Summary
The skin is the outermost layer of the body with an extensive surface area of approximately 1·8 m2, and is the first line of defence against a multitude of external pathogens and environmental insults. The skin also has important homeostatic functions such as reducing water loss and contributing to thermoregulation of the body. The structure of the skin and its cellular composition work in harmony to prevent infections and to deal with physical and chemical challenges from the outside world. In this review, we discuss how the structural cells such as keratinocytes, fibroblasts and adipocytes contribute to barrier immunity. We also discuss specialized immune cells that are resident in steady‐state skin including mononuclear phagocytes, such as Langerhans cells, dermal macrophages and dermal dendritic cells in addition to the resident memory T cells. Ageing results in an increased incidence of cancer and skin infections. As we age, the skin structure changes with thinning of the epidermis and dermis, increased water loss, and fragmentation of collagen and elastin. In addition, the skin immune composition is altered with reduced Langerhans cells, decreased antigen‐specific immunity and increased regulatory populations such as Foxp3+ regulatory T cells. Together, these alterations result in decreased barrier immunity in the elderly, explaining in part their increased susceptiblity to cancer and infections.
Keywords: ageing, immunosenescence, skin, tissue resident
Stromal cells such as keratinocytes and fibroblasts in addition to skin‐resident leucocyte populations – including Langerhans cells, macrophages, dermal dendritic cells and T resident memory cells – all contribute to skin barrier immunity. In this review, we discuss how the structure of the skin changes with age, resulting in skin thinning, increased water loss, skin wrinkling and fragmentation of the extracellular matrix. In addition to the skin structural changes, the skin‐resident immune cells are altered with age, rendering the older person more susceptible to skin infections and cancers.

Abbreviations
- DC
dendritic cells
- DETCs
dendritic epidermal γδ T cells
- IL
interleukin
- ILC
innate lymphoid cell
- LCs
Langerhans cells
- MMP
matrix metalloproteinases
- TLR
Toll‐like receptor
- Treg cells
T regulatory cells
- Trm cells
T resident memory cells
- UV
ultraviolet
- VZV
varicella zoster virus
Skin barrier
The skin is the outermost layer of the body with an extensive surface area of approximately 1·8 m2, and is the first line of defence against a multitude of external pathogens. The skin consists of three layers: the top layer is the epidermis, a thin layer (approximately 0·1 mm thick) of stratified squamous epithelium, composed of four strata of keratinocytes in progressive stages of differentiation. The stratified epithelium provides a watertight barrier from the external environment and prevents excessive water loss from the body. The epidermis is mainly composed of keratinocytes; however, there are also melanocytes, which provide a barrier from ultraviolet (UV) radiation through expression of melanin. The epidermis does not have a blood supply of its own, but instead is nourished from blood vessels below. The second layer is the dermis, a thicker layer (up to 3–4 mm depending on body site), which has a relatively low cell volume compared with the epidermis. The dermis predominantly consists of the extracellular matrix, such as collagen, which is made by fibroblasts. In addition to the extracellular matrix, the dermis contains structures such as blood vessels, lymphatics, nerves, sweat glands and pilosebaceous units. The deepest layer of the skin is the subcutaneous layer, which consists of subcutaneous fat and connective tissue.1
Skin barrier immunity
The skin is a complex organ that carries out numerous functions contributing to its barrier immunity function – the skin structure and stromal and immune cell composition can be seen in Fig. 1.
Figure 1.
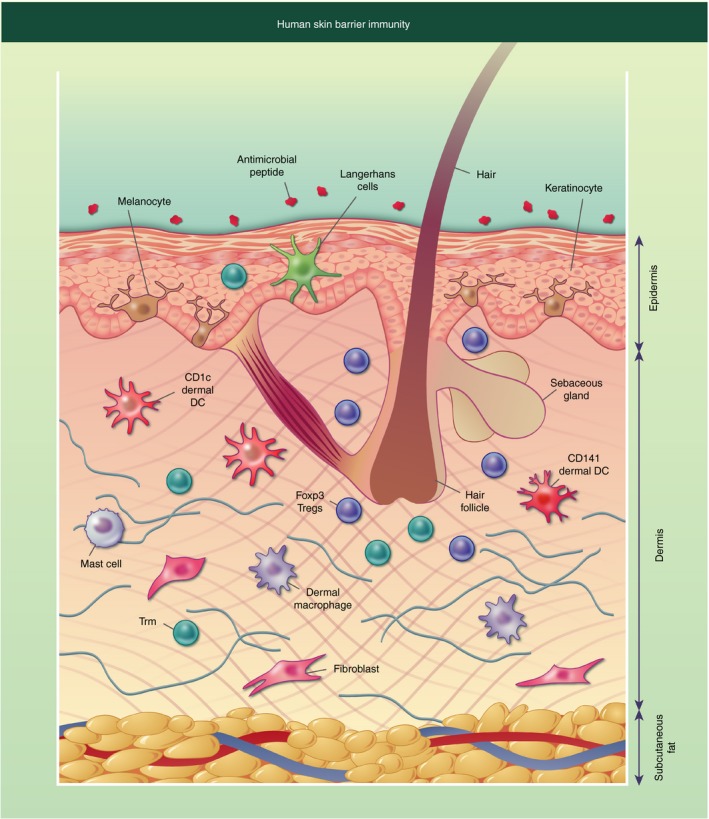
Diagrammatic representation of human skin barrier immunity. The surface of the skin is covered in antimicrobial peptides and lipids, some of which originate from the sebaceous gland located near the hair follicle. The epidermis consists of keratinocytes forming stratified corneum, with melanocytes interspersed. Langerhans cells and T resident memory cells (Trm) can also be found in the epidermis. The dermis has a more diverse collection of cells including structural cells such as fibroblasts, and immune cells such as dermal dendritic cells (DCs) and macrophages, CD4+ and CD8+ Trm, mast cells and Foxp3+ T regulatory cells (Tregs), which are often located near the hair follicle. The final layer of the skin is the subcutaneous fat, which is primarily composed of adipocytes.
Antimicrobial peptides and lipids are secreted onto the cell surface to control bacterial growth. These include dermcidin, which is secreted in human sweat and has broad antimicrobial activity against a range of pathogenic bacteria. Its antimicrobial activity is not affected by the low pH value and high salt concentrations of human sweat.2 Sebum is made by sebaceous glands found independently of or near hair follicles. Within the sebum are antimicrobial lipids, such as lauric acid and sapienic acid, which play an important role in controlling pathogenic organisms.3
However, the skin is not a sterile site, and there is extensive research showing the role that the skin microbiota plays in immunity by restricting the growth of pathogenic bacteria.4 Commensal bacteria have been shown to produce an antimicrobial peptide that synergizes with the human antimicrobial peptide LL37, which together kill the pathogenic bacterium Staphylococcus aureus.5 However, insults and pathogens are mostly controlled and prevented entry due to structure and barrier immunity in the skin.
Skin‐resident stromal cells
Keratinocytes are the main component of the epidermis. They express Toll‐like receptors (TLRs), which are crucial pathogen pattern recognition receptors that when triggered lead to the production of inflammatory cytokines and initiation of an immune response.6 Keratinocytes have been shown to constitutively express TLR1, ‐2, ‐3, ‐5, ‐6 and ‐10.7, 8 They also have the ability to sense wound damage and produce inflammatory cytokines and chemokines such as interleukin‐1β (IL‐1β), IL‐8 and CCL20 to recruit leucocytes to the site of damage.9
Keratinocytes express a raft of antimicrobial peptides that control bacterial growth, including adrenomedullin and β‐defensins.10, 11 β‐Defensin‐1 is constitutively expressed by human keratinocytes and β‐defensin‐2 and ‐4 are up‐regulated upon inflammatory challenge.11, 12, 13 Keratinocytes can express the antimicrobial peptide Cathelicidin upon stimulation and can store Cathelicidin in cytoplasmic granules until needed.14, 15 Keratinocytes also constitutively express RNase 7, which is a very potent antimicrobial ribonuclease, and upon inflammatory or bacterial challenge there is further increased expression.16
More recently, it has been proposed that keratinocytes have the ability to process and present antigen to CD4+ and CD8+ T cells, initiating an adaptive immune response.17 In addition, keratinocytes are the key site for the first step in the vitamin D metabolism pathway, when pro‐vitamin D3 (7‐dehydro‐cholesterol) is metabolized into vitamin D3, catalysed by UVB. Vitamin D is an important component of a functioning immune system and its metabolism at the skin site contributes to barrier immunity.18
Dermal fibroblasts are the structural cells of the dermis; their primary function is to secrete extracellular matrix components such as pro‐collagen. Fibroblasts express the full range of TLRs, at a higher level than keratinocytes, demonstrating their important role in the detection of pathogens.19 In vitro studies have shown that dermal fibroblasts can have differing roles in immunity, indeed TLR4 signalling results in the production of inflammatory cytokines such as IL‐6, IL‐8 and the monocyte chemoattractant CCL2.20 Conversely fibroblasts have been shown to suppress T‐cell proliferation via indoleamine 2,3‐dioxygenase production, and to skew the T cells to produce immunoregulatory cytokines such as IL‐10.21
The subcutaneous layer of the skin is predominantly composed of adipocytes – their primary function is to be a repository of energy that responds to hypothermia by producing heat. More recent work has identified the important role of adipocytes in barrier immunity as a significant source of antimicrobial peptides. In response to infection, for example with S. aureus, dermal fibroblasts can differentiate into adipocytes and produce the antimicrobial peptide cathelicidin.22
Skin‐resident immune cells
Mononuclear phagocytes
Within the epidermis there is a population of mononuclear phagocytes called Langerhans cells (LCs). These were believed to have been seeded at birth and maintained by local turnover to ensure a steady‐state population.23 However, a recent study demonstrated, in a murine model of immune injury, that repopulation of LCs from peripheral monocytes makes up for the slow repopulation from mature LCs.24 Langerhans cells are located at the interface with the external environment and as such are multifunctional sentinels of the epidermis. They sample the environment by extension and retraction of their dendrites between the keratinocytes in an amoeba‐like movement.25 They present antigen to T cells within the epidermis to initiate a local immune response and also have the capacity to migrate to the lymph node and initiate immune responses.26
Within the dermis, there is a more diverse population of mononuclear phagocytes including dermal dendritic cells (DCs) and dermal macrophage populations. Dendritic cells are the sentinels of the immune system, they sample the microenvironment and either present antigen to the resident T cells or migrate through the lymphatics to the lymph node to initiate an immune response.27 Historical assessment of dermal DCs identified that they are more activated then their blood counterparts; dermal DCs had increased expression of co‐stimulatory receptors and were strong stimulators of T‐cell proliferation relative to their peripheral blood counterparts.28 Two main populations of dermal myeloid DCs have been identified; the CD1c+ DCs and the CD141+ DCs. CD141+ DCs are the cells responsible for cross‐presenting antigens to CD8+ T cells and have homology to the mouse CD103+ DCs.29 Very few plasmacytoid DCs are observed in steady‐state skin.30
Macrophages are another type of antigen‐presenting cell resident in the dermis and they sense pathogens and damage and initiate an appropriate immune response. In addition to the immune function, macrophages maintain tissue homeostasis through increasing appropriate anti‐inflammatory mechanisms, contribute to wound healing, and heal nerves upon tissue injury.31, 32 Macrophages are thought to populate tissues early on but studies have also shown that they are replenished by circulating monocytes.33 These data are supported by a study in humans showing that CD14+ cells were a transient population of monocyte‐derived macrophages.34 CD163 has been proposed to be a good marker for dermal macrophages, as it specifically identifies skin‐specific macrophages that are not recently migrated monocytes.35
Analysis of the location of these different mononuclear phagocyte populations in the dermis have shown that DCs can be found closer to the epidermis (around 0–20 µm beneath the dermo–epidermal junction) and macrophages are located deeper in the skin (around 40–60 µm beneath the dermo–epidermal junction).36
Other innate populations
In rodent and cattle skin a population of γδ T cells has been described called dendritic epidermal γδ T cells (DETCs) – these cells are localized in the epidermis.37 The DETCs express a limited T‐cell receptor repertoire and recognize danger‐associated molecular patterns induced on damaged or dysregulated keratinocytes. In addition, DETCs have been shown to play a role in maintaining keratinocyte homeostasis as in the absence of DETCs there was increased keratinocyte apoptosis.37 However, DETCs have not been observed in human skin. Indeed, in human skin the predominant leucocyte population is αβ T cells, γδ T cells and natural killer cells were found in the skin but at very low frequencies (0·35% and 0·97%, respectively).38 Neutrophils are not present in steady‐state skin; however, upon sun exposure there is an infiltration of neutrophils that contribute to sunburn and photo‐ageing.39
Innate lymphoid cells (ILCs) are a relatively recently described immune cell population and their function in the skin is still under investigation. In steady‐state human skin, there are few ILCs, and those cells that are present tend to be ILC1 and ILC3. ILC populations are significantly increase in inflammatory conditions; there is an influx of ILC2s in atopic dermatitis, and in psoriatic plaques ILC1 and ILC3 populations have been observed.40, 41
The dermis also contains mast cells, of which there are between 77 and 108 cells/mm2.42 Mast cells contain granules with pre‐formed inflammatory mediators such as histamine that are released when receptors are crosslinked, contributing to local inflammatory responses. Mast cells also play an important role in allergic reactions and associated itching and rash.
T cells
Skin T resident memory (Trm) cells are non‐circulating T cells present in the skin that maintain immune surveillance and are crucial for initiating robust immune responses at times of infection.43, 44, 45 In steady‐state skin, there are around 1 × 106 T cells/cm2 suggesting that in an average person, there are around 2 × 1010 T cells present in the whole skin.46 The majority (80%–90%) of T cells found in the skin are Trm and the remaining T cells are recirculating T cells.47 Cutaneous Trm cells are generated after exposure to antigen and provide memory at the site of initial exposure – Trm cells are more potent effector cells compared with circulating T cells.47 Of the CD3+ Trm cells present in the skin, the ratio of CD4+ to CD8+ T cells was found to be approximately 3 : 1 in human epidermis and 6 : 1 in dermis.47
The most commonly used markers to define Trm cells are cell surface expression of CD69 and CD103.48 T cells increase CD69 expression in response to antigen exposure or type I interferon signalling, and this blocks T‐cell egress from the skin by inhibiting sphingosine‐1‐phosphate receptor function.49, 50 CD103 is an integrin that binds to E‐cadherin, it has been associated with CD8+ Trm cells present in the epidermis.47, 48 CD103 expression is believed to be partly due to the expression of E‐cadherin on the keratinocytes, which is important for retention of these cells in the epidermis.51
In addition to CD69 and CD103, CCR8 has been proposed to be a Trm cell marker.52, 53 The sole ligand for CCR8 is CCL1, which is predominantly expressed by CD1a+ LCs.52 The epidermis and in particular keratinocytes have been shown to play a role in up‐regulating CCR8 on naive T cells in the skin and generating Trm cells, through production of Vitamin D3 and prostaglandin E2.53, 54
CD4+ FoxP3 T regulatory (Treg) cells are an important regulatory cell type that plays an role in immune and tissue homeostasis.55 Foxp3+ Treg cells with a memory skin‐resident phenotype have been observed in the dermis and in particular in steady‐state conditions can be found located close to hair follicles.56 The short‐chain fatty acid sodium butyrate, which is a bacterial metabolite produced by skin commensals, can increase Foxp3 expression in non‐Treg cells driving an increase Foxp3+ Treg cells leading to increased immune tolerance to skin commensals.57 In addition, UVB light has been shown to increase the number of Foxp3+ Treg cells by facilitating the proliferation of thymically derived Foxp3+ Treg cells.58 This effect of UVB could be in part due to the production of Vitamin D3, which can drive Foxp3+ Treg cell proliferation in vitro.59 Indeed it is believed Foxp3+ Treg cells accumulate around the hair follicle because of entry of commensal bacteria to newly formed hair follicles during neonatal skin development.60
Ageing and skin structure
As we age our skin structure changes (Fig. 2), the epidermal layer is thinner due to keratinocyte atrophy.61 This leads to increased trans‐epidermal water loss in elderly individuals, resulting in increased skin dryness.62 The extracellular matrix components collagen and elastin, which provide tensile strength and elasticity respectively, are substantially changed with age. The total amount of collagen has been shown to be reduced with age.63 However, there is also increased collagen fragmentation, which is believed to be due to increased matrix metalloproteinase (MMP) expression in older skin.64 Elastin is an inert protein that is formed during early development and is not replenished, therefore any changes to elastin that occur over a lifetime tend to be permanent.65 MMPs, in particular MMP‐1, ‐3 and ‐9, target elastin for fragmentation,65 resulting in reduced skin elasticity and the classical sign of skin ageing, wrinkling.
Figure 2.
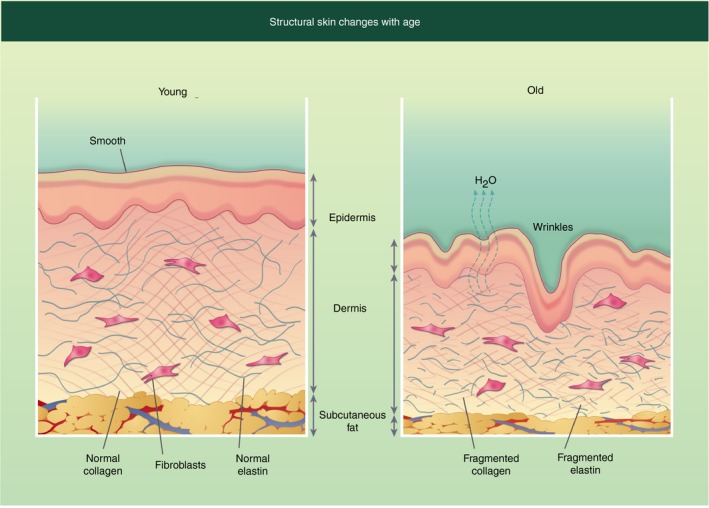
Structural changes in human skin with age. Young skin structure (left) and compared with older skin structure (right). Older skin has fragmented elastin and collagen, increasing water loss, which leads to skin dryness and increased wrinkles. In addition, the skin is thinner with all three layers being less thick then the younger counterpart.
Dermal fibroblasts contribute to age‐associated dermal thinning as they are reduced in size.66 In addition, dermal fibroblasts from elderly individuals make less pro‐collagen and have increased expression of MMP‐1, contributing to increased collagen fragmentation.66, 67, 68 Other changes in the skin that are observed with age are reduced sweat and sebum production.69 Finally, there is a thinning of the adipose tissue observed with age due to a reduction in white adipose tissue – subsequent antimicrobial protection (by the dermal fat) in response to infection is significantly decreased. This reduction in adipocytes is believed to be due in part to the inability of fibroblasts to convert to adipose tissue.70
Changes in skin structure with age are dependent upon lifestyle choices and environment challenges, including UVB exposure and the use of sunscreen, smoking and environmental pollution.71, 72 Collectively these changes render older people more susceptible to mechanical injury, alter the skin microbiome and have important implications for skin barrier immunity.
Immunological changes in the skin with age
The decrease in cutaneous immune function has been well documented in older humans. A variety of bacterial infections are more common in the elderly, including cellulitis (in particular of the lower legs), erysipelas, necrotizing fasciitis, folliculitis, impetigo, folliculitis and furunculosis.73 Staphylococcus aureus and β‐Haemolytic streptococci are the most common skin pathogens in the elderly, although other bacterial infections caused by Pseudomonas spp. and Klebsiella spp. are also elevated in older individuals.74 The prevalence of skin colonization by Proteus mirabilis and Pseudomonas aeruginosa in people over 65 years old is increased by about 25% compared with younger individuals.74 Fungal infections (such as Candida) and viral infections such as shingles, herpes simplex virus‐1 and human papillomavirus are also more common in the elderly.74, 75
Non‐melanoma skin cancer, including basal cell and squamous cell carcinomas, is more commonly diagnosed in persons older than 70 years. The highest incidence of malignant melanoma and melanoma is in individuals aged 65 years and older.75, 76, 77, 78
Together these observations provide strong evidence for age‐dependent changes in the skin barrier immunity. Although changes in peripheral immune cell populations have been well described (as reviewed previously79, 80, 81), we have focused on skin‐specific immunological differences with age (Fig. 3).
Figure 3.
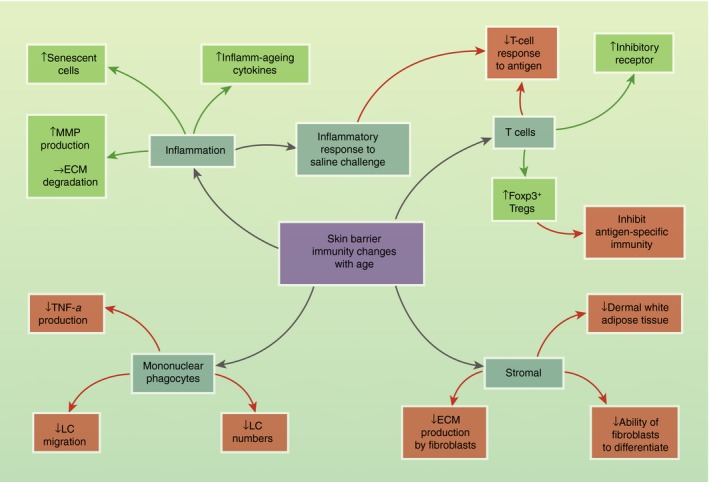
Skin barrier immunity changes with age. Schematic showing the effect of age on skin‐resident populations. Negative/inhibitory effects are shown in red and positive/enhancing effects are shown in green. ECM, extracellular matrix; LC, Langerhans cell; MMP, matrix metalloproteinases; Treg, T regulatory cells.
Mononuclear phagocytes
Langerhans cells are reduced in number in the elderly. In addition, LCs from older donors have reduced capacity to migrate to the lymph node.82 Using an ex vivo epidermal model, Pilkington et al. 83 have shown that lower levels of IL‐1β observed in elderly skin result in reduced migration of the LCs to the cytokine gradient – demonstrating that the skin microenvironment plays a detrimental role. The specific source of IL‐1β in the skin remains controversial, and both keratinocytes and LCs themselves have been proposed as the primary source. In addition, LCs from aged skin express less human β‐defensin‐3, an important antimicrobial peptide for response to infection.84
The number and phenotype of dermal DCs is comparable between young and old skin.81 However, dermal DCs from aged skin appear to be functionally impaired in terms of migration, phagocytosis and ability to stimulate T cells in a mouse B16 melanoma model.85 The effect of age on macrophage function is still contentious – some studies demonstrate reduced TLR expression and TLR‐induced cytokine production.86 In contrast other studies have shown that there is increased inflammatory cytokine production after TLR ligation.87 However, there are limited data on the effect of age on dermal macrophage populations. We have shown that CD163+ macrophages produce less tumour necrosis factor‐α in antigen‐challenged old skin, but upon removal of the macrophages from the skin environment they produce similar amounts of pro‐inflammatory cytokine in response to TLR ligands.82 This suggests that it is the skin environment itself that is altered with age rather than intrinsic dysfunction of macrophages.
T cells
Repeated antigen stimulation throughout life can have significant effects on human antigen‐specific T cells, including the induction of exhaustion and senescence. Functional exhaustion of T cells is characterized by loss of functional activity, increase in inhibitory receptor expression [such as programmed cell death protein 1 (PD‐1)]. It is a mechanism necessary for limiting the magnitude of the effector T‐cell response but it also contributes to the functional decline in adaptive immunity with age. Senescence, a loss of replicative capacity, is often induced by repeated stimulation, and is primarily induced through the process of telomere erosion. Although the age‐related changes in the circulating T‐cell pool have been well characterized and extensively reviewed,79 the age‐related changes in the skin‐resident T‐cell population have not been extensively studied. The differences in the regulation of senescence and the importance of telomere shortening between mouse and human T cells should also be taken into account when extrapolating from mouse models.88
Tissue‐resident CD8+ T cells have recently been shown to promote a long‐lasting state of equilibrium between melanoma and the immune system.89 Depletion of these Trm cells demonstrated that they actively suppress tumour progression.89 How anti‐tumour surveillance and control by skin‐resident Trm cells is affected by age and age‐related changes within the CD8 population has not been studied. It is known that skin‐resident Trm cells are vital to clear skin infections,90, 91, 92 so defects in Trm cells may explain the increased incidence of infection seen in the elderly. We and others have shown that there are decreased delayed‐type hypersensitivity responses to recall antigens such as Candida or varicella zoster virus (VZV)75, 76, 77, 78 in older adults due to a reduced infiltration of T cells at the site of antigen challenge. Our group has shown that the function of skin‐derived CD4+ T cells was not impaired with age in response to both mitogen‐ and antigen‐specific stimulation ex vivo,93 although the skin residency markers were not used for cell isolation. Interestingly old skin actually had a higher proportion of VZV‐specific T cells compared with young – possibly suggesting accumulation over a lifetime of subclinical reactivation.94 There was, however, an increase in PD‐1 expression on both CD4 and CD8 T cells in old individuals compared with young skin, suggesting that older T cells are more susceptible to inhibition via programmed death ligand 1/PD‐1 signalling.93
Foxp3+ Treg cells
The proportion of regulatory cells in normal skin is increased in older mice and humans.95, 96 People who had the highest proportion of Foxp3+ Treg cells had the worst delayed‐type hypersensitivity response to VZV recall antigen – showing that Foxp3+ Treg cells in the skin can interfere with antigen‐specific immunity.97 Indeed, in a mouse model of melanoma, Treg cells can suppress very early stages of the inflammatory response to antigen challenge.98 It is known that there is an increase in Foxp3+ Treg cell numbers in cancers such as melanoma and basal cell carcinoma.99, 100, 101 In human squamous cell carcinoma, 50% of cells have a Foxp3+ Treg phenotype, reduction of Foxp3+ Treg cell percentage in these patients and their function led to clinical improvement.102 The reasons why Foxp3+ Treg cell numbers are increased in older skin are not clear. It has been shown that UVB irradiation can lead to the induction of Foxp3+ Treg cells and that these Foxp3+ Treg cells suppress other immune cells through the production of IL‐10.58, 103 It is also tempting to postulate that Foxp3+ Treg cells could be induced or accumulate as an attempt to the immune system to control unwanted low‐grade inflammation, which accompanies ageing.
Inflamm‐ageing and senescence in the skin
Chronic low‐grade inflammation, termed inflamm‐ageing, is characterized by high serum C‐reactive protein.104 Inflamm‐ageing is known to negatively impact on immunity because older people with elevated IL‐1β had increased risk of morbidity and mortality.105 It has been postulated that innate immune cells such as macrophages are a contributor to the inflamm‐ageing phenotype, because due to changes in tissue structure – such as skin thinning – they are exposed to more bacteria, which leads to chronic activation and subsequent inflammatory cytokine production, such as is seen with increased gut permeability in an aged mouse model.106
Another contributor to inflamm‐ageing, especially in the skin, is UV damage. Repeated exposure to UVB, as would be the case in old skin, leads to the accumulation of macrophages and increase in reactive oxygen species and MMP, and subsequent damage to the extracellular matrix. Inappropriate complement activation may also be caused by the increase in oxidative stress and accumulation of damaged cells, in line with observations in atherosclerosis.107 Another contributor to increased inflammation in the old is the accumulation of senescent cells; senescence is defined as irreversible growth arrest. It is known that there is an accumulation of senescent dermal fibroblasts, as classically defined by p16 expression in the skin of old mice and humans.108, 109, 110 Senescent fibroblasts secrete a raft of inflammatory mediators such as IL‐8, IL‐6, tumour necrosis factor‐α and CCL2.110 This production of inflammatory mediators from senescent cells is termed senescence‐associated secretory phenotype, which contributes to the low‐grade inflammation observed in older individuals.111 A recent paper has shown that senescent dermal fibroblasts persist in the skin by evading recognition and killing by natural killer cells and CD8+ T cells, through increased expression of HLA‐E.110 Other skin‐resident cell populations that have been shown to be senescent include endothelial cells and melanocytes.112, 113 Although increased frequency and number of senescent T cells have been observed in the periphery,80 their contribution to the skin environment is unknown and warrants further investigation.
How this inflammation directly negatively affects cutaneous immune responses is not clear. Our studies have shown that skin from older individuals has a propensity to mount an inappropriate response to saline injection, which negatively correlates with antigen‐specific cutaneous immunity.94 Furthermore, blocking inflammation using a p38‐mitogen‐activated protein kinase inhibitor, Losmapimod, reduced this non‐specific inflammation while improving the ability of old individuals to respond to recall antigen challenge.94
Concluding remarks
Skin barrier immunity is comprised of stromal cells such as keratinocytes and adipocytes and immune cells such as Langerhans and Trm cells working in concert to prevent pathogen entry and to deal with continuous physical and chemical challenges. With increasing lifespan, it is important to understand how skin changes with age and the impact that these changes have on barrier immunity. Clearly, the skin environment is detrimental to a successful immune response of older people as removal of individual cells from the skin microenvironment results in restoration of immune function. Specifically which cells alter the ageing skin environment is unknown, certainly senescent cells such as fibroblasts will contribute greatly. However, there more research is required to understand fully which cells are responsible for the ageing skin microenvironment and which cell types, such as keratinocytes, endothelium and adipocytes, warrant further investigation. Better understanding of the inhibitory and inflammatory mechanisms that operate in older skin is crucial for the development of new strategies to combat infections and cancer.
Disclosures
The authors declare that they have no competing interests related to this work.
References
- 1. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009; 9:679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S et al Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol 2001; 2:1133–7. [DOI] [PubMed] [Google Scholar]
- 3. Wertz PW. Lipids and the permeability and antimicrobial barriers of the skin. J Lipids 2018; 2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science 2014; 346:954–9. [DOI] [PubMed] [Google Scholar]
- 5. Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T et al Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017; 9:eaah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medzhitov R. Toll‐like receptors and innate immunity. Nat Rev Immunol 2001; 1:135–45. [DOI] [PubMed] [Google Scholar]
- 7. Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll‐like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 2003; 148:670–9. [DOI] [PubMed] [Google Scholar]
- 8. Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J et al Various members of the Toll‐like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 2005; 114:531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kennedy‐Crispin M, Billick E, Mitsui H, Gulati N, Fujita H, Gilleaudeau P et al Human keratinocytes' response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J Invest Dermatol 2012; 132:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez A, Elsasser TH, Muro‐Cacho C, Moody TW, Miller MJ, Macri CJ et al Expression of adrenomedullin and its receptor in normal and malignant human skin: a potential pluripotent role in the integument. Endocrinology 1997; 138:5597–604. [DOI] [PubMed] [Google Scholar]
- 11. Fulton C, Anderson GM, Zasloff M, Bull R, Quinn AG. Expression of natural peptide antibiotics in human skin. Lancet 1997; 350:1750–1. [DOI] [PubMed] [Google Scholar]
- 12. Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L et al Human β‐defensin‐2 production in keratinocytes is regulated by interleukin‐1, bacteria, and the state of differentiation. J Invest Dermatol 2002; 118:275–81. [DOI] [PubMed] [Google Scholar]
- 13. Harder J, Meyer‐Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human β‐defensins (hBD‐1, ‐2, ‐3, and ‐4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol 2004; 123:522–9. [DOI] [PubMed] [Google Scholar]
- 14. Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol 2005; 124:394–400. [DOI] [PubMed] [Google Scholar]
- 15. Sorensen OE, Cowland JB, Theilgaard‐Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol 2003; 170:5583–9. [DOI] [PubMed] [Google Scholar]
- 16. Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem 2002; 277:46779–84. [DOI] [PubMed] [Google Scholar]
- 17. Black AP, Ardern‐Jones MR, Kasprowicz V, Bowness P, Jones L, Bailey AS et al Human keratinocyte induction of rapid effector function in antigen‐specific memory CD4+ and CD8+ T cells. Eur J Immunol 2007; 37:1485–93. [DOI] [PubMed] [Google Scholar]
- 18. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc 2012; 71:50–61. [DOI] [PubMed] [Google Scholar]
- 19. Yao C, Oh JH, Lee DH, Bae JS, Jin CL, Park CH et al Toll‐like receptor family members in skin fibroblasts are functional and have a higher expression compared to skin keratinocytes. Int J Mol Med 2015; 35:1443–50. [DOI] [PubMed] [Google Scholar]
- 20. Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A et al Toll‐like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol 2011; 226:1265–73. [DOI] [PubMed] [Google Scholar]
- 21. Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM et al Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 2007; 179:1595–604. [DOI] [PubMed] [Google Scholar]
- 22. Chen SX, Zhang LJ, Gallo RL. Dermal white adipose tissue: a newly recognized layer of skin innate defense. J Invest Dermatol 2019; 139:1002–9. [DOI] [PubMed] [Google Scholar]
- 23. Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin‐expressing dendritic cells. Nat Rev Immunol 2008; 8:935–47. [DOI] [PubMed] [Google Scholar]
- 24. Ferrer IR, West HC, Henderson S, Ushakov DS, Santos ESP, Strid J et al A wave of monocytes is recruited to replenish the long‐term Langerhans cell network after immune injury. Sci Immunol 2019; 4:eaax8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol 2006; 126:787–96. [DOI] [PubMed] [Google Scholar]
- 26. West HC, Bennett CL. Redefining the role of Langerhans cells as immune regulators within the skin. Front Immunol 2017; 8:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology 2018; 154:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLellan AD, Heiser A, Sorg RV, Fearnley DB, Hart DN. Dermal dendritic cells associated with T lymphocytes in normal human skin display an activated phenotype. J Invest Dermatol 1998; 111:841–9. [DOI] [PubMed] [Google Scholar]
- 29. Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P et al Human tissues contain CD141hi cross‐presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012; 37:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Conrad C, Meller S, Gilliet M. Plasmacytoid dendritic cells in the skin: to sense or not to sense nucleic acids. Semin Immunol 2009; 21:101–9. [DOI] [PubMed] [Google Scholar]
- 31. Kolter J, Feuerstein R, Zeis P, Hagemeyer N, Paterson N, d'Errico P et al A subset of skin macrophages contributes to the surveillance and regeneration of local nerves. Immunity 2019; 50:1482–97 e7. [DOI] [PubMed] [Google Scholar]
- 32. Mowat AM, Scott CL, Bain CC. Barrier‐tissue macrophages: functional adaptation to environmental challenges. Nat Med 2017; 23:1258–70. [DOI] [PubMed] [Google Scholar]
- 33. Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C et al Origins and functional specialization of macrophages and of conventional and monocyte‐derived dendritic cells in mouse skin. Immunity 2013; 39:925–38. [DOI] [PubMed] [Google Scholar]
- 34. McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E et al Human dermal CD14+ cells are a transient population of monocyte‐derived macrophages. Immunity 2014; 41:465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaba LC, Fuentes‐Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA‐1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest 2007; 117:2517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang XN, McGovern N, Gunawan M, Richardson C, Windebank M, Siah TW et al A three‐dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J Invest Dermatol 2014; 134:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 2017; 17:733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCully ML, Ladell K, Andrews R, Jones RE, Miners KL, Roger L et al CCR8 expression defines tissue‐resident memory T cells in human skin. J Immunol 2018; 200:1639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rijken F, Kiekens RC, Bruijnzeel PL. Skin‐infiltrating neutrophils following exposure to solar‐simulated radiation could play an important role in photoageing of human skin. Br J Dermatol 2005; 152:321–8. [DOI] [PubMed] [Google Scholar]
- 40. Bruggen MC, Bauer WM, Reininger B, Clim E, Captarencu C, Steiner GE et al In situ mapping of innate lymphoid cells in human skin: evidence for remarkable differences between normal and inflamed skin. J Invest Dermatol 2016; 136:2396–405. [DOI] [PubMed] [Google Scholar]
- 41. Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK et al Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol 2014; 134:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Janssens AS, Heide R, den Hollander JC, Mulder PG, Tank B, Oranje AP. Mast cell distribution in normal adult skin. J Clin Pathol 2005; 58:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin‐resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med 2015; 212:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N et al Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab‐treated CTCL patients. Sci Transl Med 2012; 4:117ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A et al Immune surveillance by CD8αα + skin‐resident T cells in human herpes virus infection. Nature 2013; 497:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK et al The vast majority of CLA+ T cells are resident in normal skin. J Immunol 2006; 176:4431–9. [DOI] [PubMed] [Google Scholar]
- 47. Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C et al Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 2015; 7:279ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML et al The developmental pathway for CD103+CD8+ tissue‐resident memory T cells of skin. Nat Immunol 2013; 14:1294–301. [DOI] [PubMed] [Google Scholar]
- 49. Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S et al Cutting edge: CD69 interference with sphingosine‐1‐phosphate receptor function regulates peripheral T cell retention. J Immunol 2015; 194:2059–63. [DOI] [PubMed] [Google Scholar]
- 50. Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 2013; 14:1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown DW, Furness J, Speight PM, Thomas GJ, Li J, Thornhill MH et al Mechanisms of binding of cutaneous lymphocyte‐associated antigen‐positive and α β7‐positive lymphocytes to oral and skin keratinocytes. Immunology 1999; 98:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P et al A skin‐selective homing mechanism for human immune surveillance T cells. J Exp Med 2004; 199:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCully ML, Ladell K, Hakobyan S, Mansel RE, Price DA, Moser B. Epidermis instructs skin homing receptor expression in human T cells. Blood 2012; 120:4591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCully ML, Collins PJ, Hughes TR, Thomas CP, Billen J, O'Donnell VB et al Skin metabolites define a new paradigm in the localization of skin tropic memory T cells. J Immunol 2015; 195:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sharma A, Rudra D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol 2018; 9:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW et al Memory regulatory T cells reside in human skin. J Clin Invest 2014; 124:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schwarz A, Bruhs A, Schwarz T. The short‐chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol 2017; 137:855–64. [DOI] [PubMed] [Google Scholar]
- 58. Yamazaki S, Nishioka A, Kasuya S, Ohkura N, Hemmi H, Kaisho T et al Homeostasis of thymus‐derived Foxp3+ regulatory T cells is controlled by ultraviolet B exposure in the skin. J Immunol 2014; 193:5488–97. [DOI] [PubMed] [Google Scholar]
- 59. Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabrysova L et al The role of 1α,25‐dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL‐10+ CD4+ T cells. Eur J Immunol 2012; 42:2697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong HA et al Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe 2017; 21:467–77 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol 2005; 11:221–35. [DOI] [PubMed] [Google Scholar]
- 62. Wilhelm KP, Cua AB, Maibach HI. Skin aging. Effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch Dermatol 1991; 127:1806–9. [DOI] [PubMed] [Google Scholar]
- 63. Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol 1975; 93:639–43. [DOI] [PubMed] [Google Scholar]
- 64. Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase‐1 in vitro. J Invest Dermatol 2003; 120:842–8. [DOI] [PubMed] [Google Scholar]
- 65. Le Page A, Khalil A, Vermette P, Frost EH, Larbi A, Witkowski JM et al The role of elastin‐derived peptides in human physiology and diseases. Matrix Biol 2019. 10.1016/j.matbio.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 66. Fisher GJ, Shao Y, He T, Qin Z, Perry D, Voorhees JJ et al Reduction of fibroblast size/mechanical force down‐regulates TGF‐β type II receptor: implications for human skin aging. Aging Cell 2016; 15:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xia W, Quan T, Hammerberg C, Voorhees JJ, Fisher GJ. A mouse model of skin aging: fragmentation of dermal collagen fibrils and reduced fibroblast spreading due to expression of human matrix metalloproteinase‐1. J Dermatol Sci 2015; 78:79–82. [DOI] [PubMed] [Google Scholar]
- 68. Salzer MC, Lafzi A, Berenguer‐Llergo A, Youssif C, Castellanos A, Solanas G et al Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 2018; 175:1575–90 e22. [DOI] [PubMed] [Google Scholar]
- 69. Farage MA, Miller KW, Elsner P, Maibach HI. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res 2008; 20:195–200. [DOI] [PubMed] [Google Scholar]
- 70. Zhang LJ, Chen SX, Guerrero‐Juarez CF, Li F, Tong Y, Liang Y et al Age‐related loss of innate immune antimicrobial function of dermal fat is mediated by transforming growth factor β . Immunity 2019; 50:121–36 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krutmann J, Liu W, Li L, Pan X, Crawford M, Sore G et al Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci 2014; 76:163–8. [DOI] [PubMed] [Google Scholar]
- 72. Martires KJ, Fu P, Polster AM, Cooper KD, Baron ED. Factors that affect skin aging: a cohort‐based survey on twins. Arch Dermatol 2009; 145:1375–9. [DOI] [PubMed] [Google Scholar]
- 73. Castro MCR, Ramos ESM. Cutaneous infections in the mature patient. Clin Dermatol 2018; 36:188–96. [DOI] [PubMed] [Google Scholar]
- 74. Laube S. Skin infections and ageing. Ageing Res Rev 2004; 3:69–89. [DOI] [PubMed] [Google Scholar]
- 75. Wessman LL, Andersen LK, Davis MDP. Incidence of diseases primarily affecting the skin by age group: population‐based epidemiologic study in Olmsted County, Minnesota, and comparison with age‐specific incidence rates worldwide. Int J Dermatol 2018; 57:1021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Diffey BL, Langtry JA. Skin cancer incidence and the ageing population. Br J Dermatol 2005; 153:679–80. [DOI] [PubMed] [Google Scholar]
- 77. Weiss SA, Han J, Darvishian F, Tchack J, Han SW, Malecek K et al Impact of aging on host immune response and survival in melanoma: an analysis of 3 patient cohorts. J Transl Med 2016; 14:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hoey SE, Devereux CE, Murray L, Catney D, Gavin A, Kumar S et al Skin cancer trends in Northern Ireland and consequences for provision of dermatology services. Br J Dermatol 2007; 156:1301–7. [DOI] [PubMed] [Google Scholar]
- 79. Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol 2011; 11:289–95. [DOI] [PubMed] [Google Scholar]
- 80. Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol 2016; 37:866–76. [DOI] [PubMed] [Google Scholar]
- 81. Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol 2010; 22:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cumberbatch M, Dearman RJ, Kimber I. Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin‐1β . Immunology 2002; 105:466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pilkington SM, Ogden S, Eaton LH, Dearman RJ, Kimber I, Griffiths CEM. Lower levels of interleukin‐1β gene expression are associated with impaired Langerhans' cell migration in aged human skin. Immunology 2018; 153:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pilkington SM, Dearman RJ, Kimber I, Griffiths CEM. Langerhans cells express human β‐defensin 3: relevance for immunity during skin ageing. Br J Dermatol 2018; 179:1170–1. [DOI] [PubMed] [Google Scholar]
- 85. Grolleau‐Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res 2008; 68:6341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age‐related changes in microglial function. Neurobiol Aging 2012; 33:195.e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll‐like receptor expression and function in aging. J Immunol 2002; 169:4697–701. [DOI] [PubMed] [Google Scholar]
- 88. Smithey MJ, Uhrlaub JL, Li G, Vukmanovic‐Stejic M, Akbar AN, Nikolich‐Zugich J. Lost in translation: mice, men and cutaneous immunity in old age. Biogerontology 2015; 16:203–8. [DOI] [PubMed] [Google Scholar]
- 89. Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M et al Tissue‐resident memory CD8+ T cells promote melanoma‐immune equilibrium in skin. Nature 2019; 565:366–71. [DOI] [PubMed] [Google Scholar]
- 90. Davies B, Prier JE, Jones CM, Gebhardt T, Carbone FR, Mackay LK. Cutting edge: tissue‐resident memory T cells generated by multiple immunizations or localized deposition provide enhanced immunity. J Immunol 2017; 198:2233–7. [DOI] [PubMed] [Google Scholar]
- 91. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10:524–30. [DOI] [PubMed] [Google Scholar]
- 92. Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez‐Eerland R et al Tissue‐resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci USA 2012; 109:19739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vukmanovic‐Stejic M, Sandhu D, Seidel JA, Patel N, Sobande TO, Agius E et al The characterization of varicella zoster virus‐specific T cells in skin and blood during aging. J Invest Dermatol 2015; 135:1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vukmanovic‐Stejic M, Chambers ES, Suarez‐Farinas M, Sandhu D, Fuentes‐Duculan J, Patel N et al Enhancement of cutaneous immunity during aging by blocking p38 mitogen‐activated protein (MAP) kinase‐induced inflammation. J Allergy Clin Immunol 2018; 142:844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA et al Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol 2008; 181:1835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vukmanovic‐Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR et al The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen‐specific memory response in vivo. J Clin Invest 2008; 118:3639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vukmanovic‐Stejic M, Sandhu D, Sobande TO, Agius E, Lacy KE, Riddell N et al Varicella zoster‐specific CD4+Foxp3+ T cells accumulate after cutaneous antigen challenge in humans. J Immunol 2013; 190:977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Richards H, Williams A, Jones E, Hindley J, Godkin A, Simon AK et al Novel role of regulatory T cells in limiting early neutrophil responses in skin. Immunology 2010; 131:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ahmadzadeh M, Felipe‐Silva A, Heemskerk B, Powell DJ Jr, Wunderlich JR, Merino MJ et al FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood 2008; 112:4953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF et al Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res 2010; 70:7788–99. [DOI] [PubMed] [Google Scholar]
- 101. Kaporis HG, Guttman‐Yassky E, Lowes MA, Haider AS, Fuentes‐Duculan J, Darabi K et al Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J Invest Dermatol 2007; 127:2391–8. [DOI] [PubMed] [Google Scholar]
- 102. Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M et al Human squamous cell carcinomas evade the immune response by down‐regulation of vascular E‐selectin and recruitment of regulatory T cells. J Exp Med 2008; 205:2221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schwarz A, Navid F, Sparwasser T, Clausen BE, Schwarz T. In vivo reprogramming of UV radiation‐induced regulatory T‐cell migration to inhibit the elicitation of contact hypersensitivity. J Allergy Clin Immunol 2011; 128:826–33. [DOI] [PubMed] [Google Scholar]
- 104. Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and 'Garb‐aging'. Trends Endocrinol Metab 2017; 28:199–212. [DOI] [PubMed] [Google Scholar]
- 105. Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B et al Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 2017; 23:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP et al Age‐associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017; 21:455–66 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jacinto TA, Meireles GS, Dias AT, Aires R, Porto ML, Gava AL et al Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol Res 2018; 51:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP et al Aging of mice is associated with p16(Ink4a)‐ and β‐galactosidase‐positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 2016; 8:1294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter‐Kochanek K, Jansen‐Durr P et al p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006; 5:379–89. [DOI] [PubMed] [Google Scholar]
- 110. Pereira BI, Devine OP, Vukmanovic‐Stejic M, Chambers ES, Subramanian P, Patel N et al Senescent cells evade immune clearance via HLA‐E‐mediated NK and CD8+ T cell inhibition. Nat Commun 2019; 10:2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. He S, Sharpless NE. Senescence in health and disease. Cell 2017; 169:1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR et al An essential role for senescent cells in optimal wound healing through secretion of PDGF‐AA. Dev Cell 2014; 31:722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K et al Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J 2019: e101982 10.15252/embj.2019101982 [DOI] [PMC free article] [PubMed] [Google Scholar]


