Abstract
Background
The prevalence of pediatric obesity is increasing worldwide and strongly associates with metabolic abnormalities, including inflammation, insulin resistance, and dyslipidemia. This study assessed the influence of 3 measures of adiposity on levels of routinely assessed biochemical markers in apparently healthy children and adolescents.
Methods
The influence of adiposity on 35 biochemical markers was examined in the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) cohort of healthy children and adolescents by comparing serum biomarker levels between subjects with a normal weight, overweight, and obese body mass index (BMI). The cohort comprised 1332 subjects 5.1 to 19.0 years of age with a BMI ranging from 13.4 to 65.0 kg/m2. The association between each biochemical marker and BMI, waist circumference, and waist-to-height ratio z-scores was assessed, while adjusting for age and sex. Reference intervals were established for all biochemical markers before and after removing overweight/obese subjects.
Results
In children and adolescents, levels of 13 routinely assessed biochemical markers, including alanine aminotransferase, apolipoprotein B, complement components 3 and 4, cholinesterase, high sensitivity C-reactive protein, gamma-glutamyl transferase, haptoglobin, high-density lipoprotein cholesterol, iron, transferrin, triglycerides, and uric acid, were significantly different between BMI categories. BMI, waist circumference, and/or waist-to-height ratio were significantly associated with the serum concentration of 24 of the 35 markers examined, after adjusting for age and sex.
Conclusions
Excess adiposity significantly influences circulating levels of routinely assessed laboratory markers, most notably liver enzymes, lipids/lipoproteins, inflammatory markers, and uric acid in children and adolescents. Although it is unknown whether altered biochemical marker levels in subjects with overweight/obesity reflect health or indolent disease, clinicians should be aware of the effect of weight status on several laboratory tests.
Keywords: body mass index, waist circumference, waist-to-height ratio, laboratory tests, biochemical markers
Pediatric obesity has become one of the most important public health concerns (1), with the proportion of children with overweight and obesity increasing by 47.1% between 1980 and 2013 worldwide (2). According to the US National Health and Nutrition Examination Survey (NHANES), the prevalence of overweight and obesity in the pediatric population (2-19 years of age) was estimated to be 35.1% in 2015-2016, with the highest prevalence (ie, 41.5%) in adolescents 16 to 19 years of age (3). Furthermore, the estimated prevalence of Canadian children and adolescents with overweight and obesity aged 5 to 17 years is 31.4%, according to the 2012-2013 Canadian Health Measures Survey (4). The changing population of community children and adolescents, with respect to an increasing prevalence of obesity, can pose a potential challenge to public health and laboratory test interpretation. Although excess adiposity is highly associated with the development of multiple comorbidities, including insulin resistance, dyslipidemia, and atherosclerosis, some individuals with obesity are free from metabolic complications and have relatively normal insulin sensitivity, termed metabolically healthy obesity (5). Laboratory tests are often used to assess the risk for and presence of obesity-associated comorbidities. However, the influence of excess adiposity itself on routinely assessed biochemical markers in otherwise healthy individuals can complicate laboratory test result interpretation and has been seldom assessed.
Accurate interpretation of laboratory test results is essential to appropriate clinical decision making and relies on availability of robust population-based normative ranges (ie, reference intervals [RIs]). Laboratory RIs are defined as the central 95% of the test result distribution for a reference (ie, healthy) population and are used clinically to interpret laboratory test results.(6) Although age and sex are commonly considered important covariates when establishing RIs, the effect of excess adiposity on biomarker levels in a community population remains to be comprehensively assessed. To evaluate obesity in the pediatric setting, age- and sex-specific body mass index (BMI) percentiles or z-scores are most commonly used, which strongly associate with increased body fat (7–9). Additional measures of excess adiposity, including waist circumference (WC) and waist-to-height ratio (WHtR), have been reported as being equal or superior to BMI as indicators of adiposity and cardiovascular disease (CVD) risk because they more specifically measure central adiposity and better represent visceral adipose tissue (10–14). Because children and adolescents are constantly growing and developing, the use of anthropometric measurements is more complex than in adults. For example, BMI decreases in later infancy, remains relatively stable from ages 2 to 5 years, and subsequently increases throughout childhood and adolescence (15). Therefore, age- and sex-specific z-scores for BMI, WC, and WHtR are used to assess adiposity in the pediatric population.
Determining how excess adiposity affects biochemical marker concentrations in an otherwise healthy population is essential to appropriately interpret biochemical test results in clinical practice. The present study aimed to determine the influence of adiposity (as measured by BMI, WC, and WHtR z-scores) on routinely assessed biochemical markers in the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) cohort of healthy children and adolescents (16). Serum concentrations of biochemical markers were statistically compared between pediatric subjects with a normal weight, overweight, and obese BMI, defined based on the World Health Organization (WHO) classification of BMI z-scores. We also determined the associations between BMI, WC, and WHtR z-scores and each biochemical marker, after adjusting for age and sex.
Methods
Study population
This study was approved by the Research Ethics Board at The Hospital for Sick Children (Toronto, ON). A total of 1332 community children and adolescents (age range, 5.1-<19.0 years) from the Greater Toronto Area and Hamilton completed a health questionnaire and written informed consent, provided height, weight, and WC measurements, and donated a blood sample. Subjects were not required to fast before blood sample collection. Participants were excluded if they had an underweight BMI (BMI <3rd percentile), a history of chronic illness or metabolic disease, an acute illness within the previous month, or used prescribed medication within the previous 2 weeks. Blood samples were collected in serum separator tubes (BD) and centrifuged, separated, and aliquoted within 4 hours of collection. All serum aliquots were kept frozen at -80°C until testing. Subjects from the CALIPER cohort were chosen to ensure the ethnic composition of the study participants reflected the 2006 Canadian census data for the province of Ontario (17).
Sample analysis
Serum samples were analyzed on the Abbott ARCHITECT c8000 system for 35 routine biochemical markers. These included routine chemistry markers (bilirubin direct, bilirubin total, calcium, carbon dioxide [CO2], creatinine [enzymatic], iron, magnesium, phosphorus, urea, and uric acid), enzymes (alkaline phosphatase [ALP], alanine aminotransferase [ALT; without pyridoxal phosphate], amylase, aspartate aminotransferase [AST; without pyridoxal phosphate], cholinesterase [ChE], gamma-glutamyl transferase [GGT], lactate dehydrogenase [LDH], and lipase), lipids/lipoproteins (apolipoprotein A1 [apoA1], apoB, cholesterol, high-density lipoprotein cholesterol [HDL-C], and triglycerides), and proteins (albumin [bromocresol purple method], antistreptolysin O [ASO], complement component 3 (C3), complement component 4 (C4), high-sensitivity C-reactive protein (hsCRP), haptoglobin, immunoglobulin A (IgA), IgG, IgM, prealbumin, total protein, and transferrin) (Table 1). The samples were analyzed in batches over a 6-month period. All samples underwent automated interference analysis for hemolysis, icterus, and turbidity. Data on analytical parameters, calibration, and traceability information are summarized in our previous publication (18).
Table 1.
Comparison of Biochemical Marker Reference Values Between Adiposity Groups Based on Body Mass Index z-score
| Analyte | Sex | Normal Weight | Overweight | Obese | Overweight/Obese Combined |
|---|---|---|---|---|---|
| Albumin (g/L) | B | 42 (5) | 41 (6) | 41 (5) | 41 (5) |
| F | 42 (5) | 41 (5) | 40 (7) | 41 (5) | |
| M | 42 (5) | 42 (5) | 41 (5) | 42 (5) | |
| ALPa (U/L) | B | 211 ± 106 | 203 ± 112 | 226 ± 101 | 211 ± 108 |
| F | 187 ± 96 | 183 ± 99 | 200 ± 102 | 188 ± 100 | |
| M | 235 ± 111 | 226 ± 121 | 247 ± 96 | 234 ± 112 | |
| ALTa (U/L) | B | 16 ± 6b | 17 ± 6b | 21 ± 10c | 18 ± 8d |
| F | 16 ± 7 | 16 ± 5 | 17 ± 5 | 16 ± 5 | |
| M | 17 ± 6b | 18 ± 6b | 23 ± 13c | 20 ± 9d | |
| Amylase (U/L) | B | 55 (28) | 53 (32) | 49 (28) | 52 (30) |
| F | 56 (26) | 54 (31) | 51 (23) | 54 (30) | |
| M | 54 (30) | 52 (32) | 48 (29) | 51 (29) | |
| ApoA1 (g/L) | B | 1.17 (0.35) | 1.16 (0.34) | 1.11 (0.28) | 1.14 (0.34) |
| F | 1.18 (0.33) | 1.18 (0.34) | 1.06 (0.40) | 1.15 (0.36) | |
| M | 1.13 (0.35) | 1.14 (0.35) | 1.13 (0.21) | 1.14 (0.31) | |
| ApoB (g/L) | B | 0.52 ± 0.15b | 0.56 ± 0.16c | 0.58 ± 0.17c | 0.57 ± 0.17d |
| F | 0.53 ± 0.16 | 0.58 ± 0.17 | 0.56 ± 0.16 | 0.57 ± 0.17d | |
| M | 0.51 ± 0.14b | 0.54 ± 0.16b | 0.60 ± 0.17c | 0.56 ± 0.17d | |
| ASO (IU/mL) | B | 55 (162) | 49 (153) | 49 (149) | 49 (153) |
| F | 50 (174) | 49 (152) | 49 (103) | 49 (134) | |
| M | 58 (148) | 51 (154) | 49 (159) | 49 (156) | |
| AST (U/L) | B | 24 (11) | 24 (11) | 25 (13) | 24 (12) |
| F | 23 (10) | 23 (12) | 23 (12) | 23 (11) | |
| M | 26 (13) | 24 (11) | 25 (17) | 24 (11) | |
| Bilirubin (direct) (μmol/L) | B | 3.6 (3.0) | 3.6 (3.4) | 3.1 (2.8) | 3.4 (3.0) |
| F | 3.4 (2.9) | 3.2 (2.8) | 3.2 (2.9) | 3.2 (2.8) | |
| M | 3.8 (3.3) | 4.0 (3.9) | 3.1 (2.5) | 3.7 (3.8) | |
| Bilirubin (total) (μmol/L) | B | 6.2 (5.4) | 6.3 (5.3) | 5.4 (5.4) | 6.1 (5.3) |
| F | 6.0 (4.7) | 5.9 (4.2) | 5.4 (5.3) | 5.5 (4.6) | |
| M | 6.7 (5.7) | 6.8 (6.6) | 5.5 (5.5) | 6.7 (6.1) | |
| C3 (g/L) | B | 1.10 (0.23)b | 1.23 (0.22)c | 1.39 (0.29)e | 1.28 (0.28)d |
| F | 1.11 (0.24)b | 1.24 (0.23)c | 1.34 (0.26)e | 1.27 (0.24)d | |
| M | 1.09 (0.23)b | 1.20 (0.24)c | 1.41 (0.28)e | 1.29 (0.30)d | |
| C4a (g/L) | B | 0.22 ± 0.07b | 0.24 ± 0.07c | 0.27 ± 0.09e | 0.25 ± 0.08d |
| F | 0.23 ± 0.07b | 0.24 ± 0.07b,c | 0.27 ± 0.09c | 0.25 ± 0.08 | |
| M | 0.22 ± 0.06b | 0.23 ± 0.07b | 0.27 ± 0.09c | 0.25 ± 0.08d | |
| Calcium (mmol/L) | B | 2.45 (0.13) | 2.44 (0.14) | 2.44 (0.11) | 2.44 (0.13) |
| F | 2.44 (0.13) | 2.44 (0.13) | 2.43 (0.13) | 2.43 (0.14) | |
| M | 2.45 (0.13) | 2.45 (0.12) | 2.46 (0.11) | 2.45 (0.12) | |
| ChE (U/L) | B | 10 807 (2425)b | 11 380 (2445)c | 12 828 (3007)e | 11 720 (2760)d |
| F | 10 518 (2491)b | 11 211 (2070)c | 12 376 (2584)c | 11 391 (2451)d | |
| M | 11 027 (2457)b | 11 473 (2879)b | 13 346 (2927)c | 11 871 (2991)d | |
| Cholesterol (mmol/L) | B | 3.75 ± 1.03 | 3.79 ± 1.10 | 3.77 ± 1.11 | 3.79 ± 1.10 |
| F | 3.83 ± 1.01 | 4.03 ± 1.02 | 3.78 ± 0.98 | 3.96 ± 1.01 | |
| M | 3.66 ± 1.05 | 3.55 ± 1.12 | 3.76 ± 1.20 | 3.63 ± 1.15 | |
| CO2 (mmol/L) | B | 22 (3) | 21 (4) | 22 (2) | 22 (4) |
| F | 22 (3) | 21 (3) | 22 (3) | 21 (3) | |
| M | 22 (4) | 22 (5) | 22 (2) | 22 (4) | |
| Creatinine (enzymatic)a (μmol/L) | B | 55 ± 15 | 55 ± 14 | 53 ± 14 | 54 ± 14 |
| F | 51 ± 12 | 50 ± 12 | 52 ± 12 | 51 ± 12 | |
| M | 58 ± 18 | 60 ± 15 | 54 ± 15 | 58 ± 15 | |
| hsCRP (mg/L) | B | 0.3 (0.8)b | 0.6 (1.0)c | 1.6 (3.4)e | 0.8 (1.8)d |
| F | 0.4 (0.9)b | 0.6 (1.0)c | 2.1 (4.2)e | 0.7 (1.3)d | |
| M | 0.3 (0.7)b | 0.6 (1.2)c | 1.5 (2.8)e | 0.9 (2.2)d | |
| GGT (U/L) | B | 13 (5)b | 14 (7)c | 15 (10)c | 14 (8)d |
| F | 12 (6) | 13 (5) | 14 (5) | 13 (5) | |
| M | 14 (6)b | 16 (9)c | 17 (11)c | 16 (11)d | |
| Haptoglobin (g/L) | B | 0.68 ± 0.47b | 0.79 ± 0.46c | 1.04 ± 0.57e | 0.88 ± 0.51d |
| F | 0.72 ± 0.48b | 0.88 ± 0.41c | 1.19 ± 0.57e | 0.97 ± 0.48d | |
| M | 0.63 ± 0.44b | 0.71 ± 0.49b | 0.95 ± 0.55c | 0.80 ± 0.53d | |
| HDL-C (mmol/L) | B | 1.28 ± 0.35b | 1.23 ± 0.32b | 1.10 ± 0.26c | 1.18 ± 0.31d |
| F | 1.30 ± 0.35 | 1.27 ± 0.34 | 1.15 ± 0.24 | 1.23 ± 0.32 | |
| M | 1.26 ± 0.35b | 1.18 ± 0.30b | 1.07 ± 0.27c | 1.14 ± 0.29d | |
| Iron (μmol/L) | B | 15.1 ± 6.1b | 14.9 ± 6.7b | 12.5 ± 6.1c | 14.1 ± 6.6 |
| F | 14.6 ± 5.8b | 14.4 ± 6.8b | 11.4 ± 5.6c | 13.5 ± 6.6 | |
| M | 15.6 ± 6.3 | 15.6 ± 6.5 | 13.4 ± 6.4 | 14.7 ± 6.5 | |
| IgA (g/L) | B | 1.23 (0.79) | 1.23 (0.79) | 1.35 (0.75) | 1.25 (0.79) |
| F | 1.18 (0.79) | 1.23 (0.60) | 1.43 (0.69) | 1.28 (0.67) | |
| M | 1.28 (0.78) | 1.23 (0.91) | 1.28 (0.87) | 1.24 (0.90) | |
| IgG (g/L) | B | 10.4 ± 2.5 | 10.5 ± 2.6 | 10.3 ± 2.5 | 10.5 ± 2.5 |
| F | 10.7 ± 2.4 | 10.7 ± 2.5 | 10.9 ± 2.5 | 10.7 ± 2.5 | |
| M | 10.2 ± 2.5 | 10.4 ± 2.6 | 9.9 ± 2.4 | 10.2 ± 2.5 | |
| IgM (g/L) | B | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.4 |
| F | 1.2 ± 0.5 | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.1 ± 0.4 | |
| M | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.9 ± 0.4 | 0.9 ± 0.4 | |
| LDH (U/L) | B | 226 (89) | 226 (81) | 244 (82) | 234 (84) |
| F | 218 (92) | 216 (83) | 218 (85) | 216 (84) | |
| M | 232 (79) | 236 (78) | 248 (83) | 241 (77) | |
| Lipasea (U/L) | B | 22 ± 12 | 22 ± 10 | 20 ± 9 | 21 ± 10 |
| F | 23 ± 12 | 23 ± 10 | 23 ± 9 | 23 ± 10 | |
| M | 21 ± 12 | 20 ± 10 | 18 ± 7 | 19 ± 9 | |
| Magnesium (mmol/L) | B | 1.01 (0.12) | 1.00 (0.11) | 0.99 (0.11) | 1.00 (0.11) |
| F | 1.01 (0.10) | 1.01 (0.12) | 0.97 (0.11) | 0.99 (0.12) | |
| M | 1.01 (0.12) | 0.99 (0.11) | 1.02 (0.11) | 1.00 (0.12) | |
| Phosphorus (mmol/L) | B | 1.57 (0.43) | 1.56 (0.48) | 1.59 (0.45) | 1.56 (0.47) |
| F | 1.52 (0.41) | 1.56 (0.46) | 1.52 (0.36) | 1.54 (0.43) | |
| M | 1.62 (0.44) | 1.56 (0.45) | 1.66 (0.42) | 1.59 (0.44) | |
| Prealbumin (g/L) | B | 0.21 (0.06) | 0.22 (0.06) | 0.21 (0.07) | 0.22 (0.07) |
| F | 0.21 (0.06) | 0.22 (0.06) | 0.21 (0.07) | 0.22 (0.07) | |
| M | 0.22 (0.08) | 0.22 (0.07) | 0.21 (0.07) | 0.22 (0.07) | |
| Total protein (g/L) | B | 72 (6) | 72 (6) | 72 (5) | 72 (5) |
| F | 72 (6) | 73 (6) | 73 (6) | 73 (6) | |
| M | 72 (6) | 72 (6) | 72 (5) | 72 (5) | |
| Transferrin (g/L) | B | 2.66 (0.43)b | 2.76 (0.49)c | 2.75 (0.51)c | 2.76 (0.50)d |
| F | 2.66 (0.48)b | 2.79 (0.52)c | 2.71 (0.51)b,c | 2.77 (0.48)d | |
| M | 2.66 (0.41) | 2.75 (0.45) | 2.76 (0.49) | 2.76 (0.46)d | |
| Triglyceridesa (mmol/L) | B | 1.23 ± 0.60b | 1.42 ± 0.77c | 1.67 ± 0.77e | 1.51 ± 0.80d |
| F | 1.23 ± 0.54b | 1.45 ± 0.86c | 1.56 ± 0.69c | 1.48 ± 0.81d | |
| M | 1.23 ± 0.66b | 1.39 ± 0.68c | 1.74 ± 0.82e | 1.53 ± 0.75d | |
| Urea (mmol/L) | B | 4.7 (1.9) | 4.7 (1.7) | 4.9 (1.5) | 4.7 (1.6) |
| F | 4.4 (1.8) | 4.4 (1.6) | 4.4 (1.5) | 4.4 (1.6) | |
| M | 5.1 (1.9) | 4.9 (1.8) | 5.1 (1.4) | 5.0 (1.7) | |
| Uric acid (μmol/L) | B | 237 (128)b | 261 (121)c | 270 (127)c | 266 (122)d |
| F | 219 (101)b | 248 (76)c | 263 (94)c | 251 (86)d | |
| M | 269 (151) | 309 (141) | 279 (135) | 297 (141)d |
Analytes indicated as mean ± SD were compared between NW, OW, and OB using ANOVA and Tukey post hoc test and were compared between NW and OW/OB using independent samples t test. Analytes indicated as median (interquartile ratio) were compared between NW, OW, and OB using Kruskall-Wallis and Dunn’s post hoc tests and were compared between NW and OW/OB using Mann-Whitney U test. The Benjamini-Hochberg procedure was applied to control for false discovery rate.
B, both sexes; F, female; M, male; NW, normal weight; OB, obese; OW, overweight.
a These variables were log-transformed prior to analysis, although raw values are reported.
b,c,e Values with different letters are significantly different.
d Indicates OW/OB significantly different than NW.
Statistical analysis
Calculating z-scores.
Statistical analysis was performed using R version 3.5.1. BMI was calculated as weight (in kg)/(height [in m])2 and WHtR was calculated as WC (in cm)/height (in cm). Before analysis, BMI, WC, and WHtR were converted to age- and sex-specific z-scores. BMI z-scores were calculated using age- and sex-specific least mean square values provided by the WHO growth reference standards for 5 to 19 year olds (19). WC and WHtR z-scores were determined using age- and sex-specific least mean square values developed based on data from the US NHANES III (20). For each physical parameter, y (ie, BMI, WC, or WHtR), the z-score was calculated as z = ((y/M)L-1)/(LS), where L (lambda) is the power of Box-Cox transformation for correcting the skewness, M (mu) is the median, and S (sigma) is the coefficient of variation.
Analyzing biochemical marker concentration differences between BMI categories
Subjects were grouped into 3 BMI categories: normal weight (NW; 3rd percentile < BMI < 85th percentile), overweight (OW; 85th percentile ≤ BMI ≤ 97th percentile), and obese (OB; BMI > 97th percentile) (19,21). Extreme outliers were identified and subsequently removed from each BMI category using the Dixon test (22). Briefly, the ratio D/R is calculated, where D is the absolute difference between an extreme observation and the next largest (or smallest) observation and R in the range of observations that includes extremes. Extreme observations resulting in a ratio ≥1:3, as suggested by Reed et al. (23), were removed. Assumptions of parametric tests include normality of residuals, assessed with Q-Q plots, Anderson-Darling and Shapiro-Wilk tests, and homogeneity of variances, assessed with Bartlett’s and Levene’s tests. Variables that violated assumptions for parametric tests were either log transformed before using parametric tests or analyzed using nonparametric methods if assumptions were not satisfied even after transformation.
Levels of biochemical markers were compared among all 3 BMI categories (ie, all subjects, males, and females). For biochemical markers that satisfied the parametric assumptions, one-way ANOVA followed by Tukey’s honestly significant difference post hoc test was used. For biochemical markers that violated assumptions of parametric tests even after log transformation, Kruskal-Wallis test followed by Dunn’s post hoc test were used. Additionally, levels of biochemical markers were compared between NW subjects and a combined group consisting of both OW and OB subjects. Independent-samples t-test or Mann-Whitney U test were performed for parametric or nonparametric data, respectively. The Benjamini-Hochberg procedure (24) was applied to control for false discovery rate because of multiple comparisons at an alpha level of 0.05.
Multiple regression analysis
Separate multiple regression analyses were performed with specified predictor variables (ie, BMI, WC, and WHtR z-scores), response variables (ie, concentration of each biochemical marker), and covariates (ie, age and sex). Separate analyses were performed for each sex if sex was significantly associated with biochemical marker concentration. Variables that violated assumptions of multiple regression (ie, normality and homoscedasticity of residuals) were either transformed using the natural logarithm before using parametric analyses or tested using robust linear regression if parametric assumptions were not met even after transformation. Robust regression requires less restrictive assumptions and thus provides an alternative to least squares regression. The Benjamini-Hochberg procedure (24) was applied to control for false discovery rate because of multiple comparisons at an alpha level of 0.05.
Reference interval establishment
Reference intervals were established for all analytes before and after removing OW or OB subjects. The statistical procedure used for reference interval establishment, including determining age- and sex-specific partitions, has been described in detail elsewhere (18). Briefly, outliers were removed using the Tukey (25) or adjusted Tukey (26) test twice for each age/sex partition for normally distributed and skewed data, respectively. Reference intervals were established using the nonparametric rank method for partitions with ≥120 participants and the robust method of Horn and Pesce (27) for partitions with between 40 and 120 participants. Lastly, 90% confidence intervals around each reference limit were calculated.
Results
The difference in concentration of 35 biochemical markers between NW (n = 910), OW (n = 286), and OB (n = 136) categories, as well as the association among BMI, WC, and WHtR z-scores with each biochemical marker were assessed in children and adolescents aged 5.1 to < 19.0 years. Age and sex did not significantly differ among the NW, OW, OB, and OW/OB groups (Table S1 and Fig. S1) (28). The BMI and BMI z-score across the entire cohort ranged from 13.4 to 65.0 kg/m2 and -1.97 to 7.42, respectively. The BMI and BMI z-score distributions in each category are shown in Figs S2-S3 (28). The biochemical marker concentrations for each BMI category in the combined sex, female, and male categories are reported in Table 1 and depicted in Fig. 1–4 and Figs S4–S34 (28). Overall, 13 of the 35 biochemical markers were significantly different among NW, OW, and/or OB groups (ie, ALT, apoB, C3, C4, ChE, hsCRP, GGT, haptoglobin, HDL-C, iron, transferrin, triglycerides, and uric acid).
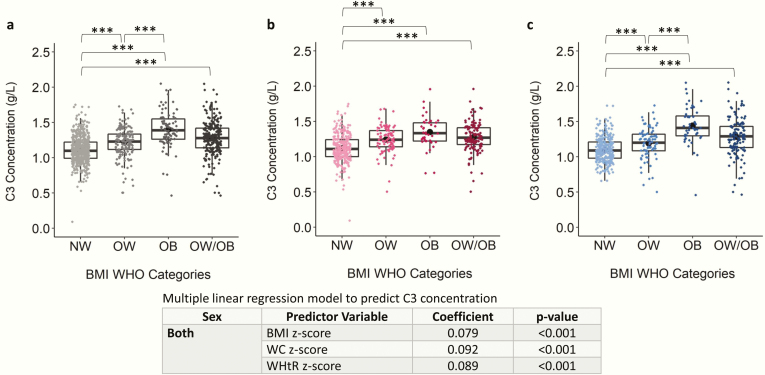
Figure 1.
Scatter boxplot of complement component 3 (C3) concentration in normal weight (NW), overweight (OW), obese (OB), and overweight and obese combined (OW/OB) groups. Plots depict data from children and adolescents aged 5 to <19 years for (A) both sexes, (B) females only, and (C) males only. C3 concentration was compared between NW, OW, and OB using Kruskal-Wallis and Dunn’s post hoc tests and were compared between NW and OW/OB using the Mann-Whitney U test. Coefficient and P values of robust multiple linear regression models to predict C3 concentration are shown. All models are adjusted by age and sex. The Benjamini-Hochberg procedure was applied to control for false discovery rate because of multiple comparisons at an alpha level of .05. *P < .05; **P < .01; ***P < .001. Abbreviations: BMI, body mass index; WHO, World Health Organization.
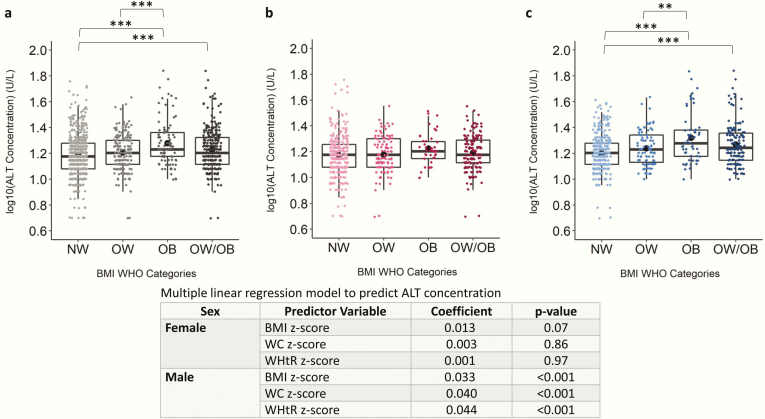
Figure 4.
Scatter boxplot of log(alanine aminotransferase [ALT]) concentration in normal weight (NW), overweight (OW), obese (OB), and overweight and obese combined (OW/OB) groups. Plots depict data from children and adolescents aged 5 to <19 years for (A) both sexes, (B) females only, and (C) males only. Log(ALT) concentration was compared between NW, OW, and OB using ANOVA and Tukey post hoc test and were compared between NW and OW/OB using independent samples t test. Coefficient and P values of multiple linear regression models are shown for both sexes because sex significantly predicted ALT concentration when added to the model. All models are adjusted by age. The Benjamini-Hochberg procedure was applied to control for false discovery rate because of multiple comparisons at an alpha level of .05. *P < .05; **P < .01; ***P < .001. Abbreviations: BMI, body mass index; WHO, World Health Organization.
Results of regression analysis are reported in Table 2. Of the 35 biochemical markers, 24 were significantly associated with BMI, WC, and/or WHtR z-scores after adjusting for age and sex. A total of 20 biochemical markers were significantly associated with BMI z-score (ie, iron, uric acid, ALP, ALT, AST, apoA1, apoB, ChE, GGT, LDH, albumin, HDL-C, triglycerides, C3, C4, hsCRP, haptoglobin, prealbumin, total protein, and transferrin), 16 were significantly associated with WC z-score (ie, iron, phosphorus, uric acid, ALT, amylase, AST, apoB, ChE, GGT, triglycerides, C3, C4, hsCRP, haptoglobin, total protein, and transferrin), and 18 were significantly associated with WHtR z-score (ie, bilirubin direct, bilirubin total, iron, phosphorus, uric acid, ALT, ChE, GGT, LDH, apoB, HDL-C, triglycerides, C3, C4, hsCRP, haptoglobin, total protein, and transferrin). Of the 24 biochemical markers significantly associated with BMI, WC, and/or WHtR z-scores, 16 were analyzed separately for each sex (ie, bilirubin direct, bilirubin total, iron, phosphorus, uric acid, ALP, ALT, AST, ChE, GGT, LDH, apoA1, HDL-C, C4, haptoglobin, and prealbumin) because sex was significantly associated with the concentrations of these biochemical markers.
Table 2.
Multiple Regression Models Predicting Analyte Concentration
| Analyte | Sex | Predictor Variable | Coefficient | Confidence Interval | P Value |
|---|---|---|---|---|---|
| Albumina (g/L) | B | BMI z-score | –0.276 | (–0.492 to –0.060) | .04 |
| WC z-score | –0.199 | (–0.526 to 0.128) | .37 | ||
| WHtR z-score | –0.343 | (–0.651 to –0.035) | .06 | ||
| ALPa (U/L) | F | BMI z-score | –6.93 | (–12.5 to –1.39) | .04 |
| WC z-score | 0.470 | (–9.17 to 10.1) | .97 | ||
| WHtR z-score | –8.20 | (–17.3 to 0.868) | .14 | ||
| M | BMI z-score | –0.835 | (–8.45 to 6.78) | .91 | |
| WC z-score | –1.99 | (–14.4 to 10.4) | .86 | ||
| WHtR z-score | –3.30 | (–16.7 to 10.1) | .75 | ||
| ALTb (U/L) | F | BMI z-score | 0.013 | (–0.502 to 0.528) | .07 |
| WC z-score | 0.003 | (–0.017 to 0.023) | .86 | ||
| WHtR z-score | 0.001 | (–0.017 to 0.019) | .97 | ||
| M | BMI z-score | 0.033 | (0.021 to 0.045) | <.001 | |
| WC z-score | 0.040 | (0.021 to 0.059) | <.001 | ||
| WHtR z-score | 0.044 | (0.026 to 0.062) | <.001 | ||
| Amylasea (U/L) | B | BMI z-score | –1.69 | (–3.19 to –0.185) | .06 |
| WC z-score | –2.64 | (–4.76 to –0.523) | .04 | ||
| WHtR z-score | –1.16 | (–3.15 to 0.819) | .39 | ||
| Apo A1 (g/L) | F | BMI z-score | –0.038 | (–0.060 to –0.016) | .003 |
| WC z-score | –0.033 | (–0.066 to 0.000) | .10 | ||
| WHtR z-score | –0.025 | (–0.056 to 0.006) | .21 | ||
| M | BMI z-score | –0.009 | (–0.031 to 0.013) | 0.53 | |
| WC z-score | –0.026 | (–0.059 to 0.007) | 0.21 | ||
| WHtR z-score | –0.023 | (–0.054 to 0.008) | 0.25 | ||
| Apo B (g/L) | B | BMI z-score | 0.013 | (0.003 to 0.023) | .01 |
| WC z-score | 0.024 | (0.010 to 0.038) | .003 | ||
| WHtR z-score | 0.028 | (0.012 to 0.044) | <.001 | ||
| ASOa (IU/L) | B | BMI z-score | 0.114 | (–5.70 to 5.93) | .98 |
| WC z-score | 3.81 | (–4.79 to 12.4) | .54 | ||
| WHtR z-score | 4.05 | (–3.78 to 11.9) | .46 | ||
| ASTa (U/L) | F | BMI z-score | –0.489 | (–0.879 to –0.099) | 0.04 |
| WC z-score | –0.648 | (–1.22 to –0.074) | 0.06 | ||
| WHtR z-score | –0.496 | (–1.03 to 0.041) | 0.13 | ||
| M | BMI z-score | –0.532 | (–1.006 to –0.058) | .06 | |
| WC z-score | –1.35 | (–2.04 to –0.672) | <.001 | ||
| WHtR z-score | –0.760 | (–1.51 to –0.015) | .09 | ||
| Bilirubin (direct)a (μmol/L) | F | BMI z-score | –0.057 | (–0.204 to 0.090) | .60 |
| WC z-score | –0.061 | (–0.290 to 0.168) | .73 | ||
| WHtR z-score | –0.166 | (–0.372 to 0.040) | .20 | ||
| M | BMI z-score | –0.151 | (–0.284 to –0.018) | .06 | |
| WC z-score | –0.101 | (–0.326 to 0.124) | .53 | ||
| WHtR z-score | –0.253 | (–0.457 to –0.049) | .04 | ||
| Bilirubin (total)a (μmol/L) | F | BMI z-score | –0.105 | (–0.375 to 0.165) | .60 |
| WC z-score | –0.035 | (–0.484 to 0.414) | .95 | ||
| WHtR z-score | –0.165 | (–0.557 to 0.227) | .56 | ||
| M | BMI z-score | –0.161 | (–0.392 to 0.070) | .28 | |
| WC z-score | –0.186 | (–0.553 to 0.181) | .47 | ||
| WHtR z-score | –0.436 | (–0.785 to –0.087) | .04 | ||
| C3a (g/L) | B | BMI z-score | 0.079 | (0.065 to 0.093) | <.001 |
| WC z-score | 0.092 | (0.072 to 0.112) | <.001 | ||
| WHtR z-score | 0.089 | (0.069 to 0.109) | <.001 | ||
| C4b (g/L) | F | BMI z-score | 0.004 | (0.002 to 0.006) | .006 |
| WC z-score | 0.004 | (0.000 to 0.008) | .06 | ||
| WHtR z-score | 0.005 | (0.001 to 0.009) | .004 | ||
| M | BMI z-score | 0.004 | (0.002 to 0.006) | <.001 | |
| WC z-score | 0.006 | (0.002 to 0.010) | .002 | ||
| WHtR z-score | 0.006 | (0.002 to 0.010) | <.001 | ||
| Calciuma (mmol/L) | B | BMI z-score | 0.002 | (–0.004 to 0.008) | .65 |
| WC z-score | 0.005 | (–0.003 to 0.013) | .31 | ||
| WHtR z-score | 0.005 | (–0.003 to 0.013) | .36 | ||
| ChEa (U/L) | F | BMI z-score | 365 | (201 to 530) | <.001 |
| WC z-score | 481 | (232 to 730) | <.001 | ||
| WHtR z-score | 516 | (291 to 741) | <.001 | ||
| M | BMI z-score | 488 | (308 to 667) | <.001 | |
| WC z-score | 687 | (385 to 988) | <.001 | ||
| WHtR z-score | 740 | (460 to 1020) | <.001 | ||
| Cholesterol (mmol/L) | F | BMI z-score | 0.003 | (–0.081 to 0.087) | .97 |
| WC z-score | 0.070 | (–0.053 to 0.193) | .41 | ||
| WHtR z-score | 0.110 | (–0.006 to 0.226) | .12 | ||
| M | BMI z-score | –0.001 | (–0.081 to 0.079) | .98 | |
| WC z-score | 0.009 | (–0.122 to 0.140) | .96 | ||
| WHtR z-score | 0.040 | (–0.085 to 0.165) | .68 | ||
| CO2a (mmol/L) | B | BMI z-score | 0.011 | (–0.169 to 0.191) | .96 |
| WC z-score | 0.067 | (–0.202 to 0.336) | .75 | ||
| WHtR z-score | 0.016 | (–0.229 to 0.261) | .96 | ||
| Creatinine (enzymatic)b (μmol/L) | F | BMI z-score | 0.007 | (–0.736 to 0.750) | .07 |
| WC z-score | 0.008 | (–0.002 to 0.018) | .18 | ||
| WHtR z-score | 0.007 | (–0.001 to 0.015) | .17 | ||
| M | BMI z-score | –0.001 | (–0.007 to 0.005) | .79 | |
| WC z-score | –0.125 | (–0.135 to –0.115) | .80 | ||
| WHtR z-score | 0.001 | (–0.009 to 0.011) | .96 | ||
| hsCRPb (mg/L) | B | BMI z-score | 0.150 | (0.119 to 0.181) | <.001 |
| WC z-score | 0.205 | (0.156 to 0.254) | <.001 | ||
| WHtR z-score | 0.203 | (0.156 to 0.250) | <.001 | ||
| GGTa (U/L) | F | BMI z-score | 0.546 | (0.279 to 0.813) | <.001 |
| WC z-score | 0.419 | (0.049 to 0.789) | .06 | ||
| WHtR z-score | 0.424 | (0.067 to 0.781) | .05 c | ||
| M | BMI z-score | 1.04 | (0.675 to 1.40) | <.001 | |
| WC z-score | 1.15 | (0.612 to 1.69) | <.001 | ||
| WHtR z-score | 1.05 | (0.549 to 1.55) | <.001 | ||
| Haptoglobin (g/L) | F | BMI z-score | 0.108 | (0.065 to 0.151) | <.001 |
| WC z-score | 0.141 | (0.080 to 0.202) | <.001 | ||
| WHtR z-score | 0.148 | (0.091 to 0.205) | <.001 | ||
| M | BMI z-score | 0.066 | (0.029 to 0.103) | .002 | |
| WC z-score | 0.108 | (0.045 to 0.171) | .003 | ||
| WHtR z-score | 0.088 | (0.029 to 0.147) | .01 | ||
| HDL-C (mmol/L) | F | BMI z-score | –0.042 | (–0.071 to –0.013) | .02 |
| WC z-score | –0.047 | (–0.090 to –0.004) | .07 | ||
| WHtR z-score | –0.019 | (–0.058 to 0.020) | .51 | ||
| M | BMI z-score | –0.045 | (–0.070 to –0.020) | .002 | |
| WC z-score | –0.047 | (–0.088 to –0.006) | .06 | ||
| WHtR z-score | –0.048 | (–0.087 to –0.009) | .04 | ||
| Irona (μmol/L) | F | BMI z-score | –0.870 | (–1.41 to –0.329) | .006 |
| WC z-score | –1.16 | (–1.99 to –0.324) | .02 | ||
| WHtR z-score | –1.69 | (–2.42 to –0.959) | <.001 | ||
| M | BMI z-score | –0.768 | (–1.31 to –0.229) | .02 | |
| WC z-score | –1.08 | (–1.97 to –0.192) | .04 | ||
| WHtR z-score | –1.25 | (–2.12 to –0.382) | .02 | ||
| IgAa (g/L) | B | BMI z-score | 0.007 | (–0.026 to 0.040) | .80 |
| WC z-score | –0.015 | (–0.072 to 0.042) | .74 | ||
| WHtR z-score | –0.019 | (–0.070 to 0.032) | .62 | ||
| IgG (g/L) | F | BMI z-score | 0.193 | (–0.013 to 0.399) | .12 |
| WC z-score | 0.177 | (–0.123 to 0.477) | .39 | ||
| WHtR z-score | 0.156 | (–0.126 to 0.438) | .42 | ||
| M | BMI z-score | –0.007 | (–0.203 to 0.189) | .97 | |
| WC z-score | 0.104 | (–0.223 to 0.431) | .68 | ||
| WHtR z-score | –0.025 | (–0.333 to 0.283) | .95 | ||
| IgM (g/L) | F | BMI z-score | –0.013 | (–0.052 to 0.026) | .68 |
| WC z-score | –0.015 | (–0.074 to 0.044) | .74 | ||
| WHtR z-score | –0.020 | (–0.075 to 0.035) | .63 | ||
| M | BMI z-score | 0.001 | (–0.030 to 0.032) | .98 | |
| WC z-score | 0.001 | (–0.050 to 0.052) | .98 | ||
| WHtR z-score | 0.024 | (–0.025 to 0.073) | .49 | ||
| LDHa (U/L) | F | BMI z-score | 2.54 | (–0.417 to 5.49) | .17 |
| WC z-score | 1.37 | (–3.67 to 6.42) | .73 | ||
| WHtR z-score | 4.99 | (0.359 to 9.62) | .07 | ||
| M | BMI z-score | 3.96 | (0.652 to 7.26) | .05 c | |
| WC z-score | 2.51 | (–2.96 to 7.98) | .53 | ||
| WHtR z-score | 6.37 | (1.65 to 11.1) | .02 | ||
| Lipaseb (U/L) | F | BMI z-score | 0.003 | (–1.03 to 1.04) | .87 |
| WC z-score | 0.001 | (–0.026 to 0.028) | .97 | ||
| WHtR z-score | 0.005 | (–0.020 to 0.030) | .80 | ||
| M | BMI z-score | –0.008 | (–0.026 to 0.010) | .56 | |
| WC z-score | –0.966 | (–0.997 to –0.935) | .11 | ||
| WHtR z-score | –0.016 | (–0.045 to 0.013) | .42 | ||
| Magnesiuma (mmol/L) | B | BMI z-score | –0.003 | (–0.009 to 0.003) | .30 |
| WC z-score | –0.004 | (–0.012 to 0.004) | .56 | ||
| WHtR z-score | –0.001 | (–0.009 to 0.007) | .89 | ||
| Phosphorusa (mmol/L) | F | BMI z-score | –0.025 | (–0.047 to –0.003) | .05 |
| WC z-score | –0.043 | (–0.076 to –0.010) | .03 | ||
| WHtR z-score | –0.041 | (–0.070 to –0.012) | .02 | ||
| M | BMI z-score | –0.013 | (–0.037 to 0.011) | .44 | |
| WC z-score | –0.041 | (–0.082 to 0.000) | .11 | ||
| WHtR z-score | –0.032 | (–0.069 to 0.005) | .18 | ||
| Prealbumina (g/L) | F | BMI z-score | 0.005 | (0.001 to 0.009) | .01 |
| WC z-score | 0.005 | (–0.001 to 0.011) | .13 | ||
| WHtR z-score | 0.005 | (0.001 to 0.009) | .08 | ||
| M | BMI z-score | 0.003 | (–0.001 to 0.007) | .12 | |
| WC z-score | 0.002 | (–0.004 to 0.008) | .69 | ||
| WHtR z-score | 0.002 | (–0.004 to 0.008) | .68 | ||
| Total proteina (g/L) | B | BMI z-score | 0.393 | (0.119 to 0.667) | .02 |
| WC z-score | 0.712 | (0.306 to 1.12) | .003 | ||
| WHtR z-score | 0.592 | (0.186 to 0.998) | .01 | ||
| Transferrina (g/L) | B | BMI z-score | 0.061 | (0.037 to 0.085) | <.001 |
| WC z-score | 0.074 | (0.037 to 0.111) | <.001 | ||
| WHtR z-score | 0.081 | (0.048 to 0.114) | <.001 | ||
| Triglyceridesb (mmol/L) | B | BMI z-score | 0.035 | (0.023 to 0.047) | <.001 |
| WC z-score | 0.048 | (0.030 to 0.066) | <.001 | ||
| WHtR z-score | 0.050 | (0.032 to 0.068) | <.001 | ||
| Ureaa (mmol/L) | F | BMI z-score | 0.027 | (–0.073 to 0.127) | .73 |
| WC z-score | 0.025 | (–0.138 to 0.188) | .86 | ||
| WHtR z-score | 0.102 | (–0.041 to 0.245) | .27 | ||
| M | BMI z-score | 0.015 | (–0.093 to 0.123) | .87 | |
| WC z-score | –0.052 | (–0.240 to 0.136) | .73 | ||
| WHtR z-score | –0.034 | (–0.195 to 0.127) | .79 | ||
| Uric acida (μmol/L) | F | BMI z-score | 14.1 | (9.30 to 18.9) | <.001 |
| WC z-score | 14.9 | (7.43 to 22.3) | <.001 | ||
| WHtR z-score | 14.7 | (8.05 to 21.4) | <.001 | ||
| M | BMI z-score | 10.3 | (4.20 to 16.4) | .004 | |
| WC z-score | 16.1 | (6.14 to 26.1) | .006 | ||
| WHtR z-score | 11.6 | (2.33 to 20.9) | .04 |
Coefficients and P values are reported for multiple linear regression models with each analyte as the dependent variable and BMI z-score, WC z-score, and WHtR z-score as the independent variables in separate regression models. All models were adjusted for age and sex or were performed separately for sex if sex significantly predicted analyte concentration when added to the multiple regression model. The Benjamini-Hochberg procedure was applied to control for false discovery rate. Bolded P values indicate those that are statistically significant (ie, P < .05).
Abbreviations: B, both sexes; BMI, body mass index; F, female; M, male; WC, waist circumference; WHtR, waist-to-height ratio.
a Robust multiple linear regression model was applied.
b Dependent variable was transformed using the natural logarithm (ln) before applying multiple linear regression.
c Value was <.05 before rounding value to 2 decimal places.
Reference intervals before and after removing subjects with overweight/obesity are shown in Table 3 for analytes that significantly differed between BMI groups, and in Table S2 (28) for analytes that did not significantly differ between BMI groups.
Table 3.
Age- and Sex-Specific Pediatric Reference Intervals for 13 Biochemical Markers Before and After Removing Subjects with Overweight/Obesity
| All Subjects | NW Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Units | Age | Lower Limit | Upper Limit | n | Lower 90% Confidence Interval | Upper 90% Confidence Interval | Lower Limit | Upper Limit | n | Lower 90% Confidence Interval | Upper 90% Confidence Interval |
| ALT | U/L | 5-<13 y | 10 | 27 | 428 | (9 to 10) | (26 to 30) | 9 | 26 | 285 | (8 to 10) | (25 to 27) |
| 13-<19 y, F | 9 | 29 | 232 | (9 to 9) | (27 to 33) | 9 | 28 | 164 | (9 to 9) | (26 to 33) | ||
| 13-<19 y, M | 10 | 42 | 238 | (9 to 10) | (37 to 51) | 11 | 31 | 137 | (11 to 11) | (29 to 34) | ||
| ApoB | g/L | 5-<19 y | 0.31 | 0.86 | 834 | (0.29 to 0.32) | (0.84 to 0.92) | 0.28 | 0.83 | 567 | (0.27 to 0.31) | (0.79 to 0.86) |
| C3 | g/L | 5-<19 y | 0.87 | 1.63 | 765 | (0.85 to 0.88) | (1.57 to 1.69) | 0.79 | 1.47 | 536 | (0.78 to 0.82) | (1.44 to 1.51) |
| C4 | g/L | 5-<19 y | 0.13 | 0.4 | 818 | (0.12 to 0.13) | (0.38 to 0.41) | 0.12 | 0.38 | 557 | (0.12 to 0.13) | (0.36 to 0.39) |
| ChE | U/L | 5-<16 y | 8554 | 15712 | 536 | (8266 to 8745) | (15 219 to 16 069) | 8915 | 15667 | 339 | (8627 to 9086) | (14 996 to 16 021) |
| 16-<19 y, F | 7717 | 12387 | 86 | (7394 to 8034) | (1 1963 to 12 820) | 7068 | 12740 | 69 | (6499 to 7558) | (12 225 to 13 271) | ||
| 16-<19 y, M | 6763 | 14131 | 100 | (6159 to 7325) | (13 667 to 14 671) | 6891 | 13579 | 72 | (6185 to 7975) | (13 106 to 14 112) | ||
| hsCRP | mg/L | 5-<15 y | 0.1 | 9.6 | 596 | (0.1 to 0.1) | (7.6 to 11.3) | 0.1 | 7.6 | 404 | (0.1 to 0.1) | (5.8 to 10.4) |
| 15-<19 y | 0.1 | 14.0 | 299 | (0.1 to 0.1) | (10.1 to 20.6) | 0.1 | 12.0 | 210 | (0.1 to 0.1) | (7.7 to 17.5) | ||
| GGT | U/L | 5-<11 y | 8 | 17 | 261 | (7 to 9) | (16 to 19) | 9 | 17 | 171 | (9 to 9) | (16 to 21) |
| 11-<19 y | 8 | 175 | 594 | (7 to 9) | (147 to 197) | 8 | 49 | 361 | (8 to 9) | (38 to 54) | ||
| Haptoglobin | g/L | 5-<12 y | 0.07 | 1.53 | 319 | (0.07 to 0.07) | (1.40 to 1.67) | 0.07 | 1.38 | 221 | (0.07 to 0.07) | (1.25 to 1.63) |
| 12-<19 y | 0.07 | 1.71 | 473 | (0.07 to 0.07) | (1.61 to 1.77) | 0.07 | 1.72 | 328 | (0.07 to 0.07) | (1.61 to 1.79) | ||
| HDL-C | mmol/L | 5-<13 y | 0.59 | 1.91 | 397 | (0.52 to 0.71) | (1.87 to 1.99) | 0.52 | 1.93 | 271 | (0.46 to 0.59) | (1.88 to 2.05) |
| 13-<19 y, F | 0.56 | 1.79 | 238 | (0.49 to 0.66) | (1.71 to 1.85) | 0.88 | 1.91 | 159 | (0.85 to 0.94) | (1.85 to 1.99) | ||
| 13-<19 y, M | 0.58 | 1.75 | 235 | (0.51 to 0.66) | (1.63 to 1.84) | 0.64 | 1.76 | 151 | (0.53 to 0.78) | (1.63 to 1.84) | ||
| Iron | μmol/L | 5-<14 y | 5.0 | 25.9 | 382 | (4.2 to 5.5) | (24.0 to 27.3) | 5.5 | 25.9 | 252 | (4.8 to 6.8) | (24.0 to 27.2) |
| 14-<19 y | 5.2 | 32.9 | 297 | (3.8 to 6.0) | (28.8 to 33.5) | 5.2 | 31.0 | 208 | (3.8 to 6.2) | (27.5, 33.8) | ||
| Transferrin | g/L | 5-<19 y | 1.93 | 3.32 | 765 | (1.83 to 2.03) | (3.30 to 3.35) | 1.56 | 3.09 | 526 | (1.42 to 1.72) | (3.06 to 3.10) |
| Triglycerides | mmol/L | 5-<19 y | 0.52 | 3.00 | 873 | (0.49 to 0.55) | (2.84 to 3.35) | 0.50 | 2.81 | 604 | (0.47 to 0.54) | (2.51 to 3.03) |
| Uric acid | μmol/L | 5-<12 y | 125 | 418 | 343 | (119 to 132) | (373 to 473) | 114 | 374 | 236 | (101 to 123) | (333 to 428) |
| 12-<19 y, F | 158 | 511 | 272 | (150 to 162) | (463 to 533) | 144 | 451 | 192 | (121 to 157) | (385 to 488) | ||
| 12-<19 M | 204 | 571 | 267 | (174 to 210) | (524 to 615) | 190 | 536 | 176 | (156 to 210) | (521 to 580) | ||
Abbreviations: ALT, alanine aminotransferase; apoB, apolipoprotein B; C3, complement component 3; C4, complement component 4; ChE, cholinesterase; F, female-specific reference interval; GGT, gamma-glutamyl transferase; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; M, male-specific reference interval; NW, normal weight.
Protein markers
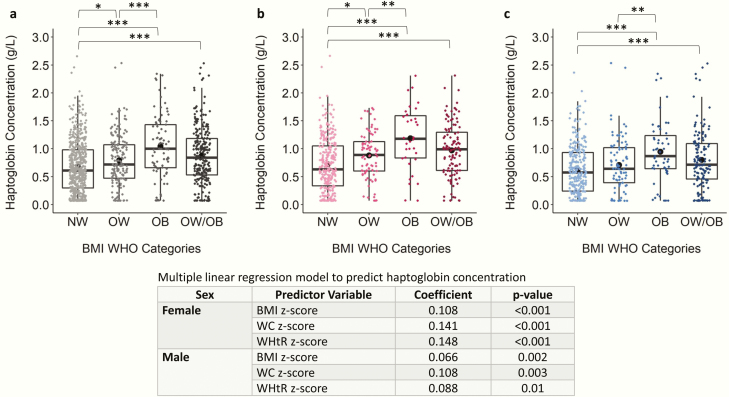
C3, C4, hsCRP, haptoglobin, and transferrin were the protein markers that differed between BMI categories. C3 and C4 were significantly higher in OW, OB, and OW/OB categories compared with NW, with the exception of females, in which C4 levels were only significantly higher in OB compared with NW (Fig. 1A; Fig. S4) (28). BMI, WC, and WHtR z-scores were significantly positively associated with C3 levels, with a 1-unit increase in BMI, WC, and WHtR z-scores associated with an increase in mean C3 levels of 0.079, 0.092, and 0.089 g/L, respectively (Fig. 1). Both hsCRP (Fig. S5) (28) and transferrin (Fig. S6) (28) levels were significantly higher in the OW/OB compared with the NW group. BMI, WC, and WHtR z-scores were positively associated with hsCRP, transferrin, and C4, with the exception that WC z-scores were not significantly associated with C4 levels in females. Haptoglobin was significantly higher in OW, OB, and OW/OB categories compared with NW in the combined sex category (Fig. 2). Accordingly, haptoglobin levels were significantly positively associated with BMI, WC, and WHtR z-scores. In females, a 1-unit increase in BMI, WC, and WHtR z-scores were associated with an increase in mean haptoglobin levels by 0.11, 0.15, and 0.15 g/L, respectively, and by 0.07, 0.11, and 0.09 g/L, respectively, in males (Fig. 2). The remaining proteins, albumin, ASO, IgA, IgG, IgM, prealbumin, and total protein were not significantly different between BMI categories (Figs S7-S13) (28), although albumin, prealbumin, and total protein were significantly associated with BMI, WC, and/or WHtR z-scores.
Figure 2.
Scatter boxplot of haptoglobin concentration in normal weight (NW), overweight (OW), obese (OB), and overweight and obese combined (OW/OB) groups. Plots depict data from children and adolescents aged 5 to <19 years for (A) both sexes, (B) females only, and (C) males only. Haptoglobin concentration was compared between NW, OW, and OB using ANOVA and Tukey post hoc test and were compared between NW and OW/OB using independent samples t test. Coefficient and P values of multiple linear regression models are shown for both sexes because sex significantly predicted haptoglobin concentration when added to the model. All models are adjusted by age. The Benjamini-Hochberg procedure was applied to control for false discovery rate because of multiple comparisons at an alpha level of .05. *P < .05; **P < .01; ***P < .001. Abbreviations: BMI, body mass index; WHO, World Health Organization.
Lipids/lipoproteins
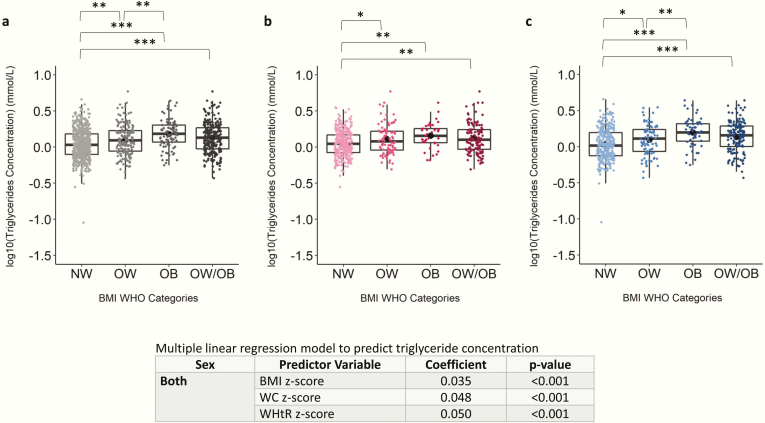
All lipid/lipoprotein markers, except apoA1 and total cholesterol (Fig. S14-S15) (28), differed between BMI categories. HDL-C levels were significantly lower in the OB group compared with OW and NW groups in all subjects and males only (Fig. S16) (28). ApoA1 (females only) and HDL-C levels were negatively associated with BMI z-score, and HDL-C levels were also negatively associated with WHtR z-score in males. ApoB levels were significantly higher in OW, OB, and OW/OB categories compared with NW in all subjects and males only (Fig. S17) (28). ApoB levels significantly increased with increasing BMI, WC, and WHtR z-scores. Similar to apoB, triglyceride levels were significantly higher in OB (1.67 ± 0.77 mmol/L), OW (1.42 ± 0.77 mmol/L), and OW/OB combined (1.51 ± 0.80 mmol/L) groups compared with NW subjects (1.23 ± 0.60 mmol/L) in the combined sex category (Fig. 3A). Levels of triglycerides were significantly positively associated with BMI, WC, and WHtR z-scores (Fig. 3).
Figure 3.
Scatter boxplot of log(triglyceride) concentration in normal weight (NW), overweight (OW), obese (OB), and overweight and obese combined (OW/OB) groups. Plots depict data from children and adolescents aged 5 to <19 years for (A) both sexes, (B) females only, and (C) males only. Log(triglyceride) concentration was compared between NW, OW, and OB using ANOVA and Tukey post hoc test and were compared between NW and OW/OB using independent samples t test. Coefficient and P values of multiple linear regression models to predict triglyceride concentration are shown. All models are adjusted by age and sex. The Benjamini-Hochberg procedure was applied to control for false discovery rate because of multiple comparisons at an alpha level of .05. *P < .05; **P < .01; ***P < .001. Abbreviations: BMI, body mass index; WHO, World Health Organization.
Enzymes
ALT, ChE, and GGT were the only enzymes that differed between BMI categories. GGT was significantly higher in the OW, OB, and combined OW/OB categories compared with NW in the combined sex and male categories (Fig. S19) (28). Similarly, GGT was significantly positively associated with BMI, WC, and WHtR z-scores, with the exception of WC z-score in females. In the combined sex and male-specific categories, ALT levels were significantly higher in the OB (mean ± SD: 21 ± 10 U/L and 23 ± 13 U/L, respectively) and OW/OB groups (18 ± 8 U/L and 20 ± 9 U/L, respectively) compared with NW (16 ± 6 U/L and 17 ± 6 U/L, respectively) (Fig. 4). In males, ALT levels were positively associated with BMI, WC, and WHtR z-scores (Fig. 4). In the combined sex, female, and male categories, ChE was significantly higher in the OW/OB and OB groups compared with the NW group (Fig. S20) (28). ChE levels were significantly associated in BMI, WC, and WHtR z-scores in both males and females. Although the remaining enzymes (ie, ALP, AST, LDH, and lipase) did not significantly differ between BMI categories (Figs S21-S24) (28), ALP was significantly negatively associated with BMI z-score in females, amylase was significantly negatively associated with WC z-score in all subjects, AST was significantly negatively associated with BMI z-score in females and WC z-score in males, and LDH was significantly positively associated with BMI and WHtR z-scores in males.
Other biochemical markers
Iron and uric acid were the only chemistry markers that differed significantly between BMI categories. Iron was significantly higher in the NW and OW groups compared with the OB group in the combined sex category and in females (Fig. S25) (28). Similarly, iron levels were negatively associated with BMI, WC, and WHtR z-scores. Uric acid concentration was significantly higher in the OW (median [interquartile range]): 261 [121] µmol/L), OB (270 [127] µmol/L), and combined OW/OB (266 [122] µmol/L) groups compared with NW (237 [128] µmol/L) in the combined sex category (Fig. S26) (28). These differences were also seen in females; however, males only had a higher uric acid concentration in the OW/OB combined category compared with NW. Accordingly, uric acid concentration was positively associated with BMI, WC, and WHtR z-score. With a 1-unit increase in BMI, WC, or WHtR z-score, mean uric acid concentration increased by 14.1, 14.9, and 14.7 μmol/L, respectively, in females and increased by 10.3, 16.1, and 11.6 μmol/L, respectively, in males. Although the remaining chemistry markers (ie, bilirubin direct, bilirubin total, calcium, CO2, creatinine, magnesium, phosphorus, and urea) did not significantly differ between BMI categories (Figs S27-S34) (28), direct and total bilirubin negatively associated with WHtR z-score in males and phosphorus negatively associated with WC and WHtR z-scores in females.
Discussion
We report the first comprehensive analysis of routinely assessed biochemical marker concentration in a large community population of children and adolescents stratified by BMI (ie, NW, OW, and OB). Furthermore, we report the association between the concentration of each of these biochemical markers with 3 measures of adiposity (ie, BMI, WC, and WHtR z-scores), while adjusting for age and sex. Lastly, reference intervals for each biochemical marker were calculated before and after removing OW and OB subjects to highlight the effect of including OW and OB subjects in a reference population for reference interval establishment. With the rising rates of overweight and obesity in the pediatric population, it is imperative that laboratorians and clinicians understand the potential influence of weight status on levels of biochemical markers when interpreting laboratory test results. In addition to BMI, the most widely used measure of obesity in children and adolescents, we used WC and WHtR z-scores as additional measures of adiposity. Furthermore z-scores, rather than absolute values, were used for all markers of adiposity to ensure the physiological variation of body measures with age and sex in the pediatric population are considered.
Thirty-one percent of biochemical markers examined were not significantly different between BMI groups and were not significantly associated with any measures of adiposity. Thus, weight status does not need to be considered when interpreting laboratory test results for a subset of markers, including ASO, calcium, cholesterol, CO2, creatinine, IgA, IgG, IgM, lipase, magnesium, and urea. An additional 10 biochemical markers (ie, albumin, ALP, apoA1, amylase, AST, bilirubin direct, bilirubin total, LDH, phosphorus, prealbumin, and total protein) did not differ between BMI categories, although they were significantly associated with at least one marker of adiposity. The majority of biochemical markers (69%) were significantly associated with BMI, WC, and/or WHtR z-score after adjusting for age and sex. Interestingly, most biochemical markers that significantly associate with adiposity tend to increase with increasing adiposity, whereas iron shows an inverse relationship. This suggests that even in an apparently healthy reference population, increasing adiposity is linked to altered circulating levels of these biochemical markers.
For routine chemistry markers, the most pronounced changes between BMI categories were evident for uric acid, which significantly increased in a stepwise manner among NW, OW, and OB categories. Uric acid is a metabolic breakdown product of purine catabolism and has recently been shown to be produced and secreted from adipose tissue in mice via the catalysis of purines by xanthine oxidoreductase (29). Xanthine oxidoreductase activity and subsequent uric acid release from adipose tissue is augmented in ob/ob mice (29) and hyperuricemia has been shown to be associated with visceral fat accumulation in humans (30–32). A study of Japanese men showed that serum uric acid levels positively correlate with visceral fat area and negatively correlate with serum adiponectin (31). Furthermore, the change in visceral fat over a 1-year period was significantly positively associated with the change in serum uric acid levels (31). In adults, hyperuricemia is associated with obesity, type 2 diabetes (T2D), as well as kidney and CVD states (reviewed in (33)). Similarly, a study of more than 300 European children and adolescents aged 5 to 18 years showed a significant association between uric acid concentration and the number of abnormal metabolic risk factors (34). A cross-sectional study of more than 1000 Japanese children and adolescents 6 to 14 years of age found that the metabolic syndrome was more prevalent in those with hyperuricemia (30.5%) compared to those with normal uric acid levels (13.6%) (35). Our study suggests that excess adiposity is positively associated with uric acid levels in a healthy population of children and adolescents; thus, a patient’s weight status should be considered when interpreting uric acid results.
Several enzymes were significantly different between BMI categories and were associated with measures of adiposity, particularly ALT and ChE. ALT, commonly measured clinically to help evaluate hepatocellular injury, including the presence of nonalcoholic fatty liver disease (36), was significantly elevated in the OB compared with the OW and NW groups. When sexes were analyzed separately, males had higher ALT levels in OW/OB and OB groups compared with NW and BMI, WC, and WHtR z-scores all significantly positively associated with ALT levels. However, these observations were absent in females. ALT has been shown to be associated with insulin resistance, the metabolic syndrome, and the development of T2D (37–39). In accordance with our study, others have shown sex differences in the association between ALT levels and metabolic complications (40,41). For example, ALT has been shown to predict coronary heart disease in men but not women (40), and a sex difference in the association between liver enzymes and insulin resistance in the adolescent population has been reported (41). Adult men are more insulin resistant than women (42), possibly because of the ability of women to expand subcutaneous fat stores, while remaining highly insulin sensitive (43). Indeed, subcutaneous fat storage capacity is significantly lower in men, driven predominantly by differential sex hormones. Therefore, as males gain weight, excess fat is placed more rapidly into other tissues (eg, liver), paralleled by a rise in ALT levels and dyslipidemia (44). ChE, another enzyme found primarily in the liver, was elevated in the OW, OB, and OW/OB combined groups compared with NW. Previous studies have shown elevated ChE levels in patients with nonalcoholic steatohepatitis and T2D (45) as well as a positive association between ChE and glycated hemoglobin (HbA1c), a sensitive long-term marker of elevated plasma glucose. (46) ChE has also been shown to be elevated in adolescents with the metabolic syndrome compared with controls (47); therefore, the influence of BMI on ALT and ChE must be considered when interpreting test results in children and adolescents.
As expected, the majority of lipids and lipoproteins significantly differed between BMI categories and/or were significantly associated with BMI, WC, and WHtR z-scores, including apoA1, apoB, HDL-C, and triglycerides. Triglycerides and apoB, a marker of atherogenic lipoproteins, were positively associated with increasing adiposity, whereas HDL-C and apoA1 (the main apolipoprotein of HDL particles) were negatively associated with increasing adiposity. Lipid/lipoprotein abnormalities (ie, dyslipidemia), commonly observed in OB and insulin resistant states, are associated with increased risk of developing CVD in adults with T2D (48). Indeed, children with obesity and T2D exhibit a very similar pattern of dyslipidemia as adults (49,50), and lipid abnormalities in childhood have been shown to associate with the initiation and early progression of the atherosclerotic process (51,52). This suggests that lipid abnormalities manifest early in metabolic disease progression.
Serum proteins most notably effected by excess adiposity (ie, BMI, WC, and WHtR) were inflammatory markers, including C3, C4, CRP, and haptoglobin. C3 and C4 have previously been reported to positively correlate with CVD risk factors, such as obesity, blood pressure, blood lipids, and the metabolic syndrome (53). Adipose tissue produces complement components and generates complement activation products in proportion to the amount of adipose tissue, further supporting the idea of the complement system as a trigger of low-grade inflammation associated with obesity (53). Our study suggests that this phenomenon is also evident in apparently healthy children and adolescents with excess adiposity. Adipose tissue is also a known source of CRP (54), a marker used to assess both inflammation and cardiovascular risk. Similar to previous studies in adults (55), CRP was found to significantly increase with excess adiposity. A previous study in adolescents assessed the ability of adiposity measures to identify abnormal levels of inflammatory markers, including C3, C4, and CRP using receiver operating characteristic curves and similarly found positive associations between these markers and obesity (56). Adipose tissue may play a role in regulating CRP levels via IL-6 production (57). In addition to increased concentration of CRP with increasing weight class, the variability in CRP levels also increased. This is likely due to the greater degree of heterogeneity of metabolic status in the OB subclass, which encompasses individuals with metabolically healthy obesity and those with inflammation and progression toward metabolic abnormalities (eg, insulin resistance, dyslipidemia). Taken together, these results suggest that children and adolescents with an increased BMI, but that are otherwise apparently healthy, are in a state of chronic low-grade subclinical inflammation; this can manifest at a very early age. Moreover, levels of haptoglobin, an acute phase reactant, is known to increase during inflammation (58). In the present study, haptoglobin levels were significantly higher in the OB, OW, and OW/OB categories compared with NW. Previous studies have reported similar findings, with haptoglobin positively associated with body fat (59). Although liver is the main source of haptoglobin, studies have shown that it is also secreted into plasma by adipose tissue (60). A study by Hamalainen et al. also showed that haptoglobin levels were significantly higher in subjects with the metabolic syndrome and those with elevated glucose or blood pressure (61), suggesting that haptoglobin may be associated with both excess adiposity and metabolic abnormalities. Similar observations have been made in pediatrics, including from a study in children with obesity (ages 5-15.5 years) that reported elevated haptoglobin levels in those with the metabolic syndrome compared with those without (62).
Our data show that several routinely assessed biochemical markers are significantly affected by weight status, most notably, uric acid, liver markers, lipids/lipoproteins, and inflammatory markers. Altered levels of biochemical markers in adolescents with overweight and obesity may indicate a subclinical disease or condition and therefore, it may be best to exclude these subjects from reference populations when establishing reference intervals. The 2017 American Academy of Pediatrics Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents (63) reported new cutoffs for blood pressure that were established using only normal weight children because of evidence of the strong association between overweight/obesity and elevated blood pressure. Similarly, the WHO growth reference for school-aged children and adolescents was established using a nonobese sample of subjects aged 5 to 19 years (19). Reference intervals for laboratory tests may also be most appropriate if established using a reference population devoid of subjects with overweight/obesity. This was further demonstrated by comparing reference intervals for 35 biochemical tests before and after removing subjects with overweight/obesity from the reference population. Indeed, for biochemical markers found to be significantly affected by excess adiposity, their reference intervals differed when based on all subjects compared with only NW subjects.
All biochemical marker measurements were obtained on nonfasting samples. Eighteen of the biochemical markers analyzed in the present study were previously shown to differ between fasting and nonfasting states in a subset of the CALIPER cohort (64). However, patients are not required to fast before blood collection for the clinical analysis of these biochemical markers, with the exception of lipids. Therefore, the values obtained in the present study are reflective of those obtained in clinical practice. Triglyceride increases and HDL-C decreases postprandially (64–66); however, nonfasting lipid measurements are becoming increasingly common in clinical practice because of the minimal change in lipid parameters following habitual food intake (67) and the potential superior ability of nonfasting triglyceride to predict CVD risk (68).
In summary, the present study highlights the important influence of BMI, WC, and WHtR on circulating pediatric biochemical markers, particularly on liver enzymes, inflammatory markers, lipids, and lipoproteins in pediatrics. Overall, the majority of biomarkers exhibited differences by weight category. Although it is unknown whether altered levels of biochemical markers in subjects with excess adiposity reflect health or indolent disease, it is important that altered analyte concentrations in subjects with overweight and/or obesity are not normalized and that clinicians are aware of the effect of weight status on several laboratory tests.
Acknowledgments
We thank all the CALIPER participants and their families; this study would not have been possible without their participation.
Financial Support: This study was supported by a Canadian Institutes of Health (CIHR) Foundations Scheme Grant and CIHR graduate scholarship.
Glossary
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- apoA1
apolipoprotein A1
- apoB
apolipoprotein B
- ASO
anti-streptolysin-O
- BMI
body mass index
- CALIPER
Canadian Laboratory Initiative for Pediatric Reference Intervals
- Che
cholinesterase
- CO2
carbon dioxide
- C3
complement component 3
- C4
complement component 4
- CVD
cardiovascular disease
- GGT
gamma-glutamyl transferase
- HDL-C
high-density lipoprotein cholesterol
- hsCRP
high-sensitivity C-reactive protein
- LDH
lactate dehydrogenase
- NW
normal weight
- OB
obese
- OW
overweight
- RI
reference interval
- T2D
type 2 diabetes
- WC
waist circumference
- WHO
World Health Organization
- WHtR
waist-to-height ratio
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. [DOI] [PubMed] [Google Scholar]
- 2. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skinner AC, Perrin EM, Skelton JA. Prevalence of obesity and severe obesity in US children, 1999-2014. Obesity (Silver Spring). 2016;24(5):1116–1123. [DOI] [PubMed] [Google Scholar]
- 4. Rao DP, Kropac E, Do MT, Roberts KC, Jayaraman GC. Childhood overweight and obesity trends in Canada. Health Promot Chronic Dis Prev Can. 2016;36(9):194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen DL, Liess C, Poljak A, et al. Phenotypic characterization of insulin-resistant and insulin-sensitive obesity. J Clin Endocrinol Metab. 2015;100(11):4082–4091. [DOI] [PubMed] [Google Scholar]
- 6.CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory. 3rd ed. CLSI document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 7. Barlow SE; Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 8. Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132(2):204–210. [DOI] [PubMed] [Google Scholar]
- 9. Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75(6):978–985. [DOI] [PubMed] [Google Scholar]
- 10. Kahn HS, Imperatore G, Cheng YJ. A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth. J Pediatr. 2005;146(4):482–488. [DOI] [PubMed] [Google Scholar]
- 11. Khoury M, Manlhiot C, McCrindle BW. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013;62(8):742–751. [DOI] [PubMed] [Google Scholar]
- 12. Mokha JS, Srinivasan SR, Dasmahapatra P, et al. Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: the Bogalusa Heart Study. BMC Pediatr. 2010;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garnett SP, Baur LA, Srinivasan S, Lee JW, Cowell CT. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr. 2007;86(3):549–555. [DOI] [PubMed] [Google Scholar]
- 14. Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114(2):e198–e205. [DOI] [PubMed] [Google Scholar]
- 15. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;(11):1–190. [PubMed] [Google Scholar]
- 16. Adeli K, Higgins V, Trajcevski K, White-Al Habeeb N. The Canadian laboratory initiative on pediatric reference intervals: a CALIPER white paper. Crit Rev Clin Lab Sci. 2017;54(6):358–413. [DOI] [PubMed] [Google Scholar]
- 17. Ethnic origins, 2006 counts, for Canada, provinces and territories - 20% sample data [Internet]. Statistics Canada; 2010. http://www12.statcan.ca/census-recensement/2006/dp-pd/hlt/97–562/pages/page.cfm?Lang=E&Geo=PR&Code=35&Data=Count&Table=2&StartRec=1&Sort=3&Display=All. Accessed October 18, 2016. [Google Scholar]
- 18. Colantonio DA, Kyriakopoulou L, Chan MK, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58(5):854–868. [DOI] [PubMed] [Google Scholar]
- 19. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5-19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 2015;78(6):723–729. [DOI] [PubMed] [Google Scholar]
- 21. BMI-for-age (5–19 years) [Internet]. World Health Organization (WHO); 2019. https://www.who.int/growthref/who2007_bmi_for_age/en/. Accessed March 23, 2016. [Google Scholar]
- 22. Dixon W. Processing date for outliers. Biometrics. 1953;9(1):74–89. [Google Scholar]
- 23. Reed AH, Henry RJ, Mason WB. Influence of statistical method used on the resulting estimate of normal range. Clin Chem. 1971;17(4):275–284. [PubMed] [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc. 1995;57(1):289–300. [Google Scholar]
- 25. Tukey J. Exploratory Data Analysis. Boston: Addison-Wesley; 1977. [Google Scholar]
- 26. Hubert M, Van der Veeken S. Outlier detection for skewed data. J Chemom. 2008;22(3–4):235–46. [Google Scholar]
- 27. Horn P, Pesce A. Reference Intervals: A User’s Guide. Washington, DC: AACC Press; 2005. [Google Scholar]
- 28. Higgins V, Omidi A, Tahmasebi H, et al. Marked influence of adiposity on laboratory biomarkers in a healthy cohort of children and adolescents - Supplemental files [Internet]. Toronto, ON: TSpace; 2019. http://hdl.handle.net/1807/96469. Accessed November 1, 2019. [Google Scholar]
- 29. Tsushima Y, Nishizawa H, Tochino Y, et al. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013;288(38):27138–27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hikita M, Ohno I, Mori Y, Ichida K, Yokose T, Hosoya T. Relationship between hyperuricemia and body fat distribution. Intern Med. 2007;46(17):1353–1358. [DOI] [PubMed] [Google Scholar]
- 31. Tamba S, Nishizawa H, Funahashi T, et al. Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern Med. 2008;47(13):1175–1180. [DOI] [PubMed] [Google Scholar]
- 32. Kim TH, Lee SS, Yoo JH, et al. The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15(3):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lurbe E, Torro MI, Alvarez-Pitti J, Redon J, Borghi C, Redon P. Uric acid is linked to cardiometabolic risk factors in overweight and obese youths. J Hypertens. 2018;36(9):1840–1846. [DOI] [PubMed] [Google Scholar]
- 35. Tang L, Kubota M, Nagai A, Mamemoto K, Tokuda M. Hyperuricemia in obese children and adolescents: the relationship with metabolic syndrome. Pediatr Rep. 2010;2(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanal MG. Biomarkers in nonalcoholic fatty liver disease-the emperor has no clothes? World J Gastroenterol. 2015;21(11):3223–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(6):1889–1895. [DOI] [PubMed] [Google Scholar]
- 38. Chen PH, Chen JD, Lin YC. A better parameter in predicting insulin resistance: obesity plus elevated alanine aminotransferase. World J Gastroenterol. 2009;15(44):5598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Lu X, Hong J, et al. Positive correlations of liver enzymes with metabolic syndrome including insulin resistance in newly diagnosed type 2 diabetes mellitus. Endocrine. 2010;38(2):181–187. [DOI] [PubMed] [Google Scholar]
- 40. Feitosa MF, Reiner AP, Wojczynski MK, et al. Sex-influenced association of nonalcoholic fatty liver disease with coronary heart disease. Atherosclerosis. 2013;227(2):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SY, Sung E, Chang Y. Elevated serum gamma-glutamyltransferase is a strong marker of insulin resistance in obese children. Int J Endocrinol. 2013;2013:578693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6 (Suppl 1):60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koster A, Stenholm S, Alley DE, et al. ; Health ABC Study. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring). 2010;18(12):2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract Res Clin Endocrinol Metab. 2013;27(4):501–507. [DOI] [PubMed] [Google Scholar]
- 45. Kojima H, Sakurai S, Uemura M, et al. Difference and similarity between non-alcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2005;29 (12 Suppl):259S–263S. [DOI] [PubMed] [Google Scholar]
- 46. Katoh S, Peltonen M, Wada T, et al. Fatty liver and serum cholinesterase are independently correlated with HbA1c levels: cross-sectional analysis of 5384 people. J Int Med Res. 2014;42(2):542–553. [DOI] [PubMed] [Google Scholar]
- 47. Duchnowicz P, Ziobro A, Rapacka E, Koter-Michalak M, Bukowska B. Changes in cholinesterase activity in blood of adolescent with metabolic syndrome after supplementation with extract from Aronia melanocarpa. Biomed Res Int. 2018;2018:5670145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lorenzo C, Hartnett S, Hanley AJ, et al. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2013;98(4):1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ludwig DS, Ebbeling CB. Type 2 diabetes mellitus in children: primary care and public health considerations. JAMA. 2001;286(12):1427–1430. [DOI] [PubMed] [Google Scholar]
- 50. American Diabetes Association. Management of dyslipidemia in children and adolescents with diabetes. Diabetes Care. 2003;26(7):2194–2197. [DOI] [PubMed] [Google Scholar]
- 51. Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–1656. [DOI] [PubMed] [Google Scholar]
- 52. Strong JP, Malcom GT, McMahan CA, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281(8):727–735. [DOI] [PubMed] [Google Scholar]
- 53. Nilsson B, Hamad OA, Ahlström H, et al. C3 and C4 are strongly related to adipose tissue variables and cardiovascular risk factors. Eur J Clin Invest. 2014;44(6):587–596. [DOI] [PubMed] [Google Scholar]
- 54. Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46(6):1112–1113. [DOI] [PubMed] [Google Scholar]
- 55. Piéroni L, Bastard JP, Piton A, Khalil L, Hainque B, Jardel C. Interpretation of circulating C-reactive protein levels in adults: body mass index and gender are a must. Diabetes Metab. 2003;29(2 Pt 1):133–138. [DOI] [PubMed] [Google Scholar]
- 56. Oliveira-Santos J, Santos R, Moreira C, et al. Ability of measures of adiposity in identifying adverse levels of inflammatory and metabolic markers in adolescents. Child Obes. 2016;12(2):135–143. [DOI] [PubMed] [Google Scholar]
- 57. Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99(16):2221–2222. [PubMed] [Google Scholar]
- 58. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. [DOI] [PubMed] [Google Scholar]
- 59. Chiellini C, Santini F, Marsili A, et al. Serum haptoglobin: a novel marker of adiposity in humans. J Clin Endocrinol Metab. 2004;89(6):2678–2683. [DOI] [PubMed] [Google Scholar]
- 60. Quaye IK. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg. 2008;102(8):735–742. [DOI] [PubMed] [Google Scholar]
- 61. Hämäläinen P, Saltevo J, Kautiainen H, Mäntyselkä P, Vanhala M. Erythropoietin, ferritin, haptoglobin, hemoglobin and transferrin receptor in metabolic syndrome: a case control study. Cardiovasc Diabetol. 2012;11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cura-Esquivel I, Cordero-Pérez P, Torres-González L, Muñoz-Espinosa LE. Acute phase markers in obese children and adolescents with metabolic disorders. Arch Argent Pediatr. 2018;116(4):275–282. [DOI] [PubMed] [Google Scholar]
- 63. Flynn JT, Kaelber DC, Baker-Smith CM, et al. ; Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):74. [DOI] [PubMed] [Google Scholar]
- 64. Pasic MD, Colantonio DA, Chan MK, Venner AA, Brinc D, Adeli K. Influence of fasting and sample collection time on 38 biochemical markers in healthy children: a CALIPER substudy. Clin Biochem. 2012;45(15):1125–1130. [DOI] [PubMed] [Google Scholar]
- 65. Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29(4):469–479. [PubMed] [Google Scholar]
- 66. De Bruin TW, Brouwer CB, Gimpel JA, Erkelens DW. Postprandial decrease in HDL cholesterol and HDL apo A-I in normal subjects in relation to triglyceride metabolism. Am J Physiol. 1991;260(3 Pt 1):E492–E498. [DOI] [PubMed] [Google Scholar]
- 67. Nordestgaard BG, Langsted A, Mora S, et al. ; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cutpoints-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2016;62(7):930–946. [DOI] [PubMed] [Google Scholar]
- 68. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.