ABSTRACT
Background and Objective
Respiratory syncytial virus (RSV) is the most significant cause of acute respiratory infection (ARI) in early life. RSV and other respiratory viruses are known to stimulate substantial outgrowth of potentially pathogenic bacteria in the upper airways of young children. However, the clinical significance of interactions between viruses and bacteria is currently unclear. The present study aimed to clarify the effect of viral and bacterial co‐detections on disease severity during paediatric ARI.
Methods
Nasopharyngeal aspirates from children under 2 years of age presenting with ARI to the emergency department were screened by quantitative PCR for 17 respiratory viruses and the bacterial pathogens Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis. Associations between pathogen detection and clinical measures of disease severity were investigated.
Results
RSV was the most common virus detected, present in 29 of 58 samples from children with ARI (50%). Detection of S. pneumoniae was significantly more frequent during RSV infections compared to other respiratory viruses (adjusted effect size: 1.8, P: 0.03), and co‐detection of both pathogens was associated with higher clinical disease severity scores (adjusted effect size: 1.2, P: 0.03).
Conclusion
Co‐detection of RSV and S. pneumoniae in the nasopharynx was associated with more severe ARI, suggesting that S. pneumoniae colonization plays a pathogenic role in young children.
Keywords: co‐infection, human respiratory syncytial virus, pneumococcus, respiratory tract infection, severity of illness index
Short abstract
http://onlinelibrary.wiley.com/doi/10.1111/resp.13209/abstract
High loads of bacteria colonizing the upper respiratory tract are often observed during paediatric respiratory syncytial virus (RSV) infections. The present study identified an association between co‐detection of RSV and Streptococcus pneumoniae and more severe disease, suggesting the bacteria has a pathogenic role in these young children.
Abbreviations
- ARI
acute respiratory infection
- ED
emergency department
- ED
emergency department
- Hi
Haemophilus influenzae
- hRV
human rhinovirus
- IQR
interquartile range
- Mc
Moraxella catarrhalis
- NPA
nasopharyngeal aspirate
- PIV
parainfluenza viruses
- PyV
polyomaviruses
- qPCR
quantitative PCR
- RCH
Royal Children's Hospital
- RSV
respiratory syncytial virus
- Sp
Streptococcus pneumoniae
INTRODUCTION
Acute respiratory infection (ARI) is a significant cause of childhood illness worldwide.1 Respiratory viruses, such as respiratory syncytial virus (RSV) and human rhinovirus (hRV) are the most common cause. While disease is often mild with cold‐like symptoms, it can progress to severe presentations such as bronchiolitis or pneumonia requiring hospitalization or resulting in death. RSV accounts for over three million paediatric hospitalizations per year.2 Outgrowth of bacteria known to asymptomatically colonize the upper airway of young children, including Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis, has been reported during viral ARI, often in association with increased disease severity.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The contribution of viral/bacterial co‐infections to disease severity has been well‐documented in co‐infections caused by influenza viruses and S. pneumoniae in adults,15 however, the role of bacteria during paediatric viral ARI is less clear.16 Synergistic interactions between respiratory viruses and bacteria have been demonstrated in mechanistic studies, including increased attachment to airway epithelium and enhancement of immune evasion strategies.17, 18, 19, 20, 21, 22, 23 Consideration of potentially pathogenic bacteria may be important in the diagnosis, treatment and prevention of paediatric ARI.
The majority of paediatric ARI studies investigating associations between specific viral/bacterial interactions and disease severity have taken place in temperate climates in the northern hemisphere.6, 7, 8, 9, 10, 11 These climates are characterized by distinct seasonal epidemics of respiratory viruses peaking in winter each year.24 The present study setting of Brisbane, Australia, has a subtropical climate with only moderate temperature variations, where the RSV seasonal epidemic usually peaks in early autumn (March–April), depending on rainfall during the preceding summer.4, 24 Differences in the environment of study locations may affect the carriage of respiratory viruses and bacteria, thereby influencing the potential for clinically significant interactions to occur between pathogens. We and others have previously reported increased co‐detection of H. influenzae and S. pneumoniae with RSV in Brisbane‐based cohorts of children with ARI3, 4; however, the resulting effect on disease severity has not been investigated. In the present study, we further characterized the respiratory viruses and colonizing bacteria present during viral ARI, and their associations with disease severity, in a cohort of young children presenting at the emergency department (ED) of the Royal Children's Hospital (RCH), Brisbane.
METHODS
Study population and sample collection
Children aged less than 2 years and presenting with symptoms of bronchiolitis, wheeze or asthma to RCH were recruited throughout 2011–2014. Participants with serious co‐morbidities, gestational age < 37 weeks, birth weight < 2.5 kg or requiring significant resuscitation were excluded. Admission to hospital, use of supplemental oxygen, use of assisted ventilation and previous medical history was recorded. Severity of illness was assessed using a modified Wood's Clinical Asthma Score (Table S1 in Appendix S1, Supplementary Information), validated for use in children up to 2 years of age.25 This scoring system resulted in a disease severity score between 0 (well) and 10 (severe disease). Nasopharyngeal aspirates (NPAs) were taken by trained personnel following routine procedures, centrifuged and the supernatant stored at −80 °C.
Six to eight weeks later, participants were requested to return for a convalescent visit. A questionnaire collecting additional demographic and medical history data was completed. Current symptoms (if any) were recorded and children with evidence of sinus or ear infections were excluded. Nasal swabs (2011–2012) or nasal washes (2013–2014) were collected by trained personnel following routine procedures and stored at −80 °C.
The present study was approved by the human research ethics committee of Children's Health Queensland Health and Hospital Service (approval number HREC/11/QRCH/47). Parents gave written consent for their children to participate in the study.
Pathogen detection
Nucleic acid extraction and screening for 17 viruses was conducted using previously described quantitative (q) PCR assays (Table S2 in Appendix S1, Supplementary Information).26 Virus detection included RSV‐A, RSV‐B, parainfluenza virus 1–3, influenza A and B viruses, hRV, metapneumovirus, human adenovirus, human bocavirus, human enterovirus, polyomaviruses WU and KI and coronaviruses HKU1, OC43, NL63 and 29E.
Bacterial loads were determined for each sample using qPCR assays for S. pneumoniae, 27 M. catarrhalis 28 and H. influenzae 29 (Table S2 in Appendix S1, Supplementary Information). Since cross‐reactivity of the S. pneumoniae assay, targeting the pneumolysin gene ply, has been reported between closely related Streptococcus species, the presence of S. pneumoniae was confirmed through a real‐time PCR assay for autolysin (lytA).30 The total microbial load of each sample was also estimated using a qPCR assay targeting the 16S ribosomal RNA gene.
Statistical analysis
Statistical analysis was carried out in R 3.0.1.31 Univariable analysis was carried out using the epicalc package.32 Variables that were significant at P < 0.05 in the univariable analysis were used in logistic regression to model factors contributing to the detection of RSV, hRV, multiple viral species in a sample, S. pneumoniae, H. influenzae and M. catarrhalis, with adjustment for confounding factors (age, sex, history of wheezing illness and season of ED visit). Firth's penalised likelihoods were used to correct for small sample bias with the logistf package.33 Variables that had P values < 0.1 in the logistic regression models were entered into a single partial correlation matrix using the ppor package.34 Partial correlations with P values < 0.05 were viewed in Cytoscape 3.3.035 as an interaction network. Such analysis techniques have been used effectively by others36 to investigate interactions between viruses and bacteria in the respiratory tract. This analysis was also repeated using siblings, childcare attendance, history of severe ARI (i.e. those requiring hospitalization) and antibiotic usage in the last year as additional confounding factors, for participants with available data.
To analyse the effect of pathogen detection and interactions on disease severity, a linear regression model for severity score, and logistic regression models for admission to hospital and use of supplemental oxygen, were used with adjustment for confounding factors. For each, a simple model was built that included the confounding factors and detection of RSV, hRV, multiple viruses, S. pneumoniae, H. influenzae and M. catarrhalis, and multiple interaction models were built to reflect the co‐detections between specific pathogens identified in the partial correlation analysis.
In the convalescent samples, there was insufficient statistical power to investigate associations between pathogens; however, the difference between bacterial loads at the acute and convalescent visit was analysed using Wilcoxon matched‐pairs signed rank tests in Prism 7 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Patient demographics and pathogen detection
NPAs were collected from 60 infants and young children with ARI symptoms between September 2011 and October 2014. Two samples were virus negative and were excluded from analysis. Demographic, clinical and pathogen detection data from the remaining 58 participants are reported in Table 1. Follow‐up questionnaires collecting additional demographic data were completed for 40 (69.0%) of the 58 participants and are also reported in Table 1. RSV and hRV were the most commonly detected viruses. Detection of multiple viral species in a patient was frequent, with up to four viruses detected in one sample. RSV was the most common sole virus observed (17 of 32 samples), while hRV was the most frequent co‐viral pathogen (20 of 26 samples). Bacterial DNA was detected in all 58 samples, and 48 (83%) of participants were colonized by at least one of the three targeted species. A total of 17 (29%) participants were colonized by all three target species.
Table 1.
Participant characteristics and pathogen detection
| Number of samples | 58 |
| Demographics | |
| Median age in months (IQR) | 4.8 (2.4,8.4) |
| Male (%) | 31 (53.4) |
| ED visit during autumn/winter (%) | 35 (60.3) |
| Diagnosed asthma (%) | 2 (3.4) |
| History of previous wheezing illness (%) | 16 (22.2) |
| Siblings (%) | 29 of 40 (72.5)* |
| Childcare attendance (%) | 12 of 40 (30.0)* |
| History of severe (hospitalized) ARI (%) | 27 of 39 (69.2)* |
| Antibiotics prescribed in the last year (%) | 12 of 38 (31.6)* |
| Clinical parameters | |
| Received supplemental oxygen in ED (%) | 11 (20.0) |
| Admitted to hospital (%) | 31 (57.4) |
| Received assisted ventilation in ED (%) | 4 (7.3) |
| Median clinical disease severity score (IQR) | 1 (0.5,2) |
| Severity score criteria | |
| Median score – oxygen saturation (IQR) | 0 (0,0) |
| Median score – inspiratory breath sounds (IQR) | 0 (0,0) |
| Median score – expiratory wheezing (IQR) | 0.5 (0,1) |
| Median score – accessory muscles (IQR) | 0.5 (0,1) |
| Median score – cerebral function (IQR) | 0 (0,0) |
| Bacterial detection | |
| S. pneumoniae (%) | 26 (44.8) |
| H. influenzae (%) | 28 (48.3) |
| M. catarrhalis (%) | 42 (72.4) |
| S. pneumoniae ply load† (IQR) | 2.1 × 101 (0, 3.5 × 103) |
| H. influenzae load† (IQR) | 0 (0, 4.0 × 103) |
| M. catarrhalis load† (IQR) | 5.6 × 102 (0, 1.3 × 104) |
| Total microbial load‡ (IQR) | 1.5 × 105 (3.9 × 104, 6.3 × 105) |
| Viral detection | |
| RSV (%) | 29 (50.0) |
| RSV‐A (%) | 21 (36.2) |
| RSV‐B (%) | 8 (13.8) |
| hRV (%) | 28 (48.3) |
| Enteroviruses (%) | 8 (13.8) |
| PIV (%) | 7 (12.1) |
| PIV‐1 (%) | 0 (0) |
| PIV‐2 (%) | 2 (3.4) |
| PIV‐3 (%) | 5 (8.6) |
| Metapneumovirus (%) | 5 (8.6) |
| Influenza A virus (%) | 2 (3.4) |
| Influenza B virus (%) | 0 (0) |
| Adenovirus (%) | 6 (10.3) |
| Bocavirus (%) | 4 (6.9) |
| Polyomaviruses (%) | 5 (8.6) |
| Coronaviruses (%) | 2 (3.4) |
| No. of viral species detected per sample (IQR) | 1 (1, 2) |
| Single virus detected (%) | 32 (55.2) |
| Multiple viruses detected (%) | 26 (44.8) |
These data were collected by questionnaire during the convalescent visit and were only available for a subset (38–40) of participants.
Median genomes per μL.
Median 16S ribosomal RNA gene copies per μL.
ARI, acute respiratory infection; ED, emergency department; hRV, human rhinovirus; IQR, interquartile range; PIV, parainfluenza viruses; RSV, respiratory syncytial virus.
Pathogen interactions
In the univariable analysis (Table S3 in Appendix S1, Supplementary Information), detection of multiple viruses, S. pneumoniae and H. influenzae were all associated with an increase in age (P = 0.021, P < 0.001 and P < 0.001, respectively). Enteroviruses, adenoviruses and bocaviruses were exclusively detected with other viruses, and hRV was also associated with the presence of multiple viruses (P < 0.001). A negative association was observed between RSV and hRV (P < 0.001). H. influenzae colonization was positively associated with M. catarrhalis and S. pneumoniae (P = 0.013 and P = 0.009, respectively). S. pneumoniae colonization was also positively associated with detection of multiple viruses (P = 0.002), while H. influenzae colonization was associated with detection of polyomaviruses (P = 0.021). S. pneumoniae colonization was increased in the presence of RSV and hRV; however the differences were not statistically significant.
After adjustment for confounding factors, RSV and hRV remained negatively associated in the multivariable logistic regression models (P < 0.001) (Fig. 1), while hRV and detection of multiple viruses remained positively associated (P = 0.007). A positive association between S. pneumoniae colonization and RSV detection was also observed (P = 0.030). Other associations identified in the univariable analysis were no longer significant at P < 0.05.
Figure 1.
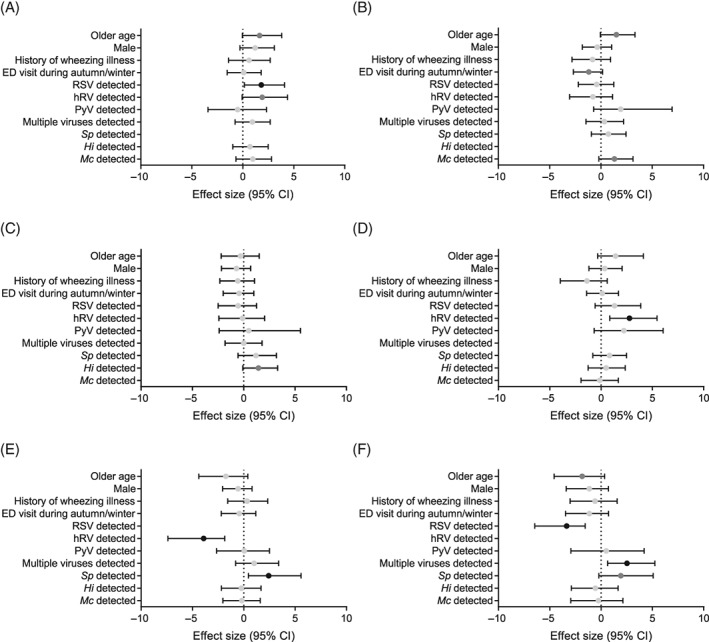
Associations between pathogen detections in multivariable logistic regression models with adjustment for confounding factors and detection of Streptococcus pneumoniae (A), Haemophilus influenzae (B), Moraxella catarrhalis (C), multiple viruses per sample (D), RSV (E) and hRV (F). Effect size (penalised maximum likelihoods) and 95% CI are shown. Black points represent likelihoods significant at P < 0.05, dark grey points P < 0.1 and light grey points P > 0.1. ED, emergency department; Hi, H. influenzae; hRV, human rhinovirus; Mc, M. catarrhalis; PyV, polyomaviruses; RSV, respiratory syncytial virus; Sp, S. pneumoniae.
All variables that had associations with a significance level P < 0.1 were included in the partial correlation matrix analysis and significant partial correlations (P < 0.05) were visualized in an interaction network (Fig. 2). Increasing age was associated with H. influenzae and S. pneumoniae colonization, while decreasing age correlated with RSV and hRV detection. All associations observed in the logistic regression models persisted, and an association between S. pneumoniae colonization and hRV was observed.
Figure 2.
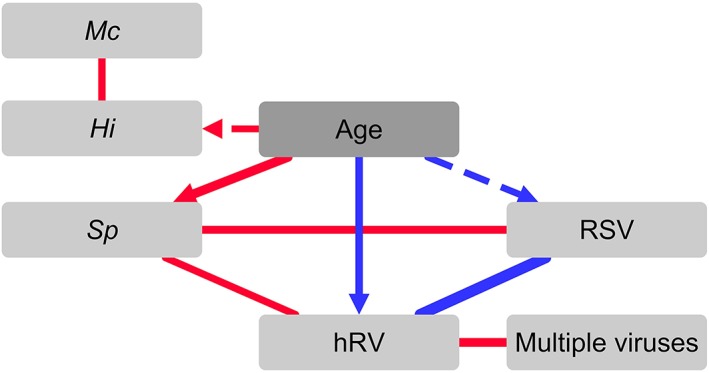
Interactions between viruses and bacteria in the upper respiratory tract during paediatric viral acute respiratory infection. Network built from output of partial correlation matrix analysis. Associations with P < 0.01 (solid line) and P < 0.05 (dashed line) are shown. Line thickness indicates magnitude of partial correlation. Positive (red lines) and negative (blue lines) correlations are shown. Age was the only confounding factor found to have a significant effect, indicated by arrow heads on edge lines. Hi, Haemophilus influenzae; hRV, human rhinovirus; Mc, Moraxella catarrhalis; RSV, respiratory syncytial virus; Sp, Streptococcus pneumoniae.
The above analyses were repeated with further adjustment for additional confounding factors using the subset of 40 samples for which this information was available, with similar results (Fig. S1 in Appendix S1, Supplementary Information). Contact with other children, through siblings or childcare attendance, was found to influence S. pneumoniae and H. influenzae colonization.
Effect on disease severity
Clinical disease severity scores for all 58 children ranged from 0 to 6 (median score 1) with 57% of participants admitted to hospital (Table 1). In the univariable analysis (Table S3 in Appendix S1, Supplementary Information), increasing severity scores were associated with increasing age (P = 0.015) and a previous history of wheezing illness (P = 0.007). Detection of RSV (P = 0.031), multiple viruses (P = 0.035) and S. pneumoniae (P = 0.01) were also associated with increased severity scores.
In the multivariable models (Table S4 in Appendix S1, Supplementary Information), higher severity scores were associated with RSV (P = 0.032), and specifically with RSV and S. pneumoniae co‐detection (P = 0.028). This association was particularly evident when the distribution of severity scores, separated by RSV and S. pneumoniae detection status, was visualized (Fig. 3). Severity scores were not associated with any other pathogen detection or patient demographic variable after adjustment for confounding factors. Admission to hospital and use of supplemental oxygen were also not associated with any variable (Table S4 in Appendix S1, Supplementary Information).
Figure 3.
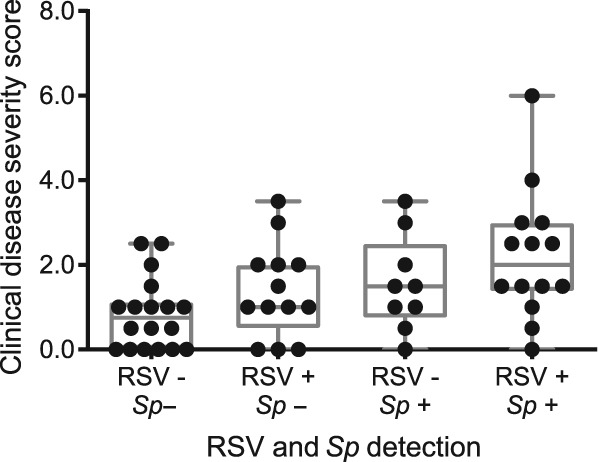
Clinical disease severity scores stratified by RSV and Sp co‐detection. Box and whisker plot with median and interquartile range is shown. + indicates positive detection of RSV or Sp, − indicates negative detection. RSV, respiratory syncytial virus; Sp, Streptococcus pneumoniae.
Pathogen detection at convalescence
Nasal samples were available from only 27 infants and young children at convalescence, collected on average 8 weeks after the acute visit. Despite the small sample size, pathogen detection assays were performed and the results reported in Figure S2 in Appendix S1, Supplementary Information. Eleven samples were virus positive and generally bacterial loads decreased between acute and convalescence visits.
DISCUSSION
There is growing recognition that interactions between viral and bacterial pathogens during ARI may affect disease severity and outcome. We have identified an association between RSV and S. pneumoniae in a Brisbane‐based paediatric cohort presenting to the ED with viral ARI. This interaction was associated with a significant enhancement of overall disease severity, as determined by criteria‐based scoring. While several other interactions between respiratory viruses and bacteria were observed, including hRV and S. pneumoniae, and hRV with other viruses, these were not strongly associated with clinical measures of disease.
While the present study determined S. pneumoniae nasopharyngeal colonization, and as such cannot comment on the occurrence of pneumococcal infection, the results provide further evidence that RSV and S. pneumoniae interact with a clinically relevant synergism. The RSV G glycoprotein has previously been shown to act as a receptor for S. pneumoniae adherence, promoting pneumococcal colonization of the airways.17, 18, 19 RSV has also been shown to enhance pneumococcal virulence in animal models, with co‐infection resulting in increased inflammation.18, 19, 20 In young children, colonization of the upper airways by Streptococcus species, such as S. pneumoniae, has also been correlated with increased disease severity and inflammation.5, 6, 7 However, the clinical relevance of paediatric RSV and S. pneumoniae co‐infections is still under debate, due to conflicting data. Some investigations have shown a clear association between positive bacterial cultures and increased admission, duration of stay and use of supportive care at hospitals and intensive care units,8, 9, 10, 14 while older and generally cross‐sectional studies have found no association between viral/bacteria co‐detection and markers of disease severity.11, 12, 13, 37, 38 The longitudinal study reported by Teo et al.,5 in which every ARI over the first year of life was captured using culture‐independent techniques, clearly demonstrated that colonization by bacteria such as S. pneumoniae, H. influenzae and M. catarrhalis was associated with more severe lower respiratory infections in the presence of respiratory viruses. Together, these data strongly suggest that specific viral/bacterial interactions are associated with increased disease severity.
In the present study, RSV and S. pneumoniae was the only co‐pathogen detection that was associated with increased disease severity. Surprisingly, while many clinical studies of paediatric ARI have reported strong positive associations between RSV and H. influenzae and a correlation with disease severity,4, 6, 7, 8 a positive association between these two pathogens, or an association with disease severity, was not found in this study. Likewise, no difference in overall severity of hRV ARI was observed when S. pneumoniae was co‐detected, despite previous reports of synergism between these two pathogens.22, 23, 39 Interactions between RSV and H. influenzae, and hRV and S. pneumoniae have also been identified in the upper airways of healthy young children,36 suggesting interaction mechanisms independent of disease.
No association was observed between the presence of multiple viruses and increased ARI severity in the present study. The prevalence and clinical significance of multiple viral infections varies depending on the viruses investigated and the cohort.40, 41, 42, 43 Studies identifying associations with increased disease severity have often used increased duration of symptoms and hospitalization stays as clinical outcomes.41, 42, 43, 44 It has been suggested that a secondary viral infection, before the primary infection has resolved, may result in an additive effect on variables like duration of symptoms.41 The majority of the participants in our study only attended the acute visit at the ED, thus longitudinal data, like duration of symptoms, could not be collected and the clinical effects of multiple viral infection could not be evaluated in more depth.
Season of ED visit had only a limited effect on H. influenzae colonization and did not affect detection of the other pathogens investigated in the present study. RSV infections are known to peak during autumn in Brisbane,4, 24 while S. pneumoniae infections tend to peak in winter45; however, the small sample size of the present study does not allow in‐depth examination of seasonal effects. Similar to the findings of other studies, in the subset of samples with available data, childcare attendance and presence of siblings were found to influence detection of RSV, S. pneumoniae and H. influenzae. 4, 46, 47
Matched, healthy controls were not available in the present study, thus the interaction dynamics and the cause‐effect relationships between the identified co‐pathogens could not be investigated. Longitudinal studies, where samples are taken before, during and after ARI episodes, such as that reported by Teo et al.,5 are ideal future such investigations.
In the present study, we have provided evidence supporting a clinically relevant synergism between RSV and S. pneumoniae in a Brisbane‐based cohort of young children with viral ARI. Understanding the complex interactions between potential respiratory pathogens, and their short and long‐term health consequences, is important for improved diagnosis, treatment and prevention of paediatric ARI.
Supporting information
Figure S1 Reanalysis of pathogen detection data after adjustment for additional confounding factors.
Figure S2 Pathogen detection results from the 27 nasal swabs and washes taken at the convalescent visit.
Table S1 Criteria‐based scoring system for quantifying clinical disease severity.
Table S2 Viral and bacterial detection qPCR assays.
Table S3 Univariable analysis of associations between pathogen detection, clinical parameters and participant demographics.
Table S4 Associations between pathogen detections and severity parameters with adjustment for confounding factors.
Acknowledgements
The authors would like to thank the following for their assistance in study management, participant recruitment, sample collection and sample processing: staff at the emergency department of the Royal Children's Hospital; Janine Di‐Masi and Emma Thomas at the Centre for Children's Health Research; and Rebecca Holding at the Queensland Paediatric Infectious Diseases Group. The study was supported in part by grants from the National Health and Medical Research Council (#1047663) and the Australian Infectious Diseases Research Centre.
Brealey, J.C. , Chappell, K.J. , Galbraith, S. , Fantino, E. , Gaydon, J. , Tozer, S. , Young, P.R. , Holt, P.G. and Sly, P.D. (2017) Streptococcus pneumoniae colonization of the nasopharynx is associated with increased severity during respiratory syncytial virus infection in young children. Respirology, 23: 220–227., doi: 10.1111/resp.13179.
(Associate Editor: Marcos Restrepo)
REFERENCES
- 1. Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010; 375: 1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nair H, Simoes EA, Rudan I, Gessner BD, Azziz‐Baumgartner E, Zhang JS, Feikin DR, Mackenzie GA, Moisi JC, Roca A et al; Severe Acute Lower Respiratory Infections Working Group . Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013; 381: 1380–90. [DOI] [PMC free article] [PubMed]
- 3. Chappell KJ, Brealey JC, Mackay IM, Bletchly C, Hugenholtz P, Sloots T, Sly PD, Young PR. Respiratory syncytial virus infection is associated with increased bacterial load in the upper respiratory tract in young children. J. Med. Microbiol. Diagn. 2013; S1: 005. [Google Scholar]
- 4. O'Grady KF, Grimwood K, Sloots TP, Whiley DM, Acworth JP, Phillips N, Goyal V, Chang AB. Prevalence, codetection and seasonal distribution of upper airway viruses and bacteria in children with acute respiratory illnesses with cough as a symptom. Clin. Microbiol. Infect. 2016; 22: 527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015; 17: 704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Steenhuijsen Piters WA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez‐Arrabal MC, Chaussabel D, Cohen DM, Sanders EA, Ramilo O et al. Nasopharyngeal microbiota, host transcriptome and disease severity in children with respiratory syncytial virus infection. Am. J. Respir. Crit. Care Med. 2016; 194: 1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suarez‐Arrabal MC, Mella C, Lopez SM, Brown NV, Hall M, Hammond S, Shiels W, Groner J, Marcon M, Ramilo O et al. Nasopharyngeal bacterial burden and antibiotics: influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J. Infect. 2015; 71: 458–69. [DOI] [PubMed] [Google Scholar]
- 8. Thorburn K, Harigopal S, Reddy V, Taylor N, van Saene HKF. High incidence of pulmonary bacterial co‐infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 2006; 61: 611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kneyber MC, Blusse van Oud‐Alblas H, van Vliet M, Uiterwaal CS, Kimpen JL, van Vught AJ. Concurrent bacterial infection and prolonged mechanical ventilation in infants with respiratory syncytial virus lower respiratory tract disease. Intensive Care Med. 2005; 31: 680–5. [DOI] [PubMed] [Google Scholar]
- 10. Resch B, Gusenleitner W, Mueller WD. Risk of concurrent bacterial infection in preterm infants hospitalized due to respiratory syncytial virus infection. Acta Paediatr. 2007; 96: 495–8. [DOI] [PubMed] [Google Scholar]
- 11. Vissers M, Ahout IM, van den Kieboom CH, van der Gaast de Jongh CE, Groh L, Cremers AJ, de Groot R, de Jonge MI, Ferwerda G. High pneumococcal density correlates with more mucosal inflammation and reduced respiratory syncytial virus disease severity in infants. BMC Infect. Dis. 2016; 16: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vu HT, Yoshida LM, Suzuki M, Nguyen HA, Nguyen CD, Nguyen AT, Oishi K, Yamamoto T, Watanabe K, Vu TD. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr. Infect. Dis. J. 2011; 30: 11–8. [DOI] [PubMed] [Google Scholar]
- 13. Wei L, Liu W, Zhang XA, Liu EM, Wo Y, Cowling BJ, Cao WC. Detection of viral and bacterial pathogens in hospitalized children with acute respiratory illnesses, Chongqing, 2009–2013. Medicine (Baltimore) 2015; 94: e742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leung TF, Lam DS, Miu TY, Hon KL, Chau CS, Ku SW, Lee RS, Chow PY, Chiu WK, Ng DK; Hong Kong Society of Paediatric Respirology RSVCG . Epidemiology and risk factors for severe respiratory syncytial virus infections requiring pediatric intensive care admission in Hong Kong children. Infection 2014; 42: 343–50. [DOI] [PMC free article] [PubMed]
- 15. McCullers JA. The co‐pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 2014; 12: 252–62. [DOI] [PubMed] [Google Scholar]
- 16. Brealey JC, Sly PD, Young PR, Chappell KJ. Viral bacterial co‐infection of the respiratory tract during early childhood. FEMS Microbiol. Lett. 2015; 10.1093/femsle/fnv062 [DOI] [PubMed] [Google Scholar]
- 17. Avadhanula V, Wang Y, Portner A, Adderson E. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J. Med. Microbiol. 2007; 56: 1133–7. [DOI] [PubMed] [Google Scholar]
- 18. Hament JM, Aerts PC, Fleer A, van Dijk H, Harmsen T, Kimpen JL, Wolfs TF. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr. Res. 2005; 58: 1198–203. [DOI] [PubMed] [Google Scholar]
- 19. Smith CM, Sandrini S, Datta S, Freestone P, Shafeeq S, Radhakrishnan P, Williams G, Glenn SM, Kuipers OP, Hirst RA et al. RSV increases the virulence of Streptococcus pneumoniae by binding to PBP1a: a new paradigm in respiratory infection. Am. J. Respir. Crit. Care Med. 2014; 190: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stark JM, Stark MA, Colasurdo GN, LeVine AM. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J. Med. Virol. 2006; 78: 829–38. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen DT, Louwen R, Elberse K, van Amerongen G, Yuksel S, Luijendijk A, Osterhaus AD, Duprex WP, de Swart RL. Streptococcus pneumoniae enhances human respiratory syncytial virus infection in vitro and in vivo . PLoS One 2015; 10: e0127098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishizuka S, Yamaya M, Suzuki T, Takahashi H, Ida S, Sasaki T, Inoue D, Sekizawa K, Nishimura H, Sasaki H. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J. Infect. Dis. 2003; 188: 1928–39. [DOI] [PubMed] [Google Scholar]
- 23. Wang JH, Kwon HJ, Jang YJ. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope 2009; 119: 1406–11. [DOI] [PubMed] [Google Scholar]
- 24. Paynter S. Humidity and respiratory virus transmission in tropical and temperate settings. Epidemiol. Infect. 2015; 143: 1110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinon‐Torres F, Rodriguez‐Nunez A, Martinon‐Sanchez JM. Heliox therapy in infants with acute bronchiolitis. Pediatrics 2002; 109: 68–73. [DOI] [PubMed] [Google Scholar]
- 26. O'Grady KA, Torzillo PJ, Rockett RJ, Whiley DM, Nissen MD, Sloots TP, Lambert SB. Successful application of a simple specimen transport method for the conduct of respiratory virus surveillance in remote Indigenous communities in Australia. Trop. Med. Int. Health 2011; 16: 766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greiner O, Day PJ, Bosshard PP, Imeri F, Altwegg M, Nadal D. Quantitative detection of Streptococcus pneumoniae in nasopharyngeal secretions by real‐time PCR. J. Clin. Microbiol. 2001; 39: 3129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunne EM, Manning J, Russell FM, Robins‐Browne RM, Mulholland EK, Satzke C. Effect of pneumococcal vaccination on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in Fijian children. J. Clin. Microbiol. 2012; 50: 1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Mair R, Hatcher C, Theodore MJ, Edmond K, Wu HM, Harcourt BH, Carvalho Mda G, Pimenta F, Nymadawa P et al. Detection of bacterial pathogens in Mongolia meningitis surveillance with a new real‐time PCR assay to detect Haemophilus influenzae . Int. J. Med. Microbiol. 2011; 301: 303–9. [DOI] [PubMed] [Google Scholar]
- 30. Carvalho Mda G, Tondella ML, McCaustland K, Weidlich L, McGee L, Mayer LW, Steigerwalt A, Whaley M, Facklam RR, Fields B et al. Evaluation and improvement of real‐time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 2007; 45: 2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2013. [Google Scholar]
- 32. Chongsuvivatwong V. Analysis of Epidemiological Data Using R and Epicalc. Hat Yai, Epidemiology Unit, Prince of Songkla University, 2008. [Accessed 27 Jun 2016.] Available from URL: https://cran.r-project.org/doc/contrib/Epicalc_Book.pdf. [Google Scholar]
- 33. Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat. Med. 2002; 21: 2409–19. [DOI] [PubMed] [Google Scholar]
- 34. Kim S. ppcor: an R package for a fast calculation to semi‐partial correlation coefficients. Commun. Stat. Appl. Methods 2015; 22: 665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13: 2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van den Bergh MR, Biesbroek G, Rossen JW, de Steenhuijsen Piters WA, Bosch AA, van Gils EJ, Wang X, Boonacker CW, Veenhoven RH, Bruin JP et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One 2012; 7: e47711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duttweiler L, Nadal D, Frey B. Pulmonary and systemic bacterial co‐infections in severe RSV bronchiolitis. Arch. Dis. Child. 2004; 89: 1155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehtinen P, Jartti T, Virkki R, Vuorinen T, Leinonen M, Peltola V, Ruohola A, Ruuskanen O. Bacterial coinfections in children with viral wheezing. Eur. J. Clin. Microbiol. Infect. Dis. 2006; 25: 463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, Gangnon RE, Bochkov YA, Jackson DJ, Lemanske RF Jr et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J. Allergy Clin. Immunol. 2014; 133: 1301–7.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greer RM, McErlean P, Arden KE, Faux CE, Nitsche A, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Do rhinoviruses reduce the probability of viral co‐detection during acute respiratory tract infections? J. Clin. Virol. 2009; 45: 10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karppinen S, Toivonen L, Schuez‐Havupalo L, Waris M, Peltola V. Interference between respiratory syncytial virus and rhinovirus in respiratory tract infections in children. Clin. Microbiol. Infect. 2016; 22: 208.e1‐6. [DOI] [PubMed] [Google Scholar]
- 42. Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J. Infect. Dis. 2013; 207: 982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, Espinola JA, Camargo CA, Jr. ; MARC‐30 Investigators . Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch. Pediatr. Adolesc. Med.. 2012;166:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kouni S, Karakitsos P, Chranioti A, Theodoridou M, Chrousos G, Michos A. Evaluation of viral co‐infections in hospitalized and non‐hospitalized children with respiratory infections using microarrays. Clin. Microbiol. Infect. 2013; 19: 772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiu C, Dey A, Wang H, Menzies R, Deeks S, Mahajan D, Macartney K, Brotherton J, Jardine A, Quinn H et al. Vaccine preventable diseases in Australia, 2005 to 2007. Commun. Dis. Intell. Q. Rep. 2010; 34(Supp.): S1–167. [PubMed] [Google Scholar]
- 46. Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J. Pediatr. 2003; 143: S118–26. [DOI] [PubMed] [Google Scholar]
- 47. Jacoby P, Carville KS, Hall G, Riley TV, Bowman J, Leach AJ, Lehmann D; Kalgoorlie Otitis Media Research Project Team . Crowding and other strong predictors of upper respiratory tract carriage of otitis media‐related bacteria in Australian Aboriginal and non‐Aboriginal children. Pediatr. Infect. Dis. J. 2011; 30: 480–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Reanalysis of pathogen detection data after adjustment for additional confounding factors.
Figure S2 Pathogen detection results from the 27 nasal swabs and washes taken at the convalescent visit.
Table S1 Criteria‐based scoring system for quantifying clinical disease severity.
Table S2 Viral and bacterial detection qPCR assays.
Table S3 Univariable analysis of associations between pathogen detection, clinical parameters and participant demographics.
Table S4 Associations between pathogen detections and severity parameters with adjustment for confounding factors.


