Abstract
The renin-angiotensin system (RAS) is a key player in the control of the cardiovascular system and hydroelectrolyte balance, with an influence on organs and functions throughout the body. The classical view of this system saw it as a sequence of many enzymatic steps that culminate in the production of a single biologically active metabolite, the octapeptide angiotensin (ANG) II, by the angiotensin converting enzyme (ACE). The past two decades have revealed new functions for some of the intermediate products, beyond their roles as substrates along the classical route. They may be processed in alternative ways by enzymes such as the ACE homolog ACE2. One effect is to establish a second axis through ACE2/ANG-(1–7)/MAS, whose end point is the metabolite ANG-(1–7). ACE2 and other enzymes can form ANG-(1–7) directly or indirectly from either the decapeptide ANG I or from ANG II. In many cases, this second axis appears to counteract or modulate the effects of the classical axis. ANG-(1–7) itself acts on the receptor MAS to influence a range of mechanisms in the heart, kidney, brain, and other tissues. This review highlights the current knowledge about the roles of ANG-(1–7) in physiology and disease, with particular emphasis on the brain.
I. INTRODUCTION
The renin-angiotensin system (RAS) is a key player in the control of blood pressure and the hydroelectrolyte balance. The first end product of this system associated with a biological activity was the octapeptide angiotensin (ANG) II, produced through the activity of the angiotensin converting enzyme (ACE) at the end of the pipeline. Years later it was discovered that the amino-terminal aspartate could be removed from ANG II to produce the heptapeptide ANG III, which had similar biological activities (67, 562). Finally, about three decades ago came the discovery that phenylalanine could be removed from the carboxy terminus of ANG II to produce another RAS peptide, ANG-(1–7) (45, 65, 85, 470, 492).
Originally the biological relevance of this finding was questioned because up to that point, ANG-(1–7) had been seen purely as a degradation product of ANG I and ANG II (221, 619). This perception has changed mainly through the discovery that the ACE homolog ACE2 is often involved in its formation and that ANG-(1–7) acts on the G protein-coupled receptor MAS (468). ANG-(1–7) and ANG II usually have opposing effects, stimulating high interest because it suggests that ANG-(1–7) or other MAS agonists might be useful in the development of therapeutic agents to counteract the negative role of ANG II in many diseases. This review will examine physiological aspects of the ACE2/ANG-(1–7)/MAS axis in different organs/systems, with special emphasis on the role of ANG-(1–7) in the brain.
II. THE DISCOVERY OF THE ACE2/ANGIOTENSIN-(1–7)/MAS AXIS
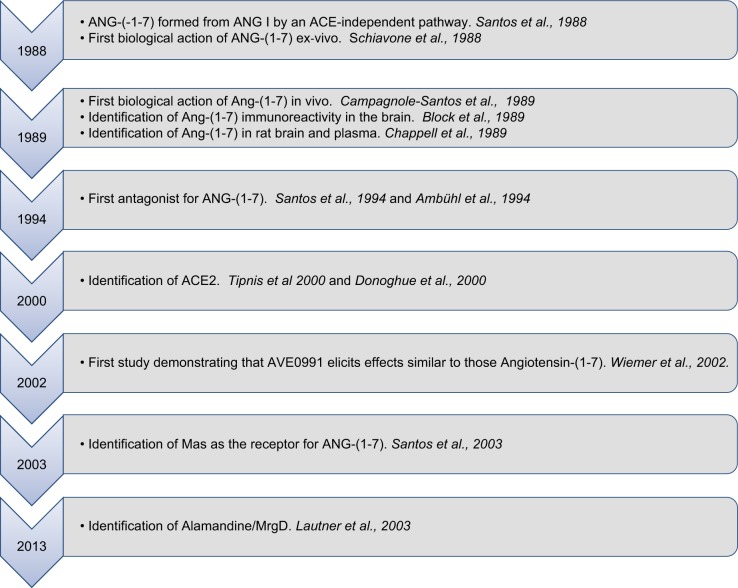
FIGURE 1 shows the main findings which contributed to the discovery of the ACE2/ANG-(1–7)/MAS axis as one of the main components of the RAS. In 1968, Yang, Erdos and Chiang (619) described the heptapeptide Des-Phe8-Angiotensin II, generated from ANG II as a route for the inactivation of ANG II. Tonnaer et al. (544) came to the same conclusion when he found this heptapeptide in brain synaptosomes. In 1988, Santos and colleagues examined tissue taken from dogs that had been treated with enalapril to determine how this affected the RAS in the brain (470). They incubated brain micro-punch homogenates with 125I-labeled ANG I and discovered a consistent formation of a radioactive peak they identified as 125I-ANG-(1–7). The peptide continued to form when they added an ACE inhibitor to the incubation. This meant that the ANG-(1–7) peptide was being formed from ANG I, but along a different route that bypassed ACE (470). The formation of 125I-ANG-(1–7) from 125I-ANG I was in keeping with an early observation made by Greene et al. (221) in whole rodent brain homogenates, who had interpreted ANG-(1–7) as the end point of an angiotensin inactivation pathway. Santos et al. (470) suggested instead that ANG-(1–7) might be another biologically active product of the RAS, achieved along this enzymatic pathway. The same year, Schiavone et al. (492) delivered proof through experiments with hypothalamus/hypophysial explants. The function they found for ANG-(1–7) in this preparation was to trigger the release of vasopressin (AVP), through dose-dependent effects that were just as potent as those of ANG II. In vivo confirmation came 1 yr later, when Campagnole-Santos et al. reported the first biological action of ANG-(1–7) in vivo (65). Microinjection of femtomole amounts of ANG-(1–7) in the nucleus tractus solitarii (NTS) produced significant reductions in the blood pressure of urethane-anesthetized rats. This effect was similar to that produced by microinjection of ANG II in the same region. These exciting observations motivated Carlos Ferrario's group at Cleveland Clinic to produce an ANG-(1–7) antibody which was then used by Block et al. (45) to describe the localization of ANG-(1–7) immunoreactivity in the brain. In 1989, Chappell et al. (85) again using this antibody confirmed these findings. In 1994, we (473) and Ambühl et al. (17) described the first selective antagonist for ANG-(1–7), its analog d-Ala7-ANG-(1–7), synthetized by Prof. Mahesch Khosla, which we named A-779. Having a selective antagonist was critical in uncovering many of the physiological actions of ANG-(1–7) including its role in baroreflex modulation (66) and in the central control of blood pressure (183). The use of A-779 was also important in postulating that ANG-(1–7) had a specific receptor (183).
FIGURE 1.
ACE2/angiotensin-(1–7)/Mas axis discovery timeline.
Despite the relatively large body of evidence that ANG-(1–7) was a biologically active component of the RAS, the idea only gained general acceptance with the discovery that ACE2 was the major enzyme involved in its formation (140, 543, 570, 647), followed by identifying MAS as its receptor years later (479). ACE2 is a membrane-anchored carboxypeptidase, which is the product of an ancient duplication of ACE that underwent a fusion with collectrin. Its discovery was reported in two papers which appeared almost simultaneously in 2000 (140, 543). The first, by Tipnis et al. (543) reported on the ability of ACE2 to convert ANG II to ANG-(1–7) and thus identified it as the key ANG-(1–7) forming enzyme. The Donoghue et al. article (140) did not test ANG II as a substrate for ACE2; they identified several other substrates, such as des-Arg9-bradykinin, apelin, and neurotensin, and first reported ANG-(1–7) generation in a subsequent paper (570). Later the Turner group also discovered that the ectodomain of ACE2 can be shed from the membrane by ADAM17 (290). Interestingly, some years later it turned out that the carboxypeptidase activity is not the only function of the enzyme. Its collectrin domain serves as trafficking adaptor for the large amino acid transporter B(O)AT1, transferring it to the apical membrane of epithelial cells in the intestine (285). This translocation is essential for the uptake of several amino acids from food, and the absence of ACE2 leads to the depletion of some, particularly of tryptophan in the blood (242). Later ACE2 was also discovered to be the cellular receptor of the SARS coronavirus (309).
MAS is a G protein-coupled receptor (GPCR) that was first described in 1986 as mas oncogene (the name is the first 3 surname letters of the patient whose tumor cells were used to identify the gene)(624) and was initially thought to be a receptor of ANG II (263). However, this hypothesis was disproved through further studies on MAS signaling (15) and also cloning of the ANG II receptor AT1 in 1991 (382, 487). It was not until 2003 that we finally demonstrated the specific binding of ANG-(1–7) to Mas-transfected cells (479). The fact that this binding was abolished in kidney sections of Mas-deficient mice demonstrated that MAS is a receptor for the heptapeptide (479).
III. ANGIOTENSIN-(1–7) FORMATION AND METABOLISM
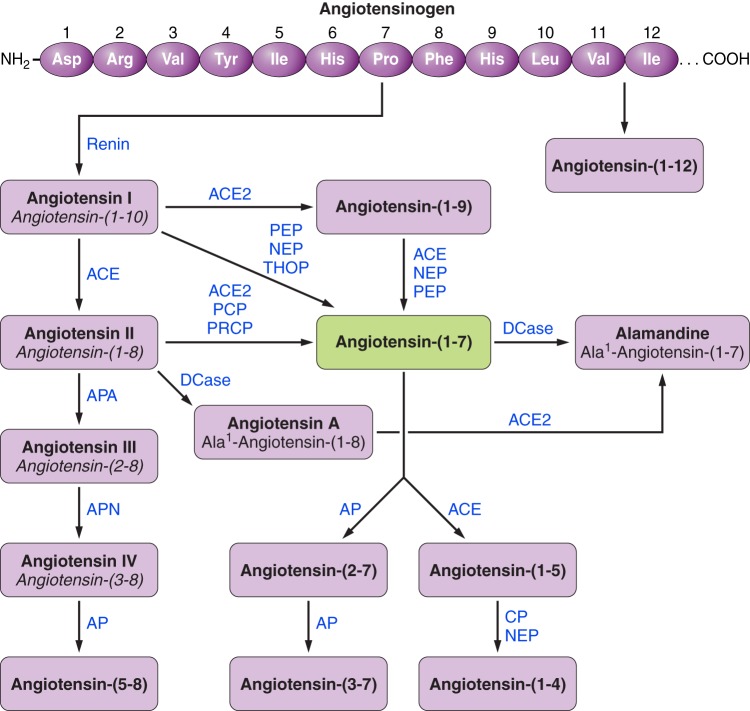
FIGURE 2 shows the main enzymatic pathways involved in the formation and catabolism of angiotensin peptides. In the classical RAS, the enzyme renin cleaves its substrate angiotensinogen to form the decapeptide ANG I, which is in turn cleaved by ACE to produce ANG II, a key player in this system. As shown in FIGURE 2, ANG-(1–7) can be generated by the cleavage of ANG I by endopeptidases or ANG II by carboxypeptidases. The main enzymes involved in the production of ANG-(1–7) from ANG I are thimet oligopeptidase (THOP1; EC 3.4.24.15), neutral endopeptidase (NEP; EC 3.4.24.11) and prolyl oligopeptidase (PEP; EC 3.4.21.26). In addition, ACE2 (EC 3.4.17.23), carboxypeptidase A (EC 3.4.17.1), and prolyl carboxypeptidase (EC 3.4.16.2) can generate ANG-(1–7) from ANG II (72, 441, 468). The formation of ANG-(1–7) from ANG I by ACE2 involves the production of the intermediate ANG-(1–9) and its subsequent cleavage by ACE or NEP (444). However, the catalytic efficiency of this pathway is much lower than that of the ACE2-dependent conversion of ANG II to ANG-(1–7) (444).
FIGURE 2.
The renin-angiotensin system cascade: updated view. ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; APA, aminopeptidase A; APN, aminopeptidase N; PRCP, prolyl endopeptidase; PCP, prolylcarboxyendopeptidase; NEP, neutral endopeptidase; PEP, prolyl endopeptidase; CP, carboxypeptidase; AP, aminopeptidase; Dcase, decarboxylase; THOP, thimet oligopeptidase.
A. Alamandine
Recently a new biologically active angiotensin, alamandine, was discovered (297) as seen in FIGURE 2. Alamandine has an Ala which replaces Asp at position 1 of ANG-(1–7). This peptide can be generated by either decarboxylation of the Asp residue or through the catalytic action of ACE2 on ANG A (264), Ala1-ANG II. Functional studies in blood vessels (235, 297) and transfected cells (297) have provided evidence that the receptor for alamandine is the MAS-related G protein-coupled receptor D (MrgD). However, despite solid functional evidence that the effects of alamandine can be at least partially mediated through binding to MrgD, there is no direct radioligand binding data or other classical pharmacological data to fully demonstrate that MrgD has this function. Even so, alamandine has attracted attention as a potential mediator of the RAS.
IV. TOOLS FOR STUDYING ANG-(1–7)
A. Pharmacological Tools
The main pharmacological tools used to study the ACE2/ANG-(1–7)/MAS axis are shown in FIGURE 3. Many are MAS agonists that stimulate NO production/release [ANG-(1–7), AVE 0991, CGEN 861, CGEN 856, CGEN 856S, cyclic ANG-(1–7)] (3, 147, 163, 281, 424, 449, 489, 490, 509, 545, 594), NorLeu3-A(1–7) (2, 448). There are also two antagonists [d-Ala7-ANG-(1–7) (A-779) and d-Pro7-ANG-(1–7)]. Other MAS ligands, the non-peptides AR234960 (agonist) and AR244555 (inverse agonist) and neuropeptide FF (NPFF) appear to act through a different signaling pathway in which ANG-(1–7) is essentially ineffective (276, 633). Therefore, the possibility that some of the non-peptide or peptidic compounds are biased MAS agonists should be considered (522).
FIGURE 3.
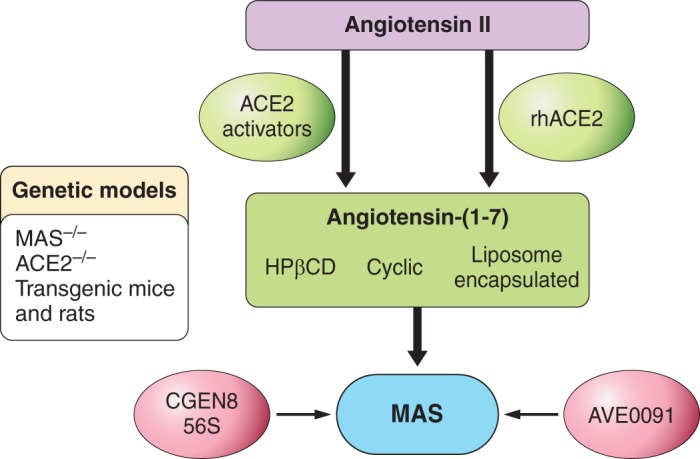
Pharmacological and genetic tools for the study of the ACE2/angiotensin-(1–7)/Mas axis.
One of the formulations for the chronic administration of ANG-(1–7) that has been well tested in animals is an inclusion compound, hydroxypropyl-β-cyclodextrin/ANG-(1–7) [HPβCD-ANG-(1–7)]. This compound protects ANG-(1–7) from inactivation by digestive tract enzymes and permits oral administration (188, 332). It should be emphasized that in this case, only ANG-(1–7) enters the bloodstream. The inclusion compound acts as a sustained-release system or, more properly, as a long-lasting releasing system. This approach has led to the description of many beneficial cardiovascular and metabolic effects of ANG-(1–7), including antithrombogenesis (185), an attenuation of cardiac remodeling induced by isoproterenol treatment (347), a reduction of the lesion area, and an attenuation of acute and chronic postinfarction cardiac dysfunction (348). There have also been reports of antihypertensive effects (37) and improvements in cases of erectile dysfunction (187), muscular dystrophy (5a, 455), and type II diabetes mellitus (486). Some of the effects in preclinical studies have been remarkable considering that the peptide was given orally once a day in doses ranging from 10 to 50 μg/kg, which are equivalent to 11 to 55 nmol·kg−1·day−1.
In addition to cyclodextrins, cyclic ANG-(1–7) is also undergoing preclinical testing (146, 281). It is more resistant to enzymatic hydrolysis than ANG-(1–7). Interestingly, the vasorelaxation produced by cyclic ANG-(1–7) in aortic rings from Sprague-Dawley rats is only partially blocked by the MAS antagonist A-779 (281), whereas this effect is completely blocked by the ANG-(1–7) analog d-Pro7-ANG-(1–7), an ANG-(1–7)/alamandine antagonist (297). This pharmacological profile suggests that cyclic ANG-(1–7) could be a dual MAS/MrgD agonist sharing ANG-(1–7) and alamandine characteristics. This possibility awaits clarification.
In addition to MAS agonists, recombinant human ACE2 (hACE2) is currently being used in physiological/pharmacological studies and tests of its potential therapeutic use (241). Prof. Raizada's group has suggested another interesting possibility: to use ACE2 activators to alter the balance between the ACE/ANG II/ AT1R and the ACE2/ANG-(1–7)/MAS axes. The group virtually screened its structure to identify small-molecule ACE2 activators (250). The first compound they found was the 1-[(2-dimethylamino)ethylamino]-4-(hydroxymethyl)-7-[(4-methylphenyl)sulfonyloxy]-9H-xanthene-9-one, or XNT. Acute administration induced a dose-dependent hypotensive response in spontaneously hypertensive rats (SHR), while long-term treatment with XNT improved cardiac function and reversed the cardiac and renal fibrosis in these animals (250). Oral administration of XNT was able to attenuate diabetes-induced heart dysfunction (381). XNT adminstration also prevented the increase in right ventricular systolic pressure and hypertrophy in a monocrotaline-induced pulmonary hypertension model (169) and attenuated thrombus formation in SHR (190). Numerous protective effects have been reported with diminazene aceturate (DIZE), another putative ACE2 activator (102, 118, 119, 184, 186, 335, 341, 357, 429, 445, 511, 513, 535, 566). It should be pointed out, however, that the beneficial effects of these small-molecule activators might also depend on ACE2-independent mechanisms (234). And results obtained in rodents should be interpreted with caution considering the important differences between the biochemical properties of rodent and human ACE2 (622). Moreover, ACE2 can cleave other substrates (140), a fact which should be taken into account when interpreting results obtained with methods involving the gain or loss of ACE2 functions.
More recently, oral delivery of ACE2 and ANG-(1–7) bioencapsulated in plant cells has been reported to attenuate pulmonary hypertension (497). This is in keeping with early studies using a similar construct, developed by the Reudelhuber group (556), showing that transgenic rats and mice which overexpress an ANG-(1–7)-forming fusion protein presented reduced heart failure/remodeling following isoproterenol treatment or ANG II infusion (366, 466).
B. Genetic Models
1. ACE2 models
a) ace2 knockout Mice.
Based on the pleiotropic actions of this protein, mice lacking ACE2 are expected to exhibit increased levels of ANG II, and decreased levels of ANG-(1–7) and tryptophan as well as alterations in other peptide levels, all of which may contribute to the phenotypes that are observed. The X-chromosomal localization of the ACE2 gene makes male mice with ACE2 gene deletion already deficient in the enzyme in the hemizygous state (ACE2-/y). The first report on ACE2-/y mice described several cardiac abnormalities, particularly at older ages (104), which were later attributed to an increased effect of ANG II. However, other groups could not confirm these effects and the issue remained controversial (231, 232, 617). Notwithstanding, several studies report a higher susceptibility of ACE2-/y and heterozygous female ACE2+/− mice to cardiac injury induced by pressure overload or diabetes (410, 598, 617). Additional cardiovascular effects of ACE2 deletion remain under debate, including hypertension. Here the strain of mice that is used seems to be important. The 129 strain of mice lacking ACE2 is normotensive and C57BL/6 and FVB/N mice are hypertensive (232, 541) (our unpublished results). The hypertensive phenotype of ACE2-deficient mice on the C57BL/6 background is exacerbated in pregnancy, and they develop a preeclampsia-like syndrome (39). The main reason for increased blood pressure in ACE2-/y mice is probably endothelial dysfunction (327), but central effects including an increased sympathetic outflow cannot be excluded (605). Moreover, endothelial dysfunction may be involved in the aggravating effect of ACE2 deficiency in two models of atherosclerosis and aortic aneurysm, apolipoprotein E (ApoE)- and low-density lipoprotein receptor-deficient mice (397, 457, 537, 538), as well as in the increased neointima formation after vascular injury (457). ACE2-deficient mice also show a worse outcome in other inflammation and injury models, such as diabetic and shock-induced kidney injury (501, 598, 621), chronic hepatic injury (406), bleomycin- or influenza virus-induced lung injury (443, 648), and in models of acute respiratory distress syndrome (258). The lung injury phenotype could be rescued by infused mesenchymal stem cells overexpressing ACE2 (243). The unifying feature of most of the injury models is an increase in oxidative stress in ACE2-/y mice, which was recently confirmed for the kidney (603), liver (own unpublished results), and vessels (412, 431) of these animals.
Due to the trafficking function of ACE2 in the gut, mice lacking this protein show reduced tryptophan and other large amino acids in the blood, alterations in their gut microflora, and intestinal inflammation (242). These effects may contribute to the metabolic changes observed in these mice, mainly in insulin resistance and impaired glucose homeostasis (397, 406), which is aggravated under high-fat diets (328). The fact that ANG-(1–7) could rescue this phenotype in the liver seems again to indicate that the dysbalance between different angiotensin peptides is crucial.
b) human ace2 overexpression in mouse.
In an effort to humanize mice regarding their susceptibility to the human severe acute respiratory syndrome (SARS) coronavirus, several transgenic mouse models have been generated expressing human ACE2 in a range of tissues using the ACE2 promoter itself (620), the ubiquitously active cytomegalovirus promoter (546, 623), or the airway-specific cytokeratin 18 promoter (355, 392). One of these mice was also used in a kidney injury model and showed a protected phenotype (621).
c) human ace2 overexpression in mouse heart.
Somewhat surprisingly, ventricular tachycardia and sudden death were observed in transgenic mice expressing human ACE2 in the heart (141). This was accompanied by a dysregulation of connexin expression. Peptides besides angiotensins are likely to be involved in this gain-of-function model, and apelin may be a candidate, since it has been shown to be important for cardiac function (287) and is also metabolized by ACE2 (570).
d) human ace2 overexpression in mouse podocytes.
In contrast to the heart, overexpression of human ACE2 in the kidney, particularly using the nephrin promoter in podocytes, turned out to be protective in diabetes-induced kidney injury (386). Again, the shifted balance between ANG II and ANG-(1–7) seemed to be critical through its regulation of the local expression of transforming growth factor β.
e) human ace2 overexpression in mouse brain.
ACE2 seems to be particularly important in the central nervous system for cardiovascular regulation. Transgenic mice expressing human ACE2 driven by the synapsin promoter exhibit protective phenotypes for cardiovascular diseases including hypertension induced by peripheral infusions of ANG II (154) and by DOCA-salt treatment (605), cardiac hypertrophy also induced by ANG II (154), chronic heart failure elicited by coronary ligation (606), and stroke triggered by middle cerebral artery occlusion (91, 637). The balance between ANG-(1–7) and ANG II in different brain regions, which determines local NO production and regulates the autonomic nervous system, seems to be critical in these effects.
f) human ace2 overexpression in rat vascular smooth muscle.
ACE2 is a candidate gene for hypertension in the spontaneously hypertensive stroke-prone rat (SHRSP) strain. This is based on a linkage study and on the fact that very low levels of the enzyme are found in these animals (104). Therefore, we restored ACE2 expression specifically in the vascular smooth muscle cells (VSMC) of these rats by expressing human ACE2 from the smooth muscle myosin heavy chain promoter. This again shifted the balance between ANG II and ANG-(1–7) to the protective side, blunted oxidative stress, improved endothelial function, and markedly reduced blood pressure in the transgenic rats (439).
2. MAS models
a) Mas knockout mice.
The first phenotypes analyzed in Mas-deficient (Mas−/−) mice concerned brain function, since this is the most important MAS expressing organ (578). Male (but not female) (579) Mas−/− mice showed increased anxiety-like behavior and long-term potentiation (LTP) in the hippocampus. Unexpectedly, despite the improved LTP, object recognition memory was impaired (299).
The discovery of MAS as the receptor for ANG-(1–7) triggered extensive cardiovascular phenotyping. On the C57BL/6 mouse background, MAS deficiency leads to cardiac fibrosis and dysfunction both in vitro (78) and in vivo (416, 476). While oxidative stress and endothelial dysfunction were observed in the C57BL/6 and FVB/N genetic backgrounds (430, 611), it resulted only in significantly increased blood pressure in FVB/N mice. MAS seems to play a major role in regional hemodynamics since vascular resistance was significantly and differentially altered in the tissues of Mas−/− mice (48). Increased vascular resistance in the corpus cavernosum is also the most likely cause of the erectile dysfunction that occurs in these mice (107).
Renal function is also impaired in Mas−/− mice, which exhibit increased urinary volume and proteinuria (423). Surprisingly, however, the absence of MAS seems to be beneficial in one kidney injury model (149), while in others the outcome is worse, confirming the protective effects of the ACE2/ANG-(1–7)/MAS axis of the RAS (507). This discrepancy remains unresolved, but anti-inflammatory actions of MAS have repeatedly been described (267, 485, 514), most recently in an endotoxic shock model (515).
Mas−/− mice develop metabolic abnormalities such as type 2 diabetes mellitus and dyslipidemia (484), which together with their increased blood pressure renders them a valuable model for the metabolic syndrome. This is accompanied by decreased PPARγ expression in adipose tissue (346) and the development of liver steatosis when the animals are bred with apolipoprotein E (ApoE)-deficient mice (505).
b) mas overexpression in retina.
MAS has a certain degree of constitutive, ligand-independent activity that may induce proliferative effects in cells when the gene is overexpressed. This may be the reason for the degeneration of photoreceptors in a transgenic mouse in which MAS is overexpressed in the retina under the control of the opsin promoter (612).
3. ANG-(1–7) models
a) transgenic rats overexpressing ang-(1–7).
Using an artificial protein which releases a predesigned peptide upon secretion from a cell (366, 368), ANG-(1–7) was overexpressed in transgenic rats using the cytomegalovirus promoter (166). Surprisingly the resulting strain, TGR(A1–7)3292, expressed the peptide mainly in the testis, which nevertheless led to significantly increased plasma levels. Despite a decrease in total peripheral resistance and increases in blood flow to several organs, the animals remained normotensive, which may be due to their improved cardiac function (48). These cardiac effects protect the heart from pressure and ischemia-induced damage (477). The high plasma levels of ANG-(1–7) also have effects in the kidney, such as a reduced urinary flow and increased urinary osmolality (166). Furthermore, the TGR(A1–7)3292 strain exhibits decreased levels of lipids in plasma, improved glucose tolerance, and less fat mass, confirming the beneficial metabolic actions of the peptide (44, 483).
b) transgenic mice and rats overexpressing ang-(1–7) in the heart.
Transgenic mice and rats expressing the ANG-(1–7) release construct specifically in the heart with the alpha cardiac myosin heavy chain promoter show normal to slightly improved cardiac function at the baseline and are protected from cardiac hypertrophy (161, 366), but not from myocardial infarction (589).
V. ACTIONS OF ANGIOTENSIN-(1–7)
A. Brain
The brain expresses all the necessary components, precursor and enzymes, to produce the active peptides of the RAS known to date: ANG II, ANG III, ANG IV, ANG-(1–7), and alamandine. The effects elicited by angiotensins in the brain are complex, site-specific, and dependent on the pathophysiological condition. ANG II, ANG III, and ANG-(1–7) can be considered the main effectors of the RAS in the brain, since ANG IV presents more restricted actions (599). These peptides interact with a certain degree of selectivity to receptor proteins including AT1, AT2, MAS, or MrgD distributed within the central and peripheral nervous systems. Although there is still a debate on whether ANG II or ANG III is the true ligand of the AT1 receptor in the brain (195), the effects described for ANG-(1–7) appear to be related to MAS (479). It is well established that in the central nervous system (CNS), ANG II/ANG III function through AT1 to induce thirst, release of AVP, an increase of sympathetic activity and blood pressure, and an impairment of the baroreflex function. In contrast, ANG-(1–7) facilitates the baroreflex and may lower or increase blood pressure depending on the specific brain area or the pathophysiological condition in which it occurs. Additional features not directly related to cardiovascular function have been described, including neuroprotection from brain ischemia or hemorrhage, improvements of memory, and an attenuation of epileptic seizures. The possibility that ANG II and ANG-(1–7) may exert opposite effects from each other in the brain, even if this only occurs under specific physiological situations, provides a more complete picture of the RAS mechanisms involved in the neural regulation of physiological functions.
1. ANG-(1–7) metabolism in the brain
The exact site at which angiotensins are generated in the brain, intracellular or extracellular, is still uncertain (269, 415, 592). Nor is it known whether the generation of ANG peptides is solely dependent on brain angiotensinogen (Angiotensinogen), ACE, ACE2 and renin, taking into account discrepancies in the location of RAS components (23, 45, 56, 160, 224, 415). However, increasing evidence suggests that RAS generation is intracellular and includes the expression of intracellular ANG II and ANG-(1–7), as well as their respective receptors (87, 233, 289, 379). Krob et al. (286) demonstrated intense ANG-(1–7) immunostaining in hypothalamic neurons of TGR(mREN2)27 transgenic rats, suggesting an intracellular localization of the peptide. Further experiments have demonstrated an intracellular expression of ACE2 based on immunohistochemistry in different areas of the brain (142) and ANG-(1–7) expression in primary neuronal cell cultures from the hypothalamic-brain stem areas (209, 211, 325). Interestingly, studies using double transgenic mice that overexpress both human Angiotensinogen and renin in either glial or neuronal cells suggest that the source of ANG II may be both cell types. Moreover, glia-derived angiotensin peptides are responsible for impairments in the baroreflex sensitivity (BRS) that controls heart rate (HR), whereas neuronal overexpression of angiotensin peptides resets the baroreceptor set point without altering BRS (458). Overexpression in both glial and neuronal cells contributes to increases in resting arterial pressure (379). On the other hand, rats that express an antisense RNA against Angiotensinogen under the control of the glial GFAP promoter indicates that the source of ANG-(1–7) is mainly non-glial cells (460).
More recently an interesting possibility has been raised: ANG-(1–12) could be a renin-independent precursor for angiotensin peptides in the rodent brain (158). ANG-(1–12) exhibits higher concentrations in the brain than ANG II and displays cardiovascular effects upon microinjection into discrete areas of the medulla and hypothalamus. These effects were partially antagonized by A-779, suggesting that ANG-(1–12) can be metabolized to ANG-(1–7) (158).
Nevertheless, ANG-(1–7) can be processed directly from ANG I by several endopeptidases including NEP, thimet oligopeptidase, and prolyl endopeptidase (84, 442, 592). Additionally, ANG-(1–7) is efficiently generated directly from ANG II by the monocarboxypeptidase, ACE2, and prolyl carboxypeptidase (136, 211). Accordingly, it has been shown that ANG-(1–7) attenuates the ANG II pressor response in the hypothalamus, and concomitant injections of ANG II and A-779 increased the response, suggesting that ANG-(1–7) may be formed from ANG II, at least in the hypothalamus (256). It is interesting to point out that although ACE constitutes the major ANG II-forming pathway, it also degrades ANG-(1–7) in a way that leads to ANG-(1–5) (89). However, so far not a single brain cell has been detected that expresses all components of the RAS; thus the formation of bioactive forms of angiotensin might require interactions between multiple cell types, or, the brain possesses enzymatic mechanisms different from the classical ones which are responsible for the formation of neuroactive forms of angiotensins (115, 116, 574).
Recently, sheep medulla and cerebrospinal fluid (351) have demonstrated a peptidase activity capable of metabolizing ANG-(1–7) to ANG-(1–4). This peptidase exhibits marked sensitivity to mercury-based inhibitors, chelating agents, and the metalloendopeptidase agent JMV-390. It also hydrolyzes ANG-(1–7) to a greater extent than ANG II and ANG I, while other bioactive peptides such as bradykinin, neurotensin, and apelin-13 are not cleaved. The peptidase appears to preferentially cleave the Tyr4-Ile5 bond of ANG-(1–7) generating ANG-(1–4). These data likely represent a novel pathway for the specific regulation of central ANG-(1–7) levels. The intracellular distribution of this ANG-(1–7) peptidase and the specific cell type that may express it within the brain (i.e., neuronal vs. glia) are not known. This peptidase may influence the local processing of ANG-(1–7) within cells or may be secreted or released from medullary tissue to degrade extracellular ANG-(1–7) or other peptides/substrates (87). The protein identity of this metalloendopeptidase-like activity, which converts ANG-(1–7) to ANG-(1–4) in the brain, has not yet been described, and its role in the physiological regulation of the brain RAS is similarly elusive.
ANG-(1–7) in the brain can also be converted to alamandine, through the decarboxylation of aspartate to alanine (297). Although a selective enzyme responsible for this decarboxylation has not yet been identified, the actions of alamandine and the receptor mediating its effects, MrgD, have been described in the brain (573).
2. ANG-(1–7) location in the brain
ANG-(1–7) immunoreactivity has been described in paraventricular, suprachiasmatic nuclei, the bed nucleus of the stria terminals, substantia innominata, median eminence, and neurohypophysial and other areas of the medulla oblongata and amygdala of normotensive rats (45). ANG-(1–7) was also identified in hypothalamic extracts (63, 85). Later, ANG-(1–7) immunoreactivity was described in neurons of the supraoptic nucleus (SON), and in the anterior (ap-), medial (mp-), and lateral (lp-) parvocellular, and posterior magnocellular (pm-) subdivisions of the paraventricular nucleus (PVN) in TGR(mREN2)27 transgenic hypertensive rats (286). Furthermore, cells immunoreactive for ANG-(1–7) were also stained for AVP, specifically in the SON and in the pmPVN, suggesting that ANG-(1–7) and AVP are colocalized, coreleased, and may carry out similar actions on common targets (286), possibly in combination. Interestingly, ANG-(1–7) levels were found to be fivefold higher in hypophysial-portal plasma than in jugular plasma of normotensive sheep, while no differences were observed for ANG II, renin, or angiotensinogen (298). In addition, it was shown that ACE2, the major enzyme involved in ANG-(1–7) formation, is present in the brain, predominantly in neurons (142). ACE2 immunostaining is in fact widespread throughout the brain, from the telencephalon to the medulla, at least in the mouse (142).
3. MAS location in the brain
The hypothesis of a receptor that selectively mediated the physiological effects of ANG-(1–7) in the brain developed from studies that showed that ANG-(1–7) and ANG II triggered distinct effects. The first antagonistic effect of ANG-(1–7) was described on the modulation of the baroreflex (66). ANG-(1–7), given intracerebroventricularly (ICV), facilitated baroflex control, while ANG II attenuated it (66). Further studies have strengthened this hypothesis by showing that ANG-(1–7) binds with low affinity to AT1 and AT2 receptors (452) and that its central and peripheral effects are different from those induced by ANG II (159, 473).
In 2003, with the identification of MAS as an ANG-(1–7) receptor (479), we showed that MAS expression was localized to specific areas of the brain (30) particularly related to cardiovascular control. There was a strong staining in the NTS, caudal and rostral ventrolateral medulla (CVLM and RVLM), inferior olive, parvo- and magnocellular portions of the PVN, SON, and lateral preoptic area (LPA) of normotensive Sprague-Dawley (SD) rats. However, other areas also stained for MAS, such as the hippocampal nucleus, different subregions of the frontal cortex, anterodorsal thalamic nucleus, basomedial and basolateral amygdaloid nucleus, and hypoglossal nucleus (nXII). In fact, MAS had already been recognized as an orphan receptor for some time and mRNA had been found in areas such as the hippocampus, the dentate gyrus, piriform cortex, and amygdala (58, 352, 369). In the murine brain, strong MAS protein expression was detected in the dentate gyrus of the hippocampus, within the piriform cortex, hippocampus, amygdala, basal ganglia, thalamus, and hypothalamus (192). Recently, Regenhardt et al. (433) have shown MAS immunostaining mainly in the soma of neurons and microglia of the adult rat cerebral cortex but not in astroglia, and in both nonnuclear and nuclear compartments. We still do not know whether AT1, AT2, and MAS may have a degree of overlapping localization or are expressed in distinct neuronal populations.
4. ANG-(1–7) actions in the brain
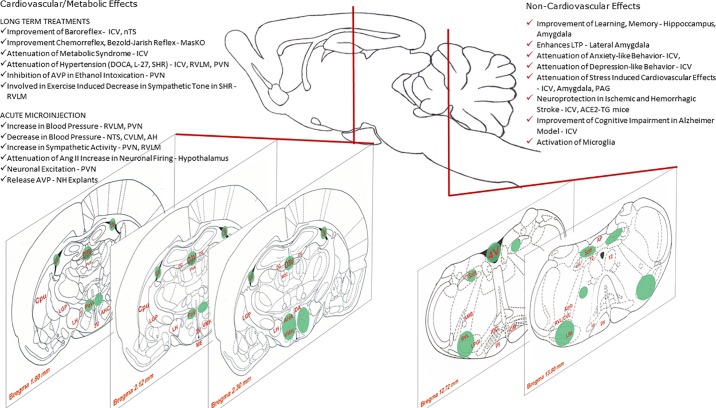
ANG-(1–7) acts as an important neuromodulator, especially in areas related to the tonic and reflex control of arterial pressure, in the hypothalamus, and in the dorsomedial and ventrolateral medulla (FIGURE 4). At these sites, the cardiovascular effects induced by ANG-(1–7) are blocked by the MAS antagonist A-779 (133, 336), suggesting that in the brain, ANG-(1–7) actions are mainly mediated by its interaction with MAS.
FIGURE 4.
Localization of Mas in the central nervous system and main actions of angiotensin-(1–7) in the brain. See text for definitions.
Among its central actions, the more consistent effects of ANG-(1–7) are related to the modulation of the baroreflex, especially through improving the bradycardic component of the baroreflex control of heart rate in normotensive (46, 66) or hypertensive animals (249, 402). This effect was initially investigated through short-term infusions of ANG-(1–7) into the lateral ventricle. On the other hand, infusion of A-779 attenuated the baroreflex sensitivity of normotensive rats but did not significantly alter the depressed baroreflex sensitivity of SHR (249, 402), suggesting an overall imbalance of ANG formation or MAS expression in SHR. However, when SHR received an ICV infusion of an ACE inhibitor, baroreflex experienced a significant increase in sensitivity that could be completely prevented by ICV A-779 (249). Moreover, the improvement in baroreflex after oral treatment with an ACE inhibitor in Goldblatt two kidney-one clip (2K1C) hypertensive rats was also reversed by ICV infusions of A-779 (52), showing that ANG-(1–7) may at least partly contribute to the beneficial effects on baroreflex from ACE inhibitor treatments. Because ACE inhibitors also affect bradykinin metabolism, we showed that ICV infusions of subeffective doses of ANG-(1–7), combined with equally subeffective doses of bradykinin, significantly facilitated baroreflex bradycardia, an effect which could be completely blocked by the bradykinin B2 receptor antagonist HOE-140 or by A-779, suggesting an interaction of both peptides in modulating baroreflex gain (46).
No alteration in blood pressure or drinking behavior was observed after short-term (up to few hours) infusions of ANG-(1–7) into the lateral ventricle (66) or microinjections into the PVN (427), in contrast to the classical stimulatory effect mediated by AT1 in the brain (339). However, when long-term infusions (14–28 days) were performed, a chronic increase in ANG-(1–7) levels in the CNS strongly attenuated the increase in arterial pressure that is observed in DOCA-salt rats (226), TGR(mREN2)27 hypertensive rats (390), or in female rats (614) subjected to DOCA-salt or ANG II hypertension (613). Similar data were observed after the delivery of an ANG-(1–7) fusion protein at the cisterna magna of TGR(mREN2)27 hypertensive rats (197). Furthermore, the blood pressure-lowering effect of ANG-(1–7) in DOCA-salt hypertensive rats was related to an improvement in baroreflex bradycardia, the restoration of the baroreflex control of renal sympathetic nerve activity (RSNA), and a regaining of the balance of cardiac autonomic tone (226). These effects were blocked by A-779, once again suggesting a mediation by MAS (273). An enhancement of the baroreflex control of the RSNA was also observed after 4 days of ICV infusion of ANG-(1–7) in rabbits subjected to chronic heart failure (274) and in transgenic mice overexpressing human ACE2 selectively in the brain (154). Additionally, mice lacking MAS presented a marked imbalance in the neural control of blood pressure, with a blunted sensitivity of not only the baroreflex but also the chemo- and Bezold-Jarisch reflexes (125). It is interesting to observe that in pathophysiological conditions, such as aortic coarctation (209), TGR(mREN2)27 hypertensive transgenic rats (493) exhibit an increase in concentrations of ANG-(1–7) in the brain.
Interestingly the facilitatory effect of ANG-(1–7) on the baroreflex control of heart rate is also consistently observed in the NTS, a key site in the brain stem which controls cardiovascular reflex functions (20, 90, 103, 135, 136, 459, 460). Microinjections of ANG-(1–7) induce facilitation, while injections of ANG II produce attenuation of the baroreflex bradycardia (90, 133). Accordingly, A-779 and losartan, selective MAS and AT1 receptor antagonists, produce opposite effects on the baroreflex in normotensive or hypertensive rats (90, 135, 460). At this site ANG-(1–7), which can be at least partially derived from ANG II through ACE2 (136), is degraded by ACE (259) and may have a preferential non-glial source (103, 460). Moreover, decreased levels of ANG-(1–7) at the NTS may be related to the attenuated baroreflex control that is observed in hypertension and aging (20, 90, 133, 249, 389, 459).
More recently studies in rats that develop metabolic syndrome after chronic fructose intake have shown that fructose-fed rats receiving ANG-(1–7) infusions into the lateral ventricle had normalized baseline mean arterial pressure, baroreflex control of heart rate (HR), and reduced cardiac sympathetic tone (225). More strikingly, alongside these cardiovascular improvements, the rats presented normalized glucose tolerance, glycemia, insulinemia, and HOMA score. Fructose-fed rats treated with ANG-(1–7) had increased HDL and normalized hepatic and muscle glycogen content (225). These data suggest that activation of ANG-(1–7)/MAS pathway in the brain may produce important benefits for cardiovascular and metabolic disorders.
Molecular analyses of the brains of fructose-fed rats treated with ANG-(1–7) ICV revealed a reduced mRNA expression of neuronal nitric oxide synthase (nNOS) and NR1/NMDAr in the hypothalamus and medullary areas of these animals (225). The major candidates for mediation of the central actions of ANG-(1–7)/MAS lie in these regions: the PVN, the RVLM, and the NTS, which are crucial for the sympathetic drive to the cardiovascular system and for baroreflex control. In agreement with this interpretation, a selective overexpression of ACE2 in the RVLM (618) or PVN (635) induces a significant decrease in blood pressure of SHR (618), attenuation of sympathetic activity, and improvement of the baroreflex function in animals with congestive heart failure (635).
Antagonizing the effect of ANG-(1–7) through ICV infusions of A-779 attenuated ethanol-induced activation of NOS in the PVN and restored hemorrhage-induced AVP in circulation (593). In congestive heart failure (CHF), restoring ACE2 induces an increase in NO and a decrease in sympathetic nerve activity (275, 605). In the ethanol-intoxicated hemorrhaged states, ICV A-779 administration decreased NOS activity and NO concentrations, partially restoring the rise in levels of circulating AVP, suggesting that MAS activation contributes to the NO-mediated inhibitory tone of AVP release (593).
5. Cardiovascular effects of angiotensin-(1–7) at specific medullary sites
At selective medullary sites, the acute stimulation of MAS induces effects similar to those triggered by ANG II (12, 14, 17, 26, 31, 64, 65, 103, 133, 170, 171, 180, 182, 183, 210, 297, 403, 425, 492, 506). In the NTS, ANG-(1–7) induces neuronal excitation (26) and decreases baseline blood pressure in normotensive (65) and hypertensive rats (90, 135). In the CVLM, an inhibitory area participating in the baroreflex arch (595), ANG-(1–7) induces decreases in mean arterial pressure similar to those observed for ANG II (13, 14, 69, 170, 581). However, the hypotensive effect of ANG-(1–7) was blocked by A-779, while ANG II was inhibited by losartan (475). Wang et al. (581) showed that the depressor response to ANG-(1–7) in the CVLM was accompanied by an increased release of glutamate and a decrease in taurine. More interestingly, we showed that the signaling mechanisms triggered by these peptides at the CVLM were distinct. ANG-(1–7) acts through a nitrergic pathway sensible to the NOS inhibitors, l-NAME and 7-NI, while ANG II acts preferably by decreasing the noradrenergic drive (14). Also in contrast to ANG II, microinjections of ANG-(1–7) into the CVLM attenuated the bradycardia and facilitated the baroreflex tachycardia (13). These effects were completely abolished by intravenous methyl-atropine, showing a dependence of a cholinergic/nitrergic peripheral mechanism (13). In SHR, the hypotensive effect of these peptides reaches a similar magnitude but involves the participation of different vascular beds (170). The hypotensive effect produced by ANG-(1–7) and ANG II at CVLM was caused by a decrease in renal vascular resistance, but did not alter femoral vascular resistance as shown for ANG-(1–7) in normotensive rats (170). This suggests a possible impairment of vasodilatory mechanisms triggered by ANG-(1–7) in the CVLM of SHR to the hindlimb. In 2K1C rats, the CVLM BP effect of ANG-(1–7) is mediated by MAS. However, A-779 at the CVLM of renovascular hypertensive rats induced a fall in BP and improved baroreflex bradycardia (69).
The RVLM represents the main relay for sympathetic output regulating cardiovascular homeostasis (112). MAS is expressed in the RVLM (29, 30) and microinjections of ANG-(1–7) induce a pressor response whose magnitude is similar to that of ANG II (172, 182, 183, 308, 316, 506, 640). Conversely, the selective blockade of endogenous ANG-(1–7) actions by A-779 results in a decrease in BP in normotensive (24, 180, 182, 315) and hypertensive rats (143, 172, 180, 307, 308, 388). The ANG-(1–7) pressor effect at the RVLM is increased after hemorrhage (316) and in hypertensive rats (172, 388). In keeping with the excitatory role of ANG-(1–7) after acute injections, A-779 or an inhibition of ACE2 with DX600 induces greater decreases in blood pressure in SHR (388) and TGR(mREN2)27 rats (180). In addition, RVLM microinjections of ANG-(1–7) induce an increase in RSNA in normotensive (425, 640) and renovascular hypertensive rats (307). Although ANG-(1–7)/MAS at this site does not alter baroreflex (13), it increases the cardiac sympathetic afferent reflex (CSAR), which contributes to sympathetic excitation in hypertension (307, 388, 640). The facilitation of CSAR seems to be mediated by MAS activation of cAMP-protein kinase A and increases in NAD(P)H oxidase activity and the superoxide anion level (307, 308).
The pressor effect induced by acute injections of ANG-(1–7) into the RVLM may involve the release of glutamate and decreases in glycine, taurine, and GABA (582). Interestingly, in astrocytes from newborn SHRs, MAS mediates ANG-(1–7)-driven increases in Ca2+ signaling (228). On the other hand, A-779 but not the AT1 receptor antagonist losartan inhibited [Ca2+]i elevations induced by ANG-(1–7), which could also be antagonized by blocking intracellular Ca2+ stores (228). ANG-(1–7) evoked no consistent changes in [Ca2+]i or membrane excitability in catecholaminergic or noncatecholaminergic neurons of slice cultures containing the RVLM in either SHR or WKY (228). Recently, in an evaluation of the peripheral mechanism involved in the ANG-(1–7) effect at the RVLM, we showed that its pressor response may involve an increase in sympathetic tone, the release of AVP and possibly the inhibition of a vasodilatory peripheral mechanism (403).
All the excitatory effects described above counter the hypothesis that ANG-(1–7) plays a counterregulatory role to ANG II and mediates a decrease in blood pressure, improvement in baroreflex, decrease in sympathetic tone, and increase in vagal tone to the periphery. These effects were triggered by acute injections of ANG-(1–7) or antagonists into the RVLM. However, inducing a long-term alteration of RAS peptides at this site led to specific results which are informative. Stress-induced hypertension, for instance, induces increases in the expression of ACE and AT1, a decrease in ACE2, and a hyperresponsiveness of the RVLM to ANG II (143). Moderate physical exercise during SHR development (7–23 wk old) attenuates hypertension, prevents increases in tumor necrosis factor (TNF)-α, interleukin (IL)-1β, ACE, and AT1 expression in the RVLM and upregulates IL-10, ACE2, and MAS at this site (6). In addition, these changes are associated with reductions in plasma ANG II levels, neuronal activity, NADPH-oxidase subunit gp91(phox), and inducible NOS in trained SHRs, all of which indicate reduced oxidative stress (6). Accordingly, exercise rescues ACE2 expression in the RVLM of animals subjected to heart failure (275). Exercise training in normotensive rats, on the other hand, induces a pressor response to A-779, suggesting that in this condition, endogenous ANG-(1–7) triggers the inhibition of pressor neurons at the RVLM (31). Nevertheless, the most striking result was obtained with lentivirus-mediated long-term ACE2 expression in the RVLM, which induced a significant and long-term reduction in blood pressure in SHR (618). This suggests that increasing the level of ANG-(1–7) may contribute to the anti-hypertensive effects of exercise training, at least in the RVLM.
6. Cardiovascular effects of angiotensin- (1–7) at specific hypothalamic sites
Biological effects of ANG-(1–7) have been described in several nuclei of the hypothalamus (80, 108, 134, 137, 138, 213, 214, 252, 253, 349, 353, 414, 504, 547, 649). Diz et al. (134) used hypothalamic slice preparations of normotensive and hypertensive TGR(mREN2)27 rats to show that ANG-(1–7) produced a significant increase in the release of substance P from the hypothalamus of normotensive but not hypertensive rats. The physiological significance of these actions is still unclear. ANG-(1–7) does not, however, change the firing of cultured hypothalamic neurons; instead, it attenuates the action of ANG II in neurons of prehypertensive SHR through MAS and PTEN (phosphatase and tensin homolog deleted on chromosome 10) signaling (370).
Microinjections of ANG-(1–7) in the anterior hypothalamus (AH) had no effect in normal rats but induced a decrease in blood pressure in baroreceptor denervated rats (252). This effect, which was blocked by A-779, was in contrast to the excitatory effect induced by ANG II in control and pressoreceptor-denervated rats. More interesting, ACE inhibitors lower blood pressure after microinjections into the AH of denervated rats, an effect that is blocked by A-779 (252). This suggests that the effect of ACE inhibition is due to the formation of ANG-(1–7), which increases in this condition.
Felix and co-workers (16, 17, 152) used a microiontophoresis methodology to show that most neurons in the PVN are excited by ANG-(1–7). In fact, Qadri et al. (427) confirmed the first study performed in neurohypophysial explants by Schiavone et al. (492), showing that ANG-(1–7) microinjections into the PVN induce a release of AVP. However, in this study ANG-(1–7) was much less potent and its effect was abolished by losartan (AT1 antagonist) and PD123319 (antagonist for AT2 and MrgD). More recently, Whitaker and Molina (593) studied hemodynamic alterations after hemorrhagic shock in a model of acute ethanol intoxication. They found a reduction in the release of AVP mediated by a decrease in central NO inhibitory tone. The decrease in AVP release is considered the main factor in aggravating hemodynamic instability in animals with ethanol intoxication that suffer hemorrhages. These authors further showed an increase in ACE2 activity and ANG-(1–7) and substantial MAS-mediated upregulation of NOS in PVN of acute ethanol intoxicated hemorrhaged animals. A blockade of MAS ICV or in the PVN partially restored circulatory levels of AVP, suggesting that NO upregulation mediated by ANG-(1–7) contributes to inhibitory tone upon AVP release during hemorrhaging in acute ethanol intoxication (593). Although these studies point to contrasting effects of ANG-(1–7) on the release of AVP, together they strengthen the concept that distinct ANG-(1–7) effects can be observed in specific brain areas depending on the existing pathophysiological condition.
In anesthetized normotensive rats, Fontes and colleagues (504) in our laboratory showed that bilateral microinjections of A-779 into the PVN decreased the RSNA, suggesting that endogenous ANG-(1–7) at the PVN contributes to the maintenance of the tonic activity of the sympathetic system and blood pressure. More recently, A-779 injections into the PVN have been shown to attenuate hypertension induced by a sleep apnea model in rats (108), which agrees with the sympathetic stimulatory effect of acute injections of ANG-(1–7) into the PVN. Nevertheless, ANG-(1–7) injections into the PVN effectively increased the RSNA, CSAR, and arterial pressure through an activation of MAS via the cAMP-PKA pathway (237, 307, 308, 527, 640). However, these studies were performed in anesthetized vagotomized and sinoaortic denervated rats, a condition that may introduce important changes in the activity of PVN neurons and their sensitivity to neuromodulators. For instance, others have reported opposite trends in other models (409, 413, 635)
7. Other angiotensin-(1–7) effects in the brain
A role for ANG-(1–7) has been reported in stress, learning, and memory processes that occur in central limbic regions such as the hippocampus and amygdala (7, 43, 246, 299, 404, 518, 607, 629). Hellner et al. (246) were the first to show that ANG-(1–7) enhances LTP in limbic structures, implicating a distinct function in learning and memory mechanisms via MAS. In 2007, Albrecht (7) showed that the LTP modulatory effect of ANG-(1–7) working through MAS in the lateral nucleus of the amygdala depended on cyclooxygenase-2 (COX-2) and NO. Moreover, Staschewski et al. (518) showed that there is a gender-dependent involvement of different isoforms of NOS in the effects of ANG-(1–7) on LTP in the amygdala. The ANG-(1–7)-induced increase in the magnitude of LTP involves nNOS in males and eNOS in females (518). In CA1 region of the hippocampus, Lazaroni et al. (299) showed that mice without MAS or subjected to MAS blockade present impaired object recognition memory (ORM). In addition, in Mas−/− mice, ANG-(1–7) concentrations are increased in the whole hippocampus, as well as in the CA1 area, suggesting the need for a functional ANG-(1–7)/MAS axis for normal ORM processing (299). Interestingly, it has been reported that in this brain region ANG-(1–7) can be formed independent of ANG II processing (417). Despite this evidence, the role of ANG-(1–7)/MAS axis in limbic-dependent memories remains poorly understood.
In 1998 Walther et al. (578) reported that Mas-deficient animals displayed an increase in anxiety-like behavior. Accordingly, years later Bild and Ciobica (43) showed an anxiolytic-like effect after chronic ICV infusions of ANG-(1–7), accompanied by a reduction in oxidative stress in the amygdala. More recently, using two different transgenic rat lines, we showed that ANG-(1–7) ICV infusions could attenuate anxiety-like behavior (11, 272). In addition, we showed that ANG-(1–7) was also effective in attenuating a depression-like phenotype (11). Interestingly, hypertensive patients who suffer from depression exhibit mood improvements after treatment with the ACE inhibitor captopril, which increases levels of ANG-(1–7) (50). Recently, we showed that the anxiety-like behavior of hypertensive rats was rescued in enalapril-treated animals after blockade, unveiling an anti-aversive role for endogenous ANG-(1–7), at least in hypertensive rats (11). More recently, similar findings have been obtained in mice by increasing ACE2 activity (583).
ANG-(1–7) is involved not only in modulating chronic stress-coping responses in psychiatric disorders, such as anxiety and depression, but can also participate in cardiovascular adjustments to acute responses to stress. Fontes and colleagues (181, 353, 404) have suggested that the ANG-(1–7)/MAS axis is a promising target to attenuate the physiological response to emotional stress and reduce the risk of cardiovascular diseases. ANG-(1–7) given in the lateral ventricle (353) or microinjected into the basolateral amygdala (404), an area of the limbic system that is involved in coordinating emotional responses, markedly attenuated the pressor and tachycardia responses evoked by air jet stress, a model to study cardiovascular changes to acute stress. Additionally, ANG-(1–7) injections into basolateral amygdala also attenuated the pressor response evoked by the cage-switch stress paradigm, another model of acute psychosocial stress (404). These effects were blocked by A-779, suggesting that they are MAS-mediated (353, 404). These findings reinforce the concept that ANG-(1–7)/MAS can modulate the cardiovascular responses evoked by emotional stress and point to the basolateral amygdala as an important site for mediating these actions.
Another area implicated in the integration of emotional behavior and sympathetic drive to the periphery is the periaqueductal gray (PAG). Xing et al. (608) showed that ANG-(1–7) interaction with MAS inhibits the neuronal activity of dorsolateral PAG which depends on a NO-dependent signaling pathway via MAS activation. More recently, these authors showed a decrease in ANG-(1–7) levels and an attenuation in MAS-nNOS pathways in the PAG of rats with chronic heart failure (609). Moreover, ANG-(1–7)'s ability to attenuate the neuronal activity of the dorsolateral PAG was significantly decreased in chronic heart failure animals (609). Whether the inhibitory effects of ANG-(1–7) at PAG are involved in modulating responses of emotional stress is an open question; however, it is very likely considering the role of the PAG in controlling sympathetic and cardiovascular system.
The ACE2/ANG-(1–7)/MAS axis also exerts a neuroprotective influence on ischemic and hemorrhagic strokes. In 2011, the Sumners group published the first (357) of a series of studies showing that ICV infusions of ANG-(1–7) or an ACE2 activator before and after endothelin-1-induced middle cerebral artery occlusion (MCAO) significantly attenuated the size of the cerebral infarct and the neurological deficits measured 72 h after the insult (32, 357, 433, 434, 526). These data were corroborated and extended by others, who showed an involvement of NO and different NOS isoforms, a decrease in cytokines, and a reduction in NFκB, COX-2, ROS, and neuronal apoptosis (267, 268, 421, 422, 637). The anti-inflammatory effects of central administrations of ANG-(1–7) were in accordance with the demonstration of a decrease in the activation of microglia both in ischemic stroke (433) and in hemorrhagic stroke induced in SHR-SP (434). All of these effects were attenuated by A-779, but were unaffected by the AT2 receptor antagonist PD123319, pointing to MAS as the receptor involved (526).
It has been reported that ischemic stroke increases ACE2 activity and MAS expression in the brain and ANG-(1–7) in the brain and circulation (329). Nevertheless, treatment with an ACE2 activator after stroke induces a decreased infarct volume and improves neurological functions without significant changes in cerebral blood flow (357). Accordingly, ACE2 overexpression in neurons of transgenic mice that overexpress human renin and Angiotensinogen (SARA, triple transgenic mice) induced neuroprotection, as demonstrated by a reduction in MCAO-induced infarct volume, an increase in cerebral blood flow, attenuation of neurological deficits, and increases in cerebral microvascular density in the peri-infarct area (636). These effects were MAS-related and independent of baseline arterial pressure. Moreover, there were increases in the ANG-(1–7)/ANG II ratio, angiogenic factors, eNOS expression, and NO production, whereas levels of NADPH oxidase subunits and ROS were decreased in the brain of SARA mice (636). The counterbalancing effect of ANG-(1–7) in the presence of high levels of ANG II was also observed in hemorrhagic stroke (42).
Other effects of ACE2/ANG-(1–7)/MAS in cerebroprotection are an attenuation of the loss of endothelial function of cerebral arteries that occurs with aging (421), increases in cerebral angiogenesis in ischemic stroke (268), and an attenuation of alterations in the integrity of the blood brain barrier after ischemia reperfusion injury (IRI), which induces deleterious alterations in brain permeability and edema (600). In this regard, ANG-(1–7) infusion restored the expression of tight junction proteins (claudin-5 and zonula occludens ZO-1) in IRI-induced blood-brain barrier damage by modulating the TIMP1-MMP9 pathway (600).
Imbalances of the two arms of the RAS in the brain favoring ANG II/AT1 activity are involved in the pathophysiology of diseases such as arterial hypertension, metabolic syndrome, heart failure, psychiatric disorders, and emotional stress. Strategies aimed at rescuing the RAS balance and inducing an increase in ANG-(1–7) in the brain can help ameliorate the problems that arise as these diseases develop. Physical exercise is an effective, nonpharmacological strategy to lower blood pressure in hypertensive patients, improving cognitive function, attenuating depression, and demonstrating other effects. Recent studies have shown that physical exercise induces changes in RAS components that lead to an increase in ACE2 activity and ANG-(1–7) in the brain in animals with heart failure or arterial hypertension (81, 275). Future studies will be necessary to reveal the mechanisms involved in exercise-triggered alteration in RAS components in the brain and their role in the beneficial effects of exercise.
8. Neurotransmitter/neuromodulator effects of ANG-(1–7)
Gironacci and colleagues have contributed to the understanding of the intracellular mechanisms triggered by ANG-(1–7) in neurons (80, 211–214, 325). These authors showed that ANG-(1–7) acting induces a decrease in presynaptic norepinephrine (NE) release in hypothalamus isolated from normotensive (214) and SHR (213) both by direct action or by blocking the release of ANG II-stimulated NE, thus balancing the stimulatory action of ANG II. They also showed that the decrease in NE release through ANG-(1–7)/MAS activation leads to bradykinin generation, which stimulates the B2 receptor and enhances NO release, activating the cGMP/PKG signaling pathway (215). In agreement, Feng and colleagues (154, 634) showed that the overexpression of ACE2 produces a significant increase in brain NO production, as do ICV administrations of ANG-(1–7). The activation of cGMP/PKG signaling in turn inhibits voltage-dependent calcium channels or induces the phosphorylation of proteins of the synaptic vesicle, decreasing the release of neurotransmitters and thus lowering their levels at the synaptic cleft. Additionally, it was shown that ANG-(1–7) decreases tyrosine hydroxylase (TH) activity and expression, the rate-limiting enzyme in catecholamine biosynthesis, in neurons from the hypothalamus and brain stem of WKY and SHR (325). This effect was unrelated to MAS, but blocked by PD123319, which led the authors to conclude that ANG-(1–7) acts via AT2 receptor activation, to stimulate the ubiquitin-proteasome system; this, in turn, induces increases in TH degradation. However, the recent finding that PD123319 also inhibits MrgD (297) means that the reduction in TH activity could be due to alamandine, a possibility yet to be explored.
Neuronal communication in the brain depends on neurotransmitter transporters in the cell membrane of neurons and/or glial cells which limit concentrations of neurotransmitters in the synaptic cleft. ANG-(1–7) does not alter acute NE neuronal uptake in the hypothalamus of SHR (212). However, through MAS, ANG-(1–7) induces both MEK1/2-ERK1/2 and phosphatidylinositol 3-kinase/AKT pathways, resulting in increase in NE transporter transcription and translation (326). This suggests that ANG-(1–7) induces a long-term stimulatory effect on NE uptake by augmenting levels of the transporter. In addition to NE, Stragier et al. (523) showed that ANG-(1–7)/MAS stimulated the release of GABA and dopamine, but not of glutamate, in the rat striatum. In contrast, ANG-(1–7)/MAS stimulation in the CVLM induces an increase in the release of glutamate and a decrease in taurine (581). Thus a site-specific regulation of levels of synaptic neurotransmitters may be an alternative mechanism by which ANG-(1–7) contributes to the control of various functions in a range pathophysiological conditions through MAS.
B. Heart
Effects of ANG-(1–7) in the heart have been reported in a large number of studies (4, 9, 10, 21, 35, 78, 79, 82, 101, 120–124, 130, 145, 146, 161, 162, 164, 165, 167, 168, 175, 193, 203, 204, 206, 208, 216, 217, 222, 227, 239, 240, 260–262, 277, 303, 311, 313, 317–319, 321, 347, 354, 356, 358, 362, 366, 370, 377, 393, 394, 398, 408, 411, 413, 465, 476, 477, 489, 494, 498, 521, 531, 532, 561, 565, 576, 585–587, 589, 641, 646). Averill et al. (21) were the first to report ANG-(1–7) immunoreactivity within myocytes in the heart. This was in line with immunoreactivity in blood collected from the canine coronary sinus (472). More recently, ANG-(1–7) and MAS were identified in the sinoatrial node, providing the morphological basis for the ANG-(1–7) antiarrythmogenic effect (164). The presence of ACE2 in cardiomyocytes further supports a local formation of ANG-(1–7) in the heart of different species (164, 646). It should be pointed out that other enzymes capable of directly or indirectly forming ANG-(1–7) are also present in the heart, including prolyl-oligopeptidase (94) and cathepsin A (262).
1. Coronary vessels
ANG-(1–7) produces vasorelaxation in the coronary vessels of dogs (53, 54) and pigs (220, 426). In rodents, the peptide generally has no effects or produces vasoconstriction (288, 371, 393, 474). However, these observations were made using relatively high concentrations of ANG-(1–7) (nano- to micromolar range). Recently, using picomolar concentrations of ANG-(1–7), Souza et al. (516) were able to detect a significant vasodilator effect of ANG-(1–7) in isolated hearts from aorta-coarcted rat. Intriguingly, the blunted ANG-(1–7)-induced vasodilation in hypertensive animals was rescued by acute or chronic AT1 blockade using losartan. These observations are in keeping with an interaction of AT1 receptors with MAS (68, 79, 284), which still needs to be addressed in more detail.
2. Cardiomyocytes
In cardiomyocytes, acute exposure to ANG-(1–7) has no demonstrable effect on Ca2+ transients but promotes NO release by activating endothelial NO synthase (eNOS) and nNOS (101, 130). On the other hand, chronic exposure to ANG-(1–7) or genetic deletion of MAS has significant effects on Ca2+-handling proteins (217, 476). Transgenic rats harboring an ANG-(1–7)-producing fusion protein in the heart show an increased Ca2+ transient amplitude, faster Ca2+ uptake, and increased expression of SERCA2 (217). Cardiomyocytes from Mas−/− mice have a smaller peak Ca2+ transient and a slower uptake of Ca2+, probably due to the decreased expression of SERCA2 (217). These changes translated into impaired heart functions in Mas−/− mice (48, 198, 476). The changes in calcium-handling proteins were paralleled by changes in the NO production machinery (130). Cardiomyocytes from Mas−/− mice have normal eNOS protein levels, but a 70% increase in caveolin-3 expression and a decrease in heat shock protein 90 (117, 130). These two alterations may induce a decrease in eNOS activity because caveolin-3 prevents interactions between calmodulin and NOS, and heat shock protein 90 acts as a scaffold protein to recruit protein kinase B (AKT) to the eNOS complex (602).
3. ANG-(1–7) and cardioprotection
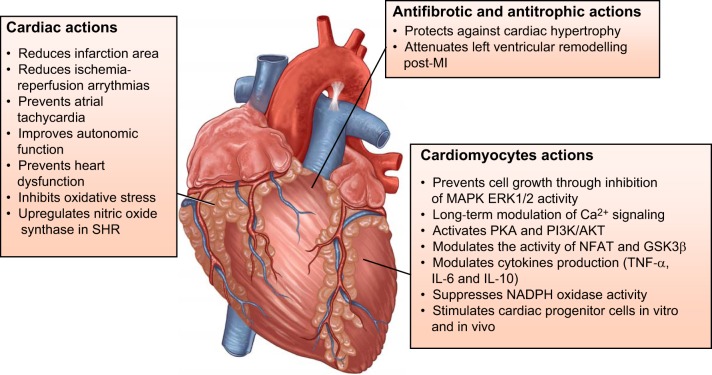
Most data related to ANG-(1–7) or other MAS agonists in the heart deal with its cardioprotective effects (FIGURE 5) (117, 168, 216, 223, 465, 477).
FIGURE 5.
Effects of ANG-(1–7) in the heart. The effects illustrated in the figure include the ones produced in coronary vessels, fibroblasts, and cardiomyocytes.
The first description of such an effect was made by Ferreira et al. (168). Low concentrations (220 pM) of ANG-(1–7) produced a significant reduction of ischemia/reperfusion-induced cardiac arrhythmias in isolated rat hearts. This was in contrast to the pro-arrhythmogenic effects of ANG-(1–7) at 10-fold higher concentrations (393). In keeping with these observations, De Mello et al. (124) reported a biphasic effect of ANG-(1–7) on impulse propagation and cardiac arrhythmias: at 10 nM, an anti-arrhythmogenic effect was observed (466), whereas at a 10-fold higher concentration, ANG-(1–7) was pro-arrhythmogenic (124). In transgenic TGR(A1–7)3292 rats which presented a moderate increase in circulating ANG-(1–7), a reduction in the duration of reperfusion cardiac arrhythmias and improved postischemic heart functions were observed (466). The mechanism of the anti-arrhythmogenic effect of ANG-(1–7) seems to involve, at least in part, the sodium pump (124).
In addition to influencing cardiac rhythm, ANG-(1–7) has a significant anti-remodeling effect in different models of cardiomyopathy. TGR(A1–7)3292 rats exhibit a marked attenuation of isoproterenol-induced cardiac fibrosis (466).
A number of later studies have described anti-remodeling effects of ANG-(1–7) (82, 117, 161, 177, 216, 223, 229, 311, 317, 348, 354, 366, 428, 440, 465, 585) and of other MAS agonists (AVE0991, CGEN-856S) (105, 165, 244, 334, 490, 628). These observations are in line with the deleterious cardiac effects of the genetic ablation of MAS in mice (48, 75, 78, 198, 476, 611). Interestingly, even an acute blockade of MAS with A-779 has been reported to produce a deterioration of function in isolated mouse hearts (78).
In contrast to the consistent antifibrotic effects of ANG-(1–7)/MAS, the picture is less clear for cardiomyocyte hypertrophy, although in general antihypertrophic effects have been described. The pro-hypertrophic effect of isoproterenol is attenuated in ANG-(1–7)- overexpressing TGR(A1–7)3292 rats (387). Similarly, treatments with ANG-(1–7) attenuated ANG II-induced cardiac hypertrophy (216). However, in the DOCA-salt model of hypertension, treatment with ANG-(1–7) attenuated cardiac fibrosis without interfering with blood pressure or cardiac hypertrophy (223), whereas the induction of DOCA-salt hypertension in TGR(A1–7)3292 rats (466) resulted in the attenuation of hypertension, myocardial fibrosis, and cardiac hypertrophy (465). An anti-hypertrophic effect of ANG-(1–7) was also observed in cultured cardiomyocytes treated with ANG II (177), AVP (177), and endothelin (193).
In contrast to several reports showing a cardioprotective effect of ANG-(1–7), Velkoska et al. (565) reported a dramatic deleterious effect of ANG-(1–7) infusions in SD rats after 5/6 nephrectomy. However, opposite effects were reported by Li et al. in the mouse (311) and, more recently, Xu et al. in the rat (610). In their studies, ANG-(1–7) prevented heart dysfunctions and left ventricular remodeling. Additional studies are needed to clarify this issue. It would be interesting, for example, to remove interfering factors such as the action of ANG-(1–7) on AT1 receptors in this condition (468). In a contrasting report, Zhang et al. (633) showed that the overexpression of human MAS in rat cardiomyocytes leads to hypertrophy. In addition to the possibility that the overexpressed receptors might be uncoupled from the usual signaling machinery activated by endogenous receptors, these data and other studies of ANG-(1–7) (393) indicate that important differences in signaling may arise in conditions where levels of MAS or ANG-(1–7) rise well above physiological concentrations.
C. Blood Vessels
The production of ANG-(1–7) in vascular endothelium was first described by Santos et al. (471). Its endothelium-dependent vasorelaxation has been reported by many authors. ANG-(1–7) relaxes the aortic rings of SD (300) and TGR(mREN2)27 (304) rats, canine (53) and porcine (426) coronary arteries, the canine middle cerebral artery (174), piglet pial arterioles (361), feline systemic vasculature (405), rabbit renal afferent arterioles (437), and mesenteric microvessels of normotensive (156) and hypertensive (155) rats. Moreover, ANG-(1–7) potentiates the vasodilator effect of bradykinin in several vascular beds, including dog (53) and rat (10) coronary vessels, rat kidney vessels (467), mesenteric arteries (156), and pancreatic microcirculation (625).
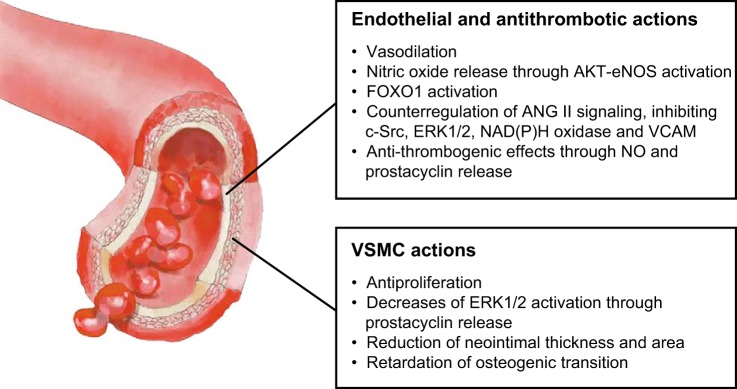
Although vasodilation is the most well-characterized action of ANG-(1–7), the ANG-(1–7)/MAS axis also induces antiproliferative (191, 530) and antithrombotic (185, 189) effects in vasculature. ANG-(1–7) and MAS are expressed in VSMCs (530) and platelets (189). The antiproliferative action of ANG-(1–7) in the vasculature has been demonstrated in VSMCs, in which ANG-(1–7) reduces the ANG II-stimulated mitogen-activated protein kinase (MAPK) activities of ERK1/2 through an increase in prostacyclin (PGI2) (530). A concurring result was observed in a rat stenting model, where ANG-(1–7) treatment produced a significant reduction in neointimal thickness, neointimal area, and stenosis (292). In rats with vascular calcification, ANG-(1–7) restored the decreased expression of lineage markers in VSMCs, including smooth muscle α-actin, SM22α, calponin, and smoothelin, and retarded the osteogenic transition of these cells by reducing the expression of bone-associated proteins (525). This antiproliferative potential was also observed in cardiac fibroblasts (354) and tumor cells (194).
As for antithrombotic effects, experiments performed in Mas−/− mice demonstrated an increased venous thrombus size and short bleeding times (189). Changes of an opposing nature were observed with the administration of an orally active form of ANG-(1–7), based on ANG-(1–7) inclusion in cyclodextrin [HPβCD-ANG-(1–7)]. The oral administration of HPβCD-ANG-(1–7) promotes an antithrombotic effect that has been associated with an increase in the plasma concentration of ANG-(1–7) (185). The antithrombotic effects of HPβCD-ANG-(1–7) were abolished in Mas−/− mice (185). This activity was also found in bradykinin B2 receptor-deleted mice (Bdkrb2−/−), in which the MAS antagonist A-779 shortens the time leading up to arterial thrombosis and tail bleeding time (150). MAS-mediated NO release from platelets and increased prostacyclin seem to mediate these antithrombogenic actions (150, 189).
Most work on the hemodynamic actions of ANG-(1–7) over the years has been focused on clarifying its effects on vessels and blood pressure. FIGURE 6 illustrates its main effects on blood vessels. Interestingly, the vasodilator effect of ANG-(1–7)/MAS is selective for specific vascular beds, contrasting with the more widespread vasoconstriction described for ANG II/AT1. In normotensive rats, ANG-(1–7) produced marked changes on regional blood flow, increasing vascular conductance in the mesenteric, cerebral, cutaneous, and renal territories. Furthermore, ANG-(1–7) simultaneously increases cardiac output (CO) by 30% and decreases total peripheral resistance (TPR) by 26%. These opposing changes lead to an absence of substantial changes in blood pressure (462). Similarly, transgenic rats with a slight increase in circulating ANG-(1–7) over their lifetime, TGR(A1–7)3292, present pronounced changes in regional blood flow, resulting in an increase in vascular conductance in the kidneys, lungs, adrenals, spleen, brain, testis, and brown fat tissue. In contrast, Mas−/− mice show a marked increase in vascular resistance in many territories such as the kidney, lung, adrenal gland, mesentery, spleen, and brown adipose tissue. This model also exhibited a parallel increase in TPR and decreased CO (48).
FIGURE 6.
Main known effects of ANG-(1–7) in blood vessels.
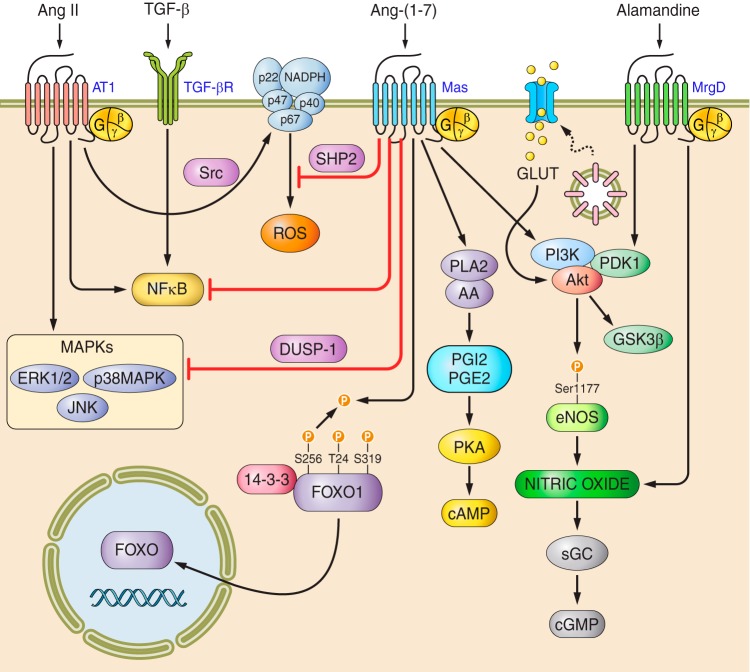
Many vascular actions of ANG-(1–7) have their effects through the release of NO. Human endothelial cells express MAS, through which ANG-(1–7) mediates an activation of eNOS and NO production via AKT-dependent pathways. ANG-(1–7) also promotes NO release in Mas-transfected Chinese hamster ovary cells via the phosphoinositide 3-kinase (PI3K)/AKT pathway (463). Recently, it has been demonstrated that ANG-(1–7) can also activate downstream components such as the FOXO1 transcription factor, a well-known negative modulator of the AKT signaling pathway (568). These results highlight the complex modulatory effects of ANG-(1–7), which probably involve downstream effectors that coordinate negative feedback loops through fine-tuned mechanisms.
Regarding its effects on NO generation, ANG-(1–7) regulates the phosphorylation of eNOS at Ser1177 and Thr495. In resting conditions, eNOS is phosphorylated on Thr495 and only weakly phosphorylated on Ser1177. The relative phosphorylation state of Ser1177 and Thr495 determines the activity of eNOS in endothelial cells (176). ANG-(1–7) increases Ser1177 phosphorylation while simultaneously decreasing the phosphorylation of Thr495; both posttranslational effects are blocked by the MAS antagonist A-779 (461). Subsequently, it was demonstrated that the ANG-(1–7)-mediated activation of PI3K/AKT counteracts the negative effects of ANG II on insulin signaling in endothelial cells and is involved in the survival and proliferation of CD34(+) cells from diabetic individuals (536). In vivo, this pathway seems to mediate the improvement of insulin sensitivity induced by ANG-(1–7) in liver, skeletal muscle, and adipose tissue from fructose-fed rats (384).
In human endothelial cells, ANG-(1–7) also counterregulates ANG II signaling, blunting the phosphorylation of c-Src and ERK1/2 as well as the activation of NAD(P)H oxidase by ANG II. This modulatory effect is mediated by the phosphorylation of SHP-2, preventing ANG II-induced SHP-2 dephosphorylation and promoting interactions between SHP-2 and c-Src. A-779 inhibited these actions, demonstrating that these effects are mediated through MAS (463). In keeping with this concept, the impairment of endothelial function in two different genetic backgrounds with MAS deficiencies, C57Bl/6 and FVB/N, indicates a crucial role of MAS in endothelial functions through its effects on the generation and metabolism of NO and ROS (430, 611). In the FVB/N background, endothelial dysfunction is associated with an increase in blood pressure (611), whereas in C57BL/6 mice, no alteration of blood pressure has been reported (430). A worsening of 2K1C Goldblatt hypertension has also been observed in Mas−/− mice (432). Conversely, short-term infusions of ANG-(1–7) improve endothelial functions, significantly increasing the hypotensive effects of administering intra-arterial acetylcholine in normotensive rats (151). In diet-induced obesity mice, chronic treatments with ANG-(1–7) induced a significant improvement in endothelial functions and reversed the elevated aortic expression of the NAD(P)H oxidase subunits p22(phox) and p47(phox) and plasma TBARS (38). Similar results were obtained in diabetic rats, in which the carotid blood flow was restored by chronic treatment with ANG-(1–7), probably through the MAS-mediated antioxidant effects that oppose AT1-activated NAD(P)H oxidase (420). Moreover, ANG-(1–7) also negatively regulates the ANG II-induced expression of VCAM-1 by attenuating the nuclear translocation of NFκB in endothelial cells (630).
The actions of ANG-(1–7) in human vasculature still require investigation to determine whether the potent vasodilator effect described in rodents applies. Although an initial examination focused on human vessels led to some controversy, the conflicting results could be due to methodological divergences, or a racial or vascular territory selectivity of ANG-(1–7). The infusion of ANG-(1–7) in patients chronically treated with ACE inhibitors had no effect on forearm blood flow, whereas the infusion of bradykinin produced a noticeable vasodilation (114). This observation was taken as evidence that ANG-(1–7) played no role in the hemodynamic effects of ACE inhibitors. However, this did not consider the fact that ACE inhibition significantly increases circulating levels of ANG-(1–7), which means that using a MAS antagonist rather than ANG-(1–7) would have been more suitable to evaluate the peptide's effects on blood flow. Similar negative results were obtained in the forearm blood flow of normotensive patients, in which ANG-(1–7) did not alter vasodilation produced by bradykinin infusion (596). In contrast, Sasaki et al. (488) observed a dose-dependent vasodilation in the forearm circulation in normotensive subjects and patients with essential hypertension. Ueda et al. (548) similarly reported a dose-dependent potentiation of bradykinin vasodilation by ANG-(1–7) in normotensive forearm resistance vessels of normotensive healthy men, which is in keeping with the well-known bradykinin-potentiating activity of ANG-(1–7) described in animal models (10, 53, 156, 467). An attenuation of the vasoconstrictor effect of ANG II, but not of NE, by ANG-(1–7) was described in the forearm of normotensive patients as well as in mammary arteries in vitro (451, 488). ANG-(1–7) also antagonized ANG II in renal vessels in vitro, but does not appear to have a pronounced effect in normal physiological regulation of renal vascular function in vivo (450). More recently, Van Twist et al. (560) observed a significant dose-dependent increase in blood flow to the kidney during intrarenal infusions of ANG-(1–7) in hypertensive patients. Interestingly, like the observation made in rat renal vessels (555), this effect was weakened in patients on a low-salt diet, probably because this diet leads to an increase in circulating angiotensin peptides including ANG-(1–7) (282). The same authors (560) demonstrated that the effect of ANG-(1–7) infusion on renal blood flow was also reduced in stenotic kidneys. Mendonça et al. (359) have recently demonstrated that ANG-(1–7) significantly attenuates ANG II-induced vasoconstriction in human mammary arteries from patients submitted to coronary revascularization, probably through direct effects on smooth muscle cells, since this action was not abolished by A-779, PD123177, or endothelium removal.
As discussed here there are numerous MAS-mediated effects of ANG-(1–7) in blood vessels. Here the ANG-(1–7)/MAS axis clearly acts as an important counterregulatory arm within the RAS. However, further studies are required to elucidate the complex interactions between RAS receptors, peptides, and enzymes in the vasculature.
D. Kidney
ANG-(1–7) has many well-established effects in the kidney. Since the initial reports, the complexity of the vascular and nonvascular actions of ANG-(1–7) in this organ have been recognized. They appear to be highly dependent on experimental conditions, gender, species, the nephron segment, and hydroelectrolyte status (83, 86, 131, 474, 508, 643). For example, in water-loaded rats, ANG-(1–7) produces a potent antidiuretic effect (469), while in baseline conditions, natriuresis or an absence of a clear effect has been reported (see TABLE 1). At least some of the controversial effects of ANG-(1–7) in the kidney may be due to an involvement of AT1 and AT2 in its actions (293, 295, 474). For instance, in water-loaded rats, the antidiuretic effects of ANG-(1–7) could be blocked by pretreatment with the AT1 antagonist losartan (25). An involvement of non-angiotensin receptors can also explain the variability of its kidney effects. For example, evidence for a participation of AVP V2 receptors in distal tubular cells was obtained by Magaldi et al. (337). In these cells, ANG-(1–7) produces a marked increase in cAMP. Interestingly, pretreatment with A-779 in this preparation abolished the effect of AVP on water absorption, while pretreatment with an AVP antagonist abolished the effects of ANG-(1–7). However, when the antagonist was added on top of the AVP or ANG-(1–7) effects, no cross-inhibition was observed. Yet as expected, A-779 reversed the effects of ANG-(1–7), and the AVP antagonist reversed the effects of AVP. Whether this phenomenon is due to a cross-internalization of MAS and AVP V2 receptors induced by the antagonists or crosstalk between the signaling mechanisms remains to be clarified.
Table 1.
Renal actions of ANG-(1–7)
| Species | ANG-(1–7) Effects | Condition | Reference Nos. |
|---|---|---|---|
| Rat | Antidiuresis | Water loaded | 469 |
| Rat | Natriuresis/diuresis | SD isolated kidneys | 128 |
| Rat | *Biphasic dose-dependent effect on fluid absorption (↑1 pM) (↓10 nM) | Isolated rat proximal straight tubule | 196 |
| Rat | Natriuresis/diuresis | SD isolated kidneys | 251 |
| Rat | Transient natriuresis/diuresis | Hypertensive | 33 |
| Rat | Antidiuresis | Water loaded | 480 |
| Rat | Natriuresis/diuresis | Na-repleted anesthetized rats with denervated kidney and rat proximal tubules in vitro | 238 |
| Rat | *Biphasic dose-dependent effect on fluid absorption (↑ 10−8 M) (↓ 10−12 to 10−10 M) | Anesthetized Wistar rats | 553 |
| Rat | Antidiuresis | Water loaded | 25 |
| Rat | Natriuresis/diuresis | Anesthetized rat | 552 |
| Dog | Natriuresis/diuresis | Anesthetized dogs | 245 |
| Pig | Modulation of Na+-ATPase ouabain-insensitive | Cortex homogenates and basolateral membranes from adult pig kidney | 76, 294 |
| Rabbit | Vasodilation | Isolated afferent arterioles | 437 |
| Rat | ↑Oxidative stress generation | Production of TBARS in rat kidneys | 218 |
| Rat | ANG II-mediated vasoconstriction | Interlobular arteries, afferent and efferent arterioles | 557 |
| Rat | Blunted the ANG II–induced decrease in Na+ excretion | Normotensive anesthetized rats | 59 |
| Rat | ↑ RBF | Urethane-anesthetized rats, using fluorescence microspheres | 462 |
| ↓ RVR | |||
| Rat | Inhibition of ANG I and ANG II-mediated norepinephrine release in kidney | Isolated kidney | 520 |
| Mouse | MAS-dependent antidiuretic effect | Water loaded | 479 |
| Rat | ↑ Water permeability involving vasopressin V2 receptors | Normotensive rat IMCD | 337 |
| Rat | ↑ NE release and inhibition of ANG I and ANG II-mediated NE release in kidney | Isolated kidneys of SHR | 519 |
| Rat | No antiproteinuric effect | Adriamycin-induced proteinuria | 554 |
| Rat | ↓ Proteinuria and ↓ histological indexes of renal damage | l-NAME-induced proteinuria in SHR | 34 |
| Rat | ↓ TGF–β1 | Proximal tubular cells | 524 |
| ↓ ANG II-stimulated phosphorylation of p38, ERK 1/2, and JNK | |||
| Rat | ↓ Urine flow | [TGR(A1–7)3292] rats with a chronic 2.5-fold increase in plasma ANG-(1–7) | 166 |
| ↑ Urinary osmolality compared with SD control | |||
| Rat | ↓ ANG-II-induced constriction of isolated renal arteries | Isolated perfused kidneys, intravital microscopy in vivo under anesthesia, electromagnetic flow probe in freely moving rats | 555 |
| No effects on ANG-II-induced constriction of glomerular afferent and efferent arterioles in vivo | |||
| No effect on ANG-II-induced renal blood flow reduction in vivo | |||
| Rat | Vasodilation and ↓ renal vascular responsiveness to ET-1, NE, and ANG II in SHR | SHR and diabetic SHR | 30 |
| ↑ Sodium excretion | |||
| ↓ Proteinuria | |||
| ↓ Renal NOX-mediated oxidative stress | |||
| Rat | ↑ Diuresis in late gestation | Pregnant and virgin rats | 271 |
| ↓ Diuresis in virgin rats | |||
| ↑ AQP1 (virgin) | |||
| ↓ AQP1 (pregnant) | |||
| Rat | ↓ Effects of ovariectomy on renal wrap-induced renal pathology (tubulointerstitial fibrosis and glomerulosclerosis) | Ovariectomized renal wrap model of hypertension | 266 |
| Rat | ↓ Renal function | Streptozotocin (STZ) injection-induced diabetic rats | 495 |
| ↑ TGF-β1, ACE, and AT1 receptor | |||
| Sheep | Minimal effects on basal GFR, renal plasma flow, and Na+ excretion in males | Prenatal betamethasone-exposed ewes and rams | 534 |
| ↑ Na+ excretion in vehicle-treated females when compared with betamethasone-exposed females | |||
| Human | ↑ Growth-stimulatory effects in human mesangial cells | Cultured human mesangial cells | 643 |
| Pig | ↓ Profibrotic effects of high glucose | LLC-PK1 proximal tubular cells | 199 |
| Mice | ↓ Urine volume and fractional sodium excretion | Mas knockout mice | 423 |
| ↑ Microalbuminuria | |||
| ↓ Renal blood flow in Mas-knockout mice | |||
| Mice | ↓ NF-κB activation and proinflammatory cytokines in Mas knockout | Unilateral ureteral obstruction (UUO) model in Mas knockout mice | 149 |
| Rat | A-779 infusion reduced RPF | 2-Kidney-1-clip model in [TGR(A1–7)3292] rats | 60 |
| Rat | ↓ Glomerulosclerosis | Monoclonal anti-Thy-1 antibody, OX-7 induced glomerulonephritis | 630 |
| Mice | Did not alter FITC-inulin clearance or urinary albumin excretion, increased relative mesangial area | Mouse model of chronic kidney disease (CKD) | 132 |
| Rat | ↓ Proteinuria, renal collagen content, blood urea nitrogen, and dyslipidemia | STZ diabetic model | 512 |
| Rat | ↓ NADPH oxidase activity | SHR-STZ combined model | 129 |
| ↑ Catalase and PPAR-gamma | |||
| Rat | ↓ Proteinuria, and structural alterations in the kidney (↑ glomerular nephrin, ↓ IL-6, TNF-α, and NF-κB) | Salt-loaded SHRSP | 207 |
| Rat | ↑ Plasma ANG-(1–7) correlated with glomerular sclerosis and ↓ renal perivascular collagen deposition | SHR given an AT1 receptor blocker | 257 |
| Rat | ↑ ERK1/2 in a MAS/cAMP/PKA/MEK-dependent way | SD mesangial cells | 320 |
| Rat | ↓ α-SMA, vimentin, E-cadherin, TGF-β1, and fibronectin | High glucose-induced epithelial to mesenchymal transition (EMT) NRK-52E cells | 637 |
| ↓ ERK, p38 phosphorylation | |||
| Mice | ↓ Neutrophil influx and CXCL1/KC chemokine and renal damage | AVE0991 treatment in renal ischemia and reperfusion (I/R) injury in Mas knockout mice | 28 |
| ↓ Serum creatinine | |||
| Rat | ↓ Triglyceridemia | Zucker diabetic fatty rats (ZDF) | 202 |
| ↓ Proteinuria | |||
| ↓ Renal fibrosis | |||
| ↑ Creatinine clearance | |||
| Rat | ↓ ERK1/2 and TGF-β1 phosphorylation | Cultured rat renal mesangial cells (MCs) | 615 |
| Mice | ↑ RVR and ↓ RBF in Mas-knockout mice | Mas knockout mice | 48 |
| Sheep | ↓ RBF | ANG-(1–7)–treated adult, uninephrectomized rams exposed to betamethasone before birth | 40 |
| ↓ Basal sodium excretion | |||
| Rat | ↓ Basal sodium excretion | Normal, low and high salt intake rats | 400 |
| ↓ Diuresis and natriuresis in high-salt intake | |||
| Rat | ↓ JHCO3− [endogenous ANG-(1–7)] | In vivo proximal tubules | 77 |
| Biphasic dose-dependent effect on JHCO3−, mediated via NHE3 | |||
| Mice | AVE0991 treatment ↓ renal injury and proteinuria | Adriamycin (ADR)-induced nephropathy in Mas-KO mice | 507 |
| No differences in adriamycin-induced nephropathy in Mas-knockout or wild-type mice | |||
| ↓ Losartan reduction in renal injury in Mas knockout | |||
| Mice | ↓ Urinary albumin excretion | Diabetic nephropathy in db/db mice | 378 |
| ↓ NADPH oxidase activity | |||
| ↓ Inflammation in perirenal adipose tissue | |||
| Rat | ↑ RBF in male and female, A-779 blocked this response only in female | Male and female Wistar rats | 378 |
| Human | ↓ LDL-induced lipid deposition | Human mesangial cells (HMCs) | 256 |
| ↓ LDLr-SREBP2-SCAP | |||
| Dual effect on TGF-β1 | |||
| Human | ↓ Podocyte injury | Podocytes incubated with serum from preeclamptic and normal pregnant women | 539 |
| ↓ MAPK (p38, ERK1/2, and JNK) phosphorylation | |||
| Rat | No effect on glomerular damage | Uni-nephrectomized fawn-hooded hypertensive rat | 564 |
| Rat | A-779 blockage ↑ urine flow rate and sodium excretion in males, but not in females | Anesthetized male and female rats | 344 |
| Sheep | ↓ 8-Isoprostane excretion | Male and female adult sheep preexposed to antenatal betamethasone | 41 |
| Human | Vasodilation in contralateral stenotic kidneys, but reduced in stenotic kidneys | Human kidneys with renal artery stenosis | 559 |
| Rat | ↓ Glomeruli sclerosis | STZ rats | 638 |
| ↓ Oxidative stress | Rat mesangial HBZY-1 cell line exposed to high-glucose | ||
| ↓ Cell proliferation, | |||
| ↓ Collagen IV, TGF-β1, VEGF, NOX4, p47phox, PKCα, and PKCβ1 | |||
| ↓ Smad3 phosphorylation | |||
| Mice | ↓ Kidney injury | Type 1 diabetic Akita mice | 499 |
| ↓ Urinary albumin/creatinine ratio, glomerular hyperfiltration, renal hypertrophy, fibrosis, and tubular apoptosis | |||
| ↓ Renal oxidative stress | |||
| Mice | ↑ Renal injury in Mas-knockout mice [endogenous ANG-(1–7) protection] and in high doses of ANG-(1–7) | Mice with unilateral ureteral obstruction | 644 |
| Mice | ↓ Glomerular damage | db/db mice | 408 |
| ↓ Renal oxidative stress | |||
| Rat | ↓ Renal apoptosis and fibrosis | Unilateral ureteral obstruction in SD rats | 278 |
| ↓ TGF-β1/Smad | |||
| Rat | Dose-dependent increase in RBF | Ovariectomized female Wistar-rats treated with ANG-(1–7) | 453 |
| Rat | In presence of AT1 and AT2 blockade, there was a tendency for an increase in RBF/kidney weight by A779 (P = 0.08) | Anesthetized male and female Wistar rats | 343 |
| Human | Intrarenal infusion of ANG-(1–7) ↑ RBF | Hypertensive patients with multifocal renal artery fibromuscular dysplasia | 558 |
| Rat | Significant increase in RBF | Hemorrhagic shock model in Wistar rats | 342 |
| Rat | ↓ Renal injury (fibronectin and plasminogen activator inhibitor-1) | SD nephrectomized rats | 610 |
| ↓ Serum creatinine, ↓ proteinuria |
SD, Sprague-Dawley rats; IMCD, inner medullary collecting duct; SHR, spontaneously hypertensive rats; SHRSP, stroke-prone spontaneously hypertensive rats; STZ, streptozotocin-induced diabetic nephropathy; NE, norepinephrine; ET1, endothelin-1; AQP1, aquaporin 1; NRK-52E, rat renal cell line; α-SMA, α-smooth muscle actin; 2K1C, two-kidney one-clip Goldblatt hypertensive rats; RPF, renal plasma flow; RBF, renal blood flow; RVR, renal vascular resistance; JHCO3−, bicarbonate reabsorption; NHE3, Na+/H+ exchanger 3; LDLr-SREBP2-SCAP, low-density lipoprotein receptor-sterol regulatory element binding proteins 2-SREBP cleavage activating protein; LLC-PK1, porcine kidney cells.
TABLE 1 lists most of the known actions of ANG-(1–7) in the kidney, illustrating its complexity and unpredictability in this organ (28, 33, 34, 36, 40, 41, 59, 60, 76, 77, 128, 129, 132, 196, 202, 218, 238, 245, 251, 256, 257, 266, 271, 278, 294, 320, 342–344, 378, 391, 400, 408, 453, 478, 480, 495, 499, 512, 519, 520, 524, 534, 552–554, 557, 558, 564, 597, 615, 631, 638, 639, 644, 645).
1. Vascular effects of ANG-(1–7) in the kidney
The first direct demonstration of a vasoactive activity of ANG-(1–7) in the kidney was provided by Ren et al. using microperfused rabbit afferent arterioles (437). ANG-(1–7) produced a NO-dependent vasodilation that was abolished by A-779 but not by AT1 (L-158809) or AT2 (PD-123319) antagonists. The blockade of cyclooxygenase had no effect on ANG-(1–7) action (437). Evidence for vasodilator action in vivo was provided by Sampaio et al. using fluorescent microspheres (462). Other approaches did not lead to any direct effects of the heptapeptide on the renal vasculature in vivo despite in vitro activity (TABLE 1). This latter observation has led to skepticism regarding the biological significance of ANG-(1–7) in vivo (57). However, more recent studies in humans clearly indicate that ANG-(1–7) promotes vasodilation in human kidneys, a phenomenon that is influenced by the Na+ balance (560) or by the presence of renal artery stenosis (559).
E. Lung
Although little is known concerning the physiological roles of the ACE2/ANG-(1–7)/MAS axis in the lung, it appears to be critically involved in pathophysiological processes in this organ. ANG-(1–7) has been reported to reduce lung inflammation, fibrosis, and pulmonary arterial hypertension (92, 310, 333, 338, 364, 445, 449, 576). MAS has been detected in the epithelium and bronchial smooth muscle, suggesting sites where the beneficial actions of ANG-(1–7) may occur (148, 338). Anti-inflammatory actions of ANG-(1–7) were first described in a model of allergic asthma by El-Hashim et al. (148). The authors demonstrate that treatments with ANG-(1–7) resulted in the inhibition of the ovalbumin-induced increase in total cell counts, eosinophils, lymphocytes, and neutrophils as well as significantly reductions in ovalbumin-induced perivascular and peribronchial inflammation, fibrosis, and goblet cell hyper/metaplasia. These effects were mediated via MAS and blocked by A-779. ANG-(1–7) further reduced ovalbumin-induced increases in phosphorylation levels of ERK 1/2 and IκB-α and also inhibited the phytohemagglutinin-stimulated proliferation of human peripheral blood mononuclear cells (HPBMC). In chronic asthma, ANG-(1–7) triggered the beneficial attenuation of three major characteristics of this disease: lung inflammation, airway remodeling, and hyperresponsiveness (338). Similar effects were achieved in a model using the ANG-(1–7) mimetic compound AVE 0991 (449). The molecular mechanisms involved in these anti-inflammatory actions include a reduction of NFκB-related signaling, reductions of transforming growth factor (TGF)-β, and cytokine modulation (reducing IL-6 and IL-1β and increasing IL-10) (312, 363) (see FIGURE 7). The pulmonary activity of ACE and ACE2 activity becomes imbalanced in acute respiratory distress syndrome, which is characterized by an enhancement in ACE activity, the main pathway to ANG-(1–7) degradation, combined with a reduction in the activity of ACE2, which mainly generates it (598a). Experimental models of acute lung injury have highlighted an important protective role for ANG-(1–7). Infusions protected rats from acute lung lesions after oleic acid administration as seen through a decrease in lung edema, myeloperoxidase activity, histological lung injury score, and pulmonary vascular resistance (280). Similar results were observed in a pulmonary injury model based on lipopolysaccharide (LPS) (92). ANG-(1–7) treatment also decreases the severity of lung damage and inflammation induced by a combination of acid aspiration and high stretch ventilation. Moreover, continuous infusions of ANG-(1–7) reduced lung fibrosis 2 wk after acid aspiration injury, indicating its potential use in therapies for acute respiratory distress syndrome (ARDS) (626). The signaling mechanisms underlying the protective effects of the ACE2/ANG (1–7)/MAS axis against lung fibroblast migration and lung fibrosis seem to involve the inhibition of the NOX4-derived ROS-mediated RhoA/Rock pathway (363). In addition to its protective actions in pulmonary inflammatory progression, ANG-(1–7) also regulates cell survival, inhibiting alveolar epithelial cell (AEC) apoptosis, a critical event that initiates and propagates lung fibrotic disease. It decreases AEC apoptosis through pathways including the inhibition of JNK activation (312), reduction of endoplasmic reticulum (ER) stress-induced apoptosis (549), inhibition of LPS-induced ADAM17 shedding activity (333), and the upregulation of MAPK phosphatase-2 (219). Altogether, these findings indicate that this peptide may hold therapeutic potential in the treatment of lung fibrosis.
FIGURE 7.
Effects of ANG-(1–7) in the lung. Most of the known effects of ANG-(1–7) in the lungs are related to inflammation.
Since the demonstration of the beneficial effects of the ACE2/ANG-(1–7)/MAS axis in pulmonary arterial hypertension (PAH) by Shenoy et al. (497), emerging evidence has suggested that ANG-(1–7) has a protective role in this condition (498). Its importance in PAH comes from the observation that in patients with PAH from congenital heart disease, the peptide is reduced in plasma (110). The significant smaller response shown by TGR(mREN2)27 rats than SD to chronic hypoxia was related to the increased expression of ACE2 in the lung, leading to ACE2 activity and especially to markedly increased lung ANG-(1–7) concentrations and MAS expression in this strain (236). Interestingly, treatments with DIZE, a putative ACE2 activator, protect rats from monocrotaline (MCT)-induced PAH and reverse the imbalance in the autonomic nervous system modulation elicited by PAH, increasing parasympathetic and decreasing sympathetic cardiac activation (445). Although the administration of ANG-(1–7) and cyclic ANG-(1–7) immediately after induction of PAH in rats demonstrated no superiority of the MAS agonists compared with conventional treatments, the route of administration as well as the high doses used, which may have effects via AT1 receptors, suggest caution in interpreting the authors' conclusions (51). On the other hand, these results are important in designing future evaluations of the therapeutic potential of ANG-(1–7) for PAH treatment. Remarkable recent results from the Touyz group demonstrated that ANG-(1–7) negatively modulates the pro-inflammatory signaling of endothelin-1, a powerful pro-fibrotic mediator and vasoconstrictor that is elevated in PAH (255). This protective effect involves a crosstalk between MAS and the ETB receptor. These data identify the endothelin system as a novel molecular mechanism involved in the putative protective actions of ANG-(1–7) in PAH and illustrate the need for more studies to clarify the therapeutic usefulness of MAS agonists in this area.
F. Endocrine System
In addition to its actions directly related to the cardiovascular system, ANG-(1–7) has widespread effects in the endocrine system. The majority of these actions can also influence the role of the RAS in cardiovascular, metabolic, and electrolyte control. As discussed below, ANG-(1–7) has physiological actions in the major endocrine organs and plays a modulatory role in metabolic disorders such as diabetes and metabolic syndrome. FIGURE 8 illustrates the beneficial effects of ANG-(1–7) in diabetes (35, 36, 47, 71, 129, 139, 147, 202, 345, 377, 438, 447, 481–483, 486, 510).
FIGURE 8.
Protective effects of ANG-(1–7) on diabetes. ANG-(1–7) improves insulin signaling, increasing AKT phosphorylation, which is a key event for GLUT translocation to membrane and glucose uptake. This effect on AKT is also related to the increase in nitric oxide release and vasodilation. In addition to the anti-inflammatory and antioxidative properties, these effects lead to the reduction in microcapillary damage and in the vascular damage. Together, these actions result in clinical benefits, including reduction in cardiomyopathy and nephropathy, decrease in peripheral insulin resistance, protection against eye microcirculation damage, and retinopathy as well as acceleration in wound dermal repair.
RAS components have been identified in both the endocrine and exocrine pancreas. Angiotensinogen, renin, AT1 and AT2 receptors, ACE, and ACE2 are all expressed in this organ (74, 88, 305, 306, 542). The pancreatic RAS appears to be important in pathological conditions such as pancreatitis, hypoxia, inflammation, and endocrine pancreatic tumors. The expression of both ANG-(1–7) (88) and ACE2 (542) has been established in the rat and canine endocrine pancreas as well as in the developing mouse pancreas, where ACE2 and MAS proteins were similarly localized in cytoplasm throughout the periods of gestation that were evaluated (E12.5 to E18.5) (580). ANG-(1–7) modulates islet function through the regulation of blood flow and inhibition of fibrosis by stimulating eNOS expression and NO release (625). Islet blood flow is critical for endocrine pancreas function, and it is important to note that pancreatic islets have a wide capillary network. Each β-cell neighbors at least one islet endothelial cell, which delivers signals for islet cell development and for adult β-cell proliferation (291). It has been reported that ANG-(1–7) attenuates palmitate-induced apoptosis in islet endothelial cells, decreasing phosphorylation levels of p38 MAPK and JNK and preventing palmitate-induced decreases in the PI3K/AKT/eNOS pathway (625). In islets isolated from neonatal mouse pancreas, treatment with ANG-(1–7) augmented mRNA levels of insulin and the pancreatic progenitor marker Ngn3 (584). These effects were attenuated by cotreatment with A-779, suggesting that ANG-(1–7), via MAS, stimulates the differentiation of pancreatic progenitors into insulin-producing cells (584). Increased plasma and pancreatic levels of ANG-(1–7) and increased ACE2 expression were observed in a murine model of severe acute pancreatitis, suggesting that the ACE2/ANG-(1–7)/MAS axis participates in the pathogenesis of severe acute pancreatitis (322, 588). Endogenous ACE2 seems to have a key role in the adaptive β-cell hyperinsulinemic response, as seen in a diet-induced diabetes type 2 model, in which β-cell mass and proliferation were significantly reduced in ACE2-/y mice compared with controls (502).
In addition to its effects on acute pancreatic conditions, the protective axis of the RAS, ACE2/ANG-(1–7)/MAS also has beneficial modulatory effects in chronic metabolic diseases such as diabetes and metabolic syndrome. In these conditions, the triad insulin resistance/hyperglycemia/ANG II is intimately involved in the pathogenesis of target organ damage (49, 73). The main triggers for the development of vascular complications are endothelial dysfunction, inflammation, and proliferation of VSMC. High levels of ANG II and hyperglycemia form a self-reinforcing positive feedback loop that accelerates vascular damage. Like other growth factors, insulin stimulates the MAPK pathway, and the stimulatory cascade starts with the phosphorylation of IRS and/or Shc proteins that interact with the Grb2 protein. It is constitutively associated with the SOS protein, which sequentially activates Ras, a small G protein. In turn, Ras triggers the sequential phosphorylation of the MAPK cascade, leading to cell proliferation and differentiation (350). The responses of ANG II in vascular cells are mediated by various and complex effector systems of the plasma membrane, such as phospholipases (A, C, and D), adenylyl cyclase, PKC, and ion channels, which are activated in conjunction with a number of microdomain proteins, mainly adapters. These proximal pathways lead to the activation of Ras/MAPK/ERK and JAK/STAT cascades which amplify the signal and extend it to the nucleus by regulating gene expression and stimulating cell proliferation (350). In addition, ANG II stimulates TGF-β, an important mediator of collagen formation, extracellular matrix (ECM) deposition and fibrosis, all of which are involved in nephropathy. Hyperglycemia enhances the vasoconstrictive and proliferative actions of ANG II, increasing vascular hyperplasia and the progression of diabetic nephropathy (49). On the other hand, ANG-(1–7) has protective actions on vascular functions, increasing the generation of NO and inhibiting both vascular proliferation and inflammation. Intracellular pathways activated by ANG-(1–7) are opposed to those stimulated by ANG II. The antiproliferative effects of ANG-(1–7) on VSMC are associated with inhibitory activity on MAPKs ERK1/2 (p44/42 MAPK) and p38 (530, 601).
An essential pathway in insulin signaling is PI3K/AKT. ANG II, via the AT1 receptor, inhibits the protective effects of insulin in the vasculature, interfering with the PI3K/AKT cascade and reducing NO availability while also reducing GLUT translocation and glucose transport (517). ANG-(1–7), on the other hand, induces the in vivo activation of GSK3β, the downstream target of AKT, in liver and skeletal muscle (385). It stimulates the phosphorylation of crucial insulin signaling mediators (AKT, GSK3β, and AS160) in liver, skeletal muscle, and adipose tissue (384). Chronic ANG-(1–7) treatment restored IR/IRS-1/PI3K/AKT activation, which was decreased in fructose-fed rats, a model of metabolic syndrome. Additionally, ANG-(1–7) ameliorates insulin resistance in this model (205). It was also demonstrated that a high sucrose intake in rats is associated with increased ACE2 and ANG-(1–7) levels in adipose tissue, suggesting a modulatory role of this axis under altered metabolic conditions (96). In TGR(A1–7)3292 rats, increased ANG-(1–7) levels enhanced glucose tolerance, insulin sensitivity, and insulin-stimulated glucose uptake and decreased levels of triglycerides, cholesterol, and abdominal fat mass, despite normal food intake (483). Moreover, the elevated levels of ANG-(1–7) protected the rats against metabolic stress induced by a high-fat diet by decreasing the proinflammatory molecules IL-1β and COX-2 in adipose tissue (485). Similar results were observed by Santos et al. (485) and Mario et al. (346), also suggesting crosstalk between ANG-(1–7) and sirtuins, particularly, SIRT1, whose levels rise in the adipose tissue of mice on a high-fat diet which are treated with ANG-(1–7) (346). In these mice, the oral administration of ANG-(1–7) improved insulin sensitivity and glucose tolerance and lowered plasma levels of fasting glucose and lipids (346). These effects were associated with an increase in GLUT4 and AMPK/FOXO1/peroxisome proliferator-activated receptor gamma (PPARγ) expression in adipose tissue (346). ANG-(1–7) and MAS are also essential in mediating the endothelium-dependent relaxation response induced by perivascular adipose tissue (302). MAS deficiency alters the response of adipocytes to insulin and decreases the expression of PPARγ in adipose tissue, which is accompanied by a lower expression of acetyl-CoA carboxylase and lower amounts of fatty acid synthase, which are the target enzymes of PPARγ (346). Oh et al. (401) showed that the effect of captopril in body weight decrease was partly related to ANG-(1–7)-stimulated NO release, since this action was attenuated by pretreatment with A-779 as well as by both PI3K and eNOS inhibitors. Interestingly, Gupte et al. (230) demonstrated that sex differences in the development of obesity-associated hypertension are related to the ACE2-mediated regulation of the ANG II/ANG-(1–7) balance. Male mice exhibited obesity hypertension associated with enhanced ANG II/AT1 effects, whereas the protection of female mice against obesity hypertension was abolished by the MAS antagonist A-779.
1. Female reproductive system
The presence of ANG-(1–7) in the ovary was initially described by Costa et al. in 2003 (100). These authors also were the first to suggest an involvement of the ANG-(1–7) peptide in pre- and postovulatory events, demonstrating higher levels of ANG-(1–7) in the proestrus and estrus as well as in equine chorionic gonadotropin (eCG)-treated rats (254). Later, the same group demonstrated that the ACE2/ANG-(1–7)/MAS axis is fully expressed in rat ovaries (419) and in all stages of follicular development in humans (436). ANG-(1–7) also plays a role in the ovulatory process, stimulating estradiol production and enhancing ovulatory efficiency. The specific ANG-(1–7) antagonist A-779 blocked these effects (569). Additionally, ANG-(1–7) promotes meiotic resumption in follicle-enclosed oocytes (FEOs), and gonadotropins upregulate the ACE2/ANG-(1–7)/MAS axis. A-779 reduced the percentage of germinal vesicle breakdown in luteinizing hormone (LH)-stimulated FEOs, suggesting that ANG-(1–7) participates in oocyte maturation as a gonadotropin intermediate (254). In addition to this action in ovaries, the expression of ANG-(1–7) in rat longitudinal myometrium and uterine serosa may indicate that the peptide is important for muscle physiology (563). Interestingly, ovarian steroids are not required for the endometrial expression of ANG-(1–7) in rats, since it remains abundant in ovariectomized animals. However, estrogen and progestin may modulate the distribution pattern of ANG-(1–7) in endometrial glands, where this peptide participates in the regulation of the endometrial response to ovarian steroids (563).
The ACE2/ANG-(1–7)/MAS axis is also differentially expressed during follicular development in cattle (27). ACE2 was upregulated in the granulosa cells of the largest follicles (F1) during and after the follicular deviation. MAS was upregulated in granulosa cells of the second-largest follicles (F2) after the establishment of follicular deviation, while its expression increased in F1 in which atresia had been induced, suggesting that MAS may be involved with the establishment of follicular dominance (27).
There is also evidence indicating that ANG-(1–7) plays an important role in pregnancy. In humans, ANG-(1–7) plasmatic and urinary levels increase during pregnancy, indicating that this peptide may have an important function in adaptations during gestation. Remarkably, lower concentrations of maternal and fetal ANG-(1–7) have been independently associated with preterm births (93). Moreover, in pregnant rats, ANG-(1–7)-induced vasodilation in mesentery resistance vessels increases, reinforcing the hypothesis that ANG-(1–7) may make an essential contribution to the regulation of blood pressure during pregnancy (55). Systemic infusions of ANG-(1–7) resulted in a greater increase in CO in normotensive pregnant women than in women who were not pregnant (616).
Merril et al. (367) were the first to demonstrate an increase in plasma ANG-(1–7) in normal pregnant women compared with nonpregnant women; they also found decreased levels of ANG-(1–7) in preeclamptic compared with normal pregnant women. Velloso et al. (567) also found a significant reduction in plasma ANG-(1–7) levels, as well as a significantly higher plasma ACE activity and a marked reduction in plasma renin activity in preeclamptic women, especially in patients presenting the ACE deletion polymorphism deletion/deletion (DD) genotype. In contrast, ANG II levels in the plasma of preeclamptic women did not differ from those of normotensive pregnant subjects (567). Pregnancy-induced increases of plasma ANG-(1–7) are also blunted in gestational diabetes (399). Following iontophoretic applications of ANG-(1–7), normotensive pregnant women exhibited a higher change in skin flow compared with preeclamptic women (616). Women who developed gestational hypertension or preeclampsia had increased ANG-(1–7) levels at 15 wk of gestation compared with women with normal pregnancies, suggesting that levels of ANG-(1–7) during early gestation could be useful indicators in predicting a woman's risk of developing hypertension during pregnancy (528).
Regarding the expression of ACE2/ANG-(1–7)/MAS axis components in human placentas of normal and pathological pregnancies, syncytial ANG-(1–7) expression in samples obtained from pathological placentas was higher than those from normal-term pregnancies. In the maternal stroma, ANG-(1–7) and ACE2 expression was found in the invading and intravascular trophoblast and in decidual cells. Furthermore, ANG-(1–7) and ACE2 are expressed in arterial and venous endothelium and smooth muscle of the umbilical cord. The expression of ACE2 is increased in umbilical arterial endothelium in preeclampsia (550). Preeclamptic women also exhibit an imbalance of local RAS in the chorionic villous in the placenta, where it is responsible for the regulation of fetal oxygen and nutrient transport. In association with the upregulation of angiotensinogen and the AT1 receptor, a significant increase in ANG II was observed, but there was no accompanying increase in ANG-(1–7) and a decrease in MAS (19). In the uteroplacental unit of pregnant rats, the distribution profile of ANG-(1–7) and ACE2 expression differs during early stages of pregnancy (decidua, luminal, and glandular epithelium, embryo, and ectoplacental cone) and late pregnancy (epithelial cells of the yolk sac and amnion). This pattern indicates that ANG-(1–7) participates in the initial events of a pregnancy, including angiogenesis, apoptosis, and growth, and it also plays a role in uteroplacental blood flow during the last phase of gestation (395). In guinea pigs, ANG-(1–7) and ACE2 have been detected in the endothelium and syncytiotrophoblast of the labyrinthine placenta, interlobium, subplacenta, giant cells, syncytial sprouts, syncytial streamers, and myometrium throughout pregnancy. Moreover, ANG-(1–7) and ACE2 expression were especially notable in giant cells, which also express kallikrein, the bradykinin B2 receptor, vascular endothelial growth factor (VEGF) and its type 2 receptor, and eNOS, which integrates ANG-(1–7) into the uteroplacental vasodilatory network (551). The expression of the ACE2/ANG-(1–7)/MAS axis was also found in two trophoblast cell lines (HTR-8/SVneo and BeWo cells), reinforcing the idea that ANG-(1–7) is involved in the early stages of pregnancy (588). ANG-(1–7) treatment attenuated podocyte injuries in cultured podocytes incubated with preeclamptic serum, which demonstrated a decrease in the expression of podocyte-specific proteins (nephrin and WT-1), a rearrangement of F-actin, and apoptosis. These protective effects of ANG-(1–7) are probably mediated through the downregulation of MAPK (p38, ERK1/2, and JNK) phosphorylation (539).
2. Male reproductive system
ANG-(1–7) also plays a role in the male reproductive system, regulating spermatogenesis and erection. The expression of MAS mRNA was detected in Leydig cells (8), and a specific binding of fluorescent-labeled ANG-(1–7) in testis occurred mainly in the intertubular compartment (likely also in Leydig cells), where ANG-(1–7) has regulatory actions on spermatogenesis (301). Studies in Mas−/− mice demonstrated that these animals exhibited a significant reduction in testis weight and a greater volume of apoptotic cells, giant cells, and vacuoles in the seminiferous epithelium (301). Moreover, Mas−/− mice also showed lower Sertoli efficiency, meiotic index, overall rate of spermatogenesis, and daily sperm production, indicating the overall importance of MAS in male reproduction (301). Neither ANG-(1–7) nor MAS was detected in the seminiferous tubules of infertile men with nonobstructive azoospermia and severely impaired spermatogenesis. Furthermore, testicular samples from infertile men with impaired spermatogenesis (nonobstructive azoospermia) expressed MAS and ACE2 mRNA at lower concentrations than samples with normal spermatogenesis (obstructive azoospermia) (435).
ANG-(1–7) also mediates penile erection through the activation of MAS. Erectile functions of Mas−/− gene-deleted mice are substantially reduced and associated with a marked increase in fibrous tissue in the corpus cavernosum (107). In rats, the MAS agonist AVE 0991 potentiates the penile erectile response in a NO-dependent manner (106). Similarly, chronic treatment with HPβCD-ANG-(1–7), an oral formulation of ANG-(1–7), reduces penile fibrosis associated with an attenuation of oxidative stress in hypercholesterolemic ApoE−/− mice) (187).
G. Skeletal Muscle System
The identification of RAS components in skeletal muscle (590) indicates that the effects of angiotensins on metabolism and skeletal muscle blood flow can also be adjusted locally. In addition, due to their large presence throughout the body, skeletal muscle cells represent important modulators of the systemic effects of angiotensins.
The local production of ANG II and its effects on skeletal muscle have been widely studied (61, 111, 126, 157, 179, 200, 270). ACE inhibition and AT1 blockade represent novel strategies that are being pursued in the treatment of various skeletal muscle disorders and cachexia induced by CHF (97, 127, 144, 464, 491, 529, 575).
An example is Duchene Muscular Dystrophy (DMD), caused by mutations in the dystrophin gene, the most common muscular dystrophy in children. The primary animal model used to study this pathology is the MDX mouse (503). Recently, rodent studies have shown beneficial effects of ANG-(1–7) in this and other muscular dystrophy models (5a, 95, 360, 372, 374, 375, 446, 454, 456) (FIGURE 9).
FIGURE 9.
Actions of ANG-(1–7) in the skeletal muscle system.
In a recent study, chronic infusion or oral treatment with ANG-(1–7) in MDX mice normalized skeletal muscle structure, drastically reducing the total amount of collagen and collagen III and improving muscle function “in vitro” and “in vivo” (5a). This study also demonstrated that MDX mice treated with A-779 or crossed with Mas−/− mice (mdxKOMas) presented a dramatic increase in damaged and inflamed tissue in comparison with MDX mice. These effects are mediated TGF-β/Smad signaling (5a).
Chronic ANG II treatment induced skeletal muscle wasting in a manner involving p38MAPK and Smad TGF-β1 signaling (376). The accompanying increases of fibronectin, collagen, TGF-β1, atrogin, and MURF1 induced by ANG II were attenuated by treatment with ANG-(1–7) (95).
Some patients with DMD carry mutations in genes that encode sarcoglycans (SGs). δ-Sarcoglycan-deficient mice (Sgcd−/−) develop a DMD-like disease and exhibit higher levels of ANG I, ANG II, and AT1 expression in skeletal muscle, whereas the levels of ANG-(1–7) and MAS expression are reduced (454). Mice treated with HPβCD-ANG-(1–7) exhibited ameliorations in skeletal muscle pathology, a normalization of autonomic activity, and an increase in spontaneous locomotor activity (455). Skeletal muscle fibrosis and oxidative stress were also markedly reduced in Sgcd−/− mice treated with HPβCD-ANG-(1–7).
Riquelme et al. (446) suggested that the expression of ACE2 regulates fibrosis in dystrophic muscle since muscle damage causes increased activity of ACE2, and ACE2 levels correlate with the degree of fibrosis. Recently it has been reported that ACE2-/y mice show reduced performance in voluntary running (380), but it is unknown whether this is a result of the genetic deletion of ACE2 in muscle cells, considering that this mouse exhibits an overall deficiency in ACE2.
MAS is also upregulated in skeletal muscle atrophies that have been induced by sepsis, immobilization, or ANG II (373). In these conditions, MAS expression in gastrocnemius and tibialis anterior muscles was upregulated, as were atrogin-1 and MURF-1. These data and the fact that ANG-(1–7) has protective effects against muscle atrophy suggest that MAS is involved in modulating the muscle wasting response (95, 360, 372–375). Mice who are submitted to a unilateral cast immobilization of the hindlimbs for a period of 14 days developed decreased muscle strength; pretreatment with ANG-(1–7) improved isometric strength and prevented the decreased fiber diameter of muscle. This also increased atrogin and MuRF1 expression, important markers of muscle atrophy (372). These anti-atrophic effects of ANG-(1–7) involve the IGF-1/IGFR-1/AKT pathway as shown by the fact that pretreatment with ANG-(1–7) prevents the decrease in AKT phosphorylation and FOXO3. All the effects of ANG-(1–7) were absent in Mas−/− mice, confirming the involvement of MAS in the actions of ANG-(1–7) in muscle tissue (372).
H. Liver
A number of reports document beneficial effects of the ACE2/ANG-(1–7)/MAS axis in the liver (FIGURE 10). They include improvements in nonalcoholic steatosis and inflammation (70, 153, 533), liver fibrosis (331, 340, 406, 418, 591), and sensitivity to insulin (71, 205). These observations are in keeping with the increase in ACE2 in chronic liver injuries in rats and humans (247, 407) and with the liver steatosis associated with MAS-deficiencies in ApoE−/− mice (505). Other reports indicate that ANG-(1–7) suppresses the growth of hepatocellular carcinoma and angiogenesis via interactions of different angiotensin receptors (323).
FIGURE 10.
Effects of ANG-(1–7) in the liver.
The liver ACE/ANG II axis is mainly activated in patients with chronic liver disease and plays important roles in hepatic fibrosis and portal hypertension (279). On the other hand, as in other tissues, the axis also has a protective role, delaying the development of hepatic fibrosis (406, 571). Lubel et al. (330) demonstrated that human patients with cirrhosis exhibit markedly elevated levels of concentrations of both plasma ANG-(1–7) and ANG II. Furthermore, ANG-(1–7) levels were higher in non-cirrhotic patients with hepatitis C than in controls. This study also showed that ANG-(1–7) is upregulated in human liver disease and has antifibrotic actions in a rat model of cirrhosis (bile-duct ligation). Hepatic stellate cells (HSCs) expressed MAS and when treated with ANG-(1–7) or the MAS agonist AVE 0991 produced less α-SMA and hydroxyproline, which was blocked by the MAS antagonist A-779 (331). In cirrhotic rat liver, ANG-(1–7) significantly inhibits vasoconstriction induced by intrahepatic ANG II or the α1 agonist methoxamine (MTX) through eNOS and guanylate cyclase-dependent NO signaling pathways (248). Recently, it has also been demonstrated that ANG-(1–7) reduces bile duct ligation-induced hepatic fibrosis via redox balance modulation. ANG-(1–7) treatment decreased H2O2 as well as NOX4 and the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, which is activated by reactive oxygen species (ROS) and is involved in ANG II-stimulated collagen synthesis (62, 632). On the other hand, ANG-(1–7) increased glutathione and the nuclear erythroid 2-related factor 2 antioxidant response element (218).
VI. INTRACELLULAR SIGNALING PATHWAYS INDUCED BY ANGIOTENSIN-(1–7)
In recent decades several aspects of ANG-(1–7)-induced signaling have been clarified. The increase in NO release is the most classic event related to the protective role of ANG-(1–7) and includes calcium-independent pathways and the posttranslational regulation of eNOS (463). ANG-(1–7), via AKT-dependent pathways, stimulates eNOS activation (reciprocal phosphorylation/dephosphorylation at Ser1177/Thr495), which triggers NO production (463) and increases levels of cGMP (174, 216, 536). ANG-(1–7)-mediated AKT activation is also involved in its metabolic actions and participates in improvements in insulin resistance (205). In addition to the phosphorylation of AKT, ANG-(1–7) mediates the phosphorylation of GSK-3β and AS160, which are crucial insulin mediators (384). It has also been demonstrated that chronic oral administration of HPβCD-ANG-(1–7) attenuates hyperglycemia in an animal model of type 2 diabetes (486), indicating the potential of ANG-(1–7) as a hypoglycemic drug.
Although AKT is an important FOXO inactivator, ANG-(1–7) is able to dephosphorylate FOXO1 at Ser256, directly activating this transcription factor (568). This effect is probably involved in the antitumor actions of ANG-(1–7) since FOXO1 is a well-known tumor suppressor (99).
ANG-(1–7) increases arachidonic acid (AA) release, phospholipase A2 (PLA2) activity and prostaglandin E2 (PGE2)/prostacyclin (PGI2) synthesis (18, 324, 383). It also counterregulates ANG II signaling, blunting the phosphorylation of c-Src and the activation of NAD(P)H oxidase by ANG II and reducing ROS generation (461). This modulatory effect is mediated by the phosphorylation of SHP-2, preventing ANG II-induced SHP-2 dephosphorylation and promoting the interaction between SHP-2 and c-Src (461). Additionally, ANG-(1–7) inhibits MAPKs (ERK1/2, p38, JNK), which are central mediators in cell proliferation, fibrosis, and remodeling. The inhibition of these pathways has been demonstrated in a number of cell types including VSMCs (642), lung cancer cells (194, 396), cardiac myocytes (531), endothelial cells (461), and kidney proximal tubule cells (LLC-PK) (199). In tumor cells (98) and myocytes (354), the inhibition of MAPK induced by ANG-(1–7) probably involves the MAPK phosphatase dual-specificity phosphatase (DUSP)-1.
ANG-(1–7) additionally decreases TGF-β/NFκB signaling (148, 267, 345, 482, 627), a key pathway in inflammation. Reductions in proinflammatory molecules such as TNF-α (5, 207, 482, 514), MCP-1, IL-8 (42), IL-1β (42, 485), and COX-2 (267, 365, 485) were observed. The anti-inflammatory role of ANG-(1–7) has been demonstrated in pathological conditions such as asthma (148, 338), arthritis (5, 109), angioplasty (627), cerebral ischemia (267), diabetes (486), and intracerebral hemorrhagic stroke (42). There is growing evidence for an activation of the GS pathway by ANG-(1–7) (320), leading to increased cAMP formation and PKA activity (337).
The signaling pathways elicited by alamandine are being clarified and also involve NO release. In ventricular myocytes, alamandine leads to the phosphorylation of 3-phosphoinositide dependent protein kinase-1 (PDK1), which is crucial for the activation of AKT and interferes with GSK3β depending on the model. In cardiac myocytes from TGR(mREN2)27 rats, alamandine increases GSK3β phosphorylation but decreases it in myocytes from healthy animals (265). FIGURE 11 summarizes the main signaling pathways involved in the ANG-(1–7) effects.
FIGURE 11.
Intracellular signaling pathways induced by ANG-(1–7). ANG-(1–7) mediates, via AKT-dependent pathways, phosphorylation (Ser 1177) and activation of eNOS, stimulating nitric oxide (NO) production. ANG-(1–7)-mediated AKT activation is also involved in its metabolic actions, through GLUT phosphorylation and translocation to the membrane, enhancing glucose uptake. Although AKT is an important FOXO inactivator, ANG-(1–7) is able to dephosphorylate (Ser256) FOXO1, directly activating this transcription factor. ANG-(1–7) also counterregulates ANG II signaling, blunting phosphorylation of c-Src and the activation of NAD(P)H oxidase by ANG II, reducing reactive oxygen species (ROS) generation. This modulatory effect is mediated by phosphorylation of SHP-2, preventing ANG II-induced SHP-2 dephosphorylation and promoting interaction between SHP-2 and c-Src. Additionally, ANG-(1–7) inhibits MAPKs (ERK1/2, p38, JNK, and ERK5), central mediators in proliferation, fibrosis, and remodeling. Furthermore, ANG-(1–7) inhibits NF-κB, a key transcription factor in inflammation. The signaling pathways elicited by alamandine also involve NO release.
Besides MAS, other GPCRs have been implicated in mediating ANG-(1–7) actions. The blockade of some functions of ANG-(1–7) by the bradykinin B2 receptor antagonist HOE-140 has been taken as evidence that these receptors are linked (1, 178). This may indicate that some vascular actions of ANG-(1–7) depend on protein-protein interactions involved in eNOS activation (283). In addition to caveolin and calmodulin, which undergo reciprocal Ca2+-dependent associations and dissociations with eNOS, the eNOS protein complex includes the B2 receptor and other proteins, including the AT1 receptor, the cationic amino acid transporter-1 arginine transporter, and the chaperone heat shock protein 90 (283). The direct heterodimerization of MAS with the B2 receptor is another possibility to explain the effect of HOE-140. However, the fact that ANG-(1–7), through MAS, is capable of producing strong actions in B2-deficient mice demonstrates that these actions of ANG-(1–7) are independent of B2 receptors (496).
The participation of the ANG II AT2 receptor in some of the ANG-(1–7) effects has also been suggested (79, 500, 572, 577). Part of the evidence for this has been derived from experiments using the putative AT2 antagonist PD123319 (572). The specificity of this compound has been challenged, however, because it can produce effects in AT2 knockout mice (113), and it can displace the binding of alamandine to human MrgD-transfected cells and the vascular effects of alamandine. On the other hand, MAS appears to heterodimerize with AT2 (572). More reports include a ligand-induced heterodimerization of MAS with endothelin ETB receptors (255). Weak interactions of ANG-(1–7) with MrgD receptors have also been described (201). Finally, the heterodimerization of MAS with the AT1 receptor has been reported, and this inhibited the actions of ANG II. In this situation, MAS acts as a physiological antagonist of AT1 (284). These observations are in keeping with the well-known heterodimerization of other GPCRs (173).
VII. CONCLUDING REMARKS
Since the initial discovery of the RAS made by Tigerstedt and Bergmann by the end of the 19th century (540), studies by many laboratories have contributed to the establishment of this system as a key player in the physiology and pathophysiology of systems throughout the body. This review covers one of the most important recent chapters in the never-ending story of this system. The compelling evidence that the stimulation of the ACE2/ANG-(1–7)/MAS axis of the RAS may represent a powerful, novel therapeutic approach in the treatment of cardiometabolic diseases and other disorders has motivated many groups to evaluate the role of ANG-(1–7) in a range of conditions related to health. In this review we have attempted to highlight relevant aspects of the role of ANG-(1–7) in a broad range of physiological and pathophysiological contexts. Ongoing clinical trials based on ACE2- and ANG-(1–7)/MAS-related strategies may soon usher in a new chapter of the RAS saga.
GRANTS
We are thankful for the support from Humboldt Foundation (Georg Forster Research Award to R. A. S. Santos); CNPq Grants INCT 573924/2008–2, 465612/2014–8, 4000990/2012–1, and 407352/2013–9; CAPES; and FAPEMIG.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are thankful to Russ Hodge, Max Delbrück Center for Molecular Medicine in the Helmholtz Association, for the careful revision of the manuscript.
Address for reprint requests and other correspondence: R. A. S. Santos or M. J. Campagnole-Santos, National Institute of Science and Technology in Nanobiopharmaceutics, Department of Physiology and Biophysics, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil (e-mail: robsonsant@gmail.com or mjcampagnole.ufmg@gmail.com).
REFERENCES
- 1.Abbas A, Gorelik G, Carbini LA, Scicli AG. Angiotensin-(1-7) induces bradykinin-mediated hypotensive responses in anesthetized rats. Hypertension 30: 217–221, 1997. doi: 10.1161/01.HYP.30.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah WF, Louie SG, Zhang Y, Rodgers KE, Sivok E, diZerega SG, Humayun MS. NorLeu3A(1-7) Accelerates Clear Corneal Full Thickness Wound Healing. Invest Ophthalmol Vis Sci 57: 2187–2194, 2016. doi: 10.1167/iovs.15-18515. [DOI] [PubMed] [Google Scholar]
- 3.Aboye T, Meeks CJ, Majumder S, Shekhtman A, Rodgers K, Camarero JA. Design of a MCoTI-Based Cyclotide with Angiotensin (1-7)-Like Activity. Molecules 21: 152, 2016. doi: 10.3390/molecules21020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abwainy A, Babiker F, Akhtar S, Benter IF. Endogenous angiotensin-(1-7)/Mas receptor/NO pathway mediates the cardioprotective effects of pacing postconditioning. Am J Physiol Heart Circ Physiol 310: H104–H112, 2016. doi: 10.1152/ajpheart.00121.2015. [DOI] [PubMed] [Google Scholar]
- 5.Açikalin Ö, Bölükbaşi Hatip FF, Tan RF, Hatip-Al-Khatib I. Effect of Angiotensin-(1-7) on Aortic Response, TNF-α, IL-1β and Receptor for Advanced Glycation Endproduct in Rat’s Adjuvant-Induced Arthritis. Pharmacology 97: 207–217, 2016. doi: 10.1159/000444188. [DOI] [PubMed] [Google Scholar]
- 5a.Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Muñoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-β signalling. Hum Mol Genet 23: 1237–1249, 2014. doi: 10.1093/hmg/ddt514. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol 106: 1069–1085, 2011. doi: 10.1007/s00395-011-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht D. Angiotensin-(1-7)-induced plasticity changes in the lateral amygdala are mediated by COX-2 and NO. Learn Mem 14: 177–184, 2007. doi: 10.1101/lm.425907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alenina N, Baranova T, Smirnow E, Bader M, Lippoldt A, Patkin E, Walther T. Cell type-specific expression of the Mas proto-oncogene in testis. J Histochem Cytochem 50: 691–696, 2002. doi: 10.1177/002215540205000510. [DOI] [PubMed] [Google Scholar]
- 9.Alghamri MS, Morris M, Meszaros JG, Elased KM, Grobe N. Novel role of aminopeptidase-A in angiotensin-(1-7) metabolism post myocardial infarction. Am J Physiol Heart Circ Physiol 306: H1032–H1040, 2014. doi: 10.1152/ajpheart.00911.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida AP, Frábregas BC, Madureira MM, Santos RJ, Campagnole-Santos MJ, Santos RA. Angiotensin-(1-7) potentiates the coronary vasodilatatory effect of bradykinin in the isolated rat heart. Braz J Med Biol Res 33: 709–713, 2000. doi: 10.1590/S0100-879X2000000600012. [DOI] [PubMed] [Google Scholar]
- 11.Almeida-Santos AF, Kangussu LM, Moreira FA, Santos RA, Aguiar DC, Campagnole-Santos MJ. Anxiolytic- and antidepressant-like effects of angiotensin-(1-7) in hypertensive transgenic (mRen2)27 rats. Clin Sci (Lond) 130: 1247–1255, 2016. doi: 10.1042/CS20160116. [DOI] [PubMed] [Google Scholar]
- 12.Alzamora A, Santos R, Campagnole-Santos M. Effect of Angiotensin-(1–7) and Angiotensin II microinjection into the caudal ventrolateral medulla on the baroreflex control of heart rate. J Hypertens 16: S181, 1998. [Google Scholar]
- 13.Alzamora AC, Santos RA, Campagnole-Santos MJ. Baroreflex modulation by angiotensins at the rat rostral and caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 290: R1027–R1034, 2006. doi: 10.1152/ajpregu.00852.2004. [DOI] [PubMed] [Google Scholar]
- 14.Alzamora AC, Santos RA, Campagnole-Santos MJ. Hypotensive effect of ANG II and ANG-(1-7) at the caudal ventrolateral medulla involves different mechanisms. Am J Physiol Regul Integr Comp Physiol 283: R1187–R1195, 2002. doi: 10.1152/ajpregu.00580.2001. [DOI] [PubMed] [Google Scholar]
- 15.Ambroz C, Clark AJ, Catt KJ. The mas oncogene enhances angiotensin-induced [Ca2+]i responses in cells with pre-existing angiotensin II receptors. Biochim Biophys Acta 1133: 107–111, 1991. doi: 10.1016/0167-4889(91)90248-V. [DOI] [PubMed] [Google Scholar]
- 16.Ambühl P, Felix D, Imboden H, Khosla MC, Ferrario CM. Effects of angiotensin analogues and angiotensin receptor antagonists on paraventricular neurones. Regul Pept 38: 111–120, 1992. doi: 10.1016/0167-0115(92)90049-Z. [DOI] [PubMed] [Google Scholar]
- 17.Ambühl P, Felix D, Khosla MC. [7-d-ALA]-angiotensin-(1-7): selective antagonism of angiotensin-(1-7) in the rat paraventricular nucleus. Brain Res Bull 35: 289–291, 1994. doi: 10.1016/0361-9230(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 18.Andreatta-van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1-7). Kidney Int 44: 932–936, 1993. doi: 10.1038/ki.1993.334. [DOI] [PubMed] [Google Scholar]
- 19.Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. Activation of local chorionic villi angiotensin II levels but not angiotensin (1-7) in preeclampsia. Hypertension 51: 1066–1072, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold AC, Sakima A, Ganten D, Ferrario CM, Diz DI. Modulation of reflex function by endogenous angiotensins in older transgenic rats with low glial angiotensinogen. Hypertension 51: 1326–1331, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Averill DB, Ishiyama Y, Chappell MC, Ferrario CM. Cardiac angiotensin-(1-7) in ischemic cardiomyopathy. Circulation 108: 2141–2146, 2003. doi: 10.1161/01.CIR.0000092888.63239.54. [DOI] [PubMed] [Google Scholar]
- 23.Bader M, Ganten D. Transgenic rats: tools to study the function of the renin-angiotensin system. Clin Exp Pharmacol Physiol 23, Suppl 3: S81–S87, 1996. doi: 10.1111/j.1440-1681.1996.tb02818.x. [DOI] [PubMed] [Google Scholar]
- 24.Baltatu O, Fontes MA, Campagnole-Santos MJ, Caligiorni S, Ganten D, Santos RA, Bader M. Alterations of the renin-angiotensin system at the RVLM of transgenic rats with low brain angiotensinogen. Am J Physiol Regul Integr Comp Physiol 280: R428–R433, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Baracho NC, Simões-e-Silva AC, Khosla MC, Santos RA. Effect of selective angiotensin antagonists on the antidiuresis produced by angiotensin-(1-7) in water-loaded rats. Braz J Med Biol Res 31: 1221–1227, 1998. doi: 10.1590/S0100-879X1998000900016. [DOI] [PubMed] [Google Scholar]
- 26.Barnes KL, Knowles WD, Ferrario CM. Angiotensin II and angiotensin (1-7) excite neurons in the canine medulla in vitro. Brain Res Bull 24: 275–280, 1990. doi: 10.1016/0361-9230(90)90215-L. [DOI] [PubMed] [Google Scholar]
- 27.Barreta MH, Gasperin BG, Ferreira R, Rovani M, Pereira GR, Bohrer RC, de Oliveira JF, Gonçalves PB. The components of the angiotensin-(1-7) system are differentially expressed during follicular wave in cattle. J Renin Angiotensin Aldosterone Syst 16: 275–283, 2015. doi: 10.1177/1470320313491996. [DOI] [PubMed] [Google Scholar]
- 28.Barroso LC, Silveira KD, Lima CX, Borges V, Bader M, Rachid M, Santos RA, Souza DG, Simões E Silva AC, Teixeira MM. Renoprotective Effects of AVE0991, a Nonpeptide Mas Receptor Agonist, in Experimental Acute Renal Injury. Int J Hypertens 2012: 808726, 2012. doi: 10.1155/2012/808726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker L, Santos R, Campagnole-Santos M. The angiotensin-(1–7) receptor Mas is present in cardiovascular-related areas of the rat brain. Hypertension 46: 873, 2005. [Google Scholar]
- 30.Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol 293: H1416–H1424, 2007. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- 31.Becker LK, Santos RA, Campagnole-Santos MJ. Cardiovascular effects of angiotensin II and angiotensin-(1-7) at the RVLM of trained normotensive rats. Brain Res 1040: 121–128, 2005. doi: 10.1016/j.brainres.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 32.Bennion DM, Haltigan EA, Irwin AJ, Donnangelo LL, Regenhardt RW, Pioquinto DJ, Purich DL, Sumners C. Activation of the Neuroprotective Angiotensin-Converting Enzyme 2 in Rat Ischemic Stroke. Hypertension 66: 141–148, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benter IF, Ferrario CM, Morris M, Diz DI. Antihypertensive actions of angiotensin-(1-7) in spontaneously hypertensive rats. Am J Physiol 269: H313–H319, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol 290: H684–H691, 2006. doi: 10.1152/ajpheart.00632.2005. [DOI] [PubMed] [Google Scholar]
- 35.Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. Angiotensin-(1-7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 292: H666–H672, 2007. doi: 10.1152/ajpheart.00372.2006. [DOI] [PubMed] [Google Scholar]
- 36.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28: 25–33, 2008. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 37.Bertagnolli M, Casali KR, De Sousa FB, Rigatto K, Becker L, Santos SH, Dias LD, Pinto G, Dartora DR, Schaan BD, Milan RD, Irigoyen MC, Santos RA. An orally active angiotensin-(1-7) inclusion compound and exercise training produce similar cardiovascular effects in spontaneously hypertensive rats. Peptides 51: 65–73, 2014. doi: 10.1016/j.peptides.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Beyer AM, Guo DF, Rahmouni K. Prolonged treatment with angiotensin 1-7 improves endothelial function in diet-induced obesity. J Hypertens 31: 730–738, 2013. doi: 10.1097/HJH.0b013e32835ecbe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bharadwaj MS, Strawn WB, Groban L, Yamaleyeva LM, Chappell MC, Horta C, Atkins K, Firmes L, Gurley SB, Brosnihan KB. Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension 58: 852–858, 2011. doi: 10.1161/HYPERTENSIONAHA.111.179358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bi J, Contag SA, Carey LC, Tang L, Valego NK, Chappell MC, Rose JC. Antenatal betamethasone exposure alters renal responses to angiotensin-(1-7) in uninephrectomized adult male sheep. J Renin Angiotensin Aldosterone Syst 14: 290–298, 2013. doi: 10.1177/1470320312465217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bi J, Contag SA, Chen K, Su Y, Figueroa JP, Chappell MC, Rose JC. Sex-specific effect of antenatal betamethasone exposure on renal oxidative stress induced by angiotensins in adult sheep. Am J Physiol Renal Physiol 307: F1013–F1022, 2014. doi: 10.1152/ajprenal.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bihl JC, Zhang C, Zhao Y, Xiao X, Ma X, Chen Y, Chen S, Zhao B, Chen Y. Angiotensin-(1-7) counteracts the effects of Ang II on vascular smooth muscle cells, vascular remodeling and hemorrhagic stroke: role of the NFкB inflammatory pathway. Vascul Pharmacol 73: 115–123, 2015. doi: 10.1016/j.vph.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bild W, Ciobica A. Angiotensin-(1-7) central administration induces anxiolytic-like effects in elevated plus maze and decreased oxidative stress in the amygdala. J Affect Disord 145: 165–171, 2013. doi: 10.1016/j.jad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Bilman V, Mares-Guia L, Nadu AP, Bader M, Campagnole-Santos MJ, Santos RA, Santos SH. Decreased hepatic gluconeogenesis in transgenic rats with increased circulating angiotensin-(1-7). Peptides 37: 247–251, 2012. doi: 10.1016/j.peptides.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Block CH, Santos RA, Brosnihan KB, Ferrario CM. Immunocytochemical localization of angiotensin-(1-7) in the rat forebrain. Peptides 9: 1395–1401, 1988. doi: 10.1016/0196-9781(88)90208-2. [DOI] [PubMed] [Google Scholar]
- 46.Bomtempo CA, Santos GF, Santos RA, Campagnole-Santos MJ. Interaction of bradykinin and angiotensin-(1-7) in the central modulation of the baroreflex control of the heart rate. J Hypertens 16: 1797–1804, 1998. doi: 10.1097/00004872-199816120-00013. [DOI] [PubMed] [Google Scholar]
- 47.Bossi F, Bernardi S, De Nardo D, Bramante A, Candido R, Carretta R, Fischetti F, Fabris B. Angiotensin 1-7 significantly reduces diabetes-induced leukocyte recruitment both in vivo and in vitro. Atherosclerosis 244: 121–130, 2016. doi: 10.1016/j.atherosclerosis.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Botelho-Santos GA, Bader M, Alenina N, Santos RA. Altered regional blood flow distribution in Mas-deficient mice. Ther Adv Cardiovasc Dis 6: 201–211, 2012. doi: 10.1177/1753944712461164. [DOI] [PubMed] [Google Scholar]
- 49.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol 287: R943–R949, 2004. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 50.Braszko JJ, Karwowska-Polecka W, Halicka D, Gard PR. Captopril and enalapril improve cognition and depressed mood in hypertensive patients. J Basic Clin Physiol Pharmacol 14: 323–343, 2003. doi: 10.1515/JBCPP.2003.14.4.323. [DOI] [PubMed] [Google Scholar]
- 51.Breitling S, Krauszman A, Parihar R, Walther T, Friedberg MK, Kuebler WM. Dose-dependent, therapeutic potential of angiotensin-(1-7) for the treatment of pulmonary arterial hypertension. Pulm Circ 5: 649–657, 2015. doi: 10.1086/683696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Britto RR, Santos RA, Fagundes-Moura CR, Khosla MC, Campagnole-Santos MJ. Role of angiotensin-(1-7) in the modulation of the baroreflex in renovascular hypertensive rats. Hypertension 30: 549–556, 1997. doi: 10.1161/01.HYP.30.3.549. [DOI] [PubMed] [Google Scholar]
- 53.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension 27: 523–528, 1996. doi: 10.1161/01.HYP.27.3.523. [DOI] [PubMed] [Google Scholar]
- 54.Brosnihan KB, Li P, Tallant EA, Ferrario CM. Angiotensin-(1-7): a novel vasodilator of the coronary circulation. Biol Res 31: 227–234, 1998. [PubMed] [Google Scholar]
- 55.Brosnihan KB, Neves LA, Anton L, Joyner J, Valdes G, Merrill DC. Enhanced expression of Ang-(1-7) during pregnancy. Braz J Med Biol Res 37: 1255–1262, 2004. doi: 10.1590/S0100-879X2004000800017. [DOI] [PubMed] [Google Scholar]
- 56.Brosnihan KB, Schiavone MT, Sprunger AE, Chappell MC, Rizzo M, Ferrario CM. In vivo release of angiotensin II from the rat hypothalamus. Hypertension 11: I158–I162, 1988. doi: 10.1161/01.HYP.11.2_Pt_2.I158. [DOI] [PubMed] [Google Scholar]
- 57.Brunner HR, Gavras H. Validation of in-vitro data by in-vivo evidence: the example of angiotensin (1-7). J Hypertens 24: 1919–1920, 2006. doi: 10.1097/01.hjh.0000244936.26922.b0. [DOI] [PubMed] [Google Scholar]
- 58.Bunnemann B, Fuxe K, Metzger R, Mullins J, Jackson TR, Hanley MR, Ganten D. Autoradiographic localization of mas proto-oncogene mRNA in adult rat brain using in situ hybridization. Neurosci Lett 114: 147–153, 1990. doi: 10.1016/0304-3940(90)90063-F. [DOI] [PubMed] [Google Scholar]
- 59.Bürgelová M, Kramer HJ, Teplan V, Velicková G, Vítko S, Heller J, Malý J, Cervenka L. Intrarenal infusion of angiotensin-(1-7) modulates renal functional responses to exogenous angiotensin II in the rat. Kidney Blood Press Res 25: 202–210, 2002. doi: 10.1159/000066340. [DOI] [PubMed] [Google Scholar]
- 60.Bürgelová M, Vanourková Z, Thumová M, Dvorák P, Opocenský M, Kramer HJ, Zelízko M, Malý J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens 27: 1988–2000, 2009. doi: 10.1097/HJH.0b013e32832f0d06. [DOI] [PubMed] [Google Scholar]
- 61.Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev 35: 437–463, 2015. doi: 10.1002/med.21343. [DOI] [PubMed] [Google Scholar]
- 62.Cai SM, Yang RQ, Li Y, Ning ZW, Zhang LL, Zhou GS, Luo W, Li DH, Chen Y, Pan MX, Li X. Angiotensin-(1-7) Improves Liver Fibrosis by Regulating the NLRP3 Inflammasome via Redox Balance Modulation. Antioxid Redox Signal 24: 795–812, 2016. doi: 10.1089/ars.2015.6498. [DOI] [PubMed] [Google Scholar]
- 63.Calka J, Block CH. Angiotensin-(1-7) and nitric oxide synthase in the hypothalamo-neurohypophysial system. Brain Res Bull 30: 677–685, 1993. doi: 10.1016/0361-9230(93)90099-W. [DOI] [PubMed] [Google Scholar]
- 64.Campagnole-Santos MJ, Diz DI, Ferrario CM. Actions of angiotensin peptides after partial denervation of the solitary tract nucleus. Hypertension 15, Suppl: I34–I39, 1990. doi: 10.1161/01.HYP.15.2_Suppl.I34. [DOI] [PubMed] [Google Scholar]
- 65.Campagnole-Santos MJ, Diz DI, Santos RA, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1-7) injected into the dorsal medulla of rats. Am J Physiol Heart Circ Physiol 257: H324–H329, 1989. [DOI] [PubMed] [Google Scholar]
- 66.Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol Regul Integr Comp Physiol 263: R89–R94, 1992. [DOI] [PubMed] [Google Scholar]
- 67.Campbell WB, Brooks SN, Pettinger WA. Angiotensin II- and angiotensin 3-induced aldosterone release vivo in the rat. Science 184: 994–996, 1974. doi: 10.1126/science.184.4140.994. [DOI] [PubMed] [Google Scholar]
- 68.Canals M, Jenkins L, Kellett E, Milligan G. Up-regulation of the angiotensin II type 1 receptor by the MAS proto-oncogene is due to constitutive activation of Gq/G11 by MAS. J Biol Chem 281: 16757–16767, 2006. doi: 10.1074/jbc.M601121200. [DOI] [PubMed] [Google Scholar]
- 69.Cangussu LM, de Castro UG, do Pilar Machado R, Silva ME, Ferreira PM, dos Santos RA, Campagnole-Santos MJ, Alzamora AC. Angiotensin-(1-7) antagonist, A-779, microinjection into the caudal ventrolateral medulla of renovascular hypertensive rats restores baroreflex bradycardia. Peptides 30: 1921–1927, 2009. doi: 10.1016/j.peptides.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 70.Cao X, Yang F, Shi T, Yuan M, Xin Z, Xie R, Li S, Li H, Yang JK. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis. Sci Rep 6: 21592, 2016. doi: 10.1038/srep21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao X, Yang FY, Xin Z, Xie RR, Yang JK. The ACE2/Ang-(1-7)/Mas axis can inhibit hepatic insulin resistance. Mol Cell Endocrinol 393: 30–38, 2014. doi: 10.1016/j.mce.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 72.Carey RM. Newly discovered components and actions of the renin-angiotensin system. Hypertension 62: 818–822, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01111. [DOI] [PubMed] [Google Scholar]
- 73.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab 14: 274–281, 2003. doi: 10.1016/S1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 74.Carlsson PO. The renin-angiotensin system in the endocrine pancreas. JOP 2: 26–32, 2001. [PubMed] [Google Scholar]
- 75.Carmos-Silva C, Almeida JF, Macedo LM, Melo MB, Pedrino GR, Santos FF, Biancardi MF, Santos RA, Carvalho AA, Mendes EP, Colugnati DB, Mazaro-Costa R, Castro CH. Mas receptor contributes to pregnancy-induced cardiac remodeling. Clin Sci (Lond) 130: 2305–2316, 2016. doi: 10.1042/CS20160095. [DOI] [PubMed] [Google Scholar]
- 76.Caruso-Neves C, Lara LS, Rangel LB, Grossi AL, Lopes AG. Angiotensin-(1-7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta 1467: 189–197, 2000. doi: 10.1016/S0005-2736(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 77.Castelo-Branco RC, Leite-Delova DC, de Mello-Aires M. Dose-dependent effects of angiotensin-(1-7) on the NHE3 exchanger and [Ca2+]i in in vivo proximal tubules. Am J Physiol Renal Physiol 304: F1258–F1265, 2013. doi: 10.1152/ajprenal.00401.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Effects of genetic deletion of angiotensin-(1-7) receptor Mas on cardiac function during ischemia/reperfusion in the isolated perfused mouse heart. Life Sci 80: 264–268, 2006. doi: 10.1016/j.lfs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 46: 937–942, 2005. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- 80.Cerrato BD, Frasch AP, Nakagawa P, Longo-Carbajosa N, Peña C, Höcht C, Gironacci MM. Angiotensin-(1-7) upregulates central nitric oxide synthase in spontaneously hypertensive rats. Brain Res 1453: 1–7, 2012. doi: 10.1016/j.brainres.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 81.Chaar LJ, Alves TP, Batista Junior AM, Michelini LC. Early Training-Induced Reduction of Angiotensinogen in Autonomic Areas-The Main Effect of Exercise on Brain Renin-Angiotensin System in Hypertensive Rats. PLoS One 10: e0137395, 2015. doi: 10.1371/journal.pone.0137395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang RL, Lin JW, Kuo WW, Hsieh DJ, Yeh YL, Shen CY, Day CH, Ho TJ, Viswanadha VP, Huang CY. Angiotensin-(1-7) attenuated long-term hypoxia-stimulated cardiomyocyte apoptosis by inhibiting HIF-1α nuclear translocation via Mas receptor regulation. Growth Factors 34: 11–18, 2016. doi: 10.3109/08977194.2016.1155150. [DOI] [PubMed] [Google Scholar]
- 83.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol 2: 2733–2752, 2012. doi: 10.1002/cphy.c120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chappell MC, Allred AJ, Ferrario CM. Pathways of angiotensin-(1-7) metabolism in the kidney. Nephrol Dial Transplant 16, Suppl 1: 22–26, 2001. doi: 10.1093/ndt/16.suppl_1.22. [DOI] [PubMed] [Google Scholar]
- 85.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem 264: 16518–16523, 1989. [PubMed] [Google Scholar]
- 86.Chappell MC, Diz DI, Yunis C, Ferrario CM. Differential actions of angiotensin-(1-7) in the kidney. Kidney Int Suppl 68: S3–S6, 1998. doi: 10.1038/sj.ki.4490555. [DOI] [PubMed] [Google Scholar]
- 87.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the Angiotensin converting enzyme 2-Angiotensin (1-7)-MAS receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 4: 201, 2014. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chappell MC, Millsted A, Diz DI, Brosnihan KB, Ferrario CM. Evidence for an intrinsic angiotensin system in the canine pancreas. J Hypertens 9: 751–759, 1991. doi: 10.1097/00004872-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 89.Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1-7) by angiotensin-converting enzyme. Hypertension 31: 362–367, 1998. doi: 10.1161/01.HYP.31.1.362. [DOI] [PubMed] [Google Scholar]
- 90.Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole-Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin-(1-7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens 18: 1841–1848, 2000. doi: 10.1097/00004872-200018120-00019. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Zhao Y, Chen S, Wang J, Xiao X, Ma X, Penchikala M, Xia H, Lazartigues E, Zhao B, Chen Y. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology 79: 550–558, 2014. doi: 10.1016/j.neuropharm.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Q, Yang Y, Huang Y, Pan C, Liu L, Qiu H. Angiotensin-(1-7) attenuates lung fibrosis by way of Mas receptor in acute lung injury. J Surg Res 185: 740–747, 2013. doi: 10.1016/j.jss.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 93.Chen YP, Lu YP, Li J, Liu ZW, Chen WJ, Liang XJ, Chen X, Wen WR, Xiao XM, Reichetzeder C, Hocher B. Fetal and maternal angiotensin (1-7) are associated with preterm birth. J Hypertens 32: 1833–1841, 2014. doi: 10.1097/HJH.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 94.Cicilini MA, Ramos PS, Vasquez EC, Cabral AM. Heart prolyl endopeptidase activity in one-kidney, one clip hypertensive rats. Braz J Med Biol Res 27: 2821–2830, 1994. [PubMed] [Google Scholar]
- 95.Cisternas F, Morales MG, Meneses C, Simon F, Brandan E, Abrigo J, Vazquez Y, Cabello-Verrugio C. Angiotensin-(1-7) decreases skeletal muscle atrophy induced by angiotensin II through a Mas receptor-dependent mechanism. Clin Sci (Lond) 128: 307–319, 2015. doi: 10.1042/CS20140215. [DOI] [PubMed] [Google Scholar]
- 96.Coelho MS, Lopes KL, Freitas RA, de Oliveira-Sales EB, Bergasmaschi CT, Campos RR, Casarini DE, Carmona AK, Araújo MS, Heimann JC, Dolnikoff MS. High sucrose intake in rats is associated with increased ACE2 and angiotensin-(1-7) levels in the adipose tissue. Regul Pept 162: 61–67, 2010. doi: 10.1016/j.regpep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 97.Coirault C, Langeron O, Lambert F, Blanc FX, Lerebours G, Claude N, Riou B, Chemla D, Lecarpentier Y. Impaired skeletal muscle performance in the early stage of cardiac pressure overload in rabbits: beneficial effects of angiotensin-converting enzyme inhibition. J Pharmacol Exp Ther 291: 70–75, 1999. [PubMed] [Google Scholar]
- 98.Cook KL, Metheny-Barlow LJ, Tallant EA, Gallagher PE. Angiotensin-(1-7) reduces fibrosis in orthotopic breast tumors. Cancer Res 70: 8319–8328, 2010. doi: 10.1158/0008-5472.CAN-10-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coomans de Brachène A, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci 73: 1159–1172, 2016. doi: 10.1007/s00018-015-2112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Costa AP, Fagundes-Moura CR, Pereira VM, Silva LF, Vieira MA, Santos RA, Dos Reis AM. Angiotensin-(1-7): a novel peptide in the ovary. Endocrinology 144: 1942–1948, 2003. doi: 10.1210/en.2002-220787. [DOI] [PubMed] [Google Scholar]
- 101.Costa MA, Lopez Verrilli MA, Gomez KA, Nakagawa P, Peña C, Arranz C, Gironacci MM. Angiotensin-(1-7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 299: H1205–H1211, 2010. doi: 10.1152/ajpheart.00850.2009. [DOI] [PubMed] [Google Scholar]
- 102.Coutinho DC, Monnerat-Cahli G, Ferreira AJ, Medei E. Activation of angiotensin-converting enzyme 2 improves cardiac electrical changes in ventricular repolarization in streptozotocin-induced hyperglycaemic rats. Europace 16: 1689–1696, 2014. doi: 10.1093/europace/euu070. [DOI] [PubMed] [Google Scholar]
- 103.Couto AS, Baltatu O, Santos RA, Ganten D, Bader M, Campagnole-Santos MJ. Differential effects of angiotensin II and angiotensin-(1-7) at the nucleus tractus solitarii of transgenic rats with low brain angiotensinogen. J Hypertens 20: 919–925, 2002. doi: 10.1097/00004872-200205000-00027. [DOI] [PubMed] [Google Scholar]
- 104.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 105.Cunha TM, Lima WG, Silva ME, Souza Santos RA, Campagnole-Santos MJ, Alzamora AC. The nonpeptide ANG-(1-7) mimic AVE 0991 attenuates cardiac remodeling and improves baroreflex sensitivity in renovascular hypertensive rats. Life Sci 92: 266–275, 2013. doi: 10.1016/j.lfs.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Da Costa Gonçalves AC, Fraga-Silva RA, Leite R, Santos RA. AVE 0991, a non-peptide Mas-receptor agonist, facilitates penile erection. Exp Physiol 98: 850–855, 2013. doi: 10.1113/expphysiol.2012.068551. [DOI] [PubMed] [Google Scholar]
- 107.Da Costa Gonçalves AC, Leite R, Fraga-Silva RA, Pinheiro SV, Reis AB, Reis FM, Touyz RM, Webb RC, Alenina N, Bader M, Santos RA. Evidence that the vasodilator angiotensin-(1-7)-Mas axis plays an important role in erectile function. Am J Physiol Heart Circ Physiol 293: H2588–H2596, 2007. doi: 10.1152/ajpheart.00173.2007. [DOI] [PubMed] [Google Scholar]
- 108.Da Silva AQ, Fontes MA, Kanagy NL. Chronic infusion of angiotensin receptor antagonists in the hypothalamic paraventricular nucleus prevents hypertension in a rat model of sleep apnea. Brain Res 1368: 231–238, 2011. doi: 10.1016/j.brainres.2010.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, Bretas TL, Bader M, de Sousa LP, da Silva TA, dos Santos RA, Simões e Silva AC, Teixeira MM. Anti-inflammatory effects of the activation of the angiotensin-(1-7) receptor, MAS, in experimental models of arthritis. J Immunol 185: 5569–5576, 2010. doi: 10.4049/jimmunol.1000314. [DOI] [PubMed] [Google Scholar]
- 110.Dai H, Gong Y, Xiao Z, Guang X, Yin X. Decreased levels of serum Angiotensin-(1-7) in patients with pulmonary arterial hypertension due to congenital heart disease. Int J Cardiol 176: 1399–1401, 2014. doi: 10.1016/j.ijcard.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 111.Dalla Libera L, Ravara B, Angelini A, Rossini K, Sandri M, Thiene G, Battista Ambrosio G, Vescovo G. Beneficial effects on skeletal muscle of the angiotensin II type 1 receptor blocker irbesartan in experimental heart failure. Circulation 103: 2195–2200, 2001. doi: 10.1161/01.CIR.103.17.2195. [DOI] [PubMed] [Google Scholar]
- 112.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. [DOI] [PubMed] [Google Scholar]
- 113.Daugherty A, Rateri DL, Howatt DA, Charnigo R, Cassis LA. PD123319 augments angiotensin II-induced abdominal aortic aneurysms through an AT2 receptor-independent mechanism. PLoS One 8: e61849, 2013. doi: 10.1371/journal.pone.0061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davie AP, McMurray JJ. Effect of angiotensin-(1-7) and bradykinin in patients with heart failure treated with an ACE inhibitor. Hypertension 34: 457–460, 1999. doi: 10.1161/01.HYP.34.3.457. [DOI] [PubMed] [Google Scholar]
- 115.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics 1: 3–9, 1999. [DOI] [PubMed] [Google Scholar]
- 116.Davisson RL. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 285: R498–R511, 2003. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- 117.De Almeida PW, de Freitas Lima R, de Morais Gomes ER, Rocha-Resende C, Roman-Campos D, Gondim AN, Gavioli M, Lara A, Parreira A, de Azevedo Nunes SL, Alves MN, Santos SL, Alenina N, Bader M, Resende RR, dos Santos Cruz J, Souza dos Santos RA, Guatimosim S. Functional cross-talk between aldosterone and angiotensin-(1-7) in ventricular myocytes. Hypertension 61: 425–430, 2013. doi: 10.1161/HYPERTENSIONAHA.111.199539. [DOI] [PubMed] [Google Scholar]
- 118.De Macedo SM, Guimarares TA, Andrade JM, Guimaraes AL, Batista de Paula AM, Ferreira AJ, Sousa Santos SH. Angiotensin converting enzyme 2 activator (DIZE) modulates metabolic profiles in mice, decreasing lipogenesis. Protein Pept Lett 22: 332–340, 2015. doi: 10.2174/0929866522666150209125401. [DOI] [PubMed] [Google Scholar]
- 119.De Maria ML, Araújo LD, Fraga-Silva RA, Pereira LA, Ribeiro HJ, Menezes GB, Shenoy V, Raizada MK, Ferreira AJ. Anti-hypertensive Effects of Diminazene Aceturate: An Angiotensin- Converting Enzyme 2 Activator in Rats. Protein Pept Lett 23: 9–16, 2016. doi: 10.2174/0929866522666151013130550. [DOI] [PubMed] [Google Scholar]
- 120.De Mello WC. Angiotensin (1-7) re-establishes heart cell communication previously impaired by cell swelling: implications for myocardial ischemia. Exp Cell Res 323: 359–365, 2014. doi: 10.1016/j.yexcr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 121.De Mello WC. Angiotensin (1-7) re-establishes impulse conduction in cardiac muscle during ischaemia-reperfusion. The role of the sodium pump. J Renin Angiotensin Aldosterone Syst 5: 203–208, 2004. doi: 10.3317/jraas.2004.041. [DOI] [PubMed] [Google Scholar]
- 122.De Mello WC. Cell swelling, impulse conduction, and cardiac arrhythmias in the failing heart. Opposite effects of angiotensin II and angiotensin (1-7) on cell volume regulation. Mol Cell Biochem 330: 211–217, 2009. doi: 10.1007/s11010-009-0135-0. [DOI] [PubMed] [Google Scholar]
- 123.De Mello WC. Intracellular angiotensin (1-7) increases the inward calcium current in cardiomyocytes. On the role of PKA activation. Mol Cell Biochem 407: 9–16, 2015. doi: 10.1007/s11010-015-2449-4. [DOI] [PubMed] [Google Scholar]
- 124.De Mello WC, Ferrario CM, Jessup JA. Beneficial versus harmful effects of Angiotensin (1-7) on impulse propagation and cardiac arrhythmias in the failing heart. J Renin Angiotensin Aldosterone Syst 8: 74–80, 2007. doi: 10.3317/jraas.2007.015. [DOI] [PubMed] [Google Scholar]
- 125.De Moura MM, dos Santos RA, Campagnole-Santos MJ, Todiras M, Bader M, Alenina N, Haibara AS. Altered cardiovascular reflexes responses in conscious Angiotensin-(1-7) receptor Mas-knockout mice. Peptides 31: 1934–1939, 2010. doi: 10.1016/j.peptides.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 126.De Resende MM, Stodola TJ, Greene AS. Role of the renin angiotensin system on bone marrow-derived stem cell function and its impact on skeletal muscle angiogenesis. Physiol Genomics 42: 437–444, 2010. doi: 10.1152/physiolgenomics.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Delafontaine P, Akao M. Angiotensin II as candidate of cardiac cachexia. Curr Opin Clin Nutr Metab Care 9: 220–224, 2006. doi: 10.1097/01.mco.0000222103.29009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DelliPizzi AM, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin(1-7). Br J Pharmacol 111: 1–3, 1994. doi: 10.1111/j.1476-5381.1994.tb14014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1-7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur J Pharmacol 638: 108–114, 2010. doi: 10.1016/j.ejphar.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dias-Peixoto MF, Santos RA, Gomes ER, Alves MN, Almeida PW, Greco L, Rosa M, Fauler B, Bader M, Alenina N, Guatimosim S. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension 52: 542–548, 2008. doi: 10.1161/HYPERTENSIONAHA.108.114280. [DOI] [PubMed] [Google Scholar]
- 131.Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. Sci World J 9: 522–535, 2009. doi: 10.1100/tsw.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dilauro M, Zimpelmann J, Robertson SJ, Genest D, Burns KD. Effect of ACE2 and angiotensin-(1-7) in a mouse model of early chronic kidney disease. Am J Physiol Renal Physiol 298: F1523–F1532, 2010. doi: 10.1152/ajprenal.00426.2009. [DOI] [PubMed] [Google Scholar]
- 133.Diz DI, Arnold AC, Nautiyal M, Isa K, Shaltout HA, Tallant EA. Angiotensin peptides and central autonomic regulation. Curr Opin Pharmacol 11: 131–137, 2011. doi: 10.1016/j.coph.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Diz DI, Falgui B, Bosch SM, Westwood BM, Kent J, Ganten D, Ferrario CM. Hypothalamic substance P release. Attenuated angiotensin responses in mRen2(27) transgenic rats. Hypertension 29: 510–513, 1997. doi: 10.1161/01.HYP.29.1.510. [DOI] [PubMed] [Google Scholar]
- 135.Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1-7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol 51: 542–548, 2008. doi: 10.1097/FJC.0b013e3181734a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann Tallant E, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol 93: 694–700, 2008. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Diz DI, Pirro NT. Differential actions of angiotensin II and angiotensin-(1-7) on transmitter release. Hypertension 19, Suppl: II41–II48, 1992. doi: 10.1161/01.HYP.19.2_Suppl.II41. [DOI] [PubMed] [Google Scholar]
- 138.Dobruch J, Paczwa P, Łoń S, Khosla MC, Szczepańska-Sadowska E. Hypotensive function of the brain angiotensin-(1-7) in Sprague Dawley and renin transgenic rats. J Physiol Pharmacol 54: 371–381, 2003. [PubMed] [Google Scholar]
- 139.Dominici FP, Burghi V, Muñoz MC, Giani JF. Modulation of the action of insulin by angiotensin-(1-7). Clin Sci (Lond) 126: 613–630, 2014. doi: 10.1042/CS20130333. [DOI] [PubMed] [Google Scholar]
- 140.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87: e1–e9, 2000. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 141.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol 35: 1043–1053, 2003. doi: 10.1016/S0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 142.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 292: R373–R381, 2007. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Du D, Chen J, Liu M, Zhu M, Jing H, Fang J, Shen L, Zhu D, Yu J, Wang J. The effects of angiotensin II and angiotensin-(1-7) in the rostral ventrolateral medulla of rats on stress-induced hypertension. PLoS One 8: e70976, 2013. doi: 10.1371/journal.pone.0070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du Bois P, Pablo Tortola C, Lodka D, Kny M, Schmidt F, Song K, Schmidt S, Bassel-Duby R, Olson EN, Fielitz J. Angiotensin II Induces Skeletal Muscle Atrophy by Activating TFEB-Mediated MuRF1 Expression. Circ Res 117: 424–436, 2015. doi: 10.1161/CIRCRESAHA.114.305393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Duncan AM, Burrell LM, Kladis A, Campbell DJ. Angiotensin and bradykinin peptides in rats with myocardial infarction. J Card Fail 3: 41–52, 1997. doi: 10.1016/S1071-9164(97)90007-5. [DOI] [PubMed] [Google Scholar]
- 146.Durik M, van Veghel R, Kuipers A, Rink R, Haas Jimoh Akanbi M, Moll G, Danser AH, Roks AJ. The effect of the thioether-bridged, stabilized Angiotensin-(1-7) analogue cyclic ang-(1-7) on cardiac remodeling and endothelial function in rats with myocardial infarction. Int J Hypertens 2012: 536426, 2012. doi: 10.1155/2012/536426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ebermann L, Spillmann F, Sidiropoulos M, Escher F, Heringer-Walther S, Schultheiss HP, Tschöpe C, Walther T. The angiotensin-(1-7) receptor agonist AVE0991 is cardioprotective in diabetic rats. Eur J Pharmacol 590: 276–280, 2008. doi: 10.1016/j.ejphar.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 148.El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF. Angiotensin-(1-7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-κB-dependent pathways. Br J Pharmacol 166: 1964–1976, 2012. doi: 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Esteban V, Heringer-Walther S, Sterner-Kock A, de Bruin R, van den Engel S, Wang Y, Mezzano S, Egido J, Schultheiss HP, Ruiz-Ortega M, Walther T. Angiotensin-(1-7) and the g protein-coupled receptor MAS are key players in renal inflammation. PLoS One 4: e5406, 2009. doi: 10.1371/journal.pone.0005406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, Nieman MT, Adams GN, LaRusch G, Zhou Y, Bilodeau ML, Mahdi F, Warnock M, Schmaier AH. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood 121: 3023–3032, 2013. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Faria-Silva R, Duarte FV, Santos RA. Short-term angiotensin(1-7) receptor MAS stimulation improves endothelial function in normotensive rats. Hypertension 46: 948–952, 2005. doi: 10.1161/01.HYP.0000174594.17052.33. [DOI] [PubMed] [Google Scholar]
- 152.Felix D, Khosla MC, Barnes KL, Imboden H, Montani B, Ferrario CM. Neurophysiological responses to angiotensin-(1-7). Hypertension 17: 1111–1114, 1991. doi: 10.1161/01.HYP.17.6.1111. [DOI] [PubMed] [Google Scholar]
- 153.Feltenberger JD, Andrade JM, Paraíso A, Barros LO, Filho AB, Sinisterra RD, Sousa FB, Guimarães AL, de Paula AM, Campagnole-Santos MJ, Qureshi M, dos Santos RA, Santos SH. Oral formulation of angiotensin-(1-7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension 62: 324–330, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00919. [DOI] [PubMed] [Google Scholar]
- 154.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res 106: 373–382, 2010. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fernandes L, Fortes Z, Nigro D, Scivoletto R, Santos R, Carvalho M. Interaction of angiotensin-(1–7) and bradykinin in mesenteric microvessels of SHR, studied in vivo-in situ. Hypertension 37: 1007, 2001. [Google Scholar]
- 156.Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Catelli De Carvalho MH. Potentiation of bradykinin by angiotensin-(1-7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension 37: 703–709, 2001. doi: 10.1161/01.HYP.37.2.703. [DOI] [PubMed] [Google Scholar]
- 157.Fernandes T, Hashimoto NY, Oliveira EM. Characterization of angiotensin-converting enzymes 1 and 2 in the soleus and plantaris muscles of rats. Braz J Med Biol Res 43: 837–842, 2010. doi: 10.1590/S0100-879X2010007500088. [DOI] [PubMed] [Google Scholar]
- 158.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, Dell’italia LJ. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond) 126: 461–469, 2014. doi: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A, Tallant EA. Angiotensin-(1-7): a new hormone of the angiotensin system. Hypertension 18, Suppl: III126–III133, 1991. doi: 10.1161/01.HYP.18.5_Suppl.III126. [DOI] [PubMed] [Google Scholar]
- 160.Ferrario CM, Santos RA, Brosnihan KB, Block CH, Schiavone MT, Khosla MC, Greene LJ. A hypothesis regarding the function of angiotensin peptides in the brain. Clin Exp Hypertens A 10, Suppl 1: 107–121, 1988. [DOI] [PubMed] [Google Scholar]
- 161.Ferreira AJ, Castro CH, Guatimosim S, Almeida PW, Gomes ER, Dias-Peixoto MF, Alves MN, Fagundes-Moura CR, Rentzsch B, Gava E, Almeida AP, Guimarães AM, Kitten GT, Reudelhuber T, Bader M, Santos RA. Attenuation of isoproterenol-induced cardiac fibrosis in transgenic rats harboring an angiotensin-(1-7)-producing fusion protein in the heart. Ther Adv Cardiovasc Dis 4: 83–96, 2010. doi: 10.1177/1753944709353426. [DOI] [PubMed] [Google Scholar]
- 162.Ferreira AJ, Hernández Prada JA, Ostrov DA, Raizada MK. Cardiovascular protection by angiotensin-converting enzyme 2: a new paradigm. Future Cardiol 4: 175–182, 2008. doi: 10.2217/14796678.4.2.175. [DOI] [PubMed] [Google Scholar]
- 163.Ferreira AJ, Jacoby BA, Araújo CA, Macedo FA, Silva GA, Almeida AP, Caliari MV, Santos RA. The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol 292: H1113–H1119, 2007. doi: 10.1152/ajpheart.00828.2006. [DOI] [PubMed] [Google Scholar]
- 164.Ferreira AJ, Moraes PL, Foureaux G, Andrade AB, Santos RA, Almeida AP. The angiotensin-(1-7)/Mas receptor axis is expressed in sinoatrial node cells of rats. J Histochem Cytochem 59: 761–768, 2011. doi: 10.1369/0022155411411712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ferreira AJ, Oliveira TL, Castro MC, Almeida AP, Castro CH, Caliari MV, Gava E, Kitten GT, Santos RA. Isoproterenol-induced impairment of heart function and remodeling are attenuated by the nonpeptide angiotensin-(1-7) analogue AVE 0991. Life Sci 81: 916–923, 2007. doi: 10.1016/j.lfs.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 166.Ferreira AJ, Pinheiro SV, Castro CH, Silva GA, Silva AC, Almeida AP, Bader M, Rentzsch B, Reudelhuber TL, Santos RA. Renal function in transgenic rats expressing an angiotensin-(1-7)-producing fusion protein. Regul Pept 137: 128–133, 2006. doi: 10.1016/j.regpep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 167.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7) improves the post-ischemic function in isolated perfused rat hearts. Braz J Med Biol Res 35: 1083–1090, 2002. doi: 10.1590/S0100-879X2002000900009. [DOI] [PubMed] [Google Scholar]
- 168.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension 38: 665–668, 2001. doi: 10.1161/01.HYP.38.3.665. [DOI] [PubMed] [Google Scholar]
- 169.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 179: 1048–1054, 2009. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferreira PM, Alzamora AC, Santos RA, Campagnole-Santos MJ. Hemodynamic effect produced by microinjection of angiotensins at the caudal ventrolateral medulla of spontaneously hypertensive rats. Neuroscience 151: 1208–1216, 2008. doi: 10.1016/j.neuroscience.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 171.Ferreira PM, Souza Dos Santos RA, Campagnole-Santos MJ. Angiotensin-(3-7) pressor effect at the rostral ventrolateral medulla. Regul Pept 141: 168–174, 2007. doi: 10.1016/j.regpep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 172.Ferreira PM, Xavier CH, Alzamora AC, Santos RA, Campagnole-Santos MJ. Differential control of vasomotion by angiotensins in the rostral ventrolateral medulla of hypertensive rats. Neuropeptides 53: 11–18, 2015. doi: 10.1016/j.npep.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 173.Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66: 413–434, 2014. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Feterik K, Smith L, Katusic ZS. Angiotensin-(1-7) causes endothelium-dependent relaxation in canine middle cerebral artery. Brain Res 873: 75–82, 2000. doi: 10.1016/S0006-8993(00)02482-3. [DOI] [PubMed] [Google Scholar]
- 175.Filho AG, Ferreira AJ, Santos SH, Neves SR, Silva Camargos ER, Becker LK, Belchior HA, Dias-Peixoto MF, Pinheiro SV, Santos RA. Selective increase of angiotensin(1-7) and its receptor in hearts of spontaneously hypertensive rats subjected to physical training. Exp Physiol 93: 589–598, 2008. [DOI] [PubMed] [Google Scholar]
- 176.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: e68–e75, 2001. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 177.Flores-Muñoz M, Godinho BM, Almalik A, Nicklin SA. Adenoviral delivery of angiotensin-(1-7) or angiotensin-(1-9) inhibits cardiomyocyte hypertrophy via the mas or angiotensin type 2 receptor. PLoS One 7: e45564, 2012. doi: 10.1371/journal.pone.0045564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Flores-Muñoz M, Smith NJ, Haggerty C, Milligan G, Nicklin SA. Angiotensin1-9 antagonises pro-hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J Physiol 589: 939–951, 2011. doi: 10.1113/jphysiol.2010.203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Folland J, Leach B, Little T, Hawker K, Myerson S, Montgomery H, Jones D. Angiotensin-converting enzyme genotype affects the response of human skeletal muscle to functional overload. Exp Physiol 85: 575–579, 2000. doi: 10.1111/j.1469-445X.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- 180.Fontes MA, Baltatu O, Caligiorne SM, Campagnole-Santos MJ, Ganten D, Bader M, Santos RA. Angiotensin peptides acting at rostral ventrolateral medulla contribute to hypertension of TGR(mREN2)27 rats. Physiol Genomics 2: 137–142, 2000. [DOI] [PubMed] [Google Scholar]
- 181.Fontes MA, Martins Lima A, Santos RA. Brain angiotensin-(1-7)/Mas axis: a new target to reduce the cardiovascular risk to emotional stress. Neuropeptides 56: 9–17, 2016. doi: 10.1016/j.npep.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 182.Fontes MA, Martins Pinge MC, Naves V, Campagnole-Santos MJ, Lopes OU, Khosla MC, Santos RA. Cardiovascular effects produced by microinjection of angiotensins and angiotensin antagonists into the ventrolateral medulla of freely moving rats. Brain Res 750: 305–310, 1997. doi: 10.1016/S0006-8993(96)01476-X. [DOI] [PubMed] [Google Scholar]
- 183.Fontes MA, Silva LC, Campagnole-Santos MJ, Khosla MC, Guertzenstein PG, Santos RA. Evidence that angiotensin-(1-7) plays a role in the central control of blood pressure at the ventro-lateral medulla acting through specific receptors. Brain Res 665: 175–180, 1994. doi: 10.1016/0006-8993(94)91171-1. [DOI] [PubMed] [Google Scholar]
- 184.Foureaux G, Nogueira JC, Nogueira BS, Fulgêncio GO, Menezes GB, Fernandes SO, Cardoso VN, Fernandes RS, Oliveira GP, Franca JR, Faraco AA, Raizada MK, Ferreira AJ. Antiglaucomatous effects of the activation of intrinsic Angiotensin-converting enzyme 2. Invest Ophthalmol Vis Sci 54: 4296–4306, 2013. doi: 10.1167/iovs.12-11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RA. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (Sao Paulo) 66: 837–841, 2011. doi: 10.1590/S1807-59322011000500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fraga-Silva RA, Costa-Fraga FP, Montecucco F, Sturny M, Faye Y, Mach F, Pelli G, Shenoy V, da Silva RF, Raizada MK, Santos RA, Stergiopulos N. Diminazene protects corpus cavernosum against hypercholesterolemia-induced injury. J Sex Med 12: 289–302, 2015. doi: 10.1111/jsm.12757. [DOI] [PubMed] [Google Scholar]
- 187.Fraga-Silva RA, Costa-Fraga FP, Savergnini SQ, De Sousa FB, Montecucco F, da Silva D, Sinisterra RD, Mach F, Stergiopulos N, da Silva RF, Santos RA. An oral formulation of angiotensin-(1-7) reverses corpus cavernosum damages induced by hypercholesterolemia. J Sex Med 10: 2430–2442, 2013. doi: 10.1111/jsm.12262. [DOI] [PubMed] [Google Scholar]
- 188.Fraga-Silva RA, Da Silva DG, Montecucco F, Mach F, Stergiopulos N, da Silva RF, Santos RA. The angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas receptor axis: a potential target for treating thrombotic diseases. Thromb Haemost 108: 1089–1096, 2012. doi: 10.1160/TH12-06-0396. [DOI] [PubMed] [Google Scholar]
- 189.Fraga-Silva RA, Pinheiro SV, Gonçalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med 14: 28–35, 2008. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fraga-Silva RA, Sorg BS, Wankhede M, Dedeugd C, Jun JY, Baker MB, Li Y, Castellano RK, Katovich MJ, Raizada MK, Ferreira AJ. ACE2 activation promotes antithrombotic activity. Mol Med 16: 210–215, 2010. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Freeman EJ, Chisolm GM, Ferrario CM, Tallant EA. Angiotensin-(1-7) inhibits vascular smooth muscle cell growth. Hypertension 28: 104–108, 1996. doi: 10.1161/01.HYP.28.1.104. [DOI] [PubMed] [Google Scholar]
- 192.Freund M, Walther T, von Bohlen und Halbach O. Immunohistochemical localization of the angiotensin-(1-7) receptor Mas in the murine forebrain. Cell Tissue Res 348: 29–35, 2012. doi: 10.1007/s00441-012-1354-3. [DOI] [PubMed] [Google Scholar]
- 193.Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 295: H2373–H2379, 2008. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gallagher PE, Tallant EA. Inhibition of human lung cancer cell growth by angiotensin-(1-7). Carcinogenesis 25: 2045–2052, 2004. doi: 10.1093/carcin/bgh236. [DOI] [PubMed] [Google Scholar]
- 195.Gao J, Marc Y, Iturrioz X, Leroux V, Balavoine F, Llorens-Cortes C. A new strategy for treating hypertension by blocking the activity of the brain renin-angiotensin system with aminopeptidase A inhibitors. Clin Sci (Lond) 127: 135–148, 2014. doi: 10.1042/CS20130396. [DOI] [PubMed] [Google Scholar]
- 196.Garcia NH, Garvin JL. Angiotensin 1-7 has a biphasic effect on fluid absorption in the proximal straight tubule. J Am Soc Nephrol 5: 1133–1138, 1994. [DOI] [PubMed] [Google Scholar]
- 197.Garcia-Espinosa MA, Shaltout HA, Gallagher PE, Chappell MC, Diz DI. In vivo expression of angiotensin-(1-7) lowers blood pressure and improves baroreflex function in transgenic (mRen2)27 rats. J Cardiovasc Pharmacol 60: 150–157, 2012. doi: 10.1097/FJC.0b013e3182588b32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gava E, de Castro CH, Ferreira AJ, Colleta H, Melo MB, Alenina N, Bader M, Oliveira LA, Santos RA, Kitten GT. Angiotensin-(1-7) receptor Mas is an essential modulator of extracellular matrix protein expression in the heart. Regul Pept 175: 30–42, 2012. doi: 10.1016/j.regpep.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 199.Gava E, Samad-Zadeh A, Zimpelmann J, Bahramifarid N, Kitten GT, Santos RA, Touyz RM, Burns KD. Angiotensin-(1-7) activates a tyrosine phosphatase and inhibits glucose-induced signalling in proximal tubular cells. Nephrol Dial Transplant 24: 1766–1773, 2009. doi: 10.1093/ndt/gfn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Effect of captopril on skeletal muscle angiogenic growth factor responses to exercise. J Appl Physiol (1985) 88: 1690–1697, 2000. [DOI] [PubMed] [Google Scholar]
- 201.Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T. Angiotensin metabolites can stimulate receptors of the Mas-related genes family. Mol Cell Biochem 319: 115–123, 2008. doi: 10.1007/s11010-008-9884-4. [DOI] [PubMed] [Google Scholar]
- 202.Giani JF, Burghi V, Veiras LC, Tomat A, Muñoz MC, Cao G, Turyn D, Toblli JE, Dominici FP. Angiotensin-(1-7) attenuates diabetic nephropathy in Zucker diabetic fatty rats. Am J Physiol Renal Physiol 302: F1606–F1615, 2012. doi: 10.1152/ajprenal.00063.2012. [DOI] [PubMed] [Google Scholar]
- 203.Giani JF, Gironacci MM, Muñoz MC, Peña C, Turyn D, Dominici FP. Angiotensin-(1 7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol 293: H1154–H1163, 2007. doi: 10.1152/ajpheart.01395.2006. [DOI] [PubMed] [Google Scholar]
- 204.Giani JF, Gironacci MM, Muñoz MC, Turyn D, Dominici FP. Angiotensin-(1-7) has a dual role on growth-promoting signalling pathways in rat heart in vivo by stimulating STAT3 and STAT5a/b phosphorylation and inhibiting angiotensin II-stimulated ERK1/2 and Rho kinase activity. Exp Physiol 93: 570–578, 2008. doi: 10.1113/expphysiol.2007.014269. [DOI] [PubMed] [Google Scholar]
- 205.Giani JF, Mayer MA, Muñoz MC, Silberman EA, Höcht C, Taira CA, Gironacci MM, Turyn D, Dominici FP. Chronic infusion of angiotensin-(1-7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am J Physiol Endocrinol Metab 296: E262–E271, 2009. doi: 10.1152/ajpendo.90678.2008. [DOI] [PubMed] [Google Scholar]
- 206.Giani JF, Muñoz MC, Mayer MA, Veiras LC, Arranz C, Taira CA, Turyn D, Toblli JE, Dominici FP. Angiotensin-(1-7) improves cardiac remodeling and inhibits growth-promoting pathways in the heart of fructose-fed rats. Am J Physiol Heart Circ Physiol 298: H1003–H1013, 2010. doi: 10.1152/ajpheart.00803.2009. [DOI] [PubMed] [Google Scholar]
- 207.Giani JF, Muñoz MC, Pons RA, Cao G, Toblli JE, Turyn D, Dominici FP. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol 300: F272–F282, 2011. doi: 10.1152/ajprenal.00278.2010. [DOI] [PubMed] [Google Scholar]
- 208.Gironacci MM, Adler-Graschinsky E, Peña C, Enero MA. Effects of angiotensin II and angiotensin-(1-7) on the release of [3H]norepinephrine from rat atria. Hypertension 24: 457–460, 1994. doi: 10.1161/01.HYP.24.4.457. [DOI] [PubMed] [Google Scholar]
- 209.Gironacci MM, Brosnihan KB, Ferrario CM, Gorzalczany S, Verrilli MA, Pascual M, Taira C, Peña C. Increased hypothalamic angiotensin-(1-7) levels in rats with aortic coarctation-induced hypertension. Peptides 28: 1580–1585, 2007. doi: 10.1016/j.peptides.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gironacci MM, Cerniello FM, Longo Carbajosa NA, Goldstein J, Cerrato BD. Protective axis of the renin-angiotensin system in the brain. Clin Sci (Lond) 127: 295–306, 2014. doi: 10.1042/CS20130450. [DOI] [PubMed] [Google Scholar]
- 211.Gironacci MM, Longo Carbajosa NA, Goldstein J, Cerrato BD. Neuromodulatory role of angiotensin-(1-7) in the central nervous system. Clin Sci (Lond) 125: 57–65, 2013. doi: 10.1042/CS20120652. [DOI] [PubMed] [Google Scholar]
- 212.Gironacci MM, Rodríguez-Fermepín M, Vatta M, Fernandez BE, Rubio M, Peña C. Angiotensin-(1-7) does not affect norepinephrine neuronal uptake or catabolism in rat hypothalamus and atria. Cell Mol Neurobiol 20: 773–779, 2000. doi: 10.1023/A:1007063111479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gironacci MM, Valera MS, Yujnovsky I, Peña C. Angiotensin-(1-7) inhibitory mechanism of norepinephrine release in hypertensive rats. Hypertension 44: 783–787, 2004. doi: 10.1161/01.HYP.0000143850.73831.9d. [DOI] [PubMed] [Google Scholar]
- 214.Gironacci MM, Vatta M, Rodriguez-Fermepín M, Fernández BE, Peña C. Angiotensin-(1-7) reduces norepinephrine release through a nitric oxide mechanism in rat hypothalamus. Hypertension 35: 1248–1252, 2000. doi: 10.1161/01.HYP.35.6.1248. [DOI] [PubMed] [Google Scholar]
- 215.Gironacci MM, Yujnovsky I, Gorzalczany S, Taira C, Peña C. Angiotensin-(1-7) inhibits the angiotensin II-enhanced norepinephrine release in coarcted hypertensive rats. Regul Pept 118: 45–49, 2004. doi: 10.1016/j.regpep.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 216.Gomes ER, Lara AA, Almeida PW, Guimarães D, Resende RR, Campagnole-Santos MJ, Bader M, Santos RA, Guatimosim S. Angiotensin-(1-7) prevents cardiomyocyte pathological remodeling through a nitric oxide/guanosine 3′,5′-cyclic monophosphate-dependent pathway. Hypertension 55: 153–160, 2010. doi: 10.1161/HYPERTENSIONAHA.109.143255. [DOI] [PubMed] [Google Scholar]
- 217.Gomes ER, Santos RA, Guatimosim S. Angiotensin-(1-7)-mediated signaling in cardiomyocytes. Int J Hypertens 2012: 493129, 2012. doi: 10.1155/2012/493129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gonzales S, Noriega GO, Tomaro ML, Peña C. Angiotensin-(1-7) stimulates oxidative stress in rat kidney. Regul Pept 106: 67–70, 2002. doi: 10.1016/S0167-0115(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 219.Gopallawa I, Uhal BD. Angiotensin-(1-7)/mas inhibits apoptosis in alveolar epithelial cells through upregulation of MAP kinase phosphatase-2. Am J Physiol Lung Cell Mol Physiol 310: L240–L248, 2016. doi: 10.1152/ajplung.00187.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gorelik G, Carbini LA, Scicli AG. Angiotensin 1-7 induces bradykinin-mediated relaxation in porcine coronary artery. J Pharmacol Exp Ther 286: 403–410, 1998. [PubMed] [Google Scholar]
- 221.Greene LJ, Spadaro AC, Martins AR, Perussi De Jesus WD, Camargo AC. Brain endo-oligopeptidase B: a post-proline cleaving enzyme that inactivates angiotensin I and II. Hypertension 4: 178–184, 1982. doi: 10.1161/01.HYP.4.2.178. [DOI] [PubMed] [Google Scholar]
- 222.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7). Am J Physiol Heart Circ Physiol 292: H736–H742, 2007. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 223.Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol 290: H2417–H2423, 2006. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- 224.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 23: 187–193, 2008. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Guimaraes PS, Oliveira MF, Braga JF, Nadu AP, Schreihofer A, Santos RA, Campagnole-Santos MJ. Increasing angiotensin-(1-7) levels in the brain attenuates metabolic syndrome-related risks in fructose-fed rats. Hypertension 63: 1078–1085, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01847. [DOI] [PubMed] [Google Scholar]
- 226.Guimaraes PS, Santiago NM, Xavier CH, Velloso EP, Fontes MA, Santos RA, Campagnole-Santos MJ. Chronic infusion of angiotensin-(1-7) into the lateral ventricle of the brain attenuates hypertension in DOCA-salt rats. Am J Physiol Heart Circ Physiol 303: H393–H400, 2012. doi: 10.1152/ajpheart.00075.2012. [DOI] [PubMed] [Google Scholar]
- 227.Guimarães GG, Santos SH, Oliveira ML, Pimenta-Velloso EP, Motta DF, Martins AS, Alenina N, Bader M, Santos RA, Campagnole-Santos MJ. Exercise induces renin-angiotensin system unbalance and high collagen expression in the heart of Mas-deficient mice. Peptides 38: 54–61, 2012. doi: 10.1016/j.peptides.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 228.Guo F, Liu B, Tang F, Lane S, Souslova EA, Chudakov DM, Paton JF, Kasparov S. Astroglia are a possible cellular substrate of angiotensin(1-7) effects in the rostral ventrolateral medulla. Cardiovasc Res 87: 578–584, 2010. doi: 10.1093/cvr/cvq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guo L, Yin A, Zhang Q, Zhong T, O’Rourke ST, Sun C. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac hypertrophy via a Sirt3-dependent mechanism. Am J Physiol Heart Circ Physiol 312: H980–H991, 2017. doi: 10.1152/ajpheart.00768.2016. [DOI] [PubMed] [Google Scholar]
- 230.Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, Karounos M, Cassis LA. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 32: 1392–1399, 2012. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 116: 2218–2225, 2006. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gurley SB, Coffman TM. Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp Physiol 93: 538–542, 2008. doi: 10.1113/expphysiol.2007.040014. [DOI] [PubMed] [Google Scholar]
- 233.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R518–R530, 2012. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Haber PK, Ye M, Wysocki J, Maier C, Haque SK, Batlle D. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: studies in vivo, ex vivo, and in vitro. Hypertension 63: 774–782, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Habiyakare B, Alsaadon H, Mathai ML, Hayes A, Zulli A. Reduction of angiotensin A and alamandine vasoactivity in the rabbit model of atherogenesis: differential effects of alamandine and Ang(1-7). Int J Exp Pathol 95: 290–295, 2014. doi: 10.1111/iep.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hampl V, Herget J, Bíbová J, Baňasová A, Husková Z, Vaňourková Z, Jíchová Š, Kujal P, Vernerová Z, Sadowski J, Červenka L. Intrapulmonary activation of the angiotensin-converting enzyme type 2/angiotensin 1-7/G-protein-coupled Mas receptor axis attenuates pulmonary hypertension in Ren-2 transgenic rats exposed to chronic hypoxia. Physiol Res 64: 25–38, 2015. [DOI] [PubMed] [Google Scholar]
- 237.Han Y, Sun HJ, Li P, Gao Q, Zhou YB, Zhang F, Gao XY, Zhu GQ. Angiotensin-(1-7) in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS One 7: e48966, 2012. doi: 10.1371/journal.pone.0048966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7): in vivo and in vitro studies. Am J Physiol 270: F141–F147, 1996. [DOI] [PubMed] [Google Scholar]
- 239.Hao P, Yang J, Liu Y, Zhang M, Zhang K, Gao F, Chen Y, Zhang C, Zhang Y. Combination of angiotensin-(1-7) with perindopril is better than single therapy in ameliorating diabetic cardiomyopathy. Sci Rep 5: 8794, 2015. doi: 10.1038/srep08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hao PP, Yang JM, Zhang MX, Zhang K, Chen YG, Zhang C, Zhang Y. Angiotensin-(1-7) treatment mitigates right ventricular fibrosis as a distinctive feature of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 308: H1007–H1019, 2015. doi: 10.1152/ajpheart.00563.2014. [DOI] [PubMed] [Google Scholar]
- 241.Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, Penninger J, Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet 52: 783–792, 2013. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 242.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487: 477–481, 2012. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.He H, Liu L, Chen Q, Liu A, Cai S, Yang Y, Lu X, Qiu H. Mesenchymal Stem Cells Overexpressing Angiotensin-Converting Enzyme 2 Rescue Lipopolysaccharide-Induced Lung Injury. Cell Transplant 24: 1699–1715, 2015. doi: 10.3727/096368914X685087. [DOI] [PubMed] [Google Scholar]
- 244.He JG, Chen SL, Huang YY, Chen YL, Dong YG, Ma H. The nonpeptide AVE0991 attenuates myocardial hypertrophy as induced by angiotensin II through downregulation of transforming growth factor-beta1/Smad2 expression. Heart Vessels 25: 438–443, 2010. doi: 10.1007/s00380-009-1213-7. [DOI] [PubMed] [Google Scholar]
- 245.Heller J, Kramer HJ, Malý J, Cervenka L, Horácek V. Effect of intrarenal infusion of angiotensin-(1-7) in the dog. Kidney Blood Press Res 23: 89–94, 2000. doi: 10.1159/000025959. [DOI] [PubMed] [Google Scholar]
- 246.Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin-(1-7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol Cell Neurosci 29: 427–435, 2005. doi: 10.1016/j.mcn.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 247.Herath CB, Lubel JS, Jia Z, Velkoska E, Casley D, Brown L, Tikellis C, Burrell LM, Angus PW. Portal pressure responses and angiotensin peptide production in rat liver are determined by relative activity of ACE and ACE2. Am J Physiol Gastrointest Liver Physiol 297: G98–G106, 2009. doi: 10.1152/ajpgi.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Herath CB, Mak K, Burrell LM, Angus PW. Angiotensin-(1-7) reduces the perfusion pressure response to angiotensin II and methoxamine via an endothelial nitric oxide-mediated pathway in cirrhotic rat liver. Am J Physiol Gastrointest Liver Physiol 304: G99–G108, 2013. doi: 10.1152/ajpgi.00163.2012. [DOI] [PubMed] [Google Scholar]
- 249.Heringer-Walther S, Batista EN, Walther T, Khosla MC, Santos RA, Campagnole-Santos MJ. Baroreflex improvement in shr after ace inhibition involves angiotensin-(1-7). Hypertension 37: 1309–1314, 2001. doi: 10.1161/01.HYP.37.5.1309. [DOI] [PubMed] [Google Scholar]
- 250.Hernández Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, Raizada MK. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 51: 1312–1317, 2008. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- 251.Hilchey SD, Bell-Quilley CP. Association between the natriuretic action of angiotensin-(1-7) and selective stimulation of renal prostaglandin I2 release. Hypertension 25: 1238–1244, 1995. doi: 10.1161/01.HYP.25.6.1238. [DOI] [PubMed] [Google Scholar]
- 252.Höcht C, Gironacci MM, Mayer MA, Schuman M, Bertera FM, Taira CA. Involvement of angiotensin-(1-7) in the hypothalamic hypotensive effect of captopril in sinoaortic denervated rats. Regul Pept 146: 58–66, 2008. doi: 10.1016/j.regpep.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 253.Höcht C, Opezzo JA, Gironacci MM, Peña C, Taira CA. Hypothalamic cardiovascular effects of angiotensin-(1-7) in spontaneously hypertensive rats. Regul Pept 135: 39–44, 2006. doi: 10.1016/j.regpep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 254.Honorato-Sampaio K, Pereira VM, Santos RA, Reis AM. Evidence that angiotensin-(1-7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp Physiol 97: 642–650, 2012. doi: 10.1113/expphysiol.2011.061960. [DOI] [PubMed] [Google Scholar]
- 255.Hood K, Yusuf H, Findlay J, Santos R, Castro C, Baillie G, Montezano A, MacLean M, Touyz R. Angiotensin 1–7 Regulation Of Endothelin-1 System In Pulmonary Arterial Hypertension. Hypertension 64: A316, 2014. [Google Scholar]
- 256.Huang W, Tang L, Cai Y, Zheng Y, Zhang L. Effect and mechanism of the Ang-(1-7) on human mesangial cells injury induced by low density lipoprotein. Biochem Biophys Res Commun 450: 1051–1057, 2014. doi: 10.1016/j.bbrc.2014.06.107. [DOI] [PubMed] [Google Scholar]
- 257.Igase M, Yokoyama H, Ferrario CM. Attenuation of hypertension-mediated glomerulosclerosis in conjunction with increased angiotensin (1-7). Ther Adv Cardiovasc Dis 5: 297–304, 2011. doi: 10.1177/1753944711429343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Isa K, Arnold AC, Westwood BM, Chappell MC, Diz DI. Angiotensin-converting enzyme inhibition, but not AT(1) receptor blockade, in the solitary tract nucleus improves baroreflex sensitivity in anesthetized transgenic hypertensive (mRen2)27 rats. Hypertens Res 34: 1257–1262, 2011. doi: 10.1038/hr.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH. Angiotensin-(1-7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 289: H2356–H2363, 2005. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 261.Iwata M, Cowling RT, Yeo SJ, Greenberg B. Targeting the ACE2-Ang-(1-7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol 51: 542–547, 2011. doi: 10.1016/j.yjmcc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jackman HL, Massad MG, Sekosan M, Tan F, Brovkovych V, Marcic BM, Erdös EG. Angiotensin 1-9 and 1-7 release in human heart: role of cathepsin A. Hypertension 39: 976–981, 2002. doi: 10.1161/01.HYP.0000017283.67962.02. [DOI] [PubMed] [Google Scholar]
- 263.Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR. The mas oncogene encodes an angiotensin receptor. Nature 335: 437–440, 1988. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- 264.Jankowski V, Vanholder R, van der Giet M, Tölle M, Karadogan S, Gobom J, Furkert J, Oksche A, Krause E, Tran TN, Tepel M, Schuchardt M, Schlüter H, Wiedon A, Beyermann M, Bader M, Todiras M, Zidek W, Jankowski J. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol 27: 297–302, 2007. doi: 10.1161/01.ATV.0000253889.09765.5f. [DOI] [PubMed] [Google Scholar]
- 265.Jesus I, Scalzo S, Rocha-Resende C, Santos R, Guatimosim S. Alamandine Signaling in Cardiomyocytes in Health and Disease. Hypertension 66: AP140, 2015. [Google Scholar]
- 266.Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol 93: 648–657, 2008. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
- 267.Jiang T, Gao L, Guo J, Lu J, Wang Y, Zhang Y. Suppressing inflammation by inhibiting the NF-κB pathway contributes to the neuroprotective effect of angiotensin-(1-7) in rats with permanent cerebral ischaemia. Br J Pharmacol 167: 1520–1532, 2012. doi: 10.1111/j.1476-5381.2012.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Lu J, Gao Q, Zhang YD, Tan L. Angiotensin-(1-7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br J Pharmacol 171: 4222–4232, 2014. doi: 10.1111/bph.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Johnson AR, Erdös EG. Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest 59: 684–695, 1977. doi: 10.1172/JCI108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jones A, Woods DR. Skeletal muscle RAS and exercise performance. Int J Biochem Cell Biol 35: 855–866, 2003. doi: 10.1016/S1357-2725(02)00342-4. [DOI] [PubMed] [Google Scholar]
- 271.Joyner J, Neves LA, Stovall K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) serves as an aquaretic by increasing water intake and diuresis in association with downregulation of aquaporin-1 during pregnancy in rats. Am J Physiol Regul Integr Comp Physiol 294: R1073–R1080, 2008. doi: 10.1152/ajpregu.00572.2007. [DOI] [PubMed] [Google Scholar]
- 272.Kangussu LM, Almeida-Santos AF, Bader M, Alenina N, Fontes MA, Santos RA, Aguiar DC, Campagnole-Santos MJ. Angiotensin-(1-7) attenuates the anxiety and depression-like behaviors in transgenic rats with low brain angiotensinogen. Behav Brain Res 257: 25–30, 2013. doi: 10.1016/j.bbr.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 273.Kangussu LM, Guimaraes PS, Nadu AP, Melo MB, Santos RA, Campagnole-Santos MJ. Activation of angiotensin-(1-7)/Mas axis in the brain lowers blood pressure and attenuates cardiac remodeling in hypertensive transgenic (mRen2)27 rats. Neuropharmacology 97: 58–66, 2015. doi: 10.1016/j.neuropharm.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 274.Kar S, Gao L, Belatti DA, Curry PL, Zucker IH. Central angiotensin (1-7) enhances baroreflex gain in conscious rabbits with heart failure. Hypertension 58: 627–634, 2011. doi: 10.1161/HYPERTENSIONAHA.111.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol (1985) 108: 923–932, 2010. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected] Pharmacol Rev 67: 754–819, 2015. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, Scholey JW, Penninger JM, Oudit GY. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail 2: 446–455, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 278.Kim CS, Kim IJ, Bae EH, Ma SK, Lee J, Kim SW. Angiotensin-(1-7) Attenuates Kidney Injury Due to Obstructive Nephropathy in Rats. PLoS One 10: e0142664, 2015. doi: 10.1371/journal.pone.0142664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kim G, Kim J, Lim YL, Kim MY, Baik SK. Renin-angiotensin system inhibitors and fibrosis in chronic liver disease: a systematic review. Hepatol Int 10: 819–828, 2016. doi: 10.1007/s12072-016-9705-x. [DOI] [PubMed] [Google Scholar]
- 280.Klein N, Gembardt F, Supé S, Kaestle SM, Nickles H, Erfinanda L, Lei X, Yin J, Wang L, Mertens M, Szaszi K, Walther T, Kuebler WM. Angiotensin-(1-7) protects from experimental acute lung injury. Crit Care Med 41: e334–e343, 2013. doi: 10.1097/CCM.0b013e31828a6688. [DOI] [PubMed] [Google Scholar]
- 281.Kluskens LD, Nelemans SA, Rink R, de Vries L, Meter-Arkema A, Wang Y, Walther T, Kuipers A, Moll GN, Haas M. Angiotensin-(1-7) with thioether bridge: an angiotensin-converting enzyme-resistant, potent angiotensin-(1-7) analog. J Pharmacol Exp Ther 328: 849–854, 2009. doi: 10.1124/jpet.108.146431. [DOI] [PubMed] [Google Scholar]
- 282.Kocks MJ, Lely AT, Boomsma F, de Jong PE, Navis G. Sodium status and angiotensin-converting enzyme inhibition: effects on plasma angiotensin-(1-7) in healthy man. J Hypertens 23: 597–602, 2005. doi: 10.1097/01.hjh.0000160217.86597.b6. [DOI] [PubMed] [Google Scholar]
- 283.Kone BC. Protein-protein interactions controlling nitric oxide synthases. Acta Physiol Scand 168: 27–31, 2000. doi: 10.1046/j.1365-201x.2000.00629.x. [DOI] [PubMed] [Google Scholar]
- 284.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111: 1806–1813, 2005. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 285.Kowalczuk S, Bröer A, Tietze N, Vanslambrouck JM, Rasko JE, Bröer S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J 22: 2880–2887, 2008. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- 286.Krob HA, Vinsant SL, Ferrario CM, Friedman DP. Angiotensin-(1-7) immunoreactivity in the hypothalamus of the (mRen-2d)27 transgenic rat. Brain Res 798: 36–45, 1998. doi: 10.1016/S0006-8993(98)00384-9. [DOI] [PubMed] [Google Scholar]
- 287.Kuba K, Zhang L, Imai Y, Arab S, Chen M, Maekawa Y, Leschnik M, Leibbrandt A, Markovic M, Schwaighofer J, Beetz N, Musialek R, Neely GG, Komnenovic V, Kolm U, Metzler B, Ricci R, Hara H, Meixner A, Nghiem M, Chen X, Dawood F, Wong KM, Sarao R, Cukerman E, Kimura A, Hein L, Thalhammer J, Liu PP, Penninger JM. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ Res 101: e32–e42, 2007. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 288.Kumagai H, Khosla M, Ferrario C, Fouad-Tarazi FM. Biological activity of angiotensin-(1-7) heptapeptide in the hamster heart. Hypertension 15, Suppl: I29–I33, 1990. doi: 10.1161/01.HYP.15.2_Suppl.I29. [DOI] [PubMed] [Google Scholar]
- 289.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab 18: 208–214, 2007. doi: 10.1016/j.tem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 290.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 294: 564–567, 2001. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 292.Langeveld B, van Gilst WH, Tio RA, Zijlstra F, Roks AJ. Angiotensin-(1-7) attenuates neointimal formation after stent implantation in the rat. Hypertension 45: 138–141, 2005. doi: 10.1161/01.HYP.0000149382.83973.c2. [DOI] [PubMed] [Google Scholar]
- 293.Lara LS, Cavalcante F, Axelband F, De Souza AM, Lopes AG, Caruso-Neves C. Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1-7). Biochem J 395: 183–190, 2006. doi: 10.1042/BJ20051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lara LS, Bica RB, Sena SL, Correa JS, Marques-Fernandes MF, Lopes AG, Caruso-Neves C. Angiotensin-(1-7) reverts the stimulatory effect of angiotensin II on the proximal tubule Na+-ATPase activity via a A779-sensitive receptor. Regul Pept 103: 17–22, 2002. doi: 10.1016/S0167-0115(01)00322-6. [DOI] [PubMed] [Google Scholar]
- 295.Lara LS, Correa JS, Lavelle AB, Lopes AG, Caruso-Neves C. The angiotensin receptor type 1-Gq protein-phosphatidyl inositol phospholipase Cbeta-protein kinase C pathway is involved in activation of proximal tubule Na+-ATPase activity by angiotensin(1-7) in pig kidneys. Exp Physiol 93: 639–647, 2008. doi: 10.1113/expphysiol.2007.040584. [DOI] [PubMed] [Google Scholar]
- 297.Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, Soares E, Barbosa C, Kjeldsen F, Oliveira A, Braga J, Savergnini S, Maia G, Peluso AB, Passos-Silva D, Ferreira A, Alves F, Martins A, Raizada M, Paula R, Motta-Santos D, Klempin F, Pimenta A, Alenina N, Sinisterra R, Bader M, Campagnole-Santos MJ, Santos RAS. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res 112: 1104–1111, 2013. doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- 298.Lawrence AC, Clark IJ, Campbell DJ. Increased angiotensin-(1-7) in hypophysial-portal plasma of conscious sheep. Neuroendocrinology 55: 105–114, 1992. doi: 10.1159/000126103. [DOI] [PubMed] [Google Scholar]
- 299.Lazaroni TL, Raslan AC, Fontes WR, de Oliveira ML, Bader M, Alenina N, Moraes MF, Dos Santos RA, Pereira GS. Angiotensin-(1-7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol Learn Mem 97: 113–123, 2012. doi: 10.1016/j.nlm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 300.Le Tran Y, Forster C. Angiotensin-(1-7) and the rat aorta: modulation by the endothelium. J Cardiovasc Pharmacol 30: 676–682, 1997. doi: 10.1097/00005344-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 301.Leal MC, Pinheiro SV, Ferreira AJ, Santos RA, Bordoni LS, Alenina N, Bader M, França LR. The role of angiotensin-(1-7) receptor Mas in spermatogenesis in mice and rats. J Anat 214: 736–743, 2009. doi: 10.1111/j.1469-7580.2009.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lee RM, Bader M, Alenina N, Santos RA, Gao YJ, Lu C. Mas receptors in modulating relaxation induced by perivascular adipose tissue. Life Sci 89: 467–472, 2011. doi: 10.1016/j.lfs.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 303.Lei ZY, Huang YS, Xiao R, Zhang BQ, Zhang Q. Effects of angiotensin (1-7) and enalaprilat on function of isolated rat heart perfused by burn serum. Zhonghua Shao Shang Za Zhi 25: 180–183, 2009. doi: 10.3760/cma.j.issn.1009-2587.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 304.Lemos VS, Côrtes SF, Silva DM, Campagnole-Santos MJ, Santos RA. Angiotensin-(1-7) is involved in the endothelium-dependent modulation of phenylephrine-induced contraction in the aorta of mRen-2 transgenic rats. Br J Pharmacol 135: 1743–1748, 2002. doi: 10.1038/sj.bjp.0704630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Leung PS, Chan HC, Wong PY. Immunohistochemical localization of angiotensin II in the mouse pancreas. Histochem J 30: 21–25, 1998. doi: 10.1023/A:1003210428276. [DOI] [PubMed] [Google Scholar]
- 306.Leung PS, Chan WP, Wong TP, Sernia C. Expression and localization of the renin-angiotensin system in the rat pancreas. J Endocrinol 160: 13–19, 1999. doi: 10.1677/joe.0.1600013. [DOI] [PubMed] [Google Scholar]
- 307.Li P, Sun HJ, Cui BP, Zhou YB, Han Y. Angiotensin-(1-7) in the rostral ventrolateral medulla modulates enhanced cardiac sympathetic afferent reflex and sympathetic activation in renovascular hypertensive rats. Hypertension 61: 820–827, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00191. [DOI] [PubMed] [Google Scholar]
- 308.Li P, Zhang F, Zhou YB, Cui BP, Han Y. Superoxide anions modulate the effects of angiotensin-(1-7) in the rostral ventrolateral medulla on cardiac sympathetic afferent reflex and sympathetic activity in rats. Neuroscience 223: 388–398, 2012. doi: 10.1016/j.neuroscience.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 309.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li Y, Cao Y, Zeng Z, Liang M, Xue Y, Xi C, Zhou M, Jiang W. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis prevents lipopolysaccharide-induced apoptosis of pulmonary microvascular endothelial cells by inhibiting JNK/NF-κB pathways. Sci Rep 5: 8209, 2015. doi: 10.1038/srep08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Li Y, Wu J, He Q, Shou Z, Zhang P, Pen W, Zhu Y, Chen J. Angiotensin (1-7) prevent heart dysfunction and left ventricular remodeling caused by renal dysfunction in 5/6 nephrectomy mice. Hypertens Res 32: 369–374, 2009. doi: 10.1038/hr.2009.25. [DOI] [PubMed] [Google Scholar]
- 312.Li Y, Zeng Z, Li Y, Huang W, Zhou M, Zhang X, Jiang W. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation. Shock 43: 395–404, 2015. doi: 10.1097/SHK.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 313.Liao XX, Guo RX, Ma H, Wang LC, Chen ZH, Yang CT, Feng JQ. Effects of angiotensin-(1-7) on oxidative stress and functional changes of isolated rat hearts induced by ischemia-reperfusion. Nan Fang Yi Ke Da Xue Xue Bao 28: 1345–1348, 2008. [PubMed] [Google Scholar]
- 315.Lima D, Fontes M, Campagnole-Santos M, Khosla M, Santos R. Effect of selective angiotensin receptor antagonists on the pressor effect of Angiotensin-I microinjection into the ventrolateral medulla. Hypertension 25: 1410, 1995. [Google Scholar]
- 316.Lima DX, Campagnole-Santos MJ, Fontes MA, Khosla MC, Santos RA. Haemorrhage increases the pressor effect of angiotensin-(1-7) but not of angiotensin II at the rat rostral ventrolateral medulla. J Hypertens 17: 1145–1152, 1999. doi: 10.1097/00004872-199917080-00014. [DOI] [PubMed] [Google Scholar]
- 317.Lin L, Liu X, Xu J, Weng L, Ren J, Ge J, Zou Y. Mas receptor mediates cardioprotection of angiotensin-(1-7) against Angiotensin II-induced cardiomyocyte autophagy and cardiac remodelling through inhibition of oxidative stress. J Cell Mol Med 20: 48–57, 2016. doi: 10.1111/jcmm.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Liu E, Xu Z, Li J, Yang S, Yang W, Li G. Enalapril, irbesartan, and angiotensin-(1-7) prevent atrial tachycardia-induced ionic remodeling. Int J Cardiol 146: 364–370, 2011. doi: 10.1016/j.ijcard.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 319.Liu E, Yang S, Xu Z, Li J, Yang W, Li G. Angiotensin-(1-7) prevents atrial fibrosis and atrial fibrillation in long-term atrial tachycardia dogs. Regul Pept 162: 73–78, 2010. doi: 10.1016/j.regpep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 320.Liu GC, Oudit GY, Fang F, Zhou J, Scholey JW. Angiotensin-(1-7)-induced activation of ERK1/2 is cAMP/protein kinase A-dependent in glomerular mesangial cells. Am J Physiol Renal Physiol 302: F784–F790, 2012. doi: 10.1152/ajprenal.00455.2011. [DOI] [PubMed] [Google Scholar]
- 321.Liu HZ, Gao CY, Wang XQ, Fu HX, Yang HH, Wang XP, Liu YH, Li MW, Niu ZM, Dai GY, Qi DT, Zhang Y. Angiotensin(1-7) attenuates left ventricular dysfunction and myocardial apoptosis on rat model of adriamycin-induced dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi 40: 219–224, 2012. [PubMed] [Google Scholar]
- 322.Liu R, Qi H, Wang J, Wang Y, Cui L, Wen Y, Yin C. Angiotensin-converting enzyme (ACE and ACE2) imbalance correlates with the severity of cerulein-induced acute pancreatitis in mice. Exp Physiol 99: 651–663, 2014. doi: 10.1113/expphysiol.2013.074815. [DOI] [PubMed] [Google Scholar]
- 323.Liu Y, Li B, Wang X, Li G, Shang R, Yang J, Wang J, Zhang M, Chen Y, Zhang Y, Zhang C, Hao P. Angiotensin-(1-7) Suppresses Hepatocellular Carcinoma Growth and Angiogenesis via Complex Interactions of Angiotensin II Type 1 Receptor, Angiotensin II Type 2 Receptor and Mas Receptor. Mol Med 21: 626–636, 2015. doi: 10.2119/molmed.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lopes AG, Soares AC, Santos DP, Fernandes MS, Leão-Ferreira LR, Quintana-Gomes E, Caruso-Neves C. PLA2/PGE2 are involved in the inhibitory effect of bradykinin on the angiotensin-(1-7)-stimulated Na(+)-ATPase activity of the proximal tubule. Regul Pept 117: 37–41, 2004. doi: 10.1016/j.regpep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 325.Lopez Verrilli MA, Pirola CJ, Pascual MM, Dominici FP, Turyn D, Gironacci MM. Angiotensin-(1-7) through AT receptors mediates tyrosine hydroxylase degradation via the ubiquitin-proteasome pathway. J Neurochem 109: 326–335, 2009. doi: 10.1111/j.1471-4159.2009.05912.x. [DOI] [PubMed] [Google Scholar]
- 326.Lopez Verrilli MA, Rodriguez Fermepín M, Longo Carbajosa N, Landa S, Cerrato BD, García S, Fernandez BE, Gironacci MM. Angiotensin-(1-7) through Mas receptor up-regulates neuronal norepinephrine transporter via Akt and Erk1/2-dependent pathways. J Neurochem 120: 46–55, 2012. doi: 10.1111/j.1471-4159.2011.07552.x. [DOI] [PubMed] [Google Scholar]
- 327.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, Slutsky AS, Peterson MD, Backx PH, Penninger JM, Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 295: H1377–H1384, 2008. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 328.Lu CL, Wang Y, Yuan L, Li Y, Li XY. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects the function of pancreatic β cells by improving the function of islet microvascular endothelial cells. Int J Mol Med 34: 1293–1300, 2014. doi: 10.3892/ijmm.2014.1917. [DOI] [PubMed] [Google Scholar]
- 329.Lu J, Jiang T, Wu L, Gao L, Wang Y, Zhou F, Zhang S, Zhang Y. The expression of angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas receptor axis are upregulated after acute cerebral ischemic stroke in rats. Neuropeptides 47: 289–295, 2013. doi: 10.1016/j.npep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 330.Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol 23: 1327–1338, 2008. doi: 10.1111/j.1440-1746.2008.05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Lubel JS, Herath CB, Tchongue J, Grace J, Jia Z, Spencer K, Casley D, Crowley P, Sievert W, Burrell LM, Angus PW. Angiotensin-(1-7), an alternative metabolite of the renin-angiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat. Clin Sci (Lond) 117: 375–386, 2009. doi: 10.1042/CS20080647. [DOI] [PubMed] [Google Scholar]
- 332.Lula I, Denadai AL, Resende JM, de Sousa FB, de Lima GF, Pilo-Veloso D, Heine T, Duarte HA, Santos RA, Sinisterra RD. Study of angiotensin-(1-7) vasoactive peptide and its beta-cyclodextrin inclusion complexes: complete sequence-specific NMR assignments and structural studies. Peptides 28: 2199–2210, 2007. doi: 10.1016/j.peptides.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 333.Ma X, Xu D, Ai Y, Zhao S, Zhang L, Ming G, Liu Z. Angiotensin-(1-7)/Mas Signaling Inhibits Lipopolysaccharide-Induced ADAM17 Shedding Activity and Apoptosis in Alveolar Epithelial Cells. Pharmacology 97: 63–71, 2016. doi: 10.1159/000441606. [DOI] [PubMed] [Google Scholar]
- 334.Ma Y, Huang H, Jiang J, Wu L, Lin C, Tang A, Dai G, He J, Chen Y. AVE 0991 attenuates cardiac hypertrophy through reducing oxidative stress. Biochem Biophys Res Commun 474: 621–625, 2016. doi: 10.1016/j.bbrc.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 335.Macedo LM, Souza ÁP, De Maria ML, Borges CL, Soares CM, Pedrino GR, Colugnati DB, Santos RA, Mendes EP, Ferreira AJ, Castro CH. Cardioprotective effects of diminazene aceturate in pressure-overloaded rat hearts. Life Sci 155: 63–69, 2016. doi: 10.1016/j.lfs.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 336.Machado-Silva A, Passos-Silva D, Santos RA, Sinisterra RD. Therapeutic uses for Angiotensin-(1-7). Expert Opin Ther Pat 26: 669–678, 2016. doi: 10.1080/13543776.2016.1179283. [DOI] [PubMed] [Google Scholar]
- 337.Magaldi AJ, Cesar KR, de Araújo M, Simões e Silva AC, Santos RA. Angiotensin-(1-7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflugers Arch 447: 223–230, 2003. doi: 10.1007/s00424-003-1173-1. [DOI] [PubMed] [Google Scholar]
- 338.Magalhães GS, Rodrigues-Machado MG, Motta-Santos D, Silva AR, Caliari MV, Prata LO, Abreu SC, Rocco PR, Barcelos LS, Santos RA, Campagnole-Santos MJ. Angiotensin-(1-7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation. Br J Pharmacol 172: 2330–2342, 2015. doi: 10.1111/bph.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mahon JM, Allen M, Herbert J, Fitzsimons JT. The association of thirst, sodium appetite and vasopressin release with c-fos expression in the forebrain of the rat after intracerebroventricular injection of angiotensin II, angiotensin-(1-7) or carbachol. Neuroscience 69: 199–208, 1995. doi: 10.1016/0306-4522(95)00238-E. [DOI] [PubMed] [Google Scholar]
- 340.Mak KY, Chin R, Cunningham SC, Habib MR, Torresi J, Sharland AF, Alexander IE, Angus PW, Herath CB. ACE2 Therapy Using Adeno-associated Viral Vector Inhibits Liver Fibrosis in Mice. Mol Ther 23: 1434–1443, 2015. doi: 10.1038/mt.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Malek M, Nematbakhsh M. The preventive effects of diminazene aceturate in renal ischemia/reperfusion injury in male and female rats. Adv Prev Med 2014: 740647, 2014. doi: 10.1155/2014/740647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Maleki M, Nematbakhsh M. Renal Blood Flow Response to Angiotensin 1-7 versus Hypertonic Sodium Chloride 7.5% Administration after Acute Hemorrhagic Shock in Rats. Int J Vasc Med 2016: 6562017, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Mansoori A, Oryan S, Nematbakhsh M. Role of Mas receptor antagonist (A779) in renal hemodynamics in condition of blocked angiotensin II receptors in rats. Physiol Int 103: 13–20, 2016. doi: 10.1556/036.103.2016.1.2. [DOI] [PubMed] [Google Scholar]
- 344.Mansoori A, Oryan S, Nematbakhsh M. Role of Mas receptor antagonist (A779) on pressure diuresis and natriuresis and renal blood flow in the absence of angiotensin II receptors type 1 and 2 in female and male rats. J Physiol Pharmacol 65: 633–639, 2014. [PubMed] [Google Scholar]
- 345.Marcus Y, Shefer G, Sasson K, Kohen F, Limor R, Pappo O, Nevo N, Biton I, Bach M, Berkutzki T, Fridkin M, Benayahu D, Shechter Y, Stern N. Angiotensin 1-7 as means to prevent the metabolic syndrome: lessons from the fructose-fed rat model. Diabetes 62: 1121–1130, 2013. doi: 10.2337/db12-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Mario EG, Santos SH, Ferreira AV, Bader M, Santos RA, Botion LM. Angiotensin-(1-7) Mas-receptor deficiency decreases peroxisome proliferator-activated receptor gamma expression in adipocytes. Peptides 33: 174–177, 2012. doi: 10.1016/j.peptides.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 347.Marques FD, Ferreira AJ, Sinisterra RD, Jacoby BA, Sousa FB, Caliari MV, Silva GA, Melo MB, Nadu AP, Souza LE, Irigoyen MC, Almeida AP, Santos RA. An oral formulation of angiotensin-(1-7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension 57: 477–483, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167346. [DOI] [PubMed] [Google Scholar]
- 348.Marques FD, Melo MB, Souza LE, Irigoyen MC, Sinisterra RD, de Sousa FB, Savergnini SQ, Braga VB, Ferreira AJ, Santos RA. Beneficial effects of long-term administration of an oral formulation of Angiotensin-(1-7) in infarcted rats. Int J Hypertens 2012: 795452, 2012. doi: 10.1155/2012/795452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Marques MB, Ribeiro-Oliveira A Jr, Guimarães J, Nascimento GF, Anjos AP, Vilas-Boas WW, Santos RA, Thomas JD, Igreja SM, Grossman AB, Kola B, Korbonits M. Modifications in basal and stress-induced hypothalamic AMP-activated protein kinase (AMPK) activity in rats chronically treated with an angiotensin II receptor blocker. Stress 15: 554–561, 2012. doi: 10.3109/10253890.2011.648673. [DOI] [PubMed] [Google Scholar]
- 350.Marrero MB, Fulton D, Stepp D, Stern DM. Angiotensin II-induced insulin resistance and protein tyrosine phosphatases. Arterioscler Thromb Vasc Biol 24: 2009–2013, 2004. doi: 10.1161/01.ATV.0000140059.04717.f3. [DOI] [PubMed] [Google Scholar]
- 351.Marshall AC, Pirro NT, Rose JC, Diz DI, Chappell MC. Evidence for an angiotensin-(1-7) neuropeptidase expressed in the brain medulla and CSF of sheep. J Neurochem 130: 313–323, 2014. doi: 10.1111/jnc.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Martin KA, Grant SG, Hockfield S. The mas proto-oncogene is developmentally regulated in the rat central nervous system. Brain Res Dev Brain Res 68: 75–82, 1992. doi: 10.1016/0165-3806(92)90249-V. [DOI] [PubMed] [Google Scholar]
- 353.Martins Lima A, Xavier CH, Ferreira AJ, Raizada MK, Wallukat G, Velloso EP, dos Santos RA, Fontes MA. Activation of angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. Am J Physiol Heart Circ Physiol 305: H1057–H1067, 2013. doi: 10.1152/ajpheart.00433.2013. [DOI] [PubMed] [Google Scholar]
- 354.McCollum LT, Gallagher PE, Tallant EA. Angiotensin-(1-7) abrogates mitogen-stimulated proliferation of cardiac fibroblasts. Peptides 34: 380–388, 2012. doi: 10.1016/j.peptides.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.McCray PB Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81: 813–821, 2007. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.McKinney CA, Fattah C, Loughrey CM, Milligan G, Nicklin SA. Angiotensin-(1-7) and angiotensin-(1-9): function in cardiac and vascular remodelling. Clin Sci (Lond) 126: 815–827, 2014. doi: 10.1042/CS20130436. [DOI] [PubMed] [Google Scholar]
- 357.Mecca AP, Regenhardt RW, O’Connor TE, Joseph JP, Raizada MK, Katovich MJ, Sumners C. Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke. Exp Physiol 96: 1084–1096, 2011. doi: 10.1113/expphysiol.2011.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Mendes AC, Ferreira AJ, Pinheiro SV, Santos RA. Chronic infusion of angiotensin-(1-7) reduces heart angiotensin II levels in rats. Regul Pept 125: 29–34, 2005. doi: 10.1016/j.regpep.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 359.Mendonça L, Mendes-Ferreira P, Bento-Leite A, Cerqueira R, Amorim MJ, Pinho P, Brás-Silva C, Leite-Moreira AF, Castro-Chaves P. Angiotensin-(1-7) modulates angiotensin II-induced vasoconstriction in human mammary artery. Cardiovasc Drugs Ther 28: 513–522, 2014. doi: 10.1007/s10557-014-6555-4. [DOI] [PubMed] [Google Scholar]
- 360.Meneses C, Morales MG, Abrigo J, Simon F, Brandan E, Cabello-Verrugio C. The angiotensin-(1-7)/Mas axis reduces myonuclear apoptosis during recovery from angiotensin II-induced skeletal muscle atrophy in mice. Pflugers Arch 467: 1975–1984, 2015. doi: 10.1007/s00424-014-1617-9. [DOI] [PubMed] [Google Scholar]
- 361.Meng W, Busija DW. Comparative effects of angiotensin-(1-7) and angiotensin II on piglet pial arterioles. Stroke 24: 2041–2044, 1993. doi: 10.1161/01.STR.24.12.2041. [DOI] [PubMed] [Google Scholar]
- 362.Meng W, Zhao W, Zhao T, Liu C, Chen Y, Liu H, Sun Y. Autocrine and paracrine function of Angiotensin 1-7 in tissue repair during hypertension. Am J Hypertens 27: 775–782, 2014. doi: 10.1093/ajh/hpt270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang MX, Li W, Li Y, Zhang WY, Li X. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxid Redox Signal 22: 241–258, 2015. doi: 10.1089/ars.2013.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Meng Y, Yu CH, Li W, Li T, Luo W, Huang S, Wu PS, Cai SX, Li X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am J Respir Cell Mol Biol 50: 723–736, 2014. doi: 10.1165/rcmb.2012-0451OC. [DOI] [PubMed] [Google Scholar]
- 365.Menon J, Soto-Pantoja DR, Callahan MF, Cline JM, Ferrario CM, Tallant EA, Gallagher PE. Angiotensin-(1-7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in cyclooxygenase-2. Cancer Res 67: 2809–2815, 2007. doi: 10.1158/0008-5472.CAN-06-3614. [DOI] [PubMed] [Google Scholar]
- 366.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res 103: 1319–1326, 2008. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 367.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine 18: 239–245, 2002. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 368.Methot D, LaPointe MC, Touyz RM, Yang XP, Carretero OA, Deschepper CF, Schiffrin EL, Thibault G, Reudelhuber TL. Tissue targeting of angiotensin peptides. J Biol Chem 272: 12994–12999, 1997. doi: 10.1074/jbc.272.20.12994. [DOI] [PubMed] [Google Scholar]
- 369.Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D. Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett 357: 27–32, 1995. doi: 10.1016/0014-5793(94)01292-9. [DOI] [PubMed] [Google Scholar]
- 370.Modgil A, Zhang Q, Pingili A, Singh N, Yao F, Ge J, Guo L, Xuan C, O’Rourke ST, Sun C. Angiotensin-(1-7) attenuates the chronotropic response to angiotensin II via stimulation of PTEN in the spontaneously hypertensive rat neurons. Am J Physiol Heart Circ Physiol 302: H1116–H1122, 2012. doi: 10.1152/ajpheart.00832.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Moltzer E, Verkuil AV, van Veghel R, Danser AH, van Esch JH. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension 55: 516–522, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145037. [DOI] [PubMed] [Google Scholar]
- 372.Morales MG, Abrigo J, Acuña MJ, Santos RA, Bader M, Brandan E, Simon F, Olguin H, Cabrera D, Cabello-Verrugio C. Angiotensin-(1-7) attenuates disuse skeletal muscle atrophy in mice via its receptor, Mas. Dis Model Mech 9: 441–449, 2016. doi: 10.1242/dmm.023390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Morales MG, Abrigo J, Meneses C, Cisternas F, Simon F, Cabello-Verrugio C. Expression of the Mas receptor is upregulated in skeletal muscle wasting. Histochem Cell Biol 143: 131–141, 2015. doi: 10.1007/s00418-014-1275-1. [DOI] [PubMed] [Google Scholar]
- 374.Morales MG, Abrigo J, Meneses C, Simon F, Cisternas F, Rivera JC, Vazquez Y, Cabello-Verrugio C. The Ang-(1-7)/Mas-1 axis attenuates the expression and signalling of TGF-β1 induced by AngII in mouse skeletal muscle. Clin Sci (Lond) 127: 251–264, 2014. doi: 10.1042/CS20130585. [DOI] [PubMed] [Google Scholar]
- 375.Morales MG, Olguín H, Di Capua G, Brandan E, Simon F, Cabello-Verrugio C. Endotoxin-induced skeletal muscle wasting is prevented by angiotensin-(1-7) through a p38 MAPK-dependent mechanism. Clin Sci (Lond) 129: 461–476, 2015. doi: 10.1042/CS20140840. [DOI] [PubMed] [Google Scholar]
- 376.Morales MG, Vazquez Y, Acuña MJ, Rivera JC, Simon F, Salas JD, Alvarez Ruf J, Brandan E, Cabello-Verrugio C. Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol 44: 1993–2002, 2012. doi: 10.1016/j.biocel.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 377.Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail 7: 327–339, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000672. [DOI] [PubMed] [Google Scholar]
- 378.Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, Lopaschuk GD, Oudit GY. Angiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol 306: F812–F821, 2014. doi: 10.1152/ajprenal.00655.2013. [DOI] [PubMed] [Google Scholar]
- 379.Morimoto S, Cassell MD, Sigmund CD. Glia- and neuron-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem 277: 33235–33241, 2002. doi: 10.1074/jbc.M204309200. [DOI] [PubMed] [Google Scholar]
- 380.Motta-Santos D, Dos Santos RA, Oliveira M, Qadri F, Poglitsch M, Mosienko V, Kappes Becker L, Campagnole-Santos MJ, M Penninger J, Alenina N, Bader M. Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res 39: 506–512, 2016. doi: 10.1038/hr.2016.28. [DOI] [PubMed] [Google Scholar]
- 381.Murça TM, Moraes PL, Capuruço CA, Santos SH, Melo MB, Santos RA, Shenoy V, Katovich MJ, Raizada MK, Ferreira AJ. Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regul Pept 177: 107–115, 2012. doi: 10.1016/j.regpep.2012.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351: 233–236, 1991. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 383.Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther 284: 388–398, 1998. [PubMed] [Google Scholar]
- 384.Muñoz MC, Giani JF, Burghi V, Mayer MA, Carranza A, Taira CA, Dominici FP. The Mas receptor mediates modulation of insulin signaling by angiotensin-(1-7). Regul Pept 177: 1–11, 2012. doi: 10.1016/j.regpep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 385.Muñoz MC, Giani JF, Dominici FP. Angiotensin-(1-7) stimulates the phosphorylation of Akt in rat extracardiac tissues in vivo via receptor Mas. Regul Pept 161: 1–7, 2010. doi: 10.1016/j.regpep.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 386.Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012. doi: 10.1038/ki.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Nadu AP, Ferreira AJ, Reudelhuber TL, Bader M, Santos RA. Reduced isoproterenol-induced renin-angiotensin changes and extracellular matrix deposition in hearts of TGR(A1-7)3292 rats. J Am Soc Hypertens 2: 341–348, 2008. doi: 10.1016/j.jash.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 388.Nakagaki T, Hirooka Y, Ito K, Kishi T, Hoka S, Sunagawa K. Role of angiotensin-(1-7) in rostral ventrolateral medulla in blood pressure regulation via sympathetic nerve activity in Wistar-Kyoto and spontaneous hypertensive rats. Clin Exp Hypertens 33: 223–230, 2011. doi: 10.3109/10641963.2011.583967. [DOI] [PubMed] [Google Scholar]
- 389.Nautiyal M, Katakam PV, Busija DW, Gallagher PE, Tallant EA, Chappell MC, Diz DI. Differences in oxidative stress status and expression of MKP-1 in dorsal medulla of transgenic rats with altered brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 303: R799–R806, 2012. doi: 10.1152/ajpregu.00566.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Nautiyal M, Shaltout HA, de Lima DC, do Nascimento K, Chappell MC, Diz DI. Central angiotensin-(1-7) improves vagal function independent of blood pressure in hypertensive (mRen2)27 rats. Hypertension 60: 1257–1265, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Nematbakhsh M, Safari T. Role of Mas receptor in renal blood flow response to angiotensin (1-7) in male and female rats. Gen Physiol Biophys 33: 365–372, 2014. doi: 10.4149/gpb_2014008. [DOI] [PubMed] [Google Scholar]
- 392.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82: 7264–7275, 2008. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Neves LA, Almeida AP, Khosla MC, Campagnole-Santos MJ, Santos RA. Effect of angiotensin-(1-7) on reperfusion arrhythmias in isolated rat hearts. Braz J Med Biol Res 30: 801–809, 1997. doi: 10.1590/S0100-879X1997000600016. [DOI] [PubMed] [Google Scholar]
- 394.Neves LA, Almeida AP, Khosla MC, Santos RA. Metabolism of angiotensin I in isolated rat hearts. Effect of angiotensin converting enzyme inhibitors. Biochem Pharmacol 50: 1451–1459, 1995. doi: 10.1016/0006-2952(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 395.Neves LA, Stovall K, Joyner J, Valdés G, Gallagher PE, Ferrario CM, Merrill DC, Brosnihan KB. ACE2 and ANG-(1-7) in the rat uterus during early and late gestation. Am J Physiol Regul Integr Comp Physiol 294: R151–R161, 2008. doi: 10.1152/ajpregu.00514.2007. [DOI] [PubMed] [Google Scholar]
- 396.Ni L, Feng Y, Wan H, Ma Q, Fan L, Qian Y, Li Q, Xiang Y, Gao B. Angiotensin-(1-7) inhibits the migration and invasion of A549 human lung adenocarcinoma cells through inactivation of the PI3K/Akt and MAPK signaling pathways. Oncol Rep 27: 783–790, 2012. doi: 10.3892/or.2011.1554. [DOI] [PubMed] [Google Scholar]
- 397.Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine 34: 56–61, 2008. doi: 10.1007/s12020-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 398.Niu X, Xue Y, Li X, He Y, Zhao X, Xu M, Zhao L. Effects of angiotensin-(1-7) on the proliferation and collagen synthesis of arginine vasopressin-stimulated rat cardiac fibroblasts: role of mas receptor-calcineurin-NF-κB signaling pathway. J Cardiovasc Pharmacol 64: 536–542, 2014. doi: 10.1097/FJC.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 399.Nogueira AI, Souza Santos RA, Simões E Silva AC, Cabral AC, Vieira RL, Drumond TC, Machado LJ, Freire CM, Ribeiro-Oliveira A Jr. The pregnancy-induced increase of plasma angiotensin-(1-7) is blunted in gestational diabetes. Regul Pept 141: 55–60, 2007. doi: 10.1016/j.regpep.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 400.O’Neill J, Corbett A, Johns EJ. Dietary sodium intake modulates renal excretory responses to intrarenal angiotensin (1-7) administration in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 304: R260–R266, 2013. doi: 10.1152/ajpregu.00583.2011. [DOI] [PubMed] [Google Scholar]
- 401.Oh YB, Kim JH, Park BM, Park BH, Kim SH. Captopril intake decreases body weight gain via angiotensin-(1-7). Peptides 37: 79–85, 2012. doi: 10.1016/j.peptides.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 402.Oliveira DR, Santos RA, Santos GF, Khosla M, Campagnole-Santos MJ. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension 27: 1284–1290, 1996. doi: 10.1161/01.HYP.27.6.1284. [DOI] [PubMed] [Google Scholar]
- 403.Oliveira RC, Campagnole-Santos MJ, Santos RA. The pressor effect of angiotensin-(1-7) in the rat rostral ventrolateral medulla involves multiple peripheral mechanisms. Clinics (Sao Paulo) 68: 245–252, 2013. doi: 10.6061/clinics/2013(02)OA20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Oscar CG, Müller-Ribeiro FC, de Castro LG, Martins Lima A, Campagnole-Santos MJ, Santos RA, Xavier CH, Fontes MA. Angiotensin-(1-7) in the basolateral amygdala attenuates the cardiovascular response evoked by acute emotional stress. Brain Res 1594: 183–189, 2015. doi: 10.1016/j.brainres.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 405.Osei SY, Ahima RS, Minkes RK, Weaver JP, Khosla MC, Kadowitz PJ. Differential responses to angiotensin-(1-7) in the feline mesenteric and hindquarters vascular beds. Eur J Pharmacol 234: 35–42, 1993. doi: 10.1016/0014-2999(93)90703-K. [DOI] [PubMed] [Google Scholar]
- 406.Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM, Brenner DA. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 50: 929–938, 2009. doi: 10.1002/hep.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 54: 1790–1796, 2005. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Papinska AM, Mordwinkin NM, Meeks CJ, Jadhav SS, Rodgers KE. Angiotensin-(1-7) administration benefits cardiac, renal and progenitor cell function in db/db mice. Br J Pharmacol 172: 4443–4453, 2015. doi: 10.1111/bph.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Patel KP, Schultz HD. Angiotensin peptides and nitric oxide in cardiovascular disease. Antioxid Redox Signal 19: 1121–1132, 2013. doi: 10.1089/ars.2012.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Patel VB, Bodiga S, Fan D, Das SK, Wang Z, Wang W, Basu R, Zhong J, Kassiri Z, Oudit GY. Cardioprotective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1-7 in experimental heart failure in angiotensin-converting enzyme 2-null mice. Hypertension 59: 1195–1203, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191650. [DOI] [PubMed] [Google Scholar]
- 411.Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, Parajuli N, Penninger JM, Grant MB, Lopaschuk GD, Oudit GY. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes 65: 85–95, 2016. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Patel VB, Zhong JC, Fan D, Basu R, Morton JS, Parajuli N, McMurtry MS, Davidge ST, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension 64: 157–164, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03388. [DOI] [PubMed] [Google Scholar]
- 413.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res 118: 1313–1326, 2016. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Pawlak R, Napiorkowska-Pawlak D, Takada Y, Urano T, Nagai N, Ihara H, Takada A. The differential effect of angiotensin II and angiotensin 1-7 on norepinephrine, epinephrine, and dopamine concentrations in rat hypothalamus: the involvement of angiotensin receptors. Brain Res Bull 54: 689–694, 2001. doi: 10.1016/S0361-9230(01)00489-0. [DOI] [PubMed] [Google Scholar]
- 415.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 57: 313–370, 1977. [DOI] [PubMed] [Google Scholar]
- 416.Peiró C, Vallejo S, Gembardt F, Azcutia V, Heringer-Walther S, Rodríguez-Mañas L, Schultheiss HP, Sánchez-Ferrer CF, Walther T. Endothelial dysfunction through genetic deletion or inhibition of the G protein-coupled receptor Mas: a new target to improve endothelial function. J Hypertens 25: 2421–2425, 2007. doi: 10.1097/HJH.0b013e3282f0143c. [DOI] [PubMed] [Google Scholar]
- 417.Pereira MG, Souza LL, Becari C, Duarte DA, Camacho FR, Oliveira JA, Gomes MD, Oliveira EB, Salgado MC, Garcia-Cairasco N, Costa-Neto CM. Angiotensin II-independent angiotensin-(1-7) formation in rat hippocampus: involvement of thimet oligopeptidase. Hypertension 62: 879–885, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01613. [DOI] [PubMed] [Google Scholar]
- 418.Pereira RM, Dos Santos RA, Teixeira MM, Leite VH, Costa LP, da Costa Dias FL, Barcelos LS, Collares GB, Simões e Silva AC. The renin-angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of Angiotensin-(1-7). J Hepatol 46: 674–681, 2007. doi: 10.1016/j.jhep.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 419.Pereira VM, Reis FM, Santos RA, Cassali GD, Santos SH, Honorato-Sampaio K, dos Reis AM. Gonadotropin stimulation increases the expression of angiotensin-(1–7) and MAS receptor in the rat ovary. Reprod Sci 16: 1165–1174, 2009. doi: 10.1177/1933719109343309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Pernomian L, Gomes MS, Restini CB, de Oliveira AM. MAS-mediated antioxidant effects restore the functionality of angiotensin converting enzyme 2-angiotensin-(1-7)-MAS axis in diabetic rat carotid. BioMed Res Int 2014: 640329, 2014. doi: 10.1155/2014/640329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Peña Silva RA, Chu Y, Miller JD, Mitchell IJ, Penninger JM, Faraci FM, Heistad DD. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 43: 3358–3363, 2012. doi: 10.1161/STROKEAHA.112.667063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Peña Silva RA, Heistad DD. Promising neuroprotective effects of the angiotensin-(1-7)-angiotensin-converting enzyme 2-Mas axis in stroke. Exp Physiol 99: 342–343, 2014. doi: 10.1113/expphysiol.2013.076836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhães JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int 75: 1184–1193, 2009. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 424.Pinheiro SV, Simões e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, Pesquero JB, Walther T, Alenina N, Bader M, Bleich M, Santos RA. Nonpeptide AVE 0991 is an angiotensin-(1-7) receptor Mas agonist in the mouse kidney. Hypertension 44: 490–496, 2004. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- 425.Potts PD, Horiuchi J, Coleman MJ, Dampney RA. The cardiovascular effects of angiotensin-(1-7) in the rostral and caudal ventrolateral medulla of the rabbit. Brain Res 877: 58–64, 2000. doi: 10.1016/S0006-8993(00)02626-3. [DOI] [PubMed] [Google Scholar]
- 426.Pörsti I, Bara AT, Busse R, Hecker M. Release of nitric oxide by angiotensin-(1-7) from porcine coronary endothelium: implications for a novel angiotensin receptor. Br J Pharmacol 111: 652–654, 1994. doi: 10.1111/j.1476-5381.1994.tb14787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Qadri F, Wolf A, Waldmann T, Rascher W, Unger T. Sensitivity of hypothalamic paraventricular nucleus to C- and N-terminal angiotensin fragments: vasopressin release and drinking. J Neuroendocrinol 10: 275–281, 1998. [PubMed] [Google Scholar]
- 428.Qi Y, Shenoy V, Wong F, Li H, Afzal A, Mocco J, Sumners C, Raizada MK, Katovich MJ. Lentivirus-mediated overexpression of angiotensin-(1-7) attenuated ischaemia-induced cardiac pathophysiology. Exp Physiol 96: 863–874, 2011. doi: 10.1113/expphysiol.2011.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Qiu Y, Shil PK, Zhu P, Yang H, Verma A, Lei B, Li Q. Angiotensin-converting enzyme 2 (ACE2) activator diminazene aceturate ameliorates endotoxin-induced uveitis in mice. Invest Ophthalmol Vis Sci 55: 3809–3818, 2014. doi: 10.1167/iovs.14-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Rabelo LA, Xu P, Todiras M, Sampaio WO, Buttgereit J, Bader M, Santos RA, Alenina N. Ablation of angiotensin (1-7) receptor Mas in C57Bl/6 mice causes endothelial dysfunction. J Am Soc Hypertens 2: 418–424, 2008. doi: 10.1016/j.jash.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 431.Rabelo LA, Todiras M, Nunes-Souza V, Qadri F, Szijártó IA, Gollasch M, Penninger JM, Bader M, Santos RA, Alenina N. Genetic Deletion of ACE2 Induces Vascular Dysfunction in C57BL/6 Mice: Role of Nitric Oxide Imbalance and Oxidative Stress. PLoS One 11: e0150255, 2016. doi: 10.1371/journal.pone.0150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Rakušan D, Bürgelová M, Vaněčková I, Vaňourková Z, Husková Z, Skaroupková P, Mrázová I, Opočenský M, Kramer HJ, Netuka I, Malý J, Alenina N, Bader M, Santos RA, Cervenka L. Knockout of angiotensin 1–7 receptor Mas worsens the course of two-kidney, one-clip Goldblatt hypertension: roles of nitric oxide deficiency and enhanced vascular responsiveness to angiotensin II. Kidney Blood Press Res 33: 476–488, 2010. doi: 10.1159/000320689. [DOI] [PubMed] [Google Scholar]
- 433.Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, Sumners C. Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke. Neuropharmacology 71: 154–163, 2013. doi: 10.1016/j.neuropharm.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, Banuelos C, Bizon JL, Reinhard MK, Sumners C. Centrally administered angiotensin-(1-7) increases the survival of stroke-prone spontaneously hypertensive rats. Exp Physiol 99: 442–453, 2014. doi: 10.1113/expphysiol.2013.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Reis AB, Araújo FC, Pereira VM, Dos Reis AM, Santos RA, Reis FM. Angiotensin (1-7) and its receptor Mas are expressed in the human testis: implications for male infertility. J Mol Histol 41: 75–80, 2010. doi: 10.1007/s10735-010-9264-8. [DOI] [PubMed] [Google Scholar]
- 436.Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril 95: 176–181, 2011. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 437.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 438.Renno WM, Al-Banaw AG, George P, Abu-Ghefreh AA, Akhtar S, Benter IF. Angiotensin-(1-7) via the mas receptor alleviates the diabetes-induced decrease in GFAP and GAP-43 immunoreactivity with concomitant reduction in the COX-2 in hippocampal formation: an immunohistochemical study. Cell Mol Neurobiol 32: 1323–1336, 2012. doi: 10.1007/s10571-012-9858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, Baltatu OC, Santos RA, Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 52: 967–973, 2008. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- 440.Reudelhuber T, Mercure C, Jain D, Bader M, Santos R. Targeted over-production of angiotensin 1–7 in the heart reverses hypertension-related cardiac hypertrophy. Circulation 112: U306, 2005. [Google Scholar]
- 441.Reudelhuber TL. A place in our hearts for the lowly angiotensin 1-7 peptide? Hypertension 47: 811–815, 2006. doi: 10.1161/01.HYP.0000209020.69734.73. [DOI] [PubMed] [Google Scholar]
- 442.Reudelhuber TL. The renin-angiotensin system: peptides and enzymes beyond angiotensin II. Curr Opin Nephrol Hypertens 14: 155–159, 2005. doi: 10.1097/00041552-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 443.Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, Loibner H, Penninger JM, Kassiri Z, Oudit GY, Thébaud B. Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med (Berl) 90: 637–647, 2012. doi: 10.1007/s00109-012-0859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383: 45–51, 2004. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Rigatto K, Casali KR, Shenoy V, Katovich MJ, Raizada MK. Diminazene aceturate improves autonomic modulation in pulmonary hypertension. Eur J Pharmacol 713: 89–93, 2013. doi: 10.1016/j.ejphar.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Riquelme C, Acuña MJ, Torrejón J, Rebolledo D, Cabrera D, Santos RA, Brandan E. ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS One 9: e93449, 2014. doi: 10.1371/journal.pone.0093449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Rodgers K, Verco S, Bolton L, Dizerega G. Accelerated healing of diabetic wounds by NorLeu(3)-angiotensin (1-7). Expert Opin Investig Drugs 20: 1575–1581, 2011. doi: 10.1517/13543784.2011.619976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Rodgers KE, Espinoza T, Felix J, Roda N, Maldonado S, diZerega G. Acceleration of healing, reduction of fibrotic scar, and normalization of tissue architecture by an angiotensin analogue, NorLeu3-A(1-7). Plast Reconstr Surg 111: 1195–1206, 2003. doi: 10.1097/01.PRS.0000047403.23105.66. [DOI] [PubMed] [Google Scholar]
- 449.Rodrigues-Machado MG, Magalhães GS, Cardoso JA, Kangussu LM, Murari A, Caliari MV, Oliveira ML, Cara DC, Noviello ML, Marques FD, Pereira JM, Lautner RQ, Santos RA, Campagnole-Santos MJ. AVE 0991, a non-peptide mimic of angiotensin-(1-7) effects, attenuates pulmonary remodelling in a model of chronic asthma. Br J Pharmacol 170: 835–846, 2013. doi: 10.1111/bph.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Roks AJ, Nijholt J, van Buiten A, van Gilst WH, de Zeeuw D, Henning RH. Low sodium diet inhibits the local counter-regulator effect of angiotensin-(1-7) on angiotensin II. J Hypertens 22: 2355–2361, 2004. doi: 10.1097/00004872-200412000-00018. [DOI] [PubMed] [Google Scholar]
- 451.Roks AJ, van Geel PP, Pinto YM, Buikema H, Henning RH, de Zeeuw D, van Gilst WH. Angiotensin-(1-7) is a modulator of the human renin-angiotensin system. Hypertension 34: 296–301, 1999. doi: 10.1161/01.HYP.34.2.296. [DOI] [PubMed] [Google Scholar]
- 452.Rowe BP, Saylor DL, Speth RC, Absher DR. Angiotensin-(1-7) binding at angiotensin II receptors in the rat brain. Regul Pept 56: 139–146, 1995. doi: 10.1016/0167-0115(95)00010-9. [DOI] [PubMed] [Google Scholar]
- 453.Saberi S, Dehghani A, Nematbakhsh M. Role of Mas receptor in renal blood flow response to angiotensin-(1-7) in ovariectomized estradiol treated rats. Res Pharm Sci 11: 65–72, 2016. [PMC free article] [PubMed] [Google Scholar]
- 454.Sabharwal R, Chapleau MW. Autonomic, locomotor and cardiac abnormalities in a mouse model of muscular dystrophy: targeting the renin-angiotensin system. Exp Physiol 99: 627–631, 2014. doi: 10.1113/expphysiol.2013.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Sabharwal R, Cicha MZ, Sinisterra RD, De Sousa FB, Santos RA, Chapleau MW. Chronic oral administration of Ang-(1-7) improves skeletal muscle, autonomic and locomotor phenotypes in muscular dystrophy. Clin Sci (Lond) 127: 101–109, 2014. doi: 10.1042/CS20130602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Sabharwal R, Weiss RM, Zimmerman K, Domenig O, Cicha MZ, Chapleau MW. Angiotensin-dependent autonomic dysregulation precedes dilated cardiomyopathy in a mouse model of muscular dystrophy. Exp Physiol 100: 776–795, 2015. doi: 10.1113/EP085066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Sahara M, Ikutomi M, Morita T, Minami Y, Nakajima T, Hirata Y, Nagai R, Sata M. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc Res 101: 236–246, 2014. doi: 10.1093/cvr/cvt245. [DOI] [PubMed] [Google Scholar]
- 458.Sakai K, Chapleau MW, Morimoto S, Cassell MD, Sigmund CD. Differential modulation of baroreflex control of heart rate by neuron- vs. glia-derived angiotensin II. Physiol Genomics 20: 66–72, 2004. doi: 10.1152/physiolgenomics.00168.2004. [DOI] [PubMed] [Google Scholar]
- 459.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii. Hypertension 46: 333–340, 2005. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 460.Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glia-derived angiotensinogen. Am J Physiol Heart Circ Physiol 292: H1412–H1419, 2007. doi: 10.1152/ajpheart.00984.2006. [DOI] [PubMed] [Google Scholar]
- 461.Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50: 1093–1098, 2007. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 462.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol 284: H1985–H1994, 2003. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 463.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49: 185–192, 2007. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 464.Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer 93: 425–434, 2005. doi: 10.1038/sj.bjc.6602725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Santiago NM, Guimarães PS, Sirvente RA, Oliveira LA, Irigoyen MC, Santos RA, Campagnole-Santos MJ. Lifetime overproduction of circulating Angiotensin-(1-7) attenuates deoxycorticosterone acetate-salt hypertension-induced cardiac dysfunction and remodeling. Hypertension 55: 889–896, 2010. doi: 10.1161/HYPERTENSIONAHA.110.149815. [DOI] [PubMed] [Google Scholar]
- 466.Santos G, Sampaio W, Reudelhurber T, Bader M, Campagnole-Santos M, Santos R. Expression of an angiotensin-(1–7)-producing fusion protein in rats produced marked changes in regional vascular resistance. Hypertension 44: 525–526, 2004. [Google Scholar]
- 467.Santos RAS, Passaglio KT, Pesquero JB, Bader M, Simões E Silva AC. Interactions between angiotensin-(1-7), kinins, and angiotensin II in kidney and blood vessels. Hypertension 38: 660–664, 2001. doi: 10.1161/01.HYP.38.3.660. [DOI] [PubMed] [Google Scholar]
- 468.Santos RA. Angiotensin-(1-7). Hypertension 63: 1138–1147, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- 469.Santos RA, Baracho NC. Angiotensin-(1-7) is a potent antidiuretic peptide in rats. Braz J Med Biol Res 25: 651–654, 1992. [PubMed] [Google Scholar]
- 470.Santos RA, Brosnihan KB, Chappell MC, Pesquero J, Chernicky CL, Greene LJ, Ferrario CM. Converting enzyme activity and angiotensin metabolism in the dog brainstem. Hypertension 11: I153–I157, 1988. doi: 10.1161/01.HYP.11.2_Pt_2.I153. [DOI] [PubMed] [Google Scholar]
- 471.Santos RA, Brosnihan KB, Jacobsen DW, DiCorleto PE, Ferrario CM. Production of angiotensin-(1-7) by human vascular endothelium. Hypertension 19, Suppl: II56–II61, 1992. doi: 10.1161/01.HYP.19.2_Suppl.II56. [DOI] [PubMed] [Google Scholar]
- 472.Santos RA, Brum JM, Brosnihan KB, Ferrario CM. The renin-angiotensin system during acute myocardial ischemia in dogs. Hypertension 15, Suppl: I121–I127, 1990. doi: 10.1161/01.HYP.15.2_Suppl.I121. [DOI] [PubMed] [Google Scholar]
- 473.Santos RA, Campagnole-Santos MJ. Central and peripheral actions of angiotensin-(1-7). Braz J Med Biol Res 27: 1033–1047, 1994. [PubMed] [Google Scholar]
- 474.Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regul Pept 91: 45–62, 2000. doi: 10.1016/S0167-0115(00)00138-5. [DOI] [PubMed] [Google Scholar]
- 475.Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen Júnior C, , et al. Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): evidence that the actions of angiotensin-(1-7) are mediated by specific angiotensin receptors. Brain Res Bull 35: 293–298, 1994. doi: 10.1016/0361-9230(94)90104-X. [DOI] [PubMed] [Google Scholar]
- 476.Santos RA, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension 47: 996–1002, 2006. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 477.Santos RA, Ferreira AJ, Nadu AP, Braga AN, de Almeida AP, Campagnole-Santos MJ, Baltatu O, Iliescu R, Reudelhuber TL, Bader M. Expression of an angiotensin-(1–7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics 17: 292–299, 2004. doi: 10.1152/physiolgenomics.00227.2003. [DOI] [PubMed] [Google Scholar]
- 478.Santos RA, Haibara AS, Campagnole-Santos MJ, Simões e Silva AC, Paula RD, Pinheiro SV, Leite MF, Lemos VS, Silva DM, Guerra MT, Khosla MC. Characterization of a new selective antagonist for angiotensin-(1-7), D-pro7-angiotensin-(1-7). Hypertension 41: 737–743, 2003. doi: 10.1161/01.HYP.0000052947.60363.24. [DOI] [PubMed] [Google Scholar]
- 479.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Santos RA, Simões e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT, Baracho NC. Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension 27: 875–884, 1996. doi: 10.1161/01.HYP.27.4.875. [DOI] [PubMed] [Google Scholar]
- 481.Santos SH, Andrade JM. Angiotensin 1-7: a peptide for preventing and treating metabolic syndrome. Peptides 59: 34–41, 2014. doi: 10.1016/j.peptides.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 482.Santos SH, Andrade JM, Fernandes LR, Sinisterra RD, Sousa FB, Feltenberger JD, Alvarez-Leite JI, Santos RA. Oral Angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed with high-fat diet. Peptides 46: 47–52, 2013. doi: 10.1016/j.peptides.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 483.Santos SH, Braga JF, Mario EG, Pôrto LC, Rodrigues-Machado MG, Murari A, Botion LM, Alenina N, Bader M, Santos RA. Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1-7). Arterioscler Thromb Vasc Biol 30: 953–961, 2010. doi: 10.1161/ATVBAHA.109.200493. [DOI] [PubMed] [Google Scholar]
- 484.Santos SH, Fernandes LR, Mario EG, Ferreira AV, Pôrto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57: 340–347, 2008. doi: 10.2337/db07-0953. [DOI] [PubMed] [Google Scholar]
- 485.Santos SH, Fernandes LR, Pereira CS, Guimarães AL, de Paula AM, Campagnole-Santos MJ, Alvarez-Leite JI, Bader M, Santos RA. Increased circulating angiotensin-(1-7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul Pept 178: 64–70, 2012. doi: 10.1016/j.regpep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 486.Santos SH, Giani JF, Burghi V, Miquet JG, Qadri F, Braga JF, Todiras M, Kotnik K, Alenina N, Dominici FP, Santos RA, Bader M. Oral administration of angiotensin-(1-7) ameliorates type 2 diabetes in rats. J Mol Med (Berl) 92: 255–265, 2014. doi: 10.1007/s00109-013-1087-0. [DOI] [PubMed] [Google Scholar]
- 487.Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray JJ, Hasegawa M, Matsuda Y, Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature 351: 230–233, 1991. doi: 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- 488.Sasaki S, Higashi Y, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. Effects of angiotensin-(1-7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension 38: 90–94, 2001. doi: 10.1161/01.HYP.38.1.90. [DOI] [PubMed] [Google Scholar]
- 489.Savergnini SQ, Beiman M, Lautner RQ, de Paula-Carvalho V, Allahdadi K, Pessoa DC, Costa-Fraga FP, Fraga-Silva RA, Cojocaru G, Cohen Y, Bader M, de Almeida AP, Rotman G, Santos RA. Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension 56: 112–120, 2010. doi: 10.1161/HYPERTENSIONAHA.110.152942. [DOI] [PubMed] [Google Scholar]
- 490.Savergnini SQ, Ianzer D, Carvalho MB, Ferreira AJ, Silva GA, Marques FD, Peluso AA, Beiman M, Cojocaru G, Cohen Y, Almeida AP, Rotman G, Santos RA. The novel Mas agonist, CGEN-856S, attenuates isoproterenol-induced cardiac remodeling and myocardial infarction injury in rats. PLoS One 8: e57757, 2013. doi: 10.1371/journal.pone.0057757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Scherrer-Crosbie M. Losartan: a new treatment for cardiac cachexia? J Mol Cell Cardiol 86: 12–13, 2015. doi: 10.1016/j.yjmcc.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 492.Schiavone MT, Santos RA, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1-7) heptapeptide. Proc Natl Acad Sci USA 85: 4095–4098, 1988. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Senanayake PD, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides 15: 919–926, 1994. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 494.Shah A, Oh YB, Shan G, Song CH, Park BH, Kim SH. Angiotensin-(1-7) attenuates hyposmolarity-induced ANP secretion via the Na+-K+ pump. Peptides 31: 1779–1785, 2010. doi: 10.1016/j.peptides.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 495.Shao Y, He M, Zhou L, Yao T, Huang Y, Lu LM. Chronic angiotensin (1-7) injection accelerates STZ-induced diabetic renal injury. Acta Pharmacol Sin 29: 829–837, 2008. doi: 10.1111/j.1745-7254.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 496.Shariat-Madar Z, Mahdi F, Warnock M, Homeister JW, Srikanth S, Krijanovski Y, Murphey LJ, Jaffa AA, Schmaier AH. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood 108: 192–199, 2006. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Díez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 182: 1065–1072, 2010. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, Shil P, Nair A, Qi Y, Li Q, Francis J, Katovich MJ, Daniell H, Raizada MK. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 64: 1248–1259, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Shi Y, Lo CS, Padda R, Abdo S, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Angiotensin-(1-7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci (Lond) 128: 649–663, 2015. doi: 10.1042/CS20140329. [DOI] [PubMed] [Google Scholar]
- 500.Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, Nagahiro S, Hashimoto T. Angiotensin-(1-7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab 35: 1163–1168, 2015. doi: 10.1038/jcbfm.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Shiota A, Yamamoto K, Ohishi M, Tatara Y, Ohnishi M, Maekawa Y, Iwamoto Y, Takeda M, Rakugi H. Loss of ACE2 accelerates time-dependent glomerular and tubulointerstitial damage in streptozotocin-induced diabetic mice. Hypertens Res 33: 298–307, 2010. doi: 10.1038/hr.2009.231. [DOI] [PubMed] [Google Scholar]
- 502.Shoemaker R, Yiannikouris F, Thatcher S, Cassis L. ACE2 deficiency reduces β-cell mass and impairs β-cell proliferation in obese C57BL/6 mice. Am J Physiol Endocrinol Metab 309: E621–E631, 2015. doi: 10.1152/ajpendo.00054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580, 1989. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 504.Silva AQ, Santos RA, Fontes MA. Blockade of endogenous angiotensin-(1-7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension 46: 341–348, 2005. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 505.Silva AR, Aguilar EC, Alvarez-Leite JI, da Silva RF, Arantes RM, Bader M, Alenina N, Pelli G, Lenglet S, Galan K, Montecucco F, Mach F, Santos SH, Santos RA. Mas receptor deficiency is associated with worsening of lipid profile and severe hepatic steatosis in ApoE-knockout mice. Am J Physiol Regul Integr Comp Physiol 305: R1323–R1330, 2013. doi: 10.1152/ajpregu.00249.2013. [DOI] [PubMed] [Google Scholar]
- 506.Silva LC, Fontes MA, Campagnole-Santos MJ, Khosla MC, Campos RR Jr, Guertzenstein PG, Santos RA. Cardiovascular effects produced by micro-injection of angiotensin-(1-7) on vasopressor and vasodepressor sites of the ventrolateral medulla. Brain Res 613: 321–325, 1993. doi: 10.1016/0006-8993(93)90920-I. [DOI] [PubMed] [Google Scholar]
- 507.Silveira KD, Barroso LC, Vieira AT, Cisalpino D, Lima CX, Bader M, Arantes RM, Dos Santos RA, Simões-E-Silva AC, Teixeira MM. Beneficial effects of the activation of the angiotensin-(1-7) MAS receptor in a murine model of adriamycin-induced nephropathy. PLoS One 8: e66082, 2013. doi: 10.1371/journal.pone.0066082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Simões E Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 107: 154–162, 2016. doi: 10.1016/j.phrs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 509.Singh K, Sharma K, Singh M, Sharma PL. Possible mechanism of the cardio-renal protective effects of AVE-0991, a non-peptide Mas-receptor agonist, in diabetic rats. J Renin Angiotensin Aldosterone Syst 13: 334–340, 2012. doi: 10.1177/1470320311435534. [DOI] [PubMed] [Google Scholar]
- 510.Singh K, Singh T, Sharma PL. Beneficial effects of angiotensin (1-7) in diabetic rats with cardiomyopathy. Ther Adv Cardiovasc Dis 5: 159–167, 2011. doi: 10.1177/1753944711409281. [DOI] [PubMed] [Google Scholar]
- 511.Singh N, Joshi S, Guo L, Baker MB, Li Y, Castellano RK, Raizada MK, Jarajapu YP. ACE2/Ang-(1-7)/Mas axis stimulates vascular repair-relevant functions of CD34+ cells. Am J Physiol Heart Circ Physiol 309: H1697–H1707, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Singh T, Singh K, Sharma PL. Ameliorative potential of angiotensin1-7/Mas receptor axis in streptozotocin-induced diabetic nephropathy in rats. Methods Find Exp Clin Pharmacol 32: 19–25, 2010. doi: 10.1358/mf.2010.32.1.1434160. [DOI] [PubMed] [Google Scholar]
- 513.Souza LK, Nicolau LA, Sousa NA, Araújo TS, Sousa FB, Costa DS, Souza FM, Pacífico DM, Martins CS, Silva RO, Souza MH, Cerqueira GS, Medeiros JV. Diminazene aceturate, an angiotensin-converting enzyme II activator, prevents gastric mucosal damage in mice: role of the angiotensin-(1-7)/Mas receptor axis. Biochem Pharmacol 112: 50–59, 2016. doi: 10.1016/j.bcp.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 514.Souza LL, Costa-Neto CM. Angiotensin-(1-7) decreases LPS-induced inflammatory response in macrophages. J Cell Physiol 227: 2117–2122, 2012. doi: 10.1002/jcp.22940. [DOI] [PubMed] [Google Scholar]
- 515.Souza LL, Duchene J, Todiras M, Azevedo LC, Costa-Neto CM, Alenina N, Santos RA, Bader M. Receptor MAS protects mice against hypothermia and mortality induced by endotoxemia. Shock 41: 331–336, 2014. doi: 10.1097/SHK.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 516.Souza ÁP, Sobrinho DB, Almeida JF, Alves GM, Macedo LM, Porto JE, Vêncio EF, Colugnati DB, Santos RA, Ferreira AJ, Mendes EP, Castro CH. Angiotensin II type 1 receptor blockade restores angiotensin-(1-7)-induced coronary vasodilation in hypertrophic rat hearts. Clin Sci (Lond) 125: 449–459, 2013. doi: 10.1042/CS20120519. [DOI] [PubMed] [Google Scholar]
- 517.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol 286: H1597–H1602, 2004. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 518.Staschewski J, Kulisch C, Albrecht D. Different isoforms of nitric oxide synthase are involved in angiotensin-(1-7)-mediated plasticity changes in the amygdala in a gender-dependent manner. Neuroendocrinology 94: 191–199, 2011. doi: 10.1159/000328128. [DOI] [PubMed] [Google Scholar]
- 519.Stegbauer J, Oberhauser V, Vonend O, Rump LC. Angiotensin-(1-7) modulates vascular resistance and sympathetic neurotransmission in kidneys of spontaneously hypertensive rats. Cardiovasc Res 61: 352–359, 2004. doi: 10.1016/j.cardiores.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 520.Stegbauer J, Vonend O, Oberhauser V, Rump LC. Effects of angiotensin-(1-7) and other bioactive components of the renin-angiotensin system on vascular resistance and noradrenaline release in rat kidney. J Hypertens 21: 1391–1399, 2003. doi: 10.1097/00004872-200307000-00030. [DOI] [PubMed] [Google Scholar]
- 521.Stewart JA Jr, Lazartigues E, Lucchesi PA. The angiotensin converting enzyme 2/Ang-(1-7) axis in the heart: a role for MAS communication? Circ Res 103: 1197–1199, 2008. doi: 10.1161/CIRCRESAHA.108.189068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Strachan RT, Sun JP, Rominger DH, Violin JD, Ahn S, Rojas Bie Thomsen A, Zhu X, Kleist A, Costa T, Lefkowitz RJ. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR). J Biol Chem 289: 14211–14224, 2014. doi: 10.1074/jbc.M114.548131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Stragier B, Hristova I, Sarre S, Ebinger G, Michotte Y. In vivo characterization of the angiotensin-(1-7)-induced dopamine and gamma-aminobutyric acid release in the striatum of the rat. Eur J Neurosci 22: 658–664, 2005. doi: 10.1111/j.1460-9568.2005.04188.x. [DOI] [PubMed] [Google Scholar]
- 524.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 69: 2212–2218, 2006. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 525.Sui YB, Chang JR, Chen WJ, Zhao L, Zhang BH, Yu YR, Tang CS, Yin XH, Qi YF. Angiotensin-(1-7) inhibits vascular calcification in rats. Peptides 42: 25–34, 2013. doi: 10.1016/j.peptides.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 526.Sumners C, Horiuchi M, Widdop RE, McCarthy C, Unger T, Steckelings UM. Protective arms of the renin-angiotensin-system in neurological disease. Clin Exp Pharmacol Physiol 40: 580–588, 2013. doi: 10.1111/1440-1681.12137. [DOI] [PubMed] [Google Scholar]
- 527.Sun HJ, Li P, Chen WW, Xiong XQ, Han Y. Angiotensin II and angiotensin-(1-7) in paraventricular nucleus modulate cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS One 7: e52557, 2012. doi: 10.1371/journal.pone.0052557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Sykes SD, Pringle KG, Zhou A, Dekker GA, Roberts CT, Lumbers ER; SCOPE Consortium . Fetal sex and the circulating renin-angiotensin system during early gestation in women who later develop preeclampsia or gestational hypertension. J Hum Hypertens 28: 133–139, 2014. doi: 10.1038/jhh.2013.51. [DOI] [PubMed] [Google Scholar]
- 529.Tabet JY, Meurin P, Ben Driss A, Logeart D, Héliès-Toussaint C, Tartière JM, Cohen-Solal A, Grynberg A, Bourdel-Marchasson I. Heart failure and cachexia. Arch Mal Coeur Vaiss 99: 1203–1209, 2006. [PubMed] [Google Scholar]
- 530.Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1-7). Hypertension 42: 574–579, 2003. doi: 10.1161/01.HYP.0000090322.55782.30. [DOI] [PubMed] [Google Scholar]
- 531.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289: H1560–H1566, 2005. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 532.Tallant EA, Ferrario CM, Gallagher PE. Cardioprotective role for angiotensin-(1-7) and angiotensin converting enzyme 2 in the heart. Future Cardiol 2: 335–342, 2006. doi: 10.2217/14796678.2.3.335. [DOI] [PubMed] [Google Scholar]
- 533.Tang A, Li C, Zou N, Zhang Q, Liu M, Zhang X. Angiotensin-(1-7) improves non-alcoholic steatohepatitis through an adiponectin-independent mechanism. Hepatol Res 47: 116–122, 2017. doi: 10.1111/hepr.12707. [DOI] [PubMed] [Google Scholar]
- 534.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009. doi: 10.1152/ajpregu.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Tao L, Qiu Y, Fu X, Lin R, Lei C, Wang J, Lei B. Angiotensin-converting enzyme 2 activator diminazene aceturate prevents lipopolysaccharide-induced inflammation by inhibiting MAPK and NF-κB pathways in human retinal pigment epithelium. J Neuroinflammation 13: 35, 2016. doi: 10.1186/s12974-016-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Tassone EJ, Sciacqua A, Andreozzi F, Presta I, Perticone M, Carnevale D, Casaburo M, Hribal ML, Sesti G, Perticone F. Angiotensin (1-7) counteracts the negative effect of angiotensin II on insulin signalling in HUVECs. Cardiovasc Res 99: 129–136, 2013. doi: 10.1093/cvr/cvt065. [DOI] [PubMed] [Google Scholar]
- 537.Thatcher SE, Zhang X, Howatt DA, Yiannikouris F, Gurley SB, Ennis T, Curci JA, Daugherty A, Cassis LA. Angiotensin-converting enzyme 2 decreases formation and severity of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 34: 2617–2623, 2014. doi: 10.1161/ATVBAHA.114.304613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res 107: 888–897, 2010. doi: 10.1161/CIRCRESAHA.110.219279. [DOI] [PubMed] [Google Scholar]
- 539.Tian J, Zhang L, Zhou Y, Xiao J, Li S, Chen Y, Qiao Z, Niu J, Gu Y. Angiotensin-(1-7) attenuates damage to podocytes induced by preeclamptic serum through MAPK pathways. Int J Mol Med 34: 1057–1064, 2014. doi: 10.3892/ijmm.2014.1870. [DOI] [PubMed] [Google Scholar]
- 540.Tigerstedt R, Bergman PG. Niere und Kreislauf. Acta Physiol (Oxf) 8: 223–271, 1898. [Google Scholar]
- 541.Tikellis C, Brown R, Head GA, Cooper ME, Thomas MC. Angiotensin-converting enzyme 2 mediates hyperfiltration associated with diabetes. Am J Physiol Renal Physiol 306: F773–F780, 2014. doi: 10.1152/ajprenal.00264.2013. [DOI] [PubMed] [Google Scholar]
- 542.Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 41: 392–397, 2003. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- 543.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 544.Tonnaer JA, Wiegant VM, de Jong W. Subcellular localization in rat brain of angiotensin I-generating endopeptidase activity distinct from cathepsin D. J Neurochem 38: 1356–1364, 1982. doi: 10.1111/j.1471-4159.1982.tb07913.x. [DOI] [PubMed] [Google Scholar]
- 545.Toton-Zuranska J, Gajda M, Pyka-Fosciak G, Kus K, Pawlowska M, Niepsuj A, Wolkow P, Olszanecki R, Jawien J, Korbut R. AVE 0991-angiotensin-(1-7) receptor agonist, inhibits atherogenesis in apoE-knockout mice. J Physiol Pharmacol 61: 181–183, 2010. [PubMed] [Google Scholar]
- 546.Tseng CT, Huang C, Newman P, Wang N, Narayanan K, Watts DM, Makino S, Packard MM, Zaki SR, Chan TS, Peters CJ. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J Virol 81: 1162–1173, 2007. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Tsuda K, Nishio I, Gironacci MM. Angiotensin-(1-7) and bradykinin in norepinephrine release in the central nervous system of hypertension. Hypertension 45: e8, 2005. doi: 10.1161/01.HYP.0000154192.64972.b8. [DOI] [PubMed] [Google Scholar]
- 548.Ueda S, Masumori-Maemoto S, Wada A, Ishii M, Brosnihan KB, Umemura S. Angiotensin(1-7) potentiates bradykinin-induced vasodilatation in man. J Hypertens 19: 2001–2009, 2001. doi: 10.1097/00004872-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 549.Uhal BD, Nguyen H, Dang M, Gopallawa I, Jiang J, Dang V, Ono S, Morimoto K. Abrogation of ER stress-induced apoptosis of alveolar epithelial cells by angiotensin 1-7. Am J Physiol Lung Cell Mol Physiol 305: L33–L41, 2013. doi: 10.1152/ajplung.00001.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Valdés G, Neves LAA, Anton L, Corthorn J, Chacón C, Germain AM, Merrill DC, Ferrario CM, Sarao R, Penninger J, Brosnihan KB. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 27: 200–207, 2006. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 551.Valdés G, Corthorn J, Bharadwaj MS, Joyner J, Schneider D, Brosnihan KB. Utero-placental expression of angiotensin-(1-7) and ACE2 in the pregnant guinea-pig. Reprod Biol Endocrinol 11: 5, 2013. doi: 10.1186/1477-7827-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Vallon V, Heyne N, Richter K, Khosla MC, Fechter K. [7-d-ALA]-angiotensin 1-7 blocks renal actions of angiotensin 1-7 in the anesthetized rat. J Cardiovasc Pharmacol 32: 164–167, 1998. doi: 10.1097/00005344-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 553.Vallon V, Richter K, Heyne N, Osswald H. Effect of intratubular application of angiotensin 1-7 on nephron function. Kidney Blood Press Res 20: 233–239, 1997. doi: 10.1159/000174151. [DOI] [PubMed] [Google Scholar]
- 554.Van der Wouden EA, Henning RH, Deelman LE, Roks AJ, Boomsma F, de Zeeuw D. Does angiotensin (1-7) contribute to the anti-proteinuric effect of ACE-inhibitors. J Renin Angiotensin Aldosterone Syst 6: 96–101, 2005. doi: 10.3317/jraas.2005.016. [DOI] [PubMed] [Google Scholar]
- 555.Van der Wouden EA, Ochodnický P, van Dokkum RP, Roks AJ, Deelman LE, de Zeeuw D, Henning RH. The role of angiotensin(1-7) in renal vasculature of the rat. J Hypertens 24: 1971–1978, 2006. doi: 10.1097/01.hjh.0000244945.42169.c0. [DOI] [PubMed] [Google Scholar]
- 556.Van Kats JP, Methot D, Paradis P, Silversides DW, Reudelhuber TL. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice. Direct and indirect effects of angiotensin II on the heart. J Biol Chem 276: 44012–44017, 2001. doi: 10.1074/jbc.M106132200. [DOI] [PubMed] [Google Scholar]
- 557.Van Rodijnen WF, van Lambalgen TA, Tangelder GJ, van Dokkum RP, Provoost AP, ter Wee PM. Reduced reactivity of renal microvessels to pressure and angiotensin II in fawn-hooded rats. Hypertension 39: 111–115, 2002. doi: 10.1161/hy1201.096817. [DOI] [PubMed] [Google Scholar]
- 558.Van Twist DJ, Houben AJ, de Haan MW, de Leeuw PW, Kroon AA. Renal hemodynamics and renin-angiotensin system activity in humans with multifocal renal artery fibromuscular dysplasia. J Hypertens 34: 1160–1169, 2016. doi: 10.1097/HJH.0000000000000917. [DOI] [PubMed] [Google Scholar]
- 559.Van Twist DJ, Houben AJ, De Haan MW, Mostard GJ, De Leeuw PW, Kroon AA. Angiotensin-(1-7)-induced renal vasodilation is reduced in human kidneys with renal artery stenosis. J Hypertens 32: 2428–2432, 2014. doi: 10.1097/HJH.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 560.Van Twist DJ, Houben AJ, de Haan MW, Mostard GJ, Kroon AA, de Leeuw PW. Angiotensin-(1-7)-induced renal vasodilation in hypertensive humans is attenuated by low sodium intake and angiotensin II co-infusion. Hypertension 62: 789–793, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01814. [DOI] [PubMed] [Google Scholar]
- 561.Varagic J, Ahmad S, Brosnihan KB, Groban L, Chappell MC, Tallant EA, Gallagher PE, Ferrario CM. Decreased cardiac Ang-(1-7) is associated with salt-induced cardiac remodeling and dysfunction. Ther Adv Cardiovasc Dis 4: 17–25, 2010. doi: 10.1177/1753944709353337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Vaughan ED Jr, Peach MJ. Letter: Regulation of aldosterone biosynthesis by “angiotensin III”. N Engl J Med 291: 1193, 1974. doi: 10.1056/NEJM197411282912220. [DOI] [PubMed] [Google Scholar]
- 563.Vaz-Silva J, Tavares RL, Ferreira MC, Honorato-Sampaio K, Cavallo IK, Santos RA, dos Reis AM, Reis FM. Tissue specific localization of angiotensin-(1-7) and its receptor Mas in the uterus of ovariectomized rats. J Mol Histol 43: 597–602, 2012. doi: 10.1007/s10735-012-9427-x. [DOI] [PubMed] [Google Scholar]
- 564.Velez JC, Janech MG, Hicks MP, Morinelli TA, Rodgers J, Self SE, Arthur JM, Fitzgibbon WR. Lack of renoprotective effect of chronic intravenous angiotensin-(1-7) or angiotensin-(2-10) in a rat model of focal segmental glomerulosclerosis. PLoS One 9: e110083, 2014. doi: 10.1371/journal.pone.0110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Velkoska E, Dean RG, Griggs K, Burchill L, Burrell LM. Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci (Lond) 120: 335–345, 2011. doi: 10.1042/CS20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Velkoska E, Patel SK, Burrell LM. Angiotensin converting enzyme 2 and diminazene: role in cardiovascular and blood pressure regulation. Curr Opin Nephrol Hypertens 25: 384–395, 2016. doi: 10.1097/MNH.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 567.Velloso EP, Vieira R, Cabral AC, Kalapothakis E, Santos RA. Reduced plasma levels of angiotensin-(1-7) and renin activity in preeclamptic patients are associated with the angiotensin I- converting enzyme deletion/deletion genotype. Braz J Med Biol Res 40: 583–590, 2007. doi: 10.1590/S0100-879X2007000400018. [DOI] [PubMed] [Google Scholar]
- 568.Verano-Braga T, Schwämmle V, Sylvester M, Passos-Silva DG, Peluso AA, Etelvino GM, Santos RA, Roepstorff P. Time-resolved quantitative phosphoproteomics: new insights into angiotensin-(1-7) signaling networks in human endothelial cells. J Proteome Res 11: 3370–3381, 2012. doi: 10.1021/pr3001755. [DOI] [PubMed] [Google Scholar]
- 569.Viana GE, Pereira VM, Honorato-Sampaio K, Oliveira CA, Santos RA, Reis AM. Angiotensin-(1-7) induces ovulation and steroidogenesis in perfused rabbit ovaries. Exp Physiol 96: 957–965, 2011. doi: 10.1113/expphysiol.2011.058453. [DOI] [PubMed] [Google Scholar]
- 570.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 571.Vilas-Boas WW, Ribeiro-Oliveira A Jr, Pereira RM, Ribeiro RC, Almeida J, Nadu AP, Simões e Silva AC, dos Santos RA. Relationship between angiotensin-(1-7) and angiotensin II correlates with hemodynamic changes in human liver cirrhosis. World J Gastroenterol 15: 2512–2519, 2009. doi: 10.3748/wjg.15.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Villela D, Leonhardt J, Patel N, Joseph J, Kirsch S, Hallberg A, Unger T, Bader M, Santos RA, Sumners C, Steckelings UM. Angiotensin type 2 receptor (AT2R) and receptor Mas: a complex liaison. Clin Sci (Lond) 128: 227–234, 2015. doi: 10.1042/CS20130515. [DOI] [PubMed] [Google Scholar]
- 573.Villela DC, Passos-Silva DG, Santos RA. Alamandine: a new member of the angiotensin family. Curr Opin Nephrol Hypertens 23: 130–134, 2014. doi: 10.1097/01.mnh.0000441052.44406.92. [DOI] [PubMed] [Google Scholar]
- 574.Von Bohlen und Halbach O, Albrecht D. The CNS renin-angiotensin system. Cell Tissue Res 326: 599–616, 2006. doi: 10.1007/s00441-006-0190-8. [DOI] [PubMed] [Google Scholar]
- 575.Von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther 121: 227–252, 2009. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 576.Wagenaar GT, Laghmani H, Fidder M, Sengers RM, de Visser YP, de Vries L, Rink R, Roks AJ, Folkerts G, Walther FJ. Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol 305: L341–L351, 2013. doi: 10.1152/ajplung.00360.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension 45: 960–966, 2005. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- 578.Walther T, Balschun D, Voigt JP, Fink H, Zuschratter W, Birchmeier C, Ganten D, Bader M. Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem 273: 11867–11873, 1998. doi: 10.1074/jbc.273.19.11867. [DOI] [PubMed] [Google Scholar]
- 579.Walther T, Voigt JP, Fink H, Bader M. Sex specific behavioural alterations in Mas-deficient mice. Behav Brain Res 107: 105–109, 2000. doi: 10.1016/S0166-4328(99)00115-1. [DOI] [PubMed] [Google Scholar]
- 580.Wang J, Liu R, Qi H, Wang Y, Cui L, Wen Y, Li H, Yin C. The ACE2-angiotensin-(1-7)-Mas axis protects against pancreatic cell damage in cell culture. Pancreas 44: 266–272, 2015. doi: 10.1097/MPA.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 581.Wang J, Peng YJ, Zhu DN. Amino acids modulate the hypotensive effect of angiotensin-(1-7) at the caudal ventrolateral medulla in rats. Regul Pept 129: 1–7, 2005. doi: 10.1016/j.regpep.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 582.Wang J, Shen LL, Cao YX, Zhu DN. Effects of electroacupuncture on pressor response to angiotensin-(1-7) by amino acid release in the rostral ventrolateral medulla. Acupunct Electrother Res 28: 25–34, 2003. doi: 10.3727/036012903815901679. [DOI] [PubMed] [Google Scholar]
- 583.Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, Ludin JA, Oh SP, Katovich MJ, Frazier CJ, Raizada MK, Krause EG. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology 105: 114–123, 2016. doi: 10.1016/j.neuropharm.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Wang L, Liang J, Leung PS. The ACE2/Ang-(1-7)/Mas Axis Regulates the Development of Pancreatic Endocrine Cells in Mouse Embryos. PLoS One 10: e0128216, 2015. doi: 10.1371/journal.pone.0128216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Wang LJ, He JG, Ma H, Cai YM, Liao XX, Zeng WT, Liu J, Wang LC. Chronic administration of angiotensin-(1-7) attenuates pressure-overload left ventricular hypertrophy and fibrosis in rats. Di Yi Jun Yi Da Xue Xue Bao 25: 481–487, 2005. [PubMed] [Google Scholar]
- 586.Wang W, Bodiga S, Das SK, Lo J, Patel V, Oudit GY. Role of ACE2 in diastolic and systolic heart failure. Heart Fail Rev 17: 683–691, 2012. doi: 10.1007/s10741-011-9259-x. [DOI] [PubMed] [Google Scholar]
- 587.Wang W, Patel VB, Parajuli N, Fan D, Basu R, Wang Z, Ramprasath T, Kassiri Z, Penninger JM, Oudit GY. Heterozygote loss of ACE2 is sufficient to increase the susceptibility to heart disease. J Mol Med (Berl) 92: 847–858, 2014. doi: 10.1007/s00109-014-1149-y. [DOI] [PubMed] [Google Scholar]
- 588.Wang Y, Pringle KG, Chen YX, Zakar T, Lumbers ER. Regulation of the renin-angiotensin system (RAS) in BeWo and HTR-8/SVneo trophoblast cell lines. Placenta 33: 634–639, 2012. doi: 10.1016/j.placenta.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 589.Wang Y, Qian C, Roks AJ, Westermann D, Schumacher SM, Escher F, Schoemaker RG, Reudelhuber TL, van Gilst WH, Schultheiss HP, Tschöpe C, Walther T. Circulating rather than cardiac angiotensin-(1-7) stimulates cardioprotection after myocardial infarction. Circ Heart Fail 3: 286–293, 2010. doi: 10.1161/CIRCHEARTFAILURE.109.905968. [DOI] [PubMed] [Google Scholar]
- 590.Ward PE, Russell JS, Vaghy PL. Angiotensin and bradykinin metabolism by peptidases identified in skeletal muscle. Peptides 16: 1073–1078, 1995. doi: 10.1016/0196-9781(95)00082-U. [DOI] [PubMed] [Google Scholar]
- 591.Warner FJ, Lubel JS, McCaughan GW, Angus PW. Liver fibrosis: a balance of ACEs? Clin Sci (Lond) 113: 109–118, 2007. doi: 10.1042/CS20070026. [DOI] [PubMed] [Google Scholar]
- 592.Welches WR, Santos RA, Chappell MC, Brosnihan KB, Greene LJ, Ferrario CM. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J Hypertens 9: 631–638, 1991. doi: 10.1097/00004872-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 593.Whitaker AM, Molina PE. Angiotensin (1-7) contributes to nitric oxide tonic inhibition of vasopressin release during hemorrhagic shock in acute ethanol intoxicated rodents. Life Sci 93: 623–629, 2013. doi: 10.1016/j.lfs.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension 40: 847–852, 2002. doi: 10.1161/01.HYP.0000037979.53963.8F. [DOI] [PubMed] [Google Scholar]
- 595.Willette RN, Krieger AJ, Barcas PP, Sapru HN. Medullary gamma-aminobutyric acid (GABA) receptors and the regulation of blood pressure in the rat. J Pharmacol Exp Ther 226: 893–899, 1983. [PubMed] [Google Scholar]
- 596.Wilsdorf T, Gainer JV, Murphey LJ, Vaughan DE, Brown NJ. Angiotensin-(1-7) does not affect vasodilator or TPA responses to bradykinin in human forearm. Hypertension 37: 1136–1140, 2001. doi: 10.1161/01.HYP.37.4.1136. [DOI] [PubMed] [Google Scholar]
- 597.Wilson BA, Cruz-Diaz N, Marshall AC, Pirro NT, Su Y, Gwathmey TM, Rose JC, Chappell MC. An angiotensin-(1-7) peptidase in the kidney cortex, proximal tubules, and human HK-2 epithelial cells that is distinct from insulin-degrading enzyme. Am J Physiol Renal Physiol 308: F594–F601, 2015. doi: 10.1152/ajprenal.00609.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438–451, 2007. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598a.Wösten-van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic J, Florquin S, Bos AP. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol 225: 618–627, 2011. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 599.Wright JW, Harding JW. Brain renin-angiotensin–a new look at an old system. Prog Neurobiol 95: 49–67, 2011. doi: 10.1016/j.pneurobio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 600.Wu J, Zhao D, Wu S, Wang D. Ang-(1-7) exerts protective role in blood-brain barrier damage by the balance of TIMP-1/MMP-9. Eur J Pharmacol 748: 30–36, 2015. doi: 10.1016/j.ejphar.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 601.Wu JG, Tang H, Liu ZJ, Ma ZF, Tang AL, Zhang XJ, Gao XR, Ma H. Angiotensin-(1-7) inhibits vascular remodelling in rat jugular vein grafts via reduced ERK1/2 and p38 MAPK activity. J Int Med Res 39: 2158–2168, 2011. doi: 10.1177/147323001103900612. [DOI] [PubMed] [Google Scholar]
- 602.Wu KK. Regulation of endothelial nitric oxide synthase activity and gene expression. Ann N Y Acad Sci 962: 122–130, 2002. doi: 10.1111/j.1749-6632.2002.tb04062.x. [DOI] [PubMed] [Google Scholar]
- 603.Wysocki J, Ortiz-Melo DI, Mattocks NK, Xu K, Prescott J, Evora K, Ye M, Sparks MA, Haque SK, Batlle D, Gurley SB. ACE2 deficiency increases NADPH-mediated oxidative stress in the kidney. Physiol Rep 2: e00264, 2014. doi: 10.1002/phy2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Xia H, Suda S, Bindom S, Feng Y, Gurley SB, Seth D, Navar LG, Lazartigues E. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS One 6: e22682, 2011. doi: 10.1371/journal.pone.0022682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension 58: 1057–1065, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Xie W, Zhu D, Ji L, Tian M, Xu C, Shi J. Angiotensin-(1-7) improves cognitive function in rats with chronic cerebral hypoperfusion. Brain Res 1573: 44–53, 2014. doi: 10.1016/j.brainres.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 608.Xing J, Kong J, Lu J, Li J. Angiotensin-(1-7) inhibits neuronal activity of dorsolateral periaqueductal gray via a nitric oxide pathway. Neurosci Lett 522: 156–161, 2012. doi: 10.1016/j.neulet.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Xing J, Lu J, Li J. Role of angiotensin-(1-7) and Mas-R-nNOS pathways in amplified neuronal activity of dorsolateral periaqueductal gray after chronic heart failure. Neurosci Lett 563: 6–11, 2014. doi: 10.1016/j.neulet.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Xu C, Ding W, Zhang M, Gu Y. Protective effects of angiotensin-(1-7) administrated with an angiotensin-receptor blocker in a rat model of chronic kidney disease. Nephrology (Carlton) 18: 761–769, 2013. doi: 10.1111/nep.12146. [DOI] [PubMed] [Google Scholar]
- 611.Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, Santos SS, Luft FC, Bader M, Gross V, Alenina N, Santos RA. Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension 51: 574–580, 2008. doi: 10.1161/HYPERTENSIONAHA.107.102764. [DOI] [PubMed] [Google Scholar]
- 612.Xu X, Quiambao AB, Roveri L, Pardue MT, Marx JL, Röhlich P, Peachey NS, Al-Ubaidi MR. Degeneration of cone photoreceptors induced by expression of the Mas1 protooncogene. Exp Neurol 163: 207–219, 2000. doi: 10.1006/exnr.2000.7370. [DOI] [PubMed] [Google Scholar]
- 613.Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am J Physiol Heart Circ Physiol 307: H191–H198, 2014. doi: 10.1152/ajpheart.01012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Xue B, Zhang Z, Johnson RF, Guo F, Hay M, Johnson AK. Central endogenous angiotensin-(1-7) protects against aldosterone/NaCl-induced hypertension in female rats. Am J Physiol Heart Circ Physiol 305: H699–H705, 2013. doi: 10.1152/ajpheart.00193.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Xue H, Zhou L, Yuan P, Wang Z, Ni J, Yao T, Wang J, Huang Y, Yu C, Lu L. Counteraction between angiotensin II and angiotensin-(1-7) via activating angiotensin type I and Mas receptor on rat renal mesangial cells. Regul Pept 177: 12–20, 2012. doi: 10.1016/j.regpep.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 616.Yamaleyeva LM, Merrill DC, Ebert TJ, Smith TL, Mertz HL, Brosnihan KB. Hemodynamic responses to angiotensin-(1-7) in women in their third trimester of pregnancy. Hypertens Pregnancy 33: 375–388, 2014. doi: 10.3109/10641955.2014.911884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 47: 718–726, 2006. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 618.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49: 926–931, 2007. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 619.Yang HY, Erdös EG, Chiang TS. New enzymatic route for the inactivation of angiotensin. Nature 218: 1224–1226, 1968. doi: 10.1038/2181224a0. [DOI] [PubMed] [Google Scholar]
- 620.Yang XH, Deng W, Tong Z, Liu YX, Zhang LF, Zhu H, Gao H, Huang L, Liu YL, Ma CM, Xu YF, Ding MX, Deng HK, Qin C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med 57: 450–459, 2007. [PubMed] [Google Scholar]
- 621.Yang XH, Wang YH, Wang JJ, Liu YC, Deng W, Qin C, Gao JL, Zhang LY. Role of angiotensin-converting enzyme (ACE and ACE2) imbalance on tourniquet-induced remote kidney injury in a mouse hindlimb ischemia-reperfusion model. Peptides 36: 60–70, 2012. doi: 10.1016/j.peptides.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 622.Ye M, Wysocki J, Gonzalez-Pacheco FR, Salem M, Evora K, Garcia-Halpin L, Poglitsch M, Schuster M, Batlle D. Murine recombinant angiotensin-converting enzyme 2: effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension 60: 730–740, 2012. doi: 10.1161/HYPERTENSIONAHA.112.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Yoshikawa N, Yoshikawa T, Hill T, Huang C, Watts DM, Makino S, Milligan G, Chan T, Peters CJ, Tseng CT. Differential virological and immunological outcome of severe acute respiratory syndrome coronavirus infection in susceptible and resistant transgenic mice expressing human angiotensin-converting enzyme 2. J Virol 83: 5451–5465, 2009. doi: 10.1128/JVI.02272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45: 711–719, 1986. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 625.Yuan L, Li Y, Li G, Song Y, Gong X. Ang(1-7) treatment attenuates β-cell dysfunction by improving pancreatic microcirculation in a rat model of Type 2 diabetes. J Endocrinol Invest 36: 931–937, 2013. doi: 10.3275/8951. [DOI] [PubMed] [Google Scholar]
- 626.Zambelli V, Bellani G, Borsa R, Pozzi F, Grassi A, Scanziani M, Castiglioni V, Masson S, Decio A, Laffey JG, Latini R, Pesenti A. Angiotensin-(1-7) improves oxygenation, while reducing cellular infiltrate and fibrosis in experimental Acute Respiratory Distress Syndrome. Intensive Care Med Exp 3: 44, 2015. doi: 10.1186/s40635-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Zeng W, Chen W, Leng X, He JG, Ma H. Chronic angiotensin-(1-7) administration improves vascular remodeling after angioplasty through the regulation of the TGF-beta/Smad signaling pathway in rabbits. Biochem Biophys Res Commun 389: 138–144, 2009. doi: 10.1016/j.bbrc.2009.08.112. [DOI] [PubMed] [Google Scholar]
- 628.Zeng WT, Chen WY, Leng XY, Tang LL, Sun XT, Li CL, Dai G. Impairment of cardiac function and remodeling induced by myocardial infarction in rats are attenuated by the nonpeptide angiotensin-(1-7) analog AVE 0991. Cardiovasc Ther 30: 152–161, 2012. doi: 10.1111/j.1755-5922.2010.00255.x. [DOI] [PubMed] [Google Scholar]
- 629.Zhang D, Xiao Q, Luo H, Zhao K. Effects of angiotensin-(1-7) on hippocampal expressions of GFAP and GDNF and cognitive function in rats with diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao 35: 646–651, 2015. [PubMed] [Google Scholar]
- 630.Zhang F, Ren J, Chan K, Chen H. Angiotensin-(1-7) regulates Angiotensin II-induced VCAM-1 expression on vascular endothelial cells. Biochem Biophys Res Commun 430: 642–646, 2013. doi: 10.1016/j.bbrc.2012.11.098. [DOI] [PubMed] [Google Scholar]
- 631.Zhang J, Noble NA, Border WA, Huang Y. Infusion of angiotensin-(1-7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am J Physiol Renal Physiol 298: F579–F588, 2010. doi: 10.1152/ajprenal.00548.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Zhang LL, Huang S, Ma XX, Zhang WY, Wang D, Jin SY, Zhang YP, Li Y, Li X. Angiotensin(1-7) attenuated Angiotensin II-induced hepatocyte EMT by inhibiting NOX-derived H2O2-activated NLRP3 inflammasome/IL-1β/Smad circuit. Free Radic Biol Med 97: 531–543, 2016. doi: 10.1016/j.freeradbiomed.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 633.Zhang T, Li Z, Dang H, Chen R, Liaw C, Tran TA, Boatman PD, Connolly DT, Adams JW. Inhibition of Mas G-protein signaling improves coronary blood flow, reduces myocardial infarct size, and provides long-term cardioprotection. Am J Physiol Heart Circ Physiol 302: H299–H311, 2012. doi: 10.1152/ajpheart.00723.2011. [DOI] [PubMed] [Google Scholar]
- 634.Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, Liu Y, Tong Q. Central administration of angiotensin-(1-7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides 42: 593–600, 2008. doi: 10.1016/j.npep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 635.Zheng H, Liu X, Patel KP. Angiotensin-converting enzyme 2 overexpression improves central nitric oxide-mediated sympathetic outflow in chronic heart failure. Am J Physiol Heart Circ Physiol 301: H2402–H2412, 2011. doi: 10.1152/ajpheart.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Zheng J, Li G, Chen S, Bihl J, Buck J, Zhu Y, Xia H, Lazartigues E, Chen Y, Olson JE. Activation of the ACE2/Ang-(1-7)/Mas pathway reduces oxygen-glucose deprivation-induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience 273: 39–51, 2014. doi: 10.1016/j.neuroscience.2014.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Zheng JL, Li GZ, Chen SZ, Wang JJ, Olson JE, Xia HJ, Lazartigues E, Zhu YL, Chen YF. Angiotensin converting enzyme 2/Ang-(1-7)/Mas axis protects brain from ischemic injury with a tendency of age-dependence. CNS Neurosci Ther 20: 452–459, 2014. doi: 10.1111/cns.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Zheng Y, Tang L, Huang W, Yan R, Ren F, Luo L, Zhang L. Anti-Inflammatory Effects of Ang-(1-7) in Ameliorating HFD-Induced Renal Injury through LDLr-SREBP2-SCAP Pathway. PLoS One 10: e0136187, 2015. doi: 10.1371/journal.pone.0136187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Zhou L, Xue H, Wang Z, Ni J, Yao T, Huang Y, Yu C, Lu L. Angiotensin-(1-7) attenuates high glucose-induced proximal tubular epithelial-to-mesenchymal transition via inhibiting ERK1/2 and p38 phosphorylation. Life Sci 90: 454–462, 2012. doi: 10.1016/j.lfs.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 640.Zhou LM, Shi Z, Gao J, Han Y, Yuan N, Gao XY, Zhu GQ. Angiotensin-(1-7) and angiotension II in the rostral ventrolateral medulla modulate the cardiac sympathetic afferent reflex and sympathetic activity in rats. Pflugers Arch 459: 681–688, 2010. doi: 10.1007/s00424-010-0793-5. [DOI] [PubMed] [Google Scholar]
- 641.Zhou P, Cheng CP, Li T, Ferrario CM, Cheng HJ. Modulation of cardiac L-type Ca2+ current by angiotensin-(1-7): normal versus heart failure. Ther Adv Cardiovasc Dis 9: 342–353, 2015. doi: 10.1177/1753944715587424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Zhu Z, Zhong J, Zhu S, Liu D, Van Der Giet M, Tepel M. Angiotensin-(1-7) inhibits angiotensin II-induced signal transduction. J Cardiovasc Pharmacol 40: 693–700, 2002. doi: 10.1097/00005344-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 643.Zimmerman D, Burns KD. Angiotensin-(1-7) in kidney disease: a review of the controversies. Clin Sci (Lond) 123: 333–346, 2012. doi: 10.1042/CS20120111. [DOI] [PubMed] [Google Scholar]
- 644.Zimmerman DL, Zimpelmann J, Xiao F, Gutsol A, Touyz R, Burns KD. The effect of angiotensin-(1-7) in mouse unilateral ureteral obstruction. Am J Pathol 185: 729–740, 2015. doi: 10.1016/j.ajpath.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 645.Zimpelmann J, Burns KD. Angiotensin-(1-7) activates growth-stimulatory pathways in human mesangial cells. Am J Physiol Renal Physiol 296: F337–F346, 2009. doi: 10.1152/ajprenal.90437.2008. [DOI] [PubMed] [Google Scholar]
- 646.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme homologue ACE2. Circulation 108: 1707–1712, 2003. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 647.Zisman LS, Meixell GE, Bristow MR, Canver CC. Angiotensin-(1-7) formation in the intact human heart: in vivo dependence on angiotensin II as substrate. Circulation 108: 1679–1681, 2003. doi: 10.1161/01.CIR.0000094733.61689.D4. [DOI] [PubMed] [Google Scholar]
- 648.Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, Ju X, Liang Z, Liu Q, Zhao Y, Guo F, Bai T, Han Z, Zhu J, Zhou H, Huang F, Li C, Lu H, Li N, Li D, Jin N, Penninger JM, Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun 5: 3594, 2014. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 126: 695–706, 2014. doi: 10.1042/CS20130294. [DOI] [PMC free article] [PubMed] [Google Scholar]