Gaucher disease (GD) is a lysosomal storage disorder caused by mutations in the glucocerebrosidase 1 (GBA1) gene encoding the lysosomal enzyme glucocerebrosidase. Patients with type 1 GD present with accumulation of glucosylceramide in macrophages leading to a range of systemic manifestations, while patients with type 2 or type 3 GD also exhibit central nervous system involvement.1,2 Importantly, mutations in GBA1 are also the main risk factor for the neurodegenerative disorders Parkinson disease and dementia with Lewy bodies.3 Mechanisms linking GBA1 mutations to neurodegeneration are not clear but are hypothesized to involve the central nervous system-resident macrophage population of microglia.4 Enzyme replacement therapy has been successful in treating systemic features of lysosomal storage disorder but, because it does not penetrate the brain, there are currently no treatments available for the neuronopathic features of type 2 and type 3 GD, although substrate reduction therapy is under evaluation.5,6 The difficulty in developing neuroprotective therapeutics for the treatment of these diseases is compounded by genetic diversity among patients, with over 300 disease-causing mutations in GBA1.7 Moreover, the distribution of disease alleles varies between ethnic groups.8 There is some therapeutic promise in brain-penetrant, small molecule chemical chaperone compounds that stabilize mutant, misfolded glucocerebrosidase in the endoplasmic reticulum, allowing efficient trafficking to lysosomes where the enzyme can function.9,10 Many of the candidate compounds do, however, fall into an inhibitory class: they bind and stabilize glucocerebrosidase to facilitate its trafficking to the lysosome but they may concomitantly inhibit its enzymatic activity even at low lysosomal pH.11 How to evaluate the downstream functional consequences of these therapeutic compounds, what these mean for patients’ treatment and how to stratify a genetically diverse population for therapeutic intervention are three key issues that remain to be solved.
Using an in vitro patient blood monocytic cell (PBMC)-derived macrophage model described by Aflaki et al.12 we explored the effectiveness of inhibitory chaperones on lipid metabolism and compared the response to such compounds in a panel of genetically heterogeneous GD patient-derived material. We found that inhibitory chaperone compounds can have positive effects on lipid metabolism despite their mode of action but, crucially, this depends on how the treatment is applied. Moreover, we found that despite genetic diversity among the patients tested, response to compounds can be similar across patients, suggesting that it may be possible to base inhibitory compound clinical trial stratification on in vitro phenotype rather than GBA1 genotype. This would allow for clinical trials with greater power. The development of a preclinical in vitro biomarker to identify treatment responders is critical for advancing therapeutics currently in the pipeline.6
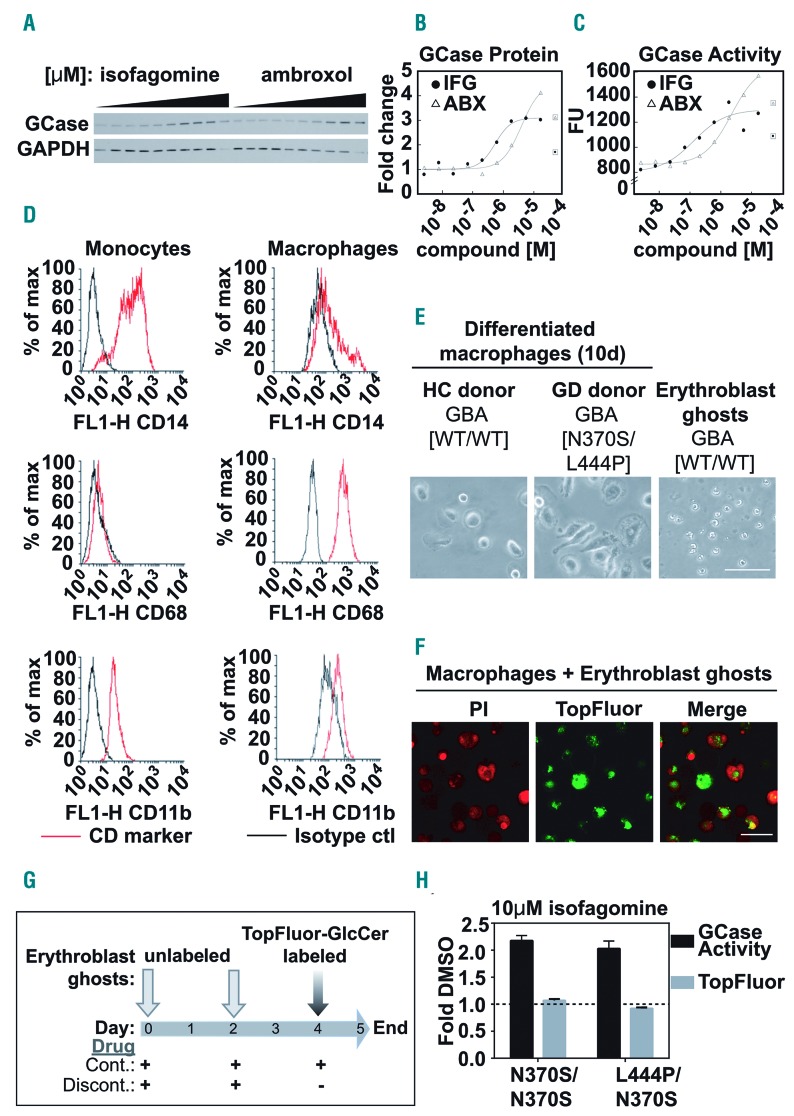
Inhibitory chaperone compound treatment has been shown to increase glucocerebrosidase activity in GD patient-derived fibroblasts via stabilization of the mutant protein.13,14 We found that in total cell extracts from a patient-derived fibroblast line, even at high concentrations of the inhibitory chaperone compounds ambroxol15 and isofagomine,11 the levels of glucocerebrosidase protein were increased (Figure 1A, B) and its enzymatic activity was concurrently elevated (Figure 1C). However, analysis of total cell extracts does not provide information about the activity of glucocerebrosidase in situ in the lysosomal compartment. It is thus not clear to what extent lysosomally localized glucocerebrosidase is affected by inhibitory compounds: i.e., whether large increases in glucocerebrosidase protein correctly targeted to the lysosome are enough to overcome any residual inhibitory effect of compound binding at low pH.
Figure 1.
Patient blood monocytic cell-derived macrophage model to assess the functional impact of glucocerebrosidase-specific inhibitory chaperone compounds. (A-C) Fibroblasts from a Gaucher disease (GD) patient [GBA1 N370S/del] were treated with increasing doses (0-50 μM) of the glucocerebrosidase (GCase) inhibitors isofagomine (IFG) or ambroxol (ABX) for 6 days before the cells were harvested. (A) Western blot of the protein levels of GCase and GAPDH, as a loading control, in whole cell lysates. (B) Dose-response curves of densitometrically quantified GCase protein levels. Boxed points are outliers removed due to observable toxicity. (C) Dose-response curves of GCase activity using whole cell lysates from ambroxol- or isofagomine-treated GD fibroblasts. Boxed points are outliers removed due to observable toxicity. (D-G) Patient-derived monocytes were isolated using a Percoll gradient and CD14+ magnetic beads and were then differentiated into patient blood monocytic cell (PBMC)-derived macrophages using granulocyte-macrophage colony-stimulating factor. Erythroblast ghosts were generated by hypo-osmotic lysis. Unlabeled erythroblast ghosts were added to the macrophages for phagocytosis at assay set up (day 0) and at 48 h (day 2), to saturate the intracellular glycolipid pool. Twenty-four hours before the assay readout (day 4) erythroblast ghosts labeled with TopFluor-glucosylceramide (GlcCer) were added to the macrophages. Remaining TopFluor-GlcCer levels in PBMC-derived macrophages were read out at 485/528 nm using a spectrophotometer.12 (D) Fluorescence activated cell sorting analysis showing enrichment of the CD68+ population of differentiated macrophages compared with CD14/CD11b+ monocytic precursors. (E) Transmitted light micrographs showing representative PBMC-derived macrophages from a healthy control (HC) donor (left) and a GD patient (middle) and erythroblast ghosts (right). (F) Confocal micrographs of propidium iodide (PI)-labeled fixed PBMC-derived macrophages (red) and TopFluor-labeled erythroblast ghosts (green) after incubation for 24 h with TopFluor-GlcCer-labeled erythroblast ghosts. (G) Schematic representation of erythroblast ghost delivery and compound treatment protocols. On day 4, compounds were either (i) replenished as part of a continuous protocol (Cont.), or (ii) removed for the 24 h period of TopFluor-GlcCer-labeled erythroblast ghost delivery in a discontinuous protocol (Discont.). (H) Two different GD PBMC-derived macrophage samples were exposed to 10 μM isofagomine in the Continuous protocol and GCase activity (black bars) and TopFluor-GlcCer (gray bars) were measured and expressed as fold change compared to those of the samples exposed to dimethylsulfoxide (DMSO), the vehicle control (dotted line at 1). Scale bar in (E) and (F) = 50 μM.
To address this issue, we used an in vitro functional model of GD that was developed to evaluate the effects of compounds on the downstream functional consequences of modulation of the enzymatic activity of glucocerebrosidase, namely substrate degradation.12 Loss of glucocerebrosidase enzymatic function leads to intracellular accumulation of its lipid substrate glucosylceramide (GlcCer); cells of the monocyte-macrophage lineage are severely affected by impaired glucocerebrosidase function, which leads to visible accumulation of glycolipids in the cell.1,16 We used human GD PBMC-derived macrophages (Figure 1D, E), pre-fed with unlabeled patient-derived erythroblast ghosts (Figure E), to measure degradation of fluorescently-conjugated glucosylceramide (TopFluor-GlcCer)-labeled erythroblast ghosts, 24 h after feeding (Figure F, G).12 Aflaki et al. showed that in this model a reduction in TopFluor signal denotes increased degradation of the labeled erythroblast ghosts, thus reflecting increased glucocerebrosidase activity in the lysosomes.12 When exposing PBMC-derived macrophages to 10 μM isofagomine in a continuous manner throughout the whole period of erythroblast ghost incubation (Figure 1G) we found a robust two-fold increase in total cellular glucocerebrosidase activity compared to that in the vehicle-treated control, but no concurrent downstream increase in erythroblast ghost degradation (Figure 1H) and thus no positive functional consequence of the observed increase in glucocerebrosidase activity. We surmised that this might be due to an overriding effect of lysosomal glucocerebrosidase inhibition, masking the effect of increased total levels of stabilized glucocerebrosidase protein available to degrade its substrate. We therefore used the model to understand whether inhibitory chaperones can be delivered in such a way that the compound-driven lysosomal inhibition of glucocerebrosidase function can be overcome.
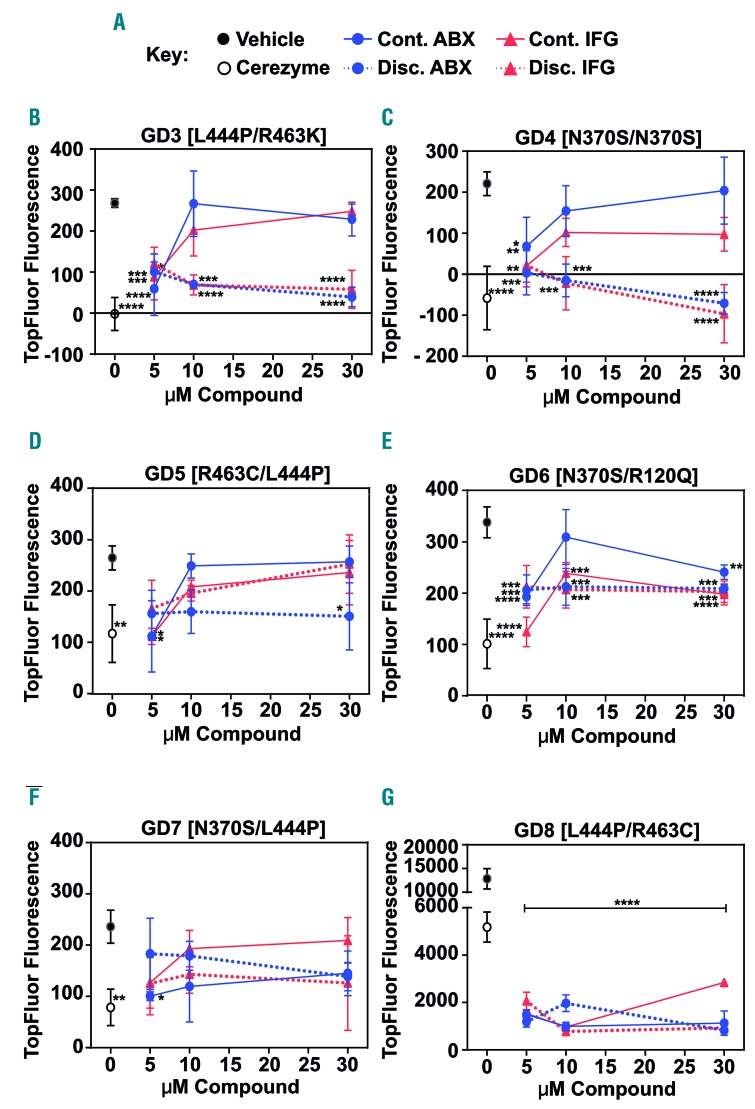
To address this issue we compared the dose-response profiles of PBMC-derived macrophages cultured in the continuous presence of an inhibitory compound, with those of PBMC-derived macrophages that were transferred to compound-free medium for the final 24 h in a discontinuous treatment protocol (Figure 1G). We found that the reduction of the TopFluor signal was far greater when the inhibitory compound was removed for the last 24 h than when it was not (Figure 2). This demonstrated that the capacity to degrade erythroblast ghosts was enhanced by chaperone compound treatment compared to vehicle control, but that removal of the compound was necessary to observe this experimentally. These results showed that by allowing a 24 h compound-free period, the functional impact of increased glucocerebrosidase protein was unmasked in the absence of lysosomal inhibition, indicating a therapeutic value of such compounds under the right treatment regimen. We therefore conclude that assessment of inhibitory compound efficacy on total glucocerebrosidase activity alone is insufficient to determine the therapeutic potential of such compounds. Additional testing of the downstream functional impact of such treatment is essential in order to understand the effect on lysosomal glucocerebrosidase function.
Figure 2.
Comparison of differential drug dosing protocols revealed a positive effect of glucocerebrosidase inhibitory chaperone compounds on lysosomal glucocerebrosidase function. Gaucher disease (GD) patient blood monocytic cell (PBMC)-derived macrophages were treated with 5, 10 or 30 μM ambroxol (ABX, blue) or isofagomine (IFG, red) or dimethylsulfoxide (DMSO) vehicle control (black filled circles) either continuously (Cont.; solid lines) for 5 days or with discontinuation of treatment (Discont.; dashed lines) 24 h before the readout of TopFluor fluorescence (excitation at 485 nm and emission at 528 nm). Continuous treatment with 0.4 U Cerezyme (open circles) for 5 days was used as a control. (A) Key to compound treatment conditions (see Figure 1G for a schematic representation of the dosing schedules). (B-G) Quantification of TopFluor fluorescence in PBMC-derived macrophages from six different GD patients who harbored different combinations of GBA1 mutant alleles as shown. All samples were assayed in triplicate. Graphs show the mean and standard deviation. Data were analyzed by one-way analysis of variance followed by the Dunnett test for multiple comparisons. *P≤0.05; **P≤0.01; ***P≤0.001, compared to the DMSO control.
It is thought that inhibitory chaperones such as isofagomine require a washout period in order to be effective,11 which would lead to a complicated dosing regimen for patients. We show that even for a mixed-type glucocerebrosidase inhibitor such as ambroxol, previously found to have no inhibitory activity at low pH in vitro,15 when assessed in live cells with continuous exposure at high concentrations, the lysosomal functional improvement is masked. Molecular mechanisms that modulate the action of ambroxol in a cellular context may contribute to this finding. Importantly, we show that, although under washout conditions (24 h compound-free protocol), the response was greater as concentration increased, the lowest concentration of compound tested (5 μM) gave an equivalent outcome independently of the treatment protocol used. However, it is also important to highlight that in some patients only a 2-fold increase in compound concentration was needed before the inhibitory mechanism was demonstrated, indicating a small therapeutic window. This highlights the importance of assessing both treatment paradigms when evaluating inhibitory compounds in vitro to understand likely responses in individual patients. Collectively, our data demonstrate an optimization point at which continuous application at sub-inhibitory concentrations could still be therapeutically effective, avoiding the need to employ a washout dosing strategy. It also underscores the need to perform such in vitro biomarker testing to understand how individual patients may respond to treatment.
Finally, inappropriate patient stratification is currently cited as a factor contributing to the failure of clinical trials on disease-modifying compounds used for the treatment of nervous system disorders.17–19 Having repeated the study in a number of GD patient cells harboring a panel of different GBA1 mutation allele combinations, we saw that individual patients did demonstrate subtly different responses to the two treatment protocols. However, importantly, there was a general trend for the lower concentrations of a compound to be effective in all patients under both treatment strategies, regardless of GBA1 allele combination. This provides promising evidence (i) for the ability to identify groups of patients who are likely to respond well to treatment and (ii) that grouping patients together for clinical trials based on their in vitro phenotypic response to candidate compounds could be a valid method for effectively stratifying cohorts. Additional studies are required to confirm that this in vitro assay is representative of an in vivo response. However, we would like to highlight the implication of our findings for improving the design and outcome of clinical trials.
In summary, we describe a potential in vitro biomarker assay for stratification in inhibitory chaperone compound clinical trials, highlight the importance of using a dual approach treatment regimen to gain mechanistic insight into the therapeutic effectiveness of inhibitory chaperones in order to identify likely responders and, importantly, show that phenotype-based patient stratification might be a plausible method for determining an inclusion or stratification criterion to ensure that the right population of patients will benefit from well-designed clinical trials.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Beutler E, Grabowski GA. Glucosylceramide lipidosis–Gaucher disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Diseases. 8th ed. 2001:3635–3668. [Google Scholar]
- 2.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. [DOI] [PubMed] [Google Scholar]
- 3.Migdalska-Richards A, Schapira AH. The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem. 2016;139(Suppl 1):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gegg ME, Schapira AHV. The role of glucocerebrosidase in Parkinson disease pathogenesis. FEBS J. 2018;285(19):3591–3603. [DOI] [PubMed] [Google Scholar]
- 5.Bennett LL, Mohan D. Gaucher disease and its treatment options. Ann Pharmacother. 2013;47(9):1182–1193. [DOI] [PubMed] [Google Scholar]
- 6.Shihabuddin LS, Brundin P, Greenamyre JT, Stephenson D, Sardi SP. New frontiers in Parkinson’s disease: from genetics to the clinic. J Neurosci. 2018;38(44):9375–9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat. 2008;29(5):567–583. [DOI] [PubMed] [Google Scholar]
- 8.Sawkar AR, D’Haeze W, Kelly JW. Therapeutic strategies to ameliorate lysosomal storage disorders–a focus on Gaucher disease. Cell Mol Life Sci. 2006;63(10):1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schapira AH, Chiasserini D, Beccari T, Parnetti L. Glucocerebrosidase in Parkinson’s disease: insights into pathogenesis and prospects for treatment. Mov Disord. 2016;31(6):830–835. [DOI] [PubMed] [Google Scholar]
- 10.McMahon B, Aflaki E, Sidransky E. Chaperoning glucocerebrosidase: a therapeutic strategy for both Gaucher disease and Parkinsonism. Neural Regen Res. 2016;11(11):1760–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steet RA, Chung S, Wustman B, et al. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci U S A. 2006;103(37):13813–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aflaki E, Stubblefield BK, Maniwang E, et al. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med. 2014;6(240):240ra273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawkar AR, Cheng WC, Beutler E, et al. Chemical chaperones increase the cellular activity of N370S beta-glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A. 2002;99(24):15428–15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendikov-Bar I, Maor G, Filocamo M, Horowitz M. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol Dis. 2013;50(2):141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maegawa GH, Tropak MB, Buttner JD, et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009;284(35):23502–23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beutler E. Gaucher’s disease. N Engl J Med. 1991;325(19):1354–1360. [DOI] [PubMed] [Google Scholar]
- 17.Vellas B, Carrillo MC, Sampaio C, et al. Designing drug trials for Alzheimer’s disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9(4):438–444. [DOI] [PubMed] [Google Scholar]
- 18.Cook D, Brown D, Alexander R, et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov. 2014;13(6):419–431. [DOI] [PubMed] [Google Scholar]
- 19.Pankevich DE, Altevogt BM, Dunlop J, Gage FH, Hyman SE. Improving and accelerating drug development for nervous system disorders. Neuron. 2014;84(3):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone DL, Tayebi N, Orvisky E, et al. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15(2):181–188. [DOI] [PubMed] [Google Scholar]
- 21.von der Heul C, Kroos MJ, de Jeu-Jaspars CM, von Eijk HG. The uptake of iron by reticulocytes. The influence of purification of the ghosts on iron-containing components in the ghost suspension. Biochim Biophys Acta. 1980;601(3):572–583. [DOI] [PubMed] [Google Scholar]
- 22.Gegg ME, Burke D, Heales SJ, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72(3):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]