Highlights
-
•
Functional random forest identified transdiagnostic ASD and ADHD subtypes.
-
•
Subtypes are directly tied to ADHD symptoms, relevant to ASD and ADHD.
-
•
Neurocognitive subtypes do not map one to one with functional connectivity trends.
-
•
There may be multiple mechanistic “pathways” to observed phenotypes.
Keywords: ASD, ADHD, Executive function, Machine learning, rs-fMRI
Abstract
Background
Those with autism spectrum disorder (ASD) and/or attention-deficit-hyperactivity disorder (ADHD) exhibit symptoms of hyperactivity and inattention, causing significant hardships for families and society. A potential mechanism involved in these conditions is atypical executive function (EF). Inconsistent findings highlight that EF features may be shared or distinct across ADHD and ASD. With ADHD and ASD each also being heterogeneous, we hypothesized that there may be nested subgroups across disorders with shared or unique underlying mechanisms.
Methods
Participants (N = 130) included adolescents aged 7–16 with ASD (n = 64) and ADHD (n = 66). Typically developing (TD) participants (n = 28) were included for a comparative secondary sub-group analysis. Parents completed the K-SADS and youth completed an extended battery of executive and other cognitive measures. A two stage hybrid machine learning tool called functional random forest (FRF) was applied as a classification approach and then subsequently to subgroup identification. We input 43 EF variables to the classification step, a supervised random forest procedure in which the features estimated either hyperactive or inattentive ADHD symptoms per model. The FRF then produced proximity matrices and identified optimal subgroups via the infomap algorithm (a type of community detection derived from graph theory). Resting state functional connectivity MRI (rs-fMRI) was used to evaluate the neurobiological validity of the resulting subgroups.
Results
Both hyperactive (Mean absolute error (MAE) = 0.72, Null model MAE = 0.8826, (t(58) = −4.9, p < .001) and inattentive (MAE = 0.7, Null model MAE = 0.85, t(58) = −4.4, p < .001) symptoms were predicted better than chance by the EF features selected. Subgroup identification was robust (Hyperactive: Q = 0.2356, p < .001; Inattentive: Q = 0.2350, p < .001). Two subgroups representing severe and mild symptomology were identified for each symptom domain. Neuroimaging data revealed that the subgroups and TD participants significantly differed within and between multiple functional brain networks, but no consistent “severity” patterns of over or under connectivity were observed between subgroups and TD.
Conclusion
The FRF estimated hyperactive/inattentive symptoms and identified 2 distinct subgroups per model, revealing distinct neurocognitive profiles of Severe and Mild EF performance per model. Differences in functional connectivity between subgroups did not appear to follow a severity pattern based on symptom expression, suggesting a more complex mechanistic interaction that cannot be attributed to symptom presentation alone.
1. Introduction
Although they co-occur sufficiently often to be clustered in the syndrome of Attention-deficit Hyperactivity disorder (ADHD), hyperactivity-impulsivity and inattention-disorganization comprise two partially separable symptom domains with distinct validation in regard to factor structure, clinical correlates, and neurobiology (Willcutt et al., 2012). The clinical significance of these problems in the adolescent period is substantial–they are associated with peer rejection (Nijmeijer et al., 2008), are strong predictors of worse academic outcomes (Breslau et al., 2010; Galéra et al., 2009) and related issues often persist throughout life (Doshi et al., 2012; Matza et al., 2005). However, although they are most pronounced and synchronous in individuals with ADHD (particularly the combined presentation), symptoms of inattention and hyperactivity are not confined to ADHD. Rather, they are an associated comorbid feature of many conditions (just as many conditions overlap with ADHD itself.)
ASD is a second neurodevelopmental population in which symptoms of hyperactivity and inattention are now recognized as a substantial problem in the DSM-5 (American Psychiatric Association, 2013). Best estimates across studies utilizing clinical, in-lab, and national samples report that adolescents with ASD and comorbid ADHD (ASD+ADHD) broadly represent anywhere from ~28 to 50% of all ASD cases (Matson & Burns, 2019). However, the number of adolescents experiencing sub-clinical hyperactive/inattentive symptoms are likely substantially higher (Stevens et al., 2016). Moreover, heritability of ASD and ADHD appears to overlap; for example, parents with ADHD have elevated rates of ASD offspring compared to parents without ADHD (Musser et al., 2014) and siblings cross aggregate within ADHD and ASD (Miller et al., 2019).
1.1. Atypical executive functions might relate to hyperactive and inattentive symptoms
A set of potentially shared mechanisms across ADHD and ASD instantiate atypical executive functioning (EF) (Karalunas et al., 2018). EF represents a collection of functions ranging in complexity, from holding two things in mind at once to complex sequential planning, but comprises abilities supporting self-monitoring and goal-oriented behavior (Welsh & Pennington, 1988). Although the best decomposition of EF into component functions is debated, a theme of unity and diversity recognizes that they have both shared and distinct elements (Friedman & Miyake, 2017). When statistically decomposed in factor analytic studies, common examples of EF include working memory, inhibition, task-control, and cognitive flexibility (Baddeley, 2003; Barkley, 1997; Diamond, 2013; Miyake et al., 2000). EF has been commonly associated with hyperactive and inattentive symptoms (Silverstein et al., 2018; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005) and the severity of EF impairments has also been linked to an increased number of ADHD symptoms for adolescents with either ADHD or ASD (Semrud-Clikeman et al., 2010). Overall, it's clear that several areas of EF functioning are correlated with symptoms of inattention and hyperactivity (Kofler, Rapport, Bolden, Sarver, & Raiker, 2010; Martel, Nigg, & von Eye, 2009; Shiels & Hawk, 2010; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). As well, several components of EF have been linked to hyperactive and/or inattentive symptom domains for those with ASD and/or ADHD (Sinzig et al., 2008).
1.2. The relationship between EF and hyperactive/inattentive symptoms are discrepant
If EF tasks are measuring the same constructs in children with ADHD or ASD, and if EF impairments are tied to hyperactive/inattentive symptoms, it is reasonable to assume that measures of EF should be able to predict the level of hyperactive/inattentive symptom severity among adolescents with ASD+/-ADHD as well as those with ADHD. Some studies have demonstrated that this may theoretically work. For example, ADHD participants showed greater impairment across all EF domains than ASD without comorbid ADHD (ASD-ADHD) participants. This points to the link between both hyperactive/inattentive symptom domains of ADHD and EF deficits. Inattentive symptoms were also significantly associated with EF deficits in metacognition for those with ASD+ADHD indicating that metacognitive EF may be linked to inattention for those with ASD+ADHD (Berenguer et al., 2018). EF tasks can also discriminate those with ADHD (i.e., a defined increase of hyperactive/inattentive symptoms) and typically developing (TD) adolescents (Holmes et al., 2010).
Conversely, some studies have demonstrated that the link between EF and hyperactive/inattentive symptoms is not so clearly defined. A meta-analysis indicated that although EF were associated with ADHD, effect sizes were moderate and findings were not universal per effected individual (Willcutt et al., 2005). This variability in the relationship between EF and ASD has also been demonstrated, but whether this is due to comorbid ASD+ADHD remains unclear (Karalunas et al., 2018). Overall, such discrepancies with regard to EF and inattentive/hyperactive symptoms across ASD and ADHD are largely unknown.
1.3. Some discrepancies in the literature might relate to shared and distinct mechanisms
It is increasingly recognized that EF are not a sole nor sufficient explanation for elevated hyperactive/inattentive symptoms (Nigg, 2006) but that multiple routes likely contribute to the expression of EF and hyperactive/inattentive symptoms across disorders (Chan et al., 2008; Feczko et al., 2019; Molitor et al., 2018; Volkow et al., 2011). For example, those with ASD-ADHD have shown measureable impairments in arousal, processing speed, working memory, and response inhibition when compared to a TD group. The deficits in working memory and processing speed also persisted when compared to an ADHD sample (Karalunas et al., 2018). Thus, an interesting possibility is that some EF are specific to ASD-ADHD but other elements may be more specific to those with elevated levels of hyperactive/inattentive symptoms, i.e. ADHD and ASD+ADHD. This suggests that it may be more useful to clarify the role of EF across ASD and ADHD both transdiagnostically as well as dimensionally across hyperactivity/inattention, rather than confined to the relatively heterogeneous syndrome of ADHD itself.
Another recent study examining the relationship of EF and hyperactivity/inattention for adolescents with ADHD, ASD, and learning deficits found that while all participants had higher scores than a TD population on inattention and hyperactivity, they clustered into unique cognitive profiles (Bathelt et al., 2018). One group exhibited more problems with hyperactivity/inattention and EF across the board, a second showed reduced, but still clinically significant hyperactive/inattentive rates with primary deficits in inattention but not hyperactivity, while a third group showed more problems with EF despite having fewer hyperactive/inattentive symptoms. Importantly, while the first group consisted of mostly participants diagnosed with ADHD, all three groups contained children from each of the three diagnostic categories.
Overall, it is unclear which EF deficits persist among those with ASD and/or ADHD in light of, or in the absence of, hyperactive/inattentive symptoms. These results indicate that while ASD and ADHD share some etiological basis, there may be more than one sole “cause” of any given phenotype (Musser et al., 2014; Reiersen & Todd, 2008) and such causes may also be shared and distinct, both between and within the disorders (Karalunas et al., 2018; Rommelse et al., 2017; Vaidya et al., 2020). We refer to this possible explanation as the heterogeneity problem (Feczko et al., 2019).
1.4. New approaches might help us better handle the “heterogeneity problem”
The fact that distinct mechanisms for ASD and ADHD for the same inattentive/hyperactive symptoms might be unique or whether these symptoms can be tied to EF transdiagnostically is an important heterogeneity problem for the field (Lombardo et al., 2019). Different mechanisms may contribute to the same symptom degree/severity, necessitating modeling approaches suited to address it. Newer machine learning techniques may offer an assistance by parsing out non-linear patterns in data that are missed with general linear model-based statistical tests and predictive modeling (Duda et al., 2016; Uluyagmur-Ozturk et al., 2017; Vaidya et al., 2020). One group approached this by using a community detection approach (Fair et al., 2012) in combination with support vector machines to identify subtypes of EF across ASD, ADHD, and TD participants (Vaidya et al., 2020). In doing so, they found three transdiagnostic subtypes with specific relevance to the engagement of different brain regions involved in EF. Here, we adopted a recently developed hybrid approach called the functional random forest (FRF) for tackling these issues. This allows us to combine supervised (machine learning) with unsupervised (graph theory) approaches in a new way that may better address the heterogeneity problem. A strength of the FRF is that it identifies profiles based on features optimized for a relevant outcome a priori, rather than post hoc. It does so by using a series of independent classifiers in a decision-tree approach to make predictions about an outcome (e.g., inattention) using input data (measures of EF) (Feczko et al., 2018). After making these predictions, it then employs a community detection approach (Rosvall & Bergstrom, 2008) to identify subgroups based on shared and unique features as tied to the outcome of interest.
In previous work, our group employed this FRF approach to distinguish ASD and TD participants with 72.7% accuracy across groups using multiple measures of affect processing, as well as identify subgroups (Feczko et al., 2018). However, the studies intentions were not to explore or define subgroups transdiagnostically. Therefore, we applied the FRF as a transdiagnostic approach to identify potential subgroups while tying EF to clinical phenotypes across disorders.
Once subgroups are identified, evaluating the clinical relevance of such subgroups necessitates additional validation metrics to determine if there are indeed measureable differences among related data Utilizing an independent dataset and testing the model is one common method for validating machine learning. Aside from testing generalizability or replication in an independent data set, within-sample cross-validation can be used and in some cases combined with secondary validation tests. Here, we conducted internal cross-validation and also looked to neurobiological correlates using brain imaging as an index of validity as suggested in the literature (Filiou & Turck, 2011). In line with behavior-based EF studies, our group has advocated a data driven approach to better account for the heterogeneity of symptom expression as tied to brain imaging (Dias et al., 2015; Karalunas et al., 2018; Ray et al., 2014). Therefore, utilizing the within and between brain-network connectivity as derived from resting state functional magnetic resonance imaging (rs-fMRI) data serves to support the existence of subgroups discovered by the FRF by informing the unique mechanistic underpinnings.
In the current report, we use measures of EF in two separate FRF models to predict either (1) hyperactive or (2) inattentive symptom domains among adolescents with ASD and/or ADHD transdiagnostically. We first identify the presence of potential sub-populations across diagnoses that do not split by DSM categories for hyperactive or inattentive symptoms. We then further validate the identified subgroups using rs-fMRI to examine group differences among the sub-populations within and between several brain networks.
2. Methods and materials
2.1. Participant demographics
All procedures were approved by the Oregon Health & Science University (OHSU) Institutional Review Board (IRB#5239 and #4817) and informed consent and assent were obtained from adolescents and parents according to protocol. One hundred and thirty participants between the ages of 7–16 with a primary ASD diagnosis (N = 64, female = 13) or ADHD diagnosis (N = 66, female = 18) were included in the analysis (See Table 1 for demographic comparisons.) Of the ASD participants, 36 met DSM criteria for ASD+ADHD, 19 exhibited subthreshold levels of ASD+ADHD (ASD+subADHD), and 9 had ASD alone (ASD–ADHD). A TD group (N = 28) was also included for descriptive purposes. These participants were demographically matched to ASD and ADHD participants with useable scan data (see: 2.4 fMRI data, below, and Table 2 for demographic comparisons. See Supplemental Materials 1.1 for additional information on Participant recruitment and diagnostics.)
Table 1.
Demographics for ASD and ADHD with significance tests.
| Participants (N = 130) | ASD (n = 64) | ADHD (n = 66) | Significance testa |
|---|---|---|---|
| Female gender, n = 31 (23.8 %) | 13 (20.3) | 18 (27.3) | p = .413 |
| Age in years (7–16y), m = 11.5 (2.2) | 12.2 (2.2) | 10.9 (1.9) | t(128) = 3.67, p < 001 |
| Est. IQ (Block design), m = 11 (3.3) | 10.6 (3.2) | 11.3 (3.3) | t(128) = −1.22, p = .225 |
Table 2.
Demographics for ASD and ADHD participants with useable scan data.
| Useable scan data for ASD and ADHD participants (n = 67) | (ASD = 26) | ADHD (n = 41) | Significance testa | TD (N = 28) | Significance testb |
|---|---|---|---|---|---|
| Female gender, n = 15 (22.4%) | 4 (15.4) | 11 (26.8) | p = .37 | 14 (39.3) | p = .13 |
| Age (7–16y), m = 12.2 (2.3) | 13.6 (1.9) | 11.2 (2.0) | t(65) = 4.9, p < .001 | 11.4 (1.7) | t(68.3) = 1.6, p = .074 |
| Est. IQ (Block design) m = 10.8 (3.2) | 10.5 (3.0) | 11 (3.2) | t(65) = −.68, p = .502 | 12.4 (2.8) | t(93) = 4.9, p = .074 |
: Significance tests comparing ASD and ADHD;
Significance tests comparing TD and all ASD and/or ADHD participants.
Although we are examining continuous measures across diagnostic categories rather than explicitly comparing groups, there is always a possibility of a batch effect due to participant age differences. Because of this, we further examined the age difference and potential relationship to the EF variables (see: Supplemental Materials, 2. Supplemental Analyses).
2.2. Neuropsychological tasks and other EF data
Because impairments may vary across all of the EF domains, including tasks that cover a variety of potential impairments may provide a more comprehensive understanding of subgroup neurocognitive profiles. Therefore we used multiple methods to assess EF including standardized cognitive batteries and a parent answered questionnaire. These measures included NEPSY Verbal Fluency (Korkman et al., 2007), the Behavior Rating Inventory of Executive Function questionnaire (Gioia et al., 2001), D-KEFS Color-Word Interference, Trails, and Tower Test (Delis et al., 2001), WISC-IV Digit Span (Wechsler, 2003), a Go/No-Go Stop Task (Logan, 1994; Nigg, 1999), and Spatial Span (Robbins et al., 1994). The battery was designed to comprehensively assess the aforementioned different domains of EF including response inhibition, working memory, task control, and cognitive flexibility (Friedman & Miyake, 2017; Nigg, 2005; Pennington, 1997; Pennington & Ozonoff, 1996). Detailed information about the task battery and variable selection, as well as the domains they cover, is included in the Supplemental Material under 1.4 Individual task descriptions and review.
Research reliable Kiddie-Schedule of Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al., 1997) interviews were completed with the parents by trained clinicians. Either total hyperactive or inattentive symptom scores were included in our models as the outcome variable, described in further detail under 2.5 Analysis Overview. Table 3 shows the distribution of total symptom scores by comorbid diagnostic category.
Table 3.
Total hyperactive/inattentive symptoms per comorbid diagnosis.
| Diagnosis | Total hyperactive symptoms | Total inattentive symptoms |
|---|---|---|
| ASD-ADHD (n = 9) | m = 1.50 (1.60) | m = 1.33 (.90) |
| ASD+subADHD (n = 19) | m = 2.89 (1.40) | m = 3.32 (1.80) |
| ASD+ADHD (n = 36) | m = 3.63 (2.74) | m = 6.19 (2.60) |
| ADHD (n = 66) | m = 4.02 (2.70) | m = 6.40 (2.36) |
2.3. Missing data
Very few data points were missing in our dataset. Of the 43 input variables used in the model for all participants, only .9% of the total data was missing. The participants averaged .7% missing data each, with the maximum amount of missing data for a single participant at 14% and only 10% of participants with any missing data at all. (See 2.5.1 Functional Random Forest and Subgroup Detection under Methods for how missing data were handled.)
2.4. fMRI data
Participants were scanned at OHSU's Advanced Imaging Research Center (AIRC) on a 3.0 T Siemens Tim Trio Magnetom scanner using a 12 channel head coil, and completed one T1 weighted structural image as well as 3 5-minute resting state scans (see Supplement Section 1.5.1 for detailed information on fMRI data acquisition). All of the data were processed using a modified version of the Human Connectome Project (HCP) image processing pipeline (Glasser et al., 2013; Mills et al., 2018). (See Supplement for more information on HCP pipeline steps and additional processing under 1.5.2 fMRI data processing.) After processing, we used a manual curation process to further assess the data quality. (See Supplemental Material, 1.5.3 Quality control.)
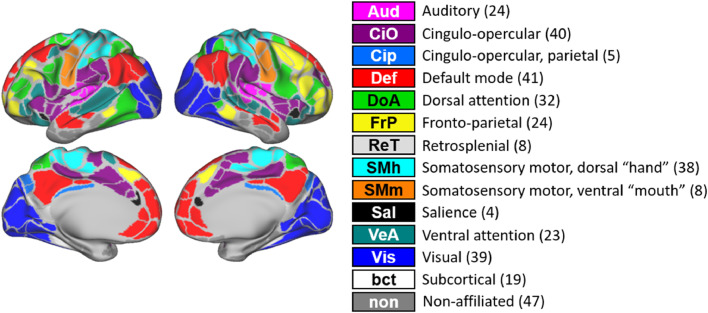
To analyze the imaging data, 352 regions of interest (ROI's), including 19 subcortical, were generated based on previously informed parcellation schemes, as seen in Fig. 1 (Gordon et al., 2014).
Fig. 1.
352 regions of interest (ROIs), including 19 subcortical, were generated based on previously informed parcellation schemes (Gordon Parcellation (Gordon et. al., 2014). Here, the identified networks are shown with the number of assigned ROIs and color coded by network.
2.5. Analysis overview
2.5.1. Functional random forest and subgroup detection
An FRF algorithm (Feczko et al., 2018) is used in two separate models, with each model containing all 130 subjects, to estimate either (1) participant's total hyperactivity or (2) total inattentive ADHD symptom scores from the K-SADS ADHD module. Input (predictive) measures include the 43 variables from the EF tasks and the EF questionnaire. (Detailed descriptions in the Supplemental Material 1.4 Individual task descriptions and review.)
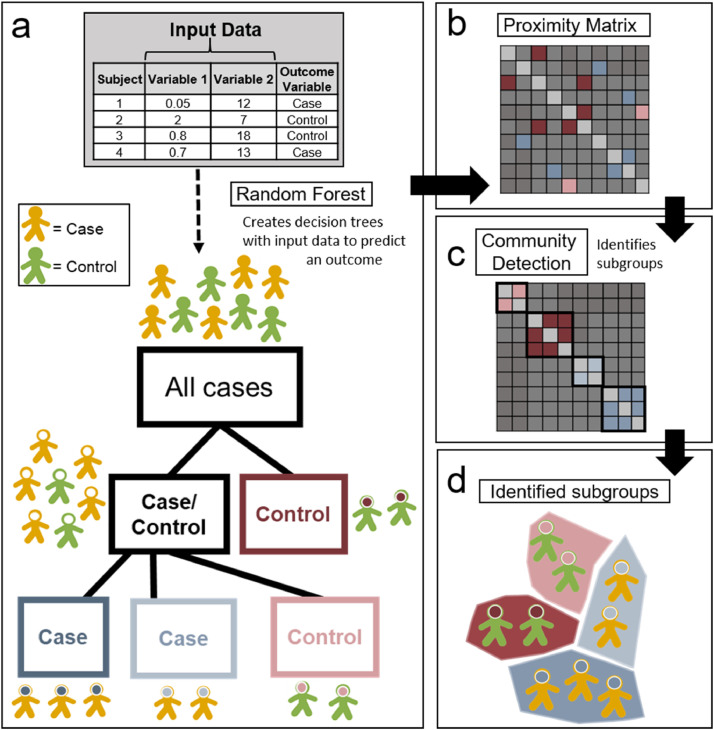
Fig. 2 provides a schematic image for the FRF model. For simplicity, the schema uses a binary outcome; case or control. In our study, we applied the FRF to continuous outcomes (hyperactivity or inattention.) The FRF algorithm constructs a series of 1000 decision trees using the 43 input EF measures to predict an outcome (hyperactivity or inattention) (2a.) Each tree is then given a bootstrapped subset of randomly selected training data to optimize performance. From there, a random selection of participants and variables excluded from the training data are used to evaluate the overall accuracy of the model using 10-fold, 3 repetition, cross-validation by comparing the accuracy distributions from 30 permutation tests against 30 null models with a t-test (Kohavi, 1995). Twenty trees were used to determine surrogate splits for each fold (Breiman, 2001).
Fig. 2.
a: The FRF uses a series of input data to predict an outcome variable with a series of decision trees. 2b: The number of times any 2 participants end up in the same terminal branch is mapped in proximity matrix. 2c: The infomap algorithm (Rosvall & Bergstrom, 2008) is then used to determine subgroup assignments. 2d: Identified subgroups are shown.
A proximity matrix is then generated from all of the decision trees (2b), wherein each cell indicates the number of times across all trees and forests that any given two participants end up in the same terminal branch. Community detection via the infomap algorithm (2c) (Rosvall & Bergstrom, 2008) is then used to identify subgroups from this proximity matrix, with nodes and edges determined in steps of a .05 threshold from .2 to 1. To determine the optimal groupings, an iterative procedure using matrix thresholds from .2 to 1 in steps of .05 was used to identify a consensus of subgroup assignments (2d) from all generated thresholds.
2.5.2. Imaging data and chi-squared test
To compare imaging data from identified subgroups as a validation metric, individual parcellation matrices (see 2.4 fMRI data) were generated per subject, then subgroup matrices were created by averaging each individual matrix across subgroups. From there, the parcellated matrices for each subgroup, two mass univariate analysis of variance (ANOVA) tests, and a novel chi-squared analysis (Eggebrecht et al., 2017) are used to identify significant differences in functional connectivity between all the subgroups ascertained from each model.
For every ROI to ROI pair, as represented in the parcellated matrices, the ANOVA tests are used to measure significant differences in correlations between (1) the identified Hyperactive Subgroups (HSG) and the TD group and (2) the identified Inattentive Subgroups (ISG) and TD group. For the chi-squared analyses, the results of the ANOVAs are then binarized at p < .05 significance. An expectancy ratio is subsequently calculated by comparing the number of expected significant and non-significant functional connections to the observed number. A chi-squared test statistic is then calculated from the observed and the expected ratio of significant connections. Permutation tests are used to construct an empirical distribution of null chi-squared tests to determine the statistical significance of the observed chi-squared test statistics. FDR correction is used to control for multiple comparisons.
3. Results
3.1. Hyperactive model EF prediction and subgroups across ASD and ADHD
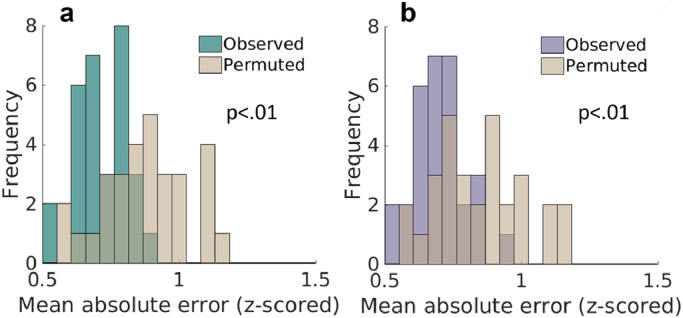
The FRF predicted the hyperactivity scores among ASD and/or ADHD participants better than the null model (Mean absolute error (MAE) = 0.72, Null model MAE = 0.8826, (t(58) = −4.9, p < .001) (Fig. 3a.) There was no significant difference observed in the model performance between subgroups (Hyperactive error: t(128) = −1.107, p = .271.) .
Fig. 3.
a: The mean absolute error (MAE) over all permutations of the Hyperactive model (teal) plotted with the MAE of the null model permutations (tan.) 3b: MAE over all permutations of the Inattentive model (purple) plotted with the MAE of the null model (tan.)
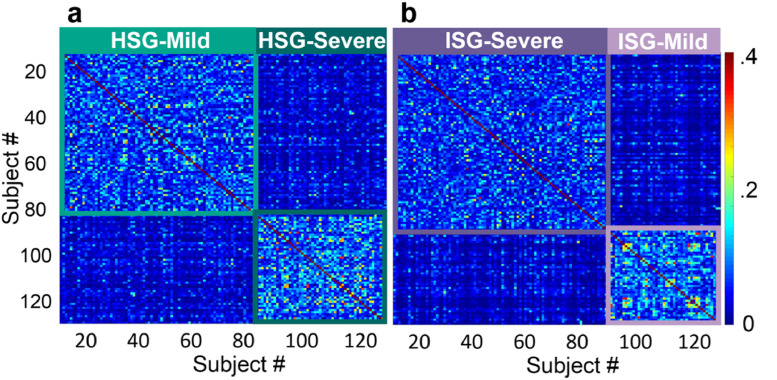
Community detection identified two unique subgroups in the Hyperactive model (Fig. 4a.) The subgroups were split with 79 participants in HSG-1 (HSG-Mild; ASD = 40, ADHD = 39) and 51 in HSG-2 (HSG-Severe; ASD = 24, ADHD = 27). There were no significant differences between the subgroups on diagnostic composition of ASD and ADHD participants per subgroup, gender, age, or estimated IQ (see Table 4.) There were also no significant differences between subgroups on comorbid diagnostic composition (ASD–ADHD, ASD+subADHD, ASD+ADHD, or ADHD) (χ2 (3, n = 130, p = .213) (Table 5.)
Fig. 4.
a: A proximity matrix produced by the Hyperactive FRF model. The participants are reorganized into subgroups (HSG = Hyperactive model subgroup) identified via Infomap and are captured in teal squares to show the subgroup boundaries. Colorbar on the far right indicates the proportion of times each participant ended up in the same terminal branch as another participant on the alternate axis over all FRF permutations. 4b: A proximity matrix for the Inattentive FRF model, reorganized into identified subgroups (ISG = Inattentive model subgroup) and captures in purple squares to show the boundaries of each subgroup.
Table 4.
Demographics for identified Hyperactive subgroups and significance tests comparing HSG-Mild and HSG-Severe.
| Demographics | HSG-Mild (n = 79) | HSG-Severe (n = 51) | Significance testa |
|---|---|---|---|
| ASD, n (%) | 40 (50.6) | 24 (47.1) | p = .72 |
| Female gender, n (%) | 15 (19) | 16 (31.4) | p = .14 |
| Age in years (7-16y) | 11.6 (2.1) | 11.4 (2.3) | t(128) = 0.663, p = .508 |
| Est. IQ (Block design) | 11.1 (3.4) | 11.3 (3.3) | t(128) = 0.553, p = .581 |
Table 5.
Comorbidities per Hyperactive subgroup.
| Diagnosis | HSG-Mild | HSG-Severe |
|---|---|---|
| ASD-ADHD | n = 8 | n = 1 |
| ASD+subADHD | n = 13 | n = 6 |
| ASD+ADHD | n = 19 | n = 17 |
| ADHD | n = 39 | n = 27 |
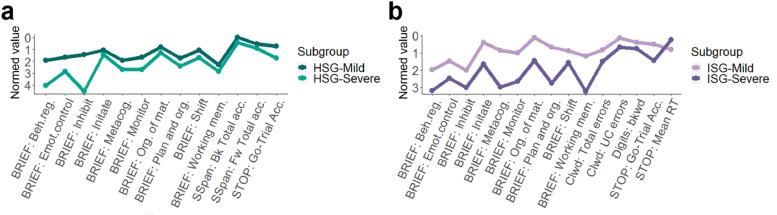
HSG-Mild had significantly better EF performance and ratings than HSG-Severe across all of the cognitive domains on several of the tasks (Fig. 5a, Table 6). (Note that the plots are scaled as z-scores relative to the TD population such that 0 = TD mean and all points below 0 are weaker performance and all scores above 0 are better.)
Fig. 5.
a: Behavioral measures used in the FRF Hyperactive model that significantly differed between identified subgroups. Normed means (y axis) from Table 6 are plotted per measure on the x axis. To better represent the true differences when compared to a normative sample, all measures are normed to the TD group. 5b: Behavioral measures used in the FRF Inattentive model that significantly differed between identified subgroups. Normed means (y axis) from Table 7 are plotted per measure on the x axis (normed to the TD group.)
Table 6.
Variables included in the Hyperactive model that significantly differed between subgroups. The table is organized by cognitive domains on the left. T-tests comparing subgroups (HSG-Severe, HSG-Mild) are shown for each variable. Columns for HSG-Severe and HSG-Mild show the subgroups means, normed to the TD sample.
| Response Inhibition | t-test | HSG-Severe | HSG-Mild |
|---|---|---|---|
| BRIEF: Inhibit | t(127.19) = 15.28, p < .001 | −4.55 | −1.43 |
| BRIEF: Emotional control | t(128) = −3.99, p < .001 | −2.84 | −1.65 |
| BRIEF: Monitor | t(127) = −4.20, p < .001 | −2.69 | −1.66 |
| Stop task: Accuracy on Go-trials | t(127) = 3.23, p = .002 | −1.72 | −0.71 |
| Cognitive Flexibility | |||
| BRIEF: Shift | t(128) = −2.96, p = .004 | −1.71 | −1.06 |
| BRIEF: Behavior regulation index | t(128) = −8.08, p < .001 | −4.07 | −1.92 |
| BRIEF: Metacognition | t(128) = −3.49, p < .001 | −2.71 | −1.93 |
| Working Memory | |||
| BRIEF: Working memory | t(128) = −2.24, p = .027 | −2.85 | −2.29 |
| Spatial span, forward: Total Accuracy | t(124) = 2.04, p = .044 | −0.92 | −0.52 |
| Spatial span, backward: Total accuracy | t(125) = 2.25, p = .026 | −0.43 | 0.03 |
| Task Control | |||
| BRIEF: Initiate | t(128) = −2.39, p = .018 | −1.42 | −1.06 |
| BRIEF: Plan and organize | t(128) = −2.88, p = .005 | −2.42 | −1.74 |
| BRIEF: Organization of materials | t(128) = −2.77, p = .006 | −1.27 | −0.79 |
Thus the graph (Fig. 5a) highlights lower scores on all measures for both subgroups. HSG-Mild had better ratings than HSG-Severe on multiple BRIEF modules involved in response inhibition (inhibit, emotional control, monitor), cognitive flexibility (shift, behavioral regulation, metacognition), working memory (working memory), and task control (initiate, plan and organize, organization of materials). HSG-Mild also showed better working memory (spatial span; backward and forward total accuracy.)
3.2. Inattentive model EF prediction and subgroups across ASD and ADHD
The FRF also predicted the inattentive scores for our participants with greater accuracy than the null model (MAE = 0.7, Null model MAE = 0.85, t(58) = −4.4, p < .001.) (Fig. 3b.) There were no significant differences observed in model performance between subgroups (Inattentive error: t(128) = −0.494, p = .622).
Community detection identified two distinct subgroups for the inattentive model (Fig. 4b). The subgroups were split with 84 participants in ISG-1 (ISG-Severe; ASD = 38, ADHD = 46) and 46 in ISG-2 (ISG-Mild; ASD = 26, ADHD = 20). There were no significant differences between the subgroups on diagnostic composition of ASD and ADHD participants per subgroup, gender, age, or estimated IQ (see Table 7.) There were however, significant differences between subgroups on comorbid diagnostic composition (χ2 (3, n = 130, p < .01), with ISG-Severe containing more subjects with ADHD and ASD+ADHD than ISG-Mild. See Table 8 for the diagnostic numbers per subgroup.
Table 7.
Demographics for identified Inattentive subgroups and significance tests comparing ISG-Mild and ISG-Severe.
| Demographics | ISG-Mild (n = 46) | ISG-Severe (n = 84) | Significance testa |
|---|---|---|---|
| ASD, n (%) | 26 (56.5) | 38 (45.2) | p = .272 |
| Female gender, n (%) | 8 (17.4) | 23 (27.4) | p = .282 |
| Age in years (7–16y) | 11.6 (2.1) | 11.5 (2.2) | t(128) = 0.189, p = .85 |
| Est. IQ (Block design) | 11.6 (3.2) | 10.6 (3.2) | t(128) = 1.58, p = .117 |
Table 8.
Comorbidities per Inattentive subgroup.
| Diagnosis | ISG-Mild | ISG-Severe |
|---|---|---|
| ASD-ADHD | n = 6 | n = 3 |
| ASD+subADHD | n = 14 | n = 5 |
| ASD+ADHD | n = 6 | n = 30 |
| ADHD | n = 20 | n = 46 |
ISG-Mild had significantly better performance and ratings than ISG-Severe across all cognitive domains on several tasks (Fig. 5b and Table 9).
Table 9.
Variables included in the Inattentive model that significantly differed between identified subgroups are organized by cognitive domains on the left. T-tests comparing identified subgroups (ISG-Severe, ISG-Mild), with degrees of freedom in parentheses, are shown for each variable. Columns for ISG-Severe and ISG-Mild show their groups means, normed to the TD sample.
| Response Inhibition | t-test | ISG-Severe | ISG-Mild |
|---|---|---|---|
| Colorword: Total errors | t(91.10) = −2.02, p = .046 | −1.50 | −0.82 |
| Colorword: Uncorrected errors | t(108.70) = −2.24, p = .02 | −0.67 | −0.13 |
| BRIEF: Inhibit | t(128) = −2.83, p = .005 | −3.00 | −2.01 |
| BRIEF: Emotional control | t(128) = −3.17, p = .002 | −2.47 | −1.48 |
| BRIEF: Monitor | t(74.76) = −6.74, p < .001 | −2.64 | −1.01 |
| Stop task: Mean reaction time | t(71.67) = 2.63, p = .01 | −0.23 | −0.80 |
| Stop task: Accuracy on Go trials | t(123) = −3.35, p = .01 | −1.45 | −0.49 |
| Cognitive Flexibility | |||
| BRIEF: Shift | t(128) = −3.10, p = .002 | −1.56 | −0.87 |
| BRIEF: Behavior regulation index | t(128) = −3.84, p < .001 | −3.19 | 1.98 |
| BRIEF: Metacognition | t(128) = −14.41, p < .001 | −2.99 | −0.86 |
| Working Memory | |||
| BRIEF: Working memory | t(128) = −11.18, p < .001 | −3.24 | −1.18 |
| Digit span: Backward | t(128) = −2.18, p = .031 | −0.76 | −0.39 |
| Task Control | |||
| BRIEF: Initiate | t(128) = −10.84, p < .001 | −1.64 | −0.40 |
| BRIEF: Plan and organize | t(128) = −12.15, p < .001 | −2.74 | −0.67 |
| BRIEF: Organization of materials | t(128) = −9.70, p < .001 | −1.45 | −0.11 |
ISG-Mild was rated better on multiple BRIEF modules involved in response inhibition (inhibit, emotional control, monitor), cognitive flexibility (shift, behavior regulation, metacognition), working memory (working memory), and task control (initiate, plan and organize, organization of materials). ISG-Mild also demonstrated better performance on tasks involved in response inhibition (stop task accuracy) and working memory (digit span backwards). Interestingly, ISG-Mild had a slower reaction time than ISG-Severe on the stop task which may reflect a speed-accuracy tradeoff (Heitz, 2014; Mulder et al., 2010), for ISG-Mild also showed better accuracy than ISG-Severe within the same task.
3.3. Participants landed in different severity subgroups depending on the model
To ensure that the two models, hyperactive or inattentive, were revealing distinctive subgroups depending on the outcome of interest, we examined the subgroup assignments across the two models. Overall, 45.4% of the participants (n = 59) swapped their subgroup assignment (Mild vs. Severe) depending on the model. Of the comorbidities, 33 with ADHD (50%), 3 with ASD-ADHD (33%), 5 with ASD+subADHD (26%), and 17 ASD+ADHD (50%) moved from a Mild to Severe (or vice versa) subgroup across the models.
3.4. Brain connectivity differences between subgroups
In the Hyperactive model, 38 participants from HSG-Mild (ASD = 16, ADHD = 22) and 29 from HSG-Severe (ASD = 10, ADHD = 19) met the requirements for analyzable imaging data (see 2.4 fMRI data and Supplemental Materials 1.5.2 fMRI data processing and 1.5.3 Quality control) and were subsequently included in the analysis along with the matched TD group (n = 28). Additional analyses examining subgroup differences in the demographic and behavioral data only for participants with usable scan data can be seen in the Supplemental Materials.
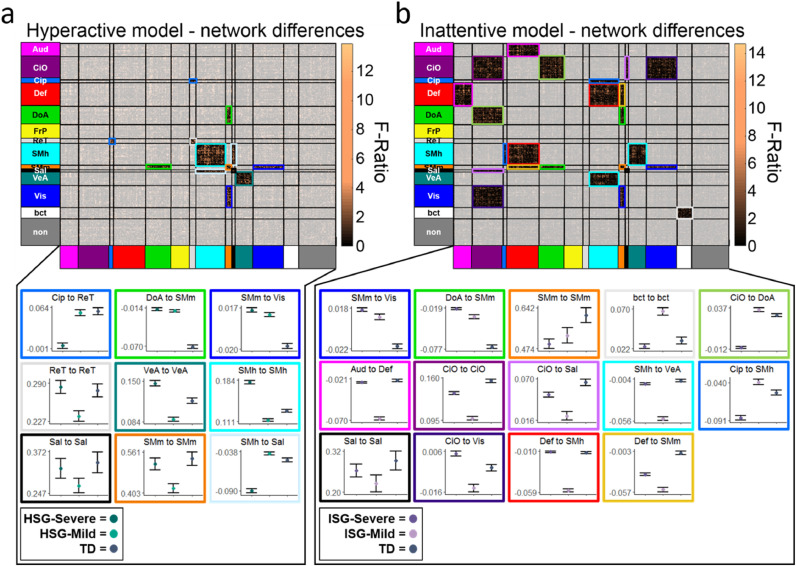
Using the ANOVA and chi-squared test, several network connections were significantly different between the three groups (Fig. 6a.) Connectivity differences did not appear to follow severity patterns based on hyperactive symptoms and EF impairment. For example, although the TD group showed significantly greater connectivity between the cingulo-opercular parietal (CiP) and retrosplenial (ReT) (Figs. 1, 5a) networks as compared to HSG-Mild, and HSG-Mild was significantly greater than HSG-Severe, nearly all other network connections showed no distinguishable trends - with the TD, Mild, and Severe groups swapping directionality depending on the between or within network connection.
Fig. 6.
a: Network connectivity that significantly differed between HSG-Severe, HSG-Mild, and TD groups are shown in the matrix representing the Gordon-parcellation. Significant regions are highlighted with colored squares, which are plotted below the matrix. Significantly different marginal means and error bars for (from left to right) HSG-Severe, HSG-Mild, and TD groups, within and between networks are shown with colors corresponding to the boxes in the matrix above. 6b: Network connectivity that significantly differed between ISG-Severe, ISG-Mild, and TD groups are shown in the matrix representing the Gordon-parcellation. Significant regions are highlighted with colored squares, which are plotted below the matrix. Significantly different marginal means and error bars (from left to right) ISG-Severe, ISG-Mild, and TD groups, within and between networks are shown with colors corresponding to the boxes in the matrix above.
In the Inattentive model, 47 participants from ISG-Severe (ASD = 17, ADHD = 30) and 20 from ISG-Mild (ASD = 9, ADHD = 11) were determined to have enough good imaging data and were analyzed along with the comparison TD group. Using the combined ANOVA and chi-squared test, many network connections were significantly different between the three groups. (Fig. 6b). A similar “non-pattern” emerged across networks, with the first three network connections on 6b showing severity trends, and the remaining 11 connections having no distinguishable patterns as related to inattentive symptoms and EF impairment.
4. Discussion
4.1. Results summary
Using EF variables in the FRF models, we predicted either hyperactive or inattentive symptom severity for participants with ASD and/or ADHD better than random chance, identified two distinct subgroups per model, and validated the subgroups via rs-fMRI
We linked behavioral variables from the EF tasks and rating scale directly to clinical hyperactive/inattentive outcomes for ASD and ADHD participants using the FRF approach. EF measures predicted hyperactive and inattentive symptom counts better than random chance for both of the models. We were also able to show that task and ratings measures both contributed, something many prior reports did not do. Because task and ratings measures that purport to measure executive functions typically do not correlate very well, it is unclear if they measure the same construct. Thus, it is reassuring that here that they both contributed to the prediction models.
We also identified subgroups and further validated them by confirming neurocognitive phenotypes. All 43 EF variables helped identify the unique profiles among participants, with different combinations informing subgroups for each model. We identified two subgroups per hyperactive/inattentive model using community detection. The identified subgroups differed significantly on measures that are purportedly related to multiple EF processes.
These results support the growing supposition in the field that there may be multiple mechanistic subgroups across diagnostic categories in these neurodevelopmental disorders (Sonuga-Barke et al., 2010). In other words, several different underlying causes may also lead to similar phenotypes that inform subgroups. In our study the subgroups did not split by primary diagnosis, indicating that features of EF may be shared transdiagnostically.
To determine if subgroups had real, measurable differences in their biological underpinnings, we further validated them via neuroimaging and compared them to a TD population. Some of the imaging results appeared to follow a severity trend (i.e. ISG-Severe showing decreased connectivity between identified regions compared to ISG-Mild, which is decreased compared to TD.) However, the entirety of the results do not follow a discernable trend. For example, we might typically expect to see high performing groups showing “greater” connectivity between related functional networks (e.g. attentional and default) as compared to a lower performing group. It appears that the subgroups cannot simply be measured on a continuum of functional-connectivity and rather, may have unique underpinnings.
4.2.1. Measures of EF estimate hyperactive and inattentive symptoms in adolescents with ASD and/or ADHD
Multiple theories have been proposed regarding the relationship between EF and hyperactive/inattentive symptoms (Castellanos et al., 2006; Corbett et al., 2009; Martel et al., 2007; Sonuga-Barke et al., 2005; Thorell, 2007). Our results support growing evidence that while EF are involved in perceived hyperactive/inattentive symptoms across ASD and ADHD, they are not the sole cause. This was evidenced by the contribution of the BRIEF to model performance (see Supplemental Material), showing that it may be measuring something slightly different than the other EF tasks which were also included in the models.
Importantly, until recently many of the prior studies have not been conducted transdiagnostically (Geurts et al., 2004; Joshi et al., 2017). Indeed the current study builds on a handful of recent studies that have employed transdiagnostic approaches (Dajani et al., 2019; Karalunas et al., 2018; Lecei et al., 2019; Vaidya et al., 2020). For example, our subtypes compliment the profiles found in a recent study (Vaidya et al., 2020); they found EF and symptom subtypes whereas we found EF subtypes tied to hyperactive/inattentive symptoms. Such studies suggest that impairments across ASD and ADHD are not entirely attributable to comorbid diagnoses (Karalunas et al., 2018; Vaidya et al., 2020). While not informing ‘causality,’ our results compliment these findings, demonstrating that EF may be tied to hyperactive/inattentive symptoms for those with ADHD and/or ASD.
4.2.2. Subgroups were identified based on unique combinations of EF features for both hyperactive and inattentive models
A growing body of evidence suggests multiple pathways lead to shared symptoms among a given disorder (Castellanos et al., 2006; Feczko et al., 2019). Yet challenges arise in understanding the association between domains (e.g. EF) and outcome (e.g. hyperactive/inattentive symptoms), while accounting for multiple pathways and nested subgroups. Knowing these challenges, the FRF was used in prior work with a different clinical question in mind. In that study the model identified 3 ASD and 4 TD subgroups (Feczko et al., 2018) based on measures of EF and facial/vocal affect recognition and processing. As such, the outcome of interest is a critical component in guiding the research question itself.
By including both ADHD and ASD participants in a transdiagnostic study, we can also characterize whether hyperactive or inattentive symptoms are related to the same underlying mechanisms across disorders. If the identified subgroups split by primary diagnostic category, it would indicate that the mechanisms leading to observed ADHD symptoms are potentially distinct. Inversely, if the subgroups share participants across ASD and ADHD, it would indicate that the mechanisms are likely to be shared. With EF features creating “pathways” to the hyperactive/inattentive outcome of interest, ASD and ADHD participants end up in the same or different subgroups.
4.2.3. We employ the FRF to tie EF to clinical outcomes and identify neurocognitive phenotypes
Thus, we applied the FRF using EF to examine their relationship to hyperactive/ inattentive symptoms across ASD and ADHD, and to determine whether more than one ‘pathway’ exists for these outcomes.
In doing so, we found the existence of sub-groups that differed on severity of multiple EF features, representing putatively mild and severe subgroups per model. The hyperactive subgroups differed significantly on measures related to multiple cognitive domains– with HSG-Mild showing improved performance across multiple measures of EF as compared to HSG-Severe. HSG-Mild also showed fewer total hyperactivity symptoms than HSG-Severe.
The inattentive subgroups showed significant differences on multiple measures of EF, with ISG-Mild out-performing/showing better ratings than ISG-Severe across tasks. Most notable was ISG-Mild's slower reaction time on the stop task as compared to ISG-Severe. This may reflect a speed-accuracy tradeoff (Heitz, 2014; Mulder et al., 2010) in which ISG-Mild is compromising speed in order to improve performance accuracy on the task. This tradeoff may also be reflected in their superior stop task accuracy score compared to ISG-Severe. As several studies have confirmed the variability of performance of ADHD participants on inhibitory tasks (Huang-Pollock et al., 2012; Karalunas et al., 2012; Mulder et al., 2010), our results may further validate the necessity for subgroup splitting.
When examined on a comorbidity level (ASD-ADHD, ASD+subADHD, ASD+ADHD, or ADHD) we found that the hyperactive subgroups did not split by comorbidity. Interestingly, the inattentive subgroups did show significant differences on comorbid diagnostic composition, with a greater number of ASD+ADHD and ADHD participants in ISG-Severe. This may indicate that mechanisms underlying hyperactive symptoms are more nested within typical development than inattention across the disorders.
Importantly, we also found that the subgroups in both analyses were not defined by primary diagnostic category (ASD or ADHD) (Tables 4 and 7). This supports the transdiagnostic approach and is consistent with previous work indicating that the underlying mechanisms leading to observed symptoms are likely shared across ASD and ADHD, even though there may be more than one (Chan et al., 2008; Feczko et al., 2019; Leitner, 2014; Molitor et al., 2018). The findings here are not that dissimilar with other modeling approaches such as latent class analysis that often derive low and high performing groups ( Karalunas et al., 2014, Katuwal et al., 2016). The difference between the FRF approach and other methods is that the FRF may discover any number of groups or none at all, as demonstrated in our prior work (Feczko et al., 2018). In addition, the discovered groups may vary depending on outcome of interest. In other words the low and high performing groups, identified in the current work, are tied to the relevant symptom dimensions, and other outcomes (e.g, prognosis, response to therapy, etc), may reveal distinct grouping characteristics.
4.2.4. The mild or severe subgroups were comprised of different participants per model and did not simply duplicate participant composition
Another important observation in the data presented here is that when we compare HSG-Severe to ISG-Severe and HSG-Mild to ISG-Mild, we find that the two Severe and two Mild subgroups were comprised of different combinations of participants: although all 130 participants were included in each model, the subject composition of HSG-Severe did not simply duplicate the subject composition of ISG-Severe. These findings highlight that the identified subgroups as tied to hyperactive or inattentive symptoms are different depending on the outcome of interest. This result is consistent with previous literature suggesting there may be different “drivers” for these two symptom dimensions (Kofler et al., 2010; Martel et al., 2009; Shiels & Hawk, 2010).
4.2.5. Subgroups showed functional connectivity differences among their brain imaging data
It's important for any machine learning model, in particular those using cross-validation, to further validate identified subgroups (Feczko et al., 2019). Ideally, this validation can be accomplished using independent participants in a unique sample. In the absence of such a cohort, independent data within the same participants can be used. Because multiple brain regions have been implicated in the various EF processes (Alexander & Nitz, 2015; Braga & Buckner, 2017; Braunlich et al., 2015; Corbetta & Shulman, 2002; Dosenbach et al., 2007; Dosenbach et al., 2006; Fox et al., 2005; Leech et al., 2011; Lin et al., 2015; Power et al., 2011; Seeley et al., 2007; Vann et al., 2009; Zhang et al., 2017), we used neuroimaging data as derived from rs-fMRI to further validate the findings.
4.2.6. The subgroups showed differences within and between many functional networks implicated in EF
We found that identified subgroups and the TD group significantly differed from one another both within and between functional networks related to EF. In the hyperactive model, notable differences were found between the HSG-Mild, HSG-Severe, and TD group within and between brain regions associated with attention (salience (Sal), DoA) (Corbetta & Shulman, 2002; Power et al., 2011; Seeley et al., 2007), response inhibition (ventral attention (VeA)) (Zhang et al., 2017) and motor activity (somatosensory motor, ventral “mouth” (SMm, somatosensory motor, dorsal “hand” (SMh)) (Fig. 6a) (Power et al., 2011). These findings highlight the potential implications for the SMm+SMh, attentional, and response inhibition networks and their relationship to EF domains such as behavioral regulation and initiation, which were shown to be different between groups (Table 6, Fig. 5).
For the inattentive model, the ISG-Mild, ISG-Severe, and TD groups showed significant differences within and between networks associated with task-positive (cingulo-opercular (CiO), DoA) (Fair et al., 2007) and attentional networks (Sal, DoA.) (Fig. 5b). This is consistent with the findings showing significant performance differences on measures related to task control initiation and organization (Table 9, Fig. 5b). There were also several differences seen between the task-negative (Def) (Braga & Buckner, 2017; Fox et al., 2005; Leech et al., 2011) and multiple sensory networks including motor (SMm, SMh), visual (Vis), and auditory (Aud). This difference in task negative and sensory networks may suggest varying strategies participants engage to manage their attention to internal and external stimuli.
4.2.7. Unlike behavior, subgroups did not follow a “severity trend” across functional brain networks
In this study the imaging data is purposed with serving as a validation metric. In doing so, it is not critical that the same ‘severity trend’ is seen among imaging data as in the behavioral phenotypes. What is important is that the biological differences exist. Our findings showed just that: there were significant differences among subgroups both within and between multiple functional brain networks, but no discernably consistent trend. This is helpful in suggesting true configural types as opposed to merely recapturing severity. At the same time it may seem counterintuitive. It might be expected, for example, that for any given network the TD group would show the most connectivity between regions, followed by high-performers, followed by low-performers, or vice-versa. However, the majority of within and between network connections did not follow this pattern. Rather, multiple patterns of connectivity were present in the data (Fig. 6a, b). These results demonstrate that despite identification of lower (HSG-Severe, ISG-Severe) and higher (HSG-Mild, ISG-Mild) performing groups, such behavioral manifestations are not entirely attributable to a global theme of over- or under-connected functional networks per group, suggesting a more intricate mechanistic interaction.
We propose that specific complex patterns of brain interactions do not map one to one with a more or less optimal state (Holmes & Patrick, 2018). To further explain, we will provide a simple example. Imagine a study whereby the goal was to identify the factors attributable to the length of time participants were able to stay upright on a balance beam. To identify characteristics, we then split participants into ‘good’ and ‘bad’ balancers. On the one hand we might find that those participants who practice balancing are better balancers than those who do not – i.e., more practice equates to a better balancer. In this case the ‘cause’ of good or bad follows the same pattern, along the dimension of more or less practice. On the other hand we might also find that the bad balancers may have just simply had their eyes closed, whereby it was the lack of visual perception that made them worse at the task. In this latter case, the ‘cause’ does not follow the same pattern, i.e. there is no dimensional relationship with the outcome. Our findings are more akin to the latter scenario wherein brain networks do not follow along one dimension from high to low connectivity (or vice versa) leading to high and low performers; rather, a fundamentally different organization is seen between the high and low performing subgroups.
4.3. Limitations and future directions
In the present report, while we aimed to match participants on various demographic phenomenon, the ASD group was significantly older than the ADHD group. However, no significant age differences were observed between identified subgroups per model (Tables 4 and 5.) Post-hoc analyses (see: Supplemental Materials; Supplemental Analysis) revealed that there was a low-likelihood of the age discrepancy influencing the models. Still, the effects of age on the models cannot be entirely ruled out.
We did our best to validate the results with secondary neuroimaging data. While our subgroup sample sizes are large enough to consider the results, further validating the analyses with a much larger dataset, such as the Adolescent Brain Cognitive Development (ABCD) study (The Adolescent Brain Cognitive Development Study, 2018), could provide both confirmatory and additional information.
The FRF approach itself presents some limitations. Subgroups were identified through a consensus community detection approach. While we are confident that the subgroups identified are indeed tied to the ADHD symptoms, adjusting parameters or including different EF data may result in new subgroups with additional meaning. Future studies should not only attempt to replicate subgroup findings, but also seek to identify best practices and standards for use of the FRF as a tool. The FRF also requires a larger sample size in order to uncover potentially smaller subgroups. While we identified two subgroups per model, it is possible that the subgroups can be further differentiated with the inclusion of more participants and data. The inclusion of larger datasets, with careful scrutiny and inclusion criteria, may improve the model results.
One strength of the FRF is the ability to parse patterns in data that are difficult to detect with other methods. Yet it does not model variable interactions in ways that detect clusters, such as with latent variable analysis. A small body of work dedicated to EF has revealed some information about EF relationships across tasks and within cognitive domains (Friedman & Miyake, 2017).
While the transdiagnostic approach has been recommended in the literature with the benefit of considering symptoms over syndrome, it is certainly not without its own potential limitations. It may be that the transdiagnostic inclusion of those with ASD and ADHD introduces greater neural heterogeneity in the data, as those with ASD have shown differences in neuroimaging studies when compared to TD and ADHD groups (Karalunas et al., 2018; Ray et al., 2014; Rommelse et al., 2017). Still, if the behavioral differences were influenced by the presence of ASD, we would expect to see subgroup splitting based on this criteria, which we did not.
By applying the FRF in an inverse manner, with neuroimaging data as the input features, we could better tie the biological metrics to behavioral data. Thus, it's possible that the varying brain-behavior relationships found among our subgroups and TD group are attributable to the issue of sample size reduction: approximately half of the ASD and ADHD participants with complete neuropsychological battery did not meet the requirements for useable scan data (67 useable scans out of 130 participants). As those with increased symptomology are also more likely to provide unusable scan data (See Supplemental Material: Discussion) they represent a significant population of interest in regards to the behavioral manifestation of hyperactive/inattentive symptoms and EF. To eliminate those without scan data from our analyses entirely, we would also lose valuable information about the behavioral phenotypes. Therefore we found it important to use all of the participants in the FRF models.
Although our stringent criteria improves the likelihood of reproducibility, the exclusion of half of the participants whose “bad scans” may also introduce unknown cohort effects (a common problem in the literature). Novel methods to overcome movement in the scanner as well as improvements in processing pipelines may help future studies overcome this issue, as well as tackling the issue of including more participants with symptoms that reduce scan quality.
Alternatively, examining the relationship between brain engagement and task may reveal new information about the subtypes as shown previously (Vaidya et al., 2020). Unlike rs-fMRI, task fMRI is more resilient to motion (Siegel et al., 2014) and doesn't require motion censoring which would make it easier to acquire more data and a larger sample size. Unfortunately, task fMRI is limited to understanding the subtypes with regard to just the tasks that were run whereas with rs-fMRI we are able to look at the whole brain.
5. Conclusions
In this study we demonstrate that the FRF can use measures of EF to predict hyperactive/inattentive symptoms for those with ADHD and/or ASD. Different combinations of EF led to similar neuropsychological profiles among individuals and the FRF identified transdiagnostic subgroups representing mild and severe subgroups per model. The subgroups and TD group differed both within and between functional networks related to EF with no discernable severity trend but rather, more complex mechanistic interactions across networks.
CRediT authorship contribution statement
Michaela Cordova: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration. Kiryl Shada: Conceptualization, Investigation, Writing - review & editing. Damion V Demeter: Software, Writing - review & editing. Olivia Doyle: Data curation, Investigation, Project administration, Writing - review & editing. Oscar Miranda-Dominguez: Software, Supervision, Writing - review & editing. Anders Perrone: Software, Data curation, Writing - review & editing. Emma Schifsky: Methodology, Data curation, Writing - review & editing. Alice Graham: Supervision, Writing - original draft, Writing - review & editing. Eric Fombonne: Investigation, Supervision, Writing - review & editing. Beth Langhorst: Investigation, Supervision. Joel Nigg: Investigation, Supervision, Funding acquisition, Writing - review & editing. Damien A Fair: Investigation, Supervision, Funding acquisition, Writing - original draft, Writing - review & editing. Eric Feczko: Conceptualization, Methodology, Supervision, Software, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors Cordova, M., Shada, K., Demeter, D.V., Doyle, O., Miranda-Dominguez, O., Perrone, A., Schifsky, E., Graham, A., Langhorst, B., Nigg, J., Fombonne, E., Fair, D.A., Feczko, E. have no financial disclosures or conflicts to declare.
Acknowledgements
The authors of this paper would like to thank the members of the Developmental Cognition and Neuroimaging (DCAN) lab under the leadership of Dr. Damien Fair, Dr. Alice Graham, Dr. Oscar Miranda-Dominguez, Dr. Lisa Karstens, and Michaela Cordova, the mentorship of Dr. Eric Feczko, and Dr. Joel Nigg's ADHD Project lab for their contributions to the ASD and ADHD research studies. We would also like to express our appreciation for the families that gave their valuable time and effort to participate in these studies. This research was supported by the National Institutes of Health (grants R01 MH096773 and K99/R00 MH091238, R01 MH115357, R01 MH086654, U24 DA04112, U01 DA041148), the Oregon Clinical and Translational Research Institute, the Gates Foundation, and the Destefano Innovation Fund. Dr. Eric Feczko was supported by the National Library of Medicine (T15LM007088).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102245.
Contributor Information
Michaela Cordova, Email: cordova@ohsu.edu.
Kiryl Shada, Email: Kiryl.Shada@UHhospitals.org.
Damion V Demeter, Email: demeter@utexas.edu.
Olivia Doyle, Email: doyleo@ohsu.edu.
Oscar Miranda-Dominguez, Email: mirandad@ohsu.edu.
Anders Perrone, Email: perronea@ohsu.edu.
Alice Graham, Email: grahaal@ohsu.edu.
Eric Fombonne, Email: fombonne@ohsu.edu.
Beth Langhorst, Email: calame@ohsu.edu.
Joel Nigg, Email: niggj@ohsu.edu.
Damien A Fair, Email: faird@ohsu.edu.
Eric Feczko, Email: feczko@ohsu.edu.
Appendix. Supplementary materials
References
- Alexander A.S., Nitz D.A. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat. Neurosci. 2015 doi: 10.1038/nn.4058. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association (APA); 2013. DSM 5 Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bathelt J., Holmes J., Astle D.E., Holmes J., Gathercole S., Astle D., Manly T., Kievit R. Data-driven subtyping of executive function-related behavioral problems in children. J. Am. Acad. Child Adolesc. Psychiatry. 2018 doi: 10.1016/j.jaac.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer C., Roselló B., Colomer C., Baixauli I., Miranda A. Children with autism and attention deficit hyperactivity disorder. Relationships between symptoms and executive function, theory of mind, and behavioral problems. Res. Dev. Disabil. 2018 doi: 10.1016/j.ridd.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Braga R.M., Buckner R.L. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron. 2017 doi: 10.1016/j.neuron.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunlich K., Gomez-Lavin J., Seger C.A. Frontoparietal networks involved in categorization and item working memory. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2014.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L.E.O. Random Forests. Mach. Learn. 2001;45(1):5–32. [Google Scholar]
- Breslau N., Breslau J., Peterson E., Miller E., Lucia V.C., Bohnert K., Nigg J. Change in teachers’ ratings of attention problems and subsequent change in academic achievement: a prospective analysis. Psychol. Med. 2010 doi: 10.1017/S0033291709005960. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J.S.S., Milham M.P., Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Shum D., Toulopoulou T., Chen E.Y.H. Assessment of executive functions: review of instruments and identification of critical issues. Arch. Clin. Neuropsychol. 2008;23(2):201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Corbett B.A., Constantine L.J., Hendren R., Rocke D., Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166(2-3):210–222. doi: 10.1016/j.psychres.2008.02.005. https://doi.org/S0165-1781(08)00052-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dias Costa, G. T., Iyer S.P., Carpenter S.D., Cary R.P., Wilson V.B., Mitchel S.H., Nigg J.T., Fair D.A., Mitchell S.H., Nigg J.T., Fair D.A. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Dev. Cogn. Neurosci. 2015;11:155–174. doi: 10.1016/j.dcn.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani D.R., Burrows C.A., Odriozola P., Baez A., Nebel M.B., Mostofsky S.H., Uddin L.Q. Investigating functional brain network integrity using a traditional and novel categorical scheme for neurodevelopmental disorders. NeuroImage. 2019;21 doi: 10.1016/j.nicl.2019.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D.C., Kaplan E., Kramer J. The Psychological Corporation; 2001. Delis Kaplan Executive Function System. [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci., USA. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.t., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. The network structure of task control. Soc. Neurosci. 2006 Abstract. [Google Scholar]
- Doshi J.A., Hodgkins P., Kahle J., Sikirica V., Cangelosi M.J., Setyawan J., Erder M.H., Neumann P.J. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(10):990–1002. doi: 10.1016/j.jaac.2012.07.008. e2. [DOI] [PubMed] [Google Scholar]
- Duda M., Ma R., Haber N., Wall D.P. Use of machine learning for behavioral distinction of autism and ADHD. Transl. Psychiatry. 2016 doi: 10.1038/tp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht A.T., Elison J.T., Feczko E., Todorov A., Wolff J.J., Kandala S., Adams C.M., Snyder A.Z., Lewis J.D., Estes A.M., Zwaigenbaum L., Botteron K.N., McKinstry R.C., Constantino J.N., Evans A., Hazlett H.C., Dager S., Paterson S.J., Schultz R.T., Pruett J.R. Joint Attention and Brain Functional Connectivity in Infants and Toddlers. Cereb. Cortex. 2017 doi: 10.1093/cercor/bhw403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Bathula D., Nikolas M.A., Nigg J.T. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc. Natl. Acad. Sci. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natil. Acad. Sci. USA. 2007;104(33):13507–13512. [Google Scholar]
- Feczko E.J., Balba N., Miranda-Dominguez O., Cordova M., Karalunas S.L., Irwin L., Demeter D.V., Hill A.P., Langhorst B.H., Grieser Painter J., Van Santen J., Fombonne E.J., Nigg J.L., Fair D.A. Subtyping cognitive profiles in Autism Spectrum Disorder using a random forest algorithm. Neuroimage. 2018;172:674–688. doi: 10.1016/j.neuroimage.2017.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feczko E., Miranda-Dominguez O., Marr M., Graham A.M., Nigg J.T., Fair D.A. The heterogeneity problem: approaches to identify psychiatric subtypes. Trends Cogn. Sci. 2019;23(7):584–601. doi: 10.1016/j.tics.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiou M.D., Turck C.W. General overview: biomarkers in neuroscience research. Int. Rev. Neurobiol. 2011 doi: 10.1016/B978-0-12-387718-5.00001-8. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N.P., Miyake A. Unity and diversity of executive functions: individual differences as a window on cognitive structure. Cortex. 2017 doi: 10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galéra C., Melchior M., Chastang J.F., Bouvard M.P., Fombonne E. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: the GAZEL youth study. Psychol. Med. 2009 doi: 10.1017/S0033291709005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts H.M., Verte S., Oosterlaan J., Roeyers H., Sergeant J.A., Verté S., Oosterlaan J., Roeyers H., Sergeant J.A. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism. J. Child Psychol. Psychiatry. 2004;45(4):836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Gioia G.A., Isquith P.K., Guy S.C., Kenworthy L., Baron I.S. Behavior rating inventory of executive function. Child Neuropsychol. 2001 doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cerebral Cortex. 2014;26(1):288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz R.P. The speed-accuracy tradeoff: History, physiology, methodology, and behavior. Front. Neurosci. 2014;(Issue 8 JUN) doi: 10.3389/fnins.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.J., Patrick L.M. The myth of optimality in clinical neuroscience. Trends Cogn. Sci. 2018;22(3):241–257. doi: 10.1016/j.tics.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J., Gathercole S.E., Place M., Alloway T.P., Elliott J.G., Hilton K.A. The diagnostic utility of executive function assessments in the identification of ADHD in children. Child Adolesc. Mental Health. 2010 doi: 10.1111/j.1475-3588.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C.L., Karalunas S.L., Tam H., Moore A.N. Evaluating vigilance deficits in ADHD: a meta-analysis of CPT performance. J. Abnorm. Psychol. 2012;121(2):360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Adolescent Brain Cognitive Development Study J. Res. Adolesc. 2018;154 doi: 10.1111/jora.12374. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G., Faraone S.V., Wozniak J., Tarko L., Fried R., Galdo M., Furtak S.L., Biederman J. Symptom Profile of ADHD in Youth With High-Functioning Autism Spectrum Disorder: A Comparative Study in Psychiatrically Referred Populations. J. Atten. Disord. 2017;21(10):846–855. doi: 10.1177/1087054714543368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas S.L., Fair D., Musser E.D., Aykes K., Iyer S.P., Nigg J.T. Subtyping Attention-Deficit/Hyperactivity Disorder Using Temperament Dimensions: Toward Biologically Based Nosologic Criteria. JAMA Psychiatry. 2014;71(9):1015–1024. doi: 10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Karalunas S.L., Hawkey E., Gustafsson H., Miller M., Langhorst M., Cordova M., Fair D., Nigg J.T. Overlapping and distinct cognitive impairments in attention-deficit/hyperactivity and autism spectrum disorder without intellectual disability. J. Abnorm. Child Psychol. 2018 doi: 10.1007/s10802-017-0394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas S.L., Nigg J.T., Huang-Pollock C.L. Decomposing attention-deficit/hyperactivity disorder (ADHD)-related effects in response speed and variability. Neuropsychology. 2012 doi: 10.1037/a0029936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katuwal G.J., Baum S.A., Cahill N.D., Michael A.M. Divide and conquer: Sub-grouping of ASD improves ASD detection based on brain morphometry. PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0153331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kofler M.J., Rapport M.D., Bolden J., Sarver D.E., Raiker J.S. ADHD and working memory: the impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. J. Abnorm. Child Psychol. 2010 doi: 10.1007/s10802-009-9357-6. [DOI] [PubMed] [Google Scholar]
- Kohavi R. International Joint Conference on Artificial Intelligence (IJCAI) 1995. A study of cross-validation and bootstrap for accuracy estimation and model selection; pp. 1137–1145. [Google Scholar]
- Korkman M., Kirk U., Kemp S. NEPSY-Second Edition (NEPSY-II) J. Psychoeduc. Assess. 2007 [Google Scholar]
- Lecei A., van Hulst B.M., de Zeeuw P., van der Pluijm M., Rijks Y., Durston S. Can we use neuroimaging data to differentiate between subgroups of children with ADHD symptoms: a proof of concept study using latent class analysis of brain activity. NeuroImage. 2019 doi: 10.1016/j.nicl.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011 doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front. Hum. Neurosci. 2014 doi: 10.3389/fnhum.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.Y., Tseng W.Y.I., Lai M.C., Matsuo K., Gau S.S.F. Altered resting-state frontoparietal control network in children with attention-deficit/hyperactivity disorder. J. Int. Neuropsychol. Soc. 2015 doi: 10.1017/S135561771500020X. [DOI] [PubMed] [Google Scholar]
- Logan G.D. Inhibitory processes in attention, memory, and language. 1994. On the ability to inhibit thought and action: A users guide to the stop-signal paradigm; pp. 189–239. [Google Scholar]
- Lombardo M.V, Lai M.-C., Baron-Cohen S. Molecular Psychiatry. 2019. Big data approaches to decomposing heterogeneity across the autism spectrum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M.M., Nigg J.T., von Eye A. How do trait dimensions map onto ADHD symptom domains? J. Abnorm. Psychol. 2009;37(3):337–348. doi: 10.1007/s10802-008-9255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M., Nikolas M., Nigg J.T. Executive function in adolescents with ADHD. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(11):1437–1444. doi: 10.1097/chi.0b013e31814cf953. [DOI] [PubMed] [Google Scholar]
- Matson, J. L., & Burns, C. O. (2019). Comorbidity and the Need for Interdisciplinary Treatments. 10.1007/978-3-030-13027-5_3.
- Matza L.S., Paramore C., Prasad M. A review of the economic burden of ADHD. Cost Effect. Resour. Alloc. 2005;3:5. doi: 10.1186/1478-7547-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Musser E.D., Young G.S., Olson B., Steiner R.D., Nigg J.T. Sibling recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatr. 2019 doi: 10.1001/jamapediatrics.2018.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B.D., Miranda-Dominguez O., Mills K.L., Earl E., Cordova M., Painter J., Karalunas S.L., Nigg J.T., Fair D.A. ADHD and attentional control: impaired segregation of task positive and task negative brain networks. Netw. Neurosci. (Cambridge, Mass.) 2018;2(2):200–217. doi: 10.1162/netn_a_00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki a H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit. Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Molitor S.J., Oddo L.E., Eadeh H.M., Langberg J.M. Executive function deficits in adolescents with ADHD: untangling possible sources of heterogeneity. J. Emot. Behav. Disord. 2018 [Google Scholar]
- Mulder M.J., Bos D., Weusten J.M.H., Van Belle J., Van Dijk S.C., Simen P., Van Engeland H., Durston S. Basic impairments in regulating the speed-accuracy tradeoff predict symptoms of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68(12):1114–1119. doi: 10.1016/j.biopsych.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Musser E.D., Hawkey E., Kachan-Liu S.S., Lees P., Roullet J.B., Goddard K., Steiner R.D., Nigg J.T. Shared familial transmission of autism spectrum and attention-deficit/ hyperactivity disorders. J. Child Psychol. Psychiatry. 2014 doi: 10.1111/jcpp.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T. The ADHD response-inhibition deficit as measured by the Stop Task: replication with DSM-IV combined type, extension, and qualification. J. Abnorm. Child Psychol. 1999;27(5):393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol. Psychiatry. 2005;57(11):1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(3–4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nijmeijer J.S., Minderaa R.B., Buitelaar J.K., Mulligan A., Hartman C.A., Hoekstra P.J. Attention-deficit/hyperactivity disorder and social dysfunctioning. Clinical Psychology Review. 2008;28(4):692–708. doi: 10.1016/j.cpr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Pennington B.F. Dimensions of executive functions in normal and abnormal developmant. In: Krasnegor N., Lyon R., Goldman-Rakic P., editors. Development of the Prefrontal Cortex: Evolution, Neurobiology, and Behavior. Brookes Publishing Co., Inc; 1997. pp. 265–281. [Google Scholar]
- Pennington B.F., Ozonoff S. Executive functions and developmental psychopathology. J. Child Psychol. Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. http://www.ncbi.nlm.nih.gov/pubmed/8655658 [DOI] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Miller M., Karalunas S., Robertson C., Grayson D.S., Cary R.P., Hawkey E., Painter J.G., Kriz D., Fombonne E., Nigg J.T., Fair D.A. Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: A rich club-organization study. Hum. Brain Mapp. 2014;35(12):6032–6048. doi: 10.1002/hbm.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen A.M., Todd R.D. Co-occurrence of ADHD and autism spectrum disorders: phenomenology and treatment. Expert. Rev. Neurother. 2008;8(4):657–669. doi: 10.1586/14737175.8.4.657. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., James M., Owen A.M., Sahakian B.J., McInnes L., Rabbitt P.M. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Rommelse N., Buitelaar J.K., Hartman C.A. Structural brain imaging correlates of ASD and ADHD across the lifespan: a hypothesis-generating review on developmental ASD-ADHD subtypes. J. Neural Transm. 2017;124(2):259–271. doi: 10.1007/s00702-016-1651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M., Bergstrom C.T. Maps of random walks on complex networks reveal community structure. Proc. Natl. Acad. Sci. USA. 2008;105(4):1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrud-Clikeman M., Walkowiak J., Wilkinson A., Butcher B. Executive functioning in children with asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. J. Autism Dev. Disord. 2010 doi: 10.1007/s10803-010-0951-9. [DOI] [PubMed] [Google Scholar]
- Shiels K., Hawk L.W. Clinical Psychology Review. 2010. Self-regulation in ADHD: The role of error processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Vogel A.C., Church J.A., Dubis J.W., Schlaggar B.L., Petersen S.E., Vogel A.C., Church J.A., Schlaggar B.L., Petersen S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014;35(5):1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein M.J., Faraone S.V., Leon T.L., Biederman J., Spencer T.J., Adler L.A. The Relationship Between Executive Function Deficits and DSM-5-Defined ADHD Symptoms. J. Atten. Disord. 2018 doi: 10.1177/1087054718804347. [DOI] [PubMed] [Google Scholar]
- Sinzig J., Morsch D., Bruning N., Schmidt M.H., Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child Adolesc. Psychiatry Mental Health. 2008;2(1):4. doi: 10.1186/1753-2000-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E., Bitsakou P., Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(4):345–355. doi: 10.1016/j.jaac.2009.12.018. http://www.ncbi.nlm.nih.gov/pubmed/20410727 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Elgie S., Hall M. More to ADHD than meets the eye: observable abnormalities in search behaviour do not account for performance deficits on a discrimination task. Behav. Brain Funct. 2005;1(1):10. doi: 10.1186/1744-9081-1-10. https://doi.org/1744-9081-1-10 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T., Peng L., Barnard-Brak L. The comorbidity of ADHD in children diagnosed with autism spectrum disorder. Res. Autism Spectr. Disord. 2016;31:11–18. [Google Scholar]
- Thorell L.B. Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? A study of early academic skill deficits. J. Child Psychol. Psychiatry. 2007 doi: 10.1111/j.1469-7610.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- Uluyagmur-Ozturk M., Arman A.R., Yilmaz S.S., Findik O.T.P., Genc H.A., Carkaxhiu-Bulut G., Yazgan M.Y., Teker U., Cataltepe Z. Proceedings - 2016 15th IEEE International Conference on Machine Learning and Applications, ICMLA 2016. 2017. ADHD and ASD classification based on emotion recognition data. [Google Scholar]
- Vaidya C.J., You X., Mostofsky S., Pereira F., Berl M.M., Kenworthy L. Data-driven identification of subtypes of executive function across typical development, attention deficit hyperactivity disorder, and autism spectrum disorders. J. Child Psychol. Psychiatry. 2020 doi: 10.1111/jcpp.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann S.D., Aggleton J.P., Maguire E.A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 2009 doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Newcorn J.H., Kollins S.H., Wigal T.L., Telang F., Fowler J.S., Goldstein R.Z., Klein N., Logan J., Wong C., Swanson J.M. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol. Psychiatry. 2011 doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 4th Edition (WISC-IV®) Harcourt Assessment; 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Welsh M.C., Pennington B.F. Assessing frontal lobe functioning in children: Views from developmental psychology. Dev. Neuropsychol. 1988;4:199–230. [Google Scholar]
- Willcutt E G, Nigg J.T., Pennington B.F., Solanto M.V, Rohde L.A., Tannock R., Loo S.K., Carlson C.L., McBurnett K., Lahey B.B. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J. Abnorm. Psychol. 2012;121(4):991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang R., Geng X., Lee T.M.C. Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct. Funct. 2017;222(9):3973–3990. doi: 10.1007/s00429-017-1443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.