Abstract
To understand oxidative stress, antioxidant defense, and redox signaling in health and disease it is essential to assess protein thiol redox state. Protein thiol redox state is seldom assessed immunologically because of the inability to distinguish reduced and reversibly oxidized thiols by Western blotting. An underappreciated opportunity exists to use Click PEGylation to realize the transformative power of simple, time and cost-efficient immunological techniques. Click PEGylation harnesses selective, bio-orthogonal Click chemistry to separate reduced and reversibly oxidized thiols by selectively ligating a low molecular weight polyethylene glycol moiety to the redox state of interest. The resultant ability to disambiguate reduced and reversibly oxidized species by Western blotting enables Click PEGylation to assess protein thiol redox state. In the present review, to enable investigators to effectively harness immunological techniques to assess protein thiol redox state we critique the chemistry, promise and challenges of Click PEGylation.
Keywords: protein thiols, click PEGylation, click chemistry, redox signaling, reactive oxygen species, oxidative stress
1. Protein Thiol Redox Biology: An Overview
Context dependent functionality reconciles the ability of chemically heterogeneous radical and non-radical oxygen, nitrogen, carbon, and sulfur species to be beneficial (e.g., signal) and deleterious (e.g., damage macromolecules) [1,2,3,4,5,6,7,8,9,10]. For example, the ability of reactive species to inflict damage [11,12,13], which was proposed by Denman Harman to cause ageing [14], is exploited by phagocytes to kill ensnared pathogens [15,16,17]. Biological context, therefore, governs whether their immutable chemistry (i.e., set species specific spectrum of chemical reactivity in a biological system) benefits or harms the cell. Or more likely benefits and harms the cell simultaneously to a varying degree (i.e., granularity) set, to a large extent, by the local microenvironment (pH, temperature, solvent accessibility, and vicinal interactome) [18]. Granular context dependent functionality provides a useful theoretical lens to interpret the interplay between reactive species and cysteine residues (i.e., protein thiols). The rich chemical biology of sulfur defined by its ability to occupy eight distinct oxidation states from −2 to +6 affords an apt interface between the heterogeneous thiol proteome and a chemically diverse panoply of reactive species [19,20,21,22,23,24]. The ability of reactive species to interact with the thiol proteome can underlie redox signaling, oxidative damage, and antioxidant defense [25,26,27,28,29,30,31,32,33,34]. None are inherently good or bad: Wayward redox signals have as much potential to cause harm as a quenched sulfur radical in an enzymes active site or antioxidant defense aberrantly silencing or amplifying redox signals.
Unsurprisingly, given their functional significance, cysteine residues are used parsimoniously (2.26% of the time yields 1 thiol per 50 kDa protein—the mean mass of a typical human protein [35]) and tend to be almost fully conserved or lost completely [36,37]. Additionally, cysteine residues seem to increase with complexity being more prevalent in mammals [38,39]. Analogous to reactive species, treating the ~214,000 cysteines (~50 mM) that comprise the thiol proteome in humans as a homogenous pool is perilous [40,41,42]. Despite possessing the same functional group (i.e., sulfur), the reactivity of protein thiols with hydrogen peroxide (H2O2) spans at least 6 orders of magnitude [43,44]. Differential kinetics stems from the ability of the local protein environment to deprotonate the thiol (electrostatic gating), stabilize a transition state and/or co-ordinate the leaving group (e.g., by providing a proton relay) [33,45,46,47,48]. For example, the reaction of peroxiredoxin (PRDX) isoforms with H2O2 (k ~105–108 M−1 s−1) is significantly faster than KEAP1 (k ~140 M−1 s−1) and most thiols (k ~10–50 M−1 s−1) [49,50,51]. Even slow reactions can, however, proceed if they are favored by the local microenvironment and/or facilitated by an enzyme. Transiently inactivating PRDX enzymes could open the floodgates for H2O2 to signal [52]. Further, PRDX enzymes can transmit redox signals by transferring H2O2 derived electrons to a target (i.e., a redox relay) [53,54,55,56]. Beyond H2O2, a role for free radicals (e.g., nitrogen dioxide radical) and other non-radical species (e.g., peroxynitrite) must be considered [57,58].
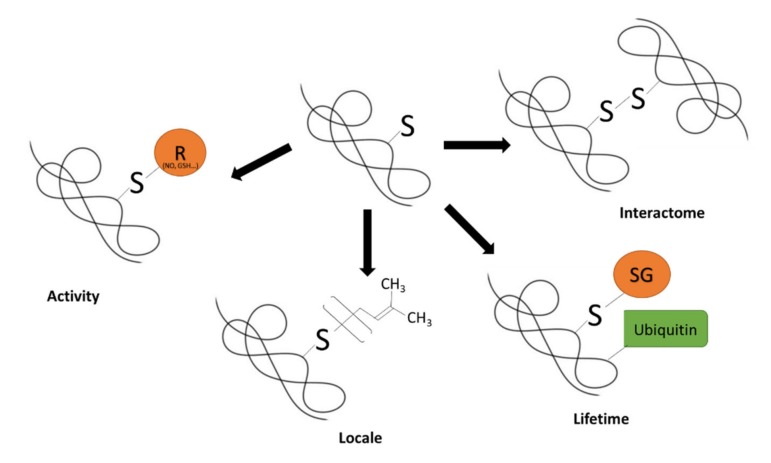
Regardless of the functional consequences, reactive species interact with the heterogenous thiol proteome by changing sulfur oxidation state via electron exchange. One major outcome is an increase in the amount of a thiol that is reversibly oxidized (i.e., a fractional increase in reversible thiol oxidation occupancy). Thiyl radicals (RS•) and sulfenic acids (SOH) define the common starting point for free radical and non-radical reactions, respectively [20,57,59,60]. RS• and SOH provide an initial platform for a rich set of chemically heterogenous modifications with disparate functionality (Table 1) [19,20]. In principle, a shift in the fractional occupancy of a thiol can enact a functional change by altering protein: activity, locale, interactome, and lifetime (Figure 1) [28,61,62]. Moreover, distinct chemical biology means different modifications can exert diametrically opposed effects even when they modify the same thiol. A redox code may exist wherein the biological outcome may differ depending on the reversible oxidation occupancy of constituent protein thiols (i.e., a shift in one thiol may tip the balance towards a given outcome) [63]. The fractional reversible thiol occupancy is dynamic: it shifts as a function of differences in the rate of formation and removal over time [64]. For example, a change in NADPH metabolism able to decrease peroxidase mediated H2O2 metabolism would suffice to increase reversible thiol oxidation occupancy even if the rate of formation stayed constant. Ultimately, residing at the strategic nexus of oxidative stress, antioxidant defense, and redox signaling the thiol proteome is central to understanding the biological role of reactive species in health and disease across the lifespan from development to ageing.
Table 1.
Major reversible thiol modifications by type. Key reactions and enzyme regulated, and selected examples are provided. Note many more important modifications (e.g., S-acetylation [65]) exist. The table merely provides a brief overview of some of the key modifications.
| Modification | Example Reaction | Enzyme Regulation | Selected Examples |
|---|---|---|---|
| Sulfenic acid (SOH) | RS− + H2O2 → RSOH + H2O | Thioredoxin/PRDX. | EGF receptor SOH at Cys797 potentates tyrosine kinase activity [66]. Src Cys185 and 277 SOH enhances protein activity [67]. |
| Thiyl radical (RS•) | RS− + NO2• → RS• + NO2− | n/a | RS. play a role in the reversible oxidation of thiols to RSSG in NDUFV1 and NDUFS1 in complex I [68], which can inhibit enzyme activity [69,70,71,72,73]. A catalytic RS. enables ribonucleotide reductase to remove an oxygen atom from the 2-OH position in ribose to yield deoxyribose [74]. |
| Disulfide bonds (RSSR) | RSOH + RSH → RSSR + H2O | Thioredoxin isoforms. | Intermolecular RSSR moieties activate ATM dimers to initiate DNA double strand break repair [75]. RSSR exchange between PRDX2 and STAT3 represses STAT3 transcriptional activity [53]. RSSR of plasma membrane bound HPLAC1 enables plants to sense and respond to extracellular H2O2 [76]. |
| Glutathionylation (RSSG) | RS− + GSSG → RSSG + GS− | Glutaredoxin isoforms. | eNOS RSSG at multiple sites enhances uncoupling mediated superoxide production [77]. SMYD2 Cys13 RSSG dissociates SYMD2 from titin leading to sarcomere instability [78]. |
| S-nitrosation (RSNO) | RS• + NO• → RSNO | Protein mediated NO• transfer. | ND3 Cys39 RNSO holds complex I inactive to prevent oxidative damage in ischemia reperfusion injury [79,80,81]. LURE1 RSNO inhibits polyspermy in flowering plants [82]. |
Figure 1.
Functional aspects of reversible thiol oxidation. The schematic depicts the four main impacts of reversible thiol oxidation on protein function: activity, locale, interactome, and lifetime. For illustrative purposes, S-prenylation is depicted and an RSSG modification leading to ubiquitination. In principle, any modification could exert an effect by any of the major functional aspects described (e.g., interactome effects are not restricted to RSSR).
2. The Case for Using Immunological Techniques to Assess Protein Thiol Redox State
The functional significance of many protein thiols reinforces the importance of assessing their redox state. Protein thiol redox state is usually assessed using redox proteomics approaches [83,84,85,86,87,88,89]. Redox proteomic affords hypothesis-free, systematic, and quantitative global thiol proteome profiling [90]. For example, state-of-the-art cysteine reactive phosphate tag technology coupled to tandem mass tag multiplexing identified and quantified 171,000 thiols modification events in mammalian tissues [91] (i.e., ~80% of the total thiol proteome was assessed). Low-throughout immunological techniques (e.g., ~2 thiols per experiment or 0.0009% of the human thiol proteome) may seem redundant when one can quantify the redox state of thousands of protein thiols in parallel in a single experiment. Analogous to Western blotting in standard proteomics [92], the value of immunological techniques stems, in part, from their ability to verify redox proteomic findings. Verifying redox proteomic findings using a complementary orthogonal technique enables protein identity to be immunologically confirmed as opposed to being assigned based on a unique peptide mass alone [93,94]. Quantifying each modified thiol relative to the entire protein is important because peptide analysis could be biased (e.g., some may be unsuitable for electron spray ionization). Further, studying each thiol enriches redox proteomic findings by placing the original finding in context. Target redox state context is important for concluding whether the protein per se (i.e., all target thiols) or an individual thiol responds to given stimuli/context (e.g., cardiovascular disease [95]). Without immunological analysis one could conclude a single thiol is reversibly oxidized in cardiovascular disease when all target thiols are. Far from being trivial, such nuances can have profound consequences for interpreting how key biological phenomena impact the thiol proteome and for developing biomarkers. Ideally, immunological assays would be performed in parallel with targeted multiple reaction monitoring (MRM) to identify the thiols (i.e., sites) modified [96,97].
The value of immunological techniques extends well beyond merely verifying redox proteomics findings. In many cases, immunological techniques represent the only viable way to assess certain targets. For example, redox proteomics studies often fail to detect hydrophobic protein thiols [98]. Even state-of-the-art cysteine reactive phosphate tag technology was unable to detect two hydrophobic complex I subunits (i.e., ND6 and ND4L [91,99]). Their hydrophobicity makes proteomics, yet alone redox proteomics, challenging [100]. Moreover, certain thiols remain unstudied because they form part of linear amino acids sequences recalcitrant to tryptic digestion. As Held [89] remarks, recalcitrance to tryptic digestion often precludes analysis of the active site thiol (Cys215) in PTP1B. Additionally, data dependent acquisition (DDA) presents difficulties for detecting thiols on low abundance proteins [90]. In DDA, low abundant thiols are effectively masked by highly abundant peptides preferentially fragmented to daughter ions in MS-MS. Immunological techniques are, therefore, required to detect many protein thiols. Above all, immunological techniques open-up new opportunities to study the thiol proteome for investigators who lack access to mass spectrometric facilities. Even when mass spectrometric facilities are available, the cost and expertise required can preclude redox proteomics. Further, when redox proteomics is possible, access to a complementary orthogonal technique can only enrich the field. Analogous to immuno-spin trapping for electron resonance spectrometry [101], the overarching goal of immunological techniques is to place protein thiol redox biology into the hands of the masses by empowering any investigator to assess the redox state of a target protein using simple, time and cost-efficient methods.
3. Novel Immunological Techniques to Assess Protein Thiol Redox State
3.1. Click PEGylation
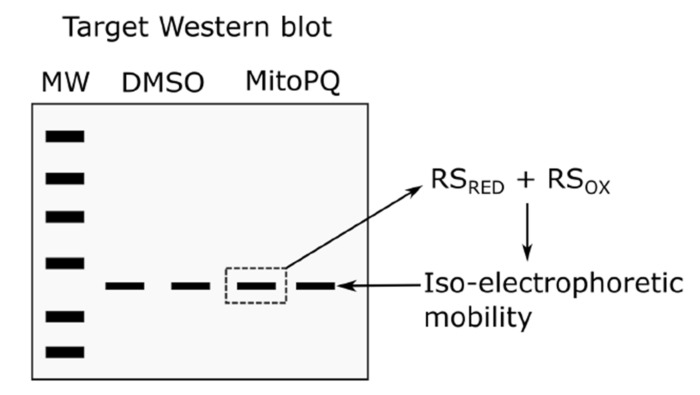
Until recently, investigators were unable to harness immunological techniques to assess protein thiol redox state. The inability to distinguish between reduced and reversibly oxidized protein species owing to their iso-electrophoretic mobility rate-limits attempts to assess protein thiol redox state by Western blot (i.e., immunologically). Unless reversible thiol oxidation alters oligomeric state (e.g., typical 2-Cys PRDX isoforms dimerize when they form intermolecular disulfide bonds [102,103,104]), then Western blotting is unlikely to report protein thiol redox state. For example, one would be unable to identify whether selectively inducing superoxide at the flavin mononucleotide group in complex I using mitochondria-targeted paraquat [105,106] suffices to reversibly oxidize a given target using Western blotting (Figure 2), which limits understanding of the sources and targets of mitochondrial superoxide [107,108,109]. Even when a change in oligomeric state makes Western blotting possible, it is often orthogonal to certain thiols and modifications. For example, in the typical 2-Cys PRDX dimer assay RSSG species could be present in the “reduced” monomeric band and the redox state of additional thiols (i.e., beyond the RSSR pair) will be missed. Moreover, unless target immunocapture is performed, SOH, RSNO, and RSSG blotting alone (e.g., avidin detection of biotinylated glutathione labelled samples [110]) fails to disclose the proteins modified and are limited by quantification issues (e.g., loading controls) and inability to resolve individual thiol modifications (i.e., number of RSSG sites). Techniques like biotinylated glutathione are, however, invaluable for global modification screening and purifying samples for redox proteomics [110].
Figure 2.
The iso-electrophoretic mobility problem. An exemplar scenario is depicted wherein Western blotting is used to detect a change in the redox state of a target in biological samples treated with and without (i.e., DMSO) the pro-oxidant mitochondria targeted paraquat (MitoPQ). For many protein thiols, Western blotting is unable to detect differences in target redox state because reduced and reversibly oxidized thiols possess similar electrophoretic mobility. MW denotes molecular weight.
To detect reversibly oxidized thiols with iso-electrophoretic mobility by Western blotting, one must ectopically achieve a mobility shift by ligating a bulky biocompatible moiety to a thiol. In 2013, Eaton’s group built on the biotin switch assay by ligating N-ethylmaleimide (NEM) functionalized polyethylene glycol (PEG) to mobility shift reversibly oxidized protein thiols, termed the PEG switch assay [111]. NEM functionalized PEG (mPEG) has proven invaluable for assessing protein thiol oxidation. For example, Murphy’s group [112] used mPEG to show that mitochondrial reactive species inactivate pyruvate dehydrogenase kinase 2. Recently, Jakob’s group [113] demonstrated that histone methyltransferase oxidation, evidenced using mPEG, decreases methylation, which enables stochastic fluxes in developmental reactive species to regulate lifespan via an epigenetic switch. The utility of mPEG is, however, limited because the bulky PEG sterically impedes thiol labelling [98]. To solve the steric problem, the Cochemé group used copper (I) catalyzed azide-alkyne Click (CuAAC) to split the labelling reaction into two steps [98]. A sterically free labelling step is achieved by alkylating protein thiols with a heterobifunctional alkyne maleimide reagent. CuAAC is deployed to conjugate alkyne labelled thiols to azide functionalized PEG via a stable triazole [114]. The necessity for a cytotoxic catalyst is, however, problematic [115]. We extended their work by using Inverse Electron Demand Diels Alder (IEDDA) chemistry to develop catalyst-free Click PEGylation workflows [116]. The following subsections critique the chemistry, promise, and challenges of catalyst-free Click PEGylation.
3.2. Underlying Principles
Catalyst-free Click PEGylation to assess reversibly oxidized thiols involves four key steps (Figure 3). First, reduced protein thiols are alkylated to render them orthogonal to subsequent labelling steps. Typically, NEM is used to form a thioether bond via Michael addition with the sulfhydryl/thiolate [19]. NEM is normally preferred to iodoacetamide (IDA) because it reacts appreciably faster with reduced thiols [20]. Care must be taken to ensure buffer pH is between 6.5 to 7.5 to prevent off-target amino acid labelling (e.g., lysine) [19,20,117]. Likewise, excess unreacted NEM, as well as, glutathione (GSH) and l-cysteine must be removed with a spin column and/or a quenching step to prevent downstream interference. Second, reversibly oxidized thiols are reduced with a generic chemical reductant like Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) or 1,4-Dithiothreitol (DTT). TCEP is preferred because DTT can autoxidize to produce superoxide when transition metal ion (e.g., Fe2+) catalysts are present (as is likely the case in complex biological samples) [74]. Additionally, commercially available TCEP reducing gels omit the need for a second spin column step. Optionally, TCEP/DTT can be substituted for selective reductants (e.g., copper and ascorbate for RSNO [118]) to unveil reversible modification type. The ability to disclose the number of thiols modified by a particular chemotype (e.g., RSNO) represents a key advantage of Click PEGylation compared to other immunological techniques (e.g., target immunocapture followed by RSNO antibody probing).
Figure 3.
Catalyst-free Click PEGylation schematic. Modified with permission from Cobley et al [116]. The left side of the circle depicts the Click PEGylation reduction (Click-PEGRED) protocol wherein reduced thiols are labelled with TCO- polyethylene glycol 3 (PEG)-maleimide (TPN); (2) before 6-methyltetrazine PEG 5 kDa (Tz-PEG5) is added to initiate the IEDDA Click reaction to mass shift reduced thiols. An optional Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) reduction step to reduce reversibly oxidized thiols before labelling them with N-ethylmaleimide (NEM) is included. The right side of the circle depicts the Click PEGylation oxidation (Click-PEGOX) protocol wherein (1) reduced thiols are labelled with NEM; (2) reversibly oxidized thiols are reduced with TCEP; (3) before being labelled with TPN; and (4) Tz-PEG5 is added to initiate IEDDA Click reaction to mass shift reversibly oxidized thiols. A subsequent Western blot of a target is depicted wherein the Click PEGylated bands are selectively mass shifted. For example, in the Click-PEGOX workflow the mass shifted bands correspond to reversibly oxidized thiols (each one being shifted by approximately 5 kDa) and the unshifted band corresponds to the reduced protein. Following densitometry, percent reversibly oxidized protein can be quantified (as depicted in the inset).
Third, newly reduced (i.e., reversibly oxidized) thiols are alkylated with trans-cyclooctene (TCO)-PEG3-maleimide (TPN) via Michael addition. The short PEG3 linker enhances TPN hydrophilicity, flexibility, and accessibility [116]. Excess TPN should be removed via a spin column to prevent unproductive competition with TPN decorated protein thiols. Care should be taken when storing TPN to prevent unclickable TCO isomers forming [119,120]. Fourth, TCO labelled thiols are PEGylated by adding 6-methyltetrazine functionalized PEG 5000 (Tz-PEG5) to achieve a 5 kDa mobility shift per modified thiol via IEDDA, which renders reversibly oxidized thiols detectable by Western blotting. Methyl substituted tetrazines are preferred owing to their enhanced stability [120]. Reciprocal reduced protein thiol analysis can be performed by starting at step 3. Mass shifted band intensity is internally normalized to unshifted band (i.e., reduced) intensity to calculate percent reversible thiol oxidation. No between-lane loading control is required because data are internally normalized [116]. Additionally, the contribution of each mass shifted band (i.e., each individual thiol) to the total reversible oxidized signal can be calculated. Click PEGylation, therefore, enables reversible thiol oxidation to be quantified by Western blotting [98,116]. Importantly, Click PEGylation is compatible with cell, isolated organelle, and tissue lysate analysis.
Redox biologists will be familiar with Diels Alder chemistry because it enables singlet dioxygen to react with unsaturated fatty acids to initiate lipid peroxidation [121]. IEDDA involves an electron rich dienophile reacting with an electron deficient diene to yield a stable 4,5-dihydropyrazine conjugate in the absence of a catalyst [122,123,124]. As Oliveira and colleagues remark [124], 1,4 addition of 6-methyltetrazine diene to the TCO alkene yields a strained catalytic intermediate able to evolve to 4,5-dihydropyrazine after releasing nitrogen (i.e., a benign byproduct). IEDDA mediated Click PEGylation confers three important advantages: (1) it obviates the need for a cytotoxic copper catalyst; (2) it affords selective, bio-orthogonal conjugation; and (3) enables rapid conjugation since IEDDA proceeds 10,000 times faster than CuAAC [122,123,124]. Table 2 summarizes the key advantages and disadvantages of Click PEGylation. In principle, therefore, IEDDA mediated Click PEGylation enables one to immunologically quantify protein thiol redox state by Western blotting.
Table 2.
Key advantages and disadvantages of Click PEGylation. Modified with permission from Cobley and colleagues [116].
| Advantage | Disadvantage |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* ng/mL may be possible with sample purification.
3.3. Click PEGylation Is A Useful Tool to Assess Protein Thiol Redox State: A Mitochondrial ATP Synthase Case Study
Recent studies substantiate the ability of Click PEGylation to assess protein thiol redox state (e.g., [98]). To highlight the advantages of Click PEGylation, we consider the mitochondrial ATP synthase (i.e., complex V). The mitochondrial ATP synthase harnesses the electrochemical proton motive force across the inner mitochondrial membrane to synthesize ATP from inorganic phosphate and ADP via a catalytic rotary mechanism [125,126,127]. Since the seminal work of Yagi and Hatefi in 1984 [128], it has been appreciated that reversible thiol oxidation can regulate mitochondrial ATP synthase activity. Subsequent studies have unraveled the identity of the reversibly oxidized subunits, as well as, the sites and types of modification [129]. To give a key example, reversible oxidation of the matrix facing ATP synthase F1 alpha subunit at two evolutionary conserved thiols (Cys244 and 294) seems to impair catalysis [129,130,131].
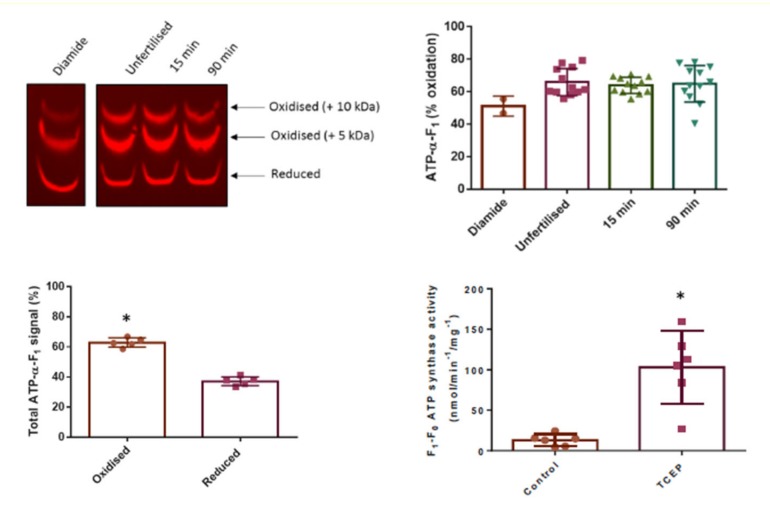
Until recently, reversible subunit alpha oxidation had only been assessed by redox proteomics usually in either isolated mitochondria or disease models [131]. Intrigued by a possible physiological redox regulatory role, we asked whether the alpha subunit is reversibly oxidized in oocytes and zygotes from the key developmental model Xenopus laevis (X. laevis) [132,133,134]. We hypothesized that the alpha subunit would be reversibly oxidized in oocytes to prevent wasteful ATP hydrolysis [135], but that fertilization induced ADP may provide an instructive cue to relieve reversible thiol oxidation to initiate embryonic mitochondrial ATP synthesis [136]. Unexpectedly, reciprocal reduced and reversibly oxidized catalyst-free Click PEGylation workflows revealed the ATP synthase is substantially (~65%) oxidized before and after fertilization (Figure 4) [116]. To place our findings in context, the median oxidation of thiols in the mammalian proteome is ~12% [41] and the Oximouse dataset (see [91]) reveals C244 and C294 are ~20% reversibly oxidized. Substantial reversible thiol oxidation is consistent with a distinct subset of thiols being persistently oxidized (≥20%) and tissue specific redox signatures [91]. Click PEGylation reveals substantial reversible oxidation of ~20% of the total available thiols (~10-11) in the X. laevis ATP synthase. Further analysis revealed a single thiol was preferentially modified. Consistent with a single thiol being modified, selective reduction experiments unveiled RSSG as the dominant reversible thiol oxidation type [116]. Reversible thiol oxidation is biologically meaningful because reducing oxidized thiols significantly increased mitochondrial ATP synthase activity in X. laevis oocytes (Figure 4) [137].
Figure 4.
Reversible oxidation regulates mitochondrial ATP synthase activity in X. laevis oocytes. Modified with permission from Cobley et al [116,137]. The top right figure shows an illustrative example of a catalyst-free IEDDA Click PEGylation blot against the alpha subunit of the mitochondrial ATP synthase. Quantifying the mass shifts (top left figure) reveals the alpha subunit is substantially oxidized before and after fertilization. The bottom figure shows reversible thiol oxidation of the alpha subunit is statistically significant (denoted by an Asterix) in oocytes. The bottom right figure shows that chemically reducing thiol oxidation significantly (statistical significance is denoted by an Asterix) increases mitochondrial ATP synthase activity in isolated X. leavis oocyte mitochondria. Click PEGylation, therefore, helped unveil a regulatory role for reversible thiol oxidation in early development.
While the identity of the critical redox switch(es) remains elusive (several subunits are reversibly oxidized), Click PEGylation was crucial for unravelling a novel redox regulatory role for reversible ATP synthase oxidation in development [138,139,140]. From a wider theoretical perspective, temporal context may govern the outcome of redox switches in the mitochondrial ATP synthase. Perhaps, they are beneficial in early life (e.g., to constrain ATP hydrolysis) but deleterious in later life when aberrant reversible thiol oxidation compromises mitochondrial ATP synthesis [141]. The mitochondrial ATP synthase example reinforces the ability of Click PEGylation to: (1) test experimental hypotheses; (2) quantify reversible thiol occupancy; (3) quantify the number of thiols modified; (4) assess the relative contribution of each modified thiol to the total reversible oxidation observed; and (5) quantify reversible thiol oxidation type in complex biological samples.
3.4. Challenges
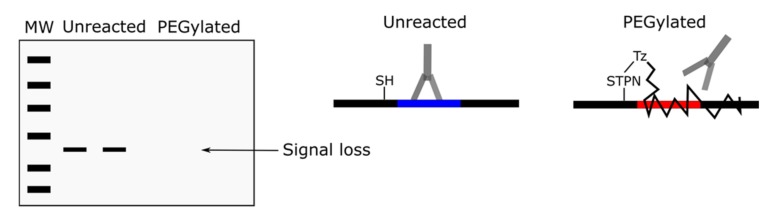
Beyond the inability to resolve the identity of the thiol oxidized (unless a protein with a lone thiol is assessed; e.g., ND3 Cys39 [142]), antibody binding concerns beset Click PEGylation (Figure 5). Click PEGylation will unilaterally fail to report protein thiol redox state if the antibody is unable to bind the PEGylated protein. The bulky PEG polymer likely sterically impedes antibody binding by physically occupying the epitope [98,116]. Epitope occupancy probability is likely to increase proportional to the number of PEGylated thiols, especially if they are evenly distributed over the linear denatured protein. Even distribution presents difficulties for polyclonal antibodies. Proximity of the PEGylated thiol to the epitope will also influence antibody binding. A protein with a lone thiol distal to a single epitope (i.e., monoclonal) should be amenable to Click PEGylation. Additionally, the probability of epitope occupancy seems to increase when PEGylation adds significant mass to a protein. We have found that PEGylation fails for ND3 (unpublished data) with Tz-PEG5000 (~38% of mass added) but successfully detects ATP synthase subunit alpha (~18% of mass added). Unfortunately, steric hinderance may persist even when smaller PEG moieties (e.g., Tz-PEG2000) are used [98]. Moreover, adding mass to a protein raises the possibility of unequal transfer of PEGylated proteins onto a PVDF membrane leading to the mass shifted signal being underestimated. The unsuitability of many strategically important protein thiols for Click PEGylation rate-limits attempts to study protein thiol redox state using immunological techniques. For example, Lee and Chang found that alkylating reduced thiols with m-PEG5000 led to an inability to detect many strategically important proteins, including glutathione reductase, calcium calmodulin kinase II, and nuclear factor kappa beta [143].
Figure 5.
Click PEGylation challenges. Left to right. Signal loss in the PEGylated compared to the unreacted lanes is often observed. In the unreacted samples, the antibody can bind to the epitope. PEGylation, however, may sterically impede epitope binding resulting in partial or complete signal loss.
Additional challenges include the ability of SDS to distort PEGylated bands, inability to multiplex by re-probing a blot, and unsuitability for certain species (e.g., Drosophila melanogaster) due to a lack of antibodies [116]. While antibodies can be raised against certain species, the biophysical interaction between PEG and SDS is difficult to surmount [144]. Native blotting is an obvious solution but relies on conformational antibody availability [144]. Additionally, native blotting is comparatively time consuming (e.g., transfer time is at least 4 h compared to 1 h for Western blotting [145]). The SDS interference can be minimized by removing excess Tz-PEG5 with a spin column and limiting [SDS] in the Laemmili buffer [116,146]. The inability to multiplex may be overcome by stripping the membrane before re-probing against a new target. Differential protein losses between the total and mass shifted bands could, however, confound the analysis. While multiplexing a single blot is problematic, PEGylated lysates yield enough material to assess multiple proteins [143].
Click PEGylation relies on the unshifted total band representing the reduced protein. Analogous to the typical 2-Cys PRDX dimer assay (or any oligomeric shift assay), TCEP/DTT irreducible sulfinic (SO2) and sulfonic acid (SO3) species will contaminate the reduced unshifted band. SO2/SO3 occupancy can be partially estimated with reciprocal reduced and reversibly oxidized Click PEGylation [98]. Strategies are required to define their specific occupancy by selective reduction and/or mass shifting reduced and reversibly oxidized thiols together—any unshifted protein should correspond to the irreversibly oxidized form. Anything beyond a binary doublet signifies partial SO2/SO3 occupancy. Accounting for SO2/SO3 occupancy is important since recent electrophilic nitrogen species based chemically selective proteomic profiling reveals 387 thiols (e.g., NDUFS1 Cys92) can be oxidized to SO2 [147]. Their sensitivity to sulfiredoxin [148] catalyzed reduction means SO2 is, in over 50 cases (e.g., Cofilin Cys39), reversible [147]. Reversibility implies an underappreciated role for SO2 in redox signaling and antioxidant defense.
Troublingly, NEM and IDA can react with SOH species [149], which implies reversible thiol oxidation could be underestimated given SOH occurs on more than 1200 thiols distributed across ~700 proteins (e.g., Src kinase Cys185 and 277) [67,150]. Furthermore, NEM and IDA can also react with biologically significant SO2 and persulfide species [147,151,152]. The second-order bimolecular reaction between an alkylating agent and SOH is, however, significantly slower than the thiol labelling reaction [149], suggesting it could be mitigated by titrating the molar mass used and reaction time. If methylsulfonyl benzothiazole is used to label reduced thiols without reacting with SOH species, then the ability of the resultant SO2 species to distort downstream analysis by reacting with RSNO should be considered [153]. As the development of tunable cysteine reactive phosphate tags attests [91], untuned alkylating agents can fail to label many proteins despite their molar excess. Incomplete labelling likely relates to an inability to tune hydrophobicity to local protein environments (e.g., hydrophobic mitochondrial membrane ensconced proteins).
A challenge is the ability of destructive analysis to distort protein thiol redox state during lysis. However, measuring any molecule, even in vivo, can change the system in accordance with the Heisenberg principle. As Paulsen and Carroll remark [19], lysing cells exposes thiols to atmospheric ground state molecular dioxygen (O2). Exposure to 21% O2 coupled to transition metal ion availability, could result in artificial protein thiol oxidation likely via free radical mechanisms able to outcompete NEM labelling. For example, molecules (e.g., dopamine [154,155,156]) autoxidized to superoxide via a transition metal catalyst could lead to hydroxyl radical (•OH) production secondary to H2O2/metal mediated Fenton chemistry (e.g., Fe2+ + H2O2 → •OH + −OH). While Fenton chemistry could be mitigated by adding chelators, the reaction between •OH and cysteine (k = 7.9 × 109 M−1 s−1) is orders of magnitude faster than NEM mediated alkylation [74]. Lysis could lead to SOH loss via condensation with a reduced thiol to form a disulfide bond (i.e., SOH + RSH → RSSR + H2O), which occurs significantly faster (k ~105 M−1 s−1) than alkylation [41]. Unless the local environment stabilized SOH, condensation would likely occur in vivo. For cases when SOH is stabilized (e.g., a binding protein can stabilize SOH in ORP1 [157]), condensation could confound reversible oxidation type and occupancy analysis. For example, condensation with GSH, present at ~10 mM in most cells [158], could be favored ex vivo leading to the misassignment of SOH as RSSG. Even in the presence of alkylating agents, the possibility of lysis induced ex vivo oxidation represents a perennial concern.
3.5. Solutions
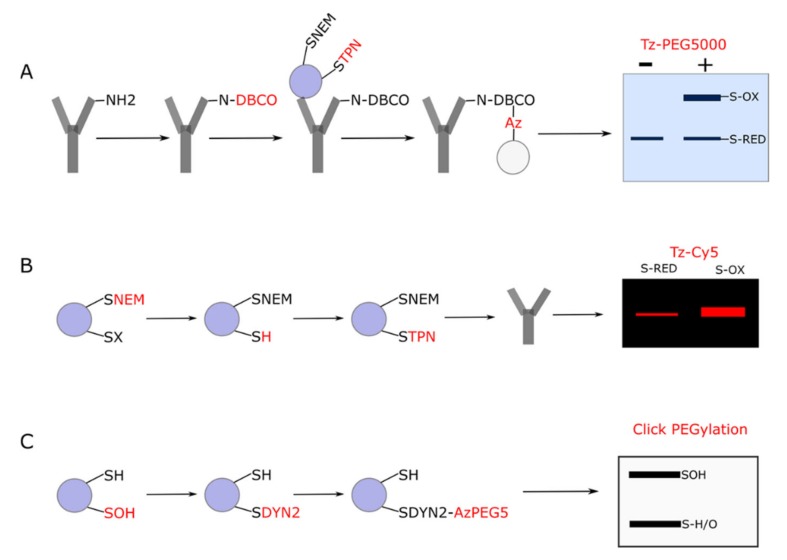
The potential of the bulky PEG moiety to occlude antibody binding can be overcome by adopting an “antibody first” immunocapture workflow (Figure 6A). To do so, additional clickable immunocapture steps precede Tz-PEG5 labelling. Dibenzocyclooctyne (DBCO) functionalized N-hydroxysuccinimide is used to label the primary antibody by forming stable amide bonds with primary amines. The labelled antibody is added to the sample before being captured with an azide functionalized resin via strain-promoted azide–alkyne cycloaddition (SPAAC) [115]. The solid support and stable triazole enable the antibody-target complex to be isolated with high stringency and for the target to be eluted without the antibody, respectively. Following elution, Tz-PEG5 is added to initiate IEDDA and resultant mass shifts are visualized in gel via Coomassie or Silver Staining. Optionally, native polyacrylamide gel electrophoresis could be used to prevent SDS distorting mass shifted bands [144]. In gel analysis eliminates differential PEGylated protein transfer. Antibody first workflows represent a promising alternative to canonical Click PEGylation, especially when they can be readily coupled to selective reduction workflows.
Figure 6.
Novel, clickable immunological approaches to assess protein thiol redox state. (A). Antibody first Click PEGylation. Dibenzocyclooctyne (DBCO) functionalized N-hydroxysuccinimide is used to label NH2 moieties in the primary antibody. The DBCO labelled primary antibody is incubated with the sample to capture the TPN labelled target. Azide functionalized resin is used to capture the antibody-target complex via SPAAC. After stringently washing the captured complex via spin cups (omitted for clarity), the eluted target is reacted with Tz-PEG5 via IEDDA and mass shifts are visualized in gel via Coomassie staining. (B). Fluorescent IEDDA. After alkylating reduced thiols with NEM, reversibly oxidized thiols are reduced with TCEP before TPN labelling. The TPN labelled target is captured immunologically (as above) before the eluted target is conjugated with 6-methyltetrazine functionalized Cy5 (Tz-Cy5). Fluorescence is visualized in gel at the appropriate excitation and emission. Comparative parallel reduced and reversibly oxidized target fluorescence is depicted. (C). Reaction based target oxidative modification type profiling. The example shown considers SOH. SOH moieties are selectively labelled with Dyn-2 (SOH reactive warhead with a clickable alkyne) before being reacted with Azide functionalized PEG5000 via SPACC. Total SOH occupancy is then quantified via Click PEGylation. If Click PEGylation failed to detect a given target, then workflow A or B could be used.
Even antibody first workflows may fail to adequately resolve relatively large proteins with many solvent exposed protein thiols. For example, the ~500 kDa ryanodine receptor 1 (RYR1) possesses over 100 thiols [159,160], which presents axiomatic difficulties for Click PEGylation. For proteins like RYR1, one could assess the weighted mean of all solvent exposed protein thiols in gel using the antibody first workflow to capture the target and substituting Tz-PEG5 for a 6-methyltetrazine functionalized fluorophore (e.g., Tz-Cy5); which could be quantified provided an equal amount of protein was loaded (Figure 6B). Alternatively, the fluorescent signal could be expressed relative to the total band after Coomassie or Silver staining. Importantly, antibody-first fluorescent workflows afford a useful tool to verify Click PEGylation findings, especially when reduced and reversibly oxidized protein thiol analysis is performed in parallel.
Beyond affording a simple method to check successful Click PEGylation, anti-PEG makes it possible to use a single antibody to assess global RSSG, RSNO, and RSSR using selective reduction strategies. Single antibody global blotting is a useful, inexpensive tool to screen reversible thiol oxidation type. Single antibody reversibly modification type screening would be advanced by the development of a Tz-PEG20 reagent to reduce band complexity. 6-methyltetrazine functionalized Biotin is, however, commercially available and can be coupled to streptavidin functionalized fluorophores [137]. Optionally, immobile anti-PEG or streptavidin supports could be used to purify a certain reversible thiol oxidation type for proteomic analysis. It is, however, stressed that excellent technologies like biotinylated NEM already exist to use one tool (i.e., a streptavidin conjugated fluorophore) to assess multiple chemotypes at the global level.
Opportunities exist to solve the alkylating agent SOH reactivity issue. Cochemé and colleagues [98] propose that: SOH could be specifically labelled with Dyn-2 (Figure 6C). Carroll’s group developed Dyn-2—a dual functional reagent containing a SOH reactive dimedone moiety coupled to a clickable alkyne group [150]. The expanded palette of carbon nucleophiles with enhanced SOH reactivity [161], set the stage to develop novel clickable tools to assess SOH using Click PEGylation. Furthermore, Fox’ group [162] have developed SOH reactive TCO, which enables Tz quenching to avoid ex vivo artefacts; such tools could be modified to enable IEDDA mediated Click PEGylation. Novel DiaAlk reagents afford similar opportunities to selectively assess SO2 [147]. Importantly, in vivo fluorescent SOH imaging compared to pan-SOH blotting enables one to gauge whether destructive lysis confounds the analysis. Cell permeable labelling reagents afford a means to label reduced thiols before lysis (mainly in the cytosol unless they are organelle targeted). However, the ability of TCO/Tz functionalized reagents to permeate the cell is unclear and should be tested before live cell labelling is undertaken. The development of SH/S- selective tools coupled to reagents to directly probe each modification type without reduction by forming a diagnostic product allied to carefully buffer preparation should make it possible to advance redox proteomic and immunological analysis [153]; especially for contexts when only destructive analysis is currently possible (e.g., human skeletal muscle). At present, many reaction based technologies (e.g., a mutant glutathione synthase able to incorporate azide functionalized alanine instead of glycine into GSH to study RSSG [163]) would need to be modified (e.g., synthesis of TCO alanine) to be compatible with IEDDA Click PEGylation. Likewise, IEDDA compatible triarylphosphine ester [164] tools for RNSO would need to be synthesized.
4. Opportunities: How to Use Click PEGylation to Advance Knowledge of Redox Biology
We define three opportunities to highlight how Click PEGylation can be used to advance knowledge of redox biology. First, Click PEGylation could be used to develop site-specific sentinels of mitochondrial superoxide production. Unravelling the providence of the superoxide detected by fluorescent reporters (e.g., MitoNeoD [165]) is challenging [166,167,168]. It is imperative to overcome existing challenges to rationally manipulate mitochondrial superoxide production to treat disease. Selective inhibitors of superoxide production at complex I and complex III make it possible to infer providence by assessing the change in superoxide reporter signal with and without the inhibitor [169,170]. While coupling selective inhibitors to a superoxide reporter is useful in cells, it is difficult to apply to tissues (e.g., due to their optical inaccessibility) and impossible to apply to humans until the inhibitors satisfy legislative requirements. Site-selective superoxide reporters would, therefore, advance the field. Using redox proteomics, Dröse group [171] have identified distinct subsets of thiols are reversibly oxidized by complex III, complex I forward, and complex I reverse electron transfer mediated superoxide production. Their findings raise the possibility of assessing site-specific superoxide production sentinels using Click PEGylation, provided subsequent works affirms their fidelity (i.e., they must faithfully respond to a single site out of over 15 known mitochondrial sites [172]). Assessing a panel of site-specific superoxide production sentinels using Click PEGylation could be used to translate literature suggesting hypoxia increases complex III mediated superoxide production to humans [173,174,175]. Such work would advance understanding of how we sense O2 [176].
Second, Click PEGylation could be used to assess exercise-induced redox signaling [177,178,179,180,181,182,183,184]. A recent comprehensive review [185] concluded that redox signaling is central to exercise responses and adaptations (e.g., mitochondrial biogenesis [186,187]). Knowledge of exercise induced redox signaling in human skeletal muscle is, however, fragmentary [188,189]. Opportunities exist to decipher exercise induced redox signals in human skeletal muscle using integrative unbiased redox proteomics, Click PEGylation and MRM. We propose using skeletal muscle biopsies to: (1) identify reversibly oxidized proteins using unbiased redox proteomics; (2) to quantify candidate reversibly oxidized proteins using Click PEGylation; (3) identify and quantify modified thiols using MRM [90,190,191]; and (4) interrogate reversible thiol oxidation type or assess function (e.g., enzyme assays with and without TCEP/DTT). Optionally, Click PEGylated lysates could be used to assess proteins that are difficult to detect by mass spectrometry (e.g., KEAP1) owing to the abundance of contractile proteins (e.g., myosin) [192]. Moreover, parallel in vitro skeletal muscle cell exercise models with and without site directed Crisper-Cas9 [193,194] thiol mutagenesis could be used to determine the biological significance of reversible thiol oxidation using exercise reporters (e.g., mitochondrial mass), provided the thiol was non-catalytic. Mutating a catalytic thiol (e.g., mutating Cys215 in PTB1B [195]) presents obvious difficulties for functional studies [38]. As a murine study attests [196], the integrated approach proposed would yield unprecedent insight into exercise induced redox signaling in humans [197].
Third, Click PEGylation could help disambiguate the molecular basis of oxidative stress in assisted reproduction technologies like in vitro fertilization (IVF) [198,199,200]. IVF induced oxidative stress stems from exposing oocytes/zygotes to 21% O2; which is exacerbated by culture conditions (e.g., nutritional antioxidant deficiency, ambient light exposure, and transition metal catalyzed autoxidation [201,202]). The molecular basis of oxidative stress in IVF is unclear. The role of reversible thiol oxidation is unconsidered. Considering reversible thiol oxidation using Click PEGylation could shift the paradigm from oxidative macromolecule damage (e.g., lipid peroxidation) alone as a driver of oxidative stress to disrupted redox signaling manifested by aberrant reversible thiol oxidation. IVF could increase fractional reversible thiol oxidation of strategically important thiols leading to impaired fertility secondary to dysregulated redox signaling; especially if the pre-existing infertility distorted the thiol proteome. For example, the early cell cycle is regulated by redox sensitive protein phosphatases (e.g., cdc25c [203]) [204]. Increased fractional reversible thiol occupancy and/or shift towards SO2/SO3 occupancy of protein phosphatases could arrest the early cell cycle setting the stage for apoptotic/necrotic mediated embryo fragmentation. Reversing increased fractional thiol oxidation occupancy may be constrained by the functionally immaturity of the glutathione and thioredoxin dependent enzyme systems [205,206]. Even if they were fully operational NADPH synthesis could be limiting [207]. From a clinical perspective, redox proteomics could be used to identify reversibly oxidized proteins secreted by viable and non-viable embryos to develop non-invasive molecular biomarkers assessed using immunological techniques to select the “best” embryos for IVF. Immunological techniques to assess the redox state of proteins secreted in the picomolar and femtomolar range would, however, be required. Until then, Click PEGylation could be used to explore a role for disrupted redox signaling in IVF.
5. A Concluding Perspective and Recommendations
Most biologically relevant reactive species appear and disappear on the nanosecond timescale. For instance, the lifetime of even chemically constrained species like superoxide [208] is limited by a set of kinetically efficient reactions (e.g., superoxide dismutase isoform catalyzed dismutation to O2 and H2O2 [208,209,210]). The analytical challenges of measuring reactive species directly are, therefore, considerable [211,212,213,214]. While significant analytical challenges have been surmounted (e.g., real time ratio-metric H2O2 and GSH measurement [215,216,217,218,219]), it is essential to measure what reactive species are doing by chemically foot-printing their biological reactivity. For many years, the scope of chemical foot-printing was narrowed by oxidative stress being viewed as solely deleterious and a resultant focus on measuring oxidative macromolecule adducts. Measuring many oxidative macromolecule adducts is prone to artefact [220]; and, moreover, unsuitable when one is interested in redox signaling [221], which is often orthogonal to oxidative macromolecule damage [222]. We contend the centrality of the heterogenous thiol proteome to oxidative stress, antioxidant defense, and redox signaling provides a conceptual mandate to reimagine chemical foot-printing. Specifically, the thiol proteome is an endogenous reactive species sensor that dynamically transforms labile signals into relatively stable sulfur oxidation signatures (i.e., chemical footprints) capable of enacting diverse biological outcomes by altering protein function. Measurement and function can coalesce into a single entity when one foot-prints the chemical biology of reactive species and indeed key redox enzymes (recall redox enzymes bidirectionally control oxidation state) by assessing protein thiol redox state. That is to say, assessing protein thiol redox state is as important as measuring reactive species directly. Considering many overtly flawed assays (e.g., DCF, see [223,224]) still plague the field, it is surprising that Click PEGylation—a technically sound assay—has seldom been used to interrogate the functional interface between reactive species and the thiol proteome. By continually refining Click PEGylation and complementary redox proteomic approaches, we believe it is possible to harness immunological techniques to significantly advance current knowledge. To do so, we provide a concluding set of Click PEGylation recommendations:
Identify the number and solvent exposure (if structural models allow) of the target protein thiol, as well as, protein mass to rationally select the PEG size and gel percentage. Note some thiols can selectively become exposed to solvent (termed cryptic thiols).
Select polyclonal antibodies if possible or monoclonal antibodies distal to the PEGylated thiols.
Carefully prepare reaction buffers (e.g., pH in lysis buffer and SDS in Laemmli buffer) and consider using TCEP as a generic reductant to avoid sulfur autoxidation artefacts.
Include an unreacted control with and without DTT in the Laemmli buffer to assess target recognition and RSSR mediated oligomeric mobility shifts, respectively.
Consider direct reaction strategies to assess SOH occupancy.
Judiciously appraise the merits of selective reduction strategies (they can affect other modification types).
Perform reciprocal reduced and reversibly oxidized Click PEGylation analysis.
If reciprocal reduced and reversibly oxidized Click PEGylation implies SO2/SO3 occupancy, then consider direct reaction strategies or mass shifting reduced and reversibly oxidized proteins together to confirm. Optionally, use a recombinant sulfiredoxin reduction system.
Use complementary redox proteomics (e.g., MRM) and functional assays (e.g., enzyme assay) if possible.
If Click PEGylation fails, consider antibody first workflows.
Author Contributions
Writing—original draft preparation, J.N.C.; writing—review and editing, J.N.C. & H.H.; funding acquisition, J.N.C. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are funded by the Highlands and Islands Enterprise (HMS 9353763).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 2.Murphy M.P., Holmgren A., Larsson N.-G., Halliwell B., Chang C.J., Kalyanaraman B., Rhee S.G., Thornalley P.J., Partridge L., Gems D., et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imlay J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson B.C., Chang C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutteridge J.M.C., Halliwell B. Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010;393:561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Oxidative Stress. Academic Press; London, UK: 1985. [Google Scholar]
- 7.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgio M., Trinei M., Migliaccio E., Pelicci P. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 10.D’Autréaux B., Toledano M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 11.Gardner P., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 12.Gerschman R., Gilbert D.L., Nye S., Dwyer P., Fenn W. Oxygen poisoning and x-irradiation: A mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 13.Lane N. Oxygen: The Molecule that Made the World. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- 14.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Winterbourn C.C., Kettle A. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 16.Babior B.M., Lambeth J.D., Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 17.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 18.Booth D.M., Enyedi B., Geiszt M., Varnai P., Hajnoczky G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Mol. Cell. 2016;63:240–248. doi: 10.1016/j.molcel.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulsen C.E., Carroll K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013;113:4633–4679. doi: 10.1021/cr300163e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvez S., Long M.J.C., Poganik J.R., Aye Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018;118:8798–8888. doi: 10.1021/acs.chemrev.7b00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuto J.M., Carrington S.J., Tantillo D.J., Harrison J.G., Ignarro L.J., Freeman B.A., Chen A., Wink D.A. Small molecule signaling agents: The integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q., Lancaster J.R. Chemical foundations of hydrogen sulfide biology. Nitric Oxide Biol. Chem. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul B.D., Snyder S.H. H 2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 24.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., Van Goor H., et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxidants Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy M.P. Mitochondrial Thiols in Antioxidant Protection and Redox Signaling: Distinct Roles for Glutathionylation and Other Thiol Modifications. Antioxid. Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 26.Collins Y., Chouchani E.T., James A.M., Menger K.E., Cocheme H.M., Murphy M.P. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012;125:1837. doi: 10.1242/jcs.110486. [DOI] [PubMed] [Google Scholar]
- 27.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 29.Cobley J.N., Fiorello M.-L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulrich K., Jakob U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019;140:14–27. doi: 10.1016/j.freeradbiomed.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toledo J., Augusto O. Connecting the Chemical and Biological Properties of Nitric Oxide. Chem. Res. Toxicol. 2012;25:975–989. doi: 10.1021/tx300042g. [DOI] [PubMed] [Google Scholar]
- 32.Wood Z.A., Poole L.B., Karplus P.A. Peroxiredoxin Evolution and the Regulation of Hydrogen Peroxide Signaling. Science (80) 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 33.Poole L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015;80:148–157. doi: 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020 doi: 10.1038/s41580-020-0230-3. In press. [DOI] [PubMed] [Google Scholar]
- 35.Kim M.-S., Pinto S., Getnet D., Nirujogi R., Manda S., Chaerkady R., Madugundu A., Kelkar D., Isserlin R., Jain S., et al. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marino S.M., Gladyshev V.N. Cysteine Function Governs Its Conservation and Degeneration and Restricts Its Utilization on Protein Surfaces. J. Mol. Biol. 2010;404:902–916. doi: 10.1016/j.jmb.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weerapana E., Wang C., Simon G.M., Richter F., Khare S., Dillon M.B.D., Bachovchin D.A., Mowen K., Baker D., Cravatt B.F. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature. 2010;468:790–797. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leichert L.I., Dick T.P. Incidence and physiological relevance of protein thiol switches. Biol. Chem. 2015;396:389–399. doi: 10.1515/hsz-2014-0314. [DOI] [PubMed] [Google Scholar]
- 39.Miseta A., Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol. Biol. Evol. 2000;17:1232–1239. doi: 10.1093/oxfordjournals.molbev.a026406. [DOI] [PubMed] [Google Scholar]
- 40.Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., Vina J., et al. Even free radicals should follow some rules: A Guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 41.Go Y.-M., Chandler J.D., Jones D.P. The cysteine proteome. Free Radic. Biol. Med. 2015;84:227–245. doi: 10.1016/j.freeradbiomed.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Requejo R., Hurd T.R., Costa N.J., Murphy M.P. Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J. 2010;277:1465–1480. doi: 10.1111/j.1742-4658.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forman H.J., Maiorino M., Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adimora N., Jones D.P., Kemp M. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxid. Redox Signal. 2010;13:731–743. doi: 10.1089/ars.2009.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peralta D., Bronowska A.K., Morgan B., Dóka É., Van Laer K., Nagy P., Gräter F., Dick T.P. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 2015;11:156–163. doi: 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 46.Nagy P., Karton A., Betz A., Peskin A.V., Pace P., O’Reilly R.J., Hampton M.B., Radom L., Winterbourn C.C. Model for the exceptional reactivity of peroxiredoxins 2 and 3 with hydrogen peroxide: A kinetic and computational study. J. Biol. Chem. 2011;286:18048–18055. doi: 10.1074/jbc.M111.232355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karplus P.A. A primer on peroxiredoxin biochemistry. Free Radic. Biol. Med. 2015;80:183–190. doi: 10.1016/j.freeradbiomed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeida A., Trujillo M., Ferrer-Sueta G., Denicola A., Estrin D.A., Radi R. Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols. Chem. Rev. 2019;119:10829–10855. doi: 10.1021/acs.chemrev.9b00371. [DOI] [PubMed] [Google Scholar]
- 49.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brigelius-Flohé R., Flohé L. Basic Principles and Emerging Concepts in the Redox Control of Transcription Factors. Antioxid. Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flohé L., Toppo S., Cozza G., Ursini F. A comparison of thiol peroxidase mechanisms. Antioxid. Redox Signal. 2011;15:763–780. doi: 10.1089/ars.2010.3397. [DOI] [PubMed] [Google Scholar]
- 52.Woo H.A., Yim S.H., Shin D.H., Kang D., Yu D.Y., Rhee S.G. Inactivation of Peroxiredoxin I by Phosphorylation Allows Localized H2O2 Accumulation for Cell Signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T., Scharf A.N.D., Dick T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015;11:64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- 54.Stöcker S., Van Laer K., Mijuskovic A., Dick T.P. The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxid. Redox Signal. 2018;28:558–573. doi: 10.1089/ars.2017.7162. [DOI] [PubMed] [Google Scholar]
- 55.Rhee S.G., Woo H.A., Kang D. The Role of Peroxiredoxins in the Transduction. Antioxid. Redox Signal. 2018;28:537–557. doi: 10.1089/ars.2017.7167. [DOI] [PubMed] [Google Scholar]
- 56.Travasso R.D.M., Sampaio dos Aidos F., Bayani A., Abranches P., Salvador A. Localized Redox Relays as a Privileged Mode of Hydrogen Peroxide Signaling. Redox Biol. 2017 doi: 10.1016/j.redox.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winterbourn C.C. Are free radicals involved in thiol-based redox signaling? Free Radic. Biol. Med. 2015;80:164–170. doi: 10.1016/j.freeradbiomed.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 58.Radi R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013;288:26464–26472. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurd T.R., Costa N.J., Dahm C.C., Beer S.M., Brown S.E., Filipovska A. Murphy Glutathionylation of Mitochondrial Proteins. Antioxid. Redox Signal. 2005;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- 60.Winterbourn C.C. Superoxide as an intracellular radical sink. Free Radic. Biol. Med. 1993;14:85–90. doi: 10.1016/0891-5849(93)90512-S. [DOI] [PubMed] [Google Scholar]
- 61.Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janssen-Heininger Y.M.W., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Rhee S.G., van der Vliet A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones D.P., Sies H. The Redox Code. Antioxid. Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antunes F., Brito P.M. Quantitative biology of hydrogen peroxide signaling. Redox Biol. 2017 doi: 10.1016/j.redox.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.James A.M., Hoogewijs K., Logan A., Hall A.R., Ding S., Fearnley I.M., Murphy M.P. Non-enzymatic N-acetylation of Lysine Residues by AcetylCoA Often Occurs via a Proximal S-acetylated Thiol Intermediate Sensitive to Glyoxalase II. Cell Rep. 2017;18:2105–2112. doi: 10.1016/j.celrep.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paulsen C.E., Truong T.H., Garcia F.J., Homann A., Gupta V., Leonard S.E., Carroll K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2012;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heppner D.E., Dustin C.M., Liao C., Hristova M., Veith C., Little A.C., Ahlers B.A., White S.L., Deng B., Lam Y.W., et al. Direct cysteine sulfenylation drives activation of the Src kinase. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-06790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang P.T., Zhang L., Chen C.L., Chen J., Green K.B., Chen Y.R. Protein thiyl radical mediates S-glutathionylation of complex i. Free Radic. Biol. Med. 2012;53:962–973. doi: 10.1016/j.freeradbiomed.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor E.R., Hurrell F., Shannon R.J., Lin T.K., Hirst J., Murphy M.P. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 70.Hurd T.R., Requejo R., Filipovska A., Brown S., Prime T.A., Robinson A.J., Fearnley I.M., Murphy M.P. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: Potential role of Cys residues in decreasing oxidative damage. J. Biol. Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mailloux R.J., Willmore W.G. S-glutathionylation reactions in mitochondrial function and disease. Front. Cell Dev. Biol. 2014;2:1–17. doi: 10.3389/fcell.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mailloux R.J., Treberg J.R. Protein S-glutathionlyation links energy metabolism to redox signaling in mitochondria. Redox Biol. 2016;8:110–118. doi: 10.1016/j.redox.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mailloux R.J. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology & Medicine. 5th ed. Oxford University Press; Oxford, UK: 2015. [Google Scholar]
- 75.Guo Z., Kozlov S., Lavin M.F., Person M.D., Paull T.T. ATM activation by oxidative stress. Science (80) 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 76.Wu F., Chi Y., Jiang Z., Xu Y., Xie L., Huang F., Wan D., Ni J., Yuan F., Wu X., et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature. 2020;578:577–581. doi: 10.1038/s41586-020-2032-3. [DOI] [PubMed] [Google Scholar]
- 77.Chen C.A., Wang T.Y., Varadharaj S., Reyes L.A., Hemann C., Talukder M.A.H., Chen Y.R., Druhan L.J., Zweier J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1120. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munkanatta Godage D.N.P., VanHecke G.C., Samarasinghe K.T.G., Feng H.Z., Hiske M., Holcomb J., Yang Z., Jin J.P., Chung C.S., Ahn Y.H. SMYD2 glutathionylation contributes to degradation of sarcomeric proteins. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-06786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chouchani E.T., Methner C., Nadtochiy S.M., Logan A., Victoria R., Ding S., James A.M., Cochemé H.M., Reinhold J., Lilley K.S., et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Emily N.J., Smith A.C., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chouchani E.T., Pell V.R., James A.M., Work L.M., Saeb-parsy K., Frezza C., Krieg T., Murphy M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Duan Q., Liu M.-C.J., Kita D., Jordan S.S., Yeh F.-L.J., Yvon R., Carpenter H., Federico A.N., Garcia-Valencia L.E., Eyles S.J., et al. FERONIA controls pectin- and nitric oxide-mediated male–female interaction. Nature. 2020;579:1–6. doi: 10.1038/s41586-020-2106-2. [DOI] [PubMed] [Google Scholar]
- 83.Yang J., Carroll K.S., Liebler D.C. The Expanding Landscape of the Thiol Redox Proteome. Mol. Cell. Proteomics. 2016;15:1–11. doi: 10.1074/mcp.O115.056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDonagh B. Detection of ROS induced proteomic signatures by mass spectrometry. Front. Physiol. 2017;8:1–7. doi: 10.3389/fphys.2017.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gould N.S., Evans P., Martínez-Acedo P., Marino S.M., Gladyshev V.N., Carroll K.S., Ischiropoulos H. Site-Specific Proteomic Mapping Identifies Selectively Modified Regulatory Cysteine Residues in Functionally Distinct Protein Networks. Chem. Biol. 2015;22:965–975. doi: 10.1016/j.chembiol.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leichert L.I., Gehrke F., Gudiseva H.V., Blackwell T., Ilbert M., Walker A.K., Strahler J.R., Andrews P.C., Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Go Y.M., Roede J.R., Walker D.I., Duong D.M., Seyfried N.T., Orr M., Liang Y., Pennell K.D., Jones D.P. Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems. Mol. Cell. Proteomics. 2013;12:3285–3296. doi: 10.1074/mcp.M113.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim H., Ha S., Lee H., Lee K. ROSics: Chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom. Rev. 2015;34:184–208. doi: 10.1002/mas.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Held J.M. Redox Systems Biology: Harnessing the Sentinels of the Cysteine Redoxome. Antioxid. Redox Signal. 2020;32:659–676. doi: 10.1089/ars.2019.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cobley J.N., Sakellariou G.K., Husi H., McDonagh B. Proteomic strategies to unravel age-related redox signalling defects in skeletal muscle. Free Radic. Biol. Med. 2019;132:24–32. doi: 10.1016/j.freeradbiomed.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 91.Xiao H., Jedrychowski M.P., Schweppe D.K., Huttlin E.L., Yu Q., Heppner D.E., Li J., Long J., Mills E.L., Szpyt J., et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell. 2020;180:968–983. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Husi H., Ward M.A., Choudhary J.S., Blackstock W.P., Grant S.G.N. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat. Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 93.Gracia K.C., Husi H. Computational Approaches in Proteomics. In: Husi H., editor. Computational Biology. Codon Publications; Brisbane, Australia: 2019. [PubMed] [Google Scholar]
- 94.Fernandes M., Sanches B., Husi H. Cheminformatics and Computational Approaches in Metabolomics. In: Husi H., editor. Computational Biology. Codon Publications; Brisbane, Australia: 2019. pp. 143–159. [PubMed] [Google Scholar]
- 95.Cervantes Gracia K., Llanas-Cornejo D., Husi H. CVD and Oxidative Stress. J. Clin. Med. 2017;6:22. doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Picotti P., Aebersold R. Selected reaction monitoring–based proteomics: Workflows, potential, pitfalls and future directions. Nat. Methods. 2012;9:555–566. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 97.Held J.M., Danielson S.R., Behring J.B., Atsriku C., Britton D.J., Puckett R.L., Schilling B., Campisi J., Benz C.C., Gibson B.W. Targeted quantitation of site-specific cysteine oxidation in endogenous proteins using a differential alkylation and multiple reaction monitoring mass spectrometry approach. Mol. Cell. Proteomics. 2010;9:1400–1410. doi: 10.1074/mcp.M900643-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Leeuwen L.A.G., Hinchy E.C., Murphy M.P., Robb E.L., Cochemé H.M. Click-PEGylation—A mobility shift approach to assess the redox state of cysteines in candidate proteins. Free Radic. Biol. Med. 2017;108:374–382. doi: 10.1016/j.freeradbiomed.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vinothkumar K.R., Zhu J., Hirst J. Architecture of mammalian respiratory complex I. Nature. 2014;515:80–84. doi: 10.1038/nature13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fandiño A.S., Rais I., Vollmer M., Elgass H., Schägger H., Karas M. LC-nanospray-MS/MS analysis of hydrophobic proteins from membrane protein complexes isolated by blue-native electrophoresis. J. Mass Spectrom. 2005;40:1223–1231. doi: 10.1002/jms.903. [DOI] [PubMed] [Google Scholar]
- 101.Mason R.P., Ganini D. Immuno-spin trapping of macromolecules free radicals in vitro and in vivo – One stop shopping for free radical detection. Free Radic. Biol. Med. 2019;131:318–331. doi: 10.1016/j.freeradbiomed.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 102.Low F.M., Hampton M.B., Peskin A.V., Winterbourn C.C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611–2617. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- 103.Woo H.A., Kang S.W., Kim H.K., Yang K.S., Chae H.Z., Rhee S.G. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem. 2003;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 104.Chae H.Z., Robison K., Poole L.B., Church G., Storz G., Rhee S.G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: Alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robb E.L., Gawel J.M., Aksentijevic D., Cocheme H.M., Stewart T.S., Shchepinova M.M., Qiang H., Prime T.A., Bright T.P., James A.M., et al. Selective superoxide generation within mitochondria by the targeted redox cycler MitoParaquat. Free Radic. Biol. Med. 2015;89:883–894. doi: 10.1016/j.freeradbiomed.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 106.Kussmaul L., Hirst J. The mechanism of superoxide production by NADH: Ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dröse S., Brandt U., Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. Biophys. Acta Proteins Proteomics. 2014;1844:1344–1354. doi: 10.1016/j.bbapap.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 108.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cox A.G., Winterbourn C.C., Hampton M.B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 110.Brennan J.P., Miller J.I.A., Fuller W., Wait R., Begum S., Dunn M.J., Eaton P. The utility of N,N-biotinyl glutathione disulfide in the study of protein S-glutathiolation. Mol. Cell. Proteomics. 2006;5:215–225. doi: 10.1074/mcp.M500212-MCP200. [DOI] [PubMed] [Google Scholar]
- 111.Burgoyne J.R., Oviosu O., Eaton P. The PEG-switch assay: A fast semi-quantitative method to determine protein reversible cysteine oxidation. J. Pharmacol. Toxicol. Methods. 2013;68:297–301. doi: 10.1016/j.vascn.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 112.Hurd T.R., Collins Y., Abakumova I., Chouchani E.T., Baranowski B., Fearnley I.M., Prime T.A., Murphy M.P., James A.M. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. J. Biol. Chem. 2012;287:35153–35160. doi: 10.1074/jbc.M112.400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bazopoulou D., Knoefler D., Zheng Y., Ulrich K., Oleson B.J., Xie L., Kim M., Kaufmann A., Lee Y.T., Dou Y., et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature. 2019;576:301–305. doi: 10.1038/s41586-019-1814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rostovtsev V., Green L., Fokin V., Sharpless K. A Stepwise Huisgen Cycloaddition Process Catalyzed by Copper (I): Regioselective Ligation of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 115.Jewett J., Bertozzi C. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cobley J.N., Noble A., Jimenez-Fernandez E., Valdivia Moya M.T., Guille M., Husi H. Catalyst-free Click PEGylation reveals substantial mitochondrial ATP synthase sub-unit alpha oxidation before and after fertilisation. Redox Biol. 2019;26:101258. doi: 10.1016/j.redox.2019.101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Müller T., Winter D. Systematic evaluation of protein reduction and alkylation reveals massive unspecific side effects by iodine-containing reagents. Mol. Cell. Proteomics. 2017;16:1173–1187. doi: 10.1074/mcp.M116.064048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hess D.T., Matsumoto A., Kim S.-O.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 119.Taylor M.T., Blackman M.L., Dmitrenko O., Fox J.M. Design and synthesis of highly reactive dienophiles for the tetrazine-trans-cyclooctene ligation. J. Am. Chem. Soc. 2011;133:9646–9649. doi: 10.1021/ja201844c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Darko A., Wallace S., Dmitrenko O., Machovina M.M., Mehl R.A., Chin J.W., Fox J.M. Conformationally strained trans-cyclooctene with improved stability and excellent reactivity in tetrazine ligation. Chem. Sci. 2014;5:3770–3776. doi: 10.1039/C4SC01348D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogilby P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010;39:3181–3209. doi: 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- 122.Diels O., Alder K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928;460:98–122. doi: 10.1002/jlac.19284600106. [DOI] [Google Scholar]
- 123.Blackman M.L., Royzen M., Fox J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels—Alder Reactivity. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oliveira B.L., Guo Z., Bernardes G.J.L. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017;46:4895–4950. doi: 10.1039/C7CS00184C. [DOI] [PubMed] [Google Scholar]
- 125.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 126.Nicholls D.G., Ferguson S.J. Bioenergetics 4. 4th ed. Academic Press; London, UK: 2013. [Google Scholar]
- 127.Walker J.E. The ATP synthase: The understood, the uncertain and the unknown. Biochem. Soc. Trans. 2013;41:1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- 128.Yagi T., Hatefi Y. Thiols in Oxidative Phosphorylation: Inhibition and Energy-Potentiated Uncoupling by Monothiol and Dithiol Modifiers. Biochemistry. 1984;23:2449–2455. doi: 10.1021/bi00306a020. [DOI] [PubMed] [Google Scholar]
- 129.Kaludercic N., Giorgio V. The dual function of reactive oxygen/nitrogen species in bioenergetics and cell death: The role of ATP synthase. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/3869610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang S.B., Foster D.B., Rucker J., O’Rourke B., Kass D.A., Van Eyk J.E. Redox regulation of mitochondrial ATP synthase: Implications for cardiac resynchronization therapy. Circ. Res. 2011;109:750–757. doi: 10.1161/CIRCRESAHA.111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang S.B., Murray C.I., Chung H.S., Van Eyk J.E. Redox Regulation of Mitochondrial ATP Synthase. Trends Cardiovasc. Med. 2013;23:18. doi: 10.1016/j.tcm.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Session A.M., Uno Y., Kwon T., Chapman J.A., Toyoda A., Takahashi S., Fukui A., Hikosaka A., Suzuki A., Kondo M., et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538:1–15. doi: 10.1038/nature19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harland R.M., Grainger R.M. Xenopus research: Metamorphosed by genetics and genomics. Trends Genet. 2011;27:507–515. doi: 10.1016/j.tig.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sidlauskaite E., Gibson J.W., Megson I.L., Whitfield P.D., Tovmasyan A., Batinic-Haberle I., Murphy M.P., Moult P.R., Cobley J.N. Mitochondrial ROS cause motor deficits induced by synaptic inactivity: Implications for synapse pruning. Redox Biol. 2018;16:344–351. doi: 10.1016/j.redox.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nalin C.M., McCarty R.E. Role of a disulfide bond in the gamma subunit in activation of the ATPase of chloroplast coupling factor 1. J. Biol. Chem. 1984;259:7275–7280. [PubMed] [Google Scholar]
- 136.Garcia J., Han D., Sancheti H., Yap L.P., Kaplowitz N., Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J. Biol. Chem. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cobley J., Noble A., Bessell R., Guille M., Husi H. Reversible Thiol Oxidation Inhibits the Mitochondrial ATP Synthase in Xenopus laevis Oocytes. Antioxidants. 2020;9:215. doi: 10.3390/antiox9030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hansen J.M., Jones D.P., Harris C. The Redox Theory of Development. Antioxid. Redox Signal. 2020;32:715–740. doi: 10.1089/ars.2019.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allen R.G., Balin A. Oxidative influence on development and differentiation: An overview of a free radical theory of development. Free Radic. Biol. Med. 1989;6:631–661. doi: 10.1016/0891-5849(89)90071-3. [DOI] [PubMed] [Google Scholar]
- 140.Hitchler M.J., Domann F.E. An epigenetic perspective on the free radical theory of development. Free Radic. Biol. Med. 2007;43:1023–1036. doi: 10.1016/j.freeradbiomed.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cobley J.N. Synapse pruning: Mitochondrial ROS with their hands on shears. BioEssays. 2018;40:e1800031. doi: 10.1002/bies.201800031. [DOI] [PubMed] [Google Scholar]
- 142.Galkin A., Meyer B., Wittig I., Karas M., Schägger H., Vinogradov A., Brandt U. Identification of the mitochondrial ND3 subunit as a structural component involved in the active/deactive enzyme transition of respiratory complex I. J. Biol. Chem. 2008;283:20907–20913. doi: 10.1074/jbc.M803190200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee Y.-J., Chang G.-D. Quantitative display of the redox status of proteins with maleimide-polyethylene glycol tagging. Electrophoresis. 2019;40:491–498. doi: 10.1002/elps.201800335. [DOI] [PubMed] [Google Scholar]
- 144.Zheng C.Y., Ma G., Su Z. Native PAGE eliminates the problem of PEG-SDS interaction in SDS-PAGE and provides an alternative to HPLC in characterization of protein PEGylation. Electrophoresis. 2007;28:2801–2807. doi: 10.1002/elps.200600807. [DOI] [PubMed] [Google Scholar]
- 145.Wittig I., Braun H.P., Schägger H. Blue native PAGE. Nat. Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 146.Laemmli U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 147.Akter S., Fu L., Jung Y., Conte M.L., Lawson J.R., Lowther W.T., Sun R., Liu K., Yang J., Carroll K.S. Chemical proteomics reveals new targets of cysteine sulfinic acid reductase. Nat. Chem. Biol. 2018;14:995–1004. doi: 10.1038/s41589-018-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Biteau B., Labarre J., Toledano M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 149.Reisz J.A., Bechtold E., King S.B., Poole L.B., Furdui C.M. Thiol-blocking electrophiles interfere with labeling and detection of protein sulfenic acids. FEBS J. 2013;280:6150–6161. doi: 10.1111/febs.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang J., Gupta V., Carroll K.S., Liebler D.C. Site-specific mapping and quantification of protein S-sulphenylation in cells. Nat. Commun. 2014;5:4776. doi: 10.1038/ncomms5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fu L., Liu K., He J., Tian C., Yu X., Yang J. Direct Proteomic Mapping of Cysteine Persulfidation. Antioxid. Redox Signal. 2019 doi: 10.1089/ars.2019.7777. [DOI] [PubMed] [Google Scholar]
- 152.Heppner D.E., Hristova M., Ida T., Mijuskovic A., Dustin C.M., Bogdándi V., Fukuto J.M., Dick T.P., Nagy P., Li J., et al. Cysteine perthiosulfenic acid (Cys-SSOH): A novel intermediate in thiol-based redox signaling? Redox Biol. 2018;14:379–385. doi: 10.1016/j.redox.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shi Y., Carroll K.S. Activity-Based Sensing for Site-Specific Proteomic Analysis of Cysteine Oxidation. Acc. Chem. Res. 2020;53:20–31. doi: 10.1021/acs.accounts.9b00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S., Santos D.P., Blanz J., Obermaier C.D., Strojny C., et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;9080:1–12. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anderson D.G., Mariappan S.V.S., Buettner G.R., Doorn J.A. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J. Biol. Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 157.Bersweiler A., D’Autréaux B., Mazon H., Kriznik A., Belli G., Delaunay-Moisan A., Toledano M.B., Rahuel-Clermont S. A scaffold protein that chaperones a cysteine-sulfenic acid in H2O2 signaling. Nat. Chem. Biol. 2017;13:909–915. doi: 10.1038/nchembio.2412. [DOI] [PubMed] [Google Scholar]
- 158.Deponte M. The Incomplete Glutathione Puzzle: Just Guessing at Numbers and Figures? Antioxidants Redox Signal. 2017;27:1130–1161. doi: 10.1089/ars.2017.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun Q.A., Wang B., Miyagi M., Hess D.T., Stamler J.S. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor/Ca2+ release channel (RyR1): Sites and nature of oxidative modification. J. Biol. Chem. 2013;288:22961–22971. doi: 10.1074/jbc.M113.480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Place N., Ivarsson N., Venckunas T., Neyroud D., Brazaitis M., Cheng A.J., Ochala J., Kamandulis S., Girard S., Volungevičius G., et al. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca 2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. USA. 2015;112:201507176. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gupta V., Yang J., Liebler D.C., Carroll K.S. Diverse Redoxome Reactivity Profiles of Carbon Nucleophiles. J. Am. Chem. Soc. 2017;139:5588–5595. doi: 10.1021/jacs.7b01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scinto S.L., Ekanayake O., Seneviratne U., Pigga J.E., Boyd S.J., Taylor M.T., Liu J., Am Ende C.W., Rozovsky S., Fox J.M. Dual-Reactivity trans-Cyclooctenol Probes for Sulfenylation in Live Cells Enable Temporal Control via Bioorthogonal Quenching. J. Am. Chem. Soc. 2019;141:10932–10937. doi: 10.1021/jacs.9b01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Samarasinghe K.T.G., Munkanatta Godage D.N.P., Vanhecke G.C., Ahn Y.H. Metabolic synthesis of clickable glutathione for chemoselective detection of glutathionylation. J. Am. Chem. Soc. 2014;136:11566–11569. doi: 10.1021/ja503946q. [DOI] [PubMed] [Google Scholar]
- 164.Seneviratne U., Godoy L.C., Wishnok J.S., Wogan G.N., Tannenbaum S.R. Mechanism-based triarylphosphine-ester probes for capture of endogenous RSNOs. J. Am. Chem. Soc. 2013;135:7693–7704. doi: 10.1021/ja401565w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shchepinova M.M., Cairns A.G., Prime T.A., Logan A., James A.M., Hall A.R., Vidoni S., Arndt S., Caldwell S.T., Prag H.A., et al. MitoNeoD: A Mitochondria-Targeted Superoxide Probe. Cell Chem. Biol. 2017;3:8–21. doi: 10.1016/j.chembiol.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cochemé H.M., Quin C., McQuaker S.J., Cabreiro F., Logan A., Prime T.A., Abakumova I., Patel J.V., Fearnley I.M., James A.M., et al. Measurement of H2O2 within living drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Logan A., Cochemé H.M., Li Pun P.B., Apostolova N., Smith R.A.J., Larsen L., Larsen D.S., James A.M., Fearnley I.M., Rogatti S., et al. Using exomarkers to assess mitochondrial reactive species in vivo. Biochim. Biophys. Acta Gen. Subj. 2014;1840:923–930. doi: 10.1016/j.bbagen.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 168.Kalyanaraman B., Darley-Usmar V., Davies K.J.A., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Orr A.L., Vargas L., Turk C.N., Baaten J.E., Matzen J.T., Dardov V.J., Attle S.J., Li J., Quackenbush D.C., Goncalves R.L.S., et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Biol. 2015;11:834–839. doi: 10.1038/nchembio.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brand M.D., Goncalves R.L.S., Orr A.L., Vargas L., Gerencser A.A., Borch Jensen M., Wang Y.T., Melov S., Turk C.N., Matzen J.T., et al. Suppressors of Superoxide-H2O2Production at Site IQof Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metab. 2016;24:582–592. doi: 10.1016/j.cmet.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bleier L., Wittig I., Heide H., Steger M., Brandt U., Drose S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic. Biol. Med. 2015;78:1–10. doi: 10.1016/j.freeradbiomed.2014.10.511. [DOI] [PubMed] [Google Scholar]
- 172.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 173.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–166. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guzy R.D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K.D., Simon M.C., Hammerling U., Schumacker P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 176.Schofield C.J., Ratcliffe P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 177.Cobley J.N., Sakellariou G.K., Owens D.J., Murray S., Waldron S., Gregson W., Fraser W.D., Burniston J.G., Iwanejko L.A., McArdle A., et al. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014;70:23–32. doi: 10.1016/j.freeradbiomed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 178.Cobley J.N., McHardy H., Morton J.P., Nikolaidis M.G., Close G.L. Influence of vitamin C and vitamin E on redox signalling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015;84:65–76. doi: 10.1016/j.freeradbiomed.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 179.Cobley J.N., Moult P.R., Burniston J.G., Morton J.P., Close G.L. Exercise improves mitochondrial and redox-regulated stress responses in the elderly: Better late than never! Biogerontology. 2015;16:249–264. doi: 10.1007/s10522-014-9546-8. [DOI] [PubMed] [Google Scholar]
- 180.Cobley J.N., Margaritelis N.V., Morton J.P., Close G.L., Nikolaidis M.G., Malone J.K. The basic chemistry of exercise-induced DNA oxidation: Oxidative damage, redox signaling, and their interplay. Front. Physiol. 2015;6:1–8. doi: 10.3389/fphys.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Margaritelis N.V., Cobley J.N., Paschalis V., Veskoukis A.S., Theodorou A.A., Kyparos A., Nikolaidis M.G. Principles for integrating reactive species into in vivo biological processes: Examples from exercise physiology. Cell. Signal. 2016;28:256–271. doi: 10.1016/j.cellsig.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 182.Mason S.A., Trewin A.J., Parker L., Wadley G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020:101471. doi: 10.1016/j.redox.2020.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Henríquez-Olguin C., Knudsen J.R., Raun S.H., Li Z., Dalbram E., Treebak J.T., Sylow L., Holmdahl R., Richter E.A., Jaimovich E., et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Henriquez-Olguin C., Meneses-Valdes R., Jensen T.E. Compartmentalized muscle redox signals controlling exercise metabolism—Current state, future challenges. Redox Biol. 2020:101473. doi: 10.1016/j.redox.2020.101473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Margaritelis N.V., Paschalis V., Theodorou A.A., Kyparos A., Nikolaidis M.G. Redox basis of exercise physiology. Redox Biol. 2020:101499. doi: 10.1016/j.redox.2020.101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cobley J.N., Bartlett J.D., Kayani A., Murray S.W., Louhelainen J., Donovan T., Waldron S., Gregson W., Burniston J.G., Morton J.P., et al. PGC-1?? transcriptional response and mitochondrial adaptation to acute exercise is maintained in skeletal muscle of sedentary elderly males. Biogerontology. 2012;13:621–631. doi: 10.1007/s10522-012-9408-1. [DOI] [PubMed] [Google Scholar]
- 187.Merry T.L., Ristow M. Mitohormesis in exercise training. Free Radic. Biol. Med. 2016;98:123–130. doi: 10.1016/j.freeradbiomed.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 188.Cobley J.N. How exercise induces oxidative eustress. In: Sies H., editor. Oxidative Stress. Elsevier; Amsterdam, The Netherlands: 2020. pp. 447–462. [Google Scholar]
- 189.Wadley A.J., Aldred S., Coles S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016;8:51–58. doi: 10.1016/j.redox.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cobley J.N., Malik Z.A., Morton J.P., Close G.L., Edwards B.J., Burniston J.G. Age- and Activity-Related Differences in the Abundance of Myosin Essential and Regulatory Light Chains in Human Muscle. Proteomes. 2016;4:15. doi: 10.3390/proteomes4020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Malik Z.A., Cobley J.N., Morton J.P., Close G.L., Edwards B.J., Koch L.G., Britton S.L., Burniston J.G. Label-Free LC-MS Profiling of Skeletal Muscle Reveals Heart-Type Fatty Acid Binding Protein as a Candidate Biomarker of Aerobic Capacity. Proteomes. 2013;1:290–308. doi: 10.3390/proteomes1030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Burniston J.G., Hoffman E.P. Proteomic responses of skeletal and cardiac muscle to exercise. Expert Rev. Proteomics. 2011;8:361–377. doi: 10.1586/epr.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science (80) 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.O’Connell M.R., Oakes B.L., Sternberg S.H., East-Seletsky A., Kaplan M., Doudna J. A Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature. 2014;516:263–266. doi: 10.1038/nature13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Van Montfort R., Congreve M., Tisi D. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 196.Kramer P.A., Duan J., Gaffrey M.J., Shukla A.K., Wang L., Bammler T.K., Qian W.J., Marcinek D.J. Fatiguing contractions increase protein S-glutathionylation occupancy in mouse skeletal muscle. Redox Biol. 2018;17:367–376. doi: 10.1016/j.redox.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cobley J.N., Close G.L., Bailey D.M., Davison G.W. Exercise redox biochemistry: Conceptual, methodological and technical recommendations. Redox Biol. 2017;12:540–548. doi: 10.1016/j.redox.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Agarwal A., Gupta S., Sekhon L., Shah R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxidants Redox Signal. 2008;10:1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- 199.Ruder E.H., Hartman T.J., Blumberg J., Goldman M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update. 2008;14:345–357. doi: 10.1093/humupd/dmn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bontekoe S., Mantikou E., van Wely M., Seshadri S., Repping S., Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD008950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Halliwell B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003;540:3–6. doi: 10.1016/S0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 202.Halliwell B. Cell culture, oxidative stress, and antioxidants: Avoiding pitfalls. Biomed. J. 2014 doi: 10.4103/2319-4170.128725. [DOI] [PubMed] [Google Scholar]
- 203.Savitsky P.A., Finkel T. Redox regulation of Cdc25C. J. Biol. Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- 204.Han Y., Ishibashi S., Iglesias-Gonzalez J., Chen Y., Love N.R., Amaya E. Ca2+-Induced Mitochondrial ROS Regulate the Early Embryonic Cell Cycle. Cell Rep. 2018;22:218–231. doi: 10.1016/j.celrep.2017.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bell K.F.S., Al-Mubarak B., Martel M.-A., McKay S., Wheelan N., Hasel P., Márkus N.M., Baxter P., Deighton R.F., Serio A., et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat. Commun. 2015;6:7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dennery P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010;49:1147–1151. doi: 10.1016/j.freeradbiomed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 207.Dick T.P., Ralser M. Metabolic Remodeling in Times of Stress: Who Shoots Faster than His Shadow? Mol. Cell. 2015;59:519–521. doi: 10.1016/j.molcel.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 208.Sheng Y., Abreu I.A., Cabelli D.E., Maroney M.J., Miller A.F., Teixeira M., Valentine J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McCord J., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 210.Buettner G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer. Agents Med. Chem. 2011;11:341–346. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Winterbourn C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta Gen. Subj. 2014;1840:730–738. doi: 10.1016/j.bbagen.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 212.Hardy M., Zielonka J., Karoui H., Sikora A., Michalski R., Podsiadły R., Lopez M., Vasquez-Vivar J., Kalyanaraman B., Ouari O. Detection and Characterization of Reactive Oxygen and Nitrogen Species in Biological Systems by Monitoring Species-Specific Products. Antioxid. Redox Signal. 2017:ars.2017.7398. doi: 10.1089/ars.2017.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zielonka J., Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic. Biol. Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Miller E.W., Chang C.J. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr. Opin. Chem. Biol. 2007;11:620–625. doi: 10.1016/j.cbpa.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Belousov V., Fradkov A., Lukyanov K., Staroverov D., Shakhbazov K., Terskikh A., Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 216.Bilan D.S., Belousov V.V. New tools for redox biology: From imaging to manipulation. Free Radic. Biol. Med. 2017;109:167–188. doi: 10.1016/j.freeradbiomed.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 217.Gutscher M., Sobotta M.C., Wabnitz G.H., Ballikaya S., Meyer A.J., Samstag Y., Dick T.P. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schwarzländer M., Dick T.P., Meye A.J., Morgan B. Dissecting Redox Biology using Fluorescent Protein Sensors. Antioxid. Redox Signal. 2016;24:680–712. doi: 10.1089/ars.2015.6266. [DOI] [PubMed] [Google Scholar]
- 219.Pak V.V., Ezeriņa D., Lyublinskaya O.G., Pedre B., Tyurin-Kuzmin P.A., Mishina N.M., Thauvin M., Young D., Wahni K., Martínez Gache S.A., et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020;31:642–653. doi: 10.1016/j.cmet.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Margaritelis N.V., Cobley J.N., Paschalis V., Veskoukis A.S., Theodorou A.A., Kyparos A., Nikolaidis M.G. Going retro: Oxidative stress biomarkers in modern redox biology. Free Radic. Biol. Med. 2016;98:2–12. doi: 10.1016/j.freeradbiomed.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 223.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 224.Bonini M.G., Rota C., Tomasi A., Mason R.P. The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: A self-fulfilling prophesy? Free Radic. Biol. Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]