Abstract
Recent advances in genetic data generation, through massive parallel sequencing (MPS), storage and analysis have fostered significant progresses in microbial forensics (or forensic microbiology). Initial applications in circumstances of biocrime, bioterrorism and epidemiology are now accompanied by the prospect of using microorganisms (i) as ancillary evidence in criminal cases; (ii) to clarify causes of death (e.g., drownings, toxicology, hospital-acquired infections, sudden infant death and shaken baby syndromes); (iii) to assist human identification (skin, hair and body fluid microbiomes); (iv) for geolocation (soil microbiome); and (v) to estimate postmortem interval (thanatomicrobiome and epinecrotic microbial community). When compared with classical microbiological methods, MPS offers a diverse range of advantages and alternative possibilities. However, prior to its implementation in the forensic context, critical efforts concerning the elaboration of standards and guidelines consolidated by the creation of robust and comprehensive reference databases must be undertaken.
Keywords: Criminal investigation, Genetics, Massive parallel sequencing, Microbial forensics, Microbiome
Introduction
Microorganisms (e.g., viruses, bacteria and many fungi) can be found nearly everywhere on Earth, establishing remarkably diverse and ubiquitous communities. From the intersection of knowledge and expertise from microbiology and forensics, microbial forensics (MF or forensic microbiology) was born (Kuiper 2016). This discipline employs microbiological methods for analyzing evidence involved in a multitude of criminal cases classically aiming forensic attribution (assigning to a source), ranging from bioterrorism, biocrime, frauds, outbreaks and transmission of pathogens, or accidental release of a biological agent and/or a toxin (Allard et al. 2017; Amorim 2010; Budowle et al. 2014; González-Candelas 2017; González-Candelas et al. 2013).
Typically, pathogens’ outbreak monitoring and toxicology are not considered as crucial elements of MF. However, these two topics are basic to MF, since pathogens’ release can be intentional or due to medical malpractice and the application of a reliable and robust surveillance protocol for pathogen monitoring would provide valuable information to distinguish between spontaneous and harmful spread of microorganisms (either related with bioterrorist or biocrime). Besides the differences between protocols and objectives of forensic investigations and epidemiological studies, it is our opinion that the investigations of the sources of outbreaks to fall in the realm of MF due to the shared aim of origins’ ascertainment.
When natural pathogens’ outbreaks are concerned, measures to prevent other cases of the disease must be implemented. The success of such measures relays in the quantity and quality of the data collected on isolate identification (to the strain level), location of possible sources, definition of possible reservoirs, vectors and intermediary host, determination of transmission routes or evolution dynamics (Schürch and Siezen 2010). Over the preceding decades, the number of epidemic and pandemic has been increasing, examples of the latest cases of pathogens’ outbreaks are (Arenas et al. 2017; Oliveira et al. 2018): Mycobacterium tuberculosis (tuberculosis; 2006, Canada; Gardy et al. 2011), Acinetobacter baumannii hospital outbreak (2008, United Kingdom; Lewis et al. 2010), swine-origin influenza A (H1N1 virus; 2009, America, 2009; Smith et al. 2009), Vibrio cholerae (cholera; 2010, Haiti; Hendriksen et al. 2011), the Middle East respiratory syndrome hospital outbreak (MERS coronavirus; 2012, Saudi Arabia; Assiri et al. 2013), avian-origin influenza A (H7N9 virus; 2013, China; Kageyama et al. 2013), Shiga toxin-producing Escherichia coli O104:H4 (2014, Germany; Grad et al. 2012), Ebola (Ebolavirus; 2014, Sierra Leone; Cenciarelli et al. 2015), poliomyelitis (Poliovirus; 2014, Middle East; Aylward and Alwan 2014), Legionnaires’ disease (Legionella pneumophila; 2014, Portugal; Borges et al. 2016) and Zika virus (2015, Brazil; Faria et al. 2016).
In the case of a bioterrorist attack, a given biological agent (e.g., bacteria, viruses, fungi and toxins) is intentionally released with the purpose to cause panic, mass casualties or economic loss, being motivated by ideological, political or religious beliefs (Jansen et al. 2014). These agents can be used as they are present in nature or be genetically engineered for improved mass dissemination, more significant mortality or resistance to the currently available medicines and/or vaccines (Pavlin 1999). When facing the possibility of a bioterrorist attack is crucial to identify the agent involved, not only to prevent panic from the population but also to contain the morbidity and mortality associated with the dissemination of such agent (Lehman 2014). The emphasis of MF on bioterrorism has emerged from the challenges brought up by the responses to the famous Amerithrax mailing attacks in 2001 (Rasko et al. 2011). Besides Bacillus anthracis, other examples of agents used in bioterrorism attacks can be presented: Yersinia pestis (bubonic plague), Salmonella enterica serovar Typhi (typhoid fever), Shigella dysenteriae (dysentery), Francisella tularensis (tularemia), human immunodeficiency virus (HIV), Variola major and Variola minor viruses (smallpox), Rickettsia prowazekii (typhus), Ascaris suum (ascariasis); and toxins such as those produced by Clostridium botulinum (botulism), Corynebacterium diphtheria (diphtheria), Staphylococcus aureus [e.g., toxic shock syndrome (TSS), staphylococcal scalded skin syndrome (SSSS), necrotizing pneumonia, or deep-seated skin infections] and V. cholerae (cholera) (Jansen et al. 2014).
Biocrime can be defined as the weaponization of a disease-causing agent or toxin with the intention to harm or cause panic in a specific individual or in a limited group of individuals (Jansen et al. 2014; Lehman 2014). The use of MF in biocrime investigations can be illustrated by the paradigmatic cases of intended dissemination of HIV (Ciesielski et al. 1992; Kovach 2008; Metzker et al. 2002; Muchmore 2013), hepatitis B (HBV) (Muchmore 2013) and C (HCV) viruses (Akehurst 1998; Bosch 1998; Muchmore 2013). In such cases is of primordial importance to establish the source of the bioagent through the comparison of the isolate found in victims and that associated with the perpetrators (Lehman 2014).
Considering the topic of toxicology, if during an autopsy the microbiome present in a given organ from a cadaver is unveiled, the presence of bioindicator microorganisms can indicate the occurrence of postmortem production or degradation of several drugs or poison that otherwise would provide misleading information concerning the cause of death. Therefore, both aspects, outbreak monitoring and toxicology, will be addressed in the present review.
Since its introduction more than 30 years ago (Gill et al. 1985), forensic DNA testing has assumed a substantial role in civil and criminal cases (Butler 2015). DNA evidence can be used as a silent witness to establish a probability-based connection between a crime scene and particular individuals (suspects and/or victims) and/or objects. These links, determined by the comparison of the genetic profiles and complemented by other means of proof, are frequently decisive aiding in acquitting or convicting a suspect (Budowle et al. 2017; Butler 2015).
Previously to the advent of DNA-based techniques, in the late 1980s, the available tools for microorganism detection and identification were restricted to phenotypic methods associated with antigenic and/or antimicrobial resistance profiles. These methods only allow resolution at the genus and/or species level (using culture-dependent techniques) (Oliveira et al. 2018).
Afterwards, a paradigm shift was observed: the traditional phenotypic methods were supplanted by nucleic acid-based molecular methods [e.g., DNA fingerprinting, whole genome sequencing and microarray analysis] (Budowle et al., 2005; Pattnaik and Sekhar 2008). These methods not only improved taxonomical resolution (identification to isolate/strain level), being unsubjected to the changes observed phenotypic characteristics due to the influence of environmental factors, but also decreased the turnaround time between sample collection and results (Pattnaik and Sekhar 2008).
The next evolutionary step, in the early 2000s, was associated with the introduction of massive parallel sequencing (MPS) technology [also referred as next-generation sequencing (NGS) or high-throughput sequencing (HTS)] (Yang et al. 2014). Due to its increased multiplexing capacity, this method complies with different approaches, such as sequencing the whole genome (WGS) of a given microorganism (viruses, bacteria or fungi) or sequencing all the microbial species present in complex sample collected from a given individual or environment (metagenomics) (Kuiper 2016; Schmedes et al. 2016).
As such, presently, the forensic science has evolved and expanded, reaching out to numerous innovative technological breakthroughs and molecular advances. These recent developments have led to an increasing use MPS over conventional Sanger sequencing (Bano et al. 2015; Clarke et al. 2017; Schmedes et al. 2017). Such advances, like the ability to sequence single isolates with de novo assembly, read mapping, targeted sequencing of specified genes or other regions of interest (e.g., hypervariable regions of 16S and 18S rRNA genes, 16S–23S rRNA intergenic spacer region, SNPs, indels) (Sijen 2015) and metagenomics (Børsting and Morling 2015; Franzosa et al. 2015), ensure a growing capacity of identification and characterization of microbial communities, paving the way for the use of microbial evidence in cases associated to human identification, geolocation and postmortem interval estimation, amongst other applications (Arenas et al. 2017; Kuiper 2016).
Although phylogenetic analyses have supported associations and have successfully been admitted as evidence in criminal cases, the fundamental limitations to the widespread application of MF are the non-existence of both standards and guidelines and the deficiency of reference databases, reference genome sequences, metadata and representative genetic diversity coverage (Arenas et al. 2017; Sjödin et al. 2013).
The main assets of MPS over classical Sanger sequencing are (i) its high-throughput capacity, hundreds of millions of sequencing reactions can be completed in parallel, permitting to sequence an entire bacterial genome in just one or two instrument runs; (ii) a unique protocol can be applied for all microorganisms for identification and genotyping; (iii) the necessity for DNA cloning is obviated, exclusively depending on libraries preparation, in a cell-free system; (iv) there is no need for an a priori knowledge about the sequence of a particular gene/genome because these new technologies can read the DNA templates randomly distributed throughout the entire genome, and then de novo genome assembly can be applied; (v) overcome the need for isolation and culture of the microorganism of interest, a particularly critical aspect once many strains are unable to grow in culture media, allowing to identify microorganisms present in trace concentrations and undetected by conventional methods; and (vi) reduces both costs (usually less than US$1000 per genome, depending on the genome length) and the turnaround time (only a few hours) (Oliveira et al. 2018; Schmedes et al. 2016).
The main shortcoming of the MPS is associated with the shorter read length for each DNA template. Nowadays, read length ranges from 35 to 500 base pairs (bp) comparing with the 1000–1200 bp obtained with Sanger sequencing (Oliveira et al. 2018; Van Dijk et al. 2014). Furthermore, the application of these approaches poses two challenging tasks: data storage and analysis (van Dijk et al. 2014; Kuiper 2016). Depending on the platform used, 30,000–500,000 sequence reads of 50–700 nucleotides can be generated in a single run. Due the extremely large amount of data collected, the process is deeply dependent not only on storage ability but also on the bioinformatics capacity to produce valuable data and to evaluate the quality of the MPS platform (Kuiper 2016). Moreover, to compel an increasingly widespread application of MF the forensic community needs to be further educated: experts must address all the requirements of transparency (e.g., application of legal standards for genetic privacy and reliable standards) and accuracy of the data produced (e.g., correct use of databases and informatics tools); while the end-users (i.e., crime scene investigators, lawyers, judges and juries) must be receptive to this new developments, acknowledging both its possibilities and limitation (Kuiper 2016; Clarke et al. 2017).
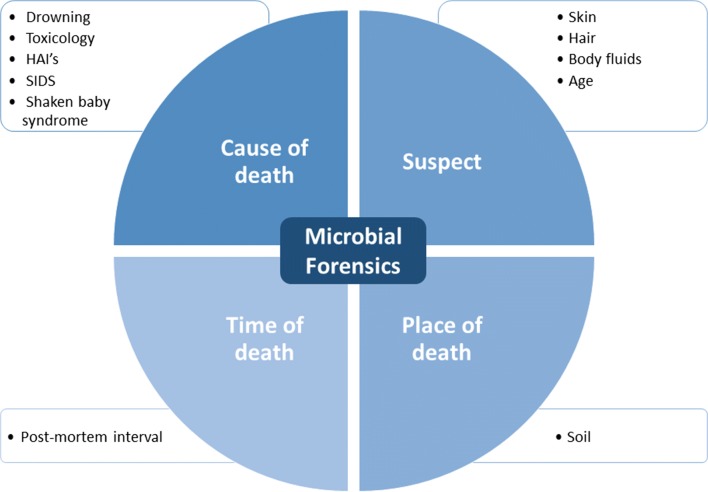
The present review on MF discusses how MPS techniques are already implemented as well as its potential applications to answer criminal and legal questions, organized in the topical issues: (i) determining cause of death; (ii) determining time of death; (iii) determining place of death; (iv) identifying suspects (Fig. 1).
Fig. 1.
Application of MF to answer criminal and legal questions
Determining cause of death
In general, the presence of specific microorganisms can constitute evidence, helping to determine the cause of a given death, acting as bioindicators of criminal cases. In the following examples, we show some cases in which MPS was already implemented while in other instances, we discuss how such techniques could improve forensic analyses.
Drowning cases
During an autopsy, a common concern is the diagnosis of drowning, principally when the cadaver reaches an advanced state of decomposition due to an extended postmortem interval. The diatoms’ presence in blood or organs (e.g., lungs, kidneys, spleen, liver and bone marrow) from the victims has been frequently considered as the gold standard indicator for drowning cases, discriminating between antemortem immersion (live entry) and postmortem submersion (dumping of a dead body) or even indicating the location of a probable drowning place (seawater vs. freshwater diatoms) (Sitthiwong et al. 2014; Spitz and Schneider 1964). However, this analysis presents several constraints: (i) diatoms may be present in low concentrations in some water sources, being difficult to detect even in some true drowning victims; (ii) some of these microorganisms present relatively large diameter/length (ranging between 2 and 500 μm), limiting their transposition of the alveolar wall and, consequently, their passage into the blood stream and organs; (iii) diatoms can be disclosed in several organs (e.g., kidney, liver and lungs) of non-drowned bodies (Calder 1984; Kakizaki et al. 2010; Kakizaki et al. 2009; Lee et al. 2017).
In the previous decade, attention has expanded to the use of other microorganisms — bacteria and cyanobacteria (Kakizaki et al. 2010; Lucci et al. 2008) and phytoplankton, including microalgae (Díaz-Palma et al. 2009) — using different approaches such as classical culture-dependent methods (Huys et al. 2012; Kakizaki et al. 2009; Lucci et al. 2008), light (Sitthiwong et al. 2014) or electron microscopy (Lunetta et al. 1998) or PCR-based techniques (He et al. 2008; Huys et al. 2012; Rácz et al. 2016; Tie et al. 2010). These other microorganisms are much more abundant and present smaller sizes (ranging between 0.2 and 2 μm) than diatoms and, therefore, at least theoretically, would provide more informative results. A recent study compared lungs, closed organs (kidney and liver) and cardiac blood between antemortem immersion and postmortem submersion, either using MPS or the 16S rRNA gene and culture-dependent identification of aquatic microorganisms using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) (Lee and Eom 2016; Lee et al. 2017). The findings revealed that the microbial community in the closed organs was a significant marker for the diagnosis of death by drowning. Likewise, the same study hypothesized that drowning is associated with higher expression levels of surfactant protein A (regarded as a valuable marker of asphyxiation and acute lung injury) in the lung of true drowning victims (Lee et al. 2017).
Also, in the case of the presence of such microorganisms, through the comparison of the microbial communities present in the victim and the known environmental samples, it might be possible to reveal the place of death (or even discriminating between primary and secondary crime scenes). Additionally, comparing the microbiomes present in the victim and trace evidence found in the possible suspect(s), the data collected could be used to infer (or not) an association between them.
Toxicology
Although subjected to an intense debate, it has been hypothesized that during decomposition some microorganisms are responsible for the degradation of drugs (e.g., antidepressants, antipsychotics, benzodiazepines, cannabinoids, cocaine, heroin and morphine glucuronides) or poisons (e.g., cyanide) and for the neoformation of other metabolites (e.g., alcohol, methamphetamines and amphetamines, 4-hydroxybutanoic acid, methadone and other potent opioids) (Byard and Butzbach 2012; Gerostamoulos et al. 2012; Han et al. 2012). Therefore, postmortem microbial activity can, at least theoretically, interfere with autopsy findings concerning pre-mortem drug consumption and, consequently, alter the interpretation of the cause of death (Butzbach 2010; Butzbach et al. 2013; Castle et al. 2017; Drummer 2004; Gunn and Pitt 2012; Sastre et al. 2017; Skopp 2010).
In high-income countries, the consumption of antidepressant drugs is significantly increasing, with the collateral risks of producing addiction or increasing the risk of self-poisoning (Gjelsvik et al. 2014; Löfman et al. 2017). In MF, it is important to bear in mind that while some antidepressant drugs (e.g., amitriptyline, doxepin, fluoxetine, imipramine) present stable concentrations, others (e.g., dothiepin, phenelzine, tranylcypromine) are known to be degraded during cadaver putrefaction due to microbial activity (Butzbach 2010; Caddy and Stead 1978; Garcia-Pardo et al. 2018; Kwon and Armbrust 2006; Monden et al. 2018; Pounder et al. 1994; Stevens 1984; Yonemitsu and Pounder 1993).
Benzodiazepines are frequently used to facilitate assaults and significantly increase the risk of road accidents (amongst others). Therefore, the accurate identification and quantification of these drugs are of primordial importance during autopsies. Several bacterial species, such as Bacillus cereus, Staphylococcus epidermidis, Clostridium perfringens and Bacteroides fragilis, are known to be responsible for bioconversion of nitrobenzodiazepines (e.g., clonazepam, diazepam, flunitrazepam, nimetazepam, nitrazepam, temazepam) into their 7-amino-metabolites under putrefying conditions, being the conversion rate dependent both on the tissue analyzed and storage conditions (Levine et al. 1983; Moriya and Hashimoto 2003; Robertson and Drummer 1998; Robertson and Drummer 1995; Stevens 1984).
The consumption of methamphetamines is rapidly increasing on a worldwide scale and, therefore, its forensic relevance. Several gastrointestinal microorganisms, such as species belonging to the genera Enterobacterium, Enterococcus, Lactobacillus, Clostridium and Bacterioides, are responsible for the N-demethylation of methamphetamines converting these compounds into amphetamines (Castle et al. 2017; Rommel et al. 2016).
The production of ethanol and other volatiles from a variety of substrates (namely carbohydrates, amino acids, glycerol and fatty acids) represents a common phenomenon amongst bacteria (more than 58 species; e.g., Corynebacterium sp., Lactococcus garviae, Escherichia coli, Enterococcus faecalis, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis), yeasts (more than 17 species; e.g., Candida albicans, Candida tropicalis, Candida tropicana, Candida glabrata, Candida parapsilosis, Saccharomyces cerevisiae) and fungi (more than 29 species; e.g., Aspergillus awamori, Aspergillus foetidus, Aspergillus nidulans, Aspergillus oryzae, Aspergillus sojae, Aspergillus tamari, Fusarium sp., Rhizopus javanicus and Rhizopus oryzae) (Boumba et al. 2012; Boumba et al. 2008; Skory et al. 1997). Therefore, the origin of postmortem ethanol can be attributed either to antemortem ingestion or postmortem microbial production (Boumba et al. 2013).
Gamma-hydroxybutyrate (GHB) has become a popular recreational drug, often consumed in combination with alcohol, being associated with several cases of drug-facilitated sexual assault. Therefore, during autopsy is frequently necessary to discriminate the cut-off concentration values between endogenous levels and exogenous exposure. It has been suggested a possible role of microorganisms in the postmortem production of GHB. Bacteria, such as Pseudomonas spp. (e.g., P. aeruginosa), present the enzymes necessary to convert gamma-aminobutyric acid (GABA; a main inhibitory neurotransmitter present in the central nervous system from mammals) to GHB (Elliott et al. 2004; Elliott 2004; Kalasinsky et al. 2001; Kintz et al. 2004; Mazarr-Proo and Kerrigan 2005; Wells 2001).
The decrease in concentration of cyanide during the postmortem period has been proven using blood and tissues (lungs, brain, liver and kidneys) of rabbits killed with potassium cyanide. This decrease is associated with the metabolism of cyanide into thiocyanates. Nevertheless, the bacteria involved in this process are still unknown (Ballantyne 2013; Ballantyne et al. 1974).
During an autopsy, the application of MPS-based technologies can provide a full screen of the microbiomes present in several body parts (e.g., blood, gut, brain). The occurrence of one or several of the previous referred microorganism delivers insights concerning possible postmortem variations in the concentration of these drugs and poisons (either by degradation, production or bioconversion) that may alter the results presented in the coroner report and misleading in the determination of the cause of death.
Hospital-acquired infections
Within the hospital environments, and despite all the imposed meticulous hygienization measures, there is circulation of bacteria (e.g., Acinetobacter baumannii, Pseudomonas aeruginosa, S. aureus, K. pneumoniae, Enterococcus faecium, Salmonella enterica and Clostridium difficile), fungi (e.g., Aspergillus ustus, Aspergillus flavus, Aspergillus fumigatus, Microsporum canis and Trichophyton tonsurans), viruses [e.g., norovirus, respiratory syncytial virus (RSV), rotavirus, adenovirus, HBV and HCV] and other pathogenic agents (e.g., C. albicans), some of them multi-resistant to antibiotics that may trigger serious infections in ill-debilitated patients subjected to an intervention or undergoing immunosuppression treatments — hospital-acquired infections (HAIs) also known as nosocomial infections (NIs). Worldwide, the HAI rates are around 7% in developed and 10% in developing countries (Khan et al. 2017). In the United States of America are reported an annual a total of 1.7 million HAIs, 99,000 deaths and costs of 20 billion USD (Klevens et al. 2007; Sodhi et al. 2013). In Europe, the numbers are 3 million HAIs, 50,000 deaths and costs of 7 billion Euros (Bryers 2008). Besides this economic cost, HAI also create user insecurity and are a vulnerability hotspot of hospital environment and health system.
Over the precedent decade, HAIs have evolved continuously to a current state of significant growth. As a result, recent attention has been given by health governmental institutions (e.g., European Centre for Disease Prevention and Control and Centre for Disease Control) that have implemented specific surveillance programs to collect data on HAIs and have issued regulations for mandatory reporting of such infections (Herzig et al. 2015; Meier et al. 2008). Nowadays, pathogen monitoring in hospital surfaces is performed using conventional microbiology methods and luminescent determination of ATP concentrations. However, these methods present several limitations and fail to reveal the true diversity of the microorganisms present, contributing to the significant worldwide rates of HAIs (Boyce et al. 2009; Shama and Malik 2013).
The main risk factors for the development of an HAI have been described as patient age, longer hospital stay duration, larger hospital size and the insertion of a central catheter. Also, several studies have indicated the potential routes of transmission of hospital-associated pathogens (HAPs) comprehend medical doctors’ and nursing staff’s clothing, stethoscopes, personal cellphones and computer keyboards (Lax and Gilbert 2015).
The use of robust and comprehensive techniques associated with MPS will allow comparing the microbiome present in hospital surfaces, patients, staff and visitors the bacteria responsible for the infection. The detection, identification and possible transmission routes of a given HAP are essential for the allocation of Infection Prevention and Control (IPC) resources and compelling constraint of infection. As well, in a case of a lawsuit, the genotyping data collected may be used to confirm or exonerate the hospital for the spread of the infection.
Sudden infant death and shaken baby syndromes
Sudden infant death syndrome (SIDS), the leading cause of post-neonatal infant mortality in developed countries, can be roughly defined as the unexpected death of a seemingly healthy infant in the postnatal period (i.e., the first year of life), remaining the cause of death unexplained after a meticulous investigation comprising autopsy, review of the clinical history and death scene investigation (Duncan and Byard 2018). The risk factors for SIDS have for long be regarded as multifactorial, resulting in the combination of factors such as the presence of an underlying vulnerability that increases susceptibility (e.g., genetics, undiagnosed pathology and immunologic disorders) and exposure to an exogenous stressor (e.g., sleeping position, exposure to tobacco smoke, alcohol and illicit drugs) (Duncan and Byard 2018). It has been reported that undiagnosed infections, attributed to several pathogens (e.g., viruses and bacteria), are responsible for 10% of the cases of SIDS (Morentin et al. 2012). The most common agents associated with SIDS are human herpes virus, Epstein Barr virus, cytomegalovirus, respiratory syncytial virus, adenovirus, human metapneumovirus, Neisseria meningitidis, Haemophilus influenzae, Streptococcus pneumoniae and Bordetella pertussis (Fernández-Rodríguez et al. 2006; Hawksworth and Wiltshire 2011). The viral infections are sometimes exacerbated by bacterial colonization and subsequent inflammatory response to endotoxins that cause hemorrhagic shock and encephalopathy. The unexpected death of children aged between 15 days and 1 year has been for long a matter of several legal investigations, to exclude the possibility of a crime. Nowadays, routine postmortem microbiology protocols for SIDS comprise a limited number of viruses and bacteria (serology tests and PCR assays) performed in small quantities of specimens collected during autopsies. Efforts should be engaged to the development of comprehensive microbial studies providing etiologically specific disease diagnoses (Brooks et al. 2015; Fernández-Rodríguez et al. 2006; Krous and Byard 2005).
Shaken baby syndrome (SBS) is usually characterized by unexplainable intracranial, retinal hemorrhages and acute encephalopathy. The well-known Sally Clark case demonstrates the need of accurate microbiological analysis. The mother was sentenced for the homicide of both her children. However, the posterior evaluation of this case demonstrated a white blood cell profile compatible with a bacterial infection due to staphylococcal septicemia and meningitis. After spending several years imprisoned, the sentence was re-evaluated (Byard 2004a; Byard 2004b; Dyer 2005; Krous and Byard 2005).
As in the previous cases, if a comprehensive MPS-based panel (including both viruses and bacteria from several body locations — blood, cerebrospinal fluid, heart, lungs, spleen and throat — and body secretions — urine and feces) run during autopsy indicates the presence of such microbiological agents, it may constitute an effective tool to ascertain the causes of death. These microbial profiles should be accompanied with histological findings, positive C-reactive protein (CRP) measurement and antemortem clinical information.
Determining time of death
For law enforcement agents and forensic researchers, determining the time of death (postmortem interval — PMI) occupies a pivotal role in criminal investigations, mainly in suspicious death cases where no witnesses are present at the crime scene. An accurate estimation of the PMI may validate witness declarations, support the trustworthiness of supplementary physical evidence, provide reliable information for developing leads, simplify information management concerning decisions on investigative resources allocation and constitute evidence for later prosecutorial action (Maile et al. 2017).
Customarily a cornerstone of all death investigations, PMI can be estimated implementing two alternative approaches: (i) the assessment of antemortem changes, pathological or physiological, observed in the cadaver and combined with conclusions from investigations by the law enforcement agents (survival time), yielding rough estimations of the PMI (e.g., wound age estimation or evaluation of the gastric contents); and (ii) the assessment of postmortem changes evaluated at the crime scene, allowing investigators to conclude about the PMI, more accurate than the previously described methods (e.g., body cooling, postmortem lividity, rigor mortis, supravital reagibility of skeletal muscle, vitreous potassium levels, autolysis, cerebrospinal fluid chemistry, H3-magnetic resonance spectroscopy, putrefaction and entomology). However, and despite all methods available, PMI can only be determined within certain probability limits (Madea 2015, 2016).
PMI estimation
For many years, forensic entomology, based on the postmortem succession of insects colonizing the cadaver, has been used for PMI estimation. In the recent years, evidence has been accumulated, proving that microbial communities can also go through successional changes postmortem, paving the ways for its use as PMI indicators (Dickson et al. 2011; Hauther et al. 2015; Heimesaat et al. 2012; Javan et al. 2016, b; Javan et al. 2017; Metcalf et al. 2013, 2016, b, 2017; Parkinson et al. 2009).
Microbiome approaches, based on the recent improvements both in sequencing platforms using phylogenetically and/or taxonomically informative gene markers (e.g., 16S rRNA, 18S rRNA, and internal transcribed spacer—ITS) and statistical and computational pipelines (e.g., machine learning), allow not merely to evaluate the microbial changes during PMI but also to analyze additional trace evidence signatures characterized by differences in microbial communities (Metcalf et al. 2017).
The postmortem microbiome — community of prokaryotic and eukaryotic microorganisms associated with the decomposition of animal carrion and human corpses — can be divided into two core components: the thanatomicrobiome and the epinecrotic microbial community. While the first is composed microorganisms, mainly bacteria and fungi, present in the blood and internal organs (e.g., brain, heart, liver, lungs, spleen); the second is composed by the microorganisms residing in and/or moving on the surface (e.g., superficial epithelial tissues, oral mucosal membranes and distal orifices of the digestive tract) of decomposing cadavers. Contrary to the epinecrotic community, the thanatomicrobiome is more stable being unbiased by either abiotic (e.g., humidity, temperature and pH) or biotic factors (e.g., gasses, insects and scavenger activities) (Javan et al. 2016, b).
Upon death, because of the cessation of the activity of the immune system, the microorganisms present in the gastrointestinal tract are able to invade not only the surrounding tissues organs but also to move along the circulatory and lymphatic systems, initiating the decomposition process in a nutrient-rich environment, mediated by tissue autolysis (Carter 2017). In general, as decomposition progresses, microbial communities’ diversity decreases with only a few genera becoming dominant. Additionally, a shift from an aerobic bacteria-based community (Phylum Bacteroidetes: Bacteroides and Parabacteroides; Phylum Firmicutes: Faecalibacterium, Phascolarctobacterium, Blautia and Lachnospiraceae incertae sedis) to an anaerobic bacteria-based community (Phylum Bacteroidetes: Clostridium; Phylum Firmicutes: Peptostreptococcus and Anaerosphaera; Phylum Gammaproteobacteria: Wohlfahrtiimonas, Ignatzschineria, Acinetobacter and Providencia) has been reported (DeBruyn and Hauther 2017). Therefore, it seems legitimate to infer that bacterial taxa present in internal organs are potentially useful for estimating the minimum PMI.
In a foreseeable future, the elaboration of a microbiome calendar produced with MPS-based technology, including samples collected at several time-points after death, will allow match the microorganisms present in a given cadaver and the time of death based on the proposed calendar.
Determining place of death
Another fundamental aspect in law enforcement and forensic researches is the distinction between primary and secondary crime scenes. While the first can be defined as the place where the criminal act was actually committed, the second is any other place (or places) related in a later time lapse, to this event (Lee et al. 2001).
As an example, the case of the massacre of civilians near Srebrenica (NE Bosnia) can be presented. In July of 1995, more than 8000 Bosnian Muslim males were executed and buried in a mass grave (primary crime scene) (Herman 2006). However, 3 months after the massacre, the cadavers were exhumed and transported to several other locations in Bosnia (secondary crime scenes) (Brown 2006). Although both scenes are critical for the crime reconstruction, the primary scene frequently contributes with more evidence to the forensic research. However, in several cases, only the secondary crime scene is available for the investigators. In this case, the identification of such mass graves was obtained using botanical and geological data. However, a similar approach, using microbial data, can be foreseen.
In forensic casework, soil analysis is frequently used as evidence, establishing a link between a suspect and an object, location or victim. This analysis studies intrinsic properties of soil such as mineralogy, chemistry, geophysics, texture and color. Soil, a complex matrix constituted by both organic and inorganic matter, although highly informative, is frequently overlooked when trying to conceal evidence. Furthermore, it adheres and persists on numerous surfaces (e.g., clothing, footwear, tires and tools) constituting a valuable trace material providing clues about the origin of an unknown sample, or to compare samples from a suspect with those from a crime scene. However, because of the significant similarity between samples collected at short distances, the substantial amounts of material required and the lack of robust soil databases, the information provided by soil analysis has been quite restricted up to now (Young et al. 2014, 2015, 2017).
Soil microbiome
In the recent years, analysis of soil microbial communities has been regarded as potentially useful in forensics providing discriminatory power at fine scale resolution. Since the structure of the microbial community is deeply determined by several factors (e.g., soil type, seasonal variation, site management, vegetation cover and environmental conditions), bacterial and fungal communities possess an extremely site-specific profile (Grantham et al. 2015). The recent advances in MPS can generate thousands of sequencing reads per sample, providing a much more comprehensive snapshot of microbial communities present in a given soil sample (Demanèche et al. 2017; Young et al. 2014, b, 2015, 2017). Depending on the type of soil, a single gram may comprehend up to 8.3 million unique sequences (Roesch et al. 2007).
Recent studies have demonstrated that during the decomposition process, the surface and buried soil communities presented diverse behaviors. While microbial communities from surface soil present a decreasing trends in taxon richness, diversity and evenness; microbial communities in close contact with buried cadavers present the opposite characteristics (i.e., increasing taxon richness, consistent diversity and decreasing evenness). Also, Proteobacteria has been referred as the most abundant phylum in grave soil samples, the relative abundance Acidobacteria decreased and Firmicutes increased in surface cadaver-soil communities, while the microbial community composition remained rather constant in buried soil communities (Finley et al. 2015, 2016).
Given the complexity of soil microbial communities, the application of MPS will significantly accelerate the identification of such communities. Although a daunting endeavor, it would be rewarding to construct comprehensive maps of the microbial communities’ geographic distribution, such as the works recently publish by Thompson and co-workers (2017) and Delgado-Baquerizo and co-workers (Delgado-Baquerizo et al. 2018); these databases would certainly be useful to identify the place of death at least in broad areas scales.
Identifying suspects
A recent study revisited the ratio of bacterial/human (B/H) cells, estimating that in a reference man (age 20–30 years; weight 70 kg, height 170 cm), there are 3.8 × 1013 bacterial and 3.0 × 1013 human cells, corresponding to a B/H = 1.3 (uncertainty of 25%, variation of 53%) and a total mass is about 0.2 kg (Sender et al. 2016a, b). This values differ from the previously proposed 10:1 ratio (Savage 1977). In those earlier studies, only nucleated human cells (0.3 × 1013) were considered, disregarding the population of non-nucleated red blood cells (Sender et al. 2016b).
The human microbiome should not be regarded as an indivisible whole but as a sum of parts. Several lines of evidence point out towards this direction: (i) these bacteria are unevenly distributed through the different anatomical sites (e.g., colon — 1014; dental plaque — 1012; ileum — 1011; saliva — 1011; skin — 1011; stomach — 107; duodenum and jejunum — 107); (ii) each anatomical site/location presents a specific taxonomic composition; (iii) these microbiomes present seasonal patterns influenced by several factors (e.g., environmental exposure, age, dietary preference and antibiotic or antisepsis use) (Cho and Blaser 2012; Ding and Schloss 2014; Lloyd-Price et al. 2017; Sender et al. 2016b). However, to be of any use in human identification, individual microbiome stability and differentiation must be evaluated, a task far from being easily performed and likely unreachable (Arumugam et al. 2011; Franzosa et al. 2015; Lax et al. 2015).
Beyond individual identity, the human microbiome research may also disclose unpredicted information. Therefore, and despite being highly regulated, human microbiome research still poses several ethical, legal and social challenges. Microbiomes may provide information on people origins or ethnic background and past exposures or countries/geographical locations previously visited. This intelligence may be used to highlight the subject as a person of interest for homeland security and law enforcement services; breaching individual’s right to privacy. Moreover, human microbiotas are associated with both health and homeostasis. The occurrence of certain microorganisms may reveal individual’s susceptibility and predispositions to certain clinical conditions such as obesity or diabetes (correlation between intestinal microbiota) and local or systemic infections (colonization of the nasopharynges by S. aureus), contributing for socioeconomic discrimination or stigmatization. Ultimately, microbial sample collection (e.g., collection of stool samples and vaginal swabs) can be regarded as invasive and culturally non-acceptable amongst certain sub-sectors of the society (McGuire et al. 2008; Hawkins and O’Doherty 2011).
Skin microbiome
The human skin surface is colonized by a substantial number of microorganisms that can be easily dispersed and transferred to surfaces upon touching (Fierer et al. 2010). The human microbiome can be used as a personal signature, establishing a link between individuals and the objects they touch, such as cell phones and computers (keyboards, touchpads and other devices’ surfaces) (Fierer et al. 2010; Lax et al. 2015; Meadow et al. 2014). The degree of diversity of the skin microbiome depends on the depth of the study, if only the phylum of the bacteria is considered, only a few distinctive taxa can be identified amongst various persons. However, increasing the taxonomic depth, if identification is achieved at the genus, species and strain population levels, the diversity is extremely specific for each person, acting as an individualized signature, with a discriminatory power similar to a fingerprint (Blaser 2010; Costello et al. 2009; Fierer et al. 2008; Gao et al. 2007; Grice et al. 2009). Additionally, each body part constitutes a specific niche, with a particular microbiome composition, dictated by several factors (e.g., skin thickness and folds, densities of hair follicles and the presence of both eccrine and apocrine glands). For instance, if Propionibacteria and Staphylococcus species dominate sebaceous sites and Corynebacteria moist sites, β-Proteobacteria and Flavobacteriales dominate dry sites. However, these differences remain relatively stable throughout time and despite of the personal hygiene. Additionally, the bacteria constituting this microbiome persist on touched surfaces for extended periods since some of these microorganisms are extremely resistant to environmental stresses (moisture, temperature and UV radiation) (Brooke et al. 2009; Kong and Segre 2012; Smith et al. 1996). It remains, however, in doubt the stability of the personal profile and changes induced by antifungal and antibiotic therapies (Langdon et al. 2016; Raymond et al. 2016).
Hair microbiome
Hairs are amongst of the most commonly found evidence collected at crime scenes. However, the vast majority lacks the anagenic root (actively growing hair), essential for DNA extraction and posterior analysis employing conventional typing methods, such as STR profiles (Tridico et al. 2014; Tridico et al. 2017).
A recent study compares the microbiome present both in from human scalp and pubic hairs and discuss the use of these traces in criminal and civil investigations (Tridico et al. 2014). The majority of hairs recovered in forensic investigations correspond to scalp hairs while pubic hairs are used in sexual assault cases. According to the authors, data collected from the microbiome analysis allowed not only the individualization of the contributor but also to discriminate between the two sources of hair. The aforementioned study also revealed that cohabiting and sexually active couples interchange microbiomes during sexual intercourse. Additionally, the microbiome present on scalp hairs has been shown as more prone to compositional oscillations than pubic hairs (Tridico et al. 2014).
Body fluid microbiome
At crime scenes, the most commonly found body fluids are blood, semen and saliva. However, other fluids (e.g., vaginal fluid, urine and sweat) can also be present and perform meaningful roles in investigations, namely as DNA evidence (Virkler and Lednev 2009). It has been proposed that each body fluid type presents a specific composition of microorganisms that can be used as bioindicators, being possible to infer the type of body fluid present based on its microbial composition (Hanssen et al. 2017). For instance, saliva could be identified by detection of specific bacteria, such as Veillonella atypica, Streptococcus mutans and Streptococcus salivarius; while vaginal fluid could be identified by detection of specific bacteria, such as Lactobacillus crispatus and Lactobacillus gasseri (Choi et al. 2014; Giampaoli et al. 2017).
When considering lower taxonomic levels (i.e., genus, species and strains), the human microbiome is utterly characteristic from a given individual (Blaser 2010). Therefore, the fine resolution of the results acquired from MPS-based techniques will allow to identify suspects comparing the microbiomes or, at least theoretically, establish an association between perpetrators and victims.
Conclusions
Over the preceding decade, the advances in MPS and related techniques have revolutionized science in many numerous fields, from individualized medicine to forensics. Nowadays is possible to sequence complete genomes or complex samples in a few hours at an affordable price. As such, these innovative techniques can be used as a complement to classical microbiological methods in a polyphasic approach (Tomaso and Neubauer 2011).
As previously suggested (Amorim 2010), one of the most prominent breakthroughs associated with microbial forensics might be reached in the cases of sexual offenses, associating offender and victim even several years after the crime has been committed and using as bioindicators sexual transmitted diseases (e.g., HIV and HCV). This possibility was proven in a case in which a step-father was convicted of abusing his 10-year-old stepdaughter, based on the phylogenetic analysis of the HIV transmitted (Banaschak et al. 2000).
However, as previously referred, prior to its wide implementation in forensic investigations, several steps should be considered (Budowle et al. 2014, 2017). Indeed, it is necessary to define standard operational protocols for sample collection, handling and preservation (Budowle et al. 2006). All methods should be further validated, concerning aspects such as sensitivity, specificity, reproducibility, repeatability and limit of detection (Budowle et al. 2014). Finally, the creation of reliable and comprehensive databases is mandatory for the accurate interpretation of the obtained results (Skopp 2010).
Author contribution
Drafting and critical revision of the manuscript: MO, AA.
Funding
This study was funded by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT - Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Akehurst C (1998) Outbreak of hepatitis C associated with two hospitals in Spain. Weekly releases (1997–2007) 2(20):1217
- Allard MW, Wilson M, Brown EW. Genetic and genomic methods of microbial taxonomic assignment. In: Amorim A, Budowle B, editors. Handbook of forensic genetics: biodiversity and heredity in civil and criminal investigation. New Jersey: World Sci; 2017. pp. 535–560. [Google Scholar]
- Amorim A. Introduction to the special issue on forensic genetics: non-human DNA. Open Forensic Sci J. 2010;3:6–8. doi: 10.2174/1874402801003010006. [DOI] [Google Scholar]
- Arenas M, Pereira F, Oliveira M, Pinto N, Lopes AM, Gomes V, Carracedo A, Amorim A. Forensic genetics and genomics: much more than just a human affair. PLoS Genet. 2017;13(9):e1006960. doi: 10.1371/journal.pgen.1006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMx130042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward RB, Alwan A. Polio in Syria. Lancet. 2014;383(9916):489–491. doi: 10.1016/S0140-6736(14)60132-X. [DOI] [PubMed] [Google Scholar]
- Ballantyne B. The forensic diagnosis of acute cyanide poisoning. In: Ballantyne B, editor. Forensic toxicology. Bristol: John Wright and Sons Limited; 2013. pp. 99–113. [Google Scholar]
- Ballantyne B, Bright J, Williams P. The post-mortem rate of transformation of cyanide. Forensic Sci. 1974;3:71–76. doi: 10.1016/0300-9432(74)90009-0. [DOI] [PubMed] [Google Scholar]
- Banaschak S, Werwein M, Brinkmann B, Hauber I. Human immunodeficiency virus type 1 infection after sexual abuse: value of nucleic acid sequence analysis in identifying the offender. Clin Infect Dis. 2000;31(4):1098–1100. doi: 10.1086/318152. [DOI] [PubMed] [Google Scholar]
- Bano H, Mohamed O, Bensaheb F, Behl S, Nazir M. Evaluating emerging technologies applied in forensic analysis. Int J Eng Res Sci Technol. 2015;4(3):146–171. [Google Scholar]
- Blaser MJ. Harnessing the power of the human microbiome. Proc Natl Acad Sci U S A. 2010;107(14):6125–6126. doi: 10.1073/pnas.1002112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges V, Nunes A, Sampaio D, Vieira L, Gomes JP (2016) Phylogenomic characterization of the causative strain of one of the largest worldwide outbreaks of legionnaires’ disease occurred in Portugal in 2014. In: 11th international meeting on microbial epidemiological markers (IMMEM XI), 9–12 March 2016
- Børsting C, Morling N. Next generation sequencing and its applications in forensic genetics. Forensic Sci Int Genet. 2015;18:78–89. doi: 10.1016/j.fsigen.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Bosch X. Hepatitis C outbreak astounds Spain. Lancet. 1998;351(9113):1415. doi: 10.1016/S0140-6736(05)79465-4. [DOI] [Google Scholar]
- Boumba VA, Economou V, Kourkoumelis N, Gousia P, Papadopoulou C, Vougiouklakis T. Microbial ethanol production: experimental study and multivariate evaluation. Forensic Sci Int. 2012;215(1–3):189–198. doi: 10.1016/j.forsciint.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Boumba VA, Kourkoumelis N, Gousia P, Economou V, Papadopoulou C, Vougiouklakis T. Modeling microbial ethanol production by E. coli under aerobic/anaerobic conditions: applicability to real postmortem cases and to postmortem blood derived microbial cultures. Forensic Sci Int. 2013;232(1–3):191–198. doi: 10.1016/j.forsciint.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Boumba VA, Ziavrou KS, Vougiouklakis T. Biochemical pathways generating post-mortem volatile compounds co-detected during forensic ethanol analyses. Forensic Sci Int. 2008;174(2):133–151. doi: 10.1016/j.forsciint.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Boyce JM, Havill NL, Dumigan DG, Golebiewski M, Balogun O, Rizvani R. Monitoring the effectiveness of hospital cleaning practices by use of an adenosine triphosphate bioluminescence assay. Infect Control Hosp Epidemiol. 2009;30(7):678–684. doi: 10.1086/598243. [DOI] [PubMed] [Google Scholar]
- Brooke JS, Annand JW, Angela Hammer M, Shulman ST. Investigation of bacterial pathogens on 70 frequently used environmental surfaces in a large urban US university. J Environ Health. 2009;71(6):17. [PubMed] [Google Scholar]
- Brooks EG, Gill JR, Buchsbaum R, Utley S, Sathyavagiswaran L, Peterson DC. Testing for infectious diseases in sudden unexpected infant death: a survey of medical examiner and coroner offices in the United States. J Pediatr. 2015;167(1):178–182.e1. doi: 10.1016/j.jpeds.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Brown AG. The use of forensic botany and geology in war crimes investigations in NE Bosnia. Forensic Sci Int. 2006;163(3):204–210. doi: 10.1016/j.forsciint.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008;100(1):1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budowle B, Churchill JD, King JL. The next state-of-the-art forensic genetics technology: massively parallel sequencing. In: Amorim A, Budowle B, editors. Handbook of forensic genetics: biodiversity and heredity in civil and criminal investigation. New Jersey: World Sci; 2017. pp. 249–291. [Google Scholar]
- Budowle B, Connell ND, Bielecka-Oder A, Colwell RR, Corbett CR, Fletcher J, Forsman M, Kadavy DR, Markotic A, Morse SA. Validation of high throughput sequencing and microbial forensics applications. Investig Genet. 2014;5(1):9. doi: 10.1186/2041-2223-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budowle B, Johnson MD, Fraser CM, Leighton TJ, Murch RS, Chakraborty R. Genetic analysis and attribution of microbial forensics evidence. Crit Rev Microbiol. 2005;31(4):233–254. doi: 10.1080/10408410500304082. [DOI] [PubMed] [Google Scholar]
- Budowle B, Schutzer SE, Burans JP, Beecher DJ, Cebula TA, Chakraborty R, Cobb WT, Fletcher J, Hale ML, Harris RB. Quality sample collection, handling, and preservation for an effective microbial forensics program. Appl Environ Microbiol. 2006;72(10):6431–6438. doi: 10.1128/AEM.01165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler John M. The future of forensic DNA analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1674):20140252. doi: 10.1098/rstb.2014.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzbach DM. The influence of putrefaction and sample storage on post-mortem toxicology results. Forensic Sci Med Pathol. 2010;6(1):35–45. doi: 10.1007/s12024-009-9130-8. [DOI] [PubMed] [Google Scholar]
- Butzbach DM, Stockham PC, Kobus HJ, Noel Sims D, Byard RW, Lokan RJ, Stewart Walker G. Bacterial degradation of risperidone and paliperidone in decomposing blood. J Forensic Sci. 2013;58(1):90–100. doi: 10.1111/j.1556-4029.2012.02280.x. [DOI] [PubMed] [Google Scholar]
- Byard RW. Lessons to be learnt from the Sally Clark case. Aust J Forensic Sci. 2004;36(1):3–10. doi: 10.1080/00450610409410590. [DOI] [Google Scholar]
- Byard RW. Unexpected infant death: lessons from the Sally Clark case. Med J Aust. 2004;181(1):52–54. doi: 10.5694/j.1326-5377.2004.tb06162.x. [DOI] [PubMed] [Google Scholar]
- Byard RW, Butzbach DM. Issues in the interpretation of postmortem toxicology. Forensic Sci Med Pathol. 2012;8(3):205–207. doi: 10.1007/s12024-011-9278-x. [DOI] [PubMed] [Google Scholar]
- Caddy B, Stead A. Three cases of poisoning involving the drug phenelzine. J Forensic Sci Soc. 1978;18(3–4):207–208. doi: 10.1016/S0015-7368(78)71205-3. [DOI] [PubMed] [Google Scholar]
- Calder IM. An evaluation of the diatom test in deaths of professional divers. Med Sci Law. 1984;24(1):41–46. doi: 10.1177/002580248402400107. [DOI] [PubMed] [Google Scholar]
- Carter DO (2017) Forensic microbiology. John Wiley & Sons
- Castle Jared W., Butzbach Danielle M., Walker G. Stewart, Lenehan Claire E., Reith Frank, Kirkbride K. Paul. Forensic Microbiology. Chichester, UK: John Wiley & Sons, Ltd; 2017. Microbial impacts in postmortem toxicology; pp. 212–244. [Google Scholar]
- Cenciarelli O, Pietropaoli S, Malizia A, Carestia M, D’Amico F, Sassolini A, Di Giovanni D, Rea S, Gabbarini V, Tamburrini A. Ebola virus disease 2013-2014 outbreak in west Africa: an analysis of the epidemic spread and response. Int J Microbiol. 2015;2015:769121. doi: 10.1155/2015/769121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A, Shin K-J, Yang WI, Lee HY. Body fluid identification by integrated analysis of DNA methylation and body fluid-specific microbial DNA. Int J Legal Med. 2014;128(1):33–41. doi: 10.1007/s00414-013-0918-4. [DOI] [PubMed] [Google Scholar]
- Ciesielski C, Marianos D, Ou C-Y, Dumbaugh R, Witte J, Berkelman R, Gooch B, Myers G, Luo C-C, Schochetman G. Transmission of human immunodeficiency virus in a dental practice. Ann Intern Med. 1992;116(10):798–805. doi: 10.7326/0003-4819-116-10-798. [DOI] [PubMed] [Google Scholar]
- Clarke TH, Gomez A, Singh H, Nelson KE, Brinkac LM. Integrating the microbiome as a resource in the forensics toolkit. Forensic Sci Int Genet. 2017;30:141–147. doi: 10.1016/j.fsigen.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyn JM, Hauther KA. Postmortem succession of gut microbial communities in deceased human subjects. PeerJ. 2017;5:e3437. doi: 10.7717/peerj.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-González A, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK, Fierer N. A global atlas of the dominant bacteria found in soil. Science. 2018;359(6373):320–325. doi: 10.1126/science.aap9516. [DOI] [PubMed] [Google Scholar]
- Demanèche S, Schauser L, Dawson L, Franqueville L, Simonet P. Microbial soil community analyses for forensic science: application to a blind test. Forensic Sci Int. 2017;270:153–158. doi: 10.1016/j.forsciint.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Díaz-Palma P, Alucema A, Hayashida G, Maidana N. Development and standardization of a microalgae test for determining deaths by drowning. Forensic Sci Int. 2009;184(1–3):37–41. doi: 10.1016/j.forsciint.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Dickson GC, Poulter RT, Maas EW, Probert PK, Kieser JA. Marine bacterial succession as a potential indicator of postmortem submersion interval. Forensic Sci Int. 2011;209(1–3):1–10. doi: 10.1016/j.forsciint.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummer OH. Postmortem toxicology of drugs of abuse. Forensic Sci Int. 2004;142(2–3):101–113. doi: 10.1016/j.forsciint.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Byard RW. Sudden infant death syndrome: an overview. In: Duncan JR, Byard RW, editors. SIDS sudden infant and early childhood death: the past, the present and the future. Adelaide: University of Adelaide Press; 2018. pp. 15–50. [Google Scholar]
- Dyer C. Pathologist in Sally Clark case suspended from court work. BMJ. 2005;330(7504):1347. doi: 10.1136/bmj.330.7504.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SP. Further evidence for the presence of GHB in postmortem biological fluid: implications for the interpretation of findings. J Anal Toxicol. 2004;28(1):20–26. doi: 10.1093/jat/28.1.20. [DOI] [PubMed] [Google Scholar]
- Elliott S, Lowe P, Symonds A. The possible influence of micro-organisms and putrefaction in the production of GHB in post-mortem biological fluid. Forensic Sci Int. 2004;139(2–3):183–190. doi: 10.1016/j.forsciint.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Faria NR, Azevedo RDSDS, Kraemer MUG, Souza R, Cunha MS, Hill SC, Thézé J, Bonsall MB, Bowden TA, Rissanen I, Rocco IM, Nogueira JS, Maeda AY, Vasami FGDS, Macedo FLL, Suzuki A, Rodrigues SG, Cruz ACR, Nunes BT, Medeiros DBA, Rodrigues DSG, Queiroz ALN, da Silva EVP, Henriques DF, da Rosa EST, de Oliveira CS, Martins LC, Vasconcelos HB, Casseb LMN, Simith DB, Messina JP, Abade L, Lourenço J, Alcantara LCJ, de Lima MM, Giovanetti M, Hay SI, de Oliveira RS, Lemos PDS, de Oliveira LF, de Lima CPS, da Silva SP, de Vasconcelos JM, Franco L, Cardoso JF, Vianez-Júnior JLDSG, Mir D, Bello G, Delatorre E, Khan K, Creatore M, Coelho GE, de Oliveira WK, Tesh R, Pybus OG, Nunes MRT, Vasconcelos PFC. Zika virus in the Americas: early epidemiological and genetic findings. Science. 2016;352(6283):345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rodríguez A, Ballesteros S, De Ory F, Echevarria J, Alvarez-Lafuente R, Vallejo G, Gómez J. Virological analysis in the diagnosis of sudden children death: a medico-legal approach. Forensic Sci Int. 2006;161(1):8–14. doi: 10.1016/j.forsciint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A. 2008;105(46):17994–17999. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N, Lauber CL, Zhou N, McDonald D, Costello EK, Knight R. Forensic identification using skin bacterial communities. Proc Natl Acad Sci U S A. 2010;107(14):6477–6481. doi: 10.1073/pnas.1000162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley SJ, Benbow ME, Javan GT. Potential applications of soil microbial ecology and next-generation sequencing in criminal investigations. Appl Soil Ecol. 2015;88:69–78. doi: 10.1016/j.apsoil.2015.01.001. [DOI] [Google Scholar]
- Finley SJ, Pechal JL, Benbow ME, Robertson B, Javan GT. Microbial signatures of cadaver gravesoil during decomposition. Microb Ecol. 2016;71(3):524–529. doi: 10.1007/s00248-015-0725-1. [DOI] [PubMed] [Google Scholar]
- Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannan BJ, Huttenhower C. Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci U S A. 2015;112(22):E2930–E2938. doi: 10.1073/pnas.1423854112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Tseng C-H, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A. 2007;104(8):2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pardo M, Platero Armero J, De la Rubia Orti J. Anxiolytics and antidepressants: problem or solution. J Neurol Neurosci. 2018;9(1):247. doi: 10.21767/2171-6625.1000247:. [DOI] [Google Scholar]
- Gardy JL, Johnston JC, Sui SJH, Cook VJ, Shah L, Brodkin E, Rempel S, Moore R, Zhao Y, Holt R. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364(8):730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- Gerostamoulos D, Beyer J, Staikos V, Tayler P, Woodford N, Drummer OH. The effect of the postmortem interval on the redistribution of drugs: a comparison of mortuary admission and autopsy blood specimens. Forensic Sci Med Pathol. 2012;8(4):373–379. doi: 10.1007/s12024-012-9341-2. [DOI] [PubMed] [Google Scholar]
- Giampaoli S, DeVittori E, Valeriani F, Berti A, Romano Spica V. Informativeness of NGS analysis for vaginal fluid identification. J Forensic Sci. 2017;62(1):192–196. doi: 10.1111/1556-4029.13222. [DOI] [PubMed] [Google Scholar]
- Gill P, Jeffreys AJ, Werrett DJ. Forensic application of DNA ‘fingerprints’. Nature. 1985;318(6046):577. doi: 10.1038/318577a0. [DOI] [PubMed] [Google Scholar]
- Gjelsvik B, Heyerdahl F, Lunn D, Hawton K. Change in access to prescribed medication following an episode of deliberate self-poisoning: a multilevel approach. PLoS One. 2014;9(5):e98086. doi: 10.1371/journal.pone.0098086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Candelas F. Molecular epidemiology and evolution concepts in microbial forensics. In: Amorim A, Budowle B, editors. Handbook of forensic genetics: biodiversity and heredity in civil and criminal investigation. New Jersey: World Sci; 2017. pp. 561–582. [Google Scholar]
- González-Candelas F, Bracho MA, Wróbel B, Moya A. Molecular evolution in court: analysis of a large hepatitis C virus outbreak from an evolving source. BMC Biol. 2013;11(1):76. doi: 10.1186/1741-7007-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad YH, Lipsitch M, Feldgarden M, Arachchi HM, Cerqueira GC, Fitzgerald M, Godfrey P, Haas BJ, Murphy CI, Russ C, Sykes S, Walker BJ, Wortman JR, Young S, Zeng Q, Abouelleil A, Bochicchio J, Chauvin S, Desmet T, Gujja S, McCowan C, Montmayeur A, Steelman S, Frimodt-Møller J, Petersen AM, Struve C, Krogfelt KA, Bingen E, Weill FX, Lander ES, Nusbaum C, Birren BW, Hung DT, Hanage WP. Genomic epidemiology of the Escherichia coli O104: H4 outbreaks in Europe, 2011. Proc Natl Acad Sci U S A. 2012;109(8):3065–3070. doi: 10.1073/pnas.1121491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham NS, Reich BJ, Pacifici K, Laber EB, Menninger HL, Henley JB, Barberán A, Leff JW, Fierer N, Dunn RR. Fungi identify the geographic origin of dust samples. PLoS One. 2015;10(4):e0122605. doi: 10.1371/journal.pone.0122605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Comparative Sequencing Program NISC, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn A, Pitt SJ. Review paper microbes as forensic indicators. Trop Biomed. 2012;29(3):311–330. [PubMed] [Google Scholar]
- Han E, Kim E, Hong H, Jeong S, Kim J, In S, Chung H, Lee S. Evaluation of postmortem redistribution phenomena for commonly encountered drugs. Forensic Sci Int. 2012;219(1–3):265–271. doi: 10.1016/j.forsciint.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Hanssen EN, Avershina E, Rudi K, Gill P, Snipen L. Body fluid prediction from microbial patterns for forensic application. Forensic Sci Int Genet. 2017;30:10–17. doi: 10.1016/j.fsigen.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Hauther KA, Cobaugh KL, Jantz LM, Sparer TE, DeBruyn JM. Estimating time since death from postmortem human gut microbial communities. J Forensic Sci. 2015;60(5):1234–1240. doi: 10.1111/1556-4029.12828. [DOI] [PubMed] [Google Scholar]
- Hawkins AK, O'Doherty KC. “Who owns your poop?”: insights regarding the intersection of human microbiome research and the ELSI aspects of biobanking and related studies. BMC Med Genet. 2011;4(1):72. doi: 10.1186/1755-8794-4-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Wiltshire PE. Forensic mycology: the use of fungi in criminal investigations. Forensic Sci Int. 2011;206(1–3):1–11. doi: 10.1016/j.forsciint.2010.06.012. [DOI] [PubMed] [Google Scholar]
- He F, Huang D, Liu L, Shu X, Yin H, Li X. A novel PCR–DGGE-based method for identifying plankton 16S rDNA for the diagnosis of drowning. Forensic Sci Int. 2008;176(2–3):152–156. doi: 10.1016/j.forsciint.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Heimesaat MM, Boelke S, Fischer A, Haag L-M, Loddenkemper C, Kühl AA, Göbel UB, Bereswill S. Comprehensive postmortem analyses of intestinal microbiota changes and bacterial translocation in human flora associated mice. PLoS One. 2012;7(7):e40758. doi: 10.1371/journal.pone.0040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen RS, Price LB, Schupp JM, Gillece JD, Kaas RS, Engelthaler DM, Bortolaia V, Pearson T, Waters AE, Upadhyay BP, Shrestha SD, Adhikari S, Shakya G, Keim PS, Aarestrup FM. Population genetics of Vibrio cholerae from Nepal in 2010: evidence on the origin of the Haitian outbreak. MBio. 2011;2(4):e00157–e00111. doi: 10.1128/mBio.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman ES. The approved narrative of the Srebrenica massacre. Int J Semiot Law. 2006;19(4):409–434. doi: 10.1007/s11196-006-9031-z. [DOI] [Google Scholar]
- Herzig CTA, Reagan J, Pogorzelska-Maziarz M, Srinath D, Stone PW. State-mandated reporting of health care-associated infections in the United States: trends over time. Am J Med Qual. 2015;30(5):417–424. doi: 10.1177/1062860614540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys G, Coopman V, Van Varenbergh D, Cordonnier J. Selective culturing and genus-specific PCR detection for identification of Aeromonas in tissue samples to assist the medico-legal diagnosis of death by drowning. Forensic Sci Int. 2012;221(1–3):11–15. doi: 10.1016/j.forsciint.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Jansen HJ, Breeveld FJ, Stijnis C, Grobusch MP. Biological warfare, bioterrorism, and biocrime. Clin Microbiol Infect. 2014;20(6):488–496. doi: 10.1111/1469-0691.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javan GT, Finley SJ, Abidin Z, Mulle JG. The thanatomicrobiome: a missing piece of the microbial puzzle of death. Front Microbiol. 2016;7:225. doi: 10.3389/fmicb.2016.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javan GT, Finley SJ, Can I, Wilkinson JE, Hanson JD, Tarone AM. Human thanatomicrobiome succession and time since death. Sci Rep. 2016;6:29598. doi: 10.1038/srep29598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javan GT, Finley SJ, Smith T, Miller J, Wilkinson JE. Cadaver thanatomicrobiome signatures: the ubiquitous nature of Clostridium species in human decomposition. Front Microbiol. 2017;8:2096. doi: 10.3389/fmicb.2017.02096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama T, Fujisaki S, Takashita E, Xu H, Yamada S, Uchida Y, Neumann G, Saito T, Kawaoka Y, Tashiro M. Genetic analysis of novel avian A (H7N9) influenza viruses isolated from patients in China, February to April 2013. Eurosurveillance. 2013;18(15):20453. [PMC free article] [PubMed] [Google Scholar]
- Kakizaki E, Kozawa S, Matsuda H, Muraoka E, Uchiyama T, Sakai M, Yukawa N. Freshwater bacterioplankton cultured from liver, kidney and lungs of a decomposed cadaver retrieved from a sandy seashore: possibility of drowning in a river and then floating out to sea. Legal Med. 2010;12(4):195–199. doi: 10.1016/j.legalmed.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Kakizaki E, Kozawa S, Tashiro N, Sakai M, Yukawa N. Detection of bacterioplankton in immersed cadavers using selective agar plates. Leg Med (Tokyo) 2009;Suppl 1:S350–S353. doi: 10.1016/j.legalmed.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Kalasinsky KS, Dixon MM, Schmunk GA, Kish S. Blood, brain, and hair GHB concentrations following fatal ingestion. J Forensic Sci. 2001;46(3):728–730. doi: 10.1520/JFS15032J. [DOI] [PubMed] [Google Scholar]
- Khan HA, Baig FK, Mehboob R. Nosocomial infections: epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478–482. doi: 10.1016/j.apjtb.2017.01.019. [DOI] [Google Scholar]
- Kintz P, Villain M, Cirimele V, Ludes B. GHB in postmortem toxicology: discrimination between endogenous production from exposure using multiple specimens. Forensic Sci Int. 2004;143(2–3):177–181. doi: 10.1016/j.forsciint.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007;122(2):160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Segre JA. Skin microbiome: looking back to move forward. J Investig Dermatol. 2012;132(3):933–939. doi: 10.1038/jid.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach GC (2008) Prison for man with HIV who spit on a police officer. New York Times:A16
- Krous HF, Byard RW. Controversies in pediatric forensic pathology. Forensic Sci Med Pathol. 2005;1(1):9–18. doi: 10.1385/FSMP:1:1:009. [DOI] [PubMed] [Google Scholar]
- Kuiper I. Microbial forensics: next-generation sequencing as catalyst: the use of new sequencing technologies to analyze whole microbial communities could become a powerful tool for forensic and criminal investigations. EMBO Rep. 2016;17:1085–1087. doi: 10.15252/embr.201642794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JW, Armbrust KL. Laboratory persistence and fate of fluoxetine in aquatic environments. Environ Toxicol Chem. 2006;25(10):2561–2568. doi: 10.1897/05-613R.1. [DOI] [PubMed] [Google Scholar]
- Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S, Gilbert JA. Hospital-associated microbiota and implications for nosocomial infections. Trends Mol Med. 2015;21(7):427–432. doi: 10.1016/j.molmed.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Lax S, Hampton-Marcell JT, Gibbons SM, Colares GB, Smith D, Eisen JA, Gilbert JA. Forensic analysis of the microbiome of phones and shoes. Microbiome. 2015;3(1):21. doi: 10.1186/s40168-015-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-Y, Eom Y-B. Analysis of microbial composition associated with freshwater and seawater. Biomed Sci Lett. 2016;22(4):150–159. doi: 10.15616/BSL.2016.22.4.150. [DOI] [Google Scholar]
- Lee HC, Palmbach T, Miller MT (2001) Henry Lee’s crime scene handbook. Academic Press
- Lee SY, Woo SK, Lee SM, Ha EJ, Lim KH, Choi KH, Roh YH, Eom YB. Microbiota composition and pulmonary surfactant protein expression as markers of death by drowning. J Forensic Sci. 2017;62(4):1080–1088. doi: 10.1111/1556-4029.13347. [DOI] [PubMed] [Google Scholar]
- Lehman DC. Forensic microbiology. Clin Microbiol Newsl. 2014;36(7):49–54. doi: 10.1016/j.clinmicnews.2014.03.001. [DOI] [Google Scholar]
- Levine B, Blanke R, Valentour J. Postmortem stability of benzodiazepines in blood and tissues. J Forensic Sci. 1983;28(1):102–115. doi: 10.1520/JFS12243J. [DOI] [PubMed] [Google Scholar]
- Lewis T, Loman N, Bingle L, Jumaa P, Weinstock G, Mortiboy D, Pallen MJ. High-throughput whole-genome sequencing to dissect the epidemiology of Acinetobacter baumannii isolates from a hospital outbreak. J Hosp Infect. 2010;75(1):37–41. doi: 10.1016/j.jhin.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, Brady A, Creasy HH, McCracken C, Giglio MG, McDonald D, Franzosa EA, Knight R, White O, Huttenhower C. Strains, functions and dynamics in the expanded human microbiome project. Nature. 2017;550(7674):61–66. doi: 10.1038/nature23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfman S, Hakko H, Mainio A, Riipinen P. Affective disorders and completed suicide by self-poisoning, trend of using antidepressants as a method of self-poisoning. Psychiatry Res. 2017;255:360–366. doi: 10.1016/j.psychres.2017.05.031. [DOI] [PubMed] [Google Scholar]
- Lucci A, Campobasso CP, Cirnelli A, Lorenzini G. A promising microbiological test for the diagnosis of drowning. Forensic Sci Int. 2008;182(1–3):20–26. doi: 10.1016/j.forsciint.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Lunetta P, Penttilä A, Hällfors G. Scanning and transmission electron microscopical evidence of the capacity of diatoms to penetrate the alveolo-capillary barrier in drowning. Int J Legal Med. 1998;111(5):229–237. doi: 10.1007/s004140050159. [DOI] [PubMed] [Google Scholar]
- Madea B (2015) Estimation of the time since death. CRC Press
- Madea B. Methods for determining time of death. Forensic Sci Med Pathol. 2016;12(4):451–485. doi: 10.1007/s12024-016-9776-y. [DOI] [PubMed] [Google Scholar]
- Maile AE, Inoue CG, Barksdale LE, Carter DO. Toward a universal equation to estimate postmortem interval. Forensic Sci Int. 2017;272:150–153. doi: 10.1016/j.forsciint.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Mazarr-Proo S, Kerrigan S. Distribution of GHB in tissues and fluids following a fatal overdose. J Anal Toxicol. 2005;29(5):398–400. doi: 10.1093/jat/29.5.398. [DOI] [PubMed] [Google Scholar]
- McGuire AL, Colgrove J, Whitney SN, Diaz CM, Bustillos D, Versalovic J. Ethical, legal, and social considerations in conducting the human microbiome project. Genome Res. 2008;18(12):1861–1864. doi: 10.1101/gr.081653.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow JF, Altrichter AE, Green JL. Mobile phones carry the personal microbiome of their owners. PeerJ. 2014;2:e447. doi: 10.7717/peerj.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier BM, Stone PW, Gebbie KM. Public health law for the collection and reporting of health care–associated infections. Am J Infect Control. 2008;36(8):537–551. doi: 10.1016/j.ajic.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf JL, Carter DO, Knight R. Microbiology of death. Curr Biol. 2016;26(13):R561–R563. doi: 10.1016/j.cub.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Metcalf JL, Parfrey LW, Gonzalez A, Lauber CL, Knights D, Ackermann G, Humphrey GC, Gebert MJ, Van Treuren W, Berg-Lyons D. A microbial clock provides an accurate estimate of the postmortem interval in a mouse model system. eLIFE. 2013;2:e01104. doi: 10.7554/eLife.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf JL, Xu ZZ, Bouslimani A, Dorrestein P, Carter DO, Knight R. Microbiome tools for forensic science. Trends Biotechnol. 2017;35(9):814–823. doi: 10.1016/j.tibtech.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Metcalf JL, Xu ZZ, Weiss S, Lax S, Van Treuren W, Hyde ER, Song SJ, Amir A, Larsen P, Sangwan N, Haarmann D, Humphrey GC, Ackermann G, Thompson LR, Lauber C, Bibat A, Nicholas C, Gebert MJ, Petrosino JF, Reed SC, Gilbert JA, Lynne AM, Bucheli SR, Carter DO, Knight R. Microbial community assembly and metabolic function during mammalian corpse decomposition. Science. 2016;351(6269):158–162. doi: 10.1126/science.aad2646. [DOI] [PubMed] [Google Scholar]
- Metzker ML, Mindell DP, Liu X-M, Ptak RG, Gibbs RA, Hillis DM. Molecular evidence of HIV-1 transmission in a criminal case. Proc Natl Acad Sci U S A. 2002;99(22):14292–14297. doi: 10.1073/pnas.222522599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monden R, Roest AM, van Ravenzwaaij D, Wagenmakers E-J, Morey R, Wardenaar KJ, de Jonge P. The comparative evidence basis for the efficacy of second-generation antidepressants in the treatment of depression in the US: a Bayesian meta-analysis of Food and Drug Administration reviews. J Affect Disord. 2018;235:393–398. doi: 10.1016/j.jad.2018.04.040. [DOI] [PubMed] [Google Scholar]
- Morentin B, Suárez-Mier MP, Aguilera B, Arrieta J, Audicana C, Fernández-Rodríguez A. Clinicopathological features of sudden unexpected infectious death: population-based study in children and young adults. Forensic Sci Int. 2012;220(1–3):80–84. doi: 10.1016/j.forsciint.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Moriya F, Hashimoto Y. Tissue distribution of nitrazepam and 7-aminonitrazepam in a case of nitrazepam intoxication. Forensic Sci Int. 2003;131(2–3):108–112. doi: 10.1016/S0379-0738(02)00421-8. [DOI] [PubMed] [Google Scholar]
- Muchmore S (2013) Former dentist faces another lawsuit. Tulsa World 18
- Oliveira M, Arenas M, Amorim A. New trends in microbial epidemiology: can an old dog learn new tricks? Ann Microbiol Immunol. 2018;1(1):1–7. [Google Scholar]
- Parkinson RA, Dias K-R, Horswell J, Greenwood P, Banning N, Tibbett M, Vass AA. Microbial community analysis of human decomposition on soil. In: Ritz K, Dawson L, Miller D, editors. Criminal and environmental soil forensics. Amsterdam: Springer; 2009. pp. 379–394. [Google Scholar]
- Pattnaik P, Sekhar K. Forensics for tracing microbial signatures: biodefence perspective and preparedness for the unforeseen. Indian J Biotecnhol. 2008;7:23–31. [Google Scholar]
- Pavlin JA. Epidemiology of bioterrorism. Emerg Infect Dis. 1999;5(4):528–530. doi: 10.3201/eid0504.990412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounder DJ, Hartley AK, Watmough PJ. Postmortem redistribution and degradation of dothiepin. Human case studies and an animal model. Am J Forensic Med Pathol. 1994;15(3):231–235. doi: 10.1097/00000433-199409000-00010. [DOI] [PubMed] [Google Scholar]
- Rácz E, Könczöl F, Tóth D, Patonai Z, Porpáczy Z, Kozma Z, Poór VS, Sipos K. PCR-based identification of drowning: four case reports. Int J Legal Med. 2016;130(5):1303–1307. doi: 10.1007/s00414-016-1359-7. [DOI] [PubMed] [Google Scholar]
- Rasko DA, Worsham PL, Abshire TG, Stanley ST, Bannan JD, Wilson MR, Langham RJ, Decker RS, Jiang L, Read TD, Phillippy AM, Salzberg SL, Pop M, Van Ert MN, Kenefic LJ, Keim PS, Fraser-Liggett CM, Ravel J. Bacillus anthracis comparative genome analysis in support of the Amerithrax investigation. Proc Natl Acad Sci U S A. 2011;108(12):5027–5032. doi: 10.1073/pnas.1016657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond F, Ouameur AA, Déraspe M, Iqbal N, Gingras H, Dridi B, Leprohon P, Plante P-L, Giroux R, Bérubé È Frenette J, Boudreau DK, Simard JL, Chabot I, Domingo MC, Trottier S, Boissinot M, Huletsky A, Roy PH, Ouellette M, Bergeron MG, Corbeil J. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016;10(3):707–720. doi: 10.1038/ismej.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MD, Drummer OH. Postmortem drug metabolism by bacteria. J Forensic Sci. 1995;40(3):382–386. doi: 10.1520/JFS13791J. [DOI] [PubMed] [Google Scholar]
- Robertson M, Drummer O. Stability of nitrobenzodiazepines in postmortem blood. J Forensic Sci. 1998;43(1):5–8. [PubMed] [Google Scholar]
- Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AK, Kent AD, Daroub SH, Camargo FA, Farmerie WG, Triplett EW. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007;1(4):283–290. doi: 10.1038/ismej.2007.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel N, Rohleder NH, Koerdt S, Wagenpfeil S, Härtel-Petri R, Wolff K-D, Kesting MR. Sympathomimetic effects of chronic methamphetamine abuse on oral health: a cross-sectional study. BMC Oral Health. 2016;16(1):59. doi: 10.1186/s12903-016-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre C, Bartoli C, Baillif-Couniou V, Leonetti G, Pelissier-Alicot A-L. Post mortem redistribution of drugs: current state of knowledge. Curr Pharm Des. 2017;23(36):5530–5541. doi: 10.2174/1381612823666170622111739. [DOI] [PubMed] [Google Scholar]
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31(1):107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Schmedes SE, Sajantila A, Budowle B. Expansion of microbial forensics. J Clin Microbiol. 2016;54:1964–1974. doi: 10.1128/JCM.00046-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmedes SE, Woerner AE, Budowle B. Forensic human identification using skin microbiomes. Appl Environ Microbiol. 2017;83(22):e01672–e01617. doi: 10.1128/AEM.01672-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürch AC, Siezen RJ. Genomic tracing of epidemics and disease outbreaks. Microb Biotechnol. 2010;3(6):628–633. doi: 10.1111/j.1751-7915.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shama G, Malik DJ. The uses and abuses of rapid bioluminescence-based ATP assays. Int J Hyg Environ Health. 2013;216(2):115–125. doi: 10.1016/j.ijheh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Sijen T. Molecular approaches for forensic cell type identification: on mRNA, miRNA, DNA methylation and microbial markers. Forensic Sci Int Genet. 2015;18:21–32. doi: 10.1016/j.fsigen.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Sitthiwong N, Ruangyuttikarn W, Vongvivach S, Peerapornpisal Y. Detection and identification of diatoms in tissue samples of drowning victims. Chiang Mai J Sci. 2014;41(5.1):1020–1031. [Google Scholar]
- Sjödin A, Broman T, Melefors Ö, Andersson G, Rasmusson B, Knutsson R, Forsman M. The need for high-quality whole-genome sequence databases in microbial forensics. Biosecur Bioterror. 2013;11(S1):S78–S86. doi: 10.1089/bsp.2013.0007. [DOI] [PubMed] [Google Scholar]
- Skopp G. Postmortem toxicology. Forensic Sci Med Pathol. 2010;6(4):314–325. doi: 10.1007/s12024-010-9150-4. [DOI] [PubMed] [Google Scholar]
- Skory CD, Freer SN, Bothast RJ. Screening for ethanol-producing filamentous fungi. Biotechnol Lett. 1997;19(3):203–206. doi: 10.1023/A:1018337003433. [DOI] [Google Scholar]
- Smith SM, Eng R, Padberg JF. Survival of nosocomial pathogenic bacteria at ambient temperature. J Med. 1996;27(5–6):293–302. [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459(7250):1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Sodhi K, Shrivastava A, Arya M, Kumar M. Knowledge of infection control practices among intensive care nurses in a tertiary care hospital. J Infect Public Health. 2013;6(4):269–275. doi: 10.1016/j.jiph.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Spitz WU, Schneider V. The significance of diatoms in the diagnosis of death by drowning. J Forensic Sci. 1964;9(1):11. [PubMed] [Google Scholar]
- Stevens H. The stability of some drugs and poisons in putrefying human liver tissues. Sci Justice. 1984;24(6):577–589. doi: 10.1016/s0015-7368(84)72350-4. [DOI] [PubMed] [Google Scholar]
- Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vázquez-Baeza Y, González A, Morton JT, Mirarab S, Xu ZZ, Jiang L, Haroon MF, Kanbar J, Zhu Q, Song SJ, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R, The Earth Microbiome Project Consortium A communal catalogue reveals Earth’s multiscale microbial diversity. Nature. 2017;551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie J, Uchigasaki S, Haseba T, Ohno Y, Isahai I, Oshida S. Direct and rapid PCR amplification using digested tissues for the diagnosis of drowning. Electrophoresis. 2010;31(14):2411–2415. doi: 10.1002/elps.200900754. [DOI] [PubMed] [Google Scholar]
- Tomaso Herbert, Neubauer Heinrich. Forensic Medicine - From Old Problems to New Challenges. 2011. Forensic Microbiology. [Google Scholar]
- Tridico SR, Murray DC, Addison J, Kirkbride KP, Bunce M. Metagenomic analyses of bacteria on human hairs: a qualitative assessment for applications in forensic science. Investig Genet. 2014;5(1):16. doi: 10.1186/s13323-014-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tridico Silvana R., Murray Dáithí C., Bunce Michael, Kirkbride K. Paul. Forensic Microbiology. Chichester, UK: John Wiley & Sons, Ltd; 2017. DNA profiling of bacteria from human hair; pp. 358–375. [Google Scholar]
- Van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends Genet. 2014;30(9):418–426. doi: 10.1016/j.tig.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Virkler K, Lednev IK. Analysis of body fluids for forensic purposes: from laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci Int. 2009;188(1–3):1–17. doi: 10.1016/j.forsciint.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Wells D. Drug administration and sexual assault: sex in a glass. Sci Justice. 2001;41(3):197–199. doi: 10.1016/S1355-0306(01)71890-4. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xie B, Yan J. Application of next-generation sequencing technology in forensic science. Genomics, Proteomics Bioinformatics. 2014;12(5):190–197. doi: 10.1016/j.gpb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemitsu K, Pounder DJ. Postmortem changes in blood tranylcypromine concentration: competing redistribution and degradation effects. Forensic Sci Int. 1993;59(2):177–184. doi: 10.1016/0379-0738(93)90157-6. [DOI] [PubMed] [Google Scholar]
- Young J. M., Austin J. J., Weyrich L. S. Soil DNA metabarcoding and high-throughput sequencing as a forensic tool: considerations, potential limitations and recommendations. FEMS Microbiology Ecology. 2016;93(2):fiw207. doi: 10.1093/femsec/fiw207. [DOI] [PubMed] [Google Scholar]
- Young JM, Rawlence NJ, Weyrich LS, Cooper A. Limitations and recommendations for successful DNA extraction from forensic soil samples: a review. Sci Justice. 2014;54(3):238–244. doi: 10.1016/j.scijus.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Young JM, Weyrich LS, Breen J, Macdonald LM, Cooper A. Predicting the origin of soil evidence: high throughput eukaryote sequencing and MIR spectroscopy applied to a crime scene scenario. Forensic Sci Int. 2015;251:22–31. doi: 10.1016/j.forsciint.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Young JM, Weyrich LS, Cooper A. Forensic soil DNA analysis using high-throughput sequencing: a comparison of four molecular markers. Forensic Sci Int Genet. 2014;13:176–184. doi: 10.1016/j.fsigen.2014.07.014. [DOI] [PubMed] [Google Scholar]