Key Points
Questions
What is the prevalence of cannabis withdrawal syndrome among individuals with regular or dependent use of cannabis, and which factors are associated with cannabis withdrawal syndrome?
Findings
In this meta-analysis of observational studies including 23 518 participants, the prevalence of cannabis withdrawal syndrome was found to be 47%. Factors that were associated with higher cannabis withdrawal syndrome were clinical settings (particularly inpatient and outpatient vs population settings), concurrent tobacco or other substance use, and daily cannabis use.
Meaning
Cannabis withdrawal syndrome appears to be common among regular users of cannabis, particularly those in outpatient and inpatient settings and individuals with substance use disorders; clinicians should be aware of the high prevalence of cannabis withdrawal syndrome to counsel patients and support individuals who are reducing their use of cannabis.
Abstract
Importance
Cannabis withdrawal syndrome (CWS)—a diagnostic indicator of cannabis use disorder—commonly occurs on cessation of heavy and prolonged cannabis use. To date, the prevalence of CWS syndrome has not been well described, nor have the factors potentially associated with CWS.
Objectives
To estimate the prevalence of CWS among individuals with regular or dependent use of cannabinoids and identify factors associated with CWS.
Data Sources
A search of literature from database inception to June 19, 2019, was performed using MEDLINE, Embase, PsycINFO, Web of Science, the Cumulative Index to Nursing and Allied Health Literature, ProQuest, Allied and Complementary Medicine, and Psychiatry online, supplemented by manual searches of reference lists of included articles.
Study Selection
Articles were included if they (1) were published in English, (2) reported on individuals with regular use of cannabinoids or cannabis use disorder as a primary study group, (3) reported on the prevalence of CWS or CWS symptoms using a validated instrument, (4) reported the prevalence of CWS, and (5) used an observational study design (eg, cohort or cross-sectional).
Data Extraction and Synthesis
All abstracts, full-text articles, and other sources were reviewed, with data extracted in duplicate. Cannabis withdrawal syndrome prevalence was estimated using a random-effects meta-analysis model, alongside stratification and meta-regression to characterize heterogeneity.
Main Outcomes and Measures
Cannabis withdrawal syndrome prevalence was reported as a percentage with 95% CIs.
Results
Of 3848 unique abstracts, 86 were selected for full-text review, and 47 studies, representing 23 518 participants, met all inclusion criteria. Of 23 518 participants included in the analysis, 16 839 were white (72%) and 14 387 were men (69%); median (SD) age was 29.9 (9.0) years. The overall pooled prevalence of CWS was 47% (6469 of 23 518) (95% CI, 41%-52%), with significant heterogeneity between estimates (I2 = 99.2%). When stratified by source, the prevalence of CWS was 17% (95% CI, 13%-21%) in population-based samples, 54% in outpatient samples (95% CI, 48%-59%), and 87% in inpatient samples (95% CI, 79%-94%), which were significantly different (P < .001). Concurrent cannabis (β = 0.005, P < .001), tobacco (β = 0.002, P = .02), and other substance use disorders (β = 0.003, P = .05) were associated with a higher CWS prevalence, as was daily cannabis use (β = 0.004, P < .001).
Conclusions and Relevance
These findings suggest that cannabis withdrawal syndrome appears to be prevalent among regular users of cannabis. Clinicians should be aware of the prevalence of CWS in order to counsel patients and support individuals who are reducing their use of cannabis.
This systematic review and meta-analysis examines the prevalence of cannabis withdrawal syndrome in individuals who use cannabinoids regularly.
Introduction
Cannabinoids are the most commonly used group of illicit drugs, and cannabis use and dependence are estimated to have increased over the past 2 decades.1 Despite common perceptions that cannabis is relatively harmless, there is substantial evidence to support an association between cannabis use and several medical, neurocognitive, functional, and psychosocial sequalae.2 The known short-term risks of cannabinoid use include impaired short-term memory and motor coordination, altered judgment, paranoia, and psychosis.3 Similarly, long-term effects of cannabinoid use include addiction, altered brain development, poor educational outcomes, cognitive impairment, diminished quality of life, increased risk of chronic respiratory tract and psychotic disorders, injuries, motor vehicle collisions, and suicide.3,4
In parallel with other substance withdrawal syndromes, a cannabis withdrawal syndrome (CWS)—originally proposed by Budney and colleagues5,6,7,8 —has received recognition in recent years. Cannabis withdrawal syndrome symptoms occur reliably following a specific time course with cessation of cannabis use, were transient, could be ameliorated by readministration of cannabis, and were clinically significant. Cannabis withdrawal syndrome was recognized by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,9 and requires the presence of at least 3 of the following symptoms developing within 7 days of reduced cannabis use: (1) irritability, anger, or aggression; (2) nervousness or anxiety; (3) sleep disturbance; (4) appetite or weight disturbance; (5) restlessness; (6) depressed mood; and (7) somatic symptoms, such as headaches, sweating, nausea, vomiting, or abdominal pain.
Several studies using varied approaches have characterized CWS, and resulting prevalence estimates have ranged from 11.1% to 94.2%.8,10,11,12 Hence, although there is concern about the risks associated with cannabinoid use and CWS, to our knowledge, there currently exists no comprehensive quantitative synthesis of the magnitude of risk and how elevated that risk might be relative to the general population among people with regular or problematic cannabinoid use.
The primary aim of this systematic review and meta-analysis was to estimate the prevalence of CWS and identify contributors to heterogeneity in reported results. We sought to produce age-specific and sex estimates of CWS prevalence where possible.
Methods
Using an a priori protocol,13 we conducted our systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.14 The need for institutional review board approval was waived by Queen’s University because this systematic review does not constitute human subject research. The search strategy was developed in consultation with a research librarian. Eight electronic databases (MEDLINE, Embase, PsycInfo, Web of Science, Allied and Complementary Medicine, Cumulative Index to Nursing and Allied Health Literature, ProQuest, and Psychiatry Online) were searched from inception to June 19, 2019, with no restriction on the year of the study. Medical Subject Headings and key words related to cannabis withdrawal, cannabis use, and prevalence of epidemiologic factors were used (eTable 1 in the Supplement). The reference lists of all included full-text articles were searched to identify any studies missed in the initial search, and the PubMed similar articles feature was used to find additional academic articles citing eligible articles. References that consisted of abstracts alone were not considered. References were compiled and managed using Zotero (George Mason University).15 Citations were then imported into the web-based screening tool Covidence (Cochrane Collaboration),16 where duplicate citations were removed.
Titles and abstracts were screened by one reviewer (A.B.), and all material marked as excluded was reviewed by a second person (R.T.) to ensure accuracy in first-pass screening. At this stage, the criteria were purposely broad to allow inclusion of any relevant studies. To be included, studies had to be published in English and report original research using any observational design (eg, cross-sectional or cohort) that reported on CWS in individuals with regular or dependent cannabis or synthetic cannabinoid use. The exact definition of regular cannabinoid use varied across cohorts, and we summarize the studies’ criteria and characteristics in Table 1.17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60 Case reports and series were excluded. Full-text articles were screened by 2 independent reviewers (A.B. and C.S.), with discrepancies resolved by consensus or via consultation with a third reviewer (R.T., E.R.H., or D.P.S.) when consensus was not reached. Articles were included if they (1) were published in English, (2) reported individuals with regular or dependent cannabinoid use as a primary study group, (3) reported CWS or CWS symptoms using a validated instrument, and (4) reported the prevalence of CWS in individuals with regular or dependent cannabinoid use. For studies that used the same sample of data, those providing the most detailed information were included, and the others were kept for reference.
Table 1. Characteristics of Included Studies.
| Source | Study setting | Criteria | No. | CUD, % | Male, % | Age, y | CWS, % | |
|---|---|---|---|---|---|---|---|---|
| CUD | CWS | |||||||
| Cottler et al,17 1995, United States | Population | DSM-III-R | CIDI-SAM | 102 | 8 | 57 | 37.0 | 15.7 |
| Wiesbeck et al,18 1996, United States | Population | DSM-IV | SSAGA | 1735 | 50.4 | 63 | 32.3 | 15.6 |
| Budney et al,19 1998, United States | Outpatient | DSM-III-R | Operationalized | 62 | 100 | 87 | 31.2 | 75.8 |
| Crowley et al,20 1998, United States | Outpatient | DSM-III-R | CIDI-SAM, DISC | 229 | 78.6 | 72 | 15.8 | 66.8 |
| Swift et al,21 1998, Australia | Population | DSM-III-R | Operationalized | 243 | 57 | 58 | 36.0 | 20.2 |
| Budney et al,6 1999, United States | Outpatient | DSM-III-R | MWC | 54 | 54 | 85 | 33.8 | 57.4 |
| Schuckit et al,22 1999, United States | Outpatient | DSM-III-R | Operationalized | 596 | 30 | 66.1 | 32.0 | 39.9 |
| Kouri and Pope et al,23 2000, United States | Outpatient | DSM-IV | Self-reported diary | 30 | 100 | 87 | 42.5 | 60.0 |
| Swift et al,24 2000, Australia | Outpatient | DSM-III-R | Operationalized | 162 | 92 | 53.7 | 30.0 | 32.1 |
| Swift et al,25 2001, Australia | Population | DSM-IV | CIDI, DSM-IV, SCID | 722 | 20.8 | NA | NA | 29.5 |
| Stephens et al,26 2002, United States | Outpatient | DSM-IV | SCID, TLFB, ASI | 450 | 100 | 68.4 | 36.1 | 77.6 |
| Budney et al,7 2003, United States | Outpatient | DSM-IV | MWC, MCQ | 18 | 100 | 61 | 30.9 | 77.8 |
| Vandrey et al,27 2005, United States | Outpatient | DSM-IV | MWC, YSR, WDS | 72 | 56.9 | 90 | 16.2 | 58.3 |
| Copersino et al,28 2006, United States | Outpatient | DSM-IV | MJQQ | 104 | 54 | 78 | 35.0 | 44.2 |
| Levin et al,29 2006, United States | Outpatient | DSM-IV | CMR, URICA, RDU | 42 | 100 | 74 | 34.3 | 69.0 |
| Nocon et al,30 2006, Germany | Population | DSM-IV | CIDI-SAM, MWC | 732 | 3.5 | NA | 19.0 | 16.1 |
| Lukasiewicz et al,31 2007, France | Population | DSM-IV | Operationalized | 278 | 26.7 | 90.1 | 39.0 | 7.6 |
| Agrawal et al,32 2008, United States | Population | DSM-IV | AUDADIS | 1603 | 12.2 | 62 | 30.8 | 8.0 |
| Chung et al,33 2008, United States | Outpatient | DSM-IV | MWC, SCID | 214 | 60.7 | 67 | 16.8 | 36.9 |
| Cornelius et al,34 2008, United States | Outpatient | DSM-IV | MWC | 170 | 100 | 54 | 20.3 | 43.5 |
| Hasin et al,35 2008, United States | Population | DSM-IV | SCID | 2613 | 57.2 | 67 | 58.5 | 34.4 |
| Jungerman et al,36 2008, Brazil | Outpatient | DSM-III-R | CIDI, TFLB, MWC | 160 | 100 | 80 | 32.3 | 51.3 |
| Milin et al,37 2008, Canada | Inpatient | DSM-IV | CWS, SCID | 21 | 100 | 67 | 17.0 | 100.0 |
| Vandrey et al,38 2008, United States | Inpatient | DSM-IV | WSC | 12 | 100 | 50 | 28.2 | 100.0 |
| Mennes et al,39 2009, United Statesa | Outpatient | DSM-IV | CIDI-SAM | 416 | 48 | 49 | 22.0 | 50.0 |
| Mennes et al,39 2009, United Statesa | Outpatient | DSM-IV | CIDI-SAM | 278 | 63 | 49 | 22.0 | 68.0 |
| Ehlers et al,40 2010, United States | Population | DSM-IV | SSAGA | 818 | 13.9 | 38 | 48.4 | 16.5 |
| Levin et al,41 2010, United States | Outpatient | DSM-IV | MJQQ | 469 | 91 | 58 | 31.2 | 42.4 |
| Preuss et al,42 2010, Germany | Inpatient | DSM-IV | MWC | 118 | 100 | 85 | 19.6 | 72.0 |
| Vorspan et al,43 2010, United Statesa | Outpatient | DSM-IV | MJQQ | 43 | 79.1 | 69.8 | 37.0 | 65.1 |
| Vorspan et al,43 2010, United Statesa | Outpatient | DSM-IV | MJQQ | 56 | 100 | 71.4 | 27.0 | 64.3 |
| Dervaux et al,44 2011, France | Inpatient | DSM-IV | DIGS | 92 | 100 | 75 | 28.7 | 84.8 |
| Gorelick et al,45 2012, United States | Outpatient | DSM-IV | MJQQ, self-report diary | 384 | 92.4 | 58.3 | 29.2 | 40.9 |
| Boggs et al,46 2013, United States | Outpatient | DSM-IV | MJQQ | 120 | 81.7 | 77 | 41.5 | 50.0 |
| Smith et al,47 2013, United Statesa | Population | DSM-IV | AUDADIS | 1712 | NA | 68 | 34.3 | 18.8 |
| Smith et al,47 2013, United Statesa | Population | DSM-IV | AUDADIS | 1187 | NA | 68 | 34.3 | 9.8 |
| Verweij et al,48 2013, Australia | Population | DSM-IV | SSAGA, CWS, MCQ | 2276 | 23.6 | 39 | 31.9 | 11.9 |
| Bonnet et al,49 2014, Germany | Inpatient | DSM-IV | MWC | 39 | 100 | 80 | 28.6 | 92.3 |
| Greene et al,50 2014, United States | Outpatient | DSM-IV | CDDR | 90 | 84.4 | 82 | 16.6 | 40.0 |
| Lee et al,51 2014, United States | Inpatient | DSM-IV | CWS, MCQ, SCL-90R | 30 | 79.3 | 100 | 28.5 | 73.3 |
| Delforterie et al,52 2015, United Statesa | Population | DSM-IV | AUDADIS, CIDI | 1568 | 11.7 | 50 | 24.8 | 29.2 |
| Delforterie et al,52 2015, the Netherlandsa | Population | DSM-IV | AUDADIS, CIDI | 359 | 16.4 | 65 | 23.9 | 12.5 |
| Herrmann et al,53 2015, United States | Outpatient | DSM-5 | MWC, WDS | 136 | 77.9 | 73 | 33.3 | 50.7 |
| Macfarlane and Christie,54 2015, New Zealand | Inpatient | DSM-IV | MWC | 47 | 100 | 63 | 31.0 | 87.2 |
| Soenksen et al,55 2015, United States | Outpatient | DSM-IV | MWC | 93 | 76.9 | 100 | 16.4 | 66.7 |
| Davis et al,56 2016, United States | Outpatient | DSM-IV | CWS | 110 | 53.4 | 93 | 19.2 | 48.2 |
| Sherman et al,57 2017, United States | Outpatient | DSM-5 | TFLB, MWC, MCQ | 302 | 100 | 72 | 30.3 | 50.3 |
| Chauchard et al,58 2018, United States | Outpatient | DSM-IV | MJQQ | 23 | 100 | 82.6 | 27.4 | 30.4 |
| Livne et al,59 2019, United States | Population | DSM-5 | DSM-5 | 1527 | 24.6 | 66 | NA | 12.1 |
| Perron et al,60 2019, United States | Outpatient | DSM-5 | MWC | 801 | 1.8 | 53.6 | 45.1 | 52.3 |
Abbreviations: ASI, Addiction Severity Index; AUDADIS, Alcohol Use Disorder and Associated Disabilities Schedule; CDDR, Customary Drinking and Drug Use Record; CIDI-SAM, Composite International Diagnostic Interview–Substance Abuse Module; CMR, Circumstances, Motivation, Readiness; CUD, cannabis use disorder; CWS, cannabis withdrawal syndrome; DIGS, Diagnostic Interview for Genetic Studies; DISC, Diagnostic Interview Schedule for Children; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders; DSM-IV, Diagnostic and Statistical Manual, Fourth Edition; DSM-5, Diagnostic and Statistical Manual, Fifth Edition; MCQ, Marijuana Cravings Questionnaire; MJQQ, Marijuana Quit Questionnaire; MWC, Marijuana Withdrawal Checklist; SCID, Structured Clinical Interview for DSM; SCL-90R, Symptom Checklist 90–Revised; SSAGA, Semi-Structured Assessment for the Genetics of Alcoholism; TFLB, time-line follow-back; WDS, Withdrawal Discomfort Scale; WSC, Withdrawal Symptom Checklist; YSR, Youth Self-Report.
These studies included 2 or more substudies.
The data extraction form was developed in Microsoft Excel 2016 (Microsoft Corp) based on previously conducted reviews12,61,62 and recommendations outlined in the STROBE statement (eTable 2 in the Supplement).63 Data were independently extracted by 1 member of the research team (A.B.) and checked by a second (C.S.). Bibliographic information was extracted in addition to study-specific data.
The following data were abstracted: study information (ie, author, journal, and year of publication), study characteristics (ie, study setting, country of study, and duration of follow-up), participant characteristics (ie, age, comorbidities, substance use, and race/ethnicity), condition information (ie, data sources, condition definition, and total number of participants), the prevalence of CWS, or the information necessary to calculate an estimate.
Data on the prevalence of CWS information were extracted and, where possible, grouped to be consistent with previous CWS rating instruments developed by cannabinoid expert groups (eTable 3 in the Supplement).64,65 If data reporting in the publications was incomplete, supplementary information and documents were searched to locate missing data. If supplementary information could not be located or did not provide the necessary data needed, primary study authors were contacted by email for additional information.
The quality of studies was assessed using the Newcastle-Ottawa Scale for observational studies.66 This scale uses a star system to evaluate nonrandomized studies regarding 3 domains of quality (selection, comparability, and outcome) using 8 criteria: representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study, comparability of cohorts on the basis of the design or analysis, assessment of outcome, sufficient length of follow-up for outcomes to occur, and adequacy of follow-up of the cohort. Individual star scores for each criterion were tallied to provide an overall quality score, where the greater the quality score, the higher the methodologic quality of the study (maximum score: 8 points). Studies that achieved a total rating of 6 points or higher were considered to be of the highest quality, studies that achieved a total rating of fewer than 2 points were considered to be of lowest quality, and those between 2 and 5 points were rated as fair quality. Study information necessary for quality assessment was extracted to the Excel template by one reviewer (A.B.) and double checked by a second (C.S.). Discrepancies were resolved via consultation with a third reviewer (R.T., E.R.H. or D.P.S.).
Statistical Analysis
Descriptive statistics were calculated using proportions and means and compared using t tests or χ2 tests where appropriate. For all tests, 2-sided P values <.05 were considered statistically significant. Study settings included nonclinical, population-based studies, outpatient clinical studies, or inpatient clinical settings. Informant-rated scales were those completed by a family member or other informant familiar with the participant. If studies used multiple cut points to calculate CWS, the lowest threshold for defining CWS was selected.
A random-effects model for meta-analysis was used because of assumed heterogeneity between the studies. The metafor package in R, version 1.1.463 (R Studio) was used to produce the pooled estimates, forest plots, and meta-regression.67 The meta-analysis of proportions uses the binomial distribution to model the within-study variability or by allowing Freeman-Tukey double arcsine transformation to stabilize the variances.68 Heterogeneity was quantified using the I2 statistic, and its significance was determined based on the accompanying Cochran Q test P value.69 An I2 value of 0% indicates no observed heterogeneity, and increasing values represent greater amounts of heterogeneity; values of 25%, 50%, and 75% indicate low, moderate, and high levels of heterogeneity, respectively.69
Subgroup analyses were planned for accessing the associations of study population source (population or clinic based), method of CWS diagnosis (informant rated, self-report, or clinician administered), geographic location, intensity of cannabis use, sex, psychiatric comorbidity, and age with the prevalence of CWS in patients with regular or dependent use of cannabinoids. However, where studies did not report subgroup-level estimates within primary studies, we applied random-effects meta-regression to assess the association between the variable and prevalence of CWS.70
Publication bias was assessed qualitatively, using funnel plot symmetry as a surrogate for low risk of publication bias, as well as quantitatively, using the Egger and trim-and-fill methods.71,72,73 Supplementary analyses are outlined in the eFigure 1 in the Supplement.
Results
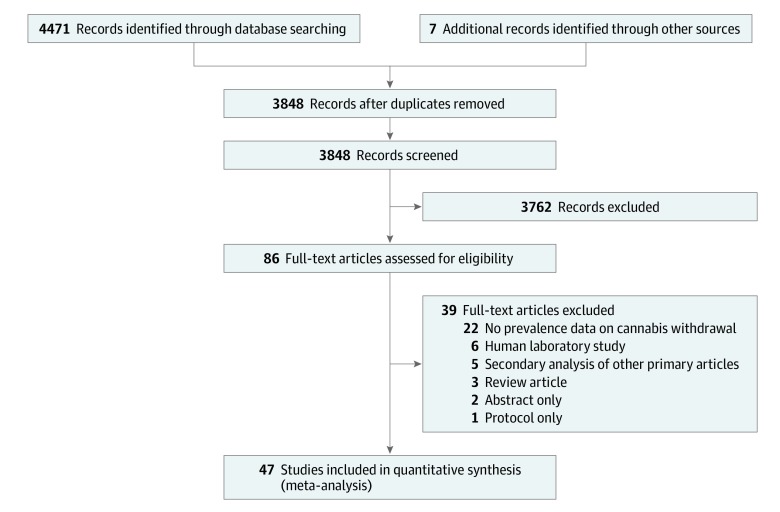
We screened a total of 3848 unique citations, of which 86 were screened in full, and 47 were included in the review (Figure 1), reporting on 50 unique cohorts. In total, 23 518 participants were represented across cohorts (median [SD] age, 29.9 [9.0] years; 16 839 white [72%]; and 14 387 men [69%]). Twenty-five cohorts (50%) were of treatment-seeking individuals. Most of the cohorts came from North America (38 [76%]), Australia (7 [14%]), or Europe (6 [12%]) (Table 1). Participants in included sources were obtained from primarily clinical samples (inpatient: 7 [14%] and outpatient: 28 [56%]) or population-based samples (15 [30%]). Individual cohorts varied widely in size (12-2613). Reporting of cohort demographics was incomplete; for example, fewer than half of the cohorts reported the baseline cannabis intake. Eighteen cohorts reported the percentage who had experienced lifetime CWS, and the remaining 32 reported current (past year) CWS prevalence.
Figure 1. PRISMA Flowchart of Study Selection.
Search and selection process applied during the systematic review.
Cannabis withdrawal syndrome was identified by a variety of clinician-administered instruments (including the Cannabis Withdrawal Scale74), self-reported rating scales (including the Marijuana Withdrawal Symptom Checklist6), and semistructured clinical interviews (involving the Time-Line-Follow-Back75 and the Structured Clinical Interview for the DSM76). Across studies, the specific instruments used were the Alcohol Use Disorder and Associated Disabilities Schedule,32,47,52 the Customary Drinking and Drug Use Record,50 the Composite International Diagnostic Interview-Substance Abuse Module,17,20,25,30,36,39 the Cannabis Withdrawal Scale,37,51,54,56 the Marijuana Quit Questionnaire,28,41,43,45,46,58 the Marijuana Withdrawal Symptom Checklist,6,7,27,33,34,42,49,53,55,60 the Semi-Structured Assessment for the Genetics of Alcoholism,18,40,48 the Structured Clinical Interview for the DSM,19,21,22,23,24,26,31,35,59 and the Time-Line-Follow-Back.57
Cannabis use disorder (CUD) or its equivalent (ie, cannabis dependence with or without cannabis abuse) was analyzed as defined by the study authors using varying criteria sets, including the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition77 or International Statistical Classification of Diseases and Related Health Problems, 11th Revision,78 with or without the use of interview guides, such as the Mini-International Neuropsychiatric Interview79 or the Structured Clinical Interview for the DSM.76 The overall proportion of participants with CUD was 34.7% (n = 8275). In population studies, estimates ranged from 8% to 34%. In outpatient-based samples, estimates ranged from 30% to 74%. In inpatient-based samples, estimates ranged from 72% to 98%.
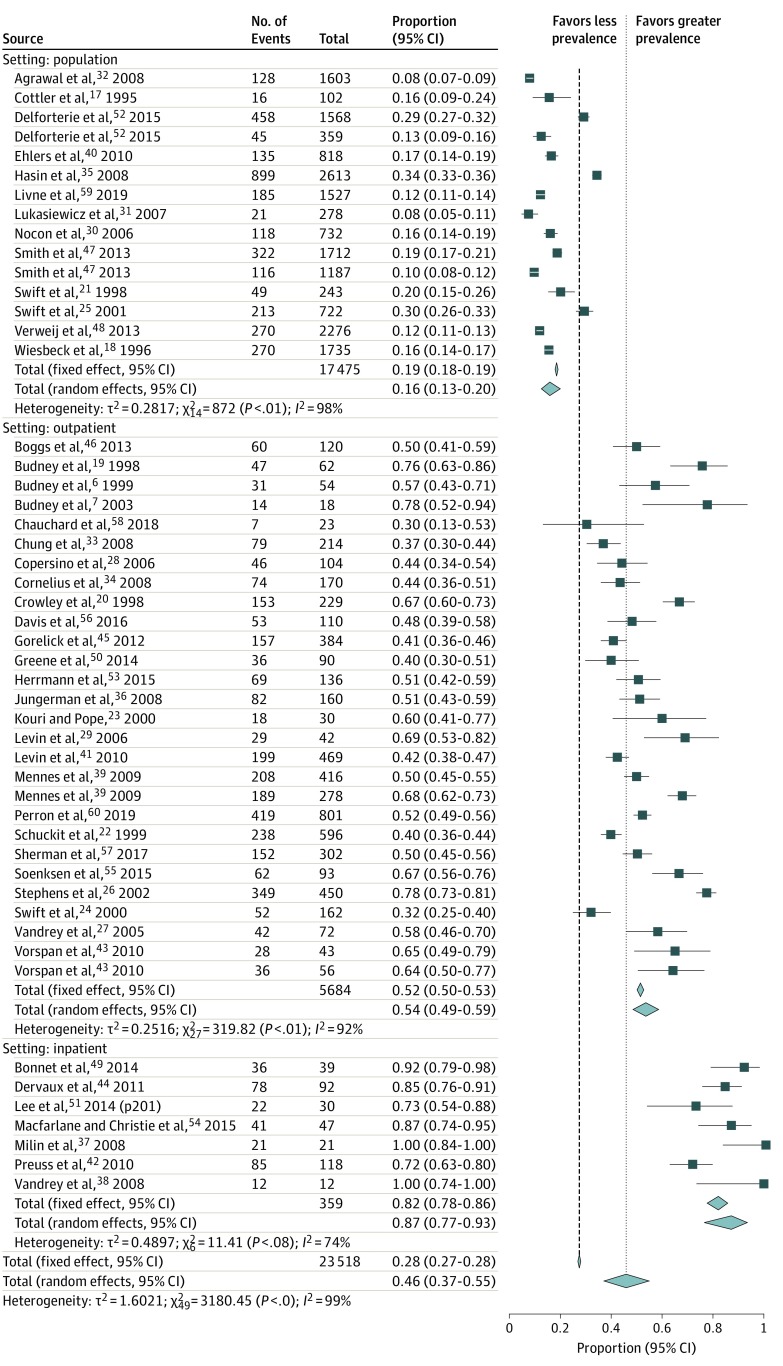
Meta-analysis identified that the overall pooled prevalence of CWS in patients with regular or dependent use of cannabinoids was 47% (95% CI, 27%-37%). There was significant heterogeneity observed in this estimate (I2 = 99.2%, P < .001; 50 studies; n = 23 518), with proportions of 0.16 (95% CI, 0.13-0.20) for population, 0.54 (95% CI, 0.49-0.59) for inpatient, and 0.87 (95% CI, 0.77-0.93) for inpatient (Figure 2). The range of CWS across studies varied from 8% to 100%.
Figure 2. Prevalence of Cannabis Withdrawal in People With Cannabis Use Disorder .
Prevalence of cannabis withdrawal symptoms across 3 clinical settings: population-level samples, outpatient clinical samples, and inpatient clinical samples. The studies by Smith et al (2013), Mennes et al (2009), and Vorspan et al (2010) included 2 or more substudies.
When stratified by study setting, the prevalence of CWS in population-based samples was 17% (95% CI, 13%-21%; n = 15 studies; n = 17 475 participants), 54% in outpatient samples (95% CI, 48%-59%; n = 28 studies; n = 5684 participants), and 87% in inpatient samples (95% CI, 79%-94%; n = 7 studies; n = 357 participants). The difference between these groups was statistically significantly different (χ2 = 0.172, P < .001) (Table 2). Significant heterogeneity existed within the estimates for population-based samples (I2 = 98%), outpatient-based samples (I2 = 92%) and inpatient-based samples (I2 = 74%). The subgroup analysis based on sex did not find any differences in the prevalence of CWS among men (27%) compared with women (26%) (χ2 = 0.172, P = .99). Similarly, there was no association between CWS prevalence and age, race/ethnicity, method of CWS ascertainment, method of CUD diagnosis, comorbid alcohol use, comorbid psychiatric disorder, or geographic region. Furthermore, CWS estimates were significantly higher among studies measuring lifetime rather than current CWS prevalence (χ2 = 0.314, P < .001), among cohort studies rather than cross-sectional surveys (χ2 = 0.194, P < .001), and among studies involving participants who were seeking treatment compared with those who were not (χ2 = 446.32, P < .001).
Table 2. Subgroup Analyses of Factors Associated With Cannabis Withdrawal Syndrome Prevalence.
| Subgroup analyses | Prevalence (95% CI), % | Studies, No. | z Value | I2, % | P value | Between-group comparison | |
|---|---|---|---|---|---|---|---|
| χ2 | P value | ||||||
| Sample sourcea | |||||||
| Population-based | 17 (13-22) | 15 | 7.866 | 98 | <.001 | 0.172 | <.001 |
| Clinical | |||||||
| Outpatient | 54 (48-59) | 28 | 20.267 | 94 | <.001 | ||
| Inpatient | 87 (79-94) | 7 | 22.346 | 94 | <.001 | ||
| Study designa | |||||||
| Cross-sectional | 19 (15.-24) | 17 | 8.733 | 99 | <.001 | 0.194 | <.001 |
| Cohort | 62 (56-68) | 33 | 19.816 | 96 | <.001 | ||
| Method of CWS diagnosis | |||||||
| Clinician-rated | 52 (41-62) | 20 | 9.725 | 99. | <.001 | 0.518 | .49 |
| Self-reported | 45 (37-53) | 23 | 11.086 | 99. | <.001 | ||
| Informant-rated | 40 (21-59) | 7 | 4.071 | 99 | <.001 | ||
| Method of CUD diagnosis | |||||||
| DSM-IV | 49 (43-56) | 37 | 14.993 | 99 | <.001 | 0.493 | .44 |
| DSM-III-R | 45 (31-58) | 8 | 6.382 | 97 | <.001 | ||
| DSM-5 | 34 (15-54) | 5 | 3.470 | 99 | <.001 | ||
| Timeline of CWSa | |||||||
| Past year | 31 (27-36) | 32 | 13.590 | 99 | <.001 | 0.314 | <.001 |
| Lifetime | 76 (70-82) | 18 | 26.659 | 86 | <.001 | ||
| Sex | |||||||
| Male | 27 (21-34) | 15 | 9.952 | 93 | <.001 | 0.172 | .99 |
| Female | 26 (19-33) | 15 | 9.200 | 96 | <.001 | ||
| Geographic region | |||||||
| North America | 48 (42-55) | 38 | 14.637 | 99 | <.001 | 0.484 | .77 |
| Europe | 47 (25-70) | 6 | 4.121 | 99 | <.001 | ||
| South America | 51 (44-59) | 1 | NA | NA | NA | ||
| Australasia | 36 (20-52) | 5 | 5.385 | 99 | <.001 | ||
Abbreviations: CUD, cannabis use disorder; CWS, cannabis withdrawal syndrome; DSM-III-R, Diagnostic and Statistical Manual, Third Edition-Revision; DSM-IV, Diagnostic and Statistical Manual, Fourth Edition; DSM-5, Diagnostic and Statistical Manual, Fifth Edition; NA, not applicable.
Statistically significant at P < .05.
We used meta-regression to explore potential variables that may have accounted for the high heterogeneity observed for CWS prevalence (Table 3; eFigure 1 in the Supplement). Several methodologic features of studies and participant characteristics were significantly associated with CWS prevalence in meta-regression. The prevalence of CWS was higher with greater proportions of participants who reported daily cannabis use (β = 0.004, P < .001), had cannabis use disorder (β = 0.005, P < .001), had comorbid tobacco use (β = 0.002, P = .02), and had comorbid drug use (β = 0.003, P = .05).
Table 3. Meta-regression Analyses of Factors Associated With Prevalence of Cannabis Withdrawal.
| Meta-regression | β | Intercept | Studies, No. | P value |
|---|---|---|---|---|
| Disorder, % | ||||
| Alcohol use | −0.000 | 0.463 | 17 | .97 |
| Psychiatric | 0.000 | 0.469 | 16 | .93 |
| Drug use | 0.003 | 0.443 | 11 | .05 |
| Tobacco use | 0.002 | 0.396 | 26 | .02 |
| Cannabis use | 0.005 | 0.128 | 48 | <.001 |
| Mean age, y | −0.007 | 0.679 | 48 | .10 |
| Daily cannabis use, % | 0.004 | 0.151 | 48 | <.001 |
| White race, % | 0.001 | 0.433 | 42 | .46 |
We explored the association of each study with pooled estimates via sensitivity analysis with leave-out-one meta-analysis, allowing the removal of each study from the evaluation. This analysis did not change the pooled prevalence of CWS substantially.
The potential for publication bias was assessed through funnel plots and by applying rank correlation tests, Egger tests, and the trim and fill method (eFigure 2 in the Supplement). The results did not suggest any evidence to support that a significant bias existed within this review.
Of the 50 studies, most (36 [72%]) had an overall rating of fair quality, while 2 studies (4%) were rated as good and 12 studies (24%) were rated as poor (eTable 3 in the Supplement). The most frequently met quality criteria were ascertainment of exposure, reported by 36 studies (72%), and comparability of cohorts on the basis of the design or analysis, reported by 26 studies (52%). A number of items were inconsistently completed, including demonstration that outcome of interest was not present at the start of the study, which was reported by 3 studies (6%), and adequacy of follow-up of cohorts, with rates of attrition and complete follow-up reported by 7 studies (14%).
Discussion
Our systematic review and meta-analysis identified 50 studies that examined the prevalence of CWS. Overall, it was estimated that nearly half (47%) of all people with regular or dependent cannabinoid use will experience cannabis withdrawal. Other factors associated with CWS included study setting; concurrent tobacco, cannabis, and drug use disorders; and intensity of cannabis use. We did not find CWS to be associated with sex, age, race/ethnicity, or psychiatric comorbidity. The quality of the literature was rated as being fair for the majority of studies considered.
Many professionals and members of the general public may not be aware of cannabis withdrawal, potentially leading to confusion about the benefits of cannabis to treat or self-medicate symptoms of anxiety or depressive disorders.80 For example, when medical marijuana clients were asked about actual symptom relief, fewer than half report such relief,81 while others82 reported return of anxiety symptoms on cessation of use, suggesting the symptoms might be due to cannabis withdrawal.83 Because many CWS criteria are depression or anxiety symptoms, regular users may seek cannabis to obtain short-term symptom relief, unaware that this use could perpetuate a longer-term withdrawal problem.77
Clinicians should be aware of CWS as it is associated with clinically significant symptoms, which can trigger resumption of cannabis use and serve as negative reinforcement for relapse during a quit attempt.28,41 The clinical significance of CWS is shown by the fact that it can be impairing,84 that cannabis or other substances are used to relieve it, by its association with trouble quitting use,28,41,85 and by its negative prognostic association.33,34,50,84 The clinical significance of CWS is also supported by epidemiologic evidence, as studies involving latent variable modeling have shown that adding withdrawal to other CUD criteria improves model fit.86 Personality traits, psychiatric comorbidity, age at onset, level of cannabis use, severity of cannabis dependence, and concurrent drug and alcohol use have been proposed as other risk factors that may play a role in increasing risk of cannabis relapse following a quit attempt.74
When the Diagnostic Manual of Mental Disorders, Fourth Edition, was published, little was known about CWS, but in the ensuing 2 decades, substantial research efforts have advanced our understanding of CWS.87,88,89 Animal models have been helpful in elucidating the potential mechanisms and causes of CWS, with rodents exhibiting both tolerance and dependence following chronic administration of cannabinoids.90 Cannabis tolerance is known to be mediated by downregulation of the cannabinoid receptor type 1,91 which occurs more rapidly in cortical regions than in subcortical regions92,93 and is reversible on abstinence.91 Inhibitors of endocannabinoid-metabolizing enzymes reduce CWS responses among cannabis-dependent mice.94 Cannabis withdrawal syndrome and CUD are moderately heritable,48 implicating both genetic and environmental factors in their occurrence.
In our study, CWS was more frequently encountered among patients with comorbid tobacco and drug use. Although our study did not identify an association between psychiatric comorbidity or alcohol use and CWS prevalence, the prevalence of CUD comorbidity is known to be substantially higher among individuals with a primary anxiety,44,95,96 mood,34,97 eating,61 or psychotic disorder46,98,99 relative to the general population. These findings are consistent with comorbidity literature, which provides further support for the notion that the nature of associations between substance use and psychiatric disorders is usually adverse.100 As well, this association may be exacerbated by potential kindling effects induced by cannabis with the occurrence of other psychiatric conditions.101 An understanding of these risks may support clinicians in providing evidence-based care and appropriate counseling to their patients, particularly regarding cannabinoid stewardship.101
The finding that the prevalence of CWS was substantially higher in clinical populations—particularly inpatient samples—is consistent with the notion of a bidirectional association between cannabis use and mental health disorders.102,103,104,105 This finding is compatible with previous reviews, which have consistently reported that one-third of regular cannabis users in the general population5,32,35 and 50% to 95% of heavy users in treatment or research studies28,33,34,41 report symptoms of CWS. This finding may indicate that people with CWS are more likely to present to clinicians for help compared with those without CWS, notwithstanding the fact that CWS can be diagnosed and untreated.10 Whether there is an interaction or cumulative association between CWS prevalence and rates of presentation for clinical care is speculative at this point and requires further study. With this in mind, if CWS reflects underlying CUD pathologic factors, it may be an indication of underlying addictive burden and increase the likelihood of people being in clinical care as opposed to having CUD in the community without clinical support.10 The association between CWS and CUD may also be related to the central theories of substance initiation, whereby cannabinoids may be used to self-medicate psychiatric symptoms106 or may precipitate or aggravate existing mental health conditions.107
Several studies have attempted to determine the best tools for diagnosing CWS,74,108 but there has generally been poor correlation between rating scales. Despite within-sample heterogeneity, CWS prevalence estimates were similar irrespective of ascertainment method in our study. Stratification of CWS ascertainment methods did not reconcile heterogeneity in prevalence estimates; however, this does not mean that all CWS instruments are equal. Until there are methodologic guidelines and consensus on the best tools to screen for CWS, to our knowledge, these are the most comprehensive available data. The treatment of CUD is particularly challenging because there are no efficacious medications currently available, even with cannabinoid replacement therapies, such as nabilone, nabiximols, or dronabinol.12,109
Strengths and Limitations
There are a number of strengths of this study. First, to our knowledge, this is the largest systematic review of cannabis withdrawal among people with CUD, and the first meta-analysis. Second, the quality of the majority of studies evaluated was fair. However, this study has limitations that should be considered in the appraisal of the evidence presented by this review. The largest limitation is the wide range of tools used to define CUD and CWS, which contributed to the large heterogeneity across studies. While the broad spectrum of included studies likely contributed to heterogeneity, the inclusion of only validated rating scales may have mitigated the heterogeneity somewhat. Although sex proportions were reported in overall samples, sex-specific prevalence estimates were only reported by a subset of studies (n = 15). As this is a study-level meta-analysis, a limitation of the methods is that individual-level characteristics were not explored. There was also limited representation of studies from all geographic regions, with only 1 study from South America and none from Africa; this limitation hampered our ability to estimate the prevalence of CWS across all continents. However, our subgroup analysis indicated that there was no significant difference in prevalence of CWS across the regions evaluated, which suggests that geographic regions may not play a substantial role in estimating CWS prevalence. There was also limited information about CWS in specific patient subgroups. There are other issues that are likely to influence CWS, which could not be addressed in this meta-analysis, including the changing products that are being used, which may affect tolerance, dependence, and CWS. However, this information is not available in most clinical studies to date. There was also a lack of individual-level analyses, which may be considered as another limitation of this study. In addition, few studies reported the amounts of concurrent substance use or cannabinoid levels in bodily fluids (eg, urine and blood), precluding a more focused analysis on the association between these measures and CWS prevalence.
Conclusions
Cannabis withdrawal syndrome appears to be common among people with regular or dependent use of cannabinoids, with an overall pooled prevalence of 47% in this meta-analysis. Cannabis withdrawal syndrome was more common in men, participants from clinical samples, individuals with comorbid drug or tobacco use, and those with a higher level of cannabis use. Clinicians should be aware of the high prevalence of CWS and should consider screening for CWS, particularly among those who are at greater risk, in order to counsel patients and support individuals who are reducing their use of cannabis.
eTable 1. Full Systematic Review Search Strategy
eTable 2. Study Characteristics
eTable 3. List of Validated Cannabis Withdrawal Instruments and Scales
eFigure 1. Subgroup Analyses and Meta-Regressions
eFigure 2. Publication Bias Analysis Using Funnel Plot for Prevalence of Cannabis Withdrawal Syndrome Against Standard Error
eTable 3. Quality Assessment Using Newcastle Ottawa Scale
eReferences
References
- 1.Degenhardt L, Charlson F, Ferrari A, et al. ; GBD 2016 Alcohol and Drug Use Collaborators . The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):-. doi: 10.1016/S2215-0366(18)30337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crean RD, Tapert SF, Minassian A, Macdonald K, Crane NA, Mason BJ. Effects of chronic, heavy cannabis use on executive functions. J Addict Med. 2011;5(1):9-15. doi: 10.1097/ADM.0b013e31820cdd57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227. doi: 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho AF, Stubbs B, Vancampfort D, et al. Cannabis use and suicide attempts among 86,254 adolescents aged 12-15 years from 21 low- and middle-income countries. Eur Psychiatry. 2019;56:8-13. doi: 10.1016/j.eurpsy.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 5.Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19(3):233-238. doi: 10.1097/01.yco.0000218592.00689.e5 [DOI] [PubMed] [Google Scholar]
- 6.Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94(9):1311-1322. doi: 10.1046/j.1360-0443.1999.94913114.x [DOI] [PubMed] [Google Scholar]
- 7.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393-402. doi: 10.1037/0021-843X.112.3.393 [DOI] [PubMed] [Google Scholar]
- 8.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161(11):1967-1977. doi: 10.1176/appi.ajp.161.11.1967 [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Association Publishing; 2013. [Google Scholar]
- 10.Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212. doi: 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anthony JC, Lopez-Quintero C, Alshaarawy O. Cannabis epidemiology: a selective review. Curr Pharm Des. 2017;22(42):6340-6352. doi: 10.2174/1381612822666160813214023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahji A, Mazhar MN. Treatment of cannabis dependence with synthetic cannabinoids: a systematic review. Can J Addict. 2016;7(4):8-13. [Google Scholar]
- 13.National Institute for Health Research PROSPERO International prospective register of systematic reviews. 2019. Accessed November 24, 2019. https://www.crd.york.ac.uk/PROSPERO/
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy Rosenzweig Center for History and New Media Zotero; 2018. Accessed March 9, 2020. https://rrchnm.org/zotero/ [Google Scholar]
- 16.Covidence: better systematic review management.Veritas Health Innovation Accessed March 9, 2020. https://www.covidence.org/home
- 17.Cottler LB, Schuckit MA, Helzer JE, et al. The DSM-IV field trial for substance use disorders: major results. Drug Alcohol Depend. 1995;38(1):59-69. doi: 10.1016/0376-8716(94)01091-X [DOI] [PubMed] [Google Scholar]
- 18.Wiesbeck GA, Schuckit MA, Kalmijn JA, Tipp JE, Bucholz KK, Smith TL. An evaluation of the history of a marijuana withdrawal syndrome in a large population. Addiction. 1996;91(10):1469-1478. doi: 10.1111/j.1360-0443.1996.tb02251.x [DOI] [PubMed] [Google Scholar]
- 19.Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. Adults seeking treatment for marijuana dependence: a comparison with cocaine-dependent treatment seekers. Exp Clin Psychopharmacol. 1998;6(4):419-426. doi: 10.1037/1064-1297.6.4.419 [DOI] [PubMed] [Google Scholar]
- 20.Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend. 1998;50(1):27-37. doi: 10.1016/S0376-8716(98)00003-9 [DOI] [PubMed] [Google Scholar]
- 21.Swift W, Hall W, Didcott P, Reilly D. Patterns and correlates of cannabis dependence among long-term users in an Australian rural area. Addiction. 1998;93(8):1149-1160. doi: 10.1046/j.1360-0443.1998.93811493.x [DOI] [PubMed] [Google Scholar]
- 22.Schuckit MA, Daeppen J-B, Danko GP, et al. Clinical implications for four drugs of the DSM-IV distinction between substance dependence with and without a physiological component. Am J Psychiatry. 1999;156(1):41-49. doi: 10.1176/ajp.156.1.41 [DOI] [PubMed] [Google Scholar]
- 23.Kouri EM, Pope HG Jr. Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8(4):483-492. doi: 10.1037/1064-1297.8.4.483 [DOI] [PubMed] [Google Scholar]
- 24.Swift W, Hall W, Copeland J. One year follow-up of cannabis dependence among long-term users in Sydney, Australia. Drug Alcohol Depend. 2000;59(3):309-318. doi: 10.1016/S0376-8716(99)00131-3 [DOI] [PubMed] [Google Scholar]
- 25.Swift W, Hall W, Teesson M. Characteristics of DSM-IV and ICD-10 cannabis dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Drug Alcohol Depend. 2001;63(2):147-153. doi: 10.1016/S0376-8716(00)00197-6 [DOI] [PubMed] [Google Scholar]
- 26.Stephens RS, Babor TF, Kadden R, Miller M; Marijuana Treatment Project Research Group . The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction. 2002;97(s1)(suppl 1):109-124. doi: 10.1046/j.1360-0443.97.s01.6.x [DOI] [PubMed] [Google Scholar]
- 27.Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depend. 2005;78(2):205-210. doi: 10.1016/j.drugalcdep.2004.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copersino ML, Boyd SJ, Tashkin DP, et al. Cannabis withdrawal among non–treatment-seeking adult cannabis users. Am J Addict. 2006;15(1):8-14. doi: 10.1080/10550490500418997 [DOI] [PubMed] [Google Scholar]
- 29.Levin FR, Brooks DJ, Bisaga A, et al. Severity of dependence and motivation for treatment: comparison of marijuana- and cocaine-dependent treatment seekers. J Addict Dis. 2006;25(1):33-41. doi: 10.1300/J069v25n01_06 [DOI] [PubMed] [Google Scholar]
- 30.Nocon A, Wittchen HU, Pfister H, Zimmermann P, Lieb R. Dependence symptoms in young cannabis users? a prospective epidemiological study. J Psychiatr Res. 2006;40(5):394-403. doi: 10.1016/j.jpsychires.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 31.Lukasiewicz M, Falissard B, Michel L, Neveu X, Reynaud M, Gasquet I. Prevalence and factors associated with alcohol and drug-related disorders in prison: a French national study. Subst Abuse Treat Prev Policy. 2007;2:1. doi: 10.1186/1747-597X-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrawal A, Pergadia ML, Lynskey MT. Is there evidence for symptoms of cannabis withdrawal in the national epidemiologic survey of alcohol and related conditions? Am J Addict. 2008;17(3):199-208. doi: 10.1080/10550490802019519 [DOI] [PubMed] [Google Scholar]
- 33.Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction. 2008;103(5):787-799. doi: 10.1111/j.1360-0443.2008.02158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33(11):1500-1505. doi: 10.1016/j.addbeh.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 2008;69(9):1354-1363. doi: 10.4088/JCP.v69n0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jungerman FS, Laranjeira R. Characteristics of cannabis users seeking treatment in São Paulo, Brazil. Rev Panam Salud Publica. 2008;23(6):384-393. doi: 10.1590/S1020-49892008000600003 [DOI] [PubMed] [Google Scholar]
- 37.Milin R, Manion I, Dare G, Walker S. Prospective assessment of cannabis withdrawal in adolescents with cannabis dependence: a pilot study. J Am Acad Child Adolesc Psychiatry. 2008;47(2):174-179. doi: 10.1097/chi.0b013e31815cdd73 [DOI] [PubMed] [Google Scholar]
- 38.Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92(1-3):48-54. doi: 10.1016/j.drugalcdep.2007.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mennes CE, Ben Abdallah A, Cottler LB. The reliability of self-reported cannabis abuse, dependence and withdrawal symptoms: multisite study of differences between general population and treatment groups. Addict Behav. 2009;34(2):223-226. doi: 10.1016/j.addbeh.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 40.Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35(2):102-110. doi: 10.1016/j.addbeh.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levin KH, Copersino ML, Heishman SJ, et al. Cannabis withdrawal symptoms in non–treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111(1-2):120-127. doi: 10.1016/j.drugalcdep.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preuss UW, Watzke AB, Zimmermann J, Wong JW, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug Alcohol Depend. 2010;106(2-3):133-141. doi: 10.1016/j.drugalcdep.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 43.Vorspan F, Guillem E, Bloch V, et al. Self-reported sleep disturbances during cannabis withdrawal in cannabis-dependent outpatients with and without opioid dependence. Sleep Med. 2010;11(5):499-500. doi: 10.1016/j.sleep.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dervaux A, Krebs MO, Laqueille X. Anxiety and depressive symptoms or disorders in patients with cannabis dependence without major psychiatric disorders. Eur Neuropsychopharmacol. 2011;21(suppl 3):S578-S579. doi: 10.1016/S0924-977X(11)70946-4 [DOI] [Google Scholar]
- 45.Gorelick DA, Levin KH, Copersino ML, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend. 2012;123(1-3):141-147. doi: 10.1016/j.drugalcdep.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boggs DL, Kelly DL, Liu F, et al. Cannabis withdrawal in chronic cannabis users with schizophrenia. J Psychiatr Res. 2013;47(2):240-245. doi: 10.1016/j.jpsychires.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith PH, Homish GG, Leonard KE, Collins RL. Marijuana withdrawal and aggression among a representative sample of US marijuana users. Drug Alcohol Depend. 2013;132(1-2):63-68. doi: 10.1016/j.drugalcdep.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verweij KJ, Agrawal A, Nat NO, et al. A genetic perspective on the proposed inclusion of cannabis withdrawal in DSM-5. Psychol Med. 2013;43(8):1713-1722. doi: 10.1017/S0033291712002735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonnet U, Specka M, Stratmann U, Ochwadt R, Scherbaum N. Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification: cannabis withdrawal syndrome and its correlation with delta-9-tetrahydrocannabinol and -metabolites in serum. Drug Alcohol Depend. 2014;143:189-197. doi: 10.1016/j.drugalcdep.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 50.Greene MC, Kelly JF. The prevalence of cannabis withdrawal and its influence on adolescents’ treatment response and outcomes: a 12-month prospective investigation. J Addict Med. 2014;8(5):359-367. doi: 10.1097/ADM.0000000000000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee D, Schroeder JR, Karschner EL, et al. Cannabis withdrawal in chronic, frequent cannabis smokers during sustained abstinence within a closed residential environment. Am J Addict. 2014;23(3):234-242. doi: 10.1111/j.1521-0391.2014.12088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delforterie M, Creemers H, Agrawal A, et al. Functioning of cannabis abuse and dependence criteria across two different countries: the United States and the Netherlands. Subst Use Misuse. 2015;50(2):242-250. doi: 10.3109/10826084.2014.952445 [DOI] [PubMed] [Google Scholar]
- 53.Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol. 2015;23(6):415-421. doi: 10.1037/pha0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macfarlane V, Christie G. Synthetic cannabinoid withdrawal: a new demand on detoxification services. Drug Alcohol Rev. 2015;34(2):147-153. doi: 10.1111/dar.12225 [DOI] [PubMed] [Google Scholar]
- 55.Soenksen S, Stein LAR, Brown JD, Stengel JR, Rossi JS, Lebeau R. Cannabis withdrawal among detained adolescents: exploring the impact of nicotine and race. J Child Adolesc Subst Abuse. 2015;24(2):119-124. doi: 10.1080/1067828X.2013.770379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis JP, Smith DC, Morphew JW, Lei X, Zhang S. Cannabis withdrawal, posttreatment abstinence, and days to first cannabis use among emerging adults in substance use treatment: a prospective study. J Drug Issues. 2016;46(1):64-83. doi: 10.1177/0022042615616431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherman BJ, McRae-Clark AL, Baker NL, et al. Gender differences among treatment-seeking adults with cannabis use disorder: clinical profiles of women and men enrolled in the Achieving Cannabis Cessation–Evaluating N-Acetylcysteine Treatment (ACCENT) study. Am J Addict. 2017;26(2):136-144. doi: 10.1111/ajad.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chauchard E, Hartwell KJ, McRae-Clark AL, Sherman BJ, Gorelick DA. Cannabis withdrawal in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2018;20(1):17m02203. doi: 10.4088/PCC.17m02203 [DOI] [PubMed] [Google Scholar]
- 59.Livne O, Shmulewitz D, Lev-Ran S, Hasin DS. DSM-5 cannabis withdrawal syndrome: demographic and clinical correlates in US adults. Drug Alcohol Depend. 2019;195:170-177. doi: 10.1016/j.drugalcdep.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perron BE, Holt KR, Yeagley E, Ilgen M. Mental health functioning and severity of cannabis withdrawal among medical cannabis users with chronic pain. Drug Alcohol Depend. 2019;194:401-409. doi: 10.1016/j.drugalcdep.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 61.Bahji A, Mazhar MN, Hudson CC, Nadkarni P, MacNeil BA, Hawken E. Prevalence of substance use disorder comorbidity among individuals with eating disorders: a systematic review and meta-analysis. Psychiatry Res. 2019;273:58-66. doi: 10.1016/j.psychres.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 62.Bahji A, Hawken ER, Sepehry AA, Cabrera CA, Vazquez G. ECT beyond unipolar major depression: systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr Scand. 2019;139(3):214-226. doi: 10.1111/acps.12994 [DOI] [PubMed] [Google Scholar]
- 63.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Degenhardt L, Ferrari AJ, Calabria B, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One. 2013;8(10):e76635. doi: 10.1371/journal.pone.0076635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Degenhardt L, Coffey C, Romaniuk H, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013;108(1):124-133. doi: 10.1111/j.1360-0443.2012.04015.x [DOI] [PubMed] [Google Scholar]
- 66.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed May 23, 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 67.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i0320808728 [DOI] [Google Scholar]
- 68.Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. 1978;32(4):138. doi: 10.1080/00031305.1978.10479283 [DOI] [Google Scholar]
- 69.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 70.Willis BH, Riley RD. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat Med. 2017;36(21):3283-3301. doi: 10.1002/sim.7372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 73.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 74.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119(1-2):123-129. doi: 10.1016/j.drugalcdep.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 75.Sobell LC, Sobell MB. Timeline follow-back In: Litten RZ, Allen JP, eds. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; 1992:41-72, . doi: 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- 76.First MB. Structured Clinical Interview for the DSM (SCID) In: The Encyclopedia of Clinical Psychology. American Cancer Society; 2015:1-6. doi: 10.1002/9781118625392.wbecp351 [DOI] [Google Scholar]
- 77.American Psychiatric Association DSM-V: Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Association; 2013. doi: 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 78.World Health Organization International Statistical Classification of Diseases and Related Health Problems; 11th Revision, 2018 version. Accessed November 24, 2019. https://icd.who.int/browse11/l-m/en
- 79.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. [PubMed] [Google Scholar]
- 80.Katz G, Lobel T, Tetelbaum A, Raskin S. Cannabis withdrawal—a new diagnostic category in DSM-5. Isr J Psychiatry Relat Sci. 2014;51(4):270-275. [PubMed] [Google Scholar]
- 81.Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse. 2014;40(1):23-30. doi: 10.3109/00952990.2013.821477 [DOI] [PubMed] [Google Scholar]
- 82.Swift W, Gates P, Dillon P. Survey of Australians using cannabis for medical purposes. Harm Reduct J. 2005;2(1):18. doi: 10.1186/1477-7517-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walsh Z, Gonzalez R, Crosby K, S Thiessen M, Carroll C, Bonn-Miller MO. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29. doi: 10.1016/j.cpr.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 84.Allsop DJ, Copeland J, Norberg MM, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7(9):e44864. doi: 10.1371/journal.pone.0044864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35(4):362-368. doi: 10.1016/j.jsat.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agrawal A, Lynskey MT, Madden PAF, Bucholz KK, Heath AC. A latent class analysis of illicit drug abuse/dependence: results from the National Epidemiological Survey on Alcohol and Related Conditions. Addiction. 2007;102(1):94-104. doi: 10.1111/j.1360-0443.2006.01630.x [DOI] [PubMed] [Google Scholar]
- 87.Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4(1):4-16. doi: 10.1151/ASCP07414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haney M, Hart CL, Vosburg SK, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29(1):158-170. doi: 10.1038/sj.npp.1300310 [DOI] [PubMed] [Google Scholar]
- 89.Lichtman AH, Martin BR. Marijuana withdrawal syndrome in the animal model. J Clin Pharmacol. 2002;42(S1):20S-27S. doi: 10.1002/j.1552-4604.2002.tb05999.x [DOI] [PubMed] [Google Scholar]
- 90.González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81(2):300-318. doi: 10.1016/j.pbb.2005.01.028 [DOI] [PubMed] [Google Scholar]
- 91.Hirvonen J, Goodwin RS, Li C-T, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17(6):642-649. doi: 10.1038/mp.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15(2):91-119. doi: 10.1615/CritRevNeurobiol.v15.i2.10 [DOI] [PubMed] [Google Scholar]
- 93.Sim-Selley LJ, Schechter NS, Rorrer WK, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70(3):986-996. doi: 10.1124/mol.105.019612 [DOI] [PubMed] [Google Scholar]
- 94.Schlosburg JE, Carlson BLA, Ramesh D, et al. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009;11(2):342-352. doi: 10.1208/s12248-009-9110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paulus DJ, Manning K, Hogan JBD, Zvolensky MJ. The role of anxiety sensitivity in the relation between anxious arousal and cannabis and alcohol use problems among low-income inner city racial/ethnic minorities. J Anxiety Disord. 2017;48:87-94. doi: 10.1016/j.janxdis.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 96.Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24(7):515-523. doi: 10.1002/hup.1048 [DOI] [PubMed] [Google Scholar]
- 97.Arias F, Szerman N, Vega P, et al. Abuse or dependence on cannabis and other psychiatric disorders: Madrid study on dual pathology prevalence. Actas Esp Psiquiatr. 2013;41(2):122-129. [PubMed] [Google Scholar]
- 98.Koola MM, Boggs DL, Kelly DL, et al. Relief of cannabis withdrawal symptoms and cannabis quitting strategies in people with schizophrenia. Psychiatry Res. 2013;209(3):273-278. doi: 10.1016/j.psychres.2013.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koola MM, Kelly DL, McMahon RP, Boggs DL, Liu F, Gorelick DA. Psychoactive substance use by adults with schizophrenia before and during cannabis withdrawal. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.16l01959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-455. doi: 10.1080/00952990.2016.1213273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacDonald K, Pappas K. Why not pot? a review of the brain-based risks of cannabis Innov Clin Neurosci. 2016;13(3-4):13-22. [PMC free article] [PubMed] [Google Scholar]
- 102.Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Bipolar disorder and co-occurring cannabis use disorders: characteristics, co-morbidities and clinical correlates. Psychiatry Res. 2013;209(3):459-465. doi: 10.1016/j.psychres.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 103.Gage SH. Cannabis and psychosis: triangulating the evidence. Lancet Psychiatry. 2019;6(5):364-365. doi: 10.1016/S2215-0366(19)30086-0 [DOI] [PubMed] [Google Scholar]
- 104.Pacek LR, Martins SS, Crum RM. The bidirectional relationships between alcohol, cannabis, co-occurring alcohol and cannabis use disorders with major depressive disorder: results from a national sample. J Affect Disord. 2013;148(2-3):188-195. doi: 10.1016/j.jad.2012.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Womack SR, Shaw DS, Weaver CM, Forbes EE. Bidirectional associations between cannabis use and depressive symptoms from adolescence through early adulthood among at-risk young men. J Stud Alcohol Drugs. 2016;77(2):287-297. doi: 10.15288/jsad.2016.77.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4(5):231-244. doi: 10.3109/10673229709030550 [DOI] [PubMed] [Google Scholar]
- 107.Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? an oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401. doi: 10.1038/npp.2015.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodgins DC, Stea JN. Psychometric evaluation of a lifetime version of the marijuana problems scale. Addict Behav Rep. 2018;8:21-24. doi: 10.1016/j.abrep.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database Syst Rev. 2014;(12):CD008940. doi: 10.1002/14651858.CD008940.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213. doi: 10.1016/0740-5472(92)90062-S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Full Systematic Review Search Strategy
eTable 2. Study Characteristics
eTable 3. List of Validated Cannabis Withdrawal Instruments and Scales
eFigure 1. Subgroup Analyses and Meta-Regressions
eFigure 2. Publication Bias Analysis Using Funnel Plot for Prevalence of Cannabis Withdrawal Syndrome Against Standard Error
eTable 3. Quality Assessment Using Newcastle Ottawa Scale
eReferences