Abstract
Evidence from randomized controlled trials (RCTs) suggests that coenzyme Q10 (CoQ10) can regulate adipokine levels to impact inflammation and oxidative stress in conditions of metabolic syndrome. Here, prominent electronic databases such as MEDLINE, Cochrane Library, and EMBASE were searched for eligible RCTs reporting on any correlation between adipokine levels and modulation of inflammation and oxidative stress in individuals with metabolic syndrome taking CoQ10. The risk of bias was assessed using the modified Black and Downs checklist, while the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool was used to evaluate the quality of evidence. Results from the current meta-analysis, involving 318 participants, showed that CoQ10 supplementation in individuals with metabolic syndrome increased adiponectin levels when compared to those on placebo (SMD: 1.44 [95% CI: −0.13, 3.00]; I2 = 96%, p < 0.00001). Moreover, CoQ10 supplementation significantly lowered inflammation markers in individuals with metabolic syndrome in comparison to those on placebo (SMD: −0.31 [95% CI: −0.54, −0.08]; I2 = 51%, p = 0.07). Such benefits with CoQ10 supplementation were related to its ameliorative effects on lipid peroxidation by reducing malondialdehyde levels, concomitant to improving glucose control and liver function. The overall findings suggest that optimal regulation of adipokine function is crucial for the beneficial effects of CoQ10 in improving metabolic health.
Keywords: coenzyme Q10, ubiquinone, metabolic syndrome, metabolic complications, non-alcoholic fatty liver disease, hypertension, adipokines, inflammation, oxidative stress, lipid peroxidation
1. Introduction
Metabolic diseases are acknowledged to greatly affect the quality of life to those affected in addition to be the leading cause of death worldwide [1,2]. Overnutrition and sedentary lifestyle are currently known to be some of the major factors driving the rapid rise in metabolic diseases [3,4], in both developed and developing countries [5,6]. In fact, the past few decades have seen a drastic increase in the number of overweight and obese individuals [7]. Of major concern is that obesity, together with other metabolic complications such as hypertension, type 2 diabetes (T2D), and non-alcoholic fatty liver disease (NAFLD) can significantly accelerate the risk of heart failure, leading to reduced lifespan of the general population [4,8,9]. Consequently, there has been a great interest in understanding the pathophysiological mechanisms associated with the development of metabolic complications such as T2D and NAFLD [10]. This is especially important to identify molecular mechanisms for therapeutic target to alleviate metabolic complications.
Complex pathological mechanisms have thus far been linked with the development and aggravation of metabolic syndrome. For instance, a state of dyslipidemia, which is characterized by abnormally elevated serum levels of triglycerides and cholesterol, has been shown to promote enhanced ectopic lipid accumulation and subsequent injury to various body organs [4,11,12]. Increased organ and arterial lipid accumulation can induce insulin resistance and cause endothelial dysfunction, mostly through exacerbating inflammation and oxidative stress. Indeed, elevated pro-inflammatory cytokines such as C-reactive protein (CRP), interleukin (IL)-6, including lipid peroxidation makers, such as malondialdehyde (MDA) levels, have been identified in patients with dyslipidemia [13,14,15]. Consequently, our group and others are scrutinizing literature on the therapeutic value of various compounds in protecting against metabolic diseases, especially their impact on adipose tissue function and ameliorative properties on inflammation and oxidative stress [16,17,18,19].
Coenzyme Q10 (CoQ10), also referred to as ubiquinone, is attracting much interest due to its envisaged health benefits [20,21]. Interestingly, CoQ10 is present in all eukaryotic cells, and it forms a crucial part of the electron transport chain. Notably, its deficiency is associated with the generation of oxidative stress and myocardial damage in the diabetic state [22]. Whereas, dietary intake of CoQ10 is directly linked with enhanced levels of ubiquinol-10 within the human body, which is further crucial for blocking lipid peroxidation by protecting against low-density lipoprotein oxidation in conditions on metabolic syndrome [23,24]. Beyond its impact on lipid peroxidation, published evidence from randomized clinical trials (RCTs) shows that CoQ10 remains important in ameliorating inflammation and improving adipokine function in conditions of metabolic syndrome [25,26,27]. Although other meta-analysis of RCTs has been conducted on the effects of CoQ10 on markers of inflammation or oxidative stress [28,29,30], including assessing adiponectin levels [31], such information remains limited for being too specific and does not collectively give a better picture on the influence of this quinone on conditions of metabolic syndrome. Therefore, this meta-analysis assesses essential evidence on the modulatory effects of CoQ10 on adipokine function, including its impact on markers of inflammation and lipid peroxidation in individuals with metabolic syndrome.
2. Results
2.1. Selection and Characteristic Features of Included RCTs
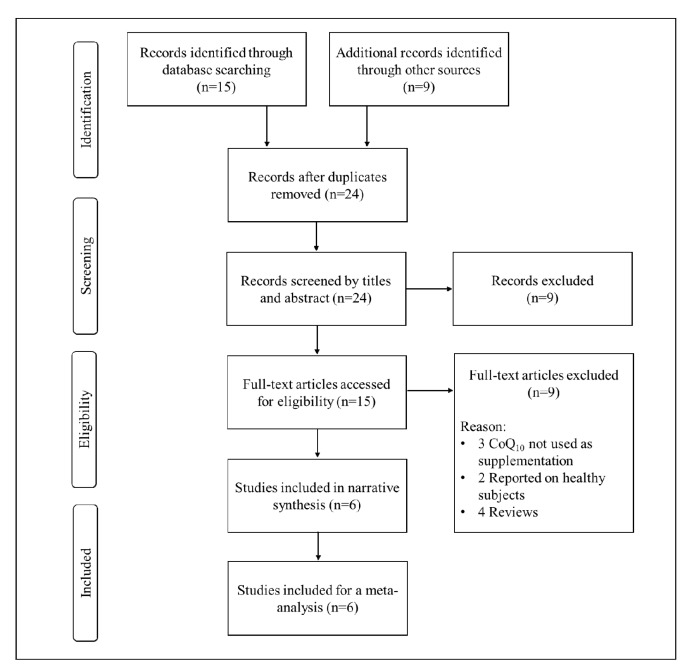
Approximately, 24 RCTs were retrieved from relevant online databases reporting on the impact of CoQ10 on adipokine levels in individuals with metabolic syndrome. Subsequently, six RCTs met the inclusion criteria as displayed in Figure 1. Other studies were excluded for reasons such as being out of scope or not reporting on primary findings. The RCTs reporting on the impact of CoQ10 on adipokine levels in individuals without the metabolic syndrome were excluded from the meta-analysis [32].
Figure 1.
An overview of flow diagram of included studies.
The general characteristic features of the included studies are summarized in Table 1. In brief, all included RCTs were published in peer-reviewed journals, each year between 2014 and 2018. Briefly, this study was composed of a total of 318 participants with an average age of 48 years, and the majority of these being female (73%). The majority of participants included in the current meta-analysis were those with T2D (n = 176), NAFLD (n = 82), and hypertension (n = 60). The prominent doses of CoQ10 used were 100 or 200 mg/daily for a minimum of four weeks, up to three months (Table 1).
Table 1.
Characteristic features of included studies and the reported impact of coenzyme Q10 (CoQ10) on adipokine function and inflammatory response.
| Study | Study Size | Male, n (%) | Age (Years) | CoQ10 Dosage and Duration | Main Findings |
|---|---|---|---|---|---|
| Farhangi et al. (2014) [25] | 41 Non-alcoholic fatty liver disease (NAFLD) patients | 31 (74) | 42.0 ± 10.8 | CoQ10 at 100 mg for 4 weeks | CoQ10 significantly reduced waist circumference and serum aspartate transaminase (AST) and total antioxidant capacity (TAC) concentrations compared to the placebo-treated group. In the stepwise multivariate linear regression model, change in serum fasting serum glucose was a significant predictor of changes in serum vaspin, chemerin, and pentraxin 3 |
| Moazen et al. (2015) [34] | 52 type 2 diabetic (T2D) patients | 28 (54) | 51.7 ± 7.3 | CoQ10 at 100 mg for 8 weeks | CoQ10 reduced malondialdehyde (MDA) levels; however, fasting blood glucose (FPG), glycated hemoglobin (HbA1c) and adiponectin levels showed no significant differences when compared to the placebo control |
| Nesami et al. (2015) [26] | 60 patients suffering from mild hypertension | 17 (28) | 48.8 ± 5.9 | CoQ10 at 100 mg for 12 weeks | CoQ10 was effective in decreasing some pro-inflammatory factors, such as IL-6 and C-reactive protein (CRP), and in increasing adiponectin levels |
| Farsi et al. (2016) [27] | 41 NAFLD patients | 28 (68) | Not reported | CoQ10 at 100 mg for 3 months | CoQ10 significantly reduced AST and gamma-glutamyl transpeptidase, CRP, tumor necrosis factor-alpha (TNF-α), and the grades of NAFLD. In addition, patients who received CoQ10 supplement had higher serum levels of adiponectin and considerable changes in serum leptin |
| Mehrdadi et al. (2017) [35] | 56 patients with T2D | 32 (57) | 47.0 ± 8 | CoQ10 at 200 mg/day for 12 weeks | CoQ10 reduced HbA1c, although interestingly, adipolin levels declined simultaneously. CoQ10 modulated glucose homeostasis, which was expected to be mediated by increasing adipolin. Similar mechanisms of action of CoQ10 and adipolin may justify lowering the effect of CoQ10 on adipolin. In addition, the possible anti-adipogenic effect of CoQ10 might explain the significant reduction in weight and waist circumference and hence the adipolin decrease |
| Gholami et al. (2018) [36] | 68 patients with T2D | Not reported | 48.8 ± 6.4 | CoQ10 at 200 mg/d for 8 weeks |
CoQ10 supplementation in women with T2D was effective in elevation of adiponectin and the adiponectin/leptin ratio (A/L), MDA and 8-isoprostane which could result in improving insulin resistance and modulating oxidative stress |
2.2. Risk of Bias and Quality of RCTs
The risk of bias and quality of six included studies was assessed by Kabelo Mokgalaboni (K.M.) and Vuyolwethu Mxinwa (V.M.), according to a modified Downs and Black’s guideline, as previously mentioned. The overall median score range of the included studies was 22 (19–24), with five of them rated excellent (21–24 scores) and one rated as good (19 scores). In addition, KM used the Cohen’s Kappa interrater to assess the degree of agreement in terms of quality assessment scores of the included studies [33]. The levels of agreement between the two reviewers (K.M. and V.M.) were scored as moderate to perfect. Furthermore, all included studies scored high in reporting bias 9 (9–10) out of ten possible scores (overall agreement 90%, Kappa = 0.89), external validity 2 (1–3) out of three possible scores (overall agreement 62%, Kappa = 0.52), internal validity 6 (5–6) out of seven possible scores, (overall agreement 79.6%, Kappa = 0.83) and selection bias with an average median scores of 5 (3–6) out of a six possible scores (overall agreement 80.95%, Kappa = 0.76).
2.3. Publication Bias
Publication bias was assessed using funnel plots for each measured outcome. Notably, visual inspection of funnel plots showed symmetry in all reported outcomes.
2.4. Data Synthesis
2.4.1. CoQ10 Supplementation Improved Adipokine Levels
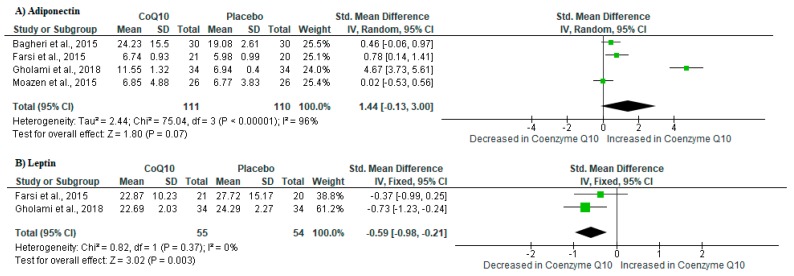
It is now well-established that adipose tissue functions as a vital organ for the secretion of adipose-derived factors or rather adipokines which play a key role in the regulation of inflammation [37]. In fact, one of these adipokines is adiponectin, an anti-inflammatory metabolite that has been shown to improve insulin sensitivity [38,39]. Interestingly, pooled estimates showed that CoQ10 supplementation in individuals with metabolic syndrome increased adiponectin levels when compared to those on placebo (SMD: 1.44 [95% CI: −0.13, 3.00]; I2 = 96%, p < 0.00001) (Figure 2A). On the other hand, leptin has been long identified as a pro-inflammatory metabolite that exacerbates inflammation in conditions of metabolic syndrome [40]. Remarkably, a meta-analysis of included studies revealed significant reduction of leptin levels in individuals on CoQ10 supplementation when compared to those on placebo (SMD: −0.59 [95% CI: −0.98, −0.21]; I2 = 0%, p = 0.37) (Figure 2B).
Figure 2.
The impact of coenzyme Q10 (CoQ10) supplementation on adipokine levels in individuals with metabolic syndrome. Briefly, the results showed that CoQ10 supplementation increased the levels of adiponectin (A) whilst reducing that of leptin (B) in included randomized controlled trials.
2.4.2. CoQ10 Supplementation Ameliorated Pro-Inflammatory Markers
Consistent with the dysregulation in adipokine levels, metabolic syndrome has been associated an aggravation in inflammation [38,39], and this state that is characterized by increased pro-inflammatory cytokines and acute phase reactants such as CRP, IL-6, and tumor necrosis factor alpha (TNF-α) [13,14]. Interestingly, pooled estimates of inflammation markers showed a significant decrease in individuals on CoQ10 supplements when compared to controls placebo (SMD: −0.31 [95% CI: −0.54, −0.08]; I2 = 51%, p = 0.07) (Figure 3).
Figure 3.
The impact of coenzyme Q10 (CoQ10) supplementation on markers of inflammation, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP) in individuals with metabolic syndrome.
2.4.3. CoQ10 Supplementation Reduced Markers of Lipid Peroxidation
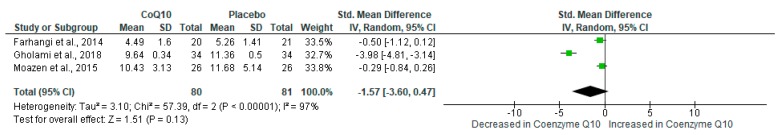
It is well-recognized that individuals with metabolic syndrome have increased levels of oxidative stress, especially lipid peroxidation [41]. The latter is also a relevant target of CoQ10 in light of its critical role as a lipophilic antioxidant [41]. Interestingly, CoQ10 supplementation reduced MDA levels, as a marker of lipid peroxidation, when compared to placebo (SMD: −1.57 [95% CI: −3.60, 0.47]; I2 = 97%, p < 0.00001) (Figure 4).
Figure 4.
The impact of coenzyme Q10 (CoQ10) supplementation on malondialdehyde (MDA) levels as a maker of lipid peroxidation in individuals with metabolic syndrome.
2.4.4. CoQ10 Supplementation Improved Blood Glucose Control
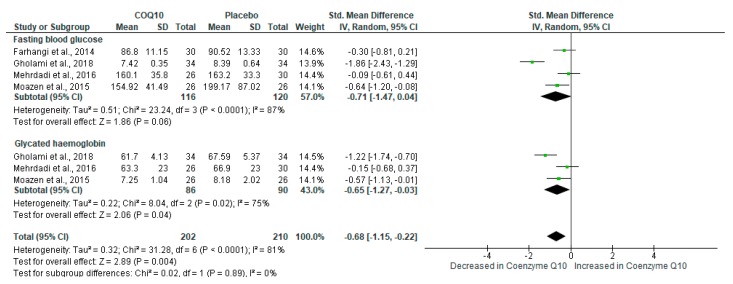
Abnormally raised blood glucose levels are a prominent feature of T2D, consistent with the development of metabolic syndrome [4,42]. Interestingly, the results showed that CoQ10 supplementation significantly improved glucose control in individuals with metabolic syndrome (SMD: −0.68 [95% CI: −1.15, −0.22]; I2 = 81%, p < 0.00001) (Figure 5). Despite substantial levels of heterogeneity, the test for subgroup differences showed no significant subgroup effect (p = 0.89).
Figure 5.
The impact of coenzyme Q10 (CoQ10) supplementation on basic metabolic markers such as fasting plasma glucose (FPG) levels and glycated hemoglobin (HbA1c).
2.4.5. CoQ10 Supplementation Did Not Impact Liver Function in Individuals with NAFLD
Briefly, NAFLD remains one of the major characteristic features of metabolic syndrome [43], and this condition is persistent with the rise in hepatic enzymes such as alanine transaminase and aspartate transaminase (AST). Here, pooled estimates showed a small effect size in the liver function among individuals on CoQ10 supplements versus those on placebo (SMD: 0.33 [95% CI: −0.94, 1.61]; I2 = 93%, p < 0.00001) (Figure 6). Despite substantial levels of heterogeneity, the test for subgroup differences showed no significant subgroup effect (p = 0.09). The overall summary of findings on the beneficial effects of CoQ10 supplementation in individuals with metabolic syndrome is provided in Table 2.
Figure 6.
The effect of coenzyme Q10 (CoQ10) supplementation on liver function measured using alanine transaminase (ALT) and aspartate transaminase (AST) in individuals with non-alcoholic fatty liver disease (NAFLD).
Table 2.
Summary of findings.
| Coenzyme Q10 (CoQ10) Supplementation Compared to Placebo | ||||||
|---|---|---|---|---|---|---|
| Patient or population: Adults (≥18 years of age) with Metabolic Syndrome Intervention: CoQ10 Supplementation Comparison: Individuals with Metabolic Syndrome Receiving Placebo | ||||||
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) |
№ of Participants (Studies) |
Certainty of the Evidence (GRADE) |
Comments | |
| Risk with Placebo | Risk with CoQ10 Supplementation | |||||
| Adipokine control measured using adiponectin levels |
- | The mean level in the intervention group was 1.44 higher (0.13 lower to 3.00 higher) |
- | 221 (4 RCT’s studies) |
⨁⨁⨁⨁ HIGH |
|
| Inflammation measured by C-reactive protein (CRP) levels |
- | The mean level in the intervention group was 0.57 lower (0.97 lower to 0.17 lower) |
101 (2 RCT’s studies) |
⨁⨁⨁⨁ HIGH |
||
| Oxidative stress measured by malondialdehyde (MDA) levels |
- | The mean level in the intervention group was 1.57 lower (3.60 lower to 0.47 higher) |
161 (3 RCT’s studies) |
⨁⨁⨁⨁ HIGH |
||
| Glucose control measured by glycated haemoglobin (Hb1Ac) levels |
- | The mean level in the intervention group was 0.65 lower (1.27 lower to 0.03 lower) |
176 (3 RCT’s studies) |
⨁⨁⨁⨁ HIGH |
||
| Liver function measured by aspartate transaminase (AST) levels |
- | The mean level in the intervention group was 0.66 lower (1.10 lower to 0.21 lower) |
82 (2 RCT’s studies) |
⨁⨁⨁⨁ HIGH |
||
* The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; OR: odds ratio; NE: not estimable GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
3. Discussion
Emerging research supports the beneficial effects of CoQ10 against metabolic complications [32,34,35,44,45]. Several published RCTs suggest that CoQ10 can regulate adipokine levels, including those of leptin and adiponectin, to impact metabolic function. As a prime example, Gholami et al. 2018 [36] reported that CoQ10 supplementation in women with T2D was effective in increasing the concentrations of adiponectin or the adiponectin/leptin ratio, including reducing MDA levels which could translate to improved insulin sensitivity and amelioration of oxidative stress. Although Mehrdadi and colleagues [35] recorded a decline in adipolin levels with CoQ10 supplementation, this dietary compound could improve glucose homeostasis by reducing HbA1c concentrations. Perhaps suggesting that the anti-adipogenic effects of CoQ10 [46] are important in improving glucose homeostasis though not affecting adipolin in these patients. Like adiponectin, adipolin can impact the metabolic syndrome by attenuating atherosclerosis and improving insulin sensitivity, as reviewed elsewhere [47]. In another RCT [34], CoQ10 could lower lipid peroxidation by reducing MDA levels, albeit not significantly affecting FPG, HbA1c or adiponectin levels in patients with T2D. Apparently, dose selection could explain such limitation since diabetic individuals were treated with a dose of 100 mg CoQ10, which was much lower than the other two RCTs [35,36], which showed beneficial effects with 200 mg dose of ubiquinone on modulating glucose metabolism.
Nevertheless, the overall pooled estimates in the current study showed that CoQ10 supplementation significantly increased adiponectin levels in individuals with metabolic syndrome, which is concomitant to improved glucose control, and reduced levels of leptin and pro-inflammatory markers such as IL-6, CRP, and TNF-α. Apparently, circulation levels of adipokines such as leptin and adiponectin can act as biomarkers to evaluate obesity-associated complications, including low-grade inflammation [48]. While leptin is considered a crucial link between the compromised metabolic state and exacerbation of inflammation [40], enhanced adiponectin levels remain essential for effective regulation of glucose and lipid metabolism to improve metabolic function [38]. Interestingly, certain pharmacological interventions such as stragaloside II and isoastragaloside I have been found to selectively enhance adiponectin secretion in primary adipocytes without any apparent effects on other adipokines [49]. Thus, making therapeutic regulation of adipokine levels, including associated pro-inflammatory responses, as explored in the current study, an interesting strategy to protect against the metabolic syndrome.
Beyond controlling hyperglycemia and makers of lipid peroxidation or inflammation in those with T2D, other RCTs support the beneficial effects of CoQ10 in patients with NAFLD. Briefly, Farsi and colleagues [27] demonstrated that CoQ10 supplementation in patients with NAFLD limits liver damage by lowering the actions of AST and gamma-glutamyl transpeptidase, in connection with reducing the levels of CRP and TNF-α. Interestingly, such findings are consistent with elevated serum levels of adiponectin in patients with NAFLD [27]. Although these benefits could be observed, pooled estimates showed that CoQ10 did not show a significant effect in improving liver function in those with NAFLD. A possible reason for not observing significant effects with CoQ10 supplementation could be the limited number of RCTs included in the current meta-analysis.
Looking at the impact of other adipokine markers, Farhangi and colleagues [25] demonstrated that CoQ10 could significantly reduce serum levels of AST and MDA when compared to the placebo group. Here, changes in serum FPG were a significant predictor of fluctuations in serum adipokines such as vaspin, chemerin and pentraxin-3. Although not widely explored as adiponectin and leptin, adequate regulation of serum levels of other adipokines such as vaspin, chemerin, and pentraxin 3 has been associated with improved insulin sensitivity and vascular function [50,51,52]. Alternatively, Nesami and co-workers [26] found CoQ10 supplementation to be effective in decreasing some pro-inflammatory factors like IL-6 and CRP, in association with increasing adiponectin levels in patients with mild hypertension. Thus, broadly indicating that by effectively regulating adipokine levels, CoQ10 can greatly impact metabolic health in connection to ameliorating inflammation and oxidative stress in patients with T2D, NAFLD, or hypertension, as increasingly discussed [28,29,30,53]. Therapeutic benefits of CoQ10 supplementation are essential important since its deficiency in the body or enhanced oxidation status has been linked with compromised metabolic function [24,54,55]. In fact, Gholami and colleagues [36] demonstrated that at the end of the intervention, CoQ10 supplementation significantly improved its serum levels, including that of adiponectin while reducing that of MDA in patients with T2D. Thus, inferring that increased intake of CoQ10 could lead to its enhanced endogenous levels, which are vital for the amelioration of oxidative stress, especially lipid peroxidation products. Also highlighting the importance of understanding the link between CoQ10 intake and its delivery in vivo, as discussed elsewhere [56].
4. Materials and Methods
The current meta-analysis was prepared in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines [57]. Subsequently, supplementary file S1 provides a PRISMA checklist for this meta-analysis.
4.1. Strategy to Search RCTs
The systematic search for eligible studies was conducted using electronic databases such as MEDLINE, Cochrane Library, and EMBASE from inception up to 29 February 2020 by two independent investigators (Phiwayinkosi V. Dludla, P.V.D.; and Tawanda M. Nyambuya, T.M.N.). In cases of disagreement, a third investigator (Bongani B. Nkambule, B.B.N.) was consulted for adjudication. The primary search was limited to RCTs, reporting the impact of CoQ10 supplementation on adipokine levels, glucose control, liver function, including makers of inflammation and oxidative stress in individuals with metabolic syndrome. Within the same cohort, individuals receiving placebo were used a comparative control. For an optimal search strategy, Medical Subject-Heading (MeSH) and text words such as “coenzyme Q10”, “metabolic syndrome”, “adipokine”, “inflammation”, and their matching synonyms and connected words or phrases were modified for each database used. In addition, a manual search was also performed through references of articles, abstracts, preprint platforms (to identify unpublished studies), which is essential to identify relevant evidence. There were no language restrictions applied in the search strategy, whilst EndNote version 10 (Clarivate Analytics, Philadelphia, USA) was used to manage the reference list and eliminate duplicates, as previously reported [58].
4.2. Inclusion and Exclusion Criteria
The current meta-analysis encompassed RCTs evaluating the impact of CoQ10 on adipokine levels in correlation with basic metabolic parameters in adults (>18 years) with metabolic syndrome. In brief, included RCTs were those that evaluating the use of CoQ10 as an intervention, contained the comparison group on placebo, and reported on adipokine levels in individuals with metabolic syndrome. Animal or in vitro studies were not included, while other exclusions included books, cohort or observational studies, letters, and case reports. Review articles were only scanned for RCTs. Furthermore, studies not reporting on the effects of CoQ10 on adipokine levels in patients without metabolic syndrome were excluded.
4.3. Data Extraction and Assessment of Quality
For data extraction, P.V.D. and T.M.N independently assessed all relevant articles and cautiously selected those that met the inclusion criteria. Any disagreements were resolved by referring a third investigator (B.B.N.). The primary outcome of the meta-analysis was to establish the impact of CoQ10 supplementation on adipokine levels in conditions of metabolic syndrome. Another significant objective was to understand whether the modulation of adipokine levels by CoQ10 supplementation related to improved inflammatory response and amelioration of oxidative stress markers. To achieve this, pertinent data items from each RCT included, such as name and year of publication, the country where the study was conducted, sample and gender distribution, in addition to CoQ10 dosage and intervention period were extracted. Moreover, the risk of bias was assessed by two investigators (V.M. and K.M.) using the modified Downs and Black checklist, which is suitable for both randomized and non-randomized studies [59,60]. Any differences were determined by referring the third investigator (T.M.N.). The same investigators were also tasked with evaluating the quality of evidence across the selected RCTs by using the Grading of Recommendations Assessment Development and Evaluation (GRADE) approach [61].
4.4. Statistical Analysis
Statistical analysis was performed using RevMan software (version 5.0; Cochrane Collaboration, Oxford, UK). Higgin’s I2 statistics was used to test for statistical heterogeneity [62]. The random or fixed-effects models were used depending on the levels of statistical heterogeneity, as previously discussed [63]. Cohen’s d method was employed to interpret effect sizes, whereby a standardized mean difference of 0.2, 0.5. and 0.8 were regarded as small, medium, and large effect, respectively [64]. This method makes use of standardized mean difference (SMD) and not actual mean differences to measure effect sizes. Briefly, SMD is calculated as per the Cochrane guidelines, where it is recommended when estimating the effect size of pooled studies which report a similar outcome using a variety scales (SI units) [65]. While a p-value <0.05 was considered statistically significant. The inter-rater reliability was evaluated for both the encompassed studies and risk of bias by means of Cohen’s kappa. For example, a kappa value of <0.00 was considered as poor strength of agreement, 0.00–0.20 as minor agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as reasonable agreement, 0.61–0.80 as significant agreement, and 0.81–1.00 as perfect agreement [33].
5. Study Limitations
The limitation of the current meta-analysis includes the low number of RCTs included, which significantly reduces the confidence in the level of the. reported findings, especially those on its beneficial properties against NAFLD. Thus, suggesting that additional RCTs are necessary to improve our understanding of the correlation between adipokine regulation and metabolic abnormalities in patients with NAFLD. Another limitation, most included RCTs did not detect serum levels of CoQ10 post-intervention, which is a crucial aspect to assess to determine its bioavailability and its therapeutic potential.
6. Conclusions
Coenzyme Q10 (CoQ10) is a dietary compound with established antioxidant properties and is increasingly investigated for its ameliorative effects against various metabolic complications. Evidence from other RCTs has been synthesized to inform on the impact of CoQ10 on inflammation, oxidative stress, or insulin resistance [28,29,30,66,67,68], including its modulation of adiponectin [31]. However, the current meta-analysis provides novel findings on the broad effects of this compound on the regulation of various adipokines on diverse metabolic complications. Indeed, summarized evidence suggests that optimal regulation of adipokine markers such as adiponectin and leptin is crucial for the beneficial effects of CoQ10 in attenuating oxidative stress and inflammation in conditions on metabolic syndrome.
Acknowledgments
B.B.N. is a University of KwaZulu-Natal Developing Research Innovation, Localisation and Leadership in South Africa (DRILL) fellow. DRILL, is a NIH D43 grant (D43TW010131) awarded to UKZN in 2015 to support a research training and induction programme for early career academics. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the funders.
Abbreviations
| ALT | Aspartate transaminase |
| CVD | Cardiovascular disease |
| CoQ10 | Coenzyme Q10 |
| CRP | C-reactive protein |
| FPG | Fasting plasma glucose |
| GRADE | Grading of Recommendations Assessment Development and Evaluation |
| HbA1c | Glycated hemoglobin |
| IL | Interleukin |
| MDA | Malondialdehyde |
| NAFLD | Non-alcoholic fatty liver disease |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analysis |
| RCT | Randomized controlled trials |
| SMD | Standardized mean difference |
| T2D | Type 2 diabetes |
| TNF-α | Tumor necrosis factor alpha |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/9/3247/s1, Supplementary file S1: Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) checklist.
Author Contributions
Conceptualization, P.V.D. and L.T.; methodology, T.M.N., V.M., K.M. and B.B.N.; validation, T.M.N. and B.B.N.; formal analysis, T.M.N., V.M., K.M., and B.B.N.; resources, R.J., C.J.F.M., J.L. and L.T.; writing—original draft preparation, P.V.D. and L.T.; writing—review and editing, P.V.D., P.O., S.S., F.M., I.C., T.M.N., B.B.N., R.J., S.E.M.-M., C.J.F.M., J.L. and L.T.; funding acquisition, P.V.D., R.J., C.J.F.M., J.L. and L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by baseline funding from the Biomedical Research and Innovation Platform of the South African Medical Research Council (SAMRC) and the National Research Foundation (Grant number: 117829). P.V.D. was partially supported as a Post-Doctoral Fellow by funding from the SAMRC through its division of Research Capacity Development under the Intra-Mural Postdoctoral Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the SAMRC or the funders.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Trikkalinou A., Papazafiropoulou A.K., Melidonis A. Type 2 diabetes and quality of life. World J. Diabetes. 2017;8:120–129. doi: 10.4239/wjd.v8.i4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization The Top 10 Causes of Death. [(accessed on 2 February 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 3.Pitsavos C., Panagiotakos D., Weinem M., Stefanadis C. Diet, exercise and the metabolic syndrome. Rev. Diabet. Stud. 2006;3:118–126. doi: 10.1900/RDS.2006.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy S.M. Overnutrition, ectopic lipid and the metabolic syndrome. J. Investig. Med. 2016;64:1082–1086. doi: 10.1136/jim-2016-000155. [DOI] [PubMed] [Google Scholar]
- 5.Saklayen M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Noncommunicable Diseases Country Profiles 2014. [(accessed on 3 February 2020)]; Available online: https://www.who.int/nmh/publications/ncd-profiles-2014/en/
- 7.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A., Marczak L., Mokdad A.H., Moradi-Lakeh M., Naghavi M., et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heather L.C., Clarke K. Metabolism, hypoxia and the diabetic heart. J. Mol. Cell. Cardiol. 2011;50:598–605. doi: 10.1016/j.yjmcc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Mosterd A., Hoes A.W. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bril F., Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: A call to action. Diabetes Care. 2017;40:419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 11.Das H., Banik S. Prevalence of dyslipidemia among the diabetic patients in southern Bangladesh: A cross-sectional study. Diabetes Metab. Syndr. 2019;13:252–257. doi: 10.1016/j.dsx.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Schofield J.D., Liu Y., Rao-Balakrishna P., Malik R.A., Soran H. Diabetes dyslipidemia. Diabetes Ther. 2016;7:203–219. doi: 10.1007/s13300-016-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waheed P., Naveed A.K., Farooq F. Levels of inflammatory markers and their correlation with dyslipidemia in diabetics. J. Coll. Physicians Surg. Pak. 2009;19:207–210. [PubMed] [Google Scholar]
- 14.Aulinas A., Ramírez M.J., Barahona M.J., Valassi E., Resmini E., Mato E., Santos A., Crespo I., Bell O., Surrallés J., et al. Dyslipidemia and chronic inflammation markers are correlated with telomere length shortening in Cushing’s syndrome. PLoS ONE. 2015;10:e0120185. doi: 10.1371/journal.pone.0120185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao V., Kiran R. Evaluation of correlation between oxidative stress and abnormal lipid profile in coronary artery disease. J. Cardiovasc. Dis. Res. 2011;2:57–60. doi: 10.4103/0975-3583.78598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dludla P.V., Nkambule B.B., Jack B., Mkandla Z., Mutize T., Silvestri S., Orlando P., Tiano L., Louw J., Mazibuko-Mbeje S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients. 2018;11:23. doi: 10.3390/nu11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dludla P.V., Mazibuko-Mbeje S.E., Nyambuya T.M., Mxinwa V., Tiano L., Marcheggiani F., Cirilli I., Louw J., Nkambule B.B. The beneficial effects of N-acetyl cysteine (NAC) against obesity associated complications: A systematic review of pre-clinical studies. Pharmacol. Res. 2019;146:104332. doi: 10.1016/j.phrs.2019.104332. [DOI] [PubMed] [Google Scholar]
- 18.Calvano A., Izuora K., Oh E.C., Ebersole J.L., Lyons T.J., Basu A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019;10:6227–6243. doi: 10.1039/C9FO01426H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salehi B., Mishra A.P., Nigam M., Sener B., Kilic M., Sharifi-Rad M., Fokou P.V.T., Martins N., Sharifi-Rad J. Resveratrol: A double-edged sword in health benefits. Biomedicines. 2018;6:91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu H., Guo M., Chai H., Wang W.T., Gao Z.Y., Shi D.Z. Effects of coenzyme Q10 on statin-induced myopathy: An updated meta-analysis of randomized controlled trials. JAHA. 2018;7:e009835. doi: 10.1161/JAHA.118.009835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez-Mariscal F.M., Yubero-Serrano E.M., Villalba J.M., Lopez-Miranda J. Coenzyme Q(10): From bench to clinic in aging diseases, a translational review. Crit. Rev. Food Sci. Nutr. 2019;59:2240–2257. doi: 10.1080/10408398.2018.1442316. [DOI] [PubMed] [Google Scholar]
- 22.Shen Q., Pierce J.D. Supplementation of coenzyme Q10 among patients with type 2 diabetes mellitus. Healthcare. 2015;3:296–309. doi: 10.3390/healthcare3020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littarru G.P., Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol. Biotechnol. 2007;37:31–37. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 24.Orlando P., Chellan N., Louw J., Tiano L., Cirilli I., Dludla P., Joubert E., Muller C.J.F. Aspalathin-rich green rooibos extract lowers LDL-cholesterol and oxidative status in high-fat diet-induced diabetic vervet monkeys. Molecules. 2019;24:1713. doi: 10.3390/molecules24091713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farhangi M.A., Alipour B., Jafarvand E., Khoshbaten M. Oral coenzyme Q10 supplementation in patients with nonalcoholic fatty liver disease: Effects on serum vaspin, chemerin, pentraxin 3, insulin resistance and oxidative stress. Arch. Med. Res. 2014;45:589–595. doi: 10.1016/j.arcmed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Bagheri Nesami N., Mozaffari-Khosravi H., Najarzadeh A., Salehifar E. The effect of coenzyme Q10 supplementation on pro-inflammatory factors and adiponectin in mildly hypertensive patients: A randomized, double-blind, placebo-controlled trial. Int. J. Vitam. Nutr. Res. 2015;85:156–164. doi: 10.1024/0300-9831/a000234. [DOI] [PubMed] [Google Scholar]
- 27.Farsi F., Mohammadshahi M., Alavinejad P., Rezazadeh A., Zarei M., Engali K.A. Functions of coenzyme Q10 supplementation on liver enzymes, markers of systemic inflammation, and adipokines in patients affected by nonalcoholic fatty liver disease: A double-blind, placebo-controlled, randomized clinical trial. J. Am. Coll. Nutr. 2016;35:346–353. doi: 10.1080/07315724.2015.1021057. [DOI] [PubMed] [Google Scholar]
- 28.Jorat M.V., Tabrizi R., Kolahdooz F., Akbari M., Salami M., Heydari S.T., Asemi Z. The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 2019;27:233–248. doi: 10.1007/s10787-019-00572-x. [DOI] [PubMed] [Google Scholar]
- 29.Farsi F., Heshmati J., Keshtkar A., Irandoost P., Alamdari N.M., Akbari A., Janani L., Morshedzadeh N., Vafa M. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;148:104290. doi: 10.1016/j.phrs.2019.104290. [DOI] [PubMed] [Google Scholar]
- 30.Zhai J., Bo Y., Lu Y., Liu C., Zhang L. Effects of coenzyme Q10 on markers of inflammation: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0170172. doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazary-Vannani A., Ghaedi E., Salamat S., Sayyaf A., Varkaneh H.K., Mohammadi H., Djalali M. Effects of coenzyme Q10 supplementation on serum adiponectin levels: A systematic review and meta-analysis of randomized controlled trials. Curr. Drug ther. 2020;15:3–11. doi: 10.2174/1574885514666190308162322. [DOI] [Google Scholar]
- 32.Gökbel H., Gergerlioğlu H.S., Okudan N., Gül I., Büyükbaş S., Belviranli M. Effects of coenzyme Q10 supplementation on plasma adiponectin, interleukin-6, and tumor necrosis factor-alpha levels in men. J. Med. Food. 2010;13:216–218. doi: 10.1089/jmf.2008.0310. [DOI] [PubMed] [Google Scholar]
- 33.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 34.Moazen M., Mazloom Z., Ahmadi A., Dabbaghmanesh M., Roosta S. Effect of coenzyme Q10 on glycaemic control, oxidative stress and adiponectin in type 2 diabetes. J. Pak. Med. Assoc. 2015;65:404–408. [PubMed] [Google Scholar]
- 35.Mehrdadi P., Kolahdouz Mohammadi R., Alipoor E., Eshraghian M.R., Esteghamati A., Hosseinzadeh-Attar M.J. The Effect of coenzyme Q10 supplementation on circulating levels of novel adipokine adipolin/CTRP12 in overweight and obese patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2017;125:156–162. doi: 10.1055/s-0042-110570. [DOI] [PubMed] [Google Scholar]
- 36.Gholami M., Zarei P., Sadeghi Sedeh B., Rafiei F., Khosrowbeygi A. Effects of coenzyme Q10 supplementation on serum values of adiponectin, leptin, 8-isoprostane and malondialdehyde in women with type 2 diabetes. Gynecol. Endocrinol. 2018;34:1059–1063. doi: 10.1080/09513590.2018.1481944. [DOI] [PubMed] [Google Scholar]
- 37.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achari A.E., Jain S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017;18:1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahima R.S., Osei S.Y. Adipokines in obesity. Front. Horm. Res. 2008;36:182–197. doi: 10.1159/000115365. [DOI] [PubMed] [Google Scholar]
- 40.Iikuni N., Lam Q.L., Lu L., Matarese G., La Cava A. Leptin and Inflammation. Curr. Immunol. Rev. 2008;4:70–79. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakhtiari A., Hajian-Tilaki K., Omidvar S., Nasiri Amiri F. Association of lipid peroxidation and antioxidant status with metabolic syndrome in Iranian healthy elderly women. Biomed. Rep. 2017;7:331–336. doi: 10.3892/br.2017.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Goblan A.S., Al-Alfi M.A., Khan M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014;7:587–591. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paschos P., Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 44.Moradi M., Haghighatdoost F., Feizi A., Larijani B., Azadbakht L. Effect of coenzyme Q10 supplementation on diabetes biomarkers: A systematic review and meta-analysis of randomized controlled clinical trials. Arch. Iran Med. 2016;19:588–596. [PubMed] [Google Scholar]
- 45.Dludla P.V., Nyambuya T.M., Orlando P., Silvestri S., Mxinwa V., Mokgalaboni K., Nkambule B.B., Louw J., Muller C.J.F., Tiano L. The impact of coenzyme Q10 on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrinol. Diab. Metab. 2020;3:e00118. doi: 10.1002/edm2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z., Huo J., Ding X., Yang M., Li L., Dai J., Hosoe K., Kubo H., Mori M., Higuchi K. Coenzyme Q10 improves lipid metabolism and ameliorates obesity by regulating CaMKII-mediated PDE4 inhibition. Sci. Rep. 2017;7:8253. doi: 10.1038/s41598-017-08899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sargolzaei J., Chamani E., Kazemi T., Fallah S., Soori H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clin. Biochem. 2018;54:1–10. doi: 10.1016/j.clinbiochem.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Inadera H. The usefulness of circulating adipokine levels for the assessment of obesity-related health problems. Int. J. Med. Sci. 2008;5:248–262. doi: 10.7150/ijms.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu A., Wang H., Hoo R.L., Sweeney G., Vanhoutte P.M., Wang Y., Wu D., Chu W., Qin G., Lam K.S. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology. 2009;150:625–633. doi: 10.1210/en.2008-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada J. Vaspin: A novel serpin with insulin-sensitizing effects. Expert Opin. Investig. Drugs. 2008;17:327–333. doi: 10.1517/13543784.17.3.327. [DOI] [PubMed] [Google Scholar]
- 51.Peri G., Introna M., Corradi D., Iacuitti G., Signorini S., Avanzini F., Pizzetti F., Maggioni A.P., Moccetti T., Metra M., et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.CIR.102.6.636. [DOI] [PubMed] [Google Scholar]
- 52.Zanetti M., Bosutti A., Ferreira C., Vinci P., Biolo G., Fonda M., Valente M., Cattin L., Guarnieri G., Barazzoni R. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: Evidence for association with atherogenic lipid profile. Clin. Exp. Med. 2009;9:243–248. doi: 10.1007/s10238-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 53.Rosenfeldt F.L., Haas S.J., Krum H., Hadj A., Ng K., Leong J.Y., Watts G.F. Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J. Hum. Hypertens. 2007;21:297–306. doi: 10.1038/sj.jhh.1002138. [DOI] [PubMed] [Google Scholar]
- 54.Quinzii C.M., DiMauro S., Hirano M. Human coenzyme Q10 deficiency. Neurochem. Res. 2007;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman S., Clarke C.F., Hirano M. 176th ENMC International Workshop: Diagnosis and treatment of coenzyme Q(1)(0) deficiency. Neuromuscul. Disord. 2012;. 22:76–86. doi: 10.1016/j.nmd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaki N.M. Strategies for oral delivery and mitochondrial targeting of CoQ10. Drug Deliv. 2016;23:1868–1881. doi: 10.3109/10717544.2014.993747. [DOI] [PubMed] [Google Scholar]
- 57.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 58.Mahlangu T., Dludla P.V., Nyambuya T.M., Mxinwa V., Mazibuko-Mbeje S.E., Cirilli I., Marcheggiani F., Tiano L., Louw J., Nkambule B.B. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine. 2019;126:154892. doi: 10.1016/j.cyto.2019.154892. [DOI] [PubMed] [Google Scholar]
- 59.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connor S.R., Tully M.A., Ryan B., Bradley J.M., Baxter G.D., McDonough S.M. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: A comparison study. BMC Res. Notes. 2015;8:224. doi: 10.1186/s13104-015-1181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 62.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 63.Schroll J.B., Moustgaard R., Gotzsche P.C. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med. Res. Methodol. 2011;11:22. doi: 10.1186/1471-2288-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan G.M., Feinn R. Using effect size-or why the p value is not enough. J. Grad. Med. Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higgins J.P.T., Wells G.A. Cochrane Handbook for Systematic Reviews of Interventions. Volume 4. John Wiley & Sons; 2011. [(accessed on 20 February 2020)]. (The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England) Available online: https://www.radioterapiaitalia.it/wp-content/uploads/2017/01/cochrane-handbook-for-systematic-reviews-of-interventions.pdf. [Google Scholar]
- 66.Mazidi M., Kengne A.P., Banach M., Lipid. Blood Pressure Meta-analysis Collaboration, G Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2018;128:130–136. doi: 10.1016/j.phrs.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Sangsefidi Z.S., Yaghoubi F., Hajiahmadi S., Hosseinzadeh M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020;8:1766–1776. doi: 10.1002/fsn3.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang H., Chi H., Liao D., Zou Y. Effects of coenzyme Q10 on cardiovascular and metabolic biomarkers in overweight and obese patients with type 2 diabetes mellitus: A pooled analysis. Diabetes Metab. Syndr. Obes. 2018;11:875–886. doi: 10.2147/DMSO.S184301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.