Abstract
Wetland plants can tolerate long-term strict hypoxia and anoxic conditions and the subsequent re-oxidative stress compared to terrestrial plants. During O2 deficiency, both wetland and terrestrial plants use NAD(P)+ and ATP that are produced during ethanol fermentation, sucrose degradation, and major amino acid metabolisms. The oxidation of NADH by non-phosphorylating pathways in the mitochondrial respiratory chain is common in both terrestrial and wetland plants. As the wetland plants enhance and combine these traits especially in their roots, they can survive under long-term hypoxic and anoxic stresses. Wetland plants show two contrasting strategies, low O2 escape and low O2 quiescence strategies (LOES and LOQS, respectively). Differences between two strategies are ascribed to the different signaling networks related to phytohormones. During O2 deficiency, LOES-type plants show several unique traits such as shoot elongation, aerenchyma formation and leaf acclimation, whereas the LOQS-type plants cease their growth and save carbohydrate reserves. Many wetland plants utilize NH+4 as the nitrogen (N) source without NH+4-dependent respiratory increase, leading to efficient respiratory O2 consumption in roots. In contrast, some wetland plants with high O2 supply system efficiently use NO−3 from the soil where nitrification occurs. The differences in the N utilization strategies relate to the different systems of anaerobic ATP production, the NO−2-driven ATP production and fermentation. The different N utilization strategies are functionally related to the hypoxia or anoxia tolerance in the wetland plants.
Keywords: Anoxia, Hypoxia, Low O2 escape and low O2 quiescence strategies (LOES and LOQS), Nitrogen acquisition strategy, Re-oxidative stress, Respiration, Wetland plants
Introduction
O2 deficiency in roots is often caused by frequent flooding during rains, submergence by excess rainfall, soil compaction, and increased microorganism activity caused by the rise in temperature. Prolonged submergence of the roots in water can even lead to O2 deficiency in shoots. These factors negatively affect the growth and survival of the whole plant, both in natural and agricultural ecosystems. The degree of O2 deficiency in plant cells is classified as either hypoxia or anoxia. Hypoxia is characterized by restriction of aerobic metabolism, in which ATP production via the mitochondrial oxidative phosphorylation and NAD+ regeneration via the mitochondrial electron transport chain (mETC) are partially restricted. Anoxia is characterized by anaerobic metabolism, in which ATP is supplied solely via glycolysis, mitochondrial oxidative phosphorylation is completely inhibited, and the cellular ATP content is extremely low (Bailey-Serres and Voesenek 2008). Under these stress conditions, plants suffer from impairments that are caused by cell acidification and accumulation of reducing equivalents that lead to the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Hebelstrup and Møller 2015; Turkan 2018). Large amounts of ROS and RNS are produced under re-oxygenated conditions following the post-hypoxic and anoxic stresses, and they can further potentially damage the organelles. Thus, aerobic metabolism is suppressed during the recovery phase from O2 deficiency owing to an inhibition of the metabolic functions (Bailey-Serres and Chang 2005; Fukao et al. 2011; Santosa et al. 2007).
Most terrestrial plants such as Arabidopsis, barley and maize, cannot survive long-term O2 deficiency and severe anaerobic conditions even though they can survive under short-term stress with their hypoxia and anoxia tolerant responses. Arabidopsis under hypoxic conditions can regenerate NAD+ via mETC and fermentation (Bucher et al. 1994; Dolferus et al. 2008; Ismond et al. 2003; Lasanthi-Kudahettige et al. 2007; Narsai and Whelan 2013), but glycolysis cannot continuously function under anoxic conditions (Lasanthi-Kudahettige et al. 2007; Loreti et al. 2005). The responses to O2 deficiency in barley and maize have been examined to maintain their high yields at O2 deficiency (Tollenaar and Lee 2002). They can oxidize NAD(P)H to NAD(P)+ via glycolysis and fermentation to avoid accumulation of reducing equivalents when they are exposed to hypoxia by flooding (Guglielminetti et al 1995, 1999). They can metabolize RNS such as NO to maintain redox states and energy levels in the cytosol and mitochondria under hypoxia conditions (Igamberdiev and Hill 2004; Igamberdiev et al. 2006; Sowa et al. 1998). Maize can also form aerenchyma to aerate O2-deficient cells under hypoxic conditions similar to wetland plants (Armstrong and Armstrong 1994; Drew et al. 2000; Evans 2004; Hu et al. 2013). However, these plants cannot tolerate severe anoxia conditions that are caused by prolonged flooding and the following re-oxygenated condition after O2 deficiency.
In contrast, wetland plants such as rice can tolerate severe anoxia and the following re-oxygenation conditions due to repeated flooding. This is because they possess high tolerant mechanisms such as advanced regulation of glycolysis in the cytosol, detoxification of ROS and RNS in the mitochondria, and maintenance of ATP production linked to NO detoxification in the mitochondria (Fukao et al. 2011; Huang et al. 2008; Voesenek and Bailey-Serres 2015). They can also acclimate to severely flooded soils through the O2 supply from the aerial organ to O2-deficient organ. Their strategy is called low O2 escape strategies (LOES). Plants with the LOES phenotypes can change their shoots and roots in response to O2 deficiency such as shoot elongation, formation of aerenchyma, barriers to radial O2 loss (ROL) from the roots, formation of adventitious roots (ARs), and maintenance of gas films on leaf surface (Colmer 2003; Eysholdt-Derzsó and Sauter 2019; Sorrell and Hawes 2009; Winkel et al. 2013; Winkel et al. 2014). Some wetland plants show another strategy, low O2 quiescence strategy (LOQS). Plants with the LOQS phenotypes can survive under sever O2-deficient conditions where their aerial parts are completely submerged by flooding. They can cease growth and save their carbohydrate reserves until normal growth condition is recovered (Fukao and Bailey-Serres 2008; Fukao et al. 2006). These cellular and tissue level responses to O2 deficiency in wetland plants permit their growth and survival under severe anoxic conditions.
Plant roots that are the sites of active nutrient absorption are often exposed to frequent and large fluctuations of O2 concentration for short and long periods compared to the other organs. Wetland plants maintain their root activity by the aeration from the aerial organs to roots through the aerenchyma, and the available O2 in their roots depends on their abilities of LOES which are enhanced under O2-deficient conditions. Many wetland species specialize in NH+4 utilization in habitats with a predominance of NH+4. However, the wetland plants with the ability to supply O2 from shoots to roots can utilize NO−3 in addition to NH+4, because of nitrification in their rhizosphere by active ROL from their root tips (Brix et al. 2002; Kirk and Kronzucker 2005). Moreover, it was recently reported that the root O2 consumption strategies related to nitrogen (N) acquisition differ among species with differences in their ability to O2 supply to the roots. The differences in strategies are associated with the differences in the O2 demand by the aerobic respiration for root growth and N acquisition (Nakamura and Nakamura 2016; Nakamura et al. 2013). The N acquisition traits linked to the O2 supply ability could relate to the hypoxia and anoxia tolerance in wild wetland species.
In this review, we summarize the previous findings on regulatory mechanisms of glycolysis, mitochondrial respiratory systems, primary metabolism in maintaining the energy production and homeostasis under O2-deficient and re-oxidative stress conditions, and developmental plasticity underlying acclimation to hypoxic and anoxic conditions in terrestrial, wild wetland, and cultivated species. Particularly, we aim to show different molecular level responses and different cellular and whole-plant level strategies between wetland species with long-term tolerance to O2-deficient conditions and terrestrial species with only short-term tolerance to the same conditions. Moreover, we summarize the effects of N sources (NH+4 and NO−3) on the root respiratory systems of wetland species, and thereafter, we discuss the characteristics of aerobic and anaerobic root respiration in wetland species associated with the utilization strategies of available inorganic N in their rhizosphere for maintaining energy production.
Regulatory mechanisms of fermentation and glycolysis under oxygen-deficient conditions
Under O2-deficient conditions, ATP production in most plants rapidly declines because the electron transfer in the mETC and flux in the tricarboxylic acid (TCA) cycle slow down and the transcripts encoding many of their enzymes are down-regulated (Narsai et al. 2011). Even under low O2 conditions, large amounts of energy are required for maintaining the various cellular components, including proteins, for survival (Mustroph and Albrecht 2003). Plants that are tolerate to O2 deficiency have a high ATP production ability which is achieved by the enhancement of fermentation and glycolysis. They can regenerate NAD(P)+ from NAD(P)H accumulated by slow electron transport to maintain normal redox level under O2-deficient conditions. In the first part of this section, we show mechanisms of ATP production and NAD(P)+ regeneration through the fermentation and glycolysis. In the latter part of this section, we show the regulations of glycolysis and cytosolic pH by the utilization of pyrophosphate (PPi) which can act as a donor of phosphate for various metabolisms similarly to ATP (Shingaki-Wells et al. 2011).
Management of energy crisis through fermentation
Ethanol and lactate fermentation are two major metabolic pathways that produce energy under O2-deficient conditions. Pyruvate derived from glycolysis is converted to ethanol through the coupled reactions catalyzed by pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) in the ethanol fermentation pathway, and to lactate by lactate dehydrogenase (LDH) in the lactate fermentation pathway with the concomitant oxidation of NADH to NAD+ (Fig. 1). As the glycolytic flux to the ethanol fermentation pathway is higher than that to the lactate fermentation pathway in several plants, ethanol fermentation is considered to strongly contribute to low O2 tolerance compared to lactate fermentation (Licausi and Perata 2009). A shift from pyruvate metabolism via the TCA cycle to the ethanol fermentation pathway is attributed to the Km of PDC, which is similar to that of the accumulated pyruvate level under O2-deficient conditions (Pronk et al. 1996). In rice plants, this shift is also associated with the inactivation of pyruvate dehydrogenase (PDH) by an up-regulation of PDH kinase (Marillia et al. 2003) and decreased translation of PDH mRNA (Branco-Price et al. 2008).
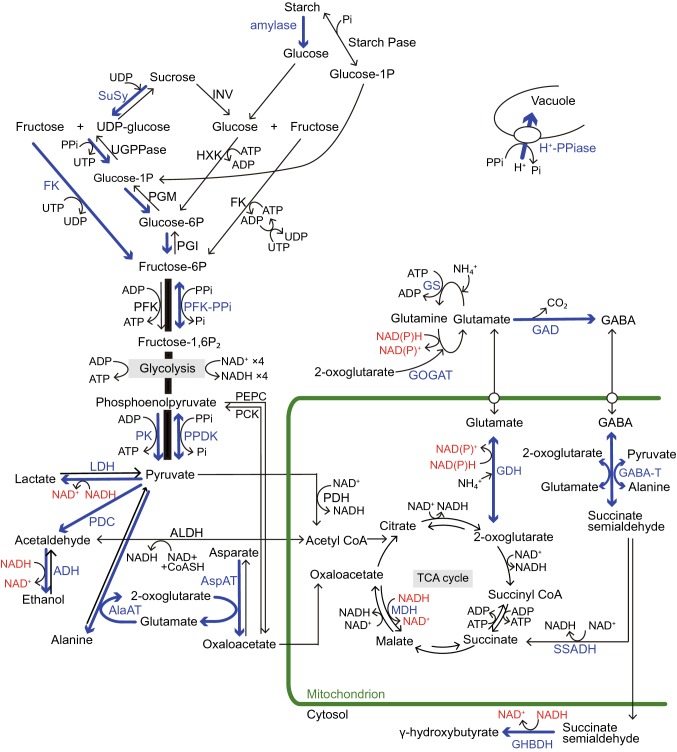
Fig. 1.
Regulations of sugar catabolism, fermentation, glycolysis, and major amino acid metabolism associated with NAD(P)+ regeneration and ATP production in terrestrial and wetland plants under O2-deficient conditions. Blue arrows and letters indicate the reactions and enzymes in the up-regulated pathways when the mitochondrial electron transport and the TCA-cycle flux decrease under O2-deficient conditions. Red letters indicate the regeneration of NAD(P)+ from NAD(P)H. In rice plants, the blue pathways contribute to their tolerance to long-term O2 deficiency compared with the terrestrial plants. Some wetland plants such as rice also have a high ability to optimally regulate the pyruvate level by activation of pyrophosphate (PPi)-dependent phosphofructokinase (PFK-PPi) and pyruvate phosphate dikinase (PPDK) that consume PPi instead of ATP for energy conservation. Besides glycolysis, PPi is consumed to regulate the cytosolic pH by the tonoplast H+-pumping pyrophosphatase (H+-PPiase) instead of H+-ATPase in wetland plants. Although two independent pathways for sucrose degradation contribute to the regulation of glycolytic flux in both terrestrial and wetland plants, the UDP-dependent sucrose synthase (SuSy) pathway is regarded as energetically more advantageous for survival under O2-deficient conditions than the invertase (INV) pathway because here, PPi is utilized instead of ATP. Sugar supply to glycolysis through starch mobilization is observed in species with developed storage organs such as tuber, rhizome, and endosperm. In NAD(P)H regeneration during the metabolisms of 2-oxoglutarate and glutamate associated with γ-aminobutyric acid (GABA) production, the glutamate dehydrogenase (GDH) pathway without ATP consumption is more efficient in energy consumption than the NAD(P)H-dependent glutamine: 2-oxoglutarate aminotransferase (GOGAT) pathway with ATP consumption. The accumulation of some amino acids such as GABA, alanine, and glutamate play an important role in avoiding carbohydrate loss not only during O2-deficient conditions but also during the recovery phase of re-oxygenation after hypoxia/anoxia. Alanine accumulation by alanine aminotransferase (AlaAT) can operate non-circular TCA-cycle and gluconeogenesis under O2 deficiency and re-oxygenation. Abbreviations are as follows: ADH, alcohol dehydrogenase; AlaAT, alanine aminotransferase; ALDH, acetaldehyde dehydrogenase; AspAT, aspartate aminotransferase; CoASH, coenzyme A; FK, fructokinase; GABA-T, GABA transaminase; GAD, glutamate decarboxylase; GHBDH, γ-aminobutyrate dehydrogenase; Glucose-1-P, glucose-1-phosphate; GS, glutamine synthetase; HXK, hexokinase; LDH lactate dehydrogenase; MDH, malate dehydrogenase; PCK, phosphoenolpyruvate carboxykinase; PDC, pyruvate decarboxylase; PDH; pyruvate dehydrogenase; PEPC, phosphoenolpyruvate carboxylase; PFK, ATP-dependent phosphofructokinase; PFK-PPi, PPi-dependent phosphofructokinase; PGI, phosphoglucoisomerase; PGM, phosphoglucomutase; Pi, phosphate; PK, pyruvate kinase; PPDK, pyruvate Pi dikinase; SSADH, succinate semialdehyde dehydrogenase; Starch Pase, starch phosphorylase; TCA, tricarboxylic acid; UDP, uridine diphosphate; UGPPase, UDP-glucose pyrophosphorylase; UTP, uridine triphosphate
The ethanol fermentation pathway is controlled by the activity level and gene expression of PDC because the maximum catalytic activity of PDC is low (Drew 1997; Ismond et al. 2003; Mithran et al. 2014; Morrell et al. 1990). PDC-overexpressed terrestrial plants such as Arabidopsis and tobacco were reported to exhibit much higher ethanol concentration in their leaves compared with the wild types, and their survival rates under low O2 conditions were enhanced (Bucher et al. 1994; Ismond et al. 2003).
In Arabidopsis plants, during O2 deficiency, PDC1 and PDC2 are up-regulated in roots and leaves, respectively (Mithran et al. 2014). In rice plants, PDC is induced at both the transcript and protein levels during O2 deficiency (Narsai and Whelan 2013). Experiments examining the effects of PDC level on the submergence tolerance of rice had revealed that the correlation between PDC activity or expression and submerge tolerance was stronger in shoots that show high growth than those in roots and endosperms that show low growth at O2 deficiency (Rahman et al. 2001). Moreover, rice varieties with high shoot elongation under anaerobic conditions showed active ethanol fermentation due to the high activity and gene expression of PDC in shoots, where the ethanol production was more active than that in roots under dark anaerobic conditions (Mustroph et al. 2006a, b). In contrast, there were no differences in root PDC activities between the varieties, and only a slight increase in activity was observed in submerged tolerant species under severe O2 deficiency at night (Mohanty and Ong 2003; Rahman et al. 2001). These results suggest that the distribution range of wild wetland species is characterized by the fermentation abilities of the species due to the PDC activities in their shoots rather than those in their roots, and that the fermentation abilities in roots to survive in hypoxic soils are similar among species.
The activation of ADH does not lead to the acceleration of ethanol production in maize (Roberts et al. 1989). As with terrestrial species, in some rice cultivars, although the expressions of ADH genes (ADH1 and ADH2) are not considered to be major determinants of the seedling vigor during hypoxic stress caused by submergence, these gene expressions respond to low O2 stress (Vu et al. 2009). These reports suggest that ADH activity is not crucial in ethanol fermentation for energy compensation for anoxia tolerance. However, increased activity of ADH may be crucial in utilizing ethanol as a carbon (C) source under conditions where the plant experiences different stress at the same time or during re-oxygenation after flooding (Gibbs and Greenway 2003; Tsuji et al. 2003). Moreover, ADH has an essential role in germination and subsequent survival of the seedling at O2 deficiency (Rahman et al. 2001).
Lactate fermentation also plays a crucial role in plant survival under anoxic conditions through the activity of LDH that reversibly catalyzes pyruvate and lactate (Fig. 1). Under O2-deficient conditions, pyruvate is converted to lactate via LDH, and under re-oxygenated conditions after flooding, the accumulated lactate quickly disappears and the glycolytic flux is regulated by the regeneration of pyruvate from lactate (Germain et al. 1997a). Additionally, as lactate can induce cytosolic acidification and the cytosolic pH is adjusted to an optimal value for the PDC activity, lactate accumulation induces metabolic change from lactate fermentation to ethanol fermentation (Davies 1980). Dolferus et al. (2008) have also reported that increased LDH activity induces ethanol fermentation in Arabidopsis. Excessive accumulation of lactate causes cell death by a sharp decline of the cell pH. Thus, many plants possess lactate efflux mechanism from their cytoplasm. In Arabidopsis, a high cytosolic accumulation of lactate is prevented by lactate excretion via a hypoxia-induced nodulin intrinsic protein NIP2;1 (Choi and Roberts 2007). A similar function was reported in some pleiotropic drug resistance (PDR) type ATP-binding cassette (ABC) transporters, whose expression in rice is regulated by lactate and other weak acids (Moons 2008).
Regulation of glycolytic flux via carbohydrate mobilization, sucrose catabolism, and amino acid metabolism under anaerobic conditions
Under anaerobic conditions, glycolysis operates for ATP production through the stable supply of carbohydrate by starch mobilization and sucrose degradation. Moreover, metabolisms of some amino acids such as glutamate, alanine and γ-aminobutyric acid (GABA) lead to the maintenance of glycolysis operation through the NAD(P)+ regeneration and the stable preservation of carbohydrates under anaerobic conditions. Under post-anoxic conditions, metabolisms of GABA and alanine contribute to the avoidance from ROS accumulation and the recovery of aerobic metabolism during re-oxygenation, respectively. Terrestrial and wetland plants show different responses in starch mobilization, sucrose degradation and amino acid metabolisms to O2 deficiency.
Starch mobilization in the maintenance of glycolysis
A crucial point in the maintenance of glycolysis when plants are exposed to O2-deficient conditions is the efficient mobilization of the reserved carbohydrates (Dixon et al. 2006; Drew 1997; Gibbs and Greenway 2003; Licausi and Perata 2009; Sato et al. 2002; van Dongen et al. 2004) (Fig. 1). α-amylase and starch phosphorylase (Starch Pase) are induced by low O2 and they convert starch to glucose and glucose-1 phosphate (P), respectively. These reactions are observed in storage organs of cereal grains, potato tubers and rhizomes for their germination and subsequent growth (Bucher and Kuhlemeier 1993; Das et al. 2000; Geigenberger 2003). Rice can germinate under low O2 conditions due to the expression of α-amylase genes in its aleurone layer. Other terrestrial cereals such as wheat and barley cannot germinate under the same low O2 conditions (Das et al. 2000; Guglielminetti et al. 1995, 1999; Narsai et al. 2009; Ricard et al. 1991). Among the three subfamilies of α-amylases genes (AMY1A-C, AMY2A, and AMY3A-F) in rice, AMY3D is anoxic-specific (Loreti et al. 2003; Park et al. 2010). Its induction under anoxia is repressed by high sugar levels, but is independent of gibberellin (GA) levels. This is because its promoter region lacks the distinctive cis-acting element that confers GA-responsiveness (Loreti et al. 2003; Park et al. 2010). In contrast, isoforms encoded by AMY1A are found in both aerobic and anaerobic seedlings, and AMY1A is induced by GA in seedlings under aerobic but not under anoxic conditions (Loreti et al. 2003; Morita et al. 1998). Thus, under aerobic conditions, GA-dependent activation of AMY1A maintains high sugar levels in the aleurone layer, and thereby AMY3D induction is prevented. The AMY3D expression depends on the cis-acting elements in its promoter region, named sugar repression core (SRC, Chen et al. 2006), and trans-acting transcription factor (TF), MYBS1 (Lu et al. 2002). Moreover, AMY3D and MYBS1 have been reported to be activated by the general regulator, sucrose non-fermenting receptor kinase 1A (OsSnRK1), which is promoted by calcineurin B-like interacting protein kinase 15 (CIPK15). They play a role in sugar and energy depletion signaling (Lee et al. 2014; Lu et al. 2002; Lu et al. 2007).
The mobilization of starch for constant carbohydrate supplementation under O2-deficient conditions is not common to all plants. Among terrestrial plants, this ability is limited to species with storage organs such as cereal grains and potato tubers (Arpagaus and Brändle 2000; Dixon et al. 2006; Guglielminetti et al. 1995). These plants can prevent starch depletion from their storage organs because they can sense the sugar level in their cells and subsequently down-regulate their metabolic rates (Arpagaus and Brändle 2000; Dixon et al. 2006). The mobilization of starch and regulation of sugar level at the germination and seedling growth stages have been poorly understood in wetland species other than rice plants. This metabolism may act as a crucial tolerance mechanism in the germination and early development of wetland species because many wetland species with relatively large endosperms (members from the Gramineae and Cyperaceae families) (Kettenring and Galatowitsch 2007; Leck and Brock 2000; Wijte and Gallagher 1996) and developed rhizomes (members from the Nymphaeaceae and Menyanthaceae families) can grow in stagnant soil with low O2 condition.
Two independent pathways for the sucrose degradation
The bidirectional UDP-dependent sucrose synthase (SuSy) and the unidirectional invertase (INV) are two distinct pathways for degradation of sucrose in plant cells (Fig. 1). SuSy consumes a net of one mol PPi per one mol sucrose when UDP glucose and fructose are substrates for glycolysis. This is because the by-product of UDP glucose pyrophosphorylase, UTP, is used for the formation of phosphorylated fructose by fructokinase (FK), and simultaneously ATP is regenerated from ADP via NDP kinase (Bailey-Serres and Voesenek 2008; Guglielminetti et al. 1999). In contrast to the consumption of PPi by SuSy, the INV reaction involves two mols ATP per mol sucrose (Mustroph et al. 2005). Therefore, SuSy is regarded as a more energetically advantageous pathway in various species for survival under O2-deficient conditions than INV. Indeed, transgenic potato tubers with elevated INV activity were unable to maintain ATP levels under low O2 conditions (8% O2) (Bologa et al. 2003).
Responses of activities and transcriptions to low O2 conditions differ between SySy and INV. The activity and mRNA transcript level of SuSy are rapidly increased by sugar starvation, in contrast to the constitutive expression of INV in various terrestrial and wetland plants (Branco-Price et al. 2008; Koch 2004; Lasanthi-Kudahettige et al. 2007; Loreti et al. 2005). Comparative analysis of gene inductions and protein expressions with or without sucrose addition revealed that SuSy gene expression is up-regulated by sensing sugar starvation as signals (Contento et al. 2004; Liu et al. 2010; Loreti et al. 2005; Nicolai et al. 2006; Rolland et al. 2006). In rice, six SuSy isoforms localized in roots, mesophylls, and phloem are tissue-specifically expressed at different developmental stages (Hirose et al. 2008; Wang et al. 1999). Particularly, the expression of SUS2 significantly increases in germinating seeds and growing seedlings under anoxic conditions (Hirose et al. 2008), indicating that SUS2 can serve not only as a housekeeper but also as the initial reaction of sucrose degradation during stress. In addition to the single hypoxia-inducible SuSy isoform, multi-expressions of the SuSy isoforms with functional redundancy are required to ensure low O2 tolerance in several species of both terrestrial and wetland plants (Bieniawska et al. 2007; Hirose et al. 2008; Wang et al. 1999).
INV catalyzes sucrose into fructose and glucose, which are then phosphorylated by hexokinase (HXK) or FK to be channeled into the glycolytic pathway (Licausi and Perata 2009) (Fig. 1). The glycolytic flux is also regulated by the activation of the INV pathway, but the main pathway of sucrose degradation under aerobic conditions may be the SuSy pathway (Fig. 1). This regulation of glycolysis is noted not only in the roots of terrestrial crops such as maize and tomato (Bouny and Saglio 1996; Germain et al. 1997b) but also in the seedlings of rice (Cho et al. 2006; Guglielminetti et al. 2006). In rice plants exposed to anoxia, OsFK2 and OsHXK7 are induced by sensing sucrose starvation as signals (Cho et al. 2006; Guglielminetti et al. 2006; Lasanthi-Kudahettige et al. 2007). Other isoforms, OsHXK5 and OsHXK6, dual-targeted to the mitochondria and nucleus, also act as glucose sensors (Cho et al. 2009; Narsai and Whelan 2013). They directly regulate the downstream factors including CIPK15, which in turn regulate the representative α-AMY3 gene (RAMY3D) and ADH2 expression in rice under low O2 conditions (Yim et al. 2012).
Metabolism of typical amino acids linked to glycolysis regulation under O2-deficient conditions
At low O2, NAD(P)+ regeneration can be achieved by amino acid metabolisms such as the metabolism of 2-oxoglutarate and glutamate associated with the production of GABA (Fig. 1). The synthetic pathway of GABA through the glutamate decarboxylation by glutamate decarboxylase (GDC) with H+ consumption can contribute to the counteraction of cytosolic acidification caused by anoxic stress (Aurisano et al. 1995). The metabolism of 2-oxoglutarate and glutamate is promoted through the glutamine synthetase (GS)-glutamine oxoglutarate aminotransferase (GOGAT) pathway or the glutamate dehydrogenase (GDH) pathway. In the former, GS and GOGAT catalyze the conversion of glutamine to glutamate with 2-oxoglutarate incorporation, whereas in the latter GDH reversibly catalyzes the reaction between 2-oxoglutarate and glutamate (Narsai et al. 2009; Rocha et al. 2010; Shingaki-Wells et al. 2011) (Fig. 1). The GDH pathway does not consume ATP in the conversion of 2-oxoglutarate to glutamate, while the GS-GOGAT pathway consumes one ATP mol per glutamate mol for the conversion of glutamine to glutamate (Gibbs and Greenway 2003) (Fig. 1). Therefore, the GDH pathway is more efficient in energy consumption. Moreover, increased glutamate can act as an amino group donner in the aspartate transamination by aspartate aminotransferase (AspAT) for the production of oxaloacetate, an intermediate product in the TCA cycle during anoxia (Fig. 1). Oxaloacetate is then converted to malate by malate dehydrogenase with NAD(P)+ regeneration (Bailey-Serres and Voesenek 2008). Glutamate is simultaneously incorporated into the pathway by alanine aminotransferase (AlaAT) (Bailey-Serres and Voesenek 2008; Ricoult et al. 2006) (Fig. 1). Under hypoxia, strong induction of mRNA levels and enzymatic activities of AspAT, AlaAT, and GDH in the cytosol and mitochondria have been reported in Arabidopsis and rice plants (Klok et al. 2002; Lasanthi-Kudahettige et al. 2007; Liu et al. 2005; Loreti et al. 2005; Mustroph et al. 2010; Narsai and Whelan 2013; Narsai et al. 2011). Thus, the synthesis of these amino acids may contribute to the regulation of glycolysis through NAD(P)+ regeneration in both terrestrial and wetland species.
Metabolisms of GABA and alanine seem to play important roles for plants in survival during low O2 stress and re-oxygenation. Kreuzwieser and Rennenberg (2014) have reported that the supply of carbohydrates to amino acid metabolism in shoots and roots is a key process that helps plants survive hypoxia. The metabolism of these amino acids does not result in carbohydrate loss compared with fermentation. In Arabidopsis, the importance of organ-specific response in these advantageous amino acid metabolisms has been pointed out. High GABA accumulation in roots was observed under hypoxia compared with that in shoots, while alanine accumulation was observed in both organs (Mustroph et al. 2014). Transcriptome and metabolome analyses of flooding-intolerant Arabidopsis and flooding-tolerant rice and poplar exposed to anoxia indicated that the accumulation of alanine and succinate and the increased activities of fermentation enzymes were observed in all the species, but that the transcriptional regulations of amino acid metabolism and anaerobic fermentation were different among species (Narsai and Whelan 2013; Narsai et al. 2011). Plant species may have specific mechanisms for signal transduction and post-transcriptional regulation in the amino acid and carbohydrate metabolisms in roots and shoots.
The increased accumulation of GABA under anoxia is metabolized by GABA transaminase (GABA-T) to succinic semialdehyde coupling with consumption of 2-oxoglutarate and additional conversion of pyruvate to alanine (Fig. 1). In the subsequent reaction, succinic semialdehyde is converted either to γ-hydroxybutyrate by the NAD(P)H-consuming reaction catalyzed by GABA dehydrogenase (GHBDH) or to succinate that can be channeled to the TCA cycle by succinate semialdehyde dehydrogenase (SSADH) by the NAD+-consuming reaction (Fig. 1). Although the high activity of SSADH with NAD+ consumption is thought to be disadvantageous for glycolytic regulation under hypoxia, this enzyme can function as one component of the bypass of the TCA cycle (GABA-shunt) to decrease ROS accumulation under re-oxygenation conditions (Bouche et al. 2003) (Fig. 1). Indeed, higher accumulation of SSADH protein was observed under re-oxygenation conditions in Arabidopsis (Bouche et al. 2003).
Besides anoxic stress, post-anoxic stress can also severely damage plant growth owing to large amounts of ROS that are produced in the cells. Particularly, wetland plants such as rice plants often suffer from post-anoxic stress after frequent floods; they have been reported to exhibit significant transcript reprogramming, which rapidly increased the expression of genes encoding TCA-cycle enzymes and levels of metabolites including citrate and 2-oxoglutarate to restore aerobic growth under post-anoxic conditions (Narsai et al. 2009). Moreover, large amounts of alanine generated by AlaAT under anaerobic conditions help plants to survive under subsequent re-oxygenation conditions because alanine can be transported through the xylem as a transportable energy source (De Sousa and Sodek 2003). AlaAT can convert the transported alanine into pyruvate, which can be used in gluconeogenesis or metabolized to acetyl-CoA (Fig. 1); both processes are important for aerobic metabolism during re-oxygenation (Rocha et al. 2010; Shingaki-Wells et al. 2014). In contrast, coleoptiles of anoxia-intolerant wheat seedlings cannot accumulate alanine when they are subjected to anoxia (Shingaki-Wells et al. 2011). These findings suggest that alanine accumulation by activated AlaAT in the flood-tolerant species plays an important role in their survival not only during hypoxia but also during the recovery phase of re-oxygenation after hypoxia.
Utilization of available PPi as the phosphate donner instead of ATP
It has been assumed that PPi is particularly favored as a phosphoryl donor compared with ATP in anoxic tissues where the cytosol is acidic (Davies et al. 1993; Felle 2005). Thus, PPi can serve an alternative energy source instead of ATP, and is utilized to maintain the glycolysis flux and regulation of cytosolic pH under low O2 conditions where ATP levels are low.
In glycolysis, the enzymes with reversible reactions, PPi-dependent phosphofructokinase (PFK-PPi) and pyruvate phosphate dikinase (PPDK), can function instead of ATP-dependent phosphofructokinase (PFK-ATP) and pyruvate kinase (PK), respectively (Fig. 1). As PFK-PPi can catalyze fructose 6-P without consumption of ATP, the PFK-PPi function can increase the net ATP production in anoxia-tolerant plants (Huang et al. 2008; Plaxton and Podestá 2006) (Fig. 1). In rice seedlings, the enzymatic activity of PFK-PPi is dramatically increased by 15-fold after 24 h in anoxia (Gibbs et al. 2000; Kato-Noguchi 2002; Mertens et al. 1990). The expression of annotated PFK-PPi genes in anoxic rice coleoptiles is complex. Some are down-regulated and others are up-regulated (Lasanthi-Kudahettige et al. 2007). In contrast, gene expression and protein amounts of the cytosol-type and plastid-type PPDKs in rice are up-regulated under anoxia. Especially the expression of cytosolic-type PPDK is up-regulated by 365-fold when the plants are exposed to anoxia (Lasanthi-Kudahettige et al. 2007; Moons et al. 1998; Shingaki-Wells et al. 2011) and is higher in roots than that in shoots (Huang et al. 2008). This suggests that roots have a higher ability to enhance anoxia tolerance than shoots because roots often experience more frequent fluctuations of O2 concentration than shoots.
Induction of PFK-PPi and PPDK is controlled by the cytosolic PPi content under short- and long-term anoxia, but not by the exogenous substrates such as starch and sucrose (Huang et al. 2008). While the PFK-ATP activity is rate-limiting for glycolysis in short-term anoxia, PFK-PPi can compensate for the ATP limitation by using PPi. In such a situation, PPi can be provided by a reaction cycle catalyzed by both PPDK and PK. This would accelerate the glycolytic flux and supply energy for survival at the early phase of anoxia. In contrast, if the plants are exposed to long-term anoxia, glycolysis may need to be down-regulated to conserve carbohydrates. Thus, PFK-PPi and PPDK may regulate the PPi level to slow down the net glycolytic flux for survival under long-term anoxia, and thereby the direction of glycolysis is changed to gluconeogenesis. Some reports indicate that this functional regulation of PPi level may strongly contribute towards maintaining glycolysis under severe anoxic conditions where the ethanol fermentation is declined (Colmer et al. 2001; Huang et al. 2008; Kato-Noguchi 2002; Loreti et al. 2005). Therefore, enzyme-mediated reactions can contribute to low O2 tolerance in either direction, towards glycolysis or gluconeogenesis, although it is difficult to experimentally show the reaction directions by PFK-PPi and PPDK because of their small free energy values (ΔG) (Huang et al. 2008).
Gene expressions of PFK-PPi and PPDK in anoxia-intolerant Arabidopsis did not show significant changes in anoxia (Lasanthi-Kudahettige et al. 2007; Loreti et al. 2005). Under these conditions, the gene encoding PFK-PPi was up-regulated by 1.9-fold, and PPDK by only 1.1-1.7-fold. This change in PPDK in Arabidopsis was much less than that in the cytosolic-type PPDK in rice plants. In rice plants, PFK-PPi and tonoplast H+-PPiase were induced during phosphate (Pi) deficiency, but the change in PPDK during Pi deficiency is unclear (Plaxton 2004). Interestingly, even under various stress environments where the levels of the nucleoside triphosphate (NTP) pools including Pi, ATP, and ADP were significantly decreased, the PPi levels were relatively stable in rice plants (Plaxton 2004). Especially the PPi concentrations in coleoptiles and cultured cells of rice plants were similar between anoxic and aerated conditions (Kato-Noguchi 2002; Mohanty et al. 1993). These results suggest that the stable level of PPi in rice plants supports a stable response to the crisis in energy production by a sudden O2 decrease via PPi-dependent enzymes. Moreover, the gene expression of inorganic pyrophosphatase (PPiase) in rice coleoptile was significantly down-regulated by 35-fold when they were exposed to anoxic conditions, whereas the gene expression of inorganic PPiases in Arabidopsis was unchanged or slightly up-regulated under anoxic conditions (Lasanthi-Kudahettige et al. 2007). Consequently, in rice plants under anoxic conditions, PPi is not degraded by inorganic PPiase and large amounts of PPi can be used for the other essential processes (Huang et al. 2008). In contrast, in Arabidopsis cells under anoxic conditions, the PPi content decreases and the plants suffer severe energy deficiency because solute transport across the tonoplast decreases and the cytosol pH is acidic, resulting in ultimately cell death (Fukao and Bailey-Serres 2004). The roles of PPi-dependent enzymes in wild wetland plants, other than rice plants, are restricted to a few studies in which the gene expression of cytosolic PPDK in Eleocharis vivipara and the activity and induction of PFK-PPi and PPDK in Potamogeton pectinatus were examined (Dixon et al. 2006; Summers et al. 2000). As these amphibious plants grow under various O2 conditions from land to deep-water wetland, it is assumed that many wild wetland plants may commonly utilize PPi-dependent enzymes in response to changes in the O2 availability.
Besides glycolysis, many plants consume PPi to regulate the cytosolic pH under O2-deficient conditions by the tonoplast H+-pumping pyrophosphatase (H+-PPiase) instead of H+-ATPase (Fig. 1). This can support the essential process of pH regulation even under ATP-deficient conditions. Anoxia-tolerant rice can suppress the pH decline in anoxia to half of that in the normal conditions, while anoxia-intolerant wheat and Arabidopsis suffer severe pH decline during anoxia (Lasanthi-Kudahettige et al. 2007; Loreti et al. 2005; Menegus et al. 1991). In rice plants, the activity of the tonoplast H+-PPiase was increased by 75-fold after 6 days in anoxia (Carystinos et al. 1995), and the gene (Os02g55890) encoding H+-PPiase was up-regulated by 35-fold in anoxia (Lasanthi-Kudahettige et al. 2007).
Mitochondrial metabolic adaptation to O2 deficiency
ROS (e.g., H2O2 and O·−2) and RNS (e.g., NO and ONOO−) can function as important physiological regulators of the intercellular signaling pathway in plant cells (Desikan et al. 2001; LiQiang 2011; Nie et al. 2006). However, they can cause disorders of oxidative phosphorylation due to oxidation and nitration of proteins. Anoxic and post-anoxic stresses by frequent flooding lead to ROS formation due to over-reduction of mETC, and these further lead to decreases in energy production (Blokhina and Fagerstedt 2010; Blokhina et al. 2000; Santosa et al. 2007; Szal et al. 2003). Under such stress conditions, non-phosphorylating components of mETC, the alternative oxidase (AOX) and type II NAD(P)H dehydrogenases (NDs) can consume the accumulated reducing equivalents for maintaining the mitochondrial homeostasis. These components are not coupled with the proton motive force (Blokhina et al. 2014; Maxwell et al. 1999; Millar et al. 2004; Møller 1997; Sweetlove et al. 2006; Szal et al. 2003; Xu et al. 2011) (Fig. 2). The AOX directly transfers an electron from ubiquinol (UQH2) to O2, and functions in the stress response (Fig. 2). AOX has lower affinity for O2 than cytochrome c oxidase (COX, complex IV) (Gupta et al. 2009), but O2 consumption through AOX does not depend on O2 concentration. In contrast, O2 consumption through COX decreases depending on the decrease in O2 concentration (Zabalza et al. 2009). Thus, AOX can consume reducing equivalents under low O2 conditions even when COX activity is inhibited. The AOX activity is controlled by its protein amount, AOX and ubiquinone (UQ) redox states, and pyruvate level (Day and Wiskich 1995; Møller 2001; Simons and Lambers 1999; Vanlerberghe and Mclntosh 1996; Vanlerberghe et al. 2002). A study in which the transcript responses to low O2 between flood-tolerant rice and poplar and flood-intolerant Arabidopsis were compared revealed differences in the response of their AOX genes. The induction of AOX in response to low O2 was observed in Arabidopsis but not in rice and poplar (Narsai et al. 2011). In the case of Arabidopsis, the expression of AOX gene is induced by citrate accumulation resulting from the inhibition of aconitase activity by NO formed under low O2 stress. Consequently, the primary metabolism shifts to amino acid biosynthesis to counteract the energy crisis under low O2 stress (Gupta et al. 2012). However, when O2 availability is a limiting factor for the O2 consumption, this AOX induction would be futile for O2 consumption and energetically burdensome. Thus, the inability of Arabidopsis to prevent the induction of AOX genes under low O2 conditions could be reasonable for its intolerance to anaerobic conditions (Shingaki-Wells et al. 2014). Interestingly, the in vivo AOX activity correlates with the relative growth rate in some wild species (Millenaar et al. 2001). It has been reported that AOX in illuminated leaves can contribute to optimizing the photosynthetic electron transport through the dissipation of excessive reducing equivalents under stress conditions, and the AOX1 gene expression and AOX capacity are often induced by the presence of exogenous H2O2 or under stress conditions with high light or high temperatures (Amor et al. 2000; Feng et al. 2010; Murakami and Toriyama 2008; Vanlerberghe and Mclntosh 1996; Vishwakarma et al. 2015; Wagner and Krab 1995; Xu et al. 2011). These results support that AOX is indispensable for the flexible control of ATP synthesis to maintain homeostasis and growth through the interaction between mitochondria and other organelles under various stress conditions (Hansen et al. 2002).
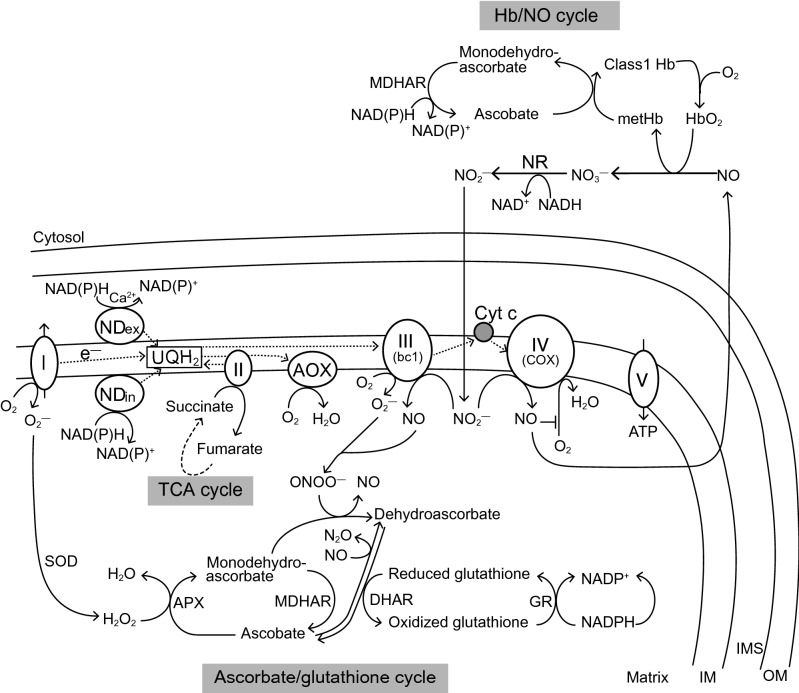
Fig. 2.
Production and elimination of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in mitochondria and cytosol. H2O2, NO, O·−2, and ONOO− produced under hypoxic stress conditions are detoxified by the mitochondrial electron transport chain (mETC) and ascorbate/glutathione cycle in the mitochondrial matrix to maintain a redox balance in the cells. The alternative oxidase (AOX) and type II NAD(P)H dehydrogenases (NDs), NDex, and NDin (NDs located at the outer and inner surfaces of the mitochondrial inner membrane, respectively), can consume the accumulated reducing equivalents for maintaining the mitochondrial homeostasis. AOX has lower affinity to O2 than cytochrome c oxidase (COX, complex IV); NDs, especially Ca2+ dependent NDin, have lower affinity to NAD(P)H than complex I and nitrate reductase (NR). In NO scavenging under hypoxic stress condition, ascorbate can contribute to the reduction of NO to N2O in the mitochondrial matrix. Ascorbate is converted to monodehydroascorbate by ascorbate peroxidase (APX), which also involves the scavenging of ONOO− and converting it into NO, The NO generated is resupplied to mETC. Ascorbate can also participate in Class 1 hemoglobin (Class 1 Hb) regeneration from methemoglobin (metHb) in the cytosol. Abbreviations are as follows: Cyt c, cytochrome c; DHAR, dehydroascorbate reductase; GR, glutathione reductase; IM, inner membrane; IMS, inter-membrane space; MDHAR, monodehydroascorbate reductase; NO, nitric oxide; NR, nitrate reductase; OM, outer membrane; SOD, superoxide dismutase; TCA, tricarboxylic acid; UQH2, ubiquinol
It has been reported that the induction of AOX is associated with the mitochondrial retrograde signaling and AOX can directly influence mitochondrial signaling by decreasing the ROS and uncoupling the electron transport from ATP synthesis (Rhoads and Subbaiah 2007). One of the TFs involving the AOX gene expression is related to abscisic acid (ABA), ABI4; it was reported to be a strong repressor of AOX expression in leaves of Arabidopsis exposed to high light and temperature stress (Giraud et al. 2009; Møller and Sweetlove 2010; Neill et al. 2003; Selinski et al. 2018; Xu et al. 2011). ABI4 is also intimately involved in sugar and plastid retrograde signaling pathways (Woodson and Chory 2008). Therefore, AOX could be controlled through ABI4 to integrate the mitochondrial retrograde signaling and respiratory regulation with other cellular anterograde and retrograde regulatory pathways. Vanlerberghe et al. (2009) indicated that AOX could buffer cellular signaling pathways, including cell death pathways, against adverse conditions. Moreover, AOX can regulate the gene expression of ROS-scavenging enzymes such as glutathione S-transferase, catalase, ascorbate peroxidase (APX), and superoxide dismutase (Giraud et al. 2009; Rhoads and Subbaiah 2007). In many plants under post-anoxic conditions with high ROS production, the AOX induction was found at both the transcript and protein levels to support a rapid response to re-oxygenation shock (Howell et al. 2007; Millar et al. 2004; Narsai et al. 2009).
Ca2+-dependent NDs located at the outer (NDex) and inner (NDin) surfaces of the mitochondrial inner membrane are not coupled with the generation of the proton motive force, and function as a bypass of complex I (Michalecka et al. 2003; Møller 1997; Rasmusson et al. 2004) (Fig. 2). The NDex can mainly utilize the cytosolic reducing equivalents (NAD(P)H) and have a higher Km for NADH than those of complex I and nitrate reductase (NR) (Møller et al. 1993). As NDex can utilize NAD(P)H independently of the other processes of mETC, it can regulate the NAD(P)H levels inside the intermembrane space and cytosol (Igamberdiev and Hill 2004) (Fig. 2). Moreover, it can be regulated by the elevated concentration of cytosolic Ca2+, due to Ca2+ release from the mitochondria during hypoxia (Fig. 2). An increase in the cytosolic Ca2+ concentration is stimulated by NO and H+/Ca2+ antiport, which is linked to the decrease in the cytosolic pH (Igamberdiev and Kleczkowski 2003; Subbaiah et al. 1998). The increase in Ca2+ concentration under anaerobic conditions functions as a signal for the regulation of many enzymes such as GDC and NAD+ kinase (Igamberdiev and Hill 2008). Interestingly, NDB2, a gene encoding the NDex, is strongly co-expressed with AOX1a in Arabidopsis because these genes share many common cis-acting regulatory elements (CAREs) in their promoter regions and are affected in a similar manner (Clifton et al. 2005; Elhafez et al. 2006). Further, some of the NDs (NDC1 and NDA1) are dual-targeted to plastids and peroxisome, therefore, their regulation is affected by the proteins outside the mitochondria (Ho et al. 2008). These indicate that important mitochondrial components involved in stress responses could provide the means for coordinating the activities between the organelles via coregulation and dual localization.
Effect of inorganic N sources on respiration in plants under the O2-deficient conditions
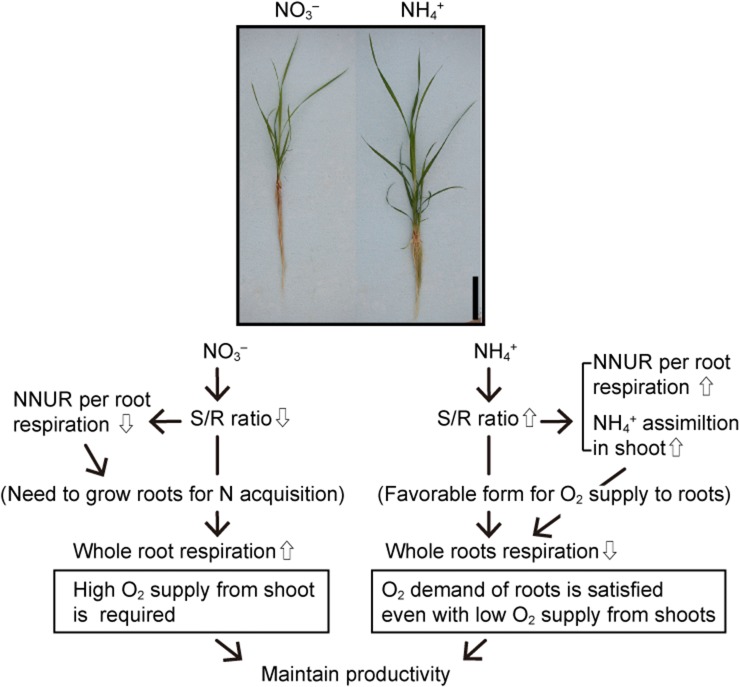
Plant roots play an important role in the absorption and assimilation of N and other essential minerals using respiratory energy. In soil, NO3− and NH4+ are found as inorganic N sources, and the energy cost for NH4+ assimilation is lower than that for NO3− (Bloom et al. 1992). Many terrestrial plants prefer to NO3− as inorganic N source, while wetland species specialize in NH4+ utilization because NH4+ predominates in flooded soils in their habitats. However, some wetland plants with the ability to supply O2 from the shoots to the roots can utilize NO3− because active radial O2 loss (ROL) from their root tips allow nitrification in their rhizosphere (Brix et al. 2002; Kirk and Kronzucker 2005). The preference of the roots for inorganic N sources affects the ATP production levels and O2 concentrations in roots. This is because the respiratory system has different responses to NO3− and NH4+ under anaerobic conditions.
Two NO3− reduction pathways in plants under O2-deficient conditions
Exogenous NO3− can act as a terminal acceptor of electrons and protons in the absence of molecular O2. NO3− can accept reducing equivalents to regenerate NAD(P)+ and prevent deteriorative effects of the cytoplasmic acidification through assimilative or catabolic NO3−-reduction pathways (Fig. 3, Fan et al. 1997; Müller et al. 1994; Vartapetian and Polyakova 1999). NAD(P)H can be oxidized by the assimilative pathway in which NO3− is reduced to NO2− and NH4+, and by the catabolic pathway involving the reductive NO2−-dependent NO production. These two pathways contribute to up-regulation of glycolysis under hypoxic and anoxic conditions due to the facilitation of glycolytic flux (Igamberdiev and Hill 2004; Reggiani et al. 1985; Stoimenova et al. 2007). Under low O2 and acidic conditions, the transcript level and activity of NR, which catalyzes the first step of NO−3 reduction to NO2− in both pathways, are increased in some terrestrial species (Lager et al. 2010). The NO3− reduction through the catabolic reduction pathway requires a large amount of NAD(P)H. In the NO2−-driven ATP synthesis cycle, about 2.5 mol NADH per 1 mol NO3− is consumed. Thus, the flux to glycolytic fermentation decreases as a result of competition for NADH oxidation (Fan et al. 1988; Sowa et al. 1998).
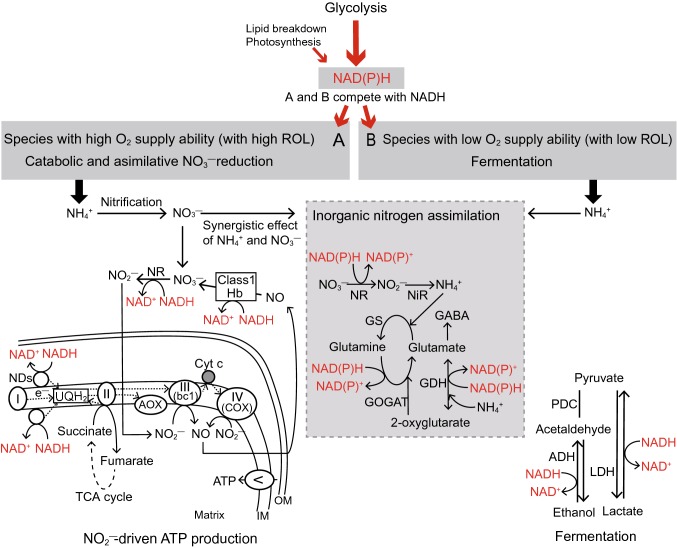
Fig. 3.
Different utilization strategies of inorganic nitrogen (N) source for the maintenance of ATP production caused by the difference in the O2 supply ability in wetland plants under O2-deficient condition. NAD(P)H produced mainly during glycolysis, lipid breakdown, and photosynthesis is oxidized to NAD(P)+ by the following two pathways competing for the oxidation, assimilation, and catabolic reduction of NO−3: NO−2-driven ATP production (A) or fermentation (B). The oxidation of NAD(P)H is shown by red letters and arrows. (A) As the species with high O2 supply ability can accelerate nitrification in their rhizosphere by high radial O2 loss (ROL) from the roots, they can utilize NO−2 produced from NO−3 by hypoxia-induced nitrate reductase (NR) as the electron acceptor in the mitochondrial electron transport chain (mETC) instead of O2. NO−2-driven ATP production enables NAD(P)H oxidation for regulating glycolysis, avoiding cytosolic anoxia, and anaerobic ATP synthesis, which is in the same order as that in the ATP through fermentation during hypoxia. Moreover, species with high potential for NR can oxidize NAD(P)H for N assimilation, and these species can acquire a large amount of N and productivity by the “synergistic effect of NH4+ and NO3−”. (B) The species with low O2 supply ability specializing in the assimilation of NH4+ that dominates the anaerobic soil may oxidize NAD(P)H through fermentation. NAD(P)H levels in the A and B pathway are regulated by glycolysis with pyrophosphate (PPi) utilization by PPi-dependent phosphofructokinase (PFK-PPi) and pyruvate phosphate dikinase (PPDK) instead of ATP and metabolisms of major amino acids such as the alanine, glutamate, 2-oxoglutarate, and γ-aminobutyric acid (GABA). Thus, in wetland plants, A and B pathways function as the N utilization strategy in maintaining the ATP production under anaerobic conditions. Abbreviations are as follows: ADH, alcohol dehydrogenase; AOX, alternative oxidase; bc1, cytochrome bc1; Class 1 Hb, class 1 hemoglobin; COX, cytochrome c oxidase; Cyt c, cytochrome c; GS, glutamine synthetase; GOGAT, glutamine oxoglutarate aminotransferase; GDH, glutamate dehydrogenase; IM, inner membrane; LDH, lactate dehydrogenase; NDs, mitochondrial NAD(P)H dehydrogenases; NiR, nitrite reductase; OM; outer membrane; PDC, pyruvate decarboxylase; TCA, tricarboxylic acid; UQH2, ubiquinol, I–V; mitochondrial complexes I–V
In maize and barley roots, an increase in the NR activity under anaerobic conditions in the presence of NO−3 was observed to be accompanied by a decrease in the ethanol accumulation (Botrel and Kaiser 1997; Fan et al. 1988). In contrast, in roots of rice and Carex species (C. pseudocyperus L. and C. sylvatica Huds.) under anaerobic conditions, exogenous NO−3 stimulated anaerobic respiration (glycolytic fermentation) due to an accelerated glycolytic flux. This stimulation results from a more effective NADH reoxidation capacity by both NO−3 reduction and fermentation compared with only fermentation (Müller et al. 1994; Reggiani et al. 1985, 1993). Moreover, high capacity to use NO−2 as an electron acceptor strongly contributes to continuous ATP production in roots of rice and barley under anoxic stress (Stoimenova et al. 2007). This is because the NO−2-driven ATP synthesis cycle is activated by the addition of NO−3 under anoxic conditions (Fig. 3). These indicate that, under O2-deficient conditions, NO−3 has a favorable effect on the energy metabolism in roots of terrestrial as well as wetland plants.
The balance of the oxidation capacity of reducing equivalents (NADH) between the fermentation and the catabolic NO−3-reduction pathways may be different among species (Fig. 3). The protective effects of NO−3 utilization in rice shoots have been confirmed by analyzing their mitochondria using electron microscopy. In this study by Vartapetian et al. (2003), the marked destructive changes in the coleoptile mitochondria ultrastructure (membrane destruction, cristae disappearance, and pale matrix) were delayed until 48 h after the onset of anaerobic incubation in the presence of exogenous NO−3. In rice plants, NO−3 is reduced through the assimilative NO−3-reduction pathway in their shoots because they show high activities and transcript levels of NR and GS when they are grown in both NO−3 and NH+4 conditions (Yun et al. 2008). In contrast, under O2-deficient conditions, NO−3 is reduced to NO−2 by the catabolic NO3−-reduction pathway in their roots. These reports imply that the capacities of the two NO−3-reduction pathways, the assimilative and catabolic pathways, vary in the different tissues of a plant under anaerobic condition. Both pathways can contribute to hypoxic-stress tolerance through favorable effects on energy metabolism and cytoplasmic pH stabilization (Fig. 3).
NO2−-driven ATP synthesis in plants under O2-deficient conditions
Under O2 deficient conditions, exogeneous NO−3 is reduced to NO−2 by hypoxia-induced NR (Lager et al. 2010). When the O2 level falls below the saturation level of COX, mETC utilizes NO−2 as the electron acceptor instead of O2 for the maintenance of ATP synthesis and O2 concentration in cells (Gupta et al. 2005; Planchet et al. 2005) (Fig. 2). The rates of this NO−2-driven anaerobic ATP synthesis are of the same order as those of glycolytic ATP production during hypoxia, and about 3–5% of the aerobic mitochondrial ATP synthesis (Stoimenova et al. 2007). As NO produced by this NO−2-driven ATP synthesis is immediately converted to NO−3 through the hypoxia-induced Class 1 hemoglobin (Class 1 Hb), mETC components including COX are not damaged (Gupta and Igamberdiev 2011; Nie et al. 2006; Taylor et al. 1994) (Fig. 2). The expression of Class 1 Hb gene is triggered by a disruption of ATP synthesis and by Ca2+ release under O2-deficient conditions (Nie et al. 2006). Class 1 Hb has an extremely high affinity to O2, and its oxidized form, oxyHb, can oxygenate NO to NO−3 even at extremely low O2 concentrations (Trevaskis et al. 1997) (Fig. 2). In such a case, homeostasis of O2 and ROS is maintained because NO can tightly control respiration via inhibiting COX, which leads to an increase in the internal O2 levels under hypoxic conditions (Gupta et al. 2014). Thereafter, NO−3 is reduced to NO−2 by hypoxia-induced NR and recycled by the operation of this Hb/NO cycle (Gupta and Igamberdiev 2011; Igamberdiev and Hill 2004; Igamberdiev and Kleczkowski 2011). In the reaction when NO is converted to NO−3, the heme iron of Hb is oxidized to its ferric form, methemoglobin (metHb) (Fig. 2). To maintain the Hb/NO cycling, Class 1 Hb is regenerated from metHb by ascorbate (Fig. 2). The oxidized form of ascorbate, monodehydroascorbate, is reduced by monodehydroascorbate reductase (MDHAR) along with the oxidation of NAD(P)H (Igamberdiev et al. 2006; Loreti et al. 2005) (Fig. 2). High ascorbate level and induction of MDHAR are observed under hypoxia (Igamberdiev et al. 2006). Besides playing a role in the above cycle, ascorbate also has an important role in the detoxification of ROS and RNS such as H2O2, ONOO−, and O−2. In the NO scavenging process under hypoxia, ascorbate reduces NO to N2O in the mitochondrial matrix while monodehydroascorbate produced from ascorbate oxidizes ONOO− to NO, and thus resupplies NO back to the cell (Alegria et al. 2004; Igamberdiev and Hill 2008) (Fig. 2). The Hb/NO cycling is influenced by the cytosolic NO−2 accumulation via high NR activation. This NR activation is induced by the decrease in ATP during hypoxia/anoxia, but inhibited by low NO−3 concentrations (Gupta et al. 2011; Planchet et al. 2005; Rockel et al. 2002; Stöhr and Mäck 2001). Thus, it seems that NO−2-driven ATP production may be an important strategy for hypoxia-tolerance in plants with high NR potential.
The turnover of NO and maintenance of the cellular redox and energy levels are strong evidence for NO−2-driven ATP production in some terrestrial plants, such as maize, alfalfa, and barley growing on NO−3 dominant soils under low O2 stress (Dordas et al. 2003; Igamberdiev and Hill 2004; Igamberdiev et al. 2006; Sowa et al. 1998). Moreover, NO−2-driven ATP production was also reported in rice plants that typically prefer NH4+ when exogenously supplied with NO−2 and NO−3 under anoxic conditions (Ohwaki et al. 2005; Stoimenova et al. 2007). The rate of anaerobic NO−2-driven mitochondrial ATP synthesis in rice was reported to be 25% of their total ATP turnover rate compared to that of 11.5% in barley during hypoxia (Stoimenova et al. 2007). These values were calculated based on the estimations that mitochondrial proteins represent 7% of the total proteins in heterotrophic plant cells (Douce 1985) and the rate of ATP turnover is 70 nmol min−1 mg−1 mitochondrial protein (Neuburger et al. 1996). This rate could be much higher in rice, 35% of the total ATP turnover rate, because the mitochondrial proteins of rice could comprise as much as 10% of the total proteins (Stoimenova et al. 2007). The ATP production per anaerobic mitochondrial NAD(P)H oxidation of rice is also higher than that of barley (Stoimenova et al. 2007). Thus, species that possess high potential of NO−2-driven ATP production system and contain abundant mitochondrial proteins such as rice plants, can increase their ATP production per anaerobic mitochondrial NAD(P)H oxidation when they utilize NO−3 as the N source. So far, the contribution of NO−2-driven ATP production system in wild wetland plants has been unnoticed because these plants prefer NH4+ in their habitats as nitrification is restricted by stagnant water. However, this system could become a crucial strategy in hypoxia-tolerant wild wetland species with a high ATP turnover rate, when NO−3 is available in their rhizosphere.
Differences in effects of NH4+ on respiration between terrestrial and wetland plants
The energy cost for NH4+ assimilation is lower than that for NO−3 (Bloom et al. 1992). However, many terrestrial plants need to assimilate NH4+ immediately after their absorption in the roots to avoid the toxicity symptoms associated with NH4+ as the sole N source (Britto and Kronzucker 2002). Concentrated NH4+ often increases the respiration rate (NH4+-dependent respiratory increase, ARI) in shoots, roots, and whole plants (Britto et al. 2001; Escobar et al. 2006; Hachiya et al. 2010). Thus, NH4+ utilization may lead to further O2 deficiency through ARI in many terrestrial plants when the N source is limited to only NH4+ by rhizosphere environmental changes such as submergence. In shoots and roots of terrestrial plants, ARI that is induced by an increase in NH4+ concentration in the external media (Britto et al. 2001) increases the ATP content and ATP/ADP ratio by inducing the phosphorylating components of mETC such as complex I, III, and IV (COX) (Curi et al. 2003; Hachiya et al. 2010; Welchen et al. 2002). However, these increases in respiratory ATP production are not related to an increase in useful energy demands such as growth. One of the main causes of ARI has been suggested to be an increase in the inward/outward flux of NH4+ across the plasma membrane, called “futile NH4+ cycling (FAC)” (Britto and Kronzucker 2002; Britto et al. 2001; Hachiya et al. 2010). As NH4+ uptake via the NH4+ transporter (AMT) is accompanied by proton extrusion from the plasma membrane H+-ATPase to maintain the cytosolic charge balance (Britto and Kronzucker 2002), the increased FAC under conditions of NH4+ as the sole N source would require more respiratory ATP (Britto et al. 2001). Consequently, ARI would occur to meet the increase in ATP demand related to increased FAC, when the plants are grown under high concentration of NH4+. Indeed, in some NH4+-intolerant terrestrial species such as maize and barley, the H+-ATPase activity is high when they are grown under conditions of NH4+ as the sole N source (Britto et al. 2001; Nielsen and Schjoerring 1998; Schubert and Yan 1997). In contrast, in the roots of NH4+-tolerant rice, ARI is not observed (Britto et al. 2001), and the activity of H+-ATPase is independent of the N source (Zhu et al. 2009). Moreover, the experiments in which NH4+ metabolism and growth rate are analyzed in rice plants have reported that the decrease in energy cost for FAC does not correlate with the optimized growth (Balkos et al. 2010). This low FAC in rice plants may reflect that they have evolved to be NH4+ tolerant without any energy cost to maintain the NH4+ balance across the plasma membrane (Karasawa et al. 1994; Kronzucker et al. 1999, 2001).
ARI is also explained by another hypothesis in which it occurs in relation to the dissipation of excess reducing equivalents in mETC. The NO3− assimilation process competes with mETC for the reducing equivalents. The shift of an available N source from NO3− to NH4+ increases the reducing equivalents that are not consumed through NO3− assimilation and are thus available to be consumed by mETC, thereby increasing the O2 uptake rate (Bloom et al. 1992; Escobar et al. 2006). In particular, under low O2 stress conditions where COX is saturated with reducing equivalents, there is a possibility that the non-phosphorylating AOX and NDs in mETC can consume the excessive reducing equivalents without being limited by adenylate control (Escobar et al. 2006; Vanlerberghe et al. 2009). In fact, AOX capacity in terrestrial plants such as Arabidopsis, pea, and spinach increases when they are transferred from NO3− to NH4+ conditions (Escobar et al. 2006; Frechilla et al. 2002; Lasa et al. 2002). NDB2, which is a major isoform of NDex, is also induced in shoots and roots of Arabidopsis under NO3−-depleted conditions (Wang et al. 2004; Watanabe et al. 2010). Although these responses have an important role in the dissipation of excessive reducing equivalents under the low O2 stress conditions, ARI itself would lead to further strict anoxic threat for NO3−-preferring terrestrial plants under O2-deficient condition.
Two contrasting adaptive strategies in flood-tolerant plants: the low oxygen escape strategy versus the low oxygen quiescence strategy
Flood tolerant plants that can survive at O2 deficiency or light-limited submergence conditions are characterized by two survival strategies. One of them is the low O2 escape strategy (LOES), and the other is the low O2 quiescence strategy (LOQS) (Fig. 4). Plants with LOES phenotypes show upward bending of leaves (hyponasty) that can enhance shoot elongation, formation of interconnected air-filled voids (aerenchyma), induction of barriers to radial O2 loss (ROL) in roots, development of adventitious roots (ARs), formation of gas films on leaf surfaces, modification in leaf anatomy, and pressurized gas flow through porous tissues under O2-deficient conditions. All of these characteristics are not necessarily found in one species (Blom 1999; Evans 2004; Jackson and Armstrong 1999; Ridge 1987; Sauter 2013) (Fig. 4). The elongation of aerial organs and the formation of aerenchyma and ARs are all ethylene dependent. The former trait is also controlled by a hormonal network, which includes ABA and GA, and the latter trait by ROS (Voesenek and Bailey-Serres 2015) (Fig. 4). In contrast, plants with LOQS phenotypes cease their growth and save their carbohydrate reserves under O2-deficient conditions.
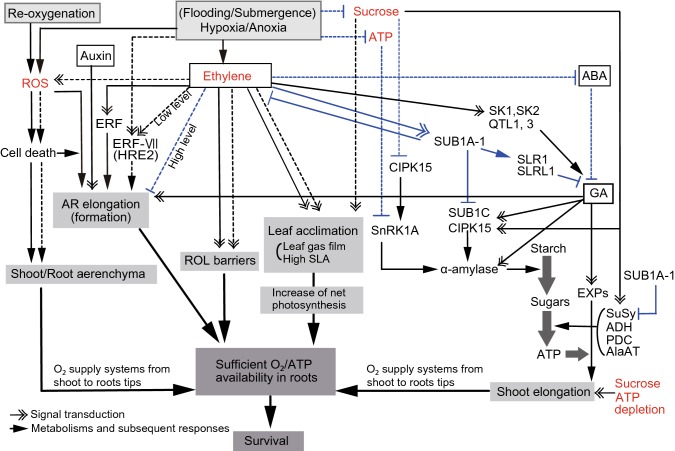
Fig. 4.
Characteristics of low O2 escape strategy (LOES) and low O2 quiescence strategy (LOQS) to hypoxia/anoxia caused by flooding/submergence in wetland and terrestrial plants. Black solid and dashed lines are the networks of LOES (aerenchyma formation, shoot elongation, radial O2 loss (ROL) barriers, and leaf acclimation) in wetland and terrestrial plants, respectively, while blue dashed lines indicate responses to suppress LOES in both plants; blue solid lines indicate the submerged regulatory network of LOQS in rice (wetland species). Four key factors, ROS accumulation, ethylene content, ATP depletion, and sucrose reserve decrease, involve the LOES and LOQS networks are shown in red letters. ROS production in hypoxic and anoxic stresses causes programmed cell death (PCD) in both plant types and involves the mechanisms of adventitious roots (ARs) emergence and aerenchyma formation. AR elongation in Arabidopsis (terrestrial plant) is promoted by the hypoxia signal and its formation is mediated by hypoxia-responsive HRE2, which is one of the group VII ethylene response transcription factors (ERFVIIs). High ethylene level inhibits the AR formation in Arabidopsis under hypoxic condition, although ARs are formed at low ethylene level. In contrast, in rice plants, ethylene has promotive effects on the AR formation and elongation. The contrasting regulation by ethylene on ARs may reflect different adaptive strategies in the flood-tolerant rice plants compared to the flooding-intolerant terrestrial species such as Arabidopsis. Leaf acclimation such as high specific leaf area (SLA), reoriented chloroplasts along with cell wall in leaf epidermis, thin cuticles and cell walls, development of dissected leaves underwater, and the maintenance of gas films can increase the net photosynthesis by decreasing the diffusion resistance for CO2. The leaf plasticity could also result from the accumulation of ethylene and a decrease in CO2 levels. Flooding/submergence causes ethylene accumulation, which triggers gibberellin (GA)-promoted cell elongation through the expansins (EXPs). In deep-water rice with LOES, ethylene promotes the induction of SNORKELs (SKs, SK1, and SK2) and GA elevation and the internodes of the shoots elongate rapidly to come out of the water surface. In the deep submergence lines of rice with LOQS, ethylene activates the submergence 1A-1 (SUB1A-1) promoting an increase in SLENDER RICE 1 (SLR1) and SLENDER RICE-Like 1 (SLRL1) transcription factors, which inhibit GA-mediated activation of gene expressions. This LOQS characteristic of rice can limit carbohydrate consumption by inhibiting shoot growth. Wetland plants develop shoot and root aerenchyma, ROL barriers, and elongated shoots elongation and these characteristics of LOES act synergistically with each other in enhancing the stability of O2 and ATP availability in roots where nitrogen (N) uptake and active N assimilation take place. Abbreviations are as follows: ABA, abscisic acid; ADH, alcohol dehydrogenase; AlaAT, alanine aminotransferase; CIPK15, calcineurin B-like interacting protein kinase 15; HRE2, hypoxia-responsive ERF 2; PDC, pyruvate decarboxylase; QTL1 and 3, quantitative trait loci on chromosomes 1 and 3; SnRK1A, sucrose non-fermenting receptor kinase 1A; SuSy, sucrose synthase; SUB1A-1, submergence 1A-1
Molecular mechanisms of LOES and LOQS
In rice varieties, both LOES and LOQS are found to counteract flooding stress. SNORKEL1 (SK1) and SNORKEL2 (SK2) of deep-water rice varieties (LOES type) are involved in the rapid internode elongation and escape of leaves near the water surface (Hattori et al. 2009), whereas submergence 1A-1 (SUB1A-1) in lowland varieties of rice (indica) (LOQS type) limits elongation, growth, and carbohydrate consumption (Fukao and Bailey-Serres 2008; Fukao et al. 2006) (Fig. 3). The key regulatory genes in both strategic responses are the ethylene-responsive TFs of the subfamily group VII (ERF-VII); the TFs act downstream of ethylene and modulate GA-mediated shoot growth (Bailey-Serres and Voesenek 2010; Voesenek and Bailey-Serres 2015). Deepwater varieties of rice (LOES type) can escape from adverse partially submerged deep-water conditions through SK1 and SK2 genes (SKs) that trigger rapid internode elongation at a rate of 25 cm day−1 (Colmer et al. 2014; Hattori et al. 2011) (Fig. 4). In contrast, these genes are absent in shallow water varieties including all japonica varieties (LOQS type). Moreover, two additional uncharacterized loci on chromosomes 1 and 3 (QTL1 and 3) are needed along with SKs for the full deep-water escape response (LOES type) (Ayano et al. 2014). Shoot and internode elongations in submerged deep-water varieties of rice are promoted by cell expansion and division, which are positively regulated by ethylene and GA (GA1 and GA4). These hormones enable expansins (EXPs) and α-amylase to drive cell elongation and starch degradation by SKs and QTL1 and 3 (Choi et al. 2004; Rzewuski and Sauter 2002; Sauter et al. 2002; van der Knaap et al. 2000) (Fig. 4).
In contrast, the indica varieties possessing SUB1A-1 (LOQS type) decrease their metabolic activities and constrain their growth to save energy consumption under shortly prolonged submergence conditions (up to only a few weeks) (Fig. 4). The indica and japonica varieties lacking the SUB1A gene or SUB1A-1 allele cannot cease their metabolic activities (Fukao et al. 2006; Xu et al. 2006). Submergence 1 (SUB1) locus of rice consists of three genes, SUB1A, SUB1B, and SUB1C. The expression of SUB1A-1 alone is sufficient to provide flood tolerance, but it exists only in flood-tolerant varieties with LOQS traits. SUB1C is present in all varieties, and responds to GA and positively regulates the expression of several EXPs (Fukao and Bailey-Serres 2008; Hattori et al. 2011; Xu et al. 2011). SUB1B is ERF similar to SUB1C (Bailey-Serres et al. 2010). The submergence-intolerant japonica cultivar Nipponbare has both SUB1B and SUB1C, but lacks SUB1A. SUB1A-1 inhibits shoot elongation by maintaining the levels of TFs, SLENDER RICE 1 (SLR1) and SLENDER RICE-Like 1 (SLRL1), to counterbalance the GA responsiveness and regulate the SUB1C mRNA level negatively (Bailey-Serres and Voesenek 2008; Fukao et al. 2006) (Fig. 4). Furthermore, SUB1A-1 negatively regulates the submergence-induced synthesis of ethylene, mRNA expression of cell-wall-loosening EXP, starch and sucrose degradation (Fukao et al. 2006), and chlorophyll degradation through zinc-finger TF encoded by DELAY OF THE ONSET OF SENESCENCE (Fukao et al. 2012; Winkel et al. 2014). The elongation processes through SUB1C require a large amount of energy during shoot submergence because elongation of aerial organs is accompanied with the rapid and efficient translocation of photosynthates and reserved carbohydrates and amino acids (Ayano et al. 2014; Hattori et al. 2011; Kende et al. 1998; Sauter 2000). In contrast, LOQS varieties with SUB1A-1 can decrease their energy utilization until the water level decreases and normoxic conditions are restored, thereby they resume growth with preserved energy under subsequently normoxic conditions (Ayano et al. 2014; Barding et al. 2012, 2013; Fukao and Bailey-Serres 2008; Hattori et al. 2011; Kende et al. 1998; Nagai et al. 2010; Sauter 2000). The rice varieties with SUB1A-1 can restrict the rate and extent of starch hydrolysis and accumulate lower concentrations of ethanol, lactate, and amino acids than the varieties without SUB1A (Barding et al. 2012, 2013). It has been assumed that repeated elongation of aerial tissues in every short-term submersion may damage the growth by serious re-oxidation and water loss in the LOQS phenotypes (Hattori et al. 2011; Nagai et al. 2010). The varieties with SUB1 can manage ROS accumulation and leaf water loss during recovery from submergence conditions to a minimum extent. This is because they have higher levels of mRNA associated with the repression of ROS accumulation during the recovery phase (Fukao et al. 2011; Mustroph et al. 2010).
In wild wetland plants such as Rumex palustris, R. acetosa. Sagittaria trifolia and Lotus tenuis, it has been suggested that there are networks in conserved flooding response that relate to growth and stress-induced catabolism of carbohydrates for the efficient ATP production. However, studies on ERF-VII TFs (SKs and SUB1) are required in wild wetland species that experience long-term flooding (Kim et al. 2000; Manzur et al. 2009; Ookawara et al. 2005; Vreeburg et al. 2005). In these plants, there are considerable genetic variations between and within species in the ethylene-induced elongation capacity under submergence conditions. It is noteworthy that the wild species R. palustris displays submergence escape by ethylene-driven shoot elongation (LOES type) (Benschop et al. 2005), and R. acetosa invokes quiescence owing to a lack of ABA down-regulation, GA up-regulation, and increased EXP expression (LOQS type), although these two species are closely related to each other (Benschop et al. 2005; Chen et al. 2009; van Veen et al. 2013; Vriezen et al. 2000). In R. palustris, it seems that the elements downstream of ethylene and upstream of ABA and GA can switch on this elongation cascade (Benschop et al. 2005; Chen et al. 2009; van Veen et al. 2013). Moreover, R. palustris exposed to dark under submergence conditions can convert their strategy from escape to quiescence for survival. This strategy is achieved by the pretreatment using ethylene, in which LOQS ability is promoted (van Veen et al. 2013). This strategy conversion in R. palustris relates to the light-signaling genes that regulate the enhancement of shoot elongation (van Veen et al. 2013), and this observation demonstrates the similarity of growth control between shade avoidance and underwater elongation. Another wetland species, L. tenuis, also elongates upon partial submergence but arrests its growth upon complete submergence. It switches from LOES to LOQS due to elevation of shoot porosity and limited consumption of soluble carbohydrates in shoots and roots (Manzur et al. 2009). Both antithetical LOES and LOQS strategies exist within a single species and are not mutually exclusive. These may be combined by the threshold of O2 level or energy deficiency. In this regard, Voesenek and Bailey-Serres (2015) indicate that three key factors, an increase of cellular ethylene content, depletion of ATP, and consumption of readily available sucrose in the submergence network, can contribute to increased induction and regulation of shoot elongation. The level of reserved carbohydrates for ATP production seems to strongly affect the strategies for sustainable and facilitative survival in various natural flooding environments (Fig. 4). Especially the LOES type may be required for the high photosynthetic capacity and translocation activity of photosynthates and reserves under submergence conditions. Therefore, the interplay among hormones (ethylene, ABA, and GA), O2 availability, and specific metabolites (ATP, sugars, and pyruvate) needs further clarification for understanding the network balancing growth and quiescence.
Avoidance strategies in LOES-type plants for improvement of O2 level within plant tissues
When plants are submerged by flooding, species with LOES phenotypes respond to O2 deficiency for improvement of cellular O2 level. Shoot elongation, formation of interconnected air-filled voids (aerenchyma), pressurized gas flow through the aerencyma and leaf acclimations for the decrease of the diffusion resistance to air can function to improve cellular O2 levels. In the roots, developed aerenchyma, formation of the ROL barrier from the roots surface and development of ARs can enhance the longitudinal O2 diffusion in root tips with the most active cells.
Aerenchyma
Aerenchyma can decrease the gas diffusion resistance from the atmospheric tissues to the O2-deficient tissues. The formation of aerenchyma and enhancement of the gas transport ability are essential strategies in LOES (Fig. 4). O2 produced during photosynthesis or taken up by the aerobic shoots diffuses inside the aerenchyma connecting the shoots and the roots; this O2 diffusion supports respiration in O2-deficient underwater organs (Fig. 4). Aerenchyma can be formed by different processes such as schizogeny and lysigeny (Drew et al. 2000; Evans 2004; Seago et al. 2005). These processes often appear simultaneously at different organs in one individual plant (Steffens et al. 2011). Although little is known about the process of schizogenous aerenchyma formation (Evans 2004), it has been hypothesized that the causal protein, NOP1, regulates the schizogenous formation of air chambers via a membrane-localized receptor-like kinase signaling pathway resulting in ubiquitylation and degradation of target proteins (Ishizaki et al. 2013). In contrast, the lysigenous formation of air chambers via programmed cell death (PCD) requires ethylene, Ca2+, and ROS signaling, which ultimately breaks down the cell walls as observed in some species such as rice, Arabidopsis, maize, and wheat (Drew et al. 2000; Evans 2004). In roots of maize and deep-water rice, studies have reported that aerenchyma formation is associated with the accumulation of ROS and down-regulation of METALLOTHIONEIN 2b mRNA encoding a ROS scavenging protein (Rajhi et al. 2011; Steffens et al. 2011). Moreover, it seems that the Ca2+-dependent plasma membrane-localized respiratory burst oxidase homologs (RBOHs) influence the ROS sources in this process as they have been reported to promote apoplastic superoxide production to amplify ROS-mediated signaling in wheat and rice (Parlanti et al. 2011; Yamauchi et al. 2013b).
Pressurized flow-through system
In addition to the inward diffusion, a mechanism of gas transport in all wetland plants, some floating plants and macrophytes have a pressurized flow-through system in stems and rhizomes to aerate the O2-deficient underwater organs (roots and rhizome). This system enables the underwater organs to keep the O2 concentration to an ambient level for maintaining oxidative phosphorylation, thereby normalizing the ATP concentration (Colmer 2003; Sorrell and Hawes 2009) (Fig. 4). Pressurized flow-through is produced by the species-specific positive pressure capacity in shoot tissues and the resistance to the flow in the aerenchyma. Also, leaf-to-air gradients of temperature and humidity affect the pressurized flow (Brix et al. 1992; Colmer 2003). This flow mechanism also contributes to an outward diffusion of ethylene generated in roots and methane generated in soils (Colmer 2003; Laanbroek 2009). Thus, pressurized flow-through that facilitates effective gas flow through developed aerenchyma seems to provide a competitive advantage to large varieties of plants in deep-water habitats (Konnerup et al. 2011).
Leaf acclimation
In terrestrial plants, the net photosynthesis of submerged leaves often decreases significantly compared with that of aerial leaves due to an exponential decrease in light intensity with increasing depth and resistance to CO2 and O2 fluxes in submerged leaves (Colmer et al. 2011; Herrera 2013). In response to submergence, some terrestrial plants develop new acclimated leaves that are characterized by higher specific leaf area (SLA) that is a ratio of leaf area to leaf mass, reoriented chloroplasts along with cell walls in leaf epidermis, thin cuticles and cell walls, development of dissections, and maintenance of gas films (Colmer et al. 2011; Mommer et al. 2005b) (Fig. 4). All of these traits can decrease the diffusion resistance to CO2, thereby enabling the leaves to increase the net rate of CO2 assimilation and decrease the CO2 compensation point under water (Mommer et al. 2006; Pedersen et al. 2013; Winkel et al. 2014).
Wetland plants, such as Rumex palustris, R. acetosa and rice, show plastic acclimation of their morphological, anatomical, and biochemical traits of leaves to submergence (Mommer et al. 2005a, b; Pedersen et al. 2009, 2013; Winkel et al. 2014). Ranunculus repens constitutively dissect their leaves when they grow underwater (He et al. 1999). In R. palustris, the new acclimating leaves developed in underwater conditions can lead to a nearly 40-fold decrease in the diffusion resistance to CO2 (Mommer et al. 2005a). Rice plants show a large variation in these leaf traits among varieties. Submergence-tolerant landrace FR13A with LOES has higher net underwater photosynthesis, longer retention of the leaf gas film and longer persistence compared with a Sub1 variety, Swarna-Sub1 (Winkel et al. 2014; Xu et al. 2006). These traits of FR13A contribute to submergence tolerance because the persistence of gas film could potentially increase net photosynthesis and internal aeration during submergence. In contrast, in Swarana-Sub1, the duration of gas film retention is shorter than that in FR13A, although Swarana-Sub1 can maintain carbohydrate levels during submergence. Since both varieties have SUB1A, genetic determinants other than SUB1A contribute to gas film formation and underwater photosynthesis. Leaf acclimation ability to submergence may be related to flood tolerance in wetland plants with LOES. In contrast, these acclimations may not be related to flood tolerance in terrestrial plants (Mommer et al. 2007). The accumulated ethylene does not necessarily function as a signal for the flood-induced leaf acclimation in terrestrial plants under flooded conditions, but other signals associated with changed photosynthetic rates and/or decreased levels of carbohydrates may induce these leaf acclimations (Bailey-Serres and Voesenek 2008) (Fig. 4).
Barrier to radial O2 loss
The O2 supplied from the aboveground to underground organs diffuses to the anaerobic soil as radial O2 loss (ROL), which contributes to protecting the roots from toxic ions (Fe2+and Mn2+) and to the nitrification in NH4+-predominant and excessive-reduced soils by the oxidized layers around the roots. Moreover, wetland plants and some terrestrial plants form an impermeable barrier to ROL from the root basal zone to the apex (ROL barrier) by the deposition of suberin in the root exodermis (Fig. 4). The suberin layer is mainly composed of long-chain fatty acids. The ROL barrier acts synergistically to enhance the longitudinal O2 diffusion in the root tips with the most active cells and enables the development of aerobic rhizosphere around the root tips for root extension (Abiko et al. 2012; Armstrong and Beckett 1987; Colmer 2003; Sauter 2013) (Fig. 4). The ROL barrier is permanently formed in some wetland species, or temporarily induced by waterlogging in rice and wheat (Colmer 2003; Kotula et al. 2009; Malik et al. 2011) (Fig. 4). Molecular investigations in rice plants have clarified that the ROL barrier formation involves the up-regulation of genes including a hypodermal cell ABC transporter (REDUCED CULM NUMBER1 [RCN1]/OsABCG5), which is proposed to export the long-chain fatty acids and/or their derivatives across the hypodermal plasma membrane into the apoplast to induce hypodermal suberization (Shiono et al. 2014). Indeed, the metabolite profile analysis in rice roots growing under barrier-forming stagnant conditions reveals that the concentrations of long-chain fatty acids and malate, which is a substrate for fatty acid biosynthesis, gradually increase from the root apex to the base (Kulichikhin et al. 2014).
Adventitious roots
Adventitious roots (ARs) are also associated with conferring developmental plasticity to plants under waterlogged condition. ARs with high porosities emerge from submerged stem nodes and hypocotyls to replace the existing and deteriorating primary root system in rice, R. palustris, Solanum lycopersicum, and Larix laricina (Calvo-Polanco et al. 2012; Dawood et al. 2014; Dawood et al. 2016; Eysholdt-Derzsó and Sauter 2019; Visser et al. 1996; Yang et al. 2018; Zhang et al. 2017) (Fig. 4). Some ARs develop chloroplasts and thus provide an additional source of O2 and carbohydrates (Rich et al. 2012) because ARs typically develop in well-aerated topsoil layers (Dawood et al. 2014; Eysholdt-Derzsó and Sauter 2019; Zhang et al. 2015). The terrestrial plant Solanum dulcamara can survive under flooding condition by replacing the original flood-sensitive root system with aerenchymatous ARs that are produced from pre-formed primordia on the stem. The AR outgrowth is involved with auxins, ABA, and jasmonic acid (Dawood et al. 2016; Vidoz et al. 2010; Yang et al. 2018). ABA is a negative regulator of AR outgrowth, but there is a highly tissue-specific response to decreased ABA levels. Auxins may be necessary for AR outgrowth because a disruption in the auxin signaling in AR primordia of S. dulcamara resulted in the abortion of AR outgrowth under complete submergence (Dawood et al. 2016) (Fig. 4). Moreover, the auxin pathways act together with decreased levels of ABA because the AR emergence in S. dulcamara was not sufficient when they were treated with auxin alone (Yang et al. 2018).
In Arabidopsis, low levels of ethylene and hypoxia signals mainly promote AR elongation due to the expression of the hypoxia-responsive HRE2 which is one of the ERFVII TFs (Bailey-Serres et al. 2012; Eysholdt-Derzsó and Sauter 2019; Hess et al. 2011) (Fig. 4). However, high levels of ethylene inhibit the initial formation of ARs because high ethylene concentration can override the hypoxia signal (Eysholdt-Derzsó and Sauter 2019). Thus, the formation and elongation of ARs in Arabidopsis are controlled by ethylene in a dose-dependent manner (Fig. 4). Although low levels of ethylene in Arabidopsis may contribute to fast elongation of ARs immediately after exposure to hypoxia, they cannot exert hypoxia tolerance. This is because they do not form subsequent ARs when exposed to long-term severe anaerobic stress associated with high ethylene accumulation. In contrast to Arabidopsis, ethylene has promotive effects on the AR emergence and growth in several wetland species including rice (Lin and Sauter 2018). The contrasting regulation of ethylene on ARs may reflect the different adaptive strategies between the flood-tolerant and intolerant species (Fig. 4).
The roles of these phytohormones in the terrestrial species would be different from those in rice plants, especially in the AR emergence pathway. The emergence of the AR primordia in rice plants involves PCD in the overlying epidermal cells, which is mediated by ethylene-promoted ROS production (Steffens et al. 2013) (Fig. 4). The developmental process of ARs involves the up-regulation of the plasma membrane RBOHs (Steffens et al. 2012), and the decrease in METALLOTHIONEIN 2b, which regulates the ROS amelioration for nodal AR emergence (Voesenek and Bailey-Serres 2015). The location of this PCD is determined by the force exerted by the outgrowing meristems, and at the same place, epidermal weakening for emergence of AR primordium can be elicited by the essential degradation of pericycle and epidermal cells by cell wall-modifying proteins such as EXPs, subtilisin-like proteases, pectate lyases, and endo-β-1,4-glucanases (Cho and Kende 1997; Kimpara et al. 2008; Laskowski et al. 2006; Steffens et al. 2012; Yamauchi et al. 2013a).
Diverse strategies for avoidance from O2 deficiency
In rice and some wetland species, ROL barrier and leaf acclimation improve their underwater photosynthesis, root aeration, and growth (Colmer and Pedersen 2008; Pedersen et al. 2009; Winkel et al. 2013, 2014) (Fig. 4). These characteristics act synergistically with each other to enhance flood tolerance in wetland species (Fig. 4). In contrast, acclimated leaves with morphological modifications and ARs cannot provide flooding tolerance to terrestrial plants because O2 cannot be transported to the underwater organs of terrestrial plants (Fig. 4). As roots consume large amounts of O2 for nutrient uptake, they often suffer from O2 deficiency. The internal O2 pressure in roots is much lower than that in shoots, especially at night when photosynthetic O2 production ceases (Pedersen et al. 2006). Therefore, effective aerenchyma is required to enable the roots to sustain the oxidative phosphorylation leading to normal ATP production and growth (Visser and Pierik 2007). However, despite the benefits of aerenchyma under flooded conditions, it is not constitutive in all plants. In this regard, Striker et al. (2007) described the significant trade-off between root porosity and mechanical strength. Hu et al. (2013) also confirmed that aerenchyma inhibits radial nutrient transport in maize roots. Therefore, it could be assumed that many wetland plants can maintain an optimal balance between their growth and low O2 tolerance in their roots, and this balance is significantly different from that in the terrestrial plants.
Traits of root N use and O2 uptake in LOES-type wetland plants under O2-deficient conditions
Differences in the rhizosphere N conditions such as sole or mixture of NO−3 and NH4+ generally change root density, extension, and whole weight. These changes alter the rhizosphere pH and redox potential, which regulate the root cell proliferation and mechanical properties (Bloom et al. 2003; Brix et al. 2002; Marschner and Römheld 1983). Because NH4+ dominates as the inorganic N source under anaerobic soil conditions due to the limitation of nitrification, NH4+-tolerant wetland species ameliorate the toxic effect of excess NH4+ by exhibiting high GS activity for the quick assimilation of NH4+ compared with that of NH4+-intolerant terrestrial plants (Balkos et al. 2010; Britto and Kronzucker 2002). As rice plants have isoenzymes of the cytosolic GS1 gene family (OsGLN1;1 and OsGLN1;2) that can be classified into high-affinity subtypes with relatively high Vmax, these GSs facilitate active NH4+ assimilation in their roots (Ishiyama et al. 2004). Studies on NH4+ metabolism in rice, maize, and tomato plants have reported that rice plants have much higher GS activity than the other species, and the GS activity increased more in the shoot tissues than that in the roots with the increase in NH4+ (Magalhaes and Huber 1991). This indicates that the GS activity is a key factor in the detoxification and assimilation of NH4+ in shoots of plant species with efficient NH4+ utilization (Magalhaes and Huber 1991). The ability to rapidly assimilate NH4+ not only in roots but also in shoots can also function as an N acquisition strategy in the wetland species growing under O2-deficient conditions. This is because the up-regulated N assimilation in shoots can lead to decrease in the demand for N assimilation in the roots, thereby decreasing root respiration and avoiding O2 deficiency in roots (Fig. 5). When wetland wild grass and Carex species (C. lyngbyei, C. lasiocarpa var. occultans, and C. middendorffii) are grown in the sole NH4+ treatment under low O2 condition, they exhibit a smaller root to shoot weight ratio (i.e., high S/R ratio) and increased net N uptake rate per unit root weight (NNUR) compared to the sole NO−3 treatment. This high S/R ratio can lead to the decrease in the whole-root O2 consumption in the sole NH4+ treatment (Nakamura and Nakamura 2016; Nakamura et al. 2010, 2013) (Fig. 5). The decreased root growth (high S/R ratio) with sole NH4+ treatment is more prominent in species with weak O2-supply system with only diffusion than in species with strong O2-supply system with pressurized gas flow (Nakamura et al. 2013) (Fig. 5). Although the high NNUR causes high root respiration rate per unit root weight in species with low O2-supply system, the whole root respiration rate per shoot weight is similar between sole NH4+ and NO−3 conditions due to the compensation for high O2 uptake in roots by their high S/R ratio under sole NH4+ condition (Fig. 5). Thus, it seems that wetland plants primarily employ the NH4+ utilization strategy for N-acquisition, which enables them to acquire sufficient N for their growth and to minimize and regulate the whole-root O2 consumption depending on the O2 supply from shoots to roots (Fig. 5).
Fig. 5.
Effects of NO−3 (left) and NH4+ (right) utilization as the sole nitrogen (N) source on root respiration and N acquisition in wetland plants. The downward (⇩) and upward (⇧) arrows indicate the decreasing and increasing responses, respectively. NO−3 utilization results in a low root to shoot weight (S/R) ratio, which is unfavorable for O2 supply. As the N uptake rate per root weight (NNUR) per root respiration rate decreases when the wetland plants utilize NO−3, they develop the roots for N acquisition, consequently increasing the respiration of the whole roots. Therefore, NO−3 utilization requires high O2 supply to maintain productivity. In contrast, NH4+ utilization results in a high S/R ratio, which is favorable for O2 supply, and high NNUR per root respiration. Moreover, when NH4+ concentrations increase, the wetland plants may assimilate NH4+ in their shoots instead of their roots. These traits contribute to a decrease in the respiration of the whole root, and thus wetland plants can ensure NH4+ utilization even under low O2 supply. Photograph of Carex lyngbyei grown in 200 µM NO3− and NH4+ treatments under hypoxic hydroponic culture for 1 month. Bar 5 cm
Even in anaerobic soil dominated by NH4+, small amounts of NO3− are produced by oxidization in the soil due to the O2 flux to the rhizosphere by species with active ROL (Brix et al. 2002; Kirk and Kronzucker 2005). When NH4+-preferring species such as rice are grown under conditions where both NH4+ and NO−3 are supplied, they show improved productivity and increase in the net N acquisition and ATP production (called “the synergistic effect of NH4+ and NO3−”) compared with only sole NH4+ conditions (Kirk and Kronzucker 2005; Kronzucker et al. 1999; Li et al. 2006; Ying-Hua et al. 2006, 2007). Some studies have also reported the underlying mechanisms by which the addition of NO−3 to an NH4+ containing soil increased the Vmax value of NH4+ uptake and plasma membrane potential due to an increase in the number of NH4+ transporters, leading to enhanced growth and N uptake in rice plants (Ying-Hua et al. 2006, 2007). Moreover, the synergistic effect of NH4+ and NO−3 in rice differs among varieties depending on the supply level of each inorganic N source and is genetically controlled (Ancheng et al. 1993; Ying-Hua et al. 2007). The synergistic effect of inorganic N has been studied in wild wetland plants. The synergistic effect of NH4+ and NO−3 may be limited to species growing in habitats where nitrification occurs in their rhizosphere. Especially, the fast-growing species with high O2-supply system may display high ATP production and N acquisition under NH4+-dominant soil conditions due to this synergistic effect (Fig. 5).
The acquisition abilities of each inorganic N source in the soil are different among species with different O2-supplying abilities even when they exist in similar habitats. Phragmites australis and Zizania latifolia are observed in the same habitat, but the abilities of O2 supply are different. P. australis has a high ability of O2 supply by the convective gas flow system and high ability of NO−3 use owing to relatively high NR activity in roots under sole NO−3 and− low O2 conditions. In contrast, Z. latifolia with only diffusion as the O2 supply system cannot survive under such conditions because of low activity of NR (Nakamura et al. 2013). Moreover, species with high ability of NO3− use can utilize the NO2−-driven ATP production system under O2-deficient conditions, but species without the ability of NO−3 use can only utilize the fermentation system for ATP production under O2-deficient conditions (Fig. 5). Thus, the utilization ability of inorganic N depending on the O2 supplying capacity might be related not only to the N acquisition strategy but also to the anaerobic ATP production pathway under O2-deficient conditions. This suggests that low O2 tolerance is characterized by the functional linkage between N utilization strategy and O2-supply capacity for the anaerobic energy conservation in wetland plants (Fig. 5). Stoimenova et al. (2007) have reported that rice plants with a low O2-supplying diffusion system can have the NO2−-driven ATP production system in the presence of exogenous NO3− under anoxic condition, and this can strongly contribute to energy maintenance under anoxic conditions.
In general, NO production is elevated under low O2 conditions via the catabolic pathway from NO2− (NO2−-dependent NO production) (Planchet et al. 2005; Rockel et al. 2002). In root cortex under hypoxic condition, NO is released from organelles when it is not scavenged by Class 1 Hb involving the NO−2-driven ATP synthesis cycle and other NO detoxification systems in the cytosol. NO increases ethylene that activates the signal transduction pathway involving phosphoinositides and Ca2+, and thereby aerenchyma formation is induced through PCD (Dordas et al. 2003; Drew et al. 2000; Voesenek and Bailey-Serres 2015; Yamauchi et al. 2013a). In transgenic alfalfa root cultures expressing the antisense barley Hb transcripts, the NO level was not changed, and cell breakdown and aerenchyma formation were induced when the cells were subjected to hypoxia (3% O2). In contrast, no cell breakdown was reported in the overexpressing Hb line under the same growth conditions (Dordas et al. 2003). Most wetland plants can develop aerenchyma in their shoots and roots, but the mechanisms of aerenchyma formation and their development level may differ among species depending on their NO−3 utilization ability and the NO levels. Further analyses are required to compare the effects of NO−3 utilization on the aerenchyma formation among species with different O2 supply capacities.
The formation of root cortical aerenchyma can also be induced by nutrient deficiency in the terrestrial species, maize (Hu et al. 2013). The formation of root cortical aerenchyma decreases the radial transport of nutrients by decreasing the living cortical tissue, which leads to a decrease in the maintenance requirements of living tissues of roots (Hu et al. 2013). Similarly, aerenchyma formation in wetland plants may contribute to not only avoid O2 deficiency in root tips but also decrease the respiratory energy required to maintain the living tissues under O2 limiting conditions. This saving of energy consumption by the aerenchyma formation may increase the allocation of the respiratory energy to other processes such as root growth and nutrient uptake. Some wetland plants with developed aerenchyma allocate their root respiratory ATP to maximize the N uptake instead of root maintenance and growth (Nakamura and Nakamura 2016). Such root responses in wetland plants could be their strategy for efficient O2 consumption and high N acquisition for adapting to O2 deficiency.
Conclusion
Wetland species with hypoxia and anoxia tolerance can regulate their carbohydrate level to maintain the glycolytic flux and reduce ATP consumption under O2-deficient condition. At low O2, NAD(P)+ regeneration by ethanol fermentation, sucrose degradation through the energy-saving SuSy pathway, and amino acid metabolisms such as glutamate, GABA, and alanine are common in both wetland and terrestrial species. Gene expression of α-amylase in the aleurone layer and storage organs at the germination and initial growth stages are limited to wetland species. Moreover, an effective tolerant function in the wetland species for surviving long-term hypoxic and anoxic conditions is caused by maintaining glycolysis through the reversible reaction catalyzed by PPDK and PFK-PPi. In rice plant, cytosolic PPDK is more abundant in their roots than that in their shoots, and this may affect their adaptive response to frequent fluctuations in O2 concentration. In post-hypoxic/anoxic stress, the metabolic change from glycolysis to gluconeogenesis and to the TCA cycle contributes to normal metabolism during the recovery phase of re-oxygenation.
In wetland plants, the quick response of non-phosphorylating components, AOX and NDs, to the consumption of excessive reducing equivalents and avoidance of ROS and RNS production for maintaining mitochondrial homeostasis is effective in recovering from post-anoxic stress. However, the activation of these components would be futile in O2 consumption and energetically burdensome under hypoxic condition. As some non-phosphorylating components are strongly co-expressed, precise coordination between the expressions and activities of these mitochondrial components can provide flexible ATP production and maintain cellular homeostasis in wetland species under more severe hypoxic and anoxic stresses.
The two strategies, LOES and LOQS, in wetland species reflect the different responses to O2 deficiency and ATP production at subsequent post-hypoxic and anoxic stresses in their habitats. The difference in strategies could be due to the difference in the requirement of the reserved carbohydrates during the stress condition. Both LOES and LOQS in a single species are also reported. In wetland species with LOES, the developed aerenchyma and high O2 supply system by the pressurized gas flow are effective in maintaining high O2 availability to the roots, but not all plants have these functions. Further research on the interaction among hormones, O2 availability, and primary metabolites is needed to understand their optimal balance among growth, escape, and quiescence to facilitate survival in their habitats. At low O2 conditions, the efficient respiratory O2 consumption in the roots of wetland species is carried out in soils with NH4+ as the sole N source because these species can utilize NH4+ without ARI. Some wetland species in which nitrification occurs due to their high O2 supply system can also efficiently use the soil NO3−. The differences in the preference for N sources among wetland species could also be ascribed to the differences in their anaerobic ATP production systems. The species with high ability of NO3− utilization can use the NO2−-driven ATP production system, and the species specialized for NH4+ utilization can use the fermentation system. The different N utilization strategies for ATP production may be functionally linked to hypoxia tolerance in wetland species. Thus, further exploration of the ecophysiological mechanisms of aerobic and anaerobic respiratory responses to the N sources in roots of wild wetland species is needed to completely understand the anaerobic stress before global climate change makes the stress more severe.
Acknowledgements
We are grateful to Prof. Ichiro Terashima, Prof. Takayoshi Tsuchiya, Asso. Prof. Takatoshi Nakamura, and laboratory members for the critical advice. The first author is also grateful to Prof. Kazuhiko Terasawa for supporting and encouragement our writing activities.
Footnotes
Ko Noguchi is the recipient of the BSJ Award for Young Scientist, 2008.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Motoka Nakamura, Email: m5nakamu@nodai.ac.jp.
Ko Noguchi, Email: knoguchi@toyaku.ac.jp.
References
- Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays) Plant Cell Environ. 2012;35:1618–1630. doi: 10.1111/j.1365-3040.2012.02513.x. [DOI] [PubMed] [Google Scholar]
- Alegria AE, Sanchez S, Quintana I. Quinone-enhanced ascorbate reduction of nitric oxide: role of quinone redox potential. Free Radic Res. 2004;38:1107–1112. doi: 10.1080/10715760400009852. [DOI] [PubMed] [Google Scholar]
- Amor Y, Chevion M, Levine A. Anoxia pretreatment protects soybean cells against H2O2-induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Lett. 2000;477:175–180. doi: 10.1016/s0014-5793(00)01797-x. [DOI] [PubMed] [Google Scholar]
- Ancheng L, Jianming X, Xiaoe Y. Effect of nitrogen (NH4NO3) supply on absorption of ammonium and nitrate by conventional and hybrid rice during reproductive growth. Plant Soil. 1993;155:395–398. [Google Scholar]
- Armstrong J, Armstrong W. Chlorophyll development in mature lysigenous and schizogenous root aerenchyma provides evidence of continuing cortical cell viability. New Phytol. 1994;126:493–497. doi: 10.1111/j.1469-8137.1994.tb04246.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Beckett P. Internal aeration and development of stela anoxia in submerged roots: a multishelled mathem mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol. 1987;105:221–245. [Google Scholar]
- Arpagaus S, Brändle R. The significance of α-amylase under anoxia stress in tolerant rhizomes (Acorus calamus L.) and non-tolerant tubers (Solanum tuberosum L., var. Désirée) J Exp Bot. 2000;51:1475–1477. [PubMed] [Google Scholar]
- Aurisano N, Bertani A, Reggiani R. Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant Cell Physiol. 1995;36:1525–1529. [Google Scholar]
- Ayano M, Kani T, Kojima M, Sakakibara H, Kitaoka T, Kuroha T, Angeles-Shim RB, Kitano H, Nagai K, Ashikari M. Gibberellin biosynthesis and signal transduction is essential for internode elongation in deepwater rice. Plant Cell Environ. 2014;37:2313–2324. doi: 10.1111/pce.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Chang R. Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot. 2005;96:507–518. doi: 10.1093/aob/mci206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LA. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LA. Life in the balance: a signaling network controlling survival of flooding. Curr Opin Plant Biol. 2010;13:489–494. doi: 10.1016/j.pbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Ronald P, Ismail A, Heuer S, Mackill D. Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice. 2010;3:137–147. [Google Scholar]
- Bailey-Serres J, Lee SC, Brinton E. Waterproofing crops: effective flooding survival strategies. Plant Physiol. 2012;160:1698–1709. doi: 10.1104/pp.112.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkos KD, Britto DT, Kronzucker HJ. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72) Plant, Cell Environ. 2010;33:23–34. doi: 10.1111/j.1365-3040.2009.02046.x. [DOI] [PubMed] [Google Scholar]
- Barding GA, Fukao T, Beni S, Bailey-Serres J, Larive CK. Differential metabolic regulation governed by the rice SUB1A gene during submergence stress and identification of alanylglycine by 1H NMR spectroscopy. J Proteome Res. 2012;11:320–330. doi: 10.1021/pr200919b. [DOI] [PubMed] [Google Scholar]
- Barding GA, Beni S, Fukao T, Bailey-Serres J, Larive CK. Comparison of GC-MS and NMR for metabolite profiling of rice subjected to submergence stress. J Proteome Res. 2013;12:898–909. doi: 10.1021/pr300953k. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Jackson MB, Guhl K, Vreeburg RA, Croker SJ, Peeters AJ, Voesenek LA. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J. 2005;44:756–768. doi: 10.1111/j.1365-313X.2005.02563.x. [DOI] [PubMed] [Google Scholar]
- Bieniawska Z, Paul Barratt DH, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. 2007;49:810–828. doi: 10.1111/j.1365-313X.2006.03011.x. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Fagerstedt KV. Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol Biochem. 2010;48:359–373. doi: 10.1016/j.plaphy.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Blokhina OB, Virolainen E, Fagerstedt KV, Hoikkala A, Wähälä K, Chirkova TV. Antioxidant status of anoxia-tolerant and-intolerant plant species under anoxia and reaeration. Physiol Plant. 2000;109:396–403. [Google Scholar]
- Blokhina OB, Törönen P, Fagerstedt KV. Oxidative stress components explored in anoxic and hypoxic global gene expression data. In: van Dongen JT, Licausi F, editors. Low-oxygen stress in plants. Vienna: Springer; 2014. pp. 19–39. [Google Scholar]
- Blom C. Adaptations to flooding stress: from plant community to molecule. Plant Biol. 1999;1:261–273. [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL. Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol. 1992;99:1294–1301. doi: 10.1104/pp.99.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Meyerhoff PA, Taylor AR, Rost TL. Root development and absorption of ammonium and nitrate from the rhizosphere. J Plant Growth Regul. 2003;21:416–431. [Google Scholar]
- Bologa KL, Fernie AR, Leisse A, Loureiro ME, Geigenberger P. A bypass of sucrose synthase leads to low internal oxygen and impaired metabolic performance in growing potato tubers. Plant Physiol. 2003;132:2058–2072. doi: 10.1104/pp.103.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botrel A, Kaiser WM. Nitrate reductase activation state in barley roots in relation to the energy and carbohydrate status. Planta. 1997;201:496–501. doi: 10.1007/s004250050094. [DOI] [PubMed] [Google Scholar]
- Bouche N, Fait A, Bouchez D, Moller SG, Fromm H. Mitochondrial succinic-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci USA. 2003;100:6843–6848. doi: 10.1073/pnas.1037532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouny JM, Saglio PH. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 1996;111:187–194. doi: 10.1104/pp.111.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco-Price C, Kaiser KA, Jang CJ, Larive CK, Bailey-Serres J. Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J. 2008;56:743–755. doi: 10.1111/j.1365-313X.2008.03642.x. [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. NH4+ toxicity in higher plants: a critical review. J Plant Physiol. 2002;159:567–584. [Google Scholar]
- Britto DT, Siddiqi MY, Glass AD, Kronzucker HJ. Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA. 2001;98:4255–4258. doi: 10.1073/pnas.061034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix H, Sorrell BK, Orr PT. Internal pressurization and convective gas flow in some emergent freshwater macrophytes. Limnol Oceanogr. 1992;37:1420–1433. [Google Scholar]
- Brix H, Dyhr-Jensen K, Lorenzen B. Root-zone acidity and nitrogen source affects Typha latifolia L. growth and uptake kinetics of ammonium and nitrate. J Exp Bot. 2002;53:2441–2450. doi: 10.1093/jxb/erf106. [DOI] [PubMed] [Google Scholar]
- Bucher M, Kuhlemeier C. Long-term anoxia tolerance (Multi-level regulation of gene expression in the amphibious plant Acorus calamus L.) Plant Physiol. 1993;103:441–448. doi: 10.1104/pp.103.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M, Brändle R, Kuhlemeier C. Ethanolic fermentation in transgenic tobacco expressing Zymomonas mobilis pyruvate decarboxylase. EMBO J. 1994;13:2755–2763. doi: 10.1002/j.1460-2075.1994.tb06569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Polanco M, Señorans J, Zwiazek JJ. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012;12:99. doi: 10.1186/1471-2229-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carystinos GD, MacDonald HR, Monroy AF, Dhindsa RS, Poole RJ. Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiol. 1995;108:641–649. doi: 10.1104/pp.108.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PW, Chiang CM, Tseng TH, Yu SM. Interaction between rice MYBGA and the gibberellin response element controls tissue-specific sugar sensitivity of α-amylase genes. Plant Cell. 2006;18:2326–2340. doi: 10.1105/tpc.105.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Huber H, de Kroon H, Peeters AJ, Poorter H, Voesenek LA, Visser EJ. Intraspecific variation in the magnitude and pattern of flooding-induced shoot elongation in Rumex palustris. Ann Bot. 2009;104:1057–1067. doi: 10.1093/aob/mcp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Kende H. Expansins and internodal growth of deepwater rice. Plant Physiol. 1997;113:1145–1151. doi: 10.1104/pp.113.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JI, Ryoo N, Ko S, Lee SK, Lee J, Jung KH, Lee YH, Bhoo SH, Winderickx J, An G, Hahn TR, Jeon JS. Structure, expression, and functional analysis of the hexokinase gene family in rice (Oryza sativa L.) Planta. 2006;224:598–611. doi: 10.1007/s00425-006-0251-y. [DOI] [PubMed] [Google Scholar]
- Cho JI, Ryoo N, Eom JS, Lee DW, Kim HB, Jeong SW, Lee YH, Kwon YK, Cho MH, Bhoo SH, Hahn TR, Park Y, Hwang I, Shen J, Jeon JS. Role of the rice hexokinases OsHXK5 and OsHXK6 as glucose sensors. Plant Physiol. 2009;149:745–759. doi: 10.1104/pp.108.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Roberts DM. Arabidopsis NIP2; 1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J Biol Chem. 2007;282:24209–24218. doi: 10.1074/jbc.M700982200. [DOI] [PubMed] [Google Scholar]
- Choi D, Kim JH, Kende H. Whole genome analysis of the OsGRF gene family encoding plant-specific putative transcription activators in rice (Oryza sativa L.) Plant Cell Physiol. 2004;45:897–904. doi: 10.1093/pcp/pch098. [DOI] [PubMed] [Google Scholar]
- Clifton R, Lister R, Parker KL, Sappl PG, Elhafez D, Millar AH, Day DA, Whelan J. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Mol Biol. 2005;58:193. doi: 10.1007/s11103-005-5514-7. [DOI] [PubMed] [Google Scholar]
- Colmer T. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003;26:17–36. [Google Scholar]
- Colmer TD, Pedersen O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytol. 2008;177:918–926. doi: 10.1111/j.1469-8137.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Huang S, Greenway H. Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia. J Exp Bot. 2001;52:1507–1517. doi: 10.1093/jexbot/52.360.1507. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Winkel A, Pedersen O (2011) A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants 2011:plr030 [DOI] [PMC free article] [PubMed]
- Colmer T, Armstrong W, Greenway H, Ismail A, Kirk G, Atwell B. Physiological mechanisms of flooding tolerance in rice: transient complete submergence and prolonged standing water. In: Lüttge U, Beyschlag W, Cushman J, editors. Progress in botany. Berlin: Springer; 2014. pp. 255–307. [Google Scholar]
- Contento AL, Kim S-J, Bassham DC. Transcriptome profiling of the response of Arabidopsis suspension culture cells to Suc starvation. Plant Physiol. 2004;135:2330–2347. doi: 10.1104/pp.104.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curi GC, Welchen E, Chan RL, Gonzalez DH. Nuclear and mitochondrial genes encoding cytochrome c oxidase subunits respond differently to the same metabolic factors. Plant Physiol Biochem. 2003;41:689–693. [Google Scholar]
- Das A, Nanda B, Sarkar R, Lodh S. Effect of complete submergence on the activity of starch phosphorylase enzyme in rice (Oryza sativa L.) leaves. J Plant Biochem Biotechnol. 2000;9:41–43. [Google Scholar]
- Davies DD. Anaerobic metabolism and the production of organic acids. In: Davies DD, editor. Metabolism and respiration. London: Academic Press; 1980. pp. 581–611. [Google Scholar]
- Davies JM, Poole RJ, Sanders D. The computed free energy change of hydrolysis of inorganic pyrophosphate and ATP: apparent significance. for inorganic-pyrophosphate-driven reactions of intermediary metabolism. Biochim Biophys Acta. 1993;1141:29–36. [Google Scholar]
- Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJ (2014) Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants 6:plt058 [DOI] [PMC free article] [PubMed]
- Dawood T, Yang X, Visser EJ, Te Beek TA, Kensche PR, Cristescu SM, Lee S, Floková K, Nguyen D, Mariani C. A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiol. 2016;170:2351–2364. doi: 10.1104/pp.15.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Wiskich JT. Regulation of alternative oxidase activity in higher plants. J Bioenerg Biomembr. 1995;27:379–385. doi: 10.1007/BF02110000. [DOI] [PubMed] [Google Scholar]
- De Sousa C, Sodek L. Alanine metabolism and alanine aminotransferase activity in soybean (Glycine max) during hypoxia of the root system and subsequent return to normoxia. Environ Exper Bot. 2003;50:1–8. [Google Scholar]
- Desikan R, Soheila A-H, Hancock JT, Neill SJ. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001;127:159–172. doi: 10.1104/pp.127.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M, Hill S, Jackson M, Ratcliffe R, Sweetlove L. Physiological and metabolic adaptations of Potamogeton pectinatus L. tubers support rapid elongation of stem tissue in the absence of oxygen. Plant Cell Physiol. 2006;47:128–140. doi: 10.1093/pcp/pci229. [DOI] [PubMed] [Google Scholar]
- Dolferus R, Wolansky M, Carroll R, Miyashita Y, Ismond K, Good A. Functional analysis of lactate dehydrogenase during hypoxic stress in Arabidopsis. Funct Plant Biol. 2008;35:131–140. doi: 10.1071/FP07228. [DOI] [PubMed] [Google Scholar]
- Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003;35:763–770. doi: 10.1046/j.1365-313x.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- Douce R. Mitochondria in higher plants: structure, function, and biogenesis. London: Academic Press; 1985. [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Drew MC, He C-J, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Elhafez D, Murcha MW, Clifton R, Soole KL, Day DA, Whelan J. Characterization of mitochondrial alternative NAD (P) H dehydrogenases in Arabidopsis: intraorganelle location and expression. Plant Cell Physiol. 2006;47:43–54. doi: 10.1093/pcp/pci221. [DOI] [PubMed] [Google Scholar]
- Escobar MA, Geisler DA, Rasmusson AG. Reorganization of the alternative pathways of the Arabidopsis respiratory chain by nitrogen supply: opposing effects of ammonium and nitrate. Plant J. 2006;45:775–788. doi: 10.1111/j.1365-313X.2005.02640.x. [DOI] [PubMed] [Google Scholar]
- Evans DE. Aerenchyma formation. New Phytol. 2004;161:35–49. [Google Scholar]
- Eysholdt-Derzsó E, Sauter M. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biol. 2019;21:103–108. doi: 10.1111/plb.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan TW, Higashi RM, Lane AN. An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Arch Biochem Biophys. 1988;266:592–606. doi: 10.1016/0003-9861(88)90292-5. [DOI] [PubMed] [Google Scholar]
- Fan TW, Higashi RM, Frenkiel TA, Lane AN. Anaerobic nitrate and ammonium metabolism in flood-tolerant rice coleoptiles. J Exp Bot. 1997;48:1655–1666. [Google Scholar]
- Felle HH. pH regulation in anoxic plants. Ann Bot. 2005;96:519–532. doi: 10.1093/aob/mci207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Wang Y, Li H, Wang R, Sun K, Jia L. Salt stress-induced expression of rice AOX1a is mediated through an accumulation of hydrogen peroxide. Biologia. 2010;65:868–873. [Google Scholar]
- Frechilla S, Lasa B, Aleu M, Juanarena N, Lamsfus C, Aparicio-Tejo PM. Short-term ammonium supply stimulates glutamate dehydrogenase activity and alternative pathway respiration in roots of pea plants. J Plant Physiol. 2002;159:811–818. [Google Scholar]
- Fukao T, Bailey-Serres J. Plant responses to hypoxia–is survival a balancing act? Trends Plant Sci. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci USA. 2008;105:16814–16819. doi: 10.1073/pnas.0807821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor–like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell. 2011;23:412–427. doi: 10.1105/tpc.110.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol. 2012;160:1795–1807. doi: 10.1104/pp.112.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P. Regulation of sucrose to starch conversion in growing potato tubers. J Exp Bot. 2003;54:457–465. doi: 10.1093/jxb/erg074. [DOI] [PubMed] [Google Scholar]
- Germain V, Raymond P, Ricard B. Differential expression of two tomato lactate dehydrogenase genes in response to oxygen deficit. Plant Mol Biol. 1997;35:711–721. doi: 10.1023/a:1005854002969. [DOI] [PubMed] [Google Scholar]
- Germain V, Ricard B, Raymond P, Saglio PH. The role of sugars, hexokinase, and sucrose synthase in the determination of hypoxically induced tolerance to anoxia in tomato roots. Plant Physiol. 1997;114:167–175. doi: 10.1104/pp.114.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol. 2003;30:1–47. doi: 10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Morrell S, Valdez A, Setter T, Greenway H. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. J Exp Bot. 2000;51:78–796. [PubMed] [Google Scholar]
- Giraud E, Van Aken O, Ho LH, Whelan J. The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol. 2009;150:1286–1296. doi: 10.1104/pp.109.139782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Yamaguchi J, Perata P, Alpi A. Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiol. 1995;109:1069–1076. doi: 10.1104/pp.109.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Loreti E, Perata P, Alpi A. Sucrose synthesis in cereal grains under oxygen deprivation. J Plant Res. 1999;112:353–359. [Google Scholar]
- Guglielminetti L, Morita A, Yamaguchi J, Loreti E, Perata P, Alpi A. Differential expression of two fructokinases in Oryza sativa seedlings grown under aerobic and anaerobic conditions. J Plant Res. 2006;119:351–356. doi: 10.1007/s10265-006-0281-3. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Igamberdiev AU. The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion. 2011;11:537–543. doi: 10.1016/j.mito.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot. 2005;56:2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Zabalza A, van Dongen JT. Regulation of respiration when the oxygen availability changes. Physiol Plant. 2009;137:383–391. doi: 10.1111/j.1399-3054.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Igamberdiev AU, Manjunatha G, Segu S, Moran JF, Neelawarne B, Bauwe H, Kaiser WM. The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci. 2011;181:520–526. doi: 10.1016/j.plantsci.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Shah JK, Brotman Y, Jahnke K, Willmitzer L, Kaiser WM, Bauwe H, Igamberdiev AU. Inhibition of aconitase by nitric oxide leads to induction of the alternative oxidase and to a shift of metabolism towards biosynthesis of amino acids. J Exp Bot. 2012;63:1773–1784. doi: 10.1093/jxb/ers053. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Hebelstrup KH, Kruger NJ, Ratcliffe RG. Nitric oxide is required for homeostasis of oxygen and reactive oxygen species in barley roots under aerobic conditions. Mol Plant. 2014;7:747–750. doi: 10.1093/mp/sst167. [DOI] [PubMed] [Google Scholar]
- Hachiya T, Watanabe CK, Boom C, Tholen D, Takahara K, Kawai-Yamada M, Uchimiya H, Uesono Y, Terashima I, Noguchi K. Ammonium-dependent respiratory increase is dependent on the cytochrome pathway in Arabidopsis thaliana shoots. Plant Cell Environ. 2010;33:1888–1897. doi: 10.1111/j.1365-3040.2010.02189.x. [DOI] [PubMed] [Google Scholar]
- Hansen LD, Church J, Matheson S, McCarlie VW, Thygerson T, Criddle RS, Smith BN. Kinetics of plant growth and metabolism. Thermochim Acta. 2002;388:415–425. [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song X, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Ashikari M. Rice growth adapting to deepwater. Curr Opin Plant Biol. 2011;14:100–105. doi: 10.1016/j.pbi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- He J, Bögemann G, Van de Steeg H, Rijnders J, Voesenek L, Blom C. Survival tactics of Ranunculus species in river floodplains. Oecologia. 1999;118:1–8. doi: 10.1007/s004420050696. [DOI] [PubMed] [Google Scholar]
- Hebelstrup KH, Møller IM. Mitochondrial signaling in plants under hypoxia: use of reactive oxygen species (ROS) and reactive nitrogen species (RNS) In: Gupta KJ, Igamberdiev AU, editors. Reactive oxygen and nitrogen species signaling and communication in plants. Cham: Springer; 2015. pp. 63–77. [Google Scholar]
- Herrera A. Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front Plant Sci. 2013;4:106. doi: 10.3389/fpls.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess N, Klode M, Anders M, Sauter M. The hypoxia responsive transcription factor genes ERF71/HRE2 and ERF73/HRE1 of Arabidopsis are differentially regulated by ethylene. Physiol Plant. 2011;143:41–49. doi: 10.1111/j.1399-3054.2011.01486.x. [DOI] [PubMed] [Google Scholar]
- Hirose T, Scofield GN, Terao T. An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci. 2008;174:534–543. [Google Scholar]
- Ho LH, Giraud E, Uggalla V, Lister R, Clifton R, Glen A, Thirkettle-Watts D, Van Aken O, Whelan J. Identification of regulatory pathways controlling gene expression of stress-responsive mitochondrial proteins in Arabidopsis. Plant Physiol. 2008;147:1858–1873. doi: 10.1104/pp.108.121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KA, Cheng K, Murcha MW, Jenkin LE, Millar AH, Whelan J. Oxygen initiation of respiration and mitochondrial biogenesis in rice. J Biol Chem. 2007;282:15619–15631. doi: 10.1074/jbc.M609866200. [DOI] [PubMed] [Google Scholar]
- Hu B, Henry A, Brown KM, Lynch JP. Root cortical aerenchyma inhibits radial nutrient transport in maize (Zea mays) Ann Bot. 2013;113:181–189. doi: 10.1093/aob/mct259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Colmer TD, Millar AH. Does anoxia tolerance involve altering the energy currency towards PPi? Trends Plant Sci. 2008;13:221–227. doi: 10.1016/j.tplants.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Hill RD. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot. 2004;55:2473–2482. doi: 10.1093/jxb/erh272. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Hill RD. Plant mitochondrial function during anaerobiosis. Ann Bot. 2008;103:259–268. doi: 10.1093/aob/mcn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igamberdiev AU, Kleczkowski LA. Membrane potential, adenylate levels and Mg2+ are interconnected via adenylate kinase equilibrium in plant cells. Biochim Biophys Acta. 2003;1607:111–119. doi: 10.1016/j.bbabio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Kleczkowski LA. Magnesium and cell energetics in plants under anoxia. Biochem J. 2011;437:373–379. doi: 10.1042/BJ20110213. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Bykova NV, Hill RD. Nitric oxide scavenging by barley hemoglobin is facilitated by a monodehydroascorbate reductase-mediated ascorbate reduction of methemoglobin. Planta. 2006;223:1033–1040. doi: 10.1007/s00425-005-0146-3. [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Inoue E, Tabuchi M, Yamaya T, Takahashi H. Biochemical background and compartmentalized functions of cytosolic glutamine synthetase for active ammonium assimilation in rice roots. Plant Cell Physiol. 2004;45:1640–1647. doi: 10.1093/pcp/pch190. [DOI] [PubMed] [Google Scholar]
- Ishizaki K, Mizutani M, Shimamura M, Masuda A, Nishihama R, Kohchi T. Essential role of the E3 ubiquitin ligase NOPPERABO1 in schizogenous intercellular space formation in the liverwort Marchantia polymorpha. Plant Cell. 2013;25:4075–4084. doi: 10.1105/tpc.113.117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismond KP, Dolferus R, De Pauw M, Dennis ES, Good AG. Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol. 2003;132:1292–1302. doi: 10.1104/pp.103.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1999;1:274–287. [Google Scholar]
- Karasawa T, Hayakawa T, Mae T, Ojima K, Yamaya T. Characteristics of ammonium uptake by rice cells in suspension culture. Soil Sci. Plant Nutr. 1994;40:333–338. [Google Scholar]
- Kato-Noguchi H. The catalytic direction of pyrophosphate: fructose 6-phosphate 1-phosphotransferase in rice coleoptiles in anoxia. Physiol Plant. 2002;116:345–350. [Google Scholar]
- Kende H, van der Knaap E, Cho H-T. Deepwater rice: a model plant to study stem elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenring KM, Galatowitsch SM. Temperature requirements for dormancy break and seed germination vary greatly among 14 wetland Carex species. Aquat Bot. 2007;87:209–220. [Google Scholar]
- Kim J, Cho HT, Kende H. α-Expansins in the semiaquatic ferns Marsilea quadrifolia and Regnellidium diphyllum: evolutionary aspects and physiological role in rachis elongation. Planta. 2000;212:85–92. doi: 10.1007/s004250000367. [DOI] [PubMed] [Google Scholar]
- Kimpara T, Aohara T, Soga K, Wakabayashi K, Hoson T, Tsumuraya Y, Kotake T. β-1, 3: 1, 4-Glucan synthase activity in rice seedlings under water. Ann Bot. 2008;102:221–226. doi: 10.1093/aob/mcn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk G, Kronzucker H. The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Ann Bot. 2005;96:639–646. doi: 10.1093/aob/mci216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell. 2002;14:2481–2494. doi: 10.1105/tpc.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol. 2004;7:235–246. doi: 10.1016/j.pbi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Konnerup D, Sorrell BK, Brix H. Do tropical wetland plants possess convective gas flow mechanisms? New Phytol. 2011;190:379–386. doi: 10.1111/j.1469-8137.2010.03585.x. [DOI] [PubMed] [Google Scholar]
- Kotula L, Ranathunge K, Schreiber L, Steudle E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J Exp Bot. 2009;60:2155–2167. doi: 10.1093/jxb/erp089. [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Rennenberg H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014;37:2245–2259. doi: 10.1111/pce.12310. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass AD, Kirk GJ. Nitrate-ammonium synergism in rice. A subcellular flux analysis. Plant Physiol. 1999;119:1041–1046. doi: 10.1104/pp.119.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT, Davenport RJ, Tester M. Ammonium toxicity and the real cost of transport. Trends Plant Sci. 2001;6:335–337. doi: 10.1016/s1360-1385(01)02022-2. [DOI] [PubMed] [Google Scholar]
- Kulichikhin K, Yamauchi T, Watanabe K, Nakazono M. Biochemical and molecular characterization of rice (O ryza sativa L.) roots forming a barrier to radial oxygen loss. Plant Cell Environ. 2014;37:2406–2420. doi: 10.1111/pce.12294. [DOI] [PubMed] [Google Scholar]
- Laanbroek HJ. Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Ann Bot. 2009;105:141–153. doi: 10.1093/aob/mcp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager I, Andréasson O, Dunbar TL, Andreasson E, Escobar MA, Rasmusson AG. Changes in external pH rapidly alter plant gene expression and modulate auxin and elicitor responses. Plant Cell Environ. 2010;33:1513–1528. doi: 10.1111/j.1365-3040.2010.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa B, Frechilla S, Aparicio-Tejo PM, Lamsfus C. Alternative pathway respiration is associated with ammonium ion sensitivity in spinach and pea plants. Plant Growth Regul. 2002;37:49–55. [Google Scholar]
- Lasanthi-Kudahettige R, Magneschi L, Loreti E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P. Transcript profiling of the anoxic rice coleoptile. Plant Physiol. 2007;144:218–231. doi: 10.1104/pp.106.093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R. Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol. 2006;47:788–792. doi: 10.1093/pcp/pcj043. [DOI] [PubMed] [Google Scholar]
- Leck MA, Brock MA. Ecological and evolutionary trends in wetlands: evidence from seeds and seed banks in New South Wales, Australia and New Jersey, USA. Plant Spec Biol. 2000;15:97–112. [Google Scholar]
- Lee KW, Chen PW, Yu SM. Metabolic adaptation to sugar/O2 deficiency for anaerobic germination and seedling growth in rice. Plant Cell Environ. 2014;37:2234–2244. doi: 10.1111/pce.12311. [DOI] [PubMed] [Google Scholar]
- Li B, Xin W, Sun S, Shen Q, Xu G. Physiological and molecular responses of nitrogen-starved rice plants to re-supply of different nitrogen sources. Plant Soil. 2006;287:145–159. [Google Scholar]
- Licausi F, Perata P. Low oxygen signaling and tolerance in plants. Adv Bot Res. 2009;50:139–198. [Google Scholar]
- Lin C, Sauter M. Control of adventitious root architecture in rice by darkness, light, and gravity. Plant Physiol. 2018;176:1352–1364. doi: 10.1104/pp.17.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiQiang J. Is reactive oxygen species (ROS) the underlying factor for inhibited root growth in Osspr1? Plant Signal Behav. 2011;6:1024–1025. doi: 10.4161/psb.6.7.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, VanToai T, Moy LP, Bock G, Linford LD, Quackenbush J. Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol. 2005;137:1115–1129. doi: 10.1104/pp.104.055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Guo T, Wan X, Wang H, Zhu M, Li A, Su N, Shen Y, Mao B, Zhai H. Transcriptome analysis of grain-filling caryopses reveals involvement of multiple regulatory pathways in chalky grain formation in rice. BMC Genom. 2010;11:730. doi: 10.1186/1471-2164-11-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E, Yamaguchi J, Alpi A, Perata P. Sugar modulation of α-amylase genes under anoxia. Ann Bot. 2003;91:143–148. doi: 10.1093/aob/mcf117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol. 2005;137:1130–1138. doi: 10.1104/pp.104.057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CA, Ho TD, Ho SL, Yu SM. Three novel MYB proteins with one DNA binding repeat mediate sugar and hormone regulation of α-amylase gene expression. Plant Cell. 2002;14:1963–1980. doi: 10.1105/tpc.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CA, Lin CC, Lee KW, Chen JL, Huang LF, Ho SL, Liu HJ, Hsing YI, Yu SM. The SnRK1A protein kinase plays a key role in sugar signaling during germination and seedling growth of rice. Plant Cell. 2007;19:2484–2499. doi: 10.1105/tpc.105.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes J, Huber D. Response of ammonium assimilation enzymes to nitrogen form treatments in different plant species. J Plant Nutr. 1991;14:175–185. [Google Scholar]
- Malik A, Islam A, Colmer T. Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): evaluation of four H. marinum—wheat amphiploids. New Phytol. 2011;190:499–508. doi: 10.1111/j.1469-8137.2010.03519.x. [DOI] [PubMed] [Google Scholar]
- Manzur M, Grimoldi A, Insausti P, Striker G. Escape from water or remain quiescent? Lotus tenuis changes its strategy depending on depth of submergence. Ann Bot. 2009;104:1163–1169. doi: 10.1093/aob/mcp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillia EF, Micallef BJ, Micallef M, Weninger A, Pedersen KK, Zou J, Taylor DC. Biochemical and physiological studies of Arabidopsis thaliana transgenic lines with repressed expression of the mitochondrial pyruvate dehydrogenase kinase1. J Exp Bot. 2003;54:259–270. doi: 10.1093/jxb/erg020. [DOI] [PubMed] [Google Scholar]
- Marschner H, Römheld V. In vivo measurement of root-induced pH changes at the soil-root interface: effect of plant species and nitrogen source. Z Pflanzenphysiol. 1983;111:241–251. [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. Response to anoxia in rice and wheat seedlings: changes in the pH of intracellular compartments, glucose-6-phosphate level, and metabolic rate. Plant Physiol. 1991;95:760–767. doi: 10.1104/pp.95.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens E, Larondelle Y, Hers HG. Induction of pyrophosphate: fructose 6-phosphate 1-phosphotransferase by anoxia in rice seedlings. Plant Physiol. 1990;93:584–587. doi: 10.1104/pp.93.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalecka AM, Svensson ÅS, Johansson FI, Agius SC, Johanson U, Brennicke A, Binder S, Rasmusson AG. Arabidopsis genes encoding mitochondrial type II NAD (P) H dehydrogenases have different evolutionary origin and show distinct responses to light. Plant Physiol. 2003;133:642–652. doi: 10.1104/pp.103.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Trend AE, Heazlewood JL. Changes in the mitochondrial proteome during the anoxia to air transition in rice focus around cytochrome-containing respiratory complexes. J Biol Chem. 2004;279:39471–39478. doi: 10.1074/jbc.M406015200. [DOI] [PubMed] [Google Scholar]
- Millenaar FF, Gonzalez-Meler MA, Fiorani F, Welschen R, Ribas-Carbo M, Siedow JN, Wagner AM, Lambers H. Regulation of alternative oxidase activity in six wild monocotyledonous species. An in vivo study at the whole root level. Plant Physiol. 2001;126:376–387. doi: 10.1104/pp.126.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithran M, Paparelli E, Novi G, Perata P, Loreti E. Analysis of the role of the pyruvate decarboxylase gene family in Arabidopsis thaliana under low-oxygen conditions. Plant Biol. 2014;16:28–34. doi: 10.1111/plb.12005. [DOI] [PubMed] [Google Scholar]
- Mohanty B, Ong BL. Contrasting effects of submergence in light and dark on pyruvate decarboxylase activity in roots of rice lines differing in submergence tolerance. Ann Bot. 2003;91:291–300. doi: 10.1093/aob/mcf050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B, Wilson PM, Rees APT. Effects of anoxia on growth and carbohydrate metabolism in suspension cultures of soybean and rice. Phytochemistry. 1993;34:75–82. [Google Scholar]
- Møller IM. The oxidation of cytosolic NAD (P) H by external NAD (P) H dehydrogenases in the respiratory chain of plant mitochondria. Physiol Plant. 1997;100:85–90. [Google Scholar]
- Møller IM. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- Møller IM, Sweetlove LJ. ROS signalling–specificity is required. Trends Plant Sci. 2010;15:370–374. doi: 10.1016/j.tplants.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Møller IM, Rasmusson AG, Fredlund KM. NAD (P) H-ubiquinone oxidoreductases in plant mitochondria. J Bioenerg Biomembr. 1993;25:377–384. doi: 10.1007/BF00762463. [DOI] [PubMed] [Google Scholar]
- Mommer L, Pons TL, Visser EJ. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. J Exp Bot. 2005;57:283–290. doi: 10.1093/jxb/erj015. [DOI] [PubMed] [Google Scholar]
- Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJ. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiol. 2005;139:497–508. doi: 10.1104/pp.105.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Lenssen JP, Huber H, Visser EJ, De Kroon H. Ecophysiological determinants of plant performance under flooding: a comparative study of seven plant families. J Ecol. 2006;94:1117–1129. [Google Scholar]
- Mommer L, Wolters-Arts M, Andersen C, Visser EJ, Pedersen O. Submergence-induced leaf acclimation in terrestrial species varying in flooding tolerance. New Phytol. 2007;176:337–345. doi: 10.1111/j.1469-8137.2007.02166.x. [DOI] [PubMed] [Google Scholar]
- Moons A. Transcriptional profiling of the PDR gene family in rice roots in response to plant growth regulators, redox perturbations and weak organic acid stresses. Planta. 2008;229:53–71. doi: 10.1007/s00425-008-0810-5. [DOI] [PubMed] [Google Scholar]
- Moons A, Valcke R, Van M. Low-oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C3 plant. Plant J. 1998;15:89–98. doi: 10.1046/j.1365-313x.1998.00185.x. [DOI] [PubMed] [Google Scholar]
- Morita A, Umemura T, Kuroyanagi M, Futsuhara Y, Perata P, Yamaguchi J. Functional dissection of a sugar-repressed α-amylase gene (RAmy1A) promoter in rice embryos. FEBS Lett. 1998;423:81–85. doi: 10.1016/s0014-5793(98)00067-2. [DOI] [PubMed] [Google Scholar]
- Morrell S, Greenway H, Davies D. Regulation of pyruvate decarboxylase in vitro and in vivo. J Exp Bot. 1990;41:131–139. [Google Scholar]
- Müller E, Albers BP, Janiesch P. Influence of NO3− and NH4+ nutrition on fermentation, nitrate reductase activity and adenylate energy charge of roots of Carex pseudocyperus L. and Carex sylvatica Huds. exposed to anaerobic nutrient solutions. Plant Soil. 1994;166:221–230. [Google Scholar]
- Murakami Y, Toriyama K. Enhanced high temperature tolerance in transgenic rice seedlings with elevated levels of alternative oxidase, OsAOX1a. Plant Biotech. 2008;25:361–364. [Google Scholar]
- Mustroph A, Albrecht G. Tolerance of crop plants to oxygen deficiency stress: fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol Plant. 2003;117:508–520. doi: 10.1034/j.1399-3054.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Mustroph A, Albrecht G, Hajirezaei M, Grimm B, Biemelt S. Low levels of pyrophosphate in transgenic potato plants expressing E. coli pyrophosphatase lead to decreased vitality under oxygen deficiency. Ann Bot. 2005;96:717–726. doi: 10.1093/aob/mci223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Boamfa EI, Laarhoven LJ, Harren FJ, Albrecht G, Grimm B. Organ-specific analysis of the anaerobic primary metabolism in rice and wheat seedlings. I: dark ethanol production is dominated by the shoots. Planta. 2006;225:103–114. doi: 10.1007/s00425-006-0333-x. [DOI] [PubMed] [Google Scholar]
- Mustroph A, Boamfa EI, Laarhoven LJ, Harren FJ, Pörs Y, Grimm B. Organ specific analysis of the anaerobic primary metabolism in rice and wheat seedlings II: light exposure reduces needs for fermentation and extends survival during anaerobiosis. Planta. 2006;225:139–152. doi: 10.1007/s00425-006-0336-7. [DOI] [PubMed] [Google Scholar]
- Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010;152:1484–1500. doi: 10.1104/pp.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Barding GA, Jr, Kaiser KA, Larive CK, Bailey-Serres J. Characterization of distinct root and shoot responses to low-oxygen stress in A rabidopsis with a focus on primary C-and N-metabolism. Plant Cell Environ. 2014;37:2366–2380. doi: 10.1111/pce.12282. [DOI] [PubMed] [Google Scholar]
- Nagai K, Hattori Y, Ashikari M. Stunt or elongate? Two opposite strategies by which rice adapts to floods. J Plant Res. 2010;123:303–309. doi: 10.1007/s10265-010-0332-7. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nakamura M. Root respiratory costs of ion uptake, root growth, and root maintenance in wetland plants: efficiency and strategy of O2 use for adaptation to hypoxia. Oecologia. 2016;182:667–678. doi: 10.1007/s00442-016-3691-5. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nakamura T, Tsuchiya T. Advantages of NH4+ on growth, nitrogen uptake and root respiration of Phragmites australis. Plant Soil. 2010;331:463–470. [Google Scholar]
- Nakamura M, Nakamura T, Tsuchiya T, Noguchi K. Functional linkage between N acquisition strategies and aeration capacities of hydrophytes for efficient oxygen consumption in roots. Physiol Plant. 2013;147:135–146. doi: 10.1111/j.1399-3054.2012.01643.x. [DOI] [PubMed] [Google Scholar]
- Narsai R, Whelan J. How unique is the low oxygen response? An analysis of the anaerobic response during germination and comparison with abiotic stress in rice and Arabidopsis. Front Plant Sci. 2013;4:349. doi: 10.3389/fpls.2013.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Howell KA, Carroll A, Ivanova A, Millar AH, Whelan J. Defining core metabolic and transcriptomic responses to oxygen availability in rice embryos and young seedlings. Plant Physiol. 2009;151:306–322. doi: 10.1104/pp.109.142026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsai R, Rocha M, Geigenberger P, Whelan J, van Dongen JT. Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. New Phytol. 2011;190:472–487. doi: 10.1111/j.1469-8137.2010.03589.x. [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Neuburger M, Rébeillé F, Jourdain A, Nakamura S, Douce R. Mitochondria are a major site for folate and thymidylate synthesis in plants. J Biol Chem. 1996;271:9466–9472. doi: 10.1074/jbc.271.16.9466. [DOI] [PubMed] [Google Scholar]
- Nicolai M, Roncato M, Canoy A, Rouquie D, Sarda X, Freyssinet G, Robaglia C. Large-scale analysis of mRNA translation states during sucrose starvation in Arabidopsis cells identifies cell proliferation and chromatin structure as targets of translational control. Plant Physiol. 2006;141:663–673. doi: 10.1104/pp.106.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Durnin DC, Igamberdiev AU, Hill RD. Cytosolic calcium is involved in the regulation of barley hemoglobin gene expression. Planta. 2006;223:542–549. doi: 10.1007/s00425-005-0094-y. [DOI] [PubMed] [Google Scholar]
- Nielsen KH, Schjoerring JK. Regulation of apoplastic NH4+ concentration in leaves of oilseed rape. Plant Physiol. 1998;118:1361–1368. doi: 10.1104/pp.118.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwaki Y, Kawagishi-Kobayashi M, Wakasa K, Fujihara S, Yoneyama T. Induction of class-1 non-symbiotic hemoglobin genes by nitrate, nitrite and nitric oxide in cultured rice cells. Plant Cell Physiol. 2005;46:324–331. doi: 10.1093/pcp/pci030. [DOI] [PubMed] [Google Scholar]
- Ookawara R, Satoh S, Yoshioka T, Ishizawa K. Expression of α-expansin and xyloglucan endotransglucosylase/hydrolase genes associated with shoot elongation enhanced by anoxia, ethylene and carbon dioxide in arrowhead (Sagittaria pygmaea Miq.) tubers. Ann Bot. 2005;96:693–702. doi: 10.1093/aob/mci221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Hk Yim, Park H, Lim J, Kim SH, Hwang Y. Interference with oxidative phosphorylation enhances anoxic expression of rice α-amylase genes through abolishing sugar regulation. J Exp Bot. 2010;61:3235–3244. doi: 10.1093/jxb/erq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlanti S, Kudahettige N, Lombardi L, Mensuali-Sodi A, Alpi A, Perata P, Pucciariello C. Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann Bot. 2011;107:1335–1343. doi: 10.1093/aob/mcr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O, Vos H, Colmer TD. Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant Cell Environ. 2006;29:1388–1399. doi: 10.1111/j.1365-3040.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Pedersen O, Rich SM, Colmer TD. Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. Plant J. 2009;58:147–156. doi: 10.1111/j.1365-313X.2008.03769.x. [DOI] [PubMed] [Google Scholar]
- Pedersen O, Colmer TD, Sand-Jensen K. Underwater photosynthesis of submerged plants–recent advances and methods. Frontier Plant Sci. 2013;4:140. doi: 10.3389/fpls.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchet E, Gupta JK, Sonoda M, Kaiser WM. Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 2005;41:732–743. doi: 10.1111/j.1365-313X.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- Plaxton WC. Plant response to stress: biochemical adaptations to phosphate deficiency. In: Goodman RM, editor. Encyclopedia of plant and crop science. New York: Marcel Dekker; 2004. pp. 976–980. [Google Scholar]
- Plaxton WC, Podestá FE. The functional organization and control of plant respiration. Crit Rev Plant Sci. 2006;25:159–198. [Google Scholar]
- Pronk JT, Yde Steensma H, van Dijken JP. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast. 1996;12:1607–1633. doi: 10.1002/(sici)1097-0061(199612)12:16<1607::aid-yea70>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rahman M, Grover A, Peacock WJ, Dennis ES, Ellis MH. Effects of manipulation of pyruvate decarboxylase and alcohol dehydrogenase levels on the submergence tolerance of rice. Funct Plant Biol. 2001;28:1231–1241. [Google Scholar]
- Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol. 2011;190:351–368. doi: 10.1111/j.1469-8137.2010.03535.x. [DOI] [PubMed] [Google Scholar]
- Rasmusson AG, Soole KL, Elthon TE. Alternative NAD (P) H dehydrogenases of plant mitochondria. Annu Rev Plant Biol. 2004;55:23–39. doi: 10.1146/annurev.arplant.55.031903.141720. [DOI] [PubMed] [Google Scholar]
- Reggiani R, Brambilla I, Bertani A. Effect of exogenous nitrate on anaerobic metabolism in excised rice roots: II. Fermentative activity and adenylic energy charge. J Exp Bot. 1985;36:1698–1704. [Google Scholar]
- Reggiani R, Mattana M, Aurisano N, Bertani A. Utilization of stored nitrate during the anaerobic germination of rice seeds. Plant Cell Physiol. 1993;34:379–383. [Google Scholar]
- Rhoads DM, Subbaiah CC. Mitochondrial retrograde regulation in plants. Mitochondrion. 2007;7:177–194. doi: 10.1016/j.mito.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. Anaerobic stress induces the transcription and translation of sucrose synthase in rice. Plant Physiol. 1991;95:669–674. doi: 10.1104/pp.95.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich SM, Ludwig M, Colmer TD. Aquatic adventitious root development in partially and completely submerged wetland plants Cotula coronopifolia and Meionectes brownii. Ann Bot. 2012;110:405–414. doi: 10.1093/aob/mcs051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricoult C, Echeverria LO, Cliquet J-B, Limami AM. Characterization of alanine aminotransferase (AlaAT) multigene family and hypoxic response in young seedlings of the model legume Medicago truncatula. J Exp Bot. 2006;57:3079–3089. doi: 10.1093/jxb/erl069. [DOI] [PubMed] [Google Scholar]
- Ridge I. Ethylene and growth control in amphibious plants. In: Crawford RMM, editor. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific; 1987. pp. 53–76. [Google Scholar]
- Roberts JK, Chang K, Webster C, Callis J, Walbot V. Dependence of ethanolic fermentation, cytoplasmic pH regulation, and viability on the activity of alcohol dehydrogenase in hypoxic maize root tips. Plant Physiol. 1989;89:1275–1278. doi: 10.1104/pp.89.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M, Licausi F, Araújo WL, Nunes-Nesi A, Sodek L, Fernie AR, Van Dongen JT. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010;152:1501–1513. doi: 10.1104/pp.109.150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot. 2002;53:103–110. [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- Rzewuski G, Sauter M. The novel rice (Oryza sativa L.) gene OsSbf1 encodes a putative member of the Na+/bile acid symporter family. J Exp Bot. 2002;53:1991–1993. doi: 10.1093/jxb/erf053. [DOI] [PubMed] [Google Scholar]
- Santosa I, Ram P, Boamfa E, Laarhoven L, Reuss J, Jackson M, Harren F. Patterns of peroxidative ethane emission from submerged rice seedlings indicate that damage from reactive oxygen species takes place during submergence and is not necessarily a post-anoxic phenomenon. Planta. 2007;226:193–202. doi: 10.1007/s00425-006-0457-z. [DOI] [PubMed] [Google Scholar]
- Sato T, Harada T, Ishizawa K. Stimulation of glycolysis in anaerobic elongation of pondweed (Potamogeton distinctus) turions. J Exp Bot. 2002;53:1847–1856. doi: 10.1093/jxb/erf036. [DOI] [PubMed] [Google Scholar]
- Sauter M. Rice in deep water: “how to take heed against a sea of troubles”. Naturwissenschaften. 2000;87:289–303. doi: 10.1007/s001140050725. [DOI] [PubMed] [Google Scholar]
- Sauter M. Root responses to flooding. Curr Opin Plant Biol. 2013;16:282–286. doi: 10.1016/j.pbi.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Sauter M, Rzewuski G, Marwedel T, Lorbiecke R. The novel ethylene-regulated gene OsUsp1 from rice encodes a member of a plant protein family related to prokaryotic universal stress proteins. J Exp Bot. 2002;53:2325–2331. doi: 10.1093/jxb/erf096. [DOI] [PubMed] [Google Scholar]
- Schubert S, Yan F. Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+ ATPase. Z Pflanz Bodenkunde. 1997;160:275–281. [Google Scholar]
- Seago JRJL, Marsh LC, Stevens KJ, Soukup A, Votrubova O, Enstone DE. A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Ann Bot. 2005;96:565–579. doi: 10.1093/aob/mci211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinski J, Scheibe R, Day DA, Whelan J. Alternative oxidase is positive for plant performance. Trends Plant Sci. 2018;23:588–597. doi: 10.1016/j.tplants.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Shingaki-Wells RN, Huang S, Taylor NL, Carroll AJ, Zhou W, Millar AH. Differential molecular responses of rice and wheat coleoptiles to anoxia reveal novel metabolic adaptations in amino acid metabolism for tissue tolerance. Plant Physiol. 2011;156:1706–1724. doi: 10.1104/pp.111.175570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingaki-Wells R, Millar AH, Whelan J, Narsai R. What happens to plant mitochondria under low oxygen? An omics review of the responses to low oxygen and reoxygenation. Plant, Cell Environ. 2014;37:2260–2277. doi: 10.1111/pce.12312. [DOI] [PubMed] [Google Scholar]
- Shiono K, Ando M, Nishiuchi S, Takahashi H, Watanabe K, Nakamura M, Matsuo Y, Yasuno N, Yamanouchi U, Fujimoto M. RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa) Plant J. 2014;80:40–51. doi: 10.1111/tpj.12614. [DOI] [PubMed] [Google Scholar]
- Simons BH, Lambers H. The alternative oxidase: is it a respiratory pathway allowing a plant to cope with stress. In: Lerner HR, editor. Plant responses to environmental stresses: from phytohormones to genome reorganization. Boca Raton: Taylor & Francis; 1999. pp. 265–286. [Google Scholar]
- Sorrell BK, Hawes I. Convective gas flow development and the maximum depths achieved by helophyte vegetation in lakes. Ann Bot. 2009;105:165–174. doi: 10.1093/aob/mcp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa AW, Duff SM, Guy PA, Hill RD. Altering hemoglobin levels changes energy status in maize cells under hypoxia. Proc Natl Acad Sci USA. 1998;95:10317–10321. doi: 10.1073/pnas.95.17.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Geske T, Sauter M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011;190:369–378. doi: 10.1111/j.1469-8137.2010.03496.x. [DOI] [PubMed] [Google Scholar]
- Steffens B, Kovalev A, Gorb SN, Sauter M. Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. Plant Cell. 2012;24:3296–3306. doi: 10.1105/tpc.112.101790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Steffen-Heins A, Sauter M. Reactive oxygen species mediate growth and death in submerged plants. Frontier Plant Sci. 2013;4:179. doi: 10.3389/fpls.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr C, Mäck G. Diurnal changes in nitrogen assimilation of tobacco roots. J Exp Bot. 2001;52:1283–1289. doi: 10.1093/jexbot/52.359.1283. [DOI] [PubMed] [Google Scholar]
- Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta. 2007;226:465–474. doi: 10.1007/s00425-007-0496-0. [DOI] [PubMed] [Google Scholar]
- Striker G, Insausti P, Grimoldi A, Vega A. Trade-off between root porosity and mechanical strength in species with different types of aerenchyma. Plant Cell Environ. 2007;30:580–589. doi: 10.1111/j.1365-3040.2007.01639.x. [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiol. 1998;118:759–771. doi: 10.1104/pp.118.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JE, Ratcliffe RG, Jackson MB. Anoxia tolerance in the aquatic monocot Potamogeton pectinatus: absence of oxygen stimulates elongation in association with an unusually large Pasteur effect. J Exp Bot. 2000;51:1413–1422. [PubMed] [Google Scholar]
- Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Eickmeier I, Fernie AR. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA. 2006;103:19587–19592. doi: 10.1073/pnas.0607751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szal B, Jolivet Y, Hasenfratz-Sauder MP, Dizengremel P, Rychter AM. Oxygen concentration regulates alternative oxidase expression in barley roots during hypoxia and post-hypoxia. Physiol Plant. 2003;119:494–502. [Google Scholar]
- Taylor ER, Nie XZ, MacGregor AW, Hill RD. A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol. 1994;24:853–862. doi: 10.1007/BF00014440. [DOI] [PubMed] [Google Scholar]
- Tollenaar M, Lee EA. Yield potential, yield stability and stress tolerance in maize. Field Crops Res. 2002;75:161–169. [Google Scholar]
- Trevaskis B, Watts RA, Andersson CR, Llewellyn DJ, Hargrove MS, Olson JS, Dennis ES, Peacock WJ. Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA. 1997;94:12230–12234. doi: 10.1073/pnas.94.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Meguro N, Suzuki Y, Tsutsumi N, Hirai A, Nakazono M. Induction of mitochondrial aldehyde dehydrogenase by submergence facilitates oxidation of acetaldehyde during re-aeration in rice. FEBS Lett. 2003;546:369–373. doi: 10.1016/s0014-5793(03)00631-8. [DOI] [PubMed] [Google Scholar]
- Turkan I. ROS and RNS: key signalling molecules in plants. J Exp Bot. 2018;69:3313–3315. doi: 10.1093/jxb/ery198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Kim JH, Kende H. A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiol. 2000;122:695–704. doi: 10.1104/pp.122.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Roeb GW, Dautzenberg M, Froehlich A, Vigeolas H, Minchin PE, Geigenberger P. Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol. 2004;135:1809–1821. doi: 10.1104/pp.104.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen H, Mustroph A, Barding GA, Vergeer-van Eijk M, Welschen-Evertman RA, Pedersen O, Visser EJ, Larive CK, Pierik R, Bailey-Serres J. Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell. 2013;25:4691–4707. doi: 10.1105/tpc.113.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Mclntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996;111:589–595. doi: 10.1104/pp.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Robson CA, Yip JY. Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol. 2002;129:1829–1842. doi: 10.1104/pp.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Cvetkovska M, Wang J. Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? Physiol Plant. 2009;137:392–406. doi: 10.1111/j.1399-3054.2009.01254.x. [DOI] [PubMed] [Google Scholar]
- Vartapetian BB, Polyakova LI. Protective effect of exogenous nitrate on the mitochondrial ultrastructure of Oryza sativa coleoptiles under strict anoxia. Protoplasma. 1999;206:163–167. [Google Scholar]
- Vartapetian BB, Andreeva IN, Generozova IP, Polyakova LI, Maslova IP, Dolgikh YI, Stepanova AY. Functional electron microscopy in studies of plant response and adaptation to anaerobic stress. Ann Bot. 2003;91:155–172. doi: 10.1093/aob/mcf244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010;63:551–562. doi: 10.1111/j.1365-313X.2010.04262.x. [DOI] [PubMed] [Google Scholar]
- Vishwakarma A, Tetali SD, Selinski J, Scheibe R, Padmasree K. Importance of the alternative oxidase (AOX) pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of the cytochrome oxidase pathway in Arabidopsis thaliana. Ann Bot. 2015;116:555–569. doi: 10.1093/aob/mcv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJ, Pierik R. Inhibition of root elongation by ethylene in wetland and non-wetland plant species and the impact of longitudinal ventilation. Plant Cell Environ. 2007;30:31–38. doi: 10.1111/j.1365-3040.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- Visser EJ, Cohen JD, Barendse GW, Blom CW, Voesenek LA. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 1996;112:1687–1692. doi: 10.1104/pp.112.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Bailey-Serres J. Flood adaptive traits and processes: an overview. New Phytol. 2015;206:57–73. doi: 10.1111/nph.13209. [DOI] [PubMed] [Google Scholar]
- Vreeburg RA, Benschop JJ, Peeters AJ, Colmer TD, Ammerlaan AH, Staal M, Elzenga TM, Staals RH, Darley CP, McQueen-Mason SJ. Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. Plant J. 2005;43:597–610. doi: 10.1111/j.1365-313X.2005.02477.x. [DOI] [PubMed] [Google Scholar]
- Vriezen WH, De Graaf B, Mariani C, Voesenek LA. Submergence induces expansin gene expression in flooding-tolerant Rumex palustris and not in flooding-intolerant R. acetosa. Planta. 2000;210:956–963. doi: 10.1007/s004250050703. [DOI] [PubMed] [Google Scholar]
- Vu H, Manangkil O, Mori N, Yoshida S, Nakamura C. Submergence-induced ADH and ALDH gene expression in japonica and indica rice with contrasting levels of seedling vigor under submergence stress. Biotechnol Biotec Eq. 2009;23:1469–1473. [Google Scholar]
- Wagner AM, Krab K. The alternative respiration pathway in plants: role and regulation. Physiol Plant. 1995;95:318–325. [Google Scholar]
- Wang AY, Kao MH, Yang WH, Sayion Y, Liu LF, Lee PD, Su JC. Differentially and developmentally regulated expression of three rice sucrose synthase genes. Plant Cell Physiol. 1999;40:800–807. doi: 10.1093/oxfordjournals.pcp.a029608. [DOI] [PubMed] [Google Scholar]
- Wang R, Tischner R, Gutiérrez RA, Hoffman M, Xing X, Chen M, Coruzzi G, Crawford NM. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004;136:2512–2522. doi: 10.1104/pp.104.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe CK, Hachiya T, Takahara K, Kawai-Yamada M, Uchimiya H, Uesono Y, Terashima I, Noguchi K. Effects of AOX1a deficiency on plant growth, gene expression of respiratory components and metabolic profile under low-nitrogen stress in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:810–822. doi: 10.1093/pcp/pcq033. [DOI] [PubMed] [Google Scholar]
- Welchen E, Chan R, Gonzalez D. Metabolic regulation of genes encoding cytochrome c and cytochrome c oxidase subunit Vb in Arabidopsis. Plant, Cell Environ. 2002;25:1605–1615. [Google Scholar]
- Wijte AH, Gallagher JL. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. I. Different germination strategies of Spartina alterniflora and Phragmites australis (Poaceae) Amer J Bot. 1996;83:1337–1342. [Google Scholar]
- Winkel A, Colmer TD, Ismail AM, Pedersen O. Internal aeration of paddy field rice (Oryza sativa) during complete submergence–importance of light and floodwater O2. New Phytol. 2013;197:1193–1203. doi: 10.1111/nph.12048. [DOI] [PubMed] [Google Scholar]
- Winkel A, Pedersen O, Ella E, Ismail AM, Colmer TD. Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. J Exp Bot. 2014;65:3225–3233. doi: 10.1093/jxb/eru166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JD, Chory J. Coordination of gene expression between organellar and nuclear genomes. Nat Rev Gen. 2008;9:383. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Xu F, Yuan S, Lin H-H. Response of mitochondrial alternative oxidase (AOX) to light signals. Plant Signal Behav. 2011;6:55–58. doi: 10.4161/psb.6.1.14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Shimamura S, Nakazono M, Mochizuki T. Aerenchyma formation in crop species: a review. Field Crops Res. 2013;152:8–16. [Google Scholar]
- Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot. 2013;65:261–273. doi: 10.1093/jxb/ert371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Jansen MJ, Zhang Q, Sergeeva L, Ligterink W, Mariani C, Rieu I, Visser EJ. A disturbed auxin signaling affects adventitious root outgrowth in Solanum dulcamara under complete submergence. J Plant Physiol. 2018;224:11–18. doi: 10.1016/j.jplph.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Yim H, Lim M, Lee S, Lim J, Lee Y, Hwang Y. Hexokinase-mediated sugar signaling controls expression of the calcineurin B-like interacting protein kinase 15 gene and is perturbed by oxidative phosphorylation inhibition. J Plant Physiol. 2012;169:1551–1558. doi: 10.1016/j.jplph.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Ying-Hua D, Ya-Li Z, Qi-Rong S, Song-Wei W. Nitrate effect on rice growth and nitrogen absorption and assimilation at different growth stages. Pedosphere. 2006;16:707–717. [Google Scholar]
- Ying-Hua D, Xiao-Ming Y, Zhang YL, Qi-Rong S. Mechanisms of enhanced rice growth and nitrogen uptake by nitrate. Pedosphere. 2007;17:697–705. [Google Scholar]
- Yun C, Xiao-Rong F, Shu-Bin S, Guo-Hua X, Jiang H, Qi-Rong S. Effect of nitrate on activities and transcript levels of nitrate reductase and glutamine synthetase in rice. Pedosphere. 2008;18:664–673. [Google Scholar]
- Zabalza A, van Dongen JT, Froehlich A, Oliver SN, Faix B, Gupta KJ, Schmälzlin E, Igal M, Orcaray L, Royuela M, Geigenberger P. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 2009;149:1087–1098. doi: 10.1104/pp.108.129288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Visser EJ, de Kroon H, Huber H. Life cycle stage and water depth affect flooding-induced adventitious root formation in the terrestrial species Solanum dulcamara. Ann Bot. 2015;116:279–290. doi: 10.1093/aob/mcv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Huber H, Beljaars SJ, Birnbaum D, de Best S, de Kroon H, Visser EJ. Benefits of flooding-induced aquatic adventitious roots depend on the duration of submergence: linking plant performance to root functioning. Ann Bot. 2017;120:171–180. doi: 10.1093/aob/mcx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q. Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant, Cell Environ. 2009;32:1428–1440. doi: 10.1111/j.1365-3040.2009.02009.x. [DOI] [PubMed] [Google Scholar]