Abstract
Chronic wasting disease (CWD) is a major concern for the management of North American cervid populations. This fatal prion disease has led to declines in populations which have high CWD prevalence and areas with both high and low infection rates have experienced economic losses in wildlife recreation and fears of potential spill-over into livestock or humans. Research from human and veterinary medicine has established that the prion protein gene (Prnp) encodes the protein responsible for transmissible spongiform encephalopathies (TSEs). Polymorphisms in the Prnp gene can lead to different prion forms that moderate individual susceptibility to and progression of TSE infection. Prnp genes have been sequenced in a number of cervid species including those currently infected by CWD (elk, mule deer, white-tailed deer, moose) and those for which susceptibility is not yet determined (caribou, fallow deer, sika deer). Over thousands of sequences examined, the Prnp gene is remarkably conserved within the family Cervidae; only 16 amino acid polymorphisms have been reported within the 256 amino acid open reading frame in the third exon of the Prnp gene. Some of these polymorphisms have been associated with lower rates of CWD infection and slower progression of clinical CWD. Here we review the body of research on Prnp genetics of North American cervids. Specifically, we focus on known polymorphisms in the Prnp gene, observed genotypic differences in CWD infection rates and clinical progression, mechanisms for genetic TSE resistance related to both the cervid host and the prion agent and potential for natural selection for CWD-resistance. We also identify gaps in our knowledge that require future research.
Keywords: cervids, chronic wasting disease, disease progression, prion disease, prion protein gene (Prnp), susceptibility
Introduction
In recent decades prion diseases have transitioned from a novel, but relatively obscure, group of diseases to one that presents substantial ecological, agricultural, and economic problems, with potential threats to human health. Prion diseases, or transmissible spongiform encephalopathies (TSEs), are invariably fatal neurodegenerative diseases caused by unique transmissible protein pathogens.1 They can occur as genetic, infectious, or spontaneous afflictions. Prion diseases first gained public notoriety as “mad cow” disease (bovine spongiform encephalopathy, BSE) emerged in the United Kingdom, and caused variant Creutzfeld-Jacob (vCJD) disease in humans.2 But TSEs were recognized before the media coverage and even before the identification of prions as a disease agent.1 Kuru, linked to ritualistic cannibalism in the Fore population of Papua New Guinea,3 and scrapie affecting sheep and goats4 have been known for decades, if not centuries. More recently an emerging TSE, chronic wasting disease (CWD), was discovered in North American wildlife populations during the 1980s and has caused great concern for captive and wild cervid populations.5
Originally discovered in the western United States, CWD now occurs in at least 19 states and 2 Canadian provinces. Currently both free-ranging and captive mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), elk (Cervus elaphus), and moose (Alces alces) are known to be affected by CWD. Disease prevalence has exceeded 30% in some free-ranging populations in western states6 and 80% in some captive deer herds.7 Given the potential for high prevalence and impacts on wildlife populations, CWD is one of the primary concerns of wildlife management agencies in the US and Canada.8,9 Though there is currently no evidence that humans can contract CWD,10 some affected regions have reported public concerns and economic losses in wildlife-related recreation.11,12
CWD is unique, and especially problematic, because it is one of the most efficiently transmitted prion diseases.13 Transmission of many TSEs depends on ingestion of tissue from infected animals. This has allowed BSE and Kuru to be largely controlled by disrupting the food chain responsible for their transmission. In contrast, scrapie and CWD are contagious and can be transmitted horizontally, making disease eradication challenging. In captive animals, CWD can be transmitted by both direct contact6 and exposure to environmental reservoirs.14 Further, some TSEs are only able to infect hosts with specific prion protein genotypes, and these genetic differences in susceptibility have formed the basis for selective breeding programs to reduce scrapie infection in sheep.15 However, there are concerns related to atypical scrapie (an uncommon strain with unique neuropathology that appears to affect all genotypes).16 In contrast, CWD appears to infect (albeit at varying levels) all currently known cervid genotypes and is transmissible among different cervid species and among genotypes.
In contrast to cellular pathogens carrying their own genome, prions contain no nucleic acid. The structure of the normal cellular prion protein (PrPC) is determined by the amino acid sequence encoded by the host prion protein gene (Prnp). In pathogenic form (PrPres), the prion protein is misfolded such that it is more resistant to degradation by proteases. The PrPres accumulates, not through cellular replication, but through a chain reaction converting additional PrPC to PrPres. This newly formed PrPres is identical in amino acid composition to the template PrPC in the infected individual.
In prion diseases affecting sheep, mice, and humans, amino acid polymorphisms in the Prnp gene influence susceptibility and incubation period.13,17 Genetic analysis of CWD affected cervid populations also suggests that Prnp variation affects susceptibility and/or disease progression. Many of these conclusions have been base on the proportion of affected and unaffected cervids in a population with respect to their Prnp genotype. However, recent studies have evaluated disease susceptibility and progression of Prnp genotypes based on tissue pathogenesis,18 infection rates in captive animals,7 disease incubation period in experimentally challenged animals,19 and determination of genotype-specific infection and survival rates in wild populations.20
Determining the role of Prnp genetics in CWD is important in understanding the epidemiology of affected species, identifying potential species barriers to infection, modeling disease risk, and evaluating potential management actions. Here we compile a wide body of research on Prnp genetics of North American cervids. Specifically we review known polymorphisms in the Prnp gene and genotype effects on CWD susceptibility and progression. We review potential mechanisms for genetic TSE resistance for both the cervid host and prion agent, identify knowledge gaps, and identify opportunities for future research.
Prions: A unique agent with a complex etiology
While we know little about the function of normal prion proteins,21,22 its conserved genetic structure among mammals and expression in numerous tissues suggests a broad functionality.13 The structure of the mature protein is consistent across taxa, with a long, flexible N-terminal tail, three α helices, and a two-stranded anti-parallel β sheet.13,23 An amino acid loop connecting the β sheet strand to the α helixes is particularly rigid in cervids, perhaps relating to the highly infectious nature of CWD in cervids.24 Structures in the loop region and the central portion of the molecule appear influential in determining efficiency of converting PrPC to β sheet-rich PrPres.1,25-27
Several steps are involved in the reconfiguration of the normal protein to the pathogenic PrPres. First the reaction must be seeded by the introduction of PrPres particles. The PrPres binds to PrPC at a site on the 3rd α helix.25 Once bound, the PrPC is converted to PrPres. This conversion stage is believed to be the slower step in the process, and potentially the most influenced by amino acid structure of the protein.28-31 Newly converted PrPres particles become bound to the seed material forming aggregate polymers which are extremely resistant to degradation.25 As these protease-resistant aggregates accumulate in the body, disease develops. CWD prions accumulate first in gastrointestinal lymphoid tissues, followed by peripheral lymphoid tissues, and eventually in the central nervous system. Amyloid prion plaques in the brain tissue cause neuronal degeneration and encephalopathy that lead to clinical signs of disease and finally to death.6
Once an animal is infected, likely by ingesting PrPres, progression of disease is primarily driven by the efficiency of the prion misfolding chain reaction, which may be related to Prnp similarity between the seed PrPres and template PrPC.13,32 One theory suggests a general heterozygote advantage against disease, where reactions between non-homologous proteins are less efficient due to competition at binding sites.1,25,26 An alternative explanation is that variation in the Prnp sequence produces prions that differ in physical properties and chemical reactivity. Prusiner (1998) has suggested that one mechanism for differential TSE susceptibility might be the different activation energy required to refold amino acid sequences formed by some Prnp types.
The role of host-specific Prnp variation in CWD epidemiology
Given the importance of the protein structure in prion pathogenicity, variation in the amino acid make-up of both PrPC and PrPres may have important implications for TSE epidemiology. Infection with, distribution, and accumulation of PrPres in cervid tissues33 are crucial steps in CWD which may produce differential susceptibility to infection and efficiency of disease progression. We focus our review on the variability in the third exon in the Prnp gene which encodes the prion protein (PrPC) sequence, based on the 256 amino acid open reading frame, ORF. A total of 16 polymorphic codons in the Prnp ORF have been reported in cervid species (Table 1). The conservation of this DNA sequence suggests strong purifying selection for maintenance of prion function.15 The greatest number of Prnp alleles and amino acid substitution occurs in white-tailed deer, red deer, caribou, and mule deer (Table 1). So far, research has demonstrated that Prnp coding variations in elk, white-tailed deer, and mule deer are associated with the rate of CWD infection or disease progression, with no evidence for a completely resistant genotype (references in Table 1).
Table 1. Variability in the PRNP genotypes of cervids found in North American.
| Variable Codons | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | PRNP type | Freq | 2 | 20 | 59 | 95 | 96 | 98 | 100 | 116 | 129 | 132 | 138 | 168 | 169 | 209 | 225 | 226 | Citation | ||
| Concensus amino acid sequence | V | D | G | Q | G | T | S | A | G | M | S | P | V | M | S | Q | |||||
| Subfamily Cervinae | |||||||||||||||||||||
| Rocky Mountain Elk | O'Rourke et al., 1999 | ||||||||||||||||||||
| 132M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | White et al., 2010 | ||||
| ++ | 132L | 37% | - | - | - | - | - | - | - | - | - | L | - | - | - | - | - | E | Perucchini et al., 2008 | ||
| Red Deer | Paletto et al., 2009 | ||||||||||||||||||||
| 59G / 98T / 168P / 226E | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |||||
| 59S | 0.2% | - | - | S | - | - | - | - | - | - | - | - | - | - | - | - | E | ||||
| 98A | 8% | - | - | - | - | - | A | - | - | - | - | - | - | - | - | - | E | ||||
| 168S | 0.2% | - | - | - | - | - | - | - | - | - | - | - | S | - | - | - | E | ||||
| 226Q | 44% | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| Sika Deer | Jeong et al., 2007 | ||||||||||||||||||||
| 100S / 226E | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | Meng et al., 2005 | ||||
| 100G | 3% | - | - | - | - | - | - | G | - | - | - | - | - | - | - | - | E | ||||
| 226Q | 48% | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| Fallow Deer | Rhyan et al., 2011 | ||||||||||||||||||||
| monomorphic | 100% | - | - | - | - | - | - | - | - | - | - | N | - | - | - | - | E | ||||
| Subfamily Capreolinae | |||||||||||||||||||||
| White-tailed Deer | Johnson et al., 2003; 2006 | ||||||||||||||||||||
| 95Q / 96G / 116A/ 138S / 226Q | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Robinson et al., not published | ||||
| ++ | 95H | 2% | - | - | - | H | - | - | - | - | - | - | - | - | - | - | - | - | Kelly et al., 2008 | ||
| ++ | 96S | 26% | - | - | - | - | S | - | - | - | - | - | - | - | - | - | - | - | O'Rourke et al., 2004 | ||
| ++ | 116G | 13% | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Wilson et al., 2009 | ||
| 226K | 0.5% | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | K | Heaton et al. 2003 | |||
| 138N psuedogene | 15% | - | - | - | - | - | - | - | - | - | - | N | - | - | - | - | - | ||||
| Mule Deer | Brayton et al., 2003 | ||||||||||||||||||||
| 20D / 138S / 225S | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Jewell et al., 2005 | ||||
| 20G | 9% | - | G | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Wilson et al., 2009 | |||
| ++ | 225F | 5% | - | - | - | - | - | - | - | - | - | - | - | - | - | - | F | - | Heaton et al. 2003 | ||
| 138N psuedogene | ~100% | - | - | - | - | - | - | - | - | - | - | N | - | - | - | - | - | ||||
| Moose | Huson, Happ, 2006 | ||||||||||||||||||||
| 209M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| 209I | 45% | - | - | - | - | - | - | - | - | - | - | - | - | - | I | - | - | ||||
| Caribou | Happ et al., 2007 | ||||||||||||||||||||
| 2V / 129G / 138S / 169V | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| 129S | 2% | - | - | - | - | - | - | - | - | S | - | - | - | - | - | - | - | ||||
| 138N | 30% | - | - | - | - | - | - | - | - | - | - | N | - | - | - | - | - | ||||
| 2M / 129S/169M | 4% | M | - | - | - | - | - | - | - | S | - | - | - | M | - | - | - | ||||
| Roe Deer | Paletto et al., 2009 | ||||||||||||||||||||
| monomorphic | 100% | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| Chinese Water Deer | Jeong, 2009 | ||||||||||||||||||||
| 100S | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| 100N | na | - | - | - | - | - | - | N | - | - | - | - | - | - | - | - | - | ||||
Amino acid codes: A = alanine, D = aspartic acid, E = glutamic acid, f = phenylalanine, G = glycine, H = histidine, I = isoleucine, K = lysine, L = leucine, M = methionine, n = asparagine, p = proline, Q = glutamine, R = arginine, S = serine, t = threonine, V = valine
++ = genotypes associated with reduced susceptibility to and/or delayed progress of CWD. We summarized known coding variations in the cervid PRNP gene. Allele are described by their characteristic variation from the consensus sequence. Frequencies are given for minor alleles. The consensus amino acid sequence shows the most common alleles at each of the variable codon positions (numbered), dashes indicate no difference from the common sequence, alternate amino acids are indicated with their abbreviation.
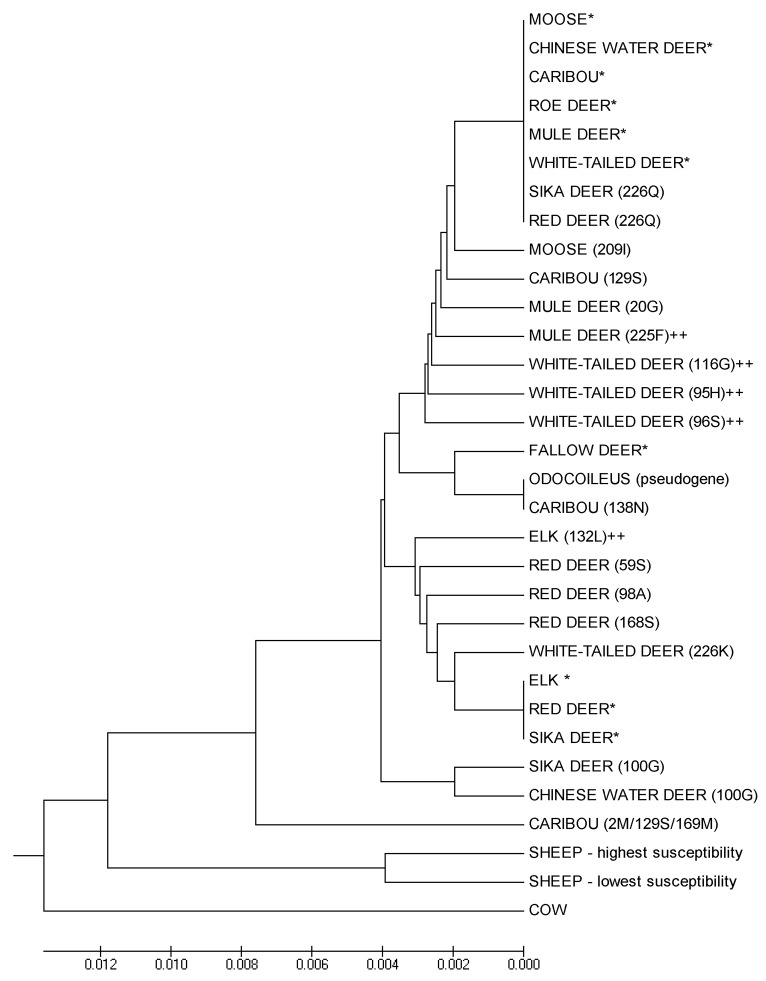
To illustrate the diversity of Prnp sequences and their relationship among taxa, we constructed a UPGMA (Unweighted Pair Group Method with Arithmetic Mean) phylogeny from available Prnp sequences (translated animo acid sequences from GenBank aligned and analyzed in Mega 5.234). The dendrogram illustrates the shallow divergence among taxa (Fig. 1). The tree is polyphyletic, with branches reflecting specific codon polymorphisms rather than taxonomic relationships. The most common Prnp genotype in deer, which is also the most susceptible to CWD infection, is widely shared across cervid taxa, and the common elk genotype differs from deer only at codon 226. None of the cervid Prnp genotypes described are highly diverged from the known susceptible genotypes (Fig. 1), suggesting that Prnp divergence alone is unlikely to provide a strong barrier to CWD transmission between species and that complete resistance is unlikely for any specific genotype.
Figure 1. Phylogeny of Prnp genotypes. We generated a UPGMA phylogeny based on translated sequences of the Prnp gene reported in GenBank for species within the family Cervidae, labels correspond to Table 1. As out groups, we include the common domestic cow genotype, and major sheep genotypes known to vary in TSE-susceptibility. (* indicates the consensus genotype for each species, ++ indicates Prnp types shown to have reduced susceptibility to TSEs.)
Mule deer
Experimental research tracking the pathological progression of CWD has demonstrated a major effect of Prnp variation on the rate of disease progression. Fox et al.18 contribute valuable information on CWD progression by examining animal tissues at systematic time points after experimental inoculation of mule deer. The accumulation and distribution of PrPres was similar in 225SS and 225SF deer, but the time course varied with genotype. Delay in accumulation in the lymphoid tissue was relatively slight, but deposition of PrPres in the central nervous system was markedly delayed in 225SF deer.18 While 225SS deer developed spongiform lesions in about 19 mo, 225SF animals were still subclinical and free of lesions at the study’s end 6 mo later.18 The researchers make the important distinction between the short lag until PrPres infection of the lymphatic system and the much slower time to clinical stages, indicating the potential to prolong shedding after 225SF animals become lymph node-positive and before they succumb to disease.18,33
Genetic screening in free ranging mule deer populations is suggestive of differential infection rates associated with Prnp genotypes, but specific results have varied by population (Table 1). In the endemic zones of Colorado and Wyoming, 225SS deer tested positive for CWD at a rate 30 times greater than 225SF deer.35 Mule deer in Canada did not have the 225F allele, but the 20G allele was over twice as likely to be associated with CWD infection than 20D.36 This finding differed from US studies where the codon 20 allele was not associated with CWD infection.
White-tailed deer
Prnp polymorphism has also been strongly associated with delayed progression of CWD in white-tailed deer. In harvested free-ranging deer, brains of infected 96GS deer showed lower accumulation of PrPres than brains from 96GG deer.37 In addition, controlled experiments using high dosage oral inoculations have demonstrated that 96S deer experience a longer incubation period before developing clinical CWD.19 In the same experiment the 95H allele had a profound effect on incubation period, more than doubling the time to clinical signs compared with 95Q.19
Several field studies of Prnp genetics in free-ranging and captive white-tailed deer have consistently reported lower infection rates in some genotypes (Table 1). In all populations studied, the 96S allele has been associated with lower prevalence compared with 96G. Epidemiological models for wild (in press20) and captive animals7 indicate 96GG deer are infected at 3 to 4 times the rate of animals with at least one copy of 96S. Lower CWD infection has also been suggested for minor alleles 95H and 116G; however, these alleles occur at very low frequencies, thus trends based on observational studies and have not been confirmed with formal statistical analysis. In Illinois, where 95H was more common (about 2%) it was significantly associated with decreased odds of CWD infection, with positive 95H animals occurring nearly five times less frequently than 95Q.38 This finding is concordant with studies where 95H has been underrepresented in CWD cases, but too rare for formal evaluation.36,37 In a captive Nebraska herd, animals with the 116A allele were over twice as likely to be infected as those with the 116G allele.39 There is also some evidence that synonymous polymorphisms might be associated with CWD, but the role of linkage with coding polymorphisms is unclear.36,38
Elk
Experimental studies have provided strong evidence for delayed CWD progression in elk with the 132L allele. Heterozygous elk (132ML) showed an approximate doubling-time to clinical disease following oral challenge compared with homozygous 132MM elk.40 Homozygosity for 132LL further extended incubation times41 and was consistent with research in transgenic mice where the 132L allele diminished propagation of PrPres and greatly extended incubation time.42 Research is inconclusive as to whether the 132L allele also resists CWD infection. CWD is rarely diagnosed in 132LL farmed elk;43 however, a survey of free-ranging elk in Colorado found each genotype represented in CWD-positive animals in proportion to their frequency in the population.44 This differs from earlier results in which O’Rourke et al.43 found an over-representation of the 132M allele in CWD-positive elk. It is likely that the discrepancy in these findings can be attributed to differences in sampling; however, temporal changes in infection rates are also possible.
Moose
To date, CWD has been reported in only 3 wild moose (A. alces shirasi) from Colorado and testing is not widespread for this species.45 The homozygous 209MM amino acid sequence of the index case in wild moose was identical to that of the most susceptible genotypes in mule and white-tailed deer (Fig. 1). However, both 209MM and 209IM moose have been experimentally infected with CWD.46 Additional genetic screening has been conducted in Alaskan moose (A. alces gigas), and suggests that Prnp divergence alone would not provide a species barrier for CWD transmission between moose and deer.47
Caribou
CWD has not yet been detected in caribou (Rangifer tarandus) or in areas were caribou are found, but the importance of herds to subsistence hunters and the potential for infectious contact within gregarious caribou herds has generated concern about their potential susceptibility to CWD.48 Prnp sequences from three Alaskan populations are similar to that of other cervids, especially mule and white-tailed deer (Fig. 1), suggesting a very weak species barrier.48
Exotic cervids
While we are primarily concerned with the ecological impacts of Prnp variation in free-ranging populations of cervids native to North America, numerous exotic species are also present in captive cervid facilities. The potential for these species to contact wild cervids and contribute to disease dynamics makes them an important part of the puzzle. Many exotic cervid species have Prnp genes largely homologous with North American species (Table 1), suggesting that Prnp divergence would not be sufficient to avoid disease. This is borne out by successful experimental infection of fallow deer49,50 and red deer.51 However, cross-species infection may still be unlikely via natural routes. For example, despite susceptibility to experimental intracerebral infection, fallow deer are relatively resistant to CWD infection when housed with infected mule deer.52 Still, potential for cross-species infection from other cervid species needs further investigation and suggests caution in the movement and management of exotic species.
Accessory genetic regions
Although the effect of critical amino acid changes in the Prnp sequence on TSE susceptibility and disease progression is well established, the role of other genetic factors is less understood. Several studies have investigated broader genomic associations with CWD, finding none. For example, research has shown no association between the Prnp promoter region, which regulates production of mRNA, and CWD.53,54 Several studies have evaluated the interaction between prion disease and the immune complement system, presumably because circulating or cell-associated β-sheet rich PrP oligomers fix components of the classical cascade.55 However, both rodent TSE models56 and studies of the C1q gene in white-tailed deer57 found no association between complement genetics and CWD. Although, one study found that microsatellite markers linked to the neurofibromin 1 gene were associated with CWD status in mule deer, indicating that there may be genomic interactions beyond the Prnp gene.58
Beyond Prnp: Other sources of variation in agent and host biology
While identity between prion molecules facilitates transmission, it is likely not required. In experimental conditions both inter- and intra-species Prnp divergence has sometimes proven inconsequential for PrPres amplification. Other risk factors such as dose, strain, and route of infection, are all likely to play important roles in the actual transmissibility of disease.32
Transmission of prions between different mammalian species is typically limited by the species barrier phenomenon.1,2 This interspecies resistance to prion infection is characterized by an extremely long incubation period on first passage into a new host species.59 Currently, the strength of this barrier between different CWD host species is unpredictable60 and even strong barriers can be broken experimentally.2,60,61 Further, prion adaptation may occur upon serial passage through a new host, or intermediate hosts might bridge the species barrier to expand host range.62
Just as host genetics influences TSE susceptibility and progression, the strain of disease agent can also impact these processes. Prion strains are defined when disease agents passed into recipients of identical genetic backgrounds produce unique phenotypes, including differing incubation time and clinical symptoms, differences in PrPres conformational stability, and glycoform ratios (for reviews63-65). While logistical challenges such as dose, route, and time of exposure make it impossible to identify strains in wild populations, laboratory studies have indicated the existence of unique CWD strains. Several researchers have reported that deer or elk CWD strains produce differences in interspecies transmission. For example fallow deer experimentally infected with PrPres from CWD-infected elk survived nearly 40% longer than those infected with PrPres from white-tailed deer.50 Similarly, other species including primates66 and cattle67,68 have show different susceptibility and survival times depending on the source of PrPres in experimental CWD infections. Rodent models have also been used to show differences in incubation time based on the source of inocculation.69,70 Angers et al.65 suggested that elk stably carry either of two strains (CWD1 or CWD2) whereas deer carry a mixture of strains. Preliminary evidence linking CWD strain to Prnp genotype is suggested by a unique PrPres electrophoretic pattern from a white-tailed deer with both the 95H and 96S alleles.19
The dose of infectious prions or their degree of infectivity may also affect transmission or progression of disease in cervids. Although there is little direct evidence to substantiate these hypotheses in cervids, experiments in transgenic mice have suggested decreasing incubation period with larger infectious doses.15,71 Prions may also bind to soil, thus increasing agent persistence in the environment72 and potentially increasing the effective infectious dose.73 Still other evidence suggests Prnp sequence and PrPC characteristics are not sufficient to explain differing infection rates and profiles of TSE disease. Gordon et al.,74 also citing Arjona et al.,75 point out that the PrPres protein alone has lower infectivity than TSE agent particles and suggest that the infectivity of PrPres might be augmented by association with other pathogenic particles such as retroviruses.76-78
In addition to variability in the agent and mode of infection, host differences beyond genotype can have substantial impacts on CWD infection. Both age and sex are important predictors of CWD infection rates in deer.79,80 Current evidence suggests that higher CWD prevalence in males is likely due to differences in behavior and exposure, rather than innate susceptibility.79,80 In both Odocoileus species infection rates increase with age, as is characteristic of a chronic disease where cumulative risk increases with time of exposure.
Implications and opportunities for future research
Disease risk
There is much debate over whether a partial genetic resistance to CWD infection, or delayed progression, might have positive or negative effects on disease dynamics on the landscape. On the one hand, CWD infection rates may be substantially lower in some genotypes, reducing the prevalence of CWD and disease impacts on the affected population. These less-susceptible genotypes may gain a survival advantage over other genotypes.20 Yet, because the infectious state may be prolonged, these animals could disproportionately contribute to environmental contamination and transmission to susceptible animals.18,81 Future research is needed to determine whether all genotypes shed infectious material at similar rates. We currently understand little about the relative importance of direct vs. environmental routes of transmission in wild cervid populations. It will be important to understand the ways in which these routes interact with Prnp types to influence CWD infection and progression of disease. In addition to the mode of contact, the biological source of infectious material may have an impact on infectivity. It is unknown whether cervid species have different sensitivities to particular agent conformations, genotypes, or strains. Additionally, though we know that cross-species infection is possible between elk, mule deer, and white-tailed deer, we do not understand the level of transmissibility of agent between these species, or to other mammals in the ecosystem.82 Newly developed laboratory methods including PMCA,27,83 shaking assays,84 and cell-free conversions32 may provide insights on this question.
Disease progression
Research has recently begun to examine the progression of CWD in infected animals, but currently little is known about the pathogenic mechanisms by which Prnp genotypes influence prion pathology. It will be important to determine whether genotypic or species-specific differences exist in profiles of prion accumulation as well as rates of disease progression, or whether differences exist in the physiological reaction or tolerance to prion accumulation. To track disease progression, most studies have used experimentally infected captive animals,18,19 which is essential for discerning the starting point of infection. Future research will benefit from incorporating realistic infection scenarios to evaluate the role of prion dose, which could affect progression rates measured in experimental settings.
Disease management or treatment
Recent progress toward a CWD vaccine has inspired optimism in wildlife management agencies.85,86 Naturally, careful development and efficacy trials will be required to evaluate new vaccines. The differential susceptibility and disease progression of varying Prnp genotypes add a level of complication to this endeavor. Vaccine developers should strive for vaccines that prevent infection and/or shedding of PrPres, rather than simply prolonging animal life by delaying CWD progression and potentially increasing shedding of PrPres. Currently there is no information whether any candidate vaccines will be efficacious across species or Prnp genotypes. It will be important to account for such variability in estimating the number of vaccinated animals needed to achieve herd immunity, and in weighing both the cost and efficacy of vaccination as a CWD management strategy.
Survival and selection
The survival advantage conferred by decreased CWD susceptibility can be sufficient to alter population dynamics and provide selective pressure favoring disease resistance (demonstrated for the 96S allele in white-tailed deer, in press20). Such selective pressure is rarely measurable in wild populations, and indicates the potential for CWD to impact cervid populations. Additional genetic work will be needed to evaluate potential selective pressure on other loci and in other species to understand future trends in CWD epidemics and deer populations. Further, we currently lack information about non-disease related fitness characteristics associated with Prnp genetics. This is fertile ground for future selection studies. Future research such as simulation modeling might be used to address questions of how selective pressure could change as disease prevalence alters infection hazard, as agent strains shift, or how animal movement affects disease dynamics in wildlife populations.
Concluding remarks
As early as prions were identified as a disease agent, it was noted that, “Investigations of prions have elucidated a previously unknown mechanism of disease in humans and animals.”1 Reviewing the body of literature describing prion genetics in cervids, we can report that subsequent investigations of Prnp have increased our understanding TSE epidemiology and the impacts of prion disease in both wild and captive animal populations. But each step forward in knowledge brings new questions, and there is still much research to be done to improve our understanding of how Prnp variation affects prion disease dynamics, and how to use this information for the management of disease on the landscape.
Glossary
Abbreviations:
- A
alanine
- BSE
bovine spongiform encephalopathy
- CJD
Creutzfeldt-Jakob disease
- CWD
chronic wasting disease
- D
aspartic acid
- E
glutamic acid
- F
phenylalanine
- G
glycine
- H
histidine
- I
isoleucine
- K
lysine
- L
leucine
- M
methionine
- N
asparagines
- P
proline
- Prnp
prion protein gene
- PrP
prion protein
- PrPC
normal cellular prion protein
- PrPres
protease-resistant prion protein
- Q
glutamine
- R
arginine
- S
serine
- T
threonine
- TSE
transmissible spongiform encephalopathy
- V
valine
- vCJD
variant Creutzfeldt-Jakob disease
Acknowledgments
We thank the USGS for funding supporting this work. Thanks to Sally Madsen-Bouterse for helpful comments on early drafts of this manuscript. Note that any use of trade, product or firm names is for descriptive purposes, and does not imply endorsement by the US Government.
References
- 1.Prusiner SB. . Prions. Proc Natl Acad Sci U S A 1998; 95:13363 - 83; http://dx.doi.org/ 10.1073/pnas.95.23.13363; PMID: 9811807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priola SA, Vorberg I. . Molecular aspects of disease pathogenesis in the transmissible spongiform encephalopathies. Mol Biotechnol 2006; 33:71 - 88; PMID: 16691009 [DOI] [PubMed] [Google Scholar]
- 3.Alpers MP. . Epidemiology and ecology of Kuru. Slow Transmissible Diseases of the Nervous System 1979; 1:67 - 90 [Google Scholar]
- 4.Detwiler L, Baylis M. The epidemiology of scrapie. Rev Sci Tech - Office International des Épizooties 2003; 22:121-43. [DOI] [PubMed] [Google Scholar]
- 5.Williams ES, Young S. . Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 1980; 16:89 - 98; PMID: 7373730 [DOI] [PubMed] [Google Scholar]
- 6.Williams ES. . Chronic wasting disease. Vet Pathol 2005; 42:530 - 49; http://dx.doi.org/ 10.1354/vp.42-5-530; PMID: 16145200 [DOI] [PubMed] [Google Scholar]
- 7.Keane DP, Barr DJ, Bochsler PN, Hall SM, Gidlewski T, O’Rourke KI, et al. . Chronic wasting disease in a Wisconsin white-tailed deer farm. J Vet Diagn Invest 2008; 20:698 - 703; http://dx.doi.org/ 10.1177/104063870802000534; PMID: 18776116 [DOI] [PubMed] [Google Scholar]
- 8.Bartelt G, Pardee J, Thiede J. Environmental Impact Statement on Rules to Eradicate Chronic Wasting Disease in Wisconsin’s Free-Ranging White-tailed Deer Herd. Wisconsin Department of Natural Resources, Madison, WI, USA 2003. [Google Scholar]
- 9.Miller MW, Williams ES, McCarty CW, Spraker TR, Kreeger TJ, Larsen CT, et al. . Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. J Wildl Dis 2000; 36:676 - 90; PMID: 11085429 [DOI] [PubMed] [Google Scholar]
- 10.Zheng M, Qing L, Huang S, Chen F, Wang M, Wang L, et al. Assessment of direct and indirect transmission of CWD from three cervid species to humans. Prion 2007: Paper No. FC5.8, 2007. [Google Scholar]
- 11.Bishop RC. . The economic impacts of chronic wasting disease (CWD) in Wisconsin. Hum Dimens Wildl 2004; 9:181 - 92; http://dx.doi.org/ 10.1080/10871200490479963 [DOI] [Google Scholar]
- 12.Williams ES, Miller MW, Kreeger TJ, Kahn RH, Thorne ET. . Chronic wasting disease of deer and elk: A review with recommendations for management. J Wildl Manage 2002; 66:551 - 63; http://dx.doi.org/ 10.2307/3803123 [DOI] [Google Scholar]
- 13.Aguzzi A, Sigurdson C, Heikenwaelder M. . Molecular mechanisms of prion pathogenesis. Annu Rev Pathol 2008; 3:11 - 40; http://dx.doi.org/ 10.1146/annurev.pathmechdis.3.121806.154326; PMID: 18233951 [DOI] [PubMed] [Google Scholar]
- 14.Miller MW, Williams ES, Hobbs NT, Wolfe LL. . Environmental sources of prion transmission in mule deer. Emerg Infect Dis 2004; 10:1003 - 6; PMID: 15207049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldmann W. . PrP genetics in ruminant transmissible spongiform encephalopathies. Vet Res 2008; 39:30 - 44; http://dx.doi.org/ 10.1051/vetres:2008010; PMID: 18284908 [DOI] [PubMed] [Google Scholar]
- 16.Benestad SL, Sarradin P, Thu B, Schönheit J, Tranulis MA, Bratberg B. . Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec 2003; 153:202 - 8; http://dx.doi.org/ 10.1136/vr.153.7.202; PMID: 12956297 [DOI] [PubMed] [Google Scholar]
- 17.Ironside JW, Bishop MT, Connolly K, Hegazy D, Lowrie S, Le Grice M, et al. . Variant Creutzfeldt-Jakob disease: prion protein genotype analysis of positive appendix tissue samples from a retrospective prevalence study. BMJ 2006; 332:1186 - 8; http://dx.doi.org/ 10.1136/bmj.38804.511644.55; PMID: 16606639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox KA, Jewell JE, Williams ES, Miller MW. . Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 2006; 87:3451 - 61; http://dx.doi.org/ 10.1099/vir.0.81999-0; PMID: 17030882 [DOI] [PubMed] [Google Scholar]
- 19.Johnson CJ, Herbst A, Duque-Velasquez C, Vanderloo JP, Bochsler P, Chappell R, et al. . Prion protein polymorphisms affect chronic wasting disease progression. PLoS One 2011; 6:e17450; http://dx.doi.org/ 10.1371/journal.pone.0017450; PMID: 21445256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson SJ, Samuel MD, Johnson CJ, McKenzie DI, Adams M. . Emerging prion disease drives host selection in a wildlife population. Ecol Appl 2011; In press PMID: 21774439 [DOI] [PubMed] [Google Scholar]
- 21.Collinge J, Whittington MA, Sidle KCL, Smith CJ, Palmer MS, Clarke AR, et al. . Prion protein is necessary for normal synaptic function. Nature 1994; 370:295 - 7; http://dx.doi.org/ 10.1038/370295a0; PMID: 8035877 [DOI] [PubMed] [Google Scholar]
- 22.Vassallo N, Herms J. . Cellular prion protein function in copper homeostasis and redox signalling at the synapse. J Neurochem 2003; 86:538 - 44; http://dx.doi.org/ 10.1046/j.1471-4159.2003.01882.x; PMID: 12859667 [DOI] [PubMed] [Google Scholar]
- 23.Brockes JP. . Topics in prion cell biology. Curr Opin Neurobiol 1999; 9:571 - 7; http://dx.doi.org/ 10.1016/S0959-4388(99)00016-1; PMID: 10508744 [DOI] [PubMed] [Google Scholar]
- 24.Gossert AD, Bonjour S, Lysek DA, Fiorito F, Wüthrich K. . Prion protein NMR structures of elk and of mouse/elk hybrids. Proc Natl Acad Sci U S A 2005; 102:646 - 50; http://dx.doi.org/ 10.1073/pnas.0409008102; PMID: 15647363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caughey B. . Prion protein conversions: insight into mechanisms, TSE transmission barriers and strains. Br Med Bull 2003; 66:109 - 20; http://dx.doi.org/ 10.1093/bmb/66.1.109; PMID: 14522853 [DOI] [PubMed] [Google Scholar]
- 26.Priola SA. . Prion protein and species barriers in the transmissible spongiform encephalopathies. Biomed Pharmacother 1999; 53:27 - 33; http://dx.doi.org/ 10.1016/S0753-3322(99)80057-2; PMID: 10221165 [DOI] [PubMed] [Google Scholar]
- 27.Kurt TD, Telling GC, Zabel MD, Hoover EA. . Trans-species amplification of PrP(CWD) and correlation with rigid loop 170N. Virology 2009; 387:235 - 43; http://dx.doi.org/ 10.1016/j.virol.2009.02.025; PMID: 19269662 [DOI] [PubMed] [Google Scholar]
- 28.DebBurman SK, Raymond GJ, Caughey B, Lindquist S. . Chaperone-supervised conversion of prion protein to its protease-resistant form. Proc Natl Acad Sci U S A 1997; 94:13938 - 43; http://dx.doi.org/ 10.1073/pnas.94.25.13938; PMID: 9391131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horiuchi M, Caughey B. . Specific binding of normal prion protein to the scrapie form via a localized domain initiates its conversion to the protease-resistant state. EMBO J 1999; 18:3193 - 203; http://dx.doi.org/ 10.1093/emboj/18.12.3193; PMID: 10369660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bessen RA, Raymond GJ, Caughey B. . In situ formation of protease-resistant prion protein in transmissible spongiform encephalopathy-infected brain slices. J Biol Chem 1997; 272:15227 - 31; http://dx.doi.org/ 10.1074/jbc.272.24.15227; PMID: 9182546 [DOI] [PubMed] [Google Scholar]
- 31.Morrissey MP, Shakhnovich EI. . Evidence for the role of PrP(C) helix 1 in the hydrophilic seeding of prion aggregates. Proc Natl Acad Sci U S A 1999; 96:11293 - 8; http://dx.doi.org/ 10.1073/pnas.96.20.11293; PMID: 10500170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raymond GJ, Bossers A, Raymond LD, O’Rourke KI, McHolland LE, Bryant PKI 3rd, et al. . Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J 2000; 19:4425 - 30; http://dx.doi.org/ 10.1093/emboj/19.17.4425; PMID: 10970836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. . Detection of CWD prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol 2011; 85:6309 - 18; http://dx.doi.org/ 10.1128/JVI.00425-11; PMID: 21525361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. . MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28:2731 - 9; http://dx.doi.org/ 10.1093/molbev/msr121; PMID: 21546353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jewell JE, Conner MM, Wolfe LL, Miller MW, Williams ES. . Low frequency of PrP genotype 225SF among free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. J Gen Virol 2005; 86:2127 - 34; http://dx.doi.org/ 10.1099/vir.0.81077-0; PMID: 16033959 [DOI] [PubMed] [Google Scholar]
- 36.Wilson GA, Nakada SM, Bollinger TK, Pybus MJ, Merrill EH, Coltman DW. . Polymorphisms at the PRNP gene influence susceptibility to chronic wasting disease in two species of deer (Odocoileus Spp.) in western Canada. J Toxicol Environ Health A 2009; 72:1025 - 9; http://dx.doi.org/ 10.1080/15287390903084264; PMID: 19697236 [DOI] [PubMed] [Google Scholar]
- 37.Johnson C, Johnson J, Vanderloo JP, Keane D, Aiken JM, McKenzie D. . Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J Gen Virol 2006; 87:2109 - 14; http://dx.doi.org/ 10.1099/vir.0.81615-0; PMID: 16760415 [DOI] [PubMed] [Google Scholar]
- 38.Kelly AC, Mateus-Pinilla NE, Diffendorfer J, Jewell E, Ruiz MO, Killefer J, et al. . Prion sequence polymorphisms and chronic wasting disease resistance in Illinois white-tailed deer (Odocoileus virginianus). Prion 2008; 2:28 - 36; http://dx.doi.org/ 10.4161/pri.2.1.6321; PMID: 19164895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Rourke KI, Spraker TR, Hamburg LK, Besser TE, Brayton KA, Knowles DP. . Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white-tailed deer. J Gen Virol 2004; 85:1339 - 46; http://dx.doi.org/ 10.1099/vir.0.79785-0; PMID: 15105552 [DOI] [PubMed] [Google Scholar]
- 40.Hamir AN, Gidlewski T, Spraker TR, Miller JM, Creekmore L, Crocheck M, et al. . Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J Vet Diagn Invest 2006; 18:110 - 4; http://dx.doi.org/ 10.1177/104063870601800118; PMID: 16566268 [DOI] [PubMed] [Google Scholar]
- 41.O’Rourke KI, Spraker TR, Zhuang D, Greenlee JJ, Gidlewski TE, Hamir AN. . Elk with a long incubation prion disease phenotype have a unique PrPd profile. Neuroreport 2007; 18:1935 - 8; http://dx.doi.org/ 10.1097/WNR.0b013e3282f1ca2f; PMID: 18007190 [DOI] [PubMed] [Google Scholar]
- 42.Green KM, Browning SR, Seward TS, Jewell JE, Ross DL, Green MA, et al. . The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. J Gen Virol 2008; 89:598 - 608; http://dx.doi.org/ 10.1099/vir.0.83168-0; PMID: 18198392 [DOI] [PubMed] [Google Scholar]
- 43.O’Rourke KI, Besser TE, Miller MW, Cline TF, Spraker TR, Jenny AL, et al. . PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol 1999; 80:2765 - 9; PMID: 10573173 [DOI] [PubMed] [Google Scholar]
- 44.Perucchini M, Griffin K, Miller MW, Goldmann W. . PrP genotypes of free-ranging wapiti (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol 2008; 89:1324 - 8; http://dx.doi.org/ 10.1099/vir.0.83424-0; PMID: 18420812 [DOI] [PubMed] [Google Scholar]
- 45.Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW. . A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J Wildl Dis 2007; 43:309 - 14; PMID: 17495319 [DOI] [PubMed] [Google Scholar]
- 46.Kreeger TJ, Montgomery DL, Jewell JE, Schultz W, Williams ES. . Oral transmission of chronic wasting disease in captive Shira’s moose. J Wildl Dis 2006; 42:640 - 5; PMID: 17092895 [DOI] [PubMed] [Google Scholar]
- 47.Huson HJ, Happ GM. . Polymorphisms of the prion protein gene (PRNP ) in Alaskan moose (Alces alces gigas). Anim Genet 2006; 37:425 - 6; http://dx.doi.org/ 10.1111/j.1365-2052.2006.01466.x; PMID: 16879366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Happ GM, Huson HJ, Beckmen KB, Kennedy LJ. . Prion protein genes in caribou from Alaska. J Wildl Dis 2007; 43:224 - 8; PMID: 17495306 [DOI] [PubMed] [Google Scholar]
- 49.Hamir AN, Kunkle RA, Nicholson EM, Miller JM, Hall SM, Schoenenbruecher H, et al. . Preliminary observations on the experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer. J Comp Pathol 2008; 138:121 - 30; http://dx.doi.org/ 10.1016/j.jcpa.2007.12.002; PMID: 18336829 [DOI] [PubMed] [Google Scholar]
- 50.Hamir AN, Greenlee JJ, Nicholson EM, Kunkle RA, Richt JA, Miller JM, et al. . Experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer by intracerebral route: final report. Can J Vet Res 2011; 75:152 - 6; PMID: 21731188 [PMC free article] [PubMed] [Google Scholar]
- 51.Balachandran A, Harrington NP, Algire J, Soutyrine A, Spraker TR, Jeffrey M, et al. . Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): early detection and late stage distribution of protease-resistant prion protein. Can Vet J 2010; 51:169 - 78; PMID: 20436863 [PMC free article] [PubMed] [Google Scholar]
- 52.Rhyan JC, Miller MW, Spraker TR, McCollum M, Nol P, Wolfe LL, et al. . Failure of fallow deer (Dama dama) to develop chronic wasting disease when exposed to a contaminated environment and infected mule deer (Odocoileus hemionus). J Wildl Dis 2011; 47:739 - 44; PMID: 21719844 [DOI] [PubMed] [Google Scholar]
- 53.Heaton MP, Leymaster KA, Freking BA, Hawk DA, Smith TPL, Keele JW, et al. . Prion gene sequence variation within diverse groups of U.S. sheep, beef cattle, and deer. Mamm Genome 2003; 14:765 - 77; http://dx.doi.org/ 10.1007/s00335-003-2283-y; PMID: 14722726 [DOI] [PubMed] [Google Scholar]
- 54.White SN, Spraker TR, Reynolds JO, O’Rourke KI. . Association analysis of PRNP gene region with chronic wasting disease in Rocky Mountain elk. BMC Res Notes 2010; 3:314; http://dx.doi.org/ 10.1186/1756-0500-3-314; PMID: 21087518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erlich P, Dumestre-Pérard C, Ling WL, Lemaire-Vieille C, Schoehn G, Arlaud GJ, et al. . Complement protein C1q forms a complex with cytotoxic prion protein oligomers. J Biol Chem 2010; 285:19267 - 76; http://dx.doi.org/ 10.1074/jbc.M109.071860; PMID: 20410306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haybaeck J, Heikenwalder M, Klevenz B, Schwarz P, Margalith I, Bridel C, et al. . Aerosols transmit prions to immunocompetent and immunodeficient mice. PLoS Pathog 2011; 7:e1001257; http://dx.doi.org/ 10.1371/journal.ppat.1001257; PMID: 21249178 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Blanchong JA, Heisey DM, Scribner KT, Libants SV, Johnson C, Aiken JM, et al. . Genetic susceptibility to chronic wasting disease in free-ranging white-tailed deer: complement component C1q and Prnp polymorphisms. Infect Genet Evol 2009; 9:1329 - 35; http://dx.doi.org/ 10.1016/j.meegid.2009.08.010; PMID: 19723593 [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto T. Association Mapping of Genetic Risk Factors for Chronic Wasting Disease in Wild Deer. Biological Sciences. Edmonton, AB, CAN: University of Alberta, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Béringue V, Vilotte JL, Laude H. . Prion agent diversity and species barrier. Vet Res 2008; 39:47; http://dx.doi.org/ 10.1051/vetres:2008024; PMID: 18519020 [DOI] [PubMed] [Google Scholar]
- 60.Barria MA, Telling GC, Gambetti P, Mastrianni JA, Soto C. . Generation of a new form of human PrP(Sc) in vitro by interspecies transmission from cervid prions. J Biol Chem 2011; 286:7490 - 5; http://dx.doi.org/ 10.1074/jbc.M110.198465; PMID: 21209079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruce ME, Dickinson AG. . Biological evidence that scrapie agent has an independent genome. J Gen Virol 1987; 68:79 - 89; http://dx.doi.org/ 10.1099/0022-1317-68-1-79; PMID: 3100717 [DOI] [PubMed] [Google Scholar]
- 62.Bartz JC, Marsh RF, McKenzie DI, Aiken JM. . The host range of chronic wasting disease is altered on passage in ferrets. Virology 1998; 251:297 - 301; http://dx.doi.org/ 10.1006/viro.1998.9427; PMID: 9837794 [DOI] [PubMed] [Google Scholar]
- 63.Collinge J, Clarke AR. . A general model of prion strains and their pathogenicity. Science 2007; 318:930 - 6; http://dx.doi.org/ 10.1126/science.1138718; PMID: 17991853 [DOI] [PubMed] [Google Scholar]
- 64.Morales R, Abid K, Soto C. The prion strain phenomenon: molecular basis and unprecedented features. BBA Molecular Basis of Disease 2007; 1772:681-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aguzzi A, Heikenwalder M, Polymenidou M. . Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol 2007; 8:552 - 61; http://dx.doi.org/ 10.1038/nrm2204; PMID: 17585315 [DOI] [PubMed] [Google Scholar]
- 66.Race B, Meade-White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, et al. . Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 2009; 15:1366 - 76; http://dx.doi.org/ 10.3201/eid1509.090253; PMID: 19788803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamir AN, Kunkle RA, Cutlip RC, Miller JM, O’Rourke KI, Williams ES, et al. . Experimental transmission of chronic wasting disease agent from mule deer to cattle by the intracerebral route. J Vet Diagn Invest 2005; 17:276 - 81; http://dx.doi.org/ 10.1177/104063870501700313; PMID: 15945388 [DOI] [PubMed] [Google Scholar]
- 68.Hamir AN, Miller JM, Kunkle RA, Hall SM, Richt JA. . Susceptibility of cattle to first-passage intracerebral inoculation with chronic wasting disease agent from white-tailed deer. Vet Pathol 2007; 44:487 - 93; http://dx.doi.org/ 10.1354/vp.44-4-487; PMID: 17606510 [DOI] [PubMed] [Google Scholar]
- 69.Raymond GJ, Raymond LD, Meade-White KD, Hughson AG, Favara C, Gardner D, et al. . Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. J Virol 2007; 81:4305 - 14; http://dx.doi.org/ 10.1128/JVI.02474-06; PMID: 17287284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, et al. . Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 2010; 328:1154 - 8; http://dx.doi.org/ 10.1126/science.1187107; PMID: 20466881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. . High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med 1994; 1:19 - 30; PMID: 8790598 [PMC free article] [PubMed] [Google Scholar]
- 72.Brown P, Gajdusek DC. . Survival of scrapie virus after 3 years’ interment. Lancet 1991; 337:269 - 70; http://dx.doi.org/ 10.1016/0140-6736(91)90873-N; PMID: 1671114 [DOI] [PubMed] [Google Scholar]
- 73.Pedersen JA, Johnson CJ, Ma X, Russo F, Benson CH, McKenzie D, et al. Fate of Prions in Soils. Proc Water Environment Federation 2007; 2007:7868-77. [Google Scholar]
- 74.Sun R, Liu Y, Zhang H, Manuelidis L. . Quantitative recovery of scrapie agent with minimal protein from highly infectious cultures. Viral Immunol 2008; 21:293 - 302; http://dx.doi.org/ 10.1089/vim.2008.0039; PMID: 18788938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arjona A, Simarro L, Islinger F, Nishida N, Manuelidis L. . Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures. Proc Natl Acad Sci U S A 2004; 101:8768 - 73; http://dx.doi.org/ 10.1073/pnas.0400158101; PMID: 15161970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murdoch GH, Sklaviadis T, Manuelidis EE, Manuelidis L. . Potential retroviral RNAs in Creutzfeldt-Jakob disease. J Virol 1990; 64:1477 - 86; PMID: 2108258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gordon PMK, Schütz E, Beck J, Urnovitz HB, Graham C, Clark R, et al. . Disease-specific motifs can be identified in circulating nucleic acids from live elk and cattle infected with transmissible spongiform encephalopathies. Nucleic Acids Res 2009; 37:550 - 6; http://dx.doi.org/ 10.1093/nar/gkn963; PMID: 19059996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gabus C, Auxilien S, Péchoux C, Dormont D, Swietnicki W, Morillas M, et al. . The prion protein has DNA strand transfer properties similar to retroviral nucleocapsid protein. J Mol Biol 2001; 307:1011 - 21; http://dx.doi.org/ 10.1006/jmbi.2001.4544; PMID: 11286552 [DOI] [PubMed] [Google Scholar]
- 79.Grear DA, Samuel MD, Langenberg JA, Keane D. . Demographic patterns and harvest vulnerability of chronic wasting disease infected white-tailed deer in Wisconsin. J Wildl Manage 2006; 70:546 - 53; http://dx.doi.org/ 10.2193/0022-541X(2006)70[546:DPAHVO]2.0.CO;2 [DOI] [Google Scholar]
- 80.Miller MW, Conner MM. . Epidemiology of chronic wasting disease in free-ranging mule deer: spatial, temporal, and demographic influences on observed prevalence patterns. J Wildl Dis 2005; 41:275 - 90; PMID: 16107661 [DOI] [PubMed] [Google Scholar]
- 81.Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, et al. . Asymptomatic deer excrete infectious prions in faeces. Nature 2009; 461:529 - 32; http://dx.doi.org/ 10.1038/nature08289; PMID: 19741608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jennelle CS, Samuel MD, Nolden CA, Berkley EA. . Deer carcass decomposition and potential scavenger exposure to chronic wasting disease. J Wildl Manage 2009; 73:655 - 62; http://dx.doi.org/ 10.2193/2008-282 [DOI] [Google Scholar]
- 83.Kurt TD, Seelig DM, Schneider JR, Johnson CJ, Telling GC, Heisey DM, et al. . Alteration of the chronic wasting disease species barrier by in vitro prion amplification. J Virol 2011; 85:8528 - 37; http://dx.doi.org/ 10.1128/JVI.00809-11; PMID: 21697475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Coulthart MB, Balachandran A, Chakrabartty A, Cashman NR. . Species barriers for chronic wasting disease by in vitro conversion of prion protein. Biochem Biophys Res Commun 2007; 364:796 - 800; http://dx.doi.org/ 10.1016/j.bbrc.2007.10.087; PMID: 17964288 [DOI] [PubMed] [Google Scholar]
- 85.Li L, Napper S, Cashman NR. . Immunotherapy for prion diseases: opportunities and obstacles. Immunotherapy 2010; 2:269 - 82; http://dx.doi.org/ 10.2217/imt.10.3; PMID: 20635933 [DOI] [PubMed] [Google Scholar]
- 86.Hedlin PD, Cashman NR, Li L, Gupta J, Babiuk LA, Potter AA, et al. . Design and delivery of a cryptic PrP(C) epitope for induction of PrP(Sc)-specific antibody responses. Vaccine 2010; 28:981 - 8; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.134; PMID: 19925901 [DOI] [PubMed] [Google Scholar]