Abstract
Objective
The detailed associations between type 2 diabetes (T2D) and total stroke and magnesium intake as well as the dose–response trend should be updated in a timely manner.
Design
Systematic review and meta-analyses.
Data sources
PubMed, Embase, Cochrane Library, Web of Science and ClinicalTrials.gov were rigorously searched from inception to 15 March 2019.
Eligibility criteria
Prospective cohort studies investigating these two diseases were included.
Data synthesis
Relative risk (RR) and 95% CI in random effects models as well as absolute risk (AR) were pooled to calculate the risk of T2D and stroke. Methodological quality was assessed by the Newcastle-Ottawa Scale.
Results
Forty-one studies involving 53 cohorts were included. The magnitude of the risk was significantly reduced by 22% for T2D (RR 0.78 (95% CI 0.75 to 0.81); p<0.001; AR reduction 0.120%), 11% for total stroke (RR 0.89 (95% CI 0.83 to 0.94); p<0.001; AR reduction 0.281%) and 12% for ischaemic stroke (RR 0.88 (95% CI 0.81 to 0.95); p=0.001; AR reduction 0.246%) when comparing the highest magnesium intake to the lowest. The inverse association still existed when studies on T2D were adjusted for cereal fibre (RR 0.79; p<0.001) and those on total stroke were adjusted for calcium (RR 0.89; p=0.040). Subgroup analyses suggested that the risk for total and ischaemic stroke was significantly decreased in females, participants with ≥25 mg/m2 body mass index and those with ≥12-year follow-up; the reduced risk in Asians was not as notable as that in North American and European populations.
Conclusions
Magnesium intake has significantly inverse associations with T2D and total stroke in a dose-dependent manner. Feasible magnesium-rich dietary patterns may be highly beneficial for specific populations and could be highlighted in the primary T2D and total stroke prevention strategies disseminated to the public.
PROSPERO registration number
CRD42018092690.
Keywords: magnesium intake, type 2 diabetes, stroke, meta-analysis
Strengths and limitations of this study.
In this study, we performed an updated comprehensive quantitative analysis focusing on the dietary effect of magnesium intake.
The study identified an inverse association between magnesium intake and T2D and stroke.
A quite number of prospective cohort studies were employed to guarantee the robust evidence.
Limitation of study was non-inclusion of randomised controlled trails to prove the causality.
Cases ascertainments are limited by food frequency questionnaires or self-reports.
Introduction
Diabetes is a global burden with an alarming increasing rate throughout the world.1 2 Stroke is an independent disorder and a typical macrovascular complication of type 2 diabetes (T2D), and it is regarded as the second leading cause of death after ischaemic heart disease.3 4 These pandemic health problems necessitate better primary prevention strategies.
Magnesium, a common cellular ion, acts as a critical cofactor for hundreds of enzymes involved in glucose metabolism, protein production and nucleic acid synthesis.5 6 Low levels of magnesium have been associated with many chronic and inflammatory diseases, such as Alzheimer’s disease, asthma, attention deficit hyperactivity disorder, insulin resistance, T2D, hypertension, cardiovascular disease (CVD; eg, stroke), migraine headaches, osteoporosis and cancer.1 5 7 8
Notably, many adults in developed countries do not consume the recommended daily amount of magnesium-rich foods such as whole grains, nuts and green leafy vegetables, and magnesium is less mentioned in dietary guidelines and in studies on T2D or stroke prevention.9 10 Thus, we chose T2D and stroke as our outcome of interest (CVD was not evaluated because there is already a wealth of research relating to CVD, and the definitions of CVD vary greatly among studies, which would increase the heterogeneity in the pooled process and impair our interpretation of the final conclusions). Emerging studies11–51 on this topic are limited, and the results remain mixed. For example, most studies have indicated that magnesium intake has an inverse association with T2D or total stroke incidence; however, several others have revealed that there is an inverse trend but not a significant association, which is possibly due to limitations related to small sample sizes and differences in the intervention duration, study design and participant characteristics. Moreover, consecutive meta-analyses52 53 have used less rigorous inclusion; the results were not comprehensive, and they did not completely address the influence of other confounders (ie, body mass index (BMI), cereal fibre, calcium, potassium) on the relationship. Accordingly, we performed a meta-analysis to (1) establish a comprehensive estimate and update the epidemiological evidence for clinical practice; (2) discuss the results of stroke subtype and the impact of several statistical and epidemiology confounders on the investigated association; and (3) highlight the details of the dose–response pattern observed among the participants analysed in the studies.
Methods
This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (online supplementary table S1) and the Meta-analysis of Observational Studies in Epidemiology Guidelines Checklist (online supplementary table S2).
bmjopen-2019-032240supp001.pdf (74.8KB, pdf)
bmjopen-2019-032240supp002.pdf (43.2KB, pdf)
Search strategy
PubMed, Embase, Cochrane Library, Web of Science and ClinicalTrials.gov were systematically reviewed through inception to 15 March 2019, for studies on magnesium intake and T2D or stroke without language restrictions. The following key words were used: “Magnesium”, “Type 2 Diabetes Mellitus”, “Type 2 Diabetes”, “Stroke”, “Cerebrovascular Stroke”, “Cohort Studies” and “Prospective Studies”. We also manually searched the reference lists of the retrieved literature (including meta-analyses and brief reports), bibliographies and grey literature (including presentations and unpublished literature) for further eligible articles. The search strategy can be found in online supplementary table S3.
bmjopen-2019-032240supp003.pdf (39.4KB, pdf)
Selection criteria
(1) Eligible populations must be composed of individuals with plausible dietary/energy intake who had no history of diabetes and/or insulin treatment for T2D analysis and no current stroke for stroke analysis. (2) Their apparent life expectancy was long enough for proper follow-up. (3) We included only prospective cohort studies that reported magnesium intake and T2D and/or various types of stroke. (4) The follow-up duration of eligible studies was at least 1 year if they provided follow-up data. Notably, magnesium intake consisted of both dietary magnesium intake and total magnesium intake (dietary and supplementary magnesium).
Only studies containing the most comprehensive information on the population or endpoints were included to avoid duplication. We excluded reviews, basic science studies, meta-analyses, studies on gestational diabetes mellitus and studies that focused only on magnesium supplementation.
Data extraction and quality assessments
Two researchers independently extracted the following information: the first author, publication year, period of cohort studies, duration of persistent exposure, basic characteristics of the enrolled participants (weight, age, region, BMI, drinking and smoking habits (previous plus current), etc), median magnesium intake for each quantile (tertile, quartile, or quintile), diabetes and total stroke cases, subtypes of total stroke, dietary and case assessments, adjusted confounding covariates. Importantly, total stroke is classified as clinical ischaemic stroke (87%), haemorrhagic stroke (13%) and undetermined stroke.54 Haemorrhagic stroke is classified as subarachnoid haemorrhage and intracerebral haemorrhage according to anatomical site or presumed aetiology.55 In cases of continuing disagreement, a final decision was reached after discussion with a third member of the panel.
Methodological quality was described by the Newcastle-Ottawa Scale (NOS), which was validated for assessment of the quality of non-randomised controlled trials (RCTs) in meta-analyses.56 For the 0–10 scale, each study was categorised as low (0–5), medium (6–7) or high (8–10) quality.
Statistical analysis
Articles providing data separately for men and women or black and white or different types of disease within an article were treated as independent studies. Multivariate relative risk (RR) and corresponding 95% CI as well as absolute risk (AR) for measuring the quantitative associations between exposure and T2D, total stroke and other wanted outcomes, particularly for the highest versus the lowest categories of magnesium intake, were estimated by the DerSimonian-Laird random effects model because the assumptions involved account for the presence of within-study and between-study variability. Statistical heterogeneity was determined with the Cochran Q χ2 test and the I2. An I2 >50% or a p value for the Q test <0.1 was considered to indicate significant heterogeneity.57 We performed sensitivity analyses to test the robustness and postsubgroup analyses to detect the source of heterogeneity. In addition, a random effects meta-regression analysis on BMI, sex, participant region and dietary assessments with RR for each trial was performed to obtain an understanding of the reasons for heterogeneity. RR and 95% CI might begin to significantly change as publication years increased in T2D and total stroke, which would be validated by cumulative meta-analyses.
The dose–response analyses for all outcomes were proposed by Greenland and Longnecker58 and Orsini et al59. The categories of magnesium intake, distributions of cases and person year, RR and 95 CI were extracted. If the number of cases and/or person years was not available, variance-weighted least squares regression was used to pool the risk estimate. For most studies, the median intake for each quantile (tertile, quartile or quintile) of magnesium intake was assigned as the representative dose. For continuous intake, which was reported as categorical data (range) in some studies, we assigned the midpoint category of the lower and upper bounds to the RR in these studies; when the highest category was open ended, we assumed the length of the open-ended interval to be 1.5 times the adjacent interval; when the lowest category was open, we assigned the adjacent interval of the category to be 1.5 times the length of the open-ended interval. We employed generalised least squares regression models to calculate study-specific RR estimates per 50 mg/day, 100 mg/day and 150 mg/day magnesium intake increment if there was evidence of a linear relationship. Nonlinear relationships between magnesium intake and all outcomes were evaluated using restricted cubic splines with four knots located at the 5th, 35th, 65th and 95th percentiles of the distribution. The p value for curve linearity or nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to zero. All results were presented using two-stage dose–response model plots (including linear and nonlinear relationships). Some results were demonstrated as forest plots for intake increments of <50 mg/day, ≥50 and<100 mg/day, ≥100 and <150 mg/day, and ≥150 mg/day.
Publication bias was assessed graphically by Begg’s adjusted rank correlation funnel plots60 and Egger’s linear regression tests.61 All analyses were performed using Stata V.14.0 (StataCorp); two-sided p<0.05 was considered statistically significant except where otherwise specified.
Patient and public involvement
No patients were involved in developing the research question or the outcome measures, and no patients were involved in planning the design or implementation of the study. Furthermore, no patients were asked to advise on the interpretation or write-up of the results. Since this study used aggregated data from previous publications, it is not easy to disseminate the results of the research to study participants directly.
Results
Study characteristics and quality assessment
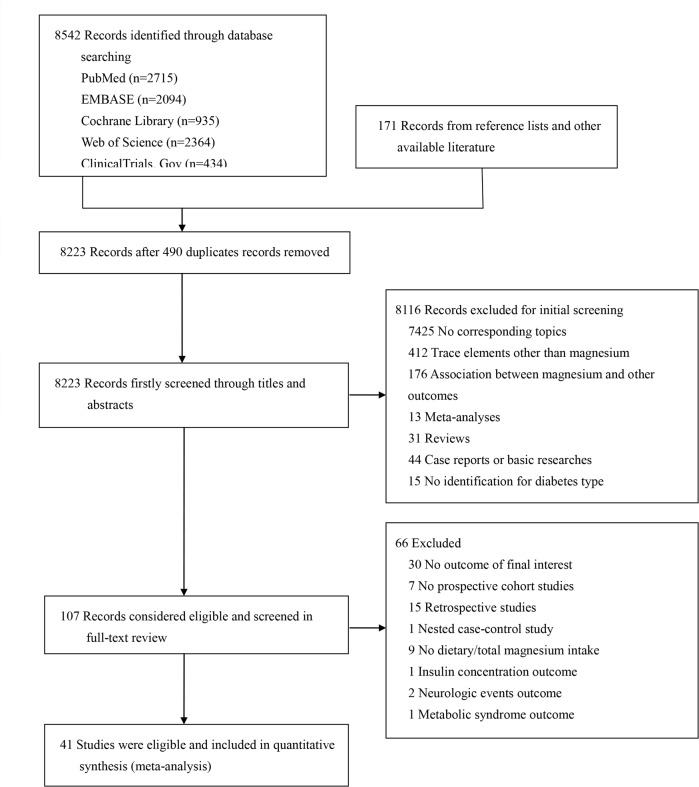
Of the 8713 studies, 107 studies were considered for eligibility after screening the titles and abstracts (figure 1). A total of 4111–51 prospective cohort studies comprising 53 cohorts, 1 912 634 participants and 76 678 cases were eligible for inclusion in the systematic review and meta-analysis (online supplementary table S4). Hodge et al18 recorded only 500 mg/day increments of magnesium for further pooled analyses; two studies33 51 failed to clearly distinguish the diabetes type, but the vast majority of cases had T2D. We computed the subtype data in three studies14 27 36 after the extraction of total stroke, and we regarded ischaemic stroke in three other studies28 30 42 as total stroke given that ischaemic stroke accounted for nearly 87% of total stroke. Participants were predominately middle aged at baseline, with a mean magnesium intake of 370 mg/day for the highest category and 232 mg/day for the lowest category. The mean duration of all eligible studies was 10.7 years. Nineteen studies were conducted in North America (America); 5 studies were conducted in Europe (Sweden, the Netherlands and Britain); 13 studies were conducted in Asia (China and Japan and Taipei); and 4 studies enrolled individuals in multiple nations. Most of the included studies used food frequency questionnaires (FFQs) or semiquantitative FFQs to assess individual dietary intake. Eighteen studies used dietary magnesium intake, and 21 studies recorded total magnesium intake (dietary and supplementary magnesium intake). Of note, supplementary magnesium intake was assessed by the use of magnesium or multivitamin supplements; nevertheless, dietary magnesium accounted for the majority of magnesium intake. Adjusted confounders were mostly similar; however, adjusted dietary confounders such as cereal fibre, potassium and calcium still varied across individual studies. It was unclear whether the included studies had adjusted for sodium because they did not provide this information. All the studies were written in English.
Figure 1.
Flow chart for the literature search and screening process.
bmjopen-2019-032240supp004.pdf (60.7KB, pdf)
After the quality assessments of the studies according to NOS, the average score was 8.85 (online supplementary table S5), and all studies were of high quality (NOS scores 8–10).
bmjopen-2019-032240supp005.pdf (20.3KB, pdf)
Magnesium intake and T2D incidence
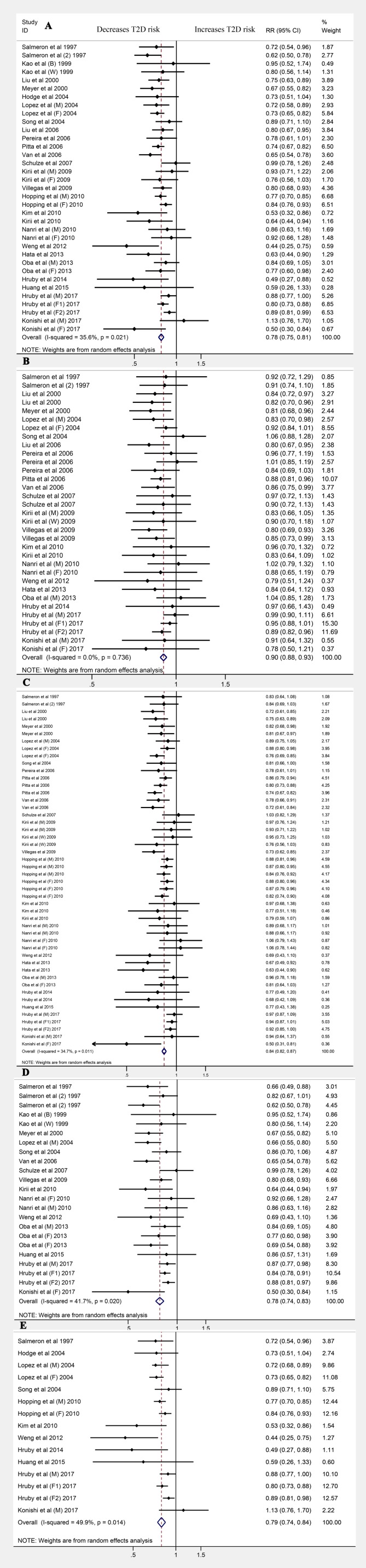
Thirty-five cohorts from 26 publications11 12 15 20 22–26 29 31–35 37 39 41 43 48 49 51 (1 219 636 participants and 56 540 T2D cases) reported that the magnitude of T2D risk was reduced by 22% (RR 0.78 (95% CI 0.75 to 0.81); p<0.001; AR reduction 0.120%), comparing the highest category of magnesium intake to the lowest, with little evidence of heterogeneity (I2=35.6%; p=0.021). The dose category-specific analysis suggested that for the <50 mg/day magnesium increment, the risk of T2D was reduced by 10% (RR 0.90 (95% CI 0.88 to 0.93); p<0.001); for the ≥50 and<100 mg/day increments, the risk was decreased by 16% (RR 0.84 (95% CI 0.82 to 0.87); p<0.001); for ≥100 and<150 mg/day increments, the risk was reduced by 22% (RR 0.78 (95% CI 0.74 to 0.83); p<0.001); and for the ≥150 mg/day increment, the risk was reduced by 21% (RR 0.79 (95% CI 0.74 to 0.84); p<0.001; figure 2). Little evidence of publication bias was found (Egger’s test: p=0.088; online supplementary figure S1A).
Figure 2.
Forest plots for the risk of type 2 diabetes (T2D) for (A) magnesium intake and for (B) <50 mg/day, (C) ≥50 and <100 mg/day, (D) ≥100 and <150 mg/day and (E) ≥150 mg/day increments (E).
bmjopen-2019-032240supp006.pdf (140.6KB, pdf)
Magnesium intake and stroke incidence
Eighteen cohorts from 15 publications13 14 21 27 28 30 36 38 40 42 44–47 50 (692 998 participants and 20 138 total stroke cases) reported that the magnitude of the risk of total stroke was decreased by 11% (RR 0.89 (95% CI 0.83 to 0.94); p<0.001; AR reduction 0.281%), comparing the highest category of magnesium intake with the lowest, with no heterogeneity (I2=0%; p=0.529). The dose category-specific analysis revealed no significant association with the <50 mg/day, ≥50 and<100 mg/day increments or the ≥100 and<150 mg/day increments. For the ≥150 mg/day increment, the risk of total stroke was decreased by 15% (RR 0.85 (95% CI 0.79 to 0.91); p<0.001; online supplementary figure S2). Publication bias was evaluated for stroke subtypes.
bmjopen-2019-032240supp007.pdf (643.3KB, pdf)
Fifteen cohorts from 12 publications14 21 27 28 30 36 38 40 42 45 46 50 reported ischaemic stroke. The magnitude of the risk of ischaemic stroke was reduced by 12% (RR 0.88 (95% CI 0.81 to 0.95); p=0.001; AR reduction 0.246%) with no significant heterogeneity (I2=16.9%; p=0.265). The dose category-specific analysis identified no significant association with the <50 mg/day, ≥50 and<100 mg/day, or ≥100 and<150 mg/day increments. A decreasing trend existed but remained non-significant. The original risk was reduced by 16% in the analysis of the ≥150 mg/day increment (RR 0.84 (95% CI 0.78 to 0.91); p<0.001; online supplementary figure S3). No publication bias was observed in terms of ischaemic stroke (Egger’s test: p=0.937; online supplementary figure S1B).
bmjopen-2019-032240supp008.pdf (517.2KB, pdf)
Ten cohorts from eight studies14 21 27 36 38 45 46 50 reported that haemorrhagic stroke was not significantly associated with magnesium intake (RR 0.93 (95% CI 0.82 to 1.06); p=0.282). The dose category-specific analysis identified no significant association (online supplementary figure S4). No significant heterogeneity or publication bias was observed in terms of haemorrhagic stroke (Egger’s test: p=0.809; online supplementary figure S1C).
bmjopen-2019-032240supp009.pdf (424.4KB, pdf)
Three publications involving three cohorts14 27 36 showed that high magnesium intake had no significant effect on reducing the risk of subarachnoid haemorrhage (RR 0.99 (95% CI 0.71 to 1.39); p=0.963). The dose category-specific analysis revealed no significant association (online supplementary figure S5).
bmjopen-2019-032240supp010.pdf (243.7KB, pdf)
With respect to intracerebral haemorrhage, the pooled results from three cohorts14 27 36 in three publications revealed no significant advantages of intracerebral haemorrhage (RR 0.92 (95% CI 0.71 to 1.20); p=0.540). The dose category-specific analysis revealed no significant association (online supplementary figure S6).
bmjopen-2019-032240supp011.pdf (237KB, pdf)
Meta-regression and cumulative meta-analysis
According to the meta-regression results, there was no evidence of BMI, sex, participant region or dietary assessment for each individual trial bias in terms of T2D (online supplementary figure S7), total stroke (online supplementary figure S8), ischaemic stroke (online supplementary figure S9) and haemorrhagic stroke events (online supplementary figure S10). The male subgroup (p=0.041) in the sex category might lead to slight heterogeneity in terms of total stroke; however, sex (p=0.112) showed no association with total stroke incidence.
bmjopen-2019-032240supp012.pdf (322KB, pdf)
bmjopen-2019-032240supp013.pdf (308.1KB, pdf)
bmjopen-2019-032240supp014.pdf (315.8KB, pdf)
bmjopen-2019-032240supp015.pdf (304.8KB, pdf)
Analyses of T2D (online supplementary figure S11), total stroke (online supplementary figure S12) and ischaemic stroke demonstrated that the RRs of the final results became robust within a narrow range and remained significant as publication years increased and more recent high-quality studies were included. After inclusion of the Iso et al14 study, the RR and 95% CI for ischaemic stroke decreased to less than 1 and then became stable (online supplementary figure S13). Although there was no significant reduction in the risk of haemorrhagic stroke, evidence clearly showed that the CI was becoming narrow, which trended towards significance (online supplementary figure S14). Thus, the risk for haemorrhagic stroke might be reduced; additional studies are warranted.
bmjopen-2019-032240supp016.pdf (185.1KB, pdf)
bmjopen-2019-032240supp017.pdf (166.2KB, pdf)
bmjopen-2019-032240supp018.pdf (75.3KB, pdf)
bmjopen-2019-032240supp019.pdf (61.9KB, pdf)
Sensitivity analysis
When three24–26 studies were excluded from the T2D analysis, the summary RR changed from 0.78 (95% CI 0.75 to 0.81) to 0.78 (95% CI 0.75 to 0.82), with the heterogeneity declining from (I2=35.6%; p=0.021) to (I2=24.0%; p=0.112). Among T2D analyses, eight studies19 22 23 26 33 39 48 49 adjusted for cereal fibre intake yielded an RR of 0.79 (95% CI 0.73 to 0.85; p<0.001), and two studies15 35 adjusted for calcium yielded an RR of 0.87 (95% CI 0.73 to 1.04; p=0.128). Among the total stroke analysis, the summary RR was 0.92 (95% CI 0.82 to 1.02; p=0.097) in five studies13 44–46 50 adjusted for potassium intake and was 0.89 (95% CI 0.80 to 0.99; p=0.040) in five studies14 44–46 50 adjusted for calcium. Only one study15 adjusted for potassium intake in T2D, and one study36 adjusted for cereal fibre in total stroke.
Subgroup analysis
Stratified analyses by characteristics of the population and study design were conducted on T2D (table 1), total stroke, ischaemic stroke and haemorrhagic stroke (table 2). The inverse association with T2D remained robust across all subgroups with little evidence of heterogeneity. For stroke incidence, a decreased risk of total stroke and ischaemic stroke was found in female participants (RR 0.91 (95% CI 0.83 to 0.99) for total stroke; 0.89 (95% CI 0.79 to 1.00) for ischaemic stroke) and individuals with ≥25 kg/m2 mean BMI (RR 0.89 (95% CI 0.82 to 0.96) for total stroke; 0.88 (95% CI 0.81 to 0.96) for ischaemic stroke). When restricted to a≥12-year follow-up, the risk of total stroke and ischaemic stroke was significantly reduced (RR 0.89 (95% CI 0.83 to 0.95) for total stroke; 0.88 (95% CI 0.81 to 0.95) for ischaemic stroke). These risks were more reduced in North American and European individuals than in Asians. Cardiovascular events (CV events, coronary heart disease, heart failure, atrial fibrillation, self-reported heart disease, etc, other than stroke), hypercholesterolaemia and diabetes would blunt the effect of magnesium on total and ischaemic stroke. However, magnesium intake could still, or at least, demonstrate the trend to decrease total and ischaemic stroke in individuals even with those risk factors. Similarly, CV events, hypercholesterolaemia and family diabetes history had no substantial impact on the inverse association between T2D incidence and magnesium intake. We did not find a significantly reduced risk of haemorrhagic stroke in the subgroup analyses.
Table 1.
Subgroup analysis relating to magnesium intake and type 2 diabetes (T2D)
| Group | T2D | |||||
| No of studies | RR (95% CI) | PES | Pheterogeneity | I2 (%) | Pinteraction | |
| Total | 26 | 0.78 (0.75 to 0.81) | <0.001 | 0.021 | 35.6 | NA |
| Participants region | 26 | 0.905 | ||||
| North America | 13 | 0.77 (0.73 to 0.82) | <0.001 | 0.048 | 39.5 | |
| Europe | 0 | NA | NA | NA | NA | |
| Asia | 9 | 0.78 (0.71 to 0.87) | <0.001 | 0.165 | 21.7 | |
| Multiple nations | 4 | 0.79 (0.71 to 0.88) | <0.001 | 0.048 | 58.3 | |
| Sex* | 34 | 0.284 | ||||
| Male | 9 | 0.81 (0.76 to 0.87) | <0.001 | 0.337 | 11.7 | |
| Female | 17 | 0.77 (0.73 to 0.81) | <0.001 | 0.055 | 37.5 | |
| Both† | 8 | 0.70 (0.57 to 0.85) | <0.001 | 0.067 | 45.3 | |
| BMI (kg/m2) | 26 | 0.716 | ||||
| ≥25 | 12 | 0.75 (0.69 to 0.81) | <0.001 | 0.135 | 31 | |
| <25 | 11 | 0.78 (0.74 to 0.83) | <0.001 | 0.022 | 45.4 | |
| Unknown | 3 | 0.81 (0.76 to 0.86) | <0.001 | 0.586 | 0 | |
| Follow-up duration (y) | 26 | 0.150 | ||||
| ≥10 | 12 | 0.80 (0.76 to 0.84) | <0.001 | 0.047 | 38.8 | |
| <10 | 14 | 0.74 (0.68 to 0.80) | <0.001 | 0.164 | 25.2 | |
| Dietary assessment | 26 | 0.281 | ||||
| FFQ/validated FFQ | 15 | 0.77 (0.73 to 0.82) | <0.001 | 0.159 | 23.7 | |
| SFFQ/validated SFFQ | 9 | 0.79 (0.74 to 0.84) | <0.001 | 0.017 | 52.5 | |
| Other | 2 | 0.55 (0.36 to 0.83) | 0.005 | 0.826 | 0 | |
| Magnesium intake type‡ | 28 | 0.335 | ||||
| Total magnesium intake§ | 15 | 0.79 (0.75 to 0.84) | <0.001 | 0.035 | 39.8 | |
| Dietary magnesium intake | 13 | 0.77 (0.72 to 0.82) | <0.001 | 0.166 | 25.0 | |
| Total energy adjustment | 26 | |||||
| Yes | 17 | 0.79 (0.74 to 0.84) | <0.001 | 0.027 | 40.4 | 0.396 |
| No | 9 | 0.76 (0.72 to 0.81) | <0.001 | 0.225 | 21.6 | 0.671 |
| Difference between top and bottom intake (mg/day)¶ | 27 | |||||
| ≥140 | 13 | 0.78 (0.74 to 0.83) | <0.001 | 0.020 | 45.3 | |
| <140 | 14 | 0.77 (0.72 to 0.82) | <0.001 | 0.209 | 21.0 | |
| Current CV events status** | 26 | 0.536 | ||||
| Yes | 13 | 0.79 (0.74 to 0.83) | <0.001 | 0.049 | 37.9 | |
| Unknown | 13 | 0.77 (0.71 to 0.82) | <0.001 | 0.082 | 35.1 | |
| Hypercholesterolaemia status†† |
26 | 0.625 | ||||
| Yes | 5 | 0.79 (0.73 to 0.85) | <0.001 | 0.021 | 57.5 | |
| Unknown | 21 | 0.77 (0.73 to 0.82) | <0.001 | 0.096 | 27.3 | |
| Family diabetes history | 26 | 0.168 | ||||
| Yes | 17 | 0.76 (0.72 to 0.80) | <0.001 | 0.021 | 41.8 | |
| Unknown | 9 | 0.81 (0.76 to 0.87) | <0.001 | 0.258 | 14.3 | |
*Male and female of T2D outcome were treated as independent cohorts within eight studies.
†Male and female participants were in independent cohorts.
‡Two studies reported total magnesium and dietary magnesium intake outcome.
§Total magnesium intake (mg/day) included the total amount of magnesium from both food (diet) and supplement.
¶Subtract the lowest category intake from the highest. Oba el al (M) was in <140 group, while Oba el al (F) was in ≥140 group.
**Grouped by whether participants with or without cardiovascular events (CV events). CV events in this part include coronary heart disease, heart failure, stroke, atrial fibrillation and self-reported heart disease.
††Grouped by whether participants with or without hypercholesterolaemia. Hypercholesterolaemia in this part means cholesterol concentration ≥240 mg/dL.
BMI, body mass index; ES, effect size; FFQ, food frequency questionnaire; NA, not available; RR, relative risk; SFFQ, semiquantitative food frequent questionnaire.
Table 2.
Subgroup analyses relating to magnesium intake and total stroke, Ischaemic stroke, haemorrhagic stroke
| Group | Total stroke | Ischaemic stroke | Haemorrhagic stroke | |||||||||
| No of studies | RR (95% CI) | I2 (%) | Pinteration | No of studies | RR (95% CI) | I2 (%) | Pinteration | No of studies | RR (95% CI) | I2 (%) | Pinteration | |
| Total | 15 | 0.89 (0.83 to 0.94) | 0.00 | NA | 12 | 0.88 (0.81 to 0.95) | 16.90 | NA | 8 | 0.93 (0.82 to 1.06) | 0.461 | NA |
| Participants region | 15 | 0.733 | 12 | 0.584 | 8 | 0.873 | ||||||
| North America | 6 | 0.87 (0.79 to 0.96) | 0.00 | 5 | 0.85 (0.76 to 0.95) | 0.00 | 4 | 0.90 (0.71 to 1.15) | 0.00 | |||
| Europe | 5 | 0.87 (0.77 to 0.98) | 14.80 | 3 | 0.86 (0.78 to 0.95) | 0.00 | 2 | 0.99 (0.79 to 1.25) | 0.00 | |||
| Asia | 4 | 0.90 (0.78 to 1.05) | 32.80 | 4 | 0.93 (0.75 to 1.14) | 45.50 | 2 | 0.89 (0.66 to 1.21) | 53.40 | |||
| Multiple nations | 0 | NA | NA | 0 | NA | NA | 0 | NA | NA | |||
| Sex* | 18 | 0.031 | 14 | 0.134 | 10 | 0.425 | ||||||
| Male | 6 | 0.95 (0.86 to 1.05) | 0.00 | 4 | 0.99 (0.82 to 1.19) | 52.80 | 4 | 0.97 (0.75 to 1.26) | 35.50 | |||
| Female | 7 | 0.91 (0.83 to 0.99) | 0.00 | 6 | 0.89 (0.79 to 1.00) | 0.00 | 6 | 0.88 (0.74 to 1.06) | 0.00 | |||
| Both† | 5 | 0.74 (0.64 to 0.85) | 0.00 | 4 | 0.76 (0.65 to 0.88) | 0.00 | 0 | NA | NA | |||
| Mean BMI (kg/m2) | 15 | 0.606 | 12 | 0.631 | 8 | 0.418 | ||||||
| ≥25 | 8 | 0.89 (0.82 to 0.96) | 0.00 | 6 | 0.88 (0.81 to 0.96) | 0.00 | 5 | 0.97 (0.81 to 1.17) | 0.00 | |||
| <25 | 5 | 0.89 (0.78 to 1.01) | 30.00 | 5 | 0.87 (0.73 to 1.03) | 44.00 | 3 | 0.88 (0.69 to 1.12) | 39.30 | |||
| Unknown | 2 | 0.80 (0.63 to 1.02) | 0.00 | 1 | 0.76 (0.57 to 1.07) | NA | 0 | NA | NA | |||
| Follow-up duration (years) | 15 | 0.798 | 12 | 0.811 | 8 | 0.808 | ||||||
| ≥12 | 11 | 0.88 (0.82 to 0.94) | 5.30 | 10 | 0.87 (0.80 to 0.95) | 19.10 | 7 | 0.93 (0.81 to 1.08) | 7.70 | |||
| <12 | 4 | 0.90 (0.77 to 1.05) | 0.00 | 2 | 0.86 (0.62 to 1.20) | 48.40 | 1 | 0.88 (0.57 to 1.36) | NA | |||
| Dietary assessment | 15 | 0.578 | 12 | NA | 8 | NA | ||||||
| FFQ/validated FFQ | 14 | 0.89 (0.83 to 0.95) | 3.80 | 12 | 0.88 (0.81 to 0.95) | 16.90 | 8 | 0.93 (0.82 to 1.06) | 0.00 | |||
| SFFQ/validated SFFQ | 0 | NA | NA | 0 | NA | NA | 0 | NA | NA | |||
| Other | 1 | 0.81 (0.61 to 1.09) | 0.00 | 0 | NA | NA | 0 | NA | NA | |||
| Magnesium intake type | 15 | 0.865 | 12 | 0.831 | 8 | 0.831 | ||||||
| Total magnesium intake‡ | 8 | 0.89 (0.82 to 0.96) | 0.00 | 6 | 0.87 (0.80 to 0.94) | 0.00 | 5 | 0.94 (0.79 to 1.12) | 0.00 | |||
| Dietary magnesium intake | 7 | 0.88 (0.81 to 0.96) | 0.44 | 0.888 | 6 12 |
0.89 (0.77 to 1.03) | 35.40 | 0.689 | 3 8 |
0.91 (0.70 to 1.18) | 39.40 | 0.538 |
| Total energy adjustment | 15 | |||||||||||
| Yes | 5 | 0.87 (0.77 to 0.99) | 27.00 | 2 | 0.86 (0.78 to 0.94) | 0.00 | 2 | 0.93 (0.82 to 1.06) | 0.00 | |||
| No | 10 | 0.89 (0.83 to 0.96) | 0.00 | 10 | 0.88 (0.79 to 0.99) | 26.60 | 6 | 0.90 (0.76 to 1.07) | 11.40 | |||
| Difference between top and bottom intake (mg/day)§ | 15 | 0.107 | 12 | 0.180 | 8 | 0.244 | ||||||
| ≥180 | 7 | 0.83 (0.76 to 0.91) | 0.00 | 5 | 0.83 (0.76 to 0.91) | 0.00 | 6 | 1.07 (0.83 to 1.37) | 0.00 | |||
| <180 | 8 | 0.93 (0.86 to 1.00) | 0.00 | 7 | 0.92 (0.81 to 1.03) | 26.20 | 2 | 0.89 (0.76 to 1.03) | 0.00 | |||
| Current CV events status¶ | 15 | 0.074 | 12 | 0.393 | 8 | NA | ||||||
| Yes | 12 | 0.90 (0.85 to 0.96) | 0.00 | 11 | 0.88 (0.81 to 0.96) | 18.20 | 8 | 0.93 (0.82 to 1.06) | 0.00 | |||
| Unknown | 3 | 0.75 (0.63 to 0.90) | 0.00 | 1 | 0.76 (0.57 to 1.01) | NA | 0 | NA | NA | |||
| Hypercholesterolaemia status** | 15 | 0.480 | 12 | 0.565 | 8 | 0.651 | ||||||
| Yes | 7 | 0.91 (0.83 to 0.99) | 0.00 | 6 | 0.90 (0.80 to 1.01) | 6.90 | 5 | 0.90 (0.76 to 1.08) | 0.00 | |||
| Unknown | 8 | 0.86 (0.79 to 0.95) | 13.10 | 6 | 0.86 (0.77 to 0.97) | 32.40 | 3 | 0.94 (0.72 to 1.22) | 40.30 | |||
| Current diabetes status†† | 15 | 0.039 | 12 | 0.159 | 8 | NA | ||||||
| Yes | 10 | 0.91 (0.82 to 0.97) | 0.00 | 10 | 0.89 (0.82 to 0.97) | 13.50 | 8 | 0.93 (0.82 to 1.06) | 0.00 | 0.00 | ||
| Unknown | 5 | 0.75 (0.64 to 0.88) | 0.00 | 2 | 0.72 (0.56 to 0.92) | 0.00 | 0 | NA | NA | NA | ||
*Several studies reported stroke outcome of male and female participants in different cohorts.
†Male and female participants were in the same cohort.
‡Total magnesium intake (mg/day) included the total amount of magnesium from both food (diet) and supplements.
§Subtract the lowest category intake from the highest.
¶Grouped by whether participants with or without cardiovascular events (CV events). CV events in this part include coronary heart disease, heart failure, atrial fibrillation and self-reported heart disease; stroke is not included.
**Grouped by whether participants with or without hypercholesterolaemia. Hypercholesterolaemia in this part means cholesterol concentration ≥240 mg/dL.
††Grouped by whether participants with or without diabetes.
BMI, body mass index; CV events, cardiovascular events; FFQ, food frequency questionnaire; NA, not available; RR, relative risk; SFFQ, semiquantitative food frequency questionnaire.
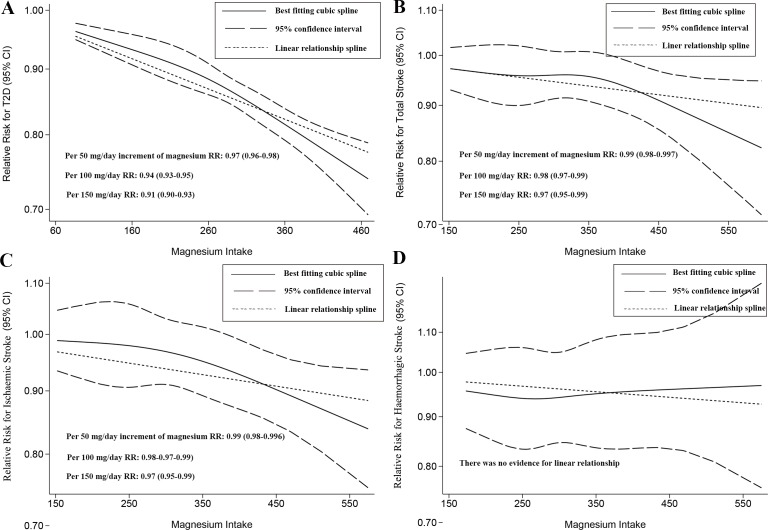
Dose–response analysis
In this part, both linear and nonlinear relationships were found in T2D (figure 3A), in total stroke (figure 3B) and in ischaemic stroke (figure 3C). However, no linear or nonlinear dose–response relationship was observed in haemorrhagic stroke (figure 3D) along with the subtypes including subarachnoid haemorrhage and intracerebral haemorrhage (online supplementary figure S15).
Figure 3.
Two-stage dose–response effect on the relationships between magnesium intake and (A) type 2 diabetes (T2D), (B) total stroke, (C) ischaemic stroke and (D) haemorrhagic stroke. RR, relative risk.
bmjopen-2019-032240supp020.pdf (181.9KB, pdf)
Specifically, we calculated the RR for the magnesium increments if a linear relationship was found. The calculated RR was 0.94 (95% CI 0.93 to 0.95) for the 100 mg/day increment for T2D. For total stroke, the summary RR was 0.98 (95% CI 0.97 to 0.99) related to a 100 mg/day increment in magnesium intake, and the RR for ischaemic stroke was 0.98 (95% CI 0.97 to 0.99) related to a 100 mg/day increment in magnesium intake. There was no RR cut-off point at which the decreasing trend reversed, but the RR decreased slightly rapidly with any slight decreases at approximately 260 mg/day for T2D and 350 mg/day for total/ischaemic stroke. However, there was substantial uncertainty in the lower range of this distribution (figure 3A–C).
Discussion
Main findings
This paper used a general and up-to-date search strategy to identify additional studies that were missed in prior meta-analyses under real-world conditions. Our results support a significant inverse association between magnesium consumption and T2D, total stroke and ischaemic stroke at the highest level versus the lowest. No significant association for haemorrhagic stroke, subarachnoid haemorrhage or intracerebral haemorrhage was detected. Female participants with obesity (mean BMI≥25 kg/m2) with a longer follow-up period (≥12 years) might obtain greater benefit from magnesium intake with a lower risk of total and ischaemic stroke incidence. In subgroup analyses, the RR of stroke risk was highly decreased among North American and European individuals. Significant risk was reduced by 6%, 2% and 2% for T2D, total stroke and ischaemic stroke, respectively, per 100 mg/day increment in magnesium intake level. Overall, our study supports the guidelines to address the role of magnesium intake in early prevention strategies to combat T2D and stroke. However, additional RCTs are needed in the future to validate the causality.
Clinical implications
Dietary nutrients are popular topics for current clinical medicine; folic acid, vitamin D and ω−3 fatty acids have been specifically recommended to pregnant women, infants and children, and the elderly.62 63 However, magnesium has been less extensively discussed. This is a noteworthy study for the following reasons. First, the current study reinforces the possible role of magnesium in the prevention and management of two chronic illnesses and invites new considerations regarding the potential avoidance of other chronic diseases through dietary strategies. Second, this comprehensive study including nearly two million individuals and possessing abundant statistical power provides confirmatory evidence for medical practitioners, health educators and policy-makers. Third, to date, no related paper has discussed such detailed stratified analyses; thus, this work helps physicians amplify dietary benefits through individualised strategies. Interestingly, North American and European participants seemed to receive more benefits from magnesium intake than Asians. Fourth, to the best of our knowledge, this is the first study in which a cumulative meta-analysis was performed to predict changes in the tendency of main risk estimates. Based on past and current cutting edge evidence about nutrition and T2D prevention, the US Diabetes Prevention Program conducted a study and demonstrated that proper lifestyle modification (exercise and Mediterranean diet) significantly reduced T2D risk irrespective of population baselines, and this benefit was enhanced with increased follow-up.64 The UK National Health Service will launch an intervention programme including weight loss, nutrition, monitoring and peer support targeting up to 10 000 people prone to develop T2D.65
The 2018 American Diabetes Association guidelines66 recommend that the intake of nuts, berries, yoghurt, coffee and tea be increased in individuals who are at high risk of diabetes. The latest guidelines by the American Heart Association/American Stroke Association9 also validate the considerable status of early management of stroke (ischaemic stroke). In fact, magnesium is a cofactor in enzyme systems that regulate diverse biomedical reactions, including protein synthesis, muscle and nerve transmission, neuromuscular conduction, signal transduction blood glucose control and blood pressure (BP) management.67 Magnesium also plays a role in transporting calcium and potassium ions across the cell membrane and is crucial for the structural function of proteins, nucleic acids or mitochondria.68 In diabetes, magnesium is involved in glucose and insulin metabolism by regulating the tyrosine kinase activity of the insulin receptor. Magnesium also influences phosphorylase B kinase activity by releasing glucose-1-phosphate from glycogen and regulates glucose translocation into the cell.69 In stroke, higher magnesium levels lead to the deregulation of glutamate and calcium cation influx by reducing N-Methyl-D-aspartic acid (NMDA) receptor activity and blocking voltage-gated calcium channels, eliminating calcium cation cytotoxicity. Additionally, the vasodilatory effects of magnesium may benefit patients who had ischaemic stroke.70 Indeed, a poor outcome of haemorrhagic stroke was observed in an RCT; however, high serum magnesium might be better for the prognosis of intracerebral haemorrhage.71
Most specific nutrients, especially macronutrients, are correlated with total energy intake. In the included free-living human studies, the variation in total energy intake originated from differences in physical activity levels, body size and energy efficiency.72 Thus, total energy intake can weaken the investigated association with considerable nutrient intake if this covariable is not properly removed. Epidemiologists should assess the reproducibility and validity of energy-adjusted nutrients as well as absolute nutrient intake. For micronutrients such as magnesium, an inverse association with T2D, total stroke and ischaemic stroke outcomes could be still found after total energy intake adjustment. In terms of other nutrients, potassium intake is proposed to lower BP and improve vascular outcomes (including stroke); dietary potassium may also be influential in glucose control and limiting the risk of diabetes.73 Vitamin D and calcium may negatively influence glycaemia, but evidence is limited and mostly based on cross-sectional observational studies.74 Calcium may be inversely associated with stroke in populations with low-to-moderate calcium intakes, but no significant association was found between calcium and CVD.75 Altogether, the results indicate that magnesium-rich food such as nuts (151–567 mg/100 g edibles), fruits (132–448 mg/100 g edibles), vegetables (132–1257 mg/100 g edibles), legumes (138–243 mg/100 g edibles), fish (143–303 mg/100 g edibles) and total grain (134–306 mg/100 g edibles) should be recommended to populations with insufficient magnesium intake.
Comparisons with other similar studies
This analysis has several differences from previous studies. Dong et al52 found that magnesium intake had an inverse association with T2D incidence (RR 0.78 (95% CI 0.73 to 0.84)), and with an intake of 100 mg/day magnesium, the risk was reduced by 14%. However, they failed to include adequate studies, and standard quality assessments of eligible studies were absent. Individuals from multiple nations were included in some studies18 25 26 32 but were incorrectly assigned to Asia or the USA in the subgroups; other minor issues also existed in the selection criteria, making it unclear whether they excluded participants with subclinical diabetes. BMI was not a potential modifier for T2D in our study due to the inclusion of more evidence with a longer follow-up period. Fang et al76 revealed that dietary magnesium was significantly associated with a reduced risk of T2D (RR 0.74 (95% CI 0.69 to 0.80)) and stroke (RR 0.88 (95% CI 0.82 to 0.95)). The results were comparable, but they focused only on dietary magnesium intake rather than overall magnesium intake (total or dietary), and subtypes of total stroke were missing. To the best of our knowledge, BMI, follow-up, family diabetes history, and so on, are crucial confounders for evaluating the association, and these factors were not addressed in their study. Moreover, other researchers have better investigated the likelihood of a linear association in the dose–response pattern (using methods by Greenland and Orsini et al). For example, Fang et al77 found that the 100 mg/day intake of dietary magnesium was associated with an 8%–13% reduction in T2D risk, and while a nonlinear relationship did not exist, a minor publication bias was present. Twenty-five studies were eligible; however, some of them focused not on dietary intake but rather on total magnesium intake. Moreover, there were two included studies focusing on red meat intake instead of magnesium intake. After excluding ineligible studies, we found no evidence of publication bias. Additionally, both linear and nonlinear relationships existed for T2D because the RRs of the highest category of magnesium intake versus the lowest in our pooled study were still used. A study by Larsson et al53 including seven studies supported a modest but statistically significant inverse association between dietary magnesium intake and stroke. However, the sample size was quite small, and there was no useful information on stroke subtypes (eg, ischaemic stroke, haemorrhagic stroke) in the main analysis. In our opinion, a well-designed subgroup analysis is compulsory, and a pooled stroke result restricted by potassium and calcium adjustment is recommended. The current study found that magnesium intake was strongly inversely associated with total stroke and ischaemic stroke, which still existed in the dose–response pattern.
Directions for future research
Future studies are needed to address some remaining questions. At first, no significant association was found for haemorrhagic stroke; however, a beneficial trend was observed in the cumulative meta-analysis, which highlights the need for more updated prospective studies and RCTs. Second, there is a key question regarding the optimal time to start prevention and methods to screen severe complications. CV events occur in more than 50% of patients with diabetes, and diabetic kidney disease occurs in 20%–40%. Additionally, CV events increase the risk of death threefold to fourfold compared with patients without such complications. A sustained period of intensive glucose control early in T2D has been confirmed to reduce complication rates.78 Most importantly, for the public, educators and policy-makers, promoting magnesium-rich food consumption can translate into considerable benefit in preventing T2D and total stroke, especially for high-risk populations.
Limitations
This work has several limitations that deserve further discussion. First, this group-level meta-analysis is insufficient. Although strong inverse associations for T2D and total stroke were reported, individual-level studies having more detection power are required. Second, several variations cannot be totally understood; for example, we cannot exclude the possibility that other nutrients and/or dietary components correlated with dietary magnesium may have been responsible, either partially or entirely, for the observed associations. Based on eligible studies, we could not quantify the impact of supplementary magnesium (not combined with dietary intake) on T2D and stroke incidence. The real effect of some dietary supplements on T2D or CVD has proven very interesting to a number of medical experts, clinicians and nutrition educators. Third, FFQs/validated FFQs mostly used in primary studies could not characterise all the nutrients, which misclarified plausible associations. It was suggested that magnesium-specific food questionnaires and/or food records should be reasonably used for accurate magnesium intake estimation. Finally, additional RCT are needed, as observational studies might only reach one conclusion (ie, magnesium intake is inversely associated with T2D incidence) and cannot prove causality.
Conclusion
Magnesium intake has a substantial inverse association with T2D and total stroke. Among these populations, magnesium consumption can be recommended as an optimisation for T2D, total stroke and ischaemic stroke primary prevention or early management. In particular, the greater the magnesium intake, the greater the reduction in risk. As patients, physicians, policy-makers and legislators debate these issues, such a cost-effective alternative is needed to inform policy decisions and aid in reforming nutritional healthcare worldwide.
Supplementary Material
Acknowledgments
The authors thank Yanhua Tang, MD (The Second Affiliated Hospital of Nanchang University) for her advice and Xiaoshu Cheng, MD, PhD (The Second Affiliated Hospital of Nanchang University) for his data collection.
Footnotes
Contributors: BZ had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: all authors. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: BZ and WZ. Critical revision of the manuscript for important intellectual content: BZ, LZ, JZ, QW, FZ, LG and YD. Statistical analysis: BZ. Supervision: WZ and YW.
Funding: This study was supported by National Natural Science Foundation of China (NSFC), number of grants (81560345), Natural Science Foundation of Jiangxi Province (Grant number: 20161BAB215237).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Centers for Disease Control and Prevention National diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017. [Google Scholar]
- 2.Zhou B, Lu Y, Hajifathalian K, et al. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke Statistics-2017 update: a report from the American heart association. Circulation 2017;135:e146–603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet 2014;383:245–55. 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbagallo M, Dominguez LJ. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch Biochem Biophys 2007;458:40–7. 10.1016/j.abb.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 6.Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018;359:1151–6. 10.1126/science.aao5774 [DOI] [PubMed] [Google Scholar]
- 7.Reffelmann T, Ittermann T, Dörr M, et al. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis 2011;219:280–4. 10.1016/j.atherosclerosis.2011.05.038 [DOI] [PubMed] [Google Scholar]
- 8.Fadelu T, Zhang S, Niedzwiecki D, et al. Nut consumption and survival in patients with stage III colon cancer: results from CALGB 89803 (Alliance). J Clin Oncol 2018;36:1112–20. 10.1200/JCO.2017.75.5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 10.Brignole M, Moya A, de Lange FJ, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:ehy037:1883–948. 10.1093/eurheartj/ehy037 [DOI] [PubMed] [Google Scholar]
- 11.Salmerón J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50. 10.2337/diacare.20.4.545 [DOI] [PubMed] [Google Scholar]
- 12.Salmerón J, Manson JE, Stampfer MJ, et al. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7. 10.1001/jama.1997.03540300040031 [DOI] [PubMed] [Google Scholar]
- 13.Ascherio A, Rimm EB, Hernán MA, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation 1998;98:1198–204. 10.1161/01.CIR.98.12.1198 [DOI] [PubMed] [Google Scholar]
- 14.Iso H, Stampfer MJ, Manson JE, et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke 1999;30:1772–9. 10.1161/01.STR.30.9.1772 [DOI] [PubMed] [Google Scholar]
- 15.Kao WH, Folsom AR, Nieto FJ, et al. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis risk in Communities study. Arch Intern Med 1999;159:2151–9. 10.1001/archinte.159.18.2151 [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Manson JE, Stampfer MJ, et al. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health 2000;90:1409–15. 10.2105/ajph.90.9.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer KA, Kushi LH, Jacobs DR, et al. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. 10.1093/ajcn/71.4.921 [DOI] [PubMed] [Google Scholar]
- 18.Hodge AM, English DR, O'Dea K, et al. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004;27:2701–6. 10.2337/diacare.27.11.2701 [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Ridaura R, Willett WC, Rimm EB, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004;27:134–40. 10.2337/diacare.27.1.134 [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Manson JE, Buring JE, et al. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 2004;27:59–65. 10.2337/diacare.27.1.59 [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Manson JE, Cook NR, et al. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol 2005;96:1135–41. 10.1016/j.amjcard.2005.06.045 [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Choi HK, Ford E, et al. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006;29:1579–84. 10.2337/dc06-0256 [DOI] [PubMed] [Google Scholar]
- 23.Pereira MA, Parker ED, Folsom AR. Coffee consumption and risk of type 2 diabetes mellitus: an 11-year prospective study of 28 812 postmenopausal women. Arch Intern Med 2006;166:1311–6. 10.1001/archinte.166.12.1311 [DOI] [PubMed] [Google Scholar]
- 24.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006;29:650–6. 10.2337/diacare.29.03.06.dc05-1961 [DOI] [PubMed] [Google Scholar]
- 25.van Dam RM, Hu FB, Rosenberg L, et al. Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 2006;29:2238–43. 10.2337/dc06-1014 [DOI] [PubMed] [Google Scholar]
- 26.Schulze MB, Schulz M, Heidemann C, et al. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007;167:956–65. 10.1001/archinte.167.9.956 [DOI] [PubMed] [Google Scholar]
- 27.Larsson SC, Virtanen MJ, Mars M, et al. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch Intern Med 2008;168:459–65. 10.1001/archinte.168.5.459 [DOI] [PubMed] [Google Scholar]
- 28.Weng L-C, Yeh W-T, Bai C-H, et al. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake? Stroke 2008;39:3152–8. 10.1161/STROKEAHA.108.524934 [DOI] [PubMed] [Google Scholar]
- 29.Kirii K, Mizoue T, Iso H, et al. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia 2009;52:2542–50. 10.1007/s00125-009-1554-x [DOI] [PubMed] [Google Scholar]
- 30.Ohira T, Peacock JM, Iso H, et al. Serum and dietary magnesium and risk of ischemic stroke: the Atherosclerosis risk in Communities study. Am J Epidemiol 2009;169:1437–44. 10.1093/aje/kwp071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villegas R, Gao Y-T, Dai Q, et al. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai women's health study. Am J Clin Nutr 2009;89:1059–67. 10.3945/ajcn.2008.27182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopping BN, Erber E, Grandinetti A, et al. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr 2010;140:68–74. 10.3945/jn.109.112441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DJ, Xun P, Liu K, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care 2010;33:2604–10. 10.2337/dc10-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirii K, Iso H, Date C, et al. Magnesium intake and risk of self-reported type 2 diabetes among Japanese. J Am Coll Nutr 2010;29:99–106. 10.1080/07315724.2010.10719822 [DOI] [PubMed] [Google Scholar]
- 35.Nanri A, Mizoue T, Noda M, et al. Magnesium intake and type II diabetes in Japanese men and women: the Japan public health Center-Based prospective study. Eur J Clin Nutr 2010;64:1244–7. 10.1038/ejcn.2010.138 [DOI] [PubMed] [Google Scholar]
- 36.Larsson SC, Virtamo J, Wolk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol 2011;174:35–43. 10.1093/aje/kwr051 [DOI] [PubMed] [Google Scholar]
- 37.Weng L-C, Lee N-J, Yeh W-T, et al. Lower intake of magnesium and dietary fiber increases the incidence of type 2 diabetes in Taiwanese. J Formos Med Assoc 2012;111:651–9. 10.1016/j.jfma.2012.07.038 [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Iso H, Ohira T, et al. Associations of dietary magnesium intake with mortality from cardiovascular disease: the JACC study. Atherosclerosis 2012;221:587–95. 10.1016/j.atherosclerosis.2012.01.034 [DOI] [PubMed] [Google Scholar]
- 39.Hata A, Doi Y, Ninomiya T, et al. Magnesium intake decreases type 2 diabetes risk through the improvement of insulin resistance and inflammation: the Hisayama study. Diabet Med 2013;30:1487–94. 10.1111/dme.12250 [DOI] [PubMed] [Google Scholar]
- 40.Lin P-H, Yeh W-T, Svetkey LP, et al. Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pac J Clin Nutr 2013;22:482–91. [PubMed] [Google Scholar]
- 41.Oba S, Nanri A, Kurotani K, et al. Dietary glycemic index, glycemic load and incidence of type 2 diabetes in Japanese men and women: the Japan public health Center-Based prospective study. Nutr J 2013;12:165 10.1186/1475-2891-12-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sluijs I, Czernichow S, Beulens JWJ. Dietary electrolytes and risk of ischemic stroke. Eur J Prev Cardiol 2013;20:S76. [Google Scholar]
- 43.Hruby A, Meigs JB, O'Donnell CJ, et al. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care 2014;37:419–27. 10.2337/dc13-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sluijs I, Czernichow S, Beulens JWJ, et al. Intakes of potassium, magnesium, and calcium and risk of stroke. Stroke 2014;45:1148–50. 10.1161/STROKEAHA.113.004032 [DOI] [PubMed] [Google Scholar]
- 45.Adebamowo SN, Spiegelman D, Flint AJ, et al. Intakes of magnesium, potassium, and calcium and the risk of stroke among men. Int J Stroke 2015;10:1093–100. 10.1111/ijs.12516 [DOI] [PubMed] [Google Scholar]
- 46.Adebamowo SN, Spiegelman D, Willett WC, et al. Association between intakes of magnesium, potassium, and calcium and risk of stroke: 2 cohorts of US women and updated meta-analyses. Am J Clin Nutr 2015;101:1269–77. 10.3945/ajcn.114.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bain LKM, Myint PK, Jennings A, et al. The relationship between dietary magnesium intake, stroke and its major risk factors, blood pressure and cholesterol, in the EPIC-Norfolk cohort. Int J Cardiol 2015;196:108–14. 10.1016/j.ijcard.2015.05.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y-C, Wahlqvist ML, Kao M-D, et al. Optimal dietary and plasma magnesium statuses depend on dietary quality for a reduction in the risk of all-cause mortality in older adults. Nutrients 2015;7:5664–83. 10.3390/nu7075244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hruby A, Guasch-Ferré M, Bhupathiraju SN, et al. Magnesium intake, quality of carbohydrates, and risk of type 2 diabetes: results from three U.S. cohorts. Diabetes Care 2017;40:1695–702. 10.2337/dc17-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kokubo Y, Saito I, Iso H, et al. Dietary magnesium intake and risk of incident coronary heart disease in men: a prospective cohort study. Clin Nutr 2018;37:1602–8. 10.1016/j.clnu.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 51.Konishi K, Wada K, Tamura T, et al. Dietary magnesium intake and the risk of diabetes in the Japanese community: results from the Takayama study. Eur J Nutr 2017;56:767–74. 10.1007/s00394-015-1122-8 [DOI] [PubMed] [Google Scholar]
- 52.Dong J-Y, Xun P, He K, et al. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 2011;34:2116–22. 10.2337/dc11-0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr 2012;95:362–6. 10.3945/ajcn.111.022376 [DOI] [PubMed] [Google Scholar]
- 54.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993;24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 55.Rannikmäe K, Woodfield R, Anderson CS, et al. Reliability of intracerebral hemorrhage classification systems: a systematic review. Int J Stroke 2016;11:626–36. 10.1177/1747493016641962 [DOI] [PubMed] [Google Scholar]
- 56.Wells GA, Shea B, O'Connell D, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of Non-Randomized studies in meta-analysis. Appl Eng Agric 2014;18:727–34. [Google Scholar]
- 57.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 59.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J 2006;6:40–57. 10.1177/1536867X0600600103 [DOI] [Google Scholar]
- 60.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 61.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manson JE, Bassuk SS. Vitamin and mineral supplements: what clinicians need to know. JAMA 2018;319:859–60. 10.1001/jama.2017.21012 [DOI] [PubMed] [Google Scholar]
- 63.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018;319:1024–39. 10.1001/jama.2018.1150 [DOI] [PubMed] [Google Scholar]
- 64.Temprosa M, Diabetes Prevention Program Research Group . Long-Term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol 2015;3:866–75. 10.1016/S2213-8587(15)00291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maruthappu M, Sood H, Keogh B. Radically upgrading diabetes prevention in England. Lancet Diabetes Endocrinol 2015;3:312–3. 10.1016/S2213-8587(15)00079-0 [DOI] [PubMed] [Google Scholar]
- 66.American Diabetes Association 5. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S51–4. 10.2337/dc18-S005 [DOI] [PubMed] [Google Scholar]
- 67.Guerrero-Romero F, Simental-Mendía LE, Hernández-Ronquillo G, et al. Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: a double-blind placebo-controlled randomized trial. Diabetes Metab 2015;41:202–7. 10.1016/j.diabet.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 68.Ramadass S, Basu S, Srinivasan AR. SERUM magnesium levels as an indicator of status of diabetes mellitus type 2. Diabetes Metab Syndr 2015;9:42–5. 10.1016/j.dsx.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 69.Eimerl S, Schramm M. The quantity of calcium that appears to induce neuronal death. J Neurochem 2010;62:1223–6. 10.1046/j.1471-4159.1994.62031223.x [DOI] [PubMed] [Google Scholar]
- 70.van den Bergh WM, Algra A, van Kooten F, et al. Magnesium sulfate in aneurysmal subarachnoid hemorrhage: a randomized controlled trial. Stroke 2005;36:1011–5. 10.1161/01.STR.0000160801.96998.57 [DOI] [PubMed] [Google Scholar]
- 71.Goyal N, Tsivgoulis G, Malhotra K, et al. Serum magnesium levels and outcomes in patients with acute spontaneous intracerebral hemorrhage. J Am Heart Assoc 2018;7:e008698 10.1161/JAHA.118.008698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8. discussion 1229S-1231S 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 73.Stone MS, Martyn L, Weaver CM. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients 2016;8:E444 10.3390/nu8070444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pittas AG, Lau J, Hu FB, et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–29. 10.1210/jc.2007-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larsson SC, Orsini N, Wolk A. Dietary calcium intake and risk of stroke: a dose-response meta-analysis. Am J Clin Nutr 2013;97:951–7. 10.3945/ajcn.112.052449 [DOI] [PubMed] [Google Scholar]
- 76.Fang X, Wang K, Han D, et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med 2016;14:210 10.1186/s12916-016-0742-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang X, Han H, Li M, et al. Dose-response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: a systematic review and meta-regression analysis of prospective cohort studies. Nutrients 2016;8:739 10.3390/nu8110739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riddle MC, Ambrosius WT, Brillon DJ, et al. Epidemiologic relationships between A1c and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the Accord trial. Diabetes Care 2010;33:983–90. 10.2337/dc09-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-032240supp001.pdf (74.8KB, pdf)
bmjopen-2019-032240supp002.pdf (43.2KB, pdf)
bmjopen-2019-032240supp003.pdf (39.4KB, pdf)
bmjopen-2019-032240supp004.pdf (60.7KB, pdf)
bmjopen-2019-032240supp005.pdf (20.3KB, pdf)
bmjopen-2019-032240supp006.pdf (140.6KB, pdf)
bmjopen-2019-032240supp007.pdf (643.3KB, pdf)
bmjopen-2019-032240supp008.pdf (517.2KB, pdf)
bmjopen-2019-032240supp009.pdf (424.4KB, pdf)
bmjopen-2019-032240supp010.pdf (243.7KB, pdf)
bmjopen-2019-032240supp011.pdf (237KB, pdf)
bmjopen-2019-032240supp012.pdf (322KB, pdf)
bmjopen-2019-032240supp013.pdf (308.1KB, pdf)
bmjopen-2019-032240supp014.pdf (315.8KB, pdf)
bmjopen-2019-032240supp015.pdf (304.8KB, pdf)
bmjopen-2019-032240supp016.pdf (185.1KB, pdf)
bmjopen-2019-032240supp017.pdf (166.2KB, pdf)
bmjopen-2019-032240supp018.pdf (75.3KB, pdf)
bmjopen-2019-032240supp019.pdf (61.9KB, pdf)
bmjopen-2019-032240supp020.pdf (181.9KB, pdf)