Abstract
Introduction
According to the Barcelona Clinic Liver Cancer (BCLC) algorithm, transarterial chemoembolization (TACE) is recommended in patients with hepatocellular carcinoma (HCC) of intermediate stage (BCLC-B), whereas partial hepatectomy (PH) is restricted to early stage A. Expanding the indication for PH to intermediate stage remains debated.
Objective
This meta-analysis aimed to analyze short- and long-term outcomes of PH compared to TACE in patients with intermediate-stage HCC.
Methods
A meta-analysis was conducted according to PRISMA guidelines. Trials comparing PH with TACE in patients with intermediate-stage HCC were selected. Only patients of BCLC-B stage were included in the analyses. Primary endpoint was overall survival (OS) and secondary endpoint was 90-day postprocedural mortality. Random-effects models were used to analyze time ratios (TRs).
Results
Seven eligible trials were analyzed, including 1,730 BCLC-B patients undergoing PH (n = 750) or TACE (n = 980). Comparison of OS between PH and TACE determined a pooled TR of 1.91 (95% CI 1.24–2.94; p < 0.001). Survival rates at 1-, 3-, and 5-year were 85, 60, and 42% after PH, compared to 73, 60, and 20% after TACE (p < 0.001). There was no difference in postprocedural mortality between PH and TACE with rates of 3.7 and 3.4%, respectively (TR 0.95; 95% CI 0.17–5.50; p = 0.879).
Conclusions
In patients with intermediate HCC, PH was associated with increased long-term survival compared to TACE, with comparable postprocedural mortality. These results suggest considering PH as treatment option in intermediate HCC and highlight the urgent need to refine the selection of patients with BCLC-B stage who may benefit from PH.
Keywords: Liver cancer, Barcelona Clinic Liver Cancer, Resection, Surgery, Transarterial chemoembolization, Loco-regional therapy
Introduction
Responsible for 750,000 new cases each year worldwide, primary liver cancer has shown a worrisome epidemiological progression during the last 2 decades. In the United States, it affects 42,000 new patients annually and became the fastest growing malignancy in term of cancer-related mortality [1, 2]. Hepatocellular carcinoma (HCC), the most common form of liver cancer, is characterized by an aggressive biology and dismal outcomes [3].
European (EASL) [4] and previous American (AASLD) [5] consensus guidelines for the treatment of HCC endorse the Barcelona Clinic Liver Cancer (BCLC) algorithm [6]. According to these clinical practices guidelines, partial hepatectomy (PH) should only be undertaken in patients at early stage (BCLC-A), while patients at intermediate stage (BCLC-B) should be treated with transarterial chemoembolization (TACE). In the Asian guidelines [7, 8, 9], the role of PH has been extended to a broader subgroup of HCC patients with acceptable short- and long- term outcomes, and the latest AASLD guidelines also nuanced their definition of resectability [10].
Recent data have suggested higher survival rates in subgroups of HCC patients of BCLC-B stage treated with PH, as opposed to TACE [11, 12]. Subsequently, meta-analyses have compared PH and TACE in intermediate-stage HCC patients [13, 14, 15]. Unfortunately, these meta-analyses included a substantial proportion of HCC patients with large single tumors who were classified as BCLC-B stage although BCLC classification categorizes these patients in the early-stage (BCLC-A) [4, 16], for whom the benefit of surgery is not debated. Therefore, the question of PH versus TACE in intermediate HCC remains unsolved [17].
To overcome this issue, the present study aimed to analyze and compare short- and long-term outcomes of PH and TACE in HCC patients with strict BCLC-B stage.
Methods
Systematic review and meta-analysis were conducted according to PRISMA guidelines [18].
Systematic Review of the Literature
A comprehensive systematic review of the literature was performed throughout Medline Ovid SP, PubMed, Embase.com, Cochrane Central Register of Controlled Trials − Wiley, Web of Science − Core collection, and Google Scholar to identify studies comparing PH and TACE in patients with intermediate-stage HCC. Search was limited to full-text manuscripts published in English, until March 12th, 2018. Main medical subject headings used were: “HCC,” “hepatectomy,” and “chemoembolization.” Detailed search strategy and algorithms with proper syntax adapted to each database are provided in online supplementary Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000505093).
Study Selection, Data Extraction, and Quality Assessment
Two investigators (D.M. and D.C.) independently assessed the eligibility of publications for selection, according to criteria of inclusion and exclusion (online suppl. Table 2). In case of disagreement, a consensus was obtained after discussion with coinvestigators (I.L. and E.M.).
Some studies included a subset of patients with single large HCC; these patients were considered as BCLC-A, and therefore excluded from the analysis [4, 16].
The following data were extracted from each selected study: design, location, number of HCC patients (total, BCLC-B, treated with PH or TACE), age (years), proportions of male and Hepatitis B virus (HBV) positive patients, median number and size of nodules, AFP level (ng/mL), as well as main findings of the study. We used the modified Newcastle-Ottawa score to estimate the risk of bias of nonrandomized controlled trials (NRCT) [19].
Variables and Endpoints of Interest
The BCLC classification was followed to stratify and select patients with intermediate HCC (B stage according to BCLC classification) [6]. Studies using alternative classification, such as Hong Kong Liver Cancer staging [20], were not included.
Primary endpoint was overall survival (OS), and secondary endpoint was postprocedural 90-day mortality [21]. Studies with unavailable survival data for BCLC-B patients were also excluded.
Statistical Analysis
Following Guyot et al. [22], based on the “DigitizeIt” software (https://www.digitizeit.de/) and “ipdfc” Stata command [23], the Kaplan-Meier data were reconstructed for each treatment arm and study. Quality of the reconstruction was assessed by visually comparing the Kaplan-Meier survival curves. At least 4 of the selected studies exhibited clear nonproportional hazards (e.g., the survival curves were crossing). Therefore, analysis based on the Cox model was not appropriate. Instead, we used parametric mixed effects (or multi-level) accelerated failure time (AFT) models [24]. In the AFT metric, the effect size is measured in terms of a time ratio (TR; or percentile ratio [25]), which can be interpreted as the ratio of the median survival time in the first arm (e.g., TACE in the present study) over the second arm (e.g., surgery). To estimate TR for each study, we adopted an Empirical Bayes approach and the mean of the distribution was used as an overall estimate. We investigated the goodness of fit of various mixed effects AFT model distributions: Gamma, Loglogistic, Weibull, and selected the best fitting one based on the Akaike criterion, as well as visual inspection of the agreement between the Kaplan-Meier curves and the predicted survival curves from the parametric model (online suppl. Fig. 1).
This one-stage approach has been contrasted with the more commonly used 2-stage approach by Der Simonian and Laird, which first estimates the TR for each study and thereafter pools the estimates. The former has the advantage of using a more exact likelihood than the latter and allows one to compute marginal survival curves in addition to TR [26]. Moreover, heterogeneity is likely to be better estimated and accounted for by the one-stage approach. Nevertheless, as a sensitivity analysis, we also performed a 2-stage analysis using the flexible parametric 3-parameter Gamma AFT model to compute the TRs (online suppl. Fig. 2, 3).
In addition, as a third alternative, we investigated another approach based on nonproportional flexible parametric models, which allows time-dependent hazard ratios (HRs) [27] (results not shown). Despite its extremely great flexibility, this approach provides less readily interpretable results as HRs vary with time and a HR curve is estimated in each study (i.e., the effect size is not a single fixed quantity but rather a curve). On the other hand, AFT metric computes a single fixed-effect size per study, and heterogeneity is more readily assessed.
Due to the heterogeneity, a summary prediction interval (line) was computed and graphed on the forest plots, in addition to the mean and its CI (diamond). When there is heterogeneity, the prediction interval allows one to predict the likely impact of the intervention when applied to the target population.
Sensitivity analyses were conducted, by excluding each study, sequentially. Overall mean TR obtained were graphed on a forest plot along with the overall mean TR computed without exclusion.
Statistical analyses were performed using the Stata 15.1 statistical package (Stata Corporation, College Station, Texas, TX, USA).
Results
Literature Review and Selection of Studies
A thorough search strategy identified 1,511 studies, after duplicates removal (online suppl. Fig. 4). After initial screening, 116 full-text manuscripts were evaluated for eligibility. A total of 109 articles were excluded based on the selection criteria. Seven studies comparing PH and TACE in HCC patients with BCLC-B stage were included in the meta-analysis.
Characteristics or Selected Studies
Seven trials (1 RCT, 1 propensity score matching-NRCT, 5 NRCT) [11, 12, 28, 29, 30, 31, 32] were analyzed, totalizing 3,174 HCC patients. Four studies only included patients with BCLC stage B HCC [11, 12, 31, 32] while 3 trials included a subset of patients with stage A [28, 29, 30]. After strict selection of BCLC-B HCC patients, a total of 1,730 patients were included for final analysis, including 750 and 980 patients treated with PH and TACE, respectively (Table 1). Selected articles were published between 2009 and 2017, with a majority of studies conducted in Asia. Included patients showed preponderance for being male, around 60 years, and HBV positive. Six studies reported increased survival rates after PH compared to TACE [11, 12, 28, 29, 30, 32], whereas one trial did not demonstrate difference in 5-year OS [31].
Table 1.
Characteristics of selected studies
| Study | Design | Location | HCC patients, n |
Patients characteristics |
Tumors characteristics |
Main findings | |||
| total | BCLC-B* | age, years | gender, male, % | n | size, cm | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ho et al. [28], 2009 | NRCT | Taipei, Taiwan | 1,065 | 285 | 59±12 | 81 | ≥2: 100% | 5–7 | OS at 1-, 3-, 5-year, PH 77, 52, 37% vs. TACE = 63, 25, 11% [HR 1.61; 95% CI 1.30–2.01 p < 0.001] |
| Zhonget al. [29], 2013 | NRCT | Guangxi, China | 392 | 89 | 48±12 | 92 | >1: 23% | 9 | OS at 1-, 3-, 5-year, PH 84, 59, 37% vs. TACE = 69, 29, 14% [HR 2.15; 95% CI 1.59–2.90 p < 0.001] |
| Jianyong et al. [30], 2014 | NRCT | Chengdu, China | 923 | 562 | 53±10 | 72 | 2–3: 38% >3: 23% |
7 | OS at 1-, 3-, 5-year, PH 84, 71, 61% vs. TACE = 85, 62, 45% [HR 2.22; 95% CI 1.78–3.02 p < 0.001) |
| Yin et al. [11], 2014 | RCT | Shangai, China | 173 | 173 | 53±9 | 93 | 2: 60% >2: 40% |
10 | OS at 1-, 3-, 5-year, PH 76, 64, 52% vs. TACE = 52, 35, 18% [HR 2.32; 95% CI 1.56–3.45 p < 0.001) |
| Ciria et al. [31], 2015 | NRCT | Cordoba, Spain | 80 | 80 | 66±10 | 76 | 2 | 6.9 | OS at 1-, 3-, 5-year, PH 83, 53, 44% vs. TACE = 68, 48, 39% No difference in 5-years OS (p = 0.229) |
| Kim et al. [32], 2016 | NRCT | Seoul, Korea | 277 | 277 | 57–58 | 87 | 2: 48% >2: 52% |
<5: 54% >5: 46% | OS at 1-, 3-, 5-year, PH 92, 65, 52% vs. TACE = 78, 39, 28% [HR 1.64; 95% CI 1.07–2.50 p = 0.021) |
| Tada et al. [12], 2017 | PSM-NRCT | Multicenter, Japan | 264 | 264 | 69±6 | 83 | 2–3 | 4–4.6 | OS at 3-, 5-, 7-year, PH 63, 53, 38% vs. TACE = 53, 34, 25% [HR 1.10; 95% CI 1.10–2.86; p = 0.012) |
| Overall | 1 RCT 1 PSM-NRCT 5 NRCT |
Asia: 6 Europe: 1 |
3,174 | 1,730 | 48–69 | 72–93 | na | 5–10 | Consistent increased survival rates after PH, compared to TACE |
Patients included in the present meta-analysis. AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HR, hazard ratio; na, not available; NRCT, nonrandomized controlled trial; OS, overall survival; PH, partial hepatectomy; PSM, propensity score matching; RCT, randomized controlled trial; TACE, transarterial chemoembolization.
Risk of bias of NRCT was assessed with modified Newcastle-Ottawa score, showing low to intermediate risk (online suppl. Table 3).
Primary Endpoint: Overall Survival
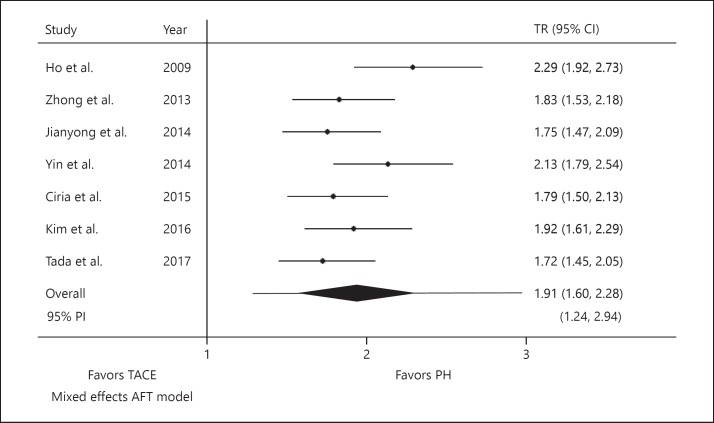
Pooled analysis showed near doubling of OS in patients undergoing PH, as opposed to TACE (TR 1.91; 95% PI 1.24–2.94; p < 0.001; Fig. 1). The estimated between-study variance (i.e., amount of heterogeneity) was 0.23 on the log-time scale.
Fig. 1.
Pooled TR for OS. For individual studies, lines indicate CIs of TR. For pooled effect (i.e., Overall), line represents the PI and diamond illustrates CI around the estimated mean effect. PH, partial hepatectomy; PI, prediction interval; TACE, transarterial chemoembolization; TR, time ratio; AFT, accelerated failure time.
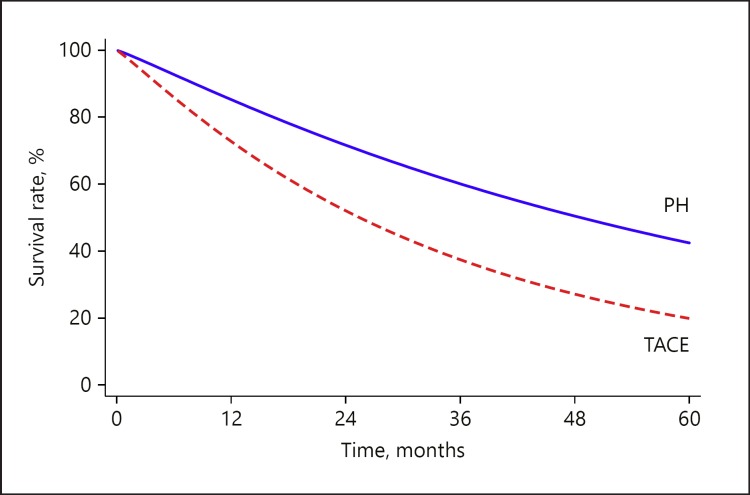
The marginal survival curves of PH and TACE, pooling all of the included studies, are provided in Figure 2. Median survival was 53 (95% CI 37–68) and 28 (95% CI 20–35) months after PH and TACE, respectively (p < 0.001). Survival rates at 1-, 3-, and 5-year were 85, 60, and 42% after PH, compared to 73, 60, and 20% after TACE (p < 0.001).
Fig. 2.
Pooled marginal survival curves of PH and TACE. PH, partial hepatectomy; TACE, transarterial chemoembolization.
Secondary Endpoint: Postprocedural Mortality
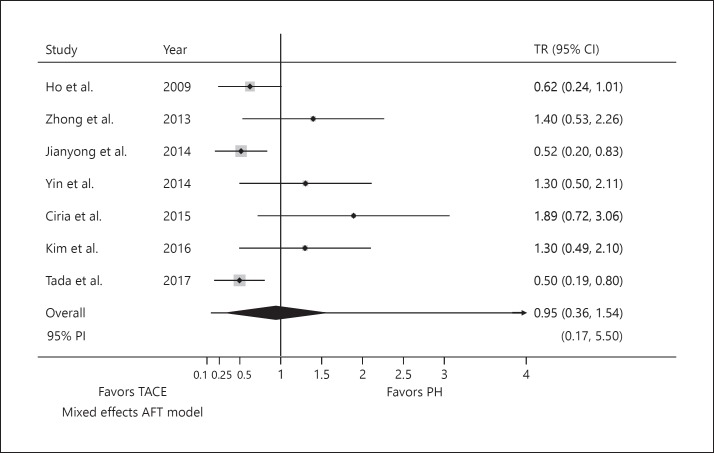
No difference was identified in postprocedural mortality at 90 day between PH and TACE, with mortality rates of 3.7 and 3.4%, respectively (TR 0.95; 95% PI 0.17–5.50; p = 0.879; Fig. 3). Again, the estimated between-study variance was 0.41.
Fig. 3.
Pooled TR for postprocedural mortality. For individual studies, lines indicate confidence intervals of TR. For pooled effect (i.e., overall), line represents the PI and diamond illustrates CI around the estimated mean effect. PH, partial hepatectomy; PI, prediction interval; TACE, transarterial chemoembolization; TR, time ratio; AFT, accelerated failure time.
Sensitivity Analyses
Sensitivity analyses with sequential exclusion of studies are detailed in online supplementary Figure 2. Results remained consistent with mean TR varying between 1.78 and 2.00 (95% CI 1.54–2.41). Moreover, the 2-stage analysis using the flexible parametric 3-parameter Gamma AFT model provided very similar results as the one-stage (TR 1.89; 95% PI 1.22–2.49; p < 0.001) except regarding the heterogeneity estimate, which was substantially smaller (estimated between-study variance = 0.006) online supplementary Figure 2. Goodness of fit is provided in online supplementary Figure 1.
Discussion
This study is the first meta-analysis exploring long-term survival after PH and TACE in intermediate-stage HCC (BCLC-B), excluding patients with single large nodule. PH was associated with a 1.9-fold increased survival over TACE with comparable postprocedural mortality.
Current management of HCC lacks clear standardized consensus, resulting in important variations across different guidelines, particularly when comparing Eastern and Western recommendations [33, 34, 35]. To date, BCLC classification remains the most widely used treatment algorithm for HCC in Western countries, endorsed by both EASL and previous AASLD guidelines. While recommendations for patients at early (BCLC-A) and advanced (BCLC-C) stages are less debated, therapeutic strategies for intermediate stage (BCLC-B) remain controversial. This controversy arises from the heterogeneity of patients grouped together into BCLC-B. To overcome this issue, a consensus suggested to substratify BCLC-B stage in 4 subgroups [36]. Albeit being of interest, this subclassification is rarely used in clinical practice.
Over recent years, the universal role of TACE in BCLC-B has been questioned [37], with studies including a randomized controlled trial suggesting increased survival after PH compared to TACE in well-defined subgroups [11]. Previous meta-analyses have compared PH and TACE in BCLC-B HCC [13, 14, 15], but they included a significant proportion of solitary large HCC (>5 cm) that were erroneously classified as BCLC-B stage instead of BCLC-A. Patients with solitary resectable HCC should be classified as BCLC-A regardless of tumor size [4, 6, 16], acknowledging that large HCC's are more frequently associated with microvascular invasion and satellites nodules [38]. Following guidelines, PH is recommended in this group of patients. The inclusion of an important subset of HCC patients with BCLC-A stage in these meta-analyses may have biased their conclusions in favor of surgical treatment. The present meta-analysis was designed with strict selection criteria, which is paramount. As an illustration, our stringent selection permitted inclusion of 1,730 patients, as opposed to 4,958 [13], 2,619 [14], and 3,417 [15] patients in the other meta-analyses; these numbers underscore the high proportion of BCLC-A patients included in those studies. Overall, our results suggest that tumor burden alone does not accurately reflect prognosis in BCLC-B HCC. It is increasingly recognized that algorithms based solely on clinical characteristics are inadequate to accurately predict HCC prognosis; while biomarkers such as AFP are being incorporated, better indices of tumor biology are urgently needed [4]. Although important efforts have been pursued in this field, molecular markers remain barely used in clinical practice to date [39, 40]. In the future, the development of new technologies such as liquid biopsy [41, 42, 43] may help in selecting patients with HCC in BCLC-B stage for surgical resection. Meanwhile, clinical markers capable to refine prognosis of patients with intermediate stage are necessary.
For the primary endpoint (e.g., OS), a statistical analysis providing TRs instead of HRs was used, suggesting the superiority of PH compared to TACE in intermediate-stage HCC patients. In addition of being clinically more intuitive and relevant, this approach is also more accurate, since survival curves were clearly nonproportional. Other statistical approaches were also investigated: A 2-stage approach, which provided very similar results and conclusions as the one-stage, and another one based on nonproportional flexible parametric models, which generate time-dependent HRs [27]. Despite its flexibility, the later approach provides less-readily-interpretable results as HRs vary with time. Moreover, one HR curve is estimated for each study (i.e., effect size is not a single fixed value but rather a curve). Conversely, the AFT metric computes a single fixed-effect size per study and facilitates the assessment of heterogeneity. Regarding the secondary endpoint (e.g., postprocedural mortality), although it is widely reported at 30-, 90-day postprocedural mortality was preferred here. There is increasing evidence supporting the relevance of this measure, particularly after HPB surgery [21]. Although increased postprocedural mortality after PH in comparison to TACE may have been suspected, the present results showed no difference. One can argue that strict selection criteria (e.g., quality of liver parenchyma, no portal hypertension) in patients undergoing PH may reflect the good outcome observed in the studies included. Nevertheless, postprocedural mortality after PH was 3.7%, confirming that surgery is feasible in this group of HCC patients, providing patients have normal liver function and no significant portal hypertension [4].
Several limitations need to be addressed. These are intrinsic limitations related to this topic and available trials.
Analyzed trials were essentially conducted in Asia (6 Asian and 1 European studies). This point raised the question whether the present findings may be extrapolated to Western patients. Also, one may hypothesize that the analyzed patients included an important proportion of noncirrhotic HCC arising on HBV-positive patients (an important subgroup in Asia) and that this subgroup may have favored PH; hence, it would mean that increasing the proportion of patients from Western countries, or of Asian patients with cirrhosis might impact the present results and rather favor TACE. First, Asian-born immigrants also represent an important subgroup of HCC patients in Western countries [44]. Second, a recent large multicentric study conducted in Asia and including 1,066 cirrhotic patients undergoing PH for multinodular HCC demonstrated that surgery can be safely performed, even with cirrhosis and multinodular disease [45]. Third, the included European study by Ciria et al. [31], albeit of small sample size, also showed increased survival after PH compared to TACE in this setting.
Most included trials were non-RCT (6 NRCT and 1 RCT). This point raised the question of potential bias. One may hypothesize that treatment arms differed and that patients with preserved liver function and good functional status might be preferentially selected as surgical candidates. Although it would be reasonable to formulate this hypothesis, it would be misleading to speculate it as a fact. First, the RCT by Yin et al. [11] demonstrated the second-largest TR and no difference in postprocedural mortality, in treatment arms showing comparable patients. This confidently supports the final results of the present meta-analysis. Second as previously discussed, the recent large multicentric study by Li et al. [45] showed that PH was safe and feasible in multinodular cirrhotic patients.
Available data in the selected studies did not permit exploration of other potential prognostic factors such as tumor size or liver function. Identifying subgroups of HCC patients within BCLC-B who may benefit from PH is paramount. This approach requires subgroup or meta-regression analyses integrating multiple variables extracted from each study. Such analyses were not performed in the present study because it would have been statistically inaccurate and associated with an important risk of false-positive results due to the limited number of included studies. In addition, potential prognostic factors were not provided in sufficient detail in all included studies [46, 47].
In conclusion, the present systematic review and meta-analysis suggests increased OS with comparable postprocedural mortality rates in HCC patients of strict BCLC-B stage treated with PH, compared to TACE. These results support the role of PH as treatment option in intermediate-stage HCC and emphasize the need to refine selection criteria of patients with HCC in BCLC-B stage who may benefit from surgery.
Statement of Ethics
Not applicable.
Disclosure Statement
The authors declare no potential conflicts of interest.
Funding Sources
No source of funding.
Author Contribution
I.L., D.M., D.C., and E.M.: study concept and design. I.L., D.M., and D.C.: acquisition of data. I.L., P.T., D.M., D.C., and E.M.: analysis and interpretation of data. I.L., P.T., and E.M.: drafting of the manuscript. I.L., P.T., D.M., D.C., M.S., N.K., A.D., N.H., N.D., and E.M.: critical revision of the manuscript for important intellectual content.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
References
- 1.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017 Sep;109((9)):109. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69((1)):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016 Apr;2((1)):16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 4.Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. European Association for the Study of the Liver Electronic address eee, European Association for the Study of the L: EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011 Mar;53((3)):1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19((3)):329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 7.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017 Jul;11((4)):317–70. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korean Liver Cancer Study Group (KLCSG) National Cancer Center, Korea (NCC) 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015 May;9((3)):267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. Liver Cancer Study Group of Japan JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014 Oct;3((3-4)):458–68. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018 Jan;67((1)):358–80. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 11.Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014 Jul;61((1)):82–8. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Tada T, Kumada T, Toyoda H, Tsuji K, Hiraoka A, Itobayashi E, et al. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci. 2017 Jul;108((7)):1414–20. doi: 10.1111/cas.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Zhou JG, Sun Y, Zhang L, Xing BC. Hepatic Resection Improved the Long-Term Survival of Patients with BCLC Stage B Hepatocellular Carcinoma in Asia: a Systematic Review and Meta-Analysis. J Gastrointest Surg. 2015 Jul;19((7)):1271–80. doi: 10.1007/s11605-015-2811-6. [DOI] [PubMed] [Google Scholar]
- 14.Liang L, Xing H, Zhang H, Zhong J, Li C, Lau WY, et al. Surgical resection versus transarterial chemoembolization for BCLC intermediate stage hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford) 2018 Feb;20((2)):110–9. doi: 10.1016/j.hpb.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology. 2018 Sep;68((3)):977–93. doi: 10.1002/hep.29883. [DOI] [PubMed] [Google Scholar]
- 16.Forner A, Gilabert M, Bruix J, Raoul JL. Reply: heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol. 2015 Jan;12((1)):10. doi: 10.1038/nrclinonc.2014.122-c2. [DOI] [PubMed] [Google Scholar]
- 17.Labgaa I, Demartines N, Melloul E. Surgical Resection Versus Transarterial Chemoembolization for Intermediate Stage Hepatocellular Carcinoma (BCLC-B): An Unsolved Question. Hepatology. 2019 Feb;69((2)):923. doi: 10.1002/hep.30338. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Jul;6((7)):e1000097. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 20.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014 Jun;146((7)):1691–700.e3. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Mise Y, Vauthey JN, Zimmitti G, Parker NH, Conrad C, Aloia TA, et al. Ninety-day Postoperative Mortality Is a Legitimate Measure of Hepatopancreatobiliary Surgical Quality. Ann Surg. 2015 Dec;262((6)):1071–8. doi: 10.1097/SLA.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012 Feb;12((1)):9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Royston P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017 Oct;17((4)):786–802. [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert P, Collett D, Kimber A, Johnson R. Parametric accelerated failure time models with random effects and an application to kidney transplant survival. Stat Med. 2004 Oct;23((20)):3177–92. doi: 10.1002/sim.1876. [DOI] [PubMed] [Google Scholar]
- 25.Siannis F, Barrett JK, Farewell VT, Tierney JF. One-stage parametric meta-analysis of time-to-event outcomes. Stat Med. 2010 Dec;29((29)):3030–45. doi: 10.1002/sim.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017 Feb;36((5)):855–75. doi: 10.1002/sim.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowther MJ, Lambert PC. A general framework for parametric survival analysis. Stat Med. 2014 Dec;33((30)):5280–97. doi: 10.1002/sim.6300. [DOI] [PubMed] [Google Scholar]
- 28.Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, et al. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. 2009 Apr;16((4)):848–55. doi: 10.1245/s10434-008-0282-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013 Jul;8((7)):e68193. doi: 10.1371/journal.pone.0068193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jianyong L, Lunan Y, Wentao W, Yong Z, Bo L, Tianfu W, et al. Barcelona clinic liver cancer stage B hepatocellular carcinoma: transarterial chemoembolization or hepatic resection? Medicine (Baltimore) 2014 Nov;93((26)):e180. doi: 10.1097/MD.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015 Sep;41((9)):1153–61. doi: 10.1016/j.ejso.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh AM, Joh JW, et al. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol. 2016 Jun;22((2)):250–8. doi: 10.3350/cmh.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, et al. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg. 2016 Jun;263((6)):1112–25. doi: 10.1097/SLA.0000000000001556. [DOI] [PubMed] [Google Scholar]
- 34.Kokudo T, Hasegawa K, Kokudo N, Kokudo T, Uldry E, Demartines N, et al. Hepatocellular Carcinoma: The Gap Between Eastern and Western Clinical Practice. Ann Surg. 2018 Feb;267((2)):e27–8. doi: 10.1097/SLA.0000000000001960. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Tabrizian P, Zhang H, Lau WY, Han J, Li ZL, et al. Comparison of Patterns and Outcomes of Liver Resection for Hepatocellular Carcinoma: east vs West. Clin Gastroenterol Hepatol. 2017 Dec;15((12)):1972–4. doi: 10.1016/j.cgh.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012 Nov;32((4)):348–59. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 37.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011 Mar;((3)):CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005 Sep;11((9)):1086–92. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 39.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015 Oct;149((5)):1226–39.e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 40.Labgaa I, Torrecilla S, Martinez-Quetglas I, Sia D. Genetics of Hepatocellular Carcinoma: Risk Stratification, Clinical Outcome, and Implications for Therapy. Dig Dis Interv. 2017;1((2)):55–65. [Google Scholar]
- 41.Labgaa I, Villanueva A. Liquid biopsy in liver cancer. Discov Med. 2015 Apr;19((105)):263–73. [PubMed] [Google Scholar]
- 42.Labgaa I, Villacorta-Martin C, D'Avola D, Craig AJ, von Felden J, Martins-Filho SN, et al. A pilot study of ultra-deep targeted sequencing of plasma DNA identifies driver mutations in hepatocellular carcinoma. Oncogene. 2018 Jul;37((27)):3740–52. doi: 10.1038/s41388-018-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut. 2018 Dec;67((12)):2204–12. doi: 10.1136/gutjnl-2017-315846. [DOI] [PubMed] [Google Scholar]
- 44.Kamath GR, Taioli E, N Egorova N, Llovet JM, Perumalswami PV, Weiss JJ, et al. Liver Cancer Disparities in New York City: A Neighborhood View of Risk and Harm Reduction Factors. Front Oncol. 2018 Jun;8:220. doi: 10.3389/fonc.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li ZL, Yu JJ, Guo JW, Sui CJ, Dai BH, Zhang WG, et al. Liver resection is justified for multinodular hepatocellular carcinoma in selected patients with cirrhosis: A multicenter analysis of 1,066 patients. Eur J Surg Oncol. 2019 May;45((5)):800–7. doi: 10.1016/j.ejso.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 46.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002 Jun;21((11)):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 47.Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI, Health Outcomes, Policy, and Economics (HOPE) Collaborative Group Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009 Oct;63((10)):1426–34. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data