Abstract
The small size of plant viral genomes, the ease with which they can be manipulated, and the simplicity of the infection process is making the viral vectors an attractive alternative to the transgenic systems for the expression of foreign proteins in plants. One use of these virus expression systems is for vaccine production. There are two basic types of viral system that have been developed for the production of immunogenic peptides and proteins in plants: epitope presentation and polypeptide expression systems. In this review, we discuss advances made in this field.
Keywords: epitope presentation, plant virus, polypeptide expression, viral vector
Introduction
The use of plants for vaccine production is emerging as an attractive alternative to traditional production systems. Plant expression systems have several advantages such as the absence of risk of contamination with animal pathogens, potentially low production costs, and the possibility of very large‐scale production. They also offer the option of producing edible vaccines.
The classical system for expressing foreign proteins in plants is stable genetic transformation. This approach involves integration of the gene of interest into the plant genome, and it has been used successfully to express a number of immunologically active proteins. An alternative to stable genetic transformation is the use of plant virus‐based vectors to achieve expression. The main advantages of using the latter strategy are that viral genomes are small and easy to manipulate, infection of plants with modified viruses is simpler and quicker than the regeneration of stably transformed plants, and the sequence inserted into a virus vector will be highly amplified. However, there are some disadvantages: the foreign gene is not heritable, there are limitations on the size and complexity of the sequences that can be expressed in a genetically stable manner, and there are concerns about the ability of modified viruses to spread in the environment. Despite these difficulties, different plant virus vectors have been used for protein expression. In the present paper, we review the development of virus‐based vectors as a potential source of novel vaccines in plants.
Types of viral expression system
The first attempts to develop plant virus vectors involved viruses with DNA genomes. Unfortunately, the complexity of the replication cycles made these viruses unsuitable for the expression of high levels of foreign proteins in plants. To gain information about these DNA virus‐based vectors, the reader is referred to Porta and Lomonossoff 1 , 2 .
The vast majority of plant viruses have genomes that consist of one or more molecules of positive‐sense RNA. These viruses can grow in a wide range of hosts, and some can reach extremely high titres. The ability of the genomes of positive‐sense RNA plant viruses to be directly translated on entering a plant cell has made this class of virus a very attractive choice to express recombinant proteins in infected plants 3 . With the development of methods that permit the manipulation of infectious cDNA clones of positive‐strand RNA viruses 4 , the exploitation of these viruses as vectors for foreign protein expression began. Since then, members of several virus families have been developed as useful vectors (Figure 1). The first strategy used for developing virus vectors was to replace existing nonessential viral genes with foreign genes. However, this replacement strategy has limitations, due to adverse consequences associated with gene deletion. A more successful approach has been to insert the foreign gene as an addition to the virus genome, avoiding the deletion of any viral genes. For reviews on the development of RNA virus‐based vectors, the reader is referred to Scholthof et al. 5 and Porta and Lomonossoff 2 , 6 .
Figure 1.
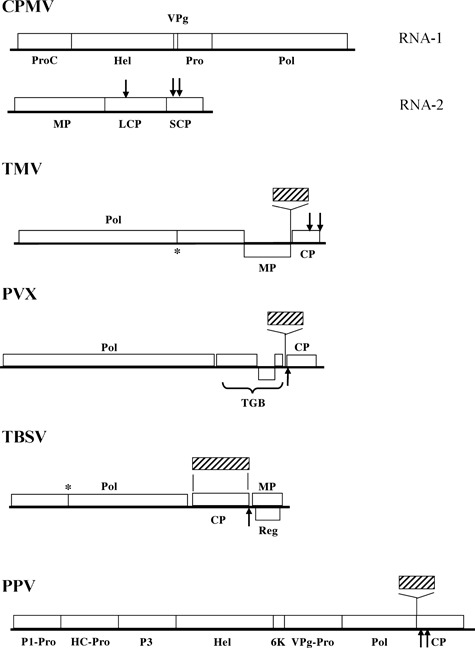
Genome organization of viruses used to express heterologous peptides and proteins in plants. Positions where epitopes have been inserted into the coat proteins of the various viruses are shown by black arrows. The positions where foreign proteins (shown hatched) have been inserted into the viral genomes are also indicated. The functions of various virus genes are shown as: CP, coat protein; HC‐Pro, helper component proteinase; Hel, helicase; MP, movement protein; LCP, large coat protein; Pol, RNA‐dependent RNA polymerase; Pro, proteinase; ProC, proteinase cofactor; Reg, regulatory protein; TGB, triple gene block; VPg, virus protein genome‐linked; P1‐Pro, P1‐Proteinase; P3, protein P3; 6K, 6 kDa protein; SCP, small coat protein; VPg‐Pro, VPg‐Proteinase. The asterisk (*) represents a leaky termination codon. CPMV, cowpea mosaic virus; PPV, plum pox virus; PVX, potato virus X; TBSV, tomato bushy stunt virus; TMV, tobacco mosaic virus.
Two basic types of expression system based on RNA plant viruses have been developed for the production of immunogenic peptides and proteins in plants. In the first type, termed epitope presentation systems, the viral vector is designed so that short antigenic peptides fused to the coat protein (CP) are displayed on the surface of assembled viral particles. These modified virions or chimeras are attractive as potential novel vaccines, because the modified particles can be readily purified and the presentation of multiple copies of an antigenic peptide on the surface of a macromolecular assembly can significantly increase its immunogenicity 7 . The second type, often referred to as polypeptide expression systems, express a whole unfused recombinant protein that accumulates within the plant.
Epitope presentation systems
In this expression strategy, the sites of insertion are chosen so that the peptide is displayed on the surface of the virus particles without interfering with the ability of the modified CP to assemble. For this reason, this system has mainly been developed for viruses where there is some information available about the topology of the CP in the assembled virions. The viral systems discussed below are the ones that are currently used for epitope presentation, but it is likely that this list will expand as structural information about more viruses becomes available.
Cowpea mosaic virus
Cowpea mosaic virus (CPMV) was the first plant virus to be developed as an epitope presentation system 1 , 8 , 9 . CPMV is a bipartite RNA virus (Figure 1), particles of which are composed of 60 copies of each of large (L) and small (S) CP arranged with icosahedral symmetry. Given the state of knowledge of the structure of its particles, the availability of infectious cDNA clones and the potential productivity of the system, CPMV was an attractive candidate for development as an epitope presentation system. Analysis of the 3‐D structure of CPMV identified exposed loops on the virus surface that would potentially be suitable sites for the insertion of foreign sequences 10 . In most cases, the foreign sequence has been inserted into the most exposed loop on the virus surface, the βB‐βC loop of the S protein 8 , 9 . However, other sites, such as the βE‐αB loop of the L protein and the βC′‐βC″ loop of the S protein, have also been used successfully 11 , 12 , 13 , 14 . Generally, provided the inserted peptide has fewer than 40 amino acids and has a pI below 9.0 14 , the yields of modified particles are similar to those obtained with wild‐type CPMV.
A number of CPMV‐based chimeras have been subjected to detailed immunological analysis. Some of them, after being purified and injected into experimental animals, have been shown to raise antibodies against the inserted peptide. These antibodies are able to bind to, and in several cases neutralize, the pathogen from which the peptide was derived. The reader is referred to Lomonossoff and Hamilton 15 , where the results of these immunological studies have been reviewed. The ability of modified plant virus particles to stimulate protective immunity, the ultimate goal of vaccine development, was first reported in 1997 16 . This study involved a CPMV chimera (CPMV‐PARVO1) that contained a 17 amino acid epitope from the N‐terminal region of the VP2 capsid protein of canine parvovirus. This peptide is also found in VP2 of related canine parvoviruses (CPV), mink enteritis virus (MEV), and feline panleukopenia virus (FPV). This CPMV‐PARVO1 chimera in an active and a UV light‐inactivated form were shown to be capable of protecting mink and dogs against MEV and CPV, respectively 16 , 17 . CPMV‐based chimeras expressing epitopes of a bacterial origin were subsequently shown to also be able to confer protective immunity 18 , 19 (Table 1).
Table 1.
Immunogenic proteins reported using the viral epitope presentation system in plants
| Epitope presentation system | Expressed epitope | Plant used for infection | Species protected | Immunological properties | Reference |
|---|---|---|---|---|---|
| CPMV | VP2 capsid protein of canine parvovirus (CPV) | Vigna unguiculata | Mink | Parenteral injection protected mink against challenge with mink enteritis virus (MEV) | 16 |
| CPMV | Canine parvovirus VP2 | Vigna unguiculata | Dog | UV light‐inactivated form of the chimera protected dogs against challenge with canine parvovirus (CPV) | 17 |
| CPMV | Pseudomonas aeruginosa outer membrane (OM) protein F | Vigna unguiculata | Mouse | Parenteral injection protected mice against challenge with P. aeruginosa | 18 |
| CPMV | Staphylococcus aureus D2 domain of fibronectin‐binding protein (FnBP) | Vigna unguiculata | Rat | Parenteral application protected rats against endocarditis | 19 |
| TMV | Glycoprotein ZP3 from the murine zona pellucida | Nicotiana tabacum | Mouse | Modified virions were capable of eliciting antibodies in parenterally immunized mice | 23 |
| TMV | Mouse hepatitis virus (MHV) | Nicotiana tabacum | Mouse | Mice parenterally and intranasally immunized were protected against challenge with MHV | 25 |
| TMV | Foot and mouth disease virus (FMDV) | Nicotiana tabacum | Guinea pig | Oral immunization was less effective than the parenteral injection against FMDV challenge | 26 |
| TMV | Pseudomonas aeruginosa outer membrane (OM) protein F | Nicotiana tabacum | Mouse | Parenterally immunized mice were protected against challenge with P. aeruginosa | 27 |
| TMV | Pseudomonas aeruginosa outer membrane (OM) protein F | Nicotiana tabacum | Mouse | Mice immunized with a mixture of a TMV chimera and a chimeric influenza virus, parenterally and nasally administered, respectively, produced antibodies against the two different epitopes and were protected against challenge with P. aeruginosa | 28 |
| TMV/AlMV | Rabies virus HIV‐1 | Nicotiana benthamiana Spinacia oleracea | Mouse | Both epitopes produced virus‐neutralizing antibodies, being the rabies ones able to protect mice when supplied either intraperitoneally or orally | 29 , 30 |
| TMV | Hepatitis C linked to the C‐terminus of the cholera toxin B subunit | Nicotiana benthamiana | Mouse | Nasal administration of crude plant material elicited the production of antibodies against both epitopes | 31 |
| TBSV | gp120 of HIV‐1 | Nicotiana benthamiana | Mouse | Parenteral immunization only gave a relatively low synthetic peptide specific primary antibodies | 32 |
| PPV | VP2 capsid protein of canine parvovirus (CPV) | Nicotiana clevelandii | Mouse Rabbit | Parenteral immunization showed the presence of neutralizing antibodies against both CPV and PPV | 34 |
| PVX | gp41 of HIV‐1 | Nicotiana benthamiana | Mouse | Mice immunized intraperitoneally or nasally produced high levels of HIV‐1 IgG and IgA antibodies; immunodeficient mice reconstituted with human peripheral lymphocytes made human primary neutralizing antibodies | 37 |
| AlMV | Respiratory syncytial virus (RSV) G protein | P12 transgenic Nicotiana tabacum | Monkey | Parenteral injection of recombinant particles generated T‐ and B‐cell responses; in vitro human dendritic cells incubated with the chimera generated strong T‐cell response | 38 |
AlMV, alfalfa mosaic virus; CPMV, cowpea mosaic virus; PPV, plum pox virus; PVX, potato virus X; TBSV, tomato bushy stunt virus; TMV, tobacco mosaic virus.
Tobacco mosaic virus
Tobacco mosaic virus (TMV) particles consist of a single molecule of genomic RNA encapsidated by 2130 copies of a single type of CP arranged with helical symmetry. The fact that TMV particles contain a large number of subunits, making the system potentially very attractive for peptide expression, is also a problem in that the subunits are very tightly packed, allowing little space on the virus surface for the expression of foreign sequences. Attempts to develop TMV as an epitope presentation system initially resulted in the production of CP subunits that were unable to assemble into virus particles. By engineering a leaky termination codon at the C‐terminus of the CP gene, a TMV vector was developed that permitted the synthesis of both native and recombinant forms of the CP from the same viral RNA 20 . As with CPMV‐based chimeras, the inserted peptides could be detected on the surface of assembled virions, being used to express epitopes of several animal pathogens 21 , 22 . Subsequently, by modifying the site of peptide insertion between amino acids 154 and 155, near but not at the C‐terminus of the CP, it has been possible to develop a TMV‐based vector in which all of the CP subunits could be modified to express foreign peptides abolishing virus viability 23 . The size of inserts that can be tolerated even at this optimized position seems to be quite small, the largest reported to date being 23 amino acids in a chimera that grew substantially more slowly than wild‐type TMV 24 .
The first analysis of the immunogenicity of a TMV chimera involved a construct expressing 13 amino acids from the glycoprotein ZP3 from the murine zona pellucida 23 (Table 1). Using the vector developed by Fitchen et al. 23 , Koo et al. 25 were able to produce chimeric TMV particles presenting an epitope from mouse hepatitis virus (MHV). Mice immunized with purified virions produced antibodies against the MHV epitope, and those with high antibody titres were protected against challenge with the virus. Recently, using the same strategy, two epitopes from foot and mouth disease virus (FMDV) were expressed on TMV 26 . As reported previously with CPMV 18 , expression of an epitope from the OM protein F of Pseudomonas aeruginosa on the surface of TMV 27 has been shown to confer protective immunity against P. aeruginosa. In the same way, a mixture of a TMV chimera and a chimeric influenza virus presenting two different epitopes from protein F of P. aeruginosa conferred immunity in mice 28 .
To overcome the limitation on the size of peptide that can be fused to the TMV CP, researchers have developed a system where an appropriately modified version of the alfalfa mosaic virus (AlMV) CP is expressed from an additional copy of the TMV CP subgenomic promoter. Using this approach, a 40 amino acid sequence containing epitopes from rabies virus and a 47 amino acid sequence from HIV‐1 were fused to the AlMV CP 29 . Both types of particle elicited the production of appropriate virus‐neutralizing antibodies; those displaying the rabies virus epitopes were able to protect mice against a normally lethal challenge with the virus 30 .
Using a similar approach, a fusion protein consisting of a potentially neutralizing epitope from hepatitis C virus linked to the C‐terminus of the cholera toxin B subunit has been expressed from TMV 31 .
Tomato bushy stunt virus
Tomato bushy stunt virus (TBSV) is a monopartite virus, particles of which contain 180 copies of a single type of CP arranged with icosahedral symmetry. Sequences derived from gp120 of HIV‐1 have been fused to the C‐terminus of the CP. A 13 amino acid epitope sequence, corresponding to the V3 loop, could be detected immunologically 32 .
Plum pox virus
Plum pox virus (PPV) has flexuous rod‐shaped particles consisting of more than 2000 copies of a single CP encapsidating a single RNA molecule. Although a detailed structure of PPV CP was not available, immunological analyses of related viruses suggested that both the N‐ and C‐termini are exposed to the surface. In addition, the demonstration that it is possible to fuse foreign sequences to the N‐terminus of the heterologously expressed CP of Johnsongrass mosaic virus (JGMV) 33 , a member of the same genus as PPV, without abolishing particle formation, has encouraged other groups to use a similar strategy in planta. The fusion of a 15 amino acid epitope, equivalent to that used by Dalsgaard et al. 16 , from VP2 of CPV to a position near the N‐terminus of PPV CP, either as a single copy or as a tandem duplication 34 , gave yields of virus particles similar to those obtained with wild‐type PPV. The site of expression of peptides on the PPV CP has subsequently been refined 35 , raising the prospect that PPV may have general use as an epitope presentation system.
Potato virus X
Potato virus X (PVX) has filamentous particles consisting of approximately 1260 CP subunits encapsidating a single RNA molecule. It has proven possible to express proteins at the surface‐exposed N‐terminus of either a proportion 36 or all of the subunits 37 .
Alfalfa mosaic virus
It has recently been reported that a 21 amino acid peptide of the respiratory syncytial virus (RSV) G protein fused with the AlMV CP generated strong T‐cell responses in human dendritic cells and both T‐ and B‐cell responses in non‐human primates to the incorporated RSV 38 .
Polypeptide expression systems
This type of viral expression system involves introducing a whole gene into the viral genome in such a manner that it is efficiently expressed in infected cells, usually as an unfused polypeptide. Although purification of the expressed protein may be necessary or desirable, this type of system could be suitable for the production of immunogens that can be supplied orally by direct feeding of plant material to animals. A number of RNA viruses – mainly those without the limitation on the size of RNA that can be packaged in the particles – have been used. Among the main ones investigated are TMV, PVX, PPV, TBSV and, to a lesser extent, CPMV. A brief account on the development of viral vectors for the expression of foreign proteins using the polypeptide expression system is given below, and examples where immunogenic proteins have been produced are further illustrated in Table 2.
Table 2.
Immunogenic proteins reported using the viral polypeptide expression system in plants
| Polypeptide expression system | Expressed sequence | Plant used for infection | Species protected | Immunological properties | Reference |
|---|---|---|---|---|---|
| TMV | VP1 from the foot and mouth disease virus (FMDV) | Nicotiana benthamiana | Mouse | Parenteral application in the presence of CFA protected all animals in two separate experiments | 47 |
| TMV | 38C13 scFv specific to the 38C13 mouse B‐cell lymphoma | Nicotiana benthamiana | Mouse | Mice vaccinated with the affinity purified 38C13 scFv generated >10 µg/mL anti‐idiotype immunoglobulins; these mice were protected from challenge by a lethal dose of the syngeneic 38C13 tumour | 43 |
| TMV | Betv1, a major birch pollen antigen | Nicotiana benthamiana | Mouse | Parenteral application with crude leaf extracts generated immunological responses comparable to those induced by the protein expressed in Escherichia coli or extracted from birch pollen | 48 |
| TMV | gDc from bovine herpes virus type 1 (BHV‐1) | Nicotiana benthamiana | Mouse Cattle | Parenteral application of oil‐based vaccines with crude extracts protected both animals | 49 |
| PVX | E7 from human papillomavirus 16 (HPV‐16) | Nicotiana benthamiana | Mouse | Parenteral application with foliar extracts in the presence of Quil A as an adjuvant developed both antibody and cell‐mediated immune responses | 54 |
| PPV | VP60 from rabbit haemorrhagic disease virus (RHDV) | Nicotiana clevelandii | Rabbit | Parenteral application with crude extracts in the presence of adjuvant fully protected rabbits against subsequent challenge with a lethal dose of RHDV | 57 |
| CPMV | Small immunogenic proteins (∈SIP) specific to transmissible gastroenteritis virus (TGEV) | Vigna unguiculata | Pig | Oral application using ∈SIP infected crude leaf powder | Lomonossoff (unpubl. data, 2005) |
CPMV, cowpea mosaic virus; PPV, plum pox virus; PVX, potato virus X; TMV, tobacco mosaic virus.
Tobacco mosaic virus
An early phase in the development of viral vectors such as those based on TMV was the expression of marker genes such as chloramphenicol acetyl transferase (CAT) 39 , 40 . Further work included the expression of an inserted sequence under the control of an additional copy of the CP subgenomic promoter 41 , with genetic stability being improved by the use of promoters from heterologous strains. This resulted in high levels (up to 2% of soluble proteins) of several valuable proteins, including the eukaryotic ribosome‐inactivating protein (RIP), α‐trichosanthin 42 , single‐chain antibodies (ScFv) 43 , or full‐length monoclonal antibodies 44 . Furthermore, it has proven possible to synthesize glycosylated proteins with TMV vectors 45 , 46 . There are a number of examples in which the TMV vector system has been used to express immunogenic proteins (Table 2). These include VP1 from FMDV 47 , a tumour‐derived scFv epitope 43 , a major birch pollen antigen (Betv1) 48 , and the cytosolic form of the bovine herpes virus gD protein 49 .
Potato virus X
The development of PVX‐based viral vectors has been carried out in two different formats. The first uses duplicated subgenomic promoters 50 similar to those used for TMV. This strategy has been used to express a number of proteins such as ScFv antibodies 51 , 52 , the major capsid protein (VP6) from a murine rotavirus 53 , and the E7 protein of human papillomavirus 16 54 . The second type involves the fusion of the foreign protein to the N‐terminus of the CP gene via the 2A catalytic peptide from FMDV. The 2A sequence promotes cotranslational cleavage between the foreign gene insert and the CP, although this is not 100% efficient, resulting in some CP subunits still bearing the inserted protein. These fusion proteins were found to retain their ability to be incorporated into virus capsids, resulting in particles that displayed the inserted polypeptide. By means of this approach it is possible, using the same construct, to produce a protein of interest in both a free (unfused) state where cleavage by 2A has occurred and as a CP fusion where it is incorporated in PVX particles. A ScFv expressed as a CP fusion was found to be functional when incorporated into virions 55 . When this system was used to express the rotavirus VP6 sequence, the uncleaved VP6‐2A‐CP was incorporated into PVX virions while the VP6‐2A cleavage product formed typical VP6 virus‐like particles (VLP) 53 .
Plum pox virus
Efforts in using the PPV‐based viral vectors have concentrated on inserting the foreign sequence into the single open reading frame (ORF) that encodes a multifunctional polyprotein that is self‐processed by proteinase domains within it to produce the mature viral proteins. Initial experiments involved inserting marker genes in such a manner that the free polypeptide would be released through the action of the VPg‐proteinase (Figure 1). This was achieved by flanking the inserted sequence with the appropriate proteinase recognition sites 56 . Subsequently, a PPV vector in which the foreign sequence was inserted between the polymerase (Pol) and CP genes (Figure 1) was used to express the VP60 structural protein from rabbit haemorrhagic disease virus (RHDV) 57 . Immunized rabbits were protected against subsequent challenge with a lethal dose of RHDV.
Tomato bushy stunt virus
The use of TBSV‐based vectors has limitations on the size of insertion that the isometric particles can tolerate. To overcome this, one group exploited the fact that the TBSV CP is not essential for infectivity, and they produced constructs in which most of the region encoding the CP was replaced with marker genes 58 . A refined version of the vector was subsequently produced in which the CP gene was replaced with a polylinker 59 . This, coupled with improvements to the infection process, permitted the facile expression of heterologous sequences in the inoculated leaves of plants. This approach has been used to express the nucleocapsid protein p24 from HIV‐1 as a fusion with the 5′‐terminal portion of the CP gene 60 , but there is no report as yet concerning the immunological properties of the plant‐expressed protein.
Cowpea mosaic virus
Regarding CPMV‐based vectors, efforts began with the expression of the green fluorescent protein (GFP) as a cytosolic form. CPMV RNA‐2 was engineered to accommodate the FMDV‐2A catalytic sequence at the C‐terminus of the small (S) CP, followed by insertion of GFP 61 . Further work involved the expression of versions of GFP that were designed either to be retained in the endoplasmic reticulum (ER) or to be secreted into the apoplastic space. The expected subcellular localizations were confirmed by confocal microscopy (Nicholson et al., unpubl. data, 2005). This encouraged the use of the CPMV system for expression of valuable proteins such as small immune proteins (SIP) specific to the coronavirus transmissible gastroenteritis virus (TGEV), whose immunological properties are now being tested.
Conclusions
The past few years have seen significant progress in the use of plant virus vectors for the production of immunogens in plants. Particularly encouraging is the fact that it is now clear that peptides or proteins expressed in this manner can confer protective immunity against a number of diseases (Table 1,Table 2), in some cases in the target animals 16 , 17 , 57 . In most of these cases, immunity was stimulated by parenteral immunization, but there are encouraging signs that mucosal immunization may be possible 25 , 29 , 62 . This raises the prospect that it may be possible to confer protective immunity by simply feeding with plant material infected with an appropriate virus construct. To achieve this, it will be necessary not only to express peptides or proteins that can stimulate mucosal immunity but also to express the material in edible plants. In this regard, the continued development of vectors that can infect edible plants – such as those based on (i) CPMV 61 , clover yellow vein virus (CIYVV) 63 and pea early browning virus (PEBV) 64 , all of which infect legumes; (ii) wheat streak mosaic virus (WSMV) 65 , which infects cereals; and (iii) zucchini yellow mosaic virus (ZYMV) 66 , which infects cucurbits – is likely to play a prominent role. In addition, the development of combined transgene/virus complementation systems, such as those described by Sanchez‐Navarro et al. 67 and Mori et al. 68 , may allow the use of defective viral replicons for the expression of foreign sequences, thereby reducing the risk of environmental spread.
Even if direct feeding is shown to be efficacious, there will still be a need to purify, at least partially, proteins and chimeric particles for certain applications. Although sufficient material for initial characterization can be obtained by laboratory‐scale extractions, a substantial scale‐up of procedures will be necessary for its widescale use. To this end, results reported on the large‐scale growth and purification of a TMV chimera expressing a 12 amino acid malarial peptide 69 show that although growth under field conditions gives a lower yield per gram (fresh weight) of tissue than growth in a greenhouse, an excess of 1 kg per acre of purified particles could potentially be obtained. Similarly, it has been shown that large quantities (up to 10 mg per gram, fresh weight) of wild‐type CPMV could be extracted from fresh or frozen cowpea leaves by methods suitable for large‐scale application 70 . The development of methods for the industrial‐scale production of plant‐derived vaccines will make an important contribution to the practical use of such material.
Acknowledgements
This review is dedicated to the memory of Kim Lomonossoff, who died on 27 November 2004. With many thanks for 25 years of love and support. The work described in this review was funded in part by the EC Framework 5 Quality of Life Programme (contract no. QLK2‐CT‐2002–01050).
References
- 1. Porta C, Lomonossoff GP. Use of viral replicons for the expression of genes in plants. Mol. Biotechnol. 1996; 5: 209–21. [DOI] [PubMed] [Google Scholar]
- 2. Porta C, Lomonossoff GP. Viruses as vectors for the expression of foreign sequences in plants. Biotechnol. Genet. Eng. Rev. 2002; 19: 245–91. [DOI] [PubMed] [Google Scholar]
- 3. Siegel A. RNA viruses as cloning vehicles. Phytopathology 1983; 73: 775. [Google Scholar]
- 4. Ahlquist P, French R, Janda M, Loesch‐Fries S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc. Natl Acad. Sci. USA 1984; 81: 7066–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scholthof HB, Scholthof KB, Jackson AO. Plant virus gene vectors for transient expression of foreign proteins in plants. Annu. Rev. Phytopathol. 1996; 34: 299–323. [DOI] [PubMed] [Google Scholar]
- 6. Porta C, Lomonossoff GP. Scope for using plant viruses to present epitopes from animal pathogens. Rev. Med. Virol. 1998; 8: 25–41. [DOI] [PubMed] [Google Scholar]
- 7. Lomonossoff GP, Johnson JE. Use of macromolecular assemblies as expression systems for peptides and synthetic vaccines. Curr. Opin. Struct. Biol. 1996; 6: 176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Usha R, Rohll JB, Spall VE et al Expression of an animal virus antigenic site on the surface of a plant virus particle. Virology 1993; 197: 366–74. [DOI] [PubMed] [Google Scholar]
- 9. Porta C, Spall VE, Loveland J, Johnson JE, Barker PJ, Lomonossoff GP. Development of cowpea mosaic virus as a high‐yielding system for the presentation of foreign peptides. Virology 1994; 202: 949–55. [DOI] [PubMed] [Google Scholar]
- 10. Lomonossoff GP, Johnson JE. Eukaryotic viral expression systems for polypeptides. Semin. Virol. 1995; 6: 257–67. [Google Scholar]
- 11. Brennan FR, Jones TD, Gilleland LB et al Pseudomonas aeruginosa outer‐membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology 1999; 145: 211–20. [DOI] [PubMed] [Google Scholar]
- 12. Taylor KM, Lin T, Porta C et al Influence of three dimensional structure on the immunogenicity of a peptide expressed on the surface of a plant virus. J. Mol. Recognit. 2000; 13: 71–82. [DOI] [PubMed] [Google Scholar]
- 13. Chatterji A, Burns LL, Taylor SS et al Cowpea mosaic virus: from the presentation of antigenic peptides to the display of active biomaterials. Intervirology 2002; 45: 362–70. [DOI] [PubMed] [Google Scholar]
- 14. Porta C, Spall VE, Findlay KC, Gergerich RC, Farrance CE, Lomonossoff GP. Cowpea mosaic virus‐based chimaeras: effects of inserted peptides on the phenotype, host‐range and transmissibility of the modified viruses. Virology 2003; 310: 50–63. [DOI] [PubMed] [Google Scholar]
- 15. Lomonossoff GP, Hamilton WD. Cowpea mosaic virus‐based vaccines. Curr. Top. Microbiol. Immunol. 1999; 240: 177–89. [DOI] [PubMed] [Google Scholar]
- 16. Dalsgaard K, Uttenthal Å, Jones TD et al Plant‐derived vaccine protects target animals against a viral disease. Nat. Biotechnol. 1997; 15: 248–52. [DOI] [PubMed] [Google Scholar]
- 17. Langeveld JP, Brennan FR, Martinez‐Torrecuadrada JL et al Inactivated recombinant plant virus protects dogs from a lethal challenge with canine parovovirus. Vaccine 2001; 19: 3661–70. [DOI] [PubMed] [Google Scholar]
- 18. Brennan FR, Gilleland LB, Staczek J, Bendig MM, Hamilton WD, Gilleland HE Jr. A chimaeric plant virus vaccine protects mice against a bacterial infection. Microbiology 1999; 145: 2061–7. [DOI] [PubMed] [Google Scholar]
- 19. Rennermalm A, Li YH, Bohaufs L et al Antibodies against a truncated Staphylococcus aureus fibronectin‐binding protein protect against dissemination of infection in the rat. Vaccine 2001; 19: 3376–83. [DOI] [PubMed] [Google Scholar]
- 20. Hamamoto H, Sugiyama Y, Nakagawa N et al A new tobacco mosaic virus vector and its use for the systemic production of angiotensin‐I‐converting enzyme inhibitor in transgenic tobacco and tomato. Biotechnology 1993; 11: 930–32. [DOI] [PubMed] [Google Scholar]
- 21. Sugiyama Y, Hamamoto H, Takemoto S, Watanabe Y, Okada Y. Systemic production of foreign peptides on the particle surface of tobacco mosaic virus. FEBS Lett. 1995; 359: 247–50. [DOI] [PubMed] [Google Scholar]
- 22. Turpen TH, Reinl SJ, Charoenvit Y, Hoffman SL, Fallarme V, Grill LK. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Biotechnology 1995; 13: 53–7. [DOI] [PubMed] [Google Scholar]
- 23. Fitchen J, Beacky RN, Hein MB. Plant virus expressing hybrid coat protein with added murine epitope elicits autoantibody response. Vaccine 1995; 13: 1051–7. [DOI] [PubMed] [Google Scholar]
- 24. Bendahmane M, Koo M, Karrer E, Beachy RN. Display of epitopes on the surface of tobacco mosaic virus: impact of charge and isoelectric point of the epitope on virus‐host interactions. J. Mol. Biol. 1999; 290: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koo M, Bendahmane M, Lettieri GA et al Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc. Natl Acad. Sci. USA 1999; 96: 7774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu L, Jiang L, Zhou Z et al Expression of foot‐and‐mouth disease virus epitopes in tobacco by a tobacco mosaic virus‐based vector. Vaccine 2003; 21: 4390–98. [DOI] [PubMed] [Google Scholar]
- 27. Staczek J, Bendahmane M, Gilleland LB, Beachy RN, Gilleland HE Jr. Immunization with a chimeric tobacco mosaic virus containing an epitope of outer membrane protein F of Pseudomonas aeruginosa provides protection against challenge with P. aeruginosa. Vaccine 2000; 18: 2266–74. [DOI] [PubMed] [Google Scholar]
- 28. Gilleland HE, Gilleland LB, Straczek J et al Chimeric animal and plant viruses expressing epitopes of outer membrane protein F as a combined vaccine against Pseudomonas aeruginosa lung infection. FEMS Immunol. Med. Microbiol. 2000; 27: 291–7. [DOI] [PubMed] [Google Scholar]
- 29. Yusibov V, Modelska A, Steplewski K et al Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV‐1. Proc. Natl Acad. Sci. USA 1997; 94: 5784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Modelska A, Dietzschold B, Sleysh N et al Immunization against rabies with plant‐derived antigen. Proc. Natl Acad. Sci. USA 1998; 95: 2481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nemchinov LG, Liang TJ, Rifaat MM, Mazyad HM, Hadidi A, Keith JM. Development of a plant‐derived subunit vaccine candidate against hepatitis C virus. Arch. Virol. 2000; 145: 2557–73. [DOI] [PubMed] [Google Scholar]
- 32. Joelson T, Åkerblom L, Oxelfelt P, Strandberg B, Tomenius K, Morris TJ. Presentation of a foreign peptide on the surface of tomato bushy stunt virus. J. Gen. Virol. 1997; 78: 1213–17. [DOI] [PubMed] [Google Scholar]
- 33. Jagadish MN, Hamilton RC, Fernandez CS et al High level production of hybrid potyvirus‐like particles carrying repetitive copies of foreign antigens in Escherichia coli. Biotechnology 1993; 11: 1166–70. [DOI] [PubMed] [Google Scholar]
- 34. Fernandez‐Fernandez MR, Martinez‐Torrecuadrada JL, Casal JI, Garcia JA. Development of an antigen presentation system based on plum pox potyvirus. FEBS Lett. 1998; 427: 229–35. [DOI] [PubMed] [Google Scholar]
- 35. Fernandez‐Fernandez MR, Martinez‐Torrecuadrada JL, Roncal F, Dominguez E, Garcia JA. Identification of immunogenic hot spots within plum pox potyvirus capsid protein for efficient antigen presentation. J. Virol. 2002; 76: 12 646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santa Cruz S, Chapman S, Roberts AG, Roberts IM, Prior DA, Oparka KJ. Assembly and movement of a plant virus carrying a green fluorescent protein overcoat. Proc. Natl Acad. Sci. USA 1996; 93: 6286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marusic C, Rizza P, Lattanzi L et al Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J. Virol. 2001; 75: 8434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yusibov V, Mett V, Mett V et al Peptide‐based candidate vaccine against respiratory syncytial virus. Vaccine 2005; 23: 2261–5. [DOI] [PubMed] [Google Scholar]
- 39. Takamatsu N, Ishikawa M, Meshi T, Okada Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV‐RNA. EMBO J. 1987; 6: 307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dawson WO, Lewandowski DJ, Hilf ME et al A tobacco mosaic virus‐hybrid expresses and loses an added gene. Virology 1989; 172: 285–92. [DOI] [PubMed] [Google Scholar]
- 41. Donson J, Kearney CM, Hilf ME, Dawson WO. Systemic expression of a bacterial gene by a tobacco mosaic virus‐based vector. Proc. Natl Acad. Sci. USA 1991; 88: 7204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumagai MH, Turpen TH, Weinzettl N et al Rapid, high‐level expression of biologically active alpha‐trichosanthin in transfected plants by an RNA viral vector. Proc. Natl Acad. Sci. USA 1993; 90: 427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCormick AA, Kumagai MH, Hanley K et al Rapid production of specific vaccines for lymphomaby expression of the tumor‐derived single‐chain Fv epitopes in tobacco plants. Proc. Natl Acad. Sci. USA 1999; 96: 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verch T, Yusibov V, Koprowski H. Expression and assembly of a full‐length monoclonal antibody in plants using a plant virus vector. J. Immunol. Methods 1998; 220: 69–75. [DOI] [PubMed] [Google Scholar]
- 45. Kumagai MH, Donson J, della‐Cioppa G, Grill LK. Rapid, high‐level expression of glycosylated rice alpha‐amylase in transfected plants by an RNA viral vector. Gene 2000; 245: 169–74. [DOI] [PubMed] [Google Scholar]
- 46. Dirnberger D, Steinkellner H, Abdennebi L, Remy J‐J, van de Wiel D. Secretion of biologically active glycoforms of bovine follicle stimulating hormone in plants. Eur. J. Biochem. 2001; 268: 4570–79. [DOI] [PubMed] [Google Scholar]
- 47. Wigdorovitz A, Perez Filgueira DM, Robertson N et al Protection of mice against challenge with foot and mouth disease virus (FMDV) by immunization with foliar extracts from plants infected with recombinant tobacco mosaic virus expressing the FMDV structural protein VP1. Virology 1999; 264: 85–91. [DOI] [PubMed] [Google Scholar]
- 48. Krebitz M, Wiedermann U, Essl D et al Rapid production of the major birch pollen allergen Betv 1 in Nicotiana benthamiana plants and its immunological in vitro and in vivo characterization. FASEB J. 2000; 14: 1279–88. [DOI] [PubMed] [Google Scholar]
- 49. Perez‐Filgueira DM, Zamorano PI, Dominguez MG et al Bovine herpes virus gD protein produced in plants using a recombinant tobacco mosaic virus (TMV) vector possesses authentic antigenicity. Vaccine 2003; 21: 4201–209. [DOI] [PubMed] [Google Scholar]
- 50. Chapman S, Kavanagh T, Baulcombe D. Potato virus X as a vector for gene expression in plants. Plant J. 1992; 2: 549–57. [DOI] [PubMed] [Google Scholar]
- 51. Hendy S, Chen ZC, Barker H et al Rapid production of single‐chain Fv fragments in plants using a potato virus X episomal vector. J. Immunol. Methods 1999; 231: 137–46. [DOI] [PubMed] [Google Scholar]
- 52. Ziegler A, Cowan GH, Torrance L, Ross HA, Davies HV. Facile assessment of cDNA constructs for expression of functional antibodies in plants using the potato virus X vector. Mol. Breed. 2000; 6: 327–35. [Google Scholar]
- 53. O'Brien GJ, Bryant CJ, Voogd C, Greenberg HB, Gardner RC, Bellamy AR. Rotavirus VP6 expressed by PVX vectors in Nicotiana benthamiana coats PVX rods and also assembles into virus like particles. Virology 2000; 270: 444–53. [DOI] [PubMed] [Google Scholar]
- 54. Franconi R, Di Bonito P, Dibello F et al Plant derived‐human papillomavirus 16 E7 oncoprotein induces immune response and specific tumour protection. Cancer Res. 2002; 62: 3654–8. [PubMed] [Google Scholar]
- 55. Smolenska L, Roberts IM, Learmonth D et al Production of a functional single chain antibody attached to the surface of a plant virus. FEBS Lett. 1998; 441: 379–82. [DOI] [PubMed] [Google Scholar]
- 56. Guo HS, Lopez‐Moya JJ, Garcia JA. Susceptibility to recombination rearrangements of a chimeric plum pox potyvirus genome after insertion of a foreign gene. Virus Res. 1998; 57: 183–95. [DOI] [PubMed] [Google Scholar]
- 57. Fernandez‐Fernandez MR, Mourino M, Rivera J, Rodriguez F, Plana‐Duran J, Garcia JA. Protection of rabbits against rabbit hemorrhagic disease virus by immunization with the VP60 protein expressed in plants with a potyvirus‐based vector. Virology 2001; 280: 283–91. [DOI] [PubMed] [Google Scholar]
- 58. Scholthof HB, Morris TJ, Jackson AO. The capsid protein gene of tomato bushy stunt virus is dispensable for systemic movement and can be replaced for localized expression of foreign genes. Mol. Plant Microbe Interact. 1993; 6: 309–22. [Google Scholar]
- 59. Scholthof HB. Rapid delivery of foreign genes into plants by direct rub‐inoculation with intact plasmid DNA of a tomato bushy stunt virus gene vector. J. Virol. 1999; 73: 7823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang GC, Leung C, Murdin L, Rovinski B, White KA. In planta expression of HIV‐1 p24 protein using an RNA plant virus‐based expression vector. Mol. Biotechnol. 2000; 14: 99–107. [DOI] [PubMed] [Google Scholar]
- 61. Gopinath K, Wellink J, Porta C, Taylor KM, Lomonossoff GP, van Kammen A. Engineering cowpea mosaic virus RNA‐2 into a vector to express heterologous proteins in plants. Virology 2000; 267: 159–73. [DOI] [PubMed] [Google Scholar]
- 62. Durrani Z, McInerney TL, McLain L et al Intranasal immunization with a plant virus expressing a peptide from HIV‐1 gp41 stimulates better mucosal and systemic HIV‐1‐specific IgA and IgG than oral immunization. J. Immunol. Methods 1998; 220: 93–103. [DOI] [PubMed] [Google Scholar]
- 63. Masuta C, Yamana T, Tacahashi Y et al Development of clover yellow vein virus as an efficient, stable gene‐expression system for legume species. Plant J. 2000; 23: 539–46. [DOI] [PubMed] [Google Scholar]
- 64. MacFarlane SA, Popovich AH. Efficient expression of foreign proteins in roots from tobravirus vectors. Virology 2000; 267: 29–35. [DOI] [PubMed] [Google Scholar]
- 65. Choi IR, Stenger DC, Morris TJ, French R. A plant virus vector for systemic expression of foreign genes in cereals. Plant J. 2000; 23: 547–55. [DOI] [PubMed] [Google Scholar]
- 66. Arazi T, Slutsky SG, Shiboleth YM et al Engineering zucchini yellow mosaic polyvirus as a non‐pathogenic vector for expression of heterologous proteins in cucurbits. J. Biotechnol. 2001; 87: 67–82. [DOI] [PubMed] [Google Scholar]
- 67. Sanchez‐Navarro J, Miglino R, Ragozzini A, Bol JF. Engineering of alfalfa mosaic virus RNA 3 into an expression vector. Arch. Virol. 2001; 146: 923–39. [DOI] [PubMed] [Google Scholar]
- 68. Mori M, Fujihara N, Mise K, Furusawa I. Inducible high‐level mRNA amplification system by viral replicase in transgenic plants. Plant J. 2001; 27: 79–86. [DOI] [PubMed] [Google Scholar]
- 69. Pogue GP, Lindbo JA, Garger SJ, Fitzmaurice WP. Making an ally from an enemy: plant virology and the new agriculture. Annu. Rev. Phytopathol. 2002; 40: 45–74. [DOI] [PubMed] [Google Scholar]
- 70. Nichols ME, Stanislaus T, Keshavarz‐Moore E, Young HA. Disruption of leaves and initial extraction of wild‐type CPMV virus as a basis for producing vaccines from plants. J. Biotechnol. 2002; 92: 229–35. [DOI] [PubMed] [Google Scholar]


