Abstract
Formins direct the elongation of unbranched actin filaments by binding their barbed ends and processively stepping onto incoming actin monomers to incorporate them into the filament. Binding of profilin to actin monomers creates profilin–actin complexes, which then bind polyproline tracts located in formin homology 1 (FH1) domains. Diffusion of these natively disordered domains enables direct delivery of profilin–actin to the barbed end, speeding the rate of filament elongation. In this study, we investigated the mechanism of coordinated actin delivery from the multiple polyproline tracts in formin FH1 domains. We found that each polyproline tract can efficiently mediate polymerization, but that all tracts do not generate the same rate of elongation. In WT FH1 domains, the multiple polyproline tracts compete to deliver profilin–actin to the barbed end. This competition ultimately limits the rate of formin-mediated elongation. We propose that intrinsic properties of the filament-binding FH2 domain tune the efficiency of FH1-mediated elongation by directly regulating the rate of monomer incorporation at the barbed end. A strong correlation between competitive FH1-mediated profilin–actin delivery and FH2-regulated gating of the barbed end effectively limits the elongation rate, thereby obviating the need for evolutionary optimization of FH1 domain sequences.
Keywords: formin, actin, profilin, fluorescence, microscopy, polyproline tract
Introduction
Dynamic actin cytoskeletal assembly supports cell growth, motility, division, the establishment of cellular polarity, and cell–cell communication. The formin family of proteins nucleates and regulates the elongation of unbranched actin filaments that are incorporated into higher-order cytoskeletal structures that support these essential processes. Examples of such structures include filopodia, cytokinetic rings, polarized actin cables, and stress fibers that play a role in adhesion maturation and nuclear positioning (1–3). Most eukaryotic organisms express multiple formin isoforms, which typically perform nonredundant biological functions (4, 5).
Formins processively regulate actin polymerization by associating with filament barbed ends via their dimeric formin homology 2 (FH2)2 domains and directly modulating the rate of subunit addition (6–12). Barbed-end–bound FH2 dimers fluctuate between polymerization-competent “open” conformations and polymerization-incompetent “closed” conformations (8, 13, 14). When a formin is in an open conformation, an actin subunit can bind the barbed end. Subsequent stepping of one of the FH2 domains onto the newly-bound subunit incorporates it into the filament without necessitating full dissociation of the formin from the barbed end (13, 15–17). The probability of finding a particular formin in an open conformation is termed the “gating factor” and ranges from near 0 to 1. A formin with a small gating factor mediates very slow filament elongation, whereas a formin with a gating factor of 1 mediates polymerization at a rate that matches the elongation of filaments with free (i.e. not bound by formin) barbed ends.
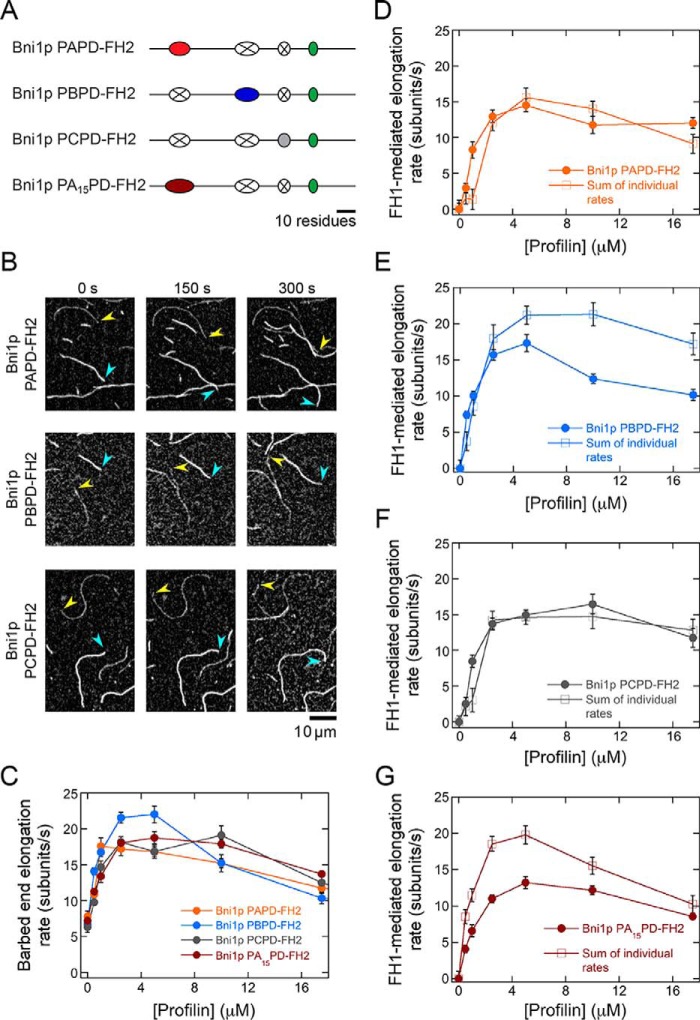
Binding of the protein profilin to actin monomers enables formins to speed elongation beyond the basal rate mediated by their FH2 domains (8, 13). Profilin–actin complexes bind to polyproline tracts located in the otherwise unstructured formin FH1 domain (18, 19), which is positioned directly N-terminal to the FH2 domain (Fig. 1). Diffusion of the flexible FH1 domain facilitates rapid delivery and transfer of bound profilin–actin complexes to the barbed end for incorporation via FH2 stepping (Fig. 1) (13, 20). Titration of profilin into polymerization reactions containing actin and formin FH1FH2 constructs increases the rate of FH1-mediated filament elongation in a profilin-dependent manner, such that maximal elongation rates are observed when the majority of actin monomers are bound by profilin (8, 13, 15, 21). The elongation rate slows in the presence of a large molar excess of profilin, which competes with profilin–actin for binding to the polyproline tracts and reduces the efficiency of the FH1 domains (8, 13).
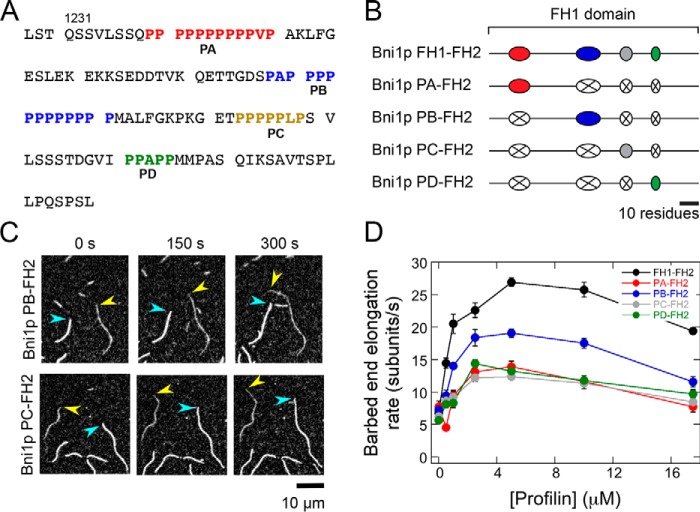
Figure 1.
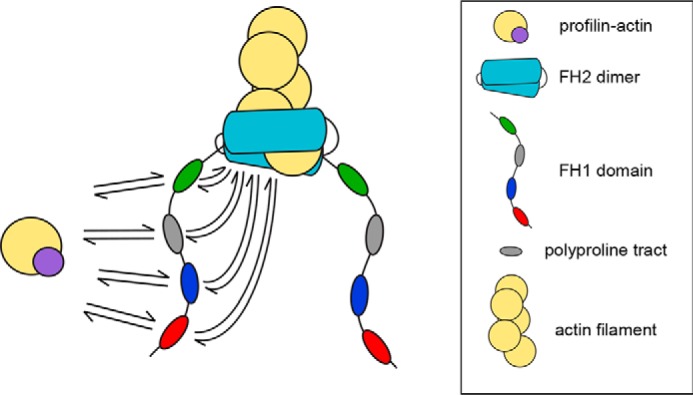
Parallel routes for FH1-mediated actin polymerization. Schematic representation of the multiple binding sites and routes to the barbed end (arrows) for profilin–actin (purple and yellow circles) presented by the FH1 domain of the S. cerevisiae formin Bni1p. The red, blue, gray, and green ovals represent each of the four polyproline tracts. The actin filament is shown in yellow, and the formin FH2 dimer is shown in cyan.
The presence of multiple polyproline tracts in formin FH1 domains gives rise to an array of parallel pathways for formin-mediated delivery of profilin–actin to the barbed end (Fig. 1). The number of polyproline tracts encoded in formin FH1 domain sequences varies widely, from as few as 1 to as many as 14 (18). It has been observed that formins whose FH1 domains contain a large number of polyproline tracts mediate faster elongation than do formins with fewer tracts (8). However, the mechanism of coordinated actin polymerization along the parallel routes presented by the FH1 domain remains poorly understood. As a result, it remains unknown why certain formins possess more polyproline tracts than do others.
In this study, we investigated the mechanism that governs the interactions of profilin–actin complexes with the multiple polyproline tracts in formin FH1 domains and their subsequent delivery to the barbed end. We found that although all polyproline tracts can efficiently mediate polymerization, competition among tracts to deliver profilin–actin to the barbed end imposes an upper limit on the rate of elongation that can be achieved by formins. We propose that intrinsic properties of the FH2 domain tune the efficiency of FH1-mediated elongation by directly regulating the rate of monomer addition at the barbed end. A strong correlation between competitive FH1-mediated profilin–actin delivery and FH2-regulated gating thus obviates the need for evolutionary optimization of FH1 domain sequences.
Results
To dissect the contributions of individual polyproline tracts to actin filament elongation mediated by formins, we made a series of variants of an FH1–FH2 construct of the Saccharomyces cerevisiae formin Bni1p. We selected Bni1p as a representative formin because its FH1 domain contains four polyproline tracts (Fig. 2A), which corresponds to the average number of tracts present in the FH1 sequences of formins whose polymerization properties have been characterized (Table 1). These tracts mediate a robust filament elongation rate in the presence of profilin, making the FH1 domain of Bni1p an excellent candidate for dissection (8, 15, 20, 21). Each of our designed variants contains the WT Bni1p FH2 domain preceded by the FH1 domain in which all but one of the polyproline tracts have been substituted with a repeated Gly–Gly–Gly–Ser sequence of the same length as the polyproline tract it replaces (Fig. 2B). Thus, the remaining tract in each construct is located at its native position within the FH1 domain, preserving its distance from the FH2 domain. We refer to each single-tract variant as Bni1p PX–FH2, where PX refers to the polyproline tract that is retained.
Figure 2.
Individual polyproline tracts robustly mediate actin polymerization by Bni1p. The experimental conditions were as follows: 0.75 μm actin (33% Oregon Green–labeled) in microscopy buffer with varying concentrations of S. cerevisiae profilin. The data were collected by TIRF microscopy. A, FH1 domain sequence, which consists of amino acids 1228–1347 of Bni1p. The four polyproline tracts are color-coded in red (PA), blue (PB), gold (PC), and green (PD). B, schematic representations of the FH1 domain of WT and single-tract variant Bni1p constructs. Red, blue, gray, and green ovals represent polyproline tracts PA, PB, PC, and PD, and black lines represent nonproline sequences. In each Bni1p single-tract variant, three tracts have been substituted with poly(Gly/Ser) sequences that correspond to the length of each replaced tract (represented by a white oval with an X). C, representative series of TIRF micrographs of elongating actin filaments in the presence of single-tract Bni1p variants and 5 μm S. cerevisiae profilin. Micrographs were collected at 150-s intervals. Yellow arrowheads indicate barbed ends of filaments bound by a Bni1p construct. Blue arrowheads indicate barbed ends of control (i.e. not formin-bound) filaments. D, dependence of the actin filament elongation rates mediated by Bni1p FH1FH2 (black circles), Bni1p PA–FH2 (red circles), Bni1p PB–FH2 (blue circles), Bni1p PC-FH2 (gray circles), and Bni1p PD–FH2 (green circles) on the concentration of profilin. Error bars are the standard errors of the mean elongation rates of at least 10 filaments.
Table 1.
Formin polyproline tract numbers and gating factors
| Formin (Organism) | No. of polyproline tractsa | Gating factor | Refs. |
|---|---|---|---|
| Bni1p (S. cerevisiae) | 4 | 0.5 | 8 |
| Cappuccino (Drosophila melanogaster) | 5 | 1 | 27 |
| Cdc12 (S. pombe) | 2 | 0.05 | 8 |
| CYK-1 (Caenorhabditis elegans) | 6 | 0.6 | 46 |
| FHOD (D. melanogaster) | 3 | 0.2 | 47 |
| FMNL3 (Homo sapiens) | 3 | 0.35 | 48 |
| For3 (S. pombe) | 4 | 0.04 | 49 |
| FORMIN-1 (Arabidopsis thaliana) | 4 | 0.125 | 50 |
| Fus1 (S. pombe) | 1 | 0.09 | 49 |
| INF2 (H. sapiens) | 7 | 0.2 | 51 |
| mDia1 (Mus musculus) | 12 | 1 | 8 |
| mDia2 (M. musculus) | 2 | 0.2 | 8 |
a A polyproline tract was included if it contains a minimum of three proline residues and is interrupted by no more than one nonproline residue.
Individual polyproline tracts mediate actin polymerization
To evaluate the ability of each polyproline tract in Bni1p's FH1 domain to mediate filament elongation, we visualized actin polymerization in the presence of our single-tract constructs using total internal reflection fluorescence (TIRF) microscopy. Each polymerization reaction contained 0.75 μm purified actin monomers, 33% of which were labeled with Oregon Green at cysteine 374, and a range of profilin concentrations (0–17.5 μm) selected to enable us to evaluate FH1 activity in the presence of sub-optimal (0–1 μm), optimal (2.5–10 μm), and excess (17.5 μm) amounts of profilin. Consistent with published observations of WT formins (8, 21), our single-tract Bni1p variants preferentially incorporated unlabeled actin subunits in the presence of profilin due to 10-fold tighter binding of profilin to unlabeled monomers compared with fluorescently-labeled monomers (22). As a result, formin-bound filaments appeared more dimly fluorescent than filaments with free (i.e. not formin-bound) barbed ends, facilitating their distinction (Fig. 2C). In conditions that produced filaments with similar fluorescence intensities, we distinguished formin-bound from free filaments by comparing their elongation rates, which typically fell into two distributions.
In the absence of profilin, all single-tract formins mediated filament elongation rates of 5–7 subunits/s (Fig. 2D). In agreement with Bni1p's gating factor of 0.5, these rates are ∼50% slower than the elongation rate of actin filaments with free barbed ends (8, 15). Thus, deletion of multiple polyproline tracts in the FH1 domain does not significantly impact gating or subunit addition mediated by the FH2 domain. Inclusion of up to 5 μm profilin increased the polymerization rate mediated by each single-tract formin, revealing that each polyproline tract is capable of binding and delivering profilin–actin to the barbed end. In the presence of profilin concentrations exceeding 5 μm, the rates of filament elongation mediated by all single-tract formins decreased (Fig. 2D). Under these conditions, the concentration of profilin exceeds the concentration of actin monomers. Thus, like formins with WT FH1 domains, competition between profilin–actin and free profilin for binding polyproline decreases the efficiency of FH1-mediated transfer of profilin–actin to the barbed end, ultimately slowing elongation (8, 13).
Polyproline tract sequences are not optimized for rapid elongation
Whereas Bni1p PA–FH2, PC–FH2, and PD–FH2 mediated similar rates of filament elongation, Bni1p PB–FH2 polymerized actin ∼50% faster at each profilin concentration than the other constructs (Fig. 2D). This suggests that individual polyproline tracts do not contribute equally to elongation and that some polyproline tract sequences may not be optimized for the most efficient delivery of profilin–actin to the barbed end. To test this hypothesis, we sought to determine whether it was possible to increase the rate at which a polyproline tract mediates elongation by systematically modifying its sequence.
Published work has established that the efficiency of profilin–actin transfer from a polyproline tract in the FH1 domain to the barbed end depends both on the affinity of the tract for profilin and the position of the tract within the FH1 domain, which dictates the frequency of collisions with the barbed end (20). A weak affinity for profilin–actin enables rapid delivery from tracts that collide frequently with the barbed end, whereas tracts that collide only infrequently with the barbed end must retain their bound profilin–actin for a longer period of time to increase the likelihood of successful delivery.
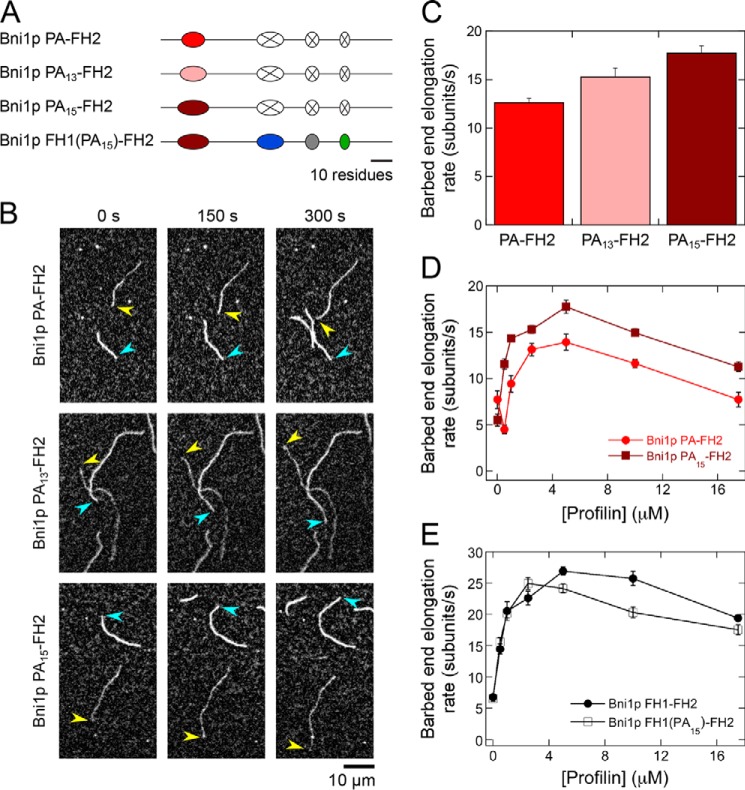
Inspection of Bni1p's FH1 domain sequence revealed that polyproline tract PA contains 11 prolines, whereas PB contains 13 prolines. Based on published binding studies that were performed using poly-l-proline peptides, the sequences of polyproline tracts PA and PB bind profilin with estimated affinities of 36 and 25 μm, respectively (Fig. 2A) (23). Experimental and theoretical studies have demonstrated that the frequency of collisions between the two ends of a disordered polypeptide chain is proportional to n−3/2, where n is the number of residues in the polypeptide (24). Thus, because of its closer proximity to the FH2 domain, the PB tract is predicted to collide 2.3 times more frequently with the barbed end than does the PA tract. Based on these calculations, we hypothesized that the PA tract interacts too weakly with profilin to promote maximally efficient rates of elongation given its position in the FH1 domain. We therefore sought to test the effect of increasing the affinity for profilin on the polymerization rate mediated by this tract. To do so, we designed two variants of Bni1p PA–FH2 in which the polyproline tract was increased in length. These variants, Bni1p PA13–FH2 and Bni1p PA15–FH2, encode modified PA tracts that contain 13 and 15 prolines, corresponding to increases of 31 and 53% in the affinity of PA for profilin (estimated Kd values of 25 and 17 μm, respectively) (Fig. 3A) (23).
Figure 3.
Optimization of the sequence of polyproline tract PA does not speed polymerization mediated by Bni1p. The experimental conditions were as follows: 0.75 μm actin (33% Oregon Green–labeled) in microscopy buffer with varying concentrations of S. cerevisiae profilin. The data were collected by TIRF microscopy. A, schematic representations of the FH1 domain of the variants of Bni1p PA–FH2 and Bni1p FH1–FH2. In Bni1p PA13–FH2 and Bni1p PA15–FH2, the native sequence of polyproline tract PA has been increased by 2 and 4 prolines (pink and dark red ovals, respectively). In Bni1p FH1(PA15)–FH2, the native sequence of polyproline tract PA has been replaced with the PA15 sequence in Bni1p FH1–FH2. B, representative series of TIRF micrographs of actin filaments elongating in the presence of 5 μm S. cerevisiae profilin and Bni1p PA–FH2 (top row), Bni1p PA13–FH2 (middle row), or Bni1p PA15–FH2 (bottom row). Micrographs were collected at 150-s intervals. Arrowheads indicate barbed ends of formin-bound (yellow) and control (blue) filaments. C, barbed end elongation rates of actin filaments bound by Bni1p PA–FH2, Bni1p PA13–FH2, and Bni1p PA15–FH2 in the presence of 5 μm S. cerevisiae profilin. Error bars are the standard errors of the mean elongation rates of at least 10 filaments. D, dependence of the barbed end elongation rates mediated by Bni1p PA–FH2 (bright red circles) and Bni1p PA15–FH2 (dark red squares) on the concentration of profilin. Error bars are the standard errors of the mean elongation rates of at least 10 filaments. E, dependence of the barbed end elongation rates mediated by Bni1p FH1–FH2 (filled circles) and Bni1p FH1(PA15)–FH2 (open squares) on the concentration of profilin. Error bars are the standard errors of the mean elongation rates of at least 10 filaments.
In the presence of 5 μm profilin, Bni1p PA13–FH2 and Bni1p PA15–FH2 generated 15 and 30% faster filament elongation than did Bni1p PA–FH2, confirming that tighter binding promotes more efficient barbed-end delivery of profilin–actin from tracts that are distal to the FH2 domain (Fig. 3, B and C). Because Bni1p PA15–FH2 contains the most efficient polyproline tract, we analyzed this protein's elongation activity in the presence of 0–17.5 μm profilin. In all reactions containing profilin, Bni1p PA15–FH2 exhibited significantly faster rates of elongation than did Bni1p PA–FH2 (Fig. 3D). Thus, the WT sequence of polyproline tract PA is not optimized to mediate a maximal polymerization rate.
We next sought to determine whether substituting polyproline tract PA with the PA15 sequence in the context of the WT FH1 domain would produce a commensurate increase in the rate of elongation mediated by Bni1p FH1–FH2 (Fig. 3A, Bni1p FH1(PA15)-FH2). We found that Bni1p FH1(PA15)-FH2–mediated filament elongation rates were nearly identical to those produced by Bni1p FH1–FH2 in the presence of low micromolar concentrations of profilin (Fig. 3E). Furthermore, Bni1p FH1(PA15)-FH2 polymerized actin less efficiently than the WT protein at profilin concentrations exceeding 2.5 μm (Fig. 3E), indicating that tight binding of profilin by the most distal tract can inhibit FH1 domain activity. Thus, WT Bni1p mediates rapid polymerization despite the fact that its polyproline tract sequences are not individually optimized for efficient elongation. These results suggest that the rate of elongation mediated by Bni1p is restricted by a mechanistic “speed limit.” This implies that optimization of individual polyproline tract sequences is unnecessary and, in some conditions, inefficient.
Competition among polyproline tracts limits the rate of filament elongation mediated by Bni1p
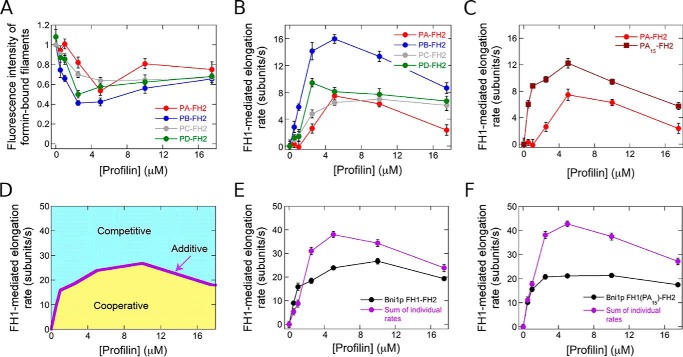
To establish the physical basis for the limit on the rate of filament elongation, we investigated the coordination of delivery of profilin–actin from all four polyproline tracts in Bni1p's FH1 domain. To do so, we separated the elongation rates mediated exclusively by the FH1 domains of the WT and single tract variants of Bni1p from the total elongation rate (which includes elongation arising from direct binding of actin monomers to FH2-bound barbed ends). We took advantage of the fact that conjugation of a fluorescent label to cysteine 374 of an actin monomer decreases its binding affinity for profilin 10-fold. Thus, whereas the FH2 domain of Bni1p incorporates labeled and unlabeled monomers with equal probability, the FH1 domain preferentially polymerizes unlabeled actin because of the requirement for profilin–actin complex formation for FH1-mediated elongation (8, 21). We quantified the fluorescence intensities of formin-bound filaments, which provides a direct measurement of the fraction of polymerized, fluorescently-labeled actin monomers (Fig. 4A). Consistent with measurements performed with WT Bni1p (21), we found that filaments polymerized by each single-tract variant were as bright as control filaments in the absence of profilin. In contrast, formin-bound filaments were dimmer than control filaments in the presence of profilin, reflecting a bias for incorporation of unlabeled actin subunits via the FH1 domain (Fig. 4A). We used these fluorescence measurements and the published affinities of S. cerevisiae profilin for labeled and unlabeled actin monomers (22, 25) to calculate the fraction of subunits incorporated by the FH1 domain of each Bni1p construct (see “Experimental procedures”) (21). Multiplication of this fraction by the overall formin-mediated elongation rate then yielded the rate produced by the FH1 (Fig. 4B).
Figure 4.
Competition among polyproline tracts limits the rate of elongation mediated by Bni1p. The data were collected by TIRF microscopy. A, dependence of the fluorescence intensity of filaments bound by Bni1p single-tract variants on the concentration of S. cerevisiae profilin. Fluorescence intensities of formin-bound filaments were normalized to the intensities of filaments that are not formin-bound. Error bars are standard errors of the mean fluorescence intensity of at least 10 filaments. B and C, dependence of the barbed end elongation rates mediated by the FH1 domains of the Bni1p single-tract variants (B) or PA–FH2 and PA15–FH2 (C) on the concentration of S. cerevisiae profilin. Error bars are standard errors of the mean elongation rate of at least 10 filaments. D, schematic graph depicting the dependence of the barbed end elongation rate mediated by the FH1 domain of Bni1p on the concentration of profilin (purple line). If the sum of the polymerization rates mediated by each individual polyproline tract coincides with the rate measured for the WT FH1 domain, the tracts contribute to elongation in an additive manner. If the sum of the polymerization rates falls below (yellow region) or above (blue region) the WT rate, the tracts contribute to elongation in a cooperative or competitive manner, respectively. E, dependence of the barbed end elongation rates mediated by the FH1 domain of Bni1p FH1–FH2 (black data) and the sum of the rates mediated by the FH1 domains of the Bni1p single-tract variants (purple data) on the concentration of profilin. Error bars are standard errors of the mean elongation rates of at least 10 filaments (Bni1p FH1–FH2) or standard errors of the sums of the mean elongation rates of 10 filaments for each single-tract variant. F, dependence of the barbed end elongation rates mediated by the FH1 domain of Bni1p FH1(PA15)–FH2 (black data) and the sum of the rates mediated by the FH1 domains of the single-tract variants that comprise Bni1p FH1(PA15)–FH2 (purple data) on the concentration of profilin. Error bars are standard errors of the mean elongation rates of at least 10 filaments (Bni1p FH1(PA15)–FH2) or standard errors of the sums of the mean elongation rates of 10 filaments for each single-tract variant.
All four single-tract variants promoted robust FH1-mediated filament elongation rates that showed a similar dependence on the concentration of profilin (Fig. 4B). Consistent with our measurements of total (i.e. both FH1- and FH2-mediated) elongation rates (Fig. 2D), polyproline tract PB coordinated faster polymerization than did tracts PA, PC, and PD at most profilin concentrations (Fig. 4B). Notably, the rates generated by tracts PA and PB were more sensitive to excess profilin concentrations than subunit addition mediated by tracts PC and PD. This likely results from slower dissociation of profilin from longer polyproline tracts, which inhibits rapid exchange of profilin for profilin–actin.
To characterize the mechanism of coordinated profilin–actin delivery from all four polyproline tracts, we compared the sum of the rates mediated by each individual tract in our single-tract mutants to that produced by the WT FH1 domain of Bni1p. To guide our interpretation of these data, we considered three possible scenarios. First, if the sum of the rates generated by the individual tracts is equal to that produced by the WT FH1 domain, the four tracts coordinate polymerization in an additive manner (Fig. 4D, purple line). In this scenario, each tract contributes an elongation rate that is independent of the other tracts. Second, if the sum of the rates generated by the individual tracts is greater than the rate produced by the WT FH1 domain, the four tracts mediate polymerization in a competitive manner (Fig. 4D, blue region). In this scenario, individual polyproline tracts mediate faster elongation in the absence of the other tracts. Third, if the sum of the rates generated by the individual tracts is smaller than the rate produced by the WT FH1 domain, the tracts coordinate polymerization in a cooperative manner (Fig. 4D, yellow region). In this case, individual polyproline tracts polymerize actin most efficiently in the presence of the other tracts.
We plotted the summed and WT elongation rates as a function of profilin concentration (Fig. 4E). At low profilin concentrations (i.e. 0.5–1 μm profilin), the rate of polymerization mediated by Bni1p is enhanced via cooperative coordination among the polyproline tracts (Fig. 4E and Fig. S1A). Thus, in these conditions, binding of profilin–actin or profilin to one polyproline tract speeds elongation mediated by another tract. In contrast, in the presence of profilin concentrations that promote the most rapid filament elongation (i.e. 2.5–17.5 μm profilin), the polyproline tracts of Bni1p competitively deliver profilin–actin to the barbed end (Fig. 4E). Thus, the four tracts mediate slower filament elongation when they are found in the context of the WT FH1 domain than they do when only one tract is present.
Optimization of an individual tract sequence increases the magnitude of competition among polyproline tracts
Competitive subunit addition mediated by the polyproline tracts of Bni1p's FH1 domain provides a possible mechanism for the establishment of an upper limit for Bni1p's elongation activity. This limit implies that optimization of individual polyproline tract sequences is unnecessary and explains why our optimized PA15 polyproline tract does not increase the elongation rate mediated by Bni1p FH1–FH2. To test this hypothesis, we compared the sum of the rates mediated by tracts PA15, PB, PC, and PD to the rate generated by the FH1 domain of Bni1p FH1(PA15)-FH2. To do so, we first quantified the fluorescence intensities of filaments polymerized by Bni1p PA15–FH2 and used these measurements to determine the FH1-mediated filament elongation rates produced by the PA15 tract within the context of this single-tract variant. Consistent with our measurements of total elongation rates (Fig. 3D), the PA15 polyproline tract mediated faster elongation than the PA tract at all concentrations of profilin (Fig. 4C).
In the presence of 2.5 μm and larger concentrations of profilin, the sum of the elongation rates mediated by the individual tracts in FH1(PA15)-FH2 (Fig. 4F, purple circles) is larger than both the rate produced by the FH1 domain of this construct (Fig. 4F, black circles) and the sum of the rates produced by the tracts comprising the WT FH1 domain (Fig. 4E, purple circles). Thus, an increase in the elongation rate mediated by an individual tract produces a corresponding increase in the magnitude of the competition among the tracts. This supports our hypothesis that competition among polyproline tracts obviates sequence optimization for each tract. At concentrations of profilin below 2.5 μm, the contribution of each tract to the elongation rate is additive, rather than cooperative as in the case of the WT FH1 domain (Fig. 4F and Fig. S1B). This suggests that polyproline tracts mediate elongation in a cooperative manner only when interactions with profilin are weak and that tighter binding to profilin eliminates this enhancement in rate.
Competition among polyproline tracts is a general feature of formin FH1 domains
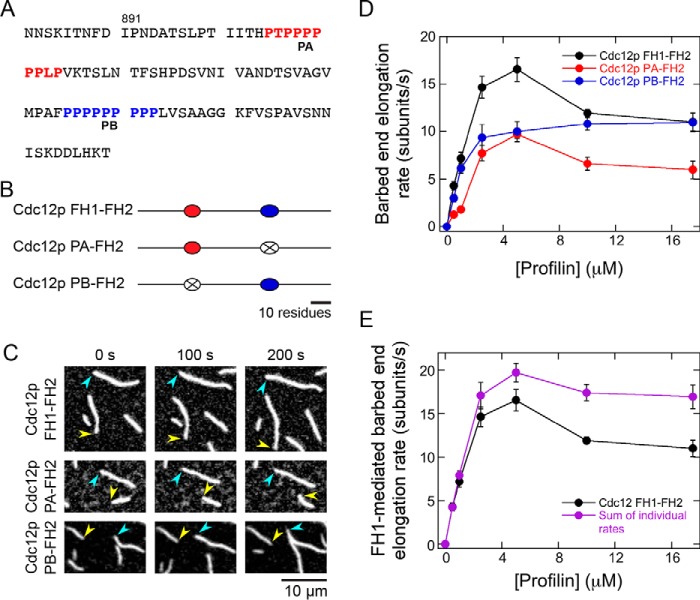
Our finding that filament elongation mediated by multiple polyproline tracts is competitive suggests that mechanistic redundancy is built into the FH1 domains of formins. To determine whether this property is a general feature of formins or whether it is unique to Bni1p, we designed single-tract variants of an FH1–FH2 construct of the Schizosaccharomyces pombe formin Cdc12p (Fig. 5, A and B). This formin's FH1 domain contains two polyproline tracts, enabling us to test whether competition takes place within the simplest context possible.
Figure 5.
Competition among polyproline tracts limits the rate of Cdc12p-mediated actin polymerization. The experimental conditions were as follows: 0.75 μm actin (33% Oregon Green–labeled) in microscopy buffer with varying concentrations of S. pombe profilin. The data were collected by TIRF microscopy. A, FH1 domain sequence, which consists of amino acids 882–979 of the S. pombe formin of Cdc12p. The two polyproline tracts are color-coded in red (PA) and blue (PB). B, schematic representations of the FH1 domain of WT and single-tract variant Cdc12p constructs. Red and blue ovals represent polyproline tracts PA and PB, and black lines depict nonproline sequences. In each Cdc12p single-tract variant, one tract has been substituted with a poly(Gly/Ser) sequence that corresponds to the length of the replaced tract (represented by a white oval with an X). C, representative series of TIRF micrographs of elongating actin filaments in the presence of single-tract Cdc12p variants and 10 μm S. pombe profilin. Micrographs were collected at 100-s intervals. Arrowheads indicate barbed ends of formin-bound (yellow) and control (blue) filaments. D, dependence of the barbed end elongation rates mediated by Cdc12p FH1–FH2 (black circles), Cdc12p PA–FH2 (red circles), and Cdc12p PB–FH2 (blue circles) on the concentration of profilin. Error bars are the standard errors of the mean elongation rates of at least 10 filaments. E, dependence of the barbed end elongation rates mediated by the FH1 domain of Cdc12p FH1–FH2 (black circles) and the sum of the rates mediated by the FH1 domains of the single-tract variants (purple circles) on the concentration of profilin. Error bars are standard errors of the mean elongation rates of at least 10 filaments (Cdc12p FH1–FH2) or standard errors of the sums of the mean elongation rates of 10 filaments for each single-tract variant.
We measured elongation rates mediated by WT and the single-tract variants of Cdc12p FH1–FH2 in the absence and presence of profilin (Fig. 5, C and D). Similar to Bni1p, inclusion of profilin increased the elongation rates mediated by all Cdc12p constructs. In most polymerization conditions, elongation directed by Cdc12p PB–FH2 was faster than that generated by Cdc12p PA–FH2, consistent with our observations that individual polyproline tract sequences do not necessarily mediate identical rates of elongation (Fig. 2D).
Cdc12p has a gating factor that is near zero (8), and therefore, this formin mediates elongation exclusively via its FH1 domain in the presence of profilin (21). To evaluate the contributions made by both polyproline tracts to Cdc12p-directed polymerization, we summed the elongation rates produced by each single-tract variant and compared these rates to those generated by the WT protein (Fig. 5E). Consistent with our findings with Bni1p, this analysis revealed that the PA and PB tracts of Cdc12p mediate filament elongation competitively at profilin concentrations that promote maximal elongation rates (Fig. 5E; 2.5–17.5 μm profilin). At limiting concentrations of profilin (i.e. 0.5–1 μm profilin), the polyproline tracts in Cdc12p's FH1 domain contribute to polymerization in an additive manner (Fig. 5E).
Polyproline tracts compete to deliver, but not to bind, profilin–actin
Our finding that competitive subunit addition occurs even within an FH1 domain that contains only two polyproline tracts suggests that this mechanism is likely a conserved feature among formins. FH1-mediated elongation involves two sequential binding reactions (Fig. 1). Therefore, competition among polyproline tracts might arise from either competitive binding of profilin–actin to neighboring tracts or competitive delivery of multiple, FH1-bound profilin–actin complexes to a single barbed-end binding site. To identify the origins of the competition among polyproline tracts, we designed three variants of Bni1p FH1–FH2 that contained combinations of two polyproline tracts. Each of these double-tract variants retained the PD tract and one other tract (Fig. 6A). We predicted that if profilin–actin binds competitively to neighboring polyproline tracts, Bni1p PCPD–FH2, which retains the two tracts located closest to one another, would exhibit the most competitive polymerization, whereas Bni1p PAPD–FH2, which retains the two most distal tracts, would exhibit little or no competition. In contrast, if polyproline tracts compete at the delivery step, Bni1p PBPD–FH2, which contains the tract that delivers actin most efficiently to the barbed end (i.e. PB), would exhibit the greatest amount of competition.
Figure 6.
Polyproline tracts compete to deliver, but not to bind, profilin–actin. The experimental conditions were as follows: 0.75 μm actin (33% Oregon Green–labeled) in microscopy buffer with varying concentrations of S. cerevisiae profilin. The data were collected by TIRF microscopy. A, schematic representations of the FH1 domain of double-tract Bni1p variants. Red, blue, gray, green, and dark red ovals represent polyproline tracts PA, PB, PC, PD, and PA15, and the black lines represent nonproline sequences. In each double-tract variant, two tracts have been substituted with poly(Gly/Ser) sequences that correspond to the length of the replaced tracts (represented by a white oval with an X). B, representative series of TIRF micrographs of actin filaments elongating in the presence of 5 μm S. cerevisiae profilin and a double-tract variant of Bni1p. Micrographs were collected at 150-s intervals. Arrowheads indicate barbed ends of formin-bound (yellow) and control (blue) filaments, respectively. C, dependence of the barbed end elongation rates of actin filaments bound by Bni1p PAPD–FH2 (orange circles), Bni1p PBPC–FH2 (blue circles), Bni1p PCPD–FH2 (gray circles), and Bni1p PA15PD–FH2 (dark red circles) on the concentration of S. cerevisiae profilin. Error bars are the standard errors of the mean elongation rates of at least 10 filaments. D–G, dependence of the barbed end elongation rates mediated by the FH1 domain of each double-tract formin (filled circles) and the sum of the rates mediated by the individual polyproline tracts (open squares) that comprise the FH1 domain of the double-tract variant (determined by summing rates mediated by single-tract formins) on the concentration of profilin. Error bars are standard errors of the mean elongation rates of at least 10 filaments (double-tract formins) or standard errors of the sums of the mean elongation rates of 10 filaments for each single-tract variant.
We measured elongation rates mediated by our double-tract Bni1p variants in a range of profilin concentrations (Fig. 6, B and C). Profilin stimulated filament elongation by all three double-tract variants. Each variant generated slower rates of filament elongation than did WT Bni1p (Fig. 2D). Consistent with the difference in the binding affinity of profilin for short and long polyproline tracts, the elongation rates produced by Bni1p PAPD–FH2 and Bni1p PBPD–FH2, which each contain a long polyproline tract, reached a peak at 2.5–5 μm profilin, whereas those mediated by Bni1p PCPD–FH2, which contains only short polyproline tracts, were fastest in the presence of 10 μm profilin (Fig. 6C).
We quantified the elongation rates mediated by the FH1 domains of each double-tract variant and compared these rates to the sum of the rates individually produced by their component tracts (Fig. 6, D–F). In the presence of limiting profilin, the polyproline tracts in all three variants cooperatively coordinate filament elongation, indicating that cooperative polymerization can occur when only two polyproline tracts are present in a formin's FH1 domain.
At profilin concentrations exceeding 1 μm, the polyproline tracts comprising the FH1 domains of Bni1p PAPD–FH2 and Bni1p PCPD–FH2 coordinate elongation in an additive manner, whereas Bni1p PBPD–FH2 exhibits competitive polymerization (Fig. 6, D–G). Thus, the tracts in PAPD–FH2 and PCPD–FH2 bind and deliver profilin–actin to the barbed end independently of one another, suggesting that neither neighboring nor distal polyproline tracts compete to bind profilin. In contrast, the competition exhibited by Bni1p PBPD–FH2 suggests the existence of a kinetic threshold for competitive profilin–actin delivery. If the individual polyproline tracts that comprise a formin's FH1 domain mediate filament elongation at a combined rate that falls below this threshold, each tract will deliver profilin–actin in an additive manner. If the combined rate of polymerization exceeds this threshold, the polyproline tracts will compete to deliver their bound profilin–actin complexes to the barbed end.
We tested this model by increasing the sum of the rates mediated by the individual polyproline tracts in Bni1p PAPD–FH2 by substituting the PA tract with our optimized PA15 sequence (Fig. 6, Bni1p PA15PD–FH2). We found that this construct also exhibits competitive profilin–actin delivery (Fig. 6G), supporting our hypothesis for a kinetic threshold for competition.
Discussion
Formin FH1 domains direct actin polymerization by binding profilin–actin complexes and delivering them to growing FH2-bound barbed ends for incorporation into filaments (8, 13, 16, 17). The existence of multiple binding sites for profilin in most FH1 domains creates a series of parallel pathways for polymerization. We dissected the contributions of each polyproline tract in the FH1 domains of the formins Bni1p and Cdc12p to determine how subunit addition along these parallel routes is coordinated. We found that each polyproline tract is capable of mediating actin polymerization when it is the only tract in the FH1 domain and that some tracts mediate significantly faster polymerization rates than others (Figs. 2D and 5D).
Competition for delivery of multiple polyproline-bound profilin–actin complexes to the barbed end establishes a speed limit for polymerization
To assess the coordination of actin polymerization along the multiple pathways presented by formin FH1 domains, we compared the elongation rates mediated by the WT FH1 domains of Bni1p and Cdc12p to the sum of the rates mediated by each of their polyproline tracts when it is the only tract present (Figs. 4D and 5E). In the presence of profilin concentrations that promote maximal polymerization rates, the sum of the elongation rates mediated by the individual polyproline tracts exceeds the rate mediated by the WT FH1 domains of both formins (Figs. 4D and 5E). Thus, in optimal polymerization conditions, subunit addition mediated by the multiple polyproline tracts in a formin's FH1 domain is competitive.
Our experiments with double-tract variants of Bni1p indicated that neighboring polyproline tracts do not compete to bind profilin–actin (Fig. 6D). This is consistent with published crystallographic and molecular modeling studies, which demonstrated that FH1 domains can accommodate simultaneous binding of multiple profilin–actin complexes to neighboring tracts (18, 19). Instead, competition arises when multiple tracts compete to deliver profilin–actin to the barbed end (Fig. 6E).
FH1-mediated delivery of a profilin–actin complex is successful only when the FH2-bound barbed end is in a polymerization-competent, open conformation. Following delivery, stepping of the FH2 dimer incorporates the newly-associated actin subunit into the filament. The FH2 domain then returns to its open conformation, making the barbed end available once again for monomer binding (15, 17). This mechanism can only accommodate binding of a single actin monomer to the barbed end during each cycle of subunit addition. It therefore follows that, if multiple profilin–actin complexes are bound simultaneously to polyproline tracts in an FH1 domain, all but one of the tracts will be less efficient at transferring their bound profilin–actin to the barbed end than they would be if they were the only tract present in the FH1 domain (Fig. 7A).
Figure 7.
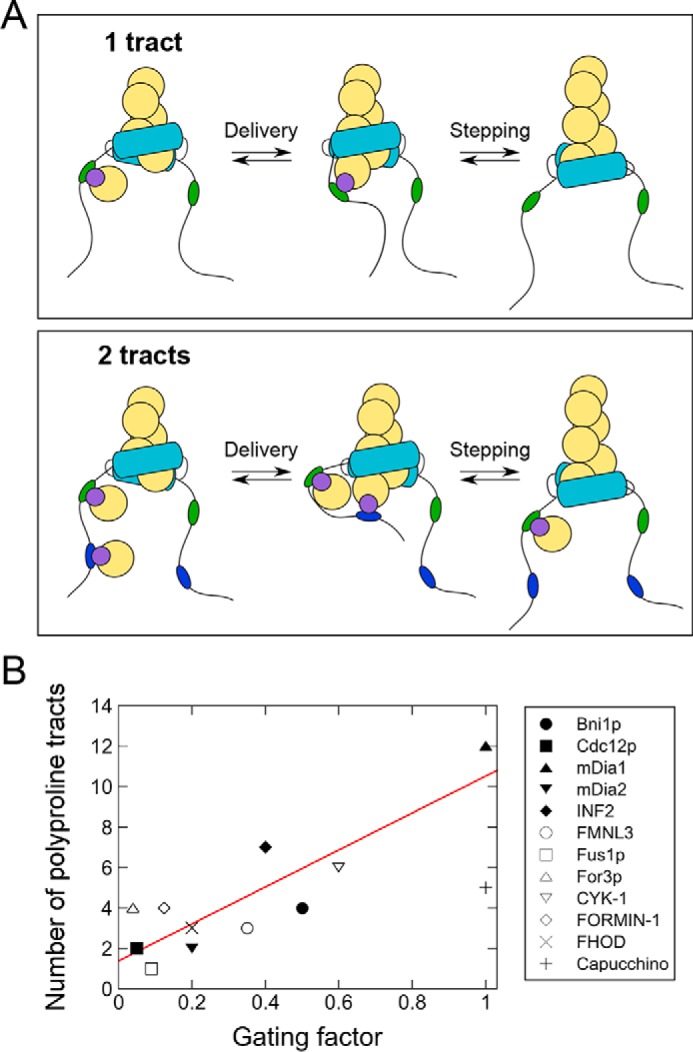
Proposed mechanism for kinetic regulation of FH1 activity by FH2 gating. A, reaction schemes depicting the delivery and incorporation of profilin–actin (purple and yellow circles) at the barbed end of a filament by formins whose FH1 domains encode a single polyproline tract (top row: green ovals) or two polyproline tracts (bottom row: green and blue ovals). When the FH1 domain contains two or more polyproline tracts, competition for delivery of profilin–actin to the barbed end binding site decreases the relative polymerization efficiency of the individual tracts. For simplicity, binding of profilin–actin to only one of the FH1 domains is depicted. B, number of polyproline tracts encoded by FH1 domains, plotted as a function of the reported FH2 gating factor for each formin. The formins represented in this graph are listed in Table 1 along with references to the publications in which their gating factors were reported. For studies that did not explicitly report a gating factor, this number was calculated by dividing the formin-mediated elongation rate measured in the absence of profilin by the elongation rate of filaments with free barbed ends. A linear fit (red line) was applied to the data to obtain the equation: y = 9.15x + 1.4. The data point for Cappuccino was omitted from the fit.
Competition among polyproline tracts establishes a limit on the rate of formin-mediated filament elongation. Consistent with this model, substitution of Bni1p's polyproline tract PA with a sequence that binds profilin more tightly yielded nearly identical rates of elongation as WT Bni1p in the presence of up to 2.5 μm profilin (Fig. 3E). This optimized formin mediated slower polymerization than did WT Bni1p in the presence of 5 μm and higher concentrations of profilin. Thus, an increase in the binding affinity of the terminal polyproline tract for profilin interferes with barbed-end delivery of profilin–actin from the other tracts. These results suggest that binding of profilin–actin at a distal position may disrupt FH1 diffusion in a way that slows the overall rate of collisions of all polyproline tracts with the barbed end. This provides a second possible mechanism for competition among polyproline tracts. Thus, individual polyproline tract sequences might be optimized not only for their profilin-binding properties but also to minimize position-specific effects on the diffusional properties of the FH1 domain when the site is bound by profilin.
Role of gating in regulating the competition among polyproline tracts
Kinetic competition for delivery limits the maximal rate at which profilin–actin can be transferred to the barbed end. In addition to obviating polyproline tract sequence optimization, this limit likely also restricts the number of polyproline tracts a formin's FH1 domain can accommodate. Consistent with this hypothesis, doubling the number of polyproline tracts in Bni1p's FH1 domain does not significantly increase the rate of filament elongation mediated by this formin (15). Some FH1 domain sequences may also possess more polyproline tracts than are strictly required to achieve a particular elongation rate. For example, an FH1-FH2 construct of the mammalian formin mDia1 that encodes five polyproline tracts elongates actin filaments at rates that are similar to those produced by the WT protein, which contains 12 tracts (8).
What determines the number of polyproline tracts encoded by a formin's FH1 domain? This number ranges among formin isoforms from as few as 1 to as many as 14 (18). Because the probability of simultaneous binding of multiple profilin–actin complexes increases with the number of polyproline tracts, competition for delivery should strongly attenuate the elongation rates mediated by formins with large numbers of tracts. However, rates of filament elongation mediated by different formin isoforms have been demonstrated to be directly proportional to the number of polyproline tracts in their FH1 domains (8). This suggests that the kinetic threshold for competitive profilin–actin delivery to the barbed end differs among formin isoforms.
The FH2 domain must adopt an open conformation to enable actin binding, so the likelihood of incorporating a new subunit following a collision with the barbed end is proportional to the formin's gating factor. Consistent with this relationship, computational studies of formin-mediated actin polymerization have revealed that collisions between profilin–actin and the barbed end are more frequently productive when the barbed end is bound by a formin with a large gating factor than a formin with a small gating factor (13, 26). The frequency of collisions between profilin–actin complexes and the barbed end is proportional to the number of binding sites for profilin–actin in each formin's FH1 domain. Therefore, the number of polyproline tracts encoded by a formin's FH1 domain might also scale with its gating factor.
Analysis of the FH1 domain sequences of 12 formins with published gating factors reveals that, in general, as a formin's gating factor increases, so does the number of polyproline tracts encoded in its FH1 domain (Table 1; Fig. 7B). Every additional polyproline tract is matched by an increase of ∼0.1 in gating, corresponding to an increase of 10% in the likelihood that the FH2-bound barbed end will be found in an open conformation. Binding affinities of individual polyproline tract sequences likely also play a role in determining the number of polyproline tracts that can be accommodated in a formin's FH1 domain. For example, the mammalian formin mDia1 and the Drosophila formin Cappuccino both possess gating factors of 1 (8, 27), but mDia1 has 12 relatively short polyproline tracts, and Cappuccino has five long tracts. Because of these particular combinations of tract numbers and lengths, it is possible that both formins are bound by similar numbers of profilin–actin complexes at any given time, and thus the tracts in each FH1 domain likely compete to the same extent for delivery of profilin–actin to the barbed end.
In suboptimal polymerization conditions, the polyproline tracts in the FH1 domain of Bni1p cooperatively mediate actin polymerization
In reactions where the concentration of profilin–actin is limiting (i.e. at 1 μm profilin and below), the rate of filament elongation mediated by the FH1 domain of Bni1p exceeds the sum of the rates mediated by its individual polyproline tracts, indicating that each polyproline tract is more efficient at polymerizing actin in the presence of the other tracts. We also observed cooperative coordination of the polyproline tracts in our double-tract Bni1p constructs, which contained only two polyproline tracts (Fig. 6, D–F).
Recent molecular dynamics simulations have demonstrated that polyproline tracts in FH1 domains adopt PPII conformations with high propensity, whereas the sequences separating the tracts are largely disordered (18). Binding of profilin to a polyproline tract causes conformational extension of the FH1 domain, which expands by ∼5–8% to sterically accommodate the volume occupied by the profilin (18, 28, 29). Profilin binding to internal polyproline tracts (e.g. tracts PB and PC in the case of Bni1p) produces a larger expansion than does binding to the most distal tract (e.g. tract PA) (18). As such, profilin binding events “untangle” the otherwise collapsed FH1 domain. This expansion may facilitate access to other polyproline tracts for successive binding events or reduce the conformational space sampled by the FH1 domain, ultimately speeding diffusion-limited delivery of profilin–actin to the barbed end. Thus, the cooperativity we observe may arise from local effects on chain flexibility and diffusion that occur upon profilin binding. The magnitude of the cooperativity likely depends on the specific sequence and intrinsic flexibility of the FH1 domain, because the polyproline tracts in Cdc12p's FH1 domain do not exhibit cooperativity under these conditions (Fig. 5E).
Optimization of Bni1p's PA tract for tighter profilin binding eliminated the cooperativity observed at low profilin concentrations and promoted additive contributions to elongation by the polyproline tracts (Fig. 4F and Fig. S1). This suggests that conformational expansion of the FH1 domain enhances its polymerization activity only when profilin–polyproline interactions are very weak. Increasing the affinity of a polyproline tract for profilin, which increases the lifetime of its profilin-bound state, might thus negate the effects an increase in tract accessibility would otherwise produce on binding and delivery of profilin–actin.
Mechanistic comparisons with other nucleation-promoting factors with multiple subunit-binding sites
Formins are unique among actin nucleation–promoting factors in their dual abilities to processively regulate the availability of actin filament barbed ends via their FH2 domains and to speed the rate of elongation by delivering subunits directly to the barbed end via multiple profilin-binding sites in their FH1 domains. However, a number of other cytoskeletal proteins promote filament assembly by binding and bringing together multiple actin or tubulin subunits. Ena/Vasp, Spire, Cobl, Junction-mediating and regulatory protein (JMY), and leiomodin all use tandem sequences to bind and promote stable interactions among multiple actin monomers (30–34), whereas Stu2/XMAP215 proteins encode multiple tumor-overexpressed gene (TOG) domains that bind tubulin heterodimers and promote their incorporation at the plus ends of microtubules (35). For some of these proteins, sequence variations among isoforms give rise to binding sites with differing affinities for actin or tubulin. These variations have been demonstrated to produce significant effects on polymer assembly rates (36–38). Similarly, modification of the number of tandem binding sites either by duplication or by increasing the oligomeric state of the protein has also been shown to modulate polymer elongation rates (38, 39).
Proteins in the Ena/Vasp family contain dual binding sites that bind actin monomers and filaments. These tetrameric proteins interact with filament barbed ends via their F-actin–binding site and mediate actin elongation by delivering actin subunits bound to their G-actin–binding (GAB) sites to the barbed end (36, 39, 40). Elongation rates mediated by Vasp isoforms are positively correlated with the affinity of the GAB sites for actin, and full saturation of all binding sites promotes a maximal elongation rate (36). In contrast, a lower affinity for filaments promotes faster elongation (36), suggesting that, like formins, interactions of Ena/Vasp proteins with the filament itself dictate the rate at which subunits can be delivered continuously from the monomer-binding sites to the barbed end. The constraint on the rate of elongation imposed by the lifetime of the Ena/Vasp-filament association might also limit the number of effective monomer-binding sites, as engineering additional GAB sites into Vasp does not increase the rate of filament elongation to the same extent as does increasing the oligomeric state (39).
In contrast to our observations with formins, the two TOG domains encoded by the S. cerevisiae microtubule-polymerizing protein Stu2p contribute to microtubule elongation in an additive manner (38). Ultimately, the rate of polymerization mediated by Stu2p is governed by a balance between lifetimes of TOG–tubulin association and interactions between a stretch of basic residues in Stu2 with the microtubule lattice (38). The competition between these two sets of interactions is similar to the interplay between FH2 gating and FH1–profilin-binding interactions in regulating actin polymerization by formins.
Sequence variations and modulation of the numbers of actin or tubulin-binding sites encoded by cytoskeletal nucleation-promoting factors influence the rates at which these proteins mediate polymer assembly. Our work with formins suggests that the optimal number of tandem subunit-binding sites encoded by a polymerization-promoting protein depends on its mechanism of interaction with the elongating filament. Together, the rate of availability of the filament end, the affinity of binding sites for actin monomers or tubulin dimers, the flexibility of intrinsically disordered regions between binding sites, the inherent processivity of the polymerization-promoting protein, and the lifetime of interactions with the filament impose practical limitations on the number of subunit-binding sites a protein might encode. Future studies will undoubtedly reveal how many aspects of the formin mechanism are conserved across divergent nucleation-promoting proteins, and the ways in which they differ.
Experimental procedures
Protein purification
Variant FH1–FH2 constructs of Cdc12p (residues 882–1375) and Bni1p (residues 1227–1776) were cloned into pGEX-4T-3 plasmids (GE Healthcare). In addition to the N-terminal GSH S-transferase (GST) tag that is encoded by pGEX vectors, constructs contained an N-terminal TEV protease recognition sequence and a C-terminal His6 tag. In variant constructs, individual polyproline tract sequences were replaced with a repetitive Gly–Gly–Gly–Ser sequence of the same length. All proteins were expressed at 16 °C overnight in 1-liter cultures of BL21 DE3 RP Codon Plus cells (Agilent Technologies). Unless otherwise specified, all chemicals were purchased from Millipore Sigma.
Cdc12p and Bni1p variant constructs were purified via GST affinity chromatography followed by overnight incubation with 2–5 μm TEV protease and further purification by nickel affinity chromatography. Cells expressing Cdc12p constructs were lysed by sonication in 20 mm Tris (pH 7.5), 200 mm NaCl, 5% glycerol, 5 mm dithiothreitol (DTT), whereas cells expressing Bni1p constructs were lysed in 50 mm Tris (pH 8.0), 500 mm NaCl, 1 mm DTT. Following centrifugation at 16,000 rpm for 40 min to remove insoluble material, lysate supernatants were incubated with 2 ml of GSH-agarose resin (Gold Biotechnology) with rotation for 1 h at 4 °C. The resin and protein solutions were then transferred to an empty column and washed with 20 ml of lysis buffer, followed by 20 ml of low-salt wash buffer (20 mm Tris (pH 7.5), 100 mm NaCl, 5% glycerol, 1 mm DTT for Cdc12p constructs, and 50 mm Tris (pH 8.0), 100 mm NaCl, 1 mm DTT for Bni1p constructs). Each protein was eluted with 6 ml of 100 mm GSH (pH 8.0) in low-salt wash buffer and incubated with 2–5 μm TEV protease overnight at 4 °C. Purified proteins were separated from residual TEV protease and GST by nickel-affinity chromatography, concentrated using 30,000 MWCO spin columns (Millipore Sigma), dialyzed into KMEI buffer (50 mm KCl, 1 mm MgCl2, 1 mm EGTA, 10 mm imidazole (pH 7.0)) with 1 mm DTT, flash-frozen, and stored at −80 °C. We used ProtParam (ProtParam Tool, RRID:SCR_018087 (41)) to calculate extinction coefficients for all constructs.
S. pombe and S. cerevisiae profilin were expressed from pMW172 vectors in BL21 DE3 pLysS cells and purified as described (15, 20). We used extinction coefficients of 19,940 and 19,060 m−1 cm−1 at λ = 280 nm for S. pombe and S. cerevisiae profilin, respectively. Skeletal muscle actin was purified from an acetone powder prepared from frozen chicken breasts (Trader Joe's) via one cycle of polymerization and depolymerization (42). Actin was labeled on cysteine 374 with Oregon Green 488 iodoacetamide (Thermo Fisher Scientific) (43). Monomers were gel-filtered on S-300 resin (GE Healthcare) in G-Buffer (2 mm Tris (pH 8.0), 0.2 mm ATP, 0.5 mm DTT, 0.1 mm CaCl2) and stored at 4 °C. We used extinction coefficients of 26,000 m−1 cm−1 at λ = 290 for unlabeled actin and 78,000 m−1 cm−1 at λ = 491 for Oregon Green, and the following relation to calculate the concentration of Oregon Green–labeled actin: [total actin] = (A290 − (A491·0.171))/26,000 m−1 cm−1.
Microscopy and data analysis
Glass slides and coverslips (22 × 50 mm; Thermo Fisher Scientific) were sonicated in 2% Hellmanex III (Millipore Sigma), rinsed extensively with water, and sonicated in water. Chambers were assembled as described previously (15). Chambers were stored at room temperature and used within 1 week.
Chambers were prepared via subsequent incubations with 0.5% Tween 20 in HS-TBS (50 mm Tris (pH 7.5), 600 mm NaCl), ∼1 μm N-ethylmaleimide–inactivated chicken skeletal muscle myosin (43) in HS-TBS, and 100 mg/ml bovine serum albumin (BSA) in HS-TBS. The chamber was washed with HS-TBS following each incubation step and with KMEI prior to the introduction of a polymerization reaction.
Prior to polymerization, mixtures of unlabeled and labeled Ca2+-ATP actin were converted to Mg2+–ATP actin by addition of 50 μm MgCl2 and 0.2 mm EGTA. Following a 5-min incubation, polymerization was initiated by addition of 2× microscopy buffer (1× microscopy buffer: 10 mm imidazole (pH 7.0), 50 mm KCl, 1 mm MgCl2, 1 mm EGTA, 50 mm DTT, 0.2 mm ATP, 15 mm glucose, 20 μg/ml catalase, 100 μg/ml glucose oxidase, 0.5% (w/v) methylcellulose (4000 cP at 2%)) with formin and profilin. Reactions were immediately introduced into the chamber for imaging upon initiation of polymerization.
Time-lapse images of elongating actin filaments were collected by through-objective TIRF microscopy on an Olympus Ti83 motorized microscope equipped with a CellTIRF system using a 60× 1.49 N.A. objective and a 488-nm laser. Images were taken every 10 s using a C9100-23B ImagEM X2 EMCCD (Hamamatsu) camera and CellSens Dimension software (Olympus).
Time-lapse movies of growing filaments were processed with ImageJ software (National Institutes of Health (44)). For each reaction, changes in length for 10–15 formin-bound and 10 control (i.e. not formin-bound) filaments were measured, typically over a span of at least 20 frames or 200 s. To determine elongation rates, linear fits were applied to plots of filament length over time using Kaleidagraph software (Synergy Software). We measured the fluorescence intensities of control and formin-bound filaments by performing line scans along filaments and neighboring background regions using ImageJ. To avoid any influence of photobleaching on our fluorescence measurements, we performed the line scans along 5–10-μm stretches at filament barbed ends.
Calculation of FH1-mediated elongation rates
In each polymerization reaction, the rate of filament elongation mediated by the formin's FH1 pathway was calculated as described previously (21). Briefly, the fluorescence intensities of formin-bound filaments were normalized to those of control filaments. The fraction of unlabeled actin subunits incorporated via the FH1 pathway (FH1black) was calculated using Equation 1,
| (Eq. 1) |
where fluorescenceformin-bound is the normalized fluorescence intensity of the formin-bound filaments in the reaction.
The fraction of labeled actin subunits incorporated via the FH1 pathway (FH1green) was then calculated using Equation 2,
| (Eq. 2) |
where green PA is the fraction of profilin-bound actin that is labeled, and black PA is the fraction of profilin-bound actin that is unlabeled. These values were calculated using published affinities of S. cerevisiae and S. pombe profilin for unlabeled actin and actin that is labeled at cysteine 374 (2.9 and 29 μm for S. cerevisiae profilin and 0.2 and 2 μm for S. pombe profilin (22, 25, 45)) and Equation 3,
| (Eq. 3) |
The total fraction of actin subunits incorporated via the FH1 pathway was then calculated by summing FH1black and FH1green, and the polymerization rate mediated through the FH1 pathway was obtained by multiplying this sum by the overall elongation rate mediated by the formin in each reaction.
Author contributions
M. E. Z. and N. C. formal analysis; M. E. Z. and N. C. investigation; M. E. Z. and N. C. methodology; M. E. Z. and N. C. writing-review and editing; N. C. conceptualization; N. C. funding acquisition; N. C. writing-original draft.
Supplementary Material
Acknowledgment
We thank Melissa Gardner for helpful comments on the manuscript.
This work was supported by National Institutes of Health Research Grant GM122787 (to N. C.) and National Institutes of Health Training Grant AR007612 (to M. E. Z.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
- FH2
- formin homology 2
- FH1
- formin homology 1
- TIRF
- total internal reflection fluorescence
- TEV
- tobacco etch virus
- TIRF
- total internal reflection fluorescence
- TOG
- tumor-overexpressed gene
- GAB
- G-actin–binding.
References
- 1. Campellone K. G., and Welch M. D. (2010) A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11, 237–251 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Faix J., and Grosse R. (2006) Staying in shape with formins. Dev. Cell 10, 693–706 10.1016/j.devcel.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 3. Goode B. L., and Eck M. J. (2007) Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76, 593–627 10.1146/annurev.biochem.75.103004.142647 [DOI] [PubMed] [Google Scholar]
- 4. Pruyne D. (2016) Revisiting the phylogeny of the animal formins: two new subtypes, relationships with multiple wing hairs proteins, and a lost human formin. PLoS ONE 11, e0164067 10.1371/journal.pone.0164067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schönichen A., and Geyer M. (2010) Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim. Biophys. Acta 1803, 152–163 10.1016/j.bbamcr.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 6. Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., Bretscher A., and Boone C. (2002) Role of formins in actin assembly: nucleation and barbed-end association. Science 297, 612–615 10.1126/science.1072309 [DOI] [PubMed] [Google Scholar]
- 7. Zigmond S. H., Evangelista M., Boone C., Yang C., Dar A. C., Sicheri F., Forkey J., and Pring M. (2003) Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 13, 1820–1823 10.1016/j.cub.2003.09.057 [DOI] [PubMed] [Google Scholar]
- 8. Kovar D. R., Harris E. S., Mahaffy R., Higgs H. N., and Pollard T. D. (2006) Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell 124, 423–435 10.1016/j.cell.2005.11.038 [DOI] [PubMed] [Google Scholar]
- 9. Pring M., Evangelista M., Boone C., Yang C., and Zigmond S. H. (2003) Mechanism of formin-induced nucleation of actin filaments. Biochemistry 42, 486–496 10.1021/bi026520j [DOI] [PubMed] [Google Scholar]
- 10. Moseley J. B., Sagot I., Manning A. L., Xu Y., Eck M. J., Pellman D., and Goode B. L. (2004) A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell 15, 896–907 10.1091/mbc.e03-08-0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Y., Moseley J. B., Sagot I., Poy F., Pellman D., Goode B. L., and Eck M. J. (2004) Crystal structures of a formin homology-2 domain reveal a tethered dimer architecture. Cell 116, 711–723 10.1016/S0092-8674(04)00210-7 [DOI] [PubMed] [Google Scholar]
- 12. Otomo T., Tomchick D. R., Otomo C., Panchal S. C., Machius M., and Rosen M. K. (2005) Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 433, 488–494 10.1038/nature03251 [DOI] [PubMed] [Google Scholar]
- 13. Vavylonis D., Kovar D. R., O'Shaughnessy B., and Pollard T. D. (2006) Model of formin-associated actin filament elongation. Mol. Cell 21, 455–466 10.1016/j.molcel.2006.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aydin F., Courtemanche N., Pollard T. D., and Voth G. A. (2018) Gating mechanisms during actin filament elongation by formins. Elife 7, e37342 10.7554/eLife.37342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paul A. S., and Pollard T. D. (2008) The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr. Biol. 18, 9–19 10.1016/j.cub.2007.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paul A. S., and Pollard T. D. (2009) Review of the mechanism of processive actin filament elongation by formins. Cell Motil. Cytoskeleton 66, 606–617 10.1002/cm.20379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Courtemanche N. (2018) Mechanisms of formin-mediated actin assembly and dynamics. Biophys. Rev. 10, 1553–1569 10.1007/s12551-018-0468-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horan B. G., Zerze G. H., Kim Y. C., Vavylonis D., and Mittal J. (2018) Computational modeling highlights the role of the disordered Formin Homology 1 domain in profilin–actin transfer. FEBS Lett. 592, 1804–1816 10.1002/1873-3468.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kursula P., Kursula I., Massimi M., Song Y. H., Downer J., Stanley W. A., Witke W., and Wilmanns M. (2008) High-resolution structural analysis of mammalian profilin 2a complex formation with two physiological ligands: the formin homology 1 domain of mDia1 and the proline-rich domain of VASP. J. Mol. Biol. 375, 270–290 10.1016/j.jmb.2007.10.050 [DOI] [PubMed] [Google Scholar]
- 20. Courtemanche N., and Pollard T. D. (2012) Determinants of formin homology 1 (FH1) domain function in actin filament elongation by formins. J. Biol. Chem. 287, 7812–7820 10.1074/jbc.M111.322958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherer L. A., Zweifel M. E., and Courtemanche N. (2018) Dissection of two parallel pathways for formin-mediated actin filament elongation. J. Biol. Chem. 293, 17917–17928 10.1074/jbc.RA118.004845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vinson V. K., De La Cruz E. M., Higgs H. N., and Pollard T. D. (1998) Interactions of Acanthamoeba profilin with actin and nucleotides bound to actin. Biochemistry 37, 10871–10880 10.1021/bi980093l [DOI] [PubMed] [Google Scholar]
- 23. Petrella E. C., Machesky L. M., Kaiser D. A., and Pollard T. D. (1996) Structural requirements and thermodynamics of the interaction of proline peptides with profilin. Biochemistry 35, 16535–16543 10.1021/bi961498d [DOI] [PubMed] [Google Scholar]
- 24. Lapidus L. J., Eaton W. A., and Hofrichter J. (2000) Measuring the rate of intramolecular contact formation in polypeptides. Proc. Natl. Acad. Sci. U.S.A. 97, 7220–7225 10.1073/pnas.97.13.7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eads J. C., Mahoney N. M., Vorobiev S., Bresnick A. R., Wen K. K., Rubenstein P. A., Haarer B. K., and Almo S. C. (1998) Structure determination and characterization of Saccharomyces cerevisiae profilin. Biochemistry 37, 11171–11181 10.1021/bi9720033 [DOI] [PubMed] [Google Scholar]
- 26. Courtemanche N., Lee J. Y., Pollard T. D., and Greene E. C. (2013) Tension modulates actin filament polymerization mediated by formin and profilin. Proc. Natl. Acad. Sci. U.S.A. 110, 9752–9757 10.1073/pnas.1308257110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vizcarra C. L., Kreutz B., Rodal A. A., Toms A. V., Lu J., Zheng W., Quinlan M. E., and Eck M. J. (2011) Structure and function of the interacting domains of Spire and Fmn-family formins. Proc. Natl. Acad. Sci. U.S.A. 108, 11884–11889 10.1073/pnas.1105703108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao C., Liu C., Hogue C. W., and Low B. C. (2014) A cooperative jack model of random coil-to-elongation transition of the FH1 domain by profilin binding explains formin motor behavior in actin polymerization. FEBS Lett. 588, 2288–2293 10.1016/j.febslet.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 29. Bryant D., Clemens L., and Allard J. (2017) Computational simulation of formin-mediated actin polymerization predicts homologue-dependent mechanosensitivity. Cytoskeleton 74, 29–39 10.1002/cm.21344 [DOI] [PubMed] [Google Scholar]
- 30. Krause M., Dent E. W., Bear J. E., Loureiro J. J., and Gertler F. B. (2003) Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 19, 541–564 10.1146/annurev.cellbio.19.050103.103356 [DOI] [PubMed] [Google Scholar]
- 31. Quinlan M. E., Heuser J. E., Kerkhoff E., and Mullins R. D. (2005) Drosophila Spire is an actin nucleation factor. Nature 433, 382–388 10.1038/nature03241 [DOI] [PubMed] [Google Scholar]
- 32. Ahuja R., Pinyol R., Reichenbach N., Custer L., Klingensmith J., Kessels M. M., and Qualmann B. (2007) Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell 131, 337–350 10.1016/j.cell.2007.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zuchero J. B., Coutts A. S., Quinlan M. E., Thangue N. B., and Mullins R. D. (2009) p53-cofactor JMY is a multifunctional actin nucleation factor. Nat. Cell Biol. 11, 451–459 10.1038/ncb1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chereau D., Boczkowska M., Skwarek-Maruszewska A., Fujiwara I., Hayes D. B., Rebowski G., Lappalainen P., Pollard T. D., and Dominguez R. (2008) Leiomodin is an actin filament nucleator in muscle cells. Science 320, 239–243 10.1126/science.1155313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slep K. C. (2009) The role of TOG domains in microtubule plus end dynamics. Biochem. Soc. Trans. 37, 1002–1006 10.1042/BST0371002 [DOI] [PubMed] [Google Scholar]
- 36. Breitsprecher D., Kiesewetter A. K., Linkner J., Vinzenz M., Stradal T. E., Small J. V., Curth U., Dickinson R. B., and Faix J. (2011) Molecular mechanism of Ena/VASP-mediated actin-filament elongation. EMBO J. 30, 456–467 10.1038/emboj.2010.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Byrnes A. E., and Slep K. C. (2017) TOG-tubulin binding specificity promotes microtubule dynamics and mitotic spindle formation. J. Cell Biol. 216, 1641–1657 10.1083/jcb.201610090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geyer E. A., Miller M. P., Brautigam C. A., Biggins S., and Rice L. M. (2018) Design principles of a microtubule polymerase. Elife 7, e34574 10.7554/eLife.34574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brühmann S., Ushakov D. S., Winterhoff M., Dickinson R. B., Curth U., and Faix J. (2017) Distinct VASP tetramers synergize in the processive elongation of individual actin filaments from clustered arrays. Proc. Natl. Acad. Sci. U.S.A. 114, E5815–E5824 10.1073/pnas.1703145114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Winkelman J. D., Bilancia C. G., Peifer M., and Kovar D. R. (2014) Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. U.S.A. 111, 4121–4126 10.1073/pnas.1322093111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M. R., Appel R. D., and Bairoch A. (2005) Protein Identification and Analysis Tools on the ExPASy Server (Walker J. M., ed), pp. 571–607), Humana Press, Totowa, NJ [Google Scholar]
- 42. Spudich J. A., and Watt S. (1971) The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 246, 4866–4871 [PubMed] [Google Scholar]
- 43. Kuhn J. R., and Pollard T. D. (2005) Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J. 88, 1387–1402 10.1529/biophysj.104.047399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu J., and Pollard T. D. (2001) Profilin binding to poly-l-proline and actin monomers along with ability to catalyze actin nucleotide exchange is required for viability of fission yeast. Mol. Biol. Cell 12, 1161–1175 10.1091/mbc.12.4.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neidt E. M., Skau C. T., and Kovar D. R. (2008) The cytokinesis formins from the nematode worm and fission yeast differentially mediate actin filament assembly. J. Biol. Chem. 283, 23872–23883 10.1074/jbc.M803734200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel A. A., Oztug Durer Z. A., van Loon A. P., Bremer K. V., and Quinlan M. E. (2018) Drosophila and human FHOD family formin proteins nucleate actin filaments. J. Biol. Chem. 293, 532–540 10.1074/jbc.M117.800888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heimsath E. G. Jr., and Higgs H. N. (2012) The C terminus of formin FMNL3 accelerates actin polymerization and contains a WH2 domain-like sequence that binds both monomers and filament barbed ends. J. Biol. Chem. 287, 3087–3098 10.1074/jbc.M111.312207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scott B. J., Neidt E. M., and Kovar D. R. (2011) The functionally distinct fission yeast formins have specific actin-assembly properties. Mol. Biol. Cell 22, 3826–3839 10.1091/mbc.e11-06-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Michelot A., Guérin C., Huang S., Ingouff M., Richard S., Rodiuc N., Staiger C. J., and Blanchoin L. (2005) The formin homology 1 domain modulates the actin nucleation and bundling activity of Arabidopsis FORMIN1. Plant Cell 17, 2296–2313 10.1105/tpc.105.030908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramabhadran V., Gurel P. S., and Higgs H. N. (2012) Mutations to the formin homology 2 domain of INF2 protein have unexpected effects on actin polymerization and severing. J. Biol. Chem. 287, 34234–34245 10.1074/jbc.M112.365122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.