Abstract
Dental health insurance coverage in the United States is either nonexistent (Medicare and the uninsured), spotty (Medicaid) and limited (most employer-based private benefit plans). Perhaps as a result, dental health in the United States is not good. What public policy makers may not appreciate is that this may well be impacting medical care costs in a way that improved dental benefits would produce a substantial return to investment in expanded dental insurance coverage.
Economics of Clinical Data Integration
A Cost Benefit Analysis of Expanding Dental Insurance Coverage
Introduction
Dental health insurance coverage in the United States is either nonexistent (Medicare and the uninsured), spotty (Medicaid) and limited (most employer-based private benefit plans). Perhaps as a result, dental health in the United States is not good. What public policy makers may not appreciate is that this may well be impacting medical care costs in a way that improved dental benefits would produce a substantial return to investment in expanded dental insurance coverage.
On the surface, it would appear to be politically and economically difficult or impossible to expand dental insurance coverage at this time. Health insurance costs have been rising at double digit rates. Most employers have been dropping health care coverage rather than expanding it (Kaiser Family Foundation 2010). Medicare trust funds are bankrupt (Social Security and Medicare Boards of Trustees 2011). Adding coverage would exacerbate an already alarming problem. Medicaid funding is a major source of state government deficits. Many states are slashing Medicaid coverage during this time of crisis (Wolf 2010). Improving Medicaid dental coverage during times of budget crisis would meet substantial political resistance.
Strikingly, strong and increasing evidence suggests relationships between oral health and a range of chronic illnesses. For example, recent findings show relationships between periodontal inflammatory conditions and diabetes, myocardial infarction, coronary artery disease, stroke, preeclampsia and rheumatoid arthritis. This suggests that improved oral health may well have the potential to reduce the incidence of chronic diseases as well as their complications. If chronic disease incidence is reduced it may be possible to avoid medical care costs related to treating them. It would be important to know more about the extent to which improved oral health could reduce health care costs and improve lives.
There are few, if any, studies of the costs of providing Medicare dental benefits, the costs of improving the Medicaid dental benefit or the cost of providing dental insurance to the uninsured. There are a few studies that indicate that periodontitis increases medical care costs, perhaps by as much as 20% (Ide et al. 2007; Albert et al. 2006).4 Ideally there should be a controlled study to assess the benefit of providing dental coverage through a government payer system. For a preliminary inquiry we can consider work already done and using some cost and benefit estimates, determine whether it is possible that benefits of extending dental coverage may outweigh costs.
Dental Insurance and Coverage in the United States
The failure of Medicare to cover dental care has engendered some (albeit not much) public debate. In 2003, Congress enacted the Medicare Prescription Drug, Improvement, and Modernization Act (Medicare Part D). By 2009 Medicare provided $56.6 billion in benefit payments for outpatient prescription drugs and Medicaid paid 15.7 billion for outpatient prescription drugs (Center for Medicare and Medicaid Services 2010). Beneficiaries provided billions more in the form of monthly Part D premiums. The expense of the Medicare prescription drug program and the controversy surrounding its enactment may well have eroded public support for increased Medicare coverage. So while there has been no shortage of effort paid to improving Medicare, the one common theme in all of the recent initiatives is that dental care has been conspicuously omitted. As a result, 43 million Medicare recipients in 2009 (US Census Bureau 2011) continue to have no dental insurance coverage through Medicare.4
Medicaid dental coverage is an optional benefit that states may or may not elect to provide. In Medicaid, both the State and the Federal government provide funds to cover healthcare services to eligible patients. The bulk of the money comes from the Federal government. Because the Medicaid dollars are limited and coverage for systemic diseases has precedence, Medicaid coverage of dental care has been spotty. Even where it has been provided, payments to dental providers have been so low as to make it difficult or impossible for Medicaid beneficiaries to obtain adequate dental care (Broadwater 2009). The 2008 recession increased the number of Medicaid eligible individuals nationwide. Further, the federal budget deficits of the past few years have reduced the federal contribution to state Medicaid programs. The combination of increases in the number of beneficiaries and diminished revenues has caused a number of states to eliminate or curtail Medicaid dental coverage (eHow 2011; Mullins et al. 2004). The result, 49 million Medicaid beneficiaries in the US (US Census Bureau 2011) in 2009 either had no dental insurance coverage or inadequate coverage.
Approximately 52 million people in the United States do not have health insurance (Kaiser Family Foundation 2010). Presumably, they have no dental insurance either. Further, not every employer provides dental insurance. A 1995 CDC survey found that 44.3% of adults do not have dental insurance coverage (Centers for Disease Control 1997). A 2006 Montana survey found that 53% of employers who offer health insurance do not offer dental insurance coverage (Montana Business Journal 2006). In 2009 there were approximately 202 million people enrolled in health insurance plans (US Census Bureau 2011). If half (a rough combination of the CDC and Montana percentages) of them do not have dental insurance it is likely that an additional 101 million (nonelderly, non-poor) people in the US do not have dental insurance coverage.
Finally, the term “dental insurance” is actually a misnomer.4 Dental policies cover routine treatments, offer discounts for more complex treatment and impose a low yearly on total payments. In fact, it has been called “part insurance, part prepayment and part large volume discount” (Manski 2001). Effectively, many (if not most) people who have dental insurance find it coverage to be quite restrictive. For example, many impose a small yearly cap ($1,500 is common) or large coinsurance amounts (50% for orthodontia, for example) (Rubenstein 2005). Even with discounts it is easy for many people to exceed the annual limit.
Given the lack of dental insurance coverage it is not surprising that the status of oral health in the US is not particularly good. In 2002 approximately 26.5% of adults between the ages of 35 and 44 had untreated caries, 42% had decayed, missing and filled tooth surfaces and more than one-half of adults had gingival bleeding (Dental, Oral and Craniofacial Data Resource Center of the National Institute of Dental and Craniofacial Research 2002). Three fourths of adults in the US have gingivitis and 35% have periodontitis (Mealey and Rose 2008). If these levels of untreated disease were applied to most systemic diseases, there would be public outcry.
The Relationship Between Dental Problems and Chronic Illness
Over the past decade evidence has been building that there is a relationship between dental disease, particularly periodontal disease, and chronic illnesses. Mealey and Rose note that there is strong evidence that “diabetes is a risk factor for gingivitis and periodontitis and that the level of glycemic control appears to be an important determinant in this relationship” (Mealey and Rose 2008). Moreover, diabetics have a six times greater risk for worsening of glycemic control over time compared to those without periodontitis and, periodontitis is associated with an increased risk for diabetic complications. For example, in one study more than 80% of diabetics with periodontitis experienced one or more major cardiovascular, cerebrovascular or peripheral vascular events compared to 21% of the diabetic subjects without periodontitis (Thorstensson et al. 1996). Also, a longitudinal study of 600 type 2 diabetics found that the death rate from ischemic heart disease was 2.3 times higher in subjects with severe periodontitis and the death rate from diabetic nephropathy was 8.5 times higher (Saremi et al. 2005). Clinical trials have demonstrated that treatment of periodontal disease improved glycemic control in diabetics (Miller et al. 1992). Moreover, investigations have found an association between periodontal disease and the development of glucose intolerance in non-diabetics (Saito et al. 2004). While it is difficult to establish causality and it is possible that other factors influence periodontal disease and medical complications, these studies suggest that treatment of periodontitis substantially improves health and greatly reduces medical complications related to diabetes.
Similarly, periodontitis is associated with cardiovascular disease and its complications including ischemia, atherosclerosis, myocardial infarction and stroke. A study by Slade and colleagues found both a relationship between periodontitis and elevated serum C- reactive protein levels (systemic marker of inflammation and documented risk factor for cardiovascular disease) as well as a relationship among body mass index, periodontitis and CRP concentrations (Slade et al. 2003). Hung and colleagues evaluated the association between baseline number of teeth and incident tooth loss and peripheral arterial disease. They determined that incident tooth loss was significantly associated with PAD, particularly among men with periodontal disease potentially implying an oral infection-inflammation pathway (Hund et al. 2003). The same group of researchers used the population enrolled in the Health Professionals’ Follow-Up Study (41,000 men free of cardiovascular disease and diabetes at baseline) to assess the relationship between tooth loss and periodontal disease and ischemic stroke. Controlling for a wide range of factors including smoking, obesity, and dietary factors, the researchers found a “modest” Association between baseline periodontal disease history and ischemic stroke (Joshipura et al. 2003). As early as 1993 DeStefano and colleagues found that among 9760 subjects, those with periodontitis had a 25% increased risk of coronary heart disease relative to those without. The association was particularly high among young men. The authors questioned whether the association was causal or not, suggesting that it might be a more general indicator of personal hygiene and possibly health care practices (DeStefano et al. 1993). In 2000 Wu and colleagues used data from the First National Health and Nutrition Examination Survey and its Epidemiologic Follow-Up Study to examine the association between periodontal disease and cerebrovascular accidents. The study found that periodontitis was a significant risk factor for total CVA, in particular, for non-hemorrhagic stroke (Wu et al. 2000).
In addition to diabetes and coronary artery disease, associations have been found between periodontal disease and rheumatoid arthritis and respiratory disease. This is not surprising given the role of periodontal disease in the production of inflammation related proteins. Dissick and colleagues conducted a pilot study of the associate ion between periodontitis and rheumatoid arthritis using multivariate regression and chi square tests. They found that periodontitis was more prevalent in patients with rheumatoid arthritis than in the control group and that patients who were seropositive for rheumatoid factor were more likely to have moderate to severe periodontitis than patients who were RF negative and also that patients who were positive for anti-cyclic citrullinated peptide antibodies were more likely to have moderate to severe periodontitis (Redman et al. 2010). Paju and Scannapeico investigated the association among oral biofilms, periodontitis and pulmonary infections. They noted that periodontitis seems to influence the incidence of pulmonary infections, particularly nosocomial pneumonia in high-risk subjects and that improved oral hygiene has been shown to reduce the occurrence of nosocomial pneumonia. They found that oral colonization by potential respiratory pathogens, for possibly fostered by periodontitis and possibly by bacteria specific to the oral cavity contribute to pulmonary infections (Paju and Scannapeico 2007).
Implications for Health Policy
The implications for these findings are profound. Professionally, they suggest that managing patients with chronic illness and periodontal disease will require teamwork and a deeper knowledge base for dentists and for physicians (Mealey and Rose 2008). Dentists will need to be alert for early signs of chronic illness among their patients and physicians will need to be alert for signs of dental disease. Both will need to consider wider treatment options than their specialty indicates. Dentistry and medicine have operated as professional silos in the past. The relationship between dental disease and chronic medical conditions suggests that continued separation is detrimental to patient centered care.
Beyond treatment implications, there are extremely important health policy concerns. If treatment of periodontitis and other dental problems leads to reduced incidence of chronic illness, fewer complications from chronic diseases and reduced morbidity among chronically ill patients, increased access to dental services could significantly reduce health care costs.
The diseases associated with periodontitis are among the most common illnesses, the fastest growing and the most expensive diseases that we treat. A recent Robert Wood Johnson report notes that approximately 141 million Americans have one or more chronic conditions, that the number of people with chronic conditions is expected to increase by 1% per year for the foreseeable future and that the most common chronic conditions include hypertension, disorders of lipid metabolism, upper respiratory disease, joint disorders, heart disease, diabetes, cardiovascular disorders, asthma and chronic respiratory infections (Anderson 2010) (see Fig. 4.1).
Fig. 4.1.
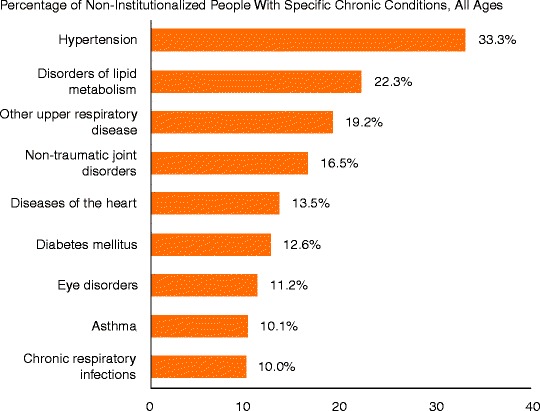
Most common chronic US illnesses. Anderson (2010) (Copyright 2010. Robert Wood Johnson Foundation. Used with permission from the Robert Wood Johnson Foundation)
One in four Americans has multiple chronic conditions. Ninety-one percent of adults aged 65 and older have at least one chronic condition and 73% have two or more of them (Anderson 2010). People with chronic conditions account for 84% of all healthcare spending. Seventy eight percent of private health insurance spending is attributable to the 48% of privately insured persons with chronic conditions. Seventy three percent of healthcare spending for the uninsured is for care received by the one third of uninsured people who have chronic conditions. Seventy nine percent of Medicaid spending goes to care for the 40% of non-institutionalized beneficiaries who have chronic conditions (Anderson 2010) (see Fig. 4.2).
Fig. 4.2.
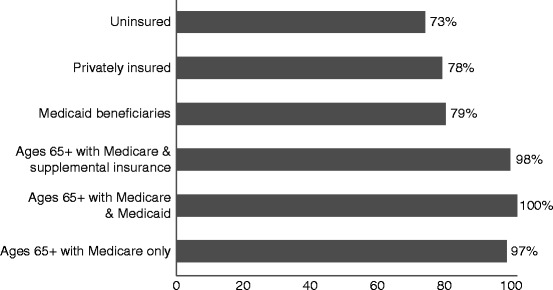
Percentage of healthcare spending for individuals with chronic conditions by type of insurance – 2006. Anderson (2010) (Copyright 2010. Robert Wood Johnson Foundation. Used with permission from the Robert Wood Johnson Foundation)
Further, health care spending increases with the number of chronic conditions (Anderson 2010) (see Fig. 4.3). More than three fifths of healthcare spending (two thirds of Medicare spending) goes to care for people with multiple chronic conditions. Those with multiple chronic conditions are more likely to be hospitalized, fill more prescriptions, and have more physician visits (Anderson 2010).
Fig. 4.3.
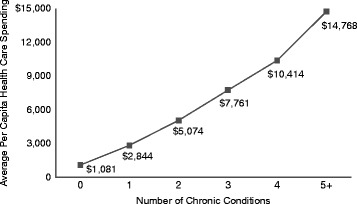
Per capita healthcare spending and number of chronic conditions – 2006. Anderson (2010) (Copyright 2010. Robert Wood Johnson Foundation. Used with permission from the Robert Wood Johnson Foundation)
In 2002 the American Diabetes Association estimated direct medical expenditures for diabetes at $91.8 billion: $23.2 billion for diabetes care, $24.6 billion for chronic complications and $44.1 billion for excess prevalence of general medical conditions. Approximately 52% of direct medical expenditures were incurred by people over 65. Indirect expenditures included lost workdays, restricted productivity mortality and permanent disability – a total of $39.8 billion. All told, diabetes was found to be responsible for $160 billion of $865 billion in total expenditures. Per capita medical expenditures totaled $13,000 annually for people with diabetes and $2600 for people without diabetes (Hogan et al. 2002). More recently, Dall and colleagues estimated that the US national economic burden of prediabetes and diabetes had reached $218 billion in 2007, $153 million in higher medical costs and $65 billion in reduced productivity. Annual cost per case was estimated at $2,900 for undiagnosed diabetes and 10,000 for type 2 diabetes (Dall et al. 2010).
The costs of caring for people with diabetes have risen both because the numbers of diabetics has been increasing and because the per capita costs of care have increased. The number of diabetics increased from 5.8 million on 1980 to 14.7 million in 2004 (Ashkenazy and Abrahamson 2006). A recent report by the UnitedHealth Group Center for Health Reform & Modernization provides a dire estimation – that more than 50% of adult Americans could have diabetes (15%) or prediabetes (37%) by 2020 at a cost of $3.35 trillion over the decade. This compares with current estimates of 12% of the population with diabetes and 28% with prediabetes, or 40%. These estimates conclude that diabetes and prediabetes will account for 10% of total healthcare spending in 2020 at an annual cost of $500 billion, up from an estimated $194 billion in 2010 (UnitedHealth Center for Health Reform and Modernization 2010). Average annual spending over the next decade by payer type is $103 billion for private health insurance, $204 billion for Medicare, $11 billion for Medicaid and $16.6 billion for the uninsured.
What about cardiovascular disease and rheumatoid arthritis? Among the top ten health conditions requiring treatment for Medicare beneficiaries in 2006 approximately 50% of beneficiaries suffered from hypertension, 25% from heart conditions, 33% had hyperlipidemia 24% had COPD, 23% had osteoarthritis and 22% had diabetes (Thorpe et al. 2010). The American Heart Association estimates the 2010 cost of cardiovascular disease and stroke to be $324 billion in direct expenditures and $41.7 billion for productivity losses due to morbidity and $137.4 billion in lost productivity due to mortality (present value of lost wages at 3%) (Lloyd-Jones et al. 2010). The Centers for Disease Control estimates that during 2007–2009 50 million Americans had self-reported doctor diagnosed arthritis, 21 million of them with activity limitations (Cheng et al. 2010). Cisternas and colleagues estimated that total expenditures by US adults with arthritis increased from $252 billion in 1997 to $353 billion in 2005. Most of the increase was attributable to people who had co-occurring chronic conditions (Cisternas et al. 2009). The Cisternas study appears to aggregate all medical care expenditures by people with arthritis (which would include expenditures to treat diabetes and cardiovascular disease). An earlier CDC study focused on the direct and indirect costs in 2003 attributable to arthritis that estimated $80.8 billion in direct costs (medical expenditures) and $47 billion in indirect costs (lost earnings) (Yelin et al. 2007).
In short, current cost estimates for direct health care expenditures (excluding productivity losses) related to diabetes are approximately $190 billion, for cardiovascular treatment, $324 billion, and for rheumatoid arthritis, approximately $111 billion (estimating that the $80.8 billion in 2003 costs have grown approximately 6% per year), a total of $625 billion of the $2.6 trillion that will be spent in the US in 2010. Moreover, given current growth in the prevalence of diabetes, the UnitedHealth estimate of $500 million in 2020 spending for diabetes alone is not unreasonable. If health care costs attributable to diabetes, cardiovascular disease and rheumatoid arthritis only increase by 100% over the next decade (even given added demand produced by the aging baby boomer population), annual costs of these chronic diseases will exceed $1.2 trillion in 2020.
If we use the UnitedHealth estimates for the proportions of diabetes costs paid by private insurance (48%), Medicare (38%), Medicaid (6%) and the uninsured (8%) and estimate total costs based on the 2010 studies projecting a 50% increase in 5 years and a 100% increase in 10 years we can obtain an estimate of future costs for treating diabetes, cardiovascular disease and arthritis. Table 4.1 set forth below, summarizes these cost estimates. By 2020 Medicare costs for these chronic illnesses would be approximately $475 billion. The estimated costs to Medicaid will be approximately $75 billion. The costs for the uninsured will be approximately $100 billion. Any intervention that has the potential to substantially reduce these costs will produce meaningful results.
Table 4.1.
US Medical care cost estimates for diabetes, cardiovascular disease and arthritis (millions of dollars)
| 2010 | 2015 | 2020 | ||
|---|---|---|---|---|
| Diabetes | 190 | 285 | 380 | |
| Cardiovascular | 324 | 486 | 648 | |
| Arthritis | 111 | 167 | 222 | |
| Total | $625 | $938 | $1,250 | |
| Private | 0.48 | 300 | 450 | 600 |
| Medicare | 0.38 | 238 | 356 | 475 |
| Medicaid | 0.06 | 38 | 56 | 75 |
| Uninsured | 0.08 | 50 | 75 | 100 |
Unfortunately, even though there had been a substantial numbers of studies that show relationships between dental disease and chronic illness that are have been very few studies that actually test whether improved dental treatment reduces the incidence of chronic illness and complications due to chronic illness. The potential for large health care cost savings through an active and aggressive program of dental care is so large that such studies are clearly indicated.
Potential Benefits of an Aggressive Dental Treatment Plan
Suppose, for example, that 10% of all medical care costs required to treat diabetes, cardiovascular disease and arthritis could be avoided through an active aggressive program of dental care.4 What this would mean is that in 2020 private health insurers could see a $60 billion reduction in healthcare costs, Medicare would see a $47.5 billion reduction and Medicaid pay $7.5 billion reduction. Recent health reform has provided for the issuance of health insurance to the uninsured by state exchanges. Aggressive dental care that saved 10% of costs attributable to diabetes, cardiovascular disease and arthritis could save the exchanges $10 billion per year. And, if greater proportions of costs can be saved or if the 2020 estimates of costs are low, potential benefits will be even larger. Once again, it would be important to know whether aggressive dental care could produce such savings and how much.
Costs of an Aggressive Dental Treatment Plan
So what do we mean by an aggressive dental treatment plan? Suppose we were to provide dental insurance to all Medicare beneficiaries at the level of current private dental insurance coverage and strongly encourage beneficiaries to receive dental treatment. Suppose we were to provide for Medicaid payment for all beneficiaries at the level of current private dental insurance coverage. Suppose health care insurers provided dental coverage in order to reduce their costs and that such coverage was consistent with current private dental insurance coverage. Suppose health insurance companies, understanding the benefits from dental care, were to require their private employer customers to cover the costs of dental care. How much would all of this cost? How would it compare to the benefits that may be available?
In order to estimate the potential costs of providing enhanced coverage for dental care we start use the CMS estimates of national health care spending for dental services and Statistical Abstract of the US estimates for Medicare enrollment, Medicaid enrollment, private health insurance enrollment and uninsured persons. Based on the estimate that half of private employers with health insurance provided dental insurance coverage we estimate that of the private health insurance enrollment one half would have dental insurance coverage and one half would not. Table 4.2 sets forth the national health care expenditures for dental services in millions and enrollment in private dental plans, Medicare, Medicaid, the uninsured without health insurance and dental insurance, the uninsured with health insurance and dual eligibles.
Table 4.2.
Estimated cost to provide full dental coverage
| Spending/millions | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|---|---|---|---|---|
| Private | 31,175 | 34,158 | 36,464 | 37,359 | 40,472 | 42,871 | 45,137 | 47,836 | 49,142 | 49,960 |
| Medicare | 81 | 86 | 79 | 70 | 71 | 86 | 103 | 164 | 222 | 290 |
| Medicaid | 2,312 | 3,124 | 3,467 | 3,745 | 4,005 | 4,229 | 4,378 | 4,758 | 5,818 | 7,147 |
| Enrollment in millions | ||||||||||
| Private dental coverage | 101 | 100 | 100 | 99 | 100 | 101 | 101 | 101 | 101 | 101 |
| Medicare | 38 | 38 | 38 | 40 | 40 | 40 | 40 | 41 | 43 | 43 |
| Medicaid | 30 | 32 | 33 | 36 | 38 | 38 | 38 | 40 | 43 | 49 |
| Uninsured | 40 | 41 | 44 | 45 | 44 | 45 | 47 | 46 | 46 | 50 |
| Uninsured w/health cov | 101 | 100 | 100 | 99 | 100 | 101 | 101 | 101 | 101 | 101 |
| Dual eligible | 12 | 11 | 11 | 11 | 8 | 9 | 9 | 9 | 9 | 9 |
| Per enrollee cost | ||||||||||
| Private | 310 | 342 | 366 | 378 | 403 | 426 | 448 | 476 | 487 | 495 |
| Medicare | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 4 | 5 | 7 |
| Medicaid | 78 | 99 | 104 | 105 | 105 | 111 | 114 | 120 | 137 | 147 |
| Pct cost increase | ||||||||||
| Private | 10.2% | 7.2% | 3.0% | 6.7% | 5.8% | 5.0% | 6.3% | 2.2% | 1.7% | |
| Medicaid | 26.2% | 5.6% | 0.7% | 0.2% | 5.3% | 3.0% | 5.1% | 13.7% | 7.5% | |
| Cost to cover/millions | ||||||||||
| Medicare | 7,913 | 9,268 | 9,839 | 10,559 | 12,635 | 13,314 | 13,929 | 15,340 | 16,368 | 16,589 |
| Medicaid | 6,835 | 7,675 | 8,700 | 9,696 | 11,305 | 12,007 | 12,764 | 14,091 | 14,909 | 16,943 |
| Uninsured | 12,340 | 14,080 | 15,978 | 16,990 | 17,526 | 19,091 | 21,036 | 21,752 | 22,527 | 24,881 |
| Uninsured w/health cov | 31,175 | 34,158 | 36,464 | 37,359 | 40,472 | 42,871 | 45,137 | 47,836 | 49,142 | 49,960 |
| Cost incl admin | ||||||||||
| Medicare 6.2% | 8,404 | 9,843 | 10,450 | 11,214 | 13,419 | 14,139 | 14,792 | 16,291 | 17,382 | 17,618 |
| Medicaid 7.6% | 7,354 | 8,258 | 9,361 | 10,433 | 12,164 | 12,919 | 13,734 | 15,162 | 16,042 | 18,231 |
| Uninsured-exchanges 7.6% | 13,278 | 15,150 | 17,193 | 18,281 | 18,858 | 20,542 | 22,634 | 23,405 | 24,240 | 26,772 |
| Uninsured w/health cov | 33,545 | 36,754 | 39,235 | 40,199 | 43,548 | 46,129 | 48,567 | 51,471 | 52,877 | 53,757 |
From this we derive a cost per enrollee for private dental insurance, Medicare dental benefits and Medicaid dental benefits. Table 4.2 also sets forth the calculations for 2000–2009. For example, per beneficiary costs in 2009 for private health dental insurance was $494.66. As expected given the lack of Medicare coverage and the low level of Medicaid coverage, per beneficiary expenditures in 2009 were $6.73 for Medicare beneficiaries and $146.75 for Medicaid beneficiaries.
In order to estimate the annual cost of providing full dental coverage to Medicare beneficiaries we subtracted dual eligibles (who receive some dental insurance) from total Medicare enrollees to determine the number of persons who would need coverage. In our 2009 example there were 43 million Medicare beneficiaries including 9 million dual eligibles. Accordingly, the estimates would cover the 34 million Medicare beneficiaries that are not dual eligible at a cost equal to the per capita cost of private dental insurance ($494.66) less amounts that Medicare is already paying for dental services ($6.73 per person). The result provides an estimate of the cost of covering all Medicare beneficiaries for dental services at a level equivalent to private health insurance. Using the 2009 example the cost of providing full dental insurance coverage to Medicare beneficiaries would have been $16.6 billion.
In addition, we used the CMS national health expenditure figures to determine administrative costs for private health insurance, Medicare and Medicaid as a percentage of program expenditures for medical care. We found that the administrative costs of the Medicare program were 6.2% on average for 1966–2009. In order to fully estimate the cost of Medicare dental coverage we added 6.2% to the cost estimates. In 2009, for example the added cost of providing full dental insurance coverage to 34 million Medicaid beneficiaries would have been $17.6 billion.
Similarly, we calculated the per person cost of bringing Medicaid payment for dental services up to the level of private dental insurance. To do this we deducted the per capita amounts provided to Medicaid beneficiaries for dental services from the amounts paid on behalf of private health insurance beneficiaries and multiplied the difference by the number of Medicaid beneficiaries in the US. For example, in 2009 there were 48.7 million Medicaid beneficiaries. The cost of upgrading their dental insurance benefits which have been 48.7 million times $494.66 less $146.75 or $16.9 billion. After adding administrative costs of 7.6% the cost of upgrading Medicaid to private insurance levels in 2009 would have been $18.2 billion.
Health insurers will be in the same position as Medicare and Medicaid regarding dental coverage. If quality dental coverage saves health care costs attributable to diabetes, cardiovascular disease and rheumatoid arthritis then the exchanges will have an incentive to provide quality dental coverage to reduce costs. Accordingly, we estimated the cost of providing dental coverage equivalent to private dental insurance coverage through the exchanges. Again we assume that the costs of such coverage will be equivalent to the number of uninsured persons multiplied by the annual per capita cost of coverage.4 For the 2009 example, this would reflect coverage for 52 million people at $494.66 per person, a total of $24.9 billion. With administrative costs, the cost of providing dental insurance coverage to the uninsured at a level equivalent to private dental coverage would be $26.8 billion.
Finally, given the evidence that improved dental care has the potential to reduce health care costs private health insurers may wish to expand health insurance to cover dental care.4 Here, we estimate the cost of providing dental insurance to the 50% of the workforce whose employers currently do not provide dental insurance benefits. Once again, we multiply the number of covered lives by the estimated annual per capita cost. For the 2009 example we estimate 101 million adults will receive dental coverage at $495 per person: $50 billion for dental services and $3.8 billion for administrative costs or a total of $53.8 billion.
Of course, as noted a number of times above, these estimates are based on providing full “universal” dental insurance coverage at levels equivalent to current benefit levels for private dental insurance. It may be that an appropriate package of dental services that deals specifically with periodontitis can be provided for less than the full cost of private dental insurance. Once again, further research should provide better information.4
Comparing Costs and Benefits
As noted in Sect. 6 above, 2010 costs for diabetes, cardiovascular disease and arthritis will be $300 billion for private health insurance, $238 billion for Medicare, $38 billion for Medicaid and $50 billion for the uninsured. Costs of providing “full” dental coverage will be $17.6 billion for Medicare, $18.2 billion for Medicaid, $26.8 billion for the uninsured and $53.8 billion for private health insurance. Given this, if 7.4% or more of the Medicare costs can be “saved” through improved dental care, Medicaid dental insurance will pay for itself and will provide a positive return on investment. See Table 4.3. Similarly, private health insurers could justify providing dental insurance coverage to employees who do not have it so long as they spend 17.9% or more of their chronic care costs for diabetes, cardiovascular disease and arthritis. On the other hand, it would appear that Medicaid expansion would require cost savings of approximately 48% and that health care insurance coverage of the uninsured would require savings of approximately 54% in order to justify coverage. While it is possible, it may not be likely that full dental coverage would be justified for these programs.
Table 4.3.
Estimated medical care costs, expanded dental coverage costs and percent of medical costs that would need to be saved to justify coverage
| Medical care costs | Insurance costs | Percent medical | |
|---|---|---|---|
| Private | 300 | 53.8 | 17.9% |
| Medicare | 238 | 17.6 | 7.4% |
| Medicaid | 38 | 18.2 | 48.5% |
| Uninsured | 50 | 26.8 | 53.6% |
Of course, these estimates do not consider indirect costs in the form of lost wages or premature death. These costs are externalities to the health insurance programs. To the extent that they represent a social benefit that a national dental insurance program might internalize, it would be appropriate to consider their impact in the cost-benefit analysis.
In any event, better understanding of the potential for deriving savings in health insurance costs related to chronic diseases like diabetes, cardiovascular disease and arthritis would be crucial to any determination whether to expand insurance coverage for dental care.
Expanded Dental Insurance Coverage
Heretofore the case for expanding Medicare coverage to include dental care has taken the form of “benefit” to patients rather than benefit to health insurance programs and society and has been cast in emotional and political terms. For example, Oral Health America grades “America’s commitment to providing oral health access to the elderly” (Oral Health America 2003). In truth, there is no American commitment to providing oral health access to any age group, much less the elderly. Rubenstein notes that “at least one commentator has suggested that the dental profession should join with senior citizen groups when the time is right to ask Congress to expand Medicare to cover oral health” (Rubenstein 2005). Rubenstein emphasizes that “calls for action” are “mere words” unless they are accompanied by political actions that health policy professionals and the dental profession must help promote (Rubenstein 2005). Another commentator has suggested that “as soon as the debate over Medicare prescription drug coverage and, the debate to provide dental care coverage for the elderly may soon begin” (Manski 2001). Rubenstein, again suggests that “the dental community must convince Americans, and particularly aging boomers, that oral health is integral to all health, and for that reason, retiree dental benefits are an important issue”.
In truth, a decade of deficit spending and public distaste for out of control program costs in the Medicare and Medicaid programs as well as the unpopularity of the process that was used to provide Medicare prescription drug coverage (with perceived abuses by the health insurance and drug lobbies) and national health reform makes it unlikely that the public would be willing to approve expansions in insurance coverage for dental care “for its own sake” or “as the right thing” or to “benefit seniors.” What this political climate has produced is an arena in which a good idea that could provide appropriate return on investment for society might well be rejected out of hand based on political history of health insurance coverage. As a result, it is incumbent on policymakers, medical and dental research scientists and health economists to investigate and confirm the potential savings that expansion of dental insurance coverage has the potential to produce and to develop hard evidence regarding potential costs of the expansion prior to, not as a part of, political efforts aimed at dental coverage expansion. A responsible, well informed effort to expand dental coverage may well go far to restore public confidence in the health policy process.
Economics of Clinical Data Integration
Introduction
The adage of “putting your money where your mouth is” is often referenced when being challenged about public statements or claims. In this instance, we use it literally. In 2008 health care costs in US were $2.2 Trillion. There have been numerous reports on health disparities, the burden of chronic diseases, increasing healthcare costs and the need for change. Long-term economic benefits associated with the cost of care are dependent upon integrating oral health with medicine. This is particularly true as it relates to the management of those conditions which impact the economics of healthcare the most. As examples, 96% of Medicare costs and 83% of Medicaid costs are in managing chronic health conditions (Partnership for Solutions National Program Office 2004). More than 40% of the U.S. population has one or more chronic condition (Cartwright-Smith 2011) and in 2006, 76% of Medicare spending was on patients with five or more chronic diseases (Swartz 2011). Effective management of health care resources and information are critical to the economic well-being of our healthcare system. We can no longer afford to manage care in isolation. Integration of care between medicine and dentistry holds much promise in terms of reducing the cost of care and an integrated Medical-Dental Electronic Healthcare Record (iEHR) is the vehicle that will lead to downstream cost savings.
The Economics of Integrated Decision Making
In the United States the Center for Medicare & Medicaid Services (CMS) has conducted demonstration projects around chronic disease management. Section 121 of the Benefits Improvement and Protection Act of 2000 mandated CMS to conduct a disease management demonstration project. April 1, 2005, as an effort to reduce the cost of care and improve quality associated with chronic diseases, CMS partnered with ten premier health systems to effectively manage chronic diseases in a Medicare Physician Group Practice Demonstration (PGP). It was the first pay-for-performance initiative for physicians under the Medicare program (Center for Medicare and Medicaid Services 2010). It involved giving additional payments to providers based on practice efficiency and improved management of chronically ill patients. Participants included ten multispecialty group practices nationwide, with a total of more than 5,000 physicians, who care for more than 200,000 Medicare beneficiaries (Frieden 2006). The chronic diseases that were targeted were based on occurrence in the population and included diabetes, heart failure, coronary artery disease, and hypertension (Frieden 2006). The partners CMS selected were; Billings Clinic, Billings, Montana Dartmouth-Hitchcock Clinic, Bedford, New Hampshire; The Everett Clinic, Everett, Washington; Forsyth Medical Group, Winston-Salem, North Carolina; Geisinger Health System, Danville, Pennsylvania; Marshfield Clinic, Marshfield, Wisconsin; Middlesex Health System, Middletown, Connecticut; Park Nicollet Health Services, St. Louis Park, Minnesota; St. John’s Health System, Springfield, Missouri; University of Michigan Faculty Group Practice, Ann Arbor, Michigan. Under the PGP, physician groups continued to be paid under regular Medicare fee schedules and had the opportunity to share in savings from enhancements in patient care management. Physician groups could earn performance payments which were divided between cost efficiency for generating savings and performance on 32 quality measures phased in during the demonstration as follows: year 1, 10 measures, year 2, 27 measures and years 3 and 4 having 32 quality measures. For each of the 4 years only the University of Michigan Faculty Group Practice and Marshfield Clinic, earned performance payments for improving the quality and cost efficiency of care. A large part of the success of this project was attributed to being able to extract, evaluate, and monitor key clinical data associated with the specific disease and to manage that data through an electronic health record (Table 4.4).
Table 4.4.
A table of the quality measures from the PGP initiative
| Diabetes mellitus | Congestive heart failure | Coronary artery disease | Preventive care |
|---|---|---|---|
| HbA1c management | Left ventricular function assessment | Antiplatelet therapy | Blood pressure screening |
| HbA1c control | Left ventricular ejection fraction testing | Drug therapy for lowering LDL cholesterol | Blood pressure control |
| Blood pressure management | Weight management | Beta-Clocker therapy – prior MI | Blood pressure control plan of care |
| Lipid measurement | Blood pressure screening | Blood pressure | Breast cancer screening |
| LDL cholesterol level | Patient education | Lipid profile | Colorectal cancer screening |
| Urine protein testing | Beta-blocker therapy | LDL cholesterol level | |
| Eye exam | Ace inhibitor therapy | Ace inhibitor therapy | |
| Foot exam | Warfarin therapy for patients HF | ||
| Influenza vaccination | Influenza vaccination | ||
| Pneumonia vaccination | Pneumonia vaccination | ||
During the third year of the demonstration project Marshfield Clinic, using a robust electronic health record succeeded in saving CMS $23 million dollars; that’s one clinic system in 1 year. As a result of such demonstration projects and as of this writing, CMS is looking to establish Accountable Care Organization’s as the medical front runners to new care delivery methods for quality and cost control. Accountable Care Organization (ACO) is a term used to describe partnerships between healthcare providers to establish accountability and improved outcomes for the patients. In a CMS workshop on October 5, 2010, Don Berwick, the administrator of CMS, stated “An ACO will put the patient and family at the center of all its activities…” An emerging model of an ACO is the patient-centered medical home (PCMH). PCMH is at the center of many demonstration projects. ACOs were derived from studies piloted by CMS. Since funds provided by CMS, do not cover routine dental care as part of the patient management or quality and cost objectives CMS ACO studies are limited if they become models for the PCMH, due to the exclusion of dental.
More recently, organizations representing the major primary care specialties – the American Academy of Family Practice, the American Academy of Pediatrics, the American Osteopathic Association, and the American College of Physicians – have worked together to develop and endorse the concept of the “patient-centered medical home,” a practice model that would more effectively support the core functions of primary care and the management of chronic disease (Fisher 2008). In 2011 Geisinger Health System, Kaiser Permanente, Mayo Clinic, Intermountain Healthcare and Group Health Cooperative announced they will be creating a project called the Care Connectivity Consortium. This project is intended to exchange patient information. Although progressive in their approach their project does not include dental.
These benefits however, are yet to be adapted in the arena of oral health. As of this writing, dentistry remains largely separate from medical reimbursement mechanisms such as shared billing, integrated consults, diagnosis, shared problem lists, and government coverage. For example, CMS does not cover routine dental care. Dentistry is also working to establish its own “dental home” with patients. However to reap the economic benefits of integrated care, a primary care “medical-dental” home is what needs to be created.
According to an Institute of Oral Health Report (2010) it is widely accepted across the dental profession that oral health has a direct impact on systemic health, and increasingly, medical and dental care providers are building to bridge relationships that create treatment solutions. The case for medical and dental professionals’ co-managing patients has been suggested for almost the past century, in 1926 William Gies reported that “The frequency of periodic examination gives dentists exceptional opportunity to note early signs of many types of illnesses outside the domain of dentistry” (Gies 1926). As described by Dr. Richard Nagelberg, DDS “The convergence of dental and medical care is underway. Our patients will be the beneficiaries of this trend. For too long, we have provided dental care in a bubble, practicing – to a large degree – apart from other health-care providers. Even when we consulted with our medical colleagues, it was to find out if premedication was necessary, get clearance for treatment of a medically compromised patient, or find out the HbA1c level of a diabetic individual, rather than providing true patient co-management. We have made diagnoses and provided treatments without the benefit of tests, reports, metrics, and other information that predict the likelihood of disease development and progression, as well as favorable treatment outcomes. We have practiced in this manner not due to negligence, but because of the limitations of tools that were available to us” (Nagelberg 2011). Integrated medical/dental records need to be a tool in a providers’ toolbox. In the case of Marshfield Clinic, dental was not included in their past CMS demonstration project as dental is not a CMS covered benefit, and thus not part of the demonstration. However, as a leader in healthcare, the Marshfield Clinic recognizes the importance of data integration for both increased quality and cost savings. “Marshfield Clinic believes the best health care comes from an integrated dental/medical approach,” said Michael Murphy, director, Business Development for Cattails Software. Integration enhances communication between providers and can ultimately lead to better management of complex diseases with oral-systemic connection, avoidance of medical errors, and improved public health.
While the CMS PGP and other demonstration projects along with independent studies have shown to improve quality and reduce costs through integration, greater results may be afforded if studies are not done in isolation from dental data. In fact, if healthcare does not find a way to manage the systemic nature of the 120 pathogens known to the oral cavity the economic impact and cost savings around chronic disease management will hit a ceiling. The economic opportunity of having clinical data for integrated decision making is readily identified by the insurance industry. The effective management of clinical data around chronic and systemic oral and medical disease as part of an iEHR is the greatest healthcare cost savings opportunity associated with such a tool.
Insurance Industry Studies Show the Way
The insurance industry sustains itself through risk management [obtaining best outcomes] using actuarial analysis [data] and controlling costs [reduction of costs] in order to ensure coverage [profitability]. As such they have pursued the economic and outcome benefits of integrated medical – dental clinical decision making. As an example, in 2009 there was a study conducted by the University of Michigan, commissioned by the Blue Cross Blue Shield of Michigan Foundation (2009), the study included 21,000 Blue Cross Blue Shield of Michigan members diagnosed with diabetes who had access to dental care, and had continuous coverage for at least 1 year. With regular periodontal care, it was observed diabetes related medical costs were reduced by 10%. When compounding chronic health complications were also examined, the study showed a 20% reduction in cost related to the treatment of cardiovascular disease in patients with diabetes and heart disease. A 30% reduction in cost related to treatment of kidney disease for patients with diabetes and kidney disease. And a 40% reduction in costs related to treating congestive heart failure for patients with diabetes and congestive heart failure. According to a joint statement by lead researchers, and Blue Cross Blue Shield of Michigan executives, “Our results are consistent with an emerging body of evidence that periodontal disease…it addresses quality of care and health care costs for all Michigan residents.”
Also, at the Institute for Oral Health conference in November 2007 Joseph Errante, D.D.S., Vice President, Blue Cross Blue Shield of MA reported that 2003 Blue Cross Blue Shield of Massachusetts claims data showed medical costs for diabetics who accessed dental care for prevention and periodontal services averaged $558/month, while medical costs for diabetics who didn’t get dental care were about $702/month (Errante 2007). Similarly insured individuals with cardiovascular diseases who accessed dental care had lower medical costs, $238/month lower than people who did not seek dental treatment (Errante 2007). The cost is $144 less per visit for those diabetics who accessed prevention and periodontal services. Those savings could be translated into access to care or additional benefits for more individuals.
In the case of neonatal health there is similar research. Over 12% of all births in the U.S. are delivered preterm, with many infants at risk of birth defects (Martin et al. 2009). According to a January 2006 statement issued by Cigna, announcing their CIGNA Oral Health Maternity Program, “the program was launched in response to mounting research indicating an increased probability of preterm birth for those with gum disease. These research-based, value-added programs are designed to help improve outcomes and reduce expense” (CIGNA 2006). The program was initially designed to offer extended dental benefits free of charge to members who were expecting mothers, citing “research supporting the negative and costly impact periodontal disease has on both mother and baby.” According to research cited by CIGNA, expecting mothers with chronic periodontal disease during the second trimester are seven times more likely to deliver preterm (before 37th week), and the costs associated with treating premature newborns is an average of 15 times more during their first year, and premature newborns have dramatically more healthcare challenges throughout their life. CIGNA also cited the correlation between periodontal disease and low birth weight, pre-eclampsia, gestational diabetes as additional rationale to support extended dental benefits to expecting mothers. Six months later CIGNA initiated Well Aware for Better Health, an extended benefits free of charge program for diabetic and cardiovascular disease patients aimed at “turning evidence into action by enhancing dental benefits for participants in disease management” programs. It is interesting to note, not only does CIGNA offer extended dental benefit to targeted groups, they also reimburse members for any out-of-pocket expenses associated to their dental care (co-pays, etc.)
In 2006, Columbia University researchers conducted a 2-year retrospective study of 116,306 Aetna PPO members with continuous medical and dental insurance, exhibiting one of three chronic conditions (diabetes mellitus, coronary artery disease, and cerebrovascular disease) (Aetna 2008). Researchers found members who received periodontal treatments incurred higher initial per member per month medical costs, but ultimately achieved significantly lower health screening (Episode Risk Group/ERG) risk scores than peers receiving little or no dental care. Convinced by the data and understanding lower risk scores ultimately leads to healthier people and cost savings, Aetna initiated the Dental/Medical Integration (DMI) Program in 2006. Aetna’s DMI program offers enhanced benefits in the form of free-of-charge extended benefit dental care to Aetna’s 37.2 million Indemnity, PPO and Managed Choice medical plan members, specifically targeting members deemed at-risk, including those who are pregnant, diabetic, and/or have cardiovascular disease and have not been to a dentist in 1 year As a result of various outreach methods during the pilot, 63% of at-risk members who had not been to a dentist in the previous 12 months, sought dental care (Aetna 2008). “The findings from this latest study we conducted continue to show that members with certain conditions who are engaged in seeking preventive care, such as regular dental visits, can improve their overall health and quality of life,” said Alan Hirschberg, head of Aetna Dental (Aetna 2008).
Delta Dental of Wisconsin understands the connection between oral and systemic health and has created a program that is designed to offer members with certain chronic health conditions the opportunity to gain additional benefits. More than 2,000 groups now offer Delta Dental of Wisconsin’s Evidence-Based Integrated Care Plan (EBICP) option (Delta Dental of Wisconsin 2011). EBICP provides expanded benefits for persons with diseases and medical conditions that have oral health implications. These benefits include increased frequency of cleanings and/or applications of topical fluoride. They address the unique oral health challenges faced by persons with these conditions, and can also play an important role in the management of an individual’s medical condition. EBICP offers additional cleanings and topical fluoride application for persons who are undergoing cancer treatment involving radiation and/or chemotherapy, persons with prior surgical or nonsurgical treatment of periodontal disease and persons with suppressed immune systems. The EBIC offers additional cleanings for persons with diabetes and those with risk factors for IE, persons with kidney failure or who are on dialysis and for women who are pregnant.
The iEHR provides the insurance industry in partnership with the healthcare industry an integrated tool to facilitate these health and subsequently economic outcomes across medicine and dentistry.
Economic Benefits of Increased Efficiency and Patient Safety Through iEHR
In addition to the anticipated savings through better outcomes using integrated clinical data, an example of a positive economic outcome associated with an integrated record as related to increased efficiency and patient safety is found in the United States Veterans Administration (VA) hospitals and clinics. The VA is one of the few institutions that have implemented the shared electronic medical–dental record successfully. The VA has the ability to be the “one stop shop” for their patients. An April 2010 press release published on the Department of Veterans Affairs website highlighted the success of VA’s health information technology in terms of cost reductions and “improvements in quality, safety, and patient satisfaction” (Department of Veterans Affairs 2010). The press release spotlighted a recent study conducted by the public health journal, Health Affairs, which focused on VA’s health IT investment from 1997 to 2007. The study confirmed that while VA has spent $4 billion on their technology initiative, a conservative estimate of cost savings was more than $7 billion. After subtracting the expense of the IT investment, there was a net savings of $3 billion for the VA during the 10 years covered by the study (McBride 2011). Furthermore, the study estimated that “more than 86 percent of the savings were due to eliminating duplicated tests and reducing medical errors. The rest of the savings came from lower operating expenses and reduced workload.” Independent studies show that the VA system does better on many measures, especially preventive services and chronic care, than the private sector and Medicare. VA officials say “its [integrated] technology has helped cut down hospitalizations and helped patients live longer” (Zhang 2009).
Recently, the Journal of Obstetrics and Gynecology reported on a tragic loss of life due to the systemic nature of oral health. A study found oral bacteria called Fusobacterium nucleatum was the likely culprit in infecting a 35-year-old woman’s fetus through her bloodstream (Carroll 2010). The doctors determined that the same strain of oral bacteria found in the woman’s mouth was in the deceased baby’s stomach and lungs. Integrated records would provide critical data to the Obstetrician including oral health issues and when the patient had her last dental exam. How does one measure the economic impact of a life not lived and another derailed by such tragedy?
In a randomized controlled study, Lopez et al. (2005) determined that periodontal therapy provided during pregnancy to women with periodontitis or gingivitis reduced the incidence of preterm and of low birth weight. The institute of Medicine and National Academies estimate that preterm births cost society at least $23billion annually (Albert et al. 2011). Data integration of the iEHR enables the effective management between the dentist and obstetrician to ensure proper periodontal therapy has been provided during pregnancy. Such management based on the Lopez et al. study, will have direct impact in reducing the prevalence per preterm births leading to reduced health care costs.
There have also been studies indicating a correlation between poorer oral hygiene or deficient denture hygiene and pneumonia or respiratory tract infection among elderly people in nursing homes or hospitals (Rosenblum 2010; Ghezzi and Ship 2000; Scannapieco 2006). One such study of 141 elderly persons in two nursing homes in Japan (Adachi et al. 2002) concluded that “the number of bacteria silently aspirating into the lower respiratory tract was lower in the group who received professional oral care, which resulted in less fatal aspiration pneumonia in that group.” Over the 24 month period of the study, of the 77 patients receiving professional oral care, 5% died of pneumonia versus 16.7% of the 64 patients that died of the same cause who maintained their own oral hygiene. Lack of access is certainly a key factor to consider. However, lack of available data respective to the interrelationship between oral health and systemic health also contributed to the apathy in these cases.
As identified above, complications are correlated to cost. As conditions compound, costs go up. Marshfield Clinic, as part of their iEHR is creating a shared problem list that identifies both oral and medical conditions and history to recent visits and medication lists for monitoring at point of care [be it a medical or dental visit], such cross access to clinical data and care management milestones serves as a tool to prevent conditions from compounding and escalating costs such as those described above.
Other Areas of Economic Impact Relative to iEHR Clinical Data
Several other areas of economic impact will be seen as iEHR’s become broadly deployed. Some of these are listed as follows:
Medication management. A great deal of provider and allied support time is spent obtaining medication information between dentistry and medicine [and vice versa] including current medications, contraindications, tolerances, etc. Marshfield Clinic Cattails software has created a dashboard that readily identifies this for both the medical and dental providers. Not is time saved but chances for complications or escalation of conditions is reduced [both of which impact cost]. For example an integrated record allows medical providers treating respiratory infections to include or exclude oral flora as the possible source of the infection which would lead to more knowledgeable prescribing decision on the antibiotic used.
Coordination of care has a direct impact on cost for the system and the patient. For example, in 2008 55.6% of the US population aged 2 years and older that was diagnosed diabetes had been to the dentist in the past year (Healthy People 2020 (2010)). The US government’s program Healthy People 2020 includes an initiative to increase the proportion of people with diagnosed diabetes who have at least an annual dental examination. The American Diabetes Association recommends that diabetic patients be seen semi-annually and more if bleeding gums or other oral issues are present. The American Diabetes Association also recommends the consultation between the dentist and doctor to decide about possible adjustments to diabetes medicines, or to decide if an antibiotic is needed before surgery to prevent infection. The target from the Healthy People 2020 is a 10% improvement at 61.2%. Integrated medical/dental records could allow for the coordination of efforts between providers to include communication of treatment plan and services leading to quicker resolution, increased patient compliance, and less patient time away from work or home and potentially less travel.
Similarly, integrated records also create a platform to integrate clinical appointing between medicine and dentistry. As such, combative patients or severely disabled patients needing anesthesia in order for care to be delivered can be treated with one hospital sedation vs. multiple sedations. Family Health Center of Marshfield, Inc. (FHC) Dental Clinics shares an iEHR with Marshfield Clinic and uses it integrated scheduling feature to complete dental care, lab work, ENT care, woman’s health, preventive studies, all in one visit.
Follow up care management can be more focused and coordinated. For example, without the knowledge or dental conditions, medical providers could spend months attempting to control diabetes with periodontal disease. However, with access to an iEHR, the practitioner or allied care manager can determine patient’s oral health status immediately to determine possible influence of periodontal disease.
Similarly an iEHR with a shared patient data dashboard brings to light history and physical examination data without having to have patients be the historian to their physician on their last dental visit or for the dentist to have to rely on the patient’s recall of medications or medical diagnosis. For example, if an integrated record saved providers 5 min per hour of patient care, that would be 40 min per day. Imagine giving a physician or dentist 40 min more a day. In a capitated system, this allows for more patients to be seen in a day for roughly the same amount of expenditure. In a production based clinic this allows more patients to be seen and more charges per day. In either case, the investment into informatics is covered. In an underserved area, more patients get care quicker, which creates the opportunity for quicker resolution, which can lead to a healthier society, which in turn may lead them back to a productive livelihood sooner.
An iEHR results in one system for acquisition, orientation, training and support. PC based owners who also own a Mac and Mac owners who also have to operate a PC can relate. Need we say more? Imagine if your PC function just like a Mac [or your Mac function just a PC]. No cross learning of software quirks. Not having to purchase two separate units to begin with. Reduced costs, increased space. Not having to jump from one computer to the other computer to get data from one data from another to create a report. Not having to call two separate computer companies for service or updates.
Third Party Coordination. Having an iEHR creates a platform for interfacing with third party payers. A common system and language for timely reimbursement. In part, the result of an iEHR is driving the diagnostic coding for dentistry. Such an integrated interface provides a tool to bridge with healthcare payors that historically kept payment as segregated as the oral and medical health professions. The iEHR overcomes that limitation. Timely payment, consolidation of payment, expansion of covered patient and provider benefits based on clinical integration, and a viable system for interfacing are all potential economic benefits of iEHR clinical data.
- The iEHR creates new horizons for research that will lead to cost saving discoveries. As example, knowing the benefits of research, Marshfield Clinic Research Foundation (MCRF) has created an Oral and Systemic Health Research Project (OSHRP). The creation of OSHRP, led by Dr. Murray Brilliant, will allow MCRF to capitalize on its existing and growing strengths in the areas of complex disease interactions and Personalized Health Care (PHC) to advance oral health and the health of the rest of the body. The OSHRP has three specific goals:
- Understand the connections between oral and systemic health (diabetes, heart disease, pre-term births)
- Understand the causes of oral diseases and determine the effect of genetics, diet, water source (well/city + fluoridation) and microbiome.
- Understand how improving oral health aids systemic health (comparative effectiveness) and bring Personalized Health Care (PHC) to the dental arena.
The OSHRP research resource will be unique in the nation. As MCRF has done with other projects, it will share this resource with qualified investigators at other academic institutions both within and outside of Wisconsin. OSHRP will advance scientific knowledge, improve healthcare and prevention, reduce the cost of oral healthcare, and create new economic opportunities. Such knowledge will have a direct economic impact on the cost of care and care management.
The iEHR creates an ability to have an integrated patient portal to comprehensively maintain their health. Portals are becoming more and more popular in the healthcare industry as a means to helping maintain compliance with care management recommendations and preventative procedures. Portals provide patents a tool to stay up to date on their care and recommendations. Portals can take iEHR clinical data, adapt it through programming, and provide creative visual reinforcement for patients as they monitor their health status. The more patients engage in owning their health status, the more preventative services are followed through with. The more medicine and dentistry can leverage the prevention potential [which insurance companies have come to realize] the more likely costly conditions can be avoided.
Conclusion
The link between oral health and systemic health is well documented. The separation of dental and medical is not a sustainable model in modern healthcare delivery. A new model of integrated care is necessary. Aristotle said, “The whole is greater than the sum of its parts.” Increased access to combined medical and dental histories and diagnosis at the providers’ fingertips makes vital information available. Shared diagnosis between physicians and dentists could aid in formulating interventions and to accelerate decision making abilities by allowing for prioritizing of medical/dental procedures. Clinical management and treatment of the patient would be expedited with immediate access to both records. Quality could be improved through a complete picture of the patient through the dashboard. All of which have a direct or indirect economic benefit.
The iEHR will be the tool that facilitates such delivery and the studies and scenarios described in these pages point to significant economic benefits to patients, payors, and providers. If increased access, multi-provider monitoring, shared problems lists with enhanced decision making abilities from iEHR could reduce healthcare costs. The greatest cost reduction will be with using the iEHR to manage chronic disease. A combined dental-medical electronic record with a shared data informatics platform is most likely to yield the best long-term economic solution while maintaining or enhancing positive patient outcomes.
Provider Viewpoints
This section reveals viewpoints from a variety of medical and dental providers. One section focuses on optimal use of ophthalmic imaging, which should show how that the challenges of clinical data integration go beyond those encountered in the effort to bring oral health and systemic health together.
Integration of Pediatric Medical and Pediatric Dental Care
Wendy E. Mouradian, Suzanne Boulter, Paul Casamassimo, Valerie J. Harvey Powell
Oral health is an important but often neglected part of overall health. Historically separate systems of education, financing and practice in medicine and dentistry fuel this neglect, contributing to poorer health outcomes for vulnerable populations such as children, while increasing costs and chances for medical error for all patients. Advances in understanding the impact of oral health on children’s overall health, changing disease patterns and demographic trends strengthen the mandate for greater integration of oral and overall healthcare, as reviewed in two recent Institute of Medicine reports (IOM 2011a, b). The pediatric population could realize substantial benefit from oral disease prevention strategies under a coordinated system of care enhanced by integrated electronic health records (EHR). This approach would benefit all children but especially young children and those from low socioeconomic, minority and other disadvantaged groups who are at higher risk for oral disease and difficulties accessing dental care.
This section focuses on the pediatric population and the need for close collaboration of pediatric medical and dental providers. First we consider how a child’s developmental position and their parents’ level of understanding might affect oral health outcomes. Next we address the importance of children’s oral health and the urgency of seizing missed opportunities to prevent disease. We then briefly outlines some steps to preventing early childhood oral disease utilizing some of the many health providers that interact with families. Finally we examine one pediatric hospital’s approach to choosing an integrated EHR technology.
A Childs-Eye View of Oral Health
Children have unique characteristics which distinguish their needs from those of adults. Children’s developmental immaturities may increase their risks for poor oral health outcomes (Fig. 4.4). For example, a child…
May not be able to communicate pain, discomfort and other symptoms,
May not recognize a particular sensation or lesion as a symptom or sign of disease,
May not grasp the consequences of poor oral health habits,
May not realize the consequences of consuming quantities of sugared foods or beverages,
Likely does not realize the consequences of chronic use of sugared medications,
Does not understand the long-term consequences of early childhood caries,
Does not know the potential for systemic spread of disease from a toothache, or for liver damage due to overuse of acetaminophen or other analgesics,
Must learn good oral health habits from parents/caregivers
Must trust parents/caregivers to judge the appropriateness of and necessity for health care, and
Does not understand how the health care system works and cannot access care without an adult.
Fig. 4.4.
Delivering oral healthcare to a child
All children, but especially young children, are limited in their ability to care for their own health and must depend upon adults. A child’s parent/caregiver may also lack basic oral health knowledge and an awareness of their child’s oral health needs, and/or suffer from poor oral health themselves. Low oral health literacy is prevalent among patients and health professionals alike in America; individuals of low socioeconomic status or from ethnically diverse backgrounds may be at particular risk for low oral health literacy (IOM 2011a). Without appropriate education, a parent….
May not correctly interpret a child’s symptoms or signs of oral disease
May not know that caries is an infectious disease that can be spread to a child by sharing spoons, for example,
May not know the potential value of chewing gum with Xylitol,
May not fully grasp the importance of good oral health hygiene habits,
May not grasp the consequences of a child consuming quantities of sugared foods or beverages,
May have difficulty controlling the child’s consumption of sugared foods or beverages in or out of the home,
May not realize the consequences of chronic use of sugared medications,
May not know the potential for systemic spread of disease from a toothache, or for liver damage due to overuse of acetaminophen or other analgesics,
May not grasp the long-term consequences of early childhood caries,
May live in a community without fluoride in the tap water and not know about alternative sources of fluoride,
May overlook oral health due to the stress of living in poverty,
May be fearful of dentists or oral health care due to their own experiences,
May have difficulty locating a dental provider accepting public insurance, or have other problems navigating the health care system.
Parents in turn depend on access to medical and dental providers with current understanding of the most effective ways to prevent caries and promote the child’s oral and overall health. An important element in helping families is the provision of culturally-sensitive care to a diverse population. Children are the most diverse segment of the population with 44% from minority backgrounds compared with 34% of the overall population (US Census Bureau 2010).
The separation of medical and dental systems and the lack of shared information can create additional barriers for families, especially for those with low health literacy or facing linguistic or cultural barriers. All pediatric health professionals have increased ethical and legal responsibilities to promote children’s health, including advocacy for them at the system level (Mouradian 1999).
Children’s Oral Health: Impact of Missed Opportunities
Although many factors can influence children’s oral health outcomes, caries is largely a preventable disease. Despite this, national trends and other data on children’s oral health attest to this persistent national problem (Mouradian et al. 2009). Some important facts include the following….
Caries is the most prevalent chronic disease of childhood,
Caries is a preventable disease unlike many chronic diseases of childhood,
Yet according to (NICDR 2011) 42% of children 2–11 have had dental caries in their primary teeth; 23% of children 2–11 have untreated dental caries. Further, “21% of children 6–11 have had dental caries in their permanent teeth; 8% of children 6–11 have untreated decay.” Overall “[c]hildren 2–11 have an average of 1.6 decayed primary teeth and 3.6 decayed primary surfaces,”
The latest epidemiologic evidence shows increasing rates of caries for youngest children, reverse from the Healthy People 2010 goal of decreasing caries. According to (NICDR 2011), overall “dental caries in the baby teeth of children 2–11 declined from the early 1970s until the mid 1990s. From the mid 1990s until the most recent (1999–2004) National Health and Nutrition Examination Survey, this trend has reversed: a small but significant increase in primary decay was found. This trend reversal was more severe in younger children.”
Disparities in children’s oral health and access to care persist by age, income level, race and ethnicity, and parental education level (Edelstein and Chinn 2009). Of concern, the latest increase was actually in a traditionally low-risk group of young children (Dye and Thornton-Evans 2010).
Children with special health care needs (CSHCN) may be at higher risk for oral disease and difficulties accessing care. Analyzing data from the National Survey of Children with Special Health Care Needs, (Lewis 2009) found that “CSHCN are more likely to be insured and to receive preventive dental care at equal or higher rates than children without special health care needs. Nevertheless, CSHCN, particularly lower income and severely affected, are more likely to report unmet dental care need compared with unaffected children.” Children who were both low-income and severely affected had 13.4 times the likelihood of unmet dental care needs,
Dental care is the highest unmet health care need of children; 4.6 million children had unmet dental care needs because families could not afford care compared with 2.8 million with unmet medical needs for the same reasons (CDC 2008),
According to the National Survey of Children’s Health, children are 2.6 times as likely to lack dental as medical insurance (Lewis et al. 2007),
There is evidence that children who get referred to a dentist early may have lower costs of care and disease. Savage et al. (2004) reported that children “who had their first preventive visit by age 1 were more likely to have subsequent preventive visits but were not more likely to have subsequent restorative or emergency visits” and concluded that preschool “children who used early preventive dental care incurred fewer dentally related costs,”
Ramos-Gomez and Shepherd (1999), in their “Cost-effectiveness Model for Prevention of Early Childhood Caries,” conclude that preventive ECC interventions could reduce ECC by 40–80% for a particularly vulnerable population of children, and that part of the costs of interventions will be offset by savings in treatment costs.
As these facts convey, and the deaths of more than one child from consequences of untreated caries make painfully clear, there is an urgent need for more attention to the oral health needs of children. A more coordinated system for oral health care including integrated EHR would be an important advance.
A Model for Intervention: Creating a System of Care
A glance at Table 4.5, an ideal model, reveals that intervention should begin before birth and that a range of medical and oral health professionals can contribute to the child’s oral health. Early intervention is necessary because of the transmissibility of cariogenic bacteria from mother/caregiver to infant, and importance of oral health practice in preventing disease. The following professionals may be involved:
- pediatric medical provider
- family physician
- pediatrician
- pediatric nurse
- nurse practitioner in pediatric/family practice
- physician assistant in pediatric /family practice
obstetrician
obstetric nurse/nurse midwife
general dentist
pediatric dentist
pediatric dental hygienist
pharmacist
other appropriate allied health professionals
Table 4.5.
Timeline of some oral health interventions to prevent early childhood caries (ECC) – birth to 3 years age (Marrs et al. 2011; Lannon et al. 2008; Han et al. 2010; Ezer et al. 2010; AAP 2008; Mouradian et al. 2000)
| Child’s age | Intervening professional(s) | Intervention, rationale, conditions and strategy |
|---|---|---|
| Planning conception, prenatal and perinatal | Family physician | The physician and/or obstetric provider educates mother-to-be about good maternal oral hygiene and infant oral health issues, including transmissibility of caries. Mother’s dentist assesses and treats caries, gingivitis or other oral health problems and educates the mother-to-be |
| Obstetrician/nurse midwife | ||
| Obstetric nurse | ||
| General dentist | ||
| Neonatal | Obstetric nurse | Obstetric nurse advises new mother to chew Xylitol gum, limit salivary contact between mother and infant, and help child avoid sugar intake (exposure) while asleep and from common sugar sources (medicines, sugared water, bottle feeding on demand at night with fluid other than water – following tooth eruption, certain foods), and to schedule dental exam at 1 year age |
| 6 months | Pediatric medical provider | First dental examination recommended by AAPD when the first tooth comes in, usually between 6 to 12 months |
| Pediatric/general dentist | Educate mother about optimal fluoride levels | |
| Dental hygienist or other allied dental professional | Anticipate window of infectivity: 17–36 months; period when deciduous teeth are erupting | |
| Caries- risk assessment tool1 | ||
| Presence of disabilities and other special health care needs may increase child’s risk of disease | ||
| 9 months | Pediatric medical provider refers to establish dental home for child | Assure child has dental home by 1 year |
| 1 year | Pediatric medical | Pediatric or general dentist able to provide infant oral health care may not be available in rural areas or for families of lower socioeconomic status |
| Professional provider delivers preventive oral healthcare if no pediatric /general dentist is available | Caries risk assessment tool1 | |
| Anticipate that ECC can affect children as soon as teeth erupt | ||
| 2 years | Pediatric medical provider | Assure child has dental home |
| Pediatric dentist | If no dental home, then administer oral health risk assessment | |
| Pediatric dental hygienist | Child may begin to use fluoride toothpaste with supervision | |
| Pediatric preventive care vital if pediatric dental care not available | ||
| If primary water source is fluoride-deficient, consider fluoride supplementation or fluoride varnish | ||
| 3 years | Pediatric medical provider | Assure child has dental home |
| Pediatric/general dentist | Pediatric preventive care vital if pediatric dental care not available | |
| Pediatric dental hygienist | If primary water source is fluoride-deficient, consider fluoride supplementation or fluoride varnish |
1Caries-Risk Assessment Tool (CAT) developed by the AAPD http://www.mchoralhealth.org/pocketguide/cat1.html
The availability of some of these professionals can be affected socioeconomic status, health insurance, place of residence, or by a child’s special health care need.
One obvious limitation on developing a “relay” as in Table 4.5, with a “hand-off” from family care to obstetric care to pediatric care is the education of the medical providers. As part of pre-conception and perinatal healthcare, providers should address oral health, but may lack the knowledge to do so. Additionally, as noted by Ressler-Maerlaender et al. (2005), “some women may believe that they or their fetus could be harmed by treatment.” During the prenatal visit, health care providers should:
assess the woman’s oral health status, oral health practices, and access to a dental home;
discuss with the woman how oral health affects general health;
offer referrals to oral health professionals for treatment;
educate the woman about oral health during pregnancy, including expected physiological changes in the mouth and interventions to prevent and relieve discomfort; and
educate the woman about diet and oral hygiene for infants and children and encourage breastfeeding
New York and California have developed state guidelines for perinatal oral health to educate providers and patients (California Dental Association 2010; New York State Department of Health 2006).
A combination of anticipatory guidance, with continuity from prenatal and perinatal care to pediatric care, can help move infant oral health from “missed opportunities” to “seized opportunities.” Others who may be of assistance to families in closing these gaps are professionals at the Women, Infants and Children’s (WIC) supplemental nutrition program, Early Head Start/Head Start and Neurodevelopmental/Birth to Three programs. Together medical, dental and community professionals can help create a system of care to improve maternal and child oral health.
Capacity of the Workforce to Prevent Childhood Caries
For the envisioned model in Table 4.5 to be realized, the mother requires access to a general dentist with accurate information on her oral health during pregnancy and on her infant’s oral health, including the need for an early dental visit. The mother and child then need access to a pediatric medical provider who will provide oral health screening/counseling, and who will guide the family to establishing the child’s dental home by age 1. Success in dental referral requires access to a pediatric or general dentist willing and able to provide infant oral health. (dela Cruz et al. 2004), in a discussion of the referral process mentioned that among the factors in assessing the likelihood of a dental referral were the medical providers’ “level of oral health knowledge, and their opinions about the importance of oral health and preventive dental care.”
Since young children are much more likely to access medical than dental care, the medical provider plays an important role in promoting children’s oral health. (Catalanotto 2010) recommends, as part of a pediatric well child checkup:
an oral screening examination,
a risk assessment, including assessment of the mother’s/caregiver’s oral health,
application of fluoride varnish
anticipatory guidance (parental education) including dietary and oral hygiene information,
attempted referral to a dental home.
The AAP recommends that child healthcare providers be trained to perform an oral health risk assessment and triage all infants and children beginning by 6 months of age to identify known risk factors for early childhood caries (ECC). The oral health component of pediatric care is integrated into the AAP’s “Recommendations for Preventive Pediatric Health Care (Periodicity Schedule)” (AAP 2008).
To what extent are medical and dental and providers aware of recommendations for a first dental visit for a child by age one, as recommended by the AAP, the American Academy of Pediatric Dentistry (AAPD), and the American Dental Association? (Wolfe et al. 2006) reported that 76% of licensed general dentists in Iowa were familiar with the AAPD age 1 dental visit recommendation and that most obtained the information through continuing education; 11% believed that the first dental visit should occur between 0 and 11 months of age. However, according to (Caspary et al. 2008), when pediatric medical residents were asked the age for the first dental visit, the average response was 2.4 years, while 35% reported received no oral health training during residency. In a national survey of pediatricians (Lewis et al. 2009) reported that less than 25% of had received oral health education in medical school, residency, or continuing education. Finally (Ferullo et al. 2010) surveyed allopathic and osteopathic schools of medicine and found that 69.3% reported offering less than 5 h of oral health curriculum, while 10.2% offered no curriculum at all.
Other workforce considerations relevant to preventing early childhood caries include the training of dentists in pediatric oral health (Seale et al. 2009), the number and diversity of the dental workforce, the number of pediatric dentists, and the use of alternative providers such as dental therapists, expanded function dental assistants and dental hygienists (Mertz and Mouradian 2009; Nash 2009).
Examples of integrated care models do exist, such as that presented by (Heuer 2007) involving school-linked and school-based clinics with an “innovative health infrastructure.” According to Heuer, “Neighborhood Outreach Action for Health (NOAH)” is staffed by two nurse practitioners and a part-time physician to provide “primary medical services to more than 3,200 uninsured patients each year” in Scottsdale, Arizona. Heuer counts caries among the “top ten” diagnoses every year.
Mabry and Mosca (2006) described community public health training of dental hygiene students for children with neurodevelopmental/intellectual disabilities. They mentioned that the dental hygiene students had worked together with school nurses and “felt they had impacted the school nurses’ knowledge of oral disease and care.”
Paper Versus EHR: Planning Pediatric EHR Acquisition: the Decision to Acquire an integrated EHR
As pediatric clinicians (both medical and dental) work more closely together, they require appropriate EHR systems that integrate a patient’s medical and dental records. Following is a set of local “best practices” from Nationwide Children’s Hospital in Columbus, Ohio, which may help other children’s hospitals in planning acquisition of an integrated pediatric EHR system. Integrated (medical-dental) EHR technologies are becoming more widely available outside the Federal government sector (see integrated models E1 and E2 in 10.1007/978-1-4471-2185-5_1). Nationwide Children’s ‘drivers’ for the acquisition process were, in 2011:
Minimize registration and dual databases. Patient registration takes time and requiring both a stand-alone dental and a medical patient registration inhibits cost-effective flow of services. Integration allows for the use of single demographics information for all clinics in the comprehensive care system serving the patient. Clinicians always have an updated health history on patients, if they have been a patient of record. If not, and for a dental clinic that sees walk-ins, a brief “critical” dental health history can be completed on paper by a parent and scanned into the eMR. In designing an integrated medical-dental record for patients of record, the system can sort essential health history elements into a brief focused dental history without the detail needed by other medical specialty clinics. Kiosk-driven electronic health histories for those children who are new to clinic similar to those used in airline travel could be considered if feasible in busy clinics.
-
For charting, no more key/mouse strokes than with paper. Some commercial dental record products try to accomplish too much. Moving from paper to electronics should be driven in part by efficiencies. The tooth chart, which is an essential part of any dental record, must be such that examination findings can be transferred quickly and accurately to either paper or electronic capture. A helpful exercise is visualization of the functionality of the charting process, including both the different types of entries (caries, existing restorations and pathology) and how these are entered in the paper world.
If charting will be able to be used for research the system should be able to translate pictures to numerical values, often a complex programming function. Dental practitioners and faculty may want to use drawings of teeth or graphics of surfaces because that is their current comfort level. A true digital charting is possible with no images of teeth, but some habits are hard to change.
Maximizing drop downs with drop down building possible. Duplication of paper chart entries using drop downs which can be upgraded as more clinical entities are found is a staple of an EMR. The paper process usually relies on a clinician’s wealth of medical-dental terms since inclusion of every possible, or even the most common findings, is prohibitive on a paper chart. The EMR drop down requires front-end loading of the most common clinical findings with opportunity for free-hand additions. Being able to add terms to any drop down is a needed capability.
Don’t design a system for uncommon contingencies, but for your bulk of work. A pediatric dental record should be primarily designed around dental caries, with secondary emphases on oral-facial development (orthodontics) and a lesser capability to record traumatic injuries and periodontal findings. These second and third level characteristics can be hot-buttoned and should not drive the design of the basic system which is caries charting for 98% of our patients. Sadly in most dental schools, the chart is slave to every teaching form, few of which ever exit with the DDS into practice! These forms may have little relationship to patient care and only create “signature black holes” that need to be addressed, usually after treatment is completed.
Progress notes should be designed for the routine entries with free-hand modification possible. Student learners tend to write too much and a carefully crafted progress note format with standard entries in required fields helps patient flow and record completion. In federally funded clinics and residencies, attending reconciliation of student/resident service delivery is a compliance requirement. A well-designed EMR system can “stack” required co-signing tasks on a computer screen, offer standard entries as well as free-hand options, and create a process far faster than paper records for an attending’s validation (same as reconciliation?).
Tie examination results to treatment planning and treatment planning into billing. A good system allows easy transfer of clinical findings needing treatment into some problem “basket” and ideally in a tabulated format. An alternative is a split screen that allows a clinician to visualize clinical findings, radiographic findings while compiling a treatment plan. Again, in clinical settings where compliance to Medicaid/Medicare regulations is required, the design of the record should give attention to auditing principles and security. A good EMR system allows portals of entry for billing and compliance personnel.
- Plan for users of different skill levels and different periods of exposure. The teaching hospital or dental school environment often involves learners and attendings with varying skill levels and computer experience who may be there for brief periods of time. This reality adds significant security and user-friendliness issues. Some medical record systems are far too complex for short-term or casual users. A well-integrated medical-dental EMR allows navigation of the depths of the medical side should a user want to explore, but should focus on the dental portion. Some suggestions in design:
- Initial opening or logging into the dental portion for dental users, rather than opening into the medical portion,
- Clearly indicated options for exploration of medical portions,
- Orientation of major dental component (examination, radiographs, treatment plan) in a logical dental treatment flow to replicate the way dentistry works rather than trying to reshape dentistry’s normal flow to the record,
- Minimization of seldom-used functions on the main dental screen, such as specialty medical clinics, old laboratory tests and hyperfunctionalities like letter writing,
- Clear identification of existing non-caries dental portions like orthodontics or trauma, so a novice user need not randomly search to see if a patient has any of these records.
Unfortunately, many pediatric hospitals do not yet have an EHR system that supports convenient communication among a pediatric patient’s medical and dental providers. Evidence of this state of affairs was provided unintentionally by (Fiks et al. 2011). Some pediatric hospitals may have an awkward mix of systems serving physicians, dentists, and orthodontists and their shared patients.
Summary
This section demonstrates how closely medical and dental professionals must collaborate to deliver appropriate oral health care for infants and children. Such collaboration is especially important given the developmental vulnerabilities of children and the urgency of the oral health needs of many children, especially those from underserved populations. Collaboration is made more difficult by the long-standing separation of medical and dental systems and poor oral health literacy of parents and medical professionals alike.
Teamwork in the delivery of pediatric care requires appropriate electronic patient record technology to facilitate sharing of patient information, to avoid patient record discrepancies between systems, and to create efficiencies by maintaining only a single repository for patient demographics. Only comparatively recently have appropriate integrated systems become available to support a range of clinical sites from pediatric special needs clinics to the largest children’s hospitals. Nationwide Children’s has given practical examples of efficient decision-making in identifying an integrated system to acquire. Much more work will be needed to develop the means to move towards integrating office and community-based care for children through the sharing of electronic health records.
Periodontal Disease and Kidney Disease
Oral health is an oft neglected area in the care of patients who have chronic kidney disease. Furthermore, the provision of care by dentists and physicians to the same patient is fragmented as communication between the two health care providers is scant. Emerging data suggesting the periodontal disease is closely linked to chronic kidney disease highlights the importance of proper oral health and the importance of communication between dentists and physicians in the care of the patient.
Investigators used data from NHANES III, including information on 11, 211 adults who had an oral examination by a dentist who categorized each patient as having no periodontal disease, periodontal disease or edentulous to examine the relationship between numerous risk factors for moderate to severe chronic kidney disease, as determined by calculation of estimated GFR through use of the MDRD formula (Fisher et al. 2011). No chronic kidney disease was defined as an estimated GFR of 60 ml per min per 1.73 m2. Three percent of the patients had CKD, 22.5% were hypertension and 4.4% had diabetes (2.4% with glycated hemoglobin of 7% or higher). Four models were constructed to examine the potential relationship between periodontal disease and CKD. In model one adults with either periodontal disease or edentulous had an adjusted odds ratio of 1.83 (with 95% confidence intervals of 1.31–2.55) of having CKD, independent of the other risk factors for CKD including of age above 60 years, ethnicity, hypertension, smoking status, female gender and C-reactive protein elevation. The fourth model contained 15 potential risk factors including the periodontal disease score and for every 1-unit increase in the score, the risk of having CKD increased by 1% controlling for the other risk factors. The authors hypothesized from their results that the relationship between periodontal disease and CKD was bidirectional in that CKD may increase the risk of periodontal disease which in turn increases the risk of CKD.
Grubbs et al. (2011) also used NHANES data to look more closely at the relationship between periodontal disease and CKD, using dental examinations obtained from 2001 to 2004 (n = 6,199 adults, 21–75 years) (Grubbs V, et al. 2011). In this analysis edentulous subjects were excluded and those with albuminuria were included in the definition of CKD. In the entire population CKD was present in 10.6%, but in those with moderate to severe periodontal disease this increased to 21.6%. Other associations with moderate to severe periodontal disease were being older, male, nonwhite, less educated and poor. There was a strong relationship between periodontal disease and CKD (2.5 unadjusted odds ratio). When adjusted for age, gender, tobacco use, hypertension, diabetes, ethnicity, poverty and educational attainment, the odds ratio for the association of periodontal disease and CKD was still significant (1.55). In some groups (Mexican American, poor, and poorly educated) dental care was not received on an annual basis in the majority of this segment of the population.
Periodontal disease has been associated with an increased risk of death in hemodialysis patients (Kshirsagar et al. 2009). This relationship has been poorly studied in peritoneal dialysis patients. This requires further study but it appears possible that periodontal disease might hasten loss of residual kidney function and perhaps contribute to atherosclerosis in dialysis patients and therefore, contribute to the high mortality in this population.
Patients who desire a kidney transplant are required to undergo a thorough evaluation beforehand including an oral examination by a dentist. Some patients on dialysis have inadequate insurance which does not cover dental care, leading to a situation in which a kidney transplant is denied because the patient cannot afford the dental examination.
Communications between dentists and physicians in the care of the patient is scant. If oral surgery is required in a dialysis patient, the surgeon generally requires a brief summary from the nephrologist with recommendations. These might include suggestions for prophylactic antibiotics, avoidance of vasoconstrictor agents to an excess locally (which can elevate blood pressure) and the increased risk of bleeding of a dialysis patient. For more routine dental examinations no information is requested which could potentially lead to drug interactions or a dangerous situation.
Most nephrologists and health care providers in the dialysis unit do not inquire of the patient concerning dental health and examination of the mouth is quite uncommon. Although the dialysis patient is seen monthly at a minimum, there is little conversation or documentation of oral health.
Connecting the electronic health records of in-patient care, the out-patient dialysis unit and the dentists’ office could potentially have a large impact in improving the care of those with end stage kidney disease.
What the Provider Needs to Know: Supporting Each Other’s Efforts
Integrating medical and dental records in EHR’s may or may not be the “golden ring.” First, we need to integrate the clinical thinking…something we both realize is important, but not likely to be solved by an inert computer. I also think that integrated records will be very cumbersome, given the fact that the language used by the separate disciplines is so different, and the kind of detail required to support good decisions and good work is so different. It could be done…but for many professionals on either “side,” they would never open the other module. To me, a more sensible solution may be to have a condensed “nugget” of information that could cross populate.
“Moderate periodontal disease” may be what the medical doctor needs to know, plus know what a treatment plan may include. She won’t need to know the number of the teeth with the deepest pockets and erosions but will need to support the patient’s determination to follow through. On the other hand, if the patient has shown remarkable initiative in gum care and has successfully migrated to a lower severity index, that would be important for congratulation and reinforcement…and also to encourage similar diligence in managing, let’s say, the hypertension that is not optimally controlled.
In the other direction, the dentist should know that a patient has been erratic in clinical follow up, does not self-test blood glucose, uses hypoglycemic drugs only intermittently, and has failed several appointments for eye exams. This would lead to a rather different set of approaches from a highly motivated grandmother who is enrolled in a community cultural center’s senior exercise club, and is learning to become a lay community teacher for diabetes.
Right now, I don’t think even this superficial degree of information is exchanged. We need to support each other’s efforts, but we probably do not need to share minute details.
Integrating Ophthalmic Imaging: Seeing the Big Picture in a Small Organ
The benefits of an electronic health record are well described. EHRs allow for legible standardized documentation and easier sharing of patient data between providers at multiple locations. They are less prone to loss and require much less space to store. They have the potential to result in a reduction in the cost of health care.
A distinct disadvantage of the EHR, in its current configuration, is the problem of information overload. Simply put, there is often too much information presented in a way that is difficult to review and digest. The EHR equivalent of thumbing through a chart quickly is not yet available. As a result we frequently see practitioners look only at the last note or two as they review a patient’s history.
We require a way to communicate information directly relevant to patient diagnosis, treatment and prognosis among subspecialists and primary care providers. We require a way to identify subclinical cerebrovascular disease in a patient, independent of blood pressure and other traditional risk factors. We require a way to recognize which patients with cerebrovascular disease are two to four times more likely than average to develop a stroke in the next 3 years. We have a way – retinal imaging.
The eye is the one place in the body we can directly observe arteries, veins and a cranial nerve in a noninvasive manner. Routine imaging of the retina and optic nerve could allow primary care providers to assess retinal, and by proxy systemic, end organ damage from atherosclerosis in an efficient manner. The key to optimal use of the medical record and efficient yet effective communication among providers may lie with the familiar adage; a picture is worth a 1,000 words.
Traditionally, when ophthalmologists communicate with primary care providers they send brief letters regarding the findings seen during a yearly dilated examination and the presence, absence or progression of diabetic retinopathy. These letters end by exhorting the virtues of improved blood sugar, blood pressure and lipid control, a sentiment that the primary care provider likely shares. This system of communication does not provide particularly useful information for the primary care provider, except to serve as a notice that the standard of care screening guidelines have been met. The box has been checked.
If primary care providers, cardiologists, nephrologists had access to routine ophthalmic imaging, they would be able to directly visualize the effect that suboptimal blood sugar control is having on their diabetic patients. As importantly, they would be equipped with information directly predictive of congestive heart failure, stroke, and cardiovascular mortality for their patient with hypertension, hyperlipidemia and for those who smoke.
Large clinical studies have shown that assessment of retinal vascular changes such as retinal hemorrhages, microaneurysms and cotton wool spots provides important information for vasculopathy risk stratification. As an example, Wong et al. showed that the presence of retinopathy indicates susceptibility to and onset of preclinical systemic vascular disease, independent of and qualitatively different from measuring blood pressure or lipids (Wong and McIntosh 2005). In the Atherosclerosis Risk in Communities (ARIC) study, individuals with hypertensive retinopathy signs such as cotton wool spots, retinal hemorrhages and microaneurysms were two to four times more likely to develop a stroke within 3 years, even when controlling for the effects of blood pressure, hyperlipidemia, cigarette smoking and other risk factors (Wong et al. 2001). In a recent study by Werther et al., patients with retinal vein occlusions were found to have a two-fold increased risk of stroke compared to controls (Werther et al. 2011).
In addition, the ARIC study group reported that individuals with retinopathy were twice as likely to develop congestive heart failure as individuals without retinopathy, even after controlling for pre-existing risk factors (Wong et al. 2005a). Interestingly, even among individuals without pre-existing coronary artery disease, diabetes or hypertension, the presence of hypertensive retinopathy was associated with a three-fold increased risk of congestive heart failure events (Wong et al. 2005a). In the Beaver Dam Eye Study, cardiovascular mortality was almost twice as high among individuals with retinal microaneurysms and retinal hemorrhages as those without these signs (Wong et al. 2003a, b). The ARIC and Beaver Dam Eye Studies have also shown that, independent of other risk factors, generalized retinal arteriolar narrowing predicts the incidence of type II diabetes among individuals initially free of the disease (Wong et al. 2002a, 2005b).
A primary care provider with access to patients’ retinal photographs may therefore have the evidence needed to suggest which patient with either established systemic vascular disease or preclinical systemic vascular disease requires a more aggressive treatment and risk factor modification. They could do this without wading through the electronic equivalent of piles of records. One photograph could reflect both acute changes in blood pressure (retinal hemorrhages, microaneurysms and cotton wool spots) and chronic changes resulting from cumulative damage from hypertension (AV nicking and generalized arteriolar narrowing) (Sharrett et al. 1999; Wong et al. 2002a; Leung et al. 2004).
In Brown et al. 23 out of 24 patients, excluding those with known diabetes, that presented with a single cotton wool spot or a predominance of cotton wool spots on examination of the retina were found to have underlying systemic disease (Brown et al. 1985). Systemic work-up revealed diagnoses including previously undiagnosed diabetes, hypertension, cardiac valvular disease, severe carotid artery obstruction, leukemia, metastatic carcinoma, systemic lupus erythematosus, AIDS and giant cell arteritis (Brown et al. 1985). These findings illustrate the importance of retinal findings on a systemic level.
The utilization and integration of ophthalmic imaging may serve to achieve more effective communication among subspecialists and primary care providers and ultimately to provide improved diagnosis and treatment for delivery of optimal quality of patient care. Moreover, the improved integration and maximal use of resources may serve to reduce overall health care cost and perhaps decrease provider frustration with the electronic health record (Fig. 4.5).
Fig. 4.5.
Nonproliferative diabetic retinopathy and hypertensive retinopathy
There are cotton wool spots, exudates, intraretinal dot-blot hemorrhages and microaneurysms. AV nicking is also present especially along the superior arcade just as the vessel leaves the optic nerve (Fig. 4.6).
Fig. 4.6.
Hypertensive retinopathy
AV nicking, tortuosity of vessels, intraretinal hemorrhages and dry exudates are seen (Fig. 4.7).
Fig. 4.7.
Central retinal vein occlusion
There is edema of the optic nerve head, with cotton wool spots and flame shaped hemorrhage along the disc margin. There are several cotton wool spots along the vascular arcades and scattered dot hemorrhages throughout the posterior pole and periphery (Fig. 4.8).
Fig. 4.8.
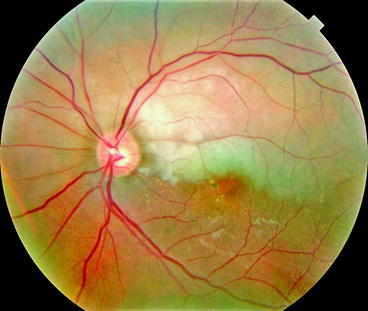
Branch retinal artery occlusion
Notice the cholesterol plaque in the vessel just as it exits the optic nerve head and the pallor in the superior macula corresponding to retinal ischemia and edema (Fig. 4.9).
Fig. 4.9.
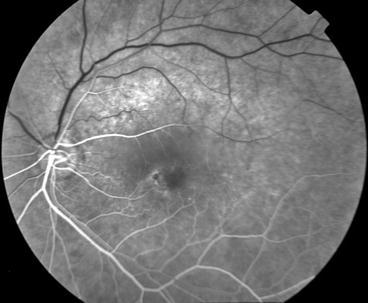
Branch retinal artery occlusion, fluorescein angiogram
The cholesterol embolus has resulted in lack of blood flow to the superior arcade (Fig. 4.10).
Fig. 4.10.
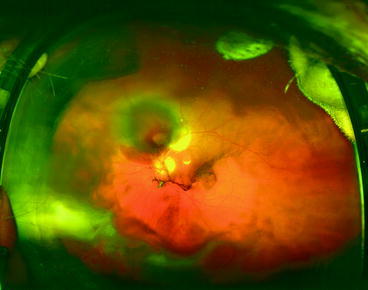
Retinal macroaneurysm
There is pooling of subretinal blood just superior to the optic disc with a central fibrin clot and associated vitreous hemorrhage (Fig. 4.11).
Fig. 4.11.
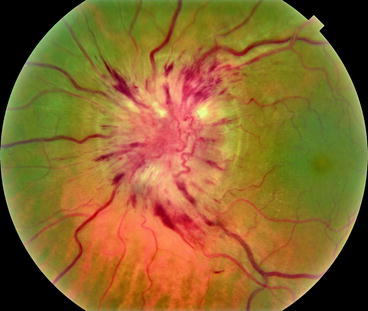
Hypertensive optic neuropathy
Optic disc edema, flame hemorrhages and venous congestion are seen in a patient with severe hypertension.
Do Dentists and Otolaryngologists Need to Collaborate? If So Does Their Patient Record System Help Them Do It?
In clinical practice, an otolaryngologist often needs a dental consult not only because of the topographically adjacent nature of the structures but also because most structures are supplied by the same neurovascular bundle and therefore there is overlapping of symptoms. The converse scenario can also apply. Apart from this, there are many systemic medical conditions (for example: bleeding diatheses, diabetes) a dentist encounters throughout his or her practice which can determine the outcome of a successful treatment. Sometimes, providers may observe a cluster of diagnostic criteria which may have to a single source. In the sections below, I will explore a few of these scenarios and conditions, and indicate where and how an integrated electronic health record (EHR) could optimize delivery of health care by dentists and otolaryngologists.
Congenital Conditions
Cleft palate/Cleft lip: Cleft lip and cleft palate (CL/CP) are congenital conditions that require multidisciplinary management by dentists, oral and maxillofacial surgeons, orthodontists, otolaryngologists, speech pathologists and plastic surgeons A number of studies report that a multidisciplinary approach is essential for better treatment outcomes (Wangsrimongkol and Jansawang 2010) and for post operative rehabilitation (Furr et al. 2011). These multidisciplinary approaches may lead to new ways to manage and treat CL/CP patients (Salyer et al. 2009).
Hutchinson’s teeth: Notching of the upper two incisors is typically seen in individuals inflicted with congenital syphilis.
Macroglossia refers to enlarged tongue in relation to oral cavity. Macroglossia is an important sign. It can indicate important systemic diseases like systemic amyloidosis, congenital hypothyroidism, acromegaly, or Down syndrome.
Headache
A common complaint that dentists and otolaryngologists encounter in their practice is the common headache. Because of the special nature of the neurovascular bundle of the head and neck this symptom can be presented to both dentists and otolaryngologists (Ram et al. 2009). Any sinus pathology can present as a headache to an otolaryngology practice. Since the maxillary sinus floor is in close proximity to the maxillary premolars and molars, it is imperative to obtain a dental evaluation in persistent cases of headache. There are a number of causes for headache from the dental and otolaryngology perspective. A mal-aligned denture patient with chronic headache, whom I saw in my practice was shuttled between departments and an array of investigations only to find at the end that an ill-fitting denture caused the intractable headache. In these cases, an integration of findings is extremely important in providing quality treatment to the patient and also saves money and time for the whole health care system. Hence it is important to have an integrated patient record for this particular symptom alone.
Trigeminal Neuralgia
Trigeminal neuralgia is facial pain of neurogenic origin experienced along the distribution of the trigeminal nerve(fifth cranial nerve). It can present as a dental pain and can also be triggered by brushing teeth among other trigger factors. As a result, patients with dental pain without obvious causes are required to have a physicians’ consultation to rule out this obscure condition. Sometimes it is diagnosed by omission (Aggarwal et al. 2011; Rodriguez-Lozano et al. 2010; Spencer et al. 2008).
Tumors of Nasal Sinuses
Any tumor of the nasal sinuses (specifically maxillary and ethmoids) can erode the lower bony wall and present in the oral cavity (usually the maxillary arch) as dental pain, loose tooth, etc. Therefore, these are areas of interest to both dentists and otolaryngologists. Such tumors most commonly present first to a dentist or could also be an accidental finding. Cancers of the naso/oro/laryngo pharynx can also present as toothache to a dentist as these structures have a common nerve supply from cranial nerves 5,7 and 9. Therefore, an integration of the patient record may even help in early diagnosis of the tumor. The same principle applies to all oral tumors, tumors of the nasopharynx, the oropharynx etc. This is especially true of malignant lesions of the oral cavity as these may help in early detection and treatment of cancer. In these cases, an early biopsy and histopathology can save the life of the patient. Therefore, it is imperative to say that a collaborative patient record can save patients’ lives.
Ulcers of the Oral Cavity
Ulcers of the oral cavity from aphthous ulcers to carcinomas can present both to a dentist and an otolaryngologist. Oral ulcers can be of dental origin. Contact ulcers from sharp edges of a mal-aligned tooth can result in intractable ulcers, where a simple smoothing of sharp edges may eradicate the ulcer and terminate it as a chronic condition and can even prevent the ulcer turning into a malignancy. If you have an integrated electronic health record (EHR) these problems are immediately addressed and managed. Otherwise, the condition will consume valuable time of both the patient and the physician concerned. In addition to this, there are a few conditions which require special attention: aphthous stomatitis (canker sore), which may indicate oral manifestation of deficiencies of iron, vitamin B12, folate deficiency and oral candidiasis, which can be a sign of diabetes mellitus or of an immunocompromised patient (e.g. AIDS).
Temporomandibular Joint Disorders
Temperomandibular joint (TMJ) disorders can present in a variety of symptoms to both dentists and otolaryngologists. They can present as a headache, earache, toothache, or as facial pain. There can be a number of causes for this including osteoarthritis of the TMJ, recurrent dislocation, bruxism, or even an ill fitting denture. There have been cases where patients have been subjected to removal of teeth for chronic toothache only to discover at the end that the symptom was a referred pain from TMJ! Therefore, an integrated EHR can prevent misdiagnoses and resulting impairment or disability to patients. Trismus (lock jaw) can indicate important diagnoses such as tetanus and rabies.It is due to a spasm of muscles of mastication, which is an important oral manifestation of widespread muscle spasm. Apart from these conditions, other causes of trismus are peritonsillar abscesses, and scleroderma.
Other problems dentists and otolaryngologists encounter in clinical practice are concurrent systemic diseases (patients with multiple problems): patients with bleeding diatheses, diabetes mellitus and a hidden primary malignancy. A non-healing ulcer in the oral cavity may hide a primary malignancy behind it. In these cases, you have to look for it specifically. Similarly, one has to be aware of oral manifestations of internal pathology. Some of them are Crohn’s disease, ulcerative colitis and gastro-intestinal tract malignancies.
Often dentists see patients after a tooth extraction with intractable bleeding to find that they have a bleeding diathesis. So, this may be the first presentation of these patients’ bleeding disorder. When this patient undergoes any elective procedure in future, it will be a great help to surgeons to be aware of this information to prevent any inadvertent complications. Therefore an integrated EHR can prevent unwanted complications where a patient’s life may be in jeopardy.
Refered Otalgia (Earache)
The source of otalgia or earache can be from a number of sites other than ear itself. Technically ear lobe and ear canal are supplied by four different cranial nerve branches (5th, 7th, 9th, 10th). Therefore, an area with a common nerve supply can present as earache. Common dental problems which present as referred otalgia are (1) dental caries (2) oro-dental diseases or abscesses (3) an impacted molar tooth (which is a common cause) (4) malocclusion (5) benign and malignant lesions of oral cavity and tongue (Kim et al. 2007). Therefore, it is essential these two departments collaborate with each other in diagnosing and treating these diseases, and one way of facilitating it is through an integrated EHR system.
Halitosis (Bad Breath)
There is a lot of overlap between dentists and otolaryngologists in the diagnosis and treatment of patients with halitosis (Delanghe et al. 1999; Bollen et al. 1999). Poor oral hygiene is the most common cause for this common complaint. Oral causes include tooth caries, oral ulcers, periodontal diseases, unhealthy mucosa of the oral cavity. It is interesting to note that a simple oral ulcer can form an abcess eroding the floor of mouth and becoming a life-threatening oral cellulitis (Ludwig angina).Once the cellulitis has developed, it becomes a medical emergency. Therefore, it is essential to prevent it before it can progress into a life-threatening condition, which of course is possible. Causes pertaining to otolaryngologists include: chronic sinusitis or mucociliary disorder, chronic laryngitis or pharyngitis, pharyngeal pouches-related pathology, tumors or ulcers of naso/oro/laryngopharynx, diseases or conditions that impair normal flow of saliva such as salivary gland diseases or stones preventing flow of saliva, medications which cause dryness of mouth: antihistamines, anti-depressants; local manifestation of systemic disorders: auto immune disorders, Sjögren syndrome, dehydration from any cause, diabetes mellitus and Gastro Esophageal Reflux Disorder (GERD).
GERD is caused by improper neuro-autonomy of the lower esophageal sphincter (LES). The LES does not close tightly after food intake which causes gastric content to enter the esophagus. Over time this can erode mucosa and cause various diseases even becoming cancerous (Friedenberg et al. 2010). This disorder is attributed to life style. Fast food consumption habits (oily fried foods) and eating habits (swallowing food without properly chewing) are partly responsible for this disorder (Lukic et al. 2010; Al-Humayed et al. 2010). Here again an early diagnosis can manage the disease process before it is fully developed.
At present there are no integrated EHR systems serving these specialties (dentistry and otolaryngology). An integrated EHR would facilitate efficient communication between a dentist and an otolaryngologist who are providing care to the same patient and addressing a problem with a shared focus between the two disciplines. Such integrated communication, may only require consulting the available medical or dental record of the patient, based on the particular circumstance. Even enabling this simple communication would avoid duplication of effort, clarify the context of certain symptoms and reduce stress endured by the patient. It also has the potential to reduce healthcare delivery costs, and in some cases, even contribute to saving the patient’s life.
Providing Collaborative, Interdisciplinary Healthcare to Patients with Neurodevelopmental Disorders and Intellectual Disabilities
Henry Hood, Allan G. Farman, Matthew Holder
Abstract
The morbidity and mortality resulting from disparities in access to basic medical and dental services for Americans with neurodevelopmental disorders and intellectual disabilities has been well-documented in both scientific monographs and in the lay press. These disparities in access to healthcare, combined with the medical fragility and complex health and social histories with which patients with a neurodevelopmental illness frequently present, create an imperative for a well-thought-out, patient-focused, interdisciplinary approach to their care.
In this chapter, the authors attempt to put forth a justification for precisely this kind of collaborative approach through a summary and discussion of a series of actual clinical cases. The protocols discussed in the management of each of these clinical cases illustrate the value in providing whole-person, interdisciplinary health care to this complex patient population.
Surveying the Landscape
There is arguably no single patient population for whom the provision of collaborative, interdisciplinary health care is more challenging than for patients with neurodevelopmental disorders and intellectual disabilities (ND/ID). In planning and delivering the generally-accepted standard of health care to this unique population, myriad biomedical, psychosocial and sociopolitical realities converge to create a landscape that is, at best, daunting for patients with these disorders, and for the clinicians who are charged with their care.
Anecdotal and scientific evidence suggest that this landscape has produced a paucity of physicians and dentists who are willing and able to provide care to patients with ND/ID, and that American medical and dental schools are providing little training focused on their care (Holder et al. 2009; Wolff et al. 2004).
In February of 2002, 16th Surgeon General David Satcher issued a report, which documented that Americans with ND/ID experience great difficulty accessing quality health care (Thompson 2002). In that same report, former Health and Human Services Secretary Tommy Thompson said, “Americans with mental retardation and their families face enormous obstacles in seeking the kind of basic health care that many of us take for granted.” (Thompson 2002) The disparities identified by Dr. Satcher and Secretary Thompson require that physicians and dentists approach this population in a spirit of collaboration, compassion, and teamwork in order to produce positive health outcomes for them.
Perhaps, an even greater imperative driving the need for collaboration between medicine and dentistry in this arena is the fact that many patients with intellectual disabilities have developed this cognitive impairment as the result of an underlying neurodevelopmental disorder that is often undiagnosed. And it is this neurodevelopmental illness and the constellation of potentially devastating complications associated with that illness that create a biomedical fragility and a vulnerability that neither begins nor ends at the oral cavity, and that leaves these patients at risk in almost every aspect of their daily lives.
When, for example, patients with ND/ID are dependent upon publicly-funded programs for their health care, and when these systems fail to provide the health services that biomedically complex cases require because they fail to account for and accommodate the link between medical and dental pathologies, the risk of a negative outcome is greatly enhanced. Such was the case for an intellectually disabled woman in Michigan who, in October of 2010, was unable to access dental services through the state’s public medical assistance program, and who fatally succumbed to a systemic bacteremia resulting from an untreated periodontal disease (Mich. Dent. Assoc. 2009).
Neurodevelopmental Disorders and Their Associated Primary Complications
The American Academy of Developmental Medicine and Dentistry (AADMD) defines a neurodevelopmental disorder as a disorder involving injury to the brain that occurs at some point between the time of conception and neurological maturation – approximately age 21 or 22 (Zelenski et al. 2008).
Examples of frequently-encountered neurodevelopmental disorders would include Fragile X syndrome, a genetically acquired neurodevelopmental disorder caused by a mutation at the distal end of the long arm of the X chromosome (see Fig. 4.12), Trisomy 21, another genetic disorder, which features extra genetic material at the chromosome 21 site (see Fig. 4.13), and Cerebral Palsy, a prenatal or perinatal, acquired neurodevelopmental disorder (see Fig. 4.14).
Fig. 4.12.

Fragile X syndrome
Fig. 4.13.
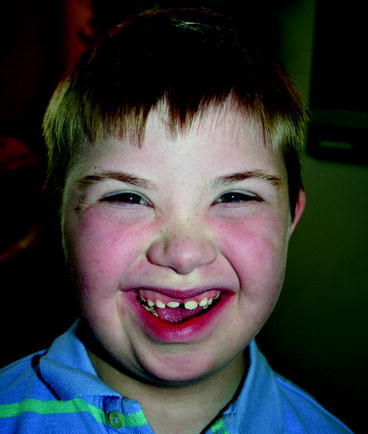
Trisomy 21
Fig. 4.14.
Cerebral palsy
Patients with neurodevelopmental disorders tend to present clinically with one or more of five frequently-encountered, objective symptom complexes or primary complications. These five, classic primary complications include intellectual disability (aka: mental retardation), neuromotor impairment, seizure disorders, behavioral disturbances, and sensory impairment (AADMD).
Additionally, multiple secondary health consequences can derive from the five primary complications; and any one of these secondary health consequences, or a combination of them, can produce profound morbidity. An example of a common secondary health consequence seen in patients with ND/ID, which is derived from intellectual disability and / or neuromotor impairment, is the patient who is unable to care for his or her own mouth, and who develops ubiquitous caries and advanced periodontal disease as a result (See: Fig. 4.15). Another example would be the patient who suffers from the secondary health consequence of gastroesophageal reflux disease (GERD) as a result of the neuromotor impairment associated with multiple neurodevelopmental disorders; and whose tooth enamel and dentinal tissues become chemically eroded as a result of the chronic intraoral acidity produced by GERD (See: Fig. 4.16).
Fig. 4.15.
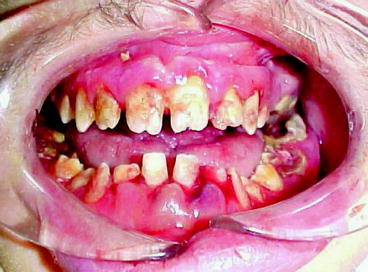
Patient with congenital syphilis
Fig. 4.16.
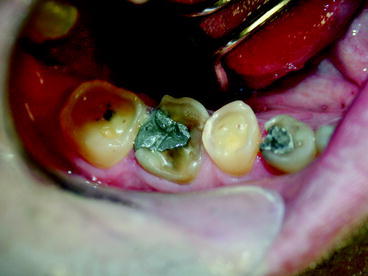
GERD-related erosion of enamel and dentinal tissues
The diagnosis and management of these secondary health consequences provide dentists and physicians with a unique opportunity to work together to improve the quality of health and quality of life for their patients by implementing a team approach, which crosses the traditional interdisciplinary lines of communication, and which expands each clinician’s ability to make meaningful treatment options available. Indeed, it is often the case that quality primary care provided in one discipline will provide potentially valuable information to an attending clinician from another discipline. Such is the case with the patients featured in Figs. 4.16 and 4.17.
Fig. 4.17.
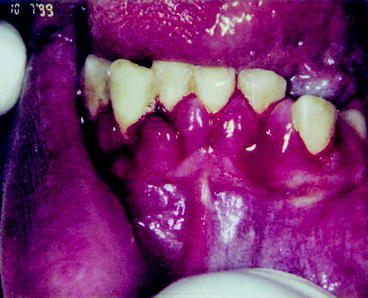
Patient on phenytoin
The Adult Patient with ND/ID and Undiagnosed Syphilis
The patient whose intraoral photograph is featured in Fig. 4.14 is a 19 year-old male patient who presented to a special needs dental clinic accompanied by his mother. The mother indicated that her son was exhibiting hand-mouthing behaviors that she believed suggested he was experiencing mouth pain. A comprehensive radiographic and intraoral exam revealed, among other maladies, notched incisors, multiple diastemas, grossly decayed mulberry molars, and advanced periodontal disease. The patient also exhibited moderate to severe intellectual disability.
These findings were all consistent with a diagnosis of congenital syphilis.6 However, in developing the medical history with the mother, it was learned that no previous diagnosis of syphilis had been discussed with the mother, nor was it included in the health history.
In cases like this, a comprehensive dental treatment plan should always include consultation with the primary care physician for purposes of moving forward with confirmation of the clinical diagnosis by serologic testing, and consultation with a cardiologist to assist in the management of potential cardiovascular sequelae.
As the dental treatment plan is being developed, consideration should also be given to Human Immunodeficiency Virus (HIV) testing for this patient, as coinfection is a common finding7. This issue could easily be attended to by a primary care physician, an internist or an infectious disease specialist. In the absence of any of these team members, the dentist should feel entirely comfortable ordering HIV testing.
The primary care physician and the developmental dentist should continue to advise each other and their respective consultant specialists of any significant developments or new information, which could in any way impact either the medical or the dental treatment plan. As treatment progresses, both the physician and the dentist should expect improvement in the patient’s periodontal status, which will likely be reflected in a decrease in the frequency of immune-related illnesses, and in the maladaptive behaviors produced by chronic oral pain.
It is quite often the case in this patient population that, with a reduction in maladaptive behaviors, comes a reduction of the use of psychotropic medications prescribed in a frequently futile attempt to manage behaviors that were born of an undiagnosed medical or dental illness.
The Adult Patient with ND/ID and Undiagnosed GERD
GERD is defined as the reflux of gastric contents into the esophagus. GERD is primarily associated with incompetence of the lower esophageal sphincter; however there are numerous co-contributors, which may predispose a patient to GERD or exacerbate an existing reflux problem. These co-contributors include a diet high in fat, neuromotor impairment associated with functional abnormalities such as dysphagia, neuromotor impairment associated with impaired ambulation and prolonged periods of recumbence, and the use of multiple medications including anxiolytics, calcium channel blockers, and anticholinergics. GERD is thought to affect approximately 25–35% of the general US population.
It has been established in the literature that the incidence of GERD in patients with intellectual disabilities is significantly higher than in the neurotypical population, and that the relative number of unreported cases of GERD is much higher in patients with a neurodevelopmental diagnosis, as well.
Patients who have gastric reflux as a function of a neurodevelopmentally-derived neuromotor impairment and a coexisting intellectual disability are impaired in their ability to voice the complaint that would, in the neurotypical patient, commonly lead to an encounter with either a family physician or a gastroenterologist and, ultimately, to a diagnosis. This inability to voice a complaint can be problematic in that, left untreated, GERD can produce maladaptive and sometimes aggressive behaviors in this population. And, of even greater concern, is the fact that undiagnosed esophageal reflux can lead to more complex conditions that can produce significant morbidity or even mortality – maladies such as Barrett’s esophagus or adenocarcinoma of the esophagus.
Chronic GERD can also produce an acidic intraoral environment, which can lead to the chemical erosion of the enamel and dentinal tissues of the teeth. Ali et al. have established a link between erosion of the enamel and dentinal tissues of the teeth and GERD. There is additional anecdotal evidence suggesting a link between tooth enamel erosion and GERD, and related maladies. A special needs dental clinic in the eastern United States serving 1,000 patients with ND/ID, has reported that, of nine patients referred to gastroenterology who presented for dental exam with a finding of either tooth enamel erosion or ubiquitous caries, two cases were diagnosed with GERD, two with Barrett’s esophagus, three with gastritis, and one with duodenitis. In all cases, medical treatment was required.
In light of all that is known about the incidence of GERD and of the GERD-related risks unique to this patient population; and in light of the link between tooth enamel erosion and GERD, it is incumbent upon any dentist encountering tooth enamel erosion in a patient with an intellectual disability to immediately refer that patient to gastroenterology for a work up, which should include esophagogastroduodenoscopy (EGD) and pH monitoring.
A dentist encountering GERD in a patient with an intellectual disability must be aware that he or she may be the first and only link between that patient and the diagnosis of a potentially life-threatening illness.
The Adult Patient with ND/ID and Phenytoin-Induced Gingival Enlargement
Phenytoin-induced gingival enlargement can appear as either an inflammatory lesion or a more dense, fibrotic hyperplastic lesion. The inflammatory lesion is one in which the gingival tissues are swollen and bleeding, and in which pain is often a component. This type of gingival enlargement is the more acute lesion, frequently seen in patients who are currently taking Phenytoin. In advanced cases of inflammatory gingival enlargement, the tissues can appear botryoid, with a characteristic grape-cluster appearance. In advanced cases of Phenytoin-induced gingival enlargement, the lesion can sometimes shroud entire sections of the dentition.
Phenytoin has long been a common medication used to treat seizure disorders in patients with neurodevelopmental disorders and intellectual disabilities. However, the gingival enlargement it produces, and the obstacle this lesion can pose to effective oral hygiene – especially in a population in which oral hygiene is typically compromised – can, over time, lead to periodontal disease, edentulism, and in advanced cases, systemic bacteremias. Gingivectomy performed to reduce Phenytoin-induced gingival enlargement will typically fail unless the patient is weaned off the offending medication, and another anti-seizure medication is titrated to effect. Multiple alternative anti-seizure medications are currently available, which do not have the side effect profile of Phenytoin, and most patients who are weaned off Phenytoin will demonstrate a virtual 100% resolution of the inflammatory lesion within a matter of 3 or 4 months.
The image in Fig. 4.18 is of a 22 year-old, microcephalic African-American male with intellectual disability, neuromotor impairment, and a seizure disorder. Figure 4.17 illustrates the appearance of this patient’s gingival tissues while he was currently on Phenytoin. Figure 4.18 features the same patient 4 months after being weaned off Phenytoin and placed on Topiramate.
Fig. 4.18.
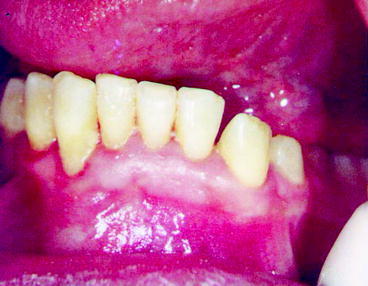
Patient 4 months post-weaning
These images illustrate the dramatic result that can be achieved when a dentist and a physician work in collaboration in the best interests of the patient. It is worth noting that this particular collaboration required only one intervention to achieve this result: The patient was weaned off Phenytoin and was placed on a safer alternate anti-seizure medication.
Any dentist caring for a patient with an intellectual disability who presents with Phenytoin induced gingival enlargement should immediately contact either the primary care physician or neurologist managing the patient’s seizure disorder, and strongly urge that the patient be weaned off Phenytoin and placed on a safer alternative anti-seizure medication. Edentulism and bacteremia need not be a side-effect of a seizure management protocol.
The patient seen in Fig. 4.19 is a 20 year old male patient with idiopathic intellectual disability who presented to an outpatient dental clinic for comprehensive dental evaluation and treatment. He was accompanied by his father. His father was referred to the clinic by the staff at his son’s day program workshop. The day program staff had observed hand-mouthing behaviors, and they had voiced concern that the patient may be in pain.
Fig. 4.19.
The adult patient suspected of having Fragile X syndrome
In the waiting room, the patient exhibited behaviors consistent with neurodevelopmental dysfunction. He was non-communicative, and his gaze aversion and tactile defensiveness were suggestive of autism. He was resistant and somewhat combative when directed to the dental chair, and effective behavior management in both the waiting room and operatory required the combined efforts of his father and two staff members. The patient’s health history was positive for attention deficit hyperactivity disorder (ADHD), and there was no history of seizure or neuromotor impairment.
The father indicated that, at age ten, the patient was admitted to an inpatient psychiatric unit for evaluation of his uncontrollable behavior. The following day, the parents were told that managing the patient’s behavior was beyond the ability of the psychiatric unit staff, and the parents were asked to take the child home. The father also indicated that the psychiatric unit staff described the child’s behavior as overwhelming.
The patient was last seen by a dentist 12 years prior to presentation; examination and treatment at that time were carried out in the operating room under general anesthesia.
Effective oral examination of this patient required utilization of papoose board and Molt mouth prop. Multiple options for behavior management, including utilization of general anesthesia in the operating room, were discussed with the father, and informed consent to utilize medical immobilization techniques for purposes of this examination was obtained and documented prior to taking the patient into the operatory. In the operatory a dental examination was performed, and a baseline panel of digital radiographs was obtained.
The head and facial features of this patient were suggestive of Fragile X syndrome (See: Fig. 4.20). The body of the mandible was somewhat elongated; the nose was prominent; the head had somewhat of a triangular shape, and the patient readily averted his gaze. Upon further inquiry, the father reported that the patient also exhibited macroorchidism, although he indicated that no physician or dentist had ever suggested a work up for Fragile X.
Fig. 4.20.
Pectus excavatum
Fragile X syndrome is a disorder with which many clinicians are unfamiliar. Yet it is the second leading genetic cause of intellectual disability in the United States, and it is the leading known cause of autism in the U.S. In addition to the phenotypic findings noted in this case, there are other frequently-encountered physical characteristics consistent with Fragile X that may move a clinician toward this diagnosis. They include pectus excavatum or funnel chest (see Fig. 4.21) and joint laxity (see Fig. 4.22).
Fig. 4.21.
Joint laxity observed in patient with Fragile X syndrome
Fig. 4.22.

Four children with autism and one neurotypical child
Gaze aversion, as previously mentioned, is a typical finding in autism and in Fragile X syndrome. Indeed, in conjunction with non-verbal behaviors, gaze aversion is often the finding that initially alerts the clinician to the possibility of a neurodevelopmental diagnosis featuring autism as a complication. Figure 4.22 features a photograph of five children at a school for children with special needs. Four of the children have been diagnosed with autism, and a fifth child is a neurotypical child who was visiting his brother on the day the photograph was taken. The reader is left to decide which child is the neurotypical child.
Any physician or dentist who encounters a patient with an obvious intellectual disability, who does not have an established underlying neurodevelopmental diagnosis, and who presents with additional findings, which may include gaze aversion, shyness, a prominent chin, pectus excavatum, a large nose or large ears, should suspect a possible Fragile X diagnosis.
The primary care clinician – physician or dentist – should discuss with the guardian or family member the importance of establishing a neurodevelopmental diagnosis. The family member or guardian should be informed that genetic counseling should be made available to all members of the extended family, since Fragile X syndrome is a genetic disorder that can be passed from parents to offspring. Once this discussion has taken place, a referral to a geneticist for a complete genetic work up is indicated. Both the dentist and physician should feel entirely comfortable making this referral. In remote areas where the services of a geneticist may not be available, the attending physician or dentist may order a high resolution chromosomal analysis and a Fragile X DNA test, and have those results sent to a remote location for interpretation by a geneticist. Consultation with a psychiatrist or a clinical psychologist may also be advisable, as patients with Fragile X can sometimes experience enhanced social integration as a benefit of behavioral therapy.
Interdisciplinary Solutions for Interdisciplinary Problems
The healthcare access problem for Americans with neurodevelopmental disorders and intellectual disabilities is, at its core, a healthcare education problem – an education problem resulting from a long-standing deficiency in professional training focused on the care of this patient population. And it is clear that the medical and dental professions share equally in responsibility for these deficiencies.
Eighty-one percent of America’s medical students will graduate without ever having rendered clinical care to a single patient with a neurodevelopmental disorder or intellectual disability; and the graduates of 90% of America’s medical residency programs will graduate from those residencies having had no formal training whatsoever – didactic or clinical – in the care of this patient population.1 Additionally, 50% of graduating dentists have never treated a single patient with a disability.2
It is no wonder that patients like those whose cases were discussed in earlier sections of this chapter have such difficulty accessing quality health care. As Robert Uchin, Dean of Nova Southeastern University College of Dental Medicine observed in a speech in 2003 to his faculty, “Not only do we not have enough doctors to care for these patients; we don’t have enough teachers to teach them how to care for them.”
As a result of these deficiencies in professional education, few clinicians with any expertise in developmental medicine or developmental dentistry are to be found in communities across America. The experts in developmental medicine and dentistry, for the most part, tend to be physicians and dentists who work at the few remaining intermediate care facilities, and at special needs outpatient clinics, psychiatric hospitals, and nursing homes. These physicians and dentists possess the knowledge and expertise in these disciplines because they are the physicians and dentists with the clinical experience. Unfortunately for the patients with neurodevelopmental disorders who are clamoring for quality care, there are too few of these clinicians.
National experts in developmental medicine and dentistry, however, have begun to collaborate in the creation of patient care protocols; and they have produced multidisciplinary curricula in both DVD and online format.
The AADMD has made available 9 hours of online curriculum in developmental medicine, developmental dentistry, and developmental psychiatry (See: List of URLs). The curriculum program is entitled, The Continuum of Quality Care, and it teaches collaborative patient care in three disciplines through an interdisciplinary format.
The AADMD, through a grant from the Wal Mart Foundation and the North Carolina Developmental Disabilities Council, and in collaboration with the North Carolina Mountain Area Health Education Center and the Family Medicine Education Consortium, has also established the National Curriculum Initiative in Developmental Medicine. This initiative, which is scheduled for completion in 2012, will develop curriculum standards for physicians in the primary care of adults with ND/ID. The curriculum stresses the importance of a collaborative approach, which includes medicine, dentistry, podiatry, optometry, and multiple ancillary health professions.
If the disparities in access to healthcare for Americans with ND/ID are to be resolved, physicians and dentists must be willing to cross professional boundaries and work together to plan and deliver whole-person healthcare to their patients with ND/ID. Interdisciplinary protocols in the diagnosis of neurodevelopmental disorders and in the management of the secondary health consequences associated with these disorders must be established. Additionally, clinicians with expertise in these arenas must be willing to work and teach in our nation’s medical and dental schools. The clinicians with expertise must be willing to develop predoctoral and postdoctoral curricula, and the deans of America’s professional schools must be willing to include these curricula as part of their larger programs in primary and specialized care.
The clinicians with expertise in developmental medicine and dentistry must also be willing to conduct patient-focused, interdisciplinary, clinical research in an effort to solve the myriad problems that create obstacles to the delivery of the standard of care for patients with ND/ID. They must be willing to obtain Institutional Review Board approval for this research, and they must be willing to make this research available to their colleagues through publication in peer-reviewed journals and text books, and in professional lecture forums.
The patient featured in Figs. 4.23 and 4.24 is a man named James. He is a 48 year old patient with idiopathic intellectual disability who presented to a dental clinic for evaluation of a painful facial swelling. A comprehensive intraoral exam revealed a cellulitis resulting from multiple grossly decayed teeth, and a generalized advanced periodontitis. No fewer than five clinicians became involved in this patient’s care. They included a general dentist, two oral surgeons, a family practice physician, and a geneticist.
Fig. 4.23.
Patient upon initial examination
Fig. 4.24.
Patient after comprehensive treatment
Over the course of several months, as the treatment plan was completed, and as the chronic dental and periodontal infections were eliminated, James experienced significant improvement in his overall state of health. A comparison of these two photographs reveals not only significant improvement in his aesthetic appearance, but also in his skin turgor and color.
These improvements in the patient’s health translated to improvements in his daily life. He found gainful employment, and his caregivers now report that he smiles constantly – at work and at home.
These photographs were entered into evidence in 2007 before a Congressional subcommittee investigating the death of a young African-American boy who died as a result of an untreated dental abscess. The photographs were intended to make the point that patients with intellectual disabilities need not die as a result of medical illnesses derived from untreated dental disease.
This patient’s case illustrates that, when physicians and dentists are willing to work together toward a common goal of whole-person health for their patients, profoundly positive outcomes can be achieved.
In a larger context, if our nation’s medical and dental professions are willing to commit to a shared agenda, one which promotes the idea of collaborative, interdisciplinary care as a foundational concept, significant improvements in quality of health and quality of life can be realized, not just for Americans with neurodevelopmental disorders, but for every patient seeking quality care.
Further Reading
Kass-Hout T, Zhang X. Biosurveillance: methods and case studies. Boca Raton: Chapman and Hall/CRC; 2010.
Lombardo JS, Buckeridge DL. Disease surveillance: a public health informatics approach. Hoboken: Wiley; 2007.
Wagner MM, Moore AW, Aryel RM. Handbook of biosurveillance. Burlington: Elsevier Academic Press; 2006.
Footnotes
A new study by Hedlund, Jeffcoat, Genco and Tanna funded by CIGNA of patients with Type II diabetes and periodontal disease found that medical costs of patients who received maintenance therapy were $2483.51 per year lower than patients who did not. CIGNA, Research from CIGNA Supports Potential Association between Treated Gum Disease and Reduced Medical Costs for People with Diabetes, http://newsroom.cigna.com/NewsReleases/research-from-cigna-supports-potential-association-between-treated-gum-disease-and-reduced-medical-costs-for-people-with-diabetes.htm, March 29, 2011, accessed June 23, 2011; Jeffcoat M (2011). Personal communication, 20 June 2011.
Some of them may have dental insurance coverage through their retirement health insurance.
Perhaps it might more accurately be called a dental plan.
Ide and colleagues found that people who were treated for periodontitis incurred 21% higher health care costs than those who were free of periodontal disease (Ide et al. 2007). Similarly, Albert, et al., found medical costs associated with diabetes, cardiovascular disease and cerebrovascular disease were significantly higher for enrollees who were treated for periodontitis than for other dental conditions (Albert et al. 2006). Additional studies of this nature would be important to support a measured approach to expanding dental coverage.
The health reform law does not attempt to provide coverage to all 52 million people without health insurance. Estimates are that only 31 million people will be covered by the bill. Even though this is the case we prepare our estimates using all 52 million uninsured Americans.
Indeed, the failure of 50% of employers to cover dental services may well constitute a classic externality in the market for health insurance. Internalizing this externality may well provide better efficiency.
It is also possible that dental care for persons with greater incidence of chronic illness as is the case with Medicare beneficiaries may require even higher levels of spending per beneficiary. Again, it would be good to know scientifically if this is the case.
Contributor Information
Valerie Powell, Email: powell@rmu.edu.
Franklin M. Din, Email: franklin.din@dbmi.columbia.edu
Amit Acharya, Email: ACHARYA.AMIT@mcrf.mfldclin.edu.
Miguel Humberto Torres-Urquidy, Email: mit7@pitt.edu.
References
- 107th Congress. Public Law 107–188. 2002. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=107_cong_public_laws&docid=f:publ188.107.pdf. Accessed May 2011.
- Acharya A, Bhavsar N, Jadav B, Parikh H. Cardioprotective effect of periodontal therapy in metabolic syndrome: a pilot study in Indian subjects. Metab Syndr Relat Disord. 2010;8(4):335–41. doi: 10.1089/met.2010.0002. [DOI] [PubMed] [Google Scholar]
- Adachi M, Ishihara K, Abe S, Okuda K, Ishikawa T. Effect of professional oral health care on the elderly living in nursing homes. Oral Surg Oral Med Oral Pathol. 2002. doi:10.1067/moe.2002.123493. [DOI] [PubMed]
- Aetna. Study shows preventive dental care may help reduce risk of preterm birth. 2008. http://www.aetna.com/news/newsReleases/2008/1001_DMI_Preterm_Birth_Risk.html. Accessed 26 June 2011.
- Aggarwal V, Joughin A, Zakrzewska J, Crawford F, Tickle M. Dentists’ and specialists’ knowledge of chronic orofacial pain: results from a continuing professional development survey. Prim Dent Care. 2011;18(1):41–4. doi: 10.1308/135576111794065838. [DOI] [PubMed] [Google Scholar]
- Albert DA, Sadowsky D, Papapanou P, Conicella ML, Ward A. An examination of periodontal treatment and per member per month (PMPM) medical costs in an insured population. BMC Health Serv Res. 2006;6(103):1–10. doi: 10.1186/1472-6963-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert DA, Begg MD, Andrews HF, Williams SZ, Ward A, Conicella ML, Rauh V, Thomson JL, Papapanou PN. An examination of periodontal treatment, dental care, and pregnancy outcomes in an insured population in the united states. Am J Public Health. 2011. doi:10.2105/AJPH.2009.185884. [DOI] [PMC free article] [PubMed]
- Al-Humayed S, Mohamed-Elbagir A, Al-Wabel A, Argobi Y. The changing pattern of upper gastro-intestinal lesions in Southern Saudi Arabia: an endoscopic study. Saudi J Gastroenterol. 2010;16(1):35–7. doi: 10.4103/1319-3767.58766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A, Singh G, Darke C, Dolby AE. Association between HLA antigens and periodontal disease. Tissue Antigens. 1988;31(2):53–8. doi: 10.1111/j.1399-0039.1988.tb02063.x. [DOI] [PubMed] [Google Scholar]
- Anderson G. Chronic care: making the case for ongoing care. Princeton: Robert Wood Johnson Foundation; 2010. [Google Scholar]
- Andraws R, Berger JS, Brown DL. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA. 2005;293(21):2641–7. doi: 10.1001/jama.293.21.2641. [DOI] [PubMed] [Google Scholar]
- Andriankaja OM, Sreenivasa S, Dunford R, DeNardin E. Association between metabolic syndrome and periodontal disease. Aust Dent J. 2010;55(3):252–9. doi: 10.1111/j.1834-7819.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- Anjomshoaa I, Cooper ME, Vieira AR. Caries is associated with asthma and epilepsy. Eur J Dent. 2009;3:297–303. [PMC free article] [PubMed] [Google Scholar]
- Ashkenazy R, Abrahamson M. Medicare coverage for patients with diabetes. J Gen Intern Med. 2006;21:386–94. doi: 10.1111/j.1525-1497.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J-F. Infections and autoimmune diseases. J Autoimmun. 2005;25(1):74–80. doi: 10.1016/j.jaut.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Bailit HL, Beazoglou TJ, Formicola AJ, Tedesco LA, Brown LJ, Weaver RG. U.S. state-supported dental schools: financial projections and implications. J Dent Educ. 2008;72(2 Suppl):98–109. [PubMed] [Google Scholar]
- Baker EL, Koplan JP. Strengthening the Nation’s public health infrastructure: historic challenge, unprecedented opportunity. Health Aff. 2002;21(6):15–27. doi: 10.1377/hlthaff.21.6.15. [DOI] [PubMed] [Google Scholar]
- Barnett WS, Brown KC. Dental health policy analysis series: issues in children’s access to dental care under Medicaid. Chicago: American Dental Association; 2000. [Google Scholar]
- Baum BJ. Inadequate training in the biological sciences and medicine for dental students. J Am Dent Assoc. 2007;138:16–25. doi: 10.14219/jada.archive.2007.0004. [DOI] [PubMed] [Google Scholar]
- Bayer R, Colgrove J. Bioterrorism, public health and the law. Health Aff. 2002;21(6):98–101. doi: 10.1377/hlthaff.21.6.98. [DOI] [PubMed] [Google Scholar]
- Begovich AB, Carlton VEH, Honigberg LA, Schrodi SJ, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75(2):330–7. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- Betts RK. Fixing intelligence, foreign affairs, January/February. 2002. URL: http://www.foreignaffairs.org/20020101faessay6556/richard-k-betts/fixing-intelligence.html?mode=print. Accessed May 2011.
- Bibikova M, Lin Z, Zhou L, Chudin E, et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res. 2006;16(3):383–93. doi: 10.1101/gr.4410706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue Cross Blue Shield of Michigan. Study links good oral care to lower diabetes care costs. 2009. http://www.bcbsm.com/pr/pr_08-27-2009_71090.shtml. Accessed 26 June 2011.
- Bollen CM, Rompen EH, Demanez JP. Halitosis: a multidisciplinary problem. Rev Med Liege. 1999;54(1):32–6. [PubMed] [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980;32(3):314–31. [PMC free article] [PubMed] [Google Scholar]
- Bretz WA, Corby PM, Schork NJ, Robinson MT, et al. Longitudinal analysis of heritability for dental caries traits. J Dent Res. 2005;84(11):1047–51. doi: 10.1177/154405910508401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz WA, Weyant RJ, Corby PM, Ren D, et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc. 2005;53:1532–7. doi: 10.1111/j.1532-5415.2005.53468.x. [DOI] [PubMed] [Google Scholar]
- Broadwater C. Attention to dental coverage lacking in health-care debate. From St. Petersburg Times. 2009. http://www.tampabay.com/news/health/attention-to-dental-coverage-lacking-in-health-care-debate/1048349. Retrieved Mar 21 2011.
- Brodeur P. Community based dental education: the pipeline, profession and practice program. Robert Wood Johnson Foundation. 2008. http://www.rwjf.org/files/research/anthology2009.chapter8.pdf. Accessed 26 June 2011.
- Brown GC, Brown MM, Hiller T, Fischer D, Benson W, Magargal L. Cotton-wool spots. Retina. 1985;5(4):206–14. doi: 10.1097/00006982-198500540-00003. [DOI] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80(2):273–90. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll L. Mother’s gum disease linked to infant’s death. 2010. http://www.msnbc.msn.com/id/34979552/ns/health-oral_health/. Accessed 26 June 2011.
- Cartwright-Smith L. Chronic disease management. Health reform GPS: navigating the implementation process. 2011. http://www.healthreformgps.org/resources/chronic-disease-management/. Accessed 25 June 2011.
- Carvalho FM, Tinoco EMB, Deeley K, Duarte PM, et al. FAM5C contributes to aggressive periodontitis. PLoS One. 2010;5(4):e10053. doi: 10.1371/journal.pone.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Medicare and Medicaid Services . Data compendium. Washington, D.C.: US Government Printing Office; 2010. [Google Scholar]
- Center for Medicare and Medicaid Services. Medicare physician group practice demonstration. 2010. http://www.cms.gov/DemoProjectsEvalRpts/downloads/PGP_Fact_Sheet.pdf. Accessed 25 June 2011.
- Centers for Disease Control Dental service use and dental insurance coverage-United States, behavioral risk factor surveillance system. MMWR Morb Mortal Wkly Rep. 1997;46(50):1199–203. [PubMed] [Google Scholar]
- Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81(12):1708–24. doi: 10.1902/jop.2010.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman WW, Bridewell W, Hanbury P, Cooper GF, Buchanan BG. Evaluation of negation phrases in narrative clinical reports. Proc AMIA Symp. 2001;105–9. [PMC free article] [PubMed]
- Chasteen JE, Murphy G, Forrey A, Heid D. The health insurance and portability and accountability act: practice of dentistry in the united states: privacy and confidentiality. J Contemp Dent Pract. 2003;1(4):059–70. [PubMed] [Google Scholar]
- Chen T, Yu W-H, Izard J, Baranova OV, et al. The human oral microbiome database: a web accessible resource for investigating oral microbiome taxonomic and genomic information. Database (Oxford). 2010;Article ID baq013. [DOI] [PMC free article] [PubMed]
- Cheng Y, Hootman J, Murphy L, Langmaid G, Helmick C, Centers for Disease Control and Prevention (CDC) Prevalence of doctor diagnosed arthritis and arthritis attributable activity limitation – United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2010;59(39):1–2. [PubMed] [Google Scholar]
- CIGNA. CIGNA dental oral health maternity program. 2006. http://www.cigna.com/our_plans/programs/dental_health/oral_health_maternity_program.html. Accessed 26 June 2011.
- Cisternas M, Murphy L, Yelin E, Foreman A, Pasta D, Helmick C. Trends in medical care expenditures of US adults with arthritis and other rheumatic conditions 1997 to 2005. J Rheumatol. 2009;36(11):2531–8. doi: 10.3899/jrheum.081068. [DOI] [PubMed] [Google Scholar]
- Cloos J, Spitz MR, Schantz SP, Hsu TC, et al. Genetic susceptibility to head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88:530–5. doi: 10.1093/jnci/88.8.530. [DOI] [PubMed] [Google Scholar]
- Connelly JJ, Shah SH, Doss JF, Gadson S, et al. Genetic and functional association of FAM5C with myocardial infarction. BMC Med Genet. 2008;9:33. doi: 10.1186/1471-2350-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey LA, Nance WE, Hofstede P, Schenkein HA. Self-reported periodontal disease in a Virginia twin population. J Periodontol. 1993;64:1205–8. doi: 10.1902/jop.1993.64.12.1205. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford PJM, Aldred M, Bloch-Zupan A. Amelogenesis imperfecta. Orphanet J Rare Dis. 2007;2:17. doi: 10.1186/1750-1172-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby AH, Scherpbier-Heddema T, Wijmenga C, Altherr MR, et al. Genetic mapping of the dentinogenesis imperfecta type II locus. Am J Hum Genet. 1995;57:832–9. [PMC free article] [PubMed] [Google Scholar]
- D’Aiuto F, Sabbah W, Netuveli G, Donos N, et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J Clin Endocrinol Metab. 2008;93(1):3989–94. doi: 10.1210/jc.2007-2522. [DOI] [PubMed] [Google Scholar]
- Dall T, Zhang Y, Chen J, Quick W, Yang W, Fogli J. The economic burden of diabetes. Health Aff. 2010;29(2):297–304. doi: 10.1377/hlthaff.2009.0155. [DOI] [PubMed] [Google Scholar]
- Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4(6):431–6. doi: 10.1016/S1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- Delanghe G, Ghyselen J, Bollen C, van Steenberghe D, Vandekerckhove BN, Feenstra L. An inventory of patients’ response to treatment at a multidisciplinary breath odor clinic. Quintessence Int. 1999;30(5):307–10. [PubMed] [Google Scholar]
- Delrose DC, Steinberg RW. The clinical significance of the digital patient record. J Am Dent Assoc. 2000;131(sup):57S–60. doi: 10.14219/jada.archive.2000.0404. [DOI] [PubMed] [Google Scholar]
- Delta Dental of Wisconsin. Evidence-based integrated care plan. 2011. http://www.deltadentalwi.com/EBICP. Accessed 26 June 2011.
- Dental, Oral and Craniofacial Data Resource Center of the National Institute of Dental and Craniofacial Research . Oral health US, 2002. Washington, D.C.: US Government Printing Office; 2002. [Google Scholar]
- Dentistry Today Buyers’ guide to dental software. Dent Today. 2003;22(9):126–39. [PubMed] [Google Scholar]
- Dentrix Dental Systems (2011) HIPAA http://www.dentrix.com/support/hipaa.aspx Accessed May 2011 Buyers’ guide to dental software. Dent Today. 2011;22(9):126–39. [Google Scholar]
- Dentrix Dental Systems. HIPAA. 2003. URL: http://www.dentrix.com/support/hipaa.aspx. Accessed May 2011.
- Department of Veterans Affairs. VA health information technology improves quality of health care while reducing costs. 2010. http://www1.va.gov/opa/pressrel/pressrelease.cfm?id=1881. Accessed 26 June 2011.
- DeStefano F, Anda R, Kahn H, Williamson D, Russell C. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688–92. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictor M. Human herpesvirus 8 and Kaposi’s sarcoma. Seim Cutan Med Surg. 1997;16(3):181–7. doi: 10.1016/S1085-5629(97)80041-7. [DOI] [PubMed] [Google Scholar]
- Dunning DG, Durham TM, Lange BM, Aksu MN. Strategic management and organizational behavior in dental education: reflections on key issues in an environment of change. J Dent Educ. 2009;73:689–95. [PubMed] [Google Scholar]
- Eaton CB, Feldman HA, Assaf AR, McPhillips JB, et al. Prevalence of hypertension, dyslipidemia, and dyslipidemic hypertension. J Fam Pract. 1994;38(1):17–23. [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- eHow. About Medicaid dental coverage. From Health. 2011. http://www.ehow.com/about_5070293_medicaid-dental-coverage.html. Retrieved Mar 21 2011
- Ehrich M, Nelson MR, Stanssens P, Zabeau M, et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA. 2005;102(44):15785–90. doi: 10.1073/pnas.0507816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Gai X, Xie HM, Perin JC, et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2010;15:637–46. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst FD, Uhr K, Teumer A, Fanghanel J, et al. Replication of the association of chromosomal region 9p21.3 with generalized aggressive periodontitis (gAgP) using an independent case-control cohort. BMC Genet. 2010;11:119. doi: 10.1186/1471-2350-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errante JV. Integration of medical and dental benefits for improved overall health. Institute for Oral Health. 2007. http://www.institutefororalhealth.org/2007/ppt/IOH07_Errante.pdf. Accessed 26 June 2011
- Etzioni A. Public health Law: a communitarian perspective. Health Aff. 2002;21(6):102–4. doi: 10.1377/hlthaff.21.6.102. [DOI] [PubMed] [Google Scholar]
- Falconer DS, MacKay TFC. Introduction to quantitative genetics. 4. Essex: Harlow; 1996. [Google Scholar]
- Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34(6):416–22. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- Field MJ. Dental education at the crossroads: challenges and change. An Institute of Medicine Report. Washington, D.C.: National Academy Press; 1995. [PubMed]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Fischer J, Blanchet-Bardon C, Prud’homme J-F, Pavek S, et al. Mapping of papillon-lefevre syndrome to the chromosome 11q14 region. Eur J Hum Genet. 1997;5:156–60. [PubMed] [Google Scholar]
- Fisher ES. Building a medical neighborhood for the medical home. N Engl J Med. 2008;359(12):1202–5. doi: 10.1056/NEJMp0806233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Taylor GW, Est BT, McCarthy ET. Bidirectional relationship between chronic kidney and periodontal disease: a study using structural equation modeling. Kidney Int. 2011;79:347–55. doi: 10.1038/ki.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Piskorowski W. Clinical outreach: seven years of a self-sustaining model. J Dent Educ. 2009;73:249. [Google Scholar]
- Flores S, Mills SE, Shackelford L. Dentistry and bioterrorism. Dent Clin North Am. 2003;47(4):733–44. doi: 10.1016/j.cden.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002;287(3):356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Formicola AJ, Bailit H, Beazoglou T, Tedesco LA. The Macy study: a framework for consensus. J Dent Educ. 2005;69:1183–5. [PubMed] [Google Scholar]
- Frieden J. CMS projects focus on chronic illnesses: three demonstration projects seek to improve efficiency and care. Caring for ages. 2006. http://www.caringfortheages.com/news/geriatric-medicine/single-article/cms-projects-focus-on-chronic-illnesses-three-demonstration-projects-seek-to-improve-efficiency-and-care/7be973db97ab1f83fd4473b96d58e48a.html. Accessed 25 June 2011.
- Friedenberg FK, Rai J, Vanar V, Bongiorno C, Nelson DB, Parepally M, et al. Prevalence and risk factors for gastroesophageal reflux disease in an impoverished minority population. Obes Res Clin Pract. 2010;4(4):e261–9. doi: 10.1016/j.orcp.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frist B. Public health and national security: the critical role of increased federal support. Health Aff. 2002;21(6):117–30. doi: 10.1377/hlthaff.21.6.117. [DOI] [PubMed] [Google Scholar]
- Furr MC, Larkin E, Blakeley R, Albert TW, Tsugawa L, Weber SM. Extending multidisciplinary management of cleft palate to the developing world. J Oral Maxillofac Surg. 2011;69(1):237–41. doi: 10.1016/j.joms.2010.06.214. [DOI] [PubMed] [Google Scholar]
- Gesteland PH, Gardner RM, Tsui FC, Espino JU, Rolfs RT, James BC, Chapman WW, Moore AW, Wagner MM. Automated syndromic surveillance for the 2002 winter olympics. J Am Med Inform Assoc. 2003;10(6):547–54. doi: 10.1197/jamia.M1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi EM, Ship JA. Systemic diseases and their treatment in the elderly: impact on oral health. J Public Health Dent. 2000;60(4):297–303. doi: 10.1111/j.1752-7325.2000.tb03337.x. [DOI] [PubMed] [Google Scholar]
- Gies W. Dental education in the United States and Canada. 1926. http://www.adeagiesfoundation.org/about/Pages/AboutWilliamJGiesandtheGiesReport.aspx. Accessed 26 June 2011.
- Goldenberg A, Shmueli G, Caruana RA, Fienberg SE. Early statistical detection of anthrax outbreaks by tracking over-the-counter medication sales. Proc Natl Acad Sci USA. 2002;99(8):5237–40. doi: 10.1073/pnas.042117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RS, Dutra WO, Moreira PR. Epigenetics and periodontal disease: future perspectives. Inflamm Res. 2009;58:625–9. doi: 10.1007/s00011-009-0041-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Kulkarni H, Bolivar H, Mangano A, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–40. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- Graham RR, Cotsapas C, Davies L, Hackett R, et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat Genet. 2008;40:1059–61. doi: 10.1038/ng.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SFA, Thorleifsson G, Reynisdottir I, Benediktsson R, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Schmidt S, Seth P, Oksenberg JR, et al. Interleukin 7 receptor α chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–91. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- Grubbs V, Plantinga LC, Crews DC, Bibbins-Domingo K, Saran R, Heung M, Patel PR, Burrows NR, Ernst KL, Powe NR, for the Centers for Disease Control and Prevention CKD Surveillance Team Vulnerable populations and the association between periodontal and chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:711–7. doi: 10.2215/CJN.08270910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay AH. Dentistry’s response to bioterrorism: a report of a consensus workshop. J Am Dent Assoc. 2002;133:1181–1187. doi: 10.14219/jada.archive.2002.0359. [DOI] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, et al. Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet. 2009;41:1122–6. doi: 10.1038/ng.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha PK, Califano JA. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol. 2006;7:77–82. doi: 10.1016/S1470-2045(05)70540-4. [DOI] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TC, Hart PS, Michalec MD, Zhang Y, et al. Localisation of a gene for prepubertal periodontitis to chromosome 11q14 and identification of a cathepsin C gene mutation. J Med Genet. 2000;37(2):95–101. doi: 10.1136/jmg.37.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid DW, Chasteen J, Forrey AW. The electronic oral health record. J Contemp Dent Pract. 2002;1(3):043–54. [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316(5830):1491–3. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- Hewitt C, McCormick D, Linden G, Turk D, et al. The role of cathepsin C in papillon-lefevre syndrome, prepubertal periodontitis, and aggressive periodontitis. Hum Mutat. 2004;23(3):222–8. doi: 10.1002/humu.10314. [DOI] [PubMed] [Google Scholar]
- Hinds DA, Stuve LL, Nilsen GB, Halperin E, et al. Whole-genome patterns of common DNA variation in three human populations. Science. 2005;307(5712):1072–9. doi: 10.1126/science.1105436. [DOI] [PubMed] [Google Scholar]
- Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4(2):45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Hogan P, Dall T, Nikolov P. Economic cost of babies in the US in 2002. Alexandria: American Diabetes Association; 2002. [Google Scholar]
- Holder M, Waldman H, Hood H. Preparing health professionals to provide care to individuals with disabilities. Int J Oral Sci. 2009;1(2):54–9. doi: 10.4248/ijos.09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tennerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299(19):2287–93. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- http://www.dentaleconomics.com/index/display/article-display/1177919242/articles/dental-economics/volume-101/issue-4/practice/the-convergence-of-dental-and-medical-carehtml. Accessed 25 June 2011.
- Hudson RR. Gene genealogies and the coalescent process. In: Futuyma D, Antonovics J, editors. Oxford surveys in evolutionary biology. New York: Oxford University Press; 1991. pp. 1–44. [Google Scholar]
- Hund H, Willett W, Merchant A, Rosner B, Ascherio A, Joshipura K. Oral health and a referral arterial disease. Circulation. 2003;107:1152–9. doi: 10.1161/01.CIR.0000051456.68470.C8. [DOI] [PubMed] [Google Scholar]
- Ide R, Hoshuyama T, Takakhashi K. The effect of periodontal disease on medical and dental costs in a middle-aged japanese population: a longitudinal worksite study. J Periodontol. 2007;78(11):2120–6. doi: 10.1902/jop.2007.070193. [DOI] [PubMed] [Google Scholar]
- Independent Task Force. Council on foreign relations “America-still unprepared, still in danger”. 2011. URL: http://www.cfr.org/pdf/Homeland_Security_TF.pdf. Accessed May 2011.
- Institute for Oral Health. Focus Groups on Oral Health in Healthcare Reform – Focus Group #2. 2010. http://www.institutefororalhealth.org/2010fg/IOH-APR2010-Focus-Group-whitepaper.pdf. Accessed 26 June 2011.
- Ivanov O, Wagner MM, Chapman WW, Olszewski RT. Accuracy of three classifiers of acute gastrointestinal syndrome for syndromic surveillance. Proc AMIA Symp. 2002; 345–9. [PMC free article] [PubMed]
- Iwamoto Y, Nishimura F, Nakagawa M, Sugimoto K, et al. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol. 2001;72(6):774–8. doi: 10.1902/jop.2001.72.6.774. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Jones HB. The relative power of linkage and association studies for the detection of genes involved in hypertension. Kidney Int. 1998;53:1446–8. doi: 10.1046/j.1523-1755.1998.00925.x. [DOI] [PubMed] [Google Scholar]
- Joshipura K, Hung H, Rimm E, Willett W, Ascherio A. Periodontal disease, tooth loss and incidence of ischemic stroke. Stroke. 2003;34:47–54. doi: 10.1161/01.STR.0000052974.79428.0C. [DOI] [PubMed] [Google Scholar]
- Josiah Macy Foundation. New models of dental education. 2004. http://www.josiahmacyfoundation.org/grantees/profile/new-models-for-dental-education. Accessed 26 June 2011.
- Kaiser Family Foundation . The uninsured. Washington, D.C.: The Henry J. Kaiser Family Foundation; 2010. [Google Scholar]
- Kaplan NL, Hill WG, Weir BS. Likelihood methods for locating disease genes in nonequilibrium populations. Am J Hum Genet. 1995;56:18–32. [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–97. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Simmer JP, Hu YY, Lin BP, et al. Amelogenin p.M1T and p.W4S mutations underlying hypoplastic X-linked amelogenesis imperfecta. J Dent Res. 2004;83(5):378–83. doi: 10.1177/154405910408300505. [DOI] [PubMed] [Google Scholar]
- Kim DS, Cheang P, Dover S, Drake-Lee AB. Dental otalgia. J Laryngol Otol. 2007;121(12):1129–34. doi: 10.1017/S0022215107000333. [DOI] [PubMed] [Google Scholar]
- Kimura M. Stochastic processes in population genetics, with special reference to distribution of gene frequencies and probability of gene fixation. In: Kojima K, editor. Mathematical topics in population genetics. Berlin/New York: Springer; 1970. pp. 178–245. [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai J-Y, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L. Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nat Genet. 1999;22(2):139–44. doi: 10.1038/9642. [DOI] [PubMed] [Google Scholar]
- Kshirsagar AV, Craig RG, Moss KL, et al. Periodontal disease adversely affects the survival of patients with end-stage renal disease. Kidney Int. 2009;75:746–51. doi: 10.1038/ki.2008.660. [DOI] [PubMed] [Google Scholar]
- Kumar PS, Griffen AL, Barton JA, Paster BJ, et al. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82(5):338–44. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Yamamoto N, Onda T, Shibahara T. Expression of the FAM5C in tongue squamous cell carcinoma. Oncol Rep. 2009;22(5):1005–11. doi: 10.3892/or_00000528. [DOI] [PubMed] [Google Scholar]
- Laass MW, Hennies HC, Preis S, Stevens HP, et al. Localisation of a gene for papillon-lefevre syndrome to chromosome 11q14-q21 by homozygosity mapping. Hum Genet. 1997;101:376–82. doi: 10.1007/s004390050645. [DOI] [PubMed] [Google Scholar]
- Laine DI, Busch-Petersen J. Inhibitors of cathepsin C (dipeptidyl peptidase I) Expert Opin Ther Pat. 2010;20(4):497–506. doi: 10.1517/13543771003657172. [DOI] [PubMed] [Google Scholar]
- Laine ML, Loos BG, Crielaard W. Gene polymorphisms in chronic periodontitis. Int J Dent. 2010;1–22. Article ID 324719. [DOI] [PMC free article] [PubMed]
- Lakshminarayanan R, Bromley KM, Lei Y-P, Snead ML, Moradian-Oldak J. Perturbed amelogenin secondary structure leads to uncontrolled aggregation in amelogenesis imperfecta mutant proteins. J Biol Chem. 2010;285:40593–603. doi: 10.1074/jbc.M110.131136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lane CH, Fauci AS. Bioterrorism on the home front a new challenge for American medicine. JAMA. 2001;286(20):2595–7. doi: 10.1001/jama.286.20.2595. [DOI] [PubMed] [Google Scholar]
- Lang N, Bartold PM, Cullinan M, Jeffcoat M, et al. Consensus report: aggressive periodontitis. Ann Periodontol. 1999;4(1):53. doi: 10.1902/annals.1999.4.1.53. [DOI] [Google Scholar]
- Letter from the President. White House. 2002. URL: http://web.archive.org/web/20031224053212/http://www.whitehouse.gov/homeland/book/letterfromthepresident.pdf. Accessed May 2011.
- Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertens. 2004;22:1543–9. doi: 10.1097/01.hjh.0000125455.28861.3f. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu L, Hasturk H, Kantarci A, et al. Localized aggressive periodontitis is linked to human chromosome 1q25. Hum Genet. 2004;114(3):291–7. doi: 10.1007/s00439-003-1065-7. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Brown T, Carnethon M, Dai S, DeSimone G, et al. Heart disease & stroke statistics – 2010 update. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Long AD, Langley CH. The power of association studies to detect the contribution of candidate genetic loci to variation in complex traits. Genome Res. 1999;9:720–31. [PMC free article] [PubMed] [Google Scholar]
- Looks JW. BioSense – a national initiative for early detection and quantification of public health emergencies. MMWR Morb Mortal Wkly Rep. 2004;53(suppl):53–5. [PubMed] [Google Scholar]
- Lopez NJ, Da Silva I, Ipinza J, Gutierrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J Periodontol. 2005;76(Suppl 11):2144–53. doi: 10.1902/jop.2005.76.11-S.2144. [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez JA. A variant of PTPN22 gene conferring risk to autoimmune diseases may protect against tuberculosis. J Postgrad Med. 2010;56:242–3. [PubMed] [Google Scholar]
- Lumpkin JR. Air, water, places, and data—public health in the information age. J Public Health Manag Pract. 2001;7(6):22–30. doi: 10.1097/00124784-200107060-00003. [DOI] [PubMed] [Google Scholar]
- Lukic M, Segec A, Segeca I, Pinotic L, Pinotic K, Atalic B, et al. The role of the nutrition in the pathogenesis of gastroesophageal reflux disease, Barrett’s oesophagus and oesophageal adenocarcinoma. Coll Antropol. 2010;34(3):905–9. [PubMed] [Google Scholar]
- Lupski JR. Structural variation in the human genome. N Engl J Med. 2007;356:1169–71. doi: 10.1056/NEJMcibr067658. [DOI] [PubMed] [Google Scholar]
- Machulla HK, Stein J, Gautsch A, Langner J, et al. HLA-A, B, Cw, DRB1, DRB3/4/5, DQB1 in German patients suffering from rapidly progressive periodontitis (RPP) and adult periodontitis (AP) J Clin Periodontol. 2002;29(6):573–9. doi: 10.1034/j.1600-051X.2002.290614.x. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manski R. Dental insurance: design, need and public policy. J Am Coll Dent. 2001;69(9):9–13. [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, et al. Births: final data for 2006. National vital statistics reports; vol 57 no 7. Hyattsville: National Center for Health Statistics. 2009.
- McBride M. VA uses integrated health informatics to produce $3 billion in savings. Daily break. 2011. http://www.darkdaily.com/va-uses-integrated-health-informatics-to-produce-3-billion-in-savings-12811. Accessed 26 June 2011.
- McPherson R, Pertsemlidis A, Kavasalar N, Stewart A, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316(5830):1488–91. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade JL, de Wynter EA, Brett P, Sharif SM, et al. A family with papillon-lefevre syndrome reveals a requirement for cathepsin C in granzyme B activation and NK cell cytolytic activity. Blood. 2006;107(9):3665–8. doi: 10.1182/blood-2005-03-1140. [DOI] [PubMed] [Google Scholar]
- Mealey B, Rose L. Diabetes mellitius and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135–41. doi: 10.1097/MED.0b013e3282f824b7. [DOI] [PubMed] [Google Scholar]
- Mertz E, O’Neil E. The growing challenge of providing oral health care services to all Americans. Health Aff. 2002;21(5):65–77. doi: 10.1377/hlthaff.21.5.65. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Aeppli D, Virag JG, Klump DG, et al. Periodontal findings in adult twins. J Periodontol. 1991;62:293–9. doi: 10.1902/jop.1991.62.5.293. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, et al. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000;71:1699–707. doi: 10.1902/jop.2000.71.11.1699. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, et al. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000;71:1699–707. doi: 10.1902/jop.2000.71.11.1699. [DOI] [PubMed] [Google Scholar]
- Miller L, Manwell M, Newbold D. The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol. 1992;63:843–8. doi: 10.1902/jop.1992.63.10.843. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Casagni F, Madianos PN. Can presence or absence of periodontal pathogens distinguish between subjects with chronic and aggressive periodontitis? A systematic review. J Clin Periodontol. 2002;29:10–21. doi: 10.1034/j.1600-051X.29.s3.1.x. [DOI] [PubMed] [Google Scholar]
- Montana Business Journal. Employer survey on health insurance in Montana. 2006. From the free library: http://www.thefreelibrary.com/2006+Employer+Survey+on+health+insurance+in+Montana.-a0172012572. Retrieved Mar 21 2011.
- Mucci LA, Bjorkman L, Douglass CW, Pedersen NL. Environmental and heritable factors in the etiology of oral diseases – a population-based study of Swedish twins. J Dent Res. 2005;84(9):800–5. doi: 10.1177/154405910508400904. [DOI] [PubMed] [Google Scholar]
- Mullins C, Cohen L, Magder L, Manski R. Medicaid coverage and utilization of adult dental services. J Health Care Poor Underserved. 2004;15(4):672–88. doi: 10.1353/hpu.2004.0068. [DOI] [PubMed] [Google Scholar]
- Nadeau JH. Transgenerational genetic effects on phenotypic variation and disease risk. Hum Mol Genet. 2009;18:R202–10. doi: 10.1093/hmg/ddp366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelberg RH (2011) The convergence of dental and medical care. Dental Economics.
- Narcisi JP. The American dental association’s commitment to electronic data interchange. J Dent Educ. 1996;60(1):28–32. [PubMed] [Google Scholar]
- National Institute of Dental and Craniofacial Research. Dentistry’s role in responding to bioterrorism and other catastrophic events. 2004. URL: http://www.nidcr.nih.gov/CareersAndTraining/DentistryCatastrophicEvents.htm. Accessed May 2011.
- National Institute of Dental and Craniofacial Research. (2011). National Institutes of Health Website: http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/DentalCaries/. Accessed 18 June 2011.
- Nesbitt MJ, Reynolds MA, Shiau H, Choe K, et al. Association of periodontitis and metabolic syndrome in the Baltimore longitudinal study of aging. Aging Clin Exp Res. 2010;22(3):238–42. doi: 10.1007/bf03324802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SB, Buckingham KJ, Lee C, Bigham AW, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42(1):30–5. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos GK, Dimou NL, Hamodrakas SJ, Bagos PG. Cytokine gene polymorphisms in periodontal disease: a meta-analysis of 53 studies including 4178 cases and 4590 controls. J Clin Periodontol. 2008;35(9):754–67. doi: 10.1111/j.1600-051X.2008.01298.x. [DOI] [PubMed] [Google Scholar]
- Oral Health America . Keep America smiling: oral health in America. Chicago: Oral Health America; 2003. [Google Scholar]
- Paju S, Scannapeico F. Oral biofilms, periodontitis and pulmonary infections. Oral Dis. 2007;13(6):508–12. doi: 10.1111/j.1601-0825.2007.01410a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. Dental leaders review roles in bioterrorism response. American Dental Association NEWS. 2003. ADA.org URL: http://web.archive.org/web/20060219190632/, http://www.ada.org/prof/resources/pubs/adanews/adanewsarticle.asp?articleid=390. Accessed May 2011.
- Partnership for Solutions National Program Office . Chronic conditions: making the case for ongoing care. Baltimore: Johns Hopkins University; 2004. [Google Scholar]
- Patir A, Seymen F, Yildirim M, Deeley K, et al. Enamel formation genes are associated with high caries experience in Turkish children. Caries Res. 2008;42(5):394–400. doi: 10.1159/000154785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poage GM, Christensen BC, Houseman EA, McClean MD, et al. Genetic and epigenetic somatic alterations in head and neck squamous cell carcinomas are globally coordinated but not locally targeted. PLoS One. 2010;5(3):e9651. doi: 10.1371/journal.pone.0009651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokholok DK, Harbison CT, Levine S, Cole M, et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–27. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Polverini P. The Ann Arbor dental deans forum: crafting a response to the emerging tiered system of dental education global. Health Nexus, College of Dentistry, New York University Summer 2010 Vol. 12, No. 2. 2010.
- Rabinowitz HK, Diamond JJ, Markham FW, Wortman JR. Medical school programs to increase the rural physician supply: a systematic review and projected impact of widespread replication. Acad Med. 2008;83:235–43. doi: 10.1097/ACM.0b013e318163789b. [DOI] [PubMed] [Google Scholar]
- Rao NV, Rao GV, Hoidal JR. Human dipeptidyl-peptidase I. J Biol Chem. 1997;272:10260–5. doi: 10.1074/jbc.272.15.10260. [DOI] [PubMed] [Google Scholar]
- Ram S, Teruel A, Kumar SK, Clark G. Clinical characteristics and diagnosis of atypical odontalgia: implications for dentists. J Am Dent Assoc. 2009;140(2):223–8. doi: 10.14219/jada.archive.2009.0136. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–74. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- Redman D, Jones M, Rangan B, Reimold R, Mikuls T, Amdur R, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81(2):223–30. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- Ren B, Robert F, Wyrick JJ, Aparicio O, et al. Genome-wide location and function of DNA binding proteins. Science. 2000;290:2306–9. doi: 10.1126/science.290.5500.2306. [DOI] [PubMed] [Google Scholar]
- Reshetin VP, Regens JL. Simulation modeling of anthrax spore dispersion in a bioterrorism incident. Risk Anal. 2003;23(6):1135–45. doi: 10.1111/j.0272-4332.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- Rhodes PR. The computer-based oral health record. J Dent Educ. 1996;60(1):14–8. [PubMed] [Google Scholar]
- Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripa LW. A half-century of community water fluoridation in the United States: review and commentary. J Public Health Dent. 1993;53(1):17–44. doi: 10.1111/j.1752-7325.1993.tb02666.x. [DOI] [PubMed] [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–7. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Robert Wood Johnson Foundation Pipeline Study. Evaluation of pipeline, profession and practice: community-based dental education. 2007. http://www.rwjf.org/pr/product.jsp?id=15455. Accessed 26 June 2011.
- Rodriguez-Lozano FJ, Sanchez-Perez A, Moya-Villaescusa MJ, Rodriguez-Lozano A, Saez-Yuguero MR. Neuropathic orofacial pain after dental implant placement: review of the literature and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):e8–12. doi: 10.1016/j.tripleo.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Rosenblum R., Jr Oral hygiene can reduce the incidence of and death resulting from pneumonia and respiratory tract infection. J Am Dent Assoc. 2010;141(9):1117–8. doi: 10.14219/jada.archive.2010.0342. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, et al. Effect of daily asprin on long-term risk of death due to cancer: analysis of individual patient data from randomized trails. Lancet. 2010;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- Rubenstein H. Access to oral health care for elders: mere words or action? J Dent Educ. 2005;69(9):1051–8. [PubMed] [Google Scholar]
- Ryan ME. Reconnecting the head to the body. Dental considerations for the optimal medical management of people with diabetes. Seattle: Institute for Oral Health; 2007. pp. 15–7. [Google Scholar]
- Safrany E, Melegh B. Functional variants of the interleukin-23 receptor gene in non-gastrointestinal autoimmune diseases. Curr Med Chem. 2009;16(28):3766–74. doi: 10.2174/092986709789104975. [DOI] [PubMed] [Google Scholar]
- Saiki RK, Scharf S, Faloona F, Mullis KB, et al. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–4. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Saikku P, Leinonen M, Mattila K, Ekman MR, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2(8618):983–6. doi: 10.1016/S0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- Saito I, Miyamura T, Ohbayashi A, Harada H, et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87(17):6547–9. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Lida M, et al. The severity of periodontal disease is associated with the element of glucose intolerance in non-diabetics. J Dent Res. 2004;83:485. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- Salyer KE, Xu H, Portnof JE, Yamada A, Chong DK, Genecov ER. Skeletal facial balance and harmony in the cleft patient: principles and techniques in orthognathic surgery. Indian J Plast Surg. 2009;42(Suppl):S149–67. doi: 10.4103/0970-0358.57196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saremi A, Nelson R, Tulloch-Reid M. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27–32. doi: 10.2337/diacare.28.1.27. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA. Pneumonia in nonambulatory patients. J Am Dent Assoc. 2006;137(2):21S–5. doi: 10.14219/jada.archive.2006.0400. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, et al. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 2009;5(2):e1000378. doi: 10.1371/journal.pgen.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Nothnagel M, Manke T, et al. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Hum Mol Genet. 2010;19(3):553–62. doi: 10.1093/hmg/ddp508. [DOI] [PubMed] [Google Scholar]
- Schleyer TK. Integrating dental office technology – the next frontier. Dent Abstr. 2003;48(3):112–3. [Google Scholar]
- Schleyer T, Eisner J. The computer-based oral health record: an essential tool for cross-provider quality management. J Calif Dent Assoc. 1997;22(11):57–64. [PubMed] [Google Scholar]
- Schleyer TK, Spallek H, Bartling WC, Corby P. The technologically well-equipped dental office. J Am Dent Assoc. 2003;134(1):30–41. doi: 10.14219/jada.archive.2003.0015. [DOI] [PubMed] [Google Scholar]
- Schleyer TK, Thyvalikakath TP, Spallek H, Torres-Urquidy MH, Hernandez P, Yuhaniak J. Clinical computing in general dentistry. J Am Med Inform Assoc. 2006;13(3):344–52. doi: 10.1197/jamia.M1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotterer C. The evolution of molecular markers – just a matter of fashion? Nat Rev Genet. 2004;5:63–70. doi: 10.1038/nrg1249. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Reynolds R, Maller J, Fagerness JA, et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Opthalmol Vis Sci. 2008;50(5):2044–53. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfikas PM. HIPAA security regulations protecting patients’ electronic health information. J Am Dent Assoc. 2003;134:640–3. doi: 10.14219/jada.archive.2003.0234. [DOI] [PubMed] [Google Scholar]
- Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, Pinsky JL, Klein R. Retinal arteriolar diameters and elevated blood pressure: the atherosclerosis risk in communities study. Am J Epidemiol. 1999;150:263–70. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev. 2010;12(5):CD004714. doi: 10.1002/14651858.CD004714.pub2. [DOI] [PubMed] [Google Scholar]
- Slade G, Ghezzi E, Heiss G, Beck J, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the atherosclerosis risk in communities study. Arch Intern Med. 2003;163:1172–9. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- Slade G, Ghezzi E, Heiss G, Beck J, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the atherosclerosis risk in communities study. Arch Intern Med. 2003;163:1172–9. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- Song YL, Wang CN, Fan MW, Su B, Bian Z. Dentin phosphoprotein frameshift mutations in hereditary dentin disorders and their variation patterns in normal human populations. J Med Genet. 2008;45:457–64. doi: 10.1136/jmg.2007.056911. [DOI] [PubMed] [Google Scholar]
- Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham heart study. Hepatology. 2010;51(6):1979–87. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CJ, Neubert JK, Gremillion H, Zakrzewska JM, Ohrbach R. Toothache or trigeminal neuralgia: treatment dilemmas. J Pain. 2008;9(9):767–70. doi: 10.1016/j.jpain.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Stark PC, Kalenderian E, White JM, Walji MF, Stewart DCL, Kimmes N, Meng TR, Jr, Willis GP, DeVries T, Chapman RJ. Consortium for oral health-related informatics: improving dental research, education, and treatment. J Dent Educ. 2010;74:1051–65. [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OPH, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbacher DM, Glick M. The dental patient with asthma: an update and oral health considerations. J Am Dent Assoc. 2001;132:1229–39. doi: 10.14219/jada.archive.2001.0365. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–10. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- Swartz K. Projected costs of chronic disease. Health care cost monitor. 2011. http://healthcarecostmonitor.thehastingscenter.org/kimberlyswartz/projected-costs-of-chronic-diseases/. loose lind Accessed 25 June 2011.
- Szekely DG, Milam S, Khademi JA. Legal issues of the electronic dental record: security and confidentiality. J Dent Educ. 1996;60(1):19–23. [PubMed] [Google Scholar]
- The International HapMap 3 Consortium Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–8. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Electronic Disease Surveillance System Working Group National Electronic Disease Surveillance System (NEDSS): a standards-based approach to connect public health and clinical medicine. J Public Health Manag Pract. 2001;7(6):42–50. [PubMed] [Google Scholar]
- The Pew Center on the States. Help wanted: a policy maker’s guide to new dental providers. National Academy for State Health Policy, WK Kellogg Foundation. 2009. http://www.pewcenteronthestates.org/uploadedFiles/Dental_Report_final_Low%20Res.pdf. Accessed 26 June 2011.
- Thorpe K, Ogden L, Galactionova K. Chronic conditions account for rise in Medicare spending from 1987 to 2006. Health Aff. 2010;29(4):718–25. doi: 10.1377/hlthaff.2009.0474. [DOI] [PubMed] [Google Scholar]
- Thorstensson H, Kuylensteima J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics. J Clin Periodontol. 1996;23:194–202. doi: 10.1111/j.1600-051X.1996.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Toomes C, James J, Wood AJ WUCL, et al. Loos-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet. 1999;23(4):421–4. doi: 10.1038/70525. [DOI] [PubMed] [Google Scholar]
- Torres-Urquidy MH, Wallstrom G, Schleyer TK. Detection of disease outbreaks by the use of oral manifestations. J Dent Res. 2009;88(1):89–94. doi: 10.1177/0022034508327546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui FC, Espino JU, Dato VM, Gesteland PH, Hutman J, Wagner MM. Technical description of RODS: a real-time public health surveillance system. J Am Med Inform Assoc. 2003;10(5):399–408. doi: 10.1197/jamia.M1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Oral health in American; surgeon general’s call to action. Rockville: USDHHS, National Institute of Dental and Craniofacial Research, Nation Institutes of Health; 2003. [Google Scholar]
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- UnitedHealth Center for Health Reform & Modernization . The United States of diabetes: challenges and opportunities in the decade ahead. Minnetonka: UnitedHealth Group; 2010. [Google Scholar]
- US Census Bureau . Statistical abstract of the US. Washington, D.C.: US Government Printing Office; 2011. [Google Scholar]
- US Department of Health and Human Services. Healthy people 2020. 2010. http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf. Accessed 26 June 2011.
- Valachovic R. Opportunities abound for new dental schools. How will we seize them? American dental education association charting progress. 2009. http://www.adea.org/about_adea/Documents/Charting%20Progress/ADEAs_Charting_Progress_August_2009.htm. Accessed 26 June 2011.
- Valachovic R. Holding ourselves to the highest standard: doing what’s best for patients. American dental education association charting progress. 2010. http://www.adea.org/about_adea/Documents/Charting%20Progress/ADEAs_Charting_Progress_October_2010.htm. Accessed 26 June 2011.
- van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73(3):672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, et al. The sequence of the human genome. Science. 2001;291(5507):1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vieira AR, Marazita ML, Goldstein-McHenry T. Genome-wide scan find suggestive caries loci. J Dent Res. 2008;87:435–9. doi: 10.1177/154405910808700506. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Hill WG, Wray NR. Heritability in the genomics era – concepts and misconceptions. Nat Rev Genet. 2008;9:255–66. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- Wagner MM, Moore AW, Aryel RM. Handbook of biosurveillance. Burlington: Elsevier Academic Press; 2006. [Google Scholar]
- Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wangsrimongkol T, Jansawang W. The assessment of treatment outcome by evaluation of dental arch relationships in cleft lip/palate. J Med Assoc Thai. 2010;93(Suppl 4):S100–6. [PubMed] [Google Scholar]
- Watson C, Alp NJ. Role of Chlamydia pneumoniae in atherosclerosis. Clin Sci. 2008;114:509–31. doi: 10.1042/CS20070298. [DOI] [PubMed] [Google Scholar]
- Weber JL, May PE. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989;44:388–96. [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werther W, Chu L, Holekamp N, Do D, Rubio R. Myocardial infarction and cerebrovascular accident in patients with retinal vein occlusion. Arch Ophthalmol. 2011;129:326–31. doi: 10.1001/archophthalmol.2011.2. [DOI] [PubMed] [Google Scholar]
- White House Office of the Press Secretary. Fact sheet project bioshield. 2003. URL: http://web.archive.org/web/20090116121142/, http://www.whitehouse.gov/news/releases/2003/02/print/20030203.html. Accessed: May 2011.
- White JM, Kalenderian E, Stark PC, Ramoni RL, Vaderhobli R, Walji MF. Evaluating a dental diagnostic terminology in an electronic health record. J Dent Educ. 2011;75:605–15. [PMC free article] [PubMed] [Google Scholar]
- Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials – fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23(3):343–62. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Wolff A, Waldman B, Milano M, Perlman S. Dental students’ experiences with and attitudes toward people with mental retardation. J Am Dent Assoc. 2004;135:353–7. doi: 10.14219/jada.archive.2004.0187. [DOI] [PubMed] [Google Scholar]
- Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and morality. Br Med Bull. 2005;73/74:57–70. doi: 10.1093/bmb/ldh050. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the atherosclerosis risk in communities study. Lancet. 2001;258:1134–40. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- Wong TY, Hubbard LD, Klein R, Marino EK, Kronmal R, Sharrett AR, Siscovick DS, Burke G, Tielsch JM. Retinal microvascular abnormalities and blood pressure in older people: the cardiovascular health study. Br J Ophthalmol. 2002;86:1007–13. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BE, Hubbard LD, Duncan BB. Retinal arteriolar narrowing and risk of diabetes in middle-aged persons. JAMA. 2002;287:2528–33. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and ten-year cardiovascular abnormality: a population-based case-control study. Ophthalmology. 2003;110:933–40. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Wong WK, Moore A, Cooper G, Wagner M. WSARE: What’s strange about recent events? J Urban Health. 2003;80(2 Suppl 1):i66–75. doi: 10.1007/PL00022317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Rosamund W, Chang PP, Couper DJ, Sharrett AR, Hubbard LD, Folsom AR, Klein R. Retinopathy and risk of congestive heart failure. JAMA. 2005;293:63–9. doi: 10.1001/jama.293.1.63. [DOI] [PubMed] [Google Scholar]
- Wong TY, Shankar A, Klein R, Klein BEK, Hubbard LD. Retinal arteriolar narrowing, hypertension and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165:1060–5. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- Wu T, Trevisan M, Genco R, Dorn J, Falkner K, Sempos C. Periodontal disease and risk of cerebrovascular disease. Arch Intern Med. 2000;160:2749–56. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- Yelin E, Cisternas M, Foreman A, Pasta D, Centers for Disease Control and Prevention (CDC) National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions – United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(1):4–7. [PubMed] [Google Scholar]
- Zackrisson AL, Lindblom B, Ahlner J. High frequency of occurrence of CYP2D6 gene duplication/multiduplication indicating ultrarapid metabolism along suicide cases. Clin Pharmacol Ther. 2010;88:354–9. doi: 10.1038/clpt.2009.216. [DOI] [PubMed] [Google Scholar]
- Zhang J. The digital pioneer. The Wall Street Journal. 2009. http://online.wsj.com/article/SB10001424052970204488304574428750133812262.html. Accessed 26 June 2011.
- Zhang Y, Syed R, Uygar C, Pallos D, et al. Evaluation of human leukocyte N-formylpeptide receptor (FPR1) SNPs in aggressive periodontitis patients. Genes Immun. 2003;4:22–9. doi: 10.1038/sj.gene.6363900. [DOI] [PubMed] [Google Scholar]
Integration of Pediatric Medical and Pediatric Dental Care
- Acs G, Shulman R, Ng MW, Chussid S. The effect of dental rehabilitation on the body weight of children with early childhood caries. Pediatr Dent. 1999;21:109–13. [PubMed] [Google Scholar]
- American Academy of Pediatrics Bright Futures Guidelines. 2008. http://brightfutures.aap.org/pdfs/AAP%20Bright%20Futures%20Periodicity%20Sched%20101107.pdf Accessed 7 June 2011.
- American Academy of Pediatrics. Recommendations for preventive pediatric health care (periodicity schedule). 2008. http://practice.aap.org/content.aspx?aid=1599 Accessed 28 June 2011.
- American Academy of Pediatric Dentistry. Dental care for your baby. 2011. http://www.aapd.org/publications/brochures/babycare.asp. Accessed 28 June 2011.
- Broadbent JM, Thomson WM, Williams SM. Does caries in primary teeth predict enamel defects in permanent teeth? A Longitudinal Study. J Dent Res. 2005. doi:10.1177/154405910508400310. [DOI] [PubMed]
- California Dental Association . Oral health during pregnancy and early childhood: evidence-based guidelines for health professionals. Sacramento: California Dental Association; 2010. [PubMed] [Google Scholar]
- Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: the human and economic cost of early childhood caries. J Am Dent Assoc. 2009;140(6):650–7. doi: 10.14219/jada.archive.2009.0250. [DOI] [PubMed] [Google Scholar]
- Caspary G, Krol DM, Boulter S, Keels MA, Romano-Clarke G. Perceptions of oral health training and attitudes toward performing oral health screenings among graduating pediatric residents. Pediatrics. 2008;122(2):e465–71. doi: 10.1542/peds.2007-3160. [DOI] [PubMed] [Google Scholar]
- Catalanotto F. Role of the medical team in preventing early childhood caries. 2010. http://allkids.mediasite.com/mediasite/Viewer/?peid=bab7ac16fa624951b3d1a4f604fc92c0. Accessed 5 Feb 2011.
- CDC. Centers for disease control and prevention. Vital and health statistics. Series 10, No. 244. 2008. http://www.cdc.gov/nchs/data/series/sr_10/sr10_244.pdf. Accessed 17 Jul 2011.
- Census Bureau (2010) Census Bureau quick facts. (2010). http://quickfacts.census.gov/qfd/states/00000.html. Accessed 17 Jul 2011.
- Dye B, Thornton-Evans G. Trends in oral health by poverty status as measured by HP 2010 objectives. Public Health Rep. 2010;125(6):817–30. doi: 10.1177/003335491012500609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein BL, Chinn CH. Update on disparities in oral health and access to dental care for America’s children. Acad Pediatr. 2009;9(6):415–9. doi: 10.1016/j.acap.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Ezer MS, Swoboda NA, Farkouh DR. Early childhood caries: the dental disease of infants. Oral Hlth J. 2010; 1–7.
- Ferullo A, Silk H, Savageau JA. Teaching oral health in U.S. Medical Schools: results of a national survey. Acad Med. 2010;86(2):226–30. doi: 10.1097/ACM.0b013e3182045a51. [DOI] [PubMed] [Google Scholar]
- Fiks AG, Alessandrini EA, Forrest CB, Khan S, Localio AR, Gerber A. Electronic medical record use in pediatric primary care. J Am Med Inform Assoc. 2011. doi:10.1136/jamia.2010.004135. [DOI] [PMC free article] [PubMed]
- Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, Shamonki JM, Redline RW. Term stillbirth caused by oral fusobacterium nucleatum. Obstet Gynecol. 2010;115(2):442–5. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer S. Integrated medical and dental health in primary care. J Spec Pediatr Nurs. 2007;12(1):61–5. doi: 10.1111/j.1744-6155.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Advancing oral health in America. Washington, D.C.: The National Academies Press; 2011. [Google Scholar]
- IOM (Institute of Medicine) Improving access to oral health care for vulnerable and underserved populations. Washington, D.C.: The National Academies Press; 2011. [Google Scholar]
- Lannon CM, Flower K, Duncan P, Moore KS, Stuart J. The bright futures training project: implementing systems to support preventive and developmental services in practice. Pediatrics. 2008. doi:10.1542/peds.2007-2700. [DOI] [PubMed]
- Lewis CW. Dental care and children with special health care needs: a population-based perspective. Acad Pediatr. 2009;9(6):420–6. doi: 10.1016/j.acap.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Johnston B, Linsenmeyer K, Williams A, Mouradian W. Preventive dental care for children in the US: a national perspective. Pediatrics. 2007;119:E544–53. doi: 10.1542/peds.2006-1958. [DOI] [PubMed] [Google Scholar]
- Lewis CW, Boulter S, Keels MA, Krol DM, Mouradian WE, O’Connor KG, Quinonez RB. Oral health and pediatricians: results of a national survey. Acad Pediatr. 2009;9(6):457–61. doi: 10.1016/j.acap.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang W. Predicting caries in permanent teeth from caries in primary teeth: an eight-year cohort study. J Dent Res. 2002;81(8):561–6. doi: 10.1177/154405910208100812. [DOI] [PubMed] [Google Scholar]
- Mabry CC, Mosca NG. Interprofessional educational partnerships in school health for children with special oral health needs. J Dent Educ. 2006;70(8):844–50. [PubMed] [Google Scholar]
- Marrs J, Trumbley S, Malik G. Early childhood caries: determining the risk factors and assessing the prevention strategies for nursing intervention. Pediatr Nurs. 2011;37(1):9–15. [PubMed] [Google Scholar]
- Mertz E, Mouradian W. Addressing children’s oral health in the new millennium: trends in the dental workforce. Acad Pediatr. 2009;9(6):443–9. doi: 10.1016/j.acap.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradian W. Making decisions for children. Angle Orthod. 1999;69(4):300–5. doi: 10.1043/0003-3219(1999)069<0300:MDFC>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Mouradian WE, Wehr E, Crall JJ. Disparities in children’s oral health and access to dental care. J Am Med Assoc. 2000;284(20):2625–31. doi: 10.1001/jama.284.20.2625. [DOI] [PubMed] [Google Scholar]
- Mouradian W, Slayton R, Maas W, Kleinman DV, Slavkin H, DePaola D, Evans C, Jr, Wilentz J. Executive summary: progress in children’s oral health since the 2000 surgeon general’s report. Acad Pediatr. 2009;9(6):374–9. doi: 10.1016/j.acap.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Nash DA. Adding dental therapists to the health care team to improve access to oral health care for children. Acad Pediatr. 2009;9(6):446–51. doi: 10.1016/j.acap.2009.08.005. [DOI] [PubMed] [Google Scholar]
- New York State Department of Health. Oral health care during pregnancy and childhood. 2006. Available at: http://www.health.state.ny.us/publications/0824.pdf.
- Otto M. For want of a dentist. 2007. Washington Post, February 28, B01.
- Ramos-Gomez FJ, Shepherd DS. Cost-effectiveness model for the prevention of early childhood caries. J Calif Dent Assoc. 1999;1999:1–11. [PubMed] [Google Scholar]
- Ressler-Maerlender J, Krishna R, Robison V. Oral health during pregnancy: current research at the Division of Oral Health, Centers for Disease Control and Prevention. J Womens Health. 2005. doi:10.1089/jwh.2005.14.880. [DOI] [PubMed]
- Savage MF, Lee JY, Kotch JB, Vann Jr WF. Early preventive dental visits: effects on subsequent utilization and costs. Pediatrics. 2004. doi:10.1542/peds.2003-0469-F. [DOI] [PubMed]
- Seale N, McWhorter A, Mouradian W. Dental education’s role in improving children’s oral health and access to care. Acad Pediatr. 2009;9(6):440–5. doi: 10.1016/j.acap.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Wolfe JD, Weber-Gasparoni JD, Kane MJ, Qian F. Survey of Iowa general dentists regarding the age 1 dental visit. Pediatr Dent. 2006;28(4):325–31. [PubMed] [Google Scholar]
Integration of Patient Records and Clinical Research: Lack of Integration as a Barrier to Needed Research; Integration as a Prerequisite to Research
- Atkinson JC, Zeller GG, Shah C. Electronic patient records for dental school clinics: more than paperless systems. J Dent Educ. 2002;66(5):634–42. [PubMed] [Google Scholar]
- Barasch A, Cunha-Cruz J, Curro FA, Hujoel P, Sung AH, Vena D, et al. Risk factors for osteonecrosis of the jaws: a case–control study from the CONDOR dental PBRN. J Dent Res. 2011;90(4):439–44. doi: 10.1177/0022034510397196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501–4. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20(2):109–17. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood) 2011;30(3):464–71. doi: 10.1377/hlthaff.2011.0178. [DOI] [PubMed] [Google Scholar]
- Chambers S, Spence J, Taylor D, Walji M, Valenza JA. Assessing user perceptions of an electronic patient record (EPR) before implementation. AMIA Annu Symp Proc. 2007;2007:896. [PubMed] [Google Scholar]
- Chute CG, Cohn SP, Campbell KE, Oliver DE, Campbell JR. The content coverage of clinical classifications. For the computer-based patient record Institute’s Work Group on codes & structures. J Am Med Inform Assoc. 1996;3(3):224–33. doi: 10.1136/jamia.1996.96310636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino JJ. From data to knowledge through concept-oriented terminologies: experience with the Medical Entities Dictionary. J Am Med Inform Assoc. 2000;7(3):288–97. doi: 10.1136/jamia.2000.0070288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congressional Budget Office. Research on the comparative effectiveness of medical treatments: issues and options for an expanded federal role Pub. No. 2975. 2007. http://www.cbo.gov/ftpdocs/88xx/doc8891/12-18-ComparativeEffectiveness.pdf. Accessed 10 June 2011.
- Day S, Fayers P, Harvey D. Double data entry: what value, what price? Control Clin Trials. 1998;19(1):15–24. doi: 10.1016/S0197-2456(97)00096-2. [DOI] [PubMed] [Google Scholar]
- Deshmukh VG, Meystre SM, Mitchell JA. Evaluating the informatics for integrating biology and the bedside system for clinical research. BMC Med Res Methodol. 2009;9:70. doi: 10.1186/1471-2288-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Emam K, Jonker E, Sampson M, Krleza-Jeric K, Neisa A. The use of electronic data capture tools in clinical trials: web-survey of 259 Canadian trials. J Med Internet Res. 2009;11(1):e8. doi: 10.2196/jmir.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows JL, Rindal DB, Barasch A, Gullion CM, Rush W, Pihlstrom DJ, et al. ONJ in Two dental practice-based research network regions. J Dent Res. 2011;90(4):433–8. doi: 10.1177/0022034510387795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LJ, Ceusters W, Eisner J, Smith B. The significance of SNODENT. Stud Health Technol Inform. 2005;116:737–42. [PubMed] [Google Scholar]
- Grabowski H, Vernon J, DiMasi JA. Returns on research and development for 1990s new drug introductions. Pharmacoeconomics. 2002;20(Suppl 3):11–29. doi: 10.2165/00019053-200220003-00002. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JD, Newhouse MM, Bagniewska A, Hawkins BS. Data collection and transcription. Control Clin Trials. 1995;16(2 Suppl):66S–103. doi: 10.1016/0197-2456(94)00094-J. [DOI] [PubMed] [Google Scholar]
- Kalenderian E, Ramoni RL, White JM, Schoonheim-Klein ME, Stark PC, Kimmes NS, et al. The development of a dental diagnostic terminology. J Dent Educ. 2010;75(1):68–76. [PMC free article] [PubMed] [Google Scholar]
- King DW, Lashley R. A quantifiable alternative to double data entry. Control Clin Trials. 2000;21(2):94–102. doi: 10.1016/S0197-2456(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Kleinman K. Adaptive double data entry: a probabilistic tool for choosing which forms to reenter. Control Clin Trials. 2001;22(1):2–12. doi: 10.1016/S0197-2456(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Langabeer JR, 2nd, Walji MF, Taylor D, Valenza JA. Economic outcomes of a dental electronic patient record. J Dent Educ. 2008;72(10):1189–200. [PubMed] [Google Scholar]
- Manheimer E, Anderson D. Survey of public information about ongoing clinical trials funded by industry: evaluation of completeness and accessibility. BMJ. 2002;325(7363):528–31. doi: 10.1136/bmj.325.7363.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray AT. Better access to information about clinical trials. Ann Intern Med. 2000;133(8):609–14. doi: 10.7326/0003-4819-133-8-200010170-00013. [DOI] [PubMed] [Google Scholar]
- Rondel RKVS, Webb CF. Clinical data management. 2. Chichester: Wileys; 2000. [Google Scholar]
- Schleyer TK, Thyvalikakath TP, Spallek H, Torres-Urquidy MH, Hernandez P, Yuhaniak J. Clinical computing in general dentistry. J Am Med Inform Assoc. 2006;13(3):344–52. doi: 10.1197/jamia.M1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SW, Meinert CL. Format-independent data collection forms. Control Clin Trials. 1995;16(6):363–76. doi: 10.1016/S0197-2456(95)00016-X. [DOI] [PubMed] [Google Scholar]
- Spence J, Valenza JA, Taylor D. Documentation of clinical workflow: a key step in a plan to facilitate implementation of an electronic patient record. AMIA Annu Symp Proc. 2007;2007:1119. [PubMed] [Google Scholar]
- Stark PC, Kalenderian E, White JM, Walji MF, Stewart DC, Kimmes N, et al. Consortium for oral health-related informatics: improving dental research, education, and treatment. J Dent Educ. 2010;74(10):1051–65. [PMC free article] [PubMed] [Google Scholar]
- Strang N, Cucherat M, Boissel JP. Which coding system for therapeutic information in evidence-based medicine. Comput Methods Programs Biomed. 2002;68(1):73–85. doi: 10.1016/S0169-2607(01)00151-1. [DOI] [PubMed] [Google Scholar]
- Taylor D, Valenza JA, Spence JM, Baber RH. Integrating electronic patient records into a multi-media clinic-based simulation center using a PC blade platform: a foundation for a new pedagogy in dentistry. AMIA Annu Symp Proc. 2007;2007:1129. [PubMed] [Google Scholar]
- Valenza JA, Walji M. Creating a searchable digital dental radiograph repository for patient care, teaching and research using an online photo management and sharing application. AMIA Annu Symp Proc. 2007;2007:1143. [PubMed] [Google Scholar]
- Walji M, Loeffelholz J, Valenza JA. A human-centered design of a dental discharge summary (DDS) for patients. AMIA Annu Symp Proc. 2007;2007:1146. [PubMed] [Google Scholar]
- Walji MF, Taylor D, Langabeer JR, 2nd, Valenza JA. Factors influencing implementation and outcomes of a dental electronic patient record system. J Dent Educ. 2009;73(5):589–600. [PubMed] [Google Scholar]
- Walji MF, Taylor D, Langabeer JR, 2nd, Valenza JA. Factors influencing implementation and outcomes of a dental electronic patient record system. J Dent Educ. 2009;73(5):589–600. [PubMed] [Google Scholar]