Abstract
Type I interferons (IFN-α/β) are potent antiviral cytokines and modulators of the adaptive immune system. They are induced by viral infection or by double-stranded RNA (dsRNA), a by-product of viral replication, and lead to the production of a broad range of antiviral proteins and immunoactive cytokines. Viruses, in turn, have evolved multiple strategies to counter the IFN system which would otherwise stop virus growth early in infection. Here we discuss the current view on the balancing act between virus-induced IFN responses and the viral counterplayers.
Abbreviations: AdV, adenovirus; BDV, Borna disease virus; BUNV, Bunyamwera virus; CSFV, classical swine fever virus; DHFV, Dengue hemorrhagic fever virus; EBOV, Ebola virus; EBV, Epstein–Barr virus; FLUAV, influenza A virus; FLUBV, influenza B virus; HCV, Hepatitis C virus; HHV-8, human herpes virus 8; HIV, human immunodeficiency virus; HPV16, human papilloma virus 16; HPV18, human papilloma virus 18; HSV-1, herpes simplex virus 1; JEV, Japanese encephalitis virus; PV, Polio virus; RV, Rabies virus; RSV, respiratory syncytial virus; RVFV, Rift valley fever virus; THOV, Thogoto virus; TMEV, Theiler's meningoencephalitis virus; VV, Vaccinia virus; VSV, vesicular stomatitis virus; WNV, West Nile virus; YFV, Yellow fever virus
Introduction
Viral infections in children and young adults are common and generally uneventful. In most instances, the patients recover and either eliminate the virus or incorporate it in a latent or persistent form without further problems. Although viruses are obligate intracellular parasites and rely entirely on the metabolic machinery of the host cell, they usually do not cause much harm. The main reason is that our body is not defenseless but makes use of numerous measures to keep viruses at bay. As we know, the type I interferon system is a major player in antiviral defense against all kinds of viruses. Virus-infected cells synthesize and secrete type I interferons (IFN-α/β) which warn the body of the dangerous intruders. Secreted IFNs circulate in the body and cause susceptible cells to express potent antiviral mechanisms which limit further viral growth and spread. IFN was discovered almost 50 years ago by Isaacs and Lindenmann as a cytokine interfering with virus replication (Isaacs and Lindenmann, 1957). Since then, much progress has been made in demonstrating how IFNs are induced and how they work. However, only now we begin to appreciate to what extent viruses modulate the IFN response they evoke in the first place. Clearly, viruses would not be successful pathogens if they had not evolved efficient escape strategies allowing them to suppress IFN production, to down-modulate IFN signaling and to block the action of antiviral effector proteins. It is becoming increasingly clear that the IFN antagonistic properties determine viral virulence to a great extent and may contribute to interspecies transmission of so-called emerging and reemerging viruses. Here, we summarize recent highlights in our understanding of how cells defend themselves against viral intruders and how viruses manage to survive in the face of the powerful IFN system.
The amazing power of the interferon system
The type I IFN system is indispensable for vertebrates to control viral infections. This is best illustrated in knockout mice which are unresponsive to IFN-α/β due to targeted deletions in the type I IFN receptor (Muller et al., 1994). These mice quickly succumb to viral infections despite having a normal adaptive immune system (Bouloy et al., 2001, Bray, 2001, Grieder and Vogel, 1999, Hwang et al., 1995, Muller et al., 1994, Ryman et al., 2000, van den Broek et al., 1995). Likewise, humans die of viral disease at an early age if they happen to acquire genetic defects of the IFN system (Dupuis et al., 2003). The importance of type I IFNs is further demonstrated in instances where disruption of a single IFN-effector gene causes a complete loss of innate immunity against a particular type of virus, leading to overwhelming infection and rapid death. A telling case is increased susceptibility to influenza and influenza-like viruses found in inbred mouse strains due to a defect in the IFN-regulated Mx1 gene (Lindenmann, 1964, Staeheli et al., 1988). Susceptible animals are equipped with the full armament of innate and adaptive immunity with the exception of Mx and resist all sorts of viruses but not orthomyxoviruses (Haller, 1981, Haller et al., 1998). Transgenic introduction of mouse or human Mx is sufficient to turn susceptible mice into resistant animals (Arnheiter et al., 1990, Pavlovic et al., 1995). More importantly, constitutive expression of human MxA in an otherwise IFN-non-responsive animal confers full protection, demonstrating the exquisite power of a single effector molecule of the human IFN system in an otherwise susceptible host (Hefti et al., 1999). A single autosomal dominant gene locus, designated Flv/Wnv, is responsible for natural resistance of mice against infection with West Nile virus (WNV) and other flaviviruses (Brinton and Perelygin, 2003). The gene was recently identified as Oas1b, a member of a large IFN-regulated gene family encoding 2′-5′-oligoadenylate synthetases (2-5 OAS) known to play an important role in antiviral defense (Mashimo et al., 2002, Perelygin et al., 2002). As in the case of the Mx GTPase, OAS1b has antiviral activity and directly mediates inhibition of WNV replication (Lucas et al., 2003, Perelygin et al., 2002). In recent years, genetic analysis of innate immune responses has exploded and has generated a wealth of new information, as discussed below.
Pathways leading to IFN gene expression
Induction of type I (α/β) IFN gene expression is tightly regulated. Recent findings suggest that cells make use of two major but distinct cellular signal transduction pathways to sense viruses and activate their type I IFN genes. Most cells of the body including fibroblasts, hepatocytes and conventional dendritic cells (cDCs) use the so-called classical pathway. They have intracellular sensors that, upon infection, detect viral components in the cytoplasm and activate the main interferon regulatory transcription factors IRF-3 and NF-kB which in turn transactivate IFN-β gene expression. Infected cells secrete mainly IFN-β as an initial response to infection but switch to IFN-α during the subsequent amplification phase of the IFN response (Marie et al., 1998). In contrast, plasmacytoid dendritic cells (pDCs) use Toll-like receptors (TLRs) expressed on the cell surface or in endosomes to sense extracellular or engulfed viral material. TLR signaling of pDCs primarily involves the adaptor protein MyD88 and activates IRF-7 which is constitutively expressed in pDCs and serves as a master regulator of IFN-α/β gene expression (Honda et al., 2005b). This cell type predominantly secretes high levels of IFN-α and represents the so-called natural interferon producing cells of the body (Colonna et al., 2002, Diebold et al., 2003). Both pathways play important roles during infection and are presently being fully explored.
Classical pathway
The classical pathway of type I IFN induction is best understood for IFN-β gene expression in fibroblasts (Fig. 1 ). In infected cells, a signaling chain is activated by double-stranded RNA (dsRNA) molecules which are generated as intermediates of viral transcription. Two intracellular RNA helicases, RIG-I (Yoneyama et al., 2004) and MDA5 (Andrejeva et al., 2004), act as sentinels for viral dsRNA detection. The two related helicases are apparently non-redundant and seem to function in parallel, having a degree of virus specificity (Yoneyama et al., 2005). RIG-I and MDA5 are ubiquitously expressed in most tissues and are inducible by IFNs which allows autocrine and paracrine amplification of the sensing system. A third member of the RIG-I helicase family, LGP2, is a natural inhibitor which presumably masks the viral dsRNA from recognition by RIG-I or MDA5 and serves as a negative regulator of IFN gene expression (Rothenfusser et al., 2005, Yoneyama et al., 2005). RIG-I and MDA5 both contain two N-terminal caspase-recruiting domain (CARD)-like regions and a C-terminal DExD/H box RNA helicase domain (Andrejeva et al., 2004, Yoneyama et al., 2004). RNA-binding to the helicase domain most likely induces a conformational change which enables that CARD domain to interact with CARD-like domains of downstream signaling partners (Kato et al., 2005, Yoneyama et al., 2004). Indeed, one interaction partner has just been identified independently by two groups. The novel protein is required to mediate RIG-I and MDA5 signals to downstream factors and is therefore called either IPS-1 for “interferon-β-promoter stimulator 1” (Kawai et al., 2005) or MAVS for “mitochondrial antiviral signaling” molecule (Seth et al., 2005). It is identical to an uncharacterized protein previously recognized to be involved in NF-kB promoter activation (Matsuda et al., 2003). IPS-1/MAVS has a CARD-like domain which binds to RIG-I and MDA5 and a C-terminal region which interacts with FADD and RIP1 which are both involved in NF-kB signaling (Kawai et al., 2005). Surprisingly, IPS-1/MAVS localizes to the outer mitochondrial membrane via a C-terminal transmembrane domain. Membrane association and localization to mitochondria is shown to be essential for function (Seth et al., 2005). These findings are intriguing, as they link IFN production and innate immune responses to mitochondrial activity. It is conceivable that the membrane localization of IPS-1/MAVS is advantageous as it may keep the interacting RNA-sensing helicases close to the viral replication factories which often develop in association with intracellullar membranes.
Fig. 1.
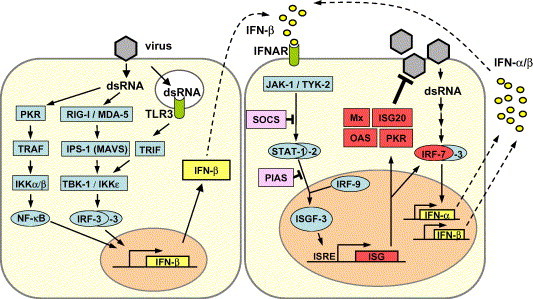
Type I IFN induction, signaling and action. Left panel: dsRNA, a characteristic by-product of virus replication, leads to activation of the transcription factors NF-κB, IRF-3 and AP-1 (not shown). The cooperative action of these factors is required for full activation of the IFN-β promoter. IRF-3 is phosphorylated by the kinases IKKε and TBK-1 which in turn are activated by the RNA-sensing complex of RIG-I, MDA5 and IPS-1/MAVS. A second signaling pathway involves endosomal TLR-3 and TRIF. Right panel: Newly synthesized IFN-β binds to the type I IFN receptor (IFNAR) and activates the expression of numerous ISGs via the JAK/STAT pathway. IRF-7 amplifies the IFN response by inducing the expression of several IFN-β subtypes. SOCS and PIAS are negative regulators of the JAK-STAT pathway. Mx, ISG20, OAS and PKR are examples of proteins with antiviral activity. For details see text.
The downstream molecules interacting with IPS-1/MAVS are not yet known. However, IPS-1/MAVS leads indirectly to activation of the IRF-3 kinases (Fig. 1) (Kawai et al., 2005, Seth et al., 2005). Two IκB kinase (IKK)-related kinases, IKKε and TANK-binding kinase-1 (TBK-1), are known to phosphorylate the transcription factor IRF-3 (Fitzgerald et al., 2003, Sharma et al., 2003). IRF-3 is a member of the IFN regulatory factor (IRF) family and plays a central role in the activation of the IFN-β promoter (Lin et al., 1998, Schafer et al., 1998, Wathelet et al., 1998, Weaver et al., 1998, Yoneyama et al., 1998). Phosphorylated IRF-3 homodimerizes and moves into the nucleus where it recruits the transcriptional coactivators p300 and CREB-binding protein (CBP) to initiate IFN-β mRNA synthesis (Hiscott et al., 1999, Suhara et al., 2002). In addition, NF-κB and AP-1 are recruited in a dsRNA-dependent way (Chu et al., 1999). Together these transcription factors strongly up-regulate IFN-α/β gene expression. This initially produced “first-wave” IFN triggers expression of a related factor, IRF-7, which is normally not present in most cells or at best in very low amounts (Sato et al., 2000). Genetic evidence has recently shown that IRF-7 is the master regulator of IFN gene expression and that IRF-3 most likely cooperates with IRF-7 for full activity (Honda et al., 2005b). IRF-7 is activated in the same way as IRF-3 (Iwamura et al., 2001, Smith et al., 2001, tenOever et al., 2004) and is responsible for a positive-feedback loop that initiates the synthesis of several IFN-α subtypes as the “second-wave” IFNs (Marie et al., 1998, Sato et al., 1998).
Some cells of the hematopoietic system such as cDCs preferentially express TLR3 and can sense viral and other dsRNA molecules in the endocytic compartment (Fig 1). Ligand-induced triggering of TLR3 by dsRNA proceeds via the adaptor molecule TRIF which bypasses IPS-1/MAVS and directly activates the kinase TBK-1. This RIG-I-independent process also leads to phosphorylation and nuclear translocation of IRF-3 and IFN-β gene expression. The main difference is that TLR-3-expressing cells do not need to get infected to produce type I IFNs but can respond to viral RNA from inactivated virus particles or dead cells provided they are taken up in the endosomal compartment (Schulz et al., 2005).
TLR pathway in plasmacytoid dendritic cells
pDCs are specialized IFN producers and represent a major source of IFN-α in humans (Colonna et al., 2002). They sense the presence of RNA or DNA viruses by TLRs expressed in endosomes (Beutler, 2004, Bowie and Haga, 2005). Human pDCs mostly express the TLR9 subfamily members TLR7, TLR8 and TLR9 which recognize viral single-stranded (ss) RNA (TLR7,8) or double-stranded CpG-rich DNA (TLR9), respectively (Iwasaki and Medzhitov, 2004). Upon activation, TLR7, 8 and 9 signal through their adaptor molecule MyD88 which forms a complex with TRAF6 and IRF-7 (Fig. 2 ). IRF-7 is phosphorylated by IRAK-1, an additional component of this receptor-associated multiprotein complex, and transcriptionally activates multiple IFN-α genes (Iwasaki and Medzhitov, 2004, Uematsu et al., 2005). A key difference between pDCs and other cell types is their capacity to constitutively express considerable amounts of IRF-7 (Kerkmann et al., 2003, Prakash et al., 2005). IRF-7 and also IRF-8 are further up-regulated in response to IFN and together generate a positive feed back loop for high IFN-α and IFN-β production (Asselin-Paturel and Trinchieri, 2005, Tsujimura et al., 2003).
Fig. 2.
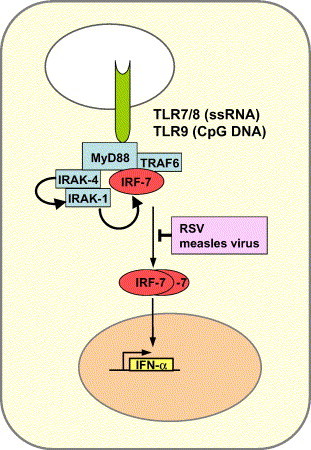
TLR signaling pathway for IFN-α production in plasmacytoid dendritic cells (pDCs). TLR7 and TLR8 respond to RNA viruses by recognizing ssRNA in endosomes. In contrast, TLR9 responds to DNA viruses by recognizing CpG-rich sequences. The receptors transmit their signals through an associated multiprotein complex containing the adaptor protein MyD88 and the transcription factor IRF-7. In contrast to most other cells, pDCs constitutively express IRF-7 (Kerkmann et al., 2003, Prakash et al., 2005). Some pathogenic viruses interfere with this pathway. See text for details.
Another distinguishing feature is the recent finding that ligand-bound TLR-9 and presumably also TLR-7/TLR8 remain active in the endosomal compartment for a long time period, whereas they are rapidly translocated to lysosomes and degraded in other cell types (Honda et al., 2005a). It is perhaps not surprising that some viruses which are known to cause prolonged immunosuppression or immune imbalances should target pDCs and inhibit IFN production induced by the TLR-MyD88-IRF-7 pathway (Fig. 2), because type I IFNs play a major role in shaping adaptive immune responses, in addition to their function in innate immunity (Le Bon and Tough, 2002). Recent examples are measles virus and respiratory syncytial virus which block IFN production in pDCs by an as yet unknown mechanism (Schlender et al., 2005).
IFN receptor signaling
The IFN receptor signaling pathway is now firmly established and has appropriately been described in comprehensive reviews (Samuel, 2001, Stark et al., 1998). IFN-β and the multiple IFN-α subspecies activate a common type I IFN receptor (IFNAR) which sends a signal to the nucleus through the so-called JAK-STAT pathway (Fig. 1). The STAT proteins are latent cytoplasmic transcription factors which become phosphorylated by the Janus kinases JAK-1 and TYK-2. Phosphorylated STAT-1 and STAT-2 recruit a third factor, IRF-9 (or p48), to form a complex known as IFN-stimulated gene factor 3 (ISGF-3) which translocates to the nucleus and binds to the IFN-stimulated response element (ISRE) in the promoter region of interferon-stimulated genes (ISGs). Specialized proteins serve as negative regulators and inhibitors of the JAK-STAT pathway. The suppressor of cytokine signaling (SOCS) proteins prevent STAT activation (Kubo et al., 2003) whereas protein inhibitor of activated STAT (PIAS) family members function as small ubiquitin-like modifier (SUMO) E3 ligases and inhibit the transcriptional activity of STATs (Shuai and Liu, 2005). Interestingly, some of these inhibitors are exploited by viruses to down-regulate IFN action (see below). Of great interest in this context is a recent genetic analysis of Drosophila C virus infection in its natural host Drosophila melanogaster, because the findings demonstrate for the first time a conserved function of the JAK-STAT signaling pathway in insect antiviral immunity (Dostert et al., 2005). It remains to be seen whether insect viruses possess evasion strategies similar to those found in vertebrate viruses.
IFN-stimulated gene products with antiviral activity
Type I IFNs activate the expression of several hundred IFN-stimulated genes (ISGs) (de Veer et al., 2001, Der et al., 1998) some of which code for antiviral proteins (Fig. 1). To date, three antiviral pathways have been firmly established. These comprise the protein kinase R (PKR) (Williams, 1999), the 2-5 OAS/RNaseL system (Silverman, 1994) and the Mx proteins (Haller and Kochs, 2002, Isaacs and Lindenmann, 1957). Mx proteins belong to the superfamily of dynamin-like large GTPases and have been discovered as mediators of genetic resistance against orthomyxoviruses in mice. Their importance for host survival following infection with certain RNA viruses has been amply demonstrated (Arnheiter et al., 1996, Hefti et al., 1999, Pavlovic et al., 1995) but their exact mode of action is still unknown. The relevance of the OAS/RNaseL and PKR systems in the IFN response to viral infection is well documented both in tissue culture and animal experiments. In addition, their importance is highlighted by the fact that most viruses have evolved specific mechanisms to counteract their activities (see below). Mice lacking one of these components show increased susceptibility to viral infections (Yang et al., 1995, Zhou et al., 1997). Nevertheless, cells from so-called triple knock-out mice lacking PKR, RNaseL and Mx still exhibit a limited IFN-induced antiviral state, indicating that additional antiviral pathways exist (Zhou et al., 1999). Additional proteins with potentially important antiviral activities are ISG20 (Espert et al., 2003), promyelocytic leukemia protein (PML) (Regad et al., 2001), guanylate-binding protein 1 (GBP-1) (Anderson et al., 1999), P56 (Guo et al., 2000, Hui et al., 2003) and RNA-specific adenosine deaminase 1 (ADAR1) (Samuel, 2001). P56 binds a subunit of the eukaryotic translation initiation factor eIF3 and thereby suppresses viral as well as cellular RNA translation (Hui et al., 2003, Wang et al., 2003). Importantly, both P56 and ADAR1 are able to limit hepatitis C virus (HCV) replication to some degree (Taylor et al., 2005, Wang et al., 2003).
Viral interference with cellular IFN responses
Most viruses need to multiply extensively to establish a solid infection in the newly infected host and to provide an outcrop of progeny virus for host-to-host transmission, or else to secure viral persistence or latency. How can a virus reach this goal in the presence of a powerful innate immune response? The answer is that viruses have learned to cope with the IFN system. Shortly after the discovery that heat-inactivated influenza viruses would induce IFN (Isaacs and Lindenmann, 1957), Jean Lindenmann reported that infection of cells with a live influenza virus inhibited the subsequent induction of IFN by an inactivated virus. He called this puzzling phenomenon “inverse interference” (Lindenmann, 1960). It is now evident that most viruses have evolved means to down-regulate IFN responses. In many cases they use non-structural viral proteins for that purpose which are otherwise non-essential for virus growth. This strategy can be exploited in the laboratory to generate mutant viruses that lack the relevant non-essential proteins. Such viruses still grow in IFN-non-responsive cells or organisms but are highly attenuated in IFN-competent hosts. Using such an approach, several laboratories have already successfully produced novel vaccine candidates lacking proteins with IFN-antagonistic activity (Ferko et al., 2004, Talon et al., 2000b, Valarcher et al., 2003). Current genetic analyses of many different viruses are revealing an ever-growing number of IFN-antagonistic proteins that target virtually all components of the IFN system. These IFN antagonists are often multifunctional proteins that interact with multiple viral or host cell components and are involved in regulating many different functions in infected cells. For example the NS1 of FLUAV is pleiotropic since it not only binds to and sequesters dsRNA molecules to prevent induction of IFNs (Garcia-Sastre, 2001, Garcia-Sastre et al., 1998, Talon et al., 2000a, Wang et al., 2000) but also inhibits the 3′ end processing of cellular pre-mRNAs, regulates the virus replication cycle and enhances translation initiation of viral mRNAs (Krug et al., 2003). Comprehensive reviews have recently dealt with these issues (Basler and Garcia-Sastre, 2002, Conzelmann, 2005, Gale and Foy, 2005, Hengel et al., 2005, Weber et al., 2004). Here we will address some general points.
Fig. 3 illustrates the range of activities mediated by IFN antagonists of various viruses. Only a few examples are listed but they demonstrate several important points. First, viral proteins or functions have been identified that cover the whole spectrum of the IFN response in infected cells. Second, a single viral protein may inhibit quite different components of the IFN induction and signaling cascade. Third, a given virus may display more than one IFN-antagonistic activity targeting different pathways. Good examples are the V proteins of some paramyxoviruses which bind to the dsRNA-sensing helicase MDA5 and thereby block induction of IFN (Andrejeva et al., 2004) but also provoke the ubiquitinylation and subsequent degradation of STAT1 in a complex reaction (Didcock et al., 1999, Palosaari et al., 2003, Precious et al., 2005, Ulane et al., 2005). The NSs protein of Rift valley fever virus is a major virulence factor (Bouloy et al., 2001) and blocks IFN production by inhibiting the basic cellular transcription machinery (Billecocq et al., 2004, Le May et al., 2004). Surprisingly, it also activates the cellular suppressor of STAT activation, SOCS-1, to impede IFN signaling (Michèle Bouloy, personal communication). Likewise, HSV-1 induces SOCS-3 to down-regulate STAT and JAK phosphorylation (Yokota et al., 2004). Interestingly, the core protein of HCV also appears to activate SOCS-3 (Bode et al., 2003), while the NS3/4A protease of HCV inhibits RIG-I signaling and has also the ability to disrupt TLR3 signaling by inducing cleavage of the adaptor protein TRIF (Breiman et al., 2005, Foy et al., 2005, Li et al., 2005). The NS3/4A protease is essential to cleave the non-structural proteins from the HCV polyprotein synthesized on intracellular membranes and may also lead to degradation of the membrane-bound cellular IPS1/MAVS complexes.
Fig. 3.
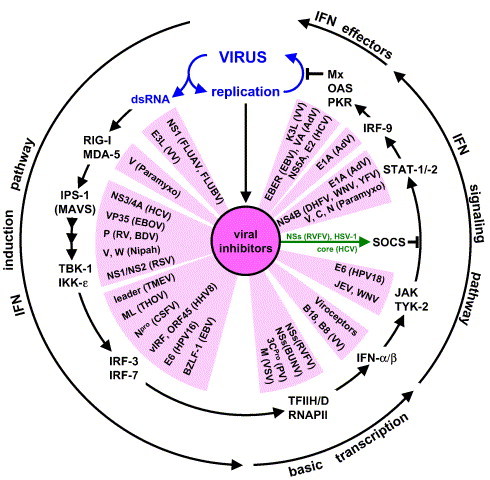
Viral inhibitors of the virus-induced IFN-α/β response loop: Viral gene products interfere with the type I IFN system at all levels. The following viral IFN antagonists are shown in clockwise order: NS1 of FLUAV (Garcia-Sastre, 2001, Garcia-Sastre et al., 1998, Talon et al., 2000a, Wang et al., 2000), NS1 of FLUBV (Dauber et al., 2004), E3L VV (Smith et al., 2001, Xiang et al., 2002), V of paramyxoviruses (Andrejeva et al., 2004), NS3/4A of HCV (Breiman et al., 2005, Foy et al., 2005), VP35 of EBOV (Basler et al., 2003), P of RV (Brzozka et al., 2005), P of BDV (Unterstab et al., 2005), V, W of Nipah virus (Park et al., 2003, Shaw et al., 2004), NS1/NS2 of RSV (Bossert et al., 2003, Spann et al., 2004, Valarcher et al., 2003), leader protein of TMEV (Delhaye et al., 2004, van Pesch et al., 2001), ML of THOV (Hagmaier, 2003 #89;Jennings, 2004 #233;Pichlmair, 2004 #247), NPro of CSFV (La Rocca et al., 2005, Ruggli et al., 2003, Ruggli et al., 2005), vIRF and ORF45 of HHV-8 (Burysek et al., 1999a, Burysek et al., 1999b, Li et al., 1998, Lubyova et al., 2004, Lubyova and Pitha, 2000, Zhu et al., 2002, Zimring et al., 1998), E6 of HPV16 (Ronco et al., 1998), BZLF-1 of EBV (Hahn et al., 2005), M of VSV (Ahmed et al., 2003, Yuan et al., 1998), 3CPro of PV (Clark et al., 1993, Yalamanchili et al., 1996), NSs of BUNV (Thomas et al., 2004, Weber et al., 2002), NSs of RVFV, (Billecocq et al., 2004, Bouloy et al., 2001, Le May et al., 2004), B18 and B8 of VV (Alcami et al., 2000, Symons et al., 1995), JEV, WNV (Guo et al., 2005, Lin et al., 2004), E6 of HPV18 (Li et al., 1999), core protein of HCV (Bode et al., 2003, Keskinen et al., 2002, Melen et al., 2004, Miller et al., 2004), HSV-1 (Yokota et al., 2004), NSs of RVFV (M. Bouloy, pers. communication), V, C, N of paramyxoviruses (Andrejeva et al., 2002, Didcock et al., 1999, Garcin et al., 2002, Garcin et al., 2004, Gotoh et al., 2003, Kato et al., 2001, Kubota et al., 2002, Palosaari et al., 2003, Parisien et al., 2001, Rodriguez et al., 2002, Rodriguez et al., 2003, Shaffer et al., 2003, Shaw et al., 2004, Takeuchi et al., 2001), NS4B of DHFV, WNV, and YFV (Jones et al., 2005, Munoz-Jordan et al., 2003, Munoz-Jordan et al., 2005), E1A AdV (Leonard and Sen, 1996, Look et al., 1998), NS5A and E2 of HCV (Gale et al., 1998, Taylor et al., 1999), EBER of EBV (Elia et al., 1996), VA of AdV (Kitajewski et al., 1986, Mathews and Shenk, 1991), K3L of VV (Davies et al., 1992). Not shown: Tat and TAR of HIV interfere with PKR activation (Gunnery et al., 1990, Roy et al., 1990). γ34.5 protein of HSV-1 reverts the translational block mediated by PKR (He et al., 1997). NS1 of influenza B virus targets the IFN-induced ubiquitin-like modifier ISG15, the significance of which remains to be established (Krug et al., 2003). The M27 gene product of mouse cytomegalovirus binds and down-regulates STAT-2 (Zimmermann et al., 2005). Unknown gene products of hepatitis A virus and SARS-coronavirus inhibit IRF-3 activation (Fensterl et al., 2005, Spiegel et al., 2005).
In many negative-strand RNA viruses, the phosphoprotein P, an essential component of the viral polymerase complex, is the main IFN antagonist. For example, the P protein of Rabies virus prevents IRF-3 phosphorylation by TBK-1 (Brzozka et al., 2005). Likewise, the P protein (called VP35) of Ebola virus (EBOV) interferes with IRF-3 activation (Basler et al., 2003). The P protein of Borna disease virus (BDV) directly binds to TBK-1 and reduces its activity (Unterstab et al., 2005). A different strategy to block cellular IRF-3 is used by certain herpesviruses. Human herpes virus 8 (HHV-8), the causative agent of Kaposi sarcoma, displays viral IRF homologues, termed vIRFs, which either mimic their cellular counterparts or exert a dominant-negative effect (Burysek et al., 1999a, Burysek et al., 1999b, Li et al., 1998, Lubyova et al., 2004, Lubyova and Pitha, 2000, Zimring et al., 1998). Finally, a far-reaching approach is used by the large poxviruses. They can afford to express soluble IFN-binding proteins which compete with the cellular receptor for its IFN ligand (Alcami et al., 2000, Symons et al., 1995). These “viroceptors” neutralize whatever IFN is secreted from cells. They prevent the autocrine IFN amplification loop and – more importantly – the establishment of an antiviral state also in the non-infected tissue surrounding the IFN-producing cells.
A fourth point worth discussing is the fact that the virus-induced IFN response is generated in a cascade-like manner (Fig. 3). Therefore, viral proteins blocking one component in this circuit also affect distant signaling or effector molecules, thereby amplifying their inhibitory effect. For example, JAK-STAT inhibitors not only suppress the production of antiviral proteins but also the expression of RIG-I, MDA5, IPS-1/MAVS, IRF-3 and IRF-7 which are all IFN-inducible proteins. As a consequence, production of the “second-wave” IFNs is reduced. This enhanced down-regulation of IFN responses acts against the IFN-activating effect of viral dsRNA molecules which progressively accumulate in infected cells. The end-result is a balance between virus-promoting and virus-inhibiting factors which may be optimal for each virus-host relationship.
Interestingly, some lytic viruses causing acute infections have adopted a radical strategy usually not found in non-lytic viruses that persist in the body. Poliovirus, vesicular stomatitis virus (VSV) and some bunyaviruses affect the basic cellular transcription machinery and shut-off host gene transcription (Ahmed et al., 2003, Billecocq et al., 2004, Clark et al., 1993, Le May et al., 2004, Thomas et al., 2004, Weber et al., 2002, Yalamanchili et al., 1996, Yuan et al., 1998). More specifically, the non-structural protein NSs of RVFV has been demonstrated to target the p44 component of the cellular transcription factor IIH (TFIIH) which is an essential cofactor of the cellular RNA polymerase II (RNAP II) (Le May et al., 2004). Likewise, the M protein of VSV inactivates TFIID, another RNAP II cofactor (Yuan et al., 1998). Hence, some viral proteins are suppressors of IFN gene expression through their general inhibitory effect on host gene transcription (Ahmed et al., 2003, Billecocq et al., 2004, Thomas et al., 2004). In addition, as already mentioned, the NSs of RVFV activates SOCS-1 and has a more specific but indirect effect on the JAK-STAT pathway. To be effective, NSs should be expressed at very early time points of infection. This task would seem difficult to achieve, because NSs of RVFV is transcribed in an ambisense coding strategy from the complementary anti-sense S segment which needs first to be synthesized in infected cells. However, a recent report finds that such anti-sense S segment RNAs are packaged into infectious virus particles and can be transcribed into NSs mRNA immediately after delivery to the cell cytoplasm. It is suggested that the immediate availability of NSs protein may provide a selective advantage in giving the virus a “head start” over the innate immune system (Ikegami et al., 2005).
Concluding remarks and outlook
The interplay between viruses and the IFN responses of their hosts, as described here, is most likely the result of an evolutionary race between the two genetic systems. The race is ongoing, as emerging viruses compete for new hosts and attempt trans-species transmission causing zoonotic infections, as illustrated by recent outbreaks of SARS-coronavirus, Hendra and Nipah viruses, Ebola and Marburg viruses, or the threat of transmission of H5N1 avian influenza viruses to humans.
An emerging virus must overcome the IFN defenses of a foreign host to be successful. There is ample evidence that viral IFN antagonists exhibit a degree of species specificity and are efficient in one species but not another (Bossert and Conzelmann, 2002, Chatziandreou et al., 2004, Parisien et al., 2002, Young et al., 2001). However, adaptations are likely to occur. This is best illustrated in arthropod-borne viruses. They replicate in their blood-feeding arthropod hosts and are then transmitted by bite to vertebrate hosts. To continue the transmission cycle, virus replication in the vertebrate host must proceed in the face of a vigorous IFN response. To succeed, arboviruses have evolved specific viral proteins to counteract the IFN system of the vertebrate host, as illustrated in bunyavirus infections (Bouloy et al., 2001, Weber et al., 2002). As invertebrates do not have equivalent IFN genes, these viral IFN antagonists are most likely an adaptation to the mammalian host, securing virus transmission. Another interesting example is Thogoto virus (THOV), an influenza-like virus transmitted by ticks to small rodents and, occasionally, man. Its replication occurs in the cell nucleus and is sensitive to inhibition by IFN-induced mouse Mx1 and human MxA GTPases (Haller et al., 1995, Kochs and Haller, 1999). The Mx block inhibits a very early step in the viral multiplication cycle that affects primary transcription of the incoming genome. Since the virus cannot transcribe and replicate its genome in the presence of Mx, generation of Mx escape mutants is virtually impossible. Therefore, the prime strategy of THOV is to suppress IFN production in the vertebrate host by virtue of its ML protein and to avoid Mx expression in potential target cells, as recently demonstrated in a mouse model (Jennings et al., 2005, Pichlmair et al., 2004).
Future applications involve the genetic manipulation of viral IFN antagonists for the development of novel candidate vaccines. Viruses with targeted deletions in genes known to code for IFN antagonists are promising candidates for live virus vaccines. They grow well in tissue culture but are highly attenuated in the host organism, due to a robust IFN and immune response. This approach has been pioneered for FLUAV (Ferko et al., 2004, Talon et al., 2000b) and for bovine RSV (Valarcher et al., 2003), and may likewise apply to other viruses.
Another fascinating development is the generation of viruses that may be used as oncolytic agents. Mutations in tumor cells often cripple the IFN system, favoring unhindered proliferation and protection from apoptosis (Stojdl et al., 2000). As a consequence, such cells become vulnerable to virus infection. For example, PKR is switched off in tumor cells due to transformation by p21ras (Mundschau and Faller, 1995). Alternatively, defects in translational regulation render tumor cells insensitive to PKR action (Balachandran and Barber, 2004). VSV or wild-type reoviruses are highly sensitive to inhibition by PKR (Balachandran et al., 2000, Strong et al., 1998) and preferentially infect cells which have an inactivated PKR pathway (Balachandran and Barber, 2004, Coffey et al., 1998). Therefore, selectivity for tumor cells can be increased using genetically engineered viruses devoid of anti-IFN proteins. Such viruses are unable to infect IFN-competent body cells, but are still capable of efficiently replicating and destroying the IFN-deficient tumor cells. A VSV variant with mutations in the M gene inactivating its IFN-antagonistic capacity was shown to specifically lyse tumors in immunocompetent mice (Stojdl et al., 2003). Similarly, an HSV-1 lacking the gene for the anti-PKR protein γ34.5 was apathogenic even when administered intracerebrally (Hunter et al., 1999), but it could destroy glioma cells (Mineta et al., 1995).
Our present knowledge of the IFN system and viral countermeasures is still limited. However, a better understanding of the intimate interplay between viruses and innate immune defenses will open new avenues for drug design, vaccine development and anti-cancer strategies.
Acknowledgments
We thank Peter Staeheli for helpful comments. Our own work described in the text was supported be grants from the Deutsche Forschungsgemeinschaft and by the Land Baden-Württemberg.
References
- Ahmed M., McKenzie M.O., Puckett S., Hojnacki M., Poliquin L., Lyles D.S. Ability of the matrix protein of vesicular stomatitis virus to suppress Beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 2003;77(8):4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcami A., Symons J.A., Smith G.L. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 2000;74(23):11230–11239. doi: 10.1128/jvi.74.23.11230-11239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.L., Carton J.M., Lou J., Xing L., Rubin B.Y. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology. 1999;256(1):8–14. doi: 10.1006/viro.1999.9614. [DOI] [PubMed] [Google Scholar]
- Andrejeva J., Young D.F., Goodbourn S., Randall R.E. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 2002;76(5):2159–2167. doi: 10.1128/jvi.76.5.2159-2167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejeva J., Childs K.S., Young D.F., Carlos T.S., Stock N., Goodbourn S., Randall R.E. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U.S.A. 2004;101(49):17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheiter H., Skuntz S., Noteborn M., Chang S., Meier E. Transgenic mice with intracellular immunity to influenza virus. Cell. 1990;62(1):51–61. doi: 10.1016/0092-8674(90)90239-b. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Frese M., Kambadur R., Meier E., Haller O. Mx transgenic mice-animal models of health. Curr. Top. Microbiol. Immunol. 1996;206:119–147. doi: 10.1007/978-3-642-85208-4_8. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C., Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J. Exp. Med. 2005;202(4):461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S., Barber G.N. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5(1):51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Balachandran S., Roberts P.C., Brown L.E., Truong H., Pattnaik A.K., Archer D.R., Barber G.N. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13(1):129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- Basler C.F., Garcia-Sastre A. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 2002;21(4-5):305–337. doi: 10.1080/08830180213277. [DOI] [PubMed] [Google Scholar]
- Basler C.F., Mikulasova A., Martinez-Sobrido L., Paragas J., Muhlberger E., Bray M., Klenk H.D., Palese P., Garcia-Sastre A. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 2003;77(14):7945–7956. doi: 10.1128/JVI.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430(6996):257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Billecocq A., Spiegel M., Vialat P., Kohl A., Weber F., Bouloy M., Haller O. NSs protein of Rift Valley Fever Virus blocks interferon production by inhibiting host gene transcription. J. Virol. 2004;78:9798–9806. doi: 10.1128/JVI.78.18.9798-9806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode J.G., Ludwig S., Ehrhardt C., Albrecht U., Erhardt A., Schaper F., Heinrich P.C., Haussinger D. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17(3):488–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- Bossert B., Conzelmann K.K. Respiratory syncytial virus (RSV) nonstructural (NS) proteins as host range determinants: a chimeric bovine RSV with NS genes from human RSV is attenuated in interferon-competent bovine cells. J. Virol. 2002;76(9):4287–4293. doi: 10.1128/JVI.76.9.4287-4293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert B., Marozin S., Conzelmann K.K. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 2003;77(16):8661–8668. doi: 10.1128/JVI.77.16.8661-8668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Janzen C., Vialat P., Khun H., Pavlovic J., Huerre M., Haller O. Genetic evidence for an interferon-antagonistic function of rift valley fever virus nonstructural protein NSs. J. Virol. 2001;75(3):1371–1377. doi: 10.1128/JVI.75.3.1371-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie A.G., Haga I.R. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 2005;42(8):859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Bray M. The role of the Type I interferon response in the resistance of mice to filovirus infection. J. Gen. Virol. 2001;82(Pt 6):1365–1373. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- Breiman A., Grandvaux N., Lin R., Ottone C., Akira S., Yoneyama M., Fujita T., Hiscott J., Meurs E.F. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKepsilon. J. Virol. 2005;79(7):3969–3978. doi: 10.1128/JVI.79.7.3969-3978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton M.A., Perelygin A.A. Genetic resistance to flaviviruses. Adv. Virus Res. 2003;60:43–85. doi: 10.1016/s0065-3527(03)60002-3. [DOI] [PubMed] [Google Scholar]
- Brzozka K., Finke S., Conzelmann K.K. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J. Virol. 2005;79(12):7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burysek L., Yeow W.S., Lubyova B., Kellum M., Schafer S.L., Huang Y.Q., Pitha P.M. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 1999;73(9):7334–7342. doi: 10.1128/jvi.73.9.7334-7342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burysek L., Yeow W.S., Pitha P.M. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) J. Hum. Virol. 1999;2(1):19–32. [PubMed] [Google Scholar]
- Chatziandreou N., Stock N., Young D., Andrejeva J., Hagmaier K., McGeoch D.J., Randall R.E. Relationships and host range of human, canine, simian and porcine isolates of simian virus 5 (parainfluenza virus 5) J. Gen. Virol. 2004;85(Pt 10):3007–3016. doi: 10.1099/vir.0.80200-0. [DOI] [PubMed] [Google Scholar]
- Chu W.M., Ostertag D., Li Z.W., Chang L., Chen Y., Hu Y., Williams B., Perrault J., Karin M. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11(6):721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- Clark M.E., Lieberman P.M., Berk A.J., Dasgupta A. Direct cleavage of human TATA-binding protein by poliovirus protease 3C in vivo and in vitro. Mol. Cell. Biol. 1993;13(2):1232–1237. doi: 10.1128/mcb.13.2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey M.C., Strong J.E., Forsyth P.A., Lee P.W. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282(5392):1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Colonna M., Krug A., Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 2002;14(3):373–379. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- Conzelmann K.K. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J. Virol. 2005;79(9):5241–5248. doi: 10.1128/JVI.79.9.5241-5248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauber B., Heins G., Wolff T. The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction. J. Virol. 2004;78(4):1865–1872. doi: 10.1128/JVI.78.4.1865-1872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M.V., Elroy-Stein O., Jagus R., Moss B., Kaufman R.J. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 1992;66(4):1943–1950. doi: 10.1128/jvi.66.4.1943-1950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veer M.J., Holko M., Frevel M., Walker E., Der S., Paranjape J.M., Silverman R.H., Williams B.R. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukocyte Biol. 2001;69(6):912–920. [PubMed] [Google Scholar]
- Delhaye S., van Pesch V., Michiels T. The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins. J. Virol. 2004;78(8):4357–4362. doi: 10.1128/JVI.78.8.4357-4362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der S.D., Zhou A., Williams B.R., Silverman R.H. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U.S.A. 1998;95(26):15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didcock L., Young D.F., Goodbourn S., Randall R.E. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 1999;73(12):9928–9933. doi: 10.1128/jvi.73.12.9928-9933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold S.S., Montoya M., Unger H., Alexopoulou L., Roy P., Haswell L.E., Al-Shamkhani A., Flavell R., Borrow P., Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424(6946):324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- Dostert C., Jouanguy E., Irving P., Troxler L., Galiana-Arnoux D., Hetru C., Hoffmann J.A., Imler J.L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005;6(9):946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Dupuis S., Jouanguy E., Al-Hajjar S., Fieschi C., Al-Mohsen I.Z., Al-Jumaah S., Yang K., Chapgier A., Eidenschenk C., Eid P., Al Ghonaium A., Tufenkeji H., Frayha H., Al-Gazlan S., Al-Rayes H., Schreiber R.D., Gresser I., Casanova J.L. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003;33(3):388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- Elia A., Laing K.G., Schofield A., Tilleray V.J., Clemens M.J. Regulation of the double-stranded RNA-dependent protein kinase PKR by RNAs encoded by a repeated sequence in the Epstein–Barr virus genome. Nucleic Acids Res. 1996;24(22):4471–4478. doi: 10.1093/nar/24.22.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espert L., Degols G., Gongora C., Blondel D., Williams B.R., Silverman R.H., Mechti N. ISG20, a new interferon-induced RNase specific for single-stranded RNA, defines an alternative antiviral pathway against RNA genomic viruses. J. Biol. Chem. 2003;278(18):16151–16158. doi: 10.1074/jbc.M209628200. [DOI] [PubMed] [Google Scholar]
- Fensterl V., Grotheer D., Berk I., Schlemminger S., Vallbracht A., Dotzauer A. Hepatitis A virus suppresses RIG-I-mediated IRF-3 activation to block induction of beta interferon. J. Virol. 2005;79(17):10968–10977. doi: 10.1128/JVI.79.17.10968-10977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferko B., Stasakova J., Romanova J., Kittel C., Sereinig S., Katinger H., Egorov A. Immunogenicity and protection efficacy of replication-deficient influenza A viruses with altered NS1 genes. J. Virol. 2004;78(23):13037–13045. doi: 10.1128/JVI.78.23.13037-13045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K.A., McWhirter S.M., Faia K.L., Rowe D.C., Latz E., Golenbock D.T., Coyle A.J., Liao S.M., Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Foy E., Li K., Sumpter R., Jr., Loo Y.M., Johnson C.L., Wang C., Fish P.M., Yoneyama M., Fujita T., Lemon S.M., Gale M., Jr. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. U.S.A. 2005;102(8):2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M., Jr., Foy E.M. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436(7053):939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- Gale M.J., Jr., Korth M.J., Katze M.G. Repression of the PKR protein kinase by the hepatitis C virus NS5A protein: a potential mechanism of interferon resistance. Clin. Diagn. Virol. 1998;10(2–3):157–162. doi: 10.1016/s0928-0197(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A. Inhibition of interferon-mediated antiviral responses by Influenza A viruses and other negative-strand RNA viruses. Virology. 2001;279(2):375–384. doi: 10.1006/viro.2000.0756. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A., Egorov A., Matassov D., Brandt S., Levy D.E., Durbin J.E., Palese P., Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252(2):324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- Garcin D., Marq J.B., Strahle L., le Mercier P., Kolakofsky D. All four Sendai Virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology. 2002;295(2):256–265. doi: 10.1006/viro.2001.1342. [DOI] [PubMed] [Google Scholar]
- Garcin D., Marq J.B., Iseni F., Martin S., Kolakofsky D. A short peptide at the amino terminus of the Sendai virus C protein acts as an independent element that induces STAT1 instability. J. Virol. 2004;78(16):8799–8811. doi: 10.1128/JVI.78.16.8799-8811.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh B., Takeuchi K., Komatsu T., Yokoo J. The STAT2 activation process is a crucial target of Sendai virus C protein for the blockade of alpha interferon signaling. J. Virol. 2003;77(6):3360–3370. doi: 10.1128/JVI.77.6.3360-3370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder F.B., Vogel S.N. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology. 1999;257(1):106–118. doi: 10.1006/viro.1999.9662. [DOI] [PubMed] [Google Scholar]
- Gunnery S., Rice A.P., Robertson H.D., Mathews M.B. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1990;87(22):8687–8691. doi: 10.1073/pnas.87.22.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Hui D.J., Merrick W.C., Sen G.C. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 2000;19(24):6891–6899. doi: 10.1093/emboj/19.24.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.T., Hayashi J., Seeger C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 2005;79(3):1343–1350. doi: 10.1128/JVI.79.3.1343-1350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A.M., Huye L.E., Ning S., Webster-Cyriaque J., Pagano J.S. Interferon regulatory factor 7 is negatively regulated by the Epstein–Barr virus immediate-early gene, BZLF-1. J. Virol. 2005;79(15):10040–10052. doi: 10.1128/JVI.79.15.10040-10052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O. Inborn resistance of mice to orthomyxoviruses. Curr. Top. Microbiol. Immunol. 1981;92:25–52. doi: 10.1007/978-3-642-68069-4_3. [DOI] [PubMed] [Google Scholar]
- Haller O., Kochs G. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic. 2002;3(10):710–717. doi: 10.1034/j.1600-0854.2002.31003.x. [DOI] [PubMed] [Google Scholar]
- Haller O., Frese M., Rost D., Nuttall P.A., Kochs G. Tick-borne thogoto virus infection in mice is inhibited by the orthomyxovirus resistance gene product Mx1. J. Virol. 1995;69(4):2596–2601. doi: 10.1128/jvi.69.4.2596-2601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Frese M., Kochs G. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Tech. 1998;17(1):220–230. doi: 10.20506/rst.17.1.1084. [DOI] [PubMed] [Google Scholar]
- He B., Gross M., Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1997;94(3):843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti H.P., Frese M., Landis H., Di Paolo C., Aguzzi A., Haller O., Pavlovic J. Human MxA protein protects mice lacking a functional alpha/beta interferon system against La Crosse virus and other lethal viral infections. J. Virol. 1999;73(8):6984–6991. doi: 10.1128/jvi.73.8.6984-6991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengel H., Koszinowski U.H., Conzelmann K.K. Viruses know it all: new insights into IFN networks. Trends Immunol. 2005;26(7):396–401. doi: 10.1016/j.it.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Pitha P., Genin P., Nguyen H., Heylbroeck C., Mamane Y., Algarte M., Lin R. Triggering the interferon response: the role of IRF-3 transcription factor. J. Interferon Cytokine Res. 1999;19(1):1–13. doi: 10.1089/107999099314360. [DOI] [PubMed] [Google Scholar]
- Honda K., Ohba Y., Yanai H., Negishi H., Mizutani T., Takaoka A., Taya C., Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434(7036):1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Hui D.J., Bhasker C.R., Merrick W.C., Sen G.C. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2, GTP.Met-tRNAi. J. Biol. Chem. 2003;278(41):39477–39482. doi: 10.1074/jbc.M305038200. [DOI] [PubMed] [Google Scholar]
- Hunter W.D., Martuza R.L., Feigenbaum F., Todo T., Mineta T., Yazaki T., Toda M., Newsome J.T., Platenberg R.C., Manz H.J., Rabkin S.D. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J. Virol. 1999;73(8):6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.Y., Hertzog P.J., Holland K.A., Sumarsono S.H., Tymms M.J., Hamilton J.A., Whitty G., Bertoncello I., Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. U.S.A. 1995;92(24):11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T., Wong S., Peters C.J., Makino S. Rift valley fever virus NSs mRNA is transcribed from an incoming anti-viral-sense RNA segment. J. Virol. 2005;79(18):12106–12111. doi: 10.1128/JVI.79.18.12106-12111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A., Lindenmann J. Virus interference: I. The interferon. Proc. R. Soc. Lond., B Biol. Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Iwamura T., Yoneyama M., Yamaguchi K., Suhara W., Mori W., Shiota K., Okabe Y., Namiki H., Fujita T. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells. 2001;6(4):375–388. doi: 10.1046/j.1365-2443.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jennings S., Martínez-Sobrido L., García-Sastre A., Weber F., Kochs G. Thogoto Virus ML protein suppresses IRF3 function. Virology. 2005;331(1):63–72. doi: 10.1016/j.virol.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Jones M., Davidson A., Hibbert L., Gruenwald P., Schlaak J., Ball S., Foster G.R., Jacobs M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 2005;79(9):5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Ohnishi Y., Kohase M., Saito S., Tashiro M., Nagai Y. Y2, the smallest of the Sendai virus C proteins, is fully capable of both counteracting the antiviral action of interferons and inhibiting viral RNA synthesis. J. Virol. 2001;75(8):3802–3810. doi: 10.1128/JVI.75.8.3802-3810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Sato S., Yoneyama M., Yamamoto M., Uematsu S., Matsui K., Tsujimura T., Takeda K., Fujita T., Takeuchi O., Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23(1):19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K.J., Takeuchi O., Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005:16127453. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Kerkmann M., Rothenfusser S., Hornung V., Towarowski A., Wagner M., Sarris A., Giese T., Endres S., Hartmann G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 2003;170(9):4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- Keskinen P., Melen K., Julkunen I. Expression of HCV structural proteins impairs IFN-mediated antiviral response. Virology. 2002;299(2):164–171. doi: 10.1006/viro.2002.1527. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R.J., Safer B., Munemitsu S.M., Samuel C.E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Kochs G., Haller O. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc. Natl. Acad. Sci. U.S.A. 1999;96(5):2082–2086. doi: 10.1073/pnas.96.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R.M., Yuan W., Noah D.L., Latham A.G. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology. 2003;309(2):181–189. doi: 10.1016/s0042-6822(03)00119-3. [DOI] [PubMed] [Google Scholar]
- Kubo M., Hanada T., Yoshimura A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003;4(12):1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Kubota T., Yokosawa N., Yokota S., Fujii N. Association of mumps virus V protein with RACK1 results in dissociation of STAT-1 from the alpha interferon receptor complex. J. Virol. 2002;76(24):12676–12682. doi: 10.1128/JVI.76.24.12676-12682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rocca S.A., Herbert R.J., Crooke H., Drew T.W., Wileman T.E., Powell P.P. Loss of interferon regulatory factor 3 in cells infected with classical swine fever virus involves the N-terminal protease, Npro. J. Virol. 2005;79(11):7239–7247. doi: 10.1128/JVI.79.11.7239-7247.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A., Tough D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 2002;14(4):432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Le May N., Dubaele S., De Santis L.P., Billecocq A., Bouloy M., Egly J.M. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell. 2004;116(4):541–550. doi: 10.1016/s0092-8674(04)00132-1. [DOI] [PubMed] [Google Scholar]
- Leonard G.T., Sen G.C. Effects of adenovirus E1A protein on interferon-signaling. Virology. 1996;224(1):25–33. doi: 10.1006/viro.1996.0503. [DOI] [PubMed] [Google Scholar]
- Li M., Lee H., Guo J., Neipel F., Fleckenstein B., Ozato K., Jung J.U. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 1998;72(7):5433–5440. doi: 10.1128/jvi.72.7.5433-5440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Labrecque S., Gauzzi M.C., Cuddihy A.R., Wong A.H., Pellegrini S., Matlashewski G.J., Koromilas A.E. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18(42):5727–5737. doi: 10.1038/sj.onc.1202960. [DOI] [PubMed] [Google Scholar]
- Li K., Foy E., Ferreon J.C., Nakamura M., Ferreon A.C., Ikeda M., Ray S.C., Gale M., Jr., Lemon S.M. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. U.S.A. 2005;102(8):2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Heylbroeck C., Pitha P.M., Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 1998;18(5):2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.J., Liao C.L., Lin E., Lin Y.L. Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus infection. J. Virol. 2004;78(17):9285–9294. doi: 10.1128/JVI.78.17.9285-9294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmann J. Interferon und inverse Interferenz. Zeitschr. Hygiene. 1960;146:287–309. [PubMed] [Google Scholar]
- Lindenmann J. Inheritance of Resistance to Influenza Virus in Mice. Proc. Soc. Exp. Biol. Med. 1964;116:506–509. doi: 10.3181/00379727-116-29292. [DOI] [PubMed] [Google Scholar]
- Look D.C., Roswit W.T., Frick A.G., Gris-Alevy Y., Dickhaus D.M., Walter M.J., Holtzman M.J. Direct suppression of Stat1 function during adenoviral infection. Immunity. 1998;9(6):871–880. doi: 10.1016/s1074-7613(00)80652-4. [DOI] [PubMed] [Google Scholar]
- Lubyova B., Pitha P.M. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 2000;74(17):8194–8201. doi: 10.1128/jvi.74.17.8194-8201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubyova B., Kellum M.J., Frisancho A.J., Pitha P.M. Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7. J. Biol. Chem. 2004;279(9):7643–7654. doi: 10.1074/jbc.M309485200. [DOI] [PubMed] [Google Scholar]
- Lucas M., Mashimo T., Frenkiel M.P., Simon-Chazottes D., Montagutelli X., Ceccaldi P.E., Guenet J.L., Despres P. Infection of mouse neurones by West Nile virus is modulated by the interferon-inducible 2′-5′ oligoadenylate synthetase 1b protein. Immunol. Cell Biol. 2003;81(3):230–236. doi: 10.1046/j.1440-1711.2003.01166.x. [DOI] [PubMed] [Google Scholar]
- Marie I., Durbin J.E., Levy D.E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17(22):6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T., Lucas M., Simon-Chazottes D., Frenkiel M.P., Montagutelli X., Ceccaldi P.E., Deubel V., Guenet J.L., Despres P. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99(17):11311–11316. doi: 10.1073/pnas.172195399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M.B., Shenk T. Adenovirus virus-associated RNA and translation control. J. Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda A., Suzuki Y., Honda G., Muramatsu S., Matsuzaki O., Nagano Y., Doi T., Shimotohno K., Harada T., Nishida E., Hayashi H., Sugano S. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22(21):3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- Melen K., Fagerlund R., Nyqvist M., Keskinen P., Julkunen I. Expression of hepatitis C virus core protein inhibits interferon-induced nuclear import of STATs. J. Med. Virol. 2004;73(4):536–547. doi: 10.1002/jmv.20123. [DOI] [PubMed] [Google Scholar]
- Miller K., McArdle S., Gale M.J., Jr., Geller D.A., Tenoever B., Hiscott J., Gretch D.R., Polyak S.J. Effects of the hepatitis C virus core protein on innate cellular defense pathways. J. Interferon Cytokine Res. 2004;24(7):391–402. doi: 10.1089/1079990041535647. [DOI] [PubMed] [Google Scholar]
- Mineta T., Rabkin S.D., Yazaki T., Hunter W.D., Martuza R.L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Mundschau L.J., Faller D.V. Platelet-derived growth factor signal transduction through the interferon-inducible kinase PKR. Immediate early gene induction. J. Biol. Chem. 1995;270(7):3100–3106. doi: 10.1074/jbc.270.7.3100. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan J.L., Sanchez-Burgos G.G., Laurent-Rolle M., Garcia-Sastre A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. U.S.A. 2003;100(24):14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Jordan J.L., Laurent-Rolle M., Ashour J., Martinez-Sobrido L., Ashok M., Lipkin W.I., Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005;79(13):8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palosaari H., Parisien J.P., Rodriguez J.J., Ulane C.M., Horvath C.M. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 2003;77(13):7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisien J.P., Lau J.F., Rodriguez J.J., Sullivan B.M., Moscona A., Parks G.D., Lamb R.A., Horvath C.M. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology. 2001;283(2):230–239. doi: 10.1006/viro.2001.0856. [DOI] [PubMed] [Google Scholar]
- Parisien J.P., Lau J.F., Horvath C.M. STAT2 acts as a host range determinant for species-specific paramyxovirus interferon antagonism and simian virus 5 replication. J. Virol. 2002;76(13):6435–6441. doi: 10.1128/JVI.76.13.6435-6441.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.S., Shaw M.L., Munoz-Jordan J., Cros J.F., Nakaya T., Bouvier N., Palese P., Garcia-Sastre A., Basler C.F. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V.W, and C proteins. J. Virol. 2003;77(2):1501–1511. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Arzet H.A., Hefti H.P., Frese M., Rost D., Ernst B., Kolb E., Staeheli P., Haller O. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J. Virol. 1995;69(7):4506–4510. doi: 10.1128/jvi.69.7.4506-4510.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelygin A.A., Scherbik S.V., Zhulin I.B., Stockman B.M., Li Y., Brinton M.A. Positional cloning of the murine flavivirus resistance gene. Proc. Natl. Acad. Sci. U.S.A. 2002;99(14):9322–9327. doi: 10.1073/pnas.142287799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A., Buse J., Jennings S., Haller O., Kochs G., Staeheli P. Thogoto virus lacking interferon-antagonistic protein ML is strongly attenuated in newborn Mx1-positive but not Mx1-negative mice. J. Virol. 2004;78:11422–11424. doi: 10.1128/JVI.78.20.11422-11424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A., Smith E., Lee C.K., Levy D.E. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J. Biol. Chem. 2005;280(19):18651–18657. doi: 10.1074/jbc.M501289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precious B., Young D.F., Andrejeva L., Goodbourn S., Randall R.E. In vitro and in vivo specificity of ubiquitination and degradation of STAT1 and STAT2 by the V proteins of the paramyxoviruses simian virus 5 and human parainfluenza virus type 2. J. Gen. Virol. 2005;86(Pt 1):151–158. doi: 10.1099/vir.0.80263-0. [DOI] [PubMed] [Google Scholar]
- Regad T., Saib A., Lallemand-Breitenbach V., Pandolfi P.P., de The H., Chelbi-Alix M.K. PML mediates the interferon-induced antiviral state against a complex retrovirus via its association with the viral transactivator. Embo J. 2001;20(13):3495–3505. doi: 10.1093/emboj/20.13.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.J., Parisien J.P., Horvath C.M. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 2002;76(22):11476–11483. doi: 10.1128/JVI.76.22.11476-11483.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.J., Wang L.F., Horvath C.M. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J. Virol. 2003;77(21):11842–11845. doi: 10.1128/JVI.77.21.11842-11845.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco L.V., Karpova A.Y., Vidal M., Howley P.M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12(13):2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfusser, S., Goutagny, N., DiPerna, G., Gong, M., Monks, B.G., Schoenemeyer, A., Yamamoto, M., Akira, S., Fitzgerald, K.A. in press. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by RIG-I. J. Immunol. [DOI] [PubMed]
- Roy S., Katze M.G., Parkin N.T., Edery I., Hovanessian A.G., Sonenberg N. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science. 1990;247(4947):1216–1219. doi: 10.1126/science.2180064. [DOI] [PubMed] [Google Scholar]
- Ruggli N., Tratschin J.D., Schweizer M., McCullough K.C., Hofmann M.A., Summerfield A. Classical swine fever virus interferes with cellular antiviral defense: evidence for a novel function of N(pro) J. Virol. 2003;77(13):7645–7654. doi: 10.1128/JVI.77.13.7645-7654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggli N., Bird B.H., Liu L., Bauhofer O., Tratschin J.D., Hofmann M.A. N(pro) of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-alpha/beta induction. Virology. 2005 doi: 10.1016/j.virol.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Ryman K.D., Klimstra W.B., Nguyen K.B., Biron C.A., Johnston R.E. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 2000;74(7):3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Hata N., Asagiri M., Nakaya T., Taniguchi T., Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441(1):106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- Sato M., Suemori H., Hata N., Asagiri M., Ogasawara K., Nakao K., Nakaya T., Katsuki M., Noguchi S., Tanaka N., Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13(4):539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Schafer S.L., Lin R., Moore P.A., Hiscott J., Pitha P.M. Regulation of type I interferon gene expression by interferon regulatory factor-3. J. Biol. Chem. 1998;273(5):2714–2720. doi: 10.1074/jbc.273.5.2714. [DOI] [PubMed] [Google Scholar]
- Schlender J., Hornung V., Finke S., Gunthner-Biller M., Marozin S., Brzozka K., Moghim S., Endres S., Hartmann G., Conzelmann K.K. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 2005;79(9):5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O., Diebold S.S., Chen M., Naslund T.I., Nolte M.A., Alexopoulou L., Azuma Y.T., Flavell R.A., Liljestrom P., Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433(7028):887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Seth R.B., Sun L., Ea C.K., Chen Z.J. Identification and Characterization of MAVS, a Mitochondrial Antiviral Signaling Protein that Activates NF-kappaB and IRF3. Cell. 2005:16125763. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Shaffer J.A., Bellini W.J., Rota P.A. The C protein of measles virus inhibits the type I interferon response. Virology. 2003;315(2):389–397. doi: 10.1016/s0042-6822(03)00537-3. [DOI] [PubMed] [Google Scholar]
- Sharma S., TenOever B.R., Grandvaux N., Zhou G.P., Lin R., Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Shaw M.L., Garcia-Sastre A., Palese P., Basler C.F. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J. Virol. 2004;78(11):5633–5641. doi: 10.1128/JVI.78.11.5633-5641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K., Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat. Rev., Immunol. 2005;5(8):593–605. doi: 10.1038/nri1667. [DOI] [PubMed] [Google Scholar]
- Silverman R.H. Fascination with 2-5A-dependent RNase: a unique enzyme that functions in interferon action. J. Interferon Res. 1994;14(3):101–104. doi: 10.1089/jir.1994.14.101. [DOI] [PubMed] [Google Scholar]
- Smith E.J., Marie I., Prakash A., Garcia-Sastre A., Levy D.E. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 2001;276(12):8951–8957. doi: 10.1074/jbc.M008717200. [DOI] [PubMed] [Google Scholar]
- Spann K.M., Tran K.C., Chi B., Rabin R.L., Collins P.L. Suppression of the induction of alpha, beta, and gamma interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 2004;78(8):4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M., Pichlmair A., Martinez-Sobrido L., Cros J., Garcia-Sastre A., Haller O., Weber F. Inhibition of beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005;79(4):2079–2086. doi: 10.1128/JVI.79.4.2079-2086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Grob R., Meier E., Sutcliffe J.G., Haller O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell. Biol. 1988;8(10):4518–4523. doi: 10.1128/mcb.8.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G.R., Kerr I.M., Williams B.R., Silverman R.H., Schreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6(7):821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F., Lichty B.D., tenOever B.R., Paterson J.M., Power A.T., Knowles S., Marius R., Reynard J., Poliquin L., Atkins H., Brown E.G., Durbin R.K., Durbin J.E., Hiscott J., Bell J.C. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Strong J.E., Coffey M.C., Tang D., Sabinin P., Lee P.W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17(12):3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara W., Yoneyama M., Kitabayashi I., Fujita T. Direct involvement of CREB-binding protein/p300 in sequence-specific DNA binding of virus-activated interferon regulatory factor-3 holocomplex. J. Biol. Chem. 2002;277(25):22304–22313. doi: 10.1074/jbc.M200192200. [DOI] [PubMed] [Google Scholar]
- Symons J.A., Alcami A., Smith G.L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81(4):551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Komatsu T., Yokoo J., Kato A., Shioda T., Nagai Y., Gotoh B. Sendai virus C protein physically associates with Stat1. Genes Cells. 2001;6(6):545–557. doi: 10.1046/j.1365-2443.2001.00442.x. [DOI] [PubMed] [Google Scholar]
- Talon J., Horvath C.M., Polley R., Basler C.F., Muster T., Palese P., Garcia-Sastre A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 2000;74(17):7989–7996. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon J., Salvatore M., O'Neill R.E., Nakaya Y., Zheng H., Muster T., Garcia-Sastre A., Palese P. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc. Natl. Acad. Sci. U.S.A. 2000;97(8):4309–4314. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.R., Shi S.T., Romano P.R., Barber G.N., Lai M.M. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285(5424):107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- Taylor D.R., Puig M., Darnell M.E., Mihalik K., Feinstone S.M. New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1. J. Virol. 2005;79(10):6291–6298. doi: 10.1128/JVI.79.10.6291-6298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tenOever B.R., Sharma S., Zou W., Sun Q., Grandvaux N., Julkunen I., Hemmi H., Yamamoto M., Akira S., Yeh W.C., Lin R., Hiscott J. Activation of TBK1 and IKK epsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J. Virol. 2004;78(19):10636–10649. doi: 10.1128/JVI.78.19.10636-10649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Blakqori G., Wagner V., Banholzer M., Kessler N., Elliott R.M., Haller O., Weber F. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 2004;279:31471–31477. doi: 10.1074/jbc.M400938200. [DOI] [PubMed] [Google Scholar]
- Tsujimura H., Tamura T., Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J. Immunol. 2003;170(3):1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- Uematsu S., Sato S., Yamamoto M., Hirotani T., Kato H., Takeshita F., Matsuda M., Coban C., Ishii K.J., Kawai T., Takeuchi O., Akira S. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J. Exp. Med. 2005;201(6):915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulane C.M., Kentsis A., Cruz C.D., Parisien J.P., Schneider K.L., Horvath C.M. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J. Virol. 2005;79(16):10180–10189. doi: 10.1128/JVI.79.16.10180-10189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterstab G., Ludwig S., Anton A., Planz O., Dauber B., Krappmann D., Heins G., Ehrhardt C., Wolff T. Viral targeting of the interferon-beta inducing Traf family member-associated NF-kB activator-(TANK) binding kinase-1. Proc. Natl. Acad. Sci. 2005;102(38):13640–13645. doi: 10.1073/pnas.0502883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valarcher J.F., Furze J., Wyld S., Cook R., Conzelmann K.K., Taylor G. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J. Virol. 2003;77(15):8426–8439. doi: 10.1128/JVI.77.15.8426-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M.F., Muller U., Huang S., Aguet M., Zinkernagel R.M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995;69(8):4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pesch V., van Eyll O., Michiels T. The leader protein of Theiler's virus inhibits immediate-early alpha/beta interferon production. J. Virol. 2001;75(17):7811–7817. doi: 10.1128/JVI.75.17.7811-7817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li M., Zheng H., Muster T., Palese P., Beg A.A., Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J. Virol. 2000;74(24):11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pflugheber J., Sumpter R., Jr., Sodora D.L., Hui D., Sen G.C., Gale M., Jr. Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication. J. Virol. 2003;77(7):3898–3912. doi: 10.1128/JVI.77.7.3898-3912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathelet M.G., Lin C.H., Parekh B.S., Ronco L.V., Howley P.M., Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell. 1998;1(4):507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- Weaver B.K., Kumar K.P., Reich N.C. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 1998;18(3):1359–1368. doi: 10.1128/mcb.18.3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Bridgen A., Fazakerley J.K., Streitenfeld H., Randall R.E., Elliott R.M. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 2002;76:7949–7955. doi: 10.1128/JVI.76.16.7949-7955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Kochs G., Haller O. Inverse interference: how viruses fight the interferon system. Viral. Immunol. 2004;17(4):498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- Williams B.R. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18(45):6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Condit R.C., Vijaysri S., Jacobs B., Williams B.R., Silverman R.H. Blockade of Interferon Induction and Action by the E3L Double-Stranded RNA Binding Proteins of Vaccinia Virus. J. Virol. 2002;76(10):5251–5259. doi: 10.1128/JVI.76.10.5251-5259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili P., Harris K., Wimmer E., Dasgupta A. Inhibition of basal transcription by poliovirus: a virus-encoded protease (3Cpro) inhibits formation of TBP-TATA box complex in vitro. J. Virol. 1996;70(5):2922–2929. doi: 10.1128/jvi.70.5.2922-2929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., Reis L.F., Pavlovic J., Aguzzi A., Schafer R., Kumar A., Williams B.R., Aguet M., Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14(24):6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Yokosawa N., Okabayashi T., Suzutani T., Miura S., Jimbow K., Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 2004;78(12):6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Suhara W., Fukuhara Y., Fukuda M., Nishida E., Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17(4):1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.M., Gale M., Jr., Akira S., Yonehara S., Kato A., Fujita T. Shared and unique functions of the DExD/H-Box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175(5):2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- Young D.F., Chatziandreou N., He B., Goodbourn S., Lamb R.A., Randall R.E. Single amino acid substitution in the V protein of simian virus 5 differentiates its ability to block interferon signaling in human and murine cells. J. Virol. 2001;75(7):3363–3370. doi: 10.1128/JVI.75.7.3363-3370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Yoza B.K., Lyles D.S. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology. 1998;251(2):383–392. doi: 10.1006/viro.1998.9413. [DOI] [PubMed] [Google Scholar]
- Zhou A., Paranjape J., Brown T.L., Nie H., Naik S., Dong B., Chang A., Trapp B., Fairchild R., Colmenares C., Silverman R.H. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16(21):6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A., Paranjape J.M., Der S.D., Williams B.R., Silverman R.H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258(2):435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- Zhu F.X., King S.M., Smith E.J., Levy D.E., Yuan Y. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. U.S.A. 2002;99(8):5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann A., Trilling M., Wagner M., Wilborn M., Bubic I., Jonjic S., Koszinowski U., Hengel H. A cytomegaloviral protein reveals a dual role for STAT2 in IFN-{gamma} signaling and antiviral responses. J. Exp. Med. 2005;201(10):1543–1553. doi: 10.1084/jem.20041401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimring J.C., Goodbourn S., Offermann M.K. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J. Virol. 1998;72(1):701–707. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


