Abstract
E2F transcription factor 1 (E2F1) is a member of the E2F family of transcription factors. E2F1 binds to DNA with dimerization partner (DP) proteins through an E2 recognition site. The dissociation of E2F1 from retinoblastoma (Rb) protein recovers its transcriptional activity, which drives the cell cycle from the G1 to S phase. E2F1 has been shown to be involved in cellular proliferation, differentiation, and apoptosis in colon cancer. It was recently found that E2F1 also participates in the metastasis and chemoresistance of colon cancer. There are abundant experimental data regarding the actions of E2F1, which can be grouped as either pro-tumorigenic or pro-apoptotic. Despite a growing interest and plentiful data, there is currently no review that focuses on the role of E2F1 in colon cancer. Research on E2F1 and colon cancer has been scattered over various genes and microRNAs (miRNAs) that affect E2F1 expression. Here, we provide the first review that aims to consider and dissect all of the elucidated complex behaviors of E2F1 in colon cancer. This review also provides an analysis and conclusion regarding the current understanding of E2F1 in colon cancer in order to facilitate the direction of future research.
Keywords: E2F1, colon cancer, proliferation, chemoresistance, apoptosis
Introduction
An enhancer box (E-box) is a DNA element that responds to basic helix-loop-helix (bHLH) transcription factors and regulates the transcription of various genes [1]. E-protein dimers bind to E-box sequences (CANNTG) with an apparent affinity for C or G in N positions. The activity of E proteins is antagonized by inhibitors of DNA-binding (Id) proteins, which transform these proteins into nonfunctional heterodimers [2]. E2F transcription factors (E2Fs), first found to bind to the promoter of adenoviruses [3], are a group of transcription factors that play a pivotal role in cellular proliferation, differentiation, and apoptosis (Figure 1). Currently, eight E2F members have been named in order of their discovery from E2F1-E2F8 [4]. Intricate machinery of cell-cycle components-such as cyclins, cyclin-dependent kinases (CDKs), CDK inhibitors, and RB1-provides impetus to downstream E2Fs, which results in their activation to enable cellular division and proliferation [4].
Figure 1.
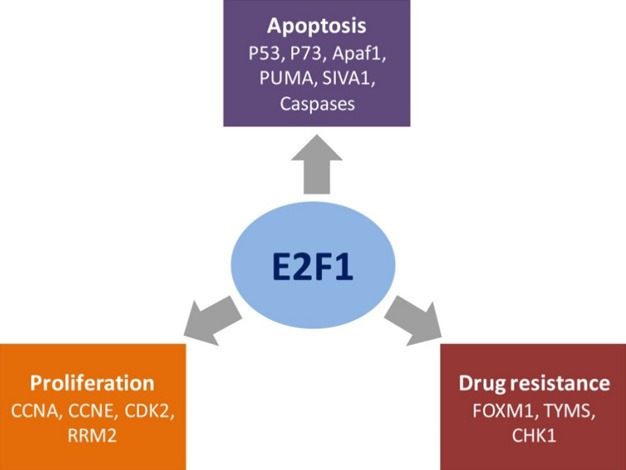
Different actions of E2F1 and their corresponding target genes in CRC. E2F1 has been reported to play crucial roles in proliferation, chemoresistance, and apoptosis of CRC through activating different downstream effectors.
Among these eight factors, E2F1 was the first member discovered [5,6]. E2F1 is a transcription activator that binds to DNA with dimerization partner (DP) proteins via the E2 recognition site, 5’-TTTC[CG]CGC-3’, found in the promoter region of various genes. It is well established that E2F1 is a downstream target of the pocket protein, retinoblastoma (Rb) [7,8], which is a master regulator of the cell cycle and a major tumor suppressor that is mutated in many human cancers [9]. A family of pocket proteins-including Rb protein (pRB), p109, and p130-has a binding specificity for E2F subunits, among which pRB preferentially binds to E2F1. The interaction of E2F1 with pRB leads to the suppression of the former in two different ways: (1) through binding and obstructing the transcriptional activation domain of E2F1; and (2) via recruiting histone deacetylases (HDACs) and SWI/SNF complexes, which change the structure of E2F-targeted genes [10-12]. Phosphorylation of Rb is indispensable for the release of active E2F1, which is required to drive the cell cycle from the G1 to S phase [13,14]. Therefore, regulation of E2F1 is closely related to DNA replication and repair [15,16]. After dissociation from pRB, E2F1 interacts with DP1 or DP2 to induce dNTP synthesis (by thymidylate synthase [TYMS] and ribonucleotide reductase M2), DNA synthesis (by DNA polymerase-α), damage repair (by ribonucleotide reductase M2b), and cell-cycle progression (by cyclin A, cyclin E, and cdk2) [17,18]. Interestingly, E2F1 is directly implicated with poor prognosis in several types of cancer and has been demonstrated to be a key cancer biomarker [19,20]. In breast cancer, overall survival and disease-free survival rates were significantly lower in E2F1-positive patients as compared to those in E2F1-negative patients [21]. Notably, in our previous work, increased E2F1 was required for the proliferation and metastasis of colorectal cancer (CRC) cells by inducing RRM2 expression, which also indicated poor survival in CRC patients [22]. Recently, we also found that high expression of E2F1 promoted the epithelial-mesenchymal transition (EMT) of CRC cells through augmenting IL4/STAT6 signaling [23]. Although E2F1 functions and their relationships with cancer have been investigated and discussed for years, we suggest that all previous studies need to be systematically reviewed and summarized to provide a better understanding of the many roles of E2F1 in CRC.
E2F1 in colon cancer
5-Fluorouracil and E2F1 in colon cancer
It is known that 5-fluorouracil (5-FU) has been widely used as a drug of choice for treating CRC [24,25]. Specifically, 5-FU typically targets TYMS, which catalyzes the reaction from 2’-deoxyuridine monophosphate to deoxythymidine-5’-monophosphate (dTMP) using 5’10-methylene tetrahydrofolate, a precursor for DNA synthesis [26]. The expressions of TYMS and FOXM1 are closely controlled by E2F1 [27-29]. In a recent study, Varghese et al. demonstrated that the expression of FOXM1, TYMS, and E2F1 were elevated in CRC cells and promoted 5-FU resistance [26]. Likewise, Osada et al. evaluated the anti-cancer properties of 5-FU in hepatocyte growth factor (HGF)-stimulated CT26 CRC cells and found that treatment with HGF increased the 5-FU-induced death signal and inhibition of cellular growth. Mechanistically, it has been suggested that HGF decreases E2F1 by reducing cyclin D or E [30]. In addition, tissue microarrays from 190 CRC patients manifested a poor prognosis for the E2F1 + thymidylate synthetase (TS) + phenotype. More aggressive or different treatments than 5-FU-based chemotherapy are suggested in such subgroups of CRC patients [31]. Furthermore, Nagaraju et al. showed more sensitivity to 5-FU in CRC after transcriptional and functional inhibition of heat shock protein 90 (HSP90). Interestingly, inhibition of HSP90 leads to the downregulation of E2F1, which may confer the demonstrated response to 5-FU [32]. Taken together, these studies suggest that the anti-tumor activities of 5-FU in CRC work at least partly by decreasing E2F1 expression, which subsequently arrests the cell cycle. Although there are many reports that indirectly associate the effect of 5-FU with E2F1 expression, direct evidence is lacking.
E2F1-incited metabolic deregulation in colon cancer
Metabolic features of E2F1 have been described in both normal cellular metabolic machinery and metabolic reprogramming in cancerous cells. In normal cells, E2F1 was found to increase the synthesis of adipogenesis, glycolysis, lipogenesis, and bile acids. On the contrary, E2F1 was highlighted to reduce lipolysis, β-oxidation, thermogenesis, and oxidative metabolism [33]. Interestingly, all of these metabolic features affected by E2F1 are independent factors for general carcinogenesis [34] and, specifically, CRC [35]. More systematically, E2F1 has been reported to contribute to Warburg effects [36], repress oxidative metabolism [37], and promote anabolic metabolism [38,39]. Consistent with these studies, Sanmartín-Salinas et al. recently found that insulin receptor substrate-4 (IRS-4) was overexpressed in CRC and promoted Rb-cyclin-dependent kinase activation via definitive involvement of E2F1 [40]. Although there is a lack of reported data on the metabolic pathways involving E2F1 in CRC, the above findings underscore a possible association among E2F1, metabolism, and CRC.
Proteins involved in E2F1 regulation in colon cancer
Many studies have highlighted different genes that contribute to the pro-tumorigenic effect of E2F1 in CRC (Figure 2). For instance, the tumor suppressor, spinophilin, participates in tumor progression and indicates a poor prognosis in many different kinds of cancers [41,42]. Ress et al. found increased cellular growth rates and anchorage-independent growth in p53 wild-type HCT116 and p53-mutated Caco-2 cells when spinophilin levels were low. Intriguingly, researchers discovered a parallel increase in E2F1 levels when spinophilin expression was low [43]. Another powerful gene, X-linked inhibitor of apoptosis (XIAP), has a well-established role in the modulation of cellular apoptosis. In 2014, Cao et al. showed that expression of XIAP with the Really Interesting New Gene (RING) domain omission (XIAPΔRING) stimulated the anchorage-independent growth and G1/S phase transition of cancer cells, in which XIAPΔRING increased binding with E2F1 in order to regulate its own transcriptional activity [44]. Another related gene, cellular inhibitor of apoptosis 1 (cIAP1), is localized in the nucleus and promotes the growth of various cancers [45,46]. The presence of cIAP1 was observed in the nucleus of undifferentiated proliferating cells, but not in differentiated cells [47,48]. A French team validated that the N-terminal part of cIAP1 interacts directly with the DNA-binding domain of E2F1 to increase the transcriptional activity of E2F1 on cyclin E (CCNE) and cyclin A (CCNA) promoters [49]. As a nucleic-acid-binding protein, Y-box-binding protein-1 (YB-1) has been demonstrated to be responsible for cancer development [50]. Knockdown of YB-1 with siRNAs significantly reduced the promoter activity of the E2F1 gene in the CRC cell line, HCT116 [51]. In our previous study, nuclear accumulation of nuclear transcription factor Y subunit β (NFYB) was found to directly activate the transcription of E2F1 in oxaliplatin-resistant CRC cells [52].
Figure 2.
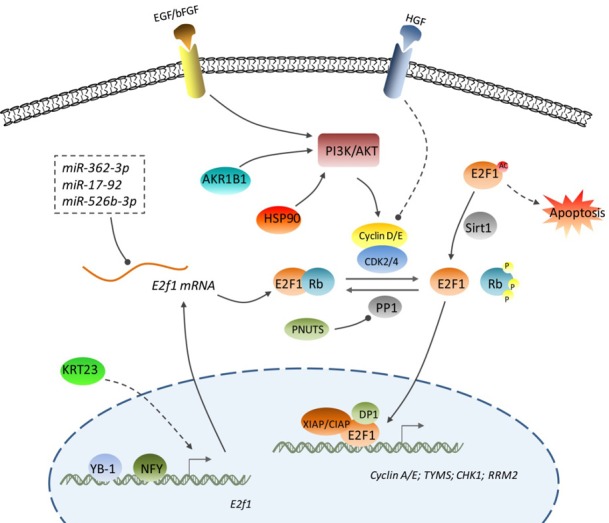
The regulatory mechanisms for E2F1 in CRC. E2F1 function in CRC is regulated at multiple levels including the transcriptional level (YB-1, NFY, and KRT23-induced transcription of the E2F1 gene), post-transcriptional level (E2F1 mRNA targeted by miR-362-3p, miR-17-92, and miR-526b-3p), post-translational level (deacetylation and acetylation of E2F1 protein exhibit opposite actions), protein-protein interaction level (dephosphorylated Rb sequesters E2F1 and hyper-phosphorylated Rb releases E2F1), and transcriptional activity level (XIAP, CIAP, and DP1 enhance the transcriptional activity of E2F1 protein). Arrowhead: promotion; Cycle: inhibition; Dashed line: indirect regulation.
Keratin is a filament-forming epithelial protein with involvement in the proliferation, invasion, metastasis, and treatment responsiveness of cancer cells [53,54]. Keratin 23 (KRT23) is a type-1 acidic keratin exhibiting tumorigenic action in hepatocellular cancer [55] and colon adenocarcinomas [56,57]. Birkenkamp-Demtröder et al. used array-based methylation profiling to reveal that normal colon mucosa had a methylated promoter of KRT23, as compared to the hypomethylated promoter in colon adenocarcinomas. Cellular proliferation and DNA damage responses were significantly curbed after KRT23 depletion in CRC cells; it was also revealed in the same report that KRT23 depletion led to a reduced expression of E2F1 [58], shedding light on a direct relationship of KRT23 and E2F1 in CRC. Moreover, the polyol pathway enzyme, aldose reductase (AR; AKR1B1), was shown to be crucial for reactive oxygen species (ROS) signals induced by growth factors (basic fibroblast growth factor [bFGF] and platelet-derived growth factor [PDGF]) and cytokines (TNF-α) [59]. Inhibition of AR prevented the growth of CRC cells, which resulted from suppressing G1/S phase proteins, such as E2F1, cdks, and cyclins [60]. Inverse relationships of E2F1 with other proteins have also been reported. Pregnane X receptor (PXR) attenuates the proliferation and tumorigenicity of CRC cells by inhibiting E2F1 expression [61]. Latini et al. reported that ABI3, a tumor suppressor, suppressed proliferation and induced senescence in cells, which was accompanied by a reduction in E2F1 levels in CRC cells [62]. A previous report demonstrated that exogenous E2F1 induction resulted in an increase in the activity of the bcl-2 promoter. Bcl-2 overexpression in PhIP-induced colon tumors was implicated along with involvement of beta-catenin, c-Myc, and E2F1 [63]. There is also evidence showing that chronic colon inflammation alone, in the absence of tumors, has higher levels of E2F1 along with hyperphosphorylated pRb, which poses a threat for cancer proliferation [64]. In addition, inflammatory bowel disease presents a potential link between inflammation and cancer due to the abnormal regulation of the cell cycle at the G1/S checkpoint. Russo et al. demonstrated increased expression of E2F1 and CycD1 in Crohn’s disease explants stimulated by lipopolysaccharides (LPS) [65]. Relatedly, Lee and colleagues analyzed 320 human tumor samples and found a link between TS and E2F1 with gastroenteropancreatic (GEP) and neuroendocrine tumors (NETs) [66].
Furthermore, growth factors-including basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factor II (IGFs), platelet derived growth factor (PDGF), transforming growth factor (TGF), and vascular endothelial growth factor (VEGF)-are usually overexpressed in the epithelial tissues of CRC [60,67]. Thus, an increasing number of studies highlight the tumor-promotive effect of growth factors by upregulating vital cell-cycle proteins, such as cyclin D1, cyclin E, cdks, PCNA, and transcription factors, including E2F1 and c-myc, through activating PI3K/AKT signals [68,69].
E2F1-associated miRNAs in colon cancer
A microRNA (miRNA) is a small non-coding RNA that functions as a post-transcriptional regulator. Previous work has described the relationship between miRNAs and E2F1 expression, which subsequently plays a pivotal role in CRC (Figure 2). This section reviews reports regarding miRNA types and their abilities to regulate E2F1. For instance, a Danish research group showed a positive correlation of miR-362-3p with improved prognosis in CRC patients and repressed expression of E2F1 when overexpressing miR-362-3p in CRC cells [70]. In addition, miR-17-92 and its target, E2F1, were found to have a similar expression pattern in human colon development and CRC, with both regulating cellular proliferation [71]. Recently, we found that yin and yang 1 (YY1)-mediated silencing of miR-526b-3p promoted CRC proliferation via increasing E2F1 expression [72]. An interesting observation regarding miRNAs and E2F1 in CRC patients was provided by Kanaan et al. in 2012. In this study, six miRNAs (miR-122, miR-181a, miR-146b-5p, let-7e, miR-17, and miR-143) were upregulated from non-neoplastic tissue to dysplasia, while being downregulated from dysplasia to cancer. The authors also found another six differentially expressed miRNAs affecting the TP53 pathway (miR-122, miR-214, miR-372, miR-15b, let-7e, and miR-17) as targets of E2F1 [73]. It is plausible that these miRNAs affect the E2F1-TP53 axis in order to exert their actions in CRC. In addition to miRNAs, the long non-coding RNA, MNX1-AS1, was upregulated by E2F1 and enhanced the malignancy of colon adenocarcinomas, in which E2F1 was bound to the promoter of MNX1-AS1 [74].
Pharmacological evidence for a role of E2F1 in colon cancer
Some pharmacological molecules that relieve colon cancer by influencing E2F1 expression provide an insightful perspective regarding the implication of E2F1 in colon cancer (Table 1). For example, tristetraprolin (TTP) is an AU-rich element-binding protein that destabilizes E2F1 mRNA [75]. It has been acknowledged that human cancers usually have lower levels of TTP. Lee et al. revealed that resveratrol inhibited the proliferation and invasion/metastasis of CRC cells via TTP-induced downregulation of E2F1 [76]. In addition, Gong et al. studied 3495 differentially expressed genes (DEGs) in cobimetinib-treated and untreated HCT116 cells and found that E2F1 was downregulated after treatment with cobimetinib, a drug that was found to increase the efficacy of 5-FU by decreasing TYMS expression in the same study [77]. In order to improve the therapeutic effect and counter 5-FU resistance in CRC, Liu et al. tested the herbal formula, Huang Qin Ge Gen Tang (HQGGT), in 5-FU-resistant cells (H630R1) and mouse colon cancer cells (MC38); HQGGT and 5-FU were found to exert synergistic effects through the modulation of the E2F1/TS pathway [78]. After studying another Chinese medicine, PHY906, Su et al. demonstrated E2F1 as a potential drug target in CRC by using the Gene Expression Omnibus (GEO) database [79]. In our recent work, a high level of E2F1 was detected in oxaliplatin-resistant CRC cells, which induced CHK1 expression, and knockdown of E2F1 undermined this resistance [52]. Furthermore, Bochicchio et al. developed nanoliposomes loaded with an siRNA against E2F1. These nanoliposomes were delivered into human colorectal adenocarcinoma cell lines and human intestinal biopsies. The vesicles loaded with siE2F1 were effective in reducing the growth of CRC cells [80]. Furthermore, triptolide is a natural compound isolated from Tripterygium wilfordi, which has a potent anti-tumor property against many types of cancers [81-83]. Oliveira et al. found that triptolide attenuated the xenograft growth and liver metastasis of CRC by suppressing the transcriptional activation of E2F1 [84]. Similarly, an ethanol extract of Innotus obliquus [85], methylselenol [86], irinotecan [87], a non-digestible fraction of beans (Phaseolus vulgaris L.) [88], SU9516 [89], ixocarpalactone [90], alpha-tocopherol succinate (TS) [91], and tetrandrine [92] yielded identical results, in which their anti-tumor properties in CRC were matched with the downregulation of E2F1.
Table 1.
Summary of E2F1-targeted drugs in CRC
| Agents | Properties | Effects on E2F1 | Anti-tumor properties | Ref. |
|---|---|---|---|---|
| Resveratrol | Polyphenolic compound | TTP-induced destabilization of E2F1 mRNA | Inhibiting the proliferation and invasion/metastasis of CRC cells | (76) |
| Cobimetinib | MEK inhibitor | Downregulation of E2F1 mRNA | Decreasing cell viability and inducing cell cycle arrest and apoptosis in CRC cells; increasing the efficacy of 5-FU | (77) |
| HQGGT | Traditional Chinese herbal medicine | Downregulation of E2F1 mRNA and protein | Suppressing the in vivo and in vitro growth of CRC cells with induction of apoptosis; enhancing the cytotoxicity of 5-FU | (78) |
| Nanoliposomes (siRNA against E2F1) | siE2F1 loaded nanoliposomes with about 40 nm size | Downregulation of E2F1 mRNA and protein | Suppressing the growth of CRC cells | (80) |
| Triptolide | diterpene triepoxide from Tripterygium wilfordi | Inhibition of transcriptional activity of E2F1 | Decreasing the in vitro growth of CRC cells; decreasing xenograft growth and liver metastasis of CRC | (84) |
| Ethanol extract of Inonotus obliquus | Bioactive compounds | Inhibition of phosphorylation of Rb and E2F1 protein | Arresting the cell cycle and inhibiting the growth of CRC cells | (85) |
| Methylselenol | Selenium metabolite | Reduction in E2F1 protein levels | Arresting the cell cycle and inhibiting the in vitro growth of mouse CRC cells; decreasing the xenograft growth of mouse CRC cells | (86) |
| Irinotecan | Samptothecin derivative; topoisomerase I inhibitor | Decrease in E2F1 protein levels | Overcoming the resistance to 5-FU; inhibiting the xenograft growth of 5-FU resistant CRC cells in combination with S-1 | (87) |
| Non-digestible fraction of beans | Extraction of beans | Downregulation of E2F1 mRNA | Contributing to the chemoprotective effect on early-stage CRC | (88) |
| SU9516 | 3-substituted indolinone compound; CDK2 inhibitor | Selective inhibition of cdk2 activity to prevent dissociation of pRb from E2F1 | May mediate cell growth arrest | (89) |
| Ixocarpalactone A | Withanolide from P. philadelphica | Downregulation of E2F-1 and DP-1 proteins | Inducing cell cycle arrest, apoptosis, and formation of multiple vacuoles in CRC cells | (90) |
| RRR-α-Tocopherol succinate | Analogue of vitamin E | Downregulation of E2F-1 protein | Inducing apoptosis and inhibiting cell proliferation and colony formation in CRC cells | (91) |
| Tetrandrine | Bis-benzylisoquinoline alkaloid from Stephania tetrandra | Induction of proteasome-dependent degradation of E2F1 | Inhibiting proliferation, arresting cell cycle, and inducing apoptosis in CRC cells | (92) |
E2F1 paradox in colon cancer
Aside from the pro-tumorigenic effects of E2F1, some studies have reported contradictory results that are worth further examination and provide an interesting counterpoint to the roles of E2F1 in colon cancer (Figure 1). In one such study, lentiviral-vector-mediated knockdown of CDK-8 led to a decreased metastasis and invasion of CRC cells with a parallel increase in E2F1 expression [93]. Lin et al. transfected an ectopic E2F1 adenoviral vector (AdCMVE2F-1) into human CRC cells, and E2F1 overexpression displayed a synergistic antitumor effect with gemcitabine [94]. It was suspected that an unwarranted entry into the S phase was responsible for the apoptotic action of E2F1. Similar results were supported by a previous study, highlighting a correlation between increased E2F1 expression and enhanced apoptosis of both SW620 and HT-29 CRC cells in vitro [95]. Earlier studies also demonstrated increased chemosensitization after E2F1 transfusion [96]. p53 is a tumor suppressor protein that plays a vital role in preventing cancers primarily through cell-cycle arrest and apoptosis [97]. It is also well known that mdm2 inversely regulates p53 expression by competing with its binding site, thereby disrupting its transcriptional activity [98]. In 2008, an American research group pointed out that Nutlin-3, an anticancer drug candidate, induced cellular apoptosis in a human CRC cell line by inhibiting pRb. Furthermore, they showed that the pro-apoptotic effect of Nutlin-3 was dependent upon p53 and E2F1 transcriptional activities partially through the inhibition of pRb [99]. Likewise, knockdown of phosphatase nuclear targeting subunit (PNUTS) in CRC cells promoted the efficacy of roscovitine by stimulating phosphatase activity toward pRb, which was dependent on the activity of E2F1 [100]. Previously, the same research group found that knockdown of PNUTS activated Rb phosphatase, subsequently causing Rb de-phosphorylation and E2F1-dependent apoptosis via caspase-8. This report also reported that the apoptotic mechanism did not rely on p53 [101]. A similar pro-apoptotic behavior of E2F1 via phosphorylation of Rb was further confirmed in zebrafish embryos [102]. Additionally, Evangelou et al. presented identical findings where E2F1 overexpression correlated with reduced proliferation and a better prognosis in colonic adenocarcinomas. However, the authors admitted a diverging result in oesophageal squamous-cell carcinomas (OSCCs), which positively correlated E2F1 with cancer progression. It was suspected that these conflicting results stemmed from the intestinal nature of the metaplastic Barrett mucosa [103]. Hao et al. found that E2F1-induced pro-apoptotic activity in CRC cells was regulated by PUMA upregulation and Bax translocation [104]. Another report indicated that E2F1 induced apoptosis of CRC cells through activating the transcription of SIVA1 apoptosis inducing factor (SIVA1) [105]. Although all of these inconsistent findings present a paradox, it is notable that they mostly occur after exogenous transfection of E2F1. In particular, our recent work showed that exogenous overexpression of E2F1 upregulated the levels of p73 and apoptotic peptidase activating factor 1 (Apaf1) and was accompanied with increased apoptosis in oxaliplatin-treated CRC cells, while endogenous E2F1 deacetylated by Sirt1 conferred the resistance in oxaliplatin-resistant CRC cells [52]. Thus, we suspect a mechanism in which exogenous E2F1 protein with different modifications activates different downstream genes as compared to its local endogenous interplay.
Conclusion and future perspectives
Undoubtedly, E2F1 is a major player in CRC, as it positively correlates with cancer development and poor prognosis. Downregulation of E2F1 has been shown to be helpful in reducing CRC burden. However, other studies have described an intriguing pro-apoptotic effect of E2F1. Although the pro-apoptotic behavior of E2F1 relies predominantly on exogenous administration of E2F1 in vitro, most of the in vivo studies have associated E2F1 with poor prognosis and development of CRC. It is likely that endogenous E2F1 neutralizes the anticipated pro-apoptotic property of E2F1 in a CRC microenvironment. Both the dual carcinogenic and pro-apoptotic effects of E2F1 represent an ongoing paradox for the scientific community. Although evidence has established an pro-tumorigenic property of E2F1, other opposing views cannot be ignored. In our opinion, future work on E2F1 in CRC should focus on the following questions:
1) How is the pro-apoptotic function of E2F1 in CRC prevented in the presence of its increased expression? 2) Does E2F1 behave differently in different kinds of cancer? 3) How do specific drug candidates against E2F1 inhibit the development of CRC?
Conclusively, E2F1 in CRC is a prospective drug target that currently lacks proper elucidation. Future research focused on answering the above questions may help to better understand the roles of E2F1 in CRC and may represent a key step in improving CRC therapeutics.
Acknowledgements
This work was mainly supported by the National Natural Science Foundation of China (81702401), Zhejiang Provincial Natural Science Foundation of China (LQ20H160004), Zhejiang Medical and Health Science and Technology Plan (2018KY920), and the Applied Basic Research Plan of Xuzhou (KC18031). We thank Letpub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–40. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, Holter W, Rauch A, Zhuang Y, Reizis B. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovesdi I, Reichel R, Nevins JR. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986;45:219–28. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZL, Bi XW, Liu PP, Lei DX, Wang Y, Li ZM, Jiang WQ, Xia Y. Expressions and prognostic values of the E2F transcription factors in human breast carcinoma. Cancer Manag Res. 2018;10:3521–3532. doi: 10.2147/CMAR.S172332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovesdi I, Reichel R, Nevins JR. Role of an adenovirus E2 promoter binding factor in E1A-mediated coordinate gene control. Proc Natl Acad Sci U S A. 1987;84:2180–4. doi: 10.1073/pnas.84.8.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yee AS, Reichel R, Kovesdi I, Nevins JR. Promoter interaction of the E1A-inducible factor E2F and its potential role in the formation of a multi-component complex. EMBO J. 1987;6:2061–8. doi: 10.1002/j.1460-2075.1987.tb02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandara LR, La Thangue NB. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351:494–7. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- 8.Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–61. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 9.Velez-Cruz R, Johnson DG. The retinoblastoma (RB) tumor suppressor: pushing back against genome instability on multiple fronts. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18081776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 11.Lees JA, Saito M, Vidal M, Valentine M, Look T, Harlow E, Dyson N, Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993;13:7813–25. doi: 10.1128/mcb.13.12.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poppy Roworth A, Ghari F, La Thangue NB. To live or let die - complexity within the E2F1 pathway. Mol Cell Oncol. 2015;2:e970480. doi: 10.4161/23723548.2014.970480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiti B, Li J, de Bruin A, Gordon F, Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W, Leone G. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2005;280:18211–20. doi: 10.1074/jbc.M501410200. [DOI] [PubMed] [Google Scholar]
- 14.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–9. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 15.Choi EH, Kim KP. E2F1 facilitates DNA break repair by localizing to break sites and enhancing the expression of homologous recombination factors. Exp Mol Med. 2019;51:106. doi: 10.1038/s12276-019-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Levine AJ. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci U S A. 1994;91:3602–6. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Li CJ, Reddy PV, Pardee AB. Cancer chemotherapy by deoxynucleotide depletion and E2F-1 elevation. Cancer Res. 2005;65:7809–14. doi: 10.1158/0008-5472.CAN-05-0888. [DOI] [PubMed] [Google Scholar]
- 18.Qi JJ, Liu L, Cao JX, An GS, Li SY, Li G, Jia HT, Ni JH. E2F1 regulates p53R2 gene expression in p53-deficient cells. Mol Cell Biochem. 2015;399:179–88. doi: 10.1007/s11010-014-2244-7. [DOI] [PubMed] [Google Scholar]
- 19.Stanelle J, Stiewe T, Theseling CC, Peter M, Putzer BM. Gene expression changes in response to E2F1 activation. Nucleic Acids Res. 2002;30:1859–67. doi: 10.1093/nar/30.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–24. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim YD, Kim HY. E2F1 expression is related with the poor survival of lymph node-positive breast cancer patients treated with fluorouracil, doxorubicin and cyclophosphamide. Breast Cancer Res Treat. 2003;82:11–6. doi: 10.1023/B:BREA.0000003843.53726.63. [DOI] [PubMed] [Google Scholar]
- 22.Fang Z, Gong C, Liu H, Zhang X, Mei L, Song M, Qiu L, Luo S, Zhu Z, Zhang R, Gu H, Chen X. E2F1 promote the aggressiveness of human colorectal cancer by activating the ribonucleotide reductase small subunit M2. Biochem Biophys Res Commun. 2015;464:407–15. doi: 10.1016/j.bbrc.2015.06.103. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Gong C, Mao H, Li Z, Fang Z, Chen Q, Lin M, Jiang X, Hu Y, Wang W, Zhang X, Chen X, Li H. E2F1/SP3/STAT6 axis is required for IL-4-induced epithelial-mesenchymal transition of colorectal cancer cells. Int J Oncol. 2018;53:567–578. doi: 10.3892/ijo.2018.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB, Danenberg PV. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–7. [PubMed] [Google Scholar]
- 25.Showalter SL, Showalter TN, Witkiewicz A, Havens R, Kennedy EP, Hucl T, Kern SE, Yeo CJ, Brody JR. Evaluating the drug-target relationship between thymidylate synthase expression and tumor response to 5-fluorouracil. Is it time to move forward? Cancer Biol Ther. 2008;7:986–94. doi: 10.4161/cbt.7.7.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varghese V, Magnani L, Harada-Shoji N, Mauri F, Szydlo RM, Yao S, Lam EW, Kenny LM. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci Rep. 2019;9:1505. doi: 10.1038/s41598-018-38017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Zheng S, Yu Q. The E2F family and the role of E2F1 in apoptosis. Int J Biochem Cell Biol. 2009;41:2389–97. doi: 10.1016/j.biocel.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Millour J, de Olano N, Horimoto Y, Monteiro LJ, Langer JK, Aligue R, Hajji N, Lam EW. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol Cancer Ther. 2011;10:1046–58. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Intuyod K, Saavedra-Garcia P, Zona S, Lai CF, Jiramongkol Y, Vaeteewoottacharn K, Pairojkul C, Yao S, Yong JS, Trakansuebkul S, Waraasawapati S, Luvira V, Wongkham S, Pinlaor S, Lam EW. FOXM1 modulates 5-fluorouracil sensitivity in cholangiocarcinoma through thymidylate synthase (TYMS): implications of FOXM1-TYMS axis uncoupling in 5-FU resistance. Cell Death Dis. 2018;9:1185. doi: 10.1038/s41419-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Osada S, Gotoh A, Yokoi R, Tsuchiya H, Sakuratani T, Sasaki Y, Okumura N, Hayashi H, Mukai T. Effective timing of surgical resection of colorectal cancer liver metastases during chemotherapy. Anticancer Res. 2018;38:737–743. doi: 10.21873/anticanres.12279. [DOI] [PubMed] [Google Scholar]
- 31.Sulzyc-Bielicka V, Domagala P, Bielicki D, Safranow K, Rogowski W, Domagala W. E2F1/TS immunophenotype and survival of patients with colorectal cancer treated with 5FU-based adjuvant therapy. Pathol Oncol Res. 2016;22:601–8. doi: 10.1007/s12253-016-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagaraju GP, Alese OB, Landry J, Diaz R, El-Rayes BF. HSP90 inhibition downregulates thymidylate synthase and sensitizes colorectal cancer cell lines to the effect of 5FU-based chemotherapy. Oncotarget. 2014;5:9980–91. doi: 10.18632/oncotarget.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denechaud PD, Fajas L, Giralt A. E2F1, a novel regulator of metabolism. Front Endocrinol (Lausanne) 2017;8:311. doi: 10.3389/fendo.2017.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long J, Zhang CJ, Zhu N, Du K, Yin YF, Tan X, Liao DF, Qin L. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res. 2018;8:778–791. [PMC free article] [PubMed] [Google Scholar]
- 35.Brown RE, Short SP, Williams CS. Colorectal cancer and metabolism. Curr Colorectal Cancer Rep. 2018;14:226–241. doi: 10.1007/s11888-018-0420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan PY, Chang CW, Duan K, Poidinger M, Ng KL, Chong YS, Gluckman PD, Stunkel W. E2F1 orchestrates transcriptomics and oxidative metabolism in wharton’s jelly-derived mesenchymal stem cells from growth-restricted infants. PLoS One. 2016;11:e0163035. doi: 10.1371/journal.pone.0163035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moniz S, Bandarra D, Biddlestone J, Campbell KJ, Komander D, Bremm A, Rocha S. Cezanne regulates E2F1-dependent HIF2alpha expression. J Cell Sci. 2015;128:3082–93. doi: 10.1242/jcs.168864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morzyglod L, Cauzac M, Popineau L, Denechaud PD, Fajas L, Ragazzon B, Fauveau V, Planchais J, Vasseur-Cognet M, Fartoux L, Scatton O, Rosmorduc O, Guilmeau S, Postic C, Desdouets C, Desbois-Mouthon C, Burnol AF. Growth factor receptor binding protein 14 inhibition triggers insulin-induced mouse hepatocyte proliferation and is associated with hepatocellular carcinoma. Hepatology. 2017;65:1352–1368. doi: 10.1002/hep.28972. [DOI] [PubMed] [Google Scholar]
- 40.Sanmartin-Salinas P, Lobo M, Noguerales-Fraguas F, Londono MT, Jimenez-Ruiz A, Guijarro LG. Insulin receptor substrate-4 is overexpressed in colorectal cancer and promotes retinoblastoma-cyclin-dependent kinase activation. J Gastroenterol. 2018;53:932–944. doi: 10.1007/s00535-018-1432-8. [DOI] [PubMed] [Google Scholar]
- 41.Carnero A. Spinophilin: a new tumor suppressor at 17q21. Curr Mol Med. 2012;12:528–35. doi: 10.2174/156652412800619987. [DOI] [PubMed] [Google Scholar]
- 42.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3:502–11. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ress AL, Stiegelbauer V, Schwarzenbacher D, Deutsch A, Perakis S, Ling H, Ivan C, Calin GA, Rinner B, Gerger A, Pichler M. Spinophilin expression determines cellular growth, cancer stemness and 5-flourouracil resistance in colorectal cancer. Oncotarget. 2014;5:8492–502. doi: 10.18632/oncotarget.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Z, Li X, Li J, Luo W, Huang C, Chen J. X-linked inhibitor of apoptosis protein (XIAP) lacking RING domain localizes to the nuclear and promotes cancer cell anchorage-independent growth by targeting the E2F1/Cyclin E axis. Oncotarget. 2014;5:7126–37. doi: 10.18632/oncotarget.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma O, Cai WW, Zender L, Dayaram T, Shen J, Herron AJ, Lowe SW, Man TK, Lau CC, Donehower LA. MMP13, Birc2 (cIAP1), and Birc3 (cIAP2), amplified on chromosome 9, collaborate with p53 deficiency in mouse osteosarcoma progression. Cancer Res. 2009;69:2559–67. doi: 10.1158/0008-5472.CAN-08-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung SA, Park YM, Hong SW, Moon JH, Shin JS, Lee HR, Ha SH, Lee DH, Kim JH, Kim SM, Kim JE, Kim KP, Hong YS, Choi EK, Lee JS, Jin DH, Kim T. Cellular inhibitor of apoptosis protein 1 (cIAP1) stability contributes to YM155 resistance in human gastric cancer cells. J Biol Chem. 2015;290:9974–85. doi: 10.1074/jbc.M114.600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plenchette S, Cathelin S, Rebe C, Launay S, Ladoire S, Sordet O, Ponnelle T, Debili N, Phan TH, Padua RA, Dubrez-Daloz L, Solary E. Translocation of the inhibitor of apoptosis protein c-IAP1 from the nucleus to the Golgi in hematopoietic cells undergoing differentiation: a nuclear export signal-mediated event. Blood. 2004;104:2035–43. doi: 10.1182/blood-2004-01-0065. [DOI] [PubMed] [Google Scholar]
- 48.Samuel T, Okada K, Hyer M, Welsh K, Zapata JM, Reed JC. cIAP1 Localizes to the nuclear compartment and modulates the cell cycle. Cancer Res. 2005;65:210–8. [PubMed] [Google Scholar]
- 49.Cartier J, Berthelet J, Marivin A, Gemble S, Edmond V, Plenchette S, Lagrange B, Hammann A, Dupoux A, Delva L, Eymin B, Solary E, Dubrez L. Cellular inhibitor of apoptosis protein-1 (cIAP1) can regulate E2F1 transcription factor-mediated control of cyclin transcription. J Biol Chem. 2011;286:26406–17. doi: 10.1074/jbc.M110.191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mertens PR, Harendza S, Pollock AS, Lovett DH. Glomerular mesangial cell-specific transactivation of matrix metalloproteinase 2 transcription is mediated by YB-1. J Biol Chem. 1997;272:22905–12. doi: 10.1074/jbc.272.36.22905. [DOI] [PubMed] [Google Scholar]
- 51.Lasham A, Samuel W, Cao H, Patel R, Mehta R, Stern JL, Reid G, Woolley AG, Miller LD, Black MA, Shelling AN, Print CG, Braithwaite AW. YB-1, the E2F pathway, and regulation of tumor cell growth. J Natl Cancer Inst. 2012;104:133–46. doi: 10.1093/jnci/djr512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang Z, Gong C, Yu S, Zhou W, Hassan W, Li H, Wang X, Hu Y, Gu K, Chen X, Hong B, Bao Y, Chen X, Zhang X, Liu H. NFYB-induced high expression of E2F1 contributes to oxaliplatin resistance in colorectal cancer via the enhancement of CHK1 signaling. Cancer Lett. 2018;415:58–72. doi: 10.1016/j.canlet.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 53.Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30:127–38. doi: 10.1038/onc.2010.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toivola DM, Boor P, Alam C, Strnad P. Keratins in health and disease. Curr Opin Cell Biol. 2015;32:73–81. doi: 10.1016/j.ceb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Xu X, Nie Y, Dai L, Wang P, Zhang J. Identification of tumor-associated antigens by using SEREX in hepatocellular carcinoma. Cancer Lett. 2009;281:144–50. doi: 10.1016/j.canlet.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 56.Birkenkamp-Demtroder K, Christensen LL, Olesen SH, Frederiksen CM, Laiho P, Aaltonen LA, Laurberg S, Sorensen FB, Hagemann R, TF OR. Gene expression in colorectal cancer. Cancer Res. 2002;62:4352–63. [PubMed] [Google Scholar]
- 57.Birkenkamp-Demtroder K, Mansilla F, Sorensen FB, Kruhoffer M, Cabezon T, Christensen LL, Aaltonen LA, Verspaget HW, Orntoft TF. Phosphoprotein Keratin 23 accumulates in MSS but not MSI colon cancers in vivo and impacts viability and proliferation in vitro. Mol Oncol. 2007;1:181–95. doi: 10.1016/j.molonc.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birkenkamp-Demtroder K, Hahn SA, Mansilla F, Thorsen K, Maghnouj A, Christensen R, Oster B, Orntoft TF. Keratin23 (KRT23) knockdown decreases proliferation and affects the DNA damage response of colon cancer cells. PLoS One. 2013;8:e73593. doi: 10.1371/journal.pone.0073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–92. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 60.Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9:813–24. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouyang N, Ke S, Eagleton N, Xie Y, Chen G, Laffins B, Yao H, Zhou B, Tian Y. Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Br J Cancer. 2010;102:1753–61. doi: 10.1038/sj.bjc.6605677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Latini FR, Hemerly JP, Freitas BC, Oler G, Riggins GJ, Cerutti JM. ABI3 ectopic expression reduces in vitro and in vivo cell growth properties while inducing senescence. BMC Cancer. 2011;11:11. doi: 10.1186/1471-2407-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q, Dashwood WM, Zhong X, Nakagama H, Dashwood RH. Bcl-2 overexpression in PhIP-induced colon tumors: cloning of the rat Bcl-2 promoter and characterization of a pathway involving beta-catenin, c-Myc and E2F1. Oncogene. 2007;26:6194–202. doi: 10.1038/sj.onc.1210438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ying L, Marino J, Hussain SP, Khan MA, You S, Hofseth AB, Trivers GE, Dixon DA, Harris CC, Hofseth LJ. Chronic inflammation promotes retinoblastoma protein hyperphosphorylation and E2F1 activation. Cancer Res. 2005;65:9132–6. doi: 10.1158/0008-5472.CAN-05-1358. [DOI] [PubMed] [Google Scholar]
- 65.Russo I, Carrizzo A, Bochicchio S, Piazza O, Lamberti G, Barba AA, Vecchione C, Zeppa P, Iovino P, Bucci C, Santonicola A, Ciacci C. siRNA delivery for control of cyclin D1 and E2F1 expression in crohn’s disease. Transl Med UniSa. 2018;17:25–33. [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HS, Chen M, Kim JH, Kim WH, Ahn S, Maeng K, Allegra CJ, Kaye FJ, Hochwald SN, Zajac-Kaye M. Analysis of 320 gastroenteropancreatic neuroendocrine tumors identifies TS expression as independent biomarker for survival. Int J Cancer. 2014;135:128–37. doi: 10.1002/ijc.28675. [DOI] [PubMed] [Google Scholar]
- 67.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–13. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 68.Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin Cancer Res. 2002;8:1228–36. [PubMed] [Google Scholar]
- 69.Ying L, Hofseth AB, Browning DD, Nagarkatti M, Nagarkatti PS, Hofseth LJ. Nitric oxide inactivates the retinoblastoma pathway in chronic inflammation. Cancer Res. 2007;67:9286–93. doi: 10.1158/0008-5472.CAN-07-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen LL, Tobiasen H, Holm A, Schepeler T, Ostenfeld MS, Thorsen K, Rasmussen MH, Birkenkamp-Demtroeder K, Sieber OM, Gibbs P, Lubinski J, Lamy P, Laurberg S, Oster B, Hansen KQ, Hagemann-Madsen R, Byskov K, Orntoft TF, Andersen CL. MiRNA-362-3p induces cell cycle arrest through targeting of E2F1, USF2 and PTPN1 and is associated with recurrence of colorectal cancer. Int J Cancer. 2013;133:67–78. doi: 10.1002/ijc.28010. [DOI] [PubMed] [Google Scholar]
- 71.Monzo M, Navarro A, Bandres E, Artells R, Moreno I, Gel B, Ibeas R, Moreno J, Martinez F, Diaz T, Martinez A, Balague O, Garcia-Foncillas J. Overlapping expression of microRNAs in human embryonic colon and colorectal cancer. Cell Res. 2008;18:823–33. doi: 10.1038/cr.2008.81. [DOI] [PubMed] [Google Scholar]
- 72.Fang Z, Yang H, Chen D, Shi X, Wang Q, Gong C, Xu X, Liu H, Lin M, Lin J, Xu C, Shao J. YY1 promotes colorectal cancer proliferation through the miR-526b-3p/E2F1 axis. Am J Cancer Res. 2019;9:2679–2692. [PMC free article] [PubMed] [Google Scholar]
- 73.Kanaan Z, Rai SN, Eichenberger MR, Barnes C, Dworkin AM, Weller C, Cohen E, Roberts H, Keskey B, Petras RE, Crawford NP, Galandiuk S. Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Hum Mutat. 2012;33:551–60. doi: 10.1002/humu.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye Y, Gu B, Wang Y, Shen S, Huang W. E2F1-mediated MNX1-AS1-miR-218-5p-SEC61A1 feedback loop contributes to the progression of colon adenocarcinoma. J Cell Biochem. 2019;120:6145–6153. doi: 10.1002/jcb.27902. [DOI] [PubMed] [Google Scholar]
- 75.Lee HH, Lee SR, Leem SH. Tristetraprolin regulates prostate cancer cell growth through suppression of E2F1. J Microbiol Biotechnol. 2014;24:287–94. doi: 10.4014/jmb.1309.09070. [DOI] [PubMed] [Google Scholar]
- 76.Lee SR, Jin H, Kim WT, Kim WJ, Kim SZ, Leem SH, Kim SM. Tristetraprolin activation by resveratrol inhibits the proliferation and metastasis of colorectal cancer cells. Int J Oncol. 2018;53:1269–1278. doi: 10.3892/ijo.2018.4453. [DOI] [PubMed] [Google Scholar]
- 77.Gong S, Xu D, Zhu J, Zou F, Peng R. Efficacy of the MEK inhibitor cobimetinib and its potential application to colorectal cancer cells. Cell Physiol Biochem. 2018;47:680–693. doi: 10.1159/000490022. [DOI] [PubMed] [Google Scholar]
- 78.Liu H, Liu H, Zhou Z, Parise RA, Chu E, Schmitz JC. Herbal formula Huang Qin Ge Gen Tang enhances 5-fluorouracil antitumor activity through modulation of the E2F1/TS pathway. Cell Commun Signal. 2018;16:7. doi: 10.1186/s12964-018-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su Z, Zhou C, Qin S, Jia E, Wu Z. The significant pathways and genes underlying the colon cancer treatment by the traditional Chinese medicine PHY906. Evid Based Complement Alternat Med. 2017;2017:8753815. doi: 10.1155/2017/8753815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bochicchio S, Dapas B, Russo I, Ciacci C, Piazza O, De Smedt S, Pottie E, Barba AA, Grassi G. In vitro and ex vivo delivery of tailored siRNA-nanoliposomes for E2F1 silencing as a potential therapy for colorectal cancer. Int J Pharm. 2017;525:377–387. doi: 10.1016/j.ijpharm.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 81.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–16. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 82.Clawson KA, Borja-Cacho D, Antonoff MB, Saluja AK, Vickers SM. Triptolide and TRAIL combination enhances apoptosis in cholangiocarcinoma. J Surg Res. 2010;163:244–9. doi: 10.1016/j.jss.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- 84.Oliveira A, Beyer G, Chugh R, Skube SJ, Majumder K, Banerjee S, Sangwan V, Li L, Dawra R, Subramanian S, Saluja A, Dudeja V. Triptolide abrogates growth of colon cancer and induces cell cycle arrest by inhibiting transcriptional activation of E2F. Lab Invest. 2015;95:648–659. doi: 10.1038/labinvest.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee HS, Kim EJ, Kim SH. Ethanol extract of Innotus obliquus (Chaga mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells. Nutr Res Pract. 2015;9:111–6. doi: 10.4162/nrp.2015.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng H, Cheng WH, Johnson LK. Methylselenol, a selenium metabolite, modulates p53 pathway and inhibits the growth of colon cancer xenografts in Balb/c mice. J Nutr Biochem. 2013;24:776–80. doi: 10.1016/j.jnutbio.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Fukushima M, Sakamoto K, Ohshimo H, Nakagawa F, Taguchi T. Irinotecan overcomes the resistance to 5-fluorouracil in human colon cancer xenografts by down-regulation of intratumoral thymidylate synthase. Oncol Rep. 2010;24:835–42. doi: 10.3892/or.2010.835. [DOI] [PubMed] [Google Scholar]
- 88.Hayde VC, Ramon GG, Lorenzo GO, Dave OB, Rosalia RC, Paul W, Guadalupe LP. Non-digestible fraction of beans (Phaseolus vulgaris L.) modulates signalling pathway genes at an early stage of colon cancer in Sprague-Dawley rats. Br J Nutr. 2012;108(Suppl 1):S145–54. doi: 10.1017/S0007114512000785. [DOI] [PubMed] [Google Scholar]
- 89.Yu B, Lane ME, Wadler S. SU9516, a cyclin-dependent kinase 2 inhibitor, promotes accumulation of high molecular weight E2F complexes in human colon carcinoma cells. Biochem Pharmacol. 2002;64:1091–100. doi: 10.1016/s0006-2952(02)01264-9. [DOI] [PubMed] [Google Scholar]
- 90.Choi JK, Murillo G, Su BN, Pezzuto JM, Kinghorn AD, Mehta RG. Ixocarpalactone a isolated from the Mexican tomatillo shows potent antiproliferative and apoptotic activity in colon cancer cells. FEBS J. 2006;273:5714–23. doi: 10.1111/j.1742-4658.2006.05560.x. [DOI] [PubMed] [Google Scholar]
- 91.Donapaty S, Louis S, Horvath E, Kun J, Sebti SM, Malafa MP. RRR-alpha-tocopherol succinate down-regulates oncogenic Ras signaling. Mol Cancer Ther. 2006;5:309–16. doi: 10.1158/1535-7163.MCT-05-0330. [DOI] [PubMed] [Google Scholar]
- 92.Meng LH, Zhang H, Hayward L, Takemura H, Shao RG, Pommier Y. Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1. Cancer Res. 2004;64:9086–92. doi: 10.1158/0008-5472.CAN-04-0313. [DOI] [PubMed] [Google Scholar]
- 93.Cai WS, Shen F, Feng Z, Chen JW, Liu QC, Li EM, Xu B, Cao J. Downregulation of CDK-8 inhibits colon cancer hepatic metastasis by regulating Wnt/beta-catenin pathway. Biomed Pharmacother. 2015;74:153–7. doi: 10.1016/j.biopha.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 94.Lin Z, Ren N, Jiang Y, Xu W, Shi Y, Liu G. Adenovirus-mediated E2F-1 gene transfer augments gemcitabine-induced apoptosis in human colon cancer cells. Clin Lab. 2015;61:1435–44. doi: 10.7754/clin.lab.2015.150104. [DOI] [PubMed] [Google Scholar]
- 95.Elliott MJ, Stilwell A, Dong YB, Yang HL, Wong SL, Wrightson WR, Martin RC, McMasters KM. C-terminal deletion mutant p21(WAF1/CIP1) enhances E2F-1-mediated apoptosis in colon adenocarcinoma cells. Cancer Gene Ther. 2002;9:453–63. doi: 10.1038/sj.cgt.7700458. [DOI] [PubMed] [Google Scholar]
- 96.Dong YB, Yang HL, McMasters KM. E2F-1 overexpression sensitizes colorectal cancer cells to camptothecin. Cancer Gene Ther. 2003;10:168–78. doi: 10.1038/sj.cgt.7700565. [DOI] [PubMed] [Google Scholar]
- 97.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 98.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–45. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 99.Kitagawa M, Aonuma M, Lee SH, Fukutake S, McCormick F. E2F-1 transcriptional activity is a critical determinant of Mdm2 antagonist-induced apoptosis in human tumor cell lines. Oncogene. 2008;27:5303–14. doi: 10.1038/onc.2008.164. [DOI] [PubMed] [Google Scholar]
- 100.De Leon G, Cavino M, D’Angelo M, Krucher NA. PNUTS knockdown potentiates the apoptotic effect of Roscovitine in breast and colon cancer cells. Int J Oncol. 2010;36:1269–75. doi: 10.3892/ijo_00000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Leon G, Sherry TC, Krucher NA. Reduced expression of PNUTS leads to activation of Rb-phosphatase and caspase-mediated apoptosis. Cancer Biol Ther. 2008;7:833–41. doi: 10.4161/cbt.7.6.5839. [DOI] [PubMed] [Google Scholar]
- 102.Cucina A, Biava PM, D’Anselmi F, Coluccia P, Conti F, di Clemente R, Miccheli A, Frati L, Gulino A, Bizzarri M. Zebrafish embryo proteins induce apoptosis in human colon cancer cells (Caco2) Apoptosis. 2006;11:1617–28. doi: 10.1007/s10495-006-8895-4. [DOI] [PubMed] [Google Scholar]
- 103.Evangelou K, Kotsinas A, Mariolis-Sapsakos T, Giannopoulos A, Tsantoulis PK, Constantinides C, Troupis TG, Salmas M, Kyroudis A, Kittas C, Gorgoulis VG. E2F-1 overexpression correlates with decreased proliferation and better prognosis in adenocarcinomas of Barrett oesophagus. J Clin Pathol. 2008;61:601–5. doi: 10.1136/jcp.2007.050963. [DOI] [PubMed] [Google Scholar]
- 104.Hao H, Dong Y, Bowling MT, Gomez-Gutierrez JG, Zhou HS, McMasters KM. E2F-1 induces melanoma cell apoptosis via PUMA up-regulation and Bax translocation. BMC Cancer. 2007;7:24. doi: 10.1186/1471-2407-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ray RM, Bhattacharya S, Johnson LR. Mdm2 inhibition induces apoptosis in p53 deficient human colon cancer cells by activating p73- and E2F1-mediated expression of PUMA and Siva-1. Apoptosis. 2011;16:35–44. doi: 10.1007/s10495-010-0538-0. [DOI] [PubMed] [Google Scholar]


