Abstract
Although the etiology and expression of psychiatric disorders are complex, mammals show biologically preserved behavioral and neurobiological responses to valent stimuli which underlie the use of rodent models of post-traumatic stress disorder (PTSD). PTSD is a complex phenotype that is difficult to model in rodents because it is diagnosed by patient interview and influenced by both environmental and genetic factors. However, given that PTSD results from traumatic experiences, rodent models can simulate stress induction and disorder development. By manipulating stress type, intensity, duration, and frequency, preclinical models reflect core PTSD phenotypes, measured through various behavioral assays. Paradigms precipitate the disorder by applying physical, social, and psychological stressors individually or in combination. This review discusses the methods used to trigger and evaluate PTSD-like phenotypes. It highlights studies employing each stress model and evaluates their translational efficacies against DSM-5, validity criteria, and criteria proposed by Yehuda and Antelman’s commentary in 1993. This is intended to aid in paradigm selection by informing readers about rodent models, their benefits to the clinical community, challenges associated with the translational models, and opportunities for future work. To inform PTSD model validity and relevance to human psychopathology, we propose that models incorporate behavioral test batteries, individual differences, sex differences, strain and stock differences, early life stress effects, biomarkers, stringent success criteria for drug development, Research Domain Criteria, technological advances, and cross-species comparisons. We conclude that, despite the challenges, animal studies will be pivotal to advances in understanding PTSD and the neurobiology of stress.
Subject terms: Depression, Physiology
Introduction
Post-traumatic stress disorder (PTSD) is an incapacitating chronic disorder. With a 3.9% lifetime prevalence rate worldwide and a 6.4–7.8% rate in the USA, PTSD’s health burden is substantial1–5. Based on the World Mental Health Surveys, 69.7% worldwide (82.7% in the USA) reported exposure to a traumatic experience. While trauma exposure is a required criterion for PTSD diagnosis, only 5.6% worldwide (8.3% in the USA) of those who experienced trauma developed the disorder1. This is due to numerous factors, including trauma type, variation in trauma response, social support, and endogenous factors of individuals. For adults, adolescents, and children older than six years, eight diagnostic criteria, defined in the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), specify measures concerning the victim’s perception of trauma and symptoms. For children six years and younger, Criteria C and D (described below) are combined, making for seven diagnostic criteria6.
Criterion A: Exposure to actual or threatened death, serious injury, or sexual violence; one or more ways (e.g., direct experience, witnessing others, learning of close family member’s or friend’s trauma, and repeated or extreme exposure to aversive details).
Criterion B: Intrusion; one or more symptoms (e.g., nightmares, flashbacks, intrusive thoughts, and physiological reactions to trauma reminders).
Criterion C: Avoidance of trauma related stimuli; one or more symptoms (e.g., avoiding thoughts, people, places, conversations, activities, objects, or situations that arouse distressing memories).
Criterion D: Negative alterations in cognition and mood; two or more symptoms (e.g., dissociative amnesia, emotional blunting, cognitive distortion, social withdrawal, and anhedonia).
Criterion E: Alterations in arousal and reactivity; two or more symptoms (e.g., irritable and aggressive behavior, reckless or self-destructive behavior, hypervigilance, exaggerated startle response, concentration problems, and sleep disturbance).
Criterion F: Symptom duration for over one month (Criteria B, C, D, and E).
Criterion G: Functional impairment (e.g., social or occupational).
Criterion H: Disturbance not attributable to substance effects or medical conditions.
Specify whether/if: With dissociative symptoms (depersonalization, derealization) or with delayed expression (full diagnostic criteria not met for at least six months).
Pretraumatic, peritraumatic, and posttraumatic risk and prognostic factors affect the prevalence of the above symptoms.
Pretraumatic factors.
Temperamental: Childhood emotional problems by age 6 and prior mental disorders.
Environmental: Lower socioeconomic status, lower education, prior trauma, childhood adversity, cultural characteristics, lower intelligence, minority racial/ethnic status, family psychiatric history, and social support (protective).
Genetic and physiological: Female, younger age at time of trauma, and certain genotypes.
Peritraumatic factors.
Environmental: Trauma severity, perceived life threat, personal injury, interpersonal violence, dissociation, and being a perpetrator, witnessing atrocities, or killing the enemy (for military personnel).
Posttraumatic factors
Temperamental: Negative appraisals, inappropriate coping strategies, and development of acute stress disorder.
Environmental: Subsequent exposure to repeated upsetting reminders, subsequent adverse life events, financial or trauma-related losses, and social support (protective).
Like for many mental disorders, animal models play a key role in deciphering the neuropathophysiology of PTSD. Although observing humans to learn about mental disorders is effective, the major obstacles to human investigations are that PTSD is variable and seldom studied throughout disorder development. Therefore, human research focuses on populations already exposed to different uncontrolled traumatic events. Animal models overcome these barriers through the ability to longitudinally monitor PTSD development pre-trauma through post-trauma with controlled stressors. In particular, rodent models are critical to understanding PTSD induction, facilitating target identification for therapies, and testing drugs for human treatment. However, these models are simplified representations of a complex condition. PTSD is challenging to model in rodents because susceptibility, an individual’s likelihood to develop long term symptomology, is influenced by the above factors. While there is no gold standard paradigm, animal models are expected to capture PTSD symptomatology (face validity), etiology (construct validity), and treatment response (predictive validity)7. As such, Yehuda and Antelman defined five criteria for the face validity of translational models: (1) the stressor induces PTSD biological and behavioral responses, (2) responses are intensity-dependent, (3) biological alterations persist or progress over time, (4) biobehavioral alterations are bidirectional, and (5) responses have inter-individual variability caused from experience, genetics, or both8. PTSD rodent models have been previously reviewed9–15. This review offers a comprehensive assessment of stress model variants against multiple criteria with a focus on behavioral assays used for model validation. Although a comprehensive rodent PTSD model is challenging to develop, the demand for more effective PTSD treatment and prevention strategies drives further research. Therefore, various stress paradigms that replicate specific disorder aspects are utilized to understand the pathophysiology of PTSD. Through a survey of recent literature, this review discusses rodent PTSD models that utilize acute stress, evaluating their translational efficacies, advantages, and challenges. The review concludes by highlighting opportunities for future work in studying the neurobiology of PTSD.
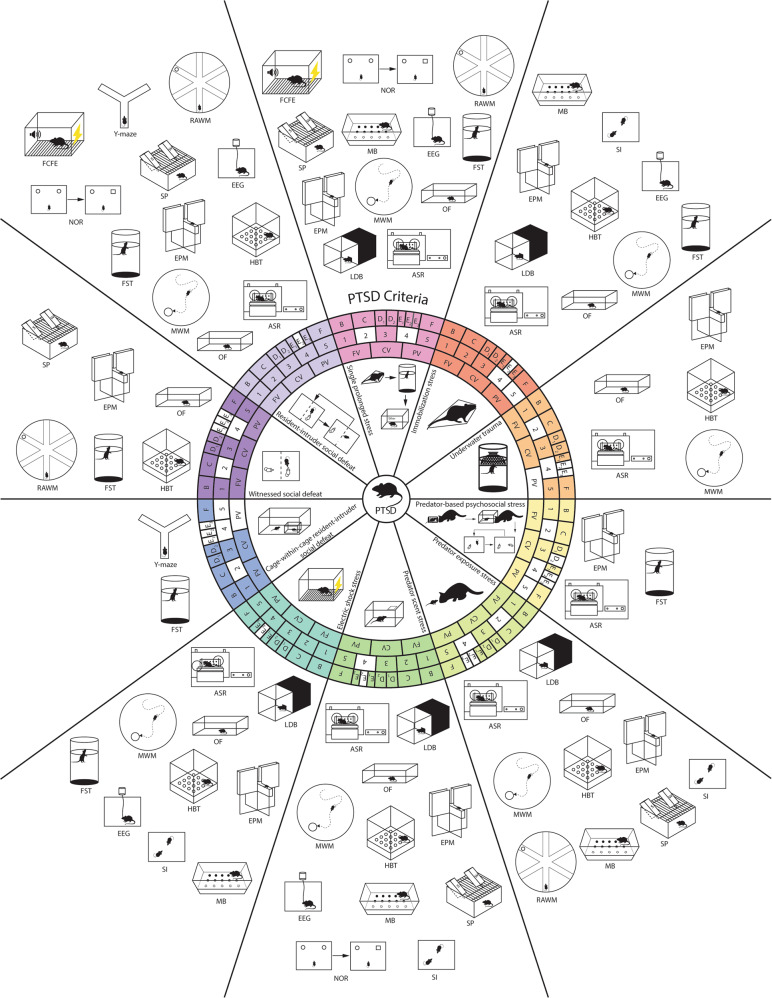
Rodent models of PTSD
Although the etiology and manifestation of psychiatric disorders are complex, mammals show biologically preserved behavioral and neurobiological responses to valent stimuli which underlie the use of rodent models of PTSD. The translational benefit of designing stress studies in rodents is supported by extensive comparative neuroanatomical studies16–18. Accordingly, the rodent models discussed reflect core PTSD phenotypes and vary by differences in stress type, intensity, duration, and frequency. Paradigms model the disorder by applying physical, social, and psychological stressors individually or in combination (Table 1). This review focuses on acute stressors and does not extensively address sub-chronic and chronic models because acute stressors are less likely to drive the symptom co-morbidity seen in other models. Behavioral tests mentioned in this survey are discussed further in Table 2. In literature, behavioral tests are frequently referred to as models. This can be misleading, and scientists should recognize their distinction, because tests elicit acute responses for measurement while models elicit pathology19.
Table 1.
Protocol and advantages/disadvantages summary of the reviewed stress paradigms.
| Animal model | Stress exposure | Control exposure | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Physical stress | |||||
| Electric shock |
1 day: −Inescapable 2–20 s, 1.0–3.0 mA foot/tail shocks delivered through a steel grid floor (shock duration and current depend on frequency) |
1 day: −Placed in shock chamber without shock; removed from vivarium; brief handling; or undisturbed |
Controllable delivery and shock parameters: current intensity, duration, number, and interstimulus interval −Reproducible context and cues −Adjustable environmental cues −Reducible stress habituation |
May result in physical injuries −Not ethologically relevant −No clear protocol distinction between fear conditioning and PTSD models |
25,28 |
| Restraint stress |
1 day: −1–2 h in a Plexiglas or wire mesh tube, restricting some locomotion |
1 day: −Removed from vivarium; brief handling; or undisturbed |
−Inexpensive |
−May result in physical injuries −Not ethologically relevant −Uncontrollable intensity |
30 |
| Immobilization stress |
1 day: −1–2 h in an immobilization bag (often a Decapicone) or attached to a wooden board with limbs and head in a prone position, preventing locomotion |
1 day: −Removed from vivarium; brief handling; or undisturbed |
−Inexpensive −More intense than restraint stress |
−May result in physical injuries −Not ethologically relevant −Uncontrollable intensity |
31,35 |
| Underwater trauma |
1 day: −1-min forced swim and 20–45 s of forced submersion in a water tank |
1 day: −1-min forced swim; removed from vivarium; brief handling; or undisturbed |
−Reproducible context −Ethologically relevant |
−May result in physical injuries −Uncontrollable intensity |
44 |
| Single prolonged stress |
1 day: −2-h restraint, 20-min forced swim, followed by diethyl ether anesthesia until loss of consciousness |
1 day: −20-min forced swim; removed from vivarium; brief handling; or undisturbed |
−Combines effects of three stressors |
−May result in physical injuries −Not ethologically relevant −Uncontrollable intensity |
50 |
| Social defeat stress | |||||
| Resident-intruder social defeat |
5–10 days: −Daily inescapable 5–10-min contact with a novel aggressive resident −24-h housing with a resident, separated by perforated screen (only sensory contact) |
5–10 days: −24-h sensory contact with novel control; 24-h housing with same control, daily 5–10-min separation by perforated screen; 30 s in a novel defeat cage without resident; 7 min in a novel clean cage; or brief handling |
−Ethologically relevant −Most intense social defeat stress |
−May result in physical injuries −Predictable stress −Uncontrollable intensity: variable resident aggression −Challenging to model female and adolescent aggression −Sub-chronic stress |
65,66 |
| Witnessed social defeat |
5–10 days: −Daily inescapable 5–15-min sensory contact during the physical social defeat of a novel intruder by a novel resident −24-h housing with a resident, separated by perforated screen (only sensory contact) |
5–10 days: −24-h sensory contact with novel control; daily 30-min sensory contact in a novel cage with novel control; or brief handling |
−Ethologically relevant −Does not result in physical injuries (psychological) |
−Predictable stress −Uncontrollable intensity: variable resident aggression −Sub-chronic stress |
67 |
| Cage-within-cage resident-intruder social defeat |
5–10 days: −Daily inescapable 6-h housing in a wire mesh cage inside a novel resident’s home cage (only sensory contact), without food/water −1–3 unpredictable 1-min physical contacts with a resident within the 6-h session |
5–10 days: −Daily inescapable 6-h housing in a wire mesh cage inside a clean larger cage without resident |
−Ethologically relevant |
−May result in physical injuries −Predictable stress −Uncontrollable intensity: variable resident aggression −Challenging to model female and adolescent aggression −Sub-chronic stress |
68 |
| Predator stress | |||||
| Predator exposure stress |
1 day: −5–60 min unprotected/protected inescapable exposure to a cat or ferret |
1 day: −5–60 min unprotected/protected inescapable exposure to context with/without toy cat; brief handling; cage transport; or undisturbed |
−Ethologically relevant |
−May result in physical injuries −Uncontrollable intensity: variable predator-rodent interaction −Expensive, may require additional facilities |
92,94 |
| Predator-based psychosocial stress |
31 days: −Days 1 (light cycle) and 11 (dark cycle): 1-h inescapable immobilization during exposure to a novel cat −Daily unstable housing conditions |
31 days: −Days 1 (light cycle) and 11 (dark cycle): 1-h inescapable exposure to context without cat; brief handling; or undisturbed |
−Combines effects of three stressors −Most intense predator stress |
−May result in physical injuries −Not ethologically relevant −Uncontrollable intensity −Chronic stress |
101 |
| Predator scent stress |
1 day: −5–10-min inescapable exposure to fox/bobcat urine or trimethylthiazoline on filter paper/cotton pad, cat-worn collar/cloth, ferret cloth, soiled cat litter, or rat scents/calls |
1 day: −5–10-min inescapable exposure to context with a neutral odor filter paper/cotton pad/ collar/cloth, unused cat litter; or undisturbed |
−Does not result in physical injuries (psychological) −Ethologically relevant: rodents use the olfactory sensory system for survival-related behaviors |
−Challenging to control olfactory cues (e.g., dosage of scent) −Olfaction is variable because it is driven by perception |
112,113 |
Table 2.
Rodent behavioral tests outlined by DSM-5 criteria for PTSD.
| Behavioral test | Description | Measures | References |
|---|---|---|---|
| Criterion B | Intrusion | ||
| Contextual and cued trauma reminders | Physiological and behavioral reactions to exposure arena | Heart rate, body temperature, systolic blood pressure, diastolic blood pressure, plasma corticosterone, time freezing, total locomotor activity | 25,224 |
| Criterion C | Avoidance | ||
| Elevated plus-maze | Elevated platform arranged in a + with four perpendicular opposite arms: two open, two closed | % time spent in open arms, % entries made into open arms | 225 |
| Elevated T-maze | Elevated platform arranged in a T with three perpendicular opposite arms: one open, two closed | Latency to leave enclosed arm, latency to enter enclosed arm | 226 |
| Elevated zero-maze | Elevated circular platform with two opposite open and closed quadrants | % time spent in open arms, frequency of head dips over edge of platform, frequency of stretched-attend postures | 227 |
| Light-dark box | Two-compartment box: one white and illuminated, the other black and dark. A small opening connects the compartments | Time spent in light, number of light entries, % distance in light, number of rears, latency to enter light, number of transitions | 228,229 |
| Open field | Square, rectangular, or circular enclosure that is bare or covered with a thin layer of bedding | Time spent in central squares, latency to enter central squares | 230 |
| Novelty suppressed feeding | Open field with novel food (e.g., sugar puffs, fruit loops) in an illuminated central area | Latency to eat | 231 |
| Hole board | Elevated and open-topped square box with evenly spaced holes in the floor | Latency to head dip, number of head dips, number of rears, time active, time spent in center | 92 |
| Modified hole board | Open field with a hole board in the middle. The hole board consists of staggered holes covered by removable lids | Latency to board entry, number of board entries, % time spent on board | 232 |
| Conditioned taste aversion | Conditioned stimulus (e.g., novel taste of saccharin) is paired with an unconditioned stimulus (e.g., lithium chloride injection that results in nausea, stress and re-stress). Recipients avoid the new taste when it is subsequently presented | Saccharin/cyclamate intake | 233 |
| Conditioned object avoidance | Conditioned stimulus (e.g., plastic prism) is paired with an unconditioned stimulus (e.g., electric footshock). In separate tests, a plastic prism or cube (novel object) is placed into the home cage | Time spent with familiar/novel object, time spent burying familiar/novel object, total locomotor activity and/or sniffing floor/walls | 234 |
| Conditioned odor avoidance | Conditioned stimulus (e.g., odor of ethanol) is paired with an unconditioned stimulus (e.g., electric footshock). Three-compartment box interconnected by guillotine doors. Center (nest) compartment: filter paper-lined Petri dish with home-cage bedding, cleaned with soapy water. Left/right compartments, counterbalanced: Petri dish with ethanol or acetate (novel neutral odor) solutions, cleaned with respective solutions. Habituation phase: rodent in nest compartment. Test phase: free exploration | Latency to first exit from nest compartment, time spent in nest/ethanol/acetate compartment | 235 |
| Conditioned odor active avoidance | Conditioned stimulus (e.g., odor of acetic acid) is paired with an unconditioned stimulus (e.g., electric footshock). Two-compartment box: start compartment cleaned with acetic acid solution, the other with ethanol solution (novel neutral odor) | % time spent in acetic acid compartment, number of rears toward the acetic acid compartment | 236 |
| Territory discrimination | Two boxes linked to a starting box with litter covering the floor. The tested animal and a novel conspecific stay in separate compartments (personal and unknown) for 24 h before testing | Time spent in personal/unknown compartment, number of entries to personal/unknown compartment | 237 |
| Criterion D | Negative alterations in cognition and mood | ||
| Morris water maze | Black circular tank filled with water in a room with visual cues on walls. The tank is conceptually divided into four quadrants and four start locations (N, S, E, W). Training sessions: animals locate or are guided to a small submerged platform (target quadrant). Probe trial: platform is removed to assess memory of its location | Time spent in target quadrant, time spent in opposite quadrant | 238 |
| Radial arm water maze | Black circular tank filled with water. The tank contains 4–12 V-shaped inserts that produce swim arms radiating from an open central area. Training trials: animals locate or are guided to a small submerged platform at the end of one arm (goal arm). Test trial: platform is removed to assess memory of its location | Number of arm entry errors | 239 |
| T-maze continuous alteration task | Elevated or enclosed platform arranged in a T with three perpendicular opposite arms: two identical goal arms and one longer start arm. Arms are separated by guillotine doors and a central partition extends into the start arm. Testing consists of one forced trial and several free-choice trials | % alternation rate | 240 |
| Novel object recognition | Open field with two objects at opposite and symmetrical corners. Habituation phase: no objects. Familiarization phase: two identical objects. Test phase: one familiar, one novel object | Time spent with familiar/novel object, number of familiar/novel object entries, discrimination index, index of global habituation, recognition index, preference index | 241 |
| Y-maze spontaneous alternation test | Platform arranged in a Y with three identical arms at 120° from each other | % alternation rate | 242 |
| Y-maze recognition memory test | Platform arranged in a Y with three identical arms at 120° from each other. Acquisition phase: access to two arms, one arm closed (novel arm). Retrieval phase: access to all arms | Time spent in familiar/novel arms, discrimination index | 243 |
| Barnes maze | Circular table with equally spaced holes around its circumference. The table’s surface is illuminated and a box is under the target hole. Acquisition trials: animals locate or are guided into the target hole. Reversal phase: target hole is moved 180° across the maze. Probe trial: target cage is removed to assess memory of its location | Latency to enter the target hole, distance traveled to the target hole, number of errors, number of entries into the former target hole during reversal/probe | 244,245 |
| Cued and contextual fear conditioning / Fear extinction | Operant chamber in a ventilated, sound-attenuated cubicle with a shock grid floor, speaker, and video camera. An unconditioned stimulus footshock is paired with a conditioned stimulus tone or context. Fear conditioning involves longer intertrial intervals and fewer stimuli than fear extinction training/testing | Time freezing | 246,247 |
| Differential contextual odor conditioning | Cue (cinnamon odor) signals reward or punishment depending on the context. Appetitive conditioning (arena 1): cue + reward (sweetened water). Aversive conditioning (arena 2): cue + conditioned cue (tone) + aversive unconditioned stimulus (electric shock). Testing (arena 3): cue | % time freezing | 248 |
| Step-through inhibitory (passive) avoidance | Two-compartment box: one white and illuminated (safe), the other black and dark (unsafe). A sliding door connects the compartments. Training phase: door closes upon entrance to the dark compartment and an unconditioned stimulus footshock is delivered. Testing phase: no shock | Latency to enter the dark compartment, time spent in dark compartment | 249 |
| Response bias probabilistic reward task | Tone discrimination training: operant testing chamber with two levers, a food receptacle, and a speaker. Rodents discriminate between two tone stimuli by pressing an associated lever. Testing: ambiguous tone durations. Correct identification of one tone (rich stimulus) is reinforced with a food pellet three times more frequently than the other tone (lean stimulus) | Response bias, discriminability, accuracy (% correct), reaction time | 250 |
| Forced swim test (Porsolt forced swim test or behavioral despair test) | Rodents are placed inside a cylindrical water tank | Time immobile | 251,252 |
| Tail suspension | Rodents are hung by their tails | Time immobile | 253 |
| Open field | Square, rectangular, or circular enclosure that is bare or covered with a thin layer of bedding | Total locomotor activity, ambulatory locomotor activity, distance traveled, frequency of rearing, frequency of sniffing | 254 |
| Flinch-jump test | Operant chamber in a ventilated, sound-attenuated cubicle with a shock grid floor, speaker, and video camera. Shock titrations are delivered in a series of ascending and descending intensities based on the rodent’s response | Flinch threshold, vocalization threshold, jump threshold | 53 |
| Hot-plate test | Rodents are placed in a glass beaker on a plate heated to a constant temperature | Latency to flinch or raise hind paws | 255,256 |
| Tail flick test | The distal third of a rodent’s tail is thermally stimulated with radiant heat (e.g., focused light from a light bulb), immersion in hot water, or direct contact with a heated surface | Latency to withdraw tail | 257 |
| von Frey test | von Frey filaments are applied to the plantar region of a rodent’s paw through a wire mesh floor. Stimulation continues in a series of ascending and descending filament forces based on the rodent’s response | Paw withdrawal threshold | 258 |
| Sucrose preference | After habituation to two drinking bottles, one water bottle is replaced with a 1–2% sucrose solution. Bottle positions (left vs. right) are alternated each day to control for side-preference bias | % sucrose preference, % sucrose intake, % water intake | 259 |
| Intracranial self-stimulation | Operant chamber in a ventilated, light-attenuated and sound-attenuated cubicle with a wheel or lever manipulandum on the wall. Bipolar electrodes are implanted into a brain region that is part of the reward system. Training: manipulandum response following noncontingent stimulation prompts an identical contingent stimulation. Testing: stimulations are delivered in a series of ascending and descending intensities based on the rodent’s response | % baseline current-intensity threshold, response latency, number of extra responses, number of time-out responses | 260,261 |
| Social interaction test | Two novel conspecific rodents (same sex, size, and age) interact freely in an open field | Time spent in active social interaction (sniffing, licking, close following, allogrooming, crawling over the partner), number of social interactions, time spent in social avoidance (escaping, keeping the partner at distance with upright forepaws) | 262 |
| Social preference/avoidance test | Open field with a wire cage centered against a wall of the arena. One trial without a rodent in the cage (no target) and one trial with a rodent in the cage (target) | Time spent in the interaction zone with the target absent/present, time spent in the two corner zones opposite the wire cage, social avoidance ratio | 263,264 |
| Partner preference test | Two boxes linked to a starting box. One female and male rodent is tethered to the rear of a goal box | Time spent with male/female, time spent in active social interaction with male/female, number of visits to male/female | 265 |
| Social approach/avoidance test | Two chambers of different widths connected by a sliding door. A large conspecific rodent (stimulus) is enclosed in a compartment of the large chamber, separated by a transparent perforated wall. The sliding door is removed after a habituation phase in the smaller chamber | % time spent in the large chamber compartment, number of entries to the large chamber | 266 |
| Three-chamber sociability and social novelty test (Crawley test) | Three-chambered box connected by sliding doors and with one wire cage in each side chamber. Habituation trial: rodent is placed in the center chamber with obstructed access to side chambers. Sociability test: novel conspecific rodent (same sex, size, and age) is placed in one of the wire cages. Social novelty test: another novel conspecific rodent is placed in the opposite cage | Time spent in empty chamber, time spent in social chamber 1, time spent in social chamber 2, social interaction ratio, social novelty preference index | 267 |
| Olfactory habituation and dishabituation test | Glass slides with drops of water (nonsocial odor) and two social odors of diluted urine from different same-sex rodents are sequentially presented into a cage. Three consecutive trials of each odor are conducted | Time spent in direct olfactory investigation (sniffing) | 268 |
| Criterion E | Alterations in arousal and reactivity | ||
| Object burying | An unfamiliar object is placed on the surface of bedding in a cage | % time spent manipulating object, % time spent burying object | 224 |
| Marble burying | 10–20 glass marbles are spaced evenly on the surface of bedding in a cage | Number of marbles buried (to 2/4 their depth), latency to dig, time spent digging, rearing count | 32,269 |
| Acoustic startle response | Cylindrical animal enclosure on a platform inside a ventilated, sound-attenuated cabinet. Speakers produce a continuous background noise and the acoustic stimuli. An acclimation period is followed by startle noise trials | Startle amplitude, average startle response | 97,270 |
| Prepulse inhibition | Cylindrical animal enclosure on a platform inside a ventilated, sound-attenuated cabinet. Speakers produce a continuous background noise and the acoustic stimuli. An acclimation period is followed by pulse-alone trials, prepulse + pulse trials, and no stimulus trials in pseudorandom order | Startle amplitude, % prepulse inhibition | 271 |
| Operant attentional set-shifting tasks | Operant chamber in a ventilated, sound-attenuated cubicle with two operandi (e.g., nose-poke holes or levers) on each side of a food dispenser and yellow lights. Correct responses are reinforced with palatable food (e.g., sucrose solution or sucrose pellets). Pretraining: rodents associate stimuli with reward. Response discrimination reversal-Response discrimination training: rodents respond on the operandum opposite to their side bias. Response discrimination reversal: rodents respond on the opposite operandum. Visual-cue-to-place set-shifting-Visual-cue discrimination training: rodents respond on the illuminated operandum. Shift to response discrimination: rodents respond on the operandum opposite of their side bias, regardless of the light’s position | Number of errors to criterion, number of errors over 20 trials, total number of trials to criterion, number of error trials, number of errors by type (pervasive, regressive, never-reinforced) | 272,273 |
Physical stressors
Physical stressors used to develop PTSD models include electric shock, underwater trauma, restraint/immobilization stress, and single prolonged stress. These stressors are advantageous for their procedural simplicity, clear symptom impact, and ease of scaling. Although widely utilized, physical stressors have not reproducibly differentiated individual variability or sex differences because most subjects display behavioral consequences. Physical stressors can also elicit injuries, pain, and/or inflammatory responses that can confound behavioral test results, including measures of motivation and movement20.
Electric shock
Electric shock is employed in animal models of anxiety, depression21, and PTSD. Inescapable and unpredictable electric shock can be administered through the animal’s tail or foot via a steel grid floor in a shock chamber. These studies combined high-intensity currents (1.0–3.0 mA) with long durations (2–20 s) to induce lasting symptoms. Currents and durations were typically larger than those applied in fear conditioning (0.5–1.5 mA, 0.5–2 s) aiming to investigate short-term fear learning. Currently, learning and trauma protocols are not clearly differentiated as there is no clear evidence for separating “nontraumatic” shocks that are within a rodent’s coping capacity from “traumatic” shocks that are beyond its coping capacity22. However, this stressor is more frequently used to study learning and memory, its initial application, rather than to model PTSD. Electric shock may be combined with contextual and/or cued trauma reminders (associative fear) through re-exposure to shock chambers and conditioned stimuli, and with neutral stimuli in a novel environment (non-associative fear). In stress-enhanced fear learning, pre-exposure to repeated intense footshocks in context A increased rodent fear response in context B when only a single shock, a less intense stressor, was administered23. This non-associative sensitization effect elevated freezing in context B for three months, even after context A extinction training, reflecting resistance to exposure therapy (extinction)24. Upon similar situational reminders, rodents exhibited behavioral responses comparable to human PTSD intrusion symptoms, such as crouching against the chamber wall, as well as increased freezing, respiratory rate, and fecal boli25–27. Since re-exposure to stressor-related environments/cues is the clinical analog of exposure therapies, the electric shock model can be studied to advance cue-based out-of-context (reminders in office) and in-context (virtual reality) therapies14. Advantages of electric shock as a PTSD model are its controllable delivery and shock parameters (current intensity, duration, number, and interstimulus interval), reproducible context and cues, adjustable environmental cues, and reducible stress habituation28. For review of electric shock, see Aliczki and Bali & Jaggi22,29.
Restraint/immobilization stress
Restraint and immobilization stresses include confining rodents in enclosed chambers to limit movement for an extended period of time. Restraint stress is generally conducted by placing animals in Plexiglas or wire mesh tubes30. Immobilization stress is achieved by either placing animals into rodent immobilization bags (often Decapicones)31–33 or attaching the animal’s limbs and head in a prone position to wooden boards34,35. Currently, restraint and immobilization protocols are not clearly differentiated and the two terms are often used interchangeably36. While restraint and immobilization are similar in that they are stressors driven by the limitation of motion, it is important to make the distinction between the two as restraint does not prevent, but only restricts, movement of the rodent’s limbs, body, and head. Accordingly, immobilization elicits stronger responses than restraint37. There are no comparative studies favoring any one acute stress protocol. Furthermore, only two studies comparing the sex-dependent effects of immobilization stress have been reported. One study found that the attribution of incentive salience toward food reward location (goal-tracking) was increased in Long-Evans males exposed to acute immobilization stress, whereas a bias toward food reward-associated cues (sign-tracking) was increased in females. This suggests sex-differences in the failure to appropriately assign motivational value as related to amotivation/anergia and anhedonia38. Another study, in agreement with one subgroup of human endocrine responses39,40, reported that immobilization stress evokes long-term hypothalamic-pituitary-adrenal (HPA) axis desensitization following re-exposure to the same (homotypic) stressor and sensitization to novel (heterotypic) stressors35. This effect was greater in female Sprague-Dawley rats41. Studies also noted delayed expression (10 days post-stress) of avoidance behavior and changes in dendritic spine density in the basolateral amygdala, a stress responsive brain region31. For review of restraint and immobilization stress, see Buynitsky42.
Underwater trauma
Underwater trauma, or submersion stress, involves 1 min of forced swim followed by 30 s of forced submersion in a water tank. Exposed rats demonstrated immediate and persistent (7–30 days post-stress) increased arousal in acoustic startle response (ASR) and anxiety-like behavior in the elevated-plus maze (EPM) tests compared to control rats that swam without submersion43–45. Learning deficits in Morris water maze (MWM) spatial memory tasks, observed three weeks after trauma exposure, demonstrated the stressor’s lasting negative effects on cognition44. However, interpretation of MWM results can be confounded by the re-exposure to water, a reminder of underwater trauma, that may influence task performance. Decreased plasma basal corticosterone (CORT) levels were found seven days after stress, indicating a lasting depression of HPA axis signaling following the trauma43. Based on successive performance in EPM and ASR, cut-off behavioral criteria demonstrated that the prevalence of maladaptive response to stress dropped from 91.6% of exposed Sprague-Dawley rats on day 1, the acute phase, to a constant rate of 41.6% by day 7 through day 30. Concomitantly, the prevalence of well-adapted rats rose from 0% to 25% over days 1–3045. This temporal pattern reaffirms that animals display an individual variation in response similar to humans46. That is, the initially large affected proportion of the exposed population decreases steadily with time as many individuals show a tendency toward symptom improvement. Some may present acute stress disorder and a minority develop PTSD. Behavioral profiling, validated by immunohistochemical assessments, uncovered three separate stress response phenotypes: an anxious, fear-based group (38%), a co-morbid, fear-anhedonic group (15%), and an exposed-unaffected group (47%). In accordance with the high anxiety trait of the posttraumatic depression model47, enhanced pretrauma freezing correlated with posttrauma saccharin preference among fear-anhedonic phenotype rats, predicting anhedonia one month after exposure48. Although its intensity cannot be easily controlled, advantages of underwater trauma as a PTSD model are its reproducible context and ethologically relevant stress.
Single prolonged stress
Single prolonged stress (SPS) involves sequential administration of three stressors—2-h restraint, 20-min forced swim, and diethyl ether anesthesia—with a 7-day or 14-day undisturbed sensitization before testing. According to time-dependent sensitization studies, the undisturbed incubation period is necessary for PTSD-like symptom manifestation49. Interestingly, studies observed most behavioral and cellular changes seven days after SPS or re-exposure, indicating time-dependent and experience-dependent sensitization50. Consistent with findings in PTSD patients, neuronal apoptosis and autophagy dysregulation in the hippocampus, amygdala, and prefrontal cortex appeared one day after stress, suggesting that morphological changes precede behavioral alterations51. Combination of the three stressors is required for the PTSD phenotype, as combinations of any two does not induce all effects observed in the SPS paradigm52. As in underwater trauma, interpretation of forced swim and MWM test results is confounded by the re-exposure to water. Although age effects on SPS susceptibility have not been evaluated to date, maternal separation was found to strengthen adult SPS-induced increases in anxiety and contextual fear53. Early life exposure to SPS caused anxiety-like and depression-like behavior at postnatal day 32 (human early-adolescence), anxiety-like behavior at postnatal day 60 (human late-adolescence), followed by depression-like behavior (stress-susceptible) or no behavioral deficits (stress-resilient) at postnatal day 90 (human adulthood), suggesting that adaptations such as behavioral and cognitive switching occur at postnatal day 6054. The two studies of sex differences in fear extinction retention following SPS report conflicting results, with one observing no effect55 and the other noting deficits56. The finding that female rats express fear by darting rather than freezing indicates that freezing alone may be misleading and motivates reinterpretation of female rodent fear conditioning studies57. SPS increased the latency of pair-housed Sprague-Dawley females to approach a novel rat in the social preference/avoidance test, implying an anxious phenotype, but decreased the latency of single housed females, implying social support seeking58. The dexamethasone suppression test revealed an exaggerated negative feedback control of the HPA axis in SPS-exposed Sprague-Dawley males, but not in females59. SPS lacks ecological validity and its stressor intensity cannot be modified. However, an advantage of SPS as a PTSD model is its combination of stressors to produce a synergistic effect. For an extensive review of SPS, see Lisieski, Souza, and Yamamoto60–62.
Social and psychological stressors
Social and psychological stressors include social defeat and predator stress. Advantageous for their ecological validity and relevance to interpersonal assault traumas63, these stressors are widely utilized in differentiating individual variability. While predator stress can be conducted on both sexes, social defeat is challenging to model in female rodents because they tend not to defend territories with aggressive behavior. When using direct exposure to a resident rodent or predator, reproducibility of the stressor can be challenging due to variation in rodent and predator aggression. If the social and psychological nature of these stressors causes physical injuries, behavioral measures may then reflect physical rather than neurological driven effects.
Social defeat stress
Social defeat stress (SDS) is used in rodent models of depression64 and PTSD. There are three variations of SDS: resident-intruder, witnessed social defeat stress (trauma witness or vicarious social defeat), and cage-within-cage resident-intruder. While in the resident-intruder paradigm, an experimental rodent (intruder) is exposed daily (5–10 days; 5–10 min per day) to a novel dominant conspecific (resident)65,66, witnessed social defeat stress is induced by daily inescapable sensory contact with novel rodents undergoing physical social defeat67. In both procedures, subordination may then be reinforced through 24 h of sensory (visual, olfactory, and auditory) contact with the resident, separated by perforated screen. The cage-within-cage resident-intruder paradigm introduces rodents to sensory contact with novel residents for 6-h sessions that include one to three 1-min unpredictable physical contact periods68. Since territoriality is established in sufficient living space and enhanced in the presence of a sexual partner and sexual experience, the intruder is either introduced to an individually housed resident or replaces a cohabitating partner in the resident cage69. Intruders with lower body weights than residents are frequently used to guarantee intruder defeat66. Aggressive behaviors, different between male and female rodents, are characterized by attacking, pushing, aggressive grooming, chasing, pinning, and upright or lateral dominant postures70. Depression models employ chronic SDS, with stress repeated for 10 days to five weeks. It has, however, been suggested that chronic SDS may not only be a depression model, but may be more appropriately used to simulate the depression, anxiety, and social avoidance dimensions of PTSD71.
Male rodents exposed to resident-intruder social defeat manifest a persistent behavioral syndrome. The social preference/avoidance test categorized 50–70% of exposed C57BL/6 J mice as susceptible to stress65,72. Interestingly, social rank predicted this individual variability through a link between response strategy and outcome, showing that dominant mice were more susceptible than subordinate mice73. Preexisting individual differences in the peripheral immune system also predicted stress susceptibility74. Morning CORT levels showed no changes among groups at day 11, but decreased in susceptible mice and increased in resilient mice at day 39 after social defeat72. In another study, stress resilience was associated with the emergence of the gram-positive bacteria Bifidobacterium. This finding is relevant to clinical applications as gut microbiota dysbiosis is found in patients with PTSD75. Many studies have also shown that environmental enrichment promotes adaptive behavior and brain function. Enriched environmental housing before social defeat conferred stress resiliency. Further investigation, through lesion of the infralimbic cortex, illustrated that the ventromedial prefrontal cortex was involved in the acquisition of protective effects76. Extending this observation, optogenetic modulation of neuron projections to/from the ventromedial prefrontal cortex77, ventral tegmental area78,79, nucleus accumbens80, and dorsal raphe nucleus81, key nodes of PTSD circuitry, exerted antidepressant-like effects in susceptible mice.
Female-female agoniztic behaviors show low levels of direct attack in resident-intruder confrontations compared to inter-male aggression, limiting studies exploring the effects of social defeat in female rodents70. Furthermore, male behavior towards female versus male intruders is not similarly motivated and results in fewer attacks. As such, resident-intruder pairings are frequently same-sex conspecifics. The rarity of female aggressive behavior has been addressed through using lactating dams82, aggressive species such as California mice and Syrian hamsters83,84, mediobasal hypothalamic lesions85, as well as testosterone treatment in neonatal86 and ovariectomized adult female rodents87,88. Female CD-1 mice exposed to lactating dams displayed elevated anxiety in the EPM, with more pronounced effects two weeks, but not 2 h, after social defeat71. Although qualitatively different from male SDS, recent studies have induced male aggression toward females through application of male odorants on females89 and chemogenetic activation of the ventrolateral subdivision of the ventromedial hypothalamus in males90. The limited number of female rodent studies using similar species, methodologies, and behavioral tests complicates result comparison. In addition, cross-sex comparison is contingent upon proof of equivalent attack magnitude toward males and females. While care is taken to minimize severe injuries, behavioral measures may be compromised when the social nature of resident-intruder and cage-within-cage resident-intruder paradigms is mixed with the stress of physical injury. SDS is also limited by the potential for adaptation to its predictable repeated stress. Although the stressor type cannot be varied, its predictability could be reduced by exposing rodents to novel residents at different times each day for different stress durations. For review of social defeat, see Hammels91.
Predator stress
Predator stress models, prioritizing ecological validity, expose rodents to species-relevant predators or their scents. Variations of this stressor include predator exposure stress, predator-based psychosocial stress (PPS), and predator scent stress (PSS).
In the predator exposure stress model, rodents are acclimated for 5 min in an inescapable exposure environment, followed by a 5–60 min exposure to an unprotected/protected (subject is free/caged) cat92,93 or ferret94. While initial acute cat exposure studies assessed overall behavior through tests of risk assessment, anxiety, and arousal92, later reports also found avoidance of trauma-reminder in the open field test95, with spatial memory retention impairments in the MWM and radial-arm water maze93,96. Like SPS, exposure enhanced dexamethasone suppression of CORT in Sprague-Dawley males, but not in females59. In addition, acute ferret exposure in Sprague-Dawley rats produced sensorimotor gating abnormalities in prepulse inhibition94. Out of the exposed Sprague-Dawley rats, 25.3% developed PTSD-like behavioral and endocrine dysregulation96. Sex differences in vulnerability were test specific, noted in EPM, open field, and ASR measures. Sex differences were also identified in hole-board and light-dark box measures which were unaffected by exposure95,97. Persistent behavioral changes, some lasting at least three weeks after exposure, corresponded to changes in the amygdala, medial prefrontal cortex, and hippocampus. These structural and molecular changes are consistent with brain regions involved in PTSD93,95,98,99. However, given predator exposure’s variable cat-rodent interaction and the challenge of controlling cat aggression, both cat and rodent behavior must be assessed. For review of predator exposure, see100.
The PPS model, composed of acute and chronic components, combines 1 h of acute immobilization during novel cat exposure on days one (during the light cycle) and 11 (during the dark cycle), with 31 days of chronic unstable housing conditions to produce a risk factor synergistic effect. Following observations that rodents directed their postures away from cats, providing them with an element of control over confrontations, immobilization was included in this model as an analog to the sense of helplessness prominent in PTSD. Repeated cat exposure was included to (1) apply to people who develop PTSD only after multiple traumas; (2) mimic intrusive trauma reminders by forcing rodents to re-experience the original stress; (3) mimic the unpredictability of re-experiences (light or dark cycle); and (4) augment stress-induced changes. Further increasing symptom prevalence, daily randomized housing conditions were included to mimic chronic mild stress and lack of social support101. Three weeks after the second predator exposure, all stressed Sprague-Dawley rats exhibited PTSD-like sequelae. PPS also caused reduced growth rate, thymus weight, and basal glucocorticoid levels, as well as increased adrenal gland weight and physiological reactivity to an acute stressor101–103. Some effects were still observed more than four months after stress onset104. Neurotransmitter changes, such as greater norepinephrine and reduced serotonin levels in the hippocampus and prefrontal cortex, are in concert with human PTSD research105–107. This paradigm was also shown to induce sex-dependent cardiovascular alterations with notable male and ovariectomized female increases in myocardial sensitivity to ischemic injury. Stressed female rats displayed anxiety-like behavior in the EPM and open field, irrespective of estrous stage or ovariectomy condition. However, stressed female rats did not demonstrate physiological effects other than a reduced growth rate108,109. For review of PPS, see Zoladz110.
The PSS model involves a 5 to 15-min inescapable exposure to fox/bobcat urine111, soiled cat litter112, a cat-worn collar/cloth113, a ferret cloth114, or trimethylthiazoline (TMT), a synthetic compound isolated from fox feces115. Similar findings in successive ferret and cat cloth exposures confirmed failure of stress-habituation114,116. Moreover, different amounts of TMT117 and sizes of cloth impregnated with cat scents118 elicited fear-related behavior in a dose-dependent manner. After application of median split criteria, the incidence of susceptible TMT-exposed Sprague-Dawley rats ranged from 14 to 21.8%119. Similarly, after cut-off behavioral criteria, extreme behavioral responses (PTSD-like) to soiled cat litter were observed among 50% of Lewis, 10% of Fischer F344, and 25% of Sprague–Dawley rats112. Although sensitive to strain differences, cut-off behavioral criteria did not distinguish PTSD prevalence among male and female Sprague-Dawley rats120. In both sexes, extreme behavioral response rats displayed inhibited cardiac autonomic system habituation and recovery after exposure121. Early life stress increased vulnerability to cardiac autonomic dysfunction, and blunted basal CORT pulse amplitude predicted post-exposure PTSD susceptibility122,123. Avoidance of bobcat urine-paired context predicted post-stress thermal hyperalgesia in Wistar rats with high stress reactivity111. Interestingly, resting-state functional magnetic resonance imaging (rsfMRI) noninvasively detected prolonged neuroadaptation within the amygdala-medial prefrontal cortex circuit in Long-Evans rats exposed to cat collar113. Another study found that pre-existing functional connectivity in olfactory and stress-related neural circuits might predispose animals to differential stress responses, linked to PTSD susceptibility, during fox urine exposure. Susceptible rats exhibited less freezing, but greater avoidance of fox urine, and displayed a prolonged CORT response, as well as higher anxiety long after exposure124. These findings indicate the importance of analyzing behavior during exposure. A high-dose of CORT injected subcutaneously 1 h before soiled cat litter exposure reduced the prevalence of extreme behavioral responses from 50% to 8% in Lewis rats, indicating that elevated CORT levels before acute stress prevent later stress effects112. Analogous to clinical trial results, high-dose CORT administered 1 h after stress reduced the prevalence of PTSD-like rats, reversing extreme behavioral disruptions along with molecular and morphological measures in the hippocampal dentate gyrus. This evidence supports the use of high-dose CORT in trauma care and suggests that there is a treatment “window of opportunity” early after trauma125. Combined, these observations indicate the protective effects of glucocorticoids against the development of PTSD. Sleep deprivation for 6 h during the first resting phase after PSS attenuated PTSD-like behaviors in EPM, ASR, and hippocampal expression of glucocorticoid receptors, demonstrating an avenue for secondary prevention of stress-related clinical disorders126,127. Since predator scent can impregnate testing rooms and influence control animals, careful handling and stressing under a fume hood is advised. Olfactory cues such as dosage of a scent are challenging to control because olfaction is variable and driven by perception. An advantage of PSS as a PTSD model is its ecologically relevant stress, as rodents use the olfactory sensory system for survival-related behaviors128. For review of PSS, see Cohen and Staples129,130.
Translational studies of PTSD
Established stress paradigms that induce PTSD-like behavioral and biological phenotypes are available (Table 1). However, since stressor severity varies, stressful experiences that are within a rodent’s coping capacity should be distinguished from traumatic experiences that are beyond its coping capacity. Rodent behavioral tests, mimicking the tests conducted in humans, are used to assess stress effects and allow researchers to make inferences about rodent psychology (Table 2). Although all stress models produce lasting general anxiety or depression effects, variety in behavior robustness makes each paradigm effective at targeting specific constructs. Behavioral tests and neurobiological changes are used to evaluate an animal model’s representation of the human disorder, satisfying DSM-5 PTSD symptom clusters (Tables 3, 4), validity criteria (Table 5), and Yehuda and Antelman’s criteria (Table 6). Unlike for the DSM-5, no criteria have been established to assess how well an animal model meets validity or Yehuda and Antelman’s criteria. Gaps in literature were addressed as no one model has been proven to satisfy all criteria.
Table 3.
Evaluation of reviewed animal models against DSM-5 criteria for PTSD–effects in males6.
| Criterion B: intrusion | Criterion C: avoidance of trauma related stimuli | Criterion D: negative alterations in cognition and mood | Criterion E: alterations in arousal and reactivity | Criterion F: lasting symptoms | ||||
|---|---|---|---|---|---|---|---|---|
| Physiological reactions to trauma reminders | Increased avoidance | Cognitive alterations | Mood alterations | Increased arousal | Concentration problems | Sleep disturbance | Symptoms present | |
|
Physical stress Electric shock |
Contextual and cued reminders25,224 | EPM, OF, CODA, conditioned object avoidance, NSF, modified hole board28,234,235,274 | MWM275 | Tail flick, SAAT, SI, FST27,28,266 | Object burying, ASR25,224 | EEG234 | >Month24,276 | |
| Immobilization stress | Contextual reminder34,35 | EPM, OF, hole board, mirror chamber, LDB31,34,277–279 | FCFE, MWM, NOR, Y-maze spontaneous alteration test34,280,281 | Incentive salience, SP, FST38,278,282 | ASR, MB32,283 | EEG, EMG, EOG33 | >1 Week31 | |
| Underwater trauma | Contextual reminder48 | EPM, hole board, OF43,284,285 | DCOC, MWM44,248 | SP48 | ASR45 | > 3 Weeks44 | ||
| Single prolonged stress | Cued reminder286 | Conditioned taste aversion, OF, EPM, elevated T-maze, LDB, cliff avoidance53,54,287–290 | NOR, FCFE, RAWM, MWM51,52,54,291,292 | Three-chamber sociability and social novelty test, flinch-jump, hot-plate, SP, FST, von Frey53,287,292–294 | ASR, MB295,296 | Set-shifting273 | EEG, EMG297 | >Month54,289,293 |
|
Social defeat stress Resident-intruder social defeat |
Contextual reminder20 | EPM, LDB, EZM72,73,298 | Response bias probabilistic reward task, MWM, Barnes maze, step-through inhibitory avoidance, FCFE, RAWM, NOR, Y-maze recognition memory test, T-maze continuous alteration task299–306 | SPAT, three-chamber sociability test, intracranial self-stimulation, SAAT, SP, FST, OF, tail suspension, olfactory habituation-dishabituation76,264,266,307–310 | ASR311 | EEG, EMG312 | >Month72 | |
| Witnessed social defeat | Contextual reminder20 | EPM, LDB67,313 | RAWM313 | Three-chamber sociability test, OF, SPAT, SP, FST67,313,314 | >Month315 | |||
| Cage-within-cage resident-intruder social defeat | Contextual reminder68,316 | Partition test68 | Y-maze spontaneous alteration test242 | FST, tail suspension317 | >Month68 | |||
|
Predator stress Predator exposure stress |
Cued reminder95 | EPM, hole board, LDB, elevated T-maze, OF92,95,97,318 | MWM, RAWM93,96 | SI92 | ASR, PPI94,97 | >3 Weeks92 | ||
| Predator-based psychosocial stress | Contextual and cued reminders102 | EPM101 | NOR101 | ASR101 | > Month104 | |||
| Predator scent stress | Contextual reminder111,119,319 | EPM, territory discrimination, LDB, OF, hole board112,116,129,320,321 | MWM, DCOC, NOR115,248,322 | Partner preference, Hargreaves test, SI111,116,129 | PPI, MB112,124 | EEG, EMG, LFP319 | >Month122 | |
Table 4.
Evaluation of reviewed animal models against DSM-5 criteria for PTSD—Effects in females6.
| Criterion B: intrusion | Criterion C: avoidance of trauma related stimuli | Criterion D: negative alterations in cognition and mood | Criterion E: alterations in arousal and reactivity | Criterion F: lasting symptoms | ||||
|---|---|---|---|---|---|---|---|---|
| Physiological reactions to trauma reminders | Increased avoidance | Cognitive alterations | Mood alterations | Increased arousal | Concentration problems | Sleep disturbance | Symptoms present | |
|
Physical stress Electric shock |
Contextual reminder26,276,323 | EPM, LDB276,323 | SI323 | > Month276,323 | ||||
| Immobilization stress | Contextual reminder41 | Incentive salience38 | >1 Week41 | |||||
| Underwater trauma | ||||||||
| Single prolonged stress | EPM324 | FCFE56 | FST, SP, SPAT, von Frey58,294,324 | > 1 Week56,294 | ||||
|
Social defeat stress Resident-intruder social defeat |
EPM71,89,90 | SP, FST, SPAT, olfactory habituation-dishabituation89,310,325,326 | ASR326 | >Month310 | ||||
| Witnessed social defeat | Contextual reminder327 | EPM328 | SP, FST, SAAT, tail suspension327,328 | >1 Week328 | ||||
| Cage-within-cage resident-intruder social defeat | ||||||||
|
Predator stress Predator exposure stress |
Contextual and cued reminders95,329 | EPM, OF, LDB95,97 | RAWM330 | ASR97 | >2 Weeks95 | |||
| Predator-based psychosocial stress | EPM, OF108 | |||||||
| Predator scent stress | EPM120,331 | MWM120 | ASR120,331 | >1 Week120 | ||||
Table 5.
Evaluation of reviewed animal models against validity criteria for animal models of human mental disorders7.
| Face validity: represents symptoms of the human disorder | Construct validity: represents the cellular and molecular mechanisms in the human patient (homologous constructs) | Predictive validity: demonstrates successful use of effective pharmacological treatments in human patients, discriminates between effective/ineffective treatments | |
|---|---|---|---|
|
Physical stress Electric shock |
234,323 | 274 | Paroxetine, fluoxetine, sertraline28,332,333 |
| Immobilization stress | 34,41 | 31,33,42 | Venlafaxine334 |
| Underwater trauma | 44,45 | 48 | |
| Single prolonged stress | 53,324 | 61,62 | Paroxetine335,336 |
|
Social defeat stress Resident-intruder social defeat |
89,307 | 72,91,312 | Fluoxetine, sertraline264,337 |
| Witnessed social defeat | 313,328 | 74 | Fluoxetine67 |
| Cage-within-cage resident-intruder social defeat | 68 | 338 | |
|
Predator stress Predator exposure stress |
96,97 | 98,339 | Fluoxetine340 |
| Predator-based psychosocial stress | 101 | 110 | Sertraline341 |
| Predator scent stress | 129,130 | 113,331 | Sertraline342 |
Table 6.
Evaluation of reviewed animal models against Yehuda and Antelman’s criteria for animal models of PTSD8.
| Induces biological and behavioral sequelae of PTSD | Produces PTSD-like sequelae in a dose-dependent manner | Produces biological alterations that persist or become more pronounced over time | Induces bidirectional biobehavioral alterations | Produces interindividual variability in response | |
|---|---|---|---|---|---|
|
Physical stress Electric shock |
234,323 | 28,224 | 323 | 332 | 28 |
| Immobilization stress | 34,41 | 30,343 | 344 | ||
| Underwater trauma | 44,45 | 345 | 45 | ||
| Single prolonged stress | 53,324 | 61,62 | 54,286 | ||
|
Social defeat stress Resident-intruder social defeat |
89,307 | 346 | 72 | 326 | 73,90 |
| Witnessed social defeat | 313,328 | 20,67 | 74 | ||
| Cage-within-cage resident-intruder social defeat | 68 | 242 | |||
|
Predator stress Predator exposure stress |
96,97 | 347 | 96,99 | ||
| Predator-based psychosocial stress | 101 | 110 | |||
| Predator scent stress | 129,130 | 118 | 348 | 112,124 |
Many behavioral tests were developed and validated in rats, and only later were adapted for mice. Rats were traditionally the species of choice in preclinical research for their performance in operant tasks and their larger size that facilitates application of invasive techniques, as well as toxicity tests of compounds. Mice, on the other hand, are advantageous for their ease of genetic modification, breeding, and group housing. Following the increasing use of mice, behavioral tests were translated into mouse versions, but with mixed success131,132. Therefore, the utility of mouse models is contingent on the availability of more behavioral tests that are optimized for use in mice.
More specific behavioral tests should be incorporated into batteries to address gaps in PTSD research. Since sucrose preference does not distinguish between affective response and motivation, more specific tests are needed such as facial reactivity analysis and measurements of reward-related ultrasonic vocalizations133,134. Other possible attention assessments that have not been conducted in the context of PTSD include the signal detection test with blank trials for sustained attention and multiple-choice serial reaction time test for sustained and selective attention135. The resident-intruder test has been used to assess irritability and aggression. However, this test is not listed in Table 2 because it incorporates the unclear stress effects of novel social interaction and could influence later behavioral time points.
One unrealized strength of rodent models of PTSD is in the discovery, development, and testing of effective pharmacological treatments. Historically, pharmaceutical companies did not invest in innovative drug discovery programs because modifying approved medications, particularly selective serotonin reuptake inhibitor (SSRI) antidepressants, was less time-consuming and more profitable136. While the SSRIs sertraline (Zoloft) and paroxetine (Paxil, Paxil CR, Brisdelle, and Pexeva), are the only medications approved for PTSD by the Food and Drug Administration (FDA) to date, off-label pharmacological treatments include fluoxetine (Prozac, Prozac Weekly, and Sarafem) and venlafaxine (Effexor XR)137–140. These drugs were tested in PTSD patients because of their effectiveness in treating depression. Many provide relief in patients, yet no medications have been approved to promote resilience. Therefore, pharmacotherapy is currently recommended as an adjunctive or next-line treatment to trauma-focused psychotherapy139,141. Interestingly, the two medications that are indicated for PTSD treatment, sertraline and paroxetine, and the two most promising drug candidates, ketamine and 3,4-methylenedioxymethamphetamine-assisted psychotherapy, did not emerge from basic research142. In addition, only some drugs have been tested in rodent models to study their targets and mechanisms of action (Table 5). One explanation for their mechanism is that medications may enhance psychotherapy’s efficacy to engage biological targets associated with recovery or resilience142. Alternatively, medications may distinguish the PTSD biological subtypes that respond to their targets143,144. Now that rodent models of PTSD are more validated and have been refined over the last 20 years, their use could elucidate human psychopathology and further reveal mechanisms driving recovery.
In rodents, as in humans, different traumas can cause different PTSD-like symptoms. Each with their advantages and disadvantages, there is no single model that serves all purposes. All reviewed animal models have phenomenological similarities to PTSD and satisfy face validity. Peripheral biologic correlates of PTSD have been evaluated for all stressors, generally meeting construct validity. For example, there are strong hypotheses regarding the neural mechanisms involved in PTSD, such as hyperactivity of the corticotropin-releasing hormone system, that have been verified in rodents145,146. However, because the biological basis of PTSD is not well understood, work needs to be done to define clear conditions for construct validity. Although preclinical research on traumatic stress has not yet resulted in drastic improvements in PTSD treatments, most models test pharmacological treatment options, providing them with predictive validity and potential for use in the development of clinical therapy. Given these results, more well-studied models such as electric shock and single prolonged stress may fit more criteria, but lack demonstration of multiplex behavioral outcomes in individual animals. While DSM-5, validity, and Yehuda and Antelman’s criteria each capture some of PTSD’s complexity, consideration of all three may be used to improve categorization of a rodent model’s trauma effects and inform stress paradigm selection. More accurately capturing the disorder in any individual model is critical, but overly limiting the types of models studied may be counterproductive in developing a comprehensive understanding of such a variable disorder.
Challenges
Symptom comorbidity
Symptom comorbidity complicates PTSD diagnosis and treatment. Although rodent models are useful for symptom analysis, there are limitations to generalizing stress in rodents to PTSD in humans since clinical diagnosis heavily relies on a patient interview rather than quantitative diagnostic measures. Individuals with PTSD are 80% more likely than those without PTSD to have symptom comorbidity. Symptoms such as avoidance, anhedonia, and exaggerated startle response may overlap with symptoms of other mental illnesses (e.g., depressive, bipolar, anxiety, or substance use disorders), making it difficult to attribute them to a specific PTSD model6. This creates challenges in distinguishing animal models of PTSD from other psychiatric illness models. The criteria used in the development and study of PTSD models will continue to evolve as scientific understanding of PTSD grows and the DSM is updated. Nevertheless, longitudinal studies are used to develop animal models with relevant PTSD symptom domains and to test causal factors in symptom development.
Anthropomorphism
The anthropomorphizing that can occur when uniquely human characteristics are attributed to animals is a danger of animal models and complicates their translatability. Many diagnostic criteria of mental disorders are subjective, involving thoughts, memories, and their interpretations. Such criteria as intrusive thoughts, emotional blunting, and cognitive distortion cannot be measured in animals without the danger of excessive anthropomorphism. Therefore, research is limited to observable behaviors with quantifiable measures, many of which are detailed in Table 2. However, even these measures have limitations, since rodent behavioral tests often do not directly correlate to human tests. The urgency to perform “translational” work can cause research groups to exaggerate a treatment’s effects and scientific foundation, ultimately hindering treatment development. Prolonged exposure, for example, is a cognitive behavioral therapy that only works for some patients and may not be based on fear extinction, yet extinction learning is so robust and explainable in animals that it is used to justify the treatment’s mechanism147. From the “translational” research opportunity and ease of modeling fear acquisition and extinction, this single aspect of PTSD has become more significant than it is clinically148. Anthropomorphizing can also result in data overinterpretation. For example, rodents that demonstrate escape or freezing behavior have been referred to as anxious rather than, more appropriately, exhibiting anxiety-related or anxiety-like behavior149. Further, this anthropomorphizing can lead to misleading interpretations of behavior, as it has been shown that behaviors like freezing are not simple quantitative measures of fear or anxiety as they are often utilized150. Therefore, to optimize the study of PTSD, rodent models should be empirically based, without anthropomorphic inference.
Non-standardized experimental designs
Experimental designs are often not standardized across laboratories, complicating inter-laboratory comparison and result replication. Studies differ in their methods of trauma exposure, behavioral test batteries, and data analysis. This variability is exacerbated through differences in experimenter sex, rodent sex, species/strain/stock, age, housing and noise conditions (e.g., stressed and control animals caged separately/together, individually ventilated cages), food, incubation time between trauma and testing, acclimation time to the testing room and its light intensity, location of trauma and testing rooms, transportation to the testing room, subject location during trauma and testing of other subjects, specific tests conducted and their order, test duration, and test timing (light or dark cycle). All of these factors have a non-trivial effect on results and illustrate the often-underestimated importance of attention to detail.
Insufficient reporting
Many publications have insufficiently reported experimental details, rendering them not fit-for-purpose and limiting their value to researchers, doctors, and policymakers151. This challenge is compounded by concerns of positive publication bias, which favors the reporting of positive results (data supporting an alternative hypothesis) and can lead to spurious claims, wasteful experimentation, and reduced meta-analysis validity. Some journals (e.g., Journal of Negative Results in BioMedicine, Journal of Pharmaceutical Negative Results, Nature Negative Results section) compensate publication bias by exclusively publishing negative results. Favoring negative results, however, can also introduce bias152. Therefore, publishing criteria should focus on a study’s design and statistical power, regardless of its outcome. Guidelines for planning and reporting animal experiments (e.g., Gold Standard Publication Checklist, Animal Research: Reporting of In Vivo Experiments, and Planning Research and Experimental Procedures on Animals: Recommendations for Excellence) have been established, but have not yet been widely adopted153–155. The lack of reproducibility in preclinical research may contribute to the failure of drugs in clinical trials156.
Ethical complications
Effective rodent models of PTSD utilize stressors that exceed the animal’s homeostatic regulatory capacity, but ethical complications remain. Studies are expected to minimize rodent suffering, improve human health, and advance general scientific knowledge. Since exposure to a significant trauma is required, emphasizing reduction in animal distress is counterproductive in PTSD models. This may result in minimizing exposure type or severity such that the model’s efficacy and scientific value is compromised10,157. Predator scent stress, for example, developed in response to safeguards limiting predator-prey interactions, is less severe and may not as effectively induce the PTSD-like phenotype as predator exposure. Therefore, stressful experiences that are within a rodent’s coping capacity should be distinguished from traumatic experiences that are beyond its coping capacity. Investigators are encouraged to collaborate with ethical committees to ensure that their selected exposure is relevant to PTSD and maximizes the probability of clinically significant findings.
Future directions
While it is apparent that well-established PTSD animal models are available, refining behavioral and neurobiological understanding of these models is still needed. Future directions include study of (1) individual variability and behavioral test batteries; (2) sex differences; (3) strain and stock differences; (4) early life stress effects; (5) biomarkers; as well as use of (6) stringent success criteria for drug development; (7) Research Domain Criteria (RDoC); (8) technological advances; and (9) cross-species comparisons.
Individual variability and behavioral test batteries
Many studies regard groups of animals under a condition as homogeneous, overlooking individual variability, and rely on a small number of tests with singular time points. Some models (e.g., immobilization stress, predator-based psychosocial stress) create a ubiquitous response in which all exposed animals present PTSD-like symptoms without the response variability inherent to human PTSD. Trends in variability have been described as “bidirectional” (Table 6), but with different meanings across research groups. Bidirectional expression has been used to mean both the concurrence or alternation between increased (i.e., intrusive re-experiencing, hyperarousal) and decreased (i.e., avoidance, numbing) responsiveness across different behavioral tests or subgroupings of animals within an experimental group. For example, a model could satisfy Yehuda and Antelman’s bidirectional criterion by showing that a group of animals display hyperarousal in ASR and anhedonia in sucrose preference or that stressed subgroups show opposite responses to the same stimulus. When only a subgroup is expected to display long-term PTSD-like phenotypes, analyzing group level statistics based on exposed and control groups can lead to weaker statistical power and would make results harder to translate. While animal behavioral studies often employ few tests post-exposure to detect symptoms, test batteries covering multiple diagnostic criteria can achieve a more reliable profiling of individual animals. With multiple tests and time points, behavioral changes measured before and after stress could provide insight into the direct effects of stress over time, controlling for subject variability. Interpreting results in a manner similar to human diagnosis (e.g., cut-off behavioral criteria96, median split criteria119, behavioral profiling48, or clustering methods) then allows for the classification of susceptible and resilient trauma-exposed individuals. By researchers considering individual variability, treatments can be evaluated on whether they decrease the proportion of maladapted animals instead of their effect on symptom severity.
Sex differences
Sex differences are another area of variability that has been poorly studied (Table 4). PTSD was introduced into the DSM-III following the high prevalence of male Vietnam veterans seeking treatment for posttrauma symptoms158. All psychiatric disorders display sex differences, yet male rodents have been predominantly studied given the historical perspective of past publications159. Over-reliance on male animals and cells conceals sex differences and may contribute to the increase of non-translationality in preclinical research156. Due to the push to address sex differences, in consideration of females’ higher prevalence of PTSD and adverse drug reactions, preclinical studies are now studying sex differences more readily6,160. Noting discrepancies in diagnostic criteria between the DSM-IV, DSM-5, and 10th Revision of the International Classification of Diseases (ICD-10), studies suggest that sex differences in symptom endorsement may make females more likely to meet DSM-5 and ICD-10 criteria for PTSD. Females were more likely to endorse intrusive thoughts, avoidance of external reminders, emotional blunting, exaggerated startle response, and sleep disturbance, while males were more likely to endorse aggressive behavior symptoms161,162. Sex differences in coping strategies, possibly mediated by differential endocrine responses (e.g., cortisol and oxytocin), have also been proposed163. Furthermore, human and rodent findings have suggested that differences in interactions between gonadal hormones testosterone or estrogen with the HPA axis or hippocampus contribute to the increased disorder risk among females164–166. Evaluations of behavioral effects of estrous cycle phase in female rodents observed trait and strain differences in test performance stability across phases, concluding that choices of behavioral paradigms, testing conditions, and genetic backgrounds are critical to controlling for hormonal effects167. However, hormone variation should be monitored as it could confound data interpretation or behavioral phenotyping performed blind to estrous cycle phase. In addition, studies should be designed with an adequate sample size to homogenize the estrous phase distribution. Further research is needed to understand the effects of hormones on PTSD and to develop hormone-sensitive treatments.
Strain and stock differences
Similar to humans, rodent genetic background modulates stress susceptibility and phenotype variation, yet strain and stock differences have not been sufficiently characterized. Inbred strains of mice (e.g., C57BL/6, BALB/c) and rats (e.g., Lewis, Fischer), genetically homogenous, are used to minimize intrastrain differences. In contrast, outbred stocks of mice (e.g., Swiss, CD-1) and rats (e.g., Long-Evans, Sprague-Dawley), genetically heterogenous, are used to model the variability of human populations. The C57Bl/6 strain is reported to be more resilient to early life stress compared to strains such as the anxious BALB/c strain168,169. In addition, strain and stock differences in baseline behavior and psychotropic treatment response have been identified170,171. Therefore, rodent strain/stock, vendor, and colony should be considered across experiments within a study to better control experimental conditions and increase result reproducibility. Further research may reveal phenotypic behavioral differences with underlying genetic bases relevant to PTSD susceptibility.
Early life stress effects
Although early life stress, such as childhood abuse, increases vulnerability to psychiatric disorders later in life, this relationship is not clearly understood172. To better understand this relationship, early life stress is modeled in rodents through interventions in mother-pup interaction time periods (e.g., early handling for 3–15 min, repeated maternal separation for 1–8 h, single maternal separation for 24 h), the quantity and/or quality of maternal care (e.g., repeated cross-fostering, naturally occurring differences in maternal care, impoverished postnatal environment), and pharmacology (e.g., postnatal glucocorticoids, postnatal lipopolysaccharide)173,174. Since the brain’s neurocircuitry matures from adolescence to early adulthood, trauma response may change over time175. In addition to effects of early life stress on brain and behavior over the developmental course, open research areas include identification of gene-environment interactions, epigenetic processes, sensitive periods, methods for disorder prevention or reversal, and beneficial effects. Different hypotheses exist to explain the interaction between early and adult stress in mental disorder susceptibility. Whereas multiple hit models, such as the cumulative-(diathesis-)stress hypothesis, state that disease risk increases with more adversity, the match/mismatch hypothesis states that risk deceases when early and adult environments match (stress inoculation), but increases upon mismatch176,177. Similarly, the differential susceptibility to environmental influence (“for-better-and-for-worse”) hypothesis states that individuals carrying alleles that are reactive to the environment display heightened sensitivity to disease risk in stressful contexts, but also to beneficial effects in supportive conditions178. Integrating hypotheses and considering individual differences in early programming sensitivity, the three-hit concept of vulnerability and resilience incorporates genetic predisposition, early life environment, and later life environment179,180. Additional assessments across the life span should be conducted to evaluate these hypotheses and clarify the relationship between maltreatment in early life and mental illness in adulthood.
Biomarkers
Potential PTSD biomarkers have been identified, but the topic remains underdeveloped as there are no biomarkers of susceptibility, disease, or therapy in clinical use. An individual’s susceptibility to developing PTSD can be evaluated before trauma through risk markers for primary prevention, to prevent exposure, and early after trauma through risk markers for secondary prevention, to begin preventative therapy before symptom expression. Reported primary risk markers in humans include: (1) high fear response to a single 35% CO2 inhalation challenge181; (2) gene × environment interactions (e.g., FK506 binding protein 5 (FKBP5) gene × childhood trauma)182; (3) HPA-axis regulators183; (4) frequent nightmares (possibly related to the hampered fear extinction memory consolidation associated with REM sleep)184; and (5) low hippocampal volume185. Few secondary risk markers, apart from increased heart rate186, have been reported because of sample size and prospective design limitations. Prominent disease diagnostic markers include HPA-axis dysregulation187, FKBP5 expression188, sympathetic nervous system hyperreactivity189, low hippocampal volume190, and high amygdala activity191. Use of biomarkers as inclusion or exclusion criteria for patient enrollment into clinical studies increases a drug candidate’s likelihood of FDA approval192. However, few PTSD therapy stratification/selection markers, used to predict treatment responses and stratify patients by therapy responder types, or progress markers, used to monitor therapy responses, have been determined. The field’s attempts to identify PTSD biomarkers has not resulted in treatments, so the aim that animal models will provide targets that will inform treatments has not yet been accomplished142. This is because biomarkers are currently limited by their interdependence, contribution to other disorders, inconsistent effect distribution, and small effect146. It is, therefore, important to determine how to use animal models to successfully translate findings. With multimodal study designs, identification of biomarkers that are homologous across species and play similar roles in the model and clinical condition will compliment behavioral measures to inform clinical trials of diagnostic and treatment targets. For reviews of PTSD biomarkers, see DePierro and Schmidt142,193.
Stringent success criteria
Implementing stringent success criteria into the preclinical stage of testing may improve upon the traditional drug development approach of expediting treatments into clinical trials to collect human data as early as possible. The unmet need for PTSD treatments may have reduced regulatory restrictions to their development and allowed drugs with suboptimal preclinical validation to enter clinical trials. Consequently, central nervous system drug candidates now have the second lowest success rate among therapeutic areas and take longer to bring to market192,194. The majority of drugs that showed initial promise in animal models failed in phases II and III of clinical development due to a lack of efficacy195–198. For example, Bionomics’ BNC210, a negative allosteric modulator of the α7 nicotinic acetylcholine receptor, failed to significantly improve symptoms compared to placebo in a Phase II PTSD trial because of insufficient exposure (bioavailability)199. Target site exposure, target binding, and expression of functional pharmacological activity (the “three pillars of survival”) all determine the likelihood of candidate survival in Phase II and progression to Phase III trials200. This underscores the importance of acquiring preclinical pharmacokinetic and pharmacodynamic data to test drug mechanisms and success. In response to the high unsustainable cost of clinical trials, pharmaceutical companies, including Bionomics, have downscaled, outsourced, or closed their neuroscience research programs201–203. The FDA estimates that a 10% improvement in failure prediction before clinical trials could save $100 million in development costs per drug204. Stringent success criteria would improve failure prediction by requiring more preclinical validation, thus shifting risk from the clinical to the preclinical stage. This may then decrease drug development costs, times to market, and prices to the public151.
Research domain criteria
PTSD is a heterogeneous disorder and the field is shifting focus from generalized diagnostic criteria toward targeting specific symptom domains. In parallel to disease-specific DSM-5 criteria, the US National Institute of Mental Health’s RDoC is a framework for subject characterization based on degrees of dysfunctions in psychological/biological systems. Therefore, the RDoC assumes that there is a dimensional continuum between health and pathology. The RDoC matrix consists of dimensional psychological constructs (or concepts), grouped into higher-level domains of human behavior and functioning, and distinguished by the units of analysis used to investigate constructs. Continuously evolving from more construct validity and utility data, the RDoC matrix currently has five domains (negative valence, positive valence, cognitive, social processes, arousal and regulatory systems) and eight units of analysis (genes, molecules, cells, circuits, physiology, behavior, self-report, paradigms)205. Additional domains that are relevant to PTSD and psychiatric research have been proposed, namely stress and emotional regulation and maintenance of consciousness206. Intended to inform future diagnostic systems, RDoC integrates multiple measures for a comprehensive understanding of matrix constructs and, consequently, their corresponding symptoms. Investigators should align studies to RDoC as it encourages scientists to seek beyond observable results to neurobiological measures. Using a more stringent criteria for what we consider as a rodent model based on the criteria discussed can improve the translatability of results.
Technological advances
Utilizing technological advances in neuroimaging and genetic manipulation in PTSD research is promising as it allows for longitudinal assessments and investigations of circuits governing PTSD etiology. In clinical practice, neuroimaging, specifically structural, is mostly used to exclude brain pathology from the cause of psychiatric symptoms. Since diagnosis is subjective, quantitative clinical measures are needed to validate the current qualitative criteria and give preclinical researchers confidence that they are modeling PTSD, rather than genetic variability. Recent technological advances may be employed to achieve this goal and improve information flow between the two fields. fMRI, for example, measures blood-oxygenation-level dependent signal as an indirect indicator of neuronal activity. It is a powerful noninvasive measure of systems-level brain function and has a higher spatial resolution than other noninvasive modalities such as electroencephalography. MRI has been used to demonstrate altered fractional anisotropy, focal neural activity, functional connectivity, as well as focal atrophy of gray matter207. Awake rat rsfMRI avoids confounding factors from anesthetics and can be correlated with behavioral measures to study functional networks implicated in stress response, discover new regions of interest, and define endophenotypes of susceptibility208. Since fMRI can be applied to both animals and humans, findings may be translated across species and the conservation of neural circuits may be compared209. Stress signaling pathways can also be selectively stimulated via optogenetics and chemogenetics (or pharmacogenetics). Optogenetics enables manipulation of neuronal activity in stress circuits through activation of light-sensitive proteins, such as microbial opsins, that are selectively inserted into intact living mammalian neurons. Using this technology, researchers can control cell firing patterns with high tissue specificity and temporal precision to study the causal relationship between circuits and behavior in PTSD210. In chemogenetics, instead of with light, cell populations and neural circuits are modulated by the injection of biologically inert ligands that activate engineered receptors. Designer Receptors Exclusively Activated by Designer Drugs are widely used in chemogenetics and act through G-protein coupled signaling cascades. Chemogenetics has a lower temporal resolution, but is relatively noninvasive compared to optogenetics, which requires fiber-optic implants211. Integrated with fMRI, these technologies can map the downstream effects of local manipulations on global brain activity212–214. The neurobiology of stress can also be studied through diffusion tensor imaging215, magnetoencephalography216, calcium-based fiber photometry217,218, and molecular fMRI214. Combined with fMRI, these multimodal methods allow researchers to collect complementary and multi-dimensional information across spatiotemporal scales on the same animal. Implementation of these tools in studies of PTSD animal models and integration with established techniques in behavioral analysis, electrophysiology, and pharmacology can unveil progressive changes after stress exposure and susceptibility endophenotypes. While reliance on qualitative assessment is still standard, the shift towards multimodal methods will allow for the determination of quantitative PTSD biomarkers that are predictive of qualitative criteria used to assess PTSD prevalence. This goal will likely require increased collaboration between clinical and preclinical researchers to be achieved.
Cross-species comparisons
Cross-species comparisons of stress responses over the phylogenetic scale, ranging from rodents to animals in the genus Mustela (e.g., weasels, ferrets, minks) and nonhuman primates, are required to truly understand the preservation of PTSD features. Neurobiological results from rodent studies have already shown relevance in other species, but with mild stress models219,220. For example, both rodent and primate research has identified brain regions, such as the bed nucleus of the stria terminalis (BNST), amygdala, prefrontal cortex, cingulate cortex, and hippocampus, that regulate stress-related behavioral mechanisms and are dysfunctional in PTSD patients146. The BNST, in particular, has been investigated for its role in fear memory neural circuitry221. In mice, optogenetic photoinhibition of neurons projecting between the BNST and ventral tegmental area during electric shock stress decreased freezing in the exposure context and closed arm entries in the EPM222. Consequently, the BNST was investigated in rhesus monkeys and its intrinsic functional connectivity with the central nucleus of the amygdala was found to be correlated with behavioral responses to no-eye-contact human intruder stress223. A possible translational roadmap for studying PTSD is to combine mechanistic and behavioral measures from rodents with multimodal neuroimaging measures from nonhuman primates and humans. Cross-species comparisons such as these show promise to elucidating the clinical relevance of stress-induced cellular, molecular, and microstructural discoveries in rodents.
Conclusion
Since a variety of traumas, producing specific symptoms, can trigger PTSD, a single rodent model will not perfectly capture a human individual’s disorder complexity. While individual animal models can replicate lack of coping and ineffective adaptation to stress, this does not necessarily represent coverage of human PTSD variability. This shortcoming is in part rooted in the modeling of general trauma conditions such as life threats, while modeling effects of particular stimuli (e.g., burning odors, sounds of gunfire) or cognitive assessments (e.g., helplessness, self-blame, coping strategy) of the general trauma condition is not further explored. As studies of animal models continue to improve, incorporation of experimental designs that can highlight the effect of specific features of a trauma or drive specific coping strategies will improve animal models’ representation of human PTSD variability. Similarly, more objective measures are needed to evaluate a rodent’s inability to self-regulate, also a cognitive aspect of responses to anxiogenic stimuli, to assess aspects of human PTSD variability further in preclinical studies. This, along with the availability of physical, social, and psychological rodent stressors, varying in length and intensity, will improve translation of results to comparable clinical cases. Each model’s strengths and limitations, reviewed here, should be considered for fit-for-purpose stress paradigm selection, hypothesis testing, and experimental design. For example, consideration of the DSM-5 risk and prognostic factors for PTSD in animal models (e.g., trauma reminders, female subjects, and early life stress) are likely to better replicate the human condition in patients with similar features. When evaluating paradigms, judging rodent models based on one set of criteria could be misleading because each focuses on different constructs. DSM-5 criteria, designed to facilitate diagnosis of human mental disorders, are used to compare stressors by their representation of PTSD symptomatology. Expanding the comparison to etiology and treatment response, validity criteria are used for animal models of human mental disorders. Yehuda and Antelman’s criteria, more specific, are used for animal models of PTSD and include factors that are essential to the induction of PTSD-like phenotypes. Therefore, all three criteria should be superimposed for the optimal reflection of a stressor’s relevance to PTSD (Fig. 1). As only a minority of individuals who experience traumatic event(s) develop the disorder, it is important to investigate animals’ individual variability in stress response and subsequent development of PTSD-like behaviors to distinguish susceptibility. It is also important to remember that the goal of this research is to expand knowledge and improve the clinical treatment of PTSD. Despite the large financial investment and the thousands of papers published annually, no treatments have been approved for PTSD since Paxil in 2000. With the incorporation of the directions discussed, rodent models of PTSD can provide more utility and translational impact as they lead to shifts in practice.
Fig. 1. Superimposition of reviewed animal models against validity criteria for animal models of human mental disorders (inner circle), Yehuda and Antelman’s criteria for animal models of PTSD (middle circle), and DSM-5 criteria for PTSD (outer circle).
Validity criteria abbreviations: face validity (FV), construct validity (CV), predictive validity (PV). Yehuda and Antelman’s criteria abbreviations: biological and behavioral sequelae of PTSD (1), dose-dependent (2), lasting symptoms (3), bidirectional (4), interindividual variability (5). DSM-5 criteria abbreviations: physiological reactions to trauma reminders (B), increased avoidance (C), cognitive alterations (D1), mood alterations (D2), increased arousal (E1), concentration problems (E2), sleep disturbance (E3), lasting symptoms (F). Behavioral test abbreviations: elevated plus maze (EPM), open field (OF), light-dark box (LDB), Morris water maze (MWM), radial arm water maze (RAWM), fear conditioning / fear extinction (FCFE), novel object recognition (NOR), social interaction (SI), forced swim test (FST), sucrose preference (SP), marble burying (MB), acoustic startle response (ASR), electroencephalogram (EEG). White: not reported. Shaded: reported.
Acknowledgements
This study was partially supported by National Institute of Neurological Disorders and Stroke Grant R01NS085200 (PI: Nanyin Zhang, PhD) and National Institute of Mental Health Grant R01MH098003 and RF1MH114224 (PI: Nanyin Zhang, PhD).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koenen KC, et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017;47:2260–2274. doi: 10.1017/S0033291717000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. JAMA Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Anxiety Disord. 2011;25:456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alegría M, et al. Prevalence, risk, and correlates of posttraumatic stress disorder across ethnic and racial minority groups in the United States. Med. Care. 2013;51:1114–1123. doi: 10.1097/MLR.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. JAMA Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5TM 5th edn. (American Psychiatric Publishing, Inc., 2013).
- 7.Willner P. Validation criteria for animal models of human mental disorders: learned helplessness as a paradigm case. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1986;10:677–690. doi: 10.1016/0278-5846(86)90051-5. [DOI] [PubMed] [Google Scholar]
- 8.Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of postraumatic stress disorder. Biol. Psychiatry. 1993;33:479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- 9.Deslauriers J, Toth M, Der-Avakian A, Risbrough VB. Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol. Psychiatry. 2018;83:895–907. doi: 10.1016/j.biopsych.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter-Levin, G., Stork, O. & Schmidt, M. V. Animal models of PTSD: a challenge to be met. Mol. Psychiatry10.1038/s41380-018-0272-5 (2018). [DOI] [PMC free article] [PubMed]
- 11.Borghans, B. Animal models for posttraumatic stress disorder: an overview of what is used in research. World J. Psychiatry10.5498/wjp.v5.i4.387 (2015). [DOI] [PMC free article] [PubMed]
- 12.Török B, Sipos E, Pivac N, Zelena D. Modelling posttraumatic stress disorders in animals. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;90:117–133. doi: 10.1016/j.pnpbp.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, L. et al. Updates in PTSD Animal Models Characterization. In Psychiatric Disorders: Methods and Protocols (ed. Kobeissy, F. H.) 331–344 (Springer New York, 2019). [DOI] [PubMed]
- 14.Flandreau, E. I. & Toth, M. Animal Models of PTSD: A Critical Review. 1–22 (Springer Berlin Heidelberg, 2017). [DOI] [PubMed]
- 15.Cohen H, Matar MA, Zohar J. Maintaining the clinical relevance of animal models in translational studies of post-traumatic stress disorder. ILAR J. 2014;55:233–245. doi: 10.1093/ilar/ilu006. [DOI] [PubMed] [Google Scholar]
- 16.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clancy B, Finlay BL, Darlington RB, Anand KJS. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos, G. & Franklin, K. B. J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates (Academic Press, 2001).
- 19.Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model?: advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Finnell, J. E. et al. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS ONE12, 10.1371/journal.pone.0172868 (2017). [DOI] [PMC free article] [PubMed]
- 21.Seligman MEP. Learned helplessness. Annu. Reviiew Med. 1972;23:407–412. doi: 10.1146/annurev.me.23.020172.002203. [DOI] [PubMed] [Google Scholar]
- 22.Aliczki, M. & Haller, J. Electric shock as model of post-traumatic stress disorder in rodents. In Comprehensive Guide to Post-Traumatic Stress Disorder (eds. Martin, C. R., Preedy, V. R. & Patel, V. B.) 1–16 (Springer International Publishing, 2014).
- 23.Rau, V., DeCola, J. P. & Fanselow, M. S. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev.10.1016/j.neubiorev.2005.04.010 (2005). [DOI] [PubMed]
- 24.Rau, V. & Fanselow, M. S. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress10.1080/10253890802137320 (2009). [DOI] [PubMed]
- 25.Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol. Psychiatry. 1996;39:129–134. doi: 10.1016/0006-3223(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 26.Diehl, L. A. et al. Long lasting sex-specific effects upon behavior and S100b levels after maternal separation and exposure to a model of post-traumatic stress disorder in rats. Brain Res. 10.1016/j.brainres.2007.01.084 (2007). [DOI] [PubMed]
- 27.Corral-Frias, N. S., Lahood, R. P., Edelman-Vogelsang, K. E., French, E. D. & Fellous, J. M. Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology10.1038/npp.2012.189 (2013). [DOI] [PMC free article] [PubMed]
- 28.Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J. Psychiatr. Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Bali, A. & Jaggi, A. S. Electric foot shock stress: a useful tool in neuropsychiatric studies. Rev. Neurosci. 10.1515/revneuro-2015-0015 (2015). [DOI] [PubMed]
- 30.Gameiro, G. H. et al. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol. Behav. 10.1016/j.physbeh.2005.12.007 (2006). [DOI] [PubMed]
- 31.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl Acad. Sci. USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kedia, S. & Chattarji, S. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J. Neurosci. Methods10.1016/j.jneumeth.2014.06.012 (2014). [DOI] [PubMed]
- 33.Hegde P, et al. Stress-induced changes in sleep and associated neuronal activity in rat hippocampus and amygdala. Neuroscience. 2008;153:20–30. doi: 10.1016/j.neuroscience.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Andero, R. et al. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci. Transl. Med. 10.1126/scitranslmed.3005656 (2013). [DOI] [PMC free article] [PubMed]
- 35.Armario A, Valles A, Dal-Zotto S, Marquez C, Belda X. A single exposure to severe stressors causes long-term desensitization of the physiological response to homotopic stressor. Stress. 2004;7:157. doi: 10.1080/10253890400010721. [DOI] [PubMed] [Google Scholar]
- 36.Şahin, Z. et al. [An evaluation of the effects of two chronic immobilization stress protocols On depression/anxiety-related behavior in male rats]. Acıbadem Üniversitesi Sağlık Bilim. Derg. 10, 10.31067/0.2019.186 (2019).
- 37.Bali A, Jaggi AS. Preclinical experimental stress studies: protocols, assessment and comparison. Eur. J. Pharmacol. 2015;746:282–292. doi: 10.1016/j.ejphar.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Fuentes, S. et al. Sex-dependent impact of early-life stress and adult immobilization in the attribution of incentive salience in rats. PLoS ONE10.1371/journal.pone.0190044 (2018). [DOI] [PMC free article] [PubMed]
- 39.Yehuda, R., Yang, R. K., Buchsbaum, M. S. & Golier, J. A. Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology10.1016/j.psyneuen.2005.10.007 (2006). [DOI] [PubMed]
- 40.Zaba M, et al. Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology. 2015;55:102–115. doi: 10.1016/j.psyneuen.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Gagliano H, Nadal R, Armario A. Sex differences in the long-lasting effects of a single exposure to immobilization stress in rats. Horm. Behav. 2014;66:793–801. doi: 10.1016/j.yhbeh.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Buynitsky, T. & Mostofsky, D. I. Restraint stress in biobehavioral research: Recent developments. Neuroscience and Biobehavioral Reviews10.1016/j.neubiorev.2009.05.004 (2009). [DOI] [PubMed]
- 43.Moore NLT, Gauchan S, Genovese RF. Differential severity of anxiogenic effects resulting from a brief swim or underwater trauma in adolescent male rats. Pharmacol. Biochem. Behav. 2012;102:264–268. doi: 10.1016/j.pbb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Richter-Levin G. Acute and long-term behavioral correlates of underwater trauma—potential relevance to stress and post-stress syndromes. Psychiatry Res. 1998;79:73–83. doi: 10.1016/s0165-1781(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 45.Cohen H, et al. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- 46.Yehuda R, McFarlane A, Shalev A. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol. Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- 47.Sandi, C. & Richter-Levin, G. From high anxiety trait to depression: a neurocognitive hypothesis. Trends Neurosci. 10.1016/j.tins.2009.02.004 (2009). [DOI] [PubMed]
- 48.Ritov G, Boltyansky B, Richter-Levin G. A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction. Mol. Psychiatry. 2016;21:630–641. doi: 10.1038/mp.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey, B. H., Naciti, C., Brand, L. & Stein, D. J. Endocrine, cognitive and hippocampal/cortical 5HT1A/2Areceptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res. 10.1016/S0006-8993(03)03033-6 (2003). [DOI] [PubMed]
- 50.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 51.Zheng S, Han F, Shi Y, Wen L, Han D. Single-prolonged-stress induced changes in autophagy-related proteins beclin-1, LC3, and p62 in the medial prefrontal cortex of rats with post-traumatic stress disorder. J. Mol. Neurosci. 2017;62:43–54. doi: 10.1007/s12031-017-0909-x. [DOI] [PubMed] [Google Scholar]
- 52.Knox D, et al. Single prolonged stress disrupts retention of extinguished fear in rats. Learn. Mem. 2012;19:43–49. doi: 10.1101/lm.024356.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imanaka, A., Morinobu, S., Toki, S. & Yamawaki, S. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav. Brain Res. 10.1016/j.bbr.2006.06.012 (2006). [DOI] [PubMed]
- 54.Liu H, Atrooz F, Salvi A, Salim S. Behavioral and cognitive impact of early life stress: Insights from an animal model. Prog. Neuro-Psychopharmacology. Biol. Psychiatry. 2017;78:88–95. doi: 10.1016/j.pnpbp.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keller, S. M., Schreiber, W. B., Staib, J. M. & Knox, D. Sex differences in the single prolonged stress model. Behav. Brain Res. 10.1016/j.bbr.2015.02.034 (2015). [DOI] [PMC free article] [PubMed]
- 56.Zer-Aviv, T. M. & Akirav, I. Sex differences in hippocampal response to endocannabinoids after exposure to severe stress. Hippocampus10.1002/hipo.22577 (2016). [DOI] [PubMed]
- 57.Gruene, T. M., Flick, K., Stefano, A., Shea, S. D. & Shansky, R. M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife10.7554/eLife.11352.001 (2015). [DOI] [PMC free article] [PubMed]
- 58.Pooley, A. E. et al. Sex differences in the traumatic stress response: The role of adult gonadal hormones. Biol. Sex Differ. 10.1186/s13293-018-0192-8 (2018). [DOI] [PMC free article] [PubMed]
- 59.Pooley, A. E. et al. Sex differences in the traumatic stress response: PTSD symptoms in women recapitulated in female rats. Biol. Sex Differ. 10.1186/s13293-018-0191-9 (2018). [DOI] [PMC free article] [PubMed]
- 60.Yamamoto, S. et al. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depression and Anxiety10.1002/da.20629 (2009). [DOI] [PubMed]
- 61.Souza, R. R., Noble, L. J. & McIntyre, C. K. Using the single prolonged stress model to examine the pathophysiology of PTSD. Front. Pharmacol. 10.3389/fphar.2017.00615 (2017). [DOI] [PMC free article] [PubMed]
- 62.Lisieski, M. J., Eagle, A. L., Conti, A. C., Liberzon, I. & Perrine, S. A. Single-prolonged stress: a review of two decades of progress in a rodent model of post-traumatic stress disorder. Front. Psychiatry9, 10.3389/fpsyt.2018.00196 (2018). [DOI] [PMC free article] [PubMed]
- 63.Daskalakis, N. P. & Yehuda, R. Principles for developing animal models of military PTSD. Eur. J. Psychotraumatol. 5, 10.3402/ejpt.v5.23825 (2014). [DOI] [PMC free article] [PubMed]
- 64.Krishnan, V. & Nestler, E. J. Animal models of depression: molecular perspectives. In Current Topics in Behavioral Neurosciences. Vol. 7, 121–147 (Springer‐Verlag Berlin, 2011). [DOI] [PMC free article] [PubMed]
- 65.Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koolhaas, J. M. et al. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J. Vis. Exp. 77, e4367 (2013). [DOI] [PMC free article] [PubMed]
- 67.Warren BL, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol. Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hammamieh R, et al. Murine model of repeated exposures to conspecific trained aggressors simulates features of post-traumatic stress disorder. Behav. Brain Res. 2012;235:55–66. doi: 10.1016/j.bbr.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 69.Albert, D. J., Dyson, E. M., Walsh, M. L. & Petrovic, D. M. Cohabitation with a female activates testosterone-dependent social aggression in male rats independently of changes in serum testosterone concentration. Physiol. Behav. 10.1016/0031-9384(88)90054-6 (1988). [DOI] [PubMed]
- 70.Clipperton Allen AE, Cragg CL, Wood AJ, Pfaff DW, Choleris E. Agonistic behavior in males and females: Effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 2010;35:1008–1022. doi: 10.1016/j.psyneuen.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobson-Pick, S., Audet, M. C., McQuaid, R. J., Kalvapalle, R. & Anisman, H. Social agonistic distress in male and female mice: changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PLoS ONE10.1371/journal.pone.0060133 (2013). [DOI] [PMC free article] [PubMed]
- 72.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Larrieu T, et al. Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following Chronic social defeat stress. Curr. Biol. 2017;27:2202–2210.e4. doi: 10.1016/j.cub.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 74.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016;22:589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lehmann, M. L. & Herkenham, M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J. Neurosci. 10.1523/jneurosci.0577-11.2011 (2011). [DOI] [PMC free article] [PubMed]
- 77.Covington, H. E. et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 10.1523/JNEUROSCI.1731-10.2010 (2010). [DOI] [PMC free article] [PubMed]
- 78.Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature10.1038/nature11713 (2013). [DOI] [PMC free article] [PubMed]
- 79.Wook Koo, J. et al. Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress–induced depressive behaviors. Biol. Psychiatry10.1016/j.biopsych.2015.12.009 (2016). [DOI] [PMC free article] [PubMed]
- 80.Francis, T. C. et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. Psychiatry10.1016/j.biopsych.2014.07.021 (2015). [DOI] [PMC free article] [PubMed]
- 81.Challis, C., Beck, S. G. & Berton, O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front. Behav. Neurosci. 10.3389/fnbeh.2014.00043 (2014). [DOI] [PMC free article] [PubMed]
- 82.Bosch, O. J. Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos. Trans. Royal Soc. B10.1098/rstb.2013.0085 (2013). [DOI] [PMC free article] [PubMed]
- 83.Payne, A. P. & Swanson, H. H. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation Area. Behaviour10.1163/156853970X00402 (1970). [PubMed]
- 84.Davis, E. S. & Marler, C. A. c-fos changes following an aggressive encounter in female California mice: a synthesis of behavior, hormone changes and neural activity. Neuroscience10.1016/j.neuroscience.2004.05.034 (2004). [DOI] [PubMed]
- 85.Haller, J., Fuchs, E., Halász, J. & Makara, G. B. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res. Bull. 10.1016/S0361-9230(99)00087-8 (1999). [DOI] [PubMed]
- 86.Bronson, F. H. & Desjardins, C. Aggression in adult mice: modification by neonatal injections of gonadal hormones. Science (80-.). 10.1126/science.161.3842.705 (1968). [DOI] [PubMed]
- 87.Simon NG, Masters DB. Activation of male-typical aggression by testosterone but not its metabolites in C57BL/6J female mice. Physiol. Behav. 1987;41:405–407. doi: 10.1016/0031-9384(87)90073-4. [DOI] [PubMed] [Google Scholar]
- 88.Whalen, R. E. & Johnson, F. Aggression in adult female mice: chronic testosterone treatment induces attack against olfactory bulbectomized male and lactating female mice. Physiol. Behav. 10.1016/0031-9384(88)90092-3 (1988). [DOI] [PubMed]
- 89.Harris, A. Z. et al. A Novel method for chronic social defeat stress in female mice. Neuropsychopharmacology10.1038/npp.2017.259 (2017). [DOI] [PMC free article] [PubMed]
- 90.Takahashi A, et al. Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 2017;7:12838. doi: 10.1038/s41598-017-12811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hammels, C. et al. Defeat stress in rodents: from behavior to molecules. Neurosci. Biobehav. Rev.10.1016/j.neubiorev.2015.10.006 (2015). [DOI] [PubMed]
- 92.Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol. Behav. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 93.Zoladz PR, et al. Differential expression of molecular markers of synaptic plasticity in the hippocampus, prefrontal cortex, and amygdala in response to spatial learning, predator exposure, and stress-induced amnesia. Hippocampus. 2012;22:577–589. doi: 10.1002/hipo.20922. [DOI] [PubMed] [Google Scholar]
- 94.Bakshi VP, Alsene KM, Roseboom PH, Connors EE. Enduring sensorimotor gating abnormalities following predator exposure or corticotropin-releasing factor in rats: A model for PTSD-like information-processing deficits? Neuropharmacology. 2012;62:737–748. doi: 10.1016/j.neuropharm.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toth M, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. 2016;41:1681–1690. doi: 10.1038/npp.2015.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol. Psychiatry. 2003;53:463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- 97.Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol. Behav. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 98.Adamec R, Hebert M, Blundell J, Mervis RF. Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav. Brain Res. 2012;226:133–146. doi: 10.1016/j.bbr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitra R, Adamec R, Sapolsky R. Resilience against predator stress and dendritic morphology of amygdala neurons. Behav. Brain Res. 2009;205:535–543. doi: 10.1016/j.bbr.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Apfelbach, R., Blanchard, C. D., Blanchard, R. J., Hayes, R. A. & McGregor, I. S. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev.10.1016/j.neubiorev.2005.05.005 (2005). [DOI] [PubMed]
- 101.Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008;11:259–281. doi: 10.1080/10253890701768613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zoladz PR, Fleshner M, Diamond DM. Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology. 2012;37:1531–1545. doi: 10.1016/j.psyneuen.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 103.O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Zoladz PR, Park CR, Fleshner M, Diamond DM. Psychosocial predator-based animal model of PTSD produces physiological and behavioral sequelae and a traumatic memory four months following stress onset. Physiol. Behav. 2015;147:183–192. doi: 10.1016/j.physbeh.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 105.Wilson CB, Ebenezer PJ, McLaughlin LD, Francis J. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS ONE. 2014;9:e89104. doi: 10.1371/journal.pone.0089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geracioti TD, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am. J. Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 107.Geracioti TD, et al. Effect of traumatic imagery on cerebrospinal fluid dopamine and serotonin metabolites in posttraumatic stress disorder. J. Psychiatr. Res. 2013;47:995–998. doi: 10.1016/j.jpsychires.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 108.Zoladz PR, et al. A predator-based psychosocial stress animal model of PTSD in females: Influence of estrous phase and ovarian hormones. Horm. Behav. 2019;115:104564. doi: 10.1016/j.yhbeh.2019.104564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rorabaugh BR, et al. Sex-dependent effects of chronic psychosocial stress on myocardial sensitivity to ischemic injury. Stress. 2015;18:645–653. doi: 10.3109/10253890.2015.1087505. [DOI] [PubMed] [Google Scholar]
- 110.Zoladz PR, Diamond DM. Predator-based psychosocial stress animal model of PTSD: preclinical assessment of traumatic stress at cognitive, hormonal, pharmacological, cardiovascular and epigenetic levels of analysis. Exp. Neurol. 2016;284:211–219. doi: 10.1016/j.expneurol.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 111.Itoga CA, et al. Traumatic stress promotes hyperalgesia via corticotropin-releasing factor-1 receptor (CRFR1) signaling in central amygdala. Neuropsychopharmacology. 2016;41:2463–2472. doi: 10.1038/npp.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cohen H, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol. Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Liang Z, King J, Zhang N. Neuroplasticity to a single-episode traumatic stress revealed by resting-state fMRI in awake rats. Neuroimage. 2014;103:485–491. doi: 10.1016/j.neuroimage.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masini CV, Sauer S, White J, Day HEW, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol. Behav. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morrow BA, Roth RH, Elsworth JD. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res. Bull. 2000;52:519–523. doi: 10.1016/s0361-9230(00)00290-2. [DOI] [PubMed] [Google Scholar]
- 116.Zangrossi H, File SE. Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Res. Bull. 1992;29:381–388. doi: 10.1016/0361-9230(92)90072-6. [DOI] [PubMed] [Google Scholar]
- 117.Wallace, K. J. & Rosen, J. B. Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav. Neurosci. 10.1037/0735-7044.114.5.912 (2000). [DOI] [PubMed]
- 118.Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci. Biobehav. Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 119.Schwendt M, et al. A novel rat model of comorbid PTSD and addiction reveals intersections between stress susceptibility and enhanced cocaine seeking with a role for mGlu5 receptors. Transl. Psychiatry. 2018;8:209. doi: 10.1038/s41398-018-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mazor A, et al. Gender-related qualitative differences in baseline and post-stress anxiety responses are not reflected in the incidence of criterion-based PTSD-like behaviour patterns. World J. Biol. Psychiatry. 2009;10:856–869. doi: 10.1080/15622970701561383. [DOI] [PubMed] [Google Scholar]
- 121.Koresh O, et al. Distinctive cardiac autonomic dysfunction following stress exposure in both sexes in an animal model of PTSD. Behav. Brain Res. 2016;308:128–142. doi: 10.1016/j.bbr.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 122.Cohen H, et al. Long-lasting behavioral effects of juvenile trauma in an animal model of PTSD associated with a failure of the autonomic nervous system to recover. Eur. Neuropsychopharmacol. 2007;17:464–477. doi: 10.1016/j.euroneuro.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 123.Danan D, Matar MA, Kaplan Z, Zohar J, Cohen H. Blunted basal corticosterone pulsatility predicts post-exposure susceptibility to PTSD phenotype in rats. Psychoneuroendocrinology. 2018;87:35–42. doi: 10.1016/j.psyneuen.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 124.Dopfel D, et al. Individual variability in behavior and functional networks predicts vulnerability using an animal model of PTSD. Nat. Commun. 2019;10:2372. doi: 10.1038/s41467-019-09926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zohar J, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. Eur. Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 126.Cohen, S. et al. Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacology10.1038/npp.2012.94 (2012). [DOI] [PMC free article] [PubMed]
- 127.Cohen, S., Kaplan, Z., Zohar, J. & Cohen, H. Preventing sleep on the first resting phase following a traumatic event attenuates anxiety-related responses. Behav. Brain Res. 10.1016/j.bbr.2016.10.039 (2017). [DOI] [PubMed]
- 128.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z. Animal model for PTSD: From clinical concept to translational research. Neuropharmacology. 2012;62:715–724. doi: 10.1016/j.neuropharm.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 130.Staples LG. Predator odor avoidance as a rodent model of anxiety: Learning-mediated consequences beyond the initial exposure. Neurobiol. Learn. Mem. 2010;94:435–445. doi: 10.1016/j.nlm.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 131.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 132.Bourin M, Petit-Demoulière B, Nic Dhonnchadha B, Hascöet M. Animal models of anxiety in mice. Fundam. Clin. Pharmacol. 2007;21:567–574. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 133.Berridge, K. C. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev.10.1016/S0149-7634(99)00072-X (2000). [DOI] [PubMed]
- 134.Knutson, B., Burgdorf, J. & Panksepp, J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 10.1037/0033-2909.128.6.961 (2002). [DOI] [PubMed]
- 135.Bushnell, P. J. & Strupp, B. J. Assessing attention in rodents. In Methods of Behavior Analysis in Neuroscience (CRC Press/Taylor & Francis, 2009). [PubMed]
- 136.Garattini S. Are me-too drugs justified? J. Nephrol. 1997;10:283–294. [PubMed] [Google Scholar]
- 137.Brady K, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 138.Marshall, R. D., Beebe, K. L., Oldham, M. & Zaninelli, R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am. J. Psychiatry10.1176/appi.ajp.158.12.1982 (2001). [DOI] [PubMed]
- 139.VA/DoD Management of Post-Traumatic Stress Working Group. VA / DoD Clinical Practice Guideline For The Management Of Posttraumatic Stress Disorder and Acute Stress Disorder. Department of Veterans Affairs Department of Defense 10.1016/j.addbeh.2017.07.010 (2016).
- 140.American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD). (APA, Guideline Development Panel for the Treatment of Posttraumatic Stress Disorder in Adults, Washington, DC, 2017).
- 141.NICE. Post-traumatic stress disorder: management. National Institute for Health and Care Excellence (2018). [PubMed]
- 142.DePierro J, Lepow L, Feder A, Yehuda R. Translating molecular and neuroendocrine findings in posttraumatic stress disorder and resilience to novel therapies. Biol. Psychiatry. 2019;86:454–463. doi: 10.1016/j.biopsych.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mushtaq, D., Ali, A., Margoob, M. A., Murtaza, I. & Andrade, C. Association between serotonin transporter gene promoter-region polymorphism and 4- and 12-week treatment response to sertraline in posttraumatic stress disorder. J. Affect. Disord. 10.1016/j.jad.2011.08.033 (2012). [DOI] [PubMed]
- 144.Guo W, et al. Exploratory genome-wide association analysis of response to ketamine and a polygenic analysis of response to scopolamine in depression. Transl. Psychiatry. 2018;8:280. doi: 10.1038/s41398-018-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Ann. NY Acad. Sci. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- 146.Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N. Engl. J. Med. 2017;376:2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- 147.Singewald N, Holmes A. Rodent models of impaired fear extinction. Psychopharmacology. 2019;236:21–32. doi: 10.1007/s00213-018-5054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.LeDoux JE. Semantics, surplus meaning, and the science of fear. Trends Cogn. Sci. 2017;21:303–306. doi: 10.1016/j.tics.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 149.Holmes P. Rodent models of depression: reexamining validity without anthropomorphic inference. Crit. Rev. Neurobiol. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- 150.Fadok JP, et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- 151.Denayer T, Stöhr T, Van Roy M. Animal models in translational medicine: validation and prediction. N. Horiz. Transl. Med. 2014;2:5–11. [Google Scholar]
- 152.Mlinarić A, Horvat M, Šupak Smolčić V. Dealing with the positive publication bias: why you should really publish your negative results. Biochem. Med. 2017;27:30201. doi: 10.11613/BM.2017.030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith AJ, Clutton RE, Lilley E, Hansen KEA, Brattelid T. PREPARE: guidelines for planning animal research and testing. Lab. Anim. 2017;52:135–141. doi: 10.1177/0023677217724823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hooijmans CR, Leenaars M, Ritskes-Hoitinga M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the three Rs, and to make systematic reviews more feasible. Altern. Lab. Anim. 2010;38:167–182. doi: 10.1177/026119291003800208. [DOI] [PubMed] [Google Scholar]
- 156.Prinz, F., Schlange, T. & Asadullah, K. Believe it or not: how much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov. 10.1038/nrd3439-c1 (2011). [DOI] [PubMed]
- 157.Koolhaas, J. M. et al. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev.10.1016/j.neubiorev.2011.02.003 (2011). [DOI] [PubMed]
- 158.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-III (3rd edn.) (1980).
- 159.Beery, A. K. & Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev.10.1016/j.neubiorev.2010.07.002 (2011). [DOI] [PMC free article] [PubMed]
- 160.Franconi, F., Brunelleschi, S., Steardo, L. & Cuomo, V. Gender differences in drug responses. Pharmacol. Res.10.1016/j.phrs.2006.11.001 (2007). [DOI] [PubMed]
- 161.Peters, L., Issakidis, C., Slade, T. & Andrews, G. Gender differences in the prevalence of DSM-IV and ICD-10 PTSD. Psychol. Med. 10.1017/S003329170500591X (2006). [DOI] [PubMed]
- 162.Carmassi C, et al. Gender differences in DSM-5 versus DSM-IV-TR PTSD prevalence and criteria comparison among 512 survivors to the L׳Aquila earthquake. J. Affect. Disord. 2014;160:55–61. doi: 10.1016/j.jad.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 163.Taylor SE, et al. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 164.Briscione, M. A., Michopoulos, V., Jovanovic, T. & Norrholm, S. D. Neuroendocrine underpinnings of increased risk for posttraumatic stress disorder in women. Vitamins Hormones103, 53–83 (2017). [DOI] [PubMed]
- 165.Maeng, L. Y. & Milad, M. R. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm. Behav. 10.1016/j.yhbeh.2015.04.002 (2015). [DOI] [PMC free article] [PubMed]
- 166.Scharfman, H. E. & Maclusky, N. J. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology10.1016/j.neuropharm.2013.04.029 (2014). [DOI] [PMC free article] [PubMed]
- 167.Meziane H, Ouagazzal A-M, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 168.Wei, L., David, A., Duman, R. S., Anisman, H. & Kaffman, A. Early life stress increases anxiety-like behavior in Balbc mice despite a compensatory increase in levels of postnatal maternal care. Horm. Behav. 10.1016/j.yhbeh.2010.01.007 (2010). [DOI] [PMC free article] [PubMed]
- 169.Savignac, H. M., Dinan, T. G. & Cryan, J. F. Resistance to early-ife stress in mice: effects of genetic background and stress duration. Front. Behav. Neurosci. 10.3389/fnbeh.2011.00013 (2011). [DOI] [PMC free article] [PubMed]
- 170.Crawley, J. N. et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology10.1007/s002130050327 (1997). [DOI] [PubMed]
- 171.Cryan, J. F., Markou, A. & Lucki, I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacol. Sci.10.1016/S0165-6147(02)02017-5 (2002). [DOI] [PubMed]
- 172.Heim, C., Shugart, M., Craighead, W. E. & Nemeroff, C. B. Neurobiological and psychiatric consequences of child abuse and neglect. Dev. Psychobiol. 10.1002/dev.20494 (2010). [DOI] [PubMed]
- 173.Schmidt, M. V., Wang, X. D. & Meijer, O. C. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology 10.1007/s00213-010-2096-0 (2011). [DOI] [PubMed]
- 174.Di Segni M, et al. Sex-dependent effects of early unstable post-natal environment on response to positive and negative stimuli in adult mice. Neuroscience. 2019;413:1–10. doi: 10.1016/j.neuroscience.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 175.Spear, L. P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev.10.1016/S0149-7634(00)00014-2 (2000). [DOI] [PubMed]
- 176.McEwen, B. S. Stress, adaptation, and disease: allostasis and allostatic load. Ann. NY Acad. Sci. USA10.1111/j.1749-6632.1998.tb09546.x (1998). [DOI] [PubMed]
- 177.Gluckman, P. D., Hanson, M. A. & Beedle, A. S. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 10.1002/ajhb.20590 (2007). [DOI] [PubMed]
- 178.Belsky, J. & Pluess, M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 10.1037/a0017376 (2009). [DOI] [PubMed]
- 179.Daskalakis, N. P., Bagot, R. C., Parker, K. J., Vinkers, C. H. & de Kloet, E. R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology10.1016/j.psyneuen.2013.06.008 (2013). [DOI] [PMC free article] [PubMed]
- 180.Nederhof, E. & Schmidt, M. V. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behavior10.1016/j.physbeh.2011.12.008 (2012). [DOI] [PubMed]
- 181.Telch, M. J., Rosenfield, D., Lee, H. J. & Pai, A. Emotional reactivity to a single inhalation of 35% carbon dioxide and its association with later symptoms of posttraumatic stress disorder and anxiety in soldiers deployed to Iraq. Arch. Gen. Psychiatry10.1001/archgenpsychiatry.2012.8 (2012). [DOI] [PubMed]
- 182.Klengel, T. et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 10.1038/nn.3275 (2013). [DOI] [PMC free article] [PubMed]
- 183.Van Zuiden, M. et al. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol. Psychiatry10.1016/j.biopsych.2011.10.026 (2012). [DOI] [PubMed]
- 184.Van Liempt, S., Van Zuiden, M., Westenberg, H., Super, A. & Vermetten, E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress. Anxiety10.1002/da.22054 (2013). [DOI] [PubMed]
- 185.Gilbertson, M. W. et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 10.1038/nn958 (2002). [DOI] [PMC free article] [PubMed]
- 186.Shalev, A. Y. et al. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch. Gen. Psychiatry10.1001/archpsyc.55.6.553 (1998). [DOI] [PubMed]
- 187.Pitman, R. K. et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci.10.1038/nrn3339 (2012). [DOI] [PMC free article] [PubMed]
- 188.Yehuda, R. et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the world trade center attacks. Biol. Psychiatry10.1016/j.biopsych.2009.02.034 (2009). [DOI] [PubMed]
- 189.Strawn, J. R. & Geracioti, T. D. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depression Anxiety10.1002/da.20292 (2008). [DOI] [PubMed]
- 190.Karl, A. et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev.10.1016/j.neubiorev.2006.03.004 (2006). [DOI] [PubMed]
- 191.Geuze, E. et al. Glucocorticoid receptor number predicts increase in amygdala activity after severe stress. Psychoneuroendocrinology10.1016/j.psyneuen.2012.03.017 (2012). [DOI] [PubMed]
- 192.Thomas, D. W. et al. Clinical development success rates 2006–2015. BIO Industry Anal.1, 16 (2016).
- 193.Schmidt U, Kaltwasser SF, Wotjak CT. Biomarkers in posttraumatic stress disorder: overview and implications for future research. Dis. Markers. 2013;35:43–54. doi: 10.1155/2013/835876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tufts Center for the Study of Drug Development. CNS Drugs Take 20% Longer to Develop and to Approve vs. Non-CNS Drugs. Tufts CSDD Impact Report. Vol. 20 (2018).
- 195.Harrison RKPhase., II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016;15:817. doi: 10.1038/nrd.2016.184. [DOI] [PubMed] [Google Scholar]
- 196.Arrowsmith J. Phase III and submission failures: 2007–2010. Nat. Rev. Drug Discov. 2011;10:87. doi: 10.1038/nrd3375. [DOI] [PubMed] [Google Scholar]
- 197.Arrowsmith J. Phase II failures: 2008–2010. Nat. Rev. Drug Discov. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- 198.Arrowsmith J, Miller P. Phase II and phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 2013;12:569. doi: 10.1038/nrd4090. [DOI] [PubMed] [Google Scholar]
- 199.Bionomics. BNC210 Update-June 26, 2019. (2019).
- 200.Morgan P, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov. Today. 2012;17:419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 201.Miller G. Is pharma running out of brainy ideas? Science. 2010;329:502 LP–502504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- 202.O’Brien PL, Thomas CP, Hodgkin D, Levit KR, Mark TL. The diminished pipeline for medications to treat mental health and substance use disorders. Psychiatr. Serv. 2014;65:1433–1438. doi: 10.1176/appi.ps.201400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bionomics. BNC210 Phase 2 PTSD Clinical Trial Results Presentation. (2018).
- 204.U.S. Department of Health and Human Services-Food and Drug Administration. Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. (2004).
- 205.Morris, S. E. & Cuthbert, B. N. Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 10.1097/ALN.0b013e318212ba87 (2012). [DOI] [PMC free article] [PubMed]
- 206.Schmidt, U. & Vermetten, E. Integrating NIMH research domain criteria (RDoC) into PTSD research. Curr. Topics Behav. Neurosci. 69–91 (2017). [DOI] [PubMed]
- 207.Kunimatsu, A., Yasaka, K., Akai, H., Kunimatsu, N. & Abe, O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imaging10.1002/jmri.26929 (2019). [DOI] [PubMed]
- 208.Zhang, N. et al. Mapping resting-state brain networks in conscious animals. J. Neurosci. Methods10.1016/j.jneumeth.2010.04.001 (2010). [DOI] [PMC free article] [PubMed]
- 209.Dopfel, D. & Zhang, N. Mapping stress networks using functional magnetic resonance imaging in awake animals. Neurobiol. Stress10.1016/j.ynstr.2018.06.002 (2018). [DOI] [PMC free article] [PubMed]
- 210.Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron10.1016/j.neuron.2011.06.004 (2011). [DOI] [PubMed]
- 211.Campbell EJ, Marchant NJ. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 2018;175:994–1003. doi: 10.1111/bph.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee, J. H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature10.1038/nature09108 (2010). [DOI] [PMC free article] [PubMed]
- 213.Giorgi A, et al. Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep. 2017;21:910–918. doi: 10.1016/j.celrep.2017.09.087. [DOI] [PubMed] [Google Scholar]
- 214.Ferenczi EA, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698–aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu X, Yuan J, Guang Y, Wang X, Feng Z. Longitudinal in vivo diffusion tensor imaging detects differential microstructural alterations in the hippocampus of chronic social defeat stress-susceptible and resilient mice. Front. Neurosci. 2018;12:613. doi: 10.3389/fnins.2018.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Uhlhaas PJ, et al. Magnetoencephalography as a tool in psychiatric research: current status and perspective. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2:235–244. doi: 10.1016/j.bpsc.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liang Z, Ma Y, Watson GDR, Zhang N. Simultaneous GCaMP6-based fiber photometry and fMRI in rats. J. Neurosci. Methods. 2017;289:31–38. doi: 10.1016/j.jneumeth.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schlegel F, et al. Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice. Nat. Protoc. 2018;13:840–855. doi: 10.1038/nprot.2018.003. [DOI] [PubMed] [Google Scholar]
- 219.Meyer JS, Hamel AF. Models of stress in nonhuman primates and their relevance for human psychopathology and endocrine dysfunction. ILAR J. 2014;55:347–360. doi: 10.1093/ilar/ilu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Coleman K, Pierre PJ. Assessing anxiety in nonhuman primates. ILAR J. 2014;55:333–346. doi: 10.1093/ilar/ilu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Miles OW, Maren S. Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Front. Behav. Neurosci. 2019;13:68. doi: 10.3389/fnbeh.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jennings JH, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fox AS, et al. Functional connectivity within the primate extended amygdala is heritable and associated with early-life anxious temperament. J. Neurosci. 2018;38:7611 LP–7617621. doi: 10.1523/JNEUROSCI.0102-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mikics E, Baranyi J, Haller J. Rats exposed to traumatic stress bury unfamiliar objects-A novel measure of hyper-vigilance in PTSD models? Physiol. Behav. 2008;94:341–348. doi: 10.1016/j.physbeh.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 225.Walf, A. A. & Frye, C. A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 10.1038/nprot.2007.44 (2007). [DOI] [PMC free article] [PubMed]
- 226.Graeff, F. G., Ferreira Netto, C. & Zangrossi, H. The elevated T-maze as an experimental model of anxiety. Neurosci. Biobehav. Rev.10.1016/S0149-7634(98)00024-4 (1998). [DOI] [PubMed]
- 227.Shepherd, J. K., Grewal, S. S., Fletcher, A., Bill, D. J. & Dourish, C. T. Behavioural and pharmacological characterisation of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology10.1007/BF02244871 (1994). [DOI] [PubMed]
- 228.Bourin M, Hascoët M. The mouse light/dark box test. Eur. J. Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 229.Arrant AE, Schramm-Sapyta NL, Kuhn CM. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013;256:119–127. doi: 10.1016/j.bbr.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gould, T. D., Dao, D. T. & Kovacsics, C. E. The Open Field Test BT-Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests (ed. Gould, T. D.) 1–20 (Humana Press, 2009).
- 231.Bodnoff, S. R., Suranyi-Cadotte, B., Aitken, D. H., Quirion, R. & Meaney, M. J. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 10.1007/BF00181937 (1988). [DOI] [PubMed]
- 232.Ohl, F., Holsboer, F. & Landgraf, R. The modified hole board as a differential screen for behavior in rodents. Behav. Res. Methods Instruments Comput. 10.3758/BF03195393 (2001). [DOI] [PubMed]
- 233.Nachman, M. & Ashe, J. H. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol. Behav. 10.1016/0031-9384(73)90089-9 (1973). [DOI] [PubMed]
- 234.Philbert J, et al. Acute inescapable stress exposure induces long-term sleep disturbances and avoidance behavior: a mouse model of post-traumatic stress disorder (PTSD) Behav. Brain Res. 2011;221:149–154. doi: 10.1016/j.bbr.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 235.Pamplona FA, et al. Prolonged fear incubation leads to generalized avoidance behavior in mice. J. Psychiatr. Res. 2011;45:354–360. doi: 10.1016/j.jpsychires.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 236.Cosentino, L. et al. Methyl-CpG binding protein 2 functional alterations provide vulnerability to develop behavioral and molecular features of post-traumatic stress disorder in male mice. Neuropharmacology160, 107664 (2019). [DOI] [PubMed]
- 237.Patin, V., Lordi, B., Vincent, A. & Caston, J. Effects of prenatal stress on anxiety and social interactions in adult rats. Dev. Brain Res. 10.1016/j.devbrainres.2005.09.010 (2005). [DOI] [PubMed]
- 238.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 240.Deacon, R. M. J. & Rawlins, J. N. P. T-maze alternation in the rodent. Nat. Protoc. 10.1038/nprot.2006.2 (2006). [DOI] [PubMed]
- 241.Antunes, M. and Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 10.1007/s10339-011-0430-z (2012). [DOI] [PMC free article] [PubMed]
- 242.Muhie S, et al. Molecular indicators of stress-induced neuroinflammation in a mouse model simulating features of post-traumatic stress disorder. Transl. Psychiatry. 2017;7:e1135. doi: 10.1038/tp.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dellu, F., Mayo, W., Cherkaoui, J., Le Moal, M. & Simon, H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 10.1016/0006-8993(92)91352-F (1992). [DOI] [PubMed]
- 244.Barnes, C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 10.1037/h0077579 (1979). [DOI] [PubMed]
- 245.Rosenfeld, C. S. & Ferguson, S. A. Barnes maze testing strategies with small and large rodent models. J. Vis. Exp. 10.3791/51194 (2014). [DOI] [PMC free article] [PubMed]
- 246.Phillips, R. G. & LeDoux, J. E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 10.1037/0735-7044.106.2.274 (1992). [DOI] [PubMed]
- 247.Curzon P, Rustay NR, Browman KE. Cued and contextual fear conditioning for rodents. Methods Behav. Anal. Neurosci. 2009;115:19–37. [PubMed] [Google Scholar]
- 248.Cohen H, Liberzon I, Richter-Levin G. Exposure to extreme stress impairs contextual odour discrimination in an animal model of PTSD. Int. J. Neuropsychopharmacol. 2009;12:291–303. doi: 10.1017/S146114570800919X. [DOI] [PubMed] [Google Scholar]
- 249.Ögren, S. O. & Stiedl, O. Passive avoidance. in Encyclopedia of Psychopharmacology (ed. Stolerman, I. P.) 960–967 (Springer Berlin Heidelberg, 2010).
- 250.Der-Avakian, A., D’Souza, M. S., Pizzagalli, D. A. & Markou, A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl. Psychiatry10.1038/tp.2013.74 (2013). [DOI] [PMC free article] [PubMed]
- 251.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 252.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012;7:1009. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 253.Steru, L., Chermat, R., Thierry, B. & Simon, P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 10.1007/BF00428203 (1985). [DOI] [PubMed]
- 254.Katz, R. J., Roth, K. A. & Carroll, B. J. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci. Biobehav. Rev. 10.1016/0149-7634(81)90005-1 (1981). [DOI] [PubMed]
- 255.Espejo, E. F. & Mir, D. Structure of the rat’s behaviour in the hot plate test. Behav. Brain Res. 10.1016/0166-4328(93)90035-O (1993). [DOI] [PubMed]
- 256.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- 257.Hole, K. & Tjølsen, A. Tail flick test. In Encyclopedia of Pain (eds. Schmidt, R. F. & Willis, W. D.) 2392–2395 (Springer Berlin Heidelberg, 2007).
- 258.Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods10.1016/0165-0270(94)90144-9 (1994). [DOI] [PubMed]
- 259.Willner, P., Towell, A., Sampson, D., Sophokleous, S. & Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology10.1007/BF00187257 (1987). [DOI] [PubMed]
- 260.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat. Protoc. 2007;2:2987. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 261.Markou, A. & Koob, G. F. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol. Behav. 10.1016/0031-9384(92)90211-J (1992). [DOI] [PubMed]
- 262.File SE, Seth P. A review of 25 years of the social interaction test. Eur. J. Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 263.Lukas M, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 265.Avitsur, R., Cohen, E. & Yirmiya, R. Effects of interleukin-1 on sexual attractivity in a model of sickness behavior. Physiol. Behav. 10.1016/S0031-9384(97)00381-8 (1997). [DOI] [PubMed]
- 266.Haller, J. & Bakos, N. Stress-induced social avoidance: a new model of stress-induced anxiety? Physiol. Behav. 10.1016/S0031-9384(02)00860-0 (2002). [DOI] [PubMed]
- 267.Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes, Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 268.Yang, M. & Crawley, J. N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 8, Unit-8.24 (2009). [DOI] [PMC free article] [PubMed]
- 269.Deacon RMJ. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006;1:122. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- 270.Pilz PKD, Schnitzler H-U. Habituation and sensitization of the acoustic startle response in rats: amplitude, threshold, and latency measures. Neurobiol. Learn. Mem. 1996;66:67–79. doi: 10.1006/nlme.1996.0044. [DOI] [PubMed] [Google Scholar]
- 271.Valsamis, B. & Schmid, S. Habituation and prepulse inhibition of acoustic startle in rodents. J. Vis. Exp. 10.3791/3446 (2011). [DOI] [PMC free article] [PubMed]
- 272.Brady, A. M. & Floresco, S. B. Operant procedures for assessing behavioral flexibility in rats. J. Vis. Exp. 96, e52387–e52387 (2015). [DOI] [PMC free article] [PubMed]
- 273.Piao, C. et al. Altered function in medial prefrontal cortex and nucleus accumbens links to stress-induced behavioral inflexibility. Behav. Brain Res. 10.1016/j.bbr.2016.09.017 (2017). [DOI] [PubMed]
- 274.Kavushansky A, Ben-Shachar D, Richter-Levin G, Klein E. Physical stress differs from psychosocial stress in the pattern and time-course of behavioral responses, serum corticosterone and expression of plasticity-related genes in the rat. Stress. 2009;12:412–425. doi: 10.1080/10253890802556081. [DOI] [PubMed] [Google Scholar]
- 275.Diehl LA, et al. Long-lasting effects of maternal separation on an animal model of post-traumatic stress disorder: effects on memory and hippocampal oxidative stress. Neurochem. Res. 2012;37:700–707. doi: 10.1007/s11064-011-0660-6. [DOI] [PubMed] [Google Scholar]
- 276.Louvart H, et al. Effects of a single footshock followed by situational reminders on HPA axis and behaviour in the aversive context in male and female rats. Psychoneuroendocrinology. 2006;31:92–99. doi: 10.1016/j.psyneuen.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 277.Machawal, L. & Kumar, A. Possible involvement of nitric oxide mechanism in the neuroprotective effect of rutin against immobilization stress induced anxiety like behaviour, oxidative damage in mice. Pharmacol. Reports10.1016/j.pharep.2013.08.001 (2014). [DOI] [PubMed]
- 278.Samad, N., Saleem, A., Yasmin, F. & Shehzad, M. A. Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in male mice. Physiol. Res. (2018). [DOI] [PubMed]
- 279.Armario, A., Gil, M., Marti, J., Pol, O. & Balasch, J. Influence of various acute stressors on the activity of adult male rats in a holeboard and in the forced swim test. Pharmacol. Biochem. Behav. 10.1016/0091-3057(91)90194-7 (1991). [DOI] [PubMed]
- 280.Andero R, et al. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am. J. Psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kim, J. W. et al. Social support rescues acute stress-induced cognitive impairments by modulating ERK1/2 phosphorylation in adolescent mice. Sci. Rep. 10.1038/s41598-018-30524-4 (2018). [DOI] [PMC free article] [PubMed]
- 282.Pastor-Ciurana, J. et al. Prior exposure to repeated immobilization or chronic unpredictable stress protects from some negative sequels of an acute immobilization. Behav. Brain Res. 10.1016/j.bbr.2014.02.028 (2014). [DOI] [PubMed]
- 283.Fuentes S, Carrasco J, Armario A, Nadal R. Behavioral and neuroendocrine consequences of juvenile stress combined with adult immobilization in male rats. Horm. Behav. 2014;66:475–486. doi: 10.1016/j.yhbeh.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 284.Adamec, R., Toth, M., Haller, J., Halasz, J. & Blundell, J. A comparison of activation patterns of cells in selected prefrontal cortical and amygdala areas of rats which are more or less anxious in response to predator exposure or submersion stress. Physiol. Behav. 10.1016/j.physbeh.2011.09.016 (2012). [DOI] [PubMed]
- 285.Ardi Z, Ritov G, Lucas M, Richter-Levin G. The effects of a reminder of underwater trauma on behaviour and memory-related mechanisms in the rat dentate gyrus. Int. J. Neuropsychopharmacol. 2014;17:571–580. doi: 10.1017/S1461145713001272. [DOI] [PubMed] [Google Scholar]
- 286.Toledano D, Gisquet-Verrier P. Only susceptible rats exposed to a model of PTSD exhibit reactivity to trauma-related cues and other symptoms: An effect abolished by a single amphetamine injection. Behav. Brain Res. 2014;272:165–174. doi: 10.1016/j.bbr.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 287.Lin C-C, Tung C-S, Liu Y-P. Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology. 2016;233:1135–1146. doi: 10.1007/s00213-015-4194-5. [DOI] [PubMed] [Google Scholar]
- 288.Brand L, Groenewald I, Stein DJ, Wegener G, Harvey BH. Stress and re-stress increases conditioned taste aversion learning in rats: Possible frontal cortical and hippocampal muscarinic receptor involvement. Eur. J. Pharmacol. 2008;586:205–211. doi: 10.1016/j.ejphar.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 289.Tanaka K-I, Yagi T, Nanba T, Asanuma M. Application of single prolonged stress induces post-traumatic stress disorder-like characteristics in mice. Acta Med. Okayama. 2018;72:479–485. doi: 10.18926/AMO/56245. [DOI] [PubMed] [Google Scholar]
- 290.Han, F., Ding, J. & Shi, Y. Expression of amygdala mineralocorticoid receptor and glucocorticoid receptor in the single-prolonged stress rats. BMC Neurosci. 10.1186/1471-2202-15-77 (2014). [DOI] [PMC free article] [PubMed]
- 291.George, S. A. et al. Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behav. Brain Res. 10.1016/j.bbr.2015.02.051 (2015). [DOI] [PubMed]
- 292.Eagle, A. L., Fitzpatrick, C. J. & Perrine, S. A. Single prolonged stress impairs social and object novelty recognition in rats. Behav. Brain Res. 10.1016/j.bbr.2013.09.014 (2013). [DOI] [PMC free article] [PubMed]
- 293.Wu, Z. et al. Behavioral changes over time in post-traumatic stress disorder: insights from a rat model of single prolonged stress. Behav. Processes10.1016/j.beproc.2016.01.001 (2016). [DOI] [PubMed]
- 294.Zhang Y, Schalo I, Durand C, Standifer KM. Sex differences in nociceptin/orphanin FQ peptide receptor-mediated pain and anxiety symptoms in a preclinical model of post-traumatic stress disorder. Front. Psychiatry. 2019;9:731. doi: 10.3389/fpsyt.2018.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Noble, L. J. et al. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl. Psychiatry10.1038/tp.2017.191 (2017). [DOI] [PMC free article] [PubMed]
- 296.Khan, S. & Liberzon, I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology. 10.1007/s00213-003-1634-4 (2004). [DOI] [PubMed]
- 297.Vanderheyden, W. M. et al. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD. Exp. Brain Res. 10.1007/s00221-015-4302-0 (2015). [DOI] [PMC free article] [PubMed]
- 298.Moshfegh, C. M., Elkhatib, S. K., Collins, C. W., Kohl, A. J. & Case, A. J. Autonomic and redox imbalance correlates with T-lymphocyte inflammation in a model of chronic social defeat stress. Front. Behav. Neurosci. 10.3389/fnbeh.2019.00103 (2019). [DOI] [PMC free article] [PubMed]
- 299.Wang XD, et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol. Dis. 2011;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Patki, G., Solanki, N., Atrooz, F., Allam, F. & Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 1539, 73-86 (2013). [DOI] [PMC free article] [PubMed]
- 301.Narayanan, V. et al. Social defeat: Impact on fear extinction and Amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS One6, 10.1371/journal.pone.0022600 (2011). [DOI] [PMC free article] [PubMed]
- 302.Der-Avakian A, et al. Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacol. (Berl.). 2017;234:1603–1614. doi: 10.1007/s00213-017-4584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Monleón S, Duque A, Vinader-Caerols C. Inhibitory avoidance learning in CD1 mice: effects of chronic social defeat stress. Behav. Process. 2015;115:64–69. doi: 10.1016/j.beproc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 304.Yu T, et al. Cognitive and neural correlates of depression-like behaviour in socially defeated mice: an animal model of depression with cognitive dysfunction. Int. J. Neuropsychopharmacol. 2011;14:303–317. doi: 10.1017/S1461145710000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Laredo, S. A. et al. Effects of defeat stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. Eur. J. Neurosci. 10.1111/ejn.12824 (2015). [DOI] [PMC free article] [PubMed]
- 306.Jianhua, F., Wei, W., Xiaomei, L. & Shao-Hui, W. Chronic social defeat stress leads to changes of behaviour and memory-associated proteins of young mice. Behav. Brain Res. 10.1016/j.bbr.2016.09.011 (2017). [DOI] [PubMed]
- 307.Rygula R, et al. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav. Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 308.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: Susceptibility, resilience, and antidepressant response. Biol. Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bharwani, A., Mian, M. F., Surette, M. G., Bienenstock, J. & Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 10.1186/s12916-016-0771-7 (2017). [DOI] [PMC free article] [PubMed]
- 310.Trainor, B. C. et al. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (peromyscus californicus). PLoS ONE10.1371/journal.pone.0017405 (2011). [DOI] [PMC free article] [PubMed]
- 311.Pulliam JVK, Dawaghreh AM, Alema-Mensah E, Plotsky PM. Social defeat stress produces prolonged alterations in acoustic startle and body weight gain in male Long Evans rats. J. Psychiatr. Res. 2010;44:106–111. doi: 10.1016/j.jpsychires.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wells AM, et al. Effects of chronic social defeat stress on sleep and circadian rhythms are mitigated by Kappa-Opioid receptor antagonism. J. Neurosci. 2017;37:7656–7668. doi: 10.1523/JNEUROSCI.0885-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Patki G, Solanki N, Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. Int. J. Neuropsychopharmacol. 2014;17:2017–2029. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li M, Xu H, Wang W. An improved model of physical and emotional social defeat: different effects on social behavior and body weight of adolescent mice by interaction with social support. Front. Psychiatry. 2018;9:688. doi: 10.3389/fpsyt.2018.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Patki G, Salvi A, Liu H, Salim S. Witnessing traumatic events and post-traumatic stress disorder: insights from an animal model. Neurosci. Lett. 2015;600:28–32. doi: 10.1016/j.neulet.2015.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gautam, A. et al. Acute and chronic plasma metabolomic and liver transcriptomic stress effects in a mouse model with features of post-traumatic stress disorder. PLoS ONE10, 10.1371/journal.pone.0117092 (2015). [DOI] [PMC free article] [PubMed]
- 317.Bian Y, et al. Identification of key genes and pathways in post-traumatic stress disorder using microarray analysis. Front. Psychol. 2019;10:302. doi: 10.3389/fpsyg.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Bulos, E. M., Pobbe, R. L. H. & Zangrossi, H. Behavioral consequences of predator stress in the rat elevated T-maze. Physiol. Behav. 10.1016/j.physbeh.2015.04.019 (2015). [DOI] [PubMed]
- 319.Sharma, R., Sahota, P. & Thakkar, M. M. Severe and protracted sleep disruptions in mouse model of post-traumatic stress disorder. Sleep 1–12 (2018). [DOI] [PubMed]
- 320.Wu, Y. P. et al. Predator stress-induced depression is associated with inhibition of hippocampal neurogenesis in adult male mice. Neural Regen. Res. 10.4103/1673-5374.244792 (2019). [DOI] [PMC free article] [PubMed]
- 321.Shallcross, J. et al. The divergent effects of CDPPB and Cannabidiol on fear extinction and anxiety in a predator scent stress model of PTSD in rats. Front. Behav. Neurosci. 10.3389/fnbeh.2019.00091 (2019). [DOI] [PMC free article] [PubMed]
- 322.Banik A, Anand A. Loss of learning in mice when exposed to rat odor: a water maze study. Behav. Brain Res. 2011;216:466–471. doi: 10.1016/j.bbr.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 323.Louvart H, Maccari S, Ducrocq F, Thomas P, Darnaudéry M. Long-term behavioural alterations in female rats after a single intense footshock followed by situational reminders. Psychoneuroendocrinology. 2005;30:316–324. doi: 10.1016/j.psyneuen.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 324.Nahvi RJ, Nwokafor C, Serova LI, Sabban EL. Single prolonged stress as a prospective model for posttraumatic stress disorder in females. Front. Behav. Neurosci. 2019;13:17. doi: 10.3389/fnbeh.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Shimamoto, A., Holly, E. N., Boyson, C. O., Debold, J. F. & Miczek, K. A. Individual differences in anhedonic and accumbal dopamine responses to chronic social stress and their link to cocaine self-administration in female rats. Psychopharmacology. 10.1007/s00213-014-3725-9 (2015). [DOI] [PMC free article] [PubMed]
- 326.Bourke, C. H. & Neigh, G. N. Exposure to repeated maternal aggression induces depressive-like behavior and increases startle in adult female rats. Behav. Brain Res. 10.1016/j.bbr.2011.11.001 (2012). [DOI] [PMC free article] [PubMed]
- 327.Finnell, J. E. et al. Essential role of ovarian hormones in susceptibility to the consequences of witnessing social defeat in female rats. Biol. Psychiatry10.1016/j.biopsych.2018.01.013 (2018). [DOI] [PMC free article] [PubMed]
- 328.Iñiguez SD, et al. Vicarious social defeat stress induces depression-related outcomes in female mice. Biol. Psychiatry. 2018;83:9–17. doi: 10.1016/j.biopsych.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Burke, H. M. et al. Sex-specific impairment of spatial memory in rats following a reminder of predator stress. Stress10.3109/10253890.2013.791276 (2013). [DOI] [PubMed]
- 330.Park, C. R., Zoladz, P. R., Conrad, C. D., Fleshner, M. & Diamond, D. M. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn. Mem. 10.1101/lm.721108 (2008). [DOI] [PMC free article] [PubMed]
- 331.Daskalakis, N. P., Cohen, H., Cai, G., Buxbaum, J. D. & Yehuda, R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc. Natl Acad. Sci. USA10.1073/pnas.1401660111 (2014). [DOI] [PMC free article] [PubMed]
- 332.Sawamura, T. et al. Effect of paroxetine on a model of posttraumatic stress disorder in rats. Neurosci. Lett. 10.1016/j.neulet.2003.12.039 (2004). [DOI] [PubMed]
- 333.Zhang L-M, et al. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacoloy. 2015;232:663–672. doi: 10.1007/s00213-014-3697-9. [DOI] [PubMed] [Google Scholar]
- 334.Kumar A, Garg R, Gaur V, Kumar P. Nitric oxide mechanism in protective effect of imipramine and venlafaxine against acute immobilization stress-induced behavioral and biochemical alteration in mice. Neurosci. Lett. 2009;467:72–75. doi: 10.1016/j.neulet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 335.Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology. 2006;189:165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- 336.Perrine SA, et al. Severe, multimodal stress exposure induces PTSD-like characteristics in a mouse model of single prolonged stress. Behav. Brain Res. 2016;303:228–237. doi: 10.1016/j.bbr.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 337.Guo L, et al. GHS-R1a deficiency alleviates depression-related behaviors after chronic social defeat stress. Front. Neurosci. 2019;13:364. doi: 10.3389/fnins.2019.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yang, R. et al. Core modular blood and brain biomarkers in social defeat mouse model for post traumatic stress disorder. BMC Syst. Biol. 7, 10.1186/1752-0509-7-80 (2013). [DOI] [PMC free article] [PubMed]
- 339.Diamond DM, et al. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- 340.Belzung C, El Hage W, Moindrot N, Griebel G. Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology. 2001;41:400–408. doi: 10.1016/s0028-3908(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 341.Wilson, C. B. et al. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Front. Behav. Neurosci. 8, 10.3389/fnbeh.2014.00256 (2014). [DOI] [PMC free article] [PubMed]
- 342.Matar MA, Cohen H, Kaplan Z, Zohar J. The effect of early poststressor intervention with sertraline on behavioral responses in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2006;31:2610–2618. doi: 10.1038/sj.npp.1301132. [DOI] [PubMed] [Google Scholar]
- 343.Belda X, Fuentes S, Nadal R, Armario A. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm. Behav. 2008;54:654–661. doi: 10.1016/j.yhbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 344.Martí O, García A, Vellès A, Harbuz MS, Armario A. Evidence that a single exposure to aversive stimuli triggers long-lasting effects in the hypothalamus-pituitary-adrenal axis that consolidate with time. Eur. J. Neurosci. 2001;13:129–136. [PubMed] [Google Scholar]
- 345.Sood R, et al. Underwater trauma causes a long-term specific increase in the expression of cyclooxygenase-2 in the ventral CA1of the hippocampus. Psychoneuroendocrinology. 2014;49:62–68. doi: 10.1016/j.psyneuen.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 346.Monleón, S., Duque, A. & Vinader-Caerols, C. Effects of several degrees of chronic social defeat stress on emotional and spatial memory in CD1 mice. Behav. Proces.10.1016/j.beproc.2015.12.002 (2016). [DOI] [PubMed]
- 347.Adamec RE, Burton P, Shallow T, Budgell J. NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure—implications for anxiety associated with posttraumatic stress disorder. Physiol. Behav. 1998;65:723–737. doi: 10.1016/s0031-9384(98)00226-1. [DOI] [PubMed] [Google Scholar]
- 348.Kozlovsky N, et al. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int. J. Neuropsychopharmacol. 2007;10:741–758. doi: 10.1017/S1461145707007560. [DOI] [PubMed] [Google Scholar]