Highlights
-
•
Detection rate of BCoV was statistically higher in dairy than in beef calves.
-
•
Argentinean strains are distant from the Mebus strain included in local vaccines.
-
•
In vitro cross-protection between Arg95 field strain and Mebus reference strain.
Keywords: Coronavirus, Neonatal calf diarrhea, Cross-protection
Abstract
Bovine coronavirus (BCoV) is an important viral pathogen associated with neonatal calf diarrhea. Our aim was to investigate the incidence of BCoV in diarrhea outbreaks in beef and dairy herds from Argentina during 1994–2010. A total of 5.365 fecal samples from diarrheic calves were screened for BCoV diagnosis by ELISA. The virus was detected in 1.71% (92/5365) of the samples corresponding to 5.95% (63/1058) of the diarrhea cases in 239 beef and 324 dairy farms. The detection rate of BCoV was significantly higher in dairy than in beef herds: 12.13% (29/239) vs. 4.32% (14/324) respectively. Phylogenetic analysis of the hypervariable S1 region of seven representative samples (from different husbandry systems, farm locations and years of sampling) indicated that BCoV strains circulating in Argentinean beef and dairy herds formed a cluster distinct from other geographical regions. Interestingly, Argentinean strains are distantly related (at both the nucleotide and amino acid levels) with the Mebus historic reference BCoV strain included in the vaccines currently available in Argentina. However, Mebus-induced antibodies were capable of neutralizing the BCoV Arg95, a field strain adapted to grow in vitro, and vice versa, indicating that both strains belong to the same CoV serotype reported in cattle. This work represents the first large survey describing BCoV circulation in Argentinean cattle.
1. Introduction
Bovine coronavirus (BCoV) is a major viral pathogen associated with neonatal calf diarrhea (NCD) (Mebus et al., 1973), winter dysentery in adult cattle (Saif et al., 1988) and respiratory tract disorders in cattle of all ages (Cho et al., 2001b, Decaro et al., 2008a). It causes important economic losses to the beef and dairy industry worldwide (Boileau and Kapil, 2010, Vlasova and Saif, 2014). Serological surveys indicate that approximately 90% of the worldwide cattle population has antibodies (Abs) against BCoV (Lin et al., 1996). Also, detection of similar CoV strains among wild ruminants, dogs and horses, with evidence for experimental interspecies transmission to calves, suggests that these species could harbor CoVs transmissible to cattle or vice versa (Barros et al., 2013, Saif, 2010).
Three antigenic groups of coronaviruses have been established and all BCoV strains characterized worldwide belonged to the subgroup initially designated as 2a (Hasoksuz et al., 2008). The International Commitee for Taxonomy Viruses (ICTV) has proposed a revision of the family Coronaviridae to create a new subfamily Coronavirinae that includes the Alpha, Beta and Gammacoronavirus genera. Following this new suggested taxonomy, BCoV belongs to the Betacoronavirus genus, cluster within the Coronavirinae subfamily, Coronaviridae family and the order Nidovirales (http://ictvonline.org/virusTaxonomy.asp).
The virus genome is comprised of single stranded non-segmented positive-sense RNA (32 kb) associated to the nucleoprotein (N) and forming a nucleocapsid with helical symmetry (Clark, 1993). Viral particles are large (100–150 nm), pleomorphic and enveloped with four major structural proteins comprising a membrane (M) glycoprotein, an envelope (E) protein, a spike (S) glycoprotein and the hemagglutinin-esterase (HE) glycoprotein (Lai, 2001). It is interesting to note that the hemagglutinating activity of the HE from BCoVs strains is lower than the hemagglutinating activity of the S glycoprotein, which forms large spike-like projections in the viral envelope (Schultze et al., 1991). Moreover, the S glycoprotein harbors domains responsible for receptor binding and induction of neutralizing antibodies, and is the most polymorphic viral protein among CoV species and also among strains of the same species. It is utilized for the molecular characterization of the isolates (Collins et al., 1982). The S glycoprotein consists of two subunits, S1 (N-terminal half) and S2 (C-terminal half). The S1 hypervariable region is useful to study the variability and evolution of this virus (Brandao et al., 2006, Hasoksuz et al., 2002).
Most of the studies assessing BCoV relevance as a primary pathogen in neonatal calf diarrhea (NCD) were conducted in the northern hemisphere (Ammar et al., 2014, Bidokhti et al., 2013, Decaro et al., 2008b, Hasoksuz et al., 2002, Jeong et al., 2005, Lu et al., 1991, Mawatari et al., 2014, Ohlson et al., 2013). In contrast, little epidemiological information is available regarding BCoV detection, incidence and characterization in cattle from Central and South American countries. In Cuba, BCoV sequences clustered with BCoV strains from USA, suggesting a common origin for these viruses (Martinez et al., 2012). In South America, most of the information comes from studies conducted in Brazil (Asano et al., 2010, Barros et al., 2013, Brandao et al., 2008, Brandao et al., 2006, Takiuchi et al., 2008). Stipp et al. (2009) reported a 15.6% detection rate of BCoV in diarrheic calves from dairy and beef farms during a survey conducted in four states of Brazil. Phylogenetic studies based on the hypervariable region of the S glycoprotein gene indicated that Brazilian BCoV strains belong to two different clusters, suggesting that at least two different BCoV strains are circulating in Brazil (Brandao et al., 2006, Takiuchi et al., 2008). Interestingly, some BCoV strains detected in Brazil showed a gap of 18 nucleotides (nt 1577–1594; aa 461–570) in the hypervariable region within the S1 encoding gene, giving rise to a paraphyletic group in the evolution of BCoV circulating in Brazil. Similar gaps were also reported in porcine and human CoV strains causing respiratory disease. The presence of this gap in the S protein from swine CoVs has been associated with a change from enteric to respiratory tropism (Saif and Sestak, 2006, St-Jean et al., 2004).
For the entire S1 encoding gene, Takiuchi et al. (2008) showed that the Brazilian BCoV strains were distant from the Mebus strain (97.8% identity for nucleotides and 96.8% identity for amino acids) and more similar to the American BCoV-ENT strain 182NS and other Canadian strains (98.7% for nucleotides and 98.7% for amino acids).
The aim of the present study was to determine the infection rates of BCoV in diarrheic calves from Argentinean farms. Additionally, to conduct a phylogenetic study with the Argentinean BCoV strains in comparison with the BCoV strains, characterized worldwide, we focused our analysis on the hypervariable region of the BCoV S1 encoding gene (330 bp -nucleotide 1381–1711 of the Mebus strain S gene U00735.2-). To confirm the results, a phylogenetic analysis using a 1555 bp (nucleotide 1066–2621 of the Mebus strain S gene U00735.2) fragment of the S glycoprotein gene from two Argentinean strains detected 18 years apart (Arg95 and 5324-2013), was also performed. Finally, to evaluate the cross-reactivity between the Arg95 isolate and the Mebus reference strain, an in vitro virus neutralization assay was conducted.
2. Materials and methods
2.1. Fecal samples and viral detection
A total of 5.365 fecal samples were collected from diarrheic calves during 1994–2010, corresponding to a total of 1.058 outbreaks or cases of NCD in dairy farms (n = 239), beef herds (n = 324) and cattle from non-specified farm types (n = 495). The survey included farms located in 10 different provinces from Argentina (Buenos Aires, Corrientes, Entre Rios, Santiago del Estero, Santa Fe, Córdoba, La Pampa, San Luis, Río Negro and Neuquén).
The detection of BCoV antigens in fecal samples was performed by an indirect antigen-capture ELISA as described elsewhere (Smith et al., 1996). Briefly, 96 well ELISA plates (Maxisorp, NUNC, Denmark) were coated with four monoclonal antibodies (MAbs BC 21 F63C, BC 22 F83C directed to HE, BC 28H1.2C directed to N and BC 29 G72C directed to S) developed against CD DB2 strain and then incubated with 10% nonfat milk in PBS-Tween 0.05% for blocking of non-specific activity. Then, samples were added and incubated for 1 h at 37 °C. The plates were later incubated with polyclonal guinea pig anti-serum to BCoV at a 1:4000 dilution, and finally with commercial HRP-labeled goat polyclonal Abs to guinea pig IgG at a 1:3000 dilution (KPL, USA) for 1 h at 37 °C. Hydrogen peroxide and ABTS were used as substrate/chromogenic system (KPL, USA).
A total of seven ELISA-positive samples were selected for molecular characterization. The selection criteria included samples representing different husbandry systems, farm locations and years of sampling. Five of these fecal samples collected from three dairy herds and two beef herds were stored at −70 °C before sequencing and one sample collected in 2013 (5324) was sequenced from fresh stool. In addition, both original and the eight passage of the tissue culture adapted Argentinean strain (Arg95) were analyzed. The studied samples were identified as CoV/Bovine-B/Argentina/1617/2001, CoV/Bovine-B/Argentina/2026/2002, CoV/Bovine D/Argentina/4041/2009, CoV/Bovine-D/Argentina/4733/2009, CoV/Bovine-D/Argentina/4583/2010, CoV/Bovine-D/Argentina/5324/2013, and both the original and the 8th passage Arg95/1995. Accession numbers were deposited in GenBank database as KP033205–KP033210 and KP059126, KP059127.
Fecal samples were also tested for group A Rotavirus (RVA) using an indirect antigen-capture ELISA as previously reported (Badaracco et al., 2013, Badaracco et al., 2012, Cornaglia et al., 1989, Garaicoechea et al., 2006).
2.2. Nested RT-PCR for BCoV S1 glycoprotein
To study the molecular variability and genetic relationship between selected Argentinean BCoV strains, the hypervariable region corresponding to the S1 subunit of the S glycoprotein gene (330 bp -nucleotide 1381–1711 of the Mebus strain S gene U00735.2-) was amplified by RT-PCR. Briefly, viral RNA extraction was conducted directly from fecal samples (1:10 dilutions in phosphate saline buffer) using the QIAamp Viral RNA mini kit (Qiagen, Germany) and following the manufacturer’s instructions. The amplification of the S1 hypervariable region was carried out in a T18 thermo cycler (Ivema Desarrollos SRL., Argentina) using a nested RT-PCR protocol and conditions previously described (Brandao et al., 2006). Bovine CoV-specific primers were designed according to the conserved regions flanking the hypervariable region of the S1 gene (Brandao et al., 2006) and are detailed in Table 1 . Amplification products were resolved in 1.8% agarose gels stained with ethidium bromide (0.5 μg/ml) and visualized under UV light.
Table 1.
Primers pairs used to amplify the S1 gene hypervariable region and S gene fragment.
| Name | Primer sequence (5′–3′) | Location (BCoV complete genome) | Amplicon size (basepairs) | References |
|---|---|---|---|---|
| S1HS | CTATACCCAATGGTAGGA | 24827–24844 | 885 bp | Brandao et al. (2006) |
| S1HA | CTGAAACACGACCGCTAT | 25694–25711 | ||
| S1NS | GTTTCTGTTAGCAGGTTTAA | 24952–24971 | 488 bp | Brandao et al. (2006) |
| S1NA | ATATTACACCTATC CCCTTG | 25420–25439 | ||
| S1For | TTGTAATTTTAATATGAGCAGCC | 24808–24830 | 908 pb | In this study |
| S1Rev | TTCTGCCAACTATTATAATAAG | 25695–25716 | ||
| S2For | TTATAATAGTTGGCAGAACC | 25699–25718 | 672 pb | In this study |
| S2Rev | ACCATTCATTAAACTATTAGC | 26351–26371 |
Primers pairs used for amplification of S1 hypervariable region: S1HS, S1HA, S1NS, S1NA, primers pairs used for amplification of S gene fragment: S1For, S1Rev, S2for, S2 Rev.
2.3. RT-PCR for a BCoV S glycoprotein fragment
The encoding gene sequence corresponding to a fragment of S glycoprotein (1555 bp -nucleotide 1066–2621 of the Mebus strain S gene U00735.2-) containing partial S1 and S2 gene was amplified using RT-PCR. We include an eighth tissue culture passage in HRT-18 cells of an Argentinean strain detected in the beginning of the study (BCoV Arg-95) and a sample collected in 2013 in a dairy farm from Santa Fe province. Viral RNA extraction was conducted with the QIAamp Viral RNA mini kit (Qiagen, Germany) following the manufacturer’s instructions. Reverse transcription (cDNA synthesis) was carried out at 42 °C for 60 min followed by 94 °C for 5 min in a reaction mix with 1X PCR Buffer (Invitrogen, Brazil), 0.5 μg of random primers, 0.2 mM of each dNTP, 5 μl of RNA template and 1.25 U of M-MLV Reverse transcriptase (Invitrogen, Brazil) in a 25 μl final reaction volume. Then, 5 μl of cDNA was added to two sets (S1 and S2) of PCR mix containing 1X Buffer (Invitrogen, Brazil), 0.2 mM of each dNTP, 0.4 μM of each primer (S1For, S1Rev for S1 mix and S2For and S2Rev for S2 mix), 2 mM of MgCl2, 13.2 of ultra-pure water and 1.5 U Taq DNA polymerase (Invitrogen, Brazil) in a 25 μl final reaction volume. Primers are detailed in Table 1. The PCR cycles were performed as follow: 94 °C for 5 min, then 35 cycles of 94 °C for 1 min, 50 °C for 1 min and 72 °C for 1 min; then a final extension step of 72 °C for 5 min. The partial S1 (908 pb) and S2 (672 pb) amplification products were resolved in 1.8% agarose gels stained with SYBR Safe DNA Gel Stain (Invitrogen, Brazil) and visualized under UV light.
2.4. DNA sequencing
The amplification products of the S1 hypervariable region were purified from agarose gels using the Qiaquick purification kit (Qiagen, Germany) and amplification products of S1 and S2 partial genes were purified from the completed PCR reaction using Illustra Exostar kit (GE, USA) following the manufactures’ instructions.
Purified samples were sequenced for both sense/antisense using an automated sequencer (ABI Prism 377, Applied Biosystems Group) from the Sequencing Service of the Biotechnology Institute (INTA, Argentina). Direct sequencing of diarrheic fecal samples and tissue culture BCoV Arg95 passage was perform using the same primers used for PCR reaction.
2.5. Phylogenetic analysis
Sequences were analyzed using nucleotide BLAST and the matrix for phylogenetic analysis were constructed comparing the hypervariable region of the S1 gene domain (330 bp -nucleotide 1381–1711 of the Mebus strain S gene U00735.2-) and partial S gene fragment (1555 bp -nucleotide 1066–2621 of the Mebus strain S gene U00735.2-) including a portion of the S1 and S2 domains of Argentinean BCoV isolates with strains available in GenBank. Considering the number of sequences available in GenBank, a total of 132 sequences included in the dataset analyzed was small fragments (330 bp) while only 70 sequences were available for the longer fragment (1555 bp).
Sequences were aligned using Clustal X (Larkin et al., 2007) and the appropriated substitution model for nucleotide level was T92 + G (MEGA6) (Tamura et al., 2013). Phylogenetic analysis were performed using the Maximum Likelihood algorithm and SPR as heuristic method with gamma distribution (G = 5) and 1000 bootstrap replicates as statistical support.
2.6. Production of strain-specific BCoV antibodies in hens and guinea pigs
Two groups of three hens each were immunized with three doses, fifteen days apart, of each BCoV strain (105 fluorescent focus forming units (FFU)/ml) emulsified in oil adjuvant (Seppic, France). After Ab seroconversion (30 days post vaccination -dpv-) detected by ELISA in hens' sera, the eggs were collected and the IgY Abs from egg yolk were extracted as previously described (Vega et al., 2011). Briefly, yolk and white from each pool of eggs were separated and the yolk was diluted 1:3 in distillated water. The IgY pools were stored at −20 °C until use. Two IgY pools were obtained: Mebus IgY with a final BCoV ELISA Ab titer of 65.536 and Arg95 IgY with a final BCoV ELISA Ab titer of 16.384. Chicken sera from both groups of animals were stored at −20 °C for further use. In parallel, two groups of 5 guinea pigs each were immunized with two doses of 0.6 ml of the same formulations (Mebus and Arg95), 21 days apart. Sera were collected at initiation and at 30 dpv (Parreño et al., 2010).
2.7. Fluorescent focus reduction virus neutralization (FFN) test
Virus neutralization (VN) Ab titers to BCoV were determined for serum samples, crude egg yolk and purified IgY from vaccinated hens in an in vitro fluorescent focus neutralization (FFN) assay conducted with each strain. Sera from vaccinated guinea pig were also included. The analysis was performed in three independent assays as follows. In 96-well flat-bottom sterile plates fourfold dilutions of each sample were mixed with an equal volume (100 μl) of each BCoV strain (Mebus and Arg95) containing 200 FFU (100 FFU in the final volume mixture). All samples were run in duplicate. Briefly, serial four-fold dilutions of each sample diluted in MEM-E + MEM-D (50% each), supplemented with 1% of penicillin–streptomycin and 2 μg/ml of trypsin (Sigma–Aldrich, Germany) were mixed with equal volume of a virus suspension containing 200 FFU and incubated at 37 °C during 1 h. Then, 100 μl of the sample-virus mixtures were transferred onto 100% confluent HRT-18 monolayers placed in 96-well flat-bottom tissue culture sterile plates and incubated at 37 °C with during 48 h in a 0.5% CO2 atmosphere. After incubation, the plates were fixed using 150 μl/well of 70% acetone in distillated water during 15 min followed by a drying period of 1–2 h at room temperature. Finally, the neutralization was revealed using a fluorescein-labeled CoVB Mebus-specific guinea pig polyclonal antiserum made in our laboratory, diluted 1/100 in Evans Blue dye and incubated at 37 °C for 1 h. The plates were washed three times with PBS buffer and kept at 4 °C until visualization under fluorescent microscope. The VN Ab titers were expressed as the reciprocal of the highest sample dilution (log 10) that reduced the number of fluorescent foci by >80% compared with the wells with the virus suspension containing 100 FFU (virus without sample).
The working dilution of the virus (containing 100 FFU of CoVB in the final volume mixture) was confirmed by back titration in each independent assay. Briefly, four replicates of serial ten-fold dilutions (pure, 1/10, 1/100 and 1/1000) of the working virus were run. The virus suspension was consider to have 100 FFU when 100% of the replicates infected with pure and 1/10 dilution show fluorescent foci, the 50% of the replicates infected with the 100% dilution were positive while no foci of viral infection were detected in replicates infected with the 1/1000 dilution.
2.8. Statistical analysis
The comparison between the BCoV detection rates found in dairy vs. beef herds was evaluated by Chi-square test using MedCalc® version 12.7.0.0 statistical software.
3. Results
3.1. Coronavirus diarrhea incidence during 1994–2010
A total of 5.365 stool samples from diarrheic calves derived from 1.058 diarrhea cases or outbreaks in 239 dairy, 324 beef and 495 from non-determined farm types, were analyzed. The samples were received at the Enteric Viruses Section of the Virology Institute for viral diagnosis.
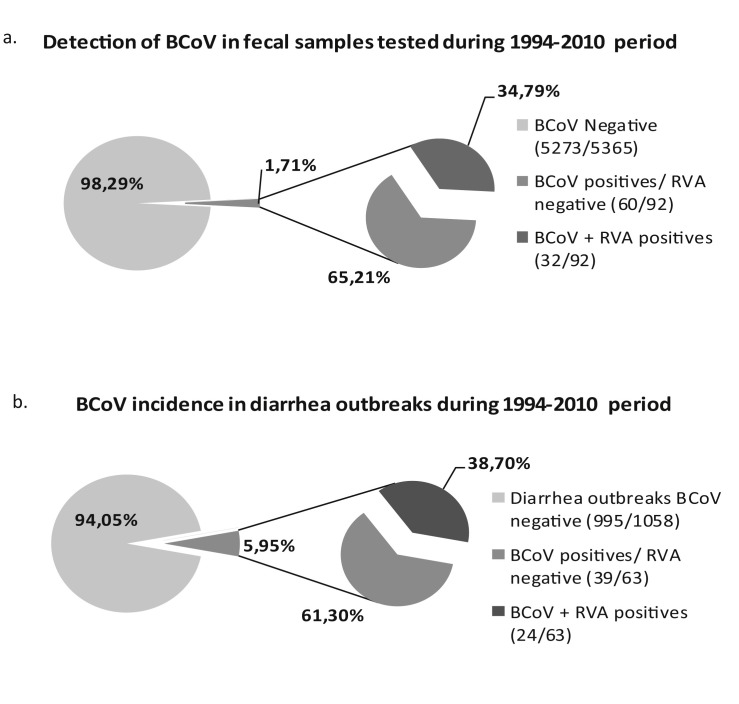
Bovine CoV was detected by ELISA in 1.71% (92/5.365) of the fecal samples tested, corresponding to 5.95% (63/1.058) of the diarrhea outbreaks studied during 1994–2010 (Table 2 , Fig. 1 a and b). Beef herds affected with BCoV diarrhea were located in Buenos Aires and Santa Fe provinces while the affected dairy farms were located in Buenos Aires, Santa Fe, Córdoba and La Pampa provinces.
Table 2.
Distribution of Coronavirus diarrhea during 1994–2010 in Argentina.
| Year | BCoV positive samples (%) | Number of BCoV + RVA positive samples | BCoV positive outbreaks (%) | BCoV positive outbreaks in dairy cattle (%) | BCoV positive outbreaks in beef cattle (%) | BCoV positive outbreaks in N/D exploitations (%) |
|---|---|---|---|---|---|---|
| 1994 | 2.02 (2/99) | 50.00 (1/2) | 2.50 (1/40) | 0.00 (0/13) | 0.00 (0/26) | 100.00 (1/1) |
| 1995 | 10.00 (2/20) | 0.00 (0/0) | 33.33 (2/6) | 0.00 (0/0) | 0.00 (0/3) | 66.67 (2/3) |
| 1996 | 0.00 (0/48) | 0.00 (0/0) | 0.00 (0/19) | 0.00 (0/2) | 0.00 (0/16) | 0.00 (0/1) |
| 1997 | 0.61 (1/163) | 0.00 (0/1) | 2.78 (1/36) | 0.00 (0/10) | 0.00 (0/21) | 20.00 (1/5) |
| 1998 | 3.25 (14/431) | 50.00 (7/14) | 6.45 (6/93) | 0.00 (0/10) | 14.29 (4/28) | 3.64 (2/55) |
| 1999 | 0.36 (2/549) | 0.00 (0/0) | 2.27 (2/88) | 0.00 (0/8) | 0.00 (0/43) | 5.41 (2/37) |
| 2000 | 1.37 (3/219) | 0.00 (0/0) | 4.76 (3/63) | 0.00 (0/4) | 0.00 (0/21) | 7.89 (3/38) |
| 2001 | 3.03 (8/264) | 12.50 (1/8) | 8.99 (8/89) | 16.67 (1/6) | 30.00 (3/10) | 5.48 (4/73) |
| 2002 | 2.13 (8/375) | 12.50 (1/8) | 6.67 (6/90) | 10.00 (1/10) | 20.83 (5/24) | 0.00 (0/56) |
| 2003 | 1.76 (3/170) | 66.67 (2/3) | 4.92 (3/61) | 0.00 (0/2) | 0.00 (0/18) | 7.32 (3/41) |
| 2004 | 0.53 (4/755) | 0.00 (0/0) | 2.33 (2/86) | 7.14 (1/14) | 2.70 (1/37) | 0.00 (0/35) |
| 2005 | 1.13 (5/443) | 20.00 (1/5) | 4.40 (4/91) | 7.69 (1/13) | 3.57 (1/28) | 4.00 (2/50) |
| 2006 | 0.36 (1/279) | 100.00 (1/1) | 1.00 (1/100) | 4.76 (1/21) | 0.00 (0/22) | 0.00 (0/57) |
| 2007 | 1.36 (4/295) | 75.00 (3/4) | 2.20 (2/91) | 5.71 (2/35) | 0.00 (0/20) | 0.00 (0/36) |
| 2008 | 0.95 (5/526) | 40.00 (2/5) | 13.51 (5/37) | 15.15 (5/33) | 0.00 (0/2) | 0.00 (0/2) |
| 2009 | 4.88 (27/553) | 40.74 (11/27) | 27.78 (15/54) | 31.25 (15/48) | 0.00 (0/1) | 0.00 (0/5) |
| 2010 | 1.70 (3/176) | 66.67 (2/3) | 14.29 (2/14) | 20.00 (2/10) | 0.00 (0/4) | 0.00 (0/0) |
| Total | 1.71 (92/5365) | 34.79 (32/92) | 5.95 (63/1058) | 12.13 (29/239) | 4.32 (14/324) | 4.04 (20/495) |
Detection of bovine Coronavirus alone and together with bovine Rotavirus in fecal samples of calves with diarrhea and outbreaks distribution among different exploitation type (dairy, beef and non-determined farms).
Fig. 1.
(a) Percentage of BCoV positive samples and from those, the BCoV + RVA co-infected samples. All samples are from animals with diarrhea. (b) Percentage of BCoV positive outbreaks and from those, the herds with BCoV + RVA mixed infections.
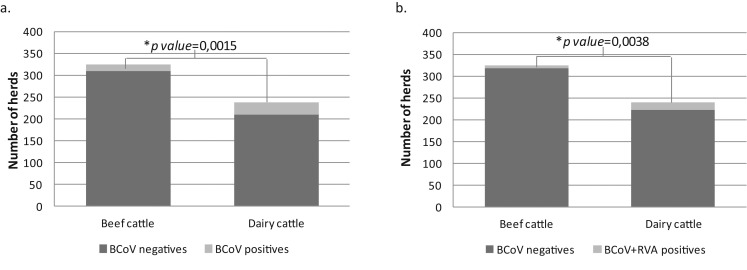
The detection rate of BCoV infection each year is detailed in Table 2. Bovine CoV incidence varies between 0% in 1996 and 10% in 1995. Globally, the BCoV detection rate in dairy herds (12.13%; 29/239) was significantly higher than the rate found in beef herds (4.32%; 14/324) during the period of study (p = 0.0015) (Fig. 2 ). The detection rate of BCoV in non-determined farm types was 4.04% (20/495) (Table 2).
Fig. 2.
(a) Distribution of BCoV-positive outbreaks in dairy and beef farms during the period of study (1994–2010). Light grey: BCoV positive outbreaks, dark grey: BCoV negative outbreaks (Chi-square test p = 0.0015). (b) Distribution of BCoV + RVA-positives outbreaks in dairy and beef farms during the period of study (1994–2010). Light grey: BCoV + RVA positive outbreaks, dark grey: BCoV negative outbreaks (Chi-square test p = 0.0038).
Examining mixed viral infections, 34.78% of BCoV positive samples were also positive for RVA by ELISA (Badaracco et al., 2012). Consequently, BCoV and RVA were detected together in 0.60% (32/5.365) of the samples tested (Table 2, Fig. 1). Co-circulation of BCoV and RVA in the same herd (in the same or in different calves) was significantly higher in dairy than in beef herds (6.8% vs. 1.9%, p = 0.0038) (Fig. 2), while for the farms of non-determined exploitation type it was 1.0% (data not shown).
3.2. Phylogenetic analysis of Argentinean Bovine Coronavirus strains
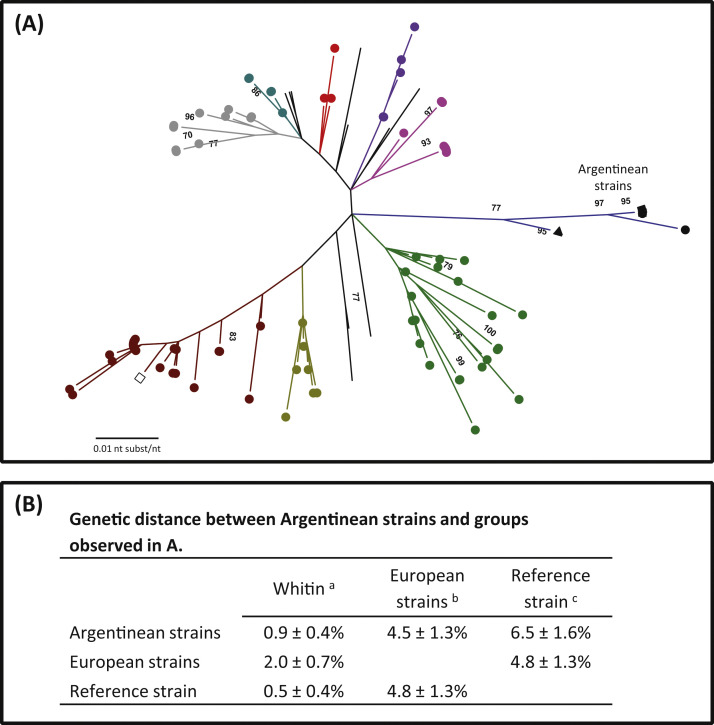
In this study two different fragments of the gene encoding the S protein of CoVB were analyzed (Fig. 3, Fig. 4 ). The analysis of a segment corresponding to the hypervariable region (330 bp and 110 aa) of the S1 domain showed that Argentinean strains formed a well-defined cluster separated from other BCoV. All Argentinean strains grouped in the same branch showing no differences between dairy and beef strains and between samples obtained in different geographical locations. Also, all Argentinean strains grouped together with the Argentine strain Arg95 and no other published sequence clustered in the same branch. Additionally, the Argentinean cluster was distant from the cluster where the Mebus reference strain is located (Fig. 3A and Fig. 1 in Supplementary material).
Fig. 3.
(A) Phylogenetic tree based on the nucleotide sequence of the hypervariable region (330 bp -nucleotide 1381–1710 of the Mebus strain S gene U00735.2-). Bootstrap values higher than 70% are shown. (●) Dairy strains, (■) beef strains, (▲) Argentinean reference strain Arg95, (□) Mebus reference strain. Each color represents a different geographical cluster. The evolutionary history was inferred by the Maximum Likelihood method based on the Tamura 3-parameter model. The tree was drawn to scale with branch lengths measured in the number of substitutions per site. The analysis involved 132 nucleotide sequences. Codification of each sample is shown in Fig. 1 from Supplementary material. (B) Genetic distance average between nucleotide sequences of Argentinean, European and reference strains expressed in percentage ± standard deviation.
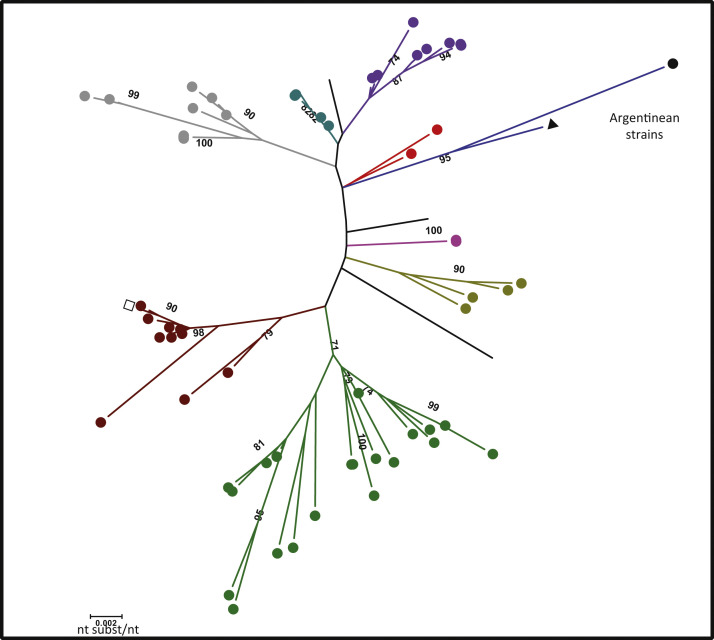
Fig. 4.
Phylogenetic tree based on the nucleotide sequence of partial S gene fragment (1555 bp -nucleotide 1066–2621 of the Mebus strain S gene U00735.2-). Bootstrap values higher than 70% are shown. (●) Dairy strains, (▲) Argentinean reference strain Arg95, (□) Mebus reference strain. Each color represents a different geographical cluster. The evolutionary history was inferred by the Maximum Likelihood method based on the Tamura 3-parameter model. The tree was drawn to scale with branch lengths measured in the number of substitutions per site. The analysis involved 70 nucleotide sequences. Codification of each sample is shown in Fig. 2 from Supplementary material.
Supplementary material related to this article found, in the online version, at http://dx.doi.org/10.1016/j.vetmic.2015.10.017.
Genetic distance between strains was studied and the Argentinean strains showed a 99% similarity between each other, even with the Argentinean strain Arg95 at nucleotide level. However, these sequences were 6.5% distant from the Mebus reference strain included in the vaccines currently available in Argentina (Fig. 3B).
Analyzing the deduced amino acid sequences we observed 23 amino acid changes between Mebus reference strain and Argentinean strains. Of these, 12 changes occur in the hypervariable region (109 amino acid fragment). Furthermore, 8 amino acid differences between tissue culture adapted Argentinean strain (Arg95) and field strains were observed (Table 3 ).
Table 3.
Amino acid substitutions in the studied S gene portion.
| Portion of 508 aa (1453 pb) used for characterization (356–873 aa) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 359 | 455 | 458 | 465 | 470 | 484 | 492 | 494 | 499 | 501 | 525 | 531 | 533 | 543 | 545 | 578 | 716 | 740 | 751 | 769 | 778 | 785 | 853 | |
| Mebus/1983 | N | T | F | V | H | S | D | S | N | P | H | N | L | S | G | T | T | S | T | A | T | N | A |
| Arg95a | Y | I | S | A | D | T | – | – | S | S | Y | D | M | A | W | S | P | I | P | S | N | – | – |
| Argentinean strains b | Y | I | S | A | D | T | N | A | S | S | Y | D | M | A | W | – | – | – | – | S | N | K | S |
Numbers are in concordance with Spike gene of Mebus reference strain (Accesion number U00735).
In bold, hypervariable region (461–570 aa) of 109 aa used for diagnostic PCR are shown. Amino acid identity marked as -:
Argentinean tissue culture cell adapted virus.
Consensus of Argentinean field strains.
Concerning the analysis of the longer fragment (1555 pb and 518 aa) including a portion of S1 and S2 domains, we confirm the distribution of sequences in regional clusters in concordance with the analysis of the S1 hypervariable region where Argentinean strains grouped separately from the rest of the sequence available in GenBank. Interestingly, the distance between the Mebus reference and Argentinean strains remained the same (Fig. 4 and Fig. 2 Supplementary material). As there are less sequences of this longer fragment (1555 bp) in GenBank than S1 hypervariable region fragment, we also analyzed the smaller dataset of 70 sequences for the S1 hypervariable region and the resultant topology of the tree was similar (Fig. 3 Supplementary material).
Supplementary material related to this article found, in the online version, at http://dx.doi.org/10.1016/j.vetmic.2015.10.017.
All these results suggest the need to evaluate if the observed diversity between the Mebus reference and the Argentinean field strains detected in the phylogenetic analysis is associated with antigenic differences. For this reason, we decided to study the degree of cross neutralizing activity among specific antisera and IgY Abs raised to the Mebus reference strain and the Argentinean Arg95 isolate adapted to tissue culture grown in HRT-18 cells.
3.3. Cross-neutralization between different Bovine coronavirus strains
Hens and guinea pigs seroconverted after immunization with both BCoV strains after 30 dpi (data not shown). Neutralizing Abs to both strains were detected in the crude egg yolk as well as in the purified IgY pools. Sera from hens and guinea pigs immunized with each BCoV strain showed the same neutralizing Ab titer to the homologous strain compared with the heterologous strain, indicating full cross reactivity between the Arg95 isolate and the historic Mebus reference strain (1969) (Table 4 ).
Table 4.
BCoV fluorescent focus reduction assay using strain-specific antibodies.
| BCoV strain (100 FFU) |
Neutralizing antibody titers to BCoV |
|||||||
|---|---|---|---|---|---|---|---|---|
| IgY to Arg95 |
IgY to Mebus |
Chicken serum to Arg95 | Chicken serum to Mebus | Guinea pig serum to Arg95 | Guinea serum to Mebus |
|||
| Crude egg yolk | Purified IgY | Crude egg yolk | Purified IgY | |||||
| Arg95 | 2.31 ± 0.35 | 3.91 ± 0.00 | 2.71 ± 0.00 | 4.21 ± 0.43 | 2.71 ± 0.00 | 3.51 ± 3547 | 2.91 ± 0.70 | 2.11 ± 0.00 |
| Mebus | 2.31 ± 0.35 | 3.91 ± 0.00 | 2.71 ± 0.00 | 4.21 ± 0.43 | 2.71 ± 0.00 | 3.51 ± 3547 | 2.91 ± 0.70 | 2.11 ± 0.00 |
A fourfold dilution of each sample (purified IgY, crude egg yolk, chicken serum and guinea pig serum) derived from animals vaccinated with inactivated BCoV strain Arg95 and Mebus was mixed with an equal volume of BCoV containing 100 FFU of each strain. Ab titers to BCoV were expressed as the inverse of the highest sample dilution (log10) reducing >80% of the number of fluorescent focus forming units (FFU) of each BCoV strain.
4. Discussion
This work represents the first large survey describing BCoV circulation in Argentinean cattle and its molecular relationship with other strains circulating worldwide. In the present study, BCoV was detected in 5.95% (63/1058) of diarrhea outbreaks in four out of ten surveyed Argentinean provinces during the period 1994–2010. Bovine CoV circulation was concentrated in four provinces (Buenos Aires, Santa Fe, Cordoba and La Pampa) which have the highest dairy livestock density (Ministerio de Agricultura and Ganadería., 2011). The number of BCoV ELISA-positive samples during the period analyzed was 1.71% (92/5365). This BCoV detection rate was consistently lower than 10% each year, although the samples included in this study were analyzed by ELISA upon arrival. Other possible explanations for this result could be the lower sensitivity of ELISA compared with RT-PCR assay (Cho et al., 2001a). However, in a survey conducted in Turkey during 2001–2002 using the same indirect antigen-capture ELISA, the BCoV detection rate was 28.1% (Hasoksuz et al., 2005), suggesting that BCoV-associated diarrhea in Argentinean calves may be lower than in other countries.
In addition, other surveys were conducted in South America, especially in Brazil where Lorenzetti et al. (2013) detected, by semi-nested PCR, BCoV in 61.5% (8/13) of the beef herds examined and in 33.3% (31/93) of the analyzed diarrheic fecal samples during 2009–2013, revealing that BCoV has an important role in neonatal diarrhea epidemiology in beef cattle reared extensively in that country. Also, Stipp et al. (2009) reported 15.6% incidence in four Brazilian states during 2004 by semi-nested PCR assay with statistically higher BCoV detection in diarrheic than in healthy calves. Another study conducted by RT-PCR in ten dairy farms from southeastern Brazil showed 17 out of 51 BCoV positive samples from diarrheic and non-diarrheic calves. All of this data indicate that further statistically designed surveys are needed to systematically evaluate BCoV as well as other enteropathogens associated with neonatal calf diarrhea in South America, similar to the ones that are being conducted in North America (Cho and Yoon, 2014).
Concerning the presence of BCoV in farms of different husbandry cattle, we observed that BCoV incidence was significantly higher in dairy than in beef farms (Chi square test: p = 0.0015). This difference may be due to the close interaction between calves in dairy farms were animals are reared in intensive management systems. Dairy calves are separated from their dams after colostrums intake and are feed with milk replacer lacking Abs. This management system increases the risk of neonatal calf diarrhea compared with beef calves, which live with their dams up to six month old with natural feeding (Bendali et al., 1999).
To compensate for the lack of passive immunity given to dairy calves, a BCoV-specific passive treatment based on milk supplementation with avian IgY has been studied as a promising therapy (Ikemori et al., 1997). The development of a spray dried whole egg powder is in progress to prevent BCoV diarrhea (Bok et al., unpublished data).
For mixed viral infections, we detected a high percentage of samples (34.79%) infected with both BCoV and RVA. This was somewhat expected, as RVA is responsible for the majority of neonatal calf diarrhea worldwide (Kapikian and Shope, 1996). RVA and BCoV co-circulation was significantly higher in dairy than in beef herds in the present survey. The co-circulation of these two viral enteropathogens was also observed in other countries such as the United States, India, Algeria, and Australia (Ammar et al., 2014, Cho et al., 2013, Izzo et al., 2011, Suresh et al., 2012).
The study of the BCoV hypervariable S1 fragment and partial S gene fragment including a region from S1 and S2 domains showed that the Argentinean field strain are quite similar considering exploitation type. Importantly, the adapted strain (Arg95) is a representative strain of the cluster being an excellent vaccine candidate. In contrast with the results reported in Brazil, none of our strains showed gaps in the genome segment analyzed (Brandao et al., 2006).
The phylogenetic analysis show that BCoV strains detected in dairy and beef cattle from different Argentinean provinces were very similar strains over the time period analyzed. In addition, all the Argentinean strains sequenced grouped separately from Mebus reference strain, which has been used for systematic vaccination of Argentinean cattle (Dr. Parreño, personal communication). Furthermore, Argentinean strains form a well-defined cluster, separate of the other geographical clusters like most of the Brazilian strains as was previously observed (Bidokhti et al., 2012, Chung et al., 2011, Fulton et al., 2013, Martinez et al., 2012, Takiuchi et al., 2008). The length of the branch indicates Argentinean cluster divergence from all the other groups of BCoVs. In the other hand, the genetic distance between the Argentinean field samples and the Mebus reference strain was observed in both analyses.
The results obtained in the phylogenetic analysis, raise the question if the vaccines currently available in Argentina (all including Mebus strain) are protective against the BCoV strains circulating in the field. To evaluate the potential efficacy of Mebus-induced Abs to neutralize BCoV field strains, we conducted a two-way in vitro virus neutralization assay using the Mebus strain and the Arg95 field isolate, adapted to grow in HRT-18 cells. The results showed that Mebus and Arg95 Abs (avian IgY and guinea pig IgG) were equally able to neutralize both BCoV strains. This confirms the asseveration that all BCoV are antigenically similar, comprising a single serotype (Hasoksuz et al., 1999a, Hasoksuz et al., 1999b, Tsunemitsu and Saif, 1995). Similarly, Cho et al. (2001a) showed that calves inoculated with calf diarrhea associated CoV, bovine respiratory CoV and winter dysentery CoV did not develop diarrhea or respiratory disease but showed subclinical BCoV infection after homologous or heterologous virus challenge. Also, Han et al. (2006) noted that gnotobiotic calves experimentally inoculated with a human CoV were protected from diarrhea after being challenged with CD-BCoV DB2. All this data suggest that current vaccines available in Argentina, which include the Mebus strain, should be effective to prevent neonatal BCoV associated diarrhea. However, there are still no animal models for BCoV vaccine potency testing in Argentina that could demonstrate this. In conclusion, it will be important to continue the epidemiological surveys of BCoV circulating strains associated with diarrhea and respiratory disease in cattle, as well to develop prevention strategies to control BCoV diarrhea, including the study of vaccine potency and the development of oral passive therapies.
Acknowledgments
We gratefully acknowledge the technical assistance of DVM Osvaldo Zábal, Nancy Suárez Pérez and José Vallejos. To Dr. Mariana Puntel for English editing. This study was supported by PICT Nro. 0328, MinCyT, Argentina. DVM, MSc Marina Bok and Dr. Viviana Parreño are members of CONICET. Dr. Sibele Souza travel grant was supported by CNPq: 140.098/2009-7.
References
- Ammar S.S., Mokhtaria K., Tahar B.B., Amar A.A., Redha B.A., Yuva B., Mohamed H.S., Abdellatif N., Laid B. Prevalence of rotavirus (GARV) and coronavirus (BCoV) associated with neonatal diarrhea in calves in western Algeria. Asian Pac. J. Trop. Biomed. 2014;4:S318–S322. doi: 10.12980/APJTB.4.2014C778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K.M., de Souza S.P., de Barros I.N., Ayres G.R., Silva S.O., Richtzenhain L.J., Brandao P.E. Multiplex semi-nested RT-PCR with exogenous internal control for simultaneous detection of bovine coronavirus and group A rotavirus. J. Virol. Methods. 2010;169:375–379. doi: 10.1016/j.jviromet.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaracco A., Garaicoechea L., Rodríguez D., Uriarte E.L., Odeón A., Bilbao G., Galarza R., Abdala A., Fernandez F., Parreño V. Bovine rotavirus strains circulating in beef and dairy herds in Argentina from 2004 to 2010. Vet. Microbiol. 2012;158:394–399. doi: 10.1016/j.vetmic.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Badaracco A., Garaicoechea L., Matthijnssens J., Louge Uriarte E., Odeón A., Bilbao G., Fernandez F., Parra G.I., Parreño V. Phylogenetic analyses of typical bovine rotavirus genotypes G6, G10, P[5] and P[11] circulating in Argentinean beef and dairy herds. Infect. Genet. Evol. 2013;18:18–30. doi: 10.1016/j.meegid.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Barros I.N., Silva S.O., Nogueira Neto F.S., Asano K.M., Souza S.P., Richtzenhain L.J., Brandao P.E. A multigene approach for comparing genealogy of Betacoronavirus from cattle and horses. Sci. World J. 2013:349702. doi: 10.1155/2013/349702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendali F., Sanaa M., Bichet H., Schelcher F. Risk factors associated with diarrhoea in newborn calves. Vet. Res. 1999;30:509–522. [PubMed] [Google Scholar]
- Bidokhti M.R.M., Tråvén M., Ohlson A., Baule C., Hakhverdyan M., Belák S., Liu L., Alenius S. Tracing the transmission of bovine coronavirus infections in cattle herds based on S gene diversity. Vet. J. 2012;193:386–390. doi: 10.1016/j.tvjl.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidokhti M.R., Traven M., Krishna N.K., Munir M., Belak S., Alenius S., Cortey M. Evolutionary dynamics of bovine coronaviruses: natural selection pattern of the spike gene implies adaptive evolution of the strains. J. Gen. Virol. 2013;94:2036–2049. doi: 10.1099/vir.0.054940-0. [DOI] [PubMed] [Google Scholar]
- Boileau M.J., Kapil S. Bovine coronavirus associated syndromes. Vet. Clin. North Am. Food Anim. Pract. 2010;26:123–146. doi: 10.1016/j.cvfa.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao P.E., Gregori F., Richtzenhain L.J., Rosales C.A., Villarreal L.Y., Jerez J.A. Molecular analysis of Brazilian strains of bovine coronavirus (BCoV) reveals a deletion within the hypervariable region of the S1 subunit of the spike glycoprotein also found in human coronavirus OC43. Arch. Virol. 2006;151:1735–1748. doi: 10.1007/s00705-006-0752-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao P.E., Scheffer K., Villarreal L.Y., Achkar S., Oliveira Rde N., Fahl Wde O., Castilho J.G., Kotait I., Richtzenhain L.J. A coronavirus detected in the vampire bat Desmodus rotundus. Braz. J. Infect. Dis. 2008;12:466–468. doi: 10.1590/s1413-86702008000600003. [DOI] [PubMed] [Google Scholar]
- Cho Y., il, Yoon K.J. An overview of calf diarrhea – infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014;15:1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.O., Hasoksuz M., Nielsen P.R., Chang K.O., Lathrop S., Saif L.J. Cross-protection studies between respiratory and calf diarrhea and winter dysentery coronavirus strains in calves and RT-PCR and nested PCR for their detection. Arch. Virol. 2001;146:2401–2419. doi: 10.1007/s007050170011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.O., Hoet A.E., Loerch S.C., Wittum T.E., Saif L.J. Evaluation of concurrent shedding of bovine coronavirus via the respiratory tract and enteric route in feedlot cattle. Am. J. Vet. Res. 2001;62:1436–1441. doi: 10.2460/ajvr.2001.62.1436. [DOI] [PubMed] [Google Scholar]
- Cho Y., Il, Han J.I., Wang C., Cooper V., Schwartz K., Engelken T., Yoon K.J. Case-control study of microbiological etiology associated with calf diarrhea. Vet. Microbiol. 2013;166:375–385. doi: 10.1016/j.vetmic.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.Y., Kim H.R., Bae Y.C., Lee O.S., Oem J.K. Detection and characterization of bovine-like coronaviruses from four species of zoo ruminants. Vet. Microbiol. 2011;148:396–401. doi: 10.1016/j.vetmic.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.A. Bovine coronavirus. Br. Vet. J. 1993;149:51–70. doi: 10.1016/S0007-1935(05)80210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.R., Knobler R.L., Powell H., Buchmeier M.J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell–cell fusion. Virology. 1982;119:358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornaglia E.M., Fitjman N., Schudel A.A.B.M. Enzyme linked immunosorbent assay, immunofluorescent test and electrophoresis analysis of rotaviral RNA in the diagnosis and characterization of the bovine rotavirus. Rev. Latinoam. Microbiol. 1989:59–62. [Google Scholar]
- Decaro N., Elia G., Campolo M., Desario C., Mari V., Radogna A., Colaianni M.L., Cirone F., Tempesta M., Buonavoglia C. Detection of bovine coronavirus using a TaqMan-based real-time RT-PCR assay. J. Virol. Methods. 2008;151:167–171. doi: 10.1016/j.jviromet.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Mari V., Desario C., Campolo M., Elia G., Martella V., Greco G., Cirone F., Colaianni M.L., Cordioli P., Buonavoglia C. Severe outbreak of bovine coronavirus infection in dairy cattle during the warmer season. Vet. Microbiol. 2008;126:30–39. doi: 10.1016/j.vetmic.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Ridpath J.F., Burge L.J. Bovine coronaviruses from the respiratory tract: antigenic and genetic diversity. Vaccine. 2013;31:886–892. doi: 10.1016/j.vaccine.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaicoechea L., Bok K., Jones L.R., Combessies G., Odeon A., Fernandez F., Parreno V. Molecular characterization of bovine rotavirus circulating in beef and dairy herds in Argentina during a 10-year period (1994–2003) Vet. Microbiol. 2006;118:1–11. doi: 10.1016/j.vetmic.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Han M.G., Cheon D.-S., Zhang X., Saif L.J. Cross-protection against a human enteric coronavirus and a virulent bovine enteric coronavirus in gnotobiotic calves. J. Virol. 2006;80:12350–12356. doi: 10.1128/JVI.00402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Lathrop S., Al-dubaib M.A., Lewis P., Saif L.J. Antigenic variation among bovine enteric coronaviruses (BECV) and bovine respiratory coronaviruses (BRCV) detected using monoclonal antibodies. Arch. Virol. 1999;144:2441–2447. doi: 10.1007/s007050050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Lathrop S.L., Gadfield K.L., Saif L.J. Isolation of bovine respiratory coronaviruses from feedlot cattle and comparison of their biological and antigenic properties with bovine enteric coronaviruses. Am. J. Vet. Res. 1999;60:1227–1233. [PubMed] [Google Scholar]
- Hasoksuz M., Sreevatsan S., Cho K.O., Hoet A.E., Saif L.J. Molecular analysis of the S1 subunit of the spike glycoprotein of respiratory and enteric bovine coronavirus isolates. Virus Res. 2002;84:101–109. doi: 10.1016/S0168-1702(02)00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Kayar A., Dodurka T., Ilgaz A. Detection of respiratory and enteric shedding of bovine coronaviruses in cattle in Northwestern Turkey. Acta Vet. Hung. 2005;53:137–146. doi: 10.1556/AVet.53.2005.1.13. [DOI] [PubMed] [Google Scholar]
- Hasoksuz M., Vlasova A., Saif L.J. Detection of group 2a coronaviruses with emphasis on bovine and wild ruminant strains. Virus isolation and detection of antibody antigen, and nucleic acid. Methods Mol. Biol. 2008;454:43–59. doi: 10.1007/978-1-59745-181-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemori Y., Ohta M., Umeda K., Icatlo F.C., Kuroki M., Yokoyama H., Kodama Y. Passive protection of neonatal calves against bovine coronavirus-induced diarrhea by administration of egg yolk or colostrum antibody powder. Vet. Microbiol. 1997;58:105–111. doi: 10.1016/s0378-1135(97)00144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo M.M., Kirkland P.D., Mohler V.L., Perkins N.R., Gunn A.A., House J.K. Prevalence of major enteric pathogens in Australian dairy calves with diarrhoea. Aust. Vet. J. 2011;89:167–173. doi: 10.1111/j.1751-0813.2011.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.H., Kim G.Y., Yoon S.S., Park S.J., Kim Y.J., Sung C.M., Shin S.S., Lee B.J., Kang M.I., Park N.Y., Koh H.B., Cho K.O. Molecular analysis of S gene of spike glycoprotein of winter dysentery bovine coronavirus circulated in Korea during 2002–2003. Virus Res. 2005;108:207–212. doi: 10.1016/j.virusres.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A.Z., Shope R.E. 4th edition. University of Texas Medical Branch at Galveston; Galveston (TX): 1996. Rotaviruses, Reoviruses, Coltiviruses, and Orbiviruses. Medical Microbiology. Chapter 63. [PubMed] [Google Scholar]
- Lai M.H.K.V. Coronaviridae: the viruses and their replication. In: Fields B.N., Howley P.M., DMK, editors. Fields Virology. Lippincott - Raven; Philadelphia: 2001. pp. 1163–1186. [Google Scholar]
- Larkin M.A., Blackshields G., Brown N.P., Chenna R., Mcgettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lin Y.J., Zhang X., Wu R.C., Lai M.M. The 3′ untranslated region of coronavirus RNA is required for subgenomic mRNA transcription from a defective interfering RNA. J. Virol. 1996;70:7236–7240. doi: 10.1128/jvi.70.10.7236-7240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti Elis, Leme Raquel de Arruda, Ribeiro Juliane, Rodrigues Almeida de Souza Vilma, Alfier Alice Fernandes, Alfieri A.A. Neonatal diarrhea by bovine coronavirus (BCoV) in beef cattle herds. Semin. Ciê ncias Agrárias. 2013;34:3795–3800. [Google Scholar]
- Lu C.P., Yao H.C., Eichhorn W. Coronavirus as an agent of neonatal calf diarrhea in a Chinese dairy cattle farm. Zentralbl. Veterinarmed. B. 1991;38:473–476. doi: 10.1111/j.1439-0450.1991.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Martinez N., Brandao P.E., de Souza S.P., Barrera M., Santana N., de Arce H.D., Perez L.J. Molecular and phylogenetic analysis of bovine coronavirus based on the spike glycoprotein gene. Infect. Genet. Evol. 2012;12:1870–1878. doi: 10.1016/j.meegid.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawatari T., Hirano K., Ikeda H., Tsunemitsu H., Suzuki T. Surveillance of diarrhea-causing pathogens in dairy and beef cows in Yamagata Prefecture, Japan from 2002 to 2011. Microbiol. Immunol. 2014;58:530–535. doi: 10.1111/1348-0421.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus C.A., Stair E.L., Rhodes M.B., Twiehaus M.J. Neonatal calf diarrhea: propagation, attenuation, and characteristics of a coronavirus-like agent. Am. J. Vet. Res. 1973;34:145–150. [PubMed] [Google Scholar]
- Ministerio de Agricultura, G. y P. de la N.S. de A., Ganadería., G. y P.S. de, 2011. Anuario 2011 | GANADOS Y CARNES.
- Ohlson A., Alenius S., Traven M., Emanuelson U. A longitudinal study of the dynamics of bovine corona virus and respiratory syncytial virus infections in dairy herds. Vet. J. 2013;197:395–400. doi: 10.1016/j.tvjl.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreño V., López M.V., Rodriguez D., Vena M.M., Izuel M., Filippi J., Romera A., Faverin C., Bellinzoni R., Fernandez F., Marangunich L. Development and statistical validation of a guinea pig model for vaccine potency testing against Infectious bovine rhinothracheitis (IBR) virus. Vaccine. 2010;28:2539–2549. doi: 10.1016/j.vaccine.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Sestak K. Transmissible gastroenteritis and porcine respiratory coronavirus. In: Straw B., Zimmerman J., D’Allaire S., editors. Diseases of Swine. Blackwell Publishing; 2006. pp. 489–516. [Google Scholar]
- Saif L.J., Redman D.R., Brock K.V., Kohler E.M., Heckert R.A. Winter dysentery in adult dairy cattle: detection of coronavirus in the faeces. Vet. Rec. 1988;123:300–301. doi: 10.1136/vr.123.11.300. [DOI] [PubMed] [Google Scholar]
- Saif L.J. Bovine respiratory coronavirus. Vet. Clin. North Am. – Food Anim. Pract. 2010 doi: 10.1016/j.cvfa.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze B., Gross H.J., Brossmer R., Herrler G. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J. Virol. 1991;65:6232–6237. doi: 10.1128/jvi.65.11.6232-6237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.R., Tsunemitsu H., Heckert R.A., Saif L.J. Evaluation of two antigen-capture ELISAs using polyclonal or monoclonal antibodies for the detection of bovine coronavirus. J. Vet. Diagn. Invest. 1996;8:99–105. doi: 10.1177/104063879600800116. [DOI] [PubMed] [Google Scholar]
- St-Jean J.R., Jacomy H., Desforges M., Vabret A., Freymuth F., Talbot P.J. Human respiratory coronavirus OC43: genetic stability and neuroinvasion. J. Virol. 2004;78:8824–8834. doi: 10.1128/JVI.78.16.8824-8834.2004. 10.1128/JVI.78.16.8824-8834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp D.T., Barry A.F., Alfieri A.F., Takiuchi E., Amude A.M., Alfieri A.A. Frequency of BCoV detection by a semi-nested PCR assay in faeces of calves from Brazilian cattle herds. Trop. Anim. Health Prod. 2009;41:1563–1567. doi: 10.1007/s11250-009-9347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh T., Rai R.B., Dhama K., Bhatt P., Sawant P.M., Sharma A.K. Prevalence of rotavirus, coronavirus and Escherichia coli: the main agents responsible for calf diarrhoea. Vet. Pract. 2012;13:160–165. [Google Scholar]
- Takiuchi E., Alfieri A.F., Alfieri A.A. Molecular analysis of the bovine coronavirus S1 gene by direct sequencing of diarrheic fecal specimens. Braz. J. Med. Biol. Res. 2008;41:277–282. doi: 10.1590/s0100-879x2008000400004. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H., Saif L.J. Antigenic and biological comparisons of bovine coronaviruses derived from neonatal calf diarrhea and winter dysentery of adult cattle. Arch. Virol. 1995;140:1303–1311. doi: 10.1007/bf01322757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C., Bok M., Chacana P., Saif L., Fernandez F., Parreno V. Egg yolk IgY: protection against rotavirus induced diarrhea and modulatory effect on the systemic and mucosal antibody responses in newborn calves. Vet. Immunol. Immunopathol. 2011;142:156–169. doi: 10.1016/j.vetimm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A.N., Saif L.J. Biological aspects of the interspecies transmission of selected coronaviruses. In: Singh S.K., editor. Viral Infections and Global Change. John Wiley & Sons, Inc.; 2014. pp. 393–418. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.