Abstract
In the sea urchin larva, most neurons lie within an ectodermal region called the ciliary band. Our understanding of the mechanisms of specification and patterning of these peripheral ciliary band neurons is incomplete. Here, we first examine the gene regulatory landscape from which this population of neural progenitors arise in the neuroectoderm. We show that ciliary band neural progenitors first appear in a bilaterally symmetric pattern on the lateral edges of chordin expression in the neuroectoderm. Later in development, these progenitors appear in a salt-and-pepper pattern in the ciliary band where they express soxC, and prox, which are markers of neural specification, and begin to express synaptotagminB, a marker of differentiated neurons. We show that the ciliary band expresses the acid sensing ion channel gene asicl, which suggests that ciliary band neurons control the larva’s ability to discern touch sensitivity. Using a chemical inhibitor of MAPK signaling, we show that this signaling pathway is required for proper specification and patterning of ciliary band neurons. Using live imaging, we show that these neural progenitors undergo small distance migrations in the embryo. We then show that the normal swimming behavior of the larvae is compromised if the neurogenesis pathway is perturbed. The developmental sequence of ciliary band neurons is very similar to that of neural crest-derived sensory neurons in vertebrates and may provide insights into the evolution of sensory neurons in deuterostomes.
Keywords: neurogenin, sea urchin, ciliary band, peripheral neurons, neuronal progenitor
Introduction
The phylogenetic position of echinoderms, a basally-branching deuterostome group which includes sea urchins, is useful for revealing shared characteristics of deuterostome development. In the last decade, there has been interest in understanding the mechanisms of nervous system development in the sea urchin embryo in order to increase our knowledge about the evolution of animal nervous systems. The sea urchin embryo produces neurons in three domains: the apical organ which includes a population of serotonergic neurons, the ciliary band which becomes rich with sensory neurons, and the endoderm which has the capacity to specify neurons that are thought to govern movement of food through the gut (Bisgrove and Burke, 1986; Nakajima et al., 2004; Wei et al., 2011). The apical organ is hypothesized to act as a central nervous system and processing center of the sea urchin larva (Garner et al., 2016; Hinman and Burke, 2018). Neurons of the ciliary band and digestive system together are considered the embryo’s peripheral nervous system. Recently it was shown that neurons within each of these domains are specified uniquely, although there is modest overlap between the developmental trajectories (McClay et al., 2018). In the last ten years, much attention has focused on the specification of serotonergic neurons in the apical organ. Early efforts were to learn how signaling establishes this region to be competent for neural specification (Wei et al., 2016, 2009; Yaguchi et al., 2016, 2006). This was followed by efforts to identify transcription factors with roles in specifying serotonergic neurons (Hinman and Burke, 2018; Slota and McClay, 2018; Wei et al., 2016, 2009; Yaguchi et al., 2012, 2011). As the number of transcription factors associated with sea urchin neurogenesis grew, more recent efforts moved toward identifying the gene regulatory networks that govern neural development in the apical organ (Wei et al., 2016).
Less attention has been paid to the development of the peripheral nervous system of the sea urchin embryo. Ciliary band neurons have been described as sensory neurons that respond to environmental stimuli and control the beating of ciliary cells (Burke et al., 2014; Garner et al., 2016; Nakajima et al., 2004). Ciliary motion is essential for larval survival because coordinated ciliary beating allows the larva to swim in a directed fashion. Sensory neurons within the ciliary band also allow the larva to detect contact with food particles and coordinate an immediate ciliary reversal behavior that directs particles toward the mouth (Nakajima et al., 2004; Strathmann, 2007). At the molecular level, a number of transcription factors, signals and markers have been identified in several studies and these have been proposed to participate in specification and differentiation of ciliary band neurons (Garner et al., 2016; McClay et al., 2018; Slota et al., 2019). How these and other molecules interact with each other as a gene regulatory network to control aspects of neurogenesis of sensory neurons in the ciliary band remains to be understood in detail. Morphological and pharmacological studies suggest that several types of neurons are present in the ciliary band (reviewed in (Hinman and Burke, 2018)), indicating that several pathways of specification may be present there. This presents a challenge to understand the specification of sensory neurons if several types of neurons are developing coincidentally in the same region.
Neural progenitors in the ciliary band express the transcription factor neurogenin (ngn) and appear to be first specified in a bilaterally symmetric pattern in the ventral ectoderm, and later in time are scattered throughout the ciliary band (Slota and McClay, 2018). This pattern in which peripheral neural progenitors are first specified in one place and then later are found in a different place throughout the embryo is reminiscent of sensory neurogenesis in other deuterostomes, and led us to investigate properties of these cells further. To do this, we were guided by a number of prior studies in the sea urchin and also by studies in other deuterostomes, especially in vertebrates where studies of sensory neuron development are extensive. Here, we use the sea urchin Lytechinus variegatus to show that ngn-expressing neural progenitors are born outside of the ciliary band on the lateral edges of chordin expression in the neuroectoderm. Later in development, we show that in the ciliary band, there is co-expression of neurogenin with soxC, synaptotagminB, and prox, providing further evidence that they of neural origin. The larval ciliary band expresses the acid sensing ion channel asicl, which suggests that these neurons in the ciliary band provide a sensory function and modulate touch sensitivity. We then show that ciliary band neurons require MAPK signaling for their specification and that these progenitors migrate small distances in the embryo. Finally, we show that when we block the specification of ciliary band neurons the larvae display an abnormal swimming behavior. Interestingly, these data suggest that neurons of the ciliary band utilize many of the same developmental processes that are used by vertebrate sensory neurons for their specification and differentiation.
Results
Ciliary band neural progenitors are born in the neuroectoderm
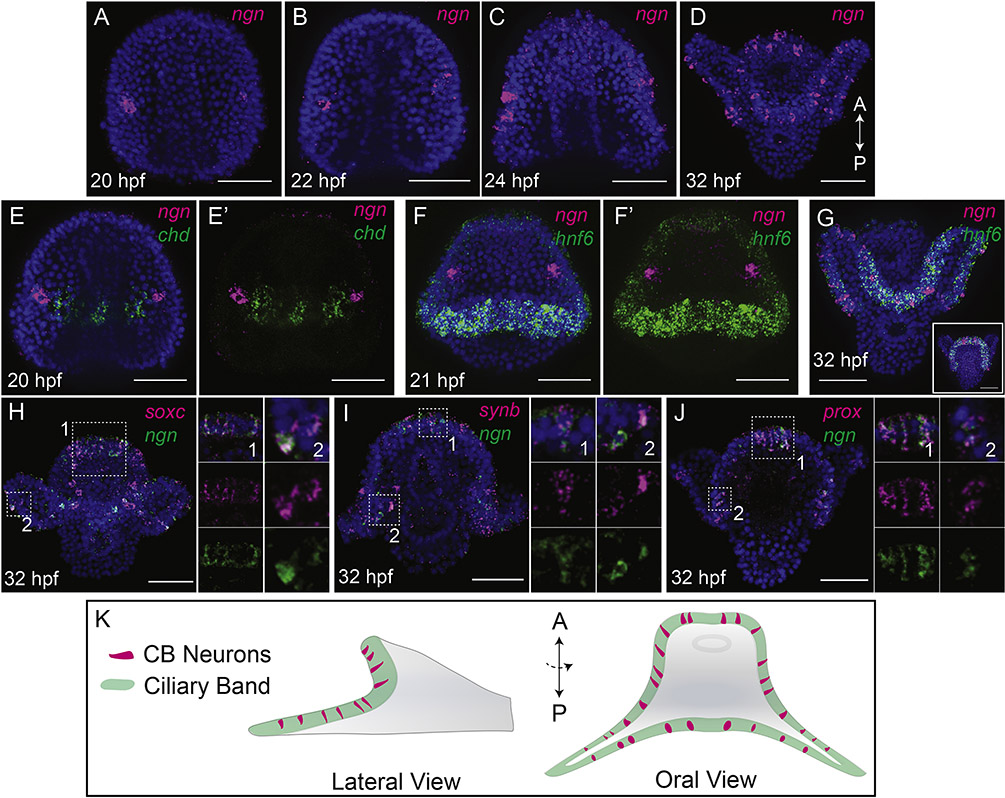
To investigate the developmental origins of ciliary band neurons, we focused on the sequential expression of the transcription factor neurogenin (ngn). The embryonic nervous system of the sea urchin is specified in an area of ectoderm with broad neurogenic potential, the ventral ectoderm, which is marked, in part, by expression of nodal and chordin (Bradham et al., 2009; Duboc et al., 2004). Previous work has shown that neurogenin expression begins at late gastrula stage in the neuroectoderm (Slota and McClay, 2018). At late gastrula stage, neurogenin-expressing neural progenitors first appear in 2 patches within the ventral ectoderm outside the ciliary band that are on the borders of chordin expression (Fig 1A–B, E–E’). At prism stage (21 hpf) cells that express ngn are close to, but not within the nascent ciliary band as marked by expression of hnf6 (Fig. 1F,F’). By 32 hpf ngn-expressing cells are no longer on the edges of chordin expression domain, but are found scattered throughout the ciliary band which houses the peripheral nervous system (Fig. 1C–D, G, K).
Figure 1: Regulatory landscape of ciliary band neurons.
(A-D) Maximum intensity projections of whole mount in situ hybridizations (WMISH) show ngn expression. At 20 hpf (late gastrula stage) and 22hpf (prism stage) ngn is expressed in bilateral patches in the neuroectoderm (A,B). Ngn expressing cells enter the ciliary band by 24 hpf (early pluteus stage), and by 32 hpf (pluteus stage) ngn is found in a salt-and-pepper pattern throughout the ciliary band. (E-E’) Maximum intensity projection of double WMISH at 20 hpf shows ngn-expressing neural cells are bilaterally symmetric in the neuroectoderm on the borders of chordin expression. (F-F’) Maximum intensity projection WMISH at prism stage shows ngn expression is in cells in the neuroectoderm outside the ciliary band, marked here by hnf6 expression. (G) By 32 hpf ngn neural cells are inside the ciliary band, within the expression domain of hnf6. Inset image shows an aboral view of a different embryo. (H-I) Maximum intensity projection WMISH shows co-expression of some ngn-expressing cells with soxC (H), synb (I), and prox (J) in the ciliary band. Panels in (H-J) show combined and split single confocal slices of the region highlighted by the white boxes. ((K) Schematic showing lateral and oral views of a pluteus larva. Green marks the ciliary band, magenta marks ngnexpressing neural cells.
To confirm that these cells within the ciliary band do in fact have a neural identity, we performed double in situ hybridizations with several neuronal markers. When ngn-expressing cells are within the ciliary band, ngn is co-expressed with soxC in some cells, a transcription factor that is established as an early specifier of neurons (Garner et al., 2016; McClay et al., 2018) (Fig. 1H). Synaptotagmin b (synb), a marker of neural differentiation (Burke et al., 2006), and prospero (prox), a transcription factor expressed in differentiating neurons in many animals (Choksi et al., 2006; Gonçalves et al., 2016), also are localized in ngn-expressing cells in the ciliary band (Fig. 1I,J) which provides further evidence that ngn-expressing cells, which are first specified within the neuroectoderm differentiate into neurons of the ciliary band. Of note, not every cell that expresses ngn in the ciliary band co-express soxC, synb, and prox, at the same time which suggests that differentiation is not synchronized in this population. In addition, earlier studies have shown co-expression of ngn with soxC and delta in the neuroectoderm (McClay et al., 2018; Slota and McClay, 2018), and co-expression of ngn and chat in the ciliary band (Slota and McClay, 2018). Consequently, the neurons that previously were reported in the ciliary band based on anatomical features (reviewed by (Hinman and Burke, 2018), and shown to express soxC, soxB2, elav, brn124 and SynB (Garner et al., 2016), are first specified at the end of gastrulation in the ventral ectoderm and later differentiate in the ciliary band.
Ciliary band neurons likely modulate touch sensitivity and require MAPK signaling for patterning
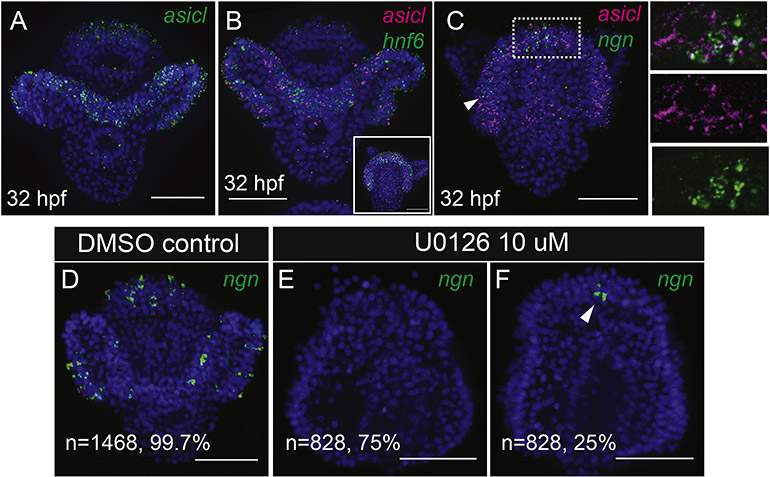
Ciliary band neurons of the sea urchin embryo have been described as afferent sensory neurons (Burke et al., 2014; Hinman and Burke, 2018). As there is a large understanding of sensory neuron development from vertebrate models, we decided to investigate whether properties of vertebrate sensory neurons are shared with sea urchin ciliary band neurons. Certain populations of sensory neurons of chordate organisms, including dorsal root ganglion neurons of vertebrates and tunicate bipolar tail neurons, express acid sensing ion channel (asic) genes that aid in depolarizing cells in response to mechanical, thermal and chemical stimuli (Deval and Lingueglia, 2015; Lingueglia, 2007; Stolfi et al., 2015). To determine whether an asic ortholog is expressed in the sea urchin peripheral nervous system and to get an idea of the function of ciliary band neurons, we cloned an asic ortholog, asicl and performed in situ hybridizations. In Lytechinus, asicl is expressed diffusely in the ciliary band in a pattern similar to the ciliary band marker hnf6 (Fig 2A–B, Supp. Fig 1A–E). Ngn-expressing neural progenitors are also found within the ciliary band at the time asicl is expressed there, although asicl is expressed in a diffuse pattern in throughout the ciliary band rather than in discreet cells like ngn expression (Fig 2C), This suggests that these ngn-expressing neurons may serve a sensory function much like vertebrate spinal neurons and tunicate bipolar tail neurons.
Figure 2: The ciliary band expresses asicl and ciliary band neuronal progenitors require MAPK signaling.
A) Maximum intensity projection WMISH shows asicl expression. (B) Maximum intensity projection WMISH shows asicl expression is in the ciliary band, marked here by hnf6. Inset image shows an aboral view of a different embryo. (C) Maximum intensity projection WMISH shows ngn-expressing neural cells are within the ciliary band at the time that asicl is expressed there. Arrowhead show ngn-expressing cells. Panels in (C) show combined and split single apotome slices of the region highlighted by the white box. (D-F) Maximum intensity projections of whole mount in situ hybridizations (WMISH) of ngn. Out of 1468 control embryos, 99.7% show ngn-expressing cells scattered throughout the ciliary band. Ngn expression is lost in 75% of embryos treated with U0126 at hatched blastula stage. In 25% of embryos treated with U0126 at hatched blastula, ngn expression is reduced to 1–2 cells in the ciliary band.
We next probed whether ciliary band neurons require similar signaling pathways as vertebrate sensory neurons for their specification. Previously it was shown that ciliary band neurons are responsive to Delta/Notch signaling, as treatment with a Delta morpholino causes an increase in cholinergic neurons in the ciliary band (Slota and McClay, 2018). We asked whether the ngn-expressing cells require MAPK/Erk signaling by inhibition of that pathway with the drug U0126. Treatment of embryos with U0126 at hatched blastula stage, which is at the onset of neurogenesis, eliminated ngn expression from the ciliary band in most embryos (Fig 2D–F). Furthermore, embryos treated with U0126 also lack axonal tracts in the ciliary band as shown with L1 (NgCAM) antibody staining (Supp Fig 2). This suggests that specification and patterning of ciliary band neurons is dependent on MAPK/ERK signaling and that this pathway is required for sea urchin sensory neuron specification.
Ciliary band neural progenitors undergo small migrations
In vertebrates, peripheral neural progenitors derived from the neural crest migrate extensively throughout the embryo. Sensory neurons in tunicates and in amphioxus have also been shown to be migratory (Kaltenbach et al., 2009; Stolfi et al., 2015; Yu, 2010). The pattern of ngn expression, originating bilaterally in two patches of ventral ectoderm at sites outside of ciliary band and then later within the ciliary band itself suggests that these neural precursors might also undergo cell migration, although this has not been reported before. Furthermore, some ngn-expressing neuronal progenitors in the anterior ciliary band express the transcription factor snail which has been shown to induce epithelial to mesenchymal transitions and migratory cell behaviors in the neural crest and other embryonic types (reviewed in (Nieto, 2002)) (Supp. Fig 1F). In a previous study, we sought evidence of neural cell migration in the sea urchin using a lineage-based analysis but failed to observe significant long range migrations of neural precursors (McClay et al., 2018). That analysis used transplanted blastomeres that were labelled with a fluorescent marker. Progeny of that future neuroectoderm cell that had been transplanted at the 32–60 cell stage developed into neurons, but in all cases those fluorescently labeled neurons remained within the patch of transplanted progeny. That analysis, therefore, ruled out long range cell migrations of the sort used by neural crest in formation of the sympathetic and parasympathetic nervous systems. However, it did not rule out shorter distance migrations of these neural progenitors to their final destinations within the ciliary band.
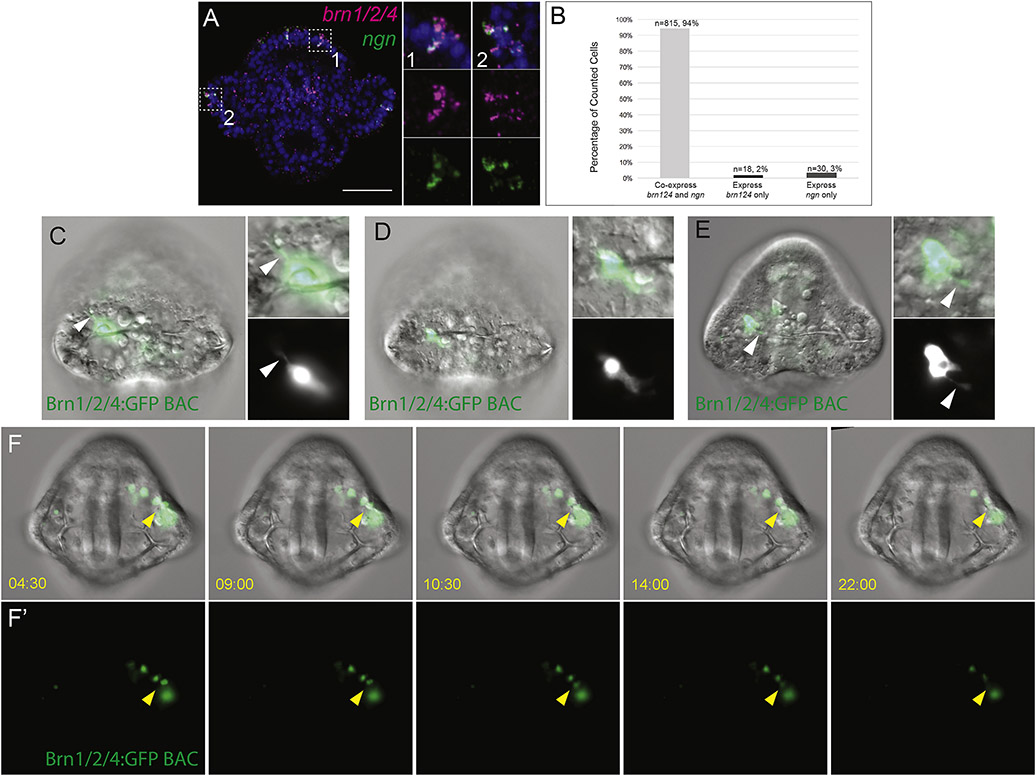
To approach that question another way and address the possibility that short distance migrations occur in the sensory neuron progenitors, we turned to BAC-expression analysis. Single-cell L. variegatus zygotes were injected with a S. purpuratus Brn1/2/4 GFP BAC reporter. Brn1/2/4 has been shown to be expressed by differentiating neural progenitors in S. purpuratus and L. variegatus (Garner et al., 2016; McClay et al., 2018). By double in situ hybridization, we show that in L. variegatus, 94% of cells in the ciliary band that express brn1/2/4 also express ngn (n=68 embryos) (Fig 3A,B). Injecting the S. purpuratus Brn1/2/4 GFP BAC into L. variegatus results in a mosaic expression that recapitulates Lv-brn1/2/4 expression by in situ hybridization (Fig 3C–E). Live imaging of embryos injected with the Brn1/2/4 GFP BAC at early pluteus stages showed neural cells undergoing small-scale migration to reach their final destination in the ciliary band (n=4 embryos) (Fig 3F–F’, Supplementary Videos 1–4). Injection of the Brn124-GFP BAC also enabled us to confirm that the GFP-expressing cells extend neurites, providing evidence that these cells are indeed neurons (Fig. 3C–E).
Figure 3: Ciliary band neural progenitors project neurites and migrate small distances.
(A) Whole mount in situ hybridization show that neural progenitors co-express ngn with brn1/2/4 in neural progenitors in the ciliary band. A single confocal section is shown. Side panels show multichannel and single channel images of highlighted regions. (B) Quantifications of cell counts done on double whole mount in situ hybridizations for ngn and brn1/2/4. A total of 68 embryos were assessed (embryos were either 26, 28, 30, or 32 hpf) and cells that expressed both genes or only ngn or brn1/2/4 were counted and scored as such. “n” represents the number of cells counted with the given condition. (C-E) Shows still images of embryos injected with Brn1/2/4 GFP BAC displaying neurites. Inset images show combined or split channel of the GFP expressing cell. Arrowhead indicates neurite projections. (F-F’) Shows still images from Supplementary Video 1 of an embryo injected with Brn1/2/4 GFP BAC. (F) Shows stills of combined DIC and GFP channels. (F’) Only the GFP fluorescent channel is shown. Time given in min:sec. Arrowhead indicates cell undergoing migration.
Ciliary band neurons are necessary for swimming behaviour
Given that it has been hypothesized that ciliary band neurons are necessary for coordination of swimming behaviour, we performed a final functional test using the MAPK inhibitor. Wildtype Lytechinus larvae swim with the arms directed upward toward the water’s surface. When the larvae come in contact with the imaging chamber or with the air-water interface, the cilia coordinately reverse and the larva falls downward in the water column. A few seconds after the ciliary reversal and fall through the water column, the larvae again begin their upward movement toward the water’s surface. This behavior is displayed by control DMSO-treated larvae as seen in Suppl. Video 5. In this assay, control larvae frequently display ciliary reversal once they come in contact with the water’s surface, or with the side of the imaging chamber. By contrast, embryos that were treated with the MAPKinhibitor from the same clutch fail to reverse ciliary-directed movement, and instead continue to move in a random direction no matter where they are located in the water column (Suppl. Video 5). This behavioral defect, in which embryos treated with MAPK inhibitor swim, yet fail to exhibit reverse ciliary behavior when they come in contact the chamber or with the water’s surface provides evidence that without ciliary band neurons, the larvae are unable to carry out their normal touch responsive behavior. It should be noted that MAPK-inhibited larva have truncated arms, which may also affect swimming behaviors, since the MAPK-Erk pathway is also required for larval skeletal growth (Röttinger et al., 2008, 2004). However, embryos treated with MAPK inhibitor still form a ciliary band and express the ciliary marker 295, which suggests the behavioral defects are due to a lack of ciliary band neurons and not cilia itself (Supp Fig 2).
Discussion
Until now, the developmental origins and properties of the peripheral nervous system of the sea urchin embryo have been largely unresolved. Our analysis indicates that neuronal progenitors that will populate the ciliary band and make up the bulk of the larval nervous system are first born in the neuroectoderm on the lateral edges of chordin expression. In the ciliary band, they express soxC, prox and synb where they likely play a role in sensing touch due to the expression of asicl in the ciliary band. We then provide evidence that ciliary band neurons require MAPK signaling for specification. We also report, for the first time, that neuronal progenitors of the sea urchin ciliary band undergo migration, albeit over small distances. We then show that those neurons are necessary for the directed locomotory behavior of the larva.
There is increasing evidence that complex sensory tissues and organs are made up of cell types that are evolutionarily ancient (Arendt et al., 2016; Schlosser, 2015). The properties of ciliary band neurons shown here are very similar to properties uncovered in bipolar tail neurons (BTNs) in the tunicate Ciona intestinalis that were proposed to be homologous to vertebrate neural crest cells (Stolfi et al., 2015). Neural crest-derived spinal neurons, tunicate BTNs, and sea urchin ciliary band neurons share at least five common properties: All three cell populations 1) are first specified on the borders of the respective neural plate or neuroectoderm 2) express neurogenin orthologs, 3) are afferent sensory neurons within the peripheral nervous system, 4) require MAPK signaling for their specification, and 5) migrate from a bilateral site of origin.
It could be argued that these shared properties provide evidence for homology of these cell types (Supp Fig 3). If so, that would indicate that the so-called sudden appearance of neural crest cells in the vertebrate lineage was not so sudden, but that the neural crest is likely a continuous character that existed in multiple states and experienced remodeling in a step wise fashion over the course of deuterostome evolution. An alternative scenario is that these migratory neural cells evolved a similar phenotype through convergent evolution in the echinoderm, tunicate, cephalochordate and vertebrate lineages (Supp Fig 4). Indeed, there are several characteristics that are not shared between these three cell types. For example, sea urchin ciliary band neurons do not have a bipolar morphology with dorsal root ganglia neurons or BTNs. A previous study has shown that lateral ganglia within the sea urchin ciliary band have multiple bifurcating projections into the posterior ectoderm as well as one or two projections into the ciliary band itself (Burke et al., 2014). Another notable difference is that some neural crest cells undergo directed migration over long distances in the embryo to their final location, while sea urchin ciliary band neurons migrate much shorter distances. The ability to migrate large distances may be a novelty that was acquired by neural crest cells within the vertebrate lineage, though it should be noted that sensory neural precursors in cephalochordate embryos also migrate significant distances (Kaltenbach et al., 2009).
If, however, a homologous migratory neural progenitor population existed in the common ancestor of all deuterostomes, a plausible scenario is that the common ancestor of tunicates and vertebrates evolved a melanocyte cell type (and perhaps other cell lineages) at the neural plate border. Then within the vertebrate lineage, a multipotency gene regulatory program was co-opted at the lateral borders of the neural plate, giving these cells a “neural crest” phenotype (Green et al., 2015; Stolfi et al., 2015).
Regardless of whether neurons of the sea urchin ciliary band share homology with neural crest cells, they offer a useful model for understanding the sequence of development of a population of sensory neurons from initial specification to function. As more properties of sensory neurons and other neuronal subtypes are uncovered in the sea urchin, there will be many opportunities for comparative studies to find both conserved and derived aspects of nervous system development. Looking forward, molecular tools such as optogenetic techniques offer the prospect of understanding how this group of neurons function to provide behaviors necessary for larval survival.
Materials and Methods
Adult animals and embryo culture
Adult L. variegatus were collected from the Duke University Marine Lab (Beaufort, NC, United States), or Pelagic Corp. (Sugarloaf Key, FL, United States). Gametes were harvested by injection of 0.5M potassium chloride into adult animals. Embryos were cultured at 23°C in filtered artificial seawater (FASW).
Cloning and in situ hybridization
Cloning of partial coding sequences for genes was carried out with primers designed against a transcriptome data set and cloned into P-GEM T-easy vector (Promega). PCR was done with Phusion High Fidelity Master Mix (NEB). Accession numbers: Asicl Partial CDS: MH996684. Whole mount in situ hybridization (ISH) was performed using RNA probes labeled with Digoxigenin-11-UTP (Roche). For double fluorescent whole mount ISH, a second probe labelled with Fluorescein-12-UTP was hybridized and ISH protocol was 298 performed as described in (Slota and McClay, 2018).
Drug Treatments
MEK inhibitor U0126 drug treatments were carried out in 6 well plates. U0126 was resuspended in dimethyl sulfoxide (DMSO), diluted with FASW to 10uM and added to embryos at hatched blastula stage. Controls were carried out in parallel in the same manner as the experimental using DMSO alone as the control treatment.
Injection of Brn1/2/4 BAC and live imaging
S. purpuratus Brn1/2/4:GFP BAC was sent to us from Echinoderm Genomics Core Facility (http://www.echinobase.org). The recombinant BAC was linearized with Not1 and injected into Lytechinus variegatus zygotes at 10 ng/ul. Embryos selected for live imaging were incubated for 10 seconds in 2X hypertonic FASW to remove cilia, and then mounted in 1X filtered FASW. Slides were sealed with a mix of Vasoline, lanolin, and paraffin wax (VALAP) and images were acquired every 30 seconds on an upright Zeiss Axiophot or an inverted Zeiss 510 confocal with 20X objectives. Images were registered using FIJI. Embryos stained with Hoechst were incubated at a dilution of 1:4,000 for 2 minutes, washed and 315 mounted in 1X FASW. Live imaging was done on embryos at early pluteus stages (between 26–32 hpf).
Larval swimming behavior assay
Vehicle control and U0126 treated larvae were mounted in a Kiehart Chamber to provide a column of sea water contained between two coverslips separated by 1mm. The behavior of the larvae was imaged using a dissecting microscope that was turned on its side to visualize the vertical movements of the larvae. Movements of the larvae were visualized at about 3X normal speed.
Immunostaining
Embryos were fixed in methanol overnight at −20°C. Primary antibodies were then diluted in blocking serum and incubated overnight at 4°C. Monoclonal 295 antibody was diluted at 1:200 and L1 antibody was diluted at 1:50. Embryos were washed in TBST, incubated at 1:200 in Cy2 and Cy3-conjugated secondary antibodies and stained with Hoechst (1:2000, Molecular Probes) to label nuclei. Imaging was performed with a Zeiss 510 Inverted confocal.
Supplementary Material
Supplemental Figure 1: Timecourse of asicl expression and ngn/snail co-expression in anterior ciliary band. (A-E) In situ hybridization shows asicl expression begins to accumulate lightly in the ciliary band at 24 hpf (hours post fertilization). By 32 hpf (E) asicl is expressed throughout the ciliary band. Inset images show an aboral view of an embryo to show expression in the posterior ciliary band. (F) Single confocal section of double whole mount in situ hybridization shows co-expression of snail (magenta) and ngn in 1–4 cells of the anterior ciliary band at 28 hpf (hours post fertilization) pluteus stage. Side panels show merged or single channels of area outlined by white box. Nuclei (blue) are stained with Hoechst. Scale bars=50 um.
Supplemental Figure 2: Embryos treated with U0126 form ciliary band but lose axonal tracts. Maximum intensity projections of immunohistochemistry show: (A) DMSO treated control larvae showing expression of 295 (ciliary marker) and L1 (NgCAM), (axonal tracts and skeletogenic cell marker) (n=8) (McClay et al., 2018). (B) Embryos treated with U0126 express the ciliary marker 295 in the ciliary band which suggests they still form ciliary cells but lack axonal tracts in ciliary band and have a reduction in skeletogenic cells (n=16). Nuclei (blue) are stained with Hoechst.
Supplemental Figure 3: Homology of sea urchin ciliary band neurons to urochordate bipolar tail neurons and vertebrate dorsal root ganglia neurons. Similarities of these cell types are listed and typogenetic tree shows proposed scenario of homology between these cell types. Dashed box indicates that bipolar morphology was either gained in the chordate lineage or lost in the clade that includes sea urchins. Black box indicates that a multipotency and/or developmental plasticity gene regulatory program was likely gained in the vertebrate lineage at the neural plate border.
Supplemental Figure 4: Alternate scenario-convergent evolution of phenotypes. In scenario two, these three cell types are not homologous, rather they evolved strikingly similar phenotypes independently within the echinoderm, urochordate and vertebrate lineages. Another, related scenario is that vertebrate dorsal root ganglion neurons and bipolar tail neurons are homologous but ciliary band neurons evolved independently.
Supplemental Video 1–3: Movement of neural progenitors in the ciliary band. Embryo injected with Brn1/2/4 GFP BAC. Left Panel shows combined DIC and fluorescent channels, right panel shows fluorescent channel only. Time shown in min:sec. Yellow arrow in first frame points to neural progenitors expressing GFP that will migrate within the ciliary band.
Supplemental Video 4: Movement of neural progenitors into the ciliary band from the oral ectoderm. Embryo injected with Brn1/2/4 GFP BAC. Left Panel shows combined channels (Hoescht in blue, GFP in green), right panel shows GFP fluorescent channel only. Time shown in min:sec. Yellow arrow in first frame points to neural progenitor expressing GFP that will migrate into the ciliary band.
Supplemental Video 5: Swimming behavior of control and MAPK-inhibited embryos. On the left 48 hpf larvae swim characteristically in a spiral upward with their arms pointed in the direction of movement. When the larva touches the surface it immediately falls backward down the water column due to ciliary reversal. Other larvae ciliary reverse when they touch another larva or the side of the imaging chamber. This coordinated behavior is missing in the MAPK-inhibited embryos to the right. These embryos swim due to ciliary motion but they move randomly and do not exhibit ciliary reversal when they touch another object. Time lapse movies about 3X normal speed.
Acknowledgements and Funding Information
We would like to thank members of the McClay lab, and Dr. Megan Martik for comments and support on this project. This work was supported by the National Science Foundation Graduate Research Fellowship Program under (Grant No. (NSF DGF 1106401) to [L.A.S], National Institute of Child Health and Human Development (NIH RO1-HD-14483, NIH PO1-HD-037105) to [D.R.M]. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Competing Interests
The authors declare no competing interest or financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendt D, Tosches MA, Marlow H, 2016. From nerve net to nerve ring, nerve cord and brain-evolution of the nervous system. Nat. Rev. Neurosci 17, 61–72. 10.1038/nrn.2015.15 [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Burke RD, 1986. Development of Serotonergic Neurons in Embryos of the Sea Urchin, Strongylocentrotus purpuratus. Dev. Growth Differ. 28, 569–574. 10.1016/j.febslet.2006.02.081 [DOI] [PubMed] [Google Scholar]
- Bradham CA, Oikonomou C, Kühn A, Core AB, Modell JW, McClay DR, Poustka AJ, 2009. Chordin is required for neural but not axial development in sea urchin embryos. Dev. Biol 328, 221–233. 10.1016/j.ydbio.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RD, Angerer LM, Elphick MR, Humphrey GW, Yaguchi S, Kiyama T, Liang S, Mu X, Agca C, Klein WH, Brandhorst BP, Rowe M, Wilson K, Churcher AM, Taylor JS, Chen N, Murray G, Wang D, Mellott D, Olinski R, Hallböök F, Thorndyke MC, 2006. A genomic view of the sea urchin nervous system. Dev. Biol 300, 434–460. 10.1016/j.ydbio.2006.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RD, Moller DJ, Krupke OA, Taylor VJ, 2014. Sea urchin neural development and the metazoan paradigm of neurogenesis. Genesis 52, 208–221. 10.1002/dvg.22750 [DOI] [PubMed] [Google Scholar]
- Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BEE, van Steensel B, Micklem G, Brand AH, 2006. Prospero Acts as a Binary Switch between SelfRenewal and Differentiation in Drosophila Neural Stem Cells. Dev. Cell 11, 775–789. 10.1016/j.devcel.2006.09.015 [DOI] [PubMed] [Google Scholar]
- Deval E, Lingueglia E, 2015. Acid-Sensing Ion Channels and nociception in the peripheral and central nervous systems. Neuropharmacology 94, 49–57. 10.1016/j.neuropharm.2015.02.009 [DOI] [PubMed] [Google Scholar]
- Duboc V, Röttinger E, Besnardeau L, Lepage T, 2004. Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev. Cell 6, 397–410. 10.1016/S1534-5807(04)00056-5 [DOI] [PubMed] [Google Scholar]
- Garner S, Zysk I, Byrne G, Kramer M, Moller D, Taylor V, Burke RD, 2016. Neurogenesis in sea urchin embryos and the diversity of deuterostome neurogenic mechanisms. Development 143, 286–297. 10.1242/dev.124503 [DOI] [PubMed] [Google Scholar]
- Gonçalves JT, Schafer ST, Gage FH, 2016. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167, 897–914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Green SA, Simoes-Costa M, Bronner ME, 2015. Evolution of vertebrates as viewed from the crest. Nature 520, 474–482. 10.1038/nature14436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman VF, Burke RD, 2018. Embryonic neurogenesis in echinoderms. Wiley Interdiscip. Rev. Dev. Biol 7, 1–15. 10.1002/wdev.316 [DOI] [PubMed] [Google Scholar]
- Kaltenbach SL, Yu JK, Holland ND, 2009. The origin and migration of the earliestdeveloping sensory neurons in the peripheral nervous system of amphioxus. Evol. Dev 11, 142–151. 10.1111/j.1525-142X.2009.00315.x [DOI] [PubMed] [Google Scholar]
- Lingueglia E, 2007. Acid-sensing ion channels in sensory perception. J. Biol. Chem 282, 17325–17329. 10.1074/jbc.R700011200 [DOI] [PubMed] [Google Scholar]
- McClay DR, Miranda E, Feinberg SL, 2018. Neurogenesis in the sea urchin embryo is initiated uniquely in three domains. Development 145 10.1242/dev.167742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Humphreys T, Kaneko H, Tagawa K, 2004. Development and neural organization of the tornaria larva of the Hawaiian hemichordate, Ptychodera flava. Zoolog. Sci 21, 69–78. 10.2108/zsj.21.69 [DOI] [PubMed] [Google Scholar]
- Nieto MA, 2002. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol 3, 155–166. 10.1038/nrm757 [DOI] [PubMed] [Google Scholar]
- Röttinger E, Besnardeau L, Lepage T, 2004. A Raf/MEK/ERK signaling pathway is required for development of the sea urchin embryo micromere lineage through phosphorylation of the transcription factor Ets. Development 131, 1075–1087. 10.1242/dev.01000 [DOI] [PubMed] [Google Scholar]
- Röttinger E, Saudemont A, Duboc V, Besnardeau L, Mcclay D, Lepage T, 2008. FGF signals guide migration of mesenchymal cells, controlskeletal morphogenesis of the skeleton and regulategastrulation during sea urchin development. Development 135, 353–365. 10.1242/dev.020016 [DOI] [PubMed] [Google Scholar]
- Schlosser G, 2015. Vertebrate cranial placodes as evolutionary innovations-The ancestor’s tale, 1st ed, Current Topics in Developmental Biology. Elsevier Inc. 10.1016/bs.ctdb.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Slota LA, McClay DR, 2018. Identification of neural transcription factors required for the differentiation of three neuronal subtypes in the sea urchin embryo. Dev. Biol. 435, 138–149. 10.1016/j.ydbio.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slota LA, Miranda EM, McClay DR, 2019. Spatial and temporal patterns of gene expression during neurogenesis in the sea urchin Lytechinus variegatus. Evodevo 10, 2 10.1186/s13227-019-0115-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi A, Ryan K, Meinertzhagen IA, Christiaen L, 2015. Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature 527, 371–374. 10.1038/nature15758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathmann RR, 2007. Time and extent of ciliary response to particles in a non-filtering feeding mechanism. Biol. Bull. 212, 93–103. 10.2307/25066587 [DOI] [PubMed] [Google Scholar]
- Wei Z, Angerer LM, Angerer RC, 2016. Neurogenic gene regulatory pathways in the sea urchin embryo. Development 143, 298–305. 10.1242/dev.125989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Angerer RC, Angerer LM, 2011. Direct development of neurons within foregut endoderm of sea urchin embryos. Proc. Natl. Acad. Sci. U. S. A 108, 9143–9147. 10.1073/pnas.1018513108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Yaguchi J, Yaguchi S, Angerer RC, Angerer LM, 2009. The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development 136, 1179–1189. 10.1242/dev.037002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi J, Angerer LM, Inaba K, Yaguchi S, 2012. Zinc finger homeobox is required for the differentiation of serotonergic neurons in the sea urchin embryo. Dev. Biol 363, 74–83. 10.1016/j.ydbio.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi J, Takeda N, Inaba K, Yaguchi S, 2016. Cooperative Wnt-Nodal Signals Regulate the Patterning of Anterior Neuroectoderm. PLoS Genet. 12, 1–27. 10.1371/journal.pgen.1006001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi S, Yaguchi J, Burke RD, 2006. Specification of ectoderm restricts the size of the animal plate and patterns neurogenesis in sea urchin embryos. Development 133, 2337–2346. 10.1242/dev.02396 [DOI] [PubMed] [Google Scholar]
- Yaguchi S, Yaguchi J, Wei Z, Jin Y, Angerer LM, Inaba K, 2011. Fez function is required to maintain the size of the animal plate in the sea urchin embryo. Development 138, 4233–4243. 10.1242/dev.069856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JKS, 2010. The evolutionary origin of the vertebrate neural crest and its developmental gene regulatory network - insights from amphioxus. Zoology 113, 1–9. 10.1016/j.zool.2009.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Timecourse of asicl expression and ngn/snail co-expression in anterior ciliary band. (A-E) In situ hybridization shows asicl expression begins to accumulate lightly in the ciliary band at 24 hpf (hours post fertilization). By 32 hpf (E) asicl is expressed throughout the ciliary band. Inset images show an aboral view of an embryo to show expression in the posterior ciliary band. (F) Single confocal section of double whole mount in situ hybridization shows co-expression of snail (magenta) and ngn in 1–4 cells of the anterior ciliary band at 28 hpf (hours post fertilization) pluteus stage. Side panels show merged or single channels of area outlined by white box. Nuclei (blue) are stained with Hoechst. Scale bars=50 um.
Supplemental Figure 2: Embryos treated with U0126 form ciliary band but lose axonal tracts. Maximum intensity projections of immunohistochemistry show: (A) DMSO treated control larvae showing expression of 295 (ciliary marker) and L1 (NgCAM), (axonal tracts and skeletogenic cell marker) (n=8) (McClay et al., 2018). (B) Embryos treated with U0126 express the ciliary marker 295 in the ciliary band which suggests they still form ciliary cells but lack axonal tracts in ciliary band and have a reduction in skeletogenic cells (n=16). Nuclei (blue) are stained with Hoechst.
Supplemental Figure 3: Homology of sea urchin ciliary band neurons to urochordate bipolar tail neurons and vertebrate dorsal root ganglia neurons. Similarities of these cell types are listed and typogenetic tree shows proposed scenario of homology between these cell types. Dashed box indicates that bipolar morphology was either gained in the chordate lineage or lost in the clade that includes sea urchins. Black box indicates that a multipotency and/or developmental plasticity gene regulatory program was likely gained in the vertebrate lineage at the neural plate border.
Supplemental Figure 4: Alternate scenario-convergent evolution of phenotypes. In scenario two, these three cell types are not homologous, rather they evolved strikingly similar phenotypes independently within the echinoderm, urochordate and vertebrate lineages. Another, related scenario is that vertebrate dorsal root ganglion neurons and bipolar tail neurons are homologous but ciliary band neurons evolved independently.
Supplemental Video 1–3: Movement of neural progenitors in the ciliary band. Embryo injected with Brn1/2/4 GFP BAC. Left Panel shows combined DIC and fluorescent channels, right panel shows fluorescent channel only. Time shown in min:sec. Yellow arrow in first frame points to neural progenitors expressing GFP that will migrate within the ciliary band.
Supplemental Video 4: Movement of neural progenitors into the ciliary band from the oral ectoderm. Embryo injected with Brn1/2/4 GFP BAC. Left Panel shows combined channels (Hoescht in blue, GFP in green), right panel shows GFP fluorescent channel only. Time shown in min:sec. Yellow arrow in first frame points to neural progenitor expressing GFP that will migrate into the ciliary band.
Supplemental Video 5: Swimming behavior of control and MAPK-inhibited embryos. On the left 48 hpf larvae swim characteristically in a spiral upward with their arms pointed in the direction of movement. When the larva touches the surface it immediately falls backward down the water column due to ciliary reversal. Other larvae ciliary reverse when they touch another larva or the side of the imaging chamber. This coordinated behavior is missing in the MAPK-inhibited embryos to the right. These embryos swim due to ciliary motion but they move randomly and do not exhibit ciliary reversal when they touch another object. Time lapse movies about 3X normal speed.