Abstract
The cellular mechanism(s) linking macrophages to norepinephrine (NE)-mediated regulation of thermogenesis has been a topic of debate. Here, we identify sympathetic neuron-associated macrophages (SAMs) as a population of cells that mediate clearance of NE via expression of Slc6a2, an NE transporter, and monoamine oxidase A (MAOa), a degradation enzyme. Optogenetic activation of the SNS upregulates NE uptake by SAMs and shifts the SAM profile to a more pro-inflammatory state. NE uptake by SAMs is prevented by genetic deletion of Slc6a2 or inhibition of the transporter. We also observed increased SAM content in the SNS of two obesity mouse models. Genetic ablation of Slc6a2 in SAMs increases brown adipose tissue (BAT) content, causes browning of white fat, increases thermogenesis, and leads to significant and sustained weight loss of obese mice. We further show that this pathway is conserved, as human sympathetic ganglia also contain SAMs expressing the analogous molecular machinery for NE clearance, thus constituting a potential target for obesity treatment.
Introduction
Sympathetic innervation of adipose tissue promotes lipolysis and fat mass reduction via norepinephrine (NE) signaling1. In obesity, chronic local inflammation underlies adipose tissue dysfunction, and macrophages have been shown to play a central role1,2. The mechanism that links macrophages in white adipose tissue (WAT) to NE remains controversial. Some groups have reported that anti-inflammatory adipose tissue macrophages (ATMs) in the WAT produce NE to sustain thermogenesis and browning. In direct contradiction, other groups have reported that ATMs do not express a key enzyme required for NE production and that genetic deletion of this enzyme in macrophages has no effect on thermogenesis and body weight3–6.
Here, we identify a previously undescribed population of sympathetic neuron-associated macrophages (SAMs) that import and degrade NE via specific proteins that are absent from ATMs. We found by transcriptional profiling of isolated SAMs that neural- and adrenergic-related genes are differentially expressed in these cells relative to other macrophage populations. SAMs accumulate intracellular NE despite lacking NE biosynthetic enzymes. Using optogenetics, we demonstrate that SNS activity increases NE content and the pro-inflammatory state of SAMs. We functionally demonstrate that SAMs import and degrade NE via, respectively, an NE transporter (Slc6a2) and a degradation enzyme (monoamine oxidase; MAOa). We further demonstrate that SAM-mediated clearance of extracellular NE contributes to obesity, as inhibiting NE import by SAMs ameliorates obesity, thermogenesis, and browning in ob/ob and high fat diet (HFD)-fed mice. Finally, we demonstrate human relevance, as we found that SAMs are also present in human sympathetic ganglia and express similar molecular machinery as mice. Thus, the identification of SAMs provides a novel contribution to the ongoing controversy surrounding the role of macrophages in thermogenesis and obesity while constituting an unforeseen immunological player in noradrenergic homeostasis with therapeutic potential for obesity.
Results
Specialized morphology and activation of SNS Cx3cr1+ cells
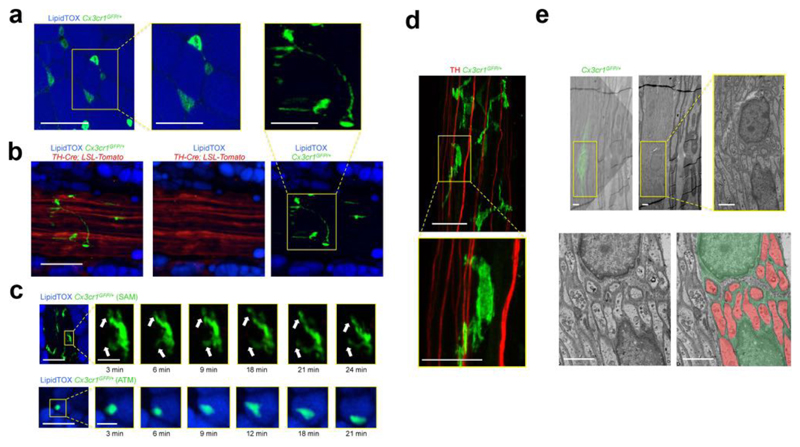
Our initial aim was to visualize the in vivo morphology of ATMs using two-photon and confocal microscopy in Cx3cr1GFP/+ mice, in which macrophages are GFP-labeled. ATMs in fat parenchyma had a regular circular shape (Fig. 1a), whereas those located on sympathetic nerve bundles exhibited profuse pseudopodia that extended over greater surface area (Fig. 1b and Supplemental Fig. 1a,b). Furthermore, we observed that sympathetic neuron-associated Cx3cr1GFP/+ cells displayed dynamic extensions and retractions of dendritiform processes over time (Fig. 1c and Supplemental Video 1). In contrast, ATMs surrounding adipocytes displayed minimal temporal plasticity or displacement (Fig. 1c and Supplemental Video 2). Using correlative light electron microscopy on WAT-derived nerve bundles, we confirmed that Cx3cr1GFP/+ cells extended thin pseudopodia processes that envelop non-myelinated SNS axons (Fig. 1d,e and Supplemental Fig. 1c).
Figure 1. Sympathetic neuron-associated Cx3cr1GFP/+ cells exhibit differentiated morphology for specific association with SNS neurons.
(a) Confocal images from white adipose tissue isolated from Cx3cr1GFP/+ mice and stained using lipid stain LipidTOX (blue) and anti-GFP (green) antibody. Images are representative of 5 similar experiments. (b) Confocal images of sympathetic nerve fibers in subcutaneous adipose tissue isolated from TH-Cre; LSL-Tomato (red) / Cx3cr1GFP/+ (green) mice and stained using lipid stain LipidTOX (blue). Images are representative of 3 similar experiments. Scale bars in a and b, 50 μm. Boxed regions in a and b represent higher magnification of the main micrographs - scale bars, 25 μm (c) Intra-vital multi-photon visualization of a neuro-adipose connection in the inguinal fat pad of a live Cx3cr1GFP/+ mouse; LipidTOX (blue) labels adipocytes. Images are depicting morphological features and cell dynamics of Cx3cr1GFP/+ cells associated with sympathetic nerve fibers (upper panels) and Cx3cr1GFP/+ cells in the parenchyma of subcutaneous fat (lower panels). Images are representative of 3 similar experiments. Scale bars, 50 μm. Boxed regions represent higher magnification of the main micrograph at time points indicated below micrographs – scale bars, 10 μm. (d) Confocal images from sympathetic nerve fibers isolated from inguinal fat pad of Cx3cr1GFP/+ mice and stained using anti-TH (red) and anti-GFP (green) antibodies. Images are representative of 5 similar experiments. Scale bar, 50 μm. Boxed region represents higher magnification of the main micrograph – scale bar, 25 μm. (e) Correlative confocal and transmission electron microscopy of the nerve fibers isolated from subcutaneous fat pad of Cx3cr1GFP/+ mice. Shown is an overlay of the Cx3cr1GFP/+ fluorescence (green) with the electron micrograph from the same section, (upper left with the lower left panel being a higher magnification), electron micrograph alone (upper middle with the yellow boxed region in the right panel being a higher magnification), and electron micrograph (lower left) with false color-coding of the same image highlighting Cx3cr1GFP/+ cells (green) and sympathetic nerves (red) (lower right). Images are representative of 2 similar experiments. Scale bars, 2 μm.
We then investigated whether sympathetic neuron-associated Cx3cr1GFP/+ cells were present in other SNS compartments, such as paravertebral sympathetic ganglia. Upon imaging superior cervical ganglia (SCG) and thoracic chains, we visualized Cx3cr1GFP/+ cells that were morphologically similar to those within WAT-derived SNS bundles (Supplemental Fig. 2). Due to established ex vivo explant potential, we used SCGs along with WAT-derived SNS nerve bundles as model systems for subsequent functional and molecular analyses.
SNS Cx3cr1+ SAMs exhibit hematopoietic characteristics
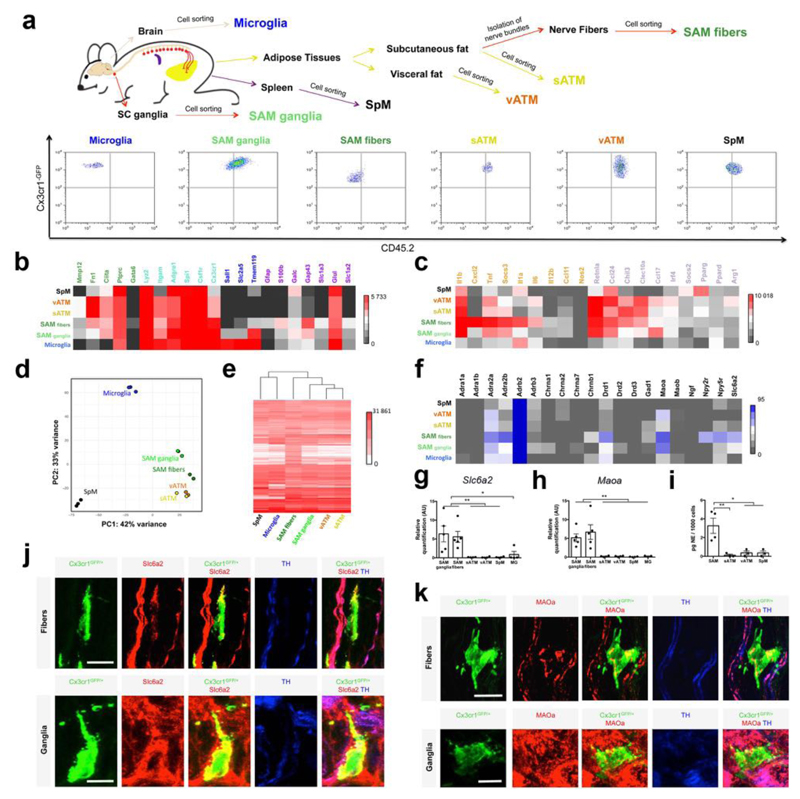
Because nearly all Cx3cr1GFP/+ cells isolated from sympathetic fibers were positive for the immune marker CD45 (Supplemental Fig. 3) and macrophage marker F4/80 (Supplemental Fig. 4a), we designate these cells sympathetic neuron-associated macrophages (SAMs). Given the specialized morphology and location of SAMs, we next explored how these cells compared to other tissue macrophages and brain microglia. We sorted F4/80+CD45+ double-positive cells from the following tissues: sympathetic ganglia (SAM ganglia), sympathetic nerve fibers from inguinal fat (SAM fibers), neighboring subcutaneous fat (sATM), visceral fat (vATM), spleen (SpM) and brain (microglia) (Fig. 2a; gating details in Supplemental Fig. 3). The relative abundance of CD45highCx3cr1-GFP+ cells was nearly four times higher within nerve fibers (SAMs) than in sWAT (sATMs; Supplemental Fig. 4b).
Figure 2. SAMs highly express macrophage-associated markers and possess the machinery for uptake and degradation of norepinephrine.
(a) Schematic representation (top) of tissue dissections and processing with macrophages isolated from the following tissues: brain, spleen, visceral fat, subcutaneous fat, sympathetic nerve fibers from subcutaneous fat, and sympathetic ganglia. Representative flow cytometry dot plots (bottom) indicating the CD45.2 status of macrophages from each tissue analyzed. (b) Heat map for genes associated with macrophage (green and cyan), microglia (blue and cyan) and glia (purple) profiles, determined by low-input RNA-seq. Values are Reads Per Kilobase of transcript per Million mapped reads (RPKM). (c) Heat map for pro-inflammatory (orange) and anti-inflammatory (purple) genes, determined by low-input RNA-seq. Values are RPKM. (d) Principal component (PC) analysis based on the top 500 most variable genes across SAM fibers (green), SAM ganglia (light green), vATM (orange), sATM (yellow), SpM (black) and microglia (blue). Each dot represents an independent experiment. (e) Heat map of transcripts (RPKM values) based on the top 5000 genes expressed by SAM fibers, determined by low-input RNA-seq. (f) Heat map of genes for neurotransmitter receptors, transporters and catalyzing enzymes. RPKM values are determined by low-input RNA-seq. Values in panels b,c,d,e and f represent 3 independent experiments (SpM, Microglia and SAM ganglia, n = 3) or 2 independent experiments (vATM, sATM and SAM fibers, n = 2). (g) Expression of mRNA for Slc6a2 determined by qRT-PCR presented normalized to Gapdh expression. Each data point represents tissues pooled from 10 mice. n = 5 experiments for SAM fibers and SAM ganglia, n = 4 experiments for SpM, vATM, sATM and Microglia (MG), *P < 0.05, **P < 0.01. (h) Expression of mRNA for Maoa determined by qRT-PCR normalized to Gapdh expression. Each data point represents tissues pooled from 10 mice. n = 5 experiments for SAM fibers and SAM ganglia, n = 4 experiments for SpM, vATM, sATM and n = 3 experiments for MG, **P < 0.01. (i) NE content in sorted CD45.2-PE, F4/80-Alexa Fluor 647-double-positive cells measured by NE ELISA. Number of cells used in NE assays were as followed: 858 ± 258 for SAMs (n = 4 experiments), and 1000 cells for sATM, vATM and SpM (n = 3 experiments), *P < 0.05, **P < 0.01. (j,k) Confocal images from sympathetic nerve fibers (upper panels) and superior cervical ganglia (lower panels) isolated from Cx3cr1GFP/+ mice and stained using anti-GFP (green), anti-Slc6a2 (j) or anti-MAOa (k) (red) and anti-TH (blue) antibodies. Images are representative of 2 experiments. Scale bars, 10 μm. Data in g, h and i were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Data are shown as average ± SEM.
CD45 is highly expressed in hematopoietic cells but expressed at low levels in microglia. Flow cytometric analysis revealed that SAMs are CD45medium/high (Supplemental Fig. 3), suggesting a hematopoietic origin of these cells. To this end, we generated bone marrow chimeras from CD45.2+Cx3cr1GFP/+ donors into irradiated CD45.1 recipient mice and observed complete repopulation of CD45+ cells derived from Cx3cr1GFP/+CD45.2 donors (Supplemental Fig. 4c). Eight weeks post-transplantation, we established that CD45.2+Cx3cr1GFP/+ SAMs repopulated sympathetic nerve bundles in WAT, whereas microglia repopulation in the brain did not occur (Supplemental Fig. 4d). This suggests that SAMs in sympathetic fibers have similar origin to other hematopoietic macrophages as opposed to microglial lineage.
SAM expression profile is more macrophage- than glia-like
Given their association with neurons, we asked how the gene expression profile of SAMs compared to other resident tissue macrophages and microglia (Fig. 2). We sorted macrophages from various tissues as described above (F4/80+CD45+ double-positive cells designated as SAM ganglia, SAM fibers, sATMs, vATMs, SpM, and microglia; Fig. 2a and Supplemental Fig. 3) and profiled gene expression by low input RNAseq (Fig. 2b-f). As expected, SAMs highly expressed markers common to both microglia and macrophages, such as Adgre1, Csf1r, Cx3cr1 (Fig. 2b). SAMs expressed macrophage-associated genes that are excluded from microglia, such as Fn1 or Ciita (Fig. 2b)7. By flow cytometric analysis, additional macrophage-specific markers that are excluded from microglia (CD68, Ly6c, MHCII, and CD11b) were also highly expressed in SAMs (Supplemental Fig. 5a,b). SAMs do not robustly express microglial- or glial-specific genes relative to macrophage-specific genes (Fig. 2b and Supplemental Fig. 5c)8–17. Sall1, a key microglia lineage-determining transcription factor, is strikingly absent from SAMs18 (Fig. 2b).
Principle component analysis (PCA) of the RNAseq data shows tight clustering across replicates, indicating low contamination and high reproducibility (Fig. 2d). The absence of tyrosine hydroxylase (Th) expression in SAMs (Supplemental Fig. 5d) further excluded the possibility of contaminating cargo from neighboring cells, as Th is highly expressed in adjacent SNS neurons (Fig. 1b,d). PCA analysis indicated that SAMs from fibers and ganglia are closely related, but both are distant from microglia and other macrophages (Fig. 2d). This is confirmed by phylogenetic analysis (Fig. 2e).
We hypothesized that the increased motility of SAMs (Fig. 1c) could indicate an activated, pro-inflammatory state. Therefore, we measured expression of a constellation of pro- and anti-inflammatory markers in SAMs by RNA-seq (Fig. 2c). Relative to other macrophage populations, SAMs highly expressed genes associated with macrophage activation, including Cxcl2, Tnf, Socs3, and Il1a (Fig. 2c), suggesting a constitutively pro-inflammatory steady state.
SAMs are phylogenically distinct from other macrophages
Consistent with the PCA analysis (Fig. 2d), Pearson correlation analyses of transcript levels indicated differential expression patterns across SAMs, sATMs, vATMs, SpMs and microglia (Supplemental Fig. 6a,b). Adipose tissue macrophages (sATMs and vATMs) showed similar expression landscapes (R = 0.92) that are distant from fiber SAMs (R = 0.63 for sATM and R = 0.61 for vATMs; Supplemental Fig. 6b). Microglia and spleen macrophages were least correlated with other groups (Supplemental Fig. 6b).
Gene ontology analyses indicated several biological processes associated with genes enriched in SAMs relative to surrounding sATMs (Supplemental Fig. 6c). SAMs preferentially expressed genes involved in synaptic signaling, cell-cell adhesion, and neuron development (Supplemental Fig. 6c), suggesting that these cells fulfill an intrinsic role in local neuronal maintenance. Taken together, these data demonstrate divergent gene expression patterns in SAMs and ATMs, constituting intra-tissue macrophage specialization.
SAMs import and degrade, but do not synthesize, NE
We next examined the specific transcripts comprising divergent macrophage gene expression landscapes. The aforementioned populations of macrophages were sorted (Fig. 2a and Supplemental Fig. 3) for transcriptome analysis via low-input RNA-seq. Given the gene ontology results (Supplemental Fig. 6c) and spatial proximity of SAMs to nerves (Fig. 1), we hypothesized differential expression of neurotransmitter receptors, transporters or catalyzing enzymes (Fig. 2f). Consistent with the ImmGen database, we detected abundant β2 adrenergic receptor (Adrb2) expression in all macrophage populations (Fig. 2f), which was confirmed by qRT-PCR (Supplemental Fig. 6d).
However, SAMs were the only population that expressed Slc6a2, the gene for the NE transporter (Fig. 2f). Similarly, Maoa, the gene encoding MAOa, was highly expressed in SAMs relative to the other macrophage types (Fig. 2f). Both results were validated by qRT-PCR (Fig. 2g,h and Supplemental Table 1). As Slc6a2 imports and MAOa degrades NE, we also tested for and detected NE by ELISA in sorted SAMs (Fig. 2i and Supplemental Fig. 6e). Consistent with our results, neither Slc6a2 nor Maoa are significantly expressed in any macrophage population listed in the ImmGen database. Furthermore, we validated Slc6a2 and MAOa protein expression by immunofluorescence in Cx3cr1GFP/+ SNS nerve fibers and SCG cryo-sections (Fig. 2j,k). Representative photomicrographs depict GFP-containing SAMs were double-positive for membrane-bound Slc6a2 (Fig. 2j) or mitochondrial-bound MAOa (Fig. 2k).
As SAMs, but not other macrophage types assessed, possess the molecular machinery for import (Fig. 2f,g,j) and degradation (Fig. 2f,h,k) of NE, as well as significantly more NE relative to other macrophages (Fig. 2I and Supplemental Fig. 6e), we tested the possibility that SAMs synthesize NE. By qRT-PCR of sorted SAMs, we did not detect expression of Th, which encodes an enzyme necessary for NE biosynthesis (Supplemental Fig. 5d). Taken together, these results indicate that SAMs possess the molecular machinery for importing and degrading NE, but not for biosynthesis.
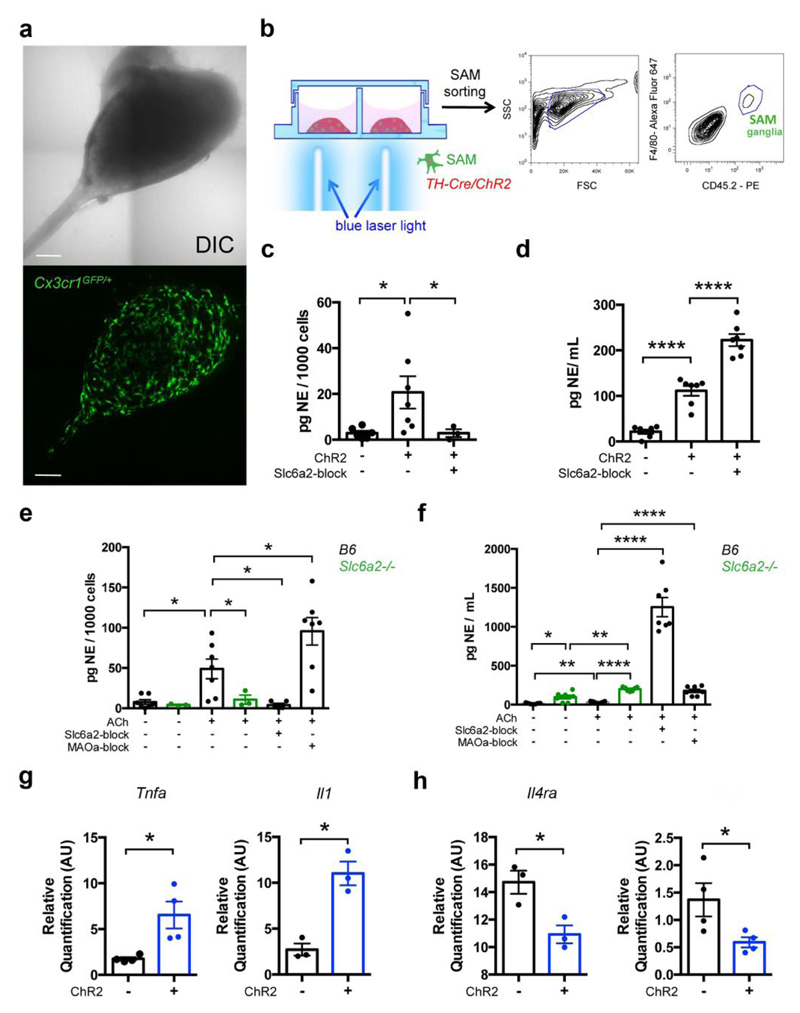
To explore the responsiveness of SAMs to NE, we optogenetically stimulated sympathetic neurons in SCG cultures from TH-Cre X Rosa26-LSL-ChR2-YFP mice1, which allowed us to visualize sympathetic neuron-macrophage interactions ex vivo (Fig. 3a,b). After optogenetic stimulation, we measured NE content of sorted CD45+F4/80+ cells. SAMs from ChR2-positive cultures exhibited significantly higher NE levels (Fig. 3c) that were proportional to NE availability in the culture medium (Fig. 3d). NE release by ChR2-positive neurons was significantly higher relative to ChR2-negative neurons (Fig. 3d). Uptake of NE by SAMs was prevented by pharmacologic blockade of Slc6a2 by the pharmacological inhibitor Nisoxetine, despite significant increase of NE in the culture medium (Fig. 3c,d).
Figure 3. SAMs import and metabolize NE via Slc6a2 and MAOa, respectively, to regulate extracellular NE availability.
(a) Representative images of ex-vivo SCG explant cultures. The area of the sympathetic ganglia is represented using reflected light differential interference contrast (DIC) channel in the upper panel, and the Cx3cr1GFP/+ cells from the same explant culture are shown in the lower panel (GFP channel). Images are representative of 20 similar experiments. (b) Schematic representation (left) of optogenetic activation of sympathetic SCG explant culture followed by CD45.2-PE, F4/80-Alexa Fluor 647-double-positive cell sorting (right). (c) NE content in CD45.2-PE, F4/80-Alexa Fluor 647-double-positive cells isolated from SCG explant cultures from TH-Cre;LSL-ChR2-YFP and LSL-ChR2-YFP mice after optogenetic activation. Each data point represents tissues pooled from 6 mice. n = 3-7 experiments, *P < 0.05. The following number of cells were used in NE assays (run in duplicates): 189 ± 30 from TH-Cre;LSL-ChR2-YFP SCG (n = 7), 126 ± 21 from LSL-ChR2-YFP SCG (n = 6), and 159 ± 19 from TH-Cre;LSL-ChR2-YFP SCG stimulated with Slc6a2-blocker (n = 3). (d) Ex vivo NE release upon optogenetic stimulation of SCG explants isolated from TH-Cre;LSL-ChR2-YFP and LSL-ChR2-YFP mice. Each data point represents medium collected from one explant culture. n = 7 per group, ****P < 0.0001. (e) NE content in CD45.2, F4/80-double-positive cells isolated from SCG of either B6 or Slc6a2-/- mice and then incubated with ACh, an ACh and Slc6a2-blocker, an ACh and MAOa-blocker, or culture medium. Each data point represents tissues pooled from 6 mice. n = 3-7 experiments, *P < 0.05. The following number of cells were used in NE assays (run in duplicates): 364 ± 128 from B6 SCG (n = 7), 238 ± 55 from Slc6a2-/- SCG (n = 3), 216 ± 58 from B6 SCG incubated with ACh (n = 7), 201 ± 63 from Slc6a2-/- SCG incubated with ACh (n = 3), 196 ± 18 from B6 SCG incubated with ACh and Slc6a2-blocker (n = 5), 133 ± 11 from B6 SCG incubated with ACh and MAOa-blocker (n = 7). (f) Ex vivo NE release from SCG of either B6 or Slc6a2-/- mice after incubation with ACh, an ACh and Slc6a2-blocker, an ACh and MAOa-blocker or culture medium. Each data point represents medium collected from one explant culture. n = 7 per group, ****P < 0.0001, **P < 0.01. (g) Expression of mRNA by qRT-PCR and relative to Gapdh for pro-inflammatory genes (Tnfa and Il1) in CD45.2, F4/80-double-positive cells isolated from SCG explant cultures from TH-Cre;LSL-ChR2-YFP (blue) and LSL-ChR2-YFP (black) mice. Prior to cell sorting, SCG explants were optogenetically stimulated. n = 3-4 experiments, *P < 0.05 (for Tnfa, n = 4, P = 0.0467; for Il1, n = 3, P = 0.011). (h) Expression of mRNA by qRT-PCR and relative to Gapdh for anti-inflammatory genes (Il4ra and Arg1) in CD45.2, F4/80-double-positive cells isolated from SCG explant cultures from TH-Cre;LSL-ChR2-YFP (blue) and LSL-ChR2-YFP (black) mice. Prior to cell sorting, SCG explants were optogenetically stimulated. n = 3-4 experiments, *P < 0.05 (for Il4ra, n = 3, P = 0.0257; for Arg1, n = 4, P = 0.0497). Data in c, d, e, f, g, h were analyzed by two-tailed unpaired Student’s t-test and are shown as average ± SEM.
To validate our optogenetic findings with a physiologically relevant stimulus, we activated SNS explants with acetylcholine (ACh), which is pre-synaptically released from spinal cord neurons to innervate SCG. ACh-treated CD45+F4/80+ cells sorted from SCG explants contained significantly higher levels of NE than vehicle controls (Fig. 3e). We validated that blockade of the NE importer Slc6a2 by Nisoxetine prevented NE accumulation in SAMs (Fig. 3e). Co-incubation with ACh and Nisoxetine further abolished NE uptake (Fig. 3e) despite the substantial increase of extracellular NE levels in the culture medium (Fig. 3f). These results, along with the negligible expression levels AChRs in SAMs (Supplemental Fig. 7a; also validated by qRT-PCR in Supplemental Fig. 7b), excluded a role for AChRs in mediating NE import.
Next, we assessed the effect of blocking MAOa on NE content in CD45+F4/80+ cells (Fig. 3e). The MAOa inhibitor clorgyline was sufficient to nearly double intracellular NE levels in SAMs (Fig. 3e). Consistently, clorgyline increased NE levels in medium (Fig. 3f), to which neuronal MAOa expression may also contribute. Genetic ablation of Slc6a2 (using SCG isolated from Slc6a2-/- mice) prevented NE uptake by SAMs regardless of the NE availability in the culture medium (Fig. 3e,f). Finally, ATMs cultured in vitro with NE did not accumulate intracellular NE (Supplemental Fig. 7c), further demonstrating the specificity of NE uptake by SAMs. Altogether, our results indicate that Slc6a2 is required for NE accumulation in SAMs.
We further probed whether the availability of NE, which can be manipulated in vivo by optogenetic activation of SNS neurons, changes the inflammatory profile of SAMs. We found that optogenetic stimulation of SCG explants correlated with an increase of pro-inflammatory gene expression as measured by changes in Tnfa and Il1 (Fig. 3g) and decrease of anti-inflammatory gene expression as measured by changes in Il4ra and Arg1 (Fig. 3h).
SAMs are recruited and activated in obesity
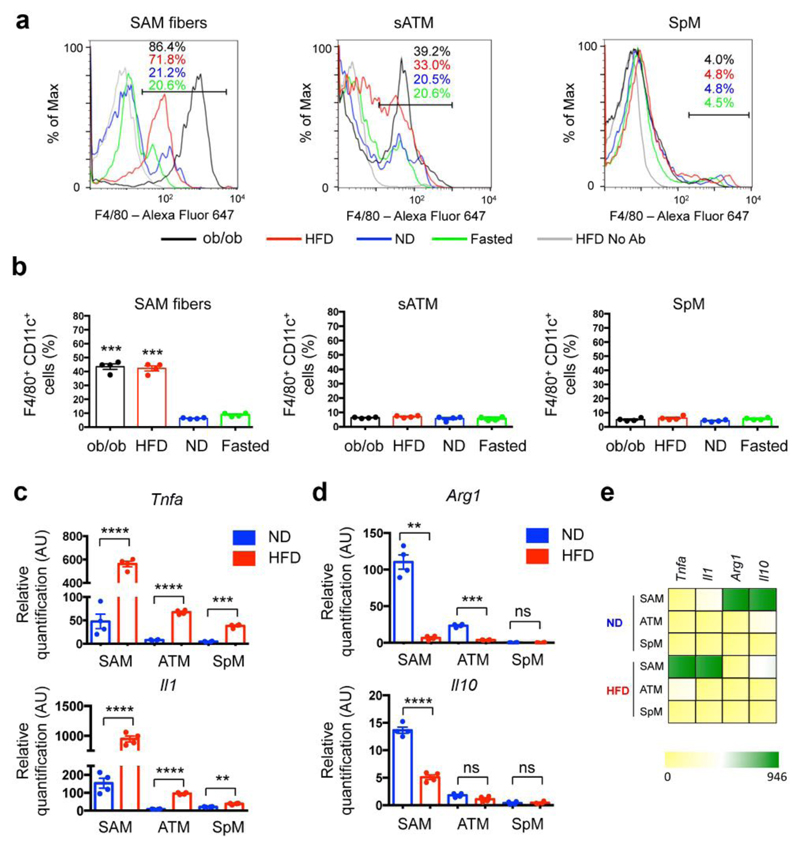
We next utilized two mouse models to characterize the effect of obesity on tissue-specific functions of SAMs. In total, we employed four experimental groups: high-fat diet (HFD)-fed, leptin-deficient (ob/ob), normal diet (ND)-fed, and 24-hr fasted ND-fed mice. Flow cytometric analysis demonstrated that both obesity models (HFD and ob/ob) exhibited significantly higher percentages of SAMs compared to lean mice (ND) (Fig. 4a and Supplemental Fig. 8a). Furthermore, the acute metabolic challenge of fasting did not result in upregulation of SAMs, suggesting an obesity-specific causation of elevated macrophage content in sympathetic fibers (Fig. 4a and Supplemental Fig. 8a). Within the F4/80+ SAM fraction in HFD and ob/ob mice, we noted a high frequency of CD11c+ cells (Fig. 4b), which are hallmarks of inflammation and insulin resistance in human obesity19. In contrast to SAM accumulation in SNS nerve fibers dissected from WAT, SAMs do not accumulate in SCG, which innervates neck structures such as salivary glands (Supplemental Fig. 8b).
Figure 4. Obesity-induced accumulation of SAMs.
(a) Representative histograms showing percentages of F4/80-Alexa Fluor 647-positive cells in sympathetic nerve fibers (left panel), subcutaneous adipose tissue (middle panel) and spleen (right panel) in mice that were genetically obese (ob/ob; black), obese due to high fat diet (red), fed normal diet (blue) or food-deprived for 24 hours (green). CD45.2-PE-positive cells were gated. Histograms are representative of 4 independent experiments. (b) Percentages of F4/80-Alexa Fluor 647- and CD11c-FITC-double-positive cells in sympathetic nerve fibers (left panel), subcutaneous adipose tissue (middle panel) and spleen (right panel) in mice that were genetically obese (ob/ob; black), obese due to high fat diet (red), fed normal diet (blue) or food-deprived for 24 hours (green). CD45.2-PE-positive cells were gated. n = 4 experiments per group, ***P < 0.001. (c) Expression of mRNA determined by qRT-PCR and relative to Gapdh for pro-inflammatory genes (Tnfa and Il1) in CD45.2-PE, F4/80-Alexa Fluor 647-double-positive cells in sympathetic nerve fibers (SAM), subcutaneous adipose tissue (ATM) and spleen (SpM) isolated from mice that were fed either normal (blue) or high fat (red) diet. n = 4 experiments per group, ****P < 0.0001, ***P < 0.001, **P < 0.01. Each data point represents tissues pooled from 10 mice. (d) Expression of mRNA determined by qRT-PCR and relative to Gapdh for anti-inflammatory genes (Arg1 and Il10) in CD45.2-PE, F4/80-Alexa Fluor 647-double-positive cells in sympathetic nerve fibers (SAM), subcutaneous adipose tissue (ATM) and spleen (SpM) isolated from mice that were fed either normal (blue) or high fat (red) diet. n = 4 experiments per group, ****P < 0.0001, **P < 0.01. Each data point represents tissues pooled from 10 mice. (e) Heat map for pro- and anti-inflammatory genes determined by the qRT-PCR analyses in c,d. Data in b were analyzed by one-way ANOVA followed by Bonferroni multiple comparison test with ND as a control group. Data in c, d were analyzed by two-tailed unpaired Student’s t-test. Data are shown as average ± SEM.
The differential distribution of macrophages in states of obesity suggested cytokine levels were also sensitive to obesity. Comparing anti- and pro-inflammatory gene profiles of SAMs, ATMs, and SpMs (Fig. 4c-e) revealed that obesity correlated with higher levels of pro-inflammatory gene expression (i.e., Tnfa or Il1; Fig. 4c,e) and lower levels of anti-inflammatory gene expression (i.e., Arg1 or Il10; Fig. 4d,e).
To determine if local proliferation contributes to SAM accumulation, we measured the proliferation marker Ki67 in SAMs by flow cytometry (Supplemental Fig. 8c,d). We observed that obesity (via HFD or ob/ob models) does not significantly increase Ki67+ SAM content, whereas (consistent with previous reports20) obesity increases Ki67+ ATMs from sWAT (Supplemental Fig. 8d).
Slc6a2 deletion in SAMs rescues obesity
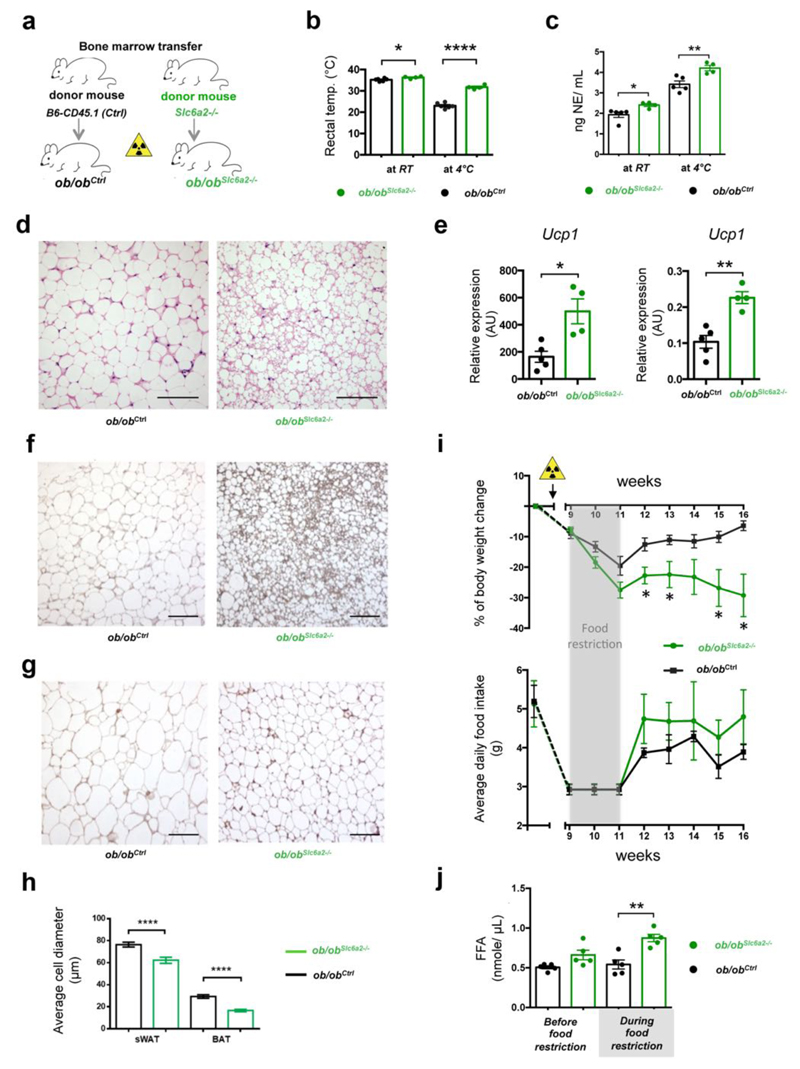
We probed how ablating Slc6a2 in SAMs affected obesity-associated pathology. We considered a Cre-Lox approach, but the established macrophage Cre lines (Cx3Cr1-Cre21,22 and LyzM-Cre23) would not allow for SAM-specificity. We thus took advantage of the cell type-specificity of Slc6a2 expression, which is high in SAMs and negligible in other macrophage and hematopoietic populations (Fig. 2b,g and ImmGen24). We validated that, besides SAMs, there did not exist another hematopoietic-derived population that expressed Slc6a2; a rare population of CD45+F4/80- cells were present in SCG (Supplemental Fig. 9a) but did not express Slc6a2 (Supplemental Fig. 9b). SAM-specific genetic ablation of Slc6a2 was attained by bone marrow transfer from Slc6a2-/- mice25 into genetically obese ob/ob recipients (ob/obSlc6a2-/-) (Fig. 5a). Control chimeras consisted of bone marrow transfer from B6-CD45.1 mice into ob/ob recipients (ob/obCtrl). Chimeras recovered for nine weeks post-transplant to allow irradiation-induced inflammation to subside.
Figure 5. Loss of function of Slc6a2 in SAMs rescues thermogenic capacities of ob/ob mice.
(a) Schematic representation of bone marrow transplant from either Slc6a2-/- or control B6 (CD45.1) mice into genetically obese ob/ob mice (ob/obSlc6a2-/- and ob/obCtrl chimeras, respectively). (b) Rectal temperature of ob/obCtrl (black) and ob/obSlc6a2-/- (green) chimeras was measured at RT and after 2 hours of cold challenge (4° C). Each data point represents one mouse. n = 4 mice for ob/obSlc6a2-/-, n = 6 mice for ob/obCtrl, *P = 0.025, ****P < 0.0001. (c) Serum NE levels of the ob/obCtrl (black) and ob/obSlc6a2-/- (green) chimeras were measured at RT and after 2 hours of cold exposure (4°C). Each data point represents one mouse. n = 4 mice per group for ob/obSlc6a2-/-, n = 5 mice per group for ob/obCtrl, *P = 0.022, **P = 0.0072. (d) Optical micrographs of BAT removed from ob/ob chimeras following 2 hours of cold challenge (4° C) and stained with H&E. Left panel represents BAT from ob/obCtrl and right panel shows BAT from the ob/obSlc6a2-/- chimeras. Images are representative of fat organs collected from 4 mice (ob/obCtrl) or 6 mice (ob/obSlc6a2-/-). (e) Expression of mRNA for Ucp1 determined by qRT-PCR and relative to Gapdh in BAT (left panel) and sWAT (right panel) dissected after 2 hours of cold (4° C) challenge. Ob/obCtrl chimeras are represented in black and ob/obSlc6a2-/- chimeras are shown in green. Each data point represents one mouse. n = 4 mice for ob/obSlc6a2-/-, n = 5 mice for ob/obCtrl, *P = 0.0269; **P = 0.0015. (f) Optical micrographs of BAT from ob/obCtrl (left) and ob/obSlc6a-/- (right) chimeras following 2 hours of cold (4° C) challenge and stained with anti-UCP1 antibody. Images are representative of fat organs collected from 4 mice (ob/obCtrl) or 6 mice (ob/obSlc6a2-/-). (g) Optical micrographs of sWAT from ob/obCtrl (left) and ob/ob SAMSlc6a2-/- (right chimeras removed from ob/ob chimeras following 2 hours of cold (4° C) challenge and stained with anti-UCP1 antibody. Images are representative of fat organs collected from 4 mice (ob/obCtrl) or 6 mice (ob/obSlc6a2-/-). (h) Average adipocyte diameter quantified from optical micrographs of sWAT and BAT from ob/ob chimeras following 2 hours of cold (4° C) challenge. Measurements are representative of 4 independent micrographs (4 ob/obSlc6a2-/- mice) or 6 micrographs (6 ob/obCtrl mice). 18-34 measurements were obtained per micrograph. n = 169 for ob/obCtrl sWAT, n = 120 for ob/obSlc6a2-/- sWAT, n = 180 for ob/obCtrl BAT, n = 120 for ob/obSlc6a2-/- BAT, ****P < 0.0001. (i) Body weight change (upper panel) and daily food intake (lower panel) of ob/obCtrl (n = 4 mice) and ob/obSlc6a2-/- (n = 6 mice) chimeras monitored for 7 weeks following 2 weeks of food intake normalization (3 gr/day – grey shade) that started 9 weeks after bone marrow transplant. Yellow triangle indicates when irradiation was performed. *P < 0.05. (j) Blood plasma non-esterified (free) fatty acid concentration in control ob/obCtrl and ob/obSlc6a2-/- chimeras measured 8 weeks after bone marrow transplant and during a 3 gr/day regimen. n = 5 mice per group, **P = 0.0022. Data in panels b, c, e, h, i were analyzed by two-tailed unpaired Student’s t-test and in panel i by multiple t-tests – Student’s t-test per row with correction for multiple comparisons using the Holm-Sidak method. Data are shown as average ± SEM. Scale bars in d, f and g, 100 μm.
As cold temperature is a robust driver of SNS activity, we challenged mice for 2 hr at 4°C and observed that ob/obSlc6a2-/- chimeras displayed superior capacity for maintaining body temperature compared to control ob/obCtrl chimeras (Fig. 5b). These thermogenic effects were accompanied by significant upregulation of NE serum levels (Fig. 5c), rescue of BAT morphology (Fig. 5d), and browning of white fat, as measured by Ucp1 mRNA and protein levels (Fig. 5e-g).
At room temperature, ob/obSlc6a2-/- transplant prevented obesity-induced hypertrophy of both BAT and WAT adipocytes (Fig. 5h) but did not affect total body weight (Fig. 5i). Because food restriction challenge drives SNS activity and mobilizes lipid stores from adipose tissue, we normalized daily food intake of the ob/ob chimeras for 2 weeks (Fig. 5i,j). After a dieting challenge ob/obSlc6a2-/- mice, relative to control chimeras, lost nearly 30% of body weight, which was stable up to 16 weeks, even after ad libitum access to food (Fig. 5i). Ob/obSlc6a2-/- mice also exhibited higher lipid mobilization during food restriction (Fig. 5j).
We analyzed wild-type B6 chimeras reconstituted with control CD45.1 bone marrow or Slc6a2-/- bone marrow (Supplemental Fig. 9c). SAMs from B6Slc6a2-/- chimeras did not accumulate NE (Supplemental Fig 9d). Consistent with the results from ob/ob chimeras (Fig. 5), B6Slc6a2-/- chimeras also exhibited increased serum NE levels, thermogenesis, and lipolysis, as well as marked weight loss, relative to B6Ctrl mice (Supplemental Fig. 9e-i). Upon HFD challenge, we observed weight gain prevention in B6Slc6a2-/- but not in B6Ctrl mice (Supplemental Fig. 9g). These results indicate a significant anti-obesity effect of SAM-specific Slc6a2 ablation.
SAMs are in BAT and act as an NE sink
In light of the enhanced thermogenic capacity of ob/obSlc6a2-/- chimeras, we questioned if SAMs are present in BAT (Supplemental Fig. 10). BAT did contain Cx3Cr1GFP cells (consistent with previous reports19) that exhibited an intermediate morphology between SAMs (multiple pseudopodia) and ATMs (round) (Supplemental Fig. 10a compared to Fig. 1c). Some of these cells appeared to make close contact with thin TH+ axons (Supplemental Fig. 10a). Because TH+ nerve fibers in BAT are too delicate for dissection, we sorted macrophages from whole BAT for qRT-PCR analysis. Slc6a2 and MAOa were expressed in BAT macrophages, although at lower levels relative to SAMs isolated from dissected SNS nerve bundles in sWAT or SCG (Supplemental Fig. 10b,c). BAT macrophages also contained NE, although at lower levels than SAMs (Supplemental Fig. 10d). The lower levels of Slc6a2, MAOa, and NE content may reflect a dilution of BAT-SAMs by BAT-ATMs since mixed (as opposed to isolated) populations were analyzed.
Finally, we used conditional LyzM-Cre;CSF1R-LSL-DTR mice to test if macrophages served as a sink for NE. After validating ablation of macrophages (Supplemental Fig. 11a,b), we observed a significant increase of NE in sWAT in vivo (Supplemental Fig. 11c). Note that, due to constant hematopoietic input, it is practically impossible to completely deplete all macrophages. This limitation notwithstanding, these results are consistent with a model in which macrophages act as sink for NE.
Human sympathetic ganglia also contain NE-degrading SAMs
Finally, we asked if SAMs exist in humans. We obtained nine human excisional biopsies of SNS or thoracolumbar ganglia that were collected during sympathectomy and/or gangliotomy. We stained tissue sections with H&E (Fig. 6a,b) or an antibody against CD68, a human macrophage marker, identifying the presence of macrophages in SNS tissues (Fig. 6c,d and Supplemental Fig. 12).
Figure 6. SAMs in the human Sympathetic Nervous System.
(a,b) Optical micrographs of human ganglia from the thoracolumbar region stained with H&E. (b) Higher magnification of the micrograph in panel a. (c,d) Optical micrographs of human ganglia from the thoracolumbar region stained with an antibody against CD68. (d) Higher magnification of the micrograph in panel c. (e) Optical micrographs of human ganglia from the thoracolumbar region stained with an antibody against CD68 and Slc6a2. Red arrows, CD68- and Slc6a2- double positive regions. (f) Optical micrographs of human ganglia from the thoracolumbar region stained with an antibody against CD68 and MAOa. Red arrows, CD68- and MAOa- double positive regions. Boxed regions in panels e and f represent higher magnification of the main micrographs. Scale bars for a and c, 1 mm, for c and d, 100 μm, for e and f, 50 μm, for boxed regions 25 μm. Images in a-f are representative of 9 different human samples.
We next determined whether SAMs in human sympathetic ganglia also contain the machinery for uptake and degradation of NE (Fig. 6e,f and Supplemental Fig. 12). The CD68 macrophage marker co-localized with staining for Slc6a2 (Fig. 6e and Supplemental Fig. 12a) and MAOa (Fig. 6f and Supplemental Fig. 12b). Both Slc6a2- and MAOa-positive neurons exist, but the background levels are low relative to control human gut-associated lymphoid tissue (GALT) samples that also contain CD68+ macrophages (Supplemental Fig. 12c,d).
Discussion
SAMs are a previously undescribed population of resident macrophages in the SNS that import and degrade NE. To fulfill their function, SAMs express a dedicated molecular machinery that is, as best we can tell, absent from neighboring macrophages and other known macrophage populations (shown by our data and ImmGen database). In SAMs, NE is imported by Slc6a2 and degraded by MAOa. This is a specialized molecular mechanism for NE uptake, the role for which is not fulfilled by canonical phagocytic mechanisms generally present in macrophages26.
Unlike most other neurons, which exclusively release neurotransmitter at a terminal synapse, SNS neurons also release NE via varicosities distributed along axons that can extend for tens of centimeters27. SAMs possibly serve to prevent NE spillover into the blood stream or neighboring tissues during high SNS activity. Indeed, we demonstrate that when SNS neurons are optogenetically activated, SAMs import increased levels of NE and become more polarized towards a pro-inflammatory phenotype. In this regard, NE can be considered a noxious stimulus that must be locally delivered in a controlled manner to a target tissue. Chronic and excessive systemic NE in serum, such as in chronic stress conditions or medullary adrenal tumors, leads to hypertension and cardiopathy due to direct action in cardiovascular tissues28.
The activated polarization state of SAMs is consistent with a model in which these cells play a tissue-protective role by acting as a sentinel and scavenger of excess levels of an endogenous neurotransmitter (i.e., NE) that, if released in excess from varicosities, could potentially be harmful. Tissue-protective immune cells have been documented in the brain and other non-neuronal systems29–34. For instance, muscularis resident macrophages in the gut induce rapid tissue-protective responses to potentially pathogenic insults via the β2-AR signaling35. This and our study indicate specialization of macrophage populations to fulfill tissue-specific tasks in response to neuronal cues. Divergent gene expression landscapes across resident-macrophage populations isolated from different tissues support the idea of local macrophage adaptations22,36,37. In this study, we use transcriptional data to molecularly characterize SAMs alongside other macrophage populations. Our results suggest that macrophages associated with the SNS have specialized molecular programs whose exploration might give further insight into mechanisms underlying SNS macrophage-neuron communication.
Although SAMs express common microglia genes and reside in proximity to nerve cells, SAM pseudopodia are morphologically distinct from the finely branching ramifications of resting microglia38,39. Moreover, SAMs are seemingly of hematopoietic origin, as suggested by our bone marrow chimera studies and high expression of CD45 and macrophage markers. Future tracing studies are necessary to definitively determine SAM origin. No reports exist on NE uptake by microglia, and we verified that machinery for NE uptake is not expressed in these cells. In this regard, only one study has reported that NE can trigger microglia to import and degrade amyloid, but not NE itself40. Neurotransmitter uptake has primarily been studied in astroglia, which are Cx3cr1-negative41.
Chimeric models require irradiation that generates inflammation. However, if given adequate recovery time (8 weeks), recruited macrophages dissipate from the brain, as represented in our chimeras by minimal residual Cx3CR1-GFP+ microglia (0.06 %). SAM levels persist at levels that greatly surmount background irradiation-induced macrophage recruitment, and regenerated SAMs are seemingly identical to those in non-irradiated mice.
We show low expression of several astroglial markers in SAMs, raising the possibility of a hybrid peripheral cell type that unites some of the features of macrophages and glia. Alternatively, mutual genes of glial cells and SAMs may be attributable to their proximity to neuron-derived signals, analogous to the observation that microglia, astrocytes and neurons share certain CNS-specific genes7,42. An alternative model is that SAMs share the lineage of satellite glial cells (SGC), which are derived from embryonic neural crest11 and also express canonical astroglial markers43. However, SGC import or degradation of NE has not been reported44.
Our study may fill a gap in the literature by demonstrating a cellular and molecular mechanism alternate to the proposed existence of NE-producing macrophages in WAT3. In this regard, our findings are consistent with other reports4–6, as we do not detect the NE biosynthetic machinery in SAMs nor in ATMs. The identification of SAMs sheds new light on this recent controversy by documenting how a particular population of macrophages can contain NE in the absence of its biosynthesis. We also document that BAT macrophages contain similar molecular machinery as SAMs for NE uptake, extending and validating the findings of our colleagues21. SAMs may play a tissue protective role by regulating regional NE levels by serving as a local sink that prevents the dangerous effects of chronically increased levels of systemic NE.
In sharp contrast to the anti-inflammatory state of intestinal nerve-associated Cx3Cr1GFP macrophages35, SAMs exhibit a pro-inflammatory profile at steady state. This could be due to the constitutive presence of a danger signal — namely, NE. Whether the polarization is caused by NE import or by adrenergic signaling remains to be established. In this regard, polarization of enteric-associated macrophages has been linked to activation of beta-2 adrenergic receptor, which is also expressed in SAMs35. Regardless, our core message is relevant: that SAMs are pro-inflammatory and act as an NE sink and that blocking NE uptake has an anti-obesity effect. Our results support a model whereby SAMs pathologically accumulate in SNS nerves of obese subjects in an organ-specific manner, thus explaining why we detect SAM accumulation in the WAT-associated SNS, but not in SCG, which innervates salivary glands and other neck structures. The NE-scavenging role of SAMs may have become evolutionarily maladaptive, as, in the past, obesity was not a common physiological stress to which humans had to adapt. In modern times, the prevalence of over-nutrition has necessitated an evolutionary need for increased lipolysis-inducing NE signaling to maintain fat stores, which is obstructed by the “original” function of SAMs to limit NE levels.
Elevation of NE in adipose tissue promotes lipolysis and fat mass reduction, which are beneficial for diseases such as obesity. We demonstrate that SAMs accumulate in the SNS and contribute to pathology in two mouse models of obesity. Furthermore, we have demonstrated that SAM-specific Slc6a2 ablation rescues BAT and adaptive thermogenesis in obese ob/ob mice, which in turn leads to sustained weight loss and lipid mobilization. Our results indicate that blocking NE import into SAMs mitigates the recidivism of obesity that is typical after dieting. Overall, our results identify SAMs as a potential new molecular and cellular target for obesity therapy.
Online Methods
General experimental approaches
No samples, mice or data points were excluded from the reported analyses. Samples were not randomized to experimental groups. Analyses were not performed in blinded fashion. More detailed information can be found in Life Sciences Reporting Summary.
Antibodies, stain reagents and drugs
The antibodies were obtained from the following vendors: anti-F4/80-Alexa Fluor 647 (BioLegend, cat# 123122, clone BM8), anti-human CD68 (Dako, cat# M 0876, clone PG-M1), anti-human Norepinephrine Transporter (Mab Technologies, cat# NET17-1, clone 3-6C1 sc H10), anti-Monoamine Oxidase A (Abcam, cat# ab126751, clone GR155892-5), anti-TH (Pel-Freez Biologicals, cat# P40101-150, lot 16736), anti-GFP (Abcam, cat# ab13970, lot GR279236-1), anti-TH (Aves Lab, cat# TYH, lot TH1205), anti-GFP (Invitrogen, cat# A11120, lot 1563696), anti-GFP (Abcam, cat# ab6556, lot: GR292567-1), Goat anti-Chicken IgY (H+L) Secondary Antibody, Alexa Fluor 488 (Molecular Probes/ Thermo Fisher Scientific, cat# A-11039, lot 1759025), Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 (Molecular Probes/ Thermo Fisher Scientific, cat# A-11012, lot 1704538), anti-Ly6c-eFluor 405 (eBioscience, cat# 48-5932-82, clone HK1.4, lot 4306743), anti-CD11c-PE (BD Pharmingen, cat#553802, clone HL3, lot 47030), anti-CD45.2-PE (Biolegend, cat# 109808, clone 104.2), anti-CD45.2-FITC (obtained from Dr. Shoji Kimura from Memorial Sloan-Kettering Cancer Center, New York, NY; clone 104.2), anti-CD11b-FITC (ATCC # TIB-128, clone M1/70), anti-MHCII-Bio (clone M5/114, ATCC # TIB-120), SAv-APC/Cy7 (Biolegend, cat# 405208, lot B215107), anti-Ki67-Alexa488 (BD Biosciences, cat# 558616, clone B56, lot 7138687), anti IgG-Alexa488, Isotype Control (BD Biosciences, cat# 557782, lot 7102576), anti-Siglec-f-BV421 (BD Biosciences, cat# 562681, lot 7047598), anti-CD68 (Bio-Rad, cat# MCA1957GA, clone FA-11), Goat anti-Rat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 (Invitrogen, cat# A-11007), Goat Anti-Chicken IgY H&L, Alexa Fluor 647 (Abcam, cat# ab150171), Goat Anti-Rabbit IgG H&L, Alexa Fluor 488, (Abcam, cat# ab150077), Goat anti-Mouse IgG (H+L), Alexa Fluor 488 (Sigma, cat# SAB4600387), Anti-Mouse IgG (whole molecule), Cy3 (Sigma, cat# C0992), anti-Rabbit to UCP1 (Abcam, cat# ab10983, lot GR249119-8), anti-mouse NET (Mab Technologies, cat# NET05-2, clone 2-3 B2 sc D7). Sytox Blue dead cell stain (Molecular Probes/ Thermo Fisher Scientific, cat# S34857, lot 1851462) was used to exclude dead cells. HCS LipidTOX™ Deep Red Neutral Lipid Stain (Molecular Probes/Thermo Fisher Scientific, cat# H34477) and HCS LipidTOX™ Red Neutral Lipid Stain (Molecular Probes/Thermo Fisher Scientific, cat# H34476) were used to stain lipids.
Acetylcholine chloride, Nisoxetine hydrochloride, Clorgyline and Norepienphrine were purchased from Sigma-Aldrich.
Mice
Cx3cr1GFP/+ mice (Cx3cr1tm1Litt/LittJ; Stock No: 008451), TH-Cre mice (Stock No: 008601), GFP-L10 mice (Stock No: 024750), LysM-Cre mice (Stock No: 004781) LSL-ChR2-YFP mice (Stock No: 012-569, Daou et al., 2013), LSL-tdTomato (Stock No: 007909), Ob/ob mice (Stock No: 000632) and CSF1R-LSL-DTR (Stock No: 024046) mice were purchased from the Jackson Laboratory (JAX). NET P/P (Slc6a2-/-) mice were kindly provided by Maureen Hahn from the University of Vanderbilt. B6 (C57BL/B6J) and B6-CD45.1 were purchased from Charles River, bred and maintained at Instituto Gulbenkian de Ciência. Both males and females were used in this study. Mice were 4-10 weeks old (for details see Life Sciences Reporting Summary). Animal procedures were approved by the ethics committee of Instituto Gulbenkian de Ciência.
Immunofluorescence and confocal microscopy
Tissues were dissected and fixed in 4% Paraformaldehyde for 2 hours (at room temperature (RT), with agitation). For images in Figure 2 j and k we employed frozen sections and the fixation step was followed by cryoprotection in 30% sucrose (Alfa Aesar). 16µm sections were obtained in a Leica Cryostat CM3050S. Both frozen sections and the whole mount tissues were incubated in a blocking/permeabilization solution (3% Bovine serum albumin, 2% Goat serum, 0.1% Tween and 0.1% Sodium azide in 1xPBS) for 1 hour at RT, with (whole mouns) or without (frozen sections) agitation. Incubations with primary antibodies were performed overnight at 4°C with (whole mount) or without (frozen sections) agitation.The following dilutions of primary antibodies were used: anti-GFP (1:500), anti-TH (1:1000), anti-Slc6a2 (1:500), anti-MAOa (1:100). Incubation with secondary antibodies was performed for 1-2 hours at RT, with or without (in case of frozen sections) agitation. Z-series stacks were acquired on a Leica TCS SP5 confocal Inverted microscope. Analysis and quantification of images were performed in FIJI.
In vivo 2-photon microscopy
Mice 2 months old were kept anesthetized with 2% isofluorane. During surgery, body temperature was maintained at 37° C with a warming pad. After application of local anesthetics (lidocaine), a sagittal incision of the skin was made above the supra-pelvic flank to expose the subcutaneous inguinal fat pad. An imaging chamber was custom built to minimize fat movement. Warm imaging solution (in mM: 130 NaCl, 3 KCl, 2.5 CaCl2, 0.6 6H2O,MgCl2, 10 HEPES without Na, 1.2 NaHCO3, 10 glucose, pH 7.45 with NaOH) (37°C) mixed with a fat dye (LipidTOX) was applied to label adipocytes, maintain tissue integrity, and to allow the use of immersion objective. Imaging experiments were performed under a two-photon laser-scanning microscope (Ultima, Prairie Instruments Inc.). Live images were acquired at 8–12 frames per second, at depths below the surface ranging from 100 to 250 mm, using an Olympus 20x 1.0 N.A. water immersion objective, with a laser tuned to 810-940 nm wavelength, and emission filters 525/50 nm and 595/50 nm for green and red fluorescence, respectively. Laser power was adjusted to be 20–25 mW at the focal plane (maximally 35 mW), depending on the imaging depth and level of expression of GFP and LipidTOX spread. Analysis and quantification of images were performed in FIJI.
Electron microscopy
Fresh tissue was perfused with 2% paraformaldehyde (Electron Microscopy Services (EMS)), 0.2% glutaraldehyde (EMS) in 0.1M phosphate buffer (PB) (pH 7.4). After perfusion, fibers were isolated and immersion fixed for 2 hours at room temperature (RT) in the same fixative. For quenching free-aldehydes auto-fluorescence, nerves were washed with 0.15% glycine (VWR), in PB for 10 minutes at RT.
Correlative Light-Electron Microscopy (CLEM)
After fixation, the fibers were stabilized with 0.1% tannic acid (EMS) and embed in 2% agarose (Omnipur) before cryoprotection in 30% sucrose (Alfa Aesar) ON at 4°C. Embed samples were placed in optimal cutting temperature (OCT) compound (Sakura) and plunge freeze in liquid nitrogen. 10µm sections were obtained in a Leica Cryostat CM3050S and placed in cover-glasses coated with 2% (3-Aminopropyl)triethoxysilane (Sigma Aldrich) in acetone. The light microscopy imaging was performed in a Leica SP5 Live microscope after mounting the sections with PB. For electron microscopy processing, samples were washed 10 times with PB and post-fixed in 1% osmium tetroxide (EMS) with 1% potassium hexacyanoferrate (Sigma Aldrich) in PB for 30 minutes, on ice. Dehydration was done in a graded ethanol series of 30%, 50%, 75%, 90% and 100%, for 10 minutes each. EPON resin (EMS) was used for embedding. 70nm serial sections were obtained in a Leica UC7 and stained with 1% uranyl acetate and lead citrate for 5 minutes each. Electron microscopy images were acquired on a Hitachi H-7650 operating at 100kV.
Single cell suspension
Tissues were dissected from 10 mice. Spleen, brain, visceral fat and subcutaneous fat were excised and digested for 30 minutes with collagenase (Sigma) at 37°C with shaking. Sympathetic nerve fibers were isolated from subcutaneous adipose tissues and digested for 30 minutes with Hyaluronidase (Sigma) at 37°C with shaking, washed and further digested with collagenase for 15 minutes. SCG were dissected and digested with collagenase for 10 minutes, washed and further digested with trypsin (Biowest) for 30 minutes at 37°C with shaking. Cell suspensions were filtered through a 70 μm sieve and centrifuged at 450 xg for 5 minutes.
Flow cytometry
Flow cytometry data were acquired on a LSR Fortessa X-20 SORP (Becton-Dickinson), FACScalibur (Becton-Dickinson) or Cyan-ADP (Beckman Coulter) and analyzed using FlowJo software package (Tree Star). Macrophages were sorted as live CD45, F4/80-double positive using a FACS Aria IIu High Speed cell sorter (Becton Dickinson) or MoFlo High-Speed Cell Sorter produced by Dako Cytomation (now owned by Beckman Coulter).
Bone marrow chimeras
B6-CD45.1 mice (8-10 weeks), B6 (C57BL/6J) mice (8-10 weeks) or ob/ob (8-10 weeks) mice were lethally irradiated (900 rad, 3.42 minutes, 137Cs source) (Gammacell 2000) and reconstituted with bone marrow cells from either Cx3cr1GFP/+ mice (6 weeks), Slc6a2-/- mice (6-8 weeks), B6 mice (6-8 weeks) or B6-CD45.1 mice (6-8 weeks). B6-CD45.1 mice and B6 mice were reconstituted with 5 x 106 total bone marrow cells and ob/ob mice were reconstituted with 3 x 107 total bone marrow cells. Chimerism was assessed 8 weeks after by flow cytometry.
Low-input RNAseq library preparation
Sequencing libraries were prepared according to the Smart-seq2 method45 with some modifications. 1715 ± 115 cells from nerve fibers, 1534 ± 85 cells from superior cervical ganglia and 5000 cells from other tissues (visceral fat, subcutaneous fat, spleen and brain) were isolated as live CD45+F4/80+ in Trizol (Thermo Fisher) and were used as starting material. RNA was extracted with the Direct-zol MicroPrep kit (Zymo Research) with on-column DNAseI treatment. 10 µL of purified RNA was mixed with 5.5 µL of SMARTScribe 5X First-Strand Buffer (Clontech), 1 µL polyT-RT primer (2.5 µM, 5’-AAGCAGTGGTATCAACGCAGAGTAC(T30)VN-3’), 0.5 µL SUPERase-IN (Ambion), 4 µL dNTP mix (10 mM, Invitrogen), 0.5 µL DTT (20 mM, Clontech) and 2 µL Betaine solution (5 M, Sigma), incubated 50°C 3 min. 3.9 µL of first strand mix, containing 0.2 µL 1% Tween-20, 0.32 µL MgCl2 (500 mM), 0.88 µL Betaine solution (5 M, Sigma), 0.5 µL SUPERase-IN (Ambion) and 2 µL SMARTScribe Reverse Transcriptase (100 U/µL, Clontech) was added and incubated one cycle 25°C 3 min., 42°C 60 min. 1.62 µL template switch (TS) reaction mix containing 0.8 µL biotin-TS oligo (10 µM, Biotin-5’-AAGCAGTGGTATCAACGCAGAGTACATrGrG+G-3’), 0.5 µL SMARTScribe Reverse Transcriptase (100 U/µL Clontech) and 0.32 µL SMARTScribe 5X First-Strand Buffer (Clontech) was added, then incubated at 50°C 2 min., 42°C 80 min., 70°C 10 min. 14.8 µL second strand synthesis, pre-amplification mix containing 1 µL pre-amp oligo (10 µM, 5’AAGCAGTGGTATCAACGCAGAGT-3’), 8.8 µL KAPA HiFi Fidelity Buffer (5X, KAPA Biosystems), 3.5 µL dNTP mix (10 mM, Invitrogen) and 1.5 µL KAPA HiFi HotStart DNA Polymerase (1U/µL, KAPA Biosystems), was added, then amplified by PCR: 95°C 3 min., 8 cycles 98°C 20 seconds, 67°C 15 sec and 72°C 6 min, final extension 72°C 5 min. The synthesized dsDNA was purified using Sera-Mag Speedbeads (Thermo Fisher Scientific) with final 8.4% PEG8000, 1.1M NaCl, then eluted with 13 µL UltraPure water (Invitrogen). The product was quantified by Qubit dsDNA High Sensitivity Assay Kit (Invitrogen) and libraries were prepared using the Nextera DNA Sample Preparation kit (Illumina). Tagmentation mix containing 11 µL 2X Tagment DNA Buffer and 1 µL Tagment DNA Enzyme was added to 10 µL purified DNA, then incubated at 55°C 15 min. 6 µL Nextera Resuspension Buffer (Illumina) was added and incubated at room temperature for 5 min. Tagmented DNA was purified using Sera-Mag Speedbeads (Thermo Fisher Scientific) with final 7.8% PEG8000, 0.98M NaCl, then eluted with 25 µL UltraPure water (Invitrogen). Final enrichment amplification was performed with Nextera primers, adding 1 µL Index 1 primers (100 µM, N7xx), 1 µL Index 2 primers (100 µM, N5xx) and 27 µL NEBNext High-Fidelity 2X PCR Master Mix (New England BioLabs), then amplified by PCR: 72°C 5 min., 98°C 30 sec., 8-13 cycles 98°C 10 seconds, 63°C 30 sec., and 72°C 1 min. Libraries were size selected, quantified Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific), pooled and sequenced on a NextSeq 500 (Illumina) for 76 cycles at a depth of 25 to 30 million single end reads per sample. To normalize for genomic DNA contamination, which occurred in some samples due to incomplete DNA removal during RNA isolation, the average intronic noise per base pair in all intronic regions per gene was calculated. The exonic reads were then normalized by subtracting the background noise per base pair for the complete length of the exonic regions. Genes without introns were not normalized, as these genes are the minority of genes and are typically short. (Code available at: https://github.com/vlink/DNA_contamination).
Fastq files from sequencing experiments were mapped to the mouse mm10 genome using default parameters for STAR46. Mapped data were analyzed with HOMER47, custom R, and Perl scripts.
Superior cervical ganglia (SCG) explant cultures
SCG were removed from 4-6 weeks old mice under a stereomicroscope and placed in Dulbecco's Modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA, U.S.A.). Ganglia were cleaned from the surrounding tissue capsule and transferred into 8-well Tissue Culture Chambers (Sarstedt, Nümbrecht, Germany) that were previously coated with poly-D-lysine (Sigma/Aldrich, Steinheim, Germany) in accordance to the manufacturer's instructions. Ganglia were then covered with 5 μl of Matrigel (BD Bioscience, San Jose, CA, U.S.A.) and incubated for 7 min at 37 °C. DMEM without phenol red (Invitrogen) supplemented with 10 % fetal bovine serum (Invitrogen), 2 mM L-Glutamine (Biowest, Nuaillé, France) and nerve growth factor (Sigma/Aldrich) were subsequently added. 12 SCG explants cultures were prepared per condition. SCG ganglia were cultured for minimum 24 hours prior to further manipulation. Stimulation protocol in Fig. 3 was performed for 2 hours with the following concentrations of drugs: 10 mM Acetylcholine chloride, 100 nM Nisoxetine hydrochloride, and 100 μM Clorgyline.
NE measurements after optogenetic stimulation ex vivo
Depolarization of sympathetic neurons in TH-Cre/LSL-ChR2-YFP explant cultures were performed on a Yokogawa CSU-X Spinning Disk confocal using the 488 nm laser line and pointing at the region of interest (ROI) for 200 μs. Stimulation was repeated 7 times using 40 % of laser intensity. NE in the SCG explant culture medium and sorted CD45,F4/80-double positive cells was determined with NE ELISA kit (Labor Diagnostika Nord GmbH, Nordhorn, Germany, cat# BA E-5200). The same procedure was performed for LSL-ChR2-YFP control mice.
NE measurements in macrophages from sWAT
CD45.2-PE, F4/80-Alexa Fluor 647 – double positive cells from sWAT were sorted as live and incubated with 2 μM Norepinephrine for 2 hours using the same culture conditions as for SCG explant cultures. Afterwards cells were washed twice with 1xPBS and NE content was measured with NE ELISA kit (Labor Diagnostika Nord GmbH, Nordhorn, Germany, cat# BA E-5200).
Quantitative PCR
Total RNA from sorted cells was isolated using RNeasy Plus Micro Kit (Qiagen, cat# 50974034). Total RNA from adipose tissues was isolated with PureLink RNA Mini Kit (Ambion, Life Technologies, cat# 12183025). cDNA was reverse transcribed using SuperScript II (Invitrogen) and random primers (Invitrogen). Quantitative PCR was performed using SYBR Green (Applied Biosystems) in ABI QuantStudio 7 (Applied Biosystems). GAPDH housekeeping gene was used to normalize samples. We used the following formula to calculate the relative expression levels:
The primers used are listed below:
Lpl-forward, 5′- CAGCTGGGCCTAACTTTGAG -3′;
Lpl-reverse, 5′- CCTCTCTGCAATCACACGAA -3′;
Pnpla2-forward, 5′- CACTTTAGCTCCAAGGATGA -3′;
Pnpla2-reverse, 5′- TGGTTCAGTAGGCCATTCCT -3′;
Gfap-forward, 5’-CCAGCTTCGAGCCAAGGA-3’;
Gfap-reverse, 5’-GAAGCTCCGCCTGGTAGACA-3’;
Gap43 forward, 5’-AGCCAAGGAGGAGCCTAAAC-3’;
Gap43 reverse, 5’-CTGTCGGGCACTTTCCTTAG-3’;
Ucp1-forward, 5′- GTGAAGGTCAGAATGCAAGC -3′;
Ucp1-reverse, 5′- AGGGCCCCCTTCATGAGGTC -3′;
Slc6a2-forward, 5′-CAGGCACCTCCATTCTGTTT-3′;
Slc6a2-reverse, 5′- GCGGCTTGAAGTTGATGATGCTG -3′;
Maoa-forward, 5′- GCCCAGTATCACAGGCCAC 3′;
Maoa-reverse, 5′-GTCCCACATAAGCTCCACCA-3′;
Chrm1-forward, 5’-CAGTCCCAACATCACCGTCTT-3’;
Chrm1-reverse, 5’-GAGAACGAAGGAAACCAACCAC-3’;
Chrm2-forward, 5’-TGTCTCCCAGTCTAGTGCAAGG-3’;
Chrm2-reverse, 5’-CATTCTGACCTGACGATCCAAC-3’;
Chrm4-forward, 5’-GCCTTCATCCTCACCTGGAC-3’;
Chrm4-reverse, 5’-AGTGGCATTGCAGAGTGCAT-3’;
Chrm5-forward, 5’-CCATGGACTGTGGGAAGTCA-3’;
Chrm5-reverse, 5’-CAGCGTCCCATGAGGATGTA-3’;
Chrna2-forward, 5’-CTCCCATCCTGCTTTCCAG-3’;
Chrna2-reverse, 5’-GTTTGAACAGGCGGTCCTC-3’;
Chrna3-forward, 5’-GCGAACAGGTCACAGTTTATG-3’;
Chrna3-reverse, 5’-GCATTTTTCTCTGGGTTTTCA-3’;
Chrna5-forward, 5’-CGCTCTTCTTCCACACACAA-3’;
Chrna5-reverse, 5’-TAGGTCCACCGTCTTTCTCG-3’;
Chrna6-forward, 5’-CTTTGTCACGCTGTCCAT-3’;
Chrna6-reverse, 5’-GCCTCCTTTGTCTTGTCC-3’;
Chrna7-forward, 5’-ACAGTACTTCGCCAGCACCA-3’;
Chrna7-reverse, 5’-AAACCATGCACACCAATTCA-3’;
Chrna9-forward, 5’-ACAAGGCCACCAACTCCA-3’;
Chrna9-reverse, 5’-ACCAACCCACTCCTCCTCTT-3’;
Chrna10-forward, 5’-TCTGACCTCACAACCCACAA-3’;
Chrna10-reverse, 5’-TCCTGTCTCAGCCTCCATGT-3’
Chrnb2-forward, 5’-GGGCAGGCACACTATTCTTC-3’;
Chrnb2-reverse, 5’-TCCAATCCTCCCTCACACTC-3’;
Chrnb3-forward, 5’-CTCCTCAGACATTGGTTCCAAGG-3’;
Chrnb3-reverse, 5’-AATGAGGTCAACCATGGT-3’;
Chrnb4- forward, 5’-TCTGGTTGCCTGACATCGTG-3’;
Chrnb4-reverse, GGGTTCACAAAGTACATGGA
Adrb2-forward, 5’- GGTTATCGTCCTGGCCATCGTGTTTG-3’;
Adrb2-reverse, 5’-TGGTTCGTGAAGAAGTCACAGCAAGTCTC-3’;
Th-forward, 5’-GGTATACGCCACGCTGAAGG-3’;
Th-reverse, 5’- TAGCCACAGTACCGTTCCAGA-3’
Tnfa-forward, 5′- ATGAGCACAGAAAGCATGATC -3′;
Tnfa-reverse, 5′- TACAGGCTTGTCACTCGAATT -3′;
Il10-forward, 5′- GCTCTTACTGACTGGCATGAG -3′;
Il10-reverse, 5′- CGCAGCTCTAGGAGCATGTG-3′;
Il1-forward, 5′- GAAGAAGAGCCCATCCTCTG -3′;
Il1-reverse, 5′- TCATCTCGGAGCCTGTAGTG -3′;
Il4ra-forward, 5’-TGACCTCACAGGAACCCAGGC-3’;
Il4ra-reverse, 5’-GAACAGGCAAAACAACGGGAT-3’;
Gapdh-forward, 5’-AACTTTGGCATTGTGGAAGG-3’;
Gapdh-reverse, 5’-ACACATTGGGGGTAGGAACA-3’.
Functional studies
We measured body rectal temperature with an electronic thermometer (Precision) when the animals were housed both at RT and at 4 °C with ND food and water ad libitum.
Free fatty acids were measured in blood plasma using Free Fatty Acid Quantitation Kit (Sigma-Aldrich, cat# MAK044-1KT).
Serum NE levels were determined with NE ELISA kit (Labor Diagnostika Nord GmbH, Nordhorn, Germany, cat# BA E-5200).
High-fat diet challenge
When B6 mice reached 8 weeks we replaced ND with HFD (Ssniff, Spezialdiäten GmbH, Soest, Germany), which contains 60 kJ% fat. Analyses in Fig. 4 were performed when mice gained 40% increase in body weight, after 3 months of HFD.
Intracellular stain with Ki67
Cells were surface stained for 30 min. Subsequently, cells were washed and fixed with fixation/permeabilization buffer (eBiosciences) and then permeabilized with permeabilization buffer (eBiosciences). Following this process cells were intracellularly stained with anti-Ki67 or isotype control.
Histopathological and immunohistochemical analysis
The human and mouse tissues were fixed in buffered formalin and the inclusion in paraffin was done according to the standard technical procedures. Histochemical and immunohistochemical studies were performed on formalin-fixed paraffin-embedded tissue sections. Sections were 2 microns (human ganglia) or 3-6 microns (mouse tissues) thick (for H&E) and 4 microns thick (for the immunohistochemical study). The following markers were used for immunohistochemistry– aminoethylcarbazole (AEC) and 3,3'-diaminobenzidine (DAB), accordingly to the usual technical procedure for the marker. For the immunohistochemical studies sections underwent antigenic recovery prior to incubation with primary antibodies – anti-CD68 (Dako; clone PG-M1; dilution 1/150) anti-human Slc6a2 (Mab Techonolgies, clone 3-6C1 sc H10; dilution 1/1000), anti-MAOa (Abcam, clone GR155892-5, dilution 1/50), anti-UCP1 (Abcam, dilution 1/500). Human tissues were analyzed under an optical microscope (Nikon Eclipse 50i) and iconography microscopic images captured using a coupled digital camera (DS Camera Control Unit DS-L2). Mouse tissues were analyzed using Leica DM LB2 microscope and images were captured with Leica DFC 250 camera.
DT-mediated macrophages depletion
We used LysM-Cre/LSL-CSF1R-DTR mice for this experiment and LSL-CSF1R-DTR as controls. Animals received injections of Diphtheria Toxin (DT) from Corynebacterium diphtheria (Calbiochem) once daily for 4 consecutive days. First dose was 500ng of DT in PBS/20g of body weight followed by three doses of 250ng of DT in PBS/20g of body weight. Depletion was assessed by flow cytometry 12 hours after the fourth injection. NE levels in adipose tissues were assayed with NE ELISA kit (Labor Diagnostika Nord GmbH, Nordhorn, Germany, cat# BA E-5200). Protein concentration was determined by the Bradford Method.
Statistics
Statistical analyses were performed using GraphPad Prism software (San Diego, CA) using unpaired Student’s t-test (two-tailed) when two groups were being compared or one-way ANOVA test when several groups were being compared. One way-ANOVA was followed by Tukey’s mulitiple comparison test, except for the data in Fig. 4b and Supplementary Fig. 8a where it was followed by Bonferroni multiple comparison test with one group indicated as a control group. A P < 0.05 was considered statistically significant. Data were represented as mean ± SEM. Sample size was predetermined based on previous studies (for more information see Life Sciences Reporting Summary). Data displayed normal variance.
Data availability
The RNA-seq data sets are available at GEO accession code. The data that support the findings herein presented are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgements
We would like to thank the Unit for Imaging and Cytometry at the IGC for assistance with flow cytometry, cell sorting and multiphoton microscopy. We also want to thank the Antibody Service at the IGC for the antibodies produced in-house and the Histopathology facility at the IGC for tissue processing and histological assessment, This work was supported by the Fundação para a Ciéncia e Tecnologia (FCT), the European Molecular Biology Organization (EMBO), the Human Frontier Science Program (HFSP), Maratona da Saude, and the National Institutes of Health (NIH). R.M.P was supported by FCT (SFRH/BD/88454/2012), J.S.S.- by American Heart Association (16PRE30980030) and a training grant (T32DK007541), B.A.A. - by Conselho Nacional de Desenvolvimento Científico e Tecnológico and N.M-S. – by Xunta de Galicia (ED481B 2016/168-0). We thank Myriam Aouadi for helpful discussions.
Footnotes
Author Contributions
A.I.D. conceptualized the study. R.M.P performed 2-photon and confocal microscopy. E.S. and R.M.P. performed flow cytometry. J.S.S and R.M.P performed low-input RNA-seq. V.M.L., J.S.S., R.M.P – analyzed the RNA-seq data. M.I., A.L.S, S.A and E.T. performed Electron Microscopy. E.S., R.M.P, N.M.S, I.M., B.A.A and C.M.L performed functional tests. N.K., I.M., R.M., V.G. performed related rodent husbandry and genotyping. F.T. and M.V. processed human ganglia. M.K.H. provided the Slc6a2-/- mice. N.J.S. developed low-input RNA-seq protocols. A.I.D, C.K.G, R.M.P wrote the original draft of the manuscript. A.I.D, C.K.G, R.M.P, C.M.L reviewed and edited final version of the manuscript.
Competing Financial Intrests
The authors declare no competing financial interests.
References
- 1.Zeng W, et al. Sympathetic Neuro-adipose Connections Mediate Leptin-Driven Lipolysis. Cell. 2015;163:84–94. doi: 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathis D. Immunological Goings-on in Visceral Adipose Tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer K, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23:623–630. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spadaro O, et al. IGF1 Shapes Macrophage Activation in Response to Immunometabolic Challenge. Cell Rep. 2017;19:225–234. doi: 10.1016/j.celrep.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reitman ML. How Does Fat Transition from White to Beige? Cell Metab. 2017;26:14–16. doi: 10.1016/j.cmet.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosselin D, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anlauf E, Derouiche A. Glutamine Synthetase as an Astrocytic Marker: Its Cell Type and Vesicle Localization. Front Endocrinol. 2013;4 doi: 10.3389/fendo.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry FA, et al. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 11.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 12.Ludwin SK, Kosek JC, Eng LF. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976;165:197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- 13.Mearow KM, Mill JF, Vitkovic L. The ontogeny and localization of glutamine synthetase gene expression in rat brain. Brain Res Mol Brain Res. 1989;6:223–232. doi: 10.1016/0169-328x(89)90068-5. [DOI] [PubMed] [Google Scholar]
- 14.Raff MC, et al. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978;274:813–816. [PubMed] [Google Scholar]
- 15.Regan MR, et al. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci Off J Soc Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusnakova V, et al. Heterogeneity of astrocytes: from development to injury - single cell gene expression. PloS One. 2013;8:e69734. doi: 10.1371/journal.pone.0069734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sensenbrenner M, Lucas M, Deloulme JC. Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells. J Mol Med Berl Ger. 1997;75:653–663. doi: 10.1007/s001090050149. [DOI] [PubMed] [Google Scholar]
- 18.Buttgereit A, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016;17:1397–1406. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- 19.Wentworth JM, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amano SU, et al. Local Proliferation of Macrophages Contributes to Obesity-Associated Adipose Tissue Inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf Y, et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat Immunol. 2017;18:665–674. doi: 10.1038/ni.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosselin D, et al. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356 doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 24.Merad M, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirey-Rice JK, et al. Norepinephrine transporter variant A457P knock-in mice display key features of human postural orthostatic tachycardia syndrome. Dis Model Mech. 2013;6:1001–1011. doi: 10.1242/dmm.012203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 27.Stjärne L. Basic mechanisms and local modulation of nerve impulse-induced secretion of neurotransmitters from individual sympathetic nerve varicosities. Rev Physiol Biochem Pharmacol. 1989;112:1–137. doi: 10.1007/BFb0027496. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;303:H1273–1282. doi: 10.1152/ajpheart.00492.2012. [DOI] [PubMed] [Google Scholar]
- 29.Filiano AJ, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galle-Treger L, et al. Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat Commun. 2016;7 doi: 10.1038/ncomms13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibiza S, et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louveau A, et al. Structural and functional features of central nervous system lymphatics. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosas-Ballina M, et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabanyi I, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crotti A, Ransohoff RM. Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity. 2016;44:505–515. doi: 10.1016/j.immuni.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 40.Kong Y, Ruan L, Qian L, Liu X, Le Y. Norepinephrine promotes microglia to uptake and degrade amyloid beta peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme. J Neurosci Off J Soc Neurosci. 2010;30:11848–11857. doi: 10.1523/JNEUROSCI.2985-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuroglia. Oxford University Press; 2013. [Google Scholar]
- 42.Butovsky O, et al. Identification of a unique TGF-[beta]-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev. 2010;64:304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Picelli S, et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- 46.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinz S, et al. Simple Combinations of Lineage-Determining Transcription Factors Prime cis-Regulatory Elements Required for Macrophage and B Cell Identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data sets are available at GEO accession code. The data that support the findings herein presented are available from the corresponding author upon reasonable request.