Significance
In this study, Kim et al. identify Drosophila CFTR (dCFTR) and show that knockdown of dCFTR in the adult intestine disrupts osmotic homeostasis and displays CF-like phenotypes that lead to intestinal stem cell hyperplasia. They show that expression of wild-type human CFTR, but not mutated forms of CFTR that affect protein production or transport, rescues the mutant phenotypes of dCFTR. Furthermore, global transcriptomic analysis of dCFTR fly intestine identified a mucin gene that is required for proper intestinal barrier protection. Altogether, their findings suggest that Drosophila can be a powerful model organism for studying CF pathophysiology.
Keywords: Drosophila, CFTR, cystic fibrosis, gut
Abstract
Cystic fibrosis (CF) is a recessive disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. The most common symptoms include progressive lung disease and chronic digestive conditions. CF is the first human genetic disease to benefit from having five different species of animal models. Despite the phenotypic differences among the animal models and human CF, these models have provided invaluable insight into understanding disease mechanisms at the organ-system level. Here, we identify a member of the ABCC4 family, CG5789, that has the structural and functional properties expected for encoding the Drosophila equivalent of human CFTR, and thus refer to it as Drosophila CFTR (Dmel\CFTR). We show that knockdown of Dmel\CFTR in the adult intestine disrupts osmotic homeostasis and displays CF-like phenotypes that lead to intestinal stem cell hyperplasia. We also show that expression of wild-type human CFTR, but not mutant variants of CFTR that prevent plasma membrane expression, rescues the mutant phenotypes of Dmel\CFTR. Furthermore, we performed RNA sequencing (RNA-Seq)-based transcriptomic analysis using Dmel\CFTR fly intestine and identified a mucin gene, Muc68D, which is required for proper intestinal barrier protection. Altogether, our findings suggest that Drosophila can be a powerful model organism for studying CF pathophysiology.
Cystic fibrosis (CF) is an autosomal recessive disorder that primarily affects individuals of European descent at a rate of 1 in 2,500 newborns (1). CF is caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a chloride channel expressed in the apical membranes of various epithelia (2–4). The most common symptom of CF includes accumulation of viscous mucus in the pulmonary and gastrointestinal tract, which is associated with bacterial infections, aberrant inflammation, and malnutrition (5, 6). Besides chloride secretion, CFTR’s regulation of other membrane proteins, including epithelial sodium channel (ENaC), plays an important role in maintaining osmotic homeostasis by controlling the movement of water through the epithelium, which is particularly important for mucous membranes. In CF, loss-of-function mutations in CFTR can elevate the activity of ENaC through a mechanism that is not fully understood (7). Up-regulation of ENaC activity can increase Na+ and water influx, which ultimately leads to dehydration of the epithelial surface and reduction in mucus transport in multiple mucin-producing organs, such as the lungs, sinuses, intestine, pancreas, and reproductive organs (8). While there has been immense progress made in understanding the basic biology of the CF, many important questions regarding the initiation and progression of the disease remains unanswered.
Animal models of human genetic diseases allow a more in-depth study of the pathophysiology of a disease than is possible in humans. CF is unique among human genetic disorders in that five animal models have been created: mouse (9), pig (10), ferret (11), zebrafish (12), and rat (13). The availability of these models, each with different features and advantages, has provided invaluable insight into understanding disease mechanisms at the organ-system level. However, widespread use of these models has been hampered due to limited accessibility to different developmental stages, high animal husbandry costs, and difficulties in genetic manipulations. Therefore, to better understand disease onset and to develop new treatments for CF, it is important to develop a more tractable model of CF that reflects the disease in humans.
Recently, we identified an evolutionarily conserved miRNA, miR-263a, that maintains intestinal stem cell (ISC) and osmotic homeostasis by directly negatively regulating the expression of ENaC in Drosophila (14). In the absence of miR-263a, the intraluminal surface of the intestine displays dehydration-like phenotypes, Na+ levels are increased in enterocytes (ECs), stress pathways are activated in ECs, and ISCs overproliferate. Furthermore, miR-263a mutants have increased bacterial load and are more susceptible to bacterial infections. Strikingly, these phenotypes are reminiscent of the pathophysiology of CF. Similar to the ENaC-overexpression mouse model of CF (15), our ENaC-overexpression fly model can be a powerful genetic model organism for studying the molecular mechanisms of CF. However, given that CF is caused by a mutation in CFTR, we decided to further establish the fly gut as a model for CF by identifying and characterizing Drosophila CFTR (Dmel\CFTR). Here, we identify a member of the ABCC4 family, CG5789, that has the structural and functional properties expected for encoding the Drosophila equivalent of human CFTR, and thus refer to it as Dmel\CFTR. We show that knockdown of Dmel\CFTR in the adult fly intestine disrupts osmotic homeostasis and activates a stress response that, in turn, activates signaling pathways required for ISC proliferation, resulting in intestinal hyperplasia. We show that expression of human CFTR (hCFTR) rescues the Dmel\CFTR mutant phenotypes, indicating that the genes are functional orthologs. Furthermore, we performed RNA sequencing (RNA-Seq)-based transcriptomic analysis using Dmel\CFTR fly intestine. Global gene expression analysis identified a mucin gene, Muc68D, which may play an analogous role to that of mammalian mucin genes, MUC5AC and MUC5B, which are commonly misregulated in CF (16, 17). Altogether, our findings establish a Drosophila model for studying CF pathophysiology.
Results
Drosophila CG5789 Is the Predicted Ortholog of hCFTR.
CFTR is the only member of the ABC superfamily of membrane transporters that function as an ion channel (18–20). More specifically, sequence similarity comparison and phylogenetic analysis indicate that CFTR is most closely related to the ABCC4 member of the ABCC family (21). Previous to this work, a Drosophila ortholog of CFTR had not been identified. Based on ortholog predictions (22), hCFTR has 11 predicted orthologs in the Drosophila genome (SI Appendix, Fig. S1A), each of which had already been tentatively categorized as Drosophila ABCC4 genes, based on sequence similarities alone (FlyBase, 2018). When we assessed the phylogenetic relationships among the candidate Dmel\CFTRs and 16 functionally characterized vertebrate CFTR and 15 functionally characterized vertebrate ABCC4 orthologs, we observed that one Drosophila gene, CG5789, branches basally to all other Dmel\CFTR candidates (SI Appendix, Fig. S1B), suggesting that it may have potentially retained sequence/function similarities to vertebrate CFTR genes. However, we note that CG5789 does not form a monophyletic group with vertebrate CFTR genes.
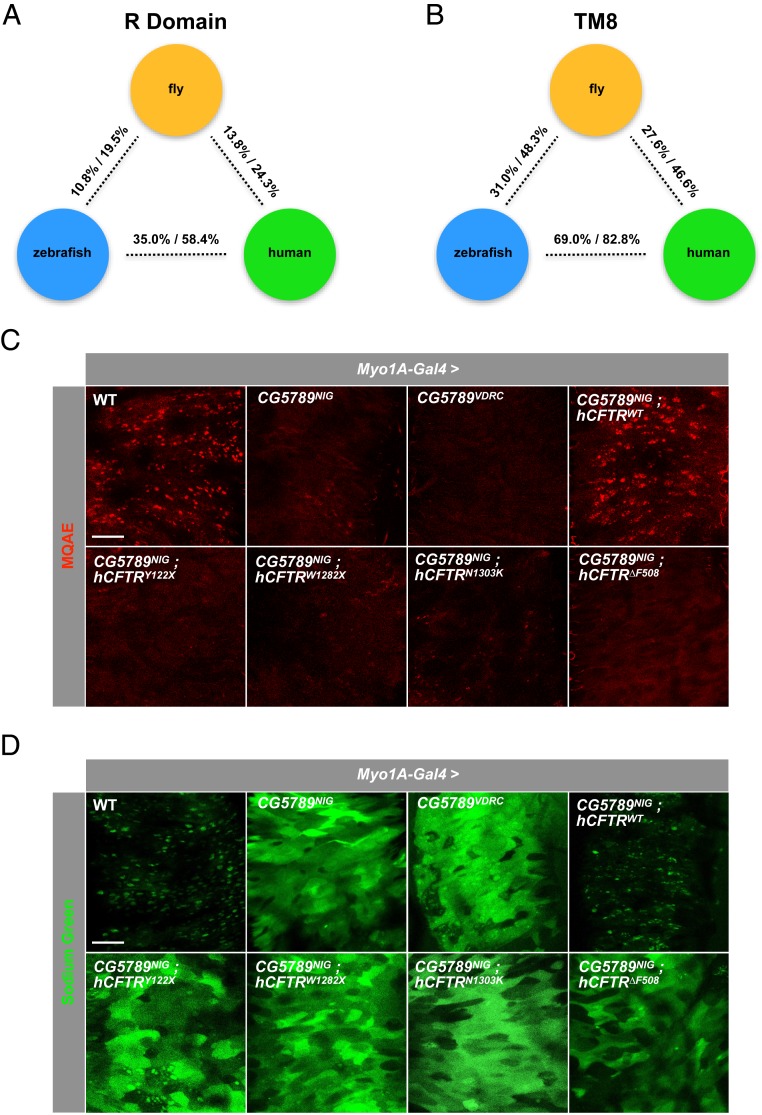
Since none of the 11 Dmel\CFTR genes shows a clear full-length sequence similarity to vertebrate CFTR orthologs, we decided to examine a specific domain that is unique to CFTR. CFTR is distinct from ABCC4 by the presence of the R domain, which contains multiple protein kinase A-dependent phosphorylation sites for full channel activity (23–25). Sequence similarity comparison and phylogenetic analysis indicate that hCFTR R domain is most closely related to the putative R domain of CG5789 (SI Appendix, Fig. S1C and Fig. 1A) while CG5789 groups with the R domains of hCFTR (SI Appendix, Fig. S1D).
Fig. 1.
Disruption of Cl− and Na+ homeostasis in the absence of CG5789. (A) Pairwise sequence identity and similarity of R domain between labeled species. (B) Pairwise sequence identity and similarity of TM8 domain between labeled species. (C) Fluorescent monitoring of intracellular Cl− levels in the midgut epithelium using MQAE. Significant quenching of fluorescence is observed when CG5789 was depleted in the ECs compared to wild-type control. (D) Fluorescent monitoring of intracellular Na+ levels in the midgut epithelium using Sodium Green. Enhancement of Sodium Green fluorescence is observed in the absence of CG5789 compared to wild-type control (C and D) (Scale bar, 50 μm.).
Next, we extended our analysis to include the transmembrane domain 8 (TM8) of hCFTR that may play a critical role in ion conduction and gating (26–28). As further evidence that CG5789 may be the functional ortholog of CFTR, hCFTR TM8 is most closely related to the putative TM8 of CG5789 (SI Appendix, Fig. S1E and Fig. 1B). However, CG5789 TM8 is slightly more identical to the TM8 of zebrafish than it is to human (SI Appendix, Fig. S1F and Fig. 1B). Furthermore, a BLAST search using either hCFTR or zebrafish CFTR (zCFTR) TM8 protein sequence both returned CG5789 as the only positive result in the Drosophila melanogaster genome (FlyBase-BLAST, 2018). Lastly, using a portal for predicting three-dimensional structure of protein from its amino acid sequence (29), we find that zCFTR TM8 is predicted to be most identical to the CG5789 TM8 based on this structural analysis (SI Appendix, Fig. S1G). Altogether, these analyses provide several lines of evidence that CG5789 may be the functional ortholog of vertebrate CFTRs.
RNAi Knockdown of CG5789 Disrupts Cl− and Na+ Homeostasis in the Intestinal Epithelium.
In CF, loss-of-function mutations in CFTR lead to buildup of Cl− inside the cell. Therefore, we asked whether depletion of CG5789 also leads to Cl− buildup in the intestinal epithelium. Using two independent RNAi lines targeting different regions of the gene, we knocked down CG5789 in the ECs where we had previously observed ENaC-dependent CF-like phenotypes (14). To monitor the amount of Cl− in the midgut epithelium, we used a quinoline-based Cl−-sensitive fluorescent dye, MQAE (N-(Ethoxycarbonylmethyl)-6-methoxyquinolinium bromide), which detects Cl− via diffusion-limited collisional quenching of fluorescence (30). When CG5789 was depleted in the ECs, we observed significantly reduced MQAE fluorescence compared to wild-type control (Fig. 1C). Quantification of MQAE fluorescence revealed ∼70 to 85% reduction in signal (SI Appendix, Fig. S2A), suggesting that more Cl− are present within the epithelium.
In CF, CFTR inhibition of ENaC is eliminated, resulting in increased Na+ transport across the epithelial cell membrane. In our previous study, we showed that up-regulation of ENaC activity causes a dramatic increase in the overall amount of intracellular Na+ in the intestinal epithelium (14). Therefore, we examined whether loss of CG5789 activity also leads to up-regulation of ENaC-mediated Na+ transport. To monitor the levels of intracellular Na+, we used a Na+-sensitive fluorescent indicator, Sodium Green (31, 32). Indeed, depletion of CG5789 resulted in higher levels of intracellular Na+ indicated by the increase in fluorescence (Fig. 1D). Quantification of Sodium Green fluorescence showed ∼4.5-fold increase in signal (SI Appendix, Fig. S2B), suggesting that these cells are allowing more Na+ across the epithelial membrane. Hereafter, all subsequent experiments were performed using the CG5789NIG RNAi line, since both the National Institute of Genetics (NIG) and Vienna Drosophila Resource Center (VDRC) RNAi lines show comparable phenotypes.
hCFTR Restores Cl− and Na+ Homeostasis in the Intestinal Epithelium.
If CG5789 is a true ortholog of hCFTR, expression of hCFTR in the presence of CG5789 RNAi may rescue the imbalance of Cl− and Na+ levels in the intestinal epithelium. To test the functional conservation between the CFTR orthologs, we generated a transgenic fly that expresses wild-type hCFTR (hCFTRWT). In addition, we generated transgenic flies that carry mutations that are commonly found in CF patients: two mutations that affect CFTR protein production (class I mutations: hCFTRY122X and hCFTRW1282X) and two mutations that affect CFTR protein transport (class II mutations: hCFTRN1303K and hCFTR∆F508). When expressed in ECs, hCFTRWT proteins are properly localized to the apical membrane of the epithelial cells (SI Appendix, Fig. S2C), whereas expression of mutant hCFTRs was not detected, which is expected since class I mutations fail to form functional CFTR proteins and class II mutations form misfolded proteins that are degraded by the ubiquitin–proteasome degradation pathway (33).
We next asked whether expression of hCFTR in the presence of CG5789 RNAi can reverse the buildup of Cl− inside the intestinal epithelium. We find that coexpression of hCFTRWT in the presence of CG5789 RNAi completely suppressed the phenotype, whereas coexpression of mutant hCFTR constructs failed to do so (Fig. 1C and SI Appendix, Fig. S2A). Consistent with our Cl− conductance results, coexpression of hCFTRWT was able to significantly reduce the amount of Na+ that entered the epithelium in the intestines of CG5789 RNAi flies, whereas mutant hCFTR constructs failed to do so (Fig. 1D and SI Appendix, Fig. S2B). Collectively, these results indicate that CG5789 is necessary for Cl− conductance across the intestinal epithelial cell membrane as well as to prevent increased influx of Na+, and that hCFTR can replace CG5789 function. Finally, since all four hCFTR mutations failed to rescue the imbalance of Cl− and Na+ levels, we chose to use the in-frame deletion of phenylalanine 508 (∆F508), a mutation that occurs in more than 70% of CF patients (34), as our negative control for all subsequent experiments.
Cell Swelling and Dehydration of Epithelial Membrane Phenotypes of CG5789 RNAi.
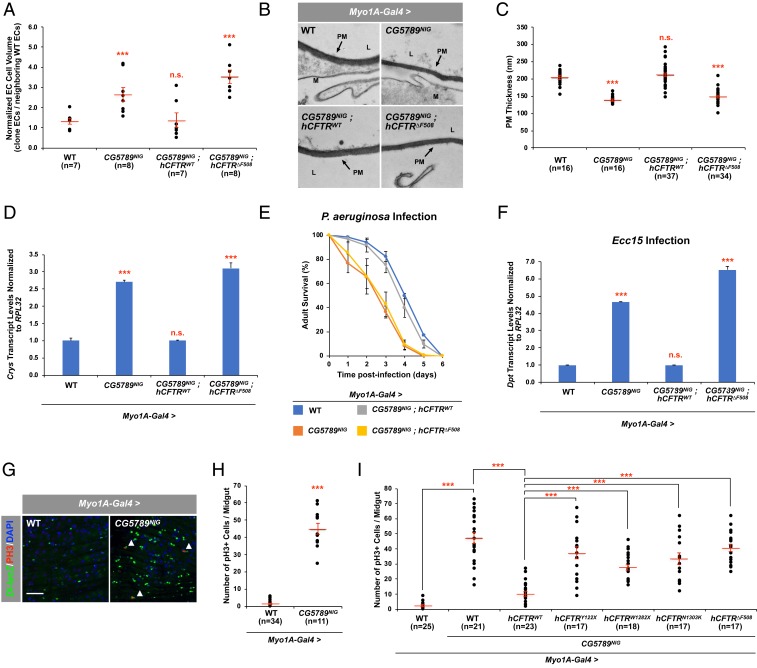
In CF patients, disruption in salt homeostasis is associated with disordered cell volume regulation in both the pulmonary and gastrointestinal tract (35–37), resulting in cell swelling. Likewise, we frequently observed large swollen ECs in the intestines of our ENaC-overexpression fly model (14). To determine whether impaired Cl− conductance in the absence of CG5789 also leads to swelling of ECs, we generated flip-out clones expressing CG5789 RNAi alone or in combination with the hCFTR constructs to measure cell volume. We find that knocking down CG5789 resulted in ECs that have nearly twice the cell volume compared to neighboring wild-type ECs (Fig. 2A). In addition, coexpression of hCFTRWT in the presence of CG5789 RNAi completely reversed the cell swelling phenotype, whereas coexpression of hCFTR∆F508 failed to do so (Fig. 2A).
Fig. 2.
Intestinal phenotypes in the absence of CG5789. (A) Quantitative measurements of the total EC cell volume. “n” denotes the number of cells of which total cell volume was measured for each genotype. (B) EM cross-sections of posterior midguts. Arrows indicate the PM, mucus (M), and lumen (L). (Scale bar, 800 nm.) (C) Quantitative measurements of the PM thickness. “n” denotes the number of PM thickness measurements for each genotype. (D) qPCR analysis of Crys using total RNA from dissected midguts of indicated genotypes. (E) Survival analysis of wild-type, CG5789 RNAi, and flies coexpressing hCFTRs upon oral infection with P. aeruginosa. Error bars indicate SEM. (F) qPCR analysis of Dpt using total RNA from dissected midguts of indicated genotypes 24 h after Ecc15 oral infection. (G) The posterior midguts of 7- to 10-d-old wild type and RNAi line against CG5789 stained anti–β-gal to mark Dl (green)-expressing ISCs and anti-pH3 to mark mitotic ISCs (red). White arrowheads mark mitotically active ISCs. (Scale bar, 50 μm.) (H) The average number of pH3+ cells in the posterior midguts expressing RNAi against CG5789. (I) The average number of pH3+ cells in the posterior midguts coexpressing wild-type or mutant hCFTRs with CG5789 RNAi. “n” denotes the number of posterior midguts examined for each genotype. Error bars indicate SEM. ***P < 0.001 (two-tailed t test). n.s., not significant.
An increase in uptake of Na+ and water by CF cells results in an abnormal mucus gel with an increased polymeric mucin concentration and altered biophysical properties in the epithelial surface (38, 39). Therefore, we examined the fly intestinal lumen and features of the peritrophic matrix (PM), which plays a role analogous to that of mucous membranes in the vertebrate digestive tract, for signs of dehydration. Interestingly, transmission electron microscopy of intestinal sections revealed that the thickness of the PM in CG5789 RNAi flies is significantly reduced and the phenotype can be completely suppressed by coexpressing hCFTRWT, whereas coexpression of hCFTR∆F508 failed to do so (Fig. 2 B and C).
To determine whether reduced PM production is responsible for the reduced PM thickness in the absence of CG5789, we measured the transcript levels of Crystallin (Crys), an integral component of the PM (40). We find that Crys levels were ∼2.5-fold higher when CG5789 is depleted compared to the control (Fig. 2D). In addition, coexpression of hCFTRWT in the presence of CG5789 RNAi completely suppressed the increased expression of Crys, whereas coexpression of hCFTR∆F508 failed to do so (Fig. 2D). These results suggest that decreased PM production is not the cause of reduced PM thickness but rather, EC swelling and reduced PM thickness result from increased influx of Na+ and water by the epithelium.
CG5789 Is Required for Defense Against Oral Bacterial Infection.
Because structurally compromising the PM is also associated with increased susceptibility to bacterial infections (14, 40), we asked whether loss of CG5789 results in increased susceptibility to bacterial infection using Pseudomonas aeruginosa, a major pathogen in the CF lung (41, 42). Oral infection of wild-type flies with P. aeruginosa causes both acute and chronic infection and the animals ultimately succumb to the infection (43, 44). Although the cause of death after chronic infection is likely dependent on other environmental factors, one study demonstrates a systemic bacteremia as the cause of death after intestinal damages (43). After oral infection, CG5789 RNAi flies exhibited a higher susceptibility than wild-type flies (Fig. 2E), which may be due to structurally compromised PM. However, as we measured survival and not hemolymph bacteria titer, we cannot rule out other factors that may contribute to the higher susceptibility observed in CG5789 RNAi flies, including formation of biofilms in the crop, as reported in flies infected with an environmental strain of P. aeruginosa (44). Consistent with our previous results, coexpression of hCFTRWT in the presence of CG5789 RNAi rescued the increased susceptibility phenotype, whereas coexpression of hCFTR∆F508 failed to do so (Fig. 2E).
The Imd pathway regulates antimicrobial peptide production in the gut and plays an important function in resistance to bacterial infections (45–47). This prompted us to investigate the effect of depleting CG5789 in the adult intestine on the Imd pathway by comparing the expression of Diptericin (Dpt), an antibacterial peptide gene tightly controlled by the Imd pathway after oral infection with the gram-negative bacterium Erwinia carotovora carotovora 15 (Ecc15). We chose Ecc15, as ingestion of this bacterium strongly induces the Imd pathway in the gut but does not kill the host (48). Real-time qPCR revealed that knocking down CG5789 leads to stronger induction of Dpt in the intestine upon oral infection with Ecc15. Coexpression of hCFTRWT in the presence of CG5789 RNAi completely suppressed this phenotype, whereas coexpression of hCFTR∆F508 failed to do so (Fig. 2F). In addition, knockdown of CG5789 in the absence of Ecc15 also leads to strong induction of Dpt (SI Appendix, Fig. S2D). Together, our results show that the structurally compromised PM of CG5789 RNAi flies allows for greater susceptibility to bacterial infection and leads to a stronger overall activation of the Imd pathway after oral infection.
CG5789 Is Required in EC to Maintain ISC Homeostasis.
Although the mechanism for the inhibition of ENaC activity by CFTR is not clear, up-regulation of ENaC activity in the ECs led to overproliferation of ISCs in the intestine (14). Therefore, we examined the ISC phenotype associated with CG5789 depletion in the ECs. We found that knockdown of CG5789 altered the basal number of ISCs in the adult intestine, as marked by increased Delta (Dl) expression (Fig. 2G). Consistent with these observations, staining with a mitotic marker, anti-phosphohistone H3 (pH3), revealed that the increased number of ISCs was due to an increase in proliferation when CG5789 was depleted (Fig. 2 G and H). Although coexpression of mutant hCFTRs failed to suppress the increased ISC proliferation phenotype of CG5789 RNAi, coexpression of hCFTRWT significantly reduced the number of pH3+ cells (Fig. 2I). Expression of hCFTRWT or all four mutant hCFTRs in an otherwise wild-type background did not perturb ISC homeostasis (SI Appendix, Fig. S3A). Interestingly, RNAi knockdown of three other Dmel\CFTR candidate genes, which are also expressed in fly intestine (49, 50), resulted in increased ISC proliferation as indicated by the increased number of pH3+ cells (SI Appendix, Fig. S3B). However, coexpression of hCFTRWT failed to suppress the increased ISC proliferation phenotype (SI Appendix, Fig. S3B), further suggesting that CG5789 is indeed a functional ortholog of hCFTR.
According to a Drosophila midgut transcriptome database (50), CG5789 is expressed broadly in all cell types, including in ISCs and progenitors (enteroblasts [EBs]) and differentiated epithelial cells (ECs and enteroendocrine cells [EEs]). To determine whether CG5789 is also required in the ISCs/progenitors and/or EEs to maintain ISC homeostasis, we performed RNAi against CG5789 using ISC/progenitor (Escargot-Gal4)- or EE (Tachykinin-gut-Gal4)-specific Gal4 drivers and measured ISC proliferation. Interestingly, depletion of CG5789 in neither ISCs/progenitors nor EEs increased the number of proliferating ISCs (SI Appendix, Fig. S3 C and D). Altogether, these results suggest that CG5789 is required in the ECs to maintain ISC homeostasis through maintenance of osmotic homeostasis, whereas it is dispensable in the other cell types.
We and others have previously shown that stress/damage triggered by cell swelling can activate the Jun N-terminal kinase (JNK) pathway (14, 51–53). Similarly, we find that CG5789 RNAi leads to activation of the JNK pathway that, in turn, activates the JAK/STAT and EGFR pathways, leading to intestinal hyperplasia (SI Appendix, Fig. S4 A–C). Consistent with our previous results, coexpression of the hCFTRWT transgene completely suppressed activation of the JNK pathway and the downstream developmental signaling pathways (SI Appendix, Fig. S4 A–C). Expression of hCFTR∆F508, however, failed to suppress these signaling pathways (SI Appendix, Fig. S4 A–C).
miR-263a Regulates ENaC and Osmotic Stress Downstream of Dmel\CFTR.
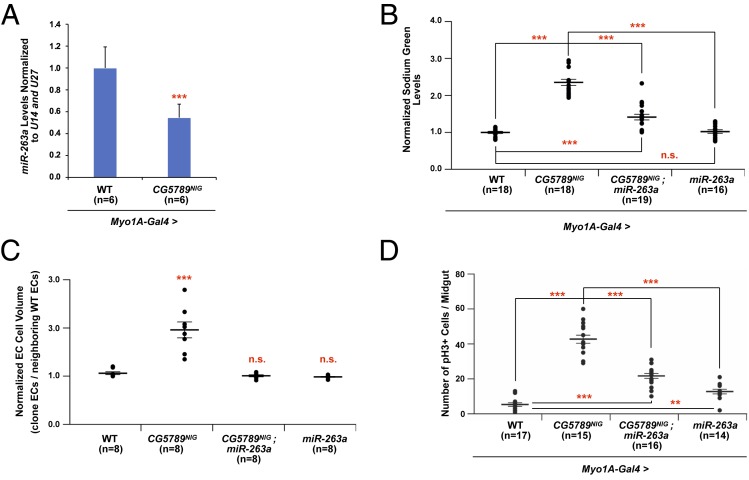
Mutations in CFTR not only alters chloride levels in the epithelia, but also has a profound effect on sodium uptake through up-regulation of ENaC by a not fully understood mechanism (7, 8). We previously identified miR-263a as a modulator of ENaC activity, that when lost recapitulates many CF phenotypes. Interestingly, miR-263a levels are reduced when CG5789 is depleted in the ECs (Fig. 3A). Therefore, we examined if the reduced levels of miR-263a in CG5789-depleted guts are responsible for the increased ENaC activity and Na+ influx into the ECs. When we overexpressed miR-263a in CG5789-depleted ECs and measured intracellular Na+, using the fluorescent indicator Sodium Green, we partially rescued the increased Na+ observed with loss of CG5789 activity (Fig. 3B). In addition, overexpression of miR-263a in CG5789-depleted cells was sufficient to rescue the cell swelling phenotype and was able to partially rescue the increased stem cell proliferation observed with loss of CG5789 (Fig. 3 C and D). Together these data suggest that miR-263a functions downstream of CG5789 to regulate ENaC activity to maintain osmotic and ISC homeostasis.
Fig. 3.
miR-263a regulates ENaC and osmotic stress downstream of Dmel\CFTR. (A) miR-263a levels are reduced when CG5789 is depleted compared to wild-type controls. (B) Fluorescent monitoring of intracellular Na+ levels in the midgut epithelium using Sodium Green. Overexpressing miR-263a in a CG5789-depleted background partially rescues increased fluorescence observed in absence of CG5789 compared to wild-type controls. (C) Quantitative measurements of the total EC cell volume. “n” denotes the number of cells of which total cell volume was measured for each genotype. (D) The number of pH3+ cells in the midguts of indicated genotype. Error bars indicate SEM. **P < 0.005 and ***P < 0.0005 two-tailed t test (A) or one-way ANOVA (B–D). n.s., not significant.
Global Gene Expression and Function Analysis of Dmel\CFTR Intestine Using RNA-Seq.
Pursuit of a one-time cure for CF using gene replacement and gene editing techniques has so far led to questionable clinical results (54, 55). In contrast, small molecules that correct or potentiate CFTR functions have shown clinical benefits, but only for patient with specific CFTR mutations (55), leaving a significant population of CF patients without any effective treatment options. Past CF twin sibling studies (56) and genome-wide association studies (GWAS) (57) have demonstrated that modifier genes contribute to the severity of the CF phenotypes, demonstrating the need to better understand the underlying pathophysiological mechanisms of CF. In an attempt to better understand the complex sequence of transcriptional events influenced by nonfunctional CFTR, we performed RNA-Seq-based transcriptomic analysis using Dmel\CFTR fly intestine.
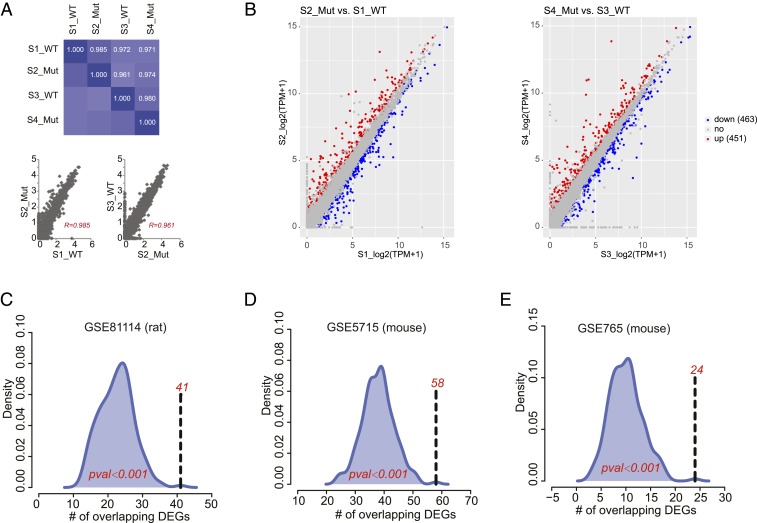
Gene expression patterns of the RNA-Seq revealed very high correlations between expression levels of any of two samples (Fig. 4A). Next, genes with high variance (SD > 0.5) were applied to cluster the samples. The results revealed that the samples were not clustered as their conditions, mutant vs. wild type, but clustered as some confounding factors, very likely due to bias from sequencing depth (SI Appendix, Fig. S5A). DESeq2, with samples having the similar sequencing depth paired, was applied to identify differentially expressed genes (DEGs) associated with CF in the intestine. The DEGs were called using P value <0.05 and fold-change ≥1.2 as the cutoff (Fig. 4B and Dataset S2). From our analysis, we identified 914 DEGs of which 451 genes were up-regulated and 463 genes were down-regulated.
Fig. 4.
RNA-Seq analysis of Dmel\CFTR intestine. (A) Pairwise Pearson correlation coefficient (R) of all samples calculated using log2(TPM) (transcript count per million) of 17,490 genes. (B) DEGs called using DESeq2 with P value <0.05 and fold-change ≥1.2 as the cutoff. (C–E) Overlaps between 914 DEGs in a fruit fly model and 151 overlapping DEGs in rat (GSE81114), and 120 (GSE5715) and 47 DEGs (GSE765) in mouse.
We next performed enrichment analyses to understand the biological processes that could be modified in Dmel\CFTR mutants (SI Appendix, Fig. S5B). For up-regulated DEGs, metabolic processes were selectively enriched (SI Appendix, Fig. S5C). More specifically, glutathione metabolism was selectively enriched in both gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses (SI Appendix, Fig. S5C). Interestingly, glutathione, a major antioxidant in the epithelial lung lining fluid, was found to be decreased in the apical fluid of CF airway epithelia due to reduced glutathione efflux (58), suggesting that Dmel\CFTR may also be necessary for glutathione efflux across the intestinal epithelium in flies. For down-regulated genes, proteolysis process was selectively enriched in the GO analysis (SI Appendix, Fig. S5D). In the case of nonresolving pathologies such as CF lung disease and chronic obstructive pulmonary disease, neutrophils are continuously recruited to the airways to release proteases and oxidants in an uncontrolled fashion, which leads to harmful oxidative stress (59–63). Although Drosophila species lack neutrophils, disruption of the proteolytic and redox processes may also cause harmful oxidative stress on the intestinal epithelium and consequently lead to CF-like phenotypes in the fly intestine. Detailed information about the enrichment functions by up-regulated or down-regulated DEGs can be found in Datasets S3 and S4, respectively.
Although expression patterns of many genes were altered in our dCFTR intestinal model, it is unclear how these changes compare to other established CF models. From 914 DEGs identified in our dataset, we identified 708 orthologous genes in rat and 710 orthologous genes in mouse. Using the hypothesis testing based on the random sampling technique, we observed statistically significant overlaps between the DEGs of Dmel\CFTR and all three mammalian datasets (Fig. 4 C–E). From these analyses, we identified 151 overlapping DEGs in rat (P < 0.001), and 120 (P < 0.001) and 47 DEGs (P < 0.001) in mouse. The detailed information of the overlapping DEGs can be found in Dataset S2. When we used the adjusted P value <0.1 as the cutoff, 448 DEGs were identified. The functions enriched by 448 DEGs were similar with those found by 914 DEGs (SI Appendix, Fig. S5 C and D). Statistically significant overlaps were still observed (Fig. 4 C–E).
Muc68D Is an Important Component of PM.
In advanced CF, airways show goblet cell hyperplasia, submucosal gland hypertrophy, and reduction in mucociliary transport (MCT), an important host defense mechanism that removes particulate from airways (64, 65). Previous reports have shown that excess mucus production and increased transcript levels of MUC5AC and MUC5B, major secreted gel-forming mucins in human airways (66, 67), are common in CF (16, 17). However, in Drosophila, besides Crys being an integral component of the PM (40), not much is known about the role of mucins in the formation of PM. Therefore, we decided to examine the role of mucin genes in the formation of PM and their role in CF pathophysiology using our fly CF model.
In the Drosophila genome, 17 mucin genes and 19 additional genes encoding mucin-related proteins have been identified (68), yet none of these genes have been studied in the context of the PM to date. Based on our RNA-Seq results, we identified four mucin (Muc14A, Muc68D, Muc55B, and Muc68E) and three mucin-related (Mur64D, Mur77A, and Mur18B) genes that were differentially expressed in CG5789 RNAi (fold change >1.2). Out of the 7 genes, only one mucin (Muc68D) and one mucin-like (Mur29B) genes are abundantly expressed in the adult intestine based on the Drosophila midgut transcriptome database (50). Since Muc68D is the only abundant gene found to be differentially expressed in CG5789 RNAi, we decided to investigate whether Muc68D is an integral component of PM and whether it has a role in CF pathophysiology.
Based on our RNA-Seq results, Muc68D transcript levels were significantly up-regulated (∼1.35-fold increase) in CG5789 RNAi (Dataset S1). Our qPCR analysis also confirmed the RNA-Seq results, revealing a ∼1.79-fold increase in its transcript levels (SI Appendix, Fig. S6A). Interestingly, we also find that Muc68D expression is significantly increased in CG5789 RNAi and hCFTR∆F508 coexpressing flies (SI Appendix, Fig. S6A). Furthermore, coexpression of hCFTRWT in the presence of CG5789 RNAi completely suppressed the increased expression of Muc68D as it did for Crys transcript levels (SI Appendix, Fig. S6A and Fig. 2D).
Upon Ecc15 infection, we also find that Crys levels are ∼17-fold higher when CG5789 is depleted after oral infection compared to the 2.5-fold increase we observed with no infection (SI Appendix, Fig. S6B and Fig. 2D). Similarly, Muc68D transcript levels were also significantly elevated after the infection when CG5789 was depleted (SI Appendix, Fig. S6B). Coexpression of hCFTRWT in the presence of CG5789 RNAi completely restored the transcript levels of both Crys and Muc68D to levels observed in wild-type animals (SI Appendix, Fig. S6B). However, coexpression of hCFTR∆F508 failed to restore the transcript levels of both genes (SI Appendix, Fig. S6B). These results are consistent with increased transcript levels of MUC5AC and MUC5B mucins in human CF airways (16, 17) as removing CG5789 from the intestine also results in increased transcript levels of Crys and Muc68D in both wild-type and infection conditions. Furthermore, the thickness of the PM in Muc68D RNAi flies is significantly reduced (SI Appendix, Fig. S6 C and D), suggesting that Muc68D is an essential component of the PM.
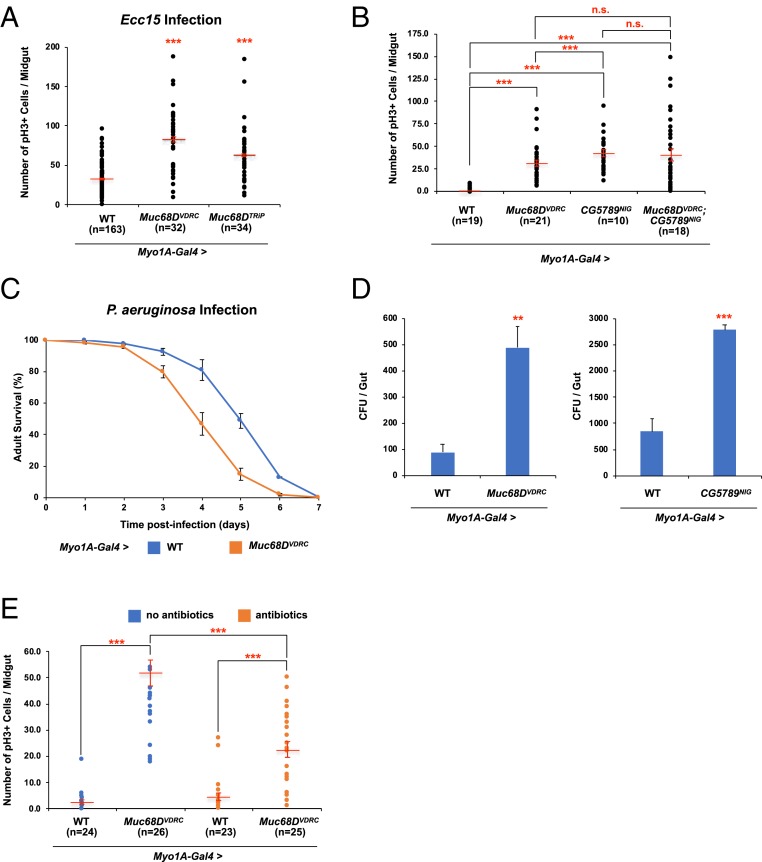
Muc68D Is Required for Maintaining ISC Homeostasis.
Since Muc68D is an integral component of PM, we asked whether knocking down Muc68D in wild-type flies can lead to greater susceptibility to bacterial infections, which consequently damages the intestinal epithelium and results in disruption of ISC homeostasis. Upon Ecc15 infection, Muc68D RNAi flies show an ∼2.6-fold increase (VDRC) and ∼2-fold increase (Transgenic RNAi Project [TRiP]) in pH3+ cell numbers compared to wild-type flies (Fig. 5A). Hereafter, all subsequent experiments were performed using the Muc68DVDRC RNAi line, since the VDRC RNAi line shows more robust pH3 phenotypes. Consistent with our results, we also find that knocking down Muc68D leads to stronger induction of Dpt in the intestine upon oral infection with Ecc15 (SI Appendix, Fig. S6E). Interestingly, in the absence of Ecc15 infection, we did not detect a significant induction of Dpt levels, suggesting that significant changes in the Dpt levels may require Ecc15 challenge in the Muc68D RNAi flies to be detected. However, we find that knockdown of Muc68D without Ecc15 infection also results in significant increase in pH3+ cell numbers (Fig. 5B), which is associated with activation of the JNK, JAK/STAT, and EGFR pathways (SI Appendix, Fig. S6 F–H). We suspect that decreased barrier protection in Muc68D RNAi intestine leads to activation of the Imd pathway that synergies with JNK signaling, as previously reported (69), to induce ISC proliferation.
Fig. 5.
Intestinal stem cell phenotypes of Muc68D RNAi. (A) The average number of pH3+ cells in the posterior midguts expressing RNAi against Muc68D 24 h after Ecc15 oral infection. (B) The average number of pH3+ cells in the posterior midguts expressing RNAi against Muc68D, CG5789, and both Muc68D and CG5789. (C) Survival analysis of wild-type and Muc68D RNAi flies upon oral infection with P. aeruginosa. Error bars indicate SEM. (D) Internal bacterial load significantly increased in Muc68D RNAi and CG5789 RNAi flies. (E) The average number of pH3+ cells in the posterior midguts expressing RNAi against Muc68D with and without antibiotics treatment. (A, B, and E) “n” denotes the number of posterior midguts examined for each genotype. Error bars indicate SEM. **P < 0.05 and ***P < 0.001 (two-tailed t test). n.s., not significant.
Muc68D RNAi also shows increased susceptibility to P. aeruginosa, which is consistent with our model that Muc68D is necessary to protect intestinal epithelium from exogenous insults as an essential component of PM (Fig. 5C). Interestingly, we also find that internal bacterial loads of Muc68D RNAi flies were ∼5.5-fold higher compared to the controls (Fig. 5D). In addition, we find that internal bacterial loads of CG5789 RNAi flies were ∼3.2-fold higher compared to the controls (Fig. 5D). To determine whether an inflammatory response due to increased internal bacterial load leads to disruption of ISC homeostasis in the Muc68D RNAi flies, we cultured control and Muc68D RNAi flies in food containing antibiotics, which eliminated all internal bacteria. Treatment with antibiotics significantly reduces the pH3+ cell numbers in Muc68D RNAi flies (Fig. 5E), indicating that structurally compromised PM leads to increased bacterial accumulation resulting in the activation of the Imd pathway and increased ISC proliferation.
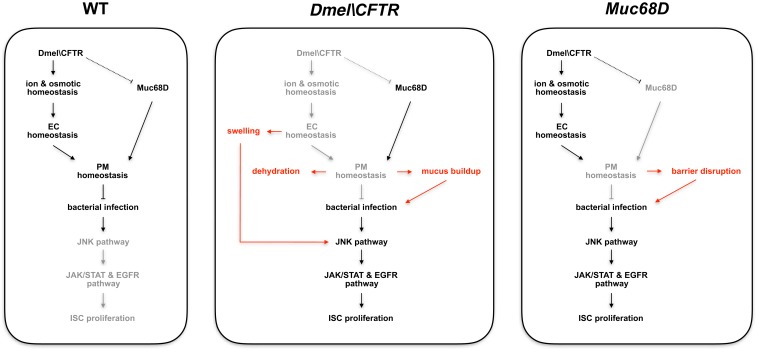
Since reduction of Dmel\CFTR leads to increase in Muc68D transcript levels in the intestine, we tested whether knocking down Muc68D in the presence of CG5789 RNAi can suppress the increased pH3+ cell numbers. Knocking down the levels of Muc68D in the CG5789 RNAi failed to suppress the increase in pH3+ cell number (Fig. 5B), suggesting that increased expression of Muc68D may be an indirect consequence of ionic and osmotic homeostatic disruption. Based on our results, our current model is that, while knockdown of Muc68D in the absence of Dmel\CFTR may reduce the increased transcription of Muc68D, the swelling of ECs caused by disruption of ionic and osmotic homeostasis can still lead to increased ISC proliferation (Fig. 6). Although the exact mechanism of Muc68D transcriptional regulation is still unknown, mucin response to nonfunctional CFTR seems similar between fly and mammalian CF models, as increased MUC5AC and MUC5B transcript levels are common in CF (16, 17).
Fig. 6.
Fly intestinal model of CF. Proposed model for the regulation of PM homeostasis, which is required for protection against pathogenic infections. Texts and arrows highlighted in red are consequences of having specific mutation as labeled.
Discussion
In this study, we identify a Drosophila transporter of the ABC superfamily that functions as a putative CFTR and refer to it as Dmel\CFTR. We show that expression of hCFTRWT fully rescues the CF-like phenotypes of CG5789 RNAi flies, whereas mutant hCFTRs failed to rescue, indicating that both are functional orthologs. Knockdown of Dmel\CFTR, CG5789, in the adult fly intestine disrupts osmotic homeostasis that activates a stress response that, in turn, activates signaling pathways required for ISC proliferation, resulting in intestinal hyperplasia. Knockdown of CG5789 impairs Cl− efflux across the intestinal epithelium, resulting in increased Na+ and water influx that leads to swelling of ECs and dehydration of the extracellular PM, which is analogous to mucous secretions in the vertebrate digestive tract. Depletion of intraluminal surface liquid increases mucus viscosity and impairs mucus clearance, which traps invading bacteria in the intestine and lungs of CF patients. This inability to clear bacteria, especially in the lungs, causes chronic infection and inflammation that can ultimately lead to death (5, 6). Consistent with this, we find greater susceptibility to bacterial infection, evident by the decreased viability after P. aeruginosa infection, and increased activation of the Imd pathway in the CG5789 RNAi lines after Ecc15 infection. Using Dmel\CFTR intestine, we performed RNA-Seq and identified Muc68D as an essential component of PM required for proper barrier protection against pathogens. We also find that increased expressions of Muc68D in Dmel\CFTR knockdown are similar to the response of several mucin genes observed in CF models. Consistent with our previous report that EC swelling can activate the JNK pathway (14), we find that CG5789 RNAi leads to activation of the JNK pathway that, in turn, activates the JAK/STAT and EGFR pathways, leading to intestinal hyperplasia. We also find that loss of Muc68D leads to increased accumulation of bacteria that activates the Imd pathway and disrupts ISC homeostasis.
Our results highlight the importance of maintaining proper barrier protection, as disrupting the balance of mucin production may exacerbate the severity of the disease such as CF. As the most common symptom of CF includes accumulation of viscous mucus in the pulmonary and gastrointestinal tract, which is associated with bacterial infections, aberrant inflammation, and malnutrition (5, 6), better understanding of mucin gene regulation is of great interest. Although we cannot address the specific questions about the role of MUC5AC and MUC5B in CF pathophysiology using our fly CF model system, we were able to show that Muc68D functions in an analogous mechanism that can be further exploited to better understand the role of mucins in the progression of CF.
CF is generally thought of as a lung disease since much of the associated morbidity and mortality is related to pulmonary complications. Indeed, lung transplantation and improved pulmonary care have improved the survival of CF patients. However, gastrointestinal complications have become an increasingly important cause of morbidity in patients with CF, in part because of improved life expectancy. As CF patients live well into adulthood, later complications from the disease, such as small-bowel bacterial overgrowth, bowel obstructions, and an increased incidence of intestinal cancers, have become a major problem. A study released in 2013 of more than 40,000 patients from 250 US CF centers showed an increased risk of colon cancer and small-bowel cancer (70). In addition, increased incidences of polyp detection and progression after age 40 have been observed (71, 72), suggesting that enhanced colon cancer screening procedures will be required as long-term patient survival rates improve. Interestingly, our fly model of CF recapitulates increased proliferation of crypt cells observed in CF mice (73). We find that knocking down Dmel\CFTR leads to increased ISC proliferation, which can be suppressed when hCFTR is coexpressed. Given its phenotypic similarities to the gastrointestinal manifestations of CF, and as the Drosophila gut is amenable to large-scale small molecule screens (74), our fly CF model may provide a cost-effective and high-throughput animal model for identifying therapeutic treatments that can rescue the activity of CFTR in vivo. There are many advantages to using Drosophila as a model organism for performing whole-animal in vivo drug screens. First, it is estimated that ∼75% of disease-related genes in humans have functional orthologs in the fly (22). The overall sequence identity at the nucleotide or protein levels between fly and mammal is ∼40% between homologs. However, in conserved functional domains, similarities can be 80 to 90% or higher (75, 76). Second, the adult fly has structures that perform the equivalent functions of the mammalian heart, lung, kidney, gut, liver, and reproductive tract. Third, about 60 compounds that were originally known for their activity in human cells were later shown to have the same molecular mechanism of action in Drosophila, which includes: actin and microtubule poisons; inhibitors of DNA topoisomerases, kinases, and phosphatases; alkylating agents; and modulators of membrane channels (77). This observation demonstrates that small compounds identified for their activity in flies may indeed work equally well in mammals, since the reverse is demonstrably true. Lastly, a Drosophila model provides powerful genetics, highly conserved disease pathways, and very low comparative costs, which are all excellent features for whole-animal high-throughput drug screens and validation studies. Moreover, although primates and other large mammals will continue to be the gold standard for assessing efficacy and safety in the late stages of drug development, model systems like Drosophila can help to test the efficacy and safety of thousands of compounds in the earlier drug discovery stage. Finally, our fly model will contribute significantly to genetically dissecting CF pathogenesis through large-scale genetic screens that are more feasible in flies than in vertebrate models.
Data Availability.
All data are available within this report and the associated SI Appendix.
Materials and Methods
Detailed materials and methods, including Drosophila stocks and genetics, immunostaining of midgut, generation of hCFTR transgenic flies, sequence conservation analysis, MQAE and Sodium Green assays, quantification of EC volume, electron microscopy, bacterial oral infection assays, qPCR, processing of RNA-Seq data, and DEG analysis can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank the TRiP, VDRC, NIG (Japan), Bloomington Drosophila Stock Center for flies, and the Developmental Studies Hybridoma Bank for monoclonal antibodies. We thank R.-J. Hung for providing the P. aeruginosa, W. Song for providing Ecc15, and P. J. Thomas for providing pCMV-CFTR-pBQ6.2. We thank M. Ericsson at the Harvard Medical School Electron Microscopy Facility for her assistance with electron microscopy imaging. We thank B. Ewen-Campen for his assistance with Dmel\CFTR sequence conservation analysis and critical comments on the manuscript. We also thank S. Mohr, L. Rabinow, and M.-L. Samson for critical comments on the manuscript. This work was supported in part by the NIH (N.P.). N.P. is a Howard Hughes Medical Institute investigator.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913127117/-/DCSupplemental.
References
- 1.Cutting G. R., Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 16, 45–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory R. J., et al. , Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature 347, 382–386 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Riordan J. R., et al. , Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 245, 1066–1073 (1989). [DOI] [PubMed] [Google Scholar]
- 4.Moran O., The gating of the CFTR channel. Cell. Mol. Life Sci. 74, 85–92 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grubb B. R., Gabriel S. E., Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am. J. Physiol. 273, G258–G266 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Mall M., Grubb B. R., Harkema J. R., O’Neal W. K., Boucher R. C., Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 10, 487–493 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Berdiev B. K., Qadri Y. J., Benos D. J., Assessment of the CFTR and ENaC association. Mol. Biosyst. 5, 123–127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhalla V., Hallows K. R., Mechanisms of ENaC regulation and clinical implications. J. Am. Soc. Nephrol. 19, 1845–1854 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Snouwaert J. N., et al. , An animal model for cystic fibrosis made by gene targeting. Science 257, 1083–1088 (1992). [DOI] [PubMed] [Google Scholar]
- 10.Rogers C. S., et al. , Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321, 1837–1841 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X., et al. , Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Invest. 120, 3149–3160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navis A., Marjoram L., Bagnat M., Cftr controls lumen expansion and function of Kupffer’s vesicle in zebrafish. Development 140, 1703–1712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuggle K. L., et al. , Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One 9, e91253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K., Hung R. J., Perrimon N., miR-263a regulates ENaC to maintain osmotic and intestinal stem cell homeostasis in Drosophila. Dev. Cell 40, 23–36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Z., et al. , The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J. Cyst. Fibros. 10 (suppl. 2), S172–S182 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Henderson A. G., et al. , Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J. Clin. Invest. 124, 3047–3060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauber H. P., et al. , Expression of HCLCA1 in cystic fibrosis lungs is associated with mucus overproduction. Eur. Respir. J. 23, 846–850 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Dassa E., Bouige P., The ABC of ABCS: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 152, 211–229 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Dean M., Annilo T., Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 6, 123–142 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Gadsby D. C., Vergani P., Csanády L., The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440, 477–483 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian A., et al. , Origin and evolution of the cystic fibrosis transmembrane regulator protein R domain. Gene 523, 137–146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y., et al. , An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics 12, 357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng S. H., et al. , Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell 66, 1027–1036 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Chang X. B., et al. , Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J. Biol. Chem. 268, 11304–11311 (1993). [PubMed] [Google Scholar]
- 25.Rich D. P., et al. , Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by negative charge in the R domain. J. Biol. Chem. 268, 20259–20267 (1993). [PubMed] [Google Scholar]
- 26.Liu F., Zhang Z., Csanady L., Gadsby D. C., Chen J., Molecular structure of the human CFTR ion channel. Cell 169, 85–95.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Doyle D. A., et al. , The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Dutzler R., Campbell E. B., Cadene M., Chait B. T., MacKinnon R., X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Biasini M., et al. , SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verkman A. S., Sellers M. C., Chao A. C., Leung T., Ketcham R., Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal. Biochem. 178, 355–361 (1989). [DOI] [PubMed] [Google Scholar]
- 31.Minta A., Tsien R. Y., Fluorescent indicators for cytosolic sodium. J. Biol. Chem. 264, 19449–19457 (1989). [PubMed] [Google Scholar]
- 32.Amorino G. P., Fox M. H., Intracellular Na+ measurements using sodium green tetraacetate with flow cytometry. Cytometry 21, 248–256 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Ward C. L., Omura S., Kopito R. R., Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83, 121–127 (1995). [DOI] [PubMed] [Google Scholar]
- 34.Watson M. S., et al. , Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet. Med. 6, 387–391 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valverde M. A., et al. , Impaired cell volume regulation in intestinal crypt epithelia of cystic fibrosis mice. Proc. Natl. Acad. Sci. U.S.A. 92, 9038–9041 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidler U., et al. , Na+/HCO3- cotransport in normal and cystic fibrosis intestine. JOP 2 (suppl. 4), 247–256 (2001). [PubMed] [Google Scholar]
- 37.Vázquez E., Nobles M., Valverde M. A., Defective regulatory volume decrease in human cystic fibrosis tracheal cells because of altered regulation of intermediate conductance Ca2+-dependent potassium channels. Proc. Natl. Acad. Sci. U.S.A. 98, 5329–5334 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boucher R. C., Cystic fibrosis: A disease of vulnerability to airway surface dehydration. Trends Mol. Med. 13, 231–240 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Button B., et al. , A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuraishi T., Binggeli O., Opota O., Buchon N., Lemaitre B., Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 108, 15966–15971 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohman D. E., Chakrabarty A. M., Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect. Immun. 37, 662–669 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer K. L., Mashburn L. M., Singh P. K., Whiteley M., Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 187, 5267–5277 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Limmer S., et al. , Pseudomonas aeruginosa RhlR is required to neutralize the cellular immune response in a Drosophila melanogaster oral infection model. Proc. Natl. Acad. Sci. U.S.A. 108, 17378–17383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulcahy H., Sibley C. D., Surette M. G., Lewenza S., Drosophila melanogaster as an animal model for the study of Pseudomonas aeruginosa biofilm infections in vivo. PLoS Pathog. 7, e1002299 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liehl P., Blight M., Vodovar N., Boccard F., Lemaitre B., Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2, e56 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nehme N. T., et al. , A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3, e173 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryu J. H., et al. , An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J. 25, 3693–3701 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchon N., Broderick N. A., Poidevin M., Pradervand S., Lemaitre B., Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Doupé D. P., Marshall O. J., Dayton H., Brand A. H., Perrimon N., Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals. Proc. Natl. Acad. Sci. U.S.A. 115, 12218–12223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dutta D., et al. , Regional cell-specific transcriptome mapping reveals regulatory complexity in the adult Drosophila midgut. Cell Rep. 12, 346–358 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Berl T., Siriwardana G., Ao L., Butterfield L. M., Heasley L. E., Multiple mitogen-activated protein kinases are regulated by hyperosmolality in mouse IMCD cells. Am. J. Physiol. 272, F305–F311 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Sinning R., Schliess F., Kubitz R., Häussinger D., Osmosignalling in C6 glioma cells. FEBS Lett. 400, 163–167 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Huangfu W. C., Omori E., Akira S., Matsumoto K., Ninomiya-Tsuji J., Osmotic stress activates the TAK1-JNK pathway while blocking TAK1-mediated NF-kappaB activation: TAO2 regulates TAK1 pathways. J. Biol. Chem. 281, 28802–28810 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burney T. J., Davies J. C., Gene therapy for the treatment of cystic fibrosis. Appl. Clin. Genet. 5, 29–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooney A. L., McCray P. B. Jr, Sinn P. L., Cystic fibrosis gene therapy: Looking back, looking forward. Genes (Basel) 9, E538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mekus F., et al. , Categories of deltaF508 homozygous cystic fibrosis twin and sibling pairs with distinct phenotypic characteristics. Twin Res. 3, 277–293 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Corvol H., et al. , Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun. 6, 8382 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao L., Kim K. J., Yankaskas J. R., Forman H. J., Abnormal glutathione transport in cystic fibrosis airway epithelia. Am. J. Physiol. 277, L113–L118 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Galli F., et al. ; Working Group on Inflammation in Cystic Fibrosis , Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim. Biophys. Acta 1822, 690–713 (2012). [DOI] [PubMed] [Google Scholar]
- 60.Cantin A. M., et al. , Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic. Biol. Med. 42, 15–31 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirkham P. A., Barnes P. J., Oxidative stress in COPD. Chest 144, 266–273 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Rahman I., Adcock I. M., Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 28, 219–242 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Hartl D., et al. , Innate immunity in cystic fibrosis lung disease. J. Cyst. Fibros. 11, 363–382 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Widdicombe J. H., Wine J. J., Airway gland structure and function. Physiol. Rev. 95, 1241–1319 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Robinson M., Bye P. T., Mucociliary clearance in cystic fibrosis. Pediatr. Pulmonol. 33, 293–306 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Hovenberg H. W., Davies J. R., Carlstedt I., Different mucins are produced by the surface epithelium and the submucosa in human trachea: Identification of MUC5AC as a major mucin from the goblet cells. Biochem. J. 318, 319–324 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davies J. R., Svitacheva N., Lannefors L., Kornfält R., Carlstedt I., Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem. J. 344, 321–330 (1999). [PMC free article] [PubMed] [Google Scholar]
- 68.Syed Z. A., Härd T., Uv A., van Dijk-Härd I. F., A potential role for Drosophila mucins in development and physiology. PLoS One 3, e3041 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhai Z., Boquete J. P., Lemaitre B., Cell-specific Imd-NF-kappaB responses enable simultaneous antibacterial immunity and intestinal epithelial cell shedding upon bacterial infection. Immunity 48, 897–910.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Maisonneuve P., Marshall B. C., Knapp E. A., Lowenfels A. B., Cancer risk in cystic fibrosis: A 20-year nationwide study from the United States. J. Natl. Cancer Inst. 105, 122–129 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Billings J. L., et al. , Early colon screening of adult patients with cystic fibrosis reveals high incidence of adenomatous colon polyps. J. Clin. Gastroenterol. 48, e85–e88 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Niccum D. E., Billings J. L., Dunitz J. M., Khoruts A., Colonoscopic screening shows increased early incidence and progression of adenomas in cystic fibrosis. J. Cyst. Fibros. 15, 548–553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gallagher A. M., Gottlieb R. A., Proliferation, not apoptosis, alters epithelial cell migration in small intestine of CFTR null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G681–G687 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Markstein M., et al. , Systematic screen of chemotherapeutics in Drosophila stem cell tumors. Proc. Natl. Acad. Sci. U.S.A. 111, 4530–4535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiter L. T., Potocki L., Chien S., Gribskov M., Bier E., A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 11, 1114–1125 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lloyd T. E., Taylor J. P., Flightless flies: Drosophila models of neuromuscular disease. Ann. N. Y. Acad. Sci. 1184, e1–e20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernández-Hernández I., Scheenaard E., Pollarolo G., Gonzalez C., The translational relevance of Drosophila in drug discovery. EMBO Rep. 17, 471–472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within this report and the associated SI Appendix.