], possibly because of improved adipose tissue signaling and subsequent less increase of fat depots [47]. It is possible that participants with pre-existing abdominal obesity may have reduced their eating frequency in an attempt to lose weight. If so, the strength of the positive association between eating frequency and high WC would be underestimated.
Also, our definition of eating frequency included both meals and snacks; thus, there was the possibility that only snacks were associated with high WC since snacking has been associated with obesity in previous studies [55, 59]. However, in sensitivity analysis, we did not find a significant association between the number of complete meals nor the number of snacks with abdominal obesity, when assessed separately. This suggests that there is no distinctive effect from just complete meals or just snacks, but rather higher number of eating occasions may be more relevant for higher abdominal obesity independently from total energy intake and diet quality.
This study has several limitations. First, non-differential measurement error in the assessment of breakfast consumption and eating frequency may have biased our results to the null. Also, misclassification could had been present due to how we defined breakfast consumption. We included occasional breakfast consumers (2–4 times/week) in the category of breakfast consumers. However, in sensitivity analysis we used a three-category definition of breakfast consumption and the results remained unchanged. Second, there was no information on the foods and nutrient composition of the breakfast consumed, and beverages consumed were not included. However, we were able to adjust for total energy intake and overall diet quality. Third, our study is cross-sectional, and it is possible that participants with abdominal obesity changed their eating behaviors as an attempt to lose weight. Due to the small sample size, we were underpowered to stratify by sex and by body mass index, which may be potential moderators. Fourth, the non-random sample design of our study may have limited the generalizability of our results. However, the study was conducted in three primary care clinics in San Juan, Puerto Rico that increased socioeconomic representation of our sample [33]. Finally, meal timing may have influenced our results. In the National Health and Nutrition Examination Survey, having one additional eating occasion per day was associated with an 8% decrease in C-reactive protein, and specifically, each 10% increase in the proportion of total energy intake consumed in the evening was associated with a 3% increase in C-reactive protein concentrations [60]. Additional longitudinal studies are needed to elucidate this association and to conduct an in-depth analysis of specific foods consumed for breakfast and meal timing.
In conclusion, adults living in Puerto Rico eating three or more times a day have higher odds of high abdominal obesity, as measured by waist circumference cutoff points recommended for this ethnic population, independent of energy intake and diet quality. Promoting less frequent meals, while maintaining a high diet quality and adequate energy intake, could prevent abdominal obesity and reduce obesity-related disparities in Puerto Ricans. This study provides information on an understudied population with a high prevalence of obesity, and our results call for a more in-depth examination of the most appropriate obesity indicators for Hispanic/Latino groups and, in addition, underscore the need for better understanding the eating patterns in this population.
Supplementary Material
Acknowledgments
The Puerto Rico Assessment of Diet, Lifestyle, and Diseases study was successful thanks to the contribution from all our interviewers, the staff at the partner clinics, and the participants. This work was supported by private anonymous donations to Harvard T.H. Chan School of Public Health; a Dry Bean Health Research Program Incentive Award from the Northarvest Bean Growers Association; institutional funds from FDI Clinical Research. MT was funded by the National Council of Science and Technology (CONACyT, Mexico), and JM was supported by a Mentored Career Development Award to Promote Faculty Diversity in Biomedical Research from the NHLBI (grant number K01-HL120951). The Northarvest Bean Growers Association, CONACyT, and NHLBI had no role in the design, analysis or writing of this article. The authors declare no conflicts of interest.
Abbreviations
- PR
Puerto Rico
- WC
waist circumference
- WHR
waist-to-hip ratio
- OR
odds ratio
- CI
confidence interval
- U.S.
United States
- PRADLAD
Puerto Rico Assessment of Diet, Lifestyle and Diseases
- FFQ
food frequency questionnaire
- IDF
International Diabetes Federation
- AHEI-2010
Alternate Healthy Eating Index 2010
- y
years
- h/d
hours per day
- kcal/d
kilocalories per day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet. 2015;115:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Haines PS, Guilkey DK, Popkin BM. Trends in breakfast consumption of US adults between 1965 and 1991. J Am Diet Assoc. 1996;96:464–70. [DOI] [PubMed] [Google Scholar]
- [3].Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. [DOI] [PubMed] [Google Scholar]
- [4].Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]
- [6].Horikawa C, Kodama S, Yachi Y, Heianza Y, Hirasawa R, Ibe Y, et al. Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: a meta-analysis. Prev Med. 2011;53:260–7. [DOI] [PubMed] [Google Scholar]
- [7].Deshmukh-Taskar P, Nicklas TA, Radcliffe JD, O’Neil CE, Liu Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2013;16:2073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Song WO, Chun OK, Obayashi S, Cho S, Chung CE. Is consumption of breakfast associated with body mass index in US adults? J Am Diet Assoc. 2005;105:1373–82. [DOI] [PubMed] [Google Scholar]
- [9].Marin-Guerrero AC, Gutierrez-Fisac JL, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. Eating behaviours and obesity in the adult population of Spain. Br J Nutr. 2008;100:1142–8. [DOI] [PubMed] [Google Scholar]
- [10].Watanabe Y, Saito I, Henmi I, Yoshimura K, Maruyama K, Yamauchi K, et al. Skipping Breakfast is Correlated with Obesity. J Rural Med. 2014;9:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berg C, Lappas G, Wolk A, Strandhagen E, Toren K, Rosengren A, et al. Eating patterns and portion size associated with obesity in a Swedish population. Appetite. 2009;52:21–6. [DOI] [PubMed] [Google Scholar]
- [12].Purslow LR, Sandhu MS, Forouhi N, Young EH, Luben RN, Welch AA, et al. Energy intake at breakfast and weight change: prospective study of 6,764 middle-aged men and women. Am J Epidemiol. 2008;167:188–92. [DOI] [PubMed] [Google Scholar]
- [13].Odegaard AO, Jacobs DR Jr., Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care. 2013;36:3100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van der Heijden AA, Hu FB, Rimm EB, van Dam RM. A prospective study of breakfast consumption and weight gain among U.S. men. Obesity (Silver Spring). 2007;15:2463–9. [DOI] [PubMed] [Google Scholar]
- [15].Centre for Public Health Excellence at NICE (UK), National Collaborating Centre for Primary Care (UK) (2006) Obesity: The prevention, identification, assessment and management of overweight and obesity in adults and children. https://www.ncbi.nlm.nih.gov/books/NBK63696/ (accessed August 2018). [PubMed]
- [16].Garaulet M, Gomez-Abellan P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- [17].McCrory MA, Campbell WW. Effects of eating frequency, snacking, and breakfast skipping on energy regulation: symposium overview. J Nutr. 2011;141:144–7. [DOI] [PubMed] [Google Scholar]
- [18].Holmback I, Ericson U, Gullberg B, Wirfalt E. A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men. Br J Nutr. 2010;104:1065–73. [DOI] [PubMed] [Google Scholar]
- [19].Mekary RA, Hu FB, Willett WC, Chiuve S, Wu K, Fuchs C, et al. The joint association of eating frequency and diet quality with colorectal cancer risk in the Health Professionals Follow-up Study. Am J Epidemiol. 2012;175:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB, et al. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation. 2013;128:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Speechly DP, Buffenstein R. Greater appetite control associated with an increased frequency of eating in lean males. Appetite. 1999;33:285–97. [DOI] [PubMed] [Google Scholar]
- [22].Speechly DP, Rogers GG, Buffenstein R. Acute appetite reduction associated with an increased frequency of eating in obese males. Int J Obes Relat Metab Disord. 1999;23:1151–9. [DOI] [PubMed] [Google Scholar]
- [23].Jenkins DJ, Wolever TM, Vuksan V, Brighenti F, Cunnane SC, Rao AV, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–34. [DOI] [PubMed] [Google Scholar]
- [24].LeBlanc J, Mercier I, Nadeau A. Components of postprandial thermogenesis in relation to meal frequency in humans. Can J Physiol Pharmacol. 1993;71:879–83. [DOI] [PubMed] [Google Scholar]
- [25].Kahleova H, Lloren JI, Mashchak A, Hill M, Fraser GE. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J Nutr. 2017;147:1722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schoenfeld BJ, Aragon AA, Krieger JW. Effects of meal frequency on weight loss and body composition: a meta-analysis. Nutr Rev. 2015;73:69–82. [DOI] [PubMed] [Google Scholar]
- [27].Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111:16647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. BRFSS Prevalence & Trends Data, https://nccd.cdc.gov/BRFSSPrevalence/rdPage.aspx?rdReport=DPH_BRFSS.ExploreByTopic&irbLocationType=StatesAndMMSA&islClass=CLASS14&islTopic=TOPIC09&islYear=2016&rdRnd=12577; 2016. [accessed 10 May 2018].
- [30].Perez CM, Guzman M, Ortiz AP, Estrella M, Valle Y, Perez N, et al. Prevalence of the metabolic syndrome in San Juan, Puerto Rico. Ethn Dis. 2008;18:434–41. [PMC free article] [PubMed] [Google Scholar]
- [31].Mattei J, Tamez M, Rios-Bedoya CF, Xiao RS, Tucker KL, Rodriguez-Orengo JF. Health conditions and lifestyle risk factors of adults living in Puerto Rico: a cross-sectional study. BMC Public Health. 2018;18:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr. 2010;64:16–22. [DOI] [PubMed] [Google Scholar]
- [33].Mattei J, Rodriguez-Orengo JF, Tamez M, Corujo F, Claudio A, Villanueva H, et al. Challenges and opportunities in establishing a collaborative multisite observational study of chronic diseases and lifestyle factors among adults in Puerto Rico. BMC Public Health. 2017;17:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. [DOI] [PubMed] [Google Scholar]
- [35].World Health Organization. Waist circumference and waist–hip ratio: report of a WHO expert consultation, http://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf;jsessionid=914B0805F1B0E043888999947484B14B?sequence=1; 2008. [accesed 10 May 2018].
- [36].International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome, https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html; 2006. [accessed 10 May 2018].
- [37].Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- [38].Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. [DOI] [PubMed] [Google Scholar]
- [40].Willett WC. Nutritional Epidemiology. 3 ed New York, NY: Oxford University Press; 2013. [Google Scholar]
- [41].Ma Y, Bertone ER, Stanek EJ 3rd, Reed GW, Hebert JR, Cohen NL, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92. [DOI] [PubMed] [Google Scholar]
- [42].Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int. 2014;31:64–71. [DOI] [PubMed] [Google Scholar]
- [43].Schlundt DG, Hill JO, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr. 1992;55:645–51. [DOI] [PubMed] [Google Scholar]
- [44].Dhurandhar EJ, Dawson J, Alcorn A, Larsen LH, Thomas EA, Cardel M, et al. The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am J Clin Nutr. 2014;100:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Geliebter A, Astbury NM, Aviram-Friedman R, Yahav E, Hashim S. Skipping breakfast leads to weight loss but also elevated cholesterol compared with consuming daily breakfasts of oat porridge or frosted cornflakes in overweight individuals: a randomised controlled trial. J Nutr Sci. 2014;3:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21:2504–12. [DOI] [PubMed] [Google Scholar]
- [47].Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr. 2014;100:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Boyle PJ, Shah SD, Cryer PE. Insulin, glucagon, and catecholamines in prevention of hypoglycemia during fasting. Am J Physiol. 1989;256:E651–61. [DOI] [PubMed] [Google Scholar]
- [49].Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. [DOI] [PubMed] [Google Scholar]
- [50].Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, et al. Myths, presumptions, and facts about obesity. N Engl J Med. 2013;368:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Levitsky DA, Pacanowski CR. Effect of skipping breakfast on subsequent energy intake. Physiol Behav. 2013;119:9–16. [DOI] [PubMed] [Google Scholar]
- [52].Halsey LG, Huber JW, Low T, Ibeawuchi C, Woodruff P, Reeves S. Does consuming breakfast influence activity levels? An experiment into the effect of breakfast consumption on eating habits and energy expenditure. Public Health Nutr. 2012;15:238–45. [DOI] [PubMed] [Google Scholar]
- [53].Sievert K, Hussain SM, Page MJ, Wang Y, Hughes HJ, Malek M, et al. Effect of breakfast on weight and energy intake: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;364:l42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Berteus Forslund H, Lindroos AK, Sjostrom L, Lissner L. Meal patterns and obesity in Swedish women-a simple instrument describing usual meal types, frequency and temporal distribution. Eur J Clin Nutr. 2002;56:740–7. [DOI] [PubMed] [Google Scholar]
- [55].Murakami K, Livingstone MB. Eating Frequency Is Positively Associated with Overweight and Central Obesity in U.S. Adults. J Nutr. 2015;145:2715–24. [DOI] [PubMed] [Google Scholar]
- [56].Murakami K, Livingstone MB. Eating frequency in relation to body mass index and waist circumference in British adults. Int J Obes (Lond). 2014;38:1200–6. [DOI] [PubMed] [Google Scholar]
- [57].Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–60. [DOI] [PubMed] [Google Scholar]
- [58].Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Keller K, Rodriguez Lopez S, Carmenate Moreno MM. Association between meal intake behaviour and abdominal obesity in Spanish adults. Appetite. 2015;92:1–6. [DOI] [PubMed] [Google Scholar]
- [60].Marinac CR, Sears DD, Natarajan L, Gallo LC, Breen CI, Patterson RE. Frequency and Circadian Timing of Eating May Influence Biomarkers of Inflammation and Insulin Resistance Associated with Breast Cancer Risk. PLoS One. 2015;10:e0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.