Abstract
Postpartum depression (PPD) is a unique subtype of major depressive disorder and a substantial contributor to maternal morbidity and mortality. In addition to affecting the mother, PPD can have short- and long-term consequences for the infant and partner. The precise etiology of PPD is unknown, but proposed mechanisms include altered regulation of stress response pathways, such as the hypothalamic-pituitary-adrenal axis, and dysfunctional gamma-aminobutyric acid (GABA) signaling, and functional linkages exist between these pathways. Current PPD pharmacotherapies are not directly related to these proposed pathophysiologies. In this review, we focus on the potential role of GABAergic signaling and the GABAA receptor positive allosteric modulator allopregnanolone in PPD. Data implicating GABAergic signaling and allopregnanolone in PPD are discussed in the context of the development of brexanolone injection, an intravenous formulation of allopregnanolone recently approved by the United States Food and Drug Administration for the treatment of adult women with PPD.
Keywords: Postpartum depression, Brexanolone injection, GABA, Allopregnanolone
1. Introduction
Major depressive disorder is one of the greatest causes of disease-related disability world-wide, and the rate of depression in women is twice that of men (Baxter et al., 2014; Whiteford et al., 2013). Postpartum depression (PPD) is a particularly common form of major depressive disorder, impacting an estimated 11.5% of new mothers in the US each year, and it is associated with substantial morbidity and mortality (Ko et al., 2017). Under- or un-treated PPD can result in multiple short- and long-term adverse outcomes for the mother, baby, and the family, and sudden and severe adverse outcomes, such as suicide and infanticide, demonstrate the need for rapid resolution of PPD symptoms (Bodnar-Deren et al., 2016; Lawrence et al., 2017; Oates, 2003; Shadigian and Bauer, 2005). PPD is associated with an increased risk for self-harm or suicidal ideation, and suicide is currently the leading cause of pregnancy-related maternal death (Bodnar-Deren et al., 2016; Oates, 2003; Shadigian and Bauer, 2005; Gressier et al., 2017). PPD can also interfere with the mother-infant dyad and negatively affect the infant's physical, mental, and emotional development, with adult children of women with PPD showing greater rates of psychological and behavioral difficulties (Balbierz et al., 2015; Netsi et al., 2018; Umboh et al., 2013; Yamaoka et al., 2016; Verkuijl et al., 2014; Woolhouse et al., 2016; Pearson et al., 2013; Koutra et al., 2013; Surkan et al., 2014; Valla et al., 2016). The PPD patient's partner is also at-risk, with 24–50% of partners experiencing paternal depression (Woolhouse et al., 2016; Goodman, 2004).
Although the time of PPD onset may differ across organization guidelines and definitions, all organizations require a diagnosis of a major depressive episode temporally related to pregnancy and childbirth. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), the diagnosis of a major depressive episode requires at least one of the first two following symptoms and at least five symptoms overall during a single two-week period: depressed mood, loss of interest or pleasure in all/almost all activities, weight loss, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue, feelings of worthlessness or guilt, diminished ability to think/concentrate, and recurrent thoughts of death/suicidal ideation (Force APAD-T, 2013). Notably, the specification for a duration of longer than two weeks differentiates PPD from the “baby blues,” which are experienced by 80% of women after childbirth and resolve within two weeks (Earls et al., 2010; Health NIoM, 2019). DSM-5 indicates that major depressive episodes during pregnancy through 4 weeks postpartum can receive a peripartum specifier, in recognition of potential onset during pregnancy or after childbirth (Force APAD-T, 2013). However, PPD is often defined by more expansive definitions, such as those used by the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, Centers for Disease Control and Prevention, and World Health Organization, which suggest that PPD can occur through one year postpartum (Force APAD-T, 2013; Earls et al., 2010; ACOG, 2015, 2018; Stewart et al., 2003; Centers for Disease C and Prevention, 2008).
There are multiple proposed etiologies for PPD, including hormonal changes during pregnancy, hypothalamic-pituitary axis (HPA) axis activation, dysfunctional gamma-aminobutyric acid (GABA) signaling, inflammation, and several others (Bloch et al., 2003; Licheri et al., 2015; Melon et al., 2018a; Osborne and Monk, 2013). In this review, we focus on the involvement of GABAergic signaling in PPD, with a particular focus on the potential role of the endogenous neuroactive steroid allopregnanolone, a potent positive allosteric modulator (PAM) of synaptic and extra-synaptic GABAA receptors (GABAARs). In addition, we highlight the intersections of allopregnanolone and GABAergic signaling with stress pathways implicated in PPD, such as the HPA axis. The potential development and application of GABAAR PAMs for PPD therapy are also discussed.
2. Etiology of PPD: the role of GABA signaling and stress
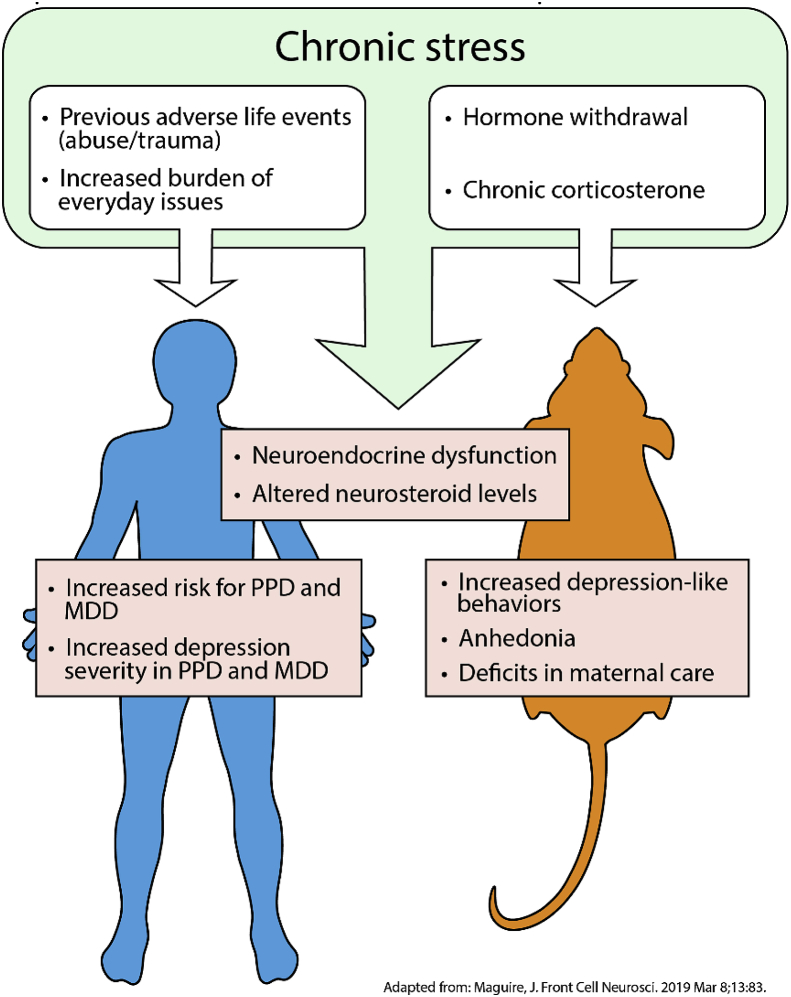
PPD is a unique form of a major depressive episode, but its underlying pathophysiology is thought to share some features with major depressive episodes outside the perinatal period (Maguire, 2019). For example, the development of major depressive disorder (MDD) is highly related to acute, chronic, and even past stress, and MDD is associated with the activation of stress pathways, including the HPA axis (Maguire, 2019). PPD development is similarly associated with certain life stressors, such as lack of social support, marital/relationship status, socioeconomic status, intimate partner violence, childhood or adult trauma, and infant health issues (Biaggi et al., 2016; Goyal et al., 2010; Hawes et al., 2016; Howard et al., 2013; Yim et al., 2015; Swendsen and Mazure, 2000), which can have long-term impacts on the regulation of the HPA axis in the postpartum period (Brand et al., 2010; Laurent et al., 2018; Meinlschmidt et al., 2010) (Fig. 1). In addition to impacting the development of PPD, stress also influences the severity of PPD symptoms (Swendsen and Mazure, 2000). Although stressors occurring in the postpartum period have been reported to have the strongest association with PPD, stressors that contribute to the development of PPD can occur at any time during a woman's life (O'Hara et al., 1984; Paykel et al., 1980).
Fig. 1.
Stress is associated with the development of depression in humans and in animal models of depression. Similar stressors have been reported for MDD and PPD.
Despite many similarities across the mechanisms of disease for PPD and MDD, these forms of depression remain fundamentally distinct from one another due to the unique physiology associated with pregnancy and childbirth. Pregnancy is typically associated with a blunting of the stress-induced activation of the HPA axis (Brunton et al., 2008; Schulte et al., 1990), and the inability to suppress the HPA axis has been proposed to play a role in the development of PPD (Bloch et al., 2003). Additionally, there are multiple neuroendocrine changes that may play a role in PPD development, and recreating hormonal changes in women with a history of PPD can produce symptoms of depression (Bloch et al., 2000). Changes in the regulation of GABAergic signaling have also been observed, including alterations in the expression of GABAAR subunits (Licheri et al., 2015) and of neuroactive steroid GABAAR positive allosteric modulators (PAMs) (Luisi et al., 2000; Paoletti et al., 2006; Pennell et al., 2015).
In support of these proposed pathophysiologies, PPD-like behaviors can be elicited in a variety of rodent models, including both wild-type and genetically-modified strains. Supporting the role of stress and the HPA axis in PPD, administration of high doses of corticosterone to mice in the postpartum period results in reduced nesting and nursing time and depression-like behaviors in forced-swim and open field tests (Brummelte and Galea, 2010). Stress from repeated long separations of rat dams from their pups can also result in forced swim test immobility (Boccia et al., 2007). Genetically-modified mice have provided support for the role of GABAergic signaling in PPD. Mice deficient in the GABAAR delta subunit exhibit PPD-like behaviors and the inability to suppress stress-induced HPA axis activation (Melon et al., 2018a; Maguire and Mody, 2008). The potassium/chloride transporter KCC2, which maintains the intracellular/extracellular chloride gradient required for GABAAR signaling (Payne et al., 2003; Rivera et al., 1999, 2005), is critical for GABAergic regulation of the paraventricular nucleus of the hypothalamus and the HPA axis, and deletion of KCC2 from corticosterone-releasing hormone neurons also results in PPD-like behaviors (Melon et al., 2018a).
In both humans and animal models, fluctuations in levels of neuroactive steroids such as allopregnanolone, which are PAMs of GABAARs in phasic and tonic signaling, have been observed throughout pregnancy and the postpartum period. Circulating levels of allopregnanolone rise dramatically through the progression of pregnancy, and decrease precipitously after childbirth (Luisi et al., 2000; Paoletti et al., 2006; Pennell et al., 2015). Allopregnanolone is a metabolite of progesterone, and its levels mimic progesterone during and after pregnancy, further linking allopregnanolone to the role of hormonal regulation in PPD (Luisi et al., 2000). Behaviorally, alterations in peripartum levels of neuroactive steroids have been associated with depressive symptoms in women at risk for PPD (Luisi et al., 2000; Deligiannidis et al., 2016, 2019a). Postpartum allopregnanolone levels are also positively correlated with altered functional connectivity in the default mode network in the brains of PPD patients, supporting the connection between behavioral changes in PPD and neuroactive steroid levels (Deligiannidis et al., 2019a). In support of the role of hormonal regulation, previous studies have demonstrated that in women with a history of PPD, abrupt withdrawal of progesterone and estrogen is associated with a significant increase in cortisol levels (i.e. HPA axis activation) and depressive symptoms (Bloch et al., 2000).
Notably, the observation that only women with a prior history of PPD demonstrated increases in depressive symptoms and cortisol levels following withdrawal of gonadal hormones suggest that although stress signaling may not be the primary mechanism for PPD development, it may contribute to the pathophysiological milieu in PPD – highlighting the complex interplay between these connected systems (Bloch et al., 2000).
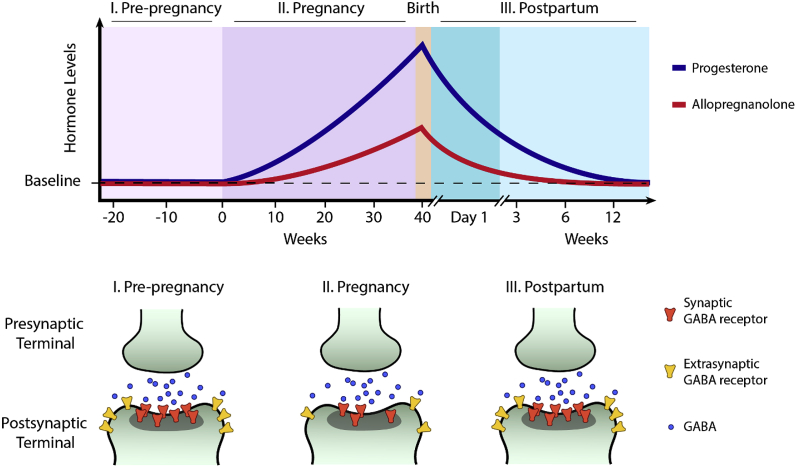
Multiple lines of evidence also offer further support for the link between GABAergic signaling and PPD. In animal models, fluctuations in the expression and function of GABAAR subunits have been observed during pregnancy and the postpartum period (Licheri et al., 2015; Maguire and Mody, 2008; Mostallino et al., 2009) (Fig. 2). Specifically, decreased GABAergic inhibition due to downregulation of the δ and ɣ2 GABAAR subunits has been observed during pregnancy in mice, and levels of these receptor subunits rebounded following birth (Maguire and Mody, 2008). Genetic deletion of the GABAAR δ subunit, or KCC2, a potassium-chloride cotransporter involved in GABAergic signaling, produce PPD-like symptoms and maternal behaviors in mice, and these behavioral changes can manifest in decreased survival of pups (Melon et al., 2018a; Maguire and Mody, 2008). There are also suggestions of links between allopregnanolone and KCC2 expression and KCC2 expression and surface expression of certain GABAARs, although these have not been specifically examined in PPD/PPD model systems (Kuver et al., 2012; Modol et al., 2014).
Fig. 2.
GABAergic signaling in the perinatal period may be altered by fluctuations in neuroactive steroid levels and changes in the expression of GABAAR subunits. Altered expression of GABAAR subunits could alter both the number and localization of GABAARs.
The hypothesis of GABA and allopregnanolone involvement in PPD also has important links to a number of other proposed mechanisms of PPD pathophysiology. For example, it is well established that GABAergic signaling controls the HPA axis at the level of corticotrophin-releasing hormone neurons in the paraventricular nucleus in the hypothalamus (Decavel and Van den Pol, 1990; Decavel and van den Pol, 1992) (Fig. 3)
Fig. 3.
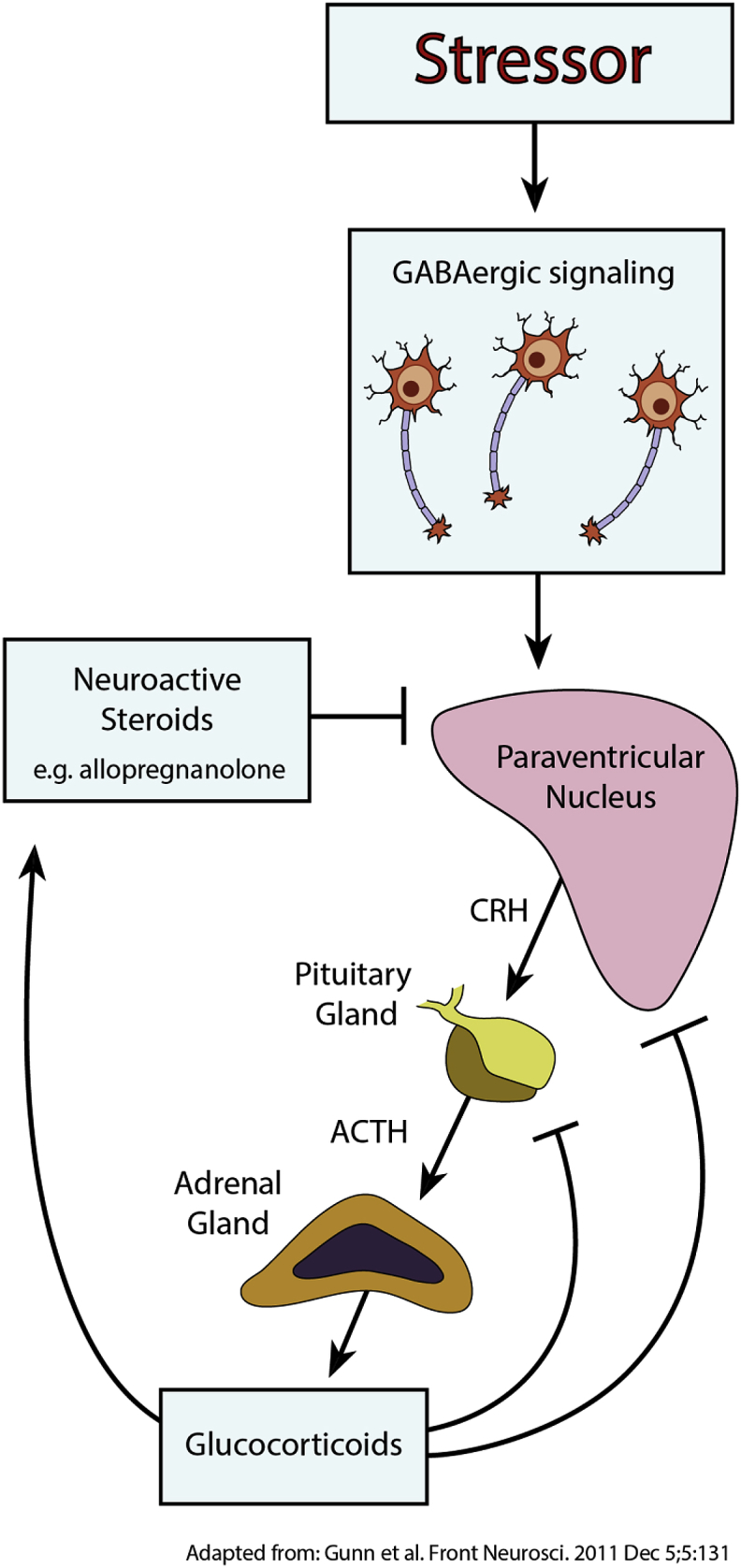
GABAergic signaling and neuroactive steroids with GABAAR positive allosteric modulator activity can act on the paraventricular nucleus of the hypothalamus to regulate the HPA axis.
Direct links between GABAergic signaling and the HPA axis have been reported in mice deficient in the GABAAR delta subunit or KCC2, both of which lack the ability to suppress stress-induced inactivation of the HPA axis and effectively model PPD-like symptoms and behaviors (Melon et al., 2018a). Furthermore, treatment of these mice with a synthetic neuroactive steroid GABAAR PAM decreases PPD-like behaviors and mitigates stress-based phenotypes associated with activation of the HPA axis (Melon et al., 2018b).
Beyond functional linkage of GABA signaling and the HPA axis, multiple observations also connect GABA signaling and allopregnanolone to stress responses. GABA levels and GABAergic signaling have been reported to be altered in the hypothalamus following acute stress, and levels of allopregnanolone have been shown to increase in response to acute stress as well (Acosta et al., 1993; Miller et al., 1987; Yoneda et al., 1983; Droogleever Fortuyn et al., 2004; Genazzani et al., 1998; Girdler et al., 2001). Chronic stress, however, is associated with alterations in GABAAR subunit expression and decreased GABA levels, as well as decreased levels and biosynthesis of neuroactive steroids, including allopregnanolone (Acosta et al., 1993; Verkuyl et al., 2004; Agis-Balboa et al., 2007; Dong et al., 2001).
Collectively, these data support the role of reduced GABAergic signaling in PPD, highlight links to other proposed PPD etiologies, and demonstrate connections between reduced GABAergic signaling and stress responses, suggesting that positive modulators of GABAergic signaling could act as potential therapies for PPD.
3. Differential pharmacology of neuroactive steroids and other therapeutic GABA modulators
The connection between GABAergic signaling dysfunction and PPD suggests that restoration of GABAergic signaling may present an attractive route for therapeutic intervention. However, although, there are several existing medications which are PAMs of GABAARs, including benzodiazepines and “Z-drugs,” there are no reported clinical trials evaluating the efficacy and safety of these drugs as monotherapy specifically in PPD (Wisden et al., 2017). In the treatment of MDD, however, benzodiazepines have been evaluated as both a monotherapy and adjunctive agents. As a monotherapy for MDD, few randomized controlled trials have examined benzodiazepines since the 1990s, and meta-analyses of these studies show no significant response rate versus placebo in depressive disorders (Benasi et al., 2018). Benzodiazepine use in major depressive disorder is often associated with specific diagnostic and symptom characteristics, such as anxiety and insomnia, and meta-analyses of benzodiazepines as an adjunct with antidepressants suggest there are some potential benefits (Davidson, 2010; Furukawa et al., 2001). In women with PPD, benzodiazepines may be used as a treatment if anxiety is a comorbidity, and in breast-feeding women, treatment guidelines recommend low doses of medications with short half-lives and no active metabolites (Cohen et al., 2010; Eberhard-Gran et al., 2006; Guille et al., 2013; Malone et al., 2004).
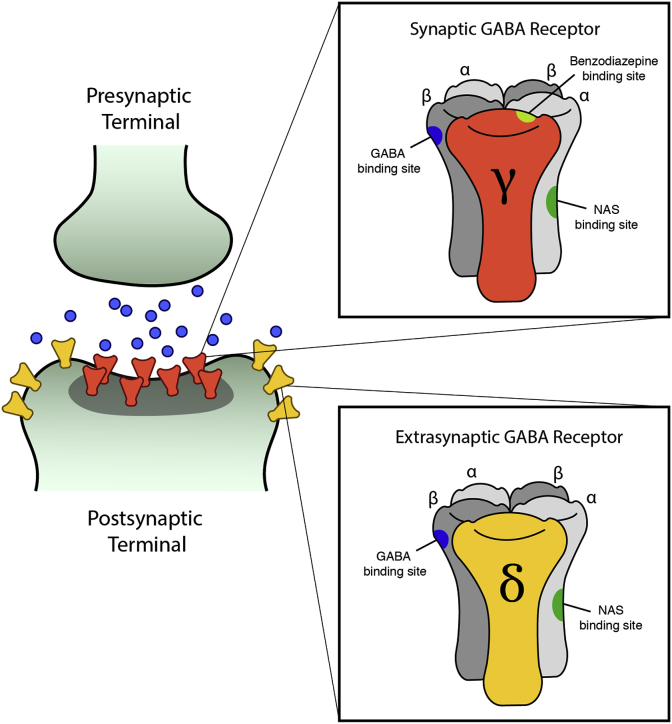
Neuroactive steroids represent an additional class of GABAAR PAMs, which are pharmacologically distinct from benzodiazepines. GABA can elicit CNS inhibition as either a short signal in response to synaptic GABAAR activation (phasic), or a longer-lasting inhibition in response to extrasynaptic GABAAR activation (tonic). Benzodiazepines bind the interface of the GABAAR ɣ2 subunit and α1-3 and α5 subunits, and GABAARs with this composition are restricted to the synapse (Sigel and Buhr, 1997; Sigel and Ernst, 2018). In contrast, neuroactive steroids bind GABAARs containing δ, α4, or α6 subunits, allowing them to act at both synaptic and extrasynaptic GABAARs through regulation of activity and/or GABAAR trafficking (Sigel and Buhr, 1997; Sigel and Ernst, 2018; Laverty et al., 2017; Abramian et al., 2014). While neuroactive steroids can bind all GABAARs, δ subunit-containing receptors show increased sensitivity at extracellular GABA concentrations, and in some brain regions, 95% of tonic currents are mediated by δ subunit-containing receptors (Glykys et al., 2008) (Fig. 4)
Fig. 4.
Neuroactive steroid GABAAR positive allosteric modulators are pharmacologically distinct from benzodiazepines. Neuroactive steroids can bind both synaptic and extrasynaptic receptors because, unlike benzodiazepines, their binding is not dependent on a gamma subunit, which is only present in synaptic receptors.
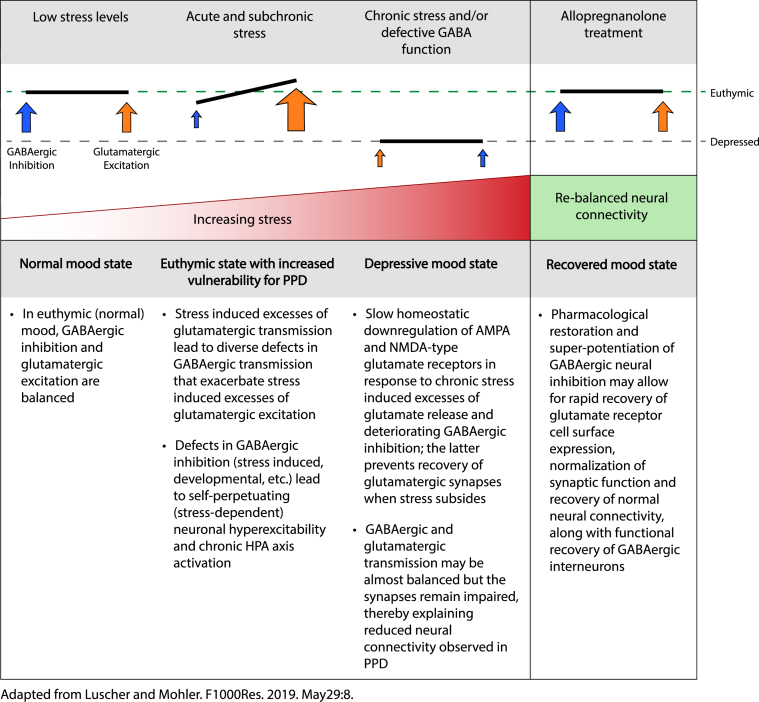
Neuroactive steroids vary in their ability to modulate GABA signaling at extrasynaptic receptors, and compared to other endogenous or synthetic neuroactive steroids, allopregnanolone is among the most potent of extrasynaptic receptor modulators (Reddy, 2018). GABAergic dysfunction is strongly linked to the etiology of PPD and although treatment with benzodiazepines has not been successful, the distinct pharmacology of neuroactive steroid GABAAR PAMs, such as allopregnanolone, offers a new opportunity to explore GABA-based treatment approach for PPD. The potential for neuroactive steroid GABAAR PAM efficacy maybe attributed to an increase in GABAergic inhibition, and stabilization of normal mood by decreasing the activity of stress-responsive dentate granule cells, thereby fostering and sustaining neuronal resilience (Luscher and Mohler, 2019) (Fig. 5)
Fig. 5.
Acute stress can result in imbalances between GABAergic and glutamatergic signaling, and further adaptation to chronic stress may result in a new balance of GABAergic and glutamatergic signaling at lower levels, contributing to depression. Restoring GABAergic signaling through brexanolone injection offers the potential to restore the original balance and level of signaling. Abbreviations: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA).
4. Relationship of pharmacologic antidepressant therapies to PPD pathophysiology
Although there are multiple proposed etiologies for PPD, existing antidepressant medications do not directly target any of the proposed mechanisms of disease. In addition, until recently, there were no pharmacological therapies approved by the US FDA specifically for the treatment of PPD. Current PPD management strategies are similar to those used for the treatment of MDD, including the use of psychotherapy and pharmacological interventions originally indicated for MDD (Yonkers et al., 2009). These pharmacotherapies typically comprise antidepressants, with selective serotonin reuptake inhibitors (SSRIs) being the most frequently prescribed medication class, followed by serotonin and norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs) (Alwan et al., 2016; Bandoli et al., 2018; Gelenberg et al., 2010). There is limited evidence, however, to support the safety and efficacy of antidepressants in treating depression specifically within the PPD population (Molyneaux et al., 2018).
A systematic literature review and meta-analysis of randomized controlled studies of patients with PPD pharmacologically treated with SSRIs found a 50% remission rate for “adequately treated” patients (Cox et al., 2016). However, given that only around 7% of PPD patients receive adequate treatment, this results in an overall weighted average remission rate of only 3% (Cox et al., 2016). Antidepressant-based therapies can take weeks to months for a potential treatment effect to take hold. Previous studies suggest that antidepressants typically require at least six weeks to achieve a positive response for the treatment of PPD, and in MDD, the time to response varies on the order of weeks to months (Hendrick et al., 2000; Rush et al., 2006a, 2006b; Trivedi et al., 2006). Existing antidepressants are associated with significant adverse effects, and their overall effectiveness in the treatment of PPD may therefore be limited by tolerability and acceptability (Cipriani et al., 2018; Jakobsen et al., 2017).
5. Neuroactive steroid administration in animal models
Neuroactive steroid GABAAR PAMs represent a potential new approach to antidepressant therapy – and PPD therapy in particular. The tool compound SGE-516 is a synthetic neuroactive steroid GABAAR PAM that has been investigated in the GABAAR δ subunit and CRH-KCC2 deficient mouse models of PPD. Mice with genetic deletion of the GABAAR δ subunit (Gabard−/−) or deletion of the KCC2 potassium/chloride co-transporter in the paraventricular nucleus of the brain (KCC/CRH), show PPD-like behaviors and the inability to suppress stress-induced activation of the HPA axis (Melon et al., 2018a; Maguire and Mody, 2008). Treatment of either Gabard−/− or KCC/CRH mice with SGE-516 during late pregnancy resulted in improvements in depression-like behaviors, as well as improvements in maternal care (Melon et al., 2018b). Treatment with SGE-516 also ameliorated the stress-related HPA axis dysregulation of this mouse model, allowing for the prevention of the stress-related increase in corticosterone (Melon et al., 2018b). Importantly, treatment of these mice with a benzodiazepine did not result in improvements in depression-like behaviors. This finding suggests that the antidepressant effects of SGE-516 are due to the unique pharmacology of neuroactive steroids, involving positive allosteric regulation and/or trafficking of both synaptic and extrasynaptic GABAARs (Melon et al., 2018b; Abramian et al., 2014; Comenencia-Ortiz et al., 2014). The findings from these genetic mouse studies corroborate clinical studies of brexanolone injection or benzodiazepine use in humans with depressive symptoms, and offer valuable mechanistic insight supporting the neuroactive steroid and GABA hypothesis in PPD.
6. Clinical studies of brexanolone injection
The GABA hypothesis of PPD and the additional links between neuroactive steroids and PPD etiology suggest the potential for allopregnanolone to act as a novel therapy for PPD (Licheri et al., 2015; Luscher et al., 2011). As a neuroactive steroid, allopregnanolone is pharmacologically distinct from other GABAA PAMs, such as benzodiazepines, binding a different site on the GABAAR that is present in both synaptic and extrasynaptic GABAARs (Laverty et al., 2017; Paul and Purdy, 1992). A proprietary, intravenous formulation of allopregnanolone (brexanolone injection) was developed as a first-in-class medication and was recently approved by the FDA for the treatment of adult women with PPD. The safety and efficacy of brexanolone injection was explored in three double-blind, randomized, placebo-controlled trials in women with PPD (Kanes et al., 2017; Meltzer-Brody et al., 2018).
6.1. Efficacy
Each of the three brexanolone injection studies achieved the primary endpoint of a reduction in depressive symptoms at Hour 60, as assessed by mean change from baseline compared to placebo in the Hamilton Rating Scale for Depression (HAM-D) (Kanes et al., 2017; Meltzer-Brody et al., 2018). The three brexanolone injection clinical trials were conducted under an umbrella protocol, allowing for the integration of the study datasets for subsequent analysis (Meltzer-Brody et al., 2018). In this integrated study population, efficacy analyses were conducted using the group which received an infusion up to the maximum dosage of 90 μg/kg/h during the 60-h infusion, corresponding to the currently (US FDA) approved dosing regimen. Safety was assessed from all women receiving any dose of brexanolone injection to provide the largest possible population for analysis (see Safety section below).
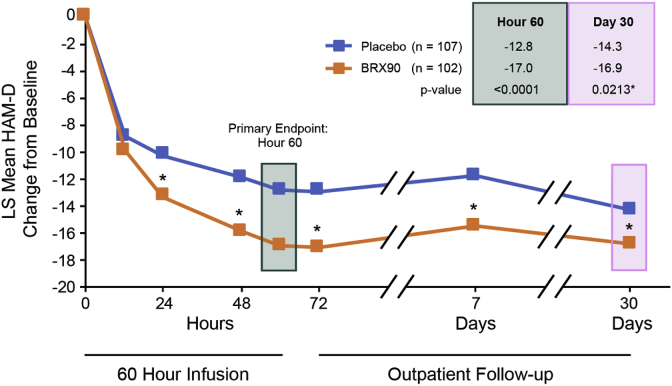
Across all three studies, brexanolone injection was associated with a rapid (by Hour 60) and sustained (through Day 30) reduction in depressive symptoms versus placebo as assessed by HAM-D (Meltzer-Brody et al., 2018) (Fig. 6)
Fig. 6.
In an integrated dataset from three double-blind, randomized, placebo-controlled trials of brexanolone injection, brexanolone injection 90 μg/kg/h (BRX90) was associated with statistically significant improvement in depressive symptoms versus placebo from the Hour 60 primary endpoint through Day 30. *p < 0.05 versus placebo; secondary endpoints were not adjusted for multiplicity.
Corresponding statistically significant differences in HAM-D response and remission favoring brexanolone injection were also observed. Additional secondary endpoints also supported the primary HAM-D findings, including Montgomery-Åsberg Depression Rating Scale total score and the Clinical Global Impression – Improvement response. Brexanolone injection had a broad antidepressant response with improvements observed in core symptoms of depression, as well as broader symptom clusters (Meltzer-Brody et al., 2018). When examined by baseline antidepressant use, which was permitted if at stable dose from 14 days prior to administration of study drug through Hour 72 assessments, brexanolone injection showed statistically significant advantages versus placebo with or without additional antidepressants, suggesting that brexanolone injection could be used as a monotherapy or in combination with other antidepressants (Meltzer-Brody et al., 2018). Additional subgroup analyses revealed there were broad responses regardless of race, ethnicity, age, PPD history (personal or family), family MDD history, time between delivery and index treatment, time of PPD onset, and depression severity (Meltzer-Brody et al., 2018).
6.2. Safety
Across the brexanolone injection integrated study population, brexanolone injection was generally well tolerated (Meltzer-Brody et al., 2018). Two serious adverse events occurred in the brexanolone injection group versus none in the placebo group. The most common adverse reactions (incidence less than or equal to 5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush (Kanes et al., 2017; Meltzer-Brody et al., 2018). The sedation-related adverse events resolved within 90 min following cessation of dosing. The overall rates of adverse events were similar in patients receiving brexanolone injection or placebo (Meltzer-Brody et al., 2018). In some patients, sedation and somnolence that required dose interruption or reduction during the infusion occurred (5% of patients treated with brexanolone injection compared with 0% of those treated with placebo). Given that patients are at potential risk of excessive sedation and sudden loss of consciousness, brexanolone injection is available only through a restricted program called the ZULRESSO Risk Evaluation and Mitigation Strategy (REMS). Notable requirements of the REMS include the need for healthcare facilities, pharmacies, patients, and wholesalers and distributors to be enrolled in the program and follow processes and procedures set forth by the program during the administration of brexanolone.
7. Potential use of neuroactive steroids in PPD
The positive results from the clinical studies of brexanolone injection in PPD and animal studies of the synthetic neuroactive steroid SGE-516 in PPD-like models support the link between GABAergic dysfunction and PPD and the further development of the proposed neuroactive steroid mechanism. Brexanolone injection demonstrated efficacy in three placebo-controlled clinical trials of PPD and is now being used commercially in the United States. Uptake of the commercial product has required health care systems to develop processes to deliver a 60-h continuous infusion of brexanolone and full compliance with the REMS requirements. Patients must also decide, in consultation with their physicians, whether to continue breastfeeding during and after treatment with brexanolone injection. Finally, successful negotiation with insurers to cover the costs of the drug must be in place before administration.
Additional neuroactive steroids are currently being examined in clinical trials of women with PPD, and these different compounds and formulations may have different pharmacokinetic and/or adverse event profiles that could reduce some barriers to treatment. Zuranolone (SAGE-217), an investigational, oral neuroactive steroid, was recently evaluated in a Phase 3 trial in women with PPD and achieved the primary endpoint of a reduction in depressive symptoms after 2 weeks as assessed by mean change from baseline in HAM-D total score compared with placebo. The effects of zuranolone were also observed as early as Day 3 and sustained through the four week follow up (Deligiannidis et al., 2019b). The neuroactive steroid ganaxolone is also currently being evaluated in trials in women with PPD (Marinus Pharmaceuticals Press Release, 2018).
8. Discussion/conclusions
PPD is a serious and common mood disorder that is most commonly treated with medications indicated for major depressive disorder. However, most currently available antidepressant therapies are associated with extended times to response and/or limited response and remission, leaving an unmet need for rapid and effective treatments for PPD. Preclinical and clinical studies in PPD have demonstrated the potential roles of dysfunctional GABAergic signaling, and in particular, allopregnanolone levels, in the development of PPD. This suggests that positive allosteric modulation of GABAARs by neuroactive steroids, such as allopregnanolone, is a promising mechanism of action for the adoption of novel pharmacotherapies in the treatment of PPD.
Declaration of competing interest
SM-B reports personal fees from MedScape and grants from Sage Therapeutics, Inc., awarded to the University of Carolina (Chapel Hill, NC, USA) during the conduct of the brexanolone injection clinical trials and grants from Janssen, PCORI, and the NIH outside the submitted work. SJK is an employee of Sage Therapeutics, Inc., with stock/stock options.
Acknowledgements
The development of this review was supported by Sage Therapeutics, Inc. The authors thank Jeffrey R. Skaar at Boston Strategic Partners (supported by Sage Therapeutics, Inc.), and Michael Craig at Sage Therapeutics, Inc., for editorial support.
References
- Abramian A.M., Comenencia-Ortiz E., Modgil A. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc. Natl. Acad. Sci. U. S. A. 2014;111(19):7132–7137. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta G.B., Otero Losada M.E., Rubio M.C. Area-dependent changes in GABAergic function after acute and chronic cold stress. Neurosci. Lett. 1993;154(1–2):175–178. doi: 10.1016/0304-3940(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Agis-Balboa R.C., Pinna G., Pibiri F., Kadriu B., Costa E., Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104(47):18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan S., Friedman J.M., Chambers C. Safety of selective serotonin reuptake inhibitors in pregnancy: a review of current evidence. CNS Drugs. 2016;30(6):499–515. doi: 10.1007/s40263-016-0338-3. [DOI] [PubMed] [Google Scholar]
- Balbierz A., Bodnar-Deren S., Wang J.J., Howell E.A. Maternal depressive symptoms and parenting practices 3-months postpartum. Matern. Child Health J. 2015;19(6):1212–1219. doi: 10.1007/s10995-014-1625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoli G., Kuo G.M., Sugathan R., Chambers C.D., Rolland M., Palmsten K. Longitudinal trajectories of antidepressant use in pregnancy and the postnatal period. Arch. Womens Ment. Health. 2018;21(4):411–419. doi: 10.1007/s00737-018-0809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A.J., Scott K.M., Ferrari A.J., Norman R.E., Vos T., Whiteford H.A. Challenging the myth of an "epidemic" of common mental disorders: trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress. Anxiety. 2014;31(6):506–516. doi: 10.1002/da.22230. [DOI] [PubMed] [Google Scholar]
- Benasi G., Guidi J., Offidani E., Balon R., Rickels K., Fava G.A. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother. Psychosom. 2018;87(2):65–74. doi: 10.1159/000486696. [DOI] [PubMed] [Google Scholar]
- Biaggi A., Conroy S., Pawlby S., Pariante C.M. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J. Affect. Disord. 2016;191:62–77. doi: 10.1016/j.jad.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M., Schmidt P.J., Danaceau M., Murphy J., Nieman L., Rubinow D.R. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatr. 2000;157(6):924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bloch M., Daly R.C., Rubinow D.R. Endocrine factors in the etiology of postpartum depression. Compr. Psychiatr. 2003;44(3):234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Boccia M.L., Razzoli M., Vadlamudi S.P., Trumbull W., Caleffie C., Pedersen C.A. Repeated long separations from pups produce depression-like behavior in rat mothers. Psychoneuroendocrinology. 2007;32(1):65–71. doi: 10.1016/j.psyneuen.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar-Deren S., Klipstein K., Fersh M., Shemesh E., Howell E.A. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219–1224. doi: 10.1089/jwh.2015.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S.R., Brennan P.A., Newport D.J., Smith A.K., Weiss T., Stowe Z.N. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35(5):686–693. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S., Galea L.A. Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm. Behav. 2010;58(5):769–779. doi: 10.1016/j.yhbeh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Brunton P.J., Russell J.A., Douglas A.J. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol. 2008;20(6):764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease C, Prevention Prevalence of self-reported postpartum depressive symptoms--17 states, 2004-2005. MMWR Morb. Mortal. Wkly. Rep. 2008;57(14):361–366. [PubMed] [Google Scholar]
- Cipriani A., Furukawa T.A., Salanti G. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L.S., Wang B., Nonacs R., Viguera A.C., Lemon E.L., Freeman M.P. Treatment of mood disorders during pregnancy and postpartum. Psychiatr. Clin. 2010;33(2):273–293. doi: 10.1016/j.psc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Comenencia-Ortiz E., Moss S.J., Davies P.A. Phosphorylation of GABAA receptors influences receptor trafficking and neurosteroid actions. Psychopharmacology. 2014;231(17):3453–3465. doi: 10.1007/s00213-014-3617-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOG opinion No. 630 The American College of Obstetricians and Gynecologists Committee on Obstetric Practice: Screening for perinatal depression. Obstet. Gynecol. 2015;125(5):1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- ACOG opinion No. 757 The American College of Obstetricians and Gynecologists Committee on Obstetric Practice: Screening for perinatal depression. Obstet. Gynecol. 2018;132(5):e208–e212. doi: 10.1097/AOG.0000000000002927. [DOI] [PubMed] [Google Scholar]
- Cox E.Q., Sowa N.A., Meltzer-Brody S.E., Gaynes B.N. The perinatal depression treatment cascade: baby steps toward improving outcomes. J. Clin. Psychiatr. 2016;77(9):1189–1200. doi: 10.4088/JCP.15r10174. [DOI] [PubMed] [Google Scholar]
- Davidson J.R. Major depressive disorder treatment guidelines in America and Europe. J. Clin. Psychiatr. 2010;71(Suppl. E1):e04. doi: 10.4088/JCP.9058se1c.04gry. [DOI] [PubMed] [Google Scholar]
- Decavel C., Van den Pol A.N. GABA: a dominant neurotransmitter in the hypothalamus. J. Comp. Neurol. 1990;302(4):1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Decavel C., van den Pol A.N. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J. Comp. Neurol. 1992;316(1):104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- Deligiannidis K.M., Kroll-Desrosiers A.R., Mo S. Peripartum neuroactive steroid and gamma-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology. 2016;70:98–107. doi: 10.1016/j.psyneuen.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis K.M., Fales C.L., Kroll-Desrosiers A.R. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546–554. doi: 10.1038/s41386-018-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Matsumoto K., Uzunova V. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. U. S. A. 2001;98(5):2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogleever Fortuyn H.A., van Broekhoven F., Span P.N., Backstrom T., Zitman F.G., Verkes R.J. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptor density. Psychoneuroendocrinology. 2004;29(10):1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Earls M.F., Committee on Psychosocial Aspects of C, Family Health American Academy of P Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126(5):1032–1039. doi: 10.1542/peds.2010-2348. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M., Eskild A., Opjordsmoen S. Use of psychotropic medications in treating mood disorders during lactation : practical recommendations. CNS Drugs. 2006;20(3):187–198. doi: 10.2165/00023210-200620030-00002. [DOI] [PubMed] [Google Scholar]
- Force APAD-T . American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [Google Scholar]
- Furukawa T.A., Streiner D.L., Young L.T. Antidepressant plus benzodiazepine for major depression. Cochrane Database Syst. Rev. 2001;(2):CD001026. doi: 10.1002/14651858.CD001026. [DOI] [PubMed] [Google Scholar]
- Gelenberg A.J., Freeman M.P., Markowitz J.C. third ed. American Psychiatric Association; 2010. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. [Google Scholar]
- Genazzani A.R., Petraglia F., Bernardi F. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J. Clin. Endocrinol. Metab. 1998;83(6):2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Girdler S.S., Straneva P.A., Light K.C., Pedersen C.A., Morrow A.L. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol. Psychiatr. 2001;49(9):788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Glykys J., Mann E.O., Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008;28(6):1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J.H. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J. Adv. Nurs. 2004;45(1):26–35. doi: 10.1046/j.1365-2648.2003.02857.x. [DOI] [PubMed] [Google Scholar]
- Goyal D., Gay C., Lee K.A. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Wom. Health Issues. 2010;20(2):96–104. doi: 10.1016/j.whi.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressier F., Guillard V., Cazas O., Falissard B., Glangeaud-Freudenthal N.M., Sutter-Dallay A.L. Risk factors for suicide attempt in pregnancy and the post-partum period in women with serious mental illnesses. J. Psychiatr. Res. 2017;84:284–291. doi: 10.1016/j.jpsychires.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Guille C., Newman R., Fryml L.D., Lifton C.K., Epperson C.N. Management of postpartum depression. J. Midwifery Wom. Health. 2013;58(6):643–653. doi: 10.1111/jmwh.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes K., McGowan E., O'Donnell M., Tucker R., Vohr B. Social emotional factors increase risk of postpartum depression in mothers of preterm infants. J. Pediatr. 2016;179:61–67. doi: 10.1016/j.jpeds.2016.07.008. [DOI] [PubMed] [Google Scholar]
- NIMH fact sheet - Postpartum depression https://www.nimh.nih.gov/health/publications/postpartum-depression-facts/index.shtml
- Hendrick V., Altshuler L., Strouse T., Grosser S. Postpartum and nonpostpartum depression: differences in presentation and response to pharmacologic treatment. Depress. Anxiety. 2000;11(2):66–72. doi: 10.1002/(sici)1520-6394(2000)11:2<66::aid-da3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Howard L.M., Oram S., Galley H., Trevillion K., Feder G. Domestic violence and perinatal mental disorders: a systematic review and meta-analysis. PLoS Med. 2013;10(5) doi: 10.1371/journal.pmed.1001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen J.C., Katakam K.K., Schou A. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatr. 2017;17(1):58. doi: 10.1186/s12888-016-1173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S., Colquhoun H., Gunduz-Bruce H. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–489. doi: 10.1016/S0140-6736(17)31264-3. [DOI] [PubMed] [Google Scholar]
- Ko J.Y., Rockhill K.M., Tong V.T., Morrow B., Farr S.L. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb. Mortal. Wkly. Rep. 2017;66(6):153–158. doi: 10.15585/mmwr.mm6606a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutra K., Chatzi L., Bagkeris M., Vassilaki M., Bitsios P., Kogevinas M. Antenatal and postnatal maternal mental health as determinants of infant neurodevelopment at 18 months of age in a mother-child cohort (Rhea Study) in Crete, Greece. Soc. Psychiatr. Psychiatr. Epidemiol. 2013;48(8):1335–1345. doi: 10.1007/s00127-012-0636-0. [DOI] [PubMed] [Google Scholar]
- Kuver A., Shen H., Smith S.S. Regulation of the surface expression of alpha4beta2delta GABAA receptors by high efficacy states. Brain Res. 2012;1463:1–20. doi: 10.1016/j.brainres.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H., Goodman S.H., Stowe Z.N. Course of ante- and postnatal depressive symptoms related to mothers' HPA axis regulation. J. Abnorm. Psychol. 2018;127(4):404–416. doi: 10.1037/abn0000348. [DOI] [PubMed] [Google Scholar]
- Laverty D., Thomas P., Field M. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat. Struct. Mol. Biol. 2017;24(11):977–985. doi: 10.1038/nsmb.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P.J., Craske M.G., Kempton C., Stewart A., Stein A. Intrusive thoughts and images of intentional harm to infants in the context of maternal postnatal depression, anxiety, and OCD. Br. J. Gen. Pract. 2017;67(661):376–377. doi: 10.3399/bjgp17X692105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licheri V., Talani G., Gorule A.A., Mostallino M.C., Biggio G., Sanna E. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi S., Petraglia F., Benedetto C. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J. Clin. Endocrinol. Metab. 2000;85(7):2429–2433. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- Luscher B., Mohler H. Brexanolone, a Neurosteroid Antidepressant, Vindicates the GABAergic Deficit Hypothesis of Depression and May Foster Resilience. F1000Res. 2019;vol. 8 doi: 10.12688/f1000research.18758.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B., Shen Q., Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatr. 2011;16(4):383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Front. Cell. Neurosci. 2019;13:83. doi: 10.3389/fncel.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J., Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone K., Papagni K., Ramini S., Keltner N.L. Antidepressants, antipsychotics, benzodiazepines, and the breastfeeding dyad. Psychiatr. Care. 2004;40(2):73–85. doi: 10.1111/j.1744-6163.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- Marinus Pharmaceuticals Press Release. 2018. https://wwwglobenewswirecom/news-release/2018/12/10/1664 282/0/en/Marinus-Pharmaceuticals-Announces-Positive-Ganaxolone-Data-in-Women-With-Postpartum-Depressionhtml
- Meinlschmidt G., Martin C., Neumann I.D., Heinrichs M. Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress. 2010;13(2):163–171. doi: 10.3109/10253890903128632. [DOI] [PubMed] [Google Scholar]
- Melon L.C., Hooper A., Yang X., Moss S.J., Maguire J. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182–193. doi: 10.1016/j.psyneuen.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon L., Hammond R., Lewis M., Maguire J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front. Endocrinol. 2018;9:703. doi: 10.3389/fendo.2018.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S., Colquhoun H., Riesenberg R. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–1070. doi: 10.1016/S0140-6736(18)31551-4. [DOI] [PubMed] [Google Scholar]
- Miller L.G., Thompson M.L., Greenblatt D.J., Deutsch S.I., Shader R.I., Paul S.M. Rapid increase in brain benzodiazepine receptor binding following defeat stress in mice. Brain Res. 1987;414(2):395–400. doi: 10.1016/0006-8993(87)90023-0. [DOI] [PubMed] [Google Scholar]
- Modol L., Casas C., Llido A., Navarro X., Pallares M., Darbra S. Neonatal allopregnanolone or finasteride administration modifies hippocampal K(+) Cl(-) co-transporter expression during early development in male rats. J. Steroid Biochem. Mol. Biol. 2014;143:343–347. doi: 10.1016/j.jsbmb.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Molyneaux E., Telesia L.A., Henshaw C., Boath E., Bradley E., Howard L.M. Antidepressants for preventing postnatal depression. Cochrane Database Syst. Rev. 2018;4:CD004363. doi: 10.1002/14651858.CD004363.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostallino M.C., Sanna E., Concas A., Biggio G., Follesa P. Plasticity and function of extrasynaptic GABA(A) receptors during pregnancy and after delivery. Psychoneuroendocrinology. 2009;34(Suppl. 1):S74–S83. doi: 10.1016/j.psyneuen.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Netsi E., Pearson R.M., Murray L., Cooper P., Craske M.G., Stein A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatr. 2018;75(3):247–253. doi: 10.1001/jamapsychiatry.2017.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara M.W., Neunaber D.J., Zekoski E.M. Prospective study of postpartum depression: prevalence, course, and predictive factors. J. Abnorm. Psychol. 1984;93(2):158–171. doi: 10.1037//0021-843x.93.2.158. [DOI] [PubMed] [Google Scholar]
- Oates M. Suicide: the leading cause of maternal death. Br. J. Psychiatry. 2003;183:279–281. doi: 10.1192/bjp.183.4.279. [DOI] [PubMed] [Google Scholar]
- Osborne L.M., Monk C. Perinatal depression--the fourth inflammatory morbidity of pregnancy?: theory and literature review. Psychoneuroendocrinology. 2013;38(10):1929–1952. doi: 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti A.M., Romagnino S., Contu R. Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABA-A receptor agonist activity. Psychoneuroendocrinology. 2006;31(4):485–492. doi: 10.1016/j.psyneuen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Paul S.M., Purdy R.H. Neuroactive steroids. Faseb. J. 1992;6(6):2311–2322. [PubMed] [Google Scholar]
- Paykel E.S., Emms E.M., Fletcher J., Rassaby E.S. Life events and social support in puerperal depression. Br. J. Psychiatry. 1980;136:339–346. doi: 10.1192/bjp.136.4.339. [DOI] [PubMed] [Google Scholar]
- Payne J.A., Rivera C., Voipio J., Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26(4):199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pearson R.M., Evans J., Kounali D. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatr. 2013;70(12):1312–1319. doi: 10.1001/jamapsychiatry.2013.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell K.D., Woodin M.A., Pennell P.B. Quantification of neurosteroids during pregnancy using selective ion monitoring mass spectrometry. Steroids. 2015;95:24–31. doi: 10.1016/j.steroids.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D.S. GABA-A receptors mediate tonic inhibition and neurosteroid sensitivity in the brain. Vitam. Horm. 2018;107:177–191. doi: 10.1016/bs.vh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Rivera C., Voipio J., Payne J.A. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rivera C., Voipio J., Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005;562(Pt 1):27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N. Engl. J. Med. 2006;354(12):1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatr. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Deligiannidis K., Lasser R., Gunduz-Bruce H. Presented at the Annual Meeting of the American Society of Clinical Psychopharmacology (ASCP). Scottsdale, AZ May 28–31. 2019. A phase 3, double-blind, placebo-controlled trial of SAGE-217 in postpartum depression: assessment of depressive symptoms across multiple measures. Poster T74. [Google Scholar]
- Schulte H.M., Weisner D., Allolio B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin. Endocrinol. 1990;33(1):99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Shadigian E., Bauer S.T. Pregnancy-associated death: a qualitative systematic review of homicide and suicide. Obstet. Gynecol. Surv. 2005;60(3):183–190. doi: 10.1097/01.ogx.0000155967.72418.6b. [DOI] [PubMed] [Google Scholar]
- Sigel E., Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol. Sci. 1997;18(11):425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Sigel E., Ernst M. The benzodiazepine binding sites of GABAA receptors. Trends Pharmacol. Sci. 2018;39(7):659–671. doi: 10.1016/j.tips.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Stewart D.E., Robertson E., Dennis C., Grace S., Wallington T. 2003. Postpartum Depression: Literature Review of Risk Factors and Interventions. [Google Scholar]
- Surkan P.J., Ettinger A.K., Hock R.S., Ahmed S., Strobino D.M., Minkovitz C.S. Early maternal depressive symptoms and child growth trajectories: a longitudinal analysis of a nationally representative US birth cohort. BMC Pediatr. 2014;14:185. doi: 10.1186/1471-2431-14-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J.D., Mazure C.M. Life stress as a risk factor for postpartum depression: current research and methodological issues. Clin. Psychol. 2000;7:17–31. [Google Scholar]
- Trivedi M.H., Rush A.J., Wisniewski S.R. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatr. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Umboh S.J., How C.H., Chen H. Postnatal depression: a family medicine perspective. Singap. Med. J. 2013;54(9):477–479. doi: 10.11622/smedj.2013167. quiz 480-471. [DOI] [PubMed] [Google Scholar]
- Valla L., Wentzel-Larsen T., Smith L., Birkeland M.S., Slinning K. Association between maternal postnatal depressive symptoms and infants' communication skills: a longitudinal study. Infant Behav. Dev. 2016;45(Pt A):83–90. doi: 10.1016/j.infbeh.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Verkuijl N.E., Richter L., Norris S.A., Stein A., Avan B., Ramchandani P.G. Postnatal depressive symptoms and child psychological development at 10 years: a prospective study of longitudinal data from the South African Birth to Twenty cohort. Lancet Psychiatr. 2014;1(6):454–460. doi: 10.1016/S2215-0366(14)70361-X. [DOI] [PubMed] [Google Scholar]
- Verkuyl J.M., Hemby S.E., Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur. J. Neurosci. 2004;20(6):1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Whiteford H.A., Degenhardt L., Rehm J. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Wisden W., Yu X., Franks N.P. GABA receptors and the pharmacology of sleep. Handb. Exp. Pharmacol. 2017 doi: 10.1007/164_2017_56. [DOI] [PubMed] [Google Scholar]
- Woolhouse H., Gartland D., Mensah F., Giallo R., Brown S. Maternal depression from pregnancy to 4 years postpartum and emotional/behavioural difficulties in children: results from a prospective pregnancy cohort study. Arch. Womens Ment. Health. 2016;19(1):141–151. doi: 10.1007/s00737-015-0562-8. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Fujiwara T., Tamiya N. Association between maternal postpartum depression and unintentional injury among 4-month-old infants in Japan. Matern. Child Health J. 2016;20(2):326–336. doi: 10.1007/s10995-015-1832-9. [DOI] [PubMed] [Google Scholar]
- Yim I.S., Tanner Stapleton L.R., Guardino C.M., Hahn-Holbrook J., Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu. Rev. Clin. Psychol. 2015;11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Kanmori K., Ida S., Kuriyama K. Stress-induced alterations in metabolism of gamma-aminobutyric acid in rat brain. J. Neurochem. 1983;40(2):350–356. doi: 10.1111/j.1471-4159.1983.tb11289.x. [DOI] [PubMed] [Google Scholar]
- Yonkers K.A., Wisner K.L., Stewart D.E. The management of depression during pregnancy: a report from the American psychiatric association and the American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2009;114(3):703–713. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]