Abstract
Nicotinamide homeostasis is a candidate common denominator to explain smooth transitions, whether demographic, epidemiological or economic. This ‘NAD world’, dependent on hydrogen-based energy, is not widely recognised as it is neither measured nor viewed from a sufficiently multi-genomic or historical perspective. Reviewing the importance of meat and nicotinamide balances during our co-evolution, recent history suggests that populations only modernise and age well with low fertility on a suitably balanced diet. Imbalances on the low meat side lead to an excess of infectious disease, short lives and boom-bust demographics. On the high side, meat has led to an excess of degenerative, allergic and metabolic disease and low fertility. A ‘Goldilocks’ diet derived from mixed and sustainable farming (preserving the topsoil) allows for high intellectual capital, height and good health with controlled population growth resulting in economic growth and prosperity. Implementing meat equity worldwide could lead to progress for future generations on ‘spaceship’ earth by establishing control over population quality, thermostat and biodiversity, if it is not already too late.
Keywords: Climate change, CO2 emissions, NAD worlds, protonopathy, Parkinson disease, demographic transition, disease transitions, deaths of despair, new levellers, metabolic rift, meat, nicotinamide, anthropocene, coronavirus, COVID-19, disease X
‘Exploration is not so much a question of covering the ground as of digging beneath the surface’.
–Claude Levi-Strauss
‘And at the end of all our exploring will be to arrive where we started and know the place for the first time’.
–TS Eliot
Introduction
Our evolution has been characterised by articulated interactions between diet and agriculture and pro-family social and sexual cultures to raise healthy innovative children (Figure 1). Ultimately, the energy needed for such progress is derived from the sun and the soil in an ‘NAD World’ (Figures 2 and 3).
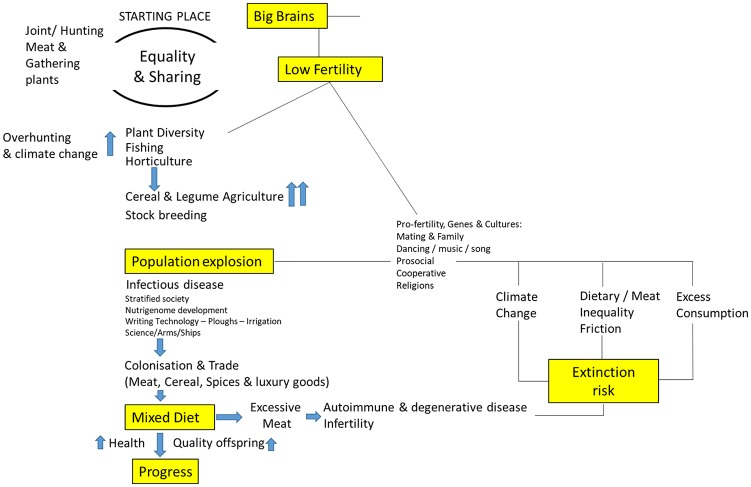
Figure 1.
One thing led to another: Factors behind high populations and cultural dominance, or extinction.
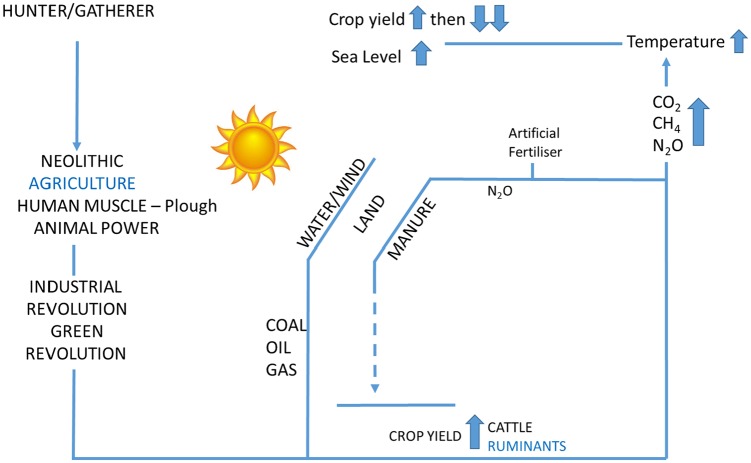
Figure 3.
Some interactions between agriculture, increasing crop yields, meat and greenhouse gases.
Figure 2.
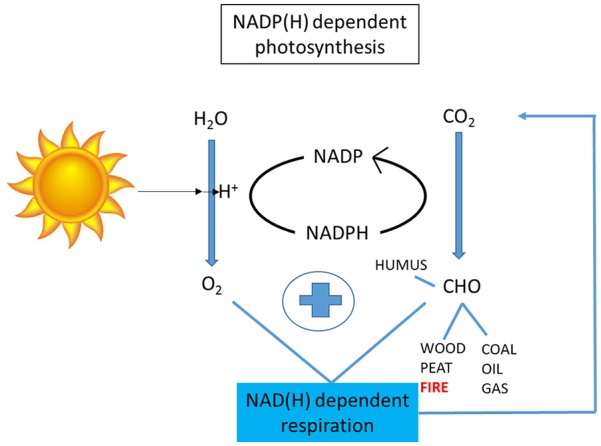
Basic formulae of photosynthesis, respiration and derived sources of topsoil and external energy. Essentially this is an ‘NAD world’.
NAD indicates nicotinamide adenine dinucleotide; NADP: nicotinamide adenine dinucleotide phosphate.
By the 4 ‘D’s’ of progress, we mean the major inter-related transitions of Demography, Domestication, Development and Disease. If these transitions were actual diseases, the lack of consensus on causation, prognosis or the value of interventions would lead to serious questions as to whether the ‘diagnosis’ was correct. Even when talking about diseases, ‘beneath the surface’ reasons behind the switch between chronic infectious and auto-immune disease and longevity as societies become wealthier are not understood – important as they represent a major preventive opportunity for otherwise ‘lost generations’. We will suggest, as a few others have done in the context of health and economic losses from malnutrition, that the aspect of modernity responsible for all these transitions is diet.1-3 Our contribution relates to emphasising the importance of a good diet (rather than simply subsistence) and generous meat, with the main active component being nicotinamide (Vitamin B3). When deficient, this vitamin causes pellagra and the 4D’s of Dementia (and low intelligence quotient), Diarrhoea (and chronic infections such as tuberculosis [TB]), and a characteristic Dermatitis (not always present) along with premature Death and a breakdown of symbiotic and social relationships. Nineteenth-century slave-owners recognised the consequent loss of productivity from poor diet and pellagra, yet we still do not fully recognise or act upon their insight when the food chain is longer extending to workers in other poorer countries.
Pellagra is, along with TB (that excretes nicotinic acid), a protean and archetypal disease of the poor: neither conditions are natural but are, by evolutionary standards, recent man-made inventions, as is poverty itself. We continue to make the case that not only is the diagnosis of pellagra missed in the poor but that nicotinamide status is the missing ‘diagnosis’ and first rung on the ladder that drives demographic, developmental and disease transitions. The upper classes and a series of empires got ahead on the meat curve driving capitalism and invention with the proletarian fertile classes, and countries, providing the labour in a fragile and misunderstood social contract. First of all, it is worth seeing this all in the context of an early ‘D’ – our Domestication.
Domesticator and Domesticated
Man was considered domesticated by Aristotle and Theophrastus and later by Blumenbach (1805), a professor of Medicine, who linked it to the emergence of culture and agriculture. Other species are domesticated by others, famous examples include leaf-cutter ants that farm fungi and, in co-evolutions, become dependent on each other’s reproduction, altering genomes and building niche-constructed environments.4-14 However, no plausible agent (some imagine supernatural forces) domesticated us (a fact that stymied Darwin) – so how did we auto-domesticate and why? We argue that a push down the food chain, forced by climate change and over-hunting of megafauna, began an unconscious selection of domesticates that merged into conscious selection, experimentation and domestication of plant, animal and even microbial domesticates pulled by higher reproductive rates of ourselves on a higher plant-based diet as well as themselves.15 So they domesticated us as we domesticated them – and all in the cause of bipartisan higher fertility, for us more babies. Illuminating wolf-dog and plant breeder experiments show how easily domestication can take place and that the pre-condition of potential tameness was necessary but the real selective and sufficient pressure was higher reproductive rates, as domesticated dogs or crops.16-19
Our cultural evolution was towards cereals and co-operation as part of a domesticated package that enabled higher fertility and the teaching and social learning of receptive and innovative children.20-34 The successful raising of children is intrinsic to our unusual metamorphoses and long life history with prolonged childhoods and adolescence through to mating, childcare and grand-parenting. This increase in fertility required a runaway sexual selection process enabling mating in an overarching social process incorporating commitment to the cause of reproduction, including art and science or state inducements and religious initiatives.35-47 Some apparent paradoxes such as violence, particularly to those outside family groupings, can be explained by protecting one’s own progeny. In other words, depending on circumstance and context being good or evil, whether as an individual or as a state, when either benefit reproductive rates or the resources needed to bring up well-fed children.48-53
Demography
‘Fecundity is totally checked by the plethoric state’.
–Thomas Doubleday in The True Law of Population: 1842
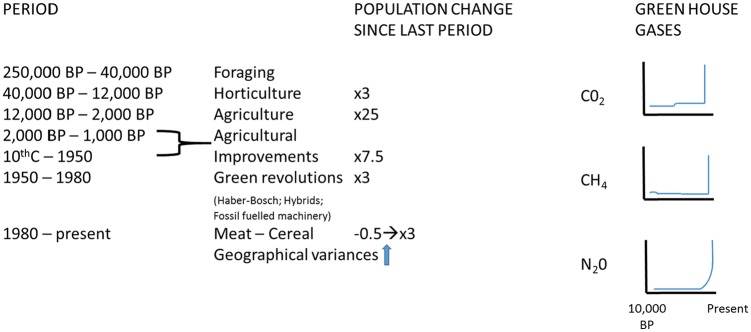
Population undoubtedly shaped the modern world, and trade-offs between quality and quantity of offspring or between fertility and longevity are evident at times of dietary hardship.54-73 Whether as hunter-gatherers or when on ‘Western’ diets, when meat intake is high, fertility falls often to below-replacement levels; whereas, whenever diet has been more cereal based, whether during the Neolithic or in sub-Saharan Africa now, populations boom.74-83 Human population growth rates and the link with agricultural revolutions and the rise in ‘green-house’ gases are shown in Figure 4. We have argued that this increased fecundity is due to relative immune tolerance of the foetus when on a high cereal diet with low nicotinamide levels, leading to induction of the tryptophan to nicotinamide adenine dinucleotide (NAD) pathway.84-89 This is the core hypothesis from which much else follows. Indeed, this move down the food chain during the Mesolithic first to horticulture and then agriculture may have saved us, but not the Neanderthals, from extinction (at the price of much disease) given that hominid populations were so low at that juncture.90-106 A pro-fertility culture with strong sexual selection and nutri-genomic adaptations contributed. Population pressure did not initiate this process but once started became part of an unchecked ratchet requiring more and more food and making it hard to reverse, leading to exponential increases on cereal diets, with contributions from cultural and religious beliefs around birth control (religions may rely more on high reproductive rates to spread than they do on converts). Now brakes may need to be applied, as although high populations were undoubtedly beneficial in the past, currently, the opposite is generally true, if we are to avoid the agricultural and subsequent Green revolutions becoming ‘the worst mistakes in the history of the human race’.83,107-113 Our suggestion for how populations expanded in the first place and how demographic transitions are hastened by increasing the meat supply is shown in Figure 5.
Figure 4.
Human population growth rates and agricultural revolutions aligned with atmospheric concentrations of greenhouse gases over the last 10 000 years in the anthropocene.
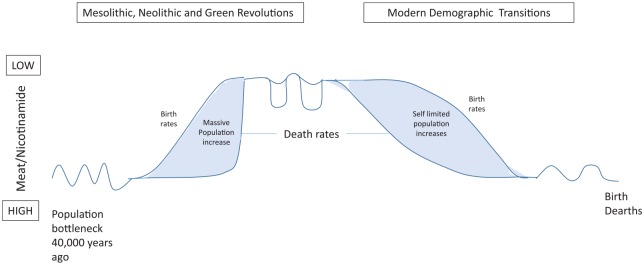
Figure 5.
Neolithic and green revolutions were similar in that more cereals can drive exponential population increases. Modern demographic transitions are different as an increase in meat drives self-limited population increases as fertility later falls.
Development
‘For when husbandry flourishes all other arts are in good fettle’. . .
–Socrates
There has been a great deal of discussion over development and the pros and cons of various forms of intervention in aid to encourage prosperity with no consensus over the benefits of aid or how to improve prospects for the future.114-127 We argue that, with rare exceptions, where valuable natural resources are present and that they are actually used to buy in a good diet, no society has ever succeeded without attention to sustainable agriculture and maintaining soil quality with mixed farming with stock breeding and husbandry so that a sufficient proportion of its citizens have a broad-based omnivorous diet. Studies from the economics of destitution and failed states support a major role for a good diet and a hunger for meat.128-135 Europe led, first in Italy, then the Netherlands and Spain and then England, France and Germany before America picked up the baton: as all by luck had a good supply of both plant and animal domesticates and a temperate climate largely on an east-west axis allowing for easier spread of farming and on a beneficial soil. With a decent investment in human capital with high intelligence and industriousness with reasonable lifespan without population explosions path-finders in these countries, well known to have higher meat diets (‘rosbifs’), one thing led to another, from technology to the arts, in a progressive and interactive manner – whereas the laggards further South or East were until recently very cereal-dependent.136
Diseases of Protons, Proles and Patricians
The ‘Big’ history of diseases, other than trauma, really starts in the Neolithic and culminates in the extraordinary shifts, within a generation at times, from chronic and acute infectious disease to allergies and later-onset diseases of modernity.137-145
We have made the case that pellagra is a man-made ‘protonopathy’ due to loss of NAD and hydrogen-carrying capacity that is of evolutionary importance: in a sense evolution in reverse, and as pointed out over 200 years ago by pellagra-ologists, such as Lombroso and Strambio, atavistic and truly degenerative with its loss of cognitive and social skills.146,147 The characteristic rash is an exaggerated sunburn so those with a darker skin will be protected, though making it harder to diagnose or self-diagnose or treat (by altering diet). Dark skin was thought to be our original state with pale skin evolving to allow more Vitamin D production in northern climes and loss of hair helping with heat control in the tropics. However, this now seems unlikely and dark skin evolving to avoid sunburn in the tropics where meat is harder to obtain, and therefore, sunburn could have been exacerbated by nicotinamide deficiency and impaired DNA repair is a possible scenario. The greater toleration of sun damage may be a mixed blessing if it makes cognitive impairment harder to recognise and/or correct that could affect progress of affected populations.148,149
There are clear links between diseases that appeared in the Neolithic, such as TB and gut dysbioses (that excrete nicotinic acid), and conditions that appeared more recently such as cancer, autoimmune and degenerative disease with nicotinamide metabolism and, in the latter cases, high meat consumption.150 Much depends on the activity of major NAD-dependent processes such as poly (ADP-ribose) polymerases (PARPs), Sirtuins and CD38 that ultimately depend on nicotinamide in the diet to produce their agonist NAD but is also an endogenous inhibitor suggesting, as does the induction of Nicotinamide N-Methyltransferase (NNMT), strong feedback mechanisms to buffer moderate extremes of dietary dosage but perhaps risking longer term pathology.151-160 Analogous to pellagra, there is an apparent mirror image effect when nicotinamide levels are high with many diseases of affluence affecting gut microbiomes, mitochondria, free radical chemistry and disturbed proteonomics, including prion-like behaviour – all downstream points where interventions would have been futile or deleterious in pellagra. We have used Parkinson disease as one example, some cancers and the metabolic syndrome may be others, that involve gut dysbioses (in a taxonomic direction suggestive of a high meat diet), T-cell and metabolic disturbance with prion-like alpha-synuclein aggregates with spreading behaviour via the vagus nerve.161-173 Dementia may also have misleading downstream metabolic, dysbiotic and amyloid and tau and other protein aggregation but may be related to low nicotinamide levels early or late in life or excessive consumption or catabolism from other stresses.174,175 Similarly, with cancer, autoimmune and metabolic syndromes, there is biochemical evidence of loss of nicotinamide homeostasis, whether dietary from increased consumption or excessive catabolism, or implied from benefits of measurement and intervention.153,176-203
Serotonin Syndromes
Moving down the food chain to a more plant-based diet would have reduced tryptophan intake and affected not only the immune tolerance system but the availability of serotonin and tryptamines – although psychedelics may have compensated and explain our addictive attraction to such secondary plant compounds.204-209 Changes in serotonin could have been important in moving from a hunter-gatherer society to a more sedentary and collectivist behaviour – gregariousness even in locusts is driven by a poorer diet and serotonin, and has been implicated in domestication syndromes.210-213 Higher meat societies tend to be more individualistic and narcissistic. The price may be effects on mood that explain an ‘anti-depressant era’ using pro-serotoninergic agents and may explain ‘deaths of despair’ arising from self-harm, chronic pain syndromes and drug addictions that are reversing previously, steadily increasing longevity trends.214-219
Genetic Advances and Late Disadvantages
Even if much progress is driven by diet, one would expect a genetic contribution shown by the known signatures in the genetic record if extracting and conserving optimal amounts of nicotinamide, while avoiding toxicity, were all so important to a ‘run-away’ process involving mitochondrial energetics aided by NAD and consumption pathways.220-223 This nutri-genomic point is well established from lactose tolerance (milk containing much nicotinamide riboside) to the use of NAD-consumers and in-house production of nicotinamide with salvage pathways and the development (in meat eaters) of the NNMT pathway.224-227 Nicotinamide riboside is of considerable interest as a potent bioavailable form of nicotinamide (including homeorhetically from both human maternal and domesticates milk) that is likely to have been important during evolution and particularly in neurogenesis and brain development, let alone is curative of overt pellagra and perhaps some other pathologies.228-231 Increasing fertility and sexual selection for not only physical but behavioural, cognitive and language prowess as secondary ‘ornaments’ is marked with many X-linked genes involved with both fertility and cognition.1-20,16-64,66-84,232 Pro-growth and pro-fertility genes, some useful when diet was poor and infection rates were high, evolved late and now are showing up as ‘antagonistic pleiotropic’ at-risk genes for late-onset cancerous and neurodegenerative disease often with a link to NAD metabolism, for instance, through impaired DNA repair mechanisms.21-34,36-49,178,199,200,202,227,233-244 DNA methylation also played a part in recent human evolution compatible with changes in diet and effects on the methylome.245,246
Diagnosis and Prescription – NAD World View
‘I thynke breakfastes necessary in this realme’.
–Thomas Elyot in Castel of Helth: 1532
The diagnosis, we feel, points to mid-range nicotinamide dosage being the recipe for success at an individual and population level. When sourced from animal products, NADH proton and electron-based mitochondrial energetics and NAD-consumer metabolism and repair mechanisms and the methylome allow a healthy metabolism, let alone resilience against many environmental stresses. We have made the case for nicotinamide being a key factor enabling bigger and better brains. Brain size may not have enlarged at the time of the creative explosion but restructured and changed its cortico-striatal neurochemistry towards a more prosocial dopaminergic and serotoninergic state.247-250 The balance with acetylcholine and cognition and oxytocin and vasopressin and reproduction and caring for each other in monogamous pairs and for infants would have been key.251-256
Climate Change and Meat
‘I see Freedom with Law and Peace, a stupendous trio all issuing forth against the idea of caste; what historic denouements are these we so rapidly approach?’
–Walt Whitman
There is no disputing that meat is expensive in monetary terms, shutting out the poor from a balanced diet, and previously was difficult and dangerous to obtain (as it still is from “bush meat”), but it is also expensive in environmental costs.254,257 Agriculture in general and meat production (such as methane emissions) specifically are a highly significant driver of the ‘Anthropocene’ as are the dramatic increases in population.258-267 Climate has had major influences on our diet and evolution and, in turn, has homeostatic mechanisms to control the amount of CO2 in the atmosphere, though how robust they are veers between the optimistic ‘Gaia’ and the pessimistic ‘Medea’ hypotheses.268-272 Agriculture and deforestation (remarkably warming of the atmosphere after deforestation was mentioned by 2 physicians in the 17th century) and many other knock-on effects such as on loss of biodiversity have crucial roles in the carbon cycle as did earlier geological disruptions from volcanic activity and rock weathering. Agriculture from the beginning intersected with greenhouse gases by affecting carbon and nitrogen cycles, and this progressively intensified to very high levels with loss of topsoil, artificial fertilisers, fossil fuel usage for mechanisation and transport, and direct excretion of methane by ruminants among several other effects compounded by the sheer number of domesticated animals. By contrast, the Great Dying in the Americas after 1492 may have arguably led to cooling of the earth from the reduced agricultural activity, and such plagues may have even been triggered by meat poverty and subclinical pellagra – these are the only possible documented example of atmospheric CO2 levels dropping in the Anthropocene from human activity.35,36,50,51,65,66,82,83,107,108,130,131,273-276
This has culminated in many calls for more plant-based diets and even taxes on meat or ‘meat retreats’ and ‘meatless Mondays’. A ‘flexitarian’ approach may well be the answer for those on a Western diet but ignores the needs of poorer individuals and countries that are stuck in a Faustian pact with often subsidised cereals stimulating population growth. We argue that meat redistribution and meat rations, enabled by reduction in wastage and the use of unconventional sources such as insects or synthetic products, is needed to control populations (with no coercion), improve health and well-being and the ‘ultimate resource’ of human capital.
New True Levellers
‘Money must not any longer be the great god that hedges in some and hedges out others’.
–Gerrard Winstanley in A Declaration from the Poor Oppressed People: 1649
We have explored under the dietary and biochemical bonnet unearthing the way that meat and nicotinamide made us clever and healthy in the first place but led to social stratification. This is still true but is why meat, cattle and pastureland should now become a common good.
Earlier attempts include the ‘Diggers and Levellers’ who were political, labour and land reform movements in the early 17th-century United Kingdom at the time of the ‘Little Ice Age’ and re-emergence of famines and the plague that influenced the course of the English civil war and later Marxian thoughts.277-281 Marx had been influenced by the chemist Liebig on metabolic rifts in society and the breakdown of some natural cycles, such as the nitrogen cycle, as animals (and their manure) were moved off the land to factory farms to feed cities. In retrospect, if such theories had stuck with the mode of sustenance, rather than expanding to the mode of industrial production, they would have had more traction by majoring on the need for a balanced diet and sustainable mixed farming and care of the soil (that then acts as a carbon sink).282,283 Similarly, as the physiocrats such as Francois Quesnay, a physician, diagnosed, the basis of economies has to be metabolic, related to land and water use, capturing the energy of the sun in a concentrated form that we then use to magnify its effect through a series of positive feedback loops or ‘zig-zags’ as illustrated in his 1750’s ‘Tableau Economique’.284 All their ideas were modernised as ‘food sovereignty’ and now should happen to the benefit of all.285,286
Action should stop an exponential population overshoot before the consequent low biodiversity ‘6th mass extinction’ event applies to ourselves – whether the coup de grace is climate change and heat or hurricanes or sea-level rises, high food prices and starvation, plague, migration, riots or war. The answer may include geo-engineering and genetic engineering of domesticates, but the larger part will need social engineering to improve equality and a reasonable diet and education and consequent fertility rate for all.287-295 A difference of just 0.25 children per couple makes all the difference to population growth over moderate timescales. Climate change forecasted to hit the poor southern ‘Tropic of chaos’ the hardest with risk of wars, plagues and mass migration could have a counter-intuitive cure in global meat justice.296-302
Engels View and Engel’s Law
Friedrich Engels pointed out the poor dietary conditions of industrialised London’s poor along with others who compared ‘darkest London’ with ‘darkest Africa’.303 These variances in meat intake narrowed in the United Kingdom due to meat imports but have widened globally maintaining disease patterns (such as TB), wrongly called ‘tropical diseases’. Action is needed to enable square meals for all, which avoids diet-induced poverty traps and automatically reconstructs societies – not fire-fighting with charity and aid often with land grabs disrupting the local peasantry, or, cheap calories without adequate wages or provision for meat – repeating damage done in colonial times.
Engel’s law states that higher wages means a lower proportion is spent on food but a higher proportion of that is spent on meat (and vegetables).37-39,58-60,73-75,92-94,117-119,141-143,152-154,161-163, 191-193,197-199,203-205,237-239,291-293,304-310 This law is distorted by competing ‘luxuries’ such as flavourful but non-nutritious ‘empty calorie’ sugars and other processed foods. An active pro-meat programme is necessary.
Despite our emphasis on nicotinamide, we would be cautious about short-cuts, except in emergencies, using vitamin supplements alone. Nicotinamide is not the only important factor in meat and on its own drains metabolism of methyl-groups also supplied by meat, as is a large proportion of the essential amino acid tryptophan. Also, carbohydrate intake affects tryptophan metabolism as does the intake of other amino acids that compete for transport across the gut and blood brain barrier. Protein in the diet and caloric restriction also affect NAD metabolism (and related mTOR, insulin-like growth factor 1 [IGF1] pathways and oxidant stress) as does aging and exercise, so this is a complex area with much potential that needs considerably more research and clinical trials, but until then, meat supplements may be safer.159,178,307-321
Conclusion
‘My interpretation is that the most sensible things to do are to hasten the arrival of a cleaner energy regime and to hasten the demographic transition’.
–JR McNeill in Something New under the Sun: 2001
NAD-dependent solar energy and other cleaner renewables replacing fossil fuels are sensible but are unlikely to be enough. It may seem extraordinary that nicotinamide and tryptophan-serotonin homeostasis could solve demographic, disease and development transitions and even climate change and migration friction in one swoop but that is the advantage of an accurate upstream preventive ‘diagnosis’.322-329 A properly constructed carrying capacity of the earth that considers the need to hasten demographic and related other transitions, rather than simply assessing the production of grain needed to provide enough calories to subsist, brings the issue of population size and human capital into sharp focus. This fits with an original suggestion 50 years ago that we need to move from ‘cowboy’ economics relying on unlimited resources and no consideration for future generations to ‘spaceman’ economics – it has been well said that the ‘invisible hand’ of traditional economics does not write the cheques (for the true environmental costs).330 Given the undoubted upfront environmental costs of meat production, it is ironic that if meat justice had been encouraged earlier, current populations would all have completed their demographic transition long ago and simultaneously removed the infectious diseases of poverty and, by redistribution and moderation, mitigated allergic, degenerative and metabolic diseases of affluence.
A real NAD world cost-benefit economic theory would readjust the true long-term cost of meat downwards and the true cost of cereals upwards given the latter’s effect on quantity rather than quality benefits to the population. By healing a divided world, meat equity would reduce tension whether from war or from emergent diseases “X” with zoonoses such as HIV, SARS, Ebola and Coronavirus being eyes of future storms hatched in meat poverty and wild bush and exotic wet meat markets – and avoids seeing recent history as a clash of civilisations, religions or populations or as an ethical tragedy (even though it is).331-337 If we do not figure this out and act upon it, basic human needs that start with diet could deteriorate fast with agriculture both being the cause and the casualty of climate change. Recent suggestions for a refreshed enlightenment as “Green New Deals” and a global co-operation to control the ‘thermostat’ with climate change mitigation and adaptations now crucially combined with ‘carbon’ taxes that pay for a reduction in inequality (egalité) and a better diet – we say with optimal meat – are promising.338-347 Increased solidarity (fraternité) ending ‘Hunger Games’ could mend dangerous metabolic, ecological and global rifts, and as TS Eliot said, we then can arrive at where we started as meat sharers enshrined by common custom and laws.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was funded by QEHB Charity, Birmingham, UK.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Author Contributions: ACW and LJH contributed equally to the writing and proofing of the article.
ORCID iD: Lisa J Hill  https://orcid.org/0000-0001-8431-7029
https://orcid.org/0000-0001-8431-7029
References
- 1. Brüne M, Schiefenhövel W. Oxford Handbook of Evolutionary Medicine. Oxford, UK: Oxford University Press; 2019. [Google Scholar]
- 2. James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. [DOI] [PubMed] [Google Scholar]
- 3. Silvertown J. Dinner With Darwin: Food, Drink, and Evolution. Chicago, IL: University of Chicago Press; 2017. [Google Scholar]
- 4. Benitez-Burraco A, Kempe V. The emergence of modern languages: has human self-domestication optimized language transmission? Front Psychol. 2018;9:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brune M. On human self-domestication, psychiatry, and eugenics. Philos Ethics Humanit Med. 2007;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blumenbach JF, Bendyshe T, Marx KFH, Flourens P, Wagner R, Hunter J. The Anthropological Treatises of Johann Friedrich Blumenbach. London, England: Anthropological Society; 1865. [Google Scholar]
- 7. Brüssow H. The Quest for Food: A Natural History of Eating. Berlin, Germany: Springer; 2007. [Google Scholar]
- 8. Darwin C. The Variation of Animals and Plants Under Domestication. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- 9. Cieri RL, Churchill SE, Franciscus RG, Tan J, Hare B. Craniofacial feminization, social tolerance, and the origins of behavioral modernity. Curr Anthropol. 2014;55:419-443. [Google Scholar]
- 10. Francis RC. Domesticated: Evolution in a Man-Made World. New York, NY: W.W. Norton; 2015. [Google Scholar]
- 11. Helen M. Human domestication reconsidered. Curr Anthropol. 2003;44:349-368. [Google Scholar]
- 12. Hemmer H, Beckhaus N. Domestication: The Decline of Environmental Appreciation. Cambridge, UK: Cambridge University Press; 1990. [Google Scholar]
- 13. Hood B. The Domesticated Brain: A Pelican Introduction. London, England: Penguin Books Limited; 2014. [Google Scholar]
- 14. Kantar MB, Bruford MW, Rieseberg LH. The genomics of domestication special issue editorial. Evol Appl. 2019;12:3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams AC, Hill LJ. Nicotinamide’s ups and downs: consequences for fertility, development, longevity and diseases of poverty and affluence [published online ahead of print October 9, 2018]. Int J Tryptophan Res. doi: 10.1177/1178646918802289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morey DF. The early evolution of the domestic dog. Am Sci. 1994;82:336-347. [Google Scholar]
- 17. Belyaev DK, Ruvinsky AO, Trut LN. Inherited activation-inactivation of the star gene in foxes: its bearing on the problem of domestication. J Hered. 1981;72:267-274. [DOI] [PubMed] [Google Scholar]
- 18. MacHugh DE, Larson G, Orlando L. Taming the past: ancient DNA and the study of animal domestication. Annu Rev Anim Biosci. 2017;5:329-351. [DOI] [PubMed] [Google Scholar]
- 19. Trut L, Oskina I, Kharlamova A. Animal evolution during domestication: the domesticated fox as a model. Bioessays. 2009;31:349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tollefson J. Advances in human behaviour came surprisingly early in Stone Age. Nature. 2018;555:424-425. [DOI] [PubMed] [Google Scholar]
- 21. Rizzolatti G, Sinigaglia C, Anderson F. Mirrors in the Brain: How Our Minds Share Actions and Emotions. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- 22. Dissanayake E. Art and Intimacy: How the Arts Began. Seattle, WA: University of Washington Press; 2015. [Google Scholar]
- 23. Aronson E. The Social Animal. New York, NY: Worth Publishers; 2004. [Google Scholar]
- 24. Blakemore SJ. The developing social brain: implications for education. Neuron. 2010;65:744-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Becker GS, Becker GS. A Treatise on the Family. Enlarged ed. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 26. Boyd R. A Different Kind of Animal: How Culture Transformed Our Species. Princeton, NJ: Princeton University Press; 2017. [Google Scholar]
- 27. Bowles S, Gintis H. A Cooperative Species: Human Reciprocity and Its Evolution. Princeton, NJ: Princeton University Press; 2011. [Google Scholar]
- 28. Bowlby J. Attachment and Loss. New York, NY: Basic Books; 1980. [Google Scholar]
- 29. Bolund E, Hayward A, Pettay JE, Lummaa V. Effects of the demographic transition on the genetic variances and covariances of human life-history traits. Evolution. 2015;69:747-755. [DOI] [PubMed] [Google Scholar]
- 30. Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344-1347. [DOI] [PubMed] [Google Scholar]
- 31. Damasio A. Self Comes to Mind: Constructing the Conscious Brain. New York, NY: Random House; 2011. [Google Scholar]
- 32. Erard M, Matacic C. Did kindness prime our species for language? Science. 2018;361:436-437. [DOI] [PubMed] [Google Scholar]
- 33. Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007;362:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laland KN. Darwin’s Unfinished Symphony: How Culture Made the Human Mind. Princeton, NJ: Princeton University Press; 2018. [Google Scholar]
- 35. Campbell BG. Sexual Selection and the Descent of Man: The Darwinian Pivot. New Brunswick, NJ: Transaction Publishers; 2017. [Google Scholar]
- 36. Gleeson BT, Kushnick G. Female status, food security, and stature sexual dimorphism: testing mate choice as a mechanism in human self-domestication. Am J Phys Anthropol. 2018;167:458-469. [DOI] [PubMed] [Google Scholar]
- 37. Iacoboni M. Mirroring People: The New Science of How We Connect With Others. New York, NY: Farrar, Straus and Giroux; 2009. [Google Scholar]
- 38. Jablonka E, Ginsburg S, Dor D. The co-evolution of language and emotions. Philos Trans R Soc Lond B Biol Sci. 2012;367:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: diet, intelligence, and longevity. Evol Anthropol. 2000;9:156-185. [Google Scholar]
- 40. Parker ST. A sexual selection model for hominid evolution. Hum Evol. 1987;2:235-253. [Google Scholar]
- 41. Mesoudi A. Cultural Evolution: How Darwinian Theory Can Explain Human Culture and Synthesize the Social Sciences. Chicago, IL: University of Chicago Press; 2011. [Google Scholar]
- 42. Lucas M. Rewire Your Brain for Love. New York, NY: Hay House; 2012. [Google Scholar]
- 43. Seabright P. The War of the Sexes: How Conflict and Cooperation Have Shaped Men and Women From Prehistory to the Present. Princeton, NJ: Princeton University Press; 2012. [Google Scholar]
- 44. van Schaik CP, Burkart JM. Social learning and evolution: the cultural intelligence hypothesis. Philos Trans R Soc Lond B Biol Sci. 2011;366:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vilarroya O, Atran S, Navarro A, Tobeña A. Values, Empathy, | Fairness Across Social Barriers. New York, NY: Wiley; 2009. [DOI] [PubMed] [Google Scholar]
- 46. Rosenthal GG. Mate Choice: The Evolution of Sexual Decision Making From Microbes to Humans. Princeton, NJ: Princeton University Press; 2017. [Google Scholar]
- 47. Ryan C, Jeth C. Sex at Dawn: The Prehistoric Origins of Modern Sexuality. Melbourne, VIC, Australia: Scribe Publications; 2011. [Google Scholar]
- 48. Boehm C. Moral Origins: The Evolution of Virtue, Altruism, and Shame. New York, NY: Basic Books; 2012. [Google Scholar]
- 49. Collins R. Violence: A Micro-Sociological Theory. Princeton, NJ: Princeton University Press; 2009. [Google Scholar]
- 50. Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation: a possible prelude to violence. Science. 2000;289:591-594. [DOI] [PubMed] [Google Scholar]
- 51. Davie MR. The Evolution of War: A Study of Its Role in Early Societies. Mineola, NY: Dover Publications; 2012. [Google Scholar]
- 52. Wrangham R. The Goodness Paradox: How Evolution Made Us Both More and Less Violent. London, England: Profile Books; 2019. [Google Scholar]
- 53. Repo J. The Biopolitics of Gender. Oxford, UK: Oxford University Press; 2016. [Google Scholar]
- 54. Meij J, van Bodegom D, Ziem JB, et al. Quality-quantity trade-off of human offspring under adverse environmental conditions. J Evol Biol. 2009;22:1014-1023. [DOI] [PubMed] [Google Scholar]
- 55. Morland P. The Human Tide: How Population Shaped the Modern World. London, England: Hodder & Stoughton; 2019. [Google Scholar]
- 56. Nenko I, Hayward AD, Simons MJP, Lummaa V. Early-life environment and differences in costs of reproduction in a preindustrial human population. PLoS ONE. 2018;13:e0207236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brown LR, Gardner G, Halweil B. Beyond Malthus: The Nineteen Dimensions of the Population Challenge. London, England: Routledge; 2014. [Google Scholar]
- 58. Bloom D, Canning D, Sevilla J. The Demographic Dividend: A New Perspective on the Economic Consequences of Population Change. Santa Monica, CA: RAND Corporation; 2003. [Google Scholar]
- 59. Bongaarts J. Human population growth and the demographic transition. Philos Trans R Soc Lond B Biol Sci. 2009;364:2985-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bongaarts J, Sobotka T. A demographic explanation for the recent rise in European fertility. Popul Dev Rev. 2012;38:83-120. [DOI] [PubMed] [Google Scholar]
- 61. Boserup E. Population and Technological Change: A Study of Long-Term Trends. Chicago, IL: University of Chicago Press; 1983. [Google Scholar]
- 62. Caldwell JC, Caldwell P. The cultural context of high fertility in sub-Saharan Africa. Popul Dev Rev. 1987;13:409-437. [Google Scholar]
- 63. Cochran G, Harpending H. The 10,000 Year Explosion: How Civilization Accelerated Human Evolution. New York, NY: Basic Books; 2009. [Google Scholar]
- 64. Dyson T. Population and Development: The Demographic Transition. London, England: Zed Books; 2013. [Google Scholar]
- 65. Lesthaeghe R. The unfolding story of the second demographic transition. Popul Dev Rev. 2010;36:211-251. [DOI] [PubMed] [Google Scholar]
- 66. Kaptijn R, Thomese F, Liefbroer AC, Van Poppel F, Van Bodegom D, Westendorp RGJ. The trade-off between female fertility and longevity during the epidemiological transition in the Netherlands. PLoS ONE. 2015;10:e0144353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Page AE, Chaudhary N, Viguier S, et al. Hunter-gatherer social networks and reproductive success. Sci Rep. 2017;7:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Page AE, Viguier S, Dyble M, et al. Reproductive trade-offs in extant hunter-gatherers suggest adaptive mechanism for the Neolithic expansion. Proc Natl Acad Sci U S A. 2016;113:4694-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bocquet-Appel JP, Demars PY. Population kinetics in the Upper Palaeolithic in Western Europe. J Archaeol Sci. 2000;27:551-570. [Google Scholar]
- 70. Turner A. Population priorities: the challenge of continued rapid population growth. Philos Trans R Soc Lond B Biol Sci. 2009;364:2977-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Van Den Bergh JCJM, Rietveld P. Reconsidering the Limits to World Population: meta-analysis and Meta-prediction. Bioscience. 2004;54:195-204. [Google Scholar]
- 72. Van Bavel J. Subreplacement fertility in the West before the baby boom: past and current perspectives. Popul Stud. 2010;64:1-18. [DOI] [PubMed] [Google Scholar]
- 73. Reher DS. Economic and social implications of the demographic transition. Popul Dev Rev. 2011;37:11-33. [Google Scholar]
- 74. Bashford A, Chaplin JE. The New Worlds of Thomas Robert Malthus: Rereading the Principle of Population. Princeton, NJ: Princeton University Press; 2016. [Google Scholar]
- 75. Bhrolcháin MN, Dyson T. On causation in demography: issues and illustrations. Popul Dev Rev. 2007;33:1-36. [Google Scholar]
- 76. Bevan A, Colledge S, Fuller D, Fyfe R, Shennan S, Stevens C. Holocene fluctuations in human population demonstrate repeated links to food production and climate. Proc Natl Acad Sci U S A. 2017;114:E10524-E10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Birdsall N. Economic approaches to population growth. In: Chenery H, Srinivasan TN, eds. Handbook of Development Economics. Amsterdam, The Netherlands: Elsevier; 1988:477-542. [Google Scholar]
- 78. Blome MW, Cohen AS, Tryon CA, Brooks AS, Russell J. The environmental context for the origins of modern human diversity: a synthesis of regional variability in African climate 150,000-30,000 years ago. J Hum Evol. 2012;62:563-592. [DOI] [PubMed] [Google Scholar]
- 79. Doubleday T. The True Law of Population: Shewn to Be Connected With the Food of the People. London, England: G. Peirce; 1846. [Google Scholar]
- 80. Gowdy J. Limited Wants, Unlimited Means: A Reader On Hunter-Gatherer Economics and The Environment. Washington, DC: Island Press; 1998. [Google Scholar]
- 81. Greenfield HJ. Animal Secondary Products: Domestic Animal Exploitation in Prehistoric Europe, the Near East and the Far East. Philadelphia, PA: Oxbow Books; 2014. [Google Scholar]
- 82. Jasienska G. Reproduction and lifespan: trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. Am J Hum Biol. 2009;21:524-532. [DOI] [PubMed] [Google Scholar]
- 83. Lunzer M, Miller SP, Felsheim R, Dean AM. The biochemical architecture of an ancient adaptive landscape. Science. 2005;310:499-501. [DOI] [PubMed] [Google Scholar]
- 84. Silvestris E, Lovero D, Palmirotta R. Nutrition and female fertility: an interdependent correlation. Front Endocrinol. 2019;10:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Spinelli P, Latchney SE, Reed JM, et al. Identification of the novel Ido1 imprinted locus and its potential epigenetic role in pregnancy loss. Hum Mol Genet. 2019;28:662-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shah NM, Herasimtschuk AA, Boasso A, et al. Changes in T cell and dendritic cell phenotype from mid to late pregnancy are indicative of a shift from immune tolerance to immune activation. Front Immunol. 2017;8:1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Williams AC, Hill LJ. Meat and nicotinamide: a causal role in human evolution, history, and demographics [published online ahead of print May 2, 2017]. Int J Tryptophan Res. doi: 10.1177/1178646917704661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chang RQ, Li DJ, Li MQ. The role of indoleamine-2, 3-dioxygenase in normal and pathological pregnancies. Am J Reprod Immunol. 2018;79:e12786. [DOI] [PubMed] [Google Scholar]
- 89. Youngson NA, Uddin GM, Das A, et al. Impacts of obesity, maternal obesity and nicotinamide mononucleotide supplementation on sperm quality in mice. Reproduction. 2019;158:171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Burroughs WJ. Climate Change in Prehistory: The End of the Reign of Chaos. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 91. Bender ML. Paleoclimate. Princeton, NJ: Princeton University Press; 2013. [Google Scholar]
- 92. Bednarik RG. Doing with less: hominin brain atrophy. Homo. 2014;65:433-449. [DOI] [PubMed] [Google Scholar]
- 93. Barton H, Denham T. Vegecultures and the social-biological transformations of plants and people. Quatern Int. 2018;489:17-25. [Google Scholar]
- 94. Cerulli T. The Mindful Carnivore: A Vegetarian’s Hunt for Sustenance. New York, NY: Pegasus Books; 2013. [Google Scholar]
- 95. Ferraro JV, Plummer TW, Pobiner BL, et al. Earliest archaeological evidence of persistent hominin carnivory. PLoS ONE. 2013;8:e62174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Henry AG, Brooks AS, Piperno DR. Plant foods and the dietary ecology of Neanderthals and early modern humans. J Hum Evol. 2014;69:44-54. [DOI] [PubMed] [Google Scholar]
- 97. Neelakantan H, Brightwell CR, Graber TG, et al. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem Pharmacol. 2019;163:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Richards MP, Pettitt PB, Stiner MC, Trinkaus E. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc Natl Acad Sci U S A. 2001;98:6528-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Posth C, Renaud G, Mittnik A, et al. Pleistocene mitochondrial genomes suggest a single major dispersal of non-Africans and a Late Glacial population turnover in Europe. Curr Biol. 2016;26:827-833. [DOI] [PubMed] [Google Scholar]
- 100. Nigst PR, Haesaerts P, Damblon F, et al. Early modern human settlement of Europe north of the Alps occurred 43,500 years ago in a cold steppe-type environment. Proc Natl Acad Sci U S A. 2014;111:14394-14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mellars P, French JC. Tenfold population increase in Western Europe at the Neandertal-to-modern human transition. Science. 2011;333:623-627. [DOI] [PubMed] [Google Scholar]
- 102. El Zaatari S, Grine FE, Ungar PS, Hublin JJ. Neandertal versus modern human dietary responses to climatic fluctuations. PLoS ONE. 2016;11:e0153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smith EI, Jacobs Z, Johnsen R, et al. Humans thrived in South Africa through the Toba eruption about 74,000 years ago. Nature. 2018;555:511-515. [DOI] [PubMed] [Google Scholar]
- 104. Smith K. The Malthusian Controversy. London, England: Taylor & Francis; 2013. [Google Scholar]
- 105. Hardy K, Brand-Miller J, Brown KD, Thomas MG, Copeland L. The importance of dietary carbohydrate in human evolution. Q Rev Biol. 2015;90:251-268. [DOI] [PubMed] [Google Scholar]
- 106. Higham T, Douka K, Wood R, et al. The timing and spatiotemporal patterning of Neanderthal disappearance. Nature. 2014;512:306-309. [DOI] [PubMed] [Google Scholar]
- 107. Ehrlich PR. The Population Bomb. New York, NY: Buccaneer Books; 1970. [Google Scholar]
- 108. Angus I, Butler S, Hartmann B. Too Many People? Population, Immigration and the Environmental Crisis. Chicago, IL: Haymarket Books; 2011. [Google Scholar]
- 109. Conway G. The Doubly Green Revolution: Food for All in the Twenty-First Century. Ithaca, NY: Comstock Pub. Associates; 1998. [Google Scholar]
- 110. Coole D. Should We Control World Population? Hoboken, NJ: Wiley; 2018. [Google Scholar]
- 111. Kaufmann EP. Shall the Religious Inherit the Earth? Demography and Politics in the Twenty-First Century. London, England: Profile Books; 2010. [Google Scholar]
- 112. O’Rourke KH. The European grain invasion, 1870-1913. J Econ Hist. 1997;57:775-801. [Google Scholar]
- 113. Lynas M. The God Species: How the Planet Can Survive the Age of Humans. London, England: Fourth Estate; 2011. [Google Scholar]
- 114. Bremmer I. The J Curve: A New Way to Understand Why Nations Rise and Fall. New York, NY: Simon & Schuster; 2006. [Google Scholar]
- 115. Clark G. A Farewell to Alms: A Brief Economic History of the World. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- 116. Clark G. In defense of the Malthusian interpretation of history. Eur Rev Econ Hist. 2008;12:175-199. [Google Scholar]
- 117. Deaton A. The Great Escape: Health, Wealth, and the Origins of Inequality. Princeton, NJ: Princeton University Press; 2013. [Google Scholar]
- 118. Easterlin RA. Will raising the incomes of all increase the happiness of all? J Econ Behav Organ. 1995;27:35-47. [Google Scholar]
- 119. Easterly W, Easterly WR. The White Man’s Burden: Why the West’s Efforts to Aid the Rest Have Done So Much Ill and So Little Good. New York, NY: Penguin Press; 2006. [Google Scholar]
- 120. Johnston BF, Mellor JW. The role of agriculture in economic development. Am Econ Rev. 1961;51:566-593. [Google Scholar]
- 121. Jones EL. Growth Recurring: Economic Change in World History. Ann Arbor, MI: University of Michigan Press; 2000. [Google Scholar]
- 122. Kuznets S. Population and economic growth. P Am Philos Soc. 1967;111:170-193. [Google Scholar]
- 123. Wilkinson RG. Poverty and Progress: An Ecological Model of Economic Development. London, England: Methuen; 1973. [Google Scholar]
- 124. Ofek H. Second Nature: Economic Origins of Human Evolution. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 125. North DC, Thomas RP. The Rise of the Western World: A New Economic History. Cambridge, UK: Cambridge University Press; 1973. [Google Scholar]
- 126. Torras M, Boyce JK. Income, inequality, and pollution: a reassessment of the environmental Kuznets Curve. Ecol Econ. 1998;25:147-160. [Google Scholar]
- 127. Trentmann F. Empire of Things: How We Became a World of Consumers, From the Fifteenth Century to the Twenty-First. London, England: Penguin Books Limited; 2016. [Google Scholar]
- 128. Hillel D. Soil in the Environment: Crucible of Terrestrial Life. San Diego, CA: Elsevier; 2007. [Google Scholar]
- 129. Hyams E. Soil and Civilization. New York, NY: Harper & Row; 1976. [Google Scholar]
- 130. Dubbin W, Museum NH. Soils. London, England: Natural History Museum; 2001. [Google Scholar]
- 131. Guttmann-Bond E. Reinventing Sustainability: How Archaeology Can Save the Planet. Philadelphia, PA: Oxbow Books; 2019. [Google Scholar]
- 132. Lemke A. Foraging in the Past: Archaeological Studies of Hunter-Gatherer Diversity. Boulder, CO: University Press of Colorado; 2019. [Google Scholar]
- 133. Ohlson K. The Soil Will Save Us: How Scientists, Farmers, and Foodies Are Healing the Soil to Save the Planet. New York, NY: Rodale Books; 2014. [Google Scholar]
- 134. Sasser JS. On Infertile Ground: Population Control and Women’s Rights in the Era of Climate Change. New York, NY: New York University Press; 2018. [Google Scholar]
- 135. Becker GS. Human Capital: A Theoretical and Empirical Analysis, With Special Reference to Education. Chicago, IL: University of Chicago Press; 2009. [Google Scholar]
- 136. Rogers B. Beef and Liberty. New York, NY: Vintage; 2004. [Google Scholar]
- 137. Williams AC, Ramsden DB. Pellagra: a clue as to why energy failure causes diseases? Med Hypotheses. 2007;69:618-628. [DOI] [PubMed] [Google Scholar]
- 138. Cartwright FF, Biddiss MD. Disease & History. Stroud, UK: Sutton; 2004. [Google Scholar]
- 139. Cook ND. Born to Die: Disease and New World Conquest, 1492-1650. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- 140. Harrison M. Disease and the Modern World: 1500 to the Present Day. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 141. Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity (The Norton History of Science). New York, NY: W.W. Norton; 1999. [Google Scholar]
- 142. Porter D. The History of Public Health and the Modern State. Amsterdam, The Netherlands: Rodopi; 1994. [PubMed] [Google Scholar]
- 143. Lieberman D. The Story of the Human Body: Evolution, Health and Disease. London, England: Penguin Books Limited; 2013. [Google Scholar]
- 144. Nunn N, Qian N. The Columbian exchange: a history of disease, food, and ideas. J Econ Perspect. 2010;24:163-188. [Google Scholar]
- 145. Steckel RH, Rose JC. The Backbone of History: Health and Nutrition in the Western Hemisphere. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- 146. Hill LJ, Williams AC. Meat intake and the dose of vitamin B3 – nicotinamide: cause of the causes of disease transitions, health divides, and health futures? [published online ahead of print May 3, 2017]. Int J Tryptophan Res. doi: 10.1177/1178646917704662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pick D. Faces of Degeneration: A European Disorder, C.1848-1918. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 148. Crawford N, Kelly DE, Hansen ME, et al. Loci associated with skin pigmentation identified in African populations [published online ahead of print November 17, 2017]. Science. doi: 10.1126/science.aan8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution? Proc Biol Sci. 2014;281:20132955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Williams AC, Dunbar RI. Big brains, meat, tuberculosis, and the nicotinamide switches: co-evolutionary relationships with modern repercussions? Int J Tryptophan Res. 2013;6:73-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Poljsak B, Kovac V, Dahmane R, Levec T, Starc A. Cancer etiology: a metabolic disease originating from life’s major evolutionary transition? Oxid Med Cell Longev. 2019;2019:7831952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hikosaka K, Yaku K, Okabe K, Nakagawa T. Implications of NAD metabolism in pathophysiology and therapeutics for neurodegenerative diseases [published online ahead of print July 7, 2019]. Nutr Neurosci. doi: 10.1080/1028415X.2019.1637504. [DOI] [PubMed] [Google Scholar]
- 154. Grignani G, Merlini A, Sangiolo D, D’Ambrosio L, Pignochino Y. Look before you leap: looking into PARP inhibition from bench to bedside and back. Pharmacol Therapeut. 2019;206:107446. [DOI] [PubMed] [Google Scholar]
- 155. Hogan KA, Chini CCS, Chini EN. The Multi-faceted Ecto-enzyme CD38: roles in immunomodulation, cancer, aging, and metabolic diseases. Front Immunol. 2019;10:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lloret A, Beal MF. PGC-1alpha, Sirtuins and PARPs in Huntington’s disease and other neurodegenerative conditions: NAD+ to rule them all. Neurochem Res. 2019;44:2423-2434. [DOI] [PubMed] [Google Scholar]
- 157. Eisemann T, Pascal JM. Poly(ADP-ribose) polymerase enzymes and the maintenance of genome integrity. Cell Mol Life Sci. 2020;77:19-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wang Y, Yang J, Hong T, Chen X, Cui L. SIRT2: controversy and multiple roles in disease and physiology. Ageing Res Rev. 2019;55:100961. [DOI] [PubMed] [Google Scholar]
- 159. Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging. 2013;5:144-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gomes P, Viana SD, Nunes S, Rolo AP, Palmeira CM, Reis F. The yin and yang faces of the mitochondrial deacetylase sirtuin 3 in age-related disorders. Ageing Res Rev. 2019;57:100983. [DOI] [PubMed] [Google Scholar]
- 161. Breen DP, Halliday GM, Lang AE. Gut-brain axis and the spread of alpha-synuclein pathology: vagal highway or dead end? Mov Disord. 2019;34:307-316. [DOI] [PubMed] [Google Scholar]
- 162. Scheperjans F, Derkinderen P, Borghammer P. The gut and Parkinson’s disease: hype or hope? J Parkinsons Dis. 2018;8:S31-S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cavanna AE, Nani A, Williams AC. Parkinsonian features in a case of pellagra: a historical report. J Parkinsons Dis. 2013;3:539-545. [DOI] [PubMed] [Google Scholar]
- 164. Parsons RB, Smith ML, Williams AC, Waring RH, Ramsden DB. Expression of nicotinamide N-methyltransferase (E.C. 2.1.1.1) in the Parkinsonian brain. J Neuropathol Exp Neurol. 2002;61:111-124. [DOI] [PubMed] [Google Scholar]
- 165. Williams A, Ramsden D. Nicotinamide: a double edged sword. Parkinsonism Relat Disord. 2005;11:413-420. [DOI] [PubMed] [Google Scholar]
- 166. Cheng CY, Gutierrez NM, Marzuki MB, et al. Host sirtuin 1 regulates mycobacterial immunopathogenesis and represents a therapeutic target against tuberculosis [published online ahead of print March, 2017]. Sci Immunol. doi: 10.1126/sciimmunol.aaj1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhou Y, Wu J, Sheng R, et al. Reduced nicotinamide adenine dinucleotide phosphate inhibits MPTP-induced neuroinflammation and neurotoxicity. Neuroscience. 2018;391:140-153. [DOI] [PubMed] [Google Scholar]
- 168. Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease: the gut-brain axis and environmental factors. Nat Rev Neurol. 2015;11:625-636. [DOI] [PubMed] [Google Scholar]
- 169. Lehmann S, Loh SH, Martins LM. Enhancing NAD+ salvage metabolism is neuroprotective in a PINK1 model of Parkinson’s disease. Biol Open. 2017;6:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Shan C, Gong YL, Zhuang QQ, et al. Protective effects of beta-nicotinamide adenine dinucleotide against motor deficits and dopaminergic neuronal damage in a mouse model of Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109670. [DOI] [PubMed] [Google Scholar]
- 171. Spielman LJ, Gibson DL, Klegeris A. Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int. 2018;120:149-163. [DOI] [PubMed] [Google Scholar]
- 172. Wekerle H. Brain autoimmunity and intestinal microbiota: 100 trillion game changers. Trends Immunol. 2017;38:483-497. [DOI] [PubMed] [Google Scholar]
- 173. Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469-1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Williams AC, Hill LJ, Ramsden DB. Nicotinamide, NAD(P)(H), and methyl-group homeostasis evolved and became a determinant of ageing diseases: hypotheses and lessons from pellagra. Curr Gerontol Geriatr Res. 2012;2012:302875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Williams AC, Ramsden DB. Nicotinamide homeostasis: a xenobiotic pathway that is key to development and degenerative diseases. Med Hypotheses. 2005;65:353-362. [DOI] [PubMed] [Google Scholar]
- 176. Larrick JW, Mendelsohn AR. Roads to the fountain of youth? Rejuvenating intestinal stem cells. Rejuvenation Res. 2019;22:342-347. [DOI] [PubMed] [Google Scholar]
- 177. Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007;129:473-484. [DOI] [PubMed] [Google Scholar]
- 178. Clement J, Wong M, Poljak A, Sachdev P, Braidy N. The plasma NAD(+) metabolome is dysregulated in ‘normal’ aging. Rejuvenation Res. 2019;22:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Dolopikou CF, Kourtzidis IA, Margaritelis NV, et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study [published online ahead of print February 6, 2019]. Eur J Nutr. doi: 10.1007/s00394-019-01919-4. [DOI] [PubMed] [Google Scholar]
- 180. Elhassan YS, Philp AA, Lavery GG. Targeting NAD+ in metabolic disease; new insights into an old molecule. J Endocr Soc. 2017;1:816-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jacobson MK, Jacobson EL. Vitamin B3 in health and disease: toward the second century of discovery. Methods Mol Biol. 2018;1813:3-8. [DOI] [PubMed] [Google Scholar]
- 182. Lefebvre L, Reader SM, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav Evol. 2004;63:233-246. [DOI] [PubMed] [Google Scholar]
- 183. Stromland O, Niere M, Nikiforov AA, VanLinden MR, Heiland I, Ziegler M. Keeping the balance in NAD metabolism. Biochem Soc Trans. 2019;47:119-130. [DOI] [PubMed] [Google Scholar]
- 184. Li S, Qiao L, Yang Z, He C. Prognostic value of nicotinamide N-methyltransferase expression in patients with solid tumors: a systematic review and meta-analysis. Front Physiol. 2018;9:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lohani M, Dhasmana A, Haque S, et al. Niacin deficiency modulates genes involved in cancer: are smokers at higher risk? J Cell Biochem. 2019;120:232-242. [DOI] [PubMed] [Google Scholar]
- 186. Pajuelo D, Gonzalez-Juarbe N, Tak U, Sun J, Orihuela CJ, Niederweis M. NAD+ depletion triggers macrophage necroptosis, a cell death pathway exploited by Mycobacterium tuberculosis. Cell Rep. 2018;24:429-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Brown K, Xie S, Qiu X, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. de Guia RM, Agerholm M, Nielsen TS, et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD(+) salvage capacity in human skeletal muscle. Physiol Rep. 2019;7:e14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Eckert MA, Coscia F, Chryplewicz A, et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature. 2019;569:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wang Y, Zeng J, Wu W, et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Res. 2019;21:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Mendelsohn AR, Larrick JW. The NAD+/PARP1/SIRT1 axis in aging. Rejuvenation Res. 2017;20:244-247. [DOI] [PubMed] [Google Scholar]
- 192. Nacarelli T, Zhang R. NAD(+) metabolism controls inflammation during senescence. Mol Cell Oncol. 2019;6:1605819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Pi C, Yang Y, Sun Y, et al. Nicotinamide phosphoribosyltransferase postpones rat bone marrow mesenchymal stem cell senescence by mediating NAD(+)-Sirt1 signaling. Aging. 2019;11:3505-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the central nervous system: an update of biological aspects and clinical applications [published online ahead of print February 23, 2019]. Int J Mol Sci. doi: 10.3390/ijms20040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Igarashi M, Guarente L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell. 2016;166:436-450. [DOI] [PubMed] [Google Scholar]
- 196. Igarashi M, Miura M, Williams E, et al. NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell. 2019;18:e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Xu J, Jackson CW, Khoury N, Escobar I, Perez-Pinzon MA. Brain SIRT1 mediates metabolic homeostasis and neuroprotection. Front Endocrinol. 2018;9:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD metabolism in cancer therapeutics. Front Oncol. 2018;8:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yaku K, Okabe K, Nakagawa T. NAD metabolism: implications in aging and longevity. Ageing Res Rev. 2018;47:1-17. [DOI] [PubMed] [Google Scholar]
- 200. Zhang N, Sauve AA. Regulatory effects of NAD(+) metabolic pathways on sirtuin activity. Prog Mol Biol Transl Sci. 2018;154:71-104. [DOI] [PubMed] [Google Scholar]
- 201. Smith AC, Holden RC, Rasmussen SM, Hoane MR, Hylin MJ. Effects of nicotinamide on spatial memory and inflammation after juvenile traumatic brain injury. Behav Brain Res. 2019;364:123-132. [DOI] [PubMed] [Google Scholar]
- 202. Sharif T, Martell E, Dai C, et al. Regulation of cancer and cancer-related genes via NAD(). Antioxid Redox Signal. 2019;30:906-923. [DOI] [PubMed] [Google Scholar]
- 203. Simon NC, Aktories K, Barbieri JT. Novel bacterial ADP-ribosylating toxins: structure and function. Nat Rev Microbiol. 2014;12:599-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44-79. [DOI] [PubMed] [Google Scholar]
- 205. Bennett AJ, Lesch KP, Heils A, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118-122. [DOI] [PubMed] [Google Scholar]
- 206. Brodie BB, Pletscher A, Shore PA. Evidence that serotonin has a role in brain function. Science. 1955;122:968. [DOI] [PubMed] [Google Scholar]
- 207. de Almeida RM, Cabral JC, Narvaes R. Behavioural, hormonal and neurobiological mechanisms of aggressive behaviour in human and nonhuman primates. Physiol Behav. 2015;143:121-135. [DOI] [PubMed] [Google Scholar]
- 208. Crockett MJ, Clark L, Apergis-Schoute AM, Morein-Zamir S, Robbins TW. Serotonin modulates the effects of Pavlovian aversive predictions on response vigor. Neuropsychopharmacology. 2012;37:2244-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Higley J, King ST, Jr, Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14:67-76. [DOI] [PubMed] [Google Scholar]
- 210. Passamonti L, Crockett MJ, Apergis-Schoute AM, et al. Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry. 2012;71:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Robbins TW. Opinion on monoaminergic contributions to traits and temperament [published online ahead of print April 19, 2018]. Philos Trans R Soc Lond B Biol Sci. doi: 10.1098/rstb.2017.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Rogers SM, Ott SR. Differential activation of serotonergic neurons during short- and long-term gregarization of desert locusts. Proc Biol Sci. 2015;282:20142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Rilling JK, Scholz J, Preuss TM, Glasser MF, Errangi BK, Behrens TE. Differences between chimpanzees and bonobos in neural systems supporting social cognition. Soc Cogn Affect Neurosci. 2012;7:369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Riley JC. Rising Life Expectancy: A Global History. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 215. Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112:15078-15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Muennig PA, Reynolds M, Fink DS, Zafari Z, Geronimus AT. America’s declining well-being, health, and life expectancy: not just a white problem. Am J Public Health. 2018;108:1626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21:10. [DOI] [PubMed] [Google Scholar]
- 218. Healy D. Let Them Eat Prozac: The Unhealthy Relationship Between the Pharmaceutical Industry and Depression. New York, NY: New York University Press; 2006. [Google Scholar]
- 219. Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry. 1998;155:373-379. [DOI] [PubMed] [Google Scholar]
- 220. Bockwoldt M, Houry D, Niere M, et al. Identification of evolutionary and kinetic drivers of NAD-dependent signaling. Proc Natl Acad Sci U S A. 2019;116:15957-15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Citarelli M, Teotia S, Lamb RS. Evolutionary history of the poly(ADP-ribose) polymerase gene family in eukaryotes. BMC Evol Biol. 2010;10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Hawks J, Wang ET, Cochran GM, Harpending HC, Moyzis RK. Recent acceleration of human adaptive evolution. Proc Natl Acad Sci U S A. 2007;104:20753-20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wallace DC. Bioenergetics in human evolution and disease: implications for the origins of biological complexity and the missing genetic variation of common diseases. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Gokhman D, Lavi E, Prufer K, et al. Reconstructing the DNA methylation maps of the Neandertal and the Denisovan. Science. 2014;344:523-527. [DOI] [PubMed] [Google Scholar]
- 225. Gokhman D, Malul A, Carmel L. Inferring past environments from ancient epigenomes. Mol Biol Evol. 2017;34:2429-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Ternes CM, Schonknecht G. Gene transfers shaped the evolution of de novo NAD+ biosynthesis in eukaryotes. Genome Biol Evol. 2014;6:2335-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Stern M. Evidence that a mitochondrial death spiral underlies antagonistic pleiotropy. Aging Cell. 2017;16:435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Trammell SA, Schmidt MS, Weidemann BJ, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Yang D, Wan Y. NR supplementation during lactation: benefiting mother and child. Trends Endocrinol Metab. 2019;30:225-227. [DOI] [PubMed] [Google Scholar]
- 230. Giroud-Gerbetant J, Joffraud M, Giner MP, et al. A reduced form of nicotinamide riboside defines a new path for NAD(+) biosynthesis and acts as an orally bioavailable NAD(+) precursor. Mol Metab. 2019;30:192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Brenner C. Why is mom stressed: homeorhesis as the potential problem and nicotinamide riboside as the potential solution [published online ahead of print August 13, 2019]. J Exp Neurosci. doi: 10.1177/1179069519869679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Dribe M. Long-term effects of childbearing on mortality: evidence from pre-industrial Sweden. Population Studies. 2004;58:297-310. [DOI] [PubMed] [Google Scholar]
- 233. Westendorp RG. Are we becoming less disposable? Evolution has programmed us for early survival and reproduction but has left us vulnerable to disease in old age. In our present affluent environment, we are better adapting to these improved conditions. EMBO Rep. 2004;5:2-6.14710174 [Google Scholar]
- 234. van Exel E, Koopman JJE, Bodegom DV, et al. Effect of APOE epsilon4 allele on survival and fertility in an adverse environment. PLoS ONE. 2017;12:e0179497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Shokeir MH. Investigation on Huntington’s disease in the Canadian Prairies. II. Fecundity and fitness. Clin Genet. 1975;7:349-353. [DOI] [PubMed] [Google Scholar]
- 236. Jasienska G, Ellison PT, Galbarczyk A, et al. Apolipoprotein E (ApoE) polymorphism is related to differences in potential fertility in women: a case of antagonistic pleiotropy? Proc Biol Sci. 2015;282:20142395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Everman ER, Morgan TJ. Antagonistic pleiotropy and mutation accumulation contribute to age-related decline in stress response. Evolution. 2018;72:303-317. [DOI] [PubMed] [Google Scholar]
- 238. Carter AJ, Nguyen AQ. Antagonistic pleiotropy as a widespread mechanism for the maintenance of polymorphic disease alleles. BMC Med Genet. 2011;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Austad SN, Hoffman JM. Is antagonistic pleiotropy ubiquitous in aging biology? Evol Med Public Health. 2018;2018:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Byars SG, Huang QQ, Gray LA, et al. Genetic loci associated with coronary artery disease harbor evidence of selection and antagonistic pleiotropy. PLoS Genet. 2017;13:e1006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Fox M. ‘Evolutionary medicine’ perspectives on Alzheimer’s disease: review and new directions. Ageing Res Rev. 2018;47:140-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Gaillard JM, Lemaitre JF. The Williams’ legacy: a critical reappraisal of his nine predictions about the evolution of senescence. Evolution. 2017;71:2768-2785. [DOI] [PubMed] [Google Scholar]
- 243. Liu Y, Clement J, Grant R, Sachdev P, Braidy N. Quantitation of NAD+: why do we need to measure it? Biochim Biophys Acta Gen Subj. 2018;1862:2527-2532. [DOI] [PubMed] [Google Scholar]
- 244. Rodriguez JA, Marigorta UM, Hughes DA, Spataro N, Bosch E, Navarro A. Antagonistic pleiotropy and mutation accumulation influence human senescence and disease. Nat Ecol Evol. 2017;1:0055. [DOI] [PubMed] [Google Scholar]
- 245. Hernando-Herraez I, Heyn H, Fernandez-Callejo M, et al. The interplay between DNA methylation and sequence divergence in recent human evolution. Nucleic Acids Res. 2015;43:8204-8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Pettay JE, Helle S, Jokela J, Lummaa V. Natural selection on female life-history traits in relation to socio-economic class in pre-industrial human populations. PLoS ONE. 2007;2:e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Baez-Mendoza R, Harris CJ, Schultz W. Activity of striatal neurons reflects social action and own reward. Proc Natl Acad Sci U S A. 2013;110:16634-16639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Bergey CM, Phillips-Conroy JE, Disotell TR, Jolly CJ. Dopamine pathway is highly diverged in primate species that differ markedly in social behavior. Proc Natl Acad Sci U S A. 2016;113:6178-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Res. 2006;1126:76-90. [DOI] [PubMed] [Google Scholar]
- 250. Holloway RL., Jr. Cranial capacity and neuron number: a critique and proposal. Am J Phys Anthropol. 1966;25:305-314. [DOI] [PubMed] [Google Scholar]
- 251. Caravaggio F, Chung JK, Gerretsen P, et al. Exploring the relationship between social attachment and dopamine D2/3 receptor availability in the brains of healthy humans using [11C]-(+)-PHNO. Soc Neurosci. 2017;12:163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Finkenwirth C, Martins E, Deschner T, Burkart JM. Oxytocin is associated with infant-care behavior and motivation in cooperatively breeding marmoset monkeys. Horm Behav. 2016;80:10-18. [DOI] [PubMed] [Google Scholar]
- 253. Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Curr Opin Neurobiol. 2000;10:784-789. [DOI] [PubMed] [Google Scholar]
- 254. Lynch Z, Laursen B. The Neuro Revolution: How Brain Science Is Changing Our World. New York, NY: St. Martin’s Press; 2009. [Google Scholar]
- 255. Pearce E, Wlodarski R, Machin A, Dunbar RI. Variation in the β-endorphin, oxytocin, and dopamine receptor genes is associated with different dimensions of human sociality. Proc Natl Acad Sci U S A. 2017;114:5300-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048-1054. [DOI] [PubMed] [Google Scholar]
- 257. Pimentel D, Marcia H, Pimentel MS. Food, Energy, and Society. 3rd ed. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 258. Shi A. The impact of population pressure on global carbon dioxide emissions, 1975-1996: evidence from pooled cross-country data. Ecol Econ. 2003;44:29-42. [Google Scholar]
- 259. Bala G, Caldeira K, Wickett M, et al. Combined climate and carbon-cycle effects of large-scale deforestation. Proc Natl Acad Sci U S A. 2007;104:6550-6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Cullather N. The Hungry World: America’s Cold War Battle Against Poverty in Asia. Cambridge, MA: Harvard University Press; 2013. [Google Scholar]
- 261. Broecker WS, Stocker TF. The Holocene CO2 rise: anthropogenic or natural? EOS Trans Am Geophys Union. 2006;87:27. [Google Scholar]
- 262. Bonan GB. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science. 2008;320:1444-1449. [DOI] [PubMed] [Google Scholar]
- 263. Goudie AS, Viles HA. The Earth Transformed: An Introduction to Human Impacts on the Environment. Oxford, UK: Wiley-Blackwell; 2013. [Google Scholar]
- 264. Houghton JT. Global Warming: The Complete Briefing. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 265. Kaplan JO, Krumhardt KM, Zimmermann N. The prehistoric and preindustrial deforestation of Europe. Quaternary Sci Rev. 2009;28:3016-3034. [Google Scholar]
- 266. Linden E. The Winds of Change: Climate, Weather, and the Destruction of Civilizations. New York, NY: Simon & Schuster; 2006. [Google Scholar]
- 267. Lewis SL, Maslin MA. Defining the anthropocene. Nature. 2015;519:171-180. [DOI] [PubMed] [Google Scholar]
- 268. Ward P. The Medea Hypothesis: Is Life on Earth Ultimately Self-Destructive? Princeton, NJ: Princeton University Press; 2009. [Google Scholar]
- 269. Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of earth’s ecosystems. Science. 1997;277:494-499. [Google Scholar]
- 270. Dietz T, Rosa EA. Effects of population and affluence on CO2 emissions. Proc Natl Acad Sci U S A. 1997;94:175-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Field CB, Raupach MR, MacKenzie SH. The Global Carbon Cycle: Integrating Humans, Climate, and the Natural World. London, England: Island Press; 2012. [Google Scholar]
- 272. Lovelock J. Gaia: A New Look at Life on Earth. Oxford, UK: Oxford University Press; 1995. [Google Scholar]
- 273. Storey R. Population decline during and after conquest. In: Nichols DL, Pool CA, eds. The Oxford Handbook of Mesoamerican Archaeology. Oxford, UK: Oxford University Press; 2012:908-915. [Google Scholar]
- 274. Faust FX, Gnecco C, Mannstein H, Stamm J. Evidence for the postconquest demographic collapse of the Americas in historical CO2 levels. Earth Interact. 2006;10:1-14. [Google Scholar]
- 275. Lewis SL, Maslin MA. The Human Planet: How We Created the Anthropocene. London, England: Penguin Books Limited; 2018. [Google Scholar]
- 276. Ruddiman WF. Plows, Plagues, and Petroleum: How Humans Took Control of Climate. Princeton, NJ: Princeton University Press; 2010. [Google Scholar]
- 277. Brockman J. This Idea Must Die: Scientific Theories That Are Blocking Progress. New York, NY: Harper Perennial; 2015. [Google Scholar]
- 278. Rees J. The Leveller Revolution: Radical Political Organisation in England, 1640-1650. New York, NY: Verso; 2016. [Google Scholar]
- 279. Phillips M. The World Turned Upside Down: The Global Battle Over God, Truth, and Power. London, England: Encounter Books; 2011. [Google Scholar]
- 280. Fagan B. The Little Ice Age: How Climate Made History 1300-1850. New York, NY: Basic Books; 2001. [Google Scholar]
- 281. Blom P. Nature’s Mutiny: How the Little Ice Age of the Long Seventeenth Century Transformed the West and Shaped the Present. New York, NY: Liveright Publishing Corporation; 2020. [Google Scholar]
- 282. Perfecto I, Vandermeer JH, Wright AL. Nature’s Matrix: Linking Agriculture, Conservation and Food Sovereignty. London, England: Earthscan; 2009. [Google Scholar]
- 283. Russell E. Evolutionary History: Uniting History and Biology to Understand Life on Earth. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- 284. Meek RL. Economics of Physiocracy. London, England: Taylor & Francis; 2013. [Google Scholar]
- 285. Banerjee A, Duflo E. Poor Economics: A Radical Rethinking of the Way to Fight Global Poverty. New York, NY: PublicAffairs; 2012. [Google Scholar]
- 286. Barrett CB, Garg T, McBride L. Well-Being Dynamics and Poverty Traps. London, England: Centre for Climate Change Economics and Policy; 2016. [Google Scholar]
- 287. Dorling D. Do We Need Economic Inequality? Cambridge, England: Wiley; 2017. [Google Scholar]
- 288. Fukuoka M, Korn L, Berry W, Lappe FM. The One-Straw Revolution: An Introduction to Natural Farming. New York, NY: New York Review Books; 2010. [Google Scholar]
- 289. Hickel J. The Divide: A Brief Guide to Global Inequality and Its Solutions. London, England: William Heinemann; 2017. [Google Scholar]
- 290. Mellor JW. Agricultural Development and Economic Transformation: Promoting Growth With Poverty Reduction. Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 291. Pearce F. Peoplequake: Mass Migration, Ageing Nations and the Coming Population Crash. London, England: Eden Project; 2011. [Google Scholar]
- 292. Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671-677. [DOI] [PubMed] [Google Scholar]
- 293. Sen A. Development as Freedom. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- 294. Rockstrom J, Steffen W, Noone K, et al. A safe operating space for humanity. Nature. 2009;461:472-475. [DOI] [PubMed] [Google Scholar]
- 295. Rieff D. The Reproach of Hunger: Food, Justice, and Money in the Twenty-First Century. New York, NY: Simon & Schuster; 2016. [Google Scholar]
- 296. Sassen S. Expulsions. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- 297. Homer-Dixon T. The Upside of Down: Catastrophe, Creativity, and the Renewal of Civilization. Washington, DC: Island Press; 2010. [Google Scholar]
- 298. Kenny C. Getting Better: Why Global Development Is Succeeding – And How We Can Improve the World Even More. New York, NY: Basic Books; 2012. [Google Scholar]
- 299. Parenti C. Tropic of Chaos: Climate Change and the New Geography of Violence. New York, NY: PublicAffairs; 2011. [Google Scholar]
- 300. Nordås R, Gleditsch NP. Climate change and conflict. Polit Geogr. 2007;26:627-638. [Google Scholar]
- 301. Stephenson AR, Edler MK, Erwin JM, et al. Cholinergic innervation of the basal ganglia in humans and other anthropoid primates. J Comp Neurol. 2017;525:319-332. [DOI] [PubMed] [Google Scholar]
- 302. Smil V. How many people can the earth feed? Popul Dev Rev. 1994;20:255-292. [Google Scholar]
- 303. Sassoon D. The Anxious Triumph: A Global History of Capitalism, 1860-1914. London, England: Penguin Books Limited; 2019. [Google Scholar]
- 304. Aime C, Verdu P, Segurel L, et al. Microsatellite data show recent demographic expansions in sedentary but not in nomadic human populations in Africa and Eurasia. Eur J Hum Genet. 2014;22:1201-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Barker G, Janowski M. Why Cultivate? Anthropological and Archaeological Approaches to Foraging-Farming Transitions in Southeast Asia. Cambridge, UK: McDonald Institute for Archaeological Research; 2011. [Google Scholar]
- 306. United Nations Economic and Social Commission for Asia and the Pacific. Sustainable Agriculture and Food Security in Asia and the Pacific. New York, NY: United Nations Economic and Social Commission for Asia and the Pacific; 2009. [Google Scholar]
- 307. Speakman JR, Mitchell SE, Mazidi M. Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp Gerontol. 2016;86:28-38. [DOI] [PubMed] [Google Scholar]
- 308. Fontana L, Cummings NE, Arriola Apelo SI, et al. Decreased consumption of branched chain amino acids improves metabolic health. Cell Rep. 2016;16:520-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Seyedsadjadi N, Berg J, Bilgin AA, Braidy N, Salonikas C, Grant R. High protein intake is associated with low plasma NAD+ levels in a healthy human cohort. PLoS ONE. 2018;13:e0201968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Braidy N, Grant R. Kynurenine pathway metabolism and neuroinflammatory disease. Neural Regen Res. 2017;12:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE. 2012;7:e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids. 2013;45:419-430. [DOI] [PubMed] [Google Scholar]
- 314. Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Choi S, Disilvio B, Fernstrom MH, Fernstrom JD. Meal ingestion, amino acids and brain neurotransmitters: effects of dietary protein source on serotonin and catecholamine synthesis rates. Physiol Behav. 2009;98:156-162. [DOI] [PubMed] [Google Scholar]
- 316. Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife healthy-diet index and late-life dementia and Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2011;1:103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta. 2006;1757:496-508. [DOI] [PubMed] [Google Scholar]
- 318. Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD(+) in brain aging and neurodegenerative disorders. Cell Metab. 2019;30:630-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Canto C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Massudi H, Grant R, Guillemin GJ, Braidy N. NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Rep. 2012;17:28-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Sorgdrager FJH, Naude PJW, Kema IP, Nollen EA, Deyn PP. Tryptophan metabolism in inflammaging: from biomarker to therapeutic target. Front Immunol. 2019;10:2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Pearson CS. On the Cusp: From Population Boom to Bust. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 323. Poston DL, Yaukey D. The Population of Modern China. New York, NY: Springer; 2013. [Google Scholar]
- 324. Barkow JH, Burley N. Human fertility, evolutionary biology, and the demographic transition. Ethol Sociobiol. 1980;1:163-180. [Google Scholar]
- 325. Giannakidis G, Karlsson K, Labriet M, Gallachóir BÓ. Limiting Global Warming to Well Below 2 °C: Energy System Modelling and Policy Development. Cham, Switzerland: Springer International Publishing; 2018. [Google Scholar]
- 326. Gietel-Basten S, Lutz W, Scherbov S. Very long range global population scenarios to 2300 and the implications of sustained low fertility. Demogr Res. 2013;28:1145-1166. [Google Scholar]
- 327. Goldstein JR, Sobotka T, Jasilioniene A. The end of ‘lowest-low’ fertility? Popul Dev Rev. 2009;35:663-699. [Google Scholar]
- 328. Harris FR. The Baby Bust: Who Will Do the Work? Who Will Pay the Taxes? Lanham, MD: Rowman & Littlefield; 2006. [Google Scholar]
- 329. Scherbov S, Lutz W, Sanderson WC. The uncertain timing of reaching 8 billion, peak world population, and other demographic milestones. Popul Dev Rev. 2011;37:571-578. [DOI] [PubMed] [Google Scholar]
- 330. Robinson KS. Forty Signs of Rain. London, England: HarperCollins; 2005. [Google Scholar]
- 331. Davis M. Planet of Slums. London, England: Verso; 2007. [Google Scholar]
- 332. Davis M. Late Victorian Holocausts: El Nino Famines and the Making of the Third World. London, England: Verso Books; 2002. [Google Scholar]
- 333. de Blij H. Why Geography Matters: More Than Ever. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 334. De Castro J. The Geography of Hunger. Boston, MA: Little, Brown and Company; 1952. [Google Scholar]
- 335. Fitouss JP, Sen AK, Stiglitz JE. Mismeasuring Our Lives: Why GDP Doesn’t Add Up. New York, NY: ReadHowYouWant.com; 2011. [Google Scholar]
- 336. Frase P. Four Futures: Visions of the World After Capitalism. Brooklyn, NY: Verso; 2016. [Google Scholar]
- 337. Kaplan RD. The Coming Anarchy: Shattering the Dreams of the Post Cold War. New York, NY: Knopf Doubleday Publishing Group; 2002. [Google Scholar]
- 338. Mbow H-OP, Reisinger A, Canadell J, O’Brien P. Special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems (SR2). Ginevra, IPCC; 2017. [Google Scholar]
- 339. O’Sullivan M. The Levelling: What’s Next After Globalization. New York, NY: PublicAffairs; 2019. [Google Scholar]
- 340. Berners-Lee M. There Is No Planet B: A Handbook for the Make or Break Years. Cambridge, UK: Cambridge University Press; 2019. [Google Scholar]
- 341. Dobson A, Eckersley R. Political Theory and the Ecological Challenge. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 342. Mazur LA, Riche MF, Sinding S, et al. A Pivotal Moment: Population, Justice, and the Environmental Challenge. Washington, DC: Island Press; 2012. [Google Scholar]
- 343. Pogge TW. World Poverty and Human Rights. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 344. Vanderheiden S. Atmospheric Justice: A Political Theory of Climate Change. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- 345. Mazower M. Governing the World: The History of an Idea. London, England: Penguin Books Limited; 2012. [Google Scholar]
- 346. Mazzucato M. The Value of Everything: Making and Taking in the Global Economy. London, England: Penguin Books Limited; 2018. [Google Scholar]
- 347. Willett W, Rockström J, Loken B, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447-492. [DOI] [PubMed] [Google Scholar]