Abstract
Study Objectives:
Mast cell activation syndrome (MCAS) is an inflammatory and allergic disorder. We determined the prevalence of restless legs syndrome (RLS) in MCAS because each common syndrome may be inflammatory in nature and associated with dysautonomia.
Methods:
Individuals with MCAS were evaluated for RLS by two standard questionnaires. Prevalence comparisons included spouse control patients and two prevalence publications. MCAS diagnosis required mast cell (MC) symptoms in ≥ 2 organs plus ≥ 1 elevated MC mediators, improvement with MC therapy, and/or increased intestinal MC density. Clinical variables were studied.
Results:
There were 174 patients with MCAS (146 female, 28 male, mean age 44.8 years) and 85 spouse control patients (12 female, 73 male, mean age 50.9 years). Patients with MCAS as a whole had a higher prevalence of RLS (40.8%) than spouse control (12.9%) (P < .0001) Male patients with MCAS had a higher prevalence of RLS (32.1%) than male controls (12.3%, odds ratio [OR] 3.4, confidence interval [CI] 1.2–9.7, P = .025), American men (8.4%, OR 5.2, CI 2.2–12.0, P < .001), and French men (5.8%, OR 7.7, CI 3.4–17.1, P < .001). Female patients with MCAS also had a higher prevalence of RLS (42.5%) than female controls (16.7%) but this did not reach statistical significance perhaps because of the sample size of the female controls. However, female patients with MCAS had a statistically higher prevalence of RLS than American women (10.0%, OR 6.7, CI 4.5–9.7, P < .0001) and French women (10.8%, OR 6.1, CI 4.4–8.6, P < .0001).
Conclusions:
RLS appears to be associated with MCAS. Effects of mast cell mediators, inflammation, immune mechanisms, dysautonomia, or hypoxia may theoretically activate RLS in MCAS.
Citation:
Weinstock LB, Walters AS, Brook JB, Kaleem Z, Afrin LB, Molderings GJ. Restless legs syndrome is associated with mast cell activation syndrome. J Clin Sleep Med. 2020;16(3):401–408.
Keywords: autonomic dysfunction, hypoxia, immune, inflammation, mast cell activation syndrome, prevalence, restless legs syndrome
BRIEF SUMMARY
Current Knowledge/Study Rationale: Restless legs syndrome (RLS) is characterized by leg discomfort that is worse at night and worse lying or sitting, leading to an urge to move the legs with at least temporary relief by activity. Mast cell activation syndrome (MCAS) is a common yet poorly recognized condition of unregulated, aberrant mast cell activation that leads to many inflammatory and allergic symptoms. Based on unpublished clinical observations by two large research centers that RLS is a common problem among their patients with MCAS, we thought that a formal, controlled prevalence study was desirable.
Study Impact: In the patients with MCAS who were studied there was a significantly higher prevalence of RLS than in the spouse control patients. The prevalence of RLS was also significantly greater than large historical groups. In addition, the prevalence of RLS in our MCAS males was statistically significantly higher than in our male spouse control patients and in males from the two historical control groups. The prevalence of RLS in our MCAS females was also higher than in the female spouse control patients, but the size of the female control group was too small to attain statistical significance. However, the prevalence of RLS in our female MCAS group was statistically higher than that for females in our two large historical samples. The relationship between these common syndromes leads to interesting theories how they may be linked by common pathophysiology.
INTRODUCTION
Restless legs syndrome (RLS) is a sensorimotor disorder that is either idiopathic or secondary and is associated with a number of conditions.1 RLS is recognized as the compelling urge to move the legs while awake, often with discomfort, and worsens at night.2 A wide prevalence of RLS is reported depending on population location, age, sex, and whether it is classified as idiopathic or secondary RLS. The pathophysiology of RLS is likely multifactorial; central and/or peripheral neuroinflammation has been considered, though the source of such inflammation has remained obscure.3
Normal mast cells (MCs) are multifunctional immune cells that play crucial roles in innate and adaptive immunity.4 MCs participate in host defense, tissue repair, wound healing, and angiogenesis. Mast cell activation syndrome (MCAS) is usually due to somatic mutations affecting MCs, but the menagerie of such mutations found in the MCAS population thus far, combined with the large number (> 200) of mediators produced by MCs and the large array of effects of each mediator, ensure extraordinary heterogeneity in clinical presentation.4 MCAS has general themes of inflammation ± allergic-type phenomena ± dystrophism (abnormalities in growth and development).5 This disorder is thought to be common, with published prevalence estimates in the range of 1% to 17% of the population.4 Symptoms can start in childhood or adulthood, depending on when the MC regulatory gene mutation occurred, and the range of symptoms experienced by the individual patient typically expands as the patient ages.5 The most frequent symptoms in 50% or more of patients with MCAS include fatigue, muscle pain, near-syncope, headaches, pruritis, urticaria, nausea, chills, edema, eye irritation, dyspnea, and heartburn.4 Because all organ systems can be involved in MCAS, the scope of symptoms can be extensive. Patients may receive a diagnosis of conversion syndrome or other functional disorders because of the waxing and waning nature of extensive symptoms over the decades and failure by physicians to diagnose a cohesive disorder.
In general, MCs are involved in many neurologic disorders including multiple sclerosis, Parkinson disease, migraine, cognitive dysfunction, autism, Alzheimer disease, and neurofibromatosis.6–9 MC-induced neurologic pathologic states are due to the direct and indirect effects of the mediators aberrantly produced and released by dysfunctional MCs. This includes inflammatory demyelination, pain, angiogenesis, and a permeable blood-brain barrier.10,11
Neurologic symptoms in MCAS are common and wide ranging, and include dizziness, headaches, numbness, tremor, vertigo, cognitive dysfunction, anxiety, panic attacks, depression, and sleep disturbances.9 In addition, MCAS has been reported to be associated with autonomic dysfunction, peripheral neuropathy, intradural adhesions, parkinsonism, intracerebral coagulopathy, seizures, tinnitus, and migraines.9
Two referral centers for MCAS have observed that RLS is a common complaint by their patients (L. Afrin, MD and G. Molderings, MD, personal communication, 2018). The prevalence, nature, and risks for RLS in patients with MCAS are presented here for the first time.
METHODS
From February 2017 through April 2019, all consecutive adult patients presenting with refractory gastrointestinal symptoms to a gastroenterologist (LBW) whose research interests includes MCAS and RLS were initially evaluated for the presence of MCAS. Starting August 2018, patients in whom MCAS had been diagnosed were invited to participate in a study of sleep disturbances in general (not just RLS). In this manner, individuals and control patients who had RLS would not be biased to participate in the study compared to those without RLS. Spouse control patients filled out a questionnaire that included the International RLS Study Group criteria.12 If these criteria were met, the control patients filled out the Cambridge-Hopkins RLS Short Form 2 diagnostic questionnaire.13 A history and physical examination of the patients ruled out RLS mimics such as muscle cramps, sciatica, sensory neuropathy, and arthritis. These patients filled out the same two questionnaires. In patients in whom RLS was diagnosed the self-administered version of the International RLS Study Group severity rating scale (sIRLS) was obtained.14 A ferritin level was obtained as part of standard of care for the patients and to determine if levels were low as an alternative factor for RLS.15 Informed consent to collect data from patients and spouse control patients was obtained. The study was approved by the Missouri Baptist Medical Center Investigational Review Board in St. Louis, Missouri.
MCAS diagnosis was based on recent diagnostic criteria, that is, typical symptoms of MC activation in two or more organ systems plus one or more of the following criteria: elevation of MC mediator(s), clinical improvement with MC-directed therapy, and intestinal MC density ≥ 20 per high-power field (HPF).5 MC mediator measurements included: (1) plasma prostaglandin D2, histamine, and heparin, (2) serum tryptase and chromogranin A, and (3) 24-hour urine prostaglandin 11-β-PGF2α, N-methylhistamine, and leukotriene E4. To help confirm a clinical diagnosis of MC activation, the MC mediator release syndrome (MCMRS) score was employed (supplemental material).4,16 The MCMRS is a questionnaire that assesses the number and weight of symptoms, laboratory, radiographic, and biopsy findings. A sum of 9 to 13 = pathological activation of mast cells as cause of the complaints. A sum ≥ 14 = clinical confirmation of a diagnosis of MC mediator release syndrome but not MCAS per se.
MC density was assessed by a standardized systematic approach where MCs were counted in multiple HPFs using the CD117 immunohistochemical stain and Nikon BX41 Plan N 40×/0.65 objective magnification. MC density was expressed as a range including < 20, 20–30, 31–40, 41–50, 51–60, 61–70, 71–80, 81–90, 91–100, and > 100 MC/HPF. The MC density was categorized as follows: (1) diffuse: MC density reported as least common denominator in any of several microscopic fields, and (2) focal: highest MC density reported in more than three mutually exclusive microscopic fields.
Additional data collection for the patients included: age, sex, height, weight, medications, complete review of systems, past medical history, and the presence of comorbid postural orthostatic tachycardia syndrome (POTS) and hypermobile Ehlers-Danlos syndrome (hEDS) determined by past history and physical examination. Irritable bowel syndrome (IBS) diagnosis used the Rome 4 criteria.17 All patients underwent a standard physical examination that included sensory examination of the legs, orthostatic pulse measurements, and joint flexibility evaluation using the Beighton criteria for hEDS.18
Exclusion criteria in patients included age younger than 18 years, Crohn disease, ulcerative colitis, and untreated celiac disease. Patients with migraines, headaches, fibromyalgia, chronic abdominal pain with fatigue, and IBS were not excluded because these are common problems of MCAS. Exclusion criteria in spouse control patients included age younger than 18 years, Crohn disease, ulcerative colitis, and untreated celiac disease. In efforts to exclude control patients for MCAS, exclusion criteria included a history of IBS, chronic abdominal pain or diarrhea, chronic nausea, chronic acid reflux requiring medications, and fibromyalgia. Pregnancy was an exclusion criterion for both groups. To account for the fact that control patients may have had conditions that excluded them from participation and they were thus not completely matched to patient exclusion criteria, two subanalyses were carried out: (1) patients with MCAS and RLS who did not have fibromyalgia, chronic abdominal pain with fatigue and IBS, headaches, and migraines were compared to the spouse control patients and the historical controls. Thus in this subanalysis the exclusion criteria for the patients and the control patients were similar and furthermore the patients were excluded if they had headaches and migraines; (2) in patients with MCAS and without RLS, variable analysis determined the effect of migraines, headaches, fibromyalgia, chronic muscle pain with chronic fatigue, IBS, and proton pump inhibitor (PPI) use on the prevalence of RLS.
Because of sex imbalance among patients with MCAS and control patients, sex-specific comparisons were performed. RLS in patients with MCAS was compared to that in spousal controls of the same sex and to published estimates of RLS in the general populations of France and the United States, which employed the International RLS Study Group criteria.19,20
The primary aim was to determine whether prevalence of RLS is higher in patients with MCAS versus spouse control patients and versus the general population matched to sex. Secondary aims included determination whether MC mediators, MC density in biopsies, medical disorders and medical traits, and medications are present that increase the risk of RLS. The use of medications used to treat MCAS and also reported in the literature to potentially exacerbate RLS was analyzed (including antidepressants and histamine-1 receptor blockers). The medicine module in the patient’s electronic medical record was used to access prescription and over-the-counter medications (which is asked of all patients).
Statistical analysis included odds ratio (OR) and 95% confidence interval (CI) calculations for comparing patients with MCAS to control patients and to the published prevalence in two general populations. The t tests and Fisher exact tests compared patients with RLS to patients without RLS. A value of P = .05 was considered statistically significant.
RESULTS
A total of 174 patients with MCAS (146 female, 28 male, age 44.8 ± 17.0 years) were compared to 85 spouse control patients (12 female, 73 male, age 50.9 ± 14.1 years) and to two published estimates of RLS prevalence in the general population.19,20 Of 174 patients, 89 were unmarried and most were young women. One additional patient with RLS was excluded because she also had Crohn disease. Three other potential patients with MCAS chose not to participate in the study. In the total of 98 spouses, 86.7% chose to participate in the study.
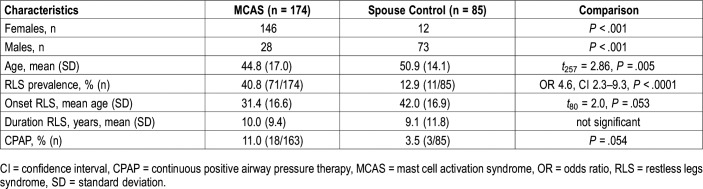
All patients in whom MCAS was diagnosed fulfilled the major criteria with multiple symptoms of MC activity with chronic fatigue, muscle pain, tinnitus, rhinitis, and conjunctivitis being most common, along with chronic gastrointestinal symptoms (abdominal pain, constipation, diarrhea, bloating, nausea, heartburn, and dysphagia). Minor criteria included: mediator tests positive in 72.9% and abnormal biopsies in 98.5% of patients. MC-directed treatment was beneficial in 81.6%, unsuccessful in 8.6%, and it was too early to determine in 9.8% at the time of data capture closure. Clinical characteristics and potential risk factors for RLS in patients with MCAS and control patients are shown in Table 1.
Table 1.
Clinical characteristics of patients with MCAS and spouse control patients.
Comparisons by sex showed that female patients with MCAS had more concomitant POTS, EDS, and both compared to male patients with MCAS (P = .003, P = .006, P = .009, respectively). Female patients were more likely to report migraines (P = .04) and gastrointestinal bloating (P = .004). The spouse control group contained a higher proportion of males than the MCAS group (P < .001). Patients with MCAS were also younger than the spouse control patients: age 44.8 ± 17.0 versus 50.9 ± 14.1 years (t257 = 2.86, P = .005). No other clinical variables showed differences by sex.
The prevalence of RLS was 40.8% (71 of 174) in patients with MCAS versus 12.9% (11 of 85) in spouse control patients, giving patients with MCAS an OR of 4.6, with 95% CI of 2.3–9.3 (P < .0001). RLS prevalence in the 174 patients with MCAS was also greater than in the American population (9.4%) (OR 6.62, 95% CI 4.71–9.31, z = 10.86, P < .0001) and the French population (8.5%) (OR 7.44, 95% CI 5.46–10.15, z = 12.68, P < .0001).
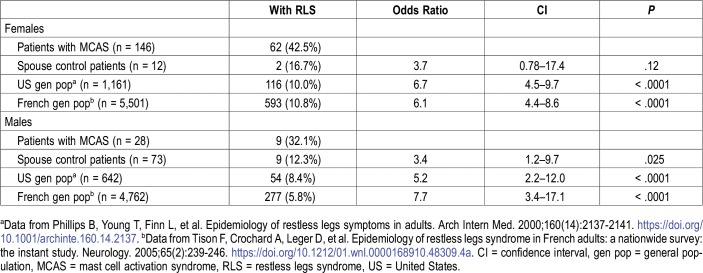
Because of the predominance of females among patients with MCAS, further comparisons were divided by sex (Table 2). Among 28 male patients with MCAS, 9 (32.1%) had RLS compared to 9 of 73 male spouse control patients (12.3%, OR 3.4, CI 1.2–9.7, P = .025). Male patients with MCAS also had higher prevalence than that estimated in the adult male general population in the United States (8.4%, OR 5.2, CI 2.2–12.0, P < .001) and France (5.8%, OR 7.7, CI 3.4–17.1, P < .001).19,20 Among the 146 female patients with MCAS, 62 (42.5%) had RLS, compared to 2 of 12 female spouse control patients (16.7%, OR 3.7, CI 0.78–17.4, P = .12). Female patients with MCAS had higher prevalence than that estimated in the adult female general population in the United States (10.0%, OR 6.7, CI 4.5–9.7, P < .0001) and France (10.8%, OR 6.1, CI 4.4–8.6, P < .0001).19,20
Table 2.
RLS prevalence comparisons by sex between patients with MCAS and three control groups.
In the subanalysis of patients with MCAS who met the same inclusion and exclusion criteria as control patients, 11/30 (36.7%) of our patients with MCAS had RLS as opposed to 11/85 control patients (12.9%) (OR = 3.89, 95% CI 1.47–10.34, z = 2.73, P = .0063). This relationship also held true when these patients with MCAS were compared to the general United States population 170/1807 (9.4%) (OR 5.56, 95% CI 2.60–11.88, z = 4.43, P < .0001) or the French population 870/10,263 (8.5%) (OR 6.25, 95% CI 2.97–13.18, z = 4.82, P < .0001). Of our female patients with MCAS who had the same exclusion criteria as control patients, 10/22 (45.5%) had RLS as compared to 2/12 (16.7%) of our female spouse control patients but this did not reach statistical significance (OR 4.17, 95% CI 0.73–23.6, z = 1.6, P = .11). However, these female patients had a higher prevalence of RLS than that estimated in the adult female general population in the United States (OR 7.5, 95% CI 3.17–17.76, z = 4.59, P < .0001) and France (OR 6.90, 95% CI 2.97–16.03, z = 4.49, P < .0001).19,20 Statistical analysis of males with MCAS and who had these additional exclusion criteria was limited by the low numbers of men (1/8 had RLS).
The second subanalysis showed there was no relationship between patients positive and negative for MCAS with respect to the risk of RLS and the presence of migraines, headaches, fibromyalgia, and PPI use. Chronic muscle pain with fatigue was more prevalent in the patients positive for MCAS-RLS (P = .04).
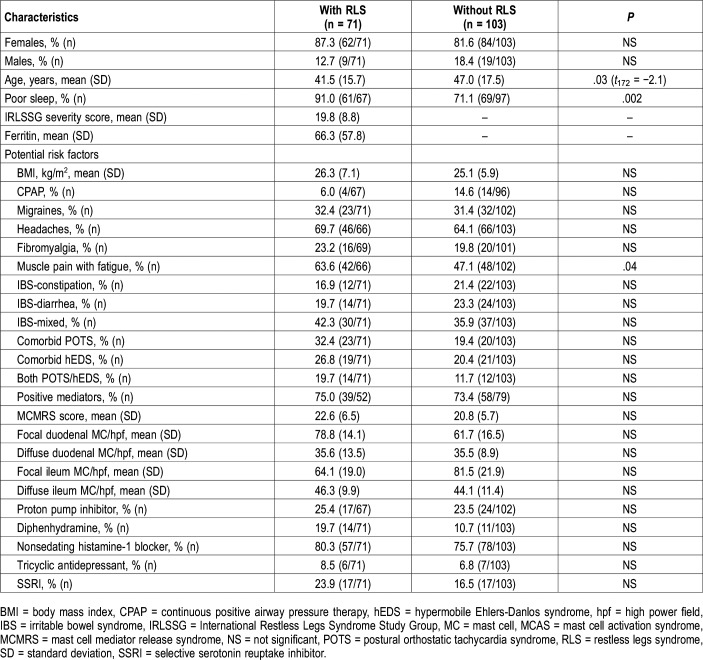
As shown in Table 3, there were few differences between the patients with MCAS with and without RLS. These conditions were common to each group, especially headaches and IBS. Comorbid POTS trended toward being more common in patients with RLS (P = .07); however, hEDS and POTS plus hEDS did not. The body mass index was not associated with RLS in patients with MCAS. The use of continuous positive airway pressure therapy was not associated with rate of RLS among patients with MCAS. There was no statistical association of the presence of RLS in patients with MCAS with either the degree of MC density in gastrointestinal biopsies, the presence of positive testing and/or specific MC mediators, or MCMRS scores. Patients positive for MCAS-RLS versus patients negative for MCAS-RLS took diphenhydramine (19.7% versus 10.7%), nonsedating histamine-1 receptor blockers (80.3% versus 75.7%), PPI (25.4% versus 23.5%), tricyclic antidepressants (8.5% versus 6.8%), and selective serotonin reuptake inhibitor antidepressants (23.9% versus 16.5%). None of these were statistically different. All of the patients positive for MCAS-RLS had RLS long before they were prescribed diphenhydramine and nonsedating histamine-1 receptor blockers.
Table 3.
Clinical characteristics of patients with MCAS with and without RLS.
Patients with MCAS and who had RLS were younger compared to patients with MCAS without RLS: 41.5 ± 15.7 versus 47.0 ± 17.5 (t172 = −2.1, P = .03). The age of onset of RLS was numerically lower in patients with MCAS compared to control patients with RLS: 31.4 ± 16.6 versus 42.0 ± 16.9 (t80 = 2.0, P = .053). The patients with MCAS had a mean sIRLS severity score of 19.8 ± 8.8, and the duration of RLS was 10.0 ± 9.4 years. In the patients with MCAS the mean ferritin level was 66.3 ± 57.8 ng/nL; range: 10–230 ng/mL (normal = 10–232 ng/mL). The percentage of patients with MCAS and who had RLS whose ferritin was ≤ 50 ng/mL was 45.7% (85.7% of these were women). The percentage of patients with MCAS and who had RLS whose ferritin was ≤ 10 ng/mL was 6.5% (all were women). Ferritin measurements for other patients and control patients were not included in the protocol.
DISCUSSION
This study is the first to investigate the prevalence of RLS in MCAS. RLS was found to be associated with MCAS. The overall prevalence of RLS in patients with MCAS was 40.8%, which was found to be statistically significantly greater than in our own control population and in comparison with two large epidemiologic studies from the literature. In women the prevalence was 42.5% and in men it was 32.1%. Each is significantly greater than the population studies with separate evaluations by sex.19,20 This degree of RLS prevalence is also higher than many of the secondary RLS conditions.3
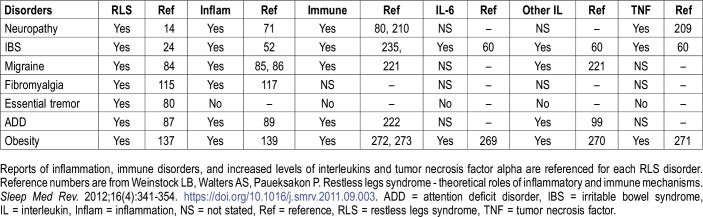
The findings of our study have clinical importance for several reasons. MCAS is emerging as the “great modern-day imitator.” It often presents with widespread systemic inflammatory and allergic manifestations and is frequently undiagnosed.4,5 Furthermore, RLS may be more effectively treated after identifying specific underlying disorders.21–23 In a 2011 study all reported causes of secondary RLS found that 42 of the 47 disorders (89%) were associated with immune or inflammatory conditions.3 At that time the extensive connection of MCAS with other syndromes had not yet been fully elucidated.4,5 Eight disorders underlying secondary RLS that are also caused by and/or associated with MCAS are shown in Table 4. The connections to inflammation and immune disorders are illustrated. Theories that potentially link RLS and MCAS include inflammatory and immune changes, MC activation, autonomic dysfunction, and hypoxia. Muscle pain and chronic fatigue are highly prevalent in MCAS. It is possible that some of these patients could have had undiagnosed fibromyalgia, which also is associated with an increased prevalence of RLS.3 Alternatively, “fibromyalgia” is often a diagnosis assigned to the yet undiagnosed patient with MCAS.
Table 4.
Disorders of secondary RLS that are also associated with mast cell activation syndrome.
The primary site of the RLS disorder is thought to be in the basal ganglia, substantia nigra, and thalamus and it is possible that local MCs may play a dysfunctional role in these areas.24–28 We theorize that central nervous system (CNS) histamine and/or other inflammatory MC mediators could cause RLS by inducing dysfunction of dopamine cells or receptors, decreasing transportation of iron into dopamine cells, and/or causing endorphin cell dysfunction, which is altered in RLS and is important in preventing malfunction of dopamine cells in the setting of iron deficiency.29,30 Furthermore, inflammatory markers have been seen in the cerebrospinal fluid and blood of patients with RLS.31 Increased levels of cytokines including tumor necrosis factor alpha and interleukin-6 are associated with activated MCs, yet interplay with MCs and T-cells may factor into this process as well.32,33
CNS iron deficiency is a major disorder in patients with RLS because, under this condition, dopamine cells do not function well.34 Blood-brain barrier alterations induced by angiogenic mediators produced by activated MCs are thought to result in vasoproliferative reactions and pathologic conditions.35 This theoretically could result in CNS iron deficiency via altered iron transportation into the CNS. Proinflammatory MC mediators could increase hepcidin production at the level of the blood-brain barrier, which theoretically could result in decreased iron transportation.36 In the current study, peripheral iron deficiency was uncommon (6.5%) and low-normal ferritin levels were common (45.7%) but were primarily found in young women (85.7%).
Autonomic dysfunction is seen in both RLS and MCAS.37–43 Patients with RLS have increased autonomic complaints compared to control patients, specifically constipation, early abdominal fullness, lightheadedness when standing, heat intolerance, hypersensitivity to light, and excessive salivation.38 On objective autonomic testing, patients with RLS demonstrate altered cardiovagal control related to the arterial baroreflex and greater peripheral vascular resistance.39 Patients with RLS also exhibit a proclivity toward hypertension and reduction of sympathetic and parasympathetic amplitude responses on the Head Up Tilt Test. Blunted parasympathetic drive to changes in blood pressure is another problem. In addition, the disorder periodic limb movements in sleep, which frequently accompanies RLS, is associated with autonomic increases in pulse and rises in systolic blood pressure.41 In the current study, the association between POTS and greater risk of RLS approached significance (P = .07). Nonetheless, in MCAS autonomic dysfunction may present with or without POTS.42,43
Finally, serum hypoxic markers are increased in patients with RLS patients compared to control patients.44–46 There is upregulation of hypoxia-inducible factor 1-alpha, vascular endothelial growth factor, and nitric oxide synthase in brains of patients with RLS compared to brains in control patients.45 Alleles of the hemoxygenase-1 gene are downregulated in patients with RLS compared to control patients.46 Importantly, histamine released by MC degranulation induces the expression of hypoxia-inducible factor 1-alpha and vascular endothelial growth factor, which theoretically could play a role in hypoxia and a higher risk of RLS.47
The current study has some limitations. There were more female than male patients, yet this is not unexpected in light of a prior large MCAS study and risks associated with sex for allergy/immune diseases in general.5,48 Accordingly, there were more male spouse control patients than females. The mean age of the control patients was higher than the mean age of the patients because many of the young women in the study were unmarried. In some RLS prevalence studies there are significant differences by sex. In more than10,000 French adults, the RLS prevalence in females was 10.8% compared with 5.8% in males.19 Yet in 1,800 Americans, RLS prevalence was 10% and did not significantly differ by sex.20 Despite the sex difference in our study, the young age at the onset and the duration of RLS are important to note. It is well known that the prevalence increases with aging and thus our findings are even more remarkable. The spouses were not tested for MCAS, yet they were excluded for common conditions seen in MCAS and pertinent well-known secondary RLS disorders. In order to account for differences in the patient and control groups, two subanalyses were studied. One difference was that more patients with MCAS and RLS had chronic fatigue with muscle pain than those without RLS. The nature of the muscle pain in MCAS was soreness and was not confused with muscle cramps or RLS at night as determined by clinical history and queries within the Cambridge-Hopkins Questionnaire.
In the other subanalysis where the inclusion and exclusion criteria were made identical for all patient and control groups, statistically significant differences were maintained for the combination of women and men. In addition, the results were similar in that the female patients with MCAS had statistically more RLS than both population control groups and numerically more than the spouse control patients. Had this circumscribed group been larger in number we would have expected a positive statistical difference between female patients and spouse control patients. Statistical analysis of males with MCAS and these additional exclusion criteria was limited by the low numbers of men (1/8 had RLS).
Another criticism might include the fact that these patients had a diagnosis of MCAS in the setting of the evaluation for refractory gastrointestinal symptoms, yet this scenario is extremely common for the patients in whom MCAS is undiagnosed.49 Although the severity of MCAS and specific MC mediators measured in our patients did not correlate with a risk for RLS, this is not unexpected. In general, the presentation of symptoms is highly variable in MCAS, which may be due to the specific mediators produced by an individual patient’s MC. The MC is capable of producing more than 200 mediators, but commercial laboratories are only able to measure a minority, and even fewer are relatively specific to the MC (eg, prostaglandin D2, leukotriene E4, heparin, and tryptase), so it could easily be the case that MC mediators responsible for directly or indirectly driving RLS are not among the very few mediators measured in the MCAS diagnostic work-up. More likely, however, is that local release of mediators in the CNS may well act locally and not be measurable in the blood. MC mediators have been shown to activate microglial cells in the CNS.50
Another potential limitation is that comparing a symptomatic clinical population to a general population and asymptomatic spouse control groups may lead to bias to higher rates of reporting of symptoms in the clinical population; there may be things that differ between patients and people who do not seek medical treatment in terms of how likely they are to report/perceive RLS symptoms and their severity. The response rate among spouses of patients with MCAS was high (86.7%) so the bias would be minimal in the comparison of patients with MCAS to control patients. In addition, in the general population studies quoted19,20 the respondents did not know that the questionnaire they were answering was specific to RLS so the bias in this case would also be minimal. A final criticism is that a family history of RLS was not elucidated.
CONCLUSIONS
This study shows that RLS appears to be associated with MCAS. Inflammation, immune mechanisms, autonomic dysfunction, and/or CNS hypoxia induced by MCAS may play pathophysiologic roles in this form of RLS. The circadian release of mediators from MCs in the evening may also have a direct relationship to RLS.51 It is paramount to look for underlying treatable causes for RLS. MCAS is generally not considered in the differential diagnosis of a patient with neurologic symptoms and syndromes. It does not appear that antihistamine medications increased the risk of exacerbating RLS in this study. We have seen patients who noted symptomatic improvement in RLS with MC-directed therapy but the current study was not designed to determine drug efficacy. Symptomatic response of RLS to inhibitors of MC activation or MC mediator production could lead to new avenues in therapy.
ACKNOWLEDGEMENTS
Author contributions: Dr. Weinstock wrote the manuscript; Dr. Kaleem performed all of the pathology; Mrs. Brook contributed statistics and review of manuscript; Drs. Walters, Afrin, Molderings, and Kaleem contributed revisions and critical review of manuscript.
ABBREVIATIONS
- CI
confidence interval
- CNS
central nervous system
- HPF
high power field
- hEDS
hypermobile Ehlers Danlos syndrome
- IBS
irritable bowel syndrome
- sIRLS
self-administered International RLS Study Group severity rating scale
- MC
mast cell
- MCAS
mast cell activation syndrome
- MCMRS
mast cell mediator release syndrome
- OR
odds ratio
- PPI
proton pump inhibitor
- RLS
restless legs syndrome
- SSRI
selective serotonin reuptake inhibitor.
DISCLOSURE STATEMENT
Dr. Walters has received research funding from the National Institutes of Health, Xenoport, Schwarz-Pharma, and Kyowa. Dr. Walters has received research funding and is on the speaker’s bureau of Glaxo Smith Kline and Boehringer-Ingelheim. Dr. Molderings is the chief medical officer of the startup company MC Sciences, Ltd. Partial funding for this study was provided by Missouri Baptist Healthcare Foundation. The other authors report no conflicts of interest.
REFERENCES
- 1.Trenkwalder C, Allen R, Högl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology. 2016;86(14):1336–1343. doi: 10.1212/WNL.0000000000002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trotti LM, Goldstein CA, Harrod CG, et al. Quality measures for the care of adult patients with restless legs syndrome. J Clin Sleep Med. 2015;11(3):293–310. doi: 10.5664/jcsm.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstock LB, Walters AS, Paueksakon P. Restless legs syndrome - theoretical roles of inflammatory and immune mechanisms. Sleep Med Rev. 2012;16(4):341–354. doi: 10.1016/j.smrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Afrin LB, Butterfield JH, Raithel M, Molderings GJ. Often seen, rarely recognized: mast cell activation disease–a guide to diagnosis and therapeutic options. Ann Med. 2016;48(3):190–201. doi: 10.3109/07853890.2016.1161231. [DOI] [PubMed] [Google Scholar]
- 5.Afrin LB, Self S, Menk J, Lazarchick J. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353(3):207–215. doi: 10.1016/j.amjms.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conti P, Kempuraj D. Important role of mast cells in multiple sclerosis. Mult Scler Relat Disord. 2016;5:77–80. doi: 10.1016/j.msard.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Kempuraj D, Selvakumar GP, Zaheer S, et al. Cross-talk between glia, neurons and mast cells in neuroinflammation associated with Parkinson’s disease. J Neuroimmune Pharmacol. 2018;13(1):100–112. doi: 10.1007/s11481-017-9766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Zhang X, Dong H, Hu Y, Qian Y. Bidirectional relationship of mast cells-neurovascular unit communication in neuroinflammation and its involvement in POCD. Behav Brain Res. 2017;322(Pt A):60–69. doi: 10.1016/j.bbr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Afrin LB, Pöhlau D, Raithel M, et al. Mast cell activation disease: an underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain Behav Immun. 2015;50:314–321. doi: 10.1016/j.bbi.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Dines KC, Powell HC. Mast cell interactions with the nervous system: relationship to mechanisms of disease. J Neuropathol Exp Neurol. 1997;56(6):627–640. [PubMed] [Google Scholar]
- 11.Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates blood-brain barrier. J Neurobiol. 1996;31(4):393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Allen RP, Picchietti DL, Garcia-Borreguero D. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Walters AS, Frauscher B, Allen R, et al. Review of diagnostic instruments for the restless legs syndrome/Willis-Ekbom Disease (RLS/WED): critique and recommendations. J Clin Sleep Med. 2014;10(12):1343–1349. doi: 10.5664/jcsm.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharon D, Allen RP, Martinez-Martin P. Validation of the self-administered version of the international Restless Legs Syndrome study group severity rating scale - the sIRLS. Sleep Med. 2019;54:94–100. doi: 10.1016/j.sleep.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Wijemanne S, Ondo W. Restless legs syndrome: clinical features, diagnosis and a practical approach to management. Pract Neurol. 2017;17(6):444–452. doi: 10.1136/practneurol-2017-001762. [DOI] [PubMed] [Google Scholar]
- 16.Molderings GJ, Kolck U, Scheurlen C, et al. [Systemic mast cell disease with gastrointestinal symptoms–a diagnostic questionnaire] Dtsch Med Wochenschr. 2006;131(38):2095–2100. doi: 10.1055/s-2006-951337. [DOI] [PubMed] [Google Scholar]
- 17.Vork L, Weerts ZZRM, Mujagic Z, et al. Rome III vs Rome IV criteria for irritable bowel syndrome: a comparison of clinical characteristics in a large cohort study. Neurogastroenterol Motil. 2018;30(2) doi: 10.1111/nmo.13189. [DOI] [PubMed] [Google Scholar]
- 18.Remvig L, Jensen DV, Ward RC. Are diagnostic criteria for general joint hypermobility and benign joint hypermobility syndrome based on reproducible and valid tests? A review of the literature. J Rheumatol. 2007;34:798–803. [PubMed] [Google Scholar]
- 19.Tison F, Crochard A, Leger D, et al. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the instant study. Neurology. 2005;65(2):239–246. doi: 10.1212/01.wnl.0000168910.48309.4a. [DOI] [PubMed] [Google Scholar]
- 20.Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 21.Avni T, Reich S, Lev N, Gafter-Gvili A. Iron supplementation for restless legs syndrome - a systematic review and meta-analysis. Eur J Intern Med. 2019;63:34–41. doi: 10.1016/j.ejim.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Weinstock LB, Myers TL. Restless Legs Syndrome. In: Elsegood L, ed. The LDN Book. 1st ed. White River Junction, VT: Chelsea Green Publishing; 2016:99-100. [Google Scholar]
- 23.Weinstock LB, Fern SE, Duntley SP. Restless legs syndrome in patients with irritable bowel syndrome: response to small intestinal bacterial overgrowth therapy. Dig Dis Sci. 2008;53(5):1252–1256. doi: 10.1007/s10620-007-0021-0. [DOI] [PubMed] [Google Scholar]
- 24.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132(9):2403–2412. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku J, Cho YW, Lee YS, et al. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting-state connectivity study using functional magnetic resonance imaging. Sleep Med. 2014;15(3):289–294. doi: 10.1016/j.sleep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Bolam JP, Ellender TJ. Histamine and the striatum. Neuropharmacology. 2016;106:74–84. doi: 10.1016/j.neuropharm.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theoharides TC, Stewart JM, Panagiotidou S, Melamed I. Mast cells, brain inflammation and autism. Eur J Pharmacol. 2016;778:96–102. doi: 10.1016/j.ejphar.2015.03.086. [DOI] [PubMed] [Google Scholar]
- 28.Theoharides TC, Tsilioni I, Bawazeer M. Mast cells, neuroinflammation and pain in fibromyalgia syndrome. Front Cell Neurosci. 2019;13:353. doi: 10.3389/fncel.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters AS, Ondo WG, Zhu W, Le W. Does the endogenous opiate system play a role in the Restless Legs Syndrome? A pilot post-mortem study. J Neurol Sci. 2009;279(1-2):62–65. doi: 10.1016/j.jns.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Sun YM, Hoang T, Neubauer JA, Walters AS. Opioids protect against substantia nigra cell degeneration under conditions of iron deprivation: a mechanism of possible relevance to the restless legs syndrome (RLS) and Parkinson’s disease. J Neurol Sci. 2011;304(1-2):93–101. doi: 10.1016/j.jns.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Bellei E, Monari E, Ozben S, et al. Discovery of restless legs syndrome plasmatic biomarkers by proteomic analysis. Brain Behav. 2018;8(10):e01062. doi: 10.1002/brb3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermans MAW, Schrijver B, van Holten-Neelen CCPA, et al. The JAK1/JAK2- inhibitor ruxolitinib inhibits mast cell degranulation and cytokine release. Clin Exp Allergy. 2018;48(11):1412–1420. doi: 10.1111/cea.13217. [DOI] [PubMed] [Google Scholar]
- 33.Salamon P, Shefler I, Hershko AY, Mekori YA. The involvement of protein kinase D in T cell-induced mast cell activation. Int Arch Allergy Immunol. 2016;171(3-4):203–208. doi: 10.1159/000452625. [DOI] [PubMed] [Google Scholar]
- 34.Earley CJ, Connor J, Garcia-Borreguero D, et al. Altered brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis-Ekbom Disease) Sleep Med. 2014;15(11):1288–1301. doi: 10.1016/j.sleep.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Ribatti D. The crucial role of mast cells in blood-brain barrier alterations. Exp Cell Res. 2015;338(1):119–125. doi: 10.1016/j.yexcr.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Marques F, Falcao AM, Sousa JC. Altered iron metabolism is part of the choroid plexus response to peripheral inflammation. Endocrinology. 2009;150(6):2822–2828. doi: 10.1210/en.2008-1610. [DOI] [PubMed] [Google Scholar]
- 37.Cikrikcioglu MA, Hursitoglu M, Erkal H, et al. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur J Clin Invest. 2011;41(7):734–742. doi: 10.1111/j.1365-2362.2010.02461.x. [DOI] [PubMed] [Google Scholar]
- 38.Shneyder N, Adler CH, Hentz JG, et al. Autonomic complaints in patients with restless legs syndrome. Sleep Med. 2013;14(12):1413–1416. doi: 10.1016/j.sleep.2013.08.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertisch SM, Muresan C, Schoerning L, Winkelman JW, Taylor JW. Impact of restless legs syndrome on cardiovascular autonomic control. Sleep. 2016;39(3):565–571. doi: 10.5665/sleep.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izzi E, Placidi F, Romigi A, et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med. 2014;15(11):1392–1397. doi: 10.1016/j.sleep.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui F, Strus J, Ming X, et al. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118(9):1923–1930. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Shibao C, Arzubiaga C, Roberts LJ, 2nd, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45(3):385–390. doi: 10.1161/01.HYP.0000158259.68614.40. [DOI] [PubMed] [Google Scholar]
- 43.Doherty TA, White AA. Postural orthostatic tachycardia syndrome and the potential role of mast cell activation. Auton Neurosci. 2018;215:83–88. doi: 10.1016/j.autneu.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Baskol G, Korkmaz S, Erdem F, et al. Assessment of nitric oxide, advanced oxidation protein products, malondialdehyde, and thiol levels in patients with restless legs syndrome. Sleep Med. 2012;13(4):414–418. doi: 10.1016/j.sleep.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Patton SM, Ponnuru P, Snyder AM, Podskalny GD, Connor JR. Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur J Neurol. 2011;18(11):1329–1335. doi: 10.1111/j.1468-1331.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Martin E, Jimenez-Jimenez FJ, Alonso-Navarro H, et al. Heme oxygenase-1 and 2 common genetic variants and risk for restless legs syndrome. Medicine (Baltimore) 2015;94(34):e1448. doi: 10.1097/MD.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong HJ, Oh HA, Nam SY. The critical role of mast cell-derived hypoxia-inducible factor-1α in human and mice melanoma growth. Int J Cancer. 2013;132(11):2492–2501. doi: 10.1002/ijc.27937. [DOI] [PubMed] [Google Scholar]
- 48.Osman M, Hansell AL, Simpson CR, Hollowell J, Helms PJ. Gender-specific presentations for asthma, allergic rhinitis and eczema in primary care. Prim Care Respir J. 2007;16(1):28–35. doi: 10.3132/pcrj.2007.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molderings GJ, Kolck UW, Scheurlen C, Brüss M, Homann J, Von Kügelgen I. Multiple novel alterations in Kit tyrosine kinase in patients with gastrointestinally pronounced systemic mast cell activation disorder. Scand J Gastroenterol. 2007;42(9):1045–1053. doi: 10.1080/00365520701245744. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Wang Y, Dong H, et al. Induction of microglial activation by mediators released from mast cells. Cell Physiol Biochem. 2016;38(4):1520–1531. doi: 10.1159/000443093. [DOI] [PubMed] [Google Scholar]
- 51.Christ P, Sowa AS, Froy O, Lorentz A. The circadian clock drives mast cell functions in allergic reactions. Front Immunol. 2018;9:1526. doi: 10.3389/fimmu.2018.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.