Abstract
As coronavirus disease 2019 (COVID-19) spreads across the world, the intensive care unit (ICU) community must prepare for the challenges associated with this pandemic. Streamlining of workflows for rapid diagnosis and isolation, clinical management, and infection prevention will matter not only to patients with COVID-19, but also to health-care workers and other patients who are at risk from nosocomial transmission. Management of acute respiratory failure and haemodynamics is key. ICU practitioners, hospital administrators, governments, and policy makers must prepare for a substantial increase in critical care bed capacity, with a focus not just on infrastructure and supplies, but also on staff management. Critical care triage to allow the rationing of scarce ICU resources might be needed. Researchers must address unanswered questions, including the role of repurposed and experimental therapies. Collaboration at the local, regional, national, and international level offers the best chance of survival for the critically ill.
Introduction
Coronavirus disease 2019 (COVID-19) is the third coronavirus infection in two decades that was originally described in Asia, after severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).1 As the COVID-19 pandemic spreads worldwide, intensive care unit (ICU) practitioners, hospital administrators, governments, policy makers, and researchers must prepare for a surge in critically ill patients. Many lessons can be learnt from the cumulative experience of Asian ICUs dealing with the COVID-19, SARS, and MERS outbreaks. In this Review, we draw on the experience of Asian ICU practitioners from a variety of settings—and available literature on the management of critically ill patients with COVID-19 and related conditions—to provide an overview of the challenges the ICU community faces and recommendations for navigating these complexities. These challenges and recommendations are summarised in Table 1, Table 2 .
Table 1.
Challenges in clinical management
| Recommendations | |
|---|---|
| Epidemiology and clinical features | |
| Prediction of disease trajectory from the time of symptom onset is difficult | Support research to develop and validate prognostic tools and biomarkers |
| Diagnosis | |
| Clinical features are non-specific; risk of missing a case early in a local outbreak is substantial | Adopt a low threshold for diagnostic testing, where available |
| Sensitivity of RT-PCR assays for critically ill patients is unknown | Repeat the sampling if necessary, preferably from lower respiratory tract |
| RT-PCR assays might not be available in many ICUs; if available, assays will take time to complete | Maintain a high index of suspicion for COVID-19 |
| Management of acute respiratory failure | |
| Benefits of NIV and HFNC, and associated risks of viral transmission through aerosolisation, are unclear | Reserve for mild ARDS, with airborne precautions, preferably in single rooms, and a low threshold for intubation |
| Intubation poses a risk of viral transmission to health-care workers | Perform intubation drills; the most skilled operator should intubate with full PPE and limited bag-mask ventilation |
| ECMO is extremely resource-intensive, even if centralised at designated centres | Balance the needs of a larger number of patients with less severe disease against the (unproven) benefit to a few |
| Other intensive care management | |
| Patients often develop myocardial dysfunction in addition to acute respiratory failure | Administer fluids cautiously for hypovolaemia, preferably with assessments for pre-load responsiveness; detect myocardial involvement early with troponin and beta-natriuretic peptide measurements and echocardiography |
| Bacterial and influenza pneumonia or co-infection are difficult to distinguish from COVID-19 alone | Consider empirical broad-spectrum antibiotics and neuraminidase inhibitors at presentation and subsequent rapid de-escalation |
| Benefits and risks of systemic corticosteroids are unclear | Avoid routine use until more evidence is available |
| Transfer out of the ICU for investigations such as CT scans poses risk of viral transmission | Minimise transfers by using alternatives such as point-of-care ultrasound |
| Viral shedding in the upper respiratory tract continues beyond 10 days after symptom onset in severe COVID-19 | De-isolate patients only after clinical recovery and two negative RT-PCR assays performed 24 h apart |
| Repurposed and experimental therapies that are not supported by strong evidence are being used | Seek expert guidance from local or international societies and enrol patients in clinical studies where possible |
ARDS=acute respiratory distress syndrome. COVID-19=coronavirus disease 2019. ECMO=extracorporeal membrane oxygenation. HFNC=high-flow nasal cannula. ICU=intensive care unit. NIV=non-invasive ventilation. PPE=personal protective equipment.
Table 2.
Challenges in infection prevention, ICU infrastructure, capacity, staffing, triage, and research
| Recommendations | |
|---|---|
| Infection prevention | |
| A global shortage of medical masks and respirators threatens efforts to prevent transmission | Consider reuse between patients and use beyond the manufacturer-designated shelf life |
| N95 respirators that do not fit facial contours might not provide the necessary protection | Conduct regular fit testing, preferably before outbreaks |
| Self-contamination often happens during removal of PPE | Train on both the donning and doffing of PPE |
| Viable virus on health-care workers' mobile phones and hospital equipment can cause nosocomial transmission | Conduct surface decontamination and consider wrapping mobile phones in disposable specimen bags |
| SARS-CoV-2 might be transmitted faecally | Practise immediate and proper disposal of soiled objects |
| ICU visits pose a risk of infection to visitors | Restrict or ban visits to minimise transmission; use video conferencing for communication between family members and patients or health-care workers |
| ICU infrastructure | |
| Airborne infection isolation rooms with negative pressure are not universally available, especially in resource-limited settings | Consider adequately ventilated single rooms without negative pressure or, if necessary, cohort cases in shared rooms with beds spaced apart |
| ICU capacity | |
| Surges in numbers of critically ill patients with COVID-19 can occur rapidly | Implement national and regional modelling of needs for intensive care |
| Low-income and middle-income countries have insufficient ICU beds in general, and even high-income countries will be put under strain in an outbreak like COVID-19 | Consider whether increasing intensive care provision is an appropriate use of resources; if so, make plans for an increase in capacity, including providing intensive care in areas outside ICUs and centralising intensive care in designated ICUs |
| Increasing ICU capacity requires more equipment (eg, ventilators), consumables, and pharmaceuticals, which might be in short supply | Pay close attention to logistical support and the supply chain; reduce the inflow of patients who do not urgently require intensive care (eg, by postponing elective surgeries) |
| Ventilators are in short supply | Consider transport, operating theatre, and military ventilators |
| ICU staffing | |
| Increasing ICU bed numbers and workload without increasing staff could result in increased mortality | Make plans for augmentation of staff from other ICUs or non-ICU areas, and provision of appropriate training (eg, with standardised short courses) |
| Risk of loss of staff to illness, medical leave, or quarantine after unprotected exposure to COVID-19, with a potentially devastating effect on morale, is high | Minimise risk of infection; consider segregation of teams and physical distancing to limit unprotected exposure of multiple team members, and travel restrictions to limit exposure to COVID-19, which is now global |
| Staff are especially vulnerable to mental health problems such as depression and anxiety during outbreaks | Reassure staff through infection prevention measures, clear communication, limitation of shift hours, provision of rest areas, and mental health support |
| ICU triage | |
| ICUs can become overwhelmed as surge strategies might not be sufficient in an emerging pandemic like COVID-19 | Consider implementing a triage policy that prioritises patients for intensive care and rations scarce resources |
| ICU research | |
| The traditional pace of research might not match the pace of the outbreak | Use and adapt pre-approved research plans and platforms |
| Studies are often single-centre and underpowered | Collaborate through international research networks and platforms |
| Rapid conduct and sharing of research might compromise scientific quality and ethical integrity | Cautiously analyse the study methodology when interpreting the literature |
COVID-19=coronavirus disease 2019. ICU=intensive care unit. PPE=personal protective equipment.
Epidemiology and clinical features of critically ill patients
The number of people diagnosed with COVID-19 worldwide crossed the one million mark on April 2, 2020; the case fatality rate across 204 countries and territories was 5·2%.2 By comparison, the SARS epidemic infected 8096 people in 29 countries from November, 2002, to July, 2003, and had a case fatality rate of 9·6%,3 whereas the MERS outbreak infected 2494 people in 27 countries from April, 2012, to November, 2019, and had a case fatality rate of 34·4%.4 These fatality rates should be interpreted with caution, because they vary across regions, are higher in strained health-care systems, and do not account for undiagnosed patients with mild disease who do not contribute to the denominator.5, 6, 7
In a review by the WHO-China Joint Mission of 55 924 laboratory-confirmed cases in China, 6·1% were classified as critical (respiratory failure, shock, and multiple organ dysfunction or failure) and 13·8% as severe (dyspnoea, respiratory rate ≥30 breaths per min, oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen [PaO2/FiO2] ratio <300 mm Hg, and increase in lung infiltrates >50% within 24–48 h).8 Not all critical cases were admitted to the ICU. Indeed, ICU admissions are dependent on the severity of illness and the ICU capacity of the health-care system. In Italy, the country outside China with the most patients with COVID-19 until March 29, 2020, up to 12% of all positive cases required ICU admission.9, 10
Critically ill patients with COVID-19 are older and have more comorbidities, including hypertension and diabetes, than do non-critically ill patients.11, 12 The most common symptoms are non-specific: fever, cough, fatigue, and dyspnoea.11, 12, 13, 14, 15, 16 The median time from symptom onset to the development of pneumonia is approximately 5 days,12, 15 and the median time from symptom onset to severe hypoxaemia and ICU admission is approximately 7–12 days.8, 13, 15, 17, 18 Most patients have bilateral opacities on chest radiograph and CT.11, 12, 13, 14, 16 Common CT findings are ground glass opacities and consolidation.19, 20 Acute hypoxaemic respiratory failure—sometimes with severe hypercapnia—from acute respiratory distress syndrome (ARDS) is the most common complication (in 60–70% of patients admitted to the ICU), followed by shock (30%), myocardial dysfunction (20–30%), and acute kidney injury (10–30%).11, 13, 15, 16 Elderly patients might develop hypoxaemia without respiratory distress.5 In one study, arrhythmia was noted in 44% of ICU patients.11
Key messages.
-
•
Clinical features of coronavirus disease 2019 (COVID-19) are non-specific and do not easily distinguish it from other causes of severe community-acquired pneumonia
-
•
As the pandemic worsens, intensive care unit (ICU) practitioners should increasingly have a high index of suspicion and a low threshold for diagnostic testing for COVID-19
-
•
Many questions on clinical management remain unanswered, including the significance of myocardial dysfunction, and the role of non-invasive ventilation, high-flow nasal cannula, corticosteroids, and various repurposed and experimental therapies
-
•
ICU practitioners, hospital administrators, governments, and policy makers must prepare early for a substantial increase in critical care capacity, or risk being overwhelmed by the pandemic
-
•
Surge options include the addition of beds to a pre-existing ICU, provision of intensive care outside ICUs, and centralisation of intensive care in designated ICUs, while considering critical care triage and rationing of resources should surge efforts be insufficient
-
•
Preparations must focus not just on infrastructure and supplies, but also on staff, including protection from nosocomial transmission and promotion of mental wellbeing
In a large report, 49% of all 2087 critically ill patients with COVID-19 in China died.21, 22 Small, single-ICU studies found mortality rates of 62% (in Wuhan, China) and 67% (in Washington State, USA), but these figures had not accounted for many who were still in the ICU.15, 16 Although 97% of patients on invasive mechanical ventilation died in a multicentre study conducted early in the Wuhan outbreak, mortality is affected by local practices, and larger studies are awaited.23 The same study reported that 53% of deaths were related to respiratory failure, 7% to shock (presumably from fulminant myocarditis), 33% to both, and 7% to unclear mechanisms.23 Mortality is associated with older age, comorbidities (including hypertension, diabetes, cardiovascular disease, chronic lung disease, and cancer), higher severity of illness scores, worse respiratory failure, higher d-dimer and C-reactive protein concentrations, lower lymphocyte counts, and secondary infections.5, 8, 12, 15, 18, 21, 22, 23, 24 Although patients older than 60 years account for more than 80% of deaths, younger patients are not spared.21, 22 The median time from symptom onset to death is 2–8 weeks, whereas the median time from symptom onset to clinical recovery is 6–8 weeks.8, 18 Prediction of the trajectory of illness from symptom onset is difficult, and prognostic tools and biomarkers are urgently needed.5
Diagnosis
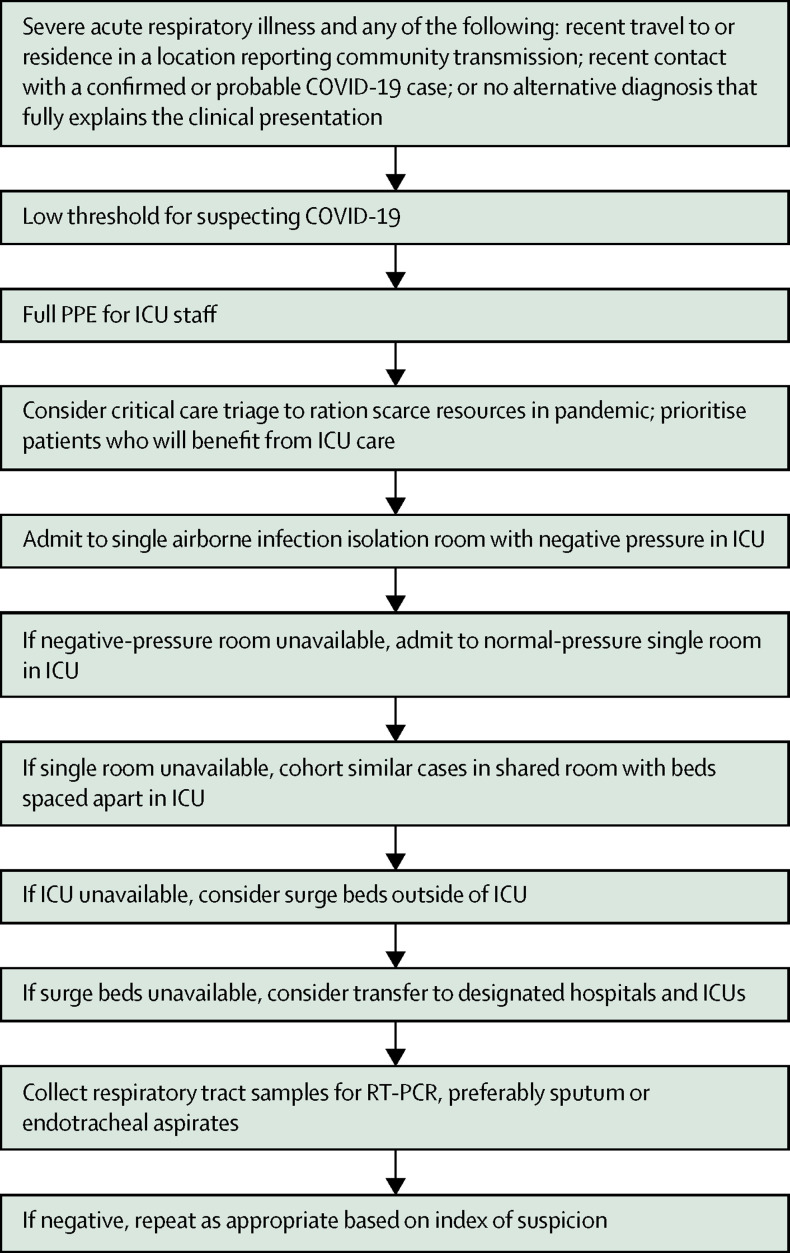
Figure 1 suggests an initial approach for ICU practitioners who are called to assess a patient with suspected COVID-19 infection. The non-specific clinical features do not easily distinguish severe COVID-19 from other causes of severe community-acquired pneumonia.25 WHO suggests that COVID-19 be suspected in patients with acute respiratory illness and fever, plus travel to or residence in a location reporting community transmission, or contact with a confirmed or probable COVID-19 case in the 14 days before symptom onset; and in patients with severe acute respiratory illness who require hospitalisation without an alternative diagnosis that fully explains the clinical presentation.26 Given the exponential rise in the number of areas with community transmission worldwide and the substantial risk of missing cases early in a local outbreak,9 ICU practitioners should increasingly have a high index of suspicion and a low threshold for diagnostic testing for any patient with severe acute respiratory infection, where available.
Figure 1.
Initial approach to critically ill patients with suspected COVID-19
COVID-19=coronavirus disease 2019. ICU=intensive care unit. PPE=personal protective equipment.
Diagnosis is based on RT-PCR assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients with pneumonia might have falsely negative upper respiratory tract samples.20 Although sampling from the lower respiratory tract is recommended by WHO, such as with sputum and endotracheal aspirates,26 this procedures potentially generate aerosol and must be performed with strict airborne precautions.8, 27 Although the diagnostic yield of bronchoalveolar lavage for COVID-19 might be high,28 bronchoscopy should generally be avoided to minimise exposure of health-care workers to SARS-CoV-2.29, 30 The sensitivity of RT-PCR assays for the critically ill is currently unknown. Repeated sampling might be required when initial tests are negative despite suspicious clinical features.31 Importantly, RT-PCR assays might be unavailable in many ICUs, and where available still take time to run. Meanwhile, serological assays are being developed.32
Management of acute respiratory failure
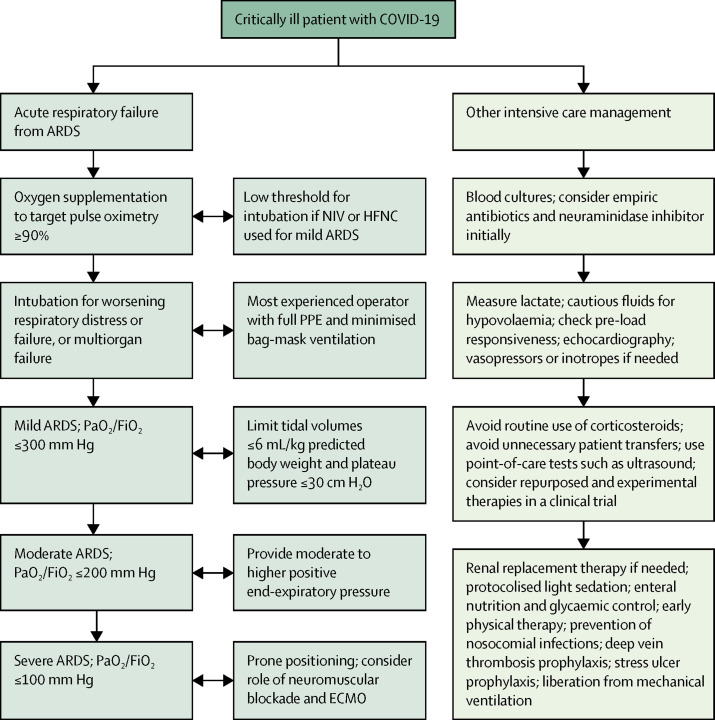
Specific data on supportive ICU care for COVID-19 are lacking, and current recommendations are based on existing evidence from other viral respiratory infections and general intensive care management (figure 2 ).33
Figure 2.
Clinical management of critically ill patients with COVID-19
ARDS=acute respiratory distress syndrome. COVID-19=coronavirus disease 2019. ECMO=extracorporeal membrane oxygenation. HFNC=high-flow nasal cannula. NIV=non-invasive ventilation. PaO2/FiO2=partial pressure of arterial oxygen to fraction of inspired oxygen. PPE=personal protective equipment.
Reports suggest that non-invasive ventilation (NIV) and high-flow nasal cannula (HFNC) were used in between one-third and two-thirds of critically ill patients with COVID-19 in China.11, 12, 13, 15 Minimal data exist to confirm or refute safety concerns regarding the risk of aerosol generation by these devices. Epidemiological data suggest that NIV was associated with nosocomial transmission of SARS;34 however, human laboratory data suggest that NIV does not generate aerosols.35 Suggestions that HFNC might be safe are questionable: studies that might be taken to support the safety of HFNC were not designed to show whether or not HFNC is aerosol generating and did not examine the spread of viruses.36, 37 Moreover, although NIV might reduce intubation and mortality in mild ARDS,38 it is associated with higher mortality in moderate-to-severe ARDS from multiple causes,39 and a high risk of failure in MERS.40 Although weak evidence suggests that HFNC might reduce intubation rates without affecting mortality in unselected patients with acute hypoxaemic respiratory failure,41 delayed intubation as a consequence of its use might increase mortality.42 Thus, NIV and HFNC should be reserved for patients with mild ARDS until further data are available, with close monitoring, airborne precautions, and preferably use of single rooms. Thresholds for intubation in the event of deterioration and the absence of single rooms should be kept low.
Extrapolating from SARS, intubation of patients with COVID-19 also poses a risk of viral transmission to health-care workers, and intubation drills are crucial.34, 43 The most skilled operator available should perform the task with full personal protective equipment (PPE) and the necessary preparation for difficult airways. The number of assistants should be limited to reduce exposure. Bag-mask ventilation, which generates aerosols, should be minimised by prolonged pre-oxygenation; a viral filter can be placed between the exhalation valve and the mask.43 Rapid sequence induction with muscle relaxants will reduce coughing. End-tidal carbon dioxide detection and observation of chest rise should be used to confirm endotracheal tube placement. The use of closed suctioning systems post-intubation will reduce aerosolisation.
A major focus of mechanical ventilation for COVID-19 is the avoidance of ventilator-induced lung injury while facilitating gas exchange via lung-protective ventilation.44, 45 Prone positioning should be applied early, given its association with reduced mortality in other causes of severe ARDS. Although outcome data on prone positioning in COVID-19 (used in 12% of patients in one ICU study from Wuhan15) are currently lacking, the tendency for SARS-CoV-2 to affect the peripheral and dorsal areas of the lungs provides the ideal conditions for a positive oxygenation response to prone positioning. Veno-venous extracorporeal membrane oxygenation (ECMO) is reserved for the most severe of ARDS patients in view of evidence that it might improve survival, including in MERS.46, 47, 48 However, the decision to provide very advanced care for fewer patients should be balanced against the requirement to provide less advanced care for more patients.49 Preliminary data for COVID-19 are not encouraging.11, 13, 15, 17 In one report, out of 28 patients who received ECMO, 14 died, nine were still on ECMO, and only five were successfully weaned.5
Other intensive care management
Patients with COVID-19 might have hypovolaemia due to anorexia, vomiting, and diarrhoea.11, 12, 13, 14, 15 Nevertheless, fluids should be administered cautiously, and preferably with assessments for pre-load responsiveness such as the passive leg raise test, given the high incidence of myocardial dysfunction in COVID-19.11, 13, 15, 16, 23 This incidence might be due to strong binding affinity of the SARS-CoV-2 spike protein to human angiotensin converting enzyme 2 (ACE2), a membrane-bound receptor crucial for host cell entry that is expressed in the heart and lungs, among other organs.50, 51 A conservative or de-resuscitative fluid strategy,52 with early detection of myocardial involvement through the measurement of troponin and beta-natriuretic peptide concentrations and echocardiography,53, 54 and early use of vasopressors and inotropes are recommended (figure 2).
Most patients with COVID-19 in China were given empirical broad-spectrum antibiotics and many, oseltamivir, because laboratory diagnosis of COVID-19 takes time, and distinguishing the disease from other bacterial and viral pneumonias is often difficult.11, 12, 13, 14, 15 One study of 201 patients with COVID-19 found only one co-infection with a different virus and none with bacteria.24 Another study of 92 patients found six co-infections by other common respiratory viruses,55 and a third study of 115 patients found five co-infections with influenza.56 Any empirical antibiotic and anti-influenza therapy should be rapidly de-escalated based on microbiology test results and clinical response.
Chinese reports also show that systemic corticosteroids were administered to approximately half of patients with COVID-19 with severe or critical illness.12, 13, 14, 15, 17 A retrospective study of 84 patients with ARDS associated with COVID-19 found lower mortality in those treated with methylprednisolone, but the findings are limited by the observational design of the study, small sample size, and possible confounders.24 Because COVID-19 might be associated with a cytokine storm like that seen in other viral infections, immunosuppression has been proposed as an approach that might be beneficial for patients with signs of hyperinflammation, such as increasing ferritin concentrations.57 Although the benefits of immunosuppression are unproven and the role of corticosteroids in COVID-19 remains unclear, a systematic review of observational studies of corticosteroids for SARS found no impact on mortality but possible harms, including avascular necrosis, psychosis, diabetes, and delayed viral clearance.58 Similarly, an observational study found that corticosteroids for MERS did not affect mortality, but did delay viral clearance.59 A systematic review of observational studies suggested that corticosteroids might increase mortality and secondary infections in influenza.60 Until further data are available, the routine use of corticosteroids in viral severe acute respiratory infections, including COVID-19, is not recommended.61
Rapid liberation from invasive mechanical ventilation to reduce the incidence of ventilator-associated pneumonia and to create ICU capacity must be balanced against the risks of premature extubation (especially without facilitative post-extubation NIV and HFNC) and subsequent re-intubation (and the attendant risks of viral transmission to health-care workers). Transfer of patients out of the ICU for investigations such as CT scans risks spreading SARS-CoV-2 and can be minimised with alternatives such as point-of-care ultrasound.62 The latter was prioritised by some Chinese ICUs, and evidence of varying degrees of an interstitial pattern and consolidation on lung ultrasonography now exists for patients with COVID-19.63, 64 Finally, the median ICU length of stay for COVID-19 was 8 days in a Chinese report;18 however, larger studies are needed to better understand the course of COVID-19 after admission to the ICU. WHO recommends that de-isolation of patients requires clinical recovery and two negative RT-PCR assays performed 24 h apart.61 Viral shedding in the upper respiratory tract continues beyond 10 days after symptom onset in severe COVID-19.65 This fact has significant implications for the use of isolation facilities.
Repurposed and experimental therapies
No proven therapy for COVID-19 exists, but several candidates—some previously used against SARS-CoV and MERS-CoV—have been used empirically and are undergoing investigation.61, 66 Table 3 summarises the evidence for some of the more prominent therapies: remdesivir,67, 68, 69, 70 lopinavir–ritonavir,71, 72, 73, 74 chloroquine,75, 76, 77 hydroxychloroquine,79, 80 intravenous immunoglobulin,81, 82 convalescent plasma,83, 84, 85 tocilizumab,57, 86 favipiravir,87 and traditional Chinese medicines.88, 89
Table 3.
Evidence for the safety and potential benefits of repurposed and experimental therapies
| Efficacy | Safety | |
|---|---|---|
| Remdesivir (nucleotide analogue) | ||
| Deemed to be the most promising candidate drug by experts convened in January, 2020, by WHO;66 relevant studies include PALM, an RCT of remdesivir and different monoclonal antibodies in 681 patients with Ebola virus disease (primary outcome: death at 28 days);67 study of remdesivir, lopinavir–ritonavir, and interferon beta in mice infected with MERS-CoV;68 in-vitro studies of remdesivir on SARS-CoV-2, MERS-CoV, and SARS-CoV69, 70 | Not efficacious for Ebola virus disease compared with other investigational therapies;67 superior activity compared with lopinavir–ritonavir in mice with MERS-CoV;68 effectively inhibited SARS-CoV-2, MERS-CoV, and SARS-CoV in vitro69, 70 | No peer-reviewed, published safety data available for SARS-CoV-2; in the PALM trial, only 1 of 175 patients randomised to remdesivir had a potentially serious adverse event (hypotension during a loading dose followed by cardiac arrest, possibly due to remdesivir or to fulminant Ebola virus disease itself)67 |
| Lopinavir–ritonavir (protease inhibitor) | ||
| Second candidate identified for rapid implementation in clinical trials, alone or in combination with interferon beta, by WHO;66 relevant studies include an RCT of lopinavir–ritonavir versus standard care in 199 hospitalised adults with SARS-CoV-2-associated pneumonia and hypoxaemia (primary outcome: time to clinical improvement);71 MIRACLE, an ongoing RCT of lopinavir–ritonavir plus interferon beta versus placebo in patients with MERS-CoV infection (primary oucome: 90-day mortality);72 case reports describing use of lopinavir–ritonavir plus interferon alfa in patients with MERS-CoV infection;73 observational study of lopinavir–ritonavir in patients with SARS-CoV74 | No significant difference in time to clinical improvement, reduction in viral load, or 28-day mortality with lopinavir–ritonavir compared with standard care in patients with severe COVID-19 (28-day mortality was numerically lower: 19·2% vs 25·0%), but median time to randomisation was 13 days after symptom onset, so effects of earlier treatment remain unknown;71 efficacy unclear in case reports of patients with MERS-CoV;73 associated with reduced viral load and mortality in an observational study of SARS-CoV74 | Gastrointestinal side-effects, including diarrhoea, nausea, and vomiting31, 71 |
| Chloroquine (antimalarial) | ||
| Studies ongoing in patients with COVID-19;75 in vitro studies of chloroquine on SARS-CoV and SARS-CoV-276, 77 | According to a news briefing,75 chloroquine slowed the progression of pneumonia and accelerated SARS-CoV-2 clearance and recovery in >100 patients with COVID-19, but results have not been published in the peer-reviewed literature and caution is advised in interpreting these findings;75 in-vitro antiviral effects reported for both SARS-CoV and SARS-CoV-276, 77 | No peer-reviewed, published safety data available for SARS-CoV-2, but concerns include the possibility of QT prolongation78 |
| Hydroxychloroquine (antimalarial) | ||
| Open label, non-randomised trial in 36 patients with COVID-19 (endpoint: presence or absence of virus at 6 days);79 in-vitro studies of hydroxychloroquine on SARS-CoV-280 | Reduced SARS-CoV-2 load in the nasopharynx of patients with COVID-19, especially when combined with azithromycin;79 more potent than chloroquine in inhibiting SARS-CoV-2 in vitro80 | No peer-reviewed, published safety data available for SARS-CoV-2, but concerns include the possibility of QT prolongation78 |
| Intravenous immunoglobulin (immunotherapy) | ||
| Phase 1 trial of human polyclonal immunoglobulin G (SAB-301) in healthy participants;81 study of human polyclonal immunoglobulin G (SAB-300) in a mouse model of MERS-CoV82 | SAB-301 found to be safe and well tolerated;81 SAB-300 reduced viral lung titres near or below the limit of detection in mice infected with MERS-CoV82 | No peer-reviewed, published safety data available for the various types of interferon (alfa and beta) for SARS-CoV-2, but generally well tolerated81 |
| Convalescent plasma (immunotherapy) | ||
| Meta-analysis of 27 studies of treatment in patients with SARS-CoV infection;83 use has been protocolised for MERS-CoV;84 uncontrolled case series of 5 patients with SARS-CoV-285 | Might reduce mortality in severe acute respiratory infections due to SARS-CoV and influenza;83 associated with reduction in viral load and improvement in fever, oxygenation, and chest imaging in a case series, but study limited by small sample size, multiple possible confounders, and absence of controls85 | No peer-reviewed, published safety data available for SARS-CoV-2, but studies of SARS-CoV have not reported serious adverse events83 |
| Tocilizumab (monoclonal antibody against interleukin-6) | ||
| Licensed for cytokine release syndrome; hypothesised to work against cytokine storm with raised ferritin and interleukin-6 levels due to SARS-CoV-257, 86 | No peer-reviewed, published efficacy data available for SARS-CoV-2 | No peer-reviewed, published safety data available for SARS-CoV-2 |
| Favipiravir (RNA-dependent RNA polymerase inhibitor) | ||
| Hypothesised to have an antiviral action on SARS-CoV-2 (RNA virus); multiple clinical studies underway for SARS-CoV-287 | No peer-reviewed, published efficacy data available for SARS-CoV-2; preliminary, unpublished trial data suggest a more potent antiviral action with favipiravir compared with lopinavir–ritonavir, but caution is advised in interpreting these results87 | No peer-reviewed, published safety data available for SARS-CoV-2; preliminary, unpublished trial data suggest fewer adverse events with favipiravir compared with lopinavir–ritonavir, but caution is advised in interpreting these results87 |
| XueBiJing and others | ||
| Traditional Chinese medicines, such as XueBiJing, suggested as candidates to treat SARS-CoV-2 infection are being studied88 | No peer-reviewed, published efficacy data available for SARS-CoV-2, but XueBiJing reported to reduce mortality in patients with severe community-acquired pneumonia with mixed aetiologies89 | No peer-reviewed, published safety data available for SARS-CoV-2 |
COVID-19=coronavirus disease 2019. MERS-CoV=Middle East respiratory syndrome coronavirus. MIRACLE=MERS-CoV Infection Treated with a Combination of Lopinavir/Ritonavir and Interferon-β1b. PALM=Pamoja Tulinde Maisha. RCT=randomised controlled trial. SARS-CoV=severe acute respiratory syndrome coronavirus. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Admittedly, therapies for which efficacy is not supported by strong evidence—not in COVID-19, and not even in SARS and MERS—are being administered in the hope of improving outcomes, before or in parallel with clinical studies. This enthusiasm to try new therapies during outbreaks must be balanced against ethical and scientific safeguards. During the Ebola outbreak, WHO experts concluded that due to “exceptional circumstances”, it was “ethically acceptable to offer unproven interventions that have shown promising results in the laboratory and in animal models but have not yet been evaluated for safety and efficacy in humans as potential treatment or prevention”.90 During the SARS outbreak, however, ribavirin was widely used, but was subsequently found to be at best ineffective and at worst harmful.58 Although expert guidance can be sought from local or international societies, patients treated with experimental therapies should be enrolled in a clinical study when possible.
Infection prevention
COVID-19 is extremely transmissible, with every case seeding more than two secondary cases.10, 91 In the WHO-China Joint Mission report, 2055 health-care workers accounted for 3·7% of cases with laboratory-confirmed COVID-19 in China.8 WHO recommends that PPE for health-care workers providing direct care to patients with COVID-19 should include medical masks, gowns, gloves, and eye protection with goggles or face shields.92 For aerosol-generating procedures (tracheal intubation, NIV, tracheostomy, cardiopulmonary resuscitation, bag-mask ventilation, and bronchoscopy), masks should be N95 or FFP2-equivalent respirators, and gowns or aprons should be fluid resistant. Although some clinicians have suggested the additional use of powered air-purifying respirators (PAPRs)—given accounts of health-care workers acquiring SARS despite wearing N95 respirators, and available albeit limited evidence that PAPRs result in less contamination of health-care workers43—their use comes with significant logistical challenges.93
There are several pitfalls related to PPE. Close attention to the supply chain is needed given the global shortage of medical masks and respirators.5, 6, 94 Reuse between patients and use beyond the manufacturer-designated shelf life might be required.95 Fit testing—preferably done before outbreaks—is crucial and should be regularly performed as facial contours change with time.96 Non-N95 reusable masks with high-efficiency particulate air (HEPA) filters that do not require fit testing might be considered.96 Although health-care workers often focus on donning PPE, data suggest a substantial risk of self-contamination when doffing PPE.97 Training on the specific steps of wearing and removing PPE, together with hand cleansing, is crucial, and references for these procedures are widely available.98 Building a safety culture and encouraging staff to point out protocol errors were useful to reduce nosocomial SARS transmission.99
Surface decontamination is also key to infection prevention. Viable SARS-CoV-2 persists on inanimate surfaces such as plastic and stainless steel for up to 72 h.27 Because more than one-third of health-care workers' mobile phones might be contaminated with common viral pathogens,100 these should be cleaned regularly or wrapped with specimen bags that are discarded after contact with patients or daily. Environmental contamination by SARS-CoV-2 was detected on furniture and equipment within a patient's room and toilet in Singapore.101 During the MERS outbreak in South Korea, viable coronavirus was detected on doorknobs, bed guardrails, air exhaust dampers, and elevators.102 Immediate and proper disposal of soiled objects is also warranted as SARS-CoV-2 might be transmitted faecally.28, 31, 101
Visits to the ICU should be restricted or banned to prevent further transmission, except perhaps for the imminently dying.63, 93 Where feasible, video conferencing via mobile phones or other interfaces can be used for communication between family members and patients or health-care workers.
ICU infrastructure
To protect other patients and health-care workers, critically ill patients with suspected or confirmed COVID-19 should ideally be admitted to an airborne infection isolation room (AIIR) that is at negative pressure relative to surrounding areas, with accessible sinks and alcohol hand gel dispensers (figure 1), especially if aerosol-generating procedures are done.103 However, a survey of 335 ICUs across 20 Asian countries showed that only 12% of ICU rooms were AIIRs, and 37% of ICUs had no AIIRs. During the SARS outbreak in Singapore, negative pressure ventilation was created by mounting industrial exhaust fans.93
If AIIRs are unavailable, patients can be placed in adequately ventilated single rooms with the doors closed, as recommended by WHO.104 In the same Asian survey, only 37% of ICU rooms were single rooms, and 13% of ICUs had no single rooms.105 The number of single rooms and AIIRs was generally lowest in low-income countries.
Where single ICU rooms are unavailable, cohorting of cases in shared rooms with dedicated staff is an alternative, with beds spaced apart.104 Although the current evidence points towards droplet rather than airborne transmission of COVID-19,8 concerns of nosocomial transmission in shared rooms remain, especially when aerosol-generating procedures are performed. Thus, PPE should be considered for patients in shared rooms. Oxygen masks with HEPA filters might provide some protection for non-intubated patients.106
ICU capacity
Controlling the community spread of COVID-19 is difficult but possible,107 and crucial for the preservation of ICU capacity. National and regional modelling of needs for intensive care is crucial.9, 10 Many countries might not have enough ICU beds in the first place, let alone isolation or single rooms. The median number of critical care beds per 100 000 population was 2·3 in ten low-income and lower-middle-income countries, 4·6 in five upper-middle-income countries, and 12·3 in eight high-income countries in Asia in one analysis,108 and 9·6 in 28 high-income countries in Europe in a 2012 report.109 China, an upper-middle-income country, has 3·6 critical care beds per 100 000 population,108 and Wuhan was initially overwhelmed by COVID-19·5, 6, 15 Italy, a high-income country with 12·5 critical care beds per 100 000 population,109 continues to struggle with the outbreak.9, 10, 110 By contrast, a low-income country such as Uganda has only 0·1 critical care bed per 100 000 population.108, 111 This raises serious concerns about the ability of resource-limited settings to manage critically ill patients with COVID-19.112
Most countries cannot match China's feat of rapidly building new hospitals and ICUs during the COVID-19 outbreak in Wuhan.15 Surges in the number of critically ill patients with COVID-19 can occur rapidly. Thus, ICU practitioners, hospital administrators, governments, and policy makers must plan in advance for a substantial increase in critical care bed capacity.9, 10, 113 Adding beds into a pre-existing ICU is a possibility, but space constraints and nosocomial transmission from crowding limit this option.6 Other options include the provision of intensive care outside ICUs, such as in high-dependency units, remodelled general wards, post-anaesthesia care units, emergency departments, or deployable field units (figure 1).6, 113 Another option is the transfer of patients to designated hospitals and ICUs. Although the centralisation of expertise and resources might improve outcomes and efficiency, these benefits must be weighed against the risks of inter-hospital transfer.6, 9 The sustainability of depending on a few centres, or scarcity thereof, even as the outbreak worsens must be considered.
A substantial increase in ICU capacity involves increases not only in bed numbers, but also in equipment (eg, ventilators), consumables, pharmaceuticals, and staffing.6, 10, 93, 113 Although focusing on bed numbers without ensuring the availability of necessary equipment is unsafe, such equipment might be in short supply. Use of transport, operating theatre, and military ventilators might be required. To reduce strain on ICUs, elective surgeries should be postponed, and lower-acuity patients discharged to other areas, including designated de-escalation wards for recovering ICU patients with COVID-19 who might still require isolation.
ICU staffing
High ICU workload-to-staffing ratios are associated with an increase in patient mortality.114 Augmentation of staff with colleagues from other ICUs or even non-ICU areas might be required.6 Training of these external staff on general intensive care management and specific COVID-19 protocols is crucial.6, 93, 113 Standardised short courses exist,115 such as the BASIC course, which incorporates a mobile app for access to course material while caring for patients. Incredibly, more than 40 000 health-care workers were deployed from other parts of China to Wuhan.8 However, as the pandemic spreads, support from other sectors of a hospital or a country might increasingly be scarce as every area starts to become overwhelmed.
Staffing of ICUs must take into account the risk that health-care workers might become infected with SARS-CoV-2.6, 93 Minimising the risk of infection is essential, not only because of the direct loss of manpower but because of the potentially devastating effect of staff infection on morale, which might result in absenteeism. Where possible, rostering of staff should consider segregation of teams to limit unprotected exposure of all team members to infected patients or colleagues, and the resultant loss of staff to illness, medical leave, or quarantine.116 Physical distancing of staff, including having meals separately, is important. Travel restrictions to limit exposure to COVID-19 are being implemented and should be considered worldwide.117
Health-care workers in ICUs are especially vulnerable to mental health problems, including depression and anxiety, during outbreaks like COVID-19, because of the constant fear of being infected and the demanding workload.118 Staff who worked in high-risk SARS units continued to suffer from post-traumatic stress disorder years later.119 Measures to prevent such problems include a focus on infection prevention to reassure staff, clear communication from hospital and ICU leadership, limitation of shift hours and provision of rest areas where feasible, and mental health support through multidisciplinary teams, including psychiatrists, psychologists, and counsellors.117, 118
ICU triage
Should ICUs become overwhelmed by COVID-19 despite surge strategies,5, 6, 9, 10, 15 critical care triage that prioritises patients for intensive care and rations scarce resources will be required (figure 1).110, 120 This applies to patients with and without COVID-19, because both groups will be competing for the same ICU resources. Critical care triage is ethically complex and can be emotionally draining. It should ideally be coordinated at a regional or national health-care systems level, and some countries have now provided guidelines for COVID-19.121, 122 A triage policy implemented by clinicians trained in triage or senior ICU practitioners, complemented by clinical decision support systems, might identify patients with such a low probability of survival that they are unlikely to benefit from ICU care.120 Although generic physiological outcome prediction scores might not accurately predict the course of illness,5 older adults with comorbidities, higher d-dimer and C-reactive protein concentrations, and lower lymphocyte counts do worse.5, 8, 12, 15, 18, 21, 22, 23, 24 Rationing of resources also involves the withholding and withdrawal of life-sustaining treatments for existing ICU patients. To this end, it is noteworthy that a quarter of patients who died early in the Wuhan outbreak did not receive invasive ventilation.5
Research questions and methodology
A search of WHO's International Clinical Trials Registry Platform on March 31, 2020, revealed 667 registered trials on COVID-19. Although many are trials of repurposed or experimental therapeutic agents, other more basic questions that are equally crucial should be addressed through research. Some of these questions have been listed as potential challenges in Table 1, Table 2. The short-term and long-term prognoses of critically ill patients have to be clarified. Data on the effectiveness of NIV and HFNC, and the associated risk of viral transmission, remain scarce.34, 35, 36, 37 The risk of nosocomial transmission in shared ICU rooms should be studied. More data on cardiac involvement and myocardial dysfunction are needed.11, 13, 15, 16, 23 The role of ECMO is unclear.49 The indications for corticosteroids should be crystallised, while considering interactions between different therapies.61 For example, although limited by confounding from differences in baseline severities, a post-hoc analysis of a non-COVID-19 ARDS trial suggested that beta-interferon use was associated with higher mortality compared with placebo in patients receiving corticosteroids, but not in those who were not on corticosteroids.123
Multiple challenges to research exist during pandemics. First, the surge of disease often outpaces the traditional steps for research, including protocol design, securing of funding, and ethics approval, all amidst busy clinical work. Pre-approved adaptable plans drawn prior to an outbreak are useful. For example, several interventions against SARS-CoV-2 are being incorporated into the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP), a pre-approved platform trial for severe community-acquired pneumonia.
Second, many ongoing studies of COVID-19 are single-centre and underpowered to detect significant differences in meaningful outcomes between arms. To this end, pandemics provide a great opportunity for collaboration. Platforms such as the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and the International Forum for Acute Care Trialists (InFACT)—formed during the 2009 H1N1 pandemic—enable large research networks to share common goals and standardise data collection globally.124 WHO has also produced a master protocol for trials on experimental therapeutics for COVID-19.125 Last, the pace of research and data sharing must be balanced with scientific quality and ethical integrity. China's rapid sharing of the SARS-CoV-2 genetic code had an immediate impact on case identification, isolation, and the spread of the virus.126 The COVID-19 pandemic also saw a ballooning of the number of preprints (manuscripts openly posted online before peer review). During the Ebola and Zika outbreaks, the median time between preprints and peer-reviewed publication was 150 days.127 Although preprints rapidly provide new knowledge, ICU practitioners should be aware of the potential compromise in data quality when the conventional peer-review process is bypassed. A systematic review also found that only 50% of Ebola intervention studies fully complied with frameworks for ethical trial conduct.128
Conclusion
As countries ramp up efforts to prevent or delay the spread of COVID-19, the world must prepare for the possibility that containment and mitigation measures might fail. Even if SARS-CoV-2 infects a small proportion of the 7·8 billion people on Earth, many thousands will still become critically ill and require ICU care. The ICU community must brace itself for this potentially overwhelming surge of patients and optimise workflows, in advance, for rapid diagnosis and isolation, clinical management, and infection prevention. Hospital administrators, governments, and policy makers must work with ICU practitioners to prepare for a substantial increase in critical care bed capacity. They must protect health-care workers from nosocomial transmission, physical exhaustion, and mental health issues that might be aggravated by the need to make ethically difficult decisions on the rationing of intensive care. Researchers must address key questions about what remains a poorly understood disease. Collaboration at the local, regional, national, and international level—with a focus on high-quality research, evidence-based practice, sharing of data and resources, and ethical integrity in the face of unprecedented challenges—will be key to the success of these efforts.
Search strategy and selection criteria
We identified the references for this Review through searches of PubMed for articles published between Jan 1, 1950, and March 22, 2020, using combinations of the terms “coronavirus”, “COVID-19”, “SARS-CoV-2”, “nCoV”, “severe acute respiratory syndrome”, “SARS”, “Middle East respiratory syndrome”, “MERS”, “outbreak”, “epidemic”, “pandemic”, “acute respiratory distress syndrome”, and “intensive care”. We reviewed guidelines for the management of COVID-19 published by WHO and the US Centers for Disease Control and Prevention. We added articles through searches of the authors' personal files. We also reviewed relevant references cited in retrieved articles. Articles published in English and Chinese were included. The final reference list was generated on the basis of relevance to the topics covered in this Review, with the aim of highlighting the multiple challenges the intensive care community might face in the management of COVID-19, and providing recommendations for navigating these complexities.
This online publication has been corrected. The corrected version first appeared at thelancet.com/respiratory on May 4, 2020
Contributors
All authors did the literature search and drafted sections of the manuscript. JP combined and edited the drafts, prepared the figures, and supervised the manuscript. All authors subsequently revised the manuscript.
Declaration of interests
This Review was not funded by any organisation. JVD reports personal fees from Edwards India, outside the submitted work. YMA reports that he is principal investigator on a clinical trial of lopinavir–ritonavir and interferon for Middle East respiratory syndrome (MERS) and that he was a non-paid consultant on therapeutics for MERS-coronavirus (CoV) for Gilead Sciences and SAB Biotherapeutics. He is a co-investigator on the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) and a board member of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). CDG reports that he is chairman of the BASIC Collaboration steering committee. BASIC Collaboration has received unrestricted educational funding from manufacturers of mechanical ventilators (Gettinge, Drager, Hamilton) and high-flow nasal oxygen devices (Fischer & Paykel). MN reports personal fees from Nihon Kohden, personal fees from Getinge Group Japan, and personal fees from Total Medical Supply, outside the submitted work. All other authors declare no competing interests.
Supplementary Materials
References
- 1.Morens DM, Daszak P, Taubenberger JK. Escaping Pandora's box—another novel coronavirus. N Engl J Med. 2020 doi: 10.1056/NEJMp2002106. published online Feb 26. [DOI] [PubMed] [Google Scholar]
- 2.Worldometer COVID-19 coronavirus pandemic. April 2, 2020. https://www.worldometers.info/coronavirus/
- 3.WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Dec 31, 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/
- 4.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) Nov 30, 2019. https://www.who.int/emergencies/mers-cov/en/
- 5.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05979-7. published online March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu H, Tong Z, Ma P. Intensive care during the coronavirus epidemic. Intensive Care Med. 2020;46:576–578. doi: 10.1007/s00134-020-05966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30195-X. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO-China Joint Mission Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) Feb 28, 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 9.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020 doi: 10.1001/jama.2020.4031. published online March 13. [DOI] [PubMed] [Google Scholar]
- 10.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020 doi: 10.1016/S0140-6736(20)30627-9. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. published online Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020 doi: 10.1001/jama.2020.4326. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao J, Hu X, Cheng W, Yu L, Tu WJ, Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05987-7. published online March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H, Han X, Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ai T, Yang Z, Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. published online Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. (in Chinese). [Google Scholar]
- 22.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. published online Feb 24. [DOI] [PubMed] [Google Scholar]
- 23.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. published online March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D, Yao F, Wang L. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa247. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance. March 20, 2020. https://www.who.int/docs/default-source/coronaviruse/global-surveillance-for-covid-v-19-final200321-rev.pdf
- 27.van Doremalen N, Bushmaker T, Morris DH. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. published online March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Xu Y, Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhopadhyay A, Tambyah PA, Singh KS, Lim TK, Lee KH. SARS in a hospital visitor and her intensivist. J Hosp Infect. 2004;56:249–250. doi: 10.1016/j.jhin.2003.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Group of Interventional Respiratory Medicine. Chinese Thoracic Society Expert consensus for bronchoscopy during the epidemic of 2019 novel coronavirus infection (Trial version) Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:199–202. doi: 10.3760/cma.j.issn.1001-0939.2020.03.012. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 31.Young BE, Ong SWX, Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. published online March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang J, Wang MX, Ang IYH. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9:623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhazzani W, Moller MH, Arabi YM. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. published online March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonds AK, Hanak A, Chatwin M. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14:131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 36.Hui DS, Chow BK, Lo T. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53 doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 37.Leung CCH, Joynt GM, Gomersall CD. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Xu XP, Zhang XC, Hu SL. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45:e727–e733. doi: 10.1097/CCM.0000000000002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellani G, Laffey JG, Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 40.Arabi YM, Arifi AA, Balkhy HH. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 41.Rochwerg B, Granton D, Wang DX. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45:563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 42.Kang BJ, Koh Y, Lim CM. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 43.Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020 doi: 10.1007/s12630-020-01591-x. published online Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan E, Del Sorbo L, Goligher EC. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 45.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30127-2. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aoyama H, Uchida K, Aoyama K. Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: a systematic review and network meta-analysis. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alshahrani MS, Sindi A, Alshamsi F. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8:3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramanathan K, Antognini D, Combes A. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30121-1. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020 doi: 10.1001/jama.2020.2342. published online Feb 19. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. published online March 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silversides JA, Major E, Ferguson AJ. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43:155–170. doi: 10.1007/s00134-016-4573-3. [DOI] [PubMed] [Google Scholar]
- 53.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. published online March 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He XW, Lai JS, Cheng J. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E011. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 55.Lin D, Liu L, Zhang M. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci. 2020 doi: 10.1007/s11427-020-1668-5. published online March 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients co-infected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25781. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta P, McAuley DF, Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. published online March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arabi YM, Mandourah Y, Al-Hameed F. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 60.Lansbury L, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2019;2 doi: 10.1002/14651858.CD010406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WHO Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. March 13, 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 62.Liew MF, Siow WT, Yau YW, See KC. Safe patient transport for COVID-19. Crit Care. 2020;24:94. doi: 10.1186/s13054-020-2828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019–2020 epidemic: preparing intensive care units—the experience in Sichuan Province, China. Intensive Care Med. 2020;46:357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study Group Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05996-6. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Yan L, Wan L. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30232-2. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WHO Informal consultation on prioritization of candidate therapeutic agents for use in novel coronavirus 2019 infection. Jan 24, 2020. https://apps.who.int/iris/bitstream/handle/10665/330680/WHO-HEO-RDBlueprint%28nCoV%29-2020.1-eng.pdf
- 67.Mulangu S, Dodd LE, Davey RT., Jr A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheahan TP, Sims AC, Leist SR. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang M, Cao R, Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheahan TP, Sims AC, Graham RL. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arabi YM, Asiri AY, Assiri AM. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21:8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- 74.Chu CM, Cheng VC, Hung IF. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 76.Vincent MJ, Bergeron E, Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang M, Cao R, Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalil AC. Treating COVID-19-Off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020 doi: 10.1001/jama.2020.4742. published online Mar 24. [DOI] [PubMed] [Google Scholar]
- 79.Gautret P, Lagier J, Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Yao X, Ye F, Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. published online March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beigel JH, Voell J, Kumar P. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis. 2018;18:410–418. doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luke T, Wu H, Zhao J. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- 83.Mair-Jenkins J, Saavedra-Campos M, Baillie JK. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arabi Y, Balkhy H, Hajeer AH. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen C, Wang Z, Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen C, Zhang XR, Ju ZY, He WF. Advances in the research of cytokine storm mechanism induced by corona virus disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. published online March 1. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 87.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 88.Chan KW, Wong VT, Tang SCW. COVID-19: An update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. Am J Chin Med. 2020 doi: 10.1142/S0192415X20500378. published online March 13. [DOI] [PubMed] [Google Scholar]
- 89.Song Y, Yao C, Yao Y. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med. 2019;47:e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.WHO Ethical considerations for use of unregistered interventions for Ebola viral disease: report of an advisory panel to WHO. 2014. https://apps.who.int/iris/handle/10665/130997
- 91.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.WHO Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19): interim guidance. Feb 27, 2020. https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf
- 93.Gomersall CD, Tai DY, Loo S. Expanding ICU facilities in an epidemic: recommendations based on experience from the SARS epidemic in Hong Kong and Singapore. Intensive Care Med. 2006;32:1004–1013. doi: 10.1007/s00134-006-0134-5. [DOI] [PubMed] [Google Scholar]
- 94.Wong JEL, Leo YS, Tan CC. COVID-19 in Singapore-current experience: critical global issues that require attention and action. JAMA. 2020 doi: 10.1001/jama.2020.2467. published online Feb 20. [DOI] [PubMed] [Google Scholar]
- 95.Centers for Disease Control and Prevention Strategies for optimizing the supply of n95 respirators: crisis/alternate strategies. March 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.html
- 96.Hui CYT, Leung CCH, Gomersall CD. Performance of a novel non-fit-tested HEPA filtering face mask. Infect Control Hosp Epidemiol. 2017;38:1260–1261. doi: 10.1017/ice.2017.105. [DOI] [PubMed] [Google Scholar]
- 97.Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: a comparison of 2 personal protective systems. CMAJ. 2006;175:249–254. doi: 10.1503/cmaj.060094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46:579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gomersall CD, Joynt GM, Ho OM. Transmission of SARS to healthcare workers. The experience of a Hong Kong ICU. Intensive Care Med. 2006;32:564–569. doi: 10.1007/s00134-006-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pillet S, Berthelot P, Gagneux-Brunon A. Contamination of healthcare workers' mobile phones by epidemic viruses. Clin Microbiol Infect. 2016;22:456 e1–456 e6. doi: 10.1016/j.cmi.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ong SWX, Tan YK, Chia PY. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim SH, Chang SY, Sung M. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. March 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Finfection-control.html
- 104.WHO Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 25, 2020. https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125
- 105.Arabi YM, Phua J, Koh Y. Structure, organization, and delivery of critical care in Asian ICUs. Crit Care Med. 2016;44:e940–e948. doi: 10.1097/CCM.0000000000001854. [DOI] [PubMed] [Google Scholar]
- 106.Wai JK, Gomersall CD. A controlled crossover human volunteer study of the in vivo filtration efficacy of a high-efficiency particulate air-filtering oxygen mask. Am J Infect Control. 2011;39:782–784. doi: 10.1016/j.ajic.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 107.Fisher D, Wilder-Smith A. The global community needs to swiftly ramp up the response to contain COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30679-6. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Phua J, Faruq MO, Kulkarni AP. Critical care bed capacity in Asian countries and regions. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004222. published online Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38:1647–1653. doi: 10.1007/s00134-012-2627-8. [DOI] [PubMed] [Google Scholar]
- 110.Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic's front line. N Engl J Med. 2020 doi: 10.1056/NEJMp2005492. published online March 18. [DOI] [PubMed] [Google Scholar]
- 111.Murthy S, Leligdowicz A, Adhikari NK. Intensive care unit capacity in low-income countries: a systematic review. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gilbert M, Pullano G, Pinotti F. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395:871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Einav S, Hick JL, Hanfling D. Surge capacity logistics: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl):e17S–e43S. doi: 10.1378/chest.14-0734. [DOI] [PubMed] [Google Scholar]
- 114.Lee A, Cheung YSL, Joynt GM, Leung CCH, Wong WT, Gomersall CD. Are high nurse workload/staffing ratios associated with decreased survival in critically ill patients? A cohort study. Ann Intensive Care. 2017;7:46. doi: 10.1186/s13613-017-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joynt GM, Zimmerman J, Li TST, Gomersall CD. A systematic review of short courses for nonspecialist education in intensive care. J Crit Care. 2011;26:533.e1–533.e10. doi: 10.1016/j.jcrc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 116.Liew MF, Siow WT, MacLaren G, See KC. Preparing for COVID-19: early experience from an intensive care unit in Singapore. Crit Care. 2020;24:83. doi: 10.1186/s13054-020-2814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020 doi: 10.1001/jama.2020.3972. published online March 12. [DOI] [PubMed] [Google Scholar]
- 118.Xiang YT, Yang Y, Li W. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu P, Fang Y, Guan Z. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54:302–311. doi: 10.1177/070674370905400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Christian MD, Sprung CL, King MA. Triage: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl):e61S–e74S. doi: 10.1378/chest.14-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vergano M, Bertolini G, Giannini A. Clinical ethics recommendations for the allocation of intensive care treatments, in exceptional, resource-limited circumstances. March 16, 2020. http://www.siaarti.it/SiteAssets/News/COVID19%20-%20documenti%20SIAARTI/SIAARTI%20-%20Covid-19%20-%20Clinical%20Ethics%20Reccomendations.pdf [DOI] [PubMed]
- 122.National Institute for Health and Care Excellence COVID-19 rapid guideline: critical care. March 20, 2020. https://www.nice.org.uk/guidance/NG159 [PubMed]
- 123.Ranieri VM, Pettila V, Karvonen MK. Effect of intravenous interferon beta-1a on death and days free from mechanical ventilation among patients with moderate to severe acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2019.22525. published online Feb 17. [DOI] [PubMed] [Google Scholar]
- 124.The InFACT Global H1N1 Collaboration InFACT: a global critical care research response to H1N1. Lancet. 2010;375:11–13. doi: 10.1016/S0140-6736(09)61792-X. [DOI] [PubMed] [Google Scholar]
- 125.WHO A multi-centre, adaptive, randomized, double-blind, placebo-controlled clinical trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized patients. Feb 24, 2020. https://www.who.int/blueprint/priority-diseases/key-action/multicenter-adaptive-RCT-of-investigational-therapeutics-for-COVID-19.pdf?ua=1
- 126.Wu F, Zhao S, Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johansson MA, Reich NG, Meyers LA, Lipsitch M. Preprints: an underutilized mechanism to accelerate outbreak science. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huynh N, Baumann A, Loeb M. Reporting quality of the 2014 Ebola outbreak in Africa: a systematic analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.