Abstract
Community‐acquired pneumonia (CAP) in adults is an infectious disease with high morbidity in China and the rest of the world. With the changing pattern in the etiological profile of CAP and advances in medical techniques in diagnosis and treatment over time, Chinese Thoracic Society of Chinese Medical Association updated its CAP guideline in 2016 to address the standard management of CAP in Chinese adults. Extensive and comprehensive literature search was made to collect the data and evidence for experts to review and evaluate the level of evidence. Corresponding recommendations are provided appropriately based on the level of evidence. This updated guideline covers comprehensive topics on CAP, including aetiology, antimicrobial resistance profile, diagnosis, empirical and targeted treatments, adjunctive and supportive therapies, as well as prophylaxis. The recommendations may help clinicians manage CAP patients more effectively and efficiently. CAP in pediatric patients and immunocompromised adults is beyond the scope of this guideline. This guideline is only applicable for the immunocompetent CAP patients aged 18 years and older. The recommendations on selection of antimicrobial agents and the dosing regimens are not mandatory. The clinicians are recommended to prescribe and adjust antimicrobial therapies primarily based on their local etiological profile and results of susceptibility testing, with reference to this guideline.
Keywords: community‐acquired pneumonia, adult, antimicrobial therapy, aetiology, diagnosis, adjunctive therapy, prevention
Abbreviations
- ATS
American Thoracic Society
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- BCYE
buffered charcoal‐yeast extract
- BUN
blood urea nitrogen
- CA‐MRSA
community‐acquired methicillin‐resistant Staphylococcus aureus
- CAP
community‐acquired pneumonia
- CARTIPS
Community‐Acquired Respiratory Tract Infection Pathogen Surveillance
- CF
complement fixation test
- CFDA
China Food and Drug Administration
- CMA
Chinese Medical Association
- CRP
C‐reactive protein
- CTS
Chinese Thoracic Society
- DFA
direct fluorescent antibody test
- DT
Sabin‐Feldman dye test
- ECMO
extracorporeal membrane oxygenation
- EIA
enzyme immunoassay
- ELISA
enzyme‐linked immunosorbent assay
- ETA
endotracheal aspiration
- GVPC
glycine‐vancomycin‐polymyxin‐cycloheximide
- HA
haemagglutination assay
- HIV
human immunodeficiency virus
- hMPV
human metapneumovirus
- ICT
immunochromatographic test
- IDSA
Infectious Diseases Society of America
- IFA
indirect immunofluorescence assay
- IGRA
interferon‐gamma release assay
- IHA
indirect haemagglutination test
- ISAGA
immunosorbent agglutination assay
- IVIG
intravenous immune globulin
- MAG
microparticle agglutination
- MAT
micro agglutination test
- MERS
Middle East respiratory syndrome
- MIC
minimum inhibitory concentration
- MIF
microimmunofluorescence assay
- MWY
modified Wadowsky Yee agar
- MRSA
methicillin‐resistant Staphylococcus aureus
- NIV
non‐invasive ventilation
- PA
particle agglutination test
- PCT
procalcitonin
- PCV
pneumococcal conjugate vaccine
- PEEP
positive end‐expiratory pressure
- PPV
pneumococcal polysaccharide vaccine
- PSB
protected specimen brush
- PSI
pneumonia severity index
- RCT
randomized, controlled trial
- RR
respiratory rate
- RSV
respiratory syncytial virus
- SBP
systolic blood pressure
- TST
tuberculin skin test
- WBC
white blood cell count
- WHO
World Health Organization
1. INTRODUCTION
This guideline is applicable for the immunocompetent community‐acquired pneumonia (CAP) patients aged 18 years and older. For immunocompromised patients, such as human immunodeficiency virus (HIV) infection, agranulocytosis, haematological tumour or solid tumour undergoing chemo‐radiotherapy, solid organ transplantation and patients receiving glucocorticoid or cytokine antagonist, this guideline may be inappropriate.
2. METHODOLOGY FOR REVISION OF THE GUIDELINE
The revision of the guideline was initiated by Chinese Thoracic Society (CTS) and Chinese Medical Association (CMA). The overall framework and main content of the updated guideline were finalized following 3 face‐to‐face work meetings and 2 online video conferences. The experts specialized in methodology provided training on standardized literature search and grading of evidence to all the specialists contributing to the guideline. Level of evidence and grading of recommendation were based on the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guidelines for CAP (2007).1 The level of evidence represents the assessment on the quality of study evidence, and the grading of recommendation refers to the assessment on the degree to which the benefits of an intervention outweighs the risks. Generally speaking, the higher the evidence level, the stronger the grade of recommendation, but they do not fully correspond to each other. The willingness and values of patients, as well as resource consumption should also be considered when making a recommendation (Table 1).
Table 1.
Evidence level and grade of recommendation
| Evidence level and grade of recommendation | Description |
|---|---|
| Evidence level | |
| Level I (high) | Evidence from well‐designed, randomized, controlled trials (RCTs), authoritative guidelines and high quality systematic reviews and meta‐analyses |
| Level II (moderate) | Evidence from RCTs with some limitations (eg, trials without allocation concealment, nonblinded, or loss to follow‐up not reported), cohort studies, case series and case‐control studies |
| Level III (low) | Evidence from case reports, expert opinions and in vitro antimicrobial susceptibility studies without clinical data |
| Grade of recommendation | |
| A (strong) | Most patients, physicians and policy makers will adopt the recommended action. |
| B (moderate) | The recommendation will be adopted by the majority, but not by some individuals. Decisions should be made with consideration of the specific condition of the patient to reflect his/her values and willingness. |
| C (weak) | Insufficient evidence; decisions must be made via mutual discussions involving the patients, physicians and policy makers. |
This guideline document is composed of 8 sections. The core panel members are responsible for 8 separate groups to prepare the first draft by searching and reviewing the relevant domestic and international literature, evaluating evidence level with the unified standard. The grading of recommendations is decided by vote of all members participating in the preparation of the guidelines.
The principal writer was responsible for summarization and modification of the first draft. In the process, 6 face‐to‐face work meetings were held to discuss revision of the draft. Three rounds of consultation were conducted to solicit advice and opinions from the specialists of the specialty groups within CTS, CMA, specialists in relevant disciplines such as infectious diseases, clinical microbiology, emergency and critical care medicine and clinical pharmacy and specialists from the United States and Europe. The guideline document was modified for 6 times based on such discussions and feedbacks.
The final revised version was approved by all the writers and consultants.
3. SECTION 1. DEFINITION AND DIAGNOSIS OF CAP
3.1. Definition
CAP refers to the infectious inflammation of lung parenchyma (including alveolar wall, ie, pulmonary interstitium in general meaning) acquired outside of hospitals, including pneumonia caused by pathogens with proven latency, the onset of disease is during the latency after the patient is admitted into hospital.
3.2. Incidence and mortality of CAP in adults
The incidence of CAP in adults is 5–11/1000 person‐year in European and North American countries,2 and increases with age. In the United States, the average incidence of CAP is 2.5/1000 person‐year in adult inpatients, 6.3/1000 person‐year in the population aged 65 to 79, and the highest 16.4/1000 person‐year in the population at least 80 years of age.3 A Japanese study showed that the incidence of CAP was 3.4/1000, 10.7/1000 and 42.9/1000 person‐year in the populations aged 15 to 64, 65 to 74 and ≥ 75, respectively.4 In China, only the proportion of CAP by age group is available at present time, but no specific data are available on the incidence of CAP in adults. A study conducted in China in 2013 showed that in the 16 585 hospitalized CAP patients, much larger proportion was found in ≤ 5 years (37.3%) and > 65 years (28.7%) of age groups compared with adults from 26 to 45 years of age (9.2%).5
The mortality of CAP increases with age of patient. In Japan, the reported mortality of hospitalized CAP patients was 1.4% in 15–44 years of age group, 3.3% in 45–64 years of age group, 6.9% in 65–74 years of age group and 9.3% in ≥75 years of age group.6 CAP mortality is also associated with the severity of disease. Data from a German CAP surveillance network showed that the 30‐day mortality of CAP in adult patients was 8.6%. The mortality rate in outpatients and inpatients was 0.8% and 12.2%, respectively.7 Additionally, the results of several studies have shown that the 30‐day mortality of moderate‐to‐severe CAP patients was up to 23%‐47% in ICU.8, 9, 10, 11
Currently, we lack the data regarding the incidence and mortality of CAP in China. According to data from the China's Health and Family Planning Statistical Yearbook 2013, in 2008, the two‐week prevalence of pneumonia was 1.1‰ in China, slight increase compared with the data in 2003 (0.9‰). In 2012, the average mortality of pneumonia was 17.46/100 000 in China; specifically, 32.07/100 000 in the population under 1 year‐old, <1/100 000 in the population aged 25 to 39, 23.55/100 000 in the population aged 65 to 69 and up to 864.17/100 000 in the population aged >85.12
3.3. Aetiology of CAP in Chinese adults
The distribution and antimicrobial resistance profile of CAP pathogens are significantly different across different countries and regions, and change over time. Currently, the results of several epidemiological surveys of CAP conducted in Chinese adults have shown that Mycoplasma pneumoniae and Streptococcus pneumoniae are important pathogens of CAP in adults in China.13, 14, 15, 16, 17 Other common pathogens include Haemophilus influenzae, Chlamydia pneumoniae, Klebsiella pneumoniae and Staphylococcus aureus; but Pseudomonas aeruginosa and Acinetobacter baumannii are infrequently isolated.13, 16, 17, 18 In China, only a small number of cases of community‐acquired methicillin‐resistant S. aureus (CA‐MRSA) pneumonia are reported in children and teenagers.19, 20, 21, 22 CA‐MRSA was not identified in the antimicrobial resistance surveillance of community‐acquired respiratory tract pathogens in adults conducted in 2009–2010.23 For special populations such as elderly patients or patients with underlying diseases (eg, congestive heart failure, cardiovascular or cerebrovascular diseases, chronic respiratory system diseases, kidney failure and diabetes mellitus), gram‐negative bacteria such as K. pneumoniae and Escherichia coli are more common.18, 24, 25
With the development and application of virus detection technology, the role of respiratory tract viruses is gradually gaining attention in the aetiology of CAP in Chinese adults. The results of several recently published multicenter studies showed that the detection rate of viruses was 15%‐34.9% in Chinese adult CAP patients, of which influenza virus accounted for the largest proportion. Other contributing viruses included parainfluenza virus, rhinovirus, adenovirus, human metapneumovirus (hMPV) and respiratory syncytial virus (RSV). Among the patients with positive test results for viruses, 5.8%‐65.7% could have concomitant infection caused by bacteria or atypical pathogens.15, 18, 26, 27
Considering the resistance profile of major pathogens, the high percentage of S. pneumoniae resistant to macrolides found in Chinese adult CAP patients is an important characteristic that differs from that in European and American countries. Two nation‐wide multicenter surveys on adult CAP conducted in 2003–2005 showed that 63.2%–75.4% of S. pneumoniae isolates were resistant to macrolides.13, 17 Recently, the results of 2 multicenter Community‐Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) studies in adults conducted in urban tertiary hospitals in China showed that 88.1%–91.3% of S. pneumoniae isolates were resistant to azithromycin, the minimum inhibitory concentration of which required to inhibit the growth of 90% of organisms (MIC90) was 32–256 mg/L and 88.2% of the isolates were resistant to clarithromycin.23, 28 While in European and American countries, 12.9%‐39% and 4.3%–33.3% of S. pneumoniae isolates were resistant to erythromycin and azithromycin, respectively.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Moreover, 24.5%–36.5% of S. pneumoniae isolates were resistant to oral penicillins, and 39.9%‐50.7% resistant to second‐generation cephalosporins in China. However, relatively low percentage of S. pneumoniae isolates were resistant to injectable penicillins and third‐generation cephalosporins (1.9% and 13.4%, respectively).23, 28
The high percentage of Mycoplasma pneumoniae strains resistant to macrolides is another important characteristic in the aetiology of CAP in China, which is different from that in most other countries. Study results showed that 58.9%‐71.7% of the mycoplasma strains isolated from Chinese adult CAP patients were resistant to erythromycin, and 54.9%‐60.4% resistant to azithromycin.35, 36, 37 The infections caused by antibiotic‐resistant mycoplasma may prolong the duration of fever and anti‐infective treatment.36 In addition to China, 25%‐46% of the mycoplasma strains isolated from Japanese adult and teenage CAP patients were resistant to macrolides. Macrolides‐resistant M. pneumoniae was also reported in France, Canada, the United States, Spain and Germany.38, 39, 40, 41, 42, 43 M. pneumoniae is highly resistant to macrolides in China, but it remains susceptible to doxycycline, minocycline and quinolones.35, 44
3.4. Clinical diagnostic criteria for CAP
Onset in community.
Relevant clinical manifestations of pneumonia: (1) New onset of cough or expectoration, or aggravation of existing symptoms of respiratory tract diseases, with or without purulent sputum, chest pain, dyspnea, or hemoptysis; (2) Fever; (3) Signs of pulmonary consolidation and/or moist rales; (4) Peripheral white blood cell count (WBC) > 10 × 109/L or < 4 × 109/L, with or without a left shift.
Chest radiograph showing new patchy infiltrates, lobar or segmental consolidation, ground‐glass opacities, or interstitial changes, with or without pleural effusion.
Clinical diagnosis can be established if a patient satisfies Criterion A, Criterion C and any one condition of Criterion B and meanwhile, tuberculosis, pulmonary tumour, non‐infectious interstitial lung disease, pulmonary edema, atelectasis, pulmonary embolism, pulmonary eosinophilia and pulmonary vasculitis are all excluded.
3.5. Diagnosis and treatment approach of CAP
Step 1: Determine whether a diagnosis of CAP is valid or not. For patients with clinically suspected CAP, the possibility of unusual infections such as tuberculosis and non‐infectious causes must be considered.
Step 2: Evaluate the severity of CAP and select the location for treatment.
Step 3: Predict the potential pathogens of CAP and risks of antibiotic resistance (Table 2): considering patient age, season of onset, underlying diseases and risk factors, symptoms or signs, characteristics of chest imaging (X‐ray film or CT), laboratory tests, severity of CAP, prior antibacterial therapies and so on.
Table 2.
Clinical manifestations of pneumonia in terms of different pathogens
| Potential pathogen | Clinical manifestations |
|---|---|
| Bacteria | Acute onset, high fever with potential shivers, purulent sputum, brown bloody sputum, chest pain, significant increase in peripheral WBC, increased C‐reactive protein (CRP), signs of pulmonary consolidation or moist rales; radiograph shows alveolar infiltrates or lobar or segmental distribution of consolidation.45, 46, 47, 48, 49 |
| Mycoplasma or Chlamydia | Under 60 years of age, with few underlying diseases; continuous cough, no sputum or no bacteria discovered in sputum smear test, few pulmonary signs, peripheral WBC <10 × 109/L; radiograph may show lesions in the upper lung field of both lungs, centrilobular nodules, tree‐in‐bud sign, ground‐glass opacities, or thickening of bronchial wall and may show signs of consolidation with disease progression.15, 46, 50, 51, 52 |
| Virus | Mostly seasonal, may have history of exposure to an epidemic or clustered outbreak, acute upper respiratory tract symptoms, myalgia, normal or decreased peripheral WBC, procalcitonin (PCT) < 0.1 ng/mL, unresponsive to treatment with antibacterial agents; radiograph shows bilateral, interstitial exudates in multiple lobes and/or ground‐glass opacities, which may be accompanied by consolidation.46, 53, 54, 55 |
Step 4: Arrange for reasonable etiological tests, and initiate empirical anti‐infective treatment in a timely manner.
Step 5: Evaluate the effectiveness of empirical anti‐infective treatment on CAP in a dynamic manner; investigate the cause if initial treatment fails, and adjust treatment protocol promptly.
Step 6: Follow up after treatment; and provide education on health maintenance.
4. SECTION 2. ASSESSMENT OF CAP SEVERITY, CRITERIA FOR HOSPITAL ADMISSION and DIAGNOSTIC CRITERIA FOR SEVERE CAP
The evaluation of CAP severity is crucial for selection of appropriate location of treatment, initial empirical antimicrobial agents, as well as adjunctive and supportive treatments.
4.1. Evaluation of CAP severity
The scoring systems of CAP severity differ from each other (Table 3). They can be used as an aid for evaluation and provide support for clinical diagnosis and treatment, but physicians should take clinical experience into consideration when making judgments, and monitor disease progression in a dynamic manner56 (II A). CURB‐65, CRB‐65 (C: disturbance of consciousness, U: urea nitrogen, R: respiratory rate, B: blood pressure, 65: age), and pneumonia severity index (PSI) scoring systems underestimate the risk of death and severity of influenza pneumonia,57, 58, 59, 60 while oxygenation index combined with absolute reduction of peripheral blood lymphocyte is superior to CURB‐65 and PSI in predicting the risk of death due to influenza pneumonia61 (II B).
Table 3.
Features of common scoring scales for evaluating CAP severity
| Scales | Indices and calculation | Risk ratings | Recommendation |
|---|---|---|---|
|
CURB‐65 score 64 |
5 indices in total; 1 pt for each criterion satisfied:
|
Mortality risk evaluation: 0–1: low risk; 2: moderate risk; 3–5: high risk |
Simple, highly sensitive, easy for clinical application |
|
CRB‐65 score 64 |
4 indices in total; 1 pt for each criterion satisfied:
|
Mortality risk evaluation: 0: low risk, outpatient treatment; 1–2: moderate risk, hospital admission or extramural treatment with close follow‐up is recommended; ≥3: high risk, patient should be hospitalized |
Suitable for medical institutions unable to perform biochemical tests |
| PSI score 65 | Sum of age (female minus 10 pts) and scores for all risk factors:
|
Evaluation of mortality risk: Low risk: Class I (<50 years of age, without underlying diseases); Class II (≤70 pts); Class III (71–90 pts); Moderate risk: Class IV (91–130 pts); High risk: Class V (>130 pts). Patients at Classes IV and V need hospitalization |
Sensitive measurement for evaluating whether a patient needs hospitalization, highly specific. Complex scoring system |
|
CURXO score 66 |
Major indices:
Minor indices:
|
Patients are diagnosed as severe CAP if any one of the major indices or two of the minor indices are met | Simple scoring method used for emergency diagnosis of severe CAP |
|
SMART‐COP Score 67 |
Sum of scores for all the following risk factors: SBP < 90 mm Hg (+2 pts); chest X‐ray showing bilateral pulmonary involvement (+1 pt); serum albumin < 35 g/L (+1 pt); RR ≥ 30 bpm (> 50 yo) or ≥ 25 bpm (≤ 50 yo) (+1 pt); heart rate ≥ 125 bpm (+1 pt); New onset of disturbance of consciousness (+1 pt); hypoxemia (+2 pts): PaO2 < 70 mm Hg, or fingertip O2 saturation ≤ 93%, or oxygenation index < 333 mm Hg (≤ 50 yo); PaO2 < 60 mm Hg, or fingertip O2 saturation ≤ 90%, or oxygenation index < 250 mm Hg (>50 yo); arterial blood pH < 7.35 (+2 pts). |
0–2: low risk 3–4: moderate risk 5–6: high risk 7–8: extremely high risk |
A score > 3 indicates the possibility that the patient needs respiratory monitoring or circulatory support therapy |
4.2. Criteria for hospital admission of CAP patients
CURB‐65 score is recommended as a standard for deciding whether a patient should be hospitalized or not. A score of 0–1 point: theoretically, patients should receive outpatient treatment; a score of 2 points: patients are recommended to receive inpatient treatment or extramural treatment with close follow‐up; a score of 3–5 points: patients should be hospitalized (I A).
However, other factors such as patient age, underlying diseases, socioeconomic status, gastrointestinal functions and treatment compliance should also be taken into account for comprehensive evaluation62 (II B).
4.3. Diagnostic criteria for severe CAP
Criteria for diagnosis of severe CAP63: patients who meet any of the major criteria or ≥ 3 minor criteria could be diagnosed as severe pneumonia and need close monitoring and active treatment; it is also recommended that the patients should be hospitalized in ICU if applicable (II A).
4.3.1. Major criteria
Requiring tracheal intubation and mechanical ventilation;
Septic shock, and still in need of vasoactive drugs after active fluid resuscitation.
4.3.2. Minor criteria
Respiratory rate (RR) ≥30 bpm;
Oxygenation index ≤ 250 mm Hg (1 mm Hg = 0.133 kPa);
Infiltrates in multiple lung lobes;
Disturbance of consciousness and (or) disorientation;
Blood urea nitrogen (BUN) ≥ 7.14 mmol/L;
Systolic blood pressure (SBP) < 90 mm Hg, requiring active fluid resuscitation.
5. SECTION 3. ETIOLOGICAL DIAGNOSIS OF CAP
5.1. Selection of method for etiological diagnosis of CAP
Unless there is a clustered outbreak of pneumonia or the clinical response to initial empirical treatment is inadequate, etiological tests are generally not required for outpatients with mild CAP1, 2, 68, 69, 70 (III B).
Hospitalized CAP patients (including patients who require monitoring at the emergency room) usually require etiological testing. The selection of etiological tests should be based on multiple factors, including patient age, underlying diseases, immune status, clinical characteristics, severity of disease and prior anti‐infective treatment. Appropriate etiological testing is especially important when antimicrobial adjustment is necessary due to insufficient efficacy of empirical anti‐infective treatment1, 2, 68 (I A).
See Table 4 for the recommended etiological tests of CAP under specific clinical situations.
-
Invasive etiological sampling is only selectively applicable for the following patients:
Patients with pneumonia and concomitant pleural effusion, especially when pleural effusion is on the same side of the infected pulmonary lesion; etiological testing could be performed with pleural effusion samples collected via thoracentesis.
Patients receiving mechanical ventilation: etiological testing could be performed with lower respiratory tract samples obtained via bronchoscopy, including endotracheal aspiration (ETA), bronchoalveolar lavage fluid (BALF) and protected specimen brush (PSB).
Patients who have inadequate response to empirical treatment and are suspected to be infected with unusual pathogens: when the cause of disease cannot be determined with respiratory tract samples obtained with regular methods, etiological testing could be performed with lower respiratory tract samples obtained via bronchoscopy (including ETA, BALF and PSB) or histological samples obtained via percutaneous needle lung biopsy.
Patients without improvement after active anti‐infective therapies, who require differential diagnosis with non‐infectious pulmonary lesions (such as tumour, vasculitis and interstitial lung disease) (III B).
Table 4.
Recommended etiological tests for CAP under specific clinical situations
| Clinical conditions | Sputum smear and culture a | Blood culture b | Pleural effusion culture | Mycoplasma/Chlamydia/Legionella screening c | Respiratory tract virus screening d | LP1 urinary antigen e | SP urinary antigen f | Fungal antigen | Tuberculosis screen g |
|---|---|---|---|---|---|---|---|---|---|
| Clustered outbreak 2 | √ | √ | √ | ||||||
| Inadequate response to initial empirical therapy 1 | √ | √ | √ | √ | √ | √ | |||
| Severe CAP 1, 2, 68 | √ | √ | √ | √ | √ | √ | |||
| Unusual radiographic manifestations 1, 2, 52, 71, 72, 73, 74, 75, 76 | |||||||||
| 1. Necrotizing pneumonia or concomitant cavity | √ i | √ | √ | √ | |||||
| 2. Concomitant pleural effusion | √ | √ | √ | √ | √ | √ | √ | ||
| 3. Lesions in multiple lobes of both lungs | √ | √ | √ | √ | √ | √ | |||
| Underlying disease | |||||||||
| 1. Concomitant chronic obstructive pulmonary disease 1, 2, 24, 25, 77 | √ | ||||||||
| 2. Concomitant structural lung disease 1, 25 | √ | √ | |||||||
| 3. Immunodeficiency h , 78, 79, 80 | √ | √ | √ | √ | √ | √ | √ | √ | |
| History of travel within 2 weeks before onset of disease j , 1, 2 | √ | ||||||||
aOther than sputum, acceptable samples also include lower respiratory tract samples and histological biopsy samples such as ETA (endotracheal aspiration), BALF (bronchoalveolar lavage fluid) and PSB (protected specimen brush).
bBlood culture should include aerobic and anaerobic bacterial cultures.
c Mycoplasma, Chlamydia and Legionella screen items are nucleic acid and serum specific antibodies.
dScreening tests are for nucleic acid, antigens, or serum specific antibodies of respiratory tract viruses.
eLP1: Legionella pneumophila serogroup 1.
fSP, Streptococcus pneumoniae.
gTuberculosis screening prefers sputum smear for the test of acid‐fast bacteria. Mycobacteria culture and nucleic acid detection should be performed if applicable.
hFor immunodeficient patients, in addition to the relatively comprehensive etiological tests listed in this table, patients should also be screened for opportunistic pathogens, such as Pneumocystis jiroveci pneumonia, cytomegalovirus and nontuberculous mycobacteria.
iSputum smears should be used to discover bacteria and fungi, while bacterial and fungal cultures should be conducted simultaneously.
jPatients with history of travel to special epidemic regions should also be screened for corresponding respiratory tract contagious diseases.
5.2. Primary testing methods for CAP pathogens and diagnostic criteria
See Table 5 for the primary testing methods for CAP pathogens and their corresponding diagnostic criteria.
Table 5.
Primary testing methods for CAP pathogens and implications for diagnosis
| Pathogen | Testing method | Samples used | Implication for diagnosis | Description |
|---|---|---|---|---|
| Aerobic bacteria and facultative anaerobic bacteria |
Direct smear microscopy (Gram staining) |
Sputum, ETA, BALF and PSB samples; blood, pleural effusion and bronchial mucosa biopsy samples; lung biopsy samples |
1. Test results that can be used as evidence for etiological diagnosis: (1) The pathogen is found in cultures of blood or other sterile samples (such as pleural effusion, lung biopsy samples, etc.)81; (2) Francisella tularensis, Yersinia pestis or Bacillus anthracis isolated from qualified lower respiratory tract samples; (3) Positive result for S. pneumoniae urinary antigen test (ICT) (except for children)81, 82, 83 2. Test results that are important reference for etiological diagnosis: (1) Significant growth of dominant bacteria (≥ +++) in qualified lower respiratory tract samples (except for normal colonization flora); (2) Small amount of bacterial growth in qualified lower respiratory tract samples, but results are consistent with smear microscopy results (S. pneumoniae, H. influenzae, or M. catarrhalis); (3) Apparent bacterial phagocytosis by neutrophils could be seen in smear microscopy of qualified lower respiratory tract samples Test results that can be used as evidence for etiological diagnosis: The pathogen is found in cultures of blood or other sterile samples (such as pleural effusion, lung biopsy samples, etc.) |
For qualified lower respiratory tract samples, sputum samples must meet the following conditions: squamous cells < 10 per low‐power field; polymorphonuclear leukocytes >25 per low‐power field, or the ratio between the two is <1:2.584 |
| Regular culture | ||||
| S. pneumoniae urinary antigen (ICT) | Fresh urine | |||
| Anaerobic bacteria |
Direct smear microscopy (Gram staining) |
Blood, pleural effusion | ||
| Anaerobial cultures | ||||
| Mycobacteria | Smear microscopy (microscopy with Ziehl‐Neelsen staining, fluorescence microscopy) | Sputum, ETA, BALF and PSB samples; blood, pleural effusion, bronchial mucosa biopsy samples; lung biopsy samples |
1. Test results that can be used as evidence for etiological diagnosis: (1) Acid‐fast bacilli discovered in smear microscopy, but cannot differentiate between tuberculosis mycobacteria or non‐tuberculosis mycobacteria85, 86; (2) A positive result for acid‐fast bacillus culture, and can differentiate between tuberculosis mycobacteria or non‐tuberculosis mycobacteria 2. Test results that are important references for etiological diagnosis: A positive result for mycobacteria nucleic acid detection, and can differentiate between tuberculosis mycobacteria or non‐tuberculosis mycobacteria85, 87 |
1. Fluorescent smear microscopy is more sensitive than Ziehl‐Neelsen staining85, 86 2. The sensitivity of mycobacteria culture is superior to that of smear microscopy; in vitro susceptibility testing can be performed, but it is more time‐consuming and complex, and has a higher biological safety requirement for laboratories85, 86 3. Xpert MTB/RIF is the method recommended by WHO for testing mycobacteria. It can provide information on rifampin resistance simultaneously85, 87. Currently, the commercial kit has been approved by the CFDA 4. A positive result for IGRAs indicates that the host has been sensitized by tuberculosis mycobacteria antigens; a positive result for TST indicates previous infection of tuberculosis, which is not recommended for diagnosis of active tuberculosis according to the WHO85, 88, 89 |
| Mycobacterial culture | ||||
| Nucleic acid detection (simultaneous mycobacteria culture is recommended) | ||||
| IGRA | Whole blood samples | |||
| TST | ||||
| Legionella | Serum specific antibody assay (IFA, ELISA) | Two sets of serum samples from acute phase and recovery phase |
1. Test results that can be used as evidence for etiological diagnosis: (1) Legionella is isolated from cultures of qualified lower respiratory tract samples, pleural effusion, bronchial mucosa biopsy samples, or lung biopsy samples81, 90, 91, 92, 93, 94; (2) A positive result for L. pneumophila serotype I urinary antigen assay (ICT)90, 91, 92, 93, 94; (3) Serum L. pneumophila Type I‐specific antibody titer (IFA or ELISA) shows a 4‐fold or higher change between the two sets of serum samples from acute phase and recovery phase90, 91, 92, 93, 94 2. Test results that are important reference for etiological diagnosis: (1) Serum L. pneumophila serotype I‐specific antibody titer in a single sample reaches the criteria for a positive result90, 91, 92, 93, 94; (2) Serum specific antibody titer of other serum types of Legionella or other Legionella strains besides L. pneumophila serotype I showing 4‐fold or higher increase90, 91, 92, 93, 94; (3) A positive result for L. pneumophila antigen assay in qualified lower respiratory tract samples, pleural effusion, bronchial mucosa biopsy samples, or lung biopsy samples (DFA)90, 91, 92, 93, 94; (4) A positive result for Legionella nucleic acid assay in qualified lower respiratory tract samples, pleural effusion, bronchial mucosa biopsy samples, or lung biopsy samples (DFA)90, 91, 92, 93, 94, 95 |
1. A positive result for Legionella culture is the gold standard for diagnosis of Legionella infection. However, the positive rate is low, and prior use of anti‐infective drugs can cause a false positive result90; BALF and lung biopsy samples can increase the positive rate 2. L. pneumophila serotype I urinary antigen assay can be used for rapid diagnosis at early stage, and the results are not affected by prior anti‐infective therapies 90, 96 3. Although Legionella antigen assay in qualified lower respiratory tract samples can be fast and convenient, and can provide differentiation between strains and subtypes, its sensitivity and specificity are not satisfying90, 91, 92, 93, 94 4. Legionella nucleic acid assay can be used for early‐stage diagnosis of Legionella pneumonia; it is highly sensitive, and can detect the subtypes of L. pneumophila.90 However, this test has not been accepted in the United States and Europe as a criteria for definite diagnosis.91, 92, 93, 94 |
| Legionella pneumophila Type I urinary antigen assay (ICT) | Urine | |||
| Nucleic acid assay | Sputum, ETA, BALF and PSB samples; blood, pleural effusion, bronchial mucosa biopsy samples; lung biopsy samples | |||
| Isolation and culture (BCYE nutrient culture medium, GVPC and MWY screening culture medium) | ||||
| Antigen assay in lower respiratory tract samples (DFA) | ||||
| Mycoplasma pneumoniae | Serum specific antibody assays (CF, PA, MAG, EIA, IFA) | Two sets of serum samples from acute phase and recovery phase |
1. Test results that can be used as evidence for etiological diagnosis: (1) M. pneumoniae is isolated from cultures of oropharyngeal or nasopharyngeal swabs, qualified lower respiratory tract samples, pleural effusion, bronchial mucosa biopsy samples, or lung biopsy samples81, 97; (2) Serum M. pneumoniae ‐ specific antibody titer shows a 4‐fold or higher change between the two sets of serum samples from acute phase and recovery phase81, 97 2. Test results that are important reference for etiological diagnosis: (1) A positive result for M. pneumoniae nucleic acid detection in oropharyngeal or nasopharyngeal swabs, qualified lower respiratory tract samples, pleural effusion, bronchial mucosa biopsy samples, or lung biopsy samples81, 95; (2) A positive result for M. pneumoniae‐specific IgM antibody in a single set of serum sample97 |
1. A positive result for M. pneumoniae culture can be used to establish definite diagnosis, but the test is time‐consuming, and the positive rate is relatively low98 2. Serum specific antibody titer obtained via CF or PA methods is largely affected by IgG, so its value for early‐stage diagnosis is limited. MAG, EIA and IFA methods can detect serum specific IgM or IgG. Serum specific IgM appears earlier, but acute infection could not be excluded by a negative result. A quadruple or higher increase in specific antibodies across two sets of serum samples is relevant for retrospective diagnosis98 3. M. pneumoniae nucleic acid assay has been approved for clinical use as an important tool for rapid early‐stage diagnosis81, 95 |
| Nucleic acid assay | Oropharyngeal swabs; nasopharyngeal swabs; sputum, ETA, BALF and PSB samples; blood, pleural effusion, bronchial mucosa biopsy samples; lung biopsy samples | |||
| Culture (special medium) | ||||
| Chlamydia pneumoniae | Serum specific antibody detection (MIF) | Two sets of serum samples from acute phase and recovery phase |
1. Test results that can be used as evidence for etiological diagnosis: (1) C. pneumoniae is isolated from cultures of oropharyngeal or nasopharyngeal swabs, qualified lower respiratory tract samples, pleural effusion, bronchial mucosa biopsy samples, or lung biopsy samples81, 99, 100; (2) Serum C. pneumoniae‐specific IgG antibody titer (MIF method) shows a 4‐fold or higher change between the two sets of serum samples from acute phase and recovery phase99, 100; (3) Serum C. pneumoniae‐specific IgM (MIF method) ≥ 1:1699, 100 2. Test results that are important reference for etiological diagnosis: (1) A positive result for C. pneumoniae nucleic acid assay in oropharyngeal or nasopharyngeal swabs, qualified lower respiratory tract samples, pleural effusion, bronchial mucosa biopsy samples, or lung biopsy samples.81, 95 (2) Serum C. pneumoniae‐specific IgG titer (MIF) in a single set of serum samples ≥ 1:512.99, 100 |
1. C. pneumoniae is an obligate intracellular pathogen, which can only be isolated in vitro via cell culture with complex technique. The method is generally not recommended for clinical diagnosis99, 100 2. Serum specific antibody assay has limited value for early‐stage diagnosis; an increase in specific IgM, or a quadruple or higher increase in IgG titer across two sets of serum samples is relevant for retrospective diagnostic99, 100 3. C. pneumoniae nucleic acid assay has been approved for clinical use; a positive result has important value for rapid early‐stage diagnosis81, 95 |
| Nucleic acid detection | Oropharyngeal swabs; nasopharyngeal swabs; sputum, ETA, BALF and PSB samples; blood, pleural effusion, bronchial mucosa biopsy samples; lung biopsy samples | |||
| Culture (cell culture) | ||||
| Coxiella burnetii | Nucleic acid assay | Pharyngeal swabs; nasal swabs; sputum, ETA, BALF and PSB samples |
1. Test results that can be used as evidence for etiological diagnosis: (1) C. burnetii is isolated from cultures of oropharyngeal or nasopharyngeal swabs or qualified lower respiratory tract samples94; (2) A positive result for C. burnetii nucleic acid assay in oropharyngeal or nasopharyngeal swabs or qualified lower respiratory tract samples91, 94; (3) C. burnetii is found in immunohistochemical staining of lung biopsy samples, with relevant inflammatory reactions91, 94; (4) Serum C. burnetii Phase II‐specific IgG antibody titer (MIF method) shows a 4‐fold or higher change between the two sets of serum samples from acute phase and recovery phase.91, 94 2. Test results that are important reference for etiological diagnosis: (1) Serum C. burnetii Phase II‐specific IgG (MIF) in a single set of serum samples ≥ 1:128 (IFA), or results by ELISA, dot‐ELISA, MAT, or CF shows an increase in serum C. burnetii Phase II‐specific antibodies (IgG, IgM or complement‐fixing antibody) titer in a single set of serum samples.91, 94 |
1. A definite diagnosis of Q fever pneumonia can be established if C. burnetii is isolated from cultures of qualified lower respiratory tract samples or if C. burnetii is found in immunohistochemical staining of lung biopsy samples,91, 94 but the sensitivity of the tests is relatively low. 2. A positive result for C. burnetii nucleic acid assay in oropharyngeal or nasopharyngeal swabs or qualified lower respiratory tract samples has been listed as evidence for definite diagnosis of Q fever pneumonia by the United States and Europe. The test is an important tool for rapid early‐stage diagnosis.91, 94 3. Serum C. burnetii Phase II‐specific IgM antibody assay is helpful for early‐stage diagnosis.91, 94 |
| Serum specific antibody assays (CF, MAT, IFA, ELISA) | Two sets of serum samples from acute phase and recovery phase | |||
| Histopathological examination | Lung biopsy samples | |||
| Virus | Nucleic acid assay | Respiratory tract samples such as oropharyngeal swabs, nasopharyngeal swabs, nasopharyngeal aspirate, airway aspirate and sputum |
1. Test results that can be used as evidence for etiological diagnosis: (1) A positive result for nucleic acid assay of influenza virus, parainfluenza virus Types 1–4, RSV, adenovirus, coronavirus, hMPV and so on in oropharyngeal or nasopharyngeal swabs, qualified lower respiratory tract samples, or lung tissue samples81, 95, 101, 102; (2) Serum specific IgG antibody titer of a respiratory tract virus such as influenza virus or RSV shows a 4‐fold or higher change between the two sets of serum samples from acute phase and recovery phase101, 102, 103; (3) A positive result for rapid antigen assay of influenza virus (DFA, colloidal gold method) in oropharyngeal or nasopharyngeal swabs or qualified lower respiratory tract samples, with supporting relevant epidemiological history101, 102; (4) A positive result for rapid antigen assay of parainfluenza virus Types 1–4, RSV, adenovirus, coronavirus, or hMPV (DFA) in oropharyngeal or nasopharyngeal swabs or qualified lower respiratory tract samples81; (5) A respiratory tract virus such as influenza virus or RSV is isolated from qualified lower respiratory tract samples 2. Test results that are important reference for etiological diagnosis: A positive result for specific IgM of respiratory tract viruses such as influenza virus or RSV.101, 102, 103 |
1. A positive result for viral isolation and culture is the gold standard for diagnosis of respiratory tract viral infection. It has important value for the discovery and diagnosis of pathogens of respiratory contagious disease with new or sudden onset. However, the test is relatively time‐consuming, and requires better laboratory conditions, so it is not a regular test for clinical setting.81, 101, 102 2. The sensitivity and specificity of real‐time PCR/rRT‐PCR (real‐time reverse transcriptase PCR) are relatively high. It is a preferred method for rapid diagnosis of respiratory tract infection with influenza virus, avian influenza virus and so on.81, 95, 101, 102 3. Viral antigen assay in qualified lower respiratory tract samples can be used as an initial screening method for rapid early‐stage diagnosis. It is less sensitive than nucleic acid assay. Patient's epidemiological history and clinical symptoms should be taken into account when interpreting the results. Nucleic acid assay or viral isolation and culture can be performed for further validation if necessary101, 102 4. Serum specific viral antibody assay is the main method for retrospective diagnosis101, 102 |
| Viral antigen assay (DFA, colloidal gold method) | ||||
| Serum specific antibody assays (IFA, ELISA, CF, haemagglutination inhibition assay) | Two sets of serum samples from acute phase and recovery phase | |||
| Viral isolation and culture | Fresh respiratory tract samples such as oropharyngeal swabs, nasopharyngeal swabs, nasopharyngeal aspirate, airway aspirate and sputum | |||
| Fungus | Smear microscopy (Gram staining, microscopy with KOH as floating fluid, Giemsa staining, GMS staining, mucicarmine staining) | Sputum, ETA, BALF and PSB samples; bronchial mucosa biopsy samples or lung biopsy samples |
1. Test results that can be used as evidence for etiological diagnosis: (1) Fungus found in cultures of blood or other sterile samples (such as pleural effusion, lung biopsy tissue samples, etc.) (note that samples with positive result of Aspergillus in blood culture due to contamination should be excluded) 81, 104; (2) Cryptococcus, mycelial fungus, or human Pneumocystis found in immunohistochemical staining of lung tissue samples, with corresponding inflammatory reactions91, 94, 104; (3) Cryptococcus or human Pneumocystis found in smear microscopy of qualified lower respiratory tract samples 81, 105; (4) Cryptococcus neoformans isolated from culture of qualified lower respiratory tract samples105, 106; (5) A positive result for serum cryptococcal capsular polysaccharide antigen107 2. Test results that are important reference for etiological diagnosis: (1) A positive result for serum or BALF galactomannan antigen; (2) A positive result for 1–3‐β‐D glucan antigen, with exclusion of factors that can potentially cause a false positive result |
1. Besides regular Gram stain microscopy, mucicarmine staining can also be used for detection of Cryptococcus. GMS staining can be used for detection of human Pneumocystis. Microscopy with KOH as floating fluid can be used to detect hypha and spores of fungi, but the strain of fungi cannot be differentiated 2. A positive result for the culture of a sample from a usually sterile site using an aseptic technique is the gold standard of diagnosis; for non‐sterile samples, the possibility of colonization or pollution should be carefully excluded 3. Serum 1–3‐β‐D glucan antigen assay has some value for the diagnosis of invasive fungal infection, except for Cryptococcus and Zygomycetes 105, 107, 108; serum or BALF galactomannan antigen assay has important value for the diagnosis of invasive aspergillosis.105, 107, 109 4. There is possibility of false negative for serum cryptococcal capsular polysaccharide antigen assay in patients with non‐disseminated cryptococcosis. The studies currently available do not support the test to be used for efficacy evaluation and prognosis prediction107 5. Although a positive result for cryptococcal capsular polysaccharide antigen in cerebrospinal fluid is not direct evidence for diagnosis of pulmonary cryptococcosis, physicians should be alert to the possibility of concomitant cryptococcosis for patients with positive results of cryptococcal capsular polysaccharide antigen in cerebrospinal fluid |
| Fungal culture | Sputum, ETA, BALF and PSB samples; pleural effusion, bronchial mucosa biopsy samples; lung biopsy samples, blood | |||
| 1–3‐β‐D glucan antigen | Serum | |||
| Galactomannan antigen | Serum, BALF | |||
| Cryptococcal capsular polysaccharide antigen (latex agglutination method, EIA) | Serum, cerebrospinal fluid | |||
| Histopathological examination | Lung biopsy samples | |||
| Parasite | Smear or tissue smear microscopy | Sputum or other lower respiratory tract samples, pleural effusion, lung tissue biopsy samples |
1. Test results that can be used as evidence for etiological diagnosis: (1) Parasite body, eggs, trophozoite, cysts, or oocysts found in smear microscopy of qualified respiratory tract samples81, 110; (2) Parasite eggs, body, trophozoite, cysts, or oocysts found in immunohistochemical staining of lung tissue samples81, 110; (3) A positive result for nucleic acid assay of Toxoplasma gondii in blood, cerebrospinal fluid, or qualified respiratory tract samples or lung tissue samples81, 111; a positive result for nucleic acid assay of Enterocytozoon bieneusi, Cryptosporidium and so on in blood, cerebrospinal fluid, or qualified respiratory tract samples or lung tissue samples81; (4) A positive result for circulating parasitic antigen in blood or other body fluids110 2. Test results that are important reference for etiological diagnosis: (1) A positive result for intradermal test with parasitic antigens110; (2) A positive result for corresponding serum specific antibodies of a parasite (IgG, IgM or IgA) 81, 110, 111 |
1. Eggs of Paragonimus and trophozoite of amebic protozoa could be detected in direct smear microscopy. Trophozoite or cysts of Toxoplasma gondii could be detected by Giemsa staining, and oocysts of Cryptosporidium by modified acid‐fast staining; Enterocytozoon bieneusi by modified three‐color staining81 2. If an opportunistic parasitic infection such as toxoplasmosis is suspected in an immunodeficient patient, nucleic acid assay can be selected as a primary testing method to obtain rapid early‐stage diagnosis112, 113, 114 3. For immunocompetent patients, serum specific antibody assay is the most commonly used initial screening test for parasitic infections. However, since serum specific antibodies continue to exist for a long time after onset of parasitic infections, a positive result for an intradermal test with parasitic antigens or a positive result for serum specific antibodies (IgG, IgM or IgA) does not necessarily indicate acute infection110, 111 |
| Histopathological examination | Lung tissue biopsy samples | |||
| Nucleic acid assay | Blood, cerebrospinal fluid, BALF, bronchial mucosa or lung biopsy samples | |||
| Serum specific antibody assays (DT, ELISA, IFA, HA, IHA, ISAGA, Western blot) | Serum | |||
| Antigen assays (ELISA, ICT) | Blood, cerebrospinal fluid, pleural effusion and so on. |
Abbreviations: BALF, bronchoalveolar lavage fluid; BCYE, buffered charcoal‐yeast extract; CF, complement fixation test; CFDA, China Food and Drug Administration; DFA, direct fluorescent antibody test; DT, Sabin‐Feldman dye test; ELISA, enzyme‐linked immunosorbent assay; EIA, enzyme immunoassay; ETA, endotracheal aspirate; Giemsa, Giemsa staining; GMS, Gomori Methenamine Silver; GVPC, glycine‐vancomycin‐polymyxin‐cycloheximide; HA, haemagglutination assay; hMPV: human Metapneumovirus; ICT, immunochromatographic test; IFA, indirect immunofluorescence assay; IGRA, interferon‐gamma release assay; IHA, indirect haemagglutination test; ISAGA, immunosorbent agglutination assay; KOH, potassium hydroxide; MAG, microparticle agglutination; MAT, micro agglutination test; MIF, microimmunofluorescence assay; MWY, modified Wadowsky Yee agar; PA, particle agglutination test; PSB, protected specimen brush; RSV, respiratory syncytial virus; TST, tuberculin skin test; WHO, World Health Organization.
6. SECTION 4. ANTI‐INFECTIVE THERAPIES FOR CAP
6.1. Empirical anti‐infective therapies for CAP
After clinical diagnosis of CAP is established, and etiological test and sampling arranged appropriately, the most potential pathogens should be assessed in terms of patient age, underlying disease, clinical characteristics, results of laboratory and radiography tests, severity of disease, hepatic and renal functions, and history of medication and antimicrobial susceptibility profile, then evaluate the risk for antibiotic resistance, select the appropriate anti‐infective agent (s) and dosing regimen (Table 6). The initial empirical antibacterial therapy should be administered promptly. It is important to note that the epidemiological distribution and antimicrobial resistance profile of pathogens may be different in different regions of China. The anti‐infective drugs listed in Table 6 are optional for initial empirical therapy. The treatment recommendations are only theoretical. The selection of therapies for specific patients must be based on the actual situation in local healthcare facilities.
Table 6.
Selection of anti‐infective agents for initial empirical therapy
| Populations | Common pathogens | Anti‐infective agents for initial empirical therapy | Comment |
|---|---|---|---|
| Outpatient treatment (Oral administration is recommended) | |||
| Young adults without underlying disease(s) | S. pneumoniae, M. pneumoniae, H. influenzae, C. pneumoniae, influenza virus, adenovirus, M. catarrhalis | (1) Aminopenicillins, penicillins‐β‐lactamase ‐inhibitor combinations; (2) I or II generation cephalosporins; (3) doxycycline or minocycline; (4) respiratory quinolones; (5) macrolides | (1) Differentiate among bacterial pneumonia, Mycoplasma, Chlamydia and viral pneumonia based on clinical characteristics; (2) Mild pneumonia caused by Mycoplasma, Chlamydia and virus is usually self‐limited |
| Patients with underlying disease(s) or elderly patients (age ≥ 65 years) | S. pneumoniae, H. influenzae, Enterobacteriaceae such as K. pneumoniae, C. pneumoniae, influenza virus, RSV, M. catarrhalis | (1) Penicillins‐β‐lactamase‐inhibitor combinations; (2) II or III generation cephalosporins (oral); (3) respiratory quinolones; (4) penicillins‐lactamase ‐inhibitor combinations, II generation cephalosporins, III generation cephalosporins combined with doxycycline or minocycline or macrolides | Monotherapy with doxycycline or minocycline or macrolides is not recommended in patients with risk factors of resistant S. pneumoniae (1), such as age > 65 years, underlying diseases (chronic cardiac, pulmonary, or renal diseases, diabetes mellitus and immunosuppression), alcoholism and β‐lactams treatment within 3 months. |
| Inpatient treatment, non‐ICU (Intravenous or oral administration) | |||
| Young adults without underlying disease(s) | S. pneumoniae, H. influenzae, M. catarrhalis, S. aureus, M. pneumoniae, C. pneumoniae, influenza virus, adenovirus, other respiratory tract viruses | (1) Penicillin G, aminopenicillins, penicillins‐β‐lactamase‐inhibitor combinations; (2) II or III generation cephalosporins, cephamycins, oxacephems; (3) the above drugs combined with doxycycline, minocycline or macrolides; (4) respiratory quinolones; (5) macrolides | (1) Only 1.9% the S. pneumoniae isolates from adult CAP are resistant to intravenous penicillins in China. The percentage of intermediate strains is only about 9%. Intravenous penicillins are still effective in hospitalized patients infected with penicillin‐intermediate S. pneumoniae when increasing the dosage23, 160; (2) When atypical pathogens are suspected, doxycycline or minocycline or respiratory quinolones are preferred. Macrolides can be used in regions with lower resistance rate to mycoplasma |
| Patients with underlying disease(s) or elderly patients (age ≥ 65 years) | S. pneumoniae, H. influenzae, Enterobacteriaceae such as K. pneumoniae, influenza virus, RSV, M. catarrhalis, anaerobic bacteria, Legionella | (1) Penicillins‐β‐lactamase‐inhibitor combinations; (2) III generation cephalosporins or their enzyme‐inhibitor combinations, carbapenems such as cephamycins, oxacephems, ertapenem; (3) monotherapy of the above drugs or in combination with macrolides; (4) respiratory quinolones | (1) Enterobacteriaceae infection must be considered in patients with underlying disease(s) and elderly patients. The patients must be further evaluated for the risk of infection with ESBLs‐producing Enterobacteriaceae; (2) Elderly patients should be monitored for the risk factors of aspiration |
| Requirement for ICU admission (Intravenous administration is recommended) | |||
| Young adults without underlying disease(s) | S. pneumoniae, S. aureus, influenza virus, adenovirus, Legionella | (1) Penicillins‐β‐lactamase‐inhibitor combinations, III generation cephalosporins, cephamycins, oxacephems, ertapenem combined with macrolides; (2) respiratory quinolones | (1) S. pneumoniae is the most common pathogen. The other pathogens such as S. aureus, Legionella, influenza virus should also be considered 1, 2, 161, 162, 163, 164, 165; (2) During influenza seasons, attention must be paid to influenza viral infections. Combination with neuraminidase inhibitors should be considered. Attention should be paid to secondary S. aureus infection.166 The agents active against MRSA can be used in combination if necessary |
| Patients with underlying disease(s) or elderly patients (age ≥ 65 years) | S. pneumoniae, Legionella, Enterobacteriaceae such as K. pneumoniae, S. aureus, anaerobic bacteria, influenza virus, RSV | (1) Penicillins‐β‐lactamase‐inhibitor combinations, III generation cephalosporins or in combination with beta‐lactamase inhibitors, carbapenems such as ertapenem combined with macrolides; (2) penicillins‐β‐lactamase‐inhibitor combinations, III generation cephalosporins or in combination with beta‐lactamase inhibitors, carbapenems such as ertapenem combined with respiratory quinolones | (1) Evaluate the risk of infection with ESBLs‐producing Enterobacteriaceae; (2) Physicians should be aware of the risk factors for aspiration and antimicrobial coverage of relevant pathogens |
| CAP with risk factors for P. aeruginosa infection and requirement for inpatient treatment or ICU admission (Intravenous administration is recommended) | |||
| Patients with structural lung disease | P. aeruginosa, S. pneumoniae, Legionella, Enterobacteriaceae such as K. pneumoniae, S. aureus, anaerobic bacteria, influenza virus, RSV virus | (1) β‐lactams with antipseudomonal activity; (2) quinolones with antipseudomonal activity; (3) β‐lactams with antipseudomonal activity combined with quinolones or aminoglycosides with antipseudomonal activity; (4) combination of β‐lactams, aminoglycosides and quinolones with antipseudomonal activity | Risk factors include: (1) airway P. aeruginosa colonization; (2) repeated doses of antibacterial drugs or glucocorticoids due to chronic airway disease. Combination therapy is recommended for patients with severe CAP or proven antimicrobial resistance |
I generation cephalosporins: cefazolin, cefradine, cephalexin, cefathiamidine and so on. II generation cephalosporins: cefuroxime, cefamandole, cefotiam, cefaclor, cefprozil, and so on. III generation cephalosporins: intravenous: ceftriaxone, cefotaxime, ceftizoxime and so on; oral: cefdinir, cefixime, cefpodoxime proxetil, cefditoren pivoxil and so on. Respiratory quinolones: levofloxacin, moxifloxacin, gemifloxacin. Aminopenicillins: amoxicillin, ampicillin. Penicillins‐β‐lactamase‐inhibitor combinations (not including penicillins with antipseudomonal activity, such as piperacillin, ticarcillin): amoxicillin‐clavulanic acid, amoxicillin‐sulbactam, ampicillin‐sulbactam and so on. Macrolides: azithromycin, clarithromycin, erythromycin. Quinolones with antipseudomonal activity: ciprofloxacin, levofloxacin. Beta‐lactams with antipseudomonal activity: ceftazidime, cefepime, aztreonam, piperacillin, piperacillin‐tazobactam, ticarcillin, ticarcillin‐clavulanic acid, cefoperazone, cefoperazone‐sulbactam, imipenem‐cilastatin, meropenem, panipenem‐betamipron, biapenem. Cephamycins: cefoxitin, cefmetazole, cefotetan, cefminox. Oxacephems: moxalactam, flomoxef. Aminoglycosides: amikacin, gentamicin, etimicin, netilmicin, tobramycin and so on. Neuraminidase inhibitors: oseltamivir, zanamivir, peramivir. Drugs for treating MRSA pneumonia: vancomycin, linezolid, teicoplanin, norvancomycin, ceftaroline.
ESBL: extended spectrum β‐lactamase; MRSA: methicillin‐resistant Staphylococcus aureus; RSV: respiratory syncytial virus.
Additionally, the pharmacokinetic and pharmacodynamic properties of antibacterial agents must be taken into consideration. For time‐dependent antibacterial agents (such as penicillins, cephalosporins, monobactams and carbapenems), their bactericidal ability is almost saturated at 4–5 times of MIC,115 and T > MIC (time above MIC) is an important determinant of efficacy.116 Better clinical efficacy can be achieved by multiple doses per day based on half‐lives. Meanwhile, the bactericidal ability of concentration‐dependent antibacterial agents, such as aminoglycosides and quinolones, increases with drug concentration. The effect improves with higher peak drug concentration.116 Therefore, these drugs are usually administered once daily in order to increase drug activity and decrease the risk of drug resistance and kidney injury caused by aminoglycosides.
Recommendations of this guideline for empirical anti‐infective treatment of CAP are provided in the following.
The first dose of anti‐infective agent should be used as early as possible after diagnosis of CAP is established in order to improve efficacy and decrease mortality and hospital stay. However, it is important to note that a correct diagnosis is a prerequisite. Physicians should not ignore necessary differential diagnosis for the purpose of early diagnosis117, 118, 119, 120 (II B).
For mild CAP outpatients, oral anti‐infective agents with high bioavailability should be used when possible. Oral treatment with amoxicillin or amoxicillin‐clavulanic acid is recommended2, 121, 122 (I B). For young patients without underlying diseases, oral doxycycline or minocycline may be considered if suspected of mycoplasma or chlamydia infection1, 123 (III B). S. pneumoniae and M. pneumoniae are highly resistant to macrolides in China.28, 36 Empirical macrolides treatment can only be used in regions with lower resistance rates122 (II B). Respiratory quinolones can be used instead in regions with higher resistance rates to macrolides or in patients who are hypersensitive or intolerant to the drugs mentioned above121, 122, 124 (II B).
For CAP patients who require hospitalization, β‐lactams monotherapy or in combination with doxycycline, minocycline or macrolides and respiratory quinolones monotherapy are recommended2, 125, 126, 127 (II B). However, compared with combination therapies, respiratory quinolones monotherapy is associated with fewer adverse reactions,128 and no skin test is required.
For young adult patients with severe CAP and without underlying diseases who require admission to ICU, penicillins‐lactamase inhibitor combinations, third generation cephalosporins, ertapenem combined with macrolides or respiratory quinolones monotherapy are recommended.1, 2, 127, 129, 130, 131, 132 For the elderly patients or patients with underlying diseases, combination antimicrobial therapy is recommended133 (II B).
For CAP patients at risk of aspiration, the optimal selection should be drugs with anti‐anaerobic activity, such as ampicillin‐sulbactam, amoxicillin‐clavulanic acid, moxifloxacin, carbapenems and so on, or therapies in combination with metronidazole, clindamycin and so on 134, 135, 136, 137, 138, 139, 140, 141 (II A).
For hospitalized patients ≥ 65 years of age and with underlying diseases (eg, congestive heart failure, cardiovascular and cerebrovascular diseases, chronic respiratory system diseases, kidney failure, diabetes mellitus, etc.), the possibility of Enterobacteriaceae infection should be considered.24 Such patients should be further evaluated for the risk of infections with extended‐spectrum beta‐lactamases (ESBLs) ‐producing bacteria (eg, history of colonization or infection with ESBLs‐producing bacteria, prior use of third generation cephalosporins, history of repeated or long‐term hospitalization, indwelling implants, renal replacement therapies).142, 143, 144 Cephamycins,145, 146 piperacillin‐tazobactam, cefoperazone‐sulbactam or ertapenem can be used in empirical therapy for high‐risk patients 24, 147 (III B).
During influenza seasons, CAP patients with suspected influenza virus infection are recommended to receive regular influenza virus antigen test or nucleic acid assay. Proactive antiviral therapy with neuraminidase inhibitors should be administered simultaneously even 48 h after disease onset. It is not necessary to wait for the results of influenza pathogen tests148, 149, 150, 151, 152 (I A). During influenza seasons, physicians must be aware of the possibility of secondary bacterial infections, especially S. pneumoniae, S. aureus and H. influenzae, which are relatively common153, 154, 155 (II A).
Anti‐infective therapy can usually be terminated 2–3 days after fever is relieved and the primary respiratory tract symptoms are improved significantly. However, the duration of therapy should differ based on the severity of disease, treatment response, complications and pathogens. It is not necessary to use chest X‐ray or CT as an indication of termination of anti‐bacterial agents. Generally, the duration of therapy should be 5–7 days for patients with mild or moderate CAP, which could be reasonably prolonged for patients with severe CAP or with extra‐pulmonary complications. The duration of therapy can be prolonged to 10–14 days for patients with atypical pathogens and/or slow response to treatment. S. aureus, P. aeruginosa, Klebsiella and anaerobic bacteria may cause necrosis of lung tissues, therefore, the duration of therapy may be prolonged to 14–21 days1, 2, 122, 156, 157, 158, 159 (I B).
6.2. Targeted anti‐infective therapies for CAP
Once aetiology of CAP is determined, targeted therapies can be delivered according to the results of in vitro susceptibility testing. See Table 7 for common pathogens of CAP, common anti‐infective agents, as well as dosage and administration.
Table 7.
Common pathogens of CAP, common anti‐infective agents, as well as dosage and administration
| Pathogens | Preferred anti‐infective agents | Alternate anti‐infective agents | Comment |
|---|---|---|---|
| Streptococcus pneumoniae | |||
|
Penicillin MIC < 2 mg/L |
Penicillin G 1.6–2.4 million units, IV q4h‐q6h; ampicillin 4–8 g IV, divided into 2–4 doses; ampicillin‐sulbactam 1.5–3 g IV q6h; amoxicillin‐clavulanic acid 1.2 g IV q8h‐q12h; cefazolin 0.5–1 g IV q6h‐q8h; cefradine 0.5–1 g IV q6h; cefuroxime 0.75–1.5 g IVq8h; moxalactam 1–2 g IV q8h; cephamycins a | Ceftriaxone; cefotaxime; clindamycin; doxycycline; quinolones b ; azithromycin; clarithromycin | |
|
Penicillin MIC ≥ 2 mg/L |
Cefotaxime 1–2 g IV q6h‐q8h; ceftriaxone 1–2 g IV q24h; levofloxacin 0.5–0.75 g IV once daily; moxifloxacin 0.4 g IV once daily; gemifloxacin 0.32 g oral, once daily | High‐dose ampicillin (2 g IV q6h); vancomycin; norvancomycin; linezolid; ceftaroline | |
| Haemophilus influenzae | |||
| Non‐β‐lactamase‐producing | Ampicillin 4–8 g/d IV, divided into 2–4 doses; ampicillin‐sulbactam 1.5–3 g IV q6h; amoxicillin‐clavulanic acid 1.2 g IV q8h‐q12h; cefuroxime 0.75–1.5 g IV q8h; moxalactam 1–2 g IV q8h; cephamycins a | Quinolones b ; doxycycline; azithromycin; clarithromycin; ceftriaxone; cefotaxime; TMP‐SMX | |
| β‐lactamase‐producing | Amoxicillin‐clavulanic acid 1.2 g IV q6h or q8h; ampicillin‐sulbactam 1.5–3 g IV q6h; cefuroxime 0.75–1.5 g IV q8h; cefotaxime 1–2 g IV q6h‐q8h; ceftriaxone 1–2 g IV q24h | Quinolones b ; azithromycin; aminoglycosides c | 25%‐35% of strains are β‐lactamase positive, and highly resistant to TMP‐SMX and doxycycline. |
| Moraxella catarrhalis | Amoxicillin‐clavulanic acid 1.2 g IV q8h‐q12h; ampicillin‐sulbactam 1.5–3 g IV q6h; cefuroxime 0.75–1.5 g IV q8h; cephamycins a ; moxalactam 1–2 g IV q8h | Ceftriaxone; cefotaxime; quinolones b ; azithromycin; clarithromycin; doxycycline; minocycline; TMP‐SMX | |
| Staphylococcus aureus | |||
| Methicillin‐susceptible | Oxacillin 1–2 g IV q4h; cloxacillin 2–4 g/d IV, divided into 2–4 doses; ampicillin 4–8 g/d IV, divided into 2–4 doses; amoxicillin‐clavulanic acid 1.2 g IV q8h‐q12h; ampicillin‐sulbactam 1.5–3 g IV q6h; cefazolin 0.5–1 g IV q6h‐q8h; cefradine 1–2 g IV q6h or q8h; cefuroxime 0.75–1.5 g IV q8h; moxalactam 1–2 g IV q8h; cephamycins a | Clindamycin; azithromycin; erythromycin; clarithromycin; doxycycline; minocycline; cefotaxime; ceftriaxone; cefepime; levofloxacin; gemifloxacin; moxifloxacin | The target trough blood concentration of vancomycin is 15–20 mg/L. Some authors recommend a loading dose of 25–30 mg/kg. Two randomized trials showed that the efficacy of linezolid was equivalent to that of vancomycin, and subgroup analysis showed that MRSA patients who showed improvement had a higher survival rate in linezolid group compared with vancomycin group. Vancomycin and linezolid should not be used together due to antagonistic effect. |
| Methicillin‐resistant | Vancomycin 1 g IV q12h or 0.5 g q6h; linezolid 600 mg IV q12h | Norvancomycin; teicoplanin; ceftaroline; tigecycline; rifampin; fosfomycin; TMP‐SMX (used in combination, not suitable for monotherapy) | If MIC of vancomycin is ≥ 2 mg/L, an alternative regimen should be used. |
| Pseudomonas aeruginosa | β‐lactams with anti‐Pseudomonas aeruginosa effect c ± ciprofloxacin 400 mg IV q8h‐q12h or ± levofloxacin 750 mg IV once daily or aminoglycosides c | Aminoglycosides d + ciprofloxacin or levofloxacin. In case of multiple‐drug resistance, polymyxin should be used | When aminoglycosides are combined with cyclosporin, vancomycin, amphotericin B, or radiographic contrast agent, the risk for renal toxicology increases. Such combined therapy are applicable for patients with severe CAP, but the therapeutic value is controversial |
| Klebsiella pneumoniae and Enterobacteriaceae | |||
| Non‐β‐lactamase‐producing | Cefuroxime 0.75–1.5 g IV q8h; cefotaxime1–2 g IV q6h‐q8h; ceftriaxone1–2 g IV q24h; β‐lactams‐β‐lactamase inhibitor combinations e ; cephamycins a | Cefepime; levofloxacin; moxifloxacin; gemifloxacin; aminoglycosides d | ESBLs can inactivate all cephalosporins. It is difficult to predict the activity of β‐lactams‐β‐lactamase combinations. ESBLs‐producing strains are also resistant to all quinolones and most aminoglycosides. |
| ESBLs‐producing Enterobacteriaceae | Carbapenems f , piperacillin‐tazobactam 4.5 g IV q6h‐q8h; cefoperazone‐sulbactam 2–4 g IV q8h‐q12h | Cefepime; tigecycline | Fourth‐generation cephalosporins and piperacillin‐ tazobactam have in‐vitro antibacterial activity, but their efficacy has not yet been completely demonstrated in animal models. |
| Enterobacteriaceae with high production of AmpC β‐lactamase | Carbapenems f | Cefepime; tigecycline | Quinolones can be effective against susceptible strains, but most strains are resistant. Some bacterial strains are susceptible to injectable II and III generation cephalosporins in vitro, but are resistant to ceftazidime. |
| Carbapenemase‐producing Enterobacteriaceae | Polymyxin B 15 000–25 000 U/kg per day, IV, in 2 separate doses | Tigecycline; drugs to which pathogens are relatively susceptible could be selected for combination therapy |
Patients infected with these bacterial strains are unresponsive to injectable II or III generation cephalosporins. Tigecycline has in vitro activity. |
| Acinetobacter | Ampicillin‐sulbactam 3 g IV q6h; cefoperazone‐sulbactam 2–4 g IV q12h or q8h; quinolones b + amikacin 15 mg/kg IV q24h or + ceftazidime 2 g IV q8h‐q12h; carbapenems f | Cefoperazone‐sulbactam + amikacin or minocycline; polymyxin B; polymyxin E; tigecycline; sulbactam g + minocycline or polymyxin E or amikacin or carbapenem f |
The sulbactam component in ampicillin‐sulbactam has antibacterial activity with an appropriate dosage of 3 g IV q6h, and has been reported to be superior to polymyxin E A. baumannii in China is highly resistant to carbapenems, which is normally used at MIC ≤ 8 mg/L. Combination therapy is recommended. |
| Anaerobic bacteria | Penicillins‐β‐lactamase‐inhibitor combinations e | Clindamycin; metronidazole; doxycycline; moxifloxacin; carbapenems f | |
| Mycoplasma pneumoniae | Doxycycline first dose 200 mg oral, followed by 100 mg oral, twice daily; minocycline 100 mg oral, twice daily; levofloxacin 500 mg IV or oral, once daily; moxifloxacin 400 mg IV or oral, once daily | Azithromycin; clarithromycin; gemifloxacin | The application of macrolides should be based on local susceptibility data. Clindamycin and β‐lactams are ineffective on M. pneumoniae. |
| Chlamydia pneumoniae | Azithromycin 500 mg IV once daily; clarithromycin 500 mg oral, twice daily; erythromycin 500 mg IV q6h; levofloxacin 500 mg IV or oral, once daily; moxifloxacin 400 mg IV or oral, once daily | Doxycycline; minocycline; gemifloxacin | |
| Legionella | Azithromycin 500 mg IV once daily or erythromycin 0.5 g IV q6h; levofloxacin 500 mg IV or oral, once daily; gemifloxacin 0.32 g oral, once daily; moxifloxacin 400 mg IV or oral, once daily | Doxycycline; clarithromycin; minocycline; TMP‐SMX; above‐mentioned quinolones + rifampin or azithromycin | When quinolones are combined with macrolides, the potential risk of abnormalities in cardiac electrophysiology should be alerted. |
| Chlamydia psittaci | Doxycycline 100 mg IV or oral, twice daily; minocycline100 mg oral, twice daily | Azithromycin; clarithromycin; erythromycin; chloramphenicol | Fever and other symptoms can normally be controlled within 48–72 h, but antibiotics should be continued for at least 10 d. |
| Coxiella burnetii | Doxycycline 200 mg oral, once daily; minocycline 100 mg oral, twice daily | Erythromycin; chloramphenicol; levofloxacin; moxifloxacin; gemifloxacin | Q fever |
| Burkholderia pseudomallei | Ceftazidime 30–50 mg/kg IVq8h; imipenem 20 mg/kg IV q8h. Treatment continued for at least 10 d. If the condition is improved, therapy may be switched to oral treatment. |
Intravenous therapy followed by oral treatment: chloramphenicol 10 mg/kg q6h × 8 weeks; doxycycline 2 mg/kg twice daily × 20 weeks; TMP‐SMX 5 mg (based on TMP) twice daily × 20 weeks Quinolones b |
Pregnant women: oral amoxicillin‐clavulanic acid sustained‐release tablets 1000/62.5 mg, 2 tabs twice daily × 20 weeks. Even with very good compliance, relapse rate is still 10%. The maximum daily dose of ceftazidime is 6 g. Tigecycline: susceptible in vitro, but no clinical data. 12%‐80% of bacterial strains are resistant to TMP‐SMX in Thailand. Quinolones are effective in vitro. Doxycycline + chloramphenicol + TMP‐SMX has better sustained efficacy compared with doxycycline monotherapy. Meropenem is also effective |
| Bordetella pertussis | Azithromycin 0.5 g IV once daily; erythromycin 0.5 g IV q6h | TMP‐SMX; clarithromycin | |
| Stenotrophomonas maltophilia | TMP‐SMX 0.48 g (80 mg + 400 mg dosage form) oral, 2–3 tablets, tid; ticarcillin‐clavulanic acid 3.2 g IV q6h‐q8h | Cefoperazone‐sulbactam; piperacillin‐tazobactam; ceftazidime; moxifloxacin; ticarcillin‐clavulanic acid + aztreonam | Ticarcillin‐clavulanic acid + TMP‐SMX; ticarcillin‐clavulanic acid + ciprofloxacin have synergetic antibacterial effect in vitro. |
| Nocardia | TMP‐SMX 15 mg/kg daily (based on TMP) oral, divided into 2–4 doses, for 3–4 weeks, followed by 60 mg/kg daily, oral, divided into 2–4 doses, for 3–4 months | Imipenem‐cilastatin + amikacin 7.5 mg/kg IV q12h, × 3–4 weeks; followed by TMP‐SMX for 3–4 months | The duration of therapy is 3–4 months for primary pulmonary nocardiosis. |
| Actinomycetes | Ampicillin 2 g IV q8h, for 4–6 weeks, followed by penicillin V potassium 2–4 g/kg per day, oral, for 3–6 weeks | Piperacillin; amoxicillin‐clavulanic acid; ampicillin‐sulbactam; piperacillin‐tazobactam; doxycycline; minocycline; ceftriaxone; clindamycin; chloramphenicol; azithromycin; erythromycin; moxifloxacin; imipenem; ertapenem | Penicillin G is an alternative to ampicillin: 10–20 million U/d, IV, divided into 4–6 separate doses, for 4–6 weeks. |
| Yersinia pestis | Gentamicin 5 mg/kg IV once daily | Doxycycline; minocycline |
TMP‐SMX can be used to prevent Yersinia pestis pneumonia. Chloramphenicol is effective but with high toxicity. Cephalosporins and quinolones are effective in animal models. |
| Anthrax pneumonia |
Ciprofloxacin 400 mg IV q12h or levofloxacin 500 mg IV once daily or doxycycline 100 mg IV q12h + clindamycin 900 mg IV q8h ± rifampin 300 mg IV q12h; Switch to oral therapy and reduce dosage after improvement: ciprofloxacin 500 mg oral, twice daily; clindamycin 450 mg oral, q8h, and rifampin 300 mg oral, twice daily. Duration of therapy is 60 d. |
Penicillin G |
Clindamycin can inhibit the production of toxins. Rifampin can enter cerebrospinal fluid and into cells. If the isolated pathogen is susceptible to penicillin, penicillin 4 million U IV q4h should be given. If structural or inductive β‐lactamase is produced, penicillin or ampicillin should not be used alone. Cephalosporins or TMP‐SMX should not be used. Erythromycin and azithromycin have borderline activity. Clarithromycin is effective. Moxifloxacin is effective, but without clinical data. |
| Influenza virus or human infections with avian influenza virus |
Oseltamivir 75 mg oral, twice daily × 5 d, for obesity patients, the dosage is increased to 150 mg oral, twice daily; for patients with severe influenza, increased dosage (150 mg twice daily) and prolonged course of treatment (eg, ≥ 10 d) should be considered. The safety of high dose therapy for pregnant women has not been established. Zanamivir 2 sprays (5 mg/spray) twice daily × 5 d |
Amantadine; rimantadine Peramivir 600 mg IV once daily for at least 5 d can be considered for patients with severe life‐threatening conditions |
For patients with chronic obstructive pulmonary disease or asthma, zanamivir can potentially cause bronchospasm. Most epidemic viral strains are resistant to amantadine and rimantadine. |
| Adenovirus | Cidofovir 1 mg/kg IV once daily × 2 weeks, and oral probenecid 2 g should be given every time before injection. And 1 g oral probenecid should be taken at 2 h and 8 h post‐infusion. Renal functions should be monitored. | The drug is contraindicated when serum creatinine >1.5 mg/dL, CrCl ≤ 55 mL/min, or urine protein ≥ 100 mg/L. | |
| Respiratory syncytial virus | No specific drug so far | Ribavirin 0.5–1 g/d IV q12h (not recommended for regular use) | Therapies are mainly symptomatic treatments, including fluid replacement and oxygen therapy. |
| Middle East respiratory syndrome coronavirus | No specific drug so far | Pegylated interferon α‐2a subcutaneous, 180 μg weekly × 2 weeks + ribavirin first dose 2000 mg po, followed by 1200 mg oral, q8h × 4 d, then 600 mg oral, q8h × 4–6 d (the dose of ribavirin should be adjusted based on liver functions, and kidney functions should be monitored)167 | Retrospective studies showed that the therapy could increase 14‐day survival in patients with severe conditions, but 28‐day survival was not be increased. It may cause decrease of hemoglobin level. |
| Aspergillus | Voriconazole 6 mg/kg IV q12h on the first day, followed by 4 mg/kg IV q12h or 200 mg oral, q12h (body weight ≥ 40 kg), or 100 mg oral, q12h (body weight < 40 kg); amphotericin B liposome 3–5mg/kg daily IV, or amphotericin B liposome compound 5 mg/kg daily IV, or amphotericin B 0.75–1 mg/kg daily IV (initial dose 1–5 mg/d) | Itraconazole; caspofungin; micafungin; posaconazole |
Voriconazole has better efficacy than amphotericin B. For patients with CrCl < 50 mL/min, the drug should only be taken orally. IV administration is contraindicated. The efficacy rate of caspofungin is about 50% for invasive pulmonary aspergillosis. It can be used as a rescue therapy. The role of combination therapy is unclear, it is not regularly recommended, but can be considered for refractory cases. The classic combination treatment is echinocandins combined with amphotericin B liposome. |
| Mucor | Amphotericin B liposome 3–5 mg/kg daily IV or amphotericin B liposome compound 5 mg/kg daily IV or amphotericin B 0.75–1 mg/kg daily IV (initial dose 1–5 mg/d) | Posaconazole | The complete or partial response rate of posaconazole rescue regimen is 60%‐80%. |
| Human pneumocystis pneumonia | |||
| Non‐acute patients who can take the drug orally and PaO2 > 70 mm Hg | TMP‐SMX (160/800 mg dosage from) 2 tabs oral, q8h × 21 d or dapsone 100 mg oral, once daily + TMP 5 mg/kg oral, tid × 21 d | Clindamycin 300–450 mg oral, q6h + primaquine base 15 mg oral, once daily, for 21 d, or atovaquone suspension 750 mg oral, twice daily, at meal time × 21 d | Patients with severe life‐threatening conditions can take glucocorticoids: start with prednisone 40 mg oral, twice daily × 5 d, followed by 40 mg oral, once daily × 5 d, then 20 mg oral, once daily × 11 d. |
| Acute patients who cannot take the drug orally and PaO2 < 70 mm Hg (dry cough, progressive dyspnea, diffuse pulmonary infiltrates) | Glucocorticoids should be given 15–30 min before TMP‐SMX which should be administered in a dosage of 15 mg/kg/d divided into separate doses once per 8 h (calculated based on TMP content) or 2 tabs once per 8 h, continued for 21 d | Clindamycin 600 mg IV q8h + primaquine base 30 mg oral, once daily; pentamidine isethionate 4 mg/kg daily IV for 21 d | Although TMP‐SMX‐resistant Pneumocystis is rarely observed, but it does exist. Caspofungin is effective in animal models. |
The selection of antimicrobial agents should ultimately depend on susceptibility testing results and the opinions of local microbiological specialists. The appropriate dosage of antimicrobial agents should be based on local data. CrCl, creatinine clearance; MIC, minimum inhibitory concentration; MRSA, methicillin‐resistant S. aureus; TMP‐SMX, trimethoprim‐sulfamethoxazole.
a Cefoxitin 1–2 g IV q8h‐q6h; cefmetazole 1–2 g q8h‐q12h; cefotetan 1–3 g IV q12h (maximum dose ≤ 6 g once daily); cefminox 1 g IV q8h.
b Levofloxacin, moxifloxacin, gemifloxacin (not as first‐line therapy for penicillin‐susceptible strains); ciprofloxacin is mainly used in treatment of gram‐negative bacteria (including H. influenzae).
c Ticarcillin 3 g IV q4h‐q6h; piperacillin 2–4 g IV q4h‐q6h; piperacillin‐tazobactam 4.5 g IV q6h‐q8h; aztreonam 1–2 g IV q8h‐q12h; ceftazidime 1–2 g IV q8h‐q12h; cefepime 1–2 g IV q8h‐q12h; cefoperazone 1–2 g IV q8h; cefoperazone‐sulbactam (2:1) 3 g q8h‐q12h; imipenem‐cilastatin (for P. aeruginosa) 500 mg (based on imipenem) IV q6h‐q8h; meropenem 1–2 g IV q8h; panipenem‐betamipron 1–2 g IV q8h‐q12h; biapenem 0.3 g IV q12h.
d Gentamicin or tobramycin 5.1 mg/kg daily IV, once daily; amikacin 15 mg/kg IV once daily; etimicin 0.2–0.3 g IV once daily; netilmicin 6.5 mg/kg IV, once daily.
e Piperacillin‐tazobactam 4.5 g IV q6h‐q8h; ticarcillin‐clavulanic acid 3.2 g IV q6h‐q8h; ampicillin‐sulbactam 1.5–3 g IV q6h or amoxicillin‐clavulanic acid 1.2 g IV q8h‐q12h.
f Imipenem‐cilastatin 500 mg (based on imipenem) IV q6h‐q8h; meropenem 1–2 g IV q8h; ertapenem 1–2 g IV q24h; panipenem‐betamipron 1–2 g IV q8h‐q12h; biapenem 0.3 g IV q12h.
g Sulbactam: 4–8 g/d IV, divided into 2–4 doses.
7. SECTION 5. ADJUNCTIVE THERAPIES FOR CAP
CAP is the primary cause of death among infectious diseases. In addition to anti‐infective treatment targeting the pathogens, it is also necessary for patients with moderate or severe CAP to receive adjunctive therapies such as rehydration, maintenance of fluid and electrolyte balance, nutrition support and physical therapy2 (II B). For patients with concomitant low blood pressure, early fluid resuscitation is an important measure to decrease the mortality of serious CAP1, 168 (II B). For patients with hypoxemia, oxygen therapy and assisted ventilation are also important to improve the outcomes of patients. Additionally, nebulization, postural drainage and chest physical therapy are also used in CAP treatment169, 170, 171 (II B). Adjunctive drugs for severe CAP also include glucocorticoids, intravenous immune globulin and statins, although currently there is no conclusive evidence for their effectiveness172 (II B).
7.1. Oxygen therapy and assisted respiration
The blood oxygen level of hospitalized CAP patients should be evaluated in a timely manner. Oxygen therapy via nasal catheter or face mask is recommended for patients with hypoxemia in order to maintain blood oxygen saturation at above 90%. Additionally, for patients with risk of hypercapnia, oxygen saturation should be maintained at 88%‐92% before obtaining the results of blood gas analysis173, 174 (III A). The results of recent studies showed that heated and humidified high‐flow oxygen therapy via nasal catheter (40–60 L/min) could also be used in clinical practice175, 176 (II B).
-
Compared with high‐concentration oxygen therapy, non‐invasive ventilation (NIV, including bilevel positive airway pressure or continuous positive pressure ventilation) can decrease the endotracheal intubation rate and mortality of CAP patients with acute respiratory failure,177, 178, 179, 180, 181 improve oxygenation index faster and more significantly,177, 178, 182, 183 and decrease the incidence of multiple organ failure,179 and septic shock.177 These benefits are more significant for patients with concomitant chronic obstructive pulmonary disease180 (II B). However, for CAP patients with acute respiratory distress syndrome (ARDS), NIV has shown high failure rate184 and it cannot improve prognosis.177 NIV is also not appropriate for CAP patients with severe hypoxemia (oxygenation index < 150 mm Hg)184 (II A).
Additionally, the failure of NIV must be recognized timely. NIV failure is indicated if NIV cannot improve respiratory rate or oxygenation state within the initial 1–2 h,180, 184, 185 or the therapy cannot decrease the blood carbon dioxide level in a patient with initial hypercapnia.180 The oxygen therapy should be switched to tracheal intubation and ventilator‐assisted ventilation immediately (II A).
Mechanical ventilation with low tidal volume (6 mL/kg ideal body weight) should be used for CAP patients with ARDS after tracheal intubation186, 187 (I A).
For patients with severe CAP and concomitant ARDS, extracorporeal membrane oxygenation (ECMO) can be used if regular mechanical ventilation cannot lead to improvement188, 189, 190, 191 (II B). Indications of ECMO include: (1) reversible respiratory failure associated with severe hypoxemia (oxygenation index < 80 mm Hg or hypoxemia that cannot be corrected even after 6 h high‐level positive end‐expiratory pressure [PEEP] assisted‐ventilation); (2) serious decompensatory acidosis (pH < 7.15); (3) excessively high plateau pressure (eg, > 35–45 cm H2O).192
7.2. Glucocorticoids
Glucocorticoids can decrease the mortality of CAP patients complicated with septic shock.193, 194, 195 Hydrocortisone succinate 200 mg/day is suggested based on the treatment of septic shock.196 The drug should be stopped promptly after septic shock is corrected. The duration of therapy is normally no more than 7 days (II C). The benefits of glucocorticoids are unclear for other severe CAP patients without septic shock. Additionally, systemic use of glucocorticoids can cause insulin‐requiring hyperglycemia.197, 198
8. SECTION 6. ASSESSMENT AFTER INITIAL THERAPY AND THE CRITERIA FOR DISCHARGE
For most CAP patients, clinical symptoms can be improved 72 h after the initial therapy, while radiographic improvement lags behind clinical symptoms.199, 200, 201, 202 Disease status should be assessed 72 h after initial therapy. Some patients are slower to respond to therapies. In such cases, it is appropriate to continue the therapy without a need to change the regimen immediately as long as no exacerbation occurs in clinical manifestations1, 203, 204, 205 (I A).
8.1. Assessment after initial therapy
The initial therapy is assessed as effective or failure based on the patient's response to treatment, and subsequent management is provided accordingly. Assessment after initial therapy should include the following 5 aspects:
Clinical manifestations: including respiratory and systemic symptoms and signs (III A).
Vital signs: general condition, consciousness, body temperature, respiratory rate, heart rate, blood pressure and so on.2 (I A).
General laboratory tests: including routine blood test, blood biochemistry, blood gas analysis, C‐reactive protein, procalcitonin and so on. It is recommended to repeat C‐reactive protein, procalcitonin and routine blood tests after 72 h for hospitalized patients in order to differentiate between treatment failure and slow response to therapy. Patients with severe conditions should be monitored closely2, 206, 207, 208, 209 (II B).
Microbiological tests: it is appropriate to repeat regular microbiological tests. Molecular biological and serological assays can be used when necessary. Efforts should be made to obtain etiological evidence210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220 (II B).
Chest radiography: it is not recommended to repeat chest radiography regularly for patients with significant improvement in clinical symptoms. When symptoms and signs persist or exacerbate, chest X‐ray or chest CT should be repeated to identify the changes of lung lesions2 (I A).
8.2. Definition of effective initial therapy and subsequent management
An effective initial therapy is defined as the situation that the clinical condition of a patient is stabilized after therapy. All the 5 criteria below must be met for clinical stability: (1) body temperature ≤ 37.8°C; (2) heart rate ≤ 100 bpm; (3) respiratory rate ≤ 24 bpm; (4) systemic blood pressure ≥ 90 mm Hg; (5) O2 saturation ≥ 90% (or arterial partial pressure of O2 ≥ 60 mm Hg, while breathing air)1, 221 (II A).
Subsequent management is recommended after an effective initial therapy: (1) For patients with significant improvement in symptoms after initial therapy, it is appropriate to continue the same anti‐infective treatment (I A). (2) For patients who have achieved clinical stability and are able to receive oral therapy, sequential therapy should be administered with pathogen‐susceptible oral preparations of the same types of antimicrobial agents or another agent with similar antibacterial spectrum1, 222 (I A).
8.3. Definition of failed initial therapy and subsequent management
-
A failed initial therapy is defined as either of the following situations in a patient: the symptoms are not improved after initial therapy, and requiring alternative antibiotics; exacerbation and disease progression after initial improvement during initial therapy (II A).
Two forms of failure are primarily observed in clinical practice223, 224: (1) Progressive pneumonia: disease progresses to acute respiratory failure requiring mechanical ventilation support or septic shock requiring vasoactive drug therapy within 72 h from arrival at the hospital223, 224; (2) Unresponsive to therapies221, 225: patient cannot achieve clinical stability 72 h after initial therapy.
-
Local or systemic complications,2, 205, 226 such as parapneumonic effusion, empyema, ARDS, phlebitis, septicemia and metastatic abscesses, are risk factors for failure of initial therapy. Other potential factors include nonbacterial infection or antibiotic‐resistant bacterial infection not covered by initial treatment, and noninfectious diseases. See the flowchart for failure of initial treatment (Figure 1) for details.1, 17, 18, 51, 223, 226, 227
Figure 1.
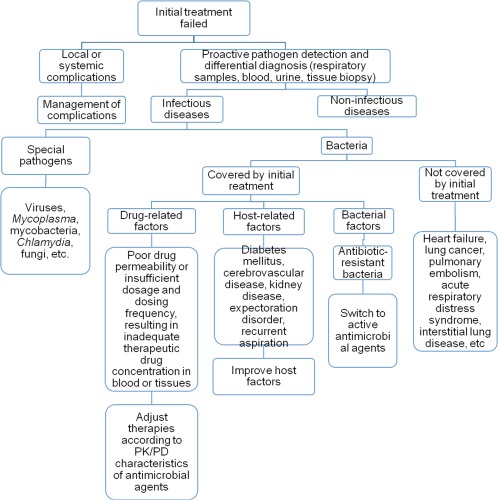 Flowchart for failure of initial treatment
Flowchart for failure of initial treatment
8.4. Criteria for hospital discharge
Discharge can be considered when a patient with clear diagnosis shows significant improvement after effective treatment, evidenced by normal body temperature for more than 24 h, and the other 4 criteria for clinical stability are satisfied. Moreover, the patient is able to receive oral treatment, and there is no other complications or disturbance of consciousness requiring further management1 (I A).
9. SECTION 7. UNUSUAL TYPES OF CAP
9.1. Unusual pathogens
9.1.1. Viral pneumonia
Respiratory tract viruses play an important role in adult CAP. They can be direct responsible pathogens for CAP, or facilitate the onset of secondary bacterial pneumonia due to S. pneumoniae, S. aureus, and so on. Both primary viral pneumonia and secondary or concomitant bacterial infections can be severe.53, 228 Viruses are reported to be detected in 15%‐34.9% of Chinese adult CAP patients with normal immune status.15, 26, 27 Common viruses in adult CAP include influenza virus, parainfluenza virus, rhinovirus, adenovirus, hMPV and RSV. Since 2009, the new H1N1 influenza A virus has become a major viral strain for seasonal influenza, along with seasonal viral strain H3N2.15, 26, 27, 229, 230, 231, 232, 233, 234 In recent years, there have also been cases of pneumonia caused by avian influenza A virus (H5N1, H7N9 and H10N8) and imported Middle East respiratory syndrome (MERS) coronavirus.235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248, 249, 250, 251, 252 Early diagnosis based on epidemiological (such as epidemic season and history of traveling to epidemic regions) and clinical characteristics, early antiviral therapy (within 48 h of the onset of the disease), and reasonable supportive therapies are critical measures to reduce mortality101, 229, 230, 253, 254, 255 (II B). See Table 8 for the epidemiological and clinical characteristics and treatment of viral pneumonia. See corresponding sections of this guideline for information on diagnosis and prophylaxis. Epidemiological clues are especially important in patients with highly contagious and newly discovered respiratory tract viruses.
Table 8.
Epidemiological and clinical characteristics and treatment of pneumonia caused by major respiratory tract viruses
| Respiratory tract virus | Key epidemiological features | Clinical characteristics | Radiographic characteristics | Antiviral treatment |
|---|---|---|---|---|
| H1N1 influenza A virus, H3N2 influenza virus | The epidemic season in the north is from November to the end of February of next year, and in the south – another peak season is from May to August. Influenza outbreak can occur in any season. High‐risk populations include the elderly (age ≥ 65 years), patients with underlying diseases, obesity, or immunosuppression and second‐ to third‐trimester pregnant women.101 The virus can be transmitted via air, saliva droplets and direct contact. The incubation period is normally 1–7 d, and most commonly 2–4 d. | Fever, cough, normal or decreased WBC, normal or decreased lymphocytes, CRP < 20 mg/L, creatine kinase or lactate dehydrogenase can increase. The disease can progress rapidly in some patients, causing persistent high fever, severe dyspnea and intractable hypoxemia.229, 230, 231, 232, 233, 234 | For patients with severe conditions, ground‐glass opacities or patchy nodule infiltrates can appear in bilateral lungs, which may be associated with consolidation | Oseltamivir, zanamivir, peramivir101, 229, 254, 256, 257, 258 (I A) |
| Human infections with avian influenza virus | Human beings lack immunity against avian influenza virus. Individuals in close contact with domestic animals dying from unknown reasons, livestock markets, or patients with confirmed diagnosis of avian influenza constitute the high exposure population.241, 255 The virus is primarily transmitted through contact with dead animals with avian influenza and the contaminated objects and environment. There is a small number of cases of person‐to‐person transmission of H5N1. The incubation period is normally no more than 7 d. | Similar to pneumonia caused by influenza virus, but decreased WBC, lymphocyte count and platelet count are more common and there is a more significant increase in alanine transaminase, lactate dehydrogenase, and/or creatine kinase. Hemoptysis and abnormal coagulation functions are more commonly seen among patients infected with H7N9.235, 239, 242, 247, 248, 249, 250 | Similar to pneumonia caused by influenza virus235, 239 | Same as pneumonia caused by influenza virus255 (I A) |
| Adenovirus | The epidemic season is from February to May. The virus is commonly seen in adults without underlying disease.26 The incubation period is 3–8 d. HAdV‐55, HAdV‐11 and HAdV‐7 are relatively common serotypes259, 260 | Similar to pneumonia caused by influenza virus; more common in immunocompetent adults259, 260, 261, 262 | Patients with severe conditions primarily show pulmonary consolidation, which may be associated with ground‐glass opacities or patchy nodule infiltrates in unilateral or bilateral lungs or multiple lobes 259, 260, 262 | Cidofovir 53, 263 (II B) |
| Respiratory syncytial virus | RSV is an important pathogen of lower respiratory tract infections in infants and young children. In adults, infections are more common in the elderly or individuals with underlying cardiac or pulmonary diseases or immunosuppression53, 264, 265. The incubation period is 4–5 d. | Similar to pneumonia caused by influenza virus 53 | Characteristic manifestations are nodule opacities or tree‐in‐bud sign associated with bronchial wall thickening266 |
Intravenous or oral ribavirin (not recommended for routine use)53, 264, 265, 267 (II B) |
| Middle East respiratory syndrome (MERS) coronavirus | The general population are generally vulnerable. Special attention should be paid to history of travel or business trip to epidemic regions such as Saudi Arabia, UAE and so on, or history of close contact with patients with confirmed diagnosis of MERS.268, 269 The incubation period is 2–14 d. | Fever, associated with chills and shivers, cough, shortness of breath, muscle soreness; gastrointestinal symptoms such as diarrhoea, nausea and vomiting and abdominal pain are relatively common. Decreased platelet count, decreased lymphocyte count and increased lactate dehydrogenase and creatinine may be observed in some patients 268, 269 | Mainly pulmonary involvement in subpleural and basal segments of lungs; broad appearance of ground‐glass opacities, which may be associated with consolidation. Pleural effusion, interlobular septal thickening may also appear 269, 270 | Ribavirin combined with interferon 168, 271 (II C) |
9.1.2. Legionella pneumonia
In China, Legionella pneumonia accounts for 5.08% of CAP.17 Legionella pneumonia usually progresses to severe condition. Almost 50% of the hospitalized patients due to Legionella infections require ICU admission,272 and the mortality is up to 5%‐30%.272 Susceptible populations include the elderly, males, smokers,273, 274 individuals with chronic underlying cardiac or pulmonary diseases,272, 274, 275, 276 diabetes mellitus,274, 275 malignant tumour, immunosuppression273, 274, 275 and use of tumour necrosis factor‐α antagonists.277 The relevant epidemiological history includes contact with contaminated air conditioners, air conditioner cooling tower, or contaminated potable water, hot recreational spa, gardening activities or plumbing repairs and the history of traveling to an area with Legionella outbreak.2, 275, 276, 278
The possibility of Legionella pneumonia should be suspected when an adult CAP patient develops the following conditions: fever but relative bradycardia, acute onset of headache, non‐drug‐induced disturbance of consciousness or sleepiness, non‐drug‐induced diarrhoea, acute renal and/or hepatic impairment, hyponatremia, hypophosphatemia and unresponsiveness to β‐lactams.164, 276, 278, 279, 280, 281, 282, 283 The relatively specific manifestations of Legionella pneumonia in chest radiograph is sharply demarcated consolidation intermingled with ground‐glass opacities. Another characteristic of Legionella pneumonia is radiographic progression within a short period of time (1 week) even though improvement in clinical symptoms. Or it may take several weeks or even months for pulmonary infiltrates to be completely absorbed.284, 285, 286
Macrolides, respiratory quinolones or doxycycline monotherapy are appropriate for immunocompetent patients with mild or moderate Legionella pneumonia. Quinolones combined with rifampin or macrolides are recommended for patients with severe conditions, or when monotherapy fails and those immunocompromised patient1, 2, 287, 288, 289, 290 (I A). When quinolones are combined with macrolides, physicians should pay close attention to the potential risk of abnormalities in cardiac electrophysiology2 (I A).
9.1.3. Community‐acquired methicillin‐resistant S. aureus pneumonia
Currently, CA‐MRSA pneumonia is relatively rare in Mainland China. Only a small number of cases are reported in children and teenagers.19, 20, 21, 22 Similarly, among the skin and soft tissue infections caused by S. aureus, MRSA only accounts for a small proportion (5/164).291 Among the pathogens of hospitalized CAP patients, the proportion of MRSA is 4.3% in Taiwan,292 3.3% in Japan293 and 6.2%‐8.9% in the United States according to a survey.294 The estimated incidence of CA‐MRSA pneumonia is 0.51–0.64/100 000 people.295 CA‐MRSA pneumonia is a severe disease associated with mortality up to 41.1%.296 Vulnerable populations include patients or individuals with close contact with a MRSA carrier or patient, individuals affected by influenza virus, prisoners, professional athletes, individuals who serve in the army recently, men who have sex with men, intravenous drug users, regular sauna users and those using antibacterial agents before infection.295
CA‐MRSA pneumonia progresses rapidly. The clinical symptoms include influenza‐like symptoms,296, 297 fever, cough, chest pain, gastrointestinal symptoms and skin rashes. For patients with serious conditions, severe pneumonia symptoms such as hemoptysis, confusion, ARDS, multiple organ failure and shock may appear, as well as complications such as acidosis, disseminated intravascular coagulation, deep vein thrombosis, pneumothorax or empyema, pneumatocele, pulmonary abscess and acute necrotic pneumonia.295 Radiographic characteristics of CA‐MRSA pneumonia include extensive pulmonary consolidation and multiple cavities in bilateral lungs.298 CA‐MRSA pneumonia should be suspected after influenza or in previously healthy young patients in case of cavitation, necrotic pneumonia associated with rapid increase of pleural effusion, massive hemoptysis, neutropenia and/or erythematous rashes.299 Glycopeptides or linezolid are the primary choice for CA‐MRSA pneumonia1, 299 (III B).
9.2. Special populations
9.2.1. CAP in the elderly
Currently, the consensus definition of CAP in the elderly (elderly CAP) is pneumonia occurring in the population aged ≥ 65 years.2, 300, 301 The incidence of elderly CAP increases with age.
The clinical manifestations of elderly CAP can be atypical.302, 303 The manifestations may only include poor appetite, urinary incontinence, tiredness and altered mental state and so on.2, 301 Typical manifestations of pneumonia such as fever, cough and increased WBC/neutrophil count may not be so evident.303 Therefore, missed diagnosis and misdiagnosis may occur. Tachypnea is a sensitive index for diagnosis of elderly CAP.304 When fever or any of the above‐mentioned atypical symptoms appear, chest radiography should be done as early as possible to confirm the diagnosis.304
S. pneumoniae is still the main pathogen for elderly CAP, but the possibility of Enterobacteriaceae infection should be considered for elderly patients with underlying diseases (congestive heart failure, cardiovascular and cerebrovascular diseases, chronic respiratory system diseases, renal failure, diabetes mellitus, etc.).24, 300, 305 These patients should be further evaluated for risk factors of ESBLs‐producing Enterobacteriaceae. Empirical treatment with cephamycins, piperacillin‐tazobactam, cefoperazone‐sulbactam, ertapenem or other carbapenems is recommended for patients with high risk of infections with ESBLs‐producing Enterobacteriaceae 145, 146, 147, 306 (III B). Relevant risk factors include history of ESBLs‐producing bacterial colonization or infection, prior use of third generation cephalosporins, history of repeated or long‐term hospitalization, indwelling medical devices, renal replacement therapies.142, 143, 144
Elderly patients are associated with reduced organ functions, which must be monitored during treatment to avoid side effects. Reduced renal excretion may cause prolonged half‐lives of drugs, so the dosage should be reasonably adjusted in terms of CrCl when treating such patients304 (II B). If no contraindication exists, hospitalized elderly CAP patients should be evaluated for risk of deep vein thrombosis and prophylaxis with low molecular weight heparin should be administered when necessary307 (II B).
The treatment failure rate is 6%‐15% for elderly CAP.308 Common reasons are concomitant severe sepsis, myocardial infarction, or progression of pneumonia.309 Cardiovascular event is common in elderly CAP, which is one of the reasons for increased mortality.308, 309
9.2.2. Aspiration pneumonia
Aspiration pneumonia is pulmonary infectious lesions caused by aspiration of food, oropharyngeal secretion, or gastric content into the throat or lower respiratory tract, not including chemical inflammation in the lung due to aspiration of sterile gastric fluid.310, 311, 312 The majority cases of aspiration pneumonia are caused by silent aspiration, accounting for around 71% of elderly CAP.312
The following points should be noted when making diagnosis of aspiration pneumonia: (1) whether there are risk factors for aspiration (eg, disturbance of consciousness due to cerebrovascular diseases or other reasons, dysphagia, periodontal diseases, or poor oral hygiene)122, 308, 313, 314, 315, 316; (2) whether chest radiograph shows primary lesions in the posterior segment of upper lobe and dorsal or basal segment of the lower lobe, just as in hypostatic pneumonia.312, 317, 318, 319
Aspiration pneumonia is mostly caused by infections with anaerobic bacteria, gram‐negative bacteria or S. aureus. The treatment should cover the above pathogens and based on the severity of disease using antimicrobial agents with anti‐anaerobic activity, such as amoxicillin‐clavulanic acid, ampicillin‐sulbactam, moxifloxacin, carbapenems or in combination with metronidazole or clindamycin 136, 139, 140, 141, 316, 320, 321 (II A). Targeted treatment can be administered after the results of sputum culture and antimicrobial susceptibility testing are available.
Intensive care is required for the elderly patients with risk factors of aspiration in order to reduce the incidence of aspiration pneumonia, specifically: (1) the head of bed should be elevated to 35–40° for long‐term bedridden patients if there is no contraindication, and the patient should be in appropriate position when feeding the patient; (2) oral hygiene should be maintained to reduce bacterial colonization in the oropharyngeal area; (3) for elderly patients with severe dysphagia who have already experienced aspiration, physicians should evaluate the risks and benefits of nasal feeding via indwelling gastric tube; (4) antipsychotic drugs, antihistamines and anticholinergic agents should be avoided or decreased135, 322, 323 (II B).
10. SECTION 8. PROPHYLAXIS
Smoking cessation,1 avoid excessive alcohol drinking, adequate nutrition and good oral health324 are all helpful in preventing pneumonia (III B). Good hand hygiene habits should be maintained. During an episode of respiratory tract symptoms such as coughing or sneezing, wearing a mask or using tissues or elbow clothes to cover the nose and mouth can reduce the dissemination of respiratory tract pathogens325 (III A).
Vaccination against S. pneumoniae can reduce the risk of pneumonia in specific populations. The S. pneumoniae vaccines currently in use include pneumococcal polysaccharide vaccine (PPV) and pneumococcal conjugate vaccine (PCV).
In China, 23‐valent pneumococcal polysaccharide vaccine (PPV23) has been on the market. It can effectively prevent invasive S. pneumoniae infections.326 PPV23 is recommended for the following populations (I B): (1) age ≥ 65 years; (2) age < 65 years, but with chronic pulmonary disease, chronic cardiovascular disease, diabetes mellitus, chronic renal failure, nephrotic syndrome, chronic hepatic disease (including hepatic cirrhosis), alcoholism, cochlear implant, cerebrospinal fluid leakage, immunodeficiency or functional or organic asplenia; (3) long‐term residents in nursing homes or other medical institutions; (4) smokers.327 For the above patients, one dose of vaccine by intramuscular or subcutaneous injection is recommended. Usually, repeat vaccination is not advised for immunocompetent individuals, although it is appropriate for individuals under 65 years of age, but with chronic renal failure, nephrotic syndrome, functional or organic asplenia or immunodeficiency. There should be at least a 5‐year interval between two doses of PPV23. Repeat vaccination is not necessary for the individuals who are at least 65 years of age at the time of first vaccination (I B).
The 13‐valent pneumococcal conjugate vaccine (PCV13) can cover 70%‐80% of S. pneumoniae serotypes in China,328, 329 associated with excellent immunogenicity,330 but it has not been available in China market. PCV13 vaccination strategy: adults aged ≥ 65 years who have not received S. pneumoniae vaccination should receive 1 dose of PCV13, and 1 dose of PPV23 within 6–12 months afterward; adults aged ≥ 65 years who have received one or more doses of PPV23 should receive 1 dose of PCV13 at least one year after the latest dose of PPV23; adults who have received PPV23 before the age of 65 should receive PCV13 after 65 years old (at least one year after the last vaccination), and can repeat PPV23 vaccination at least 6–12 months later, but there should be at least a 5‐year interval between the two doses of PPV23331 (I B).
Influenza vaccines can prevent influenza or reduce influenza‐associated symptoms.332, 333 They also have some protective effects against influenza pneumonia and bacterial pneumonia secondary to influenza.334 The target population of influenza vaccines is broader than that of S. pneumoniae vaccines. See the Chinese Guidelines for Diagnosis and Treatment of Influenza101 and visit the website for National Influenza Center335 for details. One dose of influenza vaccine is recommended per annual influenza season1 (I A). Combination of pneumococcal vaccines with influenza vaccines can decrease mortality in elderly patients336 (II B).
CONFLICT OF INTEREST
Bin Cao has been a speaker invited by Pfizer, GSK and Bayer. Jie‐Ming Qu has been a speaker on behalf of MSD China, Pfizer, Bayer, Daiichi Sankyo and Sanofi‐Aventis. All other authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Li‐Xian He and You‐Ning Liu contributed to be advisors; all authors contributed to critical revision and final approval of the manuscript.
Conception and design of the work and the drafting of Section 1: Jie‐Ming Qu, Bin Cao, Yi Huang
Drafting of Section 2: Qi‐Jian Cheng
Drafting of Section 3: Dan‐Yang She, Hui Wang
Drafting of Section 4: Hong Fan, Yi Shi
Drafting of Section 5: Xin‐Lun Tian, Xiang‐Yan Zhang
Drafting of Section 6: Jin‐Fu Xu
Drafting of Section 7: Yu Chen, Jing Zhang
Drafting of Section 8: Ning Shen, Bei He
Contributed to the methodology section: Mei Jiang
ETHICS
No ethics approval required.
ACKNOWLEDGEMENTS
The following experts provided valuable opinions in the revision of these guidelines. Their efforts are highly appreciated: Writing group members: Yusheng Chen, Xiangqun Fang, Zhancheng Gao, Chengping Hu, Guoxiang Lai, Erran Li, Yuping Li, Hong Liu, Qinghua Liu, Zhongsen Ma, Xiangdong Mu, Changzhou Shao, Xin Su, Jian Sun, Tieying Sun, Zhaohui Tong, Jing Wang, Feng Xu, Feng Ye, Qiao Zhang, Tiantuo Zhang, Wei Zhang. Secretary: Fei Zhou. Pharmacists: Rui Wang, Yuan Lv, Yuzhen Li. Infectious diseases: Minggui Wang, Bijie Hu, Xiaoju Lv. Clinical microbiology: Chao Zhuo. Critical care medicine: Yimin Li, Erzhen Chen. Emergency medicine: Xuezhong Yu, Qiming Feng. Foreign expert: Murat Akova, Lionel A. Mandell.
Cao B, Huang Y, She D‐Y, et al. Diagnosis and treatment of community‐acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. 2018;12:1320–1360. 10.1111/crj.12674
The Chinese version of this guideline was published in Chinese Journal of Tuberculosis and Respiratory Diseases (2016 April; 39: 253‐279) in Chinese. Translation and submission of this English version has got permission from Chinese Medical Association (CMA).
Contributor Information
Bin Cao, Email: caobin_ben@163.com.
Jie‐Ming Qu, Email: jmqu0906@163.com.
REFERENCES
- 1. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–ii55. [DOI] [PubMed] [Google Scholar]
- 3. Jain S, Self WH, Wunderink RG, et al. Community‐acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takaki M, Nakama T, Ishida M, et al. High incidence of community‐acquired pneumonia among rapidly aging population in Japan: a prospective hospital‐based surveillance. Jpn J Infect Dis. 2014;67:269–275. [DOI] [PubMed] [Google Scholar]
- 5. Liu H, Xiao X, Lu J, et al. [Study on epidemic characteristics and etiology of community acquired pneumonia in Guangzhou from 2009 to 2012]. Zhongguo Yufang Yixue Zazhi. 2013;47:1089–1094. [PubMed] [Google Scholar]
- 6. Takayanagi N, Hara K, Tokunaga D, et al. Etiology and outcome of community‐acquired pneumonia in relation to age and severity in hospitalized adult patients. Nihon Kokyuki Gakkai Zasshi. 2006;44:906–915. [PubMed] [Google Scholar]
- 7. Welte T, Kohnlein T. Global and local epidemiology of community‐acquired pneumonia: the experience of the CAPNETZ Network. Semin Respir Crit Care Med. 2009;30:127–135. [DOI] [PubMed] [Google Scholar]
- 8. Restrepo MI, Mortensen EM, Velez JA, et al. A comparative study of community‐acquired pneumonia patients admitted to the ward and the ICU. Chest. 2008;133:610–617. [DOI] [PubMed] [Google Scholar]
- 9. Mortensen EM, Restrepo M, Anzueto A, et al. Effects of guideline‐concordant antimicrobial therapy on mortality among patients with community‐acquired pneumonia. Am J Med. 2004;117:726–731. [DOI] [PubMed] [Google Scholar]
- 10. Restrepo MI, Mortensen EM, Rello J, et al. Late admission to the ICU in patients with community‐acquired pneumonia is associated with higher mortality. Chest. 2010;137:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hraiech S, Alingrin J, Dizier S, et al. Time to intubation is associated with outcome in patients with community‐acquired pneumonia. PLoS One. 2013;8:e74937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Health and Family Planning Commission Statistical Information Center . China Statistical Yearbook of Health and Family Planning [EB/OL] (2014–04‐26). http://www.nhfpc.gov.cn/htmlfiles/zwgkzt/ptjnj/year2013/index2013.html. Accessed November 6, 2015.
- 13. Tao LL, Hu BJ, He LX, et al. Etiology and antimicrobial resistance of community‐acquired pneumonia in adult patients in China. Chin Med J (Engl). 2012;125:2967–2972. [PubMed] [Google Scholar]
- 14. Liu YF, Gao Y, Chen MF, et al. Etiological analysis and predictive diagnostic model building of communityacquired pneumonia in adult outpatients in Beijing, China. BMC Infect Dis. 2013;13:309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao B, Ren LL, Zhao F, et al. Viral and Mycoplasma pneumoniae community‐acquired pneumonia and novel clinical outcome evaluation in ambulatory adult patients in China. Eur J Clin Microbiol Infect Dis. 2010;29:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bao Z, Yuan X, Wang L, et al. The incidence and etiology of community‐acquired pneumonia in fever outpatients. Exp Biol Med (Maywood). 2012;237:1256–1261. [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Chen M, Zhao T, et al. A multicentre study on the pathogenic agents in 665 adult patients with community‐acquired pneumonia in cities of China. Chin J Tuberc Respir Dis. 2006;29:3–8. [PubMed] [Google Scholar]
- 18. Liu Y, Chen M, Zhao T, et al. Causative agent distribution and antibiotic therapy assessment among adult patients with community acquired pneumonia in Chinese urban population. BMC Infect Dis. 2009;9:31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geng W, Yang Y, Wu D, et al. Community‐acquired, methicillin‐resistant Staphylococcus aureus isolated from children with community‐onset pneumonia in China. Pediatr Pulmonol. 2010;45:387–394. [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Luo Y, Zhang S, et al. Community‐acquired necrotizing pneumonia caused by methicillin‐resistant Staphylococcus aureus producing Panton‐Valentine leukocidin in a Chinese teenager: case report and literature review. Int J Infect Dis. 2014;26:17–21. [DOI] [PubMed] [Google Scholar]
- 21. Qu T, Feng Y, Jiang Y, et al. Whole genome analysis of a community‐associated methicillin‐resistant Staphylococcus aureus ST59 isolate from a case of human sepsis and severe pneumonia in China. PLoS One. 2014;9:e89235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bao W, Guo H, Chen Y, et al. One case of community‐acquired methicillin‐resistant necrotic Staphylococcus aureus pneumonia with concomitant bloodstream infection. Chin J Respir Crit Care. 2013;12:89–91. [Google Scholar]
- 23. Wang H, Liu Y, Chen M, et al. Antimicrobial susceptibility of community‐acquired respiratory tract pathogens isolated from adults in China during 2009 and 2010. Chin J Tuber Respir Dis. 2012;35:113–119. [PubMed] [Google Scholar]
- 24. Von Baum H, Welte T, Marre R, et al. Community‐acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35:598–605. [DOI] [PubMed] [Google Scholar]
- 25. Arancibia F, Bauer TT, Ewig S, et al. Community‐acquired pneumonia due to gram‐negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162:1849–1858. [DOI] [PubMed] [Google Scholar]
- 26. Qu JX, Gu L, Pu ZH, et al. Viral etiology of community‐acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect Dis. 2015;15:89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhan Y, Yang Z, Chen R, et al. Respiratory virus is a real pathogen in immunocompetent community‐acquired pneumonia: comparing to influenza like illness and volunteer controls. BMC Pulm Med. 2014;14:144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao C, Zhang F, Wang Z, et al. Resistance surveillance of major pathogens for adult community‐acquired respiratory tract infections in China: a multicenter study 2012. Chin J Tuber Respir Dis. 2015;38:18–22. [PubMed] [Google Scholar]
- 29. Cilloniz C, Albert RK, Liapikou A, et al. The effect of macrolide resistance on the presentation and outcome of patients hospitalized for Streptococcus pneumoniae pneumonia. Am J Respir Crit Care Med. 2015;191:1265–1272. [DOI] [PubMed] [Google Scholar]
- 30. Yayan J. The comparative development of elevated resistance to macrolides in community‐acquired pneumonia caused by Streptococcus pneumoniae . Drug Des Dev Ther. 2014;8:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torres A, Blasi F, Peetermans WE, et al. The aetiology and antibiotic management of community‐acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis. 2014;33:1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tudose C, Bumbacea D, Bogdan M. Antibiotic resistance of S. pneumoniae and H. influenzae strains isolated from patients with community acquired respiratory tract infections. BACTRO multicenter, multidisciplinary study. Pneumologia. 2011;60:30–35. [PubMed] [Google Scholar]
- 33. Castanheira M, Gales AC, Mendes RE, et al. Antimicrobial susceptibility of Streptococcus pneumoniae in Latin America: results from five years of the SENTRY Antimicrobial Surveillance Program. Clin Microbiol Infect. 2004;10:645–651. [DOI] [PubMed] [Google Scholar]
- 34. Zhanel GG, Wolter KD, Calciu C, et al. Clinical cure rates in subjects treated with azithromycin for community‐acquired respiratory tract infections caused by azithromycin‐susceptible or azithromycin‐resistant Streptococcus pneumoniae: analysis of Phase 3 clinical trial data. J Antimicrob Chemother. 2014;69:2835–2840. [DOI] [PubMed] [Google Scholar]
- 35. Yin Y, Cao B, Wang H, et al. Survey of macrolide resistance in Mycoplasma pneumoniae in adult patients with community‐acquired pneumonia in Beijing, China. Chin J Tuber Respir Dis. 2013;36:954–958. [PubMed] [Google Scholar]
- 36. Cao B, Zhao CJ, Yin YD, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis. 2010;51:189–194. [DOI] [PubMed] [Google Scholar]
- 37. Li X, Wang L, Gong G. Drug resistance of Mycoplasma pneumoniae in adult patients with community acquired pneumonia. J Clin Intern Med. 2014;31:113–115. [Google Scholar]
- 38. Caballero Jde D, Del Campo R, Mafe Mdel C, et al. First report of macrolide resistance in a Mycoplasma pneumoniae isolate causing community‐acquired pneumonia in Spain. Antimicrob Agents Chemother. 2014;58:1265–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pereyre S, Charron A, Renaudin H, et al. First report of macrolide‐resistant strains and description of a novel nucleotide sequence variation in the P1 adhesin gene in Mycoplasma pneumoniae clinical strains isolated in France over 12 years. J Clin Microbiol. 2007;45:3534–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diaz MH, Benitez AJ, Winchell JM. Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance from 2006 to 2013. J Clin Microbiol. 2015;53:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eshaghi A, Memari N, Tang P, et al. Macrolide‐resistant Mycoplasma pneumoniae in humans, Ontario, Canada, 2010–2011. Emerg Infect Dis. 2013;19:1525–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miyashita N, Kawai Y, Akaike H, et al. Macrolide‐resistant Mycoplasma pneumoniae in adolescents with community‐acquired pneumonia. BMC Infect Dis. 2012;12:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dumke R, Von Baum H, Luck PC, et al. Occurrence of macrolide‐resistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect. 2010;16:613–616. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Ye X, Zhang H, et al. Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide‐resistant strains from Shanghai, China. Antimicrob Agents Chemother. 2009;53:2160–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. [DOI] [PubMed] [Google Scholar]
- 46. Musher DM, Thorner AR. Community‐acquired pneumonia. N Engl J Med. 2014;371:1619–1628. [DOI] [PubMed] [Google Scholar]
- 47. Huijskens EG, Koopmans M, Palmen FM, et al. The value of signs and symptoms in differentiating between bacterial, viral and mixed aetiology in patients with community‐acquired pneumonia. J Med Microbiol. 2014;63:441–452. [DOI] [PubMed] [Google Scholar]
- 48. Prasad R. Community acquired pneumonia: clinical manifestations. J Assoc Physicians India. 2012;60S:10–12. [PubMed] [Google Scholar]
- 49. Hohenthal U, Hurme S, Helenius H, et al. Utility of C‐reactive protein in assessing the disease severity and complications of community‐acquired pneumonia. Clin Microbiol Infect. 2009;15:1026–1032. [DOI] [PubMed] [Google Scholar]
- 50. Von Baum H, Welte T, Marre R, et al. Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for community‐acquired pneumonia (CAPNETZ). BMC Infect Dis. 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miyashita N, Matsushima T, Oka M, et al. The JRS guidelines for the management of community‐acquired pneumonia in adults: an update and new recommendations. Intern Med. 2006;45:419–428. [DOI] [PubMed] [Google Scholar]
- 52. Shangguan Z, Sun Q, Zhang M, et al. Mycoplasma pneumoniae infection in hospitalized adult patients with community‐acquired pneumonia in China. J Infect Dev Ctries. 2014;8:1259–1266. [DOI] [PubMed] [Google Scholar]
- 53. Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. Lancet. 2011;377:1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community‐acquired pneumonia in adults. Thorax. 2008;63:42–48. [DOI] [PubMed] [Google Scholar]
- 55. Shiley KT, Van Deerlin VM, Miller WT Jr. Chest CT features of community‐acquired respiratory viral infections in adult inpatients with lower respiratory tract infections. J Thorac Imaging. 2010;25:68–75. [DOI] [PubMed] [Google Scholar]
- 56. Chen CZ, Fan PS, Lin CC, et al. Repeated pneumonia severity index measurement after admission increases its predictive value for mortality in severe community‐acquired pneumonia. J Formos Med Assoc. 2009;108:219–223. [DOI] [PubMed] [Google Scholar]
- 57. Riquelme R, Jimenez P, Videla AJ, et al. Predicting mortality in hospitalized patients with 2009 H1N1 influenza pneumonia. Int J Tuberc Lung Dis. 2011;15:542–546. [DOI] [PubMed] [Google Scholar]
- 58. Bjarnason A, Thorleifsdottir G, Love A, et al. Severity of influenza A 2009 (H1N1) pneumonia is underestimated by routine prediction rules. Results from a prospective, population‐based study. PLoS One. 2012;7:e46816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mulrennan S, Tempone SS, Ling IT, et al. Pandemic influenza (H1N1) 2009 pneumonia: CURB‐65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS One. 2010;5:e12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muller MP, Mcgeer AJ, Hassan K, et al. Evaluation of pneumonia severity and acute physiology scores to predict ICU admission and mortality in patients hospitalized for influenza. PLoS One. 2010;5:e9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shi SJ, Li H, Liu M, et al. Mortality prediction to hospitalized patients with influenza pneumonia: PO2/FiO2 combined lymphocyte count is the answer. Clin Respir J. 2017;11:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Julian‐Jimenez A, Gonzalez‐Castillo J, Candel Gonzalez FJ. When, where and how should a patient with community acquired pneumonia be admitted? Rev Clin Esp (Barc). 2013;213:99–107. [DOI] [PubMed] [Google Scholar]
- 63. Salih W, Schembri S, Chalmers JD. Simplification of the IDSA/ATS criteria for severe CAP using meta‐analysis and observational data. Eur Respir J. 2014;43:842–851. [DOI] [PubMed] [Google Scholar]
- 64. Lim WS, Van Der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250. [DOI] [PubMed] [Google Scholar]
- 66. Espana PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249–1256. [DOI] [PubMed] [Google Scholar]
- 67. Charles PG, Wolfe R, Whitby M, et al. SMART‐COP: a tool for predicting the need for intensive respiratory or vasopressor support in community‐acquired pneumonia. Clin Infect Dis. 2008;47:375–384. [DOI] [PubMed] [Google Scholar]
- 68. National Clinical Guideline Centre (UK) . Pneumonia: diagnosis and Management of Community – and Hospital Acquired Pneumonia in Adults [EB/OL]. (2014). https://www.nice.org.uk/guidance/cg191/evidence/full-guideline-193389085. Accessed November 5, 2015. [PubMed]
- 69. Theerthakarai R, El‐Halees W, Ismail M, et al. Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia. Chest. 2001;119:181–184. [DOI] [PubMed] [Google Scholar]
- 70. Ewig S, Schlochtermeier M, Goke N, et al. Applying sputum as a diagnostic tool in pneumonia: limited yield, minimal impact on treatment decisions. Chest. 2002;121:1486–1492. [DOI] [PubMed] [Google Scholar]
- 71. Tarver RD, Teague SD, Heitkamp DE, et al. Radiology of community‐acquired pneumonia. Radiol Clin North Am. 2005;43:497–512. [DOI] [PubMed] [Google Scholar]
- 72. Sharma S, Maycher B, Eschun G. Radiological imaging in pneumonia: recent innovations. Curr Opin Pulm Med. 2007;13:159–169. [DOI] [PubMed] [Google Scholar]
- 73. Reynolds JH, Mcdonald G, Alton H, et al. Pneumonia in the immunocompetent patient. Br J Radiol. 2010;83:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nambu A, Ozawa K, Kobayashi N, et al. Imaging of community‐acquired pneumonia: roles of imaging examinations, imaging diagnosis of specific pathogens and discrimination from noninfectious diseases. World J Radiol. 2014;6:779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Froudarakis ME. Diagnostic work‐up of pleural effusions. Respiration. 2008;75:4–13. [DOI] [PubMed] [Google Scholar]
- 76. Cha SI, Shin KM, Jeon KN, et al. Clinical relevance and characteristics of pleural effusion in patients with Mycoplasma pneumoniae pneumonia. Scand J Infect Dis. 2012;44:793–797. [DOI] [PubMed] [Google Scholar]
- 77. Falguera M, Carratala J, Ruiz‐Gonzalez A, et al. Risk factors and outcome of community‐acquired pneumonia due to Gram‐negative bacilli. Respirology. 2009;14:105–111. [DOI] [PubMed] [Google Scholar]
- 78. Letourneau AR, Issa NC, Baden LR. Pneumonia in the immunocompromised host. Curr Opin Pulm Med. 2014;20:272–279. [DOI] [PubMed] [Google Scholar]
- 79. Fishman JA. Infection in solid‐organ transplant recipients. N Engl J Med. 2007;357:2601–2614. [DOI] [PubMed] [Google Scholar]
- 80. Godbole G, Gant V. Respiratory tract infections in the immunocompromised. Curr Opin Pulm Med. 2013;19:244–250. [DOI] [PubMed] [Google Scholar]
- 81. Baron EJ, Miller JM, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sinclair A, Xie X, Teltscher M, et al. Systematic review and meta‐analysis of a urine‐based pneumococcal antigen test for diagnosis of community‐acquired pneumonia caused by Streptococcus pneumoniae . J Clin Microbiol. 2013;51:2303–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Horita N, Miyazawa N, Kojima R, et al. Sensitivity and specificity of the Streptococcus pneumoniae urinary antigen test for unconcentrated urine from adult patients with pneumonia: a meta‐analysis. Respirology. 2013;18:1177–1183. [DOI] [PubMed] [Google Scholar]
- 84. Chinese Thoracic Society . Guidelines for diagnosis and treatment of community‐acquired pneumonia. Chin J Tuber Respir Dis. 2006;29:651–655. [Google Scholar]
- 85. World Health Organization . Factsheet on TB diagnostics. [EB/OL]. (2014–04‐26). http://www.who.int/tb/publications/tbDiagnostics_factsheet.pdf. Accessed November 5, 2015.
- 86. World Health Organization . Implementing tuberculosis diagnostics: policy framework. [EB/OL]. (2015). http://apps.who.int/iris/bitstream/10665/162712/1/9789241508612_eng.pdf. Accessed November 5, 2015.
- 87. World Health Organization . Automated real‐time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children [EB/OL]. (2011). http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf. Accessed November 5, 2015.
- 88. Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.World Health Organization . Use of tuberculosis interferon‐gamma release assays (IGRAs) in low‐ and middle‐income countries: policy statement. [EB/OL]. (2011). http://whqlibdoc.who.int/publications/2011/9789241502672_eng.pdf. Accessed November 6, 2015. [PubMed]
- 90. Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. 2015;28:95–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Us Centers for Disease Control and Prevention . 2012 Nationally notifiable diseases and conditions and current case definitions.[EB/OL]. (2012). http://stacks.cdc.gov/view/cdc/12088/. Accessed November 5, 2015.
- 92. European Centre for Disease Control and Prevention . ECDC SURVEILLANCE REPORT: Legionnaires' disease in Europe [EB/OL]. (2013) [2015–11‐5]. http://ecdc.europa.eu/en/publications/Publications/legionnaires-disease-2015.pdf.
- 93. European Centre for Disease Control and Prevention . European Legionnaires' Disease Surveillance Network (ELDSNet): Operating Procedures. ECDC, Stockholm, Sweden: [EB/OL]. (2012) [2015–11‐5]. [Google Scholar]
- 94. European Commission . Commission Implementing Decision of 8 August 2012 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council [EB/OL]. (2012). http://eur-lex.europa.eu/legal-content/EN/TXT/?qid = 1443088458558&uri = CELEX:32012D0506. Accessed November 5, 2015.
- 95. Zumla A, Al‐Tawfiq JA, Enne VI, et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections – needs, advances, and future prospects. Lancet Infect Dis. 2014;14:1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shimada T, Noguchi Y, Jackson JL, et al. Systematic review and metaanalysis: urinary antigen tests for Legionellosis. Chest. 2009;136:1576–1585. [DOI] [PubMed] [Google Scholar]
- 97. Loens K, Goossens H, Ieven M. Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2010;29:1055–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dowell SF, Peeling RW, Boman J, et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin Infect Dis. 2001;33:492–503. [DOI] [PubMed] [Google Scholar]
- 100. Kumar S, Hammerschlag MR. Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin Infect Dis. 2007;44:568–576. [DOI] [PubMed] [Google Scholar]
- 101. Influenza Diagnosis and Treatment Expert Panel of the Chinese Ministry of Health . Chinese guidelines for diagnosis and treatment of influenza (2011). Chin J Tuber Respir Dis. 2011;34:725–734. [Google Scholar]
- 102. Us Centers for Disease Control and Prevention . Interim recommendations for clinical use of influenza diagnostic tests during the 2009–10 influenza season. [EB/OL]. (2009). http://www.cdc.gov/h1n1flu/guidance/diagnostic_tests.htm. Accessed November 5, 2015.
- 103. Falsey AR, Walsh EE. Viral pneumonia in older adults. Clin Infect Dis. 2006;42:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Arvanitis M, Anagnostou T, Fuchs BB, et al. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. 2014;27:490–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huston SM, Mody CH. Cryptococcosis: an emerging respiratory mycosis. Clin Chest Med. 2009;30:253–264. [DOI] [PubMed] [Google Scholar]
- 107. Marchetti O, Lamoth F, Mikulska M, et al. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 2012;47:846–854. [DOI] [PubMed] [Google Scholar]
- 108. Persat F, Ranque S, Derouin F, et al. Contribution of the (1→3)‐beta‐D‐glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2008;46:1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta‐analysis. Clin Infect Dis. 2006;42:1417–1427. [DOI] [PubMed] [Google Scholar]
- 110. Xu Z. Progress on the laboratory diagnosis of parasitic diseases [in Chinese]. Chin J Lab M. 2006;29:665–668. [Google Scholar]
- 111. Robert‐Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li M, Yin K, Yan G. Diagnostic techniques and study progress of toxoplasma disease [in Chinese]. J Pathogen Bio. 2011;6:942–944. [Google Scholar]
- 113. Zhang D. Laboratory diagnostic techniques for toxoplasma disease and progress in application [in Chinese]. J Tropic Dis Parasit. 2010;8:119–123. [Google Scholar]
- 114. Wong SS, Fung KS, Chau S, et al. Molecular diagnosis in clinical parasitology: when and why? Exp Biol Med (Maywood). 2014;239:1443–1460. [DOI] [PubMed] [Google Scholar]
- 115. Calbo E, Garau J. Application of pharmacokinetics and pharmacodynamics to antimicrobial therapy of community‐acquired respiratory tract infections. Respiration. 2005;72:561–571. [DOI] [PubMed] [Google Scholar]
- 116. Owens RC Jr, Ambrose PG. Antimicrobial stewardship and the role of pharmacokinetics‐pharmacodynamics in the modern antibiotic era. Diagn Microbiol Infect Dis. 2007;57(3 suppl):77S–83S. [DOI] [PubMed] [Google Scholar]
- 117. Houck PM, Bratzler DW, Nsa W, et al. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia. Arch Intern Med. 2004;164:637–644. [DOI] [PubMed] [Google Scholar]
- 118. Yahav D, Leibovici L, Goldberg E, et al. Time to first antibiotic dose for patients hospitalised with community acquired pneumonia. Int J Antimicrob Agents. 2013;41:410–413. [DOI] [PubMed] [Google Scholar]
- 119. Bader MS, Abouchehade KA, Yi Y, et al. Antibiotic administration longer than eight hours after triage and mortality of community‐acquired pneumonia in patients with diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2011;30:881–886. [DOI] [PubMed] [Google Scholar]
- 120. Menendez R, Torres A, Reyes S, et al. Initial management of pneumonia and sepsis: factors associated with improved outcome. Eur Respir J. 2012;39:156–162. [DOI] [PubMed] [Google Scholar]
- 121. Pakhale S, Mulpuru S, Verheij TJ, et al. Antibiotics for community‐acquired pneumonia in adult outpatients. Cochrane Database Syst Rev. 2014;10:CD002109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections – full version. Clin Microbiol Infect. 2011;17(suppl 6):E1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Morimoto T, Koyama H, Shimbo T, et al. Cost‐effectiveness analysis of ambulatory treatment for adult patients with community‐acquired pneumonia: according to Japanese Respiratory Society guidelines. Nihon Kokyuki Gakkai Zasshi. 2002;40:17–25. [PubMed] [Google Scholar]
- 124. Martin M, Quilici S, File T, et al. Cost‐effectiveness of empirical prescribing of antimicrobials in communityacquired pneumonia in three countries in the presence of resistance. J Antimicrob Chemother. 2007;59:977–989. [DOI] [PubMed] [Google Scholar]
- 125. Ailani RK, Agastya G, Ailani RK, et al. Doxycycline is a cost‐effective therapy for hospitalized patients with community‐acquired pneumonia. Arch Intern Med. 1999;159:266–270. [DOI] [PubMed] [Google Scholar]
- 126. Mokabberi R, Haftbaradaran A, Ravakhah K. Doxycycline vs. levofloxacin in the treatment of communityacquired pneumonia. J Clin Pharm Ther. 2010;35:195–200. [DOI] [PubMed] [Google Scholar]
- 127. Teh B, Grayson ML, Johnson PD, et al. Doxycycline vs. macrolides in combination therapy for treatment of community‐acquired pneumonia. Clin Microbiol Infect. 2012;18:E71–E73. [DOI] [PubMed] [Google Scholar]
- 128. Fan H, Liu S, Tong X. Respiratory Fluoroquinolones monotherapy versus β‐lactams plus macrolides combination therapy for non‐ICU hospitalized community‐acquired pneumonia patients: a meta‐analysis. Chin J Evid‐Based Med. 2015;15:824–832. [Google Scholar]
- 129. Ortiz‐Ruiz G, Vetter N, Isaacs R, et al. Ertapenem versus ceftriaxone for the treatment of community‐acquired pneumonia in adults: combined analysis of two multicentre randomized, double‐blind studies. J Antimicrob Chemother. 2004;53(suppl 2):ii59–ii66. [DOI] [PubMed] [Google Scholar]
- 130. Leroy O, Saux P, Bedos JP, et al. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community‐acquired pneumonia who do not require vasopressors. Chest. 2005;128:172–183. [DOI] [PubMed] [Google Scholar]
- 131. Yuan X, Liang BB, Wang R, et al. Treatment of community‐acquired pneumonia with moxifloxacin: a meta‐analysis of randomized controlled trials. J Chemother. 2012;24:257–267. [DOI] [PubMed] [Google Scholar]
- 132. Frei CR, Labreche MJ, Attridge RT. Fluoroquinolones in community‐acquired pneumonia: guide to selection and appropriate use. Drugs. 2011;71:757–770. [DOI] [PubMed] [Google Scholar]
- 133. Thiem U, Heppner HJ, Pientka L. Elderly patients with community‐acquired pneumonia: optimal treatment strategies. Drugs Aging. 2011;28:519–537. [DOI] [PubMed] [Google Scholar]
- 134. Yamasaki K, Kawanami T, Yatera K, et al. Significance of anaerobes and oral bacteria in community‐acquired pneumonia. PLoS One. 2013;8:e63103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Japanese Respiratory Society . Aspiration pneumonia. Respirology. 2009;14(suppl 2):S59–S64. [DOI] [PubMed] [Google Scholar]
- 136. Petroianni A, Ceccarelli D, Conti V, et al. Aspiration pneumonia. Pathophysiological aspects, prevention and management. A review. Panminerva Med. 2006;48:231–239. [PubMed] [Google Scholar]
- 137. Johnson JL, Hirsch CS. Aspiration pneumonia. Recognizing and managing a potentially growing disorder . Postgrad Med. 2003;113:99–102. 105–106, 111–102. [DOI] [PubMed] [Google Scholar]
- 138. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild‐to‐moderate aspiration pneumonia in elderly patients. Chest. 2005;127:1276–1282. [DOI] [PubMed] [Google Scholar]
- 139. Allewelt M, Schuler P, Bolcskei PL, et al. Ampicillin + sulbactam vs clindamycin ± cephalosporin for the treatment of aspiration pneumonia and primary lung abscess. Clin Microbiol Infect. 2004;10:163–170. [DOI] [PubMed] [Google Scholar]
- 140. Ott SR, Allewelt M, Lorenz J, et al. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36:23–30. [DOI] [PubMed] [Google Scholar]
- 141. Sun T, Sun L, Wang R, et al. Clinical efficacy and safety of moxifloxacin versus levofloxacin plus metronidazole for community‐acquired pneumonia with aspiration factors. Chin Med J (Engl). 2014;127:1201–1205. [PubMed] [Google Scholar]
- 142. Malloy AM, Campos JM. Extended‐spectrum beta‐lactamases: a brief clinical update. Pediatr Infect Dis J. 2011;30:1092–1093. [DOI] [PubMed] [Google Scholar]
- 143. Paterson DL, Bonomo RA. Extended‐spectrum beta‐lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Einhorn AE, Neuhauser MM, Bearden DT, et al. Extended‐spectrum beta‐lactamases: frequency, risk factors, and outcomes. Pharmacotherapy. 2002;22:14–20. [DOI] [PubMed] [Google Scholar]
- 145. Kerneis S, Valade S, Geri G, et al. Cefoxitin as a carbapenem‐sparing antibiotic for infections caused by extended‐spectrum beta‐lactamase‐producing Escherichia coli and Klebsiella pneumoniae . Infect Dis (Lond). 2015;47:789–795. [DOI] [PubMed] [Google Scholar]
- 146. Matsumura Y, Yamamoto M, Nagao M, et al. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended‐spectrum‐beta‐lactamase‐producing Escherichia coli bacteremia. Antimicrob Agents Chemother. 2015;59:5107–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hu F, Zhu D, Wang F, et al. 2013 CHINET surveillance of bacterial resistance in China. Chin J Infect Chemother. 2014;14:365–374. [Google Scholar]
- 148. Lee N, Leo YS, Cao B, et al. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur Respir J. 2015;45:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 150. Lee N, Choi KW, Chan PK, et al. Outcomes of adults hospitalised with severe influenza. Thorax. 2010;65:510–515. [DOI] [PubMed] [Google Scholar]
- 151. Higuera Iglesias AL, Kudo K, Manabe T, et al. Reducing occurrence and severity of pneumonia due to pandemic H1N1 2009 by early oseltamivir administration: a retrospective study in Mexico. PLoS One. 2011;6:e21838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta‐analysis of individual participant data. Lancet Respir Med. 2014;2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Morens D, Taubenberger J, Fauci A. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rice TW, Rubinson L, Uyeki TM, et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40:1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wang XY, Kilgore PE, Lim KA, et al. Influenza and bacterial pathogen coinfections in the 20th century. Interdiscip Perspect Infect Dis. 2011;2011:146376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Scalera NM, File TM. Jr., How long should we treat community‐acquired pneumonia? Curr Opin Infect Dis. 2007;20:177–181. [DOI] [PubMed] [Google Scholar]
- 157. Pinzone MR, Cacopardo B, Abbo L. Duration of antimicrobial therapy in community acquired pneumonia: less is more. ScientificWorldJournal. 2014;2014:759138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. El Moussaoui R, De Borgie CA, Van Den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Eccles S, Pincus C, Higgins B, et al. Diagnosis and management of community and hospital acquired pneumonia in adults: summary of NICE guidance. BMJ. 2014;349:g6722 [DOI] [PubMed] [Google Scholar]
- 160. Wang H, Chen M, Xu Y, et al. Antimicrobial susceptibility of bacterial pathogens associated with community‐acquired respiratory tract infections in Asia: report from the Community‐Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study, 2009–2010. Int J Antimicrob Agents. 2011;38:376–383. [DOI] [PubMed] [Google Scholar]
- 161. Ruiz M, Ewig S, Marcos MA, et al. Etiology of community‐acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. [DOI] [PubMed] [Google Scholar]
- 162. Cilloniz C, Ewig S, Ferrer M, et al. Community‐acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care. 2011;15:R209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Arancibia F, Cortes CP, Valdes M, et al. Importance of Legionella pneumophila in the etiology of severe community‐acquired pneumonia in Santiago, Chile. Chest. 2014;145:290–296. [DOI] [PubMed] [Google Scholar]
- 164. Ishiguro T, Takayanagi N, Yamaguchi S, et al. Etiology and factors contributing to the severity and mortality of community‐acquired pneumonia. Intern Med. 2013;52:317–324. [DOI] [PubMed] [Google Scholar]
- 165. Ishida T, Tachibana H, Ito A, et al. Clinical characteristics of severe community‐acquired pneumonia among younger patients: an analysis of 18 years at a community hospital. J Infect Chemother. 2014;20:471–476. [DOI] [PubMed] [Google Scholar]
- 166. Sun K, Metzger DW. Influenza infection suppresses NADPH oxidase‐dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin‐resistant Staphylococcus aureus infection. J Immunol. 2014;192:3301–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa‐2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chawla S, Demuro JP. Current controversies in the support of sepsis. Curr Opin Crit Care. 2014;20:681–684. [DOI] [PubMed] [Google Scholar]
- 169. Britton S, Bejstedt M, Vedin L. Chest physiotherapy in primary pneumonia. Br Med J (Clin Res Ed). 1985;290:1703–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mundy LM, Leet TL, Darst K, et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889. [DOI] [PubMed] [Google Scholar]
- 171. Bjorkqvist M, Wiberg B, Bodin L, et al. Bottle‐blowing in hospital‐treated patients with community‐acquired pneumonia. Scand J Infect Dis. 1997;29:77–82. [DOI] [PubMed] [Google Scholar]
- 172. Morton B, Pennington SH, Gordon SB. Immunomodulatory adjuvant therapy in severe community‐acquired pneumonia. Expert Rev Respir Med. 2014;8:587–596. [DOI] [PubMed] [Google Scholar]
- 173. Blot S, Rodriguez A, Sole‐Violan J, et al. Effects of delayed oxygenation assessment on time to antibiotic delivery and mortality in patients with severe community‐acquired pneumonia. Crit Care Med. 2007;35:2509–2514. [DOI] [PubMed] [Google Scholar]
- 174. O'driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63(suppl 6):vi1–v68. [DOI] [PubMed] [Google Scholar]
- 175. Frat JP, Thille AW, Mercat A, et al. High‐flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. [DOI] [PubMed] [Google Scholar]
- 176. Peters SG, Holets SR, Gay PC. High‐flow nasal cannula therapy in do‐not‐intubate patients with hypoxemic respiratory distress. Respir Care. 2013;58:597–600. [DOI] [PubMed] [Google Scholar]
- 177. Ferrer M, Esquinas A, Leon M, et al. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168:1438–1444. [DOI] [PubMed] [Google Scholar]
- 178. Brambilla AM, Aliberti S, Prina E, et al. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014;40:942–949. [DOI] [PubMed] [Google Scholar]
- 179. Zhan Q, Sun B, Liang L, et al. Early use of noninvasive positive pressure ventilation for acute lung injury: a multicenter randomized controlled trial. Crit Care Med. 2012;40:455–460. [DOI] [PubMed] [Google Scholar]
- 180. Confalonieri M, Potena A, Carbone G, et al. Acute respiratory failure in patients with severe community‐acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160:1585–1591. [DOI] [PubMed] [Google Scholar]
- 181. Liu Z, Feng H, Jiang Y, et al. Efficacy analysis of noninvasive positive pressure ventilation in acute respiratory failure in elderly patients with community‐acquired pneumonia [in Chinese]. Chin J Geriatrics. 2013;32:1062–1065. [Google Scholar]
- 182. Delclaux C, L'her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284:2352–2360. [DOI] [PubMed] [Google Scholar]
- 183. Cosentini R, Brambilla AM, Aliberti S, et al. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community‐acquired pneumonia: a randomized, controlled trial. Chest. 2010;138:114–120. [DOI] [PubMed] [Google Scholar]
- 184. Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi‐center study. Intensive Care Med. 2001;27:1718–1728. [DOI] [PubMed] [Google Scholar]
- 185. Carrillo A, Gonzalez‐Diaz G, Ferrer M, et al. Non‐invasive ventilation in community‐acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38:458–466. [DOI] [PubMed] [Google Scholar]
- 186. The Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 187. Eisner MD, Thompson T, Hudson LD, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. [DOI] [PubMed] [Google Scholar]
- 188. Bartlett RH, Roloff DW, Custer JR, et al. Extracorporeal life support: the University of Michigan experience. JAMA. 2000;283:904–908. [PubMed] [Google Scholar]
- 189. Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure‐controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. [DOI] [PubMed] [Google Scholar]
- 190. Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196. [DOI] [PubMed] [Google Scholar]
- 191. Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. [DOI] [PubMed] [Google Scholar]
- 192. Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–1914. [DOI] [PubMed] [Google Scholar]
- 193. Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community‐acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. [DOI] [PubMed] [Google Scholar]
- 194. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community‐acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–686. [DOI] [PubMed] [Google Scholar]
- 195. Sabry NA, Omar EE‐D. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacol Pharm. 2011;28:73–81. [Google Scholar]
- 196. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- 197. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community‐acquired pneumonia in adults: a meta‐analysis. PLoS One. 2012;7:e47926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Chen L, Chen J, Chen Y, et al. Efficacy and safety of glucocorticoids in the treatment of community‐acquired pneumonia: a meta‐analysis of randomized controlled trials. Chin J Emerg Med. 2014;23:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Oster G, Berger A, Edelsberg J, et al. Initial treatment failure in non‐ICU community‐acquired pneumonia: risk factors and association with length of stay, total hospital charges, and mortality. J Med Econ. 2013;16:809–819. [DOI] [PubMed] [Google Scholar]
- 200. Patterson CM, Loebinger MR. Community acquired pneumonia: assessment and treatment. Clin Med. 2012;12:283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Bruns AH, Oosterheert JJ, Prokop M, et al. Patterns of resolution of chest radiograph abnormalities in adults hospitalized with severe community‐acquired pneumonia. Clin Infect Dis. 2007;45:983–991. [DOI] [PubMed] [Google Scholar]
- 202. Bruns AH, Oosterheert JJ, El Moussaoui R, et al. Pneumonia recovery: discrepancies in perspectives of the radiologist, physician and patient. J Gen Intern Med. 2010;25:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ewig S, Woodhead M, Torres A. Towards a sensible comprehension of severe community‐acquired pneumonia. Intensive Care Med. 2011;37:214–223. [DOI] [PubMed] [Google Scholar]
- 204. Menendez R, Torres A. Treatment failure in community‐acquired pneumonia. Chest. 2007;132:1348–1355. [DOI] [PubMed] [Google Scholar]
- 205. Roson B, Carratala J, Fernandez‐Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community‐acquired pneumonia. Arch Intern Med. 2004;164:502–508. [DOI] [PubMed] [Google Scholar]
- 206. Chalmers JD, Singanayagam A, Hill AT. C‐reactive protein is an independent predictor of severity in community‐acquired pneumonia. Am J Med. 2008;121:219–225. [DOI] [PubMed] [Google Scholar]
- 207. Menendez R, Cavalcanti M, Reyes S, et al. Markers of treatment failure in hospitalised community acquired pneumonia. Thorax. 2008;63:447–452. [DOI] [PubMed] [Google Scholar]
- 208. Bruns AH, Oosterheert JJ, Hak E, et al. Usefulness of consecutive C‐reactive protein measurements in follow‐up of severe community‐acquired pneumonia. Eur Respir J. 2008;32:726–732. [DOI] [PubMed] [Google Scholar]
- 209. Menendez R, Martinez R, Reyes S, et al. Stability in community‐acquired pneumonia: one step forward with markers? Thorax. 2009;64:987–992. [DOI] [PubMed] [Google Scholar]
- 210. Stralin K, Tornqvist E, Kaltoft MS, et al. Etiologic diagnosis of adult bacterial pneumonia by culture and PCR applied to respiratory tract samples. J Clin Microbiol. 2006;44:643–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Lidman C, Burman LG, Lagergren A, et al. Limited value of routine microbiological diagnostics in patients hospitalized for community‐acquired pneumonia. Scand J Infect Dis. 2002;34:873–879. [DOI] [PubMed] [Google Scholar]
- 212. Campbell SG, Marrie TJ, Anstey R, et al. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study. Chest. 2003;123:1142–1150. [DOI] [PubMed] [Google Scholar]
- 213. Johansson N, Kalin M, Giske CG, et al. Quantitative detection of Streptococcus pneumoniae from sputum samples with real‐time quantitative polymerase chain reaction for etiologic diagnosis of community‐acquired pneumonia. Diagn Microbiol Infect Dis. 2008;60:255–261. [DOI] [PubMed] [Google Scholar]
- 214. Stralin K. Usefulness of aetiological tests for guiding antibiotic therapy in community‐acquired pneumonia. Int J Antimicrob Agents. 2008;31:3–11. [DOI] [PubMed] [Google Scholar]
- 215. Paganin F, Lilienthal F, Bourdin A, et al. Severe community‐acquired pneumonia: assessment of microbial aetiology as mortality factor. Eur Respir J. 2004;24:779–785. [DOI] [PubMed] [Google Scholar]
- 216. Metersky ML, Ma A, Bratzler DW, et al. Predicting bacteremia in patients with community‐acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342–347. [DOI] [PubMed] [Google Scholar]
- 217. Roson B, Fernandez‐Sabe N, Carratala J, et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis. 2004;38:222–226. [DOI] [PubMed] [Google Scholar]
- 218. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Gutierrez F, Masia M, Rodriguez JC, et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community‐acquired pneumonia in Spain. Clin Infect Dis. 2003;36:286–292. [DOI] [PubMed] [Google Scholar]
- 220. Metersky ML. Is the lateral decubitus radiograph necessary for the management of a parapneumonic pleural effusion? Chest. 2003;124:1129–1132. [DOI] [PubMed] [Google Scholar]
- 221. Menendez R, Torres A, Rodriguez De Castro F, et al. Reaching stability in community‐acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783–1790. [DOI] [PubMed] [Google Scholar]
- 222. Athanassa Z, Makris G, Dimopoulos G, et al. Early switch to oral treatment in patients with moderate to severe community‐acquired pneumonia: a meta‐analysis. Drugs. 2008;68:2469–2481. [DOI] [PubMed] [Google Scholar]
- 223. Arancibia F, Ewig S, Martinez JA, et al. Antimicrobial treatment failures in patients with community‐acquired pneumonia: causes and prognostic implications. Am J Respir Crit Care Med. 2000;162:154–160. [DOI] [PubMed] [Google Scholar]
- 224. Sialer S, Liapikou A, Torres A. What is the best approach to the nonresponding patient with community‐acquired pneumonia? Infect Dis Clin North Am. 2013;27:189–203. [DOI] [PubMed] [Google Scholar]
- 225. Chen X. Cause and management of community‐acquired pneumonia with no reaction to initial treatment [in Chinese]. Chin J Pract Intern Med. 2014;34:94–96. [Google Scholar]
- 226. Menendez R, Torres A, Zalacain R, et al. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004;59:960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ramirez JA, Anzueto AR. Changing needs of community‐acquired pneumonia. J Antimicrob Chemother. 2011;66(suppl 3):iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Karhu J, Ala‐Kokko TI, Vuorinen T, et al. Lower respiratory tract virus findings in mechanically ventilated patients with severe community‐acquired pneumonia. Clin Infect Dis. 2014;59:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507–2517. [DOI] [PubMed] [Google Scholar]
- 230. Yu H, Feng Z, Uyeki TM, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;52:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Zhang PJ, Cao B, Li XL, et al. Risk factors for adult death due to 2009 pandemic influenza A (H1N1) virus infection: a 2151 severe and critical cases analysis. Chin Med J (Engl). 2013;126:2222–2228. [PubMed] [Google Scholar]
- 232. Yang SQ, Qu JX, Wang C, et al. Influenza pneumonia among adolescents and adults: a concurrent comparison between influenza A (H1N1) pdm09 and A (H3N2) in the post‐pandemic period. Clin Respir J. 2014;8:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Cao B, Li XW, Shu YL, et al. Clinical and epidemiologic characteristics of 3 early cases of influenza A pandemic (H1N1) 2009 virus infection, People's Republic of China, 2009. Emerg Infect Dis. 2009;15:1418–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Bai L, Gu L, Cao B, et al. Clinical features of pneumonia caused by 2009 influenza A (H1N1) virus in Beijing, China. Chest. 2011;139:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368:2277–2285. [DOI] [PubMed] [Google Scholar]
- 236. Koopmans M, De Jong MD. Avian influenza A H7N9 in Zhejiang, China. Lancet. 2013;381:1882–1883. [DOI] [PubMed] [Google Scholar]
- 237. Yu H, Cowling BJ, Feng L, et al. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;382:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Zhang W, Wang L, Hu W, et al. Epidemiologic characteristics of cases for influenza A (H7N9) virus infections in China. Clin Infect Dis. 2013;57:619–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Chen Y, Liang W, Yang S, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381:1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Cowling BJ, Jin L, Lau EH, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population‐based study of laboratory‐confirmed cases. Lancet. 2013;382:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. He F, Zhang M, Wang X, et al. Distinct risk profiles for human infections with the Influenza A(H7N9) virus among rural and urban residents: Zhejiang Province, China, 2013. PLoS One. 2014;9(5):e95015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Zhang J, Zhao Y, Chen Y. Laboratory findings in patients with avian‐origin influenza A (H7N9) virus infections. J Med Virol. 2014;86:895–898. [DOI] [PubMed] [Google Scholar]
- 243. Ji H, Gu Q, Chen LL, et al. Epidemiological and clinical characteristics and risk factors for death of patients with avian influenza A H7N9 virus infection from Jiangsu Province, Eastern China. PLoS One. 2014;9:e89581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A (H7N9) virus in China. N Engl J Med. 2014;370:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Ke Y, Wang Y, Liu S, et al. High severity and fatality of human infections with avian influenza A(H7N9) infection in China. Clin Infect Dis. 2013;57:1506–1507. [DOI] [PubMed] [Google Scholar]
- 246. Hu Y, Lu S, Song Z, et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381:2273–2279. [DOI] [PubMed] [Google Scholar]
- 247. Wang C, Yu H, Horby PW, et al. Comparison of patients hospitalized with influenza A subtypes H7N9, H5N1, and 2009 pandemic H1N1. Clin Infect Dis. 2014;58:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Ip DK, Liao Q, Wu P, et al. Detection of mild to moderate influenza A/H7N9 infection by China's national sentinel surveillance system for influenza‐like illness: case series. BMJ. 2013;346:f3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Tang X, He H, Sun B, et al. ARDS associated with pneumonia caused by avian influenza A H7N9 virus treated with extracorporeal membrane oxygenation. Clin Respir J. 2015;9:380–384. [DOI] [PubMed] [Google Scholar]
- 250. Gao R, Cao B, Hu Y, et al. Human infection with a novel avian‐origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. [DOI] [PubMed] [Google Scholar]
- 251. Chen H, Yuan H, Gao R, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–721. [DOI] [PubMed] [Google Scholar]
- 252. Wan J, Zhang J, Tao W, et al. A report of first fatal case of H10N8 avian influenza virus pneumonia in the world. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:120–122. [DOI] [PubMed] [Google Scholar]
- 253. Yang SG, Cao B, Liang LR, et al. Antiviral therapy and outcomes of patients with pneumonia caused by influenza A pandemic (H1N1) virus. PLoS One. 2012;7:e29652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Yu H, Liao Q, Yuan Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. National Health and Family Planning Commission of the People's Republic of China . Diagnostic and treatment protocol for human infections with avian influenza A (H7N9) (2014). Chin J Clin Infect Dis. 2014;7:1–3. [Google Scholar]
- 256. Jefferson T, Jones M, Doshi P, et al. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Heneghan CJ, Onakpoya I, Thompson M, et al. Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Kohno S, Yen MY, Cheong HJ, et al. Phase III randomized, double‐blind study comparing single‐dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011;55:5267–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Cao B, Huang GH, Pu ZH, et al. Emergence of community‐acquired adenovirus type 55 as a cause of community‐onset pneumonia. Chest. 2014;145:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Gu L, Liu Z, Li X, et al. Severe community‐acquired pneumonia caused by adenovirus type 11 in immunocompetent adults in Beijing. J Clin Virol. 2012;54:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zhu Z, Zhang Y, Xu S, et al. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J Clin Microbiol. 2009;47:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Sun B, He H, Wang Z, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. 2014;18:456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Kim SJ, Kim K, Park SB, et al. Outcomes of early administration of cidofovir in non‐immunocompromised patients with severe adenovirus pneumonia. PLoS One. 2015;10:e0122642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Walsh EE. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2011;32:423–432. [DOI] [PubMed] [Google Scholar]
- 265. Chu HY, Englund JA. Respiratory syncytial virus disease: prevention and treatment. Curr Top Microbiol Immunol. 2013;372:235–258. [DOI] [PubMed] [Google Scholar]
- 266. Mayer JL, Lehners N, Egerer G, et al. CT‐morphological characterization of respiratory syncytial virus (RSV) pneumonia in immune‐compromised adults. Rofo. 2014;186:686–692. [DOI] [PubMed] [Google Scholar]
- 267. Ventre K, Randolph AG. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2007;(1):CD000181 [DOI] [PubMed] [Google Scholar]
- 268. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:9995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Das KM, Lee EY, Enani MA, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol. 2015;204:736–742. [DOI] [PubMed] [Google Scholar]
- 271. Shalhoub S, Farahat F, Al‐Jiffri A, et al. IFN‐alpha2a or IFN‐beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Engel MF, Van Manen L, Hoepelman AI, et al. Diagnostic, therapeutic and economic consequences of a positive urinary antigen test for Legionella spp. in patients admitted with community‐acquired pneumonia: a 7‐year retrospective evaluation. J Clin Pathol. 2013;66(9):797–802. [DOI] [PubMed] [Google Scholar]
- 273. Hilbi H, Jarraud S, Hartland E, et al. Update on Legionnaires' disease: pathogenesis, epidemiology, detection and control. Mol Microbiol. 2010;76:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch Intern Med. 1994;154:2417–2422. [PubMed] [Google Scholar]
- 275. Phin N, Parry‐Ford F, Harrison T, et al. Epidemiology and clinical management of Legionnaires' disease. Lancet Infect Dis. 2014;14:1011–1021. [DOI] [PubMed] [Google Scholar]
- 276. Diederen BM. Legionella spp. and Legionnaires' disease. J Infect. 2008;56:1–12. [DOI] [PubMed] [Google Scholar]
- 277. Lanternier F, Tubach F, Ravaud P, et al. Incidence and risk factors of Legionella pneumophila pneumonia during anti‐tumor necrosis factor therapy: a prospective French study. Chest. 2013;144:990–998. [DOI] [PubMed] [Google Scholar]
- 278. Garcia‐Vidal C, Carratala J. Current clinical management of Legionnaires' disease. Expert Rev Anti Infect Ther. 2006;4:995–1004. [DOI] [PubMed] [Google Scholar]
- 279. Morelli N, Battaglia E, Lattuada P. Brainstem involvement in Legionnaires' disease. Infection. 2006;34:49–52. [DOI] [PubMed] [Google Scholar]
- 280. Fiumefreddo R, Zaborsky R, Haeuptle J, et al. Clinical predictors for Legionella in patients presenting with community‐acquired pneumonia to the emergency department. BMC Pulm Med. 2009;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Cunha BA. Severe Legionella pneumonia: rapid presumptive clinical diagnosis with Winthrop‐University Hospital's weighted point score system (modified). Heart Lung. 2008;37:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Daumas A, El‐Mekaoui F, Bataille S, et al. Acute tubulointerstitial nephritis complicating Legionnaires' disease: a case report. J Med Case Rep. 2012;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Fernandez‐Sabe N, Roson B, Carratala J, et al. Clinical diagnosis of Legionella pneumonia revisited: evaluation of the community‐based pneumonia Incidence Study Group scoring system. Clin Infect Dis. 2003;37:483–489. [DOI] [PubMed] [Google Scholar]
- 284. Yagyu H, Nakamura H, Tsuchida F, et al. Chest CT findings and clinical features in mild Legionella pneumonia. Intern Med. 2003;42:477–482. [DOI] [PubMed] [Google Scholar]
- 285. Sakai F, Tokuda H, Goto H, et al. Computed tomographic features of Legionella pneumophila pneumonia in 38 cases. J Comput Assist Tomogr. 2007;31:125–131. [DOI] [PubMed] [Google Scholar]
- 286. Tan MJ, Tan JS, Hamor RH, et al. The radiologic manifestations of Legionnaire's disease. The Ohio Community‐Based Pneumonia Incidence Study Group. Chest. 2000;117:398–403. [DOI] [PubMed] [Google Scholar]
- 287. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138–1180. [DOI] [PubMed] [Google Scholar]
- 288. Amsden GW. Treatment of Legionnaires' disease. Drugs. 2005;65:605–614. [DOI] [PubMed] [Google Scholar]
- 289. Jacobson KL, Miceli MH, Tarrand JJ, et al. Legionella pneumonia in cancer patients. Medicine (Baltimore). 2008;87:152–159. [DOI] [PubMed] [Google Scholar]
- 290. Nakamura S, Yanagihara K, Izumikawa K, et al. The clinical efficacy of fluoroquinolone and macrolide combination therapy compared with single‐agent therapy against community‐acquired pneumonia caused by Legionella pneumophila. J Infect. 2009;59:222–224. [DOI] [PubMed] [Google Scholar]
- 291. Zhao C, Liu Y, Zhao M, et al. Characterization of community acquired Staphylococcus aureus associated with skin and soft tissue infection in Beijing: high prevalence of PVL+ ST398. PLoS One. 2012;7:e38577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Wu CL, Ku SC, Yang KY, et al. Antimicrobial drug‐resistant microbes associated with hospitalized community‐acquired and healthcare‐associated pneumonia: a multi‐center study in Taiwan. J Formos Med Assoc. 2013;112:31–40. [DOI] [PubMed] [Google Scholar]
- 293. Umeki K, Tokimatsu I, Yasuda C, et al. Clinical features of healthcare‐associated pneumonia (HCAP) in a Japanese community hospital: comparisons among nursing home‐acquired pneumonia (NHAP), HCAP other than NHAP, and community‐acquired pneumonia. Respirology. 2011;16:856–861. [DOI] [PubMed] [Google Scholar]
- 294. Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health‐care‐associated pneumonia: results from a large US database of culture‐positive pneumonia. Chest. 2005;128:3854–3862. [DOI] [PubMed] [Google Scholar]
- 295. Vardakas KZ, Matthaiou DK, Falagas ME. Incidence, characteristics and outcomes of patients with severe community acquired‐MRSA pneumonia. Eur Respir J. 2009;34:1148–1158. [DOI] [PubMed] [Google Scholar]
- 296. Li H, Zhang T, Huang J, et al. Analysis of risk factors related to mortality of patients with community‐acquired pneumonia due to methicillin‐resistant Staphylococcus aureus . Chin Crit Care Med. 2010;22(8):459–464. [PubMed] [Google Scholar]
- 297. Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton‐Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. [DOI] [PubMed] [Google Scholar]
- 298. Nguyen ET, Kanne JP, Hoang LM, et al. Community‐acquired methicillin‐resistant Staphylococcus aureus pneumonia: radiographic and computed tomography findings. J Thorac Imaging. 2008;23:13–19. [DOI] [PubMed] [Google Scholar]
- 299. Wunderink RG. How important is methicillin‐resistant Staphylococcus aureus as a cause of community‐acquired pneumonia and what is best antimicrobial therapy? Infect Dis Clin North Am. 2013;27:177–188. [DOI] [PubMed] [Google Scholar]
- 300. Stupka JE, Mortensen EM, Anzueto A, et al. Community‐acquired pneumonia in elderly patients. Aging Health. 2009;5:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J. 2008;101:1141–1145. [DOI] [PubMed] [Google Scholar]
- 302. Maruyama T, Gabazza EC, Morser J, et al. Community‐acquired pneumonia and nursing home‐acquired pneumonia in the very elderly patients. Respir Med. 2010;104:584–592. [DOI] [PubMed] [Google Scholar]
- 303. Fung HB, Monteagudo‐Chu MO. Community‐acquired pneumonia in the elderly. Am J Geriatr Pharmacother. 2010;8:47–62. [DOI] [PubMed] [Google Scholar]
- 304. Committee for the Japanese Respiratory Society Guidelines for the Management of Respiratory I . Guidelines for the management of community acquired pneumonia in adults, revised edition. Respirology. 2006;11(suppl 3):S79–133. [DOI] [PubMed] [Google Scholar]
- 305. Cilloniz C, Polverino E, Ewig S, et al. Impact of age and comorbidity on cause and outcome in communityacquired pneumonia. Chest. 2013;144:999–1007. [DOI] [PubMed] [Google Scholar]
- 306. Huang HH, Zhang YY, Xiu QY, et al. Community‐acquired pneumonia in Shanghai, China: microbial etiology and implications for empirical therapy in a prospective study of 389 patients. Eur J Clin Microbiol Infect Dis. 2006;25:369–374. [DOI] [PubMed] [Google Scholar]
- 307. Gonzalez‐Castillo J, Martin‐Sanchez FJ, Llinares P, et al. Guidelines for the management of community‐acquired pneumonia in the elderly patient. Rev Esp Quimioter. 2014;27:69–86. [PubMed] [Google Scholar]
- 308. Faverio P, Aliberti S, Bellelli G, et al. The management of community‐acquired pneumonia in the elderly. Eur J Intern Med. 2014;25:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Aliberti S, Amir A, Peyrani P, et al. Incidence, etiology, timing, and risk factors for clinical failure in hospitalized patients with community‐acquired pneumonia. Chest. 2008;134:955–962. [DOI] [PubMed] [Google Scholar]
- 310. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–671. [DOI] [PubMed] [Google Scholar]
- 311. Kwong JC, Howden BP, Charles PG. New aspirations: the debate on aspiration pneumonia treatment guidelines. Med J Aust. 2011;195:380–381. [DOI] [PubMed] [Google Scholar]
- 312. Komiya K, Ishii H, Kadota J. Healthcare‐associated pneumonia and aspiration pneumonia. Aging Dis. 2015;6:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Wang X, Gao Y, Wang Q, et al. Relevant risk factors and management of aspiration pneumonia in elderly patients [in Chinese]. Chin J Nosocomiology. 2014;24:1161–1162, 1165. [Google Scholar]
- 314. Van Der Maarel‐Wierink CD, Vanobbergen JN, Bronkhorst EM, et al. Meta‐analysis of dysphagia and aspiration pneumonia in frail elders. J Dent Res. 2011;90:1398–1404. [DOI] [PubMed] [Google Scholar]
- 315. Pace CC, Mccullough GH. The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25:307–322. [DOI] [PubMed] [Google Scholar]
- 316. Dibardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care. 2015;30:40–48. [DOI] [PubMed] [Google Scholar]
- 317. Wang J, Chang Z. Clinical risk factors and radiology analysis of aspiration pneumonia in elderly patients. J Clin Intern Med. 2013;30:270–272. [Google Scholar]
- 318. Finegold SM. Aspiration pneumonia. Rev Infect Dis. 1991;13(suppl 9):S737–S742. [DOI] [PubMed] [Google Scholar]
- 319. Komiya K, Ishii H, Umeki K, et al. Computed tomography findings of aspiration pneumonia in 53 patients. Geriatr Gerontol Int. 2013;13:580–585. [DOI] [PubMed] [Google Scholar]
- 320. Tokuyasu H, Harada T, Watanabe E, et al. Effectiveness of meropenem for the treatment of aspiration pneumonia in elderly patients. Intern Med. 2009;48:129–135. [DOI] [PubMed] [Google Scholar]
- 321. Marik PE. Pulmonary aspiration syndromes. Curr Opin Pulm Med. 2011;17:148–154. [DOI] [PubMed] [Google Scholar]
- 322. Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–336. [DOI] [PubMed] [Google Scholar]
- 323. Fan Z, Qu J, Zhu H. Study progress on aspiration pneumonia. Chin J Respir Crit Care Med. 2010;9:209–212. [Google Scholar]
- 324. Torres A, Peetermans WE, Viegi G, et al. Risk factors for community‐acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Centers for Disease Control and Prevention (CDC) . Respiratory hygiene/cough etiquette in health‐care settings[EB/OL]. (2012–02‐27). http://www.cdc.gov/flu/professionals/infectioncontrol/resphygiene.htm. Accessed May 23, 2015.
- 326. Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Bridges CB, Coyne‐Beasley T. Advisory Committee on Immunization Practices Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older: United States, 2014. Ann Intern Med. 2014;160:190–199 [DOI] [PubMed] [Google Scholar]
- 328. Wang Q, Zhang F, Zhao C, et al. Antimicrobial resistance and serotype distribution of Streptococcus pneumoniae isolated from multi‐centers across China, 2010–2011. Chin J Tuber Respir Dis. 2013;36:106–112. [PubMed] [Google Scholar]
- 329. Liu C, Zhao C, Liu Y, et al. Study of serotype distribution, antimicrobial resistance patterns and molecular epidemiology in 148 isolates of invasive Streptococcus pneumoniae . Nat Med J Chin. 2010;90:1565–1570. [PubMed] [Google Scholar]
- 330. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. [DOI] [PubMed] [Google Scholar]
- 331. Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13‐valent pneumococcal conjugate vaccine and 23‐valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 332. Wilson KC, Schunemann HJ. An appraisal of the evidence underlying performance measures for community acquired pneumonia. Am J Respir Crit Care Med. 2011;183:1454–1462. [DOI] [PubMed] [Google Scholar]
- 333. Jefferson T, Di Pietrantonj C, Rivetti A, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2014;3:CD001269 [DOI] [PubMed] [Google Scholar]
- 334. Ferdinands JM, Gargiullo P, Haber M, et al. Inactivated influenza vaccines for prevention of community‐acquired pneumonia: the limits of using nonspecific outcomes in vaccine effectiveness studies. Epidemiology. 2013;24:530–537. [DOI] [PubMed] [Google Scholar]
- 335. Guideline of seasonal influenza vaccination in China (2014‐2015) updated version. http://www.chinacdc.cn/jkzt/crb/lxxgm/ymjz/201509/t20150923_120575.html. Accessed August 14, 2017.
- 336. Mahamat A, Daures JP, De Wzieres B. Additive preventive effect of influenza and pneumococcal vaccines in the elderly: results of a large cohort study. Hum Vaccin Immunother. 2013;9:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]


