Abstract
Previously, we have revealed that prolonged administration of a polyphenol-rich 0.1% extract from the berries of Aronia melanocarpa L. (chokeberries) alone and under chronic exposure to cadmium influences the body status of zinc (Zn) and copper (Cu). The aim of this study was to evaluate, in an in vitro model, the chelating properties of the extract (0.05% and 0.1%) and its main polyphenolic ingredients (cyanidin 3-O-β-galactoside, chlorogenic acid, neochlorogenic acid, (+)-catechin, (−)-epicatechin, quercetin, and kaempferol) regarding divalent ions of Zn (Zn2+) and Cu (Cu2+) at pH reflecting physiological conditions at the gastrointestinal tract such as 2 (empty stomach), 5.5 (full stomach), and 8 (duodenum). The study has revealed that the extract from Aronia berries, as well as cyanidin 3-O-β-galactoside and quercetin, can bind Zn2+ and Cu2+, but only at pH 5.5. Moreover, kaempferol was able to chelate Zn2+ at pH 5.5; however, this ability was weaker than those of cyanidin 3-O-β-galactoside and quercetin. The ability of the chokeberry extract to chelate Zn2+ and Cu2+ may be explained, at least partially, by the presence of polyphenols such as anthocyanin derivatives of cyanidin and quercetin. The findings seem to suggest that Aronia products, used as supplements of a diet, should be consumed before meals, and particular attention should be paid to adequate intake of Zn and Cu under prolonged consumption of these products to avoid deficiency of both bioelements in the body due to their complexation by chokeberry ingredients in the lumen of the gastrointestinal tract.
Keywords: Aronia melanocarpa berries extract, chelating ability, complexation, polyphenols, cyanidin 3-O-β-galactoside, quercetin, copper, zinc
1. Introduction
Nowadays, a growing interest in the possibility of joining biologically active compounds, present in medicinal plants, into a daily diet to prevent from the development of civilization diseases and to support their treatment has been observed [1,2,3,4]. One of the most important groups of such compounds are polyphenols. These compounds, mainly due to their antioxidative and anti-inflammatory properties, are effective in the protection against disorders in the cardiovascular, digestive, nervous, and reproductive systems, as well as against hypertension, cirrhosis, overweight and obesity, diabetes, skin troubles, and cancers [1,2,3,5]. Moreover, increasing attention has been focused on polyphenol-rich products as possible effective factors in the protection against action of some xenobiotics, including common pollutants of the natural environment and dietary products such as toxic heavy metals [1,4,6,7,8].
It seems reasonable that, among plants abundant in polyphenols, special attention should be paid to Aronia melanocarpa L. (Rosaceae), the berries of which (chokeberries) are one of the richest sources of these compounds [1,2,4,9]. Chokeberries have proven beneficial impact on health and thus they are widely recommended as functional food [1,2,3,5]. Results of experimental studies indicate that Aronia products seem to be very promising agents in the protection from unfavorable effects of exposure to heavy metals, including cadmium (Cd) [1,4,6,8,10,11,12,13,14,15], belonging to the main environmental pollutants in industrialized countries [7]. In a study conducted in a rat model of low-level and moderate lifetime human exposure to Cd (1 and 5 mg Cd/kg diet, respectively, for up to 24 months) we have revealed that the administration of a 0.1% extract from the berries of A. melanocarpa (AE) during the exposure to Cd decreased the absorption of this xenobiotic from the gastrointestinal tract and its accumulation in the body [10], protected from damage to the bone [11,12] and liver [14,15], as well as completely or partially prevented most of the changes in the metabolism of zinc (Zn) and copper (Cu) [13].
Based on the available data, it can be supposed that the protective action of polyphenols and polyphenol-rich products against heavy-metal toxicity is mainly connected with chelating abilities and antioxidative properties of these compounds [1,4,6,8,12,14,15]. Polyphenols, due to the presence of hydroxyl (-OH) groups, are able to form complexes with ions of toxic metals, including Cd ions (Cd2+), as well as with ions of divalent bioelements, such as Zn (Zn2+) and Cu (Cu2+) [6,16,17]. Complexation of metals by these compounds may result in a decrease in the absorption from the gastrointestinal tract of the former, an increase in their elimination with urine, and a decrease in the concentrations of biologically available Zn2+ and Cu2+ in the extra- and intracellular fluids [6]. The complexation of metal ions may prevent from accumulation and unfavorable action of toxic metals; however, at the same time, it may unfortunately lead to bioelement deficiency in the organism [6,13]. Some, but limited thus far, data show that repeated consumption of Aronia products may negatively influence the body status of Zn and Cu [5,6]. Kowalczyk and coauthors [5] have reported that the daily consumption of 240 mg of Aronia anthocyanins for 30 days increased Zn concentration and decreased Cu concentration in the red blood cells in men with hypercholesterolemia. We have revealed that the prolonged (3–24 months) administration of the AE alone generally had no influence on the body status of Zn and Cu. However, the extract intake temporarily decreased the bioavailability of both bioelements and influenced (decreased or increased) their concentrations in some tissues and biological fluids of rats (Table S1) [13]. Moreover, as mentioned above, the administration of the 0.1% AE under the low-level and moderate exposure to Cd completely or partially protected against unfavorable impact of this toxic metal on the metabolism of Zn and Cu, but it also influenced (increased or decreased) some of the indices of the metabolism of these bioelements unchanged by this xenobiotic alone [13]. Among others, the 24-month intake of the extract under the moderate exposure to Cd decreased (by 10%) the total content of Cu in internal organs [13]. Based on our findings [13] and properties of ingredients of the AE [1,6,8], we have hypothesized that the chokeberry extract may influence the body status of Zn and Cu, including their absorption from the digestive tract, due to its ability to bind these bioelements resulting from its abundance in polyphenols.
Taking into account the above findings on the impact of Aronia products on the metabolism of Zn and Cu [5,13] together with the widely recommended consumption of these products in order to protect or improve the health status [1,2], it seems very important to recognize the possible interactions between ingredients of chokeberries and these bioelements. It is also essential due to the fact that Zn and Cu deficiencies still occur in a diet of the general population [18,19]. Thus, the aim of the present study was to investigate whether the 0.1% AE may form complexes with Zn2+ and Cu2+. Because, in the case of oral intake, the lumen of the gastrointestinal tract is theoretically the first place of interactions between ingredients of the AE and Zn2+ or Cu2+, the chelating ability of the extract was evaluated at pH reflecting the conditions noticed in different parts of the digestive system (empty or full stomach and duodenum). Due to the fact that the intake of Aronia products, Zn, and Cu with a diet may vary, the experiment was performed with the use of different concentrations of the AE and both bioelements in order to establish whether the chelating ability of the extract is dependent on these factors. Moreover, to explain which of the polyphenolic compounds present in the AE may form complexes with Zn2+ and Cu2+, the chelating abilities of the main polyphenols present in the extract towards ions of both bioelements were estimated. According to our knowledge, a similar study has not been conducted until now.
2. Results
2.1. Zn2+ Chelation by AE and Polyphenolic Compounds Present in the Extract
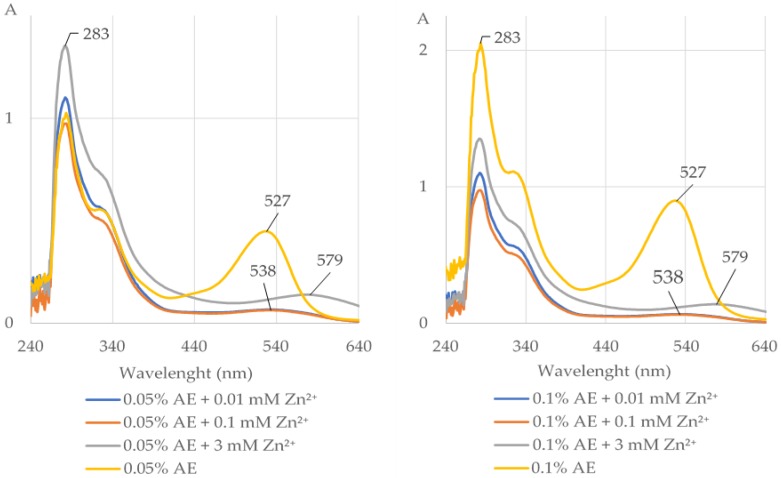
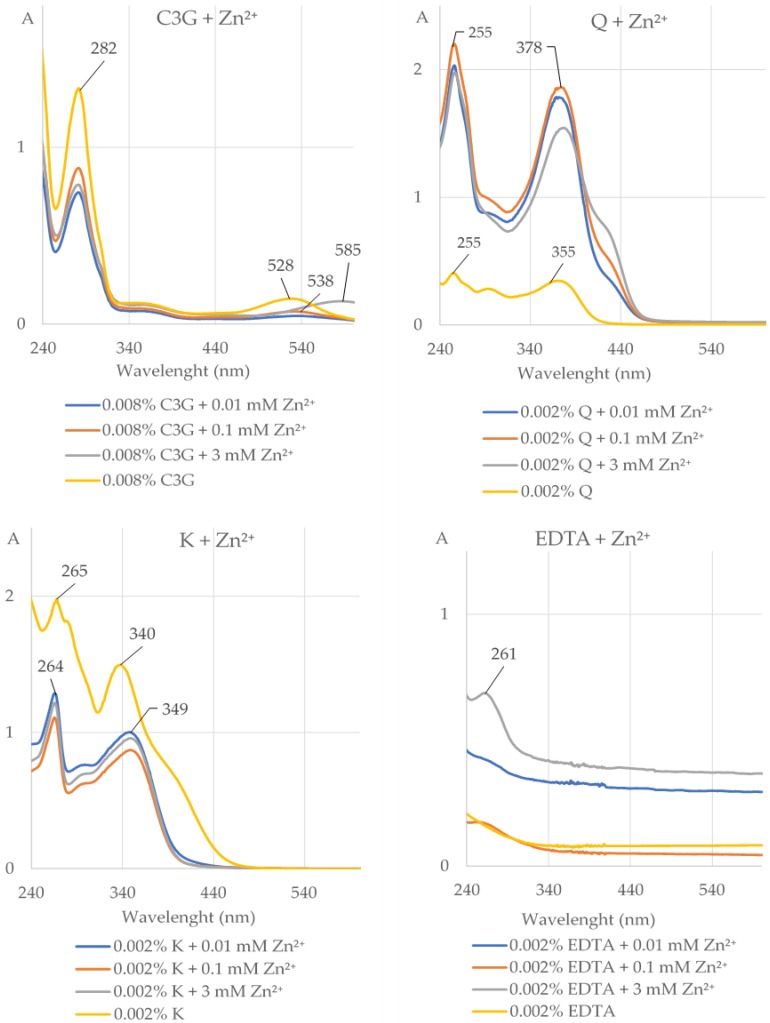
It is evident from the data presented in Figure 1 and Figure 2 and Table 1 and Tables S2–S5 that the AE (0.05% and 0.1%), cyanidin 3-O-β-galactoside (C3G), quercetin (Q), and kaempferol (K) were able to form complexes with Zn2+ at the concentrations of 0.01, 0.1, and 3 mM at pH 5.5, but not at pH 2 and 8. The length of the maximum absorption of the AE without Zn2+ (283 and 527 nm) and with Zn2+ (283 and 579 nm) corresponds well with the maximum absorption of C3G without Zn2+ (282 and 528 nm) and with Zn2+ (281 and 585 nm; Figure 1 and Figure 2, Tables S2 and S3). Chlorogenic acid (CA), neochlorogenic acid (NCA), (-)-epicatechin, and (+)-catechin did not chelate Zn2+ at pH 2, 5.5, and 8 at all studied concentrations (Figure 2, Table 1 and Tables S2–S5). Ethylenediaminetetraacetic acid (EDTA) formed complexes with Zn2+ at all used concentrations (Figure 2, Table 1, Tables S2 and S6).
Figure 1.
The UV–Vis absorption spectra (with indicated maximum absorption) of the extract from Aronia melanocarpa L. berries (0.05% and 0.1% AE) alone and after addition of divalent ions of zinc (Zn2+) at the concentrations of 0.01, 0.1, and 3 mM at pH 5.5. The shift of the maximum absorption after addition of Zn2+ to the AE indicates that the extract chelated these ions. A, absorbance.
Figure 2.
The UV–Vis absorption spectra (with indicated maximum absorption) of cyanidin 3-O-β-galactoside (0.008% C3G), quercetin (0.002% Q), kaempferol (0.002% K), and ethylenediaminetetraacetic acid (0.002% EDTA) alone and after addition of divalent ions of zinc (Zn2+) at the concentrations of 0.01, 0.1, and 3 mM at pH 5.5. The shift of the maximum absorption after addition of Zn2+ to the solution of the investigated compound indicates that this compound chelated these ions. A, absorbance.
Table 1.
Summarizing the results of the evaluation of complexation of divalent ions of zinc (Zn2+) at the concentrations of 0.01, 0.1, and 3 mM and copper (Cu2+) at the concentrations of 0.01, 0.05, and 0.5 mM by 0.1% extract from Aronia melanocarpa L. berries (AE) and the main polyphenolic compounds present in the extract, as well as by ethylenediaminetetraacetic acid (EDTA). 1, 2.
| AE, Polyphenolic Compound or EDTA | pH 2 | pH 5.5 | pH 8 | |||
|---|---|---|---|---|---|---|
| Zn2+ | Cu2+ | Zn2+ | Cu2+ | Zn2+ | Cu2+ | |
| 0.1% AE | − | − | + | + | − | − |
| 0.008% C3G | − | − | + | + | − | − |
| 0.002% Q | − | − | + | + | − | − |
| 0.002% K | − | − | + | − | − | − |
| 0.007% CA | − | − | − | − | − | − |
| 0.007% NCA | − | − | − | − | − | − |
| 0.013% (+)-catechin | − | − | − | − | − | − |
| 0.013% (−)-epicatechin | − | − | − | − | − | − |
| 0.1% EDTA | − | − | + | + | − | − |
| 0.013% EDTA | − | − | + | + | − | − |
| 0.008% EDTA | − | − | + | + | − | − |
| 0.007% EDTA | − | − | + | + | − | − |
| 0.002% EDTA | − | − | + | + | − | − |
1 The same effect was noted at all concentrations of Zn2+ (0.01, 0.1, and 3 mM). 2 The same effect was noted at all concentrations of Cu2+ (0.01, 0.05, and 0.5 mM). C3G, cyanidin 3-O-β-galactoside; Q, quercetin; K, kaempferol; CA, chlorogenic acid; NCA, neochlorogenic acid. +, complexation; −, lack of complexation.
2.2. Cu2+ Chelation by AE and Polyphenolic Compounds Present in the Extract
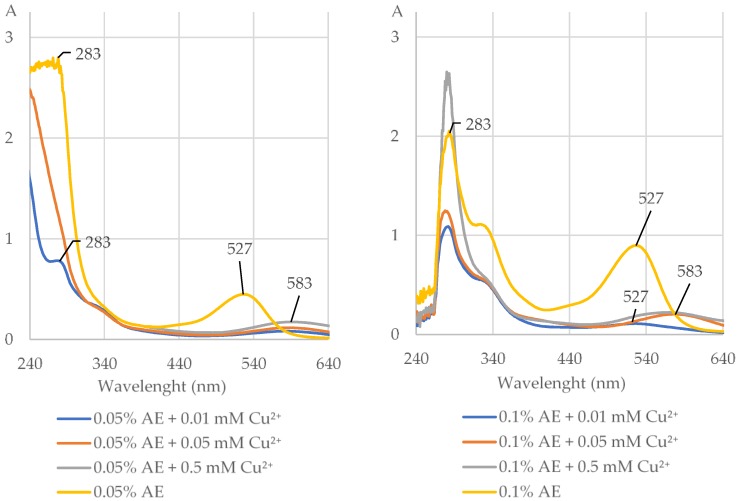
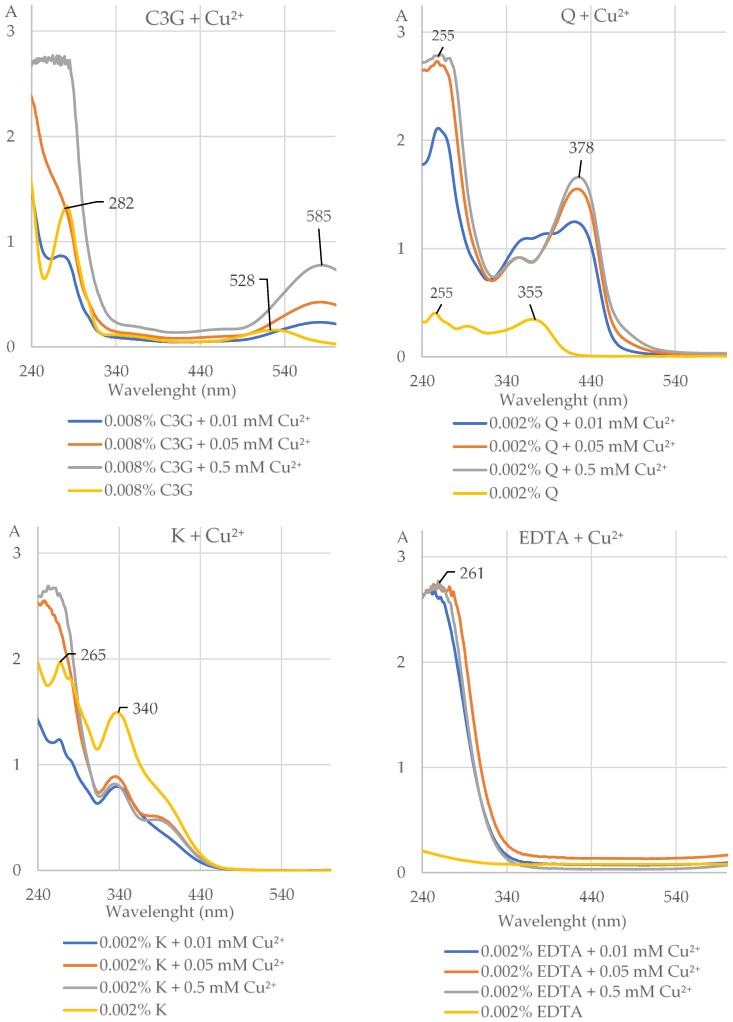
The AE (0.05% and 0.1%), C3G, and Q formed complexes with Cu2+ at the concentrations of 0.01, 0.05, and 0.5 mM at pH 5.5 but not at pH 2 and 8 (Figure 3 and Figure 4, Table 1 and Tables S2–S5). The length of the maximum absorption of the AE without Cu2+ (283 and 527 nm) and with Cu2+ (283 and 583 nm) corresponds well with the maximum absorption of C3G without Cu2+ (282 and 528 nm) and with Cu2+ at pH 5.5 (265 and 585 nm; Figure 3 and Figure 4, Tables S2 and S3). CA, NCA, (−)-epicatechin, (+)-catechin, and K did not chelate Cu2+ at pH 2, 5.5, and 8 at all studied concentrations (Figure 4, Table 1 and Tables S2–S5). EDTA formed complexes with Cu2+ at all used concentrations (Figure 4, Table 1, Tables S2 and S6).
Figure 3.
The UV–Vis absorption spectra (with indicated maximum absorption) of the extract from Aronia melanocarpa L. berries (0.05% and 0.1% AE) alone and after addition of divalent ions of copper (Cu2+) at the concentrations of 0.01, 0.05, and 0.5 mM at pH 5.5. The shift of the maximum absorption after addition of Cu2+ to the AE indicates that the extract chelated these ions. A, absorbance.
Figure 4.
The UV–Vis absorption spectra (with indicated maximum absorption) of cyanidin 3-O-β-galactoside (0.008% C3G), quercetin (0.002% Q), kaempferol (0.002% K), and ethylenediaminetetraacetic acid (0.002% EDTA) alone and after addition of divalent ions of copper (Cu2+) at the concentrations of 0.01, 0.05, and 5 mM at pH 5.5. The shift of the maximum absorption after addition of Cu2+ to the solution of the investigated compound indicates that this compound chelated these ions. A, absorbance.
3. Discussion
The most important achievement of the present study is the finding that the 0.1% AE was able to form complexes with Zn2+ and Cu2+ at pH 5.5, which seems to suggest that the extract may chelate these bioelements in the lumen of the gastrointestinal tract. The present study also provides an explanation as to which of the Aronia polyphenols may be responsible for the extract chelating ability and which of them do not have such properties. A detailed analysis of the results, taking into account the chemical structure of the used polyphenols, allows for the conclusion that the ability of the 0.1% AE to chelate Zn2+ and Cu2+ may be explained, at least partially, by the presence of anthocyanin derivatives of cyanidin and Q.
Because the aim of this study was to investigate whether the 0.1% AE may form complexes with Zn2+ and Cu2+, in order to explain the results of our earlier research concerning the influence of prolonged intake of the extract on the body status of Zn and Cu under low-level and moderate chronic exposure to Cd and without treatment with this toxic heavy metal [13], we have created an in vitro model reflecting the conditions of the gastrointestinal tract. That is why the ability of the AE to form complexes with Zn2+ and Cu2+ was evaluated at pH 2, 5.5, and 8, reflecting the conditions noticed in various parts of the digestive system in different situations (empty stomach—pH 1–2; full stomach—pH 5.5; duodenum—pH 6–8) [20,21] and the concentrations of 0.01, 0.1, and 3 mM Zn2+ and 0.01, 0.05, and 0.5 mM Cu2+ were used. Because Zn and Cu are noticed in a meal at the levels of 3–25 and 0.5–15 mM, respectively [22,23,24], these values may be the highest concentrations theoretically present in the upper parts of the lumen of the digestive tract. The concentration of 0.01 mM Zn has been detected in human gastric juice [25], while the concentration of 0.1 mM Zn was noted in the human prostate [26]. The concentration of 0.01 mM Cu corresponds to its concentration in human blood [27], while the concentration of 0.05 mM of this element was chosen as an intermediate value between that detected in diet and that determined in blood.
The use of two different concentrations of the AE (0.05% and 0.1%) in the first stage of the study allowed evaluation of the impact of the extract concentration on its chelating properties towards Zn2+ and Cu2+. Because a lack of differences between the complexing abilities of the 0.1% and 0.05% AE towards Zn2+ and Cu2+ has been revealed, the abilities of the main Aronia polyphenols to complex these ions were evaluated in the second stage only at the concentrations corresponding to their concentrations in the 0.1% AE. The use of NCA and CA, isomers belonging to the group of hydroxycinnamic acids [2,28], at the same concentrations allowed for comparison of chelating abilities of these two compounds. Because proanthocyanidins present in chokeberries constitute oligomers built from monomers that are derivatives of (−)-epicatechin and (+)-catechin [1], both (−)-epicatechin and (+)-catechin were used at the highest concentration (0.013%) in which they could theoretically be found in the 0.1% AE [11]. The applied concentration of Q (0.002%), a flavonol derivative with a dihydroxyl group in the B ring, is equivalent to the concentration of flavonol derivatives identified in the 0.1% AE (Table 2) [11]. The use of K (a flavonol derivative with the same structure as Q, but with only a single -OH group in the B ring) at the same concentration as Q (possessing a dihydroxyl group in the B ring), i.e., 0.002%, allowed to explain whether the -OH group in the B ring may influence chelating properties of polyphenols.
Table 2.
The concentrations of polyphenolic compounds in the 0.1% extract from Aronia melanocarpa L. berries (adapted from [11]).
| Polyphenolic Compounds | Concentration [μg/L] |
|---|---|
| Total polyphenols | 612.40 ± 3.33 3 |
| Total anthocyanins | 202.28 ± 1.28 |
| Total proanthocyanidins | 129.87 ± 1.12 |
| Total phenolic acids | 110.92 ± 0.89 |
| Total flavonoids | 21.94 ± 0.98 |
| Cyanidin 3-O-β-galactoside | 80.07 ± 1.05 |
| Cyanidin 3-O-α-arabinoside | 33.21 ± 0.01 |
| Cyanidin 3-O-β-glucoside | 3.68 ± 0.01 |
| Chlorogenic acid | 68.32 ± 0.08 |
3 Data are mean ± standard error (n = 3).
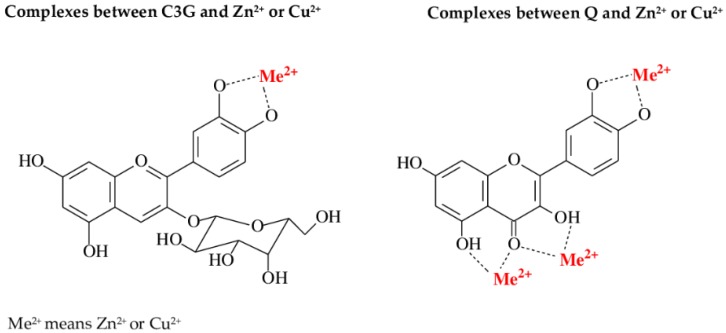
The ability of anthocyanin derivatives of cyanidin (C3G) and Q to complex Zn2+ and Cu2+ (at pH 5.5) is connected with the presence of ortho-dihydroxyl groups in the B ring in structure of these compounds (Figure 5) [6]. Our finding that both Q and cyanidin derivatives may form complexes with Zn2+ or Cu2+ are in accordance with the results of other authors [16,17]. It has been recognized that C3G may form complexes with ions of divalent metals, probably due to the presence of ortho-dihydroxyl groups in the B ring [6]. The inability revealed in the present study of NCA to complex Zn2+ and Cu2+ and of K to bind Cu2+, as they are polyphenols without an ortho-dihydroxyl group in the B ring, confirms the crucial role of this group in the B ring in the process of metal complexation (Figure 5). However, the ability of K (possessing only a single -OH group in the B ring) to bind Zn2+ at pH 5.5 shows that the presence of the ortho-dihydroxyl group in the B ring is not absolutely necessary. It should be clearly underlined that detailed analysis of the received UV-Vis absorption spectra (Figure 2, Table S2) seems to indicate that C3G and Q, possessing this structure, are stronger chelators of Zn2+ than K is. Moreover, the fact that CA was unable to bind Zn2+ and Cu2+ in all studied pH seems to indicate that the presence of a dihydroxyl group in the polyphenolic structure does not guarantee its chelating properties. According to our knowledge, there are no data in scientific literature concerning the chelating properties of CA towards bioelements. Thus, we are unable to explain why this compound was unable to complex divalent ions of Zn and Cu.
Figure 5.
The possible complexes between cyanidin 3-O-β-galactoside (C3G) and quercetin (Q) and divalent ions of zinc (Zn2+) and copper (Cu2+).
The finding that the length of the maximum absorption of the 0.05% and 0.1% AE without Zn2+ or Cu2+ and with Zn2+ or Cu2+ corresponded well with the maximum absorption of C3G without and with these ions suggests that cyanidin derivatives, belonging to anthocyanins (which constitute one of the main groups of polyphenolic compounds present in the AE (Table 2; [11]), may be responsible for the chelating properties of the extract towards Zn2+ and Cu2+. Taking into account the fact that the length of the maximum absorption of Q did not correspond well with the length of the maximum absorption of the 0.1% AE and that this polyphenolic compound is present at low concentration in the extract (total flavonoids: 21.94 ± 0.98 μg/L; Table 2; [11]), this polyphenol may be responsible to a lesser extent than cyanidin derivatives for the chelating properties of the extract towards Zn2+ and Cu2+. However, it is K that seems to be the weakest chelator of Zn2+. The lack of binding of Cu2+ by K might result from weak complexing capacity of this compound towards divalent ions of metals. It is important to underline that the noted lack of differences in the ability of binding Zn2+ and Cu2+ dependent on the used concentrations of these bioelements shows that both AE and its chosen polyphenolic ingredients (C3G, Q, and K in the case of Zn2+; C3G and Q in the case of Cu2+) are able to complex these elements within a relatively wide range of concentrations.
The results of this study are useful for the explanation of the results of our earlier research concerning the influence of prolonged intake of the 0.1% AE on the body status of Zn and Cu, as well as the impact of this extract on the metabolism of these bioelements under low-level and moderate chronic exposure to Cd [13]. The transitional decrease in the bioavailability (declined apparent absorption and retention in the body), increase in the urinary excretion and decrease in the concentrations of Zn and/or Cu in some tissues and biological fluids of rats administered with the AE alone (Table S1) [11] may be explained by formation of complexes between the extract ingredients and these bioelements in the body, including mainly the lumen of the digestive tract.
As previously mentioned, the administration of the 0.1% AE completely or partially prevented most of the changes in the metabolism of Zn and Cu caused by Cd in rats; however, the AE did not always have a protective effect against Cd-induced changes in the body status of these elements, and sometimes it also influenced some of the indices of their metabolism unchanged by Cd alone [13]. These may be connected with the complexation of Zn and Cu by ingredients of the extract, including cyanidin derivatives and Q, as well as K in the case of Zn. Our previous finding of decreased total content of Cu in internal organs due to the administration of the AE under moderate exposure to Cd for 24 months compared not only with the group exposed to Cd but also with the control group [13], together with the ability revealed in the present study of the extract to bind Cu2+, allows to conclude that prolonged consumption of Aronia products under exposure to this xenobiotic may lead to Cu deficiency in the organism.
The fact that complexation of Zn2+ and Cu2+ occurs at pH 5.5 indicates that complexes between polyphenolic compounds present in the AE and these bioelements may be formed in the stomach after a meal (when a pH value grows from strong acidic up to 5.5). This finding may suggest that the AE should not be consumed during a meal. Moreover, the complexation of Zn2+ and Cu2+ may take place not only in the lumen of the digestive tract but probably also in the internal organs and tissues [13,25]. The results of our study seem to indicate that the chelation of both bioelements by polyphenolic compounds present in the extract may occur at the concentrations in which Zn and Cu are present in the human body (gastric juice, blood, and prostate).
The knowledge on the chelating properties of the 0.1% AE in different pH may be useful also for the prediction of possible interactions between the extract or its chosen ingredients and Zn2+ or Cu2+ under not only physiological but also pathological conditions, due to the fact that various disorders may change pH in the digestive tract [20]. Despite the fact that, in the present study, the complexation of Zn2+ and Cu2+ by the extract has not been observed at pH reflecting conditions specific for duodenum, it cannot be excluded that the chelation of these ions by the 0.1% AE may take place in the duodenum under the specific conditions when the pH value in this part of the gastrointestinal tract approaches the value of 5.5. This may occur in patients with chronic pancreatitis, who have exhibited pH values of 5–6 in this part of the gastrointestinal tract [20].
Apart from polyphenols, other bioactive compounds like fibers and pectins may also be responsible for chelating properties of chokeberries [29]. It cannot be excluded that other components of Aronia berries such as dietary fiber, tannins, and pectins can also bind Zn2+ and Cu2+, decreasing their bioavailability. It has been revealed that pomace from blackcurrants rich in dietary fiber is able to bind Cu2+ at 69% and Zn2+ at 21% at pH 2.0 (what was evaluated as a sorption—a ratio between the metal content in a sample and in an adsorbate demonstrated as a percentage value) [30]. Moreover, it has been reported that the ability of fiber to bind Cu2+ decreased significantly (a drop in sorption from 69% to 8%) with a decline in pH value (from 2 to 1) [30]. However, better binding properties of dietary fibers (from potato, rye bran, wheat bran, rice bran, corn bran, soybeans, and oat hulls) towards Zn2+ and Cu2+ have generally been observed at higher pH (6.8 and 4.5) than at lower pH (0.65, 2.2, and 3.2) [31,32], which corresponds with our results concerning chelating properties of the 0.1% AE and polyphenols present in this extract, which were able to complex Zn2+ and Cu2+ at higher pH (5.5) but not at lower pH (2.0).
Wider discussion of the results of this study is presently impossible due to a lack of data on chelating properties of other plant extracts rich in polyphenolic compounds (e.g., extracts from black elderberry, blackcurrant, blackberry, blueberry, and raspberry) towards Zn2+ and Cu2+, especially in living organisms. The evaluation of interactions between plant extracts rich in polyphenols and these bioelements is particularly important at pH reflecting the environment of the gastrointestinal tract, since plant products are joined to a daily diet which may result in a decrease in trace element bioavailability from a meal. Our findings may suggest that Aronia products (not only extracts, but also juices) should be consumed on an empty stomach and not by subjects suffering from chronic pancreatitis with lower than normal pH value in duodenum. Anthocyanins—derivatives of cyanidin—are also present at high concentrations in other popular foodstuffs (blackcurrant pomace, red wine, black raspberry, cranberry, blueberry, bilberry, lingonberry, red onion, Mexican oregano, as well as cocoa powder and black chocolate) [3], so it might be expected that products made from them may also form complexes with Zn2+ and Cu2+. This is particularly important due to the fact that a shortage of Zn and/or Cu in a diet is still a current problem concerning the general population [18,19].
The knowledge about interactions between AE, as well as other food products rich in cyanidin derivatives, Q, and K and Zn2+ or Cu2+ in the digestive tract may be useful in planning a proper daily diet, and it may allow for establishing a proper menu that reduces the risk of negative consequences which may result from mixing of some food products. Presently, there is a lack of data on this issue obtained from human subjects. The results of the present study may suggest that the prolonged consumption of AE may lead to Zn and Cu deficiency as a consequence of their chelation by the extract. However, it is also very important to underline that, in the case of a diet containing excess amounts of Zn or Cu, complexation of these elements by ingredients of AE may provide protection against their excessive gastrointestinal absorption and unfavorable action in the body.
The findings of our research have great scientific and practical value. They seem to show that it is very important whether polyphenol-rich products, including Aronia extracts, currently broadly advised by nutritionists and clinicians as a functional food in the prevention and treatment of civilization diseases [1,2], are consumed before, during, or after a meal. The results may suggest that products rich in polyphenols, including extracts and juices, as a part of a daily diet or supplements, should be consumed on an empty stomach. Owing to the fact that deficiencies of bioelements in a diet still occur in the general population [18,19], this issue is particularly important in the case of consumption of polyphenols by subjects suffering from bioelement deficiency. The findings of the present study suggest that proper amounts of microelements such as Zn and Cu should be delivered into the organism during the consumption of products rich in polyphenols to prevent from their deficiency or from intensification of existing shortage of these bioelements. So far, the complexation of bioelements by polyphenols in the lumen of the gastrointestinal tract has not been studied in the living organism, but our findings show the necessity of such study in humans.
We are aware not only of novelty and achievements of our study, but also of its limitations. The main limitation of our study is the fact that the potential chelating properties of the AE and particular polyphenolic compounds towards Zn2+ and Cu2+ were investigated only with the use of one method, and they have not been estimated and confirmed by other methods. Moreover, the chemical nature (e.g., stability, ligand dissociation constants) of the formed complexes has not been assessed. The fact that we are unable to explain the lack of complexation of Zn2+ and Cu2+ by CA possessing a dihydroxyl group is also a limitation. Moreover, we were unable to explain why (−)-epicatechin and (+)-catechin possessing a free 3′,4′-dihydroxyl group in the B ring had no chelating properties towards both bioelements. This may result from the fact that proanthocyanidins in chokeberries exist in the form of oligomers that are derivatives of (−)-epicatechin and (+)-catechin [33], but in the present study we used monomers of (−)-epicatechin and (+)-catechin. We are aware that some of our considerations carried out in the discussion of the results of the present study are in the sphere of suppositions that require scientific confirmation; however, they indicate the possible health implications of bioelement complexation.
In conclusion, the study not only confirmed our hypothesis regarding the possibility of binding Zn2+ and Cu2+ by the extract from A. melanocarpa berries, but also allowed to clarify which of the polyphenolic compounds present in it can form complexes with ions of these bioelements and under what pH conditions they do so. The extract can complex Zn2+ and Cu2+ at pH 5.5, reflecting conditions noticed in a full stomach, and this ability may be explained, at least partially, by the presence of polyphenols such as anthocyanin derivatives of cyanidin and quercetin. The findings of the present study seem to suggest that Aronia products, used as supplements of a diet, should be consumed on an empty stomach, and particular attention should be paid to adequate intake of Zn and Cu under prolonged consumption of these products to avoid deficiency in both bioelements in the body due to their complexation by chokeberry ingredients in the lumen of the gastrointestinal tract. This may especially apply to people at risk of Zn and Cu deficiency. Moreover, based on the results, it can be concluded that consumption of Aronia products or another products rich in cyanidin derivatives and Q in the case of excess consumption of Zn or Cu may provide protection against their excessive gastrointestinal absorption and unfavorable action in the body due to complexation of these elements. However, further studies, including examination of the chemical nature of complexation, are needed to recognize the health implications of the complexing ability of Aronia extract and its ingredients towards Zn and Cu.
4. Materials and Methods
4.1. Chemicals
Lyophilized extract from the berries of A. melanocarpa (Rosaceae) (Adamed Consumer Healthcare, Tuszyn, Poland; Certificate KJ 4/2010, Batch No. M100703) and pure polyphenolic compounds (Sigma-Aldrich, St. Louis, MO, USA) such as C3G, CA (3-(3,4-dihydroxycinnamoyl)-quinic acid), NCA (5-O-(trans-3,4-dihydroxycinnamoyl)-D-quinic acid), (+)-catechin ((+)-trans-3,3′,4′,5,7-pentahydroxyflavane), (−)-epicatechin ((−)-cis-3,3′,4′,5,7-pentahydroxyflavane), Q (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one, 3,3′,4′,5,6-pentahydroxy-flavone), and K (3,4′,5,7-tetrahydroxyflavone, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), as well as pure EDTA (Avantor Performance Materials Poland S.A., Gliwice, Poland) were used. Zn and Cu salts such as anhydrous zinc chloride (ZnCl2) and anhydrous copper(II) sulfate(VI) (CuSO4) were purchased from Avantor Performance Materials Poland S.A (Gliwice, Poland). Hydrochloric acid (HCl) and sodium hydroxide (NaOH) were provided by Sigma-Aldrich (St. Louis, MO, USA). As a solvent of the AE, pure polyphenolic compounds, EDTA, and salts of bioelements, a solution of methanol (Avantor Performance Materials Poland S.A., Gliwice, Poland) and ultra-pure water (H2O; MAXIMA purification system; ELGA, Lane End, United Kingdom) mixed at the ratio of 7:3 was used. Buffer solutions of pH 4.01 and 10.01 were provided by Mettler Toledo (Schwarzenbach, Switzerland).
4.2. Ingredients of AE
Polyphenolic profile of the 0.1% AE used in this study is presented in Table 2 [11]. The concentrations of Zn and Cu in the extract were low and reached 1.39 ± 0.04 and 0.803 ± 0.065 μg/L, respectively [13].
According to the producer’s declaration, the extract also contained sugar, sugar alcohols (sorbitol, parasorboside), triterpenes, phytosterols, carotenoids, minerals, and vitamins. Moreover, vitamins from group B (B1, B2, B3, B5, and B6), vitamins C, E, and K, β-carotene, β-cryptoxanthin, violaxanthin, dietary fiber, tannins, organic acids (l-malic acid, citric acid), carbohydrates, proteins, as well as calcium, magnesium, and iron [1,9] are present in chokeberries. The concentration of Cd in the 0.1% AE was <0.05 μg/L [10].
4.3. Experimental Design
The experiment was divided into two stages. In the first stage, the ability of the 0.05% and 0.1% AE to form complexes with Zn2+ at the concentrations of 0.01, 0.1, and 3 mM and Cu2+ at the concentrations of 0.01, 0.05, and 0.5 mM was evaluated at pH 2, 5.5, and 8. In the second stage, the abilities of the main Aronia polyphenols at the concentrations corresponding to their concentrations in the 0.1% AE to complex these ions were evaluated. C3G (the most abundant anthocyanin in the extract) and CA were used at the concentrations of 0.008% and 0.007%, respectively, determined by us in the 0.1% AE (Table 2) [11]. Other Aronia polyphenols were used at the following concentrations: NCA—0.007%, (−)-epicatechin—0.013%, (+)-catechin— 0.013%, Q—0.002%, and K—0.002%.
In order to obtain the solutions containing Zn2+ at the concentrations of 0.01, 0.1, and 3 mM and Cu2+ at the concentrations of 0.01, 0.05, and 0.5 mM, the solutions containing 1.36, 13.6, and 408 mg ZnCl2/10 mL, respectively, as well as 1.6, 8, and 80 mg CuSO4/10 mL, respectively, were prepared in methanol:H2O (7:3) directly before the experiment. The concentrations of anhydrous salts of Zn and Cu (ZnCl2 and CuSO4) were calculated to achieve needed concentrations of Zn2+ and Cu2+, taking into account these salts’ solubility in H2O at room temperature (432 g ZnCl2/100 mL and 23 g CuSO4/100 mL; CuSO4 is suitably soluble in methanol, and this compound dissolved well in the mixture of methanol:H2O at the ratio of 7:3) and their molar mass.
The solutions of the AE, particular polyphenolic compounds, and EDTA in methanol:H2O (7:3) were prepared directly before the experiment. The needed pH values (checked with the use of buffer solutions and pH meter; Mettler Toledo, Schwarzenbach, Switzerland) were obtained by adding appropriate amounts of 5% HCl or 0.1% NaOH to stock solutions of the AE, particular polyphenols, EDTA, and bioelements. Assessment of the stability of the 0.1% AE revealed that the extract, at all used pH, is stable during the first 24 h after preparation (Supplementary Material—Assessment of the stability of the extract from the berries of Aronia melanocarpa L. (AE)).
4.4. The Rule of the Method of Estimation of Metal Ion Complexation by AE and Polyphenolic Compounds
The complexation abilities of the 0.1% and 0.05% AE, as well as of particular polyphenols towards Zn2+ and Cu2+ were evaluated based on shifts between the maximum absorption of solutions before an addition of ZnCl2 or CuSO4 and the maximum absorption of the respective solutions after addition of these salts [16,34,35]. The shift of the maximum absorption observed after the addition of the solutions containing Zn2+ or Cu2+ to the solutions with the AE (0.05% and 0.1%) or polyphenolic compounds indicates that the process of chelation of the ions occurred. A lack of the shift of the maximum absorption means that the process of chelation of metal ions by the used solution did not occur. As a positive control, EDTA solutions (with and without Zn2+ and Cu2+) were used at the concentrations analogous to the investigated concentrations of the AE (0.05% and 0.1%) and particular polyphenolic compounds (0.002%, 0.007%, 0.008%, and 0.013%).
Three hundred microliters of the solutions of AE, particular polyphenolic compounds, or EDTA, at appropriate concentrations, were mixed with 300 µL of the prepared solutions of ZnCl2 or CuSO4 in a quartz cuvette, and then the UV–Vis absorption spectrum of the mixture was recorded. Absorption spectra of the solutions were recorded within the range of 200–800 nm using a Specord 50 Plus spectrophotometer (Analytik, Jena, Germany). Each reaction was repeated three times to check the coherence of samples. Owing to the fact that the results obtained from each of the three independent models containing the AE, particular polyphenolic compounds, or EDTA with or without Zn2+ and Cu2+ were repeatable, the results have been presented for only one representative sample from each model.
Acknowledgments
The study was conducted with the use of equipment by Medical University of Bialystok as part of the OP DEP 2007–2013, Priority Axis I.3, contract No. POPW.01.00-20-001/12.
Abbreviations
| A | absorbance |
| AE | extract from the berries of Aronia melanocarpa L. |
| A. melanocarpa | Aronia melanocarpa |
| CA | chlorogenic acid |
| Cd | cadmium |
| Cu | copper |
| Cu2+ | copper(II) ion |
| CuSO4 | copper(II) sulfate(VI) |
| C3G | cyanidin 3-O-β-galactoside |
| EDTA | ethylenediaminetetraacetic acid |
| HCl | hydrochloric acid |
| H2O | water |
| K | kaempferol |
| NaOH | sodium hydroxide |
| NCA | neochlorogenic acid |
| -OH | hydroxyl group |
| Q | quercetin |
| UV | ultraviolet spectroscopy |
| Vis | visible spectroscopy |
| Zn | zinc |
| ZnCl2 | zinc chloride |
| Zn2+ | zinc ion |
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/25/7/1507/s1, Figure S1. Ultraviolet (UV) spectrum of 0.1% extract from the berries of Aronia melanocarpa L. (AE) at pH 2 after preparation (1 h) and after 24, 48, and 72 h. Figure S2. Ultraviolet (UV) spectrum of 0.05% extract from the berries of Aronia melanocarpa L. (AE) at pH 5.5 after preparation (1 h) and after 24, 48, and 72 h. Figure S3. Ultraviolet (UV) spectrum of 0.05% extract from the berries of Aronia melanocarpa L. (AE) at pH 8 after preparation (1 h) and after 24, 48, and 72 h. Table S1: The changes in zinc (Zn) and copper (Cu) metabolism after the administration of the 0.1% extract from the berries of Aronia melanocarpa L. (AE) to the female Wistar rats for up to 24 months. Table S2: The effect of divalent ions of zinc (Zn2+) and copper (Cu2+) at the studied concentrations on wavelength of absorption of the extract from Aronia melanocarpa L. berries (AE) at the concentrations of 0.05% and 0.1% and 0.1% ethylenediaminetetraacetic acid (EDTA) at pH 2, 5.5, and 8. Table S3: The effect of divalent ions of zinc (Zn2+) and copper (Cu2+) at the studied concentrations on wavelength of absorption of the main polyphenolic compounds present in the 0.1% extract from Aronia melanocarpa L. berries (AE) at pH 5.5. Table S4: The effect of divalent ions of zinc (Zn2+) and copper (Cu2+) at the studied concentrations on wavelength of absorption of polyphenolic compounds present in the 0.1% extract from Aronia melanocarpa L. berries (AE) at pH 2. Table S5: The effect of divalent ions of zinc (Zn2+) and copper (Cu2+) at the studied concentrations on wavelength of absorption of polyphenolic compounds present in the 0.1% extract from Aronia melanocarpa L. berries (AE) at pH 8. Table S6: The effect of divalent ions of zinc (Zn2+) and copper (Cu2+) at the studied concentrations on wavelength of absorption of ethylenediaminetetraacetic acid (EDTA) at pH 5.5. Supplementary Material—Assessment of the stability of the extract from the berries of Aronia melanocarpa L. (AE).
Author Contributions
Conceptualization, M.M.B., M.T., S.B.; methodology, M.M.B., M.T., S.B., J.W.S.; formal analysis, M.M.B., M.T., S.B., J.W.S.; investigation, S.B., M.T.; visualization, M.M.B., M.T., J.W.S.; writing—original draft preparation, M.M.B., S.B., M.T.; writing—review and editing, M.M.B., M.T., J.W.S.; supervision, M.M.B., M.T.; project administration, M.M.B.; funding acquisition, M.M.B., S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Bialystok (Poland), Grant Nos. N/ST/MN/16/002/2221 and N/ST/MN/18/002/2221.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the extract are not available from the authors.
References
- 1.Borowska S., Brzóska M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016;15:982–1017. doi: 10.1111/1541-4337.12221. [DOI] [PubMed] [Google Scholar]
- 2.Jurikova T., Mlcek J., Skrovankova S., Sumczynski D., Sochor J., Hlavacova I., Snopek L., Orsavova J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules. 2017;22:944. doi: 10.3390/molecules22060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manolescu B.N., Oprea E., Mititelu M., Ruta L.L., Farcasanu I.C. Dietary anthocyanins and stroke: A review of pharmacokinetic and pharmacodynamics studies. Nutrients. 2019;11:1479. doi: 10.3390/nu11071479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brzóska M.M., Borowska S., Tomczyk M. Antioxidants as a potential and therapeutic strategy for cadmium. Curr. Drug Targets. 2016;17:1350–1384. doi: 10.2174/1389450116666150506114336. [DOI] [PubMed] [Google Scholar]
- 5.Kowalczyk E., Fijałkowski P., Kura M., Krzesiński P., Błaszczyk J., Kowalski J., Śmigielski J., Rutkowski M., Kopff M. The influence of anthocyanins from Aronia melanocarpa on selected parameters of oxidative stress and microelements contents in men with hypercholesterolemia. Pol. Merkur. Lek. 2005;19:651–653. [In Polish, English abstract] [PubMed] [Google Scholar]
- 6.Borowska S., Brzóska M.M., Tomczyk M. Complexation of bioelements and toxic metals by polyphenolic compounds—implications for health. Curr. Drug Targets. 2018;19:1612–1638. doi: 10.2174/1389450119666180403101555. [DOI] [PubMed] [Google Scholar]
- 7.Mężyńska M., Brzóska M.M. Environmental exposure to cadmium—a risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. Int. 2018;25:3211–3232. doi: 10.1007/s11356-017-0827-z. [DOI] [PubMed] [Google Scholar]
- 8.Mężyńska M., Brzóska M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. Appl. Toxicol. 2019;39:117–145. doi: 10.1002/jat.3709. [DOI] [PubMed] [Google Scholar]
- 9.Sidor A., Gramza-Michałowska A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules. 2019;24:3710. doi: 10.3390/molecules24203710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brzóska M.M., Gałażyn-Sidorczuk M., Jurczuk M., Tomczyk M. Protective effect of Aronia melanocarpa polyphenols on cadmium accumulation in the body: A study in a rat model of human exposure to this metal. Curr. Drug Targets. 2015;16:1470–1487. doi: 10.2174/1389450116666150102121708. [DOI] [PubMed] [Google Scholar]
- 11.Brzóska M.M., Rogalska J., Gałażyn-Sidorczuk M., Jurczuk M., Roszczenko A., Tomczyk M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chem. Biol. Interact. 2015;229:132–146. doi: 10.1016/j.cbi.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Brzóska M.M., Rogalska J., Roszczenko A., Gałażyn-Sidorczuk M., Tomczyk M. The mechanism of the osteoprotective action of a polyphenol-rich Aronia melanocarpa extract during chronic exposure to cadmium is mediated by the oxidative defense system. Planta Med. 2016;82:621–631. doi: 10.1055/s-0042-103593. [DOI] [PubMed] [Google Scholar]
- 13.Borowska S., Brzóska M.M., Gałażyn-Sidorczuk M., Rogalska J. Effect of an extract from Aronia melanocarpa L. berries on the body status of zinc and copper under chronic exposure to cadmium: An in vivo experimental study. Nutrients. 2017;9:1374. doi: 10.3390/nu9121374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mężyńska M., Brzóska M.M., Rogalska J., Piłat-Marcinkiewicz B. Extract from Aronia melanocarpa L. berries prevents cadmium-induced oxidative stress in the liver: A study in a rat model of low-level and moderate lifetime human exposure to this toxic metal. Nutrients. 2019;11:21. doi: 10.3390/nu11010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mężyńska M., Brzóska M.M., Rogalska J., Galicka A. Extract from Aronia melanocarpa L. berries protects against cadmium-induced lipid peroxidation and oxidative damage to proteins and DNA in the liver: A study in a rat model of environmental human exposure to this xenobiotic. Nutrients. 2019;11:758. doi: 10.3390/nu11040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pliszka B., Smyk B., Mieleszko E., Oszmiański J., Drabent R. Interaction between anthocyanin compounds from chokeberries extracts and Cu(II) ions. ZPPNR. 2005;507:433–441. [In Polish, English abstract] [Google Scholar]
- 17.Tan J., Wang B., Zhu L. DNA binding, cytotoxicity, apoptotic inducing activity, and molecular modeling study of quercetin zinc(II) complex. Bioorg. Med. Chem. 2009;17:614–620. doi: 10.1016/j.bmc.2008.11.063. [DOI] [PubMed] [Google Scholar]
- 18.Kumssa D.B., Joy E.J.M., Ander E.L., Watts M.J., Young S.D., Walker S., Broadley M.R. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 2015;5:10974. doi: 10.1038/srep10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bost M., Houdart S., Oberli M., Kalonji E., Huneau J.F., Margaritis I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016;35:107–115. doi: 10.1016/j.jtemb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Geus W.P., Eddes E.H., Gielkens H.A., Gan K.H., Lamers C.B., Masclee A.A. Post-prandial intragastric and duodenal acidity are increased in patients with chronic pancreatitis. Aliment. Pharmacol. Ther. 1999;13:937–943. doi: 10.1046/j.1365-2036.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 21.Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999;46:183–196. [PubMed] [Google Scholar]
- 22.Figurska-Ciura D., Łoźna K., Styczyńska M. Cadmium, lead, zinc and copper contents in selected vegetables and fruit from garden allotments of the south-western Poland. Pol. J. Food Nutr. Sci. 2007;57:137–143. [Google Scholar]
- 23.Krebs N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000;130:1374S–1377S. doi: 10.1093/jn/130.5.1374S. [DOI] [PubMed] [Google Scholar]
- 24.Lurie D.G., Holden J.M., Schubert A., Wolf W.R., Miller-Ihli N.J. The copper content of foods based on a critical evaluation of published analytical data. J. Food Comp. Anal. 1989;2:298–316. doi: 10.1016/0889-1575(89)90002-1. [DOI] [Google Scholar]
- 25.Powell J.J., Greenfield S.M., Thompson S.M., Thompson R.P. Concentrations of metals in gastric juice in health and peptic ulcer disease. Gut. 1992;33:1617–1620. doi: 10.1136/gut.33.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldereid N.B., Thomassen Y., Attramadal A., Olaisen B., Purvis K. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J. Reprod. Fertil. 1993;99:421–425. doi: 10.1530/jrf.0.0990421. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L.L., Lu L., Pan Y.J., Ding C.G., Xu D.Y., Huang C., Pan X.F., Zheng W. Baseline blood levels of manganese, lead, cadmium, copper, and zinc in residents of Beijing suburb. Environ. Res. 2015;140:10–17. doi: 10.1016/j.envres.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng L., Gao X., Song Y., Zhao L., Guo M., Su J., Zhang L., Liu H. A rapid and sensitive UPLC-MS/MS method for quantification of two caffeoylquinic acids and four main active components in rat plasma after an intravenous administration of Qingkailing injection and its application to a pharmacokinetic study. Biomed. Chromatogr. 2014;28:601–609. doi: 10.1002/bmc.3077. [DOI] [PubMed] [Google Scholar]
- 29.Thompson A.A., Weber C. Influence of pH binding of copper, zinc and iron in six fiber sources. J. Food Sci. 1979;44:752–754. doi: 10.1111/j.1365-2621.1979.tb08492.x. [DOI] [Google Scholar]
- 30.Borycka B. Binding of the copper, zinc and iron ions to dietary fibers from black currant pomace. ŻNTJ. 2005;3:83–91. [In Polish, English abstract] [Google Scholar]
- 31.Stachowiak J., Gawęcki J. Sorption of copper, molybdenum, and selenium ions on selected dietary fibre preparations. Acta Alim. Pol. 1989;15:107–112. [Google Scholar]
- 32.Laszlo J.A. Effects of gastrointestinal conditions on the mineral-binding properties of dietary fibers. Adv. Exp. Med. Biol. 1989;249:133–145. doi: 10.1007/978-1-4684-9111-1_9. [DOI] [PubMed] [Google Scholar]
- 33.Taheri R., Connolly B.A., Brand M.H., Bolling B.W. Underutilized chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J. Agric. Food Chem. 2013;61:8581–8588. doi: 10.1021/jf402449q. [DOI] [PubMed] [Google Scholar]
- 34.Andjelković M., Van Camp J., De Meulenaer B., Depaemelaere G., Socaciu C., Verloo M., Verhe R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006;98:23–31. doi: 10.1016/j.foodchem.2005.05.044. [DOI] [Google Scholar]
- 35.Zhang L., Liu Y., Hu X., Wang Y., Xu M. Metal ion interactions with methyl gallate characterized by UV spectroscopic and computational methods. Food Chem. 2019;293:66–73. doi: 10.1016/j.foodchem.2019.04.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.