Abstract
The anterior cingulate cortex (ACC) is involved in emotion regulation and salience processing. Prior research has implicated ACC dysfunction in suicidal ideation (SI) and suicidal behavior. This study aimed to quantify ACC glutamatergic concentrations and to examine relationships with SI in a sample of healthy and depressed adolescents. Forty adolescents underwent clinical evaluation and proton magnetic resonance spectroscopy (1H-MRS) at 3 T, utilizing a 2-dimensional J-averaged PRESS sequence sampling a medial pregenual ACC voxel. Cerebrospinal fluid-corrected ACC metabolite concentrations were compared between healthy control (HC, n = 16), depressed without SI (Dep/SI−, n = 13), and depressed with SI (Dep/SI+, n = 11) youth using general linear models covarying for age, sex, and psychotropic medication use. Relationships between ACC metabolites and continuous measures of SI were examined using multiple linear regressions. ROC analysis was used to determine the ability of glutamate+glutamine (Glx) and the N-acetylaspartate (NAA)/Glx ratio to discriminate Dep/SI− and Dep/SI+ adolescents. Dep/SI+ adolescents had higher Glx than Dep/SI− participants (padj = 0.012) and had lower NAA/Glx than both Dep/SI− (padj = 0.002) and HC adolescents (padj = 0.039). There were significant relationships between SI intensity and Glx (pFDR = 0.026), SI severity and NAA/Glx (pFDR = 0.012), and SI intensity and NAA/Glx (pFDR = 0.004). ACC Glx and NAA/Glx discriminated Dep/SI− from Dep/SI+ participants. Uncoupled NAA−glutamatergic metabolism in the ACC may play a role in suicidal ideation and behavior. Longitudinal studies are needed to establish whether aberrant glutamatergic metabolism corresponds to acute or chronic suicide risk. Glutamatergic biomarkers may be promising targets for novel risk assessment and interventional strategies for suicidal ideation and behavior.
Subject terms: Predictive markers, Depression, Human behaviour, Molecular neuroscience, Diagnostic markers
Introduction
Suicide and suicide attempts have increased among adolescents and young adults during the past two decades1–3. Suicide is now the second leading cause of death in young people1,4. Suicidal behavior accounts for a substantial and increasing proportion of pediatric hospital visits5. The broad spectrum of suicidal thoughts and behaviors is remarkably common in youth; large epidemiological surveys of adolescents6,7 estimate high prevalence of suicidal ideation (SI; 12.1–17.7%), planning (4.0–14.6%), and attempts (4.1–8.6%). Adolescence represents a critical time in the development of suicidal behavior. More than half of index suicide attempts occur by the age of 258. Furthermore, childhood and adolescent suicidality predict suicidal behavior and attempts later in life9. Clinical practice is uninformed by neurobiological data and relies almost entirely on parent and adolescent reporting, which demonstrate a concerning lack of concordance10. Despite the need for objective neurobiological markers to augment the clinical assessment of suicide risk, substantial prior research in this field has not yielded reliable predictive tools11,12. There is a compelling need for better brain-based markers of suicide risk and interventions that target underlying brain dysfunction.
Numerous neurotransmitter and neuroendocrine systems have been implicated in suicide and suicidal behavior13,14. Increasing evidence indicates dysregulated glutamatergic neurotransmission in suicidal individuals. Gene association studies have linked suicidal behavior with single nucleotide polymorphisms in genes encoding subunits of the N-methyl-d-aspartate (NMDA) glutamatergic receptor15,16 and associated enzymes and transporters16. Postmortem studies in suicide victims have demonstrated altered expression of genes encoding NDMA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) ionotropic receptors17,18, metabotropic glutamate receptors18, and related proteins17,18. Other investigations in suicide attempt survivors have linked cerebrospinal fluid (CSF) levels of endogenous NMDA receptor agonists to suicidal behavior, which change over time following suicide attempts19. Further evidence for the role of glutamate in suicidality arises from the effects of ketamine, an NMDA receptor antagonist, in rapidly reducing suicidal ideation20,21.
Concentrations of glutamate (Glu) and related metabolites, including glutamine (Gln), glutamate+glutamine (Glx), N-acetylaspartate (NAA), and N-acetylaspartylglutamate (NAAG), can be quantified noninvasively using proton magnetic resonance spectroscopy (1H-MRS). Prior 1H-MRS studies of adults with unipolar depression have found reductions in Glu22–25, Glx22,26–29, Gln29, and NAA24,25 in various cortical regions. A small number of 1H-MRS studies in adults have examined cortical glutamate and NAA in depressed individuals with prior suicide attempts30–32. However, findings have been inconsistent, possibly due to divergent populations (e.g., unipolar32 vs. bipolar31 depression), different brain regions sampled, the lack of nonsuicidal depressed control groups30, and variable temporal distance between suicidal behavior and 1H-MRS measurements. Several previous 1H-MRS investigations have found that adolescents with major depression have decrements in Glu33 and Glx34,35 in the anterior cingulate cortex (ACC) and reduced NAA in the ACC and medial prefrontal cortex36 compared to healthy youth, while more recent work has examined the neurochemical basis for symptomatologic dimensions and depressive subtypes in adolescents37,38. However, to our knowledge no previous spectroscopic studies have examined glutamatergic correlates of current suicidal thoughts and behaviors among depressed adolescents.
This study sought to examine 1H-MRS-measured cortical glutamatergic metabolism in a sample of healthy and depressed youth with and without current SI. The ACC was selected as the region of interest considering (1) the robust evidence for its role in the pathophysiology of depression in adults and youth, and (2) its role in modulation of prefrontal and limbic processes39, notably the evaluation of negatively valent stimuli and their salience to the self40–44, which is highly pertinent to suicidal thoughts and behaviors45. We anticipated that depressed adolescents with current SI would demonstrate elevated Glx and reduced NAA in the ACC compared to nonsuicidal depressed and healthy youth. It was also hypothesized that Glx and NAA concentrations would correlate with continuous measures of SI intensity and severity. Finally, exploratory analyses were performed to examine the ability of ACC Glx and NAA/Glx to discriminate depressed adolescents with current SI from those without SI.
Materials and methods
Participants
Participants were adolescents between the ages of 13 and 21 years. Depressed participants were recruited from an adolescent psychopharmacology clinic and consisted of treatment-seeking youth with depressive symptoms; those who enrolled completed study assessments and 1H-MRS prior to undergoing appropriate clinical care (initiation of an antidepressant medication or change to another antidepressant). Healthy control participants were recruited from pediatric primary care clinics and through community advertising. Parents or guardians of minor participants (age < 18 years) provided written informed consent, and minor participants provided written informed assent; participants 18 years or older provided written informed consent. All study procedures were approved by the Mayo Clinic institutional review board (Rochester, MN, USA).
Clinical assessment and measures
All participants and parents/guardians underwent clinical assessment by a board-certified child and adolescent psychiatrist (P.E.C.) prior to 1H-MRS. This included evaluation on a semi-structured diagnostic interview, the K-SADS-PL46. Depressive symptom severity was rated on the Children’s Depression Rating Scale, Revised (CDRS-R)47 based on clinical interview of the adolescent participant and parent/guardian. Seventeen individual symptom items were rated by the clinician and summed for a total CDRS-R raw score ranging from 17 to 113. Additionally, since suicidality is assessed on the CDRS-R, an adjusted CDRS-R score was calculated by subtracting two items pertaining to suicidal and morbid ideation (items 12 and 13) from the total score to yield a measure of depressive symptom severity distinct from suicidal symptoms.
Current SI was assessed by clinical interview of participants using the Columbia Suicide Severity Rating Scale (C-SSRS)48. The “Severity of Ideation” and “Intensity of Ideation” subscales were used to characterize participants’ suicidal thoughts. The C-SSRS “Severity of Ideation” subscale is an ordinal scale derived from five items that assess the quality of suicidal thoughts. Severity of SI is rated as: 0 = no SI; 1 = wish to be dead; 2 = non-specific active suicidal thoughts; 3 = active SI with any method (not plan) without intent to act; 4 = active SI with some intent to act, without specific plan; 5 = active SI with specific plan and intent. The C-SSRS “Intensity of Ideation” subscale is derived from five individual items that assess intensity of suicidal thoughts across several dimensions: frequency of ideation, duration of ideation, controllability of thoughts, deterrents to suicide, and reasons for suicidal ideation. Each dimension is given a score of 0 to 5, for a total Intensity of Ideation subscale score of 0 (no SI present) to 25 (maximum intensity). Additionally, based on C-SSRS items corresponding to prior lifetime suicidal behavior (SB), participants were rated on an ordinal scale of maximal lifetime SB: 0 = no prior SB; 1 = nonsuicidal self-injurious behavior; 2 = planning or preparation for an attempt; 3 = aborted or interrupted attempt; 4 = suicide attempt.
Presence or absence of psychotropic medication use at the time of 1H-MRS was coded as a dichotomous variable (0 = no psychotropic medication; 1 = current psychotropic medication use).
Group eligibility and classification
Adolescents in the healthy control group had no current or historical psychiatric diagnosis, no current or previous psychopharmacologic or psychotherapeutic treatment, and had depression severity raw scores less than 30 on the CDRS-R. Participants in the two depressed groups had current diagnoses of unipolar depressive disorders on the K-SADS-PL diagnostic interview and had CDRS-R raw scores of 35 or greater. The Depressed without Suicidal Ideation (Dep/SI−) group had scores of zero on the current Severity of Ideation and Intensity of Ideation subscales of the C-SSRS. The Depressed with Suicidal Ideation (Dep/SI+) group consisted of depressed adolescents with current C-SSRS Severity of Ideation and Intensity of Ideation subscale scores of 1 or greater. Exclusion criteria for all participants consisted of lifetime history of mania or psychosis; presence of an active substance use disorder (except nicotine); and any contraindication to magnetic resonance imaging, such as implanted ferromagnetic material or orthodontic hardware that would cause artifact in MRI images.
1H-MRS methods
All participants underwent structural magnetic resonance imaging and 1H-MRS on a General Electric 3 T Discovery 750 scanner (GE Healthcare, Chicago, IL, USA) with an 8-channel head coil and running version 22.1 software. Structural images underwent review for incidental findings by a board-certified neuroradiologist (J.D.P.).
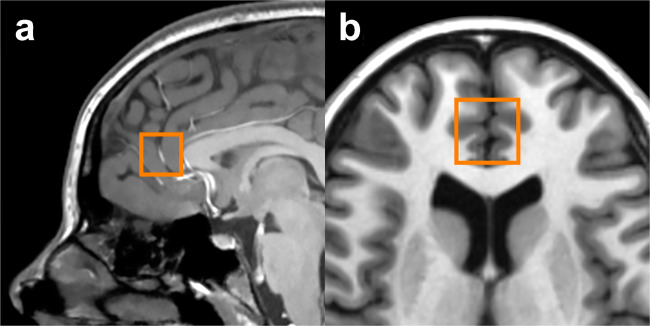
For acquisition of volumetric data, a FAST 3D SPGR sequence was used (sagittal acquisition, TR = 7.4 ms, TE = 3.0 ms, flip angle = 8°, voxel dimensions = 1.02 × 1.02 × 1.2 mm). Positioning of the ACC voxel was performed according to previously published methods49. In brief, an axial reference slice approximately 1 cm superior to the genu of the corpus callosum and permitting a continuous visualization of both anterior and posterior horns of the lateral ventricles was selected. On the reference slice, an 8-cm3 (2 × 2 × 2 cm) voxel encompassing predominantly prefrontal gray matter was centered on the interhemispheric fissure, with the posterior margin of the voxel abutting the genu of the corpus callosum. The voxel thus corresponded to the pregenual ACC (Brodmann areas 24a, 24b, and 32 as cytoarchitecturally defined)50. Voxel placement is shown in Fig. 1.
Fig. 1. Pregenual anterior cingulate cortex (ACC) voxel.
a Sagittal view; b Axial view.
Spectroscopic data were acquired using a 2-dimensional J-averaged PRESS sequence (TR = 2000 ms, TE = 35–195 ms in 16 steps, TR = 2000 ms, 8 averages, 3-way phase cycling) designed for optimal measurement of glutamate51,52. Following the scan, images and spectroscopic data were transferred to a workstation running SAGE-IDL software (GE Healthcare). Integrity of spectra was verified on visual review by the neuroradiologist, and scans with significant visible artifact were excluded. Metabolite concentrations were quantified using LCModel53 software version 6.3–1K and a vendor-provided basis set. Scans with signal-to-noise ratios less than 10 were excluded, and individual metabolite measurements were excluded if they had Cramér-Rao lower bounds (representing measurement error) > 20%.
Metabolite concentration measurements were corrected to the cerebrospinal fluid (CSF) fraction according to previously published methods49,54. In brief, segmentation of the T2-weighted anatomical images into gray matter, white matter, and CSF was performed using an in-house thresholding technique. The ACC voxel was then superimposed on the segmented anatomical images, and the number of pixels for gray matter, white matter, and CSF were quantified and normalized to the total pixels within the voxel to yield a fraction for each tissue type. The metabolite measurement (M) corrected to the CSF fraction (FCSF) was calculated as
and is expressed in institutional units. CSF-corrected Glu, Glx, and NAA concentrations were measured. Additionally, the NAA/Glx ratio was calculated. This was based on prior literature suggesting that the ratio of NAA to glutamatergic concentrations (or vice versa) allows measurement of altered glutamatergic metabolism while correcting for the effect of neuronal loss (as indexed by NAA alone)55,56 and may correspond to impairment in the metabolic cycling of glutamate–glutamine–NAA in neuropsychiatric disease states57,58.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25 (IBM Corp., Armonk, NY, USA) and JMP Pro 14.1.0 (SAS Institute, Inc., Cary, NC, USA) software. The significance level was set at α = 0.05, and p-values were adjusted for multiple comparisons according to the false-discovery rate (FDR) method59. The normality of distributions for spectroscopic outcome measures (Glu, Glx, NAA, NAA/Glx) was examined with Shapiro-Wilk tests. Distributions of all spectroscopic measures did not deviate from the normal distribution in the overall sample or within any group (all p > 0.2). Consequently, parametric statistical tests were used for the analyses.
For our primary aim, a separate fixed-effects general linear model (GLM) was conducted for each spectroscopic measure (Glu, Glx, NAA, NAA/Glx). In each GLM, main effects of the following independent variables were tested: group (HC vs. Dep/SI− vs. Dep/SI+), sex, age at time of scan, and psychotropic medication status (coded as a dichotomous variable denoting the presence or absence of psychotropic medication at the time of the 1H-MRS scan). The main effect of group was corrected for a total of four comparisons using the FDR method59. Post hoc comparisons of estimated group marginal means on each metabolite (for a total of three pairwise contrasts each) were adjusted for multiple comparisons using the Šidák correction.
For the secondary aim, the relationship between metabolite concentrations and (1) ordinal variables of SI severity, and (2) continuous variables of SI intensity were examined with multiple linear robust regressions that included age, sex, depression severity (adjusted CDRS-R total score), and psychotropic medication status as covariates.
As an exploratory sensitivity analysis, a receiver operating characteristic (ROC) analysis was used to determine the ability of ACC Glx and the NAA/Glx ratio to discriminate between Dep/SI− and Dep/SI+ groups. The analysis tested the areas under the curve (AUCs) of Glx and the NAA/Glx ratio against a nominal AUC of 0.5. The AUCs, 95% confidence intervals, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are reported for each optimal cutpoint.
Results
Participant characteristics
Forty adolescents (25 female, 15 male; mean age ± SD, 16.66 ± 1.92 years; range 13.58–20.81 years) underwent clinical evaluations and 1H-MRS scans. The three groups (HC, Dep/SI−, Dep/SI+ ) did not differ in age or sex distribution. Demographic and clinical characteristics of the three participant groups are reported in Table 1.
Table 1.
Demographic and clinical characteristics by group.
| Characteristic | HC (n = 16) |
Dep/SI− (n = 13) |
Dep/SI+ (n = 11) |
p |
|---|---|---|---|---|
| Age at time of 1H-MRS scan (years), mean ± SD | 16.90 ± 1.92 | 16.70 ± 2.08 | 16.26 ± 1.87 | 0.708a |
| Sex | 0.783a | |||
| Female, n (%) | 11 (68.75%) | 8 (61.54%) | 6 (54.55%) | |
| Male, n (%) | 5 (31.25%) | 5 (38.46%) | 5 (45.45%) | |
| Family history | ||||
| Any psychiatric illness, n (%) | 7 (50.00%)b | 13 (100%) | 11 (100%) | <0.001a |
| Mood disorder, n (%) | 7 (50.00%)b | 13 (100%) | 10 (90.91%) | 0.003a |
| Attempted or completed suicide, n (%) | 0 (0%)b | 5 (41.67%)c | 7 (63.64%) | <0.001a |
| Depression severity: CDRS-R | ||||
| Total score, mean ± SD | 18.50 ± 1.41 | 48.69 ± 7.93 | 57.64 ± 9.77 | 0.021d |
| Adjusted total score (without SI, morbid ideation items), mean ± SD | 16.50 ± 1.41 | 45.23 ± 7.24 | 49.36 ± 9.00 | 0.225d |
| Current psychotropic medication, n (%) | 0 (0%) | 4 (30.77%) | 5 (45.45%) | 0.675d |
| Number of depressive episodes, mean ± SD | n/a | 1.54 ± 0.66 | 1.73 ± 0.79 | 0.529d |
| Duration of current MDE (months), mean ± SD | n/a | 5.71 ± 5.42 | 14.82 ± 17.70 | 0.127d |
| Cumulative duration of all lifetime MDEs (months), mean ± SD | n/a | 11.48 ± 8.26 | 19.82 ± 16.35 | 0.120d |
| Duration of depressive illness (years), mean ± SD | n/a | 1.59 ± 1.58 | 2.73 ± 1.98 | 0.130d |
| Suicidal ideation/behavior: C-SSRS | ||||
| Severity of ideation (current), n (%) | <0.001d | |||
| 0 = no suicidal ideation | 16 (100%) | 13 (100%) | 0 (0%) | |
| 1 = wish to be dead | 0 (0%) | 0 (0%) | 2 (18.18%) | |
| 2 = non-specific active suicidal thoughts | 0 (0%) | 0 (0%) | 2 (18.18%) | |
| 3 = active suicidal ideation with any method (not plan) without intent to act | 0 (0%) | 0 (0%) | 2 (18.18%) | |
| 4 = active suicidal ideation with some intent to act, without specific plan | 0 (0%) | 0 (0%) | 3 (27.27%) | |
| 5 = active suicidal ideation with specific plan and intent | 0 (0%) | 0 (0%) | 2 (18.18%) | |
| Intensity of ideation (current), mean ± SD | 0 ± 0 | 0 ± 0 | 13.09 ± 3.39 | <0.001d |
| Maximal lifetime SB severity, n (%) | <0.001d | |||
| 0 = none | 16 (100%) | 11 (84.62%) | 0 (0%) | |
| 1 = nonsuicidal self-injurious behavior | 0 (0%) | 2 (15.38%) | 2 (18.18%) | |
| 2 = planning or preparation for attempt | 0 (0%) | 0 (0%) | 0 (0%) | |
| 3 = aborted or interrupted attempt | 0 (0%) | 0 (0%) | 4 (36.36%) | |
| 4 = suicide attempt | 0 (0%) | 0 (0%) | 5 (45.45%) | |
1H-MRS proton magnetic resonance spectroscopy, C-SSRS Columbia Suicide Severity Rating Scale, CDRS-R Children’s Depression Rating Scale, Revised, Dep/SI− depressed without suicidal ideation, Dep/SI+ depressed with suicidal ideation, HC healthy control, MDE major depressive episode, n/a not applicable, SB suicidal behavior, SD standard deviation, SI suicidal ideation.
aThree-group comparisons (HC vs. Dep/SI− vs. Dep/SI+) on demographic characteristics were conducted using one-way ANOVAs for continuous variables and Fisher’s exact tests for categorical variables.
bMissing two observations.
cMissing one observation.
dTwo-group comparisons (Dep/SI− vs. Dep/SI+) on clinical characteristics were conducted using independent samples t-tests for continuous variables and Fisher’s exact tests for categorical variables.
Comparing the two depressed groups, Dep/SI− and Dep/SI+ adolescents did not differ in the proportions of participants who had family histories of psychiatric illness, mood disorders, or attempted or completed suicide (Fisher’s exact tests, p > 0.99, p = 0.458, p = 0.414, respectively). Participants in the Dep/SI+ had higher CDRS-R total scores than did those in the Dep/SI− group (t = −2.477, p = 0.021). However, the adjusted depression severity score (removing CDRS-R items assessing morbid and suicidal ideation) did not differ (t = −1.247, p = 0.225). Dep/SI− and Dep/SI+ groups did not differ in number of depressive episodes (t = −0.640, p = 0.529), current episode duration (t = −1.643, p = 0.127), cumulative time depressed (t = −1.616, p = 0.120), or total depressive illness duration (t = −1.572, p = 0.130). The proportion of participants taking a psychotropic medication did not differ between the two depressed groups (p = 0.675). Medications taken by individual participants are reported in Supplemental Table S1.
Among the Dep/SI+ participants, two (18.18%) had current C-SSRS Severity of Ideation scores of 1, two (18.18%) had scores of 2, two (18.18%) had scores of 3, three (27.27%) had scores of 4, and two (18.18%) had scores of 5. On the Intensity of Ideation subscale, scores in the Dep/SI+ group ranged from 8 to 19 (mean score± SD, 13.09 ± 3.39). No adolescents in the HC group had any lifetime suicidal behavior (SB). Two participants in the Dep/SI− group (15.38%) had prior nonsuicidal self-injury; no Dep/SI− adolescents had any history of other forms of SB. In the Dep/SI+ group, two (18.18%) had prior nonsuicidal self-injury, four (36.36%) had a prior aborted or interrupted attempt, and five (45.45%) had made a suicide attempt.
Primary aim: group comparisons on 1H-MRS-measured ACC metabolites
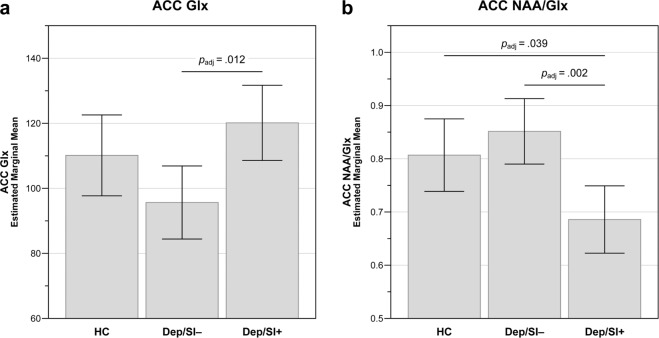
Estimated marginal means of ACC metabolites are reported in Table 2. In the GLM analyses, there were no significant main effects of group (HC vs. Dep/SI− vs. Dep/SI+ ) on Glu (F2,32 = 1.888, p = 0.168, pFDR = 0.178, = 0.106) or NAA (F2,32 = 1.821, p = 0.178, pFDR = 0.178, = 0.102). However, there were significant group main effects for ACC Glx (F2,31 = 5.003, p = 0.013, pFDR = 0.026, = 0.244) and the ACC NAA/Glx ratio (F2,31 = 7.473, p = 0.002, pFDR = 0.008, = 0.325). In post hoc pairwise contrasts (Fig. 2), Dep/SI− participants had lower mean ACC Glx than Dep/SI+ adolescents (padj = 0.012), although they did not differ from HC participants (padj = 0.190), and HC and Dep/SI+ adolescents also did not differ in mean ACC Glx (padj = 0.567). Dep/SI+ adolescents had a significantly lower mean NAA/Glx ratio than both Dep/SI− participants (padj = 0.002) and HC adolescents (padj = 0.039), whereas Dep/SI− and HC participants did not differ (padj = 0.649).
Table 2.
1H-MRS-measured anterior cingulate metabolites by group.
| Metabolite | Estimated marginal mean ±SE | p (pFDR) | |||
|---|---|---|---|---|---|
| HC (n = 16) |
Dep/SI− (n = 13) |
Dep/SI+ (n = 11) |
|||
| Glu | 85.04 ± 4.19 | 78.44 ± 3.71a | 88.43 ± 3.93a | 0.168 (0.178) | 0.106 |
| Glx | 110.13 ± 6.10 | 95.64 ± 5.51b | 120.13 ± 5.67a | 0.013 (0.026) | 0.244 |
| NAA | 85.74 ± 2.40 | 80.39 ± 2.12a | 81.07 ± 2.25a | 0.178 (0.178) | 0.102 |
| NAA/Glx | 0.807 ± 0.033 | 0.852 ± 0.030b | 0.686 ± 0.031a | 0.002 (0.008) | 0.325 |
General linear models (GLMs) were used to examine the main effect of group on metabolite concentrations, covarying for sex, age at scan, and psychotropic medication use. p-values and reported effect sizes () are for main effect of group.
1H-MRS proton magnetic resonance spectroscopy, Dep/SI− depressed without current suicidal ideation, Dep/SI+ depressed with current suicidal ideation, Glu glutamate, Glx glutamate+glutamine, HC healthy control, NAA N-acetylaspartate, pFDR p-value adjusted for multiple comparisons according to the false-discovery rate procedure59, SE standard error of the mean.
aMissing one observation (Cramér-Rao lower bound > 20%).
bMissing two observations (Cramér-Rao lower bound > 20%).
Fig. 2. Anterior cingulate cortex (ACC) metabolite differences by group.
a Glutamate+glutamine (Glx); b N-acetylaspartate/glutamate+glutamine (NAA/Glx) ratio. General linear models tested main effects of group, covarying for age, sex, and psychotropic medication use. Estimated marginal means and 95% confidence intervals for metabolites are displayed for each adolescent participant group (HC, healthy control, n = 16; Dep/SI−, depressed without suicidal ideation, n = 13; Dep/SI+ , depressed with suicidal ideation, n = 11). p-values adjusted for multiple comparisons (padj) are displayed for significant group differences in pairwise post hoc tests.
Secondary aim: relationships between glutamatergic neurochemistry and suicidal ideation
In the multiple linear regression analyses (Table 3), adjusting for age, sex, psychotropic medication, and depression severity (adjusted CDRS-R total score), there were no significant relationships between severity of SI (C-SSRS Severity of Ideation subscale) and ACC Glu (b̂ = 2.486, p = 0.140, pFDR = 0.187), Glx (b̂ = 5.653, p = 0.032, pFDR = 0.064), or NAA (b̂ = −0.262, p = 0.781, pFDR = 0.781). However, there was a significant negative relationship between SI severity and ACC NAA/Glx (b̂ = −0.044, p = 0.003, pFDR = 0.012).
Table 3.
Relationships between suicidal ideation and 1H-MRS-measured anterior cingulate metabolites.
| Metabolite | b̂ | SE | 95% CI for b̂ | p (pFDR) |
|---|---|---|---|---|
| Severity of ideation | ||||
| Glu | 2.486 | 1.644 | −0.863 to 5.834 | 0.140 (0.187) |
| Glx | 5.653 | 2.518 | 0.518 to 10.788 | 0.032 (0.064) |
| NAA | −0.262 | 0.936 | −2.168 to 1.644 | 0.781 (0.781) |
| NAA/Glx | −0.044 | 0.014 | −0.072 to −0.016 | 0.003 (0.012) |
| Intensity of ideation | ||||
| Glu | 0.655 | 0.403 | −0.167 to 1.476 | 0.114 (0.152) |
| Glx | 1.603 | 0.607 | 0.365 to 2.841 | 0.013 (0.026) |
| NAA | −0.014 | 0.231 | −0.484 to 0.457 | 0.952 (0.952) |
| NAA/Glx | −0.012 | 0.003 | −0.018 to −0.005 | 0.001 (0.004) |
Multiple linear regressions examining relationships of suicidal ideation severity and intensity with 1H-MRS-measured ACC metabolites. Regression models include sex, age at scan, psychotropic medication use, and depression severity (adjusted CDRS-R total score). The unstandardized parameter estimate (coefficient) for the SI variable (b̂), standard error of b̂, 95% confidence intervals for b̂, and p-values for the regression relationship between the suicidal ideation variable and the ACC metabolite are reported.
1H-MRS proton magnetic resonance spectroscopy, CI confidence interval, Glu glutamate, Glx glutamate+glutamine, NAA N-acetylaspartate, pFDR p-value adjusted for multiple comparisons according to the false-discovery rate procedure59, SE standard error.
In separate multiple linear regression models, again adjusting for age, sex, psychotropic medication, and depression severity (adjusted CDRS-R total score), there were no significant relationships between SI intensity (C-SSRS Intensity of Ideation subscale) and ACC Glu (b̂ = 0.655, p = 0.114, pFDR = 0.152) or NAA (b̂ = −0.014, p = 0.952, pFDR = 0.952). There was a significant positive relationship between SI intensity and Glx (b̂ = 1.603, p = 0.013, pFDR = 0.026), as well as a significant negative relationship between SI intensity and ACC NAA/Glx (b̂ = −0.012, p = 0.001, pFDR = 0.004).
Exploratory sensitivity analyses
The ROC analysis indicated that ACC Glx, using a cutpoint of ≥ 109.811, discriminated Dep/SI− participants from Dep/SI+ participants (AUC = 0.864, SE = 0.083, p = 0.005, pFDR = 0.005) with 90.00% sensitivity, 81.80% specificity, a PPV of 81.82%, and an NPV of 90.00%. The ROC analysis determined that the ACC NAA/Glx ratio, using a cutpoint of ≤ 0.73995, discriminated Dep/SI− participants from Dep/SI+ participants (AUC = 0.900, SE = 0.066, p = 0.002, pFDR = 0.004) with 81.80% sensitivity, 80.00% specificity, a PPV of 80.00%, and an NPV of 81.82%. ROC curves for the ACC Glx and NAA/Glx sensitivity analyses are displayed in Supplemental Fig. S1.
Discussion
The ACC plays essential roles in cognitive and emotional processes relevant to suicidal ideation and behavior. Through its prefrontal and limbic projections, the ACC mediates input from executive functions and motivational drives39,60. The dorsal/caudal ACC has been implicated in attentional and interpretive mechanisms used in evaluating internal and external stimuli61,62. The rostral (pregenual and subgenual) ACC, a portion of which was sampled in our study, is involved in affective regulation via inhibition of limbic and sympathetic responses to negatively valent stimuli and emotional conflict43,62. This latter ACC division, in conjunction with other areas of the medial prefrontal cortex, also appears to be involved in determining self-relevance of emotionally salient stimuli40,41. Encoding emotional valence involves implicit cognitive associations63, which have been shown to involve ACC activity in electroencephalographic63 and functional MRI64 studies. Implicit associations to suicide- and self-injury-related stimuli are stronger in adults65 and adolescents66–68 with histories of suicidality, and experimentally measured suicide- and self-injury-related implicit associations predict future suicidal ideation and self-harm in adolescents66,67,69. Altered rostral ACC activity also has been linked to other characteristics of suicidal individuals, including rumination and negative self-referential thinking42–44. Moreover, task-related ACC activation70–72 and functional connectivity to other emotion-regulating regions71 differ between adolescent suicide attempters and depressed non-attempters, suggesting that suicidality may involve ACC functions distinct from those related to depressive mood states.
Spectroscopic studies comparing depressed and healthy adolescents have found glutamatergic deficits in the ACC33–35 and diminished NAA concentrations in ACC and medial prefrontal cortex36, although, to our knowledge, none have directly compared cortical neurochemical profiles of suicidal and nonsuicidal youth. In adults, prior 1H-MRS studies have shown mixed findings on the potential roles of cortical glutamate and NAA in suicidality. Sheth et al.73 found no differences in Glu/H2O or NAA/H2O concentrations in dorsal ACC and posterior cingulate voxels between groups of military veterans with and without suicidal behavior (SB). In the same sample, Prescot et al.74 examined dorsal ACC metabolite concentrations in the overall sample, as well as in male and female subgroups; no differences in NAA/Cr+PCr or Glu/Cr+PCr were observed between veterans with and without SB in the overall sample, or within either sex group. Other 1H-MRS studies have examined potential relationships between suicidality and cortical metabolism in regions beyond the ACC. Jollant et al.32 utilized 1H-MRS to examine metabolites in the right dorsolateral prefrontal cortex (DLPFC) in healthy and depressed adults, including those with historical SB. Although no group differences survived correction for multiple comparisons, healthy control adults had lower Gln than both nonsuicidal depressed and suicide attempter groups, while NAA was lower in suicide attempters than in healthy controls. Right DLPFC NAA concentrations correlated negatively with current psychological pain, which persisted when controlling for various clinical characteristics such as age, gender, and depression severity; additionally, psychological pain mediated the relationship between DLPFC NAA and current SI32. Smesny et al.75 examined metabolite concentrations in adults with cluster B and C personality disorders, conditions with increased suicide risk, and in healthy comparators. The investigators found increased right DLPFC Glu and decreased right dorsal ACC NAA in cluster B patients, while cluster C patients demonstrated decreased NAA in bilateral DLPFC, left dorsomedial prefrontal cortex, and left dorsal ACC voxels, as well as decreased bilateral DLPFC and left dorsomedial prefrontal Glu75. By contrast, Rocha et al.31 found no difference in orbitofrontal cortical metabolite concentrations between healthy adults and groups of currently euthymic bipolar patients with and without historical suicide attempts.
When considering our results in the context of these earlier, disparate findings, it is important to note certain methodological differences. First, less mature excitatory circuitry in our younger sample may contribute to the differences in metabolite measurements between groups that we observed compared with prior adult studies. Second, metabolite concentrations differ not only between brain regions but also between heterogeneous segments of a single structure; indeed, many studies that sampled ACC voxels73–75 examined more dorsal aspects of the ACC than our pregenual voxel. The pregenual ACC is distinct in glutamatergic receptor density and microarchitecture compared to other ACC subregions, and prior 1H-MRS work has found the pregenual ACC to have higher Glu and Gln concentrations than more caudal subregions76. Furthermore, many prior studies have referenced metabolite values to total creatine (Cr+PCr). Total creatine has been found to differ in a variety of neuropsychiatric disease states in both adults54,77 and youth78,79. Thus, studies reporting metabolite concentrations relative to Cr+PCr introduce this additional confound when comparing clinical and healthy groups, and creatine-referenced metabolite values may not be directly comparable to CSF-corrected absolute concentrations as measured in our study.
Additionally, many prior studies classified patients on the basis of having a history of SB. Despite the frequent presence of prior SB in persons with current SI, it has not been established whether the neurochemical correlates of historical behavior are necessarily the same as those of current ideation. The grouping of depressed participants by presence or absence of current SI in our study is unique, and future work with larger samples of current ideators with and without prior SB is necessary to determine whether their neural metabolite profiles differ.
The most novel findings in our study were that the ACC NAA/Glx ratio was reduced in Dep/SI+ adolescents compared to those in the HC and Dep/SI− groups, correlated with current SI intensity and severity, and significantly discriminated depressed adolescents with and without current SI. Notably, these findings were observed in the absence of significant group differences in NAA or significant relationships between SI and NAA. This raises important questions about the meaning of the NAA/Glx ratio and the role that dysregulated NAA−glutamate metabolism in this crucial brain region might play in suicidality. NAA is found predominantly in neurons, and 1H-MRS-measured NAA values correspond to neuronal density80,81. Diminished NAA has been found in disease processes involving neuronal loss, and yet NAA concentrations also have been observed to recover, suggesting that NAA may index both permanent and state-dependent aspects of neuronal health, viability, and activity80–82. The 1H-MRS-measured Glx concentration is a composite of Glu and Gln signals, with γ-aminobutyric acid (GABA) and glutathione also being minor factors80,81. Glutamate both functions as the main excitatory transmitter and has roles in energy metabolism, while glutamine serves predominantly as an intermediate for glutamate and GABA synthesis, being shuttled between astrocytes and neurons in a form less reactive than these transmitters81. Glx thus indicates the combined (neuronal and glial) cytosolic pool of glutamate and glutamine that can be used for both energetic and neurotransmission functions80,81. Neuronal NAA can be converted to glutamate via a series of reactions occurring in astrocytes, oligodendrocytes, and neuronal mitochondria, and thus NAA also may serve as a reservoir for the production of glutamate and glutamine, particularly in conditions of metabolic stress57. NAA may index ATP-dependent metabolism, as well as this alternative glutamate-dependent energy production, in neuronal mitochondria82. Considering the complex relationships between these metabolites, examining their concentrations relative to one another, as indicated by a ratio, may offer insights into how cycling and metabolism of glutamate, glutamine, and NAA differ in pathological conditions57,58. Additionally, concentration ratios may be more sensitive to metabolic derangements of related molecules than single metabolite measurements alone55,83.
Local dysregulation in NAA and glutamatergic metabolism, as indicated by low NAA/Glu or NAA/Glx concentrations (or, inversely, high Glu/NAA or high Glx/NAA), has been identified as a potential marker of damage to brain structures or networks that correspond to the symptomatic processes specific to diverse neurologic and psychiatric conditions. White matter Glu/NAA was elevated in a large sample of patients with multiple sclerosis compared to healthy controls, and this ratio predicted longitudinal brain volume loss55. Hypothalamic Glx/NAA also was higher in multiple sclerosis patients than in healthy comparators, and was higher in patients with more active disease, while Glx/NAA also corresponded to symptom severity and fatigue58. Primary motor cortex NAA/Glu correlated negatively with disease duration in amyotrophic lateral sclerosis84. Glx/NAA was elevated in epileptogenic foci relative to healthy brain regions in partial epilepsy, and demonstrated utility in identifying seizure foci56. In schizophrenia, hippocampal Glx/NAA was increased compared to healthy adults83. Significant positive correlations between Glx and NAA concentrations were present in healthy controls but not in schizophrenic patients in the hippocampus83,85 right DLPFC86, and left striatum87, suggesting that the usually linked metabolism of NAA and glutamate becomes uncoupled in these regions in the disease state. Similarly, right hippocampal Glu/NAA was elevated in posttraumatic stress disorder (PTSD) patients compared to trauma-exposed controls, which correlated with re-experiencing symptoms and trauma load in patients88. It is notable that the symptomatology of both schizophrenia and PTSD are characterized by deficits in processes involving the hippocampus, and that the DLPFC and striatum have been implicated previously in schizophrenia. Altered NAA−glutamate metabolism in the ACC, by comparison, might be expected to correspond to conditions typified by impaired emotion-processing functions, such as mood disorders and suicidality. In one study, ACC NAA/Glx was lower in adults with bipolar disorder compared to healthy controls, both before treatment and after 12 weeks of lamotrigine89. This suggests the need to examine ACC glutamatergic metabolism in suicidal individuals in longitudinal studies, both for changes that occur in conjunction with natural fluctuations in suicidal risk and also for the effects (or lack thereof) of anti-suicidal interventions.
Our study has several important limitations. The sample was small, and larger, well-powered investigations are necessary to replicate these findings before they can inform clinical risk assessment and future interventions. It is particularly important for future studies to include adequate numbers of both male and female participants across broad neurodevelopmental trajectories. NAA and glutamate levels have been found to differ between age and sex groups among healthy individuals90, and patterns of suicidal behavior differ by both age and sex. The limited research examining sex-related differences in excitatory−inhibitory neurochemistry in suicidality suggests potential distinctions in ACC metabolism, but these remain poorly understood74. Regarding our spectroscopic methodology, the 2-dimensional J-averaged PRESS sequence used in this study is optimized for glutamate signal acquisition at 3 T, but it did not permit reliable measurement of Gln or GABA. It is noteworthy that while we did find significant group differences and a relationship with SI for ACC Glx, there were no significant findings for Glu. This suggests the possibility that glutamine, which accounts for the majority of the non-Glu portion of the Glx signal, could be responsible for the discrepancy between our Glu and Glx findings. This is particularly relevant considering that the pregenual ACC has a substantially higher ratio of Gln to Glu than other subregions of the cingulate gyrus76. Moreover, preliminary data suggest that these related metabolites are productive areas for further study of the intersection of mood disorders and suicidality. For example, DLPFC Gln was lower in healthy adults compared to depressed adults with and without prior suicide attempts32, and ACC GABA concentrations were found to be reduced in female veterans with SB and correlated negatively with measures of suicidality74. Further 1H-MRS studies are needed to understand how the metabolically-linked GABA and glutamate−glutamine systems relate to clinical features such as SI and SB. TE-optimized PRESS approaches91 that allow accurate measurement of Gln and MEGA-PRESS sequences designed to quantify GABA92,93, in conjunction with the use of higher field strengths, may yield more comprehensive insights into how cortical excitatory−inhibitory metabolism corresponds to acute suicidality. Furthermore, the instrument used for classifying participants and rating the intensity and severity of SI in our study, the C-SSRS, was designed to assess clinical suicide risk. Future investigations should utilize dimensional measures assessing not only overt measures of suicidality, but also specific cognitive and emotional constructs associated with suicidal thoughts and behaviors. Several promising adolescent studies have examined the role of cortical neurochemistry in symptomatologic features relevant to suicidality. Anhedonia has demonstrated strong associations with suicidality that are independent of other depressive symptoms94–97. Pregenual ACC Gln was lower in highly anhedonic depressed adults than in low-anhedonia depressed and healthy individuals, whereas ACC glutamate and NAA correlated with functional MRI-measured ACC activation in response to emotional stimuli in depressed persons98. By contrast, ACC GABA was found to be significantly reduced in anhedonic depressed youth compared to nonanhedonic depressed and healthy adolescents37,38. However, the relationships between constructs like anhedonia and complex behaviors like SB remain poorly understood at present. Future research must strive to delineate how symptom-related, cognitive, and emotional functions mediate the relationship between SB and observed neurochemical deficits in particular brain structures and circuits. Doing so will enable the development of more comprehensive and sophisticated models of the neurobiology of suicidality.
Supplementary information
Acknowledgements
This work was supported by grants from the Brain & Behavior Research Foundation (C.P.L., 2018 NARSAD Young Investigator Grant 27488, Alan G. Ross Memorial Investigator) and the National Institute of Mental Health (P.E.C., K23 MH100266 and R01 MH113700). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
C.P.L. receives grant support from the Brain & Behavior Research Foundation as the Alan G. Ross Memorial Investigator. He has been a site investigator for multicenter trials funded by Neuronetics, Inc. and NeoSync, Inc. J.D.P. serves as an imaging consultant for Takeda Pharmaceutical Company, Ltd., Biomedical Systems Corp., and Neuronetics, Inc. C.J.B. receives research support from the Mayo Foundation for Medical Education and Research Departmental Small Grant Program. M.A.F. has received grant support from Assurex Health, Inc., the Mayo Foundation for Medical Education and Research, and Medibio, Ltd.; has served as a paid consultant for Actify Neurotherapies, Allergan plc, Intra-Cellular Therapies, Inc., Janssen Pharmaceuticals, Inc., Myriad Genetics, Inc., Neuralstem, Inc., Takeda Pharmaceutical Company, Ltd., and Teva Pharmaceutical Industries, Ltd.; and has received honoraria or travel support from American Physician Institute, CME Outfitters, LLC, and Global Academy for Medical Education, LLC. P.E.C. has received research grant support from the National Institute of Mental Health and Pfizer, Inc.; equipment support from Neuronetics, Inc.; and has received supplies and genotyping services from Assurex Health, Inc. for investigator-initiated studies. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc., and he has served as a consultant for Procter & Gamble Company and Myriad Neuroscience. A.I.S., B.J.S., and J.M.L. have no financial relationships to disclose.
Footnotes
A preliminary version of this analysis was presented at the American Academy of Child and Adolescent Psychiatry, 64th Annual Meeting, Washington, DC, October 2017 (J. Am. Acad. Child. Adolesc. Psychiatry 2017; 56: S225).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-0792-z).
References
- 1.Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS), www.cdc.gov/injury/wisqars/ (2017).
- 2.Olfson M, et al. National trends in suicide attempts among adults in the United States. JAMA Psychiatry. 2017;74:1095–1103. doi: 10.1001/jamapsychiatry.2017.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miron O, Yu K-H, Wilf-Miron R, Kohane IS. Suicide rates among adolescents and young adults in the United States, 2000–2017. JAMA. 2019;321:2362–2364. doi: 10.1001/jama.2019.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Preventing Suicide: A Global Imperative. Geneva: WHO Press; 2014. [Google Scholar]
- 5.Plemmons G, et al. Hospitalization for suicide ideation or attempt: 2008-2015. Pediatrics. 2018;141:e20172426. doi: 10.1542/peds.2017-2426. [DOI] [PubMed] [Google Scholar]
- 6.Nock MK, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70:300–310. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kann L, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill. Summ. 2016;65:1–174. doi: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- 8.Bostwick JM, Pabbati C, Geske JR, McKean AJ. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am. J. Psychiatry. 2016;173:1094–1100. doi: 10.1176/appi.ajp.2016.15070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Copeland WE, Goldston DB, Costello EJ. Adult associations of childhood suicidal thoughts and behaviors: a prospective, longitudinal analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:958–965. doi: 10.1016/j.jaac.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, J. D. et al. Parent-adolescent agreement about adolescents’ suicidal thought. Pediatrics143, pii: e20181771 (2019). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6361346/pdf/PEDS_20181771.pdf. Epub 2019 Jan 14. [DOI] [PMC free article] [PubMed]
- 11.Chang BP, et al. Biological risk factors for suicidal behaviors: a meta-analysis. Transl. Psychiatry. 2016;6:e887. doi: 10.1038/tp.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin JC, et al. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol. Bull. 2017;143:187–232. doi: 10.1037/bul0000084. [DOI] [PubMed] [Google Scholar]
- 13.Oquendo MA, et al. Toward a biosignature for suicide. Am. J. Psychiatry. 2014;171:1259–1277. doi: 10.1176/appi.ajp.2014.14020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudol K, Mann JJ. Biomarkers of suicide attempt behavior: towards a biological model of risk. Curr. Psychiatry Rep. 2017;19:31. doi: 10.1007/s11920-017-0781-y. [DOI] [PubMed] [Google Scholar]
- 15.Sokolowski M, Ben-Efraim YJ, Wasserman J, Wasserman D. Glutamatergic GRIN2B and polyaminergic ODC1 genes in suicide attempts: associations and gene-environment interactions with childhood/adolescent physical assault. Mol. Psychiatry. 2013;18:985–992. doi: 10.1038/mp.2012.112. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, et al. A pilot integrative genomics study of GABA and glutamate neurotransmitter systems in suicide, suicidal behavior, and major depressive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016;171b:414–426. doi: 10.1002/ajmg.b.32423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klempan TA, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol. Psychiatry. 2009;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 18.Sequeira A, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erhardt S, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunebaum MF, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am. J. Psychiatry. 2018;175:327–335. doi: 10.1176/appi.ajp.2017.17060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson ST, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry. 2018;175:150–158. doi: 10.1176/appi.ajp.2017.17040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auer DP, et al. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 23.Horn DI, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression-the role of pregenual anterior cingulate cortex and anterior insula. Front. Syst. Neurosci. 2010;4:33. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkl A, et al. Abnormal cingulate and prefrontal cortical neurochemistry in major depression after electroconvulsive therapy. Biol. Psychiatry. 2011;69:772–779. doi: 10.1016/j.biopsych.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Portella MJ, et al. Ventromedial prefrontal spectroscopic abnormalities over the course of depression: a comparison among first episode, remitted recurrent and chronic patients. J. Psychiatr. Res. 2011;45:427–434. doi: 10.1016/j.jpsychires.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Michael N, et al. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol. Med. 2003;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- 27.Pfleiderer B, et al. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. Neuroimaging. 2003;122:185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 28.Hasler G, et al. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 29.Block W, et al. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int. J. Neuropsychopharmacol. 2009;12:415–422. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- 30.Li J, et al. [A proton magnetic spectroscopy research on hippocampus metabolisms in people withsuicide-attempted depressions] Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:59–62. [PubMed] [Google Scholar]
- 31.Rocha MV, et al. Normal metabolic levels in prefrontal cortex in euthymic bipolar I patients with and without suicide attempts. Neural Plast. 2015;2015:165180. doi: 10.1155/2015/165180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jollant, F., Near, J., Tureckiy, G. & Richard-Devantoyeva, S. Spectroscopy markers of suicidal risk and mental pain in depressed patients. Prog Neuropsychopharmacol. Biol. Psychiatry73, 64–71 (2016). https://reader.elsevier.com/reader/sd/pii/S0278584616301671?token=DB32EE1A91119BDCE0F68C6E89A730AA2C58041E2DF873CF4BCAF616D6CAAEAADC5AF892BA4D1FCF965AB6C4FCE28DE5 [Epub ahead of print]. [DOI] [PubMed]
- 33.Rosenberg DR, et al. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol. Psychiatry. 2005;58:700–704. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Mirza Y, et al. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg DR, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- 36.Olvera RL, et al. Reduced medial prefrontal N-acetyl-aspartate levels in pediatric major depressive disorder: a multi-voxel in vivo 1H spectroscopy study. Psychiatry Res. Neuroimaging. 2010;184:71–76. doi: 10.1016/j.pscychresns.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabbay V, et al. Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch. Gen. Psychiatry. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabbay V, et al. Anterior cingulate cortex γ-aminobutyric acid deficits in youth with depression. Transl. Psychiatry. 2017;7:e1216. doi: 10.1038/tp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 40.Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cogn. Neurosci. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- 41.Northoff G, et al. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura S, et al. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura S, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J. Affect. Disord. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Glenn, C. R. et al. Understanding suicide risk within the Research Domain Criteria (RDoC) framework: a meta-analytic review. Depress. Anxiety35, 1–24 (2017). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5760472/pdf/nihms904680.pdf. [DOI] [PMC free article] [PubMed]
- 46.Kaufman J, et al. Schedule for affective disorders and schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Poznanski EO, et al. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. J. Am. Acad. Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Posner K, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croarkin PE, et al. Transcranial magnetic stimulation potentiates glutamatergic neurotransmission in depressed adolescents. Psychiatry Res. Neuroimaging. 2016;247:25–33. doi: 10.1016/j.pscychresns.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogt BA, Vogt L. Cytology of human dorsal midcingulate and supplementary motor cortices. J. Chem. Neuroanat. 2003;26:301–309. doi: 10.1016/j.jchemneu.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Adalsteinsson E, et al. In vivo 2D J-resolved magnetic resonance spectroscopy of rat brain with a 3-T clinical human scanner. NeuroImage. 2004;22:381–386. doi: 10.1016/j.neuroimage.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 52.Hurd R, et al. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn. Reson. Med. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 53.Provencher SW. Automatic quantitation of localized in vivo1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 54.Port JD, Unal SS, Mrazek DA, Marcus SM. Metabolic alterations in medication-free patients with bipolar disorder: a 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Res. Neuroimaging. 2008;162:113–121. doi: 10.1016/j.pscychresns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Azevedo CJ, et al. In vivo evidence of glutamate toxicity in multiple sclerosis. Ann. Neurol. 2014;76:269–278. doi: 10.1002/ana.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savic I, et al. In vivo measurements of glutamine + glutamate (Glx) and N-acetyl aspartate (NAA) levels in human partial epilepsy. Acta Neurol. Scand. 2000;102:179–188. doi: 10.1034/j.1600-0404.2000.102003179.x. [DOI] [PubMed] [Google Scholar]
- 57.Clark JF, et al. N-acetylaspartate as a reservoir for glutamate. Med. Hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 58.Kantorová E, et al. Hypothalamic damage in multiple sclerosis correlates with disease activity, disability, depression, and fatigue. Neurol. Res. 2017;39:323–330. doi: 10.1080/01616412.2016.1275460. [DOI] [PubMed] [Google Scholar]
- 59.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- 60.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 61.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Healy GF, Boran L, Smeaton AF. Neural patterns of the Implicit Association Test. Front. Hum. Neurosci. 2015;9:605. doi: 10.3389/fnhum.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chee MW, Sriram N, Soon CS, Lee KM. Dorsolateral prefrontal cortex and the implicit association of concepts and attributes. Neuroreport. 2000;11:135–140. doi: 10.1097/00001756-200001170-00027. [DOI] [PubMed] [Google Scholar]
- 65.Glenn JJ, et al. Suicide and self-injury-related implicit cognition: a large-scale examination and replication. J. Abnorm. Psychol. 2017;126:199–211. doi: 10.1037/abn0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cha CB, et al. Using implicit and explicit measures to predict nonsuicidal self-injury among adolescent inpatients. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:62–68. doi: 10.1016/j.jaac.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Glenn CR, Kleiman EM, Cha CB, Nock MK, Prinstein MJ. Implicit cognition about self-injury predicts actual self-injurious behavior: results from a longitudinal study of adolescents. J. Child Psychol. Psychiatry. 2016;57:805–813. doi: 10.1111/jcpp.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Millner, A. J.et al. Implicit cognitions as a behavioral marker of suicide attempts in adolescents. Arch. Suicide Res23, 1–17 (2018). https://www.tandfonline.com/doi/abs/10.1080/13811118.2017.1421488?journalCode=usui20. [DOI] [PubMed]
- 69.Glenn CR, et al. Implicit identification with death predicts change in suicide ideation during psychiatric treatment in adolescents. J. Child Psychol. Psychiatry. 2017;58:1319–1329. doi: 10.1111/jcpp.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan LA, et al. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:e603. doi: 10.1016/j.jaac.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan LA, et al. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol. Med. 2013;43:2129–2142. doi: 10.1017/S0033291712002966. [DOI] [PubMed] [Google Scholar]
- 72.Harms MB, et al. Adolescent suicide attempts and ideation are linked to brain function during peer interactions. Psychiatry Res. Neuroimaging. 2019;289:1–9. doi: 10.1016/j.pscychresns.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Sheth C, et al. Alterations in anterior cingulate cortex myoinositol and aggression in veterans with suicidal behavior: a proton magnetic resonance spectroscopy study. Psychiatry Res. Neuroimaging. 2018;276:24–32. doi: 10.1016/j.pscychresns.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Prescot A, et al. Altered cortical GABA in female veterans with suicidal behavior: sex differences and clinical correlates. Chronic Stress (Thousand Oaks) 2018;2:1–12. doi: 10.1177/2470547018768771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smesny S, et al. Prefrontal glutamatergic emotion regulation is disturbed in cluster B and C personality disorders–a combined 1H/31P-MR spectroscopic study. J. Affect. Disord. 2018;227:688–697. doi: 10.1016/j.jad.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 76.Dou W, et al. Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J. Neurosci. 2013;33:12698–12704. doi: 10.1523/JNEUROSCI.1758-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frye MA, et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- 78.Mirza Y, et al. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J. Child Neurol. 2006;21:106–111. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- 79.Gabbay V, et al. Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: a proton MR spectroscopy study. Am. J. Psychiatry. 2007;164:1881–1889. doi: 10.1176/appi.ajp.2007.06122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bittšanský M, Výbohová D, Dobrota D. Proton magnetic resonance spectroscopy and its diagnostically important metabolites in the brain. Gen. Physiol. Biophys. 2012;31:101–112. doi: 10.4149/gpb_2012_007. [DOI] [PubMed] [Google Scholar]
- 81.Maddock, R. J. & Buonocore, M. H. In Brain Imaging in Behavioral Neuroscience (eds Carter, C. S. & Dalley, J. W.) 199–251 (Springer: Berlin, Heidelberg, 2012).
- 82.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sako W, Abe T, Izumi Y, Harada M, Kaji R. The ratio of N-acetyl aspartate to glutamate correlates with disease duration of amyotrophic lateral sclerosis. J. Clin. Neurosci. 2016;27:110–113. doi: 10.1016/j.jocn.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 85.Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37:2635–2642. doi: 10.1038/npp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coughlin JM, et al. Decoupling of N-acetyl-aspartate and glutamate within the dorsolateral prefrontal cortex in schizophrenia. Curr. Mol. Med. 2015;15:176–183. doi: 10.2174/1566524015666150303104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sivaraman S, et al. Neurometabolic abnormalities in the associative striatum in antipsychotic-naïve first episode psychosis patients. Psychiatry Res. Neuroimaging. 2018;281:101–106. doi: 10.1016/j.pscychresns.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosso IM, Crowley DJ, Silveri MM, Rauch SL, Jensen JE. Hippocampus glutamate and N-acetyl aspartate markers of excitotoxic neuronal compromise in posttraumatic stress disorder. Neuropsychopharmacology. 2017;42:1698–1705. doi: 10.1038/npp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Croarkin PE, et al. N-acetylaspartate normalization in bipolar depression after lamotrigine treatment. Bipolar Disord. 2015;17:450–457. doi: 10.1111/bdi.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hädel S, Wirth C, Rapp M, Gallinat J, Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J. Magn. Reson. Imaging. 2013;38:1480–1487. doi: 10.1002/jmri.24123. [DOI] [PubMed] [Google Scholar]
- 91.Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. NeuroImage. 2004;21:1762–1771. doi: 10.1016/j.neuroimage.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 92.Mullins PG, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cleve M, Gussew A, Reichenbach JR. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. NeuroImage. 2015;105:67–75. doi: 10.1016/j.neuroimage.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 94.Winer ES, Drapeau CW, Veilleux JC, Nadorff MR. The association between anhedonia, suicidal ideation, and suicide attempts in a large student sample. Arch. Suicide Res. 2016;20:265–272. doi: 10.1080/13811118.2015.1025119. [DOI] [PubMed] [Google Scholar]
- 95.Yaseen ZS, Galynker II, Briggs J, Freed RD, Gabbay V. Functional domains as correlates of suicidality among psychiatric inpatients. J. Affect. Disord. 2016;203:77–83. doi: 10.1016/j.jad.2016.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballard ED, et al. Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J. Affect. Disord. 2017;218:195–200. doi: 10.1016/j.jad.2017.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ducasse D, et al. Anhedonia is associated with suicidal ideation independently of depression: a meta-analysis. Depress. Anxiety. 2018;35:382–392. doi: 10.1002/da.22709. [DOI] [PubMed] [Google Scholar]
- 98.Walter M, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch. Gen. Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.