ABSTRACT
Seasonal influenza epidemics and periodic pandemics are important causes of morbidity and mortality. Patients with chronic co‐morbid illness, those at the extremes of age and pregnant women are at higher risks of complications requiring hospitalization, whereas young adults and obese individuals were also at increased risk during the A(H1N1) pandemic in 2009. Avian influenza A(H5N1) and A(H7N9) viruses have continued to circulate widely in some poultry populations and infect humans sporadically since 1997 and 2013, respectively. The recent upsurge in human cases of A(H7N9) infections in Mainland China is of great concern. Sporadic human cases of avian A(H5N6), A(H10N8) and A(H6N1) have also emerged in recent years while there are also widespread poultry outbreaks due to A(H5N8) in many countries. Observational studies have shown that treatment with a neuraminidase inhibitor (NAI) for adults hospitalized with severe influenza is associated with lower mortality and better clinical outcomes, especially when administered early in the course of illness. Whether higher than standard doses of NAI would provide greater antiviral effects in such patients will require further investigation. High‐dose systemic corticosteroids were associated with worse outcomes in patients with severe influenza. There is an urgent need for developing more effective antiviral therapies for treatment of influenza infections.
Keywords: avian influenza, respiratory tract infections, seasonal, treatment, viral
Abbreviations
- ACH
air changes per hour
- AGP
aerosol‐generating procedure
- ARDS
acute respiratory distress syndrome
- CAP
community‐acquired pneumonia
- CAPD
continuous ambulatory peritoneal dialysis
- CrCl
creatinine clearance
- HA
haemagglutinin
- HD
haemodialysis
- HFR
hospitalization fatality risk
- HR
hazard ratio
- IMV
invasive mechanical ventilation
- LPM
live poultry market
- LRT
lower respiratory tract
- NA
neuraminidase
- NAI
NA inhibitor
- NIV
non‐invasive ventilation
- NP
nasopharyngeal
- qd
once a day
- RCT
randomized controlled trial
- RIDT
rapid influenza diagnostic test
- rRT‐PCR
real time reverse transcription polymerase chain reaction
- RSV
respiratory syncytial virus
- URT
upper respiratory tract
- WHO
World Health Organization
INTRODUCTION
Severe acute respiratory infections such as avian influenza A(H5N1)1 and A(H7N9) viruses2 with pandemic potential have continued to circulate widely in some poultry populations and infect humans sporadically. Infection caused by these viruses may result in severe disease and high case fatality rates because most humans have no background immunity to these viruses. Sporadic human cases due to avian A(H5N6),3 A(H10N8)4 and A(H6N1)5 have also emerged in recent years. The global circulation of oseltamivir‐resistant seasonal influenza, the emergence of A(H1N1)pdm09 virus in 2009 followed by its continual circulation,6 the rising number of A(H7N9) infections in humans2 and the ongoing spread of A(H5N8) in recent months in the poultry populations in many countries in Asia, Africa, Europe and Middle East with pandemic potential7 all point to an urgent need for developing more effective antiviral therapies to reduce morbidity and mortality. This article reviews the epidemiology, clinical, diagnostic and treatment aspects of these important and emerging influenza viruses that are posing threats to human health in the Asia‐Pacific region.
EPIDEMIOLOGY
Seasonal influenza and pandemic influenza
Seasonal influenza is a major health problem. For instance, in Hong Kong, influenza A and B accounted for 39.4% and 10.2%, respectively, of hospital admissions confirmed to have viral infections.8 The average annual incidence of hospitalization with laboratory‐confirmed influenza A infection was 107.7/10 000 and 18.3/10 000 among children aged <5 years and elderly ≥65 years, respectively. Furthermore, influenza A was the most common (45.5%) virus detected from patients who died of respiratory viral infections. Although influenza B is usually associated with a less degree of disease burden, its impact on human health is substantial. Our previous study conducted in Hong Kong revealed that 24% of laboratory‐confirmed influenza‐associated hospital admissions were due to influenza B, and was much higher (41.9%) among older children and adolescents (aged 5–14 years).9 This observation is similar to data in Europe and North America.
In the temperate region, influenza exhibits an annual seasonal peak in winter. However, the seasonality in tropical and subtropical regions is more variable. For instance, in Hong Kong, a subtropical city, most of the time, influenza exhibits two peaks during January–March and June–August, and occasionally, the two peaks merge without an obvious gap.8, 10 Such bimodal and variable seasonality of influenza is also observed in countries within the subtropical and tropical regions, and poses a challenge to implement annual influenza vaccination campaign as well as a question on whether the Northern or Southern Hemisphere version of influenza vaccine would be more appropriate.
A schematic diagram of the structure of influenza virion is shown in Figure 1. Influenza represents one of the few examples of viruses with segmented genome. This, together with the widespread infection with different subtypes in various animal species, allows robust evolution through the process of ‘reassortment’. The prerequisite for reassortment is a simultaneous infection with more than one strain of viruses in a host referred to as the ‘mixing vessel’ that allows replication and swabbing of gene segments between different strains of viruses. Water birds are well‐known mixing vessels, but the reassortants are more likely to have a tropism restricted to avian species. These new reassortants may pose problems in the land birds and poultry, and subsequently be of human concern. Swine is another well‐known mixing vessel. The unique property of swine is their susceptibility to both avian and human influenza viruses. Thus, reassortants from swine are more likely to gain tropism for infection in humans. Field observations made as early as 1990s have pointed out that influenza outbreaks that began with either pigs or people were then rapidly transferred to each other.11 Swine are likely to play the role of a mixing vessel in creating pandemic strains. Although the 1918 pandemic strain was most probably an entirely avian‐like virus,12 the 1957 and 1968 pandemic strains were reassortants of human‐ and avian‐origin viruses.13
Figure 1.
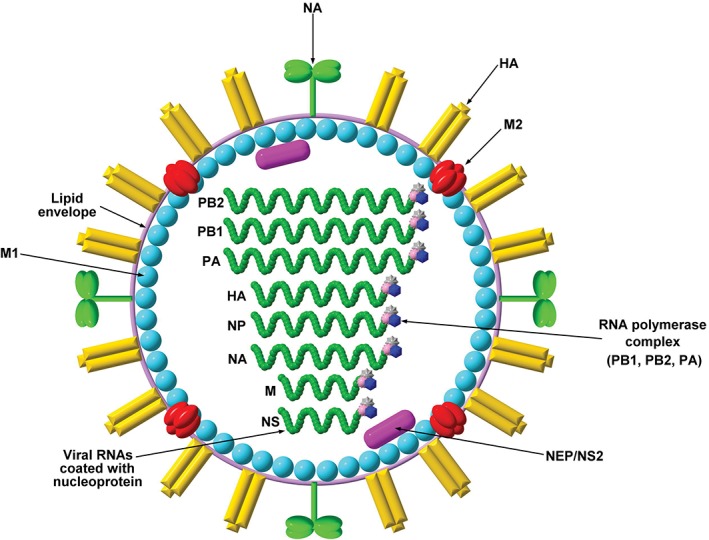
Structure of influenza A virion. The virion of influenza A contains a host‐derived lipid envelope, embedding the haemagglutinin (HA, found as trimer), neuraminidase (NA, found as tetramer) and matrix protein 2 (M2, found as tetramer). HA is required for attachment (binding between the virion and the sialic acid residues on the host cell surface). NA is used for cleaving sialic acid receptors from the host cell membrane for new virion release. M2 is an ion channel for virion internal acidification, contributing to viral uncoating. The HA : NA ratio ranges from 4:1 to 5:1. Underlying the viral envelope, there is a layer of the matrix protein 1 (M1). Inside the virion, a nuclear export protein (NEP/NS2) is also found. Eight single‐stranded, negative‐sensed viral RNA molecules are coated with nucleoproteins and bound by the RNA polymerase complex: polymerase basic protein 1 (PB1), polymerase basic protein 2 (PB2) and polymerase acidic protein (PA).
While the most recent pandemic, A(H1N1)pdm09, was not as severe as those that occurred before, there is no guarantee that the next pandemic will be as ‘mild’. At present, a few subtypes (H5, H7 and H9) are of particular concern. Cross‐species infections, especially the recent strong wave of human A(H7N9) infection in Mainland China underscores that the next pandemic could be imminent.14, 15 Countries should have pandemic preparedness in place, including antiviral stockpiling and pandemic vaccine preparations. These would be better achieved under joint multigovernment industrial partnership.
Avian influenza viruses
H5N1 viruses
Human cases of the highly pathogenic avian influenza A(H5N1) were first detected in Hong Kong in 1997.1 The number of affected countries increased between 2003 and 2008, with expansion from east and southeast Asia, then to west Asia and Africa, with a sharp rise in Egypt since November 2014.16 As of 16 March 2017, there have been 453 deaths out of 858 human cases in 16 countries since 2003.17 A Canadian tourist died of A(H5N1)‐related meningoencephalitis in Alberta in January 2014 after visiting Beijing in December 2013 without any obvious exposure to infected avian sources or environmental contamination.18 A history of exposure to dead or sick poultry/wild birds occurs in over 60% of cases of human A(H5N1) infection. The incubation period for A(H5N1) infection ranges from 2 to 8 days but may be as long as 17 days. The clinical spectrum may range from asymptomatic infection, mild influenza‐like illness to severe pneumonia, with multiorgan failure.19 Some of the A(H5N1) human cases have been linked to consumption of dishes made of raw, contaminated poultry blood. However, slaughter, defeathering, handling carcasses of infected poultry and preparing poultry for consumption, especially in household settings, appear to be important risk factors.19, 20
Among the fatal cases, the median duration from symptom onset to death was 9–10 days (range: 6–30 days).20 Viral RNA was present in the blood of fatal A(H5N1) cases and this was associated with higher pharyngeal viral loads. Nasopharyngeal swab samples, bronchoalveolar lavage and cerebrospinal fluid samples were positive in the Canadian case for influenza A(H5N1) virus by various molecular testing methods, including sequencing.18 In addition, lymphopenia and high chemokine/cytokine levels have been observed in severe A(H5N1) infection and these correlated with pharyngeal loads.21
A new reassortant genotype of A(H5N1) containing the haemagglutinin (HA) and neuraminidase (NA) genes from clade 1.1.2 and the internal genes from clade 2.3.2.1 was detected in 2013 and associated with the highest number of cases (n = 26) and deaths (n = 14) in Cambodia.22 Complete viral genome analysis of the fatal case in Canada has shown an HA gene of clade 2.3.2.1c and was a reassortant with an A(H9N2) subtype lineage polymerase basic 2 gene without mutations conferring resistance to adamantanes or NA inhibitors (NAI).18 Human–human transmission remains rare as a meta‐analysis has shown that only 1–2% of more than 12 500 study participants from 20 studies exposed to patients with A(H5N1) infection had serological evidence for prior A(H5N1) infection.23 Delay in the delivery of appropriate treatment to patients with influenza A(H5N1) infection in Indonesia was mainly related to delay in diagnosis rather than late presentation to healthcare settings.24 Age, country, per capita government health expenditure and delay from symptom onset to hospitalization were the risk factors for mortality related to A(H5N1) infection, emphasizing the importance of early diagnosis, treatment and supportive care.25 A systematic review has shown that females were at higher risk of death (OR: 1.75, 95% CI: 1.27–2.44) following A(H5N1) infection, whereas young age in particular <5 years was protective (OR: 0.44, 95% CI: 0.25–0.79).26
H7N9 viruses
Human infections with a novel avian influenza A (H7N9) virus were first reported in China in March 2013 in patients hospitalized with severe pneumonia.27 There have been five seasonal epidemics in China since the virus was first discovered in 2013. The estimated risk of death (hospitalization fatality risk, HFR) among hospitalized cases of A(H7N9) infection in the second epidemic was 48% (95% credibility interval: 42–54%), which was slightly higher than the corresponding risk of 36% in the first wave. In the second epidemic, the HFR was estimated at 36% (95% CI: 28–45%) in patients aged <60 years but at 59% (95% CI: 51–67%) among those aged ≥60 years.28
An upsurge in human A(H7N9) infections has been observed since November 2016 in Mainland China. During this fifth epidemic wave, the number of human cases is higher than during the previous two waves during 2014–2015 and 2015–2016. The majority of recently reported human cases are associated with exposure to infected live poultry or contaminated environments, including wet markets where live poultry are sold. In addition, the human cases are more geographically widespread and cases are also reported from rural areas, unlike in previous epidemics (Fig. 2).29, 30 The phylogenetic analysis on HA genes of isolates collected from humans suggested that the virus is evolving and with the more recent isolates clustered to a separate branch (Supplementary Figure S1).31, 32 As of 5 April 2017, 1364 laboratory‐confirmed cases of human infection with avian influenza A(H7N9) virus have been reported to World Health Organization (WHO).33 Human cases of A(H7N9) infection exported from the Mainland of China have been confirmed in Hong Kong (n = 21), Taiwan (n = 4), Macau (n = 2), Canada (n = 2) and Malaysia (n = 1) in visitors who developed illness after returning from the Mainland of China to their home cities.29, 30, 33
Figure 2.
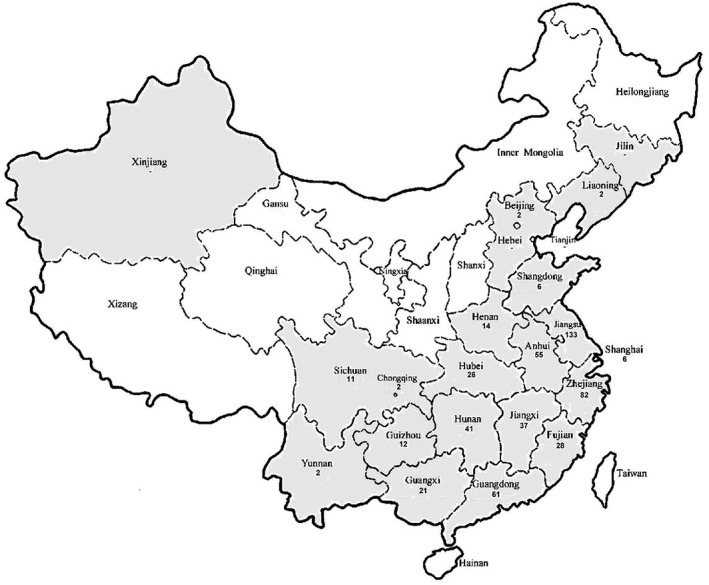
Geographic distribution of the fifth wave of human infection with avian influenza A(H7N9) in Mainland China.29 Cases include those reported from October 2016 to 25 March 2017. Two cases in Beijing were imported from Hebei and Liaoning. Two cases in Yunnan Province were imported from Jiangxi.
The incubation period of human infection with the A(H7N9) virus ranges from 1 to 10 days, with an average of 5 days. The median time from poultry exposure to disease onset was 6 days, whereas the median time from illness onset to hospitalization, acute respiratory distress syndrome (ARDS) development, antiviral therapy and death were 4, 7, 6 and 21 days, respectively.34 Preexisting co‐morbid conditions occurred in >60% of these cases. The prominent clinical features on admission were those of a severe influenza syndrome with fever, cough, fatigue and dyspnoea, whereas the most striking laboratory findings were marked lymphopenia and thrombocytopenia. Elevated cytokine levels have been observed in patients and the excessive cytokine responses may contribute to the clinical severity of A(H7N9) infection.35, 36
Limited human to human transmission with at least two epidemiologically linked cases due to close household contact with the index patients has been reported in a number of family clusters in both A(H5N1)20, 37, 38 and A(H7N9) infections.34, 39, 40 Nosocomial transmission of A(H7N9) infection between two patients sharing a hospital room,41 and co‐transmission of avian influenza A(H7N9) and A(H1N1)pdm09 viruses between two patients with haematological disorders sharing the same room with bed distance <1 m have been reported.42 Comparisons of the clinical features between human cases of A(H5N1) and A(H7N9) are shown in Table 1.34, 43
Table 1.
Comparisons of clinical and epidemiological features between A(H7N9) and A(H5N1) infections in humans16, 34, 43
| A(H7N9) (n = 139)34 | A(H5N1) (n = 907)16 | |
|---|---|---|
| Median age (years) | 61 (2–91) | 19 (5–32) |
| Male (%) | 71 | 431 (47) |
| Co‐morbid illness | 79/108 (73%) | 5/41 (12%)43 |
| Urban residence | 101 (73%) | 19 (44%)43 |
| Rural residence | 38 (27%) | 24 (56%)43 |
| Exposure to poultry | 82% | 82.5% |
| Occupational exposure to poultry | 9 (6%) | 15 (1.7%) |
| Visited wet poultry markets | 70/107 (65%) | 82/907 (9%) |
| Exposure to sick or dead poultry | 63/107 (59%) | 439/907 (48.4%) |
| Exposure to backyard poultry | NA | 188/907 (20.7%) |
| Onset of illness to hospitalization (median, days) | 4 | 4 |
| Onset of illness to ARDS (median, days) | 7 | 7.543 |
| Onset of illness to death (median, days) | 21 | 1143 |
| Case fatality rate in hospital | 34% | 53% |
ARDS, acute respiratory distress syndrome; NA, not applicable.
Closure of live poultry markets (LPM) in the Mainland of China has tremendously reduced the risk of A(H7N9) infection temporarily but closure is difficult to sustain due to cultural preference for live poultry. An ecologic modelling study estimated that closure of LPM reduced the mean daily number of A(H7N9) virus infections in the four most affected cities by 97–99%.44 A retrospective serological study of blood specimens taken during January–May and October–November in 2012 from 1544 subjects who worked in LPM, farms, slaughter houses or kept backyard poultry in Eastern China before the epidemic occurred in 2013 revealed no evidence of A(H7N9) infection.45
Other novel avian influenza subtypes
The first human case of avian A(H6N1) infection was reported in a 20‐year‐old female with pneumonia in Taiwan in May 2013, with subsequent full recovery following treatment with oseltamivir.5
The first human case of avian A(H5N6) was confirmed in a 49‐year‐old male in Sichuan Province, China, in May 2014 with a fatal outcome3 and a second case was confirmed in a 58‐year‐old male with history of exposure to live poultry in Guangdong Province in December 2014.46 The virus was a reassortant that contained seven genes from A(H5N1) and the NA gene from an A(H6N6) virus circulating in ducks.47 Influenza A(H5N6) outbreaks in birds and poultry have been reported in Laos, Vietnam and Mainland China since 2014. In 2016, A(H5N6) outbreaks in poultry have continued to occur in Vietnam and Mainland China (Guizhou, Jiangxi and Hunan). Since 2014, 16 human cases of avian influenza A(H5N6) have occurred in Mainland China with six deaths (Fig. 3).29 The latest case was reported on 1 December 2016.48
Figure 3.
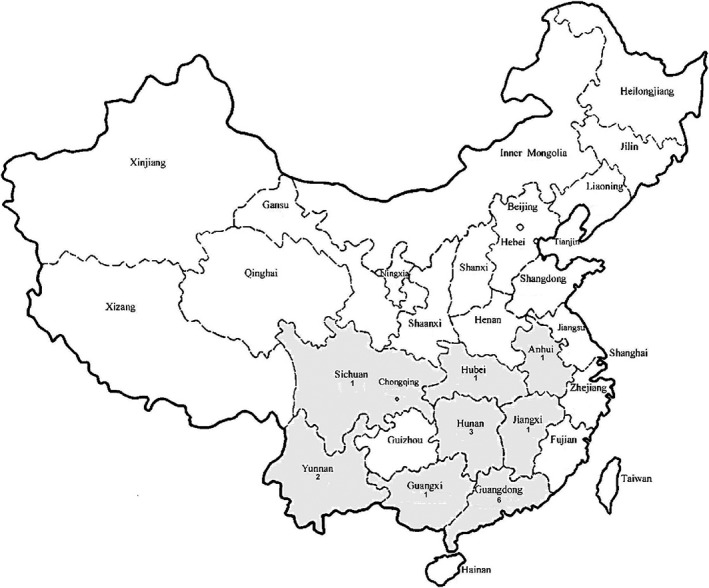
Geographic distribution of human infection with avian influenza A(H5N6) in Mainland China.29 Cases include those reported from 2014 till 25 March 2017. One case in Jiangxi was imported from Guangdong.
In addition, China has confirmed three human infections, two fatal, with avian A(H10N8) viruses that contain the internal genes from A(H9N2), as does A(H7N9).4, 49 Similar to the A(H7N9) virus, the A(H6N1) and A(H10N8) viruses have low pathogenicity in poultry and are therefore more difficult to detect in birds in contrast to the highly pathogenic A(H5N1) virus.
Highly pathogenic avian influenza A(H5N8) viruses belonging to clade 2.3.4.4 of the A/goose/Guangdong/1/1996 lineage were detected in 2014 in wild birds and poultry in China, Japan, South Korea, Netherlands, Germany, Italy, Russia, the UK and Northern Ireland. The virus was detected sporadically in Canada and the USA in wild birds and poultry until mid‐2015. The A(H5N8) viruses were also detected in Taiwan, China and in Hungary and Sweden in 2015. Since June 2016, many more countries in both Europe and Asia have detected infections in wild birds and/or domestic poultry with A(H5N8). Many of these recent detections were associated with mortality in wild birds.7, 50 Although human infection with A(H5N8) has not been detected, the ongoing spread of this virus to different countries in recent months is worrisome and may increase the risk of human infection.
CLINICAL MANAGEMENT
Initial assessment
Visitors who have returned from areas affected by avian influenza and developed respiratory symptoms and fever within 10 days after their return should be enquired about their relevant history (travel history, occupation, contact history and clustering) to facilitate early diagnosis and treatment. It is important to obtain any history of contact with poultry (live or sick/dead poultry as A(H7N9) virus may cause no or mild symptoms in poultry in contrast to A(H5N1)), exposure to wet markets or known avian influenza cases, and laboratory exposure including staff handling with spillage. Patients with influenza‐like illness who present with dyspnoea, tachypnoea, evidence of hypoxaemia and pulmonary infiltrates on chest radiograph should be hospitalized.51 Early case identification and isolation precautions would facilitate clinical management which may reduce the risk of severe infection and related complications for seasonal epidemic, zoonotic and pandemic influenza, in addition to reducing the risk of influenza transmission and mitigating the impact of outbreaks on the healthcare system.
Common findings in severe influenza infection include low or normal white cell counts, lymphopenia and thrombocytopenia, and increased serum transaminases, lactate dehydrogenase (LDH), creatine kinase and urea/creatinine.6, 20, 27, 51 In the Influenza Clinical Information Network (FLUCIN) Study, early elevation of C‐reactive protein ≥100 mg/L was an independent predictor of severe outcome. In a study of 1770 patients hospitalized with community‐acquired pneumonia (CAP), procalcitonin concentration had a strong association with need for invasive mechanical ventilation (IMV) or inotrope support (in 6.5% of those studied).52 Higher levels of certain biomarkers on presentation linked to inflammation, coagulation or immune function (interleukin 6 (IL‐6), Cluster of Differentiation 163 (CD163), interferon gamma‐induced protein 10 (IL‐10), lipopolysaccharide binding protein (LBP), IL‐2, monocyte chemoattractant protein‐1 (MCP‐1) and interferon gamma‐induced protein 10 (IP‐10)) have been associated with disease progression in both outpatients and those hospitalized with influenza.53
Laboratory investigations
Laboratory diagnosis plays an important role in the management of influenza. The narrow window of an effective antiviral intervention for both seasonal and avian influenza demands a rapid turnaround time.6, 54 Nowadays, most laboratories employ either real time reverse transcription polymerase chain reaction (rRT‐PCR)‐based methods or antigen detection‐based assays. While the rRT‐PCR approach is most sensitive and can be applied to a wide variety of specimens including the less invasive throat swab samples, it requires a certain degree of laboratory setup and technical skills, which are not readily available in many healthcare settings. The antigen‐based rapid influenza diagnostic test (RIDT), although less sensitive, is still the test of choice particularly as a point‐of‐care test. Our experience on a few RIDT suggested that the sensitivity could be lower for patients who presented more than 2 days after the onset of illness, for young children compared to elderly, and for cases with pneumonia compared to those without.55 It is therefore crucial to evaluate the performance with respect to the setting that RIDT will be applied.
The specimen of choice is critical. While in most situations, nasopharyngeal aspirate/swab is the preferred choice of specimen for seasonal influenza, false‐negative results may occur in patients with severe pneumonia where the viral load remains high in the lower respiratory tract (LRT) but has become undetectable in the upper respiratory tract (URT).56 Similarly, LRT specimens such tracheal aspirates or sputum are the specimen of choice for diagnosis of avian influenza infection in humans as the viral load in the URT is typically low. The development and availability of more affordable and reliable molecular diagnostic tests including multiplex PCR would facilitate future clinical management of patients with severe CAP due to viral or bacterial aetiology.57 The advantages and limitations of different diagnostic tests for influenza are summarized in Table 2.55, 57
Table 2.
| Testing method | Advantages | Disadvantages |
|---|---|---|
| Influenza virus | ||
| RIDT (immunoassay for antigens) |
|
|
| Immunofluorescence, direct (DFA) or indirect (IFA) antibody staining |
|
|
| Viral cell culture (conventional or shell vial) |
|
|
| Reverse transcription PCR (rRT‐PCR) |
|
|
Most RIDT are chromatographic immunoassays (some are fluorescence‐based immunoassays); applicable to NP swabs, nasal and/or throat swabs and NP aspirates/washes (training and protection equipment are required). Performance is best if applied within 48–72 h from onset before a significant drop in viral load (up to 4–5 days in selected populations). Lower sensitivity for A(H1N1)pdm09 virus has been reported.
Viral cell culture detects viable viruses, including those contained in the live‐attenuated influenza vaccines (LAIV). Isolates can be subjected to phenotypic resistance assays (e.g. neuraminidase enzyme inhibition assay). Viral load, specimen quality, transport, storage and processing techniques may affect test performance.
PCR assays can either provide universal detection of influenza A virus by targeting the matrix (M) gene or subtype‐specific virus detection (e.g. H1N1pdm09, H3N2, H5N1 and H7N9) by targeting the haemagglutinin (HA) gene. Viruses that cannot be subtyped may indicate a novel strain. Newer molecular‐based point‐of‐care tests may improve accessibility and reduce processing time and technical demands; some may allow detection of multiple viruses. Cost‐effectiveness of PCR is variable, depending on the circumstances.
Some multiplex PCR platforms may provide detection of >14 respiratory viruses (e.g. RSV, human metapneumovirus, parainfluenza virus, rhinovirus and coronavirus) and atypical pathogens (e.g. Mycoplasma pneumoniae and Chlamydophila pneumoniae).
DFA, direct fluorescent antibody test; IFA, immunofluorescence assay; NP, nasopharyngeal; RIDT, rapid influenza diagnostic test; RSV, respiratory syncytial virus.
Antiviral therapy
The M2 inhibitors (amantadine and rimantadine) and the NAI (oseltamivir, peramivir, zanamivir and laninamivir) are the main antiviral agents approved for the prevention of and treatment for influenza. In general, antiviral treatment should be commenced as soon as possible for any patient hospitalized with confirmed/suspected influenza with severe, complicated or progressive illness, and also for outpatients at higher risk for influenza complications.58
The M2 inhibitors (adamantanes) are not effective against influenza B viruses and recently circulating influenza A(H3N2) and influenza A(H1N1)pdm09 viruses, which are resistant due to an S31N mutation in the M2 ion channel. However, as some avian influenza A(H5N1) strains are still susceptible,59 combination of an adamantane with an NAI may enhance antiviral activity for susceptible isolates.60 Oseltamivir is effective in reducing mortality (OR: 0.17; P = 0.04) in influenza A(H5N1) infection when given before the onset of respiratory failure,61 and may provide some survival benefit (49% reduction in mortality) when treatment is started within 6–8 days after symptom onset.62 Several observational studies have shown that treatment with oseltamivir for adults hospitalized with severe influenza is associated with lower mortality and better clinical outcomes, especially when antiviral treatment has been initiated within 2 days of illness onset but even as late as 4–5 days after symptom onset.63, 64 A systematic review has shown that in prophylactic studies, oseltamivir could reduce the proportion of symptomatic influenza whereas in treatment studies it modestly reduced the time to first alleviation of symptoms by 16.8 h, but it caused nausea, vomiting, headaches and renal/psychiatric side effects.65 A meta‐analysis of randomized controlled trials (RCT) has shown that oseltamivir in adults with influenza shortens time to clinical symptom alleviation by 21%, reduces risks of LRT complications and hospitalization, but increases the occurrence of nausea and vomiting.66 Oseltamivir resistance is infrequent in A(H5N1), and <3% in circulating A(H1N1)pdm09, A(H3N2) and B viruses but it is important to monitor for antiviral resistance when managing patients with severe influenza.67
All H7N9 viruses are amantadine resistant due to the S31N substitution in the M2 ion channel protein. In patients hospitalized with severe A(H7N9) infection, reduction of viral load following oseltamivir treatment was associated with improved outcome, whereas the emergence of virus resistant to NAI harbouring an R292K mutation was associated with poor outcomes and lack of response to oseltamivir and peramivir, and reduced susceptibility to zanamivir and laninamivir (50‐ and 25‐fold rises in inhibitory concentration by 50% (IC50), respectively). Two patients with severe A(H7N9) infection and R292K mutation requiring extracorporeal membrane oxygenation had received systemic corticosteroid treatment which might have contributed to treatment failure and a fatal outcome.68
The recommended treatment duration of oseltamivir is generally 5 days, but longer treatment (10 days, then guided by clinical and virological testing as recommended by the WHO) is advisable for critically ill patients with respiratory failure with persistent viral replication in the LRT.51, 58, 69 Whether higher than standard dose of NAI would provide greater antiviral effects in such patients requires further investigation. One RCT of hospitalized patients (76% being children) revealed no clinical or virological advantages when comparing double dose of oseltamivir against standard dose.70 No additional benefit of higher dose oseltamivir treatment was observed in adults hospitalized with influenza A, although a faster virological response was noted in influenza B.71 However, an RCT of 18 critically ill patients with A(H1N1)pdm09 in Canada found that therapy with a triple dose of oseltamivir was associated with higher proportions of viral clearance at 5 days than standard therapy (78% vs 11%; P = 0.015).72 Intravenous (i.v.) peramivir treatment was associated with viral RNA decline as well as culture and RNA negativity, which occurred at similar rates to those on oral oseltamivir by Day 5 among adult patients hospitalized for influenza‐associated LRT complications.73 The various NAI dosages for adults with adjustment for renal failure are shown in Table 3.58
Table 3.
Dosage adjustment of oral oseltamivir, i.v. peramivir and i.v. zanamivir for adults with and without renal impairment58
| CrCl (mL/min) | Dialysis | ||||
|---|---|---|---|---|---|
| Oseltamivir | >60 to 90 | >30 to 60 | >10 to 30 | <10 | CAPD/HD |
| 75 mg bd | 30 mg bd | 30 mg qd | Data limited; may consider single dose at 30 mg |
CAPD: 30 mg single dose HD: 30 mg after every HD cycle |
|
| Peramivir | 50 to 80 | 31 to 49 | 10 to 30 | <10 | HD |
| 600 mg qd | 200 mg qd | 100 mg qd | Data limited; may consider 100 mg on Day 1, then 15 mg qd thereafter | 100 mg on Day 1, then 100 mg given 2 h after each HD session on dialysis days only | |
| Zanamivir | 50 to <80 | 30 to <50 | 15 to <30 | <15 | — |
| Initial dose 600 mg; maintenance dose for CrCl > 80 mL/min is 600 mg bd† | 400 mg bd | 250 mg bd | 150 mg bd | 60 mg bd | — |
Time interval between initial dose of zanamivir and the maintenance dose in patients with CrCl > 80 mL/min = 12 h; CrCl 15 to <30 = 24 h; CrCl <15 mL/min = 48 h.
CAPD, continuous ambulatory peritoneal dialysis; CrCl, creatinine clearance; HD, haemodialysis; qd, once a day.
Zanamivir and laninamivir have quite similar profiles of drug susceptibility. One example is that H275Y mutation, which confers high level resistance to oseltamivir carboxylate and reduced susceptibility to peramivir in N1‐containing viruses, does not reduce susceptibility to zanamivir and laninamivir significantly.74 Intravenous zanamivir was used widely on a compassionate ground for late treatment of critically ill adults with influenza A(H1N1)pdm09 and those with suspected or proven oseltamivir resistance.75 There were no drug‐related trends in safety parameters and in a subset of 93 patients with positive PCR tests at baseline for influenza, showed a median decrease in nasopharyngeal viral RNA load of 1.42 log10 copies/mL after 2 days of treatment.76 Intravenous zanamivir was used with a favourable outcome in a patient with severe A(H7N9) infection complicated by pneumonia which did not respond to oseltamivir initially.77 Favipiravir, which is an inhibitor of a viral RNA‐dependent RNA polymerase, has strong antiviral activity against NAI‐resistant and sensitive influenza viruses.67, 78
Systemic corticosteroids and other immunomodulating agents for severe influenza
Systemic corticosteroid has been used frequently for the treatment of influenza‐related ARDS. However, a meta‐analysis of data predominantly related to treatment of severe influenza A(H1N1)pdm09 has shown that systemic corticosteroid was associated with an increase in mortality (OR: 3.06, 95% CI: 1.58–5.92). Pooled subgroup analysis of adjusted estimates of mortality from four studies found OR of 2.82 and 95% CI of 1.61–4.92.79 In comparison to controls, high‐dose corticosteroids (>150 mg/day methylprednisolone eqv) was associated with increased risks in 30‐day mortality (38.5% vs 7.7%, P = 0.021) and 60‐day mortality (50% vs 15.4%, P = 0.022) and longer viral shedding (15 days vs 13 days, P = 0.039) in patients with influenza A (H7N9) viral pneumonia while there was no difference between low dose (25–150 mg/day methylprednisolone) and controls.80 In a study of 2649 adults hospitalized with influenza in Hong Kong, Singapore and Beijing during 2008–2011, 23.1% had received systemic corticosteroids. Bacterial superinfections increased risk of death (adjusted hazard ratio (HR): 2.2, 95% CI: 1.5–3.1). Systemic corticosteroids increased risks of superinfections (from 2.7% to 9.7%) and deaths when controlled for indications (adjusted HR: 1.7, 95% CI: 1.1–2.6).81 Among adults with severe sepsis not in septic shock, use of low‐dose hydrocortisone versus placebo did not reduce the risk of septic shock within 14 days. The findings do not support the use of hydrocortisone in these patients without septic shock.82 The role of low‐dose systemic corticosteroid needs further investigation by a properly planned RCT.83
In a study of critically ill patients infected with influenza A (H1N1)pdm 09 requiring IMV, addition of a mammalian target of rapamycin inhibitor, sirolimus 2 mg/day for 14 days to oseltamivir and prednisolone (n = 19), was associated with a higher frequency of liberation from IMV (84.2% vs 47.4%, P = 0.04), a shorter duration of IMV (13.8 days vs 33 days, P = 0.03) and a higher chance of achieving LRT viral RNA negativity by Day 7 (75% vs 33%, P < 0.05) than without addition of sirolimus (n = 19).84 The role of sirolimus plus oseltamivir versus oseltamivir alone in a larger sample size should be examined without systemic corticosteroid.
A recent study of adults hospitalized for A(H3N2) has shown that a triple combination treatment of 2 days of clarithromycin 500 mg, naproxen 200 mg and oseltamivir 75 mg twice daily (bd), followed by 3 days of oseltamivir reduced both 30‐ and 90‐day mortalities and length of hospital stay versus oseltamivir 75 mg bd without placebos for 5 days as control.85 Confirmatory studies of the role of this triple combination would be of interest.
Exploratory post hoc meta‐analysis of studies of severe acute respiratory syndrome (SARS) and severe influenza showed a significant reduction in the pooled odds of mortality following convalescent plasma versus placebo or no treatment (OR: 0.25; 95% CI: 0.14–0.45).86 Thus, convalescent plasma and hyperimmune globulin may provide useful alternatives for the treatment of severe novel influenza infections provided there are suitable donors with significant neutralizing antibody. Other immunomodulating agents such as acute use of statins, N‐acetylcysteine, nitazoxanide, macrolides, peroxisome proliferator activated receptor (PPAR) agonists, i.v. gammaglobulin (IVIG), celecoxib, mesalazine and the role of plasmapheresis and haemoperfusion as rescue therapy require further investigation.87
Infection control and prevention
A survey of acute care hospitals in the Canadian Nosocomial Infection Surveillance Program from 2006 to 2012 has shown that 570 (17.3%) of 3299 influenza cases were healthcare associated; 345 (60.5%) acquired in a long‐term care facility and 225 (39.5%) acquired in an acute care facility.88 Nosocomial opportunistic airborne transmission of A(H3N2) has been implicated in a medical ward with imbalanced airflow and use of non‐invasive ventilation (NIV) for an index patient with acute exacerbation of chronic obstructive pulmonary disease (COPD).89
Standard, contact and droplet precautions are recommended for routine management of patients hospitalized for influenza.90 The main infection prevention and control measures for managing influenza are droplet precaution (by wearing a surgical mask within 1 m of the patient) and contact precaution (by wearing gown and gloves on entering the room and removing them on leaving). Droplet precautions should be added to the standard precautions when providing care to all patients with symptoms of acute respiratory infection. Contact precautions and eye protection should be added when caring for probable or confirmed cases of avian influenza infection (Table 4).90
Table 4.
Infection prevention and control measures when assessing patients with complicated influenza51, 90, 91
|
AGP, aerosol‐generating procedure.
A study that measured the amount of influenza A(H1N1)pdm09 RNA in aerosols in the vicinity of H1N1‐positive patients undergoing aerosol‐generating procedures (AGP) has shown that bronchoscopy and respiratory/airway suctioning were the most definite procedures to produce aerosols above background baseline values.92 Thus, it is important for healthcare workers to take airborne precautions and perform AGP preferably in an isolation room with negative pressure. In order to reduce room contamination in the hospital setting, major health organizations have recommended the application of a minimum room ventilation rate of 6 air changes per hour (ACH) in existing facility whereas a higher ventilation of 12 ACH is recommended for new or renovated construction, especially when caring for patients receiving mechanical ventilation and during AGP.91, 93
As single circuit NIV may lead to room contamination by exhaled aerosols,94, 95 it has been recommended to apply NIV via a helmet and double circuit tubes for patients with mild to moderate respiratory failure during the influenza A(H1N1) pandemic in 2009.96 However, it is important to ensure a good seal at the neck–helmet interface to prevent nosocomial transmission through environmental contamination by exhaled aerosols.97
Antiviral prophylaxis
Post‐exposure prophylaxis with a daily dose of NAI (e.g. oseltamivir 75 mg daily for adults) for 10 days is recommended for unprotected close contacts of patients with seasonal influenza who are at risk of complicated influenza.98 WHO does not recommend routine post‐exposure antiviral chemoprophylaxis for A(H7N9) virus. However, for some asymptomatic persons in which a substantial unprotected or prolonged exposure to an ill patient with A(H7N9) infection has occurred, initiation of empiric post‐exposure antiviral treatment (e.g. oseltamivir 75 mg orally bd for 5 days), on the presumption that influenza virus infection has occurred, may be considered. This is likely to be limited to healthcare or other settings involving substantial exposure of those at higher risk for complications from influenza virus infection, including patients with severe immunosuppression, neonates and infants, pregnant and early post‐partum women, elderly adults, persons with co‐morbidities and other highly vulnerable patients; or, unprotected healthcare workers, especially those involved in AGP.99
VACCINES
Annual seasonal influenza vaccinations are recommended for the high‐risk groups, including residents of institutions for elderly and disabled, any age with chronic illness, age > 65 years; 6 months to 6 years, poultry workers in countries affected by avian influenza, healthcare workers especially those frequently in contact with high‐risk persons and household contacts of high‐risk persons. While vaccination rates among healthcare workers are highly variable in different countries,100 there is no conclusive evidence that seasonal influenza vaccination of healthcare workers may protect their patients in terms of reduction in risks of mortality and influenza‐like illness.101 A survey in Hong Kong assessing the acceptability of an additional ad hoc influenza vaccination among the healthcare professionals following seasons with significant antigenic drift has shown that the strongest factors associated with accepting an additional influenza vaccine included immunization with influenza vaccines in the past 3 years, higher perceived risk of contracting influenza and higher perceived severity of the disease impact.102
The current influenza vaccines induce a strain‐specific immunity that is not ideal for this rapidly mutating virus. A universal influenza vaccine capable of inducing a broad cross‐protection across strains is needed. To this end, the M2e antigen, a linear peptide that is conserved across influenza A strains, is being evaluated a vaccine target.103 The other approach is to prepare virus‐like‐particles based on HA, NA and M1 proteins.104, 105 Virus‐like particles with a cocktail of seasonal and potential pandemic strains have been prepared and demonstrated promising results.106
PUBLIC HEALTH MEASURES TO REDUCE ZOONOTIC AND PANDEMIC RISKS FROM AVIAN INFLUENZA IN ASIA
As A(H7N9) is now endemic in over half of the provinces in Mainland China and will continue to cause recurrent zoonotic disease in the winter months, public health measures to control the source such as some rest days every month for the poultry workers to thoroughly clean the LPM environment, and banning the stock of live poultry in markets overnight are important. Separation of live ducks and geese from land‐based (i.e. non‐aquatic) poultry in LPM systems can reduce the risk of emergence of zoonotic and epizootic viruses at source. In the long run, it is advisable to adopt a complete transition from sale of live poultry in wholesale and retail LPM to centralized slaughter and sale of chilled or frozen poultry.107
SUMMARY
Seasonal influenza epidemics and periodic pandemics are important causes of morbidity and mortality. Apart from influenza A(H5N1) virus and sporadic human cases of A(H5N6), A(H10N8) and A(H6N1) infections, infection due to A(H7N9) virus, especially the recent upsurge in the number of human infections in Mainland China, is of great concern. The widespread poultry outbreaks due to A(H5N8) in many countries increases the risk of human infection. Early isolation and treatment with an NAI for adults hospitalized with severe influenza is associated with lower mortality and better clinical outcomes. Whether higher than standard doses of NAI may provide greater antiviral effects in such patients would require further investigation. High‐dose systemic corticosteroids were associated with worse outcomes in patients with severe influenza complicated by ARDS. There is an urgent need for developing more effective antiviral therapies for the treatment of influenza infections.
Supporting information
Figure S1 Phylogenetic tree of haemagglutinin (HA) genes of H7N9 isolates collected from human infections.
Acknowledgement
This project was partially supported by the Health & Medical Research Fund (#12110392), Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. T11‐705/14N), Food & Health Bureau, HKSAR.
The Authors
D.S.C.H. is the Stanley Ho Professor of Respiratory Medicine and Chairman of Department of Medicine and Therapeutics, The Chinese University of Hong Kong. His research interests include clinical management of severe acute respiratory infections, infection control and prevention of nosocomial respiratory infections and sleep‐disordered breathing. He has published over 280 peer‐reviewed journal articles. P.K.S.C. is Clinical Professor and Chairman of Department of Microbiology at The Chinese University of Hong Kong. Professor P.K.S.C. is a renowned clinical virologist with special interest in tumour virology and human respiratory viruses. He has published more than 330 scientific papers, and attained an H‐index of 51. N.L. is Professor in Infectious Diseases in The Chinese University of Hong Kong. His main research interests include respiratory viral infections (influenza, RSV and coronavirus) and CAP, focusing on clinical trials, patient outcomes, virokinetics and host response, and infection control in the hospital settings. He is currently working on novel antivirals and adjunctive therapies against these severe diseases.
Hui, D.S.C. , Lee, N. and Chan, P.K.S. (2017) A clinical approach to the threat of emerging influenza viruses in the Asia‐Pacific region. Respirology, 22: 1300–1312. doi: 10.1111/resp.13114.
Series Editor: Grant Waterer
REFERENCES
- 1. Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, Cheung PT, To WK, Ho ET, Sung R et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998; 351: 467–71. [DOI] [PubMed] [Google Scholar]
- 2. Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K et al. Human infection with a novel avian‐origin influenza A (H7N9) virus. N. Engl. J. Med. 2013; 368: 1888–97. [DOI] [PubMed] [Google Scholar]
- 3.WHO China statement on H5N6, 2014. [Accessed 12 Feb 2017.] Available from URL: http://www.wpro.who.int/china/mediacentre/releases/2014/20140507/en/
- 4. Chen H, Yuan H, Gao R, Zhang J, Wang D, Xiong Y, Fan G, Yang F, Li X, Zhou J et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet 2014; 383: 714–21. [DOI] [PubMed] [Google Scholar]
- 5. Wei SH, Yang JR, Wu HS, Chang MC, Lin JS, Lin CY, Liu YL, Lo YC, Yang CH, Chuang JH et al. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respir. Med. 2013; 1: 771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee N, Chan PK, Lui GC, Wong BC, Sin WW, Choi KW, Wong RY, Lee EL, Yeung AC, Ngai KL et al. Complications and outcomes of pandemic 2009 influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J. Infect. Dis. 2011; 203: 1739–47. [DOI] [PubMed] [Google Scholar]
- 7. Food & Agricultural Organization of the United Nations . H5N8 HPAI global situation update, 2017. [Accessed 18 Apr 2017.] Available from URL: http://www.fao.org/ag/againfo/programmes/en/empres/H5N8/situation_update.html
- 8. Chan PK, Tam WW, Lee TC, Hon KL, Lee N, Chan MC, Mok HY, Wong MC, Leung TF, Lai RW et al. Hospitalization incidence, mortality, and seasonality of common respiratory viruses over a period of 15 years in a developed subtropical city. Medicine (Baltimore) 2015; 94: e2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan PK, Chan MC, Cheung JL, Lee N, Leung TF, Yeung AC, Wong MC, Ngai KL, Nelson EA, Hui DS. Influenza B lineage circulation and hospitalization rates in a subtropical city, Hong Kong, 2000–2010. Clin. Infect. Dis. 2013; 56: 677–84. [DOI] [PubMed] [Google Scholar]
- 10. Chan PK, Mok HY, Lee TC, Chu IM, Lam WY, Sung JJ. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J. Med. Virol. 2009; 81: 1797–806. [DOI] [PubMed] [Google Scholar]
- 11. Schultz U, Fitch WM, Ludwig S, Mandler J, Scholtissek C. Evolution of pig influenza viruses. Virology 1991; 183: 61–73. [DOI] [PubMed] [Google Scholar]
- 12. Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature 2005; 437: 889–93. [DOI] [PubMed] [Google Scholar]
- 13. Kawaoka Y, Krauss S, Webster RG. Avian‐to‐human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 1989; 63: 4603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morens DM, Taubenberger JK, Fauci AS. Pandemic influenza viruses – hoping for the road not taken. N. Engl. J. Med. 2013; 368: 2345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 2013; 381: 1916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai S, Qin Y, Cowling BJ, Ren X, Wardrop NA, Gilbert M, Tsang TK, Wu P, Feng L, Jiang H et al. Global epidemiology of avian influenza A H5N1 virus infection in humans, 1997–2015: a systematic review of individual case data. Lancet Infect. Dis. 2016; 16: e108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO . Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2017. [Accessed 12 Apr 2017.] Available from URL: http://www.who.int/influenza/human_animal_interface/2017_03_16_tableH5N1.pdf?ua=1
- 18. Pabbaraju K, Tellier R, Wong S, Li Y, Bastien N, Tang JW, Drews SJ, Jang Y, Davis CT, Fonseca K et al. Full‐genome analysis of avian influenza A(H5N1) virus from a human, North America, 2013. Emerg. Infect. Dis. 2014; 20: 887–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hui DS. Review of clinical symptoms and spectrum in humans with influenza A/H5N1 infection. Respirology 2008; 13(Suppl. 1): S10–3. [DOI] [PubMed] [Google Scholar]
- 20. Writing Committee of the second World Health Organization Consultation on clinical aspects of human infection with avian influenza A (H5N1) virus. N. Engl. J. Med. 2008; 358: 261–73. [DOI] [PubMed] [Google Scholar]
- 21. de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 2005; 352: 686–91. [DOI] [PubMed] [Google Scholar]
- 22. Hurt AC, Hui DS, Hay A, Hayden FG. Overview of the 3rd isirv‐Antiviral Group Conference – advances in clinical management. Influenza Other Respir. Viruses 2015; 9: 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang TT, Parides MK, Palese P. Seroevidence for H5N1 influenza infections in humans: meta‐analysis. Science 2012; 335: 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adisasmito W, Aisyah DN, Aditama TY, Kusriastuti R, Trihono, Suwandono A, Sampurno OD, Prasenohadi, Sapada NA, Mamahit MJ et al. Human influenza A H5N1 in Indonesia: health care service‐associated delays in treatment initiation. BMC Public Health 2013; 13: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel RB, Mathur MB, Gould M, Uyeki TM, Bhattacharya J, Xiao Y, Khazeni N. Demographic and clinical predictors of mortality from highly pathogenic avian influenza A (H5N1) virus infection: CART analysis of international cases. PLoS One 2014; 9: e91630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, Fadel SA, Tran D, Fernandez E, Bhatnagar N et al. Populations at risk for severe or complicated Avian Influenza H5N1: a systematic review and meta‐analysis. PLoS One 2014; 9: e89697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ et al. Clinical findings in 111 cases of influenza A(H7N9) virus infection. N. Engl. J. Med. 2013; 368: 2277–85. [DOI] [PubMed] [Google Scholar]
- 28. Feng L, Wu JT, Liu X, Yang P, Tsang TK, Jiang H, Wu P, Yang J, Fang VJ, Qin Y et al. Clinical severity of human infections with avian influenza A(H7N9) virus, China, 2013/14. Euro. Surveill. 2014; 19: pii: 20984 Available from URL: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Avian Influenza Report . Centre for Health Protection, Hong Kong SAR Government. 2017; 13: 15. [Accessed 17 Apr 2017.] Available from URL: http://www.chp.gov.hk/files/pdf/2017_avian_influenza_report_vol13_wk15.pdf
- 30. ECDC . Recent upsurge of A(H7N9) cases in China, updated ECDC rapid risk assessment, 2017. [Accessed 15 Feb 2017.] Available from URL: https://medicalxpress.com/news/2017-01-upsurge-ah7n9-cases-china-ecdc.html
- 31. Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003; 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 32. Katoh K, Toh H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010; 26: 1899–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. WHO . Human infection with avian influenza A(H7N9) virus – China. Disease outbreak news, 2017. [Accessed 16 Apr 2017.] Available from URL: http://www.who.int/csr/don/05-april-2017-ah7n9-china/en/
- 34. Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, Xiang N, Chen E, Tang F, Wang D et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N. Engl. J. Med. 2014; 370: 520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou J, Wang D, Gao R, Zhao B, Song J, Qi X, Zhang Y, Shi Y, Yang L, Zhu W et al. Biological features of novel avian influenza A (H7N9) virus. Nature 2013; 499: 500–3. [DOI] [PubMed] [Google Scholar]
- 36. Chi Y, Zhu Y, Wen T, Cui L, Ge Y, Jiao Y, Wu T, Ge A, Ji H, Xu K et al. Cytokine and chemokine levels in patients infected with the novel avian influenza A (H7N9) virus in China. J. Infect. Dis. 2013; 208: 1962–7. [DOI] [PubMed] [Google Scholar]
- 37. Yang Y, Halloran ME, Sugimoto JD, Longini IM Jr. Detecting human‐to‐human transmission of avian influenza A (H5N1). Emerg. Infect. Dis. 2007; 13: 1348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen, Hadisoedarsuno W, Purba W, Santoso H, Septiawati C, Tresnaningsih E, Heriyanto B et al. Three Indonesian clusters of H5N1 virus infection in 2005. N. Engl. J. Med. 2006; 355: 2186–94. [DOI] [PubMed] [Google Scholar]
- 39. Liu T, Bi Z, Wang X, Li Z, Ding S, Bi Z, Wang L, Pei Y, Song S, Zhang S et al. One family cluster of avian influenza A(H7N9) virus infection in Shandong, China. BMC Infect. Dis. 2014; 14: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yi L, Guan D, Kang M, Wu J, Zeng X, Lu J, Rutherford S, Zou L, Liang L, Ni H et al. Family clusters of avian influenza A H7N9 virus infection in Guangdong Province, China. J. Clin. Microbiol. 2015; 53: 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fang CF, Ma MJ, Zhan BD, Lai SM, Hu Y, Yang XX, Li J, Cao GP, Zhou JJ, Zhang JM et al. Nosocomial transmission of avian influenza A (H7N9) virus in China: epidemiological investigation. BMJ 2015; 351: h5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen H, Liu S, Liu J, Chai C, Mao H, Yu Z, Tang Y, Zhu G, Chen HX, Zhu C et al. Nosocomial co‐transmission of avian influenza A(H7N9) and A(H1N1)pdm09 viruses between 2 patients with hematologic disorders. Emerg. Infect. Dis. 2016; 22: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, Tsang TK, Zheng J, Fang VJ, Chang Z et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population‐based study of laboratory‐confirmed cases. Lancet 2013; 382: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu H, Wu JT, Cowling BJ, Liao Q, Fang VJ, Zhou S, Wu P, Zhou H, Lau EH, Guo D et al. Effect of closure of live poultry markets on poultry‐to‐person transmission of avian influenza A H7N9 virus: an ecological study. Lancet 2014; 383: 541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bai T, Zhou J, Shu Y. Serologic study for influenza A (H7N9) among high‐risk groups in China. N. Engl. J. Med. 2013; 368: 2339–40. [DOI] [PubMed] [Google Scholar]
- 46. WHO . Global alert and response. Human infection with avian influenza A(H5N6) virus – China. Disease outbreak news, 2014. [Accessed 12 Feb 2017.] Available from URL: http://www.who.int/csr/don/28-december-2014-avian-influenza/en/
- 47. Qi X, Cui L, Yu H, Ge Y, Tang F. Whole‐genome sequence of a reassortant H5N6 avian influenza virus isolated from a live poultry market in China, 2013. Genome Announc. 2014; 2 pii:: e00706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. WHO West Pacific Region . Emerging disease surveillance and response. Avian Influenza Weekly Update Number 571, 2017. [Accessed 15 Feb 2017.] Available from URL: http://www.wpro.who.int/emerging_diseases/AvianInfluenza/en/
- 49. Zhang T, Bi Y, Tian H, Li X, Liu D, Wu Y, Jin T, Wang Y, Chen Q, Chen Z et al. Human infection with influenza virus A(H10N8) from live poultry markets, China, 2014. Emerg. Infect. Dis. 2014; 20: 2076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. WHO . Assessment of risk associated with influenza A(H5N8) virus, 2016. [Accessed 12 Feb 2017.] Available from URL: http://www.who.int/influenza/human_animal_interface/avian_influenza/riskassessment_AH5N8_201611/en/
- 51. Hui DS, Lee N, Chan PK. Clinical management of 2009 pandemic influenza A(H1N1) infection. Chest 2010; 137: 916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Self WH, Grijalva CG, Williams DJ, Woodworth A, Balk RA, Fakhran S, Zhu Y, Courtney DM, Chappell J, Anderson EJ et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community‐acquired pneumonia. Chest 2016; 150: 819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davey RT Jr, Lynfield R, Dwyer DE, Losso MH, Cozzi‐Lepri A, Wentworth D, Lane HC, Dewar R, Rupert A, Metcalf JA et al; INSIGHT FLU 002 & 003 Study Groups . The association between serum biomarkers and disease outcome in influenza A(H1N1)pdm09 virus infection: results of two international observational cohort studies. PLoS One 2013; 8: e57121. [DOI] [PMC free article] [PubMed]
- 54. Lee N, Chan PK, Choi KW, Lui G, Wong B, Cockram CS, Hui DS, Lai R, Tang JW, Sung JJ. Factors associated with early hospital discharge of adult influenza patients. Antivir. Ther. 2007; 12: 501–8. [PubMed] [Google Scholar]
- 55. Chan MC, Lee N, Ngai KL, Leung TF, Chan PK. Clinical and virologic factors associated with reduced sensitivity of rapid influenza diagnostic tests in hospitalized elderly patients and young children. J. Clin. Microbiol. 2014; 52: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee N, Chan PK, Wong CK, Wong KT, Choi KW, Joynt GM, Lam P, Chan MC, Wong BC, Lui GC et al. Viral clearance and inflammatory response patterns in adults hospitalized for pandemic 2009 influenza A(H1N1) virus pneumonia. Antivir. Ther. 2011; 16: 237–47. [DOI] [PubMed] [Google Scholar]
- 57. Torres A, Lee N, Cilloniz C, Vila J, Van der Eerden M. Laboratory diagnosis of pneumonia in the molecular age. Eur. Respir. J. 2016; 48: 1764–78. [DOI] [PubMed] [Google Scholar]
- 58. US Centers for Disease Control and Prevention . Antiviral agents for the treatment and chemoprophylaxis of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid. Mortal. Wkly Rep. 2011; 60: 1–24. [PubMed] [Google Scholar]
- 59. Govorkova EA, Baranovich T, Seiler P, Armstrong J, Burnham A, Guan Y, Peiris M, Webby RJ, Webster RG. Antiviral resistance among highly pathogenic influenza A(H5N1) viruses isolated worldwide in 2002–2012 shows need for continued monitoring. Antiviral Res. 2013; 98: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zumla A, Memish ZA, Maeurer M, Bates M, Mwaba P, Al‐Tawfiq JA, Denning DW, Hayden FG, Hui DS. Emerging novel and antimicrobial‐resistant respiratory tract infections: new drug development and therapeutic options. Lancet Infect. Dis. 2014; 14: 1136–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chan PK, Lee N, Zaman M, Adisasmito W, Coker R, Hanshaoworakul W, Gasimov V, Oner AF, Dogan N, Tsang O et al. Determinants of antiviral effectiveness in influenza virus A subtype H5N1. J. Infect. Dis. 2012; 206: 1359–66. [DOI] [PubMed] [Google Scholar]
- 62. Adisasmito W, Chan PK, Lee N, Oner AF, Gasimov V, Aghayev F, Zaman M, Bamgboye E, Dogan N, Coker R et al. Effectiveness of antiviral treatment in human influenza A(H5N1) infections: analysis of a Global Patient Registry. J. Infect. Dis. 2010; 202: 1154–60. [DOI] [PubMed] [Google Scholar]
- 63. Lee N, Choi KW, Chan PK, Hui DS, Lui GC, Wong BC, Wong RY, Sin WY, Hui WM, Ngai KL et al. Outcomes of adults hospitalised with severe influenza. Thorax 2010; 65: 510–5. [DOI] [PubMed] [Google Scholar]
- 64. Muthuri SG, Venkatesan S, Myles PR, Leonardi‐Bee J, Al Khuwaitir TS, Al Mamun A, Anovadiya AP, Azziz‐Baumgartner E, Báez C, Bassetti M et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta‐analysis of individual participant data. Lancet Respir. Med. 2014; 2: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jefferson T, Jones MA, Doshi P, Del Mar CB, Hama R, Thompson MJ, Spencer EA, Onakpoya I, Mahtani KR, Nunan D et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst. Rev. 2014; (4): CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta‐analysis of randomised controlled trials. Lancet 2015; 385: 1729–37. [DOI] [PubMed] [Google Scholar]
- 67. Li TC, Chan MC, Lee N. Clinical implications of antiviral resistance in influenza. Viruses 2015; 7: 4929–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hu Y, Lu S, Song Z, Wang W, Hao P, Li J, Zhang X, Yen HL, Shi B, Li T et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet 2013; 381: 2273–9. [DOI] [PubMed] [Google Scholar]
- 69.WHO guidelines for pharmacological management of pandemic influenza A(H1N1) 2009 and other influenza viruses. Revised February 2010. Part I recommendations. [Accessed 19 Feb 2017.] Available from URL: http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf [PubMed]
- 70. South East Asia Infectious Disease Clinical Research Network . Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ 2013; 346: f3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee N, Hui DS, Zuo Z, Ngai KL, Lui GC, Wo SK, Tam WW, Chan MC, Wong BC, Wong RY et al. A prospective intervention study on higher‐dose oseltamivir treatment in adults hospitalized with influenza A and B infections. Clin. Infect. Dis. 2013; 57: 1511–9. [DOI] [PubMed] [Google Scholar]
- 72. Kumar A; The ROSII Study Investigators . Viral clearance with standard or triple dose oseltamivir therapy in critically ill patients with pandemic (H1N1) 2009 influenza. 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 10–13 Sept, American Society for Microbiology, Denver, CO, 2013. [Abstract V‐1470].
- 73. Lee N, Chan PK, Tam WW, Chan MC, Lui GC, Kwok AK, Ko FW, Ng SS, Yung IM, Wong RY et al. Virological response to peramivir treatment in adults hospitalised for influenza‐associated lower respiratory tract infections. Int. J. Antimicrob. Agents 2016; 48: 215–9. [DOI] [PubMed] [Google Scholar]
- 74. Yamashita M, Tomozawa T, Kakuta M, Tokumitsu A, Nasu H, Kubo S. CS‐8958, a prodrug of the new neuraminidase inhibitor R‐125489, shows long‐acting anti‐influenza virus activity. Antimicrob. Agents Chemother. 2009; 53: 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chan‐Tack KM, Gao A, Himaya AC, Thompson EG, Singer ME, Uyeki TM, Birnkrant DB. Clinical experience with intravenous zanamivir under an emergency investigational new drug program in the United States. J. Infect. Dis. 2013; 207: 196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Marty FM, Man CY, van der Horst C, Francois B, Garot D, Mánez R, Thamlikitkul V, Lorente JA, Alvarez‐Lerma F, Brealey D et al. Safety and pharmacokinetics of intravenous zanamivir treatment in hospitalized adults with influenza: an open‐label, multicenter, single‐arm, phase II study. J. Infect. Dis. 2014; 209: 542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ho PL, Sin WC, Chan JF, Cheng VC, Chan KH. Severe influenza A H7N9 pneumonia with rapid virological response to intravenous zanamivir. Eur. Respir. J. 2014; 44: 535–7. [DOI] [PubMed] [Google Scholar]
- 78. Takashita E, Ejima M, Ogawa R, Fujisaki S, Neumann G, Furuta Y, Kawaoka Y, Tashiro M, Odagiri T. Antiviral susceptibility of influenza viruses isolated from patients pre‐ and post‐administration of favipiravir. Antiviral Res. 2016; 132: 170–7. [DOI] [PubMed] [Google Scholar]
- 79. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst. Rev. 2016; 3: CD010406. [DOI] [PubMed] [Google Scholar]
- 80. Cao B, Gao H, Zhou B, Deng X, Hu C, Deng C, Lu H, Li Y, Gan J, Liu J et al. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit. Care Med. 2016; 44: e318–28. [DOI] [PubMed] [Google Scholar]
- 81. Lee N, Leo YS, Cao B, Chan PK, Kyaw WM, Uyeki TM, Tam WW, Cheung CS, Yung IM, Li H et al. Neuraminidase inhibitors, superinfection and corticosteroids affect survival of influenza patients. Eur. Respir. J. 2015; 45: 1642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, Bogatsch H, Briegel J, Engel C, Gerlach H et al; SepNet–Critical Care Trials Group . Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA 2016; 316: 1775–85. [DOI] [PubMed]
- 83. Lim WS, Brittain C, Duley L, Edwards S, Gordon S, Montgomery A, Nguyen‐Van‐Tam J, Read R, Whitham D, Whynes D et al. Blinded randomised controlled trial of low‐dose Adjuvant Steroids in Adults admitted to hospital with Pandemic influenza (ASAP): a trial ‘in hibernation’, ready for rapid activation. Health Technol. Assess. 2015; 19: 1–78, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang CH, Chung FT, Lin SM, Huang SY, Chou CL, Lee KY, Lin TY, Kuo HP. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit. Care Med. 2014; 42: 313–21. [DOI] [PubMed] [Google Scholar]
- 85. Hung IF, To KK, Chan JF, Cheng VC, Liu KS, Tam A, Chan TC, Zhang AJ, Li P, Wong TL et al. Efficacy of clarithromycin‐naproxen‐oseltamivir combination in the treatment of patients hospitalized for influenza A(H3N2) infection: an open‐label, randomized controlled, phase 2b/3 trial. Chest 2017; 151: 1069–80. [DOI] [PubMed] [Google Scholar]
- 86. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen‐Van‐Tam JS, Beck CR; Convalescent Plasma Study Group . The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J. Infect. Dis 2015; 211: 80–90. [DOI] [PMC free article] [PubMed]
- 87. Hui DS, Lee N, Chan PK. Adjunctive therapies and immunomodulatory agents in the management of severe influenza. Antiviral Res. 2013; 98: 410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Taylor G, Mitchell R, McGeer A, Frenette C, Suh KN, Wong A, Katz K, Wilkinson K, Amihod B, Gravel D; Canadian Nosocomial Infection Surveillance Program . Healthcare‐associated influenza in Canadian hospitals from 2006 to 2012. Infect. Control Hosp. Epidemiol 2014; 35: 169–75. [DOI] [PubMed]
- 89. Wong BC, Lee N, Li Y, Chan PK, Qiu H, Luo Z, Lai RW, Ngai KL, Hui DS, Choi KW et al. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin. Infect. Dis. 2010; 51: 1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Seto WH, Conly JM, Pessoa‐Silva CL, Malik M, Eremin S. Infection prevention and control measures for acute respiratory infections in healthcare settings: an update. East. Mediterr. Health J. 2013; 19(Suppl. 1): S39–47. [PubMed] [Google Scholar]
- 91. WHO . WHO guidelines on natural ventilation for infection control in health‐care settings, 2009. [Accessed 16 Apr 2017.] Available from URL: http://whqlibdoc.who.int/publications/2009/9789241547857_eng.pdf. [PubMed]
- 92. Thompson KA, Pappachan JV, Bennett AM, Mittal H, Macken S, Dove BK, Nguyen‐Van‐Tam JS, Copley VR, O'Brien S, Hoffman P et al; EASE Study Consortium . Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic – the risk of aerosol generation during medical procedures. PLoS One 2013; 8: e56278. [DOI] [PMC free article] [PubMed]
- 93. CDC . Guidelines for environmental infection control in health‐care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recommendations and Reports. US Center for Disease Control and Prevention (CDC): Atlanta, GA, 2003; 52(RR‐10): 1–42. [PubMed]
- 94. Hui DS, Hall SD, Chan MT, Chow BK, Tsou JY, Joynt GM, Sullivan CE, Sung JJ. Noninvasive positive‐pressure ventilation: an experimental model to assess air and particle dispersion. Chest 2006; 130: 730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hui DS, Chow BK, Ng SS, Chu LC, Hall SD, Gin T, Sung JJ, Chan MT. Exhaled air dispersion distances during noninvasive ventilation via different Respironics face masks. Chest 2009; 136: 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Conti G, Larrsson A, Nava S, Navalesi P. On the role of non‐invasive ventilation (NIV) to treat patients during the H1N1 influenza pandemic. ERS & ESICM, 2009. [Accessed 18 Apr 2017.] Available from URL: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.430.2475&rep=rep1&type=pdf
- 97. Hui DS, Chow BK, Lo T, Ng SS, Ko FW, Gin T, Chan MT. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest 2015; 147: 1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Public Health England . PHE guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza. Version 7.0, 2016. [Accessed 16 Feb 2017.] Available from URL: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/580509/PHE_guidance_antivirals_influenza_2016_2017.pdf
- 99. WHO . Avian influenza A(H7N9) virus: post‐exposure antiviral chemoprophylaxis of close contacts of a patient with confirmed H7N9 virus infection and/or high risk poultry/environmental exposures, 2014. [Accessed 16 Feb 2017.] Available from URL: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/13_January_2013_PEP_recs.pdf?ua=1
- 100. Haviari S, Bénet T, Saadatian‐Elahi M, André P, Loulergue P, Vanhems P. Vaccination of healthcare workers: a review. Hum. Vaccin. Immunother. 2015; 11: 2522–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long‐term care institutions. Cochrane Database Syst. Rev. 2016; (6): CD005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wong MC, Nelson EA, Leung C, Lee N, Chan MC, Choi KW, Rainer TH, Cheng FW, Wong SY, Lai CK et al. Ad hoc influenza vaccination during years of significant antigenic drift in a tropical city with 2 seasonal peaks: a cross‐sectional survey among health care practitioners. Medicine (Baltimore) 2016; 95: e3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kang SM, Song JM, Compans RW. Novel vaccines against influenza viruses. Virus Res. 2011; 162: 31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Latham T, Galarza JM. Formation of wild‐type and chimeric influenza virus‐like particles following simultaneous expression of only four structural proteins. J. Virol. 2001; 75: 6154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus‐like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 2005; 23: 5751–9. [DOI] [PubMed] [Google Scholar]
- 106. Pushko P, Pearce MB, Ahmad A, Tretyakova I, Smith G, Belser JA, Tumpey TM. Influenza virus‐like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine 2011; 29: 5911–8. [DOI] [PubMed] [Google Scholar]
- 107. Peiris JS, Cowling BJ, Wu JT, Feng L, Guan Y, Yu H, Leung GM. Interventions to reduce zoonotic and pandemic risks from avian influenza in Asia. Lancet Infect. Dis. 2016; 16: 252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Phylogenetic tree of haemagglutinin (HA) genes of H7N9 isolates collected from human infections.


