Abstract
Opioid use disorder (OUD) and alcohol use disorder (AUD) are two highly prevalent substance-related disorders worldwide. Co-use of the substances is also quite prevalent, yet there are no pharmacological treatment approaches specifically designed to treat co-morbid OUD and AUD.
Here, the authors critically summarize OUD, AUD and opioid/alcohol co-use and their current pharmacotherapies for treatment. They also review the mechanisms of action of opioids and alcohol within the brain reward circuitry and discuss potential combined mechanisms of action and resulting neuroadaptations. Pharmacotherapies that aim to treat AUD or OUD that may be beneficial in the treatment of co-use are also highlighted.
Preclinical models assessing alcohol and opioid co-use remain sparse. Lasting neuroadaptations in brain reward circuits caused by co-use of alcohol and opioids remains largely understudied. In order to fully understand the neurobiological underpinnings of alcohol and opioid co-use and develop efficacious pharmacotherapies, the preclinical field must expand its current experimental paradigms of ‘single drug’ use to encompass polysubstance use. Such studies will provide insights on the neural alterations induced by opioid and alcohol co-use, and may help develop novel pharmacotherapies for individuals with co-occurring alcohol and opioid use disorders.
Keywords: Alcohol, AUD, Co-use, Co-morbidity, Opioid, Opiate, OUD, Pharmacotherapies
1. Introduction
Recurrent substance use induces physiological dependence, leading to alterations in neural circuits in the brain that can result in the development of addiction-associated phenotypes. Substance use disorders (SUDs) negatively impact both mental (i.e. mood, memory, decision-making, psychosis) and physical health (i.e. cancer, heart and lung disease, stroke), with increased risks for contracting communicable diseases, as well as an increase the incidence of injuries as well as fatality. The severity of such consequences can increase with the use of multiple substances either sequentially or simultaneously, which is referred to as polysubstance or polydrug use. Polysubstance use is correlated with increased rates of fatal and nonfatal overdoses, increased comorbidity with mental illness, and poorer treatment outcomes as compared to single drug “mono-substance” use [1].
A global surge in the incidence of opioid use disorders (OUDs) has occurred over the past several decades, reaching epidemic status in the United States and elsewhere. In contrast, the incidence of alcohol use disorders (AUDs) has remained on a more consistent trajectory, yet still poses a significant detriment to public health as one of the leading causes of preventable death in high-income countries [2]. Numerous studies have explored the neurobiological effects of alcohol or opioids when used in conjunction with other addictive substances. For example, interactions between alcohol and nicotine have been extensively researched, as has the converging neurobiological changes produced by alcohol and cocaine [3,4]. Opioids are also commonly used in conjunction benzodiazepines and stimulants; however, as will be highlighted in this review, there has been very little research conducted on the co-use of alcohol and opioids.
OUDs and AUDs have proven difficult to treat due to a myriad of factors, including genetic vulnerability, co-occurring psychiatric disorders, socioeconomic factors, and the diverse chemical and molecular changes that opioids and alcohol induce throughout the brain. The current review evaluates the prominent pharmacological therapies used to combat OUD and AUD. These current pharmacotherapies aim to reduce relapse and prolong abstinence by alleviating negative affect that occur during withdrawal, and are most effective when administered in parallel with psychosocial support. We also review the neuroadaptive changes induced by alcohol and opioids, and the mechanism of action and clinical efficacy of common pharmacotherapies for each disorder. While there is considerable overlap in treatment therapies for AUDs and OUDs, as well as neurocircuitry modifications, this cohesion is not reflected in current preclinical models of co-use, and thus poses a considerable gap in our current understanding of this form of polysubstance use. Thus, we aim to highlight such gaps in preclinical models of polysubstance, and to propose common physiological pathways affected by opioids and alcohol, which may ultimately aide in developing effective pharmacotherapies for treating opioid and alcohol co-use.
2. Opioid Use Disorder
Misuse of opioids, a class of drugs including prescription pain relievers, heroin and related synthetic compounds (i.e., fentanyl), has risen at an alarming rate over the past few decades. Currently 1.7 million people within the United States currently suffer from opioid-related substance use disorders [5]. Nearly 8–12 percent of patients prescribed opioids develop an opioid use disorder [6], which contributes to the nearly 47,000 opioid overdose caused deaths annually [7]. While opioid use disorder encompasses many opioid drugs, the most commonly used are prescription opioids (i.e. analgesics such as oxycodone, hydrocodone, etc.), heroin, and synthetic opioids such as fentanyl. Opioid addiction is characterized by compulsive drug seeking and taking, an inability to limit intake despite negative consequences and intense drug craving [8], which is in most cases initiated during consumption of opioids prescribed to treat disorders such as chronic pain. Since opioid use within the United States and elsewhere remains an unprecedented crisis, development of effective treatments using pharmacotherapies remains a critical component for combating the opioid epidemic.
2.1. Forms of Opioid Use – Short- vs. Long-term
Opioids are the most commonly utilized prescription medication for pain treatment, yet clinical guidelines for prescribing opioids have changed vastly over time. Further, the lack of consistency between doctors, hospitals, states and other entities has contributed to inconsistent regulations and prescribing practices. Among many other factors such as increased availability of synthetic opioid derivatives and prevalent patterns of over-prescription have led to the alarming rise in opioid use, dependence, and overdoses [6]. When taken as prescribed, opioids are intended for short-term relief of pain or other symptoms, usually with a typical duration of one to three weeks [9], the risk of developing dependence is less likely. However, treatment of chronic pain with prescription opioids in opioid-naïve patients has been shown to be the largest contributor to long-term use. In fact, the likelihood of chronic opioid use increases sharply following the third day of medication supply [10]. Additionally, the longer the duration of opioid prescription, the greater the probability of continued opioid use, which is exacerbated in older adults [10]. Further, while data from 2011 show that 80 percent of patients who misuse prescription opioids will eventually transition to the use of heroin, current studies show that one-third of individuals with OUD report heroin as the first opioid used for recreational purposes [11]. Short- and long-term opioid use are both known to induce physiological alterations in the brain and periphery [12]; however, the alterations induced by chronic long-term use within the brain reward circuitry (Figure 1) are of key interest for understanding the neural basis of opioid use and addiction [12,13].
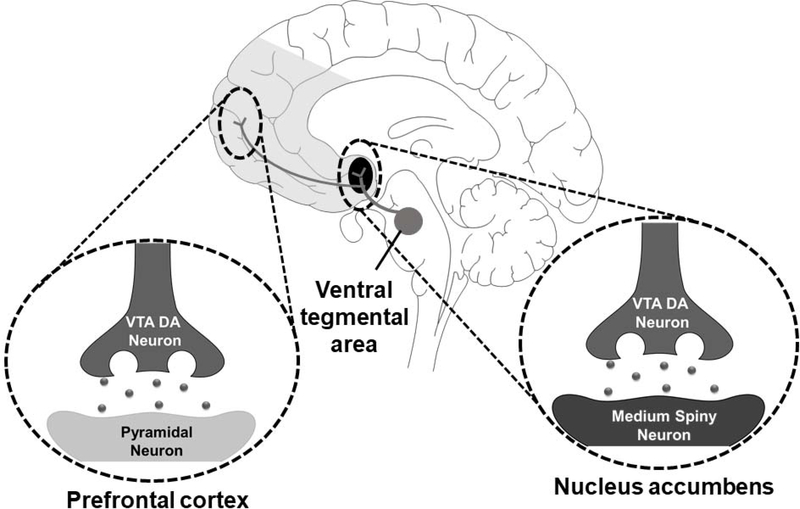
Figure 1.
Diagram of the mesolimbic dopamine reward system, which is believed to be activated alcohol and opioids. Dopamine-producing neuronal cell bodies clustered in the ventral tegmental area of the midbrain project widely to forebrain, striatal, and other subcortical structures. The primary targets of these dopaminergic neurons are pyramidal neurons of the prefrontal cortex and medium spiny neurons of the nucleus accumbens.
2.2. Mechanisms of Action of Opioids
Opioids exert their actions on neural and other cell types by acting on receptor proteins located on the plasma membrane. Belonging to the superfamily of seven-transmembrane spanning G-protein coupled receptors, three major types of opioid receptors have been extensively verified and classified as mu (μ), kappa (κ), and delta (δ) receptors [14,15]. These inhibitory (Gi/o coupled) receptors are responsible for mediating cell-cell communication by endogenous opioid peptides such as endorphins, endomorphins, enkephalins and dynorphins [8]. Other opioid receptors such as the receptor for orphanin FQ/nociceptin (NOP) have also been identified. Of these receptors, the μ-opioid receptor (MOR) has been extensively characterized for its role in mediating the rewarding effects of heroin, morphine and other opioids as reviewed elsewhere [16]. Pharmacological blockade of these receptors reduces opioid self-administration in both animals and humans, as well the ability of opioids to produce conditioned rewarding effects [17]. MORs are heavily expressed within the mesolimbic system and provide opioids (both endogenous and exogenous) the ability to modulate the excitability of mesolimbic dopaminergic neurons [18] and striatal GABAergic medium spiny neurons [19]. Because of their extensive presence on GABAergic interneurons within the substantia nigra and ventral tegmental area (VTA), MORs have the ability to disinhibit mesolimbic dopaminergic neurons when activated [20] (Figure 2). This disinhibition of dopaminergic neurons results in increased release of dopamine in projection areas, including the nucleus accumbens [21,22], where dopamine interacts with both pre- and post-synaptic receptors and elicits the euphoric effects experienced by drug users. While dopamine plays a pertinent role in modulating reward circuits and euphoric effects of abused drugs, the relationship between VTA dopamine release and opioid-induced reward remains unclear. Consistent with this, human positron-emission tomography (PET) studies have shown opioids to induce reinforcing effects despite minimal dopamine release in the striatum (see ref [23] for a comprehensive review of this topic.
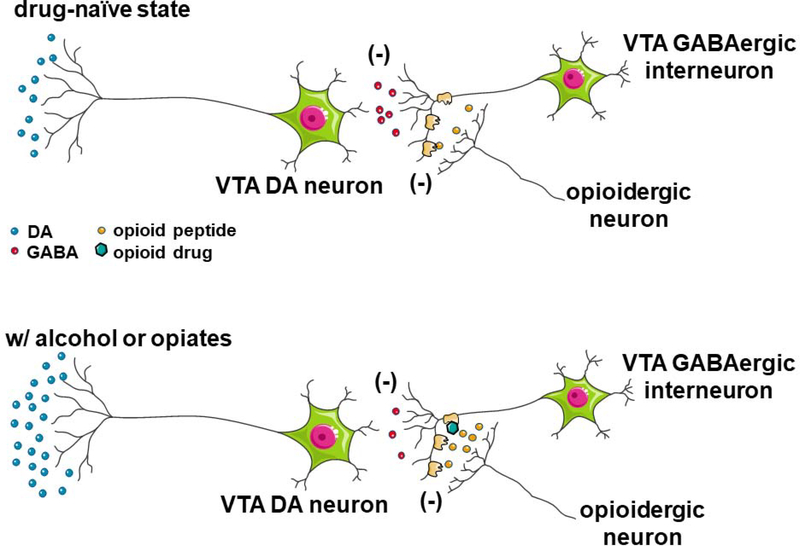
Figure 2.
Proposed mechanisms by which alcohol (top) and opioids (bottom) activate the mesolimbic dopamine reward circuits. Dopaminergic neurons in the ventral tegmental area (VTA) are tonically inhibited by local inhibitory GABAergic interneurons. Ingestion of alcohol activates opioid peptide-synthesizing neurons located elsewhere in the brain, such as endorphin-synthesizing neurons in the arcuate nucleus of the hypothalamus. Alcohol-induced release of opioid peptides into the VTA activates inhibitory MORs located on local inhibitory GABAergic interneurons, resulting in disinhibition of dopamine neuron activity. Direct activation of these inhibitory mu opioid receptors by exogenous opioids such as fentanyl or oxycodone also results in decreased activity of local inhibitory GABAergic interneurons, resulting in a similar disinhibition of dopamine neuron activity.
Many animal studies exploring the role of MORs in opioid addiction have shown that activation of MORs by opioids induces functional alterations in dopaminergic systems - primarily through long-term depression (LTD) of synaptic efficacy in striatal GABAergic cells [24] and/or long-term potentiation (LTP) of dopaminergic neurons [25,26]. Chronic opioid exposure also alters opioid receptor mRNA and surface expression levels, as well as the synthesis of various endogenous opioid peptides [27]. Opioids also have the ability to induce structural plasticity within the mesolimbic reward pathway. For example, opioids can decrease dendritic spine density and dendrite complexity of medium spiny neurons (MSNs) within the nucleus accumbens and decrease cell body size of VTA dopamine neurons [28–31], potentially gating the excitability of specific neuronal networks that contribute to OUDs. Later in this review we discuss changes in glutamatergic neurotransmission that may account for these morphological and physiological changes.
Like most G-protein-coupled-receptors, MORs can undergo rapid desensitization and internalization following exposure to opioid agonists [32–34]. Through overactivation of MORs, opioids induce MOR phosphorylation followed by binding to the intracellular protein arrestin, causing desensitization. Desensitization is then followed by clathrin-dependent receptor internalization and thought to contribute to opioid tolerance following repeated use [33]. With fewer cell surface receptors to bind to, opioids have less of an effect on cellular activity, which can lead to a compensatory increase in drug taking. Following a period of abstinence, these receptors can be trafficked from intracellular locations back to the plasma membrane. Interestingly, many cases of opioid overdose occur following bouts of abstinence from the drug, which may be explained by the upregulation of cell surface MORs compared to prior to drug use, resulting in decreased individual ‘tolerance’. Together, these findings demonstrate a crucial role of MORs in mediating OUDs, which likely rely on alterations to both reward pathways, such as dopamine signaling and subcellular receptor localization. Current pharmacotherapies to treat OUDs are primarily targeted towards MORs. Although it is beyond the scope of the current review, it should be mentioned that there are numerous efforts underway towards developing opioid analgesics and OUD therapeutics that act primarily on other opioid receptors, such as κ or NOP receptors [35,36].
It is important to note that following repeated or chronic opioid use, the functioning of some physiological systems may never return to their “pre-opioid” state. Hyperalgesia, a common symptom associated with opioid withdrawal [37], is thought to be a consequence of permanent dysregulation of antinociceptive and pronociceptive circuits in the brain and spinal cord [38]. Additionally, neuroplastic changes induced by opioids may produce very long term alterations in the brain reward circuitry, which may increase the incentive salience of attentional bias to these drugs of abuse. Further, dysregulation of mood systems may also promote hyperkatifeia. Defined as hypersensitivity to emotional distress specifically in opiate use disorder. Interestingly, high dose opioid use over extended periods of time are especially likely to induce heightened negative affect. While it is not surprising that high dosages of opioid use promote negative affect, it is important to note that acute use can also promote neuroadaptations that underlie increased pain sensitivity. In human studies, a single administration of fentanyl has been shown to increase pain sensitivity [39], further suggesting acute neuroadaptations. Thus, following acute or chronic opioid use, certain synaptic alterations may never return to their pre-drug induced state, and ultimately contribute to inappropriate drug seeking and taking behaviors.
2.3. Overview of Current Pharmacotherapies for OUD
The primary goals for treatment of OUDs include reducing opioid intake and craving, prevention of withdrawal symptoms, minimizing the chance of relapse, as well as a return of central and peripheral physiological systems to normal functional ranges. Like with other drug use disorders, the heterogeneity and variability in treatment responses also play a role in OUD treatment outcomes. The MOR agonist methadone, the partial MOR agonist buprenorphine, and the broad spectrum opioid receptor antagonist naltrexone have been shown to reduce OUD-related symptoms as well as opioid use and craving. While opioids elicit their rewarding and euphoric effects through activation of opioid receptors, as discussed above, the therapeutic efficacy of these pharmacotherapies generally rely on either substitute agonism or blockade of opioid receptors.
Methadone has been clinically used since 1947 for treatment of OUDs, and has been shown to reduce opioid use by as much as 33%, with individuals taking methadone being 4–5 times more likely to remain in treatment as compared to placebo treated controls [40]. Approved in the early 2000’s, buprenorphine and its various formulations (alone or combined with the opioid receptor antagonist naloxone) have also been shown to be effective in treatment of patients with OUD. Because buprenorphine is a partial agonist at MOR, it was believed that the likelihood of misuse would be significantly less than that of full agonist opioids. Further, buprenorphine can mitigate the symptoms of opioid withdrawal [41]. Naltrexone elicits its therapeutic efficacy by blocking MOR and other opioid receptors, thus preventing the euphoric effects of opioids. Via this mechanism, naltrexone can facilitate opioid withdrawal, but patients prescribed naltrexone must remain abstinent from opioids prior to treatment in order to avoid acute precipitated withdrawal. While methadone, buprenorphine and naltrexone are the primary treatment regimens for patients with OUD, combinations of these treatments (for example, buprenorphine + naloxone) as well as others have also been proven beneficial for preventing drug craving. Yet current treatment options are only partially effective for OUD, and the development of pharmacotherapies with improved efficacy and minimal side effects, induction of tolerance, and abuse liability are warranted. Further, despite mixed results, the use of psychosocial interventions in combination with pharmacotherapies have yielded some promise. Specifically, psychosocial interventions utilized in conjunction with methadone maintenance therapy tended to improve treatment outcomes, where 9 out of 14 reviewed studies reported beneficial effects [42]. However, a more recent review suggests that psychosocial support does not reliably improve treatment outcomes in individuals prescribed buprenorphine [43]. Thus, additional studies examining outcomes of multimodal treatments that use both pharmacotherapies and psychosocial interventions are necessary.
2.3.1. Naloxone and Naltrexone to Treat Opioid Overdose
Fatalities due to opioid overdose have increased dramatically in the past three decades [44]. In large doses, opioids can cause complete suppression of heart rate and respiration, resulting in death. Naloxone, a competitive MOR antagonist, has the ability to reverse opioid overdose by blocking MOR and other opioid receptors, preventing opioids from binding and eliciting downstream physiological effects. Originally developed in the early 1960’s, naloxone has become the emergent reversal agent for treatment of opioid overdose, and can be administered via intravenous, intramuscular, subcutaneous and intranasal routes [45]. While prominently used to treat opioid overdose, naloxone has shown some therapeutic efficacy for long-term treatment of OUD in patients who are already abstinent from drug use (this issue is further discussed below). Due to its competitive occupation of MORs, naloxone competes for binding sites with injected or consumed opioids on opioid receptors, blocking their central and peripheral effects and inducing an acute withdrawal syndrome that includes symptoms such as agitation, drug craving, vomiting and tachycardia. As a result of these negative symptoms, the patient forms associations of naloxone with dysphoria, and continuation of treatment alone is less likely than other forms of treatment. Naltrexone, like naloxone, is also a competitive antagonist at MOR and other opioid receptors and has shown promising therapeutic efficacy for patients who have not recently used opioids. While naloxone has proved beneficial for immediately treating opioid overdose, naloxone and naltrexone treatment should only be initiated following opioid abstinence, due to the rapid induction of withdrawal symptoms. Thus, opioid antagonists can be viewed as a ‘future’ therapeutics following initial treatment with buprenorphine, methadone or levo-alpha-acetyl-methadol (LAAM), which act as opioid substitutes, facilitate the tapering of opioid use, and alleviate withdrawal symptoms.
2.3.2. Opioid Replacement Therapies: Efficacy and Drawbacks
Clinically, two treatment paths for opioid dependence exist: opioid detoxification and maintenance treatment. Detoxification is best viewed as a necessary initial step of a treatment plan, rather than a treatment in and of itself. Most individuals seeking treatment for OUD are initially placed on opioid replacement therapy, consisting of opioid agonists and partial agonists, which act at the same receptors as abused opioids such as oxycodone or hydrocodone, yet have longer half-lives which reduce dosing frequencies, prevent withdrawal, and allow for gradual opioid tapering until abstinence is achieved. The most commonly utilized opioid replacements include methadone and buprenorphine. Methadone is a high affinity full MOR agonist that has a slow onset action and has a long elimination half-life (up to 120 hr) [46]. When taken orally, it has been proven beneficial for controlled opioid tapering where starting doses are high and tapered down over the course of weeks to months. Interestingly, faster opioid dose reduction has been linked to worse treatment retention rates [47], thus longer tapers may be more beneficial for long term treatment. However, while methadone reduces opioid craving, stress reactivity and withdrawal symptoms, it has multiple known drug-drug interactions that pose potential issues for inducing opioid-toxicity during treatment [48]. Known drugs that interact significantly with methadone include various monoamine oxidase inhibitors, tricyclic antidepressants, antiretrovirals, and anticonvulsants. Additionally, methadone treatment carries a substantial financial cost to treated individuals [49], and due to its long elimination half-life, daily dosing can result in drug accumulation and potential toxicity.
Buprenorphine, a partial MOR agonist, has therapeutic efficacy by competitively blocking or significantly reducing the reinforcing effects of other opioids. Due to its slow onset and long duration of action (elimination half-life 36 hr), buprenorphine can be taken less often than most therapeutic drugs, generally on alternating days [50]. As a partial MOR agonist, buprenorphine reduces the likelihood of unintentional overdose relative to full agonist medications such as methadone and LAAM. However, its partial agonism mechanism of action may limit its maximum efficacy. To reduce the potential misuse of buprenorphine, it is frequently formulated with naloxone which if ingested orally, the latter has minimal bioavailability and thus does not counteract the effects of buprenorphine. However, if buprenorphine + naloxone is ingested by unintended routes (i.e., intravenously), naloxone can counteract the euphoric effects of buprenorphine. Higher doses of buprenorphine have increased treatment retention rates and decrease illicit opioid use in patients with OUD [51]. However, some studies suggest that slow induction of buprenorphine use may result in poorer retention rates [52]. Thus, while buprenorphine has high therapeutic efficacy for reducing opioid use and withdrawal symptoms, the initiation of treatment, dosage and frequency of treatment for best efficacy may vary across patients.
2.3.3. Pitfalls of Long-Term Opioid Replacement Therapy
Currently, long-term treatment of OUD is reliant on opioid replacement therapies discussed above. These therapies have proven beneficial in treating OUD, with retention rates of over 60% in most cases over the first 2 years [49]. However, long-term use of opioid receptor agonists can have adverse consequences which include dependence on the treatment drug, sustained constipation, altered serum levels of prolactin and decreased testosterone levels [53]. In some circumstances, long-term opioid treatment has even led to unintentional overdose due long elimination half-lives and drug accumulation and toxicity. Aside from potential harmful side effects and dependence, long-term treatment is also extremely costly to the patient which contributes to decreased retention rates. Thus, opioid replacement therapies should not be considered as chronic treatment option, yet as a tapering strategy. However, while tapering of opioid agonists would be idealistic, there is significant evidence that tapering can promote opioid relapse. Therefore, optimal clinical decision-making to keep individuals on opioid agonists for as long as clinically necessary.
3. Alcohol Use Disorder (AUD)
AUD is a chronic, relapsing disease characterized by the loss of control over alcohol intake [54]. Almost 17 million Americans are afflicted with AUD, and the economic burden to society has been estimated at $249 billion, with the effects of binge drinking being responsible for over 70% of these costs [55]. Excessive alcohol use is one of the leading risk factors for health problems worldwide [56]. AUDs are one of the leading causes of disability and premature death for people between the ages of 15 and 49 [57]. Globally, the misuse of alcohol resulted in 3 million deaths in 2016 and is the third leading cause of preventable death in the United States [58]. AUD is also correlated with a higher risk of abusing other substances, including nicotine, psychostimulants, and opioids [58,59].
3.1. Mechanisms of Action of Alcohol
Alcohol is absorbed primarily through the gastrointestinal tract, where it diffuses into the bloodstream and crosses the blood brain barrier [60]. Alcohol and its primary metabolite, acetaldehyde, have dose-dependent depressive effects on the central nervous system [60–62]. Interestingly, despite decades of research, the precise mechanism of action of alcohol is still not well-defined, as no unique receptor or neurotransmitter system in the brain appears to be singularly targeted by alcohol. Alcohol can disrupt glutamatergic transmission through antagonism of the N-methyl-D-aspartate (NMDA) class of glutamate receptors, and can also potentiate γ-aminobutyric acid (GABA) signaling via facilitating the release of this inhibitory amino acid transmitter, and/or activating type A GABA (GABAA) receptors with specific subunit configurations [63]. High concentrations of alcohol can affect other classes of receptors, including serotonin type 3 receptors, nicotinic acetylcholine receptors, and various voltage-gated ion channels, and can disrupt lipid bilayer membrane fluidity [63,64].
The amount of alcohol ingested and the duration of intake plays a significant role in how it modulates the mesolimbic reward circuitry. After acute exposure, alcohol acts as a nonspecific pharmacological agent that enhances neuronal inhibition to produce sedative behavioral effects [65]. Chronic potentiation of GABAergic transmission can lead to compensatory changes in the brain to offset the depressive effects of alcohol achieved through increased glutamatergic transmission via NMDA receptor up-regulation [66]. These events disrupt brain glutamate homeostasis, a highly regulated system involving glutamate metabolism, release, and reuptake from extracellular spaces to preserve the ability of glutamatergic synapses to rapidly undergo LTP and LTD [67]. This disruption can alter synaptic plasticity, increase receptor desensitization and excitotoxicity, and ultimately promote the progression of AUD [67].
Acute exposure to alcohol is also known to increase extracellular levels of both endogenous opioid peptides and dopamine. As shown in Fig. 2, alcohol can disinhibit VTA dopaminergic neurons, which increases the release of dopamine in forebrain regions that contribute to the reinforcing properties of alcohol [68,69]. Increased extracellular levels of endorphins and other opioid peptides have been observed in the nucleus accumbens, amygdala, VTA and hypothalamus after both acute and chronic ethanol exposure [70–76], which are believed to contribute to the euphoric actions of alcohol and disinhibition of ventral midbrain dopamine neurons.
3.2. Overview of Current Pharmacotherapies for AUD
AUDs are genetically and phenotypically heterogeneous, with little likelihood that one treatment modality will be efficacious for all patients [68]. Less than one third of those suffering from AUD seek out treatments, and fewer than 9% of patients with AUD receive prescriptions for pharmacotherapies [57,77,78]. AUD pharmacotherapies aim to prevent relapse and promote abstinence, as well as reduce harm by curbing alcohol consumption in the event of relapse. Currently, only three medications are currently approved by the FDA for treating AUD. As with most pharmacotherapies, optimal outcomes may be achieved when these medications are administered in conjunction with psychosocial interventions [2,79]. However, literature addressing the conjunction of medication and psychosocial interventions have revealed contradicting results (see [80] for a comprehensive review).
3.2.1. Disulfiram
The first pharmacotherapy developed against AUD is tetraethylthiuram disulfide, a derivative of thiuram that was initially used to manufacture rubber [81]. Commonly called disulfiram, its therapeutic properties were discovered in 1937 when physicians noticed rubber plant workers exposed to the substance showed peculiar adverse physiological reactions to alcohol. This effect was independently verified in a Danish lab that was attempting to treat parasitic infections with disulfiram [82]. Disulfiram became FDA-approved for the treatment of AUD in the 1950s, and was widely known by its trade name Antabuse® [2,81].
Disulfiram is intended to provide aversive conditioning against alcohol to promote abstinence. Disulfiram causes increased sensitivity to the negative effects of alcohol by inhibiting acetaldehyde dehydrogenase 2 (ALDH2), an important enzyme for metabolizing acetaldehyde to acetate. Acetaldehyde is the first byproduct of alcohol oxidation, and when disulfiram is metabolized in the presence of alcohol, the lack of functional ALDH2 leads to the accumulation of acetaldehyde in the bloodstream. The increased concentration of acetaldehyde causes unpleasant symptoms characteristic of a severe hangover, including nausea, vomiting, headaches, and low blood pressure [2]. The severity of the reaction experienced is dependent on the dose of disulfiram and the amount of alcohol consumed, and has the potential to be fatal under some circumstances [81]. In the absence of alcohol, disulfiram induces minor side effects including drowsiness, headaches and an increased risk for heptatoxicity that is preventable if monitored properly [81]. In the same mechanistic vein, mutations in the Aldh2 gene that impede acetaldehyde metabolism appear to be protective against AUDs. For example, the ALDH2.2 allele, predominantly expressed in Asian populations, encodes a nonfunctional form of the enzyme and confers sensitivity to alcohol, most frequently characterized by facial flushing [83,84].
Disulfiram generally has a modest clinical efficacy. While it has been shown to reduce alcohol consumption, other studies report no difference between disulfiram and placebo treated groups [81,85]. While several factors obscure a definitive assessment of disulfiram’s efficacy in treating AUD, the robust malaise induced by disulfiram us the primary culprit in leading to poor compliance. Supervised treatments have resulted in positive outcomes for some patients [81,85–87]. Subcutaneous and intramuscular implants of disulfiram have also been tested in attempt to circumvent these compliance issues, but these delivery methods can yield complications at the site of injection which also reduces willingness to participate in treatment using this mode of delivery [88]. Preclinically, the efficacy of disulfiram has also been shown to be influenced by drug history. For example, chronic binge-drinking rats were more resistant to the symptoms of a disulfiram-induced aversive reaction than rats without a history of drinking [86]. Clinical reports confirm that humans do not experience uniform reactions to the same dosages of disulfiram [81]. Because of these issues, disulfiram treatment is often reserved for individuals with unsuccessful outcomes with other medications [2,85].
3.2.2. Naltrexone
Naltrexone is a broad spectrum opioid receptor antagonist initially designed to treat OUDs [2]. However, as continued research revealed important roles for the endogenous opioid system in mediating the effects of alcohol, opioid antagonists were subsequently found to be protective against alcohol consumption in animal models [2,89–92]. The first preclinical study documenting the efficacy of naltrexone in reducing alcohol self-administration was in monkeys [93], and naltrexone received FDA approval for treating AUD in humans in 1994.
The rewarding effects of alcohol are mediated in part by alcohol-induced release of opioid peptides such as endorphins and enkephalins acting at MOR and likely other opioid receptors [19]. Via competitive antagonism at opioid receptors, naltrexone decreases these effects of alcohol [94,95]. Naltrexone has also been shown to decrease alcohol-induced dopamine release in the nucleus accumbens [96–99], which disrupts the hedonic drive that increases craving and motivation for alcohol. Importantly, unlike disulfiram, naltrexone does not produce adverse physical sickness when metabolized in the presence of alcohol. Clinically, naltrexone reduces subjective craving for alcohol and the risk of relapse in patients, though it is most effective for reducing alcohol intake and the amount of heavy drinking done by those who do not abstain [2,100]. Due to its relatively high efficacy for reducing harmful drinking and mild side effect profile, naltrexone is one of the most frequently prescribed pharmacotherapies for AUD [2,100].
One of the biggest obstacles to successful AUD treatment with oral naltrexone is the failure of patients to adhere to a daily treatment regimen. One study reported only 15–20% of AUD patients refilled their prescriptions for naltrexone after 6 months [78]. While prolonged medication compliance is often mediocre for many SUD pharmacotherapies, adherence rates for naltrexone are reportedly better than those for disulfiram [81]. The problem of medication compliance led to the development of extended release naltrexone, an intramuscular injectable formulation that requires administration on a monthly basis [57]. Approved by the FDA in 2006, extended release naltrexone appears to be more efficacious than oral naltrexone in reducing alcohol intake and craving, which is primarily attributable to supervised administration, lack of daily fluctuations in drug blood levels, and more long-term cost effectiveness [57,78].
3.2.3. Acamprosate
Another pharmacological treatment for AUD is acamprosate (calcium acetylhomotaurinate), a calcium salt formulation of the amino acid N-acetylhomotaurine that is structurally similar to both glutamate and GABA [100,101]. Acamprosate was first developed in France in the late 1980’s, and gained approval in the U.S. in 2004 under the brand name Campral [102].
Despite extensive research, both clinically and preclinically, the exact mechanism of action of acamprosate is still not fully understood. Acamprosate exhibits poor bioavailability after oral ingestion, but has been shown in laboratory animals to counteract the hyperglutamatergic excitability induced by alcohol withdrawal [100,101]. A prevailing theory is that acamprosate helps dampen alcohol-induced hyperexcitability and restore the dysregulated by balance between glutamate and GABA systems caused by repeated episodes of alcohol intoxication and withdrawal [2,103]. Interestingly, acamprosate has been shown to increase extracellular dopamine levels in the nucleus accumbens on its own, while preventing alcohol-induced increases in dopamine release in this region [104,105]. It has been hypothesized that elevated dopamine levels in the nucleus accumbens induced by acamprosate prevent additional alcohol-induced activation of this circuit, which reduces alcohol intake [106]. More recently, some researchers have reported that acamprosate (N-acetylhomotaurine) is itself biologically inactive, but the relapse-preventing and anti-craving effects are primarily driven by the calcium component of the salt [107]. This hypothesis has been met with mixed support, and it remains unclear which moieties of acamprosate mediate its neurobiological effects [108,109].
A Cochrane Database review found acamprosate to be drug to a safe and effective treatment for maintaining abstinence in dependent patients after alcohol detoxification [110]. This meta-analysis showed that acamprosate significantly increased the duration of abstinence and decreased drinking levels across the trials in this review and others [110,111]. Acamprosate has also been found to ameliorate alcohol-induced disturbances in sleep patterns [112], and has no documented abuse potential or ill effects from overdoses [113]. The most common side effects associated with acamprosate use include gastrointestinal distress, headaches and dizziness. Acamprosate is not metabolized and is excreted via the kidneys, and is thus contraindicated in patients with renal insufficiency [113]. When comparing the efficacy of acamprosate to other AUD treatments such as naltrexone, specific outcome measures and recovery circumstances appear to play an important role. A meta-analysis investigating when a particular treatment is most helpful found that while naltrexone was more successful in reducing cravings and occurrences of heavy drinking, acamprosate was more efficacious for promoting abstinence [111]. However, the large multi-site COMBINE study revealed that acamprosate was not superior to placebo in reducing average number of drinking days or time to first heavy drinking day [114]. Thus, the clinical efficacy of acamprosate in treating AUD should be considered moderate at best.
4. Prevalence and Patterns of Co-morbid OUD and AUD
As mentioned previously, AUD is associated with the use of multiple substances [58,59,115], and alcohol intoxication has recently been identified as a risk factor for opioid use [116,117]. Data from the 2006 National Survey on Drug Use and Health (NSDUH) have shown that male and female drinkers were 70% and 90% more likely to have used opioids in the past year, respectively, compared to sober controls [118]. Due to its analgesic effects, alcohol use is common in patients with chronic pain [119–121], and binge drinkers specifically are almost twice as likely to misuse prescription opioids than nondrinkers [122]. Perhaps as a result of alcohol- or opioid-induced hyperalgesia, chronic pain patients with heavy drinking patterns report greater pain levels as compared to those with more moderate drinking patterns [123,124]. Recent NSDUH data analysrs pooled from 2012–2014 found that more than half of over 4 million people who used prescription opioids were also binge drinkers, and the prevalence of prescription opioid use increased significantly with the frequency of binge-drinking [116]. Between 2015 and 2017, among 2 million Americans with OUDs, one in 4 experienced comorbid AUD [125]. The misuse of prescription opioids is also highly correlated with polydrug use, including heavy drinking [126]. In a study assessing electronic health records, one-third of over 5,000 adult patients seeking treatment for OUD were found to have comorbid AUD [127]. A similar study assessing 1,397 OUD patients found that 38% also suffered from AUD [128]. Thus, co-morbid OUD and AUD appears to be fairly common dual diagnosis, and health care costs associated with this co-morbidity are greater than those of OUD alone [129].
The dangers of combining the potent depressant effects of both alcohol and opioids are severe, as interactions between these two drugs can increase risk of respiratory failure and death when co-used. Not surprisingly, alcohol use has been linked to opioid overdoses [117]. In the opioid epidemic that currently plagues the U.S., it is estimated that 130 citizens die every day from an opioid-related overdose [7]. Esser et al. reported 1 out of every 5 of these opioid-related deaths involved alcohol [116]. In addition, alcohol is also associated with 20% of opioid related hospitalizations in young adults [116].
Many factors, both genetic and environmental, influence the etiology of comorbid OUD and AUD. Risk factors include co-morbid psychiatric disorders, low socioeconomic status, delinquency, physical or sexual abuse, and family history of substance use [130,131]. Age of first drug use is also an important factor, where earlier onset of drug use is associated with increased likelihood of development and/or severity of a substance use disorder [130]. In the 2018 National Survey on Drug Use and Health, pain relief was reported as the primary motivating factor for ingesting opiates for over half of the participants who misused them [132]. In addition, cognitive dysfunction and impaired decision-making capabilities are hallmark symptoms of SUDs, and may influence the decision to initiate polydrug use [59,133].
As mentioned above, the manifestation of AUD and OUD frequently coincides with other mental illnesses. Hartzler et al. reported that OUD patients with AUD experienced more deficits in psychosocial functioning as well as more symptoms of psychiatric disorders compared to OUD patients without com-morbid AUD [128]. AUD patients often have comorbidities with affective disorders, anxiety, attention deficit hyperactive disorder (ADHD) and psychosis, and the highest rate of comorbidity between a psychiatric illnesses and AUD is in bipolar disorders (39–46%) [59,125]. Patients with SUDs in conjunction with other mental disorders tend to have poor adherence to pharmacotherapies and suffer worse prognoses and treatment successes [134,135].
AUDs have a robust heritable component estimated to account for 40–60% of the risk of developing this disorder, as evidenced through twin and pedigree studies, while a heritable predisposition can also account for a moderate (30–40%) of the potential risk for developing OUDs [130,136]. It is highly unlikely that a single gene controls the onset or progression of OUDs or AUDs, thus multiple genes are believed to participate in the creation of endophenotypes that contribute to the disease. For example, endophenotypes characteristic of AUD include low initial sensitivity to alcohol and variations in the enzymes for alcohol metabolism [136]. The specificity of these endophenotypes vary, however, with the type of primary drug used, while more general traits such as impulsivity and risk-taking may generalize across SUDs [136]. Some overlap in genes of interest have been identified in both AUDs and OUDs, such as common variants in the DRD2 gene that encodes dopamine D2 receptors [137].
Overall, the co-use of alcohol and opiates may reduce the efficacy of available treatments. Opioid misuse has been tied to poorer AUD treatment outcomes and vice versa [117,138]. The SUMMIT randomized clinical trial tested collaborative care, in which six supervised sessions of psychotherapy combined with pharmacotherapies was compared with unsupervised approach lacking psychotherapy to treat OUD and AUD [139]. While the study described participants as suffering from opioid and alcohol use disorder (OAUD), a follow-up letter clarified that only 40% of patients had comorbid OUD and AUD [139,140]. Results of the SUMMIT trial indicated that at 6 months post-treatment, there were significant increases in abstinence for AUD patients only who received the collaborative care [139]. To date there are no specific treatment approaches, pharmacological or otherwise, that effectively treat co-morbid AUD and OUD. We argue that more treatment approaches for co-morbid AUD and OUD are desperately needed, and can most likely be developed with information gathered from preclinical studies on the neurobiological substrates and mechanisms that underlie adaptations in the brain following alcohol and opioid co-use.
5. Preclinical Modeling of Opioid and Alcohol Use, Seeking, and Underlying Neuroadaptations
5.1. Preclinical models for OUD and AUD
The study of opioid and alcohol seeking and neuroadaptations induced by these drugs relies largely on preclinical animal models. In general, the most common behavioral paradigm for modeling voluntary opioid drug intake in animals is referred to as the intravenous self-administration paradigm, where animals have the ability to self-administer opioids via the intravenous route (Figure 3). Prior to self-administration sessions, animals are implanted with an catheter into an accessible vein (usually the jugular or femoral vein), as well as a vascular access port, permitting infusion of drug solutions. During self-administration sessions, animals are trained to acquire drug injections by performing a behavioral task, such as pressing a lever or nose poking into a designated aperture, which results in activation of a computer-controlled syringe pump that delivers the drug solution (i.e., morphine or fentanyl). While the pattern of responding and reinforcement schedule varies across laboratories, drug-paired cues, including stimulus lights or high frequency tones, are often used to allow for the formation of discrete drug-cues associations. It is well established that these self-administration procedures in animals model human patterns of drug intake (for review see [141]), and have the ability to predict the abuse liability of novel compounds [142]. To our knowledge, there are no reports of the development of an animal model of co-intake of alcohol and opioids, however it is highly conceivable that such models might entail the simultaneous or sequential access to i.v. opioid and oral alcohol reinforcers in a single self-administration session, which have been similarly developed as a model for nicotine and alcohol co-use [143].
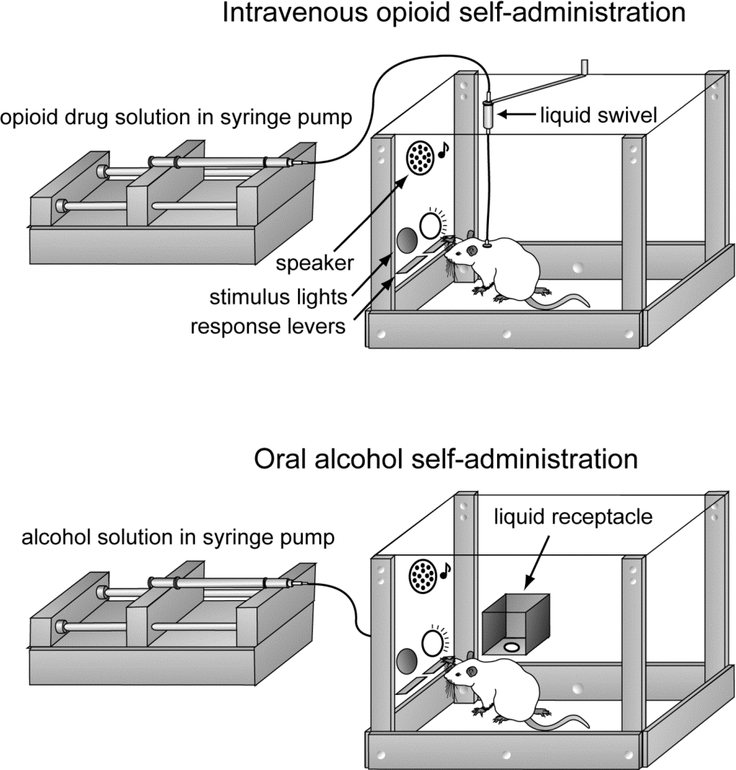
Figure 3.
Common rodent models of opioid and alcohol self-administration. In the intravenous self-administration paradigm (top), rodents are surgically implanted with a venous catheters and vascular access port, which is connected to plastic tubing and a liquid swivel that allow for intravenous delivery of an opioid (i.e., fentanyl or heroin) following completion of an operant task (i.e., correct pressing one of two available levers). During drug infusion, which is achieved by a computer-controlled syringe pump, discrete auditory and/or visual cues are presented that allow for the animal to develop drug-cue associations. In the oral self-administration paradigm (bottom), non-surgerized rodents are provided with a small amount of an alcohol-containing liquid (usually 0.1 ml of a 10–20% w/v solution) into a liquid receptacle following completion of an operant task (i.e., lever press). As with intravenous self-administration, alcohol solution delivery is accompanied by presentation of discrete auditory and/or visual cues that allow for the animal to develop alcohol-cue associations. Reproduced from [187] with permission of Springer Nature.
Humans exhibit variable patterns of opioid intake within individual days as well as across time, ranging from short-term to long-term, which in animal models can be classified as subchronic and chronic, respectively. Most traditional self-administration paradigms are conducted during short daily sessions (usually 1–3 hr/day). Short-term access conditions, which were the standard for many years, are now viewed as less relevant to human OUDs. Over the past several years, opioid self-administration models have modified to be more consistent with the human patterns of intake. The development of ‘binge’ patterns more closely mimic human binge use, where animals are allowed drug access for extended periods of time (i.e. multiple consecutive days) [144]. Incorporating binge/chronic drug self-administration paradigms more closely resemble human drug consumption; however, the study of both short-term and long-term opioid self-administration and underlying neuroadaptations are important and lend insight into prescription subchronic use as well as long-term intake.
Aside from self-administration procedures, the conditioned placed preference (CPP) paradigm has been extensively used to study the rewarding effects of opioids. The CPP paradigm is a classical conditioning approach conducted in a small hemisected chamber with distinct flooring, texture, and walls with visual patterns on either of the two sides. A drug such as heroin is administered by an experimenter and paired with one of the distinct contexts by confining the animal to that context for a short period of time (i.e., 30 min). On alternating days, placebo (i.e., saline) is administered and paired with the other context. Animals undergo repeated drug-context conditioning over multiple continuous days, which is followed by assessment of time spent in each location when free access to the entire apparatus is allowed. Generally speaking, if an animal chooses to spend a majority of its time in the context in which drug was given, the drug can be considered rewarding. While this paradigm relies on non-contingent drug administration, it allows the investigator to infer positive and negative rewarding effects of drugs of abuse.
In alcohol self-administration paradigms, oral delivery is most common as it is less invasive compared to intravenous infusions and more closely mimics the human mode of ingestion [145]. Due to the naturally aversive taste of alcohol, animals are often pretrained to consume alcohol to promote acquisition of responding. One method of pretraining is called sucrose fading, where the animal initially self-administers a sweetened alcohol solution that is gradually depleted of the sweetener until only alcohol remains. Blood alcohol concentrations (BACs) are usually determined to verify that relevant levels of alcohol are being consumed [146]. Animals can also be made dependent on alcohol via chronic alcohol vapor exposure in specialized chambers prior to commencement of oral self-administration [147].
The drinking-in-the-dark (DID) paradigm is a model for binge-like drinking that has been tested in both rats and mice [148,149]. Animals are single-housed in standard cages and receive limited access to a bottle of ethanol for a few hours during their dark cycle, as rodents have been found to drink more alcohol during this phase of the circadian period. Limited access promotes the rapid ingestion of alcohol in a binge-like fashion. Alcohol-containing bottles are weighed before and after access to track their consumption rate, and BACs are sampled on the test day. In a similar design, the two-bottle choice test allow animals to choose between either alcohol-containing or nonalcoholic solutions placed on the home cage. In this procedure, the amount of liquid consumed is typically measured over longer periods of time (i.e., every 24 hr), and is used to assess the animal’s general preference for alcohol. The primary disadvantage of DID and two-bottle choice paradigms are that the animal’s motivation for seeking alcohol is difficult to assess, as distinct operant tasks (i.e., lever presses) are not needed in order to obtain the alcohol containing solution [150].
5.2. Reinstatement Procedures
The reinstatement model is widely used in the addiction field to model relapse and/or drug-seeking behavior, and to explore their underlying mechanisms. First reported by de Wit and Stewart in 1981 [151], following extinction of drug-reinforced behavior, re-exposure to drug-paired cues can reinstate drug seeking (i.e., pressing the lever that previously delivered the drug solution). Additionally, non-contingent exposure to the drug, as well as some stressors, following extinction also induce reinstatement of drug seeking [152]. Because reinstatement in animal models includes factors such as re-exposure to drug and/or drug associated cues, which closely mimic factors known to provoke craving and relapse in humans, it serves as a tool for exploring mechanisms underlying drug relapse. Reinstatement procedures have extensively expanded our understanding of pharmacotherapies and their ability to prevent drug relapse. In general, potential pharmacotherapies are administered prior to reinstatement testing, which allows researchers the ability to observe whether a pharmacological compound has therapeutic efficacy by inhibiting drug seeking behavior in this setting. Additionally, reinstatement procedures allow researchers to identify relapse-related alterations in neuronal circuitries including physiological and morphological alterations.
5.3. Opioid- and Alcohol-induced Neuroadaptations
5.3.1. Opioids
Due to the vast expression of opioid receptors throughout the brain, it is not surprising that opioids can have profound effects on various neuronal circuitries. Like alcohol, opioids also have effects on glutamatergic and GABAergic neurotransmission. The effects of opioids on synaptic plasticity and subcellular mechanisms have been detailed in a recent review [24]. Briefly, following administration acute doses of opioids such as morphine, the ratio of amplitudes of electrical currents in neurons mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA type glutamate receptors in VTA dopamine neurons is enhanced, suggesting potentiation of glutamatergic (specifically AMPA-mediated) synaptic efficacy [24]. As well, GluR1-containing AMPA receptors are upregulated within the VTA following chronic exposure to morphine [153]. Interestingly, MOR activation depresses excitatory postsynaptic potentials (EPSPs) mediated by NMDA receptors within the nucleus accumbens, also suggesting that opioids have direct effects on glutamatergic synaptic plasticity [154]. Additionally, opioids enhance the synaptic strength of glutamatergic neurons located within the central nucleus of the amygdala, which is reliant on functional delta-opioid receptor regulation [155]. In further support of a pivotal role of glutamate in opioid actions, opioid exposure induces vast changes in NMDA receptor expression, and induces long lasting effects on both accumbal and amygdalar circuitry [156]. Opioids can exhibit bidirectional effects on neuronal plasticity within the VTA. A single exposure to morphine can block presynaptic inhibitory long-term potentiation (LTP), a form of GABAergic plasticity, in dopaminergic neurons of the VTA [157,158], an effect that is transient and absent 5 days post-injection. However, morphine can also initiate long-term depression (LTD) at GABAergic synapses within the VTA, reducing dopaminergic inhibition and promoting long-term potentiation (LTP) at excitatory synapses [159]. GABAergic LTD is shown to be dependent on D2 type dopamine receptor activation, and requires various post-synaptic scaffolding proteins [160]. Most of the literature examining the cellular effects of and neuroadaptations to opioids conclude that the majority of effects of these drugs on synaptic and circuit modifications are reliant on LTD of inhibitory GABAergic neurons [20,24,158], which can lead to enhanced glutamatergic plasticity, as well as increased dopaminergic release and tone.
While opioids have profound effects on neuronal circuitry, these effects may also play a major role in mediating increased pain sensitivity as well as pervasive negative affect states following withdrawal. Acute opioid administration has been shown to induce analgesia through activation of the anterior and posterior cingulate cortex [161], anterior insula [162], as well as the hypothalamus [163], regions well-associated with the regulation of central autonomic nervous system function. Opioid-induced hyperalgesia appears to be mediated by various glutamatergic mechanisms. For example, activation of NMDA receptors has been shown to induce hyperalgesia in animals [164–166], which is supported by additional studies reporting NMDA-receptor antagonists to reduce opioid-induced hyperalgesia or allodynia in both animals [164,165] and humans (for review see [167]). Heinl and colleagues have shown that opioid withdrawal leads to potentiation of C-fiber synapses within the dorsal horn of the spinal cord, which is reliant on activation of MORs and NMDA receptors [38] and contributes to pronociceptive effects. Withdrawal-induced LTP at C-fiber synapses is blocked in the presence of NMDA receptor antagonists [38], suggesting that opioid-induced hyperalgesia can be prevented using NMDA receptor antagonists. A complete understanding of the mechanisms of underlying opioid-induce hyperalgesia is still lacking, yet these studies demonstrate that long-term opioid-induced synaptic modifications play a significant role in mediating and promoting these effects.
While the studies described above have advanced our understanding of opioid-induced brain changes following acute exposure, the need for additional studies examining chronic opioid induced synaptic changes and modifications are warranted to fully understand the neural basis of OUDs and uncover additional therapeutic treatment options. Additionally, synaptic changes underlying withdrawal and relapse remain sparse, and studies examining these components will greatly enhance our understanding of how opioid- and withdrawal-induced neuroadaptations maintain the addicted state and/or promote relapse.
5.3.2. Alcohol
Alcohol use induces neuroadaptations within the brain reward circuitry [168]. Alcohol has profound effects on glutamatergic homeostasis, where alterations have been observed in areas heavily associated with drug reward. For example, alcohol exposure decreases glutamate uptake within the nucleus accumbens which may be mediated, in part, by alterations in the expression of glutamate transporters [169]. Further, acute ethanol intake has been shown to enhance glutamatergic tone within the periaqueductal gray [170]. As well, alcohol inhibits NMDA receptor function, and its actions at these receptors are linked to alcohol-associated phenotypes including dependence, tolerance, craving and relapse [171]. Interestingly, alcohol actions at NMDA receptors are specific for those containing NR1/NR2A and NR1/NR2B subunit configurations [172]. While the onset of alcohol- induced synaptic depression is extremely rapid, some studies suggests that depression of NMDA receptor mediated currents can be counteracted by tolerance to ethanol [173]. For example, NMDA receptor activity returns to basal levels after long periods of bath application of alcohol in hippocampal [173] and rostral ventrolateral medulla neurons [174]. In cell culture, acute and chronic ethanol exposure induces excitotoxicity in rat cerebral cortical neurons mediated by NMDA receptor stimulated lactate dehydrogenase release [175]. Thus, alcohol can have profound effects on glutamatergic transmission and can lead to long-lasting detrimental effects including neuronal cell death. Moreover, chronic alcohol consumption increases the number and density of NMDA receptors within the brain [171,172] and while currents mediated by NMDA receptors are blunted, their activation kinetics and likelihood of activation can be enhanced following chronic exposure.
In addition to glutamatergic alterations, alcohol also alters GABAergic neurotransmission, specifically through actions at the GABAA receptor. Specifically, alcohol induces transient changes in GABAA receptor levels, composition, and cellular localization [176], and in vitro studies have repeatedly shown that ethanol down-regulates GABAA–mediated phasic and inhibitory currents, which is consistent with changes in receptor surface expression [177]. Further, binge-like drinking decreases GABAA receptor levels within the dorsal raphe nucleus [178], decreases c-fos immunoreactivity within the nucleus accumbens (suggesting the reward circuitry activity to be dampened) [179]. Lastly, GABAA receptor antagonists reduce alcohol effects in vivo while GABAergic positive allosteric modulators (PAMs) mimic alcohol effects, further supporting the role of direct activation and alterations of GABAergic circuitry within the reward pathway [176,180].
As discussed earlier, the mesolimbic dopamine pathway is known to play a major role in drug reinforcement. While alcohol likely exhibits multiple modes of control over dopaminergic circuits, elevated levels of dopamine have been consistently observed following acute alcohol consumption (for review see [181]). Specifically, alcohol directly enhances VTA dopaminergic neuron excitability and increases firing frequency by enhancing the hyperpolarization activated inward cation current (Ih) [182,183], which is responsible for modulating the rhythmic firing activity displayed by VTA dopaminergic neurons. Interestingly, electrophysiological recordings have revealed that only DA neurons lacking D2 autoreceptors show enhanced excitability in the presence of acute alcohol exposure [184], suggesting specific pathways in which alcohol may induce its effects. Furthermore, through its actions on GABAergic interneurons, alcohol can disinhibit dopamine neurons, also leading to enhanced dopamine release. While acute alcohol exposure enhances dopamine release, chronic alcohol exposure produces allostatic changes within the dopaminergic circuitry, decreasing dopamine release. While less is understood about the neuroadaptations produced by prolonged alcohol exposure, one hypothesis suggest that chronic ethanol decreases Ih current density, thus decreasing dopaminergic transmission, which has been observed following alcohol withdrawal [182]. Additionally, dopamine receptor sensitization is also induced by chronic alcohol exposure, and may also play a role in the reduction of dopamine release [185]. Taken together, acute and chronic alcohol exposure has profound effects on the mesolimbic dopamine system, however these effects may be vastly different from each other. Therefore, further studies identifying the effects of alcohol at different stages of alcohol use, dependence, withdrawal and relapse may be beneficial for treatment options of AUD, which are highly reliant on stage of the disease and frequency of prior alcohol use.
5.3.3. Alcohol, Opioids and the Endogenous Opioid System
While the neuroadaptations induced by alcohol or opioids alone have been widely studied, such changes induced by co-use remains vastly unexplored. Interestingly, both alcohol and opioids are known to induce the release of endogenous opioids including β-endorphins, enkephalins and dynorphins. Acute alcohol consumption has been linked with the release of all three of these endogenous endorphins within various mesolimbic structures [70–76]. Similar to illicit opioids, β-endorphins are potent endogenous activators of MORs. Thus, alcohol and illicit opioids are capable of modulating similar signaling cascades through MORs, eliciting similar reward pathway effects and modifications. Additionally, endogenous opioids released following alcohol consumption have the ability to modulate dopamine release within the mesolimbic reward circuitry, which may account, in part, for the elevations in dopamine levels following consumption of both alcohol and opioids. In addition, MORs are located on local GABAergic interneurons in the VTA, and endorphins can produce disinhibition of mesolimbic dopaminergic neurons, promoting dopamine release (Fig. 2). Studies examining the neuroadaptations due to co-use of alcohol and opioids remain sparse, however with the abundance of studies linking the endogenous opioid system to opioids and alcohol use, it is plausible that co-use results in exacerbation of these cellular mechanisms and may drive the reinforcing properties of both drugs types.
While the current understanding of underlying synaptic alterations induced by AUD and OUD heavily relies on single-use drug pre-clinical models, we believe that the development of a co-occurring AUD and OUD animal model would provide the field a platform to (1) examine co-use induced alterations in synaptic modifications, (2) compare poly-substance use synaptic modifications with single-drug induced modifications and (3) preclinically examine the efficacy of pharmacotherapies and their effects on reducing poly-substance use.
6. Conclusion
OUD and AUD are chronic, relapsing diseases that can be, ameliorated in some cases, with pharmacological interventions. While no one treatment will provide successful recovery for all those afflicted, there are options available to increase the chances of remaining drug-free. Yet with the heterogeneity of AUDs and OUDs and treatment responses, more therapeutic options are needed to meet individual patient needs. Opioid maintenance therapies utilize partial and full opioid agonists (i.e., buprenorphine and methadone) to activate MORs in place of abused opioids, effectively reducing withdrawal symptoms and drug craving. Conversely, opioid antagonists such as naloxone and naltrexone act by preventing the activation of MORs to promote detoxification. Naltrexone is also effective for treating AUD, presumably by blocking signals from alcohol-induced release of endorphins or other opioid peptides. Thus, naltrexone is a treatment option for AUD and OUD, targeting shared mechanisms between the two disorders and may lead to better outcomes in individuals with co-occurring AUD and OUD compared to other pharmacotherapies. The efficacy of IM XR naltrexone in co-occurring OUD and AUD may be influenced by the presence or absence of psychiatric disorders or depressive symptoms. Among individuals with anhedonia, emerging data suggests opioid kappa antagonism with buprenorphine may take precedence over widespread mu-opioid blockade with naltrexone. Other AUD treatments are more mechanistically diverse: acamprosate modulates both glutamatergic and GABAergic neurotransmitter systems, whereas disulfiram produces an adverse physical reaction to alcohol by inhibiting a key metabolic enzyme. The safety and efficacy of these pharmacotherapies have been evaluated clinically, as well as preclinically in models of substance use, most notably the self-administration paradigm. Unfortunately, current research models focus primarily on mono-substance use patterns, despite the common comorbidity of AUD and OUD. The overwhelming evidence for polysubstance use disorders with regards to alcohol and opioids illuminates the need for more research to find effective treatments that will more consistently address the ongoing substance use epidemics for a wider population.
7. Expert Opinion
Current evidence supports the notion that alcohol and opioid use often go hand-in-hand. OUD and AUD have been studied extensively as independent addictions, yet polysubstance use is extremely prevalent between opioids and alcohol. While one of the many goals of OUD and AUD research is the development of effective treatment options, these developments rely in part on preclinical studies to identify: 1) misuse potential of novel medications, 2) types and patterns of use and co-use (i.e. acute versus chronic), 3) substance induced neuroadaptations, and 4) efficacy of candidate medications for use in treatment. The current lack of preclinical models exploring opioid and alcohol co-use has hindered our ability to fully understand the ensuing neuroadaptations and thus, potential treatment pharmacotherapies. As a result, there is a lack of understanding regarding additive effects of the substances, ceiling effects of these combinations, or whether synergistic or opposing compensations can occur. Thus, we believe to gain an appropriate understanding of opioid and alcohol co-use the development of preclinical models that accurately depict human consumption are necessary. Fully understanding physiological alterations induced by co-use of opioids and alcohol in preclinical models will hopefully enhance the development of pharmacotherapies that can aide in treating patients this form of polysubstance use, which otherwise may only be geared towards the more ‘severe’ addiction (i.e., AUD or OUD). While development of these models will impose challenges, such as appropriately modeling human consumption patterns, strides are currently being made to develop such models and have yielded interesting preliminary results [186]. Development of other polysubstance use models will hopefully be achieved in the near future, but will require ample time to establish appropriate and translatable polysubstance use behaviors as well as their neurophysiological underpinnings.
Further, treatment options for patients with AUD and OUD often overlap, suggesting common neurobiological bases of these disorders. However, treatment efficacies for polysubstance use in general are less well characterized. Clinical studies fully evaluating polysubstance tendencies in patients currently receiving treatment, and whether their current treatments alleviate cravings for both drugs of abuse, are clearly warranted. Such studies will lend insight into whether current pharmacotherapies and beneficial for patients engaging in co-use patterns. Together, preclinical advancements to establish behavioral models exploring phenomenon relevant to polysubstance use, in parallel with clinical advancements for evaluating current pharmacotherapies and their efficacy in treating co-use, are in great need and should be deemed necessary for advancement of the field.
Article Highlights.
The misuse of prescription opioids is heavily correlated with alcohol abuse, where binge alcohol drinkers are twice as likely to abuse prescription opioids than non-alcohol drinkers.
The co-morbidity of alcohol use disorder (AUD) and opioid use disorder (OUD) is becoming more prevalent within the United States.
While neuroadaptations induced by alcohol and opioids have been studied separately, changes observed within the brain reward circuitry overlap substantially between the two drugs.
Pharmacotherapies used to treat AUD and OUD elicit their therapeutic effects by acting on the same receptors and physiological pathways.
Treatment development for polysubstance is lacking, in part due to limitations in preclinical polysubstance abuse models.
The evaluation of preclinical findings in combination with successful clinical pharmacotherapies has the ability to advance the current understanding of OUD and AUD and lend insight into treatment options for individuals suffering from alcohol and opioid polysubstance use.
Acknowledgments
Funding:
This work was supported by Public Health Service grants DA042172, AA025590 and AA027962 from the National Institutes of Health.
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Wang L, Min JE, Krebs E, et al. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy 2017;49:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: A historical perspective of the last 50 years. Eur J Pharmacol 2018;836:89–101. [DOI] [PubMed] [Google Scholar]
- 3.Tarren JR, Bartlett SE. Alcohol and nicotine interactions: pre-clinical models of dependence. Am J Drug Alcohol Abuse 2016;43:146–154. [DOI] [PubMed] [Google Scholar]
- 4.McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone--a multiple-dose study. Biol Psychiatry 1998;44:250–9.* Pivotal study showing synergistic toxic effects of cocaine and alcohol polysubstance use.
- 5.Substance Abuse and Mental Health Services Administration. 2017 National Survey on Drug Use and Health; Rockville, MD; 2017. [Google Scholar]
- 6.Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015;156:569–76. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. Opioid Overdose: Drug Overdose Deaths. In: United States Department of Health and Human Services; Atlanta, GA: CDC; 2018. [Google Scholar]
- 8.De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J Neurosci 2002;22:3321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenan K, Mack K, Paulozzi L. Trends in prescriptions for oxycodone and other commonly used opioids in the United States, 2000–2010. Open Med 2012;6:e41–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006–2015. Morb Mortal Wkly Rep 2017;66:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicero TJ, Ellis MS, Kasper ZA. Increased use of heroin as an initiating opioid of abuse. Addict Behav 2017;74:63–66. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 2005;5:60–8. [DOI] [PubMed] [Google Scholar]
- 13.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993;18:247–91.** Highly influential theory of addiction.
- 14.Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci U S A 1993;90:5391–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharmacol 1989;36:265–72. [PubMed] [Google Scholar]
- 16.Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol 2004;14:370–8. [DOI] [PubMed] [Google Scholar]
- 17.Shippenberg TS, LeFevour A, Chefer VI. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol Disord Drug Targets 2008;7:442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolis EB, Hjelmstad GO, Fujita W, et al. Direct bidirectional μ-opioid control of midbrain dopamine neurons. J Neurosci 2014;34:14707–16.** Key study demonstrating the ability of opioids to directly activate a subset of midbrain dopamine neurons.
- 19.Gianoulakis C Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 2009;9:999–1015. [DOI] [PubMed] [Google Scholar]
- 20.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 1992;12:483–8.** One of the first studies detailing a potential cellular mechanism for activation of midbrain dopamine neurons by opiates.
- 21.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 1988;85:5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem 1990;55:1734–40. [DOI] [PubMed] [Google Scholar]
- 23.Nutt DJ, Lingford-Hughes A, Erritzoe D, et al. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci 2015;16:305–12.* Excellent review on the evolution and current state of one of the key dopamine theories of addiction.
- 24.Langlois LD, Nugent FS. Opiates and Plasticity in the Ventral Tegmental Area. ACS Chem Neurosci 2017;8:1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saal D, Dong Y, Bonci A, et al. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 2003;37:577–82.** Demonstration of synaptic plasticity in VTA dopamine neurons induced by varioud abused drugs as well as stress.
- 26.Authement ME, Langlois LD, Kassis H, et al. Morphine-induced synaptic plasticity in the VTA is reversed by HDAC inhibition. J Neurophysiol 2016;116:1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Berg DJ, Scherrer G. Beware of Undertow: Opioid Drugs Generate Additional Waves of Intracellular Signaling. Neuron 2018;98:870–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sklair-Tavron L, Shi WX, Lane SB, et al. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A 1996;93:11202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 2004;47 Suppl 1:33–46.** Excellent review describing the ability of different abused drugs to alter neuronal morphology and dendritic spine density in various brain regions.
- 30.Russo SJ, Bolanos CA, Theobald DE, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci 2007;10:93–9. [DOI] [PubMed] [Google Scholar]
- 31.Russo SJ, Dietz DM, Dumitriu D, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 2010;33:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol 2004;143:685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Zastrow M, Svingos A, Haberstock-Debic H, et al. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol 2003;13:348–53. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 2001;53:1–24. [PubMed] [Google Scholar]
- 35.Zhou Y, Leri F. Neuroscience of opiates for addiction medicine: from stress-responsive systems to behavior. Prog Brain Res 2016;223:237–51. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald MK. Anti-stress neuropharmacological mechanisms and targets for addiction treatment: A translational framework. Neurobiol Stress 2018;9:84–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carcoba LM, Contreras AE, Cepeda-Benito A, et al. Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. J Addict Dis 2011;30:258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinl C, Drdla-Schutting R, Xanthos DN, et al. Distinct mechanisms underlying pronociceptive effects of opioids. J Neurosci 2011;31:16748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyons PJ, Rivosecchi RM, Nery JP, et al. Fentanyl-induced hyperalgesia in acute pain management. J Pain Palliat Care Pharmacother 2015;29:153–60. [DOI] [PubMed] [Google Scholar]
- 40.Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009:CD002209. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Kresina TF, Campopiano M, et al. Use of pharmacotherapies in the treatment of alcohol use disorders and opioid dependence in primary care. Biomed Res Int 2015;2015:137020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dugosh K, Abraham A, Seymour B, et al. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J Addict Med 2016;10:93–103.** Outstanding review on combined psychosocial and pharmacotherapies for treadment of OUD
- 43.Carroll KM, Weiss RD. The role of behavioral interventions in buprenorphine maintenance treatment: A review. Am J Psychiatry 2017;174:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths - United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–82. [DOI] [PubMed] [Google Scholar]
- 45.Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf 2018;9:63–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilgrim JL, McDonough M, Drummer OH. A review of methadone deaths between 2001 and 2005 in Victoria, Australia. Forensic Sci Int 2013;226:216–22. [DOI] [PubMed] [Google Scholar]
- 47.Gerra G, Zaimovic A, Rustichelli P, et al. Rapid opiate detoxication in outpatient treatment: relationship with naltrexone compliance. J Subst Abuse Treat 2000;18:185–91. [DOI] [PubMed] [Google Scholar]
- 48.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict 2010;19:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother 2009;10:1727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petry NM, Bickel WK, Badger GJ. Examining the limits of the buprenorphine interdosing interval: daily, every-third-day and every-fifth-day dosing regimens. Addiction 2001;96:823–34. [DOI] [PubMed] [Google Scholar]
- 51.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess 2007;11:1–171, iii–iv. [DOI] [PubMed] [Google Scholar]
- 52.Fischer G, Gombas W, Eder H, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction 1999;94:1337–47. [DOI] [PubMed] [Google Scholar]
- 53.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry 2015;23:63–75. [DOI] [PubMed] [Google Scholar]
- 54.Crews FT, Vetreno RP. Stress and alcohol priming of brain toll-like receptor signaling in alcohol use disorder. Alcohol Alcohol 2018;53:639–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sacks JJ, Gonzales KR, Bouchery EE, et al. 2010 National and state costs of excessive alcohol consumption. Am J Prev Med 2015;49:e73–e79. [DOI] [PubMed] [Google Scholar]
- 56.Organization WH. Global status report on alcohol and health. Geneva, Switzerland: 2018. [Google Scholar]
- 57.Malone M, McDonald R, Vittitow A, et al. Extended-release vs. oral naltrexone for alcohol dependence treatment in primary care (XON). Contemp Clin Trials 2019;81:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serdarevic M, Gurka KK, Striley CW, et al. Prevalence of concurrent prescription opioid and hazardous alcohol use among older women: Results from a cross-sectional study of community members. J Community Health 2019;44:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preuss UW, Gouzoulis-Mayfrank E, Havemann-Reinecke U, et al. Psychiatric comorbidity in alcohol use disorders: results from the German S3 guidelines. Eur Arch Psychiatry Clin Neurosci 2018;268:219–229. [DOI] [PubMed] [Google Scholar]
- 60.Vasiliou V, Ziegler TL, Bludeau P, et al. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genomics 2006;16:51–8. [DOI] [PubMed] [Google Scholar]
- 61.Correa M, Manrique HM, Font L, et al. Reduction in the anxiolytic effects of ethanol by centrally formed acetaldehyde: the role of catalase inhibitors and acetaldehyde-sequestering agents. Psychopharmacology (Berl) 2008;200:455–64.**Review of the ability of ethanol as well as its metabolites to activate endogenous opioid circuits in the brain.
- 62.Font L, Luján M, Pastor R. Involvement of the endogenous opioid system in the psychopharmacological actions of ethanol: the role of acetaldehyde. Frontiers in behavioral neuroscience 2013;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crews FT, Morrow AL, Criswell H, et al. Effects of ethanol on ion channels. Int Rev Neurobiol 1996;39:283–367. [DOI] [PubMed] [Google Scholar]
- 64.You C, Savarese A, Vandegrift BJ, et al. Ethanol acts on KCNK13 potassium channels in the ventral tegmental area to increase firing rate and modulate binge-like drinking. Neuropharmacology 2019;144:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vengeliene V, Bilbao A, Molander A, et al. Neuropharmacology of alcohol addiction. Br J Pharmacol 2008;154:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rao PS, Bell RL, Engleman EA, et al. Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci 2015;9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol 2010;21:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anton RF, Schacht JP, Book SW. Pharmacologic treatment of alcoholism. Handb Clin Neurol 2014;125:527–42. [DOI] [PubMed] [Google Scholar]
- 69.Foddai M, Dosia G, Spiga S, et al. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology 2004;29:530–6. [DOI] [PubMed] [Google Scholar]
- 70.Olive MF, Koenig HN, Nannini MA, et al. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 2001;21:RC184 (1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marinelli PW, Bai L, Quirion R, et al. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res 2005;29:1821–8. [DOI] [PubMed] [Google Scholar]
- 72.Marinelli PW, Lam M, Bai L, et al. A microdialysis profile of dynorphin A1–8 release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res 2006;30:982–990. [DOI] [PubMed] [Google Scholar]
- 73.Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169:60–67. [DOI] [PubMed] [Google Scholar]
- 74.Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of b-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience 2004;127:777–784. [DOI] [PubMed] [Google Scholar]
- 75.Jarjour S, Bai L, Gianoulakis C. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol Clin Exp Res 2009;33:1033–43. [DOI] [PubMed] [Google Scholar]
- 76.Lam MP, Marinelli PW, Bai L, et al. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology 2008;201:261–71. [DOI] [PubMed] [Google Scholar]
- 77.Knox J, Wall M, Witkiewitz K, et al. Reduction in non-abstinent World Health Organization (WHO) drinking risk levels and drug use disorders: 3-year follow-up results in the US general population. Drug Alcohol Depend 2019;201:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bryson WC, McConnell J, Korthuis PT, et al. Extended-release naltrexone for alcohol dependence: persistence and healthcare costs and utilization. Am J Manag Care 2011;17 Suppl 8:S222–34. [PMC free article] [PubMed] [Google Scholar]
- 79.Plosker GL. Acamprosate: a review of its use in alcohol dependence. Drugs 2015;75:1255–68. [DOI] [PubMed] [Google Scholar]
- 80.Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: A review. JAMA 2018;320:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh JJ, Pettinati HM, Kampman KM, et al. The status of disulfiram: a half of a century later. J Clin Psychopharmacol 2006;26:290–302.** Historical review on disulfiram and its actions as an alcohol deterrent as well as a possible therapeutic for cocaine dependence.
- 82.Hald J, Jacobsen E. A drug sensitizing the organism to ethyl alcohol. Lancet 1948;2:1001–4. [DOI] [PubMed] [Google Scholar]
- 83.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 84.Peng GS, Yin JH, Wang MF, et al. Alcohol sensitivity in Taiwanese men with different alcohol and aldehyde dehydrogenase genotypes. J Formos Med Assoc 2002;101:769–74. [PubMed] [Google Scholar]
- 85.Fuller RK, Gordis E. Does disulfiram have a role in alcoholism treatment today? Addiction 2004;99:21–4. [DOI] [PubMed] [Google Scholar]
- 86.Tampier L, Quintanilla ME, Israel Y. Tolerance to disulfiram induced by chronic alcohol intake in the rat. Alcohol Clin Exp Res 2008;32:937–41. [DOI] [PubMed] [Google Scholar]
- 87.Chick J, Gough K, Falkowski W, et al. Disulfiram treatment of alcoholism. Br J Psychiatry 1992;161:84–9. [DOI] [PubMed] [Google Scholar]
- 88.Johnsen J, Morland J. Disulfiram implant: a double-blind placebo controlled follow-up on treatment outcome. Alcohol Clin Exp Res 1991;15:532–6. [DOI] [PubMed] [Google Scholar]
- 89.Czirr SA, Hubbell CL, Milano WC, et al. Selected opioids modify intake of sweetened ethanol solution among female rats. Alcohol 1987;4:157–60. [DOI] [PubMed] [Google Scholar]
- 90.Reid LD, Czirr SA, Bensinger CC, et al. Morphine and diprenorphine together potentiate intake of alcoholic beverages. Alcohol 1987;4:161–8. [DOI] [PubMed] [Google Scholar]
- 91.Hubbell CL, Czirr SA, Reid LD. Persistence and specificity of small doses of morphine on intake of alcoholic beverages. Alcohol 1987;4:149–56. [DOI] [PubMed] [Google Scholar]
- 92.Reid LD, Delconte JD, Nichols ML, et al. Tests of opioid deficiency hypotheses of alcoholism. Alcohol 1991;8:247–57. [DOI] [PubMed] [Google Scholar]
- 93.Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci 1980;26:679–88.** One of the first pharmacological studies of naltrexone and its ability to reduce alcohol intake in laboratory animals.
- 94.Hendershot CS, Wardell JD, Samokhvalov AV, et al. Effects of naltrexone on alcohol self-administration and craving: meta-analysis of human laboratory studies. Addict Biol 2017;22:1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heilig M, Goldman D, Berrettini W, et al. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci 2011;12:670–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res 1993;621:137–40. [DOI] [PubMed] [Google Scholar]
- 97.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 1992;89:2046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav 2004;81:339–58. [DOI] [PubMed] [Google Scholar]
- 99.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 1998;18:10663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bouza C, Angeles M, Magro A, et al. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 2004;99:811–28. [DOI] [PubMed] [Google Scholar]
- 101.Littleton J, Zieglgänsberger W. Pharmacological mechanisms of naltrexone and acamprosate in the prevention of relapse in alcohol dependence. Am J Addict 2003;12:s3–s11. [DOI] [PubMed] [Google Scholar]
- 102.Witkiewitz K, Saville K, Hamreus K. Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility. Ther Clin Risk Manag 2012;8:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams SH. Medications for treating alcohol dependence. Am Fam Physician 2005;72:1775–80. [PubMed] [Google Scholar]
- 104.Olive MF, Nannini MA, Ou CJ, et al. Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release. Eur J Pharmacol 2002;437:55–61. [DOI] [PubMed] [Google Scholar]
- 105.Cano-Cebrian MJ, Zornoza-Sabina T, Guerri C, et al. Local acamprosate modulates dopamine release in the rat nucleus accumbens through NMDA receptors: an in vivo microdialysis study. Naunyn Schmiedebergs Arch Pharmacol 2002;367:119–125. [DOI] [PubMed] [Google Scholar]
- 106.Chau P, Lidö HH, Söderpalm B, et al. Acamprosate’s ethanol intake-reducing effect is associated with its ability to increase dopamine. Pharmacol Biochem Behav 2018;175:101–107. [DOI] [PubMed] [Google Scholar]
- 107.Spanagel R, Vengeliene V, Jandeleit B, et al. Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology 2014;39:783–91.** Discovery of the possibility that acamprosate itself may be inert and its calcium salt may be preimarily responsible for its ability to reduce alcohol relapse.
- 108.Mann K, Hoffmann S, Pawlak CR. Does acamprosate really produce its anti-relapse effects via calcium? No support from the predict study in human alcoholics. Neuropsychopharmacology 2016;41:659–60.** Clinical refutation of evidence provided by Spanagel et al. [107] on the importance of calcium in the actions of acamprosate.
- 109.Schuster R, Koopmann A, Grosshans M, et al. Association of plasma calcium concentrations with alcohol craving: New data on potential pathways. Eur Neuropsychopharmacol 2017;27:42–47. [DOI] [PubMed] [Google Scholar]
- 110.Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev 2010:Cd004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 2013;108:275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mason BJ. Acamprosate, alcoholism, and abstinence. J Clin Psychiatry 2015;76:e224–5. [DOI] [PubMed] [Google Scholar]
- 113.Wilde MI, Wagstaff AJ. Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification. Drugs 1997;53:1038–53. [DOI] [PubMed] [Google Scholar]
- 114.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence - the COMBINE study: a randomized controlled trial. JAMA 2006;295:2003–17.* Pivotal multi-center clinical trial demonstrating efficacy of combined medical management and naltrexone, but not acamprosate, as a treatment for AUD.
- 115.Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 2005;80:105–16. [DOI] [PubMed] [Google Scholar]
- 116.Esser MB, Guy GP, Zhang K, et al. Binge drinking and prescription opioid misuse in the U.S., 2012–2014. Am J Prev Med 2019;57:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Witkiewitz K, Vowles KE. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: A critical review. Alcohol Clin Exp Res 2018;42:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Back SE, Payne RL, Simpson AN, et al. Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addict Behav 2010;35:1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vowles KE, Witkiewitz K, Pielech M, et al. Alcohol and opioid use in chronic pain: A cross-sectional examination of differences in functioning based on misuse status. J Pain 2018;19:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boissoneault J, Lewis B, Nixon SJ. Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol 2019;75:47–54. [DOI] [PubMed] [Google Scholar]
- 121.Zale EL, Maisto SA, Ditre JW. Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev 2015;37:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCabe SE, Cranford JA, Morales M, et al. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. J Stud Alcohol 2006;67:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larance B, Campbell G, Peacock A, et al. Pain, alcohol use disorders and risky patterns of drinking among people with chronic non-cancer pain receiving long-term opioid therapy. Drug Alcohol Depend 2016;162:79–87. [DOI] [PubMed] [Google Scholar]
- 124.Paulus DJ, Rogers AH, Bakhshaie J, et al. Pain severity and prescription opioid misuse among individuals with chronic pain: The moderating role of alcohol use severity. Drug Alcohol Depend 2019;204:107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend 2019;197:78–82. [DOI] [PubMed] [Google Scholar]
- 126.McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addict Behav 2008;33:1297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hser YI, Mooney LJ, Saxon AJ, et al. Chronic pain among patients with opioid use disorder: Results from electronic health records data. J Subst Abuse Treat 2017;77:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat 2010;39:114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Landsman-Blumberg PB, Katz N, Gajria K, et al. Burden of alcohol abuse or dependence among long-term opioid users with chronic noncancer pain. J Manag Care Spec Pharm 2017;23:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mistry CJ, Bawor M, Desai D, et al. Genetics of opioid dependence: a review of the genetic contribution to opioid dependence. Curr Psychiatry Rev 2014;10:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015;72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Substance Abuse and Mental Health Services Administration. (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 133.Pirastu R, Fais R, Messina M, et al. Impaired decision-making in opiate-dependent subjects: effect of pharmacological therapies. Drug Alcohol Depend 2006;83:163–8.*Key clinical trial demonstrating efficacy of brief psychotherapy and/or medication-assisted treatment for patients with AUD and/or OUD.
- 134.Gurriaran X, Rodriguez-Lopez J, Florez G, et al. Relationships between substance abuse/dependence and psychiatric disorders based on polygenic scores. Genes Brain Behav 2019;18:e12504. [DOI] [PubMed] [Google Scholar]
- 135.Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction 2004;99:1382–92. [DOI] [PubMed] [Google Scholar]
- 136.Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. Br J Pharmacol 2008;154:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang TY, Lee SY, Chen SL, et al. Association between DRD2, 5-HTTLPR, and ALDH2 genes and specific personality traits in alcohol- and opiate-dependent patients. Behav Brain Res 2013;250:285–92. [DOI] [PubMed] [Google Scholar]
- 138.Friedmann PD, Wilson D, Nunes EV, et al. Do patient characteristics moderate the effect of extended-release naltrexone (XR-NTX) for opioid use disorder? J Subst Abuse Treat 2018;85:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Watkins KE, Ober AJ, Lamp K, et al. Collaborative care for opioid and alcohol use disorders in primary care: The SUMMIT randomized clinical trial. JAMA Intern Med 2017;177:1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watkins KE, Setodji C, McCullough CM. Necessary clarifications concerning results of the SUMMIT trial-reply. JAMA Intern Med 2018;178:429. [DOI] [PubMed] [Google Scholar]
- 141.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 1996;14:375–424. [DOI] [PubMed] [Google Scholar]
- 142.Collins RJ, Weeks JR, Cooper MM, et al. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl) 1984;82:6–13. [DOI] [PubMed] [Google Scholar]
- 143.Le AD, Lo S, Harding S, et al. Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl) 2010;208:475–86.* Describes one of the first rodent models of polysubstance abuse involving intravenous nicotine and oral alcohol self-administration.
- 144.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Le AD, Kalant H. Intravenous self-administration of alcohol in rats-problems with translation to humans. Addict Biol 2017;22:1665–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blegen MB, da Silva E, Silva D, Bock R, et al. Alcohol operant self-administration: Investigating how alcohol-seeking behaviors predict drinking in mice using two operant approaches. Alcohol 2018;67:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology 2011;36:2762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holgate JY, Shariff M, Mu EW, et al. A Rat Drinking in the Dark Model for Studying Ethanol and Sucrose Consumption. Front Behav Neurosci 2017;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thiele TE, Crabbe JC, Boehm SL. “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci 2014;68:9.49.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- 151.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–43.**First of its kind study showing drug relapse-like behaviors in rodents.
- 152.Shaham Y, Shalev U, Lu L, et al. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. [DOI] [PubMed] [Google Scholar]
- 153.Fitzgerald LW, Ortiz J, Hamedani AG, et al. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci 1996;16:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martin G, Nie Z, Siggins GR. mu-Opioid receptors modulate NMDA receptor-mediated responses in nucleus accumbens neurons. J Neurosci 1997;17:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bie B, Zhu W, Pan ZZ. Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses. J Pharmacol Exp Ther 2009;329:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siggins GR, Martin G, Roberto M, et al. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci 2003;1003:196–211. [DOI] [PubMed] [Google Scholar]
- 157.Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci 2010;32:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature 2007;446:1086–90. [DOI] [PubMed] [Google Scholar]
- 159.Dacher M, Nugent FS. Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area. Neuropharmacology 2011;61:1166–71. [DOI] [PubMed] [Google Scholar]
- 160.Dacher M, Gouty S, Dash S, et al. A-kinase anchoring protein-calcineurin signaling in long-term depression of GABAergic synapses. J Neurosci 2013;33:2650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wagner KJ, Sprenger T, Kochs EF, et al. Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil. Anesthesiology 2007;106:548–56. [DOI] [PubMed] [Google Scholar]
- 162.Oertel BG, Preibisch C, Wallenhorst T, et al. Differential opioid action on sensory and affective cerebral pain processing. Clin Pharmacol Ther 2008;83:577–88. [DOI] [PubMed] [Google Scholar]
- 163.Becerra L, Harter K, Gonzalez RG, et al. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg 2006;103:208–16, table of contents. [DOI] [PubMed] [Google Scholar]
- 164.Larcher A, Laulin JP, Celerier E, et al. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience 1998;84:583–9. [DOI] [PubMed] [Google Scholar]
- 165.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 1991;251:85–7. [DOI] [PubMed] [Google Scholar]
- 166.Mao JR. NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Res Rev 1999;30:289–304. [DOI] [PubMed] [Google Scholar]
- 167.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008;24:479–96. [DOI] [PubMed] [Google Scholar]
- 168.Burnett EJ, Chandler LJ, Trantham-Davidson H. Glutamatergic plasticity and alcohol dependence-induced alterations in reward, affect and cognition. Prog Neuropsychopharmacol Biol Psychiatry 2016;65:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Melendez RI, Hicks MP, Cagle SS, et al. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res 2005;29:326–33. [DOI] [PubMed] [Google Scholar]
- 170.Li C, McCall NM, Lopez AJ, et al. Alcohol effects on synaptic transmission in periaqueductal gray dopamine neurons. Alcohol 2013;47:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hoffman PL, Rabe CS, Grant KA, et al. Ethanol and the NMDA receptor. Alcohol 1990;7:229–31. [DOI] [PubMed] [Google Scholar]
- 172.Wirkner K, Poelchen W, Köles L, et al. Ethanol-induced inhibition of NMDA receptor channels. Neurochem Int 1999;35:153–62. [DOI] [PubMed] [Google Scholar]
- 173.Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci 2003;23:3623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lai CC, Chang MC, Lin HH. Acute tolerance to ethanol inhibition of NMDA-induced responses in rat rostral ventrolateral medulla neurons. J Biomed Sci 2004;11:482–92. [DOI] [PubMed] [Google Scholar]
- 175.Chandler LJ, Newsom H, Sumners C, et al. Chronic ethanol exposure potentiates NMDA excitotoxicity in cerebral cortical neurons. J Neurochem 1993;60:1578–81. [DOI] [PubMed] [Google Scholar]
- 176.Olsen RW, Liang J. Role of GABA. Mol Brain 2017;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gonzalez C, Moss SJ, Olsen RW. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors. J Neurosci 2012;32:17874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McClintick JN, McBride WJ, Bell RL, et al. Gene expression changes in serotonin, GABA-A receptors, neuropeptides and ion channels in the dorsal raphe nucleus of adolescent alcohol-preferring (P) rats following binge-like alcohol drinking. Pharmacol Biochem Behav 2015;129:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alaux-Cantin S, Warnault V, Legastelois R, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 2013;67:521–31. [DOI] [PubMed] [Google Scholar]
- 180.Ticku MK, Mhatre M, Mehta AK. Modulation of GABAergic transmission by ethanol. Adv Biochem Psychopharmacol 1992;47:255–68. [PubMed] [Google Scholar]
- 181.Trantham-Davidson H, Chandler LJ. Alcohol-induced alterations in dopamine modulation of prefrontal activity. Alcohol 2015;49:773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol 2006;95:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res 1998;22:236–44. [PubMed] [Google Scholar]
- 184.Lammel S, Hetzel A, Häckel O, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 2008;57:760–73. [DOI] [PubMed] [Google Scholar]
- 185.Karkhanis AN, Rose JH, Huggins KN, et al. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend 2015;150:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stennett BA, Padovan-Hernandez Y, Knackstedt LA. Sequential cocaine-alcohol self-administration produces adaptations in rat nucleus accumbens core glutamate homeostasis that are distinct from those produced by cocaine self-administration alone. Neuropsychopharmacology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Olive MF and Kalivas PW. Conditioning of Addictiton. Addiction Medicine, DOI 10.1007/978-1-4419-0338-9_8, [DOI] [Google Scholar]