Abstract
Frustratingly, disease-modifying treatments for diabetic neuropathy remain elusive. Glycaemic control has a robust effect on preventing neuropathy in individuals with type 1 but not in those with type 2 diabetes, which constitute the vast majority of patients. Encouragingly, recent evidence points to new metabolic risk factors and mechanisms, and thus also at novel disease-modifying strategies, which are desperately needed. Obesity has emerged as the second most important metabolic risk factor for neuropathy (diabetes being the first) from consensus findings of seven observational studies in populations across the world. Moreover, dyslipidaemia and altered sphingolipid metabolism are emergent novel mechanisms of nerve injury that may lead to new targeted therapies. Clinical history and examination remain critical components of an accurate diagnosis of neuropathy. However, skin biopsies and corneal confocal microscopy are promising newer tests that have been used as outcome measures in research studies but have not yet demonstrated clear clinical utility. Given the emergence of obesity as a neuropathy risk factor, exercise and weight loss are potential interventions to treat and/or prevent neuropathy, although evidence supporting exercise currently outweighs data supporting weight loss. Furthermore, a consensus has emerged advocating tricyclic antidepressants, serotonin-noradrenaline (norepinephrine) reuptake inhibitors and gabapentinoids for treating neuropathic pain. Out-of-pocket costs should be considered when prescribing these medications since their efficacy and tolerability are similar. Finally, the downsides of opioid treatment for chronic, non-cancer pain are becoming increasingly evident. Despite these data, current clinical practice frequently initiates and continues opioid prescriptions for patients with neuropathic pain before prescribing guideline-recommended treatments.
Keywords: Diabetes, Dyslipidaemia, Exercise, Neuropathy, Obesity, Opioids, Pain, Skin biopsy, Sphingolipids, Weight loss
Introduction
Neuropathy is nerve injury that starts with the longest nerves that innervate the toes and progresses proximally. Common symptoms are numbness, tingling, pain and/or weakness starting in the distal lower extremities. Diabetes is well established as the most important metabolic risk factor for neuropathy, but treatment of hyperglycaemia is not enough to prevent neuropathy in those with type 2 diabetes [1]. The prevalence of neuropathy is 8–45% in those with type 2 diabetes, with about a quarter of patients experiencing pain [2]. Disease-modifying treatments for diabetic neuropathy remain elusive, but recent evidence has identified new metabolic risk factors, mechanisms and potential disease-modifying therapies. Furthermore, new diagnostic tests are available and guidelines have demonstrated the best treatments for neuropathic pain, including those to avoid. These latest advances and their implications for the future will be discussed in the following sections.
Epidemiology
Recent epidemiological studies implicate obesity as the second most influential metabolic risk factor for neuropathy after diabetes [3–9]. This evidence emerges from seven clinical studies, which were conducted in the USA (two studies) [5, 6], China (two) [4, 8], and Denmark [3] the Netherlands [7] and Germany (one each) [9]. One US study of a severely obese population (N=102, cross-sectional study design) investigated the association of neuropathy with components of the metabolic syndrome, and found diabetes and waist circumference to be the only metabolic risk factors for neuropathy [6]. Furthermore, neuropathy was more prevalent in obese normoglycaemic individuals (11.1%) than lean controls (3.8%), suggesting obesity alone may be sufficient to cause neuropathy. A second US study evaluated the association of components of the metabolic syndrome with neuropathy in an elderly population (N=2,382, cross-sectional) [5]. While diabetes was the only metabolic risk factor associated with the primary neuropathy outcome, waist circumference was associated with four of the six secondary neuropathy outcomes, which was more than any other metabolic factor. One Chinese population-based study in Pinggu revealed that diabetes and weight were the only metabolic risk factors associated with neuropathy (N=4,002, cross-sectional) [4]. A second Chinese population study in Shanghai demonstrated that non-diabetic individuals with neuropathy had a larger waist circumference and were more likely to be hypertensive (N=2035, cross-sectional) [8].
The studies conducted in Denmark, the Netherlands and Germany also highlighted obesity as a neuropathy risk factor, replicating the study findings in China and the USA. The Danish study followed individuals with screen-detected diabetes and investigated which metabolic risk factors were associated with incident neuropathy (N=1,256, longitudinal) [3]. Weight, BMI, waist circumference, and HDL- and LDL-cholesterol all associated with neuropathy. The Dutch study evaluated a community dwelling, middle-aged to elderly population (N=1,256, cross-sectional), and found waist circumference and triacylglycerols were the only metabolic factors, besides diabetes, that were associated with neuropathy [7]. Finally, the German study demonstrated that multiple obesity measures were associated with incident neuropathy in an elderly cohort (N=513, longitudinal) [9].
Overall, these five cross-sectional and two longitudinal studies in a total of 11,547 demographically diverse individuals consistently demonstrate that obesity is independently associated with neuropathy and only trails hyperglycaemia as a risk factor in terms of the strength of the association. These studies have potential implications for treating neuropathy in individuals with and without diabetes. Importantly, many obese normoglycaemic individuals diagnosed with idiopathic neuropathy may need to be re-classified as obesity-related neuropathy. In the future, treatments geared towards obesity and its downstream effects will likely be needed rather than solely focusing on hyperglycaemia.
Metabolic causes of neuropathy
The role of glucose and lipids In addition to hyperglycaemia, dyslipidaemia is increasingly viewed as a contributing pathogenic factor to neuropathy, particularly for type 2 diabetes. This more recent direction stems from a 2012 Cochrane review [1], which indicated that intense glycaemic control only marginally improved neuropathy in multiple type 2 diabetes cohorts. Rather, metabolic syndrome components, such as obesity, emerged as key players in neuropathy risk. This contention is further supported by the clinical studies discussed above. As such, in vitro, in vivo and clinical studies are focused on uncovering the mechanisms by which altered glucose and lipids converge to affect peripheral nerve function and health to support the development of potential mechanism-based therapies [10–12].
Hyperglycaemia and dyslipidaemia affect multiple cells in the peripheral nervous system, including neuronal axons, dorsal root ganglion neurons and Schwann cells. There have been decades of research on the impact of glucose overload on these cells, and consequences include excess reactive oxygen species (ROS) formation, loss of ATP production, impaired mitochondrial function, activation of stress pathways and glycation of essential proteins to form AGEs (Fig. 1). These events all further increase ROS, which in turn promote endoplasmic reticulum (ER) stress, DNA damage, apoptosis and activation of proinflammatory signalling, mechanisms that ultimately lead to nerve injury.
Fig. 1.
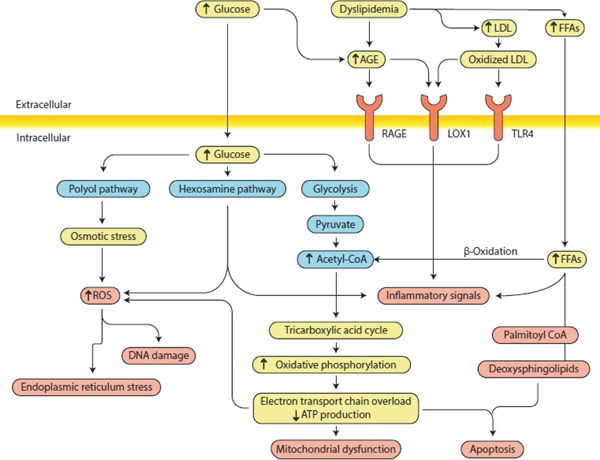
Diabetic neuropathy pathogenesis. Hyperglycaemia and dyslipidaemia lead to several pathological alterations in neurons, Schwann cells, glia and vascular cells, including ER stress, DNA damage, mitochondrial dysfunction, neurodegeneration and apoptosis and, ultimately, neuropathy. The importance of these pathways in the development of neuropathy varies with cell type, disease profile and time. Distinct cell types are more or less susceptible to injury depending on the metabolic impairments. LOX1, oxidised LDL receptor 1; RAGE, AGE-specific receptor; ROS, reactive oxygen species; TLR4, Toll-like receptor 4. Adapted from [11] with permission from Springer Nature. This figure is available as a downloadable slide
More recent emphasis, however, is focused on dyslipidaemia and elevated triacylglycerols as sources of NEFA in type 2 diabetes. NEFA are catabolised by β-oxidation in the cytosol of all cells of the peripheral nervous system. Acetyl-CoA, a β-oxidation product, can accumulate during NEFA substrate overload, and its conversion into toxic acylcarnitine species leads to further nerve injury. At the same time, compromised β-oxidation also results in extensive ROS production and inflammatory pathway activation, promoting effects similar to those observed in response to glucose overload, such as ER stress and altered mitochondrial function and impaired axonal transport, resulting in ATP deficiency [13]. Finally, oxidation of LDLs can induce ROS generation and activate receptors, such as the receptor for AGEs (RAGE), the receptor for oxidised LDLs (LOX1), and Toll-like receptor 4 (TLR4), exacerbating nerve injury, activating caspase-3 and inducing DNA degradation. Hence, inflammation, mitochondrial dysfunction, oxidative damage and impaired energy processing and utilisation converge as potential central mechanisms underlying the effects of hyperglycaemia and dyslipidaemia on peripheral nerve health (Fig. 1) [10–12]. Current research focuses on identifying the contributions from each pathway to neuropathy for developing targeted, mechanism-based therapies that can impact clinical outcomes.
Alterations in sphingolipid metabolism While hyperglycaemia and dyslipidaemia are more well-established contributors to nerve injury, increasing evidence suggests that altered sphingolipid metabolism is also an important neurotoxic factor. Specifically, altered sphingolipid metabolism in individuals with type 2 diabetes results in the formation of atypical, neurotoxic deoxysphingolipids. Sphingolipids are bioactive lipids that are essential structural components of the plasma cell membrane and important signalling molecules, particularly in the nervous system. Sphingolipid biosynthesis begins by enzymatic catalytic condensation of palmitoyl-CoA with the amino acid serine mediated by serine palmitoyltransferase (SPT). Atypical deoxysphingolipids arise when SPT metabolises alanine or glycine instead of serine. Deoxysphingolipids are toxic to neurons and to pancreatic beta cells [14, 15]. In addition, deoxysphingolipid plasma levels are elevated in individuals with the metabolic syndrome and type 1 diabetes, and the greatest elevations are observed in individuals with type 2 diabetes [15–17]. Disproportionate increases in select deoxysphingolipids are seen in diabetic individuals with neuropathy compared with those without neuropathy [17]. Elevated deoxysphingolipids are not universally associated with nerve injury, although they occur in several neuropathies other than diabetic neuropathy. They are thought to cause hereditary sensory autonomic neuropathy type I [14], a rare genetic disease that is phenotypically similar to diabetic neuropathy, including the disproportionate injury to sensory nerves, severe neuropathic pain and propensity to limb ulceration. Deoxysphingolipids are also observed in paclitaxel-induced neuropathy and neuropathy associated with mitochondrial disease [18, 19]. Altered sphingolipid metabolism is a potential novel mechanism for neuropathy that may lead to new disease-modifying therapies, but more preclinical and observational clinical studies are needed before launching future clinical trials.
Diagnostic testing A diagnosis of diabetic neuropathy should be suspected based on supportive clinical history and neurological exam in the setting of diagnosed diabetes. HbA1c, fasting glucose or a high-sensitivity OGTT should be considered for diagnosing diabetes and impaired glucose tolerance and/or impaired fasting glucose [20]. Testing may also be performed to exclude other common neuropathy causes, such as vitamin B12 levels for vitamin B12 deficiency-induced neuropathy, or serum protein electrophoresis (SPEP) or immunofixation for gammopathy-induced neuropathy [20]. Screening for alcohol abuse should also be completed since this is one of the most common causes of neuropathy.
Common neuropathy symptoms include numbness, paraesthesias and neuropathic pain, which is described as burning, electrical or sharp shooting sensations. Early exam findings include reduced or absent Achilles reflex, diminished distal sensation to small fibre function (pain/temperature), and large fibre function (vibration/proprioception). Small fibre dysfunction often precedes loss of large fibre function in diabetic neuropathy. Nerve conduction studies (NCS) demonstrate diminished sensory nerve action potential amplitudes in the distal lower extremities with mild slowing of conduction velocities [21]. In the clinical setting of a diagnosed diabetic individual with symptoms and exam findings of a distal symmetric polyneuropathy (DSP), NCS are often unnecessary for diagnosis [22, 23]. Routine NCS/electromyography (EMG) and magnetic resonance imaging have limited utility for evaluating neuropathy as these tests rarely change the diagnosis or management, but contribute substantially to healthcare costs for evaluating neuropathy [22]. The neurological history and exam lead to identification of an underlying cause of neuropathy in 64% of cases, with an additional 10% of causes identified after the simple blood tests outlined above [22]. Warning signs of atypical neuropathy, such as asymmetry, predominant weakness, non-length dependence and acute/subacute onset, should prompt additional testing, including NCS/EMG [2].
In addition to conventional diagnostic testing, newer tests have been developed to quantitatively confirm neuropathy. Skin biopsy for evaluating intraepidermal nerve fibre density (IENFD) has good test characteristics for neuropathy (AUC=0.76) and for small fibre neuropathy (AUC=0.82) based on the gold standard of the Toronto definition of probable neuropathy and small fibre neuropathy, respectively [24]. Similarly, corneal confocal microscopy (CCM) has good test characteristics for neuropathy (AUC=0.68–0.77) [25]. Importantly, CCM lacks head-to-head comparisons with skin biopsy in individuals with type 2 diabetes to determine which test performs better. However, two studies in individuals with type 1 diabetes demonstrated similar diagnostic characteristics (sensitivity and specificity) between these two techniques [26, 27]. Given the lack of data on the change of management following skin biopsy and CCM testing, these tests should remain as research trial outcomes, and not for routine clinical practice. Future studies are needed to compare these two tests and determine whether they have clinical utility.
Disease-modifying treatment
Disease-modifying treatments are particularly important as they alter the natural history of the disease. The reasons why so many diabetic neuropathy treatments have failed when studied in randomised clinical trials are multifactorial and include issues with trial design, participant selection and endpoints, along with the possibility that the therapy itself is ineffective [11]. Given the limited effect of intensive glycaemic control on preventing neuropathy, new disease-modifying treatments are needed. Since obesity is the second biggest metabolic risk factor, interventions focused on exercise and/or weight loss are promising. In terms of exercise, three uncontrolled studies and one small randomised trial have shown the potential for exercise to improve neuropathy outcomes, even though patients had minimal weight loss [28–31]. One study of 32 patients with neuropathy caused by impaired glucose tolerance revealed that IENFD significantly increased following 12 months of an exercise-focused lifestyle intervention, even though IENFD usually decreases over time without intervention [31]. Similarly, another study of 36 patients with diabetes and/or the metabolic syndrome found that cutaneous nerve regenerative capacity improved following supervised exercise for 4 months [30]. A third study of 17 individuals with diabetic neuropathy. patients demonstrated that intraepidermal nerve fibre branching significantly improved after 10 weeks of exercise [29]. Finally, a small randomised trial of type 1 or type 2 diabetes patients revealed improvements in NCS and vibration thresholds after 4 years of exercise [28]. Despite these four studies, larger randomised controlled trials are needed to determine the appropriate role of exercise for the prevention and treatment of diabetic neuropathy. Whether traditional aerobic or high intensity interval training regimens lead to better compliance and/or neuropathy outcomes also requires further study.
While multiple exercise studies exist, limited data are available on medical or surgical weight loss and their effects on diabetic neuropathy. Our group is currently conducting one medical weight loss (ClinicalTrials.gov ID NCT02689661) and one surgical weight loss observational study to determine the role of these interventions on neuropathy outcomes in obese populations. We will be able to compare and contrast neuropathy outcomes in the two populations vs controls to determine whether either is a promising disease-modifying therapy for neuropathy. Similarly, we are conducting a clinical trial ( NCT03617185) to compare the effects of bariatric surgery and/or high intensity interval training on neuropathy outcomes. Individuals will be randomised to the high intensity interval training from the group of individuals that choose to undergo bariatric surgery and from the group that attended the bariatric surgery clinic but chose not to pursue surgery. Given that exercise and weight loss require life-altering changes in behaviour, it will be important to understand if one, both or neither of these interventions can treat and/or prevent neuropathy.
Treatment of painful diabetic neuropathy
The most disabling symptom of neuropathy is often neuropathic pain, which is experienced by about a quarter of individuals with the disorder [23, 32]. Treatment of neuropathic pain does not alter the natural history of neuropathy, but is still important to improve the quality of life of patients. There are few head-to-head trials comparing the efficacy of neuropathic pain medications, all of which were originally developed for other therapeutic reasons. Very few patients experience a complete improvement in pain, and a pain reduction of 30–50% should be considered significant [33]. Both the American Academy of Neurology (AAN) and the European Federation of Neurological Societies (EFNS) have published guidelines on treating neuropathic pain [34, 35]. These guidelines and multiple other systematic reviews demonstrate consistent evidence for the efficacy of tricyclic antidepressants (TCAs; amitriptyline and nortriptyline), gabapentinoids (gabapentin and pregabalin), serotonin–noradrenaline (norepinephrine) reuptake inhibitors (SNRIs; venlafaxine and duloxetine) and opioids (see text box) [34–38]. The TCAs and gabapentin and venlafaxine have the lowest out-of-pocket costs (costs that are directly paid by patients)of the oral medications used for treating neuropathic pain and should be considered before more expensive options [39].
The opioid epidemic in the USA did not start in individuals with painful diabetic neuropathy, but it has had direct implications for this population. Although opioids have been shown to be effective for treating neuropathic pain in the short-term, these medications carry risks of serious adverse events, including overdose, tolerance and addiction. The Centers for Disease Control (CDC) and the AAN have advised caution in using opioids for non-cancer pain, including neuropathic pain [40, 41]. The CDC systematic review provides extensive evidence of the downsides of opioids, which is in contrast to the lack of data on the long-term efficacy of these medications [40]. Specific to individuals with neuropathy, chronic opioid use did not improve functional status, but was associated with an increased risk of opioid dependence and overdose [42]. Despite the risks associated with opioid medications, a recent study showed that nearly two-thirds of patients received at least one opioid prescription, and almost 9% are on chronic opioids, even when excluding patients that have other chronic pain conditions [43]. Of those individuals who were on chronic opioid therapy, only 26% received a guideline-recommended medication prior to opioid prescription. These results indicate that guideline-concordant medications are often underutilised and that opioid use in this population is extremely high. While the evidence for TCAs, SNRIs and gabapentinoids is well established, there is a clear need for new neuropathic pain medications, because these medications have small effect sizes and substantial side effects. Furthermore, with the rapidly evolving data supporting the negative effects of opioids, interventions are needed to prevent initiation and increase cessation of these medications in the neuropathy population.
Conclusions
New metabolic risk factors (obesity) and mechanisms (dyslipidaemia and deoxysphingolipids) have the potential to lead to new disease-modifying therapies for neuropathy. For example, exercise and medical and surgical weight loss are all promising new therapeutic avenues for neuropathy resulting from obesity and dyslipidaemia. While new diagnostic tests are available (IENFD and CCM), they are more well established as neuropathy research outcomes than as clinically useful tools. Finally, improved treatment of neuropathic pain will require increased utilisation of guideline-recommended medications and decreased use of opioids.
Supplementary Material
Treatment of painful diabetic neuropathy.
First-line medication: SNRI, TCA or gabapentinoid (note that pregabalin usually has the highest out-of-pocket expense amongst these medications)
Second-line medication: add or replace with a medication from a different class of first-line medication (do not attempt pregabalin after gabapentin)
Only try medications with limited or no evidence after attempting at least one medication from each class and preferably two
Avoid opioids for chronic, non-cancer pain, given the evidence supporting adverse outcomes
Acknowledgements
The authors would like to thank S. Sakowski Jacoby, M. Savelieff and P. O’Brien for editorial and graphical assistance. They are all from the Department of Neurology at the University of Michigan, Ann Arbor, MI, USA.
Funding
BCC is supported by the National Institutes of Health (NIDDK R01 DK115687). ELF is supported by the National Institutes of Health (R21NS102942, R24DK082841) and the Novo Nordisk Foundation (NNF14OC0011633).
Abbreviations
- AAN
American Academy of Neurology
- CCM
Corneal confocal microscopy
- CDC
Centers for Disease Control
- ER
Endoplasmic reticulum
- EMG
Electromyography
- IENFD
Intraepidermal nerve fibre density
- NCS
Nerve conduction studies
- ROS
Reactive oxygen species
- SNRI
Serotonin–noradrenaline (norepinephrine) reuptake inhibitor
- SPT
Serine palmitoyltransferase
- TCA
Tricyclic antidepressant
Footnotes
Duality of interest
BCC consults for a PCORI grant and for DynaMed and performs medical legal consultations including consultations for the Vaccine Injury Compensation Program. ELF consults for Novartis. GG and VF report no duality of interest associated with this manuscript.
References
- [1].Callaghan BC, Little AA, Feldman EL, Hughes RA (2012) Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev, Issue 6, Art. no.: CD007543. 10.1002/14651858.CD007543.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Callaghan BC, Price RS, Feldman EL (2015) Distal Symmetric Polyneuropathy: A Review. JAMA 314: 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andersen ST, Witte DR, Dalsgaard EM, et al. (2018) Risk factors for Incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care 41: 1068–1075 [DOI] [PubMed] [Google Scholar]
- [4].Callaghan BC, Gao L, Li Y, et al. (2018) Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol 5: 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Callaghan BC, Xia R, Banerjee M, et al. (2016) Metabolic syndrome Components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care 39: 801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Callaghan BC, Xia R, Reynolds E, et al. (2016) Association between Metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurology 73: 1468–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hanewinckel R, Drenthen J, Ligthart S, et al. (2016) Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population-based cohort study. Journal of neurology, neurosurgery, and psychiatry 87: 1336–1342 [DOI] [PubMed] [Google Scholar]
- [8].Lu B, Hu J, Wen J, et al. (2013) Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes - ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). PLoS One 8: e61053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schlesinger S, Herder C, Kannenberg JM, et al. (2019) General and abdominal obesity and incident distal sensorimotor polyneuropathy: insights into inflammatory biomarkers as potential mediators in the KORA F4/FF4 Cohort. Diabetes Care 42:240–247 [DOI] [PubMed] [Google Scholar]
- [10].Eid S, Sas KM, Abcouwer SF, et al. (2019) New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia 62: 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feldman EL, Callaghan BC, Pop-Busui R, et al. (2019) Diabetic neuropathy. Nat Rev Dis Primers 5: 41. [DOI] [PubMed] [Google Scholar]
- [12].Feldman EL, Nave KA, Jensen TS, Bennett DLH (2017) New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93: 1296–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rumora AE, LoGrasso G, Hayes JM, et al. (2019) The divergent roles of dietary saturated and monounsaturated fatty acids on nerve function in murine models of obesity. J Neurosci 39: 3770–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Penno A, Reilly MM, Houlden H, et al. (2010) Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem 285: 11178–11187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zuellig RA, Hornemann T, Othman A, et al. (2014) Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes 63: 1326–1339 [DOI] [PubMed] [Google Scholar]
- [16].Dohrn MF, Othman A, Hirshman SK, et al. (2015) Elevation of plasma 1-deoxy-sphingolipids in type 2 diabetes mellitus: a susceptibility to neuropathy? Eur J Neurol 22: 806–814, e855 [DOI] [PubMed] [Google Scholar]
- [17].Hammad SM, Baker NL, El Abiad JM, et al. (2017) Increased plasma levels of select deoxy-ceramide and ceramide species are associated with increased odds of diabetic neuropathy in type 1 diabetes: a pilot study. Neuromolecular Med 19: 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kramer R, Bielawski J, Kistner-Griffin E, et al. (2015) Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy. FASEB J 29: 4461–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferreira CR, Goorden SMI, Soldatos A, et al. (2018) Deoxysphingolipid precursors indicate abnormal sphingolipid metabolism in individuals with primary and secondary disturbances of serine availability. Mol Genet Metab 124: 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].England JD, Gronseth GS, Franklin G, et al. (2009) Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology 72: 185–192 [DOI] [PubMed] [Google Scholar]
- [21].Weisman A, Bril V, Ngo M, et al. (2013) Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PLoS One 8: e58783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Callaghan BC, Kerber KA, Lisabeth LL, et al. (2014) Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA neurology 71: 1143–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pop-Busui R, Boulton AJ, Feldman EL, et al. (2017) Diabetic neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 40: 136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Callaghan BC, Xia R, Reynolds E, et al. (2018) Better diagnostic accuracy of neuropathy in obesity: A new challenge for neurologists. Clin Neurophysiol 129: 654–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Perkins BA, Lovblom LE, Bril V, et al. (2018) Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia 61: 1856–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alam U, Jeziorska M, Petropoulos IN, et al. (2017) Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One 12: e0180175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen X, Graham J, Dabbah MA, et al. (2015) Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 38: 1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balducci S, Iacobellis G, Parisi L, et al. (2006) Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 20: 216–223 [DOI] [PubMed] [Google Scholar]
- [29].Kluding PM, Pasnoor M, Singh R, et al. (2012) The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications 26: 424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG (2015) Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Annals of neurology 77: 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smith AG, Russell J, Feldman EL, et al. (2006) Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 29: 1294–1299 [DOI] [PubMed] [Google Scholar]
- [32].Tesfaye S, Boulton AJ, Dyck PJ, et al. (2010) Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33: 2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Diabetes Canada Clinical Practice Guidelines Expert Committee, Bril V, Breiner A, Perkins BA, Zochodne D (2018) Neuropathy. Can J Diabetes 42 Suppl 1: S217–S221 [DOI] [PubMed] [Google Scholar]
- [34].Attal N, Cruccu G, Baron R, et al. (2010) EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 17: 1113-e88 [DOI] [PubMed] [Google Scholar]
- [35].Bril V, England J, Franklin GM, et al. (2011) Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 76: 1758–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Finnerup NB, Attal N, Haroutounian S, et al. (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology 14: 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Griebeler ML, Morey-Vargas OL, Brito JP, et al. (2014) Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Annals of internal medicine 161: 639–649 [DOI] [PubMed] [Google Scholar]
- [38].Waldfogel JM, Nesbit SA, Dy SM, et al. (2017) Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology 88: 1958–1967 [DOI] [PubMed] [Google Scholar]
- [39].Callaghan BC, Reynolds E, Banerjee M, et al. (2019) Out-of-pocket costs are on the rise for commonly prescribed neurologic medications. Neurology 92: e2604–e2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dowell D, Haegerich TM, Chou R (2016) CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 315: 1624–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Franklin GM (2014) Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 83: 1277–1284 [DOI] [PubMed] [Google Scholar]
- [42].Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ (2017) Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurology 74: 773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Callaghan BC, Reynolds E, Banerjee M, Kerber KA, Skolarus LE, Burke JF (2019) Longitudinal pattern of pain medication utilization in peripheral neuropathy patients. Pain 160: 592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


