Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a phthalate commonly used for its plasticizing capabilities. Because of the wide production and use of DEHP, humans are exposed to DEHP on a daily basis. Diisononyl phthalate (DiNP) is often used as a DEHP replacement chemical, and because of the increased use of DiNP, humans are increasingly exposed to DiNP over time. Of concern is that DEHP and DiNP both exhibit endocrine disrupting capabilities, and little is known about how short-term exposure to either of these phthalates affects aspects of female reproduction. Thus, this study tested the hypothesis that short-term exposure to DEHP or DiNP during adulthood has long-lasting consequences on ovarian follicles and hormones in female mice. Female CD-1 mice aged 39–40 days were orally dosed with either vehicle control (corn oil), DEHP (20 μg/kg/day–200 mg/kg/day), or DiNP (20 μg/kg/day–200 mg/kg/day) for 10 days. Ovarian follicle populations, estradiol, testosterone, progesterone, follicle stimulating hormone (FSH), and inhibin B were analyzed at time points immediately post-dosing and 3, 6, and 9 months post-dosing. The results indicate that 10 days of exposure to DEHP and DiNP changed the distribution of ovarian follicle populations and sex steroid hormones at multiple time points, including the last time point, 9 months post-dosing. Further, FSH was increased at multiple doses up to 6 months post-dosing. Inhibin B was not affected by treatment. These data show that short-term exposure to either DEHP or DiNP has long-term consequences that persist long after cessation of exposure.
Keywords: DEHP, DiNP, female reproduction, ovary, follicles, endocrine disruptors
1. INTRODUCTION
Maintenance of female fertility is a carefully controlled event with multiple inputs and feedback loops. Namely, female fertility is maintained by the cyclical rise and fall of a variety of hormones at key time points throughout the cycle. In females, the hypothalamus releases gonadotropin releasing hormone (GnRH), which travels to the pituitary via the hypophyseal portal system. Once GnRH arrives in the pituitary, it stimulates specialized cells called gonadotropes to release the gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH) (McArdle and Roberson 2015). FSH and LH are key components in proper development of the ovarian follicles, and LH initiates ovulation, which releases the mature oocyte for fertilization (Albertini 2015). The follicle is also a major player in the maintenance of female fertility. The follicle is the primary site of production of sex steroid hormones, including estradiol, testosterone, and progesterone. These hormones also contribute to the cyclic nature of female fertility, and they can exert both positive and negative feedback at the level of the hypothalamus, pituitary, or even at the level of the ovary by acting on neighboring follicles (Auchus 2015, McArdle and Roberson 2015). Additionally, they play roles in mating behavior as well as receptivity of the endometrial lining to an implanting embryo (Binder, Winuthayanon et al. 2015, Pfaus, Jones et al. 2015). Thus, disruption of the hormonal profiles may lead to disruption of follicular development and vice versa. In addition to disruption of fertility, altering levels of sex steroid hormones can elicit negative effects on somatic health. Disruption of the hormonal milieu in females has been linked to several negative health outcomes including cardiovascular disease, stroke, osteoporosis, and increased mortality (van der Schouw, van der Graaf et al. 1996, Cooper and Sandler 1998, Hu, Grodstein et al. 1999, Gallagher 2007, Rocca, Grossardt et al. 2012); thus, making reproductive health important for the overall health of the female (Chiang, Mahalingam et al. 2017).
Concerningly, a variety of environmental contaminants have been designated as endocrine disrupting chemicals that can impact the reproductive and overall health of exposed individuals. One such class of environmental endocrine disruptors is phthalates. Di(2-ethylhexyl) phthalate (DEHP) is a prominent member of the phthalate family and is commonly found in consumer products such as shower curtains, furniture and car upholstery, medical tubing, and blood storage bags (U.S. Department of Health and Human Services 2002). Because of the use of DEHP in a variety of consumer products, humans are ubiquitously exposed to DEHP on a daily basis at an estimated daily intake of 3–30 μg/kg/day for a 70kg adult (U.S. Department of Health and Human Services 2002). This is concerning because DEHP is an endocrine disruptor. Multiple studies have found that DEHP is associated with reduced anogenital distance, a marker of fetal androgens, at birth in humans (Radke, Braun et al. 2018), and DEHP has been shown to transgenerationally disrupt female reproductive parameters in animal studies (Brehm, Rattan et al. 2018, Rattan, Brehm et al. 2018). Further, DEHP is associated with reproductive disorders in women (Chiang, Mahalingam et al. 2017, Rattan, Zhou et al. 2017). One study found that women diagnosed with endometriosis had higher serum levels of DEHP than women without endometriosis (Nazir, Usman et al. 2018), and another study found that urinary DEHP metabolites were associated with increased uterine volume, which is used as a proxy for burden of uterine fibroids in women (Zota, Geller et al. 2019).
Diisononyl phthalate (DiNP) is another member of the phthalate family. Although a less prominent member of this family, DiNP production has been rising over the past several years ((ECHA) 2013), and subsequently, humans are being exposed to greater levels of DiNP as evidenced by rapidly increasing levels of DiNP metabolites in human urine samples (Zota, Calafat et al. 2014). Because DiNP is commonly used as a substitute for DEHP, it can be found in many of the same products (Commission 2010, (ECHA) 2013). However, few studies have been conducted that explore the reproductive consequences of exposure to DiNP, especially in terms of exposure during adulthood, and the scarce literature that is present indicates that DiNP has endocrine disrupting capabilities. For example, DiNP has been associated with reduced semen quality parameters and reduced testosterone in men in epidemiological studies (Radke, Braun et al. 2018). Additionally, one animal study found that in utero exposure to DiNP reduces intratesticular testosterone in fetal rats (Li, Bu et al. 2015), and previous studies have suggested the anti-androgenic effects of DiNP act through similar mechanisms of action as DEHP (Boberg, Christiansen et al. 2011). DiNP has also been shown to have negative effects in females. A study investigating reproductive outcomes in couples found that women with higher levels of serum DiNP had increased time to pregnancy (Specht, Bonde et al. 2015). Additionally, an animal study found that rats exposed through the maternal diet during gestation had reduced corpora lutea in the ovary at postnatal week 11 when compared to controls (Masutomi, Shibutani et al. 2003). Although these aforementioned studies have investigated the effects of DiNP on both male and female reproduction, a significant lack of studies that utilize environmentally relevant doses exists, and few studies have investigated the effects of DiNP exposure on follicle populations within the ovary or hormone levels in exposed females.
This study was designed to fill the gap in knowledge concerning the effects of short-term exposure to DEHP and DiNP during adulthood on major reproductive hormones and follicle populations within the ovary in female mice. Previously, our laboratory has shown that short-term exposure to both DEHP and DiNP affects cyclicity and fertility for up to nine months following completion of dosing (Hannon, Niermann et al. 2016, Chiang and Flaws 2019). However, it is still unknown if short-term exposure to DEHP or DiNP during adulthood affects follicle populations and hormones, including sex steroid hormones and peptide hormones. Thus, this study tested the hypothesis that short-term exposure to DEHP and DiNP during adulthood affects folliculogenesis and hormone levels in female mice.
MATERIALS AND METHODS
Chemicals
DEHP and DiNP were purchased from Sigma-Aldrich (St. Louis, MO). Corn oil (vehicle control) was purchased from MP Biomedicals (Solon, OH). Dosing stock solutions were created by serially diluting from the highest dose down to the lowest dose. Batches of stock solutions were stored at room temperature away from light for up to a month.
Animals and Dosing
Female CD-1 mice were purchased from Charles River (Wilmington, MA) and housed in the College of Veterinary Medicine Animal Facility at the University of Illinois at Urbana-Champaign (Urbana, IL). Mice were allowed to acclimate to the facilities for a minimum of 5 days before the dosing period started. Ambient temperature was maintained at 21.1 ± 2.2˚C, with humidity at 50 ± 20%. Mice were given ad libitum access to reverse osmosis-treated water and Teklad Rodent Diet (8604). Mice were kept on 12 hour light-dark cycles. All procedures involving animal handling were approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee (Protocol No.: 17079).
Mice were dosed orally via pipette at age 39–40 days with either vehicle control (corn oil), DEHP (20 μg/kg/day, 200 μg/kg/day, 20 mg/kg/day, 200 mg/kg/day), or DiNP (20 μg/kg/day, 100 μg/kg/day, 20 mg/kg/day, 200 mg/kg/day) for 10 consecutive days every morning at 2 hours following the start of the light cycle. Mice were weighed daily prior to dosing to determine the necessary dose volume each day. To minimize contamination between treatment groups, mice were group housed 3 to a cage, and all 3 mice within a single cage were assigned to the same treatment group. At the beginning of dosing for each time point, the original sample size for each treatment group was 6 mice per phthalate treatment and 12 mice in control groups. Final sample sizes used in statistical analyses for every result are reported in the figure legends and after every result in the results section. Sample sizes for the treatment groups less than 6 and control less than 12 are due to either unexpected death of an animal or removal of a data point as an outlier unless otherwise mentioned. Outliers were minimal throughout the study, and in no instance did any one group for any outcome have more than one outlier. Unexpected death of an animal was also minimal, and only 5 animals total were found dead or euthanized due to illness throughout the study.
The doses above were chosen because the 20 μg/kg/day DEHP dose is within the estimated range for average daily exposure for a 70kg adult (3–30 μg/kg/day) (U.S. Department of Health and Human Services 2002). The 200 μg/kg/day DEHP dose falls within the range for those that are occupationally exposed (143–286 μg/kg/day) (Kavlock, Boekelheide et al. 2002). The 20 and 200 mg/kg/day DEHP doses were chosen because they have been used in previous toxicological studies and also allow us to investigate a wide range of doses (Hannon, Peretz et al. 2014, Niermann, Rattan et al. 2015, Rattan, Brehm et al. 2018). The doses for DiNP were chosen for similar reasons. The 20 μg/kg/day DiNP dose falls within the estimated range for occupational exposure (up to 26 μg/kg/day) (Hines, Hopf et al. 2012). The 100 μg/kg/day DiNP dose is within the estimated range of exposure for infants who are chewing on plastic toys (up to 260 μg/kg/day) (CPSC 2001). Lastly, the 20 and 200 mg/kg/day DiNP doses were chosen because we wanted to directly compare the toxicity of DiNP to DEHP by using similar doses when possible.
Experimental Design
Groups of animals were euthanized via CO2 asphyxiation at different periods following completion of dosing: immediately post-dosing, 3 months post-dosing, 6 months post-dosing, and 9 months post-dosing. Females were lavaged every morning prior to euthanasia. Lavage samples were analyzed for cytology under a light microscope, and females in the diestrous stage of the cycle were designated for euthanasia and collection that day. Euthanasia during the diestrous stage was chosen because the hormone fluctuation during diestrus is minimal when compared to other stages of the estrous cycle, allowing for a better chance of lower variability in hormone endpoints (Levine 2015).
Assaying Levels of Sex Steroid Hormones and Peptide Hormones
Blood was collected in a heparinized needle from the inferior vena cava immediately following euthanasia in the diestrous stage of the cycle and allowed to clot at room temperature for a minimum of 15 minutes, followed by a minimum of 15 minutes in ice. Blood samples were then centrifuged at 4 °C at 14,000 RPM for 15 minutes. Sera that were separated out were collected and stored at −80 °C until use. Testosterone, progesterone, and estradiol ELISA kits were purchased from DRG®. Lypocheck® from Bio-Rad Laboratories was used as a control with known values for all DRG® ELISA kits. The limits of detection (LOD) for testosterone, progesterone, and estradiol were 0.083 ng/mL, 0.045 ng/mL, and 10.60 pg/mL, respectively. The inter- and intra-assay %CVs were ≤ 9.94 and ≤ 4.16, ≤ 9.96 and ≤ 6.99, and ≤ 14.91 and ≤ 9.23 for testosterone, progesterone, and estradiol, respectively. The total number of samples throughout the study that were below the LOD for testosterone, progesterone, and estradiol were 5, 4, and 1, respectively. Remaining serum samples were aliquoted and sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for analysis of levels of FSH (radioimmunoassay) and inhibin B (ELISA). The LODs for FSH and inhibin B were 1.6 ng/mL and 35 pg/mL, respectively. Intra- and inter-assay %CVs for FSH were 7.2 and 8.5, respectively, and intra- and inter-assay %CVs for inhibin B were 1.8 and 6.6, respectively (https://med.virginia.edu/research-in-reproduction/wp-content/uploads/sites/311/2019/04/2019-INTRA-INTER-ASSAY-CVs__030419.pdf). The number of samples below the LOD for FSH from immediately post-dosing, 3 months post-dosing, 6 months post-dosing, and 9 months post-dosing time points were 20, 32, 37, and 0, respectively. The only sample below the LOD for inhibin B was 1 sample from the 9 months post-dosing timepoint. Samples below the LOD were analyzed using the LOD value specific to the assay divided by the square root of 2.
Histological Evaluation of Ovarian Tissues
Ovaries were dissected out of mice, cleaned of gonadal fat, and fixed in Dietrich’s solution. Tissues remained in the fixative overnight at minimum and then were transferred to 70% ethanol before being embedded into paraffin wax. Ovaries were serially sectioned at 8 μm width onto microscope slides and stained with hematoxylin and eosin. Every 10th serial section was used to assess follicle populations using previously defined criteria (Pedersen and Peters 1968, Flaws, Doerr et al. 1994). Primordial follicles were designated as oocytes with a single layer of squamous granulosa cells, primary follicles were designated as oocytes surrounded by a single layer of cuboidal granulosa cells, preantral follicles were designated as oocytes surrounded by more than one layer of cuboidal granulosa cells, and antral follicles were designated as oocytes surrounded by multiple layers of cuboidal granulosa cells with a distinct fluid filled antrum (Hannon, Niermann et al. 2016, Rattan, Brehm et al. 2018). Nuclei must have been present in preantral and antral follicles to be counted to assure that one follicle was not counted twice across sections. Presence of the nucleus was not required for counting primordial or primary follicles because the follicles are too small to span multiple sections at this stage. Each ovary was assigned a unique ID with no information about treatment group to blind counters to treatment and prevent bias. Percentages of each follicle type were achieved by taking the raw number of a particular follicle type and dividing by the total number of follicles and multiplying by 100. All follicle types were summed together to determine the total number of follicles. Ovarian follicle population data for the DEHP treatment groups in the immediately post-dosing time point are not included herein because a study conducted by our research group previously published data on the immediate consequences of 10 days of exposure to DEHP on folliculogenesis (Hannon, Peretz et al. 2014).
Statistical Evaluation
Outliers for normally distributed data were identified and removed from analysis via Grubb’s Test (https://www.graphpad.com/quickcalcs/Grubbs1.cfm). Outliers were minimal throughout the study, and in no instance did any one group for any outcome have more than one outlier. If data met the assumptions of normality and homogeneity of variance, then a oneway analysis of variance (ANOVA) was used and followed by Dunnett T 2-sided tests. If data were non-parametric, not normally distributed, or lacked homogeneity of variance, then the Kruskal-Wallis test followed by the Mann-Whitney U test was used to compare treatment to control. All statistical analyses except those for determination of outliers were performed with SPSS statistical software (SPSS Inc., Chicago, Illinois). Significance was assigned at p ≤ 0.05.
RESULTS
Effects of DEHP and DiNP on ovarian follicle populations
Immediately Post-Dosing
A previous study in our lab found that 10 days of exposure to DEHP immediately disrupted folliculogenesis (Hannon, Peretz et al. 2014). In the present study, we investigated the immediate effects of 10 days of exposure to DiNP. Immediately post-dosing, 20 μg/kg/day of DiNP borderline increased the percent of antral follicles when compared to control (Fig. 1, n = 5–12 mice/group, p = 0.08). DiNP did not affect total follicle numbers when compared to control (data not shown).
Figure 1.
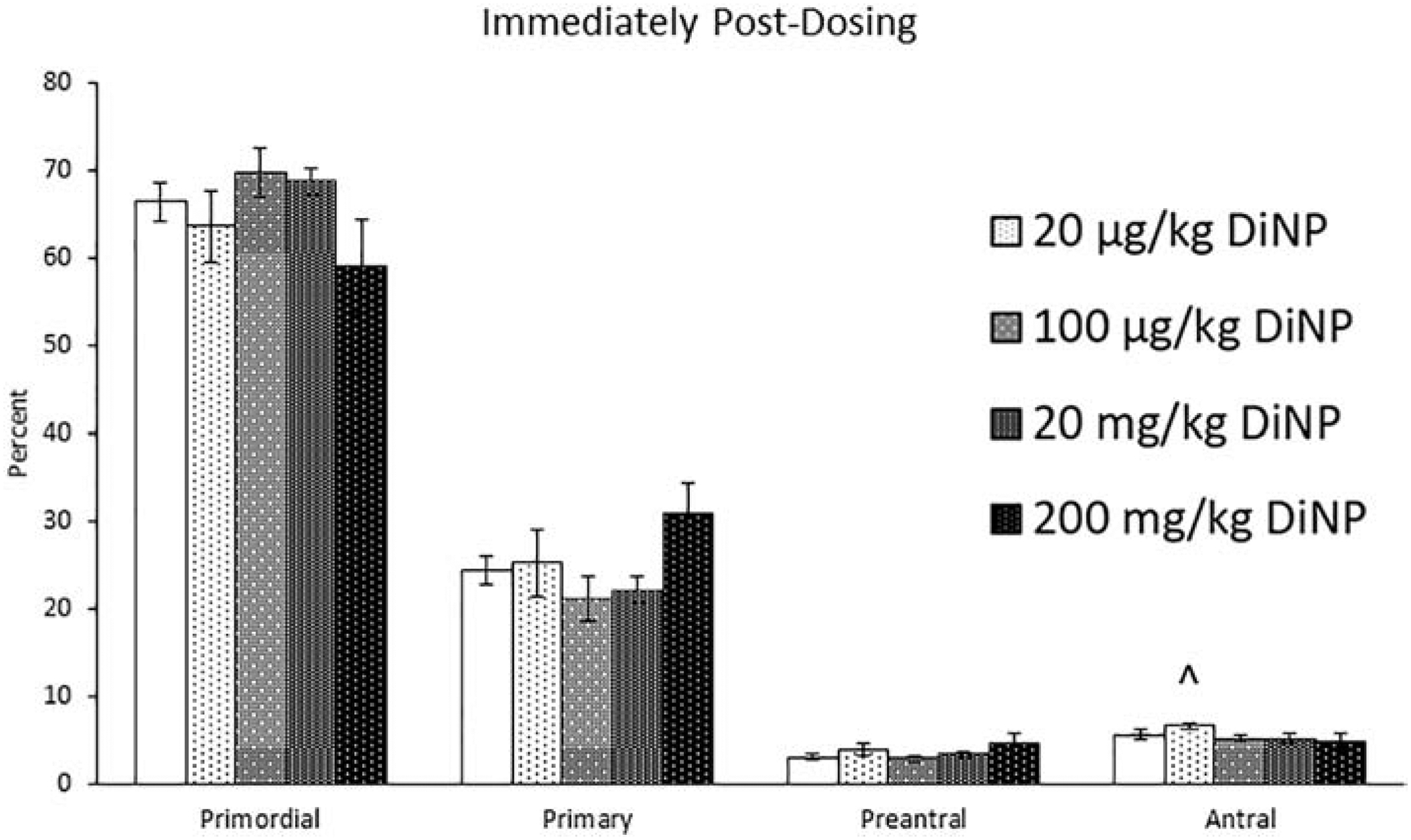
Effects of DiNP on ovarian follicle populations immediately post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil) or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle immediately following dosing and the ovaries were collected and processed for histological evaluation of the percent of healthy follicle types (n = 5–12 mice/group). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
3 Months Post-Dosing
At 3 months post-dosing, 100 μg/kg/day of DiNP decreased the percent of primordial follicles (Fig. 2, n = 4–11 mice/group, p ≤ 0.05) and borderline increased the percent of primary follicles when compared to control (Fig. 2, n = 4–11 mice/group, p = 0.08). Treatment with DEHP and DiNP did not affect total follicle numbers when compared to control (data not shown).
Figure 2.
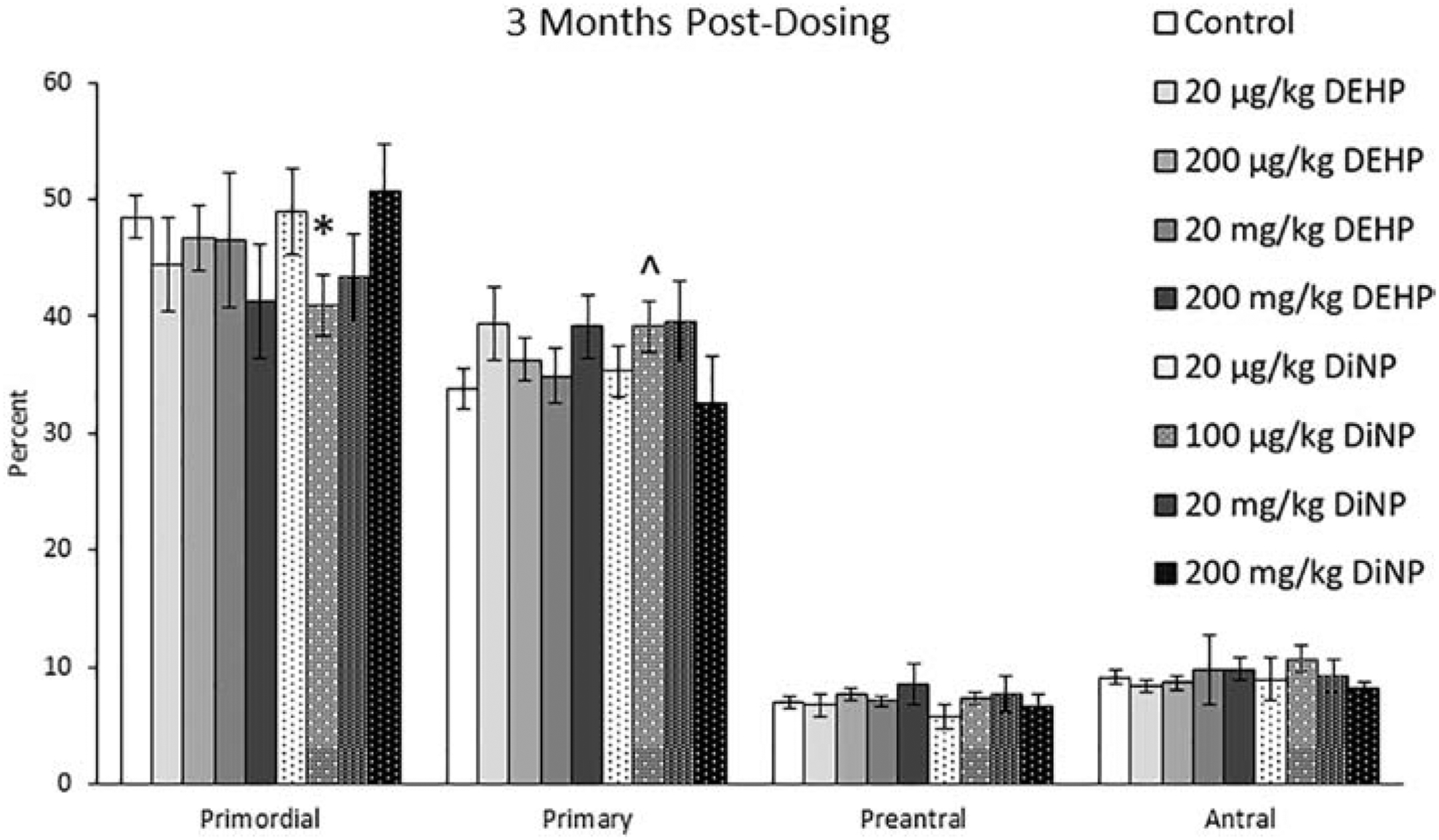
Effects of DEHP and DiNP on ovarian follicle populations at 3 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 3 months post-dosing and the ovaries were collected and processed for histological evaluation of percent of healthy follicle types (n = 4–11 mice/group). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
6 Months Post-Dosing
At 6 months post-dosing, 200 mg/kg/day of DiNP increased the percent of primary follicles (Fig. 3, n = 4–11 mice/group, p ≤ 0.05) and borderline decreased the percent of preantral follicles when compared to control (Fig. 3, n = 4–11 mice/group, p = 0.10). Further, treatment with 20 μg/kg/day DiNP and 100 μg/kg/day DiNP decreased the percentages of antral follicles when compared to control (Fig. 3, n = 4–11 mice/group, p ≤ 0.05). No effects were observed on total follicle numbers due to treatment (data not shown).
Figure 3.
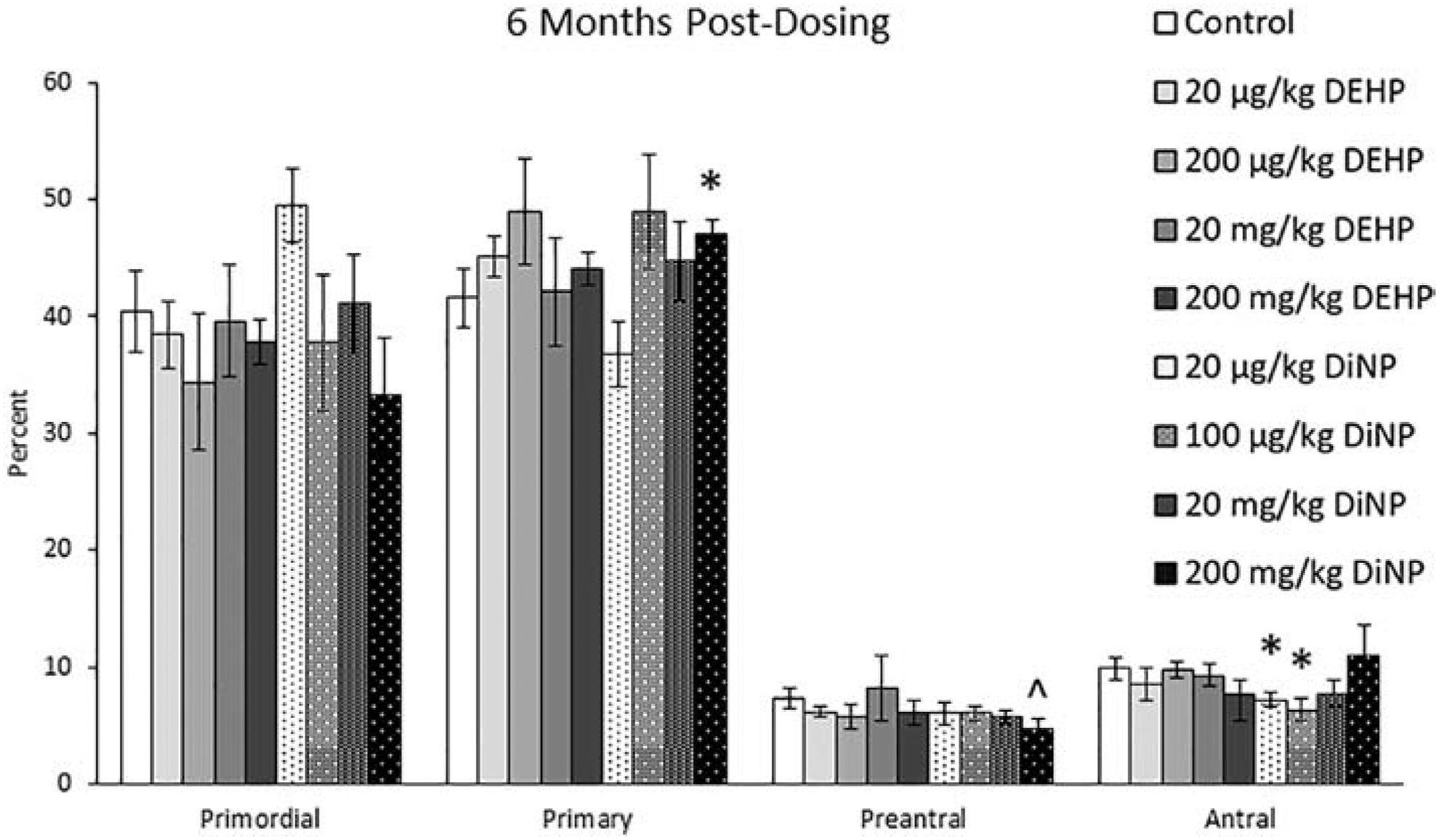
Effects of DEHP and DiNP on ovarian follicle populations at 6 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 6 months post-dosing and the ovaries were collected and processed for histological evaluation of percent of healthy follicle types (n = 4–11 mice/group). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
9 Months Post-Dosing
At 9 months post-dosing, 20 μg/kg/day of DiNP significantly decreased percentages of primary follicles when compared to control (Fig. 4, n = 4–12 mice/group, p ≤ 0.05). Further, 200 μg/kg/day (Fig. 4, n = 4–12 mice/group, p = 0.10) and 20 and 200 mg/kg/day of DEHP (Fig. 4, n = 4–12 mice/group, p ≤ 0.05) increased the percent of preantral follicles when compared to control. Additionally, 20 μg/kg/day of DEHP borderline decreased the percent of antral follicles when compared to control (Fig. 4, n = 4–12 mice/group, p = 0.07). DEHP and DiNP did not affect total follicle numbers when compared to control (data not shown).
Figure 4.
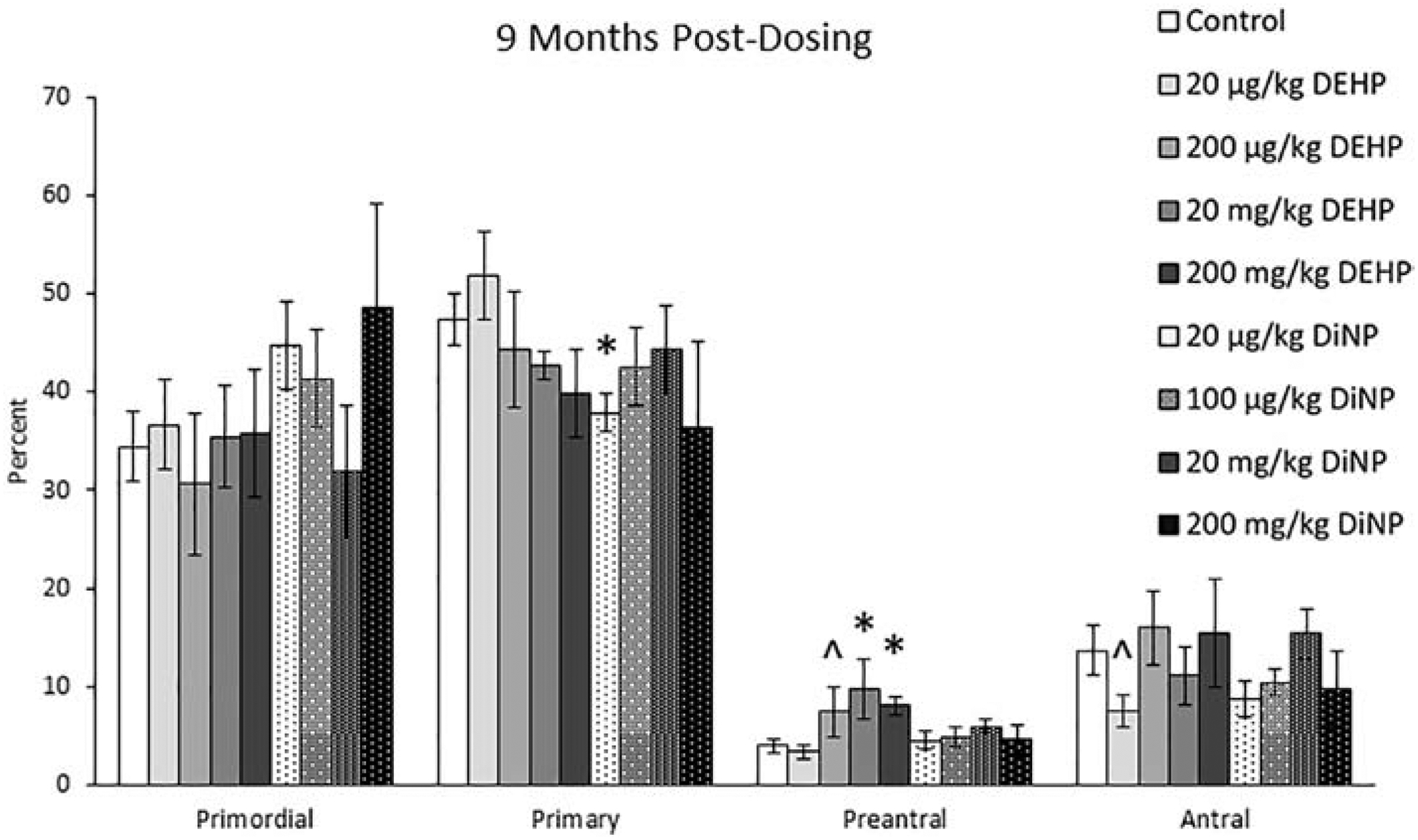
Effects of DEHP and DiNP on ovarian follicle populations at 9 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 9 months post-dosing and the ovaries were collected and processed for histological evaluation of percent of healthy follicle types (n = 4–12 mice/group). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Effects of DEHP and DiNP on hormone levels
Immediately Post-Dosing
Immediately following completion of dosing, DEHP and DiNP both altered the levels of several different hormones at multiple doses. Treatment with 20 and 100 μg/kg/day and 200 mg/kg/day of DiNP significantly reduced testosterone levels when compared to control (Fig. 5A, n = 6–12 mice/group, p ≤ 0.05). Treatment with 200 mg/kg/day of DEHP (Fig. 5B, n = 5–11 mice/group, p ≤ 0.05) and 20 mg/kg/day DiNP (Fig. 5B, n = 5–11 mice/group, p = 0.10) increased progesterone levels when compared to control. Additionally, DEHP (20 mg/kg/day) and DiNP (100 μg/kg/day and 200 mg/kg/day) significantly decreased levels of estradiol when compared to control (Fig. 5C, n = 5–12 mice/group, p ≤ 0.05), and 20 μg/kg/day of DiNP borderline decreased estradiol levels when compared to control (Fig. 5C, n = 5–12 mice/group, p = 0.10). Further, 20 μg/kg/day and 20 mg/kg/day of DEHP increased levels of FSH (Fig. 6A, n = 4–11 mice/group, p ≤ 0.05), and treatment with 100 μg/kg/day of DiNP borderline increased levels of FSH when compared to control (Fig. 6A, n = 4–11 mice/group, p = 0.09). No differences due to treatment were detected for inhibin B at this time point, although some treatment groups lacked adequate number of samples to statistically analyze due to insufficient volume of serum samples from some mice (Fig. 6B, n = 1 for 20 and 100 μg/kg/day DiNP treatment groups, all other groups n = 3–9 mice/group).
Figure 5.
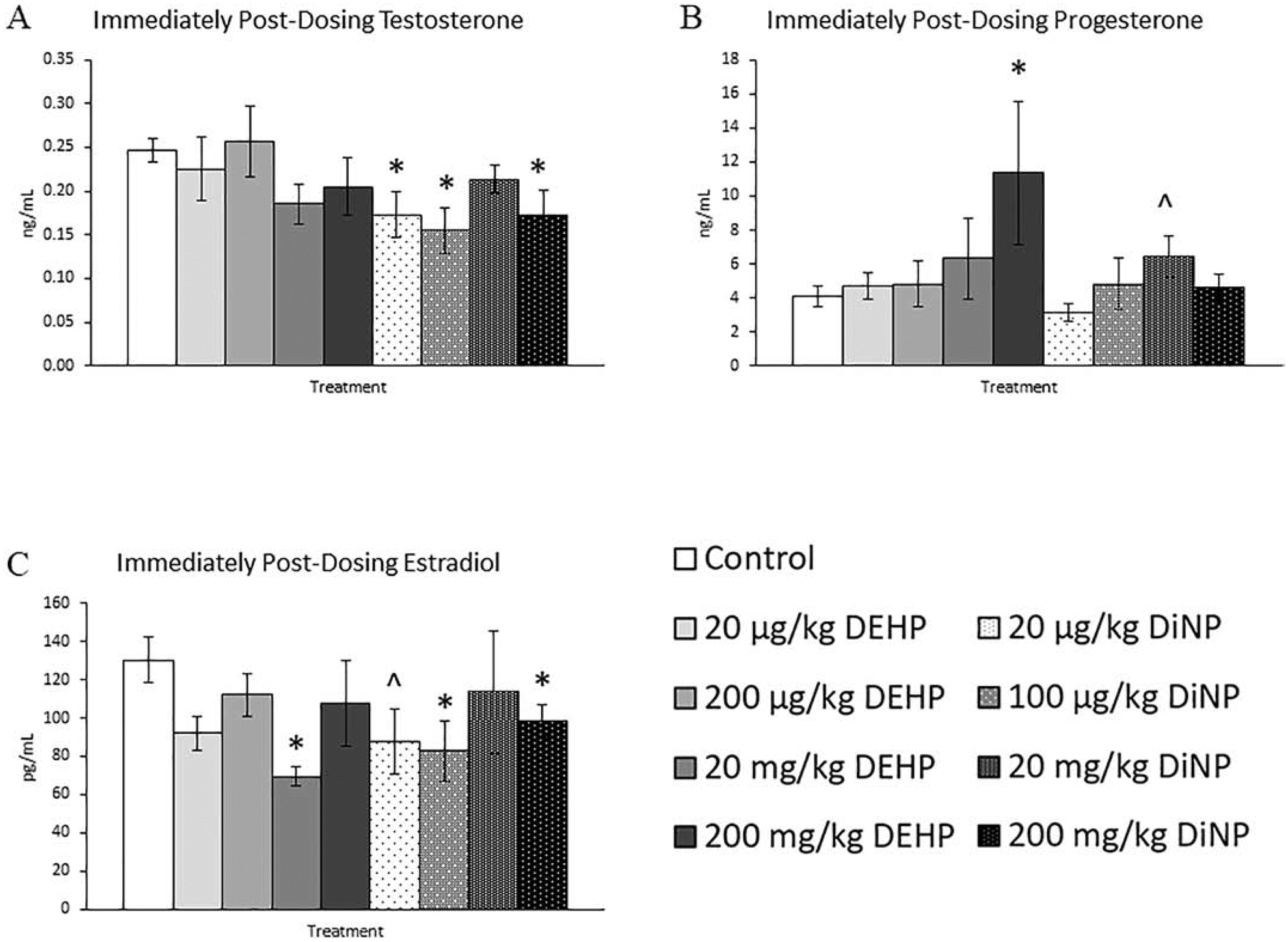
Effects of DEHP and DiNP on sex steroid hormones immediately post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle immediately post-dosing and sera were collected for analysis of testosterone (n = 6–12 mice/group) (A), progesterone (n = 5–11 mice/group) (B), and estradiol (n = 5–12 mice/group) (C). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 6.
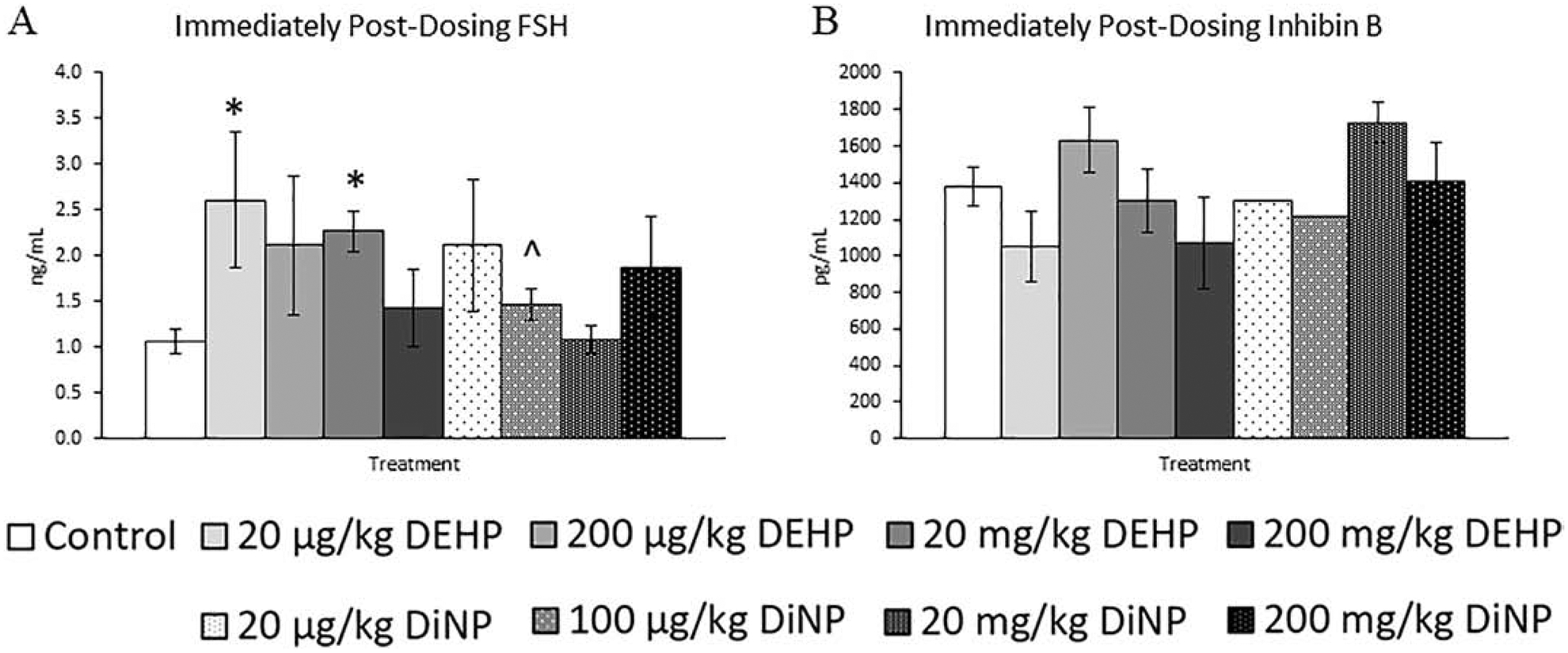
Effects of DEHP and DiNP on FSH and inhibin B immediately post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle immediately post-dosing and sera were collected for analysis of FSH (n = 4–11 mice/group) (A) and inhibin B (n = 1–9 mice/group) (B). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
3 Months Post-Dosing
After 3 months following completion of dosing, fewer effects were observed on the sex steroid hormones than at immediately post-dosing, but some effects persisted. The 100 μg/kg/day dose of DiNP significantly decreased levels of testosterone and progesterone when compared to control (Fig. 7A and 7B, n = 5–12 mice/group, p ≤ 0.05). Further, 20 μg/kg/day of DiNP borderline decreased levels of progesterone (Fig. 7B, n = 5–12 mice/group, p = 0.08), and 200 mg/kg/day of DiNP borderline increased levels of estradiol when compared to control (Fig. 7C, n = 5–12 mice/group, p = 0.08). Additionally, the 20 mg/kg/day dose of DiNP borderline increased levels of FSH when compared to control (Fig. 8A, n = 5–12 mice/group, p = 0.08), and no effects due to treatment were seen on levels of inhibin B (Fig. 8B, n = 4–11 mice/group).
Figure 7.
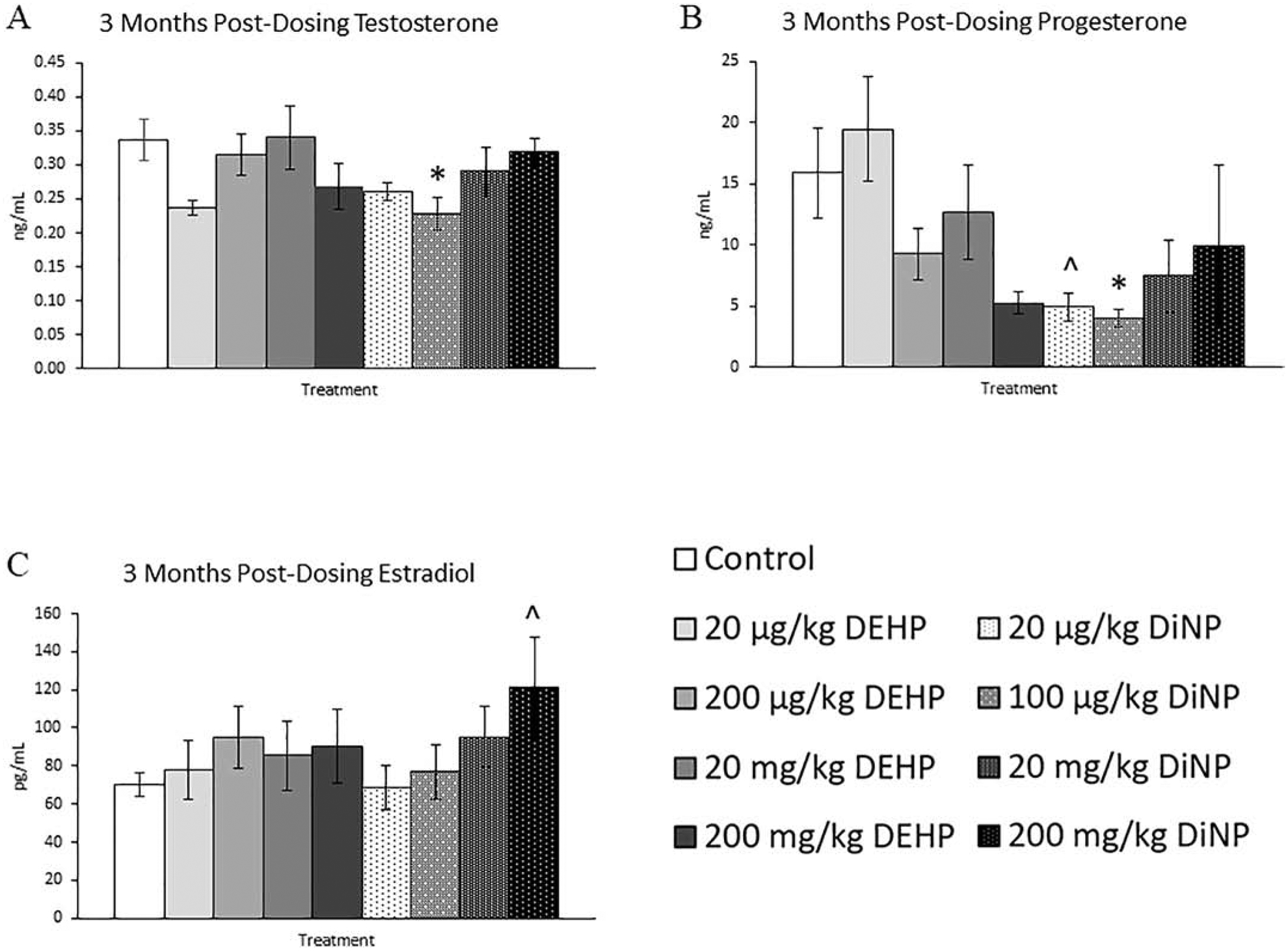
Effects of DEHP and DiNP on sex steroid hormones at 3 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 3 months post-dosing and sera were collected for analysis of testosterone (n = 5–12 mice/group) (A), progesterone (n = 5–12 mice/group) (B), and estradiol (n = 5–12 mice/group) (C). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 8.
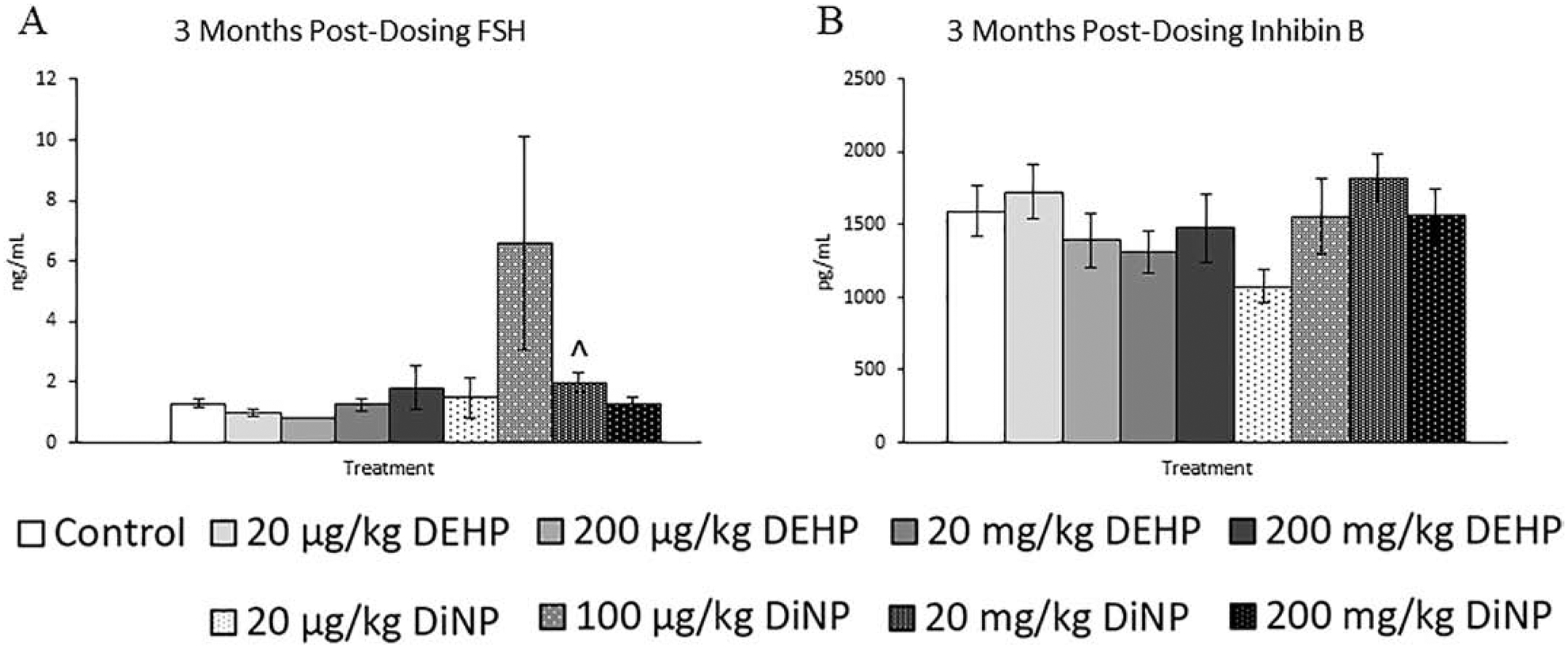
Effects of DEHP and DiNP on FSH and inhibin B at 3 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 3 months post-dosing and sera were collected for analysis of FSH (n = 5–12 mice/group) (A) and inhibin B (n = 4–11 mice/group) (B). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
6 Months Post-Dosing
Following 6 months after completion of dosing, 100 μg/kg/day of DiNP significantly reduced levels of testosterone in mice when compared to control, an effect seen at all previous time points (Fig. 9A, n = 5–11 mice/group, p ≤ 0.05). Additionally, 20 μg/kg/day and 200 μg/kg/day of DEHP and 100 μg/kg/day and 200 mg/kg/day of DiNP significantly increased estradiol when compared to control. (Fig. 9C, n = 5–11 mice/group, p ≤ 0.05). Further, 20 mg/kg/day DEHP borderline increased estradiol when compared to control (Fig. 9C, n = 5–11 mice/group, p = 0.08). However, no effects of DEHP and DiNP were seen on progesterone at any dose when compared to control (Fig. 9B, n = 6–11 mice/group). The 20 and 200 mg/kg/day doses of DEHP both significantly increased levels of FSH when compared to control (Fig. 10A, n = 4–11 mice/group, p ≤ 0.05). In contrast, inhibin B continued to be unaffected by treatment with either DEHP or DiNP (Fig. 10B, n = 3–11 mice/group)
Figure 9.
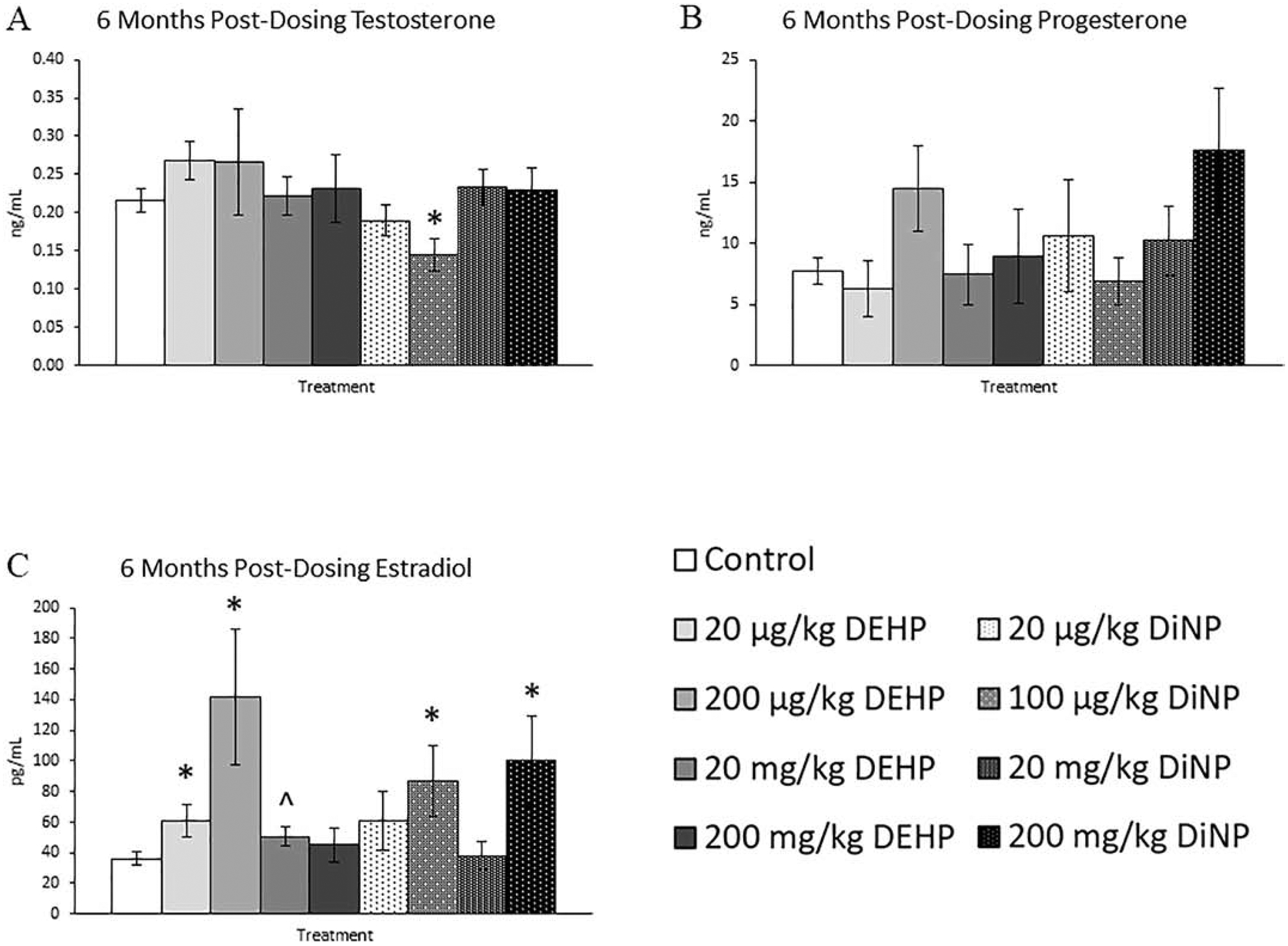
Effects of DEHP and DiNP on sex steroid hormones at 6 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 6 months post-dosing and sera were collected for analysis of testosterone (n = 5–11 mice/group) (A), progesterone (n = 5–11 mice/group) (B), and estradiol (n = 5–11 mice/group) (C). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 10.
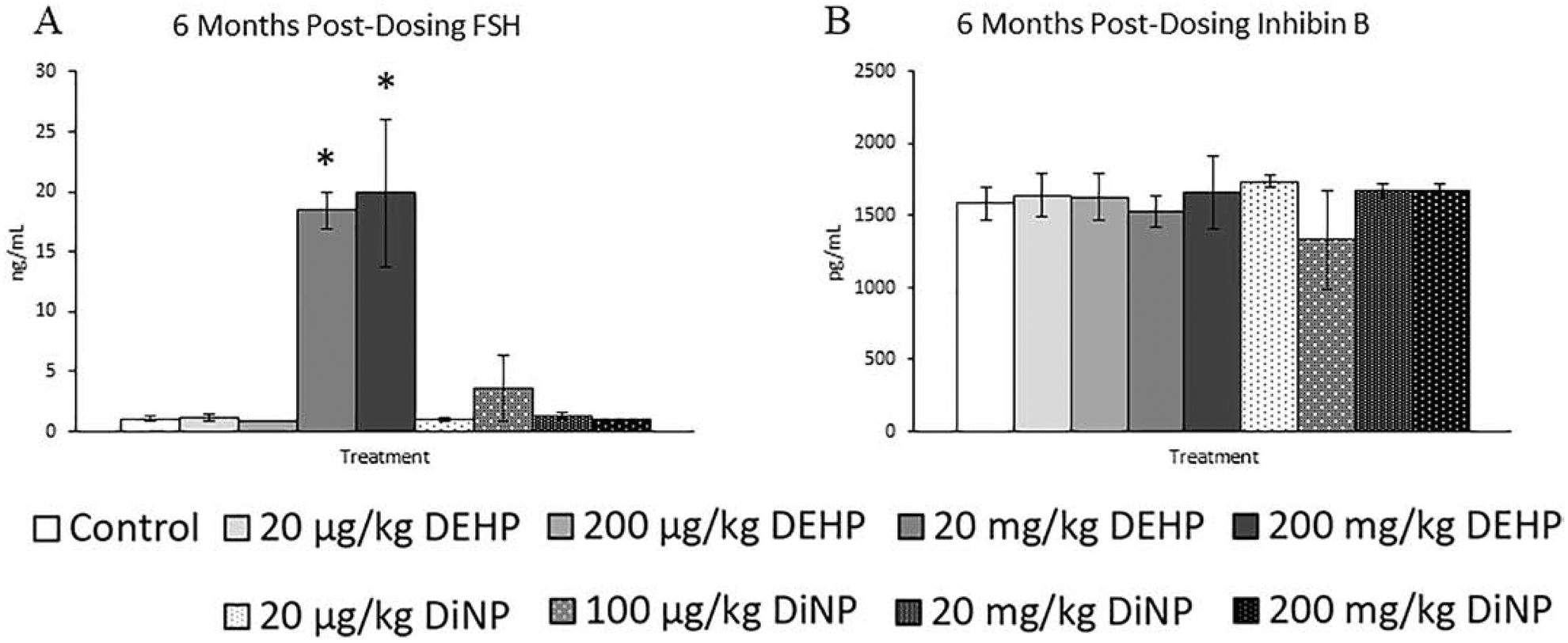
Effects of DEHP and DiNP on FSH and inhibin B at 6 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 6 months post-dosing and sera were collected for analysis of FSH (n = 4–11 mice/group) (A) and inhibin B (n = 3–11 mice/group) (B). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*).
9 Months Post-Dosing
At 9 months post-dosing, the only effect observed was a borderline increase in testosterone in the 200 μg/kg/day of DEHP treatment group when compared to control (Fig. 11A, n = 5–12 mice/group, p = 0.09). Progesterone, estradiol, FSH, and inhibin B were not affected by treatment at this time point (Figs. 11B and 11C, n = 5–12 mice/group, Figs. 12A and 12B, n = 4–12 mice/group).
Figure 11.
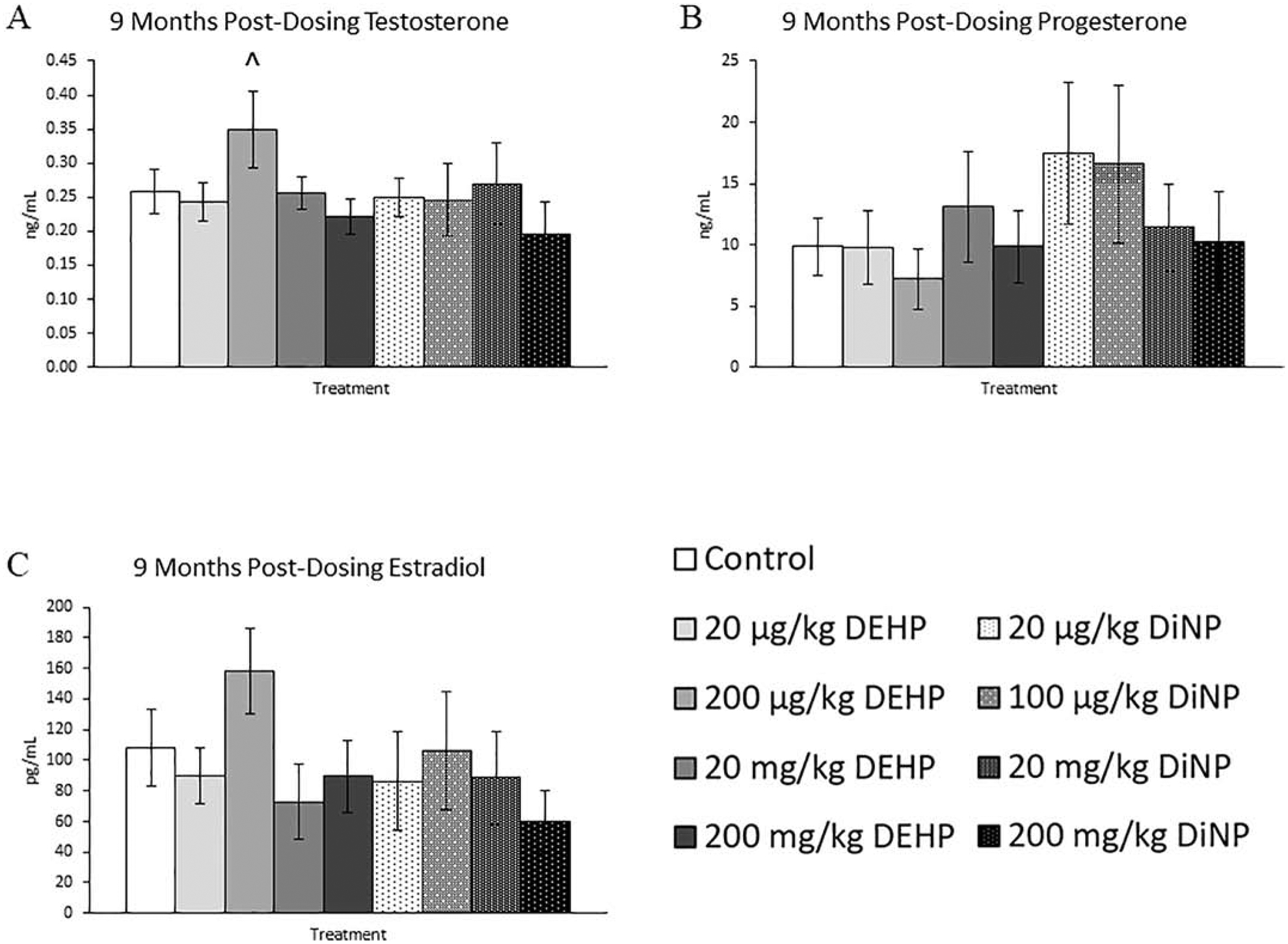
Effects of DEHP and DiNP on sex steroid hormones at 9 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 9 months post-dosing and sera were collected for analysis of testosterone (n = 5–12 mice/group) (A), progesterone (n = 5–12 mice/group) (B), and estradiol (n = 5–12 mice/group) (C). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 12.
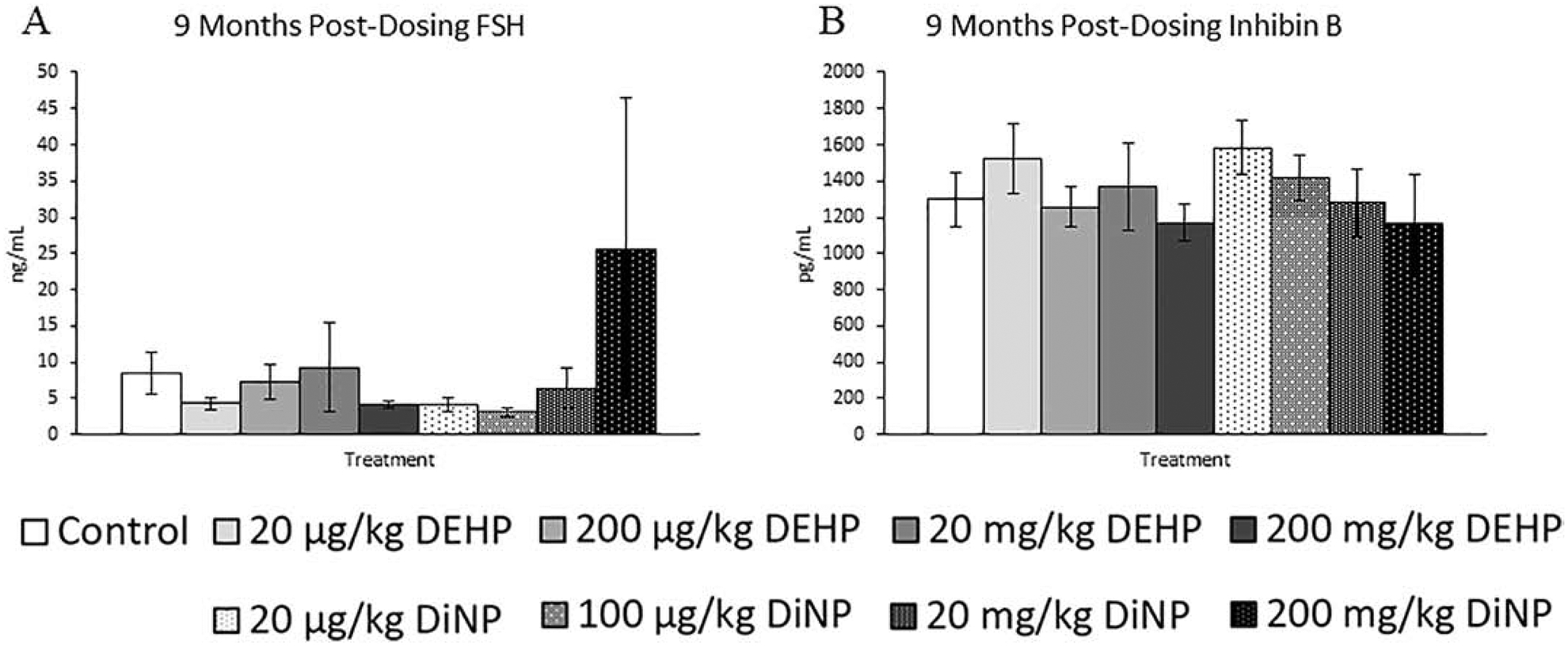
Effects of DEHP and DiNP on FSH and inhibin B at 9 months post-dosing. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 μg/kg/day – 200 mg/kg/day), or DiNP (20 μg/kg/day – 200 mg/kg/day). Mice were euthanized in the diestrous stage of the estrous cycle 9 months post-dosing and sera were collected for analysis of FSH (n = 5–12 mice/group) (A) and inhibin B (n = 4–12 mice/group) (B). Data are represented as means ± standard error. Statistically significant difference when compared to control (p ≤ 0.05) is denoted with an asterisk (*).
DISCUSSION
This study tested the hypothesis that short-term exposure to DEHP and DiNP alters ovarian follicle populations and levels of circulating hormones. Previously, we have shown that short-term exposure to DEHP during adulthood disrupts cyclicity, hormones, and follicle populations at 9 months following completion of dosing (Hannon, Niermann et al. 2016). Additionally, a previous study from our research group showed that short-term exposure to DiNP can negatively impact female reproductive aspects such as cyclicity, mating behavior, and overall fertility up to 9 months following completion of dosing (Chiang and Flaws 2019). The current study builds upon this previous knowledge by assessing the effects of short-term exposure to DEHP and DiNP on ovarian follicle populations and hormone levels at time points where fertility has been disrupted in previous studies. Further, this study fills a gap in knowledge concerning the effects of DiNP on female reproduction.
In a previous study, we found that treatment with 20 μg/kg/day of DEHP and DiNP disrupted the ability of females to achieve pregnancy at 3 months post-dosing (Chiang and Flaws 2019). Surprisingly, in the current study, we did not observe disruptions in folliculogenesis or hormones that could explain the disrupted fertility observed by Chiang and Flaws (2019). This suggests that the mechanism through which DEHP and DiNP disrupted female fertility in the study by Chiang and Flaws (2019) likely does not lie in alteration of circulating hormone levels or folliculogenesis. Thus, DEHP and DiNP likely affect an aspect other than follicular populations and hormone levels to reduce fertility. One study conducted in equine oocytes found that acute in vitro exposure to DEHP inhibits oocyte maturation (Ambruosi, Uranio et al. 2011), and another utilizing acute exposure during adulthood in dairy cattle reported that DEHP decreased developmental competence of oocytes (Kalo, Hadas et al. 2015). Thus, it is possible that DEHP and DiNP may affect oocyte quality in a manner that is undetectable via the histological evaluation utilized in the present study. Further, although the ovary is important and necessary for female reproduction, the uterus is also a key component in female fertility. DEHP and DiNP may affect aspects of uterine function that were not examined in this study. Thus, it is possible DEHP- and DiNP-induced effects on the uterus led to the decrease in fertility at 3 months post-dosing observed in the previous study by Chiang and Flaws (2019). Therefore, future studies that investigate how DEHP and DiNP exposure affect the health and quality of both the oocyte and the uterus may be able to shed light on the disruption of female fertility observed by Chiang and Flaws (2019).
The present study also builds on findings from a previous study wherein the effects of 10 days of exposure to DEHP on female reproductive outcomes were examined following 9 months post-dosing (Hannon, Niermann et al. 2016). Hannon et al. (2016) found that 10 days of DEHP exposure elicited similar modest effects on many of the same endpoints that we examined in the present study. While the majority of our 9 month post-dosing hormone and follicle results coincide with those reported in Hannon et al. (2016), we do note some differences. The most striking difference between the present study and the previous study by Hannon et al. (2016) is that we observed that multiple doses of DEHP increased the percent of preantral follicles, whereas Hannon et al. (2016) did not report any treatment-induced changes in percent of preantral follicles. The presence of slight differences between the present study and the previous was not surprising considering the experimental designs, while very similar, differ slightly. The dosing period in Hannon et al. (2016) occurred at nearly twice the age of that used in the present study. Further, the mice were group housed in the present study, whereas Hannon et al. (2016) housed all mice singly. Housing arrangement amongst mice has been shown to alter estrous cyclicity (Ryan and Schwartz 1977). Thus, it is possible that other endpoints may have been affected by the difference in housing style. Overall, while some differences are observed between the previous study by Hannon et al. (2016) and present study, both studies show modest effects of 10 days of DEHP exposure on follicle populations and hormones following 9 months after completion of dosing.
The alteration of follicle population proportions induced by 100 μg/kg/day of DiNP at 3 months post-dosing is characteristic of accelerated folliculogenesis, an event in which follicles mature at faster rates than is normal and may result in early entry into reproductive senescence if left unchecked. This is concerning because of the myriad of negative health outcomes that are associated with premature ovarian failure (van der Schouw, van der Graaf et al. 1996, Cooper and Sandler 1998, Hu, Grodstein et al. 1999, Rocca, Grossardt et al. 2012). While this effect did not persist at later time points, it is possible that longer exposure may result in more persistent acceleration of folliculogenesis. Thus, further investigation of the effects of short-term exposure to DiNP on folliculogenesis should be conducted to fully understand the risk of DiNP exposure.
The observed increases in the percent of primary follicles due to treatment with DiNP at 6 months post-dosing suggest that some disruption of folliculogenesis still persists at 6 months post-dosing. It is possible that more primordial follicles are being recruited to grow into primary follicles and fewer primary follicles are being signaled to grow into preantral follicles, leading to the observed increase in primary follicles. A delay in advancement of follicles past the primary stage could also explain the decrease in the percent of preantral and antral follicles observed in some of the DiNP treatment groups when compared to control. Finding that DiNP elicited more effects at this time point than DEHP was surprising because DiNP has been purported to be less reproductively toxic when compared to DEHP (SCENIHR 2008). Future studies should investigate how DiNP may alter expression of genes involved in folliculogenesis to help elucidate the mechanism through which DiNP acts on ovarian follicles.
At 9 months post-dosing, treatment with DEHP led to an increase in the proportion of preantral follicles in multiple treatment groups. Several genes are involved in the transition from the preantral to antral stage of the follicle. Additionally, FSH is necessary for advancement from the preantral to antral stage, and during this transition, the follicles convert from FSH-independent growth to FSH-dependent growth (Yen, Strauss et al. 2014, Albertini 2015). Some studies have found that DEHP decreases expression of FSH receptor (FSHR), suggesting that DEHP may interfere with the ability of the follicle to respond to FSH (Zhang, Zhang et al. 2013, Ernst, Jann et al. 2014). Further, one proposed mechanism of action of DEHP is that its metabolite, MEHP, may directly interfere with FSH binding to FSHR (Lovekamp-Swan and Davis 2003). Thus, DEHP may alter the ability of follicles to respond to FSH in combination with altering genes involved with follicular development, which could explain the observed pattern of folliculogenesis disruption at 9 months post-dosing.
Further evidence that treatment with DEHP may interfere with the ability of follicles to respond to FSH is the sudden and drastic reappearance of DEHP-induced increases in FSH at 6 months post-dosing. While the 20 mg/kg/day DEHP group exhibited borderline increases in estradiol to accompany the rise in FSH, we expected a much more dramatic increase in estradiol to match the sharp increase in FSH. The lack of expected FSH-induced rises in estradiol at this timepoint suggests that the follicles are not responding to the observed elevation in FSH. In support, one study reported that MEHP in culture with rat granulosa cells decreased the levels of FSH-induced estradiol (Lovekamp-Swan and Davis 2003). This reduced sensitivity of follicles to FSH could be explained by DEHP-induced modulations of FSHR and/or FSH binding to FSHR as described above. Thus, it is possible that the observed increases in FSH are compensatory and due to a lack of response of the follicle to normal levels of FSH.
One of the most persistent effects observed in this study was the 100 μg/kg/day DiNP-induced decrease in testosterone. This reduction was first noted immediately post-dosing and persisted until 6 months post-dosing. Epidemiological literature agrees with this outcome and has shown that DiNP has anti-androgenic properties and is associated with lower testosterone in men (Radke, Braun et al. 2018). With the exception of 6 months post-dosing, we did not observe concurrent increases in estradiol alongside the decreased testosterone. It is possible that increased metabolism of estradiol due to treatment could stimulate compensatory increases in aromatization of testosterone, leading to decreased testosterone while maintaining stable estradiol levels. The upregulation of metabolic enzymes in response to exogenous compounds resulting in increased hormone metabolism is a familiar concept that was exemplified by the thinning eggshells seen in birds that had been exposed to dichlorodiphenyltrichloroethane (DDT) in the mid-1900s (Peakall 1970). Thus, it is possible a similar toxicant-induced increase in hormone metabolism may have occurred in the current study. Additionally, treatment might have directly reduced the synthesis of testosterone by modulating the steroidogenic enzymes responsible for its production such as 17β-HSD. Phthalate-induced alterations in enzymes involved in the steroidogenic pathway have been observed in a multiple studies (Yuan, Zhao et al. 2012, Kay, Bloom et al. 2014). Future studies concerning the effects of DiNP exposure on metabolic and steroidogenic enzymes should be made priority, as current literature is scarce.
In terms of the sex steroid hormones, most disruptions occurred at the early time points in the study. Deviations from this pattern are most striking at 6 months post-dosing where several doses of DEHP and DiNP increased levels of circulating estradiol and two doses of DEHP sharply increased FSH levels. These data contrast with the idea that the window of adulthood is not a particularly sensitive window in terms of exposure. Due to the scarcity of experimental designs similar to the one used in the current study, comparison to previous literature is difficult. These results highlight the importance of increasing the priority of investigating exposure times that fall outside those that are classically thought to be the most sensitive such as prenatal and pubertal exposure windows.
Interestingly, the hormone and follicle count data in this study did not always correlate. This was surprising because antral follicles are the primary source of sex steroid hormones in females (Auchus 2015). These data suggest that the mechanism through which DEHP and DiNP affect hormone levels is not solely through manipulation of antral follicle numbers, and that it may involve changing the expression or activity of enzymes responsible for the synthesis and/or breakdown of hormones. However, immediately post-dosing, the effects of DEHP and DiNP on sex steroid hormones and FSH are much more correlative. Treatment groups with reduced estradiol also tended to have increased levels of FSH, suggesting that treatment directly reduced estradiol levels and alleviated the estradiol-induced suppression of FSH. Interestingly, the treatment groups that displayed increased progesterone were not the same ones that displayed an increase in FSH, indicating the treatment-induced levels of progesterone were likely not due to FSH-mediated mechanisms.
Collectively, our data show that short-term exposure to both DEHP and DiNP have negative impacts on ovarian function and folliculogenesis throughout the reproductive life of the mouse. Interestingly, throughout our study we observed changes in and sometimes inversions of effects from timepoint to timepoint, indicating the mechanism or mechanisms through which these phthalates are acting are complex. Although the antral follicle is the primary source of sex steroid hormones in females, we did not always find that antral follicle changes correlated with hormone changes, suggesting that the mechanism of action does not solely involve changes to the antral follicle population. Some studies have found that phthalates can change steroidogenic enzyme expression within the ovary (Hannon, Brannick et al. 2015, Lai, Liu et al. 2017, Tripathi, Pandey et al. 2019), and others have found that phthalates can change how the ovary responds to other endocrine signals (Lovekamp-Swan and Davis 2003, Zhang, Zhang et al. 2013, Ernst, Jann et al. 2014). Further, DEHP has been shown to be capable of causing oxidative stress in the ovary and affecting oocyte development and competency (Ambruosi, Uranio et al. 2011, Wang, Craig et al. 2012, Kalo, Hadas et al. 2015). Thus, there could be a combination of multiple pathways that contribute to the variety of effects seen in this study and the fertility effects seen in a previous study from our laboratory (Chiang and Flaws 2019). Future studies should investigate the mechanisms through which DEHP and DiNP act to disrupt these aspects of female reproduction.
DEHP and DiNP disrupt folliculogenesis up to 9 months post-dosing
Disruption in hormones is present up to 9 months post-dosing
DiNP consistently decreases testosterone up to 6 months post-dosing
In some aspects, DiNP appears to have similar toxic properties as DEHP
ACKNOWLEDGMENTS
We would like to thank the members of the Flaws laboratory group for their technical assistance. We would also like to thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core that is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934 for their work in assaying serum samples for FSH and inhibin B. This work was supported by NIH R56 ES 025147, R01 ES 028661, T32 ES 007326, and a Billie Field Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- (ECHA), E. C. A. (2013). “Evaluation of new scientific evidence concerning DINP and DIDP in relation to entry 52 of Annex XVII to REACH Regulation (EC) No 1907/2006.”
- Albertini DF (2015). Chapter 2 - The Mammalian Oocyte Knobil and Neill’s Physiology of Reproduction (Fourth Edition). Plant TM and Zeleznik AJ. San Diego, Academic Press: 59–97. [Google Scholar]
- Ambruosi B, Uranio MF, Sardanelli AM, Pocar P, Martino NA, Paternoster MS, Amati F and Dell’Aquila ME (2011). “In vitro acute exposure to DEHP affects oocyte meiotic maturation, energy and oxidative stress parameters in a large animal model.” PLoS One 6(11): e27452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ (2015). Chapter 8 - Human Steroid Biosynthesis Knobil and Neill’s Physiology of Reproduction (Fourth Edition). Plant TM and Zeleznik AJ. San Diego, Academic Press: 295–312. [Google Scholar]
- Binder AK, Winuthayanon W, Hewitt SC, Couse JF and Korach KS (2015). Chapter 25 - Steroid Receptors in the Uterus and Ovary Knobil and Neill’s Physiology of Reproduction (Fourth Edition). Plant TM and Zeleznik AJ. San Diego, Academic Press: 1099–1193. [Google Scholar]
- Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, Nellemann C and Hass U (2011). “Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats.” Reprod Toxicol 31(2): 200–209. [DOI] [PubMed] [Google Scholar]
- Brehm E, Rattan S, Gao L and Flaws JA (2018). “Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice.” Endocrinology 159(2): 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C and Flaws JA (2019). “Subchronic Exposure to Di(2-ethylhexyl) Phthalate and Diisononyl Phthalate During Adulthood Has Immediate and Long-Term Reproductive Consequences in Female Mice.” Toxicol Sci 168(2): 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Mahalingam S and Flaws JA (2017). “Environmental Contaminants Affecting Fertility and Somatic Health.” Semin Reprod Med 35(3): 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission, U. S. C. P. S. (2010). “Toxicity Review of Diisononyl Phthalate (DINP).”
- Cooper GS and Sandler DP (1998). “Age at natural menopause and mortality.” Ann Epidemiol 8(4): 229–235. [DOI] [PubMed] [Google Scholar]
- CPSC, U. S. C. P. S. C. (2001). “Chronic Hazard Advisory Panel On Diisononyl Phthalate (DiNP).”
- Ernst J, Jann JC, Biemann R, Koch HM and Fischer B (2014). “Effects of the environmental contaminants DEHP and TCDD on estradiol synthesis and aryl hydrocarbon receptor and peroxisome proliferator-activated receptor signalling in the human granulosa cell line KGN.” Mol Hum Reprod 20(9): 919–928. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG and Hoyer PB (1994). “Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide.” Reprod Toxicol 8(6): 509–514. [DOI] [PubMed] [Google Scholar]
- Gallagher JC (2007). “Effect of early menopause on bone mineral density and fractures.” Menopause 14(3 Pt 2): 567–571. [DOI] [PubMed] [Google Scholar]
- Hannon PR, Brannick KE, Wang W and Flaws JA (2015). “Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles.” Biol Reprod 92(5): 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, Niermann S and Flaws JA (2016). “Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice.” Toxicol Sci 150(1): 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, Peretz J and Flaws JA (2014). “Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice.” Biol Reprod 90(6): 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines CJ, Hopf NB, Deddens JA, Silva MJ and Calafat AM (2012). “Occupational exposure to diisononyl phthalate (DiNP) in polyvinyl chloride processing operations.” Int Arch Occup Environ Health 85(3): 317–325. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B and Stampfer MJ (1999). “Age at natural menopause and risk of cardiovascular disease.” Arch Intern Med 159(10): 1061–1066. [DOI] [PubMed] [Google Scholar]
- Kalo D, Hadas R, Furman O, Ben-Ari J, Maor Y, Patterson DG, Tomey C and Roth Z (2015). “Carryover Effects of Acute DEHP Exposure on Ovarian Function and Oocyte Developmental Competence in Lactating Cows.” PLoS One 10(7): e0130896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P and Zacharewski T (2002). “NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate.” Reprod Toxicol 16(5): 529–653. [DOI] [PubMed] [Google Scholar]
- Kay V, Bloom M and Foster W (2014). “Reproductive and developmental effects of phthalate diesters in males.” Critical Reviews in Toxicology 44: 467–498. [DOI] [PubMed] [Google Scholar]
- Lai FN, Liu JC, Li L, Ma JY, Liu XL, Liu YP, Zhang XF, Chen H, De Felici M, Dyce PW and Shen W (2017). “Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice.” Arch Toxicol 91(3): 1279–1292. [DOI] [PubMed] [Google Scholar]
- Levine JE (2015). Chapter 26 - Neuroendocrine Control of the Ovarian Cycle of the Rat Knobil and Neill’s Physiology of Reproduction (Fourth Edition). Plant TM and Zeleznik AJ. San Diego, Academic Press: 1199–1257. [Google Scholar]
- Li L, Bu T, Su H, Chen Z, Liang Y, Zhang G, Zhu D, Shan Y, Xu R, Hu Y, Li J, Hu G, Lian Q and Ge RS (2015). “Inutero exposure to diisononyl phthalate caused testicular dysgenesis of rat fetal testis.” Toxicol Lett 232(2): 466–474. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T and Davis BJ (2003). “Mechanisms of phthalate ester toxicity in the female reproductive system.” Environ Health Perspect 111(2): 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N and Hirose M (2003). “Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life.” Toxicology 192(2–3): 149–170. [DOI] [PubMed] [Google Scholar]
- McArdle CA and Roberson MS (2015). Chapter 10 - Gonadotropes and Gonadotropin-Releasing Hormone Signaling Knobil and Neill’s Physiology of Reproduction (Fourth Edition). Plant TM and Zeleznik AJ. San Diego, Academic Press: 335–397. [Google Scholar]
- Nazir S, Usman Z, Imran M, Lone KP and Ahmad G (2018). “Women Diagnosed with Endometriosis Show High Serum Levels of Diethyl Hexyl Phthalate.” J Hum Reprod Sci 11(2): 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermann S, Rattan S, Brehm E and Flaws JA (2015). “Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice.” Reprod Toxicol 53: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall DB (1970). “p,p’-DDT: effect on calcium metabolism and concentration of estradiol in the blood.” Science (New York, N.Y.) 168(3931): 592–594. [DOI] [PubMed] [Google Scholar]
- Pedersen T and Peters H (1968). “Proposal for a classification of oocytes and follicles in the mouse ovary.” J Reprod Fertil 17(3): 555–557. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Jones SL, Flanagan-Cato LM and Blaustein JD (2015). Chapter 50 - Female Sexual Behavior Knobil and Neill’s Physiology of Reproduction (Fourth Edition). Plant TM and Zeleznik AJ. San Diego, Academic Press: 2287–2370. [Google Scholar]
- Radke EG, Braun JM, Meeker JD and Cooper GS (2018). “Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence.” Environ Int 121(Pt 1): 764–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Brehm E, Gao L, Niermann S and Flaws JA (2018). “Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice.” Biol Reprod 98(1): 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E and Flaws JA (2017). “Exposure to endocrine disruptors during adulthood: consequences for female fertility.” J Endocrinol 233(3): R109–r129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Miller VM, Shuster LT and Brown RD Jr. (2012). “Premature menopause or early menopause and risk of ischemic stroke.” Menopause 19(3): 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KD and Schwartz NB (1977). “Grouped female mice: demonstration of pseudopregnancy.” Biology of reproduction 17(4): 578–583. [DOI] [PubMed] [Google Scholar]
- SCENIHR, S. C. o. E. a. N.-I. H. R. (2008). “Opinion on the Safety of Medical Devices Containing DEHP Plasticized PVC or Other Plasticizers on Neonates and Other Groups Possibly at Risk “. [DOI] [PubMed]
- Specht IO, Bonde JP, Toft G, Lindh CH, Jonsson BA and Jorgensen KT (2015). “Serum phthalate levels and time to pregnancy in couples from Greenland, Poland and Ukraine.” PLoS One 10(3): e0120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Pandey V, Sahu AN, Singh A and Dubey PK (2019). “Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells.” Toxicol Res (Camb) 8(3): 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, P. H. S., Agency for Toxic Substances and Disease Registry (2002). “TOXICOLOGICAL PROFILE FOR DI(2-ETHYLHEXYL)PHTHALATE.” [PubMed]
- van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC and Banga JD (1996). “Age at menopause as a risk factor for cardiovascular mortality.” Lancet 347(9003): 714–718. [DOI] [PubMed] [Google Scholar]
- Wang W, Craig ZR, Basavarajappa MS, Gupta RK and Flaws JA (2012). “Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway.” Toxicol Appl Pharmacol 258(2): 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SSC, Strauss JF and Barbieri RL (2014). Yen and Jaffe’s reproductive endocrinology physiology, pathophysiology, and clinical management. Philadelphia, PA, Elsevier/Saunders,: 1 online resource; (xiii, 908 p.). [Google Scholar]
- Yuan K, Zhao B, Li XW, Hu GX, Su Y, Chu Y, Akingbemi BT, Lian QQ and Ge RS (2012). “Effects of phthalates on 3beta-hydroxysteroid dehydrogenase and 17beta-hydroxysteroid dehydrogenase 3 activities in human and rat testes.” Chem Biol Interact 195(3): 180–188. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Zhang T, Wang L, Zhang HY, Chen YD, Qin XS, Feng YM, Feng YN, Shen W and Li L (2013). “Effects of diethylhexyl phthalate (DEHP) given neonatally on spermatogenesis of mice.” Mol Biol Rep 40(11): 6509–6517. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM and Woodruff TJ (2014). “Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010.” Environ Health Perspect 122(3): 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Geller RJ, Calafat AM, Marfori CQ, Baccarelli AA and Moawad GN (2019). “Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study.” Fertil Steril 111(1): 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


