Wild-type Vav1 and mutant K-Ras synergistically accelerate pancreatic acinar-to-ductal metaplasia (ADM), thus leading to pancreatic ductal adenocarcinoma (PDAC), depending on Vav1’s activity as a GEF for Rac1.
Abstract
To explore the contribution of Vav1, a hematopoietic signal transducer, to pancreatic ductal adenocarcinoma (PDAC) development, we generated transgenic mouse lines expressing, Vav1, K-RasG12D, or both K-RasG12D and Vav1 in pancreatic acinar cells. Co-expression of Vav1 and K-RasG12D synergistically enhanced acinar-to-ductal metaplasia (ADM) formation, far exceeding the number of lesions developed in K-RasG12D mice. Mice expressing only Vav1 did not develop ADM. Moreover, the incidence of PDAC in K-RasG12D/Vav1 was significantly higher than in K-RasG12D mice. Discontinuing Vav1 expression in K-RasG12D/Vav1 mice elicited a marked regression of malignant lesions in the pancreas, demonstrating Vav1 is required for generation and maintenance of ADM. Rac1–GTP levels in the K-RasG12D/Vav1 mice pancreas clearly demonstrated an increase in Rac1 activity. Treatment of K-RasG12D and K-RasG12D/Vav1 mice with azathioprine, an immune-suppressor drug which inhibits Vav1’s activity as a GDP/GTP exchange factor, dramatically reduced the number of malignant lesions. These results suggest that Vav1 plays a role in the development of PDAC when co-expressed with K-RasG12D via its activity as a GEF for Rac1GTPase.
Introduction
Vav1, a signal transducer protein which is physiologically expressed in the hematopoietic system, was first identified as an in vitro–activated oncogene (Katzav et al, 1989). It functions as a GDP/GTP exchange factor (GEF) for Rho/RacGTPases, an activity that is stringently controlled by tyrosine phosphorylation (Crespo et al, 1997). This GEF activity of Vav1 regulates cytoskeletal rearrangement during immune cell activation (Fischer et al, 1998; Holsinger et al, 1998). Vav1 also participates in GEF-independent signaling pathways, including the JNK, ERK, NF-κB, and NFATc1 pathways, and associates with numerous adapter proteins such as Shc, NCK, SLP-76, Grb2, and Crk (Tybulewicz, 2005). Although the physiological activity of Vav1 is well understood, its contribution to human cancer is only starting to emerge. Several recent studies have indicated that mutations in various domains of the Vav1 protein are present in human cancers such as adult T-cell leukemia/lymphoma (Kataoka et al, 2015), lung adenocarcinoma and squamous cell carcinomas (Campbell et al, 2016), and peripheral T-cell lymphomas (Abate, da Silva-Almeida et al, 2017). In addition, numerous studies have reported the unexpected expression of Vav1, normally found only in the hematopoietic system, in a variety of human cancers, such as neuroblastoma (Hornstein et al, 2003), lung (Lazer et al, 2009), breast (Lane et al, 2008; Sebban et al, 2013; Du et al, 2014; Grassilli et al, 2014), ovarian (Wakahashi et al, 2013), prostate (Kniazev Iu et al, 2003), esophageal (Zhu et al, 2017), and brain tumors (Lindsey et al, 2014). Notably, Vav1 expression was also identified in more than 50% of 95 examined pancreatic ductal adenocarcinoma (PDAC) tumor specimens (Fernandez-Zapico et al, 2005), a finding that was validated by Huang et al (2016). Patients with Vav1-positive tumors had a worse prognosis than patients with Vav1-negative tumors (Fernandez-Zapico et al, 2005; Huang et al, 2016). Sequence analysis of Vav1 cDNA from pancreatic cancer cell lines and tumors confirmed their expression of intact wild-type (wt) Vav1 (Fernandez-Zapico et al, 2005). The aberrant expression of Vav1 in pancreatic cancer was attributed to epigenetic changes (Fernandez-Zapico et al, 2005; Huang et al, 2016). Furthermore, Vav1 RNAi was found to abolish neoplastic cellular proliferation of human pancreatic cancer cell lines both in vitro and in vivo, even in the presence of oncogenic K-Ras (Fernandez-Zapico et al, 2005). The accumulating data, thus, clearly point to an important role of ectopically expressed wtVav1 in pancreatic cancer (Fernandez-Zapico et al, 2005; Huang et al, 2016), possibly through its activity as a GEF that regulates cytoskeletal organization and/or through its activity as a signal transducer that can affect growth factor/cytokine production. To date, however, the mechanisms that mediate this protumorigenic role of Vav1 in pancreatic cancer and the stages during tumorigenesis, at which such mediation occurs, are unknown.
The earliest identifiable precursor lesion to PDAC is acinar-to-ductal metaplasia (ADM), which progresses to a series of neoplastic precursor lesions known as pancreatic intraepithelial neoplasia (PanIN) (Morris et al, 2010; Aichler et al, 2012; Storz, 2017). The earliest and most frequent genetic alteration found in low-grade PanIN-1A lesions is mutant K-Ras, which is present in >90% of PDACs (Morris et al, 2010; Aichler et al, 2012; Kanda et al, 2012; Storz, 2017). Several groups have generated sophisticated somatic mouse models that faithfully recapitulate human pancreatic cancer pathogenesis and progression from ADM to PanIN and eventually to PDAC (Hingorani & Tuveson, 2003; Bardeesy et al, 2006; Guerra et al, 2007; Izeradjene et al, 2007). Expression of mutant K-RasG12D or K-RasG12V in the murine pancreas is sufficient to initiate the development of ADM followed by PanIN (Hingorani & Tuveson, 2003; Seidler et al, 2008; Morris et al, 2010; Guerra et al, 2011). However, the low frequency of spontaneous progression of precursor lesions to invasive PDAC suggests that additional genetic and/or epigenetic aberrations are required for disease progression, including inflammation and/or additional molecular insults (Morris et al, 2010). Other molecular components within the epithelium that drive ADM include epidermal growth factor receptor (EGFR) (Ardito et al, 2012), TGF-α (Song et al, 1999), SOX-9 (Kopp et al, 2012), MIST1 (Shi et al, 2009), KLF4 (Wei et al, 2016), Rac1 (Heid et al, 2011), and phosphoinositide-3-kinase (PI3K) (Hill et al, 2010). Development of PanINs and PDACs can be accelerated by introducing inactivating mutations in the tumor suppressor genes Cdkn2a, Trp53, or Dpc4/Smad4, all of which occur frequently in human lesions as they progress to invasive PDAC (Jones et al, 2008; Morris et al, 2010; BiankinWaddell et al, 2012) and by enhancing critical signaling pathways such as those of EGFR, Raf/Mek/Erk, PI3K/Pdk1/Akt, and Ral (Lim et al, 2005; Feldmann et al, 2010; Collisson et al, 2012; Eser et al, 2013).
Here, we report the involvement of Vav1 in pancreatic cancer development and growth by using a novel transgenic mouse model, in which Vav1 is specifically expressed in pancreatic acinar cells. Significantly, because mutant K-Ras is prevalent in PDAC, we also examined whether expression of transgenic wtVav1 (hereafter Vav1) together with mutant K-Ras enhances pancreatic malignancy. We did this by generating a novel transgenic mouse model that inducibly expresses both genes specifically in the pancreas. Our results clearly indicate that co-expression of Vav1 and K-RasG12D dramatically increases the prevalence and decreases the time course required for malignant pancreatic lesions to appear, in comparison with the expression of K-RasG12D alone, thus strongly suggesting that these two proteins synergize to enhance the development of pancreatic tumors. An important finding was the significant increase in ADM initiation observed when both proteins are present. Expression of Vav1 alone in the pancreas did not lead to development of any malignant lesions. This study adds an important layer in delineating the earliest events involved in ADM/PanIN formation and in revealing their mechanistic roles. Such knowledge can be expected to provide vital information on the molecular pathways that are instrumental in initiating PDAC.
Results
Generation of a Vav1 transgenic mouse line with inducible pancreatic-specific expression
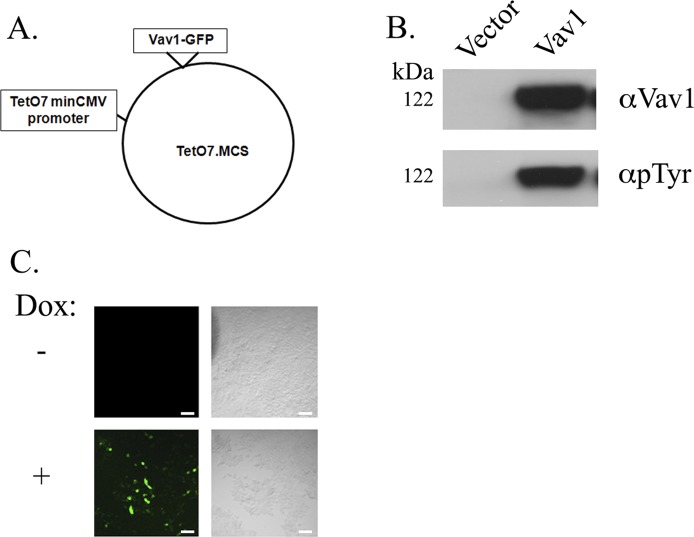
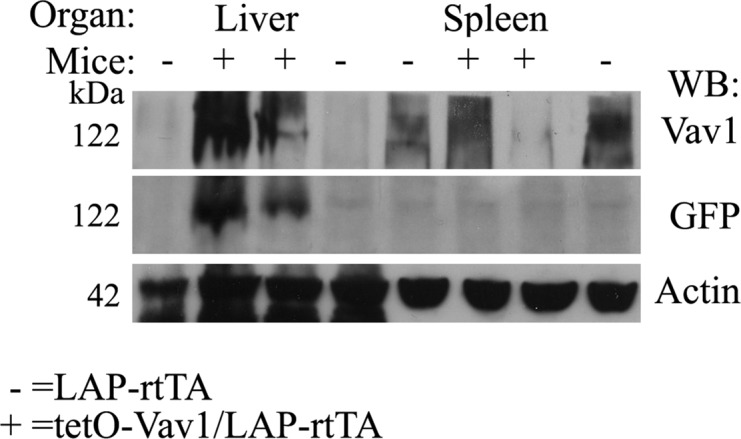
To study the contribution of Vav1 to the development of pancreatic cancer tumors, we first generated a Vav1 transgenic mouse line. We did this by using the Tet-On system, in which rtTA binds, only in the presence of Dox, to a tetO plasmid. For that purpose, we subcloned human Vav1 into a plasmid that encodes a tetO-responsive bidirectional promoter (tetO7minCMV) that drives expression of tetO–Vav1 hooked to GFP (Fig S1A). To validate the ability of our generated tetO–Vav1 plasmid to produce Vav1 upon activation, we transfected HEK293 cells with this plasmid and with a vector that encodes the rtTA. After treatment with Dox, the expression of Vav1 protein was observed, as verified by Western blotting (Fig S1B) and by immunofluorescence (Fig S1C). To validate the activity of the Vav1–tetO plasmid in vivo, we transfected it into mouse blastocysts by using an in vitro fertilization procedure to generate tetO–Vav1 mice. To ensure that these transgenic mice express Vav1 under the correct conditions, we crossed them with LAP–rtTA transgenic mice, which express rtTA in hepatocytes. As expected, Western blot analysis of tissue lysates from tetO–Vav1/LAP–tTA mice (positive mice, + in Fig S2) showed GFP expression (indicative of expression of the Vav1 transgene) in the liver but not in the spleen. LAP–rtTA transgenic mice lacking the Vav1 transgene (negative mice, Fig S2) did not express GFP in either of these tissues. Immunohistochemical analysis of livers from both positive and negative mice revealed expected staining pattern in hepatocytes (data not shown), thereby validating the tissue and cell type–specific expression of the Vav1 transgene.
Figure S1. Plasmid construction and validation.
(A) Wild-type Vav1 was subcloned into Teto7minCMV plasmid transgene plasmid fused to GFP (tetO–Vav1). (B) HEK293 cells were transfected with the tetO–Vav1 plasmid and a vector that encodes the reverse tetracycline responsive transactivator (rtTA). After treatment with Dox, the cells were lysed and analyzed by Western blotting for the presence of Vav1 and its phosphotyrosine status. The empty plasmid served as control vector. (C) HEK293 cells were transfected with the tetO–Vav1 plasmid either treated (+) or nontreated (−) with Dox were subjected to immunofluorescence. Green = GFP, which refers to Vav1 expression. Scale bar represents 50 μm.
Figure S2. Validation of tissue-specific expression of Vav1 transgene in vivo.
Western blot analysis of liver and spleen tissue from tetO–Vav1 mice crossed with LAP–rtTA mice (tetO–Vav1/LAP–rtTA; positive mice) with anti-Vav1, anti-GFP expression (indicative of the transgene Vav1 expression) and anti-actin Abs.
Source data are available for this figure.
To drive the expression of Vav1 in pancreatic acinar cells, we used a Ptf1a promoter, which participates in the maintenance of exocrine pancreas-specific gene expression (Krapp et al, 1998). We crossed Ptf1a–CreER mice with Lox-Stop-Lox (LSL)–rtTA and tetO–Vav1 mice to generate a Vav1 pancreatic mouse (Ptf1a–CreER/LSL–rtTA/tetO–Vav1; herein denoted Vav1 mouse).
The mutant K-RasG12D is present in nearly 90% of human pancreatic cancers, and is, thus, sometimes co-expressed with endogenous Vav1. To investigate potential interactions between K-RasG12D and Vav1 in PDAC development, we crossed the LSL–K-RasG12D strain (which carries an LSL termination sequence bearing the K-RasG12D point mutation) to the Ptf1a–CreER strain expressing Cre recombinase, under the control of Ptf1a. After treatment with tamoxifen, Cre recombination deletes the transcriptional termination sequence and allows K-RasG12D to be expressed in the pancreas (Ptf1a–CreER; LSL–K-RasG12D; herein denoted K-RasG12D mouse). The K-RasG12D mouse was then crossed with the Vav1 mouse to generate a transgenic mouse line that expresses both Vav1 and K-RasG12D in pancreatic acinar cells (Ptf1a–CreER; LSL–rtTA/LSL–K-RasG12D/tetO–Vav1; herein denoted K-RasG12D/Vav1 mouse). Mice expressing Ptf1a–CreER served as controls. At the age of 1 mo, all of the mice outlined were injected subcutaneously, twice over a 2-d interval, with 8 mg of tamoxifen. To ensure comparable experimental conditions, all of those mice were also treated with Dox, although this affects only Vav1 expression. Thus, whereas K-RasG12D was constitutively expressed after elimination of the LSL sequence, Vav1 could be further regulated by either addition or discontinuation of Dox.
Malignant lesions in the pancreas
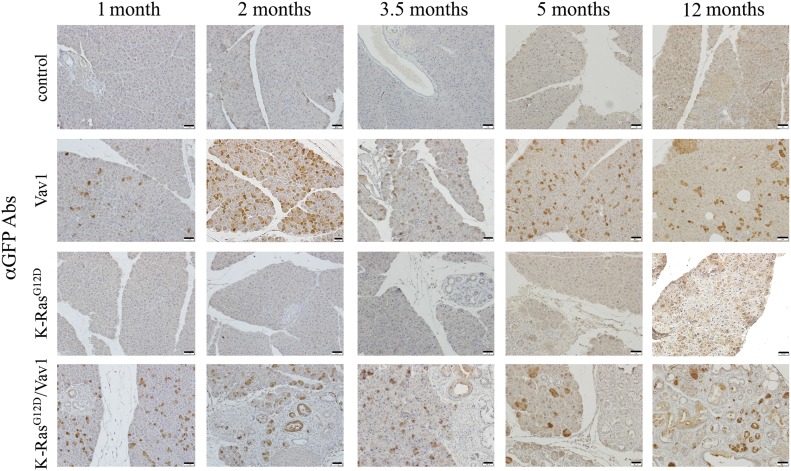
Mice were euthanized at various time points (1, 2, 3.5, 5, and 12 mo) after transgene induction was started by tamoxifen and Dox administration, and tissues removed from the pancreas, liver, lung, and spleen were analyzed for expression of the human Vav1 transgene as detected by GFP staining (Fig 1) and by anti-Vav1 Abs to detect endogenous Vav1 expression in K-RasG12D mice (Fig S3).
Fig 1. Expression of GFP in the pancreata from different mouse lines.
Representative paraffin sections of the pancreata of the various mouse lines at different time points after the onset of transgene induction (as indicated) were stained with anti-GFP Abs that identify the Vav1 transgene. Scale bar represents 25 μm. Number of mice stained in this experiment are outlined in Table S1.
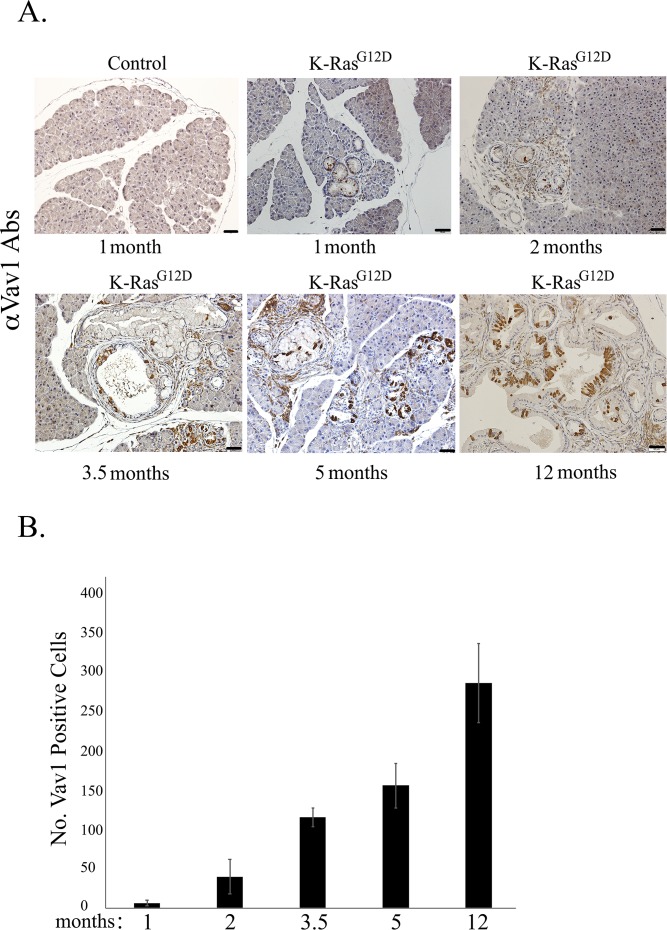
Figure S3. Expression of endogenous Vav1 in the K-RasG12Dmouse pancreas.
(A) Paraffin sections of pancreata from a control mouse (at 1-mo post tamoxifen and Dox administration) and from K-RasG12D mice at the indicated time points after transgene induction were stained with anti-Vav1 antibodies and then assessed for endogenous Vav1 expression. A representative of each of those stained sections is depicted. Scale bar represents 50 μm. (B) Endogenous Vav1 expression was quantified by counting Vav1-positive acinar and ductal cells in K-RasG12D mice at different times after transgene induction: at 1 mo (n = 2), 2 mo (n = 4), 3.5 mo (n = 3), 5 mo (n = 3), and 12 mo (n = 3). Five randomly chosen fields were counted in mouse sections (scale bar represents 50 μm). Calculated mean values of Vav1-positive acinar/ductal cells at each time point are represented in the graph. SEM are shown.
Table S1 Number of mice used in various experiments. (19.1KB, docx)
Staining for GFP (co-expressed with the Vav1 transgene) validated the expression of Vav1 in acinar pancreatic cells of Vav1 mice and in acinar and ductal pancreatic cells of K-RasG12D/Vav1 mice (Fig 1). No staining of GFP was noted in the liver, lung, and spleen (data not shown). We also surveyed the endogenous murine expression of Vav1 in the pancreas of K-RasG12D mice by staining with anti-Vav1 antibodies (Fig S3). Endogenous Vav1 expression was intensified with time in acinar and ductal cells after the start of K-RasG12D transgene induction, exhibiting an increase of ∼150-fold after 12 mo of transgene induction compared with its expression after transgene induction for 1 mo (Fig S3B). Importantly, such endogenous Vav1 expression was frequently observed in the malignant lesions that appeared in these mice (Fig S3A).
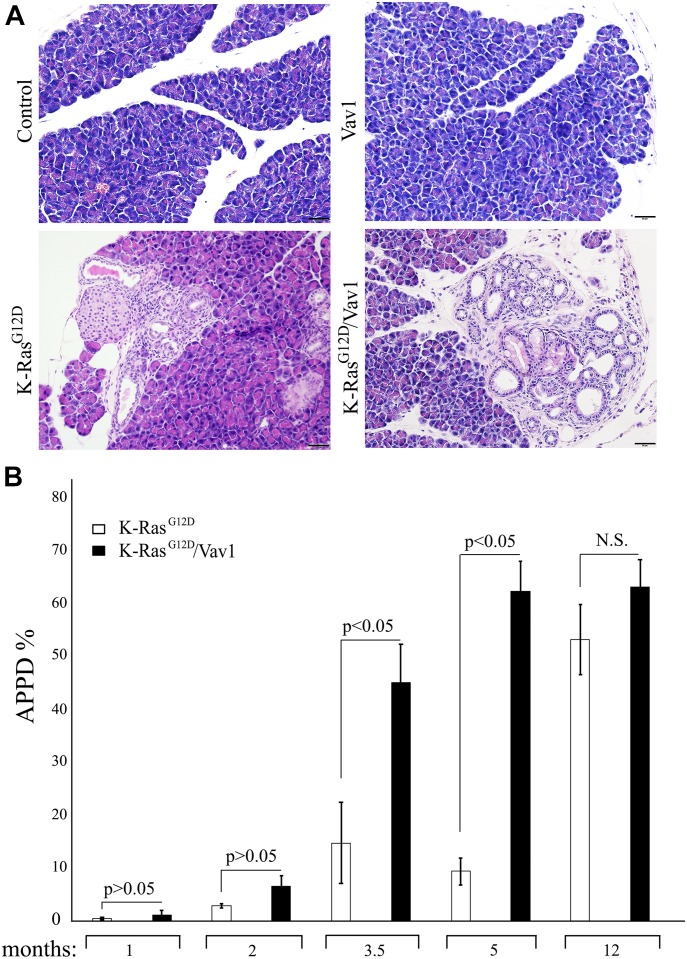
We then analyzed the appearance of premalignant and malignant lesions, including ADM, PanINs, and PDAC (APPD), by hematoxylin and eosin (H&E) staining of sections at different times after the onset of transgene induction, as indicated in Fig 2. Appearance of lesions in the pancreas were observed already at 1 mo after transgene induction and onwards. Representative examples of pancreatic sections from the tested mouse lines 3.5-mo after transgene induction are depicted in Fig 2A. For each section, we calculated the degree of malignancy (the “transformation index,” i.e., the APPD ratio expressed as APPD%), as described in the Materials and Methods section (Fig 2). APPDs were observed only in the pancreata of K-RasG12D and K-RasG12D/Vav1 mice (Fig 2). Remarkably, as seen in Fig 2B, at 3.5 mo and at 5 mo after the onset of transgene induction, co-expression of Vav1 and K-RasG12D dramatically increased the rate of development of pancreatic APPD, which progressed much faster than when either gene was expressed alone. This finding strongly suggested that mutant K-Ras and Vav1 synergize to enhance pancreatic tumor development (Fig 2B). We did not observe any malignant lesion in tissues analyzed except the pancreas. Notably, when Vav1 was expressed alone, there was no evidence of APPD in the pancreas even when examined 12 mo after Vav1 transgene induction, indicating that expression of Vav1 by itself is not sufficient for tumorigenicity (Fig 2). An increase in APPD in K-RasG12D/Vav1 mice was already observed at 1- and 2-mo post-oncogene induction, yet apparently time is needed for further enhancement of APPD, as was seen in the later time points. After 12 mo of Vav1 transgene induction, the APPD ratio in K-RasG12D mice had caught up with that in K-RasG12D/Vav1 mice, and the previously observed difference in APPD ratio between them had disappeared. This might reflect an increase in endogenous Vav1 expression in the K-RasG12D mice, as indicated in Fig S3. At 5 mo after both transgenes were induced, we compared the APPD ratio obtained from K-RasG12D/Vav1 mouse pancreata after treatment with both tamoxifen and Dox to that obtained from K-RasG12D/Vav1 mouse pancreata treated with tamoxifen (which induces K-RasG12D expression) but not with Dox (which induces the expression of Vav1 transgene) (Fig S4). As shown in Fig S4A, K-RasG12D/Vav1 mice that did not receive Dox treatment exhibited substantially fewer APPDs than their Dox-treated counterparts. Quantified results of H&E staining from several mice clearly demonstrated a significant difference in APPD ratios between Dox-treated and Dox-untreated K-RasG12D/Vav1 mice (Fig S4B), suggesting that there was no leakiness in our experimental mouse system.
Fig 2. Vav1 and K-RasG12D synergize in the generation of malignant pancreatic lesions.
Hematoxylin and eosin (H&E)–stained pancreata of control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice, at 1, 2, 3.5, 5, and 12 mo after the onset of transgene induction were analyzed for the appearance of malignant lesions. (A) Representative pictures of H&E–stained sections taken 3.5 mo after the onset of transgene induction are shown. Scale bar represents 20 μm. (B) The extent of malignant lesions generated in K-RasG12D and K-RasG12D/Vav1 mice at the indicated time points after onset of transgene induction was calculated. The sum of area occupied by ADM, PanINs, and PDAC lesions (APPD) was measured as a fraction of the total area of the pancreas (APPD%). Numbers of K-RasG12D and K-RasG12D/Vav1 mice used, respectively, were n = 4 and n = 8 at 1 mo; n = 6 and n = 8 at 2 mo; n = 13 and n = 17 at 3.5 mo; n = 9 and n = 9 at 5 mo; and n = 7 and n = 5 at 12 mo. Significant differences between the two analyzed groups (P < 0.05; t test) are indicated. N.S. refers to statistical nonsignificant differences. SEM are shown.
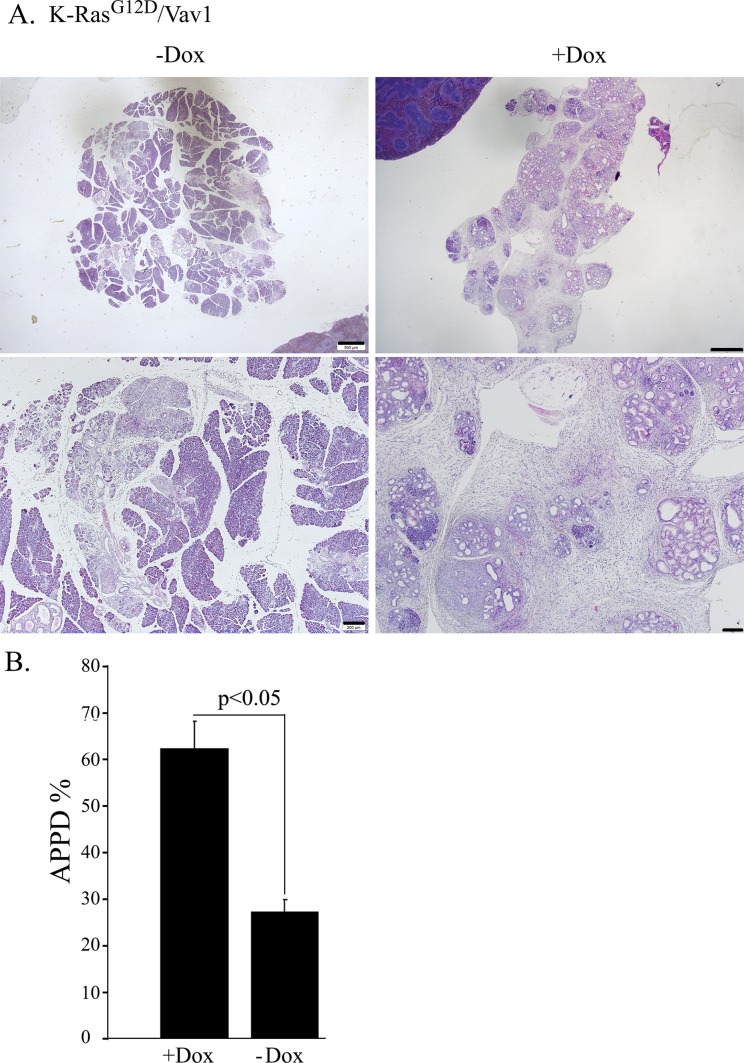
Figure S4. K-RasG12D/Vav1 mice were either treated with tamoxifen and Dox (+Dox) or treated with tamoxifen only (−Dox).
5 mo after Vav1 transgene induction, the mice were euthanized and their pancreata was analyzed for the appearance of malignant lesions. (A) A representative picture of hematoxylin and eosin (H&E) sections of pancreas from these mice at two magnifications: upper panel scale bar represents 500 μm and lower panel scale bar represents 200 μm. (B) The extent of malignant lesions generated in K-RasG12D/Vav1 mice either expressing both K-RasG12D and Vav1 (+Dox; n = 9) or expressing K-RasG12D (−Dox; n = 3) only is presented as a histogram. At the time point of 5 mo post-transgene induction. SEM and significance between the two groups analyzed are indicated (t test).
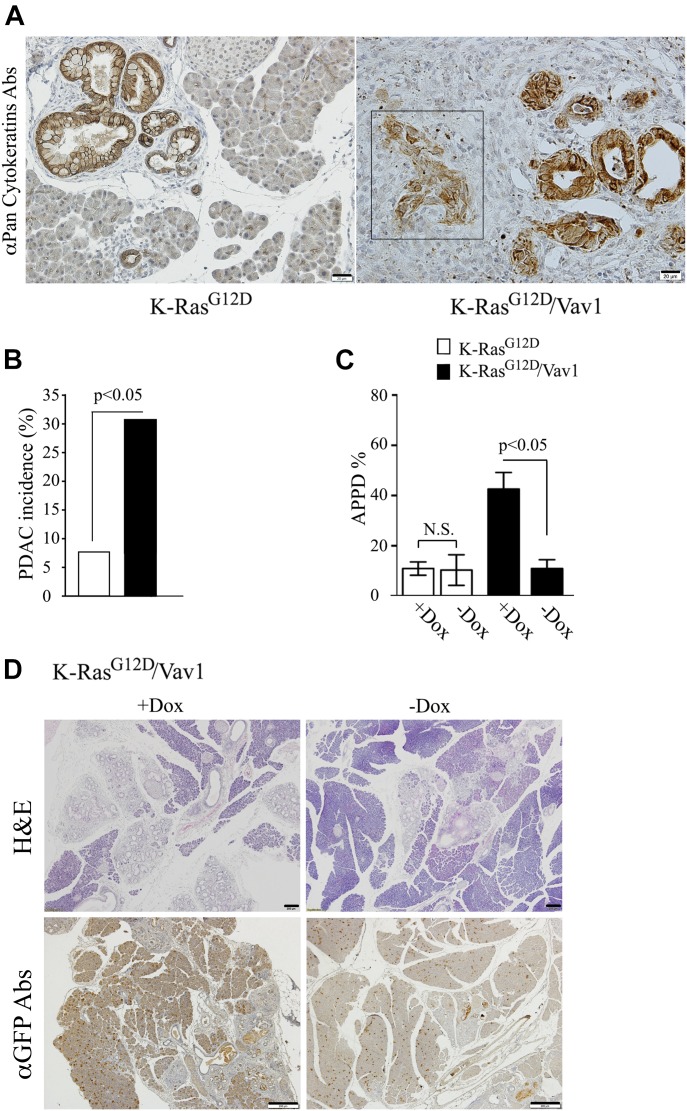
The synergistic effect of Vav1 and K-RasG12D on the development of malignancy was further highlighted by the significantly higher prevalence of PDAC in transgenic mice expressing both Vav1 and K-RasG12D than in mice expressing K-RasG12D only (Fig 3A and B). Whereas PDAC developed in K-RasG12D/Vav1 mice already at 1-mo post-transgene induction (1 case), K-RasG12D mice developed PDAC at a later stage post-oncogene induction (3.5- and 5-mo) at significant lower numbers (Fig 3B).
Fig 3. Vav1 expression enhances pancreatic ductal adenocarcinoma (PDAC) generation in the K-RasG12D/Vav1 mouse pancreas and is critical for pancreatic malignant lesions.
(A) Representative K-RasG12D (left) and K-RasG12D/Vav1 (right) stained with anti–pan-cytokeratin Abs are shown. The presence of PDAC is shown in a representative pancreatic section from a K-RasG12D/Vav1 mouse 1 mo after transgene induction (square, right panel). Scale bar represents 20 μm. (B) The extent of PDAC present in K-RasG12D and K-RasG12D/Vav1 mice at the different time points after transgene induction was assessed. The histogram summarizes the incidence of PDAC development in K-RasG12D mice and in K-RasG12D/Vav1 mice. Two of 26 K-RasG12D mice (7.7%) and 12 of 39 K-RasG12D/Vav1 mice (30.8%) had developed PDAC lesions. PDAC developed in K-RasG12D/Vav1 ranging from 1 to 12 mo post transgenes induction, whereas PDAC in K-RasG12D developed in mice of 3.5 and 5 mo post transgene induction. Significance of the difference between them (P < 0.05) was calculated using a two-tailed chi-squared test (Fisher’s exact test). (C) In some K-RasG12D/Vav1 mice (see numbers below), before completion of 3.5 mo of transgene induction, their Vav1 expression was discontinued for 20 d by removal of Dox from their drinking water (−Dox). Hematoxylin and eosin (H&E) staining of the pancreata of K-RasG12D and K-RasG12D/Vav1 either treated with Dox and tamoxifen (+Dox) or in which Dox was removed from their drinking water for 20 d before completion of 3.5 mo of transgene induction (−Dox) was performed. All the mice were then analyzed for the appearance of malignant lesions. The number of malignant lesions generated in these mice was calculated as APPD%, both for those treated with Dox (+Dox; n = 13 and n = 17, respectively) and for those in which Dox was omitted for 20 d (−Dox; n = 3 and n = 7, respectively). Significant differences between the two analyzed groups (P < 0.05; t test) are indicated. N.S. refers to statistical nonsignificant differences. SEM are shown. (D) Pancreatic sections from K-RasG12D/Vav1 mice after 3.5 mo post transgene induction either treated with Dox (+Dox) or in which Dox treatment was discontinued for 20 d (−Dox) were either stained by H&E (upper panel) or anti-GFP Abs (lower panel). Representative pictures are shown. Scale bar represents 200 μm.
To ascertain that the synergism between K-RasG12D and Vav1 in APPD development stems from expression of the Vav1 transgene, Dox (which induces Vav1 transgene expression) that was administered to K-RasG12D/Vav1 mice (in which both transgenes had been induced for 3.5 mo) was stopped 20 d before analysis (Fig 3C and D). Staining of sections of the pancreas from K-RasG12D/Vav1 mice deprived of Dox clearly indicated a marked reduction in Vav1-positive cells, visualized by GFP staining (Fig 3D). The resulting depletion of transgenic Vav1 reduced the APPD ratio to the level observed in K-RasG12D mice, that is, mice in which transgenic Vav1 was not expressed (Fig 3C and D). This finding indisputably suggests that continuous Vav1 expression is needed for maintenance of ADM lesions and/or their progression to cancer in K-RasG12D/Vav1 mice.
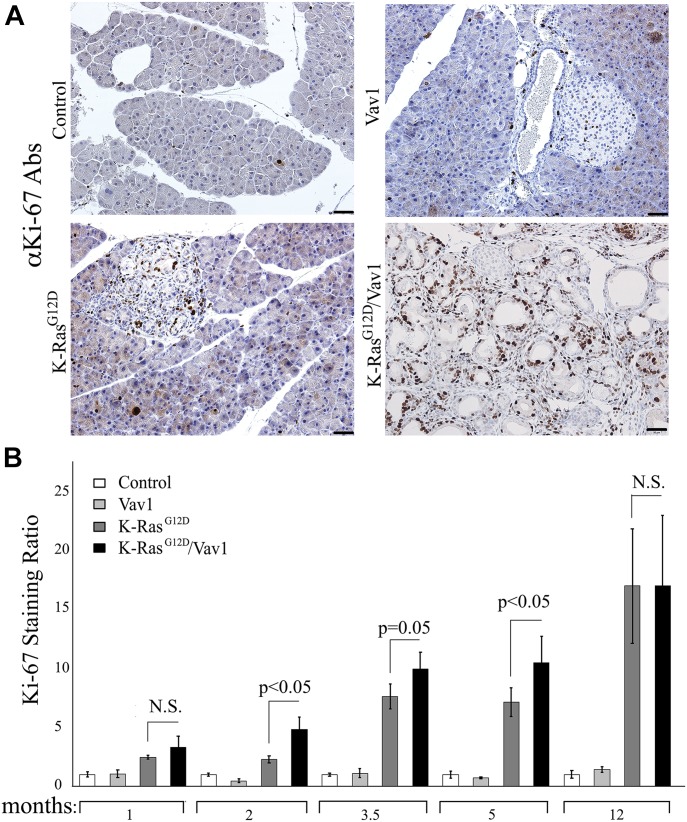
Our next step was to examine acinar and ductal cell compartments in the pancreata of control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice for differences in cell proliferation. This was done by staining for Ki-67 protein, a marker for cellular proliferation. An increase in Ki-67 staining of acinar and ductal cell compartments was observed in K-RasG12D and K-RasG12D/Vav1 mice (Fig 4A), with significant differences between these mice at 2, 3.5, and 5 mo after transgene induction (Fig 4B). No statistically significant differences in proliferation were observed between these two groups of mice at 1-mo and at 12-mo post-induction (Fig 4B). These results agree with the APPD scores observed in K-RasG12D and K-RasG12D/Vav1 mice (Fig 2B). Furthermore, the fact that no differences in Ki-67 staining were observed between K-RasG12D and K-RasG12D/Vav1 mice at the latest timepoint supports our claim that the lack of differences between these two groups is attributable to the increased expression of endogenous Vav1 (Fig S3).
Fig 4. Proliferation of pancreatic cells from control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice.
Proliferation of acinar and ductal pancreatic cells obtained, at different times after initiation of transgene induction, from control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice was assessed by anti-Ki-67 staining. (A) Representative pictures of Ki-67–stained pancreatic sections taken from mice 5 mo after the start of transgene induction. Scale bar represents 50 μm. (B) Proliferation was quantified by counting 10 fields from each pancreatic section and calculating the mean value. The numbers of mice used for Ki-67 staining calculations are recorded in Table S1. To obtain the Ki-67 staining ratio in each case, the result at each time point was divided by the result obtained for the control at the same time point. Significant differences between the two analyzed groups (P < 0.05; t test) are indicated. N.S. refers to statistical nonsignificant differences. SEM are shown.
Signaling in transgenic mouse lines
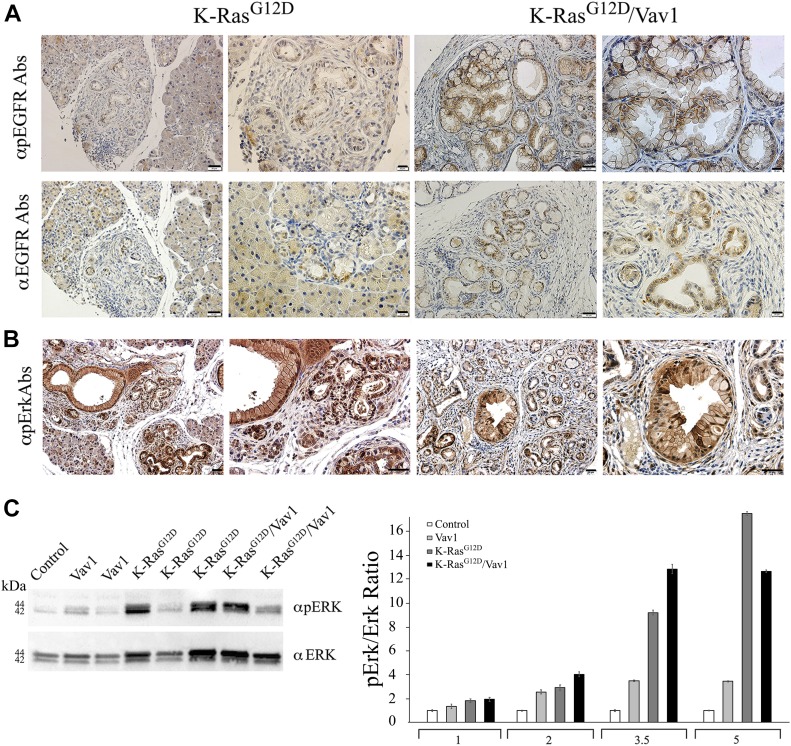
The synergy observed here between Vav1 and K-RasG12D in the development of pancreatic cancer could conceivably stem from signaling pathways in which both Vav1 and K-RasG12D participate. To examine this possibility, we analyzed the status of EGFR and Erk activation in our control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice (Fig 5). EGFR and Erk phosphorylation were similarly increased in malignant pancreatic lesions both in K-RasG12D and in K-RasG12D/Vav1 mice (Fig 5A and B). Western blot analysis of phospho-Erk and Erk in pancreatic tissues of the different mouse lines at the various time points after transgene further supported the immunohistochemistry results, indicating the absence of any synergistic effect on Erk phosphorylation when Vav1 and K-RasG12D are co-expressed (Fig 5B and C). These results raised the possibility that the contribution of Vav1 to pancreatic cancer development stems from a signaling pathway that is unique to Vav1.
Fig 5. Staining of phospho-EGFR and phospho-Erk in malignant pancreatic lesions from K-RasG12D/Vav1 mice.
(A) Pancreatic sections from 4 K-RasG12D and 8 K-RasG12D/Vav1 mice treated with tamoxifen and Dox for 5 mo were stained for pEGFR (upper panel) and total EGFR (lower panel). Representative pictures at two magnifications are shown. The scale bars represent either 50 μm (left pictures in each group) or 20 μm (right pictures in each group) as indicated. (B) Pancreatic sections from K-RasG12D and K-RasG12D/Vav1 mice treated with tamoxifen and Dox for 5 mo were stained for pERK. Representative pictures are shown. Scale bar represents 20 μm. (C) Lysates from pancreatic tissues of 5-mo post-transgene induction from 3 control, 2 Vav1, 3 K-RasG12D, and 2 K-RasG12D/Vav1 mice were Western blotted using anti–phospho-Erk and anti-Erk (left panel). A representative blot from four independent experiments is shown. Relative Erk phosphorylation in the pancreata of control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice at 1, 2, 3.5, and 5 mo after the onset of transgene induction was calculated by quantifying their Western blots using ImageJ 1.49V software (right panel). Number of mice in these experiments are outlined in Table S1. SEM are shown.
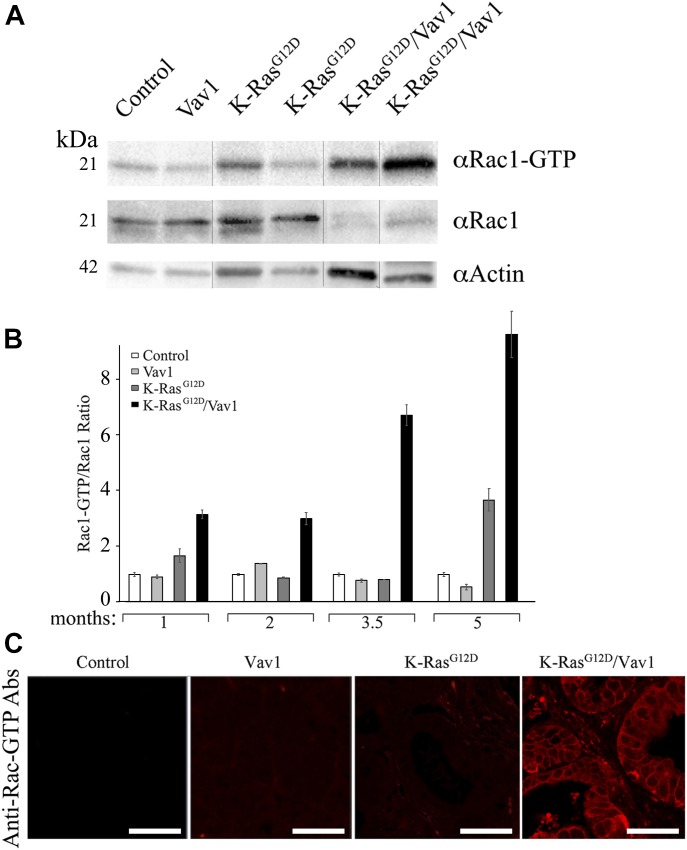
The best-known function of Vav1 is its tyrosine phosphorylation–dependent GEF activity for the Rho family of GTPases (Crespo et al, 1997). Several studies indicated that the activity of Vav1 as a GEF towards Rac1 plays an important role in Vav1’s involvement in cancer (Fernandez-Zapico et al, 2005; Lazer et al, 2009). To determine whether activation of Rac1 might play a role in the contribution of Vav1 to development of APPDs, we analyzed Rac1 activation using specific anti–Rac1–GTP antibodies (Zhou et al, 2018) (Fig 6). We found that Rac1 activity was substantially more evident in pancreatic tissues of K-RasG12D/Vav1 than in control, Vav1, or K-RasG12D pancreatic tissues (Fig 6A). Quantification of these results unequivocally demonstrated that Rac1 activation is synergistically increased in the K-RasG12D/Vav1 mouse line, where the two genes are co-expressed (Fig 6B). These results are also apparent when immunofluorescence with anti–Rac1–GTP Abs of pancreatic tissues from the mice used in our study were used (Fig 6C). Thus, although an increase in both EGFR and ERK phosphorylation was detectable in both K-RasG12D/Vav1 and K-RasG12D mice, Rac1 activation was augmented only when Vav1 was ectopically co-expressed with mutant K-Ras. Notably, an increase in Rac1 activation in the K-RasG12D/Vav1 mouse pancreas was already evident 1 mo after transgene induction, a stage at which an increase in malignant lesions in these mice is only starting to emerge. Expression of Vav1 by itself was insufficient to cause an increase in Rac1 activation, probably because, as noted in Fig 5, there is no enough activating signals to lead to Vav1 tyrosine phosphorylation. The tight correlation between Vav1-induced Rac1 activity and development of APPD suggests that the activity of Vav1 as a GEF for Rac/RhoGTPases and not another cryptic activity of Vav1 is required for maintenance or further progression of pancreatic lesions.
Fig 6. Activation of Rac–GTP in malignant pancreatic lesions from K-RasG12/Vav1 mice.
Rac1–GTP activation in the pancreata of control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice was analyzed. (A) Western blotting using anti–Rac1–GTP Abs, anti-Rac1, and anti-actin were used to evaluate Rac1–GTP levels in protein lysates from pancreatic tissues of control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice (two mice in each group) at 5 mo after transgene induction. The vertical black lines delineate sliced images that juxtapose lanes that were nonadjacent in the gel. (B) The levels of Rac1–GTP versus total Rac1 at the pancreata of control, Vav1, K-RasG12D, and K-RasG12D/Vav1 mice at 1, 2, 3.5, and 5 mo after initiation of transgene induction were calculated. Each Rac1–GTP/Rac1 was then divided by the control ratio. Band intensity of the Western blots was quantified using ImageJ 1.49V software. Number of mice in these experiments are outlined in Table S1. SEM are shown. (C) Immunofluorescence of Rac1–GTP of the pancreata from 2 control, 3 Vav1, 3 K-RasG12D, and 4 K-RasG12D/Vav1 mice, 3.5 mo post-transgene induction was performed. Representative pictures are depicted. Scale bar represents 25 μm. All sections were also stained with anti–Alexa Flour 594 dye for background analysis and found negative.
Source data are available for this figure.
Decrease in APPD after treatment with azathioprine, an inhibitor of the Rac1 pathway
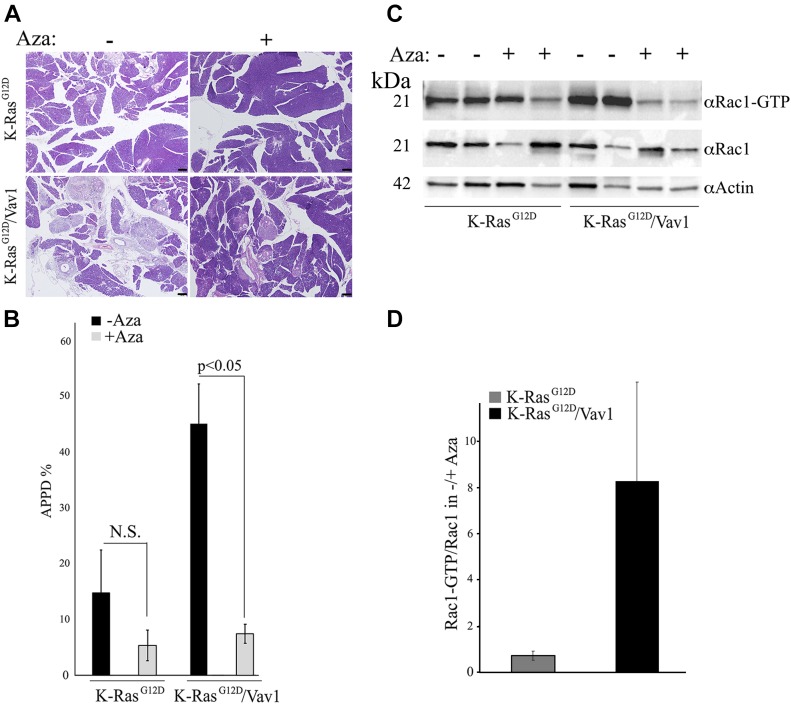
The results described above clearly point to a synergistic effect of Vav1 and K-RasG12D inducing Rac1–GTP activation. To determine the extent of involvement of this pathway in the generation of APPDs in our mouse model, we used azathioprine (Aza). This drug is one of the oldest pharmacologic immunosuppressive agents used to treat hematologic malignancies, rheumatologic diseases, solid organ transplantation, and inflammatory bowel disease (Maltzman & Koretzky, 2003). Given its structure as a purine analog, it can become incorporated into DNA replication and can also block the de novo pathway of purine synthesis. Its activity as an immunosuppressive drug stems, however, from the specific blockade of Rac1 activation through binding of azathioprine-generated 6-thioguanine triphosphate (6-thio-GTP) to Rac1 instead of GTP. Such an activity prevents GEFs such as Vav1 from converting Rac1 to Rac1–GTP (Tiede et al, 2003). 2 mo after transgene activation, K-RasG12D and K-RasG12D/Vav1 mice were injected i.p. with azathioprine (10 mg/kg) 5 d a week for 1 mo and were euthanized 15 d after the last injection (Fig 7). Remarkably, treatment with azathioprine significantly decreased APPD in the K-RasG12D/Vav1 mice, as seen in H&E–stained pancreatic sections of mice treated or untreated with azathioprine (Fig 7A). Calculation of APPD score in these experiments indicated that it is reduced from 45% in untreated K-RasG12D/Vav1 mice to 7% in Aza-treated mice (Fig 7B). We then tested whether treatment with azathioprine indeed leads to Rac1–GTP reduction. For that, we used Western blotting to analyze Rac1–GTP levels in pancreatic tissues from K-RasG12D and K-RasG12D/Vav1 mice (Fig 7C and D). Our results clearly pointed to a marked reduction in Rac1–GTP levels in azathioprine-treated (+) K-RasG12D/Vav1 mouse pancreas versus non-treated mice (−), whereas no statistically significant differences were observed in the pancreata of K-RasG12D mice treated similarly (Fig 7C). Quantification of these results revealed a ninefold decrease in Rac1 activation in azathioprine-treated K-RasG12D/Vav1 mice, whereas no such differences were observed in K-RasG12D mice (Fig 7D). Thus, our results convincingly indicate that the activity of Vav1 as a GEF towards Rac1 is essential for Vav1’s contribution to the development of malignant pancreatic lesions.
Fig 7. Treatment of K-RasG12D and K-RasG12D/Vav1 mice with azathioprine.
At 2 mo after initiation of transgene induction, K-RasG12D and K-RasG12D/Vav1 mice were injected i.p. with azathioprine (Aza; 10 mg/kg), 5 d a week for 1 mo, and euthanized 15 d after the last injection. (A) Representative pictures of hematoxylin and eosin (H&E)–stained sections of pancreata from Aza-untreated (indicated by −) and Aza-treated mice (indicated by +) are shown. Scale bar represents 200 μm. (B) The histogram shows the extent of malignant lesions (APPD%) generated in Aza-treated K-RasG12D and K-RasG12D/Vav1 mice. Numbers of K-RasG12D and K-RasG12D/Vav1 mice used without treatment (−) were n = 8 and n = 12, respectively, and numbers treated with Aza (+) were n = 6 and n = 7, respectively. Significant differences between the two analyzed groups (P < 0.05; t test) are indicated. N.S. refers to statistical nonsignificant differences. SEM are shown. (C) Western blotting using anti–Rac1–GTP Abs, anti-Rac1 and anti-actin were used to evaluate Rac1–GTP levels in protein lysates from pancreatic tissues of Aza-untreated (−) or Aza-treated (+) K-RasG12D and K-RasG12D/Vav1 mice. (D) The relative ratio of Rac1–GTP/Rac1 level was calculated for each group of Aza-untreated (−) or Aza-treated (+) mice. Band intensity of the Western blots was quantified using ImageJ 1.49V software. SEM are shown.
Discussion
In the current study of the role of Vav1 in tumor development, our novel transgenic K-RasG12D/Vav1 mouse model was used for the first time. We show that expression of Vav1 together with that of K-RasG12D in the pancreas has a synergistic effect in enhancing ADM, which leads eventually to an increase in the number of PanINs. Thus, the number of malignant lesions observed in our mice co-expressing Vav1 and K-RasG12D exceeded the number of lesions developed in mice that expressed only K-RasG12D. This finding was accompanied by a significant increase in the incidence of PDAC in K-RasG12D/Vav1 mice compared with that occurring in K-RasG12D mice and by elevated proliferation rates as indicated by Ki67-positive acinar and ductal cells. When Vav1 was the only gene expressed, it did not lead to the development of any malignant lesions in the pancreas, suggesting that Vav1 can influence tumor development only when it functions together with other signaling proteins, such as K-RasG12D. These results are in line with the observations that the survival rate of Vav1-positive human primary pancreatic adenocarcinomas was worse than that of Vav1-negative tumors (Fernandez-Zapico et al, 2005; Huang et al, 2016). Taken together, our results point to an important contribution of Vav1, when co-expressed with K-RasG12D, to the development of ADM.
ADM of the pancreas is a process in which pancreatic acinar cells differentiate into cells with ductal cell traits. Data from genetic mouse models have shown that transgenic expression of oncogenic K-RasG12D or K-RasG12V in acinar cells initiates ADM and locks the cells into a transdifferentiated duct-like state (Hingorani & Tuveson, 2003; Guerra et al, 2007; Seidler et al, 2008; Morris et al, 2010; Pylayeva-Gupta et al, 2011). However, the low frequency of spontaneous development of ADM and its progression to invasive PDAC suggests that disease progression requires additional genetic and epigenetic aberrations, such as inflammation and/or additional molecular insults (Hingorani & Tuveson, 2003; Guerra et al, 2007; Seidler et al, 2008; Morris et al, 2010; Pylayeva-Gupta et al, 2011). Appearance of other mutations observed in human PDAC, including inactivation of the P16INK4A/P19ARF, TRP53, or SMAD4 tumor suppressors as well as activation of the Hedgehog signaling pathway, significantly accelerates tumor development leading to acquisition of a metastatic phenotype (Hingorani et al, 2005; Bardeesy et al, 2006; Ijichi et al, 2006; Pasca di Magliano et al, 2006). In the present study, we found that Vav1 activation dramatically accelerates K-Ras–dependent ADM formation. Remarkably, discontinuing transgenic Vav1 expression (via removal of Dox from the drinking water of K-RasG12D/Vav1 mice) in mice with extensive established ADMs led to a marked decrease in ADM lesions in the pancreas. This indicated that Vav1-dependent signals are necessary for the maintenance of the ADM state; upon loss of Vav1 expression, the ADM lesions rapidly regained their normal cell state. Our data reveals that ADM is a highly reversible lesion that depends on ongoing singaling through Rac1. This finding may have important therapeutic and/or preventive implications in pancreatic cancer.
Vav1 can potentially contribute to ADM development through its activity as a signal transducer. Our results demonstrate an increase in phosphorylation of EGFR and of Erk in pancreatic lesions of K-RasG12D/Vav1 and K-RasG12D mice. However, these results did not point to an enhanced synergistic effect of Erk activation in K-RasG12D/Vav1 mice, as opposed to the finding in K-RasG12D mice. The fact that there was no hint of synergism in Erk activation upon co-expression of K-RasG12D and Vav1 indicates that the contribution of Vav1 might stem from its activity as a GEF towards Rac1. Analysis of the Rac1–GTP level in the pancreata from K-RasG12D/Vav1 mice compared with that from K-RasG12D or Vav1 mice indeed clearly demonstrated an increase in Rac1 activity, far exceeding the sum of its activities in the individual K-RasG12D/Vav1 and K-RasG12D mice. The well-known function of Vav1 as the tyrosine phosphorylation–dependent GEF activity for Rac1 (Crespo et al, 1997) was previously shown to be linked to an increase in tumorigenic properties of pancreatic cancer (Fernandez-Zapico et al, 2005; Lazer et al, 2010). Consistently with the notion that the activity of Vav1 as a GEF leads to its synergistic effect with K-RasG12D on ADM generation, we observed an increase in Rac1 activity in K-RasG12D/Vav1 mice, but not in Vav1 or in K-RasG12D transgenic mice. Rac1 expression is increased in mouse and human pancreatic tumors, particularly in the stroma, and is generally a consequence of enhanced upstream inputs from receptor tyrosine kinases and phosphatidylinositol 3-kinases (PI3Ks) or of reduced Rac inactivation by GTPase-activating proteins (GAPs) (Heid et al, 2011). Deletion of Rac1 in the pancreata of K-RasG12D mice reduces ADM and consequently reduces PanIN and PDAC formation, leading to prolonged survival (Heid et al, 2011). Thus, in mice, Rac1 is required for early metaplastic changes and neoplasia-associated actin rearrangements in the development of pancreatic cancer (Heid et al, 2011).
Our results are further strengthened by the significant decrease recorded in K-RasG12D/Vav1 mice treated with the Rac1 blocker azathioprine. The azathioprine metabolite 6-thio-GTP specifically blocks the activation of Rac1. Its binding to Rac1 blocks Vav1’s GEF activity upon 6-thio-GTP hydrolysis because of the accumulation of 6-thio-GDP–loaded Rac proteins, which Vav1 cannot convert to Rac–GTP (Tiede et al, 2003; Poppe et al, 2006). Furthermore, metastasis in a pancreatic cancer mouse model was shown to be inhibited with azathioprine through inhibition of the activity of Vav1 as a GEF towards Rac1 (Razidlo et al, 2015). Taken together, our results support the conclusion that the activity of Vav1 as a GEF for Rac1 is critical for the enhancement and maintaining of ADM generation.
This conclusion raises the question of why is Rac1 synergistically activated in K-RasG12D/Vav1 mice, but hardly active in mice that express either Vav1 alone or K-RasG12D alone. We consider it possible that in the pancreas of the K-RasG12D/Vav1 mouse, there is an increase in chemokine/growth factor secretion that leads to heightened activation of Vav1. The microenvironment of pancreatic cancer contains many factors such as inflammatory cytokines and tumor associated macrophages, which influence the malignant status of the tumor. Such cytokines IL-8, IL-6, IL-1β, TNF-α, IL-10, and others can be up-regulated and consequently contribute to tumor progression (Farajzadeh Valilou et al, 2018). Because Vav1 is a participant in chemokine and cytokine signaling (Matsuguchi et al, 1995; Yuo et al, 1995; Dios-Esponera et al, 2015), it is conceivable that it could further amplify K-Ras and Vav1 activities in the pancreas through such pathways, thus potentially enhancing GM-CSF, CSF1, EGF, and/or TGFα, which can function in an autocrine and/or a paracrine fashion. Our studies of lung cancer have suggested a potential cross-talk between cancer cells and the microenvironment, controlled by CSF1/Vav1 signaling pathways (Sebban et al, 2014), whereby Vav1 positively regulates the expression of CSF1, a growth factor that can lead to Vav1 activation in the microenvironment of tumor cells and in the immune cells in the microenvironment.
An additional potential possibility for the cooperation between Vav1 and mutant K-Ras involves the activity of c-Myc. Vav1 and mutant K-Ras co-operated in fibroblast transformation through overlapping downstream pathways that involve the transcription factor, c-Myc (Katzav et al, 1995). Also, Vav1 and c-Myc were shown to contribute together to the function of the immune system (Guy et al, 2013). c-Myc was found to enable transformation of embryo fibroblasts by a human Ras oncogene (Land et al, 1983). Several in vitro and in vivo data pointed to the contribution of c-Myc to mutant K-Ras pancreatic carcinogenesis (Skoudy et al, 2011). Furthermore, treatment of mutant K-Ras transgenic mice that developed PDAC with an anti-Myc drug showed an increase in cancer cell apoptosis, a reduction in cell proliferation, and a drastic attenuation of tumor growth, strongly suggesting anti-Myc drugs as potential chemotherapeutic agents for the treatment of PDAC (Stellas et al., 2014). Taken together, it is possible that c-Myc also participates as a connecting downstream pathway in enhancing malignancy in K-RasG12D/Vav1 transgenic mouse model.
The findings of our study demonstrate that when Vav1 is co-expressed with mutant K-Ras, it participates as a GEF towards Rac1 in ADM generation. Taken together, our results thus point, for the first time, to the role played by Vav1 in the initial stages of pancreatic cancer development.
Materials and Methods
Mouse strains
Four mouse strains were used in this study: 1. Control mice—pancreas transcription factor 1 complex (Ptf1a)–CreER mice purchased from Jackson Laboratories. 2. K-RasG12D mice—generated by crossing Ptf1a–CreER mice with LSL–K-RasG12D mice (Jackson Laboratories). 3. Vav1 mice—in which human Vav1 was subcloned into a plasmid encoding a tetO-responsive bidirectional promoter (tetO7minCMV), driving the expression of tetO–Vav1 hooked to GFP. TetO–Vav1–GFP mice were produced by microinjection of the plasmid into chimeric C57BL/6 mice blastocysts according to standard in vitro fertilization protocol. To ensure expression of Vav1 in pancreatic acinar cells, these mice were crossed with Ptf1a–CreER and LSL–rtTA mice. 4. K-RasG12D/Vav1 mice—produced by crossing of the K-RasG12D mice with Vav1 mice to generate a mouse line having a Ptf1a–CreER;LSL–rtTA;LSL–K-RasG12D/tetO–Vav1. Details of the genotyping of these mice, including a detailed list of primers, are shown in Table S2.
Table S2 Primers used for transgenic mice genotyping. (14.9KB, docx)
To induce expression of the K-RasG12D transgene in pancreatic acinar cells, we injected 1-mo-old mice subcutaneously with 8 mg of tamoxifen (2%; Sigma-Aldrich) dissolved in corn oil, twice over a 2-d interval. To induce Vav1 expression in these cells, doxycycline (Dox) (0.5 mg/ml; Bio Basic) was introduced into the drinking water of 1-mo-old mice as a sucrose (3% wt/vol) solution. To ensure comparable experimental conditions, all mouse strains used in this study were similarly treated with Dox and tamoxifen. All experiments were approved by the Hebrew University Ethics Committee for Animal Use (#MD-14-14199-5).
Histology, immunohistochemical, and immunofluorescence analysis
Paraffin-embedded or frozen serial sections were subjected to H&E staining using standard procedures. Antibodies used for IHC are detailed in Table S3. Immunofluorescence of anti-Rac1 GTP was performed as follows: sections from paraffin-embedded blocks were used for immunofluorescence, deparaffinized using Xylene two time for 5 min each, followed by rehydration with decreased alcohol concentrations (100%–96%–80%), and then washed three times with PBS buffer. Antigen retrieval was performed by using citrate buffer in a pressure cooker. The sections were incubated for 1 h at room temperature with a blocking solution (CAS block; Invitrogen), followed by incubation with anti Rac1–GTP primary antibodies (1:200) overnight at 4°C in humidified chambers (Table S3). Slides were washed with PBS followed by incubation with antimouse Alexa Flour 594 secondary antibody. The slides were washed once with PBS for 5 min before nuclear staining with DAPI for 5 min and were dehydrated and mounted with coverslips. Pictures were taken using confocal microscopy (Nikon).
Table S3 List of antibodies used in this study. (15.7KB, docx)
Malignancy and proliferation measurements
H&E–stained sections from the pancreata of the mouse strains used in our study were quantified for malignant lesions. The degree of malignancy, or “transformation index,” was calculated as the summed total area of ADM, PanINs, and PDAC lesions (APPDs) expressed as a percentage of the area of the entire pancreas (APPD%). PDAC cases identified in K-RasG12D/Vav1 and in K-RasG12D pancreatic sections were counted, and their incidence was calculated in a blinded manner by a certified histopathologist. Proliferation rates were calculated by counting of positive Ki-67 acinar and ductal cells from the various mouse lines at different time points after transgene induction. Ten randomly chosen fields (at 400× magnification) from each pancreatic section were used for counting using ImageJ 1.49Vi.
Western blotting
Pancreatic tissues were lysed in a lysis buffer containing 50 mM Tris–HCl, 150 mM NaCl, 25 mM EDTA, 1% NP-40, 1× phosphatase and protease inhibitors (Roche), and 1% PMSF. Tissues were fragmented using a homogenizer (Polytron Kinematica). Lysed tissues were incubated for 30 min on ice and then centrifuged at maximum speed for 20 min. Lysed proteins were quantified using Bio-Rad protein assay (Bio-Rad). Equal amounts of proteins (30 μg) were loaded on 4–15% gradient acrylamide gels, as previously described (Lazer et al, 2010). Antibodies used for Western blotting are listed in Table S3. Western blot results were quantified using ImageJ 1.49 V1.
Azathioprine treatment
Azathioprine was diluted in PBS to a final concentration of 2 mg/ml and injected i.p. (10 mg/kg) into mice, 2 mo after transgene induction, 5 d a week for 1 mo. Mice were euthanized 15 d after the last injection.
Statistical analysis
PDAC incidence was analyzed by the two-tailed Fisher’s test. Significant differences (indicated by P < 0.05) were calculated according to Student’s two-tailed test using the GraphPad Prism or Excel software.
Supplementary Material
Acknowledgements
We are indebted to Shirley Smith for editing the manuscript and to Dr Dror Gal, Prof. Ittai Ben-Porath, and Prof. Yuval Dor for advice on mouse manipulation. This work was supported, in part, by grants from the Israel Academy of Sciences, the Israel Cancer Association (ICA) with the generous assistance of the USA Friends of ICA in Honor of Laurence Holzman and Felicia Needleman; The Israel Cancer Research Fund; The Hubert H Humphrey Center for Experimental Medicine and Cancer Research; The Alex U Soyka Pancreatic Cancer Research Project; and a donation from Vibeke Lichten.
Author’s Contributions
Y Salaymeh: formal analysis, investigation, and methodology.
M Farago: investigation, methodology, project administration, and writing—original draft, review, and editing.
S Sebban: methodology.
B Shalom: formal analysis and methodology.
E Pikarsky: conceptualization and writing—original draft, review, and editing.
S Katzav: conceptualization, formal analysis, supervision, funding acquisition, and writing—original draft, review, and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Abate F, da Silva-Almeida AC, Zairis S, Robles-Valero J, Couronne L, Khiabanian H, Quinn SA, Kim MY, Laginestra MA, Kim C, et al. (2017) Activating mutations and translocations in the guanine exchange factor VAV1 in peripheral T-cell lymphomas. Proc Natl Acad Sci U S A 114: 764–769. 10.1073/pnas.1608839114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichler M, Seiler C, Tost M, Siveke J, Mazur PK, Da Silva-Buttkus P, Bartsch DK, Langer P, Chiblak S, Durr A, et al. (2012) Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: A comparative study in transgenic mice and human tissues. J Pathol 226: 723–734. 10.1002/path.3017 [DOI] [PubMed] [Google Scholar]
- Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, et al. (2012) EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 22: 304–317. 10.1016/j.ccr.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et al. (2006) Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A 103: 5947–5952. 10.1073/pnas.0601273103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491: 399–405. 10.1038/nature11547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et al. (2016) Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet 48: 607–616. 10.1038/ng.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM, Charles RP, Rabinovich BA, Hann B, Dankort D, et al. (2012) A central role for RAF→MEK→ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov 2: 685–693. 10.1158/2159-8290.cd-11-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR (1997) Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385: 169–172. 10.1038/385169a0 [DOI] [PubMed] [Google Scholar]
- Dios-Esponera A, Isern de Val S, Sevilla-Movilla S, Garcia-Verdugo R, Garcia-Bernal D, Arellano-Sanchez N, Cabanas C, Teixido J (2015) Positive and negative regulation by SLP-76/ADAP and Pyk2 of chemokine-stimulated T-lymphocyte adhesion mediated by integrin alpha4beta1. Mol Biol Cell 26: 3215–3228. 10.1091/mbc.e14-07-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du MJ, Chen XD, Zhou XL, Wan YJ, Lan B, Zhang CZ, Cao Y (2014) Estrogen induces Vav1 expression in human breast cancer cells. PloS One 9: e99052 10.1371/journal.pone.0099052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M, Hieber M, Arbeiter A, Klein S, Kong B, et al. (2013) Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell 23: 406–420. 10.1016/j.ccr.2013.01.023 [DOI] [PubMed] [Google Scholar]
- Farajzadeh Valilou S, Keshavarz-Fathi M, Silvestris N, Argentiero A, Rezaei N (2018) The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev 39: 46–61. 10.1016/j.cytogfr.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Feldmann G, Mishra A, Hong SM, Bisht S, Strock CJ, Ball DW, Goggins M, Maitra A, Nelkin BD (2010) Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation and progression through the suppression of Ras-Ral signaling. Cancer Res 70: 4460–4469. 10.1158/0008-5472.can-09-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD (2005) Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 7: 39–49. 10.1016/j.ccr.2004.11.024 [DOI] [PubMed] [Google Scholar]
- Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, et al. (1998) Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol 8: 554–562. 10.1016/s0960-9822(98)70224-6 [DOI] [PubMed] [Google Scholar]
- Grassilli S, Brugnoli F, Lattanzio R, Rossi C, Perracchio L, Mottolese M, Marchisio M, Palomba M, Nika E, Natali PG, et al. (2014) High nuclear level of Vav1 is a positive prognostic factor in early invasive breast tumors: A role in modulating genes related to the efficiency of metastatic process. Oncotarget 5: 4320–4336. 10.18632/oncotarget.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M (2011) Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19: 728–739. 10.1016/j.ccr.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M (2007) Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11: 291–302. 10.1016/j.ccr.2007.01.012 [DOI] [PubMed] [Google Scholar]
- Guy CS, Vignali KM, Temirov J, Bettini ML, Overacre AE, Smeltzer M, Zhang H, Huppa JB, Tsai YH, Lobry C, et al. (2013) Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol 14: 262–270. 10.1038/ni.2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM, Siveke JT (2011) Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology 141: 719–730, 730.e1–730.e7 10.1053/j.gastro.2011.04.043 [DOI] [PubMed] [Google Scholar]
- Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H (2010) PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res 70: 7114–7124. 10.1158/0008-5472.can-10-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Tuveson DA (2003) Ras redux: Rethinking how and where Ras acts. Curr Opin Genet Dev 13: 6–13. 10.1016/s0959-437x(02)00017-5 [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA (2005) Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7: 469–483. 10.1016/j.ccr.2005.04.023 [DOI] [PubMed] [Google Scholar]
- Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR (1998) Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol 8: 563–572. 10.1016/s0960-9822(98)70225-8 [DOI] [PubMed] [Google Scholar]
- Hornstein I, Pikarsky E, Groysman M, Amir G, Peylan-Ramu N, Katzav S (2003) The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas. J Pathol 199: 526–533. 10.1002/path.1314 [DOI] [PubMed] [Google Scholar]
- Huang PH, Lu PJ, Ding LY, Chu PC, Hsu WY, Chen CS, Tsao CC, Chen BH, Lee CT, Shan YS, et al. (2016) TGFbeta promotes mesenchymal phenotype of pancreatic cancer cells, in part, through epigenetic activation of VAV1. Oncogene 36: 2202–2214. 10.1038/onc.2016.378 [DOI] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL (2006) Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev 20: 3147–3160. 10.1101/gad.1475506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA, et al. (2007) Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 11: 229–243. 10.1016/j.ccr.2007.01.017 [DOI] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321: 1801–1806. 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, et al. (2012) Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 142: 730–733.e9. 10.1053/j.gastro.2011.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga JI, Totoki Y, Chiba K, Sato-Otsubo A, Nagae G, et al. (2015) Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 47: 1304–1315. 10.1038/ng.3415 [DOI] [PubMed] [Google Scholar]
- Katzav S, Martin-Zanca D, Barbacid M (1989) Vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J 8: 2283–2290. 10.1002/j.1460-2075.1989.tb08354.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S, Packham G, Sutherland M, Aroca P, Santos E, Cleveland JL (1995) Vav and Ras induce fibroblast transformation by overlapping signaling pathways which require c-Myc function. Oncogene 11: 1079–1088. [PubMed] [Google Scholar]
- Kniazev Iu P, Cheburkin Iu V, Spikermann K, Peter S, Jenster G, Bangma KH, Karelin MI, Shkol’nik MI, Urbanskii AI, Evtushenko VI, et al. (2003) [Gene expression profiles of protein kinases and phosphatases obtained by hybridization with cDNA arrays: Molecular portrait of human prostate carcinoma]. Mol Biol (Mosk) 37: 97–111. [PubMed] [Google Scholar]
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, Pan FC, Akiyama H, Wright CV, Jensen K, et al. (2012) Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22: 737–750. 10.1016/j.ccr.2012.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, Wellauer PK (1998) The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev 12: 3752–3763. 10.1101/gad.12.23.3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H, Parada LF, Weinberg RA (1983) Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304: 596–602. 10.1038/304596a0 [DOI] [PubMed] [Google Scholar]
- Lane J, Martin TA, Mansel RE, Jiang WG (2008) The expression and prognostic value of the guanine nucleotide exchange factors (GEFs) Trio, Vav1 and TIAM-1 in human breast cancer. Int Semin Surg Oncol 5: 23 10.1186/1477-7800-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazer G, Idelchuk Y, Schapira V, Pikarsky E, Katzav S (2009) The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role in tumourigenesis. J Pathol 219: 25–34. 10.1002/path.2579 [DOI] [PubMed] [Google Scholar]
- Lazer G, Pe’er L, Farago M, Machida K, Mayer BJ, Katzav S (2010) Tyrosine residues at the carboxyl terminus of Vav1 play an important role in regulation of its biological activity. J Biol Chem 285: 23073–23083. 10.1074/jbc.m109.094508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, Der CJ, Counter CM (2005) Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7: 533–545. 10.1016/j.ccr.2005.04.030 [DOI] [PubMed] [Google Scholar]
- Lindsey JC, Kawauchi D, Schwalbe EC, Solecki DJ, Selby MP, McKinnon PJ, Olson JM, Hayden JT, Grundy RG, Ellison DW, et al. (2014) Cross-species epigenetics identifies a critical role for VAV1 in SHH subgroup medulloblastoma maintenance. Oncogene 34: 4746–4757. 10.1038/onc.2014.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman JS, Koretzky GA (2003) Azathioprine: Old drug, new actions. J Clin Invest 111: 1122–1124. 10.1172/jci200318384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T, Inhorn RC, Carlesso N, Xu G, Druker B, Griffin JD (1995) Tyrosine phosphorylation of p95Vav in myeloid cells is regulated by GM-CSF, IL-3 and steel factor and is constitutively increased by p210BCR/ABL. EMBO J 14: 257–265. 10.1002/j.1460-2075.1995.tb06999.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Morris JP 4th, Wang SC, Hebrok M (2010) KRAS, hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 10: 683–695. 10.1038/nrc2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M (2006) Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev 20: 3161–3173. 10.1101/gad.1470806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe D, Tiede I, Fritz G, Becker C, Bartsch B, Wirtz S, Strand D, Tanaka S, Galle PR, Bustelo XR, et al. (2006) Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol 176: 640–651. 10.4049/jimmunol.176.1.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D (2011) RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer 11: 761–774. 10.1038/nrc3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razidlo GL, Magnine C, Sletten AC, Hurley RM, Almada LL, Fernandez-Zapico ME, Ji B, McNiven MA (2015) Targeting pancreatic cancer metastasis by inhibition of Vav1, a driver of tumor cell invasion. Cancer Res 75: 2907–2915. 10.1158/0008-5472.can-14-3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebban S, Farago M, Gashai D, Ilan L, Pikarsky E, Ben-Porath I, Katzav S (2013) Vav1 fine tunes p53 control of apoptosis versus proliferation in breast cancer. PloS One 8: e54321 10.1371/journal.pone.0054321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebban S, Farago M, Rabinovich S, Lazer G, Idelchuck Y, Ilan L, Pikarsky E, Katzav S (2014) Vav1 promotes lung cancer growth by instigating tumor-microenvironment cross-talk via growth factor secretion. Oncotarget 5: 9214–9226. 10.18632/oncotarget.2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler B, Schmidt A, Mayr U, Nakhai H, Schmid RM, Schneider G, Saur D (2008) A Cre-loxP-based mouse model for conditional somatic gene expression and knockdown in vivo by using avian retroviral vectors. Proc Natl Acad Sci U S A 105: 10137–10142. 10.1073/pnas.0800487105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF (2009) Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology 136: 1368–1378. 10.1053/j.gastro.2008.12.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoudy A, Hernandez-Munoz I, Navarro P (2011) Pancreatic ductal adenocarcinoma and transcription factors: Role of c-Myc. J Gastrointest Cancer 42: 76–84. 10.1007/s12029-011-9258-0 [DOI] [PubMed] [Google Scholar]
- Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ Jr, Wright CV, et al. (1999) Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology 117: 1416–1426. 10.1016/s0016-5085(99)70292-1 [DOI] [PubMed] [Google Scholar]
- Stellas D, Szabolcs M, Koul S, Li Z, Polyzos A, Anagnostopoulos C, Cournia Z, Tamvakopoulos C, Klinakis A, Efstratiadis A (2014) Therapeutic effects of an anti-Myc drug on mouse pancreatic cancer. J Natl Cancer Inst 106: 1–8. doi: 10.1093/jnci/dju320 [DOI] [PubMed] [Google Scholar]
- Storz P. (2017) Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol 14: 296–304. 10.1038/nrgastro.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, et al. (2003) CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 111: 1133–1145. 10.1172/jci16432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz VL. (2005) Vav-family proteins in T-cell signalling. Curr Opin Immunol 17: 267–274. 10.1016/j.coi.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Wakahashi S, Sudo T, Oka N, Ueno S, Yamaguchi S, Fujiwara K, Ohbayashi C, Nishimura R (2013) VAV1 represses E-cadherin expression through the transactivation of snail and slug: A potential mechanism for aberrant epithelial to mesenchymal transition in human epithelial ovarian cancer. Transl Res 162: 181–190. 10.1016/j.trsl.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Wei D, Wang L, Yan Y, Jia Z, Gagea M, Li Z, Zuo X, Kong X, Huang S, Xie K (2016) KLF4 is essential for induction of cellular identity change and acinar-to-ductal reprogramming during early pancreatic carcinogenesis. Cancer Cell 29: 324–338. 10.1016/j.ccell.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuo A, Kitagawa S, Iki S, Yagisawa M, Inuo EK, Mimura T, Minoda S, Hanazono Y, Hirai H, Urabe A, et al. (1995) Tyrosine phosphorylation of vav protooncogene product in primary human myelogenous leukemic cells stimulated by granulocyte colony-stimulating factor. Biochem Biophys Res Commun 211: 677–685. 10.1006/bbrc.1995.1865 [DOI] [PubMed] [Google Scholar]
- Zhou K, Rao J, Zhou ZH, Yao XH, Wu F, Yang J, Yang L, Zhang X, Cui YH, Bian XW, et al. (2018) RAC1-GTP promotes epithelial-mesenchymal transition and invasion of colorectal cancer by activation of STAT3. Lab Invest 98: 989–998. 10.1038/s41374-018-0071-2 [DOI] [PubMed] [Google Scholar]
- Zhu X, Jin H, Xia Z, Wu X, Yang M, Zhang H, Shang X, Cheng R, Zhan Z, Yu Z (2017) Vav1 expression is increased in esophageal squamous cell carcinoma and indicates poor prognosis. Biochem Biophys Res Commun 486: 571–576. 10.1016/j.bbrc.2017.03.091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source Data for Figure S2LSA-2020-00661_SdataFS2_1.pdf (734.6KB, pdf) LSA-2020-00661_SdataFS2_2.pdf (137.7KB, pdf) LSA-2020-00661_SdataFS2_3.pdf (416.5KB, pdf)
Table S1 Number of mice used in various experiments. (19.1KB, docx)
Source Data for Figure 6LSA-2020-00661_SdataF6A_1.pdf (2MB, pdf) LSA-2020-00661_SdataF6A_2.pdf (2.2MB, pdf) LSA-2020-00661_SdataF6A_3.pdf (1.4MB, pdf)
Table S2 Primers used for transgenic mice genotyping. (14.9KB, docx)
Table S3 List of antibodies used in this study. (15.7KB, docx)