Abstract
Quercus mongolica (QM)—a member of the Fagaceae family—has been used as traditional medicine in Korea, China and Mongolia as a treatment for inflammation of oral, genital or anal mucosa and for external inflammation of skin. To treat acne vulgaris (AV), we evaluated the inhibition of inflammatory cytokines (IL-6 and IL-8) of QM leaf extract (QML) and its main compound, pedunculagin (PD) in vitro and 5α-reductase inhibitory activity by western blotting. As results, QML and PD showed potent NO production inhibitory activity compared with the positive control (PC), NG-monomethyl-L-arginine (L-NMMA). QML and PD was also showed the decreases of IL-6 and IL-8 compared with the PC, EGCG and exhibited potent 5α-reductase type 1 inhibitory activities compared with the PC, dutasteride.
Keywords: Quercus mongolica, pedunculagin, anti-acne vulgaris
1. Introduction
Keratinocytes play a role in skin barrier function. When these keratinocytes are exposed to external stimuli like air pollution, stress and ultraviolet radiations it can induce mediator of inflammation, nitric oxide (NO) and inflammatory response such as inflammatory cytokines. Hence, NO and the inflammatory cytokines may cause acne vulgaris (AV) [1,2,3,4].
NO is a free radical that damages to cell, tissue and DNA. And NO can form peroxynitrite that is a potent cytotoxic agent damage to molecules in cells, including DNA. These damages evoke many chronic inflammatory diseases such as psoriasis and pathogenesis of AV. It also was reported that nitrate and nitrite levels which are an index of NO production were high in acne patients [5,6].
Keratinocytes produce many confirmed cytokines containing interleukin-1 (IL-1), IL-6, IL-7, IL-8, IL-10, IL-12 [4,7]. Particularly, IL-6 induce differentiation of Th17 cells. Activation of the Th17 cell produce IL-17 that the cytokine contributes significantly to the inflammatory response in AV. In addition, IL-17 found in acne lesions [8,9,10]. And IL-8 is among CXC chemokines induced AV by attracting neutrophils to pilosebaceous unit [11,12].
AV is a chronic inflammatory disease caused by excessive sebum production [13,14]. Sebum production relates to androgen. Particularly, dihydrotestosterone (DHT) more potent triggers AV approximately 5–10 times by increasing excessive sebum production. And increased DHT may act on infundibular keratinocytes leading to abnormal hyperkeratinization. If abnormal hyperkeratinization occur in keratinocytes, sebum production increases excessively in sebaceous gland [15,16,17]. 5α-reductase converts DHT from testosterone. Thus, inhibition of 5α-reductase activity improves AV by reducing DHT [18]. Several studies reported that natural sources and its active components, mainly polyphenols improve AV by inhibition of 5α-reductase activity [19,20,21,22,23].
Quercus mongolica (QM) is a deciduous oak that, has been used in oriental traditional medicine in north east Asia. It was used in Korea, China and Japan for the treatment of the inflammation of oral, genital or anal mucosa and externally for the inflammation of skin [24,25]. Leaves of QM contains flavonoids, tannins, triterpenoids and phenols. These components has been reported to possess anti-oxidative, anti-inflammatory, antitumor, anti-microbial, anti-allergic and anti-fungal activities [26]. In particular, pedunculagin (PD) which isolated QM had an effect on potent inhibitory activities of chemokine and cytokine in keratinocytes. PD has also been reported to enhance the regeneration of keratinocytes [27,28].
In spite of many studies conducted on the various effects of QM, this study was conducted to find the improving agent of AV from QM leaf extract (QML) and PD.
2. Results
2.1. Isolation of PD
QM leaves (4.69 kg) pulverized and extracted with 80% acetone for 72 h, at room temperature to obtain QML (1.4 kg). Repeated column chromatography of QML and its subfraction (45.75 g) using Sephadex LH 20 gel to yield PD (1 g). The structure of the PD was identified by analysis of 1H-NMR and 13C-NMR spectra and comparison with reference [29].
1H NMR (600 MHz, Acetone-d6+D2O): δ α-glucose 5.40 (1/2H, d, J = 3.6 Hz, H-1α), 5.02 (1/2H, dd, J = 3.6, 10.0 Hz, H-2α), 5.42 (1/2H, t, J = 10.0 Hz, H-3α), 5.03 (1/2H, t, J = 10.0 Hz, H-4α), 4.56 (1/2H, ddd, J = 1.5, 6.6, 10.0 Hz, H-5α), 5.21 (1/2H, dd, J =6.9, 13.0 Hz, H-6aα), 3.85 (1/2H, dd, J =1.5, 13.0 Hz, H-6bα), β-glucose 5.02 (1/2H, d, J =7.8 Hz, H-1β), 4.82 (1/2H, dd, J =8.0, 9.0 Hz, H-2β), 5.18 (1/2H, dd, J =9.0, 10.0 Hz, H-3β), 5.04 (1/2H, t, J =10.0 Hz, H-4β), 4.17 (1/2H, ddd, J =0.9, 6.6, 10.0 Hz, H-5β), 5.25 (1/2H, dd, J =6.3, 13.0 Hz, H-6aβ), 3.78 (1/2H, dd, J =0.9, 13.0 Hz, H-6bβ).
13C NMR (600 MHz, Acetone-d6+D2O): δ 63.08 (G-6α), 63.10 (G-6β), 66.69 (HHDP-5α), 69.12 (G-4β), 69.47 (G-4α), 71.78 (HHDP-4β), 75.05 (HHDP-4α), 75.33 (HHDP-5β), 77.13 (HHDP-2α), 77.64 (HHDP-3α), 77.9 (HHDP-3β), 78.4 (HHDP-2β), 90.98 (G-1α), 94.63 (G-1β), 106.80 (HHDP-6α), 106.82 (HHDP-6β), 106.99 (HHDP-3′), 107.04 (HHDP-3), 107.11 (C-1), 107.61 (C-1′), 107.62 (C-1′’), 113.90 (C-2′’), 114.27 (C-2′), 115.32 (C-2), 125.14 (HHDP-2), 125.21 (HHDP-2′), 125.60 (HHDP-5), 125.65 (HHDP-5′), 125.8 (C-5′’), 125.82 (C-5′), 135.62 (C-5), 135.86 (C-4′’), 135.88 (C-4′), 143.78 (C-4), 143.79 (HHDP-4), 143.87 (HHDP-4′), 143.88 (HHDP-6), 143.94 (HHDP-6′), 144.57(C-3′), 144.59 (C-3′’), 144.70 (C-3), 167.79–169.25 (-COO).
2.2. Inhibitory Activity on NO Production
Inhibitory activity on NO production of QML and PC was measured to assess the anti-inflammatory activities in RAW 246.7 cells. QML (IC50 = 1.45 ± 0.25 μg/mL) showed potent anti-inflammatory activities compared with the positive control (PC), NG-monomethyl-L-arginine (L-NMMA) (IC50 = 0.55 ± 0.49 μg/mL). PD (IC50 = 53.52 ± 9.34 µM) adequately reduced NO production compared to L-NMMA (IC50 = 14.81 ± 12.76 μg/mL) (Table 1).
Table 1.
IC50 values of Quercus mongolica leaf extract (QML) and pedunculagin (PD) on inhibitory activity of nitric oxide (NO) production.
| Samples | NO Production IC50 (μg/mL) | NO Production IC50 (µM) |
|---|---|---|
| QML | 1.45 ± 0.25 a | – |
| PD | – | 53.52 ± 9.34 b |
| L-NMMA | 0.55 ± 0.19 b | 14.81 ± 12.76 a |
Values represent means ± standard deviation (SD) of three determinations. a-b: in the same columns are significantly different (p < 0.05).
2.3. Cytotoxic Activity
Before assessing improvement effects on anti-AV, MTT assay was measured to assess the cytotoxic activity of QML and PD on RAW 264.7 cells and HaCaT cells. The cytotoxic activity of QML and PD was not observed at various concentrations (12.5, 25, 50 and 100 μg/mL or μM) (data not shown).
2.4. Inhibitory Activity on Cytokine Production
The LPS (1 μg/mL) causing the inflammation treated in HaCaT cells to evaluate the inhibitory effects of IL-6, IL-8 production. After exposure to LPS, the inhibitory activity on IL-6, IL-8 production of QML and PD was measured to assess the anti- inflammatory activities. The IL-6 concentration was decreased in the sample-treated groups. QML (IC50 = 9.37 ± 1.50 μg/mL) showed potent anti-inflammatory activities compared with the PC, EGCG (IC50 = 2.98 ± 1.47 μg/mL). PD (IC50 = 6.59 ± 1.66 µM) appeared stronger anti-inflammatory activities than EGCG (IC50 = 6.68 ± 1.86 μg/mL) (Table 2).
Table 2.
IC50 values of QML and PD against inhibitory activity on IL-6, IL-8 production.
| Samples | IL-6 IC50 (μg/mL) |
IL-6 IC50 (µM) |
IL-8 IC50 (μg/mL) |
IL-8 IC50 (µM) |
|---|---|---|---|---|
| QML | 9.37 ± 1.50 a | – | 6.38 ± 2.58 a | – |
| PD | – | 6.59 ± 1.66 b | – | 0.09 ± 0.41 b |
| EGCG | 2.98 ± 1.47 b | 6.68 ± 1.86 a | 0.74 ± 0.09 b | 0.56 ± 0.52 a |
Values represent means ± standard deviation (SD) of three determinations. a-b: in the same columns are significantly different (p < 0.05).
The IL-8 concentration was decreased in the sample-treated groups. QML (IC50 = 6.38 ± 2.58 μg/mL) showed potent anti-inflammatory activities compared with the PC, EGCG (IC50 = 0.74 ± 0.09 μg/mL). PD (IC50 = 0.09 ± 0.41 µM) appeared stronger anti-inflammatory activities than EGCG (IC50 = 0.56 ± 0.52 μg/mL) (Table 2).
2.5. 5α-Reductase Inhibitory Activity
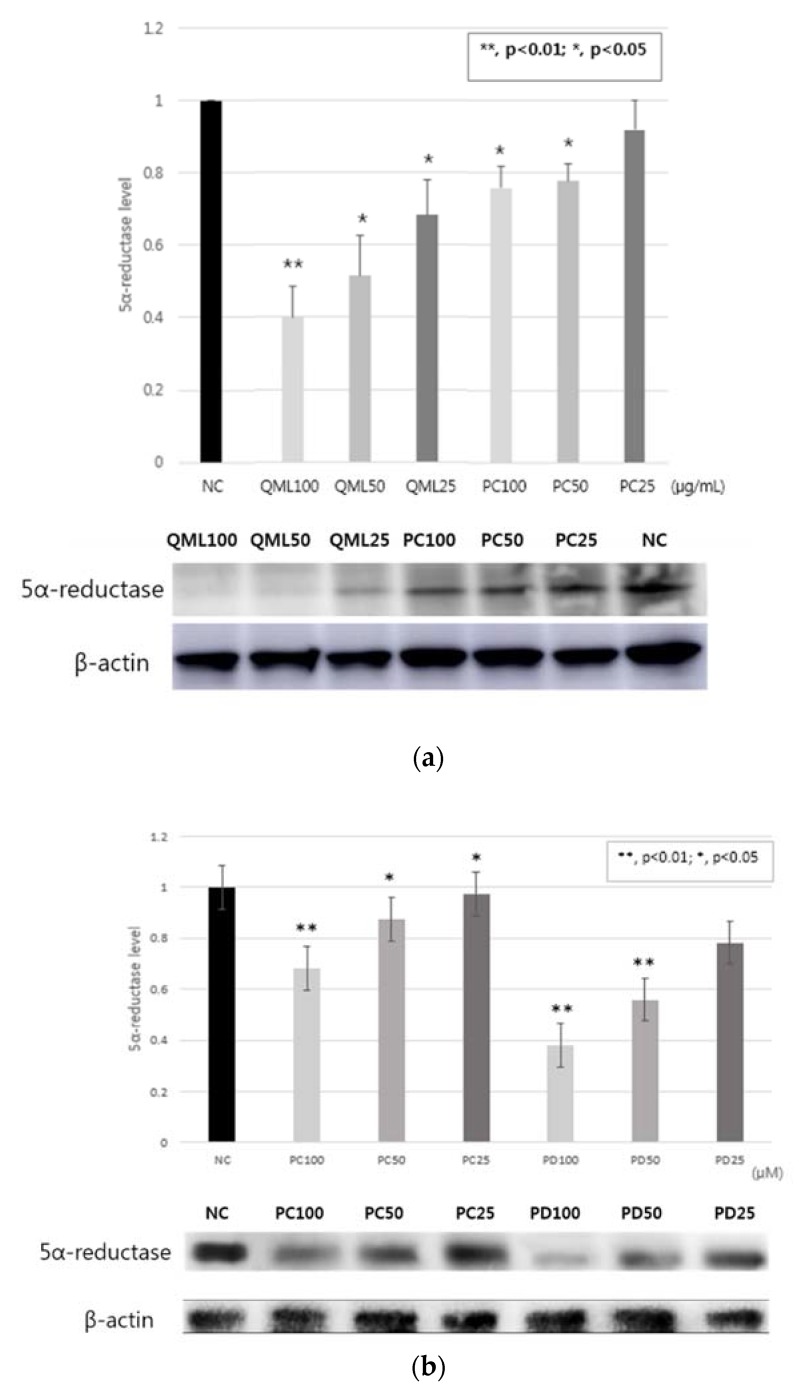
Western blotting conducted to evaluate 5α-reductase type 1inhibitory activity in HaCaT cell. 5α-reductase type 1 inhibitory activity of QML showed potent activities compared with the PC, dutasteride. (Figure 1a). And 5α-reductase type 1 inhibitory activity of PD showed great activities compared with the PC (Figure 1b).
Figure 1.
5α-reductase type 1 inhibitory activity in HaCaT cell. (a) QML; (b) PD. Data are expressed as the means ± standard deviation (SD) of three determinations. * p < 0.05; ** p < 0.01, compared with normal control. NC: normal control; PC: positive control.
3. Discussion
Inflammation triggers all types of AV. Hence, inhibition of inflammation is pathway central to anti-acne. NO, a relative free radical, is synthesized by inducible NO synthase (iNOS). NO production in acne patients can be damage to skin and cause AV [5,30,31]. In this study, QML and PD showed potent anti-inflammatory activity that QML and PD have an effect anti-acne. QML (IC50 = 1.45 ± 0.25 μg/mL) showed potent inhibitory activity on NO production compared with the PC, L-NMMA (IC50 = 0.55 ± 0.19 μg/mL). Also, PD (IC50 = 53.52 ± 9.34 µM) showed potent inhibitory activity on NO production compared with the PC, L-NMMA (IC50 = 14.81 ± 12.76 μg/mL). Thus, QML and PD have an effect on anti-AV result from inhibitory activity on NO production.
Activation of the Th17 cell that induced IL-6 secrete IL-17A and IL-17F which main effector cytokines in acne lesions. IL-17A and IL-17F activate neutrophils and can target different cell types such as keratinocytes, monocytes, fibroblasts. Therefore, IL-17A and IL-17F lead to epidermal hyperplasia on keratinocytes. In addition, IL-17A and IL-17F stimulate keratinocytes and activate vascular inflammation [8,9,32,33]. Production of IL-8 encourages destruction of keratinocytes by TLR2 and TLR4 signal. And IL-8 performs initiation of inflammatory cytokines due to production of reactive oxygen species (ROS). ROS damages to enzyme, DNA and keratinocytes In addition, ROS stimulates production of inflammatory cytokines [2,34,35]. In this study, QML and PD showed more potent anti-inflammatory activity on cytokine production. In IL-6, QML (IC50 = 9.37 ± 1.50 μg/mL) showed more potent anti-inflammatory activities compared with the PC, EGCG (IC50 = 2.98 ± 1.47 μg/mL). PD (IC50 = 6.59 ± 1.66 µM) displayed stronger anti-inflammatory activities than EGCG (IC50 = 6.68 ± 1.86 μg/mL). In IL-8, QML (IC50 = 6.38 ± 2.58 μg/mL) showed more potent anti-inflammatory activities compared with the PC, EGCG (IC50 = 0.74 ± 0.09 μg/mL). PD (IC50 = 0.09 ± 0.41 µM) displayed stronger anti-inflammatory activities than EGCG (IC50 = 0.56 ± 0.52 μg/mL). Finally, QML and PD have an effect on anti-AV result from anti-inflammatory activity on cytokine production.
5α-reductase has isoenzymes which 5α-reductase type 1 and type 2. In particular, 5α-reductase type 1 is related to sebum production and causes of AV. 5α-reductase type 1 has activity in sebaceous gland. Hence, sebum production is associated with DHT which changes from testosterone by 5α-reductase type 1. Therefore, 5α-reductase type 1 inhibitory activity decreases DHT that decreases sebum production and AV [18,36,37]. In this study, QML and PD showed great 5α-reductase type 1 inhibitory activity compare with the PC. Hence, these results showed QML and PD reduced AV.
4. Materials and Methods
4.1. Plant Material
The leaves of QM were collected from Yeoju Eco Park, Yeoju, Republic of Korea (GPS coordinates: 37.346648, 127.494519) in July 2018. The plant was identified by Dr. Sung Sik Kim (Korea National Arboretum). A voucher specimen was sited at the herbarium of the College of Pharmacy, Chung-Ang University.
4.2. Chemical and Reagents
Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS) and trypsin were purchased from Welgene (Gyeongsan, Republic of Korea). Streptomycin-penicillin was purchased from Gibco (NY, USA). Calcium-free DMEM, superscriptTM Ⅳ first-stand synthesis system and dream taq Green PCR Mix were purchased from Thermo Fisher Scientific (MA, USA). Sodium dodecyl sulfide (SDS), dithiothreitol (DTT), agarose, lipopolysaccharide (LPS), phosphate-buffered saline (PBS), 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and Griess reagent (0.1% naphthylethylenediamine and 1% sulfanilamide in 5% H3PO4 solution), NG-Methyl-L-arginine acetate salt (L-NMMA) and thiazolyl blue tetrazolium bromide (MTT) and Dutasteride were purchased from Sigma Aldrich (St. Louis, USA). Reagent set B, cytokine IL-6, IL-8 ELISA sets used for immunoassay were purchased from BD Biosciences (NJ, USA). The primary and secondary antibodies for 5α-reductase type 1 were purchased form Abcam (Cambridge, UK). PVDF membrane and Mini-protean precast gels and were purchased form Bio-Rad (Hercules, CA, USA). ECL detection reagent (GE Healthcare Life Science, NJ, USA).
4.3. Cell Culture
RAW 264.7 cells were purchased from the Korean Cell Line Bank. HaCaT cells (Human, Adult, low Calcium, High Temperature, epithelial keratinocyte cell line) were purchased form the Dermatology of Chung-Ang university hospital. These cells were grown at 37 °C in a humidified atmosphere (approximately 5% CO2) in a DMEM medium containing 10% FBS, 100 IU/mL penicillin G and 100 mg/mL streptomycin.
4.4. MTT Assay
Before the biologic assay, the cytotoxicity was measured by the mitochondrial-dependent reduction of MTT to formazan. After cells were seeded in a 96-well plate and incubated for 6 h, the cells were treated with the samples (12.5, 25, 50 and 100 μg/mL or μM). The cells were incubated for an additional 24 h and the medium was replaced with phosphate buffered saline (PBS) containing 0.5 mg/mL MTT and the incubation continued for a further 4 h at 37 °C. Then the PBS was removed, and the MTT-formazan was dissolved in 100 μL of dimethyl sulfide. The extent of the reduction of MTT to formazan within the cells was quantified by measuring the absorbance at 540 nm using an ELISA reader. The cytotoxicity was calculated as cell viability (%) = sample O.D. / blank O.D. × 100.
4.5. Measurement of Inhibitory Activity on NO Production
RAW 264.7 macrophage cells were seeded in a 96-well plate and incubated for 6 h at 37 °C in humidified atmosphere (approximately 5% CO2). The cells were incubated in a serum free medium containing each sample and 1 μg/mL LPS. After incubating for an additional 20 h, the NO contents were evaluated by Griess assay. After getting supernatant from the cells treated with the samples, the Griess reagent was added. L-NMMA was used as a positive control. Inhibitory activity on NO production was calculated as the rate of inhibition (%) = 100 − (sample O.D. − blank O.D.) / (control O.D. − blank O.D.) × 100. IC50 values were defined as the concentration that inhibit 50% of NO production.
4.6. Measurement of Inhibitory Activity on Cytokine Production
HaCaT cells were seeded in a 96-well plate and incubated for 6 h at 37 °C in humidified atmosphere (approximately 5% CO2). The cells were incubated in a medium containing 1 μg/mL LPS. After incubating for an additional 24 h, the supernatant from the cells were treated with the samples used for the assay using ELISA kit. Cytokine contents were quantified by measuring the absorbance at 450 nm using an ELISA reader.
4.7. Western Blot Assay
HaCaT cells (3 × 105 cells/mL) were pre-incubated for 16 h and then treated QML and PD. After incubation for 24 h, the cells washed with PBS and the cell lysates were prepared with RIPA buffer. The cell lysates were centrifuged at 13,000 × rpm for 15 min at 4 °C. The cell lysates were subjected to electrophoresis in SDS-polyacrylamide gels (10%) and the separated proteins were transferred on to a PVDF membrane. The membrane was blocked with blocking buffer for 60 min at room temperature. Then the membrane was probed with primary antibody for 5α-reductase for overnight at 4 °C. After washing, the blots were incubated with secondary antibody for 1 h at room temperature. The bands were visualized by LAS-4000 luminescent image analyzer (GE Healthcare Life Science, NJ, USA) using ECL detection reagent.
5. Conclusions
In this study, QML and PD showed potent anti-inflammatory activity. In additional QML and PD showed good 5α-reductase inhibitory activity. Hence, these results showed that QML and PD may have an anti-AV effect.
Acknowledgments
This study was carried out with the support of the ‘R&D Program for Forest Science Technology (Project No. 2017037A00–1919-BA01)’ provided by the Korea Forest Service (Korea Forestry Promotion Institute).
Author Contributions
Conceptualization, M.K. and M.W.L.; methodology, M.K. and I.H.H.; software, M.K., J.Y., D.H.P., E.K.L., M.J.K. and I.H.H.; formal analysis, M.K. and I.H.H.; investigation, M.K. and I.H.H.; resources, M.K., D.H.P and M.W.L.; writing—original draft preparation, M.K. and I.H.H.; writing—review and editing, M.K. and M.W.L.; supervision, M.W.L.; project administration, M.W.L.; funding acquisition, M.W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ‘R&D Program for Forest Science Technology (Project No. 2017037A00–1919-BA01)’ provided by the Korea Forest Service (Korea Forestry Promotion Institute).
Conflicts of Interest
The authors declare that there is no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Qin M., Landriscina A., Rosen J.M., Wei G., Kao S., Olcott W., Agak G.W., Paz K.B., Bonventre J., Clendaniel A., et al. Nitric Oxide-Releasing Nanoparticles Prevent Propionibacterium acnes-Induced Inflammation by Both Clearing the Organism and Inhibiting Microbial Stimulation of the Innate Immune Response. J. Investig. Dermatol. 2015;135:2723–2731. doi: 10.1038/jid.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grange P.A., Chereau C., Raingeaud J., Nicco C., Weill B., Dupin N., Batteux F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009;5:e1000527. doi: 10.1371/journal.ppat.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt N., Gans E.H. Tretinoin: A Review of Its Anti-inflammatory Properties in the Treatment of Acne. J. Clin. Aesthet. Dermatol. 2011;4:22–29. [PMC free article] [PubMed] [Google Scholar]
- 4.Farrar M.D., Ingham E. Acne: Inflammation. Clin. Dermatol. 2004;22:380–384. doi: 10.1016/j.clindermatol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Sarici G., Cinar S., Armutcu F., Altinyazar C., Koca R., Tekin N.S. Oxidative stress in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010;24:763–767. doi: 10.1111/j.1468-3083.2009.03505.x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shobaili H.A., Alzolibani A.A., Al Robaee A.A., Meki A.R., Rasheed Z. Biochemical markers of oxidative and nitrosative stress in acne vulgaris: Correlation with disease activity. J. Clin. Lab. Anal. 2013;27:45–52. doi: 10.1002/jcla.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grone A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002;88:1–12. doi: 10.1016/S0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 8.Kohda F., Koga T., Uchi H., Urabe K., Furue M. Histamine-induced IL-6 and IL-8 production are differentially modulated by IFN-γ and IL-4 in human keratinocytes. J. Dermatol. Sci. 2002;28:34–41. doi: 10.1016/S0923-1811(01)00147-5. [DOI] [PubMed] [Google Scholar]
- 9.Kelhala H.L., Palatsi R., Fyhrquist N., Lehtimaki S., Vayrynen J.P., Kallioinen M., Kubin M.E., Greco D., Tasanen K., Alenius H., et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE. 2014;9:e105238. doi: 10.1371/journal.pone.0105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agak G.W., Qin M., Nobe J., Kim M.H., Krutzik S.R., Tristan G.R., Elashoff D., Garban H.J., Kim J. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J. Investig. Dermatol. 2014;134:366–373. doi: 10.1038/jid.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takada K., Komine-Aizawa S., Hirohata N., Trinh Q.D., Nishina A., Kimura H., Hayakawa S. Poly I:C induces collective migration of HaCaT keratinocytes via IL-8. BMC Immunol. 2017;18:19. doi: 10.1186/s12865-017-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.Y., Lee W.R., Kim K.H., An H.J., Chang Y.C., Han S.M., Park Y.Y., Pak S.C., Park K.K. Effects of bee venom against Propionibacterium acnes-induced inflammation in human keratinocytes and monocytes. Int. J. Mol. Med. 2015;35:1651–1656. doi: 10.3892/ijmm.2015.2180. [DOI] [PubMed] [Google Scholar]
- 13.Tan J.K., Gold L.F.S., Alexis A.F., Harper J.C. Current concepts in acne pathogenesis: Pathways to inflammation. Semin. Cutan. Med. Surg. 2018;37:S60–S62. doi: 10.12788/j.sder2018.024. [DOI] [PubMed] [Google Scholar]
- 14.Melnik B.C. p53: Key conductor of all anti-acne therapies. J. Transl. Med. 2017;15:195. doi: 10.1186/s12967-017-1297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurokawa I., Danby F.W., Ju Q., Wang X., Xiang L.F., Xia L., Chen W., Nagy I., Picardo M., Suh D.H., et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009;18:821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 16.Barros B., Thiboutot D. Hormonal therapies for acne. Clin. Dermatol. 2017;35:168–172. doi: 10.1016/j.clindermatol.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Thiboutot D. Acne: Hormonal concepts and therapy. Clin. Dermatol. 2004;22:419–428. doi: 10.1016/j.clindermatol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Lakshmi C. Hormone therapy in acne. Indian J. Dermatol. Venereol. Leprol. 2013;79:322–337. doi: 10.4103/0378-6323.110765. [DOI] [PubMed] [Google Scholar]
- 19.Koseki J., Matsumoto T., Matsubara Y., Tsuchiya K., Mizuhara Y., Sekiguchi K., Nishimura H., Watanabe J., Kaneko A., Hattori T., et al. Inhibition of Rat 5alpha-Reductase Activity and Testosterone-Induced Sebum Synthesis in Hamster Sebocytes by an Extract of Quercus acutissima Cortex. Evid. Based Complement. Alternat. Med. 2015;2015:853846. doi: 10.1155/2015/853846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiipakka R.A., Zhang H.-Z., Dai W., Dai Q., Liao S. Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochem. Pharmacol. 2002;63:1165–1176. doi: 10.1016/S0006-2952(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 21.Mahmood T., Akhtar N., Khan B.A., Khan H.M., Saeed T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosn. J. Basic Med. Sci. 2010;10:260–264. doi: 10.17305/bjbms.2010.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon J.Y., Kwon H.H., Min S.U., Thiboutot D.M., Suh D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013;133:429–440. doi: 10.1038/jid.2012.292. [DOI] [PubMed] [Google Scholar]
- 23.Yin J., Hwang I.H., Lee M.W. Anti-acne vulgaris effect including skin barrier improvement and 5alpha-reductase inhibition by tellimagrandin I from Carpinus tschonoskii. BMC Complement. Altern. Med. 2019;19:323. doi: 10.1186/s12906-019-2734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamedov N., Gardner Z., Craker L.E. Medicinal Plants Used in Russia and Central Asia for the Treatment of Selected Skin Conditions. J. Herbs Spices Med. Plants. 2005;11:191–222. doi: 10.1300/J044v11n01_07. [DOI] [Google Scholar]
- 25.Zhou Y., Jin M., Zhou W., Li G. CHEMICAL CONSTITUENTS OF THE STEM BARKS OF Quercus mongolica. Chem. Nat. Compd. 2018;54:973–974. doi: 10.1007/s10600-018-2525-6. [DOI] [Google Scholar]
- 26.Eo H.J., Park Y., Kang J.T., Park G.H. Anti-inflammatory Effect of Branches Extracts from Quercus mongolica in LPS-induced RAW264. 7 Cells. Korean J. Plant Res. 2019;32:698–704. doi: 10.7732/kjpr.2019.32.6.698. [DOI] [Google Scholar]
- 27.Kim H.H., Kim D.H., Oh M.H., Park K.J., Heo J.H., Lee M.W. Inhibition of matrix metalloproteinase-1 and type-I procollagen expression by phenolic compounds isolated from the leaves of Quercus mongolica in ultraviolet-irradiated human fibroblast cells. Arch. Pharm. Res. 2015;38:11–17. doi: 10.1007/s12272-014-0329-1. [DOI] [PubMed] [Google Scholar]
- 28.Yin J., Kim H.H., Hwang I.H., Kim D.H., Lee M.W. Anti-Inflammatory Effects of Phenolic Compounds Isolated from Quercus Mongolica Fisch. ex Ledeb. on UVB-Irradiated Human Skin Cells. Molecules. 2019;24:3094. doi: 10.3390/molecules24173094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanbabaee K., Grosser M. An efficient total synthesis of pedunculagin by using a twofold intramolecular double esterification strategy. Eur. J. Org. Chem. 2003;2003:2128–2131. doi: 10.1002/ejoc.200300006. [DOI] [Google Scholar]
- 30.Schairer D.O., Chouake J.S., Nosanchuk J.D., Friedman A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012;3:271–279. doi: 10.4161/viru.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aslam I., Fleischer A., Feldman S. Emerging drugs for the treatment of acne. Expert Opin. Emerg. Drugs. 2015;20:91–101. doi: 10.1517/14728214.2015.990373. [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Yu X., Wu C., Jin H. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int. J. Med. Sci. 2017;14:1002–1007. doi: 10.7150/ijms.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skroza N., Proietti I., Bernardini N., Aquila E., Balduzzi V., La Viola G., Mambrin A., Muscianese M., Tolino E., Zuber S., et al. Il-17 and Its Role in Psoriasis, Hidradenitis Suppurativa And Acne. Intern. Med. Care. 2017;1 doi: 10.15761/imc.1000111. [DOI] [Google Scholar]
- 34.Nagy I., Pivarcsi A., Koreck A., Szell M., Urban E., Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J. Investig. Dermatol. 2005;124:931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- 35.Basak P.Y., Gultekin F., Kilinc I. The role of the antioxidative defense system in papulopustular acne. J. Dermatol. 2001;28:123–127. doi: 10.1111/j.1346-8138.2001.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 36.Bissonnette R., Risch J.E., McElwee K.J., Marchessault P., Bolduc C., Nigen S., Maari C. Changes in serum free testosterone, sleep patterns, and 5-alpha-reductase type I activity influence changes in sebum excretion in female subjects. Skin Res. Technol. 2015;21:47–53. doi: 10.1111/srt.12155. [DOI] [PubMed] [Google Scholar]
- 37.Leyden J., Bergfeld W., Drake L., Dunlap F., Goldman M.P., Gottlieb A.B., Heffernan M.P., Hickman J.G., Hordinsky M., Jarrett M., et al. A systemic type I 5 alpha-reductase inhibitor is ineffective in the treatment of acne vulgaris. J. Am. Acad. Dermatol. 2004;50:443–447. doi: 10.1016/j.jaad.2003.07.021. [DOI] [PubMed] [Google Scholar]