Abstract
Background
Coffee has been consistently associated with lower risk of liver cancer and chronic liver disease, suggesting that coffee affects mechanisms underlying disease development.
Methods
We measured serum metabolites using untargeted metabolomics in 1:1 matched nested case-control studies of liver cancer (n = 221 cases) and fatal liver disease (n = 242 cases) in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention cohort (n = 29 133). Associations between baseline coffee drinking and metabolites were identified using linear regression; conditional logistic regression models were used to identify associations with subsequent outcomes.
Results
Overall, 21 metabolites were associated with coffee drinking and also each subsequent endpoint; nine metabolites and trigonelline, a known coffee biomarker, were identified. Tyrosine and two bile acids, glycochenodeoxycholic acid (GCDCA) and glycocholic acid (GCA), were inversely associated with coffee but positively associated with both outcomes; odds ratios (ORs) comparing the 90th to 10th percentile (modeled on a continuous basis) ranged from 3.93 (95% confidence interval [CI] = 2.00 to 7.74) for tyrosine to 4.95 (95% CI = 2.64 to 9.29) for GCA and from 4.00 (95% CI = 2.42 to 6.62) for GCA to 6.77 (95% CI = 3.62 to 12.65) for GCDCA for liver cancer and fatal liver disease, respectively. The remaining six metabolites and trigonelline were positively associated with coffee drinking but inversely associated with both outcomes; odds ratio ranged from 0.16 to 0.37. Associations persisted following diet adjustment and for outcomes occurring greater than 10 years after blood collection.
Conclusions
A broad range of compounds were associated with coffee drinking, incident liver cancer, and liver disease death over 27 years of follow-up. These associations provide novel insight into chronic liver disease and liver cancer etiology and support a possible hepatoprotective effect of coffee.
Liver cancer is the second-leading cause of cancer death worldwide (1). Most liver cancers are preceded by chronic liver disease (2), including cirrhosis, which is also a leading cause of death in the United States, particularly among men (3). Although liver cancer rates are highest in developing countries, they have increased dramatically in the United States and Europe (4–7). This increase has been largely attributed to increasing rates of hepatitis C virus (HCV) infection (8,9) but obesity and diabetes are also likely contributors (10–13). In contrast, coffee drinking has been consistently associated with lower risk of liver cancer, with moderate coffee drinkers experiencing a 40% to 50% lower risk than nondrinkers (14,15). An earlier analysis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) cohort estimated that a one-cup-per-day increase in consumption was strongly associated with lower risk of liver cancer and chronic liver disease death (16). Additionally, coffee consumption has been consistently linked with lower risk of type 2 diabetes (17) and lower rates of liver disease progression in the context of patients with advanced hepatitis C–related liver disease (18).
The mechanisms linking coffee drinking to liver disease and liver cancer are poorly understood but may involve effects on metabolism and digestion. The liver plays a central role in human metabolism, converting dietary constituents into metabolites that can be used or stored and toxins into substances that are harmless or can be excreted. In addition, the liver produces bile, which is critical for the breakdown and absorption of fats, and it regulates blood glucose by storing and breaking down glycogen as needed. A growing body of research indicates that bile acids play an important role in the pathogenesis of chronic liver disease (19–21), but their role in the development of liver cancer is unclear.
High-resolution mass spectrometry technologies can simultaneously measure thousands of small molecules in serum and other biospecimens and may improve mechanistic understanding of liver cancer and liver disease etiology as well as the potential protective effects of coffee. The aim of our study was to prospectively evaluate the associations of serum metabolites, particularly those that may provide insight into underlying mechanisms related to coffee drinking, with liver cancer and liver disease mortality in a large cohort of men with low rates of HCV and hepatitis B virus (HBV) infection, a high prevalence of coffee drinking, and baseline serum samples collected up to 27 years prior to diagnosis or death.
Methods
Study Design
The study design, rationale, and specific aims of the ATBC study have been detailed elsewhere (22). From 1985 to 1988, the study enrolled 29 133 Finnish male smokers, age 50 to 69 years, with no prior malignancy, alcoholism, or major medical issues, to participate in a randomized, double-blinded, placebo-controlled primary prevention trial of lung cancer. Participants were passively followed during the postintervention period via linkage with the Finnish Cancer Registry, which ascertains nearly 100% of cases (23), and the Register of Causes of Death. Each participant gave written informed consent, and the study was approved by the institutional review boards of the National Cancer Institute (NCI), the National Public Health Institute of Finland, and the International Agency for Research on Cancer.
The first case-control set for this study included 229 incident liver cancer cases (International Classification of Diseases, Ninth Revision [ICD9]: 155, and ICD, Tenth Revision [ICD10]: C22), diagnosed through December 31, 2012, and 229 incidence-density matched controls. The second case-control set included 248 fatal liver disease patients (ICD9: 571, and ICD10: K70, K72, K74), who died on or before December 31, 2012, and 248 incidence-density matched controls. Approximately 90% of the fatal liver disease cases were described as alcohol related, namely alcoholic cirrhosis of the liver (ICD9: 571.2, and ICD10: K70.2/3; n = 197). Case types are mutually exclusive endpoints, so if an individual had a liver cancer diagnosis before dying of liver disease, he or she is recorded as a liver cancer case only. All controls were alive at event date and were matched on age at random assignment (+ or –5 years) and baseline serum draw date (+ or –30 days). Serum samples from patients and controls were placed next to each other in the same batch and the order of pairs was randomized. Blinded quality control (QC) samples were included at a rate of 7% and were regularly spaced throughout the batches.
Exposure Assessment
At baseline, participants reported information on demographics, diet, lifestyle, and medical history via questionnaires and donated a fasting (overnight) blood sample, which was subsequently stored at –70°C. All blood samples used in the metabolomic analyses were never thawed prior to this study. A detailed summary of fasting insulin and glucose measures as well as HBV and HCV measure have been described elsewhere (10). A subset of serum samples from ATBC participants (n = 50) were tested for the presence of aflatoxin-albumin adducts at the University of Leeds (United Kingdom); because we found no evidence for exposure, we did not measure aflatoxin exposure in our larger case-control set (10).
Untargeted Metabolomics
Mass spectrometry analyses were carried out as described in detail elsewhere (24). In brief, study samples were analyzed on a liquid chromatography-mass spectrometry system consisting of a 1290 Binary LC system, a Jet Stream electrospray ionization source, and a 6550 QTOF mass spectrometer (Agilent Technologies, Santa Clara, CA). The mass spectrometer was operated in positive ionization mode across a mass range of 50–1000 Da. Coefficients of variation of 14 known compounds in 102 pooled QC samples ranged from 3.3% to 15.5% in batch 1 and 7.0% to 18.6% in batch 2.
Acquired raw data were preprocessed using Qualitative Analysis B.06.00, DA Reprocessor and Mass Profiler Professional 12.1 software (Agilent Technologies) using a recursive workflow. First, molecular feature extraction was employed to find features as singly charged proton adducts [M + H]+ in all study samples. Features that existed in at least 2% of the study samples were combined, using 0.08 minute (min) retention time and 15 ppm + 2 mDa mass windows for alignment. These features were used as targets for recursive analysis of the data of samples, QCs, and blanks by employing a “find by formula” algorithm with match tolerances at ±10 ppm and ±0.035 min, and ions limited to [M + H]+. The resulting features were then merged to generate the final matrix of peak areas. In total, 8020 features were found; after removing batch-specific features and features whose median intensities were higher in blanks than study samples, 2879 features remained. Finally, we removed 14 samples that were extreme outliers (four liver cancer cases, five fatal liver disease cases, and five controls) using principal coordinate analysis of the 2879 spectral features (Supplementary Figure 1, available online). Our final analytic sample included 221 liver cancer and 242 fatal liver disease cases and their matched controls.
We tested whether each of the 2879 spectral features was associated with coffee intake, liver cancer, and liver disease mortality. First, we used linear regression models to test the cross-sectional associations of these 2879 features as continuous log2-transformed response variables, with coffee intake at baseline (continuous, g per day) among both case-control sets combined, adjusting for age, smoking intensity (cigarettes per day), run order, and the surrogate variables identified by surrogate variable analysis using the R package SVA version 3.32.1. (25). We then tested the associations of the spectral features as continuous log2-transformed exposure variables, with liver cancer and liver disease mortality using conditional logistic regression models, adjusting for age, smoking intensity, and run order. In total, 38 features were statistically significantly associated with coffee intake and both liver endpoints, after correcting for multiple comparisons using the Bonferroni method (P < 1.74 × 10–5). Additionally, greater than 80% of peaks associated with liver cancer were also associated with fatal liver disease (data not shown).
Feature identification was performed by grouping based on retention time similarity and intensity correlation across samples and searching their m/z values for candidate metabolites in the Human Metabolome Database 4.0 (www.hmdb.ca) using ions [M + H]+, [M+Na]+, and [M-H2O+H]+ with ±15 ppm tolerance. Retention times and tandem mass spectrometry were compared with standards when available or against library spectra. The level of confidence was as proposed by Sumner et al. (26). Chromatograms and spectra are in Supplementary Figure 2 (available online). We also identified trigonelline, a known coffee biomarker, for consideration in subsequent analyses. Intraclass correlation coefficients for blinded QCs were greater than 0.80 for all identified metabolites except lysophosphatidylcholine (lysoPC)(P-16:0) with an intraclass correlation coefficient of 0.65.
Statistical Analysis
We considered the set of potential confounders defined in Table 1. We tested for differences between cases and controls using the Wilcoxon signed-rank test for continuous variables and McNemar test for categorical variables. All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant unless indicated otherwise. Metabolite analyses use log2-transformed peaks, with missing data assigned a value of one-half the lowest observed value for a given metabolite. We calculated partial Spearman correlation coefficients (rs), adjusting for case status and batch variables, for coffee consumption and metabolites. Odds ratios (ORs) and 95% confidence intervals (CIs), comparing the 90th to the 10th percentile of metabolite values based on the distribution in the controls, were estimated using conditional logistic regression models. Letting X90 and X10 denote the 90th percentile and 10th percentile in controls, and β denote the log (OR) from the conditional logistic regression model, the odds ratio is . Multivariable models were adjusted for potential confounders including age, body mass index, smoking intensity, smoking duration, alcohol, self-reported diabetes status, and education, with indicator variables used to account for missing data. Models were further adjusted for dietary variables, including intakes of coffee, fruit and vegetables, red meat, white meat, processed meat, fish, saturated fat, and energy, using the nutrient density method of energy adjustment, also including total energy in the models. Analyses were stratified by time to diagnosis or death. We used a stepwise selection process to determine the set of metabolites that were independently associated with each examined disease endpoint, with an alpha of 0.005 to enter and 0.05 to remain in the model for each metabolite. We calculated the adjusted receiver operating characteristic (aROC) curve and adjusted area under the curve (aAUC) for each metabolite signature using the joint-modeling approach (27). Confidence intervals for the aAUC and aROC were based on normal theory with the standard errors estimated using the bootstrap procedure. We conducted sensitivity analyses (Supplementary Methods, available online) to estimate the impact of model overfitting.
Table 1.
Baseline characteristics of liver cancer cases, chronic liver disease deaths, and matched-controls in Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study
| Baseline characteristics | Liver cancer incidence |
Chronic liver disease death |
||||
|---|---|---|---|---|---|---|
| No. (%) cases (n = 221) | No. (%) controls (n = 221) | P* | No. (%) cases (n = 242) | No. (%) controls (n = 242) | P * | |
| Alcohol, >11.6 g ethanol/day† | 113 (58.3) | 92 (47.4) | .03 | 156 (77.6) | 103 (51.2) | <.001 |
| Self-reported diabetes, yes | 25 (11.3) | 5 (2.3) | <.001 | 9 (3.7) | 9 (3.7) | 1.00 |
| Education, ≤ elementary | 58 (26.2) | 45 (20.4) | .14 | 67 (27.7) | 54 (22.3) | .17 |
| HBV, anti-HBc, yes‡ | 20 (16.7) | 5 (4.2) | .003 | 12 (5.8) | 13 (6.3) | .84 |
| HBV, HBsAg, yes‡ | 2 (1.7) | 3 (2.5) | .66 | 1 (0.5) | 1 (0.5) | 1.00 |
| HCV, anti-HCV, yes‡ | 6 (5.0) | 1 (0.8) | .06 | 6 (2.9) | 2 (1.0) | .16 |
| Mean entry age (SD), y | 57.8 (4.8) | 57.3 (4.5) | .004 | 55.2 (4.0) | 55.2 (4.3) | .78 |
| Mean coffee intake (SD), 8 oz cups/day† | 2.1 (1.4) | 2.6 (1.5) | .002 | 1.6 (1.2) | 2.4 (1.4) | <.001 |
| Mean BMI (SD), kg/m2 | 27.6 (4.5) | 26.1 (3.4) | <.001 | 26.8 (4.0) | 26.9 (3.8) | .77 |
| Mean smoking intensity (SD), cigarettes/day | 21.1 (9.4) | 19.0 (8.9) | .03 | 22.2 (9.2) | 20.7 (8.1) | .06 |
| Mean smoking duration (SD), y | 36.9 (8.5) | 35.2 (8.4) | .01 | 34.0 (8.5) | 34.0 (7.7) | .48 |
| Mean glucose (SD), mg/dL§ | 111.0 (36.0) | 100.3 (12.2) | .006 | 104.6 (20.2) | 100.3 (15.3) | .02 |
| Mean insulin (SD), µU/mL§ | 9.9 (10.3) | 5.3 (3.5) | <.001 | 8.7 (13.5) | 5.5 (4.7) | <.001 |
| Mean HOMA-IR (SD)§ | 2.9 (3.8) | 1.3 (1.0) | <.001 | 2.5 (4.8) | 1.4 (1.4) | <.001 |
| Mean fruit and vegetable intake (SD), g/1000 kcal† | 144.9 (49.6) | 155.9 (59.5) | .05 | 147.3 (63.4) | 152.2 (66.2) | .56 |
| Mean red meat intake (SD), g/1000 kcal† | 28.3 (11.8) | 25.7 (10.5) | .05 | 28.0 (14.7) | 27.1 (12.1) | .89 |
| Mean processed meat intake (SD), g/1000 kcal† | 27.3 (19.2) | 26.0 (17.7) | .74 | 27.8 (16.7) | 27.3 (17.5) | .88 |
| Mean white meat intake (SD), g/1000 kcal† | 5.5 (6.8) | 5.1 (5.2) | .97 | 4.6 (4.9) | 5.4 (6.7) | .34 |
| Mean fish intake (SD), g/1000 kcal† | 15.8 (10.9) | 14.4 (10.5) | .06 | 14.6 (10.4) | 14.6 (9.4) | .89 |
| Mean saturated fat intake, (SD) g/day† | 18.5 (5.0) | 19.3 (5.0) | .16 | 18.0 (5.2) | 18.9 (4.5) | .04 |
| Mean total energy (SD), kcal† | 2617 (807) | 2729 (689) | .03 | 2687 (809) | 2732 (769) | .52 |
P value for McNemar test and Wilcoxon signed-rank test for categorical and continuous variables, respectively. BMI = body mass index; HBc = hepatitis B core antibody; HBV = hepatitis B virus; HBsAg = hepatitis B surface antigen; HCV = hepatitis C virus; HOMA-IR = homeostatic model assessment of insulin resistance.
Dietary data available for 194 liver cancer cases and matched controls and 201 fatal liver disease cases and matched controls.
Data on hepatitis B and C virus status was available for 120 liver cancer cases and matched controls and 206 fatal liver disease cases and matched controls.
Among those without a self-reported history of diabetes, data on fasting glucose and insulin were available for 107 liver cancer cases and matched controls and 190 fatal liver disease cases and matched controls.
In a series of sensitivity analyses, we excluded participants with a liver cancer that was not specified as primary or secondary (ICD9 155.2; n = 17). Among participants with data on diabetes status and fasting glucose (n = 246 for liver cancer set; n = 400 for liver disease set), we excluded those with self-reported or biochemically defined diabetes (n = 22 for liver cancer set; n = 29 for liver disease set), and among those evaluated for hepatitis B and C infections (n = 255 for liver cancer set; n = 416 for liver disease set), we excluded those who were seropositive for HBV or HCV (n = 31 for liver cancer set; n = 27 for liver disease set). Analyses were conducted with R version 3.5.2 (R Core Team, Vienna, Austria. URL https://www.R-project.org/) or with SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline characteristics for ATBC participants who developed liver cancer or who died from liver disease and their matched controls are presented in Table 1. Overall, average coffee consumption was 1.9 cups per day, and 3.6% of participants reported drinking no coffee, whereas 21.5% reported no less than 3 cups per day (data not shown). Both case groups drank less coffee but more alcohol than controls, and liver cancer cases tended to have higher body mass index than controls. Both case groups had higher median levels of glucose and insulin relative to controls, and liver cancer cases had a higher prevalence of diabetes (11.3% vs 2.3%) and HCV (5.0% vs 0.8%).
Of the 2879 spectral features, 38 features corresponding to 21 metabolites were associated with coffee intake at baseline and subsequent liver outcomes (Supplementary Table 1, available online). In total, nine metabolites, corresponding to 24 features, were identified: tyrosine, hypoxanthine, serotonin, glycochenodeoxycholic acid (GCDCA), glycocholic acid (GCA), lysoPC lysoPC[15:0], lysoPC[18:2], lysoPC[P-16:0]), and a dipeptide (leucyl-valine [leu-val] or isomer). The remaining 14 features, corresponding to 12 metabolites, could not be identified. An additional feature, identified as trigonelline, was statistically significantly associated with coffee intake and liver disease death (both P < 1.74 × 10–5). Histograms for identified metabolites by case type and status are provided (Supplementary Figure 3, available online).
In multivariable models, we observed strong positive associations with liver cancer (Table 2) and liver disease mortality (Table 3) for tyrosine (liver cancer: OR = 3.93, 95% CI = 2.00 to 7.74; liver disease mortality: OR = 4.91, 95% CI = 2.59 to 9.29) and glycine-conjugated bile acids, GCDCA (liver cancer: OR = 3.99, 95% CI = 2.22 to 7.17; liver disease mortality: OR = 6.77, 95% CI = 3.62 to 12.65) and GCA (liver cancer: OR = 4.95, 95% CI = 2.64 to 9.29; liver disease mortality OR = 4.00, 95% CI = 2.42 to 6.62). In contrast, we observed inverse associations for trigonelline, serotonin, leu-val, each of three identified glycerophospholipids, and hypoxanthine; odd ratios ranged from 0.16 to 0.37. Intriguingly, coffee consumption at baseline was negatively correlated with tyrosine (rs = −0.13), GCDCA (rs = −0.20), and GCA (rs = −0.19) but positively correlated with trigonelline (rs ≥ 0.50), serotonin (rs = 0.13), leu-val (rs = 0.20), lysoPC(15:0) (rs = 0.32), lysoPC(P16:0) (rs = 0.18), lysoPC(18:2) (rs = 0.15), and hypoxanthine (rs = 0.10) (all P < .01). Strong correlations were observed between some metabolites (Supplementary Figure 4, available online).
Table 2.
Odds ratios and 95% confidence intervals* for incident liver cancer comparing men in the 90th and 10th percentiles, based on the distribution in controls, for top metabolites, using conditional logistic regression
| Chemical class and metabolite | Unadjusted model | Model 1† | >0 to 10 years of follow-up (model 1)†,‡ | >10 years of follow-up (model 1)†,§ | Model 2 (diet adjusted)†,‖ |
|---|---|---|---|---|---|
| Alkaloid | |||||
| Trigonelline | 0.33 (0.19 to 0.56) | 0.37 (0.20 to 0.67) | 0.10 (0.03 to 0.40) | 0.59 (0.29 to 1.22) | 0.46 (0.22 to 0.96) |
| P¶ | <.001 | .001 | .001 | .15 | .04 |
| Amino Acid | |||||
| Tyrosine | 5.06 (2.74 to 9.33) | 3.93 (2.00 to 7.74) | 7.21 (1.77 to 29.31) | 3.35 (1.49 to 7.56) | 3.60 (1.72 to 7.51) |
| P¶ | <.001 | <.001 | .006 | .004 | <.001 |
| Indoleamine | |||||
| Serotonin | 0.32 (0.20 to 0.53) | 0.33 (0.19 to 0.58) | 0.28 (0.10 to 0.84) | 0.41 (0.21 to 0.82) | 0.36 (0.20 to 0.65) |
| P¶ | <.001 | <.001 | .02 | .01 | <.001 |
| Dipeptide | |||||
| Leucyl-valine | 0.24 (0.14 to 0.43) | 0.22 (0.12 to 0.41) | 0.31 (0.10 to 0.97) | 0.12 (0.05 to 0.29) | 0.20 (0.10 to 0.40) |
| P¶ | <.001 | <.001 | .04 | <.001 | <.001 |
| Bile Acid | |||||
| Glycochenodeoxycholic acid | 3.92 (2.34 to 6.59) | 3.99 (2.22 to 7.17) | 5.70 (1.91 to 17.02) | 3.76 (1.74 to 8.12) | 3.73 (1.97 to 7.05) |
| P¶ | <.001 | <.001 | .002 | <.001 | <.001 |
| Glycocholic acid | 5.00 (2.84 to 8.80) | 4.95 (2.64 to 9.29) | 8.09 (2.25 to 29.03) | 4.65 (2.03 to 10.64) | 4.43 (2.27 to 8.64) |
| P¶ | <.001 | <.001 | .001 | <.001 | <.001 |
| Glycerophospholipid | |||||
| LysoPC(15:0) | 0.20 (0.11 to 0.35) | 0.17 (0.09 to 0.34) | 0.09 (0.02 to 0.37) | 0.27 (0.12 to 0.61) | 0.15 (0.06 to 0.35) |
| P¶ | <.001 | <.001 | <.001 | <.001 | <.001 |
| LysoPC(P-16:0) | 0.15 (0.08 to 0.28) | 0.21 (0.10 to 0.40) | 0.04 (0.01 to 0.22) | 0.30 (0.14 to 0.67) | 0.21 (0.10 to 0.43) |
| P¶ | <.001 | <.001 | <.001 | .003 | <.001 |
| LysoPC(18:2) | 0.21 (0.12 to 0.37) | 0.24 (0.13 to 0.45) | 0.13 (0.03 to 0.48) | 0.30 (0.15 to 0.62) | 0.21 (0.11 to 0.42) |
| P¶ | <.001 | <.001 | .002 | .001 | <.001 |
| Purine derivative | |||||
| Hypoxanthine | 0.19 (0.10 to 0.35) | 0.19 (0.10 to 0.38) | 0.11 (0.02 to 0.47) | 0.26 (0.11 to 0.58) | 0.21 (0.10 to 0.43) |
| P¶ | <.001 | <.001 | .003 | .001 | <.001 |
ORs for 221 liver cancer cases and 221 matched controls are scaled to compare the 90th to the 10th percentile of metabolite values (modeled on a continuous basis) based on the distribution in the controls; letting X90 and X10 denote the 90th percentile and 10th percentile in controls, and β denote the log(OR) from the conditional logistic regression model, the OR is eβ(X90−X10). CI = confidence interval; LysoPC = lysophosphatidylcholine; OR = odds ratio.
Models adjusted for entry age (years), body mass index (kg/m2), smoking intensity (cigarettes per day), smoking duration (years), alcohol intake (none, <11.6 g/day, ≥11.6 g/day, or missing), self-reported diabetes status (yes or no), education (≤ or > elementary education), and run order.
n = 146 (73 cases; 73 matched controls); missing alcohol assigned to highest frequency category owing to unstable risk estimates.
n = 296 (148 cases; 148 matched controls).
Models additionally adjusted for coffee intake (none, <1, 1 to <2, 2 to <3, or ≥3 cups [8 oz] per day), fruit and vegetable intake (g/1000 kcal), red meat intake (g/1000 kcal), white meat intake (g/1000 kcal), processed meat intake (g/1000 kcal), fish intake (g/1000 kcal), saturated fat intake (g/1000 kcal), energy intake (kcal); individuals with missing food frequency questionnaire data were grouped using an indicator variable.
P-value for χ2 test obtained from conditional logistic regression model for a given metabolite (modeled on a continuous basis); all tests were two-sided.
Table 3.
Odds ratios and 95% confidence intervals* for liver disease death comparing men in the 90th and 10th percentiles, based on the distribution in controls, for top metabolites, using conditional logistic regression
| Chemical class and metabolite | Unadjusted model | Model 1† | >0 to 10 years of follow-up (model 1)†,‡ | >10 years of follow-up (model 1)†,§ | Model 2 (diet adjusted)†,‖ |
|---|---|---|---|---|---|
| Alkaloid | |||||
| Trigonelline | 0.20 (0.12 to 0.36) | 0.24 (0.13 to 0.44) | 0.25 (0.09 to 0.66) | 0.22 (0.09 to 0.55) | 0.30 (0.14 to 0.62) |
| P¶ | <.001 | <.001 | .005 | .001 | <.001 |
| Amino Acid | |||||
| Tyrosine | 4.00 (2.36 to 6.77) | 4.91 (2.59 to 9.29) | 5.36 (2.14 to 13.40) | 4.47 (1.74 to 11.49) | 4.16 (2.07 to 8.37) |
| P¶ | <.001 | <.001 | <.001 | .002 | <.001 |
| Indoleamine | |||||
| Serotonin | 0.29 (0.18 to 0.46) | 0.28 (0.17 to 0.47) | 0.24 (0.11 to 0.52) | 0.29 (0.14 to 0.63) | 0.26 (0.15 to 0.46) |
| P¶ | <.001 | <.001 | <.001 | .002 | <.001 |
| Dipeptide | |||||
| Leucyl-valine | 0.27 (0.17 to 0.44) | 0.25 (0.15 to 0.43) | 0.16 (0.07 to 0.38) | 0.40 (0.19 to 0.83) | 0.30 (0.17 to 0.53) |
| P¶ | <.001 | <.001 | <.001 | .01 | <.001 |
| Bile Acid | |||||
| Glycochenodeoxycholic acid | 6.20 (3.53 to 10.88) | 6.77 (3.62 to 12.65) | 24.54 (6.19 to 97.21) | 3.76 (1.56 to 9.06) | 8.15 (3.88 to 17.12) |
| P¶ | <.001 | <.001 | <.001 | .003 | <.001 |
| Glycocholic acid | 4.05 (2.52 to 6.51) | 4.00 (2.42 to 6.62) | 6.51 (2.83 to 14.96) | 3.21 (1.49 to 6.90) | 3.82 (2.23 to 6.53) |
| P¶ | <.001 | <.001 | <.001 | .003 | <.001 |
| Glycerophospholipid | |||||
| LysoPC(15: 0) | 0.17 (0.10 to 0.30) | 0.22 (0.12 to 0.38) | 0.15 (0.06 to 0.40) | 0.30 (0.13 to 0.66) | 0.18 (0.09 to 0.38) |
| P¶ | <.001 | <.001 | <.001 | .003 | <.001 |
| LysoPC(P-16: 0) | 0.26 (0.15 to 0.45) | 0.29 (0.16 to 0.54) | 0.32 (0.14 to 0.74) | 0.18 (0.06 to 0.52) | 0.29 (0.14 to 0.59) |
| P¶ | <.001 | <.001 | .007 | .002 | <.001 |
| LysoPC(18: 2) | 0.13 (0.07 to 0.24) | 0.16 (0.08 to 0.31) | 0.08 (0.03 to 0.25) | 0.24 (0.09 to 0.63) | 0.13 (0.06 to 0.30) |
| P¶ | <.001 | <.001 | <.001 | .004 | <.001 |
| Purine derivative | |||||
| Hypoxanthine | 0.29 (0.18 to 0.49) | 0.29 (0.17 to 0.50) | 0.18 (0.07 to 0.45) | 0.36 (0.17 to 0.80) | 0.29 (0.16 to 0.55) |
| P¶ | <.001 | <.001 | <.001 | .01 | <.001 |
ORs for 242 fatal liver disease cases and 242 matched controls are scaled to compare the 90th to the 10th percentile of metabolite values (modeled on a continuous basis) based on the distribution in the controls; letting X90 and X10 denote the 90th percentile and 10th percentile in controls, and β denote the log(OR) from the conditional logistic regression model, the OR is eβ(X90−X10). CI = confidence interval; LysoPC = lysophosphatidylcholine; OR = odds ratio.
Models adjusted for entry age (years), body mass index (kg/m2), smoking intensity (cigarettes per day), smoking duration (years), alcohol intake (none, <11.6 g/day, ≥11.6 g/day, or missing), self-reported diabetes status (yes or no), education (≤ or > elementary education), and run order.
n = 228 (114 cases; 114 matched controls).
n = 256 (128 cases; 128 matched controls).
Models additionally adjusted for coffee intake (none, <1, 1 to <2, 2 to <3, or ≥3 cups [8 oz] per day), fruit and vegetable intake (g/1000 kcal), red meat intake (g/1000 kcal), white meat intake (g/1000 kcal), processed meat intake (g/1000 kcal), fish intake (g/1000 kcal), saturated fat intake (g/1000 kcal), energy intake (kcal); individuals with missing food frequency questionnaire data were grouped using an indicator variable for missing.
P value for χ2 test obtained from conditional logistic regression model for a given metabolite (modeled on a continuous basis); all tests were two-sided.
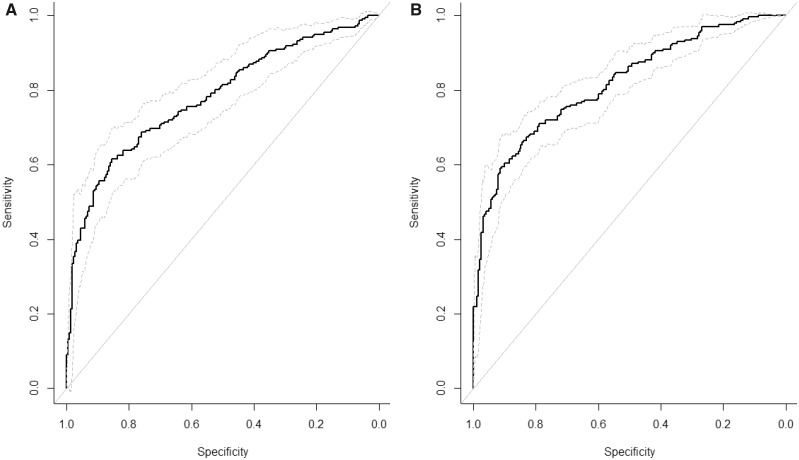
Associations were generally stronger for cases occurring within 10 years of baseline but remained strongly associated with liver outcomes for cases occurring more than 10 years after baseline. Diet-adjusted associations were moderately attenuated (5%–25%) or unchanged; all diet-adjusted associations remained statistically significant. Using a stepwise selection process, we found five metabolites (GCA, leu-val, hypoxanthine, lysoPC[18:2], and lysoPC[15:0]) and four metabolites (GCDCA, lysoPC[18:2], serotonin, and trigonelline) that were strong independent predictors of liver cancer and liver disease mortality, respectively (Table 4). The corresponding aROC curves (Figure 1) had aAUCs of 0.78 (95% CI = 0.74 to 0.83) for liver cancer and 0.82 (95% CI = 0.78 to 0.85) for liver disease mortality; sensitivity analyses suggest that the estimated aAUC and aROC were, at most, modestly influenced by selection of metabolites (Supplementary Figure 5, available online).
Table 4.
Odds ratios and 95% confidence intervals for serum metabolites* independently associated with primary incident liver cancer or liver disease mortality over 27 years of follow-up in a nested case-control study from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention cohort
| Metabolite | Order entered | Model entry P† | Mutually adjusted OR (95 % CI)‡ | Mutually adjusted P‡,† |
|---|---|---|---|---|
| Liver cancer (n = 221 cases; n = 221 controls) | ||||
| Glycocholic acid | 1 | <.001 | 2.80 (1.32 to 5.91) | .007 |
| Leu-val | 2 | <.001 | 0.30 (0.15 to 0.61) | <.001 |
| Hypoxanthine | 3 | <.001 | 0.32 (0.14 to 0.69) | .004 |
| LysoPC(18:2) | 4 | .004 | 0.33 (0.16 to 0.69) | .003 |
| LysoPC(15:0) | 5 | .003 | 0.26 (0.11 to 0.65) | .004 |
| Liver disease mortality (n = 242 cases; n = 242 controls) | ||||
| Glycochenodeoxycholic acid | 1 | <.001 | 4.49 (2.20 to 9.16) | <.001 |
| LysoPC(18:2) | 2 | <.001 | 0.28 (0.13 to 0.61) | .001 |
| Serotonin | 3 | <.001 | 0.34 (0.19 to 0.62) | <.001 |
| Trigonelline | 4 | .005 | 0.37 (0.18 to 0.75) | .006 |
ORs for cases and matched controls are scaled to compare the 90th to the 10th percentile of metabolite values (modeled on a continuous basis) based on the distribution in the controls; letting X90 and X10 denote the 90th percentile and 10th percentile in controls, and β denote the log(OR) from the conditional logistic regression model, the OR is eβ(X90−X10). CI = confidence interval; Leu-val = leucyl-valine; LysoPC = lysophosphatidylcholine; OR = odds ratio.
P value for χ2 test obtained from conditional logistic regression model for a given metabolite (modeled on a continuous basis); all tests were two-sided.
Models adjusted for entry age (years), body mass index (kg/m2), smoking intensity (cigarettes/day), smoking duration (years), alcohol intake (none, <11.6 g/day, ≥11.6 g/day, or missing), self-reported diabetes status (yes or no), education (≤ or > elementary education), run order, and all other metabolites previously in model.
Figure 1.
Adjusted receiver operating characteristic curves for metabolites independently associated with liver cancer or liver disease mortality. A) Results for liver cancer (aAUC = 0.78, 95% CI = 0.74 to 0.83) including glycocholic acid, leu-val, hypoxanthine, and lysoPC(18:2). B) Results for liver disease mortality (aAUC = 0.82, 95% CI = 0.78 to 0.85) including glycochenodeoxycholic acid, lysoPC(18:2), serotonin, and trigonelline. aAUC = adjusted area under the curve; CI = confidence interval. The solid line represents the aAUC and the dashed lines represent the upper and lower 95% confidence intervals.
We also considered the associations of 12 unidentified metabolites; of these, six were inversely associated with both endpoints and six were positively associated with both endpoints (Supplementary Table 2 and 3, available online). Results from sensitivity analyses were generally similar to those from the main analyses (Supplementary Table 4 and 5, available online). However, among those without diabetes, odds ratio estimates, adjusted for homeostatic model assessment for insulin resistance, were notably attenuated for tyrosine and GCDCA with liver cancer and for lysoPC(P-16:0) with liver cancer and fatal liver disease.
Discussion
In this prospective study with baseline serum collected up to 27 years prior to liver cancer diagnosis or liver disease death, we observed strong associations with coffee consumption, liver cancer, and liver disease mortality for 21 metabolites. We identified nine of the 21 metabolites as well as trigonelline. Metabolites positively correlated with coffee consumption were associated with lower risk of liver cancer and liver disease death. In contrast, metabolites negatively correlated with coffee consumption, including two bile acids, were associated with higher risk of liver cancer and liver disease death.
Several case-control studies have used untargeted metabolomics to identify markers to facilitate early detection of liver cirrhosis and liver cancer in high-risk populations (28–33). But, just one other study prospectively evaluated associations between serum metabolites and liver cancer. A nested case-control study in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, with 114 hepatocellular (HCC) cases, measured 44 metabolites using nuclear magnetic resonance (34) and found associations with markers of fatty acid oxidation as well as amino acid, lipid, and carbohydrate metabolism. EPIC investigators recently updated this analysis to include 220 HCC cases and matched controls (Stepien, Keski-Rahkonen, Kiss, Duarte-Salles, Murphy, Perlemuter, et al., unpublished data) using the same analytical platform as the current study. Despite distinct study populations and complementary but different endpoints, our two studies observed similar positive associations for tyrosine, GCA, and GCDCA and inverse associations for lysoPC(15:0), lysoPC(18:2), lysoPC(P-16:0), and leu-val.
Perturbations in amino acid metabolism, particularly high levels of aromatic amino acids such as tyrosine, have been implicated in the pathogenesis of chronic liver diseases (35–37), and one recent study suggested that gut dysbiosis may result in the abnormal accumulation of serum tyrosine, which may contribute to liver disease progression (32). The link between gut dysbiosis and chronic liver diseases may also be relevant to the observed associations with bile acids, which undergo biotransformation by the gut microbiota (38). A secondary bile acid, deoxycholic acid, has been shown both to cause DNA damage (39) and to promote liver tumor growth (40). Experimental studies have linked interactions between the gut microbiota and liver, termed the liver-gut axis, to the pathogenesis of liver diseases (41). Current data, primarily from rodent models, suggest that the liver-gut axis is a potential target for prevention of liver disease progression and liver cancer (19,41,42). Interestingly, the coffee compound cafestol has been shown to suppress bile acid synthesis in mice (43) and in rat hepatocytes (44), and chlorogenic acid, a phenolic compound abundant in coffee, has been shown to reduce bile acid-induced colon carcinogenesis (45). Moreover, a recent analysis of metabolomic data from the PREDIMED study also found that coffee drinking was negatively associated with GCA (46). Studies have also demonstrated that trigonelline has an inhibitory effect on nuclear factor E2-related factor 2, which may play an important role in cancer (47). Experimental evidence suggests that coffee may increase serotonin availability (48), although prospective studies are needed to clarify the metabolic role of peripheral serotonin in humans (49).
We observed inverse associations with leu-val as well as three glycerophospholipids belonging to a class of chemical compounds called lysoPCs; each of these inverse associations was also observed in the EPIC cohort (Stepien, Keski-Rahkonen, Kiss, Duarte-Salles, Murphy, Perlemuter, et al., unpublished data). Leu-val is a product of protein catabolism, and although some dipeptides have known physiological effects, their relevance to the liver is unclear. Glycerophospholipids are components of cellular membranes and play important roles in many cellular signaling pathways; lysoPCs, more specifically, have been linked to apoptosis, particularly in proliferating cells such as cancer (50), and a recent biomarker identification study for early detection of HCC found that higher serum levels of several lysoPCs, including lysoPC(15:0), were negatively correlated with liver enzymes (28). Finally, our findings for hypoxanthine, a naturally occurring purine derivative, are consistent with two small case-control studies of HCC (51) and HBV-induced HCC and cirrhosis (52). Again, however, further mechanistic studies are needed.
One key question is whether identified metabolites are a cause or result of underlying liver disease. We lacked clinical information about liver disease prevalence at baseline and incidence during follow-up; however, participants manifesting cirrhosis or who reported chronic alcohol abuse were ineligible to enroll in the ATBC trial. Nevertheless, asymptomatic cirrhosis can be present for many years prior to diagnosis. With up to 27 years of follow-up, our study was able to examine whether associations varied across follow-up time. Most associated metabolites displayed similar patterns among cases occurring more than 10 years after baseline blood draw. However, future metabolomics studies with assessment of chronic liver disease and longitudinal sample collections are needed to confirm and extend our findings.
Our study was strengthened by the availability of data on HBV and HCV status as well as fasting glucose in a subset of participants. These measures allowed us to evaluate the potential impact of known risk factors on odds ratio estimates. Our results were similar when we excluded those with positive HBV or HCV tests or those with diabetes, suggesting that the observed metabolite associations were independent of these factors. One main limitation of our study is its potential lack of generalizability given that study participants were male Finnish smokers. However, our findings support those reported in EPIC and in cross-sectional studies of liver diseases. A second limitation is that our study design and analytic approach, which focused on metabolites that were cross-sectionally associated with coffee drinking and prospectively associated with liver outcomes, does not permit a rigorous mediation analysis or establish a causal association of coffee drinking with either liver cancer or chronic liver disease mortality.
In conclusion, our study identified metabolites that were strongly associated with coffee consumption, liver cancer, and liver disease mortality. Our focus on metabolites associated with coffee drinking provides potential insight into metabolic pathways that may contribute to the strong observed inverse associations between coffee drinking, liver disease development and progression, and liver cancer. The strong positive associations of bile acids and inverse associations of glycerophospholipids warrant further exploration both in experimental and population studies.
Funding
This work was supported in part by the Intramural Research Program of the National Cancer Institute (NCI), National Institutes of Health, and by US Public Health Service contract HHSN261201500005C from the NCI, Department of Health and Human Services.
Notes
The funding agency did not have any role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors have no conflicts to disclose. Where authors are identified as personnel of the International Agency for Research on Cancer–World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer–World Health Organization. We acknowledge the authors Stepien, Keski-Rahkonen, Kiss, Duarte-Salles, Murphy, Perlemuter, et al., of the EPIC metabolomics-HCC study and have obtained written permission to cite their unpublished work.
Supplementary Material
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Siegel AB, Zhu AX.. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115(24):5651–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy S, Xu J, Kochanek K, et al. Deaths: Final Data for 2015. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 4. McGlynn KA, London WT.. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15(2):223–243, vii–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGlynn KA, Petrick JL, London WT.. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19(2):223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Center MM, Jemal A.. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2362–2368. [DOI] [PubMed] [Google Scholar]
- 7. Altekruse SF, McGlynn KA, Reichman ME.. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davila JA, Morgan RO, Shaib Y, et al. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127(5):1372–1380. [DOI] [PubMed] [Google Scholar]
- 9. Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182–1188.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loftfield E, Freedman ND, Lai GY, et al. Higher glucose and insulin levels are associated with risk of liver cancer and chronic liver disease mortality among men without a history of diabetes. Cancer Prev Res. 2016;9(11):866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. [DOI] [PubMed] [Google Scholar]
- 12. Schlesinger S, Aleksandrova K, Pischon T, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol. 2013;24(9):2449–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Serag HB, Hampel H, Javadi F.. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4(3):369–380. [DOI] [PubMed] [Google Scholar]
- 14. Bravi F, Bosetti C, Tavani A, et al. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1413–1421.e1. [DOI] [PubMed] [Google Scholar]
- 15. Sang LX, Chang B, Li XH, Jiang M. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterol. 2013;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai GY, Weinstein SJ, Albanes D, et al. The association of coffee intake with liver cancer incidence and chronic liver disease mortality in male smokers. Br J Cancer. 2013;109(5):1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding M, Bhupathiraju SN, Chen M, et al. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37(2):569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freedman ND, Everhart JE, Lindsay KL, et al. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009;50(5):1360–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puri P, Daita K, Joyce A, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67(2):534–548. doi:10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bajaj JS, Hylemon PB.. Gut-liver axis alterations in alcoholic liver disease: are bile acids the answer? Hepatology. 2018;67(6):2074–2075. [DOI] [PubMed] [Google Scholar]
- 21. Jahn D, Geier A.. Bile acids in nonalcoholic steatohepatitis: pathophysiological driving force or innocent bystanders? Hepatology. 2018;67(2):464–466. doi:10.1002/hep.29543. [DOI] [PubMed] [Google Scholar]
- 22. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 23. Korhonen P, Malila N, Pukkala E, et al. The Finnish Cancer Registry as follow-up source of a large trial cohort—accuracy and delay. Acta Oncol. 2002;41(4):381–388. [DOI] [PubMed] [Google Scholar]
- 24. Vlaanderen JJ, Janssen NA, Hoek G, et al. The impact of ambient air pollution on the human blood metabolome. Environ Res. 2017;156:341–348. [DOI] [PubMed] [Google Scholar]
- 25. Leek JT, Johnson WE, Parker HS, et al. The SVA package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sumner LW, Amberg A, Barrett D, et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics. 2007;3(3):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pepe MS, Fan J, Seymour CW.. Estimating the receiver operating characteristic curve in studies that match controls to cases on covariates. Acad Radiol. 2013;20(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo P, Yin P, Hua R, et al. A large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67(2):662–675. doi:10.1002/hep.29561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gong ZG, Zhao W, Zhang J, et al. Metabolomics and eicosanoid analysis identified serum biomarkers for distinguishing hepatocellular carcinoma from hepatitis B virus-related cirrhosis. Oncotarget. 2017;8(38):63890–63900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao H, Lu Q, Liu X, et al. Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Sci. 2009;100(4):782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen T, Xie G, Wang X, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics. 2011;10(7):M110.004945.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Wang Y, Zhang X, et al. Gut microbial dysbiosis is associated with altered hepatic functions and serum metabolites in chronic hepatitis B patients. Front Microbiol. 2017;8(2222):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Hong Z, Tan G, et al. NMR and LC/MS-based global metabolomics to identify serum biomarkers differentiating hepatocellular carcinoma from liver cirrhosis. Int J Cancer. 2014;135(3):658–668. [DOI] [PubMed] [Google Scholar]
- 34. Fages A, Duarte-Salles T, Stepien M, et al. Metabolomic profiles of hepatocellular carcinoma in a European prospective cohort. BMC Med. 2015;13(242–256). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao R, Cheng J, Fan C, et al. Serum metabolomics to identify the liver disease-specific biomarkers for the progression of hepatitis to hepatocellular carcinoma. Sci Rep. 2015;5(18175):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sands CJ, Guha IN, Kyriakides M, et al. Metabolic phenotyping for enhanced mechanistic stratification of chronic hepatitis C-induced liver fibrosis. Am J Gastroenterol. 2015;110(1):159–169. [DOI] [PubMed] [Google Scholar]
- 37. Stepien M, Duarte-Salles T, Fedirko V, et al. Alteration of amino acid and biogenic amine metabolism in hepatobiliary cancers: findings from a prospective cohort study. Int J Cancer. 2016;138(2):348–360. [DOI] [PubMed] [Google Scholar]
- 38. Louis P, Hold GL, Flint HJ.. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–672. [DOI] [PubMed] [Google Scholar]
- 39. Bernstein H, Bernstein C, Payne CM, et al. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589(1):47–65. [DOI] [PubMed] [Google Scholar]
- 40. Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. [DOI] [PubMed] [Google Scholar]
- 41. Yu LX, Schwabe RF.. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527–539. doi:10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wiest R, Albillos A, Trauner M, et al. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67(5):1084–1103. doi:10.1016/j.jhep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 43. Ricketts ML, Boekschoten MV, Kreeft AJ, et al. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21(7):1603–1616. [DOI] [PubMed] [Google Scholar]
- 44. Post SM, de Wit EC, Princen HM.. Cafestol, the cholesterol-raising factor in boiled coffee, suppresses bile acid synthesis by downregulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase in rat hepatocytes. Arterioscler Thromb Vasc Biol. 1997;17(11):3064–3070. [DOI] [PubMed] [Google Scholar]
- 45. Bernstein C, Holubec H, Bhattacharyya AK, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011;85(8):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Papandreou C, Hernández-Alonso P, Bulló M, et al. Plasma metabolites associated with coffee consumption: a metabolomic approach within the PREDIMED study. Nutrients. 2019;11(5):1032.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arlt A, Sebens S, Krebs S, et al. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene. 2013;32(40):4825–4835. [DOI] [PubMed] [Google Scholar]
- 48. Gostner JM, Schroecksnadel S, Jenny M, et al. Coffee extracts suppress tryptophan breakdown in mitogen-stimulated peripheral blood mononuclear cells. J Am Coll Nutr. 2015;34(3):212–223. [DOI] [PubMed] [Google Scholar]
- 49. Martin AM, Young RL, Leong L, et al. The diverse metabolic roles of peripheral serotonin. Endocrinology. 2017;158(5):1049–1063. [DOI] [PubMed] [Google Scholar]
- 50. van Blitterswijk WJ, Verheij M.. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14(21):2061–2074. [DOI] [PubMed] [Google Scholar]
- 51. Wu H, Xue R, Dong L, et al. Metabolomic profiling of human urine in hepatocellular carcinoma patients using gas chromatography/mass spectrometry. Anal Chim Acta. 2009;648(1):98–104. [DOI] [PubMed] [Google Scholar]
- 52. Yin P, Wan D, Zhao C, et al. A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Mol Biosyst. 2009;5(8):868–876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.