Abstract
Eukaryotic chromatin is a complex of genome DNA and associated proteins, and its structure and dynamics play a crucial role in regulating DNA functions. Chromatin takes rather irregular structures in the nucleus and exhibits heterogeneous sub-diffusive movements as polymers fluctuating in a fluid state. Using genome-wide single-nucleosome tracking data, heterogeneity of movements was statistically analyzed, which categorized chromatin into two types: slow chromatin that moves under structurally constrained environments and fast chromatin that moves with less constraints. Interactions of chromatin to various protein factors determine the motional constraints. For example, loss of the cohesin complex that bundles the chromatin chains reduces the motional constraints and increases the population of fast chromatin. Another example is the transcriptional machinery. While it was previously thought that the transcriptional activity is associated with more open and dynamic chromatin structure, recent studies suggested a more nuanced role of transcription in chromatin dynamics: dynamic association/dissociation of active RNA polymerase II (RNAPII) and other transcription factors and Mediators (TF-Meds) transiently bridges transcriptionally active DNA regions, which forms a loose network of chromatin and constrains chromatin movement, enhancing the slow chromatin population. This new view on the dynamical effects of transcription urges a reflection on the traditional model of transcription factories and invites the more recent models of condensates/phase-separated liquid droplets of RNAPII, transcription factors, and Mediators. The combined procedure of genome-wide single-nucleosome tracking and its statistical analysis would unveil heterogeneity in the chromatin movement, which should provide a key to understanding the relations among chromatin dynamics, structure, and function.
Keywords: Live-cell imaging, Statistical analyses, Nucleosome, Cohesin, RNA polymerase II, Transcription factory, Liquid droplets
Introduction
Human genome DNA amounts to 2 m in total length, which is confined in the cell nucleus of roughly around 10 − 20 μm diameter. This almost 105-fold compaction has been thought to be realized through a hierarchical packing. In the nucleus, DNA wraps around histone octamers to form nucleosomes, and the chain of nucleosome array with associated bound proteins is called chromatin. The nucleosomes were assumed to be spatially arranged in a highly ordered fashion by forming “30-nm chromatin fibers” (Finch and Klug 1976; Song et al. 2014) and further helically folded fibers. However, recent studies showed that the regular 30-nm fiber structures are stable only under exceptional conditions such as conditions of low salt concentration (Maeshima et al. 2016a, b) or they exist in an unstable transient manner (Risca et al. 2017). Instead, chromatin in cells was found to consist of more irregular and variable structures (Risca et al. 2017; Eltsov et al. 2008; Fussner et al. 2012; Nishino et al. 2012; Ricci et al. 2015; Chen et al. 2016; Ou et al. 2017; Cai et al. 2018; Ohno et al. 2019). These irregular chains should bend flexibly, which is consistent with the frequency of local contacts between chromatin loci observed with the chromatin conformation capture (3C technique) and related methods (Dekker 2008; Hsieh et al. 2015; Sanborn et al. 2015). Unlike chains regularly packed in a static ordered structure, irregular chains can take a number of different structures having similar stability, and dynamic transitions among them should be induced by gaining entropy of thermal movement (Maeshima et al. 2016a; Maeshima et al. 2019). Such dynamic fluid motion of chromatin chains is consistent (Shi and Thirumalai 2019) with the observed large cell-to-cell fluctuation of chromatin structure (Tan et al. 2018; Bintu et al. 2018; Finn et al. 2019). Thus, extensive advancement in the past 10 years has highlighted dynamical organization of chromatin in the nucleus and its significance in the regulation of DNA functions.
Highly dynamic chromatin has long been observed with live-cell imaging techniques; the movement of particular loci was monitored by using the LacO/LacI-GFP (Marshall et al. 1997; Heun et al. 2001; Chubb et al. 2002; Levi et al. 2005; Hajjoul et al. 2013) and related methods (Lucas et al. 2014; Germier et al. 2017), and by using the more recent CRISPR-dCas9-based methods, which allow us to label specific regions of the genome (Chen et al. 2013; Gu et al. 2018; Ma et al. 2019). The genome-wide chromatin movement was measured by observing the chromatin density flow reflected in the nuclear fluorescence intensity (Zidovska et al. 2013; Shaban et al. 2018; Shaban et al. 2019), and the genome-wide movement was observed with a molecular resolution by the single-nucleosome tracking techniques (Hihara et al. 2012; Nozaki et al. 2017; Nagashima et al. 2019). In these live-cell observations, mean square displacement (MSD) of chromatin, Mi(t) = < δri(t)2>, where <⋯> is the average along the trajectory, or their average over the ensemble of observed loci i showed sub-diffusive features as (Mi or ) ~tβ with β < 1 where δri(t) is the displacement of the ith locus during time t. The variability of β is indicative of the diversity of chromatin features depending on genomic position, cell type, and cell condition (Bronshtein et al. 2015; Shi et al. 2018; Shinkai et al. 2016). Indeed, theoretical analyses and simulations showed that β depends on whether the locus is on the surface or in the inner region of a chromosome (Shi et al. 2018), or whether it belongs to heterochromatin- or euchromatin-like region (Shinkai et al. 2016). In the genome-wide observations, the time averaged distribution of Mi was shown as “chromatin heat map” with the single-nucleosome measurement (Nozaki et al. 2017, Nagashima et al. 2019). More recently, spatial distribution of was measured by the flow-field monitoring (Shaban et al. 2019). Thus, heterogeneity in the chromatin movement reflects the local environment and structure of chromatin, which should provide a key to understanding the relations among chromatin dynamics, structure, and function.
Fast and slow chromatin
An important quantity to analyze the heterogeneity of chromatin motion is the distribution of MSD, , where δ(x) is a Dirac delta function; the single-nucleosome tracking methods provided genome-wide information of P(M, t). However, due to the low sample number of observed trajectories, the P(M, t) obtained by a direct sampling of Mi(t) from single-nucleosome tracking was rather noisy. This problem was overcome (Ashwin et al. 2019) by employing the method of iteration of integral equation introduced by Richardson (Richardson 1972) and Lucy (Lucy 1974) (RL). The RL method has been extensively used for reducing noise in the image “deconvolution” processing and recently applied to the data sampling problem in biophysics (Wang et al. 2012) and condensed-matter physics (Bhowmik et al. 2016; Bhowmik et al. 2018). Here, the underlying idea is to use the profile of particle diffusion as a two-dimensional point spread function; q(r, M) = (1/πM) exp(−r2/M). The displacement distribution after a time t is quantified through the so-called van Hove self-correlation (vHc);
| 1 |
One could consider expanding the vHc in the Gaussian basis q(r, M) and write it as:
| 2 |
The aim now is to extract P(M, t) from Eq. 2, which can be pursued numerically by using the iterative scheme,
| 3 |
where is the vHc directly calculated from Eq. 1 by using δri(t) observed by single-nucleosome trajectories (Nozaki et al. 2017) and
| 4 |
Starting from and P0(M, t) = exp(−M/M0)/M0 with a constant M0, Eqs. 3 and 4 were iteratively calculated under the constraint that Pn(M, t) ≥ 0 and ∫Pn(M, t)dM = 1 until the difference between Pn + 1(M, t) and Pn(M, t) became sufficiently small. By using Eq. 2, Gs(r, t) can be calculated from the thus obtained converged P(M, t), which is compared with the original in Fig. 1a, showing that the RL method efficiently reduces the noise to capture the essential distribution quantitatively.
Fig. 1.
Fast and slow nucleosomes. a The van Hove self-correlation (vHc) function, , directly calculated from the single-nucleosome trajectories observed in living HeLa cells by Nozaki et al. (2017) (red) is compared with the one obtained from the RL method, 2πrGs(r, t) (black). Here, vHc is shown by multiplying a factor 2πr for the area under the curve to be normalized. bP(M, t) obtained with the RL method for an example cell. cP(M, 0.5 s) vs M/M∗ calculated for 10 cells. M∗ is the separation point of bimodal peaks indicated. d MSD calculated for the fast (black) and slow (red) nucleosomes based on the nucleosome identification discussed in the main text. Dashed lines are MSD of individual 10 cells and real lines are the average over them. Reprinted from Ashwin et al. (2019)
As shown in Fig. 1b, P(M, t) obtained with the RL method was single peaked at a short time but showed a distinct bimodal feature at t = 0.5 s. This bimodal peak characterizes the nucleosome dynamically into fast and slow depending on the peak that contributes in the t = 0.5 s timescale. The MSD at the first minimum between the peaks is denoted by M∗. P(M, 0.5 s) from ten cell samples were scaled by M∗ and shown in Fig. 1c, indicating fast and slow peaks. The nucleosomes were then characterized as fast (Mi(0.5 s) > M∗) and slow (Mi(0.5 s) ≤ M∗). Shown in Fig. 1d are MSDs averaged separately for fast and slow nucleosomes, which are fitted to the power law tβ; the different β are suggestive of the different local environments the fast and slow nucleosomes experience (Ashwin et al. 2019).
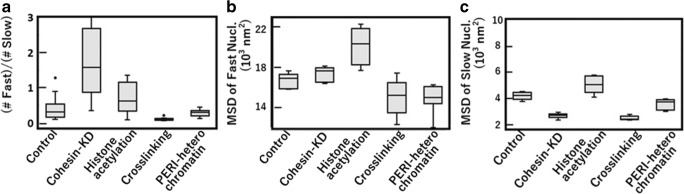
Difference between fast and slow nucleosomes was highlighted by analyzing responses of chromatin to perturbations on cells (Ashwin et al. 2019). For example, knockdown (KD) of the cohesin complex was shown to increase motion of chromatin (Ashwin et al. 2019; Nozaki et al. 2017; Dion et al. 2013). The cohesin complex can bundle chromatin chains with its ring structure and constrain the chromatin movement. Fewer chromatin constraints resulting from the cohesin KD turn the population of slow chromatin into fast chromatin (Fig. 2), which leads to the increase in the average MSD of genome chromatin (Nozaki et al. 2017). Chromatin cross-linking caused by formaldehyde (FA) addition severely decreases the population of fast chromatin and slows the chromatin motion (Fig. 2). Furthermore, when cells were treated with the histone deacetylase (HDAC) inhibitor trichostatin A (TSA), which increases acetylation of histone tails (Ricci et al. 2015; Gorisch et al. 2005), the population of slow chromatin was turned to fast chromatin. Such histone tail acetylation should weaken the nucleosome-nucleosome interactions (Kalashnikova et al. 2013; Winogradoff et al. 2015), reducing the constraint on the chromatin movement, which resulted in decrease of the slow chromatin population and the enhanced motion of both fast and slow chromatin (Fig. 2).
Fig. 2.
Effects of perturbations on HeLa cells and focusing on heterochromatin. Features of the effects on the distribution of MSD, P(M, t) at t = 0.5 s, of single nucleosomes: a the ratio of the number of fast nucleosomes to the number of slow nucleosomes, b the mean MSD of fast nucleosomes, and c the mean MSD of slow nucleosomes. Box plots of the data from 10 cells. Reprinted from Ashwin et al. (2019)
Consistently, the similar but milder effects than FA addition were found when the microscopy focus is shifted to the nuclear periphery (PERI). This region is full of heterochromatin, which is called lamina-associated chromatin domains (LADs) and has denser nucleosome-nucleosome interactions. These heterochromatin-rich regions may be tethered to the nuclear lamina (van Steensel and Belmont 2017), which should constrain the chromatin movement and increase the population of slow chromatin (Fig. 2). These regions are also enriched with the trimethylation of histone H3 lysine 9 (H3K9me3) and HP1 proteins, which can cross-link nucleosomes (Machida et al. 2018).
These results of cell perturbations and observations of the nuclear periphery regions showed that constraints on the chromatin motion arise from multiple ways: tethering or cross-linking of chromatin chains or intra-chain nucleosome-nucleosome interactions slow the chromatin motion giving rise to the population of slow chromatin which are distinguished from fast chromatin moving in the less constrained environment. Such sensitivity of chromatin motion to constraints suggests that chromatin motion is primarily driven by thermal fluctuations (Marshall et al. 1997; Levi et al. 2005) or thermal fluctuations with the effective temperature enhanced by the released free energy from chemical reactions (Di Pierro et al. 2018; Jiang and Zhang 2019). These thermal or effectively thermal movements should be less or more constrained to distinguish fast or slow chromatin.
Active RNA polymerase II globally constrains chromatin movements
Chromatin dynamics depend on the cell functional state, particularly on the transcriptional activity of the cell. On the contrary to the conventional expectation that transcription by RNA polymerase II (RNAPII) is associated with more open and dynamic chromatin, Nagashima et al. (2019) found that active RNAPII constrains chromatin chains and suppresses their movements in living human cells. The amount of active RNAPII is reduced with 5,6-dichloro-1-β-D-ribofuranosyl benzimidazole (DRB) or α-amanitin (α-AM), while active RNAPII on chromatin is stalled by actinomycin D (ActD) (Kimura et al. 2002). The averaged MSD in cells treated with DRB or α-AM increased than the control, while MSD in cells treated with ActD decreased (Fig. 2a). These data showed that active RNAPII constrains chromatin movements, and the reduction of active RNAPII enhances chromatin movements. Enhancement of chromatin dynamics by reducing active RNAPII was also confirmed by the observations that MSD of genome chromatin increased in resting G0 cells and UV-irradiated cells, which are transcriptionally less active (Nagashima et al. 2019). Consistently, some specific loci in human breast cancer cells (Germier et al. 2017), fly embryos (Chen et al. 2018), and mouse embryonic stem cells (Ochiai et al. 2015) were shown to become less dynamic when actively transcribed. On the other hand, Gu et al. (2018) reported that some transcriptional regulatory elements became more mobile upon transcriptional activation. Although further studies are needed to reconcile these seemingly incompatible results, the genome-wide results showed constraints of chromatin motion by the active RNAPII under a range of cell conditions.
Illustrated in Fig. 3b is a hypothesis explaining this movement suppression induced by active RNAPII. Recent investigations revealed that transcription factors and Mediators (TF-Meds) form dynamic condensates/clusters, which may be formed through phase separation as droplets. The active RNAPII molecules dynamically bind on and unbind from such clusters/droplets (Boehning et al. 2018, Boija et al. 2018, Cho et al. 2018, Chong et al. 2018, Lu et al. 2018, Sabari et al. 2018). Recent reports on the formation of the P-TEFb complex are consistent with this picture; P-TEFb interacts with RNAPII and forms a number of dynamic condensates/clusters/droplets in living cells (Ghamari et al. 2013; Lu et al. 2018). This picture of condensates/clusters/droplets of TF-Meds and binding of active RNAPII on them is consistent with the classical transcription factory model (Edelman and Fraser 2012; Buckley and Lis 2014; Feuerborn and Cook 2015). As shown in Fig. 3b, active RNAPII (RNAPII-Ser5P) molecules bound on these condensates/clusters/droplets can weakly connect multiple chromatin domains into a loose spatial genome chromatin network, and thereby, this loose network may globally constrain chromatin movement. These TF-Med condensates/clusters/droplets were estimated to be comparable in size with chromatin domains (Table 1).
Fig. 3.
Increased chromatin dynamics with RNAPII inhibitors. a MSD plots (±SD among n = 20 cells) of nucleosomes in the living human retinal pigment epithelium RPE-1 cells treated with RNAPII inhibitors, α-AM (pink) and ActD (brown). As untreated control, dimethyl sulfoxide (DMSO, gray) was added. b A model for the formation of a loose spatial genome chromatin network via active RNAPII-Ser5P, which can globally constrain chromatin dynamics. Chromatin domains, each formed by cohesin binding, are bound to a condensate/cluster/droplet of transcription factors and Mediators, which works as a “Hub” of loose network of chromatin. c–e Computational modeling of chromatin domain network via active RNAPII. Brownian dynamics of four chains of chromatin domains (green spheres) connected by springs (invisible) and four hubs, clusters of transcription factors/Mediators (pink spheres), were simulated. Chromatin chains bind Nglue RNAPII-Ser5P (red spheres), and transient attractive interactions were assumed between RNAPII-Ser5P-bound chromatin and hubs. c Snapshots of the Brownian dynamics with no glues (left) and with 64 glues of RNAPII-Ser5P (red spheres) (right). d The average MSD of total chromatin domains in the system calculated with various glue numbers bound on chromatin domains from Nglue = 0 to 64. e The MSD (0.5 s) distribution plots of total chromatin domains in the system with (gray) and without RNAPII-Ser5P glues (black). An arrow indicates the contribution from chromatin sites neighboring RNAPII-bound loci. Reprinted from Nagashima et al. (2019)
Table 1.
The gathering states of TF-Meds and chromatin
| Transcription factors (TFs) and Mediators (Meds) | Proteins functioning together with RNAPII to initiate transcription. | Nucleosome | Molecular complex of core histones and genome DNA with ~ 10 nm in size |
| Chromatin | A chain of nucleosomes associated with non-histone proteins | ||
| TF-Med clusters/condensates | TFs and Meds form clusters/condensates through their mutual interactions. These clusters/condensates could be regarded as droplets formed through liquid-liquid phase separation with estimated size of ~ 100 nm or larger (Cho et al. 2018) | Chromatin domains | Structurally or dynamically correlated regions in a chromatin chain formed by cohesin binding, compartmentalization, or other interactions. ~ 100 to several hundreds nm in size (Nozaki et al. 2017, Ashwin et al. 2019) |
| Compartments | Heterochromatin-like chromatin domains gather to form B compartment while euchromatin-like domains form A compartments with their size ~ μm (Finn et al. 2019) | ||
| Chromosome | An entire chain of chromatin |
The above hypothesis is consistent with the simulated results of a coarse-grained polymer model of chromatin (Nagashima et al. 2019). In Fig. 3c, snapshots of the systems simulated with the model are shown. In this simulation, RNAPII-bound sites of chromatin transiently bind on TF-Med droplets or “Hubs.” As the number of active RNAPII molecules increases, multiple chromatin sites are effectively attracted to each other through interactions between RNAPII and dynamically moving TF-Med droplets, or in other words, dynamic TF-Med droplets mediate effective attraction among multiple RNAPII-bound chromatin sites. This effective attraction among multiple chromatin sites constrains the global chromatin chain. Thus, as shown in Fig. 3d, the simulated MSD of the global system decreases as active RNAPII increases. The distribution of simulated MSD, P(M), has two peaks representing slow chromatin and fast chromatin, where the slow chromatin sites are neighbors of RNAPII-bound sites. When RNAPII is inactivated and interactions between RNAPII and TF-Med droplets are lost, slow chromatin is diminished, and the average MSD of fast chromatin increases (Fig. 3e). Similar behavior was found in the observed P(M) in cells; slow chromatin does not completely vanish upon RNAPII inhibition because there are other constraining interactions in cells, but the population of slow chromatin decreases and the average MSD of fast chromatin increases as active RNAPII is reduced, which shows the agreement between the simulated and observed results.
Heterogeneity in the chromatin motion described above is highly related to the chromatin environment and functions. While active RNA polymerase II globally constrains chromatin movements, it is interesting to note that with cohesin KD, the slow chromatin becomes even slower and fast one becomes faster (Fig. 2) (Ashwin et al. 2019). This is likely to be due to the following two factors: One is enhancement of compartments A and B, which represent “open” transcriptionally active chromatin and “closed” inactive chromatin, respectively, as found in the Hi-C contact pattern upon cohesin depletion (Schwarzer et al. 2017; Rao et al. 2017). Another factor is that cohesin loss causes co-localization of super-enhancers, which is a region comprising multiple enhancers to drive transcription, and to form hundreds of links within and across chromosomes (Rao et al. 2017), leading to more transcription-based chromatin constraints.
Perspective
Studies of chromatin dynamics, which presumably reflect chromatin organization in living cells, become more and more important to understand the genome chromatin functions. Statistical analysis described in this paper can extract valuable information from the genome-wide single-nucleosome tracking data regarding the heterogeneity and change in chromatin motion at a whole nucleus level. The combined procedure would reveal how local chromatin motions change with epigenetic modifications, the cell cycle, and cell differentiation, and how they are related to genome functions including RNA transcription, and DNA replication/repair/recombination.
Acknowledgments
We are grateful to all the collaborators Nozaki et al. (2017), Nagashima et al. (2019), and Ashwin et al. (2019) for their contribution, and Sasai and Maeshima Lab members for helpful discussion.
Funding information
This work was supported by JST-CREST Grant JPMJCR15G2; the Riken Pioneering Project; JSPS-KAKENHI Grants JP19H01860, 19H05258, JP16H04746, 16H06279 (PAGS), and 19H05273; the Takeda Science Foundation; and NIG-JOINT 2016-A2 (6).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
S. S. Ashwin, Email: ss.ashwin@gmail.com
Kazuhiro Maeshima, Email: kmaeshim@nig.ac.jp.
Masaki Sasai, Email: masakisasai@nagoya-u.jp.
References
- Ashwin SS, Nozaki T, Maeshima K, Sasai M. Organization of fast and slow chromatin revealed by single-nucleosome dynamics. Proc Natl Acad Sci U S A. 2019;116:19939–19944. doi: 10.1073/pnas.1907342116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmik BP, Das R, Karmakar S. Understanding Stokes-Einstein relation in supercooled liquids using random pinning. J Stat Mech. 2016;2016:074003. [Google Scholar]
- Bhowmik BP, Tah I, Karmakar S. Non-Gaussianity of van Hove function and dynamic heterogeneity length scale. Phys Rev E. 2018;98:022122. doi: 10.1103/PhysRevE.98.022122. [DOI] [PubMed] [Google Scholar]
- Bintu B, Mateo LJ, Su JH, Sinnott-Armstrong NA, Parker M, Kinrot S, Yamaya K, Boettiger AN, Zhuang X. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science. 2018;362:eaau1783. doi: 10.1126/science.aau1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning M, Dugast-Darzacq C, Rankovic M, Hansen AS, Yu T, Marie-Nelly H, McSwiggen DT, Kokic G, Dailey GM, Cramer P, Darzacq X, Zweckstetter M. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat Struct Mol Biol. 2018;25:833–840. doi: 10.1038/s41594-018-0112-y. [DOI] [PubMed] [Google Scholar]
- Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, Manteiga JC, Hannett NM, Abraham BJ, Afeyan LK, Guo YE, Rimel JK, Fant CB, Schuijers J, Lee TI, Taatjes DJ, Young RA. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 2018;175:1842–855.e16. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronshtein I, Kepten E, Kanter I, Berezin S, Lindner M, Redwood AB, Mai S, Gonzalo S, Foisner R, Shav-Tal Y, Garini Y. Loss of lamin A function increases chromatin dynamics in the nuclear interior. Nat Commun. 2015;6:8044. doi: 10.1038/ncomms9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MS, Lis JT. Imaging RNA polymerase II transcription sites in living cells. Curr Opin Genet Dev. 2014;25:126–130. doi: 10.1016/j.gde.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Chen C, Tan ZY, Huang Y, Shi J, Gan L. Cryo-ET reveals the macromolecular reorganization of S. pombe mitotic chromosomes in vivo. Proc Natl Acad Sci U S A. 2018;115:10977–10982. doi: 10.1073/pnas.1720476115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS, Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Lim HH, Shi J, Tamura S, Maeshima M, Surana U, et al. Budding yeast chromatin is dispersed in a crowded nucleoplasm in vivo. Mol Biol Cell. 2016;27:3357–3368. doi: 10.1091/mbc.E16-07-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Levo M, Barinov L, Fujioka M, Jaynes JB, Gregor T. Dynamic interplay between enhancer-promoter topology and gene activity. Nat Genet. 2018;50:1296–1303. doi: 10.1038/s41588-018-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WK, Spille JH, Hecht M, Lee C, Li C, Grube V, Cisse II. Mediator and RNA polymerase II clusters associate in transcription dependent condensates. Science. 2018;361:412–415. doi: 10.1126/science.aar4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S, Dugast-Darzacq C, Liu Z, Dong P, Dailey GM, Cattoglio C, Heckert A, Banala S, Lavis L, Darzacq X, Tjian R. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 2018;361:eaar2555. doi: 10.1126/science.aar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JR, Boyle S, Perry P, Bickmore WA. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol. 2002;12:439–445. doi: 10.1016/s0960-9822(02)00695-4. [DOI] [PubMed] [Google Scholar]
- Dekker J. Mapping in vivo chromatin interactions in yeast suggests an extended chromatin fiber with regional variation in compaction. J Biol Chem. 2008;283:34532–34540. doi: 10.1074/jbc.M806479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro M, Potoyan DA, Wolynes PG, Onuchic JN. Anomalous diffusion, spatial coherence, and viscoelasticity from the energy landscape of human chromosomes. Proc Natl Acad Sci U S A. 2018;115:7753–7758. doi: 10.1073/pnas.1806297115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion V, Kalck V, Seeber A, Schleker T, Gasser SM. Cohesin and the nucleolus constrain the mobility of spontaneous repair foci. EMBO Rep. 2013;14:984–991. doi: 10.1038/embor.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman LB, Fraser P. Transcription factories: genetic programming in three dimensions. Curr Opin Genet Dev. 2012;22:110–114. doi: 10.1016/j.gde.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Eltsov M, Maclellan KM, Maeshima K, Frangakis AS, Dubochet J. Analysis of cryo-electron microscopy images does not support the existence of 30-nm chromatin fibers in mitotic chromosomes in situ. Proc Natl Acad Sci U S A. 2008;105:19732–19737. doi: 10.1073/pnas.0810057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerborn A, Cook PR. Why the activity of a gene depends on its neighbors. Trends Genet. 2015;31:483–490. doi: 10.1016/j.tig.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Finch JT, Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976;73:1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn EH, Pegoraro G, Brandão HB, Valton AL, Oomen ME, Dekker J, Mirny L, Misteli T. Extensive heterogeneity and intrinsic variation in spatial genome organization. Cell. 2019;176:1502–1515. doi: 10.1016/j.cell.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussner E, Strauss M, Djuric U, Li R, Ahmed K, Hart M, et al. Open and closed domains in the mouse genome are configured as 10-nm chromatin fibres. EMBO Rep. 2012;13:992–996. doi: 10.1038/embor.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germier T, Kocanova S, Walther N, Bancaud A, Shaban HA, Sellou H, Politi AZ, Ellenberg J, Gallardo F, Bystricky K. Real-time imaging of a single gene reveals transcription-initiated local confinement. Biophys J. 2017;113:1383–1394. doi: 10.1016/j.bpj.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari A, van de Corput MP, Thongjuea S, van Cappellen WA, van Ijcken W, van Haren J, Soler E, Eick D, Lenhard B, Grosveld FG. In vivo live imaging of RNA polymerase II transcription factories in primary cells. Genes Dev. 2013;27:767–777. doi: 10.1101/gad.216200.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorisch SM, Wachsmuth M, Toth KF, Lichter P, Rippe K. Histone acetylation increases chromatin accessibility. J Cell Sci. 2005;118:5825–5834. doi: 10.1242/jcs.02689. [DOI] [PubMed] [Google Scholar]
- Gu B, Swigut T, Spencley A, Bauer MR, Chung M, Meyer T, Wysocka J. Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science. 2018;359:1050–1055. doi: 10.1126/science.aao3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjoul H, Mathon J, Ranchon H, Goiffon I, Mozziconacci J, Albert B, Carrivain P, Victor JM, Gadal O, Bystricky K, Bancaud A. High-throughput chromatin motion tracking in living yeast reveals the flexibility of the fiber throughout the genome. Genome Res. 2013;23:1829–1838. doi: 10.1101/gr.157008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun P, Laroche T, Shimada K, Furrer P, Gasser SM. Chromosome dynamics in the yeast interphase nucleus. Science. 2001;294:2181–2186. doi: 10.1126/science.1065366. [DOI] [PubMed] [Google Scholar]
- Hihara S, Pack CG, Kaizu K, Tani T, Hanafusa T, Nozaki T, et al. Local nucleosome dynamics facilitate chromatin accessibility in living mammalian cells. Cell Rep. 2012;2:1645–1656. doi: 10.1016/j.celrep.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Hsieh TH, Weiner A, Lajoie B, Dekker J, Friedman N, Rando OJ. Mapping nucleosome resolution chromosome folding in yeast by micro-C. Cell. 2015;162:108–119. doi: 10.1016/j.cell.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Zhang B. Theory of active chromatin remodeling. Phys Rev Lett. 2019;123:208102. doi: 10.1103/PhysRevLett.123.208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalashnikova AA, Porter-Goff ME, Muthurajan UM, Luger K, Hansen JC. The role of the nucleosome acidic patch in modulating higher order chromatin structure. J R Soc Interface. 2013;10:20121022. doi: 10.1098/rsif.2012.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Sugaya K, Cook PR. The transcription cycle of RNA polymerase II in living cells. J Cell Biol. 2002;159:777–782. doi: 10.1083/jcb.200206019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi V, Ruan Q, Plutz M, Belmont AS, Gratton E. Chromatin dynamics in interphase cells revealed by tracking in a two-photon excitation microscope. Biophys J. 2005;89:4275–4285. doi: 10.1529/biophysj.105.066670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Yu D, Hansen AS, Ganguly S, Liu R, Heckert A, Darzacq X, Zhou Q. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature. 2018;558:318–323. doi: 10.1038/s41586-018-0174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JS, Zhang Y, Dudko OK, Murre C. 3D trajectories adopted by coding and regulatory DNA elements: first-passage times for genomic interactions. Cell. 2014;158:339–352. doi: 10.1016/j.cell.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy LB. An iterative technique for the rectification of observed distributions. Astron J. 1974;79:745–754. [Google Scholar]
- Ma H, Tu LC, Chung YC, Naseri A, Grunwald D, Zhang S, Pederson T. Cell cycle- and genomic distance-dependent dynamics of a discrete chromosomal region. J Cell Biol. 2019;218:1467–1477. doi: 10.1083/jcb.201807162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S, Takizawa Y, Ishimaru M, Sugita Y, Sekine S, Nakayama JI, et al. Structural basis of heterochromatin formation by human HP1. Mol Cell. 2018;69:385–397 e8. doi: 10.1016/j.molcel.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Maeshima K, Ide S, Hibino K, Sasai M. Liquid-like behavior of chromatin. Curr Opin Genet Dev. 2016;37:36–45. doi: 10.1016/j.gde.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Maeshima K, Rogge R, Tamura S, Joti Y, Hikima T, Szerlong H, et al. Nucleosomal arrays self-assemble into supramolecular globular structures lacking 30-nm fibers. EMBO J. 2016;35:1115–1132. doi: 10.15252/embj.201592660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima K, Ide S, Babokhov M. Dynamic chromatin organization without the 30-nm fiber. Curr Opin Cell Biol. 2019;58:95–104. doi: 10.1016/j.ceb.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Straight A, Marko JF, Swedlow J, Dernburg A, Belmont A, Murray AW, Agard DA, Sedat JW. Interphase chromosomes undergo constrained diffusional motion in living cells. Curr Biol. 1997;7:930–939. doi: 10.1016/s0960-9822(06)00412-x. [DOI] [PubMed] [Google Scholar]
- Nagashima R, Hibino K, Ashwin SS, Babokhov M, Fujishiro S, Imai R, Nozaki T, Tamura S, Tani T, Kimura H, Shribak M, Kanemaki MT, Sasai M, Maeshima K. Single nucleosome imaging reveals loose genome chromatin networks via active RNA polymerase II. J Cell Biol. 2019;218:1511–1530. doi: 10.1083/jcb.201811090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino Y, Eltsov M, Joti Y, Ito K, Takata H, Takahashi Y, et al. Human mitotic chromosomes consist predominantly of irregularly folded nucleosome fibres without a 30-nm chromatin structure. EMBO J. 2012;31:1644–1653. doi: 10.1038/emboj.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki T, Imai R, Tanbo M, Nagashima R, Tamura S, Tani T, Joti Y, Tomita M, Hibino K, Kanemaki MT, Wendt KS, Okada Y, Nagai T, Maeshima K. Dynamic organization of chromatin domains revealed by super-resolution live-cell imaging. Mol Cell. 2017;67:282–293.e7. doi: 10.1016/j.molcel.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Sugawara T, Yamamoto T. Simultaneous live imaging of the transcription and nuclear position of specific genes. Nucleic Acids Res. 2015;43:e127. doi: 10.1093/nar/gkv624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Ando T, Priest DG, Kumar V, Yoshida Y, Taniguchi Y. Sub-nucleosomal genome structure reveals distinct nucleosome folding motifs. Cell. 2019;176:520–534.e25. doi: 10.1016/j.cell.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Ou HD, Phan S, Deerinck TJ, Thor A, Ellisman MH, O’Shea CC. ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science. 2017;357:eaag0025. doi: 10.1126/science.aag0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SSP, Huang SC, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon KR, Sanborn AL, Johnstone SE, Bascom GD, Bochkov ID, Huang X, Shamim MS, Shin J, Turner D, Ye Z, Omer AD, Robinson JT, Schlick T, Bernstein BE, Casellas R, Lander ES, Aiden EL. Cohesin loss eliminates all loop domains. Cell. 2017;171:305–320. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci MA, Manzo C, Garcia-Parajo MF, Lakadamyali M, Cosma MP. Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell. 2015;160:1145–1158. doi: 10.1016/j.cell.2015.01.054. [DOI] [PubMed] [Google Scholar]
- Richardson WH. Bayesian-based iterative method of image restoration. J Opt Soc Am. 1972;62:55–59. [Google Scholar]
- Risca VI, Denny SK, Straight AF, Greenleaf WJ. Variable chromatin structure revealed by in situ spatially correlated DNA cleavage mapping. Nature. 2017;541:237–241. doi: 10.1038/nature20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, Li CH, Guo YE, Day DS, Schuijers J, Vasile E, Malik S, Hnisz D, Lee TI, Cisse II, Roeder RG, Sharp PA, Chakraborty AK, Young RA. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361:eaar3958. doi: 10.1126/science.aar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn AL, Rao SS, Huang SC, Durand NC, Huntley MH, Jewett AI, et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci U S A. 2015;112:E6456–E6465. doi: 10.1073/pnas.1518552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer W, Abdennur N, Goloborodko A, Pekowska A, Fudenberg G, Loe-Mie Y, Fonseca NA, Huber W, Haering CH, Mirny L, Spitz F. Two independent modes of chromatin organization revealed by cohesin removal. Nature. 2017;551:51–56. doi: 10.1038/nature24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban HA, Barth R, Bystricky K. Formation of correlated chromatin domains at nanoscale dynamic resolution during transcription. Nucleic Acids Res. 2018;46:e77. doi: 10.1093/nar/gky269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban HA, Barth R, Bystricky K (2019) Nanoscale mapping of DNA dynamics in live human cells. bioRxiv. 10.1101/405969
- Shi G, Thirumalai D. Conformational heterogeneity in human interphase chromosome organization reconciles the FISH and Hi-C paradox. Nat Commun. 2019;10:3894. doi: 10.1038/s41467-019-11897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Liu L, Hyeon C, Thirumalai D. Interphase human chromosome exhibits out of equilibrium glassy dynamics. Nat Commun. 2018;9:3161. doi: 10.1038/s41467-018-05606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai S, Nozaki T, Maeshima K, Togashi Y. Dynamic nucleosome movement provides structural information of topological chromatin domains in living human cells. PLoS Comput Biol. 2016;12:e1005136. doi: 10.1371/journal.pcbi.1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Chen P, Sun D, Wang M, Dong L, Liang D, et al. Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science. 2014;344:376–380. doi: 10.1126/science.1251413. [DOI] [PubMed] [Google Scholar]
- Tan L, Xing D, Chang CH, Li H, Xie XS. Three-dimensional genome structures of single diploid human cells. Science. 2018;361:924–928. doi: 10.1126/science.aat5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, Belmont AS. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell. 2017;169:780–791. doi: 10.1016/j.cell.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Kuo J, Bae SC, Granick S. When Brownian diffusion is not Gaussian. Nat Mater. 2012;11:481–485. doi: 10.1038/nmat3308. [DOI] [PubMed] [Google Scholar]
- Winogradoff D, Echeverria I, Potoyan DA, Papoian GA. The acetylation landscape of the H4 histone tail: disentangling the interplay between the specific and cumulative effects. J Am Chem Soc. 2015;137:6245–6253. doi: 10.1021/jacs.5b00235. [DOI] [PubMed] [Google Scholar]
- Zidovska A, Weitz DA, Mitchison TJ. Micron-scale coherence in interphase chromatin dynamics. Proc Natl Acad Sci U S A. 2013;110:15555–15560. doi: 10.1073/pnas.1220313110. [DOI] [PMC free article] [PubMed] [Google Scholar]